Abstract
Human granulocytic ehrlichiosis (HGE) is an emerging tick-borne zoonosis caused by a strain of Anaplasma phagocytophila called the HGE agent, an obligatory intracellular bacterium. The agent expresses immunodominant 44-kDa outer membrane proteins (P44s) encoded by a multigene family. The present study established an experimental process for transmission of the HGE agent from infected mice (a reservoir model) to nymphal Ixodes scapularis ticks (a biological vector) and subsequently to horses (a patient model) by the adult infected ticks. Overall, a total of 20 different p44 transcripts were detected in the mammals, ticks, and cell cultures. Among them, a transcript from a p44-18 gene was major at acute stage in mice and horses but minor in ticks. Both mRNA and protein produced from the p44-18 gene were detected in the HGE agent cultivated in HL-60 cells at 37°C, but their expression levels decreased in the organisms cultivated at 24°C, suggesting that temperature is one of the factors that influence the expression of members of the p44 multigene family. Several additional p44 transcripts that were not detected in the mammals at the acute stage of infection were detected in ticks. Phylogenetic analysis of the 20 different p44 transcripts revealed that the major transcripts found in mammals and ticks were distinct, suggesting a difference in surface properties between populations of the HGE agent in different host environments. The present study provides new information for understanding the role of the p44 multigene family in transmission of the HGE agent between mammals and ticks.
Human granulocytic ehrlichiosis (HGE) is a recently discovered tick-borne zoonosis (6). More than several hundred HGE patients have been confirmed in the United States, and the disease is increasingly recognized in several other countries as well as in the United States. HGE is an acute, often severe febrile illness that requires hospitalization and can be fatal. The etiologic agent (HGE agent) is a gram-negative, obligatory intracellular bacterium that primarily infects neutrophils. The agent is a strain of Anaplasma phagocytophila that has been previously known as a ruminant or horse pathogen (2, 6). The HGE agent is transmitted by Ixodes species ticks, and a white-footed mouse (Peromyscus leucopus) is considered to be a primary reservoir in the region of endemicity of the northeastern United States (30). Other mammalian species, such as horses or dogs, are also naturally infected with the HGE agent in the region of endemicity (19, 20). Experimental inoculation of DBA/2 mice (30) and horses (13, 21) with the HGE agent leads to infection similar to infection in P. leucopus and to clinical signs compatible with human patients, respectively. Coinfections of HGE patients with the Lyme disease spirochete Borrelia burgdorferi or Babesia microti were reported, because the Ixodes tick is a common vector and P. leucopus is a common reservoir for these pathogens (12, 14, 16).
The HGE agent is cycled in nature in mammalian reservoirs through obligatory blood feeding of tick vectors, because transovarial transmission appears to be inefficient (8, 30). During horizontal transmission of other tick-borne bacterial pathogens, changes in the protein composition on the bacterial surface play a role in adaptation of the organisms to different hosts (24, 27). On the HGE agent, 44- to 49-kDa outer membrane proteins (P44s) are major antigens recognized by patients' sera (1, 9, 32, 33, 34). These proteins are encoded by a polymorphic multigene family consisting of more than 18 p44 paralogous genes, which are interspersed in the genome of the HGE agent (15, 34). These paralogs can be characterized by a central hypervariable region flanked by 5′ and 3′ conserved regions. Five paralogs are predominantly expressed by the HGE agent when it is cultivated in the HL-60 cell line at 37°C (34). However, which p44 paralogs are expressed in mammalian hosts and ticks are unknown. Passive immunization with monoclonal antibodies specific to P44 paralogous proteins of the HGE agent induces partial protection against the challenge with the HGE agent in mice, suggesting P44 paralogs as potential vaccinogens (10). Moreover, a role of P44 paralogs in HGE pathogenesis was implied by the results of our recent evidence that a recombinant P44 (rP44) protein, as well as the whole organisms, had the ability to induce production of proinflammatory cytokines by human peripheral blood leukocytes (PBLs) (11). Therefore, in order to understand the role of P44 paralogs during tick transmission and the function of P44 antigens and to explore an effective vaccine candidate, it is essential to characterize p44 paralogs expressed in mammals and ticks. The present study is the first demonstration of successful experimental transmission of the HGE agent from a mouse to a horse via blood feeding of ticks. The results may be of benefit in designing a P44-based vaccine in the future.
MATERIALS AND METHODS
Bacteria and culture.
The HGE agent (HZ strain [22]) was cultured in HL-60 cells (human promyelocytic leukemia cell line) at 37°C as previously described (22). The purification procedure of ehrlichial organisms from the infected cells using Sephacryl S-1000 chromatography was described elsewhere (23). The host cell-free organisms released by sonication of infected cells were inoculated to uninfected HL-60 cells at a 1/2 ratio (infected/uninfected cells), and the cells were cultivated at 37 or 24°C for about 5 days to reach 70% infectivity and used for transcriptional analysis. For protein analysis, organisms were purified from these cultures.
Infection of mice, ticks, and horses.
Twelve 3-week-old DBA/2 male mice (Harlan Sprague-Dawley, Indianapolis, Ind.) were inoculated intraperitoneally (i.p.) with 106 HL-60 cells infected with the HGE agent (70% of cells infected). On day 4 postinoculation, the blood specimens were collected from two mice for preparation of leukocytes. Twenty to thirty uninfected, laboratory-reared Ixodes scapularis nymphs (total, 200 to 300 nymphs) were placed on each of 10 remaining mice with a paintbrush. Each mouse was restrained in a wire cage for 24 h to protect the ticks from host grooming. Engorged nymphs were collected after detachment from the mice and were individually placed in a microcentrifuge tube with filter paper in an incubator at 25°C and 90 to 98% relative humidity until molting into adults (3 to 4 weeks to 4 months). The unfed female ticks (n = 10) were used for preparation of whole-tissue specimens. The remaining unfed ticks (n = 123) were placed in an orthopedic stockinet attached by water-soluble glue to the skin of an HGE agent-free horse (EQ001). These ticks were allowed to feed until they were engorged and detached and were used for preparation of midgut and salivary glands. On day 8 after placement of infected ticks, the blood of the horse (EQ001) was collected for preparation of leukocytes. The transmission experiment was repeated on the horse (EQ002) with a total of 300 I. scapularis molted adults infected as nymphs by attaching on infected mice. In another experiment, two HGE agent-free horses (EQ003 and EQ004) were inoculated intravenously (i.v.) with 107 HL-60 cells infected with the HGE agent (70% of cells infected). On day 8 postinoculation, the blood of these two horses was collected for preparation of leukocytes.
DNA-PCR and RT-PCR.
The PBLs from mice or horses were prepared as described previously (11). Whole tissues from the unfed female adult ticks acquisition fed as nymphs on the infected mice were prepared after homogenization of pools of 10 randomly selected ticks. The salivary glands and the midgut were separately pooled after dissection of 10 randomly selected female ticks fed on horse EQ001 or 5 ticks fed on horse EQ002. The blood samples were collected from mice on day 4 after i.p. inoculation or from horses on day 8 after i.v. inoculation or after attaching ticks. The total DNA and RNA were extracted from the PBLs or the respective tick tissues with a QIAamp Blood kit (Qiagen Inc., Chatsworth, Calif.) and TRIzol reagent (Invitrogen-Life Technologies, San Diego, Calif.), respectively (34). DNA-PCR and reverse transcription (RT)-PCR were performed as described elsewhere (34). The forward primer of p3708 was 5′-GCTAAGGAGTTAGCTTATGAT-3′, and the reverse primer of p4257 was 5′-AAGAAGATCATAACAAGCATT-3′, which were located at 5′- and 3′-end-conserved regions, respectively, among p44 paralogous genes (see Fig. 6). Therefore, the cDNA fragments including central hypervariable regions from multiple p44 transcripts can be amplified simultaneously by a single RT-PCR. The RT-PCR products were cloned into a pCRII vector (Invitrogen-Life Technologies), and the 25 cDNA clones were randomly selected in each of the samples from the PBLs or the ticks for DNA sequencing of the inserts. Sensitivity of the RT-PCR was estimated using the modified procedure as described by Shaw et al. (28). In order to prepare a DNA template for generating in vitro p44 transcripts, a forward primer having a T7 RNA polymerase binding sequence added to the 5′ end was designed. The primer (p3646) had a sequence of 5′-TAATACGACTC ACTATAGGGGTATTAGAGATAGTGG-3′, which was located 56 bp upstream from the RT-PCR forward primer of p3708. The T7 binding site is underlined. A reverse primer (p4290) used was 5′-ACATGCATAAGGAACAACACC-3′, which was located 33 bp downstream from the RT-PCR reverse primer of p4257. The PCR with the primer pair of p3646 and p4290 was done using a pHGE1221 plasmid, which was previously cloned (33, 34), as the template. The pHGE1221 carries a 6.8-kb HGE agent DNA including two tandemly arranged genes of p44-1 (previously termed p44) and p44-18 (34). Since the p3646 and p4290 primers anneal 5′- and 3′-end-conserved regions (outside of p3708 and p4257), respectively, the PCR simultaneously amplified a 550-kb DNA fragment from both p44-1 and p44-18 from this plasmid template. The amplicon was then cloned into pCRII vector (Invitrogen-Life Technologies), and two clones including the hypervariable regions of p44-1 and p44-18 were selected based on the sequence analysis. After EcoRI digestion and purification, the inserts from the two clones were used as DNA templates for generating specific in vitro runoff transcripts by using the Riboprobe in vitro transcription system (Promega Corp., Madison, Wis.). After removal of the DNA templates and purification, these two in vitro transcripts of p44-1 and p44-18 were enumerated by standard UV spectrophotometry and were used for the RT-PCR against a background of 2.5 μg of total RNA from PBLs of an uninfected horse to mimic the experimental condition.
FIG. 6.
Alignment of deduced amino acid sequences from the corresponding p44 paralogous genes or p44 cDNA clones. Aligned positions of amino acids identical to those of P44-1 are shown with dots. Gaps indicated by dashed lines were introduced for optimal alignment of all proteins. A boxed area in the middle indicates amino acid sequences deduced from nucleotide sequences of p44 cDNAs. The amino acid sequences underlined in the hypervariable regions of P44-1, P44-2, P44-12, and P44-18 indicate the sequences that were used to prepare synthetic oligopeptides Pep1, Pep2, Pep12, and Pep18, respectively. The arrows show the positions of the primers used for RT-PCR. The closed bar at bottom indicates the conserved region within the hypervariable region. The numbers on the right side indicate the positions of amino acid residues in P44 paralogs from the N terminus to the C terminus and correspond to the rightmost amino acid residues.
Quantitative-competitive (QC)-PCR and QC-RT-PCR.
Two competitor plasmids including p44-1 and p44-18 genes with deletions were constructed. To make a competitor for p44-1, two primer pairs—p3277 (5′-CCTTTTCTTTAGGAAGCGTA-3′)-p3672 (5′-CATACCGCGGACCACTATCTCTAATACCCT-3′) and p3761 (5′-CATACCGCGGTGCTCTTGCCAAAACCTC-3′)-p3913 (5′-CAGGCTCAACCGCGTACTT-3′)—were designed with SacII restriction sites (underlined). The PCRs with these two primer pairs were performed using the pHGE1221 plasmid as a template (34). After SacII digestion of p3277-p3672 and p3761-p3913 PCR products, they were ligated to each other. The ligated product was then amplified using p3277 and p3913. The truncated p44-1 PCR product was cloned into a pCRII vector (Invitrogen-Life Technologies). PCR amplification of the competitor plasmids with primers p3277-p3913 yielded a 547-bp product, whereas the amplification of the chromosomal p44-1 DNA with the same primers yielded a 636-bp product. For construction of a truncated p44-18 competitor plasmid, a p44-18 cDNA clone was used as a template for PCR. PCR was done using two primer pairs: p3277-p3672 and p3761-p4794 (5′-ACCACCACACAATGATGTAC-3′). The subsequent procedure was the same as that in the case of p44-1 competitor plasmid. PCR amplification of the p44-18 competitor plasmid with p3277-p4794 yielded a 548-bp product, whereas the amplification of p44-18 cDNA with the same primers yielded a 672-bp product. To determine the number of ehrlichial organisms by QC-PCR, total DNA was extracted from 5 × 106 PBLs of the HGE agent-infected horses or mice as well as from the infected HL-60 cells. The serially diluted competitor derived from the p44-1 gene was added into the PCR mixture, which contained 3.0 to 3.5 μg of the prepared DNA, corresponding to 6.25 × 105 infected cells. Since there is a single gene of p44-1 in the genome (34), the number of p44-1 determined by QC-PCR corresponds to that of ehrlichial genomes or to that of organisms in the respective samples. To determine the number of p44 transcripts per ehrlichial organism, the same number of cells (5 × 106) from the same specimens as those for the total DNA preparation for QC-PCR was used for the total RNA preparation. The 2.5 to 3 μg of the total RNA corresponding to 6.25 × 105 infected cells was used for the reverse transcription, and then PCR was done in 50 μl of the reaction mixture, which included 2 μl of reverse-transcribed cDNA and the serially diluted competitors derived from either p44-1 or p44-18. Amplicons were resolved on a 1.5% agarose gel stained with ethidium bromide. The gel image was digitally captured and analyzed by using a gel video system (Gel Print 2000I; BioPhotonics Corporation, Ann Arbor, Mich.) and the image analysis software (ImageQuant; Molecular Dynamics, Sunnyvale, Calif.).
Cloning of overlapping DNA fragments for assembly of full-length p44 paralogs.
Based on the sequences of hypervariable regions of the p44 transcripts, the primers specific to each gene were designed to amplify overlapping DNA fragments with unknown flanking sequences by using adapter PCR with the GenomeWalker kit (Clontech Laboratories, Inc., Palo Alto, Calif.). After amplification, the PCR products were inserted into a pCRII vector, and the sequences of the insert were assembled with known cDNA sequences.
ELISA.
The synthetic oligopeptides Pep2 (CGHSSGCTQNPKLFST), Pep12 (CGKKSGDNGSLADYTD), Pep18 (CKNQKSSDTDTGVEKA), and Pep1 (CLSNGSAEAAHKYLSK) were derived from the amino acid sequences of hypervariable regions of P44-1, P44-2, P44-12, and P44-18 (34), respectively, and were used as antigens. rP44 (33) was used as a positive control in the assay. Sera from horse EQ003 were tested by enzyme-linked immunosorbent assay (ELISA) as described elsewhere (29).
Western immunoblotting.
Western immunoblot analysis was performed as described elsewhere (33). Preparation of an anti-Pep18 serum (34) and a monoclonal antibody of 5C11 (10) was previously described.
Sequence analysis, GenBank accession numbers, and statistical analysis.
Analyses of DNA and amino acid sequences were performed as described previously (17). Phylogenetic analysis based on an amino acid sequence alignment using CLUSTAL V was carried out with PHYLIP software, version 3.5.7 (7). The phylogram was constructed using the neighbor-joining method with a Kimura formula, and bootstrap values were based on analysis of 1,000 replicates. GenBank accession numbers of the published sequences are as follows: P44-1, AF059181; P44-2, AF135254; P44-12, AF135255; P44-15, AF135256; P44-18, AF135257; and P44-19, AF135263.
Nucleotide sequence accession numbers.
The nucleotide sequence(s) reported in this paper has been submitted to the GenBank/EBI Data Bank under the following accession numbers: P44-3, AF412818; P44-4, AF412819; P44-5, AF412820; P44-6, AF412821; P44-7, AF4128122; P44-8, AF412823; P44-9, AF412824; P44-10, AF412825; P44-11, AF412826; P44-13, AF412827; P44-14, AF412828; P44-16, AF412829; P44-17, AF412830; P44-21, AF412831; p44-2b, AY062041; p44-9, AF414589; p44-11, AF414590; p44-13, AF414592; and p44-20, AF414591.
RESULTS
Sensitivity of RT-PCR.
A unique characteristic of the gene structure in a p44 multigene family of the HGE agent is that a single central hypervariable region consisting of approximately 94 amino acid residues is flanked by 5′- and 3′-end-conserved regions composed of 58 and 65 residues, respectively (33). To characterize mRNA expressed from the multiple p44 paralogous genes, we previously developed a procedure using RT-PCR with a primer pair located in the conserved regions and subsequent cloning of the amplicons followed by determination of sequences of the cloned cDNAs (34). In this study, we first examined the sensitivity of the RT-PCR analysis. Two genes of p44-1 and p44-18 were selected as representatives to generate in vitro transcripts. An RT-PCR assay using serial dilution of these in vitro transcripts was utilized to estimate detectable mRNA levels of p44 paralogs against a background of the total RNA (2.5 μg) from the PBLs of an uninfected horse. By this assay, an amplified cDNA fragment of p44-1 or p44-18 could be detected to a level of 104 transcripts (Fig. 1). The sensitivities of the RT-PCR for these two transcripts were almost identical, although p44-1 and p44-18 differed in the sequences of the central hypervariable regions (54% DNA identity). According to our standard procedure, we obtained on average 25 μg of total RNA from 5 × 106 PBLs of infected horses or mice and used 2.5 μg for each reverse-transcriptional reaction of RT-PCR. The results of QC-PCR as described below (Fig. 2) indicated that 2.5 μg of total RNA was calculated to contain an amount of ehrlichial RNA equivalent to that of 1.25 × 104 organisms. Hence, the RT-PCR would detect approximately one p44 transcript per ehrlichial organism.
FIG. 1.
Estimation of RT-PCR sensitivity in detection of p44 transcripts within total RNA samples in the infection study. A primer pair was within the in vitro transcripts and located in 5′- and 3′-conserved regions of p44-1 and p44-18 genes. The numbers of in vitro transcripts are shown at top. This assay could detect up to 104 transcripts of both P44-1 and P44-18 within a 2.5-μg total RNA background from leukocytes of uninfected horse. Symbols: +, RT-PCR analysis was performed using serial dilution of in vitro transcripts as template; −, an identical reaction without the addition of reverse transcriptase as control for DNA contamination.
FIG. 2.
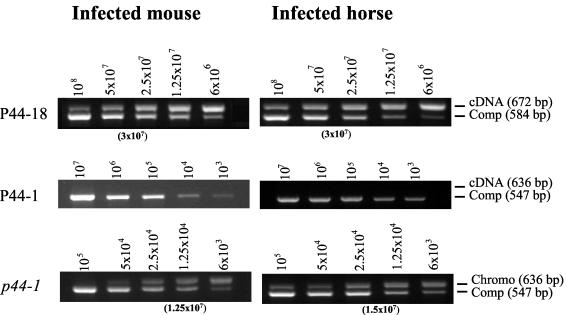
Quantitative comparison of p44 transcripts within the leukocytes from a mouse and a horse infected with the HGE agent by QC-RT-PCR. cDNA was synthesized from total RNA of the infected mouse or horse. The number of p44 transcripts in the infected cells was determined by using competitors of p44-18 or p44-1. The number of organisms in the samples was represented by the genome number determined by QC-PCR using the p44-1 competitor. The number of competitor plasmids used in each reaction is indicated at the top of each panel. The number in parentheses under each panel is the transcript number deduced by plotting the band densities of target and competitor. The result is a representative from at least three repeated experiments with similar results. Abbreviations: Comp, competitor; Chromo, chromosomal.
Next, we tested the proportion of transcripts of PCR-amplified products using a mixture of equal amounts of five different plasmids having inserts with known cDNA sequences of P44-1, P44-2, P44-12, P44-18, and P44-19, all of which were previously described (34). Within 25 randomly selected transformants, all of five cDNA sequences were detected and the number of clones containing cDNA sequence identical to P44-1, P44-2, P44-12, P44-18, and P44-19 were 7, 4, 4, 4, and 6, respectively. This result supports the notion that the random selection of transformants was not much biased toward a particular cDNA sequence and absence of crossover PCR. These results confirmed our previous study that the ratios of the transformants are proportional to the levels of transcription of p44 paralogs by Northern blot analysis using gene-specific RNA probes (34). Therefore, our procedure using a combination of the RT-PCR, TA cloning, and sequencing has sufficient reliability for identification of major transcripts of p44 paralogs.
Identification of p44 transcripts in tick transmission.
Uninfected laboratory-reared I. scapularis nymphs were allowed to feed to repletion on experimentally infected mice. After detaching and molting, the adult ticks were placed on HGE agent-free horses until they were engorged and detached. By RT-PCR, a 550-bp cDNA fragment was amplified from the leukocytes from the mice and the horses or from the tissues of ticks. No amplicon of any RT-PCRs was detected without reverse transcriptase in any of these samples, indicating the absence of the genomic DNA contamination in these RNA preparations. The p44 cDNA clones randomly selected after TA cloning of RT-PCR products were characterized based on the sequences of the central hypervariable regions. In the infected mice or horses, only two different cDNA sequences (P44-18 and P44-2) were found in their leukocytes (Table 1), although five transcripts, including P44-18 and P44-2, were detected in the HGE agent cultivated in HL-60 cells at 37°C before inoculation into the mammals (34). Of these two, P44-18 was extremely predominant, as shown in Table 1. Thus, P44-18 was a major transcript of the p44 multigene family at the early stage of mammalian infection (on day 4 post-i.p. inoculation in mice or on day 8 post-i.v. inoculation in horses or on day 8 after tick feeding in horses) regardless of animal species (mouse or horse), inoculation routes (i.p., i.v., or inoculation by tick feeding), or sources (culture or tick).
TABLE 1.
p44 transcripts identified in mammals
| p44 transcript | No. of cDNA clonesa in:
|
|||||
|---|---|---|---|---|---|---|
| Mouse 1 (i.p.)b | Mouse 2 (i.p.) | Horse EQ001 (tick) | Horse EQ002 (tick) | Horse EQ003 (i.v.) | Horse EQ004 (i.v.) | |
| P44-18 | 23 | 22 | 23 | 24 | 24 | 23 |
| P44-2 | 2 | 3 | 2 | 1 | 1 | 2 |
In a total of 25 cDNA clones.
Routes: i.p., intraperitoneal inoculation; tick, inoculated by tick feeding; i.v., intravenous inoculation.
In the HGE agent in molted acquisition-fed female ticks, seven kinds of p44 transcripts, including P44-18 and P44-2, with different cDNA clone populations were detected in the whole tissues (Table 2). After a blood meal of infected adult ticks on the horse, seven kinds of transcripts, six of which (except for P44-2) were distinct from those in the acquisition-fed ticks, were found in the midgut. In the salivary glands from both acquisition-fed and transmission-fed ticks, only two major transcripts (P44-1 and P44-2) were detected. The results showed that the major transcript represented by P44-18 in mammals became a minor transcript in ticks. Consequently, the HGE agent phenotypes based on P44 major surface antigens are likely to be more diverse in ticks than in mammals. However, before transmission to mammalian tissue, the organisms may be restricted to the phenotypes represented by at least two dominant antigens, such as P44-1 and P44-2 in the salivary glands.
TABLE 2.
p44 transcripts identified in ticks
| p44 transcript | No. of cDNA clonesa
|
||||
|---|---|---|---|---|---|
| Expt 1
|
Expt 2 (salivary gland)c | ||||
| Whole tissuesb | Midgutc | Salivary glandc | |||
| P44-1 | 2 | — | 13 | 16 | |
| P44-2 | 9 | 8 | 12 | 9 | |
| P44-6 | — | 2 | — | — | |
| P44-7 | — | 2 | — | — | |
| P44-8 | — | 2 | — | — | |
| P44-9 | — | 9 | — | — | |
| P44-10 | — | 1 | — | — | |
| P44-11 | — | 1 | — | — | |
| P44-13 | 8 | — | — | — | |
| P44-14 | 2 | — | — | — | |
| P44-16 | 1 | — | — | — | |
| P44-17 | 1 | — | — | — | |
| P44-18 | 2 | — | — | — | |
In a total of 25 cDNA clones.
From acquisition-fed female adult ticks.
From transmission-fed female adult ticks.
—, not detected in 25 cDNA clones.
Overall, a total of 150 cDNA clones from the mammals was sequenced. Among them, the cDNA sequences identical to p44-18 numbered 139 (92.7%) and the sequences identical to p44-2 numbered 11 (7.3%). In the infected ticks, a total of 100 cDNA clones was sequenced, and the numbers of sequences identical to p44-18 and p44-2 were 2 (2%) and 38 (38%), respectively. The 60 remaining cDNAs have sequences identical to those of 11 other different p44 transcripts. By determination of a confidence interval for the difference in the binomial proportions for P44-18 and P44-2 transcripts using the STATXACT software (Cytel Software Corp., Cambridge, Mass.), the number of p44-18 transcripts detected in the mammals was significantly greater than that in the ticks (P < 0.0001). In contrast, the number of p44-2 transcripts detected in the ticks was significantly greater than that in the mammals (P < 0.0001).
To further confirm the dominant p44 expression in the mammals at the acute phase of infection, we quantitatively analyzed the number of p44-18 transcripts by QC-RT-PCR using respective gene-specific primer pairs. For determination of the organism numbers by QC-PCR, we chose the p44-1 gene as a target because it had a single copy in the genome. The average number of p44-18 transcripts was 3 × 107/1.25 × 104 organisms (2,400 ± 47 transcripts/organism) in leukocytes of the infected mouse and 3 × 107/1.5 × 104 organisms (2,000 ± 59 transcripts/organism) in leukocytes of the infected horse (EQ003) (Fig. 2). However, the p44-1 transcript was not detected even at the lowest level of 1 × 103 (per 1.25 × 104 organisms) tested. These results support the argument that the p44-18 gene is dominantly transcribed but that the p44-1 gene is not transcribed in the mammalian hosts.
Antibodies against P44 paralogs at acute phase of infection in a horse.
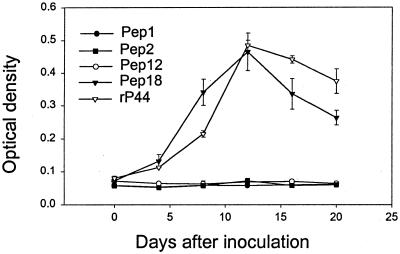
Since P44 paralogs are cross-reactive to each other, it is difficult to develop monospecific or monoclonal antibodies that can distinguish each P44 protein in the multigene family. Furthermore, the numbers of the HGE agent present in mammals are too few for its P44 proteins to be directly detected. Therefore, to determine the expression of members of the p44 multigene family at the protein level, antibodies against P44 proteins present in sera from infected horse (EQ003) at the acute phase of infection were examined by ELISA using the synthetic oligopeptides Pep1, Pep2, Pep12, and Pep18 and an rP44 as antigens. These peptides were designed based on the predicted amino acid sequences of the hypervariable region of P44-1, P44-2, P44-12, or P44-18. Antibodies raised to each peptide were previously demonstrated to react with each peptide by indirect fluorescent antibody assay and/or Western immunoblotting (34). We previously also demonstrated that sera from patients with HGE infection reacted with Pep2 and Pep18 (34). The rP44 protein was used as a positive control since this protein includes an N-terminal region highly conserved among P44 paralogs, and it is expected to react with the antibodies against most P44 proteins. The immunoglobulin M (IgM) antibody specific to P44-18 (synthetic peptide Pep18) was detected on day 4 postinoculation, and its titer subsequently elevated and peaked on day 12. The production of IgM antibody against rP44 had a pattern similar to that of Pep18 (Fig. 3). Antibodies specific for P44-1, P44-2, or P44-12 were undetectable. These results suggest that P44-18 protein was actually synthesized by the HGE agent in the horse at the early stage of infection and its linear epitope included in Pep18 was recognized by the horse immune system. This finding supports the transcriptional analysis as described above, in which the p44-18 gene is abundantly transcribed by the HGE agent at the acute phase of infection in mice and horses.
FIG. 3.
ELISA for detection of anti-P44 paralog antibodies in the horse infected with the HGE agent. Serum samples were collected every 4 days during a 20-day period from the horse (EQ003) infected with the HGE agent. The IgM antibodies against four P44 paralogs of P44-1, P44-2, P44-12, and P44-18 were examined using synthetic oligopeptides Pep1, Pep2, Pep12, and Pep18 as antigens (Fig. 4). The rP44 (33) was used as positive control in the assay.
Effect of temperature on mRNA and protein expression of p44 paralogs by the HGE agent in HL-60 cells.
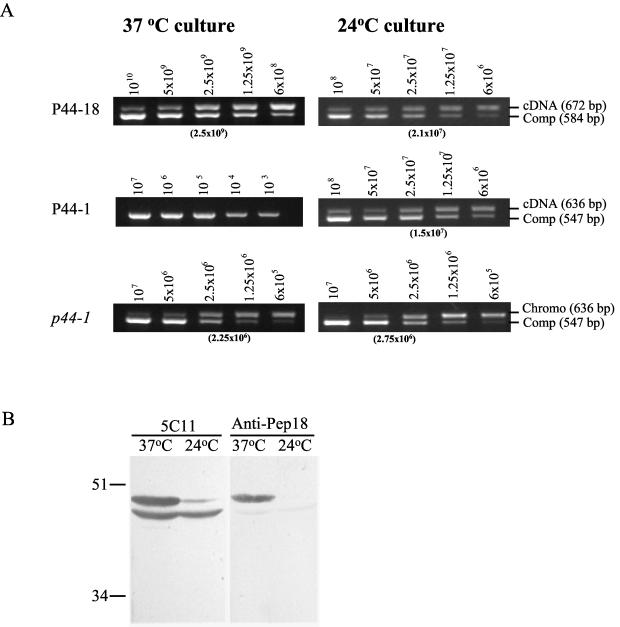
We compared the transcriptional levels of p44-18 and p44-1 for the HGE agent grown in HL-60 cells at 37 and 24°C by QC-RT-PCR because the transcriptional levels of p44-18 were much lower in ticks than in mammals but the levels of p44-1 were reversed. The average number of the p44-18 transcripts increased approximately from 2.1 × 107 transcripts/2.75 × 106 organisms (8 ± 0.2 transcripts/organism) in the cell culture at 24°C to 2.5 × 109 transcripts/2.25 × 106 organisms (1,000 ± 10 transcripts/organism) at 37°C (Fig. 4A). The P44-1 transcript was not detected previously in the HGE agent grown in HL-60 cells at 37°C by RT-PCR as well as by Northern blot analysis (34). In the present study by QC-RT-PCR, the P44-1 transcript was detected in the RNA sample only from the 24°C tissue culture as five transcripts per organism (Fig. 4A).
FIG. 4.
Effects of temperature on production of P44-18 and P44-1 transcripts and/or proteins. (A) Quantitative comparison of p44 transcripts in the HGE agent cultivated in HL-60 cells at 37 or 24°C by QC-RT-PCR. cDNA was synthesized from total RNA of the infected HL-60 cells. The number of p44 transcripts in the infected cells was determined by using competitors of p44-18 or p44-1. The number of organisms in the samples was represented by the genome number determined by QC-PCR using the p44-1 competitor. The number of competitor plasmids used in each reaction is indicated at the top of each panel. The number in parentheses under each panel indicates the competitor almost equivalent to the target based on the band intensity. Abbreviations: Comp, competitor; Chromo, chromosomal. (B) Protein production of P44 paralogs in the HGE agent cultivated in HL-60 cells at 37 or 24°C. The ehrlichial organisms that were grown in HL-60 cells at 37 or 24°C were purified and analyzed by Western blotting. The result is a representative from at least three repeated experiments with similar results.
We also analyzed the production of P44-18 protein for the HGE agent grown in HL-60 cells at 37 and 24°C by Western blotting using P44-18-specific antibody (anti-Pep18 serum) and monoclonal antibody 5C11 (10). The protein contents of the purified organisms from two different culture conditions (37 or 24°C) were adjusted. In the assay, the anti-Pep18 serum primarily reacted with a 44-kDa band in the HGE agent cultivated at 37°C, but this 44-kDa band was undetectable in the organisms cultured at 24°C (Fig. 4B). Monoclonal antibody 5C11, which recognizes the N-terminal conserved region of P44s (10), reacted with two bands of 43 and 44 kDa in the 37°C sample but lacked a 44-kDa band corresponding to P44-18 in the 37°C sample (Fig. 4B). Thus, the production of P44-18 (44-kDa) protein appears to be significantly reduced in the HGE agent in cell culture at 24°C.
Characterization of genomic loci for expressed p44 paralogs.
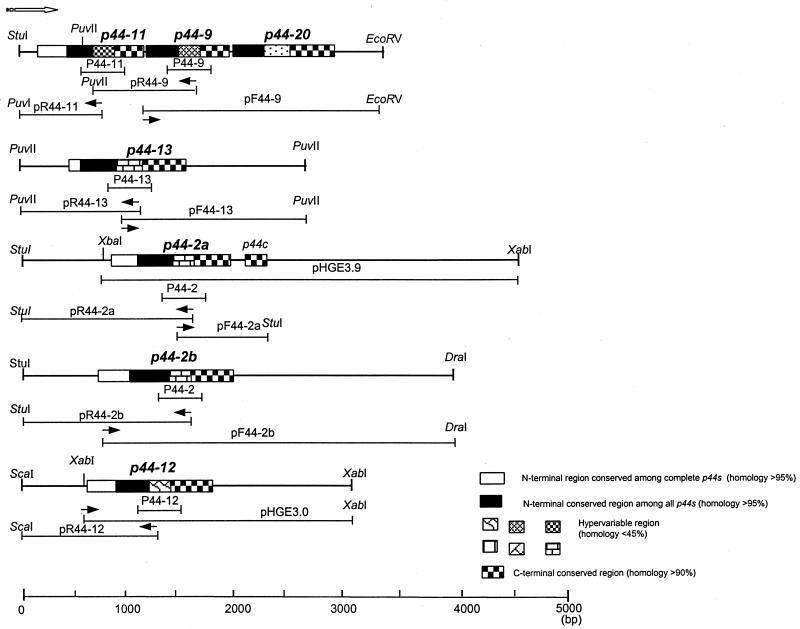
We previously characterized the gene structures of p44-1, p44-2, p44-12, and p44-18 paralogs (34). In order to compare the gene structures of additional expressed p44 paralogs found in the present study, the genome-walking procedure using an adapter PCR was performed with gene-specific primers based on the central hypervariable regions of respective cDNA sequences. With the specific primers derived from the cDNA sequences of P44-9 and P44-13 (two of the major transcripts in ticks but not in mammals), two independent loci of a p44-11-p44-9-p44-20 gene cluster and a p44-13 gene were assembled based on the several overlapping DNA sequences obtained (Fig. 5). The p44-11 gene, the transcript of which was detected in the midgut of transmission-fed ticks, and the p44-20 gene, the transcript of which was not detected in any samples used in the present study, were found upstream and downstream of p44-9, respectively, in this analysis. We previously suggested the presence of two copies for p44-2 in the HGE agent genome by using Southern blot analysis with a p44-2-specific probe (34). When p44-2-specific primers were used in the genome- walking study, we assembled another open reading frame (ORF) of the coding sequence almost identical to that of the original p44-2 gene (renamed p44-2a) except for 93 nucleotides at the 3′ end. Between the p44-2a gene and a newly identified p44-2 gene (termed p44-2b), the 5′- and 3′-noncoding sequences were different. The present analysis confirmed the presence of these two copies at the different loci in the genome and revealed the difference in primary structures of the two copies (Fig. 5). It is currently unknown whether one or both p44-2 genes are transcriptionally active. The sequence upstream from the previously identified p44-12 gene (34) was analyzed in the present study by the genome-walking procedure to determine whether other p44 paralogs exist in the region. There was no additional ORF encoding p44 paralogs within 600 bp upstream from the p44-12 gene. Based on these analyses, the gene organizations of p44 paralogs could be divided into two groups: one is an individual localization of a single gene (p44-2a, p44-2b, p44-12, and p44-13), and another is a formation of a gene cluster consisting of two or three tandemly arranged genes (p44-1-p44-18 [34] and p44-11-p44-9-p44-20 [Fig. 5]). Within the respective clusters, only the first gene, p44-1 or p44-11, is full-length with an AUG start codon and a ribosome binding site, whereas downstream genes (p44-18, p44-9, and p44-20) had coding regions shorter than those of the first genes and the first codons for all three downstream genes were TCT. It is unknown whether this first codon (TCT) functions as a translational start codon in the HGE agent. Because of the short intergenic spaces (5 bp between p44-11 and p44-9 or 9 bp between p44-9 and p44-20) or the short overlapping regions (20 bp between p44-1 and p44-18), it is possible that these downstream genes within the cluster are cotranscribed through the promoters located upstream of the first ORFs.
FIG. 5.
Schematic diagram of gene organization of p44 paralogous genes in the HGE agent genome. The solid arrows indicate the positions of the primers used for the adapter PCR, and the open arrow indicates the orientation of the ORF from the 5′ end to 3′ end. The recombinant plasmids pHGE3.9 and pHGE3.0, which contain a p44-2a gene and a p44-12 gene, respectively, were previously described (34). The thin lines below each gene indicate the DNA fragments amplified by adapter PCR or the previously cloned fragment (pHGE3.9 and pHGE3.0 [34]).
Characterization of hypervariable regions of p44 multigene family.
We previously identified five transcripts (P44-2a, P44-12, P44-15, P44-18, and P44-19) in the HGE agent cultivated in the HL-60 cell line at 37°C (34). In the present study, 11 additional different p44 transcripts were detected in mice, horses, or ticks. Moreover, when the HGE agent was cultivated in HL-60 cells at 24°C, four new different transcripts (P44-3, P44-4, P44-5, and P44-21) were found to be expressed (data not shown). Therefore, we identified a total of 20 different p44 transcripts and one new gene (p44-20) by the genome-walking experiment in the previous and present studies. A comparison of the deduced amino acid sequences from these 21 different p44s revealed the characteristic central hypervariable (hydrophilic and surface-exposed) region of approximately 94 amino acid residues corresponding to the 175th to 269th amino acids of a protein (P44-1) encoded by the p44-1 gene (Fig. 6, boxed sequences). Within the hypervariable region, there is a conservative sequence consisting of six amino acid residues, (K/G/Q)(N/H)WP(T/R)(G/S/T), which was not found in major surface protein 2 (Msp2) of Anaplasma marginale. The highest identity of amino acid sequences in all hypervariable regions was 65.6% between P44-13 and P44-16, whereas the lowest identity was 32.8% between P44-13 and P44-18. Thus, this central region of immunodominant P44 major outer membrane proteins with highly diverse sequences appears to be sufficient to lead the HGE agent to different antigenic phenotypes by producing distinctive P44 paralogous proteins during tick transmission.
Phylogenetic relationship among expressed P44 paralogs.
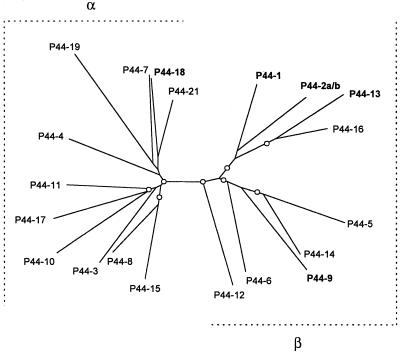
To investigate the relationship among 20 different p44 transcripts expressed in mice, horses, ticks, or tissue cultures, a phylogram was constructed based on the deduced amino acid sequence alignment of respective cDNAs (Fig. 6, boxed sequences). This region consisted of 151 to 180 amino acid residues, including the central hypervariable region of P44 paralogous proteins. Overall, 20 P44 paralogs were divided into two groups: α and β (Fig. 7). Group α consisted of 11 proteins, including P44-18, which was abundantly detected in leukocytes of mammalian hosts. Group β was composed of nine proteins, including P44-1 and P44-2, which were predominantly expressed in the salivary glands of transmission-fed ticks. The P44-9 and P44-13 proteins that were dominant in the midgut of transmission-fed ticks and in the whole tissues of acquisition-fed ticks, respectively, also belong to group β. Thus, P44 paralogs predominantly expressed in mammalian hosts and arthropod vectors are phylogenetically distinct, suggesting the significant differences of surface properties between the HGE agents in mammals and ticks due to the major antigens present.
FIG. 7.
Phylogenetic relationship of 20 different p44 transcripts based on the deduced amino acid sequence alignment. The tree was constructed using the neighbor-joining (NEIGHBOR program from PHYLIP) method based on the alignment generated with CLUSTAL V, and 1,000 bootstrap replications were performed. The nodes supported by bootstrap values greater than 55% are indicated (○). The P44s in boldface type show the transcripts that were dominantly expressed in mammals or ticks.
DISCUSSION
The progenies of ehrlichiae that survive are fittest for repeated shuttles between reservoir mammals and vector ticks through both acquisition and transmission feeding. The ehrlichial organisms require multiple physiological changes for adaptation in different host environments. The present study for the first time revealed transcript heterogeneity of the p44 multigene family in the HGE agent transmitted by the ticks. The dominant P44 phenotype of the HGE agent in the mammalian hosts at the acute phase of infection was P44-18. When the organisms were transmitted from mammals into ticks, the species of p44 transcripts became diverse. With reduction of P44-18, multiple p44 transcripts emerged in the whole tissues of acquisition-fed ticks and in the midgut of transmission-fed ticks. After dissemination of ehrlichiae into the salivary glands, only two p44 transcripts, P44-1 and P44-2, were detected. This salivary gland-associated p44 transcripts may be involved in the development of the transmissible stage of the HGE agent. When the organisms in ticks were transmitted back to a mammalian host, the process was reversed, and the ehrlichiae having P44-18 as the major transcript reemerged at the acute phase of infection. Since syringe inoculation of the HGE agent cultured at 37°C reproduced a composition of p44 transcripts in mice and horses similar to that given by tick transmission, it appears that the mammalian environment alone is sufficient to convert to the P44-18 dominant phenotype.
Temperature is one of the factors that influence the expression of members of the p44 multigene family. The transcriptional level of p44-18 was 100 times higher at 37°C than at 24°C. Furthermore, the expression of p44-1 appears to be inversely affected by temperature: P44-1 transcript was detected at 24°C but not at 37°C in cell culture. This is in agreement with the results of tick transmission: P44-1 transcript was not detected in mice or horses but was detected in ticks. In B. burgdorferi, outer surface protein C (OspC) is produced by the spirochete in culture at 32 to 37°C but not at 24°C (27). Borrelia hermsii Vmp8 is turned on at 37°C and turned off at 23°C, whereas the expression level of Vmp33 is higher at 23°C than at 37°C (26). Our recent study also showed the different expression pattern of members of the p30 multigene family of Ehrlichia canis cultivated in the dog cell line DH82 between 37 and 25°C (31).
The HGE agent is a slow-growing obligatory intracellular bacterium. The generation time of the agent was approximately 18 h when it was cultivated at 37°C (unpublished data), and it took about 5 days for a single passage in HL-60 cells at either 37 or 25°C: 20% of cells were infected at the 1st day and 70% of cells were infected at the 5th day. Within this single passage, both mRNA and protein levels expressed from the p44-18 gene were significantly decreased at 24°C compared to those at 37°C. Therefore, such a dramatic change of the p44-18 gene product within a short period of time is most likely caused at the transcriptional level rather than by a genetic selection.
The reversible change of p44 transcripts of the HGE agent detected during tick transmission is similar to that of a variable major protein (Vmp) expression in B. hermsii (26). The Vmp (Vmp7 or Vmp8) that is expressed by the spirochetes in mice is turned off and Vmp33 is turned on in the salivary glands after transmission into ticks. Subsequently, upon tick transmission back to a mammalian host, B. hermsii turns off Vmp33 expression and turned on either Vmp7 or Vmp8 expression that is previously expressed. Between vmp and p44s, however, there are several differences. In the HGE agent, we found three major p44 transcripts (P44-1, P44-2, and P44-18) during transmission between mammals and salivary glands. The P44-1 and P44-18 transcripts appear to be associated with the major change on the ehrlichial surface during the transmission. Since the P44-2 transcript was detected in all samples tested, this gene product may be essential for ehrlichial survival in nature.
Recently, we characterized a transcriptionally active gene cluster of the omp-1 multigene family (orthologs of p44s) in monocytic ehrlichiosis (ME) (canine ME [CME] and human ME) agents, E. canis and Ehrlichia chaffeensis (18). The 16S rRNA sequences were 7.5 to 7.8% divergent between the HGE agent and these two ME agents (6). The gene organization of the multigene families is significantly different between the HGE and ME agents. The HGE agent has genome-wide-distributed paralogs or small gene clusters, whereas the ME agents have a large gene cluster consisting of 22 tandemly arranged paralogs in a single locus of the genome (18). Our recent experimental transmission study of CME agent with Rhipicephalus sanguineus ticks showed that E. canis p30 multigenes are differentially expressed in infected dogs and that, unlike p44s, a transcript from only one p30 paralog is detectable in acquisition-fed as well as transmission-fed ticks (31). The difference in gene organization and gene expression regulation between the HGE and CME agents may have evolved in distinct host environments such as granulocytes versus monocytes or Ixodes ticks versus Rhipicephalus and Amblyomma ticks.
Sequence analysis revealed two kinds of gene organization in expressed p44 paralogs: a single gene locus or a gene cluster locus. The single gene and a first gene within the cluster had their own putative promoters. It is still unclear how the downstream genes within the gene cluster such as p44-18 can be predominantly expressed. As we previously suggested (34), transcription of p44-18 seems to involve posttranscriptional modification at the RNA level after polycistronic transcription of the gene cluster through a promoter located upstream of the first gene. Another possibility is a gene conversion at the genomic DNA level, in which the downstream gene is translocated into an active expression site through a recombination event. However, there is no direct evidence that such an event is involved in activation of p44 paralogous genes in the HGE agent, omp-1 multigenes of E. chaffeensis, or p30 multigenes of E. canis to date.
Another tick-borne pathogen, A. marginale, which infects bovine erythrocytes and is closely related to the HGE agent, has an Msp2 multigene family that is orthologous to the p44 multigene family. Like p44s, the msp2 multigenes are dispersed throughout the A. marginale genome. However, of several msp2 multigenes, only one is a full-length gene located in a polycistronic expression site, whereas the remaining msp2 paralogs are pseudogenes that are truncated at both 5′ and 3′ coding regions. Activation of msp2 pseudogenes by translocation into the functional expression site through a recombination event is required for emergence of a new Msp2 variant (3, 4, 5). The expression of members of the msp2 multigene family in mammals is apparently distinct from that of aforementioned p44s and vmps, because the tick salivary gland-associated Msp2s are continuously expressed in the acute phase of rickettsemia in the cattle infected by tick feeding (4, 24, 25).
It is important to point out that the number of HGE agent organisms present in ticks is substantially less than those of A. marginale or B. burgdorferi in ticks, and the number of ehrlichial organisms present in mammalian leukocytes is also substantially less than those of A. marginale present in red blood cells at the acute stage. Thus, it is difficult to apply mRNA detection techniques other than RT-PCR to determine gene expression patterns in the HGE agent in mammals or ticks. Therefore, in the present study we developed the gene-specific QC-RT-PCR. Sequencing the multiple clones of RT-PCR products provided initial estimates on levels of each transcript, and their cDNA sequences were used subsequently for designing competitors and gene-specific primers for QC-RT-PCR. This approach would allow us to compare expression of p44s or other genes among different strains of the HGE agent, in different tissues, and under different physiological conditions in the future.
The present study provides new information for understanding the role of the p44 multigene family in HGE agent transmission and establishment of infection. The data presented here would provide us an idea for development of an effective vaccine to prevent HGE agent infection using P44 paralogs as a vaccinogen. For example, a combination of three P44s (P44-1, P44-2, and P44-18) could be a new vaccine candidate because it is likely to be effective in preventing transmission of the HGE agent from the tick salivary gland to mammals (P44-1) and in preventing establishment of early infection in mammals after HGE agent transmission (P44-18), and the inhibitory effects are probably enhanced by inhibition of all HGE agents (P44-2). Alternatively, the conserved central six-amino-acid sequence, which was found in all P44s without exception but not found in Msp2s of A. marginale and which was predicted to be surface exposed and has high antigenic index, and sequences within conserved N- and C-terminal regions of P44s of similar properties could be used as targets for designing a vaccine. The present study may also lead to a new knowledge on gene expression mechanisms of multigene families during the environmental transition in a life cycle of vector-borne pathogens.
Acknowledgments
This work was supported by National Institutes of Health grants R01AI40934 and R01AI47407.
We thank Sumithra J. Mandrekar for her assistance in statistical analysis.
REFERENCES
- 1.Asanovich, K. M., J. S. Bakken, J. E. Madigan, M. Aguero-Rosenfeld, G. P. Wormser, and J. S. Dumler. 1997. Antigenic diversity of granulocytic Ehrlichia isolates from humans in Wisconsin and New York and a horse in California. J. Infect. Dis. 176:1029-1034. [DOI] [PubMed] [Google Scholar]
- 2.Bakken, J. S., J. Krueth, C. Wilson-Nordskog, R. L. Tilden, K. Asanovich, and J. S. Dumler. 1996. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA 275:199-205. [PubMed] [Google Scholar]
- 3.Barbet, A. F., A. Lundgren, J. Yi, F. R. Rurangirwa, and G. H. Palmer. 2000. Antigenic variation of Anaplasma marginale by expression of MSP2 mosaics. Infect. Immun. 68:6133-6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbet, A. F., J. Yi, A. Lundgren, B. R. McEwen, E. F. Blouin, and K. M. Kocan. 2001. Antigenic variation of Anaplasma marginale: major surface protein 2 diversity during cyclic transmission between ticks and cattle. Infect. Immun. 69:3057-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brayton, K. A., D. P. Knowles, T. C. McGuire, and G. H. Palmer. 2001. Efficient use of a small genome to generate antigenic diversity in tick-borne ehrlichial pathogens. Proc. Natl. Acad. Sci. USA 98:4130-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, S.-M., J. S. Dumler, J. S. Bakkenand, and D. H. Walker. 1994. Identification of a granulocytic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 32:589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felsenstein, J. 1995. PHYLIP, phylogeny inference package, version 3.5.7. University of Washington, Seattle.
- 8.Hodzic, E., D. Fish, C. M. Maretzki, A. M. De Silva, S. Feng, and S. W. Barthold. 1998. Acquisition and transmission of the agent of human granulocytic ehrlichiosis by Ixodes scapularis ticks. J. Clin. Microbiol. 36:3574-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ijdo, J. W., Y. Zhang, E. Hodzic, L. A. Magnarelli, M. L. Wilson, S. R. Telford III, S. W. Barthold, and E. Fikrig. 1997. The early human response in human granulocytic ehrlichiosis. J. Infect. Dis. 176:687-692. [DOI] [PubMed] [Google Scholar]
- 10.Kim, H.-Y., and Y. Rikihisa. 1998. Characterization of monoclonal antibodies to the 44-kilodalton major outer membrane protein of the human granulocytic ehrlichiosis agent. J. Clin. Microbiol. 36:3278-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, H. -Y., and Y. Rikihisa. 2000. Expression of interleukin-1β, tumor necrosis factor alpha, and interleukin-6 in human peripheral blood leukocytes exposed to human granulocytic ehrlichiosis agent or recombinant major surface protein P44. Infect. Immun. 36:3394-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leutenegger, C. M., N. Pusterla, C. N. Mislin, R. Weber, and H. Lutz. 1999. Molecular evidence of coinfection of ticks with Borrelia burgdorferi sensu lato and the human granulocytic ehrlichiosis agent in Switzerland. J. Clin. Microbiol. 37:3390-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madigan, J. E., P. J. Richter, R. B. Kimsey, J. E. Barlough, J. S. Bakken, and J. S. Dumler. 1995. Transmission and passage in horses of the agent of human granulocytic ehrlichiosis. J. Infect. Dis. Microbiol. 172:1141-1144. [DOI] [PubMed] [Google Scholar]
- 14.Magnarelli, L. A., J. S. Dumler, J. F. Anderson, R. C. Johnson, and E. Fikrig. 1995. Coexistence of antibodies to tick-borne pathogens of babesiosis, ehrlichiosis, and Lyme borreliosis in human sera. J. Clin. Microbiol. 33:3054-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy, C. I., J. R. Storey, J. Recchia, L. A. Doros-Richert, C. Gingrich-Baker, K. Munroe, J. S. Bakken, R. T. Coughlin, and G. A. Beltz. 1998. Immunodominant proteins of the agent of human granulocytic ehrlichiosis (HGE) are encoded by a multigene family. Infect. Immun. 66:3711-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadelman, R. B., H. W. Horowitz, T. C. Hsieh, J. M. Wu, M. E. Aguero-Rosenfeld, I. S. Schwartz, J. Nowakowski, S. Varde, and G. P. Wormser. 1997. Simultaneous human granulocytic ehrlichiosis and lyme borreliosis. N. Engl. J. Med. 327:27-30. [DOI] [PubMed] [Google Scholar]
- 17.Ohashi, N., N. Zhi, Y. Zhang, and Y. Rikihisa. 1998. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect. Immun. 66:132-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohashi, N., Y. Rikihisa, and A. Unver. 2001. Analysis of transcriptionally active gene clusters of major outer membrane protein multigene family in Ehrlichia canis and E. chaffeensis. Infect. Immun. 69:2083-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pusterla, N., J. B. Huder, K. Feige, and H. Lutz. 1998. Identification of a granulocytic Ehrlichia strain isolated from a horse in Switzerland and comparison with other rickettsiae of the Ehrlichia phagocytophila genogroup. J. Clin. Microbiol. 36:2035-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pusterla, N., J. B. Huder, C. Wolfensberger, B. Litschi, A. Parvis, and H. Lutz. 1997. Granulocytic ehrlichiosis in two dogs in Switzerland. J. Clin. Microbiol. 35:2307-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pusterla, N., C. M. Leutenegger, J.-S. Chae, H. Lutz, R. B. Kimsey, J. S. Dumler, and J. E. Madigan. 1999. Quantitative evaluation of ehrlichial burden in horses after experimental transmission of human granulocytic Ehrlichia agent by intravenous inoculation with infected leukocytes and by infected ticks. J. Clin. Microbiol. 37:4042-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rikihisa, Y., N. Zhi, G. P. Wormser, B. Wen, H. W. Horowitz, and K. E. Hechemy. 1997. Ultrastructural and antigenic characterization of a granulocytic ehrlichiosis agent directly isolated and stably cultivated from a patient in New York state. J. Infect. Dis. 175:210-213. [DOI] [PubMed] [Google Scholar]
- 23.Rikihisa, Y., S. A. Ewing, J. C. Fox, A. G. Siregar, F. H. Pasaribu, and M. B. Malole. 1992. Analyses of Ehrlichia canis and a canine granulocytic Ehrlichia infection. J. Clin. Microbiol. 30:143-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rurangirwa, F. R., D. Stiller, D. M. French, and G. H. Palmer. 1999. Restriction of major surface protein 2 (MSP2) variants during tick transmission of the ehrlichia Anaplasma marginale. Proc. Natl. Acad. Sci. USA 96:3171-3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rurangirwa, F. R., D. Stiller, and G. H. Palmer. 2000. Strain diversity in major surface protein 2 expression during tick transmission of Anaplasma marginale. Infect. Immun. 68:3023-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwan, T. G., and B. J. Hinnebusch. 1998. Bloodstream-versus tick-associated variants of a relapsing fever bacterium. Science 280:1938-1940. [DOI] [PubMed] [Google Scholar]
- 27.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw, E. I., C. A. Dooley, E. R. Fischer, M. A. Scidmore, K. A. Fields, and T. Hackstadt. 2000. Three temporal classes of gene expression during the Chlamydia trachomatis developmental cycle. Mol. Microbiol. 37:913-925. [DOI] [PubMed] [Google Scholar]
- 29.Tajima, T., N. Zhi, Q. Lin, Y. Rikihisa, H. W. Horowitz, J. Ralfalli, G. P. Wormser, and K. E. Hechemy. 2000. Comparison of two recombinant major outer membrane proteins of the human granulocytic ehrlichiosis agent for use in an enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 7:652-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Telford, S. R., III, J. E. Dawson, P. Katavolos, C. K. Warner, and C. P. Persing. 1996. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc. Natl. Acad. Sci. USA 93:6209-6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Unver, A., N. Ohashi, T. Tajima, R. W. Stich, D. Grover, and Y. Rikihisa. 2001. Transcriptional analysis of p30 major outer membrane multigene family of Ehrlichia canis in dogs, ticks, and cell culture at different temperatures. Infect. Immun. 69:6172-6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhi, N., Y. Rikihisa, H. Y. Kim, G. P. Wormser, and H. W. Horowitz. 1997. Comparison of major antigenic proteins of six strains of the human granulocytic ehrlichiosis agent by Western immunoblot analysis. J. Clin. Microbiol. 35:2606-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhi, N., N. Ohashi, Y. Rikihisa, G. P. Wormser, H. W. Horowitz, and K. E. Hechemy. 1998.. Cloning and expression of 44-kilodalton major outer membrane protein gene of the human granulocytic ehrlichiosis agent and application of the recombinant protein to serodiagnosis. J. Clin. Microbiol. 36:1666-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhi, N., N. Ohashi, and Y. Rikihisa. 1999. Multiple p44 genes encoding major outer membrane proteins are expressed in the human granulocytic ehrlichiosis agent. J. Biol. Chem. 274:17828-17836. [DOI] [PubMed] [Google Scholar]