Abstract
The NS1 protein of influenza A virus contributes to viral pathogenesis, primarily by enabling the virus to disarm the host cell type IFN defense system. We examined the downstream effects of NS1 protein expression during influenza A virus infection on global cellular mRNA levels by measuring expression of over 13,000 cellular genes in response to infection with wild-type and mutant viruses in human lung epithelial cells. Influenza A/PR/8/34 virus infection resulted in a significant induction of genes involved in the IFN pathway. Deletion of the viral NS1 gene increased the number and magnitude of expression of cellular genes implicated in the IFN, NF-κB, and other antiviral pathways. Interestingly, different IFN-induced genes showed different sensitivities to NS1-mediated inhibition of their expression. A recombinant virus with a C-terminal deletion in its NS1 gene induced an intermediate cellular mRNA expression pattern between wild-type and NS1 knockout viruses. Most significantly, a virus containing the 1918 pandemic NS1 gene was more efficient at blocking the expression of IFN-regulated genes than its parental influenza A/WSN/33 virus. Taken together, our results suggest that the cellular response to influenza A virus infection in human lung cells is significantly influenced by the sequence of the NS1 gene, demonstrating the importance of the NS1 protein in regulating the host cell response triggered by virus infection.
Influenza viruses are responsible for an average of 20,000 deaths and 114,000 hospitalizations per year (1). Highly pathogenic strains of influenza A virus have emerged occasionally in recent history, producing pandemics such as the one in 1918, which resulted in the death of 20–40 million people worldwide (2, 3). Although the mechanism of increased pathogenicity has been genetically traced for a few unusually virulent strains [e.g., the PB2 and hemagglutinin genes of the Hong Kong H5N1 viruses appear to contribute to their virulence in mammals (4)], the cause of severe pandemics, such as the one in 1918–1919, and the contribution of individual influenza virus genes to pathogenicity remain largely unknown.
Influenza A virus has a negative-strand RNA genome that encodes on 8 RNA segments 10 or 11 proteins, depending on the strain. Segment 8 encodes an mRNA that is alternatively spliced to express the nonstructural protein-1 (NS1) and the nuclear export protein, NEP (5). The NS1 protein, which binds double-stranded RNA and forms dimers in vivo, has been suggested to perform several important accessory functions for the optimal replication of the virus in its host (5). Importantly, the NS1 protein represses the host cell antiviral response by multiple mechanisms. These mechanisms include the inhibition of the IFN-inducible double-stranded RNA activated kinase PKR (protein kinase RNA-regulated) (6–8) and the blocking of IFN-β production by preventing NF-κB (9), IFN-regulatory factor (IRF) 3 (10), and IRF7 activation (11).
DNA microarray technology is increasingly being used to examine the effects of viral infection on host cell gene expression (12). The advantages of this discovery-based approach is that one can monitor the expression of thousands of genes simultaneously, perhaps identifying coexpressed genes from a single pathway or those that function in multiple pathways. Here, we measured the global pattern of cellular gene expression in cells infected with the wild-type (wt) influenza A/PR/8/34 virus and compared it with those in cells infected with NS1 mutant viruses lacking all or part of the NS1 gene. In addition, we have examined the effect on cell gene expression of a recombinant virus in which the NS gene of influenza A/WSN/33 virus was substituted with the NS gene of the 1918 pandemic influenza A virus. The cellular genes that were differentially regulated by these viruses and the degree of overlap between them were identified by using the resolver Expression Data Analysis System. We conclude from this analysis that NS1 expression is necessary but most likely not sufficient for evasion of the host innate defenses. Finally, we identified multiple cellular pathways and new potential antiviral genes whose expression is influenced by the presence or absence of NS1.
Materials and Methods
Viruses and Cells.
Wild-type influenza A/PR/8/34, delNS1, and NS1 (1–126) viruses were generated, propagated in 7-day-old eggs, and titrated by plaque assay as described (9, 13, 14). Influenza A/WSN/33 virus and 1918 NS WSN recombinant virus were propagated and maintained as described (15). The lung epithelial cell line A549 was purchased from American Type Culture Collection (ATCC, Manassas, VA) and maintained in DMEM containing 10% FBS, 2 mM glutamine, 100 units/ml penicillin, and 100 units/ml streptomycin at 37°C.
Viral Infections, RNA Extraction, and Immunofluorescence Assays.
Infections with wt and recombinant influenza A viruses were performed in A549 cells grown in 10-cm2 dishes or T-150 flasks. In all cases, the multiplicity of infection (moi) was adjusted before each independent experiment to ensure that at least 80% of the cells were infected 8 h postinfection as determined by immunofluorescence using a viral nucleoprotein (NP)-specific antibody. Total RNA extraction and mRNA selection procedure is described (ref. 16; http://ra.microslu.washington.edu/Website/protocol/archive_protocol.html).
Northern Blots and Electrophoretic Mobility-Shift Assay (EMSA) Analysis.
EMSAs were done to determine the activation of NF-κB as described (9), by using nuclear extracts from infected A549 cells and a DNA probe containing a mouse H2 κB binding site. Northern blot analysis of total RNA isolated from A549-infected cells using specific [32P]ATP-labeled probes was performed as previously described (9).
Microarray Conditions and Image Analysis.
Microarrays were constructed by the University of Washington's Center for Expression Array Technology with PCR products generated from the 15,000 set of sequence-verified IMAGE consortium clones (http://ra.microslu.washington.edu/Website/genelist/genelist.html). For microarray conditions, see supplemental text, which is published as supporting information on the PNAS web site, www.pnas.org. In accordance with proposed MIAME (minimum information about a microarray experiment) standards (17), raw data, including sample information, intensity measurements, error analysis, microarray content, and slide hybridization conditions will be made available in the public domain (http://expression.microslu.washington.edu).
Results
Influenza PR8 Virus Lacking the NS1 Gene Induces High Levels of IFN-β mRNA and NF-κB Activation in Human Lung Epithelial Cells.
Recent evidence in cell lines and in mice suggests that the influenza A NS1 protein antagonizes the cellular IFN response (13) in part by blocking NF-κB, IRF3, and IRF7 activation (9–11). We examined whether the same was true in A549 cells, a human lung epithelial cell line that may more accurately represent the infected cells found at the physiological sites of influenza virus infection. Both NF-κB activity (Fig. 1A) and IFN-β mRNA levels (Fig. 1B) increased dramatically by 9 h postinfection with a recombinant influenza A virus lacking the NS1 gene (delNS1 virus). In contrast, the activation of NF-κB and production of IFN-β mRNA were considerably lower in cells infected with wt PR8 virus. We confirmed that expression of IFN and NF-κB regulated genes were induced in delNS1 virus-infected cells by examining a selected set of IFN and/or NF-κB stimulated genes by Northern blot (Fig. 1B). The results demonstrate that the NS1 protein of influenza A virus down-regulates the IFN response in a human lung epithelial cell system and support the hypothesis that this viral protein has an inhibitory effect on IFN and NF-κB pathways.
Fig 1.
Increased NF-κB activation and IFN-β production in delNS1 virus-infected A549 cells results in activation of target genes. (A) Electrophoretic mobility-shift assay analysis of NF-κB binding to its cognate DNA element. An oligonucleotide corresponding to the NF-κB binding site was labeled with 32P and incubated with nuclear extracts from mock-infected or virus-infected A549 cells at 16 h postinfection, as indicated. Cells were also infected with Sendai virus as a positive control of this experiment. The position of the NF-κB/DNA complex is shown. (B) Northern blot analysis of IFN-β and cellular genes with known NF-κB or IFN responsive elements. A β-actin control Northern is also shown. The moi and the postinfection times that were used in these experiments are indicated on the top. The average fold change and P value observed by microarray analysis at 8 h postinfection of repeated experiments is shown on the right. The IFN-β gene was not present on microarrays used (N/A). The asterisk indicates the microarray results for α-actin because the β-actin cDNA was not represented on the array. It should be noted that, in contrast to some other cell lines, such as MDCK cells, no severe cytopathic effect was induced in A549 cells after influenza virus infection.
Induction of Antiviral Gene Expression in Response to Influenza PR8 Virus Infection of Lung Epithelial Cells.
We next examined global cellular gene expression levels in cells infected with viruses containing mutations in the NS1 gene and compared them with those in cells infected with the parental wt PR8 virus. Two recombinant viruses were tested, delNS1 virus (13), and NS1 (1–126) virus lacking the C-terminal 104 aa of the NS1 protein (9). A549 lung epithelial cells in monolayers were infected at an moi resulting in approximately 80% of cell infection (Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org.). We chose this moi to infect most cells with 1–2 virus particles. At 8 h postinfection, total RNA was extracted to be analyzed by microarray. Differentially expressed genes were selected based on ratio and statistical criteria from combined replica experiments. Because approximately 20% of cells are not infected, down-regulated genes in the array represent substantially down-regulated genes in infected cells or genes that are also down-regulated in noninfected neighboring cells. PR8 wt virus infection perturbed the expression of the fewest number of cellular genes (84 genes) whereas delNS1 virus infection resulted in the largest regulation of cellular genes (115 genes). The NS1 (1–126) virus regulated an intermediate number of cellular genes (93 genes). Thus, influenza A virus infection resulted in ≈0.5–1% of all of the cellular genes on the array being differentially regulated. A summary of the number of genes that are significantly regulated by each virus and the degree of overlap between gene sets is shown [Fig. 2; and see Table 1 (which is published as supporting information on the PNAS web site) for data on individual experiments]. Genes that were regulated by each virus can be classified in three primary groups: genes whose expression was similar between wt and mutant NS1 virus infections (Fig. 2, solid black and diagonal hatched), genes whose expression was similar between mutant NS1 viruses but not wt virus infections (Fig. 2, horizontal hatched), and genes whose expression is more significantly regulated by the individual viruses alone (Fig. 2, solid gray).
Fig 2.
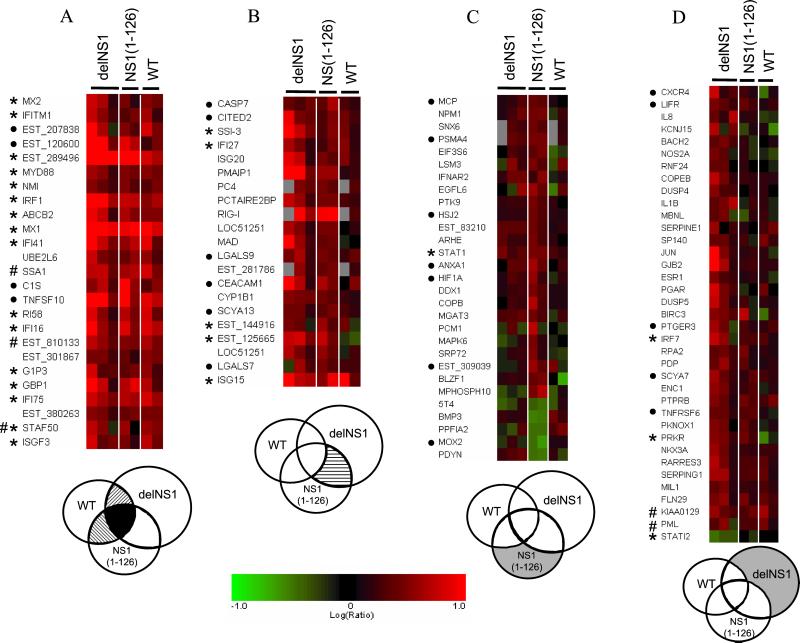
Ven diagram showing the distribution of differentially regulated genes during infection with wt or mutant NS1 influenza PR8 viruses relative to mock-infected cells. Differentially expressed genes were selected from combined data of multiple experiments (see Materials and Methods). For a gene to fall into multiple categories, it had to meet the fold change and P value selection criteria outlined in Materials and Methods (≥2.0) and P value (<0.01). Some differentially regulated genes that are shared between viruses may exhibit different degrees of regulation (see Table 2). Area shaded in black represents the number of genes that met the statistical and fold change criteria for all virus infections. Hatched areas represent the number of genes that met the criteria in two of three viruses, and areas shaded in gray represent the number of genes differentially regulated by a single virus type.
To confirm that induction of NF-κB and IFN-β regulated genes could be detected by microarray, we examined the mRNA levels for the cellular genes tested by Northern blot. The NFKBIA, IL-8, TNFRSF6, and HLA-C genes were all induced at 8 h postinfection with delNS1 virus but not by wt PR8 virus (Fig. 1B and Fig. 7, which is published as supporting information on the PNAS web site). In contrast, the levels of actin-α were unaffected (actin-β not present). It is important to stress that the Cy3/Cy5 ratio obtained by repeated microarray experiments underestimates the relative change in mRNA expression. Thus, a 2-fold change in expression by microarray is most likely a conservative cutoff for a differentially regulated gene, because the levels of the same mRNA measured by Northern blot appear to be more dramatically changed. The discrepancy between Northern and microarray results has previously been observed by using the same array platform (18).
There were 24 genes that were induced more than 2-fold with greater than 99% confidence (P ≤ 0.01) during infection with both wt and mutant PR8 viruses (Fig. 2, solid black, and Fig. 3A). There were 10 more genes whose expression was coregulated by all 3 viruses but with reduced confidence levels (P value > 0.01) in one set of experiments (Fig. 2, diagonal hatched, and Fig. 3A). Sixteen genes in this group were previously known to be stimulated by IFN and 4 more are potential antiviral genes. The IFN-stimulated genes (ISGs) up-regulated by viral infection include the influenza virus inhibitory IFN-induced gene MX1/MXA, in addition to IFI75, IFI41, IRF1, ISGF3-γ/IRF9 and others (Fig. 3A, asterisk). Note that, even though these genes were differentially regulated during infection with both wt and mutant viruses, some genes (e.g., GBP and IRF1) were induced to higher levels in delNS1 virus-infected cells (Table 2, which is published as supporting information on the PNAS web site). Other potential antiviral genes of interest that were consistently induced by PR8 virus infection include TNF-related apoptosis inducing ligand (TRAIL/TNFSF10), complement C1S component, and MYD88. The latter is an adapter molecule that participates in Toll-like receptor signaling (19).
Fig 3.
Summary of cellular genes that are regulated by wt and NS1 mutant PR8 viruses. The cellular genes represented in each panel are as follows: (A) genes regulated by all three viruses; (B) genes regulated by both mutant viruses but not by wt virus; (C) genes regulated in NS1 (1–126) mutant virus-infected cells; and (D) genes regulated in delNS1 virus-infected cells. The subset of genes that each image corresponds to is shaded in the Venn diagrams below each panel. Genes and ESTs that lacked sufficient functional annotation and those enhanced in wt virus are not shown (see Fig. 8, which is published as supporting information on the PNAS web site). Columns represent data obtained for individual replica experiments. Red, Expression was induced in infected cells relative to mock; green, expression was repressed in infected cells relative to mock; black, expression was not changed; and gray, gene was not present on the array. The shade of red or green represents the degree of change. The scale represents log10 ratios and is the same for all four images. *, IFN-stimulated gene; •, potential antiviral gene; #, tripartite motif-containing protein.
To determine whether the sequence of differentially regulated expressed sequence tags (ESTs) aligned with any known or predicted gene, a blast search of the human genome database was performed on the available sequences for each uncharacterized clone. Three ESTs aligned with regions containing known or predicted genes with potential antiviral activity (Fig. 9, which is published as supporting information on the PNAS web site). These ESTs align with an exon of cig5 similar to inflammatory response protein 6 (IMAGE ID 120600), an exon of LOC129607 which shares similarity with thymidylate kinase (IMAGE ID 207838), and the 3′ noncoding region of LOC143274 that is similar to IFIT2/ISG54 (IMAGE ID 289496).
Mutations in the NS1 Gene Lead to Enhanced Antiviral Gene Expression of IFN, Cytokine, and NF-κB-Regulated Genes During Influenza Virus Infection.
We would predict from biochemical analysis in cell culture (Fig. 1B and refs. 9 and 10) and phenotypic analysis in mice (13, 14) that the mutant NS1 viruses would induce a more pronounced antiviral response involving genes regulated by IFN and/or NF-κB. Indeed, delNS1 and NS1 (1–126) viruses induce a generally higher increase in ISGs and in NF-κB mediated gene expression than wt virus [Fig. 3A, Fig. 10 (which is published as supporting information on the PNAS web site), and Table 2]. In addition, both mutant viruses induce the expression of an additional 24 genes (Fig. 3B), 5 of which were known to be associated with the IFN pathway (including 2 ESTs revealed by blast; see Fig. 9) and 6 were potential antiviral genes based on available annotation. Furthermore, if one also considers the ISGs that are not exclusively coregulated by both mutants but enhanced during either NS1 (1–126) (1 ISG and 7 genes with potential antiviral activity, Fig. 3C) or delNS1 virus (3 ISGs and 5 genes with potential antiviral activity, Fig. 3D) infection, it is evident that the antiviral response, including IFN and NF-κB regulated genes, is more robust in the absence of the NS1 protein (Fig. 3 and Table 2). Interestingly, two genes that are induced to high levels in delNS1 virus-infected cells (PMAIP1 and GBP1) also appear to be induced by overexpression of a constitutively active form of IRF3 (20). The higher activation of IRF3 observed during infection with delNS1 virus as compared with wt PR8 virus (10) may explain these results.
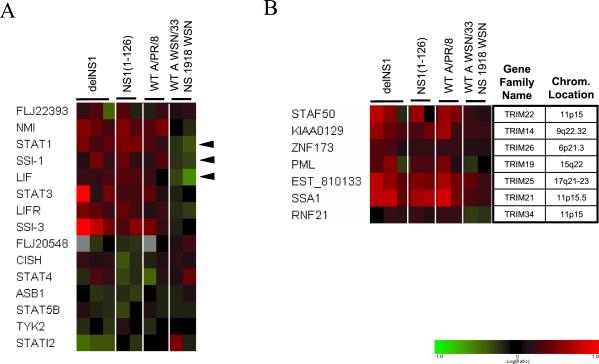
Collectively, the mutant NS1 viruses appear to have a significant impact on genes in the signal transducer and activator of transcription (STAT)-signaling pathway. For instance, the expression levels of STAT1 (Fig. 3D) and STAT3 (Table 2) were greater in cells infected with NS1 mutant viruses relative to wt A/PR/8/34 virus. In addition, the expression of two members of the suppressor of cytokine signaling (SOCS) family of proteins, STATI2/SOCS2 and SSI-3/SOCS3, whose transcription is regulated by STAT proteins (21–23), was affected in cells infected with delNS1 virus relative to wt (Table 2 and Fig. 4A). These proteins function to modulate cytokine and growth factor signaling in a negative feedback loop. Interestingly, SOCS2/STATI2 is down-regulated in delNS1 virus-infected cells (Fig. 3D), whereas SSI-3/SOCS3 is up-regulated (Fig. 3B), suggesting that the two genes may have opposing functions in modulating cytokine response, at least during influenza virus infection. The expression of another SOCS family member (SOCS1/SSI-1, Fig. 4A) and of the N-Myc interactor (NMI) (Table 2), which has also been shown to interact with STAT proteins (24), was also increased by PR8 virus infection. We examined expression of all SOCS-box-containing proteins, along with its associated cytokines and receptors that were present on the microarray (Fig. 4A). We found that, in addition to the STAT and SOCS genes discussed above, the leukemia inhibitory factor (LIF) and its receptor (LIFR), whose signaling is modulated by SOCS proteins (23, 25), were induced in PR8 virus-infected cells. The SOCS genes modulate cytokine response via different mechanisms. SOCS1/SSI-1 binds directly to Janus kinases (JAKs) and inhibits their catalytic activity (26), SOCS2/SSI-2/CIS binds to the cytoplasmic domain of cytokine receptors (27), and SOCS3/SSI-3 functions via binding to receptors in which JAKs are already bound (28).
Fig 4.
Pathways and gene families regulated by PR8 virus infection. (A) Relative mRNA levels of suppressor of cytokine gene family and associated genes during PR8, WSN, and NS 1918 virus infections. Colors represent relative change in mRNA levels relative to mock-infected cells. See legend for Fig. 3 for details on color scheme. Genes whose expression was up-regulated by PR8 virus but down-regulated by 1918 NS virus (and also by wt WSN virus, although to a lesser extent) are with solid arrow. (B) Relative mRNA levels of TRIM proteins and their corresponding chromosomal locations. Hierarchical clustering of 19 TRIM genes was performed (Fig. 12), and the cluster representing induced genes is shown. EST_810133 was identified as a TRIM gene by a blast search of the human genome. Chromosomal locations were obtained from the LocusLink database (38).
Other intriguing evidence that the STAT pathway may be regulated during influenza virus infection is an increase in dual-specificity phosphatases, DUSP4 and DUSP5, mRNAs during delNS1 virus infection (Fig. 3D). DUSP6 mRNA was also increased but its P value was 0.02 (Fig. 11, which is published as supporting information on the PNAS web site). Although the human DUSP genes, which are homologous to vaccinia virus H1 phosphatase (VH1), are known to primarily act on mitogen-activated protein (MAP) kinases [such as extracellular signal-regulated kinase 1 (ERK1)], the viral homolog VH1 has been shown to dephosphorylate and inhibit STAT proteins (29). The mRNA for several members of the DUSP gene family (e.g., DUSP1, -2, and -5) were also found to be induced in other large-scale gene expression studies of both bacterial (30) and viral infections, including influenza virus infection of dendritic cells (31). Another gene critical to IFN signaling that may also be involved in STAT signaling is the IFN-inducible gene PKR. Levels of PKR mRNA increased in cells infected with NS1 mutants relative to wt virus. We presume that increased levels of type I IFN (Fig. 1B) in the absence of NS1 result in increased PKR mRNA levels. Finally, PKR itself has been suggested to be involved in the serine phosphorylation of STAT1 (32) and therefore may also contribute to the overall regulation of STAT signaling.
Proteins containing tripartite motifs are a growing family of known and predicted proteins that have been proposed to function by forming specific subcellular compartments, for example, promyelocytic leukemia (PML) nuclear bodies (33). These genes are predicted to contain, a RING finger (3 zinc binding domains), a B-box (type 1 and/or 2), and a coiled-coil region. Tripartite motif (TRIM) genes regulated during influenza virus infections are shown in Fig. 4B (see also Fig. 12, which is published as supporting information on the PNAS web site). We propose that this set of TRIM genes may represent a new class of antiviral proteins that are coordinately regulated.
The NS Gene from Pandemic 1918 Influenza Virus Is More Effective than the NS Gene from WSN Virus at Blocking Expression of ISGs in Human Lung Cells.
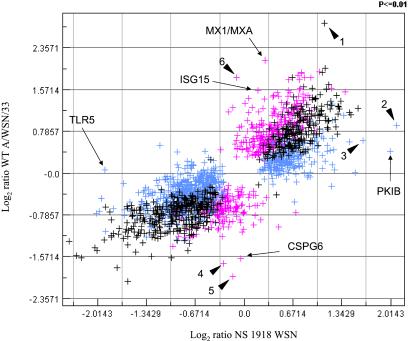
To further explore the potential contribution of the NS1 protein in influenza virus virulence, we used a recombinant WSN virus containing the NS gene from the 1918 pandemic strain (15). Because this recombinant virus was generated in the influenza A/WSN/33 virus strain background, it was also necessary to measure the effect of WSN virus infection on host cell gene expression in A549 lung cells so that a direct comparison could be made. Introduction of the 1918 NS sequence into the WSN background resulted in an overall expression pattern that was very similar to the parental WSN virus (Fig. 5). However, differences were observed for specific ISGs. Although both WSN wt and 1918 NS recombinant viruses appeared to induce lower levels of expression of IFN-regulated genes than the PR8 wt virus (Fig. 13, which is published as supporting information on the PNAS web site), the wt WSN virus did induce expression of some ISGs to high levels [MXA/MX1, ISG15, IFITM1, and ISGF3-g (IRF9)]. In contrast, infection with 1918 NS WSN virus failed to significantly induce any ISG (Figs. 5 and 13). We also noted that NS 1918 virus (and to a lesser extent wt WSN virus) had the opposite effect of PR8 virus infection on the expression of SSI-1/SOCS1, STAT1, and leukemia inhibitory factor (Fig. 4A), as well as of some NF-κB-regulated genes (e.g., SAA1 and BIRC2 and -3, Fig. 10). The compare plot in Fig. 5 also reveals that several cellular genes, in addition to ISGs, are differentially expressed between cells infected with wt or 1918 NS WSN viruses. For example, protein kinase inhibitor-β (PKIB) and Toll-like receptor 5 (TLR5) were up- and down-regulated, respectively, by 1918 NS virus but not by wt WSN. Conversely, CSPG6 is down-regulated during infection with wt WSN virus but not by 1918 NS WSN. We also found several ESTs that exhibit different expression levels between the two viruses. These results emphasize the advantage of combining recombinant virus and microarray technology to identify a few candidate genes for future study. Whether these differences in gene expression are partially responsible for the high virulence associated with the 1918 virus remains to be investigated.
Fig 5.
Comparison of expression ratios for cellular genes during infection with wt influenza A/WSN/33 and NS/1918 viruses vs. mock-infected cells. Scatter plot of log2 ratios for genes that exhibited P values ≤0.01 in at least one of the two infections. Blue, Genes that were significantly up- or down-regulated during infection with 1918 NS recombinant virus; magenta, genes that were significantly up- or down-regulated during infection with wt A/WSN/33 virus; and black, genes that had P values ≤0.01 during both infections. Examples of genes with known cellular function and whose expression differs considerably between the two viruses are indicated by arrows and Human Genome Organization (HUGO) names. ESTs that exhibited differences in gene expression between WSN and NS 1918 virus-infected cells are indicated with solid arrow heads: 1, EST_134682; 2, EST_32376 (LOC51103); 3, EST_427797; 4, EST_207649 (KIAA1272); 5, EST_283495; 6, EST_795255.
Discussion
We have shown that the influenza A virus NS1 gene has a significant impact on host cell gene expression in human lung epithelial cells. These data confirm that the NS1 protein plays a central role in inhibiting IFN, cytokine, and NF-κB pathways. Although we cannot rule out that cytokines, especially IFN, present in the egg-grown virus preparations played some role in the observed differences, chicken IFN is unlikely to signal in human cells. Interestingly, the expression of some ISGs was not affected by the presence of the NS1 gene, whereas the induction of other ISGs was reduced in wt PR8 virus-infected cells. A mutant virus containing a truncated version of the NS1 gene, lacking the C-terminal domain, exhibited an intermediate phenotype, suggesting that this region plays a significant role in NS1 function. In a different study, we have found that the C-terminal NS1 region plays mainly a structural role on NS1 function by enhancing its dimerization (X.W. and A.G.-S., unpublished results). The differential cellular response after infection with wt and mutant NS1 influenza viruses correlates well with their known virulence in mice. For instance, wt virus grows to high titers in lungs of infected mice that eventually die from infection. In contrast, delNS1 virus is attenuated in wt mice, but is lethal in mice with deficient IFN signaling (13). This finding suggests that the inability of the virus to block the initial cellular antiviral response allows the immune system to rapidly clear the delNS1 virus infection. The NS1 (1–126) virus, again, exhibits an intermediate phenotype in mice (unpublished observations).
Our experimental approach allowed us to identify genes associated with antiviral pathways. Multiple members of the SOCS and TRIM gene families are coregulated during PR8 virus infection (Fig. 4), and several genes associated with apoptosis are influenced by NS1 protein expression (Fig. 14, which is published as supporting information on the PNAS web site). blast searches against the draft human genome with EST sequences are consistent with some of these ESTs representing exons, alternatively spliced exons, or untranslated regions of expressed mRNAs (Fig. 9). However, some other ESTs appear to align with DNA sequences outside areas corresponding to predicted mRNAs (Fig. 9). In this context, two recent large-scale expression studies (34, 35) found that many cytosolic RNA species hybridize to regions outside known or predicted exon regions. We have also observed that some influenza virus coregulated genes are located in close proximity in chromosomal DNA, e.g., RI58, MPHOSPH, IFIT1, LOC143274 on chromosome 10, and TRIMs 22, 21, and 34 on chromosome 11 (Fig. 15, which is published as supporting information on the PNAS web site).
Large-scale gene expression studies are increasingly being used to compare the host cell response to infection with different pathogens. Remarkably, a comparison between host cell genes that are regulated by influenza PR8 virus infection in epithelial lung cells (this manuscript) and those regulated by a variety of pathogens (including PR8 virus, Escherichia coli, and Candida albicans) in dendritic cells (31) revealed that 27 cellular genes not only were regulated by PR8 virus infections in both cell types but also were regulated by E. coli and C. albicans in dendritic cells. This finding suggests that these genes, which consist largely of IFN-inducible genes as well as DUSP and TRIM family members, represent the core of the innate cellular immune response that is conserved in multiple cell types. In contrast, some genes do not exhibit similar trends in gene expression after PR8 virus infection in dendritic and lung cells, which presumably reflects the specialized functions of these two cell types (or other parameters that differ in the two experiments). However, because influenza virus infection is known to decrease the levels of cellular protein translation, future experiments are planned to assess the combined effects of cellular RNA changes with translational attenuation in the global pattern of protein expression in influenza virus-infected cells.
In this study, we have also explored the possibility of using microarray analysis to examine the role of the 1918 NS gene in virulence. A virus containing the NS gene from the 1918 pandemic influenza virus was more effective at inhibiting a subset of ISGs in human lung epithelial cells than the parental WSN virus strain, even though the overall cellular response was similar. These differences were most likely due to their slightly different NS1 sequences, although we cannot formally rule out the possibility that the NEP protein, whose coding sequence partially overlaps with the NS1 gene, also plays a role. Although it is tempting to speculate that the negative effect of 1918 NS on ISG expression may be responsible for its unusually high pathogenesis in humans, additional experiments will be required to assess the contributions of the NS1 and other 1918 viral proteins.
Significant differences in the IFN response to influenza WSN and PR8 infections in A549 cells were observed (Fig. 13). Although it is possible that this difference is due to their NS1 genes, which differ by 7 aa (see Fig. 16, which is published as supporting information on the PNAS web site), it is likely that additional influenza virus genes contribute to the WSN virus-mediated block of the antiviral response. Because the cellular antiviral response depends on a balance between negative and positive regulators of gene expression, it is possible that positive viral regulators are more effective in wt PR8 than in WSN virus infection. For example, PR8 virus infections may generate more double-stranded RNA than WSN virus infections. Interestingly, both viral strains represent examples of human viruses that were adapted to mice. PR8 virus, as a result of mouse adaptation, became highly attenuated in humans (36), which is consistent with the cellular gene expression pattern induced on infection of human lung cells. As of this moment, the virulence of WSN virus in humans is unknown. It will also be interesting to compare the cellular gene expression patterns induced on infection with influenza A, B, and C viruses. Although these three types of influenza viruses have common strategies of replication, influenza C viruses are believed to cause mild or asymptomatic infections in humans, and influenza B viruses have developed specific strategies to counteract the activity of some IFN-inducible genes, such as ISG15 (37).
In summary, the combination of gene profiling analysis of infected human lung epithelial cells with reverse genetics techniques has given new insights into the cell antiviral response against influenza virus and its regulation, and on the role of the NS1 gene in preventing this response. In addition, clear differences were found in cellular gene expression induced by different influenza virus strains. These differences may have a significant role in virulence. The generation of databases allowing the comparison of different viruses and viral strains with respect to their impact on cellular gene expression is likely to help to rapidly predict the pathogenicity of new or past virus strains by using high throughput gene profiling techniques.
Supplementary Material
Acknowledgments
We acknowledge Estanislao Nistal-Villan and Zhihong Wang for their excellent technical assistance, Ted Holzman and Bart Kwieciszewski for their bioinformatic support, and Jeff Furlong for developing Expression Array Management Database. We thank the University of Washington Center for Expression Arrays for providing microarray services. This work was funded in part by National Institutes of Health grants (to M.G.K., P.P., and A.G.-S.). T.M.T. was supported by U.S. Department of Agriculture, Agricultural Research Service, Current Research Information System (CRIS) Project 6612-32000-022-93.
Abbreviations
ISG, IFN-stimulated gene
moi, multiplicity of infection
wt, wild type
IRF, IFN-regulatory factor
EST, expressed sequence tag
STAT, signal transducer and activator of transcription
DUSP, dual-specificity phosphatase
PKR, protein kinase RNA-regulated
NS1, nonstructural protein-1
TRIM, tripartite motif
SOCS, suppressor of cytokine signaling
References
- 1.Simonsen L., Clarke, M. J., Williamson, G. D., Stroup, D. F., Arden, N. H. & Schonberger, L. B. (1997) Am. J. Public Health 87, 1944-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Webster R. G. (1999) Proc. Natl. Acad. Sci. USA 96, 1164-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taubenberger J. K., Reid, A. H. & Fanning, T. G. (2000) Virology 274, 241-245. [DOI] [PubMed] [Google Scholar]
- 4.Hatta M., Gao, P., Halfmann, P. & Kawaoka, Y. (2001) Science 293, 1840-1842. [DOI] [PubMed] [Google Scholar]
- 5.Lamb R. A. & Krug, R. M. (2001) in Virology, eds. Knipe, D. M. & Howley, P. M. (Lippincott, Philadelphia), pp. 1487–1531.
- 6.Tan S. L. & Katze, M. G. (1998) J. Interferon Cytokine Res. 18, 757-766. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann M., García-Sastre, A., Carnero, E., Pehamberger, H., Wolff, K., Palese, P. & Muster, T. (2000) J. Virol. 74, 6203-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatada E., Saito, S. & Fukuda, R. (1999) J. Virol. 73, 2425-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X., Li, M., Zheng, H., Muster, T., Palese, P., Beg, A. A. & García-Sastre, A. (2000) J. Virol. 74, 11566-11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talon J., Horvath, C. M., Polley, R., Basler, C. F., Muster, T., Palese, P. & García-Sastre, A. (2000) J. Virol. 74, 7989-7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith E. J., Marie, I., Prakash, A., García-Sastre, A. & Levy, D. E. (2001) J. Biol. Chem. 276, 8951-8957. [DOI] [PubMed] [Google Scholar]
- 12.Fruh K., Simmen, K., Luukkonen, B. G., Bell, Y. C. & Ghazal, P. (2001) Drug Discov. Today 6, 621-627. [DOI] [PubMed] [Google Scholar]
- 13.García-Sastre A., Egorov, A., Matassov, D., Brandt, S., Levy, D. E., Durbin, J. E., Palese, P. & Muster, T. (1998) Virology 252, 324-330. [DOI] [PubMed] [Google Scholar]
- 14.Talon J., Salvatore, M., O'Neill, R. E., Nakaya, Y., Zheng, H., Muster, T., García-Sastre, A. & Palese, P. (2000) Proc. Natl. Acad. Sci. USA 97, 4309-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basler C. F., Reid, A. H., Dybing, J. K., Janczewski, T. A., Fanning, T. G., Zheng, H., Salvatore, M., Perdue, M. L., Swayne, D. E., García-Sastre, A., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 2746-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geiss G. K., An, M. C., Bumgarner, R. E., Hammersmark, E., Cunningham, D. & Katze, M. G. (2001) J. Virol. 75, 4321-4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brazma A., Hingamp, P., Quackenbush, J., Sherlock, G., Spellman, P., Stoeckert, C., Aach, J., Ansorge, W., Ball, C. A., Causton, H. C., et al. (2001) Nat. Genet. 29, 365-371. [DOI] [PubMed] [Google Scholar]
- 18.Geiss G. K., Bumgarner, R. E., An, M. C., Agy, M. B., van't Wout, A. B., Hammersmark, E., Carter, V. S., Upchurch, D., Mullins, J. I. & Katze, M. G. (2000) Virology 266, 8-16. [DOI] [PubMed] [Google Scholar]
- 19.Alexopoulou L., Holt, A. C., Medzhitov, R. & Flavell, R. A. (2001) Nature (London) 413, 732-738. [DOI] [PubMed] [Google Scholar]
- 20.Grandvaux N., Servant, M. J., TenOever, B., Sen, G. C., Balachandran, S., Barber, G. N., Lin, R. & Hiscott, J. (2002) J. Virol. 76, 5532-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito H., Morita, Y., Fujimoto, M., Narazaki, M., Naka, T. & Kishimoto, T. (2000) J. Immunol. 164, 5833-5843. [DOI] [PubMed] [Google Scholar]
- 22.Auernhammer C. J., Bousquet, C. & Melmed, S. (1999) Proc. Natl. Acad. Sci. USA 96, 6964-6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minamoto S., Ikegame, K., Ueno, K., Narazaki, M., Naka, T., Yamamoto, H., Matsumoto, T., Saito, H., Hosoe, S. & Kishimoto, T. (1997) Biochem. Biophys. Res. Commun. 237, 79-83. [DOI] [PubMed] [Google Scholar]
- 24.Zhu M., John, S., Berg, M. & Leonard, W. J. (1999) Cell 96, 121-130. [DOI] [PubMed] [Google Scholar]
- 25.Nicholson S. E., Willson, T. A., Farley, A., Starr, R., Zhang, J. G., Baca, M., Alexander, W. S., Metcalf, D., Hilton, D. J. & Nicola, N. A. (1999) EMBO J. 18, 375-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yasukawa H., Misawa, H., Sakamoto, H., Masuhara, M., Sasaki, A., Wakioka, T., Ohtsuka, S., Imaizumi, T., Matsuda, T., Ihle, J. N. & Yoshimura, A. (1999) EMBO J. 18, 1309-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ram P. A. & Waxman, D. J. (2000) J. Biol. Chem. 275, 39487-39496. [DOI] [PubMed] [Google Scholar]
- 28.Hansen J. A., Lindberg, K., Hilton, D. J., Nielsen, J. H. & Billestrup, N. (1999) Mol. Endocrinol. 13, 1832-1843. [DOI] [PubMed] [Google Scholar]
- 29.Najarro P., Traktman, P. & Lewis, J. A. (2001) J. Virol. 75, 3185-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nau G. J., Richmond, J. F., Schlesinger, A., Jennings, E. G., Lander, E. S. & Young, R. A. (2002) Proc. Natl. Acad. Sci. USA 99, 1503-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Q., Liu, D., Majewski, P., Schulte, L. C., Korn, J. M., Young, R. A., Lander, E. S. & Hacohen, N. (2001) Science 294, 870-875. [DOI] [PubMed] [Google Scholar]
- 32.Ramana C. V., Grammatikakis, N., Chernov, M., Nguyen, H., Goh, K. C., Williams, B. R. & Stark, G. R. (2000) EMBO J. 19, 263-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reymond A., Meroni, G., Fantozzi, A., Merla, G., Cairo, S., Luzi, L., Riganelli, D., Zanaria, E., Messali, S., Cainarca, S., et al. (2001) EMBO J. 20, 2140-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saha S., Sparks, A. B., Rago, C., Akmaev, V., Wang, C. J., Vogelstein, B., Kinzler, K. W. & Velculescu, V. E. (2002) Nat. Biotechnol. 20, 508-512. [DOI] [PubMed] [Google Scholar]
- 35.Kapranov P., Cawley, S. E., Drenkow, J., Bekiranov, S., Strausberg, R. L., Fodor, S. P. & Gingeras, T. R. (2002) Science 296, 916-919. [DOI] [PubMed] [Google Scholar]
- 36.Beare A. S., Schild, G. C. & Craig, J. W. (1975) Lancet 2, 729-732. [DOI] [PubMed] [Google Scholar]
- 37.Yuan W. & Krug, R. M. (2001) EMBO J. 20, 362-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pruitt K. D., Katz, K. S., Sicotte, H. & Maglott, D. R. (2000) Trends Genet. 16, 44-47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.