ABSTRACT
Most of the short-chain fatty acids (SCFAs) are produced by Bifidobacterium, Lactobacillus, Lachnospiraceae, Blautia, Coprococcus, Roseburia, Facealibacterium and Oscillospira. Butyrate (C4H7O2−) supplies 70% of energy to intestinal epithelial cells (IECs), supports tight-junction protein formation, induces the production of inflammatory cytokines, and inhibits histone deacetylase (HDAC). Butyrate is also associated with the recovery of brain trauma, improvement of dementia, the alleviation of autoimmune encephalitis, and several intestinal disorders. Low levels of SCFAs are associated with hypertension, cardiovascular disease (CVD), strokes, obesity, and diabetes mellitus. Cis-palmitoleic acid (C16H30O2), a mono-unsaturated fatty acid (MUFA), increases insulin sensitivity and reduces the risk of developing CVD. Lipokine palmitoleic acid reduces the expression of pro-inflammatory cytokines IL-1β (pro-IL1β), tumor necrosis factor α (TNF-α), and isoleucine 6 (IL-6). Polyunsaturated fatty acids (PUFAs), such as omega-3 and omega-6, are supplied through the diet. The conversion of PUFAs by cyclooxygenases (COX) and lipoxygenases (LOX) leads to the production of anti-inflammatory prostaglandins and leukotrienes. Oxidation of linoleic acid (LA, C18H32O2), an omega-6 essential fatty acid, leads to the formation of 13-hydroperoxy octadecadienoic acid (13-HPODE, C18H32O4), which induces pro-inflammatory cytokines. Omega-3 PUFAs, such as eicosapentaenoic acid (EPA, C20H30O2) and docosahexaenoic acid (DHA, C22H32O2), lower triglyceride levels, lower the risk of developing some sort of cancers, Alzheimer’s disease and dementia. In this review, the importance of SCFAs, MUFAs, PUFAs, and saturated fatty acids (SFAs) on human health is discussed. The use of fatty acids in the treatment of diseases is investigated.
KEYWORDS: Fatty acids, human health, treatment of diseases
Introduction
The adult human gut is host to approximately 3.8 × 1013 (0.2 kg) bacteria, more or less equivalent to the estimated 3.0 × 1013 cells in a person of 70 kg.1 Most gut bacteria belong to the phyla Bacillota (Firmicutes) and Bacteroidota (Bacteroidetes)2 but are also represented by Pseudomonadota (Proteobacteria), Fusobacteriota (Fusobacteria), Verrucomicrobiota (Verrucomicrobia), Cyanobacteria, and Actinomycetota (Actinobacteria) (Figure 1). To a large extent, gut microbiota regulates the uptake of macronutrients3,4 but their development is controlled by diet, age, hormonal changes, the host’s immune system,5 and external factors such as medication, and stress (Figure 1). Western-style diets high in animal proteins have been associated with cardiovascular diseases (CVDs) such as atherosclerosis and heart failure but also obesity, type 2 diabetes mellitus,6–8 irritable bowel disease, IBD, and asthma (Figure 1). A low protein or Mediterranean diet (MD) with plant-based products such as fruit, nuts, oils, and seeds9 contains more unsaturated fatty acids and is considered healthier with fewer reports of CVDs, insulin resistance, and an imbalance in immune responses.10,11 The gut microbiome of humans on an MD is dominated by Bifidobacterium, Enterococcus, Prevotella, Bacteroides, Faecalibacterium prausnitzii, Roseburia, and Lachnospiraceae.12,13 However, low cell numbers of Ruthenibacterium lactatiformans, Flavonifractor plautii, Parabacteroides merdae, Ruminococcus torques, and Ruminococcus gnavus were reported.14 An increase in Lactobacillus 12 and Firmicutes was also noted15 (Figure 2).
Figure 1.
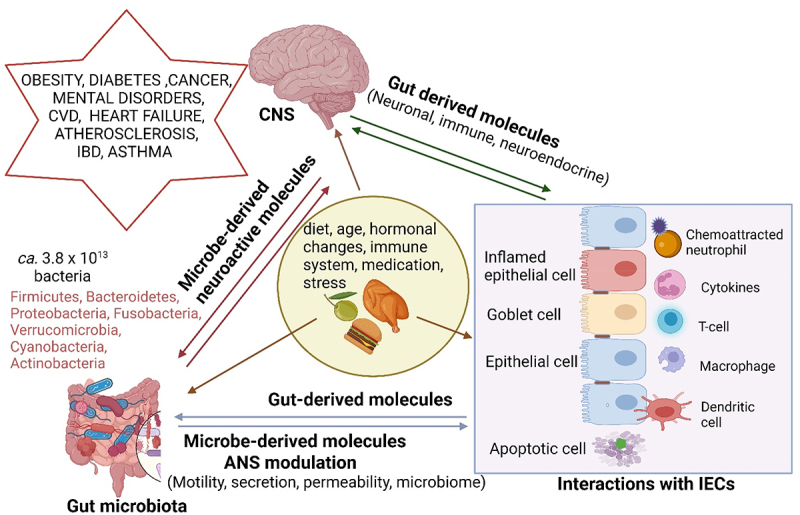
Gut microbiota, intestinal epithelial cells (IECs), the autonomic nervous system (ANS) and the brain (central nervous system, CNS) are in constant contact via bidirectional communication channels driven by gut- and microbe-derived molecules that have a direct or indirect effect on the formation of neuronal, immune, and neuroendocrine signals. These interactions regulate the composition of the gut microbiome, bowel movement, and the migration of molecules across the gut wall. Some microbe-derived molecules reach the brain via the vagus nerve or enter the systemic circulation system (bloodstream). Neuroactive molecules released from the brain affect the behavior of gut microbiota and their gene expressions. An imbalanced diet, obesity, diabetes, cancer, mental disorders, and microbial infections are examples of abnormalities that alter the composition of the gut microbiome. Metabolites produced by gut microbiota have also been implicated in some disease processes, such as cardiovascular disease (CVD). Created using Biorender.com (1 July 2024).
Figure 2.
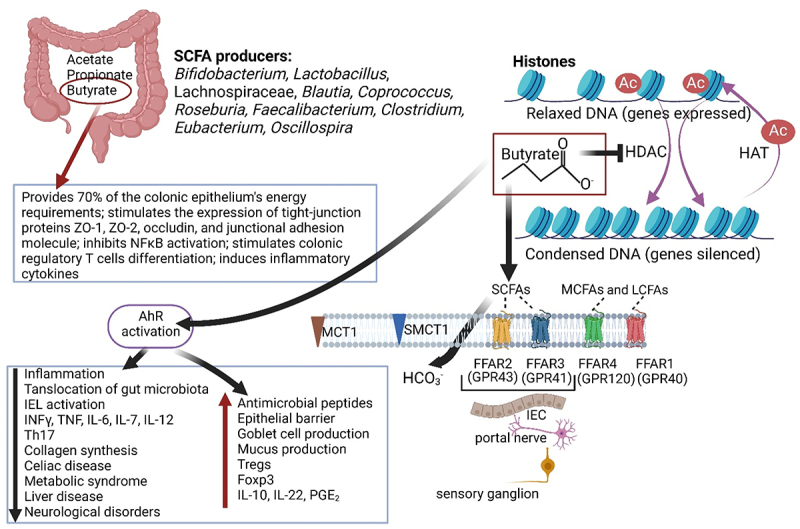
The role of short-chain fatty acids (SCFAs), especially butyrate, in inflammation, gene expressions, gut wall integrity, and disease. HDAC: histone deacetylase, HAT: histone acetyl transferase, MCFAs: medium-chain fatty acids, LCFAs: long-chain fatty acids, MCT1: monocarboxylate transporter-1, SMCT1: sodium-coupled monocarboxylate transporter-1, FFAR: free fatty acid receptor, GPR: G-protein receptor, IL: isoleucine, PGE2: prostaglandin E2, INFγ: interferon gamma, TNF: tumor necrosis factor, Th17: T-helper cell 17, nFƙB: nuclear factor kappa-B. Created using Biorender.com (1 July 2024).
Diets high in fiber support the growth of glycan-degrading gut microbiota and the production of short-chain fatty acids (SCFAs) such as butyrate (C4H7O2−), propionate (C3H5O2−), and acetate (C2H3O2−).16 Fructan and galactooligosaccharide (GOS)-rich diets stimulate the growth of Bifidobacterium and Lactobacillus.17 Some researchers claim that the consumption of grains stimulates the production of phenolic compounds that promote the growth of bifidobacteria.18 These findings were, however, not confirmed when oats were the staple diet, as shown by Kristek et al.19 Neither beta-glucans nor polyphenols stimulated the growth of bifidobacteria. It is important to support the growth of bifidobacteria and lactic acid bacteria, as they produce several SCFAs that have probiotic properties.20,21 According to McDonald et al,22 the gut microbiome of individuals who consumed more than 30 plant types weekly is dominated by SCFA producers, including F. prausnitzii and Oscillospira spp. The growth of these species is stimulated by acetate-producing Bifidobacterium and Akkermansia.23
High-molecular-weight beta-glucans stimulated the growth of Bacteriodetes and Prevotella, and repressed the growth of Firmicutes and Dorea.24 This was not observed with a diet of low-molecular-weight beta-glucans.24 In rats, beta-glucans from oats led to an increase in Lactobacillus and Bifidobacterium but a decrease in Enterobacteriaceae.25 In pigs, an oat diet led to an increase in Lactobacillus, Streptococcus, Enterococcus, Clostridium clusters I and XIVa, certain species of Bacteroides, Prevotella, Porphyromonas, and Enterobacteriaceae.26 Arabinoxylans have been associated with an increase in Bifidobacterium animalis subsp. lactis, Prevotella, F. prausnitzii, and Lactobacillus, but a decrease in Escherichia. coli, Streptococcus, Staphylococcus, Lactobacillus, Clostridium histolyticum I and II, and Enterococcus .18 Long-chain arabinoxylans also promoted the growth of Bifidobacterium longum with a concurrent increase in propionate levels.27
In this review, the importance of SCFAs, monounsaturated fatty acids (MUFAs), polyunsaturated fatty acids (PUFAs), and saturated fatty acids (SFAs) on human health is discussed. The option of using fatty acids in the treatment of diseases is also investigated.
Short-chain fatty acids (SCFAs)
Most SCFAs are produced in the colon by Bifidobacterium, Lactobacillus, Lachnospiraceae, Blautia, Coprococcus, Roseburia, Faecalibacterium, Clostridium, and Eubacterium.28,29 Of all SCFAs, butyrate is the best studied, as it supplies 70% of the energy requirements of the colonic epithelium,30 plays a critical role in the expression of tight-junction proteins ZO-1, ZO-2, occludin, and junctional adhesion molecule A,31 and has direct anti-inflammatory effects, inhibiting nuclear factor kappa-B (NFκB) activation (Figure 2). Butyrate also stimulates the differentiation of colonic regulatory T cells,32 and induces inflammatory cytokines (Figure 2).
SCFAs affect at least two systems of molecular signaling that have widespread regulatory effects, i.e., the deacetylation of histones, regulated by histone deacetylase (HDAC), and the adherence to G-protein-coupled receptors (GPCRs), also called free fatty acid receptors (FFARs) (Figure 2). G-protein receptor 43 (GPR43/FFAR2) and GPR41 (FFAR3) are located on the surface of intestinal epithelial cells (IECs),32 neurons of the enteric nervous system (ENS), portal nerve, and sensory ganglia,33,34 as shown in Figure 2. GPR43, mostly expressed in subcutaneous fat, visceral fat, and bone marrow, regulates energy expenditure in skeletal muscles and in the liver.35 GPR 41, activated by propionic acid (C3H6O2),36 transfers signals directly to the central nervous system (CNS)37 and induces the nuclear phosphoprotein Fos in the dorsal vagal complex of the brainstem, the hypothalamus, and the spinal cord.38 FFAR4 (GPR120) is expressed in adipocytes, endothelial cells, and macrophage39 and assists in the regulation of adipogenesis, insulin sensitivity, and inflammation. Dysfunction of FFAR4 is associated with insulin resistance, obesity, and eccentric remodeling.39 FFAR1 (GPR40) senses long-chain free fatty acids (FFAs) produced by lipolysis and endogenously synthesized triglycerides.40 The binding of FFAs to FFAR1 on pancreaticβ-cells and enteroendocrine cells activates signaling through the transducer protein Gq and β-arrestin.40 This releases Ca2+ into the cytosol that activates protein kinase C, which enhances the release of insulin and glucose uptake.40 Apart from regulating energy levels, FFAR1 also plays a role in regulating pain and inflammation in the brain.40 Most SCFAs are transported across the gut wall in dissociated form by an HCO3− exchanger of unknown identity, a monocarboxylate transporter-1 (MCT1) or sodium-coupled monocarboxylate transporter-1 (SMCT1) (Figure 2). Some SCFAs, however, diffuse across IEC membranes and enter the bloodstream in a non-ionized form.41 It is also noteworthy that SCFAs stimulate antimicrobial peptides through the cathelicidin LL-37 pathway, as shown in the prevention of Shigella infections.42
The acetylation and deacetylation of histones is a fundamental process in DNA coiling and the regulation of gene expression. Butyrate acts as an HDAC inhibitor (HADCi), thus preventing the deacylation of histones (Figure 2) and increasing the expression of repressed genes.43 This process is crucial in activating extrinsic and intrinsic apoptotic pathways, reactive oxygen species (ROS), and cell cycle arrest in cancer cells.44–46 The inhibition of HDAC also impacts several other diseases, such as brain trauma, dementia, and autoimmune encephalitis.47,48 By inhibiting HDAC, chromatin is exposed to aryl hydrocarbon receptor (AhR)-ligand complexes and binding sites in the promoter of AhR target genes. Butyrate thus modulates AhR activation.49 Binding to Ahr is important in several metabolic and immune processes (Figure 2), allowing the co-existence of gut microbiota and their host.50 The activation (increase) of AhR downregulates intestinal inflammation, alleviating inflammatory bowel diseases (IBD), including Crohn’s disease and ulcerative colitis (UC), but also celiac disease, metabolic syndrome, liver disease, and neurological disease, as summarized in Figure 2. Elevated levels of AhR lead to a decrease in IFNγ, IL-6, IL-12, TNF, IL-7, and IL-17, a decline in microbial translocation and fibrosis, an increase in regulatory mechanisms such as IL-10, IL-22, prostaglandin E2, and Foxp3 (scurfin), the production of antimicrobial peptides, and the restitution of damaged epithelial cells, as listed in Figure 2. An increase in deacetylated histones decreases the expression of pattern recognition receptors, kinases, transcription regulators, cytokines, and chemokines. In mice, the inhibition of HDACi in the frontal cortex and hippocampus alleviated depressive behavior,51 dementia, and brain trauma.52 Patients suffering from neurological disorders such as depression, Parkinson’s disease (PD), and schizophrenia, have higher than normal levels of HDAC.53 Parkinson’s disease is associated with increased cell numbers of enterobacteria and potentially harmful pro-inflammatory Proteobacteria, especially Ralstonia, and a decrease in Prevotella53,54 and butyrate-producing Blautia, Coprococcus, and Roseburia.55,56 In severe cases of PD, changes in the integrity of the blood-brain barrier (BBB), CNS functioning, and microglia maturation were observed.57,58 Studies conducted on germ-free (GF) mice have shown that defective microglia could be stimulated by supplementing the feed with butyrate, propionate, and acetate.59 Acetate crosses the BBB and accumulates in the hypothalamus.60,61 This stimulates the production of gamma-aminobutyric acid (GABA) in the brain.62 GABA is the most abundant neurotransmitter in the CNS of mammals and is co-transmitted with acetylcholine (ACH).63 An increase in ACH increases the expression of BDNF, encoding brain-derived neurotrophic factor (BDNF) in the frontal cortex and hippocampus.64 This stimulates brain development.65 Low levels of BDNF are associated with depression and anxiety.66,67 Neurological disorders may, thus, be prevented by keeping SCFAs and HDAC at optimal levels.
SCFAs and tryptophan precursors interact with receptors on the gut wall, muscle layers surrounding the gut, liver, pancreas, adipose tissue, and immune cells.68 In entero-epithelial cells (EECs), SCFAs stimulate the release of gut hormones69 and modulate genes encoding the cyclic adenosine monophosphate (cAMP) response element-binding (CREB) protein. The latter regulates the synthesis of catecholamine neurotransmitters such as dopamine (DA).70,71 With an increase in the expression of tyrosine hydroxylase and a decrease in DA-β-hydroxylase (DBH; EC 1.14.17.1), DA is converted to norepinephrine (NE).72,73 Elevated levels of DA caused by a deficiency in DBH may have a detrimental effect on the autonomic nervous system (ANS) that controls blood pressure and body temperature. In immune cells, SCFAs regulate T-regulatory cell differentiation59,74 and the maturation of microglial cells.75 Butyrate also activates ornithine decarboxylase, which results in the inhibition of polyamine metabolism and the activation of alkaline phosphatase.76
Low levels of SCFA have been associated with high blood pressure (hypertension), CVDs, strokes, obesity, and diabetes mellitus.77 In rats, hypertension could be prevented by restoring acetate levels in the cecum.33,76 Propionate administered to patients with obesity enhanced gut hormone secretion while reducing adiposity and overall weight gain.77,78 Propionic acid also inhibits NFκB and may improve insulin sensitivity by activating peroxisome proliferator-activated receptor gamma.79 However, despite the anti-inflammatory effects of propionic acid,79 it may have neurotoxic side effects, as reported for autism.80
SCFAs, produced by microorganisms, play a key role in microbiota-gut-brain axis (GBA) communication, protection of the intestinal barrier, and inflammatory responses. Levels of SCFAs, however, need to be carefully controlled, as several disadvantages have been reported. Acetate, for instance, promotes the production of intestinal IgA,81 stimulates the secretion of cytokine IL-6, and increases neutrophil recruitment.35
Monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs)
Monounsaturated fatty acids
Monounsaturated fatty acids (MUFAs) are found in several plants, including olives, macadamia nuts, canola seeds, avocados, pumpkin seeds, sesame seeds, almonds, cashews, peanuts, and pecans. MUFAs contain a single double bond, whereas PUFAs contain two or more double bonds. Typical examples of MUFAs are palmitoleic acid (C16H30O2) or palmitoleate, also referred to as cis-9-hexadecenoic acid and oleic- or 9-octadecanoic acid (C18H34O2). Palmitoleic acid is formed in the liver when stearoyl-CoA desaturase (SCD-1) removes two hydrogen atoms from palmitic acid (C16H32O2) at the C-9 and C-10 positions.82 Palmitoleate is present in the cis (16:1c9) or a trans (16:1t9) isomer. The cis isoform (cis-palmitoleate) is associated with increased insulin sensitivity and less lipid accumulation in the liver.83 In animal models, cis-palmitoleate repressed the expression of proinflammatory markers and adipokines, and increased carbohydrate intake and lipogenesis.84 Trans-palmitoleate, found in dairy products and partially hydrogenated oils, is not strongly associated with incident diabetes85 nor linked to blood clotting or strokes.86 Palmitoleate, converted from palmitic acid, increases insulin sensitivity (Figure 3), and reduces the risk of atherosclerosis and CVD.87,88 Lipokine palmitoleic acid has anti-inflammatory properties and reduces the expression of pro-inflammatory cytokines IL-1β (pro-IL1β), TNF-α, and IL-6 (Figure 3). In vitro studies showed that palmitoleic acid reduced lipopolysaccharide (LPS)-induced inflammation in macrophages via inflammasome and NFκB pathways.89 High concentrations of palmitoleic acid (more than 50 mm) are toxic and lower concentrations reduce human peripheral blood lymphocyte proliferation, and T helper (Th1) and Th17 responses.90 Schirmer et al.,91 however, did not report a palmitoleic acid effect on lymphocyte-associated cytokines (IFNγ, IL-17, IL-22) when studied using human peripheral blood mononuclear cells (PBMNCs). The discrepancy between these findings may be due to the use of different cell populations, i.e., isolated lymphocytes versus PBMNCs.90,91 More research is required to understand the effect MUFA has on lymphocyte responses.
Figure 3.
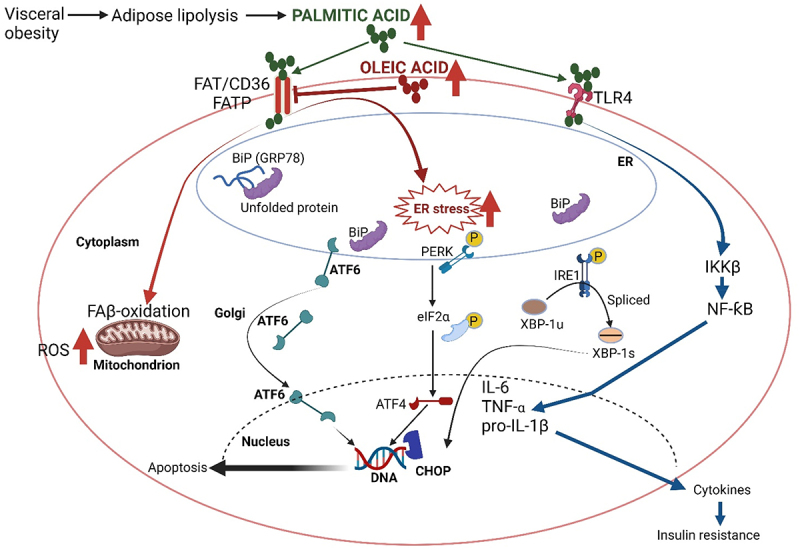
Visceral obesity and adipose lipolysis lead to the production of non-esterified fatty acids such as palmitic acid (C16H32O2). Stress induced on the mitochondrion and endoplasmic reticulum (ER) by palmitic acid results in fatty acid β (FAβ)-oxidation, an increase in reactive oxygen species (ROS), and apoptosis. Oleic acid (C18H34O2) represses the fatty acid translocase protein (FATP) FAT/CD36 and prevents an increase in ROS. Palmitic acid also triggers the transmembrane kinase protein (PERK) in the ER, which dimerizes and autophosphorylates, leading to the phosphorylation of the α subunit of eukaryotic initiation factor 2 (eIf2α) and induction of transcription factor 4 (ATF4) plus the CAAT/enhancer binding protein homologous transcription factor (CHOP), also known as GADD153, in the nucleus. CHOP is involved in DNA damage, growth arrest, and the induction of apoptosis. Under normal conditions, the three critical transmembrane proteins PERK, IRE-1 (inositol-requiring enzyme type 1), and ATF6 (an er-membrane-bound transcription factor) are associated with the major ER chaperone bip (GRP78) of the heat shock protein 70 family. Bip interacts with nonglycosylated and glycosylated proteins and er-transmembrane signaling molecules. Under ER stress conditions, bip is released and interacts with unfolded or misfolded proteins in the ER lumen. The autophosphorylation of IRE1 leads to the splicing of 26 nucleotides from the XBP1 (a transcription factor) mRNA. The smaller spliced XBP1 (XBP-1s) also promotes the transcription of CHOP. During ER stress, ATF6 is released from bip and translocates to the Golgi where it is proteolytically activated. The perk-eIf2α-ATF4-chop pathway plays an essential role in palmitic acid-triggered apoptosis. The suppression of ER stress by oleic acid and regulation of unfolded protein responses is important in preventing apoptotic cell death, especially in pancreatic β cells. Palmitic acid stimulates pro-inflammatory responses in human immune cells via Toll-like receptor 4 (TLR4). The degradation of IKKβ (IκB kinase β) activates nf-κB (nuclear factor kappa B), which induces the expression of various pro-inflammatory genes, including those encoding cytokines and chemokines. Created using Biorender.com (1 July 2024).
The effect of palmitic acid on reactive oxygen species (ROS) and apoptosis is schematically represented in Figure 3. Palmitic acid induces stress on mitochondria and the endoplasmic reticulum (ER), resulting in an increase in ROS and apoptosis.92,93 Oleic acid, in turn, prevents an increase in ROS. Under normal conditions, the three critical transmembrane proteins PERK (ER-resident transmembrane protein kinase), IRE-1 (inositol-requiring enzyme type 1), and ATF6 (ER-membrane-bound transcription factor) are linked to the major ER chaperone Bip (GRP78). Under ER stress conditions, Bip is released to interact with unfolded or misfolded proteins in the ER lumen.94 Triggering of PERK in the ER initiates the phosphorylation (activation) of the eukaryotic initiation factor 2α (eIF2α) and the induction of transcription factor ATF4 as well as the CAAT/enhancer binding protein homologous transcription factor (CHOP). The latter is involved in DNA damage, growth arrest, and the stimulation of apoptotic cell death. The autophosphorylation of IRE1 leads to the splicing of 26 nucleotides from the XBP1 mRNA. The XBP1 protein is a transcription factor that regulates gene expression in immunity and cellular stress response. The shorter spliced XBP1 (XBP-1s) also promotes the transcription of CHOP. When the ER is under stress, ATF6, released from Bip, is translocated to the Golgi and activated.95 ATF6 is an important signal transducer in cellular reprogramming that responds to protein misfolding in the endoplasmic reticulum. The mechanism by which ATF6 senses unfolded proteins and becomes activated is unknown.96 The alleviation of ER stress by oleic acid and regulation of unfolded protein responses are important in preventing apoptotic cell death, especially in pancreatic β cells.97 Palmitic acid also stimulates pro-inflammatory responses in human immune cells via Toll-like receptor 4 (TLR4).98 The degradation of IKKB (IκB kinase) activates NFκB.99 NFκB induces the expression of various pro-inflammatory genes, including those encoding cytokines and chemokines (Figure 3), and also participates in inflammasome regulation.100
Polyunsaturated fatty acids and their synthesis
Polyunsaturated fatty acids (PUFAs), such as omega-3 and omega-6, are not produced in the body but form an essential part of a diet.101 Fish oil is rich in omega-3 fatty acids such as eicosapentaenoic acid (EPA) and docosapentaenoic acid (DPA), whereas α-linolenic acid (ALA; C18H30O2), an essential omega-3 fatty acid (Figure 4), is found in flaxseed oils.102 Several bioactive mediators derived from omega-3 PUFAs are involved in the recovery of injured and infected tissue cells (summarized in Figure 4). Cell debris and bacterial cells are phagocytized by polymorphonuclear leukocytes (PMNs), which are subsequently removed by recruited monocyte-derived macrophages. These reactions are orchestrated by anti-inflammatory prostaglandins and leukotrienes produced from the conversion of PUFAs by COX and LOX (Figure 4). Prostaglandins and leukotrienes are precursors of eicosanoids, i.e., signaling molecules regulating inflammation.102 Protectin, derived from DPA (Figure 4), represses the interactions between neutrophils and endothelial cells, neutrophil chemotaxis, and recruitment but increases macrophage phagocytosis.102,103 Protectins reduce the production of inflammatory cytokines, including MCP-1/chemokine C-X-C motif ligand-2 (CXCL-2).104 Maresin 1, also derived from DPA (Figure 4), stimulates macrophage phagocytosis and the clearance of human apoptotic neutrophils, similar to maresin-1 derived from EPA.105 Concluded from these and other findings,106 the biological effects displayed by EPA and DHA also apply to DPA. DPA incorporates inflammatory cells more easily than EPA and DHA and displays stronger anti-inflammatory properties.107 Omega-3 PUFAs may thus control inflammation by mediating molecules with low or no inflammatory activity.108 Omega-3 PUFAs have also been used in treating dyslipidemic disorders, thrombosis, atherosclerosis, and myocarditis.108 An increase in the consumption of omega-3 PUFAs altered the composition of gut microbiota, led to lower levels of LPS produced, and decreased intestinal permeability.109 DHA favors the proliferation of alpha gut bacteria, especially Lachnospiraceae110 and Lactobacillus.111 PUFAs significantly increase cell numbers of Bifidobacterium, Lactobacillus, and Roseburia.111,112
Figure 4.
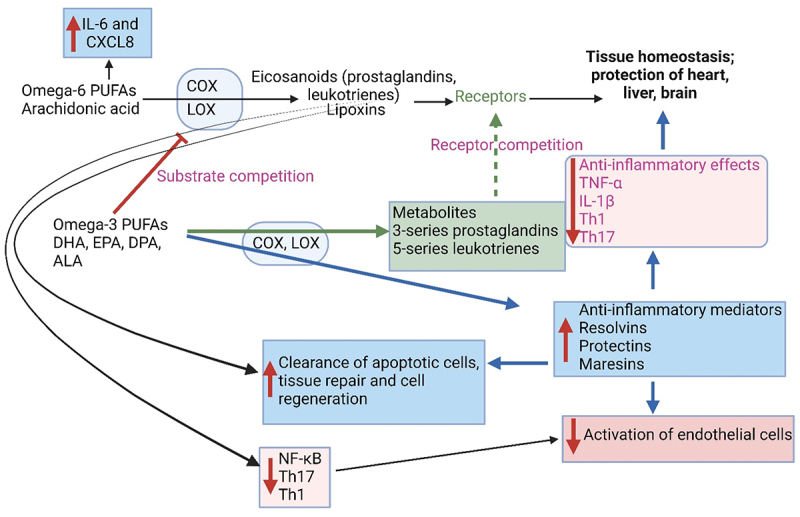
Omega-3 polyunsaturated fatty acids (PUFAs) and omega-6 PUFAs play a role in inflammation, the activation of endothelial cells, apoptosis, cell repair, and cell regeneration. IL: interleukin, CXCL8: C-X-C motif chemokine ligand 8, NFκB: nuclear factor kappa B, Th: T-helper cell, TNFα: tumor necrosis factor-alpha, CPT1A: carnitine palmitoyltransferase 1A, COX: cyclooxygenases, LOX: lipoxygenase, DHA: docosahexaenoic acid, EPA: eicosapentaenoic acid, DPA: docosapentaenoic acid, ALA: α-linolenic acid (C18H30O2). Created using Biorender.com (1 July 2024).
Pregnant rodents fed high levels of omega-3 led to a decrease in numbers of Lachnospiraceae, Anaerotruncus, and Roseburia and an increase in Blautia, Oscillibacter, Clostridiales, Robinsoniella, Lactococcus, and Eubacterium in offspring.113 The offspring of mice fed high levels of omega-3 fatty acids had lower levels of Bacteroidetes and higher levels of Firmicutes.113 In animal and human studies, a deficiency in omega-3 fatty acids early in life leads to diminished cognitive abilities, weakened attention, loss of vision, and psychological disorders such as depression, schizophrenia, and dementia.109 These conditions may be prevented when breastfeeding mothers take omega-3 fatty acid supplements.109 According to the authors, fatty acids in breast milk are only transferred to male infants. Omega-3 fatty acids are associated with improved metabolism and less weight gain in offspring.109 A reduction in maternal omega-3 acids is associated with a significant reduction in epsilon proteobacteria, Bacteroides, and Akkermansia but an increase in clostridia.109 Trans-10, cis-12 conjugated LA (t10-c12 CLA) in dairy products and red meat, and produced by Lactobacillus plantarum PL62, have antiobesity properties but may induce hepatic steatosis and hyperinsulinemia, specifically in diabetic or obese individuals.114 In mice, t10-c12 CLA reduced the Firmicutes:Bacteroidetes (F:B) ratio and decreased levels of Desulfovibrionaceae, Lachnospiraceae, Peptococcaceae, and Clostridiales Family XIII but increased Porphyromonadaceae.115 High-fat palm oil and high-fat olive oil diets led to obesity without a drastic change in gut microbiota composition. Diets rich in palm oil contain phytochemicals, lauric acid, retinoids, tocotrienols, and carotenoids. β-carotene in palm oil enhances gut immune homeostasis by modulating the production of IgA.116 Hidalgo et al.117 did, however, report an increase in Bacteroidetes when mice were fed olive oil but not when fed palm oil. This is interesting, as Bacteroidetes are associated with obesity. A high-fat palm oil diet, however, increased the F:B ratio, especially Clostridium clusters XI, XVII, and XVIII.118
Omega-6 arachidonic acid (AA, C20H32O2) is converted by COX and LOX to potential inflammatory mediators (eicosanoids; Figure 4).102 Omega-6 PUFAs are precursors of many pro-inflammatory signaling molecules that trigger inflammation.108 In the case of pulmonary infections, AA initiated the release of IL-6 and CXCL8. Cytokines produced by pulmonary fibroblasts are regulated by prostaglandin and p38 mitogen-activated protein (MAP) kinase signaling.102 Elevated levels of omega-6, typically found in a Western-style diet, may lead to more severe inflammation.119 Lipoxins (LX), also produced by the interaction of LOX with AA, (Figure 4) present anti- and pro-inflammatory reactions.120 In vitro tests have shown that LX reduces neutrophil migration121 and reduces inflammation in septic cells.122 In vivo studies have shown that LX increases neutrophil clearance.121 Lipoxin A4 (LXA4) regulates leukocyte tracking and responses,123 modulates the activation of vascular, smooth muscle, and epithelial cells,124 and reduces renal fibrosis.125 Binding of L×A4to the LX receptor (ALX) modulates the expression of adhesion molecules through inhibition of the NFκB pathway in endothelial cells.126,127
Omega-3 fatty acids have anti-inflammatory properties, whereas omega-6 fatty acids (not produced by humans) are pro-inflammatory.128 A balance between the two omega fatty acids is thus important to keep gut microbiota in a balanced state.128 The oxidation of linoleic acid (LA, C18H32O2), an omega-6 essential fatty acid, to 13-hydroperoxy octadecadienoic acid (13-HPODE), stimulates the production of TNF-α, MCP-1, IL-6 (pro-inflammatory cytokines) and cellular apoptosis.128 At the same time, barrier-forming tight junction proteins (TJPs) such as Claudin-1 and Occludin are downregulated, and pore-forming Claudin-2 is upregulated.128 This process, called “claudin switching”,129 leads to changes in the barrier function of the gut wall (IEC) and is often associated with IBD.128,130 The “switching” of TJPs is due to cytokine-mediated dysregulation.129 An increase in cytokine levels and a decrease in gut permeability were noted after 4 h when mice were fed 13-HPODE.130 After 28 days of 13-HPODE feeding, an increase in cholesterol uptake by peritoneal macrophages was noted, which was considered an indication of severe intestinal inflammation.130 PUFAs are metabolized by cyclooxygenase, lipoxygenase, and cytochrome P450 (CYP-450) to eicosanoids, lipoxygenases, and other essential metabolites (Figure 4). Linoleic 9,10-epoxy octadecenoic acid (9,10-EpOME or leukotoxin) and 12,13-epoxy octadecenoic acid (12,13-EpOME or iso-leukotoxin) are the main products derived from the metabolism of LA. Both variations of epoxy octadecenoic acids have immunomodulatory properties. Experiments with mice have shown a reduction in EpOMEs and dihydroxy octadecenoic acids (DiHOMEs) when fed a high-fat diet supplemented with the omega-3 α-linolenic acid (ALA).131 This also led to a lowering in the omega-6:omega-3 ratio, a decline in NFκB activation, divergence of M1 macrophages, and insulin resistance.131 12,13-DiHOME increased Th2 cells, which increased the risk of developing asthma.132 In children suffering from asthma, the cell numbers of Candida and Rhodotorula spp. increased and those of Bifidobacterium, Akkermansia, and Faecalibacterium spp. decreased. The role of ALA in the differentiation of M2 macrophages is poorly understood. A recent study133 has shown that 13-hydroxy9(Z),15(Z)-octadecadienoic acid (13-OH), and 13-oxo-9(Z),15(Z)-octadecadienoic acid (13-oxo) produced by lactic acid bacteria regulates M2 differentiation. This is orchestrated via the GPCR40-MAPK and PPARγ signaling pathways in the presence of IL-4 and IL-13. Mice fed ALA, 13-OH, or 13-oxo for three days showed differentiation of M2 macrophages but only in the lamina propria of the small intestinal tract. No additional formation of adipose tissue, gut-associated lymphoid tissue, and mesenteric lymph nodes was observed.133
Studies conducted by Valenzuela et al. (2023)134 on Balb/c mice have shown that the highest levels of PUFA, based on the levels and activity of desaturases Δ-6D and Δ-5D, and elongases Elovl2 and Elovl5, were synthesized in the liver. Omega-3 and omega-6 PUFAs are desaturated by Δ-6 desaturase (Δ-6D) and Δ-5D, respectively, whereas the elongation of omega-3 and omega-6 PUFAs is regulated by elongases 2 (Elovl2) and Elovl5, respectively.135 In mice, low levels of PUFA were synthesized in the brain, testicles, and kidney and no PUFA enzyme activity was reported in the heart and lung.134 The production of Δ-5D in the liver was 4.3- to 22.9-fold higher (based on protein concentration and enzyme activity) compared to Δ-5D levels in the testicle.134 This compared to Elovl2 levels in the kidney.134 Furthermore, 4.0- to 85-fold higher levels of Δ-5D activity were observed in the liver compared to Δ-6D activity in the testicle and Elovl5 activity in the kidney.134 Higher levels of omega-3 PUFAs were produced compared to omega-6 PUFAs but levels may differ depending on the physiological or pathological condition of a patient. Both processes (desaturation and elongation of PUFAs) are influenced by the availability of zinc, vitamin B, and magnesium, protein levels in the diet, and oxidative stress in the liver.135 Obese individuals and those suffering from nonalcoholic fatty liver disease (NAFLD) produce less PUFAs.136
The intermediates formed as a result of Elovl5 activity were similar in omega-3 and omega-6 production. The activity of Elovl2 was higher with omega-3 substrates (EPA and stearidonic acid, SDA) compared with omega-6 substrates (ARA and adrenic acid, ADA), as observed with recombinant Saccharomyces cerevisiae cells that expressed Elovl2.137,138 A possible explanation for this is that the fatty acid (FA) transport protein 2a/very long chain acyl-CoA synthetase 1 (FATP2a/Acsvl1) enhances the transfer, activation, and metabolism of omega-3 PUFAs.139 This may lead to an increase in dietary DHA but depends on the availability of ALA, the elongation and/or desaturation of DHA precursors, and a range of other physiological and enzymatic conditions (summarized by Valenzuela et al.134
The synthesis of PUFA is initiated by the conversion of ALA and LA to an acyl-CoA derivative by acyl-CoA synthases 3 and 4, the desaturation of acyl-CoA by Δ6D and Δ5D to form a double bond, elongation (the addition of two carbon atoms) of PUFA acyl-CoA by elongase 2/5, and the oxidation of fatty acids (FAs) by peroxisomal FA oxidase (FAO).135 The end products EPA, DHA, and AA are important in cell growth, membrane formation, and the functioning of organs. The transcription of desaturases and elongases in mammals is regulated by insulin via the sterol regulatory element binding protein 1c (SREBP-1c), under control (suppression) by omega-3 PUFAs. For further information on the synthesis of omega-3 and omega-6 PUFAs, and the influence of nutritional status on the desaturation and elongation of these fatty acids, the reader is referred to Videla et al.135
Saturated fatty acids (SFAs)
Saturated fatty acids (SFAs) are distinguished from unsaturated fatty acids by having single C – C bonds. Short-chain SFAs (C8 to C12) are found in vegetable oils, whilst SFAs with more than 12 carbons, e.g., palmitic acid and stearic acid (C18H36O2) are predominantly in eggs, animal fats, and butter87 SFAs are generally pro-inflammatory.128 The interaction of palmitic acid and other dietary SFAs with the nucleotide-binding oligomerization domain-leucine-rich repeat-pyrin domain-containing 3 (NLRP3) inflammasome leads to an increase in adiposity.128 Macrophages in adipose tissue have higher levels of the NLRP3 inflammasome, as observed in obese mice and humans. A decrease in NLRP3 inflammasome was noted when calorie intake was restricted or with an increase in exercise.140 In vitro studies have shown that diets rich in SFAs can activate TLR4 in dendritic cells and lead to an increase in NLRP3 inflammasome.140 Studies with human monocytes have shown that palmitate, myristate, and stearate, but not unsaturated fatty acids such as palmitoleate and oleate, activates TNFα and IL-1β, which promote death and increases inflammation.128 Palmitate stimulates the production of the inflammatory caspase proteins caspase-1, caspase-4, and caspase-5.87 These proteins play an important role in the production of IL-1β and the initiation of cell death.141 Palmitic acid, stearate, and lauric acid are known to regulate inflammatory responses via TLR4 and NFκB signaling in immune cells.87 The myeloid differentiation primary response 88 protein (Myd88) transduces signals from all TLRs, except TLR3.87 The toll/interleukin-1 receptor (TIR) domain contains the TIR adaptor-inducing beta interferon (TRIF) that sends signals from TLR3 and TLR4.87 TRIF protects cells from metabolic disorders and inflammation.142
Palmitic acid targets the receptor-interacting protein kinase 1 (RIPK1) in liver macrophages, leading to increased production of inflammatory cytokines (IL-1β, TNFα, and IL-6) and cell death. The condition is known as nonalcoholic steatohepatitis (NASH).143 Obese individuals and those suffering from type 2 diabetes are especially vulnerable to developing NASH. An increase in palmitic acid leads to autophagy and cellular accumulation of autophagosomes.144 Mice lacking the ability to produce the mixed lineage kinase domain-like protein (MLKL) were protected from autophagy when they were on a Westernized diet. They showed a reduction in liver injury, inflammation, and cell death.138 Palmitic acid induces the hypoxia-inducible factor-1α (hif-1 α), responsible for inflammation regulated via the NFκB pathway and the production of pro-inflammatory cytokines TNF, IL-1β, and IL-6.145,146
SFAs and a high-fat diet influence cellular processes in IECs, Paneth cells, and stem cells.147,148 Disruption of Paneth cells affects the production of antimicrobial peptides and growth factors that maintain stem cells. Previous studies have shown dysfunction in these cells in patients with IBD.149 In mice fed a high-fat diet, the dysfunction of Paneth cells led to the activation of type I interferons (IFNs) associated with nuclear farnesoid X receptor (FXR).149
Palmitic acid is converted to palmitoleic acid, oleic acid, stearic acid, and sphingolipids.128 Sphingolipids are also produced by bacteria, e.g., Bacteroides fragilis.150 Palmitic acid stimulates IgA responses, which may lead to the forming of mucosal adjuvants.151 Hepatocytes treated with palmitic acid release lipotoxic extracellular vesicles filled with sphingosine 1-phosphate (S1P). This stimulates the infiltration of macrophages and induces hepatic lipotoxicity associated with NASH.152
Can fatty acids be used in the treatment of diseases?
Fewer cases of CVDs were reported for patients following a MD.153 A low-fat diet supplemented with PUFAs reduced waist circumference, blood pressure, triglyceride levels, and the prevalence of metabolic syndrome.154 The relative abundance of Lachnospiraceae associated with an MD was inversely correlated with blood pressure and lipid profiles.155 Oleic acid was associated with an increase in the Clostridiales vadin BB60 group.155 Tryptophan, an essential amino acid found in a variety of foods, including poultry, fish, dairy products, and grains,156 typical of an MD, is metabolized by gut microbiota into small molecules that serve as ligands for AhR. This stimulates the secretion of glucagon-like peptide 1 (GLP-1) from EECs.156,157 Intestinal barrier functions are impaired with reduced AhR and less GLP-1 being released.157 Tryptophan produced by gut microbiota promotes the differentiation of neural progenitor cells into mature neurons158 and reduces inflammation of the CNS.159 Although the consumption of seafood reduces the risk for CVD,160 the production of TMA by gut microbiota and the conversion to TMAO accelerates CVD, as shown in mice.145,161 A vegetarian diet, on the other hand, favors alpha bacteria,162 especially SCFA-producing taxa such as Akkermansia,163 F. prausnitzii, Eubacterium rectale and Eubacterium biforme. 164
Lauric acid, retinoids, tocotrienols, and carotenoids in palm oil enhance gut immune homeostasis by modulating the production of IgA.116 Retinoic acid (vitamin A) triggers the production of IgA in B cells.165 Food rich in biotin (vitamin B7), such as Yam (orange sweet potato) supports the proliferation and maintenance of gut microbiota that prevents the activation of NFκB and stimulates the generation of pro-inflammatory cytokines such as tumor necrosis factor α (TNFV), IL-8, IL-6, and IL-1.166 The antioxidative, immunomodulatory, and anti-inflammatory properties of vegetable flavonoids protect the host against chronic inflammatory diseases.167,168 Innate immunity and the constant production of neutrophils are important in sustaining a balanced gut microbiome166 and fight off invading microorganisms.169
Inulin-type fructans (ITFs), typically found in wheat, onion, and chicory,170 repress appetite170 and prevent constipation.171,172 Inulin stimulates the growth of Bifidobacterium, Anaerostipes, Bacteroides, and Faecalibacterium but represses the growth of Coprococcus, Dorea, Ruminococcus, Bilophila, Blautia, Oscillibacter, and Ruminococcus.172–174 Although inulin does not affect the production of SCFAs,167,168 changes were noted in the plasma levels of tyrosine and glycine.174 Inulin propionate ester (IPE) reduced the production of IL-8, increased the secretion of insulin,174,175 and stimulated the growth of Bacteroides uniformis and Bacteroides xylanisolvens but repressed the growth of Eubacterium ruminantium and Blautia obeum. 174
Conclusions
Fatty acids are major constituents of cell membranes but are often overlooked as intracellular signaling molecules and gene expression modulators. In the past, most research on fatty acids focused on human health, especially CVDs, cancer, type 2 diabetes, and inflammatory diseases. Extensive research has been done on PUFAs, especially butyrate, and its role in IBD and CRC. Research on SCFA transports has shown that the dysregulation of monocarboxylate transporters such as MCT1, MCT4, and SMCT1 may be the answer to some gastrointestinal disorders. Acetate and propionate have similar notable effects on the GIT, with the latter demonstrating a pivotal role in weight management and the regulation of inflammation. The supplementation of a fiber-rich diet with SCFAs helps to maintain a healthy intestinal barrier and support diverse gut microbiota. More research is, however, required to explore the role intestinal microbiota play in the metabolism of SCFAs, including the mechanisms involved in the lowering of LDL-cholesterol by PUFAs such as omega-6, and the lowering of triglycerides by omega-3 PUFAs EPA and DHA. We need to understand the role of SCFAs in regulating blood flow, thrombosis, and neurological disorders. The relationship between omega-6 and omega-3 PUFAs in inflammation regulation is not fully understood. Even-numbered saturated fatty acids, such as palmitic acid, raise total and LDL cholesterol levels. Reports of saturated fatty acids that increase coagulation, inflammation, and insulin resistance necessitate in-depth research. The replacement of saturated fatty acids in a diet by cis MUFAs, such as palmitoleic and oleic acids, and ω-6 PUFA (LA) lower LDL cholesterol levels and are associated with fewer incidences of CVDs. Arachidonic acid, also a ω-6 PUFA, mainly acts as an eicosanoid precursor involved in inflammatory reactions but EPA and DHA are important mediators in signal transduction and gene expressions. Trans SCFAs raise LDL and lower HDL cholesterol levels, thus increasing the risk of CVD. Trans SCFAs also promote inflammation and are prone to play a role in metabolic diseases.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
No AI technology or ChatGPT software has been used in the writing of this review. All figures were created using Biorender.com.
References
- 1.Sender R, Fuchs S, Milo R.. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14(8):e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, Balamurugan R. The firmicutes/bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. 2020;12(5):1474. doi: 10.3390/nu12051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berthoud H-R, Münzberg H, Richards BK, Morrison CD. Neural and metabolic regulation of macronutrient intake and selection. Proc Nutr Soc. 2012;71(3):390–20. doi: 10.1017/S0029665112000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norris V, Molina F, Gewirtz AT. Hypothesis: bacteria control host appetites. J Bacteriol. 2013;195(3):411–416. doi: 10.1128/JB.01384-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, Abramson L, Katz MN, Korem T, Zmora N, et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159(3):514–529. doi: 10.1016/j.cell.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 6.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165(1):111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu W, Wang Z, Tang WHW, Hazen SL. Gut microbe-generated trimethylamine N -Oxide from dietary choline is prothrombotic in subjects. Circulation. 2017;135(17):1671–1673. doi: 10.1161/CIRCULATIONAHA.116.025338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang WHW, Hazen SL. The gut microbiome and its role in cardiovascular diseases. Circulation. 2017;135(11):1008–1010. doi: 10.1161/CIRCULATIONAHA.116.024251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwingshackl L, Hoffmann G. Monounsaturated fatty acids and risk of cardiovascular disease: synopsis of the evidence available from systematic reviews and meta-analyses. Nutrients. 2012;4(12):1989–2007. doi: 10.3390/nu4121989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gantenbein KV, Kanaka-Gantenbein C. Mediterranean diet as an antioxidant: the impact on metabolic health and overall wellbeing. Nutrients. 2021;13(6):1951. doi: 10.3390/nu13061951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz DL, Meller S. Can we say what diet is best for health? Annu Rev Public Health. 2014;35(1):83–103. doi: 10.1146/annurev-publhealth-032013-182351. [DOI] [PubMed] [Google Scholar]
- 12.Bialonska D, Ramnani P, Kasimsetty SG, Muntha KR, Gibson GR, Ferreira D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int J Food Microbiol. 2010;140(2–3):175–182. doi: 10.1016/j.ijfoodmicro.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 13.Queipo-Ortuño MI, Boto-Ordóñez M, Murri M, Gomez-Zumaquero JM, Clemente-Postigo M, Estruch R, Cardona Diaz F, Andrés-Lacueva C, Tinahones FJ. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am J Clin Nutr. 2012;95(6):1323–1334. doi: 10.3945/ajcn.111.027847. [DOI] [PubMed] [Google Scholar]
- 14.Meslier V, Laiola M, Roager HM, De Filippis F, Roume H, Quinquis B, Giacco R, Mennella I, Ferracane R, Pons N, et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut. 2020;69(7):1258–1268. doi: 10.1136/gutjnl-2019-320438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, Serrazanetti DI, Di Cagno R, Ferrocino I, Lazzi C, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65(11):1812–1821. doi: 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- 16.Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FB, Topf M, Gonzalez CG, Van Treuren W, Han S, et al. Gut-microbiota-targeted diets modulate human immune status. Cell. 2021;184(16):4137–4153.e14. doi: 10.1016/j.cell.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.So D, Whelan K, Rossi M, Morrison M, Holtmann G, Kelly JT, Shanahan ER, Staudacher HM, Campbell KL. Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. Am J Clin Nutr. 2018;107(6):965–983. doi: 10.1093/ajcn/nqy041. [DOI] [PubMed] [Google Scholar]
- 18.Gong L, Cao W, Chi H, Wang J, Zhang H, Liu J, Sun B. Whole cereal grains and potential health effects: involvement of the gut microbiota. Food Res Int. 2018;103:84–102. doi: 10.1016/j.foodres.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 19.Kristek A, Wiese M, Heuer P, Kosik O, Schär MY, Soycan G, Alsharif S, Kuhnle GGC, Walton G, Spencer JPE. Oat bran, but not its isolated bioactive β -glucans or polyphenols, have a bifidogenic effect in an in vitro fermentation model of the gut microbiota. Br J Nutr. 2019;121(5):549–559. doi: 10.1017/S0007114518003501. [DOI] [PubMed] [Google Scholar]
- 20.Dicks LMT, Grobbelaar MJ. Double-barrel shotgun: probiotic lactic acid bacteria with antiviral properties modified to serve as vaccines. Microorganisms. 2021;9(8):1565. doi: 10.3390/microorganisms9081565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dicks LMT, Geldenhuys J, Mikkelsen LS, Brandsborg E, Marcotte H. Our gut microbiota: a long walk to homeostasis. Benef Microbes. 2018;9(1):3–20. doi: 10.3920/BM2017.0066. [DOI] [PubMed] [Google Scholar]
- 22.McDonald D, Hyde E, Debelius JW, Morton JT, Gonzalez A, Ackermann G, Aksenov AA, Behsaz B, Brennan C, Chen Y, et al. American gut: an open platform for citizen science microbiome research. mSystems. 2018;3(3):3. doi: 10.1128/mSystems.00031-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blaak EE, Canfora EE, Theis S, Frost G, Groen AK, Mithieux G, Nauta A, Scott K, Stahl B, van Harsselaar J, et al. Short chain fatty acids in human gut and metabolic health. Benef Microbes. 2020;11(5):411–455. doi: 10.3920/BM2020.0057. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Ames NP, Tun HM, Tosh SM, Jones PJ, Khafipour E. High molecular weight barley β-glucan alters gut microbiota toward reduced cardiovascular disease risk. Front Microbiol. 2016;7. doi: 10.3389/fmicb.2016.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen R-L, Dang X-Y, Dong J-L, Hu X-Z. Effects of oat β-glucan and barley β-glucan on fecal characteristics, intestinal microflora, and intestinal bacterial metabolites in rats. J Agric Food Chem. 2012;60(45):11301–11308. doi: 10.1021/jf302824h. [DOI] [PubMed] [Google Scholar]
- 26.Metzler-Zebeli BU, Zijlstra RT, Mosenthin R, Gänzle MG. Dietary calcium phosphate content and oat β-glucan influence gastrointestinal microbiota, butyrate-producing bacteria and butyrate fermentation in weaned pigs. FEMS Microbiol Ecol. 2011;75(3):402–413. doi: 10.1111/j.1574-6941.2010.01017.x. [DOI] [PubMed] [Google Scholar]
- 27.Van den Abbeele P, Venema K, Van de Wiele T, Verstraete W, Possemiers S. Different human gut models reveal the distinct fermentation patterns of arabinoxylan versus inulin. J Agric Food Chem. 2013;61(41):9819–9827. doi: 10.1021/jf4021784. [DOI] [PubMed] [Google Scholar]
- 28.Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:10. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cushing K, Alvarado DM, Ciorba MA. Butyrate and mucosal inflammation: new scientific evidence supports clinical observation. Clin Transl Gastroenterol. 2015;6(8):e108. doi: 10.1038/ctg.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Preter V, Geboes KP, Bulteel V, Vandermeulen G, Suenaert P, Rutgeerts P, Verbeke K. Kinetics of butyrate metabolism in the normal colon and in ulcerative colitis: the effects of substrate concentration and carnitine on the β-oxidation pathway. Aliment Pharmacol Ther. 2011;34(5):526–532. doi: 10.1111/j.1365-2036.2011.04757.x. [DOI] [PubMed] [Google Scholar]
- 31.Wang H-B, Wang P-Y, Wang X, Wan Y-L, Liu Y-C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein claudin-1 transcription. Dig Dis Sci. 2012;57(12):3126–3135. doi: 10.1007/s10620-012-2259-4. [DOI] [PubMed] [Google Scholar]
- 32.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 33.De Vadder F, Grasset E, Mannerås Holm L, Karsenty G, Macpherson AJ, Olofsson LE, Bäckhed F. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci. 2018;115(25):6458–6463. doi: 10.1073/pnas.1720017115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egerod KL, Petersen N, Timshel PN, Rekling JC, Wang Y, Liu Q, Schwartz TW, Gautron L. Profiling of G protein-coupled receptors in vagal afferents reveals novel gut-to-brain sensing mechanisms. Mol Metab. 2018;12:62–75. doi: 10.1016/j.molmet.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4(1):1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci USA. 2011;108(19):8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nøhr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, Sichlau RM, Grunddal KV, Seier Poulsen S, Han S. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154(10):3552–3564. doi: 10.1210/en.2013-1142. [DOI] [PubMed] [Google Scholar]
- 38.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1–2):84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 39.Stuttgen GM, Sahoo D. FFAR4: a new player in cardiometabolic disease? Endocrinology. 2021;162(8):bqab111. doi: 10.1210/endocr/bqab111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumari P, Inoue A, Chapman K, Lian P, Rosenbaum DM. Molecular mechanism of fatty acid activation of FFAR1. Proc Natl Acad Sci USA. 2023;120(22):120. doi: 10.1073/pnas.2219569120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fleischer J, Bumbalo R, Bautze V, Strotmann J, Breer H. Expression of odorant receptor Olfr78 in enteroendocrine cells of the colon. Cell Tissue Res. 2015;361(3):697–710. doi: 10.1007/s00441-015-2165-0. [DOI] [PubMed] [Google Scholar]
- 42.Hase K, Eckmann L, Leopard JD, Varki N, Kagnoff MF. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect Immun. 2002;70(2):953–963. doi: 10.1128/IAI.70.2.953-963.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133(7):2485S–2493S. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- 44.Roger T, Lugrin J, Le Roy D, Goy G, Mombelli M, Koessler T, Ding XC, Chanson A-L, Reymond MK, Miconnet I. Histone deacetylase inhibitors impair innate immune responses to toll-like receptor agonists and to infection. Blood J Am Soc Hematol. 2011;117(4):1205–1217. doi: 10.1182/blood-2010-05-284711. [DOI] [PubMed] [Google Scholar]
- 45.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26(37):5541–5552. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- 46.Kim H-J, Bae S-C. Histone deacetylase inhibitors: molecular mechanisms of action and clinical trials as anti-cancer drugs. Am J Transl Res [Internet]. 2011;3:166–179. http://www.ncbi.nlm.nih.gov/pubmed/21416059. [PMC free article] [PubMed] [Google Scholar]
- 47.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10(1):32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Konsoula Z, Barile FA. Epigenetic histone acetylation and deacetylation mechanisms in experimental models of neurodegenerative disorders. J Pharmacol Toxicol Met. 2012;66(3):215–220. doi: 10.1016/j.vascn.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Modoux M, Rolhion N, Lefevre JH, Oeuvray C, Nádvorník P, Illes P, Emond P, Parc Y, Mani S, Dvorak Z. Butyrate acts through HDAC inhibition to enhance aryl hydrocarbon receptor activation by gut microbiota-derived ligands. Gut Microbes. 2022;14(1):2105637. doi: 10.1080/19490976.2022.2105637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Korecka A, Dona A, Lahiri S, Tett AJ, Al-Asmakh M, Braniste V, D’Arienzo R, Abbaspour A, Reichardt N, Fujii-Kuriyama Y, et al. Bidirectional communication between the aryl hydrocarbon receptor (AhR) and the microbiome tunes host metabolism. NPJ Biofilms Microbiomes. 2016;2(1). doi: 10.1038/npjbiofilms.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry. 2007;62(1):55–64. doi: 10.1016/j.biopsych.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 52.Galland L. The gut microbiome and the brain. J Med Food. 2014;17(12):1261–1272. doi: 10.1089/jmf.2014.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harrison IF, Dexter DT. Epigenetic targeting of histone deacetylase: therapeutic potential in Parkinson’s disease? Pharmacol Ther. 2013;140:34–52. doi: 10.1016/j.pharmthera.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 54.Scheperjans F, Aho V, Pereira PAB, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola‐Rautio J, Pohja M. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30:350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 55.Unger MM, Spiegel J, Dillmann K-U, Grundmann D, Philippeit H, Bürmann J, Faßbender K, Schwiertz A, Schäfer K-H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 56.Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, Mutlu E, Shannon KM. Colonic bacterial composition in Parkinson’s disease. Mov Disord. 2015;30:1351–1360. doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- 57.Sun M-F, Shen Y-Q. Dysbiosis of gut microbiota and microbial metabolites in Parkinson’s disease. Ageing ResearchRev. 2018;45:53–61. doi: 10.1016/j.arr.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 58.Durgan DJ, Lee J, McCullough LD, Bryan RM Jr. Examining the role of the microbiota-gut-brain axis in stroke. Stroke. 2019;50(8):2270–2277. doi: 10.1161/STROKEAHA.119.025140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Erny D, Hrabě de Angelis AL, Prinz M. Communicating systems in the body: how microbiota and microglia cooperate. Immunology. 2017;150(1):7–15. doi: 10.1111/imm.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, Anastasovska J, Ghourab S, Hankir M, Zhang S. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5(1):3611. doi: 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Sci (1979). 2012;336(6086):1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 62.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278(13):11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 63.Granger AJ, Mulder N, Saunders A, Sabatini BL. Cotransmission of acetylcholine and GABA. Neuropharmacology. 2016;100:40–46. doi: 10.1016/j.neuropharm.2015.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci. 2014;34(46):15490–15496. doi: 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol Q Publ Hellenic Soc Gastroenterol. 2015;28:203. [PMC free article] [PubMed] [Google Scholar]
- 66.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141(2):599–609.e3. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 67.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 68.Sun L-J, Li J-N, Nie Y-Z. Gut hormones in microbiota-gut-brain cross-talk. Chin Med J (Engl). 2020;133(7):826–833. doi: 10.1097/CM9.0000000000000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Larraufie P, Martin-Gallausiaux C, Lapaque N, Dore J, Gribble FM, Reimann F, Blottiere HM. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci Rep. 2018;8(1):74. doi: 10.1038/s41598-017-18259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shah P, Nankova BB, Parab S, La Gamma EF. Short chain fatty acids induce TH gene expression via erk-dependent phosphorylation of CREB protein. Brain Res. 2006;1107(1):13–23. doi: 10.1016/j.brainres.2006.05.097. [DOI] [PubMed] [Google Scholar]
- 71.Nankova BB, Agarwal R, MacFabe DF, La Gamma EF, Tsuji Y. Enteric bacterial metabolites propionic and butyric acid modulate gene expression, including creb-dependent catecholaminergic neurotransmission, in PC12 cells - possible relevance to autism spectrum disorders. PLoS One. 2014;9(8):e103740. doi: 10.1371/journal.pone.0103740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.DeCastro M, Nankova BB, Shah P, Patel P, Mally PV, Mishra R, La Gamma EF. Short chain fatty acids regulate tyrosine hydroxylase gene expression through a cAMP-dependent signaling pathway. Mol Brain Res. 2005;142(1):28–38. doi: 10.1016/j.molbrainres.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 73.Mally P, Mishra R, Gandhi S, Decastro MH, Nankova BB, Lagamma EF. Stereospecific regulation of tyrosine hydroxylase and proenkephalin genes by short-chain fatty acids in rat PC12 cells. Pediatr Res. 2004;55(5):847–854. doi: 10.1203/01.PDR.0000119365.21770.45. [DOI] [PubMed] [Google Scholar]
- 74.Arpaia N, Campbell C, Fan X, Dikiy S, Van Der Veeken J, Deroos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luu M, Visekruna A. Short‐chain fatty acids: bacterial messengers modulating the immunometabolism of T cells. Eur J Immunol. 2019;49(6):842–848. doi: 10.1002/eji.201848009. [DOI] [PubMed] [Google Scholar]
- 76.Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ganesh BP, Nelson JW, Eskew JR, Ganesan A, Ajami NJ, Petrosino JF, Bryan RM, Durgan DJ. Prebiotics, probiotics, and acetate supplementation prevent hypertension in a model of obstructive sleep apnea. Hypertension. 2018;72(5):1141–1150. doi: 10.1161/HYPERTENSIONAHA.118.11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SEK, MacDougall K, Preston T, Tedford C, Finlayson GS, et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64(11):1744–1754. doi: 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Al-Lahham SH, Peppelenbosch MP, Roelofsen H, Vonk RJ, Venema K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim Biophys Acta Mol Cell Biol Lipids. 2010;1801(11):1175–1183. doi: 10.1016/j.bbalip.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 80.Macfabe D. Autism: metabolism, mitochondria, and the microbiome. Glob Adv Health Med. 2013;2(6):52–66. doi: 10.7453/gahmj.2013.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu W, Sun M, Chen F, Cao AT, Liu H, Zhao Y, Huang X, Xiao Y, Yao S, Zhao Q, et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 2017;10(4):946–956. doi: 10.1038/mi.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Behrouzian B, Savile CK, Dawson B, Buist PH, Shanklin J. Exploring the hydroxylation− dehydrogenation connection: novel catalytic activity of castor stearoyl-acp Δ9 desaturase. J Am Chem Soc. 2002;124(13):3277–3283. doi: 10.1021/ja012252l. [DOI] [PubMed] [Google Scholar]
- 83.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134(6):933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mozaffarian D, de Oliveira Otto MC, Lemaitre RN, Fretts AM, Hotamisligil G, Tsai MY, Siscovick DS, Nettleton JA. Trans-palmitoleic acid, other dairy fat biomarkers, and incident diabetes: the multi-ethnic study of atherosclerosis (MESA). Am J Clin Nutr. 2013;97(4):854–861. doi: 10.3945/ajcn.112.045468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yakoob MY, Shi P, Hu FB, Campos H, Rexrode KM, Orav EJ, Willett WC, Mozaffarian D. Circulating biomarkers of dairy fat and risk of incident stroke in US men and women in 2 large prospective cohorts. Am J Clin Nutr. 2014;100(6):1437–1447. doi: 10.3945/ajcn.114.083097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Çimen I, Kocatürk B, Koyuncu S, Tufanlı Ö, Onat UI, Yıldırım AD, Apaydın O, Demirsoy Ş, Aykut ZG, Nguyen UT. Prevention of atherosclerosis by bioactive palmitoleate through suppression of organelle stress and inflammasome activation. Sci Transl Med. 2016;8(358):ra358126–ra358126. doi: 10.1126/scitranslmed.aaf9087. [DOI] [PubMed] [Google Scholar]
- 88.Statovci D, Aguilera M, MacSharry J, Melgar S. The impact of western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front Immunol. 2017;8:838. doi: 10.3389/fimmu.2017.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Souza CO, Teixeira AAS, Biondo LA, Silveira LS, Calder PC, Rosa Neto JC. Palmitoleic acid reduces the inflammation in LPS -stimulated macrophages by inhibition of NF κB, independently of PPAR s. Clin Exp Pharma Physio. 2017;44(5):566–575. doi: 10.1111/1440-1681.12736. [DOI] [PubMed] [Google Scholar]
- 90.Passos MEP, Alves HHO, Momesso CM, Faria FG, Murata G, Cury-Boaventura MF, Hatanaka E, Massao-Hirabara S, Gorjão R. Differential effects of palmitoleic acid on human lymphocyte proliferation and function. Lipids Health Dis. 2016;15(1):1–11. doi: 10.1186/s12944-016-0385-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA, Ter Horst R, Jansen T, Jacobs L, Bonder MJ. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell. 2016;167(4):1125–1136.e8. doi: 10.1016/j.cell.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou B, Zhang J, Zhang Q, Permatasari F, Xu Y, Wu D, Yin Z, Luo D. Palmitic acid induces production of proinflammatory cytokines interleukin-6, interleukin-1 β , and tumor necrosis factor- α via a NF- κ B-Dependent mechanism in HaCaT keratinocytes. Mediators Inflamm. 2013;2013:1–11. doi: 10.1155/2013/530429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Korbecki J, Bajdak-Rusinek K. The effect of palmitic acid on inflammatory response in macrophages: an overview of molecular mechanisms. Inflamm Res. 2019;68(11):915–932. doi: 10.1007/s00011-019-01273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pobre KFR, Poet GJ, Hendershot LM. The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: getting by with a little help from ERdj friends. J Biol Chem. 2019;294(6):2098–2108. doi: 10.1074/jbc.REV118.002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107(7):881–891. doi: 10.1016/S0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 96.Fass D. Going for the Golgi: small PDI protein helps ATF 6 perform better under stress. Embo J. 2019;38(15):e102743. doi: 10.15252/embj.2019102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ben-Dror K, Birk R. Oleic acid ameliorates palmitic acid-induced ER stress and inflammation markers in naive and cerulein-treated exocrine pancreas cells. Biosci Rep. 2019;39(5):BSR20190054. doi: 10.1042/BSR20190054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nicholas DA, Zhang K, Hung C, Glasgow S, Aruni AW, Unternaehrer J, Payne KJ, Langridge WHR, De Leon M, Miyamoto S. Palmitic acid is a toll-like receptor 4 ligand that induces human dendritic cell secretion of IL-1β. PLOS ONE. 2017;12(5):e0176793. doi: 10.1371/journal.pone.0176793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Karin M. How nf-κB is activated: the role of the IκB kinase (IKK) complex. Oncogene. 1999;18(49):6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 100.Liu T, Zhang L, Joo D, Sun S-C. Nf-κB signaling in inflammation. Sig Transduct Target Ther. 2017;2(1):1–9. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ishihara T, Yoshida M, Arita M. Omega-3 fatty acid-derived mediators that control inflammation and tissue homeostasis. Int Immunol. 2019;31(9):559–567. doi: 10.1093/intimm/dxz001. [DOI] [PubMed] [Google Scholar]
- 102.Silva AR, Moraes BPT, Gonçalves-de-Albuquerque CF. Mediterranean diet: lipids, inflammation, and malaria infection. Int J Mol Sci. 2020;21(12):4489. doi: 10.3390/ijms21124489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dalli J, Colas RA, Serhan CN. Novel n-3 immunoresolvents: structures and actions. Sci Rep. 2013;3(1):1940. doi: 10.1038/srep01940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pistorius K, Souza PR, De Matteis R, Austin-Williams S, Primdahl KG, Vik A, Mazzacuva F, Colas RA, Marques RM, Hansen TV. Pdn-3 DPA pathway regulates human monocyte differentiation and macrophage function. Cell Chem Biol. 2018;25(6):749–760.e9. doi: 10.1016/j.chembiol.2018.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tungen JE, Aursnes M, Dalli J, Arnardottir H, Serhan CN, Hansen TV. Total synthesis of the Anti-inflammatory and pro-resolving lipid mediator MaR1 n −3 DPA utilizing an sp 3 –sp 3 Negishi cross-coupling reaction. Chem A Eur J. 2014;20(45):14575–14578. doi: 10.1002/chem.201404721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Skulas-Ray AC, Flock MR, Richter CK, Harris WS, West SG, Kris-Etherton PM. Red blood cell docosapentaenoic acid (DPA n-3) is inversely associated with triglycerides and C-reactive protein (CRP) in healthy adults and dose-dependently increases following n-3 fatty acid supplementation. Nutrients. 2015;7(8):6390–6404. doi: 10.3390/nu7085291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zheng Z, Dai Z, Cao Y, Shen Q, Zhang Y. Docosapentaenoic acid (DPA, 22: 5n-3) ameliorates inflammation in an ulcerative colitis model. Food Funct. 2019;10(7):4199–4209. doi: 10.1039/C8FO02338G. [DOI] [PubMed] [Google Scholar]
- 108.Dessì M, Noce A, Bertucci P, Manca di Villahermosa S, Zenobi R, Castagnola V, Addessi E, Di Daniele N. Atherosclerosis, dyslipidemia, and inflammation: the significant role of polyunsaturated fatty acids. Int Sch Res Not. 2013;2013:1–13. doi: 10.1155/2013/191823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Robertson RC, Oriach CS, Murphy K, Moloney GM, Cryan JF, Dinan TG, Ross RP, Stanton C. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav Immun. 2017;59:21–37. doi: 10.1016/j.bbi.2016.07.145. [DOI] [PubMed] [Google Scholar]
- 110.Menni C, Zierer J, Pallister T, Jackson MA, Long T, Mohney RP, Steves CJ, Spector TD, Valdes AM. Omega-3 fatty acids correlate with gut microbiome diversity and production of N-carbamylglutamate in middle aged and elderly women. Sci Rep. 2017;7(1):11079. doi: 10.1038/s41598-017-10382-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Watson H, Mitra S, Croden FC, Taylor M, Wood HM, Perry SL, Spencer JA, Quirke P, Toogood GJ, Lawton CL. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut. 2018;67(11):1974–1983. doi: 10.1136/gutjnl-2017-314968. [DOI] [PubMed] [Google Scholar]
- 112.Vetrani C, Maukonen J, Bozzetto L, Della Pepa G, Vitale M, Costabile G, Riccardi G, Rivellese AA, Saarela M, Annuzzi G. Diets naturally rich in polyphenols and/or long-chain n-3 polyunsaturated fatty acids differently affect microbiota composition in high-cardiometabolic-risk individuals. Acta Diabetol. 2020;57(7):853–860. doi: 10.1007/s00592-020-01494-9. [DOI] [PubMed] [Google Scholar]
- 113.Myles IA, Pincus NB, Fontecilla NM, Datta SK, Schunck W-H. Effects of parental omega-3 fatty acid intake on offspring microbiome and immunity. PLoS One. 2014;9(1):e87181. doi: 10.1371/journal.pone.0087181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee K, Paek K, Lee HY, Park JH, Lee Y. Antiobesity effect of trans -10, cis -12-conjugated linoleic acid-producing lactobacillus plantarum PL62 on diet-induced obese mice. J Appl Microbiol. 2007;103(4):1140–1146. doi: 10.1111/j.1365-2672.2007.03336.x. [DOI] [PubMed] [Google Scholar]
- 115.Marques TM, Wall R, O’Sullivan O, Fitzgerald GF, Shanahan F, Quigley EM, Cotter PD, Cryan JF, Dinan TG, Ross RP. Dietary trans-10, cis-12-conjugated linoleic acid alters fatty acid metabolism and microbiota composition in mice. Br J Nutr. 2015;113(5):728–738. doi: 10.1017/S0007114514004206. [DOI] [PubMed] [Google Scholar]
- 116.Lustri BC, Sperandio V, Moreira CG, Andrews-Polymenis HL. Bacterial chat: intestinal metabolites and signals in host-microbiota-pathogen interactions. Infect Immun. 2017;85(12):10–1128. doi: 10.1128/IAI.00476-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hidalgo M, Prieto I, Abriouel H, Cobo A, Benomar N, Gálvez A, Martínez-Cañamero M. Effect of virgin and refined olive oil consumption on gut microbiota. Comparison to butter. Food Res Int. 2014;64:553–559. doi: 10.1016/j.foodres.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 118.de Wit N, Derrien M, Bosch-Vermeulen H, Oosterink E, Keshtkar S, Duval C, de Vogel-van den Bosch J, Kleerebezem M, Müller M, van der Meer R. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am J Physiol-Gastrointestinal Liver Physiol. 2012;303(5):G589–99. doi: 10.1152/ajpgi.00488.2011. [DOI] [PubMed] [Google Scholar]
- 119.Rutting S, Zakarya R, Bozier J, Xenaki D, Horvat JC, Wood LG, Hansbro PM, Oliver BG. Dietary fatty acids amplify inflammatory responses to infection through p38 MAPK signaling. Am J Respir Cell Mol Biol. 2019;60(5):554–568. doi: 10.1165/rcmb.2018-0215OC. [DOI] [PubMed] [Google Scholar]
- 120.Ryan A, Godson C. Lipoxins: regulators of resolution. Curr Opin Pharmacol. 2010;10(2):166–172. doi: 10.1016/j.coph.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 121.Wu B, Walker J, Spur B, Rodriguez A, Yin K. Effects of Lipoxin A4 on antimicrobial actions of neutrophils in sepsis. Prostaglandins Leukot Essent Fat Acids. 2015;94:55–64. doi: 10.1016/j.plefa.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 122.Walker J, Dichter E, Lacorte G, Kerner D, Spur B, Rodriguez A, Yin K. Lipoxin a4 increases survival by decreasing systemic inflammation and bacterial load in sepsis. Shock. 2011;36(4):410–416. doi: 10.1097/SHK.0b013e31822798c1. [DOI] [PubMed] [Google Scholar]
- 123.Romano M, Cianci E, Simiele F, Recchiuti A. Lipoxins and aspirin-triggered lipoxins in resolution of inflammation. Eur J Pharmacol. 2015;760:49–63. doi: 10.1016/j.ejphar.2015.03.083. [DOI] [PubMed] [Google Scholar]
- 124.Brennan EP, Mohan M, McClelland A, De Gaetano M, Tikellis C, Marai M, Crean D, Dai A, Beuscart O, Derouiche S. Lipoxins protect against inflammation in diabetes-associated atherosclerosis. Diabetes. 2018;67(12):2657–2667. doi: 10.2337/db17-1317. [DOI] [PubMed] [Google Scholar]
- 125.Brennan EP, Cacace A, Godson C. Specialized pro-resolving mediators in renal fibrosis. Mol Aspects Med. 2017;58:102–113. doi: 10.1016/j.mam.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 126.González-Herrera F, Cramer A, Pimentel P, Castillo C, Liempi A, Kemmerling U, Machado FS, Maya JD. Simvastatin attenuates endothelial activation through 15-epi-lipoxin A4 production in murine chronic Chagas cardiomyopathy. Antimicrob Agents Chemother. 2017;61(3):10–1128. doi: 10.1128/AAC.02137-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Campos-Estrada C, Liempi A, González-Herrera F, Lapier M, Kemmerling U, Pesce B, Ferreira J, López-Muñoz R, Maya JD, Burleigh BA. Simvastatin and benznidazole-mediated prevention of Trypanosoma cruzi-induced endothelial activation: role of 15-epi-lipoxin A4 in the action of simvastatin. PloS Negl Trop Dis. 2015;9(5):e0003770. doi: 10.1371/journal.pntd.0003770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Las Heras V, Melgar S, MacSharry J, Gahan CG. The influence of the Western diet on microbiota and gastrointestinal immunity. Ann Rev Food Sci Technol. 2022;13(1):489–512. doi: 10.1146/annurev-food-052720-011032. [DOI] [PubMed] [Google Scholar]
- 129.Ahmad R, Rah B, Bastola D, Dhawan P, Singh AB. Obesity-induces organ and tissue specific tight junction restructuring and barrier deregulation by Claudin Switching. Sci Rep. 2017. 11. 7(1):5125. doi: 10.1038/s41598-017-04989-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Keewan E, Narasimhulu CA, Rohr M, Hamid S, Parthasarathy S. Are fried foods unhealthy? the dietary peroxidized fatty acid, 13-HPODE, induces intestinal inflammation in vitro and in vivo. Antioxidants. 2020;9(10):926. doi: 10.3390/antiox9100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fan R, Kim J, You M, Giraud D, Toney AM, Shin S-H, Kim S-Y, Borkowski K, Newman JW, Chung S. α-linolenic acid-enriched butter attenuated high fat diet-induced insulin resistance and inflammation by promoting bioconversion of n-3 PUFA and subsequent oxylipin formation. J Nutr Biochem. 2020;76:108285. doi: 10.1016/j.jnutbio.2019.108285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, Panzer AR, LaMere B, Rackaityte E, Lukacs NW. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22(10):1187–1191. doi: 10.1038/nm.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ohue‐Kitano R, Yasuoka Y, Goto T, Kitamura N, Park S, Kishino S, Kimura I, Kasubuchi M, Takahashi H, Li Y. α‐Linolenic acid‐derived metabolites from gut lactic acid bacteria induce differentiation of anti‐inflammatory M2 macrophages through G protein‐coupled receptor 40. FASEB J. 2018;32(1):304–318. doi: 10.1096/fj.201700273R. [DOI] [PubMed] [Google Scholar]
- 134.Valenzuela R, Metherel AH, Cisbani G, Smith ME, Chouinard-Watkins R, Klievik BJ, Videla LA, Bazinet RP. Protein concentrations and activities of fatty acid desaturase and elongase enzymes in liver, brain, testicle, and kidney from mice: substrate dependency. Biofactors. 2024;50(1):89–100. doi: 10.1002/biof.1992. [DOI] [PubMed] [Google Scholar]
- 135.Videla LA, Hernandez-Rodas MC, Metherel AH, Valenzuela R. Influence of the nutritional status and oxidative stress in the desaturation and elongation of n-3 and n-6 polyunsaturated fatty acids: impact on non-alcoholic fatty liver disease. Prostaglandins Leukot Essent Fat Acids. 2022;181:102441. doi: 10.1016/j.plefa.2022.102441. [DOI] [PubMed] [Google Scholar]
- 136.Araya J, Rodrigo R, Pettinelli P, Araya AV, Poniachik J, Videla LA. Decreased liver fatty acid Δ-6 and Δ-5 desaturase activity in obese patients. Obesity. 2010;18(7):1460–1463. doi: 10.1038/oby.2009.379. [DOI] [PubMed] [Google Scholar]
- 137.Metherel AH, Lacombe RJS, Chouinard-Watkins R, Hopperton KE, Bazinet RP. Complete assessment of whole-body n-3 and n-6 PUFA synthesis-secretion kinetics and DHA turnover in a rodent model. J Lipid Res. 2018;59(2):357–367. doi: 10.1194/jlr.M081380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gregory MK, Gibson RA, Cook-Johnson RJ, Cleland LG, James MJ, Oresic M. Elongase reactions as control points in long-chain polyunsaturated fatty acid synthesis. PLoS One. 2011;6(12):e29662. doi: 10.1371/journal.pone.0029662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Melton EM, Cerny RL, Watkins PA, DiRusso CC, Black PN. Human fatty acid transport protein 2a/very long chain acyl-CoA synthetase 1 (FATP2a/Acsvl1) has a preference in mediating the channeling of exogenous n-3 fatty acids into phosphatidyl inositol. J Biol Chem. 2011;286(35):30670–30679. doi: 10.1074/jbc.M111.226316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vandanmagsar B, Youm Y-H, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pillon NJ, Chan KL, Zhang S, Mejdani M, Jacobson MR, Ducos A, Bilan PJ, Niu W, Klip A. Saturated fatty acids activate caspase-4/5 in human monocytes, triggering IL-1β and IL-18 release. Am J Physiol-Endocrinol Metab. 2016;311(5):E825–35. doi: 10.1152/ajpendo.00296.2016. [DOI] [PubMed] [Google Scholar]
- 142.Chen T, Long W, Zhang C, Liu S, Zhao L, Hamaker BR. Fiber-utilizing capacity varies in prevotella-versus bacteroides-dominated gut microbiota. Sci Rep. 2017;7(1):2594. doi: 10.1038/s41598-017-02995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tao L, Yi Y, Chen Y, Zhang H, Orning P, Lien E, Jie J, Zhang W, Xu Q, Li Y. RIP1 kinase activity promotes steatohepatitis through mediating cell death and inflammation in macrophages. Cell Death Differ. 2021;28(4):1418–1433. doi: 10.1038/s41418-020-00668-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu W, Wang X, Berleth N, Deitersen J, Wallot-Hieke N, Böhler P, Schlütermann D, Stuhldreier F, Cox J, Schmitz K. The autophagy-initiating kinase ULK1 controls RIPK1-mediated cell death. Cell Rep. 2020;31(3):31. doi: 10.1016/j.celrep.2020.107547. [DOI] [PubMed] [Google Scholar]
- 145.Wang X, de Carvalho Ribeiro M, Iracheta‐Vellve A, Lowe P, Ambade A, Satishchandran A, Bukong T, Catalano D, Kodys K, Szabo G. Macrophage‐specific hypoxia‐inducible factor‐1α contributes to impaired autophagic flux in nonalcoholic steatohepatitis. Hepatology. 2019;69(2):545–563. doi: 10.1002/hep.30215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang Z, Zhao Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell. 2018;9(5):416–431. doi: 10.1007/s13238-018-0549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Beyaz S, Mana MD, Roper J, Kedrin D, Saadatpour A, Hong S-J, Bauer-Rowe KE, Xifaras ME, Akkad A, Arias E. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature. 2016;531(7592):53–58. doi: 10.1038/nature17173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mah AT, Van Landeghem L, Gavin HE, Magness ST, Lund PK. Impact of diet-induced obesity on intestinal stem cells: hyperproliferation but impaired intrinsic function that requires insulin/IGF1. Endocrinology. 2014;155(9):3302–3314. doi: 10.1210/en.2014-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu T-C, Kern JT, Jain U, Sonnek NM, Xiong S, Simpson KF, VanDussen KL, Winkler ES, Haritunians T, Malique A, et al. Western diet induces Paneth cell defects through microbiome alterations and farnesoid X receptor and type I interferon activation. Cell Host Microbe. 2021;29(6):988–1001.e6. doi: 10.1016/j.chom.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.An C, Kuda T, Yazaki T, Takahashi H, Kimura B. Caecal fermentation, putrefaction and microbiotas in rats fed milk casein, soy protein or fish meal. Appl Microbiol Biotechnol. 2014;98(6):2779–2787. doi: 10.1007/s00253-013-5271-5. [DOI] [PubMed] [Google Scholar]
- 151.Kunisawa J, Hashimoto E, Inoue A, Nagasawa R, Suzuki Y, Ishikawa I, Shikata S, Arita M, Aoki J, Kiyono H. Regulation of intestinal IgA responses by dietary palmitic acid and its metabolism. The J Immunol. 2014;193(4):1666–1671. doi: 10.4049/jimmunol.1302944. [DOI] [PubMed] [Google Scholar]
- 152.Liao C-Y, Song MJ, Gao Y, Mauer AS, Revzin A, Malhi H. Hepatocyte-derived lipotoxic extracellular vesicle sphingosine 1-phosphate induces macrophage chemotaxis. Front Immunol. 2018;9:2980. doi: 10.3389/fimmu.2018.02980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378(25):e34. doi: 10.1056/NEJMoa1800389. [DOI] [PubMed] [Google Scholar]
- 154.Paniagua JA, Pérez-Martinez P, Gjelstad IMF, Tierney AC, Delgado-Lista J, Defoort C, Blaak EE, Risérus ULF, Drevon CA, Kiec-Wilk B. A low-fat high-carbohydrate diet supplemented with long-chain n-3 PUFA reduces the risk of the metabolic syndrome. Atherosclerosis. 2011;218(2):443–450. doi: 10.1016/j.atherosclerosis.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 155.Tindall AM, McLimans CJ, Petersen KS, Kris-Etherton PM, Lamendella R. Walnuts and vegetable oils containing oleic acid differentially affect the gut microbiota and associations with cardiovascular risk factors: follow-up of a randomized, controlled, feeding trial in adults at risk for cardiovascular disease. J Nutr. 2020;150(4):806–817. doi: 10.1093/jn/nxz289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ghiboub M, Verburgt CM, Sovran B, Benninga MA, de Jonge WJ, Van Limbergen JE. Nutritional therapy to modulate tryptophan metabolism and aryl hydrocarbon-receptor signaling activation in human diseases. Nutrients. 2020;12(9):2846. doi: 10.3390/nu12092846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Natividad JM, Agus A, Planchais J, Lamas B, Jarry AC, Martin R, Michel M-L, Chong-Nguyen C, Roussel R, Straube M. Impaired aryl hydrocarbon receptor ligand production by the gut microbiota is a key factor in metabolic syndrome. Cell Metab. 2018;28(5):737–749.e4. doi: 10.1016/j.cmet.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 158.Wei GZ, Martin KA, Xing PY, Agrawal R, Whiley L, Wood TK, Hejndorf S, Ng YZ, Low JZY, Rossant J, et al. Tryptophan-metabolizing gut microbes regulate adult neurogenesis via the aryl hydrocarbon receptor. Proc Natl Acad Sci USA. 2021;118(27):e2021091118. doi: 10.1073/pnas.2021091118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao C-C, Patel B, Yan R, Blain M, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22(6):586–597. doi: 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Innes JK, Calder PC. Marine omega-3 (N-3) fatty acids for cardiovascular health: an update for 2020. Int J Mol Sci. 2020;21(4):1362. doi: 10.3390/ijms21041362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Guasti L, Galliazzo S, Molaro M, Visconti E, Pennella B, Gaudio GV, Lupi A, Grandi AM, Squizzato A. TMAO as a biomarker of cardiovascular events: a systematic review and meta-analysis. Intern Emerg Med. 2021;16(1):201–207. doi: 10.1007/s11739-020-02470-5. [DOI] [PubMed] [Google Scholar]
- 162.Kopf JC, Suhr MJ, Clarke J, Eyun S, Riethoven J-J, Ramer-Tait AE, Rose DJ. Role of whole grains versus fruits and vegetables in reducing subclinical inflammation and promoting gastrointestinal health in individuals affected by overweight and obesity: a randomized controlled trial. Nutr J. 2018;17(1):1–13. doi: 10.1186/s12937-018-0381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Djekic D, Shi L, Brolin H, Carlsson F, Särnqvist C, Savolainen O, Cao Y, Bäckhed F, Tremaroli V, Landberg R. Effects of a vegetarian diet on cardiometabolic risk factors, gut microbiota, and plasma metabolome in subjects with ischemic heart disease: a randomized, crossover study. J Am Heart Assoc. 2020;9(18):e016518. doi: 10.1161/JAHA.120.016518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.van Soest APM, Hermes GDA, Berendsen AAM, van de Rest O, Zoetendal EG, Fuentes S, Santoro A, Franceschi C, de Groot LCPGM, de Vos WM. Associations between pro-and anti-inflammatory gastro-intestinal microbiota, diet, and cognitive functioning in Dutch healthy older adults: the nu-age study. Nutrients. 2020;12(11):1–19. doi: 10.3390/nu12113471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kim CH. Control of innate and adaptive lymphocytes by the RAR-Retinoic acid axis. In [internet]. Immune Network. 2018;18(1):0. 10.4110/in.2018.18.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yoshii K, Hosomi K, Sawane K, Kunisawa J. Metabolism of dietary and microbial vitamin b family in the regulation of host immunity. Front Nutr. 2019;6. doi: 10.3389/fnut.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ahn-Jarvis JH, Parihar A, Doseff AI. Dietary flavonoids for immunoregulation and cancer: food design for targeting disease. Antioxidants. 2019;8(7):202. doi: 10.3390/antiox8070202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ginwala R, Bhavsar R, Chigbu DGI, Jain P, Khan ZK. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants. 2019;8(2):35. doi: 10.3390/antiox8020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang D, Frenette PS. Cross talk between neutrophils and the microbiota. Blood. 2019;133(20):2168–2177. doi: 10.1182/blood-2018-11-844555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Roberfroid M. Inulin-Type Fructans [Internet]. CRC Press; 2004. https://www.taylorfrancis.com/books/9780203504932. [Google Scholar]
- 171.Reimer RA, Willis HJ, Tunnicliffe JM, Park H, Madsen KL, Soto‐Vaca A. Inulin‐type fructans and whey protein both modulate appetite but only fructans alter gut microbiota in adults with overweight/obesity: a randomized controlled trial. Mol Nutr Food Res. 2017;61(11):61. doi: 10.1002/mnfr.201700484. [DOI] [PubMed] [Google Scholar]
- 172.Vandeputte D, Falony G, Vieira-Silva S, Wang J, Sailer M, Theis S, Verbeke K, Raes J. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut. 2017;66(11):1968–1974. doi: 10.1136/gutjnl-2016-313271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Healey G, Murphy R, Butts C, Brough L, Whelan K, Coad J. Habitual dietary fibre intake influences gut microbiota response to an inulin-type fructan prebiotic: a randomised, double-blind, placebo-controlled, cross-over, human intervention study. Br J Nutr. 2018;119(2):176–189. https://www.cambridge.org/core/product/637C3E930108F079BD8DB4049562449C. [DOI] [PubMed] [Google Scholar]
- 174.Chambers ES, Byrne CS, Morrison DJ, Murphy KG, Preston T, Tedford C, Garcia-Perez I, Fountana S, Serrano-Contreras JI, Holmes E, et al. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: a randomised cross-over trial. Gut. 2019;68(8):1430–1438. doi: 10.1136/gutjnl-2019-318424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pingitore A, Chambers ES, Hill T, Maldonado IR, Liu B, Bewick G, Morrison DJ, Preston T, Wallis GA, Tedford C, et al. The diet‐derived short chain fatty acid propionate improves beta‐cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes Obes Metab. 2017;19(2):257–265. doi: 10.1111/dom.12811. [DOI] [PubMed] [Google Scholar]


