Abstract
In 1999, Wan et al. [Proc. Natl. Acad. Sci. USA 96, 6014–6019] published a pioneering paper that established the entanglement between DNA base pair motions and the transfer time of the charge carrier. The DNA assemblies contained an ethidium covalently bound via a flexible alkyl chain to the 5′ hydroxyl group of the DNA backbone. Although covalently attached, the loose way in which the ethidium was linked to DNA allowed for large degrees of conformational freedom and thus raised some concern with respect to conformational inhomogeneity. In this letter, we report studies on a different set of ethidium DNA conjugates. In contrast to the “Caltech systems,” these conjugates contain ethidium tightly incorporated (as a base pair surrogate) into the DNA base stack, opposite to an abasic site analog. Despite the tight binding, we found that charge transfer from the photoexcited ethidium base pair surrogate across two or more base pairs is several orders of magnitude slower than in case of the DNA systems bearing the tethered ethidium. To further broaden the scope of this account, we compared (oxidative) electron hole transfer and (reductive) electron transfer using the same ethidium chromophore as a charge donor in combination with two different charge acceptors. We found that both electron and hole transfer are characterized by similar rates and distance dependencies. The results demonstrate the importance of nuclear motions and conformational flexibility and underline the presence of a base gating mechanism, which appears to be generic to electronic transfer processes through π-stacked nucleic acids.
Keywords: conformational gating, electron transfer, ultrafast spectroscopy, hole transfer
Over the last decade, there were many conflicting reports on the electronic conduction properties of DNA (1–4). From the multitude of studies in various laboratories, it has emerged that DNA must be understood as a dynamic medium with nuclear fluctuations, which extend over many time scales (5–11). Since the first femtosecond time-resolved experiments on ethidium (E) nucleotide complexes were reported (12), it has become clear that charge transfer through the base stack cannot be reduced to a static “donor–bridge–acceptor” problem. Several studies followed that highlighted the importance of conformational gating for electronic transfer processes in DNA (6–11, 13) and peptides (14). The work presented here was motivated and inspired by the first publication on real-time DNA charge-transfer dynamics with photoexcited E in 1999 (5). The DNA systems investigated by the California Institute of Technology (Caltech) group contained E as chromophore that has been covalently attached to the 5′ terminal hydroxyl group of one of the oligonucleotides (15). Femtosecond anisotropy measurements revealed that the flexible alkyl chain linker warranted site-specific intercalation (within an error of 1 bp distance) without significantly restraining the orientational motions of E within the base stack (5).
In most previous experiments, E has been used as a noncovalently bound and intercalated charge donor (16–23). In principle, organic chromophores like E can be synthetically incorporated as charge donors into oligonucleotides either by covalent attachment to natural DNA bases or by replacing natural bases or even base pairs (24). Recently, we reported the successful synthetic procedure for DNA duplexes bearing the phenanthridinium heterocycle of E as a base pair surrogate (25, 26). Then, we applied the emission of the photoexcited E in combination with oxidative hole transfer (HT) to detect single base pair mismatches in duplex DNA (27).
Results and Discussion
Fig. 1 shows nine duplexes (DNA1–DNA9) bearing the synthetically incorporated base pair surrogate E. Although it was shown that the counterstrand DNA base opposite E has only a minor influence on the fluorescence properties and therefore on the stacking interactions of E (26), the abasic site analog (S) was chosen to warrant an optimal insertion of E (25). It is well established that photoexcited E does not undergo charge (i.e., electron or electron hole) transfer with any of the natural DNA bases [oxidation: E(E+*/E) ≅ 1.2 V (see ref. 29); in DNA, guanine has the lowest oxidation potential: E(dG+/dG) ≅ 1.3 V (see ref. 30); reduction: E(E2+/E+*) ≅ −0.5 V (see ref. 18); and in DNA, cytosine and thymine have the lowest reduction potential: E(dT/dT−) ≅ E(dC/dC−) ≅ −1.2 V (see ref. 31)]. Thus, suitable charge acceptors have to be provided. This task is conveniently done by choosing artificial DNA bases with similar steric demand as natural purines but alternated charge-accepting capabilities. 7-Deazaguanine (Z) is known to be a stronger electron hole acceptor than guanine [E(Z+/Z) ≅ 1.3 V (see ref. 29)] and has been used in various oxidative HT studies (5, 12, 15, 23, 27). For reductive electron transfer (ET), it was suggested that methyl viologen (MV) (16–18) or other viologens (32) represent suitable acceptors based on their redox potentials [E(MV2+/MV+) ≅ −0.44 V (see ref. 33)]. Recently, we reported ET experiments using noncovalently bound MV (25). All attempts to incorporate derivatives of MV as an artificial base synthetically into oligonucleotides failed because of their instability under basic conditions. As an alternative, 5-nitroindole (N) has similar redox properties [E(N/N−) ≅ −0.32 V (see ref. 34)] and therefore should be a suitable electron acceptor. N behaves as a universal base analog; hence, the counterbase of N can be chosen arbitrarily (35, 36). However, because Z preferentially pairs with C, C was chosen as the counterbase of both Z and N.
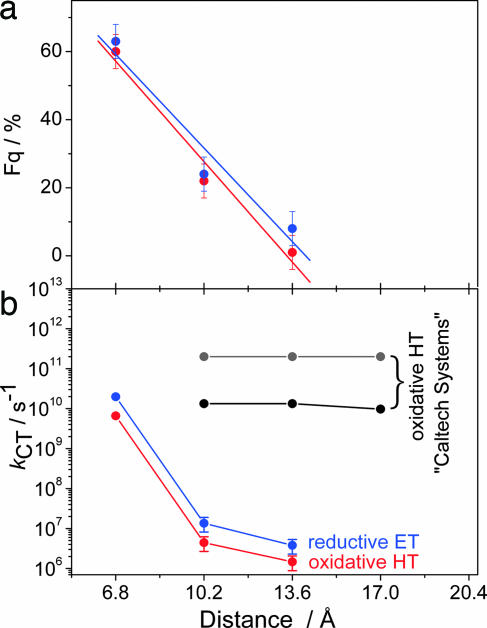
Fig. 1.
DNA conjugates. (a) Molecular models illustrating the DNA system with the tethered E intercalator (Left) [reproduced with permission from ref. 5 (Copyright 1999, The National Academy of Sciences of the USA)] in comparison with the E base pair surrogate (Right) [image generated with chimera (28)]. (b) The duplexes DNA1–DNA9. (c) The structures of the E base pair surrogate, the tethered E intercalator (5), the abasic site analog (S), and the charge acceptor nucleosides Z and N.
Note that by using this set of functionalized DNA (Fig. 1), it is possible to directly compare the dynamics of oxidative electron HT and reductive ET in structurally similar donor–DNA–acceptor assays, which has not been previously reported. It is important to note here that based on redox potentials, the driving force for both types of charge transfer (reductive ET and oxidative HT) is very similar (≈0.2 eV; 1 eV = 1.602 × 10−19 J).
The steady-state fluorescence spectra (shown in Figs. 4–6, which are published as supporting information on the PNAS web site) of DNA1–DNA3 (that serve as reference systems without a charge acceptor) exhibit very similar emission quantum yields, consistent with the absence of any charge transfer. In contrast, the emission quantum yields of the other two sets of conjugates (DNA4–DNA6 and DNA7–DNA9) show similar quenching of the fluorescence (see Fig. 3a) with a linear distance dependence of the quenching yield.
Fig. 3.
Distance dependencies. (a) Steady-state fluorescence quenching efficiencies (Fq = 1 − IN,Z/IG) for DNA7–DNA9 (red) and DNA4–DNA6 (blue). (b) Rates of HT in DNA7–DNA9 (red) and ET in DNA4–DNA6 (blue) compared with the two HT rates observed in the Caltech systems (black, gray; taken from ref. 5). Across a single base pair, the rates for HT and ET are comparable with the ones of the tethered intercalator systems. For distances of 2 and 3 bp, however, the rates differ by 4–5 orders of magnitude.
E has a spectrum of excited-state lifetimes (from 2 to 25 ns) depending on its microscopic environment and specifically on the accessibility of surrounding water molecules (37). To illuminate the ultrafast charge transfer dynamics in these conjugates, we applied femtosecond broadband pump–probe spectroscopy (38).
As expected, the G-containing duplexes (DNA1–DNA3) do not show a subnanosecond time component. Neither did we find a significant short time component in the decay of the pump–probe spectra of those conjugates where Z/N are separated by >1 bp (DNA5, DNA6 and DNA8, DNA9). Fig. 2 depicts the temporal evolutions of the transient absorption spectrum of the lowest excited E state. For DNA4 and DNA7 (Fig. 2 a and b), where charge transfer occurs rapidly across a single intervening base pair, the spectra show a pronounced decay that is absent in the corresponding reference duplex DNA1 (Fig. 2c). The transient decays (illustrated in Fig. 2d) have been fitted to 50 ps (amplitude 22%) for DNA4 and to 150 ps (amplitude 27%) for DNA7, respectively. The amplitudes of the picosecond time components reflect the fractions of reactive molecules with favorable, well stacked structures, already present in the ground state (5). The absence of ultrafast dynamics in the conjugates with larger donor/acceptor distances on the one hand, and the presence of fluorescence quenching in these systems (Fig. 3a) on the other hand, indicate that charge transfer occurs on the nanosecond time scale. To quantify the actual rates, we measured the nanosecond fluorescence lifetimes (see Tables 1–3, which are published as supporting information on the PNAS web site). The charge transfer rates were directly calculated from the mean fluorescence lifetimes¶ of the redox active duplexes DNA5, DNA6, and DNA8, DNA9 (τN,Z) and the lifetimes obtained for the reference duplexes DNA1–DNA3 that contain a G at the position of the charge acceptor
Although the multiexponential nature of the fluorescence decays renders a precise analysis of the slow charge transfer dynamics impossible, this approach allows us to specify the time scale for charge transfer across multiple base pairs.
Fig. 2.
Femtosecond time-resolved data. (a–c) Temporal evolution of the pump–probe spectra of DNA4 (a), DNA7 (b), and DNA1 (c) in the time range of 20–400 ps after excitation at 520 nm (600 μM DNA in 10 mM Na-Pi buffer, 250 mM NaCl, pH 7.0). The spectra evolve from blue (early times) to green, yellow, orange, and red (late times). (d) The normalized transient decays obtained from global-fit analysis (39).
Fig. 3b displays the measured rates for HT (red) and ET (blue) in addition to the HT rates obtained for the Caltech systems (black, gray; taken from ref. 5).¶ The difference for HT rates across >1 bp is astoundingly dramatic (4–5 orders of magnitude). It is obvious that E, when rigidly inserted as a base pair surrogate, does not facilitate ultrafast HT over a distance of >1 bp. Note that this finding is true for both types of charge transfer, reductive ET and oxidative HT.
Photoinduced charge transfer in most covalently modified DNA conjugates displays steep distance dependence at short distances. Recently, Lewis et al. (40) determined the mechanistic crossover between direct “superexchange-type” HT and an indirect “hopping” mechanism.‖ It must be noticed, however, that these two mechanistic classifications are simplistic in the sense that they do not account for explicit nuclear motions. The apparent differences in the charge transfer dynamics observed in the intercalated E duplexes (5) and in the conformationally more rigid hairpin systems are likely due to inherently different nuclear motions in these conjugates. The direct comparison between the base pair surrogate E and the intercalated tethered E conjugate demonstrates the critical role that nuclear motions play for the optimization of the various parameters that govern charge transfer rates. The fact that HT and ET across a single base pair occur on the time scale of 100-ps proves that the inserted E moiety in DNA1–DNA9 is strongly coupled within the base stack. The amplitude of the fast component signal reflects the fraction of molecules that do not need to undergo nuclear rearrangement before the transfer. High rates for charge transfer over larger distances require a sampling of the accessible configurations, which is not possible (or strongly limited) in the DNA duplexes bearing the E base pair surrogate.
Our results show that both ET and HT follow the same fundamental relationship between the nuclear motion of the base pairs and the actual charge transfer, a mechanistic concept that has been termed as “gating effect.”
Conclusion
The results presented here solidify the notion that both rates and distance dependencies of charge transfer through DNA are controlled by the nuclear base pair dynamics, an important concept that appears to be generic for all electronic transfer processes involving π-stacked nucleic acid assemblies. They also show that strong electronic coupling and base stacking must not be confused with tight binding. Efficient transfer over an extended range is only possible when assisted by nuclear motions and conformational sampling, which leads to conformational gating. The time scale for this motion is 10–100 ps. Thus, conformational flexibility that can be exerted on this time scale warrants effective interaction (and high rates) between adjacent base pairs.
Our synthetic DNA conjugates provide tight binding of the chromophore within the base stack, but they are conformationally rigid at the insertion site and do not permit sufficient nuclear motions to gate charge transfer. The DNA conjugates presented here allow for a previously undescribed direct comparison between oxidative HT and reductive ET in structurally similar donor–acceptor arrangements. Although the details of reductive ET may be different, both ET and HT are equally entangled with nuclear motions involving donor, acceptor, and the intervening medium.
Materials and Methods
For a description of the materials and methods, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Supplementary Material
Acknowledgments
This work was supported by Boston College, the Deutsche Forschungsgemeinschaft, the University of Regensburg, and the Fonds der Chemischen Industrie.
Abbreviations
- Caltech
California Institute of Technology
- E
ethidium
- ET
electron transfer
- HT
hole transfer
- MV
methyl viologen
- N
5-nitroindole
- Z
7-deazaguanine.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
As in the Caltech systems, the fluorescence lifetimes were not purely monoexponential. In addition to the major lifetime component (85–90% amplitude), which varied between 14 and 19 ns, there was a minor component between 2 and 4 ns in all conjugates. The mean fluorescence lifetime was calculated as the amplitude-weighted average of the two decay components.
The Caltech systems showed two rate constants for HT that were both independent of the E/Z distance (see ref. 5).
References
- 1.Dunlap D. D., Garcia R., Schabtach E., Bustamante C. Proc. Natl. Acad. Sci. USA. 1993;90:7652–7655. doi: 10.1073/pnas.90.16.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fink H.-W., Schönenberger C. Nature. 1999;398:407–410. doi: 10.1038/18855. [DOI] [PubMed] [Google Scholar]
- 3.Porath D., Bezryadin A., de Vries S., Dekker C. Nature. 2000;403:635–638. doi: 10.1038/35001029. [DOI] [PubMed] [Google Scholar]
- 4.Kasumov A. Y., Kociak M., Gueron S., Reulet B., Volkov V. T., Klinov D. V., Bouchiat H. Science. 2001;291:280–282. doi: 10.1126/science.291.5502.280. [DOI] [PubMed] [Google Scholar]
- 5.Wan C., Fiebig T., Kelley S. O., Treadway C. R., Barton J. K., Zewail A. H. Proc. Natl. Acad. Sci. USA. 1999;96:6014–6019. doi: 10.1073/pnas.96.11.6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berlin Y. A., Burin A. L., Siebbeles L. D. A., Ratner M. A. J. Phys. Chem. A. 2001;105:5666–5678. [Google Scholar]
- 7.O’Neill M. A., Becker H. C., Wan C. Z., Barton J. K., Zewail A. H. Angew. Chem. Int. Ed. 2003;42:5896–5900. doi: 10.1002/anie.200352831. [DOI] [PubMed] [Google Scholar]
- 8.Troisi A., Nitzan A., Ratner M. A. J. Chem. Phys. 2003;119:5782–5788. [Google Scholar]
- 9.O’Neill M. A., Barton J. K. J. Am. Chem. Soc. 2004;126:11471–11483. doi: 10.1021/ja048956n. [DOI] [PubMed] [Google Scholar]
- 10.Wagenknecht H. A., Fiebig T. In: Charge Transfer in DNA. Wagenknecht H. A., editor. Weinheim, Germany: Wiley–VCH; 2005. [Google Scholar]
- 11.Kaden P., Mayer-Enthart E., Trifonov A., Fiebig T., Wagenknecht H.-A. Angew. Chem. Int. Ed. 2005;44:1636–1639. doi: 10.1002/anie.200462592. [DOI] [PubMed] [Google Scholar]
- 12.Fiebig T., Wan C., Kelley S. O., Barton J. K., Zewail A. H. Proc. Natl. Acad. Sci. USA. 1999;96:1187–1192. doi: 10.1073/pnas.96.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trifonov A., Raytchev M., Buchvarov I., Rist M., Barbaric J., Wagenknecht H. A., Fiebig T. J. Phys. Chem. B. 2005;109:19490–19495. doi: 10.1021/jp052108c. [DOI] [PubMed] [Google Scholar]
- 14.Schlag E. W., Yang D.-Y., Sheu S.-Y., Selzle H. L., Lin S. H., Rentzepis P. M. Proc. Natl. Acad. Sci. USA. 2000;97:9849–9854. doi: 10.1073/pnas.140196597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelley S. O., Holmlin R. E., Stemp E. D. A., Barton J. K. J. Am. Chem. Soc. 1997;119:9861–9870. [Google Scholar]
- 16.Fromherz P., Rieger B. J. Am. Chem. Soc. 1986;108:5361–5362. [Google Scholar]
- 17.Atherton S. J., Beaumont P. C. J. Phys. Chem. 1987;91:3993–3997. [Google Scholar]
- 18.Dunn D. A., Lin V. H., Kochevar I. E. Biochemistry. 1992;31:11620–11625. doi: 10.1021/bi00161a048. [DOI] [PubMed] [Google Scholar]
- 19.Brun A. M., Harriman A. J. Am. Chem. Soc. 1992;114:3656–3660. [Google Scholar]
- 20.Atherton S. J., Beaumont P. C. J. Phys. Chem. 1995;99:12025–12029. [Google Scholar]
- 21.Kononov A. I., Moroshkina E. B., Tkachenko N. V., Lemmetyninen H. J. Phys. Chem. B. 2001;105:535–541. [Google Scholar]
- 22.Henderson P. T., Boone E., Schuster G. B. Helv. Chim. Acta. 2002;85:135–151. [Google Scholar]
- 23.Li H., Peng X., Seela F. Bioorg. Med. Chem. Lett. 2004;14:6031–6034. doi: 10.1016/j.bmcl.2004.09.071. [DOI] [PubMed] [Google Scholar]
- 24.Wagenknecht H.-A. Curr. Org. Chem. 2004;8:251–266. [Google Scholar]
- 25.Amann N., Huber R., Wagenknecht H. A. Angew. Chem. 2004;43:1845–1847. doi: 10.1002/anie.200353153. [DOI] [PubMed] [Google Scholar]
- 26.Huber R., Amann N., Wagenknecht H.-A. J. Org. Chem. 2004;69:744–751. doi: 10.1021/jo0355404. [DOI] [PubMed] [Google Scholar]
- 27.Valis L., Amann N., Wagenknecht H.-A. Org. Biomol. Chem. 2005;3:36–38. doi: 10.1039/b414672g. [DOI] [PubMed] [Google Scholar]
- 28.Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., Ferrin T. E. J. Comp. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 29.Kelley S. O., Barton J. K. Chem. Biol. 1998;5:413–425. doi: 10.1016/s1074-5521(98)90158-2. [DOI] [PubMed] [Google Scholar]
- 30.Steenken S., Jovanovic S. V. J. Am. Chem. Soc. 1997;119:617–618. [Google Scholar]
- 31.Steenken S., Telo J. P., Novais H. M., Candeias L. P. J. Am. Chem. Soc. 1992;114:4701–4709. [Google Scholar]
- 32.Kelley S. O., Orellana G., Barton J. K. J. Photochem. Photobiol. B. 2000;48:72–79. doi: 10.1016/s1011-1344(00)00106-8. [DOI] [PubMed] [Google Scholar]
- 33.Michaelis L., Hill E. S. J. Am. Chem. Soc. 1933;55:1481–1494. [Google Scholar]
- 34.Kokkinidis G., Kelaidopoulou A. J. Electroanal. Chem. 1996;414:197–208. [Google Scholar]
- 35.Loakes D., Brown D. M. Nucleic Acids Res. 1994;22:4039–4043. doi: 10.1093/nar/22.20.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loakes D., Hill F., Brown D. M., Salisbury S. A. J. Mol. Biol. 1997;270:426–435. doi: 10.1006/jmbi.1997.1129. [DOI] [PubMed] [Google Scholar]
- 37.Olmsted J., Kearns D. R. Biochemistry. 1977;16:3647–3654. doi: 10.1021/bi00635a022. [DOI] [PubMed] [Google Scholar]
- 38.Raytchev M., Pandurski E., Buchvarov I., Modrakowski C., Fiebig T. J. Phys. Chem. A. 2003;107:4592–4600. [Google Scholar]
- 39.Yamaguchi S., Hamaguchi H. J. Chem. Phys. 1998;109:1397–1408. [Google Scholar]
- 40.Lewis F. D., Zhu H., Daublain P., Fiebig T., Raytchev M., Wang Q., Shafirovich V. J. Am. Chem. Soc. 2006;128:791–800. doi: 10.1021/ja0540831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.