Summary
Background
Epidemiologic studies have reported an association between diesel exhaust particle (DEP) exposure, allergic sensitization, and childhood wheezing, although the mechanisms remain unclear. While DEP is known to augment allergic responses in adult animal models, its effects on sensitization and asthma severity in young animals is unknown.
Objective
To examine the impact of different doses of DEP and allergen co-exposure on allergic sensitization and asthma characteristics in young mice, and whether Th17 as well as Th2 responses are induced.
Methods
Lungs of 3-week-old wild-type Balb/c mice were exposed by pharyngeal aspiration nine times over 3 weeks to DEP at 1.2 or 6.0 mg/kg body weight, house dust mite (HDM) at 0.8, 1.2 or 6.0 mg/kg of DEP in combination with HDM, or the same volume (50 μL) of 0.9% sterile saline.
Results
In young mice, exposure to 1.2 mg/kg of DEP caused no detectable lung inflammation, but 6.0 mg/kg of DEP induced neutrophilic influx. Compared to HDM or DEP alone, mice exposed to either dose of DEP together with HDM demonstrated increased allergen-specific IgE, lung inflammation, airway hyperreactivity, goblet cell metaplasia, Th2/Th17 cytokines, dendritic cells, activated T cells, effector T cells, and IL-17pos and IL-13pos/IL-17Apos T effector cells.
Conclusions and Clinical Relevance
In young mice, co-exposure to DEP and HDM together exacerbated allergic sensitization and induced key characteristics of more severe asthma, including IL-17A, IL-17pos and IL-13pos/IL-17Apos T effector cells. While exposure to 1.2 mg/kg DEP alone caused no detectable changes, it did exacerbate allergic sensitization and asthma characteristics to a similar degree as a five-fold higher dose of DEP. This study demonstrates that exposure to DEP, even at a dose that alone causes no inflammation, exacerbates allergic asthma in young animals and suggests the importance of preventive measures to reduce the exposure of children to traffic related air pollution.
Keywords: asthma, diesel exhaust particle, air pollution, allergen, house dust mite, atopy, dendritic cells, Th2, Th17, T cells
Introduction
Asthma is a chronic inflammatory disease of the lungs characterized by restricted airflow, inflammatory cell influx, and increased mucus production. Allergic asthma is classically thought to involve CD4pos T helper 2 (Th2) cells and their associated cytokines interleukin (IL)-4, −5, and −13 [1]. Different subtypes of asthma are being defined [2–4], and new cellular and molecular mediators such as Th17 cells and IL-17A have been associated with more severe asthma phenotypes [5–7]. In addition, studies in animals have shown that Th17 cells can mediate steroid-resistant airway inflammation and airway hyper-reactivity (AHR) [8]. Despite increasing insight into the pathogenesis of the disease, the incidence and prevalence of asthma continue to rise both in the United States and globally. The prevalence of paediatric asthma is especially concerning as it is now the most common chronic disease in children [9].
While the aetiology of asthma in children is poorly understood, there is evidence that many factors play a role, including genetic predisposition and environmental exposures to viruses, smoke, allergens, and traffic-related air pollution (TRAP) [10, 11]. The major portion of particulate matter in TRAP is comprised of diesel exhaust particles (DEP). The Cincinnati Childhood Allergy and Air Pollutions Study (CCAAPS), a longitudinal prospective study, examined DEP exposure of children and assessed the effects on allergic sensitization and risk of asthma [12–15]. Data from CCAAPS demonstrated an increased prevalence for wheezing in children exposed to high levels of DEP [16, 17]. Other epidemiologic studies have also demonstrated an association between exposure to TRAP and an increased risk of allergic sensitization as well as the development of asthma and asthma symptoms in children [18, 19]. Thus, CCAAPS as well as other studies have highlighted the importance of examining early exposures to DEP and its impact on the risk of developing allergic asthma later in life.
Exposure to DEP, even short term, has been linked to adverse effects on lung function. In adult asthmatics, DEP exposure decreased lung function and increased neutrophilic biomarkers [20]. In healthy adults, DEP exposure also increased neutrophils in addition to mast cells, lymphocytes, and inflammatory cytokines [21, 22]. DEP can also synergize with allergens to augment immune responses as seen in atopic adults where a single co-exposure to DEP and ragweed increased allergen-specific IgE (1–4 days later) and Th2 cytokines (1 day later) [23]. Studies in adult animals demonstrate similar results in that exposure to DEP induced inflammatory responses [24–28] and that co-exposure with allergen increased key characteristics of allergic sensitization and asthma [29–34]. While it is clear that DEP enhances allergic responses in adult humans and animals, the effects of DEP exposure in young animals and children are poorly understood.
The aim of this study was to elucidate the impact of DEP exposure on allergic asthma in young mice. It is important to determine the cellular and molecular responses in young animals as the immune system and lungs undergo extensive developmental and functional changes with adaptation to the post-natal environment and so the effects of DEP exposure in young animals may not be identical to responses in adults [35, 36]. In epidemiologic studies such as CCAAPS, children are usually exposed to some level of DEP and allergens; therefore, the animal model utilized here allowed us to determine the effects of DEP alone and in combination with an allergen, house dust mite (HDM), compared with unexposed controls and controls only exposed to HDM. To the best of our knowledge, this study is the first to examine the effects of defined doses of DEP and allergen exposure in young animals, including features of severe asthma such as IL-17A and double-positive Th2/Th17 effector T cells. This is important because the cellular and molecular responses resulting from exposure to DEP in young animals, especially AHR, IgE production, goblet cell metaplasia, and T-cell populations, are unknown.
Methods and materials
Animals and exposures
3-week-old wild-type Balb/c mice (Charles River Laboratories) were exposed 9 times over 3 weeks to diesel exhaust particles (DEP) at 6.0 mg/kg or 1.2 mg/kg body weight, house dust mite (HDM) at 0.8 mg/kg, 6.0 mk/kg or 1.2 mg/kg of DEP in combination with HDM, or the same volume (50 μl) of 0.9% sterile saline (Fig. S1). Doses of DEP and HDM were adjusted weekly to the average body weight of the treatment group. As the children in CCAAPS had a fivefold difference between the highest and lowest DEP exposure levels, the doses of DEP for the animal study were chosen to represent a similar range. The dose of HDM used in this study was low, so that further increases in asthma and allergic responses with DEP exposure could be detected. For the exposures, mice were administered temporary anaesthesia (Isoflurane, Butler Schein Animal Health), then DEP and/or HDM were pipetted into the pharynx, and the tongue held and nostrils covered to induce aspiration into the lungs. DEP was obtained from a 4-cylinder Deutz BF4M1008 diesel engine (C-DEP) as previously described [37], resuspended in sterile saline, and sonicated prior to use. HDM (D. pteronyssinus XPB82D3A25, Greer Laboratories) was also resuspended in sterile saline. Lung mechanics and tissues were collected 24 h after the last exposure. Protocols for the animals use in this study were approved by the Institutional Animal Care and Use Committee (IACUC) at Cincinnati Children’s Hospital Medical Center.
Lung mechanics
Mice were anesthetized by intraperitoneal injection of 0.1 mL of ketamine/xylaxine/acepromazine (4:1:1), tracheostomized with an 18-g blunt aluminium needle, and then connected to the flexiVent system (Legacy Model, Scireq). Mechanical ventilation was initiated at 150 breaths/min with a tidal volume of 10 mL/kg and a positive end-expiratory pressure of 3 cmH2O. Repeated measurements, Snapshot 150 and Quickprime 3, were taken after two total lung capacity manoeuvres followed by nebulization for 10 s of 1xPBS (baseline) and then increasing doses of methacholine (acetyl-b-methylcholine chloride A2251, Sigma) at 25, 50, and 100 mg/mL.
Allergen-specific IgE
Blood was collected, and serum extracted by centrifugation and HDM-specific IgE was measured by ELISA. Plates were coated with 0.01% HDM overnight and then blocked with 1% BSA for 1 h. Samples were incubated for 1 h, and then biotin-anti-mouse IgE (1:250; 553419, BD Biosciences) was applied for 1 h and then streptavidin-HRP (1:100; DY998, R&D Systems) for 30 min. The plate was developed by adding tetramethylbenzidine substrate (555214, BD Biosciences), neutralized with 2N H2SO4, and then absorbance was measured at 450 nm using a spectrophotometer (Synergy2, BioTek).
Inflammatory cell influx, cytokines, and chemokines
Bronchoalveolar lavage fluid (BALF) was collected by washing the lungs with 1 mL of 1xPBS with a single in and out movement and then centrifuged, and the supernatant was removed and stored for measurement of cytokines and chemokines. A portion of the BALF cell suspension was centrifuged onto cytospin slides and differential staining performed with Kwik Diff Kit (9990700, Thermo Scientific). The percentage of each cell type was calculated by counting 300 cells per slide that were randomly selected. IL-4, −5, −13, −17A, −10, IFNγ, eotaxin, and KC were measured in BALF using multiplex cytokine/chemokine panel I and Luminex xMAP technology (PXMCYTO-70K, Millipore) following the manufacturer’s instructions. CCL20 was measured in BALF using an ELISA (DY760, R&D Systems) following the manufacturer’s instructions.
Lung inflations, histology, and immunostaining
The left lung was inflation-fixed at 25 cm pressure with 4% paraformaldehyde (PFA), processed in a histology tissue processor (Citadel 2000, Shandon), and embedded in paraffin, and then 6-μm sections were cut and collected onto poly-L-Lysine coated slides (M6143, Cardinal Health). Haematoxylin and eosin (H+E) staining of lung sections was performed according to the manufacturer’s instructions (PolyScientific). Masson’s trichrome staining of lung sections was also performed according to the manufacturer’s instructions (PolyScientific). Immunolocalization of Muc5ac was performed on lung sections, with primary antibody for Muc5AC (1:1000; ab3649, Abcam), secondary antibody biotinylated-goat-anti-mouse IgG1 (1:200; 1070–08, Southern Biotech), developed with diaminobenzidine and counter-stained with 10% haematoxylin. Images were acquired using a microscope and camera (Axioplan 2, Carl Zeiss).
Western blot analysis
Western blots were performed on homogenates of the lower and infracardiac lobes of the right lung. Membranes were incubated with primary antibodies for chloride channel calcium-activated family member 3 (CLCA3; 1:5000; ab46512, Abcam) and pan-Actin (C4 Actin; 1:40 000; 7 Hills Bioreagents). Secondary antibodies were goat-anti-rabbit or goat-anti-mouse (1:10 000; 401393 and 401215, Calbiochem). ECL Plus chemiluminescence (RPN2132, GE Healthcare) was used to develop blots, and a CCD camera LAS 4000 (Fujifilm) was used to acquire digital images. Densitometry measurements were performed using Multi Gauge 3.0 software (Fujifilm).
Flow cytometry
Flow cytometry was performed on lung tissue from the upper and middle right lobes. Tissues were minced in 2 mL of RPMI 1640 media containing 0.5 mg/mL Liberase TL (Roche Diagnostics) and 0.5 mg/mL DNAse I (Sigma) and incubated at 37°C for 30 min. Lung cells were then passed through a 70-mm cell strainer, centrifuged, resuspended in 2 mL of RPMI 1640 media, and counted using a haemacytometer. Approximately 500 000 lung cells were transferred to a 96-well V-bottom plate and resuspended in 1xPBS containing FcBlock (BD Bioscienes). Live and dead cells were labelled with LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Invitrogen) according to the manufacturer’s instructions, and only live cells were used to calculate data. Dendritic Cell Panel: cells were stained with Ly6G-FITC, CD11b-PE, Gr-1-APC-Cy7, MHC class II (Ia, Ie)-AF700, and CD11c-PacificBlue (BioLegend). T-cell panels: cells were stained with B220-FITC, CD62L-PE, CD3-PE/Cy7, CD4-APC, CD8-APC/Cy7, CD44-PacificBlue (BioLegend); or with CD4-FITC, CD69-PE, CD3-PECy7, CD25-AF647, and intracellular staining for Foxp3-PerCP5.5 (eBioscience); or with CD4-FITC, CD3-PECy7, CD44-Pacific-Blue and intracellular staining for IL13-PE, IFNy-PerCP5.5, and IL17A-AF647 (eBioscience) following ex vivo restimulation with 50 ng/mL phorbol 12-myristate 13-acetate (PMA; Sigma) and 500 ng/mL ionomycin (Sigma) for 1 h, and then in the presence of Brefeldin A (eBioscience) for an additional 3 h. Acquisition was performed by a FACS Canto III (Becton Dickinson) and analysed using FlowJo software (Tree Star). Full gating strategies are provided in this article’s supplementary data section (Fig. S8a–d).
Statistical analysis of data
Data analyses were performed using Prism 5 software (GraphPad) and one-way ANOVA with Tukey and Bonferroni multiple comparison post hoc tests. Statistical significance was determined to be P < 0.05 (*), P < 0.01 (**), and P < 0.001 (***), or not significant if P > 0.05 (N.S.). Error bars shown represent the standard error of mean (±SEM). Experiments were repeated three independent times, and the total number of animals per group was 15–25, for Figures 1–7, S2, S4, and S6. Experiments were repeated two independent times, and the total number of animals per group was 6–11, for Figures 8, S3, S5, and S7.
Results
Airway hyper-reactivity in young mice exposed to DEP and allergen
Airway hyper-reactivity (AHR) is an important characteristic of asthma, and so to determine the effects of DEP and allergen exposure on airway function in young mice, lung mechanics were assessed using a flexiVent system. Airway resistance, elastance, and compliance were measured before and after nebulizing methacholine (at 25, 50, and 100 mg/mL) into the airways (Fig. 1). Airway resistance and elastance were not increased with either the 1.2 mg/kg or 6.0 mg/kg dose of DEP alone (Fig. 1a,b). Resistance and elastance were moderately increased with HDM alone compared with saline controls and were further increased in young mice co-exposed to either dose of DEP and HDM together. Compliance was decreased with HDM exposure compared with saline controls, but not further decreased with either dose of DEP and HDM co-exposure even though there appeared to be a trend towards a decrease (Fig. 1c). Dose–response curves are shown in the supplemental section of this article (Fig. S2a–c). Collectively, these data demonstrate that either dose of DEP co-exposed with HDM increased AHR in young mice.
Fig. 1.
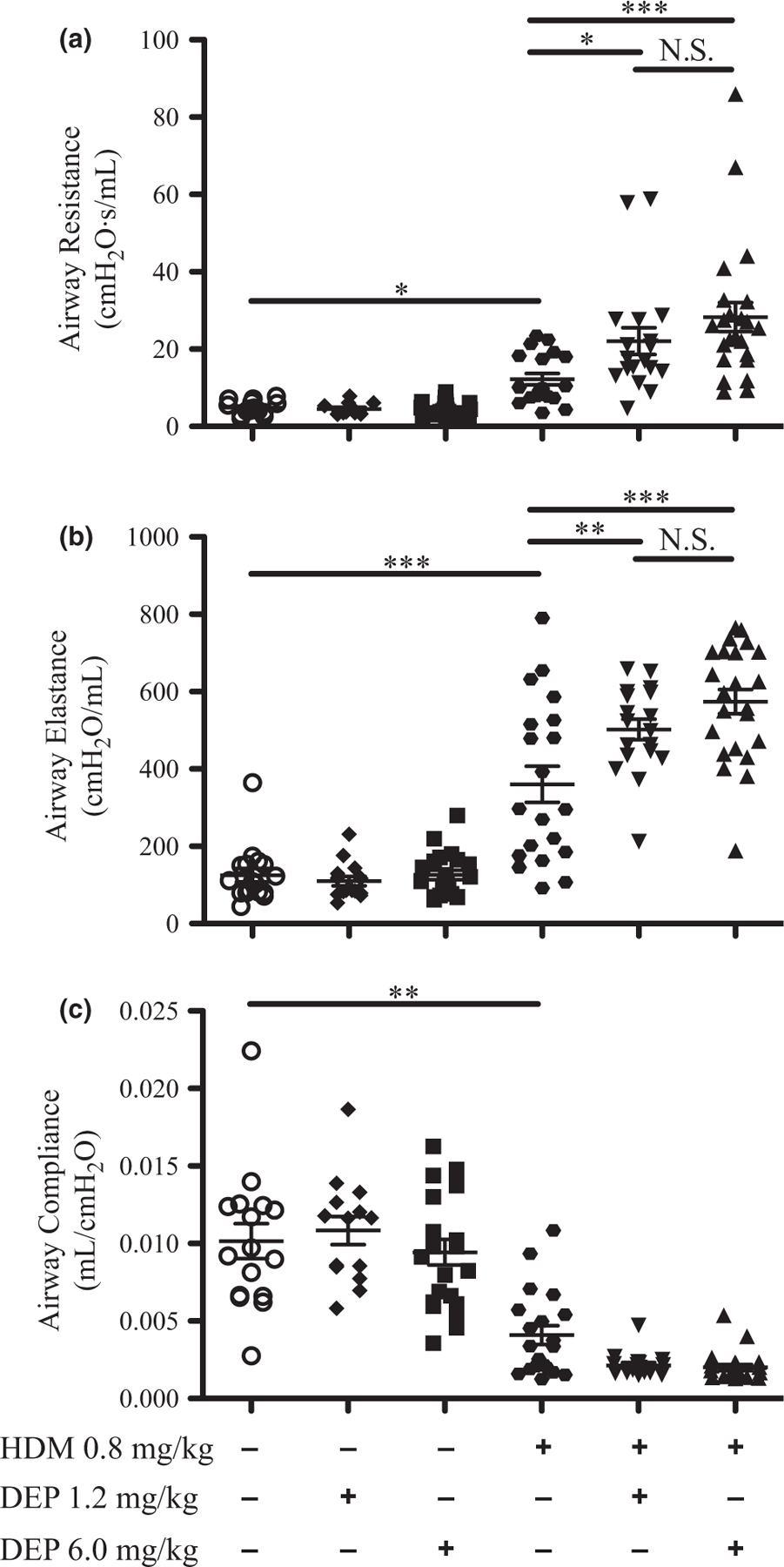
Airway hyper-reactivity is increased after DEP and allergen exposure. Airway mechanics were assessed using a flexiVent system after nebulizing PBS (baseline) and then increasing doses of methacholine (25, 50, and 100 mg/mL) into the lungs. Changes in airway resistance (a), elastance (b), and compliance (c) are shown at 100 mg/mL methacholine; dose–response curves are shown in supplemental data (Fig. S2a–c). Statistical significance was determined to be P < 0.05 (*), P < 0.01 (**), and P < 0.001 (***) or not significant if P > 0.05 (N.S.). Error bars shown represent the standard error of mean (±SEM). The number of animals per group was 15–25.
Impact of exposure to DEP on lung histology of young mice
To determine the effect of DEP exposure on lung histology, lung sections were stained with haematoxylin and eosin following exposure (Fig. 2). DEP was observed in the distal airways and alveolar regions of the lung (red arrows) (Fig. 2b,c,e,f). Inflammatory cells were observed around the distal airways (green arrows) in mice exposed to 6.0 mg/kg of DEP (Fig. 2c), but not in the 1.2 mg/kg DEP group (Fig. 2b). In mice exposed to HDM alone, inflammation around the distal airways was minor, because a low dose of HDM was used (Fig. 2d). However, inflammatory cells were more abundant around the airways and vessels of mice co-exposed to either dose of DEP and HDM together (Fig. 2e,f). To assess changes in lung structure, lung sections were stained with Masson’s trichrome. No obvious signs of fibrosis or smooth muscle thickening were seen in mice exposed to either dose of DEP or allergen alone (Fig. S3a–d), as well as in mice co-exposed to either dose of DEP and HDM (Fig. S3e,f).
Fig. 2.
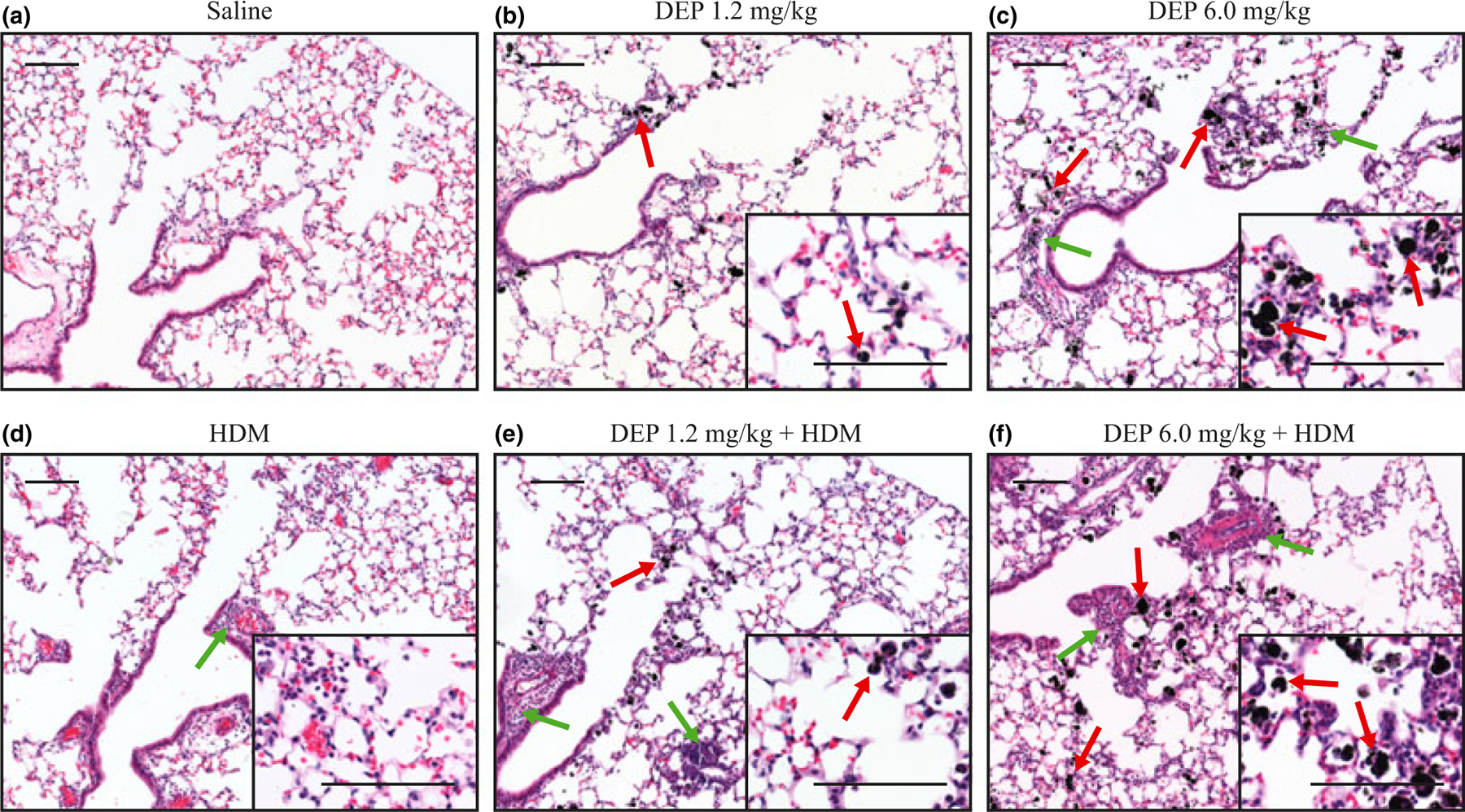
Lung histology following DEP and allergen exposure. (a–f) Haematoxylin and eosin staining of lung sections showing DEP (red arrows) and inflammation (green arrows) in the small airways and alveolar regions of the lung; scale bar = 100 μm.
Allergic sensitization and inflammation in young mice
The effects of DEP and allergen exposure on allergic sensitization were examined by measuring HDM-specific IgE in serum samples (Fig. 3). HDM-specific IgE did not increase in the HDM alone group, most likely due to the low dose of HDM that was used (Fig. 3a). However, HDM-specific IgE was similarly increased when mice were co-exposed to either dose of DEP and HDM. Inflammation was assessed by counting total inflammatory cells present in the BALF. The number of cells was not significantly increased in young mice exposed to either dose of DEP alone (Fig. 3b). However, total inflammatory cells were similarly increased when mice were co-exposed to either dose of DEP and HDM. Differential staining of BALF cells showed that neutrophils were increased in mice exposed to 6.0 mg/kg of DEP alone, but not 1.2 mg/kg, and further increases in neutrophils were seen in the groups co-exposed to DEP and HDM (Fig. 3c). Eosinophils were not significantly increased with either dose of DEP alone or HDM alone (Fig. 3d). However, eosinophils were increased with co-exposure to either dose of DEP and HDM compared with allergen alone. Only a trend towards an increase in macrophages was seen with DEP and HDM co-exposure (Fig. 3e); however, 6.0 mg/kg DEP co-exposed with HDM increased lymphocytes compared with allergen alone (Fig. 3f). The percentages of each inflammatory cell type in the BALF are shown in supplemental data (Fig. S4a–d). Taken together, these data demonstrate that young mice exposed to DEP and allergen have increased allergic sensitization and inflammation.
Fig. 3.
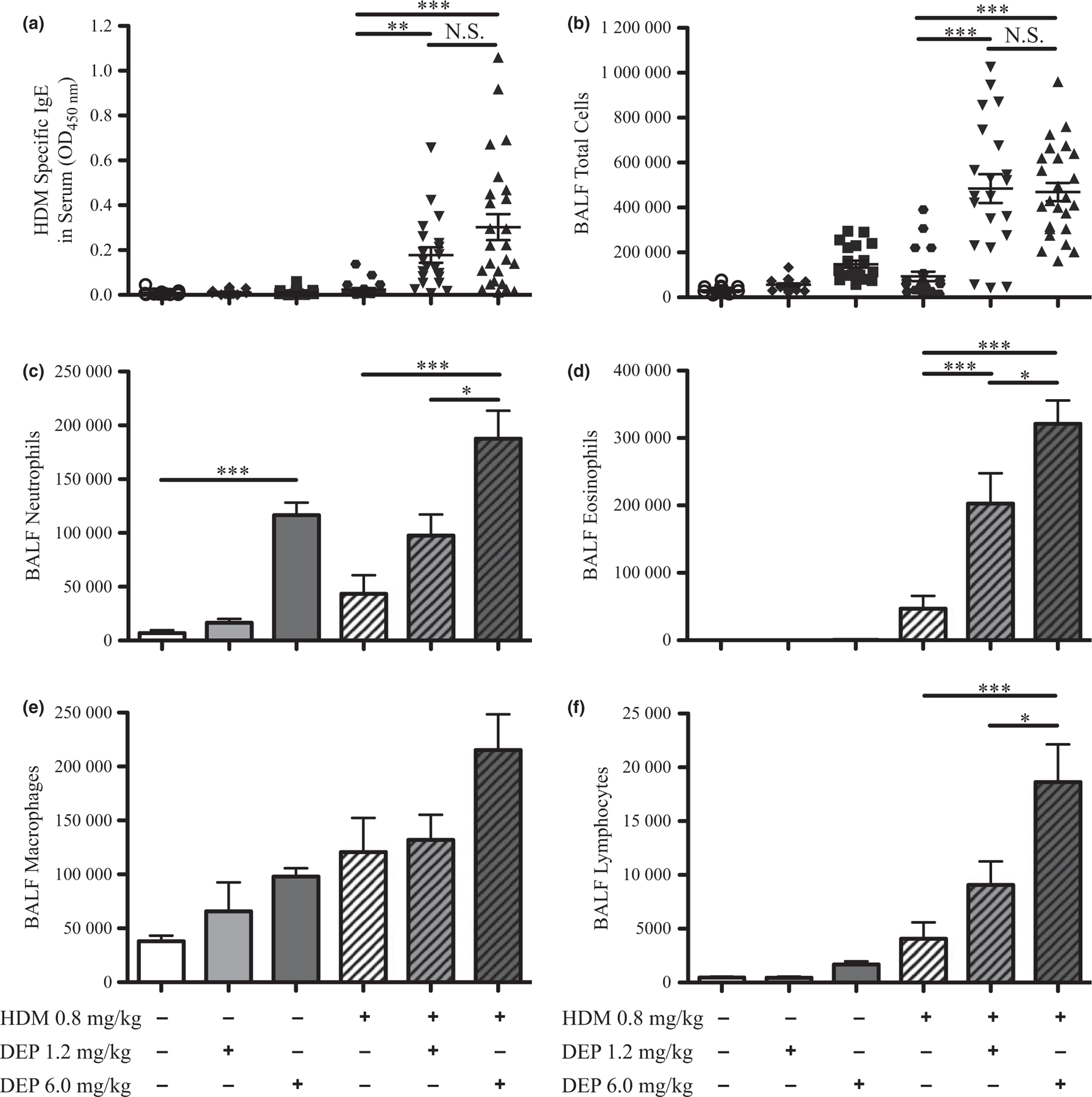
HDM-specific IgE and inflammatory cells increase with DEP and allergen exposure. (a) HDM-specific IgE in serum samples from exposure groups was measured by ELISA. (b) Inflammatory cell influx was estimated by counting cells present in BALF. Changes in the number of neutrophils (c), eosinophils (d), macrophages (e), and lymphocytes (f) were assessed after differential staining; changes in percentages of cell types are shown in supplemental data (Fig. S4). Statistical significance was determined to be P < 0.05 (*), P < 0.01 (**), and P < 0.001 (***) or not significant if P > 0.05 (N.S.). Error bars shown represent the standard error of mean (±SEM). The number of animals per group was 15–25.
Goblet Cell Metaplasia in young mice exposed to DEP and HDM
Another key characteristic of asthma is an increase in goblet cells and mucus overproduction. To assess the effects of DEP and allergen exposure on goblet cell metaplasia in proximal airways, immunohistochemistry was performed for Muc5AC, a goblet cell marker, on lung sections (Fig. 4). Occasional Muc5AC-positive cells were seen in the conducting airways of saline controls and mice treated with either dose of DEP alone (Fig. 4a–c). Mice exposed to HDM alone had increased staining for Muc5AC (Fig. 4d), and a further increase was seen when mice were co-exposed to either dose of DEP and HDM (Fig. 4e,f). To quantitate these changes, Western blot analysis was performed on lung homogenates for CLCA3, a distinct goblet cell protein. Saline controls and exposure to either dose of DEP alone showed no detectable CLCA3, but HDM exposure increased CLCA3 levels (Fig. S5a,b). CLCA3 levels were similarly increased further when mice were co-exposed to either dose of DEP and HDM. Collectively, these data demonstrate that young mice exposed to DEP and allergen have increased goblet cell metaplasia.
Fig. 4.
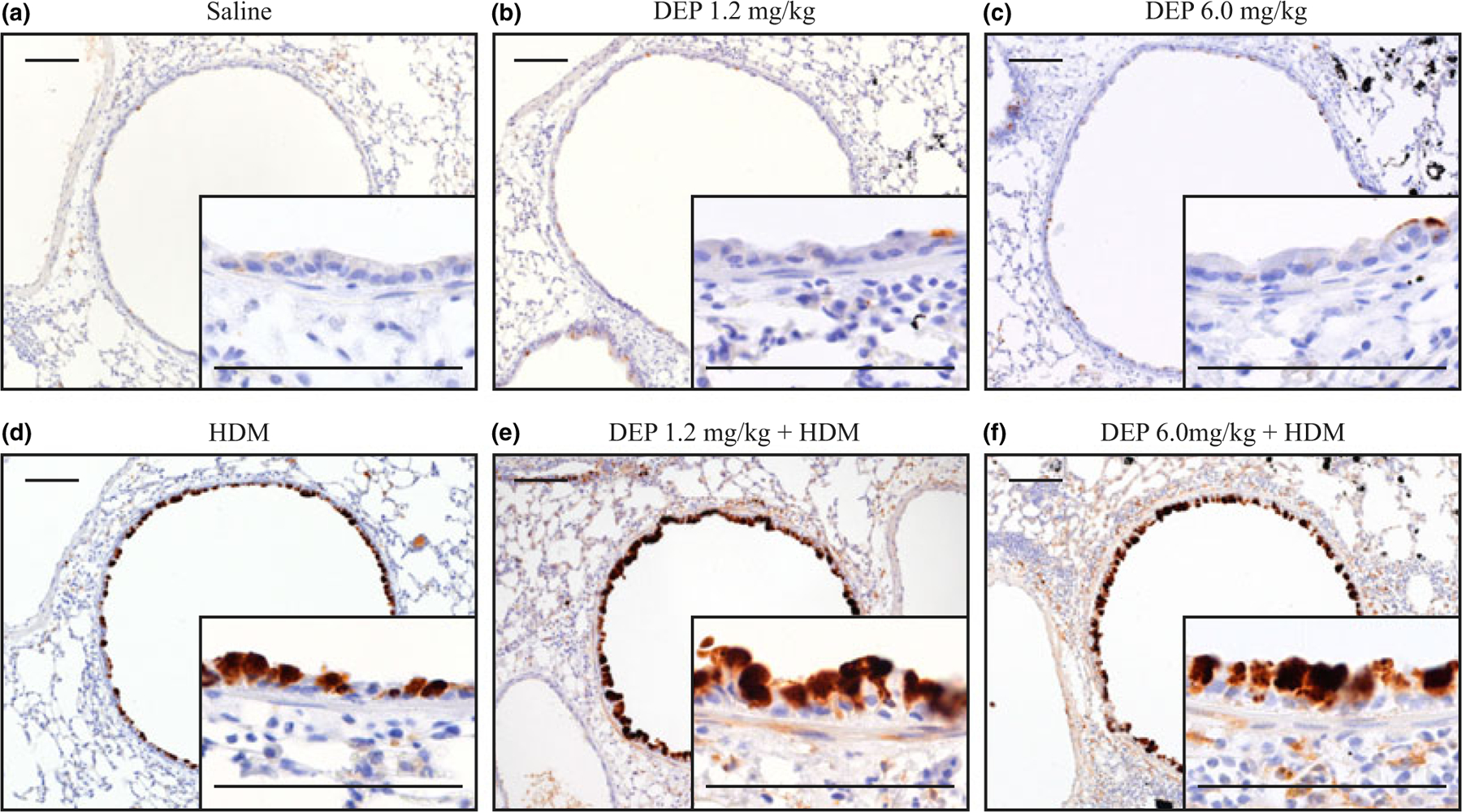
Goblet cell metaplasia following DEP and allergen exposure. (a–f) Immunohistochemistry for Muc5AC was performed on lung sections, positive staining for Muc5AC appears brown and DEP appears black; scale bar = 100 μm. Quantitative assessment of goblet cell metaplasia is shown by Western blot analysis of CLCA3 in supplemental data (Fig. S5a,b).
Cytokines and chemokines in young mice
Cytokines and chemokines are important mediators of immune responses and play a critical role in allergic asthma, and so cytokines and chemokines were measured in BALF samples by ELISA (Fig. 5). IL-4, −5, −10, −13, −17A, and CCL11 (eotaxin-1) were not induced by either dose of DEP alone (Fig. 5a–e, S6a). However, the chemokines CXCL1 (KC), CCL20 (MIP-3 alpha), and CCL2 (MCP-1) were increased with 6.0 mg/kg of DEP compared with saline controls, but not by 1.2 mg/kg (Fig. 5f, S6c,d). IL-4, −5, −10, −13, and −17A were increased in young mice exposed to either dose of DEP with HDM compared with allergen alone. CCL11 (eotaxin-1) was only increased with co-exposure to 6.0 mg/kg of DEP and HDM compared with allergen alone (Fig. 5e). IFNγ was decreased similarly with either dose of DEP co-exposed with HDM compared with allergen alone (Fig. S6b). Taken together, these data demonstrate that DEP and allergen exposure in young mice increased Th2 and Th17 cytokines.
Fig. 5.
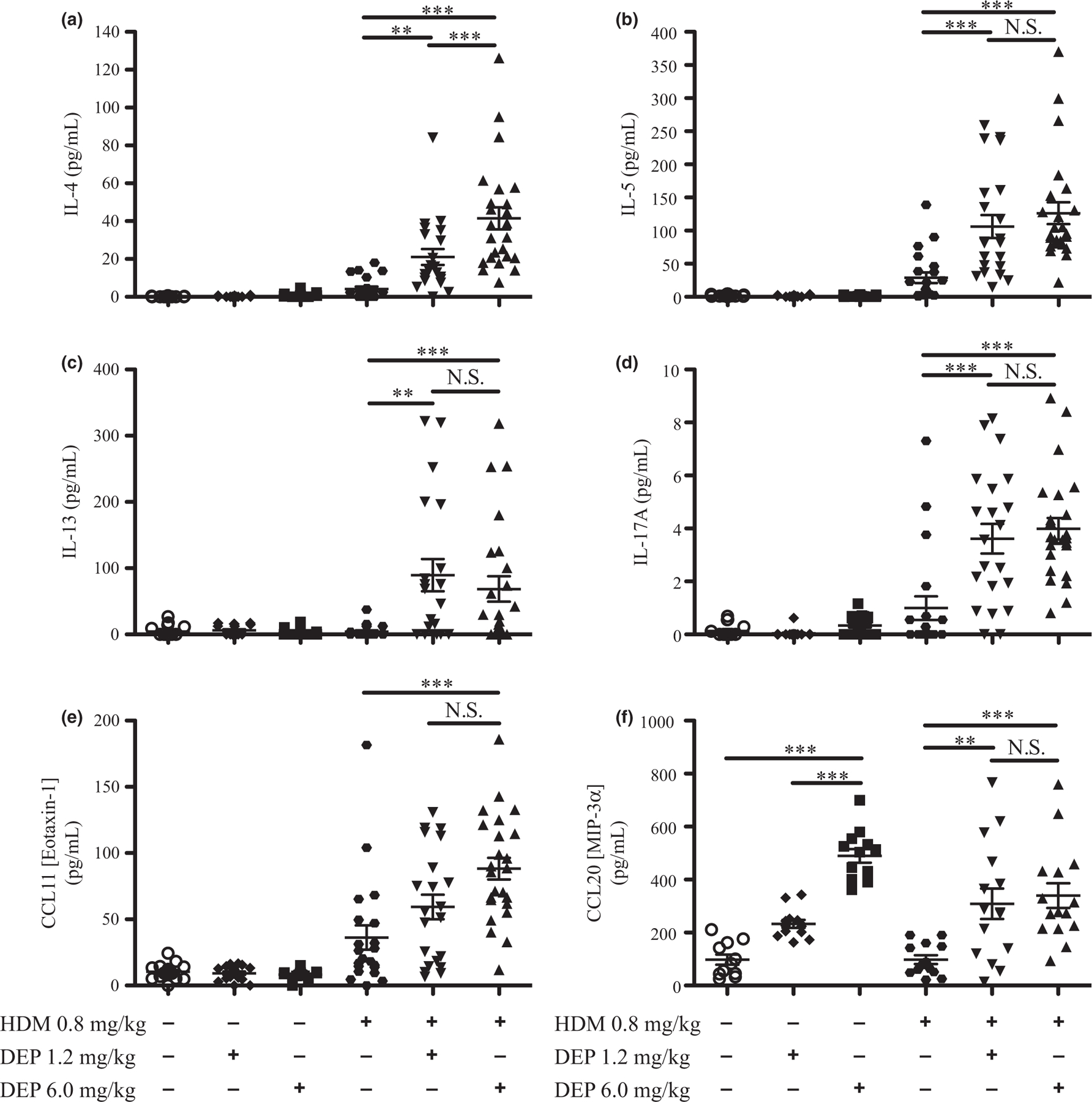
Cytokine and chemokine levels in BALF increased with DEP and allergen exposure. Cytokine and chemokine levels in BALF were measured by multiplex or ELISA (a–f). Statistical significance was determined to be P < 0.01 (**) and P < 0.001 (***) or not significant if P > 0.05 (N.S.). Error bars shown represent the standard error of mean (±SEM). The number of animals per group was 15–25.
Dendritic cells in young mice
As Th2 cytokines were increased and dendritic cells can initiate immune responses and maintain Th2 adaptive responses to allergens [38], we examined the effects of DEP and allergen exposure on dendritic cells in young mice. Flow cytometry analysis was performed for myeloid dendritic cells (mDC) on lung tissue (Fig. 6). mDCs were not induced by either dose of DEP or HDM alone. However, mDCs were similarly increased when mice were co-exposed to either dose of DEP and HDM compared with allergen alone.
Fig. 6.
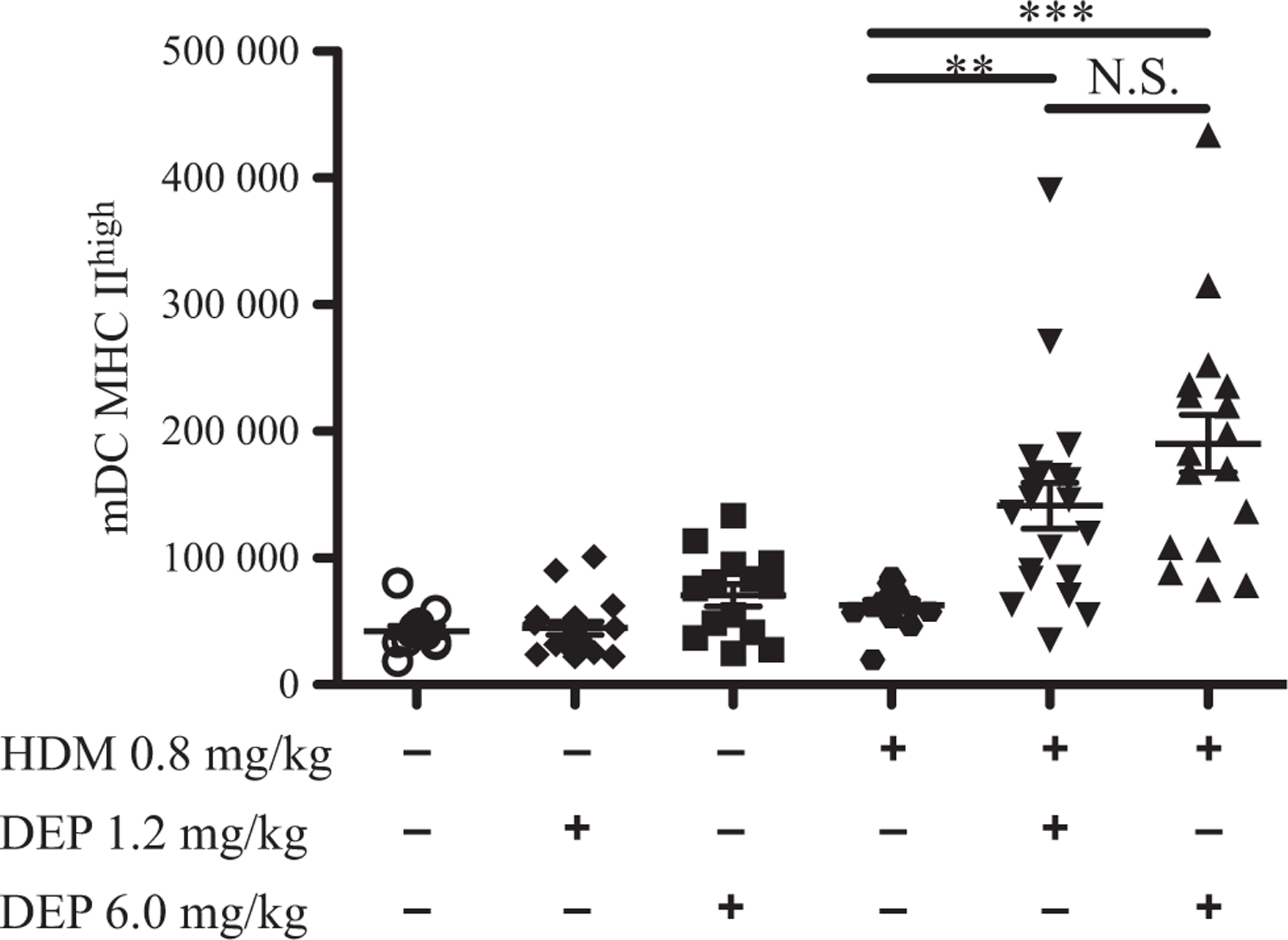
Increased myeloid dendritic cells after DEP and allergen exposure. Flow cytometry analysis was performed on lung cells from exposure groups for activated myeloid dendritic cells. The number of myeloid dendritic cells is depicted. The gating strategy is shown in supplemental data (Fig. S8a). Statistical significance was determined to be P < 0.01 (**), and P < 0.001 (***) or not significant if P > 0.05 (N.S.). Error bars shown represent the standard error of mean (±SEM). The number of animals per group was 15–25.
T-cell populations in young mice
DEP and allergen exposure increased DC numbers in these young mice, and after allergen challenge, activated DCs are involved in the activation of T cells and differentiation of regulatory T cells (Tregs) [38]. To determine the impact of DEP and allergen exposure on T-cell populations in young mice, flow cytometry analysis was performed on lung tissue to assess Tregs (CD25pos/Foxp3pos), activated T cells (CD69pos/Foxp3neg), and T effector cells (CD62Lneg/CD44high) as a percentage of CD4pos T cells (Fig. 7). CD25pos Tregs, activated T cells, and effector T cells were not increased with either dose of DEP alone (Fig. 7a–c). CD25pos Tregs and effector T cells, but not activated T cells, were increased with HDM alone. CD25pos Tregs were not further increased with co-exposure to either dose of DEP and HDM. However, activated T cells and effector T cells were similarly increased when mice were co-exposed to either dose of DEP and HDM compared with allergen alone. To further characterize these effector T cells, flow cytometry analysis was performed on effector T cells stained for intracellular cytokines IL-13 and IL-17A, after ex vivo restimulation (Fig. 8). A sample gating strategy is shown in Figure 8a. The number of effector T cells positive for IL-13, IL-17A, or both IL-13 and IL-17A was not increased by either dose of DEP alone (Fig. 8b–d). However, the number of effector T cells that were IL-13pos, IL-17Apos, and IL-13pos/IL-17Apos were increased with co-exposure to either dose of DEP and HDM compared with allergen alone. Increases in the number of IL-17Apos and IL-13pos/IL-17Apos T cells were similar with either dose of DEP together with HDM, but 6.0 mg/kg of DEP and HDM caused a greater increase in IL-13pos T cells compared with 1.2 mg/kg of DEP and HDM. The percentages of these cells as a portion of CD4pos CD44pos T cells are shown in supplemental data (Fig. S7a–c). Collectively, these data demonstrate that young mice exposed to DEP and allergen have increased T-cell activation and Th17 and Th2/Th17 effector T cells.
Fig. 7.
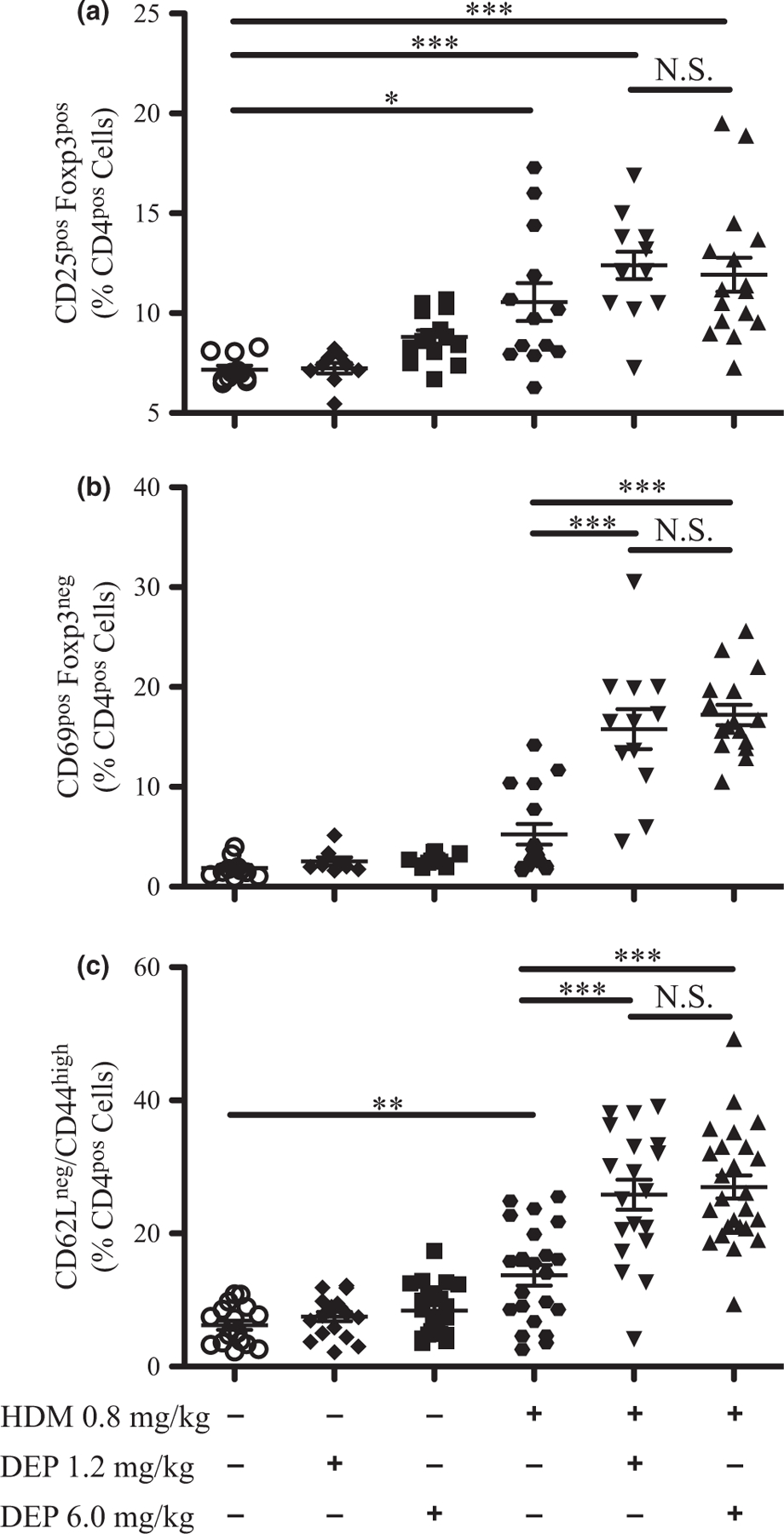
Regulatory T cells, activated T cells, and effector T cells increase with DEP and allergen exposure. Flow cytometry analysis was performed on lung cells from exposure groups for CD25pos regulatory T cells (a), activated T cells (b), and effector T cells (c). The percentages of CD4pos cells are depicted. The gating strategies are shown in supplemental data for effector T cells (Fig. S8b) and regulatory T cells and activated T cells (Fig. S8c). Statistical significance was determined to be P < 0.05 (*), P < 0.01 (**), and P < 0.001 (***) or not significant if P > 0.05 (N.S.). Error bars shown represent the standard error of mean (±SEM). The number of animals per group was 15–25.
Fig. 8.
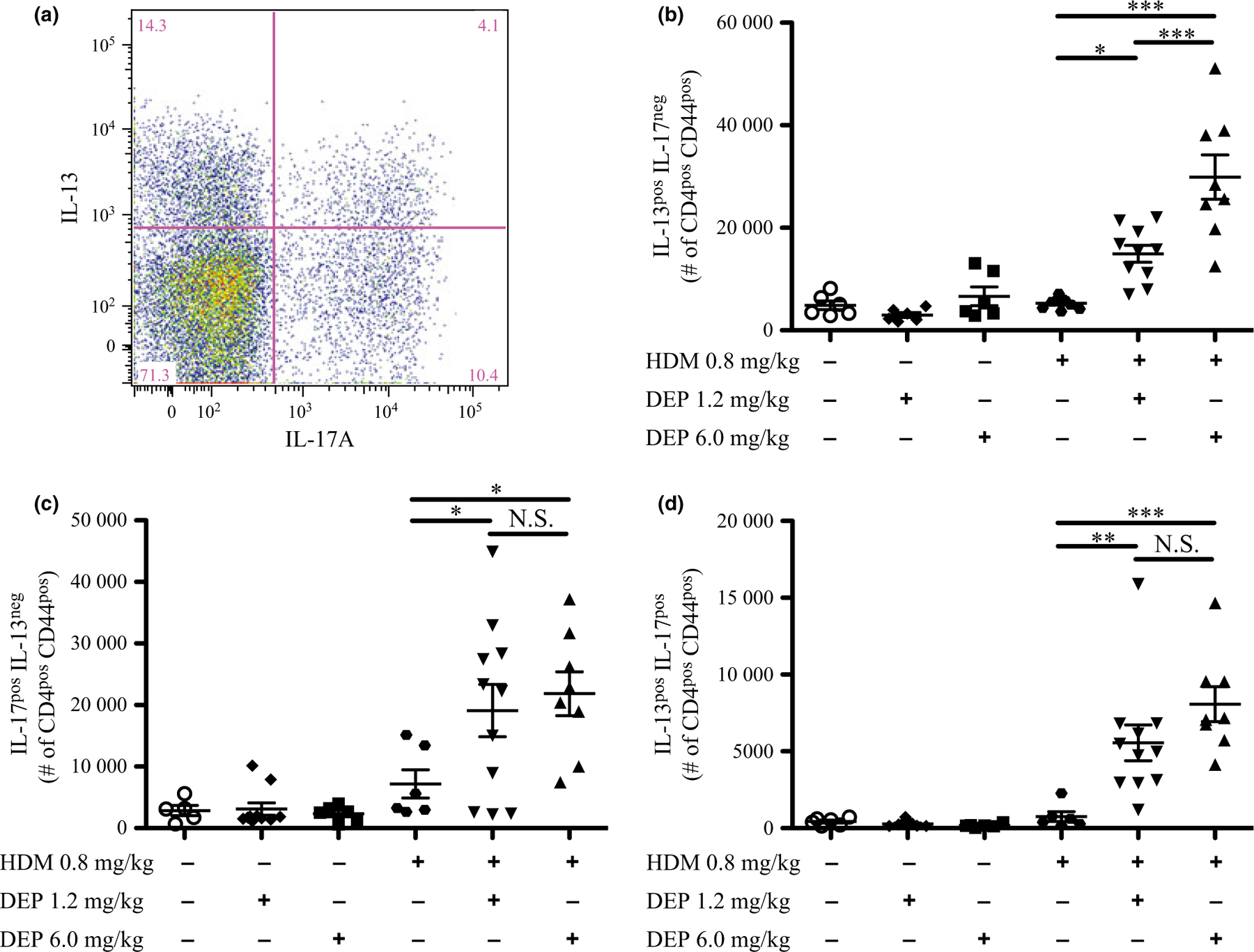
IL-13pos, IL-17Apos, and dual-positive (IL-13pos/IL-17Apos) effector T cells increase following DEP and allergen exposure. Flow cytometry analysis was performed on effector T cells from exposure groups stained for intracellular cytokines IL-13 and IL-17A, after ex vivo restimulation. A sample gating strategy (a) shows the separation of effector T cells that were only IL-13pos (b), only IL-17Apos (c), and positive for both (IL-13pos/IL-17Apos) (d). The number of each cell type is depicted. Changes in percentages of cell types are shown in supplemental data (Fig. S7a–c). The full gating strategy is also shown in supplemental data (Fig. S8d). Statistical significance was determined to be P < 0.05 (*), P < 0.01 (**), and P < 0.001 (***) or not significant if P > 0.05 (N.S.). Error bars shown represent the standard error of mean (±SEM). The number of animals per group was 6–11.
Discussion
This study was designed to examine the effects of DEP and allergen co-exposure on lung and immune responses in young mice. As data from CCAAPS and previous studies have indicated the importance of studying environmental exposures early in life [18, 19], we utilized young mice to investigate potential mediators and examine the dose-dependent impact of DEP and allergen exposure. This study demonstrated that exposure of young animals to DEP alone leads to neutrophilic influx at the 6.0 mg/kg dose, although it did not cause asthma symptoms, while the 1.2 mg/kg dose of DEP caused no detectable inflammation. However, young mice exposed to either dose of DEP and HDM together displayed exacerbated features of allergic sensitization and asthma including increased allergen-specific IgE, inflammatory cells, Th2 and Th17 cytokines, myeloid dendritic cells, activated T cells, and effector T cells. In addition to these immunological changes, exposure to DEP together with HDM increased important functional characteristics of allergic asthma, namely AHR and mucus-producing goblet cells.
Prior studies in adult animals have shown that DEP exposure alone causes inflammation and augments responses to allergens, increasing IgE levels, lung inflammation, AHR, goblet cell metaplasia, and Th2 cytokines [24–34]. Takahashi et al. exposed adult mice to 10, 30, and 100 μg (0.5, 1.5, and 5 mg/kg based on a mouse body weight of 20 g) of DEP together with allergen Der f from HDM and demonstrated that the high dose of DEP augmented allergic responses including inflammation, sensitization, AHR, and airway remodelling [31]. Based upon these prior studies as well as DEP exposure levels in the children from CCAAPS, in which the lower and upper quartiles of exposure differ by approximately fivefold, we used a 6.0 mg/kg and 1.2 mg/kg dose of DEP to examine dose-dependent effects on allergic asthma in young mice. Interestingly, while the 1.2 mg/kg dose of DEP caused no detectable increases in lung inflammation on its own, it still exacerbated many allergic asthma responses as effectively as 6.0 mg/kg of DEP and promoted sensitization when given with HDM. This is in contrast to the study by Takahashi et al. in adults in which a lower dose of DEP did not cause the same increases in asthma symptoms as the highest dose [31]. This difference suggests that mice exhibit a greater sensitivity to DEP earlier in life. As reported by Provoost et al. [27], DEP increased the maturation status of dendritic cells (DCs) and enhanced migration of DCs to the mediastinal lymph node (MLN), resulting in enhanced T-cell recruitment and differentiation. These data support the hypothesis that even a low dose of DEP might be affecting allergic responses in the lymph nodes and so enhancing sensitization, but not directly affecting the lungs.
This study demonstrates that IL-17A and IL-17Apos (Th17) and IL-13pos/IL-17Apos (Th2/Th17) effector T cells are increased in young mice co-exposed to DEP and HDM. IL-17A and Th17 cells have been associated with neutrophilic inflammation and more severe asthma phenotypes, as well as correlating with the severity of airway hyper-reactivity [5–7, 39–41]. While IL-17A can be elevated, Th17 cell numbers are not necessarily increased and must be specifically measured. Effector T cells that produce both Th2 and Th17 cytokines are a novel subset of T cells that have a potential to exacerbate allergic asthma. The transfer of dual-producing Th2/Th17 T cells into mice resulted in more severe inflammation after allergen challenge than with conventional Th2 or Th17 T cells alone [42]. Dual-producing Th2/Th17 cells are also more abundant in chronic asthmatic patients [43], suggesting that dual-producing T cells might play a role in chronic and severe phenotypes of asthma. Our study shows that exposure of young mice to both DEP and HDM results in increases in these Th2/Th17 cells in the lung. These increases are indicative of a more severe asthma phenotype and correlate with the exacerbation of inflammation, AHR, and goblet cell hyperplasia in these mice. Interestingly, exposure to 1.2 mg/kg of DEP was as effective as the 6.0 mg/kg dose, when co-exposed with HDM, at inducing IL-17A, Th17 effector T cells, and the dual-producing Th2/Th17 effector T cells. This suggests that lower doses of DEP can activate this pathway in allergic asthma.
The chemokines CCL20 (MIP-3a) and CCL2 (MCP-1) were increased in young mice exposed to DEP, but not HDM, suggesting pathways driven by DEP alone. Both CCL20 and CCL2 are released by lung epithelial cells after exposure to particulate matter in vitro [44, 45]. CCL20 is capable of recruiting immune cells, including immature dendritic cells, via its receptor CCR6 [46], and T cells up-regulate the CCR6 receptor after activation and differentiation into Th17 cells [47–49]. CCL2 can recruit dendritic cells and T cells via its receptor CCR2 [50, 51], and allergic responses after diesel particle exposure have been shown to be CCR2 dependent [52, 53]. In our study, CCL20 and CCL2 were increased in the BALF after exposure to DEP. DEP and allergen co-exposure increased myeloid dendritic cells, Th17 effector T cells, and double-positive Th2/Th17 effector T cells. These data support the concept that release of CCL20 and/or CCL2 after DEP exposure contributes to the recruitment of dendritic cells that initiate the immune response and/or recruit Th17 effector T cells that contribute to the ongoing disease pathogenesis. Future studies will determine the role of CCL20 and CCL2 in immune responses and characteristics of severe asthma resulting from exposures to DEP and allergen.
In conclusion, exposure of young mice to DEP exacerbated allergic responses characteristic of more severe asthma phenotypes, including increases in IL-17A, Th17 effector T cells, and dual-positive (Th2/Th17) effector T cells. These increases, as well as increases in IgE, eosinophils, and Th2 cytokines and chemokines from DEP and allergen co-exposure, were striking, even with a dose of DEP that alone caused no lung inflammation. These data demonstrate that exposure to DEP can exacerbate allergic responses in young animals and suggests the importance of preventive measures to reduce the exposure of children to traffic-related air pollution.
Supplementary Material
Figure S7. The percentage of dual positive effector T cells increases with DEP and HDM exposure.
Figure S6. Cytokine and chemokine levels in BALF increased with DEP and HDM exposure.
Figure S8. Gating Strategies for FACS analysis.
Figure S4. Neutrophils increased after DEP exposure and eosinophils increased after HDM and DEP exposure.
Figure S5. Goblet cell metaplasia following DEP and allergen exposure.
Figure S2. Airway hyper-reactivity increased after exposure to DEP and HDM.
Figure S1. Exposure protocol.
Figure S3. Masson’s trichrome staining of lung sections following DEP and allergen exposure.
Acknowledgements
This work was supported by NHLBI R01HL097135 (TDLC, GKKH) and NIEHS T32ES010957 (EBB). We would like to thank Patricia Pastura for her excellent technical assistance, James Bridges for his review of this article, and Ian Gilmour for providing the DEP.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
References
- 1.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med 2012; 18:673–83. [DOI] [PubMed] [Google Scholar]
- 2.Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology 2006; 11:54–61. [DOI] [PubMed] [Google Scholar]
- 3.Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP et al. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol 2010; 125:1028–36.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat Immunol 2010; 11:577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Ramli W, Préfontaine D, Chouiali F, Martin JG, Olivenstein R, Lemière C et al. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol 2009; 123:1185–7. [DOI] [PubMed] [Google Scholar]
- 6.Agache I, Ciobanu C, Agache C, Anghel M. Increased serum IL-17 is an independent risk factor for severe asthma. Respir Med 2010; 104:1131–7. [DOI] [PubMed] [Google Scholar]
- 7.Wakashin H, Hirose K, Maezawa Y, Kagami S-i, Suto A, Watanabe N et al. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med 2008; 178:1023–32. [DOI] [PubMed] [Google Scholar]
- 8.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol 2008; 181:4089–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics 2009; 123:S131–45. [DOI] [PubMed] [Google Scholar]
- 10.Subbarao P, Mandhane PJ, Sears MR. Asthma: epidemiology, etiology and risk factors. Can Med Assoc J 2009; 181:E181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gelfand EW. Pediatric asthma: a different disease. Proc Am Thorac Soc 2009; 6:278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan PH, Lemasters GK, Biagini J, Bernstein D, Grinshpun Sa, Shukla R et al. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic J Allergy Clin Immunol 2005; 116:279–84. [DOI] [PubMed] [Google Scholar]
- 13.LeMasters G, Wilson K, Levin L. High prevalence of aeroallergen sensitization among infants of atopic parents. J Pediatr 2006; 149:505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan PH, Lemasters GK, Levin L, Burkle J, Biswas P, Hu S et al. A land-use regression model for estimating microenvironmental diesel exposure given multiple addresses from birth through childhood. Sci Total Environ 2008; 404:139–47. [DOI] [PubMed] [Google Scholar]
- 15.Sahu M, Hu S, Ryan PH, Le Masters G, Grinshpun SA, Chow JC et al. Chemical compositions and source identification of PM2.5 aerosols for estimation of a diesel source surrogate. Sci Total Environ 2011; 409:2642–51. [DOI] [PubMed] [Google Scholar]
- 16.Ryan PH, Lemasters GK, Biswas P, Levin L, Hu S, Lindsey M et al. A comparison of proximity and land use regression traffic exposure models and wheezing in infants. Environ Health Perspect 2007; 115:278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan PH, Bernstein DI, Lockey J, Reponen T, Levin L, Grinshpun S et al. Exposure to traffic-related particles and endotoxin during infancy is associated with wheezing at age 3 years. Am J Respir Crit Care Med 2009; 180:1068–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gasana J, Dillikar D, Mendy A, Forno E, Ramos Vieira E. Motor vehicle air pollution and asthma in children: a meta-analysis. Environ Res 2012; 117:36–45. [DOI] [PubMed] [Google Scholar]
- 19.Bråbäck L, Forsberg B. Does traffic exhaust contribute to the development of asthma and allergic sensitization in children: findings from recent cohort studies. Environ Health 2009; 8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCreanor J, Cullinan P, Nieuwenhuijsen MJ, Stewart-Evans J, Malliarou E, Jarup L et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med 2007; 357:2348–58. [DOI] [PubMed] [Google Scholar]
- 21.Diaz-Sanchez D, Tsien A, Casillas A, Dotson AR, Saxon A. Enhanced nasal cytokine production in human beings after in vivo challenge with diesel exhaust particles. J Allergy Clin Immunol 1996; 98:114–23. [DOI] [PubMed] [Google Scholar]
- 22.Salvi SS, Blomberg A, Rudell B, Kelly FJ, Sandström T, Holgate ST et al. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med 1999; 159:702–9. [DOI] [PubMed] [Google Scholar]
- 23.Diaz-Sanchez D, Tsien A, Fleming J, Saxon A. Combined diesel exhaust particulate and ragweed allergen challenge markedly enhances human in vivo nasal ragweed-specific IgE and skews cytokine production to a T Helper Cell 2-Type Pattern. J Immunol 1997; 158:2406–13. [PubMed] [Google Scholar]
- 24.Sagai M, Furuyama A, Ichinose T. Biological effects of diesel exhaust particles (DEP). III. Pathogenesis of asthma like symptoms in mice. Free Radical Biol Med 1996; 21:199–209. [DOI] [PubMed] [Google Scholar]
- 25.Nel AE, Diaz-Sanchez D, Ng D, Hiura T, Saxon A. Enhancement of allergic inflammation by the interaction between diesel exhaust particles and the immune system. J Allergy Clin Immunol 1998; 102:539–54. [DOI] [PubMed] [Google Scholar]
- 26.Saber AT, Jacobsen NR, Bornholdt J, Kjaer SL, Dybdahl M, Risom L et al. Cytokine expression in mice exposed to diesel exhaust particles by inhalation. Role of tumor necrosis factor. Particle and Fibre. Toxicology 2006; 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Provoost S, Maes T, Willart MA, Joos GF, Lambrecht BN, Tournoy KG. Diesel exhaust particles stimulate adaptive immunity by acting on pulmonary dendritic cells. J Immunol 2010; 184:426–32. [DOI] [PubMed] [Google Scholar]
- 28.Riedl M, Diaz-Sanchez D. Biology of diesel exhaust effects on respiratory function. J Allergy Clin Immunol 2005; 115:221–8; quiz 9. [DOI] [PubMed] [Google Scholar]
- 29.Takano H, Yoshikawa T, Ichinose T, Miyabara Y, Imaoka K, Sagai M. Diesel exhaust particles enhance antigen-induced airway inflammation and local cytokine expression in mice. Am J Respir Crit Care Med 1997; 156:36–42. [DOI] [PubMed] [Google Scholar]
- 30.Miyabara Y, Takano H, Ichinose T, Lim HB, Sagai M. Diesel Exhaust Enhances Allergic Airway Inflammation and Hyperresponsiveness in Mice. Am J Respir Crit Care Med 1998; 157:1138. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi G, Tanaka H, Wakahara K, Nasu R, Hashimoto M, Miyoshi K et al. Effect of Diesel Exhaust Particles on House Dust Mite–Induced Airway Eosinophilic Inflammation and Remodeling in Mice. J Pharmacol Sci 2010; 112:192–202. [DOI] [PubMed] [Google Scholar]
- 32.Inoue K-i, Koike E, Takano H, Yanagisawa R, Ichinose T, Yoshikawa T. Effects of diesel exhaust particles on antigen-presenting cells and antigen-specific Th immunity in mice. Exp Biol Med (Maywood) 2009; 234:200–9. [DOI] [PubMed] [Google Scholar]
- 33.Kim J, Natarajan S, Vaickus LJ, Bouchard JC, Beal D, Cruikshank WW, et al. Diesel exhaust particulates exacerbate asthma-like inflammation by increasing CXC chemokines. Am J Pathol 2011; 179:2730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li N, Harkema JR, Lewandowski RP, Wang M, Bramble LA, Gookin GR et al. Ambient ultrafine particles provide a strong adjuvant effect in the secondary immune response: implication for traffic-related asthma flares. Am J Physiol Lung Cell Mol Physiol 2010; 299:374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Björkstén B The intrauterine and post-natal environments. J Allergy Clin Immunol 1999; The intrauterine and postnatal environments:1119–27. [DOI] [PubMed] [Google Scholar]
- 36.McDade TW. Life history theory and the immune system: steps toward a human ecological immunology. Am J Phys Anthropol 2003; 37:100–25. [DOI] [PubMed] [Google Scholar]
- 37.Stevens T, Cho S-H, Linak WP, Gilmour MI. Differential Potentiation of Allergic Lung Disease in Mice Exposed to Chemically Distinct Diesel Samples. Toxicol Sci 2008; 107:522–34. [DOI] [PubMed] [Google Scholar]
- 38.Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity 2009; 31:412–24. [DOI] [PubMed] [Google Scholar]
- 39.Laan M, Cui ZH, Hoshino H, Lötvall J, Sjöstrand M, Gruenert DC et al. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol 1999; 162:2347–52. [PubMed] [Google Scholar]
- 40.Hoshino H, Laan M, Sjöstrand M, Lötvall J, Skoogh BE, Linden A. Increased elastase and myeloperoxidase activity associated with neutrophil recruitment by IL-17 in airways in vivo. J Allergy Clin Immunol 2000; 105:143–9. [DOI] [PubMed] [Google Scholar]
- 41.Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X et al. IL-17A produced by ab T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med 2012; 18:547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y-H, Voo KS, Liu B, Chen C-Y, Uygungil B, Spoede W et al. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med 2010; 207:2479–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cosmi L, Maggi L, Santarlasci V, Capone M, Cardilicchia E, Frosali F et al. Identification of a novel subset of human circulating memory CD4(+) T cells that produce both IL-17A and IL-4. J Allergy Clin Immunol 2010; 125:222–30. [DOI] [PubMed] [Google Scholar]
- 44.Reibman J, Hsu Y, Chen LC, Bleck B, Gordon T. Airway epithelial cells release MIP-3alpha/CCL20 in response to cytokines and ambient particulate matter. Am J Respir Cell Mol Biol 2003; 28:648–54. [DOI] [PubMed] [Google Scholar]
- 45.Reynolds PR, Wasley KM, Allison CH. Diesel particulate matter induces receptor for advanced glycation end-products (RAGE) expression in pulmonary epithelial cells, and RAGE signaling influences NF-κB-mediated inflammation. Environ Health Perspect 2011; 119:332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thorley AJ, Goldstraw P, Young A, Tetley TD. Primary human alveolar type II epithelial cell CCL20 (macrophage inflammatory protein-3alpha)-induced dendritic cell migration. Am J Respir Cell Mol Biol 2005; 32:262–7. [DOI] [PubMed] [Google Scholar]
- 47.Lim HW, Lee J, Hillsamer P, Kim CH. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3 + regulatory T cells. J Immunol 2008; 180:122–9. [DOI] [PubMed] [Google Scholar]
- 48.Pötzl J, Botteron C, Tausch E, Pedré X, Mueller AM, Männel DN et al. Tracing functional antigen-specific CCR6 Th17 cells after vaccination. PLoS ONE 2008; 3:e2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh SP, Zhang HH, Foley JF, Hedrick MN, Farber JM. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol 2008; 180:214–21. [DOI] [PubMed] [Google Scholar]
- 50.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA 1994; 91:3652–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu LL, Warren MK, Rose WL, Gong W, Wang JM. Human recombinant monocyte chemotactic protein and other C-C chemokines bind and induce directional migration of dendritic cells in vitro. J Leukoc Biol 1996; 60:365–71. [DOI] [PubMed] [Google Scholar]
- 52.Robays LJ, Maes T, Lebecque S, Lira SA, Kuziel WA, Brusselle GG et al. Chemokine Receptor CCR2 but Not CCR5 or CCR6 Mediates the Increase in Pulmonary Dendritic Cells during Allergic Airway Inflammation. J Immunol 2007; 178:5305–11. [DOI] [PubMed] [Google Scholar]
- 53.Provoost S, Maes T, Joos GF, Tournoy KG. Monocyte-derived dendritic cell recruitment and allergic T(H)2 responses after exposure to diesel particles are CCR2 dependent. J Allergy Clin Immunol 2011; 129:483–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S7. The percentage of dual positive effector T cells increases with DEP and HDM exposure.
Figure S6. Cytokine and chemokine levels in BALF increased with DEP and HDM exposure.
Figure S8. Gating Strategies for FACS analysis.
Figure S4. Neutrophils increased after DEP exposure and eosinophils increased after HDM and DEP exposure.
Figure S5. Goblet cell metaplasia following DEP and allergen exposure.
Figure S2. Airway hyper-reactivity increased after exposure to DEP and HDM.
Figure S1. Exposure protocol.
Figure S3. Masson’s trichrome staining of lung sections following DEP and allergen exposure.


