Abstract
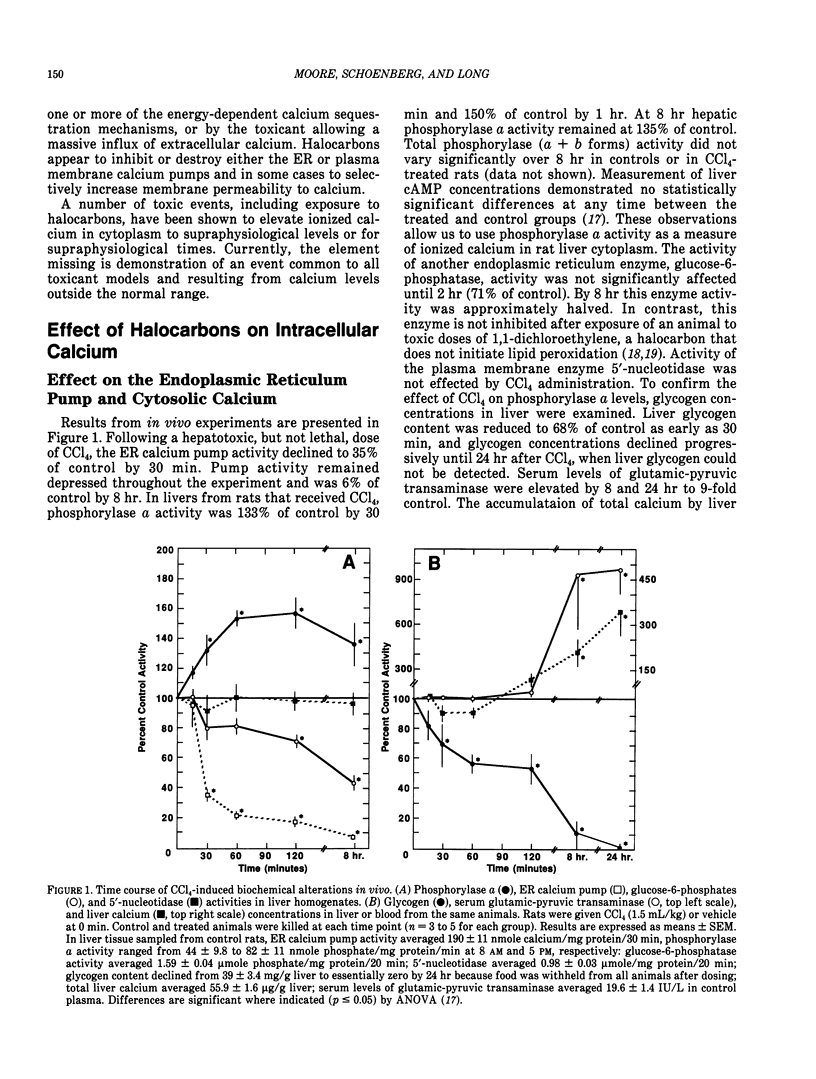
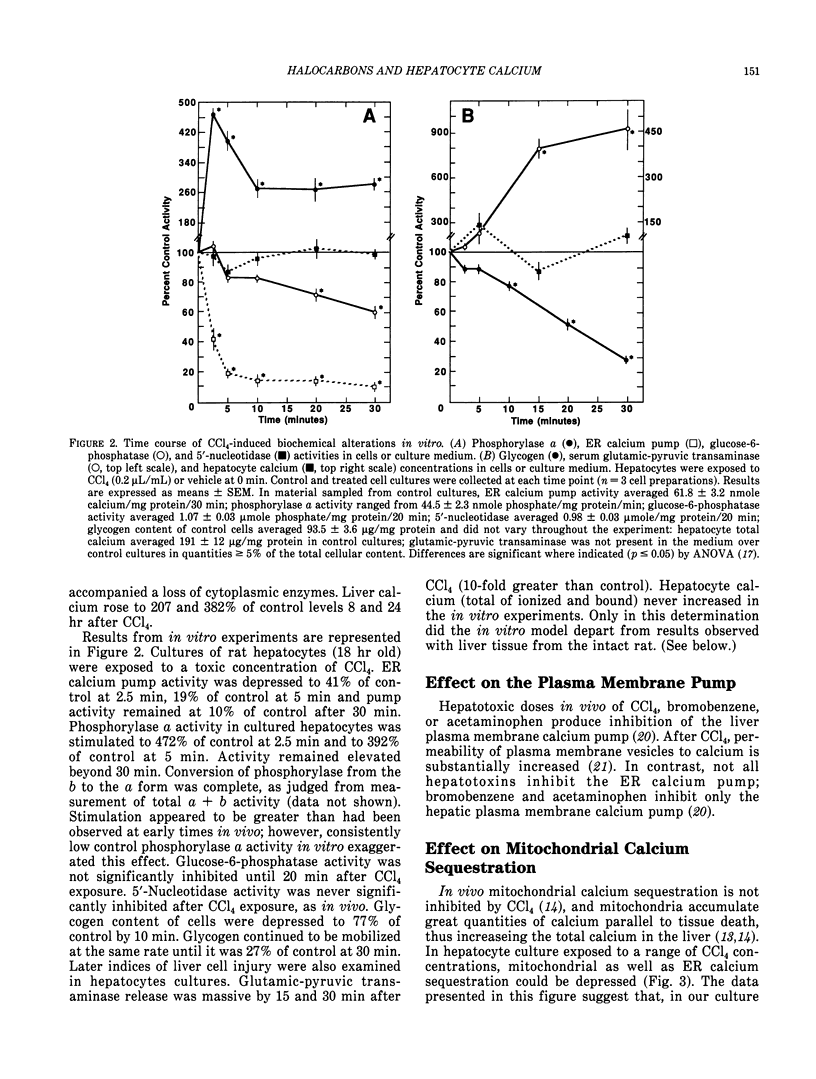
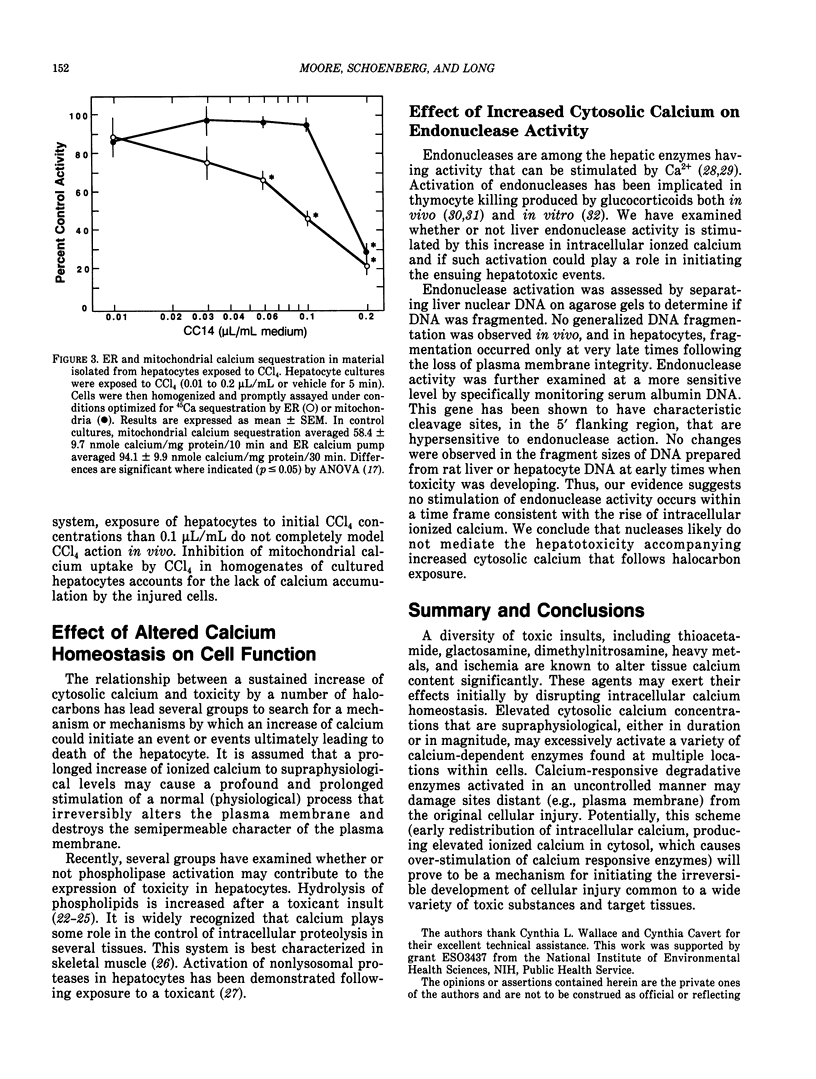
Halocarbons (CCl4, 1,1-dichlorethylene) cause a wide spectrum of effects and injury in hepatocytes. One early effect of these compounds is the inhibition and destruction of the endoplasmic reticulum (ER) calcium pump. Subsequent to inhibition of this pump, the ER calcium pool is depleted and cytosolic levels of calcium are increased for a prolonged period of time. This effect of halocarbons has been characterized and is similar in vivo and in vitro. The importance of this redistribution of cell calcium in expression of halocarbon injury of hepatocytes has not been fully resolved. Several degradative enzymes (phospholipases, proteases) have been implicated as calcium-dependent mediators in toxicity. Our preliminary studies of the effect of calcium redistribution suggest that activation of a calcium-sensitive endonuclease in liver does not play a central role in initiating the lethal effect of halocarbons on hepatocytes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellomo G., Thor H., Orrenius S. Increase in cytosolic Ca2+ concentration during t-butyl hydroperoxide metabolism by isolated hepatocytes involves NADPH oxidation and mobilization of intracellular Ca2+ stores. FEBS Lett. 1984 Mar 12;168(1):38–42. doi: 10.1016/0014-5793(84)80202-1. [DOI] [PubMed] [Google Scholar]
- Burgoyne L. A., Mobbs J. The reaction of the Ca-Mg endonuclease with the A-sites of rat nucleoprotein. Nucleic Acids Res. 1975 Sep;2(9):1551–1558. doi: 10.1093/nar/2.9.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casini A. F., Farber J. L. Dependence of the carbon-tetrachloride--induced death of cultured hepatocytes on the extracellular calcium concentration. Am J Pathol. 1981 Nov;105(2):138–148. [PMC free article] [PubMed] [Google Scholar]
- Chenery R., George M., Krishna G. The effect of ionophore A23187 and calcium on carbon tetrachloride-induced toxicity in cultured rat hepatocytes. Toxicol Appl Pharmacol. 1981 Sep 15;60(2):241–252. doi: 10.1016/0041-008x(91)90228-7. [DOI] [PubMed] [Google Scholar]
- Chien K. R., Abrams J., Serroni A., Martin J. T., Farber J. L. Accelerated phospholipid degradation and associated membrane dysfunction in irreversible, ischemic liver cell injury. J Biol Chem. 1978 Jul 10;253(13):4809–4817. [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Compton M. M., Cidlowski J. A. Rapid in vivo effects of glucocorticoids on the integrity of rat lymphocyte genomic deoxyribonucleic acid. Endocrinology. 1986 Jan;118(1):38–45. doi: 10.1210/endo-118-1-38. [DOI] [PubMed] [Google Scholar]
- Farber J. L., Young E. E. Accelerated phospholipid degradation in anoxic rat hepatocytes. Arch Biochem Biophys. 1981 Oct 1;211(1):312–320. doi: 10.1016/0003-9861(81)90459-8. [DOI] [PubMed] [Google Scholar]
- Fariss M. W., Pascoe G. A., Reed D. J. Vitamin E reversal of the effect of extracellular calcium on chemically induced toxicity in hepatocytes. Science. 1985 Feb 15;227(4688):751–754. doi: 10.1126/science.3918345. [DOI] [PubMed] [Google Scholar]
- Glende E. A., Jr, Pushpendran C. K. Activation of phospholipase A2 by carbon tetrachloride in isolated rat hepatocytes. Biochem Pharmacol. 1986 Oct 1;35(19):3301–3307. doi: 10.1016/0006-2952(86)90427-2. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. The calcium dependent endonuclease activity of isolated nuclear preparations. Relationships between its occurrence and the occurrence of other classes of enzymes found in nuclear preparations. Biochem Biophys Res Commun. 1973 May 15;52(2):475–481. doi: 10.1016/0006-291x(73)90736-5. [DOI] [PubMed] [Google Scholar]
- Ishiura S. Calcium-dependent proteolysis in living cells. Life Sci. 1981 Sep 14;29(11):1079–1087. doi: 10.1016/0024-3205(81)90194-6. [DOI] [PubMed] [Google Scholar]
- Jaeger R. J., Trabulus M. J., Murphy S. D. Biochemical effects of 1,1-dichloroethylene in rats: dissociation of its hepatotoxicity from a lipoperoxidative mechanism. Toxicol Appl Pharmacol. 1973 Mar;24(3):457–467. doi: 10.1016/0041-008x(73)90052-5. [DOI] [PubMed] [Google Scholar]
- Long R. M., Moore L. Biochemical evaluation of rat hepatocyte primary cultures as a model for carbon tetrachloride hepatotoxicity: comparative studies in vivo and in vitro. Toxicol Appl Pharmacol. 1988 Feb;92(2):295–306. doi: 10.1016/0041-008x(88)90389-4. [DOI] [PubMed] [Google Scholar]
- Long R. M., Moore L. Elevated cytosolic calcium in rat hepatocytes exposed to carbon tetrachloride. J Pharmacol Exp Ther. 1986 Jul;238(1):186–191. [PubMed] [Google Scholar]
- Long R. M., Moore L. Inhibition of liver endoplasmic reticulum calcium pump by CCl4 and release of a sequestered calcium pool. Biochem Pharmacol. 1986 Dec 1;35(23):4131–4137. doi: 10.1016/0006-2952(86)90687-8. [DOI] [PubMed] [Google Scholar]
- Moore L., Chen T., Knapp H. R., Jr, Landon E. J. Energy-dependent calcium sequestration activity in rat liver microsomes. J Biol Chem. 1975 Jun 25;250(12):4562–4568. [PubMed] [Google Scholar]
- Moore L. Inhibition of liver-microsome calcium pump by in vivo administration of CCl4, CHCl3 and 1,1-dichloroethylene (vinylidene chloride). Biochem Pharmacol. 1980 Sep 15;29(18):2505–2511. doi: 10.1016/0006-2952(80)90356-1. [DOI] [PubMed] [Google Scholar]
- Moore L., Pastan I. Energy-dependent calcium uptake activity in cultured mouse fibroblast microsomes. Regulation of the uptake system by cell density. J Biol Chem. 1977 Sep 25;252(18):6304–6309. [PubMed] [Google Scholar]
- Moore L., Rodman Davenport G., Landon E. J. Calcium uptake of a rat liver microsomal subcellular fraction in response to in vivo administration of carbon tetrachloride. J Biol Chem. 1976 Feb 25;251(4):1197–1201. [PubMed] [Google Scholar]
- Nicotera P., Hartzell P., Baldi C., Svensson S. A., Bellomo G., Orrenius S. Cystamine induces toxicity in hepatocytes through the elevation of cytosolic Ca2+ and the stimulation of a nonlysosomal proteolytic system. J Biol Chem. 1986 Nov 5;261(31):14628–14635. [PubMed] [Google Scholar]
- Pencil S. D., Brattin W. J., Jr, Glende E. A., Jr, Recknagel R. O. Evidence against involvement of calcium in carbon tetrachloride-dependent inhibition of lipid secretion by isolated hepatocytes. Biochem Pharmacol. 1984 Aug 1;33(15):2425–2429. doi: 10.1016/0006-2952(84)90714-7. [DOI] [PubMed] [Google Scholar]
- Recknagel R. O. A new direction in the study of carbon tetrachloride hepatotoxicity. Life Sci. 1983 Aug 1;33(5):401–408. doi: 10.1016/0024-3205(83)90787-7. [DOI] [PubMed] [Google Scholar]
- Reynolds E. S., Ree H. J., Moslen M. T. Liver parenchymal cell injury. IX. Phenobarbital potentiation of endoplasmic reticulum denaturation following carbon tetrachloride. Lab Invest. 1972 Mar;26(3):290–299. [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Smith M. T., Thor H., Orrenius S. Toxic injury to isolated hepatocytes is not dependent on extracellular calcium. Science. 1981 Sep 11;213(4513):1257–1259. doi: 10.1126/science.7268433. [DOI] [PubMed] [Google Scholar]
- Tsokos-Kuhn J. O., Smith C. V., Mitchell J. R., Tate C. A., Entman M. L. Evidence for increased membrane permeability of plasmalemmal vesicles from livers of phenobarbital-induced CCl4-intoxicated rats. Mol Pharmacol. 1986 Nov;30(5):444–451. [PubMed] [Google Scholar]
- Tsokos-Kuhn J. O., Todd E. L., McMillin-Wood J. B., Mitchell J. R. ATP-dependent calcium uptake by rat liver plasma membrane vesicles. Effect of alkylating hepatotoxins in vivo. Mol Pharmacol. 1985 Jul;28(1):56–61. [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]


