Abstract
To investigate whether the transcriptional activator hypoxia-inducible factor 1 (HIF-1) is required for ventilatory responses to hypoxia, we analyzed mice that were either wild type or heterozygous for a loss-of-function (knockout) allele at the Hif1a locus, which encodes the O2-regulated HIF-1α subunit. Although the ventilatory response to acute hypoxia was not impaired in Hif1a+/− mice, the response was primarily mediated via vagal afferents, whereas in wild-type mice, carotid body chemoreceptors played a predominant role. When carotid bodies isolated from wild-type mice were exposed to either cyanide or hypoxia, a marked increase in sinus nerve activity was recorded, whereas carotid bodies from Hif1a+/− mice responded to cyanide but not to hypoxia. Histologic analysis revealed no abnormalities of carotid body morphology in Hif1a+/− mice. Wild-type mice exposed to hypoxia for 3 days manifested an augmented ventilatory response to a subsequent acute hypoxic challenge. In contrast, prior chronic hypoxia resulted in a diminished ventilatory response to acute hypoxia in Hif1a+/− mice. Thus partial HIF-1α deficiency has a dramatic effect on carotid body neural activity and ventilatory adaptation to chronic hypoxia.
Oxygen homeostasis is mediated by the combined physiologic functioning of the circulatory and respiratory systems. Recent studies have demonstrated that the transcriptional activator hypoxia-inducible factor 1 (HIF-1) is required for both the establishment of the circulatory system during embryonic development and physiologic responses in postnatal life. Analysis of Hif1a−/− knockout mice, which are homozygous for a loss-of-function mutation in the Hif1a gene encoding the O2-regulated HIF-1α subunit, revealed that complete HIF-1α deficiency results in embryonic lethality at midgestation with major malformations of the heart and vasculature (1–3). Hif1a+/− heterozygous mice, which are partially HIF-1α deficient, develop normally and are indistinguishable from their wild-type littermates under normoxic conditions. However, adult Hif1a+/− mice manifest impaired physiological responses to chronic hypoxia including significantly reduced rates of erythropoiesis and pulmonary vascular remodeling (4). Thus HIF-1α plays essential roles in cardiac, erythroid, and vascular development and physiology, i.e., all three major components of the circulatory system.
An essential adaptation to both acute and chronic hypoxia is an increase in ventilation that depends on the activity of peripheral chemoreceptors, particularly those within the carotid body, which detect changes in arterial blood O2 concentration and relay sensory information to the brainstem neurons that regulate breathing (reviewed in ref. 5). Altered ventilatory responses to hypoxia may play a critical role in asthma, diabetes, Parkinson's disease, sleep-related breathing disorders, and sudden infant death syndrome (6–8). We hypothesized that HIF-1α is required for carotid body function and ventilatory adaptation to chronic hypoxia. To test this hypothesis, we studied Hif1a+/− and Hif1a+/+ adult mice and found that partial HIF-1α deficiency has dramatic effects on respiratory adaptation to chronic hypoxia and oxygen sensing by carotid body chemoreceptors.
Methods
Preparation of Animals.
Experiments were performed on age- and sex-matched wild-type (Hif1a+/+) and Hif1a+/− mice (1) by individuals blinded to the genotype. Mean weights of the mice were not significantly different (25.9 ± 0.8 g for Hif1a+/+ vs. 26.2 ± 1.7 g for Hif1a+/− mice; P > 0.05, t test). Animals were anesthetized by i.p. injection of urethane (1.2 g/kg; Sigma) (9) and allowed to breathe spontaneously after tracheal intubation. Core body temperature was monitored by a rectal thermistor probe and maintained at 37 ± 1°C by a heating pad. Mice were euthanized by intracardiac injection (0.1 ml) of Beuthanasia-D Special (Schering-Plough).
Measurements of Respiratory Variables.
In unanesthetized animals, respiration was monitored by whole body plethysmograph (10). Animals were placed in a 600-ml Lucite chamber containing an inlet port for gas administration. The chamber was connected to a high-gain differential pressure transducer (Valydine MP45, Validyne, North Ridge, CA). As the animal breathed, changes in pressure were converted to signals representing tidal volume (VT), which were amplified (BMA 830; CWE, Ardmore, PA), recorded on a strip-chart recorder (Dash 10; Astro-Med, West Warwick, RI), and stored in a computer with respiratory acquisition software for analysis. O2 consumption and CO2 production were determined by the open-circuit method by using Beckman OM-14 and LB-2 analyzers. In anesthetized animals, integrated efferent phrenic nerve activity was monitored as an index of central respiratory neuronal output. The phrenic nerve was isolated unilaterally at the level of the C3 and C4 spinal segments, cut distally, and placed on bipolar stainless steel electrodes. The electrical activity was filtered (band pass 0.3–1.0 kHz), amplified, and passed through Paynter filters (time constant of 100 ms; CWE) to obtain a moving average signal.
Carotid Body Morphology.
Mice were anesthetized, perfused at 10 ml/min with heparinized PBS, pH 7.4, followed by 4% paraformaldehyde–PBS (10 min each). Carotid artery bifurcations were placed in 4% paraformaldehyde–PBS for 1 h at 4°C, washed in PBS, and cryoprotected in 30% sucrose–PBS at 4°C for 24 h. Specimens were frozen in Tissue Tek (OCT; VWR Scientific), serially sectioned at 10 μm, washed three times in PBS, and exposed to 20% normal goat serum (NGS) and 0.2% Triton X-100 in PBS for 2 h. Endogenous biotinylated proteins were blocked with avidin and biotin (Vector Laboratories). Sections were incubated at 4°C for 16 h with anti-chromogranin A (1:1,500; Instar, Stillwater, MN) or anti-tyrosine hydroxylase (1:300; Pel-Freez Biologicals) antibody in PBS with 1% NGS and 0.2% Triton X-100. After washing in PBS, sections were incubated for 2 h with biotinylated anti-rabbit IgG (1:200; Vector Laboratories) in PBS with 1% NGS and 0.2% Triton X-100. Immunostaining was visualized by the Vectastain Elite avidin-biotinylated enzyme complex method (Vector Laboratories) by using diaminobenzidine peroxidase. Carotid body morphology, glomic volume, and glomus cell numbers were analyzed in adjacent sections using image software (Scion, Frederick, MD).
Anti-HIF-1α Antibodies.
Serum from rabbits that were immunized with a fusion protein consisting of glutathione S-transferase and residues 432–528 of human HIF-1α was passed over a protein G column, the bound fraction was eluted, passed over a glutathione-S-transferase-Sepharose column, and the flow-through was collected. Purified antibodies specifically recognized HIF-1α on immunoblot assays. Carotid body immunohistochemistry was performed as described above.
Analysis of Unanesthetized Mice.
Animals were placed in the plethysmograph chamber and acclimated for 1 h in room air. In the first set of experiments, mice (n = 8 for each genotype) were exposed to 100, 21, and 12% O2-balance N2. Each gas challenge was given for 5 min. The protocol was repeated after a 20-min interval. O2 consumption and CO2 production were measured at the end of each 5-min challenge. In the second set of experiments, mice inspired 100% O2 for 5 min followed by 3 and 5% CO2-balance O2. The protocol was repeated twice, with a 20-min interval between each protocol. In the third set of experiments, after baseline ventilatory measurements, mice were placed in a hypobaric chamber (0.4 atmospheres) for 72 h, and then respiratory responses to acute hypoxia and hyperoxic hypercapnia were monitored.
Analysis of Anesthetized Mice.
Efferent phrenic nerve activity was determined in anesthetized spontaneously breathing mice (n = 5–6 for each genotype). Respiratory activity was monitored while breathing 100, 21, and 12% O2-balance N2. Each gas challenge was maintained for 1 min. After 12% O2, inspired gas was switched back to 100% O2. Gases were administered through a needle placed near the tracheal cannula, and gas flow was controlled by a flow-meter. For vagotomy, silk thread was placed underneath the cervical vagus nerve bilaterally. After examination of the respiratory responses to 100, 21, and 12% O2, the vagi were ligated and sectioned and, after 10 min, the hypoxic ventilatory response was examined again.
Hyperoxic Challenge.
The Dejours test (11) was performed on anesthetized mice. Baseline respiration was recorded while animals breathed 21% O2 for 45 sec followed by 100% O2 for 20 sec. Respiratory variables were analyzed for 20 sec during 21% O2 and during the last 15 sec of hyperoxia (the initial 5 sec was excluded because of tubing dead space).
Ex Vivo Carotid Body Activity.
Mice were anesthetized and intubated, and the carotid bifurcation was excised and placed in ice-cold medium containing (in mM): 112 NaCl, 4.7 KCl, 2.2 CaCl2, 1.1, MgCl2, 42.0 Na glutamate, 5.0 Hepes, and 5.6 d-glucose, pH 7.4. The carotid body and sinus nerve were isolated, transferred to the recording chamber, and superfused at a flow rate of 2 ml/min. The medium was equilibrated with 100% O2, 12% O2, or 100% O2 with 5 μg/ml of NaCN. Experiments were performed at 33–34°C. Carotid sinus nerve activity was recorded with a suction electrode. Electrical signals were amplified, passed through a window discriminator, integrated for every second, and expressed as impulses/second (imp/sec).
Data Analysis.
In anesthetized mice, variables analyzed were respiratory rate (RR; number of phrenic bursts per minute), integrated phrenic nerve activity (arbitrary units), and minute neural respiration [MNR (arbitrary units/min) = RR × phrenic nerve activity]. Respiratory variables were averaged over a 5-min period of gas challenge. The variables analyzed in unanesthetized mice were RR (breaths/min), VT (μl), and minute ventilation [VE (ml/min) = RR × VT]. Respiratory variables (RR and VT) were averaged for 15 consecutive breaths over 5 min of inspired O2 and CO2 challenge. VT and VE were normalized to body weight. Metabolic variables were measured at the end of each 5-min inspired O2 challenge. Results are expressed as mean ± SEM. Increases in respiration during 21 and 12% O2 compared with 100% O2 were analyzed by paired t tests. Differences between genotypes were analyzed by unpaired t tests.
Results
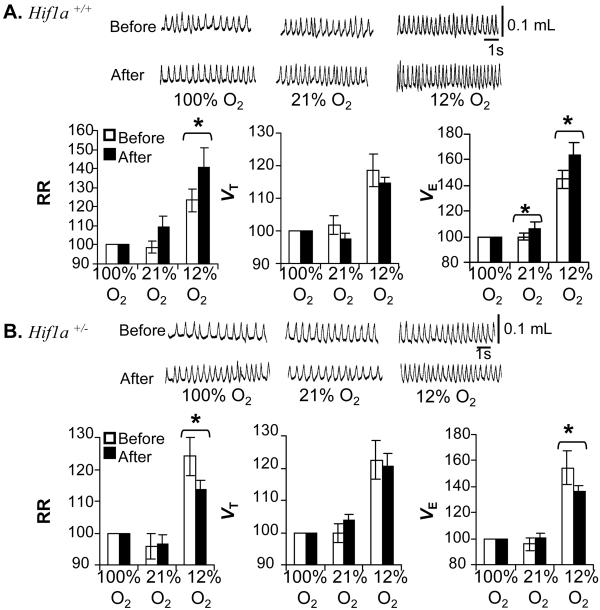
Acute Ventilatory Responses Are Maintained in Hif1a+/− Mice.
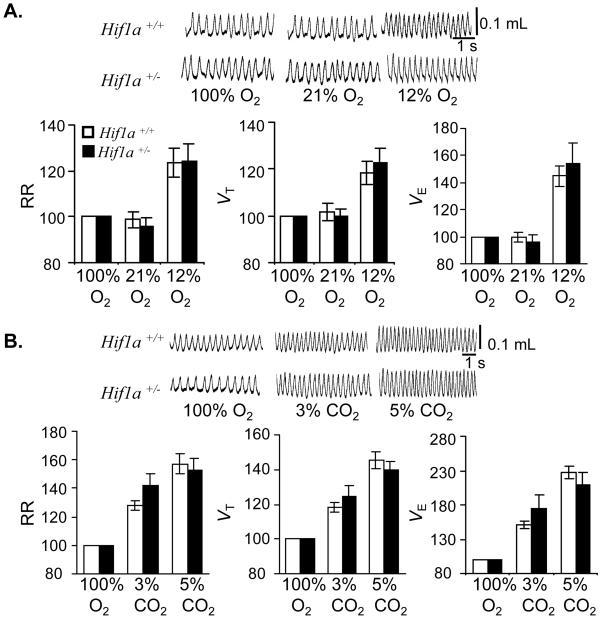
Awake nonanesthetized Hif1a+/+ and Hif1a+/− mice were exposed to 100% (hyperoxia), 21% (normoxia), or 12% (hypoxia) O2, and respiration was monitored by whole body plethysmography. Mean RR, VT, and VE under hyperoxic and normoxic conditions were similar in the two groups (percent changes are shown in Fig. 1A; raw data are presented as Table 1, which is published as supporting information on the PNAS web site, www.pnas.org). Furthermore, both groups of mice responded to hypoxia with similar increases in RR, VT, and VE. Changes in O2 consumption and CO2 production during hypoxia were also comparable in the two groups (Table 1). Both groups of mice responded to hyperoxic hypercapnia with graded increases in RR, VT, and VE on exposure to 3 and 5% CO2 (Fig. 1B and Table 2, which is published as supporting information on the PNAS web site). These results indicate that partial HIF-1α deficiency does not impair acute ventilatory responses to hypoxia or hypercapnia.
Figure 1.
Ventilatory responses to acute hypoxia and hyperoxia. RR, VT, and VE were measured in unanesthetized wild-type Hif1a+/+ (open bars) and Hif1a+/− (closed bars) mice (n = 8 each) during exposure to inspired gas mixtures containing the indicated concentrations of O2 (A) and CO2 (B). Representative tracings of VT are shown (Upper). Ventilatory data are expressed (mean ± SEM) relative to values obtained while breathing 100% O2 (Lower).
Impaired Control of Ventilation Detected by the Dejours Test.
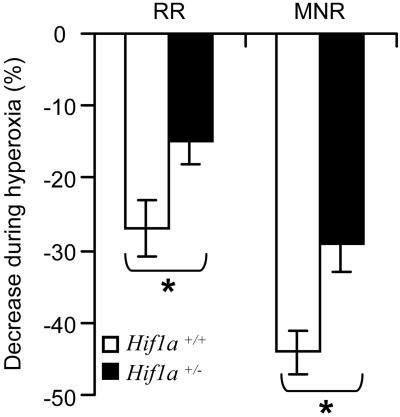
The magnitude of the transient ventilatory decline in response to a brief hyperoxic exposure is used as an index of peripheral chemoreceptor, especially carotid body, sensitivity (11). Respiratory rate and phrenic nerve activity were monitored on anesthetized mice for 5 min under normoxic conditions and then for 20 sec after a hyperoxic challenge. As compared with Hif1a+/− littermates, wild-type mice manifested a significantly greater depression of MNR and RR in response to hyperoxia (Fig. 2).
Figure 2.
Dejours test. RR and MNR (RR × integrated phrenic nerve activity) were measured in Hif1a+/+ (n = 5) and Hif1a+/− (n = 6) mice during a brief hyperoxic challenge. Ventilatory data are expressed (mean ± SEM) as % decrease from the values obtained while breathing room air. *, P < 0.01 (unpaired t test).
Impaired Ventilatory Responses in Hif1a+/− Mice After Vagotomy.
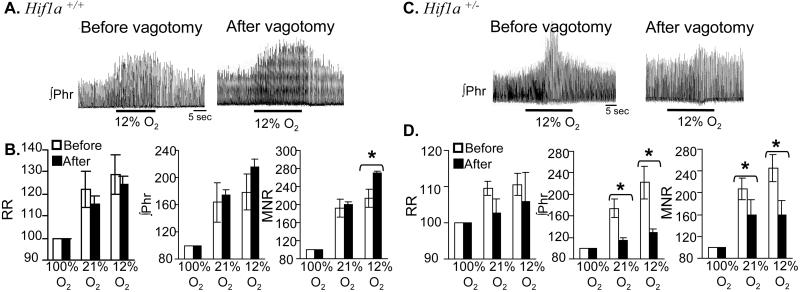
The results of the Dejours test suggest that although the ventilatory response to acute hypoxia is maintained, Hif1a+/− mice may have impaired carotid body function. Previous studies indicated that chemoreceptors innervated by the vagus nerve contribute to ventilatory responses to hypoxia (12, 13) especially under conditions of impaired carotid body function (14, 15). To test the relative contribution of vagal afferents to acute hypoxic ventilatory responses, RR and phrenic nerve activity were monitored in anesthetized wild-type and Hif1a+/− littermate mice that were exposed to 100, 21, or 12% O2 before and after bilateral vagotomy. After vagotomy, respiratory responses to hypoxia were significantly impaired in Hif1a+/− mice, whereas responses were not impaired in Hif1a+/+ mice (percent changes are shown in Fig. 3; raw data are presented in Table 3, which is published as supporting information on the PNAS web site). The differences between the two genotypes with respect to the percent increase in VT and VE in response to hypoxia postvagotomy were highly significant (P = 0.002 and 0.005, respectively, unpaired t test). The results of vagotomy and the Dejours test indicate that compared with wild-type littermates, Hif1a+/− mice depend more on O2 sensing mediated by chemoreceptors other than the carotid body.
Figure 3.
Effect of vagotomy on ventilatory responses. Integrated phrenic nerve activity (Phr) was measured as a function of inspired O2 in anesthetized Hif1a+/+ (A) and Hif1a+/− (C) mice (n = 6 each) before and after bilateral vagotomy (response to 12% O2 shown). Ventilatory data (RR, Phr, MNR) are expressed (mean ± SEM) relative to the values obtained while breathing 100% O2 (B, D). *, P < 0.01 (paired t test).
Loss of Carotid Body Response to Hypoxia in Hif1a+/− Mice.
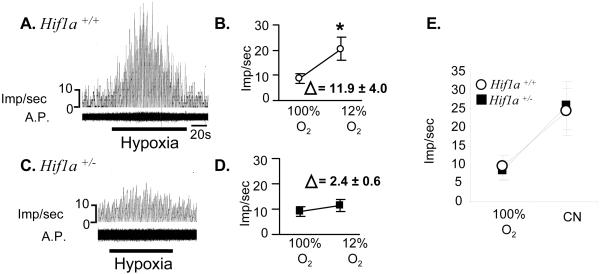
To further establish that carotid body sensitivity to hypoxia is impaired in Hif1a+/− mice, carotid bodies were isolated from Hif1a+/− and wild-type littermate mice, and carotid sinus nerve activity was recorded ex vivo. There was no significant difference between genotypes in the frequency of neural impulses recorded from carotid bodies that were superfused with 100% O2 (Fig. 4 A–D). When carotid bodies from wild-type mice were superfused with 12% O2, sinus nerve activity was markedly increased. However, in carotid bodies from Hif1a+/− mice, hypoxia-induced carotid sinus nerve activity was almost completely absent. The difference between the two genotypes with respect to the percent increase in sinus nerve activity in response to hypoxia was highly significant (P = 0.008, unpaired t test). When carotid bodies isolated from wild-type and Hif1a+/− littermate mice were exposed to 5 μg/ml of sodium cyanide, sinus nerve activity was increased to a similar degree in each genotype (Fig. 4E). Taken together, these data indicate that partial HIF-1α deficiency specifically impairs O2 sensing and/or signal transduction in the carotid body.
Figure 4.
Response of isolated carotid bodies to hypoxia. Carotid sinus nerve activity was monitored in Hif1a+/+ (A) and Hif1a+/− (C) carotid bodies exposed to 100% O2 before and after exposure to 12% O2 (hypoxia). A.P., action potentials. Mean (± SEM) impulses per second (imp/sec) are plotted and the difference (Δ) between the mean values for 100% and 12% O2 is indicated (B, D). *, P < 0.01 (paired t test). (E) Response of carotid bodies superfused with 100% O2 in the absence or presence of cyanide (CN).
Carotid Body Morphology.
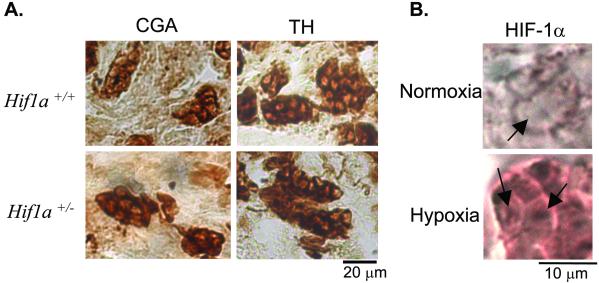
Immunohistochemistry was performed to investigate whether abnormalities in carotid body neurophysiology are associated with abnormal carotid body morphology in Hif1a+/− mice. Chromogranin A and tyrosine hydroxylase were expressed specifically within the glomus cells that mediate O2 sensing in the carotid body in both Hif1a+/+ and Hif1a+/− mice (Fig. 5A). Morphometric analysis of carotid bodies revealed no significant difference between Hif1a+/− mice and wild-type littermates with respect to the number of glomus cells per section, mean glomus cell volume, glomic volume or total volume per body, or the ratio of glomic volume to total volume (Table 4, which is published as supporting information on the PNAS web site).
Figure 5.
Immunohistochemical analysis of glomus cells. (A) Carotid body sections were stained with antibodies specific for chromogranin A (CGA) or tyrosine hydroxylase (TH). (B) Sections of carotid bodies from wild-type mice exposed to normoxia or hypoxia were subjected to immunohistochemistry by using polyclonal antibodies raised against HIF-1α. Arrows indicate increased expression of HIF-1α in the nuclei of glomus cells within carotid body from the hypoxic mouse.
Expression of HIF-1α in the Carotid Body.
To determine whether HIF-1α expression in the carotid body is induced by hypoxia, wild-type mice were exposed to 8% O2 for 45 min, anesthetized, ventilated with 8% O2, and perfused with PBS followed by 4% paraformaldehyde. Carotid body sections were analyzed by immunohistochemistry by using anti-HIF-1α antibodies. Compared with sections from normoxic mice, a marked increase in HIF-1α staining was observed in carotid bodies of hypoxic mice (Fig. 5B).
Impaired Ventilatory Adaptation to Chronic Hypoxia.
Chronic hypoxia induces ventilatory adaptation that is manifested by an augmented response to subsequent acute hypoxia. The carotid body plays a critical role in the ventilatory adaptation to chronic hypoxia (reviewed in ref. 5). Given that Hif1a+/− mice exhibit markedly reduced carotid body sensitivity to hypoxia, we hypothesized that ventilatory acclimatization to chronic hypoxia was also impaired in Hif1a+/− mice. To test this hypothesis, ventilatory responses to acute hypoxia were measured in Hif1a+/− mice and wild-type littermates either before or after a 3-day exposure to hypobaric hypoxia (0.4 atmospheres). Before hypoxic exposure, wild-type and Hif1a+/− mice manifested similar responses to acute hypoxia (percent changes are shown in Fig. 6; raw data are presented in Table 5, which is published as supporting information on the PNAS web site). Exposure of wild-type mice to chronic hypoxia resulted in an augmented ventilatory response (RR and VE) to acute hypoxia. In striking contrast, exposure of Hif1a+/− mice to chronic hypoxia was associated with a significantly diminished ventilatory response (RR and VE) to acute hypoxia. The difference between the two genotypes with respect to the change in RR in response to hypoxia was statistically significant (P = 0.04, unpaired t test). The ventilatory response of mice acutely exposed to 5% CO2 was not affected by prior chronic hypoxia in either wild-type or Hif1a+/− mice (Table 6, which is published as supporting information on the PNAS web site), again demonstrating that partial HIF-1α deficiency specifically impairs ventilatory responses to hypoxia.
Figure 6.
Effect of chronic hypoxia on ventilation. Representative tracings of VT measured as a function of inspired O2 concentration in Hif1a+/+ (A) and Hif1a+/− (B) mice before and after exposure to chronic hypoxia are shown (Upper). Ventilatory data (RR, VT, VE) are expressed (mean ± SEM) relative to the values obtained while breathing 100% O2 (Lower).
Discussion
We have demonstrated a striking absence of ventilatory adaptation to chronic hypoxia in Hif1a+/− adult mice that appears to be related to an equally striking absence of carotid body activity in response to acute hypoxia ex vivo. The observed effects are remarkable considering that these mice are only partially deficient in HIF-1α expression. Furthermore, HIF-2α, an orthologue of HIF-1α that is required for fetal catecholamine production, is also expressed in the carotid body (16). Our results indicate that HIF-1α performs unique functions in the carotid body, such that HIF-2α expression cannot compensate for reduced HIF-1α expression.
Although the carotid body is the principal mediator of the acute hypoxic ventilatory response, in Hif1a+/− mice, ventilatory responses to acute hypoxia in vivo appear to maintained via increased utilization of chemoreceptors other than the carotid body, as evidenced by the loss of these responses following vagotomy. These results are consistent with the effects of carotid sinus nerve transection, which initially abolishes acute hypoxic ventilatory responses but subsequently leads to a central reorganization of the chemoreflex pathway and recovery of the response in both rodents and large animals (14, 15, 17). Thus, in Hif1a+/− mice functional denervation of the carotid sinus nerves may lead to a similar physiological compensation.
These results provide several new insights into the control of ventilation. First, O2 sensing by carotid and noncarotid chemoreceptors appears to be controlled by distinct genetic mechanisms, with partial HIF-1α deficiency eliminating responses mediated by the former but not the latter. This conclusion is consistent with studies demonstrating distinct physiologic responses of the aortic and carotid bodies to hypoxia, hypercapnia, and carbon monoxide inhalation (18, 19). Second, carotid body chemoreceptors are essential for ventilatory responses to chronic hypoxia that are lost in Hif1a+/− mice whose carotid bodies are unresponsive to hypoxic stimulation. Third, carotid body responses to hypoxia and cyanide are controlled by distinct genetic mechanisms, with partial HIF-1α deficiency eliminating responses mediated by the former but not the latter. This difference is consistent with observations that type I cells hyperpolarize and depolarize in response to cyanide and hypoxia, respectively (reviewed in ref. 5). Thus, there is both genetic and electrophysiologic evidence indicating that the carotid body responses to cyanide and hypoxia are mechanistically distinct. Furthermore, HIF-1α expression is induced in cultured cells exposed to hypoxia but not to cyanide (20).
Expression of HIF-1α is induced by hypoxia, ischemia, or cytokine/growth factor stimulation (21). HIF-1α dimerizes with HIF-1β, which is constitutively expressed, binds to the core DNA sequence 5′-RCGTG-3′ present within hypoxia response elements of target genes, and activates their transcription. Over three dozen genes are targets for transactivation by HIF-1 (21), and several of these may be involved in O2 sensing/signal transduction by the carotid body, including endothelin-1 (22, 23) and tyrosine hydroxylase (24). Expression of endothelin-1 is induced by hypoxia in the carotid body and augments chemoreceptor responses by enhancing intracellular Ca2+ levels and stimulating proliferation of glomus cells (25, 26). However, administration of an endothelin receptor antagonist did not block ventilatory responses to hypoxia (27). Expression of tyrosine hydroxylase, the rate-limiting enzyme for catecholamine biosynthesis, is also induced by hypoxia in the carotid body (28).
In addition to the regulation of neurotransmitters, HIF-1 may also regulate more proximal steps in O2 sensing. Hypoxia inhibits K+ channel activity in glomus cells leading to membrane depolarization and increases in [Ca2+]i that trigger neurotransmitter release (5). In pulmonary artery smooth muscle cells (PASMC), hypoxia also inhibits K+ channel activity. When wild-type mice are exposed to 10% O2 for 3 weeks, there is a marked reduction in membrane potential and K+ currents in PASMC, and this response is dramatically attenuated in Hif1a+/− mice (29). A failure to down-regulate K+ channel activity may also contribute to the absence of hypoxic ventilatory adaptation in Hif1a+/− mice. Electrophysiological studies of glomus cells from wild-type and Hif1a+/− mice will be required to test this hypothesis. In PASMC, partial HIF-1α deficiency impairs both the down-regulation of K+ channel activity and development of cell hypertrophy, implicating HIF-1 in two major responses of PASMC to chronic hypoxia (29). HIF-1 may also play a multifactorial role in carotid body physiology. The results of the current study provide further evidence for the global role of HIF-1 as a master regulator of O2 homeostasis.
HIF-1 activity has been shown to decline with aging in experimental animals, which may contribute to age-related impairment of angiogenesis in ischemic tissue (30–32). Ventilatory responses to hypoxia also decline with age (33, 34), a phenomenon that is poorly characterized but is likely to be multifactorial in etiology and to affect clinical outcome in patients with disorders that result in chronic hypoxia such as congestive heart failure and chronic obstructive pulmonary disease. Whether an age-related decrease in HIF-1 activity in the carotid body contributes to this phenomenon remains to be investigated.
Supplementary Material
Acknowledgments
We are grateful to Mr. Akbar Hemadani for his assistance with carotid body histology. This work was supported by grants from the National Institutes of Health to G.L.S. (R01-HL55338) and N.R.P. (P01-HL25830) and institutional training grants supporting D.D.K. (T32-HL07887) and D.J.M. (T32-HL07534).
Abbreviations
- HIF-1
hypoxia-inducible factor 1
- VT
tidal volume
- RR
respiratory rate
- MNR
minute neural respiration
- VE
minute ventilation
- PASMC
pulmonary artery smooth muscle cells
References
- 1.Iyer N V, Kotch L E, Agani F, Leung S W, Laughner E, Wenger R H, Gassmann M, Gearhart J D, Lawler A M, Yu A Y, Semenza G L. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotch L E, Iyer N V, Laughner E, Semenza G L. Dev Biol. 1999;209:254–267. doi: 10.1006/dbio.1999.9253. [DOI] [PubMed] [Google Scholar]
- 3.Ryan H E, Lo J, Johnson R S. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu A Y, Shimoda L A, Iyer N V, Huso D L, Sun X, McWilliams R, Beaty T, Sham J S K, Wiener C M, Sylvester J T, Semenza G L. J Clin Invest. 1999;103:691–696. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prabhakar N R. J Appl Physiol. 2000;88:2287–2295. doi: 10.1152/jappl.2000.88.6.2287. [DOI] [PubMed] [Google Scholar]
- 6.Bottini P, Scionti L, Santeusanio F, Casucci G, Tantucci C. Diabetes Nutr Metab. 2000;13:165–172. [PubMed] [Google Scholar]
- 7.Kikuchi Y, Okabe S, Tamura G, Hida W, Homma M, Shirato K, Takishima T. N Engl J Med. 1994;330:1329–1334. doi: 10.1056/NEJM199405123301901. [DOI] [PubMed] [Google Scholar]
- 8.Onodera H, Okabe S, Kikuchi Y, Tsuda T, Itoyama Y. Lancet. 2000;356:739–740. doi: 10.1016/S0140-6736(00)02638-6. [DOI] [PubMed] [Google Scholar]
- 9.Buelke-Sam J, Holson J F, Bazare J J, Young J F. Lab Anim Sci. 1978;28:157–162. [PubMed] [Google Scholar]
- 10.Kline D D, Yang T, Huang P T, Prabhakar N R. J Physiol. 1998;511:273–287. doi: 10.1111/j.1469-7793.1998.273bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dejours P. Physiol Rev. 1962;42:335–358. doi: 10.1152/physrev.1962.42.3.335. [DOI] [PubMed] [Google Scholar]
- 12.Hollinshed W. Am J Physiol. 1946;147:654–660. doi: 10.1152/ajplegacy.1946.147.4.654. [DOI] [PubMed] [Google Scholar]
- 13.Howe A, Pack R J, Wise C H. J Physiol. 1981;320:309–318. doi: 10.1113/jphysiol.1981.sp013951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin-Body R L, Robson G J, Sinclair J D. J Physiol. 1986;380:61–73. doi: 10.1113/jphysiol.1986.sp016272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roux J C, Peyronnet J, Pascual O, Dalmaz Y, Pequignot J M. J Physiol. 2000;522:493–501. doi: 10.1111/j.1469-7793.2000.t01-4-00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian H, Hammer R E, Matsumoto A M, Russell D W, McKnight S L. Genes Dev. 1998;12:3320–3324. doi: 10.1101/gad.12.21.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bisgard G E, Forster H V, Klein J P. J Appl Physiol. 1980;49:964–970. doi: 10.1152/jappl.1980.49.6.964. [DOI] [PubMed] [Google Scholar]
- 18.Lahiri S, Mokashi A, Mulligan E, Nishino T. J Appl Physiol. 1981;51:55–61. doi: 10.1152/jappl.1981.51.1.55. [DOI] [PubMed] [Google Scholar]
- 19.Lahiri S, Mulligan E, Nishino T, Mokashi A, Davies R O. J Appl Physiol. 1981;50:580–586. doi: 10.1152/jappl.1981.50.3.580. [DOI] [PubMed] [Google Scholar]
- 20.Jiang B-H, Semenza G L, Bauer C, Marti H H. Am J Physiol. 1996;271:C1172–C1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 21.Semenza G L. Trends Mol Med. 2001;7:345–350. doi: 10.1016/s1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- 22.Kakinuma Y, Miyauchi T, Yuki K, Murakoshi N, Goto K, Yamaguchi I. Circulation. 2001;103:2387–2394. doi: 10.1161/01.cir.103.19.2387. [DOI] [PubMed] [Google Scholar]
- 23.Yamashita K, Discher D J, Hu J, Bishopric N H, Webster K A. J Biol Chem. 2001;276:12645–12653. doi: 10.1074/jbc.M011344200. [DOI] [PubMed] [Google Scholar]
- 24.Norris M L, Millhorn D E. J Biol Chem. 1995;270:23774–23779. doi: 10.1074/jbc.270.40.23774. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, He L, Dinger B, Fidone S. Respir Physiol. 2000;121:13–23. doi: 10.1016/s0034-5687(00)00113-4. [DOI] [PubMed] [Google Scholar]
- 26.Paciga M, Vollmer C, Nurse C. NeuroReport. 1999;10:3739–3744. doi: 10.1097/00001756-199912160-00003. [DOI] [PubMed] [Google Scholar]
- 27.McQueen D S, Dashwood M R, Cobb V J, Bond S M, Marr C G, Spyer K M. J Auton Nerv Syst. 1995;53:115–125. doi: 10.1016/0165-1838(94)00179-n. [DOI] [PubMed] [Google Scholar]
- 28.Czyzyk-Krzeska M F, Bayliss D A, Lawson E E, Millhorn D E. J Neurochem. 1992;58:1538–1546. doi: 10.1111/j.1471-4159.1992.tb11376.x. [DOI] [PubMed] [Google Scholar]
- 29.Shimoda L A, Manalo D J, Sham J S K, Semenza G L, Sylvester J T. Am J Physiol. 2001;281:L202–L208. doi: 10.1152/ajplung.2001.281.1.L202. [DOI] [PubMed] [Google Scholar]
- 30.Frenkel-Denkberg G, Gershon D, Levy A P. FEBS Lett. 1999;462:341–344. doi: 10.1016/s0014-5793(99)01552-5. [DOI] [PubMed] [Google Scholar]
- 31.Rivard A, Fabre J E, Silver M, Chen D, Murohara T, Kearney M, Magner M, Asahara T, Isner J M. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 32.Rivard A, Berthou-Soulie L, Principe N, Kearney M, Curry C, Branellec D, Semenza G L, Isner J M. J Biol Chem. 2000;275:29643–29647. doi: 10.1074/jbc.M001029200. [DOI] [PubMed] [Google Scholar]
- 33.Kronenberg R S, Drage C W. J Clin Invest. 1973;52:1812–1819. doi: 10.1172/JCI107363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson D D, Pack A I, Silage D A, Fishman A P. Am Rev Respir Dis. 1981;12:387–391. doi: 10.1164/arrd.1981.124.4.387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.