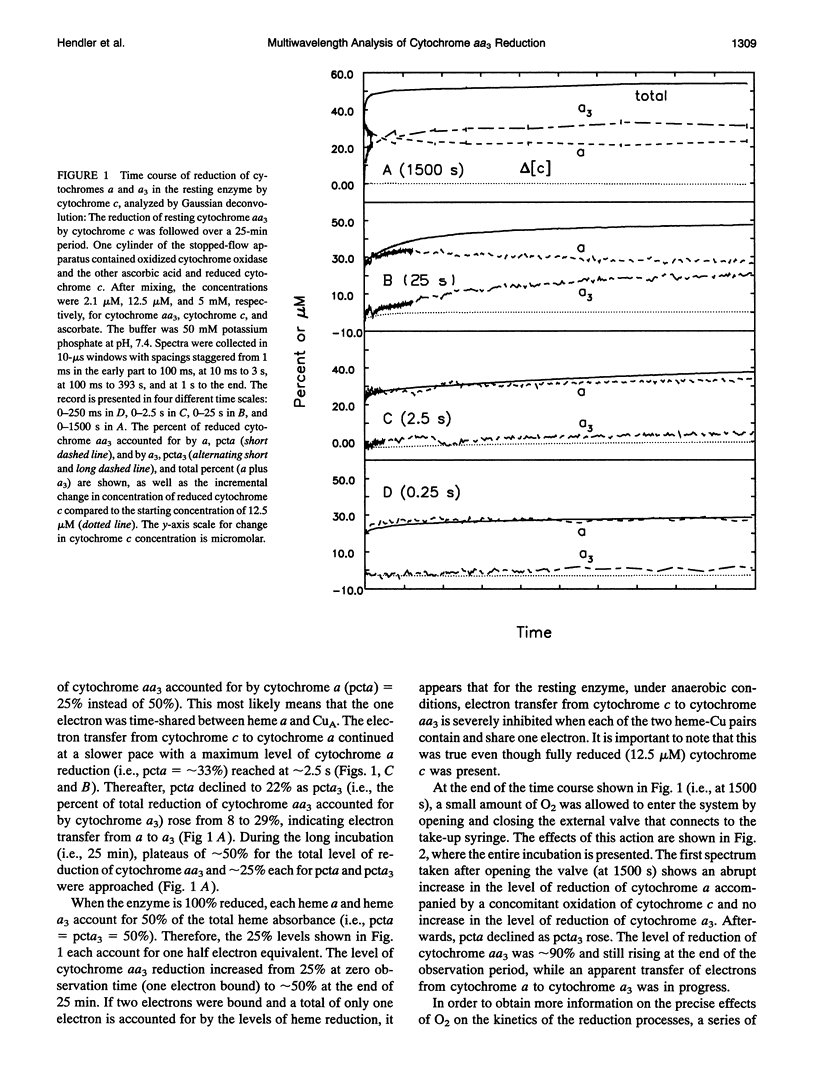
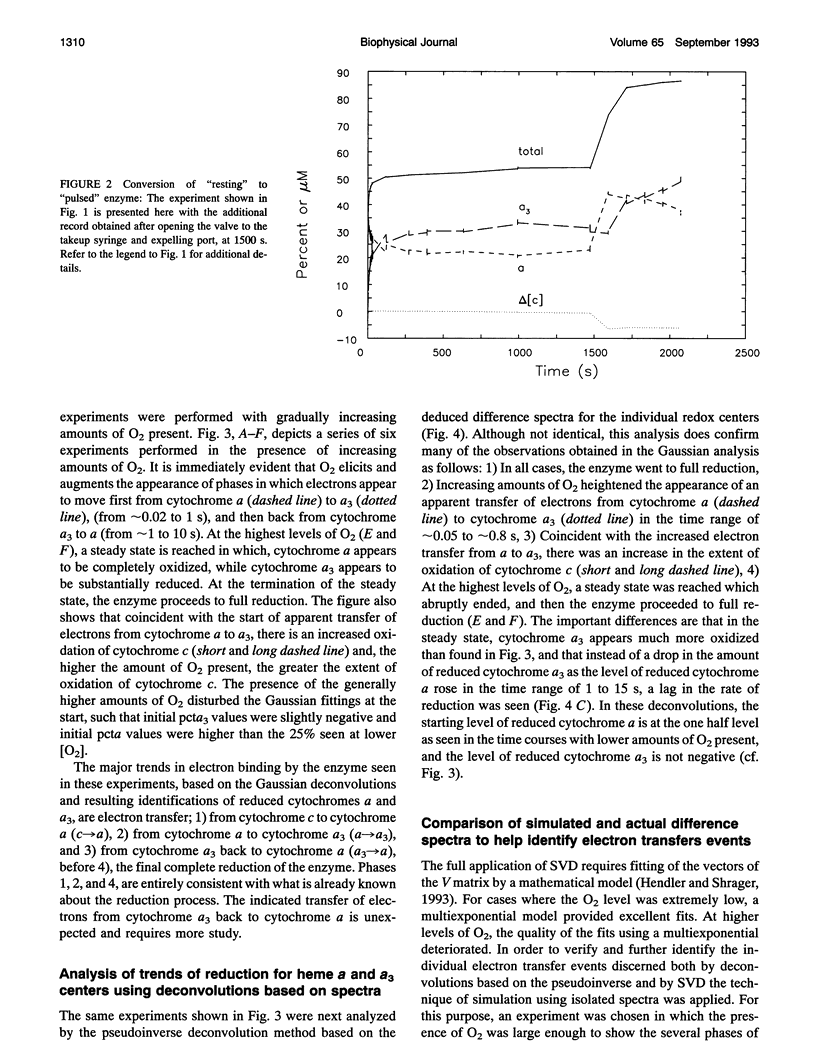
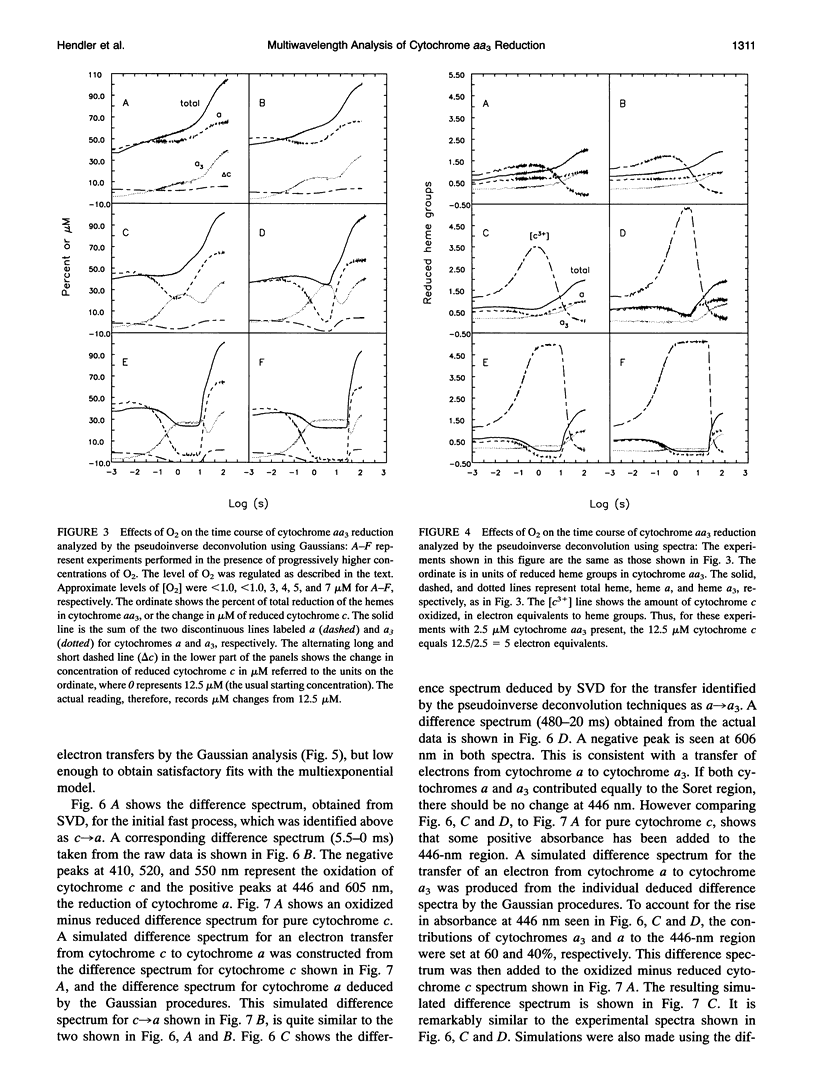
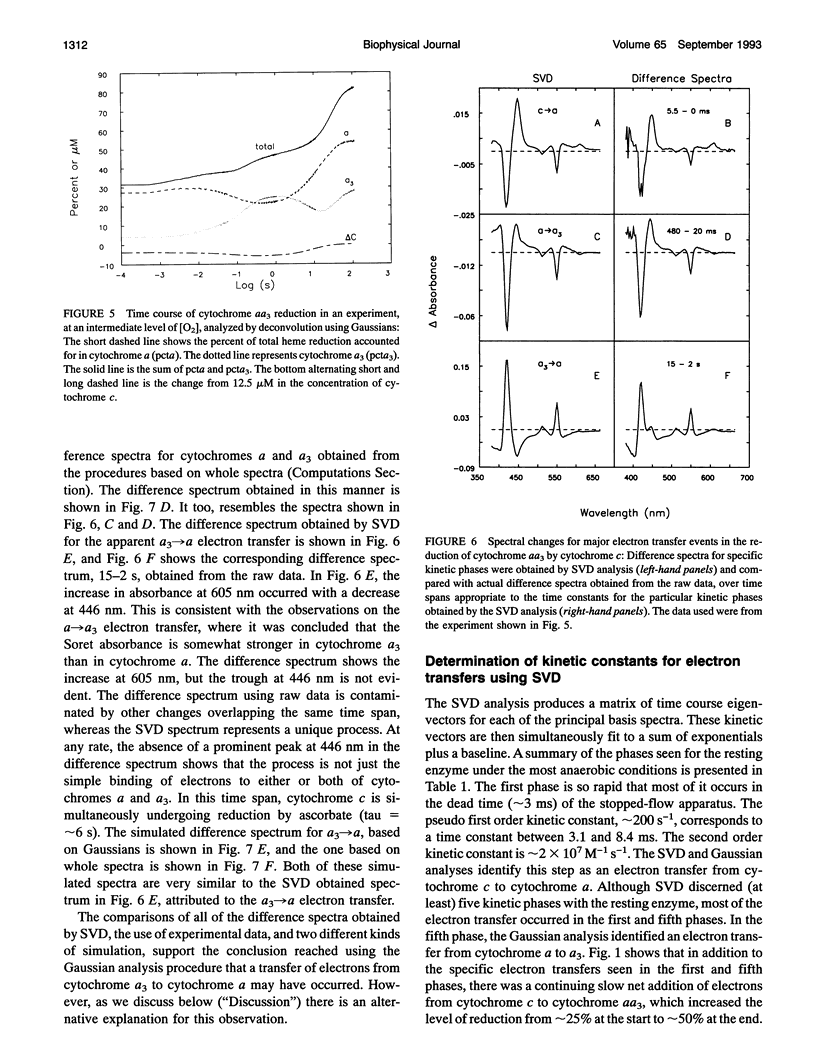
Abstract
Some new approaches to the kinetic study of the reduction of cytochrome aa3 by cytochrome c are presented. The primary innovations are the use of a spectrometer which can acquire multiwavelength data as fast as every 10 microseconds, and the application of a variety of analytical methods which can utilize simultaneously all of the time-resolved spectral data. These techniques include singular value decomposition (SVD), deconvolutions based on pure Gaussian models for absorption peaks, deconvolutions based on isolated absorption spectra for the pure components, and simulations of SVD-deduced and actual experimental difference spectra. The reduction characteristics of the anaerobic resting enzyme can be distinguished from those of pulsed forms. In the former case, only two electrons can be bound by cytochrome aa3, whereas in the latter case complete reduction of the enzyme is achieved.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andréasson L. -E., Malmström B. G., Strömberg C., Vänngård T. The reaction of ferrocytochrome c with cytochrome oxidase: A new look. FEBS Lett. 1972 Dec 15;28(3):297–301. doi: 10.1016/0014-5793(72)80735-x. [DOI] [PubMed] [Google Scholar]
- Andréasson L. E. Characterization of the reaction between ferrocytochrome c and cytochrome c oxidase. Eur J Biochem. 1975 May 6;53(2):591–597. doi: 10.1111/j.1432-1033.1975.tb04102.x. [DOI] [PubMed] [Google Scholar]
- Antalis T. M., Palmer G. Kinetic characterization of the interaction between cytochrome oxidase and cytochrome c. J Biol Chem. 1982 Jun 10;257(11):6194–6206. [PubMed] [Google Scholar]
- Brunori M., Antonini G., Malatesta F., Sarti P., Wilson M. T. Structure and function of cytochrome oxidase: a second look. Adv Inorg Biochem. 1988;7:93–153. [PubMed] [Google Scholar]
- Brzezinski P., Malmström B. G. The reduction of cytochrome c oxidase by carbon monoxide. FEBS Lett. 1985 Jul 22;187(1):111–114. doi: 10.1016/0014-5793(85)81224-2. [DOI] [PubMed] [Google Scholar]
- Di Cera E. Thermodynamics of local linkage effects. Contracted partition functions and the analysis of site-specific energetics. Biophys Chem. 1990 Aug 31;37(1-3):147–164. doi: 10.1016/0301-4622(90)88015-k. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., GREENWOOD C. THE REACTION OF CYTOCHROME OXIDASE WITH CYTOCHROME C. J Biol Chem. 1965 Feb;240:888–894. [PubMed] [Google Scholar]
- Hendler R. W. Can ferricyanide oxidize carbon monoxide-liganded cytochrome a3? J Bioenerg Biomembr. 1991 Oct;23(5):805–817. doi: 10.1007/BF00786002. [DOI] [PubMed] [Google Scholar]
- Hendler R. W., Pardhasaradhi K., Reynafarje B., Ludwig B. Comparison of energy-transducing capabilities of the two- and three-subunit cytochromes aa3 from Paracoccus denitrificans and the 13-subunit beef heart enzyme. Biophys J. 1991 Aug;60(2):415–423. doi: 10.1016/S0006-3495(91)82067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler R. W., Reddy K. V., Shrager R. I., Caughey W. S. Analysis of the spectra and redox properties of pure cytochromes aa3. Biophys J. 1986 Mar;49(3):717–729. doi: 10.1016/S0006-3495(86)83698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler R. W., Westerhoff H. V. Redox interactions in cytochrome c oxidase: from the "neoclassical" toward "modern" models. Biophys J. 1992 Dec;63(6):1586–1604. doi: 10.1016/S0006-3495(92)81748-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N., Palmer G. Further characterization of the potentiometric behavior of cytochrome oxidase. Cytochrome alpha stays low spin during oxidation and reduction. J Biol Chem. 1983 Dec 25;258(24):14908–14913. [PubMed] [Google Scholar]
- Malatesta F., Sarti P., Antonini G., Vallone B., Brunori M. Electron transfer to the binuclear center in cytochrome oxidase: catalytic significance and evidence for an additional intermediate. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7410–7413. doi: 10.1073/pnas.87.19.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls P. Control of proteoliposomal cytochrome c oxidase: the partial reactions. Biochem Cell Biol. 1990 Sep;68(9):1135–1141. doi: 10.1139/o90-169. [DOI] [PubMed] [Google Scholar]
- Nicholls P., Wrigglesworth J. M. Routes of cytochrome a3 reduction. The neoclassical model revisited. Ann N Y Acad Sci. 1988;550:59–67. doi: 10.1111/j.1749-6632.1988.tb35323.x. [DOI] [PubMed] [Google Scholar]
- Pardhasaradhi K., Ludwig B., Hendler R. W. Potentiometric and spectral studies with the two-subunit cytochrome aa3 from Paracoccus denitrificans. Comparison with the 13-subunit beef heart enzyme. Biophys J. 1991 Aug;60(2):408–414. doi: 10.1016/S0006-3495(91)82066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R. W., Hansen R. E., Beinert H. Responses of the a3 component of cytochrome c oxidase to substrate and ligand addition. Biochim Biophys Acta. 1978 Oct 11;504(1):187–199. doi: 10.1016/0005-2728(78)90017-8. [DOI] [PubMed] [Google Scholar]
- Veerman E. C., Wilms J., Casteleijn G., Van Gelder B. F. The pre-steady state reaction of ferrocytochrome c with the cytochrome c-cytochrome aa3 complex. Biochim Biophys Acta. 1980 Mar 7;590(1):117–127. doi: 10.1016/0005-2728(80)90151-6. [DOI] [PubMed] [Google Scholar]
- Wilms J., van Rijn J. L., Van Gelder B. F. The effect of pH and ionic strength on the steady-state activity of isolated cytochrome C oxidase. Biochim Biophys Acta. 1980 Nov 5;593(1):17–23. doi: 10.1016/0005-2728(80)90004-3. [DOI] [PubMed] [Google Scholar]
- Wilson M. T., Greenwood C., Brunori M., Antonini E. Kinetic studies on the reaction between cytochrome c oxidase and ferrocytochrome c. Biochem J. 1975 Apr;147(1):145–153. doi: 10.1042/bj1470145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa S., Choc M. G., O'Toole M. C., Caughey W. S. An infrared study of CO binding to heart cytochrome c oxidase and hemoglobin A. Implications re O2 reactions. J Biol Chem. 1977 Aug 10;252(15):5498–5508. [PubMed] [Google Scholar]


