Abstract
The telomere is a special functional complex at the end of linear eukaryotic chromosomes, consisting of tandem repeat DNA sequences and associated proteins. It is essential for maintaining the integrity and stability of linear eukaryotic genomes. Telomere length regulation and maintenance contribute to normal human cellular aging and human diseases. The synthesis of telomeres is mainly achieved by the cellular reverse transcriptase telomerase, an RNA-dependent DNA polymerase that adds telomeric DNA to telomeres. Expression of telomerase is usually required for cell immortalization and long-term tumor growth. In humans, telomerase activity is tightly regulated during development and oncogenesis. The modulation of telomerase activity may therefore have important implications in antiaging and anticancer therapy. This review describes the currently known components of the telomerase complex and attempts to provide an update on the molecular mechanisms of human telomerase regulation.
INTRODUCTION
The concept of the telomere had its origin in the late 1930s. Using cytogenetic approaches, Muller and McClintock independently observed that natural chromosome ends, unlike random DNA breaks, had special properties that protected them from end-to-end fusion. Due to extensive research efforts in the past few years, we now know that highly conserved telomere structures at the ends of linear chromosomes consist of tandem repeat DNA sequences and associated proteins (21).
The DNA sequence of telomeres typically consists of tandem GT-rich repeats, (TTAGGG)n, in humans and other vertebrates, with a single-stranded 3′-end overhang (155, 253). Electron microscopy has revealed that the single-stranded 3′-end overhang invades the duplex telomeric DNA repeat array to form a D-loop and T-loop structure in vitro (81, 84). Telomere binding proteins function to maintain and regulate this unique structure.
The telomere is involved in several essential biological functions. It protects chromosomes from recombination, end-to-end fusion, and recognition as damaged DNA; provides a means for complete replication of chromosomes; contributes to the functional organization of chromosomes within the nucleus; participates in the regulation of gene expression; and serves as a molecular clock that controls the replicative capacity of human cells and their entry into senescence.
Replication of chromosome ends pose a special problem for cells. Watson and Olovnikov independently hypothesized that DNA should be progressively lost from the ends of chromosomes each time cell divides, because the conventional DNA polymerase could not fully replicate the 3′ end of the lagging strands of the linear molecule (the end replication problem) (Fig. 1). Consistent with this hypothesis, telomere shortening was observed with progressive cell division in vitro and increased age in vivo (47, 95, 99, 147). Normal mammalian somatic cells proliferate a limited number of times in vitro, with the maximum number being referred to as the Hayflick limit (100). This shortening in normal human cells acts as a molecular clock that could monitor the replicative history of cells (96, 252) (Fig. 2).
FIG. 1.
End replication problem. DNA replication by the conventional polymerase proceeds in the 5′-to-3′ direction. The newly synthesized leading strands would not generate overhangs, but the newly synthesized lagging strands would lose their extreme 3′ end after RNA primers are removed. In addition, both parental strands might also be subject to nuclease processing.
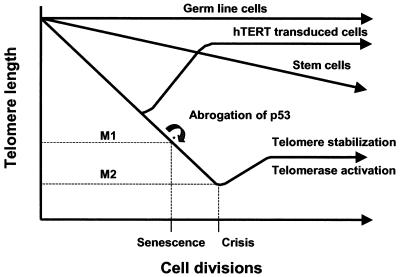
FIG. 2.
Two-step hypothesis of cellular senescence and immortalization. Unlike germ cells, in which telomere length is maintained by telomerase, most human somatic cells have lower levels of telomerase or are telomerase negative and experience telomere shortening with each cell division. Pluripotent stem cells are telomerase positive but do not maintain full telomere length. Telomere length shortens in stem cells at rates slower than that of telomerase-negative somatic cells. Critically shortened telomeres may signal cells to enter senescence at the Hayflick limit, or M1. This proliferative checkpoint can be overcome by inactivation of pRB/p16 or p53. Such cells continue to suffer telomere erosion and ultimately enter crisis, or M2, characterized by widespread cell death. Rare surviving cells acquire unlimited proliferative potential and stabilization of telomere length, almost universally by activation of telomerase. When cells are cultured in adequate conditions, ectopic expression of hTERT allows cells to bypass proliferation barriers and become immortal.
At the Hayflick limit, one or more critically shortened telomeres trigger a permanent growth arrest known as replicative senescence or mortality stage 1 (M1) (92, 211, 248). Cells that escape replicative senescence by inactivation of a critical cell cycle checkpoint gene such as p53 continue to divide and suffer further telomere loss until they reach a second proliferative block, crisis, or mortality stage 2 (M2) (39, 213, 214), characterized by massive cell death triggered by critically short and dysfunctional telomeres. Rare survivor cells escaping from crisis are able to maintain telomere length, in most cases by activation of telomerase, and this leads to unlimited proliferative capacity, i.e., cellular immortalization.
Telomerase is an RNA-dependent DNA polymerase that synthesizes telomeric DNA sequences and almost universally provides the molecular basis for unlimited proliferative potential. Since first discovered in Tetrahymena thermophila in 1985 (82), telomerase activity was found to be absent in most normal human somatic cells but present in over 90% of cancerous cells and in vitro-immortalized cells (124, 210). Telomerase consists of two essential components: one is the functional RNA component (in humans called hTR or hTERC) (64), which serves as a template for telomeric DNA synthesis; the other is a catalytic protein (hTERT) with reverse transcriptase activity (98, 123, 149, 163). hTR is highly expressed in all tissues regardless of telomerase activity (8), with cancer cells generally having fivefold-higher expression than normal cells (264). In contrast, the expression (mRNA) of the human catalytic component hTERT is estimated at less than 1 to 5 copies per cell (264) and is closely associated with telomerase activity in cells. hTERT is generally repressed in normal cells and upregulated in immortal cells, suggesting that hTERT is the primary determinant for the enzyme activity.
Recent studies demonstrated that expression of human telomerase alone is sufficient for the immortalization of diverse cell types and for allowing transformed cells to escape from crisis (24, 191, 199, 263). Importantly, telomerase can cooperate with oncogenes or with inactivation of tumor suppressor genes to induce tumorigenic conversion of normal human epithelial cells and fibroblasts (89). Taken together, these findings indicate that telomerase plays an important role in cellular aging and tumorigenesis. Since modulation of telomerase activity may have important implication for the development of diagnostic and therapeutic strategies, the mechanisms of telomerase regulation are of great interest.
FUNCTIONAL ASSEMBLY OF HUMAN TELOMERASE
Telomerase activity can be reconstituted in rabbit reticulocyte lysates from in vitro-transcribed hTR and in vitro-transcribed and -translated hTERT (17, 228, 243) and by ectopic expression of hTERT in telomerase-negative cells which express hTR (237, 244). Human telomerase activity can also be reconstituted in Saccharomyces cerevisiae by coexpressing hTR and hTERT (10, 11). These studies suggest that hTR and hTERT are the minimal requirement for telomerase activity. However, telomerase activity as measured by the telomere repeat amplification protocol (TRAP) assay (124) does not always imply telomere maintenance in vivo. Addition of a hemagglutinin (HA) epitope tag to the C terminus of hTERT preserves the catalytic activity but abolishes the ability of the enzyme to elongate telomeres in vivo (40, 190). Assembly of functional telomerase and elongation of telomeres are probably multistep processes that involve other telomerase components and associated proteins to form a functional, multisubunit telomerase holoenzyme.
RNA Subunit (hTR) of Telomerase
The RNA component (hTR) of telomerase provides the template for telomeric repeat synthesis. In humans, hTR is transcribed by RNA polymerase II and is processed at its 3′ end to produce a mature transcript of 451 nucleotides (64). The template for reverse transcription lies near the 5′ end of the molecule (nucleotides 46 to 53), which specifies synthesis of the telomeric DNA sequence. Despite divergence of the primary sequences among telomerase RNAs, hTR has a remarkably conserved secondary structure with telomerase RNAs from a variety of vertebrate species, indicating an important role for RNA structure in telomerase function (30).
The predicted secondary structure contains four conserved functional elements, including a pseudoknot domain (CR2/CR3), a CR4/CR5 domain, a box H/ACA (CR6/CR8) domain, and a CR7 domain. Interestingly, the hTR box H/ACA motif resembling a box H/ACA small nucleolar RNA (snoRNA) has been shown to be essential for hTR accumulation, hTR 3′ processing, and telomerase activity in cells (51, 55, 167, 168, 195). However, the hTR H/ACA domain is not present in lower eukaryotes. The telomerase RNAs from ciliates are small (150 to 200 nucleotides) and do not contain any H/ACA-like domain (33), while yeast telomerase RNA contains a binding site for Sm proteins, which are involved in nuclear mRNA splicing (207).
The differences in telomerase ribonucleoprotein architectures between mammalian and lower eukaryotic cells may reflect fundamental differences in the biogenesis and assembly of telomerase. It appears that the conserved domains within the hTR molecule are recognition sites for hTR binding proteins. A number of RNA binding proteins, such as hGAR, dyskerin, hNOP10, hNHP2, hStau, L22, hnRNP C1/C1, La, and hTERT, interact with hTR and are involved in hTR stability, maturation, accumulation, and functional assembly of the telomerase ribonucleoprotein complex (51, 55, 67, 68, 140, 167, 169, 195). Two regions within the hTR molecule (nucleotides 1 to 209, containing the template region, and nucleotides 241 to 330, containing the box H/ACA domain and CR4-CR5 domain) interact independently with the catalytic component of telomerase (hTERT) in a noncooperative manner (168). In agreement with this observation, in vitro telomerase reconstitution experiments indicate that a region from nucleotides 10 to 159 is the minimal sequence requirement for telomerase activity in vitro (11, 17), and two inactive fragments of hTR, nucleotides 33 to 147 and nucleotides 146 to 325, reconstitute in trans telomerase activity in vitro, suggesting that telomerase RNA sequence or the structure involved in binding to hTERT and its catalysis are functionally separated (11, 15, 168, 228).
Catalytic Subunit (hTERT) of Telomerase
The catalytic subunit of telomerase was initially purified biochemically from Euplotes aediculatus as p123 (149). Sequencing analysis revealed that p123 contains reverse transcriptase motifs and is homologous to the yeast Est2p, a protein required for telomere maintenance that was initially identified by genetic screening of yeast mutants with reduced telomere length and the senescence phenotype (143). Introduction of single-amino-acid substitutions within the reverse transcriptase motifs leads to telomere shortening and senescence in S. cerevisiae, indicating that these motifs are important for catalysis of telomere elongation in vivo (41).
Based on the conserved sequence information within the reverse transcriptase motifs from p123, the cDNA encoding the human homologue of the catalytic subunit of telomerase (hTERT) was identified nearly simultaneously by four groups (98, 123, 163, 177) and has since been identified in mice, other yeasts, ciliated protozoa, worms, and plants (26, 34, 41, 66, 78, 88, 156, 162, 183). The telomerase catalytic subunits from different organisms are phylogenetically conserved in their reverse transcriptase motifs with other reverse transcriptases (177), but are more related to each other than to other reverse transcriptases and therefore form a distinct subgroup within the reverse transcriptase family (56, 176). Several features distinguish the telomerase catalytic subunit: (i) all of the reverse transcriptase motifs are located in the C-terminal half of the proteins; (ii) a conserved telomerase-specific region, termed the T motif, is located just N-terminal to the reverse transcriptase motifs; and (iii) a large N-terminal region contains conserved, functionally important domains (4, 71, 172, 177, 256).
Experiments in both humans and S. cerevisiae indicate that telomerase might function in a complex containing more than one catalytic and one RNA subunit (15, 168, 172, 245). It is likely that elongation of telomeres by telomerase requires a multistep process and is precisely regulated, including the maturation, processing, and accumulation of hTR, nuclear transport, posttranslational modifications of hTERT, ribonucleoprotein assembly, substrate recognition, and coordinated synthesis of the C-strand (1). The telomerase-associated proteins involved in each of these processes may be required for the full activity and biological function of the enzyme.
Telomerase-Associated Proteins
Although coexpression of hTERT and hTR in rabbit reticulocyte lysates suffices to reconstitute basic telomerase enzymatic activity (17, 228, 243), this in vitro reconstitution clearly does not address some of the in vivo requirements for other factors required for the assembly of the active enzyme, since some of these factors may already be present in the rabbit reticulocyte lysates. Indeed, the molecular chaperones Hsp90 and p23, which directly associate with hTERT, are present in rabbit reticulocyte lysates and are necessary for telomerase activity (108). Both biochemical and genetic studies suggest the existence of additional protein subunits of telomerase that may be involved in the biogenesis or assembly of active telomerase and may mediate or regulate the access of telomerase to its substrate, the telomeres (Table 1).
TABLE 1.
Human telomerase-associated proteinsa
| Protein | Interacting region | Function | Reference(s) |
|---|---|---|---|
| hTERT associated | |||
| TEP1 | aa 1-350, 601-927 | Unknown | 16 |
| P23/p90 | aa 1-195 | Assembly/conformation | 108 |
| 14-3-3 | aa 1004-1132 | Nuclear localization | 206 |
| hTR associated | |||
| TEP1 | nt 1-871 | Unknown | 97 |
| hGAR1 | hTR H/ACA domain | Stability, maturation, localization | 55, 195 |
| Dyskerin/NAP57 | hTR H/ACA domain | Stability, maturation, localization | 55, 167 |
| hNOP10 | hTR H/ACA domain | Unknown | 168 |
| hNHP2 | hTR H/ACA domain | Stability, maturation, localization | 195 |
| C1/C2 | nt 33-147 | Stability, maturation, localization | 195 |
| La | nt 1-205, 250-451 | Accessibility to telomeres? | 68 |
| A1/UP1 | nt 1-208 | Unknown | 67 |
| hStau | nt 64-222 | Accessibility to telomeres? | 65 |
| L22 | nt 64-222 | hTR processing, localization? | 140 |
aa, amino acids; nt, nucleotides.
hTERT-associated proteins.
The first telomerase-associated proteins were identified by biochemical fractionation of telomerase activity from Tetrahymena thermophila (35). Two proteins, p80 and p95, were identified by copurification of telomerase activity and by association with telomerase RNA. A recent study shows that in Tetrahymena strains lacking p80 and p95, telomerase activity and levels of telomerase RNA appear to function completely normally, suggesting that these proteins are not core telomerase components and may be separate ribonucleoproteins that copurified nonspecifically with telomerase (158). However, an independent study shows that cells without p80 and p95 have elongated telomeres in both macronuclei and micronuclei and lose genetic content in their micronuclei, suggesting a role for p80 and p95 in telomere length maintenance and micronuclear genomic stability (164).
The mammalian homolog of p80, TEP1 (telomerase-associated protein 1), which is associated with telomerase activity, was identified in humans, mice, and rats (97, 178). TEP1 consists of 2,629 amino acids and is much larger than p80. The 900 amino acids at the amino terminus of TEP1, which contain the region homologous to p80, were found to interact with telomerase RNA. The carboxyl terminus of TEP1 contains 12 WD40 repeats, a motif known to be involved in protein-protein interactions. Expression of TEP1 is detected in most tissues regardless of telomerase activity. Despite its association with both the RNA and catalytic components of telomerase in cell extracts from immortalized human, mouse, and rat cells (16, 97), disruption of mouse TEP1 does not affect telomerase activity or telomere length (153). Recently, TEP1 has also been identified as a component of large cytoplasmic particles termed vaults, which are ribonucleoprotein complexes (122, 129). The functions of TEP1 in both ribonucleoproteins (telomerase and vaults) are still unknown.
Using the amino terminus (amino acids 1 to 195) of hTERT as the bait in a yeast two-hybrid screen, the molecular chaperone p23 was first found to be associated with hTERT (108). Subsequently, both p23 and p90 were detected in association with hTERT in vitro and in mammalian cells (108). The observation that p23 and p90 are required for assembly of functional telomerase from in vitro-synthesized telomerase components reveals the functional significance of these interactions. The hsp90 chaperone complex is the first known set of proteins that physically and functionally interact with human telomerase to assist in proper ribonucleoprotein assembly and the formation of active telomerase enzyme (108). It is known that other reverse transcriptases of viral origin also associate with hsp70, hsp90, and p23, but their interactions appear to be transient. However, recent studies indicate that hsp90 and p23 but not hsp70 remain stably associated with the human telomerase (70).
The human telomerase differs from many other reverse transcriptases in that it remains associated with its template RNA subunit. The telomerase recognizes and elongates telomeres through association with the hTR template region and then translocates to the next available position for hTR binding. This translocation step may require an adjustment of conformation of the assembled active telomerase, which could be mediated by the stably associated hsp90 and p23 (70).
In another yeast two-hybrid screen, the 14-3-3 proteins were also identified as hTERT binding partners and as apparent regulators of the nuclear localization of telomerase (206). The 14-3-3 family proteins play a regulatory role in signal transduction, cell cycle checkpoint, and apoptosis by binding to phosphoserine motifs and thereby regulating the subcellular localization of their binding partners (174). The C-terminal region of hTERT has been found to specifically interact with the C-terminal region of 14-3-3 proteins both in vitro and in vivo. However, this interaction is not required for telomerase activity in vitro or in intact cells (206).
The C-terminal region of hTERT that interacts with 14-3-3 proteins appears to be an amphipathic helix with serine/threonine clusters, which is a characteristic of 14-3-3 binding motifs. Mutations in these serine/theronine residues (1030Thr, 1037Ser, and 1041Ser) abolish the interaction and result in cytoplasmic localization of hTERT. In addition, a dominant negative 14-3-3 inhibits nuclear accumulation of hTERT. These results indicate that the 14-3-3 proteins promote the nuclear localization of hTERT (206).
Its structure and primary sequence suggest that hTERT contains a putative nuclear export signal, located just at the N terminus of the 14-3-3 binding region. This leads to the hypothesis that 14-3-3 binding to hTERT masks the nuclear export signal and therefore blocks nuclear export of hTERT (206). The mechanistic involvement of 14-3-3 in the nuclear localization of telomerase remains to be determined. Interestingly, 14-3-3-deficient mouse cells show frequent losses of telomere sequences and enhanced frequencies of chromosome end-to-end fusion (52). This suggests additional functions of the 14-3-3 proteins in G2/M checkpoint control and genomic integrity that may be independent of its involvement in the nuclear localization of hTERT (69, 193).
hTR-associated proteins.
The conserved domains within the hTR molecule are predicted to be recognition sites for hTR-binding proteins (30). In agreement with the structural analyses, several RNA binding proteins have been shown to interact with hTR. These proteins are components of the small ribonucleoproteins complex and heterogeneous nuclear protein complex involved in RNAs stability, maturation, pre-RNA processing and localization.
The mammalian telomerase RNA contains a 3′-terminal extension that structurally resembles H/ACA snoRNAs (30, 167). Alterations in the hTR H/ACA domain affect the accumulation of hTR and telomerase activity in vivo (168). The H/ACA box is not present in lower eukaryotes such as ciliates and S. cerevisiae. In S. cerevisiae, the telomerase RNA contains a 3′-terminal extension with a binding site for the Sm proteins (207). The presence of the H/ACA domain in mammalian telomerase RNA indicates a fundamental difference in telomerase RNA biogenesis and perhaps in assembly of telomerase between mammalian and lower eukaryotic cells.
All four common H/ACA snoRNA binding proteins (hGAR1, dyskerin/NAP57, hNOP10, and hNHP2) are found to be associated with hTR and human telomerase (51, 55, 169, 195). In addition, 7 to 8% of total nuclear hTR can be reproducibly recovered from the nucleolar fraction (167). These observations indicate that hTR is associated with small nucleolar ribonucleoprotein complexes (snoRNPs), suggesting the involvement of snoRNPs in the stability, accumulation, maturation, and localization of hTR. This association also raises questions about the location of telomerase assembly. It is of interest to determine whether the 7 to 8% of hTR nucleolar fraction is associated with active telomerase, because the nucleolus is the site of assembly of other ribonucleoproteins (188). Interestingly, a recent study shows that hTERT itself contains a nucleolar localization domain and indeed localizes to the nucleolus (61). The colocalization of hTR and hTERT within the nucleolus supports the possibility that the nucleolus might be the site of telomerase ribonucleoprotein biogenesis.
In addition to the snoRNA binding proteins, the heterogeneous nuclear ribonucleoproteins (hnRNPs) C1 and C2 and A1 and UP1 have been shown to associate with hTR in vivo (65, 68, 136). The hnRNPs C1 and C2 interact specifically with the 6-base uridylate tract within hTR and associate with the human telomerase complex; furthermore, the binding of hnRNPs C1 and C2 to telomerase correlates with the ability of telomerase to access the telomeres (68). The simultaneous interaction of A1 and UP1 with telomeric DNA and hTR strongly supports a role for A1 and UP1 in the accessibility of telomerase to telomeres. In support of this idea, overexpression of A1/UP1 results in elongation of telomeres in HeLa cells (65, 136). Other RNA binding proteins that interact with hTR and telomerase have also been identified. For example, the La antigen specifically interacts with hTR and telomerase and influences telomere length in vivo (67). Finally, hStau and L22 interact with hTR and may play a role in hTR processing and localization and telomerase assembly (140).
While the list of telomerase-associated proteins is still growing, their precise actions in the multistep processes of hTR biogenesis and telomerase assembly and their potential functions in regulating telomerase activity and in the accessibility of telomerase to telomeres for cooperative G-strand synthesis remain to be determined.
TELOMERASE ACTIVITY IN NORMAL HUMAN CELLS AND IN CANCERS
The highly sensitive TRAP assay to detect and measure the presence and level of telomerase activity from small sample sizes has allowed the evaluation of telomerase activity from a wide variety of normal human tissues and a whole spectrum of human tumors (124, 210). In normal human cells, telomerase activity appears to be strictly regulated during development (249). Telomerase activity is extinguished during embryonic differentiation in most somatic cells but remains active in some tissues, such as male germ cells, activated lymphocytes, and certain types of stem cell populations (124, 210, 249). Consistent with the telomere hypothesis, the high proliferative potential of these normal tissues would entail a special need for telomerase to maintain telomere length and genetic stability.
Deregulation of telomerase expression has been directly linked to human diseases (157). Human dyskeratosis congenita is a multiple-systems disease resulting from proliferative deficiencies that affects tissues such as skin, gut, and bone marrow, all of which require constant renewal and are normally highly regenerative. A molecular defect of dyskeratosis congenita results in dysfunction of telomerase due to mutations in either hTR or the telomerase RNA-associated protein dyskerin (169, 239). Affected patients have markedly shorter telomeres than normal individuals. Dyskeratosis congenita provides direct evidence of the importance of telomerase in normal human growth and development.
Defects in telomerase result in genetic instability and increased incidence of skin cancers, as seen in late-generation animal models lacking both p53 and telomerase (22, 141). Cells in rapidly dividing tissues that usually express telomerase would be predicted to be the most severely affected by telomerase defects. In these disorders, the more rapid and progressive telomere loss with each cell division would result in critically shortened telomeres and the induction of chromosome instability, particular in sun-exposed skin, which frequently develops p53 mutations. Indeed, chromosome end-to-end fusions have been detected both in dyskeratosis congenita and in late-generation telomerase-deficient mice (5, 54).
Most types of normal human somatic cells, however, are telomerase negative and have a limited replicative capacity (100, 212, 250). When this limit is reached, which depends on cell type and origin, cells will permanently cease proliferating. Such cells undergo an array of biochemical and morphological changes but remain viable for a long period in culture (76). It has been suggested that senescence could function as a tumor suppressor mechanism to prevent the accumulation of multiple oncogenic mutations (205, 250).
During normal human growth and development, telomerase activity is regulated to meet the proliferative demand of specific cellular functions while at the same time preserving proliferative barriers (senescence) against tumorigenesis. To date, telomerase activity has been detected in approximately 90% of tumor samples (124, 210). Recent experimental data indicate that expression of telomerase is sufficient for the escape of cells from the two barriers to proliferation (M1 and M2) and for the immortalization of many cell types (94).
In cooperation with several oncogenes, telomerase expression results in direct tumorigenic conversion of normal human epithelial cells and fibroblasts (89). The specific role of telomerase in this process is that it provides an unlimited replicative potential. It might be not needed if young cells with sufficient remaining proliferative capacity are used. Furthermore, inhibition of telomerase in immortal cells leads to telomere shortening and apoptotic cell death (90, 103, 271). All these observations indicate that telomerase activity is almost universally an essential requirement for the cellular immortalization and unlimited proliferation characteristic of cancer cells. Understanding the mechanisms of telomerase regulation would certainly have important implications for research on and management of human cancers.
REGULATION OF HUMAN TELOMERASE ACTIVITY
The regulation of telomerase activity occurs at various levels, including transcription, mRNA splicing, maturation and modifications of hTR and hTERT, transport and subcellular localization of each component, assembly of active telomerase ribonucleoprotein, and accessibility and function of the telomerase ribonucleoprotein on telomeres. A number of studies have indicated that telomerase activity is modulated under particular physiological conditions during tissue development and homeostasis (105, 134, 249). In addition to growth-related regulation, telomerase activity is subject to regulation by differentiation (78, 163, 258, 270) and by extra- and intracellular signals such as UV irradiation (91), alpha interferon (IFN-α) (257), and estrogen (133, 166).
Importantly, among the core components of human telomerase, only the catalytic component hTERT seems to be the limiting determinant of telomerase activity, as other components are usually expressed ubiquitously. In most cases, hTERT expression is closely correlated with telomerase activity and with cancer initiation and progression. It is transcriptionally repressed in many normal cells and is reactivated or upregulated during immortalization. Substantial experimental data demonstrate that the transcriptional regulation of hTERT expression represents the primary and rate-limiting step in the activation of telomerase activity in most cells (37, 110, 163, 224).
Transcriptional Regulation of hTERT Gene
Telomerase activity is extinguished in many tissues during embryonic development (249). The correlation between hTERT mRNA and telomerase activity indicates transcriptional regulation of the hTERT gene. Soon after the hTERT cDNA became available, the genomic organization of hTERT was established (37, 246). The hTERT promoter has been characterized by several laboratories (37, 110, 224, 246, 255). These materials have provided essential reagents for the molecular study of the transcriptional regulation of hTERT, which has been a major focus in the field of telomerase regulation.
Localization and organization of hTERT gene.
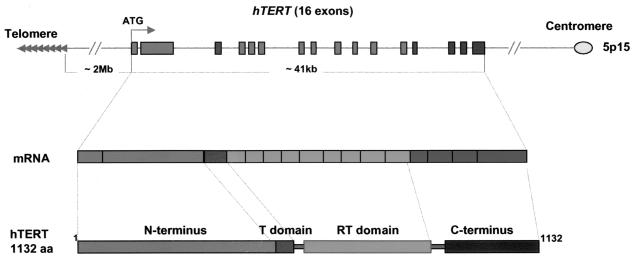
In human diploid cells, the hTERT gene is present as a single copy on chromosome band 5p15.33 (Fig. 3), the most distal band on the short arm of chromosome 5p (27, 163). The mapping of the hTERT gene to the subtelomeric region led to the speculation that telomere positional effect may contribute to the repression of hTERT gene expression. Telomere position effect, which results in the reversible silencing of a gene near the telomere, has been well characterized in the yeast S. cerevisiae (229) and was recently observed in human cells (14). However, the complete genomic sequence of hTERT indicates that the hTERT gene is more than 2 Mb away from the telomere on the short arm of chromosome 5 (142). This is much farther away from a telomere than previously thought.
FIG. 3.
Gene organization of the hTERT gene. The human hTERT gene consists of 16 exons and 15 introns located on the short arm of chromosome 5 (5p15.33), approximately 2 Mb away from the telomere. It is transcribed towards the centromere. The specific telomerase domain (T domain), reverse transcriptase domain (RT domain), and the C-terminal region of the hTERT protein are indicated.
Although the distance over which telomere position effect might act in mammalian cells is unknown, 2 Mb is sufficiently large to make it unlikely that the telomere position effect would influence hTERT expression. On other hand, normal human cells age and ultimately senesce because of progressive telomere shortening with each cell division. Cancer cells, however, usually have relatively shorter but stable telomeres. It is possible that the telomere position effect provides a mechanism to incrementally alter subtelomeric or telomeric gene expression profiles and induce cellular age-associated phenotypes (251).
The hTERT gene consists of 16 exons and 15 introns and extends over 40 kb (37, 246). A similar gene organization of telomerase catalytic genes has been reported for Tetrahymena thermophila and Schizosaccharomyces pombe, with 18 and 15 introns, respectively (26, 34), while the Oxytricha trifallax, Euplotes aediculatus, and S. cerevisiae TERT genes contain no introns (26).
The hTERT gene is differentially spliced (123), and several transcripts have been detected in human cells (123, 233-235, 266). They include the full-length transcript; the α spliced transcript, which lacks 36 nucleotides from the 5′ end of exon 6; the β spliced transcript, which lacks exon 7 and exon 8; both α and β spliced transcripts; and insertional alternative transcripts, including transcripts with a 159-nucleotide insertion of intron 14, a 38-nucleotide insertion of intron 4, a partial insertion of intron 14, and the replacement of exon 15 and part of exon 16 with the first 600 nucleotides of intron 14. All of the various transcripts are expressed during human development in a tissue-dependent and gestational age-dependent manner, but only the full-length hTERT transcript is associated with telomerase activity (233, 235).
The function of hTERT alternative spliced mRNAs is currently unknown. The protein products of these alternatively spliced transcripts have not been detected in cells, although the potential dominant negative function of a spliced product has been observed by exogenous expression (32, 265). The specific pattern of expression of splicing variants in human development indicates that splicing events are not random and could have a physiological function in cells (234). Alternative splicing of hTERT may represent an additional regulation of TERT expression and telomerase activity.
The hTERT gene is frequently amplified in human tumors and tumor cell lines (27, 268). This suggests that an increased copy number of the hTERT gene may contribute to the upregulation of telomerase expression in immortalized cells. However, gene amplification of hTERT appears to occur primarily by increasing numbers of chromosomes carrying a single copy of hTERT rather than by amplifying the gene at a single locus. Whether hTERT amplification is a mechanism for hTERT upregulation or just associated with aneuploids awaits future investigation. Interestingly, ectopic hTERT expression can restore telomerase activity in normal human cells and immortalize cells without inducing a malignant phenotype (24, 113, 171, 237). One could speculate that hTERT gene amplification are more frequent in tumors in which telomerase is activated after p53 mutation has occurred, in which short telomeres contributed to chromosomal instability and increased aneuploidy.
Features of the hTERT promoter.
Transcriptional regulation of hTERT is believed to be the major mechanism of telomerase regulation in human cells. Transient transfection experiments with hTERT promoter-luciferase reporters show that the hTERT promoter is inactive in normal and transformed preimmortal cells but, like telomerase, is activated in immortal cells (37, 224). Deletional analysis suggests that the minimum sequence requirement for promoter activity is contained within the 330 bp upstream of the ATG (the translation start site) (37, 224). However, it is possible that sequences further upstream of the minimal promoter may also be necessary for promoter activity in vivo and in certain physiological conditions. The differential activity of the hTERT promoter in mortal and immortal cells opens up the possibility of using this promoter for the selective expression of toxic genes in cancer cells for cancer therapy (85, 86, 128, 154).
Sequence analysis indicates that the hTERT promoter has no TATA or CAAT boxes but is highly GC-rich. The GC-rich region forms a large CpG island around the ATG, suggesting that methylation may be involved in regulation of hTERT expression, even though no consistent results have been reported. The transcription initiation sites map 60 to 120 bp upstream of the translational start site, depending on the methods and cell lines used in different laboratories (110, 224, 255). The hTERT promoter contains binding sites for many transcription factors that may be involved in its regulation (37). The abundance of these potential transcription factor binding sites suggests that the regulation of hTERT expression may be subject to multiple levels of control by different factors in different cellular contexts (37). Several transcription factors are known to participate in hTERT gene expression.
Activation of hTERT transcription. (i) c-Myc.
c-myc is a well-characterized oncogene which promotes proliferation, growth, and apoptosis (77). Alterations in its structure or in its expression have been linked to a wide variety of human cancers (48). The myc gene family encodes transcriptional factors containing activation domains at their N termini and a C-terminal bHLHZ (basic-helix-loop-helix-zipper) domain. Myc forms a heterodimer with Max, another bHLHZ protein, and these heterodimers recognize and bind to the sequence 5′-CACGTG, termed an E-box, or related sequences on target gene promoters (77).
The target genes of the myc family transcription factors are involved in many aspects of cellular functions, such as those involved in the cell cycle, growth, differentiation, and life span. The transcriptional activation function of Myc is thought to be mediated at least in part by recruitment of a histone acetyltransferase (160). Deregulation of c-myc function by overexpression, amplification, translocation, and mutation is frequently observed in human cancers, but the molecular mechanisms underlying c-myc oncogenic function remain unclear. In many cases, c-myc expression seems to parallel that of hTERT, in that it is upregulated in highly proliferative and immortal cells and downregulated during terminal differentiation. It has been shown that c-Myc induces hTERT expression and telomerase activity in normal human mammary epithelial cell and primary fibroblasts (241).
Sequence analysis indicates that the hTERT promoter bears two E-boxes in its core region −34 and −242 nucleotides upstream of the ATG (37, 110, 224, 246). The presence of E-boxes is compatible with a direct effect of c-Myc on hTERT activation. Indeed, overexpression of c-Myc enhances hTERT promoter activity in a promoter-luciferase reporter assay, while c-Myc fails to activate mutant hTERT promoters with a deletion in one or both E-boxes (79, 87, 135, 255). Furthermore, c-Myc-induced activation of hTERT expression is rapid and independent of cell proliferation and de novo protein synthesis (79, 185). Mobility shift and chromatin immunoprecipitation experiments revealed that c-Myc/Max heterodimers interact directly with the hTERT promoter (255, 259). Together, these results demonstrate that hTERT is a direct target of the Myc family of proteins.
The ability of c-Myc to activate hTERT gene expression and telomerase activity may contribute to c-myc-dependent cellular immortalization and transformation. However, expression of hTERT does not block the growth arrest-inducing properties of ras in the rat embryonic fibroblast cooperative assay (79). Cotransfection of c-myc and activated ras (H-rasG12V) increases the malignant transformation of early-passage rat embryonic fibroblasts, but cotransfection of hTERT and ras does not generate more transformed foci than ras alone (79). These results suggest that activation of hTERT expression by c-myc may be important in human cells but is insufficient to account for the transforming activity of c-Myc.
(ii) Sp1.
One interesting feature of the hTERT core promoter is that it contains five GC-boxes that are potential binding sites for the transcription factor Sp1 (135). These sites are located between two E-boxes, within which the major initiation sites of hTERT transcription have been mapped (−242 and −34 nucleotides upstream of the ATG). These GC-boxes could function as initiator elements of transcription. The significance of this organization has not been critically examined. However, Sp1 does cooperate with c-Myc to activate transcription of hTERT in a cell type-dependent manner (135).
Sp1 is a general transcription factor that binds to GC-boxes of promoters and enhancer and locus control regions to activate a large number of genes (222). It has been shown that Sp1 interacts with components of the general transcription machinery, such as the TATA-box binding protein (TBP) and TBP-associated factors, to help initiate transcription of TATA-less promoters (58, 106, 197). The hTERT promoter is a TATA-less promoter, and the Sp1 sites within the core promoter were found to be absolutely required for hTERT promoter activity, since mutations in these sites abolished the promoter activity in luciferase reporter assays (36, 135). While the exact mechanisms by which Sp1 contributes to hTERT transcription are unknown, the fact that Sp1 cooperates with c-Myc to activate hTERT transcription in a cell type-specific manner suggests an involvement of other transcription factors in this regulation.
(iii) Human papillomavirus 16 E6.
Telomerase activity can be induced in primary human keratinocytes and mammary epithelial cells by human papillomavirus 16 E6 protein (126). This activation is mediated by upregulation of hTERT transcription by E6, independent of degradation of p53 or induction of c-myc (75, 187, 238). Although the mechanism by which E6 activates hTERT transcription is still unknown, these studies indicate a new function for human papillomavirus 16 E6 in oncogenic transformation by activation of hTERT transcription and telomerase activity, in addition to targeting other critical cellular proteins such as p53 (74, 200).
(iv) Steroid hormones.
Human telomerase activity is detected in normal human endometrium during the menstrual cycle and is tightly correlated with the proliferative activity of endometrial cells (134, 227). These observations suggested that steroid sex hormones might regulate telomerase activity. Sequence analyses of the hTERT promoter revealed two potential estrogen response elements. One is located at −950 bp upstream of the ATG and contains an Sp1 site adjacent to an estrogen response half-site known to function as an estrogen response element. The second element is located at −2754 bp upstream of the ATG (37, 133). Recent studies have now demonstrated that estrogen activates telomerase through direct transcriptional regulation of hTERT expression in hormone-sensitive tissues (133, 166).
Studies in estrogen receptor-positive human ovary epithelial cells showed that telomerase activity is readily detectable, in parallel with induction of hTERT mRNA and protein expression, within 3 h of treatment with 17β-estradiol. Estrogen activation of hTERT transcription seems to depend on estrogen receptor α but not estrogen receptor β in these cells. In vivo DNA footprinting revealed specific modifications of the −950 estrogen response element in estrogen receptor α-positive but not -negative cells upon treatment of with 17β-estradiol. In addition, luciferase reporter assays showed that the −950 estrogen response element is directly responsible for the observed induction of hTERT transcription by estrogen and that this estrogen response element is functional when placed into the heterologous thymidine kinase promoter (166).
The activation of the hTERT promoter by estrogen was also observed in a breast cancer cell line MCF-7, in which an estrogen response element located at −2754 upstream of the ATG plays a major role in mediating hTERT activation as well as having an indirect effect on hTERT activation through induction of c-myc by estrogen (133). In breast cancer and colon cancer cell lines, tamoxifen, a nonsteroid antiestrogen drug widely used as adjuvant therapy to treat breast cancer, reduces telomerase activity (2, 179). The antagonistic effect of tamoxifen on estrogen-induced telomerase activity is consistent with its inhibitory effect on activation of the hTERT promoter by estrogen (166).
A second sex steroid hormone, progesterone, is known to antagonize estrogen's actions and inhibit estrogen-induced cell proliferation and has been used therapeutically to treat estrogen-dependent cancers (101). Evidence indicates that the hTERT promoter is a target of progesterone (242). Progesterone exerts a biphasic effect on hTERT expression, depending on the duration of exposure: hTERT mRNA is induced within 3 h but decreases after 12 h. Exposure to progesterone inhibits estrogen-induced activation of hTERT expression (242). The mechanism by which progesterone regulates hTERT expression seems complex. Although it may involve the mitogen-activated protein kinase-signaling pathway, the downstream effectors interacting directly with the hTERT promoter are unknown. On the other hand, progesterone induces expression of p21, a cyclin-dependent protein kinase inhibitor that negatively regulates the cell cycle. It is known that cell cycle exit results in downregulation of telomerase (19, 109). Therefore, progesterone's negative effects on the hTERT activation by estrogen may be indirect.
The effects of androgens on telomerase activity have also been studied in human prostate cancer cells. Telomerase activity is reduced by androgen deprivation in the androgen-sensitive cell line LNCaP but is reactivated upon treatment with testosterone. However, androgens have no effect on telomerase activity in the androgen-independent cell line DU145 (218). The mechanism underlying activation of telomerase by androgens is unknown; the conversion from androgen to estrogen could contribute to telomerase activation, or androgens could activate hTERT transcription directly. These possible mechanisms remain to be investigated
The finding that steroid hormones directly target human telomerase may provide important insights into the molecular mechanisms of tumorigenesis in hormone-dependent tissues and perhaps, in the future, clinical management of hormone-dependent cancers.
Repression of hTERT transcription.
It has been proposed that the lack of telomerase activity in most somatic human cells is due to transcriptional repression of the hTERT gene and that loss of this repression results in the upregulation of hTERT expression and telomerase activity seen during multistage carcinogenesis and associated with cellular immortality. Consistent with this hypothesis, cell fusions between normal somatic cells and some telomerase-positive immortal cells result in repression of telomerase activity (112, 247). Importantly, microcell-mediated transfer of specific human chromosomes into cancer cells results in repression of hTERT expression and downregulation of telomerase activity (189). These observations indicate that normal cells express functional transcriptional repressors of hTERT (209). As shown below, several chromosomes have been found to potentially contain transcriptional repressors of hTERT (12, 44, 59, 175, 182, 219, 226).
Evidence of hTERT transcriptional repressors in normal human chromosomes.
Transfer of chromosome 3 but not chromosome 7, 11, 8, 12, or 20 into the renal carcinoma cell line RCC23 and the breast carcinoma cell line 21NT resulted in repression of hTERT mRNA expression and downregulation of telomerase activity. This repression was associated with progressive telomere shortening and subsequently led to cellular senescence (189). Fine deletion analysis indicates two regions on the short arm of chromosome 3 (3p21.3 and 3p12-21.1) that might contain putative telomerase repressors or transcriptional repressors of hTERT (44, 226). Loss of heterozygosity or homozygous deletion in these two regions is frequently detected in human cancers (44).
In another study, normal human chromosomes 2, 4, 5, 10, and 16 were transferred individually into the telomerase-positive human hepatocellular carcinoma cell line Li7HM (182). Transfer of chromosome 10 repressed hTERT mRNA expression and telomerase activity. Transfer of a series of defined fragments from chromosome 10p successfully mapped a region of 28.9 centimorgans (cM) on 10p15.1 that caused immediate repression of telomerase and led to progressive telomere shortening, indicating that this region might contain a gene(s) encoding a repressor of hTERT (182). A recent report by the same group suggests that region 10p15.1 might also contain an hTR repressor-related gene (170). Similarly, transfer of chromosome 6 but not 11 into a human papillomavirus 16-immortalized human keratinocyte cell line and a cervical cancer cell line suggests the presence of putative telomerase repressors on chromosome 6p (219).
The observation that the transfer of a specific normal chromosome into a telomerase-positive cell represses hTERT expression and telomerase activity strongly argues that normal human cells express transcriptional repressors of hTERT and that these repressors may also have a tumor suppression function. By using approaches such as positional cloning and chromosome-specific microarrays, the mapping of those putative repressors to a specific chromosome or regions would provide important information about and reagents for identifying the corresponding genes (209). In addition, several known transcription factors have already been found to negatively regulate hTERT transcription.
Negative regulators of hTERT transcription. (i) Mad1.
The members of the c-Myc/Max/Mad network are central to the control of normal cell growth and development and regulate diverse processes such as cellular transformation, differentiation, and apoptosis (77). This family of transcription factors that dimerize and bind to the E-box of promoters are capable of either activating or repressing E-box-containing promoter activity. Both c-Myc and Mad can dimerize with the ubiquitously expressed Max. c-Myc/Max heterodimers bound to E-boxes activate gene expression, while Mad/Max heterodimers compete for binding to E-boxes and repress transcription. This antagonistic effect on hTERT promoter activity has been demonstrated during the differentiation of HL60 cells by chromatin immunoprecipitation assays (259).
In exponentially proliferating HL60 cells expressing hTERT and telomerase, the hTERT promoter E-boxes are occupied primarily by c-Myc/Max heterodimers. In contrast, in differentiated HL60 cells, the Mad1 protein was induced and bound to the E-boxes (259). This switch from c-Myc/Max to Mad1/Max results in repression of hTERT transcription and downregulation of telomerase activity. In addition to the opposite activity of c-Myc and Mad1 on hTERT transcription, c-Myc expression is often upregulated in telomerase-positive cells, such as immortal cell lines and tumor cells, while Mad expression is usually low. However, the expression levels of c-Myc and Mad1 are reversed in most normal human somatic cells and in differentiated cells, in which the hTERT gene is repressed.
In a number of tumor samples analyzed, Mad1 expression is either lost or is too low to be detected compared to the level in normal matched tissues (87). Mad1 was identified in a screen for hTERT transcriptional repressors by the expression cloning approach with a cDNA library prepared from normal human kidney cells (186). Overexpression of Mad1 resulted in decreased hTERT promoter activity in luciferase reporter assays. This repression was dependent on the E-boxes present in the hTERT promoter and was counteracted by ectopic expression of c-Myc (87, 186). These observations suggest that the interplay of c-Myc and Mad1 might play a direct role in determining either activation or repression of hTERT transcription.
(ii) p53.
The tumor suppressor protein p53 inhibits tumor formation by inducing cell cycle arrest or apoptosis in response to a variety of types of cellular damage (144). As a transcription factor, p53 regulates many specific target genes involved in the cell cycle, differentiation, senescence, and apoptosis (57). p53 function is inactivated in more than 50% of all human cancers (6, 107). The facts that telomerase activity is upregulated in most human cancers and highly proliferative somatic cells and downregulated with cell cycle exit and differentiation indicate that cell cycle regulators may also be involved in the regulation of telomerase.
Overexpression of p53, E2F, p16, p21, and p15 individually has been shown to induce premature growth arrest accompanied by inhibition of telomerase activity in head and neck squamous cell carcinoma and human glioma cell lines (73, 102, 115). Recent studies provide evidence that p53 inhibits telomerase activity through the transcriptional repression of hTERT (115, 132, 260). This repression occurs within hours after induction of p53, before cell cycle arrest or apoptosis takes place. Therefore, the transcriptional repression of hTERT by p53 might be independent of cell cycle arrest or apoptosis.
Interestingly, repression by p53 requires the transcription factor Sp1 (115, 260). Mutation in Sp1 binding sites in the core promoter abolishes the repression of the hTERT promoter by p53 (115). In addition, experiments conducted in Drosophila Schneider SL2 cells indicate that activation of the hTERT promoter is dependent on the ectopic expression of Sp1 and abolished by wild-type p53. p53 interacts with Sp1 and prevents Sp1 from binding to the hTERT promoter in vitro (260). However, the detailed mechanism by which p53 represses hTERT transcription is currently unknown. It is possible that p53 interacts with Sp1 and prevents other transcriptional activators, such as p300, impeding their access to the promoter (7). Conversely, the complex of Sp1 with p53 could bring repressor complexes, including histone deacetylases, to the promoter (173). The ability of p53 to activate or repress the transcription of genes involved in cell cycle control and tumorigenesis, such as p21, MDM-2, Bax, c-fos/jun, pRB (retinoblastoma), and Bcl2, is an important component of its tumor suppressor functions (127). In addition to its other tumor suppressor functions, p53 may represent an important barrier to the activation of human telomerase.
(iii) pRB and E2F.
Repression of telomerase activity by pRB was observed upon reestablishment of expression of pRB in pRB− p53− tumor cells and was associated with cellular growth arrest (261). Repression of telomerase activity was also observed by overexpressing pRB in a human squamous cell carcinoma cell line (43, 180) and overexpressing E2F1 in head and neck squamous cell carcinoma cell lines (102). However, these studies do not address the mechanisms by which pRB and E2F1 repress telomerase activity. It is not clear whether repression results from a secondary effect following induction of cell cycle arrest or apoptosis by pRB or E2F (272). It remains possible that pRB and E2F1 have transcriptional repression activity either independently or cooperatively.
Several mechanisms have been invoked to explain the transcriptional repression activity of pRB. First, pRB interacts with SWI/SNF nucleosome-remodeling complexes and induces alterations in higher-order chromatin structure in an ATP-dependent manner (221, 231, 269). pRB targets histone H3 methylation and chromodomain protein HP1 to the promoter and represses transcription at heterochromatic sites (181). Finally, pRB interacts with and recruits histone deacetylase complexes to DNA-bound E2F1 and other transcriptional factors and thereby promotes nucleosome formation on target genes (93).
Recent studies demonstrate that the histone deacetylases are involved in hTERT transcriptional repression (36, 111, 225, 259). Histone deacetylase complexes can be recruited to the hTERT promoter by Mad1 through the E-boxes present in the hTERT promoter (36, 111, 225). Importantly, the histone deacetylase complexes can be also recruited to the hTERT promoter independent of Mad1, presumably by still-unidentified transcription factors (36). Although the transcription factor Sp1 has been proposed to be responsible for the recruitment of histone deacetylase to the hTERT promoter, pRB interacting with DNA-bound E2F1 could be another possibility. There are two E2F1 consensus DNA binding motifs in the core promoter of hTERT. Overexpression of E2F1 represses hTERT promoter activity in a luciferase reporter assay, and this repression is dependent on an intact E2F1 binding site. Overexpression of E2F1 in a human cancer cell line (SCC25) resulted in decreased hTERT mRNA expression and telomerase activity (43).
(iv) Wilms' tumor 1 tumor suppressor.
Another protein potentially involved in the transcriptional repression of hTERT is the Wilms' tumor 1 (WT1) tumor suppressor (184). WT1 represses hTERT transcription by direct interaction with the hTERT promoter. The WT1 binding site is located in the core promoter region (−352 upstream of the ATG), and mutation of the WT1 DNA binding site increases hTERT promoter activity in 293 cells but not in HeLa cells. Overexpression of WT1 significantly reduces hTERT mRNA expression and telomerase activity in 293 cells (184). As WT1 gene is expressed in specific cell types (kidney, gonad, and spleen), the role of WT1 in hTERT repression is cell type specific. WT1 plays an important role in the differentiation and growth of kidney and gonad cells (60), WT1 may repress hTERT gene expression during differentiation, and inactivation of WT1 may contribute to the activation of telomerase during tumorigenesis in its target tissues.
(v) Myeloid cell-specific zinc finger protein 2.
Shorter hTERT promoter fragments (containing less than 500 bp upstream of ATG) appear to drive higher luciferase reporter activity in transient-transfection experiments (37, 72). This observation suggests that there may be negative regulatory elements located further upstream of the core promoter region. In reporter assays using different lengths of hTERT promoter fragments, a region of about 200 bp from −594 to −764 upstream of ATG was identified as having inhibitory effects on promoter activity. Sequence analysis indicates that there are multiple binding sites for myeloid cell-specific zinc finger protein 2 (MZF-2) within this region (72). MZF-2 specifically binds to the hTERT promoter in gel shift experiments, and overexpression of MZF-2 represses hTERT promoter activity. These studies suggest that MZF-2 might function as a negative regulator of hTERT transcription. However, it is not known whether MZF-2 has any inhibitory function on hTERT transcription in vivo. Considering that MZF-2 is stably expressed in both normal and cancer cells and during differentiation, it is unlikely that it plays a major role in hTERT transcriptional repression.
(vi) Antiproliferation and differentiation agents.
Although telomerase activity does not appear to be regulated during the cell cycle (28, 80, 109), cell cycle exit and cellular differentiation are usually associated with downregulation of human telomerase activity. The molecular basis for this is not understood at present. Several differentiation inducers and antiproliferative reagents seem to have a direct effect on hTERT transcription.
Interferon-α (IFN-α) is a well-known inhibitor of cellular proliferation and has antitumor activity in a variety of malignancies (194). IFN-α represses hTERT transcription within 4 h and thereby inhibits telomerase activity in malignant and nonmalignant human hematopoietic cell lines, primary leukemic cells and normal T lymphocytes (257). Repression of hTERT transcription by IFN-α did not depend on IFN-α-mediated cell growth arrest or inhibition of c-Myc expression, suggesting that the hTERT gene is a direct transcriptional target for the IFN-α signaling pathway.
The autocrine transforming growth factor β (TGF-β) has been shown to inhibit hTERT expression in human colon carcinoma HCT116 cells after reexpression of its receptor, and TGF-β represses hTERT promoter activity in luciferase reporter assays (262). TGF-β1 mRNA is significantly increased during trophoblast differentiation in vitro, which is associated with downregulation of telomerase activity. Lastly, treatment with TGF-β1 induces BeWo human choriocarcinoma cells to differentiate and repress hTERT transcription (198). All of these may represent indirect effects due to differentiation or cell cycle exit.
The newly synthesized vitamin D3 analog 5,6-trans-16-ene-vitamin D3 strongly inhibited cell proliferation of prostate, breast, and myeloid leukemic cells and upregulation of the cyclin-dependent protein kinase inhibitors p21 and p27, and repression of hTERT was also observed (104). Treatment of U-87MG human glioblastomas with an antagonist of growth hormone release hormone resulted in decreased telomerase activity and hTERT mRNA expression (121).
Inhibition of telomerase and repression of hTERT expression have been observed in a variety of cell lines upon induction of cellular differentiation by several reagents, such as retinoic acid and dimethyl sulfoxide (31, 109, 130, 208, 258, 270). The repression of telomerase is clearly not required for cell differentiation, since cells overexpressing an exogenous telomerase differentiate normally (230). The repression of hTERT expression may sometimes be a reflection of becoming postmitotic, and the reduction in myc activity in nondividing cells might contribute to this reduced expression. The presence of MyoD transcription factor binding sites in the hTERT promoter suggests the additional possibility that tissue-specific factors might be involved as well (36).
The regulation of telomerase activity during differentiation to a postmitotic terminal state is likely to involve different mechanisms than those that occur during commitment to specific developmental pathways. F9 embryocarcinoma and human embryonic stem cells continue to proliferate as multiple lineages after being induced to differentiate, and these lineages repress hTERT and hTERT promoter activity in luciferase reporter assays (232). This suggests that many cellular differentiation programs might be linked to hTERT transcriptional regulation. A novel transcription factor binding element, termed an MT box, has recently been identified within the core promoter of hTERT. Binding to this motif is markedly reduced by cellular differentiation, which is associated with loss of hTERT promoter activity (232). Identification of this and similar transcription factors would be helpful for understanding the mechanisms of the tissue-specific and developmental regulation of human telomerase.
Epigenetic regulation of hTERT transcription.
DNA methylation is essential for the development of mammals (114). Programmed alterations in the methylation status of genomic sequences have been seen at different stages of development from germ line cells to postembryonic cells (202). Abnormal methylation that results in upregulation of protooncogenes such as c-myc, c-fos, Ha-ras, K-ras, and Bcl-2 or in repression of tumor suppressor genes, including pRB, BRCA1, hMLH1, and p16, is often associated with human cancers (137). Developmental regulation of hTERT expression and the high GC-rich content of the hTERT promoter suggest that methylation may be involved in hTERT repression in normal human cells and in hTERT upregulation in immortal, telomerase-positive cells. However, analysis of the methylation status of the hTERT promoter in a number of cell lines and tissues failed to establish a generalized pattern of methylation associated with hTERT expression (49, 50). This suggests that changes in methylation status of the hTERT promoter may not represent a major mechanism regulating hTERT expression, at least over the regions analyzed.
Nevertheless, it has been shown that treatment with the DNA methylation inhibitor 5-azacytidine induced hTERT transcription in two telomerase-negative ALT cell lines (SUSM-1 and GM847) (49, 50). Also, a reverse correlation between the degree of methylation in the hTERT promoter and telomerase activity has been observed in B-cell lymphocytic leukemia (18). These studies suggest that methylation of the hTERT promoter may contribute to the regulation of hTERT gene expression in some cell types. As several (c-Myc, E2F, AP2, NF-κB, and CREB/ATF) transcription factor binding sites present in the hTERT promoter are methylation sensitive (29, 196), methylation in a specific site or region may affect hTERT transcription.
Transcription in eukaryotic cells is profoundly influenced by the local chromatin structure. The posttranslational modifications of nucleosomal histones dictate the dynamic transition between transcriptionally active and inactive chromatin states. Transcriptionally inactive genes tend to be associated with hypoacetylated histones, whereas transcriptionally active genes tend to be associated with hyperacetylated histones. Histone acetyltransferases and deacetylases have been demonstrated to interact with components of the transcription machinery, causing promoter-specific alterations of chromatin (77). A number of transcription factors are either associated with or are themselves histone acetyltransferases that stimulate transcription by acetylating histone amino termini and disrupting nucleosome structure. Conversely, transcriptional repressors have been shown either to be associated with or to be histone deacetylases, whose activity leads to nucleosome formation and repression of transcription (9, 45, 201).
Both Mad1 and Sp1 have been shown to be able to recruit repressor complexes containing the histone deacetylase to certain promoters through E-box and Sp1 sites, respectively (53, 201). The hTERT promoter features two E-boxes and five Sp1 sites (37, 79, 135, 224). Histone deacetylases may be involved in hTERT repression. Indeed, inhibition of histone deacetylases by a specific inhibitor, trichostatin A, in telomerase-negative normal human cells resulted in activation of telomerase and upregulation of hTERT transcription (36, 225, 259). In addition, transient-transfection experiments with a luciferase reporter showed that the hTERT promoter was activated by trichostatin A. These results demonstrated that active repression of hTERT requires histone deacetylase activity. It has been shown that Mad represses hTERT transcription (36, 87, 186). Inhibition of histone deacetylase by trichostatin A alleviated the Mad1-dependent repression of the hTERT promoter, indicating that hTERT repression by Mad1 requires the histone deacetylase (36).
Chromatin immunoprecipitation experiments have provided more direct evidence that show a switch of occupancy from Myc/Max to Mad1/Max and a decrease in histone acetylation at the hTERT promoter in vivo during leukemia cell (HL60) differentiation (259). The E-boxes of the hTERT promoter are predominantly occupied in vivo by c-Myc, and abundant levels of acetylated histones H3 and H4 are found at the hTERT promoter in proliferating HL60 cells. Given that c-Myc is part of the histone acetyltransferase SAGA (Spt-Ada-Gcn5-acetyltransferase) complex containing the histone acetyltransferase hGCN5 (160), these observations indicate that c-Myc activates hTERT transcription in part by recruiting the histone acetyltransferases to the hTERT promoter. In contrast, in differentiated HL60 cells, Mad1 expression is induced, Mad1 replaces c-Myc on the E-boxes of the hTERT promoter, and the acetylation of histones at the hTERT promoter is significantly reduced (259).
Mad1 has been shown to interact with mammalian Sin3, which associates with HDAC1, HDAC2, and a nuclear hormone transcriptional corepressor called NcoR (nuclear corepressor) (42). These observations suggest that the Myc/Max/Mad network regulates hTERT expression by modulating the properties of chromatin and thus the competence of the hTERT promoter for transcriptional activation or repression. However, the Myc/Max/Mad network is only part of the story, as an hTERT promoter with mutations in both E-boxes is still capable of responding to trichostatin A treatment to the same extent as the wild-type promoter (36). This finding suggests that the histone deacetylases may be tethered to the hTERT promoter by one or more of the still-uncharacterized hTERT repressors. Indeed, chromosome transfer experiments indicate that several human chromosomes from normal human cells are capable of repressing telomerase activity and hTERT expression in telomerase-positive cancer cells (44, 175, 219). Thus, these repressors lose expression or are modified in their activity during cellular immortalization, which leads to the activation of telomerase.
Epigenetic modification of the genome ensures the coordinated expression of genes that is essential for the development of mammals. Reactivation of telomerase activity and restoration of telomere length in cloned calves derived from the nuclear transfer of nearly senescent somatic cells indicate that epigenetic reprogramming may occur in the reconstituted embryo (20, 138, 203).
Posttranslational Modification of hTERT Modulates Telomerase Activity
The transcriptional regulation of hTERT is undoubtedly the primary mechanism in controlling telomerase activity in cells. However, substantial evidence suggests that posttranslational modifications of the hTERT protein might provide an additional layer of control of telomerase activity. In this context, it has been shown that normal ovarian tissues and uterine leiomyoma cells have no detectable telomerase activity despite expressing both hTR and full-length hTERT mRNA (235). In addition, hTERT mRNAs are present at similar levels in human lymphocytes, tonsils, and peripheral blood T and B cells regardless of the status of telomerase activity (152). Similar discordances between telomerase activity and hTERT expression are also observed in human colon and renal tissues and tumors and in human smooth muscle cells under hypoxic and normoxic culture conditions (165, 204, 223). These data suggest that expression of hTERT is not always sufficient to produce active telomerase in some cell types and that posttranslational modification of hTERT may play a role in modulating the active and inactive states of human telomerase activity. Reversible protein phosphorylation represents the most important mechanism in regulating protein structures, localization, and activities. Accumulating evidence indicates that telomerase activity can be regulated by hTERT phosphorylation.
In peripheral blood mononuclear cells, telomerase activity is enhanced by the protein kinase C (PKC) activator phorbol myristate acetate, whereas this activation is inhibited by the PKC inhibitor bisindolylmaeimide I (23). In breast cancer PMC42 cells, treatment of nuclear extracts with phosphatase 2A inhibits telomerase activity, while the phosphatase 2A inhibitor okadaic acid prevents telomerase inhibition by phosphatase 2A in vitro and stimulates telomerase activity in vivo (146). Subsequently, PKCα was implicated in hTERT phosphorylation and in the regulation of telomerase activity in human breast cancer cells (145). These studies demonstrate that human telomerase activity can be directly regulated by protein phosphorylation without involvement of transcriptional regulation. Others have shown that telomerase activity was also inhibited by the PKC inhibitors H-7 and bisindolylmaeimide I in human nasopharyngeal cancer cells (131), but PKCζ instead of PKCα seems to be involved in the activation of telomerase activity (131, 267). PKC is part of a large family of phospholipid-dependent kinases involved in cell growth, differentiation, and carcinogenesis. The family consists of at least 10 isoforms with more than 100 identified substrates (150). It is therefore possible that different PKC isoforms might be involved in the regulation of telomerase activity in different cell types under different physiological conditions.
Evidence suggests that the Akt protein kinase (also called protein kinase B or Akt kinase) may also be involved in activation of human telomerase (25, 116). Two putative Atk kinase phosphorylation sites within hTERT (protein sequences 220-GARRRGGSAS-229 and 817-AVRIRGKSYV-826) have been identified. Activated Akt kinase is able to phosphorylate these hTERT peptides and activate telomerase activity in vitro, while the Akt kinase inhibitor wortmannin inhibits telomerase activity in human melanoma cells in a dose-dependent manner (116). Recent studies have extended these observations to human umbilical cord endothelial cells, demonstrating that Akt activates human telomerase by phosphorylation of hTERT (25). As Akt kinase is a key effector of the phosphatidylinositol 3-kinase signaling pathway, the finding that it can directly activate telomerase by phosphorylation of hTERT may provide new insights into the molecular mechanisms by which the phosphatidylinositol 3-kinase pathway promotes cell proliferation and survival (139).
Recent studies have shown that the tyrosine kinase c-Abl associates with and phosphorylates hTERT both in vitro and in vivo, potentially through an interaction between the proline-rich region (308-PSTSRPPRP-316) of hTERT and the SH3 domain of c-Abl (119). In contrast to PKC and Akt kinase, phosphorylation of hTERT by c-Abl inhibits telomerase activity. Overexpression of c-Abl in 293T cells represses telomerase activity, and cells deficient in c-Abl show increased telomerase activity and telomere length. Importantly, exposure of cells to ionizing radiation induces tyrosine phosphorylation of hTERT by c-Abl-dependent mechanisms. Given that c-Abl is associated with a DNA-dependent protein kinase complex (120) involved in DNA damage response, cell cycle arrest, and proapoptosis activity, the functional interactions between c-Abl and hTERT establish an intimate connection between DNA damage and repair systems and telomere maintenance.
One interesting mechanism by which phosphorylation of hTERT regulates telomerase activity was recently proposed from studies of T-lymphocyte activation (151). It was observed that induction of telomerase activity during the activation of resting CD4 T cells was independent of net hTERT protein increase. Instead, hTERT are phosphorylated and translocated from the cytoplasm to the nucleus, indicating that the phosphorylation-dependent translocation of hTERT from the cytoplasm to its functional nuclear compartment may play a role in the regulation of telomerase activity. This idea is supported by the similar observation that phosphorylation-dependent translocation of hTERT from the cytoplasm to the nucleus leads to the induction of telomerase activity during rat vascular smooth muscle cell proliferation (165).
Control of Telomerase Holoenzyme Access to Telomeres
Human telomeres consist of long tandem arrays of TTAGGG repeats bound by proteins (21). This highly ordered telomeric DNA-protein complex allows cells not only to distinguish telomeres from damaged DNAs and to protect them from degradation and fusion, but also to sense and control telomere homeostasis by regulating telomerase accessibility. It has been proposed that the telomeric DNA-protein complex might switch stochastically between capped and uncapped states (21). In humans, telomeres end in a 3′ single-stranded overhangs that can be 75 to 300 nucleotides long (159, 253). This G-rich single-stranded overhang can be sequestered within the telomeric DNA duplex to form a t-loop in vitro (81, 84), a structure that stabilizes and protects chromosome ends. In vitro studies suggest that de novo telomere synthesis by telomerase requires an accessible 3′ overhang (148, 240). Formation of the t-loop would provide an architectural solution to protect telomeres and at the same time prevent telomerase from gaining access. In the past few years, an increasing number of telomere-associated proteins and their interacting partners have been identified (46, 63, 192). Collectively, these telomeric proteins may function to protect telomere integrity and functionality, to connect the DNA damage repair network with the controls of cellular senescence, and to monitor telomere homeostasis and modify the access of telomerase to telomeres
The regulation of telomerase access to telomeres in human cells is not yet fully understood. Recent studies indicate that telomere-associated proteins can regulate telomerase accessibility in either positive or negative ways (63). The first identified telomeric proteins, TRF1 and TRF2 (TTAGGG repeat binding factors 1 and 2), specifically bind to duplex telomeric DNA and are involved in t-loop formation (84), thus acting as negative regulators of telomere length (216, 236). Overexpression of TRF1 or TRF2 inhibits telomere elongation in telomerase-positive cells (216). Recent studies suggest that TRF1 and TRF2 also interact with the DNA damage repair complex Rad50/MRE11/NBS1 (117, 254). This observation provides a molecular basis for a direct interaction between telomere integrity and the DNA damage repair pathway.
TRF1 appears to promote parallel pairing of telomeric DNA in vitro (83), and when bound to duplex telomeric DNA, it inhibits C-strand DNA synthesis by DNA polymerase in vitro (217), which is consistent with its role in inhibiting telomere elongation by telomerase (3). Overexpression of a dominant negative TRF1, which removes endogenous TRF1 from telomeres, results in telomere lengthening in telomerase-positive cells but not in telomerase-negative cells (118, 236). TRF1 binds along the double-stranded telomeric DNA repeats and may inhibit the access of telomerase to telomeres. Since longer telomeres should bind more TRF1, this might create a negative feedback that controls telomere length and might explain, at least in part, how human telomerase might preferentially elongate the shortest telomeres (220). The TRF1-associate partners TIN2 (125) and tankyrase (38, 215) have also been shown to participate in this negative-feedback control. Interestingly, it has recently been demonstrated that the TRF1/Pin2-interacting protein PinX1 interacts directly with hTERT and is a potent telomerase inhibitor (273).
TRF2 is a critical factor for t-loop formation (84, 117). Overexpressed TRF2 causes telomere shortening in both telomerase-positive and -negative cells (118, 216). Recent studies showed that TRF2 also activates telomere degradation (3).
Both in mammals and in S. cerevisiae, double-stranded telomeric DNA binding proteins are found to negatively regulate the access of telomerase (63). In S. cerevisiae, genetic and biochemical studies indicate that the single-stranded telomeric DNA binding proteins Est1p and Cdc13p positively regulate telomere maintenance, probably by mediating access by telomerase (62, 161). The only single-stranded human telomere binding protein identified to date is POT1 (13), but its effects on human telomere length regulation have not yet been described. The hTR binding hnRNP proteins C1/C2 and A1/UP1 might be involved in the recruitment of telomerase to telomeres in mammals (65, 68).
CONCLUSIONS
Significant research efforts in the past few years have tremendously increased our understanding of the key components and complex regulation of telomerase. This unique cellular reverse transcriptase is essential for maintaining telomere stability and is required for the unlimited proliferation of almost all cancer cells. Identification of the core components of telomerase and the ability to reconstitute telomerase activity in normal human cells have allowed researchers to experimentally test and define the role of telomeres in diverse cellular processes, such as cellular senescence, aging, DNA damage/repair, cellular immortalization, and cancer. Demonstrations that ectopic expression of hTERT, the rate-limiting determinant of telomerase activity, can immortalize diverse normal human cell types and that telomerase is required for the long-term proliferation of cancer cells strongly support the role of telomerase in cell immortalization and oncogenesis.
Telomerase activity is repressed due to transcriptional repression of the catalytic subunit hTERT in most tissues prior to birth and is reactivated by upregulation of hTERT expression in over 90% of cancer cells. This indicates that transcriptional regulation of hTERT is the primary mode of control of telomerase activity in human cells. The identification of abundant regulatory elements within the hTERT promoter and transcriptional factors and pathways involved in telomerase regulation suggests that telomerase activity is subject to multiple levels of control and may be regulated by different factors in different cellular contexts. In addition, the posttranslational modification of hTERT, the assembly of the functional ribonucleoprotein telomerase holoenzyme, transport to functionally relevant nuclear compartments, and the access of the enzyme to its substrates add additional levels of complexity to the regulation of telomerase.
The mechanisms involved in telomerase regulation are still far from being fully established. Identification of additional telomerase components and associated proteins will certainly contribute to further investigations of the effect of telomerase in telomere elongation, telomere length maintenance, oncogenesis, and potentially new, unidentified cellular functions. Identification of transcriptional repressors and tumor-specific activators of hTERT should have considerable impact on our understanding of cellular senescence and immortalization and on the ability to manipulate telomerase for cancer-therapeutic and tissue-engineering applications
Acknowledgments
We are grateful to Silvia Bacchetti for insightful comments on and critical reading of the manuscript and to Brittney-Shea Herbert and Ruben Ramirez, members of the Shay-Wright lab, for helpful discussions and critical reading of the review manuscript.
W.E.W. and J.W.S. are coholders of the Southland Financial Corporation Distinguished Chair in Geriatric Research. J.W.S. is an Ellison Medical Foundation Senior Scholar.
REFERENCES
- 1.Aisner, D. L., W. E. Wright, and J. W. Shay. 2002. Telomerase regulation: not just flipping the switch. Curr. Opin. Genet. Dev. 12:80-85. [DOI] [PubMed] [Google Scholar]
- 2.Aldous, W. K., A. J. Marean, M. J. DeHart, L. A. Matej, and K. H. Moore. 1999. Effects of tamoxifen on telomerase activity in breast carcinoma cell lines. Cancer 85:1523-1529. [PubMed] [Google Scholar]
- 3.Ancelin, K., M. Brunori, S. Bauwens, C. E. Koering, C. Brun, M. Ricoul, J. P. Pommier, L. Sabatier, and E. Gilson. 2002. Targeting assay to study the cis functions of human telomeric proteins: evidence for inhibition of telomerase by TRF1 and for activation of telomere degradation by TRF2. Mol. Cell. Biol. 22:3474-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armbruster, B. N., S. S. Banik, C. Guo, A. C. Smith, and C. M. Counter. 2001. N-terminal domains of the human telomerase catalytic subunit required for enzyme activity in vivo. Mol. Cell. Biol. 21:7775-7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artandi, S. E., and R. A. DePinho. 2000. Mice without telomerase: what can they teach us about human cancer? Nat. Med. 6:852-855. [DOI] [PubMed] [Google Scholar]
- 6.Asker, C., K. G. Wiman, and G. Selivanova. 1999. p53-induced apoptosis as a safeguard against cancer. Biochem. Biophys. Res. Commun. 265:1-6. [DOI] [PubMed] [Google Scholar]
- 7.Avantaggiati, M. L., V. Ogryzko, K. Gardner, A. Giordano, A. S. Levine, and K. Kelly. 1997. Recruitment of p300/CBP in p53-dependent signal pathways. Cell 89:1175-1184. [DOI] [PubMed] [Google Scholar]
- 8.Avilion, A. A., M. A. Piatyszek, J. Gupta, J. W. Shay, S. Bacchetti, and C. W. Greider. 1996. Human telomerase RNA and telomerase activity in immortal cell lines and tumor tissues. Cancer Res. 56:645-650. [PubMed] [Google Scholar]
- 9.Ayer, D. E. 1999. Histone deacetylases: transcriptional repression with SINers and NuRDs. Trends Cell Biol. 9:193-198. [DOI] [PubMed] [Google Scholar]
- 10.Bachand, F., and C. Autexier. 1999. Functional reconstitution of human telomerase expressed in Saccharomyces cerevisiae. J. Biol. Chem. 274:38027-38031. [DOI] [PubMed] [Google Scholar]
- 11.Bachand, F., I. Triki, and C. Autexier. 2001. Human telomerase RNA-protein interactions. Nucleic Acids Res. 29:3385-3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Backsch, C., N. Wagenbach, M. Nonn, S. Leistritz, E. Stanbridge, A. Schneider, and M. Durst. 2001. Microcell-mediated transfer of chromosome 4 into HeLa cells suppresses telomerase activity. Genes Chromosomes Cancer 31:196-198. [DOI] [PubMed] [Google Scholar]
- 13.Baumann, P., and T. R. Cech. 2001. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292:1171-1175. [DOI] [PubMed] [Google Scholar]
- 14.Baur, J. A., Y. Zou, J. W. Shay, and W. E. Wright. 2001. Telomere position effect in human cells. Science 292:2075-2077. [DOI] [PubMed] [Google Scholar]
- 15.Beattie, T. L., W. Zhou, M. O. Robinson, and L. Harrington. 2001. Functional multimerization of the human telomerase reverse transcriptase. Mol. Cell. Biol. 21:6151-6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beattie, T. L., W. Zhou, M. O. Robinson, and L. Harrington. 2000. Polymerization defects within human telomerase are distinct from telomerase RNA and TEP1 binding. Mol. Biol. Cell 11:3329-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beattie, T. L., W. Zhou, M. O. Robinson, and L. Harrington. 1998. Reconstitution of human telomerase activity in vitro. Curr. Biol. 8:177-180. [DOI] [PubMed] [Google Scholar]
- 18.Bechter, O. E., W. Eisterer, M. Dlaska, T. Kuhr, and J. Thaler. 2002. CpG island methylation of the hTERT promoter is associated with lower telomerase activity in B-cell lymphocytic leukemia. Exp. Hematol. 30:26-33. [DOI] [PubMed] [Google Scholar]
- 19.Belair, C. D., T. R. Yeager, P. M. Lopez, and C. A. Reznikoff. 1997. Telomerase activity: a biomarker of cell proliferation, not malignant transformation. Proc. Natl. Acad. Sci. USA 94:13677-13682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Betts, D., V. Bordignon, J. Hill, Q. Winger, M. Westhusin, L. Smith, and W. King. 2001. Reprogramming of telomerase activity and rebuilding of telomere length in cloned cattle. Proc. Natl. Acad. Sci. USA 98:1077-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackburn, E. H. 2001. Switching and signaling at the telomere. Cell 106:661-673. [DOI] [PubMed] [Google Scholar]
- 22.Blasco, M. A., H. W. Lee, M. P. Hande, E. Samper, P. M. Lansdorp, R. A. DePinho, and C. W. Greider. 1997. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91:25-34. [DOI] [PubMed] [Google Scholar]
- 23.Bodnar, A. G., N. W. Kim, R. B. Effros, and C. P. Chiu. 1996. Mechanism of telomerase induction during T cell activation. Exp. Cell Res. 228:58-64. [DOI] [PubMed] [Google Scholar]
- 24.Bodnar, A. G., M. Ouellette, M. Frolkis, S. E. Holt, C. P. Chiu, G. B. Morin, C. B. Harley, J. W. Shay, S. Lichtsteiner, and W. E. Wright. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279:349-352. [DOI] [PubMed] [Google Scholar]
- 25.Breitschopf, K., A. M. Zeiher, and S. Dimmeler. 2001. Pro-atherogenic factors induce telomerase inactivation in endothelial cells through an Akt-dependent mechanism. FEBS Lett. 493:21-25. [DOI] [PubMed] [Google Scholar]
- 26.Bryan, T. M., J. M. Sperger, K. B. Chapman, and T. R. Cech. 1998. Telomerase reverse transcriptase genes identified in Tetrahymena thermophila and Oxytricha trifallax. Proc. Natl. Acad. Sci. USA 95:8479-8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bryce, L. A., N. Morrison, S. F. Hoare, S. Muir, and W. N. Keith. 2000. Mapping of the gene for the human telomerase reverse transcriptase, hTERT, to chromosome 5p15.33 by fluorescence in situ hybridization. Neoplasia 2:197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchkovich, K. J., and C. W. Greider. 1996. Telomerase regulation during entry into the cell cycle in normal human T cells. Mol. Biol. Cell 7:1443-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campanero, M. R., M. I. Armstrong, and E. K. Flemington. 2000. CpG methylation as a mechanism for the regulation of E2F activity. Proc. Natl. Acad. Sci. USA 97:6481-6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen, J. L., M. A. Blasco, and C. W. Greider. 2000. Secondary structure of vertebrate telomerase RNA. Cell 100:503-514. [DOI] [PubMed] [Google Scholar]
- 31.Cheng, A. J., S. K. Liao, S. E. Chow, J. K. Chen, and T. C. Wang. 1997. Differential inhibition of telomerase activity during induction of differentiation in hematopoietic, melanoma, and glioma cells in culture. Biochem. Biophys. Res. Commun. 237:438-444. [DOI] [PubMed] [Google Scholar]
- 32.Colgin, L. M., C. Wilkinson, A. Englezou, A. Kilian, M. O. Robinson, and R. R. Reddel. 2000. The hTERTalpha splice variant is a dominant negative inhibitor of telomerase activity. Neoplasia 2:426-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins, K. 1999. Cillate telomerase biochemistry. Annu. Rev. Biochem. 68:178-218. [DOI] [PubMed] [Google Scholar]
- 34.Collins, K., and L. Gandhi. 1998. The reverse transcriptase component of the Tetrahymena telomerase ribonucleoprotein complex. Proc. Natl. Acad. Sci. USA 95:8485-8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins, K., R. Kobayashi, and C. W. Greider. 1995. Purification of Tetrahymena telomerase and cloning of genes encoding the two protein components of the enzyme. Cell 81:677-686. [DOI] [PubMed] [Google Scholar]
- 36.Cong, Y. S., and S. Bacchetti. 2000. Histone deacetylation is involved in the transcriptional repression of hTERT in normal human cells. J. Biol. Chem. 275:35665-35668. [DOI] [PubMed] [Google Scholar]
- 37.Cong, Y. S., J. Wen, and S. Bacchetti. 1999. The human telomerase catalytic subunit hTERT: organization of the gene and characterization of the promoter. Hum. Mol. Genet. 8:137-142. [DOI] [PubMed] [Google Scholar]
- 38.Cook, B. D., J. N. Dynek, W. Chang, G. Shostak, and S. Smith. 2002. Role for the related poly(ADP-Ribose) polymerases tankyrase 1 and 2 at human telomeres. Mol. Cell. Biol. 22:332-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Counter, C. M., A. A. Avilion, C. E. LeFeeuvre, N. G. Stewart, C. W. Greider, C. B. Harley, and S. Bacchetti. 1992. Telomere shorthening associted with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 11:1921-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Counter, C. M., W. C. Hahn, W. Wei, S. D. Caddle, R. L. Beijersbergen, P. M. Lansdorp, J. M. Sedivy, and R. A. Weinberg. 1998. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc. Natl. Acad. Sci. USA 95:14723-14728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Counter, C. M., M. Meyerson, E. N. Eaton, and R. A. Weinberg. 1997. The catalytic subunit of yeast telomerase. Proc. Natl. Acad. Sci. USA 94:9202-9207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cress, W. D., and E. Seto. 2000. Histone deacetylases, transcriptional control, and cancer. J. Cell Physiol. 184:1-16. [DOI] [PubMed] [Google Scholar]
- 43.Crowe, D. L., and D. C. Nguyen. 2001. Rb and E2F-1 regulate telomerase activity in human cancer cells. Biochim. Biophys. Acta 1518:1-6. [DOI] [PubMed] [Google Scholar]
- 44.Cuthbert, A. P., J. Bond, D. A. Trott, S. Gill, J. Broni, A. Marriott, G. Khoudoli, E. K. Parkinson, C. S. Cooper, and R. F. Newbold. 1999. Telomerase repressor sequences on chromosome 3 and induction of permanent growth arrest in human breast cancer cells. J. Natl. Cancer Inst. 91:37-45. [DOI] [PubMed] [Google Scholar]
- 45.Davie, J. R., and V. A. Spencer. 1999. Control of histone modifications. J. Cell Biochem. Suppl. 32-33:141-148. [DOI] [PubMed] [Google Scholar]
- 46.de Lange, T. 2002. Protection of mammalian telomeres. Oncogene 21:532-540. [DOI] [PubMed] [Google Scholar]
- 47.de Lange, T., L. Shiue, R. M. Myers, D. R. Cox, S. L. Naylor, A. M. Killery, and H. E. Varmus. 1990. Structure and variability of human chromosome ends. Mol. Cell. Biol. 10:518-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DePinho, R. A., N. Schreiber-Agus, and F. W. Alt. 1991. myc family oncogenes in the development of normal and neoplastic cells. Adv. Cancer Res. 57:1-46. [DOI] [PubMed] [Google Scholar]
- 49.Dessain, S. K., H. Yu, R. R. Reddel, R. L. Beijersbergen, and R. A. Weinberg. 2000. Methylation of the human telomerase gene CpG island. Cancer Res. 60:537-541. [PubMed] [Google Scholar]
- 50.Devereux, T. R., I. Horikawa, C. H. Anna, L. A. Annab, C. A. Afshari, and J. C. Barrett. 1999. DNA methylation analysis of the promoter region of the human telomerase reverse transcriptase (hTERT) gene. Cancer Res. 59:6087-6090. [PubMed] [Google Scholar]
- 51.Dez, C., A. Henras, B. Faucon, D. Lafontaine, M. Caizergues-Ferrer, and Y. Henry. 2001. Stable expression in yeast of the mature form of human telomerase RNA depends on its association with the box H/ACA small nucleolar ribonucleoprotein proteins Cbf5p, Nhp2p and Nop10p. Nucleic Acids Res. 29:598-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dhar, S., J. A. Squire, M. P., Hande, R. J. Wellinger, and T. K. Pandita. 2000. Inactivation of 14-3-3 sigma influences telomere behavior and ionizing radiotion-induced chromosomal instability. Mol. Cell. Biol. 20:7764-7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doetzlhofer, A., H. Rotheneder, G. Lagger, M. Koranda, V. Kurtev, G. Brosch, E. Wintersberger, and C. Seiser. 1999. Histone deacetylase 1 can repress transcription by binding to Sp1. Mol. Cell. Biol. 19:5504-5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dokal, I. 2001. Dyskeratosis congenita. A disease of premature ageing. Lancet 358(Suppl.):S27. [DOI] [PubMed] [Google Scholar]
- 55.Dragon, F., V. Pogacic, and W. Filipowicz. 2000. In vitro assembly of human H/ACA small nucleolar ribonucleoproteins reveals unique features of U17. and telomerase RNAs. Mol. Cell. Biol. 20:3037-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eickbush, T. H. 1997. Telomerase and retrotransposons: which came first? Science 277:911-912. [DOI] [PubMed] [Google Scholar]
- 57.el-Deiry, W. S. 1998. Regulation of p53 downstream genes. Semin. Cancer Biol. 8:345-357. [DOI] [PubMed] [Google Scholar]
- 58.Emili, A., J. Greenblatt, and C. J. Ingles. 1994. Species-specific interaction of the glutamine-rich activation domains of Sp1 with the TATA box-binding protein. Mol. Cell. Biol. 14:1582-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.England, N. L., A. P. Cuthbert, D. A. Trott, S. Jezzard, T. Nobori, D. A. Carson, and R. F. Newbold. 1996. Identification of human tumour suppressor genes by monochromosome transfer: rapid growth-arrest response mapped to 9p21 is mediated solely by the cyclin-D-dependent kinase inhibitor gene, CDKN2A (p16INK4A). Carcinogenesis 17:1567-1575. [DOI] [PubMed] [Google Scholar]
- 60.Englert, C. 1998. WT1-more than a transcription factor? Trends Biochem. Sci. 23:389-393. [DOI] [PubMed] [Google Scholar]
- 61.Etheridge, K. T., S. S. Banik, B. N. Armbruster, Y. Zhu, R. M. Terns, M. P. Terns, and C. M. Counter. 2002. The nucleolar localization domain of the catalytic subunit of human telomerase. J. Biol. Chem. 15:15. [DOI] [PubMed] [Google Scholar]
- 62.Evans, S. K., and V. Lundblad. 1999. Est1 and Cdc13 as comediators of telomerase access. Science 286:117-120. [DOI] [PubMed] [Google Scholar]
- 63.Evans, S. K., and V. Lundblad. 2000. Positive and negative regulation of telomerase access to the telomere. J Cell Sci. 113:3357-3364. [DOI] [PubMed] [Google Scholar]
- 64.Feng, J., W. D. Funk, S. S. Wang, S. L. Weinrich, A. A. Avilion, C. P. Chiu, R. R. Adams, E. Chang, R. C. Allsopp, J. Yu, et al. 1995. The RNA component of human telomerase. Science 269:1236-1241. [DOI] [PubMed] [Google Scholar]
- 65.Fiset, S., and B. Chabot. 2001. hnRNP A1 may interact simultaneously with telomeric DNA and the human telomerase RNA in vitro. Nucleic Acids Res. 29:2268-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fitzgerald, M. S., K. Riha, F. Gao, S. Ren, T. D. McKnight, and D. E. Shippen. 1999. Disruption of the telomerase catalytic subunit gene from Arabidopsis inactivates telomerase and leads to a slow loss of telomeric DNA. Proc. Natl. Acad. Sci. USA 96:14813-14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ford, L. P., J. W. Shay, and W. E. Wright. 2001. The La antigen associates with the human telomerase ribonucleoprotein and influences telomere length in vivo. RNA 7:1068-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ford, L. P., J. M. Suh, W. E. Wright, and J. W. Shay. 2000. Heterogeneous nuclear ribonucleoproteins C1 and C2 associate with the RNA component of human telomerase. Mol. Cell. Biol. 20:9084-9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Forrest, A., and B. Gabrielli. 2001. Cdc25B activity is regulated by 14-3-3. Oncogene 20:4393-4401. [DOI] [PubMed] [Google Scholar]
- 70.Forsythe, H. L., J. L. Jarvis, J. W. Turner, L. W. Elmore, and S. E. Holt. 2001. Stable association of hsp90 and p23, but Not hsp70, with active human telomerase. J. Biol. Chem. 276:15571-15574. [DOI] [PubMed] [Google Scholar]
- 71.Friedman, K. L., and T. R. Cech. 1999. Essential functions of amino-terminal domains in the yeast telomerase catalytic subunit revealed by selection for viable mutants. Genes Dev. 13:2863-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fujimoto, K., S. Kyo, M. Takakura, T. Kanaya, Y. Kitagawa, H. Itoh, M. Takahashi, and M. Inoue. 2000. Identification and characterization of negative regulatory elements of the human telomerase catalytic subunit (hTERT) gene promoter: possible role of MZF-2 in transcriptional repression of hTERT. Nucleic Acids Res. 28:2557-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fuxe, J., G. Akusjarvi, H. M. Goike, G. Roos, V. P. Collins, and R. F. Pettersson. 2000. Adenovirus-mediated overexpression of p15INK4B inhibits human glioma cell growth, induces replicative senescence, and inhibits telomerase activity similarly to p16INK4A. Cell Growth Differ. 11:373-384. [PubMed] [Google Scholar]
- 74.Gao, Q., L. Singh, A. Kumar, S. Srinivasan, D. E. Wazer, and V. Band. 2001. Human papillomavirus type 16 E6-induced degradation of E6TP1 correlates with its ability to immortalize human mammary epithelial cells. J. Virol. 75:4459-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gewin, L., and D. A. Galloway. 2001. E box-dependent activation of telomerase by human papillomavirus type 16 E6 does not require induction of c-myc. J. Virol. 75:7198-7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goldstein, S. 1990. Replicative senescence: the human fibroblast comes of age. Science 249:1129-1133. [DOI] [PubMed] [Google Scholar]
- 77.Grandori, C., S. M. Cowley, L. P. James, and R. N. Eisenman. 2000. The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu. Rev. Cell Dev. Biol. 16:653-699. [DOI] [PubMed] [Google Scholar]
- 78.Greenberg, R. A., R. C. Allsopp, L. Chin, G. B. Morin, and R. A. De. 1998. Expression of mouse telomerase reverse transcriptase during development, differentiation and proliferation. Oncogene 16:1723-1730. [DOI] [PubMed] [Google Scholar]
- 79.Greenberg, R. A., R. C. O'Hagan, H. Deng, Q. Xiao, S. R. Hann, R. R. Adams, S. Lichtsteiner, L. Chin, G. B. Morin, and R. A. De. 1999. Telomerase reverse transcriptase gene is a direct target of c-Myc but is not functionally equivalent in cellular transformation. Oncogene 18:1219-1226. [DOI] [PubMed] [Google Scholar]
- 80.Greider, C. W. 1998. Telomerase activity, cell proliferation, and cancer. Proc. Natl. Acad. Sci. USA 95:90-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Greider, C. W. 1999. Telomeres do D-loop-T-loop. Cell 97:419-422. [DOI] [PubMed] [Google Scholar]
- 82.Greider, C. W., A. B., and E. H. Blackburn. 1985. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43:405-413. [DOI] [PubMed] [Google Scholar]
- 83.Griffith, J., A. Bianchi, and T. de Lange. 1998. TRF1. promotes parallel pairing of telomeric tracts in vitro. J. Mol. Biol. 278:79-88. [DOI] [PubMed] [Google Scholar]
- 84.Griffith, J. D., L. Comeau, S. Rosenfield, R. M. Stansel, A. Bianchi, H. Moss, and T. de Lange. 1999. Mammalian telomeres end in a large duplex loop. Cell 97:503-514. [DOI] [PubMed] [Google Scholar]
- 85.Gu, J., M. Andreeff, J. A. Roth, and B. Fang. 2002. hTERT promoter induces tumor-specific Bax gene expression and cell killing in syngenic mouse tumor model and prevents systemic toxicity. Gene Ther. 9:30-37. [DOI] [PubMed] [Google Scholar]
- 86.Gu, J., S. Kagawa, M. Takakura, S. Kyo, M. Inoue, J. A. Roth, and B. Fang. 2000. Tumor-specific transgene expression from the human telomerase reverse transcriptase promoter enables targeting of the therapeutic effects of the Bax gene to cancers. Cancer Res. 60:5359-5364. [PubMed] [Google Scholar]
- 87.Gunes, C., S. Lichtsteiner, A. P. Vasserot, and C. Englert. 2000. Expression of the hTERT gene is regulated at the level of transcriptional initiation and repressed by Mad1. Cancer Res. 60:2116-2121. [PubMed] [Google Scholar]
- 88.Guo, W., M. Okamoto, N. H. Park, and Y. M. Lee. 2001. Cloning and expression of hamster telomerase catalytic subunit cDNA. Int. J. Mol. Med. 8:73-78. [DOI] [PubMed] [Google Scholar]
- 89.Hahn, W. C., C. M. Counter, A. S. Lundberg, R. L. Beijersbergen, M. W. Brooks, and R. A. Weinberg. 1999. Creation of human tumour cells with defined genetic elements. Nature 400:464-468. [DOI] [PubMed] [Google Scholar]
- 90.Hahn, W. C., S. A. Stewart, M. W. Brooks, S. G. York, E. Eaton, A. Kurachi, R. L. Beijersbergen, J. H. Knoll, M. Meyerson, and R. A. Weinberg. 1999. Inhibition of telomerase limits the growth of human cancer cells. Nat. Med. 5:1164-1170. [DOI] [PubMed] [Google Scholar]
- 91.Hande, M. P., A. S. Balajee, and A. T. Natarajan. 1997. Induction of telomerase activity by UV-irradiation in Chinese hamster cells. Oncogene 15:1747-1752. [DOI] [PubMed] [Google Scholar]
- 92.Hara, E. T., A. Shinozaki, S. Nakada, and K. Oda. 1991. Cooperative effect of antisense-RB and antisense-p53 in the regulation of cellular senescence. Exp. Cell Res. 179:528-534. [DOI] [PubMed] [Google Scholar]
- 93.Harbour, J. W., and D. C. Dean. 2000. Chromatin remodeling and Rb activity. Curr. Opin. Cell Biol. 12:685-689. [DOI] [PubMed] [Google Scholar]
- 94.Harley, C. B. 2002. Telomerase is not an oncogene. Oncogene 21:494-502. [DOI] [PubMed] [Google Scholar]
- 95.Harley, C. B., A. B. Futcher, and C. W. Greider. 1990. Telomeres shorten during ageing of human fibroblasts. Nature 345:458-460. [DOI] [PubMed] [Google Scholar]
- 96.Harley, C. B., Vaziri, H., Counter, C., and R. C. Allsopp. 1992. The telomere hypothesis of cellular aging. Exp. Gerontol. 27:375-382. [DOI] [PubMed] [Google Scholar]
- 97.Harrington, L., T. McPhail, V. Mar, W. Zhou, R. Oulton, M. B. Bass, I. Arruda, and M. O. Robinson. 1997. A mammalian telomerase-associated protein. Science 275:973-977. [DOI] [PubMed] [Google Scholar]
- 98.Harrington, L., W. Zhou, T. McPhail, R. Oulton, D. S. Yeung, V. Mar, M. B. Bass, and M. O. Robinson. 1997. Human telomerase contains evolutionarily conserved catalytic and structural subunits. Genes Dev. 11:3109-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hastie, N. D., M. Dempster, M. G. Dunlop, A. M. Thompson, D. K. Green, and R. C. Allshire. 1990. Telomere reduction in human colorectal carcinoma and with ageing. Nature 346:866-868. [DOI] [PubMed] [Google Scholar]
- 100.Hayflick, L. 1965. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37:614-636. [DOI] [PubMed] [Google Scholar]
- 101.Henderson, B. E., R. K. Ross, and M. C. Pike. 1993. Hormonal chemoprevention of cancer in women. Science 259:633-638. [DOI] [PubMed] [Google Scholar]
- 102.Henderson, Y. C., R. L. Breau, T., J. Liu, and G. L. Clayman. 2000. Telomerase activity in head and neck tumors after introduction of wild-type p53, p21, p16 and E2F1 genes by means of recombinant adenovirus. Head Neck July:347-354. [DOI] [PubMed]
- 103.Herbert, B., A. E. Pitts, S. I. Baker, S. E. Hamilton, W. E. Wright, J. W. Shay, and D. R. Corey. 1999. Inhibition of human telomerase in immortal human cells leads to progressive telomere shortening and cell death. Proc. Natl. Acad. Sci. USA 96:14276-14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hisatake, J., T. Kubota, Y. Hisatake, M. Uskokovic, S. Tomoyasu, and H. P. Koeffler. 1999. 5,6-trans-16-ene-vitamin D3: a new class of potent inhibitors of proliferation of prostate, breast, and myeloid leukemic cells. Cancer Res. 59:4023-4029. [PubMed] [Google Scholar]
- 105.Hiyama, K., Y. Hirai, S. Kyoizumi, M. Akiyama, E. Hiyama, M. A. Piatyszek, J. W. Shay, S. Ishioka, and M. Yamakido. 1995. Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. J. Immunol. 155:3711-3715. [PubMed] [Google Scholar]
- 106.Hoey, T., R. O. Weinzierl, G. Gill, J. L. Chen, B. D. Dynlacht, and R. Tjian. 1993. Molecular cloning and functional analysis of Drosophila TAF110 reveal properties expected of coactivators. Cell 72:247-260. [DOI] [PubMed] [Google Scholar]
- 107.Hollstein, M., K. Rice, M. S. Greenblatt, T. Soussi, R. Fuchs, T. Sorlie, E. Hovig, B. Smith-Sorensen, R. Montesano, and C. C. Harris. 1994. Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res. 22:3551-3555. [PMC free article] [PubMed] [Google Scholar]
- 108.Holt, S. E., D. L. Aisner, J. Baur, V. M. Tesmer, M. Dy, M. Ouellette, J. B. Trager, G. B. Morin, D. O. Toft, J. W. Shay, W. E. Wright, and M. A. White. 1999. Functional requirement of p23 and Hsp90 in telomerase complexes. Genes Dev. 13:817-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Holt, S. E., D. L. Aisner, J. W. Shay, and W. E. Wright. 1997. Lack of cell cycle regulation of telomerase activity in human cells. Proc. Natl. Acad. Sci. USA 94:10687-10692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Horikawa, I., P. L. Cable, C. Afshari, and J. C. Barrett. 1999. Cloning and characterization of the promoter region of human telomerase reverse transcriptase gene. Cancer Res. 59:826-830. [PubMed] [Google Scholar]
- 111.Hou, M., X. Wang, N. Popov, A. Zhang, X. Zhao, R. Zhou, A. Zetterberg, M. Bjorkholm, M. Henriksson, A. Gruber, and D. Xu. 2002. The histone deacetylase inhibitor trichostatin A derepresses the telomerase reverse transcriptase (hTERT) gene in human cells. Exp Cell Res. 274:25-34. [DOI] [PubMed] [Google Scholar]
- 112.Ishii, Y., N. Tsuyama, S. Maeda, H. Tahara, and T. Ide. 1999. Telomerase activity in hybrids between telomerase-negative and telomerase-positive immortal human cells is repressed in the different complementation groups but not in the same complementation group of immortality. Mech. Ageing Dev. 110:175-193. [DOI] [PubMed] [Google Scholar]
- 113.Jiang, X. R., G. Jimenez, E. Chang, M. Frolkis, B. Kusler, M. Sage, M. Beeche, A. G. Bodnar, G. M. Wahl, T. D. Tlsty, and C. P. Chiu. 1999. Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nat. Genet. 21:111-114. [DOI] [PubMed] [Google Scholar]
- 114.Jones, P. A., and D. Takai. 2001. The role of DNA methylation in mammalian epigenetics. Science 293:1068-1070. [DOI] [PubMed] [Google Scholar]
- 115.Kanaya, T., S. Kyo, K. Hamada, M. Takakura, Y. Kitagawa, H. Harada, and M. Inoue. 2000. Adenoviral expression of p53 represses telomerase activity through downregulation of human telomerase reverse transcriptase transcription. Clin. Cancer Res. 6:1239-1247. [PubMed] [Google Scholar]
- 116.Kang, S. S., T. Kwon, D. Y. Kwon, and S. I. Do. 1999. Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit. J. Biol. Chem. 274:13085-13090. [DOI] [PubMed] [Google Scholar]
- 117.Karlseder, J., D. Broccoli, Y. Dai, S. Hardy, and T. de Lange. 1999. p53-and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science 283:1321-1325. [DOI] [PubMed] [Google Scholar]
- 118.Karlseder, J., A. Smogorzewska, and T. de Lange. 2002. Senescence induced by altered telomere state, not telomere loss. Science 295:2446-2449. [DOI] [PubMed] [Google Scholar]
- 119.Kharbanda, S., V. Kumar, S. Dhar, P. Pandey, C. Chen, P. Majumder, Z. M. Yuan, Y. Whang, W. Strauss, T. K. Pandita, D. Weaver, and D. Kufe. 2000. Regulation of the hTERT telomerase catalytic subunit by the c-Abl tyrosine kinase. Curr. Biol. 10:568-575. [DOI] [PubMed] [Google Scholar]
- 120.Kharbanda, S., P. Pandey, S. Jin, S. Inoue, A. Bharti, Z. M. Yuan, R. Weichselbaum, D. Weaver, and D. Kufe. 1997. Functional interaction between DNA-PK and c-Abl in response to DNA damage. Nature 386:732-735. [DOI] [PubMed] [Google Scholar]
- 121.Kiaris, H., and A. V. Schally. 1999. Decrease in telomerase activity in U-87MG human glioblastomas after treatment with an antagonist of growth hormone-releasing hormone. Proc. Natl. Acad. Sci. USA 96:226-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kickhoefer, V. A., A. G. Stephen, L. Harrington, M. O. Robinson, and L. H. Rome. 1999. Vaults and telomerase share a common subunit, TEP1. J. Biol. Chem. 274:32712-32717. [DOI] [PubMed] [Google Scholar]
- 123.Kilian, A., D. D. Bowtell, H. E. Abud, G. R. Hime, D. J. Venter, P. K. Keese, E. L. Duncan, R. R. Reddel, and R. A. Jefferson. 1997. Isolation of a candidate human telomerase catalytic subunit gene, which reveals complex splicing patterns in different cell types. Hum Mol. Genet. 6:2011-2019. [DOI] [PubMed] [Google Scholar]
- 124.Kim, N. W., M. A. Piatyszek, K. R. Prowse, C. B. Harley, M. D. West, P. L. Ho, G. M. Coviello, W. E. Wright, S. L. Weinrich, and J. W. Shay. 1994. Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011-2015. [DOI] [PubMed] [Google Scholar]
- 125.Kim, S. H., P. Kaminker, and J. Campisi. 1999. TIN2, a new regulator of telomere length in human cells. Nat. Genet. 23:405-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Klingelhutz, A. J., S. A. Foster, and J. K. Mc. 1996. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature 380:79-82. [DOI] [PubMed] [Google Scholar]
- 127.Ko, L. J., and C. Prives. 1996. p53: puzzle and paradigm. Genes Dev. 10:1054-1072. [DOI] [PubMed] [Google Scholar]
- 128.Koga, S., S. Hirohata, Y. Kondo, T. Komata, M. Takakura, M. Inoue, S. Kyo, and S. Kondo. 2000. A novel telomerase-specific gene therapy: gene transfer of caspase-8 utilizing the human telomerase catalytic subunit gene promoter. Hum. Gene Ther. 11:1397-1406. [DOI] [PubMed] [Google Scholar]
- 129.Kong, L. B., A. C. Siva, V. A. Kickhoefer, L. H. Rome, and P. L. Stewart. 2000. RNA location and modeling of a WD40 repeat domain within the vault. RNA 6:890-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Koyanagi, Y., D. Kobayashi, T. Yajima, K. Asanuma, T. Kimura, T. Sato, T. Kida, A. Yagihashi, H. Kameshima, and N. Watanabe. 2000. Telomerase activity is down regulated via decreases in hTERT mRNA but not TEP1 mRNA or hTERC during the differentiation of leukemic cells. Anticancer Res. 20:773-778. [PubMed] [Google Scholar]
- 131.Ku, W. C., A. J. Cheng, and T. C. Wang. 1997. Inhibition of telomerase activity by PKC inhibitors in human nasopharyngeal cancer cells in culture. Biochem. Biophys. Res. Commun. 241:730-736. [DOI] [PubMed] [Google Scholar]
- 132.Kusumoto, M., T. Ogawa, K. Mizumoto, H. Ueno, H. Niiyama, N. Sato, M. Nakamura, and M. Tanaka. 1999. Adenovirus-mediated p53 gene transduction inhibits telomerase activity independent of its effects on cell cycle arrest and apoptosis in human pancreatic cancer cells. Clin. Cancer Res. 5:2140-2147. [PubMed] [Google Scholar]
- 133.Kyo, S., M. Takakura, T. Kanaya, W. Zhuo, K. Fujimoto, Y. Nishio, A. Orimo, and M. Inoue. 1999. Estrogen activates telomerase. Cancer Res. 59:5917-5921. [PubMed] [Google Scholar]
- 134.Kyo, S., M. Takakura, T. Kohama, and M. Inoue. 1997. Telomerase activity in human endometrium. Cancer Res. 57:610-614. [PubMed] [Google Scholar]
- 135.Kyo, S., M. Takakura, T. Taira, T. Kanaya, H. Itoh, M. Yutsudo, H. Ariga, and M. Inoue. 2000. Sp1 cooperates with c-Myc to activate transcription of the human telomerase reverse transcriptase gene (hTERT). Nucleic Acids Res. 28:669-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.LaBranche, H., S. Dupuis, Y. Ben-David, M. R. Bani, R. J. Wellinger, and B. Chabot. 1998. Telomere elongation by hnRNP A1 and a derivative that interacts with telomeric repeats and telomerase. Nat. Genet. 19:199-202. [DOI] [PubMed] [Google Scholar]
- 137.Laird, P. W., and R. Jaenisch. 1994. DNA methylation and cancer. Hum. Mol. Genet. 3:1487-1495. [DOI] [PubMed] [Google Scholar]
- 138.Lanza, R. P., J. B. Cibelli, C. Blackwell, V. J. Cristofalo, M. K. Francis, G. M. Baerlocher, J. Mak, M. Schertzer, E. A. Chavez, N. Sawyer, P. M. Lansdorp, and M. D. West. 2000. Extension of cell life-span and telomere length in animals cloned from senescent somatic cells. Science 288:665-669. [DOI] [PubMed] [Google Scholar]
- 139.Lawlor, M. A., and D. R. Alessi. 2001. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J. Cell Sci. 114:2903-2910. [DOI] [PubMed] [Google Scholar]
- 140.Le, S., R. Sternglanz, and C. W. Greider. 2000. Identification of two RNA-binding proteins associated with human telomerase RNA. Mol. Biol. Cell 11:999-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee, H. W., M. A. Blasco, G. J. Gottlieb, J. W. Horner, 2nd, C. W. Greider, and R. A. DePinho. 1998. Essential role of mouse telomerase in highly proliferative organs. Nature 392:569-574. [DOI] [PubMed] [Google Scholar]
- 142.Leem, S. H., J. A. Londono-Vallejo, J. H. Kim, H. Bui, E. Tubacher, G. Solomon, J. E. Park, I. Horikawa, N. Kouprina, J. C. Barrett, and V. Larionov. 2002. The human telomerase gene: complete genomic sequence and analysis of tandem repeat polymorphisms in intronic regions. Oncogene 21:769-777. [DOI] [PubMed] [Google Scholar]
- 143.Lendvay, T. S., D. K. Morris, J. Sah, B. Balasubramanian, and V. Lundblad. 1996. Senescence mutants of Saccharomyces cerevisiae with a defect in telomere replication identify three additional EST genes. Genetics 144:1399-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Levine, A. J. 1997. p53, the cellular gatekeeper for growth and division. Cell 88:323-331. [DOI] [PubMed] [Google Scholar]
- 145.Li, H., L. Zhao, Z. Yang, J. W. Funder, and J. P. Liu. 1998. Telomerase is controlled by protein kinase Calpha in human breast cancer cells. J. Biol. Chem. 273:33436-33442. [DOI] [PubMed] [Google Scholar]
- 146.Li, H., L. L. Zhao, J. W. Funder, and J. P. Liu. 1997. Protein phosphatase 2A inhibits nuclear telomerase activity in human breast cancer cells. J. Biol. Chem. 272:16729-16732. [DOI] [PubMed] [Google Scholar]
- 147.Lindsey, J., N. I. McGill, L. A. Lindsey, D. K. Green, and H. J. Cooke. 1991. In vivo loss of telomeric repeats with age in humans. Mutat. Res. 256:45-48. [DOI] [PubMed] [Google Scholar]
- 148.Lingner, J., and T. R. Cech. 1996. Purification of telomerase from Euplotes aediculatus: requirement of a primer 3′ overhang. Proc. Natl. Acad. Sci. USA 93:10712-10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lingner, J., T. R. Hughes, A. Shevchenko, M. Mann, V. Lundblad, and T. R. Cech. 1997. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science 276:561-567. [DOI] [PubMed] [Google Scholar]
- 150.Liu, J. P. 1996. Protein kinase C and its substrates. Mol. Cell Endocrinol. 116:1-29. [DOI] [PubMed] [Google Scholar]
- 151.Liu, K., R. J. Hodes, and N. Weng. 2001. Cutting edge: telomerase activation in human T lymphocytes does not require increase in telomerase reverse transcriptase (hTERT) protein but is associated with hTERT phosphorylation and nuclear translocation. J. Immunol. 166:4826-4830. [DOI] [PubMed] [Google Scholar]
- 152.Liu, K., M. M. Schoonmaker, B. L. Levine, C. H. June, R. J. Hodes, and N. P. Weng. 1999. Constitutive and regulated expression of telomerase reverse transcriptase (hTERT) in human lymphocytes. Proc. Natl. Acad. Sci. USA 96:5147-5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu, Y., B. E. Snow, M. P. Hande, G. Baerlocher, V. A. Kickhoefer, D. Yeung, A. Wakeham, A. Itie, D. P. Siderovski, P. M. Lansdorp, M. O. Robinson, and L. Harrington. 2000. Telomerase-associated protein TEP1 is not essential for telomerase activity or telomere length maintenance in vivo. Mol. Cell. Biol. 20:8178-8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Majumdar, A. S., D. E. Hughes, S. P. Lichtsteiner, Z. Wang, J. S. Lebkowski, and A. P. Vasserot. 2001. The telomerase reverse transcriptase promoter drives efficacious tumor suicide gene therapy while preventing hepatotoxicity encountered with constitutive promoters. Gene Ther. 8:568-578. [DOI] [PubMed] [Google Scholar]
- 155.Makarov, V. L., Y. Hirose, and J. P. Langmore. 1997. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell. 88:657-666. [DOI] [PubMed] [Google Scholar]
- 156.Malik, H. S., W. D. Burke, and T. H. Eickbush. 2000. Putative telomerase catalytic subunits from Giardia lamblia and Caenorhabditis elegans. Gene 251:101-108. [DOI] [PubMed] [Google Scholar]
- 157.Marciniak, R., and L. Guarente. 2001. Human genetics. Testing telomerase. Nature 413:370-371., 373. [DOI] [PubMed] [Google Scholar]
- 158.Mason, D. X., C. Autexier, and C. W. Greider. 2001. Tetrahymena proteins p80 and p95 are not core telomerase components. Proc. Natl. Acad. Sci. USA 98:12368-12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McElligott, R., and R. J. Wellinger. 1997. The terminal DNA structure of mammalian chromosomes. EMBO J. 16:3705-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McMahon, S. B., M. A. Wood, and M. D. Cole. 2000. The essential cofactor TRRAP recruits the histone acetyltransferase hGCN5 to c-Myc. Mol. Cell. Biol. 20:556-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Meier, B., L. Driller, S. Jaklin, and H. M. Feldmann. 2001. New function of CDC13 in positive telomere length regulation. Mol. Cell. Biol. 21:4233-4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Metz, A. M., R. A. Love, G. A. Strobel, and D. M. Long. 2001. Two telomerase reverse transcriptases (TERTs) expressed in Candida albicans. Biotechnol. Appl. Biochem. 34:47-54. [DOI] [PubMed] [Google Scholar]
- 163.Meyerson, M., C. M. Counter, E. N. Eaton, L. W. Ellisen, P. Steiner, S. D. Caddle, L. Ziaugra, R. L. Beijersbergen, M. J. Davidoff, Q. Liu, S. Bacchetti, D. A. Haber, and R. A. Weinberg. 1997. hEST2, the putative human telomerase catalytic subunit gene, is upregulated in tumor cells and during immortalization. Cell 90:785-795. [DOI] [PubMed] [Google Scholar]
- 164.Miller, M. C., and K. Collins. 2000. The Tetrahymena p80/p95 complex is required for proper telomere length maintenance and micronuclear genome stability. Mol. Cell 6:827-837. [DOI] [PubMed] [Google Scholar]
- 165.Minamino, T., S. A. Mitsialis, and S. Kourembanas. 2001. Hypoxia extends the life span of vascular smooth muscle cells through telomerase activation. Mol. Cell. Biol. 21:3336-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Misiti, S., S. Nanni, G. Fontemaggi, Y. S. Cong, J. Wen, H. W. Hirte, G. Piaggio, A. Sacchi, A. Pontecorvi, S. Bacchetti, and A. Farsetti. 2000. Induction of hTERT expression and telomerase activity by estrogens in human ovary epithelium cells. Mol. Cell. Biol. 20:3764-3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mitchell, J. R., J. Cheng, and K. Collins. 1999. A box H/ACA small nucleolar RNA-like domain at the human telomerase RNA 3′ end. Mol. Cell. Biol. 19:567-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mitchell, J. R., and K. Collins. 2000. Human telomerase activation requires two independent interactions between telomerase RNA and telomerase reverse transcriptase. Mol. Cell 6:361-371. [DOI] [PubMed] [Google Scholar]
- 169.Mitchell, J. R., E. Wood, and K. Collins. 1999. A telomerase component is defective in the human disease dyskeratosis congenita. Nature 402:551-555. [DOI] [PubMed] [Google Scholar]
- 170.Miura, N., N. Onuki, A. Rathi, A. Virmani, S. Nakamoto, Y. Kishimoto, Y. Murawaki, H. Kawasaki, J. Hasegawa, M. Oshimura, W. D. Travis, and A. F. Gazdar. 2001. hTR repressor-related gene on human chromosome 10p15.1. Br J. Cancer 85:1510-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Morales, C. P., S. E. Holt, M. Ouellette, K. J. Kaur, Y. Yan, K. S. Wilson, M. A. White, W. E. Wright, and J. W. Shay. 1999. Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat. Genet. 21:115-118. [DOI] [PubMed] [Google Scholar]
- 172.Moriarty, T. J., S. Huard, S. Dupuis, and C. Autexier. 2002. Functional multimerization of human telomerase requires an RNA interaction domain in the N terminus of the catalytic subunit. Mol. Cell. Biol. 22:1253-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Murphy, M., J. Ahn, K. K. Walker, W. H. Hoffman, R. M. Evans, A. J. Levine, and D. L. George. 1999. Transcriptional repression by wild-type p53 utilizes histone deacetylases, mediated by interaction with mSin3a. Genes Dev. 13:2490-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Muslin, A. J., and H. Xing. 2000. 14-3-3 proteins: regulation of subcellular localization by molecular interference. Cell. Signal. 12:703-709. [DOI] [PubMed] [Google Scholar]
- 175.Nakabayashi, K., H. Ogino, E. Michishita, N. Satoh, and D. Ayusawa. 1999. Introduction of chromosome 7 suppresses telomerase with shortening of telomeres in a human mesothelial cell line. Exp. Cell Res. 252:376-382. [DOI] [PubMed] [Google Scholar]
- 176.Nakamura, T. M., and T. R. Cech. 1998. Reversing time: origin of telomerase. Cell 92:587-590. [DOI] [PubMed] [Google Scholar]
- 177.Nakamura, T. M., G. B. Morin, K. B. Chapman, S. L. Weinrich, W. H. Andrews, J. Lingner, C. B. Harley, and T. R. Cech. 1997. Telomerase catalytic subunit homologs from fission yeast and human. Science 277:955-959. [DOI] [PubMed] [Google Scholar]
- 178.Nakayama, J., M. Saito, H. Nakamura, A. Matsuura, and F. Ishikawa. 1997. TLP1: a gene encoding a protein component of mammalian telomerase is a novel member of WD repeats family. Cell. 88:875-884. [DOI] [PubMed] [Google Scholar]
- 179.Nakayama, Y., H. Sakamoto, K. Satoh, and T. Yamamoto. 2000. Tamoxifen and gonadal steroids inhibit colon cancer growth in association with inhibition of thymidylate synthase, survivin and telomerase expression through estrogen receptor beta mediated system. Cancer Lett. 161:63-71. [DOI] [PubMed] [Google Scholar]
- 180.Nguyen, D. C., and D. L. Crowe. 1999. Intact functional domains of the retinoblastoma gene product (pRb) are required for downregulation of telomerase activity. Biochim. Biophys. Acta 1445:207-215. [DOI] [PubMed] [Google Scholar]
- 181.Nielsen, S. J., R. Schneider, U. M. Bauer, A. J. Bannister, A. Morrison, D. O'Carroll, R. Firestein, M. Cleary, T. Jenuwein, R. E. Herrera, and T. Kouzarides. 2001. Rb targets histone H3 methylation and HP1 to promoters. Nature 412:561-565. [DOI] [PubMed] [Google Scholar]
- 182.Nishimoto, A., N. Miura, I. Horikawa, H. Kugoh, Y. Murakami, S. Hirohashi, H. Kawasaki, A. F. Gazdar, J. W. Shay, J. C. Barrett, and M. Oshimura. 2001. Functional evidence for a telomerase repressor gene on human chromosome 10p15.1. Oncogene 20:828-835. [DOI] [PubMed] [Google Scholar]
- 183.Oguchi, K., H. Liu, K. Tamura, and H. Takahashi. 1999. Molecular cloning and characterization of AtTERT, a telomerase reverse transcriptase homolog in Arabidopsis thaliana. FEBS Lett. 457:465-469. [DOI] [PubMed] [Google Scholar]
- 184.Oh, S., Y. Song, J. Yim, and T. K. Kim. 1999. The Wilms' tumor 1 tumor suppressor gene represses transcription of the human telomerase reverse transcriptase gene. J. Biol. Chem. 274:37473-37478. [DOI] [PubMed] [Google Scholar]
- 185.Oh, S., Y. H. Song, U. J. Kim, J. Yim, and T. K. Kim. 1999. In vivo and in vitro analyses of Myc for differential promoter activities of the human telomerase (hTERT) gene in normal and tumor cells. Biochem. Biophys. Res. Commun. 263:361-365. [DOI] [PubMed] [Google Scholar]
- 186.Oh, S., Y. H. Song, J. Yim, and T. K. Kim. 2000. Identification of Mad as a repressor of the human telomerase (hTERT) gene. Oncogene. 19:1485-1490. [DOI] [PubMed] [Google Scholar]
- 187.Oh, S. T., S. Kyo, and L. A. Laimins. 2001. Telomerase activation by human papillomavirus type 16 E6 protein: induction of human telomerase reverse transcriptase expression through Myc and GC-rich Sp1 binding sites. J. Virol. 75:5559-5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Olson, M. O., M. Dundr, and A. Szebeni. 2000. The nucleolus: an old factory with unexpected capabilities. Trends Cell Biol. 10:189-196. [DOI] [PubMed] [Google Scholar]
- 189.Oshimura, M., and J. C. Barrett. 1997. Multiple pathways to cellular senescence: role of telomerase repressors. Eur. J. Cancer. 33:710-715. [DOI] [PubMed] [Google Scholar]
- 190.Ouellette, M. M., D. L. Aisner, I. Savre-Train, W. E. Wright, and J. W. Shay. 1999. Telomerase activity does not always imply telomere maintenance. Biochem. Biophys. Res. Commun. 254:795-803. [DOI] [PubMed] [Google Scholar]
- 191.Ouellette, M. M., M. Liao, B. S. Herbert, M. Johnson, S. E. Holt, H. S. Liss, J. W. Shay, and W. E. Wright. 2000. Subsenescent telomere lengths in fibroblasts immortalized by limiting amounts of telomerase. J. Biol. Chem. 275:10072-10076. [DOI] [PubMed] [Google Scholar]
- 192.Pandita, T. K. 2002. ATM function and telomere stability. Oncogene 21:611-618. [DOI] [PubMed] [Google Scholar]
- 193.Peng, C. Y., P. R. Graves, R. S. Thoma, Z. Wu, A. S. Shaw, and H. Piwnica-Worms. 1997. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 277:1501-1505. [DOI] [PubMed] [Google Scholar]
- 194.Pfeffer, L. M., C. A. Dinarello, R. B. Herberman, B. R. Williams, E. C. Borden, R. Bordens, M. R. Walter, T. L. Nagabhushan, P. P. Trotta, and S. Pestka. 1998. Biological properties of recombinant alpha-interferons: 40th anniversary of the discovery of interferons. Cancer Res. 58:2489-2499. [PubMed] [Google Scholar]
- 195.Pogacic, V., F. Dragon, and W. Filipowicz. 2000. Human H/ACA small nucleolar ribonucleoproteins and telomerase share evolutionarily conserved proteins NHP2 and NOP10. Mol. Cell. Biol. 20:9028-9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Poole, J. C., L. G. Andrews, and T. O. Tollefsbol. 2001. Activity, function, and gene regulation of the catalytic subunit of telomerase (hTERT). Gene. 269:1-12. [DOI] [PubMed] [Google Scholar]
- 197.Pugh, B. F., and R. Tjian. 1991. Transcription from a TATA-less promoter requires a multisubunit TFIID complex. Genes Dev. 5:1935-1945. [DOI] [PubMed] [Google Scholar]
- 198.Rama, S., Y. Suresh, and A. J. Rao. 2001. Regulation of telomerase during human placental differentiation: a role for TGFbeta1. Mol. Cell. Endocrinol. 182:233-248. [DOI] [PubMed] [Google Scholar]
- 199.Ramirez, R. D., C. P. Morales, B. S. Herbert, J. M. Rohde, C. Passons, J. W. Shay, and W. E. Wright. 2001. Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions. Genes Dev. 15:398-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rapp, L., and J. J. Chen. 1998. The papillomavirus E6 proteins. Biochim. Biophys. Acta 1378:F1-19. [DOI] [PubMed] [Google Scholar]
- 201.Razin, A. 1998. CpG methylation, chromatin structure and gene silencing-a three-way connection. EMBO J. 17:4905-4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Reik, W., and W. Dean. 2001. DNA methylation and mammalian epigenetics. Electrophoresis 22:2838-2843. [DOI] [PubMed] [Google Scholar]
- 203.Rideout, W. M., 3rd, K. Eggan, and R. Jaenisch. 2001. Nuclear cloning and epigenetic reprogramming of the genome. Science 293:1093-1098. [DOI] [PubMed] [Google Scholar]
- 204.Rohde, V., H. P. Sattler, T. Bund, H. Bonkhoff, T. Fixemer, C. Bachmann, R. Lensch, G. Unteregger, M. Stoeckle, and B. Wullich. 2000. Expression of the human telomerase reverse transcriptase is not related to telomerase activity in normal and malignant renal tissue. Clin. Cancer Res. 6:4803-4809. [PubMed] [Google Scholar]
- 205.Sager, R. 1991. Senescence as a mode of tumor suppression. Environ. Health Perspect. 93:59-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Seimiya, H., H. Sawada, Y. Muramatsu, M. Shimizu, K. Ohko, K. Yamane, and T. Tsuruo. 2000. Involvement of 14-3-3 proteins in nuclear localization of telomerase. EMBO J. 19:2652-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Seto, A. G., A. J. Zaug, S. G. Sobel, S. L. Wolin, and T. R. Cech. 1999. Saccharomyces cerevisiae telomerase is an Sm small nuclear ribonucleoprotein particle. Nature 401:177-180. [DOI] [PubMed] [Google Scholar]
- 208.Sharma, H. W., J. A. Sokoloski, J. R. Perez, J. Y. Maltese, A. C. Sartorelli, C. A. Stein, G. Nichols, Z. Khaled, N. T. Telang, and R. Narayanan. 1995. Differentiation of immortal cells inhibits telomerase activity. Proc. Natl. Acad. Sci. USA 92:12343-12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Shay, J. W. 1999. Toward identifying a cellular determinant of telomerase repression. J. Natl. Cancer Inst. 91:4-6. [DOI] [PubMed] [Google Scholar]
- 210.Shay, J. W., and S. Bacchetti. 1997. A survey of telomerase activity in human cancer. Eur. J. Cancer 33:787-791. [DOI] [PubMed] [Google Scholar]
- 211.Shay, J. W., O. Peirera-Smith, and W. E. Wright. 1991. A role for both RB and p53 in the regulation of the cellular senescence. Exp. Cell Res. 196:33-39. [DOI] [PubMed] [Google Scholar]
- 212.Shay, J. W., and W. E. Wright. 2000. Hayflick, his limit, and cellular ageing. Nat. Rev. Mol. Cell. Biol. 1:72-76. [DOI] [PubMed] [Google Scholar]
- 213.Shay, J. W., W. E. Wright, D. Brasiskyte, and B. A. Van der Haegen. 1993. E6 of human papillomavirus type 16 can overcome the M1 stage of immortalization in human mammary epithelial cells but not in human fibroblasts. Oncogene 8:1407-1413. [PubMed] [Google Scholar]
- 214.Shay, J. W., and W. E. Wright. 1989. Quantitation of the frequency of immortalization of normal human diploid fibroblasts by SV40 larger T-antigen. Exp. Cell Res. 184:109-118. [DOI] [PubMed] [Google Scholar]
- 215.Smith, S., I. Giriat, A. Schmitt, and T. de Lange. 1998. Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science 282:1484-1487. [DOI] [PubMed] [Google Scholar]
- 216.Smogorzewska, A., B. van Steensel, A. Bianchi, S. Oelmann, M. R. Schaefer, G. Schnapp, and T. de Lange. 2000. Control of human telomere length by TRF1 and TRF2. Mol. Cell. Biol. 20:1659-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Smucker, E. J., and J. J. Turchi. 2001. TRF1 inhibits telomere C-strand DNA synthesis in vitro. Biochemistry 40:2426-2432. [DOI] [PubMed] [Google Scholar]
- 218.Soda, H., E. Raymond, S. Sharma, R. Lawrence, K. Davidson, M. Oka, S. Kohno, E. Izbicka, and D. D. Von Hoff. 2000. Effects of androgens on telomerase activity in normal and malignant prostate cells in vitro. Prostate 43:161-168. [DOI] [PubMed] [Google Scholar]
- 219.Steenbergen, R. D., D. Kramer, C. J. Meijer, J. M. Walboomers, D. A. Trott, A. P. Cuthbert, R. F. Newbold, W. J. Overkamp, M. Z. Zdzienicka, and P. J. Snijders. 2001. Telomerase suppression by chromosome 6 in a human papillomavirus type 16-immortalized keratinocyte cell line and in a cervical cancer cell line. J. Natl. Cancer Inst. 93:865-872. [DOI] [PubMed] [Google Scholar]
- 220.Steinert, S., J. W. Shay, and W. E. Wright. 2000. Transient expression of human telomerase extends the life span of normal human fibroblasts. Biochem. Biophys. Res. Commun. 273:1095-1098. [DOI] [PubMed] [Google Scholar]
- 221.Strobeck, M. W., K. E. Knudsen, A. F. Fribourg, M. F. DeCristofaro, B. E. Weissman, A. N. Imbalzano, and E. S. Knudsen. 2000. BRG-1 is required for RB-mediated cell cycle arrest. Proc. Natl. Acad. Sci. USA 97:7748-7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Suske, G. 1999. The Sp-family of transcription factors. Gene 238:291-300. [DOI] [PubMed] [Google Scholar]
- 223.Tahara, H., W. Yasui, E. Tahara, J. Fujimoto, K. Ito, K. Tamai, J. Nakayama, F. Ishikawa, and T. Ide. 1999. Immuno-histochemical detection of human telomerase catalytic component, hTERT, in human colorectal tumor and nontumor tissue sections. Oncogene 18:1561-1567. [DOI] [PubMed] [Google Scholar]
- 224.Takakura, M., S. Kyo, T. Kanaya, H. Hirano, J. Takeda, M. Yutsudo, and M. Inoue. 1999. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 59:551-557. [PubMed] [Google Scholar]
- 225.Takakura, M., S. Kyo, Y. Sowa, Z. Wang, N. Yatabe, Y. Maida, M. Tanaka, and M. Inoue. 2001. Telomerase activation by histone deacetylase inhibitor in normal cells. Nucleic Acids Res. 29:3006-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tanaka, H., M. Shimizu, I. Horikawa, H. Kugoh, J. Yokota, J. C. Barrett, and M. Oshimura. 1998. Evidence for a putative telomerase repressor gene in the 3p14.2-p21.1 region. Genes Chromosomes Cancer 23:123-133. [DOI] [PubMed] [Google Scholar]
- 227.Tanaka, M., S. Kyo, M. Takakura, T. Kanaya, T. Sagawa, K. Yamashita, Y. Okada, E. Hiyama, and M. Inoue. 1998. Expression of telomerase activity in human endometrium is localized to epithelial glandular cells and regulated in a menstrual phase-dependent manner correlated with cell proliferation. Am. J. Pathol. 153:1985-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tesmer, V. M., L. P. Ford, S. E. Holt, B. C. Frank, X. Yi, D. L. Aisner, M. Ouellette, J. W. Shay, and W. E. Wright. 1999. Two inactive fragments of the integral RNA cooperate to assemble active telomerase with the human protein catalytic subunit (hTERT) in vitro. Mol. Cell. Biol. 19:6207-6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tham, W. H., and V. A. Zakian. 2002. Transcriptional silencing at Saccharomyces telomeres: implications for other organisms. Oncogene 21:512-521. [DOI] [PubMed] [Google Scholar]
- 230.Thomas, M., L. Yang, and P. J. Hornsby. 2000. Formation of functional tissue from transplanted adrenocortical cells expressing telomerase reverse transcriptase. Nat. Biotechnol. 18:39-42. [DOI] [PubMed] [Google Scholar]
- 231.Trouche, D., C. Le Chalony, C. Muchardt, M. Yaniv, and T. Kouzarides. 1997. RB and hbrm cooperate to repress the activation functions of E2F1. Proc. Natl. Acad. Sci. USA 94:11268-11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Tzukerman, M., C. Shachaf, Y. Ravel, I. Braunstein, O. Cohen-Barak, M. Yalon-Hacohen, and K. L. Skorecki. 2000. Identification of a novel transcription factor binding element involved in the regulation by differentiation of the human telomerase (hTERT) promoter. Mol. Biol. Cell 11:4381-4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ulaner, G. A., J. F. Hu, T. H. Vu, L. C. Giudice, and A. R. Hoffman. 1998. Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts. Cancer Res. 58:4168-4172. [PubMed] [Google Scholar]
- 234.Ulaner, G. A., J. F. Hu, T. H. Vu, L. C. Giudice, and A. R. Hoffman. 2001. Tissue-specific alternate splicing of human telomerase reverse transcriptase (hTERT) influences telomere lengths during human development. Int. J. Cancer 91:644-649. [PubMed] [Google Scholar]
- 235.Ulaner, G. A., J. F. Hu, T. H. Vu, H. Oruganti, L. C. Giudice, and A. R. Hoffman. 2000. Regulation of telomerase by alternate splicing of human telomerase reverse transcriptase (hTERT) in normal and neoplastic ovary, endometrium and myometrium. Int. J. Cancer 85:330-335. [PubMed] [Google Scholar]
- 236.van Steensel, B., and T. de Lange. 1997. Control of telomere length by the human telomeric protein TRF1. Nature 385:740-743. [DOI] [PubMed] [Google Scholar]
- 237.Vaziri, H., and S. Benchimol. 1998. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr. Biol. 8:279-282. [DOI] [PubMed] [Google Scholar]
- 238.Veldman, T., I. Horikawa, J. C. Barrett, and R. Schlegel. 2001. Transcriptional activation of the telomerase hTERT gene by human papillomavirus type 16 E6 oncoprotein. J. Virol. 75:4467-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Vulliamy, T., A. Marrone, F. Goldman, A. Dearlove, M. Bessler, P. J. Mason, and I. Dokal. 2001. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature 413:432-435. [DOI] [PubMed] [Google Scholar]
- 240.Wang, H., and E. H. Blackburn. 1997. De novo telomere addition by Tetrahymena telomerase in vitro. EMBO J. 16:866-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wang, J., L. Y. Xie, S. Allan, D. Beach, and G. J. Hannon. 1998. Myc activates telomerase. Genes Dev. 12:1769-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wang, Z., S. Kyo, M. Takakura, M. Tanaka, N. Yatabe, Y. Maida, M. Fujiwara, J. Hayakawa, M. Ohmichi, K. Koike, and M. Inoue. 2000. Progesterone regulates human telomerase reverse transcriptase gene expression via activation of mitogen-activated protein kinase signaling pathway. Cancer Res. 60:5376-5381. [PubMed] [Google Scholar]
- 243.Weinrich, S. L., R. Pruzan, L. Ma, M. Ouellette, V. M. Tesmer, S. E. Holt, A. G. Bodnar, S. Lichtsteiner, N. W. Kim, J. B. Trager, R. D. Taylor, R. Carlos, W. H. Andrews, W. E. Wright, J. W. Shay, C. B. Harley, and G. B. Morin. 1997. Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nat. Genet. 17:498-502. [DOI] [PubMed] [Google Scholar]
- 244.Wen, J., Y. S. Cong, and S. Bacchetti. 1998. Reconstitution of wild-type or mutant telomerase activity in telomerase-negative immortal human cells. Hum. Mol. Genet. 7:1137-1141. [DOI] [PubMed] [Google Scholar]
- 245.Wenz, C., B. Enenkel, M. Amacker, C. Kelleher, K. Damm, and J. Lingner. 2001. Human telomerase contains two cooperating telomerase RNA molecules. EMBO J. 20:3526-3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Wick, M., D. Zubov, and G. Hagen. 1999. Genomic organization and promoter characterization of the gene encoding the human telomerase reverse transcriptase (hTERT). Gene 232:97-106. [DOI] [PubMed] [Google Scholar]
- 247.Wright, W. E., D. Brasiskyte, M. A. Piatyszek, and J. W. Shay. 1996. Experimental elongation of telomeres extends the lifespan of immortal × normal cell hybrids. EMBL J. 15:1734-1741. [PMC free article] [PubMed] [Google Scholar]
- 248.Wright, W. E., O. M. Pereira-Smith, and J. W. Shay. 1989. Reversible cellular senescence: implications for immortalization of normal human diploid fibroblasts. Mol. Cell. Biol. 9:3088-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wright, W. E., M. A. Piatyszek, W. E. Rainey, W. Byrd, and J. W. Shay. 1996. Telomerase activity in human germline and embryonic tissues and cells. Dev. Genet. 18:173-179. [DOI] [PubMed] [Google Scholar]
- 250.Wright, W. E., and J. W. Shay. 2001. Cellular senescence as a tumor-protection mechanism: the essential role of counting. Curr. Opin. Genet. Dev. 11:98-103. [DOI] [PubMed] [Google Scholar]
- 251.Wright, W. E., and J. W. Shay. 1992. Telomere positional effects and the regulation of cellular senescence. Trends Genet. 8:193-197. [DOI] [PubMed] [Google Scholar]
- 252.Wright, W. E., and J. W. Shay. 1992. The two-stage mechanism controlling cellular senescence and immortalization. Exp. Gerontol. 27:383-389. [DOI] [PubMed] [Google Scholar]
- 253.Wright, W. E., V. M. Tesmer, K. E. Huffman, S. D. Levene, and J. W. Shay. 1997. Normal human chromosomes have long G-rich telomeric overhangs at one end. Genes Dev. 11:2801-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wu, G., W. H. Lee, and P. L. Chen. 2000. NBS1 and TRF1 colocalize at promyelocytic leukemia bodies during late S/G2 phases in immortalized telomerase-negative cells. Implication of NBS1 in alternative lengthening of telomeres. J. Biol. Chem. 275:30618-30622. [DOI] [PubMed] [Google Scholar]
- 255.Wu, K. J., C. Grandori, M. Amacker, N. Simon-Vermot, A. Polack, J. Lingner, and R. Dalla-Favera. 1999. Direct activation of TERT transcription by c-MYC. Nat. Genet. 21:220-224. [DOI] [PubMed] [Google Scholar]
- 256.Xia, J., Y. Peng, I. S. Mian, and N. F. Lue. 2000. Identification of functionally important domains in the N-terminal region of telomerase reverse transcriptase. Mol. Cell. Biol. 20:5196-5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Xu, D., S. Erickson, M. Szeps, A. Gruber, O. Sangfelt, S. Einhorn, P. Pisa, and D. Grander. 2000. Interferon alpha downregulates telomerase reverse transcriptase and telomerase activity in human malignant and nonmalignant hematopoietic cells. Blood 96:4313-4318. [PubMed] [Google Scholar]
- 258.Xu, D., A. Gruber, M. Bjorkholm, C. Peterson, and P. Pisa. 1999. Suppression of telomerase reverse transcriptase (hTERT) expression in differentiated HL-60 cells: regulatory mechanisms. Br. J. Cancer 80:1156-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Xu, D., N. Popov, M. Hou, Q. Wang, M. Bjorkholm, A. Gruber, A. R. Menkel, and M. Henriksson. 2001. Switch from Myc/Max to Mad1/Max binding and decrease in histone acetylation at the telomerase reverse transcriptase promoter during differentiation of HL60 cells. Proc. Natl. Acad. Sci. USA 98:3826-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Xu, D., Q. Wang, A. Gruber, M. Bjorkholm, Z. Chen, A. Zaid, G. Selivanova, C. Peterson, K. G. Wiman, and P. Pisa. 2000. Downregulation of telomerase reverse transcriptase mRNA expression by wild type p53 in human tumor cells. Oncogene 19:5123-5133. [DOI] [PubMed] [Google Scholar]
- 261.Xu, H. J., Y. Zhou, W. Ji, G. S. Perng, R. Kruzelock, C. T. Kong, R. C. Bast, G. B. Mills, J. Li, and S. X. Hu. 1997. Reexpression of the retinoblastoma protein in tumor cells induces senescence and telomerase inhibition. Oncogene 15:2589-2596. [DOI] [PubMed] [Google Scholar]
- 262.Yang, H., S. Kyo, M. Takatura, and L. Sun. 2001. Autocrine transforming growth factor beta suppresses telomerase activity and transcription of human telomerase reverse transcriptase in human cancer cells. Cell Growth Differ. 12:119-127. [PubMed] [Google Scholar]
- 263.Yang, J., E. Chang, A. M. Cherry, C. D. Bangs, Y. Oei, A. Bodnar, A. Bronstein, C. P. Chiu, and G. S. Herron. 1999. Human endothelial cell life extension by telomerase expression. J. Biol. Chem. 274:26141-26148. [DOI] [PubMed] [Google Scholar]
- 264.Yi, X., V. M. Tesmer, I. Savre-Train, J. W. Shay, and W. E. Wright. 1999. Both transcriptional and posttranscriptional mechanisms regulate human telomerase template RNA levels. Mol. Cell. Biol. 19:3989-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Yi, X., D. M. White, D. L. Aisner, J. A. Baur, W. E. Wright, and J. W. Shay. 2000. An alternate splicing variant of the human telomerase catalytic subunit inhibits telomerase activity. Neoplasia 2:433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Yokoyama, Y., X. Wan, Y. Takahashi, A. Shinohara, and T. Tamaya. 2001. Alternatively spliced variant deleting exons 7 and 8 of the human telomerase reverse transcriptase gene is dominantly expressed in the uterus. Mol. Hum. Reprod. 7:853-857. [DOI] [PubMed] [Google Scholar]
- 267.Yu, C. C., S. C. Lo, and T. C. Wang. 2001. Telomerase is regulated by protein kinase C-zeta in human nasopharyngeal cancer cells. Biochem. J. 355:459-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Zhang, A., C. Zheng, C. Lindvall, M. Hou, J. Ekedahl, R. Lewensohn, Z. Yan, X. Yang, M. Henriksson, E. Blennow, M. Nordenskjold, A. Zetterberg, M. Bjorkholm, A. Gruber, and D. Xu. 2000. Frequent amplification of the telomerase reverse transcriptase gene in human tumors. Cancer Res. 60:6230-6235. [PubMed] [Google Scholar]
- 269.Zhang, H. S., and D. C. Dean. 2001. Rb-mediated chromatin structure regulation and transcriptional repression. Oncogene 20:3134-3138. [DOI] [PubMed] [Google Scholar]
- 270.Zhang, W., M. A. Piatyszek, T. Kobayashi, E. Estey, M. Andreeff, A. B. Deisseroth, W. E. Wright, and J. W. Shay. 1996. Telomerase activity in human acute myelogenous leukemia: inhibition of telomerase activity by differentiation-inducing agents. Clin. Cancer Res. 2:799-803. [PubMed] [Google Scholar]
- 271.Zhang, X., V. Mar, W. Zhou, L. Harrington, and M. O. Robinson. 1999. Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev. 13:2388-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Zheng, L., and W. H. Lee. 2001. The retinoblastoma gene: a prototypic and multifunctional tumor suppressor. Exp. Cell Res. 264:2-18. [DOI] [PubMed] [Google Scholar]
- 273.Zhou, X. Z., and K. P. Lu. 2001. The Pin2/TRF1-interacting protein PinX1 is a potent telomerase inhibitor. Cell 107:347-359. [DOI] [PubMed] [Google Scholar]