Abstract
Neisseria gonorrhoeae is a host-adapted pathogen that colonizes primarily the human genitourinary tract. This bacterium encounters reactive oxygen and reactive nitrogen species as a consequence of localized inflammatory responses in the urethra of males and endocervix of females and also of the activity of commensal lactobacilli in the vaginal flora. This review describes recent advances in the understanding of defense systems against oxidative stress in N. gonorrhoeae and shows that while some of its defenses have similarities to the paradigm established with Escherichia coli, there are also some key differences. These differences include the presence of a defense system against superoxide based on manganese ions and a glutathione-dependent system for defense against nitric oxide which is under the control of a novel MerR-like transcriptional regulator. An understanding of the defenses against oxidative stress in N. gonorrhoeae and their regulation may provide new insights into the ways in which this bacterium survives challenges from polymorphonuclear leukocytes and urogenital epithelial cells.
INTRODUCTION
Neisseria gonorrhoeae (also known as the gonococcus) colonizes primarily the human genitourinary tract, giving rise to the sexually transmitted infection gonorrhea. Disease caused by this organism is a significant health problem despite continual advances in treatment (24, 25, 87, 250). Worldwide there are an estimated 62 million new cases a year, with an average of 22 million cases at any given time (87).
N. gonorrhoeae inhabits mainly mucosal surfaces of the urethra in males and the cervix in females and as a consequence is exposed to a variety of oxidants, which are generated in three main ways: (i) as a by-product of the bacterium's own metabolic processes, (ii) as a key element of the innate immune response, and (iii) as a result of exposure to other factors within the host environment that promote oxidative stress, such as metal ions or commensal organisms that generate oxidants. The most commonly found and discussed oxidants in biological systems are the reactive oxygen species (ROS), which include the superoxide anion (O2·−), hydrogen peroxide (H2O2), and the hydroxyl radical (HO·), and the reactive nitrogen species (RNS), which include nitric oxide (NO·) and peroxynitrite (ONOO−). In addition, sulfur- and chlorine-containing compounds are generated by antimicrobial effector cells (103, 125, 227). Oxidative stress causes damage to DNA, proteins, and cell membranes and often results in cell death (reviewed in reference 125).
Mechanisms for coping with oxidative stress are crucial for the survival of all organisms, particularly obligate human pathogens, such as N. gonorrhoeae, that are routinely exposed to oxidative killing by the host and inhabit an environment of unremitting oxidative stress. Defenses against oxidative stress are increasingly being recognized as playing an important role in virulence (28, 67, 102, 105, 123, 206, 251, 258). A greater understanding of the oxidative stress response of microbial pathogens may aid the future development of treatment and prevention strategies for disease caused by these bacteria. Organisms possess a diverse range of defense mechanisms for sensing, avoiding, and removing oxidants, and Escherichia coli is typically used as a model for describing oxidative stress in bacterial systems (reviewed in references 79, 188, 227, and 229). However, the oxidative stress response in N. gonorrhoeae differs considerably from the E. coli paradigm (235, 236). N. gonorrhoeae has evolved to be highly adapted to its environment, and as a consequence it uses a novel combination of regulators and effectors to sense and respond to oxidative stress. An understanding of the mechanism of action of these systems may provide new insights into the biochemistry of oxidative stress defenses that lie outside the E. coli paradigm but that may be applicable to many other pathogens.
SOURCES OF OXIDATIVE STRESS
The level and type of oxidative stress that organisms are exposed to depend on the type of bacterium and the environment it inhabits. Oxidants exhibit a broad spectrum of toxic effects in biological systems, with particular species having different reactivities with DNA, proteins, and membranes (125). N. gonorrhoeae is exposed to oxidants that are produced both endogenously and exogenously, although some exogenously generated oxidants have the ability to penetrate cells and cause cytoplasmic damage, depending on their membrane permeability (103).
Endogenous Oxidative Stress: the Downside of Aerobic Respiration
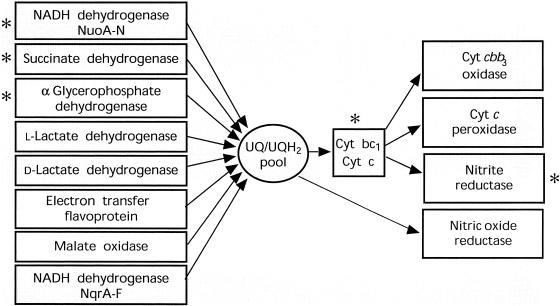
High levels of respiration in N. gonorrhoeae have long been recognized as a significant potential source of endogenous ROS (9, 133). N. gonorrhoeae gives a positive response in the classical oxidase test that is used to distinguish between enteric bacteria and those bacteria that possess a “mitochondrial-type” respiratory chain (27). This means that electron transfer from ubiquinol to oxygen in N. gonorrhoeae is catalyzed by a cytochrome bc1 complex (ubiquinol; ferricytochrome c oxidoreductase) and a cytochrome oxidase (ferrocytochrome c; oxygen oxidoreductase) (Fig. 1). Unlike almost all other aerobic bacteria, the respiratory chain of N. gonorrhoeae has only a single cytochrome oxidase of the cbb3 type (the CcoNOQP cluster, gene identification no. NG1374 to NG1371; see Table 1, footnote a, for the “NG” gene identification website) (176; investigation of available genome sequence [Fig. 1]). The cbb3-type oxidases are usually characterized by a high oxygen affinity, suggesting that N. gonorrhoeae is adapted to microaerophilic growth conditions (186). However, it is well established from research on oxidative stress in mitochondria that the cytochrome oxidase is not a major source of ROS (240).
FIG. 1.
Electron transport and sources of endogenous ROS and RNS in N. gonorrhoeae. Respiratory chains of N. gonorrhoeae and N. meningitidis are shown, as determined from genomic and biochemical information. Cyt, cytochrome; UQ, ubiquinone. Asterisks indicate potential sites of oxidant generation.
TABLE 1.
Defenses and regulators of the oxidative stress response of N. gonorrhoeae
| Protein name | Function | Gene identification no.a | Location(s)b | Sensitivity of mutant strain (assay compound[s])c | Regulation (details of interest)d | Reference(s) |
|---|---|---|---|---|---|---|
| Oxidative stress defenses | ||||||
| SodB | Superoxide dismutase B | NG0405 | C | x (PQ, X/XO) | + (Fur/high Fe, H2O2) | 9, 226, 236 |
| KatA | Catalase | NG1767 | C | s (H2O2) | + (OxyR, H2O2) | 107, 226, 262 |
| MntABC/Mn | ABC-type Mn transporter | NG0168 | P, IM | s (PQ, H2O2) | − (PerR, Mn) | 211, 236, 256 |
| Ccp | Cytochrome c peroxidase | NG1769 | P | s (H2O2) | + (FNR/low O2 + OxyR, H2O2) | 213, 239e |
| MsrA/B | Methionine sulfoxide reductase | NG2059 | C, OM | s (H2O2, X/XO) | + (H2O2, Ecf σ factor) | 221, 226f |
| BfrAB | Bacterioferritin | NG0794, NG0795 | C | s (H2O2, PQ) | NA | 49 |
| Sco | Putative thiol:disulfide oxidoreductase/peroxiredoxin | NG1237 | ND | s (PQ) | NA | 211 |
| GSH | Glutathione (GSH synthetase/glutamate-cysteine ligase) | NG1217, NG0680 | C | NA | NA | 9 |
| Gor | Glutathione reductase | NG0925 | C | x (H2O2) | + (OxyR, H2O2) | 226e |
| Prx | Peroxiredoxin | NG0926 | C | xx (H2O2) | + (OxyR) | Seib et al., submitted |
| Laz | Azurin | NG0944 | OM | s (H2O2) | NA | 255 |
| PriA | DNA replication restart helicase | NG1437 | C | s (H2O2, CH) | NA | 142 |
| RecN | DNA repair | NG0318 | C | s (H2O2) | + (H2O2) | 226 |
| NG1686 | Putative zinc metalloprotease | NG1686 | ND | s (H2O2, CH) | + (H2O2) | 226 |
| NG0554 | N. gonorrhoeae-specific predicted protein | NG0554 | ND | s (H2O2) | + (H2O2) | 226 |
| Oxidative stress regulators | ||||||
| OxyR | H2O2-dependent regulator | NG1813 | C | xx (X/XO, H2O2) | KatA, Prx, Gor | 235e |
| PerR | Mn-dependent repressor | NG0542 | C | xx (H2O2) | MntABC, RpmEJ, AdhA | 256 |
| Fur | Ferric uptake regulation protein | NG1779 | C | NA | Fur, SodB, BfrAB | 23, 210 |
| FNR | Fumarate/nitrate reductase regulator | NG1579 | C | NA | Ccp | 153, 239 |
| NmlR | Neisseria merR-like regulator | NG0602 | C | s (CH, diamide) | AdhC, TrxB, CopA | 34 |
Annotation number from the N. gonorrhoeae FA 1090 genome on the Los Alamos National Laboratory website (http://www.stdgen.lanl.gov/stdgen/bacteria/ngon/index.html), from the Gonococcal Genome Sequencing Project of the University of Oklahoma.
C, cytoplasm; P, periplasm; IM, inner membrane; OM, outer membrane; ND, not determined.
Sensitivity of mutant strains to in vitro oxidative stress killing assays relative to wild-type N. gonorrhoeae: x, same phenotype as the wild type; s, sensitive to killing; xx, resistant to killing (increased survival relative to the wild type). These assays typically involved exposure of a suspension of 104 to 106 cells over 1 h to either paraquat (PQ) (10 mM), xanthine (4.3 mM)/xanthine oxidase (300 mU/ml) (X/XO), or hydrogen peroxide (H2O2) (10 or 40 mM). Exposure to cumene hydroperoxide (CH) (0 to 1%) and diamide (0 to 50 mM) was performed with liquid cultures over 16 h. For further details, see the references cited in the table. NA, not available. PQ (PQ2+) (1,1′-4,4′-bipyridinium dichloride) is a redox compound that is reduced to the paraquat free radical (PQ·+) by low-potential electron donors within the bacterial cell. The paraquat free radical is then oxidized by dioxygen, leading to generation of the superoxide anion (O2·−). This redox cycling also depletes low-potential reducing agents within the cell, such as NADH (106).
Regulation of defenses, either activated/upregulated (+) or repressed (−). Genes of interest, under the control of regulators, are also shown. For details, see the cited references. NA, not available.
Also K. L. Seib, H. J. Wu, Y. N. Srikhanta, J. L. Edwards, T. L. Maguire, S. M. Grimmond, M. A. Apicella, A. G. McEwan, and M. P. Jennings, submitted for publication.
Also J. K. Davies, personal communication.
The major source of endogenous ROS in E. coli is a result of auto-oxidation of components of the aerobic respiratory chain (i.e., NADH dehydrogenase [complex I] and succinate dehydrogenase [complex II], as well as fumarate reductase) (124, 167, 168, 178), leading to the generation of 5 μM/s superoxide (124, 126) (intracellular concentration, 10−10 M [93]) and 5 to 14 μM/s hydrogen peroxide (208) (intracellular concentration, 10−7 to 10−6 M [91, 209, 227]). It also seems likely that in E. coli the ubiquinol oxidases are also a source of superoxide via reactions involving quinone intermediates. However, since N. gonorrhoeae lacks the ubiquinol oxidases found in E. coli, eukaryotic mitochondria (99, 149, 164, 241, 259) represent a better comparison for the purpose of identifying sources of oxidative stress arising from quinones. Recent observations have indicated that, in addition to complexes I and II, the cytochrome bc1 complex (complex III) of the mitochondrial respiratory chain is a major source of superoxide (99, 149, 164, 241, 259). Since the superoxide is produced as a consequence of the reaction of ubisemiquinol with oxygen at the Qo site of the enzyme, this means that there is considerable deposition of superoxide into the mitochondrial intermembrane space (104), equivalent to the periplasmic space in a gram-negative bacterium (125). Based on these studies of E. coli and eukaryotic mitochondria, NADH dehydrogenase (Nuo), succinate dehydrogenase, and the cytochrome bc1 complex of the respiratory chain of N. gonorrhoeae are likely to be the main sites of endogenous ROS generation (Fig. 1).
Sera from patients with gonorrheal infection recognize both the aerobically induced protein (designated Pox 1) and an anaerobically induced nitrite reductase (AniA), indicating that N. gonorrhoeae is capable of both aerobic and anaerobic respiration in vivo (55, 56, 148). N. gonorrhoeae uses nitrite (present in cervical fluid at concentrations averaging 28 μM [242]) to support anaerobic growth (55, 143), converting nitrite (NO2−) to nitrous oxide (N2O) in two reactions catalyzed by AniA (153) and nitric oxide reductase (NorB) (6, 120). The nitric oxide produced as a free intermediate during denitrification reactions is a toxic radical species (267). Nitrous oxide has been shown to be the end product of nitrite reduction (153). The genes required for formation of nitrous oxide reductase are present in the gonococcal genome (nosLYFDZR, NG1397 to NG1402). However, nonsense mutations in nosZ, nosR, and nosD indicate that an active nitrous oxide reductase is not formed in this bacterium (41).
AniA is a copper-containing nitrite reductase and is the major anaerobically induced outer membrane protein of N. gonorrhoeae (26, 55, 115, 165). An aniA null mutant is unable to grow anaerobically (119, 165). NorB of N. gonorrhoeae is unusual, as nitric oxide reductases are typically two-subunit enzymes encoded by norBC. However, as in Ralstonia eutrophus (62), no norC is seen in pathogenic Neisseria species (6, 120). A norB mutant cannot grow but can survive anaerobically, suggesting that nitric oxide is not as toxic to N. gonorrhoeae as it is to other organisms (120), perhaps as a consequence of redundant systems for defense against RNS (see below).
The toxic nature of nitric oxide requires that the genes involved in its production and removal be tightly regulated and induced only at low oxygen tensions to reduce the coincident generation of nitric oxide and superoxide, which can interact to generate other RNS, such as peroxynitrite (249, 267). In E. coli, the transcription factor FNR and the two-component regulatory systems NarQ-NarP and NarX-NarL mediate regulation of nitrate- and nitrite-induced genes (63, 193). Homologues of the E. coli fnr, narQ, and narP genes in N. gonorrhoeae have been identified (119, 153). In N. gonorrhoeae, AniA synthesis is regulated at the transcriptional level by FNR (114) and is upregulated by NarQ-NarP when nitrite is available (119, 153). NorB is induced by nitrite and nitric oxide but is not regulated by NarP, AniA, or anaerobiosis (120).
Recent in vitro studies, using either nitrite or a nitric oxide donor, have shown that N. gonorrhoeae rapidly establishes a nitric oxide steady-state level during anaerobic respiration (41). This organism is able to reduce nitric oxide levels in the surrounding medium from >1 μM (cytotoxic and proinflammatory concentration) to approximately 100 nM (anti-inflammatory concentration) (41).
Interactions of N. gonorrhoeae with Host Cells
N. gonorrhoeae infection is usually characterized by a symptomatic localized inflammatory response of the urethra in men (urethritis) (7, 58) and the endocervix in women (endocervicitis) (75). The purulent exudate typical of gonorrhoea contains predominantly activated polymorphonuclear neutrophils (PMNs) (7, 9). The PMN-mediated inflammatory response involves migration of PMNs toward sites of infection, phagocytosis of microorganisms, and elimination of these organisms by oxygen-dependent and oxygen-independent mechanisms (37; reviewed in references 103 and 155). Activation of PMNs results in a rapid increase in oxygen consumption, referred to as the “oxidative burst,” which leads to generation of superoxide (16). This species rapidly dismutates to hydrogen peroxide and molecular oxygen (158), and the former is then consumed by myeloperoxidase, generating hypochlorous acid (252). Nitric oxide is also produced by constitutive and inducible nitric oxide synthases of PMNs (44, 146, 160), although the importance of NOS to human PMN killing remains a controversial topic. Various secondary oxidants are generated from these reactive species, including chloramines, hydroxyl radicals, singlet oxygen, and peroxynitrite (44, 103). N. gonorrhoeae stimulates the PMN oxidative burst (22, 177, 219, 246). However, interactions between N. gonorrhoeae and PMNs have not yet been fully characterized: some studies have reported that N. gonorrhoeae is able to survive and replicate within PMNs, while others have reported that N. gonorrhoeae is rapidly killed within PMNs (reviewed in references 199, 218, and 219).
In a survival assay using adherent human PMNs, a significant proportion of phagocytosed N. gonorrhoeae cells survived PMN killing and replicated over time, in contrast to efficient killing of E. coli (219). Investigation of a set of N. gonorrhoeae mutant strains, deficient in various oxidative stress defense mechanisms (including superoxide dismutase [SOD] and catalase) and regulatory systems, revealed that none of the oxidative defense enzymes were required on their own for N. gonorrhoeae to survive within PMNs in this assay (212). Another study showed that mutant strains lacking the H2O2-upregulated genes recN (involved in repair of damaged DNA; see below) and NG1686 (a putative zinc metalloprotease) had increased susceptibility to PMN killing relative to the wild type (226). These findings suggest that N. gonorrhoeae, like several species of bacteria, may divert the oxidative burst of PMNs (reviewed in reference 3). Helicobacter pylori is able to evade PMN killing despite the activation of an oxidative response by targeting of the PMN NADPH oxidase so that it locates to the plasma membrane rather than the phagosomal membrane, thus releasing ROS into the extracellular environment (4). Alternatively, oxygen-independent antimicrobial mechanisms may be of greater significance than oxygen-dependent mechanisms during PMN killing of N. gonorrhoeae. In support of the significance of nonoxidative killing, it has been shown that PMNs from people with chronic granulomatous disorder (NADPH oxidase deficient) had phagocytic killing capacities identical to PMNs from healthy donors (198) and that anaerobic PMNs kill as effectively as aerobic PMNs (46). N. gonorrhoeae cells exposed to serum- or phagocyte-derived lactate have increased metabolism, including increased lactate dehydrogenase activity, and rapidly consume available molecular oxygen, reducing the ability of PMNs to mount an oxidative response (29-31, 82). N. gonorrhoeae cells are also susceptible to the oxygen-independent components of PMN granules, including cathepsin G (45, 197, 214-217; reviewed in reference 218).
The presence of a wide range of oxidative stress defenses in N. gonorrhoeae may indicate that this organism encounters significant oxidative stress from sources in vivo, in addition to PMNs. The primary sites of gonococcal infection are the ecto- and endocervical epithelia in women (75) and the urethral epithelium in men (7, 58). N. gonorrhoeae is able to survive and replicate within epithelial cells at these sites of infection (reviewed in reference 166). Intestinal and airway epithelial cells are able to kill bacteria by oxidative mechanisms (20, 64, 200, 207). There is indirect evidence that cervical epithelial cells may also have an oxidative defense capacity, since it has been observed that several mutant N. gonorrhoeae strains that are susceptible to ROS killing in vitro also have decreased survival within primary human cervical epithelial cells. Such mutants include those lacking MntC (a component of the MntABC Mn uptake system) and the oxidative stress response regulator PerR (256), as well as glutathione reductase (Gor) and its regulator OxyR (K. L. Seib, H. J. Wu, Y. N. Srikhanta, J. L. Edwards, T. L. Maguire, S. M. Grimmond, M. A. Apicella, A. G. McEwan, and M. P. Jennings, submitted for publication).
The Host Microenvironment Inhabited by N. gonorrhoeae
Several commensal organisms, some of which are a significant source of ROS, coinhabit the genitourinary tract. Lactobacillus species, which are part of the normal vaginal flora of most women (77, 113, 163, 195), are believed to control the microflora by production of hydrogen peroxide, bacteriocins, organic acids (e.g., l-lactate) (14, 113), and nitric oxide (173, 257). Hydrogen peroxide-producing Lactobacillus species inhibit N. gonorrhoeae growth and decrease catalase activity (224, 261). Women with inhibitory strains of lactobacilli are less likely to be infected with N. gonorrhoeae (122, 205). Metabolism of l-lactate (produced by lactobacilli) by N. gonorrhoeae also greatly enhances oxygen consumption (31), and this in turn may increase levels of endogenous ROS.
DEFENSES AGAINST OXIDATIVE STRESS IN N. GONORRHOEAE
Defenses against oxidative stress involve constitutive and tightly regulated adaptive mechanisms to avoid and scavenge oxidants as well as to repair damaged biomolecules. Bacterial responses to oxidative stress are well defined for E. coli (reviewed in references 79, 188, 227, and 229). Based on this enteric model system, the generally accepted and simplified paradigm of oxidative stress defenses is that superoxide is removed by SODs (SodA, SodB, SodC), generating hydrogen peroxide in the process, which is removed by catalases (KatE, KatG) and peroxidases (AhpC). However, more than 100 proteins in E. coli that are involved in oxidative stress defense have been identified (95, 187). Many of these defenses are controlled by regulators that respond to iron (e.g., Fur), oxygen tension (e.g., FNR and ArcBA), superoxide (e.g., SoxRS), and hydrogen peroxide (e.g., OxyR). The complexity of the regulation of the oxidative stress response in E. coli is highlighted by the expression of sodA, which is controlled by at least six global regulators (61), and sodB, which is controlled by at least three regulators (72). Exposure of E. coli to low concentrations of hydrogen peroxide or superoxide induces a protective response that confers resistance to subsequent exposure (79, 127). Similarly, N. gonorrhoeae was more tolerant to oxygen when grown previously in hyperbaric pO2 (9) or when exposed to low sublethal concentrations of ROS (82), indicating the presence of adaptive responses to ROS. The transcriptional response of N. gonorrhoeae to hydrogen peroxide has recently been defined; more than 150 genes are differentially regulated after transient exposure to hydrogen peroxide, and 75 of these are upregulated (226).
While E. coli serves as a useful model for the oxidative stress response, there are significant differences in the response of N. gonorrhoeae to oxidative stress. As a host-adapted pathogen, the oxidative stress response of N. gonorrhoeae may represent a more useful model than E. coli to help understand the oxidative stress responses of other human pathogens. The current state of knowledge regarding the oxidative stress defenses of N. gonorrhoeae is shown in Table 1 and Fig. 2 and is detailed below.
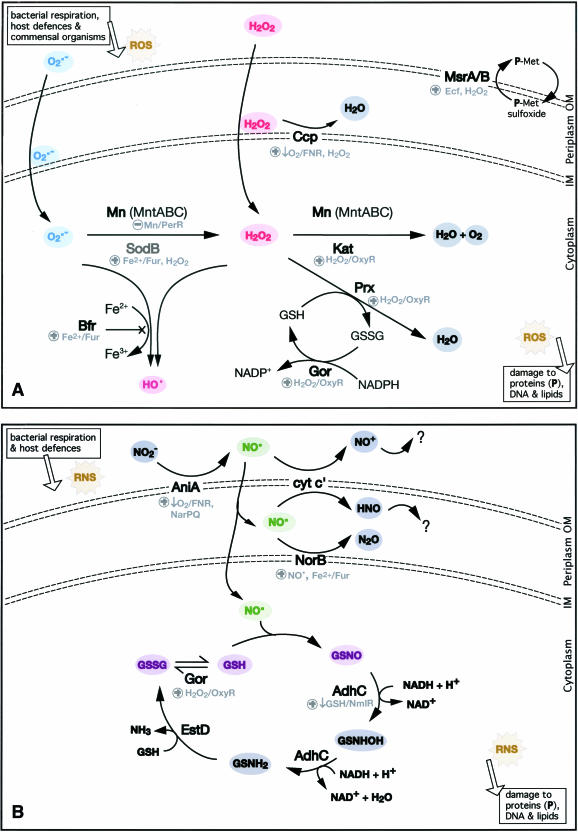
FIG. 2.
Oxidative stress responses of N. gonorrhoeae to ROS (A) and RNS (B). The following proteins involved in oxidative stress defenses, along with their activities, are shown: MntABC, manganese transporter; Mn, manganese; SodB, superoxide dismutase B; Bfr, bacterioferritin; Kat, catalase; Ccp, cytochrome c peroxidase; Prx, peroxiredoxin; Gor, glutathione reductase; GSH, reduced glutathione; GSSG, oxidized glutathione; MsrA/B; methionine sulfoxide reductase; NorB, nitric oxide reductase; cyt c′, cytochrome c′; AdhC, glutathione-dependent formaldehyde dehydrogenase; EstD, esterase D. Regulators of these defenses are shown in gray and include FNR, PerR, OxyR, Fur, NmlR, and Ecf. ROS and RNS shown include the following: O2·−, superoxide; H2O2, hydrogen peroxide; OH·, hydroxyl radical; NO·, nitric oxide; GSNO, S-nitrosoglutathione. Products of the removal of these ROS and RNS include the following: H2O, water; O2, oxygen; N2O, nitrous oxide; NH3, ammonia. P-Met, protein methionines; +, activation; −, repression; ?, area of uncertainty with respect to certain chemical pathways.
Oxidative Stress Regulons in N. gonorrhoeae: OxyR, PerR, and Hydrogen Peroxide
Recent advances in microarray technology and the availability of pan-neisserial genome microarrays have enabled detailed studies of the oxidative stress regulons of N. gonorrhoeae to be undertaken. In particular, PerR (256) and OxyR (Seib et al., submitted) regulons have been characterized through the use of N. gonorrhoeae/N. meningitidis arrays (The Institute for Genomic Research [http://pfgrc.tigr.org/]), and the transcriptome response to hydrogen peroxide (226) has been characterized using pan-neisserial arrays generated by the Neisseria Array Consortium (J. K. Davies and coworkers, personal communication).
Regulation of the peroxide stress response by OxyR is an established feature of gram-negative bacteria such as E. coli and Salmonella enterica (53, 54). On the other hand, PerR typically regulates peroxide stress responses in gram-positive organisms, including B. subtilis (36) and Staphylococcus aureus (117). The presence of both PerR and OxyR in a bacterium is rare. Apart from N. gonorrhoeae, such a situation has been found only in Streptomyces coelicolor, which has OxyR and a hydrogen peroxide-sensitive Fur-like repressor, CatR (100, 101), and Streptomyces viridosporus, which contains three peroxide sensor regulatory gene homologues, ahpA, ahpX, and oxyR (194).
Hydrogen peroxide is sensed by OxyR, a member of the LysR family of DNA-binding transcriptional modulators, which is activated by the oxidation of key cysteine residues and formation of a disulfide bond (54, 263). The OxyR regulon of N. gonorrhoeae consists of gor, prx, and katA (Table 1 and Fig. 2A; described in detail below). These three genes were differentially regulated greater than twofold between the wild type and the oxyR mutant strain; prx and gor were downregulated, and katA was upregulated in the oxyR mutant strain relative to the wild type. Expression of these genes increases under hydrogen peroxide stress, with OxyR acting as an activator of gor and prx under high hydrogen peroxide conditions but as a repressor of katA under low hydrogen peroxide conditions (Seib et al., submitted). This OxyR regulon is relatively small compared to those of E. coli and S. enterica serovar Typhimurium, in which OxyR regulates expression of at least nine genes (172, 189, 266) (265). The E. coli regulon includes katG (hydroperoxidase I), gorA, and the peroxiredoxin ahpCF (alkylhydroperoxide reductase).
PerR is a member of the Fur (for “ferric iron uptake regulator”) family of metalloregulatory proteins (169), and the PerR regulon typically includes antioxidant enzymes such as KatA, AhpCF, and the ferritin-like Dps protein MrgA (110). The PerR regulon of N. gonorrhoeae consists of 12 genes that were differentially regulated greater than twofold between the wild type and the perR mutant strain (Table 1, Fig. 2A). Eleven genes were upregulated in the perR mutant strain relative to the wild type, including the mntABC operon, responsible for Mn transport (see below); two genes encoding the ribosomal proteins RpmE (L31; NG0930) and RpmJ (L36; NG0931), which have been suggested to have an as-yet-unknown extraribosomal function (157); and two members of the TonB-dependent family (Tdf) of receptors, NG1205 and NG0925 (tdfH), which encodes a surface-exposed outer membrane protein with principal homology to heme/hemophore receptors (237). Expression of AdhA (NG1442) is reduced in the perR mutant, indicating that PerR acts as an activator of this gene. AdhA has considerable similarity with proteins that belong to the alcohol dehydrogenase subgroup, which contains the NAD(P)- and zinc-dependent alcohol dehydrogenases. Although known regulators of the peroxide defense response are present in N. gonorrhoeae, there is no evidence for the presence of SoxR in this bacterium. Thus, the pattern of gene expression in response to superoxide differs considerably from the E. coli paradigm.
The transcriptional response to hydrogen peroxide in N. gonorrhoeae involves upregulation of 75 genes and downregulation of a further 80 genes (226). Several of the genes that were upregulated encode proteins with a known role in oxidative stress defenses, including catalase, Gor and Prx (OxyR regulon), SodB (Fur regulon; see below), and MsrA/B (sigma factor Ecf regulon; see below). In addition, numerous genes involved in heat shock responses, iron acquisition (including fur), and regulation of transcription were shown to be upregulated (226). This study also led to the identification of several new oxidative stress defenses, including RecN (described below), NG1686 (a putative zinc metalloprotease), and NG0554 (an N. gonorrhoeae-specific protein of unknown function), all of which are involved in defense against peroxides.
SOD
SOD catalyzes the disproportionation of superoxide to hydrogen peroxide and water (reaction 1) (81, 161):
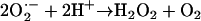 |
(1) |
Three main classes of SOD exist, SodA (a cytoplasmic Mn-SOD), SodB (a cytoplasmic Fe-SOD), and SodC (a periplasmic Cu/Zn-SOD), all of which are found in E. coli (128). E. coli mutants lacking SOD have defects in catabolism, biosynthesis, and DNA replication (42, 84, 85).
In early studies it was observed that 80 to 100% of N. gonorrhoeae strains had no measurable SOD activity, and the remainder had very low SOD activity (9, 181). This appeared to contradict the accepted view that SOD was essential for all aerobic bacteria. Genes encoding SodA or SodC are not present in N. gonorrhoeae, but the gene encoding the complete Fe-dependent SodB (sodB) is present in the gonococcal genome (Table 1, Fig. 2A) (236). However, SodB does not appear to be an essential oxidative stress defense of N. gonorrhoeae in vitro; a sodB mutant showed susceptibility to superoxide killing similar to that of wild-type cells (236). sodB expression appears to be increased under iron-replete conditions via positive regulation by Fur (210) (described below), as seen for the similar sodB of N. meningitidis (97) and E. coli (73). Expression of sodB is also upregulated after exposure to hydrogen peroxide (226). Thus, the failure to observe superoxide dismutase activity in early investigations may have been due to iron limitation and/or a lack of induction by oxidative stress. The low activity of the Fe-SodB in N. gonorrhoeae suggests that the bacterium may be adapted to grow under conditions of iron limitation as a way of minimizing the production of hydroxyl radicals via Fenton chemistry (reaction 2), which would occur if ferrous iron was in contact with hydrogen peroxide:
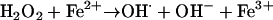 |
(2) |
SodB in E. coli has a limited role in protection against oxidative stress, which is restricted to the transition from anaerobic to aerobic conditions (135); under aerobic conditions, SodA is of key importance. This type of regulation may be of relevance to N. gonorrhoeae, since this bacterium has been proposed to occupy microaerophilic or anaerobic niches (143, 153).
In contrast to N. gonorrhoeae, the closely related organism N. meningitidis contains active SodB and SodC enzymes (9, 144, 181, 213, 251). Unlike N. gonorrhoeae, SodB of N. meningitidis has been demonstrated to play a role in protection against oxidative stress (213). The predicted SodB protein sequences of N. gonorrhoeae and N. meningitidis are 96% identical. The differences seen between sodB mutant strains in these two species (213, 236) may be due to distinctive regulatory mechanisms in these organisms. The sodC gene of N. meningitidis is absent in N. gonorrhoeae and appears to have been acquired by N. meningitidis via horizontal transfer, most likely from commensal Haemophilus species that coinhabit the upper respiratory tract (144, 145). SodC of N. meningitidis has been reported to be involved in virulence in a mouse model (251). However, contradictory results have been reported regarding its in vitro role in defense against oxidative stress (213, 251).
Manganese and the MntABC Mn Transporter
N. gonorrhoeae uses the manganous ion (Mn2+) as a chemical quenching agent of ROS in a way similar to the already- established process in Lactobacillus plantarum. Reactions 3 and 4 show examples of Mn quenching of ROS (Table 1, Fig. 2A) (11):
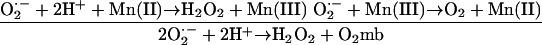 |
(3) |
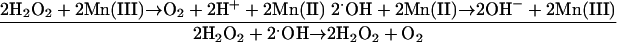 |
Lactic acid bacteria lack SOD enzymes but use manganese (Mn) accumulated to millimolar concentrations intracellularly to chemically scavenge superoxide (8, 10-13). In light of the low SOD activity seen in N. gonorrhoeae (see above), Mn accumulation is particularly significant. This defense mechanism has also been observed in Bacillus subtilis mutants lacking SodA (129). Mn(II) and Mn(III) have been shown to nonenzymatically scavenge superoxide (13) and hydrogen peroxide (13, 223). The rate constant of the interaction of Mn(II) with the peroxyl radical has been shown to be high enough to provide an antioxidant mechanism (57). In addition, the biologically relevant Mn(II)-pyrophosphate and Mn(II)-polyphosphate complexes can be effective antioxidants by indirectly decreasing or blocking hydroxyl radical production via Fenton, Haber-Weiss, xanthine oxidase-Fe-EDTA, or Fe(III)-H2O2-type reactions (51).
Growth of N. gonorrhoeae on media supplemented with Mn(II) confers resistance to in vitro oxidative killing by superoxide (236) and hydrogen peroxide (213). This phenomenon is independent of SodB and catalase, respectively, but it is dependent upon an ABC cassette-type Mn uptake system (MntABC) that is involved in this Mn accumulation and protection against ROS (236).
The MntABC Mn transporter of N. gonorrhoeae (Table 1, Fig. 2A) belongs to a group of recently characterized ABC permeases that are identified by the nature of the solute that is bound by their extracytoplasmic binding protein component (70). ABC transporters typically consist of three components: a periplasmic (or lipoprotein in the case of gram-positive bacteria) substrate-binding protein (MntC), two integral membrane permeases (MntB), and two peripheral membrane proteins that bind and hydrolyze ATP (MntA) (112). An mntC mutant of N. gonorrhoeae was shown to have lowered accumulation of Mn and was highly susceptible to superoxide (236)- and hydrogen peroxide (256)-induced oxidative killing compared to the wild type, even in the presence of added Mn. The mntC mutant grew at a reduced rate and entered stationary phase later than the wild-type strain, while an mntAB mutant strain had a severely defective growth rate, even when grown with Mn-supplemented media (256).
Regulation of mntABC expression by Mn and PerR.
MntC expression is regulated by PerR (see above) and Mn in N. gonorrhoeae (Table 1, Fig. 2A) (256). PerR is a manganese-dependent repressor that regulates the peroxide defense response in gram-positive bacteria such as Bacillus subtilis (36, 111) and Staphylococcus aureus (117). As mentioned above, although the perR gene is largely restricted to gram-positive bacteria, it has been identified in N. gonorrhoeae (256) and Campylobacter jejuni (243).
An N. gonorrhoeae perR mutant strain is more resistant to hydrogen peroxide than is the wild-type strain, and this resistance is enhanced by supplementation of growth media with Mn(II) (256). This is similar to the phenotype of a B. subtilis perR mutant strain (36). In the B. subtilis perR mutant, catalase, alkyl hydroperoxide reductase, and the DNA binding protein MrgA are all overproduced (50). In N. gonorrhoeae, PerR does not appear to have a significant regulatory role in the expression of catalase (256), which is regulated by OxyR in this organism (235) (described below). However, PerR does appear to control Mn accumulation; the perR mutant strain has resistance to oxidative killing similar to that of the wild-type strain grown on Mn-supplemented media. Northern and Western analyses indicated that the N. gonorrhoeae perR mutant had higher levels of expression of the mntC transcript and the MntC protein than did the wild-type strain. A second transcript specific to mntAB was also upregulated in the perR mutant strain. MntC expression also appears to be repressed by Mn (256).
The regulation of N. gonorrhoeae MntABC is distinct from that seen in other bacteria that have been investigated, in which MntABC is regulated by a DtxR-related transcription factor, MntR. In B. subtilis, MntR is a Mn-dependent repressor of expression of mntABC and other genes involved in Mn uptake (98). MntR is divergently transcribed from mntABC loci in B. subtilis and a number of other bacteria (36, 118, 192). In Salmonella enterica serovar Typhimurium, expression of sitABC (an mntABC homologue) is repressed by Mn via an MntR homologue but is not responsive to oxidative stress (137). No evidence for the presence of the regulatory gene mntR in N. gonorrhoeae has been found (256). It has been suggested that the sensitivity of N. gonorrhoeae to Mn(II) (236) may be related to the absence of tight control of the MntABC transporter by an MntR homologue (256).
Catalase
Catalases, widespread in aerobic bacteria, are heme-cofactored enzymes that convert hydrogen peroxide to oxygen and water (reaction 5) (162, 208, 209):
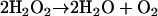 |
(5) |
N. gonorrhoeae possesses very high constitutive levels of catalase (Table 1, Fig. 2A), nearly 100 times higher than N. meningitidis (9) and E. coli (107). N. gonorrhoeae contains a single catalase, encoded by the katA gene, that is located primarily in the cytoplasm, with a small concentration potentially located in the cytoplasmic membrane (107, 262). It has been demonstrated that the presence of catalase significantly increases the ability of N. gonorrhoeae to resist in vitro killing and DNA damage by exposure to hydrogen peroxide, human PMNs, and L. acidophilus (131, 132, 261, 262). A katA mutant strain is also significantly more sensitive to hydrogen peroxide and paraquat than is the wild type (213, 222).
Regulation of Catalase: Hydrogen Peroxide and OxyR
The catalase activity of N. gonorrhoeae is inducible, increasing two- to threefold when exposed to PMNs or 1 mM hydrogen peroxide (262) and 1.5-fold when exposed to hydrogen peroxide-producing L. acidophilus (261). However, this activity is considered weakly inducible (131). Recent analysis of the transcriptome response of N. gonorrhoeae to hydrogen peroxide indicated a 51.6-fold increase in levels of katA mRNA in cells exposed to 5 mM hydrogen peroxide (226). Expression of catalase is activated by OxyR in E. coli, S. enterica serovar Typhimurium, and other gram-negative bacteria (53, 172, 189).
A typical OxyR binding site preceding the katA gene of N. gonorrhoeae has not been found (132, 235). However, it was found that catalase expression is repressed by OxyR (see above) and is induced by hydrogen peroxide via OxyR derepression (Table 1, Fig. 2) (235). An oxyR mutant strain has ninefold-higher catalase activity than constitutive levels and fourfold-higher activity than the maximally induced wild-type levels and is significantly more resistant to hydrogen peroxide killing than is the wild type (235). This is distinct from the situation in E. coli and S. enterica serovar Typhimurium, in which OxyR is a positive regulator of hydrogen peroxide-inducible genes and in which increased sensitivity to hydrogen peroxide is seen in oxyR mutants (53, 54). Analysis of OxyR from N. gonorrhoeae indicates that it contains all of the typical features of OxyR proteins. In addition, N. gonorrhoeae OxyR can complement an E. coli oxyR mutant and behave as an activator (235).
Peroxidase: Cytochrome c Peroxidase
Peroxidases, like catalases, are heme-cofactored enzymes responsible for hydrogen peroxide removal (208, 209). Peroxidases oxidize a reductant to convert hydrogen peroxide to water (reaction 6):
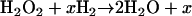 |
(6) |
where x is the reductant.
N. gonorrhoeae has high peroxidase activity, some of which may be associated with the catalase protein (9, 131). However, despite similar catalase concentrations in several N. gonorrhoeae strains, different sensitivities to hydrogen peroxide have been seen, indicating additional mechanisms of hydrogen peroxide removal (2).
A periplasmic cytochrome c peroxidase (Ccp) in N. gonorrhoeae (Table 1, Fig. 2A) (153) that is involved in defense against hydrogen peroxide-induced killing has been reported (213, 239). The ccp gene of N. gonorrhoeae is located downstream of katA (see above), distal to a conserved hypothetical gene (213). An N. gonorrhoeae ccp mutant strain showed slight sensitivity to in vitro hydrogen peroxide killing relative to the wild-type strain; however, a ccp/katA double mutant strain was significantly more sensitive to in vitro hydrogen peroxide killing than was a katA mutant strain (213, 239). Ccp belongs to class I of the peroxidase superfamily, along with catalase-peroxidases and ascorbate peroxidases (260). It is a diheme c-type cytochrome that catalyzes the reduction of hydrogen peroxide using c-type cytochromes of the respiratory chain as the electron donor (reaction 7):
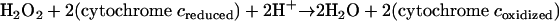 |
(7) |
In E. coli, molecular oxygen is sensed by the transcription factor FNR (21). FNR is essential for the gonococcal response to oxygen (153). Ccp of N. gonorrhoeae is expressed under conditions of low oxygen tension/anaerobiosis (153) and is dependent on FNR (239). Expression of ccp is also upregulated by hydrogen peroxide (226) and PerR (256).
Ccp of N. gonorrhoeae is believed to be a lipoprotein, with a signal peptide for cleavage by signal peptidase II, that is anchored to the membrane in the periplasm (239). The lipid modification has been suggested as a way of maintaining the close proximity of Ccp to its c-type cytochrome electron donors located in, or associated with, the cytoplasmic membrane, while also maintaining it in a location where its protective mechanism is required (239). However, N. gonorrhoeae Ccp may also be involved in energy generation by using hydrogen peroxide as an electron acceptor, as in the yeast Hansenula polymorpha (244).
In methylotrophic yeast, Ccp can substitute for catalase. Increased Ccp levels can suppress the phenotype of a catalase-deficient mutant strain (90). In addition, the presence of abundant Ccp activity has been suggested to compensate for the natural absence of catalase in Fasciola hepatica and Schistosoma mansoni. Partially purified Ccp from these organisms can inhibit damage induced by oxidative stress in vitro (38). Ccp has been identified in several bacteria, including Pseudomonas aeruginosa (83), Pseudomonas stutzeri, Paracoccus denitrificans (92), and Rhodobacter capsulatus (121), although functional roles have not yet been determined for Ccp in these organisms. Analysis of the distribution of Ccp in Neisseria spp. indicated that it is widespread in N. gonorrhoeae and commensal Neisseria strains but is absent from all N. meningitidis strains (213).
N. gonorrhoeae does not appear to contain the peroxidases present in other bacteria. E. coli and S. enterica serovar Typhimurium possess an NADPH-dependent alkyl hydroperoxide reductase (AhpC) capable of reducing organic peroxides to alcohols (130, 228). Ahp scavenges the majority of the endogenous hydrogen peroxide in E. coli (208). E. coli also possesses a thiol peroxidase (47). N. meningitidis contains a constitutively expressed cytoplasmic glutathione peroxidase (Gpx) (1, 171) that is involved in oxidative defense (170). An N. meningitidis gpx mutant strain is highly sensitive to paraquat and slightly sensitive to hydrogen peroxide (170). However, Gpx is absent from N. gonorrhoeae and the commensal Neisseria species investigated (171).
Thiol-Based Defenses
GSH and Gor.
The low-molecular-weight compound glutathione (γ-l-glutamyl-l-cysteinylglycine) (GSH) is considered one of the first lines of defense against oxidative stress (188). GSH, typically present in cells at millimolar concentrations (5 mM in E. coli) (191), is a chemical scavenger of radicals and acts as a hydrogen donor to restore oxidized macromolecules (43). Very high concentrations of GSH (reported to be >15 mM) are present in N. gonorrhoeae, which may constitute a powerful antioxidant system (9). The GSH oxidoreductase (Gor) (NG0925), which typically maintains the reduced pool of GSH (43), has recently been identified in N. gonorrhoeae as part of the OxyR regulon (Table 1, Fig. 2; described above). As in E. coli (266), Gor is upregulated by OxyR under conditions of increased hydrogen peroxide stress (Seib et al., submitted). Gor is annotated in the N. gonorrhoeae genome as a dihydrolipoamide dehydrogenase (DldH), due to its similarity to this family of proteins. The dldH gene, identified by Stohl et al. (226) to be upregulated by hydrogen peroxide, is the same gene that encodes Gor (Seib et al., submitted).
Thioredoxin and glutaredoxin.
The thiol donors thioredoxin (Trx) and glutaredoxin (Grx) are small proteins with conserved cysteine pairs that are oxidized to cystine disulfides upon reduction of cellular proteins. These thiol proteins are members of the cytoplasmic thioredoxin superfamily, the best characterized members of which are thioredoxins 1 and 2 (TrxA and TrxC, respectively) and glutaredoxins 1, 2, and 3 (GrxA, GrxB, and GrxC, respectively) (15). Reduced Trx and Grx are regenerated by an NADPH-dependent thioredoxin reductase and a GSH-dependent Gor, respectively (15). E. coli mutants lacking these pathways grow poorly under aerobic conditions (191). Several genes in the N. gonorrhoeae genome sequence (156) have been annotated as thioredoxins (NG0652, trx1, thioredoxin I; NG1923, tlpA, thioredoxin; NG0057, thioredoxin-like protein; NG0331, possible thioredoxin) and glutaredoxins (NG1381, grx2, glutaredoxin 2; NG0114, grx3, glutaredoxin 3; NG0351, probable glutaredoxin-related protein). Expression of trx1, grx2, and grx3 is upregulated by exposure to hydrogen peroxide (226). A potential thioredoxin reductase (NG0580, trxB) has also been identified. Gor of N. gonorrhoeae is described above.
Peroxiredoxin.
Peroxiredoxins (Prx) are nonheme enzymes that catalyze alkyl hydroperoxide reduction via conserved reactive cysteines, which are typically regenerated by a Trx or Grx thiol reductant (116). The cytoplasmic cofactor NADPH is essential in maintaining the reducing power of the cell for these systems, and enzymes that are involved in maintaining NADPH are upregulated by oxidative stress (187).
A hybrid peroxiredoxin/glutaredoxin (Prx) in N. meningitidis that is active in the reduction of various peroxides, including hydrogen peroxide and dehydroascorbate, in the presence of GSH has been isolated (202). The hybrid protein contains a Prx module in the N terminus and a Grx module in the C terminus joined by an eight-amino-acid linker peptide; however, the interaction of these domains remains unclear (202). The Prx protein of N. gonorrhoeae has recently been identified as part of the OxyR regulon; its expression is upregulated by hydrogen peroxide-activated OxyR (Table 1, Fig. 2A; see above) (Seib et al., submitted). A prx mutant strain has increased levels of catalase and is resistant to hydrogen peroxide killing (Seib et al., submitted).
Sco.
A homologue of the yeast Sco (for “synthesis of cytochrome oxidase”) protein in N. gonorrhoeae was identified and found to be a novel protein involved in oxidative stress defense (Table 1). N. gonorrhoeae sco mutant strains were highly sensitive to in vitro paraquat-induced oxidative killing, indicating that Sco is involved in protection against oxidative stress in these bacteria (211).
Members of the SCO1/SenC family (Pfam 02630 [19]) are considered to be involved primarily in biogenesis of the CuA center of aa3-type cytochrome oxidases via an as-yet-undefined role in copper storage or transport (68, 89, 159, 180, 184, 185, 196). However, N. gonorrhoeae does not possess an aa3-type cytochrome oxidase with a CuA center (see above), and an sco mutant strain is unaffected in cytochrome oxidase activity. It has been proposed that SCO1/SenC proteins have thiol:disulfide oxidoreductase or peroxiredoxin activity and play a catalytic role as an antioxidant protein in the periplasm (52). Primary- and secondary-structure predictions of N. gonorrhoeae Sco (211), as well as the recently determined protein structure of the B. subtilis Sco protein (17), indicate that these proteins are structurally related to peroxiredoxins and thiol:disulfide oxidoreductases. Thiol:disulfide oxidoreductases and peroxiredoxins are involved in protection against oxidative stress in several bacteria (48, 116, 140).
Methionine sulfoxide reductase.
Cysteine and methionine, the two thiol-containing amino acids that occur in proteins, play a role in several antioxidant systems. Methionine residues are highly susceptible to oxidation by superoxide, hydrogen peroxide, and nitric oxide, resulting in formation of methionine sulfoxide residues in proteins (247). Methionine sulfoxide reductase (Msr) is capable of catalyzing the reduction of methionine sulfoxide residues back to methionine (32, 33, 150, 225). N. gonorrhoeae produces two forms of Msr, a cytoplasmic form and a form with a signal sequence that is secreted to the outer membrane, which is involved in protection from hydrogen peroxide and superoxide (Table 1, Fig. 2A) (221, 230, 253). Similarly, Msr of E. coli is involved in protection from hydrogen peroxide and nitric oxide (174, 225).
MsrA and MsrB, specific for separate methionine sulfoxide epimers, are often separate proteins; however, they are encoded as a fused protein in a single open reading frame in N. gonorrhoeae, with an N-subdomain disulfide oxidoreductase, a central MsrA, and a C-subdomain thioredoxin-dependent MsrB (183, 221). MsrA/B was initially designated PilB, a regulator of pilin expression (231), but this has since been disproved (221).
Methionine oxidation has been implicated in conformational changes and inactivation of proteins (247). However, methionine residues are considered endogenous antioxidants in proteins due to the ability to be reduced and regenerated by Msr (150). In E. coli, Msr is in turn reduced by the thioredoxin system (225); however, given the location of the gonococcal Msr, the question of how it receives its reducing power is an open one. Msr is also believed to be involved in maintenance of adhesins, which are vital for colonization and virulence, in bacterial pathogens (108, 253).
Expression of msrA/B is upregulated in response to hydrogen peroxide (226), and the single extracytoplasmic function sigma factor (Ecf) of N. gonorrhoeae has also been shown to positively regulate expression of msrA/B (J. K. Davies, personal communication). The way in which oxidative stress is sensed in this system is not known, but presumably it is controlled by the interaction of an anti-sigma factor and the Ecf protein, as in other systems (reviewed in reference 18).
Azurin
Azurin is a small, blue, copper-containing protein that functions in electron transport during respiration in several different microorganisms (80, 134, 203, 204). Electrons are typically passed through the quinone pool and cytochrome bc1 and via periplasmic electron shuttles such as c-type cytochromes and azurin to nitrite reductase (71, 267). However, azurin of P. aeruginosa is not essential for denitrification but is involved in protection from oxidative stress (245). A P. aeruginosa azu mutant was proven to be very sensitive to ROS, including hydrogen peroxide and superoxide radicals.
An azurin homologue, laz (for “lipid-modified azurin”), has been identified in both N. gonorrhoeae (Table 1) (94) and N. meningitidis (254). The P. aeruginosa azurin is not a lipoprotein (40). Laz is tethered to the outer membrane via palmityl fatty acid (234, 254) and possesses an N-terminal domain consisting of five imperfect repeats of the sequence Ala-Ala-Glu-Ala-Pro (AAEAP), found in the H.8 protein, and a C-terminal domain very similar to that of azurin of other bacteria (94, 136). Laz is not believed to play a direct role in respiration, but may function in an as-yet-unidentified electron transport pathway in pathogenic Neisseria species (39). The N. gonorrhoeae and N. meningitidis laz mutants were more sensitive to hydrogen peroxide, but not superoxide, than was the wild type (255). These mutants also had increased sensitivity to copper toxicity, suggesting that Laz may play an important role in copper sequestration (255).
Iron Sequestration
Iron, although required by aerobic cells, is also problematic due to its ability to catalyze Fenton and Haber-Weiss reactions that generate ROS. Consequently, iron transport is tightly regulated and free iron is maintained at low concentrations within cells (76). In human cells, intracellular iron is sequestered mainly in ferritin while extracellular iron is bound to lactoferrin and transferrin. Bacteria produce scavenging proteins to acquire iron in iron-limited host environments. Intracellular bacterial iron is complexed primarily to ferritin or to the heme-containing bacterioferritin (179). Overloading of bacterial cells with iron leads to accelerated DNA damage (138, 233). Conversely, iron deprivation has been shown to protect N. gonorrhoeae from ROS (59).
N. gonorrhoeae is able to use multiple host iron-binding proteins as a direct source of iron, and the organism contains several high-affinity iron storage and transport proteins that maintain low levels of free intracellular iron (reviewed in references 86 and 201). Bacterioferritin (Bfr) of N. gonorrhoeae—composed of two subunits, BfrA (ferroxidase center) and BfrB (heme-binding ligand)—is involved in iron storage, growth under iron-limited conditions, and defense against iron-mediated oxidative stress generated by hydrogen peroxide and paraquat (Table 1, Fig. 2A) (49). Most bacteria possess either a ferritin or Bfr homologue (49), although E. coli possesses both (5). Investigation of genome databases has not revealed a ferritin homologue in N. gonorrhoeae (49).
Regulation of iron uptake by Fur.
Microarray analysis has indicated that 203 genes are regulated in response to iron in N. gonorrhoeae: 109 genes are upregulated and 94 genes are downregulated by iron deprivation (74). Based on analysis of putative promoter regions, 50 operons were predicted to be directly regulated by Fur (74). The expression of the majority of iron binding proteins is regulated by Fur in N. gonorrhoeae (23) in a manner similar to that of several other bacteria (78). The Fur regulon of N. gonorrhoeae includes the iron-repressible genes tbpB, lbpB, tonB, and fbpA, which are associated with iron sequestration under iron-limiting conditions (74, 86). It has proved more difficult to identify genes under Fur control that are activated in response to high levels of iron. Attempts to generate a Fur null mutant have so far been unsuccessful, which may indicate that Fur plays a critical role in gonococcal survival (232). However, fur has since been successfully deleted in N. meningitidis (65), and attempts to disrupt Fur in N. gonorrhoeae may have failed due to a polar effect on an essential downstream gene. Many iron-responsive genes that have been identified in N. gonorrhoeae may not be directly regulated by Fur. However, analysis of N. meningitidis has identified at least 15 genes whose levels are increased in the presence of high iron levels and which contain a Fur box in their promoter regions (97). These genes include sodB, recN (see below), aniA, and norB. BfrAB (see above) is also potentially regulated by Fur (49).
In E. coli, fur autoregulates its expression in response to iron levels, but it is also controlled by OxyR and the SoxR/S system and is induced by oxidative stress (264). By lowering iron uptake during oxidative stress, E. coli reduces the potential damage that would be caused by Fenton chemistry involving a combination of Fe(II) and hydrogen peroxide. Expression of fur does not appear to be regulated by OxyR or PerR in N. gonorrhoeae, as indicated by microarray analysis (see below) (256) (Seib et al., submitted). However, fur and several of the genes within the Fur regulon are upregulated in response to hydrogen peroxide (226).
The N. gonorrhoeae transcriptome response to hydrogen peroxide, described by Stohl and coworkers (226), confirms a link between iron and hydrogen peroxide regulation; however, a more detailed knowledge of individual regulons is required before the pattern of gene expression following the addition of peroxide can be understood fully. This problem is highlighted by the observation that upon the addition of hydrogen peroxide to N. gonorrhoeae there was increased expression of genes that are repressed by Fur (iron acquisition genes) and those that are probably activated by Fur (sodB, recN). Assuming that Fe-Fur is the active form of this transcription factor, it is hard to see how derepression and induction of genes can occur at the same time. It is notable that the collection of data for this experiment involved RNA isolation after a 15-min exposure to hydrogen peroxide (226). As a consequence, there is the possibility that complex changes in gene expression, not to mention changes in mRNA stability, may have been missed in this single-time-point analysis. Of particular relevance here may be changes in the intracellular iron pool in response to oxidative stress. The complex pattern of gene expression in response to hydrogen peroxide highlights the need for investigation of the kinetics of changes in gene expression at a global level and for careful analysis of these regulons.
DNA Repair Mechanisms
It has been suggested that free radicals generated during aerobic respiration are a major source of DNA damage in N. gonorrhoeae (142). N. gonorrhoeae has a network of DNA repair strategies that includes base excision, nucleotide excision, and mismatch and recombinational repair mechanisms. Comparison of Neisseria DNA repair genes with the well-characterized DNA repair mechanisms of E. coli has indicated that there are no gross differences in the repair capabilities of these organisms, with the exception of the absence of the SOS response in Neisseria (reviewed in reference 141).
PriA, a helicase of N. gonorrhoeae, is predicted to deal directly with oxidative stress-induced DNA damage through its role in restarting DNA replication at stalled replication forks (Table 1) (142). A priA mutant strain of N. gonorrhoeae has decreased DNA repair capability and is sensitive to hydrogen peroxide and cumene hydroperoxide killing relative to the wild-type parental strain (142). RecN, involved in repair of damaged DNA (220), also appears to be directly involved in defense against oxidative stress in N. gonorrhoeae (Table 1). A recN mutant strain has increased sensitivity to hydrogen peroxide and PMN killing (226). RecN is positively regulated by Fur (described above) (210) and hydrogen peroxide (226).
Defenses against RNS
Bacterial responses to RNS, while not as well characterized as the responses to ROS, are increasingly recognized as being diverse and critical for bacterial survival. In many cases, RNS resistance mechanisms overlap defenses against ROS; for example, in E. coli, SoxR is activated by both superoxide (96, 152) and nitric oxide (69, 182). The prominent nitric oxide-detoxifying enzymes in E. coli appear to be flavohemoglobin (HmpA) and the flavorubredoxin/flavorubredoxin reductase (NorVW) system, regulated by a nitric oxide response regulator, NorR (175). These systems do not seem to exist in N. gonorrhoeae. The periplasmic nitrite reductase (NrfA) of E. coli has also been shown to reduce nitric oxide (190). This enzyme is a multiheme cytochrome and is distinct from the gonococcal nitrite reductase, AniA (Fig. 2B; see above). Nitric oxide reductase, which catalyzes the reduction of nitric oxide to the less toxic compound nitrous oxide, plays a major role in protecting organisms from nitric oxide (Fig. 2B) (reviewed in reference 267). A knockout mutation of nitric oxide reductase of P. stutzeri is lethal, but the effect can be suppressed by a further mutation that inactivates the nitric oxide generator (267). The nitric oxide reductase (NorB) of N. meningitidis is able to counteract toxicity due to exogenously added nitric oxide (6). A NorB mutant of N. gonorrhoeae is still resistant to nitric oxide (120), indicating that there are additional detoxification pathways present in the bacterium. Cytochrome c′ (CycP; NG108), an outer membrane lipoprotein that binds nitric oxide in vitro, is believed to protect N. gonorrhoeae from endogenously and/or exogenously generated nitrosative stress (Fig. 2B) (238). A cycP mutant strain of N. gonorrhoeae has an extended lag phase during microaerobic growth in the presence of nitrite. It is suggested that the constitutive synthesis of cytochrome c′ provides an instant defense by binding nitric oxide until NorB has accumulated to levels sufficient to reduce nitric oxide and by preventing the generation of peroxynitrite from free nitric oxide and superoxide (238). Cytochrome c′ has also been shown to bind nitric oxide and to act as a buffer against this toxic radical species in N. meningitidis (6) and P. denitrificans (249). However, cytochrome c′ may be of limited defense against cytoplasmic nitric oxide.
AhpC is an NADPH-dependent alkyl hydroperoxide reductase, reducing an alkyl (or hydrogen) peroxide to water and alcohol (or water). In both E. coli and S. enterica serovar Typhimurium, AhpC also acts as a peroxynitrite reductase (35), but no AhpC protein is annotated in the gonococcal genome. However, the bacterioferritin comigratory protein (Bcp; NG0328) of N. gonorrhoeae is highly similar in sequence to AhpC of E. coli (27% identical and 45% similar over 104 of 167 amino acids) and may be functionally similar. The methionine sulfoxide reductase (MsrA) in the cytoplasm of E. coli has also been proposed to be involved in the repair of methionines damaged by peroxynitrite (225), representing an indirect defense against nitric oxide. It is notable that N. gonorrhoeae possesses a methionine sulfoxide reductase (MsrA/B; see above) in its outer membrane. Although the gonococcal msrA/B mutant was shown to be highly sensitive to oxidative stress, it has not been tested in its sensitivity to RNS (221).
Regulation of RNS defenses.
OxyR and SoxRS are the established regulators of the hydrogen peroxide and superoxide responses in E. coli (79). Both of these regulators have been shown to also respond to RNS (66, 109). It is worth emphasizing that the mechanisms and roles of these regulators differ among bacterial systems. Indeed, N. gonorrhoeae does not appear to possess the SoxRS system, and it is unusual in that its OxyR protein is a repressor of catalase (235) rather than an activator, as in enteric bacteria (54). Although SoxR has been suggested to have a role in nitric oxide sensing, recent microarray experiments with E. coli have shown that the response to nitrosative stress is controlled primarily by NorR (the regulator of NorV/W) and Fur (175). N. gonorrhoeae lacks the NorR regulator and NorV/W, and this has led to a search for the systems that protect this bacterium against RNS.
Recently, a novel MerR-like transcription factor, NmlR (for “Neisseria MerR-like Regulator”) (NG0602), which is an activator upon disulfide or redox stress and appears to be a key regulator in the defense against RNS, was identified in N. gonorrhoeae (Fig. 2B) (139). NmlR regulates the expression of adhC (NG0601), which encodes a zinc-dependent alcohol dehydrogenase (AdhC) that appears to be able to catalyze the reduction of S-nitrosoglutathione (GSNO) (Fig. 2A). This enzyme is present in both eukaryotes and E. coli, where it has demonstrated activity to remove GSNO (154), although its regulation is different. GSNO is a spontaneously formed adduct between reduced glutathione and nitric oxide; AdhC activity therefore controls the level of S-nitrosylated proteins and provides defense against the stresses exhibited by RNS. An N. gonorrhoeae nmlR mutant was more susceptible to killing by cumene hydroperoxide and diamide than were wild-type cells and had decreased growth under microaerophilic conditions (139). The nmlR mutant is also sensitive to killing by nitric oxide (A. J. Potter, S. P. Kidd, and A. G. McEwan, unpublished observations). The current model for the action of NmlR is that it is a Zn-dependent transcription factor which acts as a repressor of gene expression when it is in the holo form. Upon disulfide stress induced by a variety of electrophiles, such as nitric oxide, aldehydes, or peroxides, it is proposed that NmlR loses Zn and the apo form, which may contain at least one disulfide bridge, acts as an activator of gene expression. This represents a classical MerR-like response mechanism, the switch from a repressor to an activator.
NmlR also regulates a metal ion efflux pump (annotated as CopA; NG0579) and an annotated thioredoxin reductase (TrxB; NG0580) (see above), although the role of these proteins in this novel defense system is not yet defined. Analysis of microbial genomes has defined an NmlR subfamily of the MerR-like regulators, all of which are associated with adhC-like genes (139). MerR family proteins do not typically sense redox changes or ROS in the cell, with the exception of SoxR, which contains a superoxide-sensitive iron-sulfur cluster (189).
CONCLUDING REMARKS
Our understanding of the oxidative stress response of N. gonorrhoeae has greatly advanced in recent years. This understanding has continuing importance, as gonorrhea remains a serious health risk and has been linked to infertility and increased transmission and susceptibility to human immunodeficiency virus infection (60, 88, 147, 151, 248). Central to this bacterium's ability to persist and cause disease is an ability to overcome the oxidative stress generated in and around the host-pathogen environment.
It has become evident that N. gonorrhoeae has evolved complex, and in some cases novel, mechanisms to cope with oxidative and nitrosative stress (Table 1, Fig. 2). The primary defenses used by N. gonorrhoeae against ROS include accumulation of manganese by the MntABC transport system (236), unusually high catalase and peroxidase activities (9, 132, 213, 239, 262), and very high concentrations of GSH (9). However, further work is required to better understand the RNS defenses of this organism. Five environmental sensors that are involved in the oxidative stress response have been described to date: the superoxide-dependent OxyR (235), the Mn-dependent PerR (256), the Zn-dependent thiol-based NmlR (139), the iron-dependent Fur (23, 86, 210), and the oxygen-dependent FNR (153, 239). Further characterization of the regulons of these environmental sensors will provide a better understanding of the coordinated response of N. gonorrhoeae to oxidative and nitrosative stress.
It is clear that N. gonorrhoeae has mechanisms to defend against ROS and/or RNS at the cell surface, in the periplasm, and in the cytoplasm (Fig. 2). N. gonorrhoeae has the ability to defend against nitric oxide in these three cellular locations (Fig. 2B). There are no obvious dedicated defense systems against superoxide in the outer membrane, but Sco, which faces the periplasm, may provide some protection against this form of ROS (211). It is interesting that the pKa for the peroxyl-superoxide interconversion is 4.8; thus, under the acidic conditions of the cervix, the peroxyl radical (HO2·) represents about 50% of the superoxide species outside the cytoplasmic membrane. This neutral form of superoxide is much more permeable across the cytoplasmic membrane, where it forms superoxide by the loss of a proton. Adaptation to a combination of low pH and chronic oxidative stress in the female urogenital tract may be the reason the gonococcus has evolved such an unusual Mn-based defense system against superoxide. It is also interesting that the other superoxide defense system in N. gonorrhoeae (SodB) is regulated by iron and does not appear to have a key role in vitro. It is possible that under more iron-replete conditions, SodB would have a key role; it is tempting to speculate that this may be inside a PMN or cervical epithelial cell. Defense against peroxide is also critical, and again it appears that key defenses are associated with the cytoplasm (Fig. 2A). Finally, more work is required to identify the components that provide defense against secondary ROS and RNS products such as peroxynitrite and reactive aldehyde species.
The oxidative stress defenses of N. gonorrhoeae have typically been characterized based on the sensitivity of mutant strains to in vitro oxidative killing. However, recent studies using primary human cell lines have revealed interesting findings with respect to the biological significance of several of these oxidative stress responses. A set of mutant strains of N. gonorrhoeae, deficient in various oxidative stress defense mechanisms or regulatory systems, was not sensitive to PMN killing in an adherent PMN phagocytosis assay relative to the wild-type strain, despite the stimulation of the oxidative burst by N. gonorrhoeae (212). However, examination of the same panel of mutant strains in a primary human cervical epithelial cell line revealed that strains lacking PerR, MntC (256), OxyR, or Gor (Seib et al., submitted) had decreased survival. These findings raise important questions about the major species and sources of oxidative stress that N. gonorrhoeae is exposed to during infection. PMN-N. gonorrhoeae interactions have a controversial history (reviewed in references 199, 218, and 219), but it has been established that an oxidative burst is stimulated in response to infection by N. gonorrhoeae (22, 177, 219, 246). Therefore, it will be interesting to determine how N. gonorrhoeae survives PMN oxidative killing, in which several major characterized defenses in vitro do not seem to play a role in vivo. Intestinal and airway epithelial cells are able to kill bacteria by oxidative mechanisms (20, 64, 200, 207), and oxidative stress in urogenital epithelial cells may be a more significant source of oxidative stress than previously recognized. With the lack of relevant animal models, further studies using primary human cell lines may provide answers to some of the questions posed.
ADDENDUM IN PROOF
Recently, we have identified nmlR and adhC homologues in Streptococcus pneumoniae and Haemophilus influenzae. An adhC mutant of Streptococcus pneumoniae and Haemophilus influenzae is sensitive to killing by GSNO (S. P. Kidd, M. P. Jennings, and A. G. McEwan, unpublished observations). This contrasts with the situation for N. gonorrhoeae, for which an adhC mutant shows essentially the same sensitivity to GSNO as wild-type cells. This has led us to conclude either that AdhC is of minor importance in the defense of N. gonorrhoeae against NO killing or that there are redundant systems for NO defense in this bacterium. We note that Nikitovic and Holmgren (J. Biol. Chem. 271:19180-19185, 1996) have observed that GSNO can be cleaved by the reduced thioredoxin, generating NO and superoxide. It is interesting that the source of reducing power for thioredoxin, NADPH-dependent thioredoxin reductase, is encoded by a gene (trxB) that is also regulated by NmlR. Thus, an alternative route for dealing with GSNO may operate in N. gonorrhoeae.
Acknowledgments
This work was supported by Program Grant 284214 from the National Health and Medical Research Council of Australia. The laboratory of M.A.A. is supported by U.S. Public Health Service grants AI45728, AI43924, and AI38515, from NIAID.
REFERENCES
- 1.Aho, E. L., and L. P. Kelly. 1995. Identification of a glutathione peroxidase homolog in Neisseria meningitidis. DNA Seq. 6:55-60. [DOI] [PubMed] [Google Scholar]
- 2.Alcorn, T. M., H. Y. Zheng, M. R. Gunther, D. J. Hassett, and M. S. Cohen. 1994. Variation in hydrogen peroxide sensitivity between different strains of Neisseria gonorrhoeae is dependent on factors in addition to catalase activity. Infect. Immun. 62:2138-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, L. A. 2003. Mechanisms of pathogenesis: evasion of killing by polymorphonuclear leukocytes. Microbes Infect. 5:1329-1335. [DOI] [PubMed] [Google Scholar]
- 4.Allen, L. A. 2000. Modulating phagocyte activation: the pros and cons of Helicobacter pylori virulence factors. J. Exp. Med. 191:1451-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews, S. C., A. K. Robinson, and F. Rodriguez-Quinones. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215-237. [DOI] [PubMed] [Google Scholar]
- 6.Anjum, M. F., T. M. Stevanin, R. C. Read, and J. W. Moir. 2002. Nitric oxide metabolism in Neisseria meningitidis. J. Bacteriol. 184:2987-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apicella, M. A., M. Ketterer, F. K. Lee, D. Zhou, P. A. Rice, and M. S. Blake. 1996. The pathogenesis of gonococcal urethritis in men: confocal and immunoelectron microscopic analysis of urethral exudates from men infected with Neisseria gonorrhoeae. J. Infect. Dis. 173:636-646. [DOI] [PubMed] [Google Scholar]
- 8.Archibald, F. S., and M. N. Duong. 1984. Manganese acquisition by Lactobacillus plantarum. J. Bacteriol. 158:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Archibald, F. S., and M. N. Duong. 1986. Superoxide dismutase and oxygen toxicity defenses in the genus Neisseria. Infect. Immun. 51:631-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Archibald, F. S., and I. Fridovich. 1982. Investigations of the state of the manganese in Lactobacillus plantarum. Arch. Biochem. Biophys. 215:589-596. [DOI] [PubMed] [Google Scholar]
- 11.Archibald, F. S., and I. Fridovich. 1981. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J. Bacteriol. 145:442-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Archibald, F. S., and I. Fridovich. 1981. Manganese, superoxide dismutase, and oxygen tolerance in some lactic acid bacteria. J. Bacteriol. 146:928-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Archibald, F. S., and I. Fridovich. 1982. The scavenging of superoxide radical by manganous complexes: in vitro. Arch. Biochem. Biophys. 214:452-463. [DOI] [PubMed] [Google Scholar]
- 14.Aroutcheva, A., D. Gariti, M. Simon, S. Shott, J. Faro, J. A. Simoes, A. Gurguis, and S. Faro. 2001. Defense factors of vaginal lactobacilli. Am. J. Obstet. Gynecol. 185:375-379. [DOI] [PubMed] [Google Scholar]
- 15.Aslund, F., and J. Beckwith. 1999. The thioredoxin superfamily: redundancy, specificity, and gray-area genomics. J. Bacteriol. 181:1375-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babior, B. M., J. D. Lambeth, and W. Nauseef. 2002. The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 397:342-344. [DOI] [PubMed] [Google Scholar]
- 17.Balatri, E., L. Banci, I. Bertini, F. Cantini, and S. Ciofi-Baffoni. 2003. Solution structure of Sco1: a thioredoxin-like protein involved in cytochrome c oxidase assembly. Structure (Cambridge) 11:1431-1443. [DOI] [PubMed] [Google Scholar]
- 18.Bashyam, M. D., and S. E. Hasnain. 2004. The extracytoplasmic function sigma factors: role in bacterial pathogenesis. Infect. Genet. Evol. 4:301-308. [DOI] [PubMed] [Google Scholar]
- 19.Bateman, A., E. Birney, L. Cerruti, R. Durbin, L. Etwiller, S. R. Eddy, S. Griffiths-Jones, K. L. Howe, M. Marshall, and E. L. Sonnhammer. 2002. The Pfam protein families database. Nucleic Acids Res. 30:276-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Battistoni, A., F. Pacello, S. Folcarelli, M. Ajello, G. Donnarumma, R. Greco, M. G. Ammendolia, D. Touati, G. Rotilio, and P. Valenti. 2000. Increased expression of periplasmic Cu,Zn superoxide dismutase enhances survival of Escherichia coli invasive strains within nonphagocytic cells. Infect. Immun. 68:30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beinert, H., and P. J. Kiley. 1999. Fe-S proteins in sensing and regulatory functions. Curr. Opin. Chem. Biol. 3:152-157. [DOI] [PubMed] [Google Scholar]
- 22.Belland, R. J., T. Chen, J. Swanson, and S. H. Fischer. 1992. Human neutrophil response to recombinant neisserial Opa proteins. Mol. Microbiol. 6:1729-1737. [DOI] [PubMed] [Google Scholar]
- 23.Berish, S. A., S. Subbarao, C. Y. Chen, D. L. Trees, and S. A. Morse. 1993. Identification and cloning of a fur homolog from Neisseria gonorrhoeae. Infect. Immun. 61:4599-4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blake, M. S., and L. M. Wetzler. 1995. Vaccines for gonorrhea: where are we on the curve? Trends Microbiol. 3:469-474. [DOI] [PubMed] [Google Scholar]
- 25.Boslego, J. W., E. C. Tramont, R. C. Chung, D. G. McChesney, J. Ciak, J. C. Sadoff, M. V. Piziak, J. D. Brown, C. C. Brinton, Jr., S. W. Wood, et al. 1991. Efficacy trial of a parenteral gonococcal pilus vaccine in men. Vaccine 9:154-162. [DOI] [PubMed] [Google Scholar]
- 26.Boulanger, M. J., and M. E. Murphy. 2002. Crystal structure of the soluble domain of the major anaerobically induced outer membrane protein (AniA) from pathogenic Neisseria: a new class of copper-containing nitrite reductases. J. Mol. Biol. 315:1111-1127. [DOI] [PubMed] [Google Scholar]
- 27.Bovre, K. 1984. Family VIII. Neisseriaceae Prevot 1933, 199, p. 288-297. In N. R. Krieg and J. G. Hold (ed.), Bergey's manual of systemic bacteriology, vol. 1. Williams and Wilkins, Baltimore, Md. [Google Scholar]
- 28.Brenot, A., K. Y. King, B. Janowiak, O. Griffith, and M. G. Caparon. 2004. Contribution of glutathione peroxidase to the virulence of Streptococcus pyogenes. Infect. Immun. 72:408-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Britigan, B. E., Y. Chai, and M. S. Cohen. 1985. Effects of human serum on the growth and metabolism of Neisseria gonorrhoeae: an alternative view of serum. Infect. Immun. 50:738-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Britigan, B. E., and M. S. Cohen. 1986. Effects of human serum on bacterial competition with neutrophils for molecular oxygen. Infect. Immun. 52:657-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Britigan, B. E., D. Klapper, T. Svendsen, and M. S. Cohen. 1988. Phagocyte-derived lactate stimulates oxygen consumption by Neisseria gonorrhoeae. An unrecognized aspect of the oxygen metabolism of phagocytosis. J. Clin. Investig. 81:318-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brot, N., and H. Weissbach. 1983. Biochemistry and physiological role of methionine sulfoxide residues in proteins. Arch. Biochem. Biophys. 223:271-281. [DOI] [PubMed] [Google Scholar]
- 33.Brot, N., L. Weissbach, J. Werth, and H. Weissbach. 1981. Enzymatic reduction of protein-bound methionine sulfoxide. Proc. Natl. Acad. Sci. USA 78:2155-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown, N. L., J. V. Stoyanov, S. P. Kidd, and J. L. Hobman. 2003. The MerR family of transcriptional regulators. FEMS Microbiol. Rev. 27:145-163. [DOI] [PubMed] [Google Scholar]
- 35.Bryk, R., P. Griffin, and C. Nathan. 2000. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature 407:211-215. [DOI] [PubMed] [Google Scholar]
- 36.Bsat, N., A. Herbig, L. Casillas-Martinez, P. Setlow, and J. D. Helmann. 1998. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol. Microbiol. 29:189-198. [DOI] [PubMed] [Google Scholar]
- 37.Burg, N. D., and M. H. Pillinger. 2001. The neutrophil: function and regulation in innate and humoral immunity. Clin. Immunol. 99:7-17. [DOI] [PubMed] [Google Scholar]
- 38.Campos, E. G., M. Hermes-Lima, J. M. Smith, and R. K. Prichard. 1999. Characterisation of Fasciola hepatica cytochrome c peroxidase as an enzyme with potential antioxidant activity in vitro. Int. J. Parasitol. 29:655-662. [DOI] [PubMed] [Google Scholar]
- 39.Cannon, J. G. 1989. Conserved lipoproteins of pathogenic Neisseria species bearing the H.8 epitope: lipid-modified azurin and H.8 outer membrane protein. Clin. Microbiol. Rev. 2(Suppl.):S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canters, G. W. 1987. The azurin gene from Pseudomonas aeruginosa codes for a pre-protein with a signal peptide. Cloning and sequencing of the azurin gene. FEBS Lett. 212:168-172. [DOI] [PubMed] [Google Scholar]
- 41.Cardinale, J. A., and V. L. Clark. 2005. Determinants of nitric oxide steady-state levels during anaerobic respiration by Neisseria gonorrhoeae. Mol. Microbiol. 58:177-188. [DOI] [PubMed] [Google Scholar]
- 42.Carlioz, A., and D. Touati. 1986. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 5:623-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carmel-Harel, O., and G. Storz. 2000. Roles of the glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Annu. Rev. Microbiol. 54:439-461. [DOI] [PubMed] [Google Scholar]
- 44.Carreras, M. C., G. A. Pargament, S. D. Catz, J. J. Poderoso, and A. Boveris. 1994. Kinetics of nitric oxide and hydrogen peroxide production and formation of peroxynitrite during the respiratory burst of human neutrophils. FEBS Lett. 341:65-68. [DOI] [PubMed] [Google Scholar]
- 45.Casey, S. G., W. M. Shafer, and J. K. Spitznagel. 1985. Anaerobiosis increases resistance of Neisseria gonorrhoeae to O2-independent antimicrobial proteins from human polymorphonuclear granulocytes. Infect. Immun. 47:401-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Casey, S. G., W. M. Shafer, and J. K. Spitznagel. 1986. Neisseria gonorrhoeae survive intraleukocytic oxygen-independent antimicrobial capacities of anaerobic and aerobic granulocytes in the presence of pyocin lethal for extracellular gonococci. Infect. Immun. 52:384-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cha, M. K., H. K. Kim, and I. H. Kim. 1995. Thioredoxin-linked “thiol peroxidase” from periplasmic space of Escherichia coli. J. Biol. Chem. 270:28635-28641. [DOI] [PubMed] [Google Scholar]
- 48.Chae, H. Z., K. Robison, L. B. Poole, G. Church, G. Storz, and S. G. Rhee. 1994. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc. Natl. Acad. Sci. USA 91:7017-7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen, C. Y., and S. A. Morse. 1999. Neisseria gonorrhoeae bacterioferritin: structural heterogeneity, involvement in iron storage and protection against oxidative stress. Microbiology 145:2967-2975. [DOI] [PubMed] [Google Scholar]
- 50.Chen, L., L. Keramati, and J. D. Helmann. 1995. Coordinate regulation of Bacillus subtilis peroxide stress genes by hydrogen peroxide and metal ions. Proc. Natl. Acad. Sci. USA 92:8190-8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheton, P. L., and F. S. Archibald. 1988. Manganese complexes and the generation and scavenging of hydroxyl free radicals. Free Radic. Biol. Med. 5:325-333. [DOI] [PubMed] [Google Scholar]
- 52.Chinenov, Y. V. 2000. Cytochrome c oxidase assembly factors with a thioredoxin fold are conserved among prokaryotes and eukaryotes. J. Mol. Med. 78:239-242. [DOI] [PubMed] [Google Scholar]
- 53.Christman, M. F., R. W. Morgan, F. S. Jacobson, and B. N. Ames. 1985. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell 41:753-762. [DOI] [PubMed] [Google Scholar]
- 54.Christman, M. F., G. Storz, and B. N. Ames. 1989. OxyR, a positive regulator of hydrogen peroxide-inducible genes in Escherichia coli and Salmonella typhimurium, is homologous to a family of bacterial regulatory proteins. Proc. Natl. Acad. Sci. USA 86:3484-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clark, V. L., L. A. Campbell, D. A. Palermo, T. M. Evans, and K. W. Klimpel. 1987. Induction and repression of outer membrane proteins by anaerobic growth of Neisseria gonorrhoeae. Infect. Immun. 55:1359-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clark, V. L., J. S. Knapp, S. Thompson, and K. W. Klimpel. 1988. Presence of antibodies to the major anaerobically induced gonococcal outer membrane protein in sera from patients with gonococcal infections. Microb. Pathog. 5:381-390. [DOI] [PubMed] [Google Scholar]
- 57.Coassin, M., F. Ursini, and A. Bindoli. 1992. Antioxidant effect of manganese. Arch. Biochem. Biophys. 299:330-333. [DOI] [PubMed] [Google Scholar]
- 58.Cohen, M. S., J. G. Cannon, A. E. Jerse, L. M. Charniga, S. F. Isbey, and L. G. Whicker. 1994. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J. Infect. Dis. 169:532-537. [DOI] [PubMed] [Google Scholar]
- 59.Cohen, M. S., Y. Chai, B. E. Britigan, W. McKenna, J. Adams, T. Svendsen, K. Bean, D. J. Hassett, and P. F. Sparling. 1987. Role of extracellular iron in the action of the quinone antibiotic streptonigrin: mechanisms of killing and resistance of Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 31:1507-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen, M. S., I. F. Hoffman, R. A. Royce, P. Kazembe, J. R. Dyer, C. C. Daly, D. Zimba, P. L. Vernazza, M. Maida, S. A. Fiscus, J. J. Eron, Jr., and the AIDSCAP Malawi Research Group. 1997. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet 349:1868-1873. [DOI] [PubMed] [Google Scholar]
- 61.Compan, I., and D. Touati. 1993. Interaction of six global transcription regulators in expression of manganese superoxide dismutase in Escherichia coli K-12. J. Bacteriol. 175:1687-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cramm, R., R. A. Siddiqui, and B. Friedrich. 1997. Two isofunctional nitric oxide reductases in Alcaligenes eutrophus H16. J. Bacteriol. 179:6769-6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Darwin, A. J., E. C. Ziegelhoffer, P. J. Kiley, and V. Stewart. 1998. Fnr, NarP, and NarL regulation of Escherichia coli K-12 napF (periplasmic nitrate reductase) operon transcription in vitro. J. Bacteriol. 180:4192-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deitch, E. A., Y. Haskel, N. Cruz, D. Xu, and P. R. Kvietys. 1995. Caco-2 and IEC-18 intestinal epithelial cells exert bactericidal activity through an oxidant-dependent pathway. Shock 4:345-350. [DOI] [PubMed] [Google Scholar]
- 65.Delany, I., R. Ieva, C. Alaimo, R. Rappuoli, and V. Scarlato. 2003. The iron-responsive regulator Fur is transcriptionally autoregulated and not essential in Neisseria meningitidis. J. Bacteriol. 185:6032-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Demple, B. 1999. Radical ideas: genetic responses to oxidative stress. Clin. Exp. Pharmacol. Physiol. 26:64-68. [DOI] [PubMed] [Google Scholar]
- 67.Dhandayuthapani, S., M. W. Blaylock, C. M. Bebear, W. G. Rasmussen, and J. B. Baseman. 2001. Peptide methionine sulfoxide reductase (MsrA) is a virulence determinant in Mycoplasma genitalium. J. Bacteriol. 183:5645-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dickinson, E. K., D. L. Adams, E. A. Schon, and D. M. Glerum. 2000. A human SCO2 mutation helps define the role of Sco1p in the cytochrome oxidase assembly pathway. J. Biol. Chem. 275:26780-26785. [DOI] [PubMed] [Google Scholar]
- 69.Ding, H., and B. Demple. 2000. Direct nitric oxide signal transduction via nitrosylation of iron-sulfur centers in the SoxR transcription activator. Proc. Natl. Acad. Sci. USA 97:5146-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dintilhac, A., and J. P. Claverys. 1997. The adc locus, which affects competence for genetic transformation in Streptococcus pneumoniae, encodes an ABC transporter with a putative lipoprotein homologous to a family of streptococcal adhesins. Res. Microbiol. 148:119-131. [DOI] [PubMed] [Google Scholar]
- 71.Dodd, F. E., S. S. Hasnain, W. N. Hunter, Z. H. Abraham, M. Debenham, H. Kanzler, M. Eldridge, R. R. Eady, R. P. Ambler, and B. E. Smith. 1995. Evidence for two distinct azurins in Alcaligenes xylosoxidans (NCIMB 11015): potential electron donors to nitrite reductase. Biochemistry 34:10180-10186. [DOI] [PubMed] [Google Scholar]
- 72.Dubrac, S., and D. Touati. 2000. Fur-positive regulation of iron superoxide dismutase in Escherichia coli: functional analysis of the sodB promoter. J. Bacteriol. 182:3802-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dubrac, S., and D. Touati. 2002. Fur-mediated transcriptional and post-transcriptional regulation of FeSOD expression in Escherichia coli. Microbiology 148:147-156. [DOI] [PubMed] [Google Scholar]
- 74.Ducey, T. F., M. B. Carson, J. Orvis, A. P. Stintzi, and D. W. Dyer. 2005. Identification of the iron-responsive genes of Neisseria gonorrhoeae by microarray analysis in defined medium. J. Bacteriol. 187:4865-4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Edwards, J. L., J. Q. Shao, K. A. Ault, and M. A. Apicella. 2000. Neisseria gonorrhoeae elicits membrane ruffling and cytoskeletal rearrangements upon infection of primary human endocervical and ectocervical cells. Infect. Immun. 68:5354-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Emerit, J., C. Beaumont, and F. Trivin. 2001. Iron metabolism, free radicals, and oxidative injury. Biomed. Pharmacother. 55:333-339. [DOI] [PubMed] [Google Scholar]
- 77.Eschenbach, D. A., P. R. Davick, B. L. Williams, S. J. Klebanoff, K. Young-Smith, C. M. Critchlow, and K. K. Holmes. 1989. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J. Clin. Microbiol. 27:251-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Escolar, L., J. Perez-Martin, and V. de Lorenzo. 1999. Opening the iron box: transcriptional metalloregulation by the Fur protein. J. Bacteriol. 181:6223-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Farr, S. B., and T. Kogoma. 1991. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Rev. 55:561-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Farver, O., and I. Pecht. 1984. The reactivity of copper sites in the ‘blue’ copper proteins, p. 183-214. In R. Lontie (ed.), Copper proteins and copper enzymes. CRC Press Inc., Boca Raton, Fla.
- 81.Fridovich, I. 1995. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 64:97-112. [DOI] [PubMed] [Google Scholar]
- 82.Fu, H. S., D. J. Hassett, and M. S. Cohen. 1989. Oxidant stress in Neisseria gonorrhoeae: adaptation and effects on l-(+)-lactate dehydrogenase activity. Infect. Immun. 57:2173-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fulop, V., C. J. Ridout, C. Greenwood, and J. Hajdu. 1995. Crystal structure of the di-haem cytochrome c peroxidase from Pseudomonas aeruginosa. Structure 3:1225-1233. [DOI] [PubMed] [Google Scholar]
- 84.Gardner, P. R., and I. Fridovich. 1991. Superoxide sensitivity of the Escherichia coli 6-phosphogluconate dehydratase. J. Biol. Chem. 266:1478-1483. [PubMed] [Google Scholar]
- 85.Gardner, P. R., and I. Fridovich. 1991. Superoxide sensitivity of the Escherichia coli aconitase. J. Biol. Chem. 266:19328-19333. [PubMed] [Google Scholar]
- 86.Genco, C. A., and P. J. Desai. 1996. Iron acquisition in the pathogenic Neisseria. Trends Microbiol. 4:179-184. [DOI] [PubMed] [Google Scholar]
- 87.Gerbase, A. C., J. T. Rowley, D. H. Heymann, S. F. Berkley, and P. Piot. 1998. Global prevalence and incidence estimates of selected curable STDs. Sex. Transm. Infect. 74(Suppl. 1):S12-S16. [PubMed] [Google Scholar]
- 88.Ghys, P. D., K. Fransen, M. O. Diallo, V. Ettiegne-Traore, I. M. Coulibaly, K. M. Yeboue, M. L. Kalish, C. Maurice, J. P. Whitaker, A. E. Greenberg, and M. Laga. 1997. The associations between cervicovaginal HIV shedding, sexually transmitted diseases and immunosuppression in female sex workers in Abidjan, Cote d'Ivoire. AIDS 11:F85-F93. [DOI] [PubMed] [Google Scholar]
- 89.Glerum, D. M., A. Shtanko, and A. Tzagoloff. 1996. SCO1 and SCO2 act as high copy suppressors of a mitochondrial copper recruitment defect in Saccharomyces cerevisiae. J. Biol. Chem. 271:20531-20535. [DOI] [PubMed] [Google Scholar]
- 90.Gonchar, M. V., L. B. Kostryk, and A. A. Sibirny. 1997. Cytochrome c peroxidase from a methylotrophic yeast: physiological role and isolation. Appl. Microbiol. Biotechnol. 48:454-458. [DOI] [PubMed] [Google Scholar]
- 91.Gonzalez-Flecha, B., and B. Demple. 1995. Metabolic sources of hydrogen peroxide in aerobically growing Escherichia coli. J. Biol. Chem. 270:13681-13687. [DOI] [PubMed] [Google Scholar]
- 92.Goodhew, C. F., I. B. Wilson, D. J. Hunter, and G. W. Pettigrew. 1990. The cellular location and specificity of bacterial cytochrome c peroxidases. Biochem. J. 271:707-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gort, A. S., and J. A. Imlay. 1998. Balance between endogenous superoxide stress and antioxidant defenses. J. Bacteriol. 180:1402-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gotschlich, E. C., and M. E. Seiff. 1987. Identification and gene structure of an azurin-like protein with a lipoprotein signal peptide in Neisseria gonorrhoeae. FEMS Microbiol. Lett. 43:253-255. [Google Scholar]
- 95.Greenberg, J. T., and B. Demple. 1989. A global response induced in Escherichia coli by redox-cycling agents overlaps with that induced by peroxide stress. J. Bacteriol. 171:3933-3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Greenberg, J. T., P. Monach, J. H. Chou, P. D. Josephy, and B. Demple. 1990. Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in Escherichia coli. Proc. Natl. Acad. Sci. USA 87:6181-6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grifantini, R., S. Sebastian, E. Frigimelica, M. Draghi, E. Bartolini, A. Muzzi, R. Rappuoli, G. Grandi, and C. A. Genco. 2003. Identification of iron-activated and -repressed Fur-dependent genes by transcriptome analysis of Neisseria meningitidis group B. Proc. Natl. Acad. Sci. USA 100:9542-9547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guedon, E., C. M. Moore, Q. Que, T. Wang, R. W. Ye, and J. D. Helmann. 2003. The global transcriptional response of Bacillus subtilis to manganese involves the MntR, Fur, TnrA and sigmaB regulons. Mol. Microbiol. 49:1477-1491. [DOI] [PubMed] [Google Scholar]
- 99.Guo, J., and B. D. Lemire. 2003. The ubiquinone-binding site of the Saccharomyces cerevisiae succinate-ubiquinone oxidoreductase is a source of superoxide. J. Biol. Chem. 278:47629-47635. [DOI] [PubMed] [Google Scholar]
- 100.Hahn, J. S., S. Y. Oh, K. F. Chater, Y. H. Cho, and J. H. Roe. 2000. H2O2-sensitive fur-like repressor CatR regulating the major catalase gene in Streptomyces coelicolor. J. Biol. Chem. 275:38254-38260. [DOI] [PubMed] [Google Scholar]
- 101.Hahn, J. S., S. Y. Oh, and J. H. Roe. 2002. Role of OxyR as a peroxide-sensing positive regulator in Streptomyces coelicolor A3(2). J. Bacteriol. 184:5214-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Halsey, T. A., A. Vazquez-Torres, D. J. Gravdahl, F. C. Fang, and S. J. Libby. 2004. The ferritin-like Dps protein is required for Salmonella enterica serovar Typhimurium oxidative stress resistance and virulence. Infect. Immun. 72:1155-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hampton, M. B., A. J. Kettle, and C. C. Winterbourn. 1998. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92:3007-3017. [PubMed] [Google Scholar]
- 104.Han, D., E. Williams, and E. Cadenas. 2001. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem. J. 353:411-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Harris, A. G., J. E. Wilson, S. J. Danon, M. F. Dixon, K. Donegan, and S. L. Hazell. 2003. Catalase (KatA) and KatA-associated protein (KapA) are essential to persistent colonization in the Helicobacter pylori SS1 mouse model. Microbiology 149:665-672. [DOI] [PubMed] [Google Scholar]
- 106.Hassett, D. J., E. B. Bradley, B. E. Britigan, T. Svendsen, G. M. Rosen, and M. S. Cohen. 1987. Bacteria form intracellular free radicals in response to paraquat and streptonigrin. J. Biol. Chem. 262:13404-13408. [PubMed] [Google Scholar]
- 107.Hassett, D. J., L. Charniga, and M. S. Cohen. 1990. recA and catalase in H2O2-mediated toxicity in Neisseria gonorrhoeae. J. Bacteriol. 172:7293-7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hassouni, M. E., J. P. Chambost, D. Expert, F. Van Gijsegem, and F. Barras. 1999. The minimal gene set member msrA, encoding peptide methionine sulfoxide reductase, is a virulence determinant of the plant pathogen Erwinia chrysanthemi. Proc. Natl. Acad. Sci. USA 96:887-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hausladen, A., C. T. Privalle, T. Keng, J. DeAngelo, and J. S. Stamler. 1996. Nitrosative stress: activation of the transcription factor OxyR. Cell 86:719-729. [DOI] [PubMed] [Google Scholar]
- 110.Helmann, J. D., M. F. Wu, A. Gaballa, P. A. Kobel, M. M. Morshedi, P. Fawcett, and C. Paddon. 2003. The global transcriptional response of Bacillus subtilis to peroxide stress is coordinated by three transcription factors. J. Bacteriol. 185:243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Herbig, A. F., and J. D. Helmann. 2001. Roles of metal ions and hydrogen peroxide in modulating the interaction of the Bacillus subtilis PerR peroxide regulon repressor with operator DNA. Mol. Microbiol. 41:849-859. [DOI] [PubMed] [Google Scholar]
- 112.Higgins, C. F. 1992. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8:67-113. [DOI] [PubMed] [Google Scholar]
- 113.Hillier, S. L., M. A. Krohn, S. J. Klebanoff, and D. A. Eschenbach. 1992. The relationship of hydrogen peroxide-producing lactobacilli to bacterial vaginosis and genital microflora in pregnant women. Obstet. Gynecol. 79:369-373. [DOI] [PubMed] [Google Scholar]
- 114.Hoehn, G. T., and V. L. Clark. 1992. Isolation and nucleotide sequence of the gene (aniA) encoding the major anaerobically induced outer membrane protein of Neisseria gonorrhoeae. Infect. Immun. 60:4695-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hoehn, G. T., and V. L. Clark. 1992. The major anaerobically induced outer membrane protein of Neisseria gonorrhoeae, Pan 1, is a lipoprotein. Infect. Immun. 60:4704-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hofmann, B., H. J. Hecht, and L. Flohe. 2002. Peroxiredoxins. J. Biol. Chem. 383:347-364. [DOI] [PubMed] [Google Scholar]
- 117.Horsburgh, M. J., M. O. Clements, H. Crossley, E. Ingham, and S. J. Foster. 2001. PerR controls oxidative stress resistance and iron storage proteins and is required for virulence in Staphylococcus aureus. Infect. Immun. 69:3744-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Horsburgh, M. J., S. J. Wharton, A. G. Cox, E. Ingham, S. Peacock, and S. J. Foster. 2002. MntR modulates expression of the PerR regulon and superoxide resistance in Staphylococcus aureus through control of manganese uptake. Mol. Microbiol. 44:1269-1286. [DOI] [PubMed] [Google Scholar]
- 119.Householder, T. C., W. A. Belli, S. Lissenden, J. A. Cole, and V. L. Clark. 1999. cis- and trans-acting elements involved in regulation of aniA, the gene encoding the major anaerobically induced outer membrane protein in Neisseria gonorrhoeae. J. Bacteriol. 181:541-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Householder, T. C., E. M. Fozo, J. A. Cardinale, and V. L. Clark. 2000. Gonococcal nitric oxide reductase is encoded by a single gene, norB, which is required for anaerobic growth and is induced by nitric oxide. Infect. Immun. 68:5241-5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hu, W., L. De Smet, G. Van Driessche, R. G. Bartsch, T. E. Meyer, M. A. Cusanovich, and J. Van Beeumen. 1998. Characterization of cytochrome c-556 from the purple phototrophic bacterium Rhodobacter capsulatus as a cytochrome-c peroxidase. Eur. J. Biochem. 258:29-36. [DOI] [PubMed] [Google Scholar]
- 122.Hughes, V. L., and S. L. Hillier. 1990. Microbiologic characteristics of Lactobacillus products used for colonization of the vagina. Obstet. Gynecol. 75:244-248. [PubMed] [Google Scholar]
- 123.Hwang, C. S., G. E. Rhie, J. H. Oh, W. K. Huh, H. S. Yim, and S. O. Kang. 2002. Copper- and zinc-containing superoxide dismutase (Cu/ZnSOD) is required for the protection of Candida albicans against oxidative stresses and the expression of its full virulence. Microbiology 148:3705-3713. [DOI] [PubMed] [Google Scholar]
- 124.Imlay, J. A. 1995. A metabolic enzyme that rapidly produces superoxide, fumarate reductase of Escherichia coli. J. Biol. Chem. 270:19767-19777. [PubMed] [Google Scholar]
- 125.Imlay, J. A. 2003. Pathways of oxidative damage. Annu. Rev. Microbiol. 57:395-418. [DOI] [PubMed] [Google Scholar]
- 126.Imlay, J. A., and I. Fridovich. 1991. Assay of metabolic superoxide production in Escherichia coli. J. Biol. Chem. 266:6957-6965. [PubMed] [Google Scholar]
- 127.Imlay, J. A., and S. Linn. 1987. Mutagenesis and stress responses induced in Escherichia coli by hydrogen peroxide. J. Bacteriol. 169:2967-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Imlay, K. R., and J. A. Imlay. 1996. Cloning and analysis of sodC, encoding the copper-zinc superoxide dismutase of Escherichia coli. J. Bacteriol. 178:2564-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Inaoka, T., Y. Matsumura, and T. Tsuchido. 1999. SodA and manganese are essential for resistance to oxidative stress in growing and sporulating cells of Bacillus subtilis. J. Bacteriol. 181:1939-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jacobson, F. S., R. W. Morgan, M. F. Christman, and B. N. Ames. 1989. An alkyl hydroperoxide reductase from Salmonella typhimurium involved in the defense of DNA against oxidative damage. Purification and properties. J. Biol. Chem. 264:1488-1496. [PubMed] [Google Scholar]
- 131.Johnson, S. R., B. M. Steiner, D. D. Cruce, G. H. Perkins, and R. J. Arko. 1993. Characterization of a catalase-deficient strain of Neisseria gonorrhoeae: evidence for the significance of catalase in the biology of N. gonorrhoeae. Infect. Immun. 61:1232-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Johnson, S. R., B. M. Steiner, and G. H. Perkins. 1996. Cloning and characterization of the catalase gene of Neisseria gonorrhoeae: use of the gonococcus as a host organism for recombinant DNA. Infect. Immun. 64:2627-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jurtshuk, P., and T. W. Milligan. 1974. Quantitation of the tetramethyl-p-phenylenediamine oxidase reaction in Neisseria species. Appl. Microbiol. 28:1079-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kakutani, T., H. Watanabe, K. Arima, and T. Beppu. 1981. A blue protein as an inactivating factor for nitrite reductase from Alcaligenes faecalis strain S-6. J. Biochem. (Tokyo) 89:463-472. [DOI] [PubMed] [Google Scholar]
- 135.Kargalioglu, Y., and J. A. Imlay. 1994. Importance of anaerobic superoxide dismutase synthesis in facilitating outgrowth of Escherichia coli upon entry into an aerobic habitat. J. Bacteriol. 176:7653-7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kawula, T. H., S. M. Spinola, D. G. Klapper, and J. G. Cannon. 1987. Localization of a conserved epitope and an azurin-like domain in the H.8 protein of pathogenic Neisseria. Mol. Microbiol. 1:179-185. [DOI] [PubMed] [Google Scholar]
- 137.Kehres, D. G., and M. E. Maguire. 2003. Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria. FEMS Microbiol. Rev. 27:263-290. [DOI] [PubMed] [Google Scholar]
- 138.Keyer, K., and J. A. Imlay. 1996. Superoxide accelerates DNA damage by elevating free-iron levels. Proc. Natl. Acad. Sci. USA 93:13635-13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kidd, S. P., A. J. Potter, M. A. Apicella, M. P. Jennings, and A. G. McEwan. 2005. NmlR of Neisseria gonorrhoeae: a novel redox responsive transcription factor from the MerR family. Mol. Microbiol. 57:1676-1689. [DOI] [PubMed] [Google Scholar]
- 140.Kim, K., I. H. Kim, K. Y. Lee, S. G. Rhee, and E. R. Stadtman. 1988. The isolation and purification of a specific “protector” protein which inhibits enzyme inactivation by a thiol/Fe(III)/O2 mixed-function oxidation system. J. Biol. Chem. 263:4704-4711. [PubMed] [Google Scholar]
- 141.Kline, K. A., E. V. Sechman, E. P. Skaar, and H. S. Seifert. 2003. Recombination, repair and replication in the pathogenic neisseriae: the 3 R's of molecular genetics of two human-specific bacterial pathogens. Mol. Microbiol. 50:3-13. [DOI] [PubMed] [Google Scholar]
- 142.Kline, K. A., and H. S. Seifert. 2005. Mutation of the priA gene of Neisseria gonorrhoeae affects DNA transformation and DNA repair. J. Bacteriol. 187:5347-5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Knapp, J. S., and V. L. Clark. 1984. Anaerobic growth of Neisseria gonorrhoeae coupled to nitrite reduction. Infect. Immun. 46:176-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kroll, J. S., P. R. Langford, K. E. Wilks, and A. D. Keil. 1995. Bacterial [Cu,Zn]-superoxide dismutase: phylogenetically distinct from the eukaryotic enzyme, and not so rare after all! Microbiology 141:2271-2279. [DOI] [PubMed] [Google Scholar]
- 145.Kroll, J. S., K. E. Wilks, J. L. Farrant, and P. R. Langford. 1998. Natural genetic exchange between Haemophilus and Neisseria: intergeneric transfer of chromosomal genes between major human pathogens. Proc. Natl. Acad. Sci. USA 95:12381-12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kudoh, S., K. Suzuki, M. Yamada, Q. Liu, S. Nakaji, and K. Sugawara. 1999. Contribution of nitric oxide synthase to human neutrophil chemiluminescence. Luminescence 14:335-339. [DOI] [PubMed] [Google Scholar]
- 147.Laga, M., N. Nzila, and J. Goeman. 1991. The interrelationship of sexually transmitted diseases and HIV infection: implications for the control of both epidemics in Africa. AIDS 5(Suppl. 1):S55-S63. [PubMed] [Google Scholar]
- 148.Leith, D. K., and S. A. Morse. 1980. Effect of dissolved oxygen on outer membrane protein composition of Neisseria gonorrhoeae grown in continuous culture. FEMS Microbiol. Lett. 7:191-194. [Google Scholar]
- 149.Lenaz, G. 2001. The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology. IUBMB Life 52:159-164. [DOI] [PubMed] [Google Scholar]
- 150.Levine, R. L., L. Mosoni, B. S. Berlett, and E. R. Stadtman. 1996. Methionine residues as endogenous antioxidants in proteins. Proc. Natl. Acad. Sci. USA 93:15036-15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Levine, W. C., V. Pope, A. Bhoomkar, P. Tambe, J. S. Lewis, A. A. Zaidi, C. E. Farshy, S. Mitchell, and D. F. Talkington. 1998. Increase in endocervical CD4 lymphocytes among women with nonulcerative sexually transmitted diseases. J. Infect. Dis. 177:167-174. [DOI] [PubMed] [Google Scholar]
- 152.Liochev, S. I., L. Benov, D. Touati, and I. Fridovich. 1999. Induction of the soxRS regulon of Escherichia coli by superoxide. J. Biol. Chem. 274:9479-9481. [DOI] [PubMed] [Google Scholar]
- 153.Lissenden, S., S. Mohan, T. Overton, T. Regan, H. Crooke, J. A. Cardinale, T. C. Householder, P. Adams, C. D. O'Conner, V. L. Clark, H. Smith, and J. A. Cole. 2000. Identification of transcription activators that regulate gonococcal adaptation from aerobic to anaerobic or oxygen-limited growth. Mol. Microbiol. 37:839-855. [DOI] [PubMed] [Google Scholar]
- 154.Liu, L., A. Hausladen, M. Zeng, L. Que, J. Heitman, J. S. Stamler, and D. Steverding. 2001. Nitrosative stress: protection by glutathione-dependent formaldehyde dehydrogenase. Redox Rep. 6:209-210. [DOI] [PubMed] [Google Scholar]
- 155.Liu, Y., S. K. Shaw, S. Ma, L. Yang, F. W. Luscinskas, and C. A. Parkos. 2004. Regulation of leukocyte transmigration: cell surface interactions and signaling events. J. Immunol. 172:7-13. [DOI] [PubMed] [Google Scholar]
- 156.Los Alamos National Laboratory. 2005. Neisseria gonorrhoeae database (http://www.stdgen.lanl.gov/stdgen/bacteria/ngon/index.html). Los Alamos National Laboratory (University of California/U.S. Department of Energy), Los Alamos, N.Mex.
- 157.Makarova, K. S., V. A. Ponomarev, and E. V. Koonin. 2001. Two C or not two C: recurrent disruption of Zn-ribbons, gene duplication, lineage-specific gene loss, and horizontal gene transfer in evolution of bacterial ribosomal proteins. Genome Biol. 2:RESEARCH0033.1-0033.14. [Online.] http://genomebiology.com/2001/2/9/research/0033. [DOI] [PMC free article] [PubMed]
- 158.Makino, R., T. Tanaka, T. Iizuka, Y. Ishimura, and S. Kanegasaki. 1986. Stoichiometric conversion of oxygen to superoxide anion during the respiratory burst in neutrophils. Direct evidence by a new method for measurement of superoxide anion with diacetyldeuteroheme-substituted horseradish peroxidase. J. Biol. Chem. 261:11444-11447. [PubMed] [Google Scholar]
- 159.Mattatall, N. R., J. Jazairi, and B. C. Hill. 2000. Characterization of YpmQ, an accessory protein required for the expression of cytochrome c oxidase in Bacillus subtilis. J. Biol. Chem. 275:28802-28809. [DOI] [PubMed] [Google Scholar]
- 160.McCall, T. B., N. K. Boughton-Smith, R. M. Palmer, B. J. Whittle, and S. Moncada. 1989. Synthesis of nitric oxide from l-arginine by neutrophils. Release and interaction with superoxide anion. Biochem. J. 261:293-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McCord, J. M., and I. Fridovich. 1969. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244:6049-6055. [PubMed] [Google Scholar]
- 162.McCord, J. M., B. B. Keele, Jr., and I. Fridovich. 1971. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc. Natl. Acad. Sci. USA 68:1024-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.McGroarty, J. A., L. Tomeczek, D. G. Pond, G. Reid, and A. W. Bruce. 1992. Hydrogen peroxide production by Lactobacillus species: correlation with susceptibility to the spermicidal compound nonoxynol-9. J. Infect. Dis. 165:1142-1144. [DOI] [PubMed] [Google Scholar]
- 164.McLennan, H. R., and M. Degli Esposti. 2000. The contribution of mitochondrial respiratory complexes to the production of reactive oxygen species. J. Bioenerg. Biomembr. 32:153-162. [DOI] [PubMed] [Google Scholar]
- 165.Mellies, J., J. Jose, and T. F. Meyer. 1997. The Neisseria gonorrhoeae gene aniA encodes an inducible nitrite reductase. Mol. Gen. Genet. 256:525-532. [DOI] [PubMed] [Google Scholar]
- 166.Merz, A. J., and M. So. 2000. Interactions of pathogenic neisseriae with epithelial cell membranes. Annu. Rev. Cell Dev. Biol. 16:423-457. [DOI] [PubMed] [Google Scholar]
- 167.Messner, K. R., and J. A. Imlay. 1999. The identification of primary sites of superoxide and hydrogen peroxide formation in the aerobic respiratory chain and sulfite reductase complex of Escherichia coli. J. Biol. Chem. 274:10119-10128. [DOI] [PubMed] [Google Scholar]
- 168.Messner, K. R., and J. A. Imlay. 2002. Mechanism of superoxide and hydrogen peroxide formation by fumarate reductase, succinate dehydrogenase, and aspartate oxidase. J. Biol. Chem. 277:42563-42571. [DOI] [PubMed] [Google Scholar]
- 169.Mongkolsuk, S., and J. D. Helmann. 2002. Regulation of inducible peroxide stress responses. Mol. Microbiol. 45:9-15. [DOI] [PubMed] [Google Scholar]
- 170.Moore, T. D., and P. F. Sparling. 1996. Interruption of the gpxA gene increases the sensitivity of Neisseria meningitidis to paraquat. J. Bacteriol. 178:4301-4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Moore, T. D., and P. F. Sparling. 1995. Isolation and identification of a glutathione peroxidase homolog gene, gpxA, present in Neisseria meningitidis but absent in Neisseria gonorrhoeae. Infect. Immun. 63:1603-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Morgan, R. W., M. F. Christman, F. S. Jacobson, G. Storz, and B. N. Ames. 1986. Hydrogen peroxide-inducible proteins in Salmonella typhimurium overlap with heat shock and other stress proteins. Proc. Natl. Acad. Sci. USA 83:8059-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Morita, H., H. Yoshikawa, R. Sakata, Y. Nagata, and H. Tanaka. 1997. Synthesis of nitric oxide from the two equivalent guanidino nitrogens of l-arginine by Lactobacillus fermentum. J. Bacteriol. 179:7812-7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Moskovitz, J., M. A. Rahman, J. Strassman, S. O. Yancey, S. R. Kushner, N. Brot, and H. Weissbach. 1995. Escherichia coli peptide methionine sulfoxide reductase gene: regulation of expression and role in protecting against oxidative damage. J. Bacteriol. 177:502-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mukhopadhyay, P., M. Zheng, L. A. Bedzyk, R. A. LaRossa, and G. Storz. 2004. Prominent roles of the NorR and Fur regulators in the Escherichia coli transcriptional response to reactive nitrogen species. Proc. Natl. Acad. Sci. USA 101:745-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Myllykallio, H., and U. Liebl. 2000. Dual role for cytochrome cbb3 oxidase in clinically relevant proteobacteria? Trends Microbiol. 8:542-543. [DOI] [PubMed] [Google Scholar]
- 177.Naids, F. L., and R. F. Rest. 1991. Stimulation of human neutrophil oxidative metabolism by nonopsonized Neisseria gonorrhoeae. Infect. Immun. 59:4383-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Naqui, A., B. Chance, and E. Cadenas. 1986. Reactive oxygen intermediates in biochemistry. Annu. Rev. Biochem. 55:137-166. [DOI] [PubMed] [Google Scholar]
- 179.Neilands, J. B. 1981. Microbial iron compounds. Annu. Rev. Biochem. 50:715-731. [DOI] [PubMed] [Google Scholar]
- 180.Nittis, T., G. N. George, and D. R. Winge. 2001. Yeast Sco1, a protein essential for cytochrome c oxidase function is a Cu(I)-binding protein. J. Biol. Chem. 276:42520-42526. [DOI] [PubMed] [Google Scholar]
- 181.Norrod, P., and S. A. Morse. 1979. Absence of superoxide dismutase in some strains of Neisseria gonorrhoeae. Biochem. Biophys. Res. Commun. 90:1287-1294. [DOI] [PubMed] [Google Scholar]
- 182.Nunoshiba, T., T. DeRojas-Walker, J. S. Wishnok, S. R. Tannenbaum, and B. Demple. 1993. Activation by nitric oxide of an oxidative-stress response that defends Escherichia coli against activated macrophages. Proc. Natl. Acad. Sci. USA 90:9993-9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Olry, A., S. Boschi-Muller, M. Marraud, S. Sanglier-Cianferani, A. Van Dorsselear, and G. Branlant. 2002. Characterization of the methionine sulfoxide reductase activities of PILB, a probable virulence factor from Neisseria meningitidis. J. Biol. Chem. 277:12016-12022. [DOI] [PubMed] [Google Scholar]
- 184.Paret, C., A. Lode, U. Krause-Buchholz, and G. Rodel. 2000. The P(174)L mutation in the human hSCO1 gene affects the assembly of cytochrome c oxidase. Biochem. Biophys. Res. Commun. 279:341-347. [DOI] [PubMed] [Google Scholar]
- 185.Petruzzella, V., V. Tiranti, P. Fernandez, P. Ianna, R. Carrozzo, and M. Zeviani. 1998. Identification and characterization of human cDNAs specific to BCS1, PET112, SCO1, COX15, and COX11, five genes involved in the formation and function of the mitochondrial respiratory chain. Genomics 54:494-504. [DOI] [PubMed] [Google Scholar]
- 186.Pitcher, R. S., T. Brittain, and N. J. Watmough. 2002. Cytochrome cbb(3) oxidase and bacterial microaerobic metabolism. Biochem. Soc. Trans. 30:653-658. [DOI] [PubMed] [Google Scholar]
- 187.Pomposiello, P. J., M. H. Bennik, and B. Demple. 2001. Genome-wide transcriptional profiling of the Escherichia coli responses to superoxide stress and sodium salicylate. J. Bacteriol. 183:3890-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pomposiello, P. J., and B. Demple. 2002. Global adjustment of microbial physiology during free radical stress. Adv. Microb. Physiol. 46:319-341. [DOI] [PubMed] [Google Scholar]
- 189.Pomposiello, P. J., and B. Demple. 2001. Redox-operated genetic switches: the SoxR and OxyR transcription factors. Trends Biotechnol. 19:109-114. [DOI] [PubMed] [Google Scholar]
- 190.Poock, S. R., E. R. Leach, J. W. Moir, J. A. Cole, and D. J. Richardson. 2002. Respiratory detoxification of nitric oxide by the cytochrome c nitrite reductase of Escherichia coli. J. Biol. Chem. 277:23664-23669. [DOI] [PubMed] [Google Scholar]
- 191.Prinz, W. A., F. Aslund, A. Holmgren, and J. Beckwith. 1997. The role of the thioredoxin and glutaredoxin pathways in reducing protein disulfide bonds in the Escherichia coli cytoplasm. J. Biol. Chem. 272:15661-15667. [DOI] [PubMed] [Google Scholar]
- 192.Que, Q., and J. D. Helmann. 2000. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol. Microbiol. 35:1454-1468. [DOI] [PubMed] [Google Scholar]
- 193.Rabin, R. S., and V. Stewart. 1993. Dual response regulators (NarL and NarP) interact with dual sensors (NarX and NarQ) to control nitrate- and nitrite-regulated gene expression in Escherichia coli K-12. J. Bacteriol. 175:3259-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ramachandran, S., T. S. Magnuson, and D. L. Crawford. 2000. Isolation and analysis of three peroxide sensor regulatory gene homologs ahpC, ahpX and oxyR in Streptomyces viridosporus T7A—a lignocellulose degrading actinomycete. DNA Seq. 11:51-60. [DOI] [PubMed] [Google Scholar]
- 195.Reid, G., J. A. McGroarty, L. Tomeczek, and A. W. Bruce. 1996. Identification and plasmid profiles of Lactobacillus species from the vagina of 100 healthy women. FEMS Immunol. Med. Microbiol. 15:23-26. [DOI] [PubMed] [Google Scholar]
- 196.Rentzsch, A., G. Krummeck-Weiss, A. Hofer, A. Bartuschka, K. Ostermann, and G. Rodel. 1999. Mitochondrial copper metabolism in yeast: mutational analysis of Sco1p involved in the biogenesis of cytochrome c oxidase. Curr. Genet. 35:103-108. [DOI] [PubMed] [Google Scholar]
- 197.Rest, R. F. 1979. Killing of Neisseria gonorrhoeae by human polymorphonuclear neutrophil granule extracts. Infect. Immun. 25:574-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rest, R. F., S. H. Fischer, Z. Z. Ingham, and J. F. Jones. 1982. Interactions of Neisseria gonorrhoeae with human neutrophils: effects of serum and gonococcal opacity on phagocyte killing and chemiluminescence. Infect. Immun. 36:737-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rest, R. F., and W. M. Shafer. 1989. Interactions of Neisseria gonorrhoeae with human neutrophils. Clin. Microbiol. Rev. 2(Suppl.):S83-S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rochelle, L. G., B. M. Fischer, and K. B. Adler. 1998. Concurrent production of reactive oxygen and nitrogen species by airway epithelial cells in vitro. Free Radic. Biol. Med. 24:863-868. [DOI] [PubMed] [Google Scholar]
- 201.Rohde, K. H., and D. W. Dyer. 2003. Mechanisms of iron acquisition by the human pathogens Neisseria meningitidis and Neisseria gonorrhoeae. Front. Biosci. 8:d1186-d1218. [DOI] [PubMed] [Google Scholar]
- 202.Rouhier, N., and J. P. Jacquot. 2003. Molecular and catalytic properties of a peroxiredoxin-glutaredoxin hybrid from Neisseria meningitidis. FEBS Lett. 554:149-153. [DOI] [PubMed] [Google Scholar]
- 203.Ryden, L. 1984. Structure and evolution of the small blue proteins, p. 157-182. In R. Lontie (ed.), Copper proteins and copper enzymes. CRC Press Inc., Boca Raton, Fla.
- 204.Ryden, L., and J. Lundgren. 1976. Homology relationships among the small blue proteins. Nature 261:344-346. [DOI] [PubMed] [Google Scholar]
- 205.Saigh, J. H., C. C. Sanders, and W. E. Sanders, Jr. 1978. Inhibition of Neisseria gonorrhoeae by aerobic and facultatively anaerobic components of the endocervical flora: evidence for a protective effect against infection. Infect. Immun. 19:704-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sansone, A., P. R. Watson, T. S. Wallis, P. R. Langford, and J. S. Kroll. 2002. The role of two periplasmic copper- and zinc-cofactored superoxide dismutases in the virulence of Salmonella choleraesuis. Microbiology 148:719-726. [DOI] [PubMed] [Google Scholar]
- 207.Schmidt, H. H., and U. Walter. 1994. NO at work. Cell 78:919-925. [DOI] [PubMed] [Google Scholar]
- 208.Seaver, L. C., and J. A. Imlay. 2001. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 183:7173-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Seaver, L. C., and J. A. Imlay. 2001. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J. Bacteriol. 183:7182-7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sebastian, S., S. Agarwal, J. R. Murphy, and C. A. Genco. 2002. The gonococcal fur regulon: identification of additional genes involved in major catabolic, recombination, and secretory pathways. J. Bacteriol. 184:3965-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Seib, K. L., M. P. Jennings, and A. G. McEwan. 2003. A Sco homologue plays a role in defence against oxidative stress in pathogenic Neisseria. FEBS Lett. 546:411-415. [DOI] [PubMed] [Google Scholar]
- 212.Seib, K. L., M. P. Simons, H. J. Wu, A. G. McEwan, W. M. Nauseef, M. A. Apicella, and M. P. Jennings. 2005. Investigation of oxidative stress defenses of Neisseria gonorrhoeae by using a human polymorphonuclear leukocyte survival assay. Infect. Immun. 73:5269-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Seib, K. L., H. J. Tseng, A. G. McEwan, M. A. Apicella, and M. P. Jennings. 2004. Defenses against oxidative stress in Neisseria gonorrhoeae and Neisseria meningitidis: distinctive systems for different lifestyles. J. Infect. Dis. 190:136-147. [DOI] [PubMed] [Google Scholar]
- 214.Shafer, W. M. 1988. Lipopolysaccharide masking of gonococcal outer-membrane proteins modulates binding of bacterial cathepsin G to gonococci. J. Gen. Microbiol. 134:539-545. [DOI] [PubMed] [Google Scholar]
- 215.Shafer, W. M., and S. A. Morse. 1987. Cleavage of the protein III and major iron-regulated protein of Neisseria gonorrhoeae by lysosomal cathepsin G. J. Gen. Microbiol. 133:155-162. [DOI] [PubMed] [Google Scholar]
- 216.Shafer, W. M., V. Onunka, and P. J. Hitchcock. 1986. A spontaneous mutant of Neisseria gonorrhoeae with decreased resistance to neutrophil granule proteins. J. Infect. Dis. 153:910-917. [DOI] [PubMed] [Google Scholar]
- 217.Shafer, W. M., V. C. Onunka, and L. E. Martin. 1986. Antigonococcal activity of human neutrophil cathepsin G. Infect. Immun. 54:184-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Shafer, W. M., and R. F. Rest. 1989. Interactions of gonococci with phagocytic cells. Annu. Rev. Microbiol. 43:121-145. [DOI] [PubMed] [Google Scholar]
- 219.Simons, M. P., W. M. Nauseef, and M. A. Apicella. 2005. Interactions of Neisseria gonorrhoeae with adherent polymorphonuclear leukocytes. Infect. Immun. 73:1971-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Skaar, E. P., M. P. Lazio, and H. S. Seifert. 2002. Roles of the recJ and recN genes in homologous recombination and DNA repair pathways of Neisseria gonorrhoeae. J. Bacteriol. 184:919-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Skaar, E. P., D. M. Tobiason, J. Quick, R. C. Judd, H. Weissbach, F. Etienne, N. Brot, and H. S. Seifert. 2002. The outer membrane localization of the Neisseria gonorrhoeae MsrA/B is involved in survival against reactive oxygen species. Proc. Natl. Acad. Sci. USA 99:10108-10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Soler-Garcia, A. A., and A. E. Jerse. 2004. A Neisseria gonorrhoeae catalase mutant is more sensitive to hydrogen peroxide and paraquat, an inducer of toxic oxygen radicals. Microb. Pathog. 37:55-63. [DOI] [PubMed] [Google Scholar]
- 223.Stadtman, E. R., B. S. Berlett, and P. B. Chock. 1990. Manganese-dependent disproportionation of hydrogen peroxide in bicarbonate buffer. Proc. Natl. Acad. Sci. USA 87:384-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.St. Amant, D. C., I. E. Valentin-Bon, and A. E. Jerse. 2002. Inhibition of Neisseria gonorrhoeae by Lactobacillus species that are commonly isolated from the female genital tract. Infect. Immun. 70:7169-7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.St. John, G., N. Brot, J. Ruan, H. Erdjument-Bromage, P. Tempst, H. Weissbach, and C. Nathan. 2001. Peptide methionine sulfoxide reductase from Escherichia coli and Mycobacterium tuberculosis protects bacteria against oxidative damage from reactive nitrogen intermediates. Proc. Natl. Acad. Sci. USA 98:9901-9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Stohl, E. A., A. K. Criss, and H. S. Seifert. 2005. The transcriptome response of Neisseria gonorrhoeae to hydrogen peroxide reveals genes with previously uncharacterized roles in oxidative damage protection. Mol. Microbiol. 58:520-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Storz, G., and J. A. Imlay. 1999. Oxidative stress. Curr. Opin. Microbiol. 2:188-194. [DOI] [PubMed] [Google Scholar]
- 228.Storz, G., F. S. Jacobson, L. A. Tartaglia, R. W. Morgan, L. A. Silveira, and B. N. Ames. 1989. An alkyl hydroperoxide reductase induced by oxidative stress in Salmonella typhimurium and Escherichia coli: genetic characterization and cloning of ahp. J. Bacteriol. 171:2049-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Storz, G., L. A. Tartaglia, S. B. Farr, and B. N. Ames. 1990. Bacterial defenses against oxidative stress. Trends Genet. 6:363-368. [DOI] [PubMed] [Google Scholar]
- 230.Taha, M. K., B. Dupuy, W. Saurin, M. So, and C. Marchal. 1991. Control of pilus expression in Neisseria gonorrhoeae as an original system in the family of two-component regulators. Mol. Microbiol. 5:137-148. [DOI] [PubMed] [Google Scholar]
- 231.Taha, M. K., M. So, H. S. Seifert, E. Billyard, and C. Marchal. 1988. Pilin expression in Neisseria gonorrhoeae is under both positive and negative transcriptional control. EMBO J. 7:4367-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Thomas, C. E., and P. F. Sparling. 1996. Isolation and analysis of a fur mutant of Neisseria gonorrhoeae. J. Bacteriol. 178:4224-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Touati, D., M. Jacques, B. Tardat, L. Bouchard, and S. Despied. 1995. Lethal oxidative damage and mutagenesis are generated by iron in Δfur mutants of Escherichia coli: protective role of superoxide dismutase. J. Bacteriol. 177:2305-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Trees, D. L., and S. M. Spinola. 1990. Localization of and immune response to the lipid-modified azurin of the pathogenic Neisseria. J. Infect. Dis. 161:336-339. [DOI] [PubMed] [Google Scholar]
- 235.Tseng, H. J., A. G. McEwan, M. A. Apicella, and M. P. Jennings. 2003. OxyR acts as a repressor of catalase expression in Neisseria gonorrhoeae. Infect. Immun. 71:550-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tseng, H. J., Y. Srikhanta, A. G. McEwan, and M. P. Jennings. 2001. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol. Microbiol. 40:1175-1186. [DOI] [PubMed] [Google Scholar]
- 237.Turner, P. C., C. E. Thomas, I. Stojiljkovic, C. Elkins, G. Kizel, D. A. Ala'Aldeen, and P. F. Sparling. 2001. Neisserial TonB-dependent outer-membrane proteins: detection, regulation and distribution of three putative candidates identified from the genome sequences. Microbiology 147:1277-1290. [DOI] [PubMed] [Google Scholar]
- 238.Turner, S. M., J. W. Moir, L. Griffiths, T. W. Overton, H. Smith, and J. A. Cole. 2005. Mutational and biochemical analysis of cytochrome c′, a nitric oxide-binding lipoprotein important for adaptation of Neisseria gonorrhoeae to oxygen-limited growth. Biochem. J. 388:545-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Turner, S. M., E. G. Reid, H. Smith, and J. A. Cole. 2003. A novel cytochrome c peroxidase from Neisseria gonorrhoeae, a lipoprotein from a Gram-negative bacterium. Biochem. J. 373:865-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Turrens, J. F. 2003. Mitochondrial formation of reactive oxygen species. J. Physiol. 552:335-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Turrens, J. F., and A. Boveris. 1980. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem. J. 191:421-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Vaisanen-Tommiska, M., M. Nuutila, K. Aittomaki, V. Hiilesmaa, and O. Ylikorkala. 2003. Nitric oxide metabolites in cervical fluid during pregnancy: further evidence for the role of cervical nitric oxide in cervical ripening. Am. J. Obstet. Gynecol. 188:779-785. [DOI] [PubMed] [Google Scholar]
- 243.van Vliet, A. H., M. L. Baillon, C. W. Penn, and J. M. Ketley. 1999. Campylobacter jejuni contains two fur homologs: characterization of iron-responsive regulation of peroxide stress defense genes by the PerR repressor. J. Bacteriol. 181:6371-6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Verduyn, C., C. J. van Wijngaarden, W. A. Scheffers, and J. P. van Dijken. 1991. Hydrogen peroxide as an electron acceptor for mitochondrial respiration in the yeast Hansenula polymorpha. Yeast 7:137-146. [DOI] [PubMed] [Google Scholar]
- 245.Vijgenboom, E., J. E. Busch, and G. W. Canters. 1997. In vivo studies disprove an obligatory role of azurin in denitrification in Pseudomonas aeruginosa and show that azu expression is under control of RpoS and ANR. Microbiology 143:2853-2863. [DOI] [PubMed] [Google Scholar]
- 246.Virji, M., and J. E. Heckels. 1986. The effect of protein II and pili on the interaction of Neisseria gonorrhoeae with human polymorphonuclear leucocytes. J. Gen. Microbiol. 132:503-512. [DOI] [PubMed] [Google Scholar]
- 247.Vogt, W. 1995. Oxidation of methionyl residues in proteins: tools, targets, and reversal. Free Radic. Biol. Med. 18:93-105. [DOI] [PubMed] [Google Scholar]
- 248.Wasserheit, J. N. 1992. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex. Transm. Dis. 19:61-77. [PubMed] [Google Scholar]
- 249.Watmough, N. J., G. Butland, M. R. Cheesman, J. W. Moir, D. J. Richardson, and S. Spiro. 1999. Nitric oxide in bacteria: synthesis and consumption. Biochim. Biophys. Acta 1411:456-474. [DOI] [PubMed] [Google Scholar]
- 250.WHO. 1998. Control of epidemic meningococcal disease. WHO practical guidelines, 2nd ed. WHO/EMC/BAC/98.3. World Health Organization, Geneva, Switzerland.
- 251.Wilks, K. E., K. L. Dunn, J. L. Farrant, K. M. Reddin, A. R. Gorringe, P. R. Langford, and J. S. Kroll. 1998. Periplasmic superoxide dismutase in meningococcal pathogenicity. Infect. Immun. 66:213-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Winterbourn, C. C., M. C. Vissers, and A. J. Kettle. 2000. Myeloperoxidase. Curr. Opin. Hematol. 7:53-58. [DOI] [PubMed] [Google Scholar]
- 253.Wizemann, T. M., J. Moskovitz, B. J. Pearce, D. Cundell, C. G. Arvidson, M. So, H. Weissbach, N. Brot, and H. R. Masure. 1996. Peptide methionine sulfoxide reductase contributes to the maintenance of adhesins in three major pathogens. Proc. Natl. Acad. Sci. USA 93:7985-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Woods, J. P., J. F. Dempsey, T. H. Kawula, D. S. Barritt, and J. G. Cannon. 1989. Characterization of the neisserial lipid-modified azurin bearing the H.8 epitope. Mol. Microbiol. 3:583-591. [DOI] [PubMed] [Google Scholar]
- 255.Wu, H. J., K. L. Seib, J. L. Edwards, M. A. Apicella, A. G. McEwan, and M. P. Jennings. 2005. Azurin of pathogenic Neisseria spp. is involved in defense against hydrogen peroxide and survival within cervical epithelial cells. Infect. Immun. 73:8444-8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wu, H. J., K. L. Seib, Y. N. Srikhanta, S. P. Kidd, J. L. Edwards, T. L. Maguire, S. M. Grimmond, M. A. Apicella, A. G. McEwan, and M. P. Jennings. 2006. PerR controls Mn-dependent resistance to oxidative stress in Neisseria gonorrhoeae. Mol. Microbiol. 60:401-416. [DOI] [PubMed] [Google Scholar]
- 257.Xu, J., and W. Verstraete. 2001. Evaluation of nitric oxide production by lactobacilli. Appl. Microbiol. Biotechnol. 56:504-507. [DOI] [PubMed] [Google Scholar]
- 258.Yesilkaya, H., A. Kadioglu, N. Gingles, J. E. Alexander, T. J. Mitchell, and P. W. Andrew. 2000. Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae. Infect. Immun. 68:2819-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Yu, C. A., H. Tian, L. Zhang, K. P. Deng, S. K. Shenoy, L. Yu, D. Xia, H. Kim, and J. Deisenhofer. 1999. Structural basis of multifunctional bovine mitochondrial cytochrome bc1 complex. J. Bioenerg. Biomembr. 31:191-199. [DOI] [PubMed] [Google Scholar]
- 260.Zamocky, M., S. Janecek, and F. Koller. 2000. Common phylogeny of catalase-peroxidases and ascorbate peroxidases. Gene 256:169-182. [DOI] [PubMed] [Google Scholar]
- 261.Zheng, H. Y., T. M. Alcorn, and M. S. Cohen. 1994. Effects of H2O2-producing lactobacilli on Neisseria gonorrhoeae growth and catalase activity. J. Infect. Dis. 170:1209-1215. [DOI] [PubMed] [Google Scholar]
- 262.Zheng, H. Y., D. J. Hassett, K. Bean, and M. S. Cohen. 1992. Regulation of catalase in Neisseria gonorrhoeae. Effects of oxidant stress and exposure to human neutrophils. J. Clin. Investig. 90:1000-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zheng, M., F. Aslund, and G. Storz. 1998. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279:1718-1721. [DOI] [PubMed] [Google Scholar]
- 264.Zheng, M., B. Doan, T. D. Schneider, and G. Storz. 1999. OxyR and SoxRS regulation of fur. J. Bacteriol. 181:4639-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Zheng, M., X. Wang, B. Doan, K. A. Lewis, T. D. Schneider, and G. Storz. 2001. Computation-directed identification of OxyR DNA binding sites in Escherichia coli. J. Bacteriol. 183:4571-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zheng, M., X. Wang, L. J. Templeton, D. R. Smulski, R. A. LaRossa, and G. Storz. 2001. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J. Bacteriol. 183:4562-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Zumft, W. G. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61:533-616. [DOI] [PMC free article] [PubMed] [Google Scholar]