SUMMARY
Histoplasmosis is arguably the most common fungal respiratory infection worldwide, with hundreds of thousands of new infections occurring annually in the United States alone. The infection can progress in the lung or disseminate to visceral organs and can be difficult to treat with antifungal drugs. Histoplasma, the causative agent of the disease, is a pathogenic fungus that causes life-threatening lung infections and is globally distributed. The fungus has the ability to germinate from conidia into either hyphal (mold) or yeast form, depending on the environmental temperature. This transition also regulates virulence. Histoplasma and histoplasmosis have been classified as being of emergent importance, and in 2022, the World Health Organization included Histoplasma as 1 of the 19 most concerning human fungal pathogens. In this review, we synthesize the current understanding of the ecological niche, evolutionary history, and virulence strategies of Histoplasma. We also describe general patterns of the symptomatology and epidemiology of histoplasmosis. We underscore areas where research is sorely needed and highlight research avenues that have been productive.
KEYWORDS: Histoplasma, virulence, mycoses
INTRODUCTION
Fungal diseases are a growing threat to human health and agricultural productivity. The last 40 years have seen an increase in the prevalence of fungal infections (1, 2). Annually, close to one billion people are affected by mycoses (3–5). Over 11 million of these infections are life-threatening, and approximately 1 million of them are fatal (2, 4). Among acquired immunodeficiency syndrome (AIDS) patients, fungal infections have been estimated to cause more than 700,000 deaths per year (6). The burden (mortality, incidence, and prevalence) of human mycoses is similar to tuberculosis and is three times larger than the burden of malaria (4, 7). The incidence of fungal disease is also increasing over time, and modeling efforts suggest that the importance of fungal disease will increase over the next century (8–10). Other facets of the epidemiology of human fungal disease are also concerning. First, the geographic range of these diseases has expanded in the last 50 years, which suggests either changing environmental conditions and/or rapid adaptation to new niches (5, 11–13). Second, novel fungal pathogens have been documented globally, suggesting there are factors supporting emerging diseases or new routes of exposure (14–17). Finally, and arguably of most concern, strains resistant to antifungals are now found ubiquitously, and in some regions, very commonly (18–22). In 2022, the World Health Organization (WHO) introduced, for the first time, a fungal pathogens list addressing 19 groups of human fungal pathogens with significant risks of mortality or morbidity (23). This list (WHO Fungal Priority Pathogens List, WHO FPPL) sets an action plan to combat the most pressing fungal pathogens at the moment (24, 25). Histoplasma, the focus of this review, is one of the fungi on the list.
DIMORPHIC FUNGI
Histoplasma is a genus of dimorphic fungi. Members of this group have the ability to convert from a saprophytic mold state (which produces conidia, the biologically relevant infectious particle) to a parasitic yeast phase (spherule in the case of Coccidioides), with each morphology associated with temperature shifts from ~22°C to 37°C (26–28). The mold (or conidia) to yeast/spherule transition is a highly regulated process that is thought to be required for virulence (29–31). There are six genera of dimorphic fungi: Histoplasma, Blastomyces, Coccidioides, Paracoccidioides, Sporothrix schenckii, and Talaromyces marneffei (formerly known as Penicillium marneffei) (32). All these pathogens are ascomycetes. Notably, the dimorphic fungi are not a monophyletic group. Blastomyces, Paracoccidioides, Coccidioides, and Histoplasma all belong to the Onygenales, while Sporothrix and Talaromyces belong to other fungal orders, which suggests that the ability to transition to pathogenic yeast has evolved multiple times in the ascomycete phylogeny. Besides the “true dimorphic fungi,” other pathogens can also show phenotypic plasticity and adopt different morphologies, a characteristic that can be critical to causing disease. Some examples of these polymorphic changes include white to opaque switching and filamentation in Candida albicans (33–35) and titan cell formation in Cryptococcus neoformans (36, 37). Since the morphological transition is not regulated by temperature and different morphologies can coexist in the same environmental conditions, none of these pathogens are considered true dimorphic fungi (34).
All the dimorphic fungi, with the exception of Talaromyces, are primary human pathogens capable of causing disease regardless of the immune status of the host. Other fungal pathogens (e.g., Candida, Cryptococcus, or Aspergillus) are considered opportunistic pathogens because they mainly cause disease in immunosuppressed patients. Several of the pathogens (Coccidioides, Histoplasma, Blastomyces, and Paracoccidioides) cause an infection that progresses in the lung or disseminates to visceral organs and systemic infections that can be refractory to treatment with antifungal drugs. These primary human pathogens collectively are responsible for a disease burden among the highest of any disease caused by a primary fungal pathogen (38). In all certainty, these numbers are an underestimation of the true impact of the disease, as cases often go unreported (38–40). Among the dimorphic fungi, Coccidioides, Paracoccidioides, Talaromyces, and Histoplasma have all been included in the WHO FPPL (23).
HISTOPLASMOSIS
The majority of Histoplasma infections are asymptomatic. Nonetheless, histoplasmosis is one of the most notorious systemic mycoses (41–43). Estimates suggest that despite the pervasive contact of humans with the pathogen (see Epidemiology of histoplasmosis), only about ~1% of people who encounter Histoplasma develop symptoms. Histoplasmosis develops in two main forms, pulmonary and disseminated histoplasmosis (the spectrum of histoplasmosis is illustrated in Fig. 1). We describe the generalities of these two forms as follows.
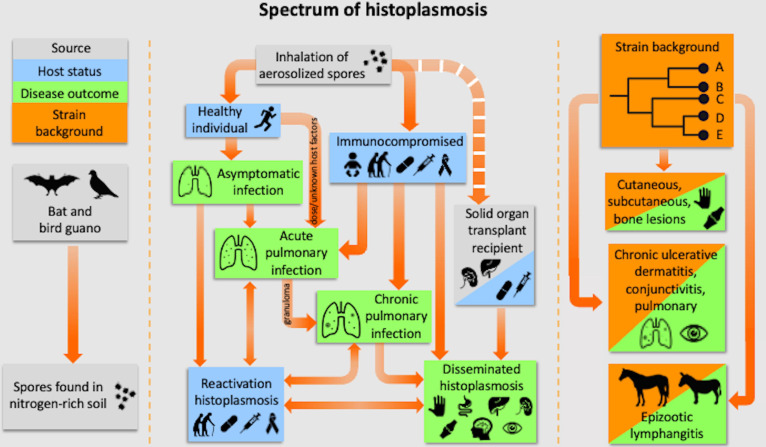
Fig 1.
Spectrum of histoplasmosis. Left: Histoplasma is found in nitrogen-rich soil, which is usually provided by bird or bat guano. Middle: the spectrum of histoplasmosis ranges from asymptomatic infection to severe disseminated disease. Disease outcome is impacted by multiple factors, including the immune status of the host and the quantity of organisms inhaled. Right: strain background also impacts the type of disease. There are strains that cause cutaneous and subcutaneous bone lesions, and other ones that infect horses and mules and cause chronic ulcerative dermatitis, conjunctivitis, and pulmonary histoplasmosis. Modified from an original figure made by Grant S. Jones (with permission). Created with BioRender.com.
Pulmonary histoplasmosis
Histoplasmosis can manifest in several forms (Fig. 1), with acute pulmonary histoplasmosis being the most common [reviewed elsewhere (40, 44, 45)]. This self-limited illness primarily affects children exposed to Histoplasma for the first time and presents with symptoms such as fever, malaise, headache, and weakness. Substernal chest discomfort and dry cough, along with patchy pneumonia on chest radiographs and enlarged hilar and mediastinal lymph nodes, are often diagnosed as acute pneumonia, even though the causal agent is rarely pursued. While improvement is prompt in most cases, fatigue may persist in some patients (46, 47).
Additionally, 5% of cases might also show rheumatologic and/or dermatologic implications, including erythema nodosum and erythema multiforme (48–50), often leading to a misdiagnosis of sarcoidosis (51). This syndrome is most common in young women (40, 48, 52). The ultimate cause is unknown but is thought to be associated with a hypersensitivity response to Histoplasma antigens. The differential diagnosis for acute pulmonary histoplasmosis includes other mycoses such as blastomycosis and atypical community-acquired bacterial pneumonia, caused by Legionella, Mycoplasma, and Chlamydia (53–55). In immunosuppressed individuals or with massive inoculum exposure, the infection can progress to severe pulmonary involvement, causing acute respiratory distress syndrome. A second form of pulmonary histoplasmosis is chronic cavitary pulmonary histoplasmosis, unique to older individuals, often associated with emphysema. This form of histoplasmosis leads to volume loss of the upper lung lobes due to increasing fibrosis, which results in persistent cavities, sometimes forming a “marching cavity” (56, 57). Systemic manifestations include fatigue, weight loss, fever, night sweats, and anorexia. Pleural thickening is common, and the differential diagnosis includes tuberculosis, cancer, and other fungal infections.
Histoplasmosis can also manifest in lung-adjacent tissues [reviewed in references (58, 59)]. In instances in which there is mediastinal lymph node involvement (granulomatous mediastinitis), there is an excess production of collagen in the mediastinum. This collagen excess can cause compression of vital structures, resulting in cough, chest pain, dyspnea, and enlargement of multiple nodes. Scar tissue usually occurs slowly (1 mm/year), and symptoms do not develop until there is a severe reduction of blood flow within an affected central airway (57, 60–66). While many patients are asymptomatic, others may experience symptoms due to the encroachment of nodes on surrounding structures. Radiographic imaging is crucial for diagnosis, and most patients ultimately calcify the involved nodes (60, 66). Mediastinal fibrosis, a rare but often lethal complication, results from an uncontrolled fibrotic response to caseous nodes infected with Histoplasma (65). In advanced cases, fibrosis slowly progresses, encasing vital mediastinal structures and causing symptoms such as dyspnea, cough, hemoptysis, and chest pain. Chest radiographs and CT scans aid in diagnosis, and involvement of both lungs is usually fatal (64). Pericardium involvement, broncholithiasis, and pleural disease are more rare but are possible in some histoplasmosis cases (67–69).
Disseminated histoplasmosis
Chronic progressive disseminated histoplasmosis refers to a slowly progressing and often fatal infection of the disease and might involve every organ in the body (40). The initial reports of the disease described it as mostly occurring in older adults without clear signs of immunosuppression (70), but currently, the disease is recognized as commonly manifesting in patients with immunosuppression. HIV-infected people can develop a progressive and disseminated Histoplasma infection with the involvement of other organs (spleen, liver, lymph nodes, bone marrow, and the central nervous system), which frequently results in death (42, 71). Disseminated histoplasmosis is common in immunosuppressed patients, including those who are under treatment with corticosteroids (22% of patients with disseminated histoplasmosis), suffering from malignancy (15% of patients with disseminated histoplasmosis), and recent solid organ transplants (10% of patients with disseminated histoplasmosis) (72). Symptoms of disseminated histoplasmosis are not exclusive to the disease and encompass fever, malaise, anorexia, and weight loss. Physical examination of patients may reveal hepatosplenomegaly (73), lymphadenopathy (74), and, in some instances, various skin manifestations, such as ulcerations, nodules, or molluscum-like papules (75, 76). Chest radiographs may show a diffuse reticulonodular infiltrate, similar to sarcoidosis, which makes the diagnosis of histoplasmosis more difficult. Laboratory findings include pancytopenia, elevated alkaline phosphatase, increased sedimentation rate, elevated C-reactive protein, high lactate dehydrogenase, and elevated ferritin levels. Severe cases can manifest as sepsis syndrome with hypotension, intravascular coagulation, renal failure, and acute respiratory symptoms, especially in infants and AIDS patients (40, 57, 77–79). Gastrointestinal involvement is common but often asymptomatic, mimicking other opportunistic infections in AIDS patients (i.e., abdominal pain and diarrhea) and leading to malabsorption in severe cases. Disseminated histoplasmosis also presents unique manifestations, including mucous membrane lesions and involvement of various organ systems. In some cases, Addison’s disease results from extensive destruction of both adrenal glands, causing symptoms like fever, orthostatic hypotension, nausea, and vomiting (80–83).
Disseminated histoplasmosis can cause a wide variety of diseases and symptoms. Endocarditis is a rare manifestation of histoplasmosis, occurring on native or prosthetic valves, while vascular graft infections have been reported in a few cases. Central nervous system involvement [reviewed in reference (84)] occurs in 5%–10% of patients with disseminated histoplasmosis with a high chance of relapse and fatality (85). The most common manifestations are chronic meningitis, focal spinal cord lesions, strokes, communicating hydrocephalus, and encephalitis (84). Magnetic resonance imaging reveals small ring-enhancing lesions in the brain and spinal cord, with symptoms including headache, mental status changes, and cranial nerve palsies. Histoplasma infection can cause endocarditis in both native and prosthetic heart valves, leading to a high rate of systemic embolization (58%) and often resulting in a delayed diagnosis compared to bacterial endocarditis (86). Histoplasmosis can also rarely manifest as an urogenital disease. In males, infections can cause testicular and prostatic abscesses, epididymitis, penile lesions, and bladder ulcerations (87–89). In females, histoplasmosis can cause ulcerated vulvar lesions similar to carcinoma (90–92). Other very uncommon manifestations such as osteoarticular histoplasmosis can present as carpal tunnel syndrome [reviewed in references (93, 94)], joint pain (90, 95), septic arthritis (96, 97), or osteomyelitis (98). These symptoms however might also be caused by tuberculosis [e.g., reference (99)]. The most effective antemortem diagnostic methods for histoplasmosis are serology (serum antibody titers or urinary antigen) or blood, bone marrow, and tissue cultures, with untreated infections uniformly fatal.
Epidemiology of histoplasmosis
Histoplasma spp. and the other dimorphic fungi are primary human pathogens that are capable of causing disease regardless of the immune status of the host (32). However, the disease outcome is more severe if the immune system of the host is deficient. Estimates of the number of cases of disseminated histoplasmosis range between 100,000 and 300,000 cases in AIDS patients (100, 101). Diagnosis among AIDS patients could lead to a disease burden reduction for disseminated histoplasmosis (6).
Histoplasma is a common soil dweller, and a large proportion of human hosts have been exposed to the pathogen. In endemic areas, 50%–80% of the population has high antibody titers against Histoplasma, indicating previous exposure or infection. More than 90% of individuals over 20 years of age residing in the United States are skin test-positive for a previous infection (102, 103). Other countries show similar levels of reactivity [Table 1 in reference (104)]. In Mexico, skin reactivity to histoplasmin, a diagnostic antigen, can be up to 80% (105), while in Nigeria it is much lower [4.4% (106)]. Obviously, these estimates are not uniform within countries. In Brazil, histoplasmin tests indicate 10-fold differences among regions [89% vs 8.9% (107)]. These patient surveys indicate that Histoplasma, as an environmental fungus, is likely to cause subclinical infections. This is one of the main challenges in the study of the epidemiology of histoplasmosis, as most patients infected with Histoplasma might show an initially mild condition that goes undetected and is often misdiagnosed. Even with the development of cell-mediated immunity, the remaining foci of viable Histoplasma may persist in various organs, which parallels the situation of tuberculosis. The fungal population is diminished by the immune response but not completely killed, which makes reactivation a possibility. Reactivation may occur months to years later, especially if the immune system becomes compromised by AIDS (108), corticosteroids, or chemotherapy (109). Cases of histoplasmosis have been reported in transplant recipients of organs from donors living in endemic areas (110, 111).
Nonetheless, exposure and reactivity are not synonymous with disease. For histoplasmosis, the CDC has now estimated that over 50,000 hospitalizations occurred due to this infection in the U.S. between 2001 and 2012, with increased proportions of infections in patients with complex illness and immunosuppression, especially due to diabetes (137, 138), transplantation (139), and autoimmune disease (140, 141). In addition, the environmental distribution of the organism has spread, and disease is increasing in non-HIV+ patients in the U.S. (108, 142). In other parts of the world, histoplasmosis remains important in people with HIV; in fact, histoplasmosis has been described as a “lethal blind spot of international health organizations” (143). The incidence and burden of HIV-associated histoplasmosis are equal to HIV-associated tuberculosis, and histoplasmosis has overtaken tuberculosis as the leading cause of death in people with HIV in Central/South America (144, 145). These numbers led to the classification of Histoplasma as a high-priority pathogen in the WHO FPPL (23, 24). Morbidity and mortality are particularly high in many regions, even in the U.S., because diagnosis remains difficult, infection is frequently unsuspected, and clinical presentations are variable (40, 42, 82, 146, 147). The potential for environmental exposure, individual reactivation, and difficulties with antifungal treatments suggest that mycoses caused by dimorphic fungi will persist as a public health problem in the years to come.
The actual disease burden of histoplasmosis remains unknown even though some local estimates have been proposed (7, 144). These are likely underestimates because disease notification is not mandatory (148) and also because a large proportion of the infections present nonspecific clinical symptoms or radiological findings that may be interpreted as tuberculosis (42, 43, 149) and other pulmonary diseases. Furthermore, diagnosis of histoplasmosis is often delayed (150). The percentage of infection in AIDS patients paralleled the general prevalence of exposure (151).
DISEASE TREATMENT
The choice of therapy management strategy for histoplasmosis depends on its form and has been extensively reviewed (152). Histoplasmosis in healthy individuals usually resolves without treatment. More complex cases require antifungal therapy. The two preferred antifungal agents for treating histoplasmosis are amphotericin B (AmB) in its many preparations and itraconazole [reviewed in references (152, 153)]. Fluconazole, isavuconazole, voriconazole, and posaconazole are also used as alternative salvage therapy (45, 77). Itraconazole and ketoconazole are effective in 75%–85% of patients, but there is a high chance of relapse (154, 155); hence, monitoring of itraconazole serum concentrations is advised due to variability in absorption [reviewed in reference (156)]. Other antifungals are not recommended, such as echinocandins (including caspofungin), due to conflicting in vitro and in vivo data on their efficacy in histoplasmosis (153, 157–159). Antifungal susceptibility testing is also complicated by non-standard methodology for optimal testing of yeast, which is the most physiologically relevant form of the organism (160).
Oral itraconazole is the preferred therapy for mild to moderate pulmonary or disseminated histoplasmosis (149) and serves as a milder option to continuing treatment started with AmB in severe cases (77). The availability of itraconazole in capsule and solution forms allows flexibility, with the solution offering better absorption but a higher likelihood of gastrointestinal upset and taste-related issues (161, 162). Dosage and frequency are discussed elsewhere (163, 164).
AmB preparations (e.g., AmB deoxycholate, liposomal AmB, and AmB lipid complex) are reserved for severe pulmonary or disseminated histoplasmosis cases, with liposomal AmB showing higher response rates and improved survival compared to AmB deoxycholate, as demonstrated in a multicenter trial involving AIDS patients (163). Other lipid formulations of AmB, or AmB deoxycholate, are considered alternatives to liposomal AmB when the latter is not accessible. AmB deoxycholate, while less expensive, poses the risk of nephrotoxicity and requires a central venous catheter. In patients with immunodeficiencies, the recommended treatment is liposomal amphotericin B in combination with itraconazole (153, 163, 165, 166). Different strains of Histoplasma show differences in their levels of antifungal resistance (167), which in turn might correspond to interspecific differences among Histoplasma species.
LIFE CYCLE
As is the case with all the dimorphic fungi, Histoplasma thrives in soil primarily as mycelia. The fungus is often associated with areas rich in bird or bat droppings [(168–170); see below]. In this mold form, Histoplasma produces infectious microconidia and larger macroconidia, facilitating airborne dissemination. When these conidia are inhaled by a host, particularly in areas endemic to Histoplasma, they reach the lungs. Upon entering the host’s lungs, the environmental mold transforms into a yeast form, adapting to the warmer body temperature. This transition is thought to be essential for Histoplasma to establish infection in the host tissues. The yeast form (more specifically, the virulence factors produced uniquely by the yeast phase), characterized by a unicellular, oval structure, enables the fungus to evade the host immune response and persist intracellularly.
Histoplasma has been traditionally studied as a haploid asexual fungus. Nonetheless, Histoplasma does reproduce sexually, although the frequency of sexual reproduction is unknown (171–174). The factors that lead to sexual reproduction in nature are completely unknown but are hypothesized to be stress-related (175). In experimental crosses, the sexual stage of Histoplasma is Ajellomyces capsulatum and occurs when mycelia fuse and form coils in the presence of their mate, then forming mature cleistothecia. The compatibility of strains is determined by the mating (MAT) locus, a region with low sequence similarity that encompasses genes for one or more transcription factors featuring structural motifs like α1 domains, amphipathic α-helices, high-mobility-group (HMG) DNA-binding domains, and metallothioneins (176–178). In filamentous ascomycetes, the initial MAT idiomorph region encoding an α1 domain transcription factor is commonly denoted as MAT1-1, while the initial MAT idiomorph region encoding an HMG DNA-binding domain is labeled as MAT1-2 (179–181). Sexual compatibility in Histoplasma, as is the case with most filamentous ascomycetes, is governed by the mating-type locus (MAT1). The mating system in Histoplasma is heterothallic bipolar, which means that sexual compatibility requires positive and negative strains. The mating events only take place in the mycelial phase and are more likely to be successful between freshly isolated strains. The reasons why isolates become more difficult to reproduce sexually as they are kept in culture remain unknown. In Histoplasma and Coccidioides, the two allelic forms of the MAT alleles encode either an α-box transcription factor or an HMG domain, similar to the structure observed in other ascomycete fungal species in which the MAT locus has been characterized [e.g., Aspergillus (178)]. Several surveys have suggested that the two loci are not equally represented in clinical samples (173, 182), but they might show a 1:1 ratio in environmental samples (174). Since the two mating types can cause disease in mouse models, these proportions have been taken as evidence of the difference in producing infectious particles (178).
The diploid stage presumably undergoes meiosis and generates haploid ascospores that might germinate into mycelia, which in turn generates haploid conidia, the infective particles. Nonetheless, this step has not been formally studied in Histoplasma. Infection of the host might also occur by inhalation of mycelial fragments, but this has not been formally proven (183).
ECOLOGY
Histoplasma is thought to be distributed globally (184), but few countries have cataloged its fine distribution. The most granular resolution map of the distribution of Histoplasma in the USA was done by Edwards et al. (185) and used histoplasmin skin tests among Navy recruits in the United States with a minor history of traveling to determine that the fungus was most common in the Ohio and Mississippi river valleys. The landscape of the United States has changed substantially since this survey (186). Histoplasmosis outbreaks have revealed that the fungus has either expanded its range or that agricultural and population changes have unmasked conditions that kept the fungus hidden in previously thought non-endemic regions (187–190).
Even though Histoplasma spends most of its life cycle in the soil (183, 191), little is known about the biotic and abiotic interactions of Histoplasma in the soil. Histoplasma is found in soil throughout the world, even in Antarctica (192) with endemic regions in Africa, Latin America, and North America. In the United States, Histoplasma is prevalent in the Ohio-Mississippi river valleys, with localized foci reported in various states including Kentucky, Minnesota, Illinois, Missouri, Mississippi, Michigan, Indiana, and Pennsylvania (40, 193). Experimental inoculation of Histoplasma in non-sterile soil suggests that the fungus can survive as conidia for more than 8 weeks, but after that, the survival starts decreasing, which might be caused by competition with soil microbes (194). The fungus is unable to survive the composting process (195).
The suite of natural hosts of Histoplasma is extensive. PCR-based surveys in wild-caught mammals (196–198), zoo animals (199), roadkill (200), pets (201, 202), and burrows (203) have revealed that the range and niche of Histoplasma are broad. Arguably, the most commonly reported infected animals are bats from different locations around the world [e.g., references (204, 205)]. A systematic review of the literature found that 49 species of bat have reports of Histoplasma infections (206). This result is important because the migratory nature of bats has been implicated with Histoplasma dispersion and speciation (207–209). Nonetheless, there may be biases in these results as the majority of the studied bat samples originate from the Americas and only a few from Asia and Africa regions known to harbor Histoplasma and cause histoplasmosis. There are 1,400 species of bats, so the 49 reported species could represent a true phylogenetic distribution of the host specificity of Histoplasma but more likely reflect a bias in sampling. The only unbiased way to test whether there is an association between the occurrence, diversification, and epidemiological patterns of Histoplasma and different species of bats is to do a systematic sampling of hundreds of bat species. Regardless of the association between bat species and Histoplasma, histoplasmosis outbreaks are often associated with proximity to guano-rich caves [reviewed in references (168, 210, 211)], presumably because the increased levels of nitrogen in the soil provide the ideal environment for Histoplasma to grow as mycelia. Consequently, cave exploration is a risk factor for histoplasmosis (168, 170, 212).
The relationship between Histoplasma and birds is less clear. A survey of 59 bird-related outbreaks revealed the involvement of chickens (41% n = 24), unspecified blackbirds (32%, n = 19), pigeons (15%, n = 9), and seagulls (2%, n = 1) (213). Chicken and pigeon dissection has found no natural infection of the animal intestines (214). Experimental inoculations of chickens suggest that the fungus can survive up to 1 week inside the birds, but the birds themselves seem to be of little epidemiological importance for the spread of the disease (214, 215). Histoplasma has been often found in chicken manure and chicken coops (169, 216), which might indicate non-specificity to the type of bird droppings.
TAXONOMY AND EVOLUTIONARY BIOLOGY
Delimiting species is an important component of evolutionary biology. The case of Histoplasma is illustrative. Even though all the species of the genus cause histoplasmosis, they show morphological, geographical, and potentially health-related differences. Histoplasma has been the subject of consistent and vigorous taxonomic exploration. Early assessments of the diversity within Histoplasma evaluated geographical distribution, host association, morphology, and clinical symptoms to characterize the extent of genetic variation in the fungus. These surveys suggested the existence of three lineages (217): Histoplasma capsulatum var. capsulatum, the New World human pathogens; var. duboisii, the African human pathogens; and var. farciminosum, the Old World horse pathogens (217, 218). Histoplasma capsulatum var. capsulatum was determined to be distributed worldwide, especially in Latin America and in the mid-west of the United States (45, 183). Histoplasma capsulatum var. duboisii causes African histoplasmosis, which, unlike var. capsulatum, was characterized by the manifestation of granulomatous lesions predominantly in subcutaneous, cutaneous, and osseous tissue and by the larger size of the yeast cells (219–221). Finally, H. capsulatum var. farciminosum was thought to infect horses, donkeys, and mules, causing epizootic lymphangitis characterized by pyogranulomatous subcutaneous nodules and ulcerated lesions of the skin, and to be found throughout Europe, India, South Asia, and North Africa (222, 223). The morphology of the yeast of the H. capsulatum var. farciminosum was hypothesized to be similar to that of H. capsulatum var. capsulatum (224, 225). The exploration of the species boundary between H. capsulatum var. duboissii and H. capsulatum var. capsulatum went beyond phenotypic surveys. Crosses between tester strains of North American lines of Histoplasma (capsulatum sensu lato) and H. duboisii produced cleistothecia and pseudo-cleistothecia with ascospores that failed to germinate (173). While this result might be interpreted as evidence of hybrid inviability between these lineages (a trademark of advanced speciation), we argue caution as the control crosses also show deficiencies in germination. This fact was recognized by Kwon-Chung (173).
The emergence of DNA sequencing led to a reclassification of Histoplasma. Two seminal and comprehensive phylogeographic studies of isolates from around the world reclassified H. capsulatum into at least seven distinct clades that correlate with their region of geographic isolation (226, 227). Using partial DNA sequences of four protein-coding genes (ADP-ribosylation factor, H antigen precursor, ∆−9 fatty acid desaturase, and Alpha-tubulin), Kasuga et al. (226) used multi-locus sequence typing (MLST) to analyze the evolutionary relationship of 46 H. capsulatum environmental and clinical isolates, including members of the three known varieties (capsulatum, duboisii, and farciminosum). This study identified six clades: (i) class 1 North American H. capsulatum var. capsulatum, (ii) class 2 North American H. capsulatum var. capsulatum, (iii) Central American H. capsulatum var. capsulatum, (iv) South American H. capsulatum var. capsulatum group A, (v) South American H. capsulatum var. capsulatum group B, and (vi) H. capsulatum var. duboisii. In comparison with the previous taxonomic proposal, var. capsulatum is composed of multiple species, var. duboisii is a monophyletic and distinct group, and var. farciminosum was found to be non-monophyletic at the typed loci. The clades were found to be genetically isolated from the others, and thus a reclassification from three into six species was proposed (226).
In an even more comprehensive phylogenetic analysis, Kasuga et al. (227) genotyped 137 isolates and constructed a more detailed phylogeographical map of Histoplasma. Again, the phylogenetic trees suggested the need to re-classify H. capsulatum into seven genetically isolated phylogenetic groups and a Eurasian clade, the only group that was not genetically isolated as it originated from the Latin American group A. The eight major identified clades were (i) North American class 1 clade; (ii) North American class 2 clade; (iii) Latin American group A clade; (iv) Latin American group B clade; (v) Australian clade; (vi) Netherlands (or Indonesian origin) clade; (vii) Eurasian clade, and (viii) African clade. Some isolates did not group with any of the eight main clades or with each other, forming lone lineages. Three strains from Panama (G186A, G186B, and G184B) grouped together to form one of these lone lineages (H81 lineage). Molecular clock estimates suggest that the radiation of Histoplasma occurred 3–13 million years ago in Latin America (227). This new classification practically abolished the use of the three varieties as a system to distinguish H. capsulatum strains. In the following decades, MLST has been widely used to describe new phylogenetic species within the Histoplasma species complex [e.g., references (3, 209)]. More recently, using a combination of whole-genome resequencing data and population genomic approaches, Sepúlveda et al. (228) analyzed 30 Histoplasma isolates representing four endemic areas of histoplasmosis to evaluate the existence of cryptic species within Histoplasma. They showed that the genus is composed of at least four species that are genetically isolated and rarely interbreed and proposed the following taxonomic reorganization of the genus: (i) Histoplasma capsulatum sensu stricto Darling 1906 (formerly known as the Panama or H81 lineage), (ii) Histoplasma mississippiense sp. nov. (formerly known as NAm 1), (iii) Histoplasma ohiense sp. nov. (formerly known as NAm 2), and (iv) Histoplasma suramericanum sp. nov. (formerly known as LAm A) (Fig. 2). Mirroring the genome-wide population genetics and phylogenetic framework used by Sepúlveda et al. (228), Jofre et al. (229) identified the existence of a well-differentiated lineage of Histoplasma occurring in the Indian subcontinent, and Almeida-Silva et al. (230) identified another one endemic to Southern Brazil, highlighting once again the power that using speciation genomics combined with the classical method of species recognition by genealogical concordance has when evaluating the potential diversity of cryptic species not only in the Histoplasma genus but in fungal pathogens overall. The expansion of genome sequencing to more than just a handful of reference strains and the use of long-read sequencing technology have also opened the possibility of evaluating the differences and similarities in genome content among the newly identified Histoplasma species (228, 229, 231, 232), leading to the identification of extensive synteny (231), low levels of admixture among species (229, 232), and extensive genetic differences along the Histoplasma genomes (228). With the imperative advances achieved with genome sequencing and population genomics, a systematic evaluation that assists in the identification of these species at the morphological level is greatly needed. In a first step to bridge that gap, Sepúlveda et al. (233) performed a phenotypic characterization of five of the described cryptic species in Histoplasma (H. capsulatum sensu stricto, H. ohiense, H. mississippiense, H. suramericanum, African lineage, for a total of 27 isolates), which included the production of extracellular proteolytic activity and colony morphology (rough vs smooth, Fig. 2). The findings suggest the existence of diagnostic traits among the cryptic species of Histoplasma, which can be used in the clinics to positively discriminate among species (233). The characterization of the genetic diversity in Histoplasma might still be in its infancy. Further MLST has suggested the existence of additional phylogenetic species in North America (234, 235). Moreover, phylogenomic trees that have included samples from other locations have revealed additional clades that fulfill the requirements to be considered phylogenetic species (229, 230). Yet, lone lineages persist [reviewed in reference (208)]. A comprehensive sampling around the world coupled with genome sequencing and phylogenetics is necessary to determine the number of species of Histoplasma and quantify the level of genetic diversity within species.
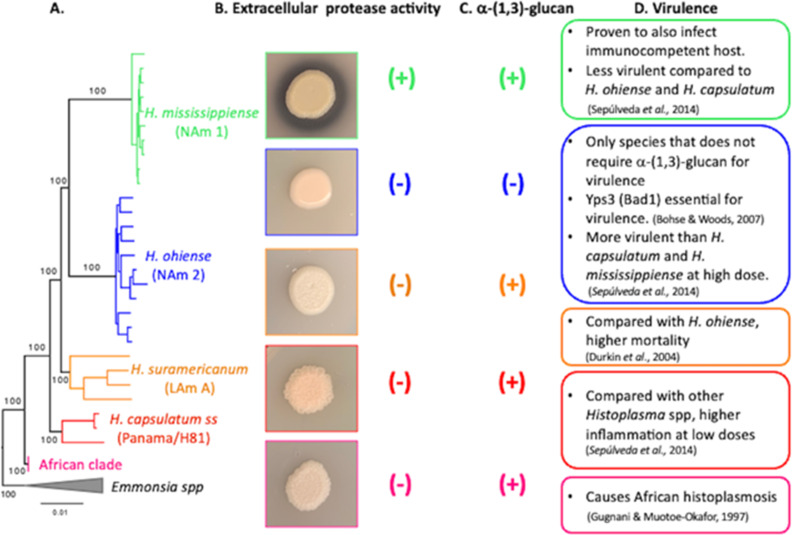
Fig 2.
(A) Histoplasma species. Phylogenetic tree using whole genome data shows that Histoplasma is composed of at least five different species [tree modified from reference (228)]. Species names represent the nomenclature proposed by Sepúlveda et al. (228) as well as the nomenclature proposed by Kasuga et al. (227) (in parenthesis). (B) Extracellular proteolytic activity in Histoplasma. Secreted proteolytic activity is visible as transparent clearance halos around fungal colonies only in H. mississippiense (NAm 1) strains. Pictures show a reference strain for each species: WU24 (H. mississippiense), G217B (H. ohiense), 3/11 (H. suramericanum), G186A (H. capsulatum), and H88 (African clade). (C) α-(1, 3)-glucan. Smooth morphology occurs in the absence of α-(1, 3)-glucan in the cell walls. H. ohiense (NAm2) is the only species that shows a smooth colony morphology (pictures on panel B) due to the lack of α-(1, 3)-glucan. (D) Virulence. Differences in virulence among Histoplasma species. Conclusions should be drawn carefully when comparing relative virulence among isolates, and strain-level variation should be considered and evaluated before making species-level conclusions. References shown are as follows: (236, 237, 219).
VIRULENCE
Histoplasma infection takes place upon inhalation of the saprophytic phase (a combination of conidia and mycelial fragments present in the soil) by the mammalian host. The temperature in the respiratory tract (37°C) induces the conversion to the parasitic yeast form. Once in its yeast phase, Histoplasma can survive and replicate within the phagolysosomes of alveolar macrophages, as it is a classical facultative intracellular pathogen (44). Intracellular pathogens must overcome a number of stressors in order to survive the harsh phagolysosome environment, including nutrient deficiency, reactive oxygen species, iron limitation, and exposure to lysosomal enzymes (238–240). Histoplasma modulates the acidification of phagolysosomes and sustains the pH near neutrality, presumably interfering with the arsenal of pH-dependent hydrolytic enzymes and promoting intracellular survival (241). Additionally, Histoplasma has developed sophisticated mechanisms to overcome the restricted amounts of metals (zinc, copper, and iron) within activated macrophages [reviewed in reference (242)].
Histoplasma is able to infect multiple species of mammals (see Ecology), and mice are a natural host of the fungus. While a variety of animal models of histoplasmosis have been developed, including invertebrates (243–245), the mouse model of infection is the preferred model to study virulence and the progression of histoplasmosis in the laboratory setting (246). Histoplasma strains from different species all cause comparable respiratory and systemic disease, but multiple studies have highlighted some differences (Fig. 2). Histoplasma ohiense was shown to be more virulent than H. capsulatum, inducing a more robust inflammation and higher tissue burdens in mice after either intranasal or intravenous inoculation with a high inoculum [at least 105 organisms (236, 247, 248)]. In contrast, H. capsulatum induces a higher infiltration of monocytic cells compared to H. mississippiense and H. ohiense when the inoculum used is lower [103 organisms (236, 249)]. A comparison of Latin American and North American isolates showed similar total lung burdens with a dissimilar host inflammatory response, where mortality was higher with H. suramericanum (formerly Histoplasma clade LAm A). Lung pathology in infections with the Latin American isolate showed large necrotizing granuloma with neutrophilic infiltration; chronic disease was unique to H. ohiense (237). The establishment, development, progression, and resolution of histoplasmosis are influenced by multiple factors, which include inoculum size, and strain background, so conclusions should be drawn carefully when comparing relative virulence among isolates, and strain-level variation should be considered and evaluated before making species-level conclusions.
A limited, yet increasing number of genes have been genetically proven (in some cases, using multiple genetic methods), to have a role in Histoplasma virulence, and they can broadly be divided into the following groups based on their biological function: secreted proteins: CBP (CBP1 locus, see next paragraph) (112); the endo-β-(1, 3)-glucanase (ENG1) performs the majority enzymatic removal of any exposed β-glucans (115), the exo-β-(1, 3)-glucanase (EXG8) also contributes to the trimming of exposed β-glucans, but to a lesser degree (116). Cell surface: the α-(1, 3)-glucan synthase (AGS1) synthesizes α-(1, 3)-glucan (see next paragraph) (113); the α-(1, 4)-amylase (AMY1) is a glycosyl hydrolase that forms α- (1, 4)-linked glucan oligomers required for α-(1, 3)-glucan production (117); Yps3 (YPS3), whose function has not been defined (118). Nutritional immunity [reviewed in reference (242)]: the suite of peroxin proteins (PEX5, PEX7, PEX10, PEX11, and PEX33) is indispensable in multiple key roles within the Histoplasma peroxisome (119); the zinc transporter (ZRT2) is necessary to combat zinc deprivation (120); the enzyme L-ornithine monooxygenase (SID1) catalyzes the first step in siderophore biosynthesis and is required for proper siderophore production (119, 121–123); the γ-glutamyltransferase protein (GGT1) catalyzes the reduction of ferric iron making it available for the yeast cells (124); the vacuolar ATPase (VMA1) is needed for growth in low-iron conditions, but its mechanism of action has not been described (125); the copper transporter (CTR3) is required for continued acquisition of essential copper and for proliferation within macrophages (126, 127); the 2,5-diamino-6-ribitylamino-4(3H)-pyrimidinone 5′-phosphate deaminase (RIB2), the pantothenate synthetase (PAN6), and the biotin synthase (BIO2) are essential for riboflavin, pantothenate, and biotin biosynthesis, respectively (128); the Tryptophan synthase (TRP5) is necessary for tryptophan synthesis (129); the bifunctional chorismate synthase and flavin reductase (ARO2) catalyze the enzymatic reaction for chorismate formation (129). Stress response: the heat shock protein 82 (HSP82) (130); the superoxide dismutase (SOD3) is the main source of extracellular superoxide dismutase activity and a key factor in response to reactive oxygen species produced by host cells (131); the extracellular catalase protein (CATB) and the intracellular catalase protein (CATP) both are important for protection against peroxide challenge from antimicrobial reactive oxygen produced by host cells (132). Mold-to-yeast transition [reviewed in reference (250)]: the histidine kinase (DRK1) is vital for yeast-phase morphology and sporulation (133); the group of Ryp (required for yeast-phase growth) transcriptional regulators [RYP1 (30), RYP2, RYP3 (134) and RYP4 (135); discussed later] and the Velvet protein (VEA1) are involved in cleistothecia formation (123, 136). Some of these factors are required for all Histoplasma species, and other ones are only required for a particular species. Table 1 summarizes the genetic tools used to identify and/or analyze each virulence factor.
TABLE 1.
Histoplasma virulence-associated genes and genetic methods used to study them
| Allelic replacement | RNA interference | Insertional mutagenesis | CRISPR/Cas9 | |
|---|---|---|---|---|
| Secreted proteins | ||||
| CBP1 | Sebghati et al. (112) | Rappleye et al. (113) | Edwards et al. (114) | |
| ENG1 | Garfoot et al. (115) | |||
| EXG8 | Garfoot et al. (116) | |||
| Cell surface | ||||
| AGS1 | Rappleye et al. (113) | Rappleye et al. (113) | Marion et al. (117) | |
| AMY1 | Marion et al. (117) | Marion et al. (117) | ||
| YPS3 | Bohse et al. (118) | |||
| Nutritional immunity | ||||
| Peroxisomal import | ||||
| PEX5 | Brechting et al. (119) | Brechting et al. (119) | ||
| PEX10 | Brechting et al. (119) | |||
| PEX33 | Brechting et al. (119) | |||
| PEX7 | Brechting et al. (119) | |||
| PEX11 | Brechting et al. (119) | |||
| Zinc acquisition | ||||
| Zrt2 | Dade et al. (120) | |||
| Iron acquisition | ||||
| SID1 | Hwang et al. (121) | Hilty et al. (122) | Brechting et al. (119) Joehnk et al. (123) | |
| GGT1 | Zarnowski et al. (124) | |||
| VMA1 | Hilty et al. (125) | |||
| Copper acquisition | ||||
| CTR3 | Shen et al. (126) | Rappleye (127) | ||
| Vitamin biosynthesis | ||||
| RIB2 | Garfoot et al. (128) | |||
| PAN6 | Garfoot et al. (128) | |||
| BIO2 | Garfoot et al. (128) | |||
| Tryptophan biosynthesis | ||||
| TRP5 | Shen et al. (129) | |||
| ARO2 | Shen et al. (129) | |||
| Stress response | ||||
| HSP82 | Edwards et al. (130) | |||
| SOD3 | Youseff et al. (131) | |||
| CATB/CATP | Holbrook et al. (132) | |||
| Mold/yeast transition | ||||
| DRK1 | Nemecek et al. (133) | |||
| RYP1 | Nguyen et al. (30) | Nguyen et al. (30) | ||
| RYP2/RYP3 | Webster et al. (134) | Webster et al. (134) | Joehnk et al. (123) | |
| RYP4 | Beyhan et al. (135) | |||
| VEA1 | Laskowski-Peak et al. (136) | Joehnk et al. (123) | ||
The case of α-(1, 3)-glucan (AGS1 locus) is intriguing. Biochemical surveys of the fungal cell wall that preceded phylogenomics found that isolates lacking the polysaccharide α-(1, 3)-glucan in their yeast cell wall formed a single group known then as chemotype I (251–253). Chemotype I isolates all belong to the North American 2 (NAm 2) clade, now known as H. ohiense. All other Histoplasma clades are classified as chemotype II and have an outer layer of α-(1, 3)-glucan in their yeast cell walls, which acts as a shield preventing recognition by the dectin-1 receptor on host phagocytes (254). Notably, when this gene (AGS1) is inactivated in H. capsulatum (113, 254), as well as in H. mississippiense (236), virulence and immune evasion are seriously compromised. For chemotype II strains, spontaneous α-(1, 3)-glucan mutants can be isolated after repeated passage in broth culture, using a procedure that enriches for non-clumping yeast cells (255, 256). This spontaneous loss of α-(1, 3)-glucan results in virulence attenuation and a “smooth” colony morphology for chemotype II strains and has also been shown to occur in Paracoccidioides brasiliensis (257–259).
Histoplasma ohiense (i.e., chemotype I) strains are virulent despite the absence of α-(1, 3)-glucan in their cell wall (113, 114). Edwards et al. (114) performed a detailed examination of the α-glucan synthase (AGS1) locus in the chemotype I/H. ohiense reference strain (G217B) and found that this interspecies difference is caused by a 2.7-kb insertion of a transposable element in the promoter of AGS1 region that reduces AGS1 expression. They also evaluated the expression of AMY1 and UGP1, both genes previously identified to be required for α-(1, 3)-glucan production (117), and proved they are fully expressed in chemotype I strain (114). These studies suggest the production of an alternative virulence factor in chemotype I strains that compensates for the lack of α-(1, 3)-glucan production and overcomes its requirements for virulence.
Besides α-(1, 3)-glucan, another yeast-specific virulence factor with differences in its requirement in Histoplasma species is Yps3, a homolog of the Blastomyces dermatitidis adhesin Bad1 (260). Yps3 has been shown to be essential for virulence in H. ohiense, but not in H. capsulatum (118). CBP was the first genetically proven virulence factor in H. capsulatum, originally identified as a secreted calcium-binding protein (112, 261, 262). Biochemical work showed CBP is a homodimer (each monomer is 8 kDa) with a strong affinity for calcium (KD ≈ 6 nM), highly resistant to proteolysis, and very stable throughout a wide pH range (263). An initial nuclear magnetic resonance (NMR) structure of the refolded Histoplasma protein showed that CBP has structural similarity to mammalian saposin B (264). Later work solved the crystal structures of the Histoplasma and Paracoccidioides CBP native proteins, which showed a distinct fold from the NMR structure with no similarity to saposins (265). This difference could be the result of solving the solution structure rather than the crystal structure. CBP has also been reported to trigger macrophage cell death (266, 267) and form an effector complex that drives macrophage lysis (265). CBP has been shown to be required for virulence in both H. capsulatum and H. ohiense (112, 114). Considering the differences in the requirement of important virulence factors such as α- (1, 3)-glucan and Yps3 among Histoplasma isolates could provide insights into how other virulence factors evolve in multiple Histoplasma species.
GENETIC TOOLS
The identification of virulence determinants in many medically important fungi has always been challenging due to the low efficacy of conventional genetic tools. A variety of molecular tools have made Histoplasma into a model for which genetic experiments can dissect the role of specific genes in the context of infection. Here, we list the most commonly used approaches in Histoplasma.
Telomeric plasmid-based genetics
Transformation of exogenous DNA into H. capsulatum results in either (i) random integration into the recipient chromosomal DNA, with tandem amplification and rearrangement of the integrated sequences, or (ii) linearization of the input DNA with telomerase addition of telomeric repeats to the ends (GGGTTA)n (268). The latter observation was the basis of engineering telomeric plasmid vectors capable of replicating in both Escherichia coli (as multicopy circular plasmids) and in Histoplasma (as multicopy linear plasmids). The introduction of these telomeric shuttle plasmids is done via electroporation of yeast cells. Transformation of Histoplasma with pre-linearized vectors has shown that the only subsequent genetic alteration to the plasmid DNA was the addition of more telomeric repeats at both ends of the linear plasmid, and all transformants maintained the DNA as linear plasmids in high copy number (268) [cf. (269, 270) for potential difficulties]. The original experiments using telomeric plasmids had the goal of studying the gain of function, with screening aided by the presence of positively selected markers. A limited number of selectable markers have been used successfully in Histoplasma. The first selectable marker was used to establish a transformation system, where mutants deficient in orotidine-5′-monophosphate pyrophosphorylase were converted to prototrophy by cloning the URA5 gene from the fungus Podospora anserina (269). The URA5 gene is particularly useful for in vivo studies, as complementation with the URA5 gene does not alter Histoplasma virulence (271, 272). A second marker, the hph gene from Escherichia coli flanked by the Aspergillus nidulans promoter and terminator sequences, confers resistance to hygromycin B (273). The G418-resistance gene apt3, another dominant selectable marker, has also been adapted to work in H. capsulatum (130) and was used in combination with the hph gene to construct a double knockout strain (132). Telomeric plasmid-based genetics has served as the scaffolding to develop all other genetic modification techniques in Histoplasma.
Gene disruption
A two-step gene disruption strategy to make isogenic mutant strains in H. capsulatum uses telomeric plasmids combined with a positive-negative selection strategy to overcome the high frequency of ectopic DNA integration (274). This process has been particularly useful to evaluate if a specific gene is required for virulence. The telomeric plasmid contains the hygromycin resistance gene hph as a selectable marker flanked by DNA homologous to the gene to be deleted. There is also a second selectable marker gene (URA5) located outside of the two homologous regions. Electroporation is used to transform the plasmid into a uracil auxotrophic strain, and the selection of allelic replacement occurs in two steps: (i) growth in the absence of uracil, selecting for the conversion of the recipient uracil auxotroph to prototrophy to maintain the plasmid at high copy number and to provide opportunity for the desired double crossover to occur; and (ii) selection with hygromycin (selects for cells having undergone homologous recombination events), 5-fluororotic acid (selects against cells retaining free URA5-containing plasmid), and uracil, as the final isogenic mutant will be a uracil auxotroph. Typically, about one-third of the recovered isolates will have undergone the desired homologous recombination event (this rate has not been formally assessed for all Histoplasma strains, including the widely used G217B strain). The first study to use this two-step gene disruption method identified the CBP1 gene as required for virulence in H. capsulatum (112). There are many other studies that have generated isogenic mutants in different genes (Table 1), most of them in H. capsulatum G186A, which is considered to be the easiest strain to genetically manipulate.
CRISPR/Cas9
An optimized CRISPR/Cas9 system for the efficient generation of targeted gene disruption and targeted gene deletion was recently implemented in Histoplasma (123, 127). This CRISPR/Cas system takes advantage of the telomeric vectors widely used in Histoplasma allowing for autonomous replication. After a desired mutation has been verified, plasmid selective pressure is removed, leading to the loss of the CRISPR/Cas9 vector. This enables multiple rounds of CRISPR/Cas9-mediated gene targeting and allows for the generation of multiple mutations in the same strain. This new system was shown to be applicable for several Histoplasma species, including H. ohiense, for which targeted gene disruption has been difficult to achieve (119, 123, 127).
RNA interference
RNA interference (RNAi) is an evolutionarily conserved mechanism normally involved in genome defense against mobile genetic elements and viruses (275). This technique is widely used in many fungal pathogens, including Histoplasma, to silence gene expression post-transcriptionally (30, 113, 117, 118, 134, 276, 277). To target H. capsulatum genes for silencing, a combination of telomeric plasmids and a green fluorescence protein(GFP) sentinel system for RNAi was developed that uses the silencing of the gfp gene as an indicator of target gene depletion (113). Briefly, the exogenous expression of double-stranded RNA homologous to the gene to be silenced triggers the degradation of the corresponding target mRNA, preventing protein synthesis. This technique is relatively rapid compared to the above methods of gene disruption or gene editing, although silenced (knockdown) strains may still have residual target gene function.
Agrobacterium-mediated mutagenesis
The bacterium Agrobacterium tumefaciens is a plant pathogen that transfers a portion of DNA (transfer DNA, T-DNA) from its tumor-inducing plasmid (Ti) to a plant cell, where it is randomly inserted into the plant chromosomal DNA causing crown gall tumors. Contact between the bacterium and the target cell must take place for the T-DNA transfer to occur (278). The A. tumefaciens-plant infection has been modified and adapted successfully to transform other eukaryotes, including dimorphic fungi (279–281). Agrobacterium tumefaciens-mediated transformation of H. capsulatum (280) [reviewed in reference (282)] was optimized to perform an insertional mutagenesis screen to identify the genes involved in α-(1, 3)-glucan production. Marion et al. (117) used an A. tumefaciens strain with the Ti plasmid lacking the tumor-producing genes but containing the genes required for successful T-DNA transfer and integration, as well as the hph gene conferring resistance to hygromycin B. The Ti plasmid also has the genes required for replication in E. coli and A. tumefaciens. Co-incubation of Agrobacterium with Histoplasma yeasts allows for the transfer of the T-DNA that randomly integrates into the yeast’s chromosomal DNA, generating thousands of colonies, each with a single T-DNA insertion at a unique site. This forward genetics technique has been used to efficiently generate mutant libraries that consist of at least 90% single random insertions in the H. capsulatum genome (282).
Genomics
Genomics has propelled a revolution in biology and has allowed for an unprecedented amount of data collection, a slew of new hypotheses, and the exploration of biological systems for which genetics has been challenging (283, 284). In the case of the dimorphic fungi, comparative studies of Histoplasma with other closely related fungal pathogens such as Blastomyces and Paracoccidioides have revealed that some genes related to the virulence of dimorphic pathogenic fungi also exist in saprophytes, but that alterations in the abundance of proteases, kinases, and transcription factors in systemic dimorphic species, as opposed to non-dimorphic counterparts, might have facilitated the development of specialized gene regulatory systems (285, 286). A myriad of genomic techniques have been used to understand the biology of Histoplasma, but we focus on the two that have arguably had the most impact in the study of Histoplasma, transcriptional profiling, and whole-genome sequencing. We also summarize progress on the study of the genetics of host susceptibility to Histoplasma.
Pathogen transcriptional analysis
Measuring differences in gene expression across tissues, individuals, and species has the potential to reveal the gene regulation strategies that lead to morphological variation (287, 288). Differences in expression must be crucial for transitioning between morphologies in a species like Histoplasma that shows temperature conditional dimorphism (272). In Histoplasma, the morphological conversion from mold to yeast is a highly regulated process that is thought to be required for virulence [reviewed in reference (250)], and differences in gene expression between the mycelium and yeast phase have been informative to identify alleles involved in virulence. Most virulence factors described to date are restricted or highly upregulated during the yeast phase (112, 113, 131, 132, 260) (Table 1). Consequently, mutants locked in the mold phase (i.e., the morphological differentiation does not take place even at 37°C) are avirulent (133, 289). The use of genomics has been key in the identification of alleles involved in virulence.
Integrative approaches using various types of mutagenesis and genomics have led to the identification of alleles and genetic interactions involved in virulence. Nguyen and Sil (30) coupled Agrobacterium-mediated mutagenesis and genomics to identify the genes necessary for the mold-to-yeast transition. First, they leveraged a library of T-DNA insertional mutants and screened for Histoplasma colonies that failed to transition to the yeast phase at 37°C. This led to the identification of the transcriptional regulator Ryp1. Ryp1 is a homolog to the Candida albicans Wor1, a transcriptional regulator required for the white-opaque switch necessary for C. albicans mating (290, 291). A subsequent whole-genome microarray analysis revealed differences in the transcriptional profile of wild-type Histoplasma RYP1 and the insertional mutant ryp1. Ryp1 was found to be a transcriptional regulator important for the morphological switch in response to temperature (30). Mit1 was identified as another homolog of Ryp1 that controls pseudohyphal formation in Saccharomyces cerevisiae (292, 293). A follow-up study using full genome chromatin immunoprecipitation showed that Mit1 in S. cerevisiae, Wor1 in C. albicans, and Ryp1 in Histoplasma recognize the same DNA sequence, showing that these widely diverged species have evolved conserved regulatory mechanism (292). Further analyses of the same T-DNA library revealed two additional virulence factors necessary for the mold-to-yeast transition (134), Ryp2 and Ryp3. These two proteins are members of the Velvet family known to be transcription factors. Ryp1, Ryp2, and Ryp3 were shown to physically interact by co-immunoprecipitation experiments in wild-type cells grown at 37°C (135). A combination of experiments, including chip-CHIP experiments, transcriptional profiling, and RNAi knockdown, revealed that Ryp4 is a fourth member of the Ryp transcriptional regulators. Ryp1, Ryp2, and Ryp3, directly interact with each other and along with Ryp4 form a temperate-responsive transcriptional network in Histoplasma that governs the mold-to-yeast transition (135). This integrative approach serves as a blueprint for the power of comparative transcriptomics to identify virulence factors.
There is evidence of genetic variation in alleles involved in the transition to the yeast phase among Histoplasma strains. Edwards et al. (294) used RNA-seq to compare the transcriptional profiles of the saprophytic mycelial phase to the pathogenic yeast phase of two phylogenetically distinct clinical isolates, which later were identified to belong to different species (228). In addition to the expected phase-specific expression differences, they found variations in the transcriptional profiles that were strain-specific (7.6% of all genes have species-specific expression) and that correlate with variations in strain virulence, highlighting the importance of studying virulence traits in a strain-specific manner. Promoter-swap experiments have shown that some differences in gene expression were due to trans-acting factors not just the cis-regulatory elements of a gene (294).
Histoplasma genome sequencing
A fosmid-based physical mapping approach for shotgun sequence assembly was developed in the mid-2000s to sequence the genomes of five Histoplasma strains from four distinct phylogenetic clades (295). The Genome Sequencing Center at Washington University in St. Louis and the Broad Institute sequenced the following Histoplasma strains (296): (i) Panamanian strain G186A, the reference strain for H. capsulatum ss, (ii) the NAm 2 strain G217B, the reference strain for H. ohiense, (iii) the NAm 1 strain WU24, the reference strain for H. mississippiense, and (iv) strains H88 and H143, which belong to the African clade. When short-read sequencing became available, genome resequencing of dozens of isolates followed, which in turn revealed the extent of genetic variation within the genus [see Evolution (228–230)]. To date, the most updated assemblies of Histoplasma used long-read sequencing to produce almost-chromosomal assemblies. Voorhies et al. (231) sequenced and assembled de novo five Histoplasma strains (H. mississippiense WU24, H. ohiense G217B, H. capsulatum ss G816A and G184A, and H88 from the African clade) using Oxford Nanopore Technologies long-read sequencing technology, providing chromosomal-level assemblies, and highlighting the advantages that complete genome assemblies provide when drawing conclusions about the evolutionary history of fungal pathogens. Chromosomal assemblies of Histoplasma genomes revealed the existence of variable repetitive regions and highly syntenic chromosomes. Granular analyses of these assemblies revealed that RYP2, a major regulator of fungal dimorphism (134), is duplicated in the H. capsulatum G186A strain relative to G184A, which in turn might contribute to virulence, but a proper detailed evaluation of their relative virulence is needed. Expression of transposon-embedded genes in the reference strains of H. ohiense (G217B) and the African clade (H88) is upregulated in the yeast phase compared to the mycelial phase, but further studies are needed to reveal the impact that transposon-rich regions have in Histoplasma’s evolutionary history and pathogenesis (231). This study highlights the need to expand chromosomal-level assemblies to other Histoplasma strains from multiple species and geographical locations. Evaluating parallels between Histoplasma strains from different locations (e.g., North America, Latin America, and Africa) may identify genes that evolve in response to specific geographic pressures.
Even though novel developments have enhanced our understanding of the genetic underpinnings of the host-pathogen interaction between mammals and Histoplasma [e.g., genetic screenings using CRISPR have allowed for a comprehensive view of the genetic architecture of immune response to Histoplasma infection (297)]. Immunological aspects of this interaction between host cells and Histoplasma have been a subject richly explored and reviewed extensively elsewhere (298).
CONCLUSIONS
Histoplasma has revealed key insights in the nature of dimorphism, intracellular parasitism, and mycotic disease. The fungus has been an outstanding model organism compared to other fungal pathogens where characteristics, such as the multinucleated status of the cells (B. dermatitidis) or the lack of genetic tools (P. brasiliensis), have constrained the identification of virulence determinants. Now that population genomics has identified different species within the genus, the time is ripe to address how fungal pathogens diverge in their genome, in their phenotypes, and in the ways they cause disease in their hosts. Although several virulence determinants have been identified and characterized, information regarding their regulation during the yeast phase, their effects in divergent Histoplasma strains, and their requirement during different phases of infection is still limited. An evolutionary-informed and mechanistic understanding of disease progression will give insight into how virulence factors are regulated and how the presence of each impacts the course of infection with different Histoplasma species. This deep level of understanding is at hand.
ACKNOWLEDGMENTS
We would like to thank our reviewers and members of the Matute lab for their helpful comments. We thank Grant S. Jones for his contribution in the preparation of Fig. 1.
V.E.S., W.E.G., and D.R.M. were supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) under award R01AI153523 to D.R.M.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Daniel R. Matute, Email: dmatute@email.unc.edu.
Joseph Heitman, Duke University, Durham, North Carolina, USA.
REFERENCES
- 1. Casadevall A. 2017. Don’t forget the fungi when considering global catastrophic biorisks. Health Secur 15:341–342. doi: 10.1089/hs.2017.0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Almeida F, Rodrigues ML, Coelho C. 2019. The still underestimated problem of fungal diseases worldwide. Front Microbiol 10:214. doi: 10.3389/fmicb.2019.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rodrigues AM, Beale MA, Hagen F, Fisher MC, Terra PPD, de Hoog S, Brilhante RSN, de Aguiar Cordeiro R, de Souza Collares Maia Castelo-Branco D, Rocha MFG, Sidrim JJC, de Camargo ZP. 2020. The global epidemiology of emerging Histoplasma species in recent years. Stud Mycol 97:100095. doi: 10.1016/j.simyco.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bongomin F, Gago S, Oladele RO, Denning DW. 2017. Lobal and multi-national prevalence of fungal diseases—estimate precision. J Fungi (Basel) 3:57. doi: 10.3390/jof3040057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv13. doi: 10.1126/scitranslmed.3004404 [DOI] [PubMed] [Google Scholar]
- 6. Denning DW. 2016. Minimizing fungal disease deaths will allow the UNAIDS target of reducing annual AIDS deaths below 500 000 by 2020 to be realized. Philos Trans R Soc Lond B Biol Sci 371:20150468. doi: 10.1098/rstb.2015.0468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Denning DW. 2017. Calling upon all public health mycologists: to accompany the country burden papers from 14 countries. Eur J Clin Microbiol Infect Dis 36:923–924. doi: 10.1007/s10096-017-2909-8 [DOI] [PubMed] [Google Scholar]
- 8. Casadevall A. 2018. Fungal diseases in the 21st century: the near and far horizons. Pathog Immun 3:183–196. doi: 10.20411/pai.v3i2.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de S Araújo GR, Souza W de, Frases S. 2017. The hidden pathogenic potential of environmental fungi. Future Microbiol 12:1533–1540. doi: 10.2217/fmb-2017-0124 [DOI] [PubMed] [Google Scholar]
- 10. Stop neglecting fungi. 2017. Nat Microbiol 2:17123. doi: 10.1038/nmicrobiol.2017.120 [DOI] [PubMed] [Google Scholar]
- 11. Gadre A, Enbiale W, Andersen LK, Coates SJ. 2022. The effects of climate change on fungal diseases with cutaneous manifestations: a report from the international society of dermatology climate change committee. J Clim Change Health 6:100156. doi: 10.1016/j.joclim.2022.100156 [DOI] [Google Scholar]
- 12. Rodrigues ML, Albuquerque PC. 2018. Searching for a change: the need for increased support for public health and research on fungal diseases. PLoS Negl Trop Dis 12:e0006479. doi: 10.1371/journal.pntd.0006479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garcia-Solache MA, Casadevall A. 2010. Global warming will bring new fungal diseases for mammals. mBio 1:e00061-10. doi: 10.1128/mBio.00061-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nnadi NE, Carter DA. 2021. Climate change and the emergence of fungal pathogens. PLoS Pathog 17:e1009503. doi: 10.1371/journal.ppat.1009503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kenyon C, Bonorchis K, Corcoran C, Meintjes G, Locketz M, Lehloenya R, Vismer HF, Naicker P, Prozesky H, van Wyk M, Bamford C, du Plooy M, Imrie G, Dlamini S, Borman AM, Colebunders R, Yansouni CP, Mendelson M, Govender NP. 2013. A dimorphic fungus causing disseminated infection in South Africa. N Engl J Med 369:1416–1424. doi: 10.1056/NEJMoa1215460 [DOI] [PubMed] [Google Scholar]
- 16. Sun S, Hoy MJ, Heitman J. 2020. Fungal pathogens. Curr Biol 30:R1163–R1169. doi: 10.1016/j.cub.2020.07.032 [DOI] [PubMed] [Google Scholar]
- 17. Friedman DZP, Schwartz IS. 2019. Emerging fungal infections: new patients, new patterns, and new pathogens. J Fungi (Basel) 5:67. doi: 10.3390/jof5030067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Berger S, El Chazli Y, Babu AF, Coste AT. 2017. Azole resistance in Aspergillus fumigatus: a consequence of antifungal use in agriculture? Front Microbiol 8:1024. doi: 10.3389/fmicb.2017.01024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scorzoni L, de Paula E Silva ACA, Marcos CM, Assato PA, de Melo WCMA, de Oliveira HC, Costa-Orlandi CB, Mendes-Giannini MJS, Fusco-Almeida AM. 2017. Antifungal therapy: new advances in the understanding and treatment of mycosis. Front Microbiol 8:36. doi: 10.3389/fmicb.2017.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. 2017. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis 17:e383–e392. doi: 10.1016/S1473-3099(17)30316-X [DOI] [PubMed] [Google Scholar]
- 21. Ghannoum MA, Rice LB. 1999. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev 12:501–517. doi: 10.1128/CMR.12.4.501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brauer VS, Rezende CP, Pessoni AM, De Paula RG, Rangappa KS, Nayaka SC, Gupta VK, Almeida F. 2019. Antifungal agents in agriculture: friends and foes of public health. Biomolecules 9:521. doi: 10.3390/biom9100521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization . 2022. WHO fungal priority pathogens list to guide research, development and public health action. WHO. [Google Scholar]
- 24. Fisher MC, Denning DW. 2023. The WHO fungal priority pathogens list as a game-changer. Nat Rev Microbiol 21:211–212. doi: 10.1038/s41579-023-00861-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parums DV. 2022. Editorial: the world health organization (WHO) fungal priority pathogens list in response to emerging fungal pathogens during the COVID-19 pandemic. Med Sci Monit 28:e939088. doi: 10.12659/MSM.939088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galgiani JN. 1999. Coccidioidomycosis: a regional disease of national importance. Rethinking approaches for control. Ann Intern Med 130:293–300. doi: 10.7326/0003-4819-130-4-199902160-00015 [DOI] [PubMed] [Google Scholar]
- 27. Klein BS, Tebbets B. 2007. Dimorphism and virulence in fungi. Curr Opin Microbiol 10:314–319. doi: 10.1016/j.mib.2007.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maresca B, Kobayashi GS. 1989. Dimorphism in Histoplasma capsulatum: a model for the study of cell differentiation in pathogenic fungi. Microbiol Rev 53:186–209. doi: 10.1128/mr.53.2.186-209.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Holbrook ED, Rappleye CA. 2008. Histoplasma capsulatum pathogenesis: making a lifestyle switch. Curr Opin Microbiol 11:318–324. doi: 10.1016/j.mib.2008.05.010 [DOI] [PubMed] [Google Scholar]
- 30. Nguyen VQ, Sil A. 2008. Temperature-induced switch to the pathogenic yeast form of Histoplasma capsulatum requires Ryp1, a conserved transcriptional regulator. Proc Natl Acad Sci U S A 105:4880–4885. doi: 10.1073/pnas.0710448105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rooney PJ, Sullivan TD, Klein BS. 2001. Selective expression of the virulence factor BAD1 upon morphogenesis to the pathogenic yeast form of Blastomyces dermatitidis: evidence for transcriptional regulation by a conserved mechanism. Mol Microbiol 39:875–889. doi: 10.1046/j.1365-2958.2001.02300.x [DOI] [PubMed] [Google Scholar]
- 32. Sil A, Andrianopoulos A. 2014. Thermally dimorphic human fungal pathogens—polyphyletic pathogens with a convergent pathogenicity trait. Cold Spring Harb Perspect Med 5:a019794. doi: 10.1101/cshperspect.a019794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Slutsky B, Staebell M, Anderson J, Risen L, Pfaller M, Soll DR. 1987. “White-opaque transition”: a second high-frequency switching system in Candida albicans. J Bacteriol 169:189–197. doi: 10.1128/jb.169.1.189-197.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Odds FC, Kerridge D. 1985. Morphogenesis in Candida albicans. Crit Rev Microbiol 12:45–93. doi: 10.3109/10408418509104425 [DOI] [PubMed] [Google Scholar]
- 35. Taschdjian CL. 1960. Rapid identification of Candida albicans by filamentation on serum and serum substitutes. Arch Pediatr Adolesc Med 99:212. doi: 10.1001/archpedi.1960.02070030214011 [DOI] [PubMed] [Google Scholar]
- 36. Okagaki LH, Strain AK, Nielsen JN, Charlier C, Baltes NJ, Chrétien F, Heitman J, Dromer F, Nielsen K. 2010. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog 6:e1000953. doi: 10.1371/journal.ppat.1000953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zaragoza O, García-Rodas R, Nosanchuk JD, Cuenca-Estrella M, Rodríguez-Tudela JL, Casadevall A. 2010. Fungal cell gigantism during mammalian infection. PLoS Pathog 6:e1000945. doi: 10.1371/journal.ppat.1000945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Joseph Wheat L. 2003. Current diagnosis of histoplasmosis. Trends Microbiol 11:488–494. doi: 10.1016/j.tim.2003.08.007 [DOI] [PubMed] [Google Scholar]
- 39. Ajello L. 1971. Coccidioidomycosis and histoplasmosis a review of their epidemiology and geographical distribution. Mycopathol Mycol Appl 45:221–230. doi: 10.1007/BF02051969 [DOI] [PubMed] [Google Scholar]
- 40. Kauffman CA. 2007. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 20:115–132. doi: 10.1128/CMR.00027-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kandi V, Vaish R, Palange P, Bhoomagiri MR. 2016. Chronic pulmonary histoplasmosis and its clinical significance: an under-reported systemic fungal disease. Cureus 8:e751. doi: 10.7759/cureus.751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Adenis A, Nacher M, Hanf M, Vantilcke V, Boukhari R, Blachet D, Demar M, Aznar C, Carme B, Couppie P. 2014. HIV-associated histoplasmosis early mortality and incidence trends: from neglect to priority. PLoS Negl Trop Dis 8:e3100. doi: 10.1371/journal.pntd.0003100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nacher M, Adenis A, Mc Donald S, Do Socorro Mendonca Gomes M, Singh S, Lopes Lima I, Malcher Leite R, Hermelijn S, Wongsokarijo M, Van Eer M, et al. 2013. Disseminated histoplasmosis in HIV-infected patients in South America: a neglected killer continues on its rampage. PLoS Negl Trop Dis 7:e2319. doi: 10.1371/journal.pntd.0002319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eissenberg LG, Goldman WE. 1991. Histoplasma variation and adaptive strategies for parasitism: new perspectives on histoplasmosis. Clin Microbiol Rev 4:411–421. doi: 10.1128/CMR.4.4.411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wheat LJ, Azar MM, Bahr NC, Spec A, Relich RF, Hage C. 2016. Histoplasmosis. Infect Dis Clin North Am 30:207–227. doi: 10.1016/j.idc.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 46. Mukherjee T, Basu A. 2015. Disseminated histoplasmosis presenting as a case of erythema nodosum and hemophagocytic lymphohistiocytosis. Med J Armed Forces India 71:S598–S600. doi: 10.1016/j.mjafi.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gangat N, Lin Y, Elkin PL. 2005. 68-year-old man with fatigue, fever, and weight loss. Mayo Clin Proc 80:939–942. doi: 10.4065/80.7.939 [DOI] [PubMed] [Google Scholar]
- 48. Medeiros AA, Marty SD, Tosh FE, Chin TDY. 1966. Erythema nodosum and erythema multiforme as clinical manifestations of histoplasmosis in a community outbreak. N Engl J Med 274:415–420. doi: 10.1056/NEJM196602242740801 [DOI] [PubMed] [Google Scholar]
- 49. Leznoff A, Frank H, Telner P, Rosensweig J, Brandt JL. 1964. Histoplasmosis in montreal during the fall of 1963, with observations on erythema multiforme. Can Med Assoc J 91:1154–1160. [PMC free article] [PubMed] [Google Scholar]
- 50. Ozols II. 1981. Erythema nodosum in an epidemic of histoplasmosis in Indianapolis. Arch Dermatol 117:709. doi: 10.1001/archderm.1981.01650110031014 [DOI] [PubMed] [Google Scholar]
- 51. Bansal A, Drewek R. 2015. Sarcoidosis and histoplasmosis: is one a consequence of the other? A case report and review of the literature. Case Rep Rheumatol 2015:108459. doi: 10.1155/2015/108459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sellers TF, Price WN, Newberry WM. 1965. An epidemic of erythema multiforme and erythema nodosum caused by histoplasmosis. Ann Intern Med 62:1244–1262. doi: 10.7326/0003-4819-62-6-1244 [DOI] [PubMed] [Google Scholar]
- 53. Plouffe JF. 2000. Importance of atypical pathogens of community-acquired pneumonia. Clin Infect Dis 31:S35–S39. doi: 10.1086/314058 [DOI] [PubMed] [Google Scholar]
- 54. Bajaj SK, Tombach B. 2017. Respiratory infections in immunocompromised patients: lung findings using chest computed tomography. Radiol Infect Dis 4:29–37. doi: 10.1016/j.jrid.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shelhamer JH, Gill VJ, Quinn TC, Crawford SW, Kovacs JA, Masur H, Ognibene FP. 1996. The laboratory evaluation of opportunistic pulmonary infections. Ann Intern Med 124:585–599. doi: 10.7326/0003-4819-124-6-199603150-00008 [DOI] [PubMed] [Google Scholar]
- 56. Baker J, Kosmidis C, Rozaliyani A, Wahyuningsih R, Denning DW. 2020. Chronic pulmonary histoplasmosis—a scoping literature review. Open Forum Infect Dis 7:faa119. doi: 10.1093/ofid/ofaa119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Linder KA, Kauffman CA. 2019. Histoplasmosis: epidemiology, diagnosis, and clinical manifestations. Curr Fungal Infect Rep 13:120–128. doi: 10.1007/s12281-019-00341-x [DOI] [Google Scholar]
- 58. Rubin SA, Winer-Muram HT. 1992. Thoracic histoplasmosis. J Thorac Imaging 7:39–50. doi: 10.1097/00005382-199209000-00007 [DOI] [PubMed] [Google Scholar]
- 59. Prager RL, Burney DP, Waterhouse G, Bender HW. 1980. Pulmonary, mediastinal, and cardiac presentations of histoplasmosis. Ann Thorac Surg 30:385–390. doi: 10.1016/s0003-4975(10)61279-9 [DOI] [PubMed] [Google Scholar]
- 60. Schade MA, Mirani NM. 2013. Fibrosing mediastinitis: an unusual cause of pulmonary symptoms. J Gen Intern Med 28:1677–1681. doi: 10.1007/s11606-013-2528-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Goodwin RA, Nickell JA, Des Prez RM. 1972. Mediastinal fibrosis complicating healed primary histoplasmosis and tuberculosis. Medicine (Baltimore) 51:227–246. doi: 10.1097/00005792-197205000-00008 [DOI] [PubMed] [Google Scholar]
- 62. Azar MM, Loyd JL, Relich RF, Wheat LJ, Hage CA. 2020. Current concepts in the epidemiology, diagnosis, and management of histoplasmosis syndromes. Semin Respir Crit Care Med 41:13–30. doi: 10.1055/s-0039-1698429 [DOI] [PubMed] [Google Scholar]
- 63. Hage CA, Azar MM, Bahr N, Loyd J, Wheat LJ. 2015. Histoplasmosis: up-to-date evidence-based approach to diagnosis and management. Semin Respir Crit Care Med 36:729–745. doi: 10.1055/s-0035-1562899 [DOI] [PubMed] [Google Scholar]
- 64. Khalid M, Khan I, Rahman Z, Alazzeh A, Youssef D. 2018. Fibrosing mediastinitis: uncommon life-threatening complication of histoplasmosis. Cureus 10:e2532. doi: 10.7759/cureus.2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Loyd JE, Tillman BF, Atkinson JB, Des Prez RM. 1988. Mediastinal fibrosis complicating histoplasmosis. Medicine (Baltimore) 67:295–310. doi: 10.1097/00005792-198809000-00002 [DOI] [PubMed] [Google Scholar]
- 66. Brcic L. 2018. Granulomatous mediastinitis. In Allen TC, Suster S (ed), Pathology of the pleura and mediastinum. Springer, Cham. [Google Scholar]
- 67. Wheat LJ, Conces D, Allen SD, Blue-Hnidy D, Loyd J. 2004. Pulmonary histoplasmosis syndromes: recognition, diagnosis, and management. Semin Respir Crit Care Med 25:129–144. doi: 10.1055/s-2004-824898 [DOI] [PubMed] [Google Scholar]
- 68. Goodwin R A, Loyd JE, Des Prez RM. 1981. Histoplasmosis in normal hosts. Medicine (Baltimore) 60:231–266. doi: 10.1097/00005792-198107000-00001 [DOI] [PubMed] [Google Scholar]
- 69. Goodwin RA, Owens FT, Snell JD, Hubbard WW, Buchanan RD, Terry RT, Des Prez RM. 1976. Chronic pulmonary histoplasmosis. Medicine 55:413–452. doi: 10.1097/00005792-197611000-00001 [DOI] [PubMed] [Google Scholar]
- 70. Parsons RJ, Zarafonetis CJ. 1945. Histoplasmosis in man: report of seven cases and a review of seventy-one cases. Arch Intern Med 75:1. doi: 10.1001/archinte.1945.00210250008001 [DOI] [Google Scholar]
- 71. Bonner JR. 1984. Disseminated histoplasmosis in patients with the acquired immune deficiency syndrome. Arch Intern Med 144:2178. doi: 10.1001/archinte.1984.04400020088014 [DOI] [PubMed] [Google Scholar]
- 72. Assi MA, Sandid MS, Baddour LM, Roberts GD, Walker RC. 2007. Systemic histoplasmosis: a 15-year retrospective institutional review of 111 patients. Medicine (Baltimore) 86:162–169. doi: 10.1097/md.0b013e3180679130 [DOI] [PubMed] [Google Scholar]
- 73. Xiong X, Fan L, Kang M, Wei J, Cheng D. 2017. Disseminated histoplasmosis: a rare clinical phenotype with difficult diagnosis. Respirol Case Rep 5:e00220. doi: 10.1002/rcr2.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Saucedo-Crespo H, Mehta T, Sakpal SV, Auvenshine C, Santella RN, Nazir J, Steers J. 2020. Histoplasma capsulatum presenting as generalized lymphadenopathy after renal transplantation. IDCases 19:e00692. doi: 10.1016/j.idcr.2019.e00692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pastor TA, Holcomb MJ, Motaparthi K, Grekin SJ, Hsu S. 2011. Disseminated histoplasmosis mimicking secondary syphilis. Dermatol Online J 17:10. [PubMed] [Google Scholar]
- 76. Harnalikar M, Kharkar V, Khopkar U. 2012. Disseminated cutaneous histoplasmosis in an immunocompetent adult. Indian J Dermatol 57:206–209. doi: 10.4103/0019-5154.96194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wheat LJ, Freifeld AG, Kleiman MB, Baddley JW, McKinsey DS, Loyd JE, Kauffman CA, Infectious Diseases Society of America . 2007. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the infectious diseases society of America. Clin Infect Dis 45:807–825. doi: 10.1086/521259 [DOI] [PubMed] [Google Scholar]
- 78. Vaidya OU, Vaidya AO, Patil HR, Huseth H. 2012. Fulminant sepsis due to disseminated histoplasmosis in renal transplantation: a diagnostic challenge. J Acute Med 2:55–57. doi: 10.1016/j.jacme.2012.03.002 [DOI] [Google Scholar]
- 79. Fernandes AR, Viana LA, Mansur JB, Françoso M de M, Santos D de C, Silva HT, Pestana JOM. 2018. Sepsis-like histoplasmosis in a kidney transplant patient. J Bras Nefrol 40:95–97. doi: 10.1590/1678-4685-JBN-3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nacher M, Valdes A, Adenis A, Blaizot R, Abboud P, Demar M, Djossou F, Epelboin L, Misslin C, Ntab B, Louvel D, Drak Alsibai K, Couppié P. 2021. Gastrointestinal disseminated histoplasmosis in HIV-infected patients: a descriptive and comparative study. PLoS Negl Trop Dis 15:e0009050. doi: 10.1371/journal.pntd.0009050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dahiya D, Kichloo A, Singh J, Albosta M, Wani F. 2021. Histoplasmosis and inflammatory bowel disease: a case report. World J Gastrointest Endosc 13:24–32. doi: 10.4253/wjge.v13.i1.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Psarros G, Kauffman CA. 2007. Colonic histoplasmosis: a difficult diagnostic problem. Gastroenterol Hepatol (N Y) 3:461–463. [PMC free article] [PubMed] [Google Scholar]
- 83. Larbcharoensub N, Boonsakan P, Aroonroch R, Rochanawutanon M, Nitiyanant P, Phongkitkarun S, Poonvutikul S, Watcharananan SP, Ngarmukos C. 2011. Adrenal histoplasmosis: a case series and review of the literature. Southeast Asian J Trop Med Public Health 42:920–925. [PubMed] [Google Scholar]
- 84. Wheat J, Myint T, Guo Y, Kemmer P, Hage C, Terry C, Azar MM, Riddell J, Ender P, Chen S, et al. 2018. Central nervous system histoplasmosis: multicenter retrospective study on clinical features, diagnostic approach and outcome of treatment. Medicine (Baltimore) 97:e0245. doi: 10.1097/MD.0000000000010245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wheat LJ, Batteiger BE, Sathapatayavongs B. 1990. Histoplasma capsulatum infections of the central nervous system: a clinical review. Medicine 69:244. doi: 10.1097/00005792-199007000-00006 [DOI] [PubMed] [Google Scholar]
- 86. Bhatti S, Vilenski L, Tight R, Smego RA. 2005. Histoplasma endocarditis: clinical and mycologic features and outcomes. J Infect 51:2–9. doi: 10.1016/j.jinf.2004.10.002 [DOI] [PubMed] [Google Scholar]
- 87. Chawla A, Chawla K, Thomas J. 2012. Genitourinary histoplasmosis in post-renal transplant patient: diagnostic dilemma. Indian J Urol 28:359–361. doi: 10.4103/0970-1591.102730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Friskel E, Klotz SA, Bartholomew W, Dixon A. 2000. Two unusual presentations of urogenital histoplasmosis and a review of the literature. Clin Infect Dis 31:189–191. doi: 10.1086/313904 [DOI] [PubMed] [Google Scholar]
- 89. Sanha V, do Valle Barbosa GRG, Miranda B, Bastos RH, Pasqualotto AC. 2022. Epididymo-orchitis caused by Histoplasma capsulatum. Med Mycol Case Rep 37:4–7. doi: 10.1016/j.mmcr.2022.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gass M, Kobayashi GS. 1969. Histoplasmosis: an illustrative case with unusual vaginal and joint involvement. Arch Dermatol 100:724–727. doi: 10.1001/archderm.100.6.724 [DOI] [PubMed] [Google Scholar]
- 91. Roy M, Roy AK, Upalakalin JN, Ahmad S. 2020. Isolated non-resolving vulvar lesion as a presentation of disseminated histoplasmosis in a woman with HIV. Eur J Case Rep Intern Med 7:001814. doi: 10.12890/2020_001814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Shine MM, Shah MM, Hanna CA, Bevis KS. 2013. Vulvar histoplasmosis as a rare cause of genital ulceration. Obstet Gynecol 122:449–452. doi: 10.1097/AOG.0b013e31829ce0f1 [DOI] [PubMed] [Google Scholar]
- 93. Strayer DS, Gutwein MB, Herbold D, Bresalier R. 1981. Histoplasmosis presenting as the carpal tunnel syndrome. Am J Surg 141:286–288. doi: 10.1016/0002-9610(81)90177-x [DOI] [PubMed] [Google Scholar]
- 94. Prasetyo M, Adistana IM, Setiawan SI. 2021. Tuberculous septic arthritis of the hip with large abscess formation mimicking soft tissue tumors: a case report. Heliyon 7:e06815. doi: 10.1016/j.heliyon.2021.e06815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Berbari HE, Gurram P, Mahmood M, Deziel PJ, Walker RC, Razonable RR. 2021. Prosthetic joint infections due to Histoplasma capsulatum: a report of 3 cases. Mayo Clin Proc Innov Qual Outcomes 5:225–229. doi: 10.1016/j.mayocpiqo.2020.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nel JS, Bartelt LA, van Duin D, Lachiewicz AM. 2018. Endemic mycoses in solid organ transplant recipients. Infect Dis Clin North Am 32:667–685. doi: 10.1016/j.idc.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rammaert B, Gamaletsou MN, Zeller V, Elie C, Prinapori R, Taj-Aldeen SJ, Roilides E, Kontoyiannis DP, Brause B, Sipsas NV, Walsh TJ, Lortholary O. 2014. Dimorphic fungal osteoarticular infections. Eur J Clin Microbiol Infect Dis 33:2131–2140. doi: 10.1007/s10096-014-2149-0 [DOI] [PubMed] [Google Scholar]
- 98. Huang L, Wu Y, Miao X. 2013. Localized Histoplasma capsulatum osteomyelitis of the fibula in an immunocompetent teenage boy: a case report. BMC Infect Dis 13:132. doi: 10.1186/1471-2334-13-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Klofkorn RW, Steigerwald JC. 1976. Carpal tunnel syndrome as the initial manifestation of tuberculosis. Am J Med 60:583–586. doi: 10.1016/0002-9343(76)90727-0 [DOI] [PubMed] [Google Scholar]
- 100. Armstrong-James D, Meintjes G, Brown GD. 2014. A neglected epidemic: fungal infections in HIV/AIDS. Trends Microbiol 22:120–127. doi: 10.1016/j.tim.2014.01.001 [DOI] [PubMed] [Google Scholar]
- 101. Denning DW. 2015. The ambitious “95-95 by 2025” roadmap for the diagnosis and management of fungal diseases. Thorax 70:613–614. doi: 10.1136/thoraxjnl-2015-207305 [DOI] [PubMed] [Google Scholar]
- 102. Chase HV, Campbell CC. 1962. Histoplasmin skin test: survey of elementary school children in Frederick County, Md. JAMA 182:335–338. doi: 10.1001/jama.1962.03050430009003 [DOI] [PubMed] [Google Scholar]
- 103. Manos NE, Ferebee SH, Kerschbaum WF. 1956. Geographic variation in the prevalence of histoplasmin sensitivity. Dis Chest 29:649–668. doi: 10.1378/chest.29.6.649 [DOI] [PubMed] [Google Scholar]
- 104. Scully MC, Baddley JW. 2018. Epidemiology of histoplasmosis. Curr Fungal Infect Rep 12:51–58. doi: 10.1007/s12281-018-0309-x [DOI] [Google Scholar]
- 105. Taylor ML, del Reyes - Montes MR, Chávez-Tapia CB, Curiel-Quesada E, Duarte-Escalante E, Rodriguez- Arellanes G, Peña-Sandoval GR, Valenzuela-Tovar F. 2000. Ecology and molecular epidemiology findings of Histoplasma capsulatum in Mexico. Res Adv Microbiol 1:29–35. [Google Scholar]
- 106. Oladele RO, Toriello C, Ogunsola FT, Ayanlowo OO, Foden P, Fayemiwo AS, Osaigbovo II, Iwuafor AA, Shettima S, Ekundayo HA, Richardson MD, Denning DW. 2018. Prior subclinical histoplasmosis revealed in Nigeria using histoplasmin skin testing. PLoS One 13:e0196224. doi: 10.1371/journal.pone.0196224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Colombo AL, Tobón A, Restrepo A, Queiroz-Telles F, Nucci M. 2011. Epidemiology of endemic systemic fungal infections in Latin America. Med Mycol:1–14. doi: 10.3109/13693786.2011.577821 [DOI] [PubMed] [Google Scholar]
- 108. Graybill JR. 1988. Histoplasmosis and AIDS. J Infect Dis 158:623–626. doi: 10.1093/infdis/158.3.623 [DOI] [PubMed] [Google Scholar]
- 109. Davies SF, Khan M, Sarosi GA. 1978. Disseminated histoplasmosis in immunologically suppressed patients: occurrence in a nonendemic area. Am J Med 64:94–100. doi: 10.1016/0002-9343(78)90183-3 [DOI] [PubMed] [Google Scholar]
- 110. Davies SF, Sarosi GA, Peterson PK, Khan M, Howard RJ, Simmons RL, Najarian JS. 1979. Disseminated histoplasmosis in renal transplant recipients. Am J Surg 137:686–691. doi: 10.1016/0002-9610(79)90050-3 [DOI] [PubMed] [Google Scholar]
- 111. Cuellar-Rodriguez J, Avery RK, Lard M, Budev M, Gordon SM, Shrestha NK, van Duin D, Oethinger M, Mawhorter SD. 2009. Histoplasmosis in solid organ transplant recipients: 10 years of experience at a large transplant center in an endemic area. Clin Infect Dis 49:710–716. doi: 10.1086/604712 [DOI] [PubMed] [Google Scholar]
- 112. Sebghati TS, Engle JT, Goldman WE. 2000. Intracellular parasitism by Histoplasma capsulatum: fungal virulence and calcium dependence. Science 290:1368–1372. doi: 10.1126/science.290.5495.1368 [DOI] [PubMed] [Google Scholar]
- 113. Rappleye CA, Engle JT, Goldman WE. 2004. RNA interference in Histoplasma capsulatum demonstrates a role for alpha-(1,3)-glucan in virulence. Mol Microbiol 53:153–165. doi: 10.1111/j.1365-2958.2004.04131.x [DOI] [PubMed] [Google Scholar]
- 114. Edwards JA, Alore EA, Rappleye CA. 2011. The yeast-phase virulence requirement for α-glucan synthase differs among Histoplasma capsulatum chemotypes. Eukaryot Cell 10:87–97. doi: 10.1128/EC.00214-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Garfoot AL, Shen Q, Wüthrich M, Klein BS, Rappleye CA. 2016. The Eng1 β-glucanase enhances Histoplasma virulence by reducing β-glucan exposure. mBio 7:e01388-15. doi: 10.1128/mBio.01388-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Garfoot AL, Dearing KL, VanSchoiack AD, Wysocki VH, Rappleye CA. 2017. Eng1 and Exg8 are the major β-glucanases secreted by the fungal pathogen Histoplasma capsulatum. J Biol Chem 292:4801–4810. doi: 10.1074/jbc.M116.762104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Marion CL, Rappleye CA, Engle JT, Goldman WE. 2006. An alpha-(1,4)-amylase is essential for alpha-(1,3)-glucan production and virulence in Histoplasma capsulatum. Mol Microbiol 62:970–983. doi: 10.1111/j.1365-2958.2006.05436.x [DOI] [PubMed] [Google Scholar]
- 118. Bohse ML, Woods JP. 2007. RNA interference-mediated silencing of the YPS3 gene of Histoplasma capsulatum reveals virulence defects. Infect Immun 75:2811–2817. doi: 10.1128/IAI.00304-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Brechting PJ, Shah C, Rakotondraibe L, Shen Q, Rappleye CA. 2023. Histoplasma capsulatum requires peroxisomes for multiple virulence functions including siderophore biosynthesis. mBio 14:e0328422. doi: 10.1128/mbio.03284-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Dade J, DuBois JC, Pasula R, Donnell AM, Caruso JA, Smulian AG, Deepe GS. 2016. HcZrt2, a zinc responsive gene, is indispensable for the survival of Histoplasma capsulatum in vivo. Med Mycol 54:865–875. doi: 10.1093/mmy/myw045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hwang LH, Mayfield JA, Rine J, Sil A. 2008. Histoplasma requires SID1, a member of an iron-regulated siderophore gene cluster, for host colonization. PLoS Pathog 4:e1000044. doi: 10.1371/journal.ppat.1000044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hilty J, George Smulian A, Newman SL. 2011. Histoplasma capsulatum utilizes siderophores for intracellular iron acquisition in macrophages. Med Mycol 49:633–642. doi: 10.3109/13693786.2011.558930 [DOI] [PubMed] [Google Scholar]
- 123. Joehnk B, Ali N, Voorhies M, Walcott K, Sil A. 2023. Recyclable CRISPR/Cas9-mediated gene disruption and deletions in Histoplasma. mSphere 8:e0037023. doi: 10.1128/msphere.00370-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zarnowski R, Cooper KG, Brunold LS, Calaycay J, Woods JP. 2008. Histoplasma capsulatum secreted gamma-glutamyltransferase reduces iron by generating an efficient ferric reductant. Mol Microbiol 70:352–368. doi: 10.1111/j.1365-2958.2008.06410.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hilty J, Smulian AG, Newman SL. 2008. The Histoplasma capsulatum vacuolar ATPase is required for iron homeostasis, intracellular replication in macrophages and virulence in a murine model of histoplasmosis. Mol Microbiol 70:127–139. doi: 10.1111/j.1365-2958.2008.06395.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Shen Q, Beucler MJ, Ray SC, Rappleye CA. 2018. Macrophage activation by IFN-γ triggers restriction of phagosomal copper from intracellular pathogens. PLoS Pathog 14:e1007444. doi: 10.1371/journal.ppat.1007444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rappleye CA. 2023. Targeted gene deletions in the dimorphic fungal pathogen Histoplasma using an optimized episomal CRISPR/Cas9 system. mSphere 8:e0017823. doi: 10.1128/msphere.00178-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Garfoot AL, Zemska O, Rappleye CA. 2014. Histoplasma capsulatum depends on de novo vitamin biosynthesis for intraphagosomal proliferation. Infect Immun 82:393–404. doi: 10.1128/IAI.00824-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Shen Q, Gonzalez-Mireles A, Ray SC, Rappleye CA. 2023. Histoplasma capsulatum relies on tryptophan biosynthesis to proliferate within the macrophage phagosome. Infect Immun 91:e0005923. doi: 10.1128/iai.00059-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Edwards JA, Zemska O, Rappleye CA. 2011. Discovery of a role for Hsp82 in Histoplasma virulence through a quantitative screen for macrophage lethality. Infect Immun 79:3348–3357. doi: 10.1128/IAI.05124-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Youseff BH, Holbrook ED, Smolnycki KA, Rappleye CA. 2012. Extracellular superoxide dismutase protects Histoplasma yeast cells from host-derived oxidative stress. PLoS Pathog 8:e1002713. doi: 10.1371/journal.ppat.1002713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Holbrook ED, Smolnycki KA, Youseff BH, Rappleye CA. 2013. Redundant catalases detoxify phagocyte reactive oxygen and facilitate Histoplasma capsulatum pathogenesis. Infect Immun 81:2334–2346. doi: 10.1128/IAI.00173-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Nemecek JC, Wüthrich M, Klein BS. 2006. Global control of dimorphism and virulence in fungi. Science 312:583–588. doi: 10.1126/science.1124105 [DOI] [PubMed] [Google Scholar]
- 134. Webster RH, Sil A. 2008. Conserved factors Ryp2 and Ryp3 control cell morphology and infectious spore formation in the fungal pathogen Histoplasma capsulatum. Proc Natl Acad Sci U S A 105:14573–14578. doi: 10.1073/pnas.0806221105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Beyhan S, Gutierrez M, Voorhies M, Sil A. 2013. A temperature-responsive network links cell shape and virulence traits in a primary fungal pathogen. PLoS Biol 11:e1001614. doi: 10.1371/journal.pbio.1001614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Laskowski-Peak MC, Calvo AM, Rohrssen J, Smulian AG. 2012. VEA1 is required for cleistothecial formation and virulence in Histoplasma capsulatum. Fungal Genet Biol 49:838–846. doi: 10.1016/j.fgb.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 137. Krunic AL, Carag H, Medenica MM, Lorincz AL. 2002. A case of primary cutaneous histoplasmosis in a patient with diabetes and multi‐infarct dementia. J Dermatol 29:797–802. doi: 10.1111/j.1346-8138.2002.tb00226.x [DOI] [PubMed] [Google Scholar]
- 138. Gupta H, Yadav Kl P, Totaganti M, Kant R, Monica Devi Y. 2023. A rare case of disseminated histoplasmosis with hemophagocytic syndrome in a patient with diabetes mellitus: a case report. Cureus 15:e36333. doi: 10.7759/cureus.36333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Grim SA, Proia L, Miller R, Alhyraba M, Costas-Chavarri A, Oberholzer J, Clark NM. 2012. A multicenter study of histoplasmosis and blastomycosis after solid organ transplantation. Transpl Infect Dis 14:17–23. doi: 10.1111/j.1399-3062.2011.00658.x [DOI] [PubMed] [Google Scholar]
- 140. Franklin AD, Larson L, Rauseo AM, Rutjanawech S, Hendrix MJ, Powderly WG, Spec A. 2021. A comparison of presentations and outcomes of histoplasmosis across patients with varying immune status. Med Mycol 59:624–633. doi: 10.1093/mmy/myaa112 [DOI] [PubMed] [Google Scholar]
- 141. Ceccato F, Gongora V, Zunino A, Roverano S, Paira S. 2007. Unusual manifestation of histoplasmosis in connective tissue diseases. Clin Rheumatol 26:1717–1719. doi: 10.1007/s10067-007-0655-5 [DOI] [PubMed] [Google Scholar]
- 142. Gnat S, Łagowski D, Nowakiewicz A, Dyląg M. 2021. A global view on fungal infections in humans and animals: infections caused by dimorphic fungi and dermatophytoses. J Appl Microbiol 131:2688–2704. doi: 10.1111/jam.15084 [DOI] [PubMed] [Google Scholar]
- 143. neglected histoplasmosis in Latin America Group . 2016. Disseminated histoplasmosis in Central and South America, the invisible elephant: the lethal blind spot of international health organizations. AIDS 30:167–170. doi: 10.1097/QAD.0000000000000961 [DOI] [PubMed] [Google Scholar]
- 144. Adenis AA, Valdes A, Cropet C, McCotter OZ, Derado G, Couppie P, Chiller T, Nacher M. 2018. Burden of HIV-associated histoplasmosis compared with tuberculosis in Latin America: a modelling study. Lancet Infect Dis 18:1150–1159. doi: 10.1016/S1473-3099(18)30354-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Adenis A, Nacher M, Hanf M, Basurko C, Dufour J, Huber F, Aznar C, Carme B, Couppie P. 2014. Tuberculosis and histoplasmosis among human immunodeficiency virus–infected patients: a comparative study. Am J Trop Med Hyg 90:216–223. doi: 10.4269/ajtmh.13-0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. de Almeida JN, Peçanha-Pietrobom PM, Colombo AL. 2018. Paracoccidioidomycosis in immunocompromised patients: a literature review. J Fungi (Basel) 5:2. doi: 10.3390/jof5010002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Bradsher RW, Chapman SW, Pappas PG. 2003. Blastomycosis. Infect Dis Clin North Am 17:21–40, doi: 10.1016/s0891-5520(02)00038-7 [DOI] [PubMed] [Google Scholar]
- 148. Nacher M, Blanchet D, Bongomin F, Chakrabarti A, Couppié P, Demar M, Denning DW, Djossou F, Epelboin L, Govender N, Leitão T, Mac Donald S, Mandengue C, Marques da Silva SH, Oladele R, Panizo MM, Pasqualotto A, Ramos R, Swaminathan S, Rodriguez-Tudela JL, Vreden S, Zancopé-Oliveira R, Adenis A. 2018. Histoplasma capsulatum antigen detection tests as an essential diagnostic tool for patients with advanced HIV disease in low and middle income countries: a systematic review of diagnostic accuracy studies. PLoS Negl Trop Dis 12:e0006802. doi: 10.1371/journal.pntd.0006802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wheat J, Hafner R, Korzun AH, Limjoco MT, Spencer P, Larsen RA, Hecht FM, Powderly W. 1995. Itraconazole treatment of disseminated histoplasmosis in patients with the acquired immunodeficiency syndrome. Am J Med 98:336–342. doi: 10.1016/s0002-9343(99)80311-8 [DOI] [PubMed] [Google Scholar]
- 150. Miller AC, Arakkal AT, Koeneman SH, Cavanaugh JE, Thompson GR, Baddley JW, Polgreen PM. 2022. Frequency and duration of, and risk factors for, diagnostic delays associated with histoplasmosis. J Fungi (Basel) 8:438. doi: 10.3390/jof8050438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Nacher M, Leitao TS, Gómez BL, Couppié P, Adenis A, Damasceno L, Demar M, Samayoa B, Cáceres DH, Pradinaud R, Sousa A de Q, Arathoon E, Restrepo A. 2019. The fight against HIV-associated disseminated histoplasmosis in the Americas: unfolding the different stories of four centers. J Fungi (Basel) 5:51. doi: 10.3390/jof5020051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Bongomin F, Kwizera R, Baruch Baluku J, Asio LG, Otu AA. 2020. Treatment of histoplasmosis, p 69–79. In Histoplasma and histoplasmosis. IntechOpen. [Google Scholar]
- 153. La Hoz RM, Loyd JE, Wheat LJ, Baddley JW. 2013. How I treat histoplasmosis. Curr Fungal Infect Rep 7:36–43. doi: 10.1007/s12281-012-0117-7 [DOI] [Google Scholar]
- 154. Slama TG. 1983. Treatment of disseminated and progressive cavitary histoplasmosis with ketoconazole. Am J Med 74:70–73. doi: 10.1016/0002-9343(83)90517-x [DOI] [PubMed] [Google Scholar]
- 155. Dismukes WE, Bradsher RW, Cloud GC, Kauffman CA, Chapman SW, George RB, Stevens DA, Girard WM, Saag MS, Bowles-Patton C. 1992. Itraconazole therapy for blastomycosis and histoplasmosis. Am J Med 93:489–497. doi: 10.1016/0002-9343(92)90575-v [DOI] [PubMed] [Google Scholar]
- 156. Goodwin ML, Drew RH. 2008. Antifungal serum concentration monitoring: an update. J Antimicrob Chemother 61:17–25. doi: 10.1093/jac/dkm389 [DOI] [PubMed] [Google Scholar]
- 157. Finquelievich J, Landaburu MF, Pinoni V, Iovannitti CA. 2011. Determination of the therapeutic activity of caspofungin compared with the amphotericin B in an animal experimental model of histoplasmosis in hamster (Mesocrisetus auratus). Rev Iberoam Micol 28:155–158. doi: 10.1016/j.riam.2011.03.006 [DOI] [PubMed] [Google Scholar]
- 158. Espinel-Ingroff A. 1998. Comparison of in vitro activities of the new triazole Sch56592 and the echinocandins MK-0991 (L-743,872) and Ly303366 against opportunistic filamentous and dimorphic fungi and yeasts. J Clin Microbiol 36:2950–2956. doi: 10.1128/JCM.36.10.2950-2956.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Kohler S, Wheat LJ, Connolly P, Schnizlein-Bick C, Durkin M, Smedema M, Goldberg J, Brizendine E. 2000. Comparison of the echinocandin caspofungin with amphotericin B for treatment of histoplasmosis following pulmonary challenge in a murine model. Antimicrob Agents Chemother 44:1850–1854. doi: 10.1128/AAC.44.7.1850-1854.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Goughenour KD, Rappleye CA. 2017. Antifungal therapeutics for dimorphic fungal pathogens. Virulence 8:211–221. doi: 10.1080/21505594.2016.1235653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Cartledge JD, Midgely J, Gazzard BG. 1997. Itraconazole solution: higher serum drug concentrations and better clinical response rates than the capsule formulation in acquired immunodeficiency syndrome patients with candidosis. J Clin Pathol 50:477–480. doi: 10.1136/jcp.50.6.477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Piérard GE, Arrese JE, Piérard-Franchimont C. 2000. Itraconazole. Expert Opin Pharmacother 1:287–304. doi: 10.1517/14656566.1.2.287 [DOI] [PubMed] [Google Scholar]
- 163. Johnson PC, Wheat LJ, Cloud GA, Goldman M, Lancaster D, Bamberger DM, Powderly WG, Hafner R, Kauffman CA, Dismukes WE, U.S. National Institute of Allergy and Infectious Diseases Mycoses Study Group . 2002. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS. Ann Intern Med 137:105–109. doi: 10.7326/0003-4819-137-2-200207160-00008 [DOI] [PubMed] [Google Scholar]
- 164. Mazi PB, Arnold SR, Baddley JW, Bahr NC, Beekmann SE, McCarty TP, Polgreen PM, Rauseo AM, Spec A. 2022. Management of histoplasmosis by infectious disease physicians. Open Forum Infect Dis 9:ofac313. doi: 10.1093/ofid/ofac313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. LeMonte AM, Washum KE, Smedema ML, Schnizlein-Bick C, Kohler SM, Wheat LJ. 2000. Amphotericin B combined with itraconazole or fluconazole for treatment of histoplasmosis. J Infect Dis 182:545–550. doi: 10.1086/315717 [DOI] [PubMed] [Google Scholar]
- 166. Thompson GR, Le T, Chindamporn A, Kauffman CA, Alastruey-Izquierdo A, Ampel NM, Andes DR, Armstrong-James D, Ayanlowo O, Baddley JW, et al. 2021. Global guideline for the diagnosis and management of the endemic mycoses: an initiative of the European confederation of medical mycology in cooperation with the international society for human and animal mycology. Lancet Infect Dis 21:e364–e374. doi: 10.1016/S1473-3099(21)00191-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Goughenour KD, Balada-Llasat J-M, Rappleye CA. 2015. Quantitative microplate-based growth assay for determination of antifungal susceptibility of Histoplasma capsulatum yeasts. J Clin Microbiol 53:3286–3295. doi: 10.1128/JCM.00795-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Diaz JH. 2018. Environmental and wilderness-related risk factors for histoplasmosis: more than bats in caves. Wilderness Environ Med 29:531–540. doi: 10.1016/j.wem.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 169. Norkaew T, Ohno H, Sriburee P, Tanabe K, Tharavichitkul P, Takarn P, Puengchan T, Bumrungsri S, Miyazaki Y. 2013. Detection of environmental sources of Histoplasma capsulatum in Chiang Mai, Thailand, by nested PCR. Mycopathologia 176:395–402. doi: 10.1007/s11046-013-9701-9 [DOI] [PubMed] [Google Scholar]
- 170. de Perio MA, Benedict K, Williams SL, Niemeier-Walsh C, Green BJ, Coffey C, Di Giuseppe M, Toda M, Park J-H, Bailey RL, Nett RJ. 2021. Occupational histoplasmosis: epidemiology and prevention measures. J Fungi (Basel) 7:510. doi: 10.3390/jof7070510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Kwon-Chung KJ. 1972. Emmonsiella capsulata: perfect state of Histoplasma capsulatum. Science 177:368–369. doi: 10.1126/science.177.4046.368 [DOI] [PubMed] [Google Scholar]
- 172. Kwon-Chung KJ. 1973. Studies on Emmonsiella capsulata. I. Heterothallism and development of the ascocarp. Mycologia 65:109–121. doi: 10.1080/00275514.1973.12019409 [DOI] [PubMed] [Google Scholar]
- 173. Kwon-Chung KJ. 1975. Perfect state (Emmonsiella capsulata) of the fungus causing large-form African histoplasmosis. Mycologia 67:980–990. doi: 10.1080/00275514.1975.12019831 [DOI] [PubMed] [Google Scholar]
- 174. Kwon-Chung KJ, Weeks RJ, Larsh HW. 1974. Studies on Emmonsiella capsulata (Histoplasma capsulatum). II. Distribution of the two mating types in 13 endemic states of the United States. Am J Epidemiol 99:44–49. doi: 10.1093/oxfordjournals.aje.a121583 [DOI] [PubMed] [Google Scholar]
- 175. Laux K, Teixeira M de M, Barker B. 2023. Love in the time of climate change: a review of sexual reproduction in the order onygenales. Fungal Genet Biol 167:103797. doi: 10.1016/j.fgb.2023.103797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Bubnick M, Smulian AG. 2007. The MAT1 locus of Histoplasma capsulatum is responsive in a mating type-specific manner. Eukaryot Cell 6:616–621. doi: 10.1128/EC.00020-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Coppin E, Debuchy R, Arnaise S, Picard M. 1997. Mating types and sexual development in filamentous ascomycetes. Microbiol Mol Biol Rev 61:411–428. doi: 10.1128/mmbr.61.4.411-428.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Fraser JA, Stajich JE, Tarcha EJ, Cole GT, Inglis DO, Sil A, Heitman J. 2007. Evolution of the mating type locus: insights gained from the dimorphic primary fungal pathogens Histoplasma capsulatum, Coccidioides immitis, and Coccidioides posadasii. Eukaryot Cell 6:622–629. doi: 10.1128/EC.00018-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Turgeon BG, Yoder OC. 2000. Proposed nomenclature for mating type genes of filamentous ascomycetes. Fungal Genet Biol 31:1–5. doi: 10.1006/fgbi.2000.1227 [DOI] [PubMed] [Google Scholar]
- 180. Glass NL, Kuldau GA. 1992. Mating type and vegetative incompatibility in filamentous ascomycetes. Annu Rev Phytopathol 30:201–224. doi: 10.1146/annurev.py.30.090192.001221 [DOI] [PubMed] [Google Scholar]
- 181. Debuchy R, Berteaux-Lecellier V, Silar P. 2014. Mating systems and sexual morphogenesis in ascomycetes, p 499–535. In Borkovich KA, Ebbole DJ (ed), Cellular and molecular biology of filamentous fungi. ASM Press, Washington, DC, USA. [Google Scholar]
- 182. Löpez Daneri G, Mujica MT. 2020. Frequency of the mating-type (MAT1) in Histoplasma capsulatum isolates from Buenos Aires, Argentina. Mycopathologia 185:169–174. doi: 10.1007/s11046-019-00402-2 [DOI] [PubMed] [Google Scholar]
- 183. Efrim ND, Dumea E, Cernat RC. 2023. Epidemiology of histoplasmosis. In Dantes E, Dumea E (ed), Histoplasmosis. IntechOpen, Rijeka. [Google Scholar]
- 184. Ashraf N, Kubat RC, Poplin V, Adenis AA, Denning DW, Wright L, McCotter O, Schwartz IS, Jackson BR, Chiller T, Bahr NC. 2020. Re-drawing the maps for endemic mycoses. Mycopathologia 185:843–865. doi: 10.1007/s11046-020-00431-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Edwards LB, Acquaviva FA, Livesay VT, Cross FW, Palmer CE. 1969. An atlas of sensitivity to tuberculin, PPD-B, and histoplasmin in the United States. Am Rev Respir Dis 99:Suppl. [PubMed] [Google Scholar]
- 186. Maiga AW, Deppen S, Scaffidi BK, Baddley J, Aldrich MC, Dittus RS, Grogan EL. 2018. Mapping Histoplasma capsulatum exposure, United States. Emerg Infect Dis 24:1835–1839. doi: 10.3201/eid2410.180032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Centers for Disease Control and Prevention . 2013. Histoplasmosis in a state where it is not known to be endemic--Montana, 2012-2013. MMWR Morb Mortal Wkly Rep 62:834–837. [PMC free article] [PubMed] [Google Scholar]
- 188. Centers for Disease Control and Prevention (CDC) . 2004. Outbreak of histoplasmosis among industrial plant workers--Nebraska, 2004. MMWR Morb Mortal Wkly Rep 53:1020–1022. [PubMed] [Google Scholar]
- 189. Baddley JW, Winthrop KL, Patkar NM, Delzell E, Beukelman T, Xie F, Chen L, Curtis JR. 2011. Geographic distribution of endemic fungal infections among older persons, United States. Emerg Infect Dis 17:1664–1669. doi: 10.3201/eid1709.101987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. McKinsey DS, Pappas PG. 2019. Histoplasmosis: time to redraw the map and up our game. Clin Infect Dis. doi: 10.1093/cid/ciz327 [DOI] [PubMed] [Google Scholar]
- 191. Emmons CW. 1962. Soil reservoirs of pathogenic fungi. J Wash Acad Sci 52:3–9. [Google Scholar]
- 192. Moreira LM, Meyer W, Chame M, Brandão ML, Vivoni AM, Portugal J, Wanke B, Trilles L. 2022. Molecular detection of Histoplasma capsulatum in Antarctica. Emerg Infect Dis 28:2100–2104. doi: 10.3201/eid2810.220046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Bagal UR, Gade L, Benedict K, Howell V, Christophe N, Gibbons-Burgener S, Hallyburton S, Ireland M, McCracken S, Metobo AK, Signs K, Warren KA, Litvintseva AP, Chow NA. 2023. A phylogeographic description of Histoplasma capsulatum in the United States. J Fungi (Basel) 9:884. doi: 10.3390/jof9090884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Goos RD. 1965. Growth and survival of Histoplasma capsulatum in soil. Can J Microbiol 11:979–985. doi: 10.1139/m65-130 [DOI] [PubMed] [Google Scholar]
- 195. Gómez Londoño LF, Pérez León LC, McEwen Ochoa JG, Zuluaga Rodriguez A, Peláez Jaramillo CA, Acevedo Ruiz JM, Taylor ML, Arango Arteaga M, Jiménez Alzate M del P. 2019. Capacity of Histoplasma capsulatum to survive the composting process. Appl Environ Soil Sci 2019:1–9. doi: 10.1155/2019/5038153 [DOI] [Google Scholar]
- 196. Canteros CE, Toranzo AI, Levis S, Salas HD, López-Joffre MC, Suárez-Alvarez RO. 2020. Use of an Argentinean wildlife tissue collection for epidemiological studies of histoplasmosis. Mycopathologia 185:905–915. doi: 10.1007/s11046-020-00430-3 [DOI] [PubMed] [Google Scholar]
- 197. de Farias DM, Leite Barros F de N, Sampaio-Júnior FD, dos Santos Cruz Vieira J, de Sousa Gonçalves T, da Costa Rodrigues A de N, de Lima Macedo RC, Duarte Cerqueira V, Mendes de Oliveira AC, Souza da Paz G, Góes-Cavalcante G, Scofield A. 2023. Molecular detection of Histoplasma capsulatum in small wild mammals, dogs, and cats from areas of remaining forest in the Brazilian amazon. Transbound Emerg Dis 2023:1–9. doi: 10.1155/2023/5943212 [DOI] [Google Scholar]
- 198. Lainson R, Shaw JJ. 1975. Pneumocystis and Histoplasma infections in wild animals from the Amazon region of Brazil. Trans R Soc Trop Med Hyg 69:505–508. doi: 10.1016/0035-9203(75)90109-1 [DOI] [PubMed] [Google Scholar]
- 199. Ahasan S, Chowdhury E, Khan M, Parvin R, Azam S, Begum J, Mohiuddin G, Uddin J, Rahman M, Rahman M. 2014. Histopathological identification of histoplasmosis in animals at Dhaka Zoo. Bangladesh J Vet Med 11:177–181. doi: 10.3329/bjvm.v11i2.19145 [DOI] [Google Scholar]
- 200. Eisenberg T, Seeger H, Kasuga T, Eskens U, Sauerwald C, Kaim U. 2013. Detection and characterization of Histoplasma capsulatum in a German badger (Meles meles) by ITS sequencing and multilocus sequencing analysis. Med Mycol 51:337–344. doi: 10.3109/13693786.2012.723831 [DOI] [PubMed] [Google Scholar]
- 201. Brömel C, Sykes JE. 2005. Histoplasmosis in dogs and cats. Clin Tech Small Anim Pract 20:227–232. doi: 10.1053/j.ctsap.2005.07.003 [DOI] [PubMed] [Google Scholar]
- 202. Brandão J, Woods S, Fowlkes N, Leissinger M, Blair R, Pucheu-Haston C, Johnson J, Elster Phillips C, Tully T. 2014. Disseminated histoplasmosis (Histoplasma capsulatum) in a pet rabbit: case report and review of the literature. J Vet Diagn Invest 26:158–162. doi: 10.1177/1040638713516623 [DOI] [PubMed] [Google Scholar]
- 203. Reyes-Montes MR, Rodríguez-Arellanes G, Pérez-Torres A, Rosas-Rosas AG, Parás-García A, Juan-Sallés C, Taylor ML. 2009. Identification of the source of histoplasmosis infection in two captive maras (Dolichotis patagonum) from the same colony by using molecular and immunologic assays. Rev Argent Microbiol 41:102–104. [PubMed] [Google Scholar]
- 204. da Silva JA, Scofield A, Barros F de N, de Farias DM, Riet-Correa G, Bezerra Júnior PS, Santos TFS, Tavares GSF, Trevelin LC, da Paz GS, Cerqueira VD. 2021. Molecular detection of Histoplasma capsulatum in bats of the Amazon biome in Pará state, Brazil. Transbound Emerg Dis 68:758–766. doi: 10.1111/tbed.13740 [DOI] [PubMed] [Google Scholar]
- 205. Emmons CW. 1958. Assocation of bats with histoplasmosis. Public Health Rep (1896-1970) 73:590. doi: 10.2307/4590196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Gugnani HC, Denning DW. 2023. Infection of bats with Histoplasma species. Med Mycol 61:myad080. doi: 10.1093/mmy/myad080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Hoff GL, Bigler WJ. 1981. The role of bats in the propagation and spread of histoplasmosis: a review. J Wildl Dis 17:191–196. doi: 10.7589/0090-3558-17.2.191 [DOI] [PubMed] [Google Scholar]
- 208. Taylor ML, Reyes-Montes MDR, Estrada-Bárcenas DA, Zancopé-Oliveira RM, Rodríguez-Arellanes G, Ramírez JA. 2022. Considerations about the geographic distribution of histoplasma species. Appl Environ Microbiol 88:e0201021. doi: 10.1128/aem.02010-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Teixeira M de M, Patané JSL, Taylor ML, Gómez BL, Theodoro RC, de Hoog S, Engelthaler DM, Zancopé-Oliveira RM, Felipe MSS, Barker BM. 2016. Worldwide phylogenetic distributions and population dynamics of the genus Histoplasma. PLoS Negl Trop Dis 10:e0004732. doi: 10.1371/journal.pntd.0004732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. González-González AE, Aliouat-Denis CM, Ramírez-Bárcenas JA, Demanche C, Pottier M, Carreto-Binaghi LE, Akbar H, Derouiche S, Chabé M, Aliouat EM, Dei-Cas E, Taylor ML. 2014. Histoplasma capsulatum and Pneumocystis spp. co-infection in wild bats from Argentina, French Guyana, and Mexico. BMC Microbiol 14:23. doi: 10.1186/1471-2180-14-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Dias MAG, Oliveira RMZ, Giudice MC, Netto HM, Jordão LR, Grigorio IM, Rosa AR, Amorim J, Nosanchuk JD, Travassos LR, Taborda CP. 2011. Isolation of Histoplasma capsulatum from bats in the urban area of São Paulo state, Brazil. Epidemiol Infect 139:1642–1644. doi: 10.1017/S095026881000289X [DOI] [PubMed] [Google Scholar]
- 212. Ordóñez N, Tobón A, Arango M, Tabares A, De Bedout C, Gómez B, Castañeda E, Restrepo A. 1997. Brotes de histoplasmosis registrados en el área andina colombiana. biomedica 17:105. doi: 10.7705/biomedica.v17i2.941 [DOI] [Google Scholar]
- 213. Benedict K, Mody RK. 2016. Epidemiology of histoplasmosis outbreaks, United States, 1938–2013. Emerg Infect Dis 22:370–378. doi: 10.3201/eid2203.151117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Schwarz J, Baum GL, Wang CJ, Bingham EL, Rubel H. 1957. Successful infection of pigeons and chickens with Histoplasma capsulatum. Mycopathol Mycol Appl 8:189–193. doi: 10.1007/BF02052831 [DOI] [PubMed] [Google Scholar]
- 215. Salfelder K, Schwarz J. 1967. Histoplasma capsulatum and chickens*. Mycoses 10:337–350. doi: 10.1111/j.1439-0507.1967.tb02886.x [DOI] [Google Scholar]
- 216. Gómez LF, Arango M, McEwen JG, Gómez OM, Zuluaga A, Peláez CA, Acevedo JM, Taylor ML, Jiménez MDP. 2019. Molecular epidemiology of Colombian Histoplasma capsulatum isolates obtained from human and chicken manure samples. Heliyon 5:e02084. doi: 10.1016/j.heliyon.2019.e02084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Ajello L. 1968. Comparative morphology and immunology of members of the genus histoplasma: a review. Mycoses 11:507–514. doi: 10.1111/j.1439-0507.1968.tb03370.x [DOI] [PubMed] [Google Scholar]
- 218. Ajello L. 1988. Histoplasmosis capsulati, p 650–653. In Balows A, Hausler WJ, Ohashi M, Turano A, Lennete EH (ed), Laboratory diagnosis of infectious diseases: principles and practice. Springer, New York. [Google Scholar]
- 219. Gugnani HC, Muotoe-Okafor F. 1997. African histoplasmosis: a review. Rev Iberoam Micol 14:155–159. [PubMed] [Google Scholar]
- 220. Loulergue P, Bastides F, Baudouin V, Chandenier J, Mariani-Kurkdjian P, Dupont B, Viard J-P, Dromer F, Lortholary O. 2007. Literature review and case histories of Histoplasma capsulatum var. duboisii infections in HIV-infected patients. Emerg Infect Dis 13:1647–1652. doi: 10.3201/eid1311.070665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Ocansey BK, Kosmidis C, Agyei M, Dorkenoo AM, Ayanlowo OO, Oladele RO, Darre T, Denning DW. 2022. Histoplasmosis in Africa: current perspectives, knowledge gaps, and research priorities. PLoS Negl Trop Dis 16:e0010111. doi: 10.1371/journal.pntd.0010111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Rezabek GB, Donahue JM, Giles RC, Petrites-Murphy MB, Poonacha KB, Rooney JR, Smith BJ, Swerczek TW, Tramontin RR. 1993. Histoplasmosis in horses. J Comp Pathol 109:47–55. doi: 10.1016/s0021-9975(08)80239-3 [DOI] [PubMed] [Google Scholar]
- 223. Weeks RJ, Padhye AA, Ajello L. 1985. Histoplasma capsulatum variety Farciminosum: a new combination for Histoplasma Farciminosum. Mycologia 77:964–970. doi: 10.1080/00275514.1985.12025187 [DOI] [Google Scholar]
- 224. Panciera RJ. 1969. Histoplasmic (Histoplasma capsulatum) infection in a horse. Cornell Vet 59:306–312. [PubMed] [Google Scholar]
- 225. Reiss E, Shadomy HJ, Lyon GM. 2011. Histoplasmosis. In Fundamental medical mycology. Wiley-Blackwell. [Google Scholar]
- 226. Kasuga T, Taylor JW, White TJ. 1999. Phylogenetic relationships of varieties and geographical groups of the human pathogenic fungus Histoplasma capsulatum darling. J Clin Microbiol 37:653–663. doi: 10.1128/JCM.37.3.653-663.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Kasuga Takao, White TJ, Koenig G, McEwen J, Restrepo A, Castañeda E, Da Silva Lacaz C, Heins-Vaccari EM, De Freitas RS, Zancopé-Oliveira RM, Qin Z, Negroni R, Carter DA, Mikami Y, Tamura M, Taylor ML, Miller GF, Poonwan N, Taylor JW. 2003. Phylogeography of the fungal pathogen Histoplasma capsulatum. Mol Ecol 12:3383–3401. doi: 10.1046/j.1365-294x.2003.01995.x [DOI] [PubMed] [Google Scholar]
- 228. Sepúlveda VE, Márquez R, Turissini DA, Goldman WE, Matute DR. 2017. Genome sequences reveal cryptic speciation in the human pathogen Histoplasma capsulatum mBio 8:e01339-17. doi: 10.1128/mBio.01339-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Jofre GI, Singh A, Mavengere H, Sundar G, D’Agostino E, Chowdhary A, Matute DR. 2022. An Indian lineage of Histoplasma with strong signatures of differentiation and selection. Fungal Genet Biol 158:103654. doi: 10.1016/j.fgb.2021.103654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Almeida-Silva F, de Melo Teixeira M, Matute DR, de Faria Ferreira M, Barker BM, Almeida-Paes R, Guimarães AJ, Zancopé-Oliveira RM. 2021. Genomic diversity analysis reveals a strong population structure in Histoplasma capsulatum LAmA (Histoplasma suramericanum). J Fungi (Basel) 7:865. doi: 10.3390/jof7100865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Voorhies M, Cohen S, Shea TP, Petrus S, Muñoz JF, Poplawski S, Goldman WE, Michael TP, Cuomo CA, Sil A, Beyhan S. 2022. Chromosome-level genome assembly of a human fungal pathogen reveals synteny among geographically distinct species. mBio 13:e0257421. doi: 10.1128/mbio.02574-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Maxwell CS, Sepulveda VE, Turissini DA, Goldman WE, Matute DR. 2018. Recent admixture between species of the fungal pathogen Histoplasma. Evol Lett 2:210–220. doi: 10.1002/evl3.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Sepúlveda VE, Rader JA, Li JJ, Goldman WE, Matute DR. 2024. Phenotypic characterization of cryptic species in the fungal pathogen Histoplasma. mSphere:e00009-24. doi: 10.1128/msphere.00009-24 [DOI] [PMC free article] [PubMed]
- 234. Arunmozhi Balajee S, Hurst SF, Chang LS, Miles M, Beeler E, Hale C, Kasuga T, Benedict K, Chiller T, Lindsley MD. 2013. Multilocus sequence typing of Histoplasma capsulatum in formalin-fixed paraffin-embedded tissues from cats living in non-endemic regions reveals a new phylogenetic clade. Med Mycol 51:345–351. doi: 10.3109/13693786.2012.733430 [DOI] [PubMed] [Google Scholar]
- 235. Vite-Garín T, Estrada-Bárcenas DA, Gernandt DS, Reyes-Montes MDR, Sahaza JH, Canteros CE, Ramírez JA, Rodríguez-Arellanes G, Serra-Damasceno L, Zancopé-Oliveira RM, Taylor JW, Taylor ML. 2021. Histoplasma capsulatum isolated from Tadarida brasiliensis bats captured in Mexico form a sister group to North American class 2 clade. J Fungi (Basel) 7:529. doi: 10.3390/jof7070529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Sepúlveda VE, Williams CL, Goldman WE. 2014. Comparison of phylogenetically distinct Histoplasma strains reveals evolutionarily divergent virulence strategies. mBio 5:e01376-14. doi: 10.1128/mBio.01376-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Durkin MM, Connolly PA, Karimi K, Wheat E, Schnizlein-Bick C, Allen SD, Alves K, Tewari RP, Keath E. 2004. Pathogenic differences between North American and Latin American strains of Histoplasma capsulatum var. capsulatum in experimentally infected mice. J Clin Microbiol 42:4370–4373. doi: 10.1128/JCM.42.9.4370-4373.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Nathan C, Shiloh MU. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci U S A 97:8841–8848. doi: 10.1073/pnas.97.16.8841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Newman SL. 1999. Macrophages in host defense against Histoplasma capsulatum. Trends Microbiol 7:67–71. doi: 10.1016/s0966-842x(98)01431-0 [DOI] [PubMed] [Google Scholar]
- 240. Garfoot AL, Rappleye CA. 2016. Histoplasma capsulatum surmounts obstacles to intracellular pathogenesis. FEBS J 283:619–633. doi: 10.1111/febs.13389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Eissenberg LG, Goldman WE, Schlesinger PH. 1993. Histoplasma capsulatum modulates the acidification of phagolysosomes. J Exp Med 177:1605–1611. doi: 10.1084/jem.177.6.1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Brechting PJ, Rappleye CA. 2019. Histoplasma responses to nutritional immunity imposed by macrophage activation. J Fungi (Basel) 5:45. doi: 10.3390/jof5020045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Singulani JL, Scorzoni L, de Oliveira HC, Marcos CM, Assato PA, Fusco-Almeida AM, Mendes-Giannini MJS. 2018. Applications of invertebrate animal models to dimorphic fungal infections. J Fungi (Basel) 4:118. doi: 10.3390/jof4040118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Trevijano-Contador N, Zaragoza O. 2018. Immune response of Galleria mellonella against human fungal pathogens. J Fungi (Basel) 5:3. doi: 10.3390/jof5010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Johnson CH, Ayyadevara S, McEwen JE, Shmookler Reis RJ. 2009. Histoplasma capsulatum and Caenorhabditis elegans: a simple nematode model for an innate immune response to fungal infection. Med Mycol 47:808–813. doi: 10.3109/13693780802660532 [DOI] [PubMed] [Google Scholar]
- 246. Sahaza JH, Pérez-Torres A, Zenteno E, Taylor ML. 2014. Usefulness of the murine model to study the immune response against Histoplasma capsulatum infection. Comp Immunol Microbiol Infect Dis 37:143–152. doi: 10.1016/j.cimid.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 247. Tewari RP, Berkhout FJ. 1972. Comparative pathogenicity of albino and brown types of Histoplasma capsulatum for mice. J Infect Dis 125:504–508. doi: 10.1093/infdis/125.5.504 [DOI] [PubMed] [Google Scholar]
- 248. Deepe GS, Gibbons RS, Smulian AG. 2008. Histoplasma capsulatum manifests preferential invasion of phagocytic subpopulations in murine lungs. J Leukoc Biol 84:669–678. doi: 10.1189/jlb.0308154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Jones GS, Sepúlveda VE, Goldman WE. 2020. Biodiverse Histoplasma species elicit distinct patterns of pulmonary inflammation following sublethal infection. mSphere 5:e00742-20. doi: 10.1128/mSphere.00742-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Sil A. 2019. Molecular regulation of Histoplasma dimorphism. Curr Opin Microbiol 52:151–157. doi: 10.1016/j.mib.2019.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Domer JE. 1971. Monosaccharide and chitin content of cell walls of Histoplasma capsulatum and Blastomyces dermatitidis. J Bacteriol 107:870–877. doi: 10.1128/jb.107.3.870-877.1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Reiss E, Miller SE, Kaplan W, Kaufman L. 1977. Antigenic, chemical, and structural properties of cell walls of Histoplasma capsulatum yeast-form chemotypes 1 and 2 after serial enzymatic hydrolysis. Infect Immun 16:690–700. doi: 10.1128/iai.16.2.690-700.1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Kanetsuna F, Carbonell LM, Gil F, Azuma I. 1974. Chemical and ultrastructural studies on the cell walls of the yeastlike and mycelial forms of Histoplasma capsulatum. Mycopathol Mycol Appl 54:1–13. doi: 10.1007/BF02055967 [DOI] [PubMed] [Google Scholar]
- 254. Rappleye CA, Eissenberg LG, Goldman WE. 2007. Histoplasma capsulatum alpha-(1,3)-glucan blocks innate immune recognition by the beta-glucan receptor. Proc Natl Acad Sci U S A 104:1366–1370. doi: 10.1073/pnas.0609848104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Klimpel KR, Goldman WE. 1987. Isolation and characterization of spontaneous avirulent variants of Histoplasma capsulatum. Infect Immun 55:528–533. doi: 10.1128/iai.55.3.528-533.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Klimpel KR, Goldman WE. 1988. Cell walls from avirulent variants of Histoplasma capsulatum lack alpha-(1,3)-glucan. Infect Immun 56:2997–3000. doi: 10.1128/iai.56.11.2997-3000.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. San-Blas G, San-Blas F. 1977. Paracoccidioides brasileensis: cell wall structure and virulence. Mycopathologia 62:77–86. doi: 10.1007/BF01259396 [DOI] [PubMed] [Google Scholar]
- 258. San-Blas G, Ordaz D, Yegres FJ. 1978. Histoplasma capsulatum: chemical variability of the yeast cell wall. Sabouraudia 16:279–284. doi: 10.1080/00362177885380381 [DOI] [PubMed] [Google Scholar]
- 259. Eissenberg LG, West JL, Woods JP, Goldman WE. 1991. Infection of P388D1 macrophages and respiratory epithelial cells by Histoplasma capsulatum: selection of avirulent variants and their potential role in persistent histoplasmosis. Infect Immun 59:1639–1646. doi: 10.1128/iai.59.5.1639-1646.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Keath EJ, Painter AA, Kobayashi GS, Medoff G. 1989. Variable expression of a yeast-phase-specific gene in Histoplasma capsulatum strains differing in thermotolerance and virulence. Infect Immun 57:1384–1390. doi: 10.1128/iai.57.5.1384-1390.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Batanghari JW, Goldman WE. 1997. Calcium dependence and binding in cultures of Histoplasma capsulatum. Infect Immun 65:5257–5261. doi: 10.1128/iai.65.12.5257-5261.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Patel JB, Batanghari JW, Goldman WE. 1998. Probing the yeast phase-specific expression of the CBP1 gene in Histoplasma capsulatum. J Bacteriol 180:1786–1792. doi: 10.1128/JB.180.7.1786-1792.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Beck M.R, DeKoster GT, Hambly DM, Gross ML, Cistola DP, Goldman WE. 2008. Structural features responsible for the biological stability of Histoplasma’s virulence factor CBP. Biochemistry 47:4427–4438. doi: 10.1021/bi701495v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Beck Moriah R, Dekoster GT, Cistola DP, Goldman WE. 2009. NMR structure of a fungal virulence factor reveals structural homology with mammalian saposin B. Mol Microbiol 72:344–353. doi: 10.1111/j.1365-2958.2009.06647.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Azimova D, Herrera N, Duvenage L, Voorhies M, Rodriguez RA, English BC, Hoving JC, Rosenberg O, Sil A. 2022. Cbp1, a fungal virulence factor under positive selection, forms an effector complex that drives macrophage lysis. PLoS Pathog 18:e1010417. doi: 10.1371/journal.ppat.1010417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Isaac DT, Berkes CA, English BC, Murray DH, Lee YN, Coady A, Sil A. 2015. Macrophage cell death and transcriptional response are actively triggered by the fungal virulence factor Cbp1 during H. capsulatum infection. Mol Microbiol 98:910–929. doi: 10.1111/mmi.13168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. English BC, Van Prooyen N, Örd T, Örd T, Sil A. 2017. The transcription factor CHOP, an effector of the integrated stress response, is required for host sensitivity to the fungal intracellular pathogen Histoplasma capsulatum. PLoS Pathog 13:e1006589. doi: 10.1371/journal.ppat.1006589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Woods JP, Goldman WE. 1992. In vivo generation of linear plasmids with addition of telomeric sequences by Histoplasma capsulatum. Mol Microbiol 6:3603–3610. doi: 10.1111/j.1365-2958.1992.tb01796.x [DOI] [PubMed] [Google Scholar]
- 269. Worsham PL, Goldman WE. 1990. Development of a genetic transformation system for Histoplasma capsulatum: complementation of uracil auxotrophy. Mol Gen Genet 221:358–362. doi: 10.1007/BF00259400 [DOI] [PubMed] [Google Scholar]
- 270. Magrini V, Goldman WE. 2001. Molecular mycology: a genetic toolbox for Histoplasma capsulatum. Trends Microbiol 9:541–546. doi: 10.1016/S0966-842X(01)02204-1 [DOI] [PubMed] [Google Scholar]
- 271. Retallack DM, Heinecke EL, Gibbons R, Deepe GS, Woods JP. 1999. The URA5 gene is necessary for Histoplasma capsulatum growth during infection of mouse and human cells. Infect Immun 67:624–629. doi: 10.1128/IAI.67.2.624-629.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Rappleye CA, Goldman WE. 2006. Defining virulence genes in the dimorphic fungi. Annu Rev Microbiol 60:281–303. doi: 10.1146/annurev.micro.59.030804.121055 [DOI] [PubMed] [Google Scholar]
- 273. Woods JP, Heinecke EL, Goldman WE. 1998. Electrotransformation and expression of bacterial genes encoding hygromycin phosphotransferase and beta-galactosidase in the pathogenic fungus Histoplasma capsulatum. Infect Immun 66:1697–1707. doi: 10.1128/IAI.66.4.1697-1707.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Woods JP, Retallack DM, Heinecke EL, Goldman WE. 1998. Rare homologous gene targeting in Histoplasma capsulatum: disruption of the URA5Hc gene by allelic replacement. J Bacteriol 180:5135–5143. doi: 10.1128/JB.180.19.5135-5143.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Cottrell TR, Doering TL. 2003. Silence of the strands: RNA interference in eukaryotic pathogens. Trends Microbiol 11:37–43. doi: 10.1016/s0966-842x(02)00004-5 [DOI] [PubMed] [Google Scholar]
- 276. Cooper KG, Woods JP. 2009. Secreted dipeptidyl peptidase IV activity in the dimorphic fungal pathogen Histoplasma capsulatum. Infect Immun 77:2447–2454. doi: 10.1128/IAI.01345-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Gomez FJ, Pilcher-Roberts R, Alborzi A, Newman SL. 2008. Histoplasma capsulatum cyclophilin A mediates attachment to dendritic cell VLA-5. J Immunol 181:7106–7114. doi: 10.4049/jimmunol.181.10.7106 [DOI] [PubMed] [Google Scholar]
- 278. Zupan JR, Zambryski P. 1995. Transfer of T-DNA from Agrobacterium to the plant cell. Plant Physiol 107:1041–1047. doi: 10.1104/pp.107.4.1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Abuodeh RO, Orbach MJ, Mandel MA, Das A, Galgiani JN. 2000. Genetic transformation of Coccidioides immitis facilitated by Agrobacterium tumefaciens. J Infect Dis 181:2106–2110. doi: 10.1086/315525 [DOI] [PubMed] [Google Scholar]
- 280. Sullivan TD, Rooney PJ, Klein BS. 2002. Agrobacterium tumefaciens integrates transfer DNA into single chromosomal sites of dimorphic fungi and yields homokaryotic progeny from multinucleate yeast. Eukaryot Cell 1:895–905. doi: 10.1128/EC.1.6.895-905.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Leal CV, Montes BA, Mesa AC, Rua AL, Corredor M, Restrepo A, McEwen JG. 2004. Agrobacterium tumefaciens-mediated transformation of Paracoccidioides brasiliensis. Med Mycol 42:391–395. doi: 10.1080/13693780410001712007 [DOI] [PubMed] [Google Scholar]
- 282. Kemski MM, Stevens B, Rappleye CA. 2013. Spectrum of T-DNA integrations for insertional mutagenesis of Histoplasma capsulatum. Fungal Biol 117:41–51. doi: 10.1016/j.funbio.2012.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Grigoriev IV, Cullen D, Goodwin SB, Hibbett D, Jeffries TW, Kubicek CP, Kuske C, Magnuson JK, Martin F, Spatafora JW, Tsang A, Baker SE. 2011. Fueling the future with fungal genomics. Mycology 2:192–209. doi: 10.1080/21501203.2011.584577 [DOI] [Google Scholar]
- 284. Cuomo CA. 2017. Harnessing whole genome sequencing in medical mycology. Curr Fungal Infect Rep 11:52–59. doi: 10.1007/s12281-017-0276-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Muñoz JF, Gauthier GM, Desjardins CA, Gallo JE, Holder J, Sullivan TD, Marty AJ, Carmen JC, Chen Z, Ding L, et al. 2015. The dynamic genome and transcriptome of the human fungal pathogen Blastomyces and close relative Emmonsia. PLoS Genet 11:e1005493. doi: 10.1371/journal.pgen.1005493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Muñoz JF, McEwen JG, Clay OK, Cuomo CA. 2018. Genome analysis reveals evolutionary mechanisms of adaptation in systemic dimorphic fungi. Sci Rep 8:4473. doi: 10.1038/s41598-018-22816-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Stark R, Grzelak M, Hadfield J. 2019. RNA sequencing: the teenage years. Nat Rev Genet 20:631–656. doi: 10.1038/s41576-019-0150-2 [DOI] [PubMed] [Google Scholar]
- 288. McDermaid A, Monier B, Zhao J, Liu B, Ma Q. 2019. Interpretation of differential gene expression results of RNA-seq data: review and integration. Brief Bioinform 20:2044–2054. doi: 10.1093/bib/bby067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Medoff G, Maresca B, Lambowitz AM, Kobayashi G, Painter A, Sacco M, Carratu L. 1986. Correlation between pathogenicity and temperature sensitivity in different strains of Histoplasma capsulatum. J Clin Invest 78:1638–1647. doi: 10.1172/JCI112757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Huang G, Wang H, Chou S, Nie X, Chen J, Liu H. 2006. Bistable expression of WOR1, a master regulator of white-opaque switching in Candida albicans. Proc Natl Acad Sci U S A 103:12813–12818. doi: 10.1073/pnas.0605270103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Zordan RE, Galgoczy DJ, Johnson AD. 2006. Epigenetic properties of white–opaque switching in Candida albicans are based on a self-sustaining transcriptional feedback loop. Proc Natl Acad Sci U S A 103:12807–12812. doi: 10.1073/pnas.0605138103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Cain CW, Lohse MB, Homann OR, Sil A, Johnson AD. 2012. A conserved transcriptional regulator governs fungal morphology in widely diverged species. Genetics 190:511–521. doi: 10.1534/genetics.111.134080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Boyce KJ, Andrianopoulos A. 2015. Fungal dimorphism: the switch from hyphae to yeast is a specialized morphogenetic adaptation allowing colonization of a host. FEMS Microbiol Rev 39:797–811. doi: 10.1093/femsre/fuv035 [DOI] [PubMed] [Google Scholar]
- 294. Edwards JA, Chen C, Kemski MM, Hu J, Mitchell TK, Rappleye CA. 2013. Histoplasma yeast and mycelial transcriptomes reveal pathogenic-phase and lineage-specific gene expression profiles. BMC Genomics 14:695. doi: 10.1186/1471-2164-14-695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Magrini V, Warren WC, Wallis J, Goldman WE, Xu J, Mardis ER, McPherson JD. 2004. Fosmid-based physical mapping of the Histoplasma capsulatum genome. Genome Res 14:1603–1609. doi: 10.1101/gr.2361404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Broad Institute . 2007. Histoplasma capsulatum. Database Broad Institute, ed. broadinstitute.org. Available from: http://www.broadinstitute.org/annotation/genome/histoplasma_capsulatum/MultiHome.html. Retrieved 14 Jan 2024. [Google Scholar]
- 297. Cohen A, Jeng EE, Voorhies M, Symington J, Ali N, Rodriguez RA, Bassik MC, Sil A. 2022. Genome-scale CRISPR screening reveals that C3aR signaling is critical for rapid capture of fungi by macrophages. PLoS Pathog 18:e1010237. doi: 10.1371/journal.ppat.1010237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Ray SC, Rappleye CA. 2019. Flying under the radar: Histoplasma capsulatum avoidance of innate immune recognition. Semin Cell Dev Biol 89:91–98. doi: 10.1016/j.semcdb.2018.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]