Abstract
Neuronal nicotinic acetylcholine (AcCho) receptors composed of α7-subunits (α7-AcChoRs) are involved in many physiological activities. Nevertheless, very little is known about their single-channel characteristics. By using outside-out patch-clamp recordings from Xenopus oocytes expressing wild-type (wt) α7-AcChoRs, we identified two classes of channel conductance: a low conductance (γL) of 72 pS and a high one (γH) of 87 pS, with mean open-times (τop) of 0.6 ms. The same classes of conductances, but longer τop (3 ms), were seen in experiments with chimeric α7 receptors in which the wtα7 extracellular C terminus was fused to the green fluorescent protein (wtα7-GFP AcChoRs). In contrast, channels with three different conductances were gated by AcCho in oocytes expressing α7 receptors carrying a Leu-to-Thr 248 mutation (mutα7) or oocytes expressing chimeric mutα7-GFP receptors. These conductance levels were significantly smaller, and their mean open-times were larger, than those of wtα7-AcChoRs. Interestingly, in the absence of AcCho, these oocytes showed single-channel openings of the same conductances, but shorter τop, than those activated by AcCho. Accordingly, human homomeric wtα7 receptors open channels of high conductance and brief lifetime, and fusion to GFP lengthens their lifetime. In contrast, mutα7 receptors open channels of lower conductance and longer lifetime than those gated by wtα7-AcChoRs, and these parameters are not greatly altered by fusing the mutα7 to GFP. All this evidence shows that GFP-tagging can alter importantly receptor kinetics, a fact that has to be taken into account whenever tagged proteins are used to study their function.
Neuronal nicotinic acetylcholine receptors (AcChoRs) are pentameric proteins that constitute a family of ligand-gated ion channels, which are widely distributed in the mammalian brain and are built by combinations of α- and β-subunits (heteromeric AcChoRs) or by an assembly of α-subunits (homomeric AcChoRs). In particular, AcChoRs composed of α7-subunits (α7 AcChoRs) are believed to be involved in various physiological activities: acting presynaptically to modulate neurotransmitter release (1); acting postsynaptically to generate postsynaptic currents (2, 3); and in cognitive functions, development, pain, and aging (4–7). Moreover, α7-AcChoRs exhibit unusual properties, such as (i) sensitivity to α-bungarotoxin (α-BuTx) (8) and β-amyloid peptide (9); (ii) having a highly Ca2+ permeability (10, 11); (iii) exerting striking antiapoptotic effects on central nervous system nerve cells (12); and (iv) transducing signals to phosphatidylinositol 3-kinase (13). Despite this pleiotropic action, relatively little is known on the behavior of the α7-containing AcChoR channels expressed in native nerve cells (14–17) and nothing is known about the channel kinetics of human homomeric α7-AcChoRs. To begin to fill this deficiency, we made single-channel recordings in outside-out patches from human homomeric α7-AcChoRs expressed in Xenopus oocytes.
To facilitate their study, ligand-gated receptors are frequently fused to the green fluorescent protein (GFP; refs. 18–22) with the notable assumption that the properties of the receptors are not modified by the fusion with this protein. We constructed a chimera by fusing the enhanced version of GFP to the C terminus region of the human α7-AcChoR (α7-GFP AcChoR) and compared the channel behavior of wild-type (wt) α7 vs. α7-GFP AcChoRs in Xenopus oocytes. Similar experiments were addressed to the Leu-to-Thr 248 mutant α7-AcChoR (mutα7 AcChoR), which represents a “gain of function” model of the homomeric α7 receptor because of its unusual pharmacological and functional properties (23–27). We report that (i) the wtα7-AcChoR exhibits short channel duration and two-channel conductance levels; (ii) the GFP fusion to the wtα7-subunit increases the channel duration; and (iii) the GFP fusion to the mutα7-subunit accelerates channel desensitization.
Materials and Methods
Oocyte Injection.
cDNAs encoding human wtα7, wtα7-GFP, mutα7, or mutα7-GFP neuronal nicotinic subunits in pcDNA3 vector were intranuclearly injected into stage VI oocytes (2 ng of cDNA in 10 nl of buffer). Preparation of oocytes and nuclear injection procedures were as detailed elsewhere (8, 25). Mutant α7-subunits and chimeras were obtained as described (28). wtα7-subunit cDNA was a gift from the Janssen Research Foundation (Belgium). All animals were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Oocytes were obtained from ovaries dissected from female Xenopus laevis anaesthetized with 0.2% 3-aminobenzoic acid ethylester (tricaine; Sigma) and killed by section of the spinal cord, in agreement with the ethics committee guidelines of the Medical Faculty, University of Rome.
Voltage-Clamp Recordings.
Membrane currents were recorded in the voltage-clamp mode, 1–4 days after injection, using two microelectrodes filled with 3 M KCl (29). The oocytes were placed in a recording chamber (0.1 ml) perfused continuously with oocyte Ringer's medium at controlled room temperature (20–22°C). AcCho was dissolved in oocyte Ringer's solution and applied by superfusing the oocyte at a flow rate of about 12 ml/min. Unless otherwise indicated, the oocyte membrane potential was held at −60 mV. T0.5 and T0.1 indicate the time interval from the current peak to a value of 50% or 90% of the peak amplitude, respectively. Oocytes that generated AcCho currents larger than 2.5 μA were selected for single-channel recording.
Patch-Clamp Recordings.
Recordings, experimental procedures, and data analysis were performed as described (27, 30, 31). Briefly, the vitelline membrane was partially removed after exposing the oocyte to a hypertonic solution for 10–20 min. Outside-out patch-clamp recordings of AcCho-activated currents were performed on excised patches continuously superfused by means of independent external tubes, with standard or drug-containing solutions positioned 50–100 μm from the outside-out patch membrane and connected to an exchanger system (11). Single-channel data were filtered at 2 kHz, sampled at 20 kHz, and analyzed on a PC computer by using PCLAMP7 software (Axon Instruments, Foster City, CA). Opening and closing transitions were detected by using a 50% threshold criterion, and the kinetic parameters were apparent, because of the filter-cut undetected shuttings and openings. Detailed analysis was performed on a subset of patches that guaranteed the minimization of noise-induced errors. The total open probability was taken as the time during which a channel remained open divided by the whole record duration, whereas the critical time (τc), used to identify a burst, was determined as reported (32). Unless otherwise indicated, measurements were carried out at −50 mV membrane holding potential.
Solutions.
The standard oocyte Ringer's solution contained (in mM): NaCl, 82.5/KCl, 2.5/CaCl2, 2.5/MgCl2, 1/Hepes-NaOH, 5 (pH 7.4). For outside-out experiments the internal patch-pipette medium contained (in mM): CsF, 80/EGTA, 5/Hepes-CsOH, 5 (pH 7.4). Drugs and chemicals were purchased from Sigma, except methyllycaconitine (Research Biochemicals, Natick, MA).
Results
Currents Activated by AcCho in Voltage-Clamped Oocytes.
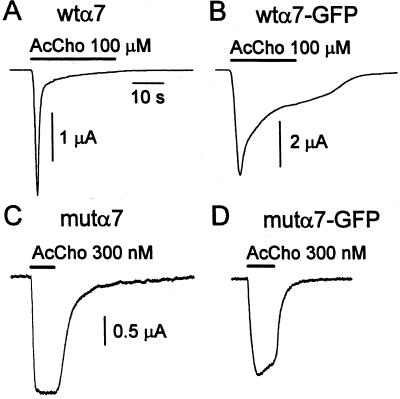
To study channel openings gated by AcCho in outside-out patch-clamp recording mode, voltage-clamped oocytes injected with α7-subunit cDNAs were routinely tested for AcChoR expression, and the most responsive oocytes were then used for patch-clamp experiments. Oocytes injected with wtα7-subunit cDNA in the voltage-clamp test responded to AcCho (100 μM) by generating an inward current with a mean peak amplitude of 3.9 μA and a T0.5 of 0.8 s (e.g., Fig. 1A). By contrast, oocytes injected with wtα7-GFP-subunit cDNA, when tested for their overall AcChoR expression with AcCho (50 μM), gave AcCho currents of 3.7 μA (mean of 4; 4 oocytes, 2 donors; 2/4) and with a much slower decay (T0.5 = 8 s; e.g., Fig. 1B) than that of wtα7-AcChoRs. Furthermore, voltage-clamped oocytes expressing mutα7 AcChoRs generated maintained AcCho currents (AcCho, 300 nM; ≈EC50; ref. 11) that had peak amplitudes of 2.5–10 μA with a mean AcCho current of 3.4 μA and a T0.1 > 10 s (e.g., Fig. 1C). Conversely, oocytes expressing the chimeric mutα7-GFP AcChoR (when voltage-clamped), responded to AcCho (300 nM) with a mean AcCho current of 2.5 μA and which decayed faster with a T0.1 of 5.4 ± 1.2 s (e.g., Fig. 1D) than oocytes expressing mutα7 AcChoRs.
Figure 1.
AcCho-current traces in voltage-clamped oocytes successively used for outside-out patch-clamp recordings. Oocytes were injected with different α7-subunit cDNAs as indicated. Horizontal bars = timing of AcCho applications. AcCho concentrations are as indicated. Note the slowing of AcChoR desensitization in B vs. A and its acceleration in D vs. C.
AcCho-Gated α7 AcChoR Channels.
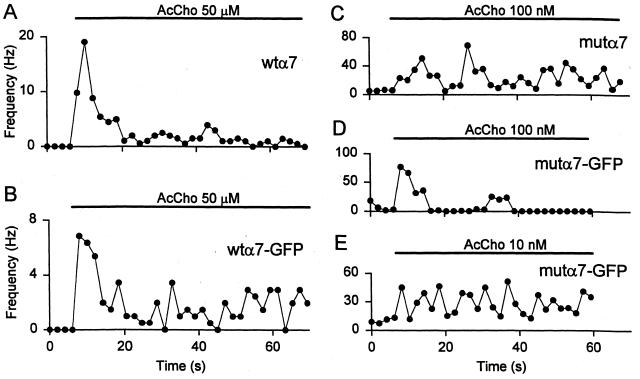
When patches from wtα7 oocytes were exposed to AcCho (50 μM), there was a rapid and strong activation of channels in five patches from four oocytes (3 donors). In contrast, channel openings were not observed before applying AcCho to less AcCho-responsive oocytes (AcCho current < 2.5 μA) or to noninjected oocytes. The channel activity decreased considerably over time. Specifically, the frequency of channel openings decreased to ≈2–4% of its initial value within 60 s after AcCho application (e.g., Fig. 2A), and full recovery required a prolonged washout (5–10 min).
Figure 2.
Time course of the frequency of channel openings from oocytes injected with α7-subunit cDNAs as indicated. Timing of AcCho application and AcCho concentrations as in Fig. 1. (E) Same patch as D. Note the decay of activity during AcCho application in A, the maintained activation throughout AcCho application in C, the activity decay during AcCho application in D, and the sustained channel activity during AcCho application in E.
Outside-out patch-clamp recordings were also performed in oocytes injected with wtα7-GFP cDNA. Channel openings were detected in 6 patches taken from 4 oocytes (2 donors). Again, openings were not observed before applying AcCho to noninjected oocytes or from oocytes that generated overall AcCho currents smaller than about 2.5 μA. When the outside-out membrane patches of the high-expression oocytes were exposed to AcCho, there was an immediate activation of channels followed by a decrease of channel-opening frequency to 30–50% within 60 s (e.g., Fig. 2B).
Patches from mutα7 AcChoR-expressing oocytes that exhibited nil spontaneous channel activity were unresponsive to subsequent AcCho applications. In contrast, patches exhibiting a clear spontaneous channel activity, i.e., blocked by the α7 receptor antagonist methyllycaconitine (1 μM, not shown), responded to AcCho (100 nM) with a marked increase in channel activity. The open-channel probability increased, on average, 9-fold at the beginning of the AcCho application, and subsequently, the channel activity was fairly stable, in agreement with the low rate of desensitization of mutα7 receptors (Fig 2C; ref. 11).
Recordings in oocytes expressing the chimeric mutα7-GFP AcChoRs evidenced spontaneous channel openings in 8 patches from 6 oocytes (2 donors). No spontaneous openings or AcCho-elicited channel openings were observed in oocytes that generated currents smaller than 2 μA (300 nM AcCho). However, when AcCho (100 nM) was applied to outside-out patches displaying spontaneous channel activity, there was an early cluster of channel openings followed by a marked decrease of openings (Fig. 2D), with long “silent” periods. This pattern may be the result of receptor desensitization (see Fig. 1D), which was almost absent if the AcCho concentration was decreased to 10 nM. Under the latter conditions, AcCho produced an enhancement of the channel activity where the open probability increased by ≈14-fold and which was substantially maintained over time during the entire transmitter application (Fig. 2E).
Taken together, these findings show that the behavior of channel activity on AcCho treatment reflected the profile of the overall AcCho currents.
AcCho-Gated wtα7-AcChoR Channels.
The AcCho-induced channel openings in oocytes expressing wtα7-AcChoRs exhibited a single-current amplitude level (Fig. 3 A–C), which remained stable during the AcCho application (1 min). The AcCho-current/voltage (I-V) relationships showed considerable rectification with depolarization, with nil current beyond 0 mV (Fig. 3D). The channel openings had a mean open-time (τop) of 0.56 ± 0.02 ms (mean ± SE; n = 471; 4 patches) and a distribution fitted by a single exponential (Fig. 3E). The closed time distribution was also fitted by a single exponential (Fig. 3F), indicating the absence of short closures and, therefore, of channel flickering. Moreover, the mean channel duration remained stable with membrane hyperpolarization of 40–70 mV (data not shown). Further analysis of AcCho-gated unitary events from the five membrane patches revealed a high and a low current level, which differed in amplitude by ≈1.2-fold, disclosing two classes of channel conductance—a low class (γL) at 72 ± 1 pS (n = 3) and a high class (γH) at 87 ± 5 pS (n = 2). Furthermore, both γL and γH could be not related to the openings of sublevels, because transitions from high to low levels were absent and each patch exhibited a homogeneous AcChoR-channel population. This channel behavior suggests that the conductances correspond to the opening of two different channel populations rather than of substates of the same channel population. Taken together, these findings indicate that human α7 receptor channels display fast kinetics and a high conductance.
Figure 3.
Single-channel properties of wtα7 receptors. (A) AcCho application to an outside-out patch-clamped membrane from the same oocyte as Fig. 1A, eliciting channel openings. (B) Enlarged single-channel traces from the same record as in A. (C) All-point histogram of amplitudes of the single-channel currents in the same patch. (D) Single-channel I-V relation from the same patch. Points represent mean values ± SE. (A) γH = 82 pS. Note current rectification beyond 0 mV. (E) Open time distribution from four outside-out membrane patches best fit by a single exponential component: τ1 = 0.37 ± 0.02 ms. (F) Closed time distribution from same four patches as E, best fit by a single exponential component: τ1 = 87 ± 6 ms. No. of channels, 471.
AcCho-Gated wtα7-GFP AcChoR Channels.
Outside-out patch-clamp recordings were also performed in oocytes injected with wtα7-GFP cDNA (Fig. 4 A–C). Similar to wtα7 receptor channels, the I-V relationships of wtα7-GFP receptor channels showed inward rectification, with nil current beyond 0 mV (e.g., Fig. 4D). In contrast, the channel-gating kinetics of the GFP chimeric receptors were clearly changed. Thus, (i) τop of 3.1 ± 0.3 ms (n = 607; 6 patches) significantly differed from that of wtα7 AcChoR (P < 0.001) and was made up of briefer and a longer exponential components (Fig. 4E), which decreased to half with 50-mV membrane hyperpolarization; (ii) a closed time distribution that was fitted by four components (Fig. 4F); and (iii) a burst duration longer than the mean open time (5.3 ± 0.7 ms; τC = 2.3 ms).
Figure 4.
Single-channel properties of wtα7-GFP receptors. (A) AcCho application (bar and dose) to an outside-out patch-clamped membrane from same oocyte as Fig. 1B, eliciting channel openings. (B) Enlarged single-channel traces from the same record as in A. (C) All-point amplitude histogram of the single-channel currents in the same patch. Note small bump at ≈−3 pA corresponding to filtered very short channels illustrated in B and A. (D) Single-channel I-V relation from the same patch. γH = 90 pS. Note current rectification beyond 0 mV. (E) Open time distribution from six outside-out membrane patches best fit by two exponential components (with weight): τ1 = 0.43 ± 0.05 ms (76%) and τ2 = 9 ± 1 ms (24%). (F) Closed time distribution from same patches as E, best fit by four exponential components: τ1 = 0.14 ± 0.02 ms (52%), τ2 = 1.2 ± 0.2 ms (18%), τ3 = 15 ± 2 ms (17%), and τ4 = 191 ± 35 ms (12%). No. of channels, 607.
Analysis of unitary events revealed two classes of high- and low-current levels, which differed in amplitude by ≈1.4-fold, disclosing again two classes of channel conductance—a low level (γL) of 70 ± 5 pS in 2 of 6 patches examined and a high level (γH) of 98 ± 3 pS in 5 of 6 patches. Furthermore, as for the wtα7 receptor channels, both classes of channel conductance may correspond to the opening of two different channel populations. Considered together, these findings indicate that AcCho activates two wtα7-GFP receptor-channel populations exhibiting slower kinetics and similar high conductance as compared with wtα7 receptors.
Spontaneous and AcCho-Gated mutα7 AcChoR Channels.
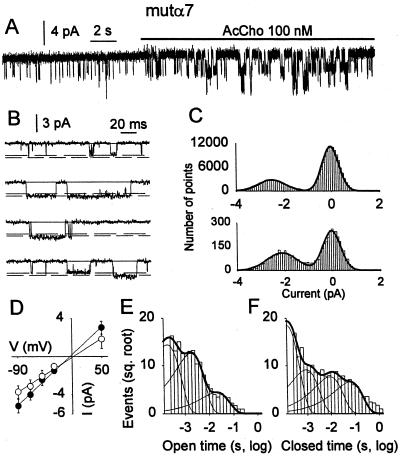
“Spontaneous” channel openings and those elicited by AcCho were detected in 8 patches from 3 oocytes expressing mutα7 AcChoR. Spontaneous openings (e.g., Fig. 5A) had a mean duration of 1.7 ± 0.1 ms (n = 540; 4 patches) made up of brief (τ1 = 0.28 ± 0.05 ms; 57%) and a long (τ2 = 2.6 ± 0.1 ms; 43%) exponential components, whereas their burst duration was of 3.2 ± 0.3 ms (τC = 2.5 ms). Analysis of unitary events disclosed three classes of current levels, exhibiting no transitions from the higher to the lower conductance in 2 of 8 patches in which two levels coexisted (Fig. 5 B and C) and three classes of channel conductance—a low (γL, n = 2) at 41 ± 3 pS, a middle (γM, n = 6) at 50 ± 1 pS, and a high one (γH, n = 2) at 60 ± 1 pS. Thus, the conductances probably correspond to the opening of three different channel populations rather than to substates of the same channel population.
Figure 5.
Single-channel properties of mutα7 receptors. (A) AcCho application to an outside-out patch-clamped membrane from same oocyte as Fig. 1C, eliciting channel openings. The open probability increased ≈13-fold (with two channels opening simultaneously) at the beginning of AcCho application. (B) Single-channel traces from the same record as in A. Note two-channel amplitude levels. (C) All-point amplitude histograms of the two-channel populations. (D) Single-channel I-V relations from the same patch. ●, γM = 50 pS; ○, γL = 42 pS. Note linearity. (E) Open time distribution from four outside-out membrane patches best fit by three exponential components (with weight): τ1 = 0.2 ± 0.03 ms (54%), τ2 = 1.8 ± 0.2 ms (41%), and τ3 = 20 ± 7 ms (5%). (F) Closed time distribution from same patches as E, best fit by the following four exponential components: τ1 = 0.13 ± 0.03 ms (65%), τ2 = 0.9 ± 0.6 ms (16%), τ3 = 9 ± 4 ms (11%), and τ4 = 62 ± 27 ms (5%). No. of channels, 1,746.
The relation between the amplitude of both spontaneous and AcCho-gated channels and the membrane potential was linear in the range +30 mV to −100 mV (Fig. 5D), as expected from voltage-clamp experiments (cf. ref. 11). The AcCho-induced openings had a τop of 2.8 ± 0.2 ms (1,746 openings from 4 patches) with a distribution made up of two exponential components (Fig. 5E), whereas the burst duration (calculated from the closed time distribution, see Fig. 5F) was 8.6 ± 1.0 ms (τc, 1.6 ms). At difference with the wtα7 receptor channels, the τop of L248T α7 receptor channels decreased to 1.3 ± 0.1 ms, as the patch-membrane was hyperpolarized by 50 mV.
Analysis of unitary events activated by AcCho identified again three classes of channel conductances, γL = 40 ± 2 pS (n = 2), γM = 50 ± 1 pS (n = 6), and γH = 60 ± 1 pS (n = 2), matching those of the spontaneous channels and coexisting together only in the two patches in which spontaneous channels exhibited the two amplitude levels. Furthermore, all conductance classes apparently could be not related to the openings of sublevels, and likely refer to distinct channel populations, as described for the chick mutα7 AcChoR (23, 27). Taken together our findings demonstrate that L248T mutation renders the α7 receptor channel activatable in the absence of AcCho, less conductive, and with slower kinetics than that of the wtα7 receptors.
Spontaneous and AcCho-Gated mutα7-GFP AcChoR Channels.
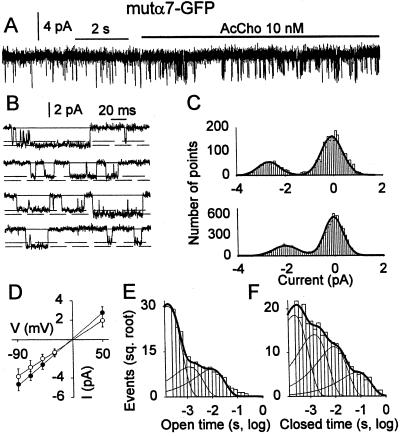
Both spontaneous and AcCho-gated channel openings from oocytes expressing mutα7-GFP AcChoRs displayed two amplitude levels (see Fig. 6 A–C) that were linearly related to membrane potential (Fig. 6D). The analysis of unitary currents identified again three classes of conductance: γL = 35 ± 3 pS (n = 3), γM = 52 ± 3 pS (n = 4), and γH = 60 ± 1 pS (n = 2), two of which coexisted in 1 of 8 patches. The conductance classes were very similar to those detected in human mutα7 receptors. As for the mutα7 receptor patches, spontaneous mutα7-GFP receptor channels showed τop of 1.0 ± 0.1 ms (n = 431; 4 patches) made up of a brief (τ1 = 0.18 ± 0.02 ms; 75%) and a longer (τ2 = 1.3 ± 0.2 ms; 25%) exponential component, whereas their mean burst duration was 3-fold longer (3.2 ± 0.5 ms; τC = 2.2 ms) than the τop. On the other hand, the τop of AcCho-activated channels was 2.4 ± 0.1 ms (n = 3694) and was fitted by three exponential components (Fig. 6E), whereas the mean burst duration (calculated from the closed time distribution, see Fig. 6F) was 9.2 ± 1.0 (τC = 2.3 ms). In contrast to the mutα7 receptor behavior, the channel duration was not affected by membrane hyperpolarization (data not shown). Considered together, these findings show that the GFP fusion to the mutα7-subunit did not significantly modify the gating and kinetics properties, except that the chimeric receptors became voltage-independent and their desensitization was accelerated after GFP fusion.
Figure 6.
Single-channel properties of mutα7-GFP receptors. (A) AcCho application to an outside-out patch-clamped membrane from same oocyte as Fig. 1D, eliciting channel openings. The open probability increased ≈10-fold after AcCho application. (B) Single-channel traces from the same record as in A. Note two-channel amplitude levels. (C) All-point amplitude histograms of the two-channel populations. (D) Single-channel I-V relations from the same patch. ●, γM = 52 pS; ○, γL = 40 pS. Note linearity. (E) Open time distribution from four outside-out membrane patches best fit by three exponential components (with weight): τ1 = 0.18 ± 0.02 ms (83%), τ2 = 1.2 ± 0.6 ms (9%), and τ3 = 9 ± 3 ms (8%). (F) Closed time distribution from same patches as E, best fit by the following four exponential components: τ1 = 0.25 ± 0.05 ms (49%), τ2 = 1.5 ± 0.4 ms (28%), τ3 = 9 ± 3 ms (18%), and τ4 = 76 ± 29 ms (5%). No. of channels, 3,694.
Discussion
The neuronal α7-AcChoR, a ligand-gated channel abundantly expressed throughout the central nervous system, is believed to play a key role in synaptic transmission and in the regulation of neuronal development, synaptic plasticity, and nerve cell differentiation (1–3, 5, 7, 33, 34). One of the central findings in this work is that human wtα7 AcChoR channels expressed in oocytes exhibit two classes of channel conductance, both much larger than the single class of wtα7 AcChoR channels described for the chick (23), a brief mean channel duration, and a channel desensitization, as expected from the fast decay of the overall AcCho current. The γL and τop values found here match well with those estimated for the native α7-containing AcChoR channels of rat hippocampal neurons (14), suggesting that the presence of the α7-subunit in the nicotinic receptors, which in the hippocampus or in the retina could be heteromeric (17, 33), determines the channel behavior. Moreover, the large conductances of the α7 AcChoR channels, which are highly permeable to Ca2+ (10, 11), may underlie many Ca2+-dependent events that follow receptor activation, such as the neuromodulation of synaptic transmission (1–3) or the triggering of cytosolic cascade pathways (13).
Fusion of ligand-gated receptors to the jellyfish GFP is generally capable of forming chimeric receptors that have properties similar to those of wt receptors and are used to visualize the receptor expression in cell systems. In a previous work we constructed human α7-GFP chimeras to study α7 receptors (28), and in the present work we have determined the single-channel properties of the chimeric homomeric α7 receptors formed in Xenopus oocytes. We confirm that chimeric receptors are functionally assembled in oocytes intranuclearly injected with α7-GFP-subunit cDNA and found that when these α7-GFP receptors are activated by the transmitter they exhibit unitary events with the same two classes of conductance but with different gating kinetics than those of the wtα7 receptors. In particular, τop and burst duration significantly increase after GFP fusion, underlying the slowing of the macroscopic AcCho-current decay (e.g., Fig. 1 A vs. B). In addition, τop becomes voltage-dependent and becomes shorter with patch hyperpolarization. These findings show that the fusion of GFP to extracellular terminus of the α7-subunit changes importantly the kinetics properties of the nicotinic α7 receptor channels.
We also addressed our attention to the mutation of the highly conserved leucine residue in the M2 channel domain of the α7-subunit (L248T), which confers many special properties to the mutant receptors, including an unconventional pharmacology (24, 25, 35), and a “gain of function” that leads to an increased affinity for AcCho, a reduced receptor desensitization and an I-V linearity (11). Furthermore, the homologous replacement of the α7-subunit gene with the L250T-mutated form (equivalent in the mouse to the chick L247T and to the human L248T mutations) causes homozygous mice to die post partum (36), representing a model of neurodegenerative disease. We report here that, similarly to the chick mutα7 receptor, the human mutα7 AcChoR when gated by AcCho opens channels that have three conductance levels that are smaller and have a longer open duration lifetime than those gated by wtα7 AcChoR. Interestingly, the mutα7 receptors are “spontaneously” gated, and this event is quite frequent. We say “spontaneous” because it is still possible that the openings observed in the absence of the neurotransmitter are activated by contaminant divalent ions, such as Zn2+ (cf. ref. 35). A constitutive activation of chick mutα7 or human mutα7 AcChoR in Xenopus oocytes has long been inferred from the reduction of a “leak” current observed after nicotinic antagonist (e.g., methyllycaconitine) administration. Whatever their origin, we have shown spontaneous openings in human cells expressing by transfection mutα7 receptors (37), and the present report shows spontaneous openings of neuronal AcChoR channels in the oocyte expression system.
We have also constructed a functional chimeric AcChoR formed by fusing GFP to the C terminus of the mutα7-subunit and found that the chimeric receptors expressed in oocytes have altered desensitization (ref. 28 and Fig. 1). In this work, we have studied the channel properties of the mutα7-GFP receptors. We report that both the conductance and the channel duration of the three channel populations activated by AcCho in mutα7 AcChoR-expressing oocytes are only slightly modified by the fusion to GFP. Moreover, the τop is independent of membrane potential, and the channels desensitize rapidly at relatively low AcCho concentrations (100 nM; see also Fig. 3 C vs. D). In conclusion, we show here that human homomeric wtα7 receptor channels exhibit high conductances and short duration and that the fusion of GFP to the C terminus produces functional modifications. Such modifications suggest complex interactions between the leucine ring at the mouth of the channel and distant extracellular domains, interactions that alter channel kinetics and desensitization.
Acknowledgments
We thank Drs. Francesca Grassi and Fabio Ruzzier for critical reading of the manuscript. The wtα7 was a gift from Janssen (Belgium). This work was supported in part by Ministero Università Ricerca Scientifica e Tecnologica (to F.E.) and by National Science Foundation (Neuronal and Glial Mechanisms) Grant 9982856 (to R.M.).
Abbreviations
- GFP
green fluorescent protein
- AcCho
acetylcholine
- AcChoR
nicotinic AcCho receptor
- wtα7 AcChoR
wild-type α7 AcChoR
- mutα7 nAcChoR
Leu-to-Thr 248 mutant α7 AcChoR
- wtα7-GFP AcChoR
wtα7 AcChoR fused to GFP
- mutα7-GFP AcChoR
Leu-to-Thr 248 mutant α7 AcChoR fused to GFP
- I-V
current/voltage
References
- 1.McGehee D, Heath M, Gelber S, Role L W. Science. 1995;269:1692–1697. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- 2.Ullian E M, McIntosh J M, Sargent P B. J Neurosci. 1997;17:7210–7219. doi: 10.1523/JNEUROSCI.17-19-07210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang K, Berg D K. J Neurosci. 1999;19:3701–3710. doi: 10.1523/JNEUROSCI.19-10-03701.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levin E D. Psychopharmacology. 1992;108:417–431. doi: 10.1007/BF02247415. [DOI] [PubMed] [Google Scholar]
- 5.Zoli M, Le Novère N, Hill J A, Changeux J P. J Neurosci. 1995;15:1912–1939. doi: 10.1523/JNEUROSCI.15-03-01912.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damaj M I, Meyer E M, Mertin B R. Neuropharmacology. 2000;39:2785–2791. doi: 10.1016/s0028-3908(00)00139-8. [DOI] [PubMed] [Google Scholar]
- 7.Mansvelder H D, McGehee D. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- 8.Couturier S, Bertrand D, Matter J M, Hernandez M C, Bertrand S, Millar N, Velera S, Barkas T, Ballivet M. Neuron. 1990;5:847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- 9.Liu Q, Berg D K. Proc Natl Acad Sci USA. 2001;98:4734–4739. doi: 10.1073/pnas.081553598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seguela P, Wadiche J, Dineley-Miller K, Dani J A, Patrick J W. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fucile S, Palma E, Mileo A M, Miledi R, Eusebi F. Proc Natl Acad Sci USA. 2000;97:3643–3648. doi: 10.1073/pnas.050582497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kihara T, Shimohama S, Sawada H, Kimura J, Kume T, Kochiyama H, Maeda T, Akaike A. Ann Neurol. 1997;42:159–163. doi: 10.1002/ana.410420205. [DOI] [PubMed] [Google Scholar]
- 13.Kihara T, Shimohama S, Sawada H, Honda K, Nakamizo T, Shibasaki H, Kume T, Akaike A. J Biol Chem. 2001;276:13541–13546. doi: 10.1074/jbc.M008035200. [DOI] [PubMed] [Google Scholar]
- 14.Castro N G, Albuquerque E X. Neurosci Lett. 1993;164:137–140. doi: 10.1016/0304-3940(93)90876-m. [DOI] [PubMed] [Google Scholar]
- 15.Mike A, Castro N G, Albuquerque E X. Brain Res. 2000;882:155–168. doi: 10.1016/s0006-8993(00)02863-8. [DOI] [PubMed] [Google Scholar]
- 16.Shao Z, Yakel J L. J Physiol. 2000;527:507–513. doi: 10.1111/j.1469-7793.2000.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu C R, Role L W. J Physiol. 1998;509:651–655. doi: 10.1111/j.1469-7793.1998.651bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Torres A, Miledi R. Proc Natl Acad Sci USA. 2000;98:1947–1951. doi: 10.1073/pnas.031584898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall J, Molloy R, Moss G W, Howe J R, Hughes T E. Neuron. 1995;14:211–215. doi: 10.1016/0896-6273(95)90279-1. [DOI] [PubMed] [Google Scholar]
- 20.David-Watine B, Shorte S L, Fucile S, Saint Jan D, Korn H, Bregestovski P. Neuropharmacology. 1999;38:785–792. doi: 10.1016/s0028-3908(99)00015-5. [DOI] [PubMed] [Google Scholar]
- 21.Zuo J, Treadaway J, Buckner T W, Fritzsch B. Proc Natl Acad Sci USA. 1999;96:14100–14105. doi: 10.1073/pnas.96.24.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dutton J L, Porronnik P, Li G H, Holding C A, Worthington R A, Vandenberg R J, Cook D I, Barden J A, Bennet M R. Neuropharmacology. 2000;39:2054–2066. doi: 10.1016/s0028-3908(00)00058-7. [DOI] [PubMed] [Google Scholar]
- 23.Revah F, Bertrand D, Galzi J L, Devillers-Thiery A, Mulle C, Hussy N, Bertrand S, Ballivet M, Changeux J P. Nature (London) 1991;353:846–849. doi: 10.1038/353846a0. [DOI] [PubMed] [Google Scholar]
- 24.Bertrand D, Devillers-Thiery A, Revah F, Galzi J L, Hussy N, Mulle C, Bertrand S, Ballivet M, Changeux J P. Proc Natl Acad Sci USA. 1992;89:1261–1265. doi: 10.1073/pnas.89.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palma E, Mileo A M, Eusebi F, Miledi R. Proc Natl Acad Sci USA. 1996;93:11231–11235. doi: 10.1073/pnas.93.20.11231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palma E, Eusebi F, Miledi R. Proc Natl Acad Sci USA. 1997;94:1539–1543. doi: 10.1073/pnas.94.4.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palma E, Maggi L, Eusebi F, Miledi R. Proc Natl Acad Sci USA. 1997;94:9915–9919. doi: 10.1073/pnas.94.18.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palma E, Mileo A M, Martínez-Torres A, Eusebi F, Miledi R. Proc Natl Acad Sci USA. 2002;99:3950–3955. doi: 10.1073/pnas.052699299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miledi R. Proc R Soc London B Biol Sci. 1982;215:491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- 30.Methfessel C, Witzemann V, Takahashi T, Mishina M, Numa S, Sakmann B. Pflügers Arch. 1986;407:577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- 31.Fucile S, Mileo A M, Grassi F, Salvatore A M, Alema' S, Eusebi F. Eur J Neurosci. 1996;8:2564–2570. doi: 10.1111/j.1460-9568.1996.tb01550.x. [DOI] [PubMed] [Google Scholar]
- 32.Colquhoun D, Sakmann B. J Physiol. 1985;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broide K G, Leslie F M. Mol Neurobiol. 1999;20:1–16. doi: 10.1007/BF02741361. [DOI] [PubMed] [Google Scholar]
- 34.Girod R, Crabtree G, Ernstrom G, Ramirez-Latorre J, McGehee D, Turner J, Role L. Ann NY Acad Sci. 1999;868:578–590. doi: 10.1111/j.1749-6632.1999.tb11331.x. [DOI] [PubMed] [Google Scholar]
- 35.Palma E, Maggi L, Miledi R, Eusebi F. Proc Natl Acad Sci USA. 1998;95:10246–10250. doi: 10.1073/pnas.95.17.10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orr-Urtreger A, Broide R S, Kasten M R, Dand H, Dani J A, Beaudet A L, Patrick J W. J Neurochem. 2000;74:2154–2166. doi: 10.1046/j.1471-4159.2000.0742154.x. [DOI] [PubMed] [Google Scholar]
- 37. Fucile, S., Palma, E., Eusebi, F. & Miledi, R. (2002) Neuroscience, in press. [DOI] [PubMed]