Abstract
Nonsteroidal anti-inflammatory drugs (NSAID) reduce the risk for cancer, due to their antiproliferative and apoptosis-inducing effects. A critical pathway for apoptosis involves the release of cytochrome c from mitochondria, which then interacts with Apaf-1 to activate caspase proteases that orchestrate cell death. In this study we found that treatment of a human cancer cell line with aspirin induced caspase activation and the apoptotic cell morphology, which was blocked by the caspase inhibitor zVAD-fmk. Further analysis of the mechanism underlying this apoptotic event showed that aspirin induces translocation of Bax to the mitochondria and triggers release of cytochrome c into the cytosol. The release of cytochrome c from mitochondria was inhibited by overexpression of the antiapoptotic protein Bcl-2 and cells that lack Apaf-1 were resistant to aspirin-induced apoptosis. These data provide evidence that the release of cytochrome c is an important part of the apoptotic mechanism of aspirin.
Keywords: aspirin, apoptosis, cytochrome c, mitochondria, NSAID
Introduction
Based on epidemiological studies and clinical observations, nonsteroidal anti-inflammatory drugs (NSAID), principally aspirin and sulindac, seem to reduce the risk for colorectal cancer [1–5] but the mechanism by which they inhibit tumorigenesis is not clear. The best defined molecular target for aspirin and other NSAIDs is cyclooxygenase (COX) [6], the key enzyme in prostaglandin biosynthesis [7]. More recently, some investigators in the field have suggested that the chemopreventive properties of NSAIDs are independent of their ability to inhibit COX activity and that they exert their antineoplastic effect by the induction of apoptosis [8–10]. Different mechanisms for the induction of apoptosis by aspirin and salicylates have been proposed, including the activation of caspases [11,12], inhibition of NF-/ΚB activation [13], ceramide pathway activation [14], and p38 MAP kinase activation [15].
Aspirin also has profound effects on mitochondria, as it uncouples mitochondrial oxidation [16] and induces the mitochondrial permeability transition [17–19]. Mitochondria play a major role in the apoptotic pathway. Many different apoptotic signals stimulate the release of cytochrome c through the mitochondrial outer membrane. Once released into the cytoplasm cytochrome c binds the apoptotic protease activating factor, Apaf-1, a human homolog of C. elegans CED-4 protein, promoting further association with procaspase-9 [20,21]. Formation of such a protein complex results in activation of caspase-9 that, in turn, activates downstream caspases such as caspase-3. When caspases are activated they cleave a number of key substrates, resulting in their activation or inactivation, which orchestrate the morphologic and biochemical features of apoptosis [22]. The release of cytochrome c from mitochondria is regulated through the Bcl-2 family of proteins. Antiapoptotic members like Bcl-2 and Bcl-Xl prevent the release of cytochrome c [23,24], whereas pro-apoptotic ones like Bax and Bid cause the release of cytochrome c [25,26] and, through this, apoptosis. Due to the connection between aspirin and mitochondria we have studied the role of mitochondria and cytochrome c release in aspirin-induced apoptosis in cancer cells.
Materials and Methods
Cell Lines
HeLa cells, HeLa cells stably transfected with cytochrome c-green fluorescent protein (GFP) (HeLa-CcGFP) [27] Bcl-2 (HeLa-CcGFP-Bcl-2) or Bax-GFP (HeLa-Bax-GFP), Apaf-1 wild type (wt), and Apaf-1 -/- (a gift from Francesco Cecconi) murine embryonic fibroblasts were maintained in Dulbecco's modified Eagle's medium (DMEM, Gibco, Gaithersburg, MD) supplemented with 10% FCS, 2 mM l-glutamine, 200 µg/ml penicillin, and 100 µg/ml streptomycin sulphate in a humidified atmosphere at 5% CO2/95% air.
Antibodies and Reagents
Detection of protein expression was performed by standard immunoblotting techniques using primary antibodies against caspase-3 (polyclonal rabbit antiserum, Pharmingen, San Diego, CA),cytochrome c (clone 7H8.2C12, Pharmingen), poly (ADP-ribose) polymerase (clone C2-10, Pharmingen), protein kinase C-δ (PKC-δ, polyclonal rabbit antisera, Santa Cruz Biotechnology, Santa Cruz, CA), and actin (clone C4, ICN Pharmaceuticals, Costa Mesa, CA). Aspirin (acetylsalicylic acid) was purchased from Sigma (St. Louis, MO) and was dissolved in DMSO. The broad-spectrum caspase inhibitor benzoyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone (zVAD-fmk) was obtained from Enzyme Systems (Livermore, CA).
Flow Cytometry
For flow cytometry, cells were harvested by trypsinization, and washed with growth medium and phosphate-buffered saline (PBS). Cell death was determined by binding of fluorescein isothiocyanate-labeled annexin V (Clontech Laboratories, Palo Alto, CA) and propidium iodide (PI) uptake using a FACSCalibur (Becton Dickinson, Sunnyvale, CA).
To count cells in which cytochrome c-GFP was released from mitochondria, cells were incubated for 5 minutes in ice-cold cell-lysis and mitochondria-intact (CLAMI) buffer (250 mM sucrose, 80 mM KCl, 50 µg/ml digitonin in PBS). The cells were immediately analyzed by FACS analysis, collecting the fluorescence intensity of the GFP of the individual cells in FL1. Control cells were 0.5log brighter than cells that had released cytochrome c.
Preparation of Whole Cell and Cytosolic Extracts
Cells were harvested by gentle scraping and washed in PBS. For preparation of whole-cell extracts the cells were incubated on ice for 20 minutes in lysis buffer (50 mM Tris (pH 8.0), 150 mM NaCl, 1% NP-40, 100 µM PMSF, and complete™ protease inhibitor (Boehringer Mannheim, Indianopolis, IN). The homogenates were centrifuged at 14,000xg for 10 minutes and the supernatants were frozen at -70°C for Western blot. For preparation of cytosolic extracts, cells were resuspended in ice-cold lysis buffer (250 mM sucrose, 80 mM KCl, 50 µg/ml digitonin, PMSF, and complete™ protease inhibitor in PBS). After 5 minutes of incubation on ice the cell suspensions were centrifuged at 10,000xg for 5 minutes and the supernatants were frozen at -70°C until analysis by Western blot.
Western Blot Analysis
The protein content of the cell extracts was determined by the Bradford assay (Bio-Rad). Samples were heated to 95°C in Laemmli buffer for 5 minutes and centrifuged at 14,000xg. The supernatants were subjected to electrophoresis on 8% or 15% SDS-polyacrylamide gels using a Bio-Rad minigel apparatus, and transferred to nitrocellulose membranes (Hybond ECL, Amersham Pharmacia Biotech). After blocking with Tris-buffered saline (TBS) containing 5% nonfat dry milk and 0.1% Tween-20 (TBS-T) for 2 hours, the membranes were incubated with primary antibodies in TBS-T overnight at 4°C on a shaker. Before and after incubation with the horseradish-peroxidase-conjugated secondary antibodies (Amersham Pharmacia Biotech, Piscataway, NJ), the membranes were washed extensively using TBS-T. Antibody binding was detected by enhanced chemiluminescence (Super Signal, Pierce, Rockford, IL).
Time-Lapse Confocal Microscopy
Cells were plated overnight in cell culture dishes containing sterilized coverslip. Cells were treated with aspirin and after a 12-hour incubation the culture dishes were placed in an MS-C Temp Controller (Narishige, New York, NY). Cytochrome c-GFP or Bax-GFP were followed by taking images every 2 minutes using a Nikon Eclipse TE 300 microscope and an MRC 1024ES laser scanning confocal microscope (Bio-Rad, Hercules, CA) as described previously [27]. Cytochrome c-GFP or Bax-GFP were excited using a 488-nm line from Ar/Kr laser attenuated at 91%. Loss of plasma membrane integrity was detected by adding 0.4 µg/ml PI. Mitochondrial membranes were stained with 40 nM tetramethylrhodamine ethyl ester (TMRE, Molecular Probes, Eugene, OR).
Results
Aspirin Induces Cell Death of HeLa Cells
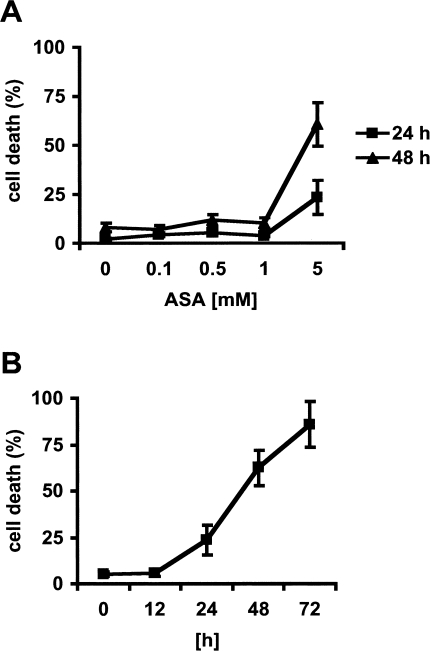
To determine the concentration of aspirin required to induce cell death in HeLa cells, we treated the cells with 0.1 to 5 mM aspirin for 24 hours and 48 hours. Cell death was determined by annexin V staining of phosphatidylserine exposure on the cell surface, assessed by flow cytometry. This method assesses the total number of cells that have exposed phosphatidylserine during apoptosis and/or lost plasma membrane integrity. Aspirin induced significant cell death in HeLa cells at concentrations greater than 1 mM (Figure 1A). Dead cells became evident after 24 hours of treatment with 5 mM aspirin, a concentration that can be measured in the serum of patients treated with aspirin for chronic inflammatory disease [28]. The number of dead cells increased with time up to 72 hours by which time the majority of cells were dead (Figure 1B).
Figure 1.
Induction of cell death in HeLa cells. (A) Cells were exposed to various concentrations of aspirin (ASA) for 24 hours (■) or 48 hours (▲). (B) Cells were exposed to aspirin for the times indicated. Death was assessed by the binding of annexin V to phosphatidylserine exposed on the outside of the apoptotic cell. The percentage annexin V-positive cell was measured using flow cytometry. Mean values±SD are given.
Aspirin-Induced Apoptosis Results in Caspase Activation
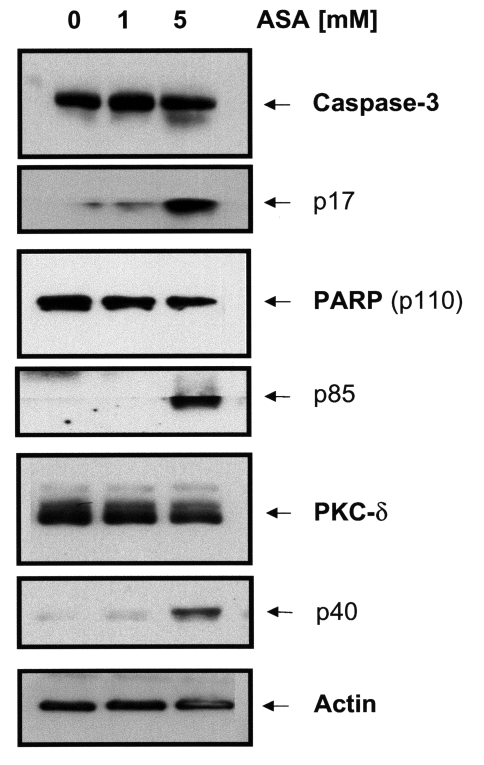
Caspase proteases are important mediators of apoptosis induced by various apoptotic stimuli. To address whether effector caspases are involved in aspirin-induced apoptosis, we examined the cleavage of caspase-3 and its substrates poly(ADP-ribose) polymerase (PARP), and PKC-δ in aspirin-treated HeLa cells by Western blotting. 5 mM aspirin induced cleavage of procaspase 3 into its active subunit p17 within 24 hours, whereas 1 mM aspirin showed no effect (Figure 2). Cleavage of the caspase substrates PARP and PKC-δ into their proteolytic fragments p85 (PARP) and p40 (PKC-δ) was also evident by this time and aspirin concentration.
Figure 2.
Aspirin induced caspase activation in HeLa cells. HeLa cells were treated with 1 and 5 mM of ASA for 24 hours. Western blotting of whole cell extracts demonstrated cleavage of procaspase 3 into its active subunit p17, and cleavage of caspase substrates PARP and PKC-δ into their proteolytic fragments p85 and p40 with 5 mM aspirin.
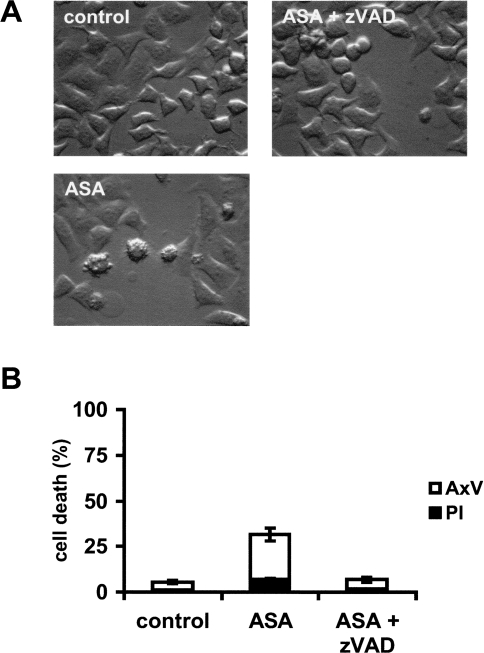
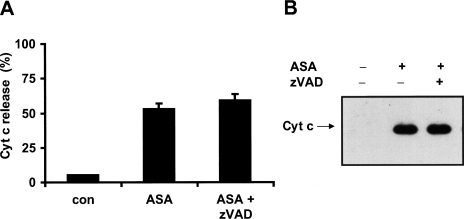
To determine whether caspase activation is required for the execution of aspirin-mediated apoptosis in HeLa cells the broad-spectrum caspase inhibitor zVAD-fmk (100 µM) was added to the cells 1 hour before treatment with 5 mM aspirin for 24 hours. Pretreatment of the cells with the caspase inhibitor prevented the morphologic appearance of apoptotic cells, i.e., blebbing and shrinkage (Figure 3A). To further confirm that aspirin induces HeLa cells to undergo apoptosis, cells were assayed for their ability to bind annexin V (to detect phosphatidylserine exposure) while excluding PI (to detect loss of plasma membrane integrity) by flow cytometry. As shown in Figure 3B, HeLa cells treated with aspirin for 24 hours resulted in a large population of cells that stained positive for annexin V, but without PI uptake. Pretreatment of the cells with the caspase inhibitor zVAD-fmk significantly reduced the number of apoptotic cells (Figure 3B).
Figure 3.
Aspirin-induced apoptosis is prevented by caspase inhibitor zVAD-fmk. HeLa cells were treated with 5 mM ASA and/or zVAD-fmk (100 µM). After 24 hours cells were analyzed by (A) microscopy or (B) FACS analysis with annexin V and PI.
Cytochrome c is Released from Mitochondria During Aspirin-Induced Apoptosis
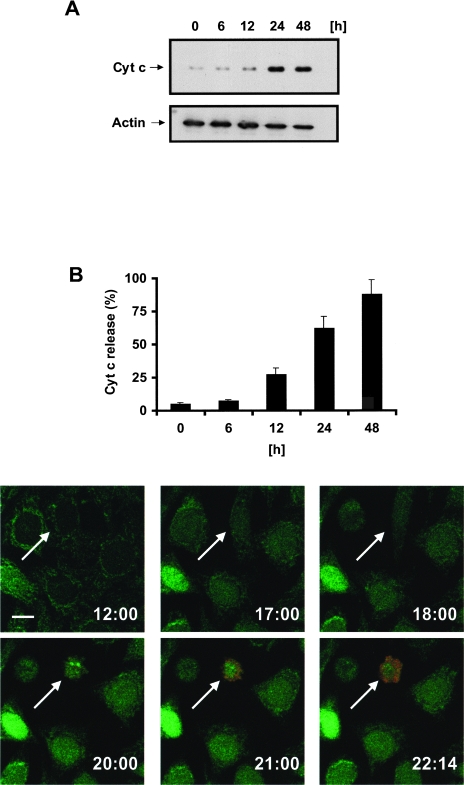
To investigate whether cytochrome c is released during aspirin-induced apoptosis, we determined cytochrome c levels in the cytosolic fractions of HeLa-Cc-GFP cells that have been exposed to 5 mM aspirin using Western blotting (Figure 4A), flow cytometry (Figure 4B), and multicolor time-lapse confocal microscopy (Figure 4C). After 12 hours of treatment the cells started to release cytochrome c into the cytosol as detected by Western blot and FACS analysis (Figure 4, A and B). After 48 hours most of the cells had released cytochrome c. Models of downstream apoptotic events begin with the release of cytochrome c and resultant caspase activation. This is followed by phosphatidylserine externalization and loss of plasma membrane integrity. We used time-lapse confocal microscopy to determine when cytochrome c-GFP release occurred during aspirin-induced apoptosis. Analysis of a single HeLa-Cc-GFP cell showed that the release of cytochrome c-GFP, as indicated by the change of punctated into diffuse expression pattern, occurred approximately 3 hours before the loss of plasma membrane integrity — as detected by uptake of propidium iodide (red) (Figure 4C). These observations suggest that the release of cytochrome c is an early event in aspirin-induced apoptosis. Pretreatment of the cells with the caspase inhibitor zVAD-fmk failed to block the release of cytochrome c (Figure 5, A and B). This is expected if caspase activation is a consequence of cytochrome c release.
Figure 4.
Aspirin induces the release of cytochrome c from HeLa cells. HeLa-Cc-GFP cells were treated with 5 mM ASA and analyzed for cytochrome c release by means of (A) Western blot with cytochrome c antibody, (B) FACS analysis (CLAMI assay), or (C) multicolor time-lapse confocal microscopy. Cytochrome c-GFP (green) moves from a mitochondrial staining pattern to a cytoplasmic distribution. Loss of plasma membrane integrity was detected by uptake of propidium iodide (red). The arrow indicates a cytochrome c-releasing cell; the number at the bottom right of each frame represents the time after treatment with aspirin. Images were taken with a x60 objective and zoomed 1.8 times. Scale bar= 15 µm.
Figure 5.
Aspirin-induced cytochrome c release is not prevented by zVAD-fmk. HeLa cells were treated with 5 mM ASA and/or zVAD-fmk (100 µM) for 24 hours and analyzed for cytochrome c release with (A) FACS analysis (CLAMI assay) or (B) Western blot.
Bcl-2 Prevents Aspirin-Induced Cytochrome c Release and Apoptosis
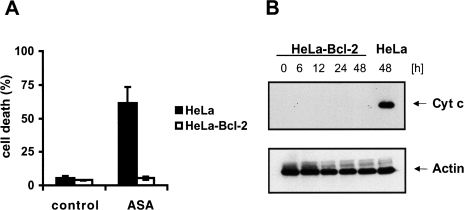
The antiapoptotic protein Bcl-2 exerts its antiapoptotic function by preventing the release of cytochrome c from the mitochondria. If cytochrome c plays a role in aspirin-induced apoptosis then Bcl-2 should be able to protect cells from aspirin-induced apoptosis. We analyzed HeLa cells, which stably overexpress cytochrome c-GFP and Bcl-2 (HeLa-Cc-GFP-Bcl-2). As shown in Figure 6A these cells were almost completely resistant to aspirin-induced apoptosis compared with their control cells. Next we examined whether Bcl-2 is capable of preventing aspirin-induced cytochrome c release. Therefore we treated HeLa-Cc-GFP-Bcl-2 cells with 5 mM aspirin up to 48 hours. As shown in Figure 6B, Bcl-2 prevented aspirin-induced cytochrome c release completely. Cells that overexpress Bcl-XL, another anti-apoptotic Bcl-2 family member, were also resistant to aspirin-induced apoptosis (data not shown).
Figure 6.
Overexpression of Bcl-2 prevented aspirin-induced cytochrome c release and apoptosis. (A) FACS analysis and (B) Western blotting of cytosolic extracts after treatment with 5 mM aspirin for the indicated time period. The percentage of annexin V positive cells was measured by FACS analysis after treatment with aspirin for 48 hours.
Apaf-1 -/- Cells are Resistant to Aspirin-Induced Apoptosis
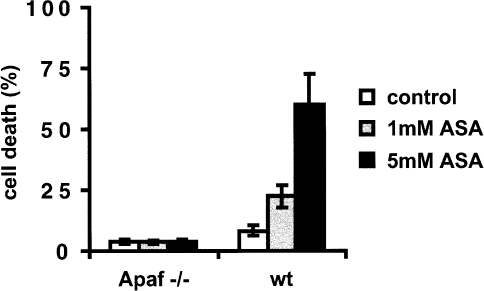
If the mitochondria and the release of cytochrome c play an active role in aspirin-induced apoptosis, then cells lacking Apaf-1, a pro-apoptotic molecule that has been shown to activate caspases and mediate apoptosis by binding cytochrome c [20], should also be resistant to aspirin-induced apoptosis. As seen in Figure 7 Apaf-1 -/- embryonic fibroblasts were almost completely resistant to aspirin-induced apoptosis (concentration 1 and 5 mM) compared to wild-type cells. In contrast, Apaf-1 -/- fibroblasts were still susceptible to TNF-induced apoptosis (data not shown), a form of apoptosis that does not require cytochrome c release for caspase activation [29].
Figure 7.
Apaf-1 -/- cells are resistant to aspirin-induced apoptosis. Wt cells and Apaf-1 -/- cells were treated with 1 and 5 mM ASA for 48 hours. After this time the percentage of annexin V positive cells was measured by FACS analysis. Note that cells that lack annexin V staining are completely intact.
Aspirin Induces Translocation of GFP-Tagged Bax from the Cytosol to the Mitochondria
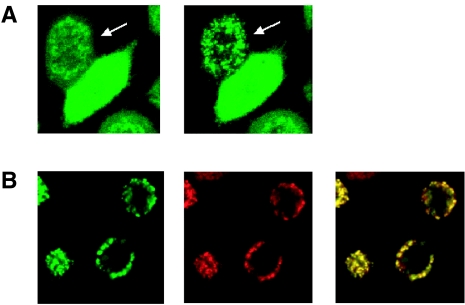
To gain insight into the mechanism by which aspirin induces cytochrome c release, we studied the pro-apoptotic Bcl-2 family member Bax in HeLa cells during aspirin-induced apoptosis. Although it is only one of several members of this family, Bax has recently been shown to be required for NSAID-induced apoptosis in a colon carcinoma line [30]. We found, however, that Bax expression did not change in response to treatment with 5 mM aspirin (data not shown). During apoptosis, Bax can translocate from the cytosol to the mitochondria [31], where it functions to mediate mitochondrial outer membrane permeability and cytochrome c release [32]. To investigate this, we stably expressed GFP-tagged Bax in HeLa cells (HeLa-Bax-GFP). When apoptosis was induced by treatment of the cells with 5 mM aspirin, Bax-GFP was redistributed from a diffuse pattern to a perinuclear, punctate pattern (Figure 8A). Following redistribution the Bax-GFP signal colocalized with the signal obtained by the mitochondrial dye TMRE (Figure 8B), arguing for a translocation of Bax-GFP from the cytosol to the mitochondria in response to aspirin treatment. Like aspirin-induced cytochrome c release, Bax translocation also occurred in the presence of the caspase inhibitor zVAD-fmk, ordering this event upstream of caspase-activation in aspirin-induced apoptosis.
Figure 8.
Aspirin induces caspase-independent translocation of Bax to mitochondria. HeLa-Bax-GFP cells were treated with 5 mM aspirin and Bax-GFP was monitored by time-lapse confocal microscopy. (A) After treatment with aspirin Bax-GFP changed from a diffuse cytoplasmic to a perinuclear pattern in the presence or absence (data not shown) of zVAD-fmk (100 µM). The arrow indicates a cell with Bax translocation to the mitochondria. Images were taken with a x40 objective and zoomed 2.0 times. (B) Left image (green) is translocated Bax-GFP after aspirin treatment. Middle image (red) is the mitochondrial marker TMRE (40 nM). The majority of translocated Bax-GFP is colocalized with TMRE as seen by overlaying the green and red images (yellow).
Discussion
Aspirin has attracted considerable attention as one of many compounds that may be of potential benefit in the chemoprevention of cancer. Recent interest is based on results from epidemiological, clinical, and animal studies, which have shown an antineoplastic effect of this drug [1,3,33–37]. The mechanisms responsible for the inhibition of tumor formation and/or growth are not completely understood. One obvious potential mechanism is the inhibition of COX activity, because prostaglandin levels are increased in human colorectal tumors [38], and a selective group of eicosanoids can increase the proliferation rate of human colon cancer cells [39,40]. In addition overexpression of COX-2 in epithelial cells inhibits apoptosis [41]. However, the chemopreventive effect of NSAIDs in general can not be explained on the basis of prostaglandin synthesis inhibition alone, as a number of NSAIDs have antiproliferative effects in cells that lack COX activity and the addition of exogenous prostaglandins to cultures failed to offset the antineoplastic and antiangiogenic effect of NSAIDs [42,43]. Therefore the chemopreventive effects of NSAIDs are also believed to involve COX-independent pathways.
Aspirin has well-documented non-COX effects, including inhibition of NF-κB activation [13], and was shown to induce apoptosis by activation of p38 MAP kinase [15], and activation of caspases [11]. There are also reports about the effects of aspirin and its active metabolite salicylate on mitochondria, such as the uncoupling of oxidative phosphorylation [16] and induction of the mitochondrial permeability transition pore [18]. These studies link the mitochondrial damage caused by aspirin to its side effects, such as gastric mucosal injury, aspirin poisoning (salicylism) and Reye's syndrome (a disease characterized by impaired metabolism and mitochondrial swelling, especially in liver and brain). Some recent studies found mitochondrial cytochrome c release and induction of apoptosis after treatment with the NSAID indomethacin [44,45]. Therefore we studied the role and the mechanism of mitochondrial cytochrome c release during aspirin-induced apoptosis in a cancer cell line.
We have found that aspirin — in concentrations that can be measured in the serum of patients treated with aspirin for chronic inflammatory disease [28] — induced cell death in a time- and dose-dependent manner. This cell death has features of apoptosis such as caspase activation, PARP and PKC-δ cleavage, phosphatidylserine externalization, and chromatin condensation. Aspirin-induced apoptosis was inhibited by the classical caspase inhibitor zVAD-fmk. To investigate the role of cytochrome c, in this death process we employed a cell line that stably expresses cytochrome c-GFP and found that cytochrome c is released from mitochondria during aspirin-induced apoptosis, which occurred before loss of plasma membrane integrity. The release of cytochrome c was not inhibited by zVAD-fmk. Therefore no caspase activation upstream of mitochondria seems to be involved. Overexpression of the antiapoptotic molecule Bcl-2, which exerts its effect by preventing the release of cytochrome c from mitochondria, protected cells from aspirin-induced cytochrome c release and apoptosis. Furthermore cells that lack Apaf-1, a molecule that mediates apoptosis through its ability to bind cytochrome c and activate caspase-9 are resistant to aspirin-mediated apoptosis. These results suggest that the release of cytochrome c is an important part of the apoptotic mechanism of aspirin and may play a role in the antineoplastic effect of this drug.
How does aspirin induce the release of cytochrome c? Cytochrome c normally resides in the intermembrane space of the mitochondria and is involved in electron transfer [46]. However, it is released into the cytosol during apoptosis, and this release is regulated by the Bcl-2 family of proteins [47]. During apoptosis the proapoptotic Bcl-2 family member Bax undergoes a conformational change, which is followed by its redistribution from the cytosol to the outer mitochondrial membrane, into which it inserts to induce the release of cytochrome c [48,49]. It has been shown that aspirin can induce Bax expression in colorectal cancer [50] and treatment with sulindac and indomethacin decrease Bcl-XL levels in colorectal cancer cells [30], thereby altering the ratio of pro- to antiapoptotic molecules to cause death. However, in our cells Bax and Bcl-XL levels remained unchanged after treatment with aspirin.
Nevertheless, aspirin is able to induce caspase-independent translocation of Bax, which may be a mechanism by which aspirin causes cytochrome c release. The translocation of Bax involves the oligomerization of this molecule, and this appears to require exposure of the hydrophobic BH3 region [51]. Therefore, aspirin might act directly to affect Bax conformation and trigger its translocation. Alternatively, such changes in Bax can be induced by other members of the Bcl-2 family, the “BH3-only” proteins [52], and therefore one or more of these may be the target of aspirin. Further studies are needed to determine how aspirin can induce translocation of Bax to the mitochondria, and whether this is the major mechanism for aspirin-induced apoptosis.
In summary, our work establishes that aspirin is capable of inducing apoptosis in a human cancer cell line. This apoptosis is accomplished by inducing the release of cytochrome c from the mitochondria and the subsequent activation of caspases. This mechanism may be part of the beneficial effect of NSAIDs in the prevention of cancer.
Acknowledgements
We thank Dr. Richard J. Youle (National Institute of Health, Bethesda, MD) for the Bax-GFP construct. This work was supported by US National Institutes of Health grants CA69381 and AI40646 awarded to D.R.G.; K.C.Z is recipient of a fellowship from the Deutscher Akademischer Austauschdienst (DAAD). This is publication no. 396 of the La Jolla Institute for Allergy and Immunology.
Abbreviations
- COX
cyclooxygenase
- Cyt c
cytochrome c
- FACS
fluorescence-activated cell sorter
- GFP
green fluorescent protein
- NSAID
nonsteroidal anti-inflammatory drug
- PBS
phosphate-buffered saline
- PI
propidium iodide
- wt
wild type
- zVAD-fmk
benzoyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone
References
- 1.Greenberg ER, Baron JA, Freeman DH, Jr, Mandel JS, Haile R. Reduced risk of large-bowel adenomas among aspirin users. The Polyp Prevention Study Group. J Natl Cancer Inst. 1993;85:912–916. doi: 10.1093/jnci/85.11.912. [DOI] [PubMed] [Google Scholar]
- 2.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 3.Giovannucci E, Egan KM, Hunter DJ, Stampfer MJ, Colditz GA, Willett WC, Speizer FE. Aspirin and the risk of colorectal cancer in women. N Engl J Med. 1995;333:609–614. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- 4.Giardiello FM. NSAID-induced polyp regression in familial adenomatous polyposis patients. Gastroenterol Clin North Am. 1996;25:349–362. doi: 10.1016/s0889-8553(05)70251-x. [DOI] [PubMed] [Google Scholar]
- 5.Thun MJ. NSAID use and decreased risk of gastrointestinal cancers. Gastroenterol Clin North Am. 1996;25:333–348. doi: 10.1016/s0889-8553(05)70250-8. [DOI] [PubMed] [Google Scholar]
- 6.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 7.Otto JC, Smith WL. Prostaglandin endoperoxide synthases-1 and -2. J Lipid Mediators Cell Signalling. 1995;12:139–156. doi: 10.1016/0929-7855(95)00015-i. [DOI] [PubMed] [Google Scholar]
- 8.Shiff SJ, Qiao L, Tsai LL, Rigas B. Sulindac sulfide, an aspirin-like compound, inhibits proliferation, causes cell cycle quiescence, and induces apoptosis in HT-29 colon adenocarcinoma cells. J Clin Invest. 1995;96:491–503. doi: 10.1172/JCI118060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piazza GA, Rahm AK, Finn TS, Fryer BH, Li H, Stoumen AL, Pamukcu R, Ahnen DJ. Apoptosis primarily accounts for the growth-inhibitory properties of sulindac metabolites and involves a mechanism that is independent of cyclooxygenase inhibition, cell cycle arrest, and p53 induction. Cancer Res. 1997;57:2452–2459. [PubMed] [Google Scholar]
- 10.Shiff SJ, Koutsos Ml, Qiao L, Rigas B. Nonsteroidal antiinflammatory drugs inhibit the proliferation of colon adenocarcinoma cells: effects on cell cycle and apoptosis. Exp Cell Res. 1996;222:179–188. doi: 10.1006/excr.1996.0023. [DOI] [PubMed] [Google Scholar]
- 11.Bellosillo B, Pique M, Barragan M, Castano E, Villamor N, Colomer D, Montserrat E, Pons G, Gil J. Aspirin and salicylate induce apoptosis and activation of caspases in B-cell chronic lymphocytic leukemia cells. Blood. 1998;92:1406–1414. [PubMed] [Google Scholar]
- 12.Klampfer L, Cammenga J, Wisniewski HG, Nimer SD. Sodium salicylate activates caspases and induces apoptosis of myeloid leukemia cell lines. Blood. 1999;93:2386–2394. [PubMed] [Google Scholar]
- 13.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 14.Chan TA, Morin PJ, Vogelstein B, Kinzler KW. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc Natl Acad Sci USA. 1998;95:681–686. doi: 10.1073/pnas.95.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwenger P, Bellosta P, Vietor I, Basilico C, Skolnik EY, Vilcek J. Sodium salicylate induces apoptosis via p38 mitogen-activated protein kinase but inhibits tumor necrosis factor-induced c-Jun N-terminal kinase/stress-activated protein kinase activation. Proc Natl Acad Sci USA. 1997;94:2869–2873. doi: 10.1073/pnas.94.7.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrescu I, Tarba C. Uncoupling effects of diclofenac and aspirin in the perfused liver and isolated hepatic mitochondria of rat. Biochim Biophys Ada. 1997;1318:385–394. doi: 10.1016/s0005-2728(96)00109-0. [DOI] [PubMed] [Google Scholar]
- 17.Somasundaram S, Rafi S, Hayllar J, Sigthorsson G, Jacob M, Price AB, Macpherson A, Mahmod T, Scott D, Wrigglesworth JM, Bjarnason I. Mitochondrial damage: a possible mechanism of the topical phase of NSAID induced injury to the rat intestine. Gut. 1997;41:344–353. doi: 10.1136/gut.41.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biban C, Tassani V, Toninello A, Siliprandi D, Siliprandi N. The alterations in the energy linked properties induced in rat liver mitochondria by acetylsalicylate are prevented by cyclosporin A or Mg2+ Biochem Pharmacol. 1995;50:497–500. doi: 10.1016/0006-2952(95)00165-v. [DOI] [PubMed] [Google Scholar]
- 19.Al-Nasser IA. Salicylate-induced kidney mitochondrial permeability transition is prevented by cyclosporin A. Toxicol Lett. 1999;105:1–8. doi: 10.1016/s0378-4274(98)00373-7. [DOI] [PubMed] [Google Scholar]
- 20.Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 21.Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 1998;94:727–737. doi: 10.1016/s0092-8674(00)81732-8. [DOI] [PubMed] [Google Scholar]
- 22.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 23.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bel-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 24.Finucane DM, Bossy-Wetzel E, Waterhouse NJ, Cotter TG, Green DR. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. J Biol Chem. 1999;274:2225–2233. doi: 10.1074/jbc.274.4.2225. [DOI] [PubMed] [Google Scholar]
- 25.Jurgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol. 2000;2:156–162. doi: 10.1038/35004029. [DOI] [PubMed] [Google Scholar]
- 28.Insel PA. Analgesic-antipyretic and anti-inflammatory agents and drugs employed in the treatment of govt. In: Harman JG, Molinoff PB, Rudden RW, Gilman AG, editors. The pharmacological basis of therapeutics. New York, NY: McGraw-Hill; 1996. pp. 617–657. [Google Scholar]
- 29.Li K, Li Y, Shelton JM, Richardson JA, Spencer E, Chen ZJ, Wang X, Williams RS. Cytochrome c deficiency causes embryonic lethality and attenuates stress-induced apoptosis. Cell. 2000;101:389–399. doi: 10.1016/s0092-8674(00)80849-1. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- 31.Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eskes R, Antonsson B, Osen-Sand A, Montessuit S, Richter C, Sadoul R, Mazzei G, Nichols A, Martinou JC. Bax-induced cytochrome c release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J Cell Biol. 1998;143:217–224. doi: 10.1083/jcb.143.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thun MJ, Namboodiri MM, Calle EE, Flanders WD, Heath CW., Jr Aspirin use and risk of fatal cancer. Cancer Res. 1993;53:1322–1327. [PubMed] [Google Scholar]
- 34.Reddy BS, Rao CV, Rivenson A, Kelloff G. Inhibitory effect of aspirin on azoxymethane-induced colon carcinogenesis in F344 rats. Carcinogenesis. 1993;14:1493–1497. doi: 10.1093/carcin/14.8.1493. [DOI] [PubMed] [Google Scholar]
- 35.Lee IM, Hennekens CH, Buring JE. Use of aspirin and other nonsteroidal antiinflammatory drugs and then risk of cancer development. In: DeVita VT, Hellmann S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. Philadelphia, PA: Lippincott-Raven; 1997. pp. 599–607. [Google Scholar]
- 36.Williams CS, Smalley W, DuBois RN. Aspirin use and potential mechanisms for colorectal cancer prevention. J Clin Invest. 1997;100:1325–1329. doi: 10.1172/JCI119651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahmoud NN, Dannenberg AJ, Mestre J, Bilinski RT, Churchill MR, Martucci C, Newmark H, Bertagnolli MM. Aspirin prevents tumors in a murine model of familial adenomatous polyposis. Surgery. 1998;124:225–231. [PubMed] [Google Scholar]
- 38.Rigas B, Goldman IS, Levine L. Altered eicosanoid levels in human colon cancer. J Lab Clin Med. 1993;122:518–523. [PubMed] [Google Scholar]
- 39.Qiao L, Kozoni V, Tsioulias GJ, Koutsos MI, Hanif R, Shiff SJ, Rigas B. Selected eicosanoids increase the proliferation rate of human colon carcinoma cell lines and mouse colonocytes in vivo. Biochim Biophys Ada. 1995;1258:215–223. doi: 10.1016/0005-2760(95)00100-q. [DOI] [PubMed] [Google Scholar]
- 40.Bortuzzo C, Hanif R, Kashfi K, Staiano-Coico L, Shiff SJ, Rigas B. The effect of leukotrienes B and selected HETEs on the proliferation of colon cancer cells. Biochim Biophys Ada. 1996;1300:240–246. doi: 10.1016/0005-2760(96)00003-3. [DOI] [PubMed] [Google Scholar]
- 41.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 42.de Mello MC, Bayer BM, Beaven MA. Evidence that prostaglandins do not have a role in the cytostatic action of anti-inflammatory drugs. Biochem Pharmacol. 1980;29:311–318. doi: 10.1016/0006-2952(80)90506-7. [DOI] [PubMed] [Google Scholar]
- 43.Hanif R, Pittas A, Feng Y, Koutsos MI, Qiao L, Staiano-Coico L, Shiff SI, Rigas B. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem Pharmacol. 1996;52:237–245. doi: 10.1016/0006-2952(96)00181-5. [DOI] [PubMed] [Google Scholar]
- 44.Fujii Y, Matsura T, Kai M, Matsui H, Kawasaki H, Yamada K. Mitochondrial cytochrome c release and caspase-3-like protease activation during indomethacin-induced apoptosis in rat gastric mucosal cells. Proc Soc Exp Biol Med. 2000;224:102–108. doi: 10.1046/j.1525-1373.2000.22407.x. [DOI] [PubMed] [Google Scholar]
- 45.Aggarwal S, Taneja N, Lin L, Orringer MB, Rehemtulla A, Beer DG. Indomethacin-induced apoptosis in esophageal adenocarcinoma cells involves upregulation of Bax and translocation of mitochondrial cytochrome c independent of COX-2 expression. Neoplasia. 2000;2:346–356. doi: 10.1038/sj.neo.7900097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cortese JD, Voglino AL, Hackenbrock CR. Persistence of cytochrome c binding to membranes at physiological mitochondrial intermembrane space ionic strength. Biochim Biophys Acta. 1995;1228:216–228. doi: 10.1016/0005-2728(94)00178-8. [DOI] [PubMed] [Google Scholar]
- 47.Antonsson B, Martinou JC. The Bcl-2 protein family. Exp Cell Res. 2000;256:50–57. doi: 10.1006/excr.2000.4839. [DOI] [PubMed] [Google Scholar]
- 48.Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci USA. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nomura M, Shimizu S, Ito T, Narita M, Matsuda H, Tsujimoto Y. Apoptotic cytosol facilitates Bax translocation to mitochondria that involves cytosolic factor regulated by Bcl-2. Cancer Res. 1999;59:5542–5548. [PubMed] [Google Scholar]
- 50.Ruschoff J, Wallinger S, Dietmaier W, Bocker T, Brockhoff G, Hofstadter F, Fishel R. Aspirin suppresses the mutator phenotype associated with hereditary nonpolyposis colorectal cancer by genetic selection. Proc Natl Acad Sci USA. 1998;95:11301–11306. doi: 10.1073/pnas.95.19.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Antonsson B, Montessuit S, Lauper S, Eskes R, Martinou JC. Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem J. 2000;345:271–278. [PMC free article] [PubMed] [Google Scholar]
- 52.Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou JC. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]