Abstract
To explore the role of plant mitochondria in the regulation of cellular redox homeostasis and stress resistance, we exploited a Nicotiana sylvestris mitochondrial mutant. The cytoplasmic male-sterile mutant (CMSII) is impaired in complex I function and displays enhanced nonphosphorylating rotenone-insensitive [NAD(P)H dehydrogenases] and cyanide-insensitive (alternative oxidase) respiration. Loss of complex I function is not associated with increased oxidative stress, as shown by decreased leaf H2O2 and the maintenance of glutathione and ascorbate content and redox state. However, the expression and activity of several antioxidant enzymes are modified in CMSII. In particular, diurnal patterns of alternative oxidase expression are lost, the relative importance of the different catalase isoforms is modified, and the transcripts, protein, and activity of cytosolic ascorbate peroxidase are enhanced markedly. Thus, loss of complex I function reveals effective antioxidant crosstalk and acclimation between the mitochondria and other organelles to maintain whole cell redox balance. This reorchestration of the cellular antioxidative system is associated with higher tolerance to ozone and Tobacco mosaic virus.
INTRODUCTION
Active oxygen species (AOS) such as the superoxide anion radical (O2.−), the hydroxyl radical (OH·), and hydrogen peroxide (H2O2) are universal products of aerobic metabolism. In plants, AOS are generated at significant rates by reactions intrinsic to photosynthesis, photorespiration, oxidative phosphorylation, fatty acid β-oxidation, and many types of oxidases (Alscher et al., 1997). H2O2 participates widely in plant metabolism (e.g., cell wall biosynthesis) and also has a signal function as a secondary messenger regulating growth and development as well as stress responses (Foyer et al., 1997). However, H2O2 can be converted via Fenton-type reactions to the dangerous hydroxyl radical, which exerts toxic effects within all cellular compartments via lipid peroxidation, protein degradation/modification, and DNA damage (Fridovich, 1986). The accumulation of AOS increases the probability of hydroxyl radical formation. Failure to control AOS accumulation leads to the phenomenon known as oxidative stress (Bartosz, 1997; Foyer and Noctor, 2000).
An appropriate intracellular balance between AOS generation and scavenging exists in all cells. This “redox homeostasis” requires the efficient coordination of reactions in different cell compartments and is governed by a complex network of prooxidant and antioxidant systems. The latter include nonenzymatic scavengers such as ascorbate, glutathione, and hydrophobic molecules (tocopherols, carotenoids, and xanthophylls) and detoxifying enzymes that operate in the different cellular organelles (Noctor and Foyer, 1998a). These enzymes include superoxide dismutases (SODs; EC 1.15.1.1), which catalyze the dismutation of O2.− to H2O2 and O2 (Scandalios, 1993). Three classes of SODs have been identified in plants on the basis of their metal cofactor content. FeSODs are found only in chloroplasts, MnSODs are found mainly in mitochondria, and Cu/ZnSODs are located in chloroplasts, cytosol, apoplast, and peroxisomes (Bowler et al., 1992). The main enzymatic H2O2 scavengers of photosynthetic cells are catalases (CAT; EC 1.11.1.6), which convert H2O2 to H2O and O2 (Scandalios, 1987), and ascorbate peroxidases (APX; EC 1.11.1.11), which use ascorbate as the electron donor for H2O2 reduction (Asada, 1992). In peroxisomes/glyoxysomes, CAT predominates. CAT isoforms are distinguished on the basis of organ specificity and responses to environmental stress (Willekens et al., 1994a). A CAT isoform has been reported to be present in maize mitochondria (Scandalios et al., 1980), but no mitochondrial form has been reported in C3 species (Foyer and Noctor, 2000). APX is part of the ascorbate-glutathione cycle, which uses reduced glutathione to regenerate ascorbate (Foyer and Halliwell, 1976), and glutathione is regenerated by glutathione reductase (GR; EC 1.6.4.2). The ascorbate/glutathione cycle is the most important H2O2-detoxifying system in the chloroplast, but it also has been identified in the cytosol (Nakano and Asada, 1981), peroxisomes, and mitochondria (Jimenez et al., 1997).
Very little is known about interorganellar redox crosstalk and homeostasis in plants. In particular, the role of mitochondria in these processes remains unclear. Indeed, unlike in animals, in which the mitochondrial electron transport chain is the major site of AOS generation (Liu et al., 2002), in plants the rates of photosynthetic electron transport and peroxisomal H2O2 formation are far higher than the capacity for H2O2 generation in mitochondria (Foyer and Noctor, 2000). Thus, despite the fact that some examples of AOS generation by plant mitochondrial electron transport enzymes (complexes I, II, and III) have been reported (Purvis et al., 1995; Braidot et al., 1999; reviewed by Møller, 2001), this production has been considered to be of secondary importance. Nevertheless, the recent demonstration of the crucial role of mitochondria in cell death in animals has led to an increased interest in parallel functions in plants. Several recent reports have suggested that plant mitochondria may be involved in the tolerance to oxidative stress induced by biotic and abiotic treatments (reviewed by Millar et al., 2001; Møller, 2001). In particular, mitochondria may play a key role in the hypersensitive response, which is a form of programmed cell death induced in response to pathogens (Jones, 2000; Swidzinski et al., 2002).
However, one of the key differences between plant and animal mitochondria is the presence of plant-specific alternative respiratory pathways, which may play a role in the control of AOS formation and scavenging. These include non-proton-pumping NAD(P)H dehydrogenases that bypass complex I (Rasmusson et al., 1998) and an alternative oxidase (AOX) that accepts electrons directly from the ubiquinone pool without the intervention of the cytochrome c oxidase pathway through complexes III and IV. These alternative pathways allow the uncoupling of electron transfer from ATP production, preventing the overreduction of the respiratory electron transport chain that otherwise could occur in situations of major flux restrictions (Day and Wiskich, 1995; Vanlerberghe and McIntosh, 1997; Wagner and Moore, 1997). This type of regulation diminishes the risk of electron transfer to O2 and AOS generation. Accordingly, AOX has been shown to be induced by H2O2 (Wagner, 1995), and inhibition or underexpression of the alternative oxidase stimulates H2O2 production (Popov et al., 1997; Maxwell et al., 1999).
To explore the role of plant mitochondria in the regulation of cellular redox homeostasis and stress resistance, we exploited a Nicotiana sylvestris mitochondrial mutant, cytoplasmic male sterile II (CMSII) (Li et al., 1988). This mutant carries a stable mitochondrial DNA mutation that affects the respiratory electron transport chain: the mitochondrial nad7 gene encoding the NAD7 subunit of complex I is deleted (Pla et al., 1995), and mitochondria are impaired in complex I structure and function (Gutierres et al., 1997). In the mutant, the activity of nonphosphorylating NAD(P)H dehydrogenases is enhanced (Sabar et al., 2000). This effect allows increased leaf respiration in CMSII (Dutilleul et al., 2003). Despite impaired photosynthesis and slower growth, the plants eventually attain biomass similar to that of the wild type and undergo reproductive development, although they are conditionally male sterile (Li et al., 1988; De Paepe et al., 1990).
Here, we show that acclimation in response to the loss of complex I function is associated with a marked spatial and temporal reorganization of defense metabolism that affords enhanced protection to oxidative stress. Diurnal patterns of antioxidant transcript abundance are modified. In addition, transcripts and activities are redistributed between cell compartments such that the oxidant/antioxidant balance is maintained, as shown by decreased H2O2 and ascorbate and glutathione redox states similar to those in the wild type. Furthermore, we show that the reorganization of the antioxidant system confers enhanced resistance to ozone and Tobacco mosaic virus (TMV). Thus, rather than inducing a state of oxidative stress, mitochondrial signals associated with the loss of complex I function switch leaves from a stress-sensitive to a stress-tolerant state.
RESULTS
The diploid tobacco N. sylvestris is a long-day species with a pattern of development very different from that of most common cultivars of its close relative Nicotiana tabacum, a tetraploid species. Under a photoperiod of 16 h, N. sylvestris wild-type plants remain at the vegetative rosette stage for ∼2 to 3 months (according to the season and environmental conditions) before bolting. The same growth pattern is followed by CMSII, but its rosette stage lasts for ∼4 to 5 months, as a result of its lower growth rate (Li et al., 1988; De Paepe et al., 1990). Unless stated otherwise, all analyses described here were performed using the second well-developed leaves of wild-type and CMSII rosette plants just before bolting. Although older than wild-type plants, CMSII plants had similar overall shape and dimensions (i.e., the plants were at similar developmental stages) (Figure 1). It must be emphasized that CMSII had undergone growth and development in the absence of a competent complex I and therefore was acclimated to this defect. Key features of this long-term acclimation process are described below.
Figure 1.
N. sylvestris Wild-Type (WT) and CMSII Plants at the Rosette Stage.
A 3-month-old wild-type plant is shown next to a 4-month-old CMSII mutant.
The Complex I Defect Does Not Result in Higher H2O2 Content or Increased Oxidation of Major Soluble Antioxidant Pools
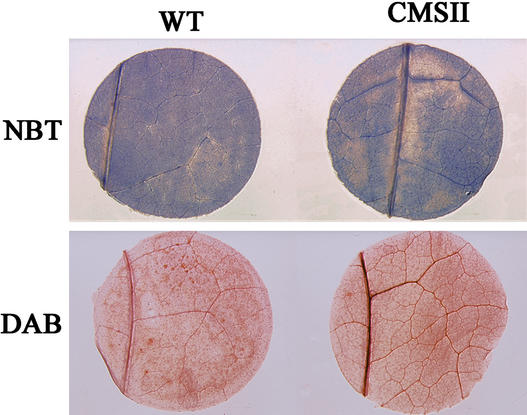
First, we analyzed the amount and distribution of key AOS in leaf tissue from wild-type and mutant plants. In situ detection of O2.− was performed using the nitroblue tetrazolium (NBT) staining method (Jabs et al., 1996), whereas H2O2 was analyzed using 3,3′-diaminobenzidine (DAB) (Thordal-Christensen et al., 1997). For both stains, the degree of staining, and hence the amounts of superoxide and H2O2 in leaf parenchyma tissue, were similar in the wild type and CMSII (Figure 2). For leaf veins, however, a stronger coloration was observed for DAB and NBT staining in CMSII (Figure 2). However, the interpretation of this finding is problematic. Because of the nature of the DAB assay, which relies on in vivo peroxidase activity (Thordal-Christensen et al., 1997), the stronger staining in the CMSII veins could be attributable to higher H2O2, greater activities of peroxidases, or both. Similarly, the degree of staining could be affected by the relative accumulation of DAB and NBT in different cell types.
Figure 2.
In Situ Detection of Leaf H2O2 and O2.−.
DAB and NBT stains were used to detect H2O2 and O2.−, respectively. The samples shown are representative of three independent experiments (six samples per experiment). In all cases, wild-type (WT) and CMSII leaf discs were harvested at the middle of the light period and stained immediately. No specific coloration was observed in controls with ascorbic acid for the DAB staining and with SOD for the NBT staining (data not shown).
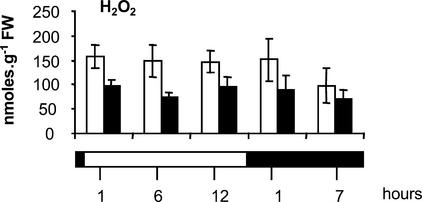
To determine in vivo AOS levels more precisely, we quantified leaf H2O2 in protein-free extracts from flash-frozen samples using an improved spectrophotometric assay (Veljovic-Jovanovic et al., 2002). This method showed that global leaf H2O2 concentrations were lower in CMSII (Figure 3), strongly suggesting that the enhanced DAB staining in CMSII veins reflects greater peroxidase activity. Because the proportional contribution of leaf mitochondria to whole cell AOS production is likely to be different in the light and in the dark, we examined leaf H2O2 contents throughout the day/night cycle. In both genotypes, global leaf H2O2 did not change significantly during the day but decreased slightly in the dark period (Figure 3). At all times, the H2O2 content was lower in CMSII than in the wild type, the difference being most significant in the light.
Figure 3.
Quantitative Comparison of Diurnal Changes in H2O2 Accumulation in CMSII and Wild-Type Leaves.
Leaf samples were harvested during the day/night cycle at the times indicated. The white horizontal bar indicates the light period, and the black horizontal bar indicates the dark period. Open columns indicate the wild type, and closed columns indicate CMSII. Values are means ± se from five independent experiments. The difference between wild-type and CMSII leaf H2O2 contents is significant in both the light (P < 0.01) and the dark (P < 0.05). FW, fresh weight.
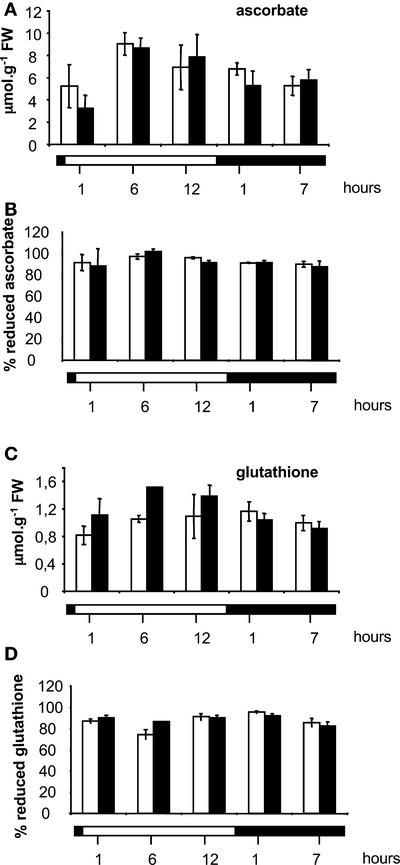
The abundance and redox state of the major nonenzymatic antioxidants of plant cells, ascorbate and glutathione, also were examined during the day/night cycle. The increase in leaf ascorbate observed between 1 and 6 h of light was similar in CMSII and the wild type (Figure 4A). Total glutathione did not show significant variation during the day/night cycle (Figure 4C). The difference between CMSII and the wild type was significant only at one time point (6 h of light). The redox state (100 × reduced/total) of both the ascorbate and glutathione pools was similar in the light and the dark and comparable in the wild type and CMSII (Figures 4B and 4D).
Figure 4.
Leaf Ascorbate and Glutathione Contents and Redox States throughout the Day/Night Cycle.
The white horizontal bar indicates the light period, and the black horizontal bar indicates the dark period. Open columns indicate the wild type, and closed columns indicate CMSII. Values are means ± se from two independent experiments. FW, fresh weight.
(A) Ascorbate content. The increase in ascorbate content between 1 and 5 h of light is significant in CMSII.
(B) Percent reduced ascorbate.
(C) Glutathione content. After 5 h of light, total glutathione content was significantly lower in CMSII than in the wild type.
(D) Percent reduced glutathione.
Together, these results strongly suggest that the oxidant/antioxidant balance is maintained in CMSII photosynthetic cells in spite of the lack of complex I function.
The Abundance and Diurnal Pattern of AOX and Antioxidant Transcripts Is Modified in CMSII
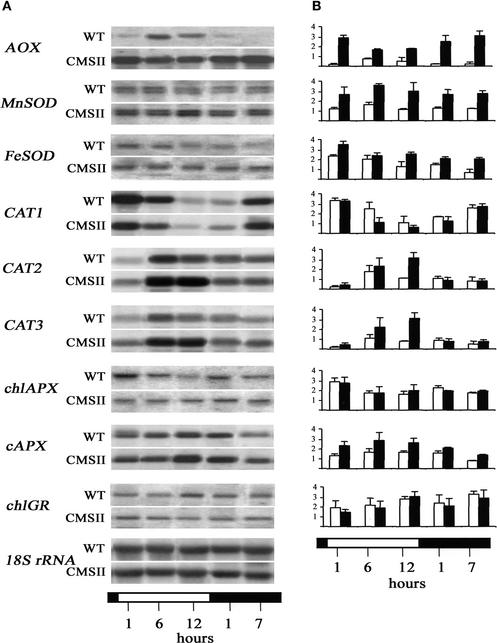
Although no evidence was obtained for oxidative stress in CMSII, transcripts that encode several major components involved in maintaining cellular redox balance were increased. AOX and MnSOD were used as marker enzymes for mitochondria; FeSOD, chlAPX, and chlGR were used as marker enzymes for chloroplasts; cAPX was used as a marker enzyme for the cytosol; and three CAT isoforms were used as marker enzymes for peroxisomes/glyoxysomes. The abundance of transcripts that encode these enzymes was measured throughout the diurnal cycle. Figure 5 shows representative RNA gel blots (Figure 5A) together with relative RNA abundance levels computed from at least three independent experiments (Figure 5B). In the wild type, the expression of most isoforms showed specific daily rhythms. The pattern of these rhythms was perturbed in CMSII.
Figure 5.
Effect of the CMSII Mutation on the Abundance and Diurnal Variation of Antioxidant Transcripts.
The white horizontal bar indicates the light period, and the black horizontal bar indicates the dark period. Open columns indicate the wild type, and closed columns indicate CMSII. Ten micrograms of total RNA from each sample was subjected to RNA gel blot analysis on the same blot. WT, wild type.
(A) Autoradiograms were obtained using AOX, MnSOD, FeSOD, CAT1, CAT2, CAT3, chlAPX, cAPX, and chlGR probes with 18S rRNA as a standard.
(B) Relative abundance of antioxidant gene transcripts in wild-type and CMSII leaves. RNA gel blot signals were scanned with the Scanalytics MasterScan software program. Results are expressed as the values of the following ratio: integrated density of the signal/integrated density of the 18S rRNA signal (arbitrary units). Values are means ± se from three independent experiments. The abundance of the following transcripts was significantly different in CMSII compared with the wild type: AOX, MnSOD, cAPX (in the light and the dark), FeSOD (in the dark), CAT2, and CAT3 (at 12 h of light).
AOX transcripts showed a marked diurnal rhythm in the wild type, with maximum values occurring in the middle of the light period and decreasing to nearly undetectable levels in darkness (Figure 5). By contrast, CMSII AOX transcripts were more abundant in the dark. Hence, the CMSII mutant had 10 times more AOX transcripts than the wild type at the beginning of the light period and during the night (Figure 5).
Although mitochondrial MnSOD transcripts did not change during the photoperiod in either genotype, CMSII values were twice those measured in the wild type (Figure 5). By contrast, the amount of chloroplastic FeSOD transcripts clearly decreased at the end of the day in the wild type (Figure 5). In the mutant, this decrease was less apparent, and in the dark, FeSOD transcript levels were higher than those in the wild type.
CAT1 transcript abundance decreased during the light period in both genotypes (Figure 5). Transcripts were very low at the end of the day and increased during the dark period. CAT2 and CAT3 transcripts (Figure 5) showed inverse diurnal changes than those observed for CAT1. They increased by as much as sixfold to sevenfold during the light period and decreased to very low levels during darkness. The increase in the light was much more apparent in CMSII, and at the end of the light period, the abundance of CAT2 and CAT3 transcripts was threefold to fourfold higher than that in the wild type (Figure 5).
In both genotypes, cAPX transcript levels increased during the light period and were significantly higher in CMSII than in the wild type (Figure 5). By contrast, values for chlAPX transcripts did not change markedly during the photoperiod and were similar in both genotypes (Figure 5). Amounts of chlGR transcripts increased slightly during the light period in both wild-type and CMSII leaves but showed no significant differences between the two genotypes (Figure 5). Cytosolic GR transcript levels, analyzed using a heterologous pea probe (Stevens et al., 1997), were similar in the wild type and CMSII (data not shown).
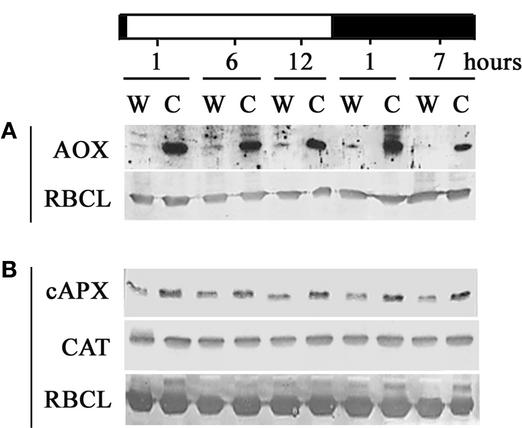
CMSII Leaves Have Greater Amounts of AOX and cAPX Proteins and Increased APX and GR Activities
We examined whether differences in transcripts also were reflected in the amounts of proteins and corresponding enzyme activities. Figure 6 shows protein gel blot analyses of AOX, CAT, and cAPX proteins throughout the diurnal cycle. Very low levels of AOX protein were found in wild-type leaves during the light period, and no protein at all was detected in darkness (Figure 6A), even using the very sensitive chemiluminescence method. By contrast, high amounts of AOX protein were found in CMSII leaves at all times (Figure 6A). cAPX protein abundance was similar in the light and in the dark in both wild-type and CMSII leaves (Figure 6B). However, cAPX protein always was more abundant in CMSII than in the wild type (Figure 6B). The rye anti-CAT antibody revealed a single protein band under our experimental conditions (Figure 6B), which did not change during the day/night cycle and was similar in both genotypes.
Figure 6.
Effect of the CMSII Mutation on the Abundance of AOX, CAT, and cAPX Proteins throughout the Day/Night Cycle.
The white horizontal bar indicates the light period, and the black horizontal bar indicates the dark period. Protein gel blot analysis was performed on total proteins (10 μg) extracted from wild-type (W) and CMSII (C) leaf samples. Results are representative of three independent experiments. The bands correspond to the following approximate molecular masses: 35 kD (AOX), 30 kD (cAPX), and 50 kD (CAT). The large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase (RBCL; 55 kD) was used as a control.
(A) AOX and RBCL proteins visualized by the chemiluminescence method.
(B) cAPX, CAT, and RBCL proteins visualized by the peroxidase reaction.
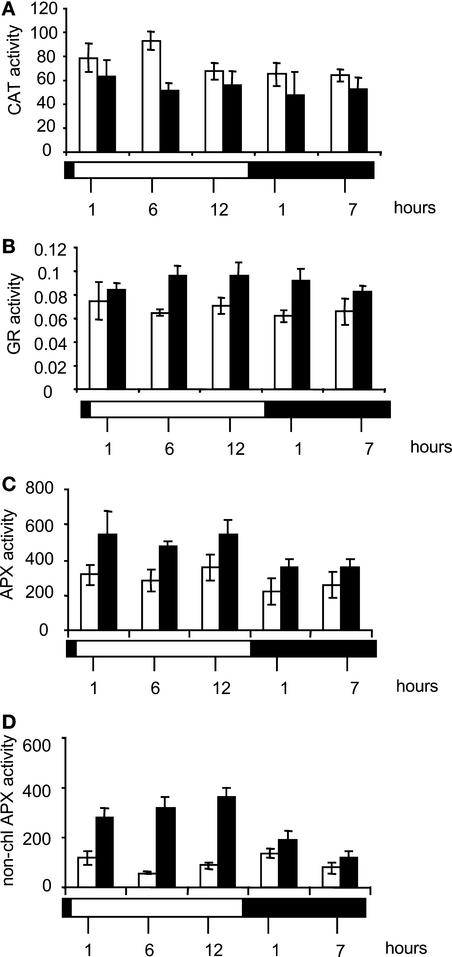
Changes in maximum extractable CAT, APX, and GR activities were measured throughout the diurnal cycle (Figure 7). In agreement with CAT protein abundance data, CAT activity was constant through the day/night cycle and was similar in both genotypes. Although values for CAT activity in CMSII were slightly lower than in the wild type, the only significant difference was recorded at one time point (6 h of light; Figure 7A). GR activity, which also was stable throughout the diurnal time course, was greater in CMSII than in wild-type leaves (Figure 7B). This increase of 30 to 40% was significant at all time points except at the end of the night period (Figure 7B).
Figure 7.
CAT, GR, and APX Activities in CMSII and Wild-Type Plants throughout the Day/Night Cycle.
The white horizontal bar indicates the light period, and the black horizontal bar indicates the dark period. Open columns indicate the wild type, and closed columns indicate CMSII. Values are means ± se from three to six independent experiments.
(A) Total CAT activity (μmol H2O2·min−1·mg−1 protein).
(B) Total GR activity (μmol NADPH oxidized·min−1·mg−1 protein).
(C) Total APX activity (nmol ascorbate oxidized·min−1·mg−1 protein).
(D) Ascorbate depletion-insensitive APX (nonchloroplastic APX) activity (nmol ascorbate oxidized·min−1·mg−1 protein).
For the measurement of leaf APX activity, samples were extracted in the presence or absence of added ascorbate. Cytosolic APX isoforms are considered to be much more resistant to ascorbate depletion than their chloroplastic counterparts, which are inactivated rapidly in the absence of ascorbate (Miyake and Asada, 1996). Therefore, a comparison of the APX activities determined in extracts prepared in the absence or presence of ascorbate provides a measure of the proportion of activity attributable to chloroplastic APX (Amako et al., 1994). In both genotypes, total soluble leaf APX activity extracted in the presence of ascorbate decreased slightly in darkness (Figure 7C). At all times, values for soluble APX activity from CMSII leaves were significantly higher than those measured in wild-type leaves (Figure 7C). This increase in total APX activity was accompanied by a marked increase in nonchloroplastic APX activity (i.e., activity resistant to ascorbate depletion; Figure 7D). This finding is consistent with the increase in cytosolic APX protein (Figure 6B) and also with the increased DAB staining in vascular tissue (Figure 2).
Transcript levels and activities also were measured in plants at two other stages of development: young plants with four well-developed leaves (2-month-old wild-type plants and 3-month-old CMSII plants) and plants at the appearance of the first flower bud (4-month-old wild-type plants and 6-month-old CMSII plants). At all three stages analyzed, transcripts for AOX, MnSOD, FeSOD, cAPX, APX, and GR activities (Table 1) were significantly more abundant in CMSII. With the exception of AOX, the difference increased with plant age.
Table 1.
Abundance of Transcripts and Total Antioxidant Enzyme Activities in Leaves of the Wild Type and CMSII at Three Developmental Stages
| Variable | Young | Rosette | Bud |
|---|---|---|---|
| Transcripts | |||
| AOX | 3.0a | 2.3a | 2.3a |
| MnSOD | 2.2a | 2.2a | 3.1a |
| FeSOD | 0.8 | 1.2 | 1.7 |
| CAT3 | 1.1a | 2.1a | 2.9a |
| cAPX | 1.5a | 1.7a | 3.2a |
| Enzyme activities | |||
| CAT | 1.1 | 0.9 | 1.0 |
| APX | 1.4a | 2.0a | 3.4a |
| GR | 1.6a | 1.5a | 2.0a |
Data are given for three developmental stages: young plants with four fully developed leaves; rosette plants (Figure 1); and mature plants at the floral bud stage. In each case, data (for a time point 6 h into the light period) are given for the second fully developed leaf. Values are means of two (young and floral stages) or three (rosette stage) independent experiments. For transcripts, results are quantified as described in the legend to Figure 5. The data show the ratios of the integrated densities of the bands in CMSII compared with those in the wild type. For enzyme activities, results are expressed as activities in CMSII divided by activities in the wild type.
Values that were significantly different between the wild type and CMSII.
Complex I Deficiency Is Associated with Improved Tolerance to Stress Generated by Both Abiotic and Biotic Treatments
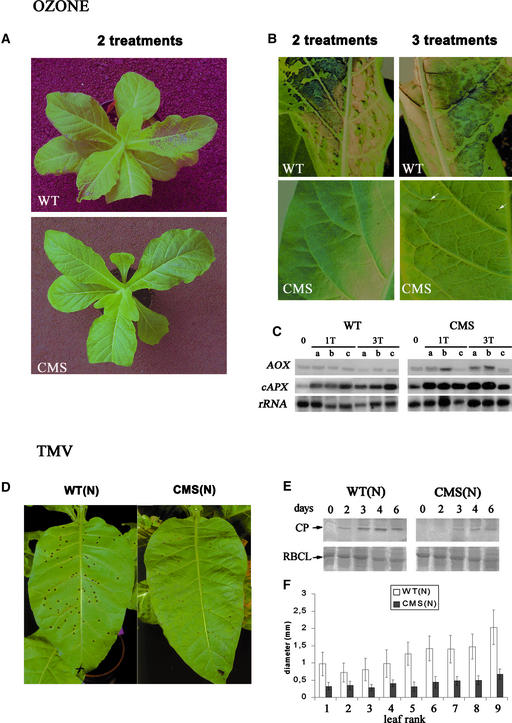
To determine whether the modulation of the antioxidant system described above results in modified tolerance to abiotic stress, we exposed CMSII and wild-type plants to ozone, an air pollutant known to induce oxidative stress (Pell et al., 1997). Plants were given up to three exposures to acute ozone (1000 parts per billion) for 4 h. Wild-type plants developed necrotic areas at the top of the most exposed leaf after a single exposure (data not shown). Two treatments resulted in severe damage, with necrotic areas first appearing at the top of the leaves and near the middle vein (Figures 8A and 8B). After the third treatment, the most damaged leaf was completely necrotic. By contrast, CMSII leaves did not show any visible damage after two treatments, and only very small necrotic points were detected after the third exposure (Figures 8A and 8B). To test the effect of ozone on antioxidant expression, we examined AOX and cAPX transcript levels 1 day after the third exposure. Samples were harvested after one or three exposures from the central part of three neighboring leaves, including the most damaged leaf. Induction of cAPX was observed in both genotypes, with a stronger induction in CMSII. Ozone treatment significantly induced AOX transcripts only in CMSII.
Figure 8.
The CMSII Mutant Is More Resistant Than the Wild Type to Ozone and TMV.
(A) Differences in symptoms in wild-type (WT) and CMSII (CMS) plants subjected to ozone (1 treatment = 1000 parts per billion, 4 h per day).
(B) Relative abundance and size of necrotic lesions developed on N. sylvestris wild-type and CMSII leaves after two or three ozone treatments. Only the wild-type leaves show extensive damage, although arrows indicate small necrotic points on the CMSII leaf after the third treatment.
(C) Induction of AOX and cAPX transcripts by ozone exposure. Ozone treatments were as in (A) and (B). 1T indicates one ozone treatment, and 3T indicates three ozone treatments. Samples were harvested 1 day after the third ozone exposure from the middle part of three successive leaves: the leaf immediately younger than the most damaged leaf (a); the most damaged leaf (b); and the leaf immediately older than the most damaged leaf (c). Ten micrograms of total RNA from each sample was subjected to RNA gel blot analysis on the same blot. AOX and cAPX were as in Figure 5. 18S rRNA was used as a standard.
(D) Hypersensitive response induced by the U1 strain of TMV in N. sylvestris × N. tabacum hybrid plants carrying the N gene of resistance with either the wild-type [WT(N)] or the mutant [CMS(N)] cytoplasm. All of the developed leaves of plants at the pre-floral-bud stage were inoculated with virus (1 μg/mL). The middle-rank leaf (5; see [F]) is shown, photographed 7 days after inoculation.
(E) Immunodetection of the TMV coat protein (CP) in inoculated WT(N) and CMS(N) plants. At each time point, protein was extracted from 10 lesions on inoculated leaf number 3. RBCL, large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase.
(F) Average TMV-induced lesion diameter in consecutive leaves from bottom (bottom leaf) to top (top leaf). At all ranks, lesion size was reduced significantly in CMS(N) compared with WT(N). Results are representative of two independent experiments, with at least 50 lesions measured per experiment.
To establish whether changes in the antioxidant system modify tolerance to biotic stress, we examined susceptibility to attack by TMV. N. sylvestris does not possess the resistance gene N, which confers hypersensitivity to the TMV U1 strain (Dawson, 1999). Thus, this gene was introduced into wild-type and CMSII cytoplasms by crossing diploid N. sylvestris plants as female with tetraploid N. tabacum cv Xanthi NN plants as male. The presence/absence of the complex I nad7 gene in the sterile triploid hybrid plants, named WT(N) and CMSII(N), respectively, was checked by DNA gel blot analyses (data not shown). Both hybrids were similar in morphology to their N. tabacum parent, but CMSII(N) plants developed more slowly than WT(N) hybrid plants, with smaller leaves and flowers. In both hybrids, inoculation with TMV U1 led to small localized necrotic lesions on the leaves after 2 days. The number of lesions per leaf was, on average, 30% lower in CMSII(N) than in WT(N) (Figure 8C). Seven days after inoculation, the average diameter of CMSII(N) lesions was 40 to 60% less (depending on leaf rank) than that of WT(N) lesions (Figures 8D and 8F). These visible effects were associated with lower abundance of the virus coat protein in inoculated CMSII(N) leaves (Figure 8E).
Together, these results show that the absence of complex I is associated with improved resistance to both abiotic (ozone) and biotic (the incompatible TMV interaction) stresses.
DISCUSSION
The role of plant mitochondria in cell death and stress resistance is receiving progressively more attention. Although our knowledge is increasing in this domain, most information comes from studies with respiratory inhibitors, and the interpretation of such studies must be done with caution because of the lack of specificity and the inherent problem of distinguishing the engagement and capacity of respiratory pathways. Very recently, Lee et al. (2002) reported that an Arabidopsis mutant lacking the 18-kD subunit of complex I is affected in cold acclimation, as indicated by increased membrane damage. By contrast, no such damage was detected in the N. sylvestris complex I mutant analyzed in this study (our unpublished results). Furthermore, the data presented here show unambiguously that the lack of complex I activity in N. sylvestris was associated with enhanced tolerance to oxidative stress induced by ozone and TMV. Comparison of these two studies is rendered difficult by the absence of complete information on the structural and functional effects of the lesion in the Arabidopsis complex.
It is possible that, in contrast to the deletion of the mitochondrial nad7 gene in CMSII, disruption of the nuclear gene that encodes the 18-kD subunit has no effect on complex I assembly and activity, as was found in several complex I mutants of Neurospora (Videira, 1998); therefore, it may not be associated with the activation of AOX and antioxidant components, as observed here. This would explain the differences between Arabidopsis and N. sylvestris mutants. It is not possible to speculate further, in the absence of information on the actual perturbation of respiration in the Arabidopsis mutant. For CMSII, we reported previously that the mutant shows modified metabolism (e.g., photosynthesis) and enhanced rotenone-resistant respiration (Sabar et al., 2000; Dutilleul et al., 2003), demonstrating that although complex I function is lost, respiratory electron flow is sustained through alternative pathways. Data presented here show that this readjustment of respiratory metabolism is associated with a temporal and spatial redistribution of the antioxidant system coupled with increased antioxidant capacity that may account for the enhanced resistance to oxidative stress. The present data allow us to draw the following conclusions.
Complex I Dysfunction Does Not Cause Oxidative Stress in N. sylvestris
Much less information is available for plants than for animals about the role of mitochondria in the maintenance of the whole cell redox state. In plants, many studies have focused on the role of the AOX, which has been proposed to be involved in the control of H2O2 levels in plant cells (Wagner and Moore, 1997). Accordingly, H2O2 levels were decreased in tobacco cells overexpressing the AOX gene (Maxwell et al., 1999). A previous study has indicated that the loss of complex I function in CMSII is associated with the induction of AOX (Gutierres et al., 1997). The present study demonstrates that the induction of AOX is associated with lower global H2O2 levels, whereas there are no marked changes in glutathione and ascorbate pools. Most importantly, these key antioxidants remain in the highly reduced state compatible with continued cell function. Because enhanced nonphosphorylating electron transport via AOX when complex I is nonfunctional is expected to decrease mitochondrial ATP yields further, our data suggest that maintenance of the cellular redox state takes precedence over ATP production. Together, these results strongly suggest that despite complex I dysfunction, there is no constitutive oxidative stress in CMSII photosynthetic tissues. Therefore, these results are in contrast to those reported for the Arabidopsis complex I mutant, in which increased DAB staining was detected (Lee et al., 2002), but they are consistent with very recent results showing no signs of protein oxidation in several mitochondrial mutants of maize (Karpova et al., 2002).
Redistribution of Compartment-Specific Antioxidants throughout the Cell Implicates Mitochondria in Whole Cell Redox Signal Transduction
As in animal mitochondria, two types of mechanisms limit AOS accumulation in plants. The first involves the avoidance of AOS production and is facilitated in plants by the presence of the nonphosphorylating respiratory enzymes NAD(P)H dehydrogenases and AOX (Wagner and Moore, 1997; Møller, 2001). Induction of AOX under stress conditions has been reported in a number of plant systems, particularly in relation to H2O2 accumulation. For example, AOX has been shown to be induced by H2O2 (Wagner, 1995), and inhibition of the alternative oxidase stimulates H2O2 production (Popov et al., 1997). Moreover, H2O2 content was decreased in N. tabacum cells overexpressing the AOX gene (Maxwell et al., 1999). The second defense strategy consists of the recruitment of AOS-scavenging enzymes. Components relating to both strategies are activated in CMSII mitochondria, because both AOX and MnSOD transcripts were increased in association with decreased H2O2. In agreement with our results, Pitkanen and Robinson (1996) have shown the induction of MnSOD in patients suffering from complex I dysfunction. Thus, our observations strongly suggest that in CMSII the expression of both MnSOD and AOX is responsive to local mitochondrial production of AOS and that signals emitted as a result cause the acclimation that increases the capacity for scavenging to prevent AOS accumulation. In any aerobic system, redox homeostasis depends on the balance between production and removal. The striking upregulation of AOX represents a local avoidance strategy, whereas that of MnSOD is part of a local defense (scavenging) tactic.
Even more importantly, our data show that the signaled redox acclimation extends far beyond the mitochondria. The induction of local mitochondrial components in CMSII was accompanied by the remote upregulation of AOS-processing systems in other cell compartments. Steady state transcript levels for CAT2 and CAT3 (targeted to microbodies such as peroxisomes and glyoxysomes), FeSOD (targeted to chloroplasts), and cAPX (targeted to the cytosol) all were more abundant in CMSII than in wild-type leaves. This finding suggests that mitochondrial signals control either rates of transcription or rates of turnover of mRNA that encodes these enzymes (or both). We recently discussed the modified redox dialog between compartments with regard to primary metabolism and the increased activation state of NADP–malate dehydrogenase activity in CMSII (Dutilleul et al., 2003). Although the sensing/signaling factors that control antioxidative gene expression remain elusive, it is generally accepted that these genes are upregulated to control AOS. Indeed, because AOS formation is virtually impossible to measure, induction of the antioxidant system frequently is taken as a marker for enhanced AOS production. If this is so, our data suggest that reactive oxygen species sensors in the mitochondria markedly influence the expression of antioxidative genes throughout the cell.
Signals Arising from the Mitochondria Cause the Loss of Wild-Type Diurnal Patterns of Antioxidant Expression
Not only is AOX induced in CMSII, but its diurnal regulation is inverted. In the wild type, AOX transcript abundance was very low during the night, and the AOX protein was hardly detectable. The light/dark rhythm in AOX expression was disrupted in the mutant, in which the AOX gene was highly expressed in darkness at both the transcript and protein levels. The daily rhythms of some other antioxidant transcripts also were altered, although to a lesser extent than that of AOX. Numerous plant antioxidant genes showed diurnal rhythms interacting with the circadian clock. Similar to N. sylvestris cAPX (Figure 5), the Arabidopsis cAPX transcripts peak during the light period (Kubo et al., 1995), and diurnal rhythms of maize CAT3 (Redinbaugh et al., 1990) and Nicotiana plumbaginifolia CAT1 (Willekens et al., 1994a) have been shown to be under the influence of a circadian clock. In wild-type N. sylvestris, the inverse relationship between the expression of CAT1 and CAT2/CAT3 was quite remarkable: transcript levels of CAT1 increased during darkness and decreased in the light, whereas those of CAT2 and CAT3 increased in the light. Clearly, the relative contributions of the different isoforms to total leaf CAT activity (which remains fairly constant) change during the day/night cycle. This diurnal pattern became even more pronounced in CMSII, in which the relative contributions of CAT2 and CAT3 were increased, especially at the end of the light period. By contrast, the diurnal rhythms of chlFeSOD and cAPX were attenuated in the mutant. These data suggest, first, that engagement of redox signaling overrides the control of diurnal rhythms by the circadian clock, and second, that mitochondria provide trig-gers for the network involved in diurnal rhythms in gene expression.
Although our data clearly show upregulation of the antioxidant system at the transcript level, several aspects suggest that antioxidant capacity can be influenced by post-transcriptional and/or post-translational events. One example is the daily rhythm of AOX transcripts, which is less apparent at the protein level. The increase in GR activity in CMSII, which is unrelated to increases in transcripts, also indicates that the readjustment of the antioxidative system in CMSII is a multilevel phenomenon that involves transcriptional and post-transcriptional events.
Although higher amounts of antioxidant transcripts/activities in CMSII are not specific to the rosette stage, the differences between the genotypes tend to increase with age (Table 1). This effect could be related to the slower growth and delayed senescence in the mutant. At the flowering stage, CMSII mature leaves remain healthy for several weeks, whereas they rapidly become senescent in the wild type. Again, these features are indicative of the absence of oxidative stress in the mutant. The relationships between aging, AOS generation, and induction of the antioxidative system have been known for many years (Thompson et al., 1987), particularly in peroxisomes and mitochondria (Droillard and Paulin, 1990). Our data indicate that mitochondria-linked control of redox state is a significant player in the regulation of leaf senescence.
Mitochondria-Driven Antioxidant Crosstalk Markedly Influences Oxidative Stress Tolerance
A number of specific antioxidant genes are induced to cope with enhanced AOS production during oxidative stress (Bartosz, 1997). For example, Arabidopsis cAPX transcripts are increased after exposure to high light (Karpinski et al., 1997), methyl viologen (Yoshimura et al., 2000), or ozone (Kubo et al., 1995; Ranieri et al., 2000). By contrast, chlAPX expression is not changed by these stresses. Enhanced tolerance to ozone has been shown in tobacco plants that overexpress MnSOD (Van Camp et al., 1994) but not in those that overexpress chlCu/ZnSOD (Pitcher et al., 1991), highlighting the importance of the intracellular localization of antioxidants. In good agreement, our data show that CMSII, with increased expression of AOX, MnSOD, and cAPX, has enhanced tolerance to ozone. Although photosynthesis and transpiration are decreased by ∼20 to 30% in CMSII compared with the wild type (Dutilleul et al., 2003), it is unlikely that ozone exclusion accounts for the very marked difference in sensitivity. The response of cAPX and AOX transcripts shows that ozone is not excluded from the leaves of either genotype and suggests that resistance to ozone is linked mainly to increased antioxidant capacity.
In addition to increased resistance to ozone, lack of complex I activity is associated with differences in hypersensitive response–induced responses to both viral and bacterial elicitors. We recently reported differences in antioxidant and defense gene expression between wild-type and CMSII leaves inoculated with a bacterial elicitor (Boccara et al., 2001; Garmier et al., 2002). Here, we show that the number and size of N gene–induced lesions were attenuated markedly in N. sylvestris × N. tabacum (N) hybrids carrying the CMSII cytoplasm. Moreover, amounts of TMV capsidial protein were reduced in the lesions. This finding suggests that CMSII(N) plants are more resistant to TMV than wild-type plants, because multiplication of the virus is hindered (Figure 8E) by the high level of leaf antioxidant enzymes.
N. tabacum NN plants, in which antioxidant enzyme levels were increased after exposure to oxidative stress, were shown to be more resistant to TMV in an incompatible interaction (Mittler et al., 1999). The possible involvement of AOX in plant resistance and programmed cell death also has given rise to much interest and debate. Increased AOX protein levels have been found in both bacterial (Simons et al., 1999) and viral (Chivasa and Carr, 1998) incompatible plant–pathogen interactions. However, although AOX protein was more abundant in N. tabacum NN inoculated with TMV, in vivo engagement of the enzyme remained unaffected (Lennon et al., 1997). Recently, the proportion of cell death was found to be related to the level of AOX expression in tobacco cell cultures (Robson and Vanlerberghe, 2002; Vanlerberghe et al., 2002), and TMV-induced lesions were slightly smaller in N. tabacum NN plants overexpressing AOX (Ordog et al., 2002). By contrast, TMV-susceptible N. tabacum plants overexpressing AOX did not show improved resistance to the virus (Ordog et al., 2002). Accordingly, we did not detect differences in viral replication between the wild type and CMSII in the compatible TMV interaction (our unpublished results). Thus, it seems that although they are possibly involved in the establishment of the hypersensitive response, AOX and other antioxidants do not necessarily confer better resistance to a compatible pathogen. It is possible that the upregulation of AOX that we observed in CMSII may be accompanied by decreased cytochrome pathway components, which may influence cell death susceptibility, because, as in animals, cytochrome c release is involved in programmed cell death in plants (Balk et al., 1999)
Concluding Remarks
Here, we have demonstrated that leaf mitochondria play a major role in the control of leaf oxidant/antioxidant balance and stress resistance. Although considered a major source of AOS in nonphotosynthetic tissues and in photosynthetic tissues in the dark, mitochondria are expected to contribute only a small fraction of total leaf AOS production in the light. Despite this fact, CMSII leaves show induction of the antioxidative system in both the light and the dark. Associated with this induction is enhanced stress resistance. Recent data show that the response to oxidative stress involves numerous genes, and not only those involved directly in the control of AOS (Vranova et al., 2002). Therefore, we expect that enhanced stress resistance may not result solely from the upregulation of antioxidative components. However, this upregulation is a clear marker for the long-term acclimatory activation of stress resistance in response to the loss of a major mitochondrial NADH dehydrogenase.
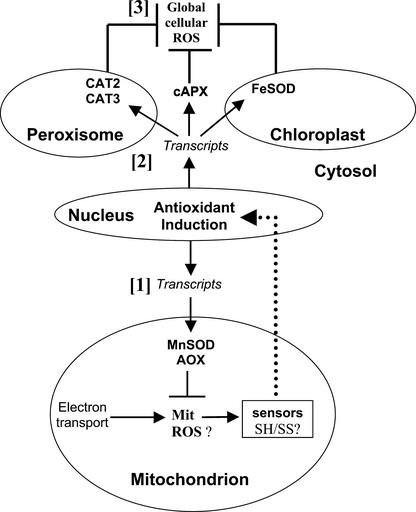
Our data are consistent with a hypothesis in which nuclear gene expression is influenced by events of mitochondrial origin, but the nature of the signal(s) to the nucleus remains to be resolved. We suggested previously that the loss of CMSII affects the shuttling of NAD(P)H between different cellular compartments (Dutilleul et al., 2003), and it is possible that the loss of complex I has repercussions for global cellular redox state, which induces changes in gene expression. Nevertheless, the present data show that neither whole leaf H2O2 content nor the oxidation of leaf antioxidants is increased in CMSII. Because of the small contribution of mitochondria to leaf AOS production, whole tissue measurements of AOS and antioxidants may not detect a localized increase in AOS production. Perception of such AOS could be linked to a localized sensor situated very close to the site of superoxide and H2O2 production within the inner mitochondrial membrane itself (Figure 9). Because the site of signal generation in the photosynthetic signaling system is close to the plastoquinone pool (Karpinski et al., 1997, 1999; Pfannschmidt et al., 2001), it is tempting to suggest that the ubiquinone pool serves a similar function in signaling for mitochondrial electron transport. The ability of the leaf cell to detect such mitochondrial signals allows these organelles to play a key role in coordinating redox homeostasis and determining stress tolerance.
Figure 9.
Whole Cell Redox Signaling by Leaf Mitochondria: Hypothesis.
The scheme proposes that alterations in redox status in the mitochondria (Mit), possibly manifested as increased AOS, are detected by as yet unidentified sensors that may involve thiol-disulfide exchange (SH/SS). These mitochondrion-specific signals are relayed to the nucleus by transducers (dotted line), leading to the effects observed in CMSII. These effects are as follows: increased abundance of transcripts of antioxidative enzymes that process locally generated AOS species (MnSOD) or decrease their rate of production (AOX) in mitochondria [1]; and whole cell induction of transcripts that encode antioxidative enzymes located in compartments (CAT1, CAT3, cAPX, and FeSOD) external to the mitochondria [2]. Together, these acclimatory responses trigger decreased whole cell reactive oxygen species (ROS) accumulation and enhance stress resistance [3].
METHODS
Plant Material
Nicotiana sylvestris is the diploid maternal ancestor of the tetraploid cultivated tobacco, Nicotiana tabacum. The N. sylvestris wild parental type is a fertile botanical line of the Institut des Tabacs (Bergerac, France). The CMSII mutant was obtained by in vitro wild-type protoplast culture (Li et al., 1988), and the variant cytoplasm was maintained by crossing with wild-type plants as male. The plants were grown in a greenhouse under a 16-h photoperiod at day/night temperatures of 23.5/17.5°C and day/night RH of 60/50%. Irrigation was supplemented with macronutrients. For analyses, wild-type and CMSII plants at the same developmental stage were transferred to a growth chamber for at least 3 days of acclimation with a 16-h photoperiod at a PPFD of 200 μmol·m−2·s−1 at day/night temperatures of 24/17°C and RH of 70%. Samples taken were from the second well-developed leaves.
RNA Isolation and Gel Blot Analysis
For RNA isolation, leaf tissue pieces (0.1 g) were harvested in liquid nitrogen during the light/dark cycle and stored at −80°C. Total RNAs were extracted by the Trizol-chloroform procedure (Gibco BRL). Ten micrograms of total RNA was fractionated on a 1.2% agarose gel, blotted onto nylon-based membranes (Appligène, Illkirsh, France), and hybridized with 32P-labeled probes. Homologous probes were Nicotiana plumbaginifolia CAT1, CAT2, and CAT3 (Willekens et al., 1994b), N. tabacum plastidial GR (Creissen and Mullineaux, 1995), N. tabacum cAPX (Orvar and Ellis, 1995), N. plumbaginifolia MnSOD1 (Bowler et al., 1989), N. plumbaginifolia FeSOD (Van Camp et al., 1990), and a N. sylvestris AOX probe (Sabar et al., 2000). An N. sylvestris chlAPX probe was generated using primers 5′-TCCAGGAAACCCTGGAGGAC-3′ and 5′-TCAGGA-GGATCAAATTTGGC-3′ designed from the N. tabacum chlAPX sequence (Yoshimura et al., 1999). All procedures for blot analysis were performed as described previously (Sabar et al., 2000). Quantification of the relative abundance of transcripts was determined using a scanning densitometer (MasterScan; Scanalytics, Billerica, MA). Results were expressed as the values of the following ratio: integrated density of the signal/integrated density of the 18S rRNA signal.
Total Protein Extraction
Leaf material (0.1 g) was ground in liquid nitrogen, and total proteins were extracted into 0.3 mL of 0.1 M Tris-HCl, pH 8.1, 10% sucrose, and 0.05% β-mercaptoethanol. To distinguish between chloroplastic and nonchloroplastic extractable APX activities, samples were extracted in the presence or absence of 5 mM ascorbate in the medium. The extract was centrifuged for 5 min at 15,000g, and protein was determined according to Bradford (1976).
Protein Gel Blot Immunodetection
Total leaf proteins (10 μg per lane) were separated on a 12% SDS-polyacrylamide gel and transferred to nitrocellulose membranes as described by Gutierres et al. (1997). The following antisera were used: mice monoclonal antiserum against Sauromatum guttatum AOX (Elthon et al., 1989) at a dilution of 1:200; rabbit antiserum directed against rye leaf catalase (Hertwig et al., 1992) at a dilution of 1:3000; rabbit antiserum directed against spinach cAPX at a dilution of 1:3000 (Saji et al., 1990); rabbit antiserum directed against sorghum ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit (RBCL); and antiserum directed against the viral coat protein (17.5 kD) of Tobacco mosaic virus (TMV) strain U1. Horseradish peroxidase–conjugated goat anti-mouse or anti-rabbit IgG was used as a secondary antibody (at a dilution of 1:2000), and immune complexes were visualized by the color reaction of peroxidase as described by Gutierres et al. (1997), except for AOX (and the corresponding RBCL detection), which was visualized by chemiluminescence (West Dura Trial Kit; Pierce).
Antioxidant Enzyme Activities
Catalase activity was determined by monitoring H2O2 removal as the decrease in A240 (ɛ = 36 M−1 cm−1) for 30 s, as described by Dorey et al. (1998). GR activity was determined by monitoring the oxidation of NADPH at 340 nm (ɛ = 6.22 M−1 cm−1), as described by Donahue et al. (1997). APX activity was determined by monitoring the oxidation of ascorbate at 290 nm (ɛ = 2.88 mM−1 cm−1), as described by Nakano and Asada (1981).
Determination of O2.− and H2O2 Content
Global leaf H2O2 was determined according to the method of Veljovic-Jovanovic et al. (2002) using the chromogenic peroxidase-coupled oxidation of 3-methyl 2-benzothiazolinone hydrazone and 3-dimethyl aminobenzoic acid.
In situ O2.− was estimated using the nitroblue tetrazolium (NBT) staining method as described by Jabs et al. (1996), with the following modifications. Leaf discs were punched out with a cork borer (2 cm in diameter) from the central area of the second fully developed leaf and vacuum-infiltrated (three cycles of 5 min) in 0.5 mg/mL NBT prepared in 10 mM potassium phosphate buffer, pH 7.8. As a control, superoxide dismutase (10 units/mL) and 10 mM MnCl2 were added to the staining medium before infiltration. Samples were incubated for 1 h in the dark at room temperature and then cleared in 90% ethanol at 70°C until complete removal of chlorophyll. O2.− was visualized as a blue color at the site of NBT precipitation. Samples were stored and examined in 70% glycerol. In situ H2O2 was estimated using the 3,3′-diaminobenzidine (DAB) staining method as described by Thordal-Christensen et al. (1997) and modified as follows. Leaf discs were vacuum-infiltrated (three cycles of 5 min) with 1 mg/mL DAB solution, pH 3.8. As a control, DAB solution was supplemented with 10 mM ascorbic acid before infiltration. After incubation in the dark at room temperature for 14 h, samples were transferred in 90% ethanol at 70°C until complete removal of chlorophyll. H2O2 was visualized as brown color at the site of DAB polymerization. Samples were stored and examined in 70% glycerol.
Ascorbate and Glutathione Determination
Leaf discs (2.4 cm in diameter) were harvested, ground in liquid nitrogen, and then ground in 1 mL of 1 M HClO4. After thawing, samples were centrifuged for 15 min at 15,000g and 4°C. The pH of the clarified supernatant was adjusted to 5.6 with K2CO3, and insoluble KClO4 was removed by centrifugation. Ascorbate and glutathione were measured in the same supernatant. Total and reduced ascorbate contents were measured as the ascorbate oxidase–dependent decrease in A265 before (reduced ascorbate) and after (total ascorbate) treatment of the sample for 15 min with 0.1 M DTT (modified from Foyer et al., 1983). The 1-mL reaction mixture contained 0.12 M NaH2PO4, pH 5.6, and 0.1 mL of extract. The difference in A265 before and after the addition of 1 unit of ascorbate oxidase was converted to ascorbate concentration (A265 = 12.6 mM−1 cm−1). Total and oxidized glutathione contents were measured using the enzymatic recycling assay, which involves the NADPH-driven glutathione-dependent reduction of 5,5-dithiobis 2-nitrobenzoic acid at 412 nm (Noctor and Foyer, 1998b).
Ozone Treatment
Wild-type and CMSII plants were exposed to acute ozone doses (1000 parts per billion) for up to 3 successive days for 4 h per day in a controlled environment, 21°C and 75% RH, as described by Cotovio et al. (2001).
TMV Inoculation
Wild-type and CMSII N. sylvestris × N. tabacum hybrid plants, at the same developmental stage (first flower bud), were inoculated with the common U1 strain of TMV. Using Carborundum as an abrasive, nine leaves of each plant were inoculated with 1 mL of 1 μg/mL viral protein prepared in 0.01 M phosphate buffer, pH 7.0. Lesions were evaluated 7 days after inoculation.
Statistical Analyses
The significance of differences was determined using Student's t test. Values are denoted as significant (P < 0.05) or highly significant (P < 0.01).
Upon request, all novel materials described in this article will be made available in a timely manner for noncommercial research purposes.
Acknowledgments
We gratefully acknowledge the following for the gifts of probes: D. Inzé (SOD and CAT cDNA probes), B. Ellis (cAPX probe), G. Creissen (chlGR probe), T. Elthon (AOX antibody), J. Feierabend (CAT antibody), and A. Kubo (cAPX antibody). We thank S. Kauffmann for providing the TMV U1 strain and Roland Boyer for photographic work. Many thanks to M. Boccara for helpful discussion and comments. This work was supported by the Centre National de la Recherche Scientifique (France), the Biotechnology and Biological Science Research Council (United Kingdom), the British Council (Alliance program funding), the European Science Foundation (fellowship award to C.D.), and the French Ministère de la Recherche et de la Technologie (award to M.G.).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.009464.
References
- Alscher, R.G., Donahue, J.L., and Cramer, C.L. (1997). Reactive oxygen species and antioxidants: Relationships in green cells. Physiol. Plant. 100, 224–233. [Google Scholar]
- Amako, K., Chen, G.X., and Asada, K. (1994). Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol. 35, 497–504. [Google Scholar]
- Asada, K. (1992). Ascorbate peroxidase: A hydrogen peroxide-scavenging enzyme in plants. Physiol. Plant. 85, 235–241. [Google Scholar]
- Balk, J., Leaver, C.J., and McCabe, P.F. (1999). Translocation of cytochrome c from the mitochondria to the cytosol occurs during heat-induced programmed cell death in cucumber plants. FEBS Lett. 463, 151–154. [DOI] [PubMed] [Google Scholar]
- Bartosz, G. (1997). Oxidative stress in plants. Acta Physiol. Plant. 19, 47–64. [Google Scholar]
- Boccara, M., Boué, C., Garmier, M., De Paepe, R., and Boccara, A.C. (2001). IR thermography revealed a role for mitochondria in presymptomatic cooling during harpin-induced hypersensitive response. Plant J. 28, 663–670. [DOI] [PubMed] [Google Scholar]
- Bowler, C., Alliotte, T., De Loose, M., Van Montagu, M., and Inzé, D. (1989). The induction of manganese superoxide dismutase in response to stress in Nicotiana plumbaginifolia. EMBO J. 8, 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler, C., Van Montagu, M., and Inzé, D. (1992). Superoxide dismutase and stress tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 43, 83–116. [Google Scholar]
- Bradford, M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 7, 248–254. [DOI] [PubMed] [Google Scholar]
- Braidot, E., Petrussa, E., Vianello, A., and Macri, F. (1999). Hydrogen peroxide generation by higher plant mitochondria oxidizing complex I or complex II substrates. FEBS Lett. 451, 347–350. [DOI] [PubMed] [Google Scholar]
- Chivasa, S., and Carr, J.P. (1998). Cyanide restores N gene–mediated resistance to tobacco mosaic virus in transgenic tobacco expressing salicylic acid hydroxylase. Plant Cell 10, 1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotovio, J., Onno, L., Justine, P., Lamure, S., and Catroux, P. (2001). Generation of oxidative stress in human cutaneous models following in vitro ozone exposure. Toxicol. in Vitro 15, 357–362. [DOI] [PubMed] [Google Scholar]
- Creissen, G., and Mullineaux, P. (1995). Cloning and characterisation of glutathione reductase cDNAs and identification of two genes encoding the tobacco enzyme. Planta 197, 422–425. [DOI] [PubMed] [Google Scholar]
- Dawson, W.O. (1999). Tobacco mosaic virus virulence and avirulence. Philos. Trans. R. Soc. Lond. B 354, 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day, D.A., and Wiskich, J.T. (1995). Regulation of alternative oxidase activity in higher plants. J. Bioenerg. Biomembr. 27, 379–385. [DOI] [PubMed] [Google Scholar]
- De Paepe, R., Chétrit, P., Vitart, V., Ambard-Bretteville, F., Prat, D., and Vedel, F. (1990). Several nuclear genes control both sterility and mitochondrial protein synthesis in Nicotiana sylvestris protoclones. Mol. Gen. Genet. 222, 206–210. [DOI] [PubMed] [Google Scholar]
- Donahue, J.L., Okpodu, C.M., Cramer, C.L., Grabau, E.A., and Alscher, R.G. (1997). Responses of antioxidants to paraquat in pea leaves. Plant Physiol. 113, 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorey, S., Baillieul, F., Saindrenan, P., Fritig, B., and Kauffmann, S. (1998). Tobacco class I and II catalases are differentially expressed during elicitor-induced hypersensitive cell death and localized acquired resistance. Mol. Plant-Microbe Interact. 11, 1102–1109. [Google Scholar]
- Droillard, M.J., and Paulin, A. (1990). Isozymes of superoxide dismutase in mitochondria and peroxisomes isolated from petals of carnation (Dianthus caryophyllus) during senescence. Plant Physiol. 94, 1187–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutilleul, C., Driscoll, S., Cornic, G., De Paepe, R., Foyer, C.H., and Noctor, G. (2003). Tobacco leaves require functional mitochondrial complex I for optimal photosynthetic performance in photorespiratory conditions and during transients. Plant Physiol. 131, 264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon, T.E., Nickels, R.L., and McIntosh, L. (1989). Monoclonal antibodies to the alternative oxidase of higher plant mitochondria. Plant Physiol. 89, 1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer, C.H., and Halliwell, B. (1976). The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 133, 21–25. [DOI] [PubMed] [Google Scholar]
- Foyer, C.H., Lopez-Delgado, H., Dat, J.F., and Scott, I.M. (1997). Hydrogen peroxide and glutathione–associated mechanisms of acclimatory stress tolerance and signalling. Physiol. Plant. 100, 241–254. [Google Scholar]
- Foyer, C.H., and Noctor, G. (2000). Tansley Review No. 112. Oxygen processing in photosynthesis: Regulation and signalling. New Phytol. 146, 359–388. [Google Scholar]
- Foyer, C.H., Rowell, J., and Walker, D. (1983). Measurement of the ascorbate content of spinach leaf protoplasts and chloroplasts during illumination. Planta 157, 239–244. [DOI] [PubMed] [Google Scholar]
- Fridovich, I. (1986). Biological effects of the superoxide radical. Arch. Biochem. Biophys. 247, 1–11. [DOI] [PubMed] [Google Scholar]
- Garmier, M., Dutilleul, C., Mathieu, C., Chétrit, P., Boccara, M., and De Paepe, R. (2002). Changes in antioxidant expression and harpin-induced hypersensitive response in a Nicotiana sylvestris mitochondrial mutant. Plant Physiol. Biochem. 40, 561–566. [Google Scholar]
- Gutierres, S., Sabar, M., Lelandais, C., Chétrit, P., Diolez, P., Degand, H., Boutry, M., Vedel, F., de Kouchkovsky, Y., and De Paepe, R. (1997). Lack of mitochondrial and nuclear-encoded subunits of complex I and alteration of the respiratory chain in Nicotiana sylvestris mitochondrial deletion mutants. Proc. Natl. Acad. Sci. USA 94, 3436–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertwig, B., Streb, P., and Feierabend, J. (1992). Light dependence of catalase synthesis and degradation in leaves and the influence of interfering stress conditions. Plant Physiol. 100, 1547–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs, T., Dietrich, R.A., and Dangl, J.L. (1996). Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 27, 1853–1856. [DOI] [PubMed] [Google Scholar]
- Jimenez, A., Hernandez, J.A., del Rio, L.A., and Sevilla, F. (1997). Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol. 114, 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, A. (2000). Does the plant mitochondrion integrate cellular stress and regulate programmed cell death? Trends Plant Sci. 5, 225–230. [DOI] [PubMed] [Google Scholar]
- Karpinski, S., Escobar, C., Karpinska, B., Creissen, G., and Mullineaux, P.M. (1997). Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell 9, 627–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski, S., Reynolds, H., Karspinka, B., Wingsle, G., Creissen, G., and Mullineaux, P. (1999). Systemic signalling and acclimation in response to excess excitation energy. Science 23, 654–657. [DOI] [PubMed] [Google Scholar]
- Karpova, O.V., Kuzmin, E.V., Elthon, T.E., and Newton, K.J. (2002). Differential expression of alternative oxidase genes in maize mitochondrial mutants. Plant Cell 14, 3271–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo, A., Saji, H., Tanaka, K., and Kondo, N. (1995). Expression of cytosolic ascorbate peroxidase gene in response to ozone or sulfur dioxide. Plant Mol. Biol. 29, 479–489. [DOI] [PubMed] [Google Scholar]
- Lee, B.H., Lee, H., Xiong, L., and Zhu, J.K. (2002). A mitochondrial complex I defect impairs cold-regulated nuclear gene expression. Plant Cell 14, 1235–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon, A.M., Neuenschwander, U.H., Ribas-Carbo, M., Giles, L., Ryals, J.A., and Siedow, J.N. (1997). The effects of salicylic acid and tobacco mosaic virus infection on the alternative oxidase of tobacco. Plant Physiol. 115, 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X.Q., Chétrit, P., Mathieu, C., Vedel, F., De Paepe, R., Rémy, R., and Ambard-Bretteville, F. (1988). Regeneration of cytoplasmic male sterile protoclones of Nicotiana sylvestris with mitochondrial variations. Curr. Genet. 13, 261–266. [Google Scholar]
- Liu, Y., Fiskum, G., and Schubert, D. (2002). Generation of reactive oxygen species by the mitochondrial electron transport chain. J. Neurochem. 80, 780–787. [DOI] [PubMed] [Google Scholar]
- Maxwell, D.P., Wang, Y., and McIntosh, L. (1999). The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc. Natl. Acad. Sci. USA 96, 8271–8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar, H., Considine, M.G., Day, D.A., and Whelan, J. (2001). Unraveling the role of mitochondria during oxidative stress in plants. IUBMB Life 51, 201–205. [DOI] [PubMed] [Google Scholar]
- Mittler, R., Herr, E.H., Orvar, B.J., van Camp, W., Willekens, H., Inzé, D., and Ellis, B.E. (1999). Transgenic tobacco plants with reduced capability to detoxify reactive oxygen intermediates are hyperresponsive to pathogen infection. Proc. Natl. Acad. Sci. USA 96, 14165–14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake, C., and Asada, K. (1996). Inactivation mechanism of ascorbate peroxidase at low concentrations of ascorbate: Hydrogen peroxide decomposes compound I of ascorbate peroxidase. Plant Cell Physiol. 37, 423–430. [Google Scholar]
- Møller, I.M. (2001). Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 561–591. [DOI] [PubMed] [Google Scholar]
- Nakano, Y., and Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22, 867–880. [Google Scholar]
- Noctor, G., and Foyer, C.H. (1998. a). Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 249–279. [DOI] [PubMed] [Google Scholar]
- Noctor, G., and Foyer, C.H. (1998. b). Simultaneous measurement of foliar glutathione, γ-glutamylcysteine, and amino acids by high-performance liquid chromatography: Comparison with two other assay methods for glutathione. Anal. Biochem. 264, 98–110. [DOI] [PubMed] [Google Scholar]
- Ordog, S.H., Higgins, V.J., and Vanlerberghe, G.C. (2002). Mitochondrial alternative oxidase is not a critical component of plant viral resistance but may play a role in the hypersensitive response. Plant Physiol. 129, 1858–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvar, B.L., and Ellis, B.E. (1995). Isolation of a cDNA encoding cytosolic ascorbate peroxidase in tobacco. Plant Physiol. 108, 839–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pell, E.J., Schlagnhaufer, C.D., and Arteca, R.N. (1997). Ozone-induced oxidative stress: Mechanisms of action and reaction. Physiol. Plant. 100, 264–273. [Google Scholar]
- Pfannschmidt, T., Schütze, K., Brost, M., and Oelmüller, R. (2001). A novel mechanism of nuclear photosynthesis gene regulation by redox signals from the chloroplast during photosystem stoichiometry adjustment. J. Biol. Chem. 276, 36125–36130. [DOI] [PubMed] [Google Scholar]
- Pitcher, L.H., Brennan, E., Hurley, A., Dunsmuir, P., Tepperman, J.M., and Zilinskas, B.A. (1991). Overproduction of petunia copper/zinc superoxide dismutase does not confer ozone tolerance in transgenic tobacco. Plant Physiol. 97, 452–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen, S., and Robinson, B.H. (1996). Mitochondrial complex I deficiency leads to increased production of superoxide radicals and induction of superoxide dismutase. J. Clin. Invest. 98, 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pla, M., Mathieu, C., De Paepe, R., Chétrit, P., and Vedel, F. (1995). Deletion of the last two exons of the mitochondrial nad7 gene results in lack of the NAD7 polypeptide in a Nicotiana sylvestris CMS mutant. Mol. Gen. Genet. 248, 279–288. [DOI] [PubMed] [Google Scholar]
- Popov, V.N., Simonian, R.A., Skulachev, V.P., and Starkov, A.A. (1997). Inhibition of the alternative oxidase stimulates H2O2 production in plant mitochondria. FEBS Lett. 415, 87–90. [DOI] [PubMed] [Google Scholar]
- Purvis, A.C., Shewfelt, R.L., and Gegogeine, J.W. (1995). Superoxide production by mitochondria isolated from green bell pepper fruit. Physiol. Plant. 94, 743–749. [Google Scholar]
- Ranieri, A., Castagna, A., and Soldatini, G.F. (2000). Differential stimulation of ascorbate peroxidase isoforms by ozone exposure in sunflower plants. J. Plant Physiol. 156, 266–271. [Google Scholar]
- Rasmusson, A.G., Heiser, V., Zabaleta, E., Brennicke, A., and Grohmann, L. (1998). Physiological, biochemical and molecular aspects of mitochondrial complex I in plants. Biochim. Biophys. Acta 1364, 1401–1411. [DOI] [PubMed] [Google Scholar]
- Redinbaugh, M.G., Sabre, M., and Scandalios, J.G. (1990). Expression of the maize Cat3 catalase gene is under the influence of a circadian rhythm. Proc. Natl. Acad. Sci. USA 87, 6853–6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson, C.A., and Vanlerberghe, G.C. (2002). Transgenic plant cells lacking mitochondrial alternative oxidase have increased susceptibility to mitochondria-dependent and -independent pathways of programmed cell death. Plant Physiol. 129, 1908–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabar, M., De Paepe, R., and de Kouchkovsky, Y. (2000). Complex I impairment, respiratory compensations, and photosynthetic decrease in nuclear and mitochondrial male sterile mutants of Nicotiana sylvestris. Plant Physiol. 124, 1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saji, H., Tanaka, K., and Kondo, N. (1990). Monoclonal antibodies to spinach ascorbate peroxidase and immunochemical detection of the enzyme in eight different plant species. Plant Sci. 69, 1–9. [Google Scholar]
- Scandalios, J.G. (1987). Isozymes. In Current Topics in Biological and Medical Research, Vol. 14, M.C. Rattazi, J.G. Scandalios, and G.S. Whitt, eds (New York: Liss), pp. 19–44.
- Scandalios, J.G. (1993). Oxygen stress and superoxide dismutases. Plant Physiol. 101, 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandalios, J.G., Tong, W.F., and Roupakias, D.G. (1980). Cat3, a third gene locus coding for a tissue-specific catalase in maize: Genetics, intracellular location, and some biochemical properties. Mol. Gen. Genet. 179, 33–41. [Google Scholar]
- Simons, B.H., Millenaar, F.F., Mulder, L., Van Loon, L.C., and Lambers, H. (1999). Enhanced expression and activation of the alternative oxidase during infection of Arabidopsis with Pseudomonas syringae pv tomato. Plant Physiol. 120, 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, R.G., Creissen, G.P., and Mullineaux, P.M. (1997). Cloning and characterisation of a cytosolic glutathione reductase cDNA from pea (Pisum sativum L.) and its expression in response to stress. Plant Mol. Biol. 5, 641–654. [DOI] [PubMed] [Google Scholar]
- Swidzinski, J.A., Sweetlove, L.J., and Leaver, C.J. (2002). A custom microarray analysis of gene expression during programmed cell death in Arabidopsis thaliana. Plant J. 30, 431–446. [DOI] [PubMed] [Google Scholar]
- Thompson, J.E., Ledge, R.L., and Barber, R.F. (1987). The role of free radicals in senescence and wounding. New Phytol. 105, 317–344. [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen, H., Zhang, Z., Wei, Y., and Collinge, D.B. (1997). Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 11, 1187–1194. [Google Scholar]
- Van Camp, W., Bowler, C., Villarroel, R., Tsang, E.W., Van Montagu, M., and Inzé, D. (1990). Characterization of iron superoxide dismutase cDNAs from plants obtained by genetic complementation in Escherichia coli. Proc. Natl. Acad. Sci. USA 87, 9903–9907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Camp, W., Willekens, H., Bowler, C., Van Montagu, M., Inzé, D., Reupold-Popp, P., Sandermann, H., Jr., and Langebartels, C. (1994). Elevated levels of superoxide dismutase protect transgenic plants against ozone damage. Biotechnology 12, 165–168. [Google Scholar]
- Vanlerberghe, G.C., and McIntosh, L.C. (1997). Alternative oxidase: From gene to function. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 703–734. [DOI] [PubMed] [Google Scholar]
- Vanlerberghe, G.C., Robson, C.A., and Yip, J.Y.H. (2002). Induction of mitochondrial alternative oxidase in response to a cell signal pathway down-regulating the cytochrome pathway prevents programmed cell death. Plant Physiol. 129, 1829–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veljovic-Jovanovic, S.D., Noctor, G., and Foyer, C.H. (2002). Are leaf hydrogen peroxide concentrations commonly over-estimated? The potential influence of artefactual interference by tissue phenolics and ascorbate. Plant Physiol. Biochem. 40, 501–508. [Google Scholar]
- Videira, A. (1998). Complex I from the fungus Neurospora crassa. Biochim. Biophys. Acta 1364, 89–100. [DOI] [PubMed] [Google Scholar]
- Vranova, E., Atichartpongkul, S., Villarroel, R., Van Montagu, M., Inzé, D., and Van Camp, W. (2002). Comprehensive analysis of gene expression in Nicotiana tabacum leaves acclimated to oxidative stress. Proc. Natl. Acad. Sci. USA 99, 10870–10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, A.M. (1995). A role for active oxygen species as second messengers in the induction of alternative oxidase gene expression in Petunia hybrida cells. FEBS Lett. 368, 339–342. [DOI] [PubMed] [Google Scholar]
- Wagner, A.M., and Moore, A.L. (1997). Structure and function of the plant alternative oxidase: Its putative role in the oxygen defence mechanism. Biosci. Rep. 17, 319–333. [DOI] [PubMed] [Google Scholar]
- Willekens, H., Langebartels, C., Tire, C., Van Montagu, M., Inzé, D., and Van Camp, W. (1994. a). Differential expression of catalase genes in Nicotiana plumbaginifolia (L.). Proc. Natl. Acad. Sci. USA 91, 10450–10454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willekens, H., Villarroel, R., Van Montagu, M., Inze, D., and Van Camp, W. (1994. b). Molecular identification of catalases from Nicotiana plumbaginifolia (L.). FEBS Lett. 352, 79–83. [DOI] [PubMed] [Google Scholar]
- Yoshimura, K., Yabuta, Y., Ishikawa, T., and Shigeoka, S. (2000). Expression of spinach ascorbate peroxidase isoenzymes in response to oxidative stresses. Plant Physiol. 123, 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura, K., Yabuta, Y., Tamoi, M., Ishikawa, T., and Shigeoka, S. (1999). Alternative spliced mRNA variants of chloroplast ascorbate peroxidase isoenzymes in spinach leaves. Biochem. J. 338, 41–48. [PMC free article] [PubMed] [Google Scholar]