INTRODUCTION
Signal-transducing GTPases in plants include small G proteins, heterotrimeric G proteins, and, potentially, several unique types of GTP binding proteins that are not members of either of the aforementioned classes. This review will focus on recent discoveries concerning the roles of heterotrimeric and “unconventional” G proteins in plant cell signaling. Small G proteins are reviewed elsewhere in this issue (Yang, 2002). Plant heterotrimeric G proteins have been the subject of several other recent reviews to which the reader is also referred (Ma, 1994; Assmann, 1996; Hooley, 1998; Bischoff et al., 1999; Fujisawa et al., 2001). Ma's review (1994) does a particularly good job of summarizing the early, mostly biochemical and immunological, progress toward identifying plant G proteins, which will not be covered here.
HETEROTRIMERIC G PROTEINS: THE MAMMALIAN PARADIGM
Much of the conceptual framework for signaling by heterotrimeric G proteins has been provided by extensive research in mammalian systems. To provide a context and a framework for comparison, the mammalian paradigm (Figure 1) is first reviewed briefly. Heterotrimeric G proteins are GTPases composed of α, β, and γ subunits. These GTPases are classically associated with plasma membrane receptors containing seven transmembrane domains (heptahelical receptors, 7-TMS receptors, or G protein–coupled receptors [GPCRs]). Receptor activation activates the G protein by inducing the exchange of GTP for GDP at a binding site on Gα. Gα and/or Gβγ then goes on to interact with effector proteins. Endogenous GTPase activity of Gα eventually returns the α subunit to its inactive, GDP-bound form, resulting in reassociation of the trimer. In exceptional cases, the dissociation of Gα and Gβγ may not be obligatory for activation of the G protein (Klein et al., 2000).
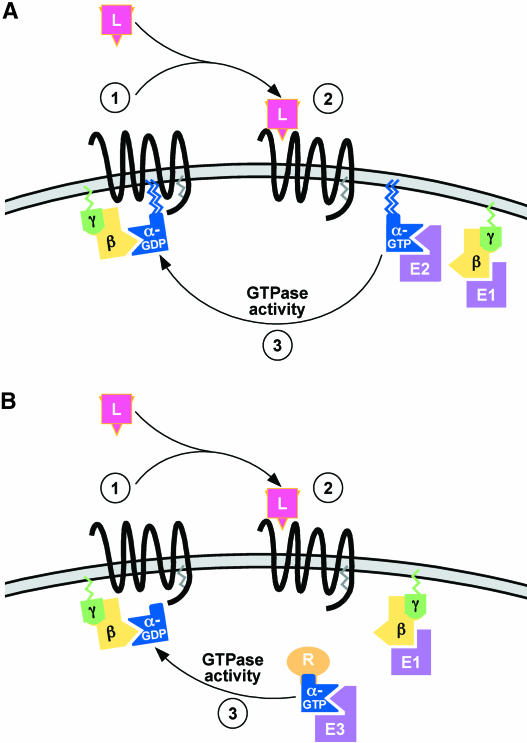
Figure 1.
Mammalian Heterotrimeric G-Protein Cycle.
(A) Classic heterotrimeric GTPase cycle. Ligand (L) binding to a GPCR activates the associated G protein, promoting Gα and/or Gβγ interaction with downstream effectors (E). Intrinsic GTPase activity of the Gα subunit eventually returns the G protein to an inactive state. The Gα subunit remains closely associated with the plasma membrane.
(B) Certain Gαs (von Zastrow and Mostov, 2001; Zheng et al., 2001) can show dissociation from the plasma membrane upon GPCR activation, contingent upon the absence or removal of lipid modification and the presence of regulatory (R) proteins.
Mammalian Gα subunits (39 to 46 kD) contain ∼20% highly conserved amino acids (Morris and Malbon, 1999). Gαs also exhibit conservation of several important structural domains: a Ras-like GTPase domain, an α-helical domain that influences the spontaneous GDP release rate, and an Asp/Glu-rich loop, which is involved in conformational changes upon GTP binding (Bourne et al., 1991). The presence of the latter two domains is one of the characteristics that distinguish Gα proteins from small GTPases. In mammals, ∼20 Gα subunits have been identified. Four classes of mammalian Gαs exist, designated Gαi, Gαs, Gαq, and Gα12/13 (Ma, 1994; Wilkie and Yokoyama, 1994). Gαi and Gαs were the initial members, identified by biochemical means, and their subscripts refer to inhibitory or stimulatory effects on the enzyme adenylate cyclase. Some species of Gα undergo lipid modifications that promote membrane association (Figure 2A) (Casey, 1994).
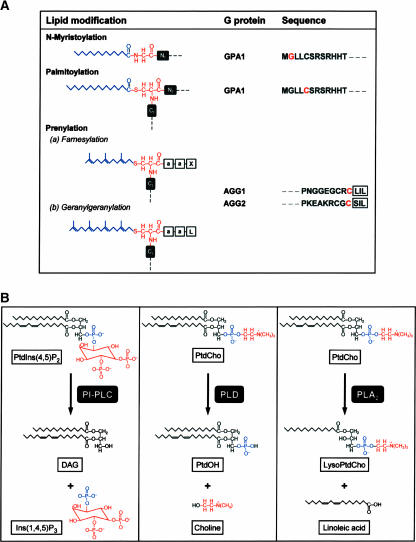
Figure 2.
Lipid Modifiers and Effectors of G Proteins.
(A) Three types of G protein lipid modifications are shown. N-Myristoylation occurs by the attachment of the saturated fatty acid myristate (blue) to a conserved acceptor Gly (red) next to the initiator Met via a stable amino bond. In palmitoylated proteins, the saturated 16-carbon fatty acid palmitate (blue) is attached to Cys residues (red) via a labile thioester bond. Protein prenylation involves the attachment of the 15- and 20-carbon isoprenes farnesyl and geranylgeranyl (blue), respectively, to conserved Cys residues (red) at the C-terminal ends of proteins via a nonreversible thioester bond. The acceptor Cys residues are part of a conserved CaaX box motif in which C indicates Cys, a represents an aliphatic amino acid, and X is usually Ser, Met, Cys, Ala, Gln, or Leu. Ct and Nt indicate C- and N-terminal amino acid positions, respectively, relative to the acceptor amino acid. Proteolysis removes the final three amino acids, and the new C-terminal Cys is then prenylated. The Arabidopsis prototypical GPA1 contains a conserved myristoylation motif and a putative sequence for palmitoylation in the N-terminal region. The two Arabidopsis G protein γ subunits (AGG1 and AGG2) contain a prenyl group binding site in the C-terminal region.
(B) Cleavage sites and hydrolysis products of phospholipases regulated by G proteins. PLA2 is an acylhydrolase that specifically removes the acyl chain from the sn-2 position of the glycerol backbone (black). PLD and PI-PLC are phosphodiesterases that generate similar products, except that the phosphate group (blue) either stays with the lipid moiety (black) or goes with the head group (red). DAG, sn-1-palmitoyl,2-linoleoyl diacylglycerol; Ins(1,4,5)P3, inositol 1,4,5-trisphosphate; LysoPtdCho, sn-1-lysophosphatidylcholine; PtdCho, sn-1-palmitoyl,2-linoleoyl phosphatidylcholine; PtdIns(4,5)P2, phosphatidylinositol 4,5-bisphosphate; PtdOH, sn-1-palmitoyl,2-linoleoyl phosphatidate.
Mammals also possess ∼5 distinct Gβ and at least 12 Gγ subunits (Seack et al., 1998; Cook et al., 2001). A hallmark of the ∼35-kD Gβ proteins is the WD-40 motif, consisting of seven or eight tandem repeats and a conserved Trp-Asp (WD) motif. These repeats assemble into a seven-bladed β-propeller structure (Lambright et al., 1996; Sondek et al., 1996). Gγ proteins range in mass from 7 to 10 kD and are not highly conserved. However, all Gγs possess the C-terminal CaaX (where “a” is an aliphatic amino acid) site for isoprenylation (Figure 2A), which confers membrane association. A coiled-coil structure formed between Gβ and Gγ results in a noncovalent but very tight interaction between these two subunits, such that they function as a nondissociable dimer.
A nonexhaustive list of some Gα subunit effectors in mammalian systems includes adenylate cyclase, cyclic GMP phosphodiesterase, phosphoinositide 3-kinase, the phosphoinositide (PI)-phospholipase Cβ (PI-PLCβ; Figure 2B) (Singer et al., 1997), phospholipase D (PLD; Figure 2B) (Singer et al., 1997), Na+/H+ exchange transporter (Voyno-Yasenetskaya et al., 1994; Voyno-Yasenetskaya, 1998), the TUBBY transcription factor (Santagata et al., 2001), and K+, Ca2+, and, to a lesser extent, Cl− and Na+ channels (Brown and Birnbaumer, 1990; Morris and Malbon, 1999; Reddy et al., 2001). Gβγ targets include phospholipase A2 (PLA2; Figure 2B), calcium channels, and some isoforms of adenylate cyclase and PI-PLCβ (Jelsema and Axelrod, 1987; Logothetis et al., 1987; Kim et al., 1989; Katz et al., 1992; de Waard et al., 1997; Morris and Malbon, 1999).
A nonexhaustive list of some ligands that interact with GPCRs to activate mammalian heterotrimeric G protein signaling includes light (Gt, members of the Gαi class), odorants (Golf, members of the Gs subfamily), sweet and bitter molecules (Ggust, members of the Gi subfamily), and numerous hormones and neurotransmitters (many different Gαs). Given this list, it is not surprising that mutations in G protein signaling components are responsible for numerous human genetic diseases. In addition, some bacterial diseases target G protein pathways. Indeed, the toxins from Bordetella pertussis (causative agent of whooping cough) and Vibrio cholerae (causative agent of cholera) are important experimental tools.
Cholera toxin (CTX)–induced ADP-ribosylation of an Arg residue on Gα inhibits its GTPase activity, resulting in persistent Gα activation. Although this residue is conserved among all Gαs, only some types of Gαs undergo ADP-ribosylation by CTX, possibly because of differential susceptibility to ADP-ribosylation factor (ARF). Pertussis toxin (PTX)–induced Gα ADP-ribosylation on a Cys near the Gα C terminus induces inactivation of signaling by interfering with Gα/receptor coupling. Other important experimental compounds include nonhydrolyzable GTPγS, which locks G proteins in their active state, nonhydrolyzable GDPβS, which promotes the inactive state, and the wasp venom mastoparan, which activates G proteins by mimicking the conformation of an activated receptor (Higashijima et al., 1988).
PLANT HETEROTRIMERIC G PROTEIN SUBUNITS
In contrast to mammals and invertebrates such as Caenorhabditis elegans, the Arabidopsis genome contains only one canonical Gα gene, GPA1 (Ma et al., 1990). GPA1 is 36% identical to Gis and transducins (Gts). It lacks the conserved ribosylation site for PTX but contains the sites for CTX and for N-myristoylation (Ma et al., 1990). Our sequence analysis (S. Coursol and S.M. Assmann, unpublished data) also suggests a possible palmitoylation site (Figure 2A). Homologs of GPA1 have been cloned from several dicots and monocots (Table 1) (Ma et al., 1991; Ma, 1994; Ishikawa et al., 1995; Gotor et al., 1996; Kusnetsov and Oelmueller, 1996b; Jones et al., 1998; Perroud et al., 2000). These proteins generally have 70 to 90% identity to each other and lesser identity (34 to 42%) to nonplant Gα subunits (Plakidou-Dymock et al., 1998).
Table 1.
Plant G Protein Designations
| Gene | Species | Classification | Reference |
|---|---|---|---|
| GPA1 | Arabidopsis | Gα | Ma et al., 1990 |
| TGA1 | Tomato | Gα | Ma et al., 1991 |
| LjGPA1 | Lotus | Gα | Poulsen et al., 1994 |
| RGA1/D1 | Rice | Gα | Ishikawa et al., 1995; Seo et al., 1995 |
| SGA1 | Soybean | Gα | Kim et al., 1995 |
| SGA2 | Soybean | Gα | Gotor et al., 1996 |
| NtGPα1 | Tobacco | Gα | Saalbach et al., 1999 |
| NtGA2 | Tobacco | Gα | Ando et al., 2000 |
| LGPα1 | Lupin | Gα | Kusnetsov and Oelmueller, 1996b |
| AfGα1 | Wild oat | Gα | Jones et al., 1998 |
| PGA1, PGA2 | Pea | Gα | Marsh and Kaufman, 1999 |
| SOGA1 | Spinach | Gα | Perroud et al., 2000 |
| NPGPA1 | Nicotiana plumbaginifolia | Gα | Kaydamov et al., 2000 |
| AGB1 | Arabidopsis | Gβ | Weiss et al., 1994 |
| ZGB1 | Maize | Gβ | Weiss et al., 1994 |
| TGB1 | Tobacco | Gβ | Kusnetsov and Oelmueller, 1996a |
| RGB1 | Rice | Gβ | Ishikawa et al., 1996 |
| AfGβ1 | Wild oat | Gβ | Jones et al., 1998 |
| AfGβ2 | Wild oat | Possible Gβ | Jones et al., 1998 |
| NPGPB1 | Nicotiana plumbaginifolia | Gβ | Kaydamov et al., 2000 |
| AGG1 | Arabidopsis | Gγ | Mason and Botella, 2000 |
| AGG2 | Arabidopsis | Gγ | Mason and Botella, 2001 |
| GCR1 | Arabidopsis | Potential heterotrimeric G protein receptor |
Josefsson and Rask, 1997; Plakidou-Dymock et al., 1998 |
| MLO | Barley | Potential heterotrimeric G protein receptor | Devoto et al., 1999 |
| AtXLG1 | Arabidopsis | Extra large GTP binding protein | Lee and Assmann, 1999 |
| PsDRG | Pea | Developmentally regulated G protein | Devitt et al., 1999 |
| AtDRG | Arabidopsis | Developmentally regulated G protein | Etheridge et al., 1999; Devitt et al., 1999 |
| RDH3 | Arabidopsis | Root hair defective (putative GTP-binding protein) |
Schiefelbein and Somerville, 1990 |
| ATGB1 | Arabidopsis | GTP-binding protein | Biermann et al., 1996 |
| fw2.2/ORFX | Tomato | Fruit weight 2.2 (putative GTP-binding protein) |
Frary et al., 2000 |
GPA1 is a single-copy gene in Arabidopsis, but some polyploid species such as soybean have been shown to have two closely related GPA1 genes (Kim et al., 1995; Gotor et al., 1996). There is also a partial cDNA clone from wild oat aleurone, AfGα1, that is only 40% identical to GPA1 and could represent a second class of plant Gα genes (Jones et al., 1998). Detailed enzymological characterization of Arabidopsis GPA1 has not been performed, although recombinant GPA1 has been shown to bind GTPγS with a nanomolar dissociation constant (Wise et al., 1997). The rice G protein α subunit, RGA1, has been confirmed to function as a Gα by classic biochemical tests, including those for binding specificity for GTPγS over other nucleotides, specific GTPase activity, and ADP-ribosylation by CTX (Seo et al., 1995, 1997; Iwasaki et al., 1997).
GPA1 transcript and protein are found in all tissues except mature seed (Weiss et al., 1993), and GPA1 expression is particularly abundant in vascular tissue and actively dividing cells such as meristems (shoot, floral, and root), young embryos, and organ primordia (Weiss et al., 1993; Huang et al., 1994). A similar pattern has been observed for spinach SOGA1 transcript (Perroud et al., 2000). Rice RGA1 is highly expressed in internodes and florets (Fujisawa et al., 1999). Subcellularly, GPA1 has been immunolocalized to the plasma membrane and endoplasmic reticulum in both Arabidopsis and Nicotiana plumbaginifolia (Weiss et al., 1997; Kaydamov et al., 2000), whereas RGA1 has been immunolocalized in total membrane proteins (Seo et al., 1997). A putative Gα subunit also has been immunolocalized to the protonemal cell junctions of the moss Physcomitrella patens (Hutton et al., 1998), where it may regulate intercellular transport (Hutton et al., 1998) or Ca2+ channels (Schumaker and Gizinski, 1996).
The regulation of Gα gene expression by environmental factors has been examined in several species, although not yet in Arabidopsis. In N. plumbaginifolia, the synthetic auxin naphthylacetic acid increases NPGPA1 transcript expression in leaf discs, whereas gibberellins have no effect, abscisic acid (ABA) weakly reduces expression, and salicylic acid strongly reduces transcript levels (Kaydamov et al., 2000). Wounding and osmotic shock also have no effect on transcript levels. RGA1 expression in vegetative tissues is stimulated by light (Seo et al., 1995). Although transcriptional regulation of RGA1 by ABA has not been reported to date, the RGA1 promoter does contain an ABA-responsive element (ABRE) sequence (Seo et al., 1995). In hairy roots of tobacco, which have abundant root hairs resulting from infection by Agrobacterium rhizogenes, NtGPA2 transcript also is abundant (Ando et al., 2000), consistent with Arabidopsis GPA1 abundance in proliferating tissues. In spinach, circadian regulation of SOGA1 gene expression has been suggested (Perroud et al., 2000).
The Arabidopsis genome also contains one canonical Gβ gene, AGB1, with ∼42% similarity to mammalian Gβ subunits (Ma, 1994; Weiss et al., 1994), and homologs have been identified in maize, rice, wild oat, tobacco, and N. plumbaginifolia (Weiss et al., 1994; Ishikawa et al., 1996; Jones et al., 1998; Kusnetsov and Oelmueller, 1996a; Kaydamov et al., 2000). Arabidopsis and maize Gβ transcripts are expressed in roots, shoots, and floral structures (Weiss et al., 1994). Expression of rice RGβ1 is higher in roots than in leaves (Ishikawa et al., 1996). NPGPB1 transcript levels closely follow the hormonal regulation described for NPGPA1, with the exception that ABA increases transcript abundance. NPGPB1 has been immunolocalized to the plasma membrane as well as the endoplasmic reticulum, in parallel with the localization of Gα (Kaydamov et al., 2000).
In tobacco leaves, Gβ has been detected in both plasma membrane and isolated nuclei by protein immunoblot analysis (Peskan and Oelmueller, 2000), with abundance inversely correlated with leaf age. Tobacco Gβ preferentially localizes to lipid rafts within the plasma membrane (Peskan et al., 2000). Lipid rafts are fleeting microdomains of unique lipid composition that are speculated to preferentially sequester certain proteins, thereby perhaps promoting “signalsome” association. Interestingly, mammalian Gi and Gs also were found recently to associate with lipid rafts, whereas Gq is concentrated in caveolae, which are cell surface invaginations that are rich in the protein caveolin (Oh and Schnitzer, 2001).
Gγ genes cannot be identified on the basis of sequence homology alone. Recently, however, two Arabidopsis Gγ subunit genes, AGG1 and AGG2, were identified by virtue of their interaction with a tobacco Gβ protein in the yeast two-hybrid assay (Mason and Botella, 2000, 2001). The predicted Gγ subunits are 48% identical to each other and display 24 to 31% identity with selected mammalian Gγ subunits. AGG1 and AGG2 both possess certain common Gγ characteristics, including small size (10.8 and 11.1 kD), a C-terminal CaaX motif, and an N-terminal region with the appropriate structure to interact with a Gβ subunit. AGG1 and AGG2 also interact with Arabidopsis AGB1 in both yeast two-hybrid and in vitro binding assays (Mason and Botella, 2000, 2001). AGG1 and AGG2 transcripts are expressed in all tissues, with an expression pattern for the most part parallel to that of AGB1 (Mason and Botella, 2000, 2001).
AUXINS, CELL PROLIFERATION, AND DEVELOPMENT
Several early studies implicated G proteins in auxin signal transduction. Auxin was found to promote both GTPγS association with rice coleoptile membrane vesicles and GTP hydrolysis by those vesicles, as would be predicted if auxin activated a G protein cycle (Zaina et al., 1990, 1991). Although not direct confirmation of a role for GPA1 in auxin response, recent studies are consistent with such a role. These studies identified two T-DNA mutant alleles of GPA1 and characterized their phenotypes (Ullah et al., 2001). The gpa1 null mutants exhibit normal root growth but show reduced cell division in both hypocotyls and leaves. Results obtained from the use of the mitotic reporter cyc1At-CDB-GUS in the gpa1 background are suggestive of prolongation of the G1 phase of the cell division cycle.
The high level of GPA1 expression reported in meristematic tissue is consistent with a role for GPA1 in cell division. Also consistent with this possibility is the observation that tobacco BY2 cells that overexpress GPA1 progress more rapidly through the cell cycle (Ullah et al., 2001). Interestingly, the expression of pea Gα subunits in yeast also promotes cell division (budding) of that organism (Marsh and Kaufman, 1999), and this occurs through a pathway independent of yeast mating pheromone signaling (which controls cell division via an endogenous G protein–dependent pathway). Thus, the role of Gα in regulating cell division may result from an interaction with downstream proteins that share a conserved function. One possible downstream target is PLA2, a known G protein effector in mammalian systems that mediates cell proliferation in many (but not all) cell types (Capper and Marshall, 2001). PLA2 is activated by auxin in soybean cell cultures (Scherer and Andre, 1989; Scherer, 1994), and auxin initiates more rapid progression through the cell cycle in cultured plant cells (Hobbie and Estelle, 1994).
gpa1 null mutants also exhibit an alteration in leaf shape to a more rounded, rotundifolia-type leaf. The rotundifolia3 mutant is deficient in a cytochrome P450 that may be involved in steroid biosynthesis (Kim et al., 1998). Ullah et al. (2001) note a reduced sensitivity to brassinolide in the gpa1 mutants, leading Ma (2001) to speculate that GPA1 also may play a role in brassinosteroid synthesis and signaling. Similarly, the Gβ null mutant agb1-1 exhibits a rotundifolia leaf shape (Lease et al., 2001), suggesting that a functional G protein cycle may be important in the control of leaf shape. In addition, the agb1-1 mutant shows alterations in silique morphology similar to those conferred by the erecta mutation, including shorter, wider siliques with blunt tips and shorter, more tightly clustered floral buds.
GIBBERELLINS AND ABA
In keeping with the hypothesis that G protein–based signaling participates in the positive regulation of plant growth, heterotrimeric G proteins have been implicated in gibberellic acid (GA) responses in monocots. Recent breakthroughs in our understanding of the role of RGA1 in rice development have arisen from mutational analyses. The dwarfing mutation d1 maps to a G protein α subunit gene, RGA1, as confirmed by allelism tests among several independent d1 mutants and by complementation. Antisense RGA1 rice plants phenocopy the d1 phenotype (Ashikari et al., 1999; Fujisawa et al., 1999), which includes not only reduced plant height as a result of reduced internode length but also broader, dark green leaf blades and sheaths, reduced elongation of panicles, and round grains (Ashikari et al., 1999).
In the germinating seed of grasses, GA plays an important role in stimulating secretion by the aleurone layer of hydrolytic enzymes such as α-amylase that break down the endosperm, releasing resources that promote the growth of the young seedling. The d1 mutant was classified originally as GA insensitive based on the observation that GA application (10−8 M) did not result in α-amylase production (Mitsunaga et al., 1994). In d1 mutant plants, 10−7 M GA only weakly stimulates expression of the α-amylase gene itself and of the Myb transcription factor believed to activate this gene (Ueguchi-Tanaka et al., 2000; Fujisawa et al., 2001).
Consistent with this observation, earlier studies using wild oat aleurone had shown that Mas7 and GTPγS promote expression of an α-amylase–β-glucuronidase (GUS) reporter gene and α-amylase secretion, whereas GDPβS opposes α-amylase–GUS induction by GA (Jones et al., 1998). These researchers also demonstrated that Gα and the wild oat Gβ gene AfGβ1 are expressed in the wild oat aleurone layer. A second gene expressed in this tissue, AfGβ2, also might encode a Gβ subunit, although its sequence homology with known Gβs is not as high (Jones et al., 1998). In addition to regulating the expression of α-amylase, small or heterotrimeric G proteins (Wang et al., 1993) also may regulate its secretion, because GDPβS inhibits Ca2+-stimulated exocytosis in barley aleurone protoplasts (Homann and Tester, 1997).
Despite the data favoring G protein involvement in GA signaling in aleurone, the Gα subunit does not appear obligatory for this response: in d1 rice, high (10−4 M) GA concentrations can stimulate the production of wild-type levels of α-amylase activity, suggesting RGA1-independent as well as RGA1-dependent pathways of GA action (Ueguchi-Tanaka et al., 2000). In addition, and at first glance surprisingly, G protein activation also appears to play a role in inhibiting the aleurone GA response by ABA. One of the important downstream effectors of the ABA response is PLD, which produces phosphatidic acid and a head group from structural phospholipids (Figure 2B) (Pappan and Wang, 1999). In vitro, GTPγS stimulates PLD activity in barley aleurone membranes, and GDPβS and PTX each inhibits the ABA stimulation of this activity. PTX also reduces the ABA inhibition of GA-stimulated α-amylase production (Ritchie and Gilroy, 2000).
Although identification at the molecular level of a heterotrimeric G protein involved in this response is still awaited, such a demonstration would be consistent with other studies that have provided pharmacological evidence for G protein regulation of PLD in the deflagellation response of Chlamydomonas eugametos and in PLD activation in carnation petals (Munnik et al., 1995). Surprisingly, however, recombinant tobacco GPA1 inhibits recombinant α-type PLD in biochemical assays of PLD activity (Lein and Saalbach, 2001). This inhibition is reduced when NtGPA1 is bound with GTPγS, and the authors speculate that perhaps G protein activation promotes the release of active PLDα from the membrane.
PLD also is activated by ABA in stomatal guard cells (Jacob et al., 1999). Recent studies, although not yet showing a direct interaction of Gα and PLD in this system, provide convincing evidence that ABA signals through G proteins in this cell type as well. Experiments using the same gpa1 null lines used by Ullah et al. (2001) demonstrated that ABA inhibition of light-stimulated stomatal opening is eliminated in these genotypes (Wang et al., 2001). Likewise, ABA inhibition of the inward K+ channels that mediate K+ uptake during stomatal opening also is abolished.
However, not all guard cell ABA responses are eliminated: ABA stimulation of stomatal closure is normal. In patch-clamped guard cells from the gpa1 lines, ABA activation of anion channels that mediate malate2− and Cl− loss during stomatal closure occurs normally if cytosolic pH is allowed to change but is absent if cytosolic pH is strongly buffered. These results suggest that a pathway of ABA-stimulated anion channel regulation dependent on ABA-stimulated changes in cytosolic pH (Blatt, 2000) functions in parallel with, or as a backup to, a GPA1-dependent response during stomatal closure. This example of anion channel regulation by a G protein is unusual, given that only a few mammalian anion channels have been shown to be regulated by G proteins (Reddy et al., 2001).
The results with the gpa1 lines are consistent with an early study of G protein regulation of plant ion channels, in which it was shown that GTPγS, mas7, and CTX inhibit inward K+ channels of guard cells, whereas GDPβS promotes inward K+ channel activity (Fairley-Grenot and Assmann, 1991; Armstrong and Blatt, 1995). The effect of GTPγS was prevented by buffering cytosolic Ca2+ to low concentrations, suggesting that ABA may act through GPA1 to increase cytosolic Ca2+. Biochemical studies (Lee et al., 1996) have shown that ABA activates PLC in guard cells, which produces inositol trisphosphate and diacylglycerol (Figure 2B); the former molecule releases Ca2+ from intracellular stores. Like ABA and GTPγS, Ca2+ strongly inhibits the inward K+ channels (Schroeder and Hagiwara, 1989). In mammalian systems, G proteins function upstream of the β-isoforms of PLC. It will be interesting to determine whether ABA activation of guard cell PLC is eliminated in the gpa1 lines, consistent with this model. In guard cells, ABA appears to signal, perhaps redundantly, through numerous secondary messengers, including not only PLC and PLD but also cyclic ADP-ribose and reactive oxygen species (McAinsh et al., 2000; Assmann and Wang, 2001; Schroeder et al., 2001). Regulation of these signals by GPA1 awaits investigation.
We also have observed the inhibition of inward K+ channel activity by GTPγS, PTX, and CTX and the activation by GDPβS in isolated patches of guard cell plasma membrane (Wu and Assmann, 1994) in experiments in which the solutions bathing both sides of the isolated membrane were provided by the experimenter. One would not expect changes in Ca2+ concentration to occur in this situation, unless Ca2+-permeable channels colocalize with the K+ channels and are activated by GTPγS to provide a transient local increase of Ca2+ concentrations in the vicinity of the K+ channel. (In this regard, it is interesting that ABA activates guard cell Ca2+ channels in isolated membrane patches [Hamilton et al., 2000].) It is possible that GPA1 bypasses cytosolic secondary messengers to regulate the inward K+ channel either by direct physical interaction with the channel or through membrane-associated proteins or lipids (Brown, 1993). This theory is lent credence by the observation that ABA can activate outward K+ channels (Lemtiri-chlieh, 1998) in isolated membrane patches; a similar test needs to be performed for the inward K+ channel. The presence of both cytosol-dependent and membrane-delimited, functionally redundant pathways may indicate the importance of a “fail-safe” regulation of the guard cell response to ABA and drought.
There also is some pharmacological evidence consistent with a role for G protein activation in mediating stomatal opening (Lee et al., 1993; Kelly et al., 1995; Cousson and Vavasseur, 1998). Although these data have yet to be supported by genetic manipulations, a precedent for counteracting G proteins acting on the same process is well established. For example, in mammalian systems, Gs and Gi act in opposition to regulate adenylate cyclase. However, the paucity of plant G protein α subunits suggests that this opposing regulation may derive in plants either from regulation via an unconventional G protein (see below) or from different “set points” of the cellular status of the guard cells that differentially dictate the outcome of G protein activation. For example, it has been demonstrated that guard cells rely on different osmotica to generate stomatal aperture changes dependent on the nature of incoming environmental stimuli (Poffenroth et al., 1992), suggesting that guard cell metabolism can exist in many different states. Likewise in the aleurone, there is evidence for G protein involvement in opposing pathways (see above).
Pharmacology also implicates G proteins in ion channel regulation in other types of plant cells. Thus, GTPγS and CTX both reduce outward K+ currents in Vicia faba mesophyll cells, whereas GDPβS enhances current magnitude and PTX has no effect (Li and Assmann, 1993). In mesophyll cells of this species, ABA reduces outward K+ current (Sutton et al., 2000), which is an effect analogous to that observed with GTPγS and CTX. It will be interesting to determine whether this ABA response, like that of the inward K+ currents of guard cells, is affected in gpa1 knockout plants. In tobacco mesophyll cells, expression of the CTX A1 subunit reduces outward K+ currents, as does the overexpression of NtGPA1 sense RNA. However, overexpression of NtGPA1 antisense RNA has the identical result: a reduction in outward K+ current. These apparently conflicting results suggest the need to assess NtGPA1 protein levels in transgenic plants, an experiment that the authors report was technically problematic (Saalbach et al., 1999). Wegner and de Boer (1997) also have used pharmacological tools to implicate a G protein in a membrane-delimited pathway of activation of two inward K+ channels in xylem parenchyma, one of which shows instantaneous activation and the other of which shows slow, time-dependent current profiles.
LIGHT RESPONSES
Light signaling is one of the first pathways in which heterotrimeric G protein involvement was implicated in plants. A detailed biochemical study by Kaufman and colleagues demonstrated that a low fluence of blue light (<10−1 μmol m−2) excites GTPase activity in isolated plasma membrane–enriched fractions from apical buds of etiolated pea. The relevant G protein is thought to be a 40-kD membrane-associated protein that binds a GTP analog in a light-dependent manner, is recognized by transducin antibody, and is ADP-ribosylated by CTX and PTX (Warpeha et al., 1991). Subsequent research in pea has identified two G protein α subunit cDNAs (Marsh and Kaufman, 1999); which of these is activated by blue light remains to be elucidated. Using similar assays, G protein mediation of blue-green light perception by rhodopsin in a flagellate green algae also has been proposed (Calenberg et al., 1998).
Other studies have implicated G proteins in cellular responses and gene regulation mediated by phytochrome. Early studies showed that the introduction of GDPβS into the cytosol of wheat mesophyll protoplasts by electroporation inhibited protoplast swelling stimulated by red light. Conversely, the introduction of GTPγS elicited swelling of protoplasts exposed to darkness or far-red light (Bossen et al., 1990). Red and blue light treatment of etiolated oat seedlings enhanced subsequent GTPγS binding by plant extracts, and the red light effect was reversed by far-red light (Romero et al., 1991). CTX, which activates G proteins, upregulated CAB gene expression in etiolated oat seedlings and soybean cell cultures (Romero et al., 1991; Romero and Lam, 1993), mimicking the effects of red light on these transcripts.
Chua and colleagues (Neuhaus et al., 1993; Bowler et al., 1994a, 1994b) combined cell biological, molecular, and genetic approaches to more thoroughly address the role of heterotrimeric G proteins in phytochrome responses. They used the phytochrome A (PhyA)–deficient tomato mutant aurea to investigate whether G protein activation could initiate known phytochrome responses—chloroplast development, anthocyanin biosynthesis, and CAB gene expression—in a PhyA-deficient genetic background. Microinjection of purified oat PhyA into individual hypocotyl cells was shown to partially restore these responses in a cell autonomous and red/far-red light–reversible manner. Coinjection of either GDPβS or PTX with PhyA eliminated the responses, whereas injection of GTPγS alone initiated them. Analogous manipulations implicated calcium/calmodulin as acting downstream of the G protein. One caveat to this study is the low percentage (8% or less) of cells showing the responses described above. The extent to which this value may represent simply the difficulty of injecting a cell without damaging or killing it is not known.
Recently, Deng's group transformed Arabidopsis with constructs composed of a glucocorticoid-inducible promoter driving either GPA1 or this cDNA mutated to produce a constitutively active Gα (Okamoto et al., 2001). They concluded that Gα overexpression leads to hypersensitivity of hypocotyl growth inhibition by red, blue, and far-red light but does not alter responsiveness to exogenous GA application. PhyB is primarily responsible for red light–mediated inhibition, and in a PhyB genetic null background, the hypersensitivity to red light conferred by GPA1 overexpression is no longer observed. Similarly, PhyA primarily mediates far-red light inhibition of hypocotyl elongation, and GPA1 promotion of far-red light–induced hypocotyl inhibition was not observed when the GPA1 constructs were introduced into a PhyA mutant. However, the effect of GPA1 overexpression still occurred under blue light in a cry1 mutant, indicating that CRY1 is not a required photoreceptor for this blue light response.
These results indicating that active GPA1 inhibits hypocotyl elongation seem at odds with the results of Ullah et al. (2001), who reported that gpa1 null plants also exhibited a short hypocotyl phenotype. Furthermore, Okamoto et al. (2001) concluded from morphometric analysis that GPA1 overexpression affects cell elongation and not cell division, whereas Ullah et al. (2001) concluded that knocking out GPA1 affects hypocotyl length by reducing cell division without affecting cell elongation. One possibility, as Okamoto et al. (2001) acknowledge, is that the high levels of GPA1 overexpression confer upon this protein a function that it does not normally assume in wild-type cells. For example, high levels of overexpression of some mammalian Gαs lead to an altered subcellular distribution of the subunit (Helms, 1995).
Another possible way to reconcile these observations is to assume that knocking out GPA1 or overexpressing GPA1 (particularly constitutively active GPA1) may result in an abundance of free Gβγ subunits, and perhaps it is these free Gβγ subunits that mediate the growth inhibition. However, this explanation does not reconcile the fact that one group reported an effect on cell division and the other group reported an effect on cell elongation. Dose-dependent auxin promotion of either cell expansion or cell division has been quantified in cultured plant cells and intact leaves (Chen et al., 2001a), and perhaps differential auxin sensitivity of the gpa1 null versus overexpressing lines is responsible for the divergent cellular phenotypes.
PATHOGEN RESPONSES
Fungal G proteins appear to be vital regulators of pathogenic status in plant fungal diseases (Choi et al., 1995; Liu and Dean, 1997; Regenfelder et al., 1997; Boelker, 1998; Yun et al., 1998; Coca et al., 2000). Conversely, evidence is accumulating for the importance of plant heterotrimeric G proteins in response to both bacterial and fungal pathogens. Thus, Beffa et al. (1995) showed that stable transformation of tobacco plants with the A1 subunit of CTX resulted in reduced susceptibility to Pseudomonas tabaci, accumulation of salicylic acid, and constitutive expression of pathogenesis-related genes.
A role of plant G proteins in plant responses to fungal pathogens was suggested by the work of Legendre et al. (1992), who showed that mastoparan elicits an oxidative burst in soybean cell suspensions, whereas CTX enhances the burst initiated by the elicitor Verticillium dahliae. They demonstrated subsequently that the elicitor polygalacturonic acid and the G protein activator mastoparan both stimulate PLC activity in this preparation, leading to an increase in inositol trisphosphate (Legendre et al., 1993).
Blumwald's group has demonstrated in tomato that race-specific elicitors from the fungus Cladosporium fulvum activate membrane redox reactions, leading to increased NADH oxidase activity and ferricyanide reduction in purified plasma membrane vesicles. Elicitor also stimulates plasma membrane H+-ATPase activity. GTPγS or mastoparan application likewise initiates these responses, whereas GDPβS prevents their elicitation (Vera-Estrella et al., 1994a, 1994b). H+-ATPase activation by elicitor occurs concurrently with a decrease in the transporter's phosphorylation status, and GTPγS and CTX cause dephosphorylation as well. Elicitor treatment also dissociates from a multimeric complex a 42-kD protein that cross-reacts with Gα antibodies, providing biochemical evidence for elicitor activation of a G protein (Xing et al., 1997). In addition, Blumwald's group has published elegant data showing that a constitutively active version of tomato GPA1 increases the activity of a Ca2+ channel that they propose is involved in pathogen-mediated increases in cytosolic calcium (Aharon et al., 1998).
Elicitors also can induce the production of plant secondary metabolites. Roos et al. (1999) demonstrated that yeast elicitor triggers the formation of benzophenanthridine alkaloids in cultured cells of California poppy. Both elicitor and mastoparan activated a phospholipase activity, and PLA2 products such as lysophosphatidylcholine and linolenic acid enhanced alkaloid production in response to elicitor.
TIP-GROWING CELLS
Approaches using pharmacological regulators have been used to implicate heterotrimeric G proteins in root hair responses to rhizobial nodulation (Nod) factors, which initiate nodulation responses in legumes. ENOD12 is a gene whose expression is stimulated by Nod factor. Mastoparan stimulates expression of an ENOD12-GUS reporter; CTX has no effect but PTX opposes induction by Nod factor. Mastoparan also stimulates root hair deformation in Vicia sativa (den Hartog et al., 2001), possibly by reorganizing the actin cytoskeleton (Braun et al., 1999), and biochemical analyses suggest that both mastoparan and Nod factor stimulate PLD and PLC activity (den Hartog et al., 2001).
G proteins also have been implicated in the development of another tip-growing cell, the pollen tube (Ma et al., 1999). PTX inhibits tip growth in this system, whereas CTX stimulates pollen tube growth, as does microinjection of GTPγS. A 41-kD plasma membrane protein from pollen tubes can be recognized by antibodies raised against conserved peptides of mammalian Gα subunits and can undergo ADP-ribosylation. Furthermore, pollen plasma membrane vesicles contain a GTPase activity that is stimulated by CTX. It will be helpful to conduct analogous experiments using Arabidopsis pollen tubes, given the well-characterized G protein complement and the wealth of mutants available in that species.
Ca2+ has been implicated as an important signaling component in both the Nod response and pollen tube growth. Nod factor elicits both Ca2+ spikes (Ehrhardt et al., 1996) and more stable increases in cytosolic calcium (Gehring et al., 1997), whereas pollen tubes exhibit well-characterized Ca2+ oscillations. Pharmacological tests have implicated PLC and Ca2+ as functioning downstream of mastoparan in the regulation of ENOD12 expression in the root epidermis (Pingret et al., 1998). Apparently, mastoparan does not elicit Ca2+ spikes in root hairs (Walker et al., 2000), but the possibility that G proteins regulate basal Ca2+ levels has not been evaluated. Similarly, no evaluation has been made concerning the effect of G protein regulators on Ca2+ oscillations in pollen tubes. However, Ma et al. (1999) present evidence that a heterotrimeric G protein could mediate pollen tube responses to extracellular calmodulin. Thus, the intriguing possibility is raised that G proteins might mediate extracellularly based as well as intracellularly based CaM signaling (Bossen et al., 1990; Neuhaus et al., 1993; Romero and Lam, 1993; Shiina et al., 1997).
OTHER FUNCTIONS OF HETEROTRIMERIC G PROTEINS?
The GPA1 subunit immunolocalizes to both the plasma membrane and the endoplasmic reticulum, whereas AGB1 is present in nuclear fractions. These unusual sites of G protein distribution suggest additional possible functions of plant heterotrimers. Heterotrimeric G protein subunits, and even GPCRs, have been localized to the nucleus in mammalian systems, in which functions in cell division, nuclear protein import, adipogenesis, and nuclear PLC and Ca2+ influxes have been posited (for review, see Willard and Crouch, 2000). At least one mammalian Gα subunit also has been localized to the rough endoplasmic reticulum, although it appears to play no role in protein translocation (Audigier et al., 1988). Other locales include the cytoskeleton, the trans-Golgi network, and the cytoplasm, where functions in vesicle trafficking and cytoskeletal rearrangement have been sought (Audigier et al., 1988; Helms, 1995; von Zastrow and Mostov, 2001; Zheng et al., 2001). Particularly given the dearth of prototypical GPCRs in the Arabidopsis genome (see below), the functions of GPA1 and AGB1 within the endoplasmic reticulum and nucleus are worth pursuing.
UNCONVENTIONAL GTP BINDING PROTEINS
Plants have several classes of “unconventional” GTP binding proteins whose signaling functions have only begun to be elucidated. One group of unconventional G proteins is the “extra-large G proteins,” which in Arabidopsis comprise three genes: AtXLG1, AtXLG-like, and Gα-putative. AtXLG-like and Gα putative show 41 and 42% similarity to AtXLG1 at the amino acid level; Gα-putative, despite its name, is more similar to AtXLG1 than to GPA1. Only AtXLG1 has been characterized (Lee and Assmann, 1999). AtXLG1 is expressed throughout the plant and encodes a 99-kD protein with a C-terminal half harboring significant homology with Gα proteins, including motifs required for GTP binding as well as an Asp/Glu-rich loop and a predicted helical domain characteristic of Gαs as opposed to small G proteins. Recombinant AtXLG1 protein binds GTP preferentially over other nucleotides, although the instability of the recombinant protein has thwarted efforts to assess its GTPase activity (Y.-R.J. Lee and S.M. Assmann, unpublished data).
It is interesting that AtXLG1 binds GTP, because it lacks some of the conserved amino acids thought from studies on mammalian systems to be critical for GTP binding. It is possible that the tertiary structure of the protein provides compensation for the functions of these residues. A unique aspect of AtXLG1 is its N-terminal half, which contains a Cys-rich region that is similar to zinc finger domains, and a TonB box. The TonB box is a consensus sequence found in proteins of the bacterial outer membrane involved in the transport of macromolecules such as vitamin B-12 and iron-chelator complexes.
Because of the presence of porins in the bacterial outer membrane, this membrane cannot build up an electrochemical gradient to drive active transport. The TonB box interacts with TonB proteins in the bacterial inner membrane, and this interaction is hypothesized to mediate energy transfer to the outer membrane for use in macromolecular transport (Postle, 1993; Larsen et al., 1999). Whether the TonB box of AtXLG1 plays an analogous role awaits further characterization. The TonB box is not found in the other members of this gene family, and to date, AtXLG1 homologs have not been found outside the plant kingdom. We are currently identifying T-DNA insertional mutations of the XLG genes to characterize phenotypes associated with XLG null mutants (L. Ding and S.M. Assmann, unpublished data).
Another large protein in Arabidopsis with potential GTP binding capability is RHD3 (root hair defective). Cloning of the RHD3 gene revealed that it encodes an 89-kD protein with two motifs that are conserved in GTP binding proteins (Wang et al., 1997). rhd3 mutants are so named because their initial characterization focused on the phenotype of short roots and short wavy root hairs with reduced vacuole size (Schiefelbein and Somerville, 1990). However, further study (Wang et al., 1997) showed that the RHD3 protein is important for cell expansion in shoots as well as roots, with hypocotyl cell length in the rhd3-1 mutant reaching only 45% of control wild-type lengths, a shoot phenotype similar to that seen in transgenic Arabidopsis lines with altered levels of GPA1 protein (Okamoto et al., 2001; Ullah et al., 2001).
Another set of interesting GTP binding proteins found in plants is the developmentally regulated G proteins (DRGs). Plant DRGs are expressed in all tissues at the mRNA and protein levels, with higher levels of transcript and protein found in actively growing tissues such as root apices and young stems (Devitt et al., 1999; Etheridge et al., 1999). The expression of pea and Arabidopsis DRG genes appears to be regulated by the cell cycle (Devitt et al., 1999), and Arabidopsis AtDRG1 protein has been immunolocalized to cytoplasmic vesicles, leading Etheridge et al. (1999) to speculate that DRGs may play a role in vesicle transport. The most striking feature of this class of G proteins is their remarkable sequence conservation, which spans archael and eukaryotic genomes (Mittenhuber, 2001). For example, AtDRG1 is >40% identical to archaebacterial members of this family. The strong evolutionary conservation of DRGs suggests a ubiquitous and fundamental function for this class of GTP binding proteins (Li and Trueb, 2000).
Two smaller G proteins also are worth mentioning: Arabidopsis ATGB1 (Biermann et al., 1996) and tomato ORFX at the fw2.2 locus (Frary et al., 2000). ATGB1 was identified in a screen for expression library clones that bound radiolabeled GTP. Despite its small size, phylogenetic analysis shows equal relatedness of ATGB1 to Gαs and to the ARF/ARL/Sar superfamily. The function of ATGB1 remains unknown. fw2.2 was identified as a quantitative trait locus that regulates fruit size (Alpert and Tanksley, 1996). When the fw2.2 locus from a small-fruited tomato is introduced into a large-fruited variety, a decrease in fruit size occurs. ORFX, the gene responsible for the fw2.2 quantitative trait locus phenotype, encodes a product with no sequence homology with any protein of known function (Frary et al., 2000). However, molecular modeling has provided important insights, yielding an overall structure for ORFX similar to that of Gα subunits. ORFX is only 22 kD (predicted) and shows regions of conservation with RAS/RAN/RAD domains. Allelic variation at fw2.2 affects the number of cells in the carpel, and Frary et al. (2000) speculate that the ORFX protein may affect fruit size by regulating cell division, a role similar to that played by many mammalian small G proteins as well as Arabidopsis GPA1.
GPCRs
More than 1000 heptahelical receptors have been estimated to exist in mammals (Dohlman et al., 1991; Wess, 1997; Bockaert and Pin, 1999). GPCRs have three extracellular and three intracellular loops; their N termini are extracellular, and their C termini are intracellular (Figure 1). GPCRs constitute the dominant members of a still more general class of proteins, the guanine nucleotide exchange factors (GEFs), which promote the exchange of GTP for GDP on the G protein (Table 2). In the case of heterotrimeric G proteins, this activates Gα dissociation from Gβγ.
Table 2.
Types of Mammalian Proteins That Regulate Heterotrimeric G Protein Signaling Pathways
| Function | Mammalian Protein | Known Targets |
|---|---|---|
| Receptor/GEF | GPCR | Heterotrimeric G proteins |
| GEF | AGS1 | Gαi |
| Activates G protein signaling by unknown mechanism; also light-chain component of the motor protein, dynein |
AGS2 | Gβγs |
| GDI | AGS3 | Gαi |
| Phosphorylate GPCRs, leading to desensitization | PKAs | Many targets, including GPCRs |
| Phosphorylate GPCRs, leading to desensitization | PKCs | Many targets, including GPCRs |
| GPCR kinases (GRKs) phosphorylate GPCRs, leading to desensitization via recruitment of β-arrestins |
GRKs | GPCRs |
| GRK and GAP; GRK2 has GAP activity toward Gαq if GPCR is also present | GRK2 | Gαq |
| Bind GRK-phosphorylated GPCRs, leading to uncoupling of GPCR and G protein and/or promotion of receptor internalization |
β-arrestins | GPCRs |
| Bind and sequester Gβγ complexes (major function); inhibit GTPase activity of Gα (minor function). Both functions impede restoration of a functional heterotrimer | Phosducins | Gαs and Gβs |
| GAP and effector | PLCβs | Gαq |
| GAP; some also compete with downstream effectors for binding to G protein subunits or act as scaffolding proteins | RGS proteins (over 20 identified); contain a 130-amino acid conserved “RGS box” | Most attenuate signals from Gαi and Gαq; individual members also have been found with specificity to Gαs and Gαz subunits |
| GAP for heterotrimeric G proteins; GEF for Rho small G proteins | p115RhoGEF | G12/13 |
Just as for Gα, Gβ, and Gγ, the abundance of mammalian GPCRs has proven to be a poor predictor of the abundance of this class of proteins in plants. There are three sequences annotated as putative GPCRs in Arabidopsis; of these, only one, GCR1, shows marked homology with GPCRs (Josefsson and Rask, 1997; Plakidou-Dymock et al., 1998; Josefsson, 1999). Homology is greatest with the Dictyostelium cAMP receptor CAR1 (Josefsson and Rask, 1997; Plakidou-Dymock et al., 1998); within the 7-TMS region, GCR1 has 20 to 23% identity with the CARs. GCR1 also is predicted to have an extracellular N terminus and a cytosolic C terminus, as expected for GPCRs, and shows conservation of several important amino acid residues as well as an extracellular N-linked glycosylation site that is found in many GPCRs.
GCR1 is expressed at a low level in leaves, stems, and roots (Plakidou-Dymock et al., 1998). GCR1 was thought initially to be involved in cytokinin signaling because GCR1 antisense plants showed a cytokinin-resistant phenotype (Plakidou-Dymock et al., 1998), but recent analysis indicates that the cytokinin-associated phenotype was actually conferred by an unrelated mutation (Humphrey and Botella, 2001; Plakidou-Dymock et al., 2001). Thus, the functional role of GCR1 remains unknown.
Even among the six families of mammalian 7-TMS receptors, distantly related receptors may show only 20 to 25% identity in the transmembrane domains, which are the most highly conserved regions of these proteins (Strader et al., 1994; Bockaert and Pin, 1999). Thus, the possibility remains that genes not yet annotated as GPCRs will be found to play this role in plants. One set of candidates is the MLOs, which possess no significant sequence similarity to mammalian GPCRs but have been demonstrated experimentally to have a 7-TMS topology (Devoto et al., 1999). There are estimated to be 35 MLO genes in Arabidopsis (Devoto et al., 1999), a number in keeping with the apparent multiple functions of G protein signaling in this species. The barley mlo mutant is resistant to powdery mildew and shows spontaneous lesions. Regardless of whether a G protein is found to be involved in this particular response, other members of the MLO family may turn out to be G protein receptors.
RECEPTOR-INDEPENDENT G PROTEIN ACTIVATION
Despite the dominance of GPCR sequences in mammalian genomes and in the literature, recent results have indicated that not all mammalian heterotrimeric G proteins require GPCRs for activation (and, arguably, not all heptahelical receptors couple with heterotrimeric G proteins [Hall et al., 1999; Druey, 2001]). In a genetic screen conducted in yeast, three non-GPCR mammalian proteins, AGS1 to AGS3 (for activators of G protein signaling), were found to activate G protein signaling (Cismowski et al., 2001) (Table 2). AGS1 is a Ras-related protein that, like GPCRs, seems to function as a GEF, increasing GTP binding to Gαi. In plants, the proteins related most closely to AGS1 are found in the ROP family of small G proteins (Yang, 2002). AGS2 is a light-chain component of the motor protein dynein and interacts with Gβγ subunits. Reddy and Day (2001) note that the Arabidopsis genome harbors sequences that have homology with dynein light chains.
AGS3 is a tetratricopeptide motif–containing protein that inhibits the dissociation of GDP from some Gαis; this interferes with GTP binding to Gα and GPCR/Gα reassociation (Peterson et al., 2000). Therefore, AGS3 functions as a guanine dissociation inhibitor (GDI), a class of regulatory proteins identified previously only for small G proteins (de Vries et al., 2000a; Natochin et al., 2000; Peterson et al., 2000). Although the GDI effects of AGS3 would inhibit Gα effector pathways, these effects, plus the additional observation that AGS3 competes with Gβγ for binding to Gαs (Bernard et al., 2001), would allow for prolonged signaling through free Gβγ, presumably accounting for the active nature of AGS3. The Arabidopsis SPY gene product, which acts as a negative regulator of GA signal transduction according to genetic analysis (Jacobsen et al., 1996; Izhaki et al., 2001), is a protein with 10 tetratricopeptide repeats (Jacobsen et al., 1996; Tseng et al., 2001) and has limited sequence homology with AGS3. Could SPY modulate signaling by targeting a G protein component of the GA pathway?
PROTEIN REGULATORS OF G PROTEIN PATHWAYS
Mammalian G protein signaling pathways are regulated both at the level of the receptor (GPCR) and at the level of the heterotrimer. Major classes of regulatory proteins are summarized in Table 2. Phosphorylation is an important mechanism of regulation at the receptor level. cAMP-dependent protein kinase A and PKC, both of which can be activated by non-G protein pathways, phosphorylate GPCRs and thereby reduce receptor affinity to the G protein, a process known as heterologous desensitization. Homologous desensitization is mediated by GPCR kinases (GRKs) that phosphorylate only ligand-bound (i.e., activated) receptors. GRK-induced phosphorylation recruits β-arrestins, which are inhibitory proteins, to the receptor complex (Hall et al., 1999; Morris and Malbon, 1999; Ferguson, 2001; Pierce and Lefkowitz, 2001).
At the level of the heterotrimer, phosducin-mediated sequestration of Gβγ provides a key inhibitory mechanism (Table 2) (Schulz, 2001). Two candidate phosducins that have limited sequence homology with mammalian phosducins are encoded by the Arabidopsis genome, according to PSI-BLAST analysis using default parameters and the current nonredundant database of Viridiplantae sequences.
GAPs (GTPase-activating proteins), which bind to Gαs, provide the most common G protein regulatory mechanism found in nonplant systems. By binding to and altering the conformation of Gα-GTP, GAPs increase the rate at which Gα hydrolyzes GTP by as much as 2000-fold (Ross and Wilkie, 2000). For the majority of GAPs, this leads to the inhibition of G protein signaling. Some GAPs, however, accelerate signal termination once GPCR activation is removed, but without attenuating the amplitude of the G protein signal generated in the presence of an activated GPCR. This effect may seem counterintuitive, but it can occur because the increased GDP-for-GTP exchange rate catalyzed by GAPs tends to keep the receptor, the G protein, and the GAP associated, such that not only deactivation but also reactivation occurs at a faster rate. Some GAPs can even accelerate activation of the G protein by acting as scaffolding proteins that organize the GPCR, G protein, and GAP in a stable signaling complex that is maintained even in the absence of receptor activation (de Vries et al., 2000b; Ross and Wilkie, 2000).
As summarized in Table 2, there are many different types of mammalian GAP proteins, including PI-PLCβs, RGS proteins, and p115RhoGEF. Arabidopsis has numerous PI-PLC genes, but these most likely encode PI-PLCs of the δ isoform, an isoform homologous with PLCβs but lacking the 40-kD C-terminal regulatory domain that is associated with GAP activity in PLCβs. It is particularly striking that the Arabidopsis genome appears not to encode any RGS proteins (Table 1) (Sierra et al., 2002). RGS proteins constitute a gene family of >20 members in mammals (Muallem and Wilkie, 1999; Zheng et al., 1999; de Vries et al., 2000b; Ross and Wilkie, 2000) and regulate G proteins via their GAP activity and, in some cases, via competition with downstream effectors for binding to Gα (Tesmer et al., 1997). Nor is there any evidence for higher plant sequence homologs of p115RhoGEF (Hall, 1998; Hart et al., 1998; Kozasa et al., 1998), based on protein–protein comparisons using PSI-BLAST. However, the lack of orthologous genes is not sufficient to exclude the possibility that functionally analogous proteins may be present in plants. For example, mammalian RGS and RhoGEF have no sequence similarity even at the protein level, yet an elegant structural study has shown that these proteins have regions of structural homology that can account for the fact that these dissimilar proteins both possess GAP activity (Chen et al., 2001b). Similarly, new types of GAPs may exist in plants that harbor little or no sequence homology with mammalian GAPs.
LIPID MODIFICATIONS OF G PROTEIN SUBUNITS
Some GPCRs can be palmitoylated (Morello and Bouvier, 1996). This lipid modification consists of the attachment of palmitate (C16:0) via a thioester bond and occurs at a conserved Cys residue in the C-terminal intracellular tail. Heterotrimeric G protein α and γ subunits also are subject to lipid modification (Figure 2A). Myristoylation consists of the cotranslational attachment of myristate (C14:0) to an N-terminal Gly residue in Gα via the amide bond. Myristoylation of Gα promotes both its palmitoylation at a Cys residue near the N terminus and its interaction with Gβγ. All three of these processes promote Gα targeting to the plasma membrane (Casey, 1994; Yalovsky et al., 1999; Chen and Manning, 2001). Depending on the particular Gα, these lipid modifications also may be essential for appropriate modulation by regulatory proteins (Tu et al., 1997). Palmitoylation is reversible, implicating this lipid modification as a mechanism by which Gα localization and activity could be modulated (Chen and Manning, 2001; Zheng et al., 2001).
As shown in Figure 2A, GPA1 possesses both myristoylation and potential palmitoylation sites. Myristoylation is catalyzed by myristoyl CoA:N-myristoyl transferase. This enzyme has yet to be identified at the biochemical level in plants, but several plant proteins contain myristoylation sites (Yalovsky et al., 1999; Ishitani et al., 2000), and genetic experiments have confirmed that disruption of the myristoylation site in the SOS3 protein, which functions in salt tolerance, renders plants hypersensitive to salt stress (Ishitani et al., 2000). In vivo palmitoylation has been confirmed biochemically for the chloroplast D1 protein (Mattoo and Edelman, 1987). These experiments have no direct bearing on G protein signaling but are important because they imply that plants possess N-myristoyl transferase and palmitoyl acyl transferases that could use GPA1 as a substrate.
Gγ subunits are subject to isoprenylation: either farnsylation (attachment of a 15-carbon isoprene) or geranylgeranylation (attachment of a 20-carbon isoprene). Gβ association with Gγ occurs in the cytosol, and prenylation occurs subsequent to this association (Higgins and Casey, 1996). Prenylation is required for high-affinity interactions with Gα and promotes membrane association. In addition, prenylation has been implicated as vital to interactions with Gβγ effectors; for example, Gβγ activation of PI-PLCβ2 requires prenylation (Higgins and Casey, 1996).
As shown in Figure 2A, AGG1 and AGG2 contain the conserved CaaX sequence for isoprenylation. Isoprenylation is catalyzed by farnesyl transferase (FTase) and type I (GGTase-I) and type II (GGTase-II or Rab-specific GGTase) geranylgeranyl transferases. FTase and GGTase-I share a common α subunit and have distinct β subunits (Yalovsky et al., 1999). Enzymatic activities for all three of these prenylases have been detected in plants (Randall et al., 1993; Biermann et al., 1996; Loraine et al., 1996; Yalovsky et al., 1999).
cDNAs for FTaseα/GGTase-Iα have been identified in pea and tomato (Qian et al., 1996; Caldelari et al., 2001). A cDNA for the GGTase-Iβ subunit was cloned recently from Arabidopsis (Caldelari et al., 2001). The FTaseβ subunit cDNA was cloned from pea (Yang et al., 1993), and an Arabidopsis FTaseβ gene, ERA1 (enhanced response to ABA), was identified by genetic methods (Cutler et al., 1996). It is the β subunits that yield substrate specificity, and interesting phenotypes have been associated with mutations (era1-1, era1-2, and era1-3) in ERA1, including hypersensitivity to ABA in seed germination and stomatal regulation (Cutler et al., 1996; Pei et al., 1998). The era1-2 deletion mutant has been analyzed further and shown to exhibit enlarged vegetative and inflorescence meristems and several floral phenotypes (Yalovsky et al., 2000). Given the roles ascribed to heterotrimeric G protein signaling in ABA response and cell division, it would be of particular interest to assess the prenylation status, localization, and functionality of AGG1 and AGG2 in the era1 mutant background.
CONCLUSIONS AND PERSPECTIVES
A major unexpected finding given the mammalian paradigm is the discovery that the Arabidopsis genome encodes only one prototypical Gα subunit, one prototypical Gβ subunit, and two (possibly more) Gγ subunits. It is remarkable that given this paucity of G proteins, null mutations of GPA1 and AGB1 are not lethal. At least under the conditions studied to date, these mutations give rise to altered phenotypes but not to reduced plant viability or fertility. Other proteins, which must be nonhomologous by sequence, may play redundant roles in signaling that allow retention of viability. The viability of gpa1 and agb1 mutants is even more striking given the diversity of processes in which GPA1 and AGB1 have been implicated, including auxin and ABA signaling, phytochrome responses, and leaf and silique morphology. Such multifunctionality of the Arabidopsis heterotrimer presumably is conferred by a wide range of G protein regulators, of which we know essentially nothing as yet. Predominant among these must be the receptors that couple G proteins to their upstream signals, yet only one Arabidopsis gene product, GCR1, exhibits convincing similarity to GPCRs, suggesting that plant-unique pathways of G protein activation remain to be discovered.
One caveat is that many of the studies implicating heterotrimeric G proteins in a signaling pathway were conducted before the cloning of plant heterotrimer genes and relied heavily on pharmacological agents. Many studies report responses to PTX, yet plant Gαs do not appear to contain the conserved PTX ADP-ribosylation site. It is possible that PTX targets another site on the plant Gα; for example, neither of the two cloned pea Gα subunits contains a classic PTX site (Marsh and Kaufman, 1999), yet Warpeha et al. (1991) reported PTX-dependent ribosylation of a pea protein that cross-reacts with Gα antibody. Alternatively, PTX may affect G protein–dependent pathways elsewhere in the signal transduction chain than at the Gα subunit, or PTX may modulate non-G protein pathways, in which case it is not a useful diagnostic tool for heterotrimeric G protein signaling in plants. If this last possibility proves true, then the number of pathways in which heterotrimeric G proteins have been implicated may be less than is thought at present.
Ca2+-based signaling appears to be a common if not universal theme in plant G protein responses, having been implicated in G protein–based pathways not only in nodulation and pollen tube responses but also in guard cell responses to ABA, α-amylase production and secretion by aleurone, phytochrome regulation of gene expression, and pathogen responses. Lipid-based signaling via PLC and/or PLD also has been suggested in several studies to operate downstream of plant heterotrimeric G proteins. Signaling via PLD is of special interest because most (although not all) mammalian PLD regulatory pathways signal via small rather than heterotrimeric G proteins.
Finally, clues exist suggesting the phylogenetic specificity of G protein action. For example, Okamoto et al. (2001) reported no alteration of GA response in their GPA1-overexpressing Arabidopsis, yet rice RGA1 clearly is involved in regulating the GA response of the aleurone. The difference may arise from functions associated with the Gα C terminus, which is divergent in rice and Arabidopsis (Ishikawa et al., 1995). Sequencing of the Arabidopsis and rice genomes provides fertile ground for the elucidation of genes encoding proteins that regulate plant heterotrimeric G proteins and will provide a starting point for addressing the extent to which plant G proteins have acquired distinct roles in dicots versus graminaceous monocots.
Acknowledgments
I thank Dr. Sylvie Coursol for the creation of Figures 1 and 2 and Dr. Claude dePamphilis for advice on bioinformatic methods. I also thank Dr. Alan Jones for many valuable discussions on plant G proteins during the past 3 years. Support by the National Science Foundation (Grants MCB-98-74438 and MCB-00-86315) and the U.S. Department of Agriculture (Grant 01-35304-09916) of G protein research in my laboratory is gratefully acknowledged.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.001792.
References
- Aharon, G.S., Snedden, W.A., and Blumwald, E. (1998). Activation of a plant plasma membrane Ca2+ channel by TGα1, a heterotrimeric G protein α-subunit homologue. FEBS Lett. 424 17–21. [DOI] [PubMed] [Google Scholar]
- Alpert, K.B., and Tanksley, S.D. (1996). High-resolution mapping and isolation of a yeast artificial chromosome contig containing fw2.2, a major fruit weight quantitative trait locus in tomato. Proc. Natl. Acad. Sci. USA 93 15503–15507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando, S., Takumi, S., Ueda, Y., Ueda, T., Mori, N., and Nakamura, C. (2000). Nicotiana tabacum cDNAs encoding α and β subunits of a heterotrimeric GTP-binding protein isolated from hairy root tissues. Genes Genet. Syst. 75 211–221. [DOI] [PubMed] [Google Scholar]
- Armstrong, F., and Blatt, M.R. (1995). Evidence for K+ channel control in Vicia guard cells coupled by G-proteins to a 7TMS receptor mimetic. Plant J. 8 187–198. [Google Scholar]
- Ashikari, M., Wu, J., Yano, M., Sasaki, T., and Yoshimura, A. (1999). Rice gibberellin-insensitive dwarf mutant gene Dwarf 1 encodes the α-subunit of GTP-binding protein. Proc. Natl. Acad. Sci. USA 96 10284–10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann, S.M. (1996). G-protein regulation of plant K+ channels. In Signal Transduction in Plant Development, D.P.S. Verma, ed (Vienna: Springer), pp. 39–61.
- Assmann, S.M., and Wang, X.Q. (2001). From milliseconds to millions of years: Guard cells and environmental responses. Curr. Opin. Plant Biol. 4 421–428. [DOI] [PubMed] [Google Scholar]
- Audigier, Y., Nigam, S.K., and Blobel, G. (1988). Identification of a G protein in rough endoplasmic reticulum of canine pancreas. J. Biol. Chem. 263 16352–16357. [PubMed] [Google Scholar]
- Beffa, R., Szell, M., Meuwly, P., Pay, A., Voegeli-Lange, R., Metraux, J., Neuhaus, G., Meins, F., Jr., and Nagy, F. (1995). Cholera toxin elevates pathogen resistance and induces pathogenesis-related gene expression in tobacco. EMBO J. 14 5753–5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard, M.L., Peterson, Y.K., Chung, P., Jourdan, J., and Lanier, S.M. (2001). Selective interaction of AGS3 with G-proteins and the influence of AGS3 on the activation state of G-proteins. J. Biol. Chem. 276 1585–1593. [DOI] [PubMed] [Google Scholar]
- Biermann, B., Randall, S.K., and Crowell, D.N. (1996). Identification and isoprenylation of plant GTP-binding proteins. Plant Mol. Biol. 31 1021–1028. [DOI] [PubMed] [Google Scholar]
- Bischoff, F., Molendijk, A., Rajendrakumar, C.S.V., and Palme, K. (1999). GTP-binding proteins in plants. Cell. Mol. Life Sci. 55 233–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt, M.R. (2000). Cellular signaling and volume control in stomatal movements in plants. Annu. Rev. Cell Dev. Biol. 16 221–224. [DOI] [PubMed] [Google Scholar]
- Bockaert, J., and Pin, J.P. (1999). Molecular tinkering of G protein-coupled receptors: An evolutionary success. EMBO J. 18 1723–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelker, M. (1998). Sex and crime: Heterotrimeric G proteins in fungal mating and pathogenesis. Fungal Genet. Biol. 25 143–156. [DOI] [PubMed] [Google Scholar]
- Bossen, M.E., Kendrick, R.E., and Vredenberg, W.J. (1990). The involvement of a G-protein in phytochrome-regulated, Ca2+-dependent swelling of etiolated wheat protoplasts. Physiol. Plant. 80 55–62. [Google Scholar]
- Bourne, H.R., Sanders, D.A., and McCormick, F. (1991). The GTPase superfamily: Conserved structure and molecular mechanism. Nature 349 117–127. [DOI] [PubMed] [Google Scholar]
- Bowler, C., Neuhaus, G., Yamagata, H., and Chua, N.H. (1994. a). Cyclic GMP and calcium mediate phytochrome phototransduction. Cell 77 73–81. [DOI] [PubMed] [Google Scholar]
- Bowler, C., Yamagata, H., Neuhaus, G., and Chua, N.H. (1994. b). Phytochrome signal transduction pathways are regulated by reciprocal control mechanisms. Genes Dev. 8 2188–2202. [DOI] [PubMed] [Google Scholar]
- Braun, M., Baluska, F., von Witsch, M., and Menzel, D. (1999). Redistribution of actin, profilin and phosphatidylinositol-4,5-bisphosphate in growing and maturing root hairs. Planta 209 435–443. [DOI] [PubMed] [Google Scholar]
- Brown, A.M. (1993). Membrane-delimited cell signaling complexes: Direct ion channel regulation by G proteins. J. Membr. Biol. 131 93–104. [DOI] [PubMed] [Google Scholar]
- Brown, A.M., and Birnbaumer, L. (1990). Ionic channels and their regulation by G protein subunits. Annu. Rev. Physiol. 52 197–213. [DOI] [PubMed] [Google Scholar]
- Caldelari, D., Sternberg, H., Rodriguez-Concepcion, M., Gruissem, W., and Yalovsky, S. (2001). Efficient prenylation by a plant geranylgeranyl-transferase-I requires a functional CaaL box motif and a proximal polybasic domain. Plant Physiol. 126 1416–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calenberg, M., Brohsonn, U., Zedlacher, M., and Kreimer, G. (1998). Light- and Ca2+-modulated heterotrimeric GTPases in the eyespot apparatus of a flagellate green alga. Plant Cell 10 91–103. [Google Scholar]
- Capper, E.A., and Marshall, L.A. (2001). Mammalian phospholipases A(2): Mediators of inflammation, proliferation, and apoptosis. Prog. Lipid Res. 40 167–197. [DOI] [PubMed] [Google Scholar]
- Casey, P.J. (1994). Lipid modifications of G proteins. Curr. Opin. Cell Biol. 6 219–225. [DOI] [PubMed] [Google Scholar]
- Chen, C.A., and Manning, D.R. (2001). Regulation of G proteins by covalent modification. Oncogene 20 1643–1652. [DOI] [PubMed] [Google Scholar]
- Chen, J.-G., Ullah, H., Young, J.C., Sussman, M.R., and Jones, A.M. (2001. a). ABP1 is required for organized cell elongation and division in Arabidopsis embryogenesis. Genes Dev. 15 902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z., Wells, C.D., Sternweis, P.C., and Sprang, S.R. (2001. b). Structure of the rgRGS domain of p115RhoGEF. Nat. Struct. Biol. 8 805–809. [DOI] [PubMed] [Google Scholar]
- Choi, G.H., Chen, B., and Nuss, D.L. (1995). Virus-mediated or transgenic suppression of a G-protein α subunit and attenuation of fungal virulence. Proc. Natl. Acad. Sci. USA 92 305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cismowski, M.J., Takesono, A., Bernard, M.L., Duzic, E., and Lanier, S.M. (2001). Receptor-independent activators of heterotrimeric G-proteins. Life Sci. 68 2301–2308. [DOI] [PubMed] [Google Scholar]
- Coca, M.A., Damsz, B., Yun, D.J., Hasegawa, P.M., Bressan, R.A., and Narasimhan, M.L. (2000). Heterotrimeric G-proteins of a filamentous fungus regulate cell wall composition and susceptibility to a plant PR-5 protein. Plant J. 22 61–69. [DOI] [PubMed] [Google Scholar]
- Cook, L.A., Schey, K.L., Cleator, J.H., Wilcox, M.D., Dingus, J., and Hildebrandt, J.D. (2001). Identification of a region in G protein γ subunits conserved across species but hypervariable among subunit isoforms. Protein Sci. 10 2548–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousson, A., and Vavasseur, A. (1998). Putative involvement of cytosolic Ca2+ and GTP-binding proteins in cyclic-GMP-mediated induction of stomatal opening by auxin in Commelina communis L. Planta 206 308–314. [Google Scholar]
- Cutler, S., Ghassemian, M., Bonetta, D., Cooney, S., and McCourt, P. (1996). A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science 273 1239–1241. [DOI] [PubMed] [Google Scholar]
- den Hartog, M., Musgrave, A., and Munnik, T. (2001). Nod factor-induced phosphatidic acid and diacylglycerol pyrophosphate formation: A role for phospholipase C and D in root hair deformation. Plant J. 25 55–65. [DOI] [PubMed] [Google Scholar]
- Devitt, M.L., Maas, K.J., and Stafstrom, J.P. (1999). Characterization of DRGs, developmentally regulated GTP-binding proteins, from pea and Arabidopsis. Plant Mol. Biol. 39 75–82. [DOI] [PubMed] [Google Scholar]
- Devoto, A., Piffanelli, P., Nilsson, I., Wallin, E., Panstruga, R., von Heijne, G., and Schulze-Lefert, P. (1999). Topology, subcellular localization, and sequence diversity of the Mlo family in plants. J. Biol. Chem. 274 34993–35004. [DOI] [PubMed] [Google Scholar]
- de Vries, L., Fischer, T., Tronchere, H., Brothers, G.M., Strockbine, B., Siderovski, D.P., and Farquhar, M.G. (2000. a). Activator of G protein signaling 3 is a guanine dissociation inhibitor for Gαi subunits. Proc. Natl. Acad. Sci. USA 97 14364–14369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries, L., Zheng, B., Fischer, T., Elenko, E., and Farquhar, M.G. (2000. b). The regulator of G protein signaling family. Annu. Rev. Pharmacol. Toxicol. 40 235–271. [DOI] [PubMed] [Google Scholar]
- de Waard, M., Liu, H., Walker, D., Scott, V.E., Gurnett, C.A., and Campbell, K.P. (1997). Direct binding of G-protein βγ complex to voltage-dependent calcium channels. Nature 385 446–450. [DOI] [PubMed] [Google Scholar]
- Dohlman, H.G., Thorner, J., Caron, M.G., and Lefkowitz, R.J. (1991). Model systems for the study of seven-transmembrane-segment receptors. Annu. Rev. Biochem. 60 653–688. [DOI] [PubMed] [Google Scholar]
- Druey, K.M. (2001). Bridging with GAPs: Receptor communication through RGS proteins. Science's STKE. www.stke.org/cgi/content/full/OC_sigtrans;2001/104/re14. [DOI] [PubMed]
- Ehrhardt, D.W., Wais, R., and Long, S.S. (1996). Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell 85 673–681. [DOI] [PubMed] [Google Scholar]
- Etheridge, N., Trusov, Y., Verbelen, J.P., and Botella, J.R. (1999). Characterization of AtDRG1, a member of a new class of GTP-binding proteins in plants. Plant Mol. Biol. 39 1113–1116. [DOI] [PubMed] [Google Scholar]
- Fairley-Grenot, K., and Assmann, S.M. (1991). Evidence for G-protein regulation of inward K+ channel current in guard cells of fava bean. Plant Cell 3 1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, S.S. (2001). Evolving concepts in G protein-coupled receptor endocytosis: The role in receptor desensitization and signaling. Pharmacol. Rev. 53 1–24. [PubMed] [Google Scholar]
- Frary, A., Nesbitt, T.C., Frary, A., Grandillo, S., van der Knapp, E., Cong, B., Liu, J., Meller, J., Elber, R., Alpert, K.B., and Tanksley, S.D. (2000). fw2.2: A quantitative trait locus key to the evolution of tomato fruit size. Science 289 85–88. [DOI] [PubMed] [Google Scholar]
- Fujisawa, Y., Kato, H., and Iwasaki, Y. (2001). Structure and function of heterotrimeric G proteins in plants. Plant Cell Physiol. 42 789–794. [DOI] [PubMed] [Google Scholar]
- Fujisawa, Y., Kato, T., Ohki, S., Ishikawa, A., Kitano, H., Sasaki, T., Asahi, T., and Iwasaki, Y. (1999). Suppression of the heterotrimeric G protein causes abnormal morphology, including dwarfism, in rice. Proc. Natl. Acad. Sci. USA 96 7575–7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring, C.A., Irving, H.R., Kabbara, A.A., Parish, R.W., Boukli, N.M., and Broughton, W.J. (1997). Rapid, plateau-like increases in intracellular free calcium are associated with Nod factor-induced root hair deformation. Mol. Plant-Microbe Interact. 10 791–802. [Google Scholar]
- Gotor, C., Lam, E., Cejudo, F.R., and Romero, L.C. (1996). Isolation and analysis of the soybean SGA2 gene (cDNA), encoding a new member of the plant G-protein family of signal transducers. Plant Mol. Biol. 32 1227–1234. [DOI] [PubMed] [Google Scholar]
- Hall, A. (1998). G proteins and small GTPases: Distant relatives keep in touch. Science 280 2074–2075. [DOI] [PubMed] [Google Scholar]
- Hall, R.A., Premont, R.T., and Lefkowitz, R.J. (1999). Heptahelical receptor signaling: Beyond the G protein paradigm. J. Cell Biol. 145 927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, D.W.A., Hills, A., Koehler, B., and Blatt, M.R. (2000). Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc. Natl. Acad. Sci. USA 97 4967–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart, M.J., Jiang, X., Kozasa, T., Roscoe, W., Singer, W.D., Gilman, A.F., Sternweis, P.C., and Bollag, G. (1998). Direct stimulation of the guanine nucleotide exchange activity of p115RhoGEF by Gα13. Science 280 2112–2114. [DOI] [PubMed] [Google Scholar]
- Helms, J.B. (1995). Role of heterotrimeric GTP binding proteins in vesicular protein transport: Indications for both classical and alternative G protein cycles. FEBS Lett. 369 84–88. [DOI] [PubMed] [Google Scholar]
- Higashijima, T., Uzu, S., Nakajima, T., and Ross, E.M. (1988). Mastoparan, a peptide toxin from wasp venom, mimics receptors by activating GTP-binding regulatory proteins (G proteins). J. Biol. Chem. 263 6491–6494. [PubMed] [Google Scholar]
- Higgins, J.B., and Casey, P.J. (1996). The role of prenylation in G-protein assembly and function. Cell. Signal. 8 433–437. [DOI] [PubMed] [Google Scholar]
- Hobbie, L., and Estelle, M. (1994). Genetic approaches to auxin action. Plant Cell Environ. 17 525–540. [DOI] [PubMed] [Google Scholar]
- Homann, U., and Tester, M. (1997). Ca2+-independent and Ca2+/GTP-binding protein-controlled exocytosis in a plant cell. Proc. Natl. Acad. Sci. USA 94 6565–6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooley, R. (1998). Plant hormone perception and action: A role for G-protein signal transduction? Philos. Trans. R. Soc. Lond. Ser. B. 353 1425–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H., Weiss, C.A., and Ma, H. (1994). Regulated expression of the Arabidopsis Gα gene GPA1. Int. J. Plant Sci. 155 3–14. [Google Scholar]
- Humphrey, T.V., and Botella, J.R. (2001). Re-evaluation of the cytokinin receptor role of the Arabidopsis gene GCR1. J. Plant Physiol. 158 645–653. [Google Scholar]
- Hutton, J.L., Knight, C.D., and Millner, P.A. (1998). The Physcomitrella patens GPα1 homologue is located at protonemal cell junctions. J. Exp. Bot. 49 1113–1118. [Google Scholar]
- Ishikawa, A., Isasaki, Y., and Asahi, T. (1996). Molecular cloning and characterization of a cDNA for the beta subunit of a G protein from rice. Plant Cell Physiol. 37 223–228. [DOI] [PubMed] [Google Scholar]
- Ishikawa, A., Tsubouchi, H., Iwasaki, Y., and Asahi, T. (1995). Molecular cloning and characterization of a cDNA for the α subunit of a G protein from rice. Plant Cell Physiol. 36 353–359. [DOI] [PubMed] [Google Scholar]
- Ishitani, M., Liu, J., Halfter, U., Kim, C., Shi, W., and Zhu, J.K. (2000). SOS3 function in plant salt tolerance requires N-myristoylation and calcium binding. Plant Cell 12 1667–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki, Y., Kato, T., Daidoh, T., Ishikawa, A., and Asahi, T. (1997). Characterization of the putative α subunit of a heterotrimeric G protein in rice. Plant Mol. Biol. 34 563–572. [DOI] [PubMed] [Google Scholar]
- Izhaki, A., Swain, S.M., Tsent, T.S., Borochov, A., Olszewski, N.E., and Weiss, D. (2001). The role of SPY and its TPR domain in the regulation of gibberellin action throughout the life cycle of Petunia hybrida plants. Plant J. 28 181–190. [DOI] [PubMed] [Google Scholar]
- Jacob, T., Ritchie, S., Assmann, S.M., and Gilroy, S. (1999). Abscisic acid signal transduction in guard cells is mediated by phospholipase D activity. Proc. Natl. Acad. Sci. USA 96 12192–12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen, S.E., Binkowski, K.A., and Olszewski, N.E. (1996). SPINDLY, a tetratricopeptide repeat protein involved in gibberellin signal transduction in Arabidopsis. Proc. Natl. Acad. Sci. USA 93 9292–9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelsema, C.L., and Axelrod, J. (1987). Stimulation of phospholipase A2 activity in bovine rod outer segments by the βγ subunits of transducin and its inhibition by the α subunit. Proc. Natl. Acad. Sci. USA 84 3623–3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, H.D., Smith, S.J., Desikan, R., Plakidou-Dymock, S., Lovegrove, A., and Hooley, R. (1998). Heterotrimeric G proteins are implicated in gibberellin induction of α-amylase gene expression in wild oat aleurone. Plant Cell 10 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson, L. (1999). Evidence for kinship between diverse G-protein coupled receptors. Gene 239 333–340. [DOI] [PubMed] [Google Scholar]
- Josefsson, L.G., and Rask, L. (1997). Cloning of a putative G-protein-coupled receptor from Arabidopsis thaliana. Eur. J. Biochem. 249 415–420. [DOI] [PubMed] [Google Scholar]
- Katz, A., Wu, D., and Simon, M.I. (1992). Subunits βγ of heterotrimeric G protein activate β2 isoform of phospholipase C. Nature 360 686–689. [DOI] [PubMed] [Google Scholar]
- Kaydamov, D., Tewes, A., Adler, K., and Manteuffel, R. (2000). Molecular characterization of cDNAs encoding G protein α and β subunits and study of their temporal and spatial expression patterns in Nicotiana plumbaginifolia Viv. Biochim. Biophys. Acta 149 143–160. [DOI] [PubMed] [Google Scholar]
- Kelly, W.B., Esser, J.E., and Schroeder, J.I. (1995). Effects of cytosolic calcium and limited, possible dual, effects of G protein modulators on guard cell inward potassium channels. Plant J. 8 479–489. [Google Scholar]
- Kim, D., Lewis, D.L., Graziadei, L., Neer, E.J., Bar-Sagi, D., and Clapham, D.E. (1989). G-protein βγ subunits activate the cardiac muscarinic K+ channel via phospholipase A2. Nature 337 557–560. [DOI] [PubMed] [Google Scholar]
- Kim, G.-T., Tsukaya, H., and Uchimiya, H. (1998). The ROTUNDIFOLIA3 gene of Arabidopsis thaliana encodes a new member of the cytochrome P-450 family that is required for the regulated polar elongation of leaf cells. Genes Dev. 12 2381–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, W.Y., Cheong, N.E., Lee, D.C., Je., D.Y., Bahk, J.D., Cho, M.J., and Lee, S.Y. (1995). Cloning and sequencing analysis of a full-length cDNA encoding a G protein α subunit, SGA1, from soybean. Plant Physiol. 108 1315–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, S., Reuveni, H., and Levitzki, A. (2000). Signal transduction by a nondissociable heterotrimeric yeast G protein. Proc. Natl. Acad. Sci. USA 97 3219–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozasa, T., Jiang, X., Hart, M.J., Sternweis, P.M., Singer, W.D., Gilman, A.G., Bollag, G., and Sternweis, P.C. (1998). p115RhoGEF, a GTPase activating protein for Gα12 and Gα13. Science 280 2109–2111. [DOI] [PubMed] [Google Scholar]
- Kusnetsov, V.V., and Oelmueller, R. (1996. a). Isolation and characterization of cDNAs encoding the subunit β of heterotrimeric G proteins from N. tabacum (accession No. X98161). Plant Physiol. 111 948. [Google Scholar]
- Kusnetsov, V.V., and Oelmueller, R. (1996. b). Isolation of cDNAs encoding the subunit α of heterotrimeric G proteins from Lupinus luteus (accession No. X99485). Plant Physiol. 112 1399. [Google Scholar]
- Lambright, D.G., Sondek, J., Bohm, A., Skiba, N.P., Hamm, H.E., and Sigler, P.B. (1996). The 2.0 angstrom crystal structure of a heterotrimeric G protein. Nature 379 311–319. [DOI] [PubMed] [Google Scholar]
- Larsen, R.A., Thomas, M.G., and Postle, K. (1999). Protonmotive force, ExbB and ligand-bound FepA drive conformational changes in TonB. Mol. Microbiol. 31 1809–1824. [DOI] [PubMed] [Google Scholar]
- Lease, K.A., Wen, J., Li, J., Doke, J.T., Liscum, E., and Walker, J.C. (2001). A mutant Arabidopsis heterotrimeric G-protein β subunit affects leaf, flower, and fruit development. Plant Cell 13 2631–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H.J., Tucker, E.B., Crain, R.C., and Lee, Y. (1993). Stomatal opening is induced in epidermal peels of Commelina communis L. by GTP analogs or pertussis toxin. Plant Physiol. 102 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y., Choi, Y.B., Suh, S., Lee, J., Assmann, S.M., Joe, C.O., Kelleher, J.F., and Crain, R.C. (1996). Abscisic acid-induced phosphoinositide turnover in guard cell protoplasts of Vicia faba. Plant Physiol. 110 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y.-R.J., and Assmann, S.M. (1999). Arabidopsis thaliana ‘extra-large GTP-binding protein’ (AtXLG1): A new class of G-protein. Plant Mol. Biol. 40 55–64. [DOI] [PubMed] [Google Scholar]
- Legendre, L., Heinstein, P.F., and Low, P.S. (1992). Evidence for participation of GTP-binding proteins in elicitation of the rapid oxidative burst in cultured soybean cells. J. Biol. Chem. 267 20140–20147. [PubMed] [Google Scholar]
- Legendre, L., Yueh, Y.G., Crain, R., Haddock, N., Heinstein, P.F., and Low, P.S. (1993). Phospholipase C activation during elicitation of the oxidative burst in cultured plant cells. J. Biol. Chem. 268 24559–24563. [PubMed] [Google Scholar]
- Lein, W., and Saalbach, G. (2001). Cloning and direct G-protein regulation of phospholipase D from tobacco. Biochim. Biophys. Acta 153 172–183. [DOI] [PubMed] [Google Scholar]
- Lemtiri-chlieh, F. (1998). Direct effects of ABA on single outward potassium channels in guard cells. In 11th International Workshop on Plant Membrane Biology, Cambridge, UK, abstract 266. http://link.springer.de/link/service/journals/00898/free/meeting/cmbrdg98/sess08.htm#08sess10.
- Li, B., and Trueb, B. (2000). DRG represents a family of two closely related GTP-binding proteins. Biochim. Biophys. Acta 1491 196–204. [DOI] [PubMed] [Google Scholar]
- Li, W., and Assmann, S.M. (1993). Characterization of a G-protein-regulated outward K+ current in mesophyll cells of Vicia faba L. Proc. Natl. Acad. Sci. USA 90 262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S., and Dean, R.A. (1997). G protein α subunit genes control growth, development, and pathogenicity of Magnaporthe grisea. Mol. Plant-Microbe Interact. 10 1075–1086. [DOI] [PubMed] [Google Scholar]
- Logothetis, D.E., Kurachi, Y., Galper, J., Neer, E.J., and Clapham, D.E. (1987). The βγ subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature 325 321–326. [DOI] [PubMed] [Google Scholar]
- Loraine, A.E., Yalovsky, S., Fabry, S., and Gruissem, S. (1996). Tomato Rab1A homologs as molecular tools for studying Rab geranylgeranyl transferase in plant cells. Plant Physiol. 110 1337–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, H. (1994). GTP-binding proteins in plants: New members of an old family. Plant Mol. Biol. 26 1611–1636. [DOI] [PubMed] [Google Scholar]
- Ma, H. (2001). Plant G proteins: The different faces of GPA1. Curr. Biol. 11 R869–R871. [DOI] [PubMed] [Google Scholar]
- Ma, H., Yanofsky, M.F., and Huang, H. (1991). Isolation and sequence analysis of TGA1 cDNAs encoding a tomato G protein α subunit. Gene 107 189–195. [DOI] [PubMed] [Google Scholar]
- Ma, H., Yanofsky, M.F., and Meyerowitz, E.M. (1990). Molecular cloning and characterization of GPA1, a G protein α subunit gene from Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 87 3821–3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, L., Xu, X., Cui, S., and Sun, D. (1999). The presence of a heterotrimeric G protein and its role in signal transduction of extracellular calmodulin in pollen germination and tube growth. Plant Cell 11 1351–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, J.F., III, and Kaufman, L.S. (1999). Cloning and characterization of PGA1 and PGA2: Two G protein α-subunits from pea that promote growth in the yeast Saccharomyces cerevisiae. Plant J. 19 237–247. [DOI] [PubMed] [Google Scholar]
- Mason, M.G., and Botella, J.R. (2000). Completing the heterotrimer: Isolation and characterization of an Arabidopsis thaliana G protein γ-subunit cDNA. Proc. Natl. Acad. Sci. USA 97 14784–14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, M.G., and Botella, J.R. (2001). Isolation of a novel G-protein γ subunit from Arabidopsis thaliana and its interaction with Gβ. Biochim. Biophys. Acta. 1520 147–153. [DOI] [PubMed] [Google Scholar]
- Mattoo, A.K., and Edelman, M. (1987). Intramembrane translocation and posttranslational palmitoylation of the chloroplast 32-kDa herbicide-binding protein. Proc. Natl. Acad. Sci. USA 84 1497–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh, M.R., Gray, J.E., Hetherington, A.M., Leckie, C.P., and Ng, C. (2000). Ca2+ signaling in stomatal guard cells. Biochem. Soc. Trans. 28 476–481. [PubMed] [Google Scholar]
- Mitsunaga, S., Rashiro, T., and Yamaguchi, J. (1994). Identificatiion and characterization of gibberellin-insensitive mutants selected from among dwarf mutants of rice. Theor. Appl. Genet. 87 705–712. [DOI] [PubMed] [Google Scholar]
- Mittenhuber, G. (2001). Comparative genomics of prokaryotic GTP-binding proteins (the Era, Obg, EngA, ThdF (TrmE), YchF and YihA families) and their relationship to eukaryotic GTP-binding proteins (the DRG, ARF, RAB, RAN, RAS and RHO families). J. Mol. Microbiol. Biotechnol. 3 21–35. [PubMed] [Google Scholar]
- Morello, J.P., and Bouvier, M. (1996). Palmitoylation: A post-translational modification that regulates signaling from G-protein coupled receptors. Biochem. Cell Biol. 74 449–457. [DOI] [PubMed] [Google Scholar]
- Morris, A.J., and Malbon, C.C. (1999). Physiological regulation of G protein-linked signaling. Physiol. Rev. 79 1373–1430. [DOI] [PubMed] [Google Scholar]
- Muallem, S., and Wilkie, T.M. (1999). G protein-dependent Ca2+ signaling complexes in polarized cells. Cell Calcium 26 173–180. [DOI] [PubMed] [Google Scholar]
- Munnik, T., Arisz, S.A., de Vrije, R., and Musgrave, A. (1995). G protein activation stimulates phospholipase D signaling in plants. Plant Cell 7 2197–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natochin, M., Lester, B., Peterson, Y.K., Bernard, M.L., Lanier, S.M., and Artemyev, N.O. (2000). AGS3 inhibits GDP dissociation from Gα subunits of the Gi family and rhodopsin-dependent activation of transducin. J. Biol. Chem. 275 40981–40985. [DOI] [PubMed] [Google Scholar]
- Neuhaus, G., Bowler, C., Kern, R., and Chua, N.H. (1993). Calcium/calmodulin-dependent and -independent phytochrome signal transduction pathways. Cell 73 937–952. [DOI] [PubMed] [Google Scholar]
- Oh, P., and Schnitzer, J.E. (2001). Segregation of heterotrimeric G proteins in cell surface microdomains. Mol. Biol. Cell 12 685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto, H., Matsui, M., and Deng, X.W. (2001). Overexpression of the heterotrimeric G-protein α-subunit enhances phytochrome-mediated inhibition of hypocotyl elongation in Arabidopsis. Plant Cell 13 1639–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappan, K., and Wang, X. (1999). Molecular and biochemical properties and physiological roles of plant phospholipase D. Biochim. Biophys. Acta 1439 151–166. [DOI] [PubMed] [Google Scholar]
- Pei, Z.-M., Ghassemian, M., Kwak, C.M., McCourt, P., and Schroeder, J.I. (1998). Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science 282 287–290. [DOI] [PubMed] [Google Scholar]
- Perroud, P.F., Diogon, T., Crevecoeur, M., and Greppin, H. (2000). Molecular cloning, spatial and temporal characterization of spinach SOGA1 cDNA, encoding an α subunit of G protein. Gene 248 191–201. [DOI] [PubMed] [Google Scholar]
- Peskan, T., and Oelmueller, R. (2000). Heterotrimeric G-protein β-subunit is localized in the plasma membrane and nuclei of tobacco leaves. Plant Mol. Biol. 42 915–922. [DOI] [PubMed] [Google Scholar]
- Peskan, T., Westermann, M., and Oelmueller, R. (2000). Identification of low-density Triton X-100-insoluble plasma membrane microdomains in higher plants. Eur. J. Biochem. 267 6989–6995. [DOI] [PubMed] [Google Scholar]
- Peterson, Y.K.K., Bernard, M.L., Ma, H., Hazard, S., 3rd, Graber, S.G., and Lanier, S.M. (2000). Stabilization of the GDP-bound conformation of Giα by a peptide derived from the G-protein regulatory motif of AGS3. J. Biol. Chem. 275 33193–33196. [DOI] [PubMed] [Google Scholar]
- Pierce, K.L., and Lefkowitz, R.J. (2001). Classical and new roles of β-arrestins in the regulation of G-protein-coupled receptors. Nat. Rev. 2 727–733. [DOI] [PubMed] [Google Scholar]
- Pingret, J.-L., Journet, E.-P., and Barker, D.G. (1998). Rhizobium Nod factor signaling: Evidence for a G protein-mediated transduction mechanism. Plant Cell 10 659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plakidou-Dymock, S., Dymock, D., and Hooley, R. (1998). A higher plant seven-transmembrane receptor that influences sensitivity to cytokinins. Curr. Biol. 8 315–324. [DOI] [PubMed] [Google Scholar]
- Plakidou-Dymock, S., Dymock, D., and Hooley, R. (2001). A higher plant seven-transmembrane receptor that influences sensitivity to cytokinins. Erratum. Curr. Biol. 11 535. [DOI] [PubMed] [Google Scholar]
- Poffenroth, M., Green, D.B., and Tallman, G. (1992). Sugar concentrations in guard cells of Vicia faba illuminated with red or blue light. Plant Physiol. 98 1460–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle, K. (1993). TonB protein and energy transduction between membranes. J. Bioenerg. Biomembr. 25 591–601. [DOI] [PubMed] [Google Scholar]
- Poulsen, C., Mai, X.M., and Borg, S. (1994). A Lotus japonicus cDNA encoding an α subunit of a heterotrimeric G-protein. Plant Physiol. 105 1453–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, D., Zhou, D., Ju, R., Cramer, C.L., and Yang, Z. (1996). Protein farnesyltransferase in plants: Molecular characterization and involvement in cell cycle control. Plant Cell 8 2381–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall, S.K., Marshall, M.S., and Crowell, D.N. (1993). Protein isoprenylation in suspension-cultured tobacco cells. Plant Cell 5 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, A.S., and Day, I.S. (2001). Analysis of the myosins encoded in the recently completed Arabidopsis thaliana genome sequence. Genome Biol. http://genomebiology.com/2001/2/7/research/0024.1. [DOI] [PMC free article] [PubMed]
- Reddy, M.M., Sun, D., and Quinton, P.M. (2001). Apical heterotrimeric G-proteins activate CFTR in the native sweat duct. J. Membr. Biol. 179 51–61. [DOI] [PubMed] [Google Scholar]
- Regenfelder, E., Spellig, T., Hartmann, A., Lauenstein, S., Bolker, M., and Kahmann, R. (1997). G proteins in Ustilago maydis: Transmission of multiple signals? EMBO J. 16 1934–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie, S., and Gilroy, S. (2000). Abscisic acid stimulation of phospholipase D in the barley aleurone is G-protein-mediated and localized to the plasma membrane. Plant Physiol. 124 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero, L.C., and Lam, E. (1993). Guanine nucleotide binding protein involvement in early steps of phytochrome-regulated gene expression. Proc. Natl. Acad. Sci. USA 90 1465–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero, L.C., Sommer, D., Gotor, C., and Song, P.-S. (1991). G-proteins in etiolated Avena seedlings. FEBS Lett. 282 341–346. [DOI] [PubMed] [Google Scholar]
- Roos, W., Dordschbla, B., Steighardt, J., Hieke, M., Weiss, D., and Saalbach, G. (1999). A redox-dependent, G-protein-coupled phospholipase A of the plasma membrane is involved in the elicitation of alkaloid biosynthesis in Eschscholtzia californica. Biochim. Biophys. Acta 1448 390–402. [DOI] [PubMed] [Google Scholar]
- Ross, E.M., and Wilkie, T.M. (2000). GTPase-activating proteins for heterotrimeric G proteins: Regulators of G protein signaling (RGS) and RGS-like proteins. Annu. Rev. Biochem. 69 795–827. [DOI] [PubMed] [Google Scholar]
- Saalbach, G., Natura, B., Lein, W., Buschmann, P., Dahse, I., Rohrbeck, M., and Nagy, F. (1999). The α-subunit of a heterotrimeric G-protein from tobacco, NtGPα1, functions in K+ channel regulation in mesophyll cells. J. Exp. Bot. 50 53–61. [Google Scholar]
- Santagata, S., Boggon, T.J., Baird, C.L., Gomez, C.A., Zhao, J., Shan, W.S., Myszka, D.G., and Shapiro, L. (2001). G-protein signaling through tubby proteins. Science 292 2041–2050. [DOI] [PubMed] [Google Scholar]
- Scherer, G.F. (1994). Phospholipid signaling by phospholipase A2 in plants: The role of mastoparan and lysophospholipids as weak ‘auxin-like’ agonists. Symp. Soc. Exp. Biol. 48 229–242. [PubMed] [Google Scholar]
- Scherer, G.F., and Andre, B. (1989). A rapid response to a plant hormone: Auxin stimulates phospholipase A2 in vivo and in vitro. Biochem. Biophys. Res. Commun. 163 111–117. [DOI] [PubMed] [Google Scholar]
- Schiefelbein, J.W., and Somerville, C. (1990). Pollen tube and root hair tip growth is disrupted in a mutant of Arabidopsis thaliana. Plant Cell 2 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, J.I., and Hagiwara, S. (1989). Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature 338 427–430. [Google Scholar]
- Schroeder, J.I., Kwak, J.M., and Allen, G.J. (2001). Guard cell abscisic acid signaling and engineering drought hardiness in plants. Nature 410 327–330. [DOI] [PubMed] [Google Scholar]
- Schulz, R. (2001). The pharmacology of phosducin. Pharm. Res. 43 1–10. [DOI] [PubMed] [Google Scholar]
- Schumaker, K.S., and Gizinski, M.J. (1996). G proteins regulate dihydropyridine binding to moss plasma membranes. J. Biol. Chem. 271 21292–21296. [DOI] [PubMed] [Google Scholar]
- Seack, J., Kruse, M., and Muller, W.E. (1998). Evolutionary analysis of G-proteins in early metazoans: Cloning of α and β subunits from the sponge, Geodia cydonium. Biochim. Biophys. Acta 141 93–103. [DOI] [PubMed] [Google Scholar]
- Seo, H., Choi, C.H., Lee, S.-Y., Cho, M.-J., and Bahk, J.-D. (1997). Biochemical characteristics of a rice (Oryza sativa L. IR-36) G-protein a-subunit expressed in Escherichia coli. Biochem. J. 324 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, H.-S., Kim, H.-Y., Jeong, J.-Y., Lee, S.-Y., Cho, M.-J., and Bahk, J.-D. (1995). Molecular cloning and characterization of RGA1 encoding a G protein α subunit from rice (Oryza sativa L. IR-36). Plant Mol. Biol. 27 1119–1131. [DOI] [PubMed] [Google Scholar]
- Shiina, T., Nishii, A., Toyoshima, Y., and Bogorad, L. (1997). Identification of promoter elements involved in the cytosolic Ca2+-mediated photoregulation of maize cab-m1 expression. Plant Physiol. 115 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra, D.A., et al. (2002). Evolution of the regulators of G-protein signaling multigene family in mouse and human. Genomics 79 177–185. [DOI] [PubMed] [Google Scholar]
- Singer, W.D., Brown, H.A., and Sternweis, P.C. (1997). Regulation of eukaryotic phosphatidylinositol-specific phospholipase C and phospholipase D. Annu. Rev. Biochem. 66 475–509. [DOI] [PubMed] [Google Scholar]
- Sondek, J., Bohm, A., Lambright, D.G., Hamm, H.E., and Sigler, P.B. (1996). Crystal structure of a G-protein βγ dimmer at 2.1 angstrom resolution. Nature 379 369–374. [DOI] [PubMed] [Google Scholar]
- Strader, C.D., Fong, T.M., Tota, M.R., Underwood, D., and Dixon, R.A. (1994). Structure and function of G protein-coupled receptors. Annu. Rev. Biochem. 63 101–132. [DOI] [PubMed] [Google Scholar]
- Sutton, F., Paul, S.S., Wang, X.Q., and Assmann, S.M. (2000). Distinct abscisic acid signaling pathways for modulation of guard cell versus mesophyll cell potassium channels revealed by expression studies in Xenopus laevis oocytes. Plant Physiol. 124 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesmer, J.J., Berman, D.M., Gilman, A.G., and Sprang, S.R. (1997). Structure of RGS4 bound to AlF4-activated Giα1: Stabilization of the transition state for GTP hydrolysis. Cell 89 251–261. [DOI] [PubMed] [Google Scholar]
- Tseng, T.S., Swain, S.M., and Olszewski, N.E. (2001). Ectopic expression of the tetratricopeptide repeat domain of SPINDLY causes defects in gibberellin response. Plant Physiol. 126 1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu, Y., Wang, J., and Ross, E.M. (1997). Inhibition of brain Gz GAP and other RGS proteins by palmitoylation of G protein α subunits. Science 278 1132–1135. [DOI] [PubMed] [Google Scholar]
- Ueguchi-Tanaka, M., Fujisawa, Y., Kobayashi, M., Ashikari, M., Iwasaki, Y., Kitano, H., and Matsuoka, M. (2000). Rice dwarf mutant d1, which is defective in the α subunit of the heterotrimeric G protein, affects gibberellin signal transduction. Proc. Natl. Acad. Sci. USA 97 11638–11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah, H., Chen, J.-G., Youg, J.C., Im, K.-H., Sussman, M.R., and Jones, A.M. (2001). Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science 292 2066–2069. [DOI] [PubMed] [Google Scholar]
- Vera-Estrella, R., Barkla, B.J., Higgins, V.J., and Blumwald, E. (1994. a). Plant defense response to fungal pathogens. I. Plant Physiol. 104 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Estrella, R., Higgins, V.J., and Blumwald, E. (1994. b). Plant defense response to fungal pathogens. II. Plant Physiol. 106 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zastrow, M., and Mostov, K. (2001). Signal transduction: A new thread in an intricate web. Science 294 1845–1847. [DOI] [PubMed] [Google Scholar]
- Voyno-Yasenetskaya, T.A. (1998). G proteins and Na+/H+ exchange. Biol. Signals Recept. 7 118–124. [DOI] [PubMed] [Google Scholar]
- Voyno-Yasenetskaya, T.A., Conklin, B.R., Gilbert, R.L., Hooley, R., Bourne, H.R., and Barber, D.L. (1994). Gα13 stimulates Na-H exchange. J. Biol. Chem. 269 4721–4724. [PubMed] [Google Scholar]
- Walker, S.A., Viprey, V., and Downie, J.A. (2000). Dissection of nodulation signaling using pea mutants defective for calcium spiking induced by Nod factors and chitin oligomers. Proc. Natl. Acad. Sci. USA 97 13413–13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H., Lockwood, S.K., Hoeltzel, M.F., and Schiefelbein, J.W. (1997). The ROOT HAIR DEFECTIVE 3 gene encodes an evolutionarily conserved protein with GTP-binding motifs and is required for regulated cell enlargement in Arabidopsis. Genes Dev. 11 799–811. [DOI] [PubMed] [Google Scholar]
- Wang, M., Sedee, N.J.A., Heideamp, F., and Snaar-Jagalska, B.E. (1993). Detection of GTP-binding proteins in barley aleurone protoplasts. FEBS Lett. 329 245–248. [DOI] [PubMed] [Google Scholar]
- Wang, X.Q., Ullah, H., Jones, A.M., and Assmann, S.M. (2001). G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science 292 2070–2072. [DOI] [PubMed] [Google Scholar]
- Warpeha, K.M.F., Hamm, H.E., Rasenick, M.M., and Kaufman, L.S. (1991). A blue-light-activated GTP-binding protein in the plasma membranes of etiolated peas. Proc. Natl. Acad. Sci. USA 88 8925–8929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner, L.H., and de Boer, A.H. (1997). Two inward K+ channels in the xylem parenchyma cells of barley roots are regulated by G-protein modulators through a membrane-delimited pathway. Planta 203 506–516. [Google Scholar]
- Weiss, C.A., Garnaat, C.W., Mukai, K., Hu, Y., and Ma, H. (1994). Isolation of cDNAs encoding guanine nucleotide-binding protein β-subunit homologues from maize (ZGB1) and Arabidopsis (AGB1). Proc. Natl. Acad. Sci. USA 91 9554–9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, C.A., Huang, H., and Ma, H. (1993). Immunolocalization of the G protein α subunit encoded by the GPA1 gene in Arabidopsis. Plant Cell 5 1513–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, C.A., White, E., Huang, H., and Ma, H. (1997). The G protein α subunit (GPα1) is associated with the ER and the plasma membrane in meristematic cells of Arabidopsis and cauliflower. FEBS Lett. 407 361–367. [DOI] [PubMed] [Google Scholar]
- Wess, J. (1997). G-protein-coupled receptors: Molecular mechanisms involved in receptor activation and selectivity of G-protein recognition. FASEB J. 11 346–354. [PubMed] [Google Scholar]
- Wilkie, T.M., and Yokoyama, S. (1994). Evolution of the G protein α subunit multigene family. Soc. Gen. Physiol. 49 249–270. [PubMed] [Google Scholar]
- Willard, F.S., and Crouch, M.F. (2000). Nuclear and cytoskeletal translocation and localization of heterotrimeric G-proteins. Immunol. Cell Biol. 78 387–394. [DOI] [PubMed] [Google Scholar]
- Wise, A., Thomas, P.G., Carr, T.H., Murphy, G.A., and Millner, P.A. (1997). Expression of the Arabidopsis G-protein GPα1: Purification and characterization of the recombinant protein. Plant Mol. Biol. 33 723–728. [DOI] [PubMed] [Google Scholar]
- Wu, W.H., and Assmann, S.M. (1994). A membrane-delimited pathway of G-protein regulation of the guard-cell inward K+ channel. Proc. Natl. Acad. Sci. USA 91 6310–6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, T., Higgins, V.J., and Blumwald, E. (1997). Identification of G proteins mediating fungal elicitor-induced dephosphorylation of host plasma membrane H+ ATPases. J. Exp. Bot. 48 229–237. [Google Scholar]
- Yalovsky, S., Kulukian, A., Rodriguez-Concepcion, M., Young, C.A., and Gruissem, W. (2000). Functional requirement of plant farnesyltransferase during development in Arabidopsis. Plant Cell 12 1267–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalovsky, S., Rodriguez-Concepcion, M., and Gruissem, W. (1999). Lipid modifications of proteins: Slipping in and out of membranes. Trends Plant Sci. 4 439–445. [DOI] [PubMed] [Google Scholar]
- Yang, Z. (2002). Small GTPases: Versatile signaling switches in plants. Plant Cell 14 (suppl.), S375–S388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z., Cramer, C.L., and Watson, J.C. (1993). Protein farnesyltransferase in plants: Molecular cloning and expression of a homolog of the β subunit from the garden pea. Plant Physiol. 101 667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun, D.J., Ibeas, J.I., Lee, H., Coca, M.A., Narasimhan, M.L., Uesono, Y., Hasegawa, P.M., Pardo, J.M., and Bressan, R.A. (1998). Osmotin, a plant antifungal protein, subverts signal transduction to enhance fungal cell susceptibility. Mol. Cell 1 807–817. [DOI] [PubMed] [Google Scholar]
- Zaina, S., Mapelli, S., Reggiani, R., and Bertani, A. (1991). Auxin and GTPase activity in membranes from aerobic and anaerobic rice coleoptile. J. Plant Physiol. 138 760–762. [Google Scholar]
- Zaina, S., Reggiani, R., and Bertani, A. (1990). Preliminary evidence for involvement of GTP-binding protein(s) in auxin signal transduction in rice (Oryza sativa L.) coleoptile. J. Plant Physiol. 136 653–658. [Google Scholar]
- Zheng, B., de Vries, L., and Farquhar, M.G. (1999). Divergence of RGS proteins: Evidence for the existence of six mammalian RGS subfamilies. Trends Biochem. Sci. 24 411–414. [DOI] [PubMed] [Google Scholar]
- Zheng, B., Ma, Y.C., Ostrom, R.S., Lavoie, C., Gill, G.N., Insel, P.A., Huang, X.Y., and Farquhar, M.G. (2001). RGS-PX1, a GAP for Gαs and sorting nexin in vesicular trafficking. Science 294 1939–1942. [DOI] [PubMed] [Google Scholar]