Abstract
Helicobacter pylori infects nearly half the world's population and is associated with a spectrum of gastric maladies. Infections with cytotoxin-associated gene pathogenicity island (cag PAI)-containing strains are associated with an increased risk for gastric cancer. The cag PAI contains genes encoding a type IV secretion system (T4SS) and a delivered effector, CagA, that becomes tyrosine phosphorylated upon delivery into host cells and initiates changes in cell signaling. Although some cag PAI genes have been shown to be required for CagA delivery, a subset of which are homologues of T4SS genes from Agrobacterium tumefaciens, the majority have no known function or homologues. We have performed a detailed investigation of one such cag PAI protein, CagN, which is encoded by the gene HP0538. Our results show that CagN is not delivered into host cells and instead is associated with the bacterial membrane. We demonstrate that CagN is cleaved at its C terminus by a mechanism that is independent of other cag PAI proteins. Finally, we show that a ΔcagN mutant is not impaired in its ability to deliver CagA to gastric epithelial cells and initiate cell elongation.
Helicobacter pylori infects the human stomach and is associated with an increased risk for diseases ranging from gastritis to peptic ulcer and gastric cancer. While some aspects of infection outcome are related to diet and host genetic factors, the strongest indicator of a severe disease risk is infection with a bacterial strain harboring the cytotoxin-associated gene pathogenicity island (cag PAI) (5). This 40-kb cassette of approximately 28 genes encodes a type IV secretion system (T4SS) and its substrate, CagA. During infection, CagA is delivered into host cells in a T4SS-dependent manner and induces multiple changes in host cell signaling which are thought to contribute directly to disease (reviewed in reference 6).
The correlation between cag PAI+ strains and disease has generated great interest in the function of the H. pylori T4SS. Several bacterial pathogens and symbionts alike employ T4SS for delivery of protein or DNA effectors into host cells (10). Of the 28 genes carried by the cag PAI, 6 have homology to the T4SS of Agrobacterium tumefaciens, the best-studied model of type IV secretion. The remaining genes are unique to the H. pylori cag PAI, and a function has been proposed for only a few of these (8, 26, 37).
Infection of cultured gastric epithelial cells with cag PAI+ H. pylori strains results in interleukin-8 (IL-8) induction and a dramatic cellular elongation. The discovery of mutations that blocked CagA delivery but not IL-8 induction led to the hypothesis that a second secreted effector molecule existed that was responsible for IL-8 induction. However, systematic deletion of cag PAI genes identified only four classes of mutants: those that had no effect on CagA delivery or IL-8 induction, those that blocked both CagA delivery and IL-8 induction, those that blocked CagA delivery but not IL-8 induction, and those that had an intermediate and variable effect on both CagA delivery and IL-8 induction (16, 31). No cag PAI mutants that allowed CagA delivery but blocked IL-8 induction were identified, ruling out any cag-encoded proteins as candidates for an IL-8-specific inducing effector. Recent experiments have implicated peptidoglycan, acting through the cytoplasmic receptor Nod1, as the secreted factor that induces IL-8 (39), while others have shown that in some strain backgrounds CagA itself is responsible for some IL-8 induction (7).
These deletion studies were an important first step in understanding the functions of individual members of the cag PAI, yet many questions about the functions of individual cag PAI genes remain unanswered. The cag PAI genes whose deletion does not alter CagA delivery and IL-8 secretion in tissue culture cells may encode delivered effector proteins with unknown activities in infection. Other pathogens that employ a T4SS in their infection process, such as A. tumefaciens, Legionella pneumophila, and Bartonella henselae, deliver multiple effector proteins into host cells (22, 29, 38). Alternatively, these genes may encode proteins with redundant functions in type IV secretion. Mutations that resulted in intermediate and variable levels of CagA delivery and IL-8 induction are promising candidates for proteins important in T4SS assembly and function. These genes may encode accessory proteins required for substrate translocation (such as chaperones) or may even encode additional substrates themselves.
Here, we report a detailed characterization of one such gene, HP0538 or cagN. A cagN mutant was reported by Fischer et al. to have an intermediate and variable CagA delivery and IL-8 induction phenotype (16). We found that CagN is localized to the bacterial membrane and is not a substrate for the T4SS. We then analyzed the CagN protein itself and found that it is cleaved at its C terminus. This processing event is independent of other cag PAI proteins and is only partially blocked by mutations of the predicted cleavage site. Finally, we show that a cagN mutant is not deficient for CagA-induced AGS cell elongation. We conclude that CagN either serves a redundant function in T4SS or is required for some other, uncharacterized aspect of pathogenesis.
MATERIALS AND METHODS
Strains and growth conditions.
All H. pylori strains used in this study are listed in Table 1. Liquid media for growth of H. pylori consisted of brucella broth supplemented with 10% fetal bovine serum (FBS) and 10 mg ml−1 vancomycin. H. pylori was maintained on blood agar plates consisting of Columbia agar (Difco) and 5% defibrillated horse blood (Hemostat) supplemented with 0.02 mg ml−1 β-cyclodextrin, 8 mg ml−1 amphotericin B, and 10 mg ml−1 vancomycin in a humidified 10% CO2 incubator. AGS cells were obtained from ATCC, cultured in Dulbecco modified Eagle medium (Gibco) supplemented with 10% FBS and 10 mg ml−1 vancomycin, and grown in a humidified 5% CO2 incubator.
TABLE 1.
Strains
| Relevant mutation | Description | Source or reference |
|---|---|---|
| None (wild type) | H. pylori strain G27 | ATCC |
| ΔcagA | cagA::aphA3 | 19 |
| ΔcagE | cagE::Tn3Kan | 11 |
| ΔcagN | Clean deletion of cagN (Hp0538) | This study |
| Δ27cagN | HA-tagged CagN with N-terminal truncation | This study |
| ΔPAI | Complete PAI deletion | 14 |
| ΔPAI rdxA::cagN | Reintroduction of cagN | This study |
| cagNS216A | Serine 216 to alanine | This study |
| cagNR217A | Arginine 217 to alanine | This study |
| cagNR217D | Arginine 217 to aspartic acid | This study |
| cagNS216D,R216D | Serine 216 and arginine 217 to aspartic acid | This study |
| rdxA::htrAS196A-HA | Protease-null C-terminal HA-tagged HtrA | This study |
Bacterial mutants.
The chromosomal copy of cagN in H. pylori strain G27 was interrupted with the KanSacB construct, encoding kanamycin resistance and sucrose sensitivity (12). Sucrose selection was then used to isolate recombinants with a clean deletion of the cagN locus as listed in Table 1. Introduction of cagN and htrAS196A-HA at the rdxA locus was achieved using the pRdxA plasmid (33), which was modified as follows. DNA corresponding to the 750-bp region directly upstream of the H. pylori ureA (HP0073) gene (which includes all of the upstream region of HP0073 as well as a 3′ portion of HP0074) was cloned into the 5′ end of the multiple cloning site. The gene of interest was then cloned into the 3′ end of the multiple cloning site adjacent to the urease promoter, which allowed for robust expression in H. pylori.
Recombinant CagN.
Because we found that full-length CagN was insoluble due to an N-terminal hydrophobic region, we generated recombinant CagN protein in which the first 27 amino acids were removed. The nucleotide sequence of HP0538 corresponding to amino acids 28 to 306 of CagN was amplified by PCR from H. pylori strain G27 and cloned into a pBH-based vector for expression in Escherichia coli strain BL21(DE3). The pBH vector contains a hexahistidine sequence followed by a tobacco etch virus protease cleavage site. After purification using Ni-nitrilotriacetic acid resin, the hexahistidine tag was removed by incubation with tobacco etch virus protease. CagN was further purified by elecroelution and dialysis before immunization of rabbits at the Monoclonal Antibody Facility at the University of Oregon.
Antibodies and cell stains.
A rabbit polyclonal antibody to CagN was created as described above and used at a 1:10,000 dilution. Mouse anti-CagA (Austral) and antiphosphotyrosine (Cell Signal Technologies) antibodies were used at a 1:2,000 dilution. Mouse antihemagglutinin (anti-HA) antibody (Covance) was used at 1:1,000. Horseradish peroxidase-conjugated donkey anti-rabbit and sheep anti-mouse antibodies (Amersham Biosciences) were used at 1:5,000. Bound antibodies were detected using ECL (Amersham Biosciences) for Western blot analysis. For microscopy, mouse anti-H. pylori antibody (Monosan) was used at 1:200, followed by Marina Blue-conjugated goat anti-mouse antibody (Molecular Probes) at 1:80. Actin was visualized with tetramethyl rhodamine isocyanate-conjugated phalloidin (Sigma) at 1:500.
Purification of endogenous CagN.
An anti-CagN antibody column was constructed by affinity purifying anti-CagN antibodies from CagN-immunized rabbit serum on a recombinant CagN antigen column. Purified antibodies were then covalently attached to protein A-Sepharose beads (Amersham). Large liquid cultures of H. pylori were grown to saturation, harvested by centrifugation (5,000 × g, 10 min, 4°C), and resuspended in ice-cold NENT lysis buffer (1% NP-40, 5 mM EDTA, 250 mM NaCl, 25 mM Tris-HCl [pH 8.0], and Complete protease inhibitor [Roche]). After 30 min of incubation on ice with gentle agitation, lysate was precleared by centrifugation (20,000 × g, 15 min, 4°C). Precleared lysate was then passed over the anti-CagN column. Purified CagN was released by elution in 100 mM glycine, pH 2.5. Combined fractions were dialyzed against phosphate-buffered saline and concentrated before sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transfer to polyvinylidene difluoride (PVDF). CagN was visualized on PVDF with Coomassie blue staining. The band was then excised and sent for N-terminal Edman degradation analysis at the Molecular Structure Facility at the University of California, Davis (http://msf.ucdavis.edu/). Bands corresponding to additional proteins that coimmunoprecipitated with CagN were excised and subjected to in-gel tryptic digestion. The resulting peptides were submitted to the University of Oregon Genomics and Proteomics Lab for one-dimensional liquid chromatography/tandem mass spectrometry peptide sequencing on a ThermoFinnigan LCQ Deca XP Plus mass spectrometer. Spectra were analyzed using SEQUEST software; peptides were scored using support vector machine learning analysis (1).
Coimmunoprecipitation.
H. pylori strain G27 rdxA::htrAS196A-HA (Table 1) was grown and lysed as described above. In some experiments bacteria were pretreated with 25 mM dithiobis(succinimidyl propionate) to cross-link proteins before lysis as described previously (13). Lysate was then incubated with anti-HA or anti-CagN antibodies coupled to Protein A-Sepharose beads (Amersham Biosciences) for 30 min at 4°C. Beads containing protein complexes were washed three times in 500 μl lysis buffer and then boiled in 2× SDS sample buffer and elecrophoresed on SDS-polyacrylamide gels. Proteins were detected by Western blotting with appropriate antibodies after transfer to PVDF.
Fractionation of H. pylori.
Bacterial cells were fractionated as described previously (17). Briefly, an overnight liquid culture of H. pylori was harvested by centrifugation (5,000 × g, 10 min, 4°C) and resuspended in ice-cold PBS. Cells were lysed in a French pressure cell at 20,000 lb/in2, and the lysate was cleared of unlysed cells by centrifugation (5,000 × g, 10 min, 4°C). To separate the membrane fractions from the cytosolic fractions, the lysate was further ultracentrifuged (100,000 × g, 1 h, 4°C). The supernatant containing cytosolic fractions was decanted from the pellet that contained the membranes. Each sample was then boiled in 2× SDS sample buffer and separated via SDS-PAGE. CagN was detected by Western blotting after transfer of proteins to PVDF and probing with anti-CagN antibody. As a control for the fractionation process, blots were also probed with an antibody against CagA, which is localized to the bacterial inner membrane (13).
CagA tyrosine phosphorylation.
In preparation for infection experiments, H. pylori cultures grown on plates were used to inoculate liquid infection medium (Dulbecco modified Eagle medium supplemented with 10% FBS, 10 μg/ml vancomycin, and 10% Brucella broth) and passaged twice overnight. Before infection, medium on AGS cells was also changed to infection medium. Cells were infected at a multiplicity of infection of 1:100 and then gently rinsed 15 min later. Infections proceeded for 8 h before cells were lysed in 2× SDS sample buffer and electrophoresed via SDS-PAGE. Proteins were transferred to PVDF membranes, probed with the appropriate antibodies, and then detected by ECL (Amersham).
Fractionation of infected AGS cells.
AGS cells were infected as described above. Cells were fractionated at 8 h postinfection as described previously (36). Briefly, cells were scraped from tissue culture dishes and resuspended in lysis buffer (250 mM sucrose, 0.5 mM EDTA, and 3 mM imidazole, pH 8.0). Cells were mechanically lysed by four passages through a 0.23-gauge needle. After centrifugation (16,000 × g, 10 min, 4°C), both the pellet (containing intact H. pylori) and supernatant (containing AGS cytosolic proteins) were dissolved in sample buffer, electrophoresed via SDS-PAGE, and transferred to PVDF. Proteins were visualized by probing with the appropriate antibodies. To verify that the AGS cell lysate was not contaminated with lysed bacteria, experiments were performed in parallel with a cagE mutant, which is unable to deliver CagA to AGS cells.
Elongation assay.
AGS cells were seeded on glass coverslips and transiently transfected with pEGFP-C3 (Clontech) using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. At approximately 24 h posttransfection, cells were infected as described above for 8 h. Coverslips were fixed for 10 min in fixative solution (2% paraformaldehyde, 10 mM sodium periodate, 750 mM lysine, 37 mM sodium phosphate, pH 7.4) and examined by fluorescence microscopy. To assay elongation, cells from approximately 100 random fields of view at a magnification of ×20 (three to seven cells per field) were captured with a Photometrics Cool Snap FX digital camera on a Nikon TE2000 inverted microscope, and their length-to-breadth ratios were measured using Metamorph imaging software.
RESULTS AND DISCUSSION
CagN is not translocated into host cells and is localized to the bacterial membrane.
The cag PAI gene HP0538 encodes CagN, a protein of unknown function. Analysis of the CagN amino acid sequence by using SignalP 3.0 (4) revealed that CagN contains a predicted N-terminal hydrophobicity region that could be a signal peptide and a predicted cleavage site between amino acids 24 and 25 but no other recognizable motifs or domain structure. Previous experiments have shown that CagN is not absolutely required for T4SS function but may have a variable effect on CagA delivery and IL-8 secretion (16). These results suggested that CagN could be delivered into host cells and alter CagA function, or it could be a component of the T4SS itself.
Other pathogens that employ a T4SS deliver multiple effector proteins. For example, VirE2 and VirF are transported by A. tumefaciens into plant cells in addition to the T-DNA/VirB2 complex. For L. pneumophila, recent studies have brought the total number of secreted proteins to at least 24, many of which have no known function or no clear effect on intracellular bacterial survival (22). In B. henselae, a conserved C-terminal domain which directs seven PAI proteins to secretion into host cells via a T4SS has been identified (29). Therefore, it is reasonable to hypothesize that the cag PAI may encode substrates in addition to CagA.
First, we tested whether CagN is translocated into host cells as a substrate of the cag PAI-encoded type IV secretion system. In order to follow the protein during infection, we raised CagN antisera against recombinant protein generated in E. coli. Antiserum was generated against a recombinant CagN protein lacking the hydrophobic N terminus, which we found to be more soluble than the full-length protein (see Materials and Methods). We then used this antibody to test whether CagN protein could be found in H. pylori-infected host cells. Initially we attempted to look for CagN delivery by using microscopy, but we found that the CagN antibody is not suitable for immunofluorescence (C. Botham, unpublished data). Instead, infected AGS monolayers were selectively lysed, leaving whole bacteria intact, and then fractionated by centrifugation (36). As a control, we also followed CagA delivery, which is dependent on CagE, the VirB4 ATPase homologue that is thought to provide energy for CagA translocation (16, 31). Whereas CagA was readily detected in supernatants of host cells infected with wild-type but not cagE mutant H. pylori in this assay, CagN remained associated with the bacterial pellet and thus did not appear to be translocated into host cells to any appreciable level (Fig. 1A).
FIG. 1.
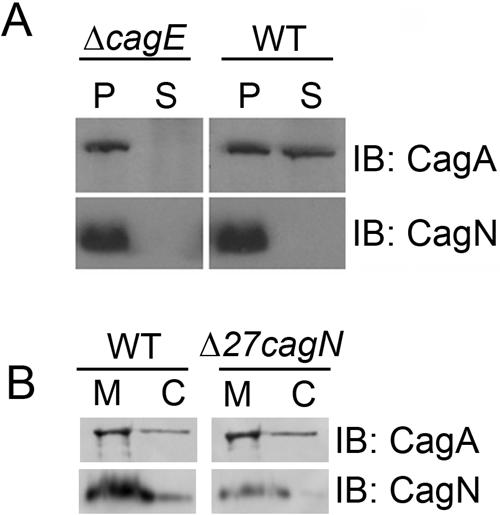
CagN is not delivered into host cells and remains localized to the bacterial membrane. A) Lysates from ΔcagE and wild-type (WT) H. pylori-infected AGS cells were fractionated into a supernatant containing AGS cell cytosol (lanes S) or a pellet containing bacteria (lanes P). B) Fractionated H. pylori lysate shows CagN and Δ27CagN localized mainly at the bacterial membrane (lanes M) rather than the bacterial cytosol (lanes C) as is seen for the inner bacterial membrane-localized protein CagA. IB, immunoblotting.
We next used cell fractionation experiments to determine CagN′s subcellular localization in H. pylori. Bacterial cultures were lysed using a French press and separated into cytosolic and membrane fractions following a procedure used to identify the membrane topology of the H. pylori NixA protein (17). Western blot analysis of these fractions revealed the vast majority of CagN to be membrane localized, with only a small amount seen in the cytosolic fraction (Fig. 1B). This may result from a small amount of membrane contamination in the cytosolic fraction, since a similar distribution was seen for CagA, which is associated with the bacterial inner membrane (13). We tested whether the N-terminal hydrophobic region of CagN, which contains a predicted signal sequence, was required for membrane localization by generating a strain in which the wild-type copy of CagN was replaced with an HA epitope-tagged CagN lacking the first 27 amino acids (Δ27cagN). Truncated CagN was still localized to the bacterial membrane; however, it appeared to be less abundant than the wild-type protein relative to the amount of CagA, suggesting that the N terminus may be important for protein stability (Fig. 1B).
CagN protein is proteolytically processed at the C terminus.
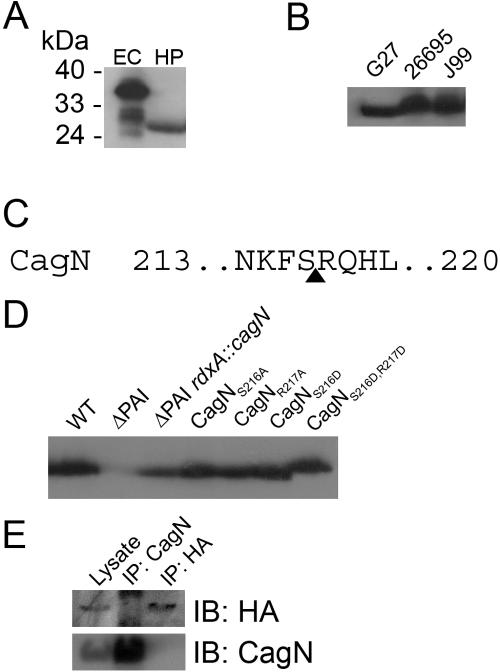
cagN in strain G27 encodes a 306-amino-acid protein with a predicted molecular mass of 34,690.83 Da (GenBank accession number DQ307685). Upon Western analysis of CagN protein using the anti-CagN antibody, we observed that endogenous CagN in H. pylori lysates appeared to be approximately 10 kDa smaller than the expected size. This 24-kDa species is not observed when recombinant CagN is expressed in E. coli (Fig. 2A). CagN is also expected to be approximately 35 kDa in the sequenced strains 26695 and J99. We found that CagN is similarly processed in these strains as well (Fig. 2B).
FIG. 2.
A) CagN in H. pylori lysates (lane HP) is 10 kDa smaller than the expected full-length protein expressed in E. coli (lane EC). B) CagN is similarly processed in strains G27, 26695, and J99. C) Predicted cleavage site between serine 216 and arginine 217 (arrow). D) CagN processing is normal in the absence of other PAI proteins. Single amino acid substitutions of the predicted cleavage site do not affect CagN processing, but the protein migrates slightly slower when both sites are mutated to aspartic acid (CagNS216D,R217D). WT, Wild type. E) Coimmunoprecipitation of HtrAS196A-HA (HA) and CagN from the rdxA::HtrAS196A-HA strain shows that HtrAS196A-HA does not precipitate CagN, nor does CagN precipitate HtrAS196A-HA. The extra bands in the lane designated IP:CagN and IP:HA resulted from the secondary antibody binding to heavy and light chains of the CagN antibody used for immunoprecipitation. IP, immunoprecipitation; IB, immunoblotting.
Processing of some T4SS proteins is essential for virulence. In A. tumefaciens, VirB1 is processed to N-terminal and C-terminal fragments which appear to have different functions in T-complex delivery (3). Similarly, the pilus protein VirB2 is cleaved and cyclized, which is essential for its function in T-pilus biogenesis (21). In H. pylori, the cag PAI proteins CagY (HP0527, a VirB10 homologue) and CagT (HP0532, a VirB7 homologue) are also processed and required for CagA delivery (26). We further investigated CagN cleavage by determining if cleavage occurred at the N or C terminus. Endogenous CagN was immunoprecipitated from H. pylori strain G27 and analyzed by Edman degradation. Given the predicted cleavage site between amino acids 24 and 25, we were surprised to find the N terminus of CagN intact in this endogenous protein. We therefore hypothesize that CagN is cleaved at its C terminus. We were unable to purify enough endogenous CagN for C-terminal sequencing, which is considerably less sensitive then Edman degradation. Instead, endogenous CagN was further analyzed by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry and was found to have a molecular mass of 24,552 Da, which exactly corresponds to a CagN peptide composed of amino acids 1 to 216 (Fig. 2C). Consistent with the endogenous CagN protein corresponding to amino acids 1 to 216, trypsin digestion and MALDI-TOF analysis of the major protein immunoprecipitated by the CagN antibody identified peptides corresponding to CagN amino acids 52 to 70, 86 to 93, 86 to 100, 133 to 145, and 197 to 204.
CagN processing is independent of the cag PAI and the predicted cleavage site sequence.
Although there are degradation products of recombinant CagN expressed in E. coli, these do not correspond to the size seen in H. pylori (Fig. 2A), suggesting that the protease(s) acting upon CagN is H. pylori specific. The cleaved H. pylori cag PAI proteins CagY and CagT require Cagγ, a putative lytic transglycosylase, for maturation (26). Therefore, we hypothesized that CagN might be cleaved by a cag PAI-encoded protein rather than a general bacterial protease. To test this hypothesis, CagN was introduced at the rdxA locus in a ΔPAI strain. However, CagN was still found to be processed even in the absence of other PAI genes (Fig. 2D).
We then performed site-directed mutagenesis of the predicted CagN cleavage site of serine 216 and arginine 217. We replaced the wild-type copy of CagN with several substitutions at this site, using combinations of alanine and aspartic acid. Single amino acid substitutions at either serine 216 or arginine 217 did not alter CagN processing (Fig. 1C). A double aspartic acid substitution of both serine 216 and arginine 217 resulted in a slightly higher-molecular-weight molecule (Fig. 1C). This shift in molecular weight could result from endoprotease processing at an alternative cleavage site (for example S231, S232). Although we were unable to visualize a 10-kDa fragment corresponding to the CagN C terminus on Western blots, we did find evidence for the existence of this fragment from mass spectrometric analysis of immunopurified CagN. A fast-migrating protein that immunoprecipitated with the anti-CagN antibody was subjected to trypsin digestion and MALDI-TOF analysis, which revealed peptides corresponding to CagN amino acids 285 to 305 and 287 to 305. This suggests that the C terminus of CagN is cleaved by an endopeptidase and may subsequently be degraded.
During purification of CagN for Edman analysis, we observed a coimmunoprecipitated protein with a molecular mass of approximately 50 kDa. MALDI-TOF analysis of trypsin-digested fragments of this band identified them as peptides corresponding to amino acids 102 to 120, 167 to 197, and 242 to 262 of HtrA, a DegP homologue. DegP is a conserved heat shock protein that functions as both a chaperone and an endopeptidase in E. coli (34) and is an important virulence determinant of several bacterial pathogens (15, 20, 23). We could not test whether CagN processing requires HtrA because htrA is an essential gene in H. pylori, as suggested by our inability to isolate an htrA mutant and confirmed by Salama and coworkers (28). Instead, we tested whether we could detect binding between CagN and a mutant HtrA (HtrAS196A) engineered to lack protease activity but retain its chaperone function, based on the characterized DegPS210A mutant of E. coli (34). HtrA from E. coli and other species is known to undergo multiple steps of N-terminal processing (32), and this processing is likely conserved in H. pylori given the observation of various-sized HtrA proteins discovered during proteome analysis (2). Therefore, we introduced an HA epitope tag at the C terminus of an HtrAS196A mutant at the rdxA locus. Bacterial lysate from this strain was immunoprecipitated with anti-HA or anti-CagN antibodies, and Western blots were probed for the presence of CagN or HA-tagged HtrAS196A. Both HA-tagged HtrAS196A and CagN were detected in the lysate. However, immunoprecipitated HtrAS196A-HA did not precipitate CagN, nor did CagN precipitate HtrAS196A-HA (Fig. 2E). We were also unable to show an interaction using bacterial lysates pretreated with a cross-linking reagent (13) or in vitro expressing wild-type or mutant HtrA with CagN in rabbit reticulocyte lysates (data not shown). We also tested CagN cleavage in an E. coli degP mutant background (34) but found no change in the CagN degradation pattern compared to CagN expressed in wild-type E. coli (data not shown). We therefore have no evidence that CagN is proteolytically processed by HtrA.
CagN is not required for CagA delivery into host cells.
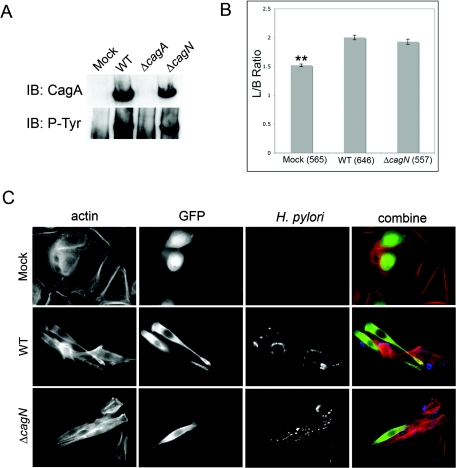
CagN is localized to the bacterial membrane and is not a T4SS substrate itself. Therefore, we tested the hypothesis that CagN functions in the T4SS machinery by modulating CagA delivery. We compared the levels of CagA delivered into host cells infected with wild-type H. pylori or a ΔcagA mutant. CagA is tyrosine phosphorylated at EPIYA motifs by c-Src upon delivery into host cells (30, 35), which can be detected with an antiphosphotyrosine antibody. We found that the amounts of tyrosine-phosphorylated CagA from infected cell lysates were similar following infection with both the wild type and the ΔcagN mutant (Fig. 3A), demonstrating that there was no qualitative difference in CagA delivery as measured by this assay.
FIG. 3.
A) Western blot showing CagA tyrosine phosphorylation after delivery into host cells. CagA delivery is not disrupted in a cagN mutant. WT, wild type; IB, immunoblotting. B) Image analysis of infected AGS cells shows that wild-type and ΔcagN H. pylori cause similar levels of cell elongation compared to mock-infected control cells. **, significantly different relative to the wild-type H. pylori-infected cells (P < 0.001). Error bars represent standard errors and the numbers of cells counted per condition are in parentheses. L/B, length to breadth. C) Images (magnification, ×600) of infected AGS cells, showing actin, green fluorescent protein-transfected cells, and H. pylori.
Although we could demonstrate CagA delivery in the absence of CagN, we wished to test whether a ΔcagN mutant exhibited a subtle defect in delivery with a more sensitive assay for CagA activity by measuring cell elongation. We transiently transfected AGS cells with a green fluorescent protein reporter construct to visualize individual green fluorescent elongated cells during infection (Fig. 3C). We used Metamorph software to quantify the extents of elongation of individual cells by measuring their lengths and breadths. Mock-infected cells were compared to cells infected with wild-type and ΔcagN H. pylori. The length-to-breadth ratios of several hundred cells were measured for each condition and are shown in Fig. 3B. Both wild-type and ΔcagN H. pylori induced AGS cell elongation compared to the mock-infected control (P < 0.0001). However, there was no statistically significant difference between wild-type- and ΔcagN-infected cells (P = 0.11). Therefore, we conclude that CagN does not play a role in T4SS function that relates to CagA delivery. Fischer et al. (16) reported that a cagN mutant of strain 26695 had a variable effect on CagA delivery by measuring CagA tyrosine phosphorylation. Although our results appear to contradict their findings, this discrepancy could be due to strain differences between 26695 and G27, differences in the infection protocol, or our use of the length-to-breadth ratio to measure the functional activity of CagA rather than tyrosine phosphorylation.
We have carried out a detailed characterization of the cag PAI protein CagN. We have shown that CagN is not a secreted effector and appears to be localized to the bacterial membrane. Processing of CagN is cag PAI independent and does not appear to require a specific cleavage site. HtrA, the H. pylori homologue of DegP, a periplasmic dual-function chaperone and protease which cleaves misfolded proteins at high temperature in E. coli (34), was a candidate for the CagN-processing protease, based on its identification by coimmunoprecipitation with CagN. However, we found no evidence of a physical interaction between CagN and HtrA in vivo or in vitro, even when we used a protease-null form of HtrA and added cross-linking agents to the lysates. Furthermore, limited proteolysis analysis of osmotically shocked H. pylori cells suggests that CagN is localized to the bacterial inner membrane rather than the periplasm, the predicted subcellular location of HtrA (unpublished results). We suspect that our initial finding of HtrA coimmunoprecipitation with CagN was an artifact because HtrA has been identified in several mass spectrometry analyses of H. pylori proteins under a variety of conditions (2, 9). Finally, we have shown that CagN is not required for T4SS control of CagA delivery. We cannot exclude the possibility that CagN serves a redundant function in T4SS function or is required for the delivery of another secreted effector that has yet to be identified.
Multiple T4SS substrates of the bacterial pathogen L. pneumophila have recently been identified which, when mutated, have no detectable phenotype in a cell culture model of infection (22). These findings point to the limitations of our current infection models and our understanding of the selective pressures experienced by pathogens throughout the infectious cycle. Even in existing animals models of H. pylori infection, the importance of the cag PAI has been controversial (24, 25). The origin of the cag PAI, which was acquired by horizontal transfer, is unknown, and most of the proteins it encodes are novel and have no known function. It is possible that many of these genes served functions in the bacterium from which the PAI originated and that they are not important in the context of H. pylori infection and disease outcome. Alternatively, because CagN has been retained by cag PAI strains and is generally conserved (18, 27), it may provide a fitness advantage to H. pylori during some aspects of its infectious cycle that we cannot readily detect in cell culture.
Acknowledgments
We thank Dave Anderson, Leslie Gay, and Dan Graham for technical assistance. We also thank Dave Baltrus and Kyle Mouery for assistance with genetic manipulations of H. pylori, Crystal Botham for help with microscopy, Benjamin Shepherd and Nina Salama for providing the rdx::ureA construct, and Michael Ehrmann of Cardiff University for generously providing a degP mutant E. coli strain.
K.G. is a recipient of a Burroughs Wellcome Fund Career Award in the Biomedical Sciences and is supported by Research Scholar Grant RSG-03-101-01-MBC from the American Cancer Society.
Editor: D. L. Burns
REFERENCES
- 1.Anderson, D. C., W. Li, D. G. Payan, and W. S. Noble. 2003. A new algorithm for the evaluation of shotgun peptide sequencing in proteomics: support vector machine classification of peptide MS/MS spectra and SEQUEST scores. J. Proteome Res. 2:137-146. [DOI] [PubMed] [Google Scholar]
- 2.Backert, S., T. Kwok, M. Schmid, M. Selbach, S. Moese, R. M. Peek, Jr., W. Konig, T. F. Meyer, and P. R. Jungblut. 2005. Subproteomes of soluble and structure-bound Helicobacter pylori proteins analyzed by two-dimensional gel electrophoresis and mass spectrometry. Proteomics 5:1331-1345. [DOI] [PubMed] [Google Scholar]
- 3.Baron, C., M. Llosa, S. Zhou, and P. C. Zambryski. 1997. VirB1, a component of the T-complex transfer machinery of Agrobacterium tumefaciens, is processed to a C-terminal secreted product, VirB1. J. Bacteriol. 179:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 5.Blaser, M. J., and J. C. Atherton. 2004. Helicobacter pylori persistence: biology and disease. J. Clin. Investig. 113:321-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourzac, K. M., and K. Guillemin. 2005. Helicobacter pylori-host cell interactions mediated by type IV secretion. Cell Microbiol. 7:911-919. [DOI] [PubMed] [Google Scholar]
- 7.Brandt, S., T. Kwok, R. Hartig, W. Konig, and S. Backert. 2005. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc. Natl. Acad. Sci. USA 102:9300-9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buhrdorf, R., C. Forster, R. Haas, and W. Fischer. 2003. Topological analysis of a putative virB8 homologue essential for the cag type IV secretion system in Helicobacter pylori. Int. J. Med. Microbiol. 293:213-217. [DOI] [PubMed] [Google Scholar]
- 9.Bumann, D., S. Aksu, M. Wendland, K. Janek, U. Zimny-Arndt, N. Sabarth, T. F. Meyer, and P. R. Jungblut. 2002. Proteome analysis of secreted proteins of the gastric pathogen Helicobacter pylori. Infect. Immun. 70:3396-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cascales, E., and P. J. Christie. 2003. The versatile bacterial type IV secretion systems. Nat. Rev. Microbiol. 1:137-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Censini, S., C. Lange, Z. Xiang, J. E. Crabtree, P. Ghiara, M. Borodovsky, R. Rappuoli, and A. Covacci. 1996. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 93:14648-14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Copass, M., G. Grandi, and R. Rappuoli. 1997. Introduction of unmarked mutations in the Helicobacter pylori vacA gene with a sucrose sensitivity marker. Infect. Immun. 65:1949-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Couturier, M. R., E. Tasca, C. Montecucco, and M. Stein. 2006. Interaction with CagF is required for translocation of CagA into the host via the Helicobacter pylori type IV secretion system. Infect. Immun. 74:273-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Covacci, A., and R. Rappuoli. 2000. Tyrosine-phosphorylated bacterial proteins: Trojan horses for the host cell. J. Exp. Med. 191:587-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farn, J., and M. Roberts. 2004. Effect of inactivation of the HtrA-like serine protease DegQ on the virulence of Salmonella enterica serovar Typhimurium in mice. Infect. Immun. 72:7357-7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer, W., J. Puls, R. Buhrdorf, B. Gebert, S. Odenbreit, and R. Haas. 2001. Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island: essential genes for CagA translocation in host cells and induction of interleukin-8. Mol. Microbiol. 42:1337-1348. [DOI] [PubMed] [Google Scholar]
- 17.Fulkerson, J. F., Jr., and H. L. Mobley. 2000. Membrane topology of the NixA nickel transporter of Helicobacter pylori: two nickel transport-specific motifs within transmembrane helices II and III. J. Bacteriol. 182:1722-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gressmann, H., B. Linz, R. Ghai, K. P. Pleissner, R. Schlapbach, Y. Yamaoka, C. Kraft, S. Suerbaum, T. F. Meyer, and M. Achtman. 2005. Gain and loss of multiple genes during the evolution of Helicobacter pylori. PLOS Genet. 1:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guillemin, K., N. R. Salama, L. S. Tompkins, and S. Falkow. 2002. Cag pathogenicity island-specific responses of gastric epithelial cells to Helicobacter pylori infection. Proc. Natl. Acad. Sci. USA 99:15136-15141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibrahim, Y. M., A. R. Kerr, J. McCluskey, and T. J. Mitchell. 2004. Role of HtrA in the virulence and competence of Streptococcus pneumoniae. Infect. Immun. 72:3584-3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai, E. M., R. Eisenbrandt, M. Kalkum, E. Lanka, and C. I. Kado. 2002. Biogenesis of T pili in Agrobacterium tumefaciens requires precise VirB2 propilin cleavage and cyclization. J. Bacteriol. 184:327-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo, Z. Q., and R. R. Isberg. 2004. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc. Natl. Acad. Sci. USA 101:841-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyon, W. R., and M. G. Caparon. 2004. Role for serine protease HtrA (DegP) of Streptococcus pyogenes in the biogenesis of virulence factors SpeB and the hemolysin streptolysin S. Infect. Immun. 72:1618-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monack, D. M., A. Mueller, and S. Falkow. 2004. Persistent bacterial infections: the interface of the pathogen and the host immune system. Nat. Rev. Microbiol. 2:747-765. [DOI] [PubMed] [Google Scholar]
- 25.O'Rourke, J. L., and A. Lee. 2003. Animal models of Helicobacter pylori infection and disease. Microbes Infect. 5:741-748. [DOI] [PubMed] [Google Scholar]
- 26.Rohde, M., J. Puls, R. Buhrdorf, W. Fischer, and R. Haas. 2003. A novel sheathed surface organelle of the Helicobacter pylori cag type IV secretion system. Mol. Microbiol. 49:219-234. [DOI] [PubMed] [Google Scholar]
- 27.Salama, N., K. Guillemin, T. K. McDaniel, G. Sherlock, L. Tompkins, and S. Falkow. 2000. A whole-genome microarray reveals genetic diversity among Helicobacter pylori strains. Proc. Natl. Acad. Sci. USA 97:14668-14673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salama, N. R., B. Shepherd, and S. Falkow. 2004. Global transposon mutagenesis and essential gene analysis of Helicobacter pylori. J. Bacteriol. 186:7926-7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schulein, R., P. Guye, T. A. Rhomberg, M. C. Schmid, G. Schroder, A. C. Vergunst, I. Carena, and C. Dehio. 2005. A bipartite signal mediates the transfer of type IV secretion substrates of Bartonella henselae into human cells. Proc. Natl. Acad. Sci. USA 102:856-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selbach, M., S. Moese, C. R. Hauck, T. F. Meyer, and S. Backert. 2002. Src is the kinase of the Helicobacter pylori CagA protein in vitro and in vivo. J. Biol. Chem. 277:6775-6778. [DOI] [PubMed] [Google Scholar]
- 31.Selbach, M., S. Moese, T. F. Meyer, and S. Backert. 2002. Functional analysis of the Helicobacter pylori cag pathogenicity island reveals both VirD4-CagA-dependent and VirD4-CagA-independent mechanisms. Infect. Immun. 70:665-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skorko-Glonek, J., D. Zurawa, F. Tanfani, A. Scire, A. Wawrzynow, J. Narkiewicz, E. Bertoli, and B. Lipinska. 2003. The N-terminal region of HtrA heat shock protease from Escherichia coli is essential for stabilization of HtrA primary structure and maintaining of its oligomeric structure. Biochim. Biophys. Acta 1649:171-182. [DOI] [PubMed] [Google Scholar]
- 33.Smeets, L. C., J. J. Bijlsma, S. Y. Boomkens, C. M. Vandenbroucke-Grauls, and J. G. Kusters. 2000. comH, a novel gene essential for natural transformation of Helicobacter pylori. J. Bacteriol. 182:3948-3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spiess, C., A. Beil, and M. Ehrmann. 1999. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97:339-347. [DOI] [PubMed] [Google Scholar]
- 35.Stein, M., F. Bagnoli, R. Halenbeck, R. Rappuoli, W. J. Fantl, and A. Covacci. 2002. c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA motifs. Mol. Microbiol. 43:971-980. [DOI] [PubMed] [Google Scholar]
- 36.Stein, M., R. Rappuoli, and A. Covacci. 2000. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc. Natl. Acad. Sci. USA 97:1263-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka, J., T. Suzuki, H. Mimuro, and C. Sasakawa. 2003. Structural definition on the surface of Helicobacter pylori type IV secretion apparatus. Cell Microbiol. 5:395-404. [DOI] [PubMed] [Google Scholar]
- 38.Vergunst, A. C., M. C. van Lier, A. den Dulk-Ras, T. A. Stuve, A. Ouwehand, and P. J. Hooykaas. 2005. Positive charge is an important feature of the C-terminal transport signal of the VirB/D4-translocated proteins of Agrobacterium. Proc. Natl. Acad. Sci. USA 102:832-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viala, J., C. Chaput, I. G. Boneca, A. Cardona, S. E. Girardin, A. P. Moran, R. Athman, S. Memet, M. R. Huerre, A. J. Coyle, P. S. DiStefano, P. J. Sansonetti, A. Labigne, J. Bertin, D. J. Philpott, and R. L. Ferrero. 2004. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 5:1166-1174. [DOI] [PubMed] [Google Scholar]