Abstract
The GTPases Rab3a and Rab27a and their effectors Granuphilin/Slp4 and Noc2 are essential regulators of neuroendocrine secretion. Chronic exposure of pancreatic β-cells to supraphysiological glucose levels decreased selectively the expression of these proteins. This glucotoxic effect was mimicked by cAMP-raising agents and blocked by PKA inhibitors. We demonstrate that the transcriptional repressor ICER, which is induced in a PKA-dependent manner by chronic hyperglycemia and cAMP-raising agents, is responsible for the decline of the four genes. ICER overexpression diminished the level of Granuphilin, Noc2, Rab3a and Rab27a by binding to cAMP responsive elements located in the promoters of these genes and inhibited exocytosis of β-cells in response to secretagogues. Moreover, the loss in the expression of the genes of the secretory machinery caused by glucose and cAMP-raising agents was prevented by an antisense construct that reduces ICER levels. We propose that induction of inappropriate ICER levels lead to defects in the secretory process of pancreatic β-cells possibly contributing, in conjunction with other known deleterious effects of hyperglycemia, to defective insulin release in type 2 diabetes.
Keywords: cAMP, exocytosis, GTPase, ICER, pancreatic islet
Introduction
The release of appropriate amounts of insulin from pancreatic β-cells is an essential prerequisite for the achievement of blood glucose homeostasis. Defects in this process can cause profound metabolic disorders and, eventually, lead to diabetes mellitus. Fine-tuning of insulin release necessitates a tight control of the final events directing insulin exocytosis. During the last few years, many of the proteins playing a key role in the regulation of these events have been identified. These proteins include the SNAREs SNAP25, Syntaxin-1 and VAMP-2, several components controlling the assembly of the SNARE complex such as Munc18 and complexin-1 and members of the Synaptotagmin family that couple Ca2+ changes to vesicle fusion (for a review see Lang, 1999; Easom, 2000; Rorsman and Renström, 2003). In addition, Rab3a and Rab27a, two members of the Rab GTPase family associated with insulin-containing secretory granules, and their effectors RIM2, MyRIP/Slac2c, Noc2 and Granuphilin/Slp4 were found to be necessary for proper regulation of insulin release (Iezzi et al, 1999; Ozaki et al, 2000; Coppola et al, 2002; Yi et al, 2002; Waselle et al, 2003; Cheviet et al, 2004a). The involvement of Rab3a, Rab27a, Noc2 and Granuphilin in the control of blood glucose levels has been confirmed also in vivo (Yaekura et al, 2003; Matsumoto et al, 2004; Gomi et al, 2005; Kasai et al, 2005). Despite the demonstration of their roles in insulin secretion, the mechanisms determining the expression of these key regulators of hormone and neurotransmitter release is poorly understood and the capacity of the secretory machinery of β-cells to adapt to various physiological and pathological conditions has not been explored in detail.
Glucose is the main physiological stimulus of insulin secretion but prolonged exposure of pancreatic β-cell to high glucose levels leads to alterations in the secretory function, including increased insulin release under basal conditions and reduced capacity to respond to secretagogues (Eizirik et al, 1992). Long-term exposure of β-cells to physiologically elevated glucose concentrations is known to have profound impacts on gene expression, but the effect of prolonged hyperglycemia on the expression of the components of the secretory machinery of β-cells has not been investigated in detail. Moreover, the promoters of the key genes involved in insulin exocytosis and the transcription factors susceptible to control their expression have not been characterized.
In this study, we found that incubation of the insulin-secreting cell line INS-1E in the presence of elevated glucose concentrations results in a selective decrease in the expression of Rab3a, Rab27a and of two of their effectors, Noc2 and Granuphilin. The promoters of the four genes involved in exocytosis that are affected by glucose were found to contain consensus elements for the binding of ICER (inducible cAMP early repressor), a transcriptional repressor whose expression is increased under hyperglycemic and hyperlipidemic conditions (Zhou et al, 2003) and in animal models of type 2 diabetes (Inada et al, 1998). Indeed, we found that ICER is a potent inhibitor of Granuphilin, Noc2, Rab3a and Rab27a synthesis. Our data indicate that inappropriate levels of ICER can repress the expression of these components of the machinery of insulin exocytosis potentially contributing to the secretory defects observed after chronic exposure of pancreatic β-cells to high glucose concentrations. In view of the wide range of cells in which Rab3a, Rab27a, Noc2 or Granuphilin regulate secretion, we propose that ICER induction may contribute to modulate the function of the exocytotic machinery in other secretory systems and be part of adaptive processes such as synaptic plasticity.
Results
Prolonged exposure of pancreatic β-cells to elevated glucose concentrations is known to lead to secretory defects, including excessive insulin release under basal conditions and diminished secretory capacity in response to stimuli (Eizirik et al, 1992). Incubation of the rat β-cell line INS-1E for 2 days in the presence of 20 mM glucose resulted in a similar impairment in the secretory process. Indeed, incubated for 45 min at basal conditions INS-1E cells cultured at 2 mM glucose released 2.1±0.1% (n=3) of their hormone content, while the corresponding value for the cells cultured at 20 mM glucose was 4.4±1.2% (n=3). Moreover, high glucose culture reduced hormone release elicited by glucose from 24.2±1.7% (n=3) to 7.8±0.6% (n=3) and secretion triggered by depolarizing K+ concentrations (30 mM) from 17.2±1.1% (n=3) to 7.2±0.8% (n=3).
In view of these findings, we determined by Northern blotting the expression level of several key components of the secretory machinery of pancreatic β-cells. We found that after 2 days culture in the presence of 20 mM glucose, the expression of the Rab GTPases Rab3a and Rab27a and of their effectors, Granuphilin and Noc2 was drastically diminished (Figure 1A). As expected, the expression of other control genes such as MIF (Waeber et al, 1997; Plaisance et al, 2002) was increased. The decrease in the mRNA level of Rab GTPases and of their effectors was associated with a reduction in the protein content. Indeed, Western blot analysis demonstrated that the expression of Granuphilin, Noc2 and Rab3a is strongly reduced after culture at 20 mM glucose (Figure 1B). Unfortunately, INS-1E cells contain relatively low levels of Rab27a and using our antibodies we were unable to detect the protein by Western blotting (data not shown). However, immunocytochemical analysis confirmed that prolonged exposure to elevated glucose levels diminishes the cellular content of all four proteins (Supplementary Figure 1). The effect of glucose was specific for Rab3a, Rab27a, Granuphilin and Noc2. In fact, the expression of Rab8, another member of the Rab family, was not altered by hyperglycemia (Figure 1B). Moreover, the level of other Rab3a and Rab27a effectors such as MyRIP and RIM2 and of two proteins regulating the activation state of Rab3a, Rab3-GAP and Rab3-GEF (Oishi et al, 1998) was not significantly different from cells incubated at low glucose. Several other proteins known to be involved in the regulation of insulin exocytosis, including the SNAREs Syntaxin-1, SNAP-25 and VAMP-2 and the SNARE-interacting proteins Complexin-1, Munc18.1 and Tomosyn, were also not affected by the incubation at 20 mM glucose. The expression of Synaptotagmin IX that functions as a Ca2+ sensor in insulin exocytosis (Iezzi et al, 2004) was slightly increased after culture at 20 mM glucose (Figure 1B). To verify if glucose affects the expression of the components of the secretory machinery also in primary β-cells, we incubated rat pancreatic islets for 96 h at 33 mM glucose, a condition that is known to result in secretory defects analogous to those observed in INS-1E cells (Zhou et al, 2003). Real-time PCR analysis revealed that prolonged incubation of rat pancreatic islets at elevated glucose concentration caused a significant reduction in the expression of Granuphilin, Noc2, Rab3a and Rab27a (Figure 2). This effect was not the result of a generalized decrease in gene expression because the level of VAMP-2 and Rab3-GAP (data not shown) was not altered and the expression of other genes was augmented (see below).
Figure 1.
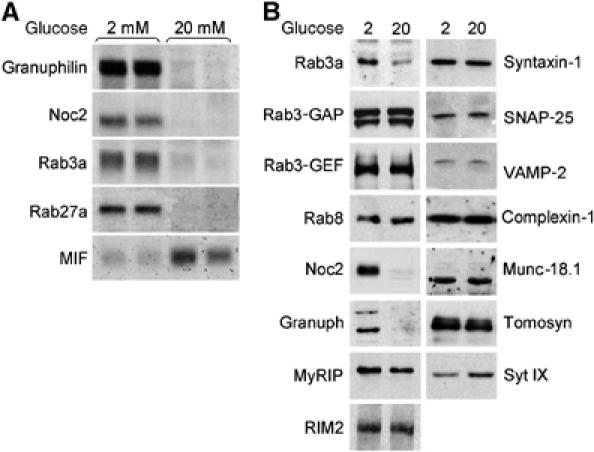
Effect of prolonged exposure to elevated glucose concentrations on mRNA and protein levels of Granuphilin, Noc2, Rab3a and Rab27a. (A) INS-1E cells were cultured in the presence of 2 or 20 mM glucose. After 2 days, total RNA was extracted and the expression of the indicated genes analyzed by Northern blotting. (B) INS-1E cells were cultured for 2 days at 2 or 20 mM glucose. Expression of the indicated proteins involved in the regulation of insulin exocytosis was determined by Western blotting. The figure shows representative results obtained in at least three independent experiments.
Figure 2.
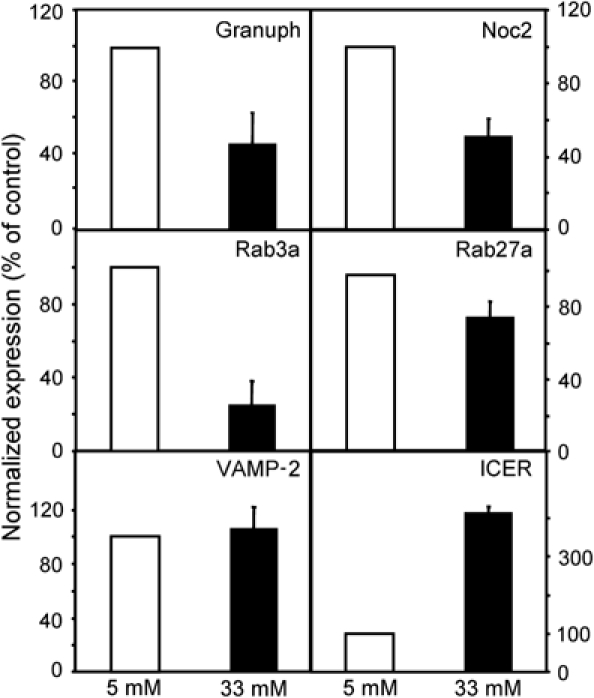
Effect of high glucose on the expression of key regulators of insulin exocytosis in rat pancreatic islets. Freshly isolated rat pancreatic islets were cultured for 96 h at 5 (open bars) or 33 mM glucose (filled bars). Expression of the indicated genes was assessed by real-time PCR analysis. The results were normalized with the values obtained for tubulin in the same sample. Normalization using Rab3GAP values gave similar results. The data obtained in control islets were set to 100%. The results are the mean±s.e.m. of four to five independent experiments performed in triplicates. The mRNA levels of Granuphilin, Noc2, Rab3a Rab27a and ICER at 33mM glucose are significantly different (P<0.001, n=4–5, Student's t-test) than in control cells. The expression of VAMP-2 at 5 and 33 mM glucose was not significantly altered.
In INS-1E cells, a decrease in the expression of Granuphilin, Noc2 and Rab3a was already detectable in the presence of 10 mM glucose and was maximal at 15 mM (Figure 3A). The effect of 20 mM glucose was apparent after 24 h incubation and was maximal after 2 days (Figure 3B). The reduction in Granuphilin, Noc2 and Rab3a expression was not the result of irreversible cell damage. In fact, 36 h incubation at 2 mM glucose after 2 days exposure to 20 mM glucose was sufficient to recover normal levels of the three proteins (Figure 4A) and to restore exocytosis (data not shown).
Figure 3.
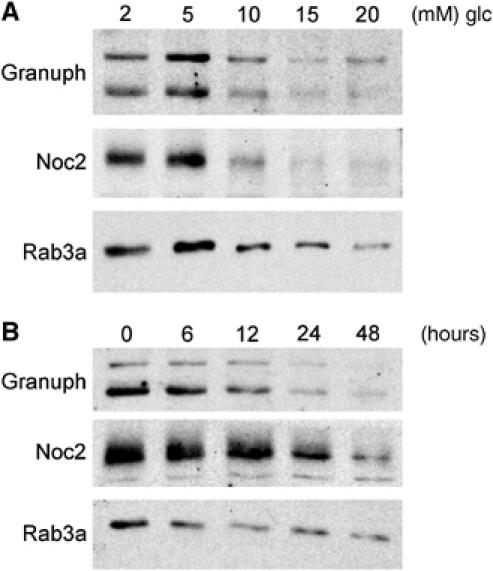
Dose and time dependency of the effect of glucose on Granuphilin, Noc2 and Rab3a expression. (A) INS-1E cells were cultured for 2 days in the presence of the indicated glucose (glc) concentrations. The level of Granuphilin, Noc2 and Rab3a was assessed by Western blotting. (B) INS-1E cells were incubated for the indicated periods in the presence of 20 mM glucose. The amount of Granuphilin, Noc2 and Rab3a remaining in the cells was estimated by Western blotting.
Figure 4.
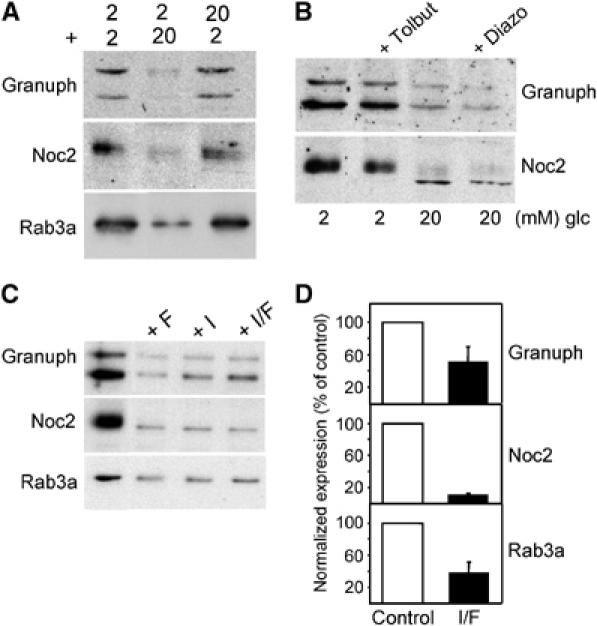
The effect of glucose is reversible and can be mimicked by cAMP-raising agents but not with pharmacological agents that affect insulin secretion. (A) The cells were incubated either at 2 mM (2) or 20 mM (20) glucose for 36 h. The medium was then replaced and the cells incubated for a second period of 36 h at 2 or 20 mM glucose. At the end of the experiment, the cells were homogenized and the extracts analyzed by Western blotting with the indicated antibodies. (B) The cells were cultured for 2 days at 2 mM glucose in the presence or absence of 100 μM Tolbutamide (Tolbut) or at 20 mM glucose with or without 200 μM Diazoxide (Diazo). The expression of Granuphilin and Noc2 was analyzed by Western blotting. (C) INS-1E cells were cultured for two days at 2 mM glucose with or without Forskolin (10 μM) (F) and IBMX (100 μM) (I). The level of Granuphilin, Noc2 and Rab3a was assessed by Western blotting. (D) Freshly isolated rat pancreatic islets were incubated with (filled bars) or without (open bars) Forskolin (10 μM) (F) and IBMX (100 μM) (I) for 72 h. Expression of Granuphilin, Noc2 and Rab3a was assessed by real-time PCR analysis. The values obtained in control islets were normalized to 100%. The results are the mean±s.e.m. of three independent experiments performed in triplicates.
Glucose is the most important physiological stimulus of insulin secretion. To test whether the inhibition of Granuphilin, Noc2 and Rab3a synthesis is linked to chronic stimulation of insulin release, INS-1E were cultured in the presence of Tolbutamide, a pharmacological agent that triggers insulin secretion by closing ATP-sensitive K+-channels and depolarizing the cell membrane (Doyle and Egan, 2003). At the concentrations used in this study (100 μM), the amount of insulin released in the presence Tolbutamide was comparable to that secreted in response to 20 mM glucose (not shown). However, Tolbutamide did not affect expression of Granuphilin and Noc2 (Figure 4B). Moreover, 200 μM Diazoxide, a drug that inhibits insulin release by impeding the closure of ATP-sensitive K+-channels (Doyle and Egan, 2003) did not prevent the drop in the expression of the two Rab GTPase effectors (Figure 4B).
We then tested whether the effect of glucose was mimicked by incubation with cAMP-raising agents. Indeed, chronic exposure to Forskolin and IBMX resulted in a strong reduction in Granuphilin, Noc2 and Rab3a expression in INS-1E cells (Figure 4C). A similar decrease in the expression of these genes was also observed in MIN6 cells (data not shown) and isolated rat pancreatic islets by real-time PCR analysis (Figure 4D). The effect of glucose and cAMP-raising agents on Granuphilin expression was reproduced using a luciferase reporter gene under the control of the human Granuphilin promoter. In fact, 2 days culture in the presence of glucose, Forskolin or IBMX resulted in a sizable decrease in luciferase activity (Figure 5A). Incubation of the cells in the presence of the protein kinase A (PKA) inhibitor H89 prevented the effect of glucose and Forskolin/IBMX on the activity of the Granuphilin promoter (Figure 5B) and restored the expression of Granuphilin and Noc2 (Figure 5C), confirming that the effect of glucose involves a PKA-mediated event.
Figure 5.
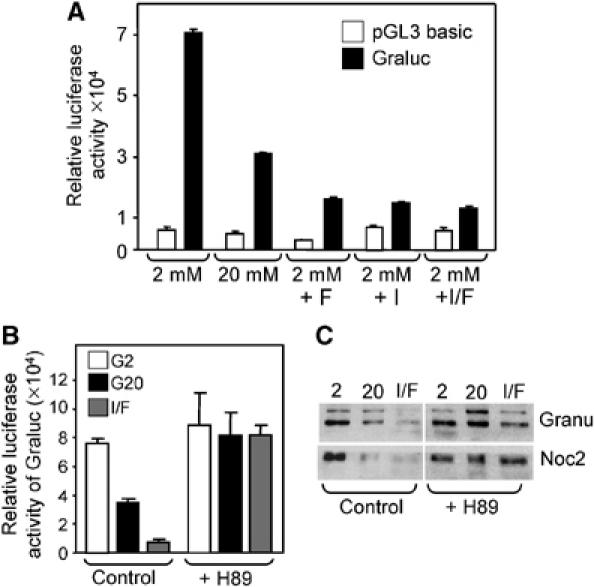
The effect of glucose on the activity of the Granuphilin promoter is mimicked by cAMP-raising agents and is prevented by the PKA inhibitor H89. (A) INS-1E cells were transiently transfected either with a plasmid encoding a luciferase reporter gene controlled by a minimal promoter (pGL3 basic, open bars) or driven by the human Granuphilin promoter (Graluc, filled bars). After transfection the cells were cultured for 2 days either at 2 mM glucose with or without Forskolin/IBMX or at 20 mM glucose. (B) INS-1E cells transiently transfected with a plasmid encoding a luciferase reporter gene controlled by the human Granuphilin promoter were cultured for 2 days either at 2 mM glucose (G2) with or without Forskolin/IBMX (I/F) or at 20 mM glucose (G20). Half of the cells were treated with the PKA inhibitor H89 (10 μM) while control cells were incubated with vehicle. All results from transfection experiments are the mean±s.e.m. of three independent experiments performed in triplicates. (C) INS-1E cells were cultured for 2 days at 2 mM glucose (2), 20 mM glucose (20) or at 2 mM glucose in the presence of Forskolin/IBMX (I/F). Where indicated, the PKA inhibitor H89 (10 μM) was added to the culture medium. The expression of Granuphilin (Granu) and Noc2 was assessed by Western blotting. The results are representative of three independent experiments.
Close inspection of the promoter region of Granuphilin, Noc2, Rab3a and Rab27a revealed the presence of putative cAMP-responsive elements (CRE) potentially capable of binding the transcriptional repressor ICER (Table I). Interestingly, ICER expression has been shown to be increased in pancreatic islets exposed to hyperglycemic conditions (Zhou et al, 2003). Indeed, we found that in pancreatic islets cultured at elevated glucose concentration, the decrease in the expression of Granuphilin, Noc2, Rab3a and Rab27a is associated with a four-fold increase in the expression of ICER (Figure 2). Similar data were obtained after 72 h at high glucose. In contrast, 48 h culture of pancreatic islets at 33 mM glucose changed neither the expression of ICER nor that of the components of the secretory machinery (data not shown), suggesting a direct relationship between these events. Western blot analysis performed after culture at high glucose concentration or in the presence of IBMX/Forskolin confirmed that the reduction in the expression Granuphilin, Noc2, Rab3a and Rab27a is paralleled by the induction of ICER also in INS-1E cells (Figure 6A). As expected, the increase in ICER levels triggered by glucose and IBMX/Forskolin was inhibited by H89 (data not shown). The induction of ICER and its ability to bind to the elements identified in the genes involved in insulin exocytosis was further investigated by EMSA (Figures 6B and 7). Incubation of nuclear extracts of INS-1E with a radiolabeled probe corresponding to the consensus sequence recognized by ICER resulted in the same binding pattern observed in other cell systems (Lamas et al, 1997). This complex binding pattern is likely to be due to the interaction of the probe with different CREB and cAMP response element modulator (CREM) isoforms. Treatment of the cells with Forskolin/IBMX or with 20 mM glucose resulted in a strong induction of two fast migrating complexes (Figure 6B). The major band was identified as ICER because it comigrates with exogenously expressed ICER-Iγ, and the formation of this complex is selectively prevented by the addition of an anti-CREM antibody (Delmas et al, 1993) (Figure 6B). The other minor complex induced by Forskolin/IBMX and glucose was also disrupted by the anti-CREM antibody, and is likely to correspond to a different CREM isoform. Antibodies directed against two unrelated transcription factors, Beta2 and REST did not affect the formation of the complexes with the radiolabeled probe. We then performed competition experiments to assess whether ICER binds to the putative CRE identified in the Granuphilin promoter. Indeed, oligonucleotides sequences derived from rat and human promoters efficiently displaced the labeled probe known to be recognized by ICER (Figure 7A). Replacement of two nucleotides in the putative CRE of human Granuphilin (H mut) did not compete for the binding of ICER with the wild-type labeled sequence, confirming the specificity of the interaction with the transcriptional repressor. The binding activity of the putative CRE found in the promoters of Rab3a, Rab27a and Noc2 was tested using competition experiments. As shown in Figure 7B, oligonucleotides containing the putative CRE identified in the three promoters were able to compete for the binding of ICER, suggesting that Rab3a, Rab27a and Noc2 are also targets of the transcriptional repressor.
Table 1.
Promoters of Granuphilin, Rab3a, Rab27a and Noc2 contain consensus elements for the binding of the transcriptional repressor ICER
| Gene | Species | Sequence |
|---|---|---|
| G6pase CRE-1 | TTGCATCA | |
| G6pase CRE-2 | TTACGTAA | |
| Consensus | TGACGTCA | |
| Granuphilin | R | GCAGAGACGTACGGGCAA |
| H | ATTTTTATGTCAGTACGT | |
| M | CTGCTGACGTGAAGCAAA | |
| Rab3a | R | ATGGTGACGTCATGGCGC |
| H | CTCCTGACCTCAAGGTCA | |
| M | CTGATGACGTGAGTTCCA | |
| Rab27a | R | GCCTGACGAGAGTGAGG |
| H | AAGTTGACATAAATCTAG | |
| M | TAGCTGACTTCAGAT GTA | |
| Noc2 | R | CAGATGACATCAATCTAA |
| H | GCTCTGACGTCTGCATCG | |
| |
M |
AAGATGACCTTAGAGATC |
| The consensus for the binding of ICER was obtained by combining the sequences of the cAMP-response element 1 (CRE-1) and 2 (CRE-2) of glucose 6 phosphatase (G6pase). The putative binding elements identified in the promoters are shown in bold. The nucleotides matching the consensus are underlined. R, rat; H, human; M, mouse | ||
Figure 6.
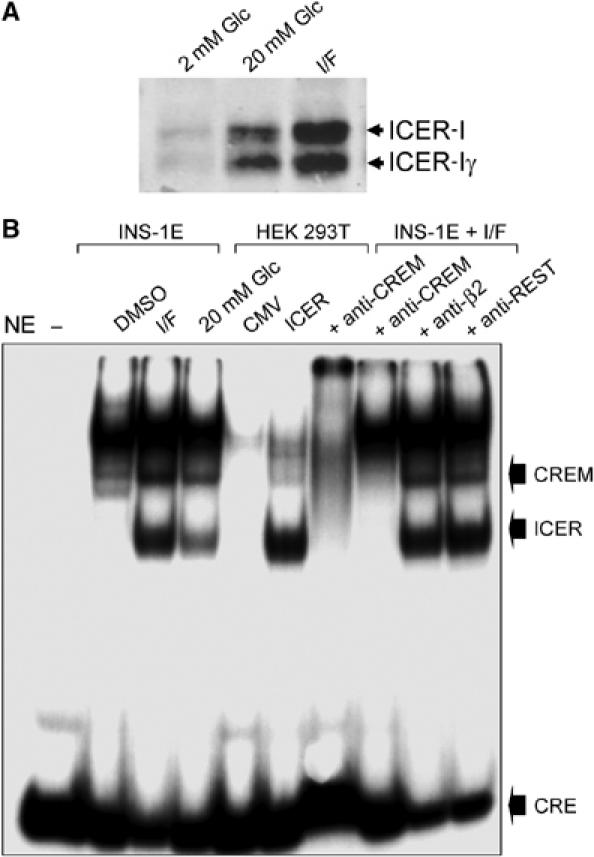
Induction of ICER protein and binding activity in INS-1E cells exposed to cAMP-raising agents or to elevated glucose concentrations. (A) INS-1E cells were cultured for 2 days at 2 mM glucose (2 mM Glc), 20 mM glucose (20 mM Glc) or at 2 mM Glc in the presence of IBMX/Forkolin (I/F). The expression of the different ICER isoforms was assessed by Western blotting. (B) Nuclear extracts (NE) obtained from INS-1E cells incubated with vehicle (DMSO), treated for 48 h with IBMX and Forskolin (I/F) or 20 mM glucose were incubated with a radioactively labeled oligonucleotide containing the consensus sequence for the binding of ICER. For comparison, nuclear extracts of HEK 293T cells transfected with an empty vector (CMV) or with a plasmid producing ICER-Iγ (ICER) were incubated in parallel with the probe. The radioactive complexes formed during the incubation were analyzed by electrophoresis and visualized by autoradiography. In the sample loaded in the first lane, the nuclear extract was omitted (−). In the last four lanes, before the addition of the labeled oligonucleotide, the nuclear extracts were preincubated with antibodies recognizing all CREM isoforms (anti-CREM), the transcription factor Beta2 (anti-Beta2) or the transcriptional repressor REST (anti-REST). The positions of ICER, the other CREM isoform increased by glucose and of the unbound CRE probe are indicated. The results are representative of three independent experiments.
Figure 7.
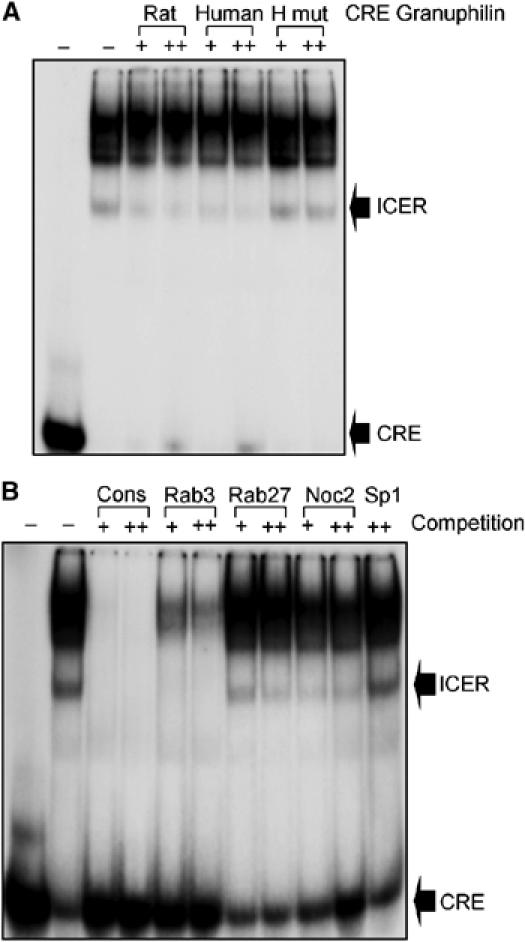
ICER binds to the putative CRE identified in the promoters of Granuphilin, Rab3a, Rab27a and Noc2. (A) Nuclear extracts (NE) of INS-1E cells treated with IBMX and Forskolin were incubated with a radioactive probe containing the consensus sequence for the binding of ICER. In the first lane, the nuclear extracts were omitted. Where indicated, the incubation was performed in the presence of 100- (+) or 400-fold (++) molar excess of unlabeled oligonucleotides corresponding to the putative CRE of rat or human Granuphilin promoters. In the last two lanes, competition was performed with a mutated CRE sequence of human Granuphilin promoter (H mut). The radioactive complexes were analyzed by electrophoresis and visualized by autoradiography. The positions of the ICER complex and of the unbound probe are indicated. (B) Nuclear extracts of INS-1E cells treated with IBMX and Forskolin were incubated with a radioactive probe containing the consensus sequence for the binding of ICER. Where indicated the incubation was performed in the presence of 100- (+) or 400-fold (++) molar excess of unlabeled oligonucleotides corresponding to the consensus sequence (cons), to rat Rab3a CRE, rat Rab27a CRE, rat Noc2 CRE or to an unrelated sequence (Sp1). In the first lane the nuclear extracts were omitted. The positions of the ICER complex and of the free probe are indicated. The results are representative of three independent experiments.
The binding of ICER to the CRE element identified in the promoter of Granuphilin was further investigated by co-transfecting INS-1E cells with the plasmid containing a luciferase reporter gene driven by the human Granuphilin promoter and with a plasmid encoding ICER-Iγ. As shown in Figure 8A, overexpression of ICER-Iγ led to strong reduction in the activity of the promoter. Introduction of two point mutations within the human Granuphilin CRE sequence (H mut) reduced the efficacy of the promoter, indicating that this element contributes to the control of the expression of the Rab effector. As expected from the binding experiments in Figure 7A, the expression of the luciferase construct containing the point mutations in human Granuphilin CRE was not repressed by co-transfection of ICER-1γ (Figure 8A) or by incubation of the cells with Forskolin and IBMX (data not shown). The involvement of the transcriptional repressor was further demonstrated by assessing the expression of Granuphilin and Noc2 in INS-1E cells transiently transfected with the ICER-Iγ-overexpressing plasmid. Under our experimental conditions, the transfection efficiency is estimated to be about 30–50%. Despite this relatively low transfection rate, we were able to detect a significant decrease in Granuphilin and Noc2 but not of Tomosyn in cells transfected with ICER-Iγ (Figure 8B). To visualize the amount of Granuphilin, Noc2, Rab3a and Rab27a remaining selectively in the cells overexpressing the transcriptional repressor, INS-1E cells were transiently co-transfected with the ICER-Iγ construct and with a plasmid encoding the green fluorescent protein (GFP). After 2 days, the fluorescent cells coexpressing GFP and ICER-Iγ were analyzed by immunofluorescence with antibodies against Granuphilin, Noc2, Rab3a and Rab27a. In agreement with the data in Figure 8B, in the cells overexpressing ICER-Iγ, the level of these four proteins was strongly reduced (Supplementary Figure 2).
Figure 8.
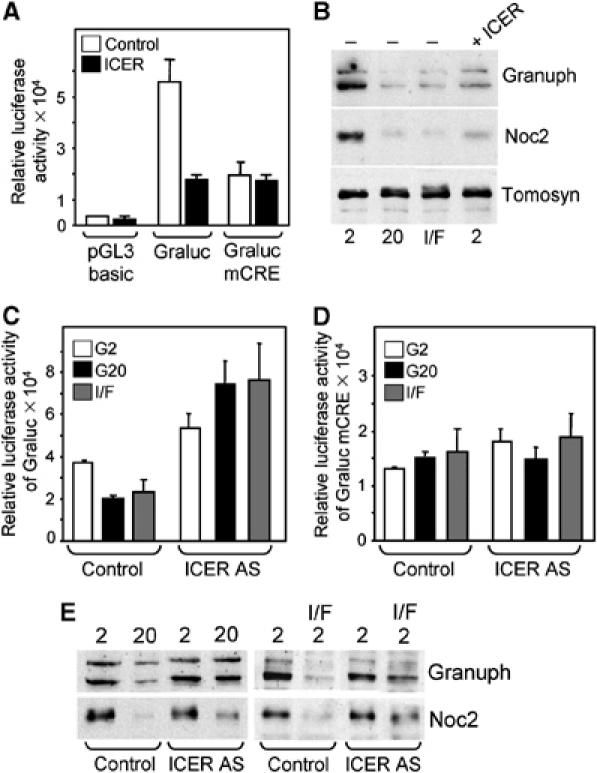
The transcriptional activity of the Granuphilin promoter and the cellular content of Granuphilin and Noc2 are linked to the expression level of ICER. (A) INS-1E cells were transiently transfected with plasmids encoding a luciferase reporter gene driven by a minimal promoter (pGL3 basic), by wild-type human Granuphilin promoter (Graluc) or by the human Granuphilin promoter in which the putative CRE was mutated (Graluc mCRE). These plasmids were co-transfected either with an empty vector (empty bars) or with a plasmid leading to the overexpression of ICERIγ (filled bars). Luciferase activity was measured 2 days later. (B) INS-1E cells transiently transfected with an empty vector (−) or with an ICER-Iγ expression plasmid (ICER) were incubated for 2 days at 2 mM glucose, 20 mM glucose or at 2 mM glucose in the presence IBMX/Forskolin (I/F). The expression of Granuphilin, Noc2 and Tomosyn was assessed by Western blotting. (C) INS-1E cells were transiently transfected with the plasmid encoding a luciferase reporter gene under the control of the Granuphilin promoter (Graluc) and with an empty vector or a vector encoding ICER antisense (ICER AS). The cells were culture at 2 mM glucose (G2), 20 mM glucose (G20) or at 2 mM in the presence of Forskolin and IBMX (I/F). The luciferase activity was measured 2 days later. (D) Same conditions as in (C) except that the luciferase reporter gene is driven by a Granuphilin promoter in which the CRE element is mutated (mCRE). (E) INS-1E cells transfected with an empty vector or with ICER antisense (ICER AS) were incubated at 2 mM glucose, 20 mM glucose or at 2 mM glucose in the presence of IBMX and Forskolin (I/F). The expression of Granuphilin and Noc2 was assessed by Western blotting. The results are representative of three independent experiments.
If the decrease in the expression of the genes controlling insulin exocytosis is mediated by ICER than silencing of the transcriptional repressor should prevent the effect of glucose and Forskolin/IBMX. Indeed, co-transfection of an antisense plasmid that specifically reduces ICER level (Lamas et al, 1997; Pfeffer et al, 1998; Maronde et al, 1999; Hussain et al, 2000) abolished the inhibition of the activity of the Granuphilin promoter caused by glucose and Forskolin/IBMX (Figure 8C). In cells expressing the antisense plasmid, the luciferase activity was significantly higher than in control cells suggesting relieve of a constitutive inhibition of the Granuphilin promoter. Transfection of the antisense plasmid had no effect on the luciferase activity driven by a Granuphilin promoter containing a mutated CRE element, confirming the specificity of the effect (Figure 8D). In agreement with these data, Western blot analysis demonstrated that transient transfection of INS-1E cells with the ICER antisense construct prevents the reduction in Granuphilin and Noc2 expression caused by glucose and Forskolin/IBMX (Figure 8E). Very similar results were obtained by transfecting the cells with a small interfering RNA directed against a sequence that is unique to ICER (Supplementary Figure 3).
Finally, we determined whether overexpression of ICER-Iγ affects the secretory process of insulin-secreting cells. For this purpose, INS-1E cells were transiently cotransfected with the ICER-Iγ-overexpressing plasmid and with a plasmid encoding the human growth hormone (hGH). We have previously shown that hGH is targeted to secretory granules and is co-released with insulin during exocytosis (Iezzi et al, 1999). This approach allows a direct assessment of the secretory activity of transiently transfected cells independently of their capacity to produce insulin. At 3 days after transfection, the cells were incubated under resting conditions or under conditions that stimulate insulin release. We found that overexpression of ICER-Iγ results in a significant decrease in unstimulated secretion and an even more pronounced reduction in hormone release in the presence of secretagogues (Figure 9). These findings demonstrate that inappropriate levels of ICER have deleterious effects on the secretory capacity of pancreatic β-cells.
Figure 9.
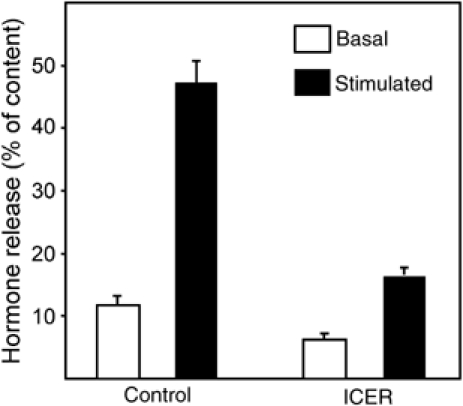
Effect of ICER overexpression on β-cell exocytosis. INS-1E cells were transiently co-transfected with an hGH encoding plasmid and with an empty plasmid (control) or a plasmid leading to the overexpression of ICER-Iγ (ICER). After 3 days culture in normal RPMI medium, the cells were incubated during 45 min at 2 mM glucose (basal condition, open bars) or in the presence of 20 mM glucose, 10 μM Forskolin and 100 μM IBMX (stimulatory condition, filled bars). The total amount of hGH present in the cells and the fraction released in the medium during the 45 min incubation period were measured by ELISA. The results are the means±s.e.m. of three independent experiments performed in triplicates.
Discussion
Type 2 diabetes is characterized by insulin resistance combined with a progressive loss of pancreatic β-cell function. Prolonged hyperglycemia and hyperlipidemia have detrimental effects on insulin-secreting cells and accelerate β-cell failure. Long-term exposure of pancreatic islets to physiologically elevated glucose concentrations lead to modification in the expression of many genes, including that of insulin (Weir et al, 2001). These important alterations in β-cell function are further exacerbated by the appearance of major defects in the secretory process of β-cells and, in particular, a decreased capability to release insulin in response to secretagogues (Eizirik et al, 1992; Zhou et al, 2003). Excessively elevated glucose and fatty acids downregulate the expression of important genes controlling insulin synthesis and β-cell metabolism, partially explaining the loss in the secretory response (Deeney et al, 2000). However, the release of insufficient amounts of insulin could also result from defects in the exocytotic process. Despite recent progress in the identification of the major components controlling insulin exocytosis (Lang, 1999; Easom, 2000; Rorsman and Renström, 2003), the capacity of the secretory machinery to adapt to physiological and pathological conditions has been poorly investigated. In Goto-Kakizaki (GK) rats, a spontaneous model of type 2 diabetes, the number of secretory granules docked at the plasma membrane is reduced and insulin exocytosis is defective (Nagamatsu et al, 1999). This finding was attributed to dysregulation in the expression of SNARE proteins (Nagamatsu et al, 1999; Gaisano et al, 2002). However, only the expression of few constituents of the secretory machinery was analyzed. The Rab GTPases Rab3a and Rab27a and their effectors Noc2 and Granuphilin are associated with secretory vesicles of endocrine and neuroendocrine cells and are major players in the regulation of hormone and neurotransmitter release (Izumi et al, 2003; Cheviet et al, 2004b; Südhof, 2004). Evidence for a central role for these proteins in pancreatic β-cell exocytosis has been provided both in vitro (Iezzi et al, 1999; Ozaki et al, 2000; Coppola et al, 2002; Yi et al, 2002; Waselle et al, 2003; Cheviet et al, 2004a) and in vivo (Yaekura et al, 2003; Matsumoto et al, 2004; Gomi et al, 2005; Kasai et al, 2005). Indeed, mice lacking Rab3a, Rab27a or Noc2 display impaired glucose-induced insulin release and elevated plasma glucose levels. In this study, we demonstrate that the production of these proteins is dynamically regulated by glucose. Chronic exposure of INS-1E cells to elevated glucose concentrations leads to a dramatic decrease in the expression of the genes encoding for these proteins. The effect of glucose is not the result of irreversible cell damage because, after returning the cells to resting conditions, normal levels of Granuphilin, Noc2 and Rab3a and normal secretory rates can be recovered within few hours. Although the effect was quantitatively less pronounced, prolonged exposure of pancreatic islets to elevated glucose concentrations led to similar results. Several factors could account for the quantitative difference between the two systems. It should be noted that Rab3a, Rab27a and Noc2 are not expressed exclusively in insulin-secreting cells. In the other cells of the pancreatic islet, the expression of these genes may not be modulated by glucose. It is also possible that INS-1E cells display a higher sensitivity to glucose compared to primary β-cells.
The effect of glucose on the expression of Rab GTPases and of their effectors was mimicked by Forskolin and IBMX and was prevented by a pharmacological PKA inhibitor, suggesting the involvement of the cAMP pathway. Glucose is known to raise cAMP both in insulin-secreting cell lines and in primary β-cells (Hellqvist et al, 1984; Briaud et al, 2003; Costes et al, 2004; Allagnat et al, 2005). The increase in cAMP elicited by glucose is relatively small compared to pharmacological stimuli such as Forskolin (Allagnat et al, 2005). However, even minor elevations in cAMP levels that persist for several hours may be sufficient to trigger long-term effects on gene expression such as the one described in this study. In addition, glucose elicits a variety of other signaling cascades that could potentially synergize with cAMP and contribute to the activation of PKA.
Using a combination of approaches, we provide strong evidence that the effect of glucose is mediated by the inducible transcription factor ICER, a member of the CREM family of basic leucine zipper transcription factors that is thought to serve as a dominant-negative repressor of cAMP-dependent gene expression. First, prolonged exposure to high glucose increases ICER transcriptional activity and ICER mRNA level confirming recent data obtained by others (Zhou et al, 2003). Second, we have identified functional elements for the binding of ICER in the promoters of Granuphilin, Noc2, Rab3a and Rab27a. Third, we demonstrate that overexpression of ICER-Iγ suppresses the expression of the two Rab GTPases and of their effectors, and that the decrease in the level of these proteins elicited by glucose can be prevented by transfection of the cells with an ICER antisense construct. Finally, we show that exocytosis is impaired in INS-1E cells transfected with ICER-Iγ, a result in good agreement with a recent study reporting a decrease in stimulus-induced secretion in rat pancreatic islets overexpressing a CREM isoform closely related to ICER-Iγ (Zhou et al, 2003). Taken together these data identify ICER as a central regulator of the secretory function of pancreatic β-cells. Alterations in the level of this transcriptional repressor are expected to result in defects in insulin exocytosis and could predispose to diabetes. In line with this assumption, ICER-Iγ expression was found to be increased in pancreatic islets of type 2 diabetic rats (Inada et al, 1998) and transgenic mice with β-cell-directed overexpression of ICER-Iγ suffer from severe diabetes (Inada et al, 2004). Plasma insulin concentrations in these transgenic animals are extremely low due to abnormal islet morphology, reduced β-cells mass and low insulin production. Expression of proteins regulating insulin exocytosis was not investigated in this mice model but glucose-induced insulin release from ICER-Iγ overexpressing β-cells was abolished (Inada et al, 2004), pointing to an additional defect in the secretory machinery. Therefore, the presence of inappropriate levels of one or more members of the ICER family have deleterious impacts on several important pancreatic β-cell functions, including proliferation, insulin production and insulin secretion. Because of the combination of adverse effects of ICER on β-cells even relatively low but persistent increases in the amount of these transcriptional repressors are likely to have major consequences on glucose homeostasis and could, added to the other detrimental effects resulting from chronic hyperglycemia, contribute to the development of type 2 diabetes. Induction of ICER in response to physiological agonists such as glucagon is normally transient (Hussain et al, 2000). However, prolonged exposure of β-cells to glucose leads to an elevation of the transcriptional repressor both in cell lines and in primary β-cells that persists for several days (Zhou et al, 2003; present study). The causes of the alteration in the control of ICER expression after prolonged exposure to supraphysiological glucose levels are still poorly understood. ICER expression has been well described to be coupled to the cAMP/PKA pathway in several cell types (Mukherjee et al, 1998; Nervina et al, 2003). In agreement with the data obtained in other cell systems, we found that inhibition of the PKA-dependent pathway prevents both the induction of ICER and the decline in the expression of the components of the exocytotic machinery caused by glucose and cAMP-raising agents. Our observations indicate that the effect of glucose is at least dependent on the activation of PKA. The determination of the precise signaling cascade underlying the long-term effects of glucose is a central issue and will be one of the main challenges for future studies investigating the contribution of ICER to β-cell failure.
The results presented in this study have relevant implications not only for elucidating the mechanisms controlling insulin release under normal and disease states but also for understanding the regulation of exocytosis in other cell systems. In fact, the secretory process of most endocrine and exocrine glands, neurons and cells of hematopoïetic origin are regulated by one or more ICER targets identified in this study (Izumi et al, 2003; Cheviet et al, 2004b; Südhof, 2004; Tolmachova et al, 2004). In view of our findings, induction of ICER expression is anticipated to have profound impacts on exocytosis in all these secretory systems. Indeed, ectopic expression of ICER in a pituitary corticotroph cell line has already been shown to cause a complete block of hormone release (Lamas et al, 1997). A variety of physiological and pathological stimuli are known to induce the expression of ICER in endocrine glands and in different brain regions (Mioduszewska et al, 2003). We propose that a rise in ICER expression is part of adaptive processes in which the activity of the exocytotic machinery needs to be modified. These processes could include certain forms of synaptic plasticity and circadian fluctuations in hormone release.
In conclusion, we highlighted a role for the transcriptional repressor ICER in the control of the expression of four key components of the exocytotic machinery regulating hormone and neurotransmitter release and demonstrated its potential involvement in physiopathological conditions affecting β-cell function. Future studies will have to determine the precise signaling cascade leading to persistent induction of ICER in the presence of elevated glucose concentrations and to pinpoint the role of ICER in the modulation of exocytosis in other secretory systems.
Materials and methods
Materials
The antibodies against Noc2, MyRIP/Slac2-c, Tomosyn, SNAP-25 and CREM were provided by Dr RD Burgoyne (University of Liverpool, UK), Dr A El-Amraoui (Institut Pasteur, Paris, France), Dr R Jahn (University of Göttingen, Germany) Dr H Hirling (EPFL, Lausanne, Switzerland) and Dr E Lalli (IPMC Nice University), respectively. The antibodies directed against Rab3-GAP and Rab3-GEP were generous gifts of Dr U Blank (Inserm U 699, Paris, France). The following antibodies were obtained from commercial sources: Rab3a, RIM2, VAMP-2, Complexin-1 were from Synaptic System (Göttingen, Germany); Rab27a, Rab8, Munc-18.1 and Synaptotagmin IX were from BD Biosciences (San Jose, USA); Syntaxin-1a was from Sigma Aldrich (St-Louis, MI). Generation of the antibody against Granuphilin has been described previously (Coppola et al, 2002). The pSV-ICER expression plasmid (Molina et al, 1993) was provided by Dr Sassone-Corsi, Strasbourg, France.
Plasmid construction
The human Granuphilin promoter was amplified by PCR on human genomic DNA (Sigma, St Louis MI). Complementary single-stranded oligodeoxynucleotides were synthesized to incorporate KpnI and XhoI sites to the sequences located in the region −4001 to −1, 5′ of the transcription start site of the human Granuphilin gene (GenBank Accession No. NT_011651.15) with the following primers sense: 5′-GGGGTACCGAGAGTGGTTCAAATTGT-3′ (KpnI site underlined, Granuphilin gene in bold) and antisense 5′-CGGCTCGAGGATTTACTCAACTTTTTC-3′ (XhoI site underlined, Granuphilin gene in bold). The PCR reaction was performed using the Expand PCR system (Roche, Rotkreuz, Switzerland), following supplier conditions. The PCR product was cut with KpnI and XhoII and cloned into the corresponding sites of the luciferase reporter plasmid pGL3-Basic (Promega, Madison, WI). Mutation in the CRE sequence of the human Granuphilin promoter (Graluc) was generated by PCR-site-directed mutagenesis using high fidelity Pfu DNA polymerase, according to the manufacturer's protocol (QuikChange™, Stratagene, La Jolla, CA). In vitro mutagenesis was carried out on full-length Graluc construct from double-stranded 8.9 kb plasmid DNA using two oligonucleotides primers each complementary to opposite strands of the vector (sense: 5′-GGCCATATTTTTATTTTAGTACGTGTGTATCT ACCACC-3′ and antisense: 5′-GGTGGTAGATACACACGTACTAAAATAAAAAT ATGGCC-3′). The CRE sequence is underlined and the mutated nucleotides are in bold. The plasmid encoding ICER antisense (ICER AS) was constructed by inserting PCR-amplified fragment of ICER from pSV-ICER. Primers were: Sense 5′-AGAAAGTCTAGACATGGCTGTAACTGGAGATG AA-3′ and antisense 5′- ACTGTGCAGGATCCCTGGTGAGGCAGC-3′. The PCR fragment was inserted between the BamHI and XbaI cloning sites of the pcDNA3 vector. The siRNA duplex against the rat ICER sequence CTGGAGATGAAACTGCTGC was obtained from Eurogentec (Liege, Belgium).
Cell culture and transfection
The insulin-secreting cell line INS-1E (Merglen et al, 2004) was cultured in RPMI 1640 medium supplemented with 5% fetal calf serum, 50 UI/ml penicillin, 50 μg/ml streptomycin, 0.1 mM sodium pyruvate and 0.001% β-mercaptoethanol. Transient transfection experiments were performed using the Lipofectamine 2000 transfection Kit (Invitrogen, Carlsbad, CA).
Preparation of rat islets
Rat islets were isolated from the pancreas of male Sprague–Dawley rats weighing 250–350 g by ductal injection of collagenase. The purification of islets was conducted as described (Sutton et al, 1986).
Immunocytochemistry
INS-1E cells were seeded on glass coverslips coated with 20 μg/ml laminin and 2 mg/ml poly-L-Lysine. The day after, they were incubated for two days in glucose-free RPMI 1640 medium supplemented with the indicated concentrations of D-Glucose. The cells were fixed in 4% paraformaldehyde and incubated for 2 h with the first antibody diluted in PBS, pH 7.5, 0.1% goat serum, 0.3% Triton-X-100 and 20 mg/ml bovine serum albumin (BSA). The coverslips were rinsed with PBS, incubated for 30 min with the secondary antibody diluted in the same buffer and mounted for confocal microscopy (Leica, model TCS NT, Lasertechnik, Heidelberg, Germany).
Northern and Western blots
Total RNA extraction and Northern blotting were performed as previously described (Abderrahmani et al, 2001). For Western blotting, the cells were scraped and lysed by brief sonication. The cell extracts were separated by SDS–PAGE and blotted onto nitrocellulose membranes. The proteins were detected using specific antibodies and were visualized by chemiluminescence using horseradish peroxidase coupled secondary antibody.
Real-time PCR
Total RNA from pancreatic islets was extracted using the RNA purification kit (Ambion, Austin, Texas), according to the manufacturer's protocol. Reverse transcription reactions were performed as previously described (Plaisance et al, 2005). Real-time PCR assays were carried out on a BioRad MyiQ Single-Color Real-Time PCR Detection System using the BioRad iQ SYBR Green Supermix, with 100 nM primers and 1 μl of template (RT reaction) per 20 μl of PCR and an annealing temperature of 59°C. Melting curve analyses were performed on all PCRs to rule out nonspecific amplification. Reactions were performed in triplicates. Primers sequences for PCR were: β-tubulin, sense 5′-GGAGGATGCTGCCAATAACT-3′ and antisense 5′-GGTGGTGAGGATGGAATTGT-3′; Granuphilin, sense 5′-GAGAATGCCGAGTTCTGGAG-3′ and antisense 5′-GTCTGCTGAAGGAGGGACTG-3′; Noc2, sense 5′-GGCACACTCTCTGGAGGAAG-3′ and antisense 5′-GGGGAGGGGCAATAAATAAA-3; Rab3a, sense 5′-GTCAGCACTGTGGGCATAGA-3′ and antisense 5′-TGCACTGCATTGAAGGACTC-3′; Rab3GAP, sense 5′-ATGCAGAGTGCCTGTCTCCT-3′ and antisense 5′-ACATGTTGCTGGGGATCTTC-3′; VAMP-2, sense 5′-TCACTGCCTCTGCCAAGTC-3′ and antisense 5′-CTCCAGGACCTTGTCCACAT-3′; ICER, sense 5′- ATGGCTGTAACTGGAGATGAAACTG-3′ and antisense 5′-CACCTTGTGGCAAAGCAGTA-3′.
The values obtained were normalized to the amount of tubulin that was measured in parallel for each sample.
Nuclear protein extract preparation and electromobility shift assays (EMSA)
Nuclear protein extracts EMSA and supershift experiments were performed as previously reported (Abderrahmani et al, 2001). Primers used as labelled probes were as follows: CRE consensus: sense 5′-GGACGTAGTCTGACGTCAGCGGA-3′ and antisense 5′-CATCAGACTGCAGTCGCCTCCGA-3′; for competition experiments, primers used were: rat CRE Rab3a: sense, 5′-ATGGTGACGTCATG-3′ and antisense 5′-CATGACGTCACCAT-3′; rat CRE Granuphilin: sense 5′-GCAGAGACGTACGGG-3′ and antisense: 5′-CCCGTACGTCTCTGC-3′; human CRE Granuphilin: sense 5′- ATTTTTATGTCAGTACGT-3′ and antisense 5′-ACGTACTGACATAAAAAT-3′; mutated human CRE Granuphilin: sense 5′-ATTTTTATTTTAGTACGT-3′ and antisense 5′- ACGTACTAAAATAAAAAT-3′; rat CRE Rab27: sense 5′-AGCCTGACGAGAGT-3′ and antisense 5′-ACTCTCGTCAGGCT-3; rat CRE Noc2: sense 5′-CAGATGACATCAAT-3′ and antisense 5′-ATTGATGTCATCTG-3′.
Secretion experiments
INS-1E cells (3 × 105) plated in 24-wells dishes were transiently cotransfected with a construct encoding the hGH (Nicholls, San Juan Capistrano, CA) and with a plasmid permitting constitutive expression of ICER-Iγ (Molina et al, 1993). After 3 days, the cells were washed and preincubated for 30 min in Krebs–Ringer/bicarbonate–HEPES buffer (KRBH: 140 mM NaCl, 3.6 mM KCl, 0.5 mM NaH2PO4, 0.5 mM MgSO4, 1.5 mM CaCl2, 2 mM NaHCO3, 10 mM HEPES and 0.1% BSA) containing 2 mM glucose (basal conditions). The medium was then removed and the cells incubated for 45 min in the same buffer (basal conditions) or in a buffer containing 20 mM glucose, 10 μM Forskolin and 100 μM IBMX (stimulatory conditions). The total amount of hGH produced by transfected cells and the fraction released into the medium during the incubation period were determined by ELISA (Roche Diagnostics, Rotkreuz, CH).
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Acknowledgments
We are indebted to Miss Sonia Gattesco and Guy Niederhauser for skilled technical assistance. We thank Drs RD Burgoyne, A El-Amraoui, R Jahn, H Hirling, U Blank, E Lalli and P Sassone-Corsi for supplying materials. This work was supported by the Swiss National Science Foundation grants 3100A0-105425 (AA), 310000-109281/1 (GW) and 3200B0-101746 (RR).
References
- Abderrahmani A, Steinmann M, Plaisance V, Niederhauser G, Haefliger JA, Mooser V, Bonny C, Nicod P, Waeber G (2001) The transcriptional repressor REST determines the cell-specific expression of the human MAPK8IP1 gene encoding IB1 (JIP-1). Mol Cell Biol 21: 7256–7267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allagnat F, Martin D, Condorelli DF, Waeber G, Haefliger JA (2005) Glucose represses connexin36 in insulin-secreting cells. J Cell Sci 118: 5335–5344 [DOI] [PubMed] [Google Scholar]
- Briaud I, Lingohr MK, Dickson LM, Wrede CE, Rhodes CJ (2003) Differential activation mechanisms of Erk-1/2 and p70(S6K) by glucose in pancreatic beta-cells. Diabetes 52: 974–983 [DOI] [PubMed] [Google Scholar]
- Cheviet S, Coppola T, Haynes LP, Burgoyne RD, Regazzi R (2004a) The Rab-binding protein Noc2 is associated with insulin-containing secretory granules and is essential for pancreatic beta-cell exocytosis. Mol Endocrinol 18: 117–126 [DOI] [PubMed] [Google Scholar]
- Cheviet S, Waselle L, Regazzi R (2004b) Noc-king out exocrine and endocrine secretion. Trends Cell Biol 14: 525–528 [DOI] [PubMed] [Google Scholar]
- Coppola T, Frantz C, Perret-Menoud V, Gattesco S, Hirling H, Regazzi R (2002) Pancreatic beta-cell protein granuphilin binds Rab3 and Munc-18 and controls exocytosis. Mol Biol Cell 13: 1906–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costes S, Longuct C, Broca C, Faruque O, Hani el H, Bataille D, Dalle S (2004) Cooperative effects between protein kinase A and p44/p42 mitogen-activated protein kinase to promote cAMP-responsive element binding protein activation after beta cell stimulation by glucose and its alteration due to glucotoxicity. Ann NY Acad Sci 1030: 230–242 [DOI] [PubMed] [Google Scholar]
- Deeney JT, Prentki M, Corkey BE (2000) Metabolic control of beta-cell function. Semin Cell Dev Biol 11: 267–275 [DOI] [PubMed] [Google Scholar]
- Delmas V, van der Hoorn F, Mellstrom B, Jegou B, Sassone-Corsi P. (1993) Induction of CREM activator proteins in spermatids: down-stream targets and implications for haploid germ cell differentiation. Mol Endocrinol 7: 1502–1514 [DOI] [PubMed] [Google Scholar]
- Doyle ME, Egan JM (2003) Pharmacological agents that directly modulate insulin secretion. Pharmacol Rev 55: 105–131 [DOI] [PubMed] [Google Scholar]
- Easom RA (2000) Beta-granule transport and exocytosis. Semin Cell Dev Biol 11: 253–266 [DOI] [PubMed] [Google Scholar]
- Eizirik DL, Korbutt GS, Hellerstrom C (1992) Prolonged exposure of human pancreatic islets to high glucose concentrations in vitro impairs the β-cell function. J Clin Invest 90: 1263–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaisano HY, Ostenson CG, Sheu L, Wheeler MB, Efendic S (2002) Abnormal expression of pancreatic islet exocytotic soluble N-ethylmaleimide-sensitive factor attachment protein receptors in Goto-Kakizaki rats is partially restored by phlorizin treatment and accentuated by high glucose treatment. Endocrinology 143: 4218–4226 [DOI] [PubMed] [Google Scholar]
- Gomi H, Mizutani S, Kasai K, Itohara S, Izumi T (2005) Granuphilin molecularly docks insulin granules to the fusion machinery. J Cell Biol 171: 99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellqvist LN, Rhodes CJ, Taylor KW (1984) Long-term biochemical changes in the islets of Langerhans in mice following infection with encephalomyocarditis virus. Diabetologia 26: 370–374 [DOI] [PubMed] [Google Scholar]
- Hussain MA, Daniel PB, Habener JF (2000) Glucagon stimulates expression of the inducible cAMP early repressor and suppresses insulin gene expression in pancreatic beta-cells. Diabetes 49: 1681–1690 [DOI] [PubMed] [Google Scholar]
- Iezzi M, Escher G, Meda P, Charollais A, Baldini G, Darchen F, Wollheim CB, Regazzi R (1999) Subcellular distribution and function of Rab3A, B, C, and D isoforms in insulin-secreting cells. Mol Endocrinol 13: 202–212 [DOI] [PubMed] [Google Scholar]
- Iezzi M, Kouri G, Fukuda M, Wollheim CB (2004) Synaptotagmin V and IX isoforms control Ca2+-dependent insulin exocytosis. J Cell Sci 117: 3119–3127 [DOI] [PubMed] [Google Scholar]
- Inada A, Hamamoto Y, Tsuura Y, Miyazaki J, Toyokuni S, Ihara Y, Nagai K, Yamada Y, Bonner-Weir S, Seino Y (2004) Overexpression of inducible cyclic AMP early repressor inhibits transactivation of genes and cell proliferation in pancreatic beta cells. Mol Cell Biol 24: 2831–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada A, Yamada Y, Someya Y, Kubota A, Yasuda K, Ihara Y, Kagimoto S, Kuroe A, Tsuda K, Seino Y (1998) Transcriptional repressors are increased in pancreatic islets of type 2 diabetic rats. Biochem Biophys Res Commun 253: 712–718 [DOI] [PubMed] [Google Scholar]
- Izumi T, Gomi H, Kasai K, Mizutani S, Torii S (2003) The roles of Rab27 and its effectors in the regulated secretory pathways. Cell Struct Funct 28: 465–474 [DOI] [PubMed] [Google Scholar]
- Kasai K, Ohara-Imaizumi M, Takahashi N, Mizutani S, Zhao S, Kikuta T, Kasai H, Nagamatsu S, Gomi H, Izumi T (2005) Rab27a mediates the tight docking of insulin granules onto the plasma membrane during glucose stimulation. J Clin Invest 115: 388–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas M, Molina C, Foulkes NS, Jansen E, Sassone-Corsi P (1997) Ectopic ICER expression in pituitary corticotroph AtT20 cells: effects on morphology, cell cycle, and hormonal production. Mol Endocrinol 11: 1425–1434 [DOI] [PubMed] [Google Scholar]
- Lang J (1999) Molecular mechanisms and regulation of insulin exocytosis as a paradigm of endocrine secretion. Eur J Biochem 259: 3–17 [DOI] [PubMed] [Google Scholar]
- Maronde E, Pfeffer M, Olcese J, Molina CA, Schlotter F, Dehghani F, Korf HW, Stehle JH (1999) Transcription factors in neuroendocrine regulation: rhythmic changes in pCREB and ICER levels frame melatonin synthesis. J Neurosci. 9: 3326–3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Miki T, Shibasaki T, Kawaguchi M, Shinozaki H, Nio J, Saraya A, Koseki H, Miyazaki M, Iwanaga T, Seino S (2004) Noc2 is essential in normal regulation of exocytosis in endocrine and exocrine cells. Proc Natl Acad Sci USA 101: 8313–8318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merglen A, Theander S, Rubi B, Chaffard G, Wollheim CB, Maechler P (2004) Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology 145: 667–678 [DOI] [PubMed] [Google Scholar]
- Mioduszewska B, Jaworski J, Kaczmarek L (2003) Inducible cAMP early repressor (ICER) in the nervous system: a transcriptional regulator of neuronal plasticity and programmed cell death. J Neurochem 87: 1313–1320 [DOI] [PubMed] [Google Scholar]
- Molina CA, Foulkes NS, Lalli E, Sassone-Corsi P (1993) Inducibility and negative autoregulation of CREM: an alternative intronic promoter directs the expression of ICER, an early response repressor. Cell 75: 875–886 [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Urban J, Sassone-Corsi P, Mayo KE (1998) Gonadotropins regulate inducible cyclic adenosine 3′,5′-monophosphate early repressor in the rat ovary: implications for inhibin alpha subunit gene expression. Mol Endocrinol 12: 785–800 [DOI] [PubMed] [Google Scholar]
- Nagamatsu S, Nakamichi Y, Yamamura C, Matsushima S, Watanabe T, Ozawa S, Furukawa H, Ishida H (1999) Decreased expression of t-SNARE, syntaxin 1, and SNAP-25 in pancreatic beta-cells is involved in impaired insulin secretion from diabetic GK rat islets: restoration of decreased t-SNARE proteins improves impaired insulin secretion. Diabetes 48: 2367–2373 [DOI] [PubMed] [Google Scholar]
- Nervina JM, Tetradis S, Huang YF, Harrison D, Molina C, Kream BE (2003) Expression of inducible cAMP early repressor is coupled to the cAMP-protein kinase A signaling pathway in osteoblasts. Bone 32: 483–490 [DOI] [PubMed] [Google Scholar]
- Oishi H, Sasaki T, Nagano F, Ikeda W, Ohya T, Wada M, Ide N, Nakanishi H, Takai Y (1998) Localization of the Rab3 small G protein regulators in nerve terminals and their involvement in Ca2+-dependent exocytosis. J Biol Chem 273: 34580–34585 [DOI] [PubMed] [Google Scholar]
- Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, Sunaga Y, Yano H, Matsuura Y, Iwanaga T, Takai Y, Seino S (2000) cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol 2: 805–811 [DOI] [PubMed] [Google Scholar]
- Pfeffer M, Kuhn R, Krug L, Korf HW, Stehle JH (1998) Rhythmic variation in beta1-adrenergic receptor mRNA levels in the rat pineal gland: circadian and developmental regulation. Eur J Neurosci. 10: 2896–2904 [DOI] [PubMed] [Google Scholar]
- Plaisance V, Niederhauser G, Azzouz F, Lenain V, Haefliger JA, Waeber G, Abderrahmani A (2005) The repressor element silencing transcription factor (REST)-mediated transcriptional repression requires the inhibition of Sp1. J Biol Chem 280: 401–407 [DOI] [PubMed] [Google Scholar]
- Plaisance V, Thompson N, Niederhauser G, Haefliger JA, Nicod P, Waeber G, Abderrahmani A (2002) The mif gene is transcriptionally regulated by glucose in insulin-secreting cells. Biochem Biophys Res Commun 295: 174–181 [DOI] [PubMed] [Google Scholar]
- Rorsman P, Renström E (2003) Insulin granule dynamics in pancreatic beta cells. Diabetologia 46: 1029–1045 [DOI] [PubMed] [Google Scholar]
- Südhof TC (2004) The synaptic vesicle cycle. Annu Rev Neurosci 27: 509–547 [DOI] [PubMed] [Google Scholar]
- Sutton R, Peters M, McShane P, Gray DW, Morris PJ (1986) Isolation of rat pancreatic islets by ductal injection of collagenase. Transplantation 42: 689–691 [DOI] [PubMed] [Google Scholar]
- Tolmachova T, Anders R, Stinchcombe J, Bossi G, Griffiths GM, Huxley C, Seabra MC (2004) A general role for Rab27a in secretory cells. Mol Biol Cell 15: 332–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselle L, Coppola T, Fukuda M, Iezzi M, El-Amraoui A, Petit C, Regazzi R (2003) Involvement of the Rab27 binding protein Slac2c/MyRIP in insulin exocytosis. Mol Biol Cell 14: 4103–4113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waeber G, Calandra T, Roduit R, Haefliger JA, Bonny C, Thompson N, Thorens B, Temler E, Meinhardt A, Bacher M, Metz CN, Nicod P, Bucala R (1997) Insulin secretion is regulated by the glucose-dependent production of islet beta cell macrophage migration inhibitory factor. Proc Natl Acad Sci USA 94: 4782–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir GC, Laybutt DR, Kaneto H, Bonner-Weir S, Sharma A (2001) b-cell adaptation and decompensation during the progression of diabetes. Diabetes 50: S154–S159 [DOI] [PubMed] [Google Scholar]
- Yaekura K, Julyan R, Wicksteed BL, Hays LB, Alarcon C, Sommers S, Poitout V, Baskin DG, Wang Y, Philipson LH, Rhodes CJ (2003) Insulin secretory deficiency and glucose intolerance in Rab3A null mice. J Biol Chem 278: 9715–9721 [DOI] [PubMed] [Google Scholar]
- Yi Z, Yokota H, Torii S, Aoki T, Hosaka M, Zhao S, Takata K, Takeuchi T, Izumi T (2002) The Rab27a/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules. Mol Cell Biol 22: 1858–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YP, Marlen K, Palma JF, Schweitzer A, Reilly L, Gregoire FM, Xu GG, Blume JE, Johnson JD (2003) Overexpression of repressive cAMP response element modulators in high glucose and fatty acid-treated rat islets. A common mechanism for glucose toxicity and lipotoxicity? J Biol Chem 278: 51316–51323 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3


