Abstract
The evolutionary conservation of the NF-κB transcription factors, from Drosophila to humans, underscores its pivotal role in immune response. Unexpectedly, the canonical NF-κB signaling pathway is not functional in the immune system of Caenorhabditis elegans. Therefore, the ancient origin of the NF-κB signaling pathway is still unknown. Here, we report the discovery and characterization of a primitive and functional NF-κB/IκB pathway in the immune defense of a “living fossil,” the horseshoe crab, Carcinoscorpius rotundicauda. The ancient NF-κB/IκB homologues, CrNFκB, CrRelish, and CrIκB, share numerous signature motifs with their vertebrate orthologues. CrNFκB recognizes both horseshoe crab and mammalian κB response elements. CrIκB interacts with CrNFκB and inhibits its nuclear translocation and DNA-binding activity. The activation of the CrNFκB is autoregulated by a feedback mechanism mediated by CrIκB, the natural inhibitor of CrNFκB. We further show that Gram-negative bacteria infection causes rapid degradation of CrIκB and nuclear translocation of CrNFκB. Infection also leads to an increase in the κB-binding activity and up-regulation of immune-related gene expression, like inducible nitric oxide synthase and Factor C, an LPS-activated serine protease. Altogether, our study shows that, although absent in C. elegans, the NF-κB/IκB signaling cascade remains well conserved from horseshoe crab to humans, playing an archaic but fundamental role in regulating the expression of critical immune defense molecules.
Keywords: conservation and coevolution, horseshoe crab, infection and immune response, transcriptional control
The family of NF-κB transcription factors plays an indispensable role in immunity, inflammation, apoptosis, development, and differentiation (1, 2). NF-κB dimers are held in an inactive cytoplasmic complex with a family of inhibitory proteins, the IκBs. Degradation of IκBs permits nuclear translocation of NF-κB, where they stimulate the transcription of various immune-related genes (1). In Drosophila melanogaster, NF-κB homologues (Dorsal, Dif, and Relish) are responsible for regulating several biological roles, including humoral immunity and development (3, 4). These important and diverse functions make NF-κB one of the best-studied transcriptional factors in biology (2). Unexpectedly, in Caenorhabditis elegans, the NF-κB protein is absent, and similar functional homologues (Toll, Traf, Cactus) are not involved in innate immune response (5). This led to the suggestion that the p38 mitogen-activated protein kinase pathway, which mediates the immune response in C. elegans, is the ancestral signaling pathway of the common ancestor of nematodes, arthropods, and vertebrates, predating the evolution of the NF-κB immune signaling pathway (6). Thus, the ancient origin of the NF-κB signaling pathway is still a conundrum, and it remains unclear whether the similarities between Drosophila and the human NF-κB pathway have resulted from convergent evolution or reflect common ancestral pathways. Therefore, additional information on NF-κB-mediated responses in other invertebrate groups that are more ancient than Drosophila will shed light on this mystery (7).
The horseshoe crab, commonly known as Limulus, is the most ancient arthropod, which has survived unchanged for ≈550 million years (8) and has evolved a formidable host defense system (9). Its scientific and medical importance is evidenced by the worldwide use of its hemocyte lysate for endotoxin detection (10). Recently, a Toll-like receptor (TLR) was found in Limulus; however, no functional characterization is available (11). Thus, it is unclear whether the horseshoe crab TLR is involved in innate immune response. Furthermore, the existence of TLR does not necessarily suggest the presence of NF-κB proteins, as was observed in C. elegans (6). Therefore, whether the horseshoe crab possesses the functional NF-κB homologue remains unknown, provoking us to trace the ancient origin of an NF-κB signaling cascade and its involvement in the transcriptional regulation of immune-related genes in this species.
Herein, we identify NF-κB and IκB homologues in a species of Limulus, the Carcinoscorpius rotundicauda, and show that their activation mechanism and transactivation properties are evolutionarily entrenched. We further show that an activated horseshoe crab NF-κB pathway can regulate the expression of immune-related genes in vivo, including inducible nitric oxide synthase (iNOS) and C. rotundicauda Factor C (CrFC), the LPS-activated enzyme that triggers the coagulation cascade in immune defense.
Results
Cloning and Characterization of C. rotundicauda NF-κB (CrNFκB and CrRelish) and IκB (CrIκB) Homologues.
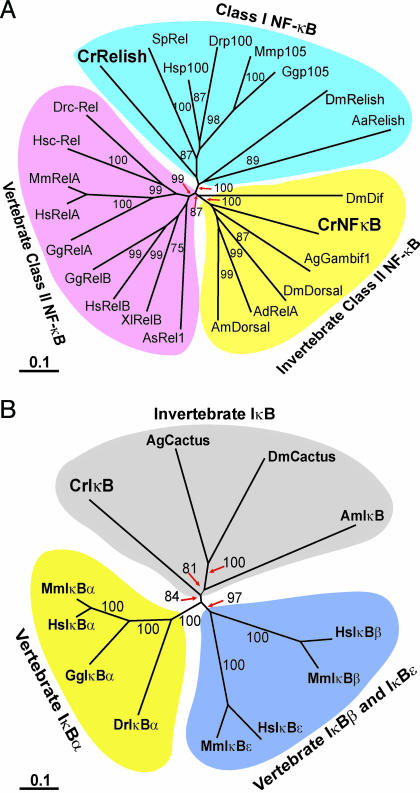
To isolate the C. rotundicauda NF-κB homologue, degenerate primers were used for RT-PCR. Full length CrNFκB cDNA was isolated by 3′ and 5′ RACE. With an ORF of 1,686 bp, it encodes a protein of 562 amino acids. Amino acid analysis revealed that CrNFκB possesses the characteristic organization of NF-κB proteins. Specifically, the CrNFκB contains an N-terminal Rel homology domain (RHD) (residues 19–301) and a C-terminal transactivation domain (TD) (residues 315–562) (see Fig. 5, which is published as supporting information on the PNAS web site). CrNFκB contains two conserved motifs in the RHD: the DNA-binding motif (RXXRXRXXC) and the nuclear localization signal (NLS) (RKRQK) which characterize all NF-κB family of proteins. Interestingly, the TD of CrNFκB lacks the polyglutamine, polyalanine, and polyasparagine stretches, which characterizes the TD of several insect Dorsal-like proteins (12). A phylogenetic analysis shows that CrNFκB clustered among Dorsal-like proteins that belong to the invertebrate class II NF-κB (Fig. 1A) (13). This cluster differs from the vertebrate class II NF-κB, which includes the RelA, RelB, and c-Rel proteins.
Fig. 1.
Phylogenetic analysis of CrNFκB, CrRelish, and CrIκB. Multiple sequence alignments were produced with clustal x by using Gonnet series protein weight matrix. The unrooted phylogenetic tree was constructed by using the neighbor-joining method based on the alignments. The confidence scores (percent) of a bootstrap test of 1,000 replicates are indicated for major branching nodes. GenBank accession nos. of the sequences are listed in Table 1, which is published as supporting information on the PNAS web site. (A) Unrooted phylogenetic tree of CrNFκB and CrRelish proteins. (B) Unrooted phylogenetic tree of CrIκB proteins.
A class I NF-κB homologue (CrRelish) was similarly isolated. It shows high homology with Drosophila Relish and mammalian p100 (Fig. 1A) and contains 3,405 base pair encoding 1,135 amino acids. The CrRelish contains an N-terminal RHD and a C-terminal IκB-like domain with six ankyrin repeats (Fig. 6, which is published as supporting information on the PNAS web site). Similar to mosquito Relish and mammalian p100 and p105 (14), a death domain is located at the C terminus of CrRelish.
The C. rotundicauda IκB (CrIκB) cDNA was cloned by using primers designed from the ankyrin repeat regions. The full length CrIκB cDNA contains 1,566 base pair encoding a 439-amino acid protein. The CrIκB protein (Fig. 7, which is published as supporting information on the PNAS web site) contains several features found in IκB members: five ankyrin repeats with homology to the mammalian and Drosophila IκB counterparts, two serine residues that are critical for its degradation at the N-terminal serine-rich region, and the C-terminal PEST domain necessary for constitutive phosphorylation and intrinsic stability of the IκB protein (15). Furthermore, at the C-terminal PEST domain, several putative casein kinase II phosphorylation sites were identified that are required for efficient signaling and its degradation in vivo (16). It shows highest homology to the Drosophila IκB, Cactus. A phylogenetic analysis revealed three main clusters: invertebrate IκB, vertebrate IκBα, and vertebrate IκBβ and IκBε (Fig. 1B).
CrNFκB Binding to the κB Response Element Is Inhibited by CrIκB.
To examine whether CrNFκB can recognize the κB response element, EMSA was done by using a recombinant RHD of CrNFκB on the κB site of CrFC promoter. The results showed that the RHD of the CrNFκB can interact with the CrFC κB response element (Fig. 2A, lane 1), which was also recognized by the human NF-κB and Drosophila Dorsal (17). The presence of κB motif is critical to the binding, because mutations to the 5′ end of the κB motif (−143 to −141), from GGG to ATT, abolished the binding (Fig. 2A, lane 8). The interaction is specific, because the binding was markedly reduced by excess cold competitor oligonucleotide, whereas the mutant competitor had no effect (Fig. 2A, lanes 2, 3, 6, and 7). CrNFκB also recognizes a consensus mammalian κB motif (data not shown), and thus it may serve as a functional substitute for vertebrate NF-κB or vice versa. Our results suggest that the specific recognition sequence of NF-κB was acquired early and maintained during evolution.
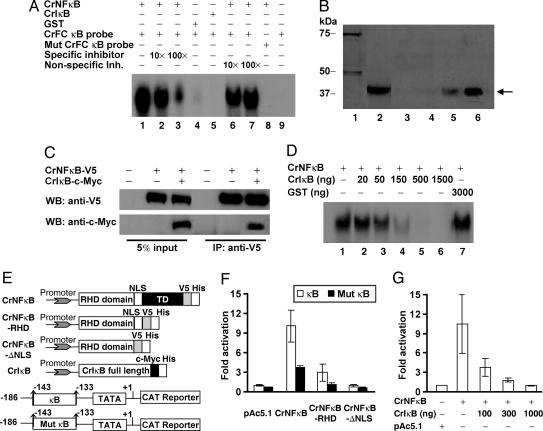
Fig. 2.
In vitro characterization of CrNFκB and CrIκB. (A) Binding of CrNFκB protein to the Factor C (CrFC) κB probe. (B) GST pull-down assay. Lane 1, the purified GST-CrIκB protein. Expression of His-tagged CrNFκB-RHD was confirmed by probing bacteria lysates with anti-His antibody (lane 2). CrNFκB-RHD (←) expression bacteria lysates were incubated with glutathione-Sepharose beads (lane 3) or glutathione-Sepharose beads loaded with GST (lane 4) or GST-CrIκB (lane 5). One-tenth volume of the CrNFκB-RHD was also electrophoresed as positive control (lane 6). (C) Immunoprecipitation (IP) of CrNFκB and CrIκB. S2 cells were transfected with the indicated combination of plasmids. Five percent volume of the cell lysates used for IP was electrophoresed as control. (D) CrIκB inhibits the CrNFκB DNA-binding activity. A known amount of CrNFκB was titrated with increasing amounts of CrIκB. GST was used as negative control. (E) Schematic representation of the expression vectors (Upper) and reporters (Lower) used in transfection. (F) S2 cells were cotransfected with wild-type (κB) and mutant (Mut κB) CrFC-CAT reporters (1 μg), a β-galactosidase expression plasmid (50 ng), together with full length or truncated CrNFκB constructs (500 ng). CAT expression level was normalized against the levels of β-galactosidase expression. (G) Cotransfection of CrNFκB (500 ng) and increasing amounts of CrIκB. Results are expressed as relative fold induction in CAT expression as compared with control cells transfected with vector backbone. Data are presented as mean ± SD of three independent experiments.
To investigate whether CrIκB can interact with CrNFκB, an in vitro pull-down assay was performed. A specific complex between these two proteins was obtained (Fig. 2B, lane 5), because no interaction was observed with either control Sepharose beads or GST protein with CrNFκB (Fig. 2B, lanes 3 and 4). To determine whether CrNFκB interacts with CrIκB in vivo, we coexpressed CrNFκB and CrIκB in Drosophila S2 cells. Indeed, CrIκB interacted with CrNFκB to form an immunoprecipitate complex (Fig. 2C). To verify whether CrIκB could inhibit the DNA-binding activity of CrNFκB, we performed EMSA, which showed that CrIκB inhibited the DNA-binding activity of CrNFκB in a dose-dependent manner (Fig. 2D, lanes 2–6). Altogether, the results show that CrIκB interacts with and specifically inhibits the DNA-binding activity of CrNFκB.
Functional Activation of the CrNFκB/CrIκB Cascade.
We next examined the ability of CrNFκB to regulate gene transcription by using transient cotransfection studies with the CrFC promoter (−186 to +1)-chloramphenicol/acetyltransferase (CAT) reporter, which harbored a potential NF-κB-binding site (−143 to −133) and wild-type or truncated CrNFκB expression constructs (Fig. 2E). The overexpression of full length CrNFκB resulted in a 10-fold increase in CAT reporter expression (Fig. 2F), whereas that of CrNFκB-RHD (amino acids 1–321) and CrNFκB-ΔNLS (amino acids 1–266) led to significantly reduced CAT expression, suggesting that the C-terminal TD of CrNFκB is essential for gene activation. The deletion of the 5′ end of CrFC κB motif (−143 to −138) resulted in significantly reduced CAT induction (Fig. 2F, Mut κB), suggesting that the κB site of CrFC is functional. In the presence of increasing amounts of CrIκB (the endogenous natural inhibitor of CrNFκB), a dose-dependent reduction in CAT reporter expression was observed (Fig. 2G).
To distinguish whether the reduced gene activation by truncated CrNFκB was attributable to the lack of transactivation activity or to impaired nuclear translocation, the subcellular localization of full length and truncated CrNFκB was examined. Immunofluorescence showed that both the full length CrNFκB and CrNFκB-RHD were evenly distributed in the cytoplasm and the nucleus (Fig. 8, which is published as supporting information on the PNAS web site), indicating that TD does not affect the localization of CrNFκB. This suggests the atypical C-terminal TD of CrNFκB is functional and essential for transcriptional activation. The NLS of CrNFκB is also functional, because the truncated CrNFκB-ΔNLS remained localized to the cytoplasm. Immunofluorescence revealed that the overexpressed CrIκB was exclusively located in the cytoplasm. We next examined the effect of coexpressed CrIκB on the localization of CrNFκB. Similar to mammalian IκBα, CrIκB overexpression resulted in the sequestration of CrNFκB to the cytoplasm (Fig. 8).
Altogether, these results show that the interaction between CrIκB and CrNFκB interferes with the latter’s ability to translocate into the nucleus, to bind DNA, and to stimulate gene transactivation. Interestingly, the activation of CrNFκB is analogous to the canonical activation cascade observed in the vertebrate (18), thus lending support to our proposal of the ancient origin and coevolution of the NF-κB/IκB signaling.
The Biological Significance of a Primitive CrNFκB/CrIκB Cascade.
To check the relevance of the NF-κB cascade in vivo, we examined κB-binding activity in the hemocytes, the major immune cell in this invertebrate. EMSA using whole hemocyte lysates suggests the presence of proteins that bind specifically to the κB site of the CrFC promoter, because mutation of the κB motif abolished binding (Fig. 3A). These gel-shift complexes were partially reduced by increasing doses of CrIκB and helenalin, a specific inhibitor of human NF-κB p65 (19), further suggesting that the complexes were formed by NF-κB-related proteins (Fig. 3A). Anti-CrNFκB caused a partial supershift of the κB-binding complex (Fig. 3B), confirming that CrNFκB binds κB and suggesting there are probably other κB-binding proteins in the horseshoe crab hemocytes. To examine the in vivo activation of CrNFκB by infection, horseshoe crabs were injected with Pseudomonas aeruginosa. Upon infection, the EMSA signal increased markedly in the hemocyte nuclear extract (Fig. 3B), and the CrIκB protein was rapidly degraded (Fig. 3C), suggesting that infection activates the NF-κB pathway. We next examined the subcellular localization of CrNFκB and CrIκB in hemocytes. The cytoplasmic CrNFκB in the naïve hemocytes was enriched in the nucleus within 30 min of infection (Fig. 9, which is published as supporting information on the PNAS web site). Although the CrIκB remained in the cytoplasm with or without infection, its intensity decreased significantly upon infection (Fig. 9). This is in agreement with the Western blot (Fig. 3C), further lending support that bacteria infection activates the CrNFκB signaling pathway.
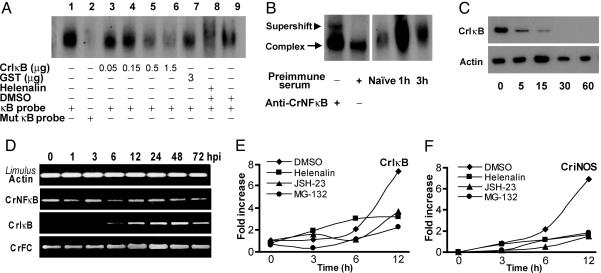
Fig. 3.
In vivo characterization of CrNFκB and CrIκB. (A) Hemocyte extracts were used for EMSA. Approximately 20 μg of extract was incubated with the CrFC κB probe (lanes 1 and 3–9) or mutant (Mut) CrFC κB probe (lane 2). Binding complexes were progressively abolished by CrIκB (lanes 3–6), although it remained unaffected by GST (lane 7). Helenalin (lane 8) decreases the intensity of the binding compared to DMSO (lane 9). (B Left) Supershift of the κB-binding complex by the anti-CrNFκB antibody. (B Right) Naïve and 1- and 3-hpi Pseudomonas-challenged horseshoe crab hemocyte nuclear extracts were incubated with the CrFC κB probe. (C) Degradation of CrIκB after bacteria infection. Hemocyte extracts were prepared from naïve and infected animals according to Ding et al. (30). Western blots show proteins extracted from hemocytes over time (min) of infection. Equal protein loading and transfer were verified by using Limulus actin. (D) Expression of CrNFκB, CrIκB, and CrFC. The hemocytes were collected 1–72 hpi. The Limulus actin 11 gene was the internal control. (E and F) CrNFκB affects the expression of immune-related genes. One hour after treatment with DMSO (♦), helenalin (■), MG-132 (•), or JSH-23 (▴), the horseshoe crabs were either left unstimulated (0 h) or challenged with P. aeruginosa, and the hemocytes were collected at the indicated hpi. Results are expressed as relative fold increase as compared with naïve control (0 h), which was set to 1. Without infection, the expression of CriNOS was undetectable. Thus, the expression level of CriNOS at 3 hpi was set to 1.
To determine the expression pattern of CrNFκB and CrIκB upon P. aeruginosa infection, RT-PCR was performed with total RNA from hemocytes collected at indicated time points after challenge. CrNFκB mRNA was constitutively expressed in the hemocytes and remained unchanged throughout the course of infection. In contrast to CrNFκB, expression of CrIκB was significantly induced after bacteria challenge (Fig. 3D). This is consistent with studies on Drosophila and humans in which activation of the NF-κB pathway increases expression of IκB and negatively autoregulates NF-κB activity (1). In comparison, CrFC exhibited a slight up-regulation over the same time frame as CrIκB, suggesting that both CrIκB and CrFC are NF-κB-responsive genes (Fig. 3D). To examine whether the expression of CrIκB is indeed affected by the NF-κB pathway, we studied the effect of NF-κB-specific inhibitors on the up-regulation of CrIκB. Injection of DMSO (vehicle) did not affect activation of CrIκB during infection (Fig. 3E), whereas treatment with three unrelated NF-κB-specific inhibitors, helenalin, MG-132, or NF-κB activation inhibitor II (JSH-23) (20), prior to infection consistently suppressed the up-regulation of CrIκB. This indicates a possible role of the NF-κB pathway in the regulation of CrIκB expression in vivo. To investigate whether NF-κB signaling also plays a role in regulating other immune-related gene transcription, we analyzed the expression of C. rotundicauda iNOS, CriNOS. iNOS is a classical NF-κB target gene required for a robust innate immune response both in the Drosophila and vertebrates (21, 22). The expression of CriNOS was induced after infection by P. aeruginosa (Fig. 3F). When cells were treated with the NF-κB-specific inhibitors, there was negligible increase of CriNOS mRNA (Fig. 3F), clearly showing that NF-κB inhibitors blocked the increase of CriNOS transcription. To further demonstrate that the NF-κB inhibitors affect only the NF-κB pathway, we analyzed the expression of transglutaminase, which has been shown to be under the control of Sp1 and CREB/AP-1 in the vertebrate (23). Indeed, injection of NF-κB inhibitors did not affect the activation of transglutaminase transcription upon infection (data not shown).
Discussion
The phenomenal success of the immune defense of the horseshoe crab, the most ancient living arthropod, has contributed to its survival for ≈550 million years (8) and makes this species an excellent model to understand the origin of innate immunity. Herein, we identified the C. rotundicauda homologue of NF-κB and IκB called CrNFκB, CrRelish, and CrIκB, respectively. To the best of our knowledge, except for Drosophila Cactus, CrIκB is the only IκB characterized in the invertebrate, and CrNFκB is the only NF-κB identified in a noninsect species of arthropods. Despite the huge evolutionary distance between the horseshoe crab and vertebrates, we showed that CrNFκB, CrRelish, and CrIκB displayed similar signature motifs found in the vertebrate orthologues, notably the DNA-binding motif and the NLS of CrNFκB (Fig. 5), the five ankyrin repeats, and the N-terminal potential phosphorylation sites of CrIκB (Fig. 7). Like human p100 and p105 and insect relish, the CrRelish is a mosaic protein that contains both RHD and the inhibitory IκB domain (Fig. 6). That human p100 and Drosophila Relish are cleaved to release its N-terminal activation domains suggests that CrRelish probably undergoes a similar process upon bacterial infection (Fig. 4). However, the functional difference(s) between CrNFκB and CrRelish need to be addressed further. Interestingly, the activation and DNA recognition of CrNFκB are reminiscent of that of more advanced species. Furthermore, CrIκB can specifically inhibit the binding of CrNFκB to the κB motif of the CrFC promoter and reduce its transcriptional activity (Fig. 2 D and G). Immunofluorescence also revealed that the NLS in CrNFκB permits its translocation into the nucleus, which can be inhibited by the overexpression of CrIκB, the natural inhibitor of CrNFκB activity. This suggests that the activity of CrNFκB is modulated by an autoregulatory feedback mechanism via CrIκB, whose expression is also controlled by the NF-κB pathway (Fig. 3E). Thus, NF-κB signaling in the horseshoe crab is functionally comparable with that of mammals, suggesting that the roles of NF-κB and IκB have coevolved and remained conserved through evolution (Fig. 4). Our findings further support the view that a signaling mechanism mediated via the NF-κB family of proteins, which controls the expression of immune defense genes, probably originated from a common ancestor and was already present in Urbilateria (7).
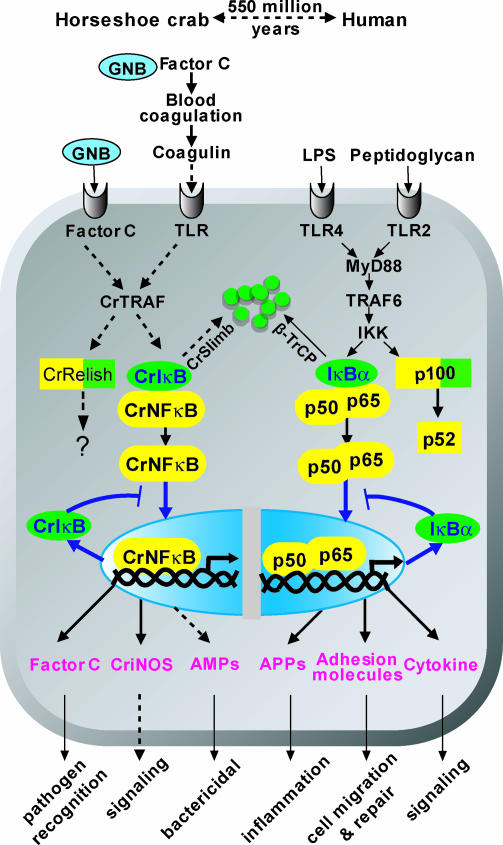
Fig. 4.
Conservation of the NF-κB signaling pathway from horseshoe crab to human. In the horseshoe crab (Left), solid and dashed arrows annotate the established and suggested pathways. The GNB-activated CrFC initiates the serine protease cascade to proteolytically process coagulogen to coagulin (a homologue of Spaetzle), which probably binds to the horseshoe crab TLR. Factor C also exists on the hemocyte surface as a biosensor for GNB (31). Ligand binding to TLR or Factor C on the membrane might activate the CrTRAF, a homologue of vertebrate TRAF, and lead to the degradation of CrIκB through CrSlimb (26), via a Slimb/β-TrCP-mediated proteosome pathway (32). The released CrNFκB translocates to the nucleus to activate target genes, including CrIκB. The newly synthesized CrIκB inhibits the nuclear translocation of CrNFκB, creating an autoregulatory pathway (blue arrows). The NF-κB pathway also controls the expression of Factor C and CriNOS and probably affects the synthesis of antimicrobial peptides (AMPs). Similarly, the human p100 homologue, CrRelish, may also be activated by pathogen infection. However, whether CrRelish undergoes degradation and functions like human p100 remains to be ascertained. The schematic representation of the human TLR/NF-κB signaling pathway is shown (Right) (APPs, acute phase proteins) (1, 33). The familiar relationship between the human and horseshoe crab homologues forming the NF-κB cascade suggests they probably coevolved.
Striking similarities between the Drosophila dorsoventral determination cascade and the horseshoe crab LPS-sensitive blood coagulation cascade have been observed (24). In Drosophila, the dorsoventral cascade can activate the Toll-ligand, Spaetzle, which then binds to Toll and triggers the intracellular NF-κB signaling pathway. Our findings herein and the recent cloning of TLR in the horseshoe crab (11) have provided compelling evidence for a functional intracellular TLR/NF-κB signaling cascade. Although the direct ligand for horseshoe crab TLR activation is unclear, it has been proposed that the end product of a LPS-sensitive blood coagulation cascade, coagulin (a homologue of Spaetzle), serves as a ligand of the horseshoe crab TLR (Fig. 4) (25). Furthermore, from a subtractive library, we have recently isolated two cDNA fragments with homology to other components of the NF-κB pathway, including the TNF receptor-associated factor, CrTRAF, and CrSlimb (26). Although the biological functions of these components remain to be further elucidated, their existence nonetheless provides additional evidence for a functional TLR/NF-κB cascade and the coevolution of this signaling cascade, which originated ≈550 million years ago.
In conclusion, the NF-κB/IκB signaling pathway was entrenched >500 million years ago and has coevolved and remained well conserved from the horseshoe crab to humans (Fig. 4), playing an archaic but crucial and fundamental role in innate immune response to regulate the expression of critical immune defense molecules.
Materials and Methods
Animals and Bacterial Challenge.
Horseshoe crabs (C. rotundicauda) were collected from the Kranji Estuary, Singapore. P. aeruginosa (American Type Culture Collection 27853) was cultured in tryptic soy broth (Difco). Bacteria was pelleted at 5,000 × g for 10 min, washed, and resuspended in 0.9% saline to 1 × 107/ml. The horseshoe crabs were injected with 1.2 × 107 colony-forming units per kilogram of body weight (27). The hemocytes were collected at indicated hours postinfection (hpi).
Construction of Expression Vectors.
pAc5.1/V5-HisA (Invitrogen) was used for expression of full length and truncated CrNFκB proteins (Fig. 2E). c-Myc-tagged full length CrIκB was similarly cloned into the pAc5.1/V5-HisA. For bacterial recombinant protein expression, the N-terminal half of CrNFκB (amino acids 1–353) and full length CrIκB were subcloned into pET15b (Novagen) and pGEX-4T-1 (GE Healthcare, Chalfont St. Giles, U.K.) expression vectors, respectively.
Pull-Down Assay.
One microgram of GST-CrIκB fusion protein was bound to 25 μl of glutathione Sepharose 4B beads (GE Healthcare) in PBS. The beads were washed five times with PBS after incubating with recombinant His-tagged CrNFκB-RHD. Bound proteins were eluted with 30 μl of SDS/PAGE sample buffer, resolved by SDS/PAGE, and detected by anti-His antibody. Immunoprecipitation assays were performed as described (28).
Immunoreagents.
Anti-CrNFκB and -CrIκB antibodies were raised in rabbits against keyhole limpet hemocyanin-conjugated peptides (CrNFκB, LPVNRDPEGLSRKR; CrIκB, VSSHSHHSPQKEYK) by BioGenes (Berlin). The antibodies were affinity-purified by using specific peptide as ligand. All antibodies were tested for specificity by Western blot by using recombinant CrNFκB and CrIκB.
Whole Hemocyte and Nuclear Extract Preparations and EMSA.
Hemocytes were washed with PBS and homogenized in binding buffer (50 mM NaCl/2 mM MgCl2/2 mM DTT/1 mM EDTA/10% glycerol/10 mM Hepes, pH 7.8). Whole hemocyte extracts were centrifuged at 4°C for 10 min at 13,000 × g, and the resulting supernatants were used for EMSA (17). Nuclear extracts were prepared as described (29). For supershift assays, hemocyte extracts were incubated with the respective antibody for 30 min on ice before adding the probe. Sequences of the oligonucleotides used in EMSA are listed in Supporting Text, which is published as supporting information on the PNAS web site.
Cell Culture and Transfection.
Drosophila Schneider S2 cells were maintained at 25°C in Drosophila Serum-Free Medium supplemented with 20 mM l-glutamine/5% FBS (Invitrogen). Twelve hours before transfection, cells were seeded in a six-well plate at 1.2 × 106 cells per well. Transfections were conducted by using CellFectin (Invitrogen). At 48 h after transfection, CAT and β-galactosidase activities were measured by ELISA (Roche Diagnostics). We performed the transfection in S2 cells on the basis that both the horseshoe crab and Drosophila are members of the arthropod family.
Inhibitor Treatments and RT-PCR.
NF-κB inhibitors, helenalin (10 mM), MG-132 (25 mM), and NF-κB activation inhibitor II (JSH-23, 25 mM), were dissolved in DMSO. DMSO (vehicle) or inhibitors was given intracardially at 500 μl/kg body weight. After 1 h, the horseshoe crabs were injected with 1.2 × 107 colony-forming units of P. aeruginosa per kilogram of body weight. Reverse transcription was performed by using the Invitrogen kit with 3 μg of total RNA and oligo(dT). Semiquantitative RT-PCR was performed with a reaction profile of 95°C for 3 min, 19–25 cycles of 56°C for 30 s, 72°C for 1 min, and 95°C for 30 s. The RT-PCR products were analyzed on gels and quantified relative to the levels of Limulus actin-11 mRNA. Sequences of all primers are in Supporting Text.
Supplementary Material
Acknowledgments
This work was supported by a Biomedical Research Council grant (03/1/21/17/227) from the Agency for Science, Technology, and Research, Singapore (A*STAR). X.W.W. is a recipient of a Graduate Research Scholarship from the National University of Singapore.
Abbreviations
- CrFC
C. rotundicauda Factor C
- CrNFκB
C. rotundicauda NFκB
- CrIκB
C. rotundicauda IκB
- GNB
Gram-negative bacteria
- iNOS
inducible nitric oxide synthase
- RHD
Rel homology domain
- TLR
Toll-like receptor
- TD
transactivation domain
- NLS
nuclear localization signal
- hpi
hours postinfection
- CAT
chloramphenicol/acetyltransferase
Footnotes
References
- 1.Ghosh S., May M. J., Kopp E. B. Annu. Rev. Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 2.Dixit V., Mak T. W. Cell. 2002;111:615–619. doi: 10.1016/s0092-8674(02)01166-2. [DOI] [PubMed] [Google Scholar]
- 3.Wasserman S. A. Curr. Opin. Genet. Dev. 2000;10:497–502. doi: 10.1016/s0959-437x(00)00118-0. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann J. A. Nature. 2003;426:33–38. doi: 10.1038/nature02021. [DOI] [PubMed] [Google Scholar]
- 5.Pujol N., Link E. M., Liu L. X., Kurz C. L., Alloing G., Tan M. W., Ray K. P., Solari R., Johnson C. D., Ewbank J. J. Curr. Biol. 2001;11:809–821. doi: 10.1016/s0960-9822(01)00241-x. [DOI] [PubMed] [Google Scholar]
- 6.Kim D. H., Ausubel F. M. Curr. Opin. Immunol. 2005;17:4–10. doi: 10.1016/j.coi.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann J. A., Reichhart J. M. Nat. Immunol. 2002;3:121–126. doi: 10.1038/ni0202-121. [DOI] [PubMed] [Google Scholar]
- 8.Størmer L. J. Paleontol. 1952;26:630–639. [Google Scholar]
- 9.Iwanaga S. Curr. Opin. Immunol. 2002;14:87–95. doi: 10.1016/s0952-7915(01)00302-8. [DOI] [PubMed] [Google Scholar]
- 10.Ding J. L., Ho B. Trends Biotechnol. 2001;19:277–281. doi: 10.1016/s0167-7799(01)01694-8. [DOI] [PubMed] [Google Scholar]
- 11.Inamori K., Ariki S., Kawabata S. Immunol. Rev. 2004;198:106–115. doi: 10.1111/j.0105-2896.2004.0131.x. [DOI] [PubMed] [Google Scholar]
- 12.Shin S. W., Kokoza V., Bian G., Cheon H. M., Kim Y. J., Raikhel A. S. J. Biol. Chem. 2005;280:16499–16507. doi: 10.1074/jbc.M500711200. [DOI] [PubMed] [Google Scholar]
- 13.Gilmore T. D. Oncogene. 1999;18:6842–6844. doi: 10.1038/sj.onc.1203237. [DOI] [PubMed] [Google Scholar]
- 14.Shin S. W., Kokoza V., Ahmed A., Raikhel A. S. Proc. Natl. Acad. Sci. USA. 2002;99:9978–9983. doi: 10.1073/pnas.162345999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernst M. K., Dunn L. L., Rice N. R. Mol. Cell. Biol. 1995;15:872–882. doi: 10.1128/mcb.15.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Z. P., Galindo R. L., Wasserman S. A. Genes Dev. 1997;11:3413–3422. doi: 10.1101/gad.11.24.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L. H., Ho B., Ding J. L. J. Biol. Chem. 2003;278:49428–49437. doi: 10.1074/jbc.M306641200. [DOI] [PubMed] [Google Scholar]
- 18.Chen L. F., Greene W. C. Nat. Rev. Mol. Cell. Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 19.Lyss G., Knorre A., Schmidt T. J., Pahl H. L., Merfort I. J. Biol. Chem. 1998;273:33508–33516. doi: 10.1074/jbc.273.50.33508. [DOI] [PubMed] [Google Scholar]
- 20.Shin H. M., Kim M. H., Kim B. H., Jung S. H., Kim Y. S., Park H. J., Hong J. T., Min K. R., Kim Y. FEBS Lett. 2004;571:50–54. doi: 10.1016/j.febslet.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 21.Foley E., O’Farrell P. H. Genes Dev. 2003;17:115–125. doi: 10.1101/gad.1018503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bogdan C. Nat. Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 23.Medvedev A., Saunders N. A., Matsuura H., Chistokhina A., Jetten A. M. J. Biol. Chem. 1999;274:3887–3896. doi: 10.1074/jbc.274.6.3887. [DOI] [PubMed] [Google Scholar]
- 24.Ding J. L., Wang L. H., Ho B. Curr. Genom. 2004;5:147–155. [Google Scholar]
- 25.Osaki T., Kawabata S. Cell. Mol. Life Sci. 2004;61:1257–1265. doi: 10.1007/s00018-004-3396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding J. L., Tan K. C., Thangamani S., Kusuma N., Seow W. K., Bui T. H., Wang J., Ho B. Genes Immunol. 2005;6:557–574. doi: 10.1038/sj.gene.6364240. [DOI] [PubMed] [Google Scholar]
- 27.Ng P. M., Jin Z., Tan S. S., Ho B., Ding J. L. J. Endotoxin Res. 2004;10:163–174. doi: 10.1179/096805104225004833. [DOI] [PubMed] [Google Scholar]
- 28.Kawai N., Shimada M., Kawahara H., Satoh N., Yokosawa H. Eur. J. Biochem. 2003;270:4459–4468. doi: 10.1046/j.1432-1033.2003.03838.x. [DOI] [PubMed] [Google Scholar]
- 29.Lin C. C., Chou C. M., Hsu Y. L., Lien J. C., Wang Y. M., Chen S. T., Tsai S. C., Hsiao P. W., Huang C. J. J. Biol. Chem. 2004;279:3308–3317. doi: 10.1074/jbc.M309749200. [DOI] [PubMed] [Google Scholar]
- 30.Ding J. L., Navas M. A., 3rd, Ho B. Biochim. Biophys Acta. 1993;1202:149–156. doi: 10.1016/0167-4838(93)90076-4. [DOI] [PubMed] [Google Scholar]
- 31.Ariki S., Koori K., Osaki T., Motoyama K., Inamori K., Kawabata S. Proc. Natl. Acad. Sci. USA. 2004;101:953–958. doi: 10.1073/pnas.0306904101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spencer E., Jiang J., Chen Z. J. Genes Dev. 1999;13:284–294. doi: 10.1101/gad.13.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonizzi G., Karin M. Trends. Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.