Abstract
Dendritic cells (DC) efficiently cross-present exogenous antigen on MHC class I molecules to CD8+ T cells. However, little is known about cross-presentation by Langerhans cells (LC), the DCs of the epidermis. Therefore, we investigated this issue in detail. Isolated murine LCs were able to cross-present soluble ovalbumin protein on MHC-class I molecules to antigen-specific CD8+ T cells, albeit less potently than the CD8+ DC subsets from spleen. Furthermore, LCs cross-presented cell-associated ovalbumin peptide and protein expressed by neighboring keratinocytes. Use of transporter associated with antigen processing (TAP-1)-deficient mice suggested a TAP-dependent pathway. Similar observations were made with migratory LC. Antigen expressed in the epidermis was ingested by LCs during migration from the epidermis and presented to antigen-specific T cells in vitro. Cross-presentation of ovalbumin protein by LCs induced IFN-γ production and cytotoxicity in antigen-specific CD8+ T cells. Additionally, epicutaneous application of ovalbumin protein induced in vivo proliferation of OT-I T cells in the draining lymph nodes; this was markedly enhanced when antigen was applied to inflamed, barrier-disrupted skin. Thus, LCs cross-present exogenous antigen to CD8+ T cells and induce effector functions, like cytokine production and cytotoxicity, and may thereby critically contribute in epicutaneous vaccination approaches.
Keywords: cross-presentation, dendritic cells, epidermis, mouse or murine, epicutaneous
Cross-presentation is an important mechanism for generating immunity to viruses and tumors as well as for the induction of tolerance against self antigens (1, 2). Dendritic cells (DCs) (3–5) cross-present soluble exogenous antigen on MHC-class I to antigen-specific CD8+ T cells. Cell-associated antigen, like virus-infected cells (6), transfected tumor cells (7), protein-coated cells (8), and dying cells (9) can be taken up and cross-presented by DCs to CD8+ T cells. Importantly, DCs proved to be more efficient in cross-presenting exogenous antigen than macrophages (10). A particular subset of the DC, the CD8α+ DCs in the spleen, seems to be superior in cross-presentation to CD8α-negative DCs (11, 12).
Many pathogens enter the body via the skin. However, little is known about the ability of Langerhans cells (LC) to capture and process exogenous antigen in intact skin and present it on MHC-class I to CD8+ T cells. In vitro generated human LCs derived from CD34+ precursors are able to cross-present exogenous antigen to CD8+ T cells (13, 14), and human Langerhans-like cells are more potent than dermal-like and monocyte-derived DCs (15). Two recent reports showed that epicutaneous immunization with an MHC-class I-restricted peptide or with ovalbumin protein onto tape-stripped skin induces cytotoxic T cell activity in the draining lymph node which can be further increased by coadministration of cholera toxin (16, 17). Others reported that these responses can also be boosted with Toll-like receptor ligands, such as oligonucleotides and imiquimod (18, 19). Thus, epicutaneous immunization elicits T cell responses in the draining lymph nodes, but inflammation in the skin is necessary for an optimal response. However, the precise role of LCs is still unclear.
In this study, we focused on LC, the DCs of the epidermis (20), and investigated their ability to cross-present soluble and cell-associated antigen to antigen-specific CD8+ T cells in vitro, a precondition for their being a potential target for epicutaneous immunization strategies.
Results
In Vitro Cultured LCs Cross-Present Exogenous Soluble Antigen to CD8+ T Cells.
LCs were isolated by trypsinization from the epidermis and pulsed for 6 h with different amounts of ovalbumin (OVA) protein. Thereafter, the cells were washed extensively and further cultured for 3 days until mature. In the subsequent cocultures with antigen-specific CD8+ (from OT-I mice) and CD4+ T cells (from OT-II mice), LCs induced proliferation of both T cell types (Fig. 1A). This ability to cross-present exogenous antigen depended on the antigen-dose used to pulse the LCs as described earlier for DCs (21).
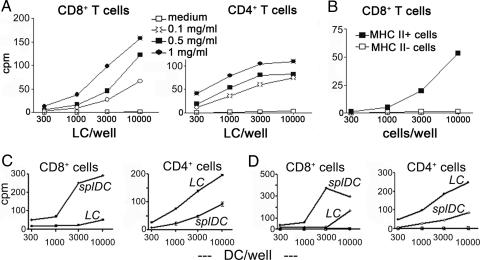
Fig. 1.
Isolated LCs cross-present OVA protein on MHC-class I. (A) LCs were isolated from the epidermis by trypsinization and cultured for 6 h with different concentrations of OVA protein. After extensive washing, cells were further cultured until day 3 and enriched on a Nycodenz gradient. Graded doses of LCs were cocultured with antigen-specific CD8+ T cells (OT-I) or CD4+ T cells (OT-II) for 60 h. Experiments were done with different concentrations of OVA protein, a total of 13 times. (B) Isolated LCs were pulsed with 0.5 mg/ml OVA protein for 6 h at the onset of culture and sorted on day 3 with magnetic beads conjugated to an MHC-class II mAb. Positive and negative fractions were compared for their ability to induce proliferation of OT-I T cells as described above (n = 2). (C and D) Isolated spleen DCs (“splDC”) were pulsed with 0.5 mg/ml OVA protein for 6 h before overnight culture and FACS sorted to purify CD8α+ DCs. LCs were isolated, pulsed for 6 h with OVA protein and matured until day 3. Both populations were used for proliferation assays as described in A (n = 3). Representative experiments are shown in each panel. C and D are two separate experiments. Y axes indicate cpm in thousands.
It was also dependent on the time of exposure to the antigen. LCs pulsed with OVA protein for 1 h vs. 6 h elicited somewhat lower proliferation of CD8+ T cells; LCs pulsed for 24 h induced stronger proliferation. However, these kinetics were not as pronounced as those observed in parallel with presentation on MHC II (Fig. 7, which is published as supporting information on the PNAS web site). Because LCs greatly reduce antigen uptake upon culture (22) and degradation of the protein in the culture medium might occur with time, we did not pulse for longer periods; rather, we decided to perform all subsequent experiments with the 6-h antigen pulse.
Because we used LCs enriched by Nycodenz-gradient centrifugation (containing ≈50% LC) for most experiments, we wished to confirm these results with highly purified LCs to exclude possible interference by keratinocytes. Mature OVA protein pulsed LCs were sorted with MHC-class II magnetic beads, and both the positive and negative fractions were cocultured with OT-I cells. As expected, only the LC-containing fraction could cross-present OVA (Fig. 1B).
CD8α+ spleen DCs were described as the most potent cross-presenting DCs subset (12). In a side-by-side comparison CD8α+ spleen DCs were indeed more potent in inducing CD8+ T cell proliferation. Inversely, LCs were better in stimulating CD4+ T cells (Fig. 1 C and D).
LCs Take Up and Process OVA Protein for Presentation on MHC-Class I Molecules.
Reis e Sousa et al. (23) reported that OVA protein preparations often contain free peptides, which may cause problems when investigating cross-presentation because free peptides can bind to MHC-class I molecules without a need for processing. Therefore, we first examined the OVA protein solutions used for pulsing the LCs for the presence of contaminating peptides by reverse phase high-performance liquid chromatography (HPLC). We could not detect peptides corresponding in size and hydrophobic properties to the OVA peptides specific for H2-Kb (amino acids 257–264; SIINFEKL) or I-Ab (amino acids 323–339; ISQAVHAAHAEINEAGR). The detection threshold for peptides was between 0.5 and 0.2 μM (Fig. 8, which is published as supporting information on the PNAS web site). Because this might be sufficient for stimulation of CD8+ T cells, we performed additional experiments to exclude the presence of peptide contaminations in OVA protein. Freshly isolated epidermal cells were pulsed with dialyzed and nondialyzed OVA protein for 6 h and cultured until day 3. LCs pulsed with either form of OVA protein were equally potent in inducing CD8+ T cell proliferation indicating the absence of peptide contamination in the OVA protein solutions (Fig. 9A, which is published as supporting information on the PNAS web site).
The stability of peptide/MHC-class I complexes on maturing DCs for almost 3 days (24) also became evident when we pulsed freshly isolated epidermal cells with SIINFEKL peptide for 6 h at 37°C followed by extensive washing and transfer of cells into fresh medium for further culture. Peptide-pulsed mature LCs induced substantial proliferation of CD8+ T cells (Fig. 9B). Next, we sought to detect newly formed MHC-class I/SIINFEKL complexes on the cell surface of LCs by means of mAb 25D1.16 (25). When we stained LCs pulsed on day 0 with either SIINFEKL peptide or OVA protein for 6 h and then cultured for 3 days, we were unable to detect any peptide/MHC-class I complexes on the cell surface despite the observed strong induction of CD8+ T cell proliferation. These complexes were only detectable when fresh or cultured LCs were analyzed immediately after the pulse with SIINFEKL (Fig. 9 C and D). The number of complexes was obviously too small to be detectable with a mAb but enough to induce CD8+ T cell proliferation.
Ex Vivo Emigrated LCs Cross-Present Soluble OVA Protein Taken Up in the Skin.
Epidermal skin explants were incubated with OVA protein overnight and, after washing for 1 h on fresh medium, further cultured for 3 days. This step was performed to ensure that the migratory LCs would only take up antigen that has diffused into the epidermis during the overnight incubation. These LCs were potent inducers of CD4+ and CD8+ T cell proliferation (Fig. 2A).
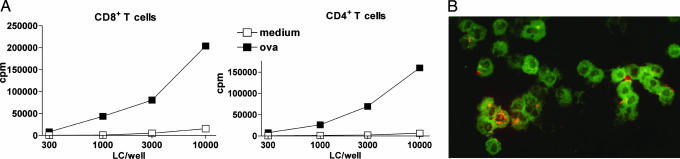
Fig. 2.
Ex vivo emigrated LCs cross-present OVA protein on MHC-class I molecules. (A) Epidermal skin explants were prepared from mouse ear skin with dispase and pulsed with 0.5 mg/ml OVA protein overnight. After washing, the explants were further cultured until day 3, and emigrated LCs were used for coculture with OT-I and OT-II T cells for 60 h (n = 5). One representative experiment is shown. (B) Migratory LC, identified by MHC II expression (green fluorescence) take up Texas red-conjugated OVA (red fluorescence) into distinct intracellular vesicles.
Uptake of fluorescently labeled OVA into vesicles could be readily observed in LCs emigrated from epidermal explants pulsed with OVA protein overnight as described above (Fig. 2B). In contrast, using mAb 25-D1.16, we were unable to detect MHC-class I/peptide complexes on the cell surface (data not shown).
LCs Cross-Present in a TAP-Dependent Manner.
The experiments described thus far do not exclude a possible extracellular processing mechanism. Therefore, we performed experiments with TAP-1-deficient mice to further elucidate the mechanism of cross-presentation. LCs from TAP-1-deficient mice were pulsed with OVA protein for 6 h, and on day 3 no cross-presentation of exogenous antigen was detected (Fig. 3A). In contrast, presentation of OVA peptides on MHC-class II molecules occurred in an undisturbed fashion. When mature LCs were pulsed with SIINFEKL peptide, both TAP-1-deficient and wild-type cells showed comparable stimulation of CD8+ T cells, indicating that the lower expression of MHC-class I molecules on the surface of TAP-1-deficient cells cannot be the reason for the absence of T cell stimulation (data not shown). Furthermore, these data confirm that there are no relevant peptide contaminations in our OVA protein solutions.
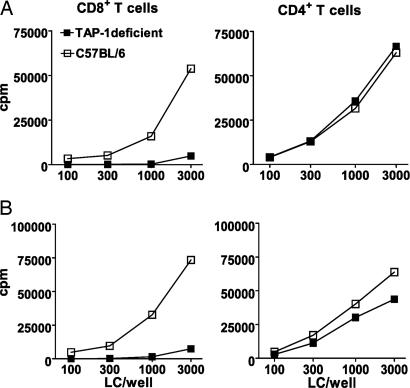
Fig. 3.
LCs use a TAP-dependent mechanism for cross-presentation. (A) LCs were isolated from epidermis of TAP-1-deficient and C57BL/6 wild-type mice, pulsed for 6 h with 0.5 mg/ml OVA protein, and transferred to fresh medium. After 3 days of culture, LCs were cocultured with OT-I or OT-II T cells for 60 h (n = 5). (B) Epidermal explants were prepared from mouse ear skin with dispase and pulsed with OVA protein overnight. After extensive washing, the explants were further cultured for 3 days, and emigrated LCs were used for coculture as described above (n = 3). Representative experiments are shown in each panel.
Migratory LCs from TAP-1-deficient mice behaved similarly to isolated LC. Emigrated mature LCs on day 3 of culture induced minimal proliferation of CD8+ T cells (but strong proliferation of CD4+ T cells), suggesting that migratory LCs need the TAP machinery to cross-present antigen taken up in the skin (Fig. 3B).
LCs Can Cross-Present Cellular Antigen from the Epidermis.
We investigated whether LCs can take up and cross-present cell-bound antigen. For this purpose, we tested LCs from mice expressing under the K14 promoter in the epidermis either the OVA peptide SIINFEKL (K14-OVAp transgenic mice; ref. 26) or the whole OVA protein (K14mOVA transgenic mice). When we compared LCs isolated from K14-OVAp mice with LCs from C57BL/6 wild-type mice, we indeed observed antigen-specific CD8+ T cell proliferation (Fig. 4A). To exclude direct presentation by keratinocytes, LCs were sorted on day 3 of culture by using MHC-class II magnetic beads and cocultured with T cells. As expected, T cell proliferation occurred only with LCs (MHC-class II+ cell fraction), but not with keratinocytes (MHC-class II− fraction, data not shown). To mimick a more “in vivo-like” situation, epidermal skin explants from C57BL/6 wild-type and K14-OVAp as well as from K14mOVA mice were cultured for 3 days, and the emigrated LCs were used for proliferation assays with OT-I T cells. LCs from transgenic mice were able to cross-present keratinocyte-derived OVA peptide and protein (Fig. 4 B and C). As in the previous experiments, we were unable to detect any staining with mAb 25D1.16 binding to SIINFEKL/MHC-class I complexes (data not shown).
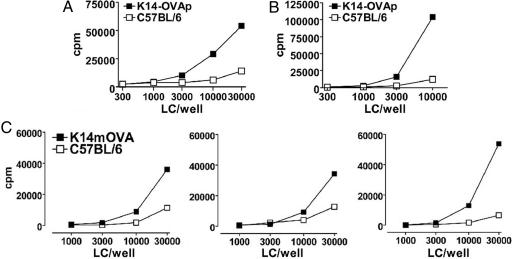
Fig. 4.
LCs cross-present keratinocyte-derived peptide and protein on MHC-class I to CD8+ T cells. (A) LCs were isolated from epidermis of K14-OVAp and C57BL/6 wild-type mice and cultured for 3 days. These mature cells were cocultured with OT-I T cells for 60 h (n = 5). (B and C) Epidermal skin explants were prepared with dispase from either K14-OVAp (B) or K14mOVA (C) and C57BL/6 wild-type mice and cultured for 3 days. Emigrated LCs were cocultured with OT-I T cells as described above (n = 3 each). Representative experiments are shown for A and B; all three experiments are shown in C.
Cross-Presentation of Exogenous Antigen Induces Cytokine Production and Cytotoxicity in CD8+ T Cells.
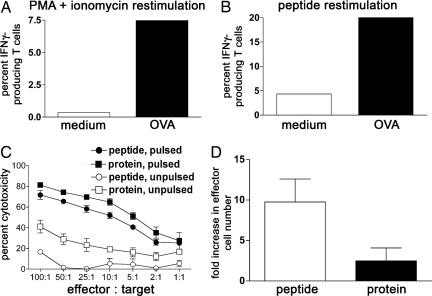
Isolated LCs were pulsed with OVA protein for 6 h, matured, and cocultured with OT-I T cells for 48 h. Thereafter, T cells were restimulated with phorbol myristate acetate and ionomycin or SIINFEKL peptide for 6 h and analyzed by intracellular cytokine FACS. We detected increased levels of IFN-γ when LCs had been pulsed with OVA protein, suggesting that cross-presentation results in the development of CD8+ T cell effector function. We never found significant production of IL-4 and IL-10 (Fig. 5A and B).
Fig. 5.
Cross-presenting LCs increase IFN-γ production and cytotoxicity in antigen-specific CD8+ T cells. (A and B) Freshly isolated LCs were pulsed for 6 h with 0.5 mg/ml OVA protein, washed, and transferred to fresh medium. After 3 days of maturation, LCs were cocultured with OT-I T cells for 48 h. T cells were restimulated for 6 h with phorbol myristate acetate and ionomycin (n = 3) or SIINFEKL peptide (n = 1) in the presence of Brefeldin A. Percentages of cytokine-positive activated T cells are shown. (C and D) LCs were either pulsed with OVA protein or peptide before coculture with antigen-specific CD8+ T cells for 5 days. Effector cells generated in these cultures were incubated with peptide pulsed or unpulsed radioactive target cells at the indicated effector/target ratios for 3.5 h, and specific lysis was measured (mean ± SD of triplicates of one representative experiment; n = 3). (D) CD8+ effector T cells generated in the 5-day cocultures of OT-I T cells with OVA protein or SIINFEKL-pulsed LCs were counted in the hemocytometer (mean ± SD; n = 3).
In a next step, we tested whether the activated CD8+ T cells developed cytotoxicity when stimulated with cross-presenting LC. To this end, OVA protein-pulsed LCs were cocultured with antigen-specific CD8+ T cells for 5 days, and then the T cells were tested for their ability to kill target cells pulsed with SIINFEKL in a cytotoxicity assay in vitro (27). The effector T cells generated in these cocultures were potent killers for pulsed but not unpulsed target cells (Fig. 5C). Cytotoxic T cells elicited by cross-presenting LCs induced equal degrees of specific lysis of target cells as cytotoxic T cells elicited by peptide-pulsed LC. However, the numbers of cytotoxic T cells generated during the 5-day culture were higher when LCs had been pulsed with the SIINFEKL peptide (Fig. 5D).
Application of OVA Protein Onto the Epidermis Induces CD8+ T Cell Proliferation in Skin-Draining Lymph Nodes.
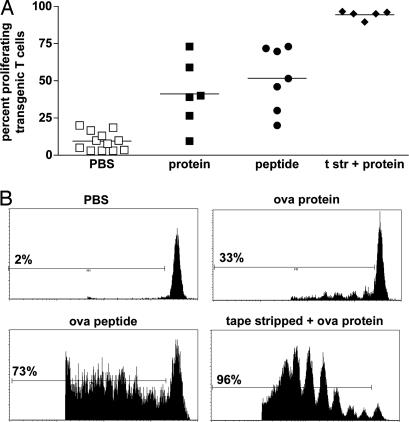
To extend these in vitro observations, we examined in vivo proliferation of antigen-specific T cells after epicutaneous application of OVA protein. CSFE-labeled OT-I T cells were injected intravenously into C57BL/6 mice, and 24 h later, either different concentrations of OVA protein or 10 μg of SIINFEKL peptide were applied onto the skin. Three days later, we observed proliferation of transgenic CD8+ T cells. Proliferation in response to 0.5 mg (Fig. 6) and 1 mg (not shown) of OVA protein was similar. Identical observations were made in experiments using specifically LPS-free OVA (not shown). Application of SIINFEKL induced a slightly higher OT-I proliferation in skin-draining lymph nodes when compared to the protein. Because there are reports that presentation of OVA protein might be better when there is inflammation in the skin [for example, after tape stripping (16) or coadministration of cholera toxin (17) or Toll-like receptor ligands (18, 19)], we applied OVA protein also onto tape-stripped ear skin. Indeed, when skin was inflamed after tape-stripping, the response to OVA protein was increased dramatically such that nearly all of the antigen-specific T cells were proliferating in the skin-draining lymph node (Fig. 6).
Fig. 6.
Epicutaneously applied OVA protein is cross-presented in skin draining lymph nodes. Purified CFSE-labeled OT-I T cells were injected intravenously into C57BL/6 mice. Twenty-four hours later, either 0.5 mg of OVA protein or 10 μg of SIINFEKL peptide in creme was applied onto untreated or tape-stripped (24 h before antigen application) ear skin. T cell proliferation in the skin-draining lymph nodes was analyzed 3 days later. Experiments were done two to five times for different groups. A summary of all mice is shown in A. Representative histograms from the FACS analyses are shown in B. Percentages of proliferating T cells are indicated. Lines in A indicate means; t str, tape-stripped.
Discussion
Our results directly show that LCs isolated from the skin can cross-present exogenous soluble and cell-associated antigen to CD8+ T cells. Furthermore, we show that migratory LCs can take up material from keratinocytes and cross-present it. Cross-presentation by LCs depends on the TAP machinery, as suggested by the use of TAP-1-deficient mice. The final outcome of cross-presentation by LCs is the induction of IFN-γ production and cytotoxicity in antigen-specific CD8+ T cells. Thus, cross-presenting LCs are able to induce an efficient immune response in CD8+ T cells that may be harnessed for clinical purposes.
Mechanism of Cross-Presentation by LC.
Reis e Sousa et al. (23) have detected peptide contaminations in different batches of OVA protein. Several experiments rule out this concern in our study. (i) OVA protein was biochemically tested for the presence of peptides by HPLC. We could not detect any peptides corresponding in size and hydrophobic properties to the immunogenic SIINFEKL peptide in different batches of dialyzed and nondialyzed OVA protein solutions. (ii) Dialyzed OVA did not lose T cell stimulatory activity, also indicating the absence of functional levels of free peptides in the protein solution. (iii) OT-I cells did not proliferate in response to OVA protein-pulsed LCs from TAP-1-deficient mice. Free peptides in the OVA protein solution would have bound to MHC-I in a TAP-independent manner and induced T cell proliferation. This may be regarded as a sensitive read-out because, despite lower total MHC I expression levels, cells from TAP-1-deficient mice are known to express more MHC I molecules available for peptide binding (28).
In Vivo Relevance of Cross-Presentation by LCs.
In this study, LCs were able to cross-present exogenous protein antigen in vitro. When compared to the CD8α+ DC subset, they were less efficient. Although this finding indicates some interesting differences in their ability to cross-present, it remains to be seen whether this holds true for the in vivo situation, i.e., LCs compared to CD8α+ DC, both isolated from lymph nodes after epicutaneous immunization. In the meantime, we compared isolated LCs with the ex vivo-derived migratory LCs from skin explants as a more physiologic model for LC behavior. Indeed, LCs emigrated from epidermal skin explants, which had been incubated with soluble OVA protein, were able to pick up the antigen in the skin and process and cross-present it to antigen-specific CD8+ T cells, similar to enzymatically isolated LC.
In further experiments, we used a model in which either the OVA peptide SIINFEKL or the whole OVA protein was expressed under the K14 promoter in surrounding keratinocytes. Both enzymatically isolated (K14-OVAp) and emigrated LCs (both strains) could cross-present keratinocyte-derived SIINFEKL on MHC-class I to antigen-specific T cells. Our results are supported by two recent reports. Shibaki et al. (29) used mice expressing the whole OVA protein under the K14 promoter and showed that DCs emigrating from skin explants cross-presented OVA peptides. DCs emigrating from whole skin explants are a mixture of dermal DCs and LCs (30), and therefore, the relative contribution of LCs could not be determined in this report. In the second report by Mayerova et al. (31), LCs were sorted from skin draining lymph nodes of mice expressing SIINFEKL under the K14 promoter. Only 60% of the sorted cells were E-cadherin-positive, and thus, these cells did not represent a pure population of LC. Nevertheless, the lymph node population containing the LCs was able to cross-present the OVA peptide. More recent observations by the same authors in a similar experimental model indicate that LCs cross-present also the whole OVA protein when it is expressed in keratinocytes (L.B. and K. Hogquist, data not shown). In summary, we conclude that LCs have the potential to take up antigen in the skin, process and cross-present it to antigen-specific CD8+ T cells in vivo. However, our data do not provide a clue as to how LCs acquire the antigen, be it by uptake of apoptotic material, transfer via gap junctions (32), or “nibbling” (33).
It is not clear whether cross presentation by LCs is operative in all situations of antigen encounter. There is evidence that, in herpes virus models, LCs do not cross-present viral antigens in vivo (34, 35). Generally, cross-presentation by DCs is down-regulated by systemic infections involving Toll-like receptor engagement (36). The skin explant model used in our study is a highly inflammatory but nondestructive and noninfectious model that leaves LCs intact and presumably strongly enhances their cross-presentation capacity (37). However, the detailed composition of the various DCs maturation stimuli operative in the explant model has not been defined.
Clinical Relevance of Cross-Presentation by LC.
Epicutaneous or transmucosal immunization with protein and peptides has been reported to be efficient in inducing IFN-γ production and cytotoxicity in T cells (38), especially when combined with inflammatory stimuli like tape stripping, cholera toxin, and Toll-like receptor ligands, such as oligonucleotides and imiquimod (16–19). Seo et al. (16) showed that LCs from tape-stripped skin are activated and express higher levels of costimulatory molecules and MHC-class I. When these LCs were peptide pulsed, they were able to induce cyotoxicity in CD8+ T cells, indicating that they might be involved in this process (16). However, this was not formally proven, and therefore, the precise role of LCs still remains unclear. Our data suggest a critical role for LCs because they fulfill one important requirement, namely cross-presenting capacity. For further discussion, see Supporting Text, which is published as supporting information on the PNAS web site.
Methods
Mice.
Mice of inbred strains C57BL/6, OT-I (39), and OT-II (40), and TAP-1-deficient (41) mice were purchased from Charles River Laboratories and used at 2–4 months of age. T cells from OT-I mice and OT-II mice express a transgenic Vα2 Vβ5.1/5.2 T cell receptor (TCR) specific for the OVA peptides presented on H2-Kb (amino acids 257–264; SIINFEKL) or on I-Ab (amino acids 323–339; ISQAVHAAHAEINEAGR), respectively. Mice expressing the SIINFEKL peptide under the K14 promoter, i.e., in the keratinocytes, were kindly provided by K. Hogquist (26). Analogous mice expressing the whole OVA protein under the K14 promoter and fused to the transmembrane portion of the transferrin receptor (42) were generated by B. E. Rich (Harvard University, Cambridge, MA).
Media and Reagents.
Culture medium was Iscove′s Minimum Essential Medium (PAN-Biotech, Aidenbach, Germany) supplemented with 10% FCS (Biochrom, Berlin), 2 mM l-glutamine (Cambrex-Biowhittaker, Verviers, Belgium), 50 μg/ml gentamycin (Invitrogen-Gibco, Paisley, U.K.) and 50 μM 2-mercaptoethanol (Sigma). The following antibodies were purchased from BD-PharMingen: MHC-class II-fluorescein (FITC, clone 2G9), CD8α (clone Lyt-2)-allophycocyanin (APC) and -phycoerythrin (PE), CD11c-APC (clone HL-3), IL-4-PE (clone BVD4–24G2), IFN-γ-FITC (clone XMG1.2), IL-10-PE (clone JES5–16E3), Vα2 TCR-PE (clone B20.1), Vβ5.1/5.2 TCR-biotinylated (clone MR9-4). For detection of MHC-class I/peptide-complexes (H2-Kb+SIINFEKL) we used mAb 25D1.16 (mouse Ig; kind gift from J. W. Yewdell, National Institutes of Health, Bethesda) (25). This antibody was visualized with a PE-conjugated anti-mouse Ig (Dako, Glostrup, Denmark).
Antigens.
OVA protein was purchased from Sigma and, in some experiments, dialyzed for 36 h at 4°C with several changes of the buffer (PBS) to exclude possible contaminating peptides (D-0405 MWC 12000 tubes from Sigma; exclusion limit, 12 kDa). The biochemical characterization of OVA is described in the supporting information.
Isolation of LC.
Pieces of mouse ear and trunk skin were incubated on 0.8% trypsin (Merck) for 25–45 min. Epidermis was peeled off and incubated for 30 min at 37°C. Resulting epidermal cell suspensions contained 1–3% LC. These populations were incubated for 6 h with OVA protein at different concentrations, then thoroughly washed and transferred into fresh medium. LCs mature spontaneously in such bulk cultures, i.e., together with keratinocytes. On day 3 of culture, the mature LCs were enriched on a Nycodenz gradient (Sigma) as described (43) resulting in an enrichment of at least 50% LC. In some experiments we further purified LCs by sorting with anti-MHC-class II magnetic beads (Miltenyi Biotec) on day 3 of culture (purity, 70–90%).
Isolation of CD8α+ Spleen DC.
Cell suspensions from spleens were prepared by digestion with 0.5 mg/ml collagenase P (Roche, Mannheim, Germany) for 30 min at 37°C and subsequent pressing of the tissue through cell strainers (70 μm; Falcon Labware, Oxnard, CA), essentially as described recently for spleen cell suspensions (44). DCs were enriched by Nycodenz gradient centrifugation (Sigma-Aldrich) as described (43). Low-density cells were pulsed for 6 h with 0.5 mg/ml OVA protein and then extensively washed before being cultured overnight in the presence of GM-CSF. As a control, spleen cells were cultured in medium alone. On the next day the spleen cells were harvested, stained for CD8α and CD11c, and cells coexpressing both markers were sorted on a FACSVantage-SE (Becton Dickinson). Cell purity was routinely >98%.
Skin Explant Culture.
Epidermal explants were procured from ear skin as described (45). Explants were incubated with different concentrations of OVA protein overnight, placed onto fresh medium for 1 h to wash off the OVA protein and transferred to another well with fresh medium for further culture. Epidermal sheets were cultured for a total of 72 h to obtain fully mature LC. Emigrated LCs were collected and cocultured with T cells.
For further methods, see Supporting Text.
Supplementary Material
Acknowledgments
We thank Alexander D. McLellan (Otago University, Dunedin, New Zealand) and Jose A. Villadangos for helpful discussions throughout this project. For expert management of mouse breeding, we are indebted to Franz Koch (Department of Dermatology, Innsbruck Medical University). We thank Kristin Hogquist (University of Minnesota, Minneapolis) for advice, encouragement, and K14-OVAp and K14mOVA mice, and Elke Scandella and Burkhart Ludewig (Canton Hospital, St. Gallen, Switzerland) and Valeska Heib and Edgar Schmitt (University of Mainz, Mainz, Germany) for the supply of TAP-1-deficient and OT-I mice. The continuous support of Peter Fritsch, Chairman of the Department of Dermatology (Innsbruck Medical University), is greatly appreciated. This work was supported Austrian Science Fund FWF Grants P-14949 and L120-B13 (to N.R.) and Erwin-Schrödinger-Fellowship J2479 (to P.S.).
Abbreviations
- DC
dendritic cell
- LC
Langerhans cell
- OVA
ovalbumin.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Heath W. R., Carbone F. R. Annu. Rev. Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 2.Guermonprez P., Amigorena S. Springer Semin. Immunopathol. 2005;26:257–271. doi: 10.1007/s00281-004-0176-0. [DOI] [PubMed] [Google Scholar]
- 3.Shen Z. H., Reznikoff G., Dranoff G., Rock K. L. J. Immunol. 1997;158:2723–2730. [PubMed] [Google Scholar]
- 4.Mitchell D. A., Nair S. K., Gilboa E. Eur. J. Immunol. 1998;28:1923–1933. doi: 10.1002/(SICI)1521-4141(199806)28:06<1923::AID-IMMU1923>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Norbury C. C., Chambers B. J., Prescott A. R., Ljunggren H. G., Watts C. Eur. J. Immunol. 1997;27:280–288. doi: 10.1002/eji.1830270141. [DOI] [PubMed] [Google Scholar]
- 6.Sigal L. J., Crotty S., Andino R., Rock K. L. Nature. 1999;398:77–80. doi: 10.1038/18038. [DOI] [PubMed] [Google Scholar]
- 7.Huang A. Y. C., Golumbek P., Ahmadzadeh M., Jaffee E., Pardoll D., Levitsky H. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 8.Carbone F. R., Bevan M. J. J. Exp. Med. 1990;171:377–387. doi: 10.1084/jem.171.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albert M. L., Sauter B., Bhardwaj N. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 10.Brossart P., Bevan M. J. Blood. 1997;90:1594–1599. [PMC free article] [PubMed] [Google Scholar]
- 11.Pooley J. L., Heath W. R., Shortman K. J. Immunol. 2001;166:5327–5330. doi: 10.4049/jimmunol.166.9.5327. [DOI] [PubMed] [Google Scholar]
- 12.Den Haan J. M. M., Lehar S. M., Bevan M. J. J. Exp. Med. 2000;192:1685–1695. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuo M., Nagata Y., Sato E., Atanackovic D., Valmori D., Chen Y. T., Ritter G., Mellman I., Old L. J., Gnjatic S. Proc. Natl. Acad. Sci. USA. 2004;101:14467–14472. doi: 10.1073/pnas.0405947101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagata Y., Ono S., Matsuo M., Gnjatic S., Valmori D., Ritter G., Garrett W., Old L. J., Mellman I. Proc. Natl. Acad. Sci. USA. 2002;99:10629–10634. doi: 10.1073/pnas.112331099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratzinger G., Baggers J., de Cos M. A., Yuan J., Dao T., Reagan J. L., Münz C., Heller G., Young J. W. J. Immunol. 2004;173:2780–2791. doi: 10.4049/jimmunol.173.4.2780. [DOI] [PubMed] [Google Scholar]
- 16.Seo N., Tokura Y., Nishijima T., Hashizume H., Furukawa F., Takigawa M. Proc. Natl. Acad. Sci. USA. 2000;97:371–376. doi: 10.1073/pnas.97.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahlon R., Hu Y. X., Orteu C. H., Kifayet A., Trudeau J. D., Tan R. S., Dutz J. P. Vaccine. 2003;21:2890–2899. doi: 10.1016/s0264-410x(03)00141-5. [DOI] [PubMed] [Google Scholar]
- 18.Klimuk S. K., Najar H. M., Semple S. C., Aslanian S., Dutz J. P. J. Invest. Dermatol. 2004;122:1042–1049. doi: 10.1111/j.0022-202X.2004.22411.x. [DOI] [PubMed] [Google Scholar]
- 19.Rechtsteiner G., Warger T., Osterloh P., Schild H., Radsack M. P. J. Immunol. 2005;174:2476–2480. doi: 10.4049/jimmunol.174.5.2476. [DOI] [PubMed] [Google Scholar]
- 20.Romani N., Holzmann S., Tripp C. H., Koch F., Stoitzner P. APMIS. 2003;111:725–740. doi: 10.1034/j.1600-0463.2003.11107805.x. [DOI] [PubMed] [Google Scholar]
- 21.Kurts C., Miller J. F. A. P., Subramaniam R. M., Carbone F. R., Heath W. R. J. Exp. Med. 1998;188:409–414. doi: 10.1084/jem.188.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reis e Sousa C., Stahl P. D., Austyn J. M. J. Exp. Med. 1993;178:509–519. doi: 10.1084/jem.178.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reis e Sousa C., Germain R. N. J. Exp. Med. 1995;182:841–851. doi: 10.1084/jem.182.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kukutsch N. A., Rössner S., Austyn J. M., Schuler G., Lutz M. B. J. Invest. Dermatol. 2000;115:449–453. doi: 10.1046/j.1523-1747.2000.00084.x. [DOI] [PubMed] [Google Scholar]
- 25.Porgador A., Yewdell J. W., Deng Y., Bennink J. R., Germain R. N. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 26.McGargill M. A., Mayerova D., Stefanski H. E., Koehn B., Parke E. A., Jameson S. C., Panoskaltsis-Mortari A., Hogquist K. A. J. Immunol. 2003;169:2141–2147. doi: 10.4049/jimmunol.169.4.2141. [DOI] [PubMed] [Google Scholar]
- 27.Matzinger P. J. Immunol. Methods. 1991;145:185–192. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- 28.Day P. M., Esquivel F., Lukszo J., Bennink J. R., Yewdell J. W. Immunity. 1995;2:137–147. doi: 10.1016/s1074-7613(95)80014-x. [DOI] [PubMed] [Google Scholar]
- 29.Shibaki A., Sato A., Vogel J. C., Miyagawa F., Katz S. I. J. Invest. Dermatol. 2004;123:109–115. doi: 10.1111/j.0022-202X.2004.22701.x. [DOI] [PubMed] [Google Scholar]
- 30.Stoitzner P., Holzmann S., McLellan A. D., Ivarsson L., Stössel H., Kapp M., Kämmerer U., Douillard P., Kämpgen E., Koch F., et al. J. Invest. Dermatol. 2003;120:266–274. doi: 10.1046/j.1523-1747.2003.12042.x. [DOI] [PubMed] [Google Scholar]
- 31.Mayerova D., Parke E. A., Bursch L. S., Odumade O. A., Hogquist K. A. Immunity. 2004;21:391–400. doi: 10.1016/j.immuni.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Neijssen J., Herberts C., Drijfhout J. W., Reits E., Janssen L., Neefjes J. Nature. 2005;434:83–88. doi: 10.1038/nature03290. [DOI] [PubMed] [Google Scholar]
- 33.Harshyne L. A., Zimmer M. I., Watkins S. C., Barratt-Boyes S. M. J. Immunol. 2003;170:2302–2309. doi: 10.4049/jimmunol.170.5.2302. [DOI] [PubMed] [Google Scholar]
- 34.Allan R. S., Smith C. M., Belz G. T., Van Lint A. L., Wakim L. M., Heath W. R., Carbone F. R. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 35.Zhao X. Y., Deak E., Soderberg K., Linehan M., Spezzano D., Zhu J., Knipe D. M., Iwasaki A. J. Exp. Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson N. S., Behrens G. M., Lundie R. J., Smith C. M., Waithman J., Young L., Forehan S. P., Mount A., Steptoe R. J., Shortman K. D., et al. Nat. Immunol. 2006;7:165–172. doi: 10.1038/ni1300. [DOI] [PubMed] [Google Scholar]
- 37.Delamarre L., Holcombe H., Mellman I. J. Exp. Med. 2003;198:111–122. doi: 10.1084/jem.20021542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belyakov I. M., Hammond S. A., Ahlers J. D., Glenn G. M., Berzofsky J. A. J. Clin. Invest. 2004;113:998–1007. doi: 10.1172/JCI20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hogquist K. A., Jameson S. C., Heath W. R., Howard J. L., Bevan M. J., Carbone F. R. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 40.Barnden M. J., Allison J., Heath W. R., Carbone F. R. Immunol. Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 41.Van Kaer L., Ashton-Rickardt P. G., Ploegh H., Tonegawa S. Cell. 1992;71:1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 42.Kurts C., Heath W. R., Carbone F. R., Allison J., Miller J. F., Kosaka H. J. Exp. Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McLellan A. D., Starling G. C., Hart D. N. J. J. Immunol. Methods. 1995;184:81–89. doi: 10.1016/0022-1759(95)00077-n. [DOI] [PubMed] [Google Scholar]
- 44.McLellan A. D., Kapp M., Eggert A., Linden C., Bommhardt U., Bröcker E. B., Kämmerer U., Kämpgen E. Blood. 2002;99:2084–2093. doi: 10.1182/blood.v99.6.2084. [DOI] [PubMed] [Google Scholar]
- 45.Ratzinger G., Stoitzner P., Ebner S., Lutz M. B., Layton G. T., Rainer C., Senior R. M., Shipley J. M., Fritsch P., Schuler G., et al. J. Immunol. 2002;168:4361–4371. doi: 10.4049/jimmunol.168.9.4361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.