Abstract
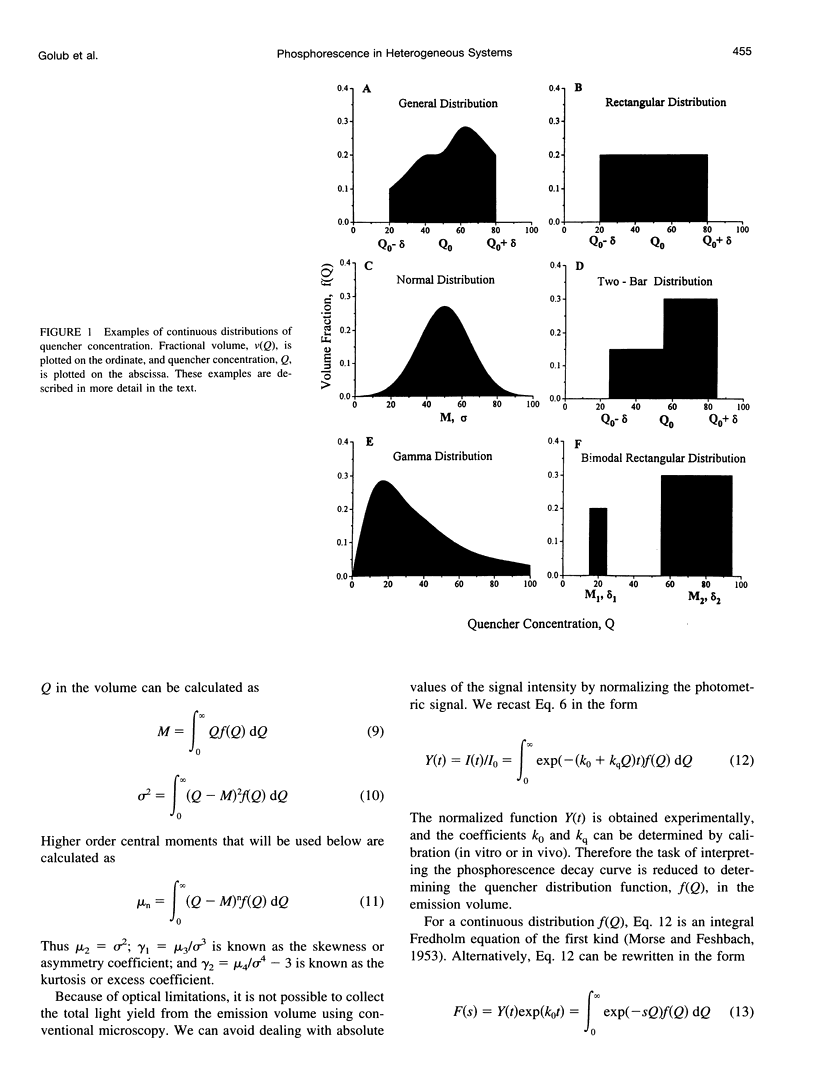
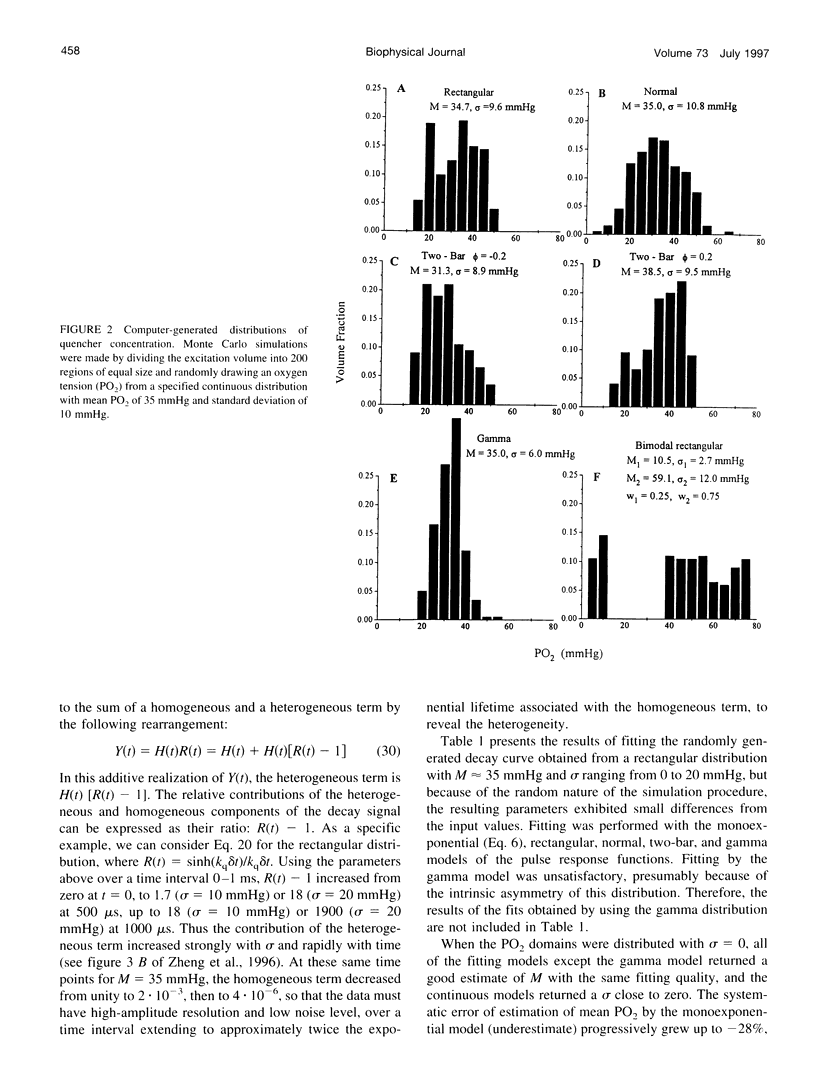
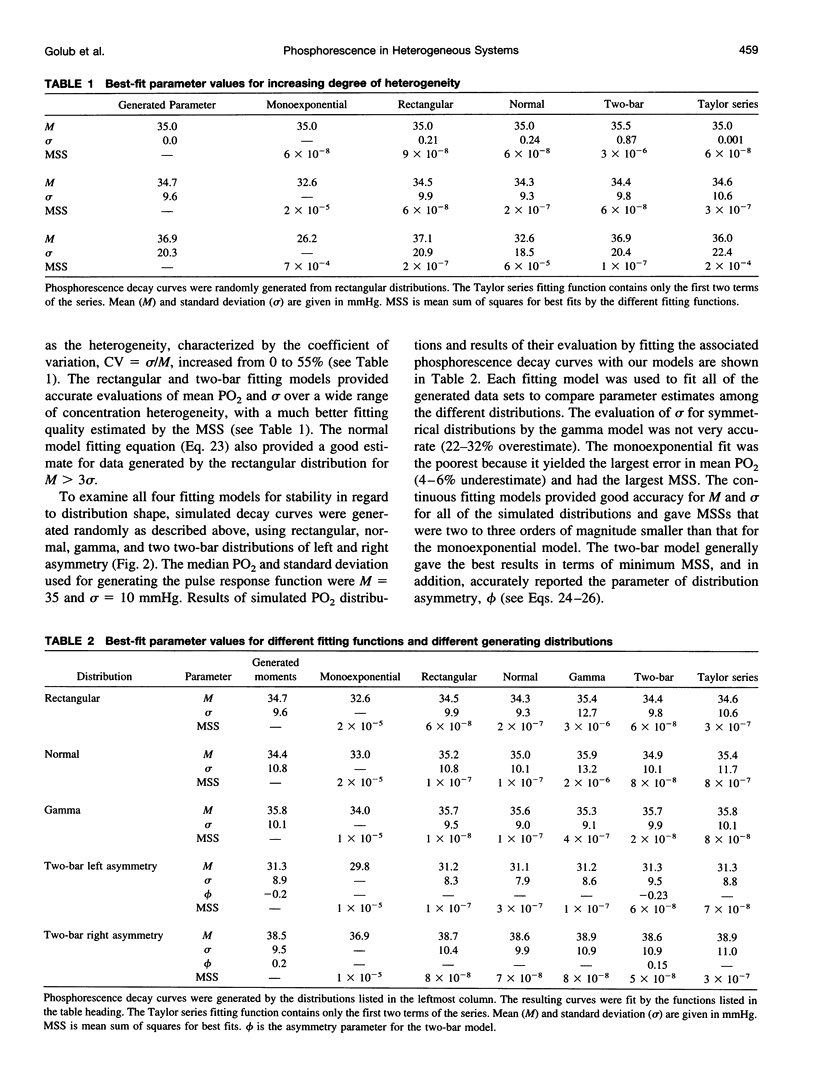
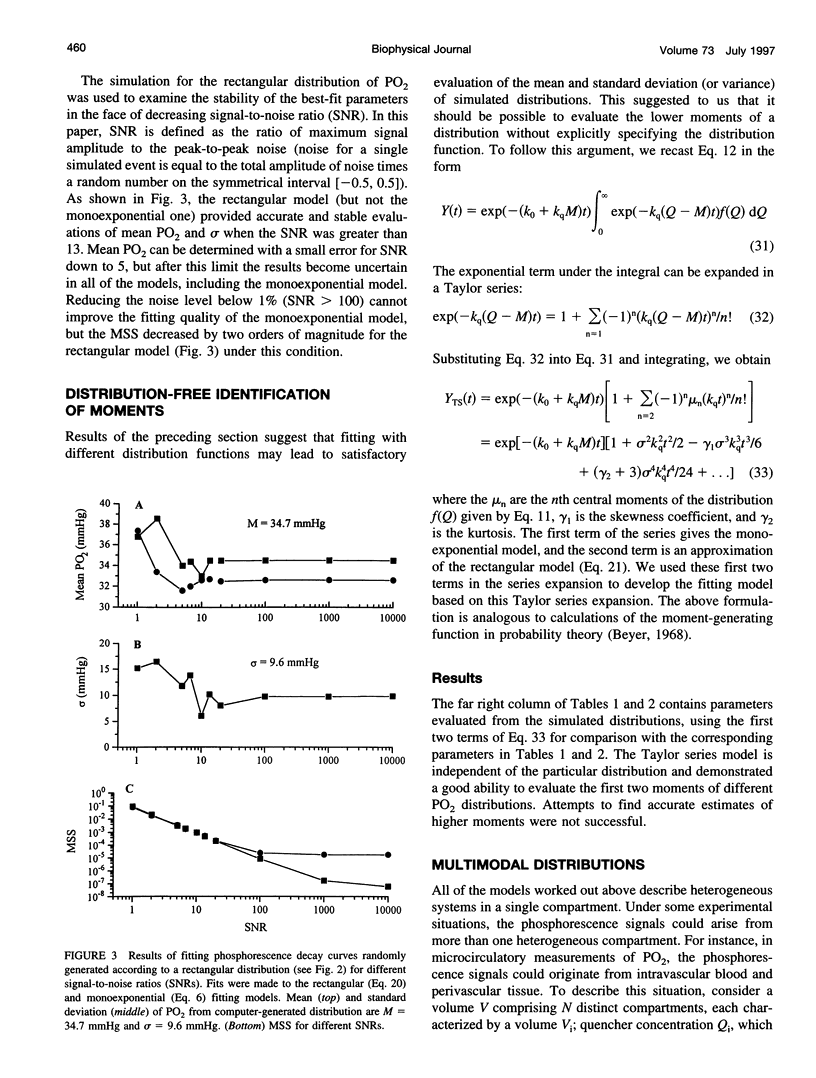
A continuous distribution approach, instead of the traditional mono- and multiexponential analysis, for determining quencher concentration in a heterogeneous system has been developed. A mathematical model of phosphorescence decay inside a volume with homogeneous concentration of phosphor and heterogeneous concentration of quencher was formulated to obtain pulse-response fitting functions for four different distributions of quencher concentration: rectangular, normal (Gaussian), gamma, and multimodal. The analysis was applied to parameter estimates of a heterogeneous distribution of oxygen tension (PO2) within a volume. Simulated phosphorescence decay data were randomly generated for different distributions and heterogeneity of PO2 inside the excitation/emission volume, consisting of 200 domains, and then fit with equations developed for the four models. Analysis using a monoexponential fit yielded a systematic error (underestimate) in mean PO2 that increased with the degree of heterogeneity. The fitting procedures based on the continuous distribution approach returned more accurate values for parameters of the generated PO2 distribution than did the monoexponential fit. The parameters of the fit (M = mean; sigma = standard deviation) were investigated as a function of signal-to-noise ratio (SNR = maximum signal amplitude/peak-to-peak noise). The best-fit parameter values were stable when SNR > or = 20. All four fitting models returned accurate values of M and sigma for different PO2 distributions. The ability of our procedures to resolve two different heterogeneous compartments was also demonstrated using a bimodal fitting model. An approximate scheme was formulated to allow calculation of the first moments of a spatial distribution of quencher without specifying the distribution. In addition, a procedure for the recovery of a histogram, representing the quencher concentration distribution, was developed and successfully tested.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcala J. R., Gratton E., Prendergast F. G. Resolvability of fluorescence lifetime distributions using phase fluorometry. Biophys J. 1987 Apr;51(4):587–596. doi: 10.1016/S0006-3495(87)83383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth M. L., Pittman R. N. Evaluation of photometric methods for quantifying convective mass transport in microvessels. Am J Physiol. 1986 Oct;251(4 Pt 2):H869–H879. doi: 10.1152/ajpheart.1986.251.4.H869. [DOI] [PubMed] [Google Scholar]
- Ellsworth M. L., Popel A. S., Pittman R. N. Assessment and impact of heterogeneities of convective oxygen transport parameters in capillaries of striated muscle: experimental and theoretical. Microvasc Res. 1988 May;35(3):341–362. doi: 10.1016/0026-2862(88)90089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander S. W., Calhoun D. B., Englander J. J. Biochemistry without oxygen. Anal Biochem. 1987 Mar;161(2):300–306. doi: 10.1016/0003-2697(87)90454-4. [DOI] [PubMed] [Google Scholar]
- Federspiel W. J., Popel A. S. A theoretical analysis of the effect of the particulate nature of blood on oxygen release in capillaries. Microvasc Res. 1986 Sep;32(2):164–189. doi: 10.1016/0026-2862(86)90052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg I., Dyson R. D. The analysis of fluorescence decay by a method of moments. Biophys J. 1969 Nov;9(11):1337–1350. doi: 10.1016/S0006-3495(69)86456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerger H., Saltzman D. J., Menger M. D., Messmer K., Intaglietta M. Systemic and subcutaneous microvascular Po2 dissociation during 4-h hemorrhagic shock in conscious hamsters. Am J Physiol. 1996 Mar;270(3 Pt 2):H827–H836. doi: 10.1152/ajpheart.1996.270.3.H827. [DOI] [PubMed] [Google Scholar]
- Kerger H., Torres Filho I. P., Rivas M., Winslow R. M., Intaglietta M. Systemic and subcutaneous microvascular oxygen tension in conscious Syrian golden hamsters. Am J Physiol. 1995 Feb;268(2 Pt 2):H802–H810. doi: 10.1152/ajpheart.1995.268.2.H802. [DOI] [PubMed] [Google Scholar]
- Kuo L., Pittman R. N. Effect of hemodilution on oxygen transport in arteriolar networks of hamster striated muscle. Am J Physiol. 1988 Feb;254(2 Pt 2):H331–H339. doi: 10.1152/ajpheart.1988.254.2.H331. [DOI] [PubMed] [Google Scholar]
- Kuo L., Pittman R. N. Influence of hemoconcentration on arteriolar oxygen transport in hamster striated muscle. Am J Physiol. 1990 Dec;259(6 Pt 2):H1694–H1702. doi: 10.1152/ajpheart.1990.259.6.H1694. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R., Laczko G., Cherek H., Gratton E., Limkeman M. Analysis of fluorescence decay kinetics from variable-frequency phase shift and modulation data. Biophys J. 1984 Oct;46(4):463–477. doi: 10.1016/S0006-3495(84)84043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey A. K., Brochon J. C. Analyzing the distribution of decay constants in pulse-fluorimetry using the maximum entropy method. Biophys J. 1987 Nov;52(5):693–706. doi: 10.1016/S0006-3495(87)83264-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky H. J., Ransnas L. A. Fitting curves to data using nonlinear regression: a practical and nonmathematical review. FASEB J. 1987 Nov;1(5):365–374. [PubMed] [Google Scholar]
- Popel A. S. Theory of oxygen transport to tissue. Crit Rev Biomed Eng. 1989;17(3):257–321. [PMC free article] [PubMed] [Google Scholar]
- Provencher S. W. A Fourier method for the analysis of exponential decay curves. Biophys J. 1976 Jan;16(1):27–41. doi: 10.1016/S0006-3495(76)85660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain D. P., Pittman R. N. Oxygen exchange in the microcirculation of hamster retractor muscle. Am J Physiol. 1989 Jan;256(1 Pt 2):H247–H255. doi: 10.1152/ajpheart.1989.256.1.H247. [DOI] [PubMed] [Google Scholar]
- Torres Filho I. P., Intaglietta M. Microvessel PO2 measurements by phosphorescence decay method. Am J Physiol. 1993 Oct;265(4 Pt 2):H1434–H1438. doi: 10.1152/ajpheart.1993.265.4.H1434. [DOI] [PubMed] [Google Scholar]
- Torres Filho I. P., Kerger H., Intaglietta M. pO2 measurements in arteriolar networks. Microvasc Res. 1996 Mar;51(2):202–212. doi: 10.1006/mvre.1996.0021. [DOI] [PubMed] [Google Scholar]
- Torres Filho I. P., Leunig M., Yuan F., Intaglietta M., Jain R. K. Noninvasive measurement of microvascular and interstitial oxygen profiles in a human tumor in SCID mice. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2081–2085. doi: 10.1073/pnas.91.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderkooi J. M., Maniara G., Green T. J., Wilson D. F. An optical method for measurement of dioxygen concentration based upon quenching of phosphorescence. J Biol Chem. 1987 Apr 25;262(12):5476–5482. [PubMed] [Google Scholar]
- Vinogradov S. A., Lo L. W., Jenkins W. T., Evans S. M., Koch C., Wilson D. F. Noninvasive imaging of the distribution in oxygen in tissue in vivo using near-infrared phosphors. Biophys J. 1996 Apr;70(4):1609–1617. doi: 10.1016/S0006-3495(96)79764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S. A., Wilson D. F. Phosphorescence lifetime analysis with a quadratic programming algorithm for determining quencher distributions in heterogeneous systems. Biophys J. 1994 Nov;67(5):2048–2059. doi: 10.1016/S0006-3495(94)80688-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. F., Pastuszko A., DiGiacomo J. E., Pawlowski M., Schneiderman R., Delivoria-Papadopoulos M. Effect of hyperventilation on oxygenation of the brain cortex of newborn piglets. J Appl Physiol (1985) 1991 Jun;70(6):2691–2696. doi: 10.1152/jappl.1991.70.6.2691. [DOI] [PubMed] [Google Scholar]
- Zheng L., Golub A. S., Pittman R. N. Determination of PO2 and its heterogeneity in single capillaries. Am J Physiol. 1996 Jul;271(1 Pt 2):H365–H372. doi: 10.1152/ajpheart.1996.271.1.H365. [DOI] [PubMed] [Google Scholar]


