Abstract
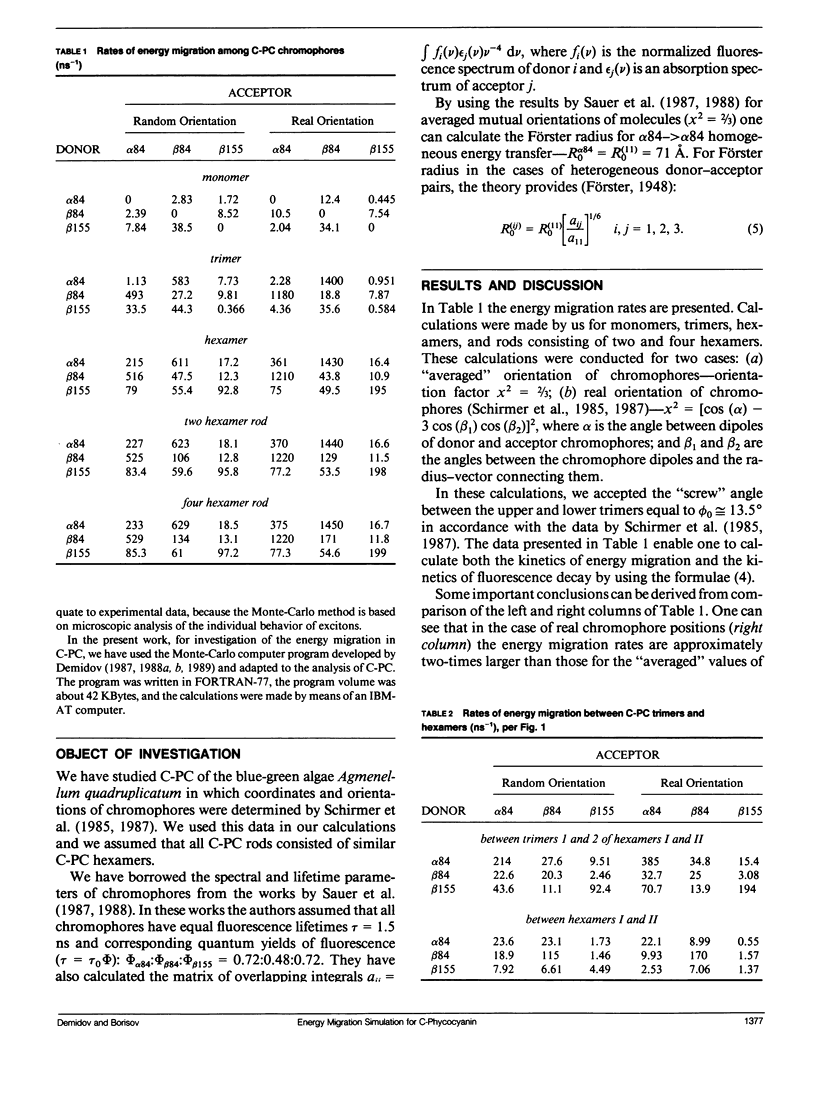
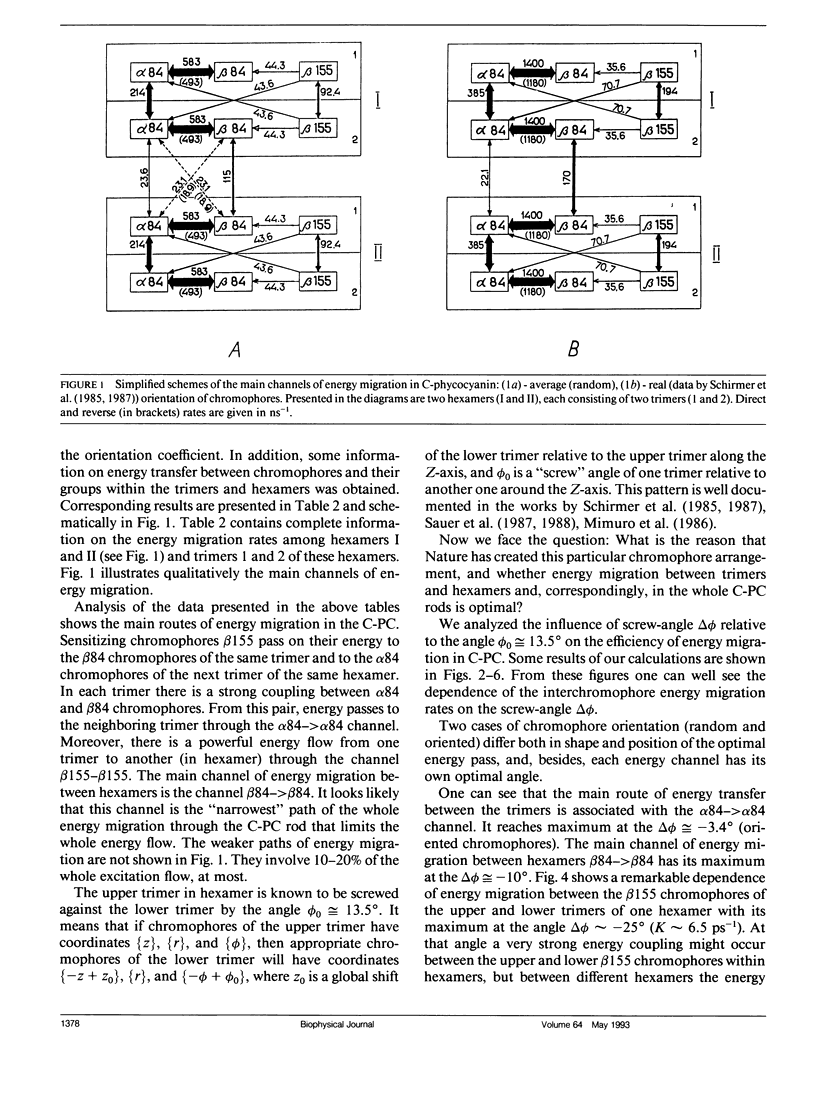
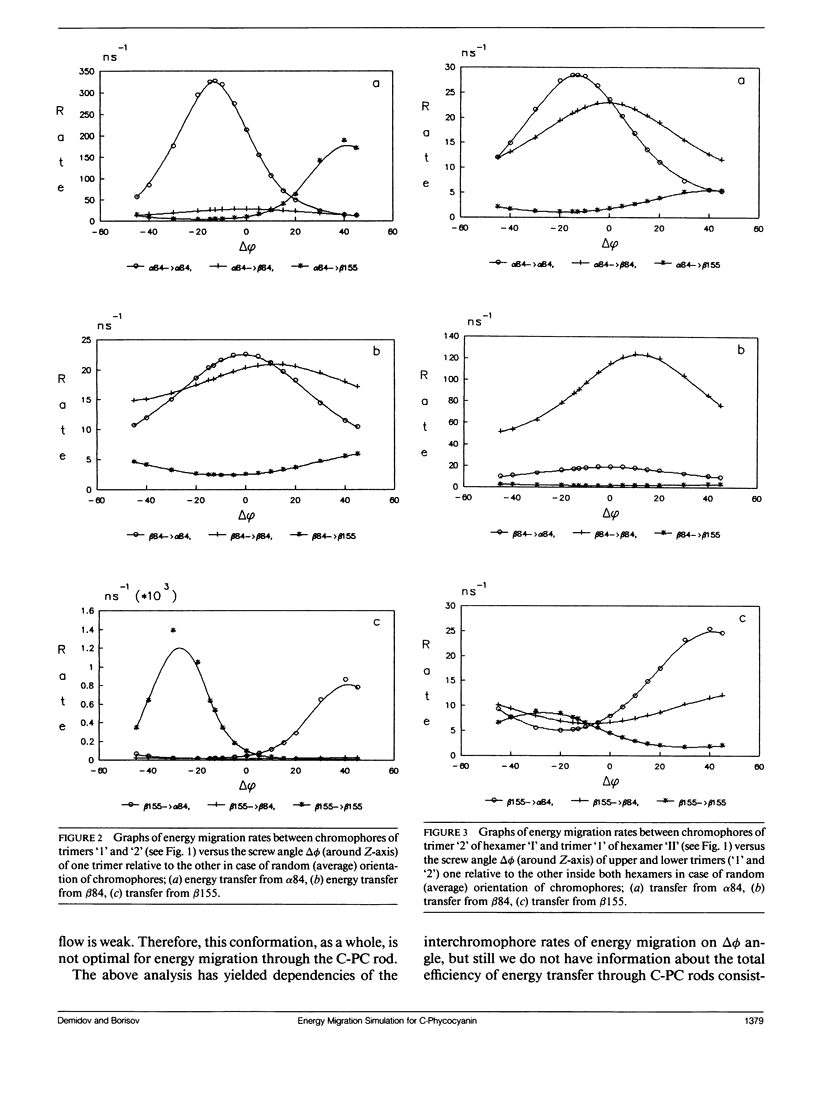
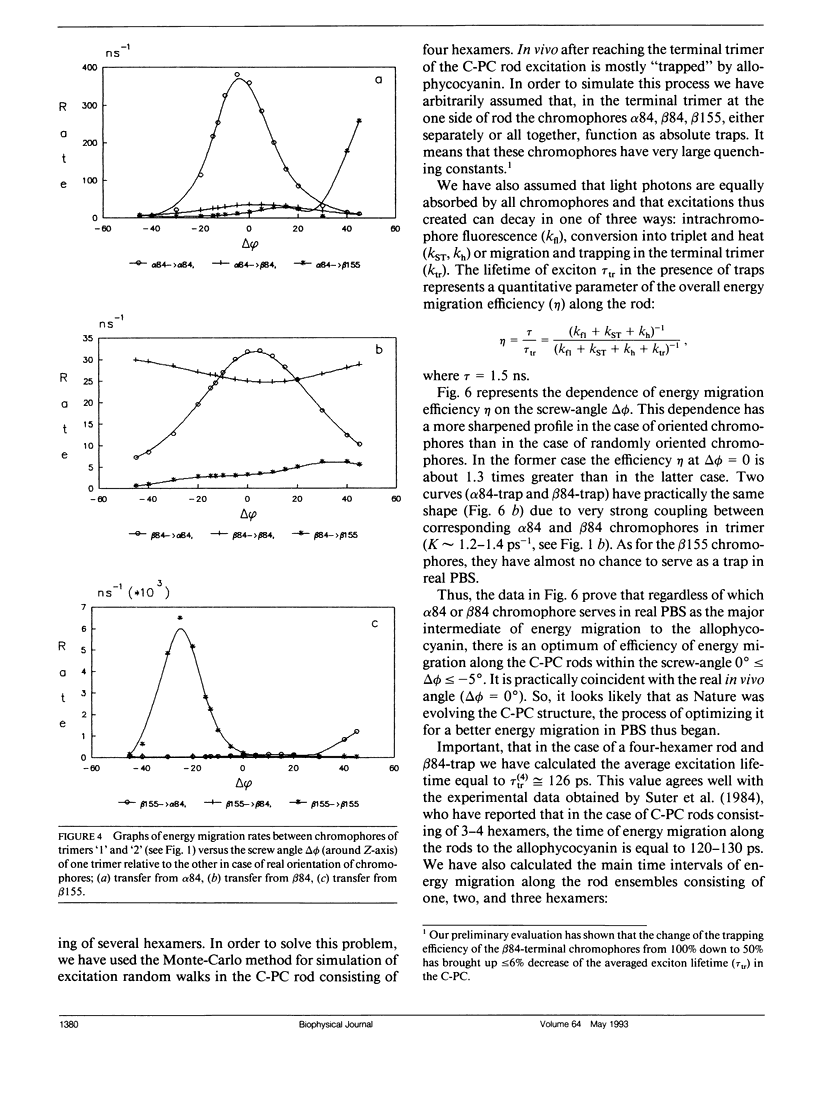
Two methods for simulation of energy migration in the C-phycocyanin fragments of PBS were developed. Both methods are based on the statistical analysis of an excitation behavior in modeling complexes with a limited number (up to hundreds) of chromophores using the Monte-Carlo approach and calculation of migration rates for the system of linear balance equations. Energy migration rates were calculated in the case of C-phycocyanin of the blue-green algae Agmenellum quadruplicatum. The main channels of energy migration were determined in a monomer, trimer, hexamer, and in the rods consisting of 2-4 hexamers. The influence of the “screw” angle between two adjoining trimers of hexamer on the rates of energy migration and on its efficiencies in 1-4 hexamers was also estimated. The analysis was made for the average (random) and real orientation of chromophores in the C-phycocyanin. For both cases the optimal angle values were determined and the one for real C-phycocyanin structure was found to be very close (Δø ≤ 5°) to the optimal angle calculated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dale R. E., Teale F. W. Number and distribution of chromophore types in native phycobiliproteins. Photochem Photobiol. 1970 Aug;12(2):99–117. doi: 10.1111/j.1751-1097.1970.tb06044.x. [DOI] [PubMed] [Google Scholar]
- Fisher R. G., Woods N. E., Fuchs H. E., Sweet R. M. Three-dimensional structures of C-phycocyanin and B-phycoerythrin at 5-A resolution. J Biol Chem. 1980 Jun 10;255(11):5082–5089. [PubMed] [Google Scholar]
- Glazer A. N., Fang S., Brown D. M. Spectroscopic properties of C-phycocyanin and of its alpha and beta subunits. J Biol Chem. 1973 Aug 25;248(16):5679–5685. [PubMed] [Google Scholar]
- Hackert M. L., Abad-Zapatero C., Stevens S. E., Jr, Fox J. L. Crystallization of C-phycocyanin from the marine blue-green alga Agmenellum quadruplicatum. J Mol Biol. 1977 Apr 15;111(3):365–369. doi: 10.1016/s0022-2836(77)80058-2. [DOI] [PubMed] [Google Scholar]
- Holzwarth A. R., Wendler J., Suter G. W. Studies on Chromophore Coupling in Isolated Phycobiliproteins: II. Picosecond Energy Transfer Kinetics and Time-Resolved Fluorescence Spectra of C-Phycocyanin from Synechococcus 6301 as a Function of the Aggregation State. Biophys J. 1987 Jan;51(1):1–12. doi: 10.1016/S0006-3495(87)83306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Degenkolb E. O., Bersohn R., Rentzepis P. M., MacColl R., Berns D. S. Energy transfer among the chromophores in phycocyanins measured by picosecond kinetics. Biochemistry. 1979 Nov 13;18(23):5073–5078. doi: 10.1021/bi00590a008. [DOI] [PubMed] [Google Scholar]
- Porter G., Tredwell C. J., Searle G. F., Barber J. Picosecond time-resolved energy transfer in Porphyridium cruentum. Part I. In the intact alga. Biochim Biophys Acta. 1978 Feb 9;501(2):232–245. doi: 10.1016/0005-2728(78)90029-4. [DOI] [PubMed] [Google Scholar]
- Scheer J. K. Effect of placement in the order of competition on scores of Nebraska high school students. Res Q. 1973 Mar;44(1):79–85. [PubMed] [Google Scholar]
- Schirmer T., Bode W., Huber R. Refined three-dimensional structures of two cyanobacterial C-phycocyanins at 2.1 and 2.5 A resolution. A common principle of phycobilin-protein interaction. J Mol Biol. 1987 Aug 5;196(3):677–695. doi: 10.1016/0022-2836(87)90040-4. [DOI] [PubMed] [Google Scholar]
- Schirmer T., Bode W., Huber R., Sidler W., Zuber H. X-ray crystallographic structure of the light-harvesting biliprotein C-phycocyanin from the thermophilic cyanobacterium Mastigocladus laminosus and its resemblance to globin structures. J Mol Biol. 1985 Jul 20;184(2):257–277. doi: 10.1016/0022-2836(85)90379-1. [DOI] [PubMed] [Google Scholar]
- Stryer L., Haugland R. P. Energy transfer: a spectroscopic ruler. Proc Natl Acad Sci U S A. 1967 Aug;58(2):719–726. doi: 10.1073/pnas.58.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]


