Abstract
Galectin-3, well characterized as a glycan binding protein, has been identified as a putative RNA binding protein, possibly through participation in pre-mRNA maturation through interactions with splicosomes. Given recent developments with cell surface RNA biology, the putative dual-function nature of galectin-3 evokes a possible non-classical connection between glycobiology and RNA biology. However, with limited functional evidence of a direct RNA interaction, many molecular-level observations rely on affinity reagents and lack appropriate genetic controls. Thus, evidence of a direct interaction remains elusive. We demonstrate that antibodies raised to endogenous human galectin-3 can isolate RNA-protein crosslinks, but this activity remains insensitive to LGALS3 knock-out. Proteomic characterization of anti-galectin-3 IPs revealed enrichment of galectin-3, but high abundance of hnRNPA2B1, an abundant, well-characterized RNA-binding protein with weak homology to the N-terminal domain of galectin-3, in the isolate. Genetic ablation of HNRNPA2B1, but not LGALS3, eliminates the ability of the anti-galectin-3 antibodies to isolate RNA-protein crosslinks, implying either an indirect interaction or cross-reactivity. To address this, we introduced an epitope tag to the endogenous C-terminal locus of LGALS3. Isolation of the tagged galectin-3 failed to reveal any RNA-protein crosslinks. This result suggests that the galectin-3 does not directly interact with RNA and may be misidentified as an RNA-binding protein, at least in HeLa where the putative RNA associations were first identified. We encourage further investigation of this phenomenon employ gene deletions and, when possible, endogenous epitope tags to achieve the specificity required to evaluate potential interactions.
Keywords: GALECTINS, Galectin-3, hnRNPA2B1, RNA-binding proteins
Introduction
Prior targeted and untargeted reports have identified members of the Galectin family as possible RNA binding proteins (Wang et al. 1995; Castello et al. 2012; Huang et al. 2018; Queiroz et al. 2019). Commonly understood to function on the cellular surface and in the extracellular matrix (Kuwabara et al. 2003; Vasta 2009; Ruvolo 2016; Thiemann and Baum 2016), galectins lack signal sequences, are synthesized on cytosolic ribosomes (Cho and Cummings 1995), and secreted through unconventional secretion mechanisms (Roff and Wang 1983; Cooper and Barondes 1990; Lindstedt et al. 1993; Sato et al. 1993; Mehul and Hughes 1997; Bänfer et al. 2018; Popa et al. 2018; Zhang et al. 2020; Davuluri et al. 2021). Cytosolic galectins are understood to sense and respond to endolysosomal membrane damage (Aits et al. 2015; Jia et al. 2018; Jia, Bissa et al. 2020a; Jia, Claude-Taupin et al. 2020b). The association between galectins and RNA was postulated in the early 1990s after the discovery of weak homology between the intrinsically disordered N-terminal domain of galectin-3 and members of the hnRNP series of RNA binding proteins (RBPs) (Jia and Wang 1988). This report was followed by a series of studies identifying galectins in the nucleus (Seve et al. 1985; Moutsatsos et al. 1986; Moutsatsos et al. 1987; Wang et al. 1991: 198; Sève et al. 1993; Hubert et al. 1995; Vyakarnam et al. 1998) and suggesting a role for galectin-3 in pre-mRNA splicing (Dagher et al. 1995; Wang et al. 1995; Vyakarnam et al. 1998; Park et al. 2001; Wang et al. 2006; Gray et al. 2008; Haudek et al. 2009; Voss et al. 2012; Patterson et al. 2015; Haudek et al. 2016). Further, recent RBP screens have identified galectin-3 as a candidate RNA binding protein (Castello et al. 2012; Huang et al. 2018; Queiroz et al. 2019).
However, unlike most RNA-binding proteins, galectin-3 lacks a canonical RNA-recognition motif suggesting a non-canonical mode of interaction (LGALS3 - Homo sapiens (Human) | UniProt). The potential RNA-binding function of galectins could represent a tantalizing link between cell-surface glycobiology and nuclear RNA biology, especially in light of recently identified cell-surface glycoRNAs (Flynn et al. 2021).
To investigate the possibility of a direct interaction between galectin-3 and RNA, we pursued an UV-Crosslinking and Immunoprecipitation (irCLIP) approach leveraging zero-distance UV-crosslinking, an infrared-dye-conjugated and biotinylated ligation adaptor, and commercial anti-galectin-3 antibodies to isolate RNA-protein crosslinks (Zarnegar et al. 2016). Here, we show that while capture of endogenous galectin-3 can generate irCLIP signal, genetic ablation of LGALS3 does not eliminate this RNAse-sensitive signal. Further, we show that some commercial anti-galectin-3 antibodies cross-react with well-characterized RNA-binding proteins and deletion of one of these RNA-binding proteins, HNRNPA2B1, eliminates the observed galectin-3 RNA-association. Finally, we use CRISPR-Cas9 gene editing (Jinek et al. 2012; Lin et al. 2014; Cho et al. 2022) to insert an epitope tag, demonstrating that galectin-3 does not associate with RNA directly.
Materials and methods
Cell culture
ATCC reference HeLa (ATCC CCL-2) and HEK-293 T (ATCC CRL-1573) cells were passaged in DMEM (Gibco 11965092) with 10% FBS in the absence of antibiotics and frozen in complete growth medium +10% DMSO (Sigma). All cell lines were maintained at 37 °C and 5% CO2. Cells were routinely tested for mycoplasma using Lonza MycoAlert Plus (Lonza) and a PCR-based test (Uphoff and Drexler 2005).
CRISPR-Cas9 editing
Three non-overlapping gRNAs targeting a conserved early exon were selected using Synthego’s CRISPR Design Tool and synthesized as modified sgRNAs (Synthego). For each clone, gene knock-out was verified at the DNA level with sanger sequencing (Elim Bio, Hayward, CA) and at the protein level by immunoblot. See Supplementary Methods for additional detail.
Endogenous tag
Based on the cell engineering pipeline from OpenCell (Cho et al. 2022). See Supplementary Methods for additional detail.
irCLIP
See Supplementary Methods for details of Adaptor synthesis, UV Crosslinking, Sub-cellular fractionation., Immunoprecipitation, and Adaptor Ligation. Following ligation elution, the total volume of the sample was analyzed SDS-PAGE with an Odyssey CLx Imager (LI-COR), visualizing ligated RNA in the 800 channel. Target proteins run ~15 kDa above expected MW (RNA fragment+preA-L3-800 adaptor). See Supplementary Methods for additional details.
Mass-spectrometry
MS Data Acquisition. See Supplementary Methods. The same MS method was used for CLIP-MS, GeLC-MS/MS, and IP-MS experiments. MS Data Analysis. Peptide spectral matches were made against a target-decoy human reference proteome database downloaded from Uniprot (Elias and Gygi 2007). For quantitative comparisons, Peptides were identified with MaxQuant label-free quantitation. Relative enrichment of log2-transformed intensities was assessed on a per-protein basis with an FDR computed by a Benjamini-Hochberg adjusted t-test. Peptides were analyzed on a per-protein basis and plotted and annotated using the ggplot2 package in R (Wickham 2009).
Results
Anti-galectin-3 antibodies recognize RNA-crosslinked proteins in irCLIP
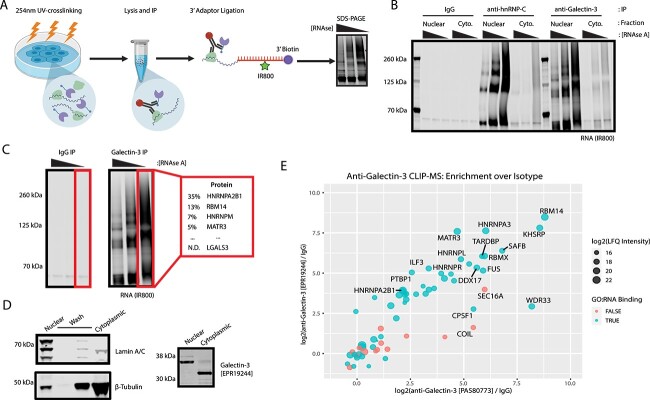
To probe for an in cellulo interaction between galectin-3 and RNA, we pursued an irCLIP approach (Fig. 1A). In this approach, the target galectin protein is isolated from UV-crosslinked cell lysate and partially digested with RNAse to enable ligation of a pre-adenylated DNA adaptor with T4 RNA ligase (Zarnegar et al. 2016). We assessed a series of anti-galectin-3 antibodies for their ability to isolate an RNA-protein crosslinks. Anti-galectin-3 antibodies isolated protein crosslinks from a variety of oncogenic cell lines (Fig. S1, Fig. S2). Intriguingly, multiple commercial anti-galectin-3 antibodies demonstrated RNA signal in irCLIP SDS-PAGE gel assays (Fig. 1B). Furthermore, sub-cellular fractionation of UV-crosslinked cells revealed that the anti-galectin-3 irCLIP signal localized to the nuclear fraction, consistent with prior reports of nuclear localization of galectin-3 (Fig. 1C) (Seve et al. 1985; Moutsatsos et al. 1986; Moutsatsos et al. 1987; Wang et al. 1991: 198; Sève et al. 1993; Hubert et al. 1995; Vyakarnam et al. 1998). However, the nuclear band recognized by anti-galectin-3 antibodies appeared ~8 kDa above the cytosolic band recognized by the same antibody, suggesting a possible post-translational modification or off-target reactivity (Fig. 1D).
Fig. 1.
A) irCLIP workflow adapted from Zarnegar et al. 2016 (Zarnegar et al. 2016). B) irCLIP in nuclear and cytosolic fraction of HeLa. Anti-hnRNPA2B1 and anti-Galectin-3 identify RNA primarily in the nuclear fraction. C) irCLIP-MS in-gel digest and proteomic identification of proteins associated with irCLIP signal. Galectin-3 is not detected in the region of the gel containing RNA signal. D) Fractionation of HeLa cells. Demonstration of separation of nuclei (Lamin A/C) from cytosol (Tubulin). Anti-Galectin-3 mAb [EPR19244] detects bands in both the cytoplasmic and nuclear fractions. E) Enrichment profiles of two anti-galectin-3 antibodies by IP-MS. Both antibodies enrich well characterized RNA-binding proteins.
Anti-galectin-3 antibodies isolate RNA-binding proteins
To evaluate the specificity of the anti-galectin-3 irCLIP, which is performed under high salt washes usually enabling stringent isolation of target proteins, we performed in-gel digest of proteins associated with RNA signal. Surprisingly, using mass spectrometry, we did not detect any galectin-3 peptides overlapping with the RNA-associated signal (Fig. 1C). Rather, the most abundant proteins associated with the RNA signal were nuclear splicing factors, including the highly abundant hnRNPA2B1 (Fig. 1E, Fig. S3). hnRNPA2B1 has been observed as a putative interactor of galectin-3, in another anti-galectin-3 co-IP experiment (Fritsch et al. 2016). IP-MS with anti-galectin-3 in cell fractions confirms enrichment of galectin-3 in addition to splicing factors, including hnRNPA2B1 (Fig. S4). While there is little structural homology between hnRNPA2B1 and galectin-3, both proteins possess an intrinsically disordered domain rich in prolines and tyrosines, with semi-regular spacing (Fig. S5) (Lin et al. 2017; Martin et al. 2020). Additionally, as tyrosine commonly forms crosslinks with nucleobases following UV-irradiation, their abundance should improve crosslinking efficiency if galectin-3 binds RNA directly (Kunkel et al. 1981; Stützer et al. 2020). Therefore, the proposed interaction is either indirect in nature or a result of off-target binding of classical RNA-binding proteins hnRNPA2B1 by the anti-galectin-3 antibodies. Specificity issues of commercial affinity reagents have been well documented with estimates suggesting ~50% of commercial antibodies recognize the wrong target (Berglund et al. 2008; Bradbury and Plückthun 2015).
Loss of hnRNPA2B1, but not galectin-3, depletes irCLIP signal
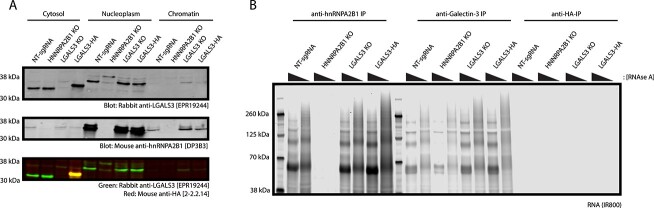
To control for off-target binding of the commercial affinity reagents, we used a multi-guide RNP-based CRISPR-Cas9 editing approach to knock-out expression of galectin-3 and hnRNPA2B1 from HeLa cells. Following CRISPR-KO and clonal selection by limiting dilution, we verified the loss of galectin-3 and hnRNPA2B1 expression by immunoblot (Fig. S6). In the LGALS3 KO background, anti-galectin-3 irCLIP retains the associated RNA signal. However, anti-galectin-3 irCLIP in the HNRNPA2B1 KO background does not identify the associated RNA signal, suggesting that galectin-3 does not bind RNA directly and may not associate with RNA at all (Fig. 2B).
Fig. 2.
A) Sub-cellular fractionation of HeLa following CRISPR KO or endogenous tagging of LGALS3. Loss of HNRNPA2B1 eliminates the nuclear band detected by the anti-galectin-3 mAb [EPR19244]. Loss of LGALS3 does not impact the nuclear band detected by anti-galectin-3 mAb [EPR19244]. B) irCLIP of HeLa following CRISPR KO and endogenous tag. RNA signal is preserved in the anti-galectin-3 irCLIP in the LGALS3 KO background, but lost in the HNRNPA2B1 KO background. Anti-HA irCLIP fails to produce any RNA signal in the endogenously tagged LGALS3-HA background.
irCLIP of endogenously tagged galectin-3 does not isolate RNA-protein crosslinks
To test this directly, we introduced an HA-tag to the endogenous LGALS3 locus at its C-terminal end. In characterizing RNA-binding proteins, endogenous tags are preferred to maintain the endogenous expression levels and preserve native RNA-binding patterns as many of these low-affinity high-valency RNA-protein interactions are highly sensitive to context and concentration (Ule et al. 2018; Alberti et al. 2019; Hafner et al. 2021). Further, this tag lets us test if the postulated galectin-3-RNA interaction is direct or an artifact of antibody cross-reactivity. Using CRISPR-Cas9 mediated HDR-directed editing, we inserted an HA epitope tag to the C-terminal region of LGALS3, without disrupting the 3’UTR (Lin et al. 2014; Feng et al. 2017; Cho et al. 2022). HA-tagged galectin-3 retains glycan binding activity (Fig. S10) and nucleocytosolic localization via IF (Fig. S11). Rather conclusively, the anti-HA IP isolates HA-tagged galectin-3, but an anti-HA irCLIP in the HA-tagged galectin-3 background identified no irCLIP signal above the non-tagged control (Fig. 2B). In addition, an orthogonal physical-chemical method for isolating RNA-protein crosslinks (Fig. S7) also failed to identify a direct interaction between galectin-3 and RNA (Figs S8, S9) (Queiroz et al. 2019; Villanueva et al. 2020). Therefore, the association of galectin-3 with RNA is likely not direct and should be reevaluated in light of observed mAb reactivity.
Some anti-galecitn-3 mAbs enrich known RPBs via IP-MS in LGALS3 KO HeLa
To assess the specificity of selected galectin-3 mAbs, rat anti-LGALS3 [Mac2] and mouse anti-LGALS3 [A3A12], previously used to study galectin-3 (Liu et al. 1996; Vyakarnam et al. 1998; Gray et al. 2008). We assayed the specificity of these mAbs, and the rabbit anti-LGALS3 [EPR19422], by IP-MS to characterize their enrichment in the presence and absence of galectin-3. IP-MS of the selected mAbs revealed enrichment of known, well-characterized RBPs in both the NT-sgRNA and LGALS3 KO backgrounds (Figs S12–S15).
Discussion
The context-dependent nature of many RNA-protein interactions makes proving non-interaction a Sisyphean task (Castello et al. 2012; Kramer et al. 2014; Hentze et al. 2018; Trendel et al. 2019; Backlund et al. 2020; Huppertz et al. 2022; Perez-Perri et al. 2023). Validation of candidate RBPs is essential. Galectin-3 in appeared in multiple RBP screens, co-isolates with RNA-protein crosslinks, may participate pre-mRNA maturation, yet fails to bind RNA directly when assessed under stringent conditions.
This study does not, and cannot, rule out the possibility of a non-classical RNA binding function for other galectins in other contexts. Investigators should proceed with caution and include positive (canonical RBPs) and negative (genetic deletions) controls for future in cellulo exploration of putative galectin-RNA interactions. As many RBPs not only self-associate, but also associate with other RBPs, attribution of RNA interactions to a target RBP requires comparison to an RBP-depleted sample (e.g. genetic deletion) or demonstration of the exclusion of other RBPs (e.g. proteomics).
While this investigation used high stringency methods to probe for direct RNA-protein interactions, indirect but functional interactions may exist. Reports proposing indirect interactions use non-zero distance formaldehyde crosslinking to capture these interactions (Coppin et al. 2017). However, recent investigations of the RNA-binding properties of galectins discover phenotypic evidence suggesting RNA binding and thus infer an RNA-protein interaction, but often lack direct evidence in vitro or in cellulo (Coppin et al. 2017; Wei et al. 2021). At minimum, RNA interaction claims would require comparison to enrichment in a knock-out background, proteomic characterization of enrichments, or an epitope tag enabling antibody specificity would be necessary for causal interpretation of the contribution of a galectin to RNA binding. Without controlling for the specificity of the enrichment, it remains possible that the RNA fragments identified in an RNA-IP are not sensitive to genetic deletion, as was the case with anti-galectin-3 in HeLa. Further, high-sequencing depth (sensitivity) in workflows with high variability and few replicates, especially in non-blinded experiments as exploratory RNA-interaction experiments often are, can create non-meaningful yet statistically-significant differences through overpowered hypothesis testing of small variations in transcripts across samples. We recommend carefully assessing effect sizes and employing nonparametric statistical testing, such as bootstrapping, to control for stochastic and batch variability in untargeted, highly-powered RNA-IP sequencing studies (Kulkarni et al. 2022).
Supplementary Material
Acknowledgments
We thank Profs. Joanna Wysocka, Jonathan Z. Long, and Nicole Martinez for their input to and feedback on this project.
Contributor Information
Egan L Peltan, Department of Chemical and Systems Biology, Stanford University School of Medicine, 269 Campus Drive CCSR 4145 Stanford, CA 94305, United States; Sarafan ChEM-H, Stanford University, Stanford ChEM-H Building 290 Jane Stanford Way Stanford, CA 94305, United States.
Nicholas M Riley, Sarafan ChEM-H, Stanford University, Stanford ChEM-H Building 290 Jane Stanford Way Stanford, CA 94305, United States; Department of Chemistry, Stanford University, 333 Campus Drive Stanford, CA 94305, United States.
Ryan A Flynn, Stem Cell Program and Division of Hematology/Oncology, Boston Children’s Hospital, 1 Blackfan Circle, Boston, MA 02445, United States; Department of Stem Cell and Regenerative Biology, Harvard University, 7 Divinity Ave, Cambridge, MA 02138, United States.
David S Roberts, Sarafan ChEM-H, Stanford University, Stanford ChEM-H Building 290 Jane Stanford Way Stanford, CA 94305, United States; Department of Chemistry, Stanford University, 333 Campus Drive Stanford, CA 94305, United States.
Carolyn R Bertozzi, Sarafan ChEM-H, Stanford University, Stanford ChEM-H Building 290 Jane Stanford Way Stanford, CA 94305, United States; Department of Chemistry, Stanford University, 333 Campus Drive Stanford, CA 94305, United States; Howard Hughes Medical Institute, Stanford University, 279 Campus Drive Room B202 Stanford, CA 94305-5323, United States.
Author contributions
E.L.P., experimental design, data collection and analysis, manuscript preparation and editing; N.M.R., mass spectrometry experimental design, data acquisition and processing; D.S.R mass spectrometry experimental design, data acquisition and processing; R.A.F., experimental design, manuscript editing, and irCLIP adaptor synthesis; C.R.B., experimental design, manuscript editing, and funding.
CRediT author statement
Egan Peltan (Conceptualization [lead], Data curation-Lead, Formal analysis [lead], Funding acquisition [supporting], Investigation [lead], Methodology [equal], Project administration [equal], Supervision [equal], Validation [lead], Visualization [lead], Writing—original draft [lead], Writing—review & editing [equal]), Nicholas M. Riley (Data curation [supporting], Funding acquisition [supporting], Investigation [supporting], Methodology [supporting], Software [equal], Writing—review & editing [equal]), Ryan A. Flynn (Methodology [supporting], Resources [supporting], Supervision [supporting], Writing—review & editing [supporting]), David Roberts (Data curation [supporting], Investigation [supporting], Writing—review & editing [supporting]), Carolyn Bertozzi (Conceptualization [supporting], Funding acquisition [lead], Project administration[supporting], Writing—review & editing [supporting])
Funding
This work was supported by National Institutes of Health Grant GM058867 (C.R.B). and the Howard Hughes Medical Institute. N.M.R was supported by the National Institutes of Health under award K99GM147304. E.L.P was National Science Foundation Graduate Research Fellowship, a Stanford Graduate Fellowship, and the Stanford ChEM-H Chemistry/Biology Interface Predoctoral Training Program.
Conflict of interest statement: None declared.
Declaration of interests
C.R.B. is a co-founder and Scientific Advisory Board member of Redwood Bioscience (a subsidiary of Catalent), Enable Biosciences, OliLux Bio, Palleon Pharmaceuticals, InterVenn Bio, and Lycia Therapeutics.
Data availability
All relevant data and the raw mass spectrometry protein identifications are included in the online version of this article.
References
- Aits S, Kricker J, Liu B, Ellegaard A-M, Hämälistö S, Tvingsholm S, Corcelle-Termeau E, Høgh S, Farkas T, Holm Jonassen A, et al. Sensitive detection of lysosomal membrane permeabilization by lysosomal galectin puncta assay. Autophagy. 2015:11(8):1408–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti S, Gladfelter A, Mittag T. Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell. 2019:176(3):419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backlund M, Stein F, Rettel M, Schwarzl T, Perez-Perri JI, Brosig A, Zhou Y, Neu-Yilik G, Hentze MW, Kulozik AE. Plasticity of nuclear and cytoplasmic stress responses of RNA-binding proteins. Nucleic Acids Res. 2020:48(9):4725–4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bänfer S, Schneider D, Dewes J, Strauss MT, Freibert S-A, Heimerl T, Maier UG, Elsässer H-P, Jungmann R, Jacob R. Molecular mechanism to recruit galectin-3 into multivesicular bodies for polarized exosomal secretion. Proc Natl Acad Sci. 2018:115(19):E4396–E4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund L, Björling E, Oksvold P, Fagerberg L, Asplund A, al-Khalili Szigyarto C, Persson A, Ottosson J, Wernérus H, Nilsson P, et al. A Genecentric human protein atlas for expression profiles based on antibodies. Mol Cell Proteomics. 2008:7(10):2019–2027. [DOI] [PubMed] [Google Scholar]
- Bradbury A, Plückthun A. Reproducibility: standardize antibodies used in research. Nature. 2015:518(7537):27–29. [DOI] [PubMed] [Google Scholar]
- Castello A, Fischer B, Eichelbaum K, Horos R, Beckmann BM, Strein C, Davey NE, Humphreys DT, Preiss T, Steinmetz LM, et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell. 2012:149(6):1393–1406. [DOI] [PubMed] [Google Scholar]
- Cho M, Cummings RD. Galectin-1, a β-Galactoside-binding lectin in Chinese hamster ovary cells: II. Localization and biosynthesis (*). J Biol Chem. 1995:270(10):5207–5212. [DOI] [PubMed] [Google Scholar]
- Cho NH, Cheveralls KC, Brunner A-D, Kim K, Michaelis AC, Raghavan P, Kobayashi H, Savy L, Li JY, Canaj H, et al. OpenCell: endogenous tagging for the cartography of human cellular organization. Science. 2022:375(6585):eabi6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DN, Barondes SH. Evidence for export of a muscle lectin from cytosol to extracellular matrix and for a novel secretory mechanism. J Cell Biol. 1990:110(5):1681–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppin L, Vincent A, Frénois F, Duchêne B, Lahdaoui F, Stechly L, Renaud F, Villenet C, van Seuningen I, Leteurtre E, et al. Galectin-3 is a non-classic RNA binding protein that stabilizes the mucin MUC4 mRNA in the cytoplasm of cancer cells. Sci Rep. 2017:7(1):43927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher SF, Wang JL, Patterson RJ. Identification of galectin-3 as a factor in pre-mRNA splicing. Proc Natl Acad Sci. 1995:92(4):1213–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davuluri GVN, Chen C-C, Chiu Y-C, Tsai H-W, Chiu H-C, Chen Y-L, Tsai P-J, Kuo W-T, Tsao N, Lin Y-S, et al. Autophagy drives Galectin-1 secretion from tumor-associated macrophages facilitating hepatocellular carcinoma progression. Front Cell Dev Biol. 2021:9. [accessed 2023 Aug 28]. 10.3389/fcell.2021.741820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias JE, Gygi SP. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods. 2007:4(3):207–214. [DOI] [PubMed] [Google Scholar]
- Feng S, Sekine S, Pessino V, Li H, Leonetti MD, Huang B. Improved split fluorescent proteins for endogenous protein labeling. Nat Commun. 2017:8(1):370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn RA, Pedram K, Malaker SA, Batista PJ, Smith BAH, Johnson AG, George BM, Majzoub K, Villalta PW, Carette JE, et al. Small RNAs are modified with N-glycans and displayed on the surface of living cells. Cell. 2021:184(12):3109–3124.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch K, Mernberger M, Nist A, Stiewe T, Brehm A, Jacob R. Galectin-3 interacts with components of the nuclear ribonucleoprotein complex. BMC Cancer. 2016:16(1):502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RM, Davis MJ, Ruby KM, Voss PG, Patterson RJ, Wang JL. Distinct effects on splicing of two monoclonal antibodies directed against the amino-terminal domain of galectin-3. Arch iochem Biophys. 2008:475(2):100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner M, Katsantoni M, Köster T, Marks J, Mukherjee J, Staiger D, Ule J, Zavolan M. CLIP and complementary methods. Nat Rev Methods Primer. 2021:1(1):1–23. [Google Scholar]
- Haudek KC, Voss PG, Locascio LE, Wang JL, Patterson RJ. A mechanism for incorporation of Galectin-3 into the spliceosome through its association with U1 snRNP. Biochemistry. 2009:48(32):7705–7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haudek KC, Voss PG, Wang JL, Patterson RJ. A 10S galectin-3–U1 snRNP complex assembles into active spliceosomes. Nucleic Acids Res. 2016:44(13):6391–6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze MW, Castello A, Schwarzl T, Preiss T. A brave new world of RNA-binding proteins. Nat Rev Mol Cell Biol. 2018:19(5):327–341. [DOI] [PubMed] [Google Scholar]
- Huang R, Han M, Meng L, Chen X. Transcriptome-wide discovery of coding and noncoding RNA-binding proteins. Proc Natl Acad Sci. 2018:115(17):E3879–E3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert M, Wang S-Y, Wang JL, Sève A-P, Hubert J. Intranuclear distribution of Galectin-3 in mouse 3T3 fibroblasts: comparative analyses by immunofluorescence and Immunoelectron microscopy. Exp Cell Res. 1995:220(2):397–406. [DOI] [PubMed] [Google Scholar]
- Huppertz I, Perez-Perri JI, Mantas P, Sekaran T, Schwarzl T, Russo F, Ferring-Appel D, Koskova Z, Dimitrova-Paternoga L, Kafkia E, et al. Riboregulation of enolase 1 activity controls glycolysis and embryonic stem cell differentiation. Mol Cell. 2022:82(14):2666–2680.e11. [DOI] [PubMed] [Google Scholar]
- Jia S, Wang JL. Carbohydrate binding protein 35. Complementary DNA sequence reveals homology with proteins of the heterogeneous nuclear RNP. J Biol Chem. 1988:263(13):6009–6011. [PubMed] [Google Scholar]
- Jia J, Abudu YP, Claude-Taupin A, Gu Y, Kumar S, Choi SW, Peters R, Mudd MH, Allers L, Salemi M, et al. Galectins control mTOR in response to endomembrane damage. Mol Cell. 2018:70(1):120–135.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Bissa B, Brecht L, Allers L, Choi SW, Gu Y, Zbinden M, Burge MR, Timmins G, Hallows K, et al. AMPK, a regulator of metabolism and autophagy, is activated by lysosomal damage via a novel galectin-directed ubiquitin signal transduction system. Mol Cell. 2020a:77(5):951–969.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Claude-Taupin A, Gu Y, Choi SW, Peters R, Bissa B, Mudd MH, Allers L, Pallikkuth S, Lidke KA, et al. Galectin-3 coordinates a cellular system for lysosomal repair and removal. Dev Cell. 2020b:52(1):69–87.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science. 2012:337(6096):816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer K, Sachsenberg T, Beckmann BM, Qamar S, Boon K-L, Hentze MW, Kohlbacher O, Urlaub H. Photo-cross-linking and high-resolution mass spectrometry for assignment of RNA-binding sites in RNA-binding proteins. Nat Methods. 2014:11(10):1064–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni RU, Wang CL, Bertozzi CR. Analyzing nested experimental designs—a user-friendly resampling method to determine experimental significance. PLoS Comput Biol. 2022:18(5):e1010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel GR, Mehrabian M, Martinson HG. Contact-site cross-linking agents. Mol Cell Biochem. 1981:34(1):3–13. [DOI] [PubMed] [Google Scholar]
- Kuwabara I, Sano H, Liu F-T. Functions of galectins in cell adhesion and chemotaxis. In: Lee YC, Lee RT editors, Methods in enzymology. Vol. 363. Academic Press; 2003. (Recognition of Carbohydrates in Biological Systems, Part B: Specific Applications). p. 1–625. [accessed 22 February 2023]. https://www.sciencedirect.com/science/article/pii/S0076687903010784. [DOI] [PubMed] [Google Scholar]
- LGALS3 - Galectin-3 - Homo sapiens (Human) | UniProtKB | UniProt . [accessed 22 February 2023]. https://www.uniprot.org/uniprotkb/P17931/entry.
- Lin S, Staahl BT, Alla RK, Doudna JA. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. elife. 2014:3:e04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Currie SL, Rosen MK. Intrinsically disordered sequences enable modulation of protein phase separation through distributed tyrosine motifs. J Biol Chem. 2017:292(46):19110–19120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstedt R, Apodaca G, Barondes SH, Mostov KE, Leffler H. Apical secretion of a cytosolic protein by Madin-Darby canine kidney cells. Evidence for polarized release of an endogenous lectin by a nonclassical secretory pathway. J Biol Chem. 1993:268(16):11750–11757. [PubMed] [Google Scholar]
- Liu F-T, Hsu DK, Zuberi RI, Hill PN, Shenhav A, Kuwabara I, Chen S-S. Modulation of functional properties of Galectin-3 by monoclonal antibodies binding to the non-lectin domains. Biochemistry. 1996:35(19):6073–6079. [DOI] [PubMed] [Google Scholar]
- Martin EW, Holehouse AS, Peran I, Farag M, Incicco JJ, Bremer A, Grace CR, Soranno A, Pappu RV, Mittag T. Valence and patterning of aromatic residues determine the phase behavior of prion-like domains. Science. 2020:367(6478):694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehul B, Hughes RC. Plasma membrane targetting, vesicular budding and release of galectin 3 from the cytoplasm of mammalian cells during secretion. J Cell Sci. 1997:110(10):1169–1178. [DOI] [PubMed] [Google Scholar]
- Moutsatsos IK, Davis JM, Wang JL. Endogenous lectins from cultured cells: subcellular localization of carbohydrate-binding protein 35 in 3T3 fibroblasts. J Cell Biol. 1986:102(2):477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsatsos IK, Wade M, Schindler M, Wang JL. Endogenous lectins from cultured cells: nuclear localization of carbohydrate-binding protein 35 in proliferating 3T3 fibroblasts. Proc Natl Acad Sci. 1987:84(18):6452–6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Voss PG, Grabski S, Wang JL, Patterson RJ. Association of galectin-1 and galectin-3 with Gemin4 in complexes containing the SMN protein. Nucleic Acids Res. 2001:29(17):3595–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson RJ, Haudek KC, Voss PG, Wang JL. Examination of the role of galectins in pre-mRNA splicing. In: Stowell SR, Cummings RD, editors. Galectins: methods and protocols. New York, NY: Springer; 2015. (Methods in Molecular Biology). p. 431–449. [accessed 2023 Feb 22]. 10.1007/978-1-4939-1396-1_28. [DOI] [PubMed] [Google Scholar]
- Perez-Perri JI, Ferring-Appel D, Huppertz I, Schwarzl T, Sahadevan S, Stein F, Rettel M, Galy B, Hentze MW. The RNA-binding protein landscapes differ between mammalian organs and cultured cells. Nature Communications. 2023:14(1):2074. 10.1038/s41467-023-37494-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa SJ, Stewart SE, Moreau K. Unconventional secretion of annexins and galectins. Semin Cell Dev Biol. 2018:83:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiroz RML, Smith T, Villanueva E, Marti-Solano M, Monti M, Pizzinga M, Mirea D-M, Ramakrishna M, Harvey RF, Dezi V, et al. Comprehensive identification of RNA–protein interactions in any organism using orthogonal organic phase separation (OOPS). Nat Biotechnol. 2019:37(2):169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff CF, Wang JL. Endogenous lectins from cultured cells. Isolation and characterization of carbohydrate-binding proteins from 3T3 fibroblasts. J Biol Chem. 1983:258(17):10657–10663. [PubMed] [Google Scholar]
- Ruvolo PP. Galectin 3 as a guardian of the tumor microenvironment. Biochim Biophys Acta. 2016:1863(3):427–437. [DOI] [PubMed] [Google Scholar]
- Sato S, Burdett I, Hughes RC. Secretion of the baby hamster kidney 30-kDa galactose-binding lectin from polarized and nonpolarized cells: a pathway independent of the endoplasmic reticulum-golgi complex. Exp Cell Res. 1993:207(1):8–18. [DOI] [PubMed] [Google Scholar]
- Seve AP, Hubert J, Bouvier D, Bouteille M, Maintier C, Monsigny M. Detection of sugar-binding proteins in membrane-depleted nuclei. Exp Cell Res. 1985:157(2):533–538. [DOI] [PubMed] [Google Scholar]
- Sève A-P, Felin M, Doyennette-Moyne M-A, Sahraoui T, Aubery M, Hubert J. Evidence for a lactose-mediated association between two nuclear carbohydrate-binding proteins. Glycobiology. 1993:3(1):23–30. [DOI] [PubMed] [Google Scholar]
- Stützer A, Welp LM, Raabe M, Sachsenberg T, Kappert C, Wulf A, Lau AM, David S-S, Chernev A, Kramer K, et al. Analysis of protein-DNA interactions in chromatin by UV induced cross-linking and mass spectrometry. Nat Commun. 2020:11(1):5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemann S, Baum LG. Galectins and immune responses—just how do they do those things they do? Annu Rev Immunol. 2016:34(1):243–264. [DOI] [PubMed] [Google Scholar]
- Trendel J, Schwarzl T, Horos R, Prakash A, Bateman A, Hentze MW, Krijgsveld J. The human RNA-binding proteome and its dynamics during translational arrest. Cell. 2019:176(1-2):391–403.e19. [DOI] [PubMed] [Google Scholar]
- Ule J, Hwang H-W, Darnell RB. The future of cross-linking and immunoprecipitation (CLIP). Cold Spring Harb Perspect Biol. 2018:10(8):a032243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uphoff CC, Drexler HG.. Detection of mycoplasma contaminations. In: Helgason CD, Miller CL, editors. Basic cell culture protocols. Totowa, NJ: Humana Press; 2005. (Methods in Molecular Biology™). p. 13–23. [accessed 2023 Apr 8]. 10.1385/1-59259-838-2:013. [DOI] [Google Scholar]
- Vasta GR. Roles of galectins in infection. Nat Rev Microbiol. 2009:7(6):424–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva E, Smith T, Queiroz RML, Monti M, Pizzinga M, Elzek M, Dezi V, Harvey RF, Ramakrishna M, Willis AE, et al. Efficient recovery of the RNA-bound proteome and protein-bound transcriptome using phase separation (OOPS). Nat Protoc. 2020:15(8):2568–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss PG, Haudek KC, Patterson RJ, Wang JL. Inhibition of cell-free splicing by saccharides that bind galectins and SR proteins. J Carbohydr Chem. 2012:31(4–6):519–534. [Google Scholar]
- Vyakarnam A, Lenneman AJ, Lakkides KM, Patterson RJ, Wang JL. A comparative nuclear localization study of Galectin-1 with other splicing components. Exp Cell Res. 1998:242(2):419–428. [DOI] [PubMed] [Google Scholar]
- Wang JL, Laing JG, Anderson RL. Lectins in the cell nucleus. Glycobiology. 1991:1(3):243–252. [DOI] [PubMed] [Google Scholar]
- Wang L, Inohara H, Pienta KJ, Raz A. Galectin-3 is a nuclear matrix protein which binds RNA. Biochem Biophys Res Commun. 1995:217(1):292–303. [DOI] [PubMed] [Google Scholar]
- Wang W, Park JW, Wang JL, Patterson RJ. Immunoprecipitation of spliceosomal RNAs by antisera to galectin-1 and galectin-3. Nucleic Acids Res. 2006:34(18):5166–5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Li DK, Hu X, Cheng C, Zhang Y. Galectin-1–RNA interaction map reveals potential regulatory roles in angiogenesis. FEBS Lett. 2021:595(5):623–636. [DOI] [PubMed] [Google Scholar]
- Wickham H. ggplot2: elegant graphics for data analysis. New York, NY: Springer New York; 2009. [accessed 2023 Apr 14]. 10.1007/978-0-387-98141-3. [DOI] [Google Scholar]
- Zarnegar BJ, Flynn RA, Shen Y, Do BT, Chang HY, Khavari PA. irCLIP platform for efficient characterization of protein–RNA interactions. Nat Methods. 2016:13(6):489–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Liu L, Lin X, Wang Y, Li Y, Guo Q, Li S, Sun Y, Tao X, Zhang D, et al. A translocation pathway for vesicle-mediated unconventional protein secretion. Cell. 2020:181(3):637–652.e15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data and the raw mass spectrometry protein identifications are included in the online version of this article.