Abstract
The tumor suppressor phosphatase and tensin homolog deleted on chromosome 10 (PTEN) regulates diverse cellular functions by dephosphorylating the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate (PIP3). Recent study revealed that PICT-1/GLTSCR2 bound to and stabilized PTEN protein in cells, implicating its roles in PTEN-governed PIP3 signals. In this study, we demonstrate that RNA interference-mediated knockdown of PICT-1 in HeLa cells down-regulated endogenous PTEN and resulted in the activation of PIP3 downstream effectors, such as protein kinase B/Akt. Furthermore, the PICT-1 knockdown promoted HeLa cell proliferation; however the proliferation of PTEN-null cells was not altered by the PICT-1 knockdown, suggesting its dependency on PTEN status. In addition, apoptosis of HeLa cells induced by staurosporine or serum-depletion was alleviated by the PICT-1 knockdown in the similar PTEN-dependent manner. Most strikingly, the PICT-1 knockdown in HeLa and NIH3T3 cells promoted anchorage-independent growth, a hallmark of tumorigenic transformation. Furthermore, PICT-1 was aberrantly expressed in 18 (41%) of 44 human neuroblastoma specimens, and the PICT-1 loss was associated with reduced PTEN protein expression in spite of the existence of PTEN mRNA. Collectively, these results suggest that PICT-1 plays a role in PIP3 signals through controlling PTEN protein stability and the impairment in the PICT-1–PTEN regulatory unit may become a causative factor in human tumor(s).
INTRODUCTION
Phosphoinositide 3-kinase (PI3K) plays pivotal roles in regulating cell proliferation and apoptosis by producing the lipid second messenger phosphatidylinositol 3,4,5-trisphosphate (PIP3), in response to various stimuli (Cantley, 2002). The resulting PIP3 lipid product activates diverse signaling pathways by recruiting its downstream effector proteins to the plasma membrane through its binding to specific protein domains, such as pleckstrin homology domain (Lemmon and Ferguson, 2000; Vanhaesebroeck et al., 2001). The tumor suppressor phosphatase and tensin homolog deleted on chromosome 10 (PTEN) dephosphorylates PIP3 to negatively regulate PI3K/PIP3 signals (Maehama and Dixon, 1998; Myers et al., 1998; Cantley and Neel, 1999); therefore, PTEN inactivation results in the hyperactivation of PIP3 downstream signals and provides enormous impact on diverse cellular functions, leading to tumorigenesis (Di Cristofano and Pandolfi, 2000; Leslie and Downes, 2004). Moreover, inactivation of PTEN has been found associated with the hyperactivation of PIP3 downstream signals in a variety of human tumors, including glioblastoma and endometrial carcinoma (Ali et al., 1999). Because accumulating body of evidence unequivocally indicates the biological significance of PTEN and its relevance to tumorigenesis, it has been speculated that the impairment in the regulatory system for PTEN function may influence PTEN-governed cellular functions and become potential causative factor in tumorigenesis. In support of this notion, recent studies have revealed that several PTEN-binding proteins and kinases that directly phosphorylate PTEN regulate PTEN protein stability, enzymatic activity, and localization in cells (Vazquez et al., 2000; Wu et al., 2000a, b; Vazquez et al., 2001; Miller et al., 2002; Das et al., 2003; Lu et al., 2003; Sumitomo et al., 2004; Li et al., 2005; Valiente et al., 2005); however, their contribution to tumorigenesis remains elusive.
Protein interacting with the carboxy terminus-1/glioma tumor suppressor candidate region 2 (PICT-1/GLTSCR2) gene was originally identified as a candidate tumor suppressor gene located at human chromosome 19q13.32 (Smith et al., 2000b). Although several groups have proven that the 19q13.32 locus is frequently altered in a variety of human tumors, a tumor suppressor gene(s) specifically encoded in this region has yet to be identified (Smith et al., 2000a, b; Mora et al., 2001; Hartmann et al., 2002). We recently unveiled that PICT-1 was able to bind to PTEN and was required for maintaining PTEN protein stability in cells (Okahara et al., 2004). PICT-1 inactivation induced by RNA interference (RNAi) renders PTEN protein unstable and leads to a rapid degradation of PTEN in cells (Okahara et al., 2004). These observations raise the possibility that a loss of PICT-1 function may induce the activation of PI3K/PIP3-mediated signals through the inactivation of PTEN, implicating that PICT-1 may function as the tumor suppressor locating at 19q13.32 locus. In this study, we demonstrated that the PICT-1 inactivation promoted both anchorage-dependent and anchorage-independent cell proliferation through the activation of PIP3 downstream effectors. Moreover, the expression of PICT-1 was impaired with significant frequency in human neuroblastoma, in which PTEN down-regulation was associated with impaired PICT-1 expression. Our findings provide insight into an intimate link between impaired PTEN regulation and human tumor(s).
MATERIALS AND METHODS
RNA Interference Constructs
Twenty-one- or 23-nucleotide small interfering RNAs (siRNAs) with UU overhangs at both 3′ ends were prepared as described previously (Yu et al., 2002). Target sequences of PIC247, PIC749, and PT1084 siRNAs corresponded to nucleotides 247–267 and 749–769 of human PICT-1/GLTSCR2 (GenBank accession no. AF182076) and nucleotides 1084–1106 of human PTEN (GenBank accession no. U92436), respectively. GFP5 control siRNA was described previously (Yu et al., 2002). For the construction of a gene silencing vector for human PTEN (PTEN/pSilencer), oligo DNA encoding short hairpin RNA that shares core target sequence with PT1084 siRNA was cloned into pSilencer 3.1-H1 hygro vector (Ambion, Austin, TX) according to the manufacturer's protocol. A gene silencing vector for PICT-1/GLTSCR2 (GLT318SH/pSilencer) was described previously (Okahara et al., 2005). For the construction of gene silencing vectors for mouse Pict-1, Pic675/pSilencer, and Pic1343/pSilencer, oligo DNAs encoding short hairpin RNAs that target nucleotides 675–695 and nucleotides 1343–1363 of mouse Pict-1 (GenBank accession no. NM_133831), respectively, were cloned into pSilencer 3.1-H1 hygro vector.
Cell Culture and Transfections
HeLa cervical carcinoma cells and U87MG glioblastoma cells were maintained at 37°C with 5% CO2 in DMEM supplemented with 5% fetal bovine serum, 50 U/ml penicillin, and 50 μg/ml streptomycin. Transfections of cells cultured on ø35-mm dish (1.6–2.0 × 105 cells) were performed using RNAiFect (QIAGEN, Valencia, CA) or FuGENE6 (Roche Diagnostics, Indianapolis, IN) according to the manufacturers' protocol. Either RNAiFect (9 μl) with 2 μg of siRNA or FuGENE6 (3 μl) with 2.5 μg of plasmid DNA was used for each transfection. NIH3T3 cells were maintained at 37°C with 5% CO2 in DMEM supplemented with 10% calf serum, 50 U/ml penicillin, and 50 μg/ml streptomycin. Lipofectamine 2000 (Invitrogen) was used for the transfection of NIH3T3 cells according to the protocol deposited on manufacturer's Web site (www.invitrogen.com).
Immunoblots
To prepare samples for immunoblot analyses, cells/proteins were precipitated in 10% trichloroacetic acid; then, precipitates were collected, washed, and dissolved in the solubilizing solution consisting of 9 M urea, 2% Nonidet-P40, and 65 mM dithiothreitol. After the determination of protein concentration, SDS, bromophenol blue, and Tris base were added to the lysate to 2.5%, 0.1%, and 20 mM, respectively. Immunoblots were conducted as described previously (Okahara et al., 2004, 2005). Antibodies used were anti-PICT-1/GLTSCR2 (Okahara et al., 2005), anti-PTEN (Cascade Bioscience, Winchester, MA), anti-actin (Sigma-Aldrich, St. Louis, MO), anti-phospho-Akt [S473] (Cell Signaling Technology, Beverly, MA), anti-Akt1/2 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-phospho-glycogen synthase kinase (GSK)3β [S9] (Cell Signaling Technology), anti-phospho-p70 S6 kinase (S6K) [T389] (Cell Signaling Technology), anti-cleaved caspase-3 [D175] (Cell Signaling Technology), and anti-p70 S6K (Cell Signaling Technology). The relative intensity of immunoreactive bands was measured by NIH Image 1.62 (http://rsb.info.nih.gov/nih-image/). Typical images from repeated experiments are represented in each figure.
Proliferation Assay
After the transfection with pSilencer or PTEN/pSilencer, HeLa cells were cultured and expanded for 7–14 d in the presence of 300 μg/ml hygromycin B to enrich cells harboring the silencing constructs. These transformants or U87MG cells were further transfected with either PIC247 or GFP5 siRNA and then cultured for 3 d. Cells were trypsinized and seeded onto 96-well plates (1.0 × 104 cells/well) with serum-free medium, followed by the incubation for 4 h to allow cells settle onto the wells. Proliferation assay was conducted in sextuplicate by using Cell Proliferation Kit II (Roche Diagnostics) according to the manufacturer's protocol. Briefly, after the addition of chromogen solution, cells were incubated at 37°C for 2 h to allow color develops; then, absorbance at 450 and 655 nm was measured. Unpaired Student's t test was used to calculate statistical significance, and typical data from three independent experiments are represented.
Apoptosis Assay
HeLa cells transfected with siRNA (GFP5, PIC749, or PT1084) were cultured for 2 d, and then 2.0 × 104 cells were seeded onto a coverslip (ø15 mm). After the incubation for 8–12 h to allow cells settle onto the coverslip, the cells were treated with 1 μM staurosporine for 4 h, serum starved for 24 h, or left untreated. Apoptotic cells were evaluated by the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) method using DeadEnd fluorometric system (Promega, Madison, WI) according to the manufacturer's protocol. Propidium iodide was used for the counterstaining and at least 500 cells were examined for each evaluation. Typical data from four independent experiments are represented.
Soft-Agar Assay
After the transfection with pSilencer or GLT318SH/pSilencer, HeLa cells were cultured and expanded for 7–14 d in the presence of 300 μg/ml hygromycin B. Then, the cells (2.0 × 105 cells) were suspended in 3 ml of top agar (DMEM containing 2% fetal bovine serum, 300 μg/ml hygromycin B, and 0.4% SeaPlaque GTG agarose [Cambrex Bio Science Walkersville, Walkersville, MD]) and added onto prelayered bottom agar (3 ml of DMEM containing 2% fetal bovine serum, 300 μg/ml hygromycin B, and 0.5% SeaPlaque GTG agarose) in a ø60-mm dish. For the NIH3T3 cells, cells were transfected with pSilencer, Pic675/pSilencer, or Pic1343/pSilencer, and then the same assay was performed except using hygromycin at 100 μg/ml, fetal bovine serum at 10%, bottom agar at 0.5%, and top agar at 0.4%. After the incubation at 37°C with 5% CO2 for 16 d, the diameter of colonies was measured. Unpaired Student's t test was used to calculate statistical significance.
Human Neuroblastoma Specimens
All tumor samples were collected through institutional review board-approved protocol after obtaining informed consents. RNA samples from randomly selected 44 patients with neuroblastoma were transcribed into cDNA using Moloney murine leukemia virus reverse transcriptase (Toyobo Engineering, Osaka, Japan), followed by polymerase chain reaction (PCR) analyses. To prepare protein samples, sectioned frozen tumor specimens were homogenized in 10% trichloroacetic acid at 4°C; then, precipitates were dissolved and used for the immunoblot analyses as described above.
Polymerase Chain Reaction
To analyze the expression of PICT-1 and PTEN transcripts, PCR was conducted in a 10-μl reaction mixture by using 0.2 μg of cDNA as a template and PrimeSTAR polymerase (Takara, Kyoto, Japan). Primer sequences used for PTEN were CCTTTTGAAGACCATAACCCACC (forward) and ATCACCACACACAGGTAACGGC (reverse); for PICT-1, CATTCCAGGAGCTGTGCGA (forward) and GCGAGTCTCCGGCATCTG (reverse); for actin, GGAGAAAATCTGGCACCACACCT (forward) and AGGAAGGAAGGCTGGAAGAGTG (reverse); and for mouse Pict-1, AGGCGAAAGGAGGAGTTA (forward) and TTCTGCCTCTTTCTCACG (reverse).
RESULTS
PICT-1 Knockdown Activates PI3K/PIP3 Signals
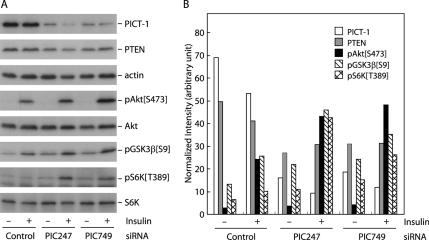
In the previous study, we demonstrated that PICT-1 stabilized PTEN protein in cells and a loss of PICT-1 rendered PTEN protein unstable, leading to a decrease in PTEN protein level in MCF7 breast carcinoma cells (Okahara et al., 2004). Because PTEN constrains the PI3K/PIP3 signaling, these observations raise the possibility that the PICT-1 loss activates PIP3 downstream signals by virtue of the PTEN down-regulation. To explore this possibility, we knocked down PICT-1 in PICT-1– and PTEN-expressing cells and examined the effect of the knockdown on PI3K/PIP3 signals. As shown in Figure 1, transfection of PICT-1-targeted siRNAs (PIC247 and PIC749) into HeLa cells induced robust reduction (79 and 75%, respectively) in PICT-1 protein levels. The residual PICT-1 protein observed in PIC247- and PIC749-treated samples is likely to be derived from cells into which siRNAs were not effectively delivered, because the transfection efficiency was ∼70% under this condition (our unpublished data). Levels of PTEN protein were concomitantly decreased (36 and 32%, respectively) after the PICT-1 knockdown; this observation was comparable to our previous results observed in MCF7 cells (Okahara et al., 2004). Insulin-induced phosphorylation of Akt at serine-473, which reflects the activation of the PIP3 signal and is required for Akt kinase activity, was significantly enhanced in PICT-1-knocked down cells, compared with that in control cells (Figure 1). As well as the insulin-induced Akt phosphorylation, consequent phosphorylation of GSK3β at serine-9, a direct phosphorylation site for Akt, was also enhanced by the PICT-1 knockdown (Figure 1). Most strikingly, in PICT-1-knocked down cells, insulin stimulation induced robust phosphorylation of p70 S6K at threonine-389, a phosphorylation site for another Akt downstream kinase, mammalian target of rapamycin; whereas control cells displayed very small increase in the phosphorylation under this condition (Figure 1). These results clearly indicate PICT-1 plays a role in PI3K/PIP3 signals and suggest that PICT-1 functions as a negative regulator for this signaling pathway.
Figure 1.
PICT-1 knockdown induces PTEN down-regulation and activation of PIP3 downstream signals. HeLa cells were transfected with GFP5 siRNA (Control) or PICT-1–directed siRNAs (PIC247 and PIC749) and cultured for 2 d. After the serum-starvation for 24 h, cells were stimulated with 0.5 μg/ml insulin for 3 min (±) or left untreated (−). Cell lysates were prepared and subjected to immunoblot analyses by indicated antibodies as described in Materials and Methods. (A) The band intensity of PICT-1, PTEN, pAkt[S473], pGSK3β [S9], and pS6K[T398] was measured by NIH Image 1.62. (B) Values normalized to that of actin are represented.
PICT-1 Knockdown Promotes Cell Proliferation and Suppresses Apoptosis in PTEN-dependent Manner
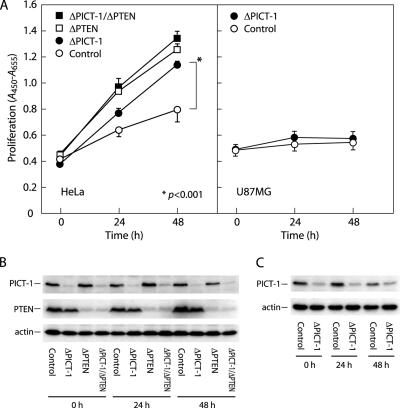
Several studies have defined that a modulation of PI3K/PIP3 signals primarily affects cell proliferation and apoptosis. Therefore, we next tested whether the proliferation would be influenced by the PICT-1 knockdown. HeLa cells were subjected to the RNAi for PICT-1, PTEN, or both simultaneously, and their proliferation was then monitored by colorimetric 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide proliferation assay. Control cells (GFP5 siRNA-transfected cells) exhibited slow proliferation under low- and no-serum conditions (Figure 2; our unpublished data), and the proliferation was significantly promoted (1.7-fold at 48 h) by siRNA-mediated PICT-1 knockdown (Figure 2; our unpublished data). Similar enhancement in the proliferation was observed in all cases in which PICT-1 was knocked down by several different siRNAs (Supplemental Figure S1), excluding the possibility of “off-target” effect of PICT-1–targeted siRNAs. These results, together with the result that forced expression of PICT-1 suppressed HeLa cell proliferation (Supplemental Figure S2), suggest the critical role of PICT-1 in regulating cell proliferation. Knockdown of PTEN by the vector-based RNAi as well as siRNA-mediated RNAi (our unpublished data) in HeLa cells also promoted the proliferation; however, additional knockdown of PICT-1 over the PTEN-knocked down cells showed no further effect on the proliferation (Figure 2). Impact of the PICT-1 knockdown on HeLa cell proliferation thus seemed to depend on the existence of PTEN. As supporting evidence for this possibility, knockdown of PICT-1 in PTEN-null U87MG glioblastoma cells also exhibited no effect on their proliferation (Figure 2); reintroduction of PTEN in PTEN-null PC3 cells restored the effect of PICT-1 knockdown on cell proliferation (Supplemental Figure S3). We further tested several cell lines and observed same dependency of the PICT-1 effect onto the PTEN status (our unpublished data).
Figure 2.
Effects of PICT-1 and PTEN knockdown on the proliferation of HeLa and U87MG cells. (A) HeLa cells (left) were transfected with pSilencer (circles; Control and ΔPICT-1) or PTEN/pSilencer (squares; ΔPTEN and ΔPICT-1/ΔPTEN) and cultured in the presence of hygromycin B. Cells harboring the gene silencing construct were further transfected with GFP5 siRNA for control knockdown (open symbols; Control and ΔPTEN) or PIC749 siRNA for PICT-1 knockdown (closed symbols; ΔPICT-1 and ΔPICT-1/ΔPTEN). The proliferation at indicated time was monitored as described in Materials and Methods. U87MG cells (right) were transfected with GFP5 (open circles; Control) or PIC247 (closed circles; ΔPICT-1) siRNAs and the proliferation was monitored. Mean ± SD from sextuplicated experiment is represented. HeLa cell lysates (B) and U87MG cell lysates (C) were prepared and subjected to immunoblot analyses by indicated antibodies as described in Materials and Methods.
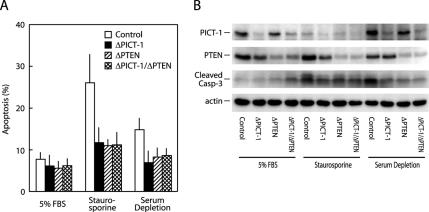
In addition to the effect on cell proliferation, the PICT-1 knockdown affected apoptosis in the similar PTEN-dependent manner. As shown in Figure 3, staurosporine, a protein kinase inhibitor, induced robust apoptotic cell death (3.3-fold increase) when evaluated by the TUNEL method, and serum depletion for 24 h also induced substantial increase (1.8-fold) in the apoptosis (Figure 3A). Cleavage of caspase-3, which indicated its activation, was also induced by staurosporine and serum depletion associated with increased apoptosis (Figure 3B). Knockdown of PICT-1 clearly suppressed both apoptosis and caspase-3 activation induced by staurosporine and serum depletion (Figure 3). It is of note that staurosporine treatment (4 h) also induced robust down-regulation of PICT-1 by unknown mechanism; however, 4-h incubation may not be enough to induce PTEN down-regulation even after complete disappearance of PICT-1 (Figure 3B). The PTEN knockdown also suppressed apoptosis and caspase-3 activation; however, combinational knockdown of PICT-1 and PTEN displayed no additional effect on the suppression, similar to their effects on cell proliferation (Figures 2 and 3). These observations collectively indicate that PICT-1 may be involved in cell proliferation and survival signals and may function through regulating PTEN.
Figure 3.
PICT-1 knockdown induces decreased susceptibility to apoptotic cell death. (A) HeLa cells were transfected with GFP5 (Control; open columns), PIC749 (ΔPICT-1; closed columns), PT1084 (ΔPTEN; hatched columns), or a combination of PIC749 and PT1084 (ΔPICT-1/ΔPTEN; cross-hatched columns) and cultured for 2 d. Cell were then transferred onto a coverslip and incubated in the presence of 5% serum or 1 μM staurosporine or in the absence of serum as indicated. Apoptosis was evaluated by the TUNEL method as described in Materials and Methods. All data represent mean ± SD. (B) Cell lysates were prepared and subjected to immunoblot analyses by indicated antibodies as described in Materials and Methods.
Tumorigenic Transformation by the PICT-1 Inactivation
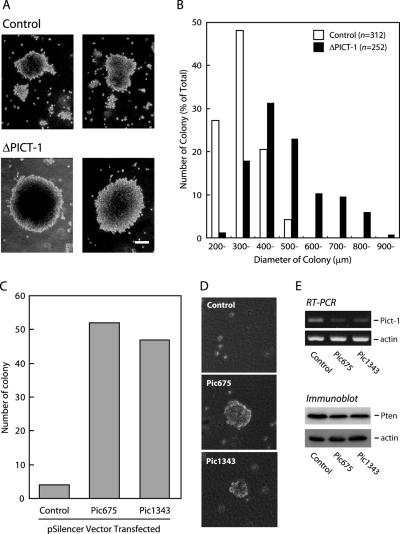
Tumor cells usually display anchorage-independent growth, whereas nontransformed cells do not; therefore, this characteristic is commonly used as a criterion for tumorigenic transformation of cells. One question arising here is whether a loss of PICT-1 is responsible for the alteration in this characteristic, because PTEN, of which stability is regulated by PICT-1, is known to suppress the tumorigenic transformation. To address this question, we used colony formation assay to evaluate the effect of the PICT-1 knockdown on anchorage-independent growth. HeLa cells exhibited very slow growth under low-serum (2%) condition and only formed tiny colonies in the soft-agar (Figure 4A). To accomplish the knockdown of PICT-1 during 16-d culture, we transfected HeLa cells with PICT-1 short hairpin RNA-encoded vector (GLT318SH/pSilencer), which was previously shown to knock down PICT-1 effectively (Okahara et al., 2005). Because the pSilencer vector contained hygromycin resistance gene, cells harboring the vector thereby grew in the soft-agar in the presence of hygromycin B. As shown in Figure 4, A and B, the GLT318SH/pSilencer-transfected cells conspicuously formed large colonies (average diameter 527 μm) in the soft-agar, whereas control vector-transfected cells barely formed small colonies (average diameter 349 μm). The number of colonies formed in the soft-agar was comparable in both control and PICT-1-knocked down cells, indicating comparable transfection efficiency. It is of note that the knockdown of PICT-1 in PTEN-positive MCF7 cells also promoted the colony formation in the soft-agar and the PTEN knockdown also increased colony formation activity (Supplemental Figure S4). We further tested the effect of PICT-1 knockdown on tumorigenic transformation in noncancerous NIH3T3 cell (Figure 4, C–E). Knockdown of mouse Pict-1 by two different RNAi constructs (Pic675/pSilencer and Pic1343/pSilencer) strikingly promoted colony formation in the soft-agar, whereas control cells formed few very tiny colonies under this condition (Figure 4, C–E). Although we cannot conclude that PICT-1 functions as a critical tumor suppressor solely from these observations, it is pronounced that PICT-1 exerts its effect on tumorigenic transformation in vitro.
Figure 4.
PICT-1 knockdown promotes anchorage-independent growth in soft-agar. (A and B) HeLa cells were transfected with pSilencer (Control) or GLT318SH/pSilencer (ΔPICT-1), and then colony formation in the soft-agar was assayed as described in Materials and Methods. (A) Typical images of formed colonies of control cells (Control; top) and ΔPICT-1 cells (ΔPICT-1; bottom). Bar, 200 μm. (B) Distribution of colony diameter from control cells (n = 312) and ΔPICT-1 cells (n = 252) is represented as a histogram. (C–E) NIH3T3 cells were transfected with pSilencer (Control), Pic675/pSilencer (Pic675), or Pic1343/pSilencer (Pic1343). (C) Number of colonies formed after 16-d culture was determined. Typical images of formed colony are represented in D. (E) Protein and RNA fractions were prepared from each transfectant and subjected to immunoblot analysis and reverse transcription (RT)-PCR to detect indicated protein and transcript, respectively.
Impaired PICT-1 Function in Human Neuroblastoma
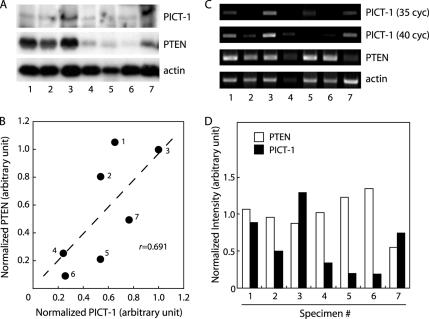
The aforementioned in vitro effects of the PICT-1 knockdown give rise to the possibility that a loss of PICT-1 function participates in the genesis of tumors and/or tumor progression through the PTEN down-regulation. In support of this notion, genetic studies have indeed demonstrated that human chromosome 19q13.32 locus where PICT-1 gene locates was frequently altered in a variety of human tumors, including low-grade glioma and neuroblastoma (Smith et al., 2000a, b; Mora et al., 2001; Hartmann et al., 2002). Therefore, we next asked whether the PTEN down-regulation would be associated with impaired PICT-1 expression in human tumors. To address this question, we first analyzed expression levels of PICT-1 and PTEN proteins and their correlation in human neuroblastoma specimens (Figure 5, A and B). Among seven specimens we tested, three specimens (4–6) displayed nearly complete loss of PTEN protein expression (Figure 5); although these specimens retained PTEN mRNA expression to an extent similar to others (Figure 5, C and D). Immunoblot and RT-PCR analyses revealed that these specimens showed lower PICT-1 protein/mRNA expression compared with the others. Furthermore, expression levels of PICT-1 and PTEN proteins from seven specimens exhibited significant correlation (r = 0.691) (Figure 5B). These results suggest that PICT-1 regulates PTEN protein levels and that PICT-1 inactivation leads to the PTEN down-regulation in vivo, reflecting the in vitro observations (Figure 1 and Okahara et al., 2004).
Figure 5.
Expression of PICT-1 and PTEN in human neuroblastomas. Protein and RNA fractions were prepared from seven human neuroblastoma specimens (1–7) and subjected to immunoblot analysis (A and B) and RT-PCR (C and D), respectively. (A) For the immunoblot analysis, 20 μg of proteins for each sample was used to detect PICT-1 and PTEN protein expression. (B) The relative intensity of immunoreactive bands was measured by NIH Image 1.62, and normalized values of PICT-1 and PTEN proteins (to actin) are plotted. Pearson r (r = 0.691) is represented in the plot. (C) Expression of PICT-1, PTEN, and actin transcripts was analyzed by RT-PCR as described in Materials and Methods. (D) Signal intensity of PCR products was measured by NIH Image 1.62, and values normalized to that of actin are represented.
We further analyzed the expression of PICT-1 transcript by using RNA samples from randomly selected 44 neuroblastomas, including 17 stage 1 tumors, seven stage 2 tumors, four stage 3 tumors, and 14 stage 4a/4s tumors; tumor stage of others was not defined/confirmed (see Supplemental Table S1 for details). As expected, these tissues showed aberrant expression of PICT-1 (loss of expression or altered splicing) with significant frequency (Figure 6); six in stage 1 tumors (35%), two in stage 2 tumors (29%), two in stage 3 tumors (50%), and eight in stage 4a/4s tumors (57%). In contrast, the loss of PTEN transcript expression was observed only in four of 44 specimens (Figure 6), supporting previous evidences of low frequent PTEN deletion (0–5%) in human neuroblastomas (Moritake et al., 2001; Munoz et al., 2004). These observations, which indicate frequent inactivation of PICT-1 gene expression in neuroblastoma and the role of PICT-1 in regulating cellular functions, suggest that PICT-1 is a potential causal factor in particular type of human tumor, such as neuroblastoma.
Figure 6.
Aberrant expression of PICT-1 transcript in human neuroblastomas. PCR analyses were performed to detect PICT-1 (top) and PTEN (middle) transcript levels in human neuroblastoma samples. Actin expression (bottom) was used as an internal control. RNA from MCF7 cells was used as a positive control (PC). M, standard size marker.
DISCUSSION
In this study, we have demonstrated that PICT-1 down-regulation in HeLa cells induces the activation of PIP3 downstream kinases and promotes cell proliferation and survival (Figures 1–3). These results, together with our previous observations, suggest that PTEN down-regulation seems to be the primary effect of the PICT-1 knockdown and major cause of these phenomena. We previously observed that the PICT-1 knockdown in MCF7 cells reduced the phosphorylation of PTEN at serine-380, one of the phosphorylation sites in the carboxyl-terminal region of PTEN, and that the reduction in the serine-380 phosphorylation was critical for the PTEN protein stability. Several studies have shown that the carboxyl-terminal phosphorylation of PTEN, including the serine-380 phosphorylation, has diverse effects in addition to promoting PTEN protein stability (Vazquez et al., 2000; Vazquez et al., 2001; Miller et al., 2002; Das et al., 2003; Valiente et al., 2005); therefore, the underlying biochemistry of how the PICT-1 knockdown exerts its effect onto cell functions could be very complex. For example, the carboxyl-terminal phosphorylation is absolutely required to maintain the cellular level of PTEN; however, the phosphorylation also inhibits PTEN phosphatase activity and targeting to the plasma membrane, both of which are essential for its biological function. Reduced phosphorylation resulted from the PICT-1 inactivation could augment the PTEN function by facilitating its membrane localization and the phosphatase activity. However, it is also pronounced that the PICT-1 inactivation eventually unstabilizes PTEN protein and induces its rapid degradation. Extensive decrease in PTEN protein levels may overcome the stimulatory effects caused by reduced phosphorylation, thereby resulting in a loss of PTEN function. It is of note that the effect of the PICT-1 knockdown on the serine-380 phosphorylation in HeLa cells was less obvious, compared with that observed in MCF7 cells (our unpublished data). Although the reason for this discrepancy is unknown, this might contribute to shift the balance in favor of the inhibition toward the PTEN function by the PICT-1 knockdown. Our result indeed indicates that relatively small change (30–40% reduction; Figure 1) in the expression level of PTEN protein seemed to be enough to down-regulate PTEN function and give severe impact on the PIP3 signals in HeLa cells. This observation further indicates that PTEN functions in a dose-dependent manner in HeLa cells, which seem to be very sensitive to the alteration in PTEN protein levels. In support of this notion, haploinsufficiency of PTEN has been recently suggested to affect several signaling pathways and tumor progression in mouse models (Di Cristofano et al., 1998, 1999; Sun et al., 1999; Kwabi-Addo et al., 2001; Fox et al., 2002; Xiao et al., 2002; Moody et al., 2004; Ma et al., 2005; Manning et al., 2005), indicating a dose-dependent effect of PTEN on diverse cellular functions in vivo. These data collectively raise the possibility that the regulatory system for PTEN protein levels, such as the PICT-1–PTEN system, may function as more dynamic regulatory system and participate in PTEN-mediated diverse signaling pathways in addition to maintaining PTEN protein in cells.
We have also demonstrated that PICT-1 down-regulation promotes the tumorigenic transformation of cells (Figure 4) and that impaired PICT-1 expression is associated with PTEN down-regulation in human neuroblastoma (Figure 5). Intriguingly, PTEN gene disruption is uncommon and not the major underlying cause of human neuroblastoma (Moritake et al., 2001; Munoz et al., 2004), whereas previous report (Mora et al., 2001) and our observations (Figures 5 and 6) indicate frequent inactivation of PICT-1 gene expression in this tumor. This mutually exclusive tissue/tumor specificity represented by PICT-1 and PTEN lesions may expand and emphasize the importance of PTEN in tumorigenesis. The impairment in the PICT-1 gene may result in PTEN inactivation without any alteration in PTEN gene and potentially gives the same impact on cellular functions as impaired PTEN. Significant population of neuroblastoma indeed displays these characteristics (Figures 5 and 6), with which tumorigenesis might proceed in a PTEN-dependent manner despite no PTEN gene alteration. In addition, low or absent PTEN protein expression, even in the presence of PTEN mRNA, has been observed in several leukemia cell lines (Dahia et al., 1999). Furthermore, human nonsmall-cell lung cancer displays inconsistent correlation of PTEN protein down-regulation and genetic/epigenetic alteration in the PTEN gene (Marsit et al., 2005). It could be conceivable that impaired regulatory system for PTEN protein stability may participate in the genesis and/or the progression of these human tumors. These results together suggest a novel itinerary for the tumorigenic transformation that is PTEN dependent but no longer requires PTEN gene alterations. Expanded genetic analyses of PICT-1 to identify its implication in human tumors are currently under investigation. Further study will be required to understand the mechanism of how tumorigenesis proceeds by the PICT-1 inactivation and how PICT-1 controls cell fate in PTEN-dependent manner.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Scientific Research Fund of the Ministry of Education, Science, Sports and Culture of Japan. We thank Dr. Jack E. Dixon for providing PC3-Ec-PTEN cells. NIH3T3 cells were provided by Cell Resource Center for Biomedical Research Institute of Development, Aging and Cancer, Tohoku University, Sendai, Japan.
Footnotes
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-04-0301) on September 13, 2006.
REFERENCES
- Ali I. U., Schriml L. M., Dean M. Mutational spectra of PTEN/MMAC1 gene: a tumor suppressor with lipid phosphatase activity. J. Natl. Cancer Inst. 1999;91:1922–1932. doi: 10.1093/jnci/91.22.1922. [DOI] [PubMed] [Google Scholar]
- Cantley L. C. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Neel B. G. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl. Acad. Sci. USA. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahia P. L., et al. PTEN is inversely correlated with the cell survival factor Akt/PKB and is inactivated via multiple mechanisms in haematological malignancies. Hum. Mol. Genet. 1999;8:185–193. doi: 10.1093/hmg/8.2.185. [DOI] [PubMed] [Google Scholar]
- Das S., Dixon J. E., Cho W. Membrane-binding and activation mechanism of PTEN. Proc. Natl. Acad. Sci. USA. 2003;100:7491–7496. doi: 10.1073/pnas.0932835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristofano A., Kotsi P., Peng Y. F., Cordon-Cardo C., Elkon K. B., Pandolfi P. P. Impaired Fas response and autoimmunity in Pten+/− mice. Science. 1999;285:2122–2125. doi: 10.1126/science.285.5436.2122. [DOI] [PubMed] [Google Scholar]
- Di Cristofano A., Pandolfi P. P. The multiple roles of PTEN in tumor suppression. Cell. 2000;100:387–390. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- Di Cristofano A., Pesce B., Cordon-Cardo C., Pandolfi P. P. Pten is essential for embryonic development and tumour suppression. Nat. Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- Fox J. A., Ung K., Tanlimco S. G., Jirik F. R. Disruption of a single Pten allele augments the chemotactic response of B lymphocytes to stromal cell-derived factor-1. J. Immunol. 2002;169:49–54. doi: 10.4049/jimmunol.169.1.49. [DOI] [PubMed] [Google Scholar]
- Hartmann C., Johnk L., Kitange G., Wu Y., Ashworth L. K., Jenkins R. B., Louis D. N. Transcript map of the 3.7-Mb D19S112–D19S246 candidate tumor suppressor region on the long arm of chromosome 19. Cancer Res. 2002;62:4100–4108. [PubMed] [Google Scholar]
- Kwabi-Addo B., Giri D., Schmidt K., Podsypanina K., Parsons R., Greenberg N., Ittmann M. Haploinsufficiency of the Pten tumor suppressor gene promotes prostate cancer progression. Proc. Natl. Acad. Sci. USA. 2001;98:11563–11568. doi: 10.1073/pnas.201167798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon M. A., Ferguson K. M. Signal-dependent membrane targeting by pleckstrin homology (PH) domains. Biochem. J. 2000;350:1–18. [PMC free article] [PubMed] [Google Scholar]
- Leslie N. R., Downes C. P. PTEN function: how normal cells control it and tumour cells lose it. Biochem. J. 2004;382:1–11. doi: 10.1042/BJ20040825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., et al. Regulation of PTEN by Rho small GTPases. Nat. Cell Biol. 2005;7:399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]
- Lu Y., et al. Src family protein-tyrosine kinases alter the function of PTEN to regulate phosphatidylinositol 3-kinase/AKT cascades. J. Biol. Chem. 2003;278:40057–40066. doi: 10.1074/jbc.M303621200. [DOI] [PubMed] [Google Scholar]
- Ma L., Teruya-Feldstein J., Behrendt N., Chen Z., Noda T., Hino O., Cordon-Cardo C., Pandolfi P. P. Genetic analysis of Pten and Tsc2 functional interactions in the mouse reveals asymmetrical haploinsufficiency in tumor suppression. Genes Dev. 2005;19:1779–1786. doi: 10.1101/gad.1314405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T., Dixon J. E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- Manning B. D., Logsdon M. N., Lipovsky A. I., Abbott D., Kwiatkowski D. J., Cantley L. C. Feedback inhibition of Akt signaling limits the growth of tumors lacking Tsc2. Genes Dev. 2005;19:1773–1778. doi: 10.1101/gad.1314605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsit C. J., Zheng S., Aldape K., Hinds P. W., Nelson H. H., Wiencke J. K., Kelsey K. T. PTEN expression in non-small-cell lung cancer: evaluating its relation to tumor characteristics, allelic loss, and epigenetic alteration. Hum. Pathol. 2005;36:768–776. doi: 10.1016/j.humpath.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Miller S. J., Lou D. Y., Seldin D. C., Lane W. S., Neel B. G. Direct identification of PTEN phosphorylation sites. FEBS Lett. 2002;528:145–153. doi: 10.1016/s0014-5793(02)03274-x. [DOI] [PubMed] [Google Scholar]
- Moody J. L., Xu L., Helgason C. D., Jirik F. R. Anemia, thrombocytopenia, leukocytosis, extramedullary hematopoiesis, and impaired progenitor function in Pten+/− SHIP−/− mice: a novel model of myelodysplasia. Blood. 2004;15:4503–4510. doi: 10.1182/blood-2003-09-3262. [DOI] [PubMed] [Google Scholar]
- Mora J., Cheung N. K., Chen L., Qin J., Gerald W. Loss of heterozygosity at 19q13.3 is associated with locally aggressive neuroblastoma. Clin. Cancer Res. 2001;7:1358–1361. [PubMed] [Google Scholar]
- Moritake H., Horii Y., Kuroda H., Sugimoto T. Analysis of PTEN/MMAC1 alteration in neuroblastoma. Cancer Genet. Cytogenet. 2001;125:151–155. doi: 10.1016/s0165-4608(00)00378-2. [DOI] [PubMed] [Google Scholar]
- Munoz J., Lazcoz P., Inda M. M., Nistal M., Pestana A., Encio I. J., Castresana J. S. Homozygous deletion and expression of PTEN and DMBT1 in human primary neuroblastoma and cell lines. Int. J. Cancer. 2004;109:673–679. doi: 10.1002/ijc.20055. [DOI] [PubMed] [Google Scholar]
- Myers M. P., Pass I., Batty I. H., Van der Kaay J., Stolarov J. P., Hemmings B. A., Wigler M. H., Downes C. P., Tonks N. K. The lipid phosphatase activity of PTEN is critical for its tumor suppressor function. Proc. Natl. Acad. Sci. USA. 1998;95:13513–13518. doi: 10.1073/pnas.95.23.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okahara F., Ikawa H., Kanaho Y., Maehama T. Regulation of PTEN phosphorylation and stability by a tumor suppressor candidate protein. J. Biol. Chem. 2004;279:45300–45303. doi: 10.1074/jbc.C400377200. [DOI] [PubMed] [Google Scholar]
- Okahara F., Itoh K., Ebihara M., Kobayashi M., Maruyama H., Kanaho Y., Maehama T. Production of research-grade antibody by in vivo electroporation of DNA-encoding target protein. Anal. Biochem. 2005;336:138–140. doi: 10.1016/j.ab.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Smith J. S., et al. Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J. Clin. Oncol. 2000a;18:636–645. doi: 10.1200/JCO.2000.18.3.636. [DOI] [PubMed] [Google Scholar]
- Smith J. S., et al. A transcript map of the chromosome 19q-arm glioma tumor suppressor region. Genomics. 2000b;64:44–50. doi: 10.1006/geno.1999.6101. [DOI] [PubMed] [Google Scholar]
- Sumitomo M., Iwase A., Zheng R., Navarro D., Kaminetzky D., Shen R., Georgescu M. M., Nanus D. M. Synergy in tumor suppression by direct interaction of neutral endopeptidase with PTEN. Cancer Cell. 2004;5:67–78. doi: 10.1016/s1535-6108(03)00331-3. [DOI] [PubMed] [Google Scholar]
- Sun H., Lesche R., Li D. M., Liliental J., Zhang H., Gao J., Gavrilova N., Mueller B., Liu X., Wu H. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc. Natl. Acad. Sci. USA. 1999;96:6199–6204. doi: 10.1073/pnas.96.11.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente M., Andres-Pons A., Gomar B., Torres J., Gil A., Tapparel C., Antonarakis S. E., Pulido R. Binding of PTEN to specific PDZ domains contributes to PTEN protein stability and phosphorylation by microtubule-associated serine/threonine kinases. J. Biol. Chem. 2005;280:28936–28943. doi: 10.1074/jbc.M504761200. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B., Leevers S. J., Ahmadi K., Timms J., Katso R., Driscoll P. C., Woscholski R., Parker P. J., Waterfield M. D. Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- Vazquez F., Grossman S. R., Takahashi Y., Rokas M. V., Nakamura N., Sellers W. R. Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex. J. Biol. Chem. 2001;276:48627–48630. doi: 10.1074/jbc.C100556200. [DOI] [PubMed] [Google Scholar]
- Vazquez F., Ramaswamy S., Nakamura N., Sellers W. R. Phosphorylation of the PTEN tail regulates protein stability and function. Mol. Cell. Biol. 2000;20:5010–5018. doi: 10.1128/mcb.20.14.5010-5018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Hepner K., Castelino-Prabhu S., Do D., Kaye M. B., Yuan X. J., Wood J., Ross C., Sawyers C. L., Whang Y. E. Evidence for regulation of the PTEN tumor suppressor by a membrane-localized multi-PDZ domain containing scaffold protein MAGI-2. Proc. Natl. Acad. Sci. USA. 2000a;97:4233–4238. doi: 10.1073/pnas.97.8.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Dowbenko D., Spencer S., Laura R., Lee J., Gu Q., Lasky L. A. Interaction of the tumor suppressor PTEN/MMAC with a PDZ domain of MAGI3, a novel membrane-associated guanylate kinase. J. Biol. Chem. 2000b;275:21477–21485. doi: 10.1074/jbc.M909741199. [DOI] [PubMed] [Google Scholar]
- Xiao A., Wu H., Pandolfi P. P., Louis D. N., Van Dyke T. Astrocyte inactivation of the pRb pathway predisposes mice to malignant astrocytoma development that is accelerated by PTEN mutation. Cancer Cell. 2002;1:157–168. doi: 10.1016/s1535-6108(02)00029-6. [DOI] [PubMed] [Google Scholar]
- Yu J. Y., DeRuiter S. L., Turner D. L. RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc. Natl. Acad. Sci. USA. 2002;99:6047–6052. doi: 10.1073/pnas.092143499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.