Abstract
The function of the Rhesus (Rh) complex in the human red cell membrane has been unknown for six decades. Based on the organismal, organ, and tissue distribution of Rh proteins, and on our evidence that their only known paralogues, the ammonium and methylammonium transport proteins (also called methylammonium permeases), are gas channels for NH3, we recently speculated that Rh proteins are biological gas channels for CO2. Like NH3, CO2 differs from other gases in being readily hydrated. We have now tested our speculation by studying expression of the RH1 gene in the photosynthetic microbe Chlamydomonas reinhardtii. Expression of RH1 was high for cells grown in air supplemented with 3% CO2 or shifted from air to high CO2 (3%) for 3 h. Conversely, RH1 expression was low for cells grown in air (0.035% CO2) or shifted from high CO2 to air for 3 h. These results make viable the hypothesis that Rh1 and Rh proteins generally are gas channels for CO2.
The Rhesus (Rh) blood group substance is the second most abundant protein in human red cell membranes (≈105 copies per cell) (1). The related RhAG and Rh30 proteins, which constitute this complex (2, 3), have only one known paralogue, the ammonium and methylammonium transport (Amt) proteins [also called methylammonium permeases (MEP)] (4). Marini and colleagues (5) reported that both Amt/MEP proteins and the human RhAG and RhCG proteins are active transporters for NH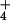 . Their conclusion regarding Rh proteins was based on the properties of Saccharomyces cerevisiae strains lacking function of its three MEP proteins and carrying cloned human Rh genes. Contrary to the views of Marini et al., we have provided several lines of evidence that Amt and MEP proteins are gas channels for NH3 and have speculated that Rh proteins are gas channels for CO2 (6–9). To test the viability of our speculation regarding Rh, we have studied expression of the RH1 gene in the green alga Chlamydomonas reinhardtii, one of the few microbes to have RH genes.
. Their conclusion regarding Rh proteins was based on the properties of Saccharomyces cerevisiae strains lacking function of its three MEP proteins and carrying cloned human Rh genes. Contrary to the views of Marini et al., we have provided several lines of evidence that Amt and MEP proteins are gas channels for NH3 and have speculated that Rh proteins are gas channels for CO2 (6–9). To test the viability of our speculation regarding Rh, we have studied expression of the RH1 gene in the green alga Chlamydomonas reinhardtii, one of the few microbes to have RH genes.
Materials and Methods
Media and Growth Conditions.
C. reinhardtii strains CC125 (137c; nit1 nit2 mt+) (10), 4A+ (nit1 nit2 mt+), and CC124 (nit1 nit2 mt−) were maintained at 24°C in TAP medium (11), under continuous illumination (40 μmol photons m−2 s−1). Strain 4A+ was kindly provided by J.-D. Rochaix (Univ. of Geneva, Switzerland). For growth in high CO2, cells were cultured in 1 l bottles containing 700 ml of TP(-N) medium (TAP medium without acetate and nitrogen; ref. 11) under constant illumination (170 μmol photons m−2 s−1) and were bubbled with air enriched with 3% (vol/vol) CO2. The nitrogen source was NH4Cl (10 mM), arginine (2.5 mM), or hypoxanthine (2.5 mM), as indicated. For growth in low CO2, cultures were bubbled with ordinary air [0.035% (vol/vol) CO2]. Chlorophyll a+b content was estimated after extracting cells with 96% (vol/vol) ethanol (12).
Sequencing of the C. reinhardtii RH (Cr-RH) cDNAs and Cloning of their Genes.
The cDNA and gene sequences of Cr-RH1 were deposited in GenBank by Liu and Huang (GenBank accession nos. AY013257 and AY013258, respectively) (3). We searched the C. reinhardtii expressed sequence tag (EST) Index (Kazusa DNA Research Institute, Chiba, Japan; http://www.kazusa.or.jp/en/plant/chlamy/EST/) for homologues of Rh proteins. Clone CL31f08 (accession no. AV395002) and clone HCL086h08 (accession no. AV644354) carried the full-length cDNA sequences of Cr-RH1 and Cr-RH2, respectively. EST clone HCL086h08 carried a fragment of 3,053 bp containing an ORF that was 73% identical to Cr-RH1 (designated Cr-RH2; GenBank accession nos. AF411248 and AF500098). It also carried 986 bp of noncoding sequence downstream of the first stop codon and 153 bp upstream of the first ATG. The Cr-RH2 gene was cloned by PCR from chromosomal DNA of strain 4A+ with the following pair of primers: forward primer h08–5 (5′-CACACGTAGACAGTTCCC-3′); reverse primer h08–6 (5′-GCGGGAGTACTTGCCATC-3′). PCR was performed by using the Expand Long Template system (Roche-Boerhinger) in a 100-μl reaction mixture (500 ng of sheared chromosomal DNA, 300 nM of each primer, 500 μM dNTPs, 2.25 mM MgCl2, and 2.5 units of proof-reading Expand Taq polymerase) under the following amplification conditions: 2 min at 94°C, 29 cycles of denaturation [(10 s at 94°C)/annealing (30 s at 55°C)/polymerization (6 min at 68°C)], and 7 min at 68°C. A unique PCR fragment of ≈6 kbp was recovered from agarose gels and cloned by using a TOPO TA cloning kit (Invitrogen). The sequence of the Cr-RH2 gene was determined by primer walking and was deposited at GenBank (accession no. AF500097).
Isolation of RNA and Northern Analysis.
RNA was isolated as described (13) from cells grown to a chlorophyll a+b content of 8 μg/ml with high CO2 or 4 μg/ml with low CO2. Cells were quickly chilled by mixing 2/3 volume of culture with 1/3 volume of crushed ice and kept on ice. After centrifugation at 8,000 × g at 4°C for 4 min, the pellet was suspended in cold water at 1/100 of the original culture volume. An equal volume of 2-fold concentrated lysis buffer (0.6 M NaCl/10 mM EDTA/100 mM Tris⋅HCl, pH 8/4% SDS) was added to the cell suspension, mixed, and the lysate was frozen in liquid nitrogen and stored at −80°C. For RNA extraction, the lysate was thawed in a water bath at 65°C, proteinase K was added to 40 μg/ml, and the lysate was incubated for 5 min at 65°C. Then 0.132 volumes of cold KCl (2 M) was added and the lysate was incubated on ice for 20 min. After centrifugation at 12,000 × g for 10 min at 4°C, the supernatant was extracted with phenol and RNA was precipitated with 0.33 volumes of LiCl (8 M) as described (13).
Northern blot hybridizations were performed as described (13). All probes were generated by random primed labeling with the High-Prime System (Roche-Boerhinger) and [α-32P]dCTP following instructions of the manufacturer. The RH1 specific probe was obtained by PCR amplification of a 1,401-bp fragment of the last exon (exon 12) using the forward primer Cr-RHp >11 (5′-CGTGTAGAGCGGCAGACT-3′) and the reverse primer Cr-RHp <20 (5′-GGATGTGCCTGTTCGATGTGT-3′). The RH2 specific probe was obtained by PCR amplification of a 1,075-bp fragment of the last exon (exon 13) using the forward primer h08–7 (5′-CGTAATCGGAATGGAGAC-3′) and the reverse primer h08–6 (5′-GCGGGAGTACTTGCCATC-3′). The CAH1-specific probe was obtained by PCR amplification of a 758-bp fragment of the last exon using the forward primer cah-11 (5′-ACTTCCCAGTAGTTAGTCACG-3′) and the reverse primer cah-12 (5′-GTCTCTGCCTCAACTGCACCG-3′). The RBCS probe was obtained by PCR amplification of a 664-bp fragment using the forward primer rbc-21 (5′-ATGGCCGCCGTCATTGCCAAG-3′) and the reverse primer rbc-22 (5′-CATCCACCGCCGTTCGTCAGG-3′).
Western Analysis.
From cultures grown for isolation of RNA, 10 ml of cells were harvested by centrifugation at 8,000 g for 5 min at 4°C. The cells were suspended to a chlorophyll a+b content of 200 μg/ml by addition of an appropriate amount of SDS loading buffer [60 mM Tris⋅HCl, pH 6.8/2% SDS/0.7 mM 2-mercaptoethanol/0.1 mM phenylmethylsulfonyl fluoride/10% glycerol/0.001% bromophenol blue] supplemented with 1% n-octylβ-d-glucopyranoside (Sigma), and were disrupted by incubation at 37°C for 30 min. Extract corresponding to 4 μg chlorophyll a+b was subjected to SDS/PAGE, and proteins were transferred to a nitrocellulose membrane. The Rh1 protein was detected with affinity-purified rabbit antibodies raised against a unique C-terminal peptide (Zymed) (Fig. 1A), and the periplasmic carbonic anhydrase Cah1 was detected with affinity-purified rabbit antibodies directed against the intact protein (14) (generous gift of M. H. Spalding).
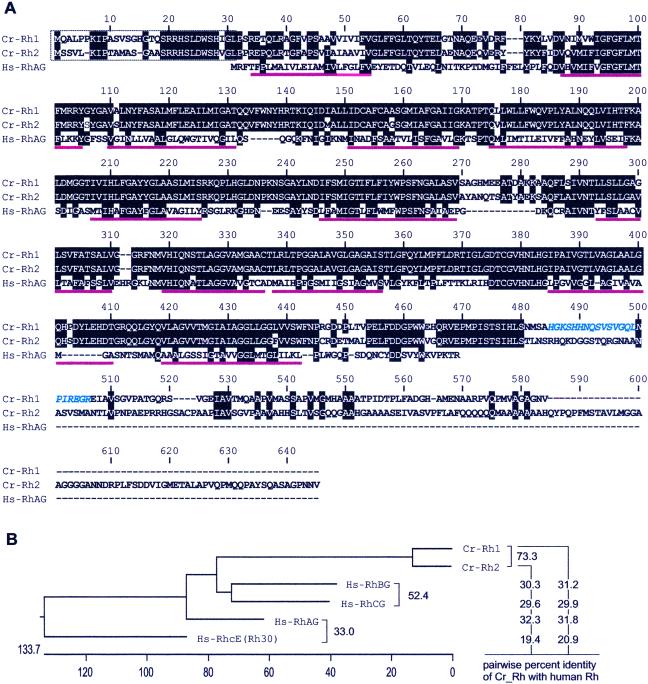
Figure 1.
Alignment of the predicted sequences of the C. reinhardtii Rh1 and Rh2 proteins with the sequence of human erythrocyte RhAG (A) and their percent sequence identity with all known human Rh proteins (B). Residues that are identical in any two of the proteins are shaded and the Rh1 peptide against which antiserum was raised (residues 485–506) is indicated in blue italics. The dotted box at the N terminus encloses a predicted chloroplast transit peptide for Rh1 and a possible transit peptide for Rh2 (ExPASy Molecular Biology Server, Swiss Institute of Bioinformatics; http://ca.expasy.org/tools/#ptm). Putative transmembrane spanning segments (3) are underlined in red.
Methylammonium Uptake Assays.
Assays for transport of [14C]methylammonium were performed as described (6) at pH 7.2 and 24°C. Cells were grown in the medium indicated and harvested at a chlorophyll a+b content of ≈4 μg/ml. Cells were washed twice at room temperature in assay buffer [50 mM Hepes, pH 7.2/20 mM acetate] and were then concentrated 2-fold in this buffer and prewarmed for 30 min at 24°C under light.
Results
Chlamydomonas Rh1 and Rh2 Proteins.
Chlamydomonas Rh1 and Rh2 were identified by sequence homology to all known human Rh proteins. These include the erythrocyte proteins RhAG and RhcE (Rh30) and nonerythroid Rh proteins (Fig. 1). Chlamydomonas Rh1 is a protein of 574 amino acid residues with 12 predicted transmembrane spanning segments (3). It has both N- and C-terminal extensions with respect to human Rh proteins. In silico analysis of the Rh1 sequence predicted that the N-terminal extension is a chloroplast transit peptide (ExPASy Molecular Biology Server, Swiss Institute of Bioinformatics, http://ca.expasy.org/tools/#ptm). Rh2 is a protein of 638 residues and is 73% identical to Rh1. It is also predicted to have 12 transmembrane spanning segments and may carry a chloroplast transit peptide at its amino terminus. The unique C-terminal extension of Rh2 is 64 residues longer than that of Rh1 and accounts for most of their sequence divergence. Both Rh1 and Rh2 have similar degrees of sequence identity with each of the four human Rh proteins (Fig. 1B).
Levels of RH1 mRNA Are Controlled by CO2 Availability.
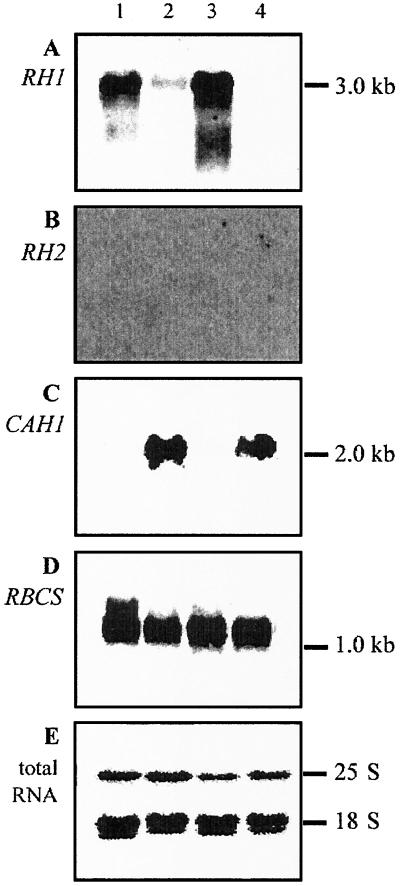
Northern analysis of total RNA indicated that wild-type C. reinhardtii strain CC125 had high levels of RH1 mRNA when it was grown in air supplemented with 3% CO2 (high CO2) and low levels when it was grown in air (0.035% CO2) (Fig. 2A, lanes 1 and 2, respectively). Levels of RH1 mRNA were high when cells were shifted from air to high CO2 for 3 h (lane 3) and low when cells were subjected to the opposite shift (lane 4). Control experiments indicated that mRNA levels for the periplasmic carbonic anhydrase Cah1, whose expression is controlled by the carbon concentrating mechanism of Chlamydomonas (15–21), varied in the opposite direction from those for Rh1 (Fig. 2C). The high levels of CAH1 mRNA in cells grown in air or shifted to air were as observed (15–17, 20, 21). Levels of mRNA for the small subunit of Rubisco (ribulose bisphosphate carboxylase/oxygenase), RbcS2, were approximately constant under different conditions of carbon availability (Fig. 2D). Similar results were obtained for a second isolate of CC125 obtained recently from the Chlamydomonas Genetics Center, for wild-type strain 4A+, which exhibits faster growth on acetate in the dark, and for wild type strain CC124, which is mating type minus (data not shown). RH2 mRNA was not detected by Northern analysis of total RNA from strain CC125 (Fig. 2B).
Figure 2.
Northern analysis of RH1 mRNA levels. Five μg of total RNA was used for each lane. Membranes were hybridized to probes for RH1 (A), RH2 (B), CAH1 (C), or RBCS (D). The gel in E was stained with ethidium bromide. Lane 1, cells grown in high CO2; lane 2, cells grown in air; lane 3, cells shifted from air to high CO2 for 3 h; lane 4, cells shifted from high CO2 to air for three hours. In all cases the nitrogen source was NH4Cl. Sizes of ribosomal RNAs and approximate positions of 1- to 3-kb transcripts are indicated on the right.
Levels of Rh1 Protein Are Controlled by CO2 Availability.
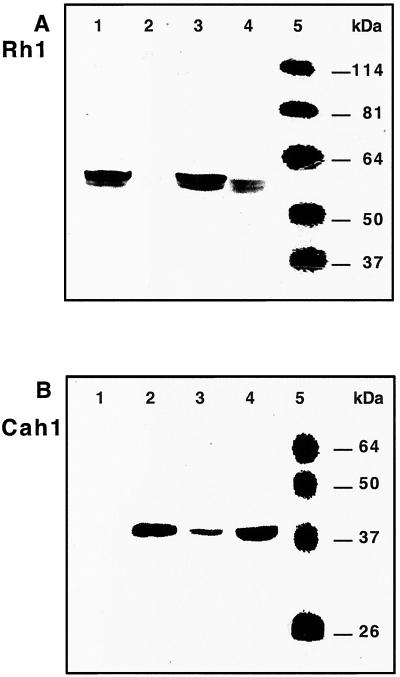
Western analysis of crude cell extracts using an antibody prepared against a unique C-terminal peptide of Rh1 (Fig. 1A; see Materials and Methods) indicated that the Rh1 protein was expressed in cells grown in high CO2 (Fig. 3, lane 1). Levels of Rh1 protein varied in parallel to levels of mRNA (lanes 2–4). The only difference was that the protein disappeared less rapidly than the mRNA on downshift from high CO2 to air (lane 3) and appeared less rapidly on upshift (lane 4). The immunoreactive band identified as Rh1 had an apparent molecular mass of 60 kDa, which was comparable to the predicted mass of 62 kDa. This band was not detected in a control experiment in which the synthetic C-terminal peptide was present as a competitor during antibody binding (data not shown). Additional control experiments indicated that protein levels for the periplasmic carbonic anhydrase Cah1 varied in the opposite direction from those for Rh1 (Fig. 1B). The immuno-reactive band had an apparent molecular mass of ≈40 kDa. The molecular mass of the Cah1 precursor is 41.6 kDa, whereas processed, glycosylated forms of Cah1 have apparent molecular masses of 35–37 kDa (14).
Figure 3.
Western analysis of levels of Rh1 protein (A) and the periplasmic carbonic anhydrase Cah1 (B). Whole cell samples were prepared, subjected to SDS-PAGE (8.5% gel, A, and 12% gel, B) and analyzed as described in Materials and Methods. The nitrocellulose membrane was hybridized with antiserum directed against a unique C-terminal peptide of Rh1 (see Fig. 1) (A) or Cah1 (B). The sera were diluted 1,000-fold. Lane 1, cells grown in high CO2; lane 2, cells grown in air; lane 3, cells shifted from high CO2 to air for 3 h; lane 4, cells shifted from air to high CO2 for 3 h; lane 5, molecular mass standards (Benchmark Prestained, GIBCO/BRL).
Levels of RH1 mRNA and Protein Are Low Under Nitrogen-Limiting Conditions.
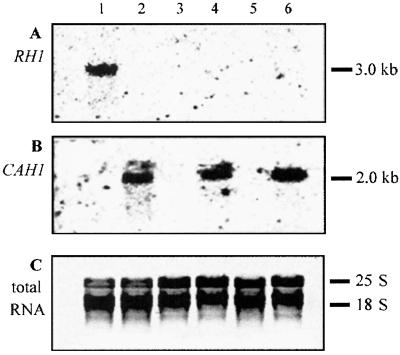
Under nitrogen-limiting conditions, i.e., with arginine as sole nitrogen source instead of NH4Cl, levels of RH1 mRNA and protein were low not only for air-grown cells but for cells grown in high CO2 (Fig. 4A, lanes 4 and 3, respectively, and data not shown). Low expression of RH1 under the latter circumstance may be explained by the fact that Chlamydomonas seldom encounters high CO2 and nitrogen limitation simultaneously and responds as if it were nitrogen-limited (22). As expected based on the initial findings, RH1 mRNA was not detected when cells were shifted from air to high CO2 with arginine as nitrogen source or when they were subjected to the opposite shift (lanes 5 and 6 respectively). Replacing NH4Cl with arginine as nitrogen source did not affect expression of CAH1. With either nitrogen source, levels of CAH1 mRNA were high for cells grown in air or shifted to air (Fig. 4B, lanes 4 and 6, respectively) and low for cells grown in high CO2 or shifted to high CO2 (lanes 3 and 5, respectively).
Figure 4.
Northern analysis of RH1 mRNA levels in nitrogen-limited cells. Five micrograms of total RNA was used for each lane. Membranes were hybridized to probes for RH1 (A) or CAH1 (B). The gel in C was stained with ethidium bromide. Lane 1, cells grown in high CO2 with NH4Cl as nitrogen source (control); lane 2, cells shifted from high CO2 with NH4Cl to air for 3 h (control); lane 3, cells grown in high CO2 with arginine as nitrogen source; lane 4, cells grown in air with arginine; lane 5, cells shifted from air with arginine to high CO2 for 3 h; lane 6, cells shifted from high CO2 with arginine to air for 3 h.
Rh1 Does Not Appear to Substitute for Amt Functionally.
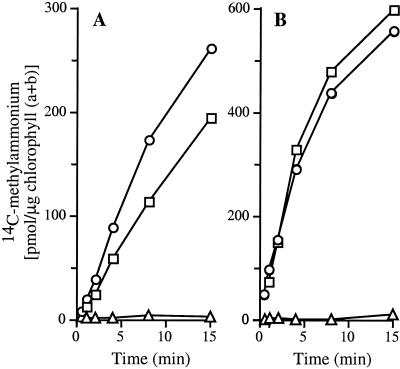
Function of Amt proteins can be detected by uptake of the ammonium analogue [14C]methylammonium (23). C. reinhardtii CC125 could take up [14C]methylammonium (6- to 600-μM external concentrations) only when it was grown under nitrogen-limiting conditions (Fig. 5 and data not shown). [14C]Methylammonium uptake was detected for cells grown with arginine or hypoxanthine as nitrogen source but not for cells grown with NH4Cl. The results were independent of whether cells were grown in air (Fig. 5A) or high CO2 (Fig. 5B). Under conditions in which [14C]methylammonium uptake was high (nitrogen-limiting conditions), RH1 expression was low (Figs. 4 and 5). Conversely, when RH1 expression was high (high CO2 with NH4Cl as nitrogen source), [14C]methylammonium uptake was not detected (Figs. 2, 3, and 5). These results provide no evidence that Rh1 can substitute for Amt in an organism that contains both proteins naturally.
Figure 5.
Uptake of [14C]methylammonium. Cells were grown in air (A) or high CO2 (B). They were grown with NH4Cl (triangles) or arginine (circles),or hypoxanthine (squares) as sole nitrogen source.
Discussion
Regulation of expression of the RH1 gene of C. reinhardtii by CO2 availability provides experimental evidence that Rh proteins, including those of humans, may be biological gas channels for CO2. Such regulation provides no support for the view that Rh proteins are active transporters for NH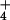 .
.
The hypothesis that the substrate for Rh proteins is CO2 rather than NH3 is in accord with the distribution of Rh proteins among organisms and with the profound differences between their distribution and that of the Amt and MEP proteins (Table 1) (refs. 2, 3, and 24–27; and the National Center for Biotechnology Information blast website, http://www.ncbi.nlm.nih.gov/blast/). Rh proteins appear to be absent from the bacteria and archaea, which may be too small to require them, and are rare in eukaryotic microbes. Within the latter group they occur in the algae, where their physiological role is presumably in acquisition of CO2 for photosynthesis, and in the slime molds, where they may function to modulate internal pH (C.-H.H., unpublished results). Rh proteins appear to be absent in higher plants, which obtain CO2 directly from air. They are found in many animals—vertebrate and invertebrate—and are prominent in mammals. The organ and tissue distribution of Rh proteins in animals (footnote to Table 1) (refs. 25, 28, and 29; and C.-H.H., unpublished data) is in agreement with the view that they function in disposal of CO2 as a waste product and/or in maintaining or establishing the pH of various fluids.
Table 1.
Organismal distribution of Rh and Amt proteins
| Group | Rh | Amt |
|---|---|---|
| Bacteria | – | + |
| Archaea | – | + |
| Microbial eukarya | Rare | + |
| Higher plants | – | + |
| Invertebrates | + | + |
| Vertebrates | + | – |
| Prominent in mammals* | Absent in mammals |
–, not found; +, found (see text) (refs. 2, 3, and 24–27, and the NCBI blast web site (http://www.ncbi.nlm.nih.gov/blast/).
The RhAG and Rh30 proteins of humans and mice are found in erythrocytes. The RhBG protein is found in kidney (convoluted tubules and Henle's loops), skin, liver, and ovary. The RhCG protein is found in kidney (collecting ducts), brain, pancreas, prostate, and testis (seminiferous tubules) (25, 28, 29).
By contrast to Rh proteins, Amt and MEP proteins are widespread among the bacteria and archaea and among eukaryotic microbes and higher plants (4, 25, 30). They occur in invertebrates but appear to be absent in vertebrates, notably so in mammals. As discussed above, regulation of the Rh1 protein of C. reinhardtii is difficult to rationalize with its being an ammonia channel, and preliminary studies of [14C]methylamine uptake provided no evidence for this being the case. Moreover, although the two Rh proteins of C. reinhardtii are 73% identical to one another and the two Amt proteins of this organism (GenBank accession nos. AF479643 and AV394025) are 57% identical to one another, the Rh and Amt proteins of C. reinhardtii share only ≈20% identities.
The carbon concentrating mechanism of C. reinhardtii allows this aquatic organism to carry out rapid photosynthesis despite the fact that diffusion of CO2 in water is 10,000 times slower than in air (31). Although the carbon concentrating mechanism (15–21) has been studied extensively, adaptations of C. reinhardtii to high CO2 availability are less well explored. Recent classification of ESTs indicated that C. reinhardtii expresses almost 2,000 genes more highly under conditions of high CO2 availability than in air (32). RH1 is certainly one of these genes. The Rh1 protein may facilitate rapid equilibration of CO2 when this nutrient is readily available.
Acknowledgments
We thank Sabeeha Merchant and Jannette Quinn for Chlamydomonas DNA and other materials. We thank Christoph Beck, Gerry Fink, and Don Weeks for advice and encouragement, and Bob Buchanan and Jack Meeks for critical reading of the manuscript. This work was supported by National Institutes of Health Grant HL66274 (to C.-H.H.), and National Science Foundation Grant MCB 9874443 and a grant from the Torrey Mesa Research Institute, Syngenta Research and Technology, San Diego (to S.K.).
Abbreviations
- Amt
ammonium and methylammonium transport proteins
- Cah
carbonic anhydrase
- Cr–RH
Chlamydomonas reinhardtii RH
- MEP
methylammonium permease
- Rh
Rhesus
- EST
expressed sequence tag
Footnotes
References
- 1.Anstee D J, Tanner M J. Baillieres Clin Haematol. 1993;6:401–422. doi: 10.1016/s0950-3536(05)80152-0. [DOI] [PubMed] [Google Scholar]
- 2.Huang C-H, Liu P Z, Cheng J G. Semin Hematol. 2000;37:150–165. doi: 10.1016/s0037-1963(00)90040-4. [DOI] [PubMed] [Google Scholar]
- 3.Huang C H, Liu P Z. Blood Cells Mol Dis. 2001;27:90–101. doi: 10.1006/bcmd.2000.0355. [DOI] [PubMed] [Google Scholar]
- 4.Marini A-M, Urrestarazu A, Beauwens R, Andre B. Trends Biochem Sci. 1997;22:460–461. doi: 10.1016/s0968-0004(97)01132-8. [DOI] [PubMed] [Google Scholar]
- 5.Marini A-M, Matassi G, Raynal V, Andre B, Cartron J-P, Cherif-Zahar B. Nat Genet. 2000;26:341–344. doi: 10.1038/81656. [DOI] [PubMed] [Google Scholar]
- 6.Soupene E, He L, Yan D, Kustu S. Proc Natl Acad Sci USA. 1998;95:7030–7034. doi: 10.1073/pnas.95.12.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soupene E, Ramirez R M, Kustu S. Mol Cell Biol. 2001;21:5733–5741. doi: 10.1128/MCB.21.17.5733-5741.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soupene E, Lee H, Kustu S. Proc Natl Acad Sci USA. 2002;99:3926–3931. doi: 10.1073/pnas.062043799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soupene, E., Chu, T., Corbin, R. W., Hunt, D. F. & Kustu, S. (2002) J. Bacteriol., in press. [DOI] [PMC free article] [PubMed]
- 10.Ebersold W T. Am J Bot. 1956;43:408–410. [Google Scholar]
- 11.Harris E H. The Chlamydomonas Sourcebook: A Comprehensive Guide To Biology and Laboratory Use. San Diego: Academic; 1989. [DOI] [PubMed] [Google Scholar]
- 12.Wintermans J F, de Mots A. Biochim Biophys Acta. 1965;109:448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- 13.Gromoff E D V, Treier U, Beck C F. Mol Cell Biol. 1989;9:3911–3918. doi: 10.1128/mcb.9.9.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts C S, Spalding M H. Plant Mol Biol. 1995;29:303–315. doi: 10.1007/BF00043654. [DOI] [PubMed] [Google Scholar]
- 15.Badger M R, Price G D. Annu Rev Plant Physiol Plant Molec Biol. 1994;45:369–392. [Google Scholar]
- 16.Fukuzawa H, Miura K, Ishizaki K, Kucho K I, Saito T, Kohinata T, Ohyama K. Proc Natl Acad Sci USA. 2001;98:5347–5352. doi: 10.1073/pnas.081593498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moroney J V, Somanchi A. Plant Physiol. 1999;119:9–16. doi: 10.1104/pp.119.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pronina N A, Semenenko V E. In: Current Research in Photosynthesis. Baltscheffsky M, editor. Vol. 4. Dordrecht, The Netherlands: Kluwer Academic; 1990. pp. 489–492. [Google Scholar]
- 19.Raven J A. Plant Cell Environ. 1997;20:147–154. [Google Scholar]
- 20.Spalding M H. In: The Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas. Rochaix J-D, Goldschmidt-Clermont M, Merchant S, editors. Vol. 7. Dordrecht, The Netherlands: Kluwer Academic; 1998. pp. 529–547. [Google Scholar]
- 21.Xiang Y, Zhang J, Weeks D P. Proc Natl Acad Sci USA. 2001;98:5341–5346. doi: 10.1073/pnas.101534498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beardall J, Johnston A, Raven J. Can J Bot. 1998;76:1010–1017. [Google Scholar]
- 23.Hackette S L, Skye G E, Burton C, Segel I H. J Biol Chem. 1970;245:4241–4250. [PubMed] [Google Scholar]
- 24.Kitano T, Saitou N. Immunogenetics. 2000;51:856–862. doi: 10.1007/s002510000202. [DOI] [PubMed] [Google Scholar]
- 25.Liu Z, Peng J, Mo R, Hui C-c, Huang C-H. J Biol Chem. 2001;276:1424–1433. doi: 10.1074/jbc.M007528200. [DOI] [PubMed] [Google Scholar]
- 26.Matassi G, Cherif-Zahar B, Pesole G, Raynal V, Cartron J-P. J Mol Evol. 1999;48:151–159. doi: 10.1007/pl00006453. [DOI] [PubMed] [Google Scholar]
- 27.Seack J, Pancer Z, Mueller I M, Mueller W E G. Immunogenetics. 1997;46:493–498. doi: 10.1007/s002510050310. [DOI] [PubMed] [Google Scholar]
- 28.Liu Z, Huang C-H. Biochem Genet. 1999;37:119–138. doi: 10.1023/a:1018726303397. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z, Chen Y, Mo R, Hui C-c, Cheng J-F, Mohandas N, Huang C-H. J Biol Chem. 2000;275:25641–25651. doi: 10.1074/jbc.M003353200. [DOI] [PubMed] [Google Scholar]
- 30.Howitt S M, Udvardi M K. Biochim Biophys Acta. 2000;1465:152–170. doi: 10.1016/s0005-2736(00)00136-x. [DOI] [PubMed] [Google Scholar]
- 31.Lide D R, editor. CRC Handbook of Chemistry and Physics. Boca Raton, FL: CRC Press; 1998–1999. p. 5.94. and 6.179. [Google Scholar]
- 32.Asamizu E, Miura K, Kucho K, Inoue Y, Fukuzawa H, Ohyama K, Nakamura Y, Tabata S. DNA Res. 2000;7:305–307. doi: 10.1093/dnares/7.5.305. [DOI] [PubMed] [Google Scholar]