Abstract
Adults provisioning dependent young are in conflict with their partners, who would prefer a greater level of effort, and with their offspring, who would prefer a greater supply of food. To what extent, then should adults negotiate their provisioning behaviour with other family members? We used experimental manipulations of brood size, and targeted playback of begging calls to determine the extent to which adult great tits Parus major adjust their provisioning rates in response to the behaviour of their partner and their brood. We found that males and females behaved similarly, both responding more to each other's behaviour than to chick calling. We also found that the degree to which adults negotiated their provisioning rates with each other varied between years. A review of the literature suggests that the extent of negotiation over provisioning is likely to vary not only between species of diverse taxa, but also between and within (this study) populations of the same species. We suggest that provisioning behaviour lies on a ‘negotiation continuum’, which describes the extent to which parents respond to the actions of other family members. We argue that an individual's location on the ‘negotiation continuum’ is determined partly by the extent to which it can physically respond to the behaviour shown by other members of the family and partly by the quality of information on offer.
Keywords: sexual conflict, parent–offspring conflict, sibling conflict, begging, biparental care, negotiation
1. Introduction
The amount of care that parents devote to young is typically limited by an associated cost (Trivers 1972; Gustafsson & Sutherland 1988; Gustafsson et al. 1995; Heaney & Monaghan 1995). As a result, offspring must compete for limited parental resources (Mock & Parker 1997) and either parent may attempt to offload the cost of care on their partner, by encouraging them to work harder (Lessells 1999). Thus, the stage is set for conflicts over the division of parental investment (Parker et al. 2002). When Hamilton's rule is used to calculate the extent to which optimal levels of investment differ between family members, three forms of evolutionary conflict are revealed (Hamilton 1964; Trivers 1974; MacNair & Parker 1979; Queller 1994; Mock & Parker 1997): sexual conflict, in which one parent prefers a greater level of investment than the other prefers to supply (Lessells 1999); interbrood conflict, in which present offspring attempt to extract investment which parents would prefer their future offspring to have (Trivers 1974); and intrabrood conflict, in which present offspring seek to obtain investment which parents wish to give to other present brood members (Macnair & Parker 1979; Mock & Parker 1997).
With conflicting interests, each parent faces the challenge of meeting the needs of their young without being exploited by excessively demanding offspring or a lazy partner. To what extent, then, do adults respond to other family members when negotiating their own provisioning rate at the nest? Empirical evidence spans the entire range of possibilities, but it is not clear why there is so much variation. At one extreme, adults appear to be entirely insensitive to the behaviour of other individuals, following rules for feeding young that are fixed and independent of their partner's or offspring's behaviour (e.g. Schwagmeyer et al. 2002; Laurien-Kehnen & Trillmich 2003). At the other extreme, adult provisioning behaviour seems to be highly sensitive to the actions of other family members. In several passerine species, for example, when nestlings beg with greater vigour, parents increase the frequency of food delivery at the nest (Smith et al. 1988; Kilner 1995; Ottoson et al. 1997; Kilner et al. 1999). Furthermore, the rate at which one parent supplies food can change the provisioning behaviour of its partner, independent of the nestlings' behaviour. Increased attentiveness to the brood by the mother, for example, may allow the father to relax his own provisioning rate (Houston & Davies 1985; Wright & Cuthill 1989; Markman et al. 1995; Ottoson et al. 1997) or it may induce him to work harder, if he thereby infers that the brood are in greater need, or of a greater quality (Hinde 2006; Johnstone & Hinde 2006).
The degree to which adults respond to others to negotiate provisioning rates at the nest is likely to have a profound influence on the outcome of the various intrafamilial conflicts (McNamara et al. 1999; Parker et al. 2002), but has seldom been considered beyond a dyadic interaction with either the partner or the offspring, either theoretically or empirically. The extent to which adults negotiate their behaviour with their partners and offspring is therefore unknown. This is what we aimed to quantify with the experiments on great tits Parus major described here. We manipulated cues that are known to affect parental work rates and that are under the control of one or more family members (table 1). Our analyses of the parents' response to our manipulations ranked the relative importance of these variables in influencing parental-provisioning rate, and so allowed us to determine the extent to which males and females respond to the behaviour of their partner and offspring when determining their own rate of provisioning at the nest.
Table 1.
The cues that we manipulated, their predicted effect on parental-provisioning rate, and the family member primarily in control of that cue.
| cue; increase in | effect on provisioning rate | under control of | references |
|---|---|---|---|
| brood size | positive | female | Smith et al. (1988), Wright & Cuthill (1990a,b), Martins & Wright (1993), Moreno et al. (1995), Richner et al. (1995), Verhulst & Tinbergen (1997), Wright et al. (1998), Sanz & Tinbergen (1999) and Neuenschwander et al. (2003) |
| chick vocalizations | positive | chicks (and female, §4) | Ottoson et al. (1997), Burford et al. (1998), Clark & Lee (1998), Price (1998), Wright (1998) and Kölliker et al. (2000) |
| male-provisioning rate | negative | male | Wright & Cuthill (1989) and Markman et al. (1995) |
| positive | Hinde (2006) | ||
| female-provisioning rate | negative | female | Wright & Cuthill (1989) and Markman et al. (1995) |
| positive | Hinde (2006) |
2. Material and methods
In April and May 1999, we ran experiments in Emily's Wood, Norfolk, an area covering 20 ha of mixed deciduous woodland (52°47′ N, 0°0.64′ W) and containing 90 ‘woodcrete’ nesting boxes (Schwegler brand type 2M). In April and May of 2000–2002, we continued experiments in Burnt Farm Plantation and the adjoining Short Nursery Plantation (52°23′ N, 0°0.04′ W), and Madingley Wood, Cambridge (52°22′ N, 0°05′ W). Both are areas of mixed deciduous woodland, separated by approximately 1 mile, covering a total area of 23 ha and containing 140 similar nest boxes. There was no effect of study site in these analyses (experiment 1: β=−0.91, confidence intervals: lower=−0.46, upper=0.21, p=0.51; experiment 2: β=−0.05, confidence intervals: lower=−0.62, upper=0.54, p=0.87).
(a) Experimental manipulations
We ran two different experiments; each ranked the relative influence of the mother, father and brood on nestling-provisioning rates by manipulating cues presented in table 1.
(b) Experiment 1
This experiment manipulated brood size and nestling begging calls (1999–2001; N=45 nests). On day 8 (hatching=day 0) chicks were swapped between nests to create brood sizes of 3, 5, 7, 9 and 11 (mean population brood size=6.7; Hinde 2006) with nests containing approximately half their own and half fostered chicks. Experimental brood sizes were allocated sequentially with respect to date where possible, and were therefore independent of date and original clutch size. Furthermore, brood size before experimental manipulation could not explain significant variation in adult behaviour (β=0.08, confidence intervals: lower=−0.06, upper=0.22, p=0.28). On day 9, when the parents visited the nest, the begging calls of each brood were supplemented with three playback treatments (described in detail later) of 1 h each: no playback, playback of two chicks begging and playback of four chicks begging. Brood sizes of nine received playback of two chicks begging only and brood sizes of eleven did not receive any playback treatments, as this would have mimicked a brood size larger than is encountered naturally. By separately manipulating brood size and the number of chicks heard calling, we could tease apart the relative influence of visual and vocal signals on adult provisioning behaviour (see also Kilner et al. 1999). Additionally, we measured the relative importance of partner-provisioning rate on provisioning frequency, using the statistical methods outlined later. Although experimental brood size and partner-provisioning rate were correlated, this was not at a level high enough to violate the assumptions of the model (Pearson's r=0.56, variance inflation factors: 1.67 and 1.60, respectively, tolerance statistics are 0.60 and 0.64, respectively; see Field 2000).
(c) Experiment 2
This experiment was designed to investigate experimentally the cause of any correlation between partner work rate and provisioning frequency detected with experiment 1. Nestling begging calls and partner-provisioning rate (2002, N=17 nests) were manipulated while holding brood size constant as described in detail elsewhere (Hinde 2006). In earlier analyses, Hinde (2006) showed that parents respond positively to partner-provisioning rates in the short term, independently of chick begging. Here, we present new analyses of the data to assess the relative importance of begging and partner work levels on provisioning for comparison with experiment 1. On day 2, nests were manipulated to create a brood size of seven (as in Brinkhof et al. 1999) so that each brood contained nestlings from two donor broods of the same age (three from one and four from the other), and none of their own chicks. On day 9, the begging calls of each brood were supplemented with three playback treatments of 1 h each: no playback, playback only to the male of four chicks calling and playback only to the female of four chicks calling. Therefore, for each adult, we could assess the independent influence of nestling begging calls and the provisioning rate of their partner on their own provisioning behaviour.
Begging call amplitude, number of gapes displayed, posture levels and prey size were not affected by playback (Hinde 2006).
(i) Playback protocol for both experiments
In 2000–2002, a dummy speaker, camera and microphone were positioned inside each nest box 8 days after hatching, and a hide set up approximately 12 m away. In 1999, only a speaker and microphone were used, since begging was not filmed and the observer sat approximately 15 m away without a hide. The dummy equipment was replaced before the experiment began on day 9. A miniature loud speaker (adapted from a small RS 250-687 speaker, as described by Davies et al. 1998) was concealed between the side of the nest and the inside wall of the nest box, at the same height as the chicks. Additionally, a miniature camera (2000–2002 only, adapted from an infrared security camera, Maplins) and microphone (Sony Electret Condenser Microphone ECM-T6) were held within the conical dome at the top of the nest box with magnets, so that the camera pointed directly down into the nest. The cables ran down to the ground through a groove in the side of the box front and connected to a video recorder (Sony Handycam DCR-TRV310E) in the hide.
Recordings of chick begging were broadcast either to both parents (experiment 1) or to the target parent (experiment 2) at each nest visit while the chicks in the nest were observed with the nest camera to be begging. Playback experiments were begun 30–60 min after setting up the equipment, when parents were visiting the nest regularly and showing no signs of hesitation or alarm. The volume was selected to be as similar as possible to that of the chicks in the nest, heard via headphones. Parents were subjected to the playback for three visits before observations began and there was a period of at least 30 min between treatments. Provisioning rates were scored from the hide during the experiments, and double-checked later from the videotapes in 2000–2002. The order in which playback treatments were run, and the time of day (morning versus afternoon) when they were performed were sequentially rotated within and between nests, respectively.
(ii) Making the playback tapes
For both experiments, playback tapes were made by recording begging calls at foreign nests during three parental visits to two and four chicks on day 9. Recordings were transferred to a computer and spliced together using the software package Canary 1.2.1 (Cornell Laboratory of Ornithology 1993) to create 15 s of begging which was looped and recorded continuously onto audiotape. Recordings from different foreign nests were used at each nest in experiment 1, and for each adult in experiment 2.
(iii) Effect of manipulation on begging scores and prey size
The number of gapes on display, begging posture and prey size were measured from video for 82 playback treatments at 33 nests in experiment 1 and 45 playback treatments at 15 nests in experiment 2. The call rate and amplitude (dB) were measured for 36 playback treatments at 15 nests in experiment 1 and 39 playback treatments at 13 nests in experiment 2, as in Hinde (2006). Measures for begging posture and call levels were taken per nest, since there was no difference in begging to males and females (p>0.33). Prey size was measured per adult.
Manipulations affected begging as intended, since a linear mixed effects model showed that the number of gapes on display was related to brood size (N=82, 33 nests with one to three playback treatments per nest, Wald/d.f.=96.54, p<0.001), but not to playback (Wald/d.f.=2.56, p=0.11). Mean begging posture was not related to brood size (N=82, 33 nests with one to three playback treatments per nest, Wald/d.f.=1.82, p=0.18) or playback (Wald/d.f.=3.28, p=0.07). Since the non-significant trends relating playback to the number of gapes on display and posture are in a negative direction, an increase in begging with playback could not have explained the positive parental response. Prey size was related to sex (N=164, 66 individuals at 33 nests with one to three playback treatments per nest, Wald/d.f.=6.15, p=0.013), since males brought larger prey than females, but not to brood size (Wald/d.f.=0.67, p=0.41) or playback (Wald/d.f.=0.52, p=0.47) and there were no significant interactions. Begging call rate was positively related to both brood size (N=36, 15 nests with one to three playback treatments per nest, Wald/d.f.=17.51, p<0.001), and playback (Wald/d.f.=13.83, p<0.001) as intended. Call amplitude was related to neither brood size (N=36, 15 nests with one to three playback treatments per nest, Wald/d.f.=2.2, p=0.34), nor playback treatment (Wald/d.f.=0.54, p=0.46). Hatch date, year and interactions were not significant in any of the above tests.
(d) Statistics
Data for each experiment were analysed separately with the statistical package R (R Development Core Team 2006). In experiment 1, brood size, playback treatment, partner-provisioning rate and hatch date were variates and parental sex and year were factors. In experiment 2, partner-provisioning rate, playback heard by the focal parent and playback heard by partner were variates and parental sex was a factor. Non-significant terms were sequentially deleted to yield the minimal model. In both experiments, a random factor (nest identity) was included to control for repeated measures at each nest. All continuous variables were standardized as Z-scores (Zar 1999) before analysis.
For each experiment, a linear mixed effects model was fitted using restricted maximum likelihood (REML) to compare the relative influence of each variable in the analysis on provisioning behaviour, by examining the slopes of the relationships between the dependent and independent variables. The relative influence of each independent variable was then assessed by calculating the change in Akaike's information criterion (AIC) when each significant variable was dropped from the minimal model, and when each non-significant variable was added to the minimal model. When comparing models, the model with the lowest AIC is the model with the best fit. For these comparisons, maximum likelihood was used to fit the mixed effects model.
A potential pseudoreplication problem may have arisen in these analyses, in that the provisioning behaviour of each adult could appear twice, once as the dependent variable and again as an independent variable, explaining partner-provisioning rate. To avoid this danger for each experiment, we randomly selected one parent from each nest so that for half of the nests female-provisioning rate was the dependent variable and male-provisioning rate the independent variable, while for the other half, the reverse was true. This procedure was repeated and for each analysis we present the average results from 1000 iterations.
The effect of brood size and playback on begging levels and prey size (see §2) were analysed in Genstat sixth edition (Genstat 2002) using a linear mixed effects model fitted using REML. Nest identity was a random factor and in each case hatch date, year and interactions were included in the full model.
3. Results
(a) Experiment 1
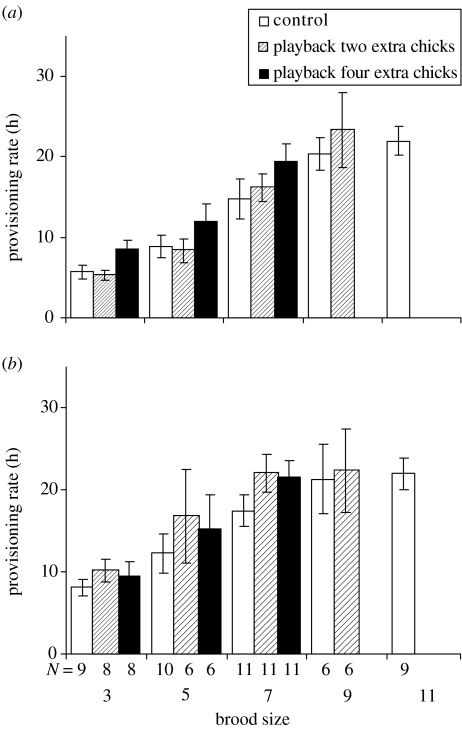
Both parents increased their provisioning rate to a similar extent when confronted with larger broods, and when exposed to playback of recorded nestling begging calls (figure 1), and males provisioned at higher levels than females. For both sexes, the most important factor determining provisioning behaviour was brood size (β and change in AIC table 2). Next came the positive response to partner-provisioning rate which varied between years (interaction table 2). Of least importance was the relative intensity of the begging call (table 2). All two-way interactions were investigated and found to be non-significant except for that between partner visit rate and year. Partner visit rate had a similar, positive effect on provisioning in 1999 and 2001, but was not important in 2000.
Figure 1.
Provisioning (per hour) by (a) females and (b) males to experimentally manipulated brood sizes during the control treatment (white bars), when recordings of two extra chicks calling were broadcast at the nest (hatched bars) and when recordings of four extra chicks calling were broadcast at the nest (black bars). Error bars represent standard errors of the mean. N=45 nests, with one, two or three playback treatments at each nest.
Table 2.
A mixed effects model (REML) showing the relationship between provisioning rate and brood size, partner-provisioning rate, playback of begging calls and parental sex. (Nest identity was included as a random factor. The change in AIC values was calculated by fitting models using maximum likelihood. Change in AIC values for the minimal model represent AIC value of model without the term; a greater negative value therefore indicates a better contribution to model fit. Change in AIC values for the full model shows the change in AIC value with the term added, so a greater negative value indicates a negative impact on model fit. All continuous variables were standardized as described in the text. All two-way interactions were investigated; all significant interactions are reported as well as non-significant interactions of interest. p values are given for continuous variables, although in all cases an AIC value of more than 2 is accepted as significant. For factor ‘year’ results are given in contrast to those for 1999, and for ‘sex’, results are given for males in contrast to females. (N=45 individuals at 45 nests, with one, two or three treatments at each nest. There was significant repeatability of provisioning rate within nests (ΔAIC=−22.23, p<0.001).).)
| β | lower confidence limit | upper confidence limit | change in AIC | p | |
|---|---|---|---|---|---|
| minimal model | |||||
| intercept | 0.29 | ||||
| brood size | 0.42 | 0.23 | 0.57 | −13.85 | <0.001 |
| partner prov. rate×year | −0.50 | −0.79 | −0.25 | −6.60 | significant |
| −0.01 | −0.34 | 0.27 | |||
| playback | 0.12 | 0.04 | 0.19 | −2.90 | 0.001 |
| sex | −0.39 | −0.67 | −0.13 | −2.43 | 0.004 |
| partner prov. rate | 0.55 | 0.32 | 0.85 | 0.02−14 | <0.001 |
| year | −0.07 | −0.41 | 0.29 | 2.56 | n.s. |
| −0.12 | −0.50 | 0.27 | |||
| terms dropped from model | |||||
| hatch date | −0.07 | −0.42 | 0.21 | 1.40 | 0.68 |
| sex (male)×brood size | −0.07 | −0.30 | 0.14 | −1.37 | 0.52 |
| partner prov. rate×brood size | −0.04 | −0.18 | 0.10 | −1.50 | 0.60 |
| brood size×playback | −0.04 | −0.13 | 0.05 | −1.10 | 0.39 |
| sex (male)×playback | −0.05 | −0.20 | 0.09 | −1.19 | 0.47 |
| partner prov. rate×playback | 0.01 | −0.07 | 0.08 | −1.51 | 0.94 |
| sex (male)×partner prov. rate | −0.09 | −0.42 | 0.22 | −0.79 | 0.60 |
(b) Experiment 2
This experiment tests the correlation between visit rate and partner visit rate found in experiment 1. Brood size was held constant and so its relative influence on provisioning rates could not be investigated. In all other respects, however, the results of this experiment supported those of experiment 1. Males again visited the nest more frequently with food than females, but the relative influence of the factors influencing provisioning did not differ significantly between the sexes (table 3). Partner-provisioning rate was the most important predictor of chick-feeding rate, with an increase in partner nest attendance causing an increase in provisioning by the focal parent (table 3). The focal parent responded to partner behaviour alone because variation in its provisioning rate could not be explained by the taped begging calls broadcast to its partner (table 3). The begging calls broadcast to the focal parent were less influential than the provisioning behaviour of its partner in determining its rate of food delivery at the nest (table 3).
Table 3.
A mixed effects model (REML) showing the relationship between provisioning rate and partner-provisioning rate, playback of begging calls heard by the focal parent, playback of begging calls heard by partner and parental sex, when begging calls were directed at one parent in each treatment. (Nest identity was included as a random factor. The change in AIC values was calculated by fitting models using maximum likelihood. Change in AIC values for the minimal model represent AIC value of model without the term; a greater negative value therefore indicates a better contribution to model fit. Change in AIC values for the full model shows the change in AIC value with the term added, so a greater negative value indicates a negative impact on model fit. All continuous variables were standardized as described in the text. All two-way interactions were investigated and were not significant. (N=17 individuals at 17 nests with three experimental treatments per nest (no playback, playback to focal parent and playback to partner). There was significant repeatability of provisioning rate within nest (ΔAIC=−11.38, p<0.001).).)
| β | lower confidence limit | upper confidence limit | change in AIC | p | |
|---|---|---|---|---|---|
| minimal model | |||||
| Intercept | −0.31 | −0.61 | −0.03 | 0.04 | |
| partner prov. rate | 0.61 | 0.50 | 0.74 | −24.32 | <0.001 |
| playback heard by focal bird | 0.23 | 0.16 | 0.31 | −6.56 | <0.001 |
| sex | 0.61 | 0.36 | 0.84 | −1.70 | <0.001 |
| terms dropped from model | |||||
| playback heard by partner | 0.09 | −0.06 | 0.25 | −0.62 | 0.28 |
| hatch date | −0.06 | −0.52 | 0.38 | 0.79 | 0.82 |
| playback heard by focal bird×playback heard by partner | 0.09 | −0.03 | 0.20 | −0.32 | 0.16 |
| sex×partner prov. rate | 0.20 | −0.16 | 0.54 | −0.18 | 0.27 |
| sex×playback heard by focal bird | 0.04 | −0.23 | 0.29 | −0.99 | 0.79 |
4. Discussion
The combined results of the two experiments show that for both adults, brood size had the greatest influence on brood-provisioning rate, followed by partner-provisioning rate (which varied between year), with nestling begging calls playing a relatively minor role in affecting the rate of food delivery at the nest (tables 2 and 3). We can use these findings to determine the parental provisioning rules, and the extent to which adults respond to behaviour of other family members when deciding their level of investment.
(a) Negotiated responses to partners and offspring
Our first conclusion from this snapshot of great tit family life is that males and females respond in a similar way to cues at the nest to determine their provisioning rate, each flexibly adjusting their feeding frequency in response to the size of their brood and to the behaviour of their partner and offspring. Both respond more to each other's behaviour than to changes in chick begging calls. In addition, we suggest that females have more influence on male visit rate than vice versa because females control clutch size (e.g. Kroodsma 1976), and thus play a major role in determining brood size, the principal determinant of male provisioning behaviour. Females might even influence their partner's provisioning through the begging behaviour of their young (tables 2 and 3; Kölliker et al. 2000), perhaps through the addition of small doses of androgen to the yolk before laying (Schwabl 1996). When determining the rate at which they bring food to the nest, males are therefore choosing to respond to female decisions made during egg-laying, as well as those made during nestling provisioning.
Both parents were more responsive to the provisioning behaviour of their partner than they were to offspring begging calls when adjusting their own supply of food to the nest. Even though we more than doubled the number of chicks heard calling in some experimental treatments, nestling begging intensity played a relatively minor role in determining adult provisioning behaviour in our study population. Perhaps, partner-provisioning rate offers a more reliable source of information about brood condition than is advertised with nestling begging vocalizations (Johnstone & Hinde 2006). If the costs of begging calls are relatively low, as is probable in a cavity-nesting species at low risk from attack by predators (Briskie et al. 1999), and with brief nest visits (Kilner 2001), then nestling begging calls are unlikely to relay the offspring's true condition accurately (Parker & MacNair 1979; Godfray 1991; Bergstrom & Lachmann 1998; Johnstone 1999). Adults would then do better to seek an alternative source of information about the value of their brood, such its size or the rate at which their partner is visiting the nest with food. It would be interesting to test whether adults are more responsive to offspring behaviour than partner behaviour (or brood size) in open-nesting species, where presumably loud begging calls are associated with a greater risk of attack by predators (Briskie et al. 1999). Similarly, it would be interesting to test whether parents feeding at open nests show greater variability from year to year in their response to nestlings than in their response to their partner's provisioning rate.
The information model (Johnstone & Hinde 2006) might explain why great tit parents varied from year to year in the extent to which they responded to partner visit rate, but maintained a similar degree of responsiveness to their nestlings year after year, since response to partner work rate is expected to vary according to the quality of information on offer.
(b) A ‘negotiation continuum’
Our experimental results provide clear evidence that adults respond to the behaviour of other family members when deciding how hard to work to raise a brood. Our results do not indicate how the underlying conflicts of interest within the family are resolved, but indicate the behavioural mechanisms that might be incorporated in theoretical analyses of conflict outcome. The relatively high degree of flexibility we have uncovered in both male and female provisioning rules cannot be captured by the traditional ‘sealed bid’ approach to modelling intrafamilial conflicts, which describes variation in behaviour on an evolutionary time-scale and so allows no variation in parental strategies in response to either the behaviour of partners or offspring. Great tit provisioning behaviour is better described by the ‘negotiation’ models developed by McNamara et al. (1999), which specify rules for responding to other family members in real time.
Nevertheless, the evidence to date suggests that not all species are as flexible as this in their response to the behaviour of other family members and, even within species, there can be variation both between and within (this study) populations in the degree to which provisioning rules are negotiated. For example, despite showing a comparatively weak reaction to taped begging calls broadcast at the nest, parents in our study population were relatively more responsive to offspring behaviour than those in a Swiss population of great tits, where males were apparently entirely insensitive to the begging calls of their young (Kölliker et al. 2000). We suggest that behaviour within families lies on a ‘negotiation continuum’, ranging from families in which behavioural rules are fixed and unchanged by the interactions of the different family members, to families in which behaviour is highly flexible.
The behavioural mechanisms underlying all three forms of family conflict (sexual, interbrood and intrabrood conflicts) can lie at any point on this continuum, but at present we can only make qualitative comparisons between existing studies because differing experimental methodologies may account for much of the variation in the results obtained. There is a wide range in flexibility of the provisioning rules that govern sexual conflict, with contrasting behaviours shown by house sparrows Passer domesticus, which appear to respond little to changes in partner work rate (Schwagmeyer et al. 2002) and great tits (Hinde 2006; this study), where partner behaviour is more influential. A similar pattern prevails with behaviours that mediate interbrood conflict. In some species, adults respond to the calls of their young when regulating the delivery of care (e.g. pigs Sus scrofa Weary et al. 1996; great tits Kölliker et al. 2000; meerkats Suricatta suricatta Manser & Avey 2000; superb fairy-wrens Malurus cyaneus Langmore et al. 2003), but in others they appear insensitive (e.g. Leach's Storm-petrel Oceanodroma leucorhoa Ricklefs 1987, 1992; red-winged blackbirds Agelaius phoeniceus Clark & Lee 1998). Likewise, offspring of some species adjust their begging to the quality of care on offer (coot Fulica atra Horsfall 1984; house sparrow Kedar et al. 2000; great tit Kölliker et al. 2000) while others continue to beg persistently, regardless (great-spotted cuckoo Clamator glandarius Redondo 1993). Even the behaviours that underpin intrabrood conflict may be negotiated by family members, although again the extent of negotiation is variable. For example, while parents of some species facultatively adjust the extent to which they intervene in sibling rivalry (e.g. blue-footed boobies Sula nebouxii Lougheed & Anderson 1999 and canaries Serinus canaria Kilner 2002), others do not (e.g. masked boobies Sula dactylatra Anderson 1995). Offspring may flexibly adjust their competitiveness to suit the quality or quantity of their rivals (e.g. tree swallows Tachycineta bicolor Leonard & Horn 1996; yellow-headed blackbirds Xanthocephalus xanthocephalus Price 1996; Price et al. 1996; Rodriguez-Girones et al. 1996; parasitoid wasps Copidosoma floridanum Giron et al. 2004) or always fight to the death, irrespective of the competition that they face (e.g. obligately siblidical birds Mock & Parker 1997; solitary parasitoid wasps Pexton & Mayhew 2002).
In negotiating their rules of engagement, family members benefit because they can incorporate information about the quality of other individuals that will affect the costs and benefits of their own actions (McNamara et al. 1999). Why, then, do family members vary in the flexibility of their behavioural rules? One possibility is that the extent to which rules are negotiated has been strategically chosen in relation to the quality of information on offer. For example, although male Smith's longspurs Calcarius pictus would benefit by directing care to offspring they have sired, they are unable to identify their young in broods of mixed paternity. They cannot preferentially allocate food to their offspring, and so divide food roughly evenly among the brood, essentially following a fixed provisioning rule with respect to kinship (Briskie et al. 1998). In other situations, the quality of information on offer is much higher and here we observe greater behavioural flexibility. When resources are scarce within a host caterpillar, for example, larvae of the parasitoid wasp C. floridanum gain most by targeting attacks on unrelated rivals and their behavioural rules vary according to information about kinship that is advertized in the larval extraembryonic membrane (Giron & Strand 2004; Giron et al. 2004).
A second explanation for variation in behavioural flexibility is that individuals vary in the extent to which they are physically able to adjust their behaviour in response to others (Parker et al. 2002; Royle et al. 2002). For example, a parasitoid wasp mother can neither physically intervene in the battles waged between her larval offspring (but see Pexton & Mayhew 2005) and nor does it seem likely that a pregnant mouse selectively directs resources to one of her developing embryos. Two key variables can thus explain a family member's location on the ‘negotiation continuum’: the degree to which it is able to physically respond to the behaviour of other individuals (its position on the ‘power continuum’, described by Royle et al. (2002) and Parker et al. (2002)) and the quality of information on offer from other family members. Just as with reproductive conflicts in animal societies (Sundstrom & Boomsma 2001; Beekman & Ratnieks 2003; Beekman et al. 2003), information and physical control combine to influence the behavioural rules followed by conflicting individuals and hence both factors play important roles in determining conflict outcome.
Acknowledgments
We would like to thank Richard Johnston, Megan Dickens and Philip Precey for help in the field and Glenn Harrison for technical support. We are very grateful to Rufus Johnstone for discussion and for Kavita Isvaran's invaluable statistical advice, and to Nick Davies, Trevor Price, Mathias Kölliker and two anonymous referees for helpful comments on the manuscript. CAH was funded by a Biotechnology and Biological Sciences Research Council studentship and a post-doctoral research associate position on Natural Environment Research Council grant NER/A/S/2002/00776. The research was funded by an Early Career Project Grant from the British Ecological Society awarded to RMK, who started the work when supported by a Royal Society Dorothy Hodgkin Research Fellowship, sponsored by the Wolfson Foundation, and completed it while in the tenure of a Royal Society University Research Fellowship.
References
- Anderson D.J. The role of parents in sibilicidal brood reduction of two booby species. Auk. 1995;112:860–869. [Google Scholar]
- Beekman M, Ratnieks F.L.W. Power over reproduction in social Hymenoptera. Phil. Trans. R. Soc. B. 2003;358:1741–1753. doi: 10.1098/rstb.2002.1262. doi:10.1098/rstb.2002.1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman M, Komdeur J, Ratnieks F.L.W. Reproductive conflicts in social animals: who has power? Trends Ecol. Evol. 2003;18:277–282. doi:10.1016/S0169-5347(03)00068-5 [Google Scholar]
- Bergstrom C, Lachmann M. Signalling among relatives. III. Talk is cheap. Proc. Natl Acad. Sci. USA. 1998;95:5100–5105. doi: 10.1073/pnas.95.9.5100. doi:10.1073/pnas.95.9.5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkhof M, Heeb P, Kölliker M, Richner H. Immunocompetence of nestling great tits in relation to rearing environment and parentage. Proc. R. Soc. B. 1999;266:2315–2322. doi:10.1098/rspb.1999.0925 [Google Scholar]
- Briskie J.V, Montgomerie R, Poldmaa T, Boag P.T. Paternity and paternal care in the polygynandrous Smith's longspur. Behav. Ecol. Socoiobiol. 1998;43:181–190. doi:10.1007/s002650050479 [Google Scholar]
- Briskie J, Martin P, Martin T. Nest predation and the evolution of nestling begging calls. Proc. R. Soc. B. 1999;226:2153–2159. [Google Scholar]
- Burford J, Friedrich T, Yasukawa K. Response to playback of nestling begging in the red-winged blackbird, Agelaius phoeniceus. Anim. Behav. 1998;56:555–561. doi: 10.1006/anbe.1998.0830. doi:10.1006/anbe.1998.0830 [DOI] [PubMed] [Google Scholar]
- Clark A.B, Lee W.H. Red-winged blackbird females fail to increase feeding in response to begging call playbacks. Anim. Behav. 1998;56:563–570. doi: 10.1006/anbe.1998.0831. doi:10.1006/anbe.1998.0831 [DOI] [PubMed] [Google Scholar]
- Cornell Laboratory of Ornithology. 1993 CANARY. Version 1.2. Bioacoustics Research Program, Cornell University, Ithaca, NY.
- Davies N.B, Kilner R.M, Noble D.G. Nestling cuckoos, Cuculus canorus, exploit hosts with begging calls that mimic a brood. Proc. R. Soc. B. 1998;265:673–678. doi:10.1098/rspb.1998.0346 [Google Scholar]
- Field A. Sage Publications; London, UK: 2000. Discovering statistics using SPSS for windows. Introducing statistical methods. [Google Scholar]
- Genstat. 6th edn. Lawes Agricultural Trust, VSN International Ltd; Oxford, UK: 2002. Genstat. [Google Scholar]
- Giron D, Strand M.R. Host resistance and the evolution of kin recognition in polyembryonic wasps. Proc. R. Soc. B. 2004;271(Suppl.):S395–S398. doi: 10.1098/rsbl.2004.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giron D, Dunn D.W, Hardy I.C.W, Strand M.R. Aggression by polyembryonic wasp soldiers correlates with kinship but not resource competition. Nature. 2004;430:676–679. doi: 10.1038/nature02721. doi:10.1038/nature02721 [DOI] [PubMed] [Google Scholar]
- Godfray H.C.J. Signalling of need by offspring to their parents. Nature. 1991;352:328–330. doi:10.1038/352328a0 [Google Scholar]
- Gustafsson L, Sutherland W.J. The costs of reproduction in the collared flycatcher Ficedula albicollis. Nature. 1988;335:813–815. doi:10.1038/335813a0 [Google Scholar]
- Gustafsson L, Qvarnstrom A, Sheldon B.C. Trade-offs between life-history traits and a secondary sexual character in male collared flycatchers. Nature. 1995;375:311–313. doi:10.1038/375311a0 [Google Scholar]
- Hamilton W.D. The genetical evolution of social behaviour. J. Theor. Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. doi:10.1016/0022-5193(64)90038-4 [DOI] [PubMed] [Google Scholar]
- Heaney V, Monaghan P. A within-clutch trade-off between egg-production and rearing in birds. Proc. R. Soc. B. 1995;261:361–365. [Google Scholar]
- Hinde C.A. Negotiation over offspring care?—a positive response to partner-provisioning rate in great tits. Behav. Ecol. 2006;17:6–12. doi:10.1093/beheco/ari092 [Google Scholar]
- Horsfall J.A. Food supply amd egg mass variation in the European Coot. Ecology. 1984;65:89–95. doi:10.2307/1939461 [Google Scholar]
- Houston A.I, Davies N.B. The evolution of cooperation and life history in the dunnock Prunella modularis. In: Sibly R.M, Smith R.H, editors. Behavioural ecology: ecological consequences of adaptive behaviour. Blackwell Scientific Publications; Oxford, UK: 1985. pp. 471–487. [Google Scholar]
- Johnstone R. Signaling of need, sibling competition, and the cost of honesty. Proc. Natl Acad. Sci. USA. 1999;96:12 644–12 649. doi: 10.1073/pnas.96.22.12644. doi:10.1073/pnas.96.22.12644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone R.A, Hinde C.A. Negotiation over offspring care—how should parents respond to each other's efforts? Behav. Ecol. 2006;17:818–827. [Google Scholar]
- Kedar H, Rodriguez-Girones M.A, Yedvab S, Winkler D.W, Lotem A. Experimental evidence for offspring learning in parent-offspring communication. Proc. R. Soc. B. 2000;267:1723–1727. doi: 10.1098/rspb.2000.1201. doi:10.1098/rspb.2000.1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner R. When do canary parents respond to nestling signals of need. Proc. R. Soc. B. 1995;260:343–348. [Google Scholar]
- Kilner R.M. A growth cost of begging in captive canary chicks. Proc. Natl Acad. Sci. USA. 2001;98:11 394–11 398. doi: 10.1073/pnas.191221798. doi:10.1073/pnas.191221798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner R.M. Sex differences in canary (Serinus canaria) provisioning rules. Behav. Ecol. Sociobiol. 2002;52:400–407. doi:10.1007/s00265-002-0533-8 [Google Scholar]
- Kilner R.M, Noble D.G, Davies N.B. Signals of need in parent–offspring communication and their exploitation by the cuckoo. Nature. 1999;397:667–672. doi:10.1038/17746 [Google Scholar]
- Kölliker M, Brinkhof M, Heeb P, Fitze P, Richner H. The quantative genetic basis of offspring solicitation and parental response in a passerine bird with parental care. Proc. R. Soc. B. 2000;267:2127–2132. doi: 10.1098/rspb.2000.1259. doi:10.1098/rspb.2000.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroodsma D.E. Reproductive development in a female songbird: differential stimulation by quality of male song. Science. 1976;192:574–575. doi: 10.1126/science.192.4239.574. [DOI] [PubMed] [Google Scholar]
- Langmore N.E, Hunt S, Kilner R.M. Escalation of a co-evolutionary arms race through host rejection of brood parasitic young. Nature. 2003;422:157–160. doi: 10.1038/nature01460. doi:10.1038/nature01460 [DOI] [PubMed] [Google Scholar]
- Laurien-Kehnen C, Trillmich F. Lactation performance of guinea pigs (Cavia porcellus) does not respond to experimental manipulation of pup demands. Behav. Ecol. Sociobiol. 2003;53:145–152. [Google Scholar]
- Leonard M, Horn A. Provisioning rules in tree swallows. Behav. Ecol. Sociobiol. 1996;38:341–347. doi:10.1007/s002650050250 [Google Scholar]
- Lessells C.M. Sexual conflict. In: Keller L, editor. Levels of selection in evolution. Monographs in behaviour and ecology. Princeton University Press; Princeton, NJ: 1999. pp. 75–99. [Google Scholar]
- Lougheed L.W, Anderson D.J. Parent blue-footed boobies suppress siblicidal behaviour of their offspring. Behav. Ecol. Sociobiol. 1999;45:11–18. doi:10.1007/s002650050535 [Google Scholar]
- Macnair M.R, Parker G.A. Models of parent–offspring conflict III. Intra-brood conflict. Anim. Behav. 1979;27:1202–1209. doi:10.1016/0003-3472(79)90067-8 [Google Scholar]
- Manser M.B, Avey G. The effect of pup vocalisations on food allocation in a cooperative mammal, the meerkat (Suricata suricatta) Behav. Ecol. Sociobiol. 2000;48:429–437. doi:10.1007/s002650000248 [Google Scholar]
- Markman S, Yom-Tov Y, Wright J. Male parental care in the orange-tufted sunbird: behavioural adjustments in provisioning and nest guarding effort. Anim. Behav. 1995;50:655–669. doi:10.1016/0003-3472(95)80127-8 [Google Scholar]
- Martins T.L.F, Wright J. Brood reduction in response to manipulated brood sizes in the common swift. Behav. Ecol. Sociobiol. 1993;32:61–70. doi:10.1007/BF00172224 [Google Scholar]
- McNamara J, Gasson C, Houston A. Incorporating rules for responding into evolutionary games. Nature. 1999;401:368–371. doi: 10.1038/43869. [DOI] [PubMed] [Google Scholar]
- Mock D.W, Parker G.A. Oxford University Press; Oxford, UK: 1997. The evolution of sibling rivalry. [Google Scholar]
- Moreno J, Cowie R.J, Sanz J.J, Williams R.R. Differential response by males and females to brood manipulations in the pied flycatcher: energy expenditure and nestling diet. J. Anim. Ecol. 1995;64:721–732. doi:10.2307/5851 [Google Scholar]
- Neuenschwander S, Brinkhof M.W.G, Kolliker M, Richner H. Brood size, sibling competition, and the cost of begging in great tits (Parus major) Behav. Ecol. 2003;14:457–462. doi:10.1093/beheco/arg025 [Google Scholar]
- Ottoson U, Backman J, Smith H.G. Begging affects parental effort in the pied flycatcher, Ficedula hypoleuca. Behav. Ecol. Sociobiol. 1997;41:381–384. doi:10.1007/s002650050399 [Google Scholar]
- Parker G.A, MacNair M.R. Models of parent–offspring conflict IV suppression: evolutionary retaliation by the parent. Anim. Behav. 1979;27:1210–1235. doi:10.1016/0003-3472(79)90068-X [Google Scholar]
- Parker G.A, Royle N, Hartley I. Intrafamilial conflict and parental investment: a synthesis. Phil. Trans. R. Soc. B. 2002;357:295–307. doi: 10.1098/rstb.2001.0950. doi:10.1098/rstb.2001.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pexton J.J, Mayhew P.J. Siblicide and life-history evolution in parasitoids. Behav. Ecol. 2002;13:690–695. doi:10.1093/beheco/13.5.690 [Google Scholar]
- Pexton J.J, Mayhew P.J. Clutch size adjustment, information use and the evolution of gregarious development in parasitoid wasps. Behav. Ecol. Sociobiol. 2005;58:99–110. doi:10.1007/s00265-004-0881-7 [Google Scholar]
- Price K. Begging as competition for food in yellow-headed blackbirds. Auk. 1996;113:963–967. [Google Scholar]
- Price K. Benefits of begging for yellow-headed blackbird nestlings. Anim. Behav. 1998;56:571–577. doi: 10.1006/anbe.1998.0832. doi:10.1006/anbe.1998.0832 [DOI] [PubMed] [Google Scholar]
- Price K, Harvey H, Ydenberg R. Begging tactics of nestling yellow-headed blackbirds, Xanthocephalus xanthocephalus, in relation to need. Anim. Behav. 1996;51:421–435. doi:10.1006/anbe.1996.0039 [Google Scholar]
- Queller D.C. Male–female conflict and parent-offspring conflict. Am. Nat. 1994;144:S84–S99. doi:10.1086/285654 [Google Scholar]
- R Development Core Team. R Foundation for Statistical Computing; Vienna, Austria: 2006. R: a language and environment for statistical computing.http://www.R-project.org [Google Scholar]
- Redondo T. Exploitation of host mechanisms for parental care by avian brood parasites. Etologia. 1993;3:235–297. [Google Scholar]
- Richner H, Oppliger A, Christe P. Paternal investment affects prevalence of malaria. Proc. Natl Acad. Sci. USA. 1995;92:1192–1194. doi: 10.1073/pnas.92.4.1192. doi:10.1073/pnas.92.4.1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs R.E. Response of adult Leach's storm petrels to increased food demand at the nest. Auk. 1987;104:750–756. [Google Scholar]
- Ricklefs R.E. The roles of parent and chick in determining feeding rates in Leach's storm-petrel. Anim. Behav. 1992;43:895–906. doi:10.1016/0003-3472(92)90003-R [Google Scholar]
- Rodriguez-Girones M.A, Cotton P.A, Kacelnik A. The evolution of begging: signalling and sibling competition. Proc. Natl Acad. Sci. USA. 1996;93:14 637–14 641. doi: 10.1073/pnas.93.25.14637. doi:10.1073/pnas.93.25.14637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle N.J, Hartley I.R, Parker G.A. Begging for control: when are offspring solicitation behaviours honest? Trends Ecol. Evol. 2002;17:434–440. doi:10.1016/S0169-5347(02)02565-X [Google Scholar]
- Sanz J.J, Tinbergen J.M. Energy expenditure, nestling age, and brood size: an experimental study of parental behavior in the great tit Parus major. Behav. Ecol. 1999;10:598–606. doi:10.1093/beheco/10.5.598 [Google Scholar]
- Schwabl H. Environment modifies the testosterone levels of a female bird and its eggs. J. Exp. Zool. 1996;276:157–163. doi: 10.1002/(SICI)1097-010X(19961001)276:2<157::AID-JEZ9>3.0.CO;2-N. doi:10.1002/(SICI)1097-010X(19961001)276:2<157::AID-JEZ9>3.0.CO;2-N [DOI] [PubMed] [Google Scholar]
- Schwagmeyer P, Mock D.W, Parker G.A. Biparental care in house sparrows: negotiation or sealed bid? Behav. Ecol. 2002;13:713–721. doi:10.1093/beheco/13.5.713 [Google Scholar]
- Smith H, Kallander H, Fontell K, Ljungstrom M. Feeding frequency and parental division of labour in the double-brooded great tit Parus major—effects of manipulating brood size. Behav. Ecol. Sociobiol. 1988;22:447–453. doi:10.1007/BF00294983 [Google Scholar]
- Sundstrom L, Boomsma J.J. Conflicts and alliances in insect families. Heredity. 2001;86:515–521. doi: 10.1046/j.1365-2540.2001.00884.x. [DOI] [PubMed] [Google Scholar]
- Trivers R.L. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the decent of man 1871–1971. Aldine Press; Chicago, IL: 1972. pp. 136–179. [Google Scholar]
- Trivers R.L. Parent–offspring conflict. Am. Zool. 1974;14:249–264. [Google Scholar]
- Verhulst S, Tinbergen J.M. Clutch size and parental effort in the great tit Parus major. Ardea. 1997;85:111–126. [Google Scholar]
- Weary D.M, Lawson G.L, Thompson B.K. Sows show stronger responses to isolation calls of piglets associated with greater levels of piglet need. Anim. Behav. 1996;52:1247–1253. doi:10.1006/anbe.1996.0272 [Google Scholar]
- Wright J. Helpers-at-the-nest have the same provisioning rule as parents: experimental evidence from play-backs of chick begging. Behav. Ecol. Sociobiol. 1998;42:423–429. doi:10.1007/s002650050456 [Google Scholar]
- Wright J, Cuthill I. Manipulations of sex differences in parental care. Behav. Ecol. Sociobiol. 1989;25:171–181. doi:10.1007/BF00302916 [Google Scholar]
- Wright J, Cuthill I. Biparental care: short-term manipulation of partner contribution and brood size in the starling, Sturnus vulgaris. Behav. Ecol. 1990a;1:116–124. [Google Scholar]
- Wright J, Cuthill I. Manipulations of sex differences in parental care: the effect of brood size. Anim. Behav. 1990b;40:462–471. doi:10.1016/S0003-3472(05)80526-3 [Google Scholar]
- Wright J, Both C, Cotton P.A, Bryant D. Quality vs. quantity: energetic and nutritional trade-offs in parental provisioning strategies. J. Anim. Ecol. 1998;67 [Google Scholar]
- Zar J.H. 4th edn. Prentice-Hall International; London, UK: 1999. Biostatistical analysis. [Google Scholar]