Abstract
Classical ideas for early eukaryotic evolution often posited a period of anaerobic evolution producing a nucleated phagocytic cell to engulf the mitochondrial endosymbiont, whose presence allowed the host to colonize emerging aerobic environments. This idea was given credence by the existence of contemporary anaerobic eukaryotes that were thought to primitively lack mitochondria, thus providing examples of the type of host cell needed. However, the groups key to this hypothesis have now been shown to contain previously overlooked mitochondrial homologues called hydrogenosomes or mitosomes; organelles that share common ancestry with mitochondria but which do not carry out aerobic respiration. Mapping these data on the unfolding eukaryotic tree reveals that secondary adaptation to anaerobic habitats is a reoccurring theme among eukaryotes. The apparent ubiquity of mitochondrial homologues bears testament to the importance of the mitochondrial endosymbiosis, perhaps as a founding event, in eukaryotic evolution. Comparative study of different mitochondrial homologues is needed to determine their fundamental importance for contemporary eukaryotic cells.
Keywords: mitochondria, hydrogenosomes, mitosomes, anaerobic eukaryote evolution
1. Introduction
Many classical theories for eukaryotic origins suggested that eukaryotes originated under anaerobic conditions, and were primitively without mitochondria (e.g. Goksoyr 1967; Cavalier-Smith 1983b). A member of this anaerobic eukaryotic community is posited to have subsequently phagocytosed a eubacterium that could carry out aerobic respiration and which subsequently became an endosymbiont (John & Whatley 1975). The presence of this eubacterial endosymbiont allowed the host to colonize aerobic environments. A variety of mechanisms have been suggested as to why this should be so; the most popular, and one that features in a variety of different formulations for mitochondrial origins (Woese 1977; Martin & Müller 1998; Vellai et al. 1998; Kurland & Andersson 2000), being the removal of cytosolic oxygen by the endosymbionts' respiratory activity. The feasibility of this idea, at least as a mechanism, is supported by a modern analogue among contemporary ciliate protozoa. Strombidium purpureum lives in anaerobic marine sands and contains facultatively aerobic photosynthetic endosymbiotic bacteria. In the light, Strombidium avoids even traces of oxygen (less than 1% atmospheric saturation) and the endosymbionts probably use host substrates for anoxygenic photosynthesis. In the dark, the ciliates, protected against O2-toxicity by the endosymbionts' aerobic respiration, accumulate in water with a partial pressure of O2 of 1–4% atmospheric saturation (Fenchel & Bernard 1993a,b; Bernard & Fenchel 1994).
Despite the plausibility of the above scenario we will probably never know why two disparate ancient cells came, and then stayed, together. Consequently, opinions are diverse concerning the ecological context and selective advantages for host and endosymbiont (e.g. John & Whatley 1975; Martin & Müller 1998; Cavalier-Smith 2002; Embley & Martin 2006). What does seem well founded is that the resulting host–endosymbiont consortium forged the common ancestor of all of the mitochondrion-containing eukaryotes that are seen today. Thus, a single common origin for all mitochondria is supported by phylogenetic analyses of the genes encoded by mitochondrial genomes (Yang et al. 1985; Gray et al. 2004). The anaerobic eukaryotes that did not participate in the mitochondrial endosymbiosis, have been posited either to have gone extinct, due to an inability to deal with rising oxygen levels or some other catastrophe (Philippe & Adoutte 1998; Vellai et al. 1998), or to have persisted in anaerobic habitats to the present day (Cavalier-Smith 1983b; Margulis et al. 2005).
The persistence hypothesis is plausible for several reasons. Anaerobic habitats have existed throughout Earth's history and they harbour anaerobic bacteria, providing food for phagotrophic eukaryotes. Extinction in the microbial world may also be rare because of large population sizes, ease of dispersal and the occurrence of resistant stages (Fenchel 1993). Even transient anaerobic habitats generally possess protist inhabitants. As ancient oxygen levels rose, the occupants of anaerobic habitats on the margins of the aerobic world would actually benefit from the reduced carbon derived from oxygenic photosynthesis. In modern situations, the activities of oxygenic phototrophs often provide the reducing power, in the form of organic material, to maintain anoxic environments; contemporary anaerobic life is most rich in the vicinity of aerobic life (Fenchel & Finlay 1995). In conclusion, there are no compelling reasons drawn from contemporary ecosystems, to suppose that primitively amitochondriate eukaryotes, if they ever existed, would have gone extinct simply because they did not participate in the mitochondrial endosymbiosis.
The persistence hypothesis was supported by the discovery of contemporary eukaryotes that apparently lacked mitochondria. These eukaryotes, called Archezoa to indicate that the absence of mitochondria was a primitive state, not a derived state resulting from secondary loss, included Entamoeba, Giardia, Trichomonas and Microsporidia, and their various close relatives (Cavalier-Smith 1983a,b). As well as not having mitochondria, these species were thought to lack other features common to most eukaryotes, including Golgi dictyosomes and peroxisomes, supporting their early emerging status (Cavalier-Smith 1987a; Patterson & Sogin 1992). Archezoa or Hypochondria as they were also known (Patterson & Sogin 1992), thus became models for studying early eukaryotic evolution and the features of the first eukaryotic cells. Some suggested that the peculiar features of Archezoa could also be a derived state (Wolters 1991; Siddall et al. 1992), caused, or perhaps facilitated, by the adaptation of Archezoa to their parasitic or anaerobic lifestyles. But that debate was unresolved because there was no clear comparative evidence that convincingly linked the Archezoa to eukaryotic groups that possessed mitochondria or the other debated features.
The Archezoa hypothesis was founded in comparative cytology as a taxonomic hypothesis designed to draw attention to a group of eukaryotes which might provide a glimpse into a pre-mitochondrial phase of eukaryotic evolution (Cavalier-Smith 1983a,b). It was thus enormously useful as a guide to study. It gained considerable support when the first phylogenetic trees based upon molecular sequence data (Vossbrinck et al. 1987; Sogin et al. 1989; Leipe et al. 1993; Hashimoto et al. 1997) did indeed place key archezoans before other eukaryotes with mitochondria. Doubts were expressed about the veracity of these trees (e.g. Wolters 1991; Siddall et al. 1992), but the apparent agreement between molecules and morphology in establishing the timing of the mitochondrial endosymbiosis, one of the pivotal events in eukaryotic evolution, was compelling and generally seen as a major breakthrough.
2. The demise of Archezoa
In an area of research where speculation and conjecture abound, the Archezoa hypothesis for early eukaryotic evolution stood out as testable in obvious ways. It could be rejected for individual archezoans if they were shown to contain genes of mitochondrial ancestry, to branch among eukaryotes with mitochondria, i.e. the trees placing them deep were wrong, or to contain previously overlooked mitochondria. Using these criteria the Archezoa hypothesis has now been rejected for all of the best-studied archezoans. The data and arguments underpinning the conclusion that the Archezoa hypothesis can now be rejected, for the key species for which it was formulated, have been extensively reviewed (Embley et al. 2003b; van der Giezen et al. 2005; Embley & Martin 2006), so only a few points will be made here in relation to each type of evidence and to controversies, where they exist, surrounding the interpretation of these data.
All of the best-studied archezoans have now been shown to contain host–nuclear-encoded genes of putative mitochondrial ancestry (table 1). That is, they contain genes that encode proteins that in aerobes typically function in mitochondria and, when the proteins are subjected to phylogenetic analysis, they form a monophyletic group with the alpha-proteobacteria. This is the group of bacteria from which the mitochondrial endosymbiont is held to have originated based upon analyses of genes that are encoded by mitochondrial genomes (Yang et al. 1985; Gray et al. 2001). One criticism of using the genes in table 1 to reject the Archezoa hypothesis is that none of them have ever been found on a mitochondrial genome (Gray et al. 2004; Gray 2005). Such a location, which is found for some of the proteins involved in oxidative phosphorylation, the canonical function of aerobic mitochondria, would make a direct link between gene and organelle. To explain the localization of genes of mitochondrial ancestry on host nuclear genomes, it is necessary to posit that they were transferred from the endosymbiont genome to the host nuclear genome during the process of transforming endosymbiont into an organelle (John & Whatley 1975; Viale & Arakaki 1994). This process has been called endosymbiotic gene transfer and it is still ongoing (Timmis et al. 2004). For example, among plants there are examples where particular genes appear on the mitochondrial genome in some lineages, but in other lineages they are encoded by the host nuclear genome (Adams & Palmer 2003). Despite the need to invoke such gene transfer, the hypothesis that the genes in table 1 came from the mitochondrial endosymbiont is probably now the standard interpretation of these data (Boorstein et al. 1994; Viale & Arakaki 1994; Clark & Roger 1995).
Table 1.
Proteins of mitochondrial ancestry in former Archezoa.
| species | protein | references for tree | location in cell | reference for location |
|---|---|---|---|---|
| Entamoeba histolytica | Mitochondrial 60-kDa chaperonin (Cpn60) | Clark & Roger 1995 | Mitosome | Tovar et al. 1999 |
| Mitochondrial 70-kDa heat shock protein (Hsp70) | Bakatselou et al. 2000 | Not determined (ND) | ||
| Giardia intestinalis | Cpn60 | Roger et al. 1998 | Mitosome | Regoes et al. 2005 |
| Horner & Embley 2001 | ||||
| Hsp70 | Morrison et al. 2001 | Mitosome | Regoes et al. 2005 | |
| Cysteine desulphurase (IscS) | Tachezy et al. 2001 | Mitosome | Tovar et al. 2003 | |
| Emelyanov 2003 | ||||
| Trichomonas vaginalis | Cpn60 | Horner et al. 1996 | Hydrogenosome | Bui et al. 1996 |
| Bui et al. 1996 | Bozner 1997 | |||
| Roger et al. 1996 | ||||
| Hsp70 | Bui et al. 1996 | Hydrogenosome | Bui et al. 1996 | |
| Germot et al. 1996 | Bozner 1997 | |||
| IscS | Tachezy et al. 2001 | Hydrogenosome | Sutak et al. 2004 | |
| Emelyanov 2003 | ||||
| Mitochondrial NADH-dehydrogenase | Hrdy et al. 2004 | Hydrogenosome | Hrdy et al. 2004 | |
| Dyall et al. 2004a,b | Dyall et al. 2004a,b | |||
| Microsporidia: | ||||
| Nosema locustae | Hsp70 | Germot et al. 1997 | ND | |
| Pyruvate dehydrogenase | Fast & Keeling 2001 | ND | ||
| Vairimorpha necatrix | Hsp70 | Hirt et al. 1997 | ND | |
| Encephatitozoon cuniculi | Hsp70 | Peyretaillade et al. 1998 | ND | |
| IscS | Emelyanov 2003 | ND | ||
| Encephalitozoon hellem | Hsp 70 | Peyretaillade et al. 1998 | ND | |
| Trachipleistophora hominis | Hsp70 | Williams et al. 2002 | Mitosome | Williams et al. 2002 |
It has also been suggested that archezoans could have acquired the genes in table 1 from a source other than the mitochondrial endosymbiont. Alternative sources that have been posited include alpha-proteobacterial food bacteria (Doolittle 1998) and bacteria related to, but distinct from, the mitochondrial endosymbiont (Sogin 1997). Although these ideas have been presented as credible alternatives to the standard interpretation discussed above (Dyall & Johnson 2000; Kurland & Andersson 2000; Knight 2004; Dyall et al. 2004b; Margulis et al. 2005), there is no unambiguous support from phylogenetic analyses for the separate origin of these Archezoan genes (Horner & Embley 2001; Williams et al. 2002; Hrdy et al. 2004).
The strongest evidence that archezoans are not primitively without mitochondria has come from discoveries that some of the bacterial proteins in table 1 are housed in double-membraned organelles. These organelles are called hydrogenosomes (Müller 1993; Bui et al. 1996), remnant mitochondria (Williams et al. 2002) or mitosomes (Tovar et al. 1999, 2003), with the latter two terms sometimes used interchangeably for the same organelle. There are only two double-membraned organelles, mitochondria and primary plastids, of undisputed bacterial origin in eukaryotes. Thus the simplest, and hence preferable, hypothesis to explain the observations that proteins typically found in mitochondria are present in double-membrane-bounded Archezoan organelles is that all such organelles share common ancestry with mitochondria (Bui et al. 1996; Tovar et al. 1999, 2003; Williams et al. 2002). Mitochondria, mitosomes and hydrogenosomes are evolutionary homologues sensu Owen and Darwin (Owen 1843; Darwin 1859), and the differences between them are the products of descent with modification from a common ancestral organelle. The hypothesis that mitochondria, mitosomes and hydrogenosomes are homologues, predicts that, as the organelles are studied more deeply, additional similarities between them will emerge to corroborate the hypothesis. So far this is what has happened.
With one notable exception (Boxma et al. 2005), hydrogenosomes and mitosomes lack a genome, presumably because they no longer require the proteins it encodes for oxidative phosphorylation. Any proteins functioning within hydrogenosomes and mitosomes must therefore be synthesized in the cytosol, and then correctly targeted and imported into the organelle. Although the mitochondrion contains a genome, most of its proteins are encoded by the host nuclear genome, so there is a similar requirement for a protein import machinery. Mitochondria have evolved two main protein import pathways (Pfanner & Geissler 2001). Proteins that are destined for the inner mitochondrial membrane are guided to their destination by internal targeting signals. Others, destined for the mitochondrial matrix, are synthesized as pre-proteins carrying a targeting sequence at their amino-terminus that is cleaved during import into the organelle. The development of a system for protein import must have been an early and critical step in the evolution of the mitochondrial organelle (Cavalier-Smith 1987b). The resulting mitochondrial protein import machinery is sufficiently complicated, and fidelity of import sufficiently important, that the same pathways are very unlikely to have evolved in different organelles.
The hydrogenosomes of Trichomonas vaginalis import proteins using both mitochondrial pathways. Hydrogenosomal ferredoxin carries an N-terminal targeting sequence that guides it into isolated Trichomonas hydrogenosomes, and the same targeting sequence can also sort a marker protein into yeast mitochondria (Plumper et al. 1998). Trichomonas hydrogenosomes also use a member of the mitochondrial carrier family (MCF) to transport ADP and ATP (Tjaden et al. 2004). Members of the MCF are eukaryotic proteins that are inserted into the inner mitochondrial membrane by internal targeting signals, where they mediate the import and export of substrates, including ADP and ATP, required or produced by the mitochondrion (Kunji 2004). The insertion of an ADP/ATP carrier into the protomitochondrion membrane was a key step in its transition into an organelle, because it made symbiont-generated ATP available to the host cell (John & Whatley 1975). The Trichomonas ADP and ATP carrier is correctly targeted to yeast mitochondria in heterologous transfection experiments, suggesting that targeting signals for import have been conserved between the Trichomonas hydrogenosome and yeast mitochondria (Dyall et al. 2000).
Trichomonas hydrogenosomes have other features in common with mitochondria. They catalyse the enzymatic assembly and insertion of Fe–S centres into apoproteins, using the same enzymes, inherited from the mitochondrial endosymbiont, as do yeast mitochondria (Sutak et al. 2004). Trichomonas hydrogenosomes also contain the NADH dehydrogenase module of complex I of the mitochondrial respiratory chain (Hrdy et al. 2004), although the reader should be aware that this last conclusion is controversial (see Gray 2005), because an alternative origin from another bacterium had previously been suggested for this module (Dyall et al. 2004b). While these data are clearly difficult to analyse using conventional phylogenetic methods, it is important to appreciate that they cannot reject a common origin of Trichomonas and mitochondrial proteins using standard tests (Hrdy et al. 2004). Moreover, when corrections are made to mitigate obvious problems with the data, such as amino acid differences between sequences, the trees do support a mitochondrial origin (Hrdy et al. 2004). The discovery of the NADH dehydrogenase activity solves the long-standing puzzle of how the Trichomonas hydrogenosome regenerates NAD+ after malate oxidation (Müller 2003).
Entamoeba mitosomes (Tovar et al. 1999) also contain a mitochondrial carrier protein that transports ADP and ATP, and in heterologous transfection experiments it too is translocated to the inner mitochondrial membrane of yeast (Chan et al. 2005). Unlike typical mitochondrial ATP/ADP carriers (Klingenberg 1985), the Entamoeba carrier does not require a positive-outside membrane potential, to transport ADP and ATP (Chan et al. 2005). This can be rationalized by reference to biochemical data (Reeves 1984) and to the recently published Entamoeba genome (Loftus et al. 2005), which show that Entamoeba lacks the electron transport chain necessary to make this gradient.
Import pathways are also conserved between Giardia mitosomes and mitochondria (Dolezal et al. 2005; Regoes et al. 2005). For example, the N-terminal presequence from the Giardia mitosomal ferredoxin is both necessary and sufficient to guide green fluorescent protein into mammalian mitochondria (Regoes et al. 2005). Study of the microsporidian mitosome has lagged behind study of other mitochondrial homologues, because Microsporidia are obligate intracellular parasites, and there are no homologous transfection methods like those established for Entamoeba, Giardia and Trichomonas. Nevertheless the published genome of the microsporidian Encephalitozoon cuniculi (Katinka et al. 2001) already provides clues to putative shared functions, e.g. homologous mechanisms of Fe–S cluster assembly, between its mitosome and mitochondria (Vivares et al. 2002).
Phylogenetic analyses previously taken to support the deep branching positions of archezoans have also come under increasing scrutiny with the result that they are no longer viewed as reliable (Stiller & Hall 1999; Hirt et al. 1999; Philippe et al. 2000; Inagaki et al. 2004; Thomarat et al. 2004; Roger & Hug 2006). The molecular sequences of the former Archezoa often evolve differently to those of other eukaryotes to which they are being compared. However, most methods of phylogenetic analysis assume a homogeneous process, i.e. that all sequences evolve in the same way, so the aberrant behaviour of Archezoan sequences can make their phylogenetic position difficult to infer reliably (see Roger & Hug 2006). The difficulty experienced when trying to resolve the origins of the Trichomonas hydrogenosomal NADH dehydrogenase provides just one example of the kind of problems that can be encountered (Dyall et al. 2004b; Hrdy et al. 2004; Gray 2005). The case for a rethink is clear-cut for Microsporidia because most data and analyses now agree in placing them with fungi rather than as deep branching eukaryotes (Hirt et al. 1999; Keeling et al. 2000; Inagaki et al. 2004; Thomarat et al. 2004). The hypothesis that Entamoeba is related to Dictyostelium, an aerobic amoeba that contains mitochondria, is also now well supported by a number of proteins (Horner & Embley 2001; Bapteste et al. 2002). The position of Giardia and Trichomonas is uncertain, but there are sufficient rooted trees that argue otherwise, to be wary of making strong claims that they branch before other eukaryotes (Horner & Embley 2001; Emelyanov 2003; Arisue et al. 2005).
Freed from the prejudice that Archezoa must be ‘early branching’ or in some sense ‘primitive’, their mitochondrial homologues fit perfectly comfortably within the spectrum of ‘non-Archezoan’ eukaryote biology.
3. Remnant mitochondria in Cryptosporidium
Cryptosporidium parvum is an apicomplexan parasite related to the malaria parasite Plasmodium, and together they are part of a broader predominantly aerobic group comprising apicomplexans, ciliates and dinoflagellates, called the alveolates (Cavalier-Smith 2002; Adl et al. 2005). Cryptosporidium parvum contains a double-membraned organelle that has been called a relict (Riordan et al. 2003) or degenerate mitochondrion (Abrahamsen et al. 2004), but from its known features it could just as easily be called a mitosome. The Cryptosporidium mitochondrion has lost its genome and the capacity for oxidative phosphorylation (Abrahamsen et al. 2004), but it still imports mitochondrial heat shock proteins (Riordan et al. 2003; Slapeta & Keithly 2004). There is also circumstantial evidence that it makes Fe–S clusters, but in situ localization of the enzymes has not been done (LaGier et al. 2003).
4. Hydrogenosomes: mitochondria that make hydrogen
Hydrogenosomes were originally discovered (Lindmark & Müller 1973) in the cattle parasite Tritrichomonas foetus, a close relative of T. vaginalis, but they are also found in anaerobic chytrid fungi and diverse lineages of ciliate protozoa (Müller 1993). The evidence for common ancestry of chytrid and ciliate hydrogenosomes with mitochondria is now very strong (van der Giezen et al. 2002, 2003; Voncken et al. 2002a; Boxma et al. 2005). Among ciliates, mitochondria- and hydrogenosome-containing groups freely intermingle; there are at least four and probably more ciliate lineages that possess hydrogenosomes within this predominantly aerobic group (Fenchel & Finlay 1995; Embley et al. 1995). The transformation of mitochondrion to hydrogenosome can occur over relatively short genetic distances as illustrated by the genus Cyclidium, which contains Cyclidium glaucoma with mitochondria and Cyclidium porcatum with hydrogenosomes (Esteban et al. 1993; Embley et al. 1995). Given the ease by which ciliates have evolved hydrogenosomes it was perhaps inevitable (Embley et al. 1997) that the strongest evidence for the common ancestry of hydrogenosomes and mitochondria would eventually come from this group. The hydrogenosomes of the anaerobic ciliate Nyctotherus ovalis uniquely retain a mitochondrial genome (Boxma et al. 2005), providing the long-sought direct link between the two organelles (Martin 2005).
Like those of Trichomonas (Clemens & Johnson 2000), the hydrogenosomes of the anaerobic fungus Neocallimastix apparently lack a genome (van der Giezen et al. 1997). Neocallimastix also contains a member of the MCF on its genome (van der Giezen et al. 2002; Voncken et al. 2002a). This protein has been shown to import and export ADP and ATP in vitro and, crucially, it can restore the function of mutant yeast mitochondria that lack their own ADP/ATP carrier (van der Giezen et al. 2002). Thus, fungal hydrogenosomes and yeast mitochondria use the same pathway for ADP/ATP exchange and they import the relevant protein in the same way. Neocallimastix hydrogenosomes also contains mitochondrial Cpn60 and Hsp70 and these proteins contain targeting signals that are capable of sorting them, or a green fluorescent reporter protein, into mammalian mitochondria (van der Giezen et al. 2003).
Anaerobic eukaryotic diversity is poorly sampled for organelle function so it is unlikely that these groups will be the final additions to the list of eukaryotes shown to possess hydrogenosomes (Fenchel & Finlay 1995). Transforming mitochondria into hydrogenosomes appears to be something that diverse eukaryotes have accomplished, raising the question of how it has been done.
5. Evolutionary origins of the enzymes used to make hydrogen
Hydrogenosomes are defined by their ability to make hydrogen so it is the source of the biochemistry to do this, i.e. key to the transformation of mitochondria into hydrogenosomes (Embley et al. 1997). In Trichomonas hydrogenosomes, which are the best studied for their biochemistry, pyruvate:ferredoxin oxidoreductase (PFO) carries out the metabolism of pyruvate, and the electrons generated are transferred via ferredoxin to [Fe]-hydrogenase, producing hydrogen as the reduced end product (Müller 1993; Hrdy & Müller 1995a,b). There are biochemical data consistent with the presence of both enzymes in the hydrogenosomes of anaerobic ciliates and fungi (Yarlett et al. 1981, 1984, 1986). By contrast, aerobic eukaryotes use the non-homologous pyruvate dehydrogenase, located in the mitochondrion, to decarboxylate pyruvate and the electrons flow via the electron transport chain to oxygen.
The presence in hydrogenosomes of PFO and [Fe]-hydrogenase, enzymes typical of anaerobic bacteria, prompted the early suggestion, recently restated in modified form, that Trichomonas hydrogenosomes were descended from an endosymbiotic anaerobic bacterium (Whatley et al. 1979; Dyall et al. 2004a). However, neither PFO nor [Fe]-hydrogenase is uniquely associated with hydrogenosomes, and thus they are not reliable indicators of a separate organelle origin. The mitochondria of Euglena contain pyruvate:NADP oxidoreductase, a fusion protein containing PFO domains linked to a C-terminal NADPH-cytochrome P450 reductase domain. The same fusion is found in C. parvum, but its localization is unknown (Rotte et al. 2001). Large fragments of PFO have also been found in Saccharomyces cerevisiae where they combine with other redox proteins to participate in methionine biosynthesis (Horner et al. 1999). PFO also occurs in Entamoeba, Giardia and Spironucleus (a close relative of Giardia), but the location of the protein in these species has not been unambiguously demonstrated (Reeves et al. 1977; Townson et al. 1996; Brown et al. 1998; Rodriguez et al. 1998).
Hydrogenase genes have now been cloned from T. vaginalis (Bui & Johnson 1996; Horner et al. 2000), the ciliates N. ovalis (Akhmanova et al. 1998) and Trimyema sp. (Embley et al. 2003a), and the chytrid fungi Neocallimastix frontalis L2 and Piromyces sp. E2 (Davidson et al. 2002; Voncken et al. 2002b). All of them encode iron-only [Fe]-hydrogenases of a type that is found in eubacteria, but not in archaebacteria. Green algae, such as Chlorella fusca, Chlamydomonas reinhardtii and Scenedesmus obliquus also produce hydrogen, using [Fe]-hydrogenases located in their chloroplasts (Horner et al. 2002). The presence of a [Fe]-hydrogenase in green algal plastids is surprising, since cyanobacteria, the endosymbiotic progenitor of plastids, use the non-homologous [NiFe]-hydrogenase to make hydrogen. The original [NiFe]-hydrogenase brought by the endosymbiont appears to have been replaced by a host-nuclear encoded [Fe]-hydrogenase of non-cyanobacterial origin.
Genes encoding [Fe]-hydrogenases similar in structure to Trichomonas enzymes, were also recently discovered in Giardia, in its relative Spironucleus, and in Entamoeba histolytica (Horner et al. 2000; Nixon et al. 2003). The intracellular location of these enzymes is unknown at present. Giardia can actually make small amounts of hydrogen, the elimination of which may help to maintain redox balance in this species (Lloyd et al. 2002). Given that the structures of the Entamoeba and Spironucleus genes closely resemble the one in Giardia, it seems likely that these species can also make hydrogen.
Most surprisingly, genes encoding short proteins related to [Fe]-hydrogenases also occur in the human genome (Barton & Worman 1999; Horner et al. 2002). These genes have been called NARF-like, after human nuclear prelamin A recognition factor (Barton & Worman 1999). In humans, the Narf protein is thought to be involved in the processing of lamin, a protein required for maintaining the structural integrity of the nucleus (Barton & Worman 1999). However, detailed studies on the yeast homologue Nar1p, suggest a rather different role for this protein. In yeast, which lacks lamin, Nar1p appears to be an essential protein located in the cytosol where it functions in the synthesis of extramitochondrial Fe–S proteins (Balk et al. 2004, 2005). Database searches have revealed that NARF-like genes are present in all of the available eukaryotic genomes (Horner et al. 2002; Balk et al. 2004), including the smallest eukaryote genome yet sequenced, from the intracellular microsporidian parasite E. cuniculi (Katinka et al. 2001). Once thought to be exotic exceptions, it seems entirely possible that [Fe]-hydrogenases, in the form of Narf-like proteins, have an important role to play in all eukaryotes.
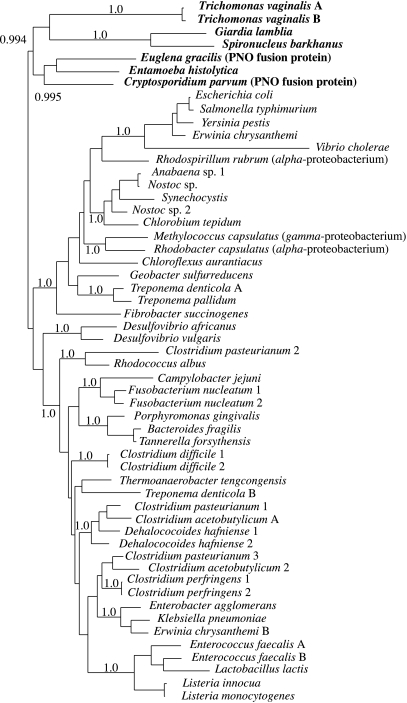
It has been suggested that the genes for PFO and [Fe]-hydrogenase could have originated from the mitochondrial endosymbiont, as both genes are common among proteobacteria (Embley et al. 1997; Martin & Müller 1998). Phylogenetic analyses have not provided any support for this hypothesis. Like many eukaryotic metabolic enzymes (Ribeiro & Golding 1998; Rivera et al. 1998; Esser et al. 2004), eukaryotic PFOs are more similar in structure to eubacterial enzymes than to those from archaebacteria. Moreover, published analyses of PFO (Horner et al. 1999; Rotte et al. 2001; Embley et al. 2003a), recover eukaryotic sequences as a single cluster consistent with an ancient common origin for eukaryotic enzymes (figure 1). However, the eukaryotic PFO sequences are not the nearest neighbours of sequences from alpha-proteobacteria. Interestingly, the two alpha-proteobacterial sequences do not cluster together in the tree, suggesting that the evolution of eubacterial PFO genes is more complex than can be explained by vertical inheritance of a single copy gene (Horner et al. 1999).
Figure 1.
Phylogenetic tree showing the evolutionary relationships between pyruvate:ferredoxin oxidoreductase (PFO) sequences from eukaryotes and eubacteria. Eukaryotes are shown in bold. The tree is a consensus tree from a Bayesian analysis of aligned protein sequences using custom software (available from p.foster@nhm.ac.uk). Aligned positions (715 sites) were recoded into the six Dayhoff groups: C, STPAG, NDEQ, HRK, MILV and FYW to reduce the effects of mutational saturation (Hrdy et al. 2004). Posterior probabilities for some groups are shown, with a value of 1.0 representing maximum support. The strongly supported groups have also been recovered in previously published analyses using different methods, including maximum-likelihood, and different support measures including bootstrapping (Horner et al. 1999; Rotte et al. 2001).
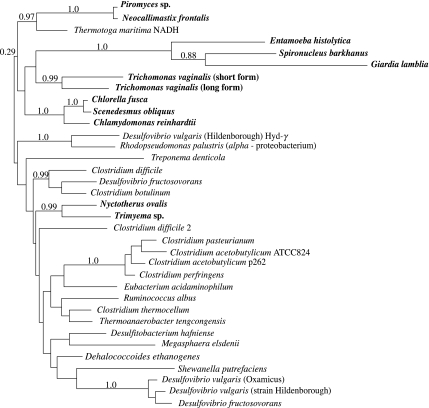
The relationships between eukaryotic [Fe]-hydrogenase sequences are not well resolved, to which saturation of variable sites is probably a contributing factor (Horner et al. 2000). Thus, although they do not cluster together in the tree (figure 2), the data cannot decisively reject the hypothesis of a common and early origin for eukaryotic sequences using standard tests (Horner et al. 2000). There is strong support for the coherence of individual clusters of eukaryotic sequences, consistent with the hypothesis that the respective ancestors of the sampled green algae and chytrid fungi already contained a gene for [Fe]-hydrogenase. The observation (Embley et al. 2003a) that the enzymes from the two ciliates Nyctotherus and Trimyema cluster together is particularly interesting, since these two morphologically distinct species are separated by aerobic mitochondria-containing lineages in the ciliate tree (Embley et al. 1995; Akhmanova et al. 1998). This is consistent with the hypothesis that the common ancestor they shared with aerobic ciliates, already contained a [Fe]-hydrogenase (Embley et al. 2003a), thus helping to explain the apparent ease by which diverse ciliates have made hydrogenosomes (Embley et al. 1995). The tree provides no support for a close relationship between eukaryotic [Fe]-hydrogenases and the only sequence from an alpha-proteobacterium, that of Rhodopseudomonas palustris (Davidson et al. 2002). The Rhodopseudomonas sequence clusters strongly with one of two different [Fe]-hydrogenases from Desulfovibrio vulgaris (strain Hildenborough), a member of the delta-proteobacteria. As for the PFO tree, there is evidence that gene duplications and/or potential horizontal gene transfers have influenced the tree topology for prokaryotic sequences. For example, the [Fe]-hydrogenases from Desulfovibrio species do not cluster together (figure 2), as their common classification suggests they should.
Figure 2.
Phylogenetic tree showing the evolutionary relationships between [Fe]-hydrogenase sequences from eukaryotes and eubacteria. Eukaryotes are shown in bold. Details of the analysis on 196 aligned positions are given in the legend to figure 1. The strongly supported groups have also been recovered in previously published analyses using different methods, including maximum-likelihood, and different support measures including bootstrapping (Horner et al. 2000).
6. Conclusions, speculations and outlook
The idea that primitively amitochondriate protists—Archezoa—were alive and well was an attractive one, not least because it lent credibility to theories for eukaryote origins that required a host of this kind to engulph the mitochondrial endosymbiont. Better trees, the discovery of genes of mitochondrial origin and, latterly, the discovery of mitochondrial homologues have allowed the rejection of the Archezoa hypothesis for the key species for which it was formulated. It thus seems possible that all eukaryotes might contain an organelle of mitochondrial ancestry, emphasizing the pivotal role that the mitochondrial endosymbiosis has played in eukaryotic evolution. Although we still need a reliable root for the eukaryotic tree (see Cavalier-Smith 2006; Roger & Hug 2006), it is evident that anaerobic eukaryotes have repeatedly arisen from within ancestrally aerobic groups with mitochondria. Anaerobic eukaryotes are neither rare nor primitive as once thought.
Most of what is known about mitochondrial function and the importance of mitochondria for the eukaryotic cell is drawn from the study of yeast, mammal and plant mitochondria. Apart from oxidative phosphorylation, other important reactions include the Krebs cycle, haem biosynthesis, beta-oxidation of fatty acids, amino acid biosynthesis and the formation and export of iron–sulphur (Fe/S) clusters (Reichert & Neupert 2004; Lill & Muhlenhoff 2005). It is already evident, from biochemical and genomic data, that hydrogenosomes and mitosomes can have retained only a limited subset of these reactions (Katinka et al. 2001; Müller 2003; Abrahamsen et al. 2004; Loftus et al. 2005). This raises important questions concerning the fundamental importance of this compartment of endosymbiotic ancestry for the eukaryotic cell, its biochemical flexibility and the limits of organelle reduction. For example, MCF protein diversity closely mirrors important facets of mitochondrial metabolic diversity; non-parasitic aerobic eukaryotes typically have between 30 and 60 MCF genes, to transport different substrates required or produced by the mitochondrion (Kunji 2004). By contrast, E. histolytica has lost all but a single member of the MCF which functions in its mitosome to transport ATP and ADP (Chan et al. 2005; Loftus et al. 2005). The microsporidian Encephalitozoon, an obligate intracellular parasite, has gone even further, because its genome lacks any MCF genes (Katinka et al. 2001). This raises the intriguing question of how the Encephalitozoon mitosome acquires ATP.
The maturation of Fe/S clusters, for insertion into the Fe/S proteins that are crucial for all cellular life, is to date the only biosynthetic function for which mitochondria are essential to the yeast cell (Lill et al. 1999). Key proteins in this pathway; cysteine desulphurase (IscS) and scaffolding protein (IscU), appear to have originated from the mitochondrial endosymbiont (Tachezy et al. 2001; Emelyanov 2003; van der Giezen et al. 2004). The maturation and export of Fe/S clusters has been suggested as a possible common function of all mitochondrial homologues (Tachezy et al. 2001; Embley et al. 2003a). Key genes for this process are indeed present on all eukaryotic genomes examined (Katinka et al. 2001; LaGier et al. 2003; Lill & Muhlenhoff 2005) but only the Trichomonas hydrogenosome (Sutak et al. 2004) and Giardia mitosome (Tovar et al. 2003) have actually been demonstrated to contain the pathway. Entamoeba is so far unique among eukaryotes in that it has lost the mitochondrial homologues of IscS and IscU (Loftus et al. 2005), and instead it possesses genes for the homologous proteins NifS and NifU, acquired through horizontal gene transfer from a bacterium related to Campylobacter (Ali et al. 2004; van der Giezen et al. 2004). The location of this pathway is central to testing the hypothesis that Fe/S cluster assembly is the common function of all mitochondrial homologues.
So far there is no evidence that the genes for [Fe]-hydrogenase and PFO, which mediate the conversion of mitochondria to hydrogenosomes, originated from the mitochondrial endosymbiont (Embley et al. 1997; Martin & Müller 1998). However, phylogenetic analyses do suggest that early eukaryotes likely contained both PFO and [Fe]-hydrogenase, and thus it is possible that they could make hydrogen. The two proteins, in various forms, have been found in diverse eukaryotes, where they are targeted to the cytosol, hydrogenosomes, mitochondria, nucleus and plastids. Although hydrogenase and PFO fail to display a unique affinity for the ‘mitochondrial’ compartment, their widespread retention among eukaryotes has undoubtedly contributed to the facility by which eukaryotes have repeatedly evolved hydrogenosomes (Embley et al. 1997). The origins of eukaryotic [Fe]-hydrogenase and PFO are also part of a bigger question concerning the origins of the eubacterial-like genes that encode much of eukaryote metabolism (Ribeiro & Golding 1998; Rivera et al. 1998; Esser et al. 2004). There are a number of imaginative hypotheses to explain these genes; as the product of multiple lateral transfers from different prokaryotes (Doolittle 1998), or the legacy of different eubacteria that participated in eukaryogenesis (Martin & Müller 1998; Moreira & LopezGarcia 1998; Rivera & Lake 2004; Cavalier-Smith 2006). Genomics coupled with more sophisticated phylogenetic analyses, should in principle be able to resolve gene origins, or at least reveal the limits of resolution that the data can provide (Penny et al. 2001; Ho & Jermiin 2004; Roger & Hug 2006). Published work on whole genomes has so far failed to provide strong support for any of the hypotheses for eukaryogenesis in common currency (Rivera & Lake 2004; Cavalier-Smith 2006).
The discovery that all eukaryotes, or at least the ones that have been studied in any detail, contain a mitochondrial homologue, potentially places the mitochondrial endosymbiosis at the very dawn of eukaryotic evolution. The demise of Archezoa also means that we can no longer be confident that the host for the mitochondrial endosymbiont was already a eukaryote. Prokaryote-host models for the mitochondrial endosymbiont (e.g. Searcy 1992; Martin & Müller 1998; Vellai & Vida 1999), which the existence of Archezoa had seemed to exclude, can now be judged on their merits as predictive hypotheses that can be tested. Of course, many anaerobic habitats and the eukaryotes that populate them remain poorly characterized and a bona fide Archezoan might have evaded discovery. However, given what is known about the ubiquity of anaerobic habitats and their persistence over time, it is difficult to see why archezoans should have proved so elusive.
Acknowledgments
Work in the author's laboratory on this topic has been supported by funding from the European Molecular Biology Organization (EMBO), the European Union Marie Curie Fellowship Programme, the Leverhulme Trust and the Wellcome Trust and from the Natural History Museum in London.
Footnotes
One contribution of 14 to a Discussion Meeting Issue ‘Major steps in cell evolution’.
References
- Abrahamsen M.S, et al. Complete genome sequence of the apicomplexan Cryptosporidium parvum. Science. 2004;304:441–445. doi: 10.1126/science.1094786. 10.1126/science.1094786 [DOI] [PubMed] [Google Scholar]
- Adams K.L, Palmer J.D. Evolution of mitochondrial gene content: gene loss and transfer to the nucleus. Mol. Phylogenet. Evol. 2003;29:380–395. doi: 10.1016/s1055-7903(03)00194-5. 10.1016/S1055-7903(03)00194-5 [DOI] [PubMed] [Google Scholar]
- Adl S.M, et al. The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. J. Eukaryot. Microbiol. 2005;52:399–451. doi: 10.1111/j.1550-7408.2005.00053.x. 10.1111/j.1550-7408.2005.00053.x [DOI] [PubMed] [Google Scholar]
- Akhmanova A, Voncken F, vanAlen T, vanHoek A, Boxma B, Vogels G, Veenhuiss M, Hackstein J.H.P. A hydrogenosome with a genome. Nature. 1998;396:527–528. doi: 10.1038/25023. 10.1038/25023 [DOI] [PubMed] [Google Scholar]
- Ali V, Shigeta Y, Tokumoto U, Takahashi Y, Nozaki T. An intestinal parasitic protist Entamoeba histolytica, possesses a non-redundant nitrogen fixation-like system for iron–sulfur cluster assembly under anaerobic conditions. J. Biol. Chem. 2004;279:16 863–16 874. doi: 10.1074/jbc.M313314200. 10.1074/jbc.M313314200 [DOI] [PubMed] [Google Scholar]
- Arisue N, Hasegawa M, Hashimoto T. Root of the Eukaryota tree as inferred from combined maximum likelihood analyses of multiple molecular sequence data. Mol. Biol. Evol. 2005;22:409–420. doi: 10.1093/molbev/msi023. 10.1093/molbev/msi023 [DOI] [PubMed] [Google Scholar]
- Bakatselou C, Kidgell C, Graham Clark C. A mitochondrial-type hsp70 gene of Entamoeba histolytica. Mol. Biochem. Parasitol. 2000;110:177–182. doi: 10.1016/s0166-6851(00)00264-4. 10.1016/S0166-6851(00)00264-4 [DOI] [PubMed] [Google Scholar]
- Balk J, Pierik A.J, Netz D.J, Muhlenhoff U, Lill R. The hydrogenase-like Nar1p is essential for maturation of cytosolic and nuclear iron–sulphur proteins. EMBO J. 2004;23:2105–2115. doi: 10.1038/sj.emboj.7600216. 10.1038/sj.emboj.7600216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk J, Pierik A.J, Aguilar Netza D.J, Muhlenhoff U, Lill R. Nar1p, a conserved eukaryotic protein with similarity to Fe—only hydrogenases, functions in cytosolic iron–sulphur protein biogenesis. Biochem. Soc. Trans. 2005;33:86–89. doi: 10.1042/BST0330086. 10.1042/BST0330086 [DOI] [PubMed] [Google Scholar]
- Bapteste E, et al. The analysis of 100 genes supports the grouping of three highly divergent amoebae: Dictyostelium, Entamoeba, and Mastigamoeba. Proc. Natl Acad. Sci. USA. 2002;99:1414–1419. doi: 10.1073/pnas.032662799. 10.1073/pnas.032662799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton R.M, Worman H.J. Prenylated prelamin A interacts with Narf, a novel nuclear protein. J. Biol. Chem. 1999;274:30 008–30 018. doi: 10.1074/jbc.274.42.30008. 10.1074/jbc.274.42.30008 [DOI] [PubMed] [Google Scholar]
- Bernard C, Fenchel T. Chemosensory behavior of Strombidium purpureum, an anaerobic oligotrich with endosymbiotic purple nonsulfur bacteria. J. Eukaryot. Microbiol. 1994;41:391–396. [Google Scholar]
- Boorstein W.R, Ziegelhoffer T, Craig E.A. Molecular evolution of the HSP70 multigene family. J. Mol. Evol. 1994;38:1–7. doi: 10.1007/BF00175490. 10.1007/BF00175490 [DOI] [PubMed] [Google Scholar]
- Boxma B, et al. An anaerobic mitochondrion that produces hydrogen. Nature. 2005;434:74–79. doi: 10.1038/nature03343. 10.1038/nature03343 [DOI] [PubMed] [Google Scholar]
- Bozner P. Immunological detection and subcellular localization of HSP70 and HSP60 homologues in Trichomonas vaginalis. J. Parasitol. 1997;83:224–229. [PubMed] [Google Scholar]
- Brown D.M, Upcroft J.A, Edwards M.R, Upcroft P. Anaerobic bacterial metabolism in the ancient eukaryote Giardia duodenalis. Int. J. Parasitol. 1998;28:149–164. doi: 10.1016/s0020-7519(97)00172-0. 10.1016/S0020-7519(97)00172-0 [DOI] [PubMed] [Google Scholar]
- Bui E.T.N, Johnson P.J. Identification and characterisation of [Fe]-hydrogenases in the hydrogenosome of Trichomonas vaginalis. Mol. Biochem. Parasitol. 1996;76:305–310. doi: 10.1016/0166-6851(96)02567-4. 10.1016/0166-6851(96)02567-4 [DOI] [PubMed] [Google Scholar]
- Bui E.T.N, Bradley P.J, Johnson P.J. A common evolutionary origin for mitochondria and hydrogenosomes. Proc. Natl Acad. Sci. USA. 1996;93:9651–9656. doi: 10.1073/pnas.93.18.9651. 10.1073/pnas.93.18.9651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier-Smith T. A 6 kingdom classification and a unified phylogeny. In: Schwemmler W, Schenk H.E.A, editors. Endocytobiology II. De Gruyter; Berlin, Germany: 1983a. pp. 1027–1034. [Google Scholar]
- Cavalier-Smith T. Endosymbiotic origin of the mitochondrial envelope. In: Schwemmler W, Schenk H.E.A, editors. Endocytobiology II. De Gruyter; Berlin, Germany: 1983b. pp. 265–279. [Google Scholar]
- Cavalier-Smith T. Eukaryotes with no mitochondria. Nature. 1987a;326:332–333. doi: 10.1038/326332a0. 10.1038/326332a0 [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. The simultaneous symbiotic origin of mitochondria, chloroplasts, and microbodies. In: Lee J.L, Frederick J.F, editors. Endocytobiology III. New York Academy of Sciences; New York, NY: 1987b. pp. 55–71. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. The phagotrophic origin of eukaryotes and phylogenetic classification of protozoa. Int. J. Syst. Evol. Microbiol. 2002;52:297–354. doi: 10.1099/00207713-52-2-297. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Cell evolution and Earth history: stasis and revolution. Phil. Trans. R. Soc. B. 2006;361:969–1006. doi: 10.1098/rstb.2006.1842. 10.1098/rstb.2006.1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.W, et al. A Novel ADP/ATP transporter in the mitosome of the microaerophilic human parasite Entamoeba histolytica. Curr. Biol. 2005;15:737–742. doi: 10.1016/j.cub.2005.02.068. 10.1016/j.cub.2005.02.068 [DOI] [PubMed] [Google Scholar]
- Clark C.G, Roger A.J. Direct evidence for secondary loss of mitochondria in Entamoeba histolytica. Proc. Natl Acad. Sci. USA. 1995;92:6518–6521. doi: 10.1073/pnas.92.14.6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens D.L, Johnson P.J. Failure to detect DNA in hydrogenosomes of Trichomonas vaginalis by nick translation and immunomicroscopy. Mol. Biochem. Parasitol. 2000;106:307–313. doi: 10.1016/s0166-6851(99)00220-0. 10.1016/S0166-6851(99)00220-0 [DOI] [PubMed] [Google Scholar]
- Darwin, C. 1859 The origin of species Penguin Classics, 1985 reprint of 1859 edition. London: Penguin Books.
- Davidson E.A, van der Giezen M, Horner D.S, Embley T.M, Howe C.J. An [Fe]-hydrogenase from the anaerobic hydrogenosome-containing fungus Neocallimastix frontalis L2. GENE. 2002;296:45–52. doi: 10.1016/s0378-1119(02)00873-9. 10.1016/S0378-1119(02)00873-9 [DOI] [PubMed] [Google Scholar]
- Dolezal P, Smid O, Rada P, Zubacova Z, Bursac D, Sutak R, Nebesarova J, Lithgow T, Tachezy J. Giardia mitosomes and trichomonad hydrogenosomes share a common mode of protein targeting. Proc. Natl Acad. Sci. USA. 2005;102:10 924–10 929. doi: 10.1073/pnas.0500349102. 10.1073/pnas.0500349102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle W.F. You are what you eat: a gene transfer ratchet could account for bacterial genes in eukaryotic nuclear genomes. Trends Genet. 1998;14:307–311. doi: 10.1016/s0168-9525(98)01494-2. 10.1016/S0168-9525(98)01494-2 [DOI] [PubMed] [Google Scholar]
- Dyall S.B, Johnson P.J. Origins of hydrogenosomes and mitochondria: evolution and organelle biogenesis. Curr. Opin. Microbiol. 2000;3:404–411. doi: 10.1016/s1369-5274(00)00112-0. 10.1016/S1369-5274(00)00112-0 [DOI] [PubMed] [Google Scholar]
- Dyall S.D, Koehler C.M, Delgadillo-Correa M.G, Bradley P.J, Plumper E, Leuenberger D, Turck C.W, Johnson P.J. Presence of a member of the mitochondrial carrier family in hydrogenosomes: conservation of membrane-targeting pathways between hydrogenosomes and mitochondria. Mol. Cell Biol. 2000;20:2488–2497. doi: 10.1128/mcb.20.7.2488-2497.2000. 10.1128/MCB.20.7.2488-2497.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall S.D, Brown M.T, Johnson P.J. Ancient invasions: from endosymbionts to organelles. Science. 2004a;304:253–257. doi: 10.1126/science.1094884. 10.1126/science.1094884 [DOI] [PubMed] [Google Scholar]
- Dyall S.D, Yan W, Delgadillo-Correa M.G, Lunceford A, Loo J.A, Clarke C.F, Johnson P.J. Non-mitochondrial complex I proteins in a hydrogenosomal oxidoreductase complex. Nature. 2004b;431:1103–1107. doi: 10.1038/nature02990. 10.1038/nature02990 [DOI] [PubMed] [Google Scholar]
- Embley T.M, Martin W. Eukaryotic evolution, changes and challenges. Nature. 2006;440:623–630. doi: 10.1038/nature04546. 10.1038/nature04546 [DOI] [PubMed] [Google Scholar]
- Embley T.M, Finlay B.J, Dyal P.L, Hirt R.P, Wilkinson M, Williams A.G. Multiple origins of anaerobic ciliates with hydrogenosomes within the radiation of aerobic ciliates. Proc. R. Soc. B. 1995;262:87–93. doi: 10.1098/rspb.1995.0180. [DOI] [PubMed] [Google Scholar]
- Embley T.M, Horner D.S, Hirt R.P. Anaerobic eukaryote evolution: hydrogenosomes as biochemically modified mitochondria? Trends Ecol. Evol. 1997;12:437–441. doi: 10.1016/s0169-5347(97)01208-1. 10.1016/S0169-5347(97)01208-1 [DOI] [PubMed] [Google Scholar]
- Embley T.M, van der Giezen M, Horner D.S, Dyal P.L, Bell S, Foster P.G. Hydrogenosomes, mitochondria and early eukaryotic evolution. IUBMB Life. 2003a;55:387–395. doi: 10.1080/15216540310001592834. [DOI] [PubMed] [Google Scholar]
- Embley T.M, van der Giezen M, Horner D.S, Dyal P.L, Foster P. Mitochondria and hydrogenosomes are two forms of the same fundamental organelle. Phil. Trans. R. Soc. B. 2003b;358:191–203. doi: 10.1098/rstb.2002.1190. 10.1098/rstb.2002.1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emelyanov V.V. Phylogenetic affinity of a Giardia lamblia cysteine desulfurase conforms to canonical pattern of mitochondrial ancestry. FEMS Microbiol. Lett. 2003;226:257–266. doi: 10.1016/S0378-1097(03)00598-6. 10.1016/S0378-1097(03)00598-6 [DOI] [PubMed] [Google Scholar]
- Esser C, et al. A genome phylogeny for mitochondria among alpha-proteobacteria and a predominantly eubacterial ancestry of yeast nuclear genes. Mol. Biol. Evol. 2004;21:1643–1660. doi: 10.1093/molbev/msh160. 10.1093/molbev/msh160 [DOI] [PubMed] [Google Scholar]
- Esteban G, Guhl B.E, Ken J.C, Embley T.M, Finlay B.J. Cyclidium porcatum n.sp.: a free-living anaerobic scuticociliate containing a stable complex of hydrogenosomes, eubacteria and archaeobacteria. Eur. J. Protistol. 1993;29:262–270. doi: 10.1016/S0932-4739(11)80281-6. [DOI] [PubMed] [Google Scholar]
- Fast N.M, Keeling P.J. Alpha and beta subunits of pyruvate dehydrogenase E1 from the microsporidian Nosema locustae: mitochondrion-derived carbon metabolism in microsporidia. Mol. Biochem. Parasitol. 2001;117:201–209. doi: 10.1016/s0166-6851(01)00356-5. 10.1016/S0166-6851(01)00356-5 [DOI] [PubMed] [Google Scholar]
- Fenchel T. There are more small than large species? Oikos. 1993;68:375–378. [Google Scholar]
- Fenchel T, Bernard C. Endosymbiotic purple non-sulphur bacteria in an anaerobic ciliated protozoon. FEMS Microbiol. Ecol. 1993a;110:21–25. [Google Scholar]
- Fenchel T, Bernard C. A purple protist. Nature. 1993b;362:300. doi: 10.1038/362300a0. 10.1038/362300a0 [DOI] [PubMed] [Google Scholar]
- Fenchel T, Finlay B.J. Oxford series in ecology and evolution. Oxford University Press; Oxford, UK: 1995. Ecology and evolution in anoxic worlds. [Google Scholar]
- Germot A, Philippe H, Le Guyader H. Presence of a mitochondrial-type HSP70 in Trichomonas suggests a very early mitochondrial endosymbiosis in eukaryotes. Proc. Natl Acad. Sci. USA. 1996;93:14 614–14 617. doi: 10.1073/pnas.93.25.14614. 10.1073/pnas.93.25.14614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germot A, Philippe H, Le Guyader H. Loss of mitochondria in microsporidia: evidence from a mitochondrial-type HSP-70 in Nosema locustae. Mol. Biochem. Parasitol. 1997;87:159–168. doi: 10.1016/s0166-6851(97)00064-9. 10.1016/S0166-6851(97)00064-9 [DOI] [PubMed] [Google Scholar]
- Goksoyr J. Evolution of eucaryotic cells. Nature. 1967;214:1161. doi: 10.1038/2141161a0. [DOI] [PubMed] [Google Scholar]
- Gray M.W. Evolutionary biology: the hydrogenosome's murky past. Nature. 2005;434:29–31. doi: 10.1038/434029a. 10.1038/434029a [DOI] [PubMed] [Google Scholar]
- Gray M.W, Burger G, Lang B.F. The origin and early evolution of mitochondria. Genome Biol. 2001;2:10 181–10 185. doi: 10.1186/gb-2001-2-6-reviews1018. 10.1186/gb-2001-2-6-reviews1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M.W, Lang B.F, Burger G. Mitochondria of protists. Annu. Rev. Genet. 2004;38:477–524. doi: 10.1146/annurev.genet.37.110801.142526. 10.1146/annurev.genet.37.110801.142526 [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Nakamura Y, Kamaishi T, Hasegawa M. Early evolution of eukaryotes inferred from the amino acid sequences of elongation factors 1a and 2. Arch. Protistenkd. 1997;148:287–295. [Google Scholar]
- Hirt R.P, Healy B, Vossbrinck C.R, Canning E.U, Embley T.M. A mitochondrial HSP70 orthologue in Vairimorpha necatrix: molecular evidence that microsporidia once contained mitochondria. Curr. Biol. 1997;7:995–998. doi: 10.1016/s0960-9822(06)00420-9. 10.1016/S0960-9822(06)00420-9 [DOI] [PubMed] [Google Scholar]
- Hirt R.P, Logsdon J.M, Healy B, Dorey M.W, Doolittle W.F, Embley T.M. Microsporidia are related to fungi: evidence from the largest subunit of RNA polymerase II and other proteins. Proc. Natl Acad. Sci. USA. 1999;96:580–585. doi: 10.1073/pnas.96.2.580. 10.1073/pnas.96.2.580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S.Y.W, Jermiin L.S. Tracing the decay of the historical signal in biological sequence data. Syst. Biol. 2004;53:623–637. doi: 10.1080/10635150490503035. 10.1080/10635150490503035 [DOI] [PubMed] [Google Scholar]
- Horner D.S, Embley T.M. Chaperonin 60 phylogeny provides further evidence for secondary loss of mitochondria among putative early-branching eukaryotes. Mol. Biol. Evol. 2001;18:1970–1975. doi: 10.1093/oxfordjournals.molbev.a003737. [DOI] [PubMed] [Google Scholar]
- Horner D, Hirt R.P, Kilvington S, Lloyd D, Embley T.M. Molecular data suggest an early acquisition of the mitochondrion endosymbiont. Proc. R. Soc. B. 1996;263:1053–1059. doi: 10.1098/rspb.1996.0155. [DOI] [PubMed] [Google Scholar]
- Horner D.S, Hirt R.P, Embley T.M. A single eubacterial origin of eukaryotic pyruvate:ferredoxin oxidoreductase genes: implications for the evolution of anaerobic eukaryotes. Mol. Biol. Evol. 1999;16:1280–1291. doi: 10.1093/oxfordjournals.molbev.a026218. [DOI] [PubMed] [Google Scholar]
- Horner D.S, Foster P.G, Embley T.M. Iron hydrogenases and the evolution of anaerobic eukaryotes. Mol. Biol. Evol. 2000;17:1695–1709. doi: 10.1093/oxfordjournals.molbev.a026268. [DOI] [PubMed] [Google Scholar]
- Horner D.S, Heil B, Happe T, Embley T.M. Iron hydrogenases—ancient enzymes in modern eukaryotes. Trends Biochem. Sci. 2002;27:148–153. doi: 10.1016/s0968-0004(01)02053-9. 10.1016/S0968-0004(01)02053-9 [DOI] [PubMed] [Google Scholar]
- Hrdy I, Müller M. Primary structure and eubacterial relationships of the pyruvate:ferredoxin oxidoreductase of the amitochondriate eukaryote Trichomonas vaginalis. J. Mol. Evol. 1995a;41:388–396. [PubMed] [Google Scholar]
- Hrdy I, Müller M. Primary structure of the hydrogenosomal malic enzyme of Trichomonas vaginalis and its relationship to homologous enzymes. J. Eukaryot. Microbiol. 1995b;42:593–603. doi: 10.1111/j.1550-7408.1995.tb05913.x. [DOI] [PubMed] [Google Scholar]
- Hrdy I, Hirt R.P, Dolezal P, Bardonova L, Foster P.G, Tachezy J, Embley T.M. Trichomonas hydrogenosomes contain the NADH dehydrogenase module of mitochondrial complex I. Nature. 2004;432:618–622. doi: 10.1038/nature03149. 10.1038/nature03149 [DOI] [PubMed] [Google Scholar]
- Inagaki Y, Susko E, Fast N.M, Roger A.J. Covarion shifts cause a long-branch attraction artifact that unites microsporidia and archaebacteria in EF-1alpha phylogenies. Mol. Biol. Evol. 2004;21:1340–1349. doi: 10.1093/molbev/msh130. 10.1093/molbev/msh130 [DOI] [PubMed] [Google Scholar]
- John P, Whatley F.R. Paracoccus denitrificans and the evolutionary origin of the mitochondrion. Nature. 1975;254:495–498. doi: 10.1038/254495a0. 10.1038/254495a0 [DOI] [PubMed] [Google Scholar]
- Katinka M.D, et al. Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi. Nature. 2001;414:450–453. doi: 10.1038/35106579. 10.1038/35106579 [DOI] [PubMed] [Google Scholar]
- Keeling P.J, Luker M.A, Palmer J.D. Evidence from beta-tubulin phylogeny that microsporidia evolved from within the fungi. Mol. Biol. Evol. 2000;17:23–31. doi: 10.1093/oxfordjournals.molbev.a026235. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. The ADP/ATP carrier in mitochondrial membranes. In: Martonosi A.N, editor. The enzymes of biological membranes. Plenum Press; New York, NY: 1985. pp. 511–553. [Google Scholar]
- Knight J. Giardia: not so special, after all? Nature. 2004;429:236–237. doi: 10.1038/429236a. 10.1038/429236a [DOI] [PubMed] [Google Scholar]
- Kunji E.R. The role and structure of mitochondrial carriers. FEBS Lett. 2004;564:239–244. doi: 10.1016/S0014-5793(04)00242-X. 10.1016/S0014-5793(04)00242-X [DOI] [PubMed] [Google Scholar]
- Kurland C.G, Andersson S.G. Origin and evolution of the mitochondrial proteome. Microbiol. Mol. Biol. Rev. 2000;64:786–820. doi: 10.1128/mmbr.64.4.786-820.2000. 10.1128/MMBR.64.4.786-820.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGier M.J, Tachezy J, Stejskal F, Kutisova K, Keithly J.S. Mitochondrial-type iron–sulfur cluster biosynthesis genes (IscS and IscU) in the apicomplexan Cryptosporidium parvum. Microbiology. 2003;149:3519–3530. doi: 10.1099/mic.0.26365-0. 10.1099/mic.0.26365-0 [DOI] [PubMed] [Google Scholar]
- Leipe D.D, Gunderson J.H, Nerad T.A, Sogin M.L. Small subunit ribosomal RNA of Hexamita inflata and the quest for the first branch in the eukaryotic tree. Mol. Biochem. Parasitol. 1993;59:41–48. doi: 10.1016/0166-6851(93)90005-i. 10.1016/0166-6851(93)90005-I [DOI] [PubMed] [Google Scholar]
- Lill R, Muhlenhoff U. Iron-sulfur-protein biogenesis in eukaryotes. Trends Biochem. Sci. 2005;30:133–141. doi: 10.1016/j.tibs.2005.01.006. 10.1016/j.tibs.2005.01.006 [DOI] [PubMed] [Google Scholar]
- Lill R, Diekert K, Kaut A, Lange H, Pelzer W, Prohl C, Kispal G. The essential role of mitochondria in the biogenesis of cellular iron–sulfur proteins. Biol. Chem. 1999;380:1157–1166. doi: 10.1515/BC.1999.147. 10.1515/BC.1999.147 [DOI] [PubMed] [Google Scholar]
- Lindmark D.G, Müller M. Hydrogenosome, a cytoplasmic organelle of the anaerobic flagellate, Tritrichomonas foetus, and its role in pyruvate metabolism. J. Biol. Chem. 1973;248:7724–7728. [PubMed] [Google Scholar]
- Lloyd D, Ralphs J.R, Harris J.C. Giardia intestinalis, a eukaryote without hydrogenosomes, produces hydrogen. Microbiology. 2002;148:727–733. doi: 10.1099/00221287-148-3-727. [DOI] [PubMed] [Google Scholar]
- Loftus B, et al. The genome of the protist parasite Entamoeba histolytica. Nature. 2005;433:865–868. doi: 10.1038/nature03291. 10.1038/nature03291 [DOI] [PubMed] [Google Scholar]
- Margulis L, Dolan M.F, Whiteside J.H. “Imperfections and oddities” in the origin of the nucleus. Paleobiology. 2005;31:175–191. 10.1666/0094-8373(2005)031%5B0175:IAOITO%5D2.0.CO;2 [Google Scholar]
- Martin W. The missing link between hydrogenosomes and mitochondria. Trends Microbiol. 2005;13:457–459. doi: 10.1016/j.tim.2005.08.005. 10.1016/j.tim.2005.08.005 [DOI] [PubMed] [Google Scholar]
- Martin W, Müller M. The hydrogen hypothesis for the first eukaryote. Nature. 1998;392:37–41. doi: 10.1038/32096. 10.1038/32096 [DOI] [PubMed] [Google Scholar]
- Moreira D, LopezGarcia P. Symbiosis between methanogenic archaea and delta-proteobacteria as the origin of eukaryotes: the syntrophic hypothesis. J. Mol. Evol. 1998;47:517–530. doi: 10.1007/pl00006408. 10.1007/PL00006408 [DOI] [PubMed] [Google Scholar]
- Morrison H.G, Roger A.J, Nystul T.G, Gillin F.D, Sogin M.L. Giardia lamblia expresses a proteobacterial-like DnaK homolog. Mol. Biol. Evol. 2001;18:530–541. doi: 10.1093/oxfordjournals.molbev.a003832. [DOI] [PubMed] [Google Scholar]
- Müller M. The hydrogenosome. J. Gen. Microbiol. 1993;139:2879–2889. doi: 10.1099/00221287-139-12-2879. [DOI] [PubMed] [Google Scholar]
- Müller M. Energy metabolism. Part 1: Anaerobic protozoa. In: Marr J.J, Nilsen T.W, Komuniecki R.W, editors. Molecular medical parasitolgy. Academic Press; Amsterdam, The Netherlands: 2003. pp. 125–139. [Google Scholar]
- Nixon J.E, Field J, McArthur A.G, Sogin M.L, Yarlett N, Loftus B.J, Samuelson J. Iron-dependent hydrogenases of Entamoeba histolytica and Giardia lamblia: activity of the recombinant entamoebic enzyme and evidence for lateral gene transfer. Biol. Bull. 2003;204:1–9. doi: 10.2307/1543490. [DOI] [PubMed] [Google Scholar]
- Owen R. Longman, Brown, Green and Longmans; London: 1843. Lectures on the comparative physiology of the invertebrate animals. [Google Scholar]
- Patterson D.J, Sogin M.L. Eukaryotic origins and protistan diversity. In: Hartman H, Matsuno K, editors. The origin and evolution of the cell. World Scientific; Singapore: 1992. pp. 13–46. [Google Scholar]
- Penny D, McComish B.J, Charleston M.A, Hendy M.D. Mathematical elegance with biochemical realism: the covarion model of molecular evolution. J. Mol. Evol. 2001;53:711–723. doi: 10.1007/s002390010258. 10.1007/s002390010258 [DOI] [PubMed] [Google Scholar]
- Peyretaillade E, Broussole V, Peyret P, Metenier G, Gouy M, Vivares C.P. Microsporidia, amitochondriate protists, possess a 70-kDa heat shock protein gene of mitochondrial origin. Mol. Biol. Evol. 1998;15:683–689. doi: 10.1093/oxfordjournals.molbev.a025971. [DOI] [PubMed] [Google Scholar]
- Pfanner N, Geissler A. Versatility of the mitochondrial protein import machinery. Nat. Rev. Mol. Cell Biol. 2001;2:339–349. doi: 10.1038/35073006. 10.1038/35073006 [DOI] [PubMed] [Google Scholar]
- Philippe H, Adoutte A. The molecular phylogeny of protozoa: solid facts and uncertainties. In: Coombs G.H, Vickerman K, Sleigh M.A, Warren A, editors. Evolutionary relationships among protozoa. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1998. pp. 25–56. [Google Scholar]
- Philippe H, Lopez P, Brinkmann H, Budin K, Germot A, Laurent J, Moreira D, Muller M, Le Guyader H. Early-branching or fast-evolving eukaryotes? An answer based on slowly evolving positions. Proc. R. Soc. B. 2000;267:1213–1221. doi: 10.1098/rspb.2000.1130. 10.1098/rspb.2000.1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumper E, Bradley P.J, Johnson P.J. Implications of protein import on the origin of hydrogenosomes. Protist. 1998;149:303–311. doi: 10.1016/S1434-4610(98)70037-9. [DOI] [PubMed] [Google Scholar]
- Reeves R.E. Metabolism of Entamoeba histolytica Schaudinn, 1903. Adv. Parasitol. 1984;23:105–142. doi: 10.1016/s0065-308x(08)60286-9. [DOI] [PubMed] [Google Scholar]
- Reeves R.E, Warren L.G, Suskind B, Lo H.S. An energy conserving pyruvate to acetate pathway in Entamoeba histolytica. J. Biol. Chem. 1977;252:726–731. [PubMed] [Google Scholar]
- Regoes A, Zourmpanou D, Leon-Avila G, van der Giezen M, Tovar J, Hehl A.B. Protein import, replication and inheritance of a vestigial mitochondrion. J. Biol. Chem. 2005;280:30 557–30 563. doi: 10.1074/jbc.M500787200. 10.1074/jbc.M500787200 [DOI] [PubMed] [Google Scholar]
- Reichert A.S, Neupert W. Mitochondriomics or what makes us breathe. Trends Genet. 2004;20:555–562. doi: 10.1016/j.tig.2004.08.012. 10.1016/j.tig.2004.08.012 [DOI] [PubMed] [Google Scholar]
- Ribeiro S, Golding G.B. The mosaic nature of the eukaryotic nucleus. Mol. Biol. Evol. 1998;15:779–788. doi: 10.1093/oxfordjournals.molbev.a025983. [DOI] [PubMed] [Google Scholar]
- Riordan C.E, Ault J.G, Langreth S.G, Keithly J.S. Cryptosporidium parvum Cpn60 targets a relict organelle. Curr. Genet. 2003;44:138–147. doi: 10.1007/s00294-003-0432-1. 10.1007/s00294-003-0432-1 [DOI] [PubMed] [Google Scholar]
- Rivera M.C, Lake J.A. The ring of life provides evidence for a genome fusion origin of eukaryotes. Nature. 2004;431:152–155. doi: 10.1038/nature02848. 10.1038/nature02848 [DOI] [PubMed] [Google Scholar]
- Rivera M.C, Jain R, Moore J.E, Lake J.A. Genomic evidence for two functionally distinct gene classes. Proc. Natl Acad. Sci. USA. 1998;95:6239–6244. doi: 10.1073/pnas.95.11.6239. 10.1073/pnas.95.11.6239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M.A, Garcia-Perez R.M, Mendoza L, Sanchez T, Guillen N, Orozco E. The pyruvate:ferredoxin oxidoreductase enzyme is located in the plasma membrane and in a cytoplasmic structure in Entamoeba. Microb. Pathog. 1998;25:1–10. doi: 10.1006/mpat.1998.0202. 10.1006/mpat.1998.0202 [DOI] [PubMed] [Google Scholar]
- Roger A.J, Hug L.A. The origin and diversification of eukaryotes: problems with molecular phylogenetics and molecular clock estimation. Phil. Trans. R. Soc. B. 2006;361:1039–1054. doi: 10.1098/rstb.2006.1845. 10.1098/rstb.2006.1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger A.J, Clark C.G, Doolittle W.F. A possible mitochondrial gene in the early-branching amitochondriate protist Trichomonas vaginalis. Proc. Natl Acad. Sci. USA. 1996;93:14 618–14 622. doi: 10.1073/pnas.93.25.14618. 10.1073/pnas.93.25.14618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger A.J, Svard S.G, Tovar J, Clark C.G, Smith M.W, Gillin F.D, Sogin M.L. A mitochondrial-like chaperonin 60 gene in Giardia lamblia: evidence that diplomonads once harbored an endosymbiont related to the progenitor of mitochondria. Proc. Natl Acad. Sci. USA. 1998;95:229–234. doi: 10.1073/pnas.95.1.229. 10.1073/pnas.95.1.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotte C, Stejskal F, Zhu G, Keithly J.S, Martin W. Pyruvate:NADP oxidoreductase from the mitochondrion of Euglena gracilis and from the apicomplexan Cryptosporidium parvum: a biochemical relic linking pyruvate metabolism in mitochondriate and amitochondriate protists. Mol. Biol. Evol. 2001;18:710–720. doi: 10.1093/oxfordjournals.molbev.a003853. [DOI] [PubMed] [Google Scholar]
- Searcy D.G. Origin of mitochondria and chloroplasts from sulfur-based symbioses. In: Matsuno H.H, Matsuno K, editors. The origin and evolution of the cell. World Scientific Press; Singapore: 1992. pp. 47–78. [Google Scholar]
- Siddall M.E, Hong H, Desser S.S. Phylogenetic analysis of the Diplomonadida (Wenyon, 1926) Brugerolle, 1975: evidence for heterochrony in protozoa and against Giardia lamblia as a “missing link”. J. Protozool. 1992;39:361–367. doi: 10.1111/j.1550-7408.1992.tb01465.x. [DOI] [PubMed] [Google Scholar]
- Slapeta J, Keithly J.S. Cryptosporidium parvum mitochondrial-type HSP70 targets homologous and heterologous mitochondria. Eukaryot. Cell. 2004;3:483–494. doi: 10.1128/EC.3.2.483-494.2004. 10.1128/EC.3.2.483-494.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M.L. History assignment: when was the mitochondrion founded? Curr. Opin. Genet. Dev. 1997;7:792–799. doi: 10.1016/s0959-437x(97)80042-1. 10.1016/S0959-437X(97)80042-1 [DOI] [PubMed] [Google Scholar]
- Sogin M.L, Gunderson J.H, Elwood H.J, Alonso R.A, Peattie D.A. Phylogenetic meaning of the kingdom concept: an unusual ribosomal RNA from Giardia lamblia. Science. 1989;243:75–77. doi: 10.1126/science.2911720. [DOI] [PubMed] [Google Scholar]
- Stiller J.W, Hall B.D. Long-branch attraction and the rDNA model of early eukaryotic evolution. Mol. Biol. Evol. 1999;16:1270–1279. doi: 10.1093/oxfordjournals.molbev.a026217. [DOI] [PubMed] [Google Scholar]
- Sutak R, Dolezal P, Fiumera H.L, Hrdy I, Dancis A, Delgadillo-Correa M, Johnson P.J, Muller M, Tachezy J. Mitochondrial-type assembly of FeS centers in the hydrogenosomes of the amitochondriate eukaryote Trichomonas vaginalis. Proc. Natl Acad. Sci. USA. 2004;101:10 368–10 373. doi: 10.1073/pnas.0401319101. 10.1073/pnas.0401319101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachezy J, Sanchez L.B, Muller M. Mitochondrial type iron-sulfur cluster assembly in the amitochondriate eukaryotes Trichomonas vaginalis and Giardia intestinalis, as indicated by the phylogeny of IscS. Mol. Biol. Evol. 2001;18:1919–1928. doi: 10.1093/oxfordjournals.molbev.a003732. [DOI] [PubMed] [Google Scholar]
- Thomarat F, Vivares C.P, Gouy M. Phylogenetic analysis of the complete genome sequence of Encephalitozoon cuniculi supports the fungal origin of microsporidia and reveals a high frequency of fast-evolving genes. J. Mol. Evol. 2004;59:780–791. doi: 10.1007/s00239-004-2673-0. 10.1007/s00239-004-2673-0 [DOI] [PubMed] [Google Scholar]
- Timmis J.N, Ayliffe M.A, Huang C.Y, Martin W. Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes. Nat. Rev. Genet. 2004;5:123–135. doi: 10.1038/nrg1271. 10.1038/nrg1271 [DOI] [PubMed] [Google Scholar]
- Tjaden J, Haferkamp I, Boxma B, Tielens A.G, Huynen M, Hackstein J.H. A divergent ADP/ATP carrier in the hydrogenosomes of Trichomonas gallinae argues for an independent origin of these organelles. Mol. Microbiol. 2004;51:1439–1446. doi: 10.1111/j.1365-2958.2004.03918.x. 10.1111/j.1365-2958.2004.03918.x [DOI] [PubMed] [Google Scholar]
- Tovar J, Fischer A, Clark C.G. The mitosome, a novel organelle related to mitochondria in the amitochondrial parasite Entamoeba histolytica. Mol. Microbiol. 1999;32:1013–1021. doi: 10.1046/j.1365-2958.1999.01414.x. 10.1046/j.1365-2958.1999.01414.x [DOI] [PubMed] [Google Scholar]
- Tovar J, Leon-Avila G, Sanchez L.B, Sutak R, Tachezy J, van der Giezen M, Hernandez M, Muller M, Lucocq J.M. Mitochondrial remnant organelles of Giardia function in iron–sulphur protein maturation. Nature. 2003;426:172–176. doi: 10.1038/nature01945. 10.1038/nature01945 [DOI] [PubMed] [Google Scholar]
- Townson S.M, Upcroft J.A, Upcroft P. Characterisation and purification of pyruvate:ferredoxin oxidoreductase from Giardia duodenalis. Mol. Biochem. Parasitol. 1996;79:183–193. doi: 10.1016/0166-6851(96)02661-8. 10.1016/0166-6851(96)02661-8 [DOI] [PubMed] [Google Scholar]
- van der Giezen M, Sjollema K.A, Artz R.R.E, Alkema W, Prins R.A. Hydrogenosomes in the anaerobic fungus Neocallimastix frontalis have a double membrane but lack an associated organelle genome. FEBS Lett. 1997;408:147–150. doi: 10.1016/s0014-5793(97)00409-2. 10.1016/S0014-5793(97)00409-2 [DOI] [PubMed] [Google Scholar]
- van der Giezen M, Slotboom D.J, Horner D.S, Dyal P.L, Harding M, Xue G.P, Embley T.M, Kunji E.R. Conserved properties of hydrogenosomal and mitochondrial ADP/ATP carriers: a common origin for both organelles. EMBO J. 2002;21:572–579. doi: 10.1093/emboj/21.4.572. 10.1093/emboj/21.4.572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Giezen M, Birdsey G.M, Horner D.S, Lucocq J, Dyal P.L, Benchimol M, Danpure C.J, Embley T.M. Fungal hydrogenosomes contain mitochondrial heat-shock proteins. Mol. Biol. Evol. 2003;20:1051–1061. doi: 10.1093/molbev/msg103. 10.1093/molbev/msg103 [DOI] [PubMed] [Google Scholar]
- van der Giezen M, Cox S, Tovar J. The iron-sulfur cluster assembly genes iscS and iscU of Entamoeba histolytica were acquired by horizontal gene transfer. BMC Evol. Biol. 2004;4:7. doi: 10.1186/1471-2148-4-7. 10.1186/1471-2148-4-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Giezen M, Tovar J, Clark C.G. Mitochondrion-derived organelles in protists and fungi. Int. Rev. Cytol. 2005;244:175–225. doi: 10.1016/S0074-7696(05)44005-X. [DOI] [PubMed] [Google Scholar]
- Vellai T, Vida G. The origin of eukaryotes: the difference between prokaryotic and eukaryotic cells. Proc. R. Soc. B. 1999;266:1571–1577. doi: 10.1098/rspb.1999.0817. 10.1098/rspb.1999.0817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellai T, Takacs K, Vida G. A new aspect to the origin and evolution of eukaryotes. J. Mol. Evol. 1998;46:499–507. doi: 10.1007/pl00006331. 10.1007/PL00006331 [DOI] [PubMed] [Google Scholar]
- Viale A.M, Arakaki A.K. The chaperone connection to the origins of the eukaryotic organelles. FEBS Lett. 1994;341:146–151. doi: 10.1016/0014-5793(94)80446-x. 10.1016/0014-5793(94)80446-X [DOI] [PubMed] [Google Scholar]
- Vivares C.P, Gouy M, Thomarat F, Metenier G. Functional and evolutionary analysis of a eukaryotic parasitic genome. Curr. Opin. Microbiol. 2002;5:499–505. doi: 10.1016/s1369-5274(02)00356-9. [DOI] [PubMed] [Google Scholar]
- Voncken F, et al. Multiple origins of hydrogenosomes: functional and phylogenetic evidence from the ADP/ATP carrier of the anaerobic chytrid Neocallimastix sp. Mol. Microbiol. 2002a;44:1441–1454. doi: 10.1046/j.1365-2958.2002.02959.x. 10.1046/j.1365-2958.2002.02959.x [DOI] [PubMed] [Google Scholar]
- Voncken F.G, Boxma B, van Hoek A.H, Akhmanova A.S, Vogels G.D, Huynen M, Veenhuis M, Hackstein J.H. A hydrogenosomal [Fe]-hydrogenase from the anaerobic chytrid Neocallimastix sp. L2. Gene. 2002b;284:103–112. doi: 10.1016/s0378-1119(02)00388-8. 10.1016/S0378-1119(02)00388-8 [DOI] [PubMed] [Google Scholar]
- Vossbrinck C.R, Maddox J.V, Friedman S, Debrunner-Vossbrinck B.A, Woese C.R. Ribosomal RNA sequence suggests microsporidia are extremely ancient eukaryotes. Nature. 1987;326:411–414. doi: 10.1038/326411a0. 10.1038/326411a0 [DOI] [PubMed] [Google Scholar]
- Whatley J.M, John P, Whatley F.R. From extracellular to intracellular: the establishment of mitochondria and chloroplasts. Proc. R. Soc. B. 1979;204:165–187. doi: 10.1098/rspb.1979.0020. [DOI] [PubMed] [Google Scholar]
- Williams B.A, Hirt R.P, Lucocq J.M, Embley T.M. A mitochondrial remnant in the microsporidian Trachipleistophora hominis. Nature. 2002;418:865–869. doi: 10.1038/nature00949. 10.1038/nature00949 [DOI] [PubMed] [Google Scholar]
- Woese C.R. Endosymbionts and mitochondrial origins. J. Mol. Evol. 1977;10:93–96. doi: 10.1007/BF01751802. 10.1007/BF01751802 [DOI] [PubMed] [Google Scholar]
- Wolters J. The troublesome parasites—molecular and morphological evidence that Apicomplexa belong to the dinoflagellate-ciliate clade. Biosystems. 1991;25:75–83. doi: 10.1016/0303-2647(91)90014-c. 10.1016/0303-2647(91)90014-C [DOI] [PubMed] [Google Scholar]
- Yang D, Oyaizu Y, Oyaizu H, Olsen G.J, Woese C.R. Mitochondrial origins. Proc. Natl Acad. Sci. USA. 1985;82:4443–4447. doi: 10.1073/pnas.82.13.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarlett N, Lloyd D, Williams A. Hydrogenosomes in the rumen protozoon Dasytricha ruminantium Schuberg. Biochem. J. 1981;200:365–372. doi: 10.1042/bj2000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarlett N, Coleman G.S, Williams A.G, Lloyd D. Hydrogenosomes in known species of rumen entodiniomorphid protozoa. FEMS Microbiol. Lett. 1984;21:15–19. [Google Scholar]
- Yarlett N, Orpin C.G, Munn E.A, Yarlett N.C, Greenwood C.A. Hydrogenosomes in the rumen fungus Neocallimastix patriciarum. Biochem. J. 1986;236:729–739. doi: 10.1042/bj2360729. [DOI] [PMC free article] [PubMed] [Google Scholar]