Abstract
Brain development during adolescence and early adulthood coincides with shifts in emotion regulation and sleep. Despite this, few existing datasets simultaneously characterize affective dynamics, sleep variation, and multimodal measures of brain development. Here, we describe the study protocol and initial release (n = 10) of an open data resource of neuroimaging paired with densely sampled behavioral measures in adolescents and young adults. All participants complete multi-echo functional MRI, compressed-sensing diffusion MRI, and advanced arterial spin-labeled MRI. Behavioral measures include ecological momentary assessment, actigraphy, extensive cognitive assessments, and detailed clinical phenotyping focused on emotion regulation. Raw and processed data are openly available without a data use agreement and will be regularly updated as accrual continues. Together, this resource will accelerate research on the links between mood, sleep, and brain development.
Keywords: adolescence, development, fMRI, dMRI, ASL, EMA, actigraphy, emotion regulation, affective instability, sleep
Introduction
Affective instability is characterized by frequent mood shifts and disturbances in emotional intensity;1 affective instability is related to similar constructs such as mood instability and emotional dysregulation.2 Affective instability is present in multiple psychiatric disorders, including borderline personality disorder (BPD), attention-deficit hyperactivity disorder (ADHD), post-traumatic stress disorder (PTSD), and major depressive disorder (MDD).3 Typically emerging when youth learn to self-regulate changes in their affective states or mood,1 affective instability has been associated with suicidality and suicide attempts.4 Despite increasing rates of mental disorders and suicide in youth,5 the neurodevelopmental underpinnings of affective instability remain sparsely studied and poorly understood.6 Here, we describe the study protocol and first data release of a new open data resource focused on how variation in brain development may relate to affective instability in youth.
Affective instability is challenging to measure. While there are numerous self-report scales (e.g., Affective Control Scale,7 Difficulties in Emotion Regulation Scale,8 etc.) and semi-structured interviews (e.g., Emotion Regulation Interview,9 Structured Clinical Interview for DSM-V Axis II Personality Disorders,10 etc.) that assess aspects of affective instability, their retrospective nature limits accuracy and can be susceptible to memory distortion.11 Ecological momentary assessment (EMA) offers a promising alternative by measuring participants’ self-reported symptoms and activities as they occur in daily life, providing contextual richness and naturalistic data.12 These strengths have led to increasing use of EMA in studies of BPD,13,14 MDD,15 and other related disorders in childhood.16
Sleep quality is closely tied to affective dynamics and emotion regulation in youth. Substantial evidence indicates that sleep disruption (e.g., sleep deprivation, insomnia) drives emotion dysregulation and predicts the onset of mood disorders in adolescence.17–22 Additionally, sleep is thought to be linked to critical plasticity mechanisms of healthy brain development,23–26 with impaired sleep contributing to variation in brain structure and function.27–33 Daytime physical activity has also been associated with affective dynamics, where lower levels of physical activity tend to worsen emotion regulation and mood.34,35 While sleep and physical activity are often assessed with parent or self-report questionnaires, research-grade actigraphy recordings provide sensor-based data that overcome questionnaire-related limitations of recall bias. Actigraphy tracks sleep and physical activity for up to several weeks in real-world settings, providing a unique opportunity to collect more accurate, dense, and ecologically valid measures of a person’s lifestyle.36–38
Neuroimaging data can be integrated with the high-quality behavioral measures described above to further characterize the neurodevelopmental underpinnings of affective dynamics. However, recent evidence suggests that due to small effect sizes, large samples are necessary to uncover generalizable brain-behavior relationships, posing a significant methodological challenge to understanding how variation in brain development connects to affective instability and mood dysregulation.39,40 Small effect sizes observed may be due, in part, to poor reliability and inaccurate measurements of key behavioral phenotypes,39,41,42 un-modeled individual variation in neuroimaging measures,43,44 and biological heterogeneity.45–47 However, studies can improve signal and minimize noise by collecting precise individualized measures of behavioral and brain data.44 The recent advent of multi-echo functional MRI (ME-fMRI)48,49 and other enhanced imaging sequences (like compressed sensing diffusion spectrum imaging; CS-DSI)50 may allow for identification of person-specific variation with less data.48 In particular, multi-echo fMRI facilitates accurate characterization of personalized functional networks (PFNs). PFNs delineated using ME-fMRI hold significant promise in improving the effect size and generalizability of translational studies.48 Thus, pairing contemporary EMA and actigraphy procedures that densely sample mood and sleep with more precise imaging methods may hold promise for linking variation in affective dynamics to brain development in youth.
In this paper, we describe the protocol and initial data release from a new study that leverages recent advances in characterizing complex behavior and non-invasive brain imaging to investigate affective instability in youth. Specifically, we employ a combination of EMA techniques and specialized self-report scales to assess affective instability and emotion regulation. Furthermore, the study measures sleep via wearable actigraphy devices in addition to EMA and other self-report measures. Perhaps most importantly, the study includes advanced imaging methods – such as ME-fMRI, CS-DSI, and advanced arterial spin labeled (ASL) perfusion MRI – to characterize structural and functional brain development. Notably, both raw and processed data are fully de-identified and openly shared without a data use agreement. We anticipate that this data resource will accelerate translational research on affective instability in youth.
Methods
Participants.
This study aims to recruit 100 individuals between the ages of 13 and 23. Participants must be proficient in English, able to understand the study procedures, and agree to participate by giving written informed consent or assent. Exclusion criteria include any significant medical or neurological illness that may impact brain function or a history of pervasive developmental disorder, psychosis, bipolar disorder, or clinically significant current substance misuse. Furthermore, participants who present with acute intoxication with alcohol or other substances or lack a mobile device with capabilities to complete the study procedures are excluded. Additional exclusion criteria include pregnancy, implanted ferrous metal, claustrophobia, and other contraindications to MRI. Initial recruitment was focused on young adults as study procedures were being finalized; the initial data release includes 10 participants (mean age = 21.5, SD = 1.33; M:F 4:6). Recruitment is ongoing to reach the full sample size of 100 participants.
Participants are primarily recruited through medical record review at the Children’s Hospital of Pennsylvania (CHOP) and Penn Medicine. In addition, individuals who had previously participated in research within the Department of Psychiatry may be recontacted to participate in the current study. Other recruitment strategies include physician referrals within the Penn Medicine and CHOP systems, announcements on the lab website, and advertisements around Philadelphia. All study procedures are approved by the Institutional Review Boards of both the University of Pennsylvania and CHOP; all participants provide written informed consent. For participants under the age of 18, a parent or legal guardian provides informed consent and the minor provides informed assent.
Behavioral and Clinical Assessments.
This study includes an array of assessments. These include self-report measures, clinical assessments, a cognitive battery, mobile phenotyping with ecological momentary assessments (EMA), actigraphy via a wearable wristwatch-like device, and MRI. Figure 1 presents the study workflow of assessments. First, the study team identifies and recruits participants through a phone call screener. Second, participants complete the in-person imaging visit at the Hospital of the University of Pennsylvania. Participants also begin the EMA procedures and actigraphy procedures following the in-person imaging visit. Participants are provided the actigraphy device fully charged as well as a prepaid shipping envelope to return the device after three weeks. Finally, participants complete a remote clinical assessment session in the days following the imaging visit.
Figure 1. Study workflow.
Self-Report Scales
A series of self-report scales assess demographic information, local environment, sleep, emotion regulation, and other domains of mental health. Table 1 presents the self-report questionnaire battery administered during the remote clinical assessment visit.
Table 1. Self-report scales.
| Scale Administered | Short Name | Outcome Assessed | Reference |
|---|---|---|---|
| Difficulties in Emotion Regulation Scale | DERS | Emotion Regulation | Gratz & Roemer (2004) |
| Affective Reactivity Index | ARI | Irritability | Stringaris et al. (2012) |
| Borderline Evaluation of Severity over Time | BEST | Borderline Personality Disorder | Pfohl et al. (2009) |
| Extended Strengths and Weaknesses Assessment of DMDD Symptoms and Normal Behavior | E-SWAN DMDD | Disruptive Mood Dysregulation Disorder | Alexander et al. (2020) |
| Sleep Reduction Screening Questionnaire | SRSQ | Sleep Reduction | van Maanen et al. (2014) |
| Ultra-Short Munich ChronoType Questionnaire | uMCTQ | Sleep-wake Behavior | Ghotbi et al. (2020) |
| Emotion Regulation Questionnaire - Short Form | ERQ-S | Emotion Regulation, Cognitive Reappraisal, Expressive Suppression | Preece et al. (2023) |
| Patient Health Questionnaire 8-item | PHQ-8 | Depression | Kroenke et al. (2009) |
| Extended Strengths and Weaknesses Assessment of ADHD Symptoms and Normal Behavior | E-SWAN ADHD | Attention-deficit/Hyperactivity Disorder | Alexander et al. (2017) |
| PROMIS Pediatric Sleep Disturbance [Short Form 4a] | PROMIS SD | Sleep Disturbance, Sleep and Wake Function | Yu et al. (2012) |
| PRIME Screen Revised 5-item | PRIME-5 | Early Subthreshold Psychosis Symptoms | Calkins (2025) |
| Behavioral Inhibition/Activation System Reward Subscale Child | BIS/BAS Reward Child | Motivational Systems | Carver & White (1994) |
| Pubertal Development Scale [Male] | PDS-TannerM | Male Adolescent Development | Morris & Udry (1980) |
| Pubertal Development Scale [Female] | PDS-TannerF | Female Adolescent Development | Morris & Udry (1980) |
| Perceived Stress Scale-4 | PSS-4 | Nonspecific Appraised Stress | Cohen et al. (1983) |
| Child and Adolescent Trauma Screen | CATS | Posttraumatic Stress Symptoms | Sachser et al. (2017) |
| Neighborhood Safety and Crime Short-Form | NSC | Residential Environment, Socioeconomic Position | Mujahid et al. (2007) |
| Neighborhood Community Cohesion Short-Form | NCC | Residential Environment, Socioeconomic Position | Mujahid et al. (2007) |
| Accountable Health Communities Health-Related Social Needs Screening Tool | AHC-HRSN | Housing Instability, Food Insecurity, Exposure to Interpersonal Violence | Billioux et al. (2017) |
Pubertal measures
Participants under the age of 18 complete the Pubertal Developmental Scale,51 a self-report measure that uses Tanner’s images52 to assess pubertal status in adolescent populations. Male and female participants receive distinct versions of the scale. Both versions are seven items and include two items where participants indicate how their development compares to Tanner’s images; the items differ between male and female versions asking either about male pubertal changes (e.g., facial hair growth, deepening voice, etc.) or female pubertal changes (e.g., menstruation).
Measures of the childhood environment
Five scales assess childhood environment. First, the Childhood and Adolescent Trauma Screen (CATS)53 is a self-report questionnaire that examines participants’ exposure to potentially traumatic events (PTEs) and post-traumatic stress symptoms. An abbreviated 9-item version includes a structured PTE checklist asking about traumatic events (e.g., natural disasters, accidents, etc.) reflected in the DSM-5 criteria for post-traumatic stress disorder (PTSD). Furthermore, participants complete the Neighborhood Safety and Crime Short-Form (NSC; 3-item questionnaire)54 and the Neighborhood Community Cohesion Short-Form (NCC; 5-item questionnaire),54 two brief self-report scales that address neighborhood socioeconomic position. Finally, the Accountable Health Communities Health-Related Social Needs Screening Tool (AHC-HRSN)55 is a 14-item self-report scale that screens for non-medical health-related needs in the following domains: housing instability, food insecurity, transportation difficulties, utility assistance needs, interpersonal safety, financial strain, family and community support, education, physical activity, and disabilities. An additional 12 items are asked to obtain demographic and socioeconomic information from participants related to income, education, and household factors.
Self-report sleep measures
Three scales assess sleep. First, the Sleep Reduction Screening Questionnaire (SRSQ)56 is a 9-item self-report questionnaire measuring individual sleep need through daytime symptoms and interrogating sleep problems in order to assess perceived symptoms of sleep reduction (e.g., insufficient/poor sleep). Second, the Ultra-Short Munich ChronoType Questionnaire (uMCTQ)57 is a 6-item self-report questionnaire designed to evaluate sleep-wake-behavior. Finally, the PROMIS Pediatric Sleep Disturbance Short Form (PROMIS SD Short Form 4a)58 is a 4-item self-report measure evaluating sleep and wake function.
Measures of emotion lability, reactivity, and regulation
All participants complete an extensive battery including five complementary measures related to emotion regulation. First, the current study uses an abbreviated 5-item version of the Difficulties in Emotion Regulation Scale (DERS)8 assessing clinically relevant difficulties in emotion regulation. Second, the Emotion Regulation Questionnaire-Short Form (ERQ-S)59 is a 6-item self-report scale assessing two distinct emotion regulation strategies: cognitive reappraisal and expression suppression, designed for time-pressured settings. Third, the Affective Reactivity Index (ARI)60 is a 7-item self-report questionnaire that evaluates irritability over the past six months, focusing on feelings and behaviors specifically relevant to irritability, as well as overall impairment due to irritability. Fourth, the Extended Strengths and Weaknesses Assessment of DMDD Symptoms and Normal Behavior (E-SWAN DMDD)61 is a 30-item self-report questionnaire that measures elements of disruptive mood dysregulation disorder (DMDD). Fifth, participants complete a 10-item version of the Borderline Evaluation of Severity over Time (BEST)62 scale which measures symptoms of borderline personality disorder (BPD), including the Thoughts and Feelings (e.g., changes in self-identity, feelings of emptiness, etc.) and Behaviors-Negative (e.g., self-injury, impulsive sexual behavior, etc.) subscales. Sixth, participants complete the 5-item Reward Responsiveness subscale of the Behavioral Inhibition System and Behavioral Activation System Scales (BIS/BAS).63
Additional clinical self-report measures
In addition to detailed measures of emotion regulation, the study collects data on stressful life events, the symptoms of major depressive disorder (MDD), attention-deficit hyperactivity disorder (ADHD), and subthreshold symptoms of psychosis in three assessments. First, the Perceived Stress Scale-4 (PSS-4)64 is a 4-item self-report measure that assesses the degree of appraised stress associated with life events over the last month. Second, the Patient Health Questionnaire (PHQ-8)65 is an 8-item self-report measure of depression. Derived from the PHQ-9,66 the PHQ-8 includes all equivalent items except for item 9, which addresses thoughts of death or self-harm. Of note, item 9 was not administered due to issues of monitoring self-harm virtually. Third, the Extended Strengths and Weaknesses Assessment of ADHD Symptoms and Normal Behavior (E-SWAN ADHD)61 is an 18-item self-report questionnaire that measures ADHD. Lastly, the PRIME Screen Revised 5-item (PRIME-5)67 is a brief self-report measure that serves as an age-normed subthreshold psychosis screening tool.
Structured assessment of clinical domains
Participants complete the Computerized Adaptive Testing (CAT) GOASSESS68 psychopathology screener. The CAT GOASSESS is an adapted version of the highly-structured GOASSESS screening interview,69 and includes five domains: mood/anxiety, phobias, externalizing, psychosis, and pathological personality characteristics. While the full GOASSESS takes an hour on average to administer, the CAT GOASSESS is designed for rapid administration – often requiring only minutes – and minimal proctoring.68 Table 2 compares the interview sections captured within the full and CAT GOASSESS versions.
Table 2. GOASSESS structured clinical interview.
Elements of the Full and CAT versions of the GOASSESS are detailed.
| Interview Component | Full GOASSESS | CAT GOASSESS |
|---|---|---|
|
| ||
| Timeline | ✓ | |
| Demographics and Medical History | ✓ | |
| Psychopathology Screener | ||
| Anxiety | ||
| Generalized Anxiety | ||
| Separation Anxiety | ✓ | ✓ |
| Specific Phobia | ✓ | ✓ |
| Social Anxiety | ✓ | ✓ |
| Panic | ✓ | ✓ |
| Agoraphobia | ✓ | ✓ |
| Obsessive-Compulsive | ✓ | ✓ |
| Post-traumatic Stress | ✓ | ✓ |
| Mood | ||
| Major Depression | ✓ | ✓ |
| Mania/Hypomania | ✓ | ✓ |
| Behavioral | ✓ | ✓ |
| Attention | ||
| Deficit/Hyperactivity | ||
| Oppositional Defiant | ✓ | ✓ |
| Conduct | ✓ | ✓ |
| Psychosis | ||
| Psychosis | ||
| Prodromal Screen | ✓ | ✓ |
| (PRIME/SIPS) | ||
| Eating | ||
| Anorexia | ✓ | ✓ |
| Bulimia | ✓ | ✓ |
| Other | ||
| Treatment History | ✓ | ✓ |
| Suicide | ✓ | ✓ |
| Substance | ||
| Other Disorders | ||
| ✓ | ||
| ✓ | ||
| ✓ | ||
| ✓ | ||
| ✓ | ||
| ✓ | ||
| Child Global Assessment of Function | ✓ | |
| Personality Interview for DSM-5 Brief Form | ✓ | ✓ |
| Interviewer Observations | ✓ | |
Cognitive Battery
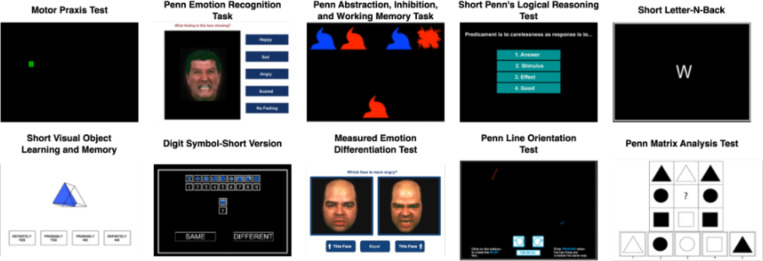
We measure cognition using the Penn Computerized Neurocognitive Battery (CNB;70 see Figure 2). The CNB contains a series of computerized tests that are administered by a member of the research team. Participants typically complete the entire battery within an hour. The CNB evaluates performance accuracy and speed on neurobehavioral domains including: executive control, episodic memory, complex cognition, social cognition, sensorimotor speed, and reward decision-making.71 The CNB tests used in this study include the Abstraction, Inhibition, and Working Memory Test, Psychomotor Vigilance Test (3 Minute Version), Short Penn Continuous Performance Task (Adaptive), Digit Symbol Test, Penn Trailmaking Test (B Version), Short Letter-N-Back Test (2 Back Version), Short Visual Object Learning Test, Short Penn Logical Reasoning Test, Penn Emotion Recognition Task, and the Motor Praxis Test.
Figure 2. Cognitive battery.
Exemplar CNB tasks featured across the CNB and CAT-CCNB batteries are displayed.
In addition to the CNB tasks described above, participants complete further cognitive assessments that use computerized adaptive testing (CAT) versions of the CNB. The CAT-CCNB evaluates the same domains, adding two measures of complex cognition (the Penn Matrix Analysis Test and the Penn Line Orientation Test), another measure of social cognition (the Measured Emotion Differentiation Test), and two reward/decision-making tests (Delay Discounting and Risk Discounting).72 See Figure 2 for displays of ten exemplar cognitive tests included across the CNB and CAT-CCNB batteries; Table 3 details the complete battery of cognitive tests and relevant domains.
Table 3. Cognitive battery domains.
| Test | Domain | Adaptive Design |
|---|---|---|
|
| ||
| Motor Praxis Test | Sensorimotor speed | |
| Penn Emotion Recognition Task | Emotion recognition | |
| Abstraction, Inhibition, and Memory Test | Executive Function | |
| Short Penn Logical Reasoning Test | Verbal intellectual ability | |
| Psychomotor Vigilance Test - 3 Minute Version | Sustained attention | |
| Short Letter-N-Back Test - 2 Back Version | Working memory | |
| Short Visual Object Learning Test | Visual object learning | |
| Short Penn Continuous Performance Task | Visual attention | |
| Digit Symbol Test | Processing speed | |
| Penn Trailmaking Test B | Executive function | |
| Delayed Discounting Task | Reward decision-making | ✓ |
| Measured Emotion Differentiation Test | Emotion discrimination | ✓ |
| Penn Line Orientation Test | Spatial ability | ✓ |
| Penn Matrix Analysis Test | Abstraction & Mental flexibility | ✓ |
| Risk Discounting Task | Reward decision-making | ✓ |
Ecological Momentary Assessment
Following the in-person imaging visit, participants begin a 2-week ecological momentary assessment (EMA) procedure with a mobile app entitled the “Real-time Ecological Assessment of the Context of mental and physical Health (REACH),” developed through a collaborative effort between the NIMH Intramural Research Program and the Child Mind Institute, adapted on the MindLogger Platform.73 MindLogger allows studies to design mental health assessments and interventions that are distributed to participants through customizable mobile or web activities.
EMA assesses participants’ emotions at the time of sampling rather than gathering a retrospective report on how they felt over the past week or month. Computerized EMA allows studies to verify the exact time when survey responses occurred;74 it thus reduces retrospective bias, provides real-time tracking of dynamic processes, and contextualizes relationships between symptoms and behaviors.73 REACH emerged after more than a decade of development and expansion of ecological assessment of emotional states with the mood circumplex75 as well as correlates of mood including sleep, physical activity, and energy.76 REACH includes modules on the mood circumplex, negative thoughts, sleep, context, screen and social media usage, life events, physical activity, food and drink intake, pain, and physical health. Descriptions of the specific items, procedures, and attribution are available through a Creative Commons License (Attribution-Non Commercial-Share Alike 4.0 International Creative Commons license agreement; CC BY-NC-SA 4.0). Versions of REACH are now being employed at several of the sites involved in an international collaborative effort on actigraphy and mood disorders (see below) that can enhance comparability and generalizability of the findings.
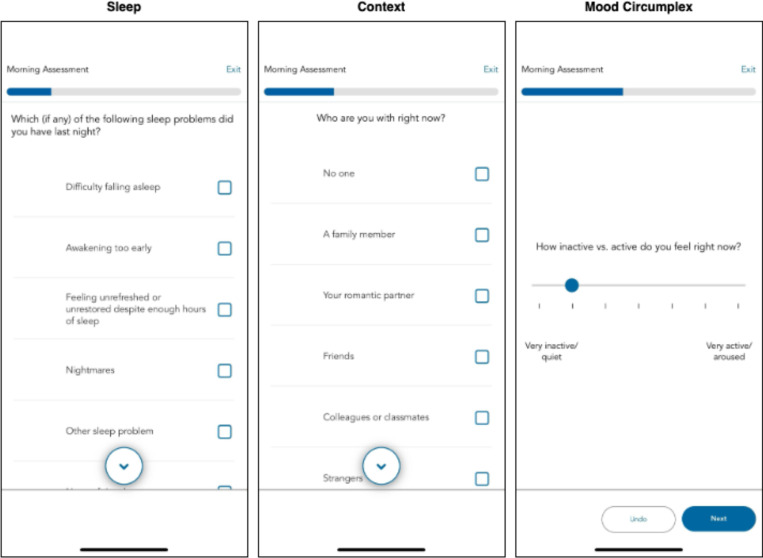
We configure the MindLogger app to send notification reminders prompting participants to complete the surveys at four daily time points: morning, mid-day, afternoon, and evening. Each participant determines the exact time when they receive these notification reminders based on their individual schedule. Participants have a 60-minute window to complete each survey. The MindLogger app allows participants to skip or exit the survey if they choose. See Figure 3 for exemplar screenshots from MindLogger; the specific item prompts assigned across each of the four surveys are detailed in Supplementary Table 1.
Figure 3. MindLogger EMA.
Examples of three EMA prompts featured within the daily morning activity are displayed.
Actigraphy
To measure physical activity and sleep,77 participants wear the GENEActiv actigraphy device (GENEActiv, Activinsights Ltd, Kimbolton, UK). The GENEActiv is a waterproof wristwatch-like device that participants are instructed to wear continuously for three weeks after the imaging visit. The GENEActiv devices are configured to collect raw acceleration data at 66.7 Hz for 21 days.
As part of our initial data release, we provide raw binary files containing acceleration data along the three x, y, and z movement axes. Binary files are extracted with the GENEActiv software version 4.0.12. Raw accelerometer data are processed with the GGIR R package (version 3.2.0),78 which performs auto-calibration, non-wear detection, and calculation of relevant sleep and physical activity variables. All processing is based on the Euclidean Norm Minus One (ENMO) metric with automated calibration. Acceleration angle metrics are calculated over 5-second epochs. Non-wear detection is performed using the 2023 algorithm, with an epoch length of 900 seconds for non-wear and signal clipping, and an epoch of 3600 seconds for non-wear detection. GGIR also evaluates the validity of each day and night of the study, requiring minimum valid wear time of 16 hours per day (midnight-midnight) and 12 hours per night (noon-noon). Sleep and wake are detected using the van Hees 2015 algorithm,79,80 which identifies sustained inactivity periods as intervals of at least five minutes during which the arm angle variability remains lower than five degrees. The sleep period window is detected using the HDCZA guider algorithm,79 which identifies the longest period of low movement and posture change to find the main daily sleep period. The sleep period time (SPT) is then used to calculate sleep variables, including sleep duration, defined as the total time spent in sustained inactivity bouts within the SPT window; wake after sleep onset (WASO), defined as the total time spent awake between the start and end of SPT; and sleep efficiency, calculated as the ratio of sleep duration to the total length of the SPT. Daytime physical activity levels are defined using GGIR’s default acceleration cutoff points of 40 mg for light, 100 mg for moderate, and 400 mg for vigorous activity. Sleep variables highlighted in the results section include total sleep duration (hours), WASO (hours), and sleep efficiency (%). Physical activity variables include time spent in light, moderate, and vigorous activity (in minutes). GGIR outputs several data quality variables, which we use to apply additional quality control. We first exclude recordings with device issues (e.g., clipping or failed calibration). Next, we exclude days or nights with poor data quality (e.g., >20% invalid data, >30% non-wear), as well as nights with abnormal features (extreme sleep durations < 2 or > 14 hours, extreme episode counts of < 5 or > 40, and daysleeper patterns). Finally, participants are excluded from each analysis if they have fewer than three valid days for activity analyses, and fewer than three valid nights for sleep analyses. Data from this study will be included in a collaborative effort with the Motor Activity Research Consortium for Health at the NIMH (mMARCH);81 that was established to harmonize the processing, data extraction, analytic methods and collection of common ancillary data for studies of mood disorders in adults and youth. In particular, mMARCH has made advances in processing accelerometry data81 and statistical analytic methods.82 Use of common procedures for actigraphy and EMA is now underway in several international settings (including the US, Brazil, Canada, Korea, and Switzerland) that will enhance the generalizability and interpretability of this research.
Imaging Acquisition
MRI data is acquired on a 3T Siemens Magnetom Prisma (Erlangen, Germany) MRI scanner with the product 64-channel receiver array at the University of Pennsylvania. Sequence parameters and file naming conventions are summarized in Table 4; a full protocol PDF and the EXAR file is also available (see Data Availability, below).
Table 4. Imaging Parameters.
| Sequence | Slices | % FOV Phase | Resolution (mm) | TR (ms) | TE (ms) | TI (ms) | Flip Angle (°) | Multi Band Accel | Phase Partial Fourier |
|---|---|---|---|---|---|---|---|---|---|
| anat-T1w | 176 | 100% | 1.0×1.0×1.0 | 2500 | 2.9 | 1070 | 8 | N/A | Off |
| anat-T2w | 176 | 100% | 1.0×1.0×1.0 | 3200 | 565 | N/A | 120 | N/A | Allowed |
| fmap-epi_acq-func_dir-AP | 72 | 100% | 2.0×2.0×2.0 | 13690 | 20.80, 58.48, 96.16, 133.84, 171.52 |
N/A | 90 | N/A | 6/8 |
| fmap-epi_acq-func_dir-PA | 72 | 100% | 2.0×2.0×2.0 | 13693 | 20.80, 58.48, 96.16, 133.84, 171.52 |
N/A | 90 | N/A | 6/8 |
| func-bold_task-bao_dir-AP | 72 | 100% | 2.0×2.0×2.0 | 1761 | 14.20, 38.93, 63.66, 88.39, 113.12 |
N/A | 68 | 6 | 6/8 |
| func-bold_task-rat_dir-PA | 72 | 100% | 2.0×2.0×2.0 | 1761 | 14.20, 38.93, 63.66, 88.39, 113.12 |
N/A | 68 | 6 | 6/8 |
| dwi-dwi_acq-HASC92_dirAP | 84 | 100% | 1.7×1.7×1.7 | 4300 | 90 | N/A | 90 | 4 | 7/8 |
| fmap-epi_acq-dwi_dir-PA | 84 | 100% | 1.7×1.7×1.7 | 4300 | 90 | N/A | 90 | 4 | 7/8 |
| perf-asl | 44 | 100% | 3.0×3.0×3.0 | 5000 | 10.8 | N/A | 120 | N/A | N/A |
| anat-MEGRE_acq-1p5mm | 96 | 75% | 1.5×1.5×1.5 | 35 | 7.5, 15, 22.5, 30 |
N/A | 15 | N/A | Off |
Structural MRI
The imaging protocol begins with one run of T1-weighted MPRAGE structural MRI (named anat-T1w) that is aligned with the ABCD study83 (176 slices; repetition time, TR = 2500 ms; echo time, TE = 2.9 ms; flip angle, FA = 8°; field of view, FOV = 256×256 mm; matrix size = 256×256; voxel size = 1×1×1 mm; phase encoding direction = A >> P; acquisition time = 7:12). We also acquire the T2-weighted SPACE structural image (named anat-T2w) used in the ABCD protocol (176 slices; TR = 3200 ms; TE = 565 ms; FA = 120°; FOV = 256×256 mm; matrix size = 256×256; voxel size = 1×1×1 mm; phase encoding direction = A >> P; acquisition time 6:35). Both scans use embedded volumetric navigators to perform real-time motion correction, reducing motion artifacts in the resulting anatomical images.84
Functional MRI
This study includes two runs of multi-echo EPI. Previous work has shown that multi-echo EPI scans facilitate the identification of person-specific variation in BOLD fMRI with far less data.48 Before the fMRI time series are acquired, we collect PEpolar-style field maps for distortion correction of the fMRI data. These field maps (named fmap-epi_acq-func_dir-AP and fmap-epi_acq-func_dir-PA) consist of two multi-echo EPI acquisitions with the same acquisition parameters as the fMRI scans with opposite phase encoding directions (72 slices; TR = 13,690 ms; TE = 20.80, 58.48, 96.16, 133.84, 171.52 ms; FA = 90°; FOV = 220×220 mm; matrix size = 110×110; voxel size = 2×2×2 mm; acquisition time = 1:22). Current software cannot leverage multiple echoes in PEpolar-style field maps, so we retain the first echo from each multi-echo field map with `acq-func` (e.g., `sub-01_ses-01_acq-func_dir-AP_epi.nii.gz`). The multi-echo versions of the field maps are retained in the dataset for potential future use.
Two runs of functional MRI (named func-bold_task-bao_dir-AP and func-bold_task-rat_dir-PA) are acquired with a multiband EPI sequence (CMRR, University of Minnesota). Each functional run is acquired with the same protocol (72 slices; TR = 1761 ms; TE = 14.20, 38.93, 63.66, 88.39, 113.12 ms; FA = 68°; FOV = 220×220 mm; matrix size = 110×110; voxel size = 2×2×2 mm; multiband factor = 6; in-plane acceleration factor = 2). This protocol is highly similar to the one used by Moser et al. (2025)85 and Siegel et al. (2024).86 During each run, participants view Pixar short animated movies: “Bao” and “Your Friend the Rat.” For “Bao,” the phase encoding direction is anterior-to-posterior and each run is 7:13, during which 246 volumes are acquired. For “Your Friend the Rat,” the phase encoding direction is posterior-to-anterior and each run is 10:45 minutes long, during which 366 volumes are acquired. For both runs, a single-band reference image is also acquired for each echo to assist with co-registration. Furthermore, three no-excitation noise volumes are also acquired at the end of each run to allow for implementation of denoising with NORDIC.85,87,88 Finally, we acquire all fMRI data with both magnitude and phase reconstruction enabled; this complex data is helpful for denoising with NORDIC, improves T2* estimation, and allows for cutting edge distortion correction methods (e.g., MEDIC).89
Diffusion MRI
This study includes compressed-sensing diffusion spectrum imaging (CS-DSI). The vast majority of dMRI images sample many directions at one or more b-values (or shells). In contrast, DSI scans densely sample q-space on a Cartesian grid. This enables the direct estimation of the diffusion Ensemble Average Propagator (EAP), the physical process driving biologically meaningful derivatives of dMRI.90 Previous research has demonstrated that DSI achieves greater biological fidelity in tractography when compared to ground-truth anatomic tracings through more accurate ODF estimates.91,92 DSI scans have also been shown to result in improved tractography93 and enhanced gray-white contrast.94 While available research emphasizes massive potential for DSI, the dense Cartesian sampling requires very long scan times (30+ minutes), which has prohibited the wide use of DSI.
To mitigate the need for long DSI scan times, recent work has shown that CS-DSI acquisitions can undersample q-space and still accurately estimate the underlying EAPs.95–100 Our team recently published the first in-depth study that compared CS-DSI and full DSI scans in humans.50 We found that a 7.4-minute CS-DSI scan provided highly similar accuracy and reliability to a full DSI scan. This study acquires a 92-direction CS-DSI scan (named dwi-dwi_acq-HASC92_dir-AP) that uses a homogenous angular sampling scheme (HA-SC;101 TR = 4300 ms; TE = 90.00 ms; FOV = 230×230 mm; voxel size = 1.7×1.7×1.7 mm; 84 interleaved slices acquired anterior to posterior; a multiband factor of 4; acquisition time = 9:02).98 In addition to 95 diffusion-weighted images, 9 b = 0 images are acquired (104 volumes total). For full details on the CS-DSI sampling schemes see Data Availability, below. dMRI data is acquired with both magnitude and phase reconstruction enabled; this complex data is helpful for denoising with Marchenko-Pastur principal components analysis (MP-PCA) (as described below). For distortion correction, we acquire a reverse phase-encoded scan (named fmap-epi_acq-dwi_dir-PA) that includes 7 volumes (TR = 4300 ms; TE = 90.00 ms; FOV = 230×230 mm; voxel size = 1.7×1.7×1.7 mm; 84 interleaved slices acquired posterior to anterior; multiband factor = 4; acquisition time = 2:09).
Arterial spin-labeled MRI
To measure brain perfusion, we acquire a state-of-the-art arterial spin-labeled (ASL) MRI scan. Specifically, we use a background-suppressed unbalanced PCASL scan (named perf-asl) using a stack of spirals turbo spin echo (Labeling duration = 1.8 s, Post-labeling delay (PLD) = 1.8s, TR = 5000.0 ms; TE = 10.8 ms; FOV = 240×240 mm; voxel size = 3.0×3.0×3.0 mm; acquisition time = 4:24).102,103 We also acquire a reference scan to assist in ASL calibration (M0 and T1 estimation) and co-registration, using the same sequence to acquire a presaturated, unsuppressed proton-density weighted volume (Tsat = 5 s) and presaturated inversion recovery volume (Tsat = 5 s, TI = 1.978 s). This reference scan can be used to calculate a low-resolution quantitative T1 map.104
MEGRE
We acquire one run of multi-echo gradient-recalled echo (MEGRE; named anat-MEGRE_acq-1p5mm) scan with magnitude and phase reconstruction (96 slices; repetition time, TR = 35 ms; echo times, TEs = 7.5, 15, 22.5, 30 ms; flip angle, FA = 15°; field of view, FOV = 180×240 mm; matrix size = 120×160; voxel size = 1.5×1.5×1.5 mm; phase encoding direction=A >> P; acquisition time = 3:59). This scan can be used for quantitative susceptibility mapping (QSM), which is sensitive to developmental changes in brain iron and myelin.
Image Processing
As part of our initial data release, we provide processed T1-weighted, fMRI, dMRI, and ASL images. sMRIPrep is used for processing T1-weighted (T1w) and T2-weighted (T2w) images. fMRIPrep is used to minimally preprocess fMRI data;105 fMRI post-processing is performed with fMRIPost-AROMA,106 tedana,107 and XCP-D.108 dMRI is processed with QSIPrep and QSIRecon.109 ASL images are processed with ASLPrep.110 As of the current data release, we do not provide processed MEGRE/QSM images.
Structural image processing
Preprocessing of T1-weighted images uses sMRIPrep 0.16.0, as implemented in fMRIPrep 24.1.1105 using Nipype 1.8.6.111,112 The T1w image undergoes correction for intensity non-uniformity with N4BiasFieldCorrection with ANTs 2.5.3,113,114 skull-stripping with a Nipype implementation of the ANTs brain extraction workflow, and brain tissue segmentation with FSL’s FAST 6.0.7.7.115 Brain surfaces are reconstructed using FreeSurfer 7.3.2,116 and the brain mask estimated previously is refined with a custom variation of the method to reconcile ANTsderived and FreeSurfer-derived segmentations.117 The T2-weighted image is used to improve pial surface refinement. Volume-based spatial normalization to two standard spaces (MNI152NLin6Asym and MNI152NLin2009cAsym, accessed through TemplateFlow 24.2.0)118 is performed through nonlinear registration with antsRegistration (ANTs 2.5.3), using brain-extracted versions of both T1w reference and the T1w template. A CIFTI grayordinate file containing 91k vertices is resampled onto the fsLR template using Connectome Workbench.119,120
Functional image preprocessing
fMRI data is preprocessed using fMRIPrep 24.1.1,105,121 which is based on Nipype 1.8.6.111,112 First, a B0-nonuniformity map is estimated based on echo-planar imaging (EPI) references using FSL’s TOPUP.122 For each BOLD run, the following preprocessing is performed. First, a reference volume is generated from the shortest echo of the BOLD run for use in head-motion correction. Head-motion parameters with respect to the BOLD reference (transformation matrices, and six corresponding rotation and translation parameters) are estimated before any spatiotemporal filtering using FSL’s mcflirt.123 The estimated fieldmap is aligned with rigid-registration to the target EPI reference run. The BOLD reference is then co-registered to the T1w reference using FreeSurfer’s bbregister124 with six degrees of freedom. The aligned T2w image is also used for initial co-registration. The BOLD time series are resampled onto the left/right-symmetric template “fsLR” using Connectome Workbench.119,120 A “goodvoxels” mask is applied during volume-to-surface sampling in fsLR space, excluding voxels whose time series have a locally high coefficient of variation. Grayordinates files119 containing 91k samples are also generated with surface data transformed directly to fsLR space and subcortical data transformed to 2 mm resolution MNI152NLin6Asym space.
Post-processing of functional images
Following preprocessing with fMRIPrep, images are post-processed with complementary software. We seek to decompose the processed fMRI time series into “noise” and “signal” components using two methods. First, ICA-AROMA106,125 is implemented using fMRIPost-AROMA 0.0.10, which is based on Nipype 1.9.2.111,112 Noise and signal components from ICA-AROMA are compared to tedana,126 which is run using the “minimal” decision tree – a simplified version of the MEICA decision tree. Tedana fits a monoexponential model to the data at each voxel using nonlinear model fitting to estimate T2* and S0 maps, using T2*/S0 estimates from a log-linear fit as initial values. For each voxel, the value from the adaptive mask is used to determine which echoes would be used to estimate T2* and S0. In cases of model fit failure, T2*/S0 estimates from the log-linear fit are retained instead. Multi-echo data are then optimally combined using the T2* combination method.127 Next, component selection is performed to identify BOLD (TE-dependent) and non-BOLD (TE-independent) components using a decision tree. Rejected components’ time series are then orthogonalized with respect to accepted components’ time series. Minimum image regression is then applied to the data to remove spatially diffuse noise.126 The tedana workflow uses NumPy,128 SciPy,129 pandas,130 scikit-learn,131 Nilearn,132 Bokeh,133 Matplotlib,134 and Nibabel.135
After tedana is applied, the component classifications from fMRIPost-AROMA and tedana are combined, such that any component that is rejected by either ICA-AROMA or MEICA is labeled as rejected. The rejected components’ time series are then orthogonalized with respect to accepted components’ time series. Minimum image regression is then applied to the mixing matrix in order to remove spatially diffuse noise.126
Following identification of noise components using ICA-AROMA and tedana, images are processed using XCP–D.108,136,137 XCP-D is built with Nipype 1.9.2.111,112 Many internal operations of XCP-D use AFNI,138,139 Connectome Workbench,119,120 ANTS,140 TemplateFlow 24.2.2,118 Matplotlib 3.10.0,134 Nibabel 5.3.2,135 Nilearn 0.11,132 NumPy 2.2.1,141 pybids 0.18.1,142 and SciPy 1.15.1.129
Non-steady-state volumes are extracted from the preprocessed confounds and are discarded from both the BOLD data and nuisance regressors. The set of orthogonalized nuisance components from the tedana step is selected for the nuisance regression, along with mean white matter signal, mean cerebrospinal fluid signal, and mean global signal.137,143 The BOLD data are converted to NIfTI format, despiked with AFNI’s 3dDespike, and converted back to CIFTI format. Nuisance regressors are regressed from the BOLD data using a denoising method based on Nilearn’s approach. The time series are band-pass filtered using a second-order Butterworth filter, in order to retain signals between 0.01–0.08 Hz. The same filter is applied to the confounds. The resulting time series are then denoised using linear regression. The denoised BOLD is then smoothed using Connectome Workbench with a Gaussian kernel (FWHM = 6 mm).
Processed functional time series are extracted from residual BOLD using Connectome Workbench119,120 for the atlases. Corresponding pairwise functional connectivity between all regions is computed for each atlas, which is operationalized as the Pearson’s correlation of each parcel’s unsmoothed time series with the Connectome Workbench. In cases of partial coverage, uncovered vertices (values of all zeros or NaNs) are either ignored (when the parcel had >50.0% coverage) or the whole parcel is set to zero (when the parcel had <50.0% coverage). The following atlases are used in the workflow: the Schaefer Supplemented with Subcortical Structures (4S) atlas119,144–147 at 10 different resolutions (156, 256, 356, 456, 556, 656, 756, 856, 956, and 1056 parcels), the Glasser atlas,148 the Gordon atlas,149 the Tian subcortical atlas,150 the HCP CIFTI subcortical atlas,119 and the MIDB precision brain atlas derived from ABCD data and thresholded at 75% probability.151 In addition, XCP-D calculates two scalar maps. First, the amplitude of low-frequency fluctuation (ALFF)152 is computed by transforming the mean-centered, standard deviation-normalized, denoised BOLD time series to the frequency domain. The power spectrum is computed within the 0.01–0.08 Hz frequency band and the mean square root of the power spectrum is calculated at each voxel to yield voxel-wise ALFF measures. The resulting ALFF values are then multiplied by the standard deviation of the denoised BOLD time series to retain the original scaling. The ALFF maps are smoothed with the Connectome Workbench using a Gaussian kernel (FWHM = 6 mm). Second, for each hemisphere, regional homogeneity (ReHo)153 is computed using surface-based 2dReHo.154 Specifically, for each vertex on the surface, Kendall’s coefficient of concordance (KCC) is computed with nearest-neighbor vertices to yield ReHo. For the subcortical, volumetric data, ReHo is computed with neighborhood voxels using AFNI’s 3dReHo.155
dMRI Processing
dMRI preprocessing is performed using QSIPrep 1.0.0,109 which is based on Nipype 1.9.1;111,112 (RRID:SCR_002502). Many internal operations of QSIPrep use Nilearn 0.10.1132 (RRID:SCR_001362) and Dipy.156 QSIPrep uses a slightly different anatomical workflow than sMRIPrep/fMRIPrep. Specifically, the T1w image is corrected for intensity non-uniformity (INU) and used as the T1w-reference map. The anatomical reference image is first reoriented into AC-PC alignment using the 6-degrees-of-freedom (rigid) component of a full affine registration to MNI152NLin2009cAsym, and a symmetric nonlinear registration (SyN) using antsRegistration to further refine alignment to the MNI152NLin2009cAsym template. Brain extraction is performed on the T1w image using SynthStrip157 and automated segmentation is performed using SynthSeg158 from FreeSurfer version 7.3.1.
Next, any diffusion images with a b-value less than 100 s/mm2 are treated as a b = 0 image. Magnitude and phase diffusion-weighted imaging data are combined into a complex-valued file, then denoised using the Marchenko-Pastur PCA method implemented in MRtrix3’s dwidenoise159–161 with a 3-voxel window. After denoising, the complex-valued data are split back into magnitude and phase. The mean intensity of the diffusion-weighted series is adjusted so all the mean intensity of the b = 0 images matched across each separate DWI scanning sequence.
Initial motion correction is performed using only the b = 0 images. An unbiased b = 0 template is constructed over two iterations of 3dSHORE registrations. To estimate head motion in b>0 images, the SHORELine method50,109 is used to iteratively leave out each b>0 image and reconstruct the remaining images using 3dSHORE;96 the signal for the left-out image served as the registration target. The reconstructed, model-generated images are transformed into alignment with each b>0 image. Both slicewise and whole-brain quality control measures (cross correlation and R2) are calculated.
Susceptibility distortion correction is performed using DRBUDDI,162 part of the TORTOISE163 software package. Reverse phase-encoding EPI-based fieldmaps are collected, resulting in pairs of images with distortions going in opposite directions. DRBUDDI uses b = 0 reference images with reversed phase encoding directions to estimate the susceptibility-induced off-resonance field. A T2-weighted image is included in the multimodal registration. The DWI time series are resampled to AC-PC, generating a preprocessed DWI run in AC-PC space with 1.7 mm isotropic voxels.
Following preprocessing, the preprocessed dMRI images are reconstructed using QSIRecon (1.0.1).109 Diffusion orientation distribution functions (ODFs) are reconstructed using generalized q-sampling imaging (GQI)164 with a ratio of mean diffusion distance of 1.250000 in DSI Studio (version 94b9c79). Automatic Tractography is run in DSI Studio (version 94b9c79) and bundle shape statistics are calculated.165
ASL image processing
Arterial spin-labeled MRI images are preprocessed using ASLPrep 0.7.5,110,166 which is based on fMRIPrep105,167 and Nipype 1.8.6.111 Many internal operations of ASLPrep use Nilearn 0.11.1,132 NumPy,141 and SciPy.129 In large part, ASLPrep uses the precomputed structural image processing from sMRIPrep, as described above. The ASL reference scan is co-registered to the T1w reference using Freesurfer’s bbregister which implements boundary-based registration124 with six degrees of freedom. All resampling in ASLPrep uses a single interpolation step that concatenates all transformations. Gridded (volumetric) resampling is performed using antsApplyTransforms, configured with Lanczos interpolation to minimize the smoothing effects of other kernels.168
Head-motion parameters are estimated for the ASL data using FSL’s mcflirt.123 Motion correction is performed separately for label and control volumes in order to account for intensity differences between different contrasts; when these volumes are motion corrected together, intensity differences can be conflated with head motions.169 Next, ASLPrep concatenates the motion parameters across volume types and re-calculates relative root mean-squared deviation. Several confounding time series are calculated, including both framewise displacement (FD) and DVARS. FD and DVARS are calculated using the implementations in Nipype (following the definition by Power et al., 2014170) for each ASL run. ASLPrep summarizes in-scanner motion as the mean framewise displacement and relative root-mean square displacement.
ASLPrep is used to calculate cerebral blood flow (CBF) from the single-delay PCASL using a single-compartment general kinetic model.171 Calibration (M0) volumes associated with the ASL scan are smoothed with a Gaussian kernel (FWHM = 5 mm) and the average calibration image is calculated and scaled by 10.0. The quality evaluation index (QEI) is computed for each CBF map.172 QEI is based on the similarity between the CBF and the structural images, the spatial variability of the CBF image, and the percentage of gray matter voxels containing negative CBF values.
Parcellated CBF estimates are extracted for multiple atlases, including the Schaefer Supplemented with Subcortical Structures (4S) atlas119,144–147 at 10 different resolutions (156, 256, 356, 456, 556, 656, 756, 856, 956, and 1056 parcels), the Glasser atlas,148 the Gordon atlas,149 the Tian subcortical atlas,150 and the HCP CIFTI subcortical atlas.119 In cases of partial coverage, either uncovered voxels (values of all zeros or NaNs) are ignored (when the parcel has >50.0% coverage) or the whole parcel is set to zero (when the parcel has <50.0% coverage).
Results
Here we present initial data from the first participants enrolled in the study protocol. As described below, we focus on EMA, actigraphy, and neuroimaging data. Notably, all processed data described below are openly available on OpenNeuro; all processing code and software is available on GitHub (see Data and Code Availability, below). Data will be updated regularly as study accrual continues.
Ecological Momentary Assessment (EMA)
A total of n = 7 completed the 14-day EMA procedure in this preliminary data release; EMA was not available for the first three study participants enrolled. We examined participant compliance with the EMA procedure according to time of day over the study period by calculating the percentage of prompts to which participants responded for each time point (Figure 4). The current study’s EMA adherence averaged 72% across all activities, with compliance of 74% in the morning, 82% at mid-day, 82% in the afternoon, and 71% in the evening. In addition to adherence, we examined mean scores and daily patterns in mood circumplex items (Figures 5 & 6). Out of a 7-point scale, we found a mean sadness score of 3.50 (SD = 0.91), a mean anxiousness score of 3.67 (SD = 0.96), a mean positive thoughts score of 4.30 (SD = 1), a mean negative thoughts score of 3.49 (SD = 0.86), a mean active score of 3.20 (SD = 1.16), and a mean tiredness score of 3.46 (SD = 1.18).
Figure 4. Ecological momentary assessment (EMA) adherence.
Average adherence for each survey across the 14-day EMA period is displayed for each of the four daily timepoints.
Figure 5. Ecological momentary assessment (EMA) mood scores.
The proportion of mood circumplex scores across participants and timepoints are displayed. Each mood score is represented by a horizontal bar, with colors indicating rating intensity on a 1–7 scale. The length of each colored segment shows the proportion of responses at each rating level across participants and activity types (morning, mid-day, afternoon, and evening assessments). Green represents lower ratings (1–2), light gray represents neutral ratings (3–5), and purple represents higher ratings (6–7). The specific meaning of high and low ratings varies for each measure as indicated by the labels on either side of the bars. For example, in ‘Sadness,’ a longer green segment indicates more responses of ‘Very cheerful/happy’ while a longer purple segment indicates more responses of ‘Very sad/depressed/unhappy.’
Figure 6. Ecological momentary assessment (EMA) daily mood patterns.
Daily patterns for each participant across the 14-day EMA period are shown for mood circumplex items: (A) sadness, (B) anxiousness, (C) positive thoughts, (D) active, (E) tired, and (F) negative thoughts). For each mood circumplex item, the left-side figure displays the score of 1 through 7 for each of the 14 days, with individual trajectories (gray lines), the mean group trajectory (colored line), and standard errors (colored shade). The right-side figure displays the distribution density of each score for that mood circumplex item across all days and participants.
Actigraphy
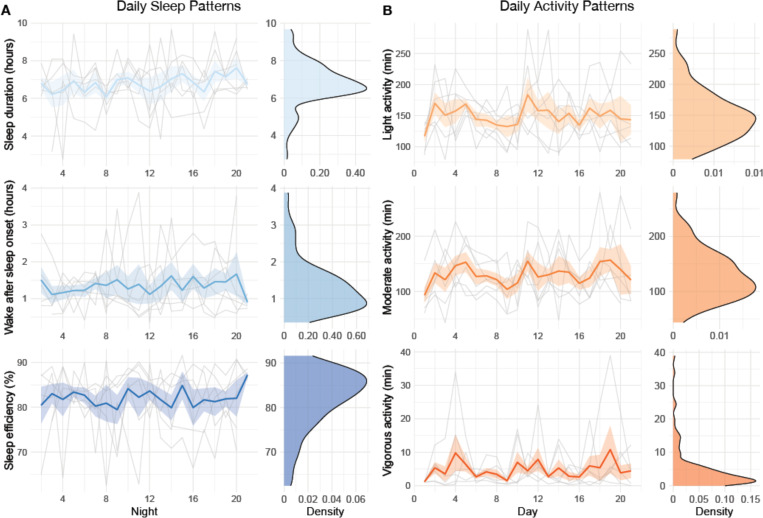
A sample of n = 6 participants completed the 21-day actigraphy procedure; actigraphy devices were not available for the first four participants enrolled in the study. We examined participants’ 24-hour sleep timing patterns through two measures: sleep onset and wake up (Figure 7). The preliminary data revealed differences in monophasic (i.e., single peak in the density plot of sleep onset) and biphasic (i.e., two peaks in the density plot of sleep onset) sleep patterns between participants. The occurrence of multiple peaks in a participant’s density plot suggests irregularity in sleep onset and wake up times. In addition to 24-hour sleep timing patterns, we explored daily sleep and physical activity patterns over multiple days and nights (Figure 8). Daily sleep metrics included sleep duration, wake after sleep onset (WASO; time spent awake after sleep onset but before the official wake up time), and sleep efficiency (the percentage of time asleep while in bed). The data collected for the 21-day actigraphy period revealed a mean sleep duration of 6.78 hours (SD = 1.22), a mean wake after sleep onset period of 1.34 hours (SD = 0.73), and mean sleep efficiency of 82% across participants (SD = 6.68). The data also highlighted distinct levels of physical activity (e.g., light activity, moderate activity, and vigorous activity). Light activity refers to an acceleration greater than 40 milli-g (mg), moderate activity refers to an acceleration greater than 100 mg, and vigorous activity refers to an acceleration greater than 400 mg. The actigraphy data revealed an across subject average of mean light activity level of 150 minutes per day (SD = 41.6), a mean moderate activity level of 130 minutes per day (SD = 43.7), and a mean vigorous activity level of 4.79 minutes per day (SD = 6.31).
Figure 7. Sleep onset and wake timing.
Distributions of sleep onset and wake times over a 24-hour period are displayed, with purple indicating sleep onset and green indicating wake up for each participant who completed the actigraphy procedure.
Figure 8. Daily sleep and physical activity patterns.
Daily patterns for each participant across the 21-day actigraphy period are shown for: (A) daily sleep patterns (sleep duration, wake after sleep onset, and sleep efficiency) and (B) daily activity patterns (light activity, moderate activity, and vigorous activity). For each actigraphy measure, the left-side figure displays the measure across the 21 days, with individual trajectories (gray lines), the mean group trajectory (colored line), and standard errors (colored shade). The right-side figure displays the distribution density of the actigraphy measure.
Neuroimaging
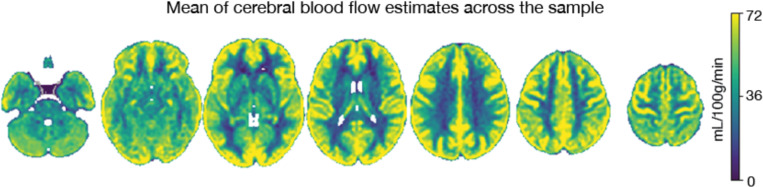
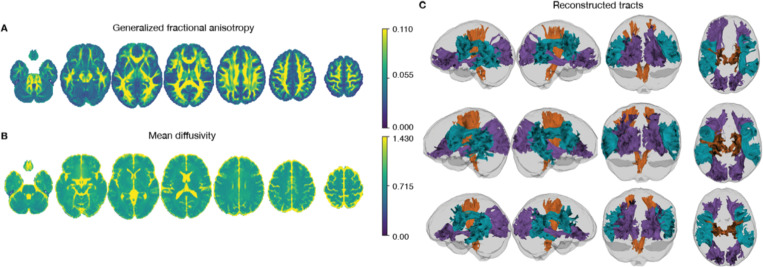
Neuroimaging data was collected on all n = 10 participants. Preprocessed structural MRI data exhibited expected mean cortical thickness, curvature, and sulcal depth across the sample (Figure 9). In addition, cerebral blood flow estimates from the ASL images displayed expected patterns of perfusion, with greater cerebral blood flow in cortical gray matter than white matter (Figure 10). For the multi-echo fMRI data, as expected, T2* did not change dramatically by including or excluding NORDIC denoising (Supplementary Figure 1). Notably, tedana and AROMA showed relatively high levels of agreement on the classification of noise components (Supplementary Figure 2A). However, tedana appeared to be more sensitive, identifying some noise components that AROMA did not. In contrast, AROMA classified very few components as noise that tedana did not identify. The noise components only identified by tedana explained somewhat lower variance than the components that both methods identified as noise (Supplementary Figure 2B). Mean correlation matrices for the “Bao” and “Your Friend the Rat” video watching tasks derived from fully processed fMRI data revealed expected patterns of functional connectivity across all runs (Figure 11). Finally, diffusion imaging data collected using CS-DSI displayed expected patterns of generalized fractional anisotropy (GFA) and mean diffusivity (MD), with GFA being higher in white matter and MD being higher in gray matter (Figure 12). Reconstructed white matter bundles in three exemplar participants revealed excellent delineation of individual-specific white matter tracts (Figure 12C).
Figure 9. Anatomical MRI.
Mean cortical thickness, curvature, and sulcal depth across the initial sample are displayed.
Figure 10. ASL cerebral blood flow.
The mean of cerebral blood flow estimates across the initial sample is displayed.
Figure 11. Multi-echo fMRI.

The mean correlation matrices from (A) “Bao” and (B) “Your Friend the Rat” videos following complete preprocessing are displayed.
Figure 12. Compressed sensing diffusion spectrum MRI.
Average generalized fractional anisotropy (A) and mean diffusivity (B) from the initial sample are displayed. Examples of reconstructed white matter tracts from three participants (participants 24683, 24053, and 60295) are displayed in C.
Discussion
This study aims to provide high-quality brain and behavioral data to investigate affective dynamics in youth. Specifically, measures include advanced multimodal imaging, ecological momentary assessment (EMA), retrospective self-report questionnaires, computerized cognitive assessments, and actigraphy measures. Below we briefly discuss relevant context, limitations, and important future directions.
Difficulties in affective regulation during childhood may confer increased risk for psychopathology in adulthood. Children rely on social interactions to regulate their mood by seeking reassurance from others, eventually developing an internal self-regulation mechanism to manage their affective state.1 Deficits in the child-caregiver relationship and early exposure to trauma may contribute to the development of affective instability.1,173 Affective instability involves acute fluctuations in affect and is linked to aberrant development of psychological and psychosocial capabilities (i.e., shortfalls in self-esteem, social interactions, and sense of identity) and increased vulnerability to psychopathology later on.1,174
While affective instability is present in multiple psychiatric disorders, it has typically been studied within a single diagnosis using a case-control design, limiting the ability to examine common brain circuits impacted across diagnoses.175,176 Affective instability is not only a diagnostic criterion of borderline personality disorder (BPD)177 but is also observed in diverse conditions seen among adolescents and young adults, including bipolar disorder,2 major depressive disorder (MDD),2 and attention-deficit hyperactivity disorder (ADHD).2 Existing evidence suggests that affective instability is associated with suicide threats and attempts among participants with BPD4 as well as suicidal ideation in adults with other psychiatric disorders (e.g., depression, anxiety).178 To extend prior work studying affective instability beyond traditional diagnostic categories, this study characterizes affective instability through a transdiagnostic, dimensional approach. Dimensional approaches to studying psychopathology have the potential to enhance the understanding of how maladaptive behaviors gradually evolve across development.176 By recruiting a community sample of youth and collecting moment-to-moment information on affective dynamics, passive physical activity and sleep data, and high-quality neuroimaging data, the data from this study may accelerate research linking brain circuits to affective instability as a transdiagnostic construct.
In addition to densely sampled behavioral and clinical measures, this study leverages cutting-edge neuroimaging methods -- such as multi-echo fMRI -- to examine the neural substrates of affective dynamics. As a result of small effect sizes, previous neuroimaging work on mental illness has often required very large sample sizes to reliably identify relationships between brain features and behavioral phenotypes.39,41,179,180 However, by adopting measures to enhance reliability and detection of individual-specific variation, studies may potentially mitigate the need to rely on large samples to identify meaningful brain-behavior associations.44 One promising approach is using personalized mapping of individuals’ functional neuroanatomy, which has been shown to better predict behavioral phenotypes than group average parcellations.181,182 Personalized functional networks (PFNs) account for individual variation in brain organization and have the potential to enhance the sensitivity of fMRI data to diagnosis-specific variation.182,183 For example, expanded topography of the frontostriatal salience network defined using PFNs has been shown in individuals with depression.184 In developmental cohorts, PFN topography is refined through childhood and adolescence and exhibits prominent sex differences.185,186 These findings highlight the potential for studying PFNs in a youth population. As multi-echo fMRI allows PFNs to be identified with smaller amounts of neuroimaging data, an advance that significantly improves the feasibility of scanning youth populations,48 we anticipate that the multi-echo fMRI data generated from this study will allow accurate delineation of PFNs. When coupled with densely-sampled behavioral EMA and actigraphy data, we hope that multiecho fMRI will enhance our ability to detect brain-behavior relationships relevant for affective instability.
Although the current study introduces important advances, several limitations should be acknowledged. The study aims to capture the developmental relevance of affective instability through a community sample of 13- to 23-year-old participants. However, participants are only measured at one time point, precluding inference on within-individual development. Furthermore, because the current participant pool is collected through community sampling, and participants do not complete a diagnostic interview, the study does not examine specific clinical diagnoses. Finally, our target sample is only 100 individuals; such a sample may still have limited statistical power to find brain-behavior links despite advances in behavioral assessment (e.g., EMA) and neuroimaging methods (e.g., multi-echo fMRI).
Taken together, this study provides a novel data resource to examine affective dynamics using precision brain and behavioral approaches – including EMA sampling, actigraphy data collection, and multi-echo fMRI. Moving forward, future longitudinal studies of brain development that examine participants pre- and post-adolescence may more fully characterize the developmental progression of affective instability, especially in clinical populations. We hope that this data resource will contribute to the identification of early markers of affective instability and emotion dysregulation before they develop into more severe psychopathology.
Supplementary Material
Acknowledgments:
This study was supported by the AE Foundation, The Dean’s Innovation Fund at the University of Pennsylvania Perelman School of Medicine, and the Penn/CHOP Lifespan Brain Institute. Additional support was provided by grants from the National Institute of Health: R01MH113550 (T.D.S.), R01MH112847 (R.T.S.), R01EB022573 (T.D.S.), R37MH125829 (T.D.S & D.A.F.), R01MH117014 and R01MH119219 (R.C.G., R.E.G.), K23 MH133118 (E.B.B.), BBRF #31319 (E.B.B.), R01EB031080 (J.A.D., M.D.T, & M.T.), BBRF #30837 (S.S.), BWF 1022955 CAMS (S.S.), DP5OD036142 (S.S.), U24NS130411 (D.W.), S10OD023495 (D.W.), F31MH136685 (J.B.).
Data availability:
All raw and processed imaging data is available on OpenNeuro: Raw https://openneuro.org/datasets/ds006131; MRIQC https://openneuro.org/datasets/ds006143; QSIPrep https://openneuro.org/datasets/ds006182; QSIRecon https://openneuro.org/datasets/ds006184; fMRIPrep https://openneuro.org/datasets/ds006185; ASLPrep https://openneuro.org/datasets/ds006188; fMRIPost-AROMA https://openneuro.org/datasets/ds006189; tedana https://openneuro.org/datasets/ds006190; tedana & AROMA https://openneuro.org/datasets/ds006191; XCP-D https://openneuro.org/datasets/ds006192}.
Footnotes
Code availability: All analysis code for this project is openly available on GitHub: https://github.com/PennLINC/affective-instability.
Conflicts of interest: MT is employed by Siemens Medical Solutions USA
References
- 1.Koenigsberg H. W. Affective Instability: Toward an Integration of Neuroscience and Psychological Perspectives. Journal of Personality Disorders 24, 60–82 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Broome M. R., He Z., Iftikhar M., Eyden J. & Marwaha S. Neurobiological and behavioural studies of affective instability in clinical populations: A systematic review. Neuroscience & Biobehavioral Reviews 51, 243–254 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Broome M. R., He Z., Iftikhar M., Eyden J. & Marwaha S. Neurobiological and behavioural studies of affective instability in clinical populations: A systematic review. Neuroscience & Biobehavioral Reviews 51, 243–254 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Koenigsberg H. W. et al. Are the Interpersonal and Identity Disturbances in the Borderline Personality Disorder Criteria Linked to the Traits of Affective Instability and Impulsivity? Journal of Personality Disorders 15, 358–370 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Ruch D. A. et al. Trends in Suicide Among Youth Aged 10 to 19 Years in the United States, 1975 to 2016. JAMA Netw Open 2, e193886 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silvers J. A. et al. Affective lability and difficulties with regulation are differentially associated with amygdala and prefrontal response in women with Borderline Personality Disorder. Psychiatry Res 254, 74–82 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams K. E., Chambless D. L. & Ahrens A. Are emotions frightening? An extension of the fear of fear construct. Behaviour Research and Therapy 35, 239–248 (1997). [DOI] [PubMed] [Google Scholar]
- 8.Gratz K. L. & Roemer L. Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the difficulties in emotion regulation scale. Journal of Psychopathology and Behavioral Assessment 26, 41–54 (2004). [Google Scholar]
- 9.Gross J. J. The Emerging Field of Emotion Regulation: An Integrative Review. Review of General Psychology 2, 271–299 (1998). [Google Scholar]
- 10.First M. B., Williams J. B. W., Benjamin L. S. & Spitzer R. Structured Clinical Interview for DSM-5® Personality Disorders (SCID-5-PD). [Google Scholar]
- 11.Stone A. A. & Shiffman S. Capturing momentary, self-report data: A proposal for reporting guidelines. ann. behav. med. 24, 236–243 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Lamers F. et al. Mood reactivity and affective dynamics in mood and anxiety disorders. Journal of Abnormal Psychology 127, 659–669 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Trull T. J. et al. Affective instability: Measuring a core feature of borderline personality disorder with ecological momentary assessment. Journal of Abnormal Psychology 117, 647–661 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Santangelo P. S. et al. The temporal interplay of self-esteem instability and affective instability in borderline personality disorder patients’ everyday lives. Journal of Abnormal Psychology 126, 1057–1065 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Janssen L. H. C. et al. A closer look into the affect dynamics of adolescents with depression and the interactions with their parents: An ecological momentary assessment study. Eur Child Adolesc Psychiatry 33, 4259–4272 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith A. R. et al. The role of anxiety and gender in anticipation and avoidance of naturalistic anxiety-provoking experiences during adolescence: An ecological momentary assessment study. JCPP Advances 2, e12084 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer C. A., Oosterhoff B., Bower J. L., Kaplow J. B. & Alfano C. A. Associations among adolescent sleep problems, emotion regulation, and affective disorders: Findings from a nationally representative sample. Journal of Psychiatric Research 96, 1–8 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Gradisar M. et al. Sleep’s role in the development and resolution of adolescent depression. Nat Rev Psychol 1, 512–523 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritter P. S. et al. Disturbed sleep as risk factor for the subsequent onset of bipolar disorder – Data from a 10-year prospective-longitudinal study among adolescents and young adults. Journal of Psychiatric Research 68, 76–82 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Baum K. T. et al. Sleep restriction worsens mood and emotion regulation in adolescents. J Child Psychol Psychiatry 55, 180–190 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstone A. et al. Sleep Disturbance Predicts Depression Symptoms in Early Adolescence: Initial Findings From the Adolescent Brain Cognitive Development Study. Journal of Adolescent Health 66, 567–574 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer C. A. & Alfano C. A. Sleep and emotion regulation: An organizing, integrative review. Sleep Med Rev 31, 6–16 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Anastasiades P. G., de Vivo L., Bellesi M. & Jones M. W. Adolescent sleep and the foundations of prefrontal cortical development and dysfunction. Progress in Neurobiology 218, 102338 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maret S., Faraguna U., Nelson A. B., Cirelli C. & Tononi G. Sleep and waking modulate spine turnover in the adolescent mouse cortex. Nat Neurosci 14, 1418–1420 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tononi G. & Cirelli C. Sleep and the Price of Plasticity: From Synaptic and Cellular Homeostasis to Memory Consolidation and Integration. Neuron 81, 12–34 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank M. G. Sleep and Synaptic Plasticity in the Developing and Adult Brain. in Sleep, Neuronal Plasticity and Brain Function (eds. Meerlo P., Benca R. M. & Abel T.) 123–149 (Springer, Berlin, Heidelberg, 2015). doi: 10.1007/7854_2014_305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng W. et al. Sleep duration, brain structure, and psychiatric and cognitive problems in children. Mol Psychiatry 26, 3992–4003 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jalbrzikowski M. et al. Associations between brain structure and sleep patterns across adolescent development. Sleep 44, zsab120 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hehr A., Huntley E. D. & Marusak H. A. Getting a Good Night’s Sleep: Associations Between Sleep Duration and Parent-Reported Sleep Quality on Default Mode Network Connectivity in Youth. Journal of Adolescent Health 72, 933–942 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lunsford-Avery J. R., Damme K. S. F., Engelhard M. M., Kollins S. H. & Mittal V. A. Sleep/Wake Regularity Associated with Default Mode Network Structure among Healthy Adolescents and Young Adults. Sci Rep 10, 509 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tashjian S. M., Goldenberg D., Monti M. M. & Galván A. Sleep quality and adolescent default mode network connectivity. Social Cognitive and Affective Neuroscience 13, 290–299 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krause A. J. et al. The sleep-deprived human brain. Nat Rev Neurosci 18, 404–418 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Souders M. C. et al. Sleep difficulties related to psychopathology and neurocognition in people with 22q11.2 deletion syndrome. Psychiatry Res 344, 116336 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korczak D. J., Madigan S. & Colasanto M. Children’s Physical Activity and Depression: A Meta-analysis. Pediatrics 139, e20162266 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Fuentealba-Urra S., Rubio A., González-Carrasco M., Oyanedel J. C. & Céspedes-Carreno C. Mediation effect of emotional self-regulation in the relationship between physical activity and subjective well-being in Chilean adolescents. Sci Rep 13, 13386 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Short M. A., Gradisar M., Lack L. C., Wright H. R. & Chatburn A. Estimating adolescent sleep patterns: parent reports versus adolescent self-report surveys, sleep diaries, and actigraphy. NSS 5, 23–26 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Zambotti M. et al. Measures of sleep and cardiac functioning during sleep using a multisensory commercially-available wristband in adolescents. Physiology & Behavior 158, 143–149 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haghayegh S., Khoshnevis S., Smolensky M. H., Diller K. R. & Castriotta R. J. Accuracy of Wristband Fitbit Models in Assessing Sleep: Systematic Review and Meta-Analysis. Journal of Medical Internet Research 21, e16273 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marek S. et al. Reproducible brain-wide association studies require thousands of individuals. Nature 603, 654–660 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gell M. et al. How measurement noise limits the accuracy of brain-behaviour predictions. Nat Commun 15, 10678 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milham M. P. Open Neuroscience Solutions for the Connectome-wide Association Era. Neuron 73, 214–218 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Milham M. P., Vogelstein J. & Xu T. Removing the Reliability Bottleneck in Functional Magnetic Resonance Imaging Research to Achieve Clinical Utility. JAMA Psychiatry 78, 587–588 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Laumann T. O. et al. Functional System and Areal Organization of a Highly Sampled Individual Human Brain. Neuron 87, 657–670 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee H. J., Dworetsky A., Labora N. & Gratton C. Using precision approaches to improve brain-behavior prediction. Trends in Cognitive Sciences 29, 170–183 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drysdale A. T. et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med 23, 28–38 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feczko E. et al. The Heterogeneity Problem: Approaches to Identify Psychiatric Subtypes. Trends in Cognitive Sciences 23, 584–601 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Satterthwaite T. D., Feczko E., Kaczkurkin A. N. & Fair D. A. Parsing Psychiatric Heterogeneity Through Common and Unique Circuit-Level Deficits. Biol Psychiatry 88, 4–5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lynch C. J. et al. Rapid Precision Functional Mapping of Individuals Using Multi-Echo fMRI. Cell Reports 33, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kundu P. et al. Multi-echo fMRI: A review of applications in fMRI denoising and analysis of BOLD signals. Neuroimage 154, 59–80 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Cieslak M. et al. Diffusion MRI head motion correction methods are highly accurate but impacted by denoising and sampling scheme. Human Brain Mapping 45, e26570 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morris N. M. & Udry J. R. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolescence 9, 271–280 (1980). [DOI] [PubMed] [Google Scholar]
- 52.Tanner J. M. Growth and endocrinology of the adolescent. in Endocrine and Diseases of Childhood 14–64 (W. B. Saunders, Philadelphia: ). [Google Scholar]
- 53.Sachser C. et al. International development and psychometric properties of the Child and Adolescent Trauma Screen (CATS). J Affect Disord 210, 189–195 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Mujahid M. S., Diez Roux A. V., Morenoff J. D. & Raghunathan T. Assessing the measurement properties of neighborhood scales: from psychometrics to ecometrics. Am J Epidemiol 165, 858–867 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Centers for Medicare and Medicaid Services et al. Standardized Screening for Health-Related Social Needs in Clinical Settings: The Accountable Health Communities Screening Tool. NAM Perspectives 7, (2017). [Google Scholar]
- 56.Van Maanen A. et al. Screening for Sleep Reduction in Adolescents Through Self-report: Development and Validation of the Sleep Reduction Screening Questionnaire (SRSQ). Child Youth Care Forum 43, 607–619 (2014). [Google Scholar]
- 57.Ghotbi N. et al. The μMCTQ: An Ultra-Short Version of the Munich ChronoType Questionnaire. J Biol Rhythms 35, 98–110 (2020). [DOI] [PubMed] [Google Scholar]
- 58.Yu L. et al. Development of Short Forms From the PROMISTM Sleep Disturbance and Sleep-Related Impairment Item Banks. Behavioral Sleep Medicine 10, 6–24 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Preece D. A., Petrova K., Mehta A. & Gross J. J. The Emotion Regulation Questionnaire-Short Form (ERQ-S): A 6-item measure of cognitive reappraisal and expressive suppression. Journal of Affective Disorders 340, 855–861 (2023). [DOI] [PubMed] [Google Scholar]
- 60.Stringaris A. et al. The Affective Reactivity Index: a concise irritability scale for clinical and research settings. J Child Psychol Psychiatry 53, 1109–1117 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alexander L. M., Salum G. A., Swanson J. M. & Milham M. P. Measuring strengths and weaknesses in dimensional psychiatry. J Child Psychol Psychiatry 61, 40–50 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pfohl B. et al. Reliability and Validity of the Borderline Evaluation of Severity Over Time (Best): A Self-Rated Scale to Measure Severity and Change in Persons With Borderline Personality Disorder. Journal of Personality Disorders 23, 281–293 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carver C. S. & White T. L. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology 67, 319–333 (1994). [Google Scholar]
- 64.Cohen S., Kamarck T. & Mermelstein R. A Global Measure of Perceived Stress. Journal of Health and Social Behavior 24, 385–396 (1983). [PubMed] [Google Scholar]
- 65.Kroenke K. et al. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 114, 163–173 (2009). [DOI] [PubMed] [Google Scholar]
- 66.Kroenke K. & Spitzer R. L. The PHQ-9: A New Depression Diagnostic and Severity Measure. Psychiatric Annals 32, 509–515 (2002). [Google Scholar]
- 67.Calkins M. E. et al. Development and Validation of a Brief Age-Normed Screening Tool for Subthreshold Psychosis Symptoms in Youth. Schizophr Bull sbae224 (2025) doi: 10.1093/schbul/sbae224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zoupou E. et al. Validation of the structured interview section of the penn computerized adaptive test for neurocognitive and clinical psychopathology assessment (CAT GOASSESS). Psychiatry Research 335, 115862 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Calkins M. E. et al. The Philadelphia Neurodevelopmental Cohort: constructing a deep phenotyping collaborative. J Child Psychol Psychiatry 56, 1356–1369 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gur R. C. et al. A cognitive neuroscience based computerized battery for efficient measurement of individual differences: Standardization and initial construct validation. J Neurosci Methods 187, 254–262 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moore T. M., Reise S. P., Gur R. E., Hakonarson H. & Gur R. C. Psychometric Properties of the Penn Computerized Neurocognitive Battery. Neuropsychology 29, 235–246 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moore T. M. et al. Construction of a computerized adaptive test (CAT-CCNB) for efficient neurocognitive and clinical psychopathology assessment. Journal of Neuroscience Methods 386, 109795 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Klein A. et al. Remote Digital Psychiatry for Mobile Mental Health Assessment and Therapy: MindLogger Platform Development Study. Journal of Medical Internet Research 23, e22369 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Granholm E., Loh C. & Swendsen J. Feasibility and Validity of Computerized Ecological Momentary Assessment in Schizophrenia. Schizophrenia Bulletin 34, 507–514 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Larsen R. J. & Diener E. Promises and problems with the circumplex model of emotion. in Emotion 25–59 (Sage Publications, Inc, Thousand Oaks, CA, US, 1992). [Google Scholar]
- 76.Merikangas K. R. et al. Real-time Mobile Monitoring of the Dynamic Associations Among Motor Activity, Energy, Mood, and Sleep in Adults With Bipolar Disorder. JAMA Psychiatry 76, 190 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mitchell J. A. et al. Variation in Actigraphy-Estimated Rest-Activity Patterns by Demographic Factors. Chronobiol Int 34, 1042–1056 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Migueles J. H., Rowlands A. V., Huber F., Sabia S. & Hees V. T. van. GGIR: A Research Community–Driven Open Source R Package for Generating Physical Activity and Sleep Outcomes From Multi-Day Raw Accelerometer Data. (2019). [Google Scholar]
- 79.van Hees V. T. et al. Estimating sleep parameters using an accelerometer without sleep diary. Sci Rep 8, 12975 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hees V. T. van et al. A Novel, Open Access Method to Assess Sleep Duration Using a Wrist-Worn Accelerometer. PLOS ONE 10, e0142533 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo W. et al. Processing of Accelerometry Data with GGIR in Motor Activity Research Consortium for Health. Journal for the Measurement of Physical Behaviour 6, 37–44 (2023). [Google Scholar]
- 82.Kang S. J. et al. Integrative Modeling of Accelerometry-Derived Sleep, Physical Activity, and Circadian Rhythm Domains With Current or Remitted Major Depression. JAMA Psychiatry 81, 911–918 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Casey B. J. et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Developmental Cognitive Neuroscience 32, 43–54 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tisdall M. D. et al. Volumetric Navigators (vNavs) for Prospective Motion Correction and Selective Reacquisition in Neuroanatomical MRI. Magn Reson Med 68, 389–399 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moser J. et al. Multi-echo acquisition and thermal denoising advances precision functional imaging. Imaging Neuroscience 3, imag_a_00426 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Siegel J. S. et al. Psilocybin desynchronizes the human brain. Nature 632, 131–138 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vizioli L. et al. Lowering the thermal noise barrier in functional brain mapping with magnetic resonance imaging. Nat Commun 12, 5181 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moeller S. et al. NOise reduction with DIstribution Corrected (NORDIC) PCA in dMRI with complex-valued parameter-free locally low-rank processing. Neuroimage 226, 117539 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van A. N. et al. Framewise multi-echo distortion correction for superior functional MRI. bioRxiv 2023.11.28.568744 (2023) doi: 10.1101/2023.11.28.568744. [DOI] [Google Scholar]
- 90.Wedeen V. J., Hagmann P., Tseng W.-Y. I., Reese T. G. & Weisskoff R. M. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med 54, 1377–1386 (2005). [DOI] [PubMed] [Google Scholar]
- 91.Daducci A., Van De Ville D., Thiran J.-P. & Wiaux Y. Sparse regularization for fiber ODF reconstruction: From the suboptimality of and priors to. Medical Image Analysis 18, 820–833 (2014). [DOI] [PubMed] [Google Scholar]
- 92.Maffei C. et al. Using diffusion MRI data acquired with ultra-high gradient strength to improve tractography in routine-quality data. Neuroimage 245, 118706 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yendiki A. et al. Post mortem mapping of connectional anatomy for the validation of diffusion MRI. Neuroimage 256, 119146 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jones R. et al. High-fidelity approximation of grid- and shell-based sampling schemes from undersampled DSI using compressed sensing: Post mortem validation. NeuroImage 244, 118621 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Menzel M. I. et al. Accelerated diffusion spectrum imaging in the human brain using compressed sensing. Magn Reson Med 66, 1226–1233 (2011). [DOI] [PubMed] [Google Scholar]
- 96.Merlet S. L. & Deriche R. Continuous diffusion signal, EAP and ODF estimation via Compressive Sensing in diffusion MRI. Med Image Anal 17, 556–572 (2013). [DOI] [PubMed] [Google Scholar]
- 97.Michailovich O., Rathi Y. & Dolui S. Spatially regularized compressed sensing for high angular resolution diffusion imaging. IEEE Trans Med Imaging 30, 1100–1115 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Paquette M., Merlet S., Gilbert G., Deriche R. & Descoteaux M. Comparison of sampling strategies and sparsifying transforms to improve compressed sensing diffusion spectrum imaging. Magn Reson Med 73, 401–416 (2015). [DOI] [PubMed] [Google Scholar]
- 99.Pu L. et al. Model-based compressive diffusion tensor imaging: 2011 8th IEEE International Symposium on Biomedical Imaging: From Nano to Macro, ISBI’11. 2011 8th IEEE International Symposium on Biomedical Imaging 254–257 (2011) doi: 10.1109/ISBI.2011.5872400. [DOI] [Google Scholar]
- 100.Ramirez-Manzanares A., Rivera M., Vemuri B. C., Carney P. & Mareci T. Diffusion basis functions decomposition for estimating white matter intravoxel fiber geometry. IEEE Trans Med Imaging 26, 1091–1102 (2007). [DOI] [PubMed] [Google Scholar]
- 101.Paquette M., Merlet S., Gilbert G., Deriche R. & Descoteaux M. Comparison of sampling strategies and sparsifying transforms to improve compressed sensing diffusion spectrum imaging. Magn Reson Med 73, 401–416 (2015). [DOI] [PubMed] [Google Scholar]
- 102.Zhao L., Vidorreta M., Soman S., Detre J. A. & Alsop D. C. Improving the robustness of pseudo-continuous arterial spin labeling to off-resonance and pulsatile flow velocity. Magn Reson Med 78, 1342–1351 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vidorreta M. et al. Whole-brain background-suppressed pCASL MRI with 1D-accelerated 3D RARE Stack-Of-Spirals readout. PLOS ONE 12, e0183762 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dai W., Robson P. M., Shankaranarayanan A. & Alsop D. C. Reduced resolution transit delay prescan for quantitative continuous arterial spin labeling perfusion imaging. Magnetic Resonance in Medicine 67, 1252–1265 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Esteban O. et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods 16, 111–116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pruim R. H. R. et al. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. NeuroImage 112, 267–277 (2015). [DOI] [PubMed] [Google Scholar]
- 107.DuPre E. et al. TE-dependent analysis of multi-echo fMRI with *tedana*. Journal of Open Source Software 6, 3669 (2021). [Google Scholar]
- 108.Mehta K. et al. XCP-D: A Robust Pipeline for the post-processing of fMRI data. bioRxiv 2023.11.20.567926 (2023) doi: 10.1101/2023.11.20.567926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cieslak M. et al. QSIPrep: an integrative platform for preprocessing and reconstructing diffusion MRI data. Nat Methods 18, 775–778 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Adebimpe A. et al. ASLPrep: a platform for processing of arterial spin labeled MRI and quantification of regional brain perfusion. Nat Methods 19, 683–686 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gorgolewski K. et al. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinform 5, 13 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gorgolewski K. J. et al. Nipype. Software (2018) doi: 10.5281/zenodo.596855. [DOI] [Google Scholar]
- 113.Avants B. B., Epstein C. L., Grossman M. & Gee J. C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 12, 26–41 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tustison N. J. et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 29, 1310–1320 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang Y., Brady M. & Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 20, 45–57 (2001). [DOI] [PubMed] [Google Scholar]
- 116.Dale A. M., Fischl B. & Sereno M. I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9, 179–194 (1999). [DOI] [PubMed] [Google Scholar]
- 117.Klein A. et al. Mindboggling morphometry of human brains. PLoS Comput Biol 13, e1005350 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ciric R. et al. TemplateFlow: FAIR-sharing of multi-scale, multi-species brain models. Nat Methods 19, 1568–1571 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Glasser M. F. et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80, 105–124 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marcus D. S. et al. Informatics and data mining tools and strategies for the human connectome project. Front Neuroinform 5, 4 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Esteban O. et al. fMRIPrep 24.1.1. Software (2018) doi: 10.5281/zenodo.852659. [DOI] [Google Scholar]
- 122.Andersson J. L. R., Skare S. & Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20, 870–888 (2003). [DOI] [PubMed] [Google Scholar]
- 123.Jenkinson M., Bannister P., Brady M. & Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841 (2002). [DOI] [PubMed] [Google Scholar]
- 124.Greve D. N. & Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 48, 63–72 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pruim R. H. R., Mennes M., Buitelaar J. K. & Beckmann C. F. Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. NeuroImage 112, 278–287 (2015). [DOI] [PubMed] [Google Scholar]
- 126.Kundu P. et al. Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proceedings of the National Academy of Sciences 110, 16187–16192 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Posse S. et al. Enhancement of BOLD-contrast sensitivity by single-shot multi-echo functional MR imaging. Magn Reson Med 42, 87–97 (1999). [DOI] [PubMed] [Google Scholar]
- 128.van der Walt S., Colbert S. C. & Varoquaux G. The NumPy Array: A Structure for Efficient Numerical Computation. Computing in Science & Engineering 13, 22–30 (2011). [Google Scholar]
- 129.Virtanen P. et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods 17, 261–272 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.team, T. pandas development. pandas-dev/pandas: Pandas. Zenodo 10.5281/zenodo.13819579 (2024). [DOI] [Google Scholar]
- 131.Pedregosa F. et al. Scikit-learn: Machine Learning in Python. Journal of Machine Learning Research 12, 2825–2830 (2011). [Google Scholar]
- 132.Abraham A. et al. Machine learning for neuroimaging with scikit-learn. Front. Neuroinform. 8, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bokeh documentation. Bokeh https://docs.bokeh.org/en/latest/index.html.
- 134.Hunter J. D. Matplotlib: A 2D Graphics Environment. Computing in Science & Engineering 9, 90–95 (2007). [Google Scholar]
- 135.Brett M. et al. nipy/nibabel: 5.3.1. Zenodo 10.5281/zenodo.13936989 (2024). [DOI] [Google Scholar]
- 136.Ciric R. et al. Mitigating head motion artifact in functional connectivity MRI. Nat Protoc 13, 2801–2826 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Satterthwaite T. D. et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage 64, 240–256 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cox R. W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29, 162–173 (1996). [DOI] [PubMed] [Google Scholar]
- 139.Cox R. W. & Hyde J. S. Software tools for analysis and visualization of fMRI data. NMR Biomed 10, 171–178 (1997). [DOI] [PubMed] [Google Scholar]
- 140.Avants B. B., Tustison N. & Johnson H. Advanced Normalization Tools (ANTS). [Google Scholar]
- 141.Harris C. R. et al. Array programming with NumPy. Nature 585, 357–362 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yarkoni T. et al. PyBIDS: Python tools for BIDS datasets. Journal of Open Source Software 4, 1294 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ciric R. et al. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. NeuroImage 154, 174–187 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schaefer A. et al. Local-Global Parcellation of the Human Cerebral Cortex from Intrinsic Functional Connectivity MRI. Cereb Cortex 28, 3095–3114 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pauli W. M., Nili A. N. & Tyszka J. M. A high-resolution probabilistic in vivo atlas of human subcortical brain nuclei. Sci Data 5, 180063 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.King M., Hernandez-Castillo C. R., Poldrack R. A., Ivry R. B. & Diedrichsen J. Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat Neurosci 22, 1371–1378 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Najdenovska E. et al. In-vivo probabilistic atlas of human thalamic nuclei based on diffusion- weighted magnetic resonance imaging. Sci Data 5, 180270 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Glasser M. F. et al. A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gordon E. M. et al. Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations. Cereb Cortex 26, 288–303 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tian Y., Margulies D. S., Breakspear M. & Zalesky A. Topographic organization of the human subcortex unveiled with functional connectivity gradients. Nat Neurosci 23, 1421–1432 (2020). [DOI] [PubMed] [Google Scholar]
- 151.Hermosillo R. J. M. et al. A precision functional atlas of personalized network topography and probabilities. Nat Neurosci 27, 1000–1013 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zou Q.-H. et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods 172, 137–141 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jiang L. & Zuo X.-N. Regional Homogeneity: A Multimodal, Multiscale Neuroimaging Marker of the Human Connectome. Neuroscientist 22, 486–505 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang B. et al. Surface-based regional homogeneity in bipolar disorder: A resting-state fMRI study. Psychiatry Research 278, 199–204 (2019). [DOI] [PubMed] [Google Scholar]
- 155.Taylor P. A. & Saad Z. S. FATCAT: (an efficient) Functional and Tractographic Connectivity Analysis Toolbox. Brain Connect 3, 523–535 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Garyfallidis E. et al. Dipy, a library for the analysis of diffusion MRI data. Frontiers in neuroinformatics 8, 8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hoopes A., Mora J. S., Dalca A. V., Fischl B. & Hoffmann M. SynthStrip: skull-stripping for any brain image. NeuroImage 260, 119474 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Billot B. et al. Robust machine learning segmentation for large-scale analysis of heterogeneous clinical brain MRI datasets. Proceedings of the National Academy of Sciences 120, e2216399120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Veraart J. et al. Denoising of diffusion MRI using random matrix theory. NeuroImage 142, 394–406 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cordero-Grande L., Christiaens D., Hutter J., Price A. N. & Hajnal J. V. Complex diffusion-weighted image estimation via matrix recovery under general noise models. Neuroimage 200, 391–404 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tournier J.-D. et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 202, 116137 (2019). [DOI] [PubMed] [Google Scholar]
- 162.Irfanoglu M. O. et al. DR-BUDDI (Diffeomorphic Registration for Blip-Up blip-Down Diffusion Imaging) method for correcting echo planar imaging distortions. Neuroimage 106, 284–299 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Irfanoglu M. O., Nayak A., Jenkins J. & Pierpaoli C. TORTOISE v3: Improvements and new features of the NIH diffusion MRI processing pipeline. in Program and proceedings of the ISMRM 25th annual meeting and exhibition, Honolulu, HI, USA: (2017). [Google Scholar]
- 164.Yeh F.-C., Wedeen V. J. & Tseng W.-Y. I. Generalized q-sampling imaging. IEEE transactions on medical imaging 29, 1626–1635 (2010). [DOI] [PubMed] [Google Scholar]
- 165.Yeh F.-C. Shape analysis of the human association pathways. Neuroimage 223, 117329 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Adebimpe A. et al. ASLPrep <version>. Software (2023) doi: 10.5281/zenodo.3759082. [DOI] [Google Scholar]
- 167.Esteban O. et al. Analysis of task-based functional MRI data preprocessed with fMRIPrep. Nature protocols 15, 2186–2202 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lanczos C. Evaluation of Noisy Data. Journal of the Society for Industrial and Applied Mathematics Series B Numerical Analysis 1, 76–85 (1964). [Google Scholar]
- 169.Wang Z. et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magnetic resonance imaging 26, 261–269 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Power J. D. et al. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage 84, 320–341 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Buxton R. B. et al. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 40, 383–396 (1998). [DOI] [PubMed] [Google Scholar]
- 172.Dolui S., Wolf R., Nabavizadeh S. A., Wolk D. A. & Detre J. A. Automated Quality Evaluation Index for 2D ASL CBF Maps. International Society for Magnetic Resonance in Medicine (2016) doi:http://indexsmart.mirasmart.com/ISMRM2017/PDFfiles/0682.html. [Google Scholar]
- 173.Marwaha S. et al. Affective instability, childhood trauma and major affective disorders. Journal of Affective Disorders 190, 764–771 (2016). [DOI] [PubMed] [Google Scholar]
- 174.Cole P. M., Llera S. J. & Pemberton C. K. Emotional instability, poor emotional awareness, and the development of borderline personality. Development and Psychopathology 21, 1293–1310 (2009). [DOI] [PubMed] [Google Scholar]
- 175.Insel T. et al. Research Domain Criteria (RDoC): Toward a New Classification Framework for Research on Mental Disorders. AJP 167, 748–751 (2010). [DOI] [PubMed] [Google Scholar]
- 176.Casey B. J., Oliveri M. E. & Insel T. A Neurodevelopmental Perspective on the Research Domain Criteria (RDoC) Framework. Biological Psychiatry 76, 350–353 (2014). [DOI] [PubMed] [Google Scholar]
- 177.Spindler G., Stopsack M., Aldinger M., Grabe H. J. & Barnow S. What about the “ups and downs” in our daily life? The influence of affective instability on mental health. Motiv Emot 40, 148–161 (2016). [Google Scholar]
- 178.Marwaha S., Parsons N. & Broome M. Mood instability, mental illness and suicidal ideas: results from a household survey. Soc Psychiatry Psychiatr Epidemiol 48, 1431–1437 (2013). [DOI] [PubMed] [Google Scholar]
- 179.Zuo X.-N. et al. An open science resource for establishing reliability and reproducibility in functional connectomics. Sci Data 1, 140049 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xu T. et al. ReX: an integrative tool for quantifying and optimizing measurement reliability for the study of individual differences. Nat Methods 20, 1025–1028 (2023). [DOI] [PubMed] [Google Scholar]
- 181.Kong R. et al. Individual-Specific Areal-Level Parcellations Improve Functional Connectivity Prediction of Behavior. Cerebral Cortex 31, 4477–4500 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gratton C. et al. Functional Brain Networks Are Dominated by Stable Group and Individual Factors, Not Cognitive or Daily Variation. Neuron 98, 439–452.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gordon E. M. et al. Precision Functional Mapping of Individual Human Brains. Neuron 95, 791–807.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lynch C. J. et al. Frontostriatal salience network expansion in individuals in depression. Nature 633, 624–633 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cui Z. et al. Individual Variation in Functional Topography of Association Networks in Youth. Neuron 106, 340–353.e8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shanmugan S. et al. Sex differences in the functional topography of association networks in youth. Proceedings of the National Academy of Sciences 119, e2110416119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw and processed imaging data is available on OpenNeuro: Raw https://openneuro.org/datasets/ds006131; MRIQC https://openneuro.org/datasets/ds006143; QSIPrep https://openneuro.org/datasets/ds006182; QSIRecon https://openneuro.org/datasets/ds006184; fMRIPrep https://openneuro.org/datasets/ds006185; ASLPrep https://openneuro.org/datasets/ds006188; fMRIPost-AROMA https://openneuro.org/datasets/ds006189; tedana https://openneuro.org/datasets/ds006190; tedana & AROMA https://openneuro.org/datasets/ds006191; XCP-D https://openneuro.org/datasets/ds006192}.