Abstract
Post-traumatic stress disorder (PTSD) is a relatively common but complex mental illness with a range of diverse risk factors. Typical symptoms include the re-experience or avoidance of traumatic events, cognitive impairment, and hypervigilance. While the exact pathogenesis of PTSD is unclear, many studies indicate that epigenetic regulation plays a key role in its development. Specifically, numerous studies have indicated that the levels of histone acetylation and methylation, DNA methylation, and noncoding RNA are altered in PTSD patients. Further to this, natural products have been found to achieve epigenetic regulation of PTSD by regulating the expression of epigenetic enzymes, long noncoding RNA (lncRNA), and miRNA, thereby playing a role in improving PTSD symptoms. To date, however, no epigenetic regulation related drugs have been used in the treatment of PTSD. Furthermore, while natural products that can epigenetically regulate PTSD have received increasing levels of attention, there have not yet been any systematic reports on the topic. Here, we summarized the roles and mechanisms of natural products in the epigenetic regulation of PTSD, providing a novel and unique perspective that will help to guide the development and application of new PTSD treatments.
Keywords: DNA methylation, epigenetic regulation, histone modification, natural products, noncoding RNA, post-traumatic stress disorder
1. Introduction
Post-traumatic stress disorder (PTSD) is both a psychiatric and psychological disease that persists after individuals have experienced a severe trauma, stimulation, or death threat (Pitman et al., 2012, Schoner et al., 2017). The core symptoms are the re-experience or avoidance of traumatic events, decreased fear memory extinction, cognitive impairment, and hypervigilance (Shalev et al., 2017, Yabuki and Fukunaga, 2019), which can further result in experiences of agony or disturbance. The incidence of PTSD is also associated with an individuals’ social background, personal experience, and sex; for example, the prevalence of PTSD in women is twice that in men (Yehuda et al., 2015). More than 70 % of people worldwide have reportedly suffered from a traumatic experience (Benjet et al., 2016), and some of these people will eventually develop PTSD, and perhaps even lifetime PTSD, the prevalence of which is approximately 3.9 % (Watson, 2019).
Individuals who have personally experienced a traumatic event such as fire, war, earthquake, motor vehicle accident, sex intrusion, child maltreatment, or terrorist attack are more likely to develop PTSD (Liriano, Hatten, & Schwartz, 2019). However, people who have suffered major traumatic events indirectly or as witnesses may also be affected by these traumas, such as frontline police officers, firefighters, or those involved in earthquake rescues. Some studies have shown that in addition to the aforementioned factors, direct severe trauma can induce PTSD, and childhood adversity and psychiatric history may also increase the risk of its development (Watson, 2019). Medical comorbidities may also lead to a higher probability of developing PTSD, such as inflammation, cardiovascular disease, or coronavirus disease 2019 (COVID-19) (Edmondson and von Kanel, 2017, Hori and Kim, 2019, Tarsitani et al., 2021). Related research has shown that the risk factors for PTSD are always present, before, during, and after the trauma, such as low social status and poor social support, severity of trauma, and post-traumatic unemployment (Maes, Delmeire, Mylle, & Altamura, 2001). Moreover, some patients may choose to self-harm or commit suicide in the worst situations, which decreases quality of life for both the patients and their caregivers, thereby putting a huge burden on the social economy and those providing medical care (Watson, 2019). In summary, PTSD is complicated as it has many triggers and symptoms; furthermore, patients with PTSD may not show any symptoms if they do not experience triggering events or places, and this phenomenon increases the difficulty of developing effective treatments.
Animal and clinical studies have reported the presence of specific neural circuits, functional structures, hormones, and other neurobiological systems that are associated with the pathophysiological basis of PTSD; however, the underlying pathogenesis is still unclear. Pathophysiological abnormalities of the disease mainly include the dysregulation of the hypothalamus–pituitary–adrenal (HPA) axis, increased inflammation, abnormal release of neurotransmitters, reduced neurogenesis, abnormal apoptosis, and autophagy processes. PTSD is regarded as a very complex psychiatric disease, as its onset involves different brain areas including the peripheral and central regions, which limits our understanding of an exact cause, and thus further research is required.
Recently, PTSD was recognized as a global public health concern (Watson, 2019); making the development of effective treatments a priority for medical research. Current methods to treat PTSD include psychotherapy and pharmacotherapy, however, chemical and Chinese medicine-based therapies have also been incorporated into pharmacotherapy strategies. Numerous articles investigating the effects of chemical medicines and natural products for the treatment of PTSD have been published, as well as several studies investigating the effects of epigenetics on PTSD. Moreover, there are several related articles available on natural products that can reportedly be used to treat PTSD; however, there are no reports that natural products modulate PTSD from an epigenetic perspective. This review aims to outline the effects of natural products on epigenetic regulations in PTSD, with an emphasis on their epigenetic regulation mechanisms.
2. Role of epigenetics in PTSD
PTSD is a complicated syndrome caused by multiple factors, including related traumatic events, the external environment, and genetics. In the traditional sense, heredity refers to patient characteristics that are reflected in their offspring. At the molecular level, heredity is the transmission of DNA-encoded genetic information from parents to their offspring through meiosis (Daskalakis, Rijal, King, Huckins, & Ressler, 2018). There is also a theory of epigenetics, which was originally proposed by Waddington (Martinez Fernandez, 1959). The epigenetic mechanism describes the encoding of biochemical information obtained from an individual's experience and environment into the genomic structure of each cell, thereby affecting and regulating subsequent gene regulation and expression, cell function and phenotype, and ultimately, individual performance (Kim, Smith, Nievergelt, & Uddin, 2018). In this process, the epigenome plays an important role that cannot be ignored. The epigenome adapts to environmental influences in the form of chemical and protein modifications to the chromatin made up of DNA, proteins, and RNA (Daskalakis, Rijal, King, Huckins, & Ressler, 2018), thereby providing the genome with continuous regulatory feedback caused by environmental changes (Kim, Smith, Nievergelt, & Uddin, 2018). An epigenetic perspective states that both the environment and genes interact to regulate PTSD (Howie, Rijal, & Ressler, 2019). Furthermore, epigenetic regulations create central regulatory mechanisms in such interactions. As the name suggests, epigenetic regulation functions as environmental changes are “written” into the genome, thus mediating alterations in gene expression, rather than directly changing the coding sequence (Howie et al., 2019, Zannas et al., 2015). Epigenetics is linked to PTSD, as to a certain extent, it can mediate changes in brain function and the gene expression of related nerves caused by the external environment or trauma exposure (Zannas, Provencal, & Binder, 2015). There are three main mechanisms of epigenetic regulation: histone modification, DNA modification, and noncoding RNA (Hwang, Aromolaran, & Zukin, 2017). These three factors are not independent in the regulation of PTSD, but are interrelated and influence each other. For example, histone methylation, acetylation, DNA methylation, and non-coding RNA can all play a role in neurogenesis (Yao et al., 2016).
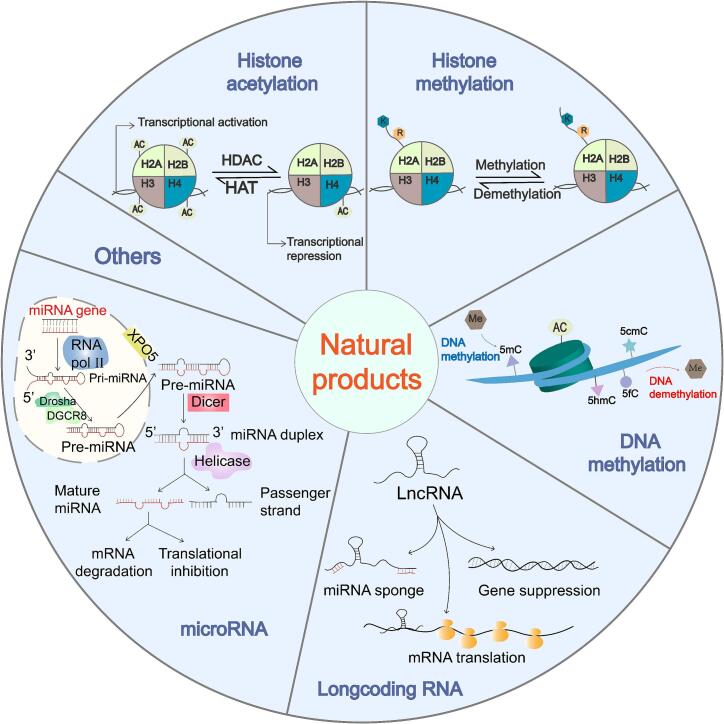
Medicinal plants are often ancient and have commonly been used as medicines for thousands of years in multiple countries (Marrelli, 2021). According to previous reports, traditional medicinal plants have been found to have more or less therapeutic or preventive effects on a variety of neurological diseases, such as neuropsychiatric and neurodegenerative diseases (Luthra and Roy, 2022, Rauf and Rahman, 2022). For example, ginseng, Withania somnifera (L.) Dunal, Bacopa monnieri (L.) Wettst, and Ginkgo biloba L. are medicinal plants that are commonly used to prevent neurological symptoms (Luthra & Roy, 2022). While natural products can be sourced from a variety of plants, animals, microorganisms, and minerals, those used for medicinal purposes are often derived from plants and are usually characterized as having a low toxicity and general benefits to human health (Gunesch et al., 2020). Medicinal plants are an important source of a wide variety of bioactive chemicals that contribute to the development of increasingly efficient multi-target drugs (Marrelli, 2021). Some natural products, such as gastrodin (GAS), are also reportedly effective at protecting nerves (Dash et al., 2021). Consequently, they are often used to treat or prevent neurological disorders such as PTSD, depression, and neurodegenerative diseases. Some studies indicate that mangiferin can exert neuroprotective effects by activating Nrf2 downstream genes (Li et al., 2020). In addition, it has also been discovered that water extracts obtained from Cymbopogon citratus (D.C.) Stapf can also alleviate the occurrence of neurological diseases (Umukoro, Ben-Azu, Ajayi, Adebesin, & Emokpae, 2020). In conclusion, the use of natural products is now in its golden age, and holds great promise for epigenetic drug development and discovery, and some natural epigenetic modulators have recently been used in clinical studies. Natural products are currently more widely used to treat cancer tumors, but this may be in part because there have been relatively few studies on neurological diseases, such as PTSD. Therefore, the use of natural products as an entry point for more in-depth research into PTSD will aid in the development of new drugs and treatments for PTSD. The epigenetic regulatory mechanisms of common natural products for the treatment of PTSD are summarized in Fig. 1.
Fig. 1.
Therapeutic mechanisms of natural products for PTSD. Natural products can exert epigenetic regulatory effects through histone acetylation, histone methylation, DNA methylation, miRNA, LncRNA, and other therapeutic mechanisms. In addition, abnormal histone acetylation or methylation levels and abnormal gene expression levels can also be rescued through the epigenetic effects of natural products, thus playing a therapeutic role.
2.1. Histone modification
Histones are basic proteins present in chromatin that bind to DNA (Huang & Bonner, 1962), and usually consist of five components, H1, H2A, H2B, H3, and H4. Histone proteins bind to DNA to form chromatin, which can then also be interpreted as a repetition unit consisting of nucleosomes, a single nucleosome consisting of a core octomer, a 147-bp length DNA fragment, and a monomer histone H1. In addition, the core octamer is composed of two H2A, H2B, H3, and H4 monomers (Luger, Mader, Richmond, Sargent, & Richmond, 1997). The nucleosomes are mainly connected by 50-bp splice DNA, the site of the connection is the monomer histone H1 on the nucleosome (Wu et al., 2022), and the nucleosomes are closely connected to form the so-called chromosome. In general, nucleosomes hinder DNA transcription, but the presence of histones can affect the conformation and activity of chromatin through post-translational modifications (Lawrence, Daujat, & Schneider, 2016), thus altering gene transcription, DNA replication, repair, recombination, and other processes (Wu et al., 2022).
Histone modifications regulate alterations in gene expression, primarily by affecting the structure of chromatin. The main mechanisms by which histones are modified are as follows: acetylation, methylation, phosphorylation, and ubiquitination (Howie et al., 2019, Strahl and Allis, 2000), SUMOylation, ADP ribosylation, and deamination (Kouzarides, 2007). Of the histone modifications listed, histone acetylation is the most widely studied, and has a basic effect on the processes of transcriptional regulation (Strahl and Allis, 2000, Struhl, 1998, Zannas et al., 2015). In addition to acetylation, there have been numerous studies on the regulation of diseases by natural products through the epigenetic mechanisms of histone methylation, but relatively few studies on histone phosphorylation and ubiquitination.
It is widely acknowledged that PTSD is caused in part by a memory association, known as memory impairment, and that histone modifications can regulate the transcription and translation processes required for memory. For example, histone acetylation represents the formation of memory (Levenson et al., 2004), and when it is blocked or inhibited, this may lead to episodic fear memory disorders and long-term memory deficits (Sillivan, Vaissiere, & Miller, 2015). Therefore, starting from the point of histone modification, it is more conducive for us to understand the pathogenesis of PTSD, and further facilitate the association of natural products that play a therapeutic role through histone modification with PTSD, to understand the treatment and prevention of PTSD from a new perspective.
2.1.1. Histone acetylation
Histone acetylation involves the addition of acetyl groups to the lysine residues at the N-terminus of histones, a process catalyzed by histone acetyltransferases (HATs). This process weakens the binding of histones to DNA, forming a decondensed chromatin structure that is more likely to facilitate transcription. In contrast, histone deacetylation is catalyzed by histone deacetylases (HDACs) and can inhibit gene expression (Graff and Tsai, 2013, Zannas et al., 2015).
The role of epigenetic regulation in neurodevelopment has been confirmed in several different studies, and the results indicate that histone acetylation is associated with memory and cognition (Peixoto and Abel, 2013, Starr, 2019). For example, increased histone H3 acetylation was found to promote long-term memory formation (Takei et al., 2011). Animal experiments have also shown that histone acetylation levels are reduced in animals with PTSD-like symptoms (Alzoubi, Al Subeh, & Khabour, 2019). One animal study that examined the HDAC2, H3K9ac, and brain-derived neurotrophic factor (BDNF) contents found the same results as in previous BDNF-related studies, in that the level of BDNF was reduced in PTSD. However, the study also found that a reduction in BDNF levels was associated with a reduction in H3K9ac expression, and this was mainly reflected by reduced acetylation of histone H3 at the BDNF promoter, leading to lower BDNF expression, and this promoted the development of PTSD (Alzoubi, Al Subeh, & Khabour, 2019). Histone acetylation levels are thought to affect synaptic plasticity, which in turn affects some behaviors associated with PTSD, such as depression-like behaviors (Sun et al., 2021). Furthermore, HDAC inhibitors have been found to enhance fear extinction and ameliorate PTSD symptoms. An experiment using rats showed that the HDAC inhibitor vorinostat, which blocked the activity of HDAC, could enhance the levels of histone acetylation in the hippocampus by increasing the expression of histones H3 and H4 at the promoter of the NR2B genes in rats with PTSD, and thus exert a certain therapeutic effect (Matsumoto et al., 2013). These results are consistent with the conclusion that histone acetylation levels are reduced in patients with PTSD (Alzoubi et al., 2019, Matsumoto et al., 2013). Some additional traits associated with histone acetylation are listed in Table 1.
Table 1.
Preclinical studies investigating role of histone acetylation in PTSD.
| Disease | Cases | Markers | Main results | Main mechanisms | References |
|---|---|---|---|---|---|
| Preclinical studies | Control (M, n = 12; F, n = 12)PTSD (M, n = 10; F, n = 10)MS3h-PTSD (M, n = 10; F, n = 10)MS6h-PTSD (M, n = 10; F, n = 10) |
Western blotting HDAC2 H3K9ac |
PTSD vs control: ↑HDAC2 protein and mRNA expression in HIP ↓H3K9ac protein in HIP |
HDAC | Sun et al., 2021 |
| SPS + DMSO (n = 10) SPS + Vorinostat (n = 10) |
Western blotting Ac-H3 Ac-H4 |
Vorinostat vs DMSO: ↑Ac-H3 and Ac-H4 in HIP |
HDAC | Matsumoto et al., 2013 | |
| Control (n = 4–5) ELS ELS + EE |
ELISA Western blotting Ac-H3K9 Ac-H4K12 |
ELS vs ELS + EE: ↓Ac-H3K9 and Ac-H4K12 in Hip ↓HAT in Hip ↑HDAC in AMY |
HAT HDAC |
Xu et al., 2022 |
Note:a ↓decreased, ↑ increased. b Abbreviations: M, male; F, female; ac, acetylation; EE, environmental enrichment; ELS, early life stress; me, methylation; MS, maternal separation.
Most natural products are extracted from medicinal plants, and they affect various diseases in different ways. For example, natural products can alleviate or worsen the occurrence or development of cancer by targeting a variety of genetic or epigenetic changes (Huang et al., 2016). Numerous reviews have shown that natural products, such as rosmarinic acid (RA), tetramethylpyrazine (TMP), oleuropein (OLE), trans-resveratrol (trans-RE), genistein (GEN), and curcumin (CUR) can regulate the occurrence of different diseases or exert therapeutic effects by affecting the levels of histone acetylation or HDAC activity.
For example, studies have shown that RA can inhibit the expression of HDAC2 in animals, while HDAC inhibition can alter apoptotic gene changes and produce pro-apoptotic effects (Jang, Hwang, & Choi, 2018). It has also been reported that RA can promote hippocampal cell proliferation and improve PTSD-like symptoms (Nie et al., 2014). It was thus hypothesized that RA may influence some hippocampal genes, such as those related to inflammation, cell proliferation or autophagy, and apoptosis, through epigenetic mechanisms such as the inhibition of HDAC, leading to changes in animal behavior. TMP and OLE also have similar inhibitory effects on HDAC as the RA. TMP is extracted from a Chinese herbal medicine called Ligusticum chuanxiong Hort., which have functions to promote neurogenesis, and specifically, the differentiation of neural stem cells into neurons during neurogenesis. It was also found that TMP may change the structure of the topoisomerase IIβ (TopIIβ) gene through its HDAC inhibition effects, increasing the acetylation of histones H3 and H4 near the TopIIβ promoter region, promoting the transcription of topoisomerase IIβ gene, and promoting neurogenesis (Yan et al., 2014). For OLE, the same is true as it inhibits HDAC and promotes histone H3H4 acetylation, thus reversing the memory and cognitive impairment caused by HDAC overexpression due to disease (Luccarini et al., 2015). For histone deacetylases, trans-RE also has a regulatory effect. Resveratrol exists in both formal and trans-isomer forms, of which only the latter shows biological activity, primarily as an anti-inflammatory, antioxidant, and antitumor agent (Dutt, Gupta, Dabur, Injeti, & Mittal, 2015). Studies have found that trans-RE can downregulate the expression of proinflammatory factors, such as interleukin-1β and interleukin-6 by increasing the activity of sirtuin 1 (SIRT1), and can also exert anti-inflammatory and neuroprotective effects by regulating the expression of miRNA (Dutt et al., 2015, Isac et al., 2017).
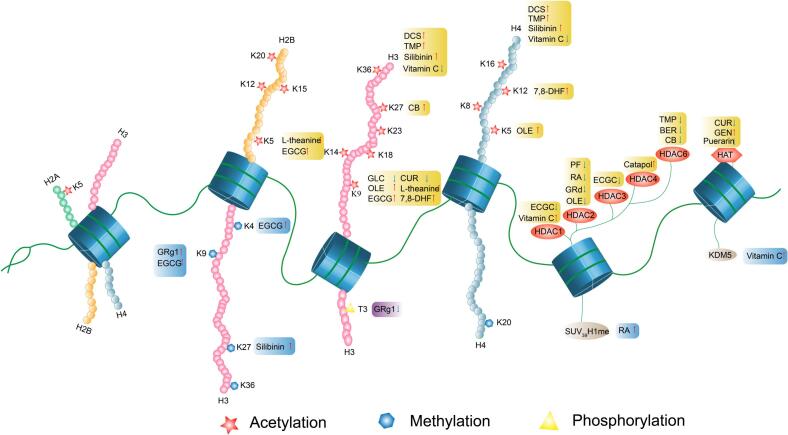
In addition to natural products acting on HDAC, GEN and CUR can also regulate histone acetylation levels by altering the expression of HAT (Majid et al., 2008, Yan et al., 2017). For example, it was found that when GEN was used to treat tumors, it could induce the transcriptional activation of tumor suppressor genes and change gene expression by upregulating HAT, and then catalyze the acetylation of histones H3 and H4 near the transcription start sites of p21, p16, and other tumor suppressors, thus achieving certain anti-tumor effects (Majid et al., 2008). In contrast, CUR, as a natural HAT inhibitor, could reduce H3K9 acetylation by inhibiting the expression of HAT, and then downregulating the expression of apoptosis-related genes, such as Caspase-3, to promote apoptosis and maintain the normal operation of organs and tissues (Yan et al., 2017). Combined with the epigenetic regulation mechanisms of the natural products mentioned above, we have hypothesized that changing the histone acetylation levels of certain gene loci may alleviate the symptoms of PTSD patients to some extent in the PTSD model. Hence, natural products with HDAC and HAT regulation may improve PTSD-like symptoms and provide a new safe option for future patient treatments. The histone acetylation sites of natural products are summarized in Fig. 2, and some common natural products that may be used to treat PTSD by regulating histone acetylation are summarized in Table 2.
Fig. 2.
Effects of various natural products on histone acetylation and methylation in PTSD. Histone modification mainly includes histone acetylation, methylation and phosphorylation. Firstly, for histone acetylation, the modification site is mainly at the relatively conserved lysine site of the N terminal of histone, and the process is mainly coordinated by histone acetyltransferase and histone deacetylase. Secondly, the main modification sites of histone methylation are arginine and lysine. In addition, different modifications at different sites also play different effects on transcription and gene expression. Moreover, natural products can exert therapeutic effects by acting on different sites. For example, EGCG can up-regulate the methylation level of H3K27 site and also up-regulate the acetylation level of H3K9 site. In short, different natural products can play different regulatory roles by acting on different sites.
Table 2.
Common natural products that can be used to regulate histone acetylation in PTSD.
| Chemicals | Main results | References |
|---|---|---|
| RA | ↓HDAC2 | Jang, Hwang, & Choi, 2018 |
| Catalpol | ↑HDAC4 ↑SIRT1 |
Zou, Zhong, Wu, Wang, & Liu, 2019 |
| GRd | ↓HDAC2 | Wan et al., 2017 |
| PF | ↓HDAC2 | Liu et al., 2022, Zhang et al., 2018 |
| trans-RE | ↑SIRT1 | Dutt et al., 2015 |
| CUR | ↓HAT ↓H3K9ac |
Montagud-Romero et al., 2016 |
| Puerarin | ↓HAT | Han et al., 2015 |
| EGCG | ↑H3K9/14ac and H3ac ↓HDAC1 and HDAC3 |
Ciesielski et al., 2020, Pan et al., 2017 |
| Silibinin | ↓HDAC activity ↑H3ac and H4ac |
Mateen et al., 2012 |
| GEN | ↑HAT expression | Li, Liu, Andrews, & Tollefsbol, 2009 |
| OLE | ↑H3K9 and H4K5 ↓HDAC2 |
Luccarini et al., 2015 |
| 7,8-DHF | ↑H3K9ac and H4K12ac | Zeng et al., 2011 |
| GLC | ↓H3K9ac | Thakur, Sadanandan, & Chattopadhyay, 2020 |
| Vitamin C | ↓H4ac and H3ac ↑HDAC1 |
Mustafi et al., 2019 |
| TMP | ↑H3ac and H4ac ↓HDAC |
Yan et al., 2014 |
| L-theanine | ↑H3K9ac and H3K14ac | Kikuchi et al., 2022 |
| BER | ↓HDAC | Kalaiarasi et al., 2016 |
| CB | ↑H3K27ac ↓HDAC6 |
Scherma et al., 2020 |
| DCS | ↑H3ac and H4ac | Wang et al., 2012 |
Note: a ↓ decreased, ↑ increased. b Abbreviations: BER, berberine; CB, cannabinoids; CUR, curcumin; DCS: D-Cycloserine, EGCG, epigallocatechin gallate; GEN, genistein; GLC, glycyrrhizin; GRb1, ginsenoside Rb1; GRd, ginsenoside Rd; HAT, histone acetyltransferase; HDAC, histone deacetylase; OLE, oleuropein; PF, paeoniflorin; RA, rosmarinic acid; SIRT1, sirtuin 1; TMP, tetramethylpyrazine; trans-RE: trans-resveratrol. 7,8-DHF, 7,8-dihydroxyflavone.
2.1.2. Histone methylation
Histone methylation, which were first discovered in the 1960s (Murray, 1964), is another epigenetic regulatory mechanism and is distinct from histone acetylation. Histone methylation can occur at different sites on the histones, but the main sites of action include lysine residues and arginine residues (Jambhekar, Dhall, & Shi, 2019). The methylation process requires the assistance of histone methyltransferases (HMTs) such as lysine methyltransferases (KMTs) or protein arginine methyltransferases (PRMTs) to transfer methyl groups to the lysine or arginine residues of histones (Howie et al., 2019, Li et al., 2022). When histone amino residues bind to methyl groups, chromatin relaxes, which facilitates gene expression. Conversely, histone demethylation inhibits transcription, a process catalyzed by histone demethylases (HMDs). However, different methylation and demethylation sites have different functions. For example, H3K4me3 serves as a marker for promoting transcription (Barski et al., 2007), while H3K9me3 represents gene silencing and heterochromatin (Bannister et al., 2001, Barski et al., 2007). Hence histone methylation is a complicated process. Moreover, it plays an imperative role in the development and treatment of psychiatric diseases (Li et al., 2019, Peter and Akbarian, 2011). Histone methylation alters BDNF levels, thereby changing brain function and neuronal development. A study observed a trend of increasing BDNF with a decrease in H3K9me2 following the administration of Unc0642, which was found to work by inhibiting an enzyme called EHMT2 (histone methyltransferase). The process promoted the transcription and expression of BDNF-related mRNA and proteins by reducing H3K9me2 at the BDNF promoter in the hippocampus and prefrontal cortex (Zhao et al., 2020). However, histone methylation was also connected with inflammation. For instance, the association among histone methylation, wingless (WNT) signaling, and inflammatory cytokines such as interleukins and interferons has been demonstrated in a series of preclinical experiments, and studies have found that the addition of H3K9me3, H3K4me3, and H3K36me3 expression led to the elevation of IL-12, further augmenting inflammation in patients with PTSD (Bam et al., 2020, Bam et al., 2016). Specifically, the overexpression of wingless 10b (WNT10B) is associated with the high expression of histone H3K4me3 around the WNT10B promoter. That is, WNT signaling regulates histone methylation by affecting HMD, which in turn regulates the expression of inflammatory cytokine genes, such as interferon-γ (INF-γ) and IL-12, and results in the level of inflammation detected in people with PTSD (Bam et al., 2020). Overall, understanding histone methylation will contribute to a deeper understanding of the pathogenesis of PTSD.
Current research on PTSD and histone modifications has predominantly focused on the above two protein modification methods, and data relating to histone phosphorylation and ubiquitination is currently lacking. However, epigenetic studies on PTSD are still rare, and most attention has been focused on inflammation and BDNF in both preclinical and clinical trials. Moreover, data has shown that histone modification-related enzymes, such as HDAC or HMT, always play a major role during the process of histone modification. Many drugs or natural products may affect discrepant modification sites by regulating different enzymes (Bam et al., 2020, Zhao et al., 2020). In conclusion, only BDNF and inflammation were involved in the above two aspects, yet we can speculate that multiple pathophysiologies involved in the pathogenesis of PTSD may be regulated by epigenetic mechanisms. Furthermore, the specific regulatory mechanisms are not yet fully understood. The histone methylation sites of natural products are summarized in Fig. 2. A summary of current preclinical and clinical trials associated with histone methylation is provided in Table 3.
Table 3.
Preclinical and clinical studies investigating role of histone methylation in PTSD.
| Diseases | Cases | Markers | Main results | Main mechanisms | References |
|---|---|---|---|---|---|
| Preclinical studies | Adolescent control (n = 12)Adult control (n = 12)Adolescent PTSD (n = 12)Adult PTSD (n = 12) Adolescent PTSD + Unc0642 (n = 12) Adult PTSD + Unc0642 (n = 12) |
Western blotting H3K9me2 |
PTSD vs Control: ↑H3K9me2 expression in HIP and PFC |
EHMT2 | Zhao et al., 2020 |
| Clinical studies | Control (n = 6)PTSD (n = 6) |
H3K4me3 | PTSD vs Control: ↑H3K4me3 around promoter region of WNT10B, WNT10A, WNT7A, DVL1 and TCF7 |
HMD | Bam et al., 2020 |
| Control (n = 17)PTSD (n = 16) |
ChIP-seq H3K4me3 H3K27me3 H3K36me3 H3K9me3 |
PTSD vs Control: ↑H3K4me3, H3K36me3 and H3K9me3 ↔H3K27me3 |
KDM5B | Bam et al., 2016 |
Note: a ↓ decreased, ↑ increased, ↔ unchanged. b Abbreviations: ac, acetylation; ChIP-seq, chromatin immunoprecipitation sequence; DMSO, dimethyl sulfoxide; EE, environmental enrichment; ELS, early life stress; me, methylation; KDM5B, lysine demethylase 5B; MS, maternal separation.
Although most of the relevant studies on histone methylation are related to cancer, there are some reports on the role of histone methylation in neuropathic diseases, including learning, memory, and neuroinflammation. A large number of animal experiments and clinical studies have shown that histone methylation levels in PTSD patients show an upward trend, and the enhanced methylation of some histones is associated with BDNF and inflammation levels (Bam et al., 2016, Zhao et al., 2020). By reviewing the abundance of available literature, some natural products were also found to affect the methylation levels of histones, such as silibinin, epigallocatechin gallate (EGCG), ginsenoside g1 (GRg1), and extracted vitamin C. For example, silibinin and EGCG are both HDAC inhibitors, but both also regulate histone methylation. Silibinin can increase the level of H3K27me3, which has an inhibitory effect on transcription, and EGCG can increase the methylation level of several sites, including H3K4me3 (promoting transcription) and H3K9me3 (inhibiting transcription), and extracted vitamin C, which is discussed later, has a similar effect (Anestopoulos et al., 2016, Ciesielski et al., 2020, Coker et al., 2022). Silibinin can not only interfere with histone modifications, but also improves DNA methyltransferase (DNMT) activity. Linking the effects that silibinin has on anxiety and depression and reducing fear behavior with its effects on increasing H3K27me3, we have proposed that it may inhibit transcription by increasing H3K27 trimethylation at promoters or other loci of genes related to PTSD symptoms, and thus improve disease symptoms (Anestopoulos et al., 2016). Similarly, some studies suggested that EGCG alleviated PTSD mainly by inhibiting neuroinflammation. EGCG can affect the methylation of multiple histone sites, among which H3K9me3 is a transcriptional repressor. Therefore, EGCG may increase the histone H3K9me3 of the inflammatory cytokine gene, and then suppress the expression of inflammatory cytokines, producing anti-inflammatory effects and alleviating PTSD symptoms (Ciesielski et al., 2020). However, the connection between the two has not yet been fully elucidated.
GRg1 also promotes H3K9 methylation, and can regulate noncoding RNA (Kwok et al., 2017). Extracted vitamin C was also found to have epigenetic regulatory effects, such as increasing lysine demethylases (KDM) activity and mediating histone demethylation (Coker, Smith-Diaz, Dyson, Vissers, & Berry, 2022). However, there is limited information available regarding their regulatory effects on histone methylation, and their precise mechanisms must be elucidated in future studies.
Furthermore, we have found that some natural products can also affect BDNF levels and related inflammatory factors in PTSD patients; for example, GRg1 can increase BDNF while EGCG can reduce interleukin 1β (IL-1β) and tumor necrosis factor-α (TNF-α) in the hippocampus (Lee et al., 2016, Lee et al., 2018). However, the exact histone modification mechanisms of these natural products in relation to PTSD is not well understood, and further research is required to address this. Other types of histone modification, such as phosphorylation and ubiquitination also require further investigation. The histone methylation sites of several common natural products and the different site modifications are described in Fig. 2. In addition, this review has provided a summary of natural products that can regulate histone changes through epigenetic regulation, such as histone methylation in Table 4.
Table 4.
Common natural products that can regulate histone methylation.
| Chemicals | Epigenetic regulation | References |
|---|---|---|
| RA | ↑SUV39H1 | Lee, Park, & Han, 2021 |
| GRg1 | ↑H3K9me | Gao et al., 2020 |
| EGCG | ↑H3K4me3 and H3K9me3 | Ciesielski et al., 2020 |
| Silibinin | ↑H3K27me3 | Anestopoulos et al., 2016 |
| Vitamin C | ↑KDM5B | Coker, Smith-Diaz, Dyson, Vissers, & Berry, 2022 |
Note: a ↓ decreased, ↑ increased. b Abbreviations: EGCG, epigallocatechin gallate; GRg1, ginsenoside Rg1; RA, rosmarinic acid.
2.2. DNA methylation
In recent decades, epigenetic mechanisms have received increasing attention in relation to neurological diseases. In addition, recent studies have shown that DNA methylation is associated with the development of diseases and clinical symptoms. Due to its reversible nature, DNA methylation has become a hot and valuable target for therapeutics or interventions (Jones, Ohtani, Chakravarthy, & De Carvalho, 2019). DNA methylation is a kind of epigenetic mark that directly modifies DNA (Younesian, Yousefi, Momeny, Ghaffari, & Bashash, 2022), and this process is mainly involved in gene expression via the recruitment of proteins that participate in the inhibition of DNA binding with transcription factors and the inhibition of genes to further regulate gene function, thus affecting normal neurodevelopment (Moore, Le, & Fan, 2013). DNA methylation is the process of adding a methyl group to the C5 position of cytosine, which is mediated by DNA methyltransferases (DNMTs) and DNA demethylases, and is associated with gene silencing (Morrison, Miller, Logue, Assef, & Wolf, 2019).
DNA methylation, in addition to being widely studied in relation to cancer, has also been a focus of neurological research, including PTSD. A previous study found that differential expression of methylation in some regions can be detected in PTSD. For example, a clinical study found two differentially methylated regions (DMR) in PTSD, HLA-APB1 and SPATC1L, and the degree of methylation in these regions could be used to assess the risk of PTSD (Katrinli et al., 2021). In addition to the above DMRs, the methylation level of some loci was found to be abnormal in PTSD. For example, spindle and kinetochore-associated protein 2 (SKA2) methylation is often used as a marker for assessing suicide risk. An analysis of blood samples from more than 200 veterans found that the methylation levels of the SKA2 gene affect the thickness of the brain's prefrontal cortex and correlate with the severity of PTSD symptoms (Sadeh et al., 2016). These data provide a basis by which to evaluate the severity of PTSD.
Numerous studies have also been conducted to determine which PTSD symptoms can be affected by DNA methylation, and a reciprocal relationship between DNA methylation and cognition and inflammation was identified. For example, klotho (KL) methylation has been used as a predictor of inflammation. By measuring the KL DNA methylation level in PTSD patients, it was found that the more severe the PTSD symptoms, the higher the DNA methylation level and the lower the KL gene expression. Conversely, the levels of related inflammatory markers such as C-reactive protein (CRP) tended to increase (Wolf et al., 2020). This, in turn, leads to aggravated inflammation, and the patient’s cognition is also damaged to a certain extent. In addition to KL gene methylation being associated with inflammation, absent in melanoma 2 (AIM2) gene methylation also affects inflammation, and Mc et al. (Mc et al., 2021) and Hawn et al. (Hawn et al., 2022) found that AIM2 gene methylation could affect the content of CRP and other inflammatory factors in vivo, and indicated that there was a certain relationship between PTSD and AIM2 methylation. In addition, DNA methylation is known to be active in the development and maturation of the central nervous system, so external stimuli or stress may induce changes in gene methylation in the central nervous system. Among them, BDNF is common and is associated with cognitive impairment in patients. Abnormal methylation of the hippocampal BDNF gene is also found to be a major cause of hippocampal dysfunction in PTSD patients, as DNA methylation of the BDNF gene can reduce BDNF expression, resulting in cognitive deficits in patients (Roth, Zoladz, Sweatt, & Diamond, 2011). Apart from the previously described association of DNA methylation with PTSD symptoms, such as cognitive impairment and increased inflammation, DNA methylation and DNA demethylation have also been connected with fear extinction, and this correlation is also related to the activation of neurons in various regions of the brain (Shan, Guo, van den Heuvel, van Heerikhuize, & Homberg, 2018). As for DNA methylation and demethylation, previous studies have shown that abnormal methylation and methylation-related enzymes such as DNMT often cause deficient fear memory and impaired fear extinction. For example, when DNMT is inhibited, the decrease of 5-mc expression can promote high expression levels of related genes, and then interfere with the consolidation phase of fear memory (Shan, Guo, van den Heuvel, van Heerikhuize, & Homberg, 2018). Although impaired fear extinction is not completely regulated by DNA methylation/demethylation, it can be concluded that they influence fear behavior in PTSD mice to some extent. The remaining experiments are summarized in Table 5.
Table 5.
Preclinical and clinical studies investigating role of DNA methylation in PTSD.
| Diseases | Cases | Markers | Main results | Main mechanisms | References |
|---|---|---|---|---|---|
| Preclinical studies |
Control (n = 10) Susceptibility (n = 7–16) Resilient (n = 7–16) |
Western blotting DNMT3a |
Susceptibility vs Resilient: ↓DNMT3a protein and mRNA expression in NAc |
DNMT | Warhaftig et al., 2021 |
| Extinction: 5-HTT-/-; 5-HTT+/+ (n = 8) No-extinction: 5-HTT-/-; 5-HTT+/+ (n = 8) |
Immunofluorescence 5-mC 5-hmC |
Extinction vs No-extinction: ↓5-hmC in BLA and CA in 5-HTT-/- rats ↔5-hmC in BLA and CA in 5-HTT+/+ rats ↔5-mC in 5-HTT+/+ and 5-HTT-/- rats |
DNMT TET |
Shan, Guo, van den Heuvel, van Heerikhuize, & Homberg, 2018 | |
| Stress (n = 10) No stress (n = 10) |
BSP BDNF DNA methylation |
Stress vs no stress:↓BDNF exon IV DNA methylation in ventral CA3 ↑BDNF exon IV DNA methylation in dorsal DG and dorsal CA1 ↔BDNF exon IV DNA methylation in dorsal CA3, ventral CA1 and DG |
DNMT | Roth, Zoladz, Sweatt, & Diamond, 2011 | |
| Clinical studies | PTSD (n = 199) | SKA2 | ↑SKA2 DNA methylation with the increasing of PTSD severity | DNMT | Sadeh et al., 2016 |
| PTSD (n = 309) | KL | ↑KL DNA methylation with the increasing of PTSD severity | DNMT | Wolf et al., 2020 | |
| Control (n = 47)PTSD (n = 47) |
AIM2 | PTSD vs Control: ↓AIM2 methylation ↑AIM2 gene expression |
DNMT | Mc et al., 2021 | |
| Control (n = 23) PTSD + tf-CBT with EMDR (n = 23) PTSD + tf-CBT without EMDR (n = 11) |
ZFP57 | EMDR vs no EMDR ↑ZFP57 methylation |
DNMT | Vinkers et al., 2021 |
Note: a ↓ decreased, ↑ increased, ↔ unchanged. b Abbreviations: AIM2, absent in melanoma 2; BSP, bisulfite sequencing PCR; EMDR, eye movement desensitization reprocessing; NAc, nucleus accumbens; KL, klotho; SKA2, spindle and kinetochore-associated protein 2; TET, translocation methylcytosine dioxygenases; tf-CBT, trauma-focused cognitive behavioral therapy; ZFP57, zinc-finger protein 57; 5-HTT+/+, serotonin transporter no-knockout; 5-HTT-/-, serotonin transporter knockout; 5-mC, 5-methylcytosine; 5-hmC, 5-hydroxymethylcytosine.
In summary, genes with abnormal DNA methylation were found to possibly play a role in the development of PTSD, but whether these genes directly contribute to the onset or persistence of PTSD is not currently known. However, it is promising that we can understand the pathogenesis and related pathological changes of PTSD from the perspective of epigenetics. In addition, the effects of DNA methylation on PTSD are multifaceted, and the effects of DNA methylation may be passed on from parents to offspring. Therefore, further molecular studies of PTSD will help to elucidate the pathogenesis of PTSD.
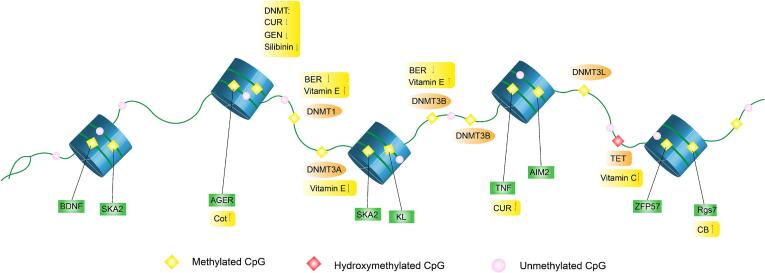
In addition to histone modifications, numerous lines of evidence support the important role of DNA methylation in PTSD, which can be regulated by DNA methylation-related enzymes. The modification sites for DNA methylation in several common natural products and their different modifications are summarized in Fig. 3. The role of natural products in PTSD from the perspective of DNA methylation was reviewed. While some natural products were found to interfere with DNA methylation levels, their effects on PTSD have not yet been reported. Here, a summary of the natural products that can be used to treat PTSD and the changes in the epigenetic regulations is provided. Epigenetic regulations in PTSD affect the immune system as well as the stress response system (Morrison, Miller, Logue, Assef, & Wolf, 2019). Lycopene, EGCG, extracted vitamin C, vitamin E, and curcumin are known to improve PTSD symptoms by regulating DNA methylation. For example, lycopene, an extract with anti-inflammation and anti-oxidation effects, has been implicated in the pathogenesis of various diseases such as PTSD, and it can thus be used to treat a diverse array of disorders. Studies have also found that in addition to enhancing the level of DNA methylation (King-Batoon, Leszczynska, & Klein, 2008), lycopene can also reduce the expression of inflammatory factors, such as interleukin 6 (IL-6), IL-1β, and TNF-α (Li et al., 2020). In this process, lycopene may regulate DNA methylation-related enzymes, and induce changes in a series of inflammatory factors, thereby improving the behaviors associated with PTSD to a certain extent.
Fig. 3.
Effects of various natural products on DNA methylation in PTSD. DNA methylation occurs under the action of DNA methyltransferase, and DNA methylation has multiple sites of action. Reports related to PTSD have shown that BDNF, TNF, AIM2, SKA2, KL and other gene loci have different levels of DNA methylation. In addition, natural products can regulate methylation levels at different sites by affecting the expression of DNA methyltransferase, which in turn affects the expression of related factors such as BDNF or TNF in PTSD patients.
EGCG can also regulate DNA methylation, mostly by inhibiting DNMT. Further to this, it can inhibit the activity of dihydrofolate reductase, and then inhibit DNA synthesis to alter DNA methylation (Navarro-Peran et al., 2005). In conclusion, the epigenetic regulation mechanism of EGCG in the treatment of PTSD is not only limited to histone modifications but also involved DNA methylation and noncoding RNA. In addition, extracted vitamin C has also been used in the treatment of PTSD. Studies have shown that in addition to increasing the activity of KDM, extracted vitamin C can also increase translocation methylcytosine dioxygenases (TET), mediate the demethylation process, promote transcription, catalyze the oxidative removal of methyl groups of histone lysine residues, and maintain the normal progression of development (Coker, Smith-Diaz, Dyson, Vissers, & Berry, 2022). A reduction in oxidative stress in the hippocampus was also shown to prevent memory impairment in PTSD mice. In conclusion, the effects of vitamin C on reducing oxidative stress in PTSD may be related to its effect on epigenetic enzymes, but further verification is required. Vitamin E can also act as an epigenetic regulator to interfere with oxidative stress-induced disease. For example, vitamin E in obesity can regulate the methylation status of the DNA repair gene MLH1 and control its expression by upregulating DNMT1 expression, thereby reducing DNA damage (Remely et al., 2017). In a tumor-related study, BER was also found to inhibit DNMT1 and DNMT3B. For example, curcumin, extracted from turmeric, has a major effect on the aging process, and can significantly improve the process of memory formation in aging animals (Flores, 2017). Furthermore, there is evidence that curcumin has a role in impairing fear memory consolidation and reconsolidation processes in PTSD (Monsey et al., 2015). Curcumin can regulate several different pathways, including DNMT inhibition, HAT, and HDAC activity regulation and miRNA expression regulation, and it also plays a role in gene expression modification (Guo et al., 2018, Momtazi et al., 2016, Montagud-Romero et al., 2016, Yan et al., 2017). Furthermore, curcumin has anti-inflammatory effects and can reduce the expression of several genes regulated by NF-κB, such as interleukin and TNF. Hence, it is possible that curcumin treatments for PTSD may work through one or more of these pathways, which need to be further elucidated. Other natural products capable of regulating the onset or progression of disease by regulating DNA methylation levels are summarized in Table 6. Similar with the histone modifications, the mechanisms of DNA methylation in response to natural products in PTSD remains unclear.
Table 6.
Common natural products that can regulate DNA methylation.
| Chemicals | Epigenetic regulation | References |
|---|---|---|
| CUR | ↑TNF DNA methylation ↓DNMT |
Guo et al., 2018, Yan et al., 2017 |
| HSD | ↓DNA methylation | Fernandez-Bedmar et al., 2017 |
| Lycopene | ↑DNA demethylation | King-Batoon, Leszczynska, & Klein, 2008 |
| Silibinin | ↓DNMT | Anestopoulos et al., 2016 |
| GEN | ↓DNMT | Li, Liu, Andrews, & Tollefsbol, 2009 |
| Vitamin E | ↑DNMT1 and DNMT3B ↓DNMT3A |
Coupland et al., 2014 |
| BER | ↓DNMT1 and DNMT3B | Liu, Meng, Wu, Qiu, & Luo, 2019 |
| Vitamin C | ↑TET | Coker, Smith-Diaz, Dyson, Vissers, & Berry, 2022 |
| CB | ↑Rgs7 DNA methylation | Tomas-Roig et al., 2017 |
| Cot | ↑AGER DNA methylation | Fuemmeler et al., 2021 |
Note: a ↓ decreased, ↑ increased. b Abbreviations: BER, berberine; CB, cannabinoids; Cot, cotinine; CUR, curcumin; DNMT, DNA methyltransferase; GEN, genistein; HSD, hesperidin; TET, Ten-eleven translocation.
2.3. Noncoding RNA
Noncoding RNAs (ncRNAs) are the products of DNA transcription, and ncRNAs are different from other RNAs as they are not translated into proteins to participate in physiological processes. However, ncRNAs, are functional RNAs, as they are involved in processes such as RNA processing and regulation (Howie et al., 2019, Schmidt et al., 2015). There are a huge number of ncRNA genes, including RNAs with relatively small molecular weights, such as microRNAs (miRNAs), piwi-interaction RNAs (pi RNAs), small interfering RNAs (siRNAs), small nuclear RNAs (snRNAs), and long noncoding RNAs (lncRNAs) with relatively large molecular weights and retrotransposons (Daskalakis et al., 2018, Eddy, 1999, Howie et al., 2019). miRNAs usually work by inhibiting the expression of post-transcriptional genes (Mendell & Olson, 2012). In addition, long noncoding RNAs mainly bind to chromatin modification proteins and are involved in altering chromatin states and influencing subsequent gene expression. This type of RNA is often an ideal drug target as it can be used to achieve time and space specificity (Sandberg, Samson, & Ji, 2013). Previous studies of noncoding RNAs in psychiatric diseases have focused more on miRNAs, lncRNAs, and retrotransposons in relation to PTSD and these are often used as biomarkers.
Approximately 70 % of miRNAs are expressed in the human nervous system (Cao, Yeo, Muotri, Kuwabara, & Gage, 2006), and they can regulate RNA stability and RNA translation, mainly by inhibiting gene expression (Daskalakis, Provost, Hunter, & Guffanti, 2018). It is thus possible that miRNAs may affect the development of multiple brain regions. In addition, there is increasing evidence that abnormal miRNA expression may lead to a series of neurological diseases, and they may also play a critical role in neurodevelopment or other functions. For example, the dysregulation of miRNAs in BDNF and FKBP5 genes can affect PTSD susceptibility and resilience (Maurel et al., 2021).
According to clinical and animal studies, multiple systems, such as PTSD patients are affected to some extent in patient systems. The effect of miRNAs on the immune system has been implicated in the development of PTSD. For instance, the downregulation of miRNAs may cause changes in the expression levels of related inflammatory factors. A preclinical experiment that dissected the relationship between the level of miR-142 and inflammatory factors found that inhibiting the expression of miR-142 could reduce the level of inflammatory cytokines such as IL-6, IL-1β, and TNF-α. They also suggested that lower miR-142 levels could improve PTSD-like symptoms in rats (Nie et al., 2021). Another study found that the levels of epigenetic regulation in certain genes such as TP53 affect susceptibility to inflammation in patients with PTSD (Busbee et al., 2021). There is thus a need to understand the epigenetic mechanisms of PTSD from the perspective of inflammation. Furthermore, miRNAs play a critical role in the regulation of PTSD. In addition to the aforementioned effects of miR-142 on inflammation, miR-132 may also modulate PTSD symptoms by affecting vulnerable X-family RNA-binding proteins (Nie et al., 2021). In a clinical experimental study, the expression of an enzyme that regulates miRNA production was found to affect the function of AMY, as reduced DICER1 can increase the activation of AMY to fearful stimuli (Wingo et al., 2015). Currently, the use of miRNAs as therapeutic targets to alleviate fear or other symptoms holds great promise. In a previous animal experiment, the administration of fluoxetine to mice was found to relieve PTSD symptoms. At the same time, the experimental results indicated that the reduction of PTSD-like symptoms was associated with the reduction of miRNA levels (Schmidt et al., 2013). Therefore, a better understanding of the underlying mechanisms by which miRNAs function in the body will aid in the development of more effective and promising drugs for PTSD. Additional studies on miRNAs in animals and humans are presented in Table 7. As mentioned, in addition to miRNAs in the noncoding RNAs involved in the development of PTSD, lncRNAs also play a role in this process. LncRNAs can modulate splicing and affect the stability of messenger RNA (mRNA), are highly abundant in the brain, and are closely related to neurodevelopment. An animal study found that lncRNAs affected fear extinction function in patients (Malan-Muller et al., 2020). Further animal studies also showed that multiple lncRNAs were differentially expressed between PTSD and control rats (Qing et al., 2016). However, overall, research on lncRNAs associated with PTSD is lacking.
Table 7.
Preclinical and clinical studies investigating the role of noncoding RNA in PTSD.
| Diseases | Cases | Markers | Main results | Main mechanisms | References |
|---|---|---|---|---|---|
| Preclinical studies | Control (n = 12) Susceptible (n = 7)Resilient (n = 11) |
RT-qPCR miR-15a-5p miR-497a-5p miR-511-5p |
Susceptible vs Resilient: ↓miR-511-5p, miR-15a-5p and miR-497a-5p expression in HIP and hypothalamus |
miRNA | Maurel et al., 2021 |
| Control (n = 24)PTSD (n = 24) Sh-miR-NC (n = 12)Sh-miR (n = 12) |
RT-qPCR miR-132 |
PTSD vs Control: ↑miR-132 in HIP |
miRNA | Nie et al., 2021 | |
| Control (n = 12)PTSD (n = 12) Sh-miR-NC (n = 12) Sh-miR-antisense (n = 12) |
RT-qPCR miR-142 |
PTSD vs Control: ↑miR-142 in HIP Sh-miR-antisense vs Sh-miR-NC: ↓miR-142 and PTSD-like behavior |
miRNA | Nie et al., 2021 | |
| Vehicle-no shock (n = 6) Vehicle-shock (n = 6)Fluoxetine-no shock (n = 6) Fluoxetine-shock (n = 6) |
RT-qPCR miR-1971 miR-1947-3p miR-33-5p |
Fluoxetine treatment vs shock: ↓miR-1971 in mPFC ↓miR-1947-3p in mPFC ↑miR-33-5p in mPFC |
miRNA | Schmidt et al., 2013 | |
| Control (n = 10)PTSD (n = 10) |
RT-qPCR lncRNA |
PTSD vs Control:↑143 lncRNAs in hippocampus ↓150 lncRNAs in hippocampus |
lncRNA | Qingzhen et al., 2016 | |
| Clinical studies | Control (n = 24)PTSD (n = 24) |
RT-PCR Has-miR-7113-5p |
PTSD vs Control: ↓ Has-miR-7113-5p |
miRNA | Bam et al., 2020 |
| Control (n = 17)PTSD (n = 16) |
qRT-PCR Has-miR-193a-5p |
PTSD vs Control: ↓ Has-miR-193a-5p |
miRNA | Bam et al., 2016 | |
| Control (n = 4)PTSD (n = 8) |
qRT-PCR miRNA let-7a miR-145 miR-16 miR-221 miR-320b |
PTSD vs Control: ↓miRNA let-7a, miR-145, miR-16, miR-221, miR-320b |
miRNA | Busbee et al., 2021 | |
| Control (n = 5)PTSD (n = 5) |
qRT-PCR miRNA |
PTSD vs control: ↓most of the miRNA in PMBC |
miRNA | Bam et al., 2017 | |
| Control (n = 72) PTSD + depression (n = 112) |
RT-PCR miRNA |
PTSD vs Control: ↓miRNA |
miRNA | Wingo et al., 2015 |
Note: a ↓ decreased, ↑ increased. b Abbreviations: lncRNA, long noncoding RNA; miR, microRNA; HIP, hippocampus; PMBC, peripheral blood mononuclear cell; qRT-PCR, quantitative real-time PCR; RT-qPCR, reverse transcription quantitative real-time PCR.
A review of numerous studies found that miRNA regulation by natural products is relatively common in cancer treatments. For example, natural products can be used to treat various diseases such as leukemia, liver cancer, pancreatic cancer, and cervical cancer (Chen et al., 2020, Liao et al., 2014, Ma et al., 2021), as well as PTSD. In addition to the anti-inflammation and anti-oxidation stress effects mentioned, some products also have certain regulatory effects on apoptosis and autophagy. This can adjust miRNA expression levels to regulate apoptosis and autophagy-related factors in vivo, thereby affecting the development of disorders (Park et al., 2017). GAS, catapol, puerarin, hesperidin (HSD), crocin (CROC), and glycyrrhizin (GLC) reportedly have miRNA regulatory effects, and have been shown to inhibit the expression of inflammatory factors and the occurrence of inflammation.
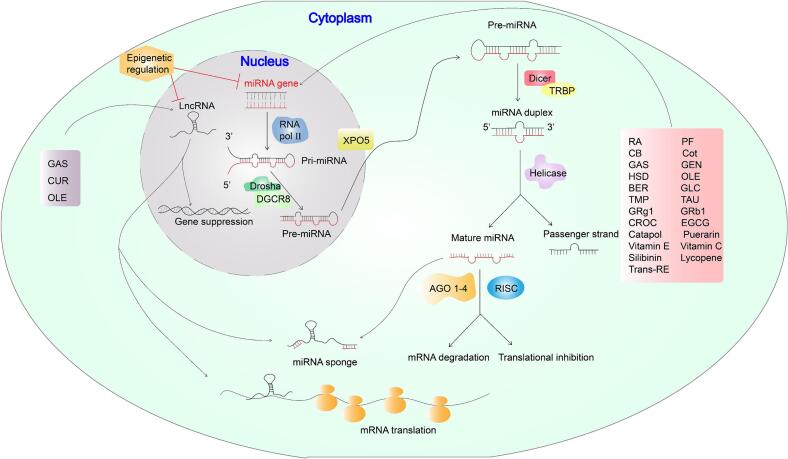
Multiple studies have indicated that GAS can alter the content of various noncoding RNAs, including long noncoding RNAs and miRNAs, and the main mechanism of action is that lncRNA binds to endogenous miRNA, which, as a noncoding RNA, mainly inhibits the expression of target mRNA (Li et al., 2022, Shu et al., 2021). Like GAS, it also has anti-inflammatory, anti-oxidative stress, and anti-apoptotic effects. GAS increases beclin-1, Bax, and light chain 3 (LC3) and reduces bcl-1 and p6 in specific brain regions through its anti-apoptotic effects. Meanwhile, GAS can also regulate miRNA497 and play a role in regulating neuronal death and survival through autophagy (Li, Wang, Yan, & Zhang, 2022). In addition, GAS can also regulate lncRNA NEAT1 and miRNA-22-3p to reduce inflammation and brain injury (Zhang, Ouyang, Ji, & Liu, 2021). In conclusion, GAS has a certain neuroprotective role and can effectively treat memory disorders and fear disorders after trauma. Some studies have indicated that GAS can improve the learning and spatial memory ability of PTSD patients to a certain extent (Lei, Yuan, & Zou, 2020). Therefore, the regulation of noncoding RNAs by natural products may be involved in the regulation of PTSD through apoptosis or autophagy. Catapol has been implicated to alleviate PTSD symptoms, mainly by inhibiting the expression of lncRNA Neat1, which reduces miR-140-5p transcription and upregulates HDAC4 expression (Zou et al., 2019). In addition, puerarin, HSD, and CROC can upregulate the levels of miR-140-5p, miR-132, and miR-122, respectively, to inhibit the expression of proinflammatory related factors and exert anti-inflammatory effects (Li et al., 2016, Liu et al., 2019, Xu et al., 2020). In addition, GLC functions mainly by reducing the expression of miR-222 (Ito, Loucas, Suzuki, Kobayashi, & Suzuki, 2020). Although the specific mechanisms of PTSD treatment are unclear, we have speculated that these natural products may affect the expression of genes related to PTSD symptoms through the regulation of miRNA, which needs to be further studied. The roles of basic noncoding RNAs and common natural products that regulate noncoding RNAs are summarized in Fig. 4. In addition, the epigenetic regulatory utility of natural products used to treat PTSD has been summarized in Table 8 (Fig. 5).
Fig. 4.
Various natural products and their effects on noncoding RNA in PTSD. The regulation of non-coding RNA mainly includes the regulation of miRNA and LncRNA. Both affect transcriptional activation and inhibition in different ways of action. A large number of natural products, including RA, GAS, GRg1, GRb1, EGCG, can regulate and change the levels of different types of miRNAs through epigenetic regulation, so as to achieve the purpose of transcriptional activation or inhibition, and then regulate the level of miRNA in PTSD patients. Natural products such as GAS, CUR and OLE can also exert therapeutic effects by regulating LncRNA.
Table 8.
Common natural products that can regulate noncoding RNA.
| Chemicals | Epigenetic regulation | References |
|---|---|---|
| RA | ↓miR-642a-3p and miR-6785-5p | Yu et al., 2019 |
| Catalpol | ↓miR-140-5p ↓miR-132 |
Xiong et al., 2017 |
| GAS | ↑miR-22-3p ↓miR-142a ↓miR-497 ↑lncRNA Gm7237 ↓lncRNA NEAT1 |
Li et al., 2022, Shu et al., 2021, Zhang et al., 2021 |
| GRg1 | ↓miR-21 ↓miR-15b, miR-23a and miR-214 |
Kwok et al., 2017, Zhai et al., 2021 |
| GRb1 | ↓miR-134 ↑miR-208 |
Wang et al., 2022, Wilkes et al., 2021 |
| PF | ↓miR-15b ↑miR-124 |
Huang et al., 2021, Wang et al., 2021 |
| trans-RE | ↓miR-132 and miR-15a ↑miR-155, miR-134 and miR-124 |
Eseberri et al., 2017, Isac et al., 2017 |
| CUR | ↓oncomiRs | Momtazi et al., 2016 |
| Puerarin | ↓miR-140-5p ↓miR-21 |
Xu et al., 2020 |
| HSD | ↑miR-132 ↓miR-155 and miR-21 in MCF-7 |
Li et al., 2016, Magura et al., 2021 |
| EGCG | ↑miR-16 | Tsang & Kwok, 2010 |
| Silibinin | ↓miR-155 | Dupuis et al., 2018 |
| GEN | ↑miR-451 | Gan et al., 2019 |
| OLE | ↑miR-137, miR-181b and Let-7d | Tezcan et al., 2019 |
| GLC | ↓miR-222 ↑LncRNA-Gas5 |
Ito, Loucas, Suzuki, Kobayashi, & Suzuki, 2020 |
| Vitamin E | ↓miR-126, miR146a and miR-124 | Ambrogini et al., 2018 |
| TMP | ↑miR-182 | Li et al., 2019 |
| BER | ↓miR-152, miR-29a and miR429 | Liu et al., 2019 |
| Lycopene | ↑miR-21 | Ahn, Lee, Jung, & Ha, 2012 |
| TAU | ↑miR-122-5p | Nabi, Atta, El-Ahwany, Elzayat, & Saleh, 2021 |
| CB | ↑miR-29b1 | Scherma et al., 2020 |
| Cot | ↑miR-223 | Herberth et al., 2014 |
| CROC | ↑miR-9 and miR-29 ↓miR-122 |
Khedr et al., 2020, Vahdati Hassani et al., 2017 |
Note: a ↓ decreased, ↑ increased. b Abbreviations: BER, berberine; CB, cannabinoids; CROC, crocin; Cot, cotinine; CUR, curcumin; EGCG, epigallocatechin gallate; GAS, gastrodin; GEN, genistein; GLC, glycyrrhizin; GRb1, ginsenoside Rb1; GRg1, ginsenoside Rg1; HSD, hesperidin; OLE, oleuropein; PF, paeoniflorin; RA, rosmarinic acid; TAU, taurine; TMP, tetramethylpyrazine.
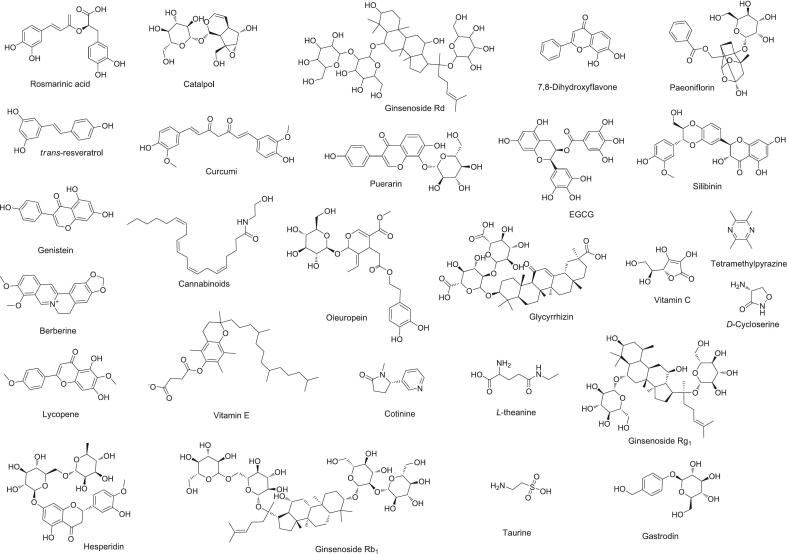
Fig. 5.
Chemical sructures of various natural products on histone acetylation and methylation in PTSD.
3. Toxicology and side effects
Reports of the toxic side effects that occur when using natural products as therapeutics for PTSD are rare. When chemical drugs are used to treat diseases, the onset of their effects is fast and perhaps also significantly curative, but they also have unavoidable toxic side effects on the human body. Consequently, the use of medicinal plants and some natural products has gradually been increasing in popularity due to their perceived safety. At present, although chemical drugs occupy a larger pharmaceutical market, with the increase in human health needs, natural products have also received increasing attention owing to their low toxicity and low levels of side effects. In addition, natural products have been shown to be effective against various diseases, including PTSD. Currently, natural products are predominantly used on a large scale for the treatment of cancer and tumors, for which chemotherapy and Western medicine are indispensable, but these often result in significant side effects or even irreversible damage to the human body. The excellent characteristics of plant extracts are often used to alleviate or prevent the occurrence of side effects. Although natural products are generally characterized as having fewer side effects and lower toxicity levels, they cannot be considered completely harmless to the human body. For example, berberine may cause insufficient glucose-6-phosphate dehydrogenase levels in pregnant women and neonates, leading to hemolysis. In addition, the combination of berberine and other Western medicines may produce a series of gastrointestinal reactions (Pang et al., 2015). Moreover, some extracts are developmentally toxic and neurotoxic, such as cannabinoids (Huestis et al., 2019). The side effects of these natural products are often dose-related, and only when taken within a safe dose range will achieve their expected efficacy. For example, resveratrol has been shown to be a safe compound when in the human body, but there are also reports in the literature that it may have negative side effects, such as cytotoxicity (Shaito et al., 2020). Therefore, the use of natural products or medicinal plants requires that people understand dosage so that they know how to best avoid any toxic side effects.
4. Discussion and conclusion
Sudden violent incidents and natural disasters are increasing globally (Raman et al., 2021), and consequently, so are mental illnesses such as PTSD. PTSD is a very complex psychiatric disorder, and while its pathogenesis is still unclear, there is sufficient evidence that epigenetics can regulate its occurrence. Here, we have focused on the role of epigenetic regulations in PTSD and the mechanisms by which common natural products are involved in these processes and can thus be utilized in treatment strategies.
Since ancient times, the natural environment has been a rich source of medical resources, as evidenced by the documentation of Chinese herbs and plants that have medicinal effects. The biologically active natural products in some medicinal plants can thus be used to help develop new drugs. Consequently, screening desirable natural products from traditional Chinese medicines and medicinal plants is an ongoing process. With the development and progress of science and technology, researchers have found that simply screening new drugs through traditional drug discovery and design methods is very unrealistic, as the process is time-consuming, human and material resources can easily be wasted, and the effects may not be significant. However, in recent years, computer technologies in the field of virtual screening and drug design have rapidly developed with continuous innovation, and the results have been remarkable (Lin, Zhang, Wang, Yang, & Shen, 2022). In addition, affinity-based screening has also notably added in the identification of active ingredients from complex ingredients and further promoted drug discovery. For example, affinity chromatography, biological chromatography, affinity electrophoresis, magnetic screening, fluorescence polarization, surface ionization resonance, and other methods are now more accurate and effective for use with drug discovery; however there are also certain shortcomings (Hou et al., 2020). The screening of natural products requires more researchers to carry out careful step-by-step investigations, and develop a more comprehensive and efficient screening method. Moreover, natural products have proven to be effective at treating and ameliorating different diseases, and while most research has been conducted on cancer, there is also evidence that natural extracts can improve PTSD symptoms. When compared with chemical treatments, natural product treatments are widely perceived as being safer. PTSD is complicated by many influencing factors, such as the traumatic event itself, the external environment of the patient, and their familial genetic history. However, the pathophysiological basis of PTSD is also complex, and multiple systems in the body are related to the occurrence of disease. The results of this literature review show that epigenetic regulation is mainly divided into three parts: histone modification, DNA methylation, and noncoding RNA, while changes in the histone, DNA, and noncoding RNA also affect the occurrence of PTSD to a certain extent. Although there are few reports on the side effects of the natural products that can be used to treat PTSD due to their low toxicity, it has been reported in the literature that natural products can have toxic side effects in the treatment of other diseases, so its disadvantages cannot be ignored. In addition, although the number of articles on the role of natural products in PTSD from the perspective of epigenetic regulation is limited or even scarce, the number of articles related to the epigenetic regulation of natural products in the treatment of other diseases is sufficient. The use of natural products to treat PTSD is gradually being adopted, and there is a plethora of available literature and reviews on how to understand PTSD from the perspective of epigenetic regulation. Consequently, studying the effects of natural products on PTSD is a promising field of research. In summary, although there are unresolved problems regarding the use of natural products, in view of their advantages such as low toxicity, few adverse reactions, and ability to regulate epigenetics in numerous ways, they have great developmental potential for the field of psychiatric disease treatment, such as for PTSD.
CRediT authorship contribution statement
Meijing Xu: Conceptualization, Investigation, Visualization, Writing – original draft. Minghui Cui: Investigation, Writing – review & editing. Yu Wang: Conceptualization, Investigation, Visualization, Writing – original draft. Boru Li: Investigation, Methodology. Lijin Feng: Investigation, Methodology. Hang Xing: Supervision, Writing – review & editing. Kuo Zhang: Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by the Project of National Natural Science Foundation of China (No. 82074041, 82004051, 81803508), Funded by China Postdoctoral Science Foundation (No. 2020M670674), the Scientific Research Fund from Liaoning Education Department (No. 2020LQN02, LJKZ0923, LJKQZ2021029), Natural Science Foundation of Liaoning Province (No. 2021-MS-216), Fund for distinguished young scholars of Shenyang Pharmaceutical University (No. ZQ202202), Fund for outstanding young scholars of Shenyang Pharmaceutical University (No. YQ202103) and rolling funding of young and middle-aged teachers’ career development project of Shenyang Pharmaceutical University.
Contributor Information
Hang Xing, Email: xinghangsyphu@163.com.
Kuo Zhang, Email: kzhangchn@163.com.
References
- Ahn J., Lee H., Jung C.H., Ha T. Lycopene inhibits hepatic steatosis via microRNA-21-induced downregulation of fatty acid-binding protein 7 in mice fed a high-fat diet. Molecular Nutrition Food Research. 2012;56(11):1665–1674. doi: 10.1002/mnfr.201200182. [DOI] [PubMed] [Google Scholar]
- Alzoubi K.H., Al Subeh Z.Y., Khabour O.F. Molecular targets for the interactive effect of etazolate during post-traumatic stress disorder: Role of oxidative stress, BDNF and histones. Behavioural Brain Research. 2019;369 doi: 10.1016/j.bbr.2019.111930. [DOI] [PubMed] [Google Scholar]
- Ambrogini P., Albertini M.C., Betti M., Galati C., Lattanzi D., Savelli D.…Cuppini R. Neurobiological correlates of alpha-tocopherol antiepileptogenic effects and microRNA expression modulation in a rat model of kainate-induced seizures. Molecular Neurobiology. 2018;55(10):7822–7838. doi: 10.1007/s12035-018-0946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anestopoulos I., Sfakianos A.P., Franco R., Chlichlia K., Panayiotidis M.I., Kroll D.J., Pappa A. A novel role of silibinin as a putative epigenetic modulator in human prostate carcinoma. Molecules. 2016;22(1):62. doi: 10.3390/molecules22010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bam M., Yang X., Busbee B.P., Aiello A.E., Uddin M., Ginsberg J.P.…Nagarkatti M. Increased H3K4me3 methylation and decreased miR-7113-5p expression lead to enhanced Wnt/beta-catenin signaling in immune cells from PTSD patients leading to inflammatory phenotype. Molecular Medicine. 2020;26(1):110. doi: 10.1186/s10020-020-00238-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bam M., Yang X., Zhou J., Ginsberg J.P., Leyden Q., Nagarkatti P.S., Nagarkatti M. Evidence for epigenetic regulation of pro-Inflammatory cytokines, interleukin-12 and interferon gamma, in peripheral blood mononuclear cells from PTSD patients. Journal Neuroimmune Pharmacology. 2016;11(1):168–181. doi: 10.1007/s11481-015-9643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bam M., Yang X., Zumbrun E.E., Ginsberg J.P., Leyden Q., Zhang J.…Nagarkatti M. Decreased AGO2 and DCR1 in PBMCs from war veterans with PTSD leads to diminished miRNA resulting in elevated inflammation. Translation Psychiatry. 2017;7(8):e1222. doi: 10.1038/tp.2017.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister A.J., Zegerman P., Partridge J.F., Miska E.A., Thomas J.O., Allshire R.C., Kouzarides T. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410(6824):120–124. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- Barski A., Cuddapah S., Cui K., Roh T.Y., Schones D.E., Wang Z.…Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129(4):823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Benjet C., Bromet E., Karam E.G., Kessler R.C., McLaughlin K.A., Ruscio A.M.…Koenen K.C. The epidemiology of traumatic event exposure worldwide: Results from the World Mental Health Survey Consortium. Psychological Medicine. 2016;46(2):327–343. doi: 10.1017/S0033291715001981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busbee P.B., Bam M., Yang X., Abdulla O.A., Zhou J., Ginsberg J.P.J.…Nagarkatti P.S. Dysregulated TP53 among PTSD patients leads to downregulation of miRNA let-7a and promotes an inflammatory th17 phenotype. Frontiers in Immunology. 2021;12 doi: 10.3389/fimmu.2021.815840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Yeo G., Muotri A.R., Kuwabara T., Gage F.H. Noncoding RNAs in the mammalian central nervous system. Annual Review Neuroscience. 2006;29:77–103. doi: 10.1146/annurev.neuro.29.051605.112839. [DOI] [PubMed] [Google Scholar]
- Chen F., Zhong Z., Tan H.Y., Guo W., Zhang C., Cheng C.S.…Feng Y. Suppression of lncRNA MALAT1 by betulinic acid inhibits hepatocellular carcinoma progression by targeting IAPs via miR-22-3p. Clinical and Translational Medicine. 2020;10(6):e190. doi: 10.1002/ctm2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesielski O., Biesiekierska M., Balcerczyk A. Epigallocatechin-3-gallate (EGCG) alters histone acetylation and methylation and impacts chromatin architecture profile in human endothelial cells. Molecules. 2020;25(10):2326. doi: 10.3390/molecules25102326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker S.J., Smith-Diaz C.C., Dyson R.M., Vissers M.C.M., Berry M.J. The epigenetic role of vitamin C in neurodevelopment. International Journal Molecular Sciences. 2022;23(3):1208. doi: 10.3390/ijms23031208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland K.G., Mellick G.D., Silburn P.A., Mather K., Armstrong N.J., Sachdev P.S.…Kwok J.B. DNA methylation of the MAPT gene in parkinson's disease cohorts and modulation by vitamin E in vitro. Movement Disorders. 2014;29(13):1606–1614. doi: 10.1002/mds.25784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash R., Jahan I., Ali M.C., Mitra S., Munni Y.A., Timalsina B.…Moon I.S. Potential roles of natural products in the targeting of proteinopathic neurodegenerative diseases. Neurochemistry International. 2021;145 doi: 10.1016/j.neuint.2021.105011. [DOI] [PubMed] [Google Scholar]
- Daskalakis N.P., Provost A.C., Hunter R.G., Guffanti G. Noncoding RNAs: Stress, glucocorticoids, and posttraumatic stress disorder. Biological Psychiatry. 2018;83(10):849–865. doi: 10.1016/j.biopsych.2018.01.009. [DOI] [PubMed] [Google Scholar]
- Daskalakis N.P., Rijal C.M., King C., Huckins L.M., Ressler K.J. Recent genetics and epigenetics approaches to PTSD. Current Psychiatry Reports. 2018;20(5):30. doi: 10.1007/s11920-018-0898-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis M.L., Conti F., Maselli A., Pagano M.T., Ruggieri A., Anticoli S.…Pierdominici M. The natural agonist of estrogen receptor beta silibinin plays an immunosuppressive role representing a potential therapeutic tool in rheumatoid arthritis. Frontiers in Immunology. 2018;9:1903. doi: 10.3389/fimmu.2018.01903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt V., Gupta S., Dabur R., Injeti E., Mittal A. Skeletal muscle atrophy: Potential therapeutic agents and their mechanisms of action. Pharmacological Research. 2015;99:86–100. doi: 10.1016/j.phrs.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Eddy S.R. Noncoding RNA genes. Current Opinion in Genetic and Development. 1999;9(6):695–699. doi: 10.1016/s0959-437x(99)00022-2. [DOI] [PubMed] [Google Scholar]
- Edmondson D., von Kanel R. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry. 2017;4(4):320–329. doi: 10.1016/S2215-0366(16)30377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eseberri I., Lasa A., Miranda J., Gracia A., Portillo M.P. Potential miRNA involvement in the anti-adipogenic effect of resveratrol and its metabolites. Public Library of Science One. 2017;12(9) doi: 10.1371/journal.pone.0184875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Bedmar Z., Anter J., Alonso-Moraga A., de Las M., Mulas J., Millan-Ruiz Y., Guil-Luna S. Demethylating and anti-hepatocarcinogenic potential of hesperidin, a natural polyphenol of Citrus juices. Molecular Carcinogenesis. 2017;56(6):1653–1662. doi: 10.1002/mc.22621. [DOI] [PubMed] [Google Scholar]
- Flores G. Curcuma longa L. extract improves the cortical neural connectivity during the aging process. Neural Regeneration Research. 2017;12(6):875–880. doi: 10.4103/1673-5374.208542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuemmeler B.F., Dozmorov M.G., Do E.K., Zhang J.J., Grenier C., Huang Z.…Murphy S.K. DNA methylation in babies born to nonsmoking mothers exposed to secondhand smoke during pregnancy: An epigenome-wide association study. Environment Health Perspective. 2021;129(5):57010. doi: 10.1289/EHP8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan M., Zheng T., Shen L., Tan Y., Fan Y., Shuai S.…Zhu L. Genistein reverses isoproterenol-induced cardiac hypertrophy by regulating miR-451/TIMP2. Biomedicine and Pharmacotherapy. 2019;112 doi: 10.1016/j.biopha.2019.108618. [DOI] [PubMed] [Google Scholar]
- Gao Y., Li J., Chu S., Zhang Z., Chen N., Li L., Zhang L. Ginsenoside Rg1 protects mice against streptozotocin-induced type 1 diabetic by modulating the NLRP3 and Keap1/Nrf2/HO-1 pathways. European Journal of Pharmacology. 2020;866 doi: 10.1016/j.ejphar.2019.172801. [DOI] [PubMed] [Google Scholar]
- Graff J., Tsai L.H. Histone acetylation: Molecular mnemonics on the chromatin. Nature Reviews Neuroscience. 2013;14(2):97–111. doi: 10.1038/nrn3427. [DOI] [PubMed] [Google Scholar]
- Gunesch S., Hoffmann M., Kiermeier C., Fischer W., Pinto A.F.M., Maurice T.…Decker M. 7-O-Esters of taxifolin with pronounced and overadditive effects in neuroprotection, anti-neuroinflammation, and amelioration of short-term memory impairment in vivo. Redox Biology. 2020;29 doi: 10.1016/j.redox.2019.101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Wu R., Gaspar J.M., Sargsyan D., Su Z.Y., Zhang C.…Kong A.N. DNA methylome and transcriptome alterations and cancer prevention by curcumin in colitis-accelerated colon cancer in mice. Carcinogenesis. 2018;39(5):669–680. doi: 10.1093/carcin/bgy043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P., Gao D., Zhang W., Liu S., Yang S., Li X. Puerarin suppresses high glucose-induced MCP-1 expression via modulating histone methylation in cultured endothelial cells. Life Sciences. 2015;130:103–107. doi: 10.1016/j.lfs.2015.02.022. [DOI] [PubMed] [Google Scholar]
- Hawn S.E., Neale Z., Wolf E.J., Zhao X., Pierce M., Fein-Schaffer D.…Miller M.W. Methylation of the AIM2 gene: An epigenetic mediator of PTSD-related inflammation and neuropathology plasma biomarkers. Depression and Anxiety. 2022;39(4):323–333. doi: 10.1002/da.23247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberth G., Bauer M., Gasch M., Hinz D., Roder S., Olek S.…Lehmann I. Maternal and cord blood miR-223 expression associates with prenatal tobacco smoke exposure and low regulatory T-cell numbers. Journal of Allergy and Clinical Immunology. 2014;133(2):543–550. doi: 10.1016/j.jaci.2013.06.036. [DOI] [PubMed] [Google Scholar]
- Hori H., Kim Y. Inflammation and post-traumatic stress disorder. Psychiatry and Clinical Neurosciences. 2019;73(4):143–153. doi: 10.1111/pcn.12820. [DOI] [PubMed] [Google Scholar]
- Hou X., Sun M., Bao T., Xie X., Wei F., Wang S. Recent advances in screening active components from natural products based on bioaffinity techniques. Acta Pharmaceutica Sinica B. 2020;10(10):1800–1813. doi: 10.1016/j.apsb.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie H., Rijal C.M., Ressler K.J. A review of epigenetic contributions to post-traumatic stress disorder. Dialogues in Clinical Neuroscience. 2019;21(4):417–428. doi: 10.31887/DCNS.2019.21.4/kressler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D., Li Z., Chen Y., Fan Y., Yu T. Paeoniflorin reduces the inflammatory response of THP-1 cells by up-regulating microRNA-124: Paeoniflorin reduces the inflammatory response of THP-1 cells through microRNA-124. Genes Genomics. 2021;43(6):623–631. doi: 10.1007/s13258-021-01083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R.C., Bonner J. Histone, a suppressor of chromosomal RNA synthesis. Proceedings of the National Academy of Sciences of the United States of America. 1962;48(7):1216–1222. doi: 10.1073/pnas.48.7.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Huang Q., Ji L., Wang Y., Qi X., Liu L.…Lu L. Epigenetic regulation of active Chinese herbal components for cancer prevention and treatment: A follow-up review. Pharmacological Research. 2016;114:1–12. doi: 10.1016/j.phrs.2016.09.023. [DOI] [PubMed] [Google Scholar]
- Huestis M.A., Solimini R., Pichini S., Pacifici R., Carlier J., Busardo F.P. Cannabidiol adverse effects and toxicity. Current Neuropharmacology. 2019;17(10):974–989. doi: 10.2174/1570159X17666190603171901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J.Y., Aromolaran K.A., Zukin R.S. The emerging field of epigenetics in neurodegeneration and neuroprotection. Nature Reviews Neuroscience. 2017;18(6):347–361. doi: 10.1038/nrn.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isac S., Panaitescu A.M., Spataru A., Iesanu M., Totan A., Udriste A.…Zagrean A.M. Trans-resveratrol enriched maternal diet protects the immature hippocampus from perinatal asphyxia in rats. Neuroscience Letters. 2017;653:308–313. doi: 10.1016/j.neulet.2017.06.003. [DOI] [PubMed] [Google Scholar]
- Ito I., Loucas B.D., Suzuki S., Kobayashi M., Suzuki F. Glycyrrhizin protects gamma-irradiated mice from gut bacteria-associated infectious complications by improving miR-222-associated gas5 RNA reduction in macrophages of the bacterial translocation Site. Journal of Immunology. 2020;204(5):1255–1262. doi: 10.4049/jimmunol.1900949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambhekar A., Dhall A., Shi Y. Roles and regulation of histone methylation in animal development. Nature Reviews Molecular Cell Biology. 2019;20(10):625–641. doi: 10.1038/s41580-019-0151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y.G., Hwang K.A., Choi K.C. Rosmarinic acid, a component of rosemary tea, induced the cell cycle arrest and apoptosis through modulation of HDAC2 expression in prostate cancer cell lines. Nutrients. 2018;10(11):1784. doi: 10.3390/nu10111784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P.A., Ohtani H., Chakravarthy A., De Carvalho D.D. Epigenetic therapy in immune-oncology. Nature Reviews Cancer. 2019;19(3):151–161. doi: 10.1038/s41568-019-0109-9. [DOI] [PubMed] [Google Scholar]
- Kalaiarasi A., Anusha C., Sankar R., Rajasekaran S., John Marshal J., Muthusamy K., Ravikumar V. Plant isoquinoline alkaloid berberine exhibits chromatin remodeling by modulation of histone deacetylase to induce growth arrest and apoptosis in the A549 cell line. Journal of Agricultural and Food Chemistry. 2016;64(50):9542–9550. doi: 10.1021/acs.jafc.6b04453. [DOI] [PubMed] [Google Scholar]
- Katrinli S., Zheng Y., Gautam A., Hammamieh R., Yang R., Venkateswaran S.…Smith A.K. PTSD is associated with increased DNA methylation across regions of HLA-DPB1 and SPATC1L. Brain Behavior and Immunity. 2021;91:429–436. doi: 10.1016/j.bbi.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr L.H., Rahmo R.M., Farag D.B., Schaalan M.F., El Magdoub H.M. Crocin attenuates cisplatin-induced hepatotoxicity via TLR4/NF-kappaBp50 signaling and BAMBI modulation of TGF-beta activity: Involvement of miRNA-9 and miRNA-29. Food and Chemical Toxicology. 2020;140 doi: 10.1016/j.fct.2020.111307. [DOI] [PubMed] [Google Scholar]
- Kikuchi H., Harata K., Akiyoshi S., Sagara T., Madhyastha H., Kuribayashi F. Potential role of green tea amino acid l-theanine in the activation of innate immune response by enhancing expression of cytochrome b558 responsible for the reactive oxygen species-generating ability of leukocytes. Microbiology and Immunology. 2022;66(6):342–349. doi: 10.1111/1348-0421.12977. [DOI] [PubMed] [Google Scholar]
- Kim G.S., Smith A.K., Nievergelt C.M., Uddin M. Neuroepigenetics of post-traumatic stress disorder. Progress in Molecular Biology and Translational Science. 2018;158:227–253. doi: 10.1016/bs.pmbts.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Batoon A., Leszczynska J.M., Klein C.B. Modulation of gene methylation by genistein or lycopene in breast cancer cells. Environmental and Molecular Mutagenesis. 2008;49(1):36–45. doi: 10.1002/em.20363. [DOI] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kwok H.H., Poon P.Y., Mak K.H., Zhang L.Y., Liu P., Zhang H.…Wong R.N. Role of G3BP1 in glucocorticoid receptor-mediated microRNA-15b and microRNA-23a biogenesis in endothelial cells. Cellular and Molecular Life Sciences. 2017;74(19):3613–3630. doi: 10.1007/s00018-017-2540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M., Daujat S., Schneider R. Lateral thinking: How histone modifications regulate gene expression. Trends in Genetics. 2016;32(1):42–56. doi: 10.1016/j.tig.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Lee B., Shim I., Lee H., Hahm D.H. Effects of epigallocatechin gallate on behavioral and cognitive impairments, hypothalamic-pituitary-adrenal axis dysfunction, and alternations in hippocampal BDNF expression under single prolonged stress. Journal of Medicinal Food. 2018;21(10):979–989. doi: 10.1089/jmf.2017.4161. [DOI] [PubMed] [Google Scholar]
- Lee B., Sur B., Cho S.G., Yeom M., Shim I., Lee H., Hahm D.H. Ginsenoside Rb1 rescues anxiety-like responses in a rat model of post-traumatic stress disorder. Journal of Natural Medicines. 2016;70(2):133–144. doi: 10.1007/s11418-015-0943-3. [DOI] [PubMed] [Google Scholar]
- Lee J.E., Park S.Y., Han P.L. Aging-dependent downregulation of SUV39H1 histone methyltransferase increases susceptibility to stress-induced depressive behavior. Molecular Neurobiology. 2021;58(12):6427–6442. doi: 10.1007/s12035-021-02529-0. [DOI] [PubMed] [Google Scholar]
- Lei X., Yuan Y., Zou Q. The role and mechanism of gastrodin in the medial prefrontal cortex autophagy of PTSD rats. International Journal of Clinical and Experimental Pathology. 2020;13(5):989–994. [PMC free article] [PubMed] [Google Scholar]
- Levenson J.M., O'Riordan K.J., Brown K.D., Trinh M.A., Molfese D.L., Sweatt J.D. Regulation of histone acetylation during memory formation in the hippocampus. Journal of Biological Chemistry. 2004;279(39):40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Li F., Xiang H., Lu J., Chen Z., Huang C., Yuan X. Lycopene ameliorates PTSD-like behaviors in mice and rebalances the neuroinflammatory response and oxidative stress in the brain. Physiology Behavior. 2020;224 doi: 10.1016/j.physbeh.2020.113026. [DOI] [PubMed] [Google Scholar]
- Li H.W., Lan T.J., Yun C.X., Yang K.D., Du Z.C., Luo X.F.…Deng J.G. Mangiferin exerts neuroprotective activity against lead-induced toxicity and oxidative stress via Nrf2 pathway. Chinese Herbal Medicines. 2020;12(1):36–46. doi: 10.1016/j.chmed.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Shao H., Zhang X., Qin B. Hesperidin alleviates lipopolysaccharide-induced neuroinflammation in mice by promoting the miRNA-132 pathway. Inflammation. 2016;39(5):1681–1689. doi: 10.1007/s10753-016-0402-7. [DOI] [PubMed] [Google Scholar]
- Li M., Xiao L., Chen X. Histone acetylation and methylation underlie oligodendroglial and myelin susceptibility in schizophrenia. Frontiers in Cellular Neuroscience. 2022;16 doi: 10.3389/fncel.2022.823708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wang Q., Ren Y., Wang X., Cheng H., Yang H., Wang B. Tetramethylpyrazine protects retinal ganglion cells against H2O2induced damage via the microRNA182/mitochondrial pathway. International Journal of Molecular Medicine. 2019;44(2):503–512. doi: 10.3892/ijmm.2019.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Liu L., Andrews L.G., Tollefsbol T.O. Genistein depletes telomerase activity through cross-talk between genetic and epigenetic mechanisms. International Journal of Cancer. 2009;125(2):286–296. doi: 10.1002/ijc.24398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.G., Wang X.W., Yan H.C., Zhang Y.C. Gastrodin promotes the regeneration of peripheral nerves by regulating miR-497/BDNF axis. BMC Complement Medicine Therapies. 2022;22(1):45. doi: 10.1186/s12906-021-03483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y.C., Lin T.H., Chen C.Y., Lin S.B., Au L.C. The antileukemia activity of natural product HQ17(3) is possibly associated with downregulation of miR-17-92 cluster. Biomed Research International. 2014;2014 doi: 10.1155/2014/306718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Zhang Y., Wang D., Yang B., Shen Y.Q. Computer especially AI-assisted drug virtual screening and design in traditional Chinese medicine. Phytomedicine. 2022;107 doi: 10.1016/j.phymed.2022.154481. [DOI] [PubMed] [Google Scholar]
- Liriano F., Hatten C., Schwartz T.L. Ketamine as treatment for post-traumatic stress disorder: A review. Drugs in Context. 2019;8 doi: 10.7573/dic.212305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Meng X., Wu D., Qiu Z., Luo H. A Natural isoquinoline alkaloid with antitumor activity: Studies of the biological activities of berberine. Frontiers in Pharmacology. 2019;10:9. doi: 10.3389/fphar.2019.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.F., Zhang L., Wu Q., Feng L.Y. Paeoniflorin ameliorates ischemic injury in rat brain via inhibiting cytochrome c/caspase3/HDAC4 pathway. Acta Pharmacological Sinica. 2022;43(2):273–284. doi: 10.1038/s41401-021-00671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luccarini I., Grossi C., Rigacci S., Coppi E., Pugliese A.M., Pantano D.…Casamenti F. Oleuropein aglycone protects against pyroglutamylated-3 amyloid-ss toxicity: Biochemical, epigenetic and functional correlates. Neurobiology of Aging. 2015;36(2):648–663. doi: 10.1016/j.neurobiolaging.2014.08.029. [DOI] [PubMed] [Google Scholar]
- Luger K., Mader A.W., Richmond R.K., Sargent D.F., Richmond T.J. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389(6648):251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- Luthra R., Roy A. Role of medicinal plants against neurodegenerative diseases. Current Pharmaceutical Biotechnology. 2022;23(1):123–139. doi: 10.2174/1389201022666210211123539. [DOI] [PubMed] [Google Scholar]
- Ma D., Chen S., Wang H., Wei J., Wu H., Gao H.…Song G. Baicalein induces apoptosis of pancreatic cancer cells by regulating the expression of miR-139-3p and miR-196b-5p. Frontiers in Oncology. 2021;11 doi: 10.3389/fonc.2021.653061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M., Delmeire L., Mylle J., Altamura C. Risk and preventive factors of post-traumatic stress disorder (PTSD): Alcohol consumption and intoxication prior to a traumatic event diminishes the relative risk to develop PTSD in response to that trauma. Journal of Affective Disorders. 2001;63(1–3):113–121. doi: 10.1016/s0165-0327(00)00173-7. [DOI] [PubMed] [Google Scholar]
- Magura J., Hassan D., Moodley R., Mackraj I. Hesperidin-loaded nanoemulsions improve cytotoxicity, induce apoptosis, and downregulate miR-21 and miR-155 expression in MCF-7. Journal of Microencapsulation. 2021;38(7–8):486–495. doi: 10.1080/02652048.2021.1979673. [DOI] [PubMed] [Google Scholar]
- Majid S., Kikuno N., Nelles J., Noonan E., Tanaka Y., Kawamoto K.…Dahiya R. Genistein induces the p21WAF1/CIP1 and p16INK4a tumor suppressor genes in prostate cancer cells by epigenetic mechanisms involving active chromatin modification. Cancer Research. 2008;68(8):2736–2744. doi: 10.1158/0008-5472.CAN-07-2290. [DOI] [PubMed] [Google Scholar]
- Malan-Muller S., de Souza V.B.C., Daniels W.M.U., Seedat S., Robinson M.D., Hemmings S.M.J. Shedding light on the transcriptomic dark matter in biological psychiatry: Role of long noncoding RNAs in D-cycloserine-induced fear extinction in posttraumatic stress disorder. OMICS-A Journal of Integrative Biology. 2020;24(6):352–369. doi: 10.1089/omi.2020.0031. [DOI] [PubMed] [Google Scholar]
- Marrelli M. Medicinal plants. Plants (Basel) 2021;10(7):1355. doi: 10.3390/plants10071355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez Fernandez J. Report for the year 1958. Acta Pediatrica Esp. 1959;17(196):232–247. [PubMed] [Google Scholar]
- Mateen S., Raina K., Jain A.K., Agarwal C., Chan D., Agarwal R. Epigenetic modifications and p21-cyclin B1 nexus in anticancer effect of histone deacetylase inhibitors in combination with silibinin on non-small cell lung cancer cells. Epigenetics. 2012;7(10):1161–1172. doi: 10.4161/epi.22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Morinobu S., Yamamoto S., Matsumoto T., Takei S., Fujita Y., Yamawaki S. Vorinostat ameliorates impaired fear extinction possibly via the hippocampal NMDA-CaMKII pathway in an animal model of posttraumatic stress disorder. Psychopharmacology (Berl) 2013;229(1):51–62. doi: 10.1007/s00213-013-3078-9. [DOI] [PubMed] [Google Scholar]
- Maurel O.M., Torrisi S.A., Barbagallo C., Purrello M., Salomone S., Drago F.…Leggio G.M. Dysregulation of miR-15a-5p, miR-497a-5p and miR-511-5p is associated with modulation of BDNF and FKBP5 in brain areas of PTSD-related susceptible and resilient mice. International Journal Molecular Sciences. 2021;22(10):5157. doi: 10.3390/ijms22105157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc D.Y.R., Lawford B., Mellor R., Morris C.P., Voisey J., Initiative P. Investigation of C-reactive protein and AIM2 methylation as a marker for PTSD in Australian Vietnam veterans. Gene. 2021;803 doi: 10.1016/j.gene.2021.145898. [DOI] [PubMed] [Google Scholar]
- Mendell J.T., Olson E.N. MicroRNAs in stress signaling and human disease. Cell. 2012;148(6):1172–1187. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momtazi A.A., Shahabipour F., Khatibi S., Johnston T.P., Pirro M., Sahebkar A. Curcumin as a microRNA regulator in cancer: A review. Reviews of Physiology Biochemisty and Pharmacology. 2016;171:1–38. doi: 10.1007/112_2016_3. [DOI] [PubMed] [Google Scholar]
- Monsey M.S., Gerhard D.M., Boyle L.M., Briones M.A., Seligsohn M., Schafe G.E. A diet enriched with curcumin impairs newly acquired and reactivated fear memories. Neuropsychopharmacology. 2015;40(5):1278–1288. doi: 10.1038/npp.2014.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagud-Romero S., Montesinos J., Pascual M., Aguilar M.A., Roger-Sanchez C., Guerri C.…Rodriguez-Arias M. Up-regulation of histone acetylation induced by social defeat mediates the conditioned rewarding effects of cocaine. Progress in Neuropsychopharmacology and Biology Psychiatry. 2016;70:39–48. doi: 10.1016/j.pnpbp.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Moore L.D., Le T., Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38(1):23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison F.G., Miller M.W., Logue M.W., Assef M., Wolf E.J. DNA methylation correlates of PTSD: Recent findings and technical challenges. Progress in Neuropsychopharmacology and Biology Psychiatry. 2019;90:223–234. doi: 10.1016/j.pnpbp.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K. The occurrence of epsilon-N-methyl lysine in histones. Biochemistry. 1964;3:10–15. doi: 10.1021/bi00889a003. [DOI] [PubMed] [Google Scholar]
- Mustafi S., Camarena V., Qureshi R., Yoon H., Volmar C.H., Huff T.C.…Wang G. Vitamin C supplementation expands the therapeutic window of BETi for triple negative breast cancer. EBioMedicine. 2019;43:201–210. doi: 10.1016/j.ebiom.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi A.A., Atta S.A., El-Ahwany E., Elzayat E., Saleh H. Taurine upregulates miRNA-122-5p expression and suppresses the metabolizing enzymes of glycolytic pathway in hepatocellular carcinoma. Molecular Biology Reports. 2021;48(7):5549–5559. doi: 10.1007/s11033-021-06571-y. [DOI] [PubMed] [Google Scholar]
- Navarro-Peran E., Cabezas-Herrera J., Garcia-Canovas F., Durrant M.C., Thorneley R.N., Rodriguez-Lopez J.N. The antifolate activity of tea catechins. Cancer Research. 2005;65(6):2059–2064. doi: 10.1158/0008-5472.CAN-04-3469. [DOI] [PubMed] [Google Scholar]
- Nie H., Peng Z., Lao N., Wang H., Chen Y., Fang Z.…Tan Q. Rosmarinic acid ameliorates PTSD-like symptoms in a rat model and promotes cell proliferation in the hippocampus. Progress in Neuropsychopharmacology and Biological Psychiatry. 2014;51:16–22. doi: 10.1016/j.pnpbp.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Nie P.Y., Ji L.L., Fu C.H., Peng J.B., Wang Z.Y., Tong L. miR-132 Regulates PTSD-like behaviors in rats following single-prolonged stress through fragile X-related p rotein 1. Cellular and Molecular Neurobiology. 2021;41(2):327–340. doi: 10.1007/s10571-020-00854-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie P.Y., Tong L., Li M.D., Fu C.H., Peng J.B., Ji L.L. miR-142 downregulation alleviates rat PTSD-like behaviors, reduces the level of inflammatory cytokine expression and apoptosis in hippocampus, and upregulates the expression of fragile X mental retardation protein. Journal of Neuroinflammation. 2021;18(1):17. doi: 10.1186/s12974-020-02064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B., Quan J., Liu L., Xu Z., Zhu J., Huang X., Tian J. Epigallocatechin gallate reverses cTnI-low expression-induced age-related heart diastolic dysfunction through histone acetylation modification. Journal of Cellular and Molecular Medicine. 2017;21(10):2481–2490. doi: 10.1111/jcmm.13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang B., Zhao L.H., Zhou Q., Zhao T.Y., Wang H., Gu C.J., Tong X.L. Application of berberine on treating type 2 diabetes mellitus. International Journal of Endocrinology. 2015;2015 doi: 10.1155/2015/905749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M.N., Song H.S., Kim M., Lee M.J., Cho W., Lee H.J.…Kim B. Review of natural product-derived compounds as potent antiglioblastoma drugs. Biomed Research International. 2017;2017:8139848. doi: 10.1155/2017/8139848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto L., Abel T. The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology. 2013;38(1):62–76. doi: 10.1038/npp.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter C.J., Akbarian S. Balancing histone methylation activities in psychiatric disorders. Trends in Molecular Medicine. 2011;17(7):372–379. doi: 10.1016/j.molmed.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman R.K., Rasmusson A.M., Koenen K.C., Shin L.M., Orr S.P., Gilbertson M.W.…Liberzon I. Biological studies of post-traumatic stress disorder. Nature Reviews Neuroscience. 2012;13(11):769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qingzhen L., Jiehua M., Zhiyang Y., Hongjun L., Chunlong C., Weiyan L. Distinct hippocampal expression profiles of lncRNAs in rats exhibiting a PTSD-like syndrome. Molecular Neurobiology. 2016;53(4):2161–2168. doi: 10.1007/s12035-015-9180-8. [DOI] [PubMed] [Google Scholar]
- Raman U., Bonanno P.A., Sachdev D., Govindan A., Dhole A., Salako O.…Kennedy C.A. Community violence, PTSD, hopelessness, substance use, and perpetuation of violence in an urban environment. Community Mental Health Journal. 2021;57(4):622–630. doi: 10.1007/s10597-020-00691-8. [DOI] [PubMed] [Google Scholar]
- Rauf A., Rahman M.M. Potential therapeutics against neurological disorders: Natural products-based drugs. Frontiers in Pharmacology. 2022;13 doi: 10.3389/fphar.2022.950457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remely M., Ferk F., Sterneder S., Setayesh T., Kepcija T., Roth S.…Haslberger A. Vitamin E modifies high-fat diet-induced increase of DNA strand breaks, and changes in expression and DNA methylation of dnmt1 and MLH1 in C57BL/6J male mice. Nutrients. 2017;9(6):607. doi: 10.3390/nu9060607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T.L., Zoladz P.R., Sweatt J.D., Diamond D.M. Epigenetic modification of hippocampal Bdnf DNA in adult rats in an animal model of post-traumatic stress disorder. Journal of Psychiatric Research. 2011;45(7):919–926. doi: 10.1016/j.jpsychires.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh N., Spielberg J.M., Logue M.W., Wolf E.J., Smith A.K., Lusk J.…Miller M.W. SKA2 methylation is associated with decreased prefrontal cortical thickness and greater PTSD severity among trauma-exposed veterans. Molecular Psychiatry. 2016;21(3):357–363. doi: 10.1038/mp.2015.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg K., Samson W.K., Ji H. Decoding noncoding RNA: Da Vinci redux? Circulation Research. 2013;113(3):240–241. doi: 10.1161/CIRCRESAHA.113.301865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherma M., Qvist J.S., Asok A., Huang S.C., Masia P., Deidda M.…Melas P.A. Cannabinoid exposure in rat adolescence reprograms the initial behavioral, molecular, and epigenetic response to cocaine. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(18):9991–10002. doi: 10.1073/pnas.1920866117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt U., Herrmann L., Hagl K., Novak B., Huber C., Holsboer F.…Buell D.R. Therapeutic action of fluoxetine is associated with a reduction in prefrontal cortical miR-1971 expression levels in a mouse model of posttraumatic stress disorder. Frontiers in Psychiatry. 2013;4:66. doi: 10.3389/fpsyt.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt U., Keck M.E., Buell D.R. miRNAs and other non-coding RNAs in posttraumatic stress disorder: A systematic review of clinical and animal studies. Journal of Psychiatric Research. 2015;65:1–8. doi: 10.1016/j.jpsychires.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Schoner J., Heinz A., Endres M., Gertz K., Kronenberg G. Post-traumatic stress disorder and beyond: An overview of rodent stress models. Journal of Cellular and Molecular Medicine. 2017;21(10):2248–2256. doi: 10.1111/jcmm.13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaito A., Posadino A.M., Younes N., Hasan H., Halabi S., Alhababi D.…Pintus G. Potential adverse effects of resveratrol: A literature review. International Journal of Molecular Sciences. 2020;21(6) doi: 10.3390/ijms21062084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev A., Liberzon I., Marmar C. Post-traumatic stress disorder. New England Journal of Medicine. 2017;376(25):2459–2469. doi: 10.1056/NEJMra1612499. [DOI] [PubMed] [Google Scholar]
- Shan L., Guo H.Y., van den Heuvel C., van Heerikhuize J., Homberg J.R. Impaired fear extinction in serotonin transporter knockout rats is associated with increased 5-hydroxymethylcytosine in the amygdala. CNS Neuroscience and Therapeutics. 2018;24(9):810–819. doi: 10.1111/cns.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y., Luo T., Wang M., Zhang Y., Zhang L., Xiao Z.…Yu S. Gastrodin promotes CNS myelination via a lncRNA Gm7237/miR-142a/MRF pathway. RNA Biology. 2021;18(9):1279–1290. doi: 10.1080/15476286.2020.1841976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillivan S.E., Vaissiere T., Miller C.A. Neuroepigenetic regulation of pathogenic memories. Neuroepigenetics. 2015;1:28–33. doi: 10.1016/j.nepig.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr J.M. Ageing and epigenetics: Linking neurodevelopmental and neurodegenerative disorders. Developmental Medicine and Child Neurology. 2019;61(10):1134–1138. doi: 10.1111/dmcn.14210. [DOI] [PubMed] [Google Scholar]
- Strahl B.D., Allis C.D. The language of covalent histone modifications. Nature. 2000;403(6765):41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes and Development. 1998;12(5):599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- Sun H., Zhang X., Kong Y., Gou L., Lian B., Wang Y.…Sun L. Maternal separation-induced histone acetylation correlates with BDNF-programmed synaptic changes in an animal model of PTSD with sex differences. Molecular Neurobiology. 2021;58(4):1738–1754. doi: 10.1007/s12035-020-02224-6. [DOI] [PubMed] [Google Scholar]
- Takei S., Morinobu S., Yamamoto S., Fuchikami M., Matsumoto T., Yamawaki S. Enhanced hippocampal BDNF/TrkB signaling in response to fear conditioning in an animal model of posttraumatic stress disorder. Journal of Psychiatric Research. 2011;45(4):460–468. doi: 10.1016/j.jpsychires.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Tarsitani L., Vassalini P., Koukopoulos A., Borrazzo C., Alessi F., Di Nicolantonio C.…d'Ettorre G. Post-traumatic stress disorder among COVID-19 survivors at 3-month follow-up after hospital discharge. Journal of General Internal Medicine. 2021;36(6):1702–1707. doi: 10.1007/s11606-021-06731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezcan G., Aksoy S.A., Tunca B., Bekar A., Mutlu M., Cecener G.…Taskapilioglu M.O. Oleuropein modulates glioblastoma miRNA pattern different from olea europaea leaf extract. Human and Experimental Toxicology. 2019;38(9):1102–1110. doi: 10.1177/0960327119855123. [DOI] [PubMed] [Google Scholar]
- Thakur V., Sadanandan J., Chattopadhyay M. High-mobility group box 1 protein signaling in painful diabetic neuropathy. International Journal of Molecular Sciences. 2020;21(3):881. doi: 10.3390/ijms21030881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas-Roig J., Benito E., Agis-Balboa R.C., Piscitelli F., Hoyer-Fender S., Di Marzo V., Havemann-Reinecke U. Chronic exposure to cannabinoids during adolescence causes long-lasting behavioral deficits in adult mice. Addiction Biology. 2017;22(6):1778–1789. doi: 10.1111/adb.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang W.P., Kwok T.T. Epigallocatechin gallate up-regulation of miR-16 and induction of apoptosis in human cancer cells. Journal of Nutrition Biochemistry. 2010;21(2):140–146. doi: 10.1016/j.jnutbio.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Umukoro S., Ben-Azu B., Ajayi A.M., Adebesin A., Emokpae O. Cymbopogon citratus aqueous leaf extract attenuates neurobehavioral and biochemical changes induced by social defeat stress in mice. Chinese Herbal Medicines. 2020;12(3):303–309. doi: 10.1016/j.chmed.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahdati Hassani F., Mehri S., Abnous K., Birner-Gruenberger R., Hosseinzadeh H. Protective effect of crocin on BPA-induced liver toxicity in rats through inhibition of oxidative stress and downregulation of MAPK and MAPKAP signaling pathway and miRNA-122 expression. Food and Chemical Toxicology. 2017;107(Pt A):395–405. doi: 10.1016/j.fct.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Vinkers C.H., Geuze E., van Rooij S.J.H., Kennis M., Schur R.R., Nispeling D.M.…Boks M.P. Successful treatment of post-traumatic stress disorder reverses DNA methylation marks. Molecular Psychiatry. 2021;26(4):1264–1271. doi: 10.1038/s41380-019-0549-3. [DOI] [PubMed] [Google Scholar]
- Wan Q., Ma X., Zhang Z.J., Sun T., Xia F., Zhao G., Wu Y.M. Ginsenoside reduces cognitive impairment during chronic cerebral hypoperfusion through brain-derived neurotrophic factor regulated by epigenetic modulation. Molecular Neurobiology. 2017;54(4):2889–2900. doi: 10.1007/s12035-016-9868-4. [DOI] [PubMed] [Google Scholar]
- Wang G., An T., Lei C., Zhu X., Yang L., Zhang L., Zhang R. Antidepressant-like effect of ginsenoside Rb1 on potentiating synaptic plasticity via the miR-134-mediated BDNF signaling pathway in a mouse model of chronic stress-induced depression. Journal of Ginseng Research. 2022;46(3):376–386. doi: 10.1016/j.jgr.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.S., Kang S., Liu W.T., Li M., Liu Y., Yu C.…Liu J.G. Extinction of aversive memories associated with morphine withdrawal requires ERK-mediated epigenetic regulation of brain-derived neurotrophic factor transcription in the rat ventromedial prefrontal cortex. Journal of Neuroscience. 2012;32(40):13763–13775. doi: 10.1523/JNEUROSCI.1991-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang Q., Li X., Luo G., Shen M., Shi J.…Tang L. Paeoniflorin sensitizes breast cancer cells to tamoxifen by downregulating microRNA-15b via the FOXO1/CCND1/beta-catenin axis. Drug Design Development and Therapy. 2021;15:245–257. doi: 10.2147/DDDT.S278002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warhaftig G., Zifman N., Sokolik C.M., Massart R., Gabay O., Sapozhnikov D.…Yadid G. Reduction of DNMT3a and RORA in the nucleus accumbens plays a causal role in post-traumatic stress disorder-like behavior: Reversal by combinatorial epigenetic therapy. Molecular Psychiatry. 2021;26(12):7481–7497. doi: 10.1038/s41380-021-01178-y. [DOI] [PubMed] [Google Scholar]
- Watson P. PTSD as a public mental health priority. Current Psychiatry Reports. 2019;21(7):61. doi: 10.1007/s11920-019-1032-1. [DOI] [PubMed] [Google Scholar]
- Wilkes M.C., Jung K., Lee B.E., Saxena M., Sathianathen R.S., Mercado J.D.…Sakamoto K.M. The active component of ginseng, ginsenoside Rb1, improves erythropoiesis in models of diamond-blackfan anemia by targeting nemo-like kinase. Journal of Biological Chemistry. 2021;297(3) doi: 10.1016/j.jbc.2021.100988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingo A.P., Almli L.M., Stevens J.S., Klengel T., Uddin M., Li Y.…Ressler K.J. DICER1 and microRNA regulation in post-traumatic stress disorder with comorbid depression. Nature Communications. 2015;6:10106. doi: 10.1038/ncomms10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf E.J., Logue M.W., Zhao X., Daskalakis N.P., Morrison F.G., Escarfulleri S.…Miller M.W. PTSD and the klotho longevity gene: Evaluation of longitudinal effects on inflammation via DNA methylation. Psychoneuroendocrinology. 2020;117 doi: 10.1016/j.psyneuen.2020.104656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M.S., Li X.J., Liu C.Y., Xu Q., Huang J.Q., Gu S., Chen J.X. Effects of histone modification in major depressive disorder. Current Neuropharmacology. 2022;20(7):1261–1277. doi: 10.2174/1570159X19666210922150043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Shi L., Wang L., Zhou Z., Wang C., Lin Y.…Chen D. Activation of sirtuin 1 by catalpol-induced down-regulation of microRNA-132 attenuates endoplasmic reticulum stress in colitis. Pharmacological Research. 2017;123:73–82. doi: 10.1016/j.phrs.2017.05.030. [DOI] [PubMed] [Google Scholar]
- Xu H., Li B., Li L., Fan Z., Gong X., Wu L., Yan C. Environmental enrichment mitigates PTSD-like behaviors in adult male rats exposed to early life stress by regulating histone acetylation in the hippocampus and amygdala. Journal of Psychiatric Research. 2022;155:120–136. doi: 10.1016/j.jpsychires.2022.07.067. [DOI] [PubMed] [Google Scholar]
- Xu Y., Xiong Y., Xu C., Xu C. Standard puerarin prevents diabetic renal damage by inhibiting miRNA-140-5p expression. Diabetes Metabolic Syndrome and Obesity. 2020;13:3947–3958. doi: 10.2147/DMSO.S273952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuki Y., Fukunaga K. Clinical therapeutic strategy and neuronal mechanism underlying post-traumatic stress disorder (PTSD) International Journal of Molecular Sciences. 2019;20(15) doi: 10.3390/ijms20153614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Pan B., Lv T., Liu L., Zhu J., Shen W.…Tian J. Inhibition of histone acetylation by curcumin reduces alcohol-induced fetal cardiac apoptosis. Journal of Biomedical Science. 2017;24(1):1. doi: 10.1186/s12929-016-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Zhao J., Cao C., Jia Z., Zhou N., Han S.…Cui H. Tetramethylpyrazine promotes SH-SY5Y cell differentiation into neurons through epigenetic regulation of topoisomerase IIbeta. Neuroscience. 2014;278:179–193. doi: 10.1016/j.neuroscience.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Yao B., Christian K.M., He C., Jin P., Ming G.L., Song H. Epigenetic mechanisms in neurogenesis. Nature Reviews Neuroscience. 2016;17(9):537–549. doi: 10.1038/nrn.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R., Hoge C.W., McFarlane A.C., Vermetten E., Lanius R.A., Nievergelt C.M.…Hyman S.E. Post-traumatic stress disorder. Nature Reviews Disease Primers. 2015;1:15057. doi: 10.1038/nrdp.2015.57. [DOI] [PubMed] [Google Scholar]
- Younesian S., Yousefi A.M., Momeny M., Ghaffari S.H., Bashash D. The DNA methylation in neurological diseases. Cells. 2022;11(21):3439. doi: 10.3390/cells11213439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Chen D.Q., Liu H.X., Li W.B., Lu J.W., Feng J.F. Rosmarinic acid reduces the resistance of gastric carcinoma cells to 5-fluorouracil by downregulating FOXO4-targeting miR-6785-5p. Biomedicine and Pharmacotherapy. 2019;109:2327–2334. doi: 10.1016/j.biopha.2018.10.061. [DOI] [PubMed] [Google Scholar]
- Zannas A.S., Provencal N., Binder E.B. Epigenetics of posttraumatic stress disorder: Current evidence, challenges, and future directions. Biological Psychiatry. 2015;78(5):327–335. doi: 10.1016/j.biopsych.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Zeng Y., Tan M., Kohyama J., Sneddon M., Watson J.B., Sun Y.E., Xie C.W. Epigenetic enhancement of BDNF signaling rescues synaptic plasticity in aging. Journal of Neuroscience. 2011;31(49):17800–17810. doi: 10.1523/JNEUROSCI.3878-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhai K., Duan H., Wang W., Zhao S., Khan G.J., Wang M.…Wei Z. Ginsenoside Rg1 ameliorates blood-brain barrier disruption and traumatic brain injury via attenuating macrophages derived exosomes miR-21 release. Acta Pharmaceutica Sinica B. 2021;11(11):3493–3507. doi: 10.1016/j.apsb.2021.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.S., Ouyang B., Ji X.Y., Liu M.F. Gastrodin alleviates cerebral ischaemia/reperfusion injury by inhibiting pyroptosis by regulating the lncRNA NEAT1/miR-22-3p axis. Neurochemical Research. 2021;46(7):1747–1758. doi: 10.1007/s11064-021-03285-2. [DOI] [PubMed] [Google Scholar]
- Zhang J.W., Li L.X., Wu W.Z., Pan T.J., Yang Z.S., Yang Y.K. Anti-tumor effects of paeoniflorin on epithelial-to-mesenchymal transition in human colorectal cancer cells. Medical Science Monitor. 2018;24:6405–6413. doi: 10.12659/MSM.912227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Wang W., Jiang Z., Zhu Z., Liu D., Pan F. Long-term effect of post-traumatic stress in adolescence on dendrite development and H3K9me2/BDNF expression in male rat hippocampus and prefrontal cortex. Frontiers in Cell and Developmental Biology. 2020;8:682. doi: 10.3389/fcell.2020.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou G., Zhong W., Wu F., Wang X., Liu L. Catalpol attenuates cardiomyocyte apoptosis in diabetic cardiomyopathy via Neat1/miR-140-5p/HDAC4 axis. Biochimie. 2019;165:90–99. doi: 10.1016/j.biochi.2019.05.005. [DOI] [PubMed] [Google Scholar]