Abstract
Although signals for vacuolar sorting of soluble proteins are well described, we have yet to learn how the plant vacuolar sorting receptor BP80 reaches its correct destination and recycles. To shed light on receptor targeting, we used an in vivo competition assay in which a truncated receptor (green fluorescent protein-BP80) specifically competes with sorting machinery and causes hypersecretion of BP80-ligands from tobacco (Nicotiana tabacum) leaf protoplasts. We show that both the transmembrane domain and the cytosolic tail of BP80 contain information necessary for efficient progress to the prevacuolar compartment (PVC). Furthermore, the tail must be exposed on the correct membrane surface to compete with sorting machinery. Mutational analysis of conserved residues revealed that multiple sequence motifs are necessary for competition, one of which is a typical Tyr-based motif (YXXΦ). Substitution of Tyr-612 for Ala causes partial retention in the Golgi apparatus, mistargeting to the plasma membrane (PM), and slower progress to the PVC. A role in Golgi-to-PVC transport was confirmed by generating the corresponding mutation on full-length BP80. The mutant receptor was partially mistargeted to the PM and induced the secretion of a coexpressed BP80-ligand. Further mutants indicate that the cytosolic tail is likely to contain other information besides the YXXΦ motif, possibly for endoplasmic reticulum export, endocytosis from the PM, and PVC-to-Golgi recycling.
INTRODUCTION
In contrast with soluble proteins that are delivered to the extracellular matrix by default, soluble and membrane proteins designated to a specific intracellular compartment require information for their sorting and/or retention (Jurgens, 2004). The Golgi apparatus is the site where a large number of decisions are made regarding the transport of proteins in the secretory pathway, and the vacuole/lysosome is one of the target compartments that can be reached from this organelle.
In plants, vacuolar transport appears to be particularly complex due to the wide range of functions performed by vacuoles (Marty, 1999) and the existence of at least two distinct types of vacuolar compartments (Robinson et al., 2005). In addition to the central lytic vacuole, thought to be the equivalent of the yeast vacuole and the mammalian lysosome, protein storage vacuoles were shown to coexist in the same cells (Hohl et al., 1996; Paris et al., 1996; Bassham and Raikhel, 2000). To maintain their highly distinct protein composition, these compartments need to be efficiently distinguished by mechanisms of cargo segregation and subsequent targeting. Sorting to the lytic vacuole is Golgi-mediated and dependent on a type I transmembrane protein, originally termed BP80 (80-kD binding protein), which was first isolated from clathrin-coated vesicle extracts of pea (Pisum sativum) cotyledons (Kirsch et al., 1994). Recently, a related membrane spanning protein with a shorter lumenal domain and a longer cytosolic tail (CT) bearing a RING-H2 domain (RMR) was proposed to carry out a similar function for Golgi-mediated sorting to the storage vacuole (Jiang et al., 2000; Park et al., 2005).
Unlike RMR, which accompanies its ligands to the storage vacuole (Jiang et al., 2000; Park et al., 2005), BP80 is thought to recycle from the prevacuolar compartment (PVC) to the Golgi to avoid degradation in the lytic vacuole. This is proposed to allow renewed cargo collection at the Golgi membrane, similar to vacuolar sorting receptors in yeast and lysosomal sorting receptors in mammalian cells (Seaman, 2005). In support of this model, BP80 was shown to localize to the trans-Golgi network and the PVC (Paris et al., 1997; Sanderfoot et al., 1998; Li et al., 2002). The latter organelle often contains electron-dense internal vesicles and is also termed the multivesicular body (Tse et al., 2004). To function according to the model, BP80 must specifically interact with at least two different types of cytosolic components needed for vesicle traffic. One is required for cargo-loaded anterograde traffic from the Golgi membrane, whereas the second is needed for retrograde recycling from the PVC after cargo release. BP80 must bind ligands in the Golgi apparatus, release them in the PVC, and ensure that it is recycled without the ligand. How these intricate steps are accomplished in vivo is not understood.
Although BP80 was first postulated as a specific receptor for lytic vacuolar cargo, it has been suggested that BP80-related receptors could also mediate transport to the storage vacuole (Shimada et al., 2003; Jolliffe et al., 2004; Vitale and Hinz, 2005). Considering the premise that plants have two distinct types of vacuoles within the same cells (Paris et al., 1996), different ligand binding specificities of the lumenal portion of BP80 would be required to distinguish storage proteins from hydrolases destined to the lytic vacuole. Equally important, a storage vacuole–specific BP80 should exhibit differences in the CT to recognize distinct adaptor molecules and coat components.
On first sight, BP80 sorting appears to occur in a similar fashion as in mammalian cells and yeast, where active cargo selection is mediated by type I membrane spanning proteins that contain a lumenal domain to recruit ligands and a cytosolic domain to form clathrin-coated vesicles for transport to the PVC or prelysosome (Marcusson et al., 1994; Ghosh et al., 2003). Likewise, the plant PVC is thought to be the equivalent of the late endosome in mammals and yeast, which serves to rescue sorting receptors from the lysosomes or vacuoles. This occurs by transport via the retromer complex back to the Golgi apparatus, and receptors are thus recycled for new rounds of cargo selection (Seaman et al., 1997, 1998; Seaman, 2005).
A highly conserved Tyr motif (YMPL/YIPL), present in all Arabidopsis thaliana BP80 isoforms (Hadlington and Denecke, 2000), was shown to be important for in vitro interaction with the μ-subunit of mammalian AP1 (Sanderfoot et al., 1998) and to a Golgi-localized μ-adaptin from Arabidopsis (Happel et al., 2004). Together with the initial purification of BP80 from a clathrin-coated vesicle–enriched fraction (Kirsch et al., 1994), these findings suggest that BP80 recruits adaptor protein (AP) complexes to the Golgi membranes and integrates clathrin coat assembly with its inclusion into the nascent vesicle (Robinson et al., 2005). These reports appear to suggest an active role for the BP80 CT in the clathrin-mediated anterograde transport from the Golgi apparatus to the PVC.
Surprisingly, it was shown that the transmembrane domain (TMD) of BP80 without the CT was sufficient for targeting of a hybrid type I membrane protein to a lytic compartment (Jiang and Rogers, 1998). In addition, the tonoplast was proposed to be the default destination for transmembrane proteins in plants (Barrieu and Chrispeels, 1999). However, the length of the membrane-spanning domain was shown to control the final destination of transmembrane proteins on the plant secretory pathway, and in the case of BP80, this was localized to the Golgi apparatus when no cytosolic domains were present (Brandizzi et al., 2002b). The discrepancies among the published findings call for a thorough investigation into the domains responsible for BP80 sorting in vivo.
We have recently introduced a novel approach to monitor receptor traffic in vivo using a truncated BP80 molecule that permits in vivo imaging and interferes with the sorting of endogenous BP80 (daSilva et al., 2005). A chimeric protein in which the TMD and CT of BP80 were fused to the C terminus of secreted green fluorescent protein (GFP-BP80) or secreted yellow fluorescent protein (YFP-BP80) is able to use receptor transport machinery to reach the PVC (Tse et al., 2004; daSilva et al., 2005). Due to this property, it competes with endogenous receptors, particularly at the retrograde transport step from the PVC, and causes receptor leakage to the vacuole and depletion in the Golgi apparatus. This manifests itself by the strongly induced secretion of BP80-ligands, which is easy to quantify (daSilva et al., 2005), and permits the monitoring of BP80-related sorting events by in vivo imaging and via cargo transport assays. It is also possible to reconstitute vacuolar sorting in the presence of constant GFP-BP80 levels by reintroducing full-length BP80 molecules (daSilva et al., 2005). The use of these tools allowed us to demonstrate that recycling of BP80 from the PVC to the Golgi is crucial in maintaining receptor function and that this step is wortmannin-sensitive (daSilva et al., 2005).
Here, a combination of these complementary assays was further explored and expanded to identify sorting determinants of BP80. We could show that both the TMD and the CT play a role in proper sorting, including endoplasmic reticulum (ER) export, Golgi-to-PVC transport, and recycling. In addition, a Tyr-based motif in the CT was shown to be important in vivo for efficient Golgi-to-PVC transport but not essential for ultimately reaching the PVC due to the presence of an alternative pathway via the plasma membrane.
RESULTS
A Role for the CT of BP80 in Receptor Sorting
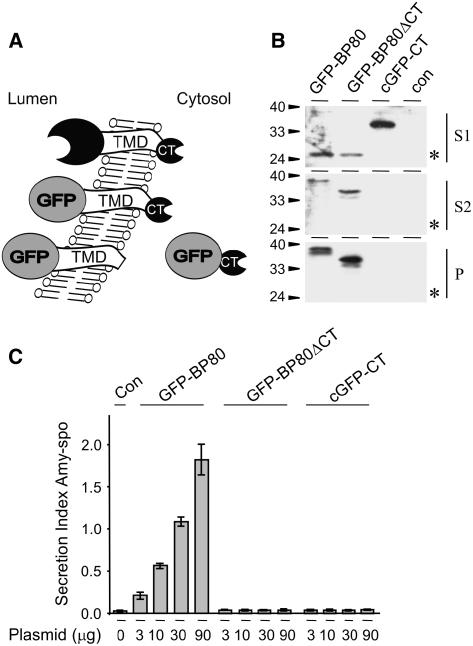
Two studies have addressed the role of the TMD and the CT of BP80, with contradictory findings (Jiang and Rogers, 1998; Brandizzi et al., 2002b). To directly test if the CT contains targeting information, we have generated a derivative of the competitor GFP-BP80, in which the entire CT was deleted (Figure 1A; GFP-BP80ΔCT). We have previously shown that the arrival of GFP-BP80 in the lytic vacuole can be monitored by the detection of a characteristic GFP degradation product, termed the GFP-core (daSilva et al., 2005). In addition, glycosylated GFP is deglycosylated in transit to the vacuole. Therefore, GFP-BP80 generally gives rise to three polypeptides that can be detected: the high molecular weight glycosylated precursor, the deglycosylated precursor, and the low molecular weight GFP-core.
Figure 1.
Competition with the Endogenous Receptor Depends on Membrane-Anchored CT.
(A) Schematic representation comparing the topology of the additional BP80-truncated fusion proteins in relation to full-length BP80 and the previously characterized competitor in which the complete lumenal ligand binding domain of Arabidopsis BP80 isoform a (Hadlington and Denecke, 2000) was replaced by GFP (GFP-BP80; daSilva et al., 2005). The entire sequence encoding the BP80 CT in GFP-BP80 was deleted, yielding the tailless protein GFP-BP80ΔCT. The cytosolic protein cGFP-CT was generated by fusion of the CT of the same BP80 isoform to the C-terminal end of the GFP sequence lacking the signal peptide for ER translocation.
(B) Transient expression experiment to compare the intracellular partitioning of the three BP80 chimeras. Tobacco mesophyll protoplasts were mock transfected (con) or transfected with 30 μg of plasmid encoding either GFP-BP80, GFP-BP80ΔCT, or cGFP-CT and incubated for 24 h, after which medium and cells were harvested. Cells were fractionated to obtain extracts enriched in soluble proteins released by osmotic shock (S1), proteins released after sonicating the first pellet (S2), and membrane spanning proteins (P). These cell fractions together with the medium were analyzed by protein gel blotting and probed with anti-GFP antibodies. Molecular weight markers are indicated in kilodaltons. Notice that the vacuolar degradation product, the GFP-core (asterisk), is present in lower amounts in the case of GFP-BP80ΔCT compared with GFP-BP80, and it is absent for cGFP-CT.
(C) Influence of GFP-BP80, GFP-BP80ΔCT, or cGFP-CT on the secretion index of amy-spo. Protoplasts were transfected with a constant amount (10 μg) of plasmid-encoded amy-spo alone (Con) or cotransfected with increasing concentrations of plasmid encoding either of the three BP80 chimeras. Plasmid concentrations are shown below each lane. The secretion index was calculated as the ratio between the extracellular and intracellular α-amylase activities (Phillipson et al., 2001). Standard errors are from four independent protoplast transfections. Notice that in sharp contrast with GFP-BP80, neither GFP-BP80ΔCT nor cGFP-CT induces secretion of amy-spo.
To compare cell partitioning, protoplasts were transfected with plasmids encoding either GFP-BP80 or GFP-BP80ΔCT. After 24 h of incubation, cells were fractionated into extracts enriched in (1) soluble proteins released after osmotic shock (S1), (2) microsomal proteins (S2) that were extracted after sonicating the pellet, and (3) membrane-spanning proteins that remained in the final pellet (P). Figure 1B shows that the truncated protein GFP-BP80ΔCT exhibits a reduced processing to the soluble vacuolar GFP-core (indicated by asterisks) detected in S1 (cf. the first two lanes). The full-length precursor of GFP-BP80ΔCT, detected in S2 and P, is more abundant compared with GFP-BP80. This suggests that anterograde transport of GFP-BP80ΔCT is compromised but not abolished, and the result is intermediate to previous reports (Jiang and Rogers, 1998; Barrieu and Chrispeels, 1999; Brandizzi et al., 2002b).
We then performed the competition assay using an α-amylase fusion protein carrying the sorting signal of sweet potato (Ipomoea batatas) sporamin (amy-spo), which is an established BP80-ligand and vacuolar cargo (Pimpl et al., 2003; daSilva et al., 2005). Tobacco (Nicotiana tabacum) leaf protoplasts were transfected with equal amounts of amy-spo encoding plasmids and an increasing concentration of either GFP-BP80 or GFP-BP80ΔCT plasmids. After 24 h, medium and cells were harvested, and the α-amylase activity was measured for each fraction as the change in optical density in function of time and volume of the cell fraction used. This allowed us to calculate the secretion index, which is the ratio between the activity in the medium and in the cells (Phillipson et al., 2001; Pimpl et al., 2003).
Confirming our previous results (daSilva et al., 2005), GFP-BP80 strongly induced the secretion of amy-spo in a dosage-dependent manner (Figure 1C). However, the fusion protein lacking the CT failed to exhibit any measurable effect regardless of the concentration. Comparable levels of the two effector molecules were produced from each plasmid (Figure 1B). This result illustrates that the CT is crucially necessary for the ability of the chimera to interfere with endogenous BP80 function and that induced secretion is not an indirect effect of a coexpressed type I membrane spanning protein.
Although we have shown previously that competition mainly involves the retrograde route of the receptor (daSilva et al., 2005), it is not clear whether this is due to titration of limiting cytosolic components or to overload of transport carriers. To distinguish between these two possibilities, we fused the CT alone to the C terminus of cytosolic GFP (Figure 1A; cGFP-CT) where it would have access to cytosolic machinery components such as adaptor molecules. The resulting protein is highly expressed in the cytosol and is not proteolytically trimmed to yield the core fragment (Figure 1B). To test if the resulting CT can titrate sorting machinery, we repeated the competition experiment using amy-spo as a marker. Figure 1C shows no detectable competing activity on BP80-mediated sorting of amy-spo, even in the highest concentration. This shows that the CT alone is not sufficient to recruit sorting machinery. Probably, the tail must be membrane anchored to mediate effective competition with the endogenous receptor.
The CT of BP80 Is Required for Efficient Anterograde Transport
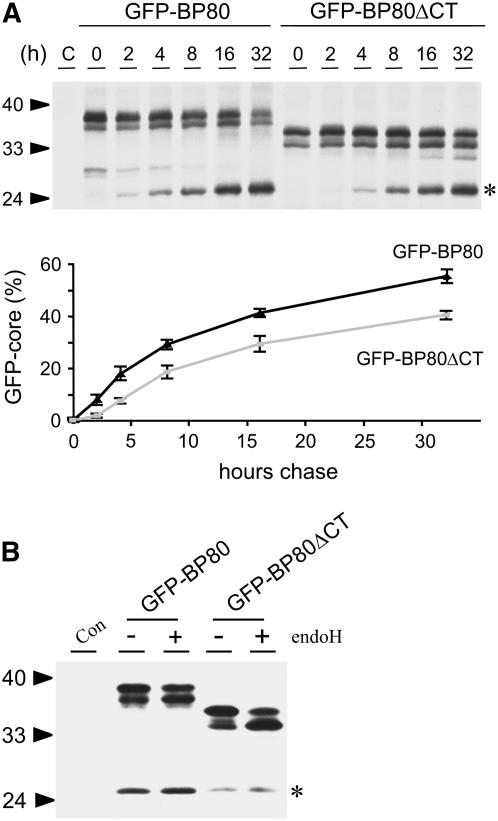
To investigate the influence of the CT on the kinetics of proteolysis, we performed pulse-chase experiments on GFP-BP80– and GFP-BP80ΔCT–expressing cells. Figure 2A shows that the latter construct chases into the typical vacuolar degradation product at a slower rate than GFP-BP80. The bottom panel represents the percentage of the core fragment in relation to the total signal from the three bands in each lane, calculated from line scanning of phosphor imaging data from two independent pulse-chase experiments. This clearly confirms the difference between the two constructs at each time point of the chase. For instance, after a 16-h chase, the GFP-core fragment accounts for ∼40% of the total GFP-BP80 protein, and similar progress of processing is only reached after 32 h for the deletion mutant GFP-BP80ΔCT. This suggests that the CT contains positive information for anterograde transport toward a lytic compartment. However, its presence is not absolutely required, confirming the notion that transport to the lytic compartment may be the default for type I membrane spanning proteins (Barrieu and Chrispeels, 1999).
Figure 2.
The C Terminus of BP80 Promotes Anterograde Transport.
(A) Pulse-chase analysis demonstrating that the BP80 CT influences the speed with which GFP-BP80 reaches the lytic vacuole. Protoplasts cotransfected with either GFP-BP80 or GFP-BP80ΔCT plasmids were pulse labeled for 1 h, after which incubations in chase buffer were performed for the periods of time indicated above each lane. Cell extracts were immunoprecipitated with anti-GFP antiserum and used for SDS-PAGE separation. The bottom panel shows the percentage of signal derived from the core fragment (asterisk) in terms of the total signal detected on each chase point for GFP-BP80 (black line) and GFP-BP80ΔCT (gray line). Line scanning of the various chase points from two independent pulse-chase experiments was conducted using AIDA software (Raytest). The signal from the GFP-core band was quantified in arbitrary units and calculated as a percentage of the total surface area from all peaks in each lane. Error bars represent standard error from two independent experiments. Notice that the percentage of GFP-core derived from GFP-BP80 is approximately twofold higher than that for GFP-BP80ΔCT at each time point in the analysis.
(B) The absence of the CT compromises acquisition of endoH-resistant glycans. Protoplasts mock transfected (Con) or transfected with 30 μg of either GFP-BP80 or GFP-BP80ΔCT plasmids were incubated for 24 h for the proteins to reach a steady state distribution. Cell extracts were incubated for 1 h in the presence (+) or absence (−) of endoH and verified by protein gel blot analysis. The top two bands represent glycosylated and deglycosylated full-length precursors, and the bottom band represents GFP-core (asterisk). All other annotations are as in Figure 1. Notice a clear shift from the glycosylated to the deglycosylated band with endoH treatment of GFP-BP80ΔCT, indicating partial ER retention of the deletion construct.
It should be noted that proteolytic processing monitors progress to the lytic vacuole because GFP-core is found in the lytic vacuole and not the PVC (daSilva et al., 2005). GFP-core formation can thus be influenced by the rate of anterograde transport as well as the efficiency of recycling from the PVC. If removal of the CT leads to poor recycling in addition to slower anterograde transport, the two effects may partially compensate for each other. To gain further insights into the effects of the CT on the distribution within the secretory pathway, we took advantage of the presence of an engineered glycosylation site (Batoko et al., 2000) on the GFP portion of the two BP80 chimeras. At the Golgi apparatus, the glycan moieties are modified by mannosidase II, which confers resistance to endoglycosidase H (endoH) digestion. Thus, resistance to endoH indicates transit of the glycoprotein through the Golgi apparatus.
To test endoH resistance, protoplasts were transfected with equal amounts of plasmid encoding either GFP-BP80 or GFP-BP80ΔCT. Following transformation, each protoplast suspension was incubated for 24 h, after which protein extracts were prepared and split into two equal aliquots. These were subjected to either mock (−) or endoH (+) digestion. Figure 2B shows that most of the GFP-BP80 molecules are resistant to endoH digestion, indicating that this protein is efficiently exported to the Golgi. In the case of GFP-BP80ΔCT, a larger proportion of the molecules are sensitive to the enzyme, as seen by a clearly reduced signal from the upper band and a corresponding increase in intensity for the deglycosylated polypeptide (Figure 2B, last lane). This suggests that the slower anterograde transport of GFP-BP80ΔCT is, at least to some extent, caused by a less efficient ER-to-Golgi transport. The BP80 CT could thus contain sorting information for ER export, but further experiments are required to identify the putative sorting signal.
The CT of BP80 Directs Increased Anterograde Transport to a Lytic Compartment
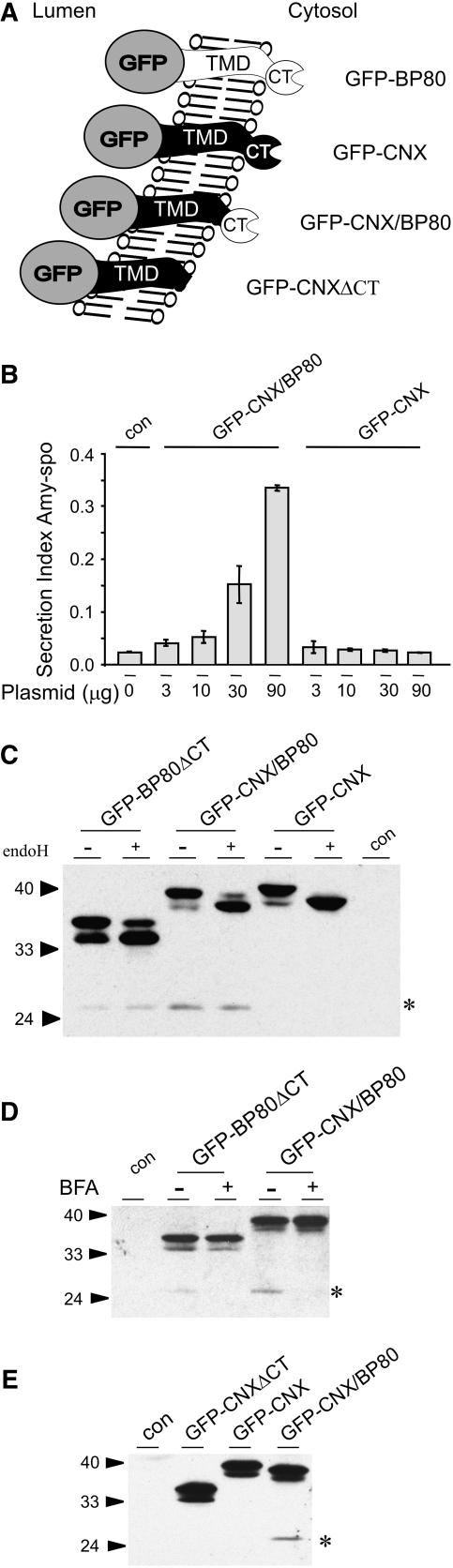
We have previously shown that a fusion protein in which the TMD and CT of calnexin were fused to GFP (GFP-CNX) did not cause any disturbance of the BP80-mediated vacuolar transport route (daSilva et al., 2005). Consistently with this observation, GFP-CNX did not produce the vacuolar GFP-core, and its subcellular distribution was unaffected by the drug wortmannin, which is in sharp contrast with GFP-BP80 (daSilva et al., 2005). To investigate how membrane anchoring contributes to the recruitment of sorting machinery that interacts with the CT of BP80, we modified GFP-CNX through replacing the CT of CNX by the corresponding region of BP80, yielding the hybrid molecule GFP-CNX/BP80 (Figure 3A). If membrane anchoring of the BP80 CT alone is sufficient to facilitate interaction with sorting machinery, GFP-CNX/BP80 should exhibit strong competition activity in our cargo secretion assay.
Figure 3.
The TMD of BP80 Is Required for Efficient Competition with the Endogenous Receptor.
(A) Schematic representation to compare the BP80 and CNX chimeras with their derivative hybrid molecule. The CT of CNX in the noncompetitor GFP-CNX (daSilva et al., 2005) was replaced by the CT of BP80, yielding the hybrid GFP-CNX/BP80. A further control was created from GFP-CNX by deleting the CT (GFP-CNXΔCT).
(B) Competition assay comparing the effects of hybrid GFP-CNX/BP80 and GFP-CNX on the secretion index of amy-spo. Protoplasts were transfected with a constant amount of amy-spo plasmid alone (con) or together with dilution series of either GFP-CNX/BP80 or GFP-CNX plasmids (concentration is shown below each lane). The amy-spo activity was obtained for both medium and cell fractions, and the secretion index was calculated. Standard errors are from four independent protoplast transfections. Notice that the induced secretion index is much lower compared with the GFP-BP80–mediated effect in Figure 1C and that the secretion index is shown at a more sensitive scale.
(C) Glycan processing analysis to compare progress through the secretory pathway of the BP80 chimeras. Cell extracts of protoplasts expressing either GFP-BP80ΔCT, GFP-CNX/BP80, or GFP-CNX for 24 h were prepared. Two equal aliquots from each cell extract were subjected to either mock (−) or endoH (+) treatment for 1 h. The resulting samples were separated by SDS-PAGE, followed by immunodetection analysis with anti-GFP antibodies. Mock-transfected protoplasts were used as control for the protein gel blot analysis (con). Further annotations are as in Figures 1 and 2. Notice the lower degree of endoH resistance and increased amount of GFP-core (asterisk) in the GFP-CNX/BP80 lane compared with the GFP-BP80ΔCT lane.
(D) Evidence for passage through the Golgi apparatus. Transfected protoplasts producing either GFP-BP80ΔCT or GFP-CNX/BP80 were split in two equal portions and incubated with (+) or without (−) 10 μM BFA. Mock-transfected protoplasts were used as controls for the protein gel blot analysis (con). Notice that GFP-core (asterisk) is only visible in the absence of the drug.
(E) Control experiment to show that the presence of BP80 CT, rather than the absence of the CNX CT, causes increased anterograde transport of the hybrid GFP-CNX/BP80. Cells were transfected with constructs encoding either GFP-CNXΔCT, GFP-CNX, or GFP-CNX/BP80. Annotations are as above. Notice that deletion of the tail from CNX does not yield GFP-core (asterisk) formation.
Figure 3B shows the effect of the hybrid molecule on the vacuolar transport of amy-spo in comparison with the noncompetitive CNX fusion GFP-CNX that served as a negative control. Increasing concentrations of either GFP-CNX– or GFP-CNX/BP80–encoding plasmids were cotransfected with a constant amount of amy-spo, and protoplasts were incubated for 24 h. Analysis of the secretion index confirmed that GFP-CNX does not affect amy-spo trafficking as shown before (daSilva et al., 2005) but that the hybrid GFP-CNX/BP80 induces the secretion of amy-spo at high effector dosage (30 and 90 μg of plasmid). The effect is much weaker compared with the original GFP-BP80 (cf. Figure 1C with 3B, note the difference in the scales) but is clearly measurable and evident when compared with the noncompetitive GFP-CNX (Figure 3B) or GFP-BP80ΔCT (Figure 1C).
To test whether the BP80 CT contains ER export information, we compared the endoH sensitivity of GFP-BP80ΔCT with the hybrid GFP-CNX/BP80 and GFP-CNX. Figure 3C shows that the truncated molecule GFP-BP80ΔCT displayed the highest level of endoH resistance. GFP-CNX/BP80 showed only a very small proportion of the endoH resistant form, and GFP-CNX was completely endoH sensitive.
One possible explanation is that the hybrid is exported from the ER faster than GFP-CNX but slower than GFP-BP80ΔCT. Interestingly, increased endoH resistance was not directly proportional to the degree of proteolytic trimming to the core fragment. Despite a higher degree of endoH resistance, GFP-BP80ΔCT showed lower levels of the core fragment. This apparent paradox could be explained if the hybrid molecule reaches the Golgi slower but proceeds to the PVC faster compared with the deletion mutant. This would result in low steady state levels of the hybrid in the Golgi apparatus.
To test that GFP-core formation is due to traffic through the Golgi apparatus in both cases, we tested the effect of the drug brefeldin A (BFA). Figure 3D shows that proteolytic processing to the core fragment is sensitive to the drug and thus rules out that the hybrid bypasses the Golgi apparatus.
One further control experiment was performed to test if the difference between GFP-CNX and GFP-CNX/BP80 was due to the presence of the BP80 CT or due to the absence of the CNX CT itself. This was important because the latter is known to contain a di-Arg motif suggested to mediate ER retention (Barrieu and Chrispeels, 1999). For this purpose, a GFP-CNXΔCT construct was generated (Figure 3A) to allow a direct comparison with GFP-CNX. Figure 3E shows that deletion of the CT alone does not confer GFP-core formation as seen for the hybrid.
Together, the results strongly suggest that the CNX TMD appears to prevent efficient ER export. Moreover, they indicate that efficient anterograde BP80 sorting is likely to be dependent on both the TMD and the CT of the vacuolar sorting receptor.
Both the TMD and the CT of BP80 Contribute to Efficient Targeting to the PVC
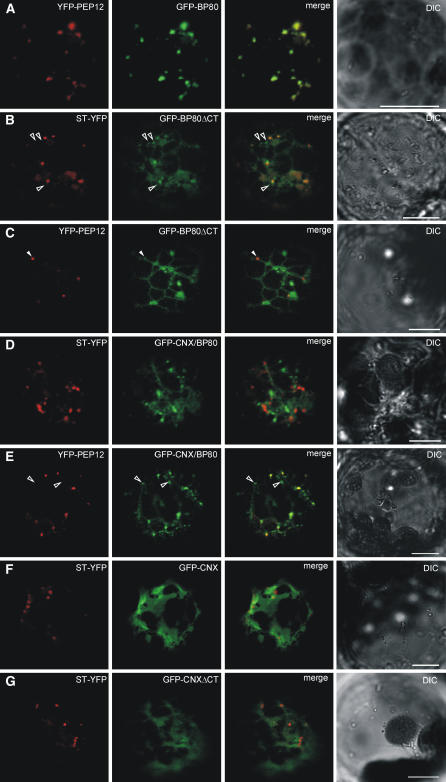
The combination of biochemical assays described above provides strong evidence, but further analysis of individual transport steps from the ER via the Golgi apparatus to the PVC could be complemented by in vivo imaging. To directly observe the intracellular localization of the GFP fusions, they were coexpressed with established YFP protein markers followed by confocal laser scanning microscopy (CLSM). The sialyl-transferase fusion ST-YFP (Brandizzi et al., 2002a) and a PEP12/SYP21 fusion, YFP-PEP12 (Ueda et al., 2004; Uemura et al., 2004), were used to label the Golgi apparatus and the PVC, respectively.
We have previously shown that GFP-BP80 labels punctate structures of variable sizes, which rarely colocalize with the Golgi marker (Figure 6 in daSilva et al., 2005). Here, predominant PVC localization of GFP-BP80 was confirmed by the high degree of colabeling with YFP-Pep12 (Figure 4A). In contrast with GFP-BP80, GFP-BP80ΔCT is partially retained in the ER and causes increased formation of membrane sheets (Figure 4B). This explains the increased endoH sensitivity. However, the deletion mutant does colocalize significantly with the Golgi marker ST-YFP, although extra-Golgi punctate structures can be detected (Figure 4B, open arrowheads). Consistently, GFP-BP80ΔCT also exhibits a low degree of colocalization with the PVC marker (Figure 4C). The partial accumulation in the Golgi apparatus corresponds with the finding that the deletion mutant still acquires some endoH resistance (Figure 3C). However, in comparison with GFP-BP80, the deletion mutant is significantly retained in the ER, which suggests a role of the CT in ER export.
Figure 6.
The YMPL Motif Is Crucial for the GFP-BP80 Effect.
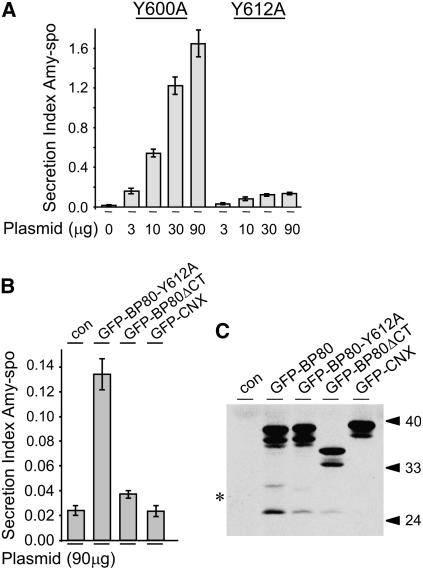
(A) The concentration-dependent influence of GFP-BP80-Y600A and GFP-BP80-Y612A coexpression on the secretion index of amy-spo. Tobacco mesophyll protoplasts were transfected with a constant amount of amy-spo plasmid alone or together with a dilution series of either GFP-BP80-Y600A or GFP-BP80-Y612A encoding plasmids. Concentrations of plasmid encoding the effector proteins are given below each lane. Standard errors are from five independent protoplast transfections. Note that competition is much reduced for GFP-BP80-Y612A, while the control mutation GFP-BP80-Y600A behaves similarly to wild-type GFP-BP80 (cf. with Figure 1A).
(B) Similar to (A) but using maximal concentrations (90 μg) of plasmid encoding either GFP-BP80-Y612A, GFP-BP80ΔCT, or GFP-CNX to allow direct comparison. The amy-spo secretion index is shown with a 10-fold more sensitive scale compared with (A) to appreciate residual competition activity of the Y612A mutant. Standard errors are from five independent protoplast transfections.
(C) Total cell extracts from protoplasts expressing either GFP-BP80, GFP-BP80-Y612A, GFP-BP80ΔCT, or GFP-CNX for 24 h were prepared and analyzed via protein gel blots to investigate the comparative ratio of GFP-core fragment for each molecule. Note that GFP-BP80-Y612A gives rise to an intermediate level of GFP-core fragment (asterisk) compared with GFP-BP80 and GFP-BP80ΔCT.
Figure 4.
Subcellular Localization of the Receptor Chimeras via CLSM.
Tobacco leaf protoplasts were cotransfected with 10 μg of plasmids encoding either of the GFP or YFP fusion proteins used in the different experiments below. Cells were incubated for 24 h before analysis. Shown are cross sections through the cell cortex to allow visualization of the cytosol with the intermediate organelles of the secretory pathway. The GFP and YFP fusions are pseudocolored in green and red, respectively. Colocalization is pseudocolored in yellow. Images in bright field (differential interference contrast [DIC]) are shown on the right side. Bars = 10 μm.
(A) Protoplast cotransfected with GFP-BP80 and the PVC marker YFP-PEP12–encoding plasmids. Notice that GFP-BP80 almost totally colocalizes with YFP-PEP12, illustrating its predominant localization at the PVC.
(B) Protoplasts were transfected with GFP-BP80ΔCT plasmid together with the Golgi marker ST-YFP–encoding plasmid. GFP-BP80ΔCT is noticeably retained in the ER and also accumulates in the Golgi apparatus. The deletion mutant also reaches punctate extra Golgi structures (open arrowheads).
(C) Protoplasts were transfected with GFP-BP80ΔCT plasmid together with YFP-PEP12–encoding plasmid. GFP-BP80ΔCT is noticeably retained in the ER and reaches the PVC inefficiently, illustrated by its partial colocalization with YFP-PEP12 (closed arrowhead).
(D) and (E) Protoplasts coexpressing the hybrid molecule GFP-CNX/BP80 together with either ST-YFP (D) or YFP-PEP12 (E). Notice that GFP-CNX/BP80 is partially retained in the ER and clearly shows a higher degree of colocalization with the PVC marker than with the Golgi marker. Notice that most punctate signals from GFP-CNX/BP80 are separate from the ST-YFP structures, whereas only few punctuate GFP-CNX/BP80 structures are separate from the PVC marker YFP-pep12 ([E], open arrowheads).
(F) and (G) Similar to previous panels, but protoplasts are coexpressing either GFP-CNX (F) or GFP-CNXΔCT (G) with the Golgi marker ST-YFP. No punctuate structures were observed, and the two GFP constructs are found in ER tubules and sheets.
The hybrid molecule GFP-CNX/BP80 was also partially retained in the ER, but in contrast with the deletion mutant GFP-BP80ΔCT, little colocalization with the Golgi apparatus was observed (Figure 4D). Instead, the hybrid molecule highlights punctate structures that colocalize with the PVC marker (Figure 4E). This was much more readily observed for the hybrid than for the deletion mutant. The control construct GFP-CNX highlights a reticular and sheet-like pattern typical of ER labeling for this construct (Runions et al., 2006) and did not show any punctate colocalization with the Golgi marker ST-YFP (Figure 4F). Likewise, the deletion mutant GFP-CNXΔCT also displayed an ER labeling (Figure 4G), confirming that the TMD contributes significantly to the retention of CNX.
The frequency of detectable ER labeling versus punctate labeling is shown in Supplemental Table 1 online for the various constructs used in Figure 4. The results strongly suggest that the CNX TMD appears to prevent efficient ER export, while the presence of the BP80 CT stimulates export as well as arrival in the PVC. Statistical analysis of the percentage of colocalization showed that extra ER punctate structures labeled with GFP-CNX/BP80 were mostly colocalized with the PVC marker, similar to GFP-BP80 (see Supplemental Figure 1 online). Although it was hard to detect the hybrid GFP-CNX/BP80 in the Golgi apparatus, Golgi-dependent traffic was shown using the drug BFA (Figure 3D). Together, the results suggest that the BP80 CT confers the ability to compete with endogenous BP80 (Figure 3B) and progress to a lytic compartment yielding GFP-core (Figure 3C) and extra Golgi punctuate structures that represent PVCs (Figures 4D and 4E).
Multiple Sequence Motifs of the BP80 CT Contribute to Receptor Sorting
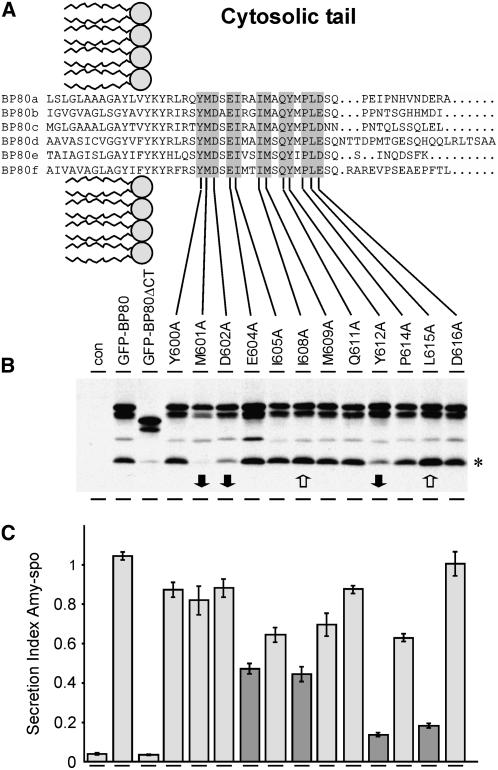
The cytosolic domain of Arabidopsis BP80a used in this study contains 37 amino acid residues, which is small compared with the long tails of the mannose-6-phosphate receptors in mammals (Ghosh et al., 2003) or VPS10p in yeast (Marcusson et al., 1994). This relatively small size and the high degree of sequence similarity among BP80 isoforms makes it an attractive system to investigate the role of putative cytosolic motifs on its sorting. The BP80 C terminus may contain several sorting signals for the various transport steps, including ER-to-Golgi transport, Golgi-to-PVC transport, and recycling from the PVC. It was expected that interference with any of these transport steps would diminish the ability to compete with endogenous BP80 for sorting machinery. This would manifest itself in a reduced induction of amy-spo secretion, thus forming a simple and reproducible test system to score mutants.
We thus mutagenized all the residues within the tail that are conserved between the known BP80-like molecules in plants (Figure 5A). Recombinant plasmids were first tested for their ability to produce GFP-BP80 fusion proteins and to monitor processing to the GFP-core fragment (Figure 5B). In a second step, the mutants were screened by scoring the ability to induce amy-spo secretion (Figure 5C), in direct comparison with the highly competitive GFP-BP80 fusion or the noncompetitive deletion mutant (GFP-BP80ΔCT). Figure 5B shows several examples of mutants in which the processing to the characteristic vacuolar GFP-core fragment was either reduced (M601A, D602A, and Y612A) or increased (I608A and L615A). Reduced core formation could be due to impaired anterograde transport or increased recycling. Increased core formation would suggest the opposite: accelerated anterograde transport or defective recycling from the PVC.
Figure 5.
Identification of Amino Acid Residues on the BP80 CT That Interfere with the Ability to Compete with the Endogenous Receptor.
(A) Sequence alignment of the predicted CT on various BP80-like molecules identified in Arabidopsis. Conserved amino acid residues in the tail, highlighted in gray, were individually replaced by an Ala residue via site-directed mutagenesis, as indicated below.
(B) Protoplasts were transfected with a constant concentration of amy-spo plasmid alone (con) or together with 30 μg of plasmid encoding either GFP-BP80, the deletion mutant GFP-BP80ΔCT, lacking the entire CT, or one of the various GFP-BP80 mutants (indicated by the usual mention of the position in the protein sequence; BP80 isoform a). Protoplast suspensions were incubated for 24 h, after which cell and medium were harvested. A portion of the protoplasts was extracted for total cellular contents and analyzed by protein gel blotting to compare the ratio between full-length precursors and the lower molecular weight GFP-core fragment (asterisk). After scanning several protein gel blots, reproducible decreases in core/precursor ratio (closed arrows) or increases in the core/precursor ratio (open arrows) were scored. The wild-type control GFP-BP80 exhibited an average of 20% ± 6% GFP-core of the sum of all signals in the lane and was used as a reference. Significantly increased processing was observed for I608A (35% ± 8% core) and L615A (37% ± 7% core), while decreased processing was seen for M601A (1% ± 0.6% core) and D602A (6% ± 3% core).
(C) Screening of mutants via the receptor competition assay. Protoplast suspensions in (B) were harvested to obtain cells and medium separately. The amy-spo activity was measured for both medium and cell fractions, and the secretion index was calculated. Standard errors are from three independent protoplast transfections. Secretion index values that were significantly below levels obtained with the positive control GFP-BP80 are indicated in dark gray.
Figure 5C reveals that some of the mutants exhibited significantly reduced competing activity. These mutants can be classified in modest competitors (E604A and I608A) and weak competitors (Y612A and L615A). However, it should be noted that none of the point mutations reduced the competing activity to the background levels of the deletion mutant GFP-BP80ΔCT (third lane), which is comparable to the complete absence of competitor (first lane). This either suggests that multiple sorting signals coexist in the CT or that single point mutations alone are not sufficient to completely abolish a sorting signal.
The YXXφ Motif of the BP80 Tail Is Required for in Vivo Competition with Sorting Machinery
Particularly noteworthy were the point mutations Y612A and L615A that displayed strongly reduced activity in the receptor competition assay (Figure 5C). Interestingly, the two mutations did not show the same effect on the formation of the soluble GFP-core. While the Tyr-to-Ala substitution reduced core formation and suggests reduced leakage to the vacuoles, the Leu-to-Ala mutation increased the leakage to the vacuole, as indicated by an increase in the core signal (Figure 5B).
The Tyr and Leu residues mentioned above are thought to be part of the typical YXXφ signature of a sorting motif known to interact with the μ-subunit of AP complexes in mammalian cells (Ohno et al., 1998; Owen and Evans, 1998). The data in our plant system strongly suggest that this motif has a biological function in vivo. However, the results also show that the residues at the beginning and the end of the YXXφ motif may not have the same functions, despite being part of the same perceived consensus sequence. This finding may reveal an unknown complexity of the YXXφ motif, but for the remainder of this work, we have concentrated on the effects of the Tyr-to-Ala substitution on this motif (mutant Y612A).
Mutation of another Tyr (Y600A), in a region closer to the TMD, had no measurable effect on receptor competition (Figure 5C), illustrating the specificity of the assay. These two Tyr mutants were directly compared in a dose–response experiment. Figure 6A shows that the Y600A mutant essentially behaved like nonmutated GFP-BP80 (cf. with Figure 1), whereas the second mutant (Y612A) only showed weak competition, as indicated by a much reduced induction of amy-spo secretion.
To test the weak competition activity of the Y612A mutant further, we compared it with the complete deletion of the CT (GFP-BP80ΔCT) or the CNX fusion (GFP-CNX) in a dose–response experiment. This analysis confirmed that the Y612A mutant still exhibited a weak but reproducible competition (Figure 6B, note the different scale compared with 6A). Similar levels of the three fusion proteins were produced for the plasmid concentration used (Figure 6C, last three lanes). Interestingly, the levels of GFP-core fragment yielded by the mutant (GFP-BP80-Y612A) are intermediate between the levels resulting from GFP-BP80 and GFP-BP80ΔCT. The results indicate that this Tyr-based motif plays a crucial role in the targeting of BP80 in vivo.
GFP-BP80-Y612A Is Partially Missorted to the Plasma Membrane
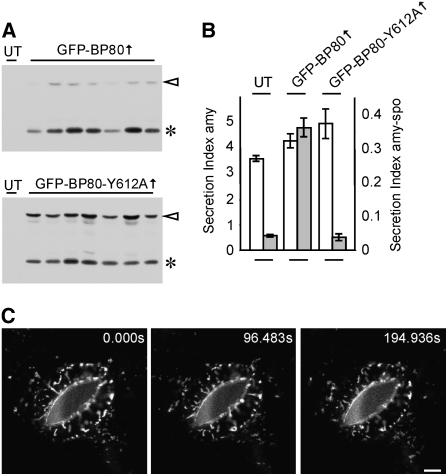
To confirm our results with transient expression, we compared the effects of GFP-BP80 with that of the mutant (GFP-BP80-Y612A) in stable transformed tobacco plants. It was noted that transformation with GFP-BP80 yielded many kanamycin-resistant lines that failed to express any detectable amount of recombinant protein. Those lines that expressed the fusion protein did so at low levels and contained mainly the GFP-core fragment resulting from the leakage to the vacuoles. By contrast, transformation with GFP-BP80-Y612A was more frequent and yielded higher expression levels. Figure 7A shows a comparison of selected transgenic lines expressing either GFP-BP80 or the Y612A derivative at similar levels. It is clearly noticeable that the mutant shows a much increased level of the full-length precursor compared with the GFP-core fragment. This confirms the transient expression data on GFP-BP80-Y612A (Figure 6C), suggesting that anterograde transport followed by proteolytic cleavage is reduced but not abolished.
Figure 7.
Effects of GFP-BP80 and GFP-BP80Y612A Production in Stably Transformed Tobacco Plants.
(A) Screening of tobacco plants transformed with either GFP-BP80 or GFP-BP80Y612A construct, resulting in overexpression of the GFP fusion proteins. Extracts were prepared from leaves of untransformed plants (UT) as controls and from independent transgenic plants overexpressing either GFP-BP80 or GFP-BP80-Y612A. Bands of the full-length GFP fusion proteins (open arrowheads) and the corresponding GFP-core degradation product (asterisks) are indicated. Note the increase in the full-length molecule at the expense of the GFP-core in the case of plants expressing the mutant molecule.
(B) Transient expression to test the capacity of vacuolar sorting in plants stably producing GFP-BP80 or GFP-BP80Y612A. Leaf protoplast from untransformed (UT) and stably transformed lines obtained from each of the two constructs and expressing similar levels of recombinant proteins were transformed with a constant concentration of plasmids encoding either the secretory marker α-amylase (amy) or the BP80-ligand amy-spo. The secretion index is given in different scales for amy (left y axis) and amy-spo (right y axis) to simplify comparison. Standard errors are from three independent protoplast transfections. Note that secretion of the vacuolar cargo amy-spo (gray bars) is induced in protoplasts from GFP-BP80 plants, while amy (white bars) is secreted at comparable levels by the three protoplast populations.
(C) Still images from Supplemental Movie 1 online, showing the first (0.000 s), middle (96.483 s), and last (194.936 s) frame. Shown is a guard cell and parts of adjacent epidermis cells analyzed by CLSM in single channel, allowing rapid frame capture. Notice the variable shapes and mobility of the highlighted structures, ranging from punctate to tubular.
The poor performance of GFP-BP80 overproduction could be due to toxicity when vacuolar sorting is compromised. Indeed, vacuolar sorting is considered essential for plant development (Rojo et al., 2001; Surpin and Raikhel, 2004). However, we tested the few lines exhibiting detectable GFP-BP80 expression and compared them with GFP-BP80-Y612A–producing lines in a transient expression assay with the BP80 cargo amy-spo. Interestingly, GFP-BP80–producing lines exhibited induced amy-spo secretion compared with untransformed tobacco or GFP-BP80-Y612A–producing tobacco (Figure 7B, gray bars). By contrast, the secretory marker amy showed comparable secretion in all three cell lines (Figure 7B, white bars), showing that there was no general increase in anterograde transport in the GFP-BP80–producing plants.
The biochemical phenotype of the transgenic plants confirms the competition data with transient expression, showing that GFP-BP80 has an inhibitory effect on BP80-mediated vacuolar sorting and that such a property is greatly compromised by the disruption of the YMPL motif. It should be noted that the observed induction of secretion in the stable transformed lines is ∼10-fold lower compared with the strongest induction seen with transient cotransfection (i.e., Figure 1C, Y612A). Most likely, high GFP-BP80–expressing transgenic lines exhibiting more pronounced missorting of BP80-ligands were not viable and failed to regenerate. This is not an issue with the transient expression assay, allowing a more sensitive and also more rapid analysis (i.e., Figure 6A).
In leaf epidermis cells, GFP-BP80 labeled highly mobile and morphologically diverse structures. These are punctate, tubular, and sometimes tubular-branched and generally are smaller and faster moving than the typical Golgi bodies (Figure 7C; see Supplemental Movie 1 online). This corresponds well with the size and variable morphology reported for multivesicular bodies of tobacco bright yellow 2 (BY2) cells (Tse et al., 2004). Since stable transformed GFP-BP80–producing plants only exhibited low expression levels, this material may be ideal for further studies on PVC movement.
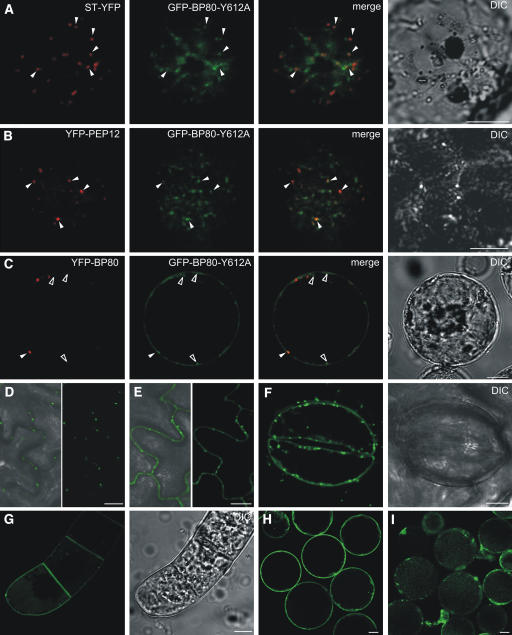
Next, we compared the intracellular localization of GFP-BP80-Y612A by fluorescence microscopy in transiently expressing protoplasts or in the leaf epidermis of stable transformed lines. GFP-BP80-Y612A was partially retained in the Golgi apparatus, as shown by partial colocalization with the Golgi marker ST-YFP (Figure 8A). However, the mutant also showed partial colocalization with the coexpressed PVC marker YFP-pep12 (Figure 8B). These results suggest that anterograde transport to the PVC is compromised but not abolished in the Y612A mutant.
Figure 8.
GFP-BP80Y612A Is Partially Missorted to the Plasma Membrane.
(A) and (B) CLSM showing that mutation of the YMPL motif causes changes in subcellular distribution of GFP-BP80. Similar conditions and annotations as in Figure 4, but protoplasts were transfected with plasmid encoding GFP-BP80-Y612A, together with either the Golgi marker ST-YFP (A) or the PVC marker YFP-PEP12 (B).
(C) Protoplast cotransfected with GFP-BP80-Y612A and YFP-BP80 plasmids. Shown is a cross section through the center of the protoplast to appreciate labeling of the plasma membrane. A colocalizing signal is indicated by a closed arrowhead, and nonoverlapping signals are indicated by open arrowheads. Notice that only GFP-BP80-Y612A highlights an uninterrupted line surrounding the entire protoplast border and also highlights punctuate structures that are not seen with YFP-BP80.
(D) to (F) Leaf epidermal cells of transgenic lines expressing GFP-BP80 (D) and GFP-BP80-Y612A (E). Notice that in addition to punctate structures, epidermal cells of GFP-BP80-Y612A plants show a diffuse staining of the apoplast. This is also seen in guard cells from plants expressing GFP-BP80-Y612A (F).
(G) Transgenic BY2 line stably transformed with the GFP-BP80-Y612A construct. Notice again the apoplastic staining.
(H) and (I) Cross section through the center of several leaf protoplasts prepared from stable transformed lines expressing GFP-BP80-Y612A. Prior to imaging, protoplasts were incubated for 48 h in TEX medium to allow accumulation of full-length GFP-BP80-Y612A at the plasma membrane. The suspension was then split in two portions and incubated for a further 2 h either with (I) or without (H) wortmannin (10 μM). Notice the drug-induced redistribution of plasma membrane fluorescence to diffuse staining of the vacuolar lumen.
To directly compare the effect of the Tyr mutation, we replaced the GFP portion of GFP-BP80 by YFP (YFP-BP80). The two latter molecules show perfect colocalization when coexpressed in protoplasts (data not shown). However, GFP-BP80-Y612A only partially colocalizes with YFP-BP80 (Figure 8C), confirming the different transport behavior of the two proteins. Interestingly, a weak labeling of the plasma membrane was also observed for GFP-BP80-Y612A but not for YFP-BP80. Out of 58 protoplasts expressing GFP-BP80 alone, only two showed weak plasma membrane labeling (see Supplemental Table 1 online). By contrast, 24 out of 32 GFP-BP80-Y612A–expressing protoplasts showed plasma membrane labeling (see Supplemental Table 1 online). Plasma membrane localization is only visible when focusing on the central part of the protoplast (Figure 8C). When focusing on the cortex, as in Figure 8A, the fluorescence is spread out over the entire surface and is below the detection limit.
It was also observed that GFP-BP80-Y612A accumulated in additional punctate structures compared with YFP-BP80 (Figure 8C, open arrowheads). This suggests that compromised Golgi-to-PVC transport could lead to missorting to the cell surface and accumulation in different compartments, such as the Golgi apparatus, through which the molecule transits.
Statistical analysis of the percentage of colocalization between either GFP-BP80, GFP-BP80ΔCT, GFP-CNX/BP80, or GFP-BP80-Y612A in combination with either the Golgi marker ST-YFP or the PVC marker YFP-pep12 confirmed a role of the Tyr motif in anterograde transport. GFP-BP80ΔCT and GFP-BP80-Y612A both showed much stronger colocalization with the Golgi marker and a concomitant lower colocalization with the PVC marker (see Supplemental Figure 1 online). By contrast, the constructs carrying the wild-type BP80 CT (GFP-BP80 and GFP-CNX/BP80) exhibited mostly PVC localization and little Golgi retention. The two constructs showed very similar distributions (see Supplemental Figure 1 online) with the exception of the earlier documented ER labeling for the hybrid (Figure 4; see Supplemental Table 1 online). These findings are consistent with the model that both deletion of the tail and point mutation of Y612 lead to reduced Golgi export and, thus, increased Golgi retention.
Analysis of the leaf epidermis of stably transformed tobacco lines also revealed a clear difference in the localization of GFP-BP80 and the mutant protein. Figure 8D shows an overview of the fluorescence in the epidermis. In contrast with GFP-BP80, GFP-BP80-Y612A–producing plants showed diffuse apoplastic GFP fluorescence and intracellular punctate structures, confirming a significant amount of missorting to the apoplast, followed by cleavage of GFP (Figures 8E and 8F). Stable transformed tobacco BY2 suspension cultures showed similar apoplastic fluorescence with the mutant (Figure 8G). This was not observed for the GFP fusion carrying the wild-type BP80 tail (data not shown).
To avoid proteolysis of GFP at the plasma membrane, we took advantage of our experience with protoplast suspensions from transgenic plants. Due to the dilution, the medium of washed protoplasts is less lytic compared with the concentrated apoplast fluid of leaf epidermis cells. When protoplasts were prepared from the leaves of transgenic plants, washed repeatedly, and then incubated in fresh medium, plasma membrane fluorescence increased gradually over time and reached maximum levels after 48 h in the case of cells expressing GFP-BP80-Y612A (Figure 8H). Moreover, no soluble cleaved GFP-core fragment was seen in the culture medium (data not shown). This confirms that GFP-BP80-Y612A reaches the plasma membrane as an intact membrane protein but is cleaved in the apoplast of intact tissues. The diluted environment of the protoplast incubation medium does not appear to be sufficiently lytic to mediate cleavage and thus reveals traffic to the plasma membrane.
Wortmannin-Induced Rapid Redistribution of Plasma Membrane–Localized GFP-BP80-Y612A to the Vacuole Suggests an Endocytic Route for the Receptor
Missorting to the plasma membrane could be explained by a variety of scenarios. One could assume that BP80 normally traffics via the plasma membrane to reach the PVC by endocytosis and that this is disrupted by the Y612A mutation. However, it was shown that the YXXL motif of BP80 interacts in vitro with the μ-subunit of mammalian AP1, normally associated with budding of clathrin-coated vesicles from the Golgi apparatus (Sanderfoot et al., 1998), but did not interact with the equivalent subunit of AP2 (associated with clathrin-coated vesicles budding from the plasma membrane). More recently, the YXXL motif was shown to interact with a μ-subunit of Arabidopsis that localized to the Golgi apparatus (Happel et al., 2004). The current state of the field therefore favors a model in which normal BP80 traffic occurs from the Golgi apparatus directly to the PVC and depends on information within the YMPL motif.
Here, we have observed that the Y612A mutation results in mistargeting to the plasma membrane and inefficient progress to the PVC. There are at least three explanations for this observation. One possibility is the existence of a clathrin-independent Golgi-to-PVC route in plants that is slower but can be used as an alternative. This could be the previously proposed default pathway for membrane proteins (Barrieu and Chrispeels, 1999). It is also possible that the clathrin-mediated route may carry bulk flow in addition to selectively recruited cargo. A third explanation could be provided by recent findings with the yeast Saccharomyces cerevisiae. In the absence of the direct clathrin-mediated Golgi-to-PVC route, the yeast vacuolar sorting receptor VPS10 was shown to reach the PVC by a detour via the plasma membrane (Deloche and Schekman, 2002), a route not normally followed by this receptor.
To test these possibilities experimentally, we took advantage of our previous observation that the drug wortmannin is a strong inhibitor of the route taken by BP80 to recycle from the PVC to the Golgi apparatus (daSilva et al., 2005). If the first two models are correct, arrival of GFP-BP80-Y612A at the plasma membrane would result from saturation of the nonselective Golgi-to-PVC route. In that case, plasma membrane fluorescence would not be influenced by wortmannin because wortmannin does not affect the constitutive ER-to-Golgi plasma membrane route (Pimpl et al., 2003). If the third model is correct, endocytosed GFP-BP80-Y612A would not recycle from the PVC to the Golgi apparatus in the presence of the drug. This would lead to accumulation of the fusion protein in the vacuole at the expense of the amount in the plasma membrane.
Therefore, leaf protoplasts from the GFP-BP80-Y612A–expressing transgenic plants were prepared and incubated in protoplast culture medium for 48 h to reach easily detectable steady state levels of the fusion protein at the plasma membrane. The sample was then split into two equal aliquots, and one of which was supplemented with wortmannin to a 10 μM final concentration. Both samples were incubated for up to 4 h, and GFP fluorescence was compared through CLSM. Drug treatment caused a rapid reduction in the fluorescence at the plasma membrane, which was accompanied by an increase in the labeling of the central vacuole lumen (Figure 8I). This redistribution is rapid and involves the majority of the plasma membrane fluorescence that required 48 h to build up. It is therefore unlikely that increased vacuolar fluorescence is due to de novo synthesis.
The results suggest that BP80 carries signals for endocytosis that may be used when BP80 reaches the plasma membrane. These must still be present in the Y612A mutant and may be found by analyzing individual point mutations found in our screen (Figure 5). It may be necessary to combine individual mutations to identify the corresponding signal.
The YXXφ Motif of the BP80 Tail Is Required for in Vivo BP80 Function
To substantiate the data obtained with the GFP fusion proteins, we tested the influence of the Tyr motif on the natural Arabidopsis receptor BP80a. We thus created the Y612A mutant within the context of the entire BP80 coding region and tested the ability to reconstitute vacuolar sorting of the BP80-ligand amy-spo in the presence of GFP-BP80 as competitor. It was shown previously that excess of wild-type receptor can rescue vacuolar sorting under these conditions, providing a positive assay for receptor function (daSilva et al., 2005). Figure 9A confirms that wild-type BP80 coexpression can overcome competition by GFP-BP80 (gray bars). By contrast, BP80-Y612A aggravated induced secretion of amy-spo (Figure 9A, white bars).
Figure 9.
Tyr-Based Motif of Full-Length BP80 Is Required to Alleviate GFP-BP80 Effect.
(A) Transient expression experiment to reconstitute vacuolar sorting with full-length BP80. Protoplasts were electroporated with a constant concentration of amy-spo plasmid, either alone (black bar, first lane), with GFP-BP80 (black bar, second lane), or with GFP-BP80 supplemented with a dilution series of either wild-type BP80 (gray bars) or mutant BP80 (Y612A) (white bars). Concentrations of GFP-BP80 and the two BP80 constructs are indicated below each lane. Standard errors are from four independent protoplast transfections. Notice that wild-type BP80 reconstitutes vacuolar sorting as seen by a reduced secretion index, while the Tyr mutant aggravates the effect of competitor GFP-BP80 and increases amy-spo secretion even further.
(B) Overexpression of Arabidopsis full-length BP80 and its Y612A mutant in stable transformed tobacco. Total leaf extracts from one untransformed plant (UT), two BP80 (BP80↑), and two BP80Y612A mutant (BP80Y612A↑) overproducing transgenic lines were compared directly (left panel). Proteins were separated by SDS-PAGE followed by gel blotting analysis with polyclonal anti-BP80 serum anti-VSRAt-1 (Tse et al., 2004). The right panel shows gel blotting analysis of total cell extracts from protoplasts prepared from the same plant material shown in the left panel. Notice that the BP80-Y612A overproducers yield several lower molecular weight degradation products, which are not detectable in protoplasts from the same material.
(C) Leaf protoplasts prepared from untransformed tobacco (UT), BP80↑, and BP80-Y612A↑ plants were transfected with a constant amount of plasmid encoding the cargo vacuolar cargo amy-spo on its own (−) or together (+) with a constant concentration of GFP-BP80 plasmid. Standard errors are from three independent protoplast transfections. Notice that protoplasts from BP80-Y612A↑ plants exhibit a comparatively higher secretion index for amy-spo, which is further increased by coexpression of GFP-BP80.
Next, we generated transgenic tobacco stably transformed with genes encoding either wild-type BP80 or BP80-Y612A. It proved easier to generate transgenic lines producing the wild-type BP80 compared with the mutant. Far more BP80-overexpressing transgenic lines were obtained compared with the mutant, and the expression levels were generally higher. We concluded that the mutant full-length receptor exhibited a detrimental effect. This was the opposite behavior compared with the transformation with fusion proteins GFP-BP80 and GFP-BP80-Y612A. For further experiments, we selected transgenic plants producing comparable levels of BP80 and BP80-Y612A.
Figure 9B shows leaf extracts from transgenic lines expressing the two recombinant proteins at comparable levels. Interestingly, the extracts from the plants expressing the mutant BP80 exhibited characteristic lower molecular weight degradation products (Figure 9B, left panel, compare BP80↑ with BP80-Y612A↑). These were water-soluble and could only be detected in extracts from entire leaf tissues but not isolated protoplasts (Figure 9B, right panel). This suggests that proteolytic cleavage did not occur intracellularly and originated from apoplastic proteases. The results confirm that in the absence of a functional YXXφ motif, a portion of the mutant receptor reaches the plasma membrane, where a lower pH and secreted proteases could cause cleavage of the receptor domain in plant tissues. This corresponds well to the diffuse apoplast staining observed for GFP-BP80-Y612A.
To test BP80 targeting to the apoplast in a functional way, we analyzed the transport properties of the BP80-ligand amy-spo in protoplasts prepared from either untransformed plants, wild-type BP80, or BP80-Y612A–producing plants. The different protoplast suspensions were transfected with plasmid encoding the cargo amy-spo in the presence or the absence of the competitor GFP-BP80. Figure 9C shows that BP80-overproducing plants exhibited increased resistance to the competitor GFP-BP80, as seen by a reduced induction of amy-spo secretion (cf. lane 2 with 4, white bars). However, mutant BP80 expression leads to a strongly induced amy-spo secretion under control conditions (cf. lane 1 with 5, gray bars) and aggravated the effect of the competitor GFP-BP80, as seen in Figure 9A in the transient assay.
The biochemical phenotype of the transgenic plants indicates that mutant BP80 binds to amy-spo within the secretory pathway and releases a portion of them after reaching the plasma membrane, probably due to the lower pH of the medium. Transport of the control cargo amy was not affected by either of the transgene products in experiments of Figures 9A and 9C (data not shown), confirming that BP80 affects only its ligands and does not change the fate of constitutively secreted proteins.
The confirmation of the findings with the native receptor strongly support all data obtained with the GFP fusions and illustrate that receptor sorting information is mainly restricted to the TMD and the CT of the receptor. Besides the typical YXXφ signature that is thought to be responsible for clathrin-mediated anterograde Golgi-to-PVC transport (Happel et al., 2004), multiple sequence motifs are likely to contribute to receptor sorting. Among numerous arguments, this was most convincingly supported by the fact that residual competition was observed with either Y612A or L615A mutants that were well above control levels or the effect of the complete deletion of the tail (Figure 5C). Further signals may control steps as diverse as ER export, endocytosis, and receptor recycling from the PVC and leave plenty of work for the future.
DISCUSSION
Receptor Competition: A Powerful Tool to Study Putative Sorting Signals in Vivo
In previous work, we established that a truncated BP80 molecule lacking its ligand binding domain could act as a potent inhibitor of vacuolar sorting by interfering with endogenous BP80 sorting (daSilva et al., 2005). The effect was semidominant because it could be fully reconstituted by wild-type BP80 overexpression. The truncated molecule contained the TMD and CT of BP80, but the lumenal domain was replaced by GFP. It was concluded that sorting information for the receptor must be localized within those two domains. Here, we have systematically explored this quantitative competition assay to study the effects of modifications in those two domains.
It could be established that competition is fully prevented when the entire BP80 CT is removed (GFP-BP80ΔCT). Figure 1 shows that in contrast with GFP-BP80, the deletion mutant sustains no measurable competence to increase the secretion of the vacuolar reporter amy-spo. These data are consistent with the notion that the CT carries information necessary for the recruitment and incorporation of receptors into transport carriers. Such a feature is similar for the mammalian mannose-6-phosphate receptors, which have been shown to contain multiple motifs on their cytosolic domain that interact with components of sorting machinery in the cytosol (Honing et al., 1997; Diaz and Pfeffer, 1998; Wan et al., 1998; Puertollano et al., 2001; Ghosh et al., 2003; Ghosh and Kornfeld, 2004).
We could also show that cytosolic display of the CT alone is not sufficient for titration of sorting machinery and does not compromise BP80-mediated vacuolar sorting (Figure 1). Moreover, display on the cytosolic face of a membrane surface alone is no guarantee for efficient competition either. We have created a fusion protein consisting of GFP, followed by the TMD of CNX and the CT of BP80. The resulting hybrid GFP-CNX/BP80 acts only as a weak competitor and induces the secretion of the vacuolar reporter amy-spo ∼10-fold lower compared with the original GFP-BP80 fusion (Figure 3). The weak effect is due to an efficient ER retention of the hybrid molecule mediated by the CNX TMD.
GFP fusions carrying the CNX TMD with or without its CT are retained in the ER and do not reach a lytic compartment (Figure 3E). However, the hybrid GFP-CNX/BP80 reaches a lytic compartment. This occurs via traffic through the Golgi apparatus because GFP-core formation was shown to be sensitive to BFA (Figure 3D). The portion of the hybrid that escapes the ER proceeds further and competes with endogenous BP80, leading to weak but measurable competition (Figure 3B). Together, the results strongly suggest that display of the BP80 CT at the Golgi apparatus is necessary to compete with endogenous BP80 for sorting machinery. This is consistent with the current concept that recruitment of specific sorting machinery components is dependent on the recognition of surface determinants characteristic for individual membrane subdomains (Munro, 2004).
The results also provide insight into the retention of CNX. While it was shown that the CT of CNX contains a di-Arg motif that contributes to retention (Barrieu and Chrispeels, 1999), our data indicate that the TMD of CNX also plays a role in reducing ER export. The two findings are not mutually exclusive, and it is possible that CNX uses both true retention and signal-mediated retrieval to reach high steady state levels in ER membranes. We cannot completely rule out that malfolding of hybrid constructs contributes to our findings, but we consider it very unlikely because all constructs were fluorescent. In addition, the export of two independent constructs (GFP-BP80ΔCT and GFP-CNXΔCT) could be stimulated by the addition of the BP80 CT. Likewise, the presence of the CNX TMD consistently reduced the anterograde transport (cf. GFP-BP80 with GFP-CNX/BP80 and GFP-BP80ΔCT with GFP-CNXΔCT). It is therefore more plausible that the CNX TMD reduces export, while the BP80CT promotes it. The mechanism by which the CNX TMD triggers the ER retention of GFP-CNX/BP80 is not within the scope of this article, but it does not seem to be merely dependent on its length and calls for further investigation.
Although it was proposed that the competition would mainly occur at the limiting recycling step from the PVC (daSilva et al., 2005), the results obtained suggested that interference with any step in the traffic of the competitor between the ER, the Golgi apparatus, the PVC, and its putative recycling route would manifest itself in a reduced ability to compete with endogenous BP80. This prompted us to use this assay to study the BP80 C terminus and establish which sorting motifs are important for its normal traffic (Figure 5).
The assay proved to be very powerful and informative because several conserved residues were found to be important for competition (Figure 5C), a full analysis of which was beyond the scope of this study. However, we could distinguish between those mutants that compromised anterograde flow and those that compromised the recycling step to rescue receptors from degradation in the vacuole. This was possible by scoring an increase or a decrease in the formation of the GFP-core, indicative of arrival in the vacuole (Figure 5B).
In this work, we present our results on the full characterization of the role of the Tyr residue within the YXXφ motif using this competition assay in conjunction with in vivo imaging and complementary biochemical assays involving the entire BP80 molecule. We are currently dissecting the various other motifs and their potential interplay within this short 37–amino acid sequence using a similar thorough approach.
The competition assay could certainly be used to study other molecules. For instance, recent work suggests that a GFP fusion derivative of At RMR (Park et al., 2005) should lead to specific interference with the sorting route to the storage vacuole in plants. The approach could also be useful to study the TMD and CT of the BP80-related pumpkin (Cucurbita maxima) receptor PV72 (Watanabe et al., 2002) and the CT of αTIP (Gomez and Chrispeels, 1993; Jiang and Rogers, 1998). Both have been implicated in mediating traffic to the storage vacuole, which is a route that requires further biochemical dissection.
Many Routes Lead to the Plant PVC
Although the highly conserved Tyr motif (YMPL/YIPL) was shown to be important for in vitro interaction with the μ-subunits of mammalian AP1 (Sanderfoot et al., 1998) and to a Golgi-localized μ-adaptin from Arabidopsis (Happel et al., 2004), other data argued against an active role of the CT in anterograde transport in vivo (Jiang and Rogers, 1998; Barrieu and Chrispeels, 1999). Among those, the observation that the transmembrane of BP80 was sufficient to target pro-aleurain to the PVC (Jiang and Rogers, 1998) certainly suggests that the tail is dispensable. Also in S. cerevisiae, the vacuolar sorting receptor VPS10 was shown to be faster degraded when the CT was absent (Cereghino et al., 1995) and continued to enter clathrin coated vesicles under these conditions (Deloche et al., 2001). It was concluded that the tail was mostly important for the recycling from the yeast endosome back to the Golgi apparatus. However, it should be noted that increased degradation can either be caused by increased anterograde transport or a block in retrograde cycling. If signals for both routes are present on the tail, deletion would have overlapping consequences.
Our results show that the CT is important for the efficient anterograde transport of GFP-BP80 to the PVC (Figures 2 and 4). However, despite the slower transport, GFP-BP80ΔCT is still able to reach the lytic vacuole via the PVC. This suggests that type I membrane proteins can reach the PVC and the lytic vacuole without specific sorting information, as long as the nature of the TMD permits ER export. Slow transport could occur by default through nonspecific membrane flow (Barrieu and Chrispeels, 1999), either within clathrin-coated vesicles or perhaps within another kind of membrane carrier that has yet to be identified.
However, it should be noted that GFP-BP80ΔCT no longer displays any measurable competition (Figure 1C). This means that its traffic toward the PVC no longer employs limiting sorting machinery that controls the flow of endogenous BP80. Since it was shown that competition is mainly at the retrograde recycling route, it could be argued that the tail also plays a role in the recycling. Deletion of the tail may thus lead to faster disposal in the vacuole and compensates for the reduced anterograde transport. This may lead to underestimating the true impact on anterograde transport when the tail is deleted. Figure 2A showed only a modest reduction in the rate of core formation, but if all molecules reaching the PVC at low rate fail to recycle and are quantitatively degraded in the vacuole, the apparent weak effect becomes more plausible. By introducing the competition assay to the tools available to study receptor traffic, it could be clearly established that the tail is crucial for the sorting of BP80.
From the various conserved amino acids of the BP80 CT, we concentrated on a YXXφ motif (YMPL) for an in-depth analysis. The Y612A or L615A mutants exhibited the strongest reduction in receptor competition (Figure 5C), although none of the mutants abolished competition completely. In yeast and mammalian cells, Tyr-based motifs are well-characterized interacting partners of the μ-subunity of cytosolic AP complexes (Ohno et al., 1998; Owen and Evans, 1998). Currently, four distinct AP complexes have been described (Robinson and Bonifacino, 2001). Two of these, AP1 and AP2, are well-established components of clathrin-coated vesicles that mediate attachment of clathrin to the trans-Golgi network and plasma membrane, respectively. Our results support the notion that the YMPL motif is essential for interaction with receptor sorting machinery in vivo.
Although consistent with the CT deletion mutant, point mutations within the CT domain did not completely prevent arrival at a lytic compartment. Comparison of tobacco transgenic lines expressing GFP-BP80 and GFP-BP80Y612A allowed us to appreciate a clear difference in the stability of the two proteins. The data revealed that GFP-BP80-Y612A is more stable than GFP-BP80 in planta, suggesting that these molecules reach the vacuole with different efficiencies. Generation of transgenic plants also revealed that the Y612A mutation leads to transport to the plasma membrane. This was shown for point mutations in the GFP-BP80 fusion (Figure 8) and the full-length BP80 gene (Figure 9).
The results open up several alternative explanations for the arrival of GFP-BP80-Y612A in the PVC. One possibility is that the Y612A mutant is transported from the Golgi apparatus to the PVC via bulk flow (Barrieu and Chrispeels, 1999) and is inefficient, causing a portion of the mutant molecules to be mistargeted to the plasma membrane. Bulk flow could either occur through nonselective incorporation into Golgi-derived clathrin-coated vesicles or within an as yet unknown clathrin-independent carrier. Finally, the mutant could also reach the PVC via the plasma membrane followed by endocytosis. The latter scenario has been suggested to explain how yeast VPS10 can reach the PVC in clathrin deletion mutants (Deloche and Schekman, 2002).
To obtain evidence that mutants can cycle from the Golgi apparatus via the plasma membrane and the PVC back to the Golgi apparatus, we disrupted the PVC-to-Golgi route with wortmannin (daSilva et al., 2005). The drug caused a rapid decline in plasma membrane fluorescence accompanied by a fast increase in the diffuse fluorescence in the vacuole, originating from cleaved GFP-core (Figures 8H and 8I). Since this drug does not disrupt constitutive transport to the plasma membrane (Pimpl et al., 2003), the data obtained support the notion of a detour via the plasma membrane and suggest that BP80 carries signals for endocytosis (Figure 10). While under normal conditions this could merely represent a backup system to recycle mistargeted receptors, the Y612A mutant may reach the plasma membrane more frequently as it may not gain access to the efficient clathrin-mediated Golgi-to-PVC route. From the plasma membrane, it could be recycled by endocytosis to reach the PVC. The circle could be closed by recycling from the PVC to the Golgi apparatus, from which the mutant can possibly reach the plasma membrane again (Figure 10). In the presence of wortmannin, this cycle would be disrupted at the retrograde PVC-to-Golgi step (daSilva et al., 2005).
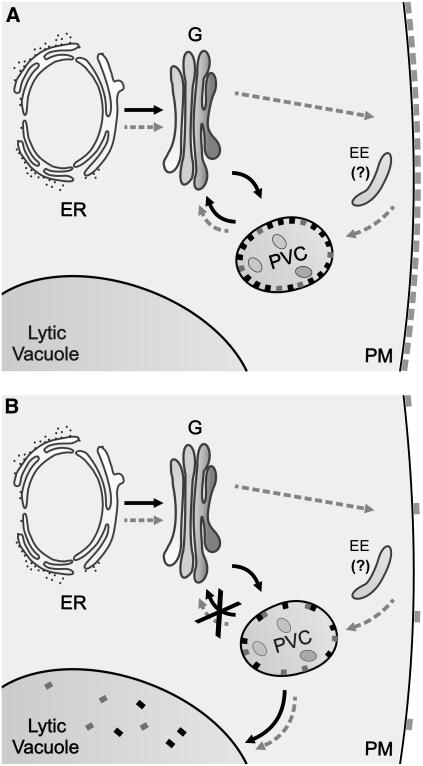
Figure 10.
Model Describing the Proposed Targeting Pathway of BP80, the Y612A Mutant, and the Effect of Wortmannin.
(A) Under normal physiological conditions, BP80 (black rectangles) is proposed to target directly from the ER via the Golgi apparatus (G) to the PVC and recycling back (black arrows). The Y612A mutant (gray rectangles) also reaches the Golgi apparatus from the ER but is not effectively targeted to the PVC via the direct route. Instead, the mutant cycles via the plasma membrane (PM) to the PVC (gray arrows). From the PVC, recycling to the Golgi still occurs, and the mutant can thus complete full transport circles from the Golgi apparatus to the PM, followed by the PVC and back to the Golgi apparatus. Possible involvement of the plant equivalent of the early endosome (EE) in either anterograde transport to the PM or endocytic traffic from the PM is not excluded but has not been illustrated by specific arrows.
(B) Wortmannin disrupts the PVC-to-Golgi (G) route and causes a rapid redistribution of BP80 from the PVC to the vacuole, where the lumenal portion is cleaved (black rectangles in the lumen). Wortmannin also disrupts the targeting circle of the mutant and causes a rapid redistribution from the PM to the vacuole, followed by proteolysis of the lumenal portion (gray rectangles).
Although wortmannin has also been shown to disrupt endocytosis in plants (Emans et al., 2002), this could be an indirect long-term effect. It has been shown in mammalian cells that wortmannin inhibits ligand internalization indirectly due to reduced recycling of cell surface receptors from the endosomes (Kundra and Kornfeld, 1998). The authors show that the rate of receptor internalization is not affected by the drug, but due to lack of recycling from the endosome, receptor steady state levels reduce drastically at the level of the plasma membrane. Our results on the rapid redistribution of GFP-BP80Y612A would strongly support this notion. The model in Figure 10 predicts that the mutant is still competent for endocytosis. The model also suggests that the Y612A mutation causes leakage to the plasma membrane from the Golgi apparatus because the direct route to the PVC is not available. The secondary endocytic route from the plasma membrane, which may only play a minor role for the wild-type receptor, could lead through the plant equivalent of the early endosome (Ueda et al., 2004), but further research is needed to confirm this.
The BP80 Reconstitution Assay: A Powerful Assay to Measure BP80 Activity in Vivo
The methods discussed so far involved the quantitative receptor competition assay, the measurement of glycan processing, or proteolytic trimming of hybrid receptors to monitor progress in the secretory pathway and in vivo imaging of steady state levels within the cells. Although complementary, they would not be as convincing on their own if they had not been complemented by one further biochemical approach: the work on the full-length receptor.
It was shown previously that overexpression of wild-type BP80 molecules can restore vacuolar sorting quantitatively when it is challenged by competing GFP-BP80 and partially during wortmannin stress. This feature was exploited to confirm the role of the YXXφ motif on BP80 itself rather than a GFP fusion. Instead of restoring vacuolar sorting, overexpressed BP80 carrying a single point mutation led not only to its missorting to the plasma membrane but caused increased secretion of the BP80-ligand amy-spo under control conditions (Figure 9). Finally, we can rule out that significant levels of wild-type BP80 reach the plasma membrane because overexpression of wild-type BP80 did not increase amy-spo secretion.
The results show that full-length BP80-Y612A represents a new semidominant mutant that directly influences BP80-ligands (exemplified by amy-spo) but not constitutively secreted proteins (exemplified by amy). In contrast with the competition by GFP-BP80, which is indirect by compromising receptor traffic, the full-length BP80-Y612A mutant causes ligand deviation because it binds to them and delivers them to the cell surface instead of the vacuole. The resulting secretion is therefore a direct effect of the mutant molecule. We do not completely rule out that a portion of amy-spo can remain bound on mutant BP80 while endocytosis occurs, but this remains to be shown experimentally.
The combination of both approaches demonstrates that our observations rely on very specific BP80-dependent transport assays in vivo and cannot be due to indirect membrane flow effects. In addition, the arrival of BP80-Y612A to the plasma membrane demonstrates that the Tyr motif is a bona fide sorting signal that functions on the receptor itself in vivo.
Multiple Functions for a Short CT
Experimental results presented in this manuscript strongly suggest that the Tyr motif is only one of several sorting signals. Deletion of the entire tail completely abolished competition with endogenous receptors, whereas none of the point mutations exhibited such a drastic effect.
Although the YMPL motif resembles the typical YXXφ signature, Y612A and L615A substitutions did not have identical phenotypes. Both mutants displayed reduced competition, but the first showed decreased vacuolar degradation, whereas the second behaved the opposite way (Figure 5B). Such heterogeneity within the sorting motif was certainly not expected and calls for future work.
The BP80 C terminus also contains a di-acidic DXE motif, recently found to be responsible for ER export in plants (Hanton et al., 2005). While mutation of the second Glu residue (E604A) reduced competition significantly, it did not seem to inhibit GFP-core formation, and future experiments will have to show which step of the transport route is compromised.
Another mutant identified (I608A) is part of a very conserved Ile-Met motif present in all BP80 genes identified so far and could be involved in the recycling of BP80. This could be deduced from the fact that GFP-core formation was increased and competition was reduced. Orthologs of yeast retromer components (Seaman et al., 1997, 1998; Seaman, 2005) were identified in mammals (Haft et al., 2000), and evidence for the involvement of this complex in the retrieval of mannose-6-phosphate receptors has recently been obtained (Arighi et al., 2004; Seaman, 2004, 2005). A retrograde route has been suggested to operate in plants (daSilva et al., 2005), and Arabidopsis orthologs of retromer components were recently characterized (Oliviusson et al., 2006). It will now be interesting to investigate if the I608A mutant fails to interact with retromer components.
Finally, it is also possible that one of the identified sequence motifs could play a role in the proposed endocytosis. This could be tested in combination with the Y612A substitution that results in defective Golgi-to-PVC transport (Figures 5 to 9). Such combinations of mutants, together with the various biochemical and cytological approaches used in this study, will help to elucidate the various sorting signals present on this important class of receptor molecules.
METHODS
Plasmids for Transient Expression of Cargo Molecules and Organelle Markers
DNA manipulations were done according to established procedures. All plasmids were amplified in Escherichia coli strain MC1061 (Casadaban and Cohen, 1980). Previously established plasmids were used encoding α-amylase amy (Crofts et al., 1999) and the previously established BP80-ligand amy-spo (Pimpl et al., 2003; daSilva et al., 2005). The Golgi marker (ST-YFP) encoding plasmid was described earlier (daSilva et al., 2005). The full-length coding region of PEP12 (SYP21) was amplified using sense oligo 5′-GACTAGCATCGATGAGTTTTCAAGATTTAGAATCA-3′ and antisense oligo 5′-TTGATGTCTAGACCAAGACAACGATGATGAC-3′ from first-strand cDNA prepared as described previously (Pimpl et al., 2003), except that 5-d-old Arabidopsis thaliana seedlings were used for RNA extraction. The underlined regions refer to engineered restriction sites for insertion in pLL4 (Brandizzi et al., 2003) between the cauliflower mosaic virus 35S (CaMV35S) promoter at the ClaI site overlapping with the start codon and the XbaI site overlapping with the stop codon preceeding the 3′-untranslated end of the nopaline synthase gene (3′nos). This created the chimeric expression plasmid pOF19 to produce untagged natural wild-type PEP12 in plant cells. To engineer a PEP12 fusion to YFP, we amplified the YFP coding region using sense oligo 5′-GACTAGCCATGGTGAGCAAGGGCGAGGAGC-3′ and antisense oligo 5′-GAAACTCATCGATGCTCCACCCTTGTACAGCTCGTCCATGCC-3′. Underlined regions refer to engineered restriction sites for subcloning as an NcoI-ClaI fragment. The antisense oligo introduces a tetrapeptide (GlyGlyAlaSer) between the natural C-terminal Lys codon of YFP and the N-terminal Met codon of the PEP12 coding region. After cutting with NcoI and ClaI, the obtained fragment was ligated together with the above-described ClaI-XbaI PEP12 fragment into the expression plasmid pAmy-HDEL (Phillipson et al., 2001). The latter was cut with NcoI and XbaI, followed by dephosphorylation, for insertion of both fragments between the CaMV35S promoter and 3′nos, yielding pOF21 to express YFP-PEP12.
Plasmids for Transient Expression of Effector Molecules
Plasmids encoding GFP-BP80 and GFP-CNX were generated as described previously (daSilva et al., 2005). Briefly, they respectively consist of the coding region of the TMD and CT of Arabidopsis BP80a (Hadlington and Denecke, 2000) or the TMD and CT of Arabidopsis CNX fused to the C-terminal end of an N-glycosylated variant of GFP5 (Batoko et al., 2000). In both plasmids, the coding sequence of the GFP fusions is flanked by CaMV35S and the 3′-untranslated end of the nos gene. To obtain a version of the competitor without the BP80 CT, the sequence encoding GFP:TMD of BP80a was amplified by PCR from the pLL38 plasmid (daSilva et al., 2005), using the 35S-sense oligo 5′-CACTATCCTTCGCAAGACC-3′ in combination with the δCT-antisense oligo 5′-TAAAGCTCTAGACTACCTCAATCTATATTTGTAAAC-3′. The resulting fragment was digested with NcoI and XbaI and inserted into the plasmid encoding GFP-CNX previously cut with NcoI-XbaI and dephosphorylated with calf intestine phosphatase. This replaced the CNX domains in GFP-CNX by the BP80 CT, yielding the plasmid pLL53. To produce the plasmid encoding the cytosolic protein GFP-CT, the fragment encoding the CT of BP80a was obtained through PCR amplification using the GFP/CT-sense oligo 5′-ACCTCGAGATCTCCCAATACATGGACTCAGAGATCAGA-3′ and the pUCOF-antisense oligo 5′-CGGCTCGTATGTTGTGTGG-3′. The resulting DNA fragment was digested with BglII-SphI and inserted downstream the coding region of cytosolic GFP5 present in the pSLH11 plasmid, producing pLL56. To generate the DNA fragment encoding the hybrid fusion comprising the CNX TMD sequence upstream the sequence encoding the BP80 CT, an assembly PCR strategy was performed. The CNX TMD sequence was amplified by PCR from the GFP-CNX encoding plasmid, using the oligo 35S-sense in combination with the oligo TMCNX-antisense (5′-TCTGATCTCTGAGTCCATGTATTGCGCCTTTTTGCCACCAAAGAT-3′). This generates a fragment that encodes the entire GFP-CNX/TMD with an additional sequence at the 3′-end, which overlaps with the 5′-end of the BP80 CT encoding sequence. The CT of BP80 was amplified using the oligos CTBP80-sense 5′-CAATACATGGACTCAGAGATCAGA-3′ and pUCOF-antisense. Both fragments were annealed and reamplified using the oligos 35S-sense and pUCOF-antisense, which produced the GFP-TMD/CNX-CTBP80 fragment. The fragment was used to replace the CNX sequences by the hybrid as a BglII and SphI insert. To generate GFP-CNXΔCT, truncated CNX sequence was amplified from pSLH6 (daSilva et al., 2005) using 35S-sense primer and antisense primer 5′-TGACGGTCTAGACTACTTTTTGCCACCAAAGATAAGC-3′. The PCR fragment was cut with NcoI and XbaI, ligated into pSLH6, previously cut with the same two enzymes, and dephosphorylated, yielding pSLH8.
The generation of the recombinant plasmid (pLL50) encoding YFP-BP80 was achieved as follows. A fragment encoding the TMD/CT of BP80 was isolated through HindIII and partial BamHI digestion, and a YFP coding region on ST-YFP was amplified by PCR using the oligo pair YFP/NcoI-sense 5′-CTGCCCGTGCCATGGCCCACCCTCGTGACCACC-3′ and YFP/BamHI-antisense 5′-ACAAATCAGATCTCGGATCCTTCTGCTCCCTTGTACAGCTCGTCCATGCC-3′ and subjected to NcoI/BamHI digestion. Both fragments were inserted into the GFP-CNX plasmid, which had been cut with NcoI/HindIII and dephosphorylated.
To produce the various Ala substitutions of the selected conserved amino acid residues of the BP80 tail (Figure 5A), the QuickChange method (Stratagene) was used with pairs of oligonucleotides encoding for the mutated triplet and 15 bases extending on either side of the mutated codon, either for site-directed mutagenesis of the GFP-BP80 encoding plasmid or the full-length BP80 molecule in case of the Y612A mutation.
Plasmids for Stable Plant Transformation
The DNA coding sequence of the chimeric genes GFP-BP80, GFP-BP80Y612A, FL-BP80, and FLBP80Y612A, flanked by the coding regions of the CaMV35S promoter and the 3′-untranslated end of the nos gene, were inserted as an EcoRI-HindIII fragment into the polylinker of the Agrobacterium tumefaciens transformation vector pDE1001 (Denecke et al., 1992). The resulting plant transformation plasmids were transfected into the Agrobacterium rifampicin-resistant strain C58 (pGV2260) as described previously (Denecke et al., 1992).
Plant Material and Stable Tobacco Transformation
Tobacco plants, Nicotiana tabacum cv Petit Havana SR1 (Maliga et al., 1973), were grown in Murashige and Skoog (MS) medium (Murashige and Skoog, 1962) and 2% sucrose in a controlled room at 25°C with a 16-h daylength at a light irradiance of 200 μE m2 s. Stably transformed plants were obtained by Agrobacterium infection of tobacco SR1 leaf disks as described by Denecke et al. (1990) (1992). Selection of transformants was accomplished in MS medium supplemented with 3% sucrose and containing 100 μg/mL kanamycin and 250 μg/mL cefotaxime. The transgenic lines most productive were chosen through comparative protein gel blotting experiments using total protein extracts from leaf material. Detection was achieved using either anti-GFP or anti-VSRAt-1 (Tse et al., 2004) antiserum.
Tobacco BY2 cells were transformed by cocultivation with Agrobacterium carrying the appropriated recombinant plasmid. Selection was achieved by pouring the infected cell cultures onto solid BY2 medium (MS medium supplemented with 30 g/L sucrose, 100 mg/L myo-inositol, 200 mg KH2PO4, 1 mg/L thiamine, and 400 mg/L 2,4D), containing kanamycin (100 μg/mL) and cefotaxine (250 μg/mL), followed by incubation in the dark for 3 to 4 weeks. Grown calli were transferred onto plates with fresh culture medium, and samples were taken to screen for transformants through checking the levels of recombinant protein via protein blot analysis (see below). Selected cell lines, at least five per construct, were cultivated in liquid medium and used for microscopy analysis.
Leaf Protoplast Preparation and Transient Expression
Preparation of tobacco leaf protoplasts and transfection via electroporation were previously described in great detail (Hadlington and Denecke, 2001; Pimpl et al., 2006), and plasmid concentrations used are given in the figure legends. Protoplasts were analyzed after 24-h incubation unless otherwise indicated. The harvesting of cells and culture medium from the standard 2.5-mL protoplast suspensions was initiated by centrifugation for 5 min at 100g using a swing-out rotor, which causes flotation of the protoplasts. Harvesting consisted of recovery of 1 mL of clear cell culture medium from underneath the floating protoplast with a refined Pasteur pipette (100 μm diameter). The medium was further cleared by centrifugation in a refrigerated microfuge (4°C, 18,000g, 10 min), and the obtained supernatant was kept on ice for further analysis (see below). To recover the total cell population of the remaining suspension, the cells were diluted 10-fold with 250 mM NaCl in 15-mL Falcon tubes. This allows recovery of washed cells in a compact pellet after 3 min of centrifugation at 200g. The resulting supernatant is removed with a peristaltic pump, and the pellets were kept on ice for subsequent extraction and analysis (see below).
α-Amylase Assay and Calculation of Secretion Index
Harvested cells and medium from protoplast suspensions were processed to allow quantitative α-amylase detection. Medium was twofold diluted in α-amylase extraction buffer (50 mM acid malic, 50 mM sodium chloride, 2 μM calcium chloride, and 0.02% [w/v] sodium azide). Cell pellets were resuspended in α-amylase extraction buffer to obtain a final volume of precisely 500 μL (including the volume of the resuspended cells). Soluble proteins were extracted by sonication for 5 s (amplitude 10 μ), followed by centrifugation for 10 min at 18,000g at 4°C and recovery of the cleared supernatant.
α-Amylase assays were done routinely using Megazyme substrate (R-CAAR4) consisting of blocked P-nitrophenyl maltoheptaoside (BPNPG7, 54.5 mg) and thermostable α-glucosidase (125 units at pH 6.0), dissolved according to the manufacturer's instructions in 10 mL of distilled autoclaved water and stocked at −80°C as 1-mL aliquots. Essays were started by adding 30 μL of substrate to 30 μL of sample, followed by incubation at 42°C, and the reaction was stopped by addition of 150 μL of 1% (w/v) Trizma base. A precise volume of 200 μL of each reaction was transferred into wells of a microtitre plate, and the absorbance was read at λ = 405 nm. Negative controls for correction of absorbance were obtained using medium and cell extracts from mock-transformed protoplasts.
The α-amylase activity was calculated in terms of change in OD (ΔOD), divided per milliliter of extract used (taking into account the twofold dilution of the medium and the fivefold concentration of the cell extract relative to the original 2.5-mL suspension), divided by the length of the assay in minutes. Only readings within the linear range between ΔOD 0.1 and 1.0 were used for calculations to avoid inaccuracy or substrate limitations. Once appropriate dilutions and incubation times were established, the assay was repeated at least three times for each extract, and the average activity was calculated. In all figures, such experiments were repeated at least three or up to five times using independent protoplast transfections including all the controls.
The secretion index in each protoplast suspension was calculated as the ratio between the extracellular and the intracellular activity and thus represents digits without units. The error bars were calculated as the standard error from at least three or up to five independent protoplast transfections, as indicated in the legends.
Drug Incubations
For wortmannin treatment, protoplasts prepared from GFP-BP80– and GFP-BP80Y612A–overexpressing lines were kept diluted in TEX medium (Hadlington and Denecke, 2001) for 48 h with a washing step with fresh TEX medium after the first 24-h incubation. After the total incubation time, each protoplast suspension was split into two equal aliquots and supplemented with TEX medium with or without wortmannin for a 10-μM final concentration of the drug (daSilva et al., 2005). After an additional 4-h incubation, the samples were observed through CLSM. We found that with 10-μM concentrations, possible degradation of wortmannin in TEX medium at the standard temperature of 25°C is negligible during 24-h incubation periods as it did not compromise the ability to inhibit vacuolar sorting of the established marker amy-spo for the BP80 route (Pimpl et al., 2003; daSilva et al., 2005).
For incubation with BFA, electroporated protoplast suspensions were initially diluted with 1 mL and supplemented with another 1 mL of TEX medium, which contains twice the final concentration of the drug (10 μM). After a 20-h incubation, total cell extracts were prepared and used for immunoblot analysis as described below.
Cell Partitioning by Osmotic Shock
Cell partitioning through osmotic shock treatment was conducted via an established procedure (Denecke et al., 1992; daSilva et al., 2005), except that a different extraction buffer was used. Cells from 2.5 mL of protoplast suspension were pelleted with 250 mM NaCl and gently resulpended in a fourfold volume of the extraction buffer (50 mM Tris, pH 7.5, 2 mM EDTA, and 1 mM β-mercaptoetanol, supplemented with pepstatin as protease inhibitor) followed by 5-min incubation on ice. Subsequently, the samples were spun at 25,000g for 15 min, resulting in osmotically released soluble proteins in the supernatant (termed fraction S1). These are mainly cytoplasmic and vacuolar solutes, whereas microsomes remained in the pellet. The pellet was resuspended in the same volume of the extraction buffer as S1, sonicated and pelleted at 25,000g for 15 min. This yields all remaining soluble microsomal proteins and some membrane proteins (termed fraction S2). The resulting pellet (P) is highly enriched in membranes and was resuspended in the same volume of the extraction buffer as S1 or S2, sonicated, and left in suspension until analyzed by SDS-PAGE.
Protein Extraction, EndoH Digestion, and Gel Blot Analysis
Cellular proteins were analyzed as total protein extracts (soluble + membrane proteins). Protoplasts from 2.5-mL suspensions were pelleted with 250 mM NaCl and resuspended in 250 μL of leaf extraction buffer (100 mM Tris-HCl, pH 7.8, 200 mM NaCl, 1 mM EDTA, 0.2% Triton X-100, and 2% β-mercaptoethanol), followed by brief sonication and 10-min centrifugation at 25,000g at 4°C. The supernatant containing both soluble and membrane proteins was recovered and subsequently used for protein gel blotting analysis. When indicated in the figure legends, these extracts were subjected to treatment with endoH prior to gel analysis.
For endoH digestion, 45-μL aliquots of total cell extracts were supplemented with 5 μL of reaction buffer supplied with the enzyme (New England BioLabs) and 1 μL of endoH (New England BioLabs; 500,000 units/mL). Control digestions were performed using extract and enzyme buffer alone. The reactions were incubated at 37°C for 1 h and were stopped by the addition of 50 μL of 2× SDS-PAGE loading buffer (Crofts et al., 1999).
Protein extracts supplemented with 2× SDS loading buffer were briefly boiled (5 min, 95°C), and equal volumes were loaded in SDS-polyacrylamide gels. Proteins were transferred onto a nitrocellulose membrane and then blocked with PBS, 05% Tween 20, and 5% milk powder for 1 h. After gel blotting, immunodetections were performed using enhanced chemiluminescence, using rabbit polyclonal antiserum raised against GFP (1:5000 dilution) or anti-VSRAt-1 (1:5000 dilution) (Tse et al., 2004).
α-Amylase Assay
For the α-amylase assays, cells and medium from protoplast suspensions were harvested as described above. Subsequently, cells were extracted in α-amylase extraction buffer (Crofts et al., 1999) via sonication for 5 s. The extracts were centrifuged 10 min at 25,000g at 4°C, and the clean soluble supernatant was recovered. The culture medium was also spun 10 min at 25,000g at 4°C to remove residual cell debris. The α-amylase assays and calculation of the secretion index as the ratio between the extracellular and intracellular activity were described previously (Denecke et al., 1990; Crofts et al., 1999; Phillipson et al., 2001; Pimpl et al., 2003).
CLSM
Protoplasts expressing the various GFP and YFP fusions were harvested 24 h after DNA transfection, washed in TEX medium, and concentrated to a density of ∼2 × 106/mL to facilitate identification of transfected protoplasts. Aliquots of the protoplast suspensions were transferred onto glass slides in which a central slot (0.5 × 1 cm) was created using electrical tape (150-μm thickness) to accommodate the cells between the slide and the cover glass (22 × 50 mm; No. 0). Confocal imaging was performed using an inverted Zeiss LSM 510 META laser scanning microscope with a Plan-Neofluar ×40/1.3 oil DIC objective. For imaging expression of GFP constructs alone or in combination with YFP fusions, excitation lines of an argon laser of 458 nm for GFP and 514 nm for YFP were used alternately with line switching using the multitrack facility of the microscope. The fluorescence was detected using a 545-nm dichroic beam splitter and a 475- to 525-nm band-pass filter for GFP and a 560- to 615-nm band-pass filter for YFP. Appropriate controls have been performed to exclude the possibility of crosstalk between the two fluorochromes prior to the experiments. To avoid overexpression artifacts, only protoplasts exhibiting similar and moderate levels of fluorescence were compared.
For direct observation of leaf epidermis tissue from plants stably expressing either GFP-BP80 or GFP-BP80Y612A, small leaf segments (0.5 × 0.5 cm) were cut and immediately placed into MS medium prior to microscopy analysis. Imaging conditions were the same as above, except that a 488-nm laser line was used in combination with a narrower band-pass filter set (505 to 530 nm) to decrease the detection of autofluorescence from the plant material. Post-acquisition image processing was performed with LSM 5 Image Browser (Zeiss) and Adobe Photoshop Elements software.
In Vivo Labeling and Immunoprecipitation
For pulse-chase analysis of GFP-BP80 and GFP-BP80ΔCT, 10 standard electroporations were pooled, washed, and concentrated in 500 μL of TEX buffer supplemented with 100 μCi/mL Pro-mix (Amersham Life Sciences). After pulse labeling for 1 h, protoplasts were diluted with chase buffer (10 mM Met and 5 mM Cys in TEX buffer). The protoplast suspensions were split into equal portions and incubated at 25°C for an appropriate period of time (see legend of Figure 2). Cells were precipitated with 250 mM NaCl and extracted in homogenization buffer (200 mM Tris-HCl, pH 8.0, 300 mM NaCl, 1% Triton X-100, and 1 mM EDTA). The material was used for immunoprecipitation with anti-GFP antiserum (Molecular Probes; 1/1000 dilution) as previously described (daSilva et al., 2005) and analyzed by SDS-PAGE. Labeled proteins were detected by a phosphor imager (FLA 500; Fuji Photo Film) and AIDA software (Raytest) to allow quantification. Exposures on x-ray films were used to obtain higher resolution images for visual display (Figure 2A, top panel). The percentage of processing was determined for each lane by dividing the peak surface area of the GFP-core band by the total surface area of all peaks and multiplied by 100 to yield percentages (Figure 2A, bottom panel).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL/AGI data libraries under accession numbers U79960/AT2G14720.1 (BP80a) and L41651/AT5G16830 (PEP12/SYP21).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Movie 1. Guard Cells of Transgenic Tobacco Producing GFP-BP80.
Supplemental Figure 1. Colocalization of BP80 Fusion Proteins with the Golgi Apparatus or the PVC.
Supplemental Table 1. Distribution of Labeled Organelles in Transfected Protoplasts.
Supplementary Material
Acknowledgments
This work was supported by grants from the Biotechnology and Biological Sciences Research Council and the European Union (HPRN-CT-2002-00262: BioInteractions). We thank Liwen Jiang (Chinese University of Hong Kong, China) for the most generous gift of a superior quality antiserum to detect BP80 (anti-VSRAT1) without background bands. We also thank Gareth Howell for help at the BioImaging Facility at Leeds and S. Hanton (University of Saskatoon, Canada) for preparing plasmid pSLH8. L.L.P.d. is indebted to the CAPES Foundation Brazilian Ministry of Education for a PhD studentship. O.F. is indebted to the European Union and the Biotechnology and Biological Sciences Research Council for a PhD studentship.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Jurgen Denecke (j.denecke@leeds.ac.uk).
Online version contains Web-only data.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.105.040394.
References
- Arighi, C.N., Hartnell, L.M., Aguilar, R.C., Haft, C.R., and Bonifacino, J.S. (2004). Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J. Cell Biol. 165 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrieu, F., and Chrispeels, M.J. (1999). Delivery of a secreted soluble protein to the vacuole via a membrane anchor. Plant Physiol. 120 961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham, D.C., and Raikhel, N.V. (2000). Unique features of the plant vacuolar sorting machinery. Curr. Opin. Cell Biol. 12 491–495. [DOI] [PubMed] [Google Scholar]
- Batoko, H., Zheng, H.Q., Hawes, C., and Moore, I. (2000). A rab1 GTPase is required for transport between the endoplasmic reticulum and Golgi apparatus and for normal Golgi movement in plants. Plant Cell 12 2201–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandizzi, F., Frangne, N., Marc-Martin, S., Hawes, C., Neuhaus, J.M., and Paris, N. (2002. b). The destination for single-pass membrane proteins is influenced markedly by the length of the hydrophobic domain. Plant Cell 14 1077–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandizzi, F., Hanton, S., DaSilva, L.L., Boevink, P., Evans, D., Oparka, K., Denecke, J., and Hawes, C. (2003). ER quality control can lead to retrograde transport from the ER lumen to the cytosol and the nucleoplasm in plants. Plant J. 34 269–281. [DOI] [PubMed] [Google Scholar]
- Brandizzi, F., Snapp, E.L., Roberts, A.G., Lippincott-Schwartz, J., and Hawes, C. (2002. a). Membrane protein transport between the endoplasmic reticulum and the Golgi in tobacco leaves is energy dependent but cytoskeleton independent: Evidence from selective photobleaching. Plant Cell 14 1293–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban, M.J., and Cohen, S.N. (1980). Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol. 138 179–207. [DOI] [PubMed] [Google Scholar]
- Cereghino, J.L., Marcusson, E.G., and Emr, S.D. (1995). The cytoplasmic tail domain of the vacuolar protein sorting receptor Vps10p and a subset of VPS gene products regulate receptor stability, function, and localization. Mol. Biol. Cell 6 1089–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts, A.J., Leborgne-Castel, N., Hillmer, S., Robinson, D.G., Phillipson, B., Carlsson, L.E., Ashford, D.A., and Denecke, J. (1999). Saturation of the endoplasmic reticulum retention machinery reveals anterograde bulk flow. Plant Cell 11 2233–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- daSilva, L.L., Taylor, J.P., Hadlington, J.L., Hanton, S.L., Snowden, C.J., Fox, S.J., Foresti, O., Brandizzi, F., and Denecke, J. (2005). Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting. Plant Cell 17 132–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deloche, O., and Schekman, R.W. (2002). Vps10p cycles between the TGN and the late endosome via the plasma membrane in clathrin mutants. Mol. Biol. Cell 13 4296–4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deloche, O., Yeung, B.G., Payne, G.S., and Schekman, R. (2001). Vps10p transport from the trans-Golgi network to the endosome is mediated by clathrin-coated vesicles. Mol. Biol. Cell 12 475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke, J., Botterman, J., and Deblaere, R. (1990). Protein secretion in plant cells can occur via a default pathway. Plant Cell 2 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke, J., De Rycke, R., and Botterman, J. (1992). Plant and mammalian sorting signals for protein retention in the endoplasmic reticulum contain a conserved epitope. EMBO J. 11 2345–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz, E., and Pfeffer, S.R. (1998). TIP47: A cargo selection device for mannose 6-phosphate receptor trafficking. Cell 93 433–443. [DOI] [PubMed] [Google Scholar]
- Emans, N., Zimmermann, S., and Fischer, R. (2002). Uptake of a fluorescent marker in plant cells is sensitive to brefeldin A and wortmannin. Plant Cell 14 71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, P., Dahms, N.M., and Kornfeld, S. (2003). Mannose 6-phosphate receptors: New twists in the tale. Nat. Rev. Mol. Cell Biol. 4 202–212. [DOI] [PubMed] [Google Scholar]
- Ghosh, P., and Kornfeld, S. (2004). The cytoplasmic tail of the cation-independent mannose 6-phosphate receptor contains four binding sites for AP-1. Arch. Biochem. Biophys. 426 225–230. [DOI] [PubMed] [Google Scholar]
- Gomez, L., and Chrispeels, M.J. (1993). Tonoplast and soluble vacuolar proteins are targeted by different mechanisms. Plant Cell 5 1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlington, J.L., and Denecke, J. (2000). Sorting of soluble proteins in the secretory pathway of plants. Curr. Opin. Plant Biol. 3 461–468. [DOI] [PubMed] [Google Scholar]
- Hadlington, J.L., and Denecke, J. (2001). Transient expression, a tool to address questions in plant cell biology. In Plant Cell Biology, C. Hawes and B. Satiat-Jeunemaitre, eds (Oxford, UK: Oxford University Press), pp. 107–125.
- Haft, C.R., de la Luz Sierra, M., Bafford, R., Lesniak, M.A., Barr, V.A., and Taylor, S.I. (2000). Human orthologs of yeast vacuolar protein sorting proteins Vps26, 29, and 35: Assembly into multimeric complexes. Mol. Biol. Cell 11 4105–4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanton, S.L., Renna, L., Bortolotti, L.E., Chatre, L., Stefano, G., and Brandizzi, F. (2005). Diacidic motifs influence the export of transmembrane proteins from the endoplasmic reticulum in plant cells. Plant Cell 17 3081–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel, N., Honing, S., Neuhaus, J.M., Paris, N., Robinson, D.G., and Holstein, S.E. (2004). Arabidopsis micro A-adaptin interacts with the tyrosine motif of the vacuolar sorting receptor VSR-PS1. Plant J. 37 678–693. [DOI] [PubMed] [Google Scholar]
- Hohl, I., Robinson, D.G., Chrispeels, M.J., and Hinz, G. (1996). Transport of storage proteins to the vacuole is mediated by vesicles without a clathrin coat. J. Cell Sci. 109 2539–2550. [DOI] [PubMed] [Google Scholar]
- Honing, S., Sosa, M., Hille-Rehfeld, A., and von Figura, K. (1997). The 46-kDa mannose 6-phosphate receptor contains multiple binding sites for clathrin adaptors. J. Biol. Chem. 272 19884–19890. [DOI] [PubMed] [Google Scholar]
- Jiang, L., Phillips, T.E., Rogers, S.W., and Rogers, J.C. (2000). Biogenesis of the protein storage vacuole crystalloid. J. Cell Biol. 150 755–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, L., and Rogers, J.C. (1998). Integral membrane protein sorting to vacuoles in plant cells: Evidence for two pathways. J. Cell Biol. 143 1183–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe, N.A., Brown, J.C., Neumann, U., Vicre, M., Bachi, A., Hawes, C., Ceriotti, A., Roberts, L.M., and Frigerio, L. (2004). Transport of ricin and 2S albumin precursors to the storage vacuoles of Ricinus communis endosperm involves the Golgi and VSR-like receptors. Plant J. 39 821–833. [DOI] [PubMed] [Google Scholar]
- Jurgens, G. (2004). Membrane trafficking in plants. Annu. Rev. Cell Dev. Biol. 20 481–504. [DOI] [PubMed] [Google Scholar]
- Kirsch, T., Paris, N., Butler, J.M., Beevers, L., and Rogers, J.C. (1994). Purification and initial characterization of a potential plant vacuolar targeting receptor. Proc. Natl. Acad. Sci. USA 91 3403–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundra, R., and Kornfeld, S. (1998). Wortmannin retards the movement of the mannose 6-phosphate/insulin-like growth factor II receptor and its ligand out of endosomes. J. Biol. Chem. 273 3848–3853. [DOI] [PubMed] [Google Scholar]
- Li, Y.B., Rogers, S.W., Tse, Y.C., Lo, S.W., Sun, S.S., Jauh, G.Y., and Jiang, L. (2002). BP-80 and homologs are concentrated on post-Golgi probable lytic prevacuolar compartments. Plant Cell Physiol. 43 726–742. [DOI] [PubMed] [Google Scholar]
- Maliga, P., Sz-Breznovits, A., and Marton, L. (1973). Streptomycin-resistant plants from callus culture of haploid tobacco. Nat. New Biol. 244 29–30. [DOI] [PubMed] [Google Scholar]
- Marcusson, E.G., Horazdovsky, B.F., Cereghino, J.L., Gharakhanian, E., and Emr, S.D. (1994). The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell 77 579–586. [DOI] [PubMed] [Google Scholar]
- Marty, F. (1999). Plant vacuoles. Plant Cell 11 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro, S. (2004). Organelle identity and the organization of membrane traffic. Nat. Cell Biol. 6 469–472. [DOI] [PubMed] [Google Scholar]
- Murashige, R., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 15 473–497. [Google Scholar]
- Ohno, H., Aguilar, R.C., Yeh, D., Taura, D., Saito, T., and Bonifacino, J.S. (1998). The medium subunits of adaptor complexes recognize distinct but overlapping sets of tyrosine-based sorting signals. J. Biol. Chem. 273 25915–25921. [DOI] [PubMed] [Google Scholar]
- Oliviusson, P., Heinzerling, O., Hillmer, S., Hinz, G., Tse, Y.C., Jiang, L., and Robinson, D.G. (2006). Plant retromer, localized to the prevacuolar compartment and microvesicles in Arabidopsis, may interact with vacuolar sorting receptors. Plant Cell 18 1239–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen, D.J., and Evans, P.R. (1998). A structural explanation for the recognition of tyrosine-based endocytotic signals. Science 282 1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris, N., Rogers, S.W., Jiang, L., Kirsch, T., Beevers, L., Phillips, T.E., and Rogers, J.C. (1997). Molecular cloning and further characterization of a probable plant vacuolar sorting receptor. Plant Physiol. 115 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris, N., Stanley, C.M., Jones, R.L., and Rogers, J.C. (1996). Plant cells contain two functionally distinct vacuolar compartments. Cell 85 563–572. [DOI] [PubMed] [Google Scholar]
- Park, M., Lee, D., Lee, G.J., and Hwang, I. (2005). AtRMR1 functions as a cargo receptor for protein trafficking to the protein storage vacuole. J. Cell Biol. 170 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson, B.A., Pimpl, P., daSilva, L.L., Crofts, A.J., Taylor, J.P., Movafeghi, A., Robinson, D.G., and Denecke, J. (2001). Secretory bulk flow of soluble proteins is COPII dependent. Plant Cell 13 2005–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimpl, P., Hanton, S.L., Taylor, J.P., Pinto-daSilva, L.L., and Denecke, J. (2003). The GTPase ARF1p controls the sequence-specific vacuolar sorting route to the lytic vacuole. Plant Cell 15 1242–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimpl, P., Taylor, J.P., Snowden, C.J., Hillmer, S., Robinson, D.G., and Denecke, J. (2006). Golgi-mediated vacuolar sorting of the ER chaperone BiP may play an active role in quality control within the secretory pathway. Plant Cell 18 198–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puertollano, R., Aguilar, R.C., Gorshkova, I., Crouch, R.J., and Bonifacino, J.S. (2001). Sorting of mannose 6-phosphate receptors mediated by the GGAs. Science 292 1712–1716. [DOI] [PubMed] [Google Scholar]
- Robinson, D.G., Oliviusson, P., and Hinz, G. (2005). Protein sorting to the storage vacuoles of plants: A critical appraisal. Traffic 6 615–625. [DOI] [PubMed] [Google Scholar]
- Robinson, M.S., and Bonifacino, J.S. (2001). Adaptor-related proteins. Curr. Opin. Cell Biol. 13 444–453. [DOI] [PubMed] [Google Scholar]
- Rojo, E., Gillmor, C.S., Kovaleva, V., Somerville, C.R., and Raikhel, N.V. (2001). VACUOLELESS1 is an essential gene required for vacuole formation and morphogenesis in Arabidopsis. Dev. Cell 1 303–310. [DOI] [PubMed] [Google Scholar]
- Runions, J., Brach, T., Kuhner, S., and Hawes, C. (2006). Photoactivation of GFP reveals protein dynamics within the endoplasmic reticulum membrane. J. Exp. Bot. 57 43–50. [DOI] [PubMed] [Google Scholar]
- Sanderfoot, A.A., Ahmed, S.U., Marty-Mazars, D., Rapoport, I., Kirchhausen, T., Marty, F., and Raikhel, N.V. (1998). A putative vacuolar cargo receptor partially colocalizes with AtPEP12p on a prevacuolar compartment in Arabidopsis roots. Proc. Natl. Acad. Sci. USA 95 9920–9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman, M.N. (2004). Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J. Cell Biol. 165 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman, M.N. (2005). Recycle your receptors with retromer. Trends Cell Biol. 15 68–75. [DOI] [PubMed] [Google Scholar]
- Seaman, M.N., Marcusson, E.G., Cereghino, J.L., and Emr, S.D. (1997). Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J. Cell Biol. 137 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman, M.N., McCaffery, J.M., and Emr, S.D. (1998). A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J. Cell Biol. 142 665–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada, T., Fuji, K., Tamura, K., Kondo, M., Nishimura, M., and Hara-Nishimura, I. (2003). Vacuolar sorting receptor for seed storage proteins in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 100 16095–16100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surpin, M., and Raikhel, N. (2004). Traffic jams affect plant development and signal transduction. Nat. Rev. Mol. Cell Biol. 5 100–109. [DOI] [PubMed] [Google Scholar]
- Tse, Y.C., Mo, B., Hillmer, S., Zhao, M., Lo, S.W., Robinson, D.G., and Jiang, L. (2004). Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell 16 672–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda, T., Uemura, T., Sato, M.H., and Nakano, A. (2004). Functional differentiation of endosomes in Arabidopsis cells. Plant J. 40 783–789. [DOI] [PubMed] [Google Scholar]
- Uemura, T., Ueda, T., Ohniwa, R.L., Nakano, A., Takeyasu, K., and Sato, M.H. (2004). Systematic analysis of SNARE molecules in Arabidopsis: Dissection of the post-Golgi network in plant cells. Cell Struct. Funct. 29 49–65. [DOI] [PubMed] [Google Scholar]
- Vitale, A., and Hinz, G. (2005). Sorting of proteins to storage vacuoles: How many mechanisms? Trends Plant Sci. 10 316–323. [DOI] [PubMed] [Google Scholar]
- Wan, L., Molloy, S.S., Thomas, L., Liu, G., Xiang, Y., Rybak, S.L., and Thomas, G. (1998). PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell 94 205–216. [DOI] [PubMed] [Google Scholar]
- Watanabe, E., Shimada, T., Kuroyanagi, M., Nishimura, M., and Hara-Nishimura, I. (2002). Calcium-mediated association of a putative vacuolar sorting receptor PV72 with a propeptide of 2S albumin. J. Biol. Chem. 277 8708–8715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.