Abstract
Summary: The establishment of cell polarity is critical for the development of many organisms and for the function of many cell types. A large number of studies of diverse organisms from yeast to humans indicate that the conserved, small-molecular-weight GTPases function as key signaling proteins involved in cell polarization. The budding yeast Saccharomyces cerevisiae is a particularly attractive model because it displays pronounced cell polarity in response to intracellular and extracellular cues. Cells of S. cerevisiae undergo polarized growth during various phases of their life cycle, such as during vegetative growth, mating between haploid cells of opposite mating types, and filamentous growth upon deprivation of nutrition such as nitrogen. Substantial progress has been made in deciphering the molecular basis of cell polarity in budding yeast. In particular, it becomes increasingly clear how small GTPases regulate polarized cytoskeletal organization, cell wall assembly, and exocytosis at the molecular level and how these GTPases are regulated. In this review, we discuss the key signaling pathways that regulate cell polarization during the mitotic cell cycle and during mating.
INTRODUCTION
Cell polarity is central to the development of most eukaryotes. It is also critical for the function of many cell types involved in vectored processes such as nutrient transport, neuronal signaling, and cell motility. Cell polarization in response to extracellular or intracellular cues appears to follow a common plan (128). First, a spatial cue marks the site of polarized growth. Signaling molecules relay the spatial information to the downstream components of polarity establishment, leading to asymmetric organization of the cytoskeleton. Polarity is then reinforced with targeted secretion that leads to the deposition of molecules needed for growth at the chosen site. It is apparent from a large number of studies of diverse organisms that the small-molecular-weight GTPases function as key signaling molecules in polarity development and that there is a remarkable conservation of these GTPases from yeast to humans at both structural and functional levels (61, 62, 137, 203).
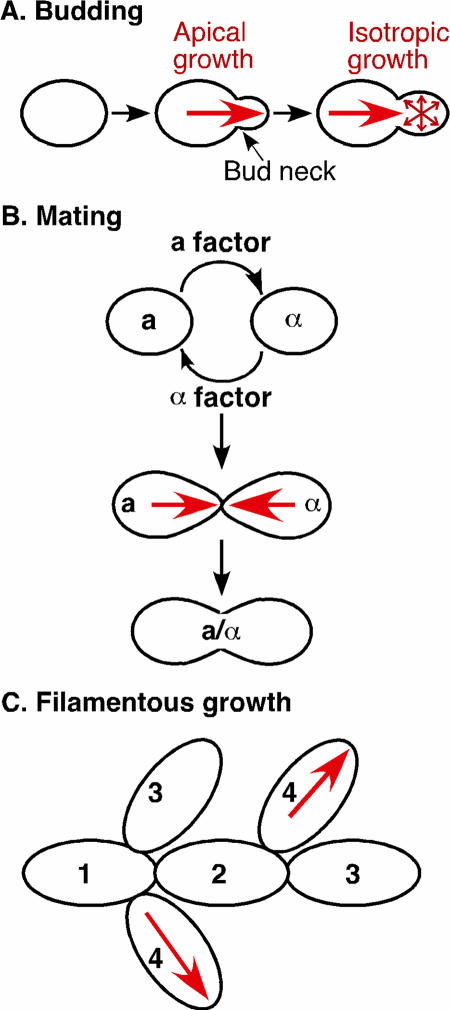
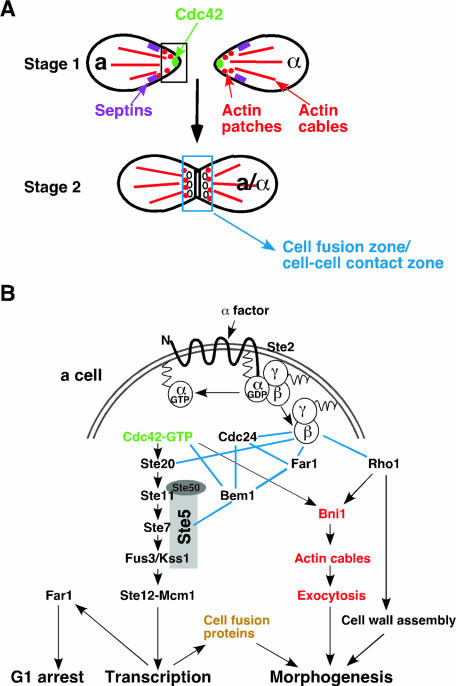
The budding yeast Saccharomyces cerevisiae is a particularly attractive model organism because it displays pronounced cell polarity in response to intracellular and extracellular cues. Cells of budding yeast undergo polarized growth during various phases of their life cycle, such as budding during vegetative growth, mating between haploid cells of opposite mating types, and filamentous growth (FG) upon deprivation of nutrients such as nitrogen (Fig. 1). There are three cell types in yeast (here we sometimes refer to S. cerevisiae as “yeast,” although we realize that other yeasts such as Schizosaccharomyces pombe are somewhat different from S. cerevisiae): a and α cells (such as normal haploids) and a/α cells (such as normal diploids), which are determined by their mating-type loci (220). Both haploid and diploid cells initiate budding when they reach the critical cell size in the late G1 phase of the cell cycle. Bud growth is initially targeted to the bud tip (apical growth) and then throughout the bud (isotropic growth) until nuclear division and cytokinesis take place (Fig. 1A). The second form of polarized growth occurs when haploid a and α cells encounter each other and undergo mating to form diploid a/α cells (Fig. 1B). During both budding and mating, the overall cellular organization is similar, although budding cells have a constriction between mother and bud called the bud neck (Fig. 1A). A specific site for cell polarization during budding is determined by the cell type, whereas cell polarization during mating is chemotropic; i.e., a cell of one mating type responds to a gradient of a peptide mating pheromone secreted by a cell of the opposite mating type (221). The most prominent feature in both processes is the organization of the polarized actin cytoskeleton, which guides secretion towards the bud site or the tip of a mating projection, resulting in polarized cell growth (126, 457).
FIG. 1.
Polarized growth in the life cycle of the budding yeast S. cerevisiae. Polarized growth occurs during budding, mating, and nutritional starvation. Nutritional starvation triggers filamentous growth, which can be divided into invasive growth (exhibited by haploid cells) and pseudohyphal growth (exhibited by diploid cells). Numbers in panel C indicate the order of each budding event. The direction of growth is indicated by red arrows.
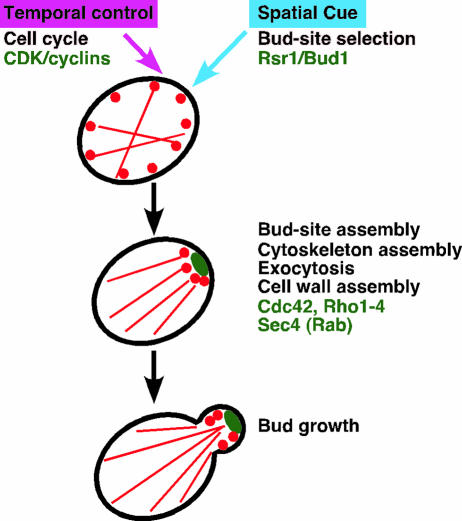
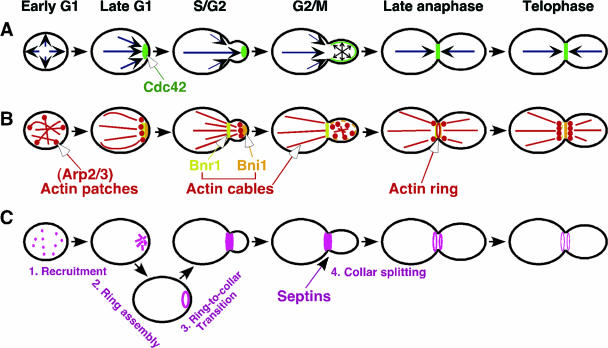
The process of budding is controlled both spatially and temporally: bud emergence occurs at a particular site in the cell cortex and at a particular time in the cell cycle. The early stage of budding can be viewed as a number of sequential, coordinated events orchestrated by a cascade of small GTPase modules (Fig. 2) (85, 126, 221, 454). First, a specific site for polarized growth is determined in the bud site selection step. The Ras family GTPase Rsr1/Bud1 (hereafter called Rsr1) and its regulators are known to play a key role in this step. Second, the assembly of components required for bud formation takes place at the chosen site to restrict cell growth to that position. Unlike the bud site selection step, this bud site assembly is an essential step for growth, which requires a group of proteins including the Rho family GTPase Cdc42 and its regulators. Cdc42 interacts with several proteins to trigger downstream processes, including polarization of the actin cytoskeleton and secretion towards the sites of cell growth. Rho GTPases are also involved in the polarized organization of the actin cytoskeleton and cell wall biogenesis. Finally, according to the polarization cue directed by Cdc42 and Rho GTPases, the Rab family GTPase Sec4 regulates secretion or exocytosis from the Golgi apparatus to the plasma membrane, resulting in the emergence and growth of the bud. The purpose of this article is to review the molecular mechanisms underlying the development of cell polarity in budding yeast, with a particular focus on the roles of small GTPases. We will discuss GTPase signaling pathways and their coordination mechanisms that are central to polarized growth. We will also discuss the regulatory mechanisms involved in the spatial and temporal control of cell polarity. Finally, we will discuss a few key concepts that may define unified intellectual frameworks for studying cell polarizations in diverse organisms.
FIG. 2.
Small GTPases and the major events in the early stage of budding in S. cerevisiae. Two major structures of actin filaments, actin cables and actin patches, are indicated with red lines and red dots, respectively, to highlight the polarized organization of the actin cyto-skeleton.
ESTABLISHMENT AND MAINTENANCE OF Cdc42 POLARIZATION
Yeast cells organize the polarity establishment machinery in response to the spatial cues from budding landmarks or mating pheromones. The key player in polarity establishment is Cdc42 GTPase, which is involved in actin organization, septin organization, and exocytosis (8, 259, 335) (Fig. 3A). In this section, we will first describe the components of the Cdc42 GTPase module. We will then discuss how the establishment and maintenance of Cdc42 polarization at the sites of polarized growth are regulated. This part includes a discussion of the concept of “symmetry breaking,” which may explain the development of cell polarity in the absence of spatial cues. In wild-type cells, the establishment of Cdc42 polarization occurs at a specific site in response to a spatial cue, which is discussed in depth below (see “Coupling of the Rsr1 GTPase Module to the Cdc42 GTPase Module”).
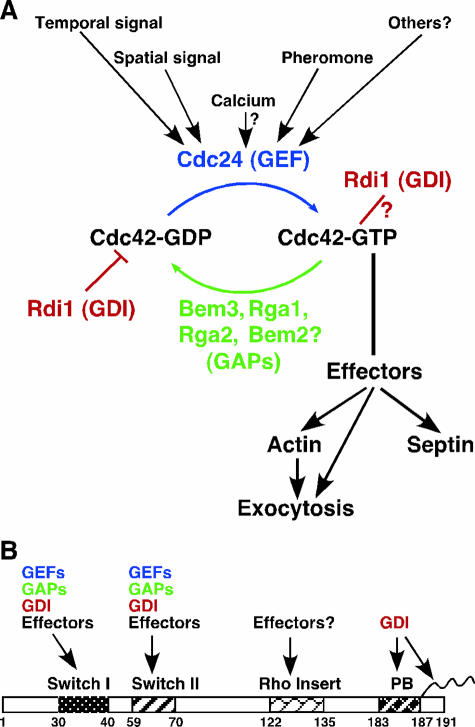
FIG. 3.
Regulation of Cdc42 in S. cerevisiae. (A) Cdc42 activity is regulated by at least three types of regulators: the GEF Cdc24; the GAPs Bem3, Rga1, Rga2, and Bem2; and the GDI Rdi1. The GEF and perhaps the GAPs are likely to mediate the regulation of Cdc42 by most internal and external signals. Once activated, Cdc42 regulates the organization of the actin cytoskeleton and the septins and interacts with components of the exocytic machinery. The polarized actin cytoskeleton guides exocytosis, leading to polarized cell growth. (B) Structure motifs of S. cerevisiae Cdc42 and their potential binding partners based on studies of Cdc42 from yeast to mammals. The switch I region (which is also known as the Ras-like effector region), the switch II region, the Rho insert domain (which is present only in Rho-type GTPases), the polybasic (PB) region (183KKSKK187), the CAAX box (188CAIL191), and their binding partners are indicated. The cysteine residue in the CAAX box is modified by prenylation (wavy line), and the last three amino acids are cleaved off, followed by carboxymethylation (see the text for details).
The Rho Family GTPase Cdc42 and Its Regulators
Cdc42 GTPase.
After being identified first in S. cerevisiae, Cdc42 and its homologs have been found in various other eukaryotes including Caenorhabditis elegans, Drosophila melanogaster, and humans (for a review, see reference 259). Cdc42, which belongs to the Rho subfamily of the Ras superfamily GTPases, is highly conserved from yeast to humans at both the sequence (80 to 95% identity in the predicted amino acid sequence) and functional (259) levels. Like all GTPases in the Ras superfamily, Cdc42 contains the putative Ras-like effector motif (also known as “switch I”) near the N terminus (Fig. 3B). The binding partners of Cdc42 through this effector domain are proteins containing the CRIB (for “Cdc42/Rac interactive binding”) domain (also known as the p21-binding domain or the GTPase-binding domain [GBD]). The CRIB domain is found in many Cdc42 downstream effectors, including the p21-activated kinase (PAK) family of protein kinases, which preferentially interact with GTP-bound Cdc42 (515). Mutational studies of CDC42 in S. cerevisiae indicate that the effector domain may differentially interact with multiple CRIB domain-containing effectors (see below).
Cdc42 also contains the “Rho insert domain” of ∼13 amino acid residues, which is unique to Rho-type GTPases (Fig. 3B). This domain has been implicated in interactions with some of its downstream effectors such as IQGAPs and guanine nucleotide dissociation inhibitors (GDIs) for human Cdc42 (375, 621). However, the crystallographic structure of the Cdc42/Rho GDI complex reveals that the switch I and switch II domains and the geranylgeranyl moiety of Cdc42 interact with Rho GDI (229). Cdc42 also contains the C-terminal CAAX (A is aliphatic; X is any amino acid) box, CTIL, for prenylation with the geranylgeranyl isoprene group at the Cys residue (651). Geranylgeranylation has been found in Cdc42s from all other organisms examined. This posttranslational modification is important for the membrane attachment and function of Cdc42. Cell fractionation experiments indicate that Cdc42 is found in both soluble and particulate pools, with most in the latter fraction, which includes the plasma membrane, secretory vesicles, and other dense materials (651). The particulate pool of Cdc42 is eliminated by mutagenizing the Cys188 in the C-terminal 188CTIL and is decreased in the geranylgeranyltransferase mutant cdc43 (651). Lipid modification of Cdc42 is thus required for its targeting to the particulate pool. Indeed, Cdc42C188S fused to green fluorescent protein (GFP) fails to localize to any internal and plasma membranes (466). A GFP fused to the CAAX motif alone (GFP-188CTIL) localizes to the internal membranes but not to the plasma membranes, while GFP-KKSKKCTIL (the polybasic motif plus the CAAX box) is sufficient for targeting to both membranes. However, GFP-KKSKKCTIL still fails to cluster at sites of polarized growth (466), supporting the idea that Cdc42 clustering may require guanine nucleotide binding (468). Thus, it appears that the CAAX box and its immediate upstream polybasic domain are sufficient for the targeting of Cdc42 to the plasma membrane, while other regions of Cdc42 are likely to be involved in its clustering at sites of polarized growth.
Cdc42 localizes to the plasma membrane and to sites of polarized growth, first to the incipient bud site, the tips of growing buds, and then the mother-bud neck in large-budded cells (466, 651). It is noteworthy that Cdc42 localization to the incipient bud site is not disrupted by incubation with latrunculin A, which sequesters G actin and thus causes a rapid depolymerization of the actin filaments (30). Thus, Cdc42 is likely to arrive to the presumptive bud site independently of the localization or integrity of the actin cytoskeleton during budding (see below for a further discussion of Cdc42 polarization).
The phenotype of a temperature-sensitive (Ts) cdc42 mutant, cdc42-1, has provided the first clue for the function of Cdc42 (8, 217). This mutant fails to form a bud at 37°C but continues DNA replication, nuclear division, and the increase in cell mass and volume, resulting in an enlarged unbudded cell. The actin cytoskeleton is haphazardly distributed in the mutant cells, indicating that Cdc42 is required for the polarized organization of the actin cytoskeleton (8). Mutations of CDC42 or CDC24, which encodes a GDP-GTP exchange factor (GEF) for Cdc42, also prevent targeted secretion so that new cell surface growth is not limited to the bud or mating projection (8, 535). Null mutants of CDC42 or CDC24 are not viable, as expected from their essential role in budding (260). Analyses of many cdc42 mutants have provided much information about the functional domains of Cdc42 and have uncovered a variety of processes in which Cdc42 is involved (84, 183, 259, 293, 394, 465) (see below).
Cdc24, a GEF for Cdc42.
As discussed above, CDC24 is also required for bud emergence or the establishment of cell polarity (215, 216). Temperature-sensitive mutations in CDC24 result in the formation of large, round, unbudded cells at the restrictive temperature (215, 216, 534, 535), as is the case with some of the cdc42-Ts mutants, suggesting that CDC42 and CDC24 are likely to function in the same process, i.e., bud emergence (8, 260). These cdc24 mutants exhibit an overall defect in cell polarity resulting in delocalized chitin and mannan deposition throughout the cell surface (473, 534, 535).
Cdc24 contains a Dbl homology (DH) domain (388), which is generally associated with its GEF activity (228). Indeed, Cdc24 displays GEF activity towards Cdc42 (563, 645). Because a hyperactive mutation in CDC42 (cdc42G60D) can bypass the requirement for Cdc24, it is likely that the GEF activity towards Cdc42 is the sole essential function for Cdc24 in polarized growth (84). Cdc24 also contains other highly conserved domains including a calponin homology (CH) domain, which has been implicated in binding to actin in some proteins (551), and the pleckstrin homology (PH) domain, which is thought to bind phosphoinositides (310). A recent genome-wide characterization of all potential PH domains in yeast indicates that the PH domain of Cdc24, like most of the other yeast PH domains, binds phosphoinositides but with no headgroup specificity and with low binding affinity (635). In addition, the PH domain of Cdc24 alone fails to localize to the membrane (635). Thus, the functional significance of the PH domain of Cdc24 is not clear. Cdc24 also has two calcium binding domains (residues 649 to 658 and 820 to 831). It has been reported previously that Bem1, another protein important for polarity establishment, binds directly to Cdc24 and that this interaction is inhibited by Ca2+ in vitro (644), which may explain why CDC24 was also identified from a screen for mutants sensitive to a high level of exogenous calcium (421, 422).
Despite no obvious transmembrane domain or hydrophobic stretches, Cdc24 fractionates to a particulate pool (383, 388, 454). It is possible that Cdc24 associates with membrane through its PH domain and/or through interactions with other proteins on the plasma membrane. Analyses of deletion and point mutations of CDC24 have identified a 56-amino-acid domain (amino acids 647 to 703) as being necessary and sufficient for its localization to sites of polarized growth (572). This domain alone, however, is unable to anchor Cdc24 at these sites or is unable to support a tight association of Cdc24 with the plasma membrane. Anchoring of Cdc24 requires the Cdc24 carboxyl-terminal PC (phox and Cdc) domain that interacts with Bem1 and also requires Bem1, Rsr1, or the potential transmembrane protein Tos2/Ygr221C (572). Not much is known about Tos2 except that it exhibits a two-hybrid interaction with Cdc24 and also localizes to sites of polarized growth (123). Together, these data suggest that Cdc24 localization requires both membrane-specific targeting and subsequent anchoring within a multiprotein complex.
Cdc24 interacts with a number of proteins including Rsr1, Cdc42, Bem1, and Far1 (47, 70, 434, 644). Domains of Cdc24 that interact with some of its binding partners have been mapped. The conserved PB1 (phox and Bem) domain of Bem1 interacts with the PC motif-containing region at the C terminus of Cdc24 (47, 69, 249, 565). The mating-specific alleles of cdc24, which fail to interact with Far1 but are not defective in budding, have been mapped in the CH domain, suggesting that Far1 interacts with the CH domain of Cdc24 (70, 408, 409). Which domain of Cdc24 interacts with Rsr1 is less clear. Rsr1 in its GTP-bound state can interact with the C-terminal half of Cdc24 in vitro (434), and this interaction is necessary for the localization of Cdc24 to the presumptive bud site (436). Consistent with these data, the C-terminal half of Cdc24 is important for its localization to the site of polarized growth, whereas the N-terminal region is required for its localization to the nucleus (571, 572). On the other hand, it has been shown that Rsr1 exhibits a two-hybrid interaction with the CH domain of Cdc24 (523), which overlaps with the Far1 binding domain located near the N terminus. It is possible that more than one domain of Cdc24 interacts with Rsr1 and that each study may have overlooked the other binding site. Several questions remain. For example, does the CH domain interact directly with Rsr1 in a GTP-dependent manner? Does Rsr1 interact with more than one domain of Cdc24 at the same time in vivo? Is the Rsr1-Cdc24 interaction important only for guiding Cdc24 to the proper bud site, or does it also affect the GEF activity of Cdc24? It has been reported that Cdc24 inhibits both the intrinsic and the GTPase-activating protein-stimulated GTPase activity of Rsr1, suggesting that Cdc24 acts as a GTPase inhibitor protein for Rsr1 (644). However, this notion is somewhat surprising given that Rsr1 does not exhibit a high intrinsic GTPase activity in vitro (even in the absence of Cdc24) (435), and thus, the physiological significance of this observation is unclear.
Cdc24 localizes to the presumptive bud site in the late G1 phase and to sites of polarized growth during the cell cycle (408, 522, 571). In wild-type cells, the localization of Cdc24 to the presumptive bud site is likely to occur through the interaction with Rsr1-GTP (434, 436, 644). However, Cdc24 still localizes to a single site, although at a random location, in the absence of Rsr1 in the late G1 phase, indicating that other mechanisms operate in the Cdc24 clustering in cells lacking Rsr1. In the late M and early G1 phases, Cdc24 localizes to the nucleus through the interaction with Far1 (411, 522). The export of Cdc24 from the nucleus is triggered either by entry into the cell cycle or by mating pheromones. Activation of the cyclin-dependent kinase (CDK) Cdc28 by G1 cyclin Cln2 triggers the degradation of Far1, and as a result, Cdc24 is relocated from the nucleus to the presumptive bud site (196). This Cdc28/Cln2-triggered relocation of Cdc24 defines one step for the temporal regulation of polarity establishment (see Temporal Control of Polarity Establishment during Yeast Budding for further discussion).
Isolation of the mating-specific alleles of CDC24 uncovers important roles of Cdc24 in polarity establishment as well as cell fusion during mating (see below [“Cell Polarization during Mating”]). Certain alleles of CDC24 also exhibit the bud site selection defect (534, 535), while others display sensitivity to high-calcium growth media (421, 422). In addition, certain cdc24 alleles are also sensitive to high-Na+ growth media and exhibit synthetic lethality with a vacuolar ATPase subunit mutant, vma5 (611), suggesting that Cdc24 might be involved in Na+ tolerance and in vacuole function. However, the role of Cdc24 in calcium-mediated regulation or in vacuole function is not well established. The genetic interaction between CDC24 and the genes involved in vacuole morphology or function is likely to be related to its role as a GEF for Cdc42 since Cdc42 is implicated in vacuole membrane fusion (402). Cdc42 promotes the assembly of the actin cytoskeleton (see below), which is also involved in vacuole inheritance or movement (224).
Cdc42 GAPs.
There are four potential GTPase-activating proteins (GAPs) for Cdc42: Bem2 (46, 282), Bem3 (645), Rga1/Dbm1 (91, 550), and Rga2 (181, 536). In vitro GAP assays indicate that Bem2 acts on Cdc42 (357) and Rho1 (446); Bem3 acts mainly on Cdc42 and, to a lesser extent, on Rho1 (645, 646); and both Rga1 and Rga2 act on Cdc42 (181, 536). These in vitro assays, together with two-hybrid interaction data (536, 550), suggest that Bem3, Rga1, and Rga2 are more specific to Cdc42, whereas Bem2 is a GAP for both Cdc42 and Rho1 (Fig. 3A) (see the section on Rho1 GAPs below for more discussion on Bem2).
Why does Cdc42 need multiple GAPs? Are the GAPs involved in distinct functions, or do they share a redundant role? Can they also carry out a specific biological function as a part of the Cdc42 effector? Answers to these questions are not yet clear. In contrast to CDC42, none of the genes encoding the putative Cdc42 GAPs is essential. BEM3 was originally isolated as a multicopy suppressor of a bem2-Ts mutant, which is defective in bud emergence (645). RGA1/DBM1 was identified in a genetic screen designed to isolate mutants that activate the pheromone response pathway in the absence of the Ste4 Gβ subunit (550) and also as a dominant suppressor of a bem2-Ts mutant (91). Deletion of RGA1 results in increased expression of a FUS1::lacZ reporter gene. Another potential Cdc42 GAP, Rga2, was identified through its homology to Rga1 (536, 550). The rga1Δ bem3Δ mutant in some strain backgrounds produces a high percentage of elongated cells, while the single mutant of either rga1Δ or rga2Δ does not produce such abnormally shaped cells (83, 181, 536). A bem3Δ mutant also produces a low percentage of abnormally shaped cells, such as cells that are peanut or finger shaped, depending on strain backgrounds. The rga1Δ bem3Δ double mutant and the rga1Δ rga2Δ bem3Δ triple mutant show more misshapen cells than the bem3Δ single mutant. Deletion of RGA1 also leads to an increase in the bipolar budding pattern in haploids instead of the axial budding pattern (91, 536), suggesting that Rga1 has a distinct function in bud site selection that is not shared by Rga2 or Bem3. It is not clear why a mutation of a Cdc42 GAP leads to a defect specifically in the axial budding pattern. Distinct phenotypes of strains lacking RGA1, RGA2, or BEM3 led to a suggestion that each GAP may regulate different functions of Cdc42, although quantitative differences in the GAP activities of the mutants may contribute to the overall phenotype (536). Importantly, the strain lacking all three GAPs, rga1Δ rga2Δ bem3Δ, displays a much more elongated bud morphology and increased induction of FUS1-LacZ than any single or double GAP mutants (although the extent of these defects seems to vary depending on strain backgrounds) (83, 181, 536), both of which are consistent with more activation of Cdc42. Rga1, Rga2, and Bem3 may thus play some overlapping roles in regulating morphogenesis and mitogen-activated protein (MAP) kinase (MAPK) activation during the mating response, presumably via their GAP activity towards Cdc42.
The localization of Bem3 and Rga2 is similar to that of Cdc42: both Bem3 and Rga2 were observed at the presumptive bud site, the tips of small buds, and the mother-bud necks of cells late in the cell cycle, although no distinct signal was detected at intermediate stages (83). Rga1 exhibits a distinct localization pattern. It localizes to the presumptive bud site and to the cortex of a tiny bud rather than only at the bud tip. Unlike Bem3 and Rga2, Rga1 localizes as a ring to the bud site of the neck in cells with a medium or large bud. Later in the cell cycle, Rga1 localizes to the neck as a double ring, with approximately equal intensities for both rings. Localization of these Cdc42 GAPs to the neck, but not to the presumptive bud site or bud tip, depends on septins (83). A large-scale localization study indicated that Bem2 is found at the bud neck and the cytoplasm (237). It remains to be determined whether the localization pattern of each GAP contributes to the functional differences in Cdc42 GAPs. In summary, Cdc42 GAPs are likely to play differential and overlapping roles in regulating polarized cell growth via their GAP activity. An important question is how Cdc42 GAPs are temporally and spatially regulated in the cell cycle so that they can coordinately regulate Cdc42 activity.
Rho GDI.
Rho GDI (GDP dissociation inhibitor) is known to display three biochemical activities: inhibiting the dissociation of GDP from Cdc42, Rho, and Rac (95, 165, 312); inhibiting the intrinsic and GAP-stimulated GTPase activity of Cdc42, Rho, and Rac (95, 208, 214); and extracting Cdc42, Rac, and Rho from cellular membranes into cytosol (232, 416). The first and third activities inhibit GDP/GTP exchange and decrease the membrane pool of small GTPases, respectively, leading to the perception that Rho GDIs act as negative regulators of these GTPases. In contrast, the second activity maintains Cdc42, Rho, and Rac in their GTP-bound form, suggesting that Rho GDI may also play a positive role in the functions of these GTPases. The C-terminal lipid modification of Rho GTPases is essential for their binding to the GDIs (208, 229, 232) (Fig. 3B). In addition, the switch I and switch II regions of Rho GTPases, which bind to their GEFs, GAPs, and effectors, and the polybasic region, which is required for their targeting to the plasma membrane, are also involved in their interactions with the GDIs (110, 229, 259) (Fig. 3B). Unlike Rab GDIs, which bind preferentially to the GDP-bound form of Rab GTPases (17, 448), Rho GDIs bind equally well to both the GTP- and the GDP-bound forms of Cdc42, Rho, and Rac (208, 416). Rab GDIs also play an important role in delivering and/or loading Rab GTPases to target membranes (177, 447, 448), whereas such a role may not exist for Rho GDIs (120), as Cdc42 and Rac mutants deficient in their interactions with Rho GDIs appear to target to plasma membranes and elicit normal biological responses (167, 174, 175).
Rdi1, the only known Rho GDI in S. cerevisiae (365), can efficiently extract Cdc42 and Rho1 from the vacuolar membrane (133) and can also extract Cdc42 from other membranes including the plasma membrane (468, 563). Deletion of RDI1 does not produce any detectable phenotypes (365) and does not affect the clustering of Cdc42 at the sites of polarized growth (468). Rho1 and Cdc42 were found in the cytosol of the rdi1Δ cells to a similar extent as in wild-type cells, suggesting that other mechanisms or other GDI-like activities are responsible for the cycling of these GTPases between membranes and the cytosol (288). Overexpression of Rdi1 causes a slightly rounder cell morphology in some strain backgrounds (563) but causes lethality in other strain backgrounds (365) for reasons that are not known. In a cdc24-Ts mutant where the activation of Cdc42 is compromised, overexpression of Rdi1 causes lethality with cells arrested as large, round, unbudded cells, which is indicative of a loss of cell polarity (563). These overexpression phenotypes are consistent with Rdi1 being a negative regulator of Cdc42.
Rdi1 localizes to the sites of polarized growth in the cell cycle, the tip of a small bud and the mother-bud neck during cytokinesis (468). Unlike Cdc42 (466), Rdi1 does not appear to localize to the presumptive bud site and internal membranes (468). The Rdi1 localization pattern is consistent with the possibility that there might be a pool of Cdc42-GDP at sites of polarized growth. This notion is supported by the observation that the GDP-locked form of Cdc42, Cdc42D57Y, clusters at the presumptive bud site as efficiently as the wild type and the active form of Cdc42 (605).
Cycling of Cdc42 between the GDP- and GTP-bound states.
CDC24, which encodes the only known GEF for Cdc42 (563, 645), is an essential gene (215, 388, 534), suggesting that the exchange of GTP for GDP is essential for Cdc42 function. Three cdc42 alleles, cdc42G12V, cdc42Q61L, and cdc42D118A, which contain mutations in the putative GTP-binding and hydrolysis domains, cause dominant dosage-dependent lethality, suggesting that GTP hydrolysis by Cdc42 is essential for its normal function (650). Consistent with this interpretation, the triple mutant of Cdc42 GAPs, rga1Δ rga2Δ bem3Δ, is defective in bud morphogenesis and septin organization (see below for details) (83, 181, 536). Cdc42 still possesses GTPase activity to some extent in the rga1Δ rga2Δ bem3Δ mutant (645), consistent with the fact that the phenotype of the GAP triple mutant is less severe than that of the mutants expressing the GTP-locked form of Cdc42. It is possible that there is an additional GAP (such as Bem2) for Cdc42 and/or that the intrinsic GTPase activity of Cdc42 is sufficient for cell survival.
Cdc42 GTPase appears to function in two different modes: one, like Ras, to turn on signaling pathways in its GTP-bound state and another, like the translation elongation factor EF-Tu (EF-1 in eukaryotes), to assemble a macromolecular structure such as the septin ring (181). The cycle of nucleotide binding and GTP hydrolysis is critical for the latter mode of action, as was also proposed for Sec4 (601) and Rsr1 (434, 435, 484). The cycling feature of Cdc42 can best explain the paradoxical phenotypes of the cdc42G60D mutation, which makes Cdc42 hyperactive and causes multiple buddings per cell cycle yet is completely recessive in terms of the budding phenotype (84). Perhaps the Cdc42G60D mutant protein may cycle more slowly than wild-type Cdc42. If rapid cycling of Cdc42 is required for establishing and maintaining a single site for budding in each cell, wild-type Cdc42 should outcompete the mutant Cdc42 for recruiting effectors and other downstream components to a single site, thus suppressing the mutant phenotype (84). This hypothesis may explain, at least in part, the recessiveness of the cdc42G60D allele. This hypothesis is also supported by a recent observation: fluorescence recovery after photobleaching (FRAP) analysis indicates that wild-type Cdc42 at the presumptive bud site recovers much more quickly after photobleaching than Cdc42Q61L and Cdc42D57Y, which are expected to be the GTP- or GDP-locked Cdc42, respectively (605). However, it is likely that there are some intrinsic differences between the cdc42G60D and cdc42Q61L mutants, since the former is recessive, whereas the latter is dominant.
Regulation of Cdc42 Clustering at Sites of Polarized Growth
One of the key issues concerning the role of Cdc42 in polarity development is to understand how Cdc42 itself becomes polarized and how its polarization state is maintained during the cell cycle (Fig. 4). Cdc42 localizes to the presumptive bud site, which is determined by the bud site selection machinery. However, Cdc42 localizes to sites of polarized growth even in the absence of spatial cues (245, 466, 604, 605, 651). Although Cdc42 polarization at a random bud site occurs under nonphysiological conditions, it has allowed us to decipher the mechanisms of cell polarization and appreciate the concept of “symmetry breaking” in budding yeast. In this section, we will discuss how Cdc42 polarization is established and maintained. Conceptually, it is useful to distinguish the establishment of Cdc42 polarization from the maintenance of its polarization. It is, however, difficult to separate these two processes, because they are occurring almost simultaneously at the same site. For the purpose of discussion, we will separate the two issues here.
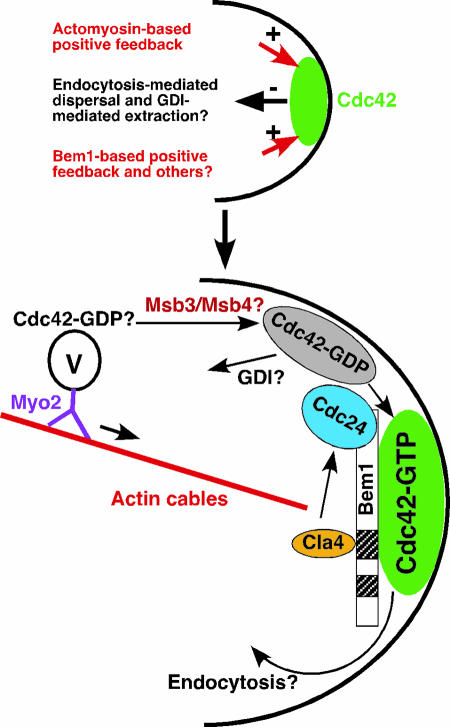
FIG. 4.
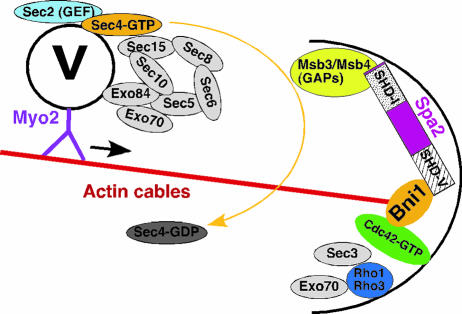
Model for the establishment and maintenance of Cdc42 polarization in the absence of a spatial cue. (Top panel) It is thought that activated Cdc42 clusters spontaneously and transiently and becomes stabilized by interactions among its effectors and/or regulators. To enhance and/or maintain the initial Cdc42 polarization at a growth site, at least two positive feedback mechanisms are operating: an actomyosin-based delivery of Cdc42 and a Bem1-based activation of Cdc42. These positive actions are counteracted by endocytosis-mediated dispersal and GDI-mediated extraction of Cdc42 away from the plasma membrane. (Bottom panel) A molecular model for Cdc42 polarization at a growth site. Cdc42 in its GDP-bound state is carried on secretory vesicles, which are transported along actin cables to the growth site by the type V myosin Myo2. The “Cdc42-GDP” cargo is captured by Msb3 and Msb4 or other factors capable of binding to Cdc42-GDP, resulting in the local accumulation of Cdc42-GDP, poised for activation by the GEF Cdc24. The Bem1-based complex functions to bring Cdc42, Cdc24, and the PAK Cla4 together, resulting in the phosphorylation of Cdc24 by Cla4 and in the further accumulation of Cdc42-GTP at the growth site. How endocytosis mediates the dispersal of Cdc42 from the polarization site remains unknown.
Establishment of Cdc42 polarization.
Establishment of Cdc42 polarization in wild-type cells occurs at a spatially defined site, which requires the coupling of the Cdc42 module to the Rsr1 GTPase module (see below [“Coupling of the Rsr1 GTPase to the Cdc42 GTPase Module”]). Activation of Cdc42 by its GEF Cdc24 is required for its polarization. When the spatial regulatory mechanism of cell polarity is absent, such as in rsr1Δ mutants, the budding process does not seem to be compromised, except that the mutant cells bud at a random site (45, 87). This spontaneous cell polarization process in the apparent absence of spatial cues is called “symmetry breaking.” Cdc42 polarization also occurs normally once per cell cycle at a random location in rsr1Δ cells (245, 466, 604, 605, 651). Thus, there is a default pathway leading to Cdc42 polarization in the absence of putative upstream events such as bud site selection. Other mechanisms must operate in rsr1Δ (and presumably also in wild-type) cells to establish and maintain Cdc42 polarization. Cdc42 polarization can occur in the rsr1Δ and bem1 single mutants but not in the rsr1Δ bem1 double mutant (245) or in bem1Δ cells in which filamentous actin (F-actin) has been disrupted by latrunculin A (LatA) (605). Cdc42 still polarizes to a single random site when bud site selection, F-actin, and microtubules are simultaneously disrupted (245). Thus, the predetermined spatial cue, F-actin, and the microtubule are not essential for Cdc42 polarization per se, although it does not rule out the possibility that these components act cooperatively to enhance Cdc42 polarization. Taken together, these results indicate that bud site selection proteins, the signaling protein Bem1, which binds to Cdc42-GTP (58), and F-actin all share an essential role in achieving a polarization state of Cdc42 (245, 605). One possibility is that the spontaneous clustering of activated Cdc42 in the absence of a spatial cue initiates Cdc42 polarization, but Bem1 or F-actin plays a crucial role in stabilizing, amplifying, and/or maintaining this initial polarization state.
Various active forms of Cdc42, Cdc42G12V (196, 466) and Cdc42Q61L (604), are able to polarize in G1-arrested cells in the presence of endogenous Cdc42. Cdc42G60D, as the sole source of Cdc42 in the cell, can also cluster on the plasma membrane randomly at more than one site, resulting in multiple budding events per nuclear cycle (84). Thus, it has been proposed that the activated Cdc42 becomes clustered through stochastic movement on the plasma membrane (84, 604) and that this initial clustering of Cdc42 could be stabilized by the interactions with Cdc42 effectors in the cell cortex (84).
Maintenance of Cdc42 polarization.
Cdc42 is highly dynamic at the sites of polarized growth, as indicated by FRAP analysis (605), suggesting that the Cdc42 concentration at these sites has to be dynamically maintained. Because GTP- or GDP-locked Cdc42 recovers more slowly than wild-type Cdc42 after photobleaching, cycling between the two nucleotide-bound states of Cdc42 is likely to be important for its dynamic accumulation at sites of polarized growth (605).
(i) The central role of Bem1 in maintaining Cdc42 polarization.
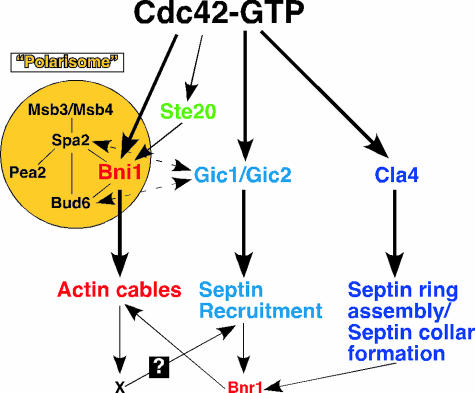
Bem1 binds to Cdc24, Cdc42-GTP, and Cla4, a PAK known to be an effector of Cdc42 (58, 196, 446, 644). It has been suggested that this protein complex enhances Cdc42 polarization and is thus involved in both the establishment and the maintenance of Cdc42 polarization. Cdc28/G1 cyclin complexes trigger Cdc42 activation indirectly through Cdc24, a GEF for Cdc42. Activated Cdc42 binds to Bem1, which, in turn, binds to Cdc24 and Cla4, and Cla4 phosphorylates Cdc24 (58, 69, 196, 245). This cascade of events may result in the accumulation of more Cdc24 and Cdc42 at sites of polarized growth. Thus, it has been proposed that the components of the Bem1-mediated protein complex constitute a positive feedback loop to establish and maintain Cdc42 polarization (69, 245) (see below [Temporal Control of Polarity Establishment during Yeast Budding] for further discussion).
(ii) Role of F-actin in maintaining Cdc42 polarization.
Two major filamentous actin structures found in yeast are actin cables and actin patches, which are required for polarized exocytosis and endocytosis, respectively (457). Cdc42 polarizes normally in cells treated with latrunculin A (30, 246), which disrupts all F-actin structures, suggesting that Cdc42 polarization can be established and maintained in the absence of F-actin. However, when cells are treated with a less potent drug, latrunculin B (which disrupts all actin cables but not all actin patches), Cdc42 fails to cluster at the sites of polarized growth, suggesting that endocytosis may be involved in Cdc42 dispersal from its polarization site. In addition, in mutants conditionally defective in actin cable formation or in post-Golgi vesicle transport, Cdc42 polarization can occur initially but cannot be maintained (246). Thus, it appears that once Cdc42 polarization is established in an F-actin-independent manner, actin cable-mediated delivery of Cdc42 is required to counteract the actin patch-mediated dispersal of Cdc42 such that a dynamic pool of Cdc42 can be maintained at the sites of polarized growth.
(iii) Role of exocytosis in maintaining Cdc42 polarization.
Cdc42 polarization is not maintained in a number of mutants that are defective at various stages of exocytosis. For example, Cdc42 fails to maintain polarization in the tropomyosin mutant tpm2Δ tpm1-2 (246, 460, 604, 637), the type V myosin mutants myo2-16 (246) and myo2-66 (604), and the late sec mutants sec4-8 and sec5-24 (637). These data indicate that Cdc42 is delivered to sites of polarized growth through exocytosis and that actomyosin-based vesicle transport and the subsequent vesicle tethering and/or fusion are required for maintaining Cdc42 polarization. It is noteworthy that the establishment of Cdc42 polarization is independent of polarized exocytosis, as Cdc42 polarization can occur in cells treated with LatA (30, 246) as well as in cells carrying tropomyosin and myo2 mutations (246, 460, 604, 637), all of which block polarized secretion but not secretion per se. In summary, current data support the view that Cdc42 polarization initiates polarized growth, while polarized exocytosis reinforces or maintains polarized growth by delivering more polarity factors, including Cdc42, to sites of polarized growth.
(iv) A possible mechanism of concentrating Cdc42-GDP at sites of polarized growth.
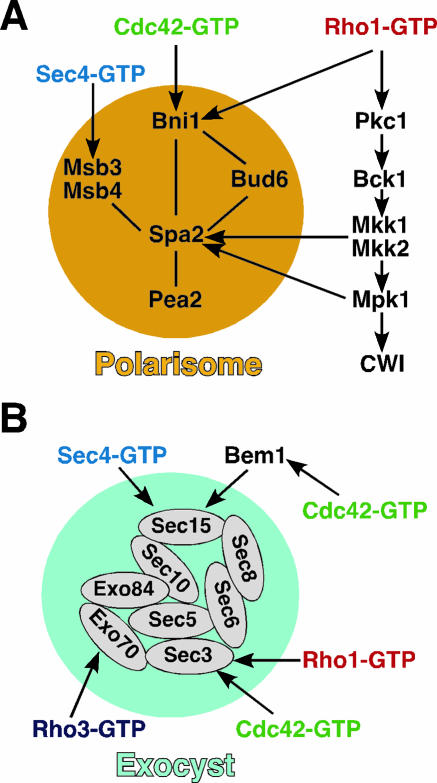
The Rsr1 GTPase module is likely to be involved in the recruitment of Cdc42 to the proper bud site (292). In rsr1Δ cells, however, a mechanism involving Msb3 and Msb4 is likely to operate. Msb3 and Msb4 may also carry out a similar function in wild-type cells as well, but it becomes more apparent in rsr1Δ cells. Msb3 and Msb4, a pair of homologous proteins each containing a Rab GAP domain (12, 13, 168), function as dosage-dependent suppressors of cdc24 and cdc42 mutants (51, 168, 563). Although a deletion of either MSB3 or MSB4 does not produce any obvious defect, the deletion of both genes results in a large, round mother with small buds, and a significant fraction of the double mutant cells has a disorganized actin cytoskeleton (32, 51). Thus, Msb3 and Msb4 may function in the same pathway as Cdc42. Msb3 and Msb4 interact with Spa2, a scaffold protein of the “polarisome” (563) (see below). Spa2 localizes to the presumptive bud site prior to Start in the late G1 phase, and this localization is dependent upon Cdc42 but not its GEF Cdc24, suggesting that Cdc42-GDP may play some role in bud site assembly (470). Interestingly, Msb3 and Msb4 bind specifically to Cdc42-GDP and Rho1-GDP but not Rho3 and Rho4 (563). Like Cdc42, Msb3 and Msb4 localize to sites of polarized growth (51, 168). Together, these results have led to a hypothesis that Msb3 and Msb4 are involved in recruiting Cdc42 from the cytosol and/or capturing Cdc42 from the secretory pathway, increasing a local pool of Cdc42-GDP at the sites of polarized growth, which is poised for activation by the GEF Cdc24 (563). Other functions of Msb3 and Msb4, including their GAP activity towards Rab GTPases and their functions in the Rho1 pathway, will be discussed below.
DETERMINATION OF THE AXIS FOR CELL POLARIZATION DURING BUDDING
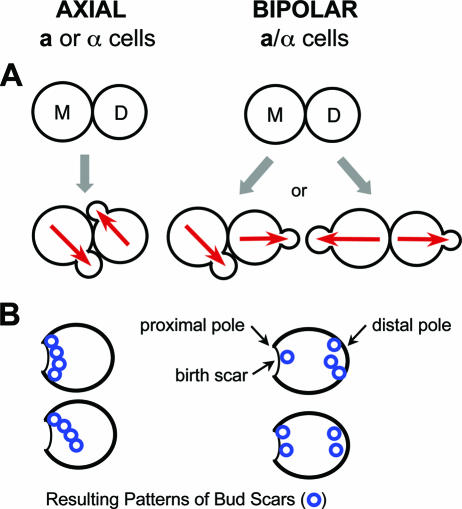
Different cell types in S. cerevisiae display distinct patterns in the selection of a cortical site (bud site) for polarized growth. Haploid a and α cells bud in the axial pattern in which both mother and daughter cells select a bud site immediately adjacent to their previous division site. In contrast, diploid a/α cells bud in the bipolar pattern: mother cells select a bud site adjacent to their daughter or on the opposite end of the cell, whereas daughter cells almost exclusively choose a bud site directed away from their mother (89, 158, 222) (Fig. 5A). These two distinct patterns of budding reflect genetic programming of cell polarization. The choice of a bud site determines the axis of cell polarity and ultimately the cell division plane, which is perpendicular to the axis of cell polarity. These patterns of cell division result in characteristic shapes of microcolonies on a solid surface (Fig. 5A) and distinct patterns of bud scars, which mark the sites of cell division on the mother cell surface (Fig. 5B). In cells undergoing axial budding, the division site is likely marked by a spatial signal(s) that specifies the location of the new bud site (89). Since the starvation and refeeding of axially budding cells result in the formation of a new bud at a nonaxial site, the spatial signal for the axial budding pattern appears to be transient in that it lasts only from one budding event to the next (89). Despite the transient nature of the axial spatial cues, haploid a or α cells exhibit the axial pattern with remarkably high fidelity during continuous logarithmic growth. Unlike the transient nature of the axial spatial cue, the bipolar spatial cue(s) appears to consist of persistent cortical markers that are present at both poles of diploid a/α cells (89).
FIG. 5.
Cell type-specific budding patterns in S. cerevisiae. (A) Axial and bipolar patterns of cell division as observed in cells growing on a solid surface. The axes of cell polarity are indicated with red arrows. (B) Patterns of bud scars on the yeast cell surface resulting from the two modes of budding. On each cell, a single birth scar marks the pole at which the cell was attached to its mother. A bud scar shown as a blue ring marks a division site on the mother cell surface. Bud scars can be visualized by staining with calcofluor dye or by scanning electron microscopy. In the axial pattern, scars form a continuous chain as shown in the two cells on the left. In the bipolar pattern, scars cluster around the poles: the birth pole (proximal pole) and the pole opposite the birth end (distal pole). (Modified from reference 85 with permission. © 1999 by Annual Reviews.)
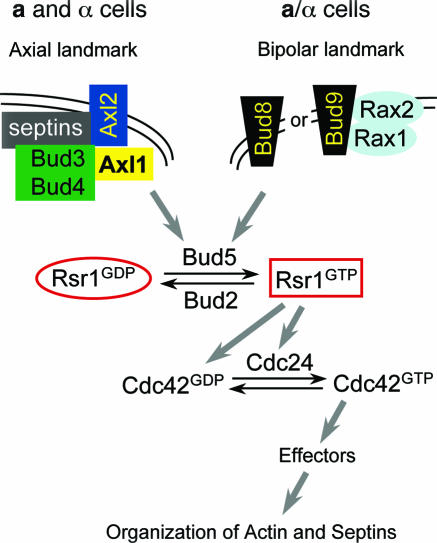
The different budding patterns are likely to occur in response to cell type-specific cortical markers, which are associated with the plasma membrane. A large number of genes are required for producing these specific budding patterns (45, 87, 163, 207, 476, 636). These genes can be divided into three groups based on their requirement for each budding pattern. The first group of genes, which includes RSR1/BUD1, BUD2, and BUD5, is required for both budding patterns and thus encodes proteins that constitute the “general site selection machinery” (89). The second group, which includes BUD3, BUD4, AXL1, and AXL2/BUD10, is required only for the axial pattern. The third group, which includes BUD7 to BUD9, is required only for bipolar budding. The deletion of any of these genes, collectively called “BUD genes,” results in a bud site selection defect but no obvious growth defect. Thus, the BUD gene products are involved in marking the site for polarized growth or directing growth at a specific location. In this section, we will first discuss the Ras-like GTPase Rsr1 and its regulators Bud2 and Bud5, which play a key role in linking the spatial cues to the downstream polarity machinery. We will then discuss how the Rsr1 GTPase module may be coupled to the Cdc42 module. Finally, we will discuss the molecular nature of the spatial landmarks and models for the cell type-specific budding patterns.
The Rsr1 GTPase Module—Rsr1, Bud2, and Bud5
Each component of the Rsr1 GTPase module, Rsr1, Bud2, and Bud5, belongs to a highly conserved family of proteins (45, 86, 87, 435). Rsr1 belongs to the Ras family of GTPases (45). The putative effector region of Rsr1 is identical to that of Ras (45) and is necessary for the interaction with its downstream target, Cdc24 (434). Bud2 is a large polypeptide of 1,104 amino acid resides containing a domain similar to that of the Ras GAP family of proteins such as NF1 (435). Bud2 activates GTP hydrolysis by Rsr1, and thus Bud2 acts as a GAP for Rsr1 (44, 435, 438). Bud5 is likely to encode a polypeptide of 608 amino acid residues (272) containing a domain similar to that of the GEFs for the Ras family of GTPases (86, 453). Bud5 acts as a GEF for Rsr1 (44, 644). Thus, Rsr1 GTPase, the GAP Bud2, and the GEF Bud5 constitute a functional GTPase module involved in the selection of a proper site for growth (435) (Fig. 6).
FIG. 6.
Model for the molecular pathway governing axial and bipolar budding in haploid a or α cells and in diploid a/α cells. Although physical interactions have been demonstrated for some proteins, many of them are postulated based on genetic and localization data. The figure also does not imply that all the proteins shown necessarily interact at the same time (see the text for details and Fig. 11 for Cdc42 effectors and their downstream components). (Modified from the supporting online material from reference 273 with permission of the publisher.)
Phenotypes of the rsr1 mutants provided the first clue for the mechanism by which the Rsr1 GTPase module functions. Expression of rsr1G12V or rsr1K16N, which is expected to encode Rsr1 constitutively in the GTP-bound state or in the GDP-bound (or nucleotide-empty) state, respectively, leads to random budding (484). Consistent with this observation, the deletion of BUD2 or BUD5 results in random budding (86, 435). The cycling of Rsr1 between the GTP- and GDP-bound states is therefore critical for its function in bud site selection. Rsr1 interacts with specific binding partners depending on its GTP- or GDP-bound state (292, 434, 644), as discussed below (see Coupling of the Rsr1 GTPase Module to the Cdc42 GTPase Module).
The localization of Rsr1, Bud2, and Bud5 is consistent with their functions at the presumptive bud site in each cell type. Rsr1 fused to GFP localizes to the plasma membrane and then becomes concentrated at sites of polarized growth, first to the presumptive bud site, the bud tip, and then the mother-bud neck at a later stage of the cell cycle (436). GFP-Rsr1 is also present at the internal organelle membranes, particularly the vacuolar membrane (436). Subcellular fractionation of Rsr1 is also consistent with its localization pattern (434). Localization of Rsr1 to the plasma membrane and to the sites of polarized growth requires both the CAAX box and the polylysine residues near the C terminus (436). The replacement of Cys269 with Ser in the CAAX box of Rsr1 abolishes all membrane association of Rsr1, whereas the replacement of all Lys residues at positions 260 to 264 with Ser disrupts the localization of Rsr1 to the plasma membrane and to the sites of polarized growth but not to the internal organelle membrane (436). Since both mutations cause random budding (436), localization of Rsr1 to the plasma membrane and to the sites of polarized growth is necessary for its function in bud site selection, whereas its localization to the internal organelle membrane is not sufficient for its function. It is not known whether the localization of Rsr1 to the internal membrane indicates other unknown functions of Rsr1 or reflects intermediate locations during its delivery to the plasma membrane.
Bud2 localizes in a patch at the incipient bud site in the late G1 phase: an axial bud site in haploid a or α cells or either the proximal or distal pole of diploid a/α cells (437). Bud2 localizes to the mother-bud neck after bud emergence and then delocalizes around the G2/M phase in all cell types (358, 437). In haploid a or α cells, Bud5 localizes to the presumptive bud site in G1, to the tip of growing buds after bud emergence, and then to the mother-bud neck around the G2/M phase. In the late M phase, Bud5-GFP appears as a double ring at the neck, which splits into two single rings upon cell separation, and both mother and daughter cells inherit a single ring. Thus, most of the newly born G1 cells have the Bud5 ring at the division site (273, 358). This localization pattern of Bud5 is similar to that of Axl2, which is required for the axial budding pattern (see below), throughout the cell cycle. Bud5-GFP exhibits distinct localization patterns in diploid a/α cells, particularly during G1 and M phases. Before bud emergence, Bud5-GFP is present at both poles: as a ring at one pole, which is the previous division site, and in a patch at the opposite pole, which becomes a new bud site. After bud emergence, Bud5-GFP localizes throughout the periphery of the bud, as seen in haploid cells. At a later stage of the cell cycle, Bud5-GFP localizes to the neck and one pole of the mother cell (and/or bud tip), while a small percentage of cells shows a Bud5-GFP signal only at the neck (273, 358). Taken together, these specific patterns of localization of Bud2 and Bud5 are probably important for proper bud site selection, since the overexpression of Bud2 or Bud5 results in the mislocalization of each protein and causes random budding (273). Consistent with this notion, some alleles of BUD5 that disrupt the proper localization of Bud5 only in a/α cells are specifically defective in the bipolar budding pattern (273). Mislocalization of Bud5 in other bud mutants suggests that Bud5 localizes to specific sites in each cell type through the interaction with the cell type-specific landmark (272, 273) (see below).
Coupling of the Rsr1 GTPase Module to the Cdc42 GTPase Module
Interaction between Rsr1 and the Cdc42 module.
Numerous genetic interactions between the BUD genes and genes involved in bud site assembly have suggested a functional interaction between the Rsr1 GTPase module and the Cdc42 GTPase module (for reviews, see references 126 and 221) (Fig. 6). RSR1 was isolated as a multicopy suppressor of a cdc24-Ts (cdc24-4) mutant (45). Certain alleles of CDC24, including cdc24-4, exhibit a bud site selection defect (534, 535). RSR1 also interacts with CDC42 (292) (see below). A bud5 mutation exacerbates the phenotype of a bem1 mutant (bem1-2): the bud5 bem1-2 double mutant fails to undergo bud emergence at the nonpermissive temperature, while the single mutants can (86). Rsr1 also physically interacts with proteins involved in bud site assembly in a guanine nucleotide-dependent manner. Rsr1-GTP interacts with Cdc24 (434, 644) through its putative “Ras-like effector domain” (434) and also with Cdc42 (292), whereas Rsr1-GDP interacts with Bem1 (434).
The interaction between Rsr1 GTPase and Cdc42 GTPase is particularly interesting. RSR1 was identified as an allele-specific dosage suppressor of a cdc42 allele (cdc42-118) that is defective in polarity establishment (292). This suppression requires the “Ras-like effector domain” of Rsr1 and the cycling of Rsr1 between its GDP- and GTP-bound states (292). In addition, an rsr1 deletion mutant was found to be synthetic lethal at 30°C with cdc42-118 (292). Rsr1 also physically interacts with Cdc42, and this interaction appears to be enhanced in the presence of Cdc24 (292). Interestingly, the rsr1-7 mutant, which carries mutations in the polylysine repeat near the C terminus of Rsr1, suppresses a cdc24 mutant but fails to suppress a cdc42 mutant. The mutation may thus disrupt the interaction between Rsr1 and Cdc42 but not the interaction between Rsr1 and Cdc24. These data are consistent with the idea that the interaction between Rsr1 and Cdc42 is likely to be direct, rather than being bridged by Cdc24, a GEF for Cdc42 (292). These findings provide a novel mechanism of action of GTPases controlling polarity establishment. Interestingly, GTPase heterodimerization as a mechanism for activating GTPase signaling in bacteria (518) and plants (607) has recently been reported.
What would the physiological significance of the interaction between Rsr1 and Cdc42 be? The interaction between Rsr1 and Cdc42 may contribute to the localization of Cdc42 to the proper bud site, although it has not been directly addressed. The genetic interaction between RSR1 and CDC42 suggests that Rsr1 functions not only in selection of a growth site but also in polarity establishment. The latter role of Rsr1 becomes phenotypically apparent when polarity establishment is compromised as in a cdc42-Ts mutant (292). In addition, RSR1 is essential in the absence of GIC1 and GIC2, which encode two related targets of Cdc42 that are involved in polarity establishment (278). Cells lacking all three genes, RSR1, GIC1, and GIC2, fail to undergo bud emergence. A detailed analysis of live cells by high-resolution microscopy also indicates that Rsr1 is required for selecting and stabilizing the polarity axis in the G1 phase of the cell cycle (426). Thus, it is likely that Rsr1 has a role in polarity establishment, although the exact role of Rsr1 in polarity establishment is yet to be determined.
Model for coupling bud site selection to polarity establishment.
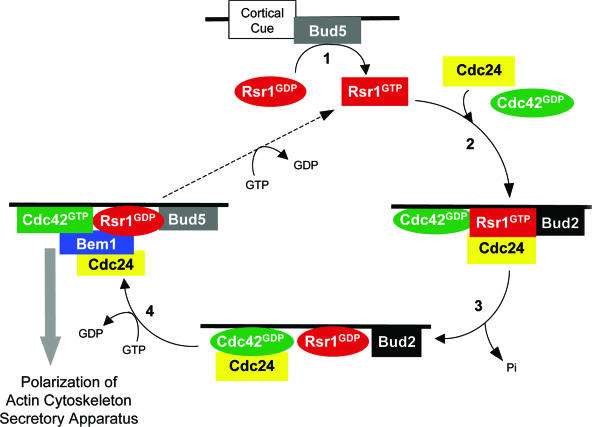
By analogy to Sec4 guiding vesicle targeting to the plasma membrane (61, 601), a scheme in which the Rsr1 GTPase cycle orchestrates bud site assembly to the proper bud site has been proposed (Fig. 7) (292, 434). The model hypothesizes that the cycle between Rsr1-GTP and Rsr1-GDP coupled with the differential affinities of each of these species for binding partners may trigger the ordered assembly of a complex at the bud site. First, Bud5 catalyzes the conversion of Rsr1 from the GDP-bound state to the GTP-bound state at the site where a cortical cue is located (step 1). Rsr1-GTP associates with Cdc24 and Cdc42 and guides these proteins to the presumptive bud site where Bud2 (and Bud5) localizes (step 2). Bud2 activates GTP hydrolysis by Rsr1, resulting in the dissociation of Cdc24 from Rsr1 (step 3). Cdc24 then catalyzes the conversion of GDP-Cdc42 to GTP-Cdc42 (step 4), which then triggers actin cytoskeleton assembly and targeted secretion. Finally, the action of Bud5 may recycle Rsr1, thus allowing further shuttling of the essential components for bud site assembly. Bem1 might join the complex through an interaction with Cdc24, Cdc42, or Rsr1-GDP (58, 69, 434, 446, 644). Several rounds of this cycle may be necessary to assemble critical levels of Cdc42-GTP and its associated proteins at the proper bud site. The model hypothesizes that the ordered assembly of a complex through multiple protein-protein interactions might ensure the establishment of polarity at a correct location. This model provides a simplified view for linking the bud site selection machinery to the Cdc42 module, but the details remain to be tested.
FIG. 7.
Model of how the Rsr1/Bud1 GTPase cycle directs polarity establishment to a specific site. In step 1, Bud5 exchanges GDP for GTP on Rsr1. In step 2, Rsr1-GTP associates with Cdc24 and Cdc42 and guides them to the bud site. In step 3, Bud2 activates Rsr1 to hydrolyze the GTP bound to Rsr1. In step 4, Dissociation of GDP-bound Rsr1 from Cdc24 may activate Cdc24, which catalyzes the exchange of GDP for GTP on Cdc42. Cdc42-GTP then triggers actin assembly and exocyst localization to establish an axis of polarity. Bud5 at the bud site may convert Rsr1 to a GTP-bound state (dashed line), allowing for another cycle of signal transduction (see the text for details). (Adapted from reference 292 with permission of the publisher.)
In the absence of the Rsr1 GTPase module, localization of Cdc24 and Cdc42 to a random bud site may occur through a distinct default pathway yet to be identified or by a “symmetry-breaking” mechanism (245, 604, 605) as discussed above.
Spatial Landmarks That Specify the Site for Polarized Growth
The Rsr1 GTPase module is coupled not only to the key polarity machinery but also to the spatial landmark that specifies the site for polarized growth in each cell type. In this section, we first will describe proteins that are involved in the determination of the axis for cell polarization in each cell type. We will then discuss how the spatial landmarks are linked to the Rsr1 GTPase module and how the cell type-specific budding patterns might be determined.
Axial landmarks.
The axial budding pattern depends on a transient cortical marker that involves a group of proteins such as Bud3, Bud4, Axl1, and Axl2/Bud10 (hereafter called Axl2) (4, 87, 88, 163, 207, 476, 491). Mutations in BUD3, BUD4, AXL1, and AXL2 result in bipolar budding in haploid a or α cells but do not affect normal bipolar budding of diploid a/α cells. Genetic and localization data support the view that the cycle of assembly and disassembly of a protein complex at the mother-bud neck provides a spatial memory of the position from one cell cycle to the next, acting as an inherited landmark for axial budding.
(i) Bud3, Bud4, and septins.
Bud3 and Bud4 localize to the mother-bud neck at or after the G2 phase (88, 491). Prior to cytokinesis, Bud3 and Bud4 appear as a double ring encircling the mother-bud neck, which splits into two single rings, one on each progeny, after cell division (88, 491). Septins, a family of related proteins including Cdc3, Cdc10, Cdc11, Cdc12, and Shs1/Sep7 (335), also play an important role in axial budding. Some alleles of septin genes, such as cdc10-10 and cdc11-6, are defective in axial budding (88, 152). Extra copies of BUD4 suppress the temperature-sensitive growth of a cdc12 mutant (491). Localization of Bud3 and Bud4 depends on the integrity of septins (88, 491), which localize as a ring to the incipient bud site and as a collar to the neck of budded cells (153, 202, 279, 336) (see below). Thus, Bud3 and Bud4 are likely to assemble at the mother-bud neck through a direct interaction with septins during the G2 and M phases, whereas the assembly of septins and the bud site complex at the axial bud site in the subsequent division cycle is likely to occur through the action of the Rsr1 and Cdc42 GTPase modules during the G1 phase (88, 181, 491). However, molecular details of the assembly of the axial landmark and the mechanisms by which the septins determine the localization of Bud3 and/or Bud4 are not known. Understanding the biochemical properties of Bud3 and Bud4 will shed light on their structural and/or regulatory role in axial budding.
(ii) Axl1 and Axl2.
Axl1 and Axl2 are also required for the axial pattern but not for the bipolar pattern (163, 207, 476). Interestingly, Axl1 is expressed in a and α cells but not in a/α cells, and ectopic expression of AXL1 increases axial budding in a/α cells (163). Although Axl1 is likely to be a key component for the cell type-specific budding patterns, how Axl1 functions in axial budding is yet to be established. Axl1 shares homology with the insulin-degrading enzyme family of endoproteases and is also required for processing of the mating pheromone a-factor precursor (4). Amino acid substitutions within the presumptive active site of Axl1 cause defects in the processing of the a-factor precursor but do not perturb bud site selection (4), suggesting that the protease activity of Axl1 is not important for bud site selection.
Axl2 is a transmembrane glycoprotein with an N-terminal signal sequence and a transmembrane domain in the middle. Axl2 is thus predicted to have the type I membrane topology, similar to that of integrin (476). As expected from the predicted structure, Axl2 is heavily glycosylated at the N-terminal half of the protein. Axl2 exhibits no similarity to the ligand-binding or catalytic domains of known transmembrane receptors. It is possible that Axl2 functions in a manner analogous to that of noncatalytic receptors, such as integrins, for which clustering appears to be important for sending a signal to the downstream components (207). However, it remains a possibility that a specific extracellular ligand for Axl2 exists, such as a component of the cell wall. It is also not known whether Axl2 undergoes clustering and the formation of oligomeric complex like the integrin or other extracellular matrix receptors and whether such clustering is required for the axial pattern.
Unlike Bud3 and Bud4, Axl1 and Axl2 are detectable before the G2 phase: the Axl2 signal is most intense in cells with emerging buds and appears at the periphery of small buds (207, 476). The localized Axl1 signal is absent in late G1 phase and weak in the S phase but is prominent in newly divided cells in early G1 phase and in medium- and large-budded cells (341). Both Axl1 and Axl2 localize to the mother-bud neck as a ring in cells with medium- or large-sized buds. Axl1 and Axl2 appear as a double ring encircling the mother-bud neck prior to cytokinesis, and this double ring splits into two single rings after cytokinesis (207, 476). Axl1 localizes normally in the absence of a component of the Rsr1 GTPase module, which is predicted to function downstream of the axial landmark. In contrast, the Axl1-GFP signal is completely lost or diminished in the absence of Bud4 or Bud3, respectively (340). Neither Bud3 nor Axl2 requires the other for localization to the mother-bud neck, although Bud3 seems to be necessary for the efficient assembly of tight double rings of Axl2 at the neck (207). Bud4 seems to localize to the mother-bud neck normally in the absence of Axl2 (476).
Axl2 is delivered to the cell surface via the secretory pathway (452, 490). Since secretion is directed to the incipient bud site, this would suggest that the spatial cue directing targeted secretion is already present at the site before delivery of Axl2 in late G1 phase. In this view, localization of Axl2 to the mother-bud neck during M phase and its subsequent inheritance at the division site after cytokinesis are likely to be more important for the axial pattern than its localization to the incipient bud site in late G1 phase. It is noteworthy that Axl2 fails to localize specifically to the bud side of the mother-bud neck in the pmt4 mutants, which are defective in O-linked glycosylation of some secretory and cell surface proteins, and daughter cells of the pmt4 mutants exhibit a specific defect in the axial pattern (490). These data support the notion that the localization of Axl2 to the mother-bud neck is important for proper bud site selection in the subsequent cell division cycle. On the other hand, a series of experiments addressing the significance of cell cycle-dependent expression of AXL2 indicates that a G1 pulse of AXL2 expression plays a direct role in the localization and function of Axl2 (341), suggesting that the localization of Axl2 depends on the timing of its passage through the secretory pathway. It would be important to understand how the axial landmark is assembled and when/how the axial landmark is linked to the downstream component, i.e., the Rsr1 GTPase module (see below).
Bipolar landmarks.
The bipolar budding pattern appears to depend on the persistent cortical markers that are present at both poles of diploid a/α cells (89). A group of genes including BUD7 to BUD9, RAX1, and RAX2 is specifically required for bipolar budding (93, 162, 271, 636). A mutation in any of these genes disrupts the bipolar budding pattern of a/α cells but does not affect the axial budding pattern of a or α cells.
(i) Bud8 and Bud9.
BUD8 and BUD9 appear to have highly specific roles in the bipolar pattern. The bud8 mutants bud almost exclusively at the proximal pole (the birth pole) (Fig. 5B), whereas bud9 mutants bud predominantly at the distal pole (the pole opposite the birth end) (636). These unipolar patterns are different from the axial pattern, since bud sites do not appear in a sequential chain as viewed by the staining of bud scars. Rather, they occur as a cluster in the vicinity of either pole in no particular order. These distinct phenotypes of bud8 and bud9 mutants indicate that BUD8 and BUD9 encode the key components that mark the poles distal and proximal to the birth pole of the daughter cell, respectively (636). Indeed, GFP fusions of Bud8 and Bud9 localize to the distal pole and the proximal pole of daughter cells, respectively (93, 210, 271, 495). Although one report suggests that both Bud8 and Bud9 localize to the distal pole of daughter cells (553), this discrepancy is likely due to the overexpression of the proteins or differences in strain backgrounds.
Bud8 is delivered to the presumptive bud site (i.e., the distal pole of a newly born cell) just before bud emergence, while Bud9 is delivered to the bud side of the mother-bud neck (which becomes the proximal pole of the daughter cell) just before cytokinesis (495). However, the levels of BUD8 mRNA and BUD9 mRNA peak in late G2/M and G1 phase, respectively, suggesting that their translation and/or delivery to the cell surface are delayed and presumably regulated in a cell cycle-dependent manner (495). This timing of transcription of Bud8 and Bud9 is important for their localization, as shown by a promoter swap experiment (495). The delivery of Bud8 and Bud9 is dependent on actin, and the delivery of Bud9, but not that of Bud8, is also dependent on septins (210, 495). Bud8 fails to localize in cells lacking the formin Bni1, which nucleates the formation of actin cables (458), indicating that the bud tip-directed actin cables are necessary for Bud8 localization (210, 415, 563). Bud8 also fails to localize to the bud tip in the absence of RAX1, whose localization is also dependent on Bni1 (271) (see below). It is puzzling, however, that daughter a/α cells of actin mutants are not defective in distal-pole budding despite the fact that the localization of Bud8 is dependent on actin. It is possible that these actin mutants still have enough actin cables and thus Bud8 directed towards the bud tip (see also below [Other proteins necessary for the bipolar budding pattern]).
Both Bud8 and Bud9 contain a predicted N-terminal extracellular domain, which appears to be heavily glycosylated, and a short cytoplasmic domain that is sandwiched by two transmembrane domains. The cytoplasmic domains of Bud8 and Bud9 are very similar to each other, unlike their extracellular domains. It has been postulated that the similar cytoplasmic domains may provide essentially the same signal at both poles of a diploid cell, thus being recognized by a common downstream target such as a component of the Rsr1 GTPase module (Fig. 6) (210) (see below).
(ii) Rax1 and Rax2.
RAX1 and RAX2 were originally identified as extragenic suppressors of the axl1 mutant (163). Although a functional linkage between Axl1 and Rax1 (or Rax2) still remains to be examined, recent studies indicate that Rax1 and Rax2 are important for bipolar budding (93, 162, 271, 415). The budding patterns of the rax1 and rax2 mutants suggest that both proteins are involved in selecting a bud site at the distal pole of daughter cells as well as at the distal or proximal pole of mother cells (271). Both Rax1 and Rax2 appear to be integral membrane proteins. Rax2 has a type I orientation, with its long N-terminal domain in the extracytoplasmic space (271). GFP-tagged Rax1 and Rax2 localize to the distal pole as well as to the division site on both mother and daughter cells, and their localization to the division sites is persistent through multiple cell division cycles (93, 162, 271). In fact, Rax1 and Rax2 appear to be very stable proteins, unlike Axl2, which undergoes a rapid turnover (93, 271). These properties of Rax1 and Rax2 fit well with the persistent nature of the bipolar landmark, which has been postulated based on physiological observations (89). Localization and biochemical studies suggest that Rax1 and Rax2 interact closely with each other and with Bud8 and Bud9 in the establishment and/or maintenance of the cortical landmarks for bipolar budding (271). Rax1 may be necessary for the efficient delivery of Bud8 and Bud9 to the proper sites.
Several questions regarding the bipolar budding pattern remain unanswered. In particular, what provides the spatial signal for bipolar budding? Although Bud9 at the proximal pole of daughter cells was detectable in their first budding cycle after cell division, and GFP-Bud8 was sometimes detected on the mother side of the mother-bud neck, Bud8 or Bud9 is rarely present at the division sites on mother cells (210, 495). If Rax1 and Rax2 by themselves can provide the spatial signal at these sites as persistent bipolar markers, it is puzzling that they seem unable to do so at the proximal pole of daughter cells.
(iii) Other proteins necessary for the bipolar budding pattern.
The actin cytoskeleton is likely to play a direct role in the placement of bipolar budding cues, since specific actin mutations that have little or no effect on cell growth, such as act1-116 and act1-117, nevertheless perturb the bipolar budding pattern (629). Actin is required for the proper localization of the potential bipolar landmarks Bud8 and Bud9 (210, 495). Interestingly, in a/α cells carrying specific mutations in the ACT1 gene, daughter cells correctly position their first bud at the distal pole of the cell, unlike the mother cells, which bud randomly, supporting the notion that different rules govern bud site selection of mother and daughter cells in a/α diploids (629). Mutations in several other genes, including those that encode components of the “polarisome,” such as BNI1, SPA2, PEA2, and BUD6/AIP3, are defective in the bipolar budding pattern, but the wild-type gene products of these mutants are also involved in other cellular processes (415, 521, 538, 588, 636). Mutations in RVS161, RVS167, AIP3/BUD6, SEC3, SEC4, SEC9, END3, VRP1, PHO85, MSB3, or MSB4 also convert the bipolar pattern to a random pattern without affecting the axial pattern (51, 150, 533, 538, 563, 564, 584, 588, 629, 636). In addition, a genome-wide screen for mutants defective in bipolar budding has identified many other genes, including genes involved in protein modification, lipid metabolism, gene transcription, and translation (415). It is unlikely that all of these gene products have a direct role in bipolar budding. Some of these genes are likely to control the actin cytoskeleton or secretory pathway and thus affect the delivery of bipolar landmarks such as Bud8, Bud9, Rax1, or Rax2. Others may be involved in the expression of a component of the bipolar landmark.
Regulation of Cell Type-Specific Budding Patterns
Coupling of spatial landmarks to the Rsr1 GTPase module.
Bud2 and Bud5 are likely linked to the cell type-specific landmarks. Bud5 mislocalizes in haploid a cells carrying a deletion of AXL2 (axl2Δ) but not in diploid a/α cells homozygous for axl2Δ. Similarly, Bud5 mislocalizes in haploid cells lacking Bud3 and, to a lesser extent, in cells lacking Bud4 (273, 358). Bud5 also fails to localize properly in a/α cells homozygous for bud8Δ but not in a bud8Δ cells (273). These localization studies suggest that the interaction of Bud5 with the axial or bipolar landmark determines its localization in haploid or diploid cells, respectively (Fig. 6). Consistent with this notion, Bud5 copurifes with Axl2 and Bud8 (272, 273). This idea is further supported by the isolation of bipolar-specific alleles of BUD5, which are defective in the bipolar budding pattern but not in the axial pattern (272, 273, 636). These bipolar-specific bud5 mutant proteins fail to localize in diploid a/α cells but not in haploid a cells (272, 273).
The distinct localization of Bud2, a GAP for Rsr1, and the isolation of the bipolar-specific alleles of BUD2 suggest that Bud2 localizes to the presumptive bud site independently of Bud5. Although Bud2 and Bud5 appear to localize to the presumptive bud site independently, localization of each protein to the proper bud site cannot be maintained in the absence of the other protein (273, 437). Mutations in the conserved residues of the putative GAP domain of Bud2 lead to random budding in all cell types (A. Sanson and H.-O. Park, unpublished data), whereas specific bud2 mutants that are defective in the bipolar pattern but not in the axial pattern have been isolated (636). Thus, the Bud2 GAP activity is necessary for both budding patterns, whereas a distinct domain of Bud2 is likely to be involved in the interaction with the bipolar landmark. It is not known yet whether Bud2 directly interacts with any spatial landmark. Localization studies suggest that Bud2 is likely to arrive at the presumptive bud site after Bud5 during the G1 phase (437). Thus, Bud5 may be important for initiating the Rsr1 GTPase cycle by activating Rsr1 to the GTP-bound state, whereas Bud2 may be more important for targeting the bud site assembly proteins, which are brought to the presumptive bud site through the interaction with Rsr1-GTP, to the proper bud site (see below).
Model for cell type-specific budding patterns.
Budding patterns depend on the cell types rather than ploidy (158, 222). Cell types are controlled by transcriptional regulators encoded by the mating loci MATa and MATα (220). How is the spatial information inherited from one cell division cycle to the next, thus allowing cells to exhibit the specific budding patterns with such high fidelity? Despite our current knowledge of several proteins that control or constitute the spatial landmarks, the mechanism by which the cell type-specific budding pattern is determined is mostly unknown. Here, we consider the two simplest models to explain the budding patterns, although many other models are also possible.
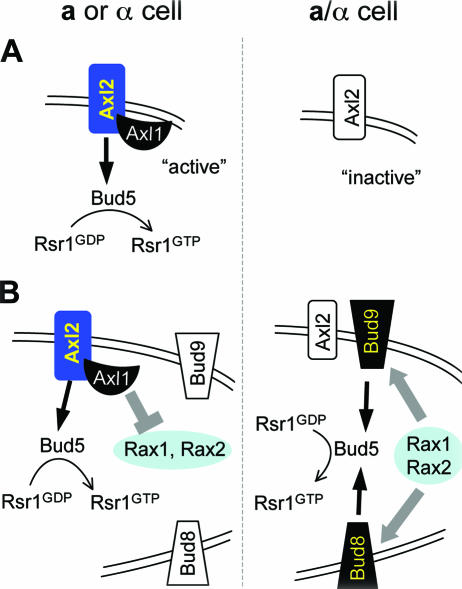
It has been hypothesized that the cell type-specific budding pattern is produced by the transcriptional repression of some genes critical to axial budding by the corepressor a1-α2 present in diploid a/α cells (87). AXL1 may be such a gene, because Axl1, which is necessary only for axial budding, is expressed in a or α cells but not in a/α cells (163). Furthermore, ectopic expression of AXL1 in a/α cells reverts bipolar budding to axial budding to some extent (163). Thus, Axl1 may be a key component of the axial landmark or may facilitate the usage of the axial landmark in a or α cells. According to this model, an axial spatial landmark such as Axl2 would be active only in the presence of Axl1. In a/α cells where AXL1 is not expressed, the axial landmark is no longer active. This model predicts that bipolar budding may occur by default (Fig. 8A). However, some recent data suggest that the determination of the cell type-specific budding pattern is more complex than this model would predict. In the absence of both putative bipolar landmarks, Bud8 and Bud9, diploid a/α cells exhibit a partially randomized budding pattern with an increased tendency to bud at the proximal pole rather than at random sites, suggesting that a/α cells have some ability to use the axial cues in the absence of both putative bipolar landmarks. In fact, when a potential axial landmark is also absent in the a/α bud8 bud9 mutant, a more fully randomized budding pattern is observed (210). These observations suggest that bipolar budding does not occur simply by default. The second model hypothesizes that the bipolar budding pattern is established by the concerted action of multiple proteins including Bud8, Bud9, Rax1, and Rax2, which are specifically required for bipolar budding. According to this model, Axl1 may block the function of Rax1 and/or Rax2, thus inhibiting bipolar budding in haploid a or α cells. In diploid a/α cells where AXL1 is not expressed, Rax1 and Rax2 would be active for the establishment of the bipolar landmark (Fig. 8B). Several aspects of these models remain to be tested. Many factors other than those considered in these models are also likely to contribute to cell type-specific budding patterns.
FIG. 8.
Models for cell type control of budding patterns. (A) Axl1 may act positively on the axial landmark proteins, and bipolar budding may occur as a default in the absence of Axl1 in a/α cells. (B) Axl1 may act indirectly by blocking the function of Rax1 and Rax2, which play a positive role in promoting bipolar budding in the absence of Axl1 in a/α cells. For simplicity, only Axl2, which interacts with Bud5, is shown as an axial landmark (see the text for details).
TEMPORAL CONTROL OF POLARITY ESTABLISHMENT DURING YEAST BUDDING
Control of the cell division cycle and several cell cycle-regulated events such as DNA replication, mitosis, and cellular morphogenesis in budding yeast have been well described elsewhere previously (317). Here, we will limit our discussion to potential regulatory mechanisms for the control of bud emergence. An important, but still poorly understood, aspect of budding is how bud emergence is triggered at the right time in the cell cycle and also only once per cell cycle. Activation of the cyclin-dependent protein kinase Cdc28 complexed with a G1 cyclin, Cln1 or Cln2 (G1 CDK), is crucial for the polarization of the actin cytoskeleton in the late G1 phase (316). Since bud site selection and bud site assembly are likely to precede polarized actin organization (Fig. 2) (126), a protein functioning in these steps may be regulated by G1 CDKs. Consistent with this notion, recent studies suggest that the localization of Cdc24, a GEF for Cdc42, to the incipient bud site requires the G1 CDK (196, 390). In addition, Cdc24 is phosphorylated by Cdc28/Cln kinase in vitro (390). However, mutations in six putative CDK phosphorylation sites of Cdc24 do not seem to affect its function in vivo (196). It is thus unlikely that G1 CDKs directly regulate the GEF activity or localization of Cdc24 by phosphorylating Cdc24 in vivo. Analyses of some of cdc42 mutants such as cdc42G60D and cdc42D38E have also suggested that Cdc42 plays a critical role in ensuring that bud emergence occurs once per cell cycle (84, 465). Regulation of Cdc24 GEF activity is thus likely to be crucial for polarity establishment at the right time in the cell cycle. However, how Cdc24 is regulated is still not clear. It has been proposed that Cdc24 is autoinhibited and that the inhibition is released by its interaction with Rsr1 and Bem1 during vegetative growth and with Far1 during mating (523, 614). Although these are intriguing possibilities, whether Rsr1 and/or Far1 activates Cdc24 has not been directly tested.
Although Cdc24 is unlikely to be a direct target of G1 CDKs, it is phosphorylated by the PAK Cla4, which is a target of Cdc42 (58, 69). There are opposing views about the functional significance of Cdc24 phosphorylation. One study suggests that the phosphorylation of Cdc24 by Cla4 leads to the dissociation of Cdc24 from Bem1, ending polarized bud growth (196). On the other hand, another group found no difference in the association of phosphorylated Cdc24 and Bem1. In addition, overexpression of a B-type cyclin mutant, clb1Δ152, which is known to trigger the depolarizing switch effectively, did not alter the association of Cdc24 with Bem1 (58). Instead, that study argued that Cdc24 phosphorylation by Cla4 is likely to be a part of the positive feedback loop to promote polarized growth. Thus, it is not clear whether phosphorylation is involved in the initial activation of Cdc24 at bud emergence or termination of polarized growth.
Bem1 may also play an important role in the temporal regulation of polarity establishment. Bem1 might be a direct target of CDK since recent proteomic data using an analog-sensitive cdc28 mutant (cdc28-as) identified Bem1 as one of many proteins phosphorylated by Cdc28 (580). A positive feedback loop has been proposed in which Cdc42-GTP interacts with Bem1, which localizes to the incipient bud site and stabilizes Cdc24 at the site, and Cdc24 in turn activates Cdc42, leading to apical growth (58, 69). However, the temporal order of these events at the bud site is not completely understood. Several questions need to be addressed in order to assess the role of Bem1 in polarized growth. For example, is Bem1 phosphorylated by CDK in vivo? If so, when does it occur in the cell cycle? Is Bem1 phosphorylation important for bud emergence or is it involved in the termination of polarized growth?
Some of the potential landmark proteins for bud site selection may be regulated in a cell cycle-dependent manner. A proteomics study using cdc28-as indicates that Bud3, Bud4, and Axl2 are phosphorylated by CDK (580). Although the functional significance of the phosphorylation of these Bud proteins is not known, it is tempting to speculate that a landmark protein whose activity is regulated by CDK triggers the activation of the Rsr1 GTPase module at a specific time. The Rsr1 GTPase module may then promote polarity establishment at the right time and at the right place. Another potential target of cell cycle-dependent regulation is the Rsr1 GAP Bud2, since a bud2 mutant is lethal in the absence of two G1 cyclins, CLN1 and CLN2 (48, 103). Recent genome-wide two-hybrid screens also identified interactions between BUD2 and CLN2 (123, 581). However, whether Bud2 is a target of the G1 CDK is not known. In summary, despite the existence of many pieces of thought-provoking data, how polarity establishment is regulated in a cell cycle-dependent manner is not well understood.
Cdc42 EFFECTORS AND THEIR ROLES IN POLARITY DEVELOPMENT
Cdc42 is required for the independent polarization of the actin and septin cytoskeletons. Cdc42 is also required for polarized exocytosis, mainly through its role in actin organization (66, 247, 259, 454, 456) but also through its direct interactions with the secretory components (5, 641). There are three classes of Cdc42 effectors: the formin Bni1 (138); the PAKs Ste20 (532, 643), Cla4 (102), and Skm1 (361); and the pair of CRIB motif-containing, yeast-specific proteins Gic1 and Gic2 (67, 68, 90). In the following section, we will describe the roles of the actin and septin cytoskeletons in polarized cell growth and how Cdc42 regulates the polarized organization of these cytoskeletons through its effectors. We will also discuss exocytosis and other biological processes in which Cdc42 is involved.
Actin Organization
There are three filamentous actin structures in S. cerevisiae: actin patches, actin cables, and actin rings (398) (Fig. 9B). Actin patches are concentrated at sites of polarized growth, and actin cables are polarized towards these sites. The actomyosin-based ring assembles at the mother-bud neck in late anaphase (9, 274). Mutations in ACT1, the only gene encoding actin in S. cerevisiae, or the disruption of F-actin by latrunculin A causes depolarized growth but does not block secretion per se (30, 418). Together, these data firmly establish that the polarized actin cytoskeleton is required for polarized exocytosis. The key question is how Cdc42 regulates the polarized organization of these actin structures.
FIG. 9.
Localization of Cdc42 and Cdc42-controlled actin and septin organization. (A) Cdc42 localization (green) and the directionality of cell growth (blue arrows) in the cell cycle are indicated. Components of the exocytic machinery such as the exocyst display a similar localization pattern as Cdc42. A cell cycle-triggered switch from apical growth to isotropic growth occurs around the G2/M transition. (B) Actin organization in the cell cycle. Actin patches (red spots), actin cables (red lines), and the actin ring (red circle) are indicated. The short, branched actin filaments in the patches are nucleated by the Arp2/3 complex and its activators, whereas the linear actin filaments in the cables and the rings are nucleated by the formins Bni1 (brown) and Bnr1 (yellow), whose localization patterns are also indicated. Presumably, Bni1 exists in the bud cortex during the isotropic growth phase at G2/M phase, but its concentration is too low to be detected by fluorescence microscopy. Thus, Bni1 is drawn as a dashed line around the bud cortex at the G2/M phase. It is not clear whether Bnr1 still localizes at the bud neck after the actomyosin ring contraction and is thus indicated as a dashed line in telophase cells. (C) Septin organization in the cell cycle. Septin collar or hourglass (purple) formation involves at least three steps: septin recruitment, which requires Cdc42-GTP, its effectors Gic1 and Gic2, and possibly the polarisome; septin ring assembly, which requires Cdc42 cycling between the GDP- and GTP-bound states, its GAPs, and possibly its effector Cla4; and maturation of the septin ring into a collar, which requires Cla4 and GTP binding of the septins.
Actin patches and endocytosis.
Signaling pathways critical for cellular processes such as cell growth, cell polarity, cell motility, stress responses to environmental stimuli, nutrient uptake, and ion homeostasis are all initiated at the plasma membrane. Many proteins and lipids involved in these processes are recycled back to the plasma membrane or are targeted for degradation through endocytosis (131, 135). In S. cerevisiae, there is receptor-mediated endocytosis as well as fluid-phase endocytosis, both of which require the actin cytoskeleton for the internalization step (135, 403). Recent studies have led to a much clearer picture of the molecular pathways underlying receptor-mediated endocytosis (263, 267, 268, 413), whereas the mechanisms underlying fluid-phase endocytosis remain unclear. The best-studied receptor-mediated endocytosis in S. cerevisiae involves the internalization and degradation of Ste2, the receptor for the mating pheromone α-factor (135, 403). Endocytosis also regulates cell wall assembly by controlling the levels of the plasma membrane-associated, cell wall synthetic enzymes such as chitin synthase III (Chs3) (585, 649) and 1,3-β-glucan synthase (Fks1) (135, 583).
Actin patches are motile, short-lived, cortical foci with a diameter of ∼200 nm (475) and a life span of ∼10 s (76, 121, 274, 537, 599). The patches are made of short, branched actin filaments (475, 634). These filaments are nucleated by the evolutionarily conserved Arp2/3 complex (451, 615, 616), which is activated by Las17 (also known as Bee1), a yeast homolog of Wiskott-Aldrich syndrome protein (WASP) (319, 350, 615), Pan1 (129, 560), the type I myosins Myo3 and Myo5 (139, 171, 172, 190, 303), Abp1 (127, 189), and F-actin (for a review, see reference 398). The Arp2/3 complex is also required for actin patch motility (267, 616). Accumulating evidence indicates that actin patches are the sites of endocytosis. First, there are approximately 50 genes involved in receptor internalization, and most of these gene products colocalize with the actin patches. Mutations in these genes cause various defects in endocytosis (135, 457). Approximately one-third of these endocytic proteins regulate actin dynamics, i.e., the assembly and the disassembly of the actin patches (135). Second, actin patches are associated with the invaginated plasma membrane (401, 475), consistent with the role of actin at the internalization step of endocytosis (299). Finally, vesicles (endosomes) labeled with the lipophilic membrane dye FM4-64 colocalize with actin patch components (236, 268).
Various cell biological approaches involving live-cell imaging of fluorophore-tagged proteins in wild-type and endocytic mutant strains have led to a working model of how the modular endocytic machinery is assembled and functions at the internalization step of clathrin- and actin-mediated endocytosis (267, 268, 398). The first module, called the coat module, consists of clathrin, Sla1 (contains four SH3 domains), Pan1 (contains two Eps15-homologous domains), End3 (contains two Eps15-homologous domains), and Sla2 (contains one epsin N-terminal homology [ENTH] domain, which may bind to phosphoinositides, and one talin-like domain) (131, 188, 384). This module may also include other ENTH domain-containing proteins such as Ent1/Ent2 and Yap1801/Yap1802 (putative clathrin adaptor proteins in yeast) (131, 384). These adaptor proteins, which bind to PIP2 (phosphatidylinositol bisphosphate) via their ENTH domains (11, 250, 251), are required for the cortical association of clathrin (413). Sla1 and End3 may regulate the initiation of actin polymerization by binding to the activators of the Arp2/3 complex, such as Pan1 (560) and Las17 (413). Sla2 interacts with PIP2, clathrin, and F-actin via its N-terminal ENTH domain, the central coiled-coil region, and the C-terminal talin-like domain and thus potentially links the endocytic machinery to the actin cytoskeleton (267). Overall, the coat module likely recruits cargoes and regulates actin dynamics.
The second module, called the actin module (268), is thought to regulate coat internalization. The actin module includes actin, the capping proteins Cap1 and Cap2, which function as an obligatory heterodimer (18, 19, 281), the fimbrin Sac6 (7), the actin-binding protein Abp1 (127), and the Arp2/3 complex (617). These components target to the actin patches in an F-actin-dependent manner (268). The third module, called the WASP/Myo module, includes the yeast WASP Las17 (319, 350, 615), the type I myosins Myo3/Myo5 (139, 171, 172, 190, 303), and Bbc1/Mti1 (389, 541). This module remains immotile at the plasma membrane and is thought to regulate actin filament nucleation via the Arp2/3 complex. The last module, called the amphiphysin module, consists of Rvs161 and Rvs167 (57, 100, 160, 334), both of which contain BAR domains that are known to tubulate membranes in vitro (442). The timing of the recruitment of the Rvs161-Rvs167 heterodimer (160) to the actin patches is consistent with a role in endocytic vesicle scission (382). Type I myosins and phosphoinositides are also thought to be involved in the scission process (263, 548). These four modules function in a highly coordinated manner to carry out the internalization step of endocytosis.
Cdc42 effectors and actin patches.
Actin patches are concentrated at sites of polarized growth in the cell cycle (9, 457). The major question is how does Cdc42 trigger the polarization of the patches at these sites? Cdc42 is required for the docking and/or the fusion of vacuolar membranes in S. cerevisiae (133, 402) but does not seem to play an important role at the internalization stage of endocytosis, although this issue has not been comprehensively examined. Most of the known cdc42-Ts and cdc24-Ts mutants still form numerous actin patches at the nonpermissive temperature, but these patches are randomly distributed in the cell cortex in unbudded and budded cells (8, 9, 260), suggesting that Cdc42 is required for the polarized organization, but not the assembly, of the actin patches. However, it is still possible that Cdc42 plays a minor role in the regulation of actin patch assembly and/or dynamics in wild-type cells. This conclusion is supported by the following facts. First, permeabilized cdc42-Ts cells are defective in actin patch assembly in vitro, and this defect can be corrected by purified recombinant GTP-locked Cdc42 (320) or the recombinant PAK Ste20 (132). Second, the PAKs Ste20 and Cla4 are known to phosphorylate the type I myosins Myo3 and Myo5 (618, 619), which are thought to function downstream of Cdc42 and act together with Las17 and Vrp1 (yeast WIP, WASP interacting protein) to regulate actin patch assembly (139, 171, 303). These results led to a hierarchal pathway starting from Cdc42-GTP to PAKs, to type I myosins plus Las17 and Vrp1, and to the Arp2/3 complex, leading to actin patch assembly (302). This pathway explains why the yeast WASP Las17 does not have a Cdc42-GTP-binding motif like its mammalian counterparts. However, this PAK-mediated pathway does not play a major role in either actin patch assembly or the polarized organization of the patches, because conditional lethal alleles (Ts or ATP analog sensitive) of CLA4 in an ste20Δ background do not affect the overall assembly or the polarized organization of actin patches (102, 186, 608). It is not clear whether the PAK-activated, myosin I-mediated pathway regulates the dynamics of the actin patches. In shmoos of myo3Δ myo5Δ cells, the life span of actin patches is increased threefold, and the number of motile patches is decreased significantly, although the velocity of the moving patches is unchanged (537). These results are consistent with the suggestion that type I myosins may be involved in the scission process of the endocytic vesicles (263).
What mediates the Cdc42-dependent polarization of the actin patches? There are two general possibilities. First, the earliest localized endocytic components, for example, those of the coat module, are good candidates for receiving the Cdc42-initiated polarization signal. Indeed, a recent study indicates that the ENTH domain of Ent1 and Ent2, redundant epsin-like proteins that are required for endocytosis and that are thought to be components of the coat module, interacts with Cdc42 GAPs Rga1, Rga2, and Bem3 (10). These interactions are independent of the endocytic role of Ent1 and Ent2 and are believed to temporally and spatially link polarity machinery to endocytosis machinery. Ent2 also interacts with Cdc24 in a two-hybrid assay (123), although the physiological significance of this interaction is not clear. Second, a putative factor involved in actin patch clustering may be delivered via post-Golgi vesicles and actin cables to the sites of polarized growth (168). This hypothesis explains why actin patches are always clustered at the ends of actin cables (9, 274) and why a defect in secretion or actin cable assembly can cause defects in actin patch organization (168, 461). This hypothesis also implies that Cdc42 does not have to interact directly with actin patch components. Formins (Bni1 and Bnr1), which are required for the formation of actin cables (140, 458, 486, 487) and actin rings (573) (see below), are also required for the targeting of the yeast WASP Las17 to the sites of polarized growth (302). In addition, the yeast WIP Vrp1 is required for the targeting of Las17 to the actin patches (302, 537). However, it is not clear whether the formins interact directly with Vrp1 or other patch components or whether formins affect actin patch polarization indirectly through their function in actin cable formation.
Actin cables and exocytosis.
Actin cables are dynamic structures that disappear within ∼15 s in cells treated with the actin monomer-sequestering drug latrunculin A (LatA) (30, 274, 628). Actin cables are aligned along the cortex of the cell from the mother cell compartment to the presumptive bud site or to the daughter cell compartment. Actin cables are polarized towards sites of active cell growth, where actin patches are clustered (9, 274, 418) (Fig. 9B). For this reason, actin cables and patches are thought to mediate polarized exocytosis (9). There are two arrays of actin cables in budding yeast: one nucleated by the formin Bni1, which localizes to the bud tip, and the other nucleated by the formin Bnr1, which localizes to the bud neck (138, 140, 242, 289, 398, 399, 458, 459, 486, 487, 628, 636) (Fig. 9B). Deletion of either BNI1 or BNR1 is tolerated by the cell, but double deletion is lethal (138, 242, 270, 289, 587), suggesting that cells can survive with one array of actin cables directed towards either the bud tip or the bud neck. Cells carrying bni1-Ts bnr1Δ alleles lose actin cables rapidly at the nonpermissive temperature without affecting actin patch assembly, although the polarization of the patches is lost over time. As a result of the cable loss, most cells become large, round, and unbudded (140, 487). The current thought is that the formins Bni1 and Bnr1 nucleate actin filaments independently of the Arp2/3 complex. These filaments are stabilized by tropomyosins and other actin-binding proteins such as Sac6 (yeast fimbrin) (7) and ABP140 (23, 628), forming actin cables.
Exocytosis is a multistep process in which an intracellular vesicle moves to the plasma membrane, and the fusion of the vesicular membrane and plasma membrane ensues, resulting in the release of vesicular content into the extracellular space (Fig. 10). Each step of exocytosis is regulated by distinct Rab GTPases and their effector pathways (199, 419, 544). When exocytosis is instructed by a polarization signal, polarized exocytosis occurs, suggesting intimate interactions between the polarity machinery and exocytosis during polarized cell growth. The final step of exocytosis, i.e., the transport, tethering, and fusion of the post-Golgi vesicles with the plasma membrane, should provide ample targets for such interactions. The post-Golgi vesicles are transported by the type V myosin Myo2 using actin cables as tracks (66, 460), and the transport also depends on the Rab GTPase Sec4 and its GEF, Sec2, both of which are associated with the vesicles (192, 600). Vesicle tethering is carried out by an evolutionarily conserved complex called the exocyst, which consists of eight subunits (Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84) in S. cerevisiae (566). The exocyst is an effector of Sec4 (198) and is thought to form dynamically during each round of exocytosis (63). Sec4 and seven subunits of the exocyst, including Exo70, are associated with the post-Golgi vesicles, whereas Sec3 and a fraction of Exo70 are associated with the plasma membrane at sites of polarized growth (63). Upon the arrival of a vesicle at the plasma membrane, the entire exocyst is formed and tethers the vesicle to the plasma membrane for the subsequent membrane fusion event that is mediated by the SNARE proteins (63, 480). The target SNAREs for the post-Golgi fusion, including Sec9 (65, 309) and Sso1 (1, 591), localize uniformly to the plasma membrane. The vesicle SNARE Snc1 is carried on the vesicles (455) and localizes to sites of polarized growth (318). Sec1, a protein required for exocytosis (420, 508), localizes to sites of polarized growth (77) and interacts with both the exocyst and the SNARE complexes (612). These components of the vesicle tethering and fusion machinery represent excellent targets for regulation by polarity machinery.
FIG. 10.
Role of Cdc42 in polarization of actin cables and exocytosis. Cdc42 regulates the polarization of actin cables by directly interacting with the formin Bni1 and regulating its localization and/or activation. Cdc42 controls polarized secretion mainly via its role in actin cable organization, but it also interacts directly with the exocyst subunit Sec3, endowing the exocyst with one polarization signal. Rho1 and Rho3 also interact with Sec3 and Exo70, respectively, providing the exocyst additional polarization signals. Sec4 (brown) and its GEF, Sec2 (light blue), associate with the secretory vesicles to guarantee Sec4 in its GTP-bound form, which is required for vesicle transport from the mother cell to the bud and also for the interaction with the vesicle-associated partial exocyst through a Sec4-Sec15 interaction (all exocyst subunits are shown in light gray). Upon vesicle arrival at the bud tip, the exocyst components on the vesicle interact with Sec3 and the fraction of Exo70 already localized at the bud tip, resulting in the assembly of a complete exocyst, which is required for vesicle tethering. Thus, an exocyst is formed dynamically for each round of vesicle transport. After fulfilling its function at the plasma membrane, Sec4-GTP is hydrolyzed with the help of its GAPs Msb3 and Msb4, and Sec4-GDP returns to the cytosol. By segregating its GEF and GAPs at distinct locations, the Sec4 GTPase cycle is employed to carry out its exocytic functions efficiently. Msb3, Msb4, and Bni1 are brought to close proximity by interacting with distinct domains of Spa2. SHD, Spa2 homology domain.
In the last decade, compelling evidence indicates that actin cables direct polarized secretion (66, 275, 461), whereas actin patches are involved in endocytosis (see above) (135, 398, 457). Tropomyosins are the only proteins known to decorate actin cables specifically (457). There are two isoforms of tropomyosins, the major isoform Tpm1 and the minor isoform Tpm2, both of which together define an essential role in maintaining the actin cable structures (122, 330, 331, 461). Shifting tpm1-2 tpm2Δ mutant cells to the nonpermissive temperature for ∼1 min results in the loss of actin cables and the depolarization of exocytosis and cell surface growth, without affecting the polarization of actin patches and endocytosis, suggesting that actin cables are responsible for exocytosis (461). In contrast, the las17Δ mutant is defective in actin patch polarization and endocytosis (404, 540) but not exocytosis (275). Actin cables are thought to function as tracks along which the type V myosin Myo2 moves secretory vesicles (193, 261, 503, 505) and various organelles, including vacuoles (80-82, 224, 248), peroxisomes (227), late compartments of the Golgi apparatus (478, 479), nucleus (40, 305, 567, 630), and, perhaps, mitochondria (251, 252) from the mother cell to the daughter cell. Another type V myosin, Myo4, also uses actin cables to transport specific mRNAs from the mother cell to the daughter cells (56, 254, 530, 555). More detailed discussions of the roles of actin cables and myosin V's in exocytosis and organelle segregation can be found in several excellent reviews (66, 247, 398, 460, 504).
Cdc42 effectors and actin cables.
Formins are a family of proteins that nucleate actin cables in yeast and stress fibers in mammalian cells (15, 141, 211, 223, 398, 423, 647). This family of proteins, in general, has a small GBD at the N terminus and a conserved formin homology 2 (FH2) domain at the C terminus. The FH2 domain is necessary and sufficient for the nucleation activity of the formins (458, 486), and FH2 forms a dimer, which associates with the barbed ends of actin filaments (399, 458, 626). Formins can function as a processive “leaky” cap that allows the elongation of the filament while capping the barbed end, as in the case of Bni1 in S. cerevisiae (647, 648). In other cases, such as the formin Cdc12 in the fission yeast S. pombe, it functions as a “tight” cap that blocks elongation but can be opened by binding of profilin (291). The nucleation activity of FH2 is thought to be negatively regulated by an autoinhibitory loop formed between the N-terminal GBD and the diaphanous-autoregulatory domain (DAD), which is located within the C-terminal end of the FH2 domain. Binding of an active Rho GTPase to the GBD opens up the loop, leading to the activation of formins (15, 16, 141, 211, 223, 423, 647). FH2 activity is also regulated positively by profilin, which binds to the upstream FH1 domain (291, 458, 486). In the case of Bni1, but not Bnr1, the actin-binding protein Bud6 binds to a region of Bni1 immediately downstream of the DAD and regulates FH2 activity (397, 399). Bni1 also binds to the polarity protein Spa2 (164) and to translation elongation factor 1α (EF1α), which binds and bundles F-actin (582). The functional significance of these interactions is not known.
The regulation of formins by Rho GTPases has been best illustrated with RhoA and the mammalian formin mDia2 (mammalian diaphanous) (16). In S. cerevisiae, the GBD of Bni1 has been shown to interact with Cdc42 (138) and Rho1 (289), while the GBD of Bnr1 has been shown to interact with Rho4 (242, 270). As discussed in previous sections, Cdc42 is involved mainly in the polarized organization, but not the assembly, of the actin cables and patches. Cdc42 may play a role in actin cable polarization, at least in part, by regulating Bni1 localization. The localization of Bni1 at sites of active cell growth is completely lost in a cdc42-Ts mutant grown at the nonpermissive temperature (425). Interestingly, multicopy CDC42 suppresses both the lethality and the actin cable assembly defect in rho3 rho4 mutant cells, suggesting that Cdc42 also plays a minor, but important, role in the local activation of Bni1 in the cell (116, 370). In addition to the direct interaction between Cdc42 and Bni1, Cdc42 also regulates the phosphorylation of Bni1 through its effector, the PAK Ste20 (187), although in vitro studies indicate that the phosphorylation of the C-terminal half of Bni1 (from FH1 to COOH) is not required for its nucleation activity (397). The functional significance of this phosphorylation is not known. It also has yet to be established whether Cdc42 affects the assembly and/or organization of the actin cables directly or indirectly via other pathways.
How does Cdc42 direct polarized exocytosis? Cdc42 is likely to contribute to this process by directing the polarized organization of the actin cables (see above) (66, 460). Accumulating evidence, however, suggests that Cdc42 may play a more direct role in exocytosis that is independent of its role in actin organization. A temperature-sensitive mutation in CDC42, cdc42-6, causes post-Golgi vesicle accumulation at 33°C, without displaying apparent defects in actin organization. Thus, Cdc42 may regulate the vesicle docking and fusion apparatus to provide maximal function at sites of polarized growth (5). In addition, Cdc42 interacts with the N-terminal domain of Sec3, a component of the exocyst, which localizes to the sites of polarized growth independently of F-actin (149), and this interaction is required for the polarized localization of Sec3 (641). Sec3 is unique among the exocyst components in that a deletion of SEC3 does not cause cell lethality or a significant defect in secretion at 25°C (150, 613). Overexpression of Sec1 or Sec4 can suppress the lethality and secretion defect of sec3Δ cells at 37°C (612), suggesting that other components of the exocytic machinery, such as Sec4, Sec1, and perhaps the vesicle SNARE Snc1, may also interact with Cdc42. Msb3 and Msb4, which display GAP activity towards Sec4 (12, 13, 168), localize to sites of polarized growth, and their localization depends on Cdc42. In addition, multicopy MSB3 and MSB4 suppress cdc24 and cdc42 mutants, and Msb3 and Msb4 also interact specifically with Cdc42-GDP (51, 168, 563). These results suggest that Msb3 and Msb4 define another linkage between Cdc42 and exocytosis.
Cdc42 is likely involved in exocytosis in mammalian cells as well. Mammalian Cdc42 controls protein sorting to the basolateral plasma membrane of epithelial cells (297), and Cdc42 localizes predominantly to a brefeldin A-sensitive compartment of the Golgi apparatus (136). Mammalian Cdc42 interacts with the coatomer at the Golgi apparatus (622) and recruits the Arp2/3 complex to stimulate actin assembly, which thus promotes Golgi-derived vesicle formation (161, 547). Coatomer-bound Cdc42 also inhibits dynein recruitment to the COPI vesicles (92). Although both yeast and mammalian Cdc42s function in exocytosis, their predominant locations of action and mechanisms of action may be different in these systems.
Septin Organization
General properties and functions of the septins.
Septins are a family of GTP-binding, filament-forming proteins that are conserved from yeast to humans but are noticeably absent in plants and Dictyostelium (99, 144, 148, 180, 184, 205, 276, 283, 335, 336, 347, 391, 414, 485, 543, 575, 594). The number of septin genes varies among organisms, with 7 in S. cerevisiae and S. pombe, 2 in Caenorhabditis elegans, 5 in Drosophila, and 13 in mammals (594). All septins share a similar structure, including a central GTP-binding domain, a polybasic region, which binds to phosphatidylinositol polyphosphates (PIPs), and a septin-unique domain. Most septins have a coiled-coil region at their C termini and have variable-length N termini (485, 594). Septins purified from yeast, Drosophila, and mammals or recombinant septins form heteromeric complexes, which polymerize into filaments in vitro (143, 147, 157, 234, 284, 381, 519). The role of GTP binding and hydrolysis in septin functions is not well established. Some data indicate that GTP binding is essential for septin filament formation (381), while other data indicate that GTP binding and hydrolysis are not required for this process (143, 387, 519). GTP binding may regulate septin organization in S. cerevisiae (593), septin-septin interactions (78, 143), and septin interactions with nonseptin proteins, such as the interactions with PIPs for membrane association (78, 640).
In S. cerevisiae, five vegetatively expressed septins, Cdc3, Cdc10, Cdc11, Cdc12, and Shs1 (also known as Sep7), form heteromeric complexes with a stoichiometry of Cdc3:Cdc10:Cdc11:Cdc12 of 2:2:2:2 (143, 157, 392, 592, 598). The interactions among individual septins in the complexes have been determined by a variety of in vitro and in vivo approaches (592). The septin-septin interaction patterns within the complexes are remarkably conserved between S. cerevisiae and the fission yeast S. pombe (21, 594). In S. cerevisiae, CDC3 and CDC12 are essential for cell viability and are thus considered the core subunits, whereas SHS1, CDC10, and CDC11 can be deleted under certain growth conditions. The growth defect of the deletion mutants varies from mild (in the case of the shs1Δ mutants) to very severe (in the case of the cdc11Δ mutants). CDC11 is essential for cell viability in some strain backgrounds (592). When one of the essential septins is inactivated by a temperature-sensitive mutation, the entire septin structure is lost at the bud neck (153, 202, 279). This property makes the characterization of certain mutations in septin and in septin-associated proteins feasible.
Recent studies on live cells with the GFP-tagged septins indicate that septin collar formation involves at least three steps: septin recruitment, ring assembly, and ring maturation (Fig. 9C) (83, 253, 335). Septins are first recruited to the presumptive bud site as unorganized septin clouds or patches, which are then transformed into the first organized structure, the septin ring, within minutes (253). Indirect immunofluorescence with antibodies against native septins showed that a cortical septin ring forms in the late G1 phase, approximately 15 min before bud emergence. Around bud emergence, the septin ring expands into a collar spanning the entire bud neck. During cytokinesis, the collar is split into two distinct rings marking the division sites at both the mother and daughter sides (153, 202, 279, 327). FRAP studies indicate that the septin structures prior to bud emergence are highly dynamic (83, 113). Upon bud emergence, the septin collar at the bud neck is stabilized. During cytokinesis, the split septin rings are dynamic again. This cell cycle-regulated septin dynamic may explain why septin-containing “neck filaments” are found only in small- to medium-budded cells but not in unbudded cells or cells undergoing cytokinesis by electron microscopy (71-73). Presumably, the labile septin structures in the latter cells are not maintained under the experimental conditions for electron microscopy but can be detected by GFP-tagged septins or indirect immunofluorescence.
Septins are best known for their role in cytokinesis. However, their requirement in this process varies tremendously from one organism to another. For example, septins play an essential role in cytokinesis in S. cerevisiae (215), presumably by functioning as a scaffold that tethers cytokinetic components, such as those involved in the formation of the actomyosin contractile ring and of the septum, at the bud neck (52, 325, 326, 587) and/or as a diffusion barrier to restrict the lateral movement of the cytokinetic components (112). In contrast, deletion of all four vegetatively expressed septins in the fission yeast S. pombe causes only a mild defect in cell separation (49, 561), perhaps by affecting the precise positioning of the septum degradation enzymes at the division site (364). Septins also play important roles in cytokinesis in other organisms such as Drosophila (3, 412), C. elegans (414), and mammals (285). In addition to cytokinesis, septins are involved in exocytosis (41, 42, 234), mitosis (542, 543, 552), integrity and motility of spermatozoa (240, 286), and apoptosis (191, 300). Recently, increased expression of septins in diverse tumor cells has been reported (205, 485, 509).
How are the septin heteromeric complexes involved in diverse cellular functions? Studies of yeast suggest that the septin collar (or hourglass) at the bud neck may serve as a scaffold (184, 335, 336) for many proteins involved in diverse cellular processes such as bud site selection (88, 491), chitin deposition (108), cytokinesis (52, 325, 326, 477, 500, 587), and the morphogenetic checkpoint (39, 338, 528). Mammalian septins are also thought to form diverse cellular scaffolds at discrete locations to perform specific functions (283, 543). For example, mammalian septin Sept2 is associated with cleavage furrow during cytokinesis (285) and with the kinetochore during mitosis (542). The Sept2/6/7 complexes regulate microtubule dynamics through an interaction of Sept2 with MAP4, a microtubule binding and bundling protein (295). The putative scaffold role of the mammalian septins in the cellular functions described above has not been extensively examined. In S. cerevisiae, accumulating evidence also suggests that septins form a diffusion barrier at the bud neck, which restricts the movement of membrane and membrane-associated proteins between the mother and daughter cell compartments (38, 554). These proteins include the putative factors involved in actin patch stability (38), the membrane protein Ist2, which may be involved in ion homeostasis (554), and the bud cortex-localized Lte1, the putative GEF for the small GTPase Tem1, which is essential for mitotic exit (37, 79, 439). It is worth emphasizing that the role of septins in cytokinesis is dependent on the cell cycle. Prior to the actomyosin ring contraction (from late G1 phase to telophase), the septin collar functions as a scaffold to recruit and maintain cytokinesis components such as the type II myosin Myo1, Iqg1 (IQGAP), the S. pombe Cdc15 homology protein Hof1, and the formin Bnr1 at the bud neck. In telophase, the split septin rings are thought to function as diffusion barriers that sandwich the actomyosin ring and the membrane trafficking components at the bud neck (112). The septin barrier is also thought to restrict endoplasmic reticulum membrane proteins from diffusing across the bud neck (344).
Many important questions regarding septin functions remain unanswered. Although many proteins in S. cerevisiae are known to localize to the bud neck in a septin-dependent manner, it is not clear whether any of these proteins directly interact with the septin scaffold. In addition, the high-order structures of septins are poorly understood. Which structural organization of the septins, such as septin heteromeric complexes or the highly stable septin collar, functions as a scaffold? Several septin-associated proteins are asymmetrically localized at the mother-bud neck, while some proteins such as Bud3 and Bud4 are localized to the entire septin collar (88, 491). The proteins involved in the synthesis of bud scar chitin, such as Bni4, Chs3, and Chs4, are localized to the mother side of the bud neck (108), whereas the proteins involved in the morphogenetic checkpoint are localized to the daughter side of the bud neck (39, 338, 528). What is the structural basis for the asymmetric localization of these septin-associated proteins? Are septin filaments intrinsically polarized like actin filaments and microtubules? None of the septins possesses a transmembrane domain unlike the transmembrane domain-containing proteins in the tight junction of epithelial cells, which is a proven diffusion barrier. How do the septins then form a physical barrier at the inner surface of the plasma membrane without tightly zipping the septin structure to the plasma membrane?
Role of Cdc42 in septin organization.
Because the septin collar plays important roles in numerous cellular processes as described above, it is crucial to understand how this collar structure is initially formed. The Cdc42 GTPase module appears to regulate all three stages of the formation of the septin collar (Fig. 11). Temperature-sensitive mutations in CDC42 and CDC24 block the recruitment of the septins to the presumptive bud site at the nonpermissive temperature, suggesting that Cdc42-GTP is required for the recruitment step (253). This role of Cdc42 is mediated at least in part by Gic1 and Gic2, a pair of Cdc42 effectors (67, 90) that interact directly with the septin Cdc12 (253). The gic1Δ gic2Δ mutant is temperature sensitive for growth (67, 90) and fails to recruit septins at 37°C but appears to do so normally at lower temperatures (253). It is thus likely that another pathway is involved in septin recruitment at lower temperatures (253). This parallel pathway appears to consist of the “polarisome” (521), a poorly defined network of interacting proteins, which includes the scaffold protein Spa2 (520, 521, 590), the formin Bni1 (another effector of Cdc42) (138), the actin-binding protein Bud6 (20, 399), and Pea2, a protein whose function is unknown (588). Thus, Cdc42 appears to regulate septin recruitment through the Gic proteins and the polarisome (Fig. 11). How the polarisome functions in this process is yet to be established at the molecular level.
FIG. 11.
Model for the role of Cdc42 in the polarized organization of the actin cytoskeleton and septins. Cdc42 controls the polarized organization of the actin cables by directly interacting and possibly activating the formin Bni1 and also by indirectly affecting the bud neck localization of the other formin Bnr1 via its role in septin recruitment and/or ring/collar assembly. Cdc42 controls polarized septin organization in late G1 via its effectors Gic1/Gic2, Bni1, and Cla4. Ste20, another effector of Cdc42, could participate in both the actin and the septin organization by phosphorylating Bni1. Actin cables may mediate the role of Bni1 and perhaps the polarisome in septin organization by guiding the delivery of a putative factor, “X,” which may be directly involved in septin recruitment as indicated by the question mark. Cdc42 effectors could also be involved in enhancing Cdc42 polarization at growth sites by interacting with each other. For simplicity, this type of potential positive feedback mechanism is not depicted here (see the text for a detailed description of the model).
After septin recruitment, Cdc42, its GAPs (Rga1, Rga2, and Bem3), and the PAK Cla4, an effector of Cdc42, are thought to play a role in septin ring formation (Fig. 11). Mutations affecting the cycling of Cdc42 between its GDP- and GTP-bound states confer profound defects in septin ring assembly. These mutations include cdc42Q61L and cdc42V36T,K94E, both of which possess decreased intrinsic GTPase activity, and cdc42K186R, which increases the intrinsic GTPase activity (181). Deletion of all Cdc42 GAPs, rga1Δ rga2Δ bem3Δ, delays septin ring formation without affecting septin recruitment (83, 181, 536). The role of Cdc42 GAPs in promoting septin ring assembly may explain the surprising observation that multicopy RGA1 and, to a lesser extent, BEM3 and RGA2 suppress the growth and septin ring assembly defects of the cdc12-6 mutant at the minimal restrictive temperature (30 to 32°C), in which septins are otherwise recruited to the bud tip (83, 454). It is, however, not clear why overexpression of the genes that are required for Cdc42 cycling suppresses defects in septin ring assembly. The cycling of Cdc42 may be required for efficient septin recruitment to the presumptive bud site (181). GTP hydrolysis by Cdc42 may control the quantity of localized “free septins” (released from Cdc42 effectors or other downstream pathways) that are competent for ring assembly (83, 253, 335). Alternatively, Cdc42 GAP may function as a part of the effector for septin assembly (83, 253, 335). In addition to the cdc42 mutants, cla4Δ cells fail to assemble a normal septin ring, in some cases (102, 182, 187, 265, 593, 608), suggesting that Cla4 plays a role in the initial septin ring assembly. Two other mutations in the effector domain of CDC42, cdc42V44A and cdc42D38E, also cause defects in septin ring formation (465, 467). Because these Cdc42 mutant proteins are defective in their interactions with Cdc42 effectors such as Cla4, Gic1, and Gic2 and also with Cdc42 regulators such as the GAP Bem3 (465, 467), it is difficult to attribute the septin defects associated with these cdc42 mutants to a particular biochemical defect. Nevertheless, the studies described above indicate that Cdc42, its three GAPs, and its effector, Cla4, are involved in septin ring assembly.
Upon bud emergence, the septin ring expands into a collar structure and becomes stabilized, as indicated by FRAP (83, 113). This ring-to-collar transition in septin organization appears to be regulated by Cla4 (113, 593) and by GTP binding of the septins (593). Cla4 directly phosphorylates certain septins and efficiently suppresses the septin collar assembly defects of a cdc10 cdc12 double mutant that is defective in GTP binding and in septin filament assembly of the septins (593). Thus, Cdc42 appears to regulate septin collar formation via Cla4. Several other kinases such as Gin4 (337) and Elm1 (60) as well as septin-interacting proteins such as Bni5 (306) localize to the bud neck in a septin-dependent manner and are also involved in septin collar formation. Cdc42 and Cla4 are required for the activation of the Gin4 kinase (568), indicating another route through which Cla4 regulates septin collar assembly.
It is worth emphasizing that the septin collar assembly is a continuous and efficient process. It is thus sometimes difficult to assign a protein function at a particular stage of septin collar assembly. However, the three-step model (Fig. 9C) helps to define the structure-function correlations for different high-order septin structures and also helps to define the functions of specific septin regulators. For example, when septins are not organized into a ring structure, such as those at the bud tip of certain cdc42 mutants and the triple mutant lacking Cdc42 GAPs, the septins are strictly associated with elongated bud morphology (83, 182). In contrast, a highly organized septin structure, such as the septin collar in wild-type cells, is associated with normal, oval-shaped bud morphology (83, 182). Cdc42 appears to be involved in all stages of septin collar assembly. A number of other proteins described above are involved in later stages of this process. However, several questions regarding the molecular pathways involved in regulation of the high-order septin structures remain. What is the role of GTP binding and hydrolysis in septin filament formation? When are the septin filaments formed: at the recruitment stage, the ring formation stage, or the collar formation stage? What are the in vivo septin structures, at least at the electron microscopy level, corresponding to the three stages in septin collar assembly?
Is the role of Cdc42 in septin organization universal?
Cdc42 regulates septin recruitment partly via its effectors, Gic1 and Gic2 (253), both of which contain a conserved CRIB domain, which specifically interacts with Cdc42-GTP (67, 90). Gic1 and Gic2 also interact directly with the septin Cdc12 (253). However, Gic1 and Gic2 homologs are not found anywhere else, except in a very few yeast species belonging to Ascomycetes (http://db.yeastgenome.org/homolog/images2/chr8/YHR061C.tree.gif). Mammalian Cdc42 regulates septin organization, at least in part, via its effector, Borg3 (258). Borg3 contains a CRIB domain and interacts directly with septin complexes containing Sept6 and Sept7 (519). Cdc42 appears to inhibit the interaction between Borg3 and the septins in vitro (258, 519). Ectopic expression of Borg3 disrupts septin organization in MDCK cells (258), but the precise role of Borg3 in this process remains unknown. Interestingly, Borg3 homologs are limited to metazoans (258). Why do the Gic proteins in S. cerevisiae and the Borg proteins in mammals, which are capable of interacting with the highly conserved proteins Cdc42 and septins, bear strong species-specific features? The answer is likely to reflect the need to organize septins into distinct structures in different organisms, so that other organism-specific processes can be coordinated.
Model for the Roles of Cdc42 in Actin and Septin Organization
Here, we propose a model to explain how Cdc42 controls polarized organization of the actin cytoskeleton and of the septins at the beginning of the cell cycle (Fig. 11). This model synthesizes the vast and fragmented pool of information collected on individual polarity proteins into the molecular pathways that govern Cdc42-mediated cytoskeletal polarization. According to the model, Cdc42 controls the polarized organization of the actin cytoskeleton and the septins through two genetically separable, biochemically cross talking pathways: one involving the polarisome with the scaffold Spa2 and the formin Bni1 as the centerpiece and the other involving Gic1 and Gic2. The polarisome pathway mainly leads to the polarized organization of actin cables through the regulation of cellular location and perhaps the activity of Bni1. This pathway also fine-tunes septin recruitment, possibly through its role in actin organization and/or polarized exocytosis. The Gic-mediated pathway mainly regulates septin recruitment via a direct interaction with the septin complex. This pathway also affects actin cable organization by regulating the initial localization of Bnr1, which depends on the septins. In addition, the polarisome components Spa2 and Bud6 interact with the Gic proteins, allowing cross talk between these two pathways (255) (S. E. Tcheperegine and E. Bi, unpublished data) (Fig. 11).
This model is consistent with and/or can explain the following observations. (i) Polarized actin and septin organizations can occur independently (30, 153, 279, 454), but both require Cdc42 function (96, 183, 253, 454). (ii) The deletion of individual polarisome components is synthetically lethal with gic1Δ gic2Δ (51, 255). (iii) Mutants in the genes encoding polarisome components and the cla4 mutant exhibit a synthetic lethal interaction (574). The deletion of any polarisome components in combination with a conditional lethal allele of CLA4 arrests cells with septins concentrated at the bud tip under restrictive conditions (187, 265). Given that the polarisome and Cla4 affect septin recruitment and ring/collar assembly, respectively, it is not surprising to see that the cells carrying mutations for polarisome components and CLA4 display severe defects in septin organization. Similarly, gic1Δ gic2Δ is synthetically sick with cla4Δ (90), and the triple mutants display severe defects in septin organization (M. Iwase and E. Bi, unpublished results). (iv) Ste20 phosphorylates Bni1 and may activate the polarisome (187). This result is also consistent with the observations that ste20Δ and cla4Δ are synthetically lethal and that the conditional inactivation of CLA4 in the ste20Δ background results in severe defects in septin organization (102, 187, 265, 608). (v) Recent studies indicate that F-actin may play a fine-tuning role in septin organization (253, 265, 294). Septin recruitment and ring assembly appear to be delayed in cells treated with LatA (253). This model predicts that the simultaneous loss of F-actin and the Gic1/Gic2 effectors might completely block septin recruitment. (vi) Septins are required for the targeting of Bnr1 to the bud neck (459), suggesting that Bnr1 may function downstream of the septins. Consistent with the model, bni1Δ and bnr1Δ are synthetically lethal (587), and bni1 mutations are synthetically lethal with the septin mutation cdc12-5 (H. Fares and J. R. Pringle, personal communication) (459).
This model also raises several questions. Are the interactions between the Gic proteins and the polarisome (Gic-Spa2 and Gic-Bud6) essential at 37°C but dispensable at lower temperatures, given that the gic1Δ gic2Δ mutant is temperature sensitive for growth (67, 90)? How are these interactions regulated at the molecular level? How does F-actin affect septin recruitment? How do the septins interact with Bnr1? Do the Gic proteins affect actin organization independently of the septins and Bnr1?
The Mitotic Exit Network and Cytokinesis
The exit from mitosis, the contraction of the actomyosin ring and septum formation, and the entry into the next G1 phase in S. cerevisiae are all controlled by a signaling cascade called the mitotic exit network (MEN) (Fig. 12) (for recent reviews, see references 59, 376, 531, 549, and 556). The counterpart of this network in the fission yeast S. pombe is called the septum initiation network (376, 531), and several key components of the MEN are also conserved in mammalian systems (34, 531, 549). The MEN starts with a small GTPase module consisting of the GTPase Tem1 (526), its putative GEF, Lte1 (524, 525), and its bipartite GAP, Bub2-Bfa1 (Fig. 12A) (173). In normal anaphase cells, Tem1 and the Bub2-Bfa1 complex localize to the spindle pole body (SPB) destined to a daughter cell (also the old SPB) (37, 156, 441), whereas Lte1 localizes to the daughter cell cortex (37, 439). This spatial segregation of the GAP and the GEF for Tem1 ensures that the MEN is not activated until the daughter-destined SPB passes through the bud neck (Fig. 12B) (37, 439). The spatially restricted activation of the MEN is further safeguarded by the protein kinase Kin4, which localizes to the SPB and the cortex of the mother cell in anaphase (104, 440). Kin4 is thought to inhibit MEN activation by counteracting the activity of the polo kinase Cdc5, which activates Tem1 by inhibiting its GAP (235). When a spindle is misaligned in the mother cell, both Kin4 and the Bub2-Bfa1 complex localize to both SPBs, inactivating Tem1 and preventing mitotic exit (104, 440). Thus, factors involved in the spatial regulation of MEN activation link spindle position to mitotic exit, which defines the spindle position checkpoint (SPOC) (54). Once Tem1 is activated, it binds to Cdc15 kinase (37, 526), which in turn activates a protein kinase complex consisting of the kinases Dbf2 and Dbf20 and their regulatory subunit, Mob1 (290, 343, 353). This cascade of kinase activation leads to the release of the protein phosphatase Cdc14 (194) from the nucleolus into the nucleus (31, 527, 597), which promotes mitotic exit by inactivating Cdc28/cyclin B (Cdk1) activity (256, 602) and also by reversing Cdk1-dependent phosphorylation (596). Cdc15 (625), Dbf2/20-Mob1 (159, 343, 633), and Cdc14 (43) also localize to the bud neck in late anaphase and play an essential role in septum formation independent of their role in mitotic exit (43, 324, 327). The lack of septum formation in MEN mutants or in mutants lacking the septum synthetic enzyme Chs2 does not affect the assembly of the actomyosin ring (50, 324, 327, 500, 587) but affects the contraction of the ring (50, 324, 500). Thus, the MEN is required for both mitotic exit and cytokinesis, and these two functions bifurcate at the level of Cdc14 (Fig. 12A). While the mechanisms underlying the role of the MEN in mitotic exit are well established, its targets in cytokinesis remain unknown.
FIG. 12.
Role of Cdc42 in mitotic exit. (A) The MEN in S. cerevisiae. The activation of Tem1 leads to the activation of a kinase cascade, resulting in the release of the phosphatase Cdc14 from the nucleolus to the nucleus, which regulates mitotic exit and cytokinesis independently. The regulators for Tem1 GTPase cycling and the localization of the key components of the MEN are indicated. (B) In normal anaphase cells, Tem1 and its GAP localize to the daughter-bound SPB (old SPB) (SPB is indicated by a solid black circle). This localization pattern ensures that Tem1 is activated only after the spindle penetrates into the bud, where Lte1 (+), the putative GEF for Tem1, is localized at the cortex. The septins and the mother cell cortex-localized inhibitor Kin4 (−) provide additional layers of regulation to further restrict the activation of Tem1 in the bud. (C) Cdc42 regulates the MEN by multiple means through its role in septin ring assembly (see the section on Cdc42 and septin organization) and its effectors Cla4, Gic1, and Gic2 to affect the localization of Lte1, through Ste20 to activate the MEN in parallel to Lte1 by an undefined mechanism, and through blocking the binding of the GAP Bub2 to Tem1 (see the text for further details).
What is the role of Cdc42 in regulating the MEN? Cdc42 affects MEN activation at multiple levels (Fig. 12C). Cdc42 and its GEF, Cdc24, are required for the asymmetric localization of Lte1 (230, 257, 517). Ras and the Cdc42 effector Cla4 act together to target Lte1 to the bud cortex (516, 632). The PAK Cla4 phosphorylates Lte1 either directly or indirectly (230, 517), thus promoting the interaction between Lte1 and Ras (516). This interaction is thought to stabilize the anchoring of Lte1 at the bud cortex. Ste20, another Cdc42 effector and a PAK, may function in parallel to Lte1 to promote mitotic exit (230), although the molecular basis for the role of Ste20 in this process remains unknown. Gic1 and Gic2 also promote MEN activation by interfering with the binding of the Bub2-Bfa1 GAP to Tem1 (231). Gic1 and Gic2 are also required for the bud cortex localization of Lte1 at 37°C (257). Septins are thought to function as a diffusion barrier at the bud neck (38, 554), which is required for restricting the localization of Lte1 to the bud cortex (79, 230, 257). Because Cdc42 is required for the initial formation of the septin ring through multiple effectors including the PAKs and the Gics (see previous sections), Cdc42 could affect Lte1 localization indirectly via septin ring assembly. Because LTE1 is essential only at lower temperatures (524), other factors that display an Lte1-like localization and/or activity may be required for MEN activation at higher temperatures. It is not known whether Cdc42 plays any role in restricting Kin4 to the mother cell cortex. The spatial regulation of Tem1 activation represents an excellent example of a modified version of the global inhibition and local activation mechanism discussed below.
Although Cdc42 localizes to the mother-bud neck in large-budded cells (466, 651), the function of Cdc42 at the neck of large-budded cells remains unknown, as Cdc42 is not required for actin ring formation during cytokinesis (573). It has been assumed that Cdc42 may be involved in targeted membrane deposition at the bud neck to promote septum formation and/or degradation. Such a hypothesis remains to be tested. Cdc42 is indirectly involved in cytokinesis through its role in the formation of the septin ring, which is essential for the tethering of cytokinesis proteins such as the type II myosin Myo1 to the bud neck prior to late anaphase or telophase (52, 326). Septins also function as a two-ring diffusion barrier that sandwiches and restricts the lateral movement of proteins involved in cytokinesis and cell separation at the bud neck during telophase (112).
Cdc42 interacts with Boi1 (47), which has been implicated in cell polarity and actin cytoskeleton reorganization. Boi1 and its functional homolog, Boi2, also interact with Bem1 and other Rho GTPases (47, 368). Boi1 and Boi2 localize to the bud neck in late anaphase (206), and their localizations depend on spindle midzone-localized Aurora kinase Ipl1 (417). Based on this and other functional analyses, Boi1 and Boi2 are proposed to function as “abscission inhibitors” in a “NoCut pathway” that delays the completion of cytokinesis in response to spindle midzone defects to prevent chromosome breakage (417). Whether this novel role of Boi1 and Boi2 is linked to their function in polarity and how exactly they inhibit cytokinesis remain to be determined.
OTHER TYPES OF CELL POLARIZATION
Cell Polarization during Mating
Haploid yeast cells are able to redirect their polarization to mate with a cell of the opposite mating type. During mating, cell polarity is determined by a pheromone gradient that is established by the position of the appropriate mating partner (Fig. 1B). Cell polarization can be induced toward an artificially produced gradient of the peptide pheromone, demonstrating that the pheromone is a sufficient chemotropic signal (510). Mating pheromones (a and α factors) are sensed by seven transmembrane receptors, α-factor receptor (encoded by STE2) of a cells and a-factor receptor (encoded by STE3) of α cells. Both receptors activate a G-protein heterotrimer consisting of an α subunit (Gpa1) and a βγ subunit dimer (Ste4 and Ste18). Binding of a pheromone to the appropriate receptor promotes the exchange of GDP for GTP on the Gα subunit, leading to the dissociation of the Gβγ heterodimer. Upon activation, Gβγ orchestrates several well-characterized cellular responses, including signal transduction pathways, leading to transcriptional induction, cell cycle arrest, and morphological changes, leading to the formation of a mating projection called the shmoo (Fig. 13). A major target of the Gβγ is a MAP kinase cascade, which includes Ste20 (a PAK-like kinase, a MAPK kinase kinase kinase), Ste5 (a scaffold protein for the MAP kinase cascade), Ste11 (a MAPK kinase kinase), Ste7 (a MAPK kinase), Fus3 (a MAP kinase), and Ste12 (a transcription factor). Much is known about the signal transduction pathways governing these responses, and it has been extensively reviewed elsewhere (114, 545). Here, we will first discuss how cells change the direction of cell polarization from the bud site towards the mating partner and then discuss how Cdc42 is involved in MAPK signaling and polarized cell growth, leading to shmoo formation.
FIG. 13.
Role of Cdc42 in cell polarization during mating. (A) In response to a gradient of mating pheromones secreted by cells of the opposite mating type, haploid a or α cells polarize to form the mating projection (stage 1). Cdc42 and other polarity proteins localize to the tip of the mating projection. Actin cables and patches are also polarized towards the tip. Septins, which may be involved in defining membrane domains, localize as patches at the base of the mating projection. The signaling events at the shmoo tip, indicated by the black box in the a cell at stage 1, are illustrated in panel B. During cell fusion (stage 2), the membrane protein Fus1 localizes to the cell-cell contact zone. Together with Cdc42, Bni1, and the polarisome component Spa2, Fus1 is involved in the localized delivery of secretory vesicles (black circles) to the cell-cell contact zone, leading to the eventual cell fusion. (B) Upon pheromone binding, the receptor Ste2 clusters and activates the heterotrimeric G protein, which anchors to the plasma membrane via the lipid-modified tails (indicated by the wavy line) of its α (Gpa1) and γ (Ste18) subunits. The GTP-bound α subunit dissociates from the β (Ste4)-γ complex. The βγ complex then triggers the activation of the MAPK cascade, resulting in transcriptional activation and G1 arrest. The activation of Gβγ also triggers polarized growth towards the pheromone gradient. The blue lines indicate protein-protein interactions (see the text for details). Rho1 has been shown to interact with the β subunit and is thus recruited to the shmoo tip. See Fig. 15 for the Rho1 pathway. For simplicity, extensive cross talks between the MAPK cascade and actin organization are not indicated here.
The Cdc42 GTPase module, which is coupled to the spatial landmark for budding by the Rsr1 GTPase module during vegetative growth, also plays a critical role in linking Gβγ activation to cell polarization (see below). The adaptor Far1 is necessary for linking Gβγ activation to the Cdc42 GEF Cdc24 (70, 408). Although the external pheromone signal normally overrides the internal signal from the bud site, the bud site is used for shmooing in the absence of a chemotropic system as in mating-specific mutants of CDC24 or FAR1 or in the presence of uniform pheromone concentrations. These cdc24 (cdc24-m1, cdc24-m2, and cdc24-m3) and far1 (far1-s) mutants still form the mating projections at wild-type efficiencies but in random directions with respect to the pheromone gradient, because these cells are defective in orienting their axes chemotropically (409, 589). When both systems that affect bud positioning and chemotropism are lacking, as in the case of the double mutant cdc24-m1 rsr1Δ, cells are defective in shmoo formation. It is therefore likely that the stabilization of the growth axis during mating requires positional signaling from either the pheromone receptor or the specific bud site selection proteins in the absence of chemotropism.
During mating, cells ignore the preexisting intrinsic spatial cues and establish a new landmark in response to receptor activation. How is the spatial information of the bud site overridden in the presence of a mating partner? It is noteworthy that Axl2 and Bud4 are down-regulated in response to pheromones (476, 491). This decreased expression of the potential axial landmarks may allow cells to ignore the spatial cue for axial budding and polarize towards a mating partner, but it is not clear how cells also override the bipolar signal, which is apparently used for bipolar budding of axl2 or bud4 mutants. Thus, down-regulation of Axl2 and Bud4 would not be sufficient for cells to override the spatial information of the bud site. Although the underlying mechanism is not completely understood, several recent findings provide some insights into the problem. Far1 appears to play a dual role during mating: Far1 triggers cell cycle arrest as an inhibitor of the G1 cyclin-Cdc28 protein kinase (443, 444) and promotes cell polarization as an adaptor protein linking Gβγ and Cdc24 for polarity establishment (70, 408, 589). In haploid G1 cells, Cdc24 is in the nucleus through an association with Far1 (411, 522, 571). In the absence of the mating pheromone, activation of the cell cycle kinase Cdc28 complexed with a G1 cyclin (Cln1 or Cln2) triggers the degradation of Far1 at bud emergence (522), thus allowing Cdc24 to interact with Rsr1 for polarity establishment toward the proper bud site (434, 644). It has been proposed that Rsr1 also activates Cdc24 during budding (523). Upon pheromone treatment, the inactivation of Cdc28-Cln kinase leads to the stabilization of Far1 and the relocation of the Far1-Cdc24 complex into the cytoplasm by the exportin Msn5 (55). Far1 may recruit Cdc24 to the site of activation of the heterotrimeric G protein, thus leading to the activation of Cdc24 at the site. Cdc24 exhibits a two-hybrid interaction with Rsr1, and the interaction is diminished by a mutation in the region that is also involved in the interaction with Far1, thus suggesting that Far1 and Rsr1 bind to the overlapping domains of Cdc24 (523). This overlapping binding site may account for the competitive binding of Far1 and Rsr1 to Cdc24. Thus, it has been proposed that Cdc24, when complexed with Far1, is unable to interact with Rsr1, thereby allowing the targeting of Cdc24 to the mating site instead of the bud site (197). Although this is an interesting possibility, some available data conflict with this notion, and some issues are controversial (see “Cdc24, a GEF for Cdc42” and Temporal Control of Polarity Establishment during Yeast Budding for further discussion). Cdc24 also interacts with Bem1 (47) and activates Cdc42 at the location where pheromone receptors are clustered (29). Activated Cdc42 presumably directs polarized actin organization, exocytosis, endocytosis, and cell wall assembly as it does during budding. Thus, it appears that the same Cdc42-based machinery functions in polarized morphogenesis during mating and budding in response to distinct signals, an external signal during mating and an internal signal (or cell cycle clock) during budding.
Cdc42 regulates the mating response via at least two distinct pathways: the activation of the MAPK pathway and the formation of the mating projection (Fig. 13). It is not clear whether and/or to what extent there is cross talk between these two pathways at the molecular level. Cdc42 and its GEF, Cdc24, are required for MAPK activation (532, 643) and morphogenesis, leading to the formation of the mating projection (see below). Cdc42 is likely to activate the PAK Ste20, as specific pheromone-resistant cdc42 alleles that do not affect budding but that affect MAPK signaling and Ste20 localization were isolated (24, 400). It has also been shown that Cdc42-GTP interacts with Ste20 in vitro and in vivo (445). The polarity protein Bem1 also interacts with Ste5 (307, 345), a scaffold protein that interacts with multiple components of the MAPK pathway and endows the shared MAPK core components with signaling specificity in the mating response (64, 134, 507). This Bem1-Ste5 interaction and an interaction between Cdc42 and Bem1 are thought to enhance MAPK signal transduction (307, 345, 400). Once activated by Cdc42, Ste20 in turn phosphorylates an autoinhibitory domain at the N-terminal region of Ste11, leading to its activation (125) and consequently turning on the MAPK pathway. Ste20 interacts directly with the G-protein β-subunit Ste4, thus linking the MAPK cascade to the receptor-coupled G protein. This interaction between Ste20 and Ste4 is essential for MAPK activation (308).
In addition to shmoo formation, Cdc24 and Cdc42 are also required for the subsequent cell fusion by restricting and/or maintaining cell fusion proteins such as Fus1 to the cell-cell contact zone during mating. The cell fusion zone during mating can be viewed as two shmoo tips facing each other (stage 2) (Fig. 13A) (35, 36). Fus1, an O-glycosylated membrane protein (577), is required for the normal localization of vesicles to the cell fusion zone (166). These vesicles presumably carry enzymes for the formation and subsequent degradation of the apposing cell wall (or septum) of a prezygote, leading to cell fusion. The cytoplasmic domain of Fus1 interacts with Chs5, a late-Golgi protein required for delivering chitin synthase Chs3-containing vesicles to the plasma membrane during budding (494). The chs5 mutants are defective in targeting Fus1 to the shmoo tip (406, 493) and fail to mate at the cell fusion step (492). This fusion defect can be partially suppressed by increasing the dosage of Fus1 in the cell (492). Fus1 also interacts with Cdc42-GTP and the formin Bni1 in two-hybrid assays (406). Mutants lacking Bni1 or other polarisome components including Spa2 and Pea2 are also defective in cell fusion (117). Spa2 is required for clustering vesicles at the cell fusion zone during mating (166). Cells lacking the tropomyosin Tpm1 are severely defective in maintaining actin cables and also fail at the cell fusion step during mating (328). Cdc42 and Bni1 are responsible for the localized formation of actin cables, which are stabilized by Tpm1. The polarized actin cables guide the Myo2-powered delivery of the Chs5-escorted, Chs3-containing vesicles to the cell fusion zone. Fus1 may be delivered to the plasma membrane via the Chs5-marked vesicles. Cdc42 then maintains Fus1 at the plasma membrane via a direct or an indirect interaction.
Two mating-specific mutants of CDC24, cdc24-m5 and cdc24-m6, were isolated and were found to have mutated residues in the conserved catalytic domain of Cdc24 GEF (36). Interestingly, the cdc24-m6 mutant responds normally to pheromones and orients its growth towards a mating partner, yet it accumulates prezygotes during mating. Despite normal exocytosis, cdc24-m6 mutant cells fail to localize proteins required for cell fusion. Thus, Cdc24 GEF activity is likely to be required for maintaining or restricting specific proteins essential for cell fusion to the cell-cell contact region. However, it is not clear why certain residues of the GEF domain are important only for mating, since Cdc24 GEF activity is also essential for budding. It remains to be seen whether these Cdc24 mutant proteins are partially defective in their GEF activity and whether higher GEF activity is required during mating in comparison to budding.
Despite the involvement of common players such as Cdc42 and its downstream targets in the polarization of the actin cytoskeleton during both budding and mating, it appears that there are some intrinsic differences between these processes. In contrast to budding in which localization of Cdc42 does not depend on actin (30), an intact actin cytoskeleton is required to maintain the polarized localization of Cdc42 and Bem1 at the shmoo tip (29). This is consistent with the finding that even after an axis of polarization is established, constant reinforcement is required to stabilize and/or maintain the polarity during mating (410). When F-actin is disrupted by LatA, Cdc42, Bem1, Spa2, and septin Cdc10 are all dispersed from shmoo tips, and pheromone receptors are no longer clustered even in the presence of their ligands (29). Receptor clustering is presumably required for the localization of many downstream components including the polarity proteins mentioned above. This result also implies that it is nearly impossible to separate the role of Cdc42 in polarized actin organization from its role in MAPK signaling. Consistent with this notion, the MAPK Fus3 phosphorylates Bni1 and is required for Bni1 localization and polarized actin organization during mating (367).
Filamentous Growth
Haploid cells penetrate agar in response to nutritional limitation. This mode of growth is termed invasive growth. Upon nitrogen starvation, diploid cells undergo a dimorphic transition known as pseudohyphal growth, which results in the formation of chains of cells with separated cytoplasms and a cell wall linkage between different cellular compartments (178, 296). Collectively, these modes of cell growth are called FG (Fig. 1C) (64), although all three terms and additional ones have been used interchangeably in many papers. The physiological function of FG is thought to enable yeast cells to forage for nutrients (178, 296). The regulatory networks involved in filamentous growth are complex and have been extensively discussed in several excellent reviews (64, 428, 483, 507). Here, we will briefly review the major molecular events involved in pseudohyphal formation.
Upon nitrogen starvation, several important cellular changes occur in a coordinated fashion: a change in the budding pattern from bipolar to unipolar (178), a G2 delay coupled with cell elongation (296), the adhesion between cellular compartments as the result of the expression of new adhesive molecules on the cell surface (332, 333), and an inhibition of the expression of enzymes that degrade the cell wall and septa (428) (Fig. 14). The nutritional signal is sensed by two membrane proteins, the osmosensor Sho1 and the mucin-related glycoprotein Msb2, both of which interact with each other to regulate filamentous growth (101). In addition, Msb2 interacts with Cdc42-GTP preferentially, and this interaction is thought to trigger the activation of the FG-specific MAPK pathway (101), which consists of the same core components involved in the mating response (329). The scaffold protein Ste50 associates with Ste11, and this interaction is required for optimal invasive growth and hyperosmotic stress (high-osmolarity glycerol) signaling but has a lesser role in the pheromone response (576). Ste50 also interacts with Cdc42 via its C-terminal “RA” (Ras association) domain (but this RA domain does not bind to Ras2) (576) and thus serves as an adaptor to tether Ste11 to the plasma membrane where Cdc42 and activated Ste20 are located, leading to the activation of Ste11 by Ste20 (576). The activation of the FG-specific MAPK pathway leads to the expression of Flo11, a glycosylphosphatidylinositol-linked cell surface protein, which mediates cell-cell adhesion (332, 333). The nutritional signal from the membrane sensors Sho1 and Msb2 is somehow sensed by Ras2 (178), which also activates the MAPK cascade (178, 396). At the same time, Ras2 activates the cyclic AMP-dependent protein kinase A (PKA), which directly regulates two functionally antagonistic transcriptional factors, Flo8 and Sfl1, to control Flo11 expression (430). Thus, Ras2 regulates Flo11 expression through both MAPK and PKA pathways. It is thought that the MAPK Fus3 is mainly involved in mating by activating the transcriptional heterodimer Ste12-Mcm1, whereas the MAPK Kss1 is mainly involved in filamentous growth by activating the transcriptional heterodimer Ste12-Tec1 (98, 352). However, genome-wide studies indicate that the expression profiles specified by Fus3 and Kss1 overlap more than previously thought (64, 472, 639). The PKA pathway also affects the budding pattern. Both PKA and MAPK pathways may contribute to the G2 delay, which is responsible for cell elongation (428, 483).
FIG. 14.
Signaling pathways leading to pseudohyphal growth. Nitrogen starvation is sensed by Ras2, which controls the expression of the cell surface flocculin Flo11 via two distinct pathways, the cAMP-dependent PKA pathway (in blue) (AC, adenylyl cyclase) and the MAPK pathway (in green). PKA activates the transcriptional activator Flo8 and inactivates the transcriptional repressor Sfl1, both of which contribute to the increased expression of Flo11. Ras2 somehow activates Cdc42, which activates the MAPK cascade via its effector, Ste20. The MAP kinase Kss1 inactivates the repressors Dig1 and Dig2, thereby allowing Ste12 and Tec1 to activate the expression of Flo11 as well as of other proteins involved in cell elongation. As in budding and mating, Cdc42 regulates polarized actin organization via its effectors (in red), thereby contributing to the elongated cell morphology. In response to the nitrogen starvation signal, Sok2 negatively regulates the expression of the transcription factors Phd1 and Swi5, which activate Flo11 expression directly or via the daughter cell-specific transcription factor Ash1. The two transcription factors Swi5 and Ace2 activate the expression of the endoglucanase Egt2 and the endochitinase Cts1. Decreased expression of these key enzymes that are required for cell separation after cytokinesis causes cell attachment during filamentous growth.
Epistasis analyses indicate that Cdc42 functions downstream of Ras2 and activates the MAPK cascade via Ste20 (396). Specific mutations in CDC42 that affect filamentous growth, but not budding, were isolated. Analysis of these mutations indicate that Cdc42 effectors, including the CRIB motif-containing proteins Gic1 and Gic2 and the PAK Skm1, may be involved in filamentous growth (394). Cdc42 interacts with Sho1 and also with Msb2 to promote differential activation of the FG-specific MAPK Kss1 (101). Screens for mutations that affect pseudohyphal growth indicate that the formin Bni1, the scaffold Spa2, and the tropomyosin Tpm1 are also required for pseudohyphal growth (393). Thus, the same Cdc42-controlled polarity machinery is operating in filamentous growth as well. However, it is not clear how Ras2 activates Cdc42 and whether the role of Cdc42 in MAPK signaling can be separated from its role in polarized actin organization during filamentous growth.
OTHER SMALL GTPases AND THEIR ROLES IN POLARIZED GROWTH
Besides Cdc42, there are five other Rho proteins, Rho1 to Rho5, in S. cerevisiae. Among them, Rho1 has been the most extensively studied Rho protein, and its role in cell wall assembly has been discussed in detail in several excellent reviews (218, 313). Here, we will briefly discuss the functions of Rho1 in polarized cell growth. We will also discuss Rho3 and Rho4, whose functions in actin organization and exocytosis have been studied to some extent. Very little is known about Rho2 (351) and Rho5 (481), two nonessential Rho GTPases in S. cerevisiae. Multicopy RHO1 or RHO2 suppresses the temperature-sensitive growth of cells carrying a deletion of ROM2, which encodes a GEF for Rho1 (424) (see below). In addition, multicopy RHO2 suppresses the growth defects of profilin (356) and tor2 deletion strains (497), both of which are defective in actin organization (201, 498). The tor2 deletion strain is also suppressed by multicopy RHO1 or ROM2 (497). Furthermore, multicopy RHO2 plus RHO1 suppresses the temperature-sensitive growth of a mutant allele of BEM2 (282), which encodes a GAP for Rho1 (446, 646). Together, these genetic data suggest that Rho2 may share some functions with Rho1 and may regulate actin organization by an unknown mechanism. Rho5, which possesses an effector domain very similar to that of Cdc42/Rac1 (481), has been reported to regulate transient actin depolarization during a temperature shift to 37°C and also to down-regulate the Pkc1-dependent cell wall integrity CWI pathway (501). However, the physiological significance of these observations is not clear, as the phenotypes of rho5 mutants appear to vary depending on the strain backgrounds (K. Singh and H.-O. Park, unpublished observations).
Rho1 Regulators
Rho1 GEFs.
Like Cdc42 and other small GTPases, the interconversion of Rho1 between its GDP- and GTP-bound states is regulated by at least three types of regulators: GEFs, GAPs, and GDI. There are at least three known GEFs, which include Rom1, Rom2, and Tus1 (Fig. 15). Multicopy ROM1 or ROM2 suppresses the cold-sensitive growth phenotype caused by a dominant negative allele of RHO1, rho1G22S,D125N (424). Like most Rho GEFs, Rom1 and Rom2 contain the DH domain, which is responsible for GEF activity for Rho GTPases, and the PH domain, which is implicated in the membrane association of the proteins and is also commonly found in Rho GEFs (424). Like Rho1 (627), Rom2 localizes to sites of polarized growth during budding and mating (355). A deletion of ROM1 does not produce any obvious phenotype, whereas a deletion of ROM2 causes temperature-sensitive growth at 37°C in most strain backgrounds (355, 424). The deletion of ROM1 and ROM2 together causes cell lethality. Like cells depleted for Rho1, cells depleted for both Rom1 and Rom2 (424) and rom2Δ cells grown at 37°C (355) arrest as small-budded cells. The temperature sensitivity of a rom2Δ strain is suppressed strongly by multicopy RHO1 or RHO2 and weakly by RHO3 or RHO4, but not by CDC42, suggesting that Rom2 functions as a GEF mainly for Rho1 and Rho2. Indeed, Rom2 displays GEF activity towards Rho1 (424, 497). As described below, Rom1 and Rom2 are mainly involved in cell wall integrity by activating Rho1. In addition, multicopy ROM2 or RHO2 suppresses the temperature sensitivity (355) caused by the deletion of KAR3 (380) and CIK1 (94, 354, 427, 546), which encode a microtubule minus-end-directed kinesin and its targeting subunit, respectively. It is not clear how Rom2 and Rho2 regulate microtubule-related functions. Besides Rom1 and Rom2, Tus1 functions as a GEF for Rho1. Tus1 has a DH and a PH domain and has GEF activity towards Rho1 in vitro (496). Diploid strains homozygous for tus1Δ are temperature sensitive for growth at 39°C, and this temperature sensitivity is suppressed by 1 M sorbitol, by multicopy ROM2 or RHO2, or by activated versions of genes encoding kinases (Pkc1, Bck1, and Mkk1) constituting the CWI pathway (496) (see below). Tus1 thus appears to regulate cell wall assembly by activating Rho1 and Rho2.
FIG. 15.
Signaling pathways mediating the roles of Rho1 in cell wall assembly and actin organization. Cell wall stresses are sensed by the five type I membrane proteins Wsc1, Wsc2, Wsc3, Mid2, and Mtl1, with the major players indicated by the thick lines and boldface type. These sensors then activate the GEFs Rom1 and Rom2, which in turn activate Rho1. Activated Rho1 controls cell wall assembly in two distinct ways: as the regulatory subunit of the glucan synthases Fks1 and Fks2 and by activating the Pkc1-MAPK-mediated CWI pathway, which increases the expression of cell wall synthetic enzymes. Rho1-GTP also controls polarized exocytosis as indicated in Fig. 10. Rho1-GTP also regulates actin ring formation via Bni1 to effect cytokinesis. Mss4, the PI4P-5K, synthesizes the plasma membrane pool of PI4,5P2, which recruits the Rho1 GEFs to the plasma membrane, thus contributing to Rho1 activation. PI4,5P2 and Tor2 are involved in recruiting and/or maintaining the pair of PH domain-containing proteins, Slm1 and Slm2, at the plasma membrane. These Slm proteins are thought to function in actin organization via Pkc1 but not the MAPK pathway. In response to heat stress, Rho1-Pkc1 is thought to function in transient actin depolarization, whereas the MAPK pathway is thought to function in subsequent actin repolarization during the recovery phase (see the text for further details). SBF, Swi4/Swi6 cell cycle box binding factors.
Rho1 GAPs.
There are four known GAPs for Rho1: Bem2, Lrg1, Sac7, and Bag7 (Fig. 15). Bem2 was identified from a genetic screen for mutations (46) that display synthetic lethality with the deletion of MSB1, a common multicopy suppressor for several cdc24-Ts and cdc42-Ts mutants (45, 169). Bem2 has a Rho GAP domain, which is preceded by a PH domain, and displays GAP activity towards both Rho1 and Cdc42 in vitro (357, 446, 646). The deletion of BEM2 causes temperature-sensitive growth at 37°C, and the mutant cells arrest mostly as large, round, unbudded cells and sometimes as large, round cells with a small bud (46, 282, 446). This phenotype resembles that caused by a loss of Cdc42 function, which is intuitively opposite to what is expected for the deletion of a Cdc42 GAP (91). The bem2Δ mutation is synthetically lethal with rga1Δ. The temperature sensitivity of bem2Δ cells is suppressed by increasing the dosage of the Cdc42 effector Gic1 (90) and also by simultaneously increasing the dosage of Rho1 and Rho2 (282). Like rho1 mutants, bem2 mutants display cell wall defects (97), and the overexpression of Bem2 down-regulates the Pkc1-Mpk1-mediated cell wall integrity pathway downstream of Rho1 (499). Together, these results suggest that Bem2 functions in both Cdc42- and Rho1-mediated processes, but the underlying mechanisms remain obscure. Lrg1, which contains a Rho GAP domain in its C-terminal region and three LIM domains in its N-terminal region, interacts with Rho1 (603) and has GAP activity towards Rho1 in vitro but not for any other Rhos and Cdc42 (151). Among other Rho GAPs tested, Lrg1 is uniquely required for the negative regulation of Rho1-mediated glucan synthase activity (603). Lrg1 localizes to the tip of the mating projection and is required for efficient cell fusion by regulating Rho1-mediated localized glucan synthesis at the shmoo tip (151). Sac7 was originally identified from a genetic screen for mutations that suppress the temperature-sensitive growth of the actin allele act1-4 (130). The deletion of SAC7 causes a cold-sensitive defect in growth and actin organization (130, 497). Sac7 contains a Rho GAP domain and displays GAP activity specifically for Rho1 but not for Rho2 and Cdc42. Null mutations of SAC7 and ROM2 suppress each other, and the deletion of SAC7 or overexpression of ROM2 suppresses the temperature-sensitive mutant of TOR2, which is known to regulate the Rho1-Pkc1-Mpk1 cell wall integrity pathway and actin organization (497). Together, these results suggest that Sac7 is a Rho1-specific GAP. Bag7 contains a Rho GAP domain but no other obvious motifs. Bag7 interacts with Rho1, but not Rho2-Rho5 and Cdc42, and has GAP activity towards Rho1 (481, 499). A deletion of BAG7 does not exhibit any obvious phenotype. However, the overexpression of BAG7 suppresses the cold sensitivity of sac7Δ cells, suggesting that Bag7 and Sac7 may share some functions, perhaps in actin organization (497, 499). The deletion of individual Rho1 GAPs produces different growth defects, suggesting that these GAPs have differentiated roles in regulating Rho1-mediated processes. Indeed, Bem2 and Sac7 are known to regulate the Rho1-Pkc1-Mkp1 signaling pathway (362, 499), whereas Bag7 and Sac7 are thought to regulate some aspect of Rho1-mediated actin organization (499), and Lrg1 is required for Rho1-mediated regulation of glucan synthase activity (603).
Rho1 is involved in cell wall synthesis, stress responses, actin organization, and exocytosis (see below). The challenge is to figure out how the activity of Rho1 is regulated to control these distinct processes. Specifically, where and how are different Rho1 regulators such as the GEFs and GAPs localized in the cell cycle? How are the activities of the GEFs and GAPs of Rho1 regulated? Are the GEFs and GAPs regulated in responses to different environmental stimuli? Knowledge on the shared or unique role for each GEF and GAP and their regulatory mechanisms would be crucial for understanding the diverse roles of Rho1.
Rho1 Effectors and Biological Responses
Rho1 and cell wall assembly.
The rigidity of the cell wall and the internal turgor pressure against the plasma membrane offer yeast cells excellent physical and chemical protection against a sudden change in osmolarity in their natural living environment (313). The cell wall is also elastic, ideal for maintaining cell shape and allowing localized surface expansion. Like the polarity proteins and the actin cytoskeleton discussed above, cell wall remodeling, including its synthesis and breakdown, must occur dynamically at sites of polarized cell growth so that plasma membrane and cell wall expansion are coordinated. The cell wall of S. cerevisiae consists of an electron-transparent inner layer and an electron-dense outer layer. The inner layer is composed of 1,3-β-glucan and chitin (N-acetylglucosamine polymers), accounting for ∼50 to 60% of the wall dry weight, and this layer is mainly responsible for the mechanical strength of the wall. The outer layer is composed of heavily glycosylated mannoproteins, accounting for 35 to 40% of the wall dry weight, and this layer mediates cell-cell interactions during mating and controls the accessibility of extracellular substances such as wall-degrading enzymes in plant tissues to the inner layer and the plasma membrane (287). The wall also consists of 1,6-β-glucan, accounting for 5 to 10% of the dry weight, whose main function is to link glycosylphosphatidylinositol-dependent cell wall proteins to β-1,3-glucan and possibly chitin to form a branched cell wall network (74, 287). The β-1,3-glucan is synthesized at the plasma membrane, and β-1,6-glucan synthesis may also occur at the plasma membrane, but it depends on the function of an intact secretory pathway (74, 287). Chitin, accounting for 1 to 2% of the wall dry weight, is synthesized at localized areas of the plasma membrane, mainly at the base of the bud from late G1 to G2/M phases and at the division septum in late anaphase and telophase (74). Here, we focus on how Rho1 controls β-1,3-glucan synthesis during bud growth by regulating the level and the activity of the β-1,3-glucan synthase under normal and stressed conditions.
Two related genes, FKS1 and FKS2, encode the catalytic subunits of β-1,3-glucan synthase. Fks1 is the predominantly expressed form under normal growth conditions, while Fks2 is induced mainly under stress conditions such as high temperature, nutritional starvation, and mating pheromone treatment (313). Both Fks1 and Fks2 contain multiple membrane-spanning domains (119, 244, 372). The deletion of FKS1 or FKS2 does not cause cell lethality, but the deletion of both genes is lethal (244, 372). Rho1 regulates β-1,3-glucan synthesis via at least two distinct pathways, as a direct regulatory subunit of glucan synthase and as an upstream activator of the CWI pathway (Fig. 15) (313). These two pathways can be genetically separated from each other by specific mutations in RHO1 (488). Temperature-sensitive mutants of rho1 are defective in GTP-stimulated glucan synthase activity (124, 462). Both Rho1 and Fks1 localize to sites of polarized growth (462, 627), Rho1 copurifies with Fks1, and GTP-bound Rho1 stimulates its enzymatic activity in vitro (124, 371, 462). Rho1-GDP and Fks1 are transported via secretory vesicles to the sites of polarized growth (2, 374). The activation of Fks1 at the plasma membrane is thought to be controlled by the membrane-associated GEFs Rom1 and Rom2 (2). Rho1 and Fks1, particularly the latter, appear to colocalize with actin patches (462, 583, 627). The actin patch-associated movement of Fks1 may be involved in localized cell wall remodeling (583) as well as endocytosis (106). Thus, Rho1 directly regulates β-1,3-glucan synthase activity under normal growth conditions. An in vitro assay based on crude membrane preparations from S. cerevisiae indicates that Rho1, but not other Rhos and Cdc42, may also regulate β-1,6-glucan synthase activity (595).
Rho1 also regulates the expression of cell wall-related genes such as FKS1, FKS2, and CHS3, which encodes the catalytic subunit of chitin synthase III via the CWI pathway (74, 218, 313) (Fig. 15). The major components of the CWI pathway function as follows. Rho1-GTP activates its effector Pkc1 (the sole protein kinase C in S. cerevisiae), which activates a MAPK cascade from Bck1 (MAPK kinase kinase), Mkk1, and Mkk2 (two functionally redundant MAPK kinases) to Mpk1 (MAPK; also called Slt2). Mpk1 then activates at least two transcriptional factors, the Swi4/Swi6 cell cycle box binding factors and Rlm1, to regulate the expression of genes required for the G1/S transition, such as G1 cyclins (Cln1, Cln2, Pcl1, and Pcl2), and genes required for cell wall biogenesis, such as the enzymes mentioned above. Like Rho1, Pkc1 (109), Mkk1/2, and Mpk1 (590) localize to sites of polarized growth. In addition, Pkc1 localizes to the nucleus and the mitotic spindle (109), and Mpk1 also localizes to the nucleus (590). This evidence and other evidence suggest that Pkc1 may play an Mpk1-dependent and an Mpk1-independent role in regulating microtubule-related functions (313). PKC1 is an essential gene, and the conditional depletion of Pkc1 results in cell cycle-specific cell lysis at all growth temperatures, which is suppressed by high osmolarity, such as the presence of 1 M sorbitol (314, 432). In contrast, cells lacking each component of the MAPK cascade (or Mkk1 and Mkk2 together) exhibit a temperature-sensitive cell lysis defect, which is suppressed by osmotic stabilizing agents (304), indicating that Pkc1 has additional targets besides the MAPK pathway (313) (see below).
The CWI pathway responds to various extracellular stimuli. For example, cell wall stresses caused by calcofluor, Congo red, zymolyase, or caffeine are sensed by five known type I membrane proteins, Wsc1 (also called Hcs77 and Slg1), Wsc2, Wsc3, Mid2, and Mtl1, with the first three and last two defining two groups based mainly on sequence homology (313). Among them, Wsc1 and Mid2 play a dominant role, as the double mutant is inviable but can be rescued by high osmolarity (449, 463). The intracellular domains of Wsc1 and Mid2 interact with and activate Rom2 (449), triggering the activation of the CWI pathway. The activation of CWI is also regulated by phospholipids (218, 313). There are two essential phosphatidylinositol-4 kinases (PI4Ks) in S. cerevisiae, the plasma membrane-localized Stt4 and the Golgi-localized Pik1, which synthesize phosphatidylinositol-4-phosphate (PI4P) at both locations. PI4P is further converted to phosphatidylinositol-4,5-bisphosphate (PI4,5P2) by the single essential PI4P-5 kinase (PI4P-5K), Mss4. The plasma membrane pool of PI4,5P2 is required for the localization and the activation of the Rho1 GEF Rom2 via an interaction between the PIP2 and the PH domain of Rom2. This interaction is essential for the activation of the CWI pathway (25-27).
Rho1 and actin organization.
Rho1 regulates actin organization via two distinct pathways: the CWI pathway and the formin Bni1 (Fig. 15). Specific rho1-Ts alleles display actin organization defects at the nonpermissive temperature (200, 219). Cells lacking Mpk1 also cannot polarize their actin cytoskeleton at 37°C (373). Thus, the Rho1-mediated CWI pathway is required for actin organization, at least, at 37°C. Upon a shift to 37°C, wild-type cells depolarize actin patches and cables transiently, reaching the peak at approximately 30 to 45 min after the shift, and cells repolarize their actin cytoskeleton at between 60 and 120 min (111, 322). The heat stress is thought to weaken the cell wall and thus activate the CWI pathway (269). Further analysis indicates that the CWI pathway bifurcates at Pkc1 to regulate actin depolarization and repolarization. The depolarization phase does not involve the MAPK cascade, but the repolarization phase does (107) (Fig. 15). Disruption of the actin cytoskeleton by latrunculin B also activates the CWI pathway (213). It is yet to be established how the CWI pathway regulates actin organization at the molecular level.
The CWI pathway also functions in Tor (target of rapamycin)-mediated actin organization. Tor1 and Tor2, a pair of highly conserved Ser/Thr kinases, control cell growth in response to nutrients. Both Tor1 and Tor2 possess multiple domains that mediate protein-protein interactions to form Tor complexes, TORC1 and TORC2, respectively (339, 360, 623). These two complexes share essential functions in regulating translation and G1 progression. They also perform distinct functions. TORC1 controls cell growth via a rapamycin-sensitive pathway regulating transcription, translation, and ribosome biogenesis. In contrast, TORC2 signals through a rapamycin-insensitive pathway to regulate the actin cytoskeleton (498). In S. cerevisiae, the CWI pathway is thought to mediate signaling from TORC2 to the actin cytoskeleton, because the overexpression of Pkc1, Bck1, Mkk1, or Mpk1 suppresses the growth and actin organization defects of the tor2-Ts cells (219). Because Mpk1 activation is not impaired in the tor2-Ts cells, it is equally possible that Tor2 and the CWI pathway signal in parallel to the actin cytoskeleton (313).
The formins Bni1 and Bnr1 are required for actin cable formation during polarized growth (140, 486) and are required for actin ring formation during cytokinesis (573, 587). Rho1 binds to Bni1 (138, 289) and is required for the activation of formin at 37°C (116). This activation requires Pkc1 but not the downstream MAPK cascade of the CWI pathway (116). Thus, it appears that Rho1-Pkc1 controls transient actin depolarization during heat stress (107) and also controls formin-mediated actin cable formation at 37°C (116). It is not clear how these two functions of Pkc1 are coordinated temporally and spatially during the temperature shift.
Phospholipids and TORC2 also act in concert to regulate actin organization. Mss4, the PI4P-5K, is required for actin organization at 37°C, suggesting that PI4,5P2 plays an essential role in this process (111). Slm1 and Slm2, a pair of functionally redundant proteins carrying the PH domains, are targeted to the plasma membrane in a PIP2-dependent manner. Conditional inactivation of Slm1 and Slm2 (e.g., slm1-Ts slm2Δ) results in a depolarized actin cytoskeleton, secretion, and Cdc42 and Rho1 localization (28, 142). The growth and actin organization defects of slm1-Ts slm2Δ cells are suppressed by a deletion of the Rho1 GAP Sac7 or overexpression of Pkc1 but not by its downstream components of the CWI pathway (28). In addition, both Slm1 and Slm2 physically associate with the components of the TORC2 complex and are directly phosphorylated by the Tor2 kinase (28, 142). Furthermore, temperature-sensitive mutations in TOR2 (tor1Δ tor2-Ts) are suppressed by the overexpression of MSS4, ROM2, or PKC1 (219). Together, these data suggest that PIP2 and TORC2 coordinately regulate the membrane association of Slm1 and Slm2, which regulates actin organization via Pkc1, but not the MAPK cascade.
Rho1 and exocytosis.
As discussed above, Rho1 regulates polarized exocytosis by regulating actin cable assembly via the formin Bni1. In addition, Rho1 binds to Sec3, a component of the exocyst that is essential for the tethering of the post-Golgi vesicles to the plasma membrane. The Rho1-Sec3 interaction is thought to play a role in the spatial regulation of exocytosis (200). In addition, during heat stress, Rho1 and Pkc1, but not the MAPK components of the CWI pathway, regulate the exit of Chs3, the catalytic subunit of chitin synthase III, from the trans-Golgi network/early endosomes, which is essential for its targeting to the plasma membrane (585, 586).
Rho3, Rho4, and Polarized Cell Growth
Rho3 and Rho4 are another two small GTPases involved in polarized growth in S. cerevisiae. Among the nine predicted Rho GAPs (506), only Rgd1 is shown to display in vitro GAP activity towards Rho3 and Rho4 (115) (Fig. 16). Rgd1 contains a C-terminal Rho GAP domain and N-terminal FCH (FER/CIP4 homology) (238, 578) and DEP (disheveled, EGL-10, and pleckstrin) (359) domains, both of which are involved in membrane association. No GEFs for Rho3 and Rho4 have been identified. It is also not known whether Rdi1, the only Rho GDI in S. cerevisiae, acts on Rho3 and Rho4. Thus, the regulation of Rho3 and Rho4 is far from clear. The deletion of RHO3 causes severe defects in cell growth, whereas the deletion of RHO4 does not produce any obvious phenotype. However, the deletion of both RHO3 and RHO4 causes lethality at 30°C, and the overexpression of RHO4 suppresses the growth defects of rho3Δ, suggesting that Rho3 and Rho4 have some overlapping functions, even though the two GTPases share only ∼35% identity in their amino acid sequences (369). Depletion of Rho3 and Rho4 together results in large, round cells with a small bud and with a disorganized actin cytoskeleton (369, 370). Thus, Rho3 and Rho4 are thought to be required for the maintenance, but not the initiation, of polarized cell growth. Because the growth defects of cells depleted for Rho3 and Rho4 are suppressed by an increased dosage of CDC42, BEM1, or SEC4, Rho3 and Rho4 are thought to play a role in polarized actin organization and exocytosis (241, 370).
FIG. 16.
Model for roles of Rho3 in exocytosis and actin organization. Activated Rho3 interacts with Myo2 and Exo70 to effect vesicle transport and vesicle tethering during exocytosis. Activated Rho3 and Rho4 (not depicted here) may activate the formins directly via their binding to the RBD of the formins (dashed line with a question mark). No physical interaction between these Rho proteins and the formins, except for the Rho4-Bnr1 interaction, has been reported. Alternatively, Rho3 and Rho4 could activate the formins indirectly and also affect actin patch polarization via their role in exocytosis (see the section on Cdc42 polarization). The specific GEFs and the GAPs, except Rgd1, of Rho3 and Rho4 are not known.
Rho3, Rho4, and actin organization.
The depletion of both Rho3 and Rho4 or specific mutations in the effector domains of Rho3 (rho3Y40C and rho3E46G) causes the depolarization of actin patches and the loss of actin cables (6, 370). The deletion of the Rho-binding domain (RBD) of the formin Bni1 or Bnr1 suppresses the growth and actin cable formation defects of rho3Δ rho4Δ cells, suggesting that Rho3 and Rho4 may activate the formins by binding to the RBDs and thus relieving a putative autoinhibitory loop formed between the RBD region at the N terminus and the DAD at the C terminus of formins (116). This idea remains to be tested. It has been shown that Rho4 interacts with Bnr1, but not with Bni1, by two-hybrid and in vitro protein-binding assays (242), but no data are available for the interaction of Rho3 with either Bni1 or Bnr1.
Rho3, Rho4, and exocytosis.
Overexpression of the Rab Sec4 suppresses the growth defects of rho3Δ rho4Δ cells (241), suggesting that Rho3 and Rho4 are involved in exocytosis. Indeed, Rho3 interacts with both the type V myosin Myo2 and the exocyst subunit Exo70 (474). A specific mutation in the Rho3 effector domain (rho3E51V) causes the accumulation of post-Golgi vesicles preferentially in the daughter cells at the permissive temperature and in the entire cells at the nonpermissive temperature (6). This mutant Rho3 no longer interacts with either Myo2 or Exo70. These data suggest that Rho3 plays a role in vesicle transport and tethering (6) (Fig. 16).
Polarized actin organization and exocytosis are intimately linked in S. cerevisiae. Depolarized actin organization such as that in cdc24 and cdc42 mutants results in depolarized exocytosis. Mutants defective in the late secretory pathway also exhibit a depolarized actin cytoskeleton (22, 168). Even cdc42 and rho3 mutants that were initially thought to display specific defects in exocytosis (5, 6) were found to have defects in actin organization (22). One intriguing possibility is that Rho3 regulates the delivery of other small GTPases that are required for Bni1 activation and/or localization via a secretory pathway and thus regulates actin organization indirectly. Although it remains to be tested, this hypothesis is consistent with the following observations. First, no direct interaction between Rho3 and Bni1 has been observed. Second, the growth defects of rho3Δ rho4Δ cells are suppressed by the overexpression of Cdc42 (370), which reinforces itself at the sites of polarized growth via the actomyosin-based delivery or secretory pathway (604). Rho1 is also delivered via the secretory pathway (2, 374). Finally, Cdc42 and Rho1 bind to Bni1, resulting in the localization and/or activation of Bni1.
There are many questions concerning the mechanisms by which Rho3 and Rho4 function. For example, where does Rho3 or Rho4 localize in the cell cycle? What are the GEFs and GAPs, in addition to Rgd1, for Rho3 and/or Rho4? What is the underlying mechanism for the cross talk between Rho3- and Rho4-mediated functions and the Rho1-Pkc1-mediated CWI pathway, as suggested by a number of genetic studies? For example, the deletion of RGD1 is synthetically lethal with the deletion of WSC1, which encodes one of the membrane sensors for the CWI pathway (105). The lethality of rgd1Δ wsc1Δ cells requires the presence of RHO3 or RHO4 (146) and is suppressed by multicopy RHO1, RHO2, MPK1, and MTL1, encoding a membrane sensor (105). As hypothesized for the coupling of the functions of Rho3 in actin organization and exocytosis described above, the genetic interactions between Rho3/Rho4 and the CWI pathway might also indicate the involvement of Rho3/Rho4 in the delivery of membrane sensors of the CWI pathway via the secretory pathway.
What Is the Role of Ras in Polarized Growth?
Ras1 and Ras2 (hereafter referred to as Ras for simplicity), two closely related Ras proteins in S. cerevisiae (170, 277, 562), are involved in choosing a developmental program or life style such as budding, filamentous growth, or sporulation by sensing and responding to nutrients such as carbon and nitrogen sources (502). During budding, Ras is required for cell growth and for the passage through the G1/S transition, two intimately linked processes (311, 502, 606). Ras is regulated by the GEF Cdc25 (209, 262) and the GAPs Ira1 and Ira2 (557-559). In response to nutrients, Ras activates adenylyl cyclase to increase the cyclic AMP (cAMP) concentration, which in turn activates cAMP-dependent PKA by releasing the three catalytic subunits Tpk1 to Tpk3 from the single regulatory subunit Bcy1 (75, 569, 570), and Tpks then activate several transcriptional factors, thus increasing the expression of genes involved in stress responses, ribosome biogenesis, and filamentous growth (311, 502, 606). One of the major outputs of the Ras-cAMP pathway is to increase the cell size critical for passage through Start (264). During this period, the G1 cyclin Cln3 is increased at the protein level, perhaps due to the increased rate of translation as a result of Ras-cAMP pathway activation (204). Ras triggers filamentous growth via two pathways, the Cdc42-MAPK pathway and the cAMP-PKA pathway (see above) (Fig. 14) (311, 395). The cellular cAMP concentration is down-regulated by two cAMP phospodiesterases: the low-affinity Pde1 and the high-affinity Pde2 (311). Pde1 functions mainly during glucose readdition or intracellular acidification (346), whereas Pde2 functions mainly during filamentous growth (298, 342, 429).
The Ras-cAMP pathway and the Rho1-Pkc1 CWI pathway appear to have an antagonistic relationship, which is mediated in part by a putative transcriptional factor, Rpi1 (280, 539). There are also cross talks between the Ras-cAMP pathway and other signaling pathways involved in growth controls (311, 502, 606). In the fission yeast S. pombe, Ras1 regulates cell morphogenesis during vegetative growth and mating by regulating Cdc42 and the mating MAPK pathway, respectively (431). These Ras1-mediated pathways are initiated by two distinct Ras1 GEFs (431). Similarly, Ras in Dictyostelium is also involved in cytoskeleton regulation and chemotaxis (323). Does Ras play a major role in polarized growth during budding in S. cerevisiae? A deletion of RAS2 is synthetically lethal with a deletion of TPM1 at 35°C, which encodes the major isoform of tropomyosin in S. cerevisiae (225). In addition, Ras2 and the Cdc42 effector Cla4 function together to target Lte1 appropriately to the bud cortex to regulate the mitotic exit network (516) (see the discussion on Cdc42 and MEN). Thus, Ras may play a more direct role in the regulation of polarized actin organization and/or growth during budding.
COORDINATION OF Cdc42, Rho, AND Rab DURING POLARIZED GROWTH
Polarized growth occurs at discrete regions of the cell surface in S. cerevisiae, the presumptive bud site (late G1), the tip of a small bud (S phase to G2 phase), the entire bud surface (G2/M phase to anaphase), and the bud neck of large-budded cells (late anaphase to telophase). Cdc42, different Rhos, and Rabs orchestrate the polarization of the actin cytoskeleton, cell wall assembly, and exocytosis towards these sites. Thus, the activities of these small GTPases and their regulated processes must be coordinated in time and space to generate the defined shape of the yeast cell. As discussed above, the functions of individual small GTPases in these processes are not completely understood. The coordination mechanisms among Cdc42, Rhos, and Rabs are even less clear. Nonetheless, we will attempt to summarize what is known about the possible coordination mechanisms here because of the central significance of this issue in polarized growth.
Polarisome-Based Signaling Hub
The “polarisome” consists of the scaffold protein Spa2, the formin Bni1, the actin-binding protein Bud6, Pea2, a protein of unknown function, and perhaps the Sec4 GAPs Msb3 and Msb4 (521, 563, 590) (Fig. 17A). Because the polarisome has never been consistently purified as a discrete protein complex like the Arp2/3 complex or the exocyst and because the “polarisome” simply represents a small network of physically interacting, functionally related proteins, it is sometimes difficult to determine whether a protein is a component of or just peripherally associated with the polarisome. The polarisome and the Gic1/Gic2 proteins act in parallel to regulate the processes downstream of Cdc42, such as the organization of the actin and septin cytoskeleton and exocytosis (Fig. 11 and 17A). At the bud tip, the polarisome coordinately regulates polarized growth (563). The bud tip is analogous to a focal adhesion site of an animal cell because both structures involve the polarized formation of actin cables or stress fibers, protein trafficking, which is dictated by the Rab Sec4 or Arf6, and perhaps localized activation of Cdc42, Rac, and Rho (563). The polarisome is a hub that integrates signals from Cdc42, Rho1, and the Rab Sec4 (247) (Fig. 17A). Msb3 and Msb4, a pair of dosage suppressors of cdc24 and cdc42 mutants (51), bind directly to the N-terminal Spa2 homology domain I (563) and also function as GAPs for Sec4 (12, 14, 168), thus linking Cdc42 to Sec4 via the polarisome. In addition, Cdc42-GTP and Rho1-GTP bind to Bni1 (138, 289), whereas these GTPases in the GDP-bound state bind to Msb3 and Msb4, thus linking Cdc42 and Rho1 together via the polarisome (563). The same Spa2 homology domain I region of Spa2 binds to Mkk1/Mkk2 and Mpk1 of the CWI pathway, thus linking Cdc42 to Rho1 again via the polarisome (521, 590).
FIG. 17.
Possible coordination mechanisms among small GTPases during polarized cell growth. (A) Polarisome-based signaling hub. Linkages between Cdc42 and Rho1 that are suggested by preliminary genetic and two-hybrid interactions are not depicted here. (B) Exocyst-based signaling hub (see the text for details).
A web of genetic interactions suggests that the function of Cdc42 and Rho1 is coordinated by several potential pathways during polarized cell growth. ZDS1 and ZDS2, encoding two closely related proteins that localize to sites of polarized growth, appear to negatively regulate CDC42 (53). A large-scale two-hybrid assay suggests that Zds1 and Zds2 interact with both Gic1 and Gic2, two closely related targets of Cdc42, and that Zds2 interacts with Rho1-GTP and Pkc1 as well (123). In addition, the overexpression of ZDS1, ZDS2, PKC1, or GIC1 (but not GIC2 or CDC42) suppresses the temperature-sensitive growth of tif51A-1, a mutant of the yeast translation initiation factor eIF5A (638). The actin organization defect of tif51A-1 is also partially recovered by the overexpression of Pkc1 and Zds1 as well as Gic1. Taken together, these data suggest that the Pkc1-Zds-Gic interaction coordinates the function of Cdc42 and Rho1, although the underlying mechanism of this coordination is not clear. It is also unclear how eIF5A might be involved in the establishment of polarity in coordination with Pkc1 or Gic1. Given that GIC1 encodes a putative effector of Cdc42, whereas ZDS1 is a potential negative regulator of Cdc42, the similar phenotype of overexpression of each gene is perplexing. Interestingly, the overexpression of PXL1, encoding a paxillin-like protein in S. cerevisiae, suppresses two cdc42-Ts mutants but inhibits a rho1-Ts mutant, suggesting that Cdc42 and Rho1 may play an antagonistic role during polarized growth (169, 349). In addition, the overexpression of ZDS1 and MSB1, a dosage suppressor of cdc42-Ts mutants (45, 169), suppresses the temperature-sensitive growth of a glucan synthase mutant, fks2Δ fks1-Ts (514). Several genetic screens for genes that functionally interact with CDC42 and CDC24 led to the identification of BEM4 (348), whose gene product interacts with multiple small GTPases including Rsr1, Cdc42, Rho1, Rho2, and Rho4 by two-hybrid assays (123, 348). Again, these genetic data suggest multiple cross talks between Cdc42 and Rho1, but the mechanistic details require further investigation.
Exocyst-Based Signaling Hub
The development of cell polarity is ultimately to establish distinct cellular domains or cellular asymmetry, which requires polarized secretion. The exocyst, an effector of Sec4 that consists of eight subunits (Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84), plays an essential role in the tethering of the post-Golgi vesicles (199, 419, 544). The current idea is that Sec4, the Rab GTPase that is essential for protein trafficking from the Golgi apparatus to the plasma membrane, is required for vesicle transport and tethering and, indirectly, for the assembly of SNARE complexes leading to membrane fusion (199, 419). It has been proposed that Sec4 regulates exocytosis via two coordinated pathways (195). In one pathway, Sec4, along with its GEF, Sec2, and an incomplete version of the exocyst, is associated with the secretory vesicles and is transported towards sites of polarized growth via the actin cables and the type V myosin Myo2. Upon the arrival of the vesicles at their destination at the cell cortex, a complete exocyst complex is formed by joining the vesicle-associated and the plasma membrane-associated subunits of the exocyst (63), leading to vesicle tethering. Sec1 then mediates the binding of the exocyst to the assembled SNAREs by interacting with both of them (77, 612), leading to membrane fusion. In a parallel pathway, Sec4 interacts with another effector, Sro7 (and perhaps with its paralog, Sro77), which belongs to the lethal giant larva (lgl) tumor suppressor family (266, 301, 309), which in turn interacts with the target SNARE Sec9 (309) to somehow promote the function of SNARE complexes. These two pathways also talk to each other via direct interactions between Exo84 and Sro7/Sro77 (642). This hypothesis may explain complex genetic interactions observed among the components depicted in Fig. 17B.
The exocyst defines another hub that integrates the signals from many small GTPases during polarized growth (Fig. 17B). The Sec4-Sec15 interaction is thought to promote the formation and/or function of the exocyst (63, 198). Sec3 interacts with both Rho1 (200) and Cdc42 (641) in a competitive manner, and these interactions provide temporally regulated polarization signals to the exocyst. Another component of the exocyst, Exo70, interacts with Rho3 (6, 474, 482). This interaction is thought to positively regulate exocytosis, but the underlying mechanism remains unclear. In addition, Sec15 interacts with Bem1 (123, 155, 196), which interacts with Cdc42 and several other proteins involved in polarity establishment (58). The Sec15-Bem1 interaction is required for the efficient targeting of Sec15, particularly at an early stage of budding (155). Thus, the exocyst coordinates the signaling from Cdc42, Rho1, Rho3, and Sec4 during polarized growth. As in yeast, Rab and Rho family proteins have also been found to interact with the exocyst in mammalian cells (419), suggesting that the regulation of the secretion machinery by small GTPases is evolutionarily conserved. The challenge now is to figure out how all these small GTPases regulate the exocyst at the biochemical level.
Cdc42 and Rho3 are also linked by two structurally related proteins, Boi1 and Boi2, both of which carry an SH3 domain at their N termini and a PH domain at their C termini (47, 368). It is not clear whether this Cdc42-Rho3 communication occurs through the exocyst directly, a point worthy of further investigation. Both Boi1 and Boi2 interact with the second SH3 domain of Bem1 (47), and the N-terminal region containing the first SH3 domain of Bem1 interacts with Sec15 (155), suggesting that the Boi proteins are linked to the exocyst via the Sec15-Bem1 interaction. Like other polarity proteins, Boi1 and Boi2 localize to the presumptive bud site and to the bud cortex early in the cell cycle and to the bud neck in late anaphase (206, 417). The PH domain of Boi1 is responsible for the bud cortex localization, whereas the SH3 domain is responsible for the bud neck localization (206). The deletion of either BOI1 or BOI2 does not cause any detectable phenotype, but the deletion of both genes causes temperature-sensitive growth, with many cells arrested as large, round mother cells with or without a bud, and the double mutant cells are prone to lysis even at the permissive temperature. Overexpression of the PH domain-containing region of Boi1 dramatically decreases cell growth, which is suppressed by the overexpression of Cdc42, and Cdc42 interacts with this region of Boi1 by a two-hybrid assay. In addition, multicopy RHO3 effectively suppresses the temperature-sensitive growth of boi1Δ boi2Δ cells (47, 368). Together, these data suggest that Cdc42 and Rho3 are functionally linked via Boi1 and Boi2, although the underlying mechanism remains unknown.
UNIFYING CONCEPTS IN CELL POLARIZATIONS IN DIFFERENT ORGANISMS
So far, we have discussed the key players in polarity development in S. cerevisiae. Comparative studies of cell polarization in multiple organisms have indicated that the core machinery involved in the establishment and maintenance of cell polarity is conserved from yeast to humans (128, 407). Here, we outline a few key concepts for understanding cell polarization and the central roles of small GTPases in polarity development in diverse organisms.
Global and Local Cell Polarity
Global cell polarity (the mother-bud polarity) in S. cerevisiae is determined by intrinsic signals that direct polarized actin organization, which leads to polarized secretion for bud growth. Such polarity is maintained until late anaphase or telophase, when the growth machinery is redirected to the bud neck to promote cytokinesis and cell separation (Fig. 9) (315, 457). Local polarity (the polarity within the bud) is a result of the fine-tuning of the global polarity by several factors, such as the polarisome in response to cell cycle signals, and is responsible for the concrete bud shape (563).
In filamentous fungi, polarized growth is established during germ tube initiation from a spore and also during hyphal branching (212, 610). Sustained polarized growth at these sites results in the elongation of the germ tube or the growing hyphae. The major elements involved in polarized growth in filamentous fungi are similar to those in S. cerevisiae (212, 450, 609, 610): the conserved, small GTPase-based signaling networks required for polarity establishment (212, 609); the polarized actin cytoskeleton (33, 226, 471, 579), which presumably guides secretory vesicles carrying cell wall synthetic and digestive enzymes to the growth sites; and the “Spitzenkorper” (apical body in German) (179), which may function as a vesicle supply center near the hyphal tip to control the fine shape of the tip (176, 464). The conserved polarity establishment proteins are centered on the Cdc42 GTPase module (212, 609). The polarized actin cables (sometimes called actin fibers or arrays in filamentous fungi) are thought to direct vesicle transport from the cell body to the apical region of the hypha (33) and are thus responsible for the global polarity of the hypha. As in S. cerevisiae, vesicles carrying cell wall synthetic and digestive enzymes are delivered to the apex of a hypha, making the hyphal tip the most active area in cell wall remodeling (529). Local cell polarity, which shapes the hyphal tip, is determined by the mode of cell wall deposition and cross-linking at the apical region, which creates a favorite site for turgor pressure to push the plasma membrane against an elastic wall at the hyphal tip. The turgor pressure may also drive the vesicles from the Spitzenkorper to the cell surface.
Despite the similarities of the major elements involved in polarity development in yeast and filamentous fungi, there are several key differences between bud and hyphal morphogenesis. First, cell cycle control of polarized growth is important in S. cerevisiae (315, 316) but not in filamentous fungi such as Ashbya gossypii (a plant pathogen whose genome is closely related to that of S. cerevisiae) (450, 609, 610). Second, the role of microtubules is not apparent in polarized growth in S. cerevisiae (9) and even in hyphal elongation in C. albicans (631). In contrast, microtubules and/or motors such as dynein and kinesin seem to be important in the morphogenesis of filamentous fungi (118, 243, 511-513). Microtubules modulate polarized growth in terms of the hyphal elongation rate and growth direction either by directly participating in vesicle transport (511) or by transporting some polarity factors to the hyphal tip to influence actin assembly, as was also shown for the fission yeast Schizosaccharomyces pombe (145, 185, 363, 366). Finally, most of the cortical markers for bud site selection in S. cerevisiae are either missing or poorly conserved in most filamentous fungi (212), suggesting that the mechanisms for selecting the axis of cell polarization differ significantly between S. cerevisiae and filamentous fungi.
Local Activation and Global Inhibition
All polarized cells, including budding yeast and human neutrophils, must develop mechanisms by which cell polarity is restricted to a single cortical site, while the rest of the cell surface is prevented from becoming additional sites of cell polarization. Although there are several possible explanations, the singularity of a polarity axis can be best explained by the hypothesis of “local activation and global inhibition,” a concept that was extensively used to explain the chemotactic behaviors of human neutrophils and the social amoeba Dictyostelium (239, 379, 433). The essence of the hypothesis is that local activation at the leading edge is coupled with delivery-based positive feedback to amplify the activating signal. At the same time, a global inhibitor in the cell prevents other portions of the cell surface from becoming another leading edge. The combined actions of the localized, amplified positive signal and of the global negative signal lead to a highly polarized cell along a single anterior-posterior axis. At the molecular level, the local activators at the leading edge in both mammalian neutrophils and Dictyostelium are likely to be phosphoinositides such as PIP3 and small GTPases such as Cdc42 (154, 469, 620), whereas the global inhibitor is represented, at least in part, by PTEN (phosphatase and tensin homolog), a phosphatase that dephosphorylates protein and phosphoinositides including PIP3 (321, 377, 378, 624). According to the same idea, the local activators for polarized growth in S. cerevisiae are Cdc42 and the proteins involved in its polarization, while the global inhibitor remains to be identified. It is interesting that compartmentalized activators and inhibitors, which can be viewed as a modified version of the local activation and global inhibition model, are involved in the Cdc42-regulated mitotic exit network (Fig. 12).
Self-Organization
Self-organization has emerged as a central theme in cell biology. It can be defined simply as the capacity of a macromolecular complex or organelle to organize its own structure using a limited number of interacting components (385). Self-organized structures are in a constant state of nonequilibrium and exhibit oscillatory behaviors at the expense of energy. Thus, self-organization is intrinsically dynamic and endows biological systems with great flexibility and robustness (233, 385). Self-organization has been analyzed extensively in cytoskeleton and motor proteins. Microtubules and actin filaments, together with their motor proteins such as kinesins and myosins, can self-organize into dynamic macrostructures such as the mitotic spindle and the actomyosin contractile apparatus, which drive nuclear division, cell migration, and cytokinesis (386, 405, 489). Self-organization is also a key feature in the selection of cell division sites in Escherichia coli (233) and in the establishment of cell polarity in mammalian chemotactic cells (624).
The establishment and maintenance of cell polarity in S. cerevisiae likely involve self-organization. As discussed in the sections above, activated Cdc42 can spontaneously polarize at the cell cortex in the absence of temporal and spatial cues (84, 196, 604). The major role of Cdc42 in polarized cell growth is to direct the formation of a polarized actomyosin system, which has the features of a self-organized structure and is involved in positive feedback to reinforce Cdc42 polarization (460, 604, 605). Thus, the minimal polarity machinery in S. cerevisiae could include two self-organizing structures, a localized Cdc42-based signaling cap at the bud tip and a global actomyosin-based transport system that interprets and amplifies the polarization signal from the Cdc42-based cap. The validity of this hypothesis requires extensive experimental investigation and theoretical modeling.
CONCLUDING REMARKS
Substantial progress in deciphering the molecular basis of cell polarity in budding yeast has been made. Key signaling pathways that regulate polarization during the mitotic cell cycle and during mating have been identified and extensively characterized. These studies have uncovered the central roles of small GTPases in the development of cell polarity. GTPases have numerous molecular interactions with effectors and regulators and can be involved in multiple distinct signaling pathways. Some of the machinery is specific to yeast, but the general principles underlying cell polarity and the core components in the signaling pathways appear to be highly conserved throughout evolution. An understanding of the spatial and temporal control of the GTPases and their regulators in yeast is thus undoubtedly relevant to other eukaryotes. There are, however, many unanswered questions, as we tried to highlight in each section. One important, but still poorly understood, aspect is the regulation of each component, particularly regulators of GTPases such as GEFs and GAPs, involved in polarity development. Another important aspect is to understand how different GTPases are coordinated at the molecular level during polarized cell growth. The recent development of new technologies in genomics and proteomics and of fluorescence-based technology has provided a better understanding of overall as well as detailed pictures of cellular networks controlling polarity development. The major challenges are to integrate all the fragmented information into cohesive molecular pathways that can be easily understood in terms of cell physiology and function and to determine how these pathways are wired into an elegant machinery that operates with such tremendous fidelity and flexibility during the development of cell polarity.
Acknowledgments
We acknowledge many contributions from the members of the Park and Bi laboratories. We thank Wei Guo for valuable discussions regarding exocytosis and E. Oakley for careful editing. We are also grateful to the anonymous reviewers for many insightful comments. We apologize for not citing all relevant work because of the broad scope and the space limitation of this review.
Our research has been supported by National Institutes of Health grants (GM56997 and GM76375) and an American Heart Association grant (0555303B) to H.-O.P. and by a National Institutes of Health grant (GM59216) and an American Cancer Society grant (RSG-02-039-05-CSM) to E.B.
REFERENCES
- 1.Aalto, M. K., H. Ronne, and S. Keranen. 1993. Yeast syntaxins Sso1p and Sso2p belong to a family of related membrane proteins that function in vesicular transport. EMBO J. 12:4095-4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe, M., H. Qadota, A. Hirata, and Y. Ohya. 2003. Lack of GTP-bound Rho1p in secretory vesicles of Saccharomyces cerevisiae. J. Cell Biol. 162:85-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adam, J. C., J. R. Pringle, and M. Peifer. 2000. Evidence for functional differentiation among Drosophila septins in cytokinesis and cellularization. Mol. Biol. Cell 11:3123-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adames, N., K. Blundell, M. N. Ashby, and C. Boone. 1995. Role of yeast insulin-degrading enzyme homologs in propheromone processing and bud site selection. Science 270:464-467. [DOI] [PubMed] [Google Scholar]
- 5.Adamo, J. E., J. J. Moskow, A. S. Gladfelter, D. Viterbo, D. J. Lew, and P. J. Brennwald. 2001. Yeast Cdc42 functions at a late step in exocytosis, specifically during polarized growth of the emerging bud. J. Cell Biol. 155:581-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adamo, J. E., G. Rossi, and P. Brennwald. 1999. The Rho GTPase Rho3 has a direct role in exocytosis that is distinct from its role in actin polarity. Mol. Biol. Cell 10:4121-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams, A. E., D. Botstein, and D. G. Drubin. 1991. Requirement of yeast fimbrin for actin organization and morphogenesis in vivo. Nature 354:404-408. [DOI] [PubMed] [Google Scholar]
- 8.Adams, A. E., D. I. Johnson, R. M. Longnecker, B. F. Sloat, and J. R. Pringle. 1990. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J. Cell Biol. 111:131-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams, A. E. M., and J. R. Pringle. 1984. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J. Cell Biol. 98:934-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguilar, R. C., S. A. Longhi, J. D. Shaw, L.-Y. Yeh, S. Kim, A. Schon, E. Freire, A. Hsu, W. K. McCormick, H. A. Watson, and B. Wendland. 2006. Epsin N-terminal homology domains perform an essential function regulating Cdc42 through binding Cdc42 GTPase-activating proteins. Proc. Natl. Acad. Sci. USA 103:4116-4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguilar, R. C., H. A. Watson, and B. Wendland. 2003. The yeast epsin Ent1 is recruited to membranes through multiple independent interactions. J. Biol. Chem. 278:10737-10743. [DOI] [PubMed] [Google Scholar]
- 12.Albert, S., and D. Gallwitz. 2000. Msb4p, a protein involved in Cdc42p-dependent organization of the actin cytoskeleton, is a Ypt/Rab-specific GAP. Biol. Chem. 381:453-456. [DOI] [PubMed] [Google Scholar]
- 13.Albert, S., and D. Gallwitz. 1999. Two new members of a family of Ypt/Rab GTPase activating proteins. Promiscuity of substrate recognition. J. Biol. Chem. 274:33186-33189. [DOI] [PubMed] [Google Scholar]
- 14.Albert, S., E. Will, and D. Gallwitz. 1999. Identification of the catalytic domains and their functionally critical arginine residues of two yeast GTPase-activating proteins specific for Ypt/Rab transport GTPases. EMBO J. 18:5216-5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alberts, A. S. 2002. Diaphanous-related formin homology proteins. Curr. Biol. 12:R796. [DOI] [PubMed] [Google Scholar]
- 16.Alberts, A. S. 2001. Identification of a carboxyl-terminal diaphanous-related formin homology protein autoregulatory domain. J. Biol. Chem. 276:2824-2830. [DOI] [PubMed] [Google Scholar]
- 17.Alory, C., and W. E. Balch. 2001. Organization of the Rab-GDI/CHM superfamily: the functional basis for choroideremia disease. Traffic 2:532-543. [DOI] [PubMed] [Google Scholar]
- 18.Amatruda, J. F., J. F. Cannon, K. Tatchell, C. Hug, and J. A. Cooper. 1990. Disruption of the actin cytoskeleton in yeast capping protein mutants. Nature 344:352-354. [DOI] [PubMed] [Google Scholar]
- 19.Amatruda, J. F., and J. A. Cooper. 1992. Purification, characterization, and immunofluorescence localization of Saccharomyces cerevisiae capping protein. J. Cell Biol. 117:1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amberg, D. C., J. E. Zahner, J. W. Mulholland, J. R. Pringle, and D. Botstein. 1997. Aip3p/Bud6p, a yeast actin-interacting protein that is involved in morphogenesis and the selection of bipolar budding sites. Mol. Biol. Cell 8:729-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.An, H., J. L. Morrell, J. L. Jennings, A. J. Link, and K. L. Gould. 2004. Requirements of fission yeast septins for complex formation, localization, and function. Mol. Biol. Cell 15:5551-5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aronov, S., and J. E. Gerst. 2004. Involvement of the late secretory pathway in actin regulation and mRNA transport in yeast. J. Biol. Chem. 279:36962-36971. [DOI] [PubMed] [Google Scholar]
- 23.Asakura, T., T. Sasaki, F. Nagano, A. Satoh, H. Obaishi, H. Nishioka, H. Imamura, K. Hotta, K. Tanaka, H. Nakanishi, and Y. Takai. 1998. Isolation and characterization of a novel actin filament-binding protein from Saccharomyces cerevisiae. Oncogene 16:121-130. [DOI] [PubMed] [Google Scholar]
- 24.Ash, J., C. Wu, R. Larocque, M. Jamal, W. Stevens, M. Osborne, D. Y. Thomas, and M. Whiteway. 2003. Genetic analysis of the interface between Cdc42p and the CRIB domain of Ste20p in Saccharomyces cerevisiae. Genetics 163:9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Audhya, A., and S. D. Emr. 2003. Regulation of PI4,5P2 synthesis by nuclear-cytoplasmic shuttling of the Mss4 lipid kinase. EMBO J. 22:4223-4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Audhya, A., and S. D. Emr. 2002. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev. Cell 2:593-605. [DOI] [PubMed] [Google Scholar]
- 27.Audhya, A., M. Foti, and S. D. Emr. 2000. Distinct roles for the yeast phosphatidylinositol 4-kinases, Stt4p and Pik1p, in secretion, cell growth, and organelle membrane dynamics. Mol. Biol. Cell 11:2673-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Audhya, A., R. Loewith, A. B. Parsons, L. Gao, M. Tabuchi, H. Zhou, C. Boone, M. N. Hall, and S. D. Emr. 2004. Genome-wide lethality screen identifies new PI4,5P2 effectors that regulate the actin cytoskeleton. EMBO J. 23:3747-3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayscough, K. R., and D. G. Drubin. 1998. A role for the yeast actin cytoskeleton in pheromone receptor clustering and signalling. Curr. Biol. 8:927-930. [DOI] [PubMed] [Google Scholar]
- 30.Ayscough, K. R., J. Stryker, N. Pokala, M. Sanders, P. Crews, and D. G. Drubin. 1997. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 137:399-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azzam, R., S. L. Chen, W. Shou, A. S. Mah, G. Alexandru, K. Nasmyth, R. S. Annan, S. A. Carr, and R. J. Deshaies. 2004. Phosphorylation by cyclin B-Cdk underlies release of mitotic exit activator Cdc14 from the nucleolus. Science 305:516-519. [DOI] [PubMed] [Google Scholar]
- 32.Bach, S., O. Bouchat, D. Portetelle, and M. Vandenbol. 2000. Co-deletion of the MSB3 and MSB4 coding regions affects bipolar budding and perturbs the organization of the actin cytoskeleton. Yeast 16:1015-1023. [DOI] [PubMed] [Google Scholar]
- 33.Bachewich, C., and I. B. Heath. 1998. Radial F-actin arrays precede new hypha formation in Saprolegnia: implications for establishing polar growth and regulating tip morphogenesis. J. Cell Sci. 111:2005-2016. [DOI] [PubMed] [Google Scholar]
- 34.Balasubramanian, M. K., E. Bi, and M. Glotzer. 2004. Comparative analysis of cytokinesis in budding yeast, fission yeast and animal cells. Curr. Biol. 14:R806-R818. [DOI] [PubMed] [Google Scholar]
- 35.Barale, S., D. McCusker, and R. A. Arkowitz. 2006. Cdc42p GDP/GTP cycling is necessary for efficient cell fusion during yeast mating. Mol. Biol. Cell 17:2824-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barale, S., D. McCusker, and R. A. Arkowitz. 2004. The exchange factor Cdc24 is required for cell fusion during yeast mating. Eukaryot. Cell 3:1049-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bardin, A. J., R. Visintin, and A. Amon. 2000. A mechanism for coupling exit from mitosis to partitioning of the nucleus. Cell 102:21-31. [DOI] [PubMed] [Google Scholar]
- 38.Barral, Y., V. Mermall, M. S. Mooseker, and M. Snyder. 2000. Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol. Cell 5:841-851. [DOI] [PubMed] [Google Scholar]
- 39.Barral, Y., M. Parra, S. Bidlingmaier, and M. Snyder. 1999. Nim1-related kinases coordinate cell cycle progression with the organization of the peripheral cytoskeleton in yeast. Genes Dev. 13:176-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beach, D. L., J. Thibodeaux, P. Maddox, E. Yeh, and K. Bloom. 2000. The role of the proteins Kar9 and Myo2 in orienting the mitotic spindle of budding yeast. Curr. Biol. 10:1497-1506. [DOI] [PubMed] [Google Scholar]
- 41.Beites, C. L., K. A. Campbell, and W. S. Trimble. 2005. The septin Sept5/CDCrel-1 competes with alpha-SNAP for binding to the SNARE complex. Biochem. J. 385:347-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beites, C. L., H. Xie, R. Bowser, and W. S. Trimble. 1999. The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nat. Neurosci. 2:434-439. [DOI] [PubMed] [Google Scholar]
- 43.Bembenek, J., J. Kang, C. Kurischko, B. Li, J. R. Raab, K. D. Belanger, F. C. Luca, and H. Yu. 2005. Crm1-mediated nuclear export of Cdc14 is required for the completion of cytokinesis in budding yeast. Cell Cycle 4:961-971. [DOI] [PubMed] [Google Scholar]
- 44.Bender, A. 1993. Genetic evidence for the roles of the bud-site-selection genes BUD5 and BUD2 in control of the Rsr1p (Bud1p) GTPase in yeast. Proc. Natl. Acad. Sci. USA 90:9926-9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bender, A., and J. R. Pringle. 1989. Multicopy suppression of the cdc24 budding defect in yeast by CDC42 and three newly identified genes including the ras-related gene RSR1. Proc. Natl. Acad. Sci. USA 86:9976-9980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bender, A., and J. R. Pringle. 1991. Use of a screen for synthetic lethal and multicopy suppressee mutants to identify two new genes involved in morphogenesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 11:1295-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bender, L., H. S. Lo, H. Lee, V. Kokojan, V. Peterson, and A. Bender. 1996. Associations among PH and SH3 domain-containing proteins and Rho-type GTPases in yeast. J. Cell Biol. 133:879-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benton, B., A. H. Tinkelenberg, D. Jean, S. D. Plump, and F. R. Cross. 1993. Genetic analysis of Cln/Cdc28 regulation of cell morphogenesis in budding yeast. EMBO J. 12:5267-5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berlin, A., A. Paoletti, and F. Chang. 2003. Mid2p stabilizes septin rings during cytokinesis in fission yeast. J. Cell Biol. 160:1083-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bi, E. 2001. Cytokinesis in budding yeast: the relationship between actomyosin ring function and septum formation. Cell Struct. Funct. 26:529-537. [DOI] [PubMed] [Google Scholar]
- 51.Bi, E., J. B. Chiavetta, H. Chen, G. C. Chen, C. S. Chan, and J. R. Pringle. 2000. Identification of novel, evolutionarily conserved Cdc42p-interacting proteins and of redundant pathways linking Cdc24p and Cdc42p to actin polarization in yeast. Mol. Biol. Cell 11:773-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bi, E., P. Maddox, D. J. Lew, E. D. Salmon, J. N. McMillan, E. Yeh, and J. R. Pringle. 1998. Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J. Cell Biol. 142:1301-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bi, E., and J. R. Pringle. 1996. ZDS1 and ZDS2, genes whose products may regulate Cdc42p in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:5264-5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bloecher, A., G. M. Venturi, and K. Tatchell. 2000. Anaphase spindle position is monitored by the BUB2 checkpoint. Nat. Cell Biol. 2:556-558. [DOI] [PubMed] [Google Scholar]
- 55.Blondel, M., P. M. Alepuz, L. S. Huang, S. Shaham, G. Ammerer, and M. Peter. 1999. Nuclear export of Far1p in response to pheromones requires the export receptor Msn5p/Ste21p. Genes Dev. 13:2284-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bobola, N., R. P. Jansen, T. H. Shin, and K. Nasmyth. 1996. Asymmetric accumulation of Ash1p in postanaphase nuclei depends on a myosin and restricts yeast mating-type switching to mother cells. Cell 84:699-709. [DOI] [PubMed] [Google Scholar]
- 57.Bon, E., P. Recordon-Navarro, P. Durrens, M. Iwase, E. A. Toh, and M. Aigle. 2000. A network of proteins around Rvs167p and Rvs161p, two proteins related to the yeast actin cytoskeleton. Yeast 16:1229-1241. [DOI] [PubMed] [Google Scholar]
- 58.Bose, I., J. E. Irazoqui, J. J. Moskow, E. S. Bardes, T. R. Zyla, and D. J. Lew. 2001. Assembly of scaffold-mediated complexes containing Cdc42p, the exchange factor Cdc24p, and the effector Cla4p required for cell cycle-regulated phosphorylation of Cdc24p. J. Biol. Chem. 276:7176-7186. [DOI] [PubMed] [Google Scholar]
- 59.Bosl, W. J., and R. Li. 2005. Mitotic-exit control as an evolved complex system. Cell 121:325-333. [DOI] [PubMed] [Google Scholar]
- 60.Bouquin, N., Y. Barral, R. Courbeyrette, M. Blondel, M. Snyder, and C. Mann. 2000. Regulation of cytokinesis by the Elm1 protein kinase in Saccharomyces cerevisiae. J. Cell Sci. 113:1435-1445. [DOI] [PubMed] [Google Scholar]
- 61.Bourne, H. R., D. A. Sanders, and F. McCormick. 1990. The GTPase superfamily: a conserved switch for diverse cell functions. Nature 348:125-132. [DOI] [PubMed] [Google Scholar]
- 62.Bourne, H. R., D. A. Sanders, and F. McCormick. 1991. The GTPase superfamily: conserved structure and molecular mechanism. Nature 349:117-126. [DOI] [PubMed] [Google Scholar]
- 63.Boyd, C., T. Hughes, M. Pypaert, and P. Novick. 2004. Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J. Cell Biol. 167:889-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Breitkreutz, A., and M. Tyers. 2002. MAPK signaling specificity: it takes two to tango. Trends Cell Biol. 12:254-257. [DOI] [PubMed] [Google Scholar]
- 65.Brennwald, P., B. Kearns, K. Champion, S. Keranen, V. Bankaitis, and P. Novick. 1994. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell 79:245-258. [DOI] [PubMed] [Google Scholar]
- 66.Bretscher, A. 2003. Polarized growth and organelle segregation in yeast: the tracks, motors, and receptors. J. Cell Biol. 160:811-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown, J. L., M. Jaquenoud, M. P. Gulli, J. Chant, and M. Peter. 1997. Novel Cdc42-binding proteins Gic1 and Gic2 control cell polarity in yeast. Genes Dev. 11:2972-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burbelo, P. D., D. Drechsel, and A. Hall. 1995. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J. Biol. Chem. 270:29071-29074. [DOI] [PubMed] [Google Scholar]
- 69.Butty, A.-C., N. Perrinjaquet, A. Petit, M. Jaquenoud, J. E. Segall, K. Hofmann, C. Zwahlen, and M. Peter. 2002. A positive feedback loop stabilizes the guanine-nucleotide exchange factor Cdc24 at sites of polarization. EMBO J. 21:1565-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Butty, A.-C., P. M. Pryciak, L. S. Huang, I. Herskowitz, and M. Peter. 1998. The role of Far1p in linking the heterotrimeric G protein to polarity establishment proteins during yeast mating. Science 282:1511-1516. [DOI] [PubMed] [Google Scholar]
- 71.Byers, B. 1981. Cytology of the yeast life cycle, p. 59-96. In J. R. Broach (ed.), The molecular biology of the yeast Saccharomyces: life cycle and inheritance. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 72.Byers, B., and L. Goetsch. 1976. A highly ordered ring of membrane-associated filaments in budding yeast. J. Cell Biol. 69:717-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Byers, B., and L. Goetsch. 1976. Loss of the filamentous ring in cytokinesis-defective mutants of budding yeast. J. Cell Biol. 70:35a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cabib, E., D. H. Roh, M. Schmidt, L. B. Crotti, and A. Varma. 2001. The yeast cell wall and septum as paradigms of cell growth and morphogenesis. J. Biol. Chem. 276:19679-19682. [DOI] [PubMed] [Google Scholar]
- 75.Cannon, J. F., and K. Tatchell. 1987. Characterization of Saccharomyces cerevisiae genes encoding subunits of cyclic AMP-dependent protein kinase. Mol. Cell. Biol. 7:2653-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carlsson, A. E., A. D. Shah, D. Elking, T. S. Karpova, and J. A. Cooper. 2002. Quantitative analysis of actin patch movement in yeast. Biophys. J. 82:2333-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carr, C. M., E. Grote, M. Munson, F. M. Hughson, and P. J. Novick. 1999. Sec1p binds to SNARE complexes and concentrates at sites of secretion. J. Cell Biol. 146:333-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Casamayor, A., and M. Snyder. 2003. Molecular dissection of a yeast septin: distinct domains are required for septin interaction, localization, and function. Mol. Cell. Biol. 23:2762-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Castillon, G. A., N. R. Adames, C. H. Rosello, H. S. Seidel, M. S. Longtine, J. A. Cooper, and R. A. Heil-Chapdelaine. 2003. Septins have a dual role in controlling mitotic exit in budding yeast. Curr. Biol. 13:654-658. [DOI] [PubMed] [Google Scholar]
- 80.Catlett, N. L., J. E. Duex, F. Tang, and L. S. Weisman. 2000. Two distinct regions in a yeast myosin-V tail domain are required for the movement of different cargoes. J. Cell Biol. 150:513-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Catlett, N. L., and L. S. Weisman. 2000. Divide and multiply: organelle partitioning in yeast. Curr. Opin. Cell Biol. 12:509-516. [DOI] [PubMed] [Google Scholar]
- 82.Catlett, N. L., and L. S. Weisman. 1998. The terminal tail region of a yeast myosin-V mediates its attachment to vacuole membranes and sites of polarized growth. Proc. Natl. Acad. Sci. USA 95:14799-14804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Caviston, J. P., M. Longtine, J. R. Pringle, and E. Bi. 2003. The role of Cdc42p GTPase-activating proteins in assembly of the septin ring in yeast. Mol. Biol. Cell 14:4051-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caviston, J. P., S. E. Tcheperegine, and E. Bi. 2002. Singularity in budding: a role for the evolutionarily conserved small GTPase Cdc42p. Proc. Natl. Acad. Sci. USA 99:12185-12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chant, J. 1999. Cell polarity in yeast. Annu. Rev. Cell Dev. Biol. 15:365-391. [DOI] [PubMed] [Google Scholar]
- 86.Chant, J., K. Corrado, J. R. Pringle, and I. Herskowitz. 1991. Yeast BUD5, encoding a putative GDP-GTP exchange factor, is necessary for bud site selection and interacts with bud formation gene BEM1. Cell 65:1213-1224. [DOI] [PubMed] [Google Scholar]
- 87.Chant, J., and I. Herskowitz. 1991. Genetic control of bud site selection in yeast by a set of gene products that constitute a morphogenetic pathway. Cell 65:1203-1212. [DOI] [PubMed] [Google Scholar]
- 88.Chant, J., M. Mischke, E. Mitchell, I. Herskowitz, and J. R. Pringle. 1995. Role of Bud3p in producing the axial budding pattern of yeast. J. Cell Biol. 129:767-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chant, J., and J. R. Pringle. 1995. Patterns of bud-site selection in the yeast Saccharomyces cerevisiae. J. Cell Biol. 129:751-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen, G. C., Y. J. Kim, and C. S. Chan. 1997. The Cdc42 GTPase-associated proteins Gic1 and Gic2 are required for polarized cell growth in Saccharomyces cerevisiae. Genes Dev. 11:2958-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen, G. C., L. Zheng, and C. S. Chan. 1996. The LIM domain-containing Dbm1 GTPase-activating protein is required for normal cellular morphogenesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:1376-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen, J. L., R. V. Fucini, L. Lacomis, H. Erdjument-Bromage, P. Tempst, and M. Stamnes. 2005. Coatomer-bound Cdc42 regulates dynein recruitment to COPI vesicles. J. Cell Biol. 169:383-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen, T., T. Hiroko, A. Chaudhuri, F. Inose, M. Lord, S. Tanaka, J. Chant, and A. Fujita. 2000. Multigenerational cortical inheritance of the Rax2 protein in orienting polarity and division in yeast. Science 290:1975-1978. [DOI] [PubMed] [Google Scholar]
- 94.Chu, H. M., M. Yun, D. E. Anderson, H. Sage, H. W. Park, and S. A. Endow. 2005. Kar3 interaction with Cik1 alters motor structure and function. EMBO J. 24:3214-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chuang, T. H., X. Xu, U. G. Knaus, M. J. Hart, and G. M. Bokoch. 1993. GDP dissociation inhibitor prevents intrinsic and GTPase activating protein-stimulated GTP hydrolysis by the Rac GTP-binding protein. J. Biol. Chem. 268:775-778. [PubMed] [Google Scholar]
- 96.Cid, V. J., L. Adamikova, M. Sanchez, M. Molina, and C. Nombela. 2001. Cell cycle control of septin ring dynamics in the budding yeast. Microbiology 147:1437-1450. [DOI] [PubMed] [Google Scholar]
- 97.Cid, V. J., R. Cenamor, M. Sanchez, and C. Nombela. 1998. A mutation in the Rho1-GAP-encoding gene BEM2 of Saccharomyces cerevisiae affects morphogenesis and cell wall functionality. Microbiology 144:25-36. [DOI] [PubMed] [Google Scholar]
- 98.Cook, J. G., L. Bardwell, and J. Thorner. 1997. Inhibitory and activating functions for MAPK Kss1 in the S. cerevisiae filamentous-growth signalling pathway. Nature 390:85-88. [DOI] [PubMed] [Google Scholar]
- 99.Cooper, J. A., and D. P. Kiehart. 1996. Septins may form a ubiquitous family of cytoskeletal filaments. J. Cell Biol. 134:1345-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Crouzet, M., M. Urdaci, L. Dulau, and M. Aigle. 1991. Yeast mutant affected for viability upon nutrient starvation: characterization and cloning of the RVS161 gene. Yeast 7:727-743. [DOI] [PubMed] [Google Scholar]
- 101.Cullen, P. J., W. Sabbagh, Jr., E. Graham, M. M. Irick, E. K. van Olden, C. Neal, J. Delrow, L. Bardwell, and G. F. Sprague, Jr. 2004. A signaling mucin at the head of the Cdc42- and MAPK-dependent filamentous growth pathway in yeast. Genes Dev. 18:1695-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cvrckova, F., C. De Virgilio, E. Manser, J. R. Pringle, and K. Nasmyth. 1995. Ste20-like protein kinases are required for normal localization of cell growth and for cytokinesis in budding yeast. Genes Dev. 9:1817-1830. [DOI] [PubMed] [Google Scholar]
- 103.Cvrckova, F., and K. Nasmyth. 1993. Yeast G1 cyclins CLN1 and CLN2 and a GAP-like protein have a role in bud formation. EMBO J. 12:5277-5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.D'Aquino, K. E., F. Monje-Casas, J. Paulson, V. Reiser, G. M. Charles, L. Lai, K. M. Shokat, and A. Amon. 2005. The protein kinase Kin4 inhibits exit from mitosis in response to spindle position defects. Mol. Cell 19:223-234. [DOI] [PubMed] [Google Scholar]
- 105.de Bettignies, G., D. Thoraval, C. Morel, M. F. Peypouquet, and M. Crouzet. 2001. Overactivation of the protein kinase C-signaling pathway suppresses the defects of cells lacking the Rho3/Rho4-GAP Rgd1p in Saccharomyces cerevisiae. Genetics 159:1435-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.deHart, A. K., J. D. Schnell, D. A. Allen, J. Y. Tsai, and L. Hicke. 2003. Receptor internalization in yeast requires the Tor2-Rho1 signaling pathway. Mol. Biol. Cell 14:4676-4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Delley, P. A., and M. N. Hall. 1999. Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J. Cell Biol. 147:163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.DeMarini, D. J., A. E. M. Adams, H. Fares, C. De Virgilio, G. Valle, J. S. Chuang, and J. R. Pringle. 1997. A septin-based hierarchy of proteins required for localized deposition of chitin in the Saccharomyces cerevisiae cell wall. J. Cell Biol. 139:75-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Denis, V., and M. S. Cyert. 2005. Molecular analysis reveals localization of Saccharomyces cerevisiae protein kinase C to sites of polarized growth and Pkc1p targeting to the nucleus and mitotic spindle. Eukaryot. Cell 4:36-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.DerMardirossian, C., and G. M. Bokoch. 2005. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 15:356-363. [DOI] [PubMed] [Google Scholar]
- 111.Desrivieres, S., F. T. Cooke, P. J. Parker, and M. N. Hall. 1998. MSS4, a phosphatidylinositol-4-phosphate 5-kinase required for organization of the actin cytoskeleton in Saccharomyces cerevisiae. J. Biol. Chem. 273:15787-15793. [DOI] [PubMed] [Google Scholar]
- 112.Dobbelaere, J., and Y. Barral. 2004. Spatial coordination of cytokinetic events by compartmentalization of the cell cortex. Science 305:393-396. [DOI] [PubMed] [Google Scholar]
- 113.Dobbelaere, J., M. S. Gentry, R. L. Hallberg, and Y. Barral. 2003. Phosphorylation-dependent regulation of septin dynamics during the cell cycle. Dev. Cell 4:345-357. [DOI] [PubMed] [Google Scholar]
- 114.Dohlman, H. G., and J. W. Thorner. 2001. Regulation of G protein-initiated signal transduction in yeast: paradigms and principles. Annu. Rev. Biochem. 70:703-754. [DOI] [PubMed] [Google Scholar]
- 115.Doignon, F., C. Weinachter, O. Roumanie, and M. Crouzet. 1999. The yeast Rgd1p is a GTPase activating protein of the Rho3 and Rho4 proteins. FEBS Lett. 459:458-462. [DOI] [PubMed] [Google Scholar]
- 116.Dong, Y., D. Pruyne, and A. Bretscher. 2003. Formin-dependent actin assembly is regulated by distinct modes of Rho signaling in yeast. J. Cell Biol. 161:1081-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dorer, R., C. Boone, T. Kimbrough, J. Kim, and L. H. Hartwell. 1997. Genetic analysis of default mating behavior in Saccharomyces cerevisiae. Genetics 146:39-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Doshi, P., C. A. Bossie, J. H. Doonan, G. S. May, and N. R. Morris. 1991. Two alpha-tubulin genes of Aspergillus nidulans encode divergent proteins. Mol. Gen. Genet. 225:129-141. [DOI] [PubMed] [Google Scholar]
- 119.Douglas, C. M., F. Foor, J. A. Marrinan, N. Morin, J. B. Nielsen, A. M. Dahl, P. Mazur, W. Baginsky, W. Li, M. El-Sherbeini, J. A. Clemas, S. M. Mandala, B. R. Frommer, and M. B. Kurtz. 1994. The Saccharomyces cerevisiae FKS1 (ETG1) gene encodes an integral membrane protein which is a subunit of 1,3-β-D-glucan synthase. Proc. Natl. Acad. Sci. USA 91:12907-12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dovas, A., and J. R. Couchman. 2005. RhoGDI: multiple functions in the regulation of Rho family GTPase activities. Biochem. J. 390:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Doyle, T., and D. Botstein. 1996. Movement of yeast cortical actin cytoskeleton visualized in vivo. Proc. Natl. Acad. Sci. USA 93:3886-3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Drees, B., C. Brown, B. G. Barrell, and A. Bretscher. 1995. Tropomyosin is essential in yeast, yet the TPM1 and TPM2 products perform distinct functions. J. Cell Biol. 128:383-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Drees, B. L., B. Sundin, E. Brazeau, J. P. Caviston, G.-C. Chen, W. Guo, K. G. Kozminski, M. W. Lau, J. J. Moskow, A. Tong, L. R. Schenkman, A. McKenzie III, P. Brennwald, M. Longtine, E. Bi, C. Chan, P. Novick, C. Boone, J. R. Pringle, T. N. Davis, S. Fields, and D. G. Drubin. 2001. A protein interaction map for cell polarity development. J. Cell Biol. 154:549-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Drgonova, J., T. Drgon, K. Tanaka, R. Kollar, G. C. Chen, R. A. Ford, C. S. Chan, Y. Takai, and E. Cabib. 1996. Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science 272:277-279. [DOI] [PubMed] [Google Scholar]
- 125.Drogen, F., S. M. O'Rourke, V. M. Stucke, M. Jaquenoud, A. M. Neiman, and M. Peter. 2000. Phosphorylation of the MEKK Ste11p by the PAK-like kinase Ste20p is required for MAP kinase signaling in vivo. Curr. Biol. 10:630-639. [DOI] [PubMed] [Google Scholar]
- 126.Drubin, D. 1991. Development of cell polarity in budding yeast. Cell 65:1093-1096. [DOI] [PubMed] [Google Scholar]
- 127.Drubin, D. G., K. G. Miller, and D. Botstein. 1988. Yeast actin-binding proteins: evidence for a role in morphogenesis. J. Cell Biol. 107:2551-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Drubin, D. G., and W. J. Nelson. 1996. Origins of cell polarity. Cell 84:335-344. [DOI] [PubMed] [Google Scholar]
- 129.Duncan, M. C., M. J. Cope, B. L. Goode, B. Wendland, and D. G. Drubin. 2001. Yeast Eps15-like endocytic protein, Pan1p, activates the Arp2/3 complex. Nat. Cell Biol. 3:687-690. [DOI] [PubMed] [Google Scholar]
- 130.Dunn, T. M., and D. Shortle. 1990. Null alleles of SAC7 suppress temperature-sensitive actin mutations in Saccharomyces cerevisiae. Mol. Cell. Biol. 10:2308-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dupre, S., D. Urban-Grimal, and R. Haguenauer-Tsapis. 2004. Ubiquitin and endocytic internalization in yeast and animal cells. Biochim. Biophys. Acta 1695:89-111. [DOI] [PubMed] [Google Scholar]
- 132.Eby, J. J., S. P. Holly, F. van Drogen, A. V. Grishin, M. Peter, D. G. Drubin, and K. J. Blumer. 1998. Actin cytoskeleton organization regulated by the PAK family of protein kinases. Curr. Biol. 8:967-970. [DOI] [PubMed] [Google Scholar]
- 133.Eitzen, G., N. Thorngren, and W. Wickner. 2001. Rho1p and Cdc42p act after Ypt7p to regulate vacuole docking. EMBO J. 20:5650-5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Elion, E. A. 2000. Pheromone response, mating and cell biology. Curr. Opin. Microbiol. 3:573-581. [DOI] [PubMed] [Google Scholar]
- 135.Engqvist-Goldstein, A. E., and D. G. Drubin. 2003. Actin assembly and endocytosis: from yeast to mammals. Annu. Rev. Cell Dev. Biol. 19:287-332. [DOI] [PubMed] [Google Scholar]
- 136.Erickson, J. W., C. Zhang, R. A. Kahn, T. Evans, and R. A. Cerione. 1996. Mammalian Cdc42 is a brefeldin A-sensitive component of the Golgi apparatus. J. Biol. Chem. 271:26850-26854. [DOI] [PubMed] [Google Scholar]
- 137.Etienne-Manneville, S., and A. Hall. 2002. Rho GTPases in cell biology. Nature 420:629-635. [DOI] [PubMed] [Google Scholar]
- 138.Evangelista, M., K. Blundell, M. S. Longtine, C. J. Chow, N. Adames, J. R. Pringle, M. Peter, and C. Boone. 1997. Bni1p, a yeast formin linking Cdc42p and the actin cytoskeleton during polarized morphogenesis. Science 276:118-122. [DOI] [PubMed] [Google Scholar]
- 139.Evangelista, M., B. M. Klebl, A. H. Tong, B. A. Webb, T. Leeuw, E. Leberer, M. Whiteway, D. Y. Thomas, and C. Boone. 2000. A role for myosin-I in actin assembly through interactions with Vrp1p, Bee1p, and the Arp2/3 complex. J. Cell Biol. 148:353-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Evangelista, M., D. Pruyne, D. C. Amberg, C. Boone, and A. Bretscher. 2002. Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nat. Cell Biol. 4:32-41. [DOI] [PubMed] [Google Scholar]
- 141.Evangelista, M., S. Zigmond, and C. Boone. 2003. Formins: signaling effectors for assembly and polarization of actin filaments. J. Cell Sci. 116:2603-2611. [DOI] [PubMed] [Google Scholar]
- 142.Fadri, M., A. Daquinag, S. Wang, T. Xue, and J. Kunz. 2005. The pleckstrin homology domain proteins Slm1 and Slm2 are required for actin cytoskeleton organization in yeast and bind phosphatidylinositol-4,5-bisphosphate and TORC2. Mol. Biol. Cell 16:1883-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Farkasovsky, M., P. Herter, B. Voss, and A. Wittinghofer. 2005. Nucleotide binding and filament assembly of recombinant yeast septin complexes. Biol. Chem. 386:643-656. [DOI] [PubMed] [Google Scholar]
- 144.Faty, M., M. Fink, and Y. Barral. 2002. Septins: a ring to part mother and daughter. Curr. Genet. 41:123-131. [DOI] [PubMed] [Google Scholar]
- 145.Feierbach, B., F. Verde, and F. Chang. 2004. Regulation of a formin complex by the microtubule plus end protein tea1p. J. Cell Biol. 165:697-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fernandes, H., O. Roumanie, S. Claret, X. Gatti, D. Thoraval, F. Doignon, and M. Crouzet. 2006. The Rho3 and Rho4 small GTPases interact functionally with Wsc1p, a cell surface sensor of the protein kinase C cell-integrity pathway in Saccharomyces cerevisiae. Microbiology 152:695-708. [DOI] [PubMed] [Google Scholar]
- 147.Field, C. M., O. Al-Awar, J. Rosenblatt, M. L. Wong, B. Alberts, and T. J. Mitchison. 1996. A purified Drosophila septin complex forms filaments and exhibits GTPase activity. J. Cell Biol. 133:605-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Field, C. M., and D. Kellogg. 1999. Septins: cytoskeletal polymers or signalling GTPases? Trends Cell Biol. 9:387-394. [DOI] [PubMed] [Google Scholar]
- 149.Finger, F. P., T. E. Hughes, and P. Novick. 1998. Sec3p is a spatial landmark for polarized secretion in budding yeast. Cell 92:559-571. [DOI] [PubMed] [Google Scholar]
- 150.Finger, F. P., and P. Novick. 1997. Sec3p is involved in secretion and morphogenesis in Saccharomyces cerevisiae. Mol. Biol. Cell 8:647-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fitch, P. G., A. E. Gammie, D. J. Lee, V. B. de Candal, and M. D. Rose. 2004. Lrg1p is a Rho1 GTPase-activating protein required for efficient cell fusion in yeast. Genetics 168:733-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Flescher, E. G., K. Madden, and M. Snyder. 1993. Components required for cytokinesis are important for bud site selection in yeast. J. Cell Biol. 122:373-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ford, S. K., and J. R. Pringle. 1991. Cellular morphogenesis in the Saccharomyces cerevisiae cell cycle: localization of the CDC11 gene product and the timing of events at the budding site. Dev. Genet. 12:281-292. [DOI] [PubMed] [Google Scholar]
- 154.Franca-Koh, J., and P. N. Devreotes. 2004. Moving forward: mechanisms of chemoattractant gradient sensing. Physiology 19:300-308. [DOI] [PubMed] [Google Scholar]
- 155.France, Y. E., C. Boyd, J. Coleman, and P. J. Novick. 2006. The polarity-establishment component Bem1p interacts with the exocyst complex through the Sec15p subunit. J. Cell Sci. 119:876-888. [DOI] [PubMed] [Google Scholar]
- 156.Fraschini, R., C. D'Ambrosio, M. Venturetti, G. Lucchini, and S. Piatti. 2006. Disappearance of the budding yeast Bub2-Bfa1 complex from the mother-bound spindle pole contributes to mitotic exit. J. Cell Biol. 172:335-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Frazier, J. A., M. L. Wong, M. S. Longtine, J. R. Pringle, M. Mann, T. J. Mitchison, and C. Field. 1998. Polymerization of purified yeast septins: evidence that organized filament arrays may not be required for septin function. J. Cell Biol. 143:737-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Freifelder, D. 1960. Bud position in Saccharomyces cerevisiae. J. Bacteriol. 80:567-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Frenz, L. M., S. E. Lee, D. Fesquet, and L. H. Johnston. 2000. The budding yeast Dbf2 protein kinase localises to the centrosome and moves to the bud neck in late mitosis. J. Cell Sci. 113:3399-3408. [DOI] [PubMed] [Google Scholar]
- 160.Friesen, H., C. Humphries, Y. Ho, O. Schub, K. Colwill, and B. Andrews. 2006. Characterization of the yeast amphiphysins Rvs161 and Rvs167 reveals roles for the Rvs heterodimer in vivo. Mol. Biol. Cell 17:1306-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fucini, R. V., J. L. Chen, C. Sharma, M. M. Kessels, and M. Stamnes. 2002. Golgi vesicle proteins are linked to the assembly of an actin complex defined by mAbp1. Mol. Biol. Cell 13:621-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fujita, A., M. Lord, T. Hiroko, F. Hiroko, T. Chen, C. Oka, Y. Misumi, and J. Chant. 2004. Rax1, a protein required for the establishment of the bipolar budding pattern in yeast. Gene 327:161-169. [DOI] [PubMed] [Google Scholar]
- 163.Fujita, A., C. Oka, Y. Arikawa, T. Katagai, A. Tonouchi, S. Kuhara, and Y. Misumi. 1994. A yeast gene necessary for bud-site selection encodes a protein similar to insulin-degrading enzymes. Nature 372:567-570. [DOI] [PubMed] [Google Scholar]
- 164.Fujiwara, T., K. Tanaka, A. Mino, M. Kikyo, K. Takahashi, K. Shimizu, and Y. Takai. 1998. Rho1p-Bni1p-Spa2p interactions: implication in localization of Bni1p at the bud site and regulation of the actin cytoskeleton in Saccharomyces cerevisiae. Mol. Biol. Cell 9:1221-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fukumoto, Y., K. Kaibuchi, Y. Hori, H. Fujioka, S. Araki, T. Ueda, A. Kikuchi, and Y. Takai. 1990. Molecular cloning and characterization of a novel type of regulatory protein (GDI) for the rho proteins, ras p21-like small GTP-binding proteins. Oncogene 5:1321-1328. [PubMed] [Google Scholar]
- 166.Gammie, A. E., V. Brizzio, and M. D. Rose. 1998. Distinct morphological phenotypes of cell fusion mutants. Mol. Biol. Cell 9:1395-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gandhi, P. N., R. M. Gibson, X. Tong, J. Miyoshi, Y. Takai, M. Konieczkowski, J. R. Sedor, and A. L. Wilson-Delfosse. 2004. An activating mutant of Rac1 that fails to interact with Rho GDP-dissociation inhibitor stimulates membrane ruffling in mammalian cells. Biochem. J. 378:409-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gao, X. D., S. Albert, S. E. Tcheperegine, C. G. Burd, D. Gallwitz, and E. Bi. 2003. The GAP activity of Msb3p and Msb4p for the Rab GTPase Sec4p is required for efficient exocytosis and actin organization. J. Cell Biol. 162:635-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gao, X. D., J. P. Caviston, S. E. Tcheperegine, and E. Bi. 2004. Pxl1p, a paxillin-like protein in Saccharomyces cerevisiae, may coordinate Cdc42p and Rho1p functions during polarized growth. Mol. Biol. Cell 15:3977-3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Garcia-Ranea, J. A., and A. Valencia. 1998. Distribution and functional diversification of the ras superfamily in Saccharomyces cerevisiae. FEBS Lett. 434:219-225. [DOI] [PubMed] [Google Scholar]
- 171.Geli, M. I., R. Lombardi, B. Schmelzl, and H. Riezman. 2000. An intact SH3 domain is required for myosin I-induced actin polymerization. EMBO J. 19:4281-4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Geli, M. I., and H. Riezman. 1996. Role of type I myosins in receptor-mediated endocytosis in yeast. Science 272:533-535. [DOI] [PubMed] [Google Scholar]
- 173.Geymonat, M., A. Spanos, S. J. Smith, E. Wheatley, K. Rittinger, L. H. Johnston, and S. G. Sedgwick. 2002. Control of mitotic exit in budding yeast. In vitro regulation of Tem1 GTPase by Bub2 and Bfa1. J. Biol. Chem. 277:28439-28445. [DOI] [PubMed] [Google Scholar]
- 174.Gibson, R. M., P. N. Gandhi, X. Tong, J. Miyoshi, Y. Takai, M. Konieczkowski, J. R. Sedor, and A. L. Wilson-Delfosse. 2004. An activating mutant of Cdc42 that fails to interact with Rho GDP-dissociation inhibitor localizes to the plasma membrane and mediates actin reorganization. Exp. Cell Res. 301:211-222. [DOI] [PubMed] [Google Scholar]
- 175.Gibson, R. M., and A. L. Wilson-Delfosse. 2001. RhoGDI-binding-defective mutant of Cdc42Hs targets to membranes and activates filopodia formation but does not cycle with the cytosol of mammalian cells. Biochem. J. 359:285-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gierz, G., and S. Bartnicki-Garcia. 2001. A three-dimensional model of fungal morphogenesis based on the vesicle supply center concept. J. Theor. Biol. 208:151-164. [DOI] [PubMed] [Google Scholar]
- 177.Gilbert, P. M., and C. G. Burd. 2001. GDP dissociation inhibitor domain II required for Rab GTPase recycling. J. Biol. Chem. 276:8014-8020. [DOI] [PubMed] [Google Scholar]
- 178.Gimeno, C. J., P. O. Ljungdahl, C. A. Styles, and G. R. Fink. 1992. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell 68:1077-1090. [DOI] [PubMed] [Google Scholar]
- 179.Girbardt, M. 1957. Der Spitzenkorper von Polystictus versicolor (L). Planta 50:47-59. [Google Scholar]
- 180.Gladfelter, A. S. 2006. Control of filamentous fungal cell shape by septins and formins. Nat. Rev. Microbiol. 4:223-229. [DOI] [PubMed] [Google Scholar]
- 181.Gladfelter, A. S., I. Bose, T. R. Zyla, E. S. G. Bardes, and D. J. Lew. 2002. Septin ring assembly involves cycles of GTP loading and hydrolysis by Cdc42p. J. Cell Biol. 156:315-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gladfelter, A. S., L. Kozubowski, T. R. Zyla, and D. J. Lew. 2005. Interplay between septin organization, cell cycle and cell shape in yeast. J. Cell Sci. 118:1617-1628. [DOI] [PubMed] [Google Scholar]
- 183.Gladfelter, A. S., J. J. Moskow, T. R. Zyla, and D. J. Lew. 2001. Isolation and characterization of effector-loop mutants of CDC42 in yeast. Mol. Biol. Cell 12:1239-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gladfelter, A. S., J. R. Pringle, and D. J. Lew. 2001. The septin cortex at the yeast mother-bud neck. Curr. Opin. Microbiol. 4:681-689. [DOI] [PubMed] [Google Scholar]
- 185.Glynn, J. M., R. J. Lustig, A. Berlin, and F. Chang. 2001. Role of bud6p and tea1p in the interaction between actin and microtubules for the establishment of cell polarity in fission yeast. Curr. Biol. 11:836-845. [DOI] [PubMed] [Google Scholar]
- 186.Goeckeler, Z. M., and R. B. Wysolmerski. 1995. Myosin light chain kinase-regulated endothelial cell contraction: the relationship between isometric tension, actin polymerization, and myosin phosphorylation. J. Cell Biol. 130:613-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Goehring, A. S., D. A. Mitchell, A. H. Tong, M. E. Keniry, C. Boone, and G. F. Sprague, Jr. 2003. Synthetic lethal analysis implicates Ste20p, a p21-activated protein kinase, in polarisome activation. Mol. Biol. Cell 14:1501-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Goode, B. L., and A. A. Rodal. 2001. Modular complexes that regulate actin assembly in budding yeast. Curr. Opin. Microbiol. 4:703-712. [DOI] [PubMed] [Google Scholar]
- 189.Goode, B. L., A. A. Rodal, G. Barnes, and D. G. Drubin. 2001. Activation of the Arp2/3 complex by the actin filament binding protein Abp1p. J. Cell Biol. 153:627-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Goodson, H. V., B. L. Anderson, H. M. Warrick, L. A. Pon, and J. A. Spudich. 1996. Synthetic lethality screen identifies a novel yeast myosin I gene (MYO5): myosin I proteins are required for polarization of the actin cytoskeleton. J. Cell Biol. 133:1277-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gottfried, Y., A. Rotem, R. Lotan, H. Steller, and S. Larisch. 2004. The mitochondrial ARTS protein promotes apoptosis through targeting XIAP. EMBO J. 23:1627-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Goud, B., A. Salminen, N. C. Walworth, and P. J. Novick. 1988. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell 53:753-768. [DOI] [PubMed] [Google Scholar]
- 193.Govindan, B., R. Bowser, and P. Novick. 1995. The role of Myo2, a yeast class V myosin, in vesicular transport. J. Cell Biol. 128:1055-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gray, C. H., V. M. Good, N. K. Tonks, and D. Barford. 2003. The structure of the cell cycle protein Cdc14 reveals a proline-directed protein phosphatase. EMBO J. 22:3524-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Grosshans, B. L., A. Andreeva, A. Gangar, S. Niessen, J. R. Yates III, P. Brennwald, and P. Novick. 2006. The yeast lgl family member Sro7p is an effector of the secretory Rab GTPase Sec4p. J. Cell Biol. 172:55-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gulli, M. P., M. Jaquenoud, Y. Shimada, G. Niederhauser, P. Wiget, and M. Peter. 2000. Phosphorylation of the Cdc42 exchange factor Cdc24 by the PAK-like kinase Cla4 may regulate polarized growth in yeast. Mol. Cell 6:1155-1167. [DOI] [PubMed] [Google Scholar]
- 197.Gulli, M. P., and M. Peter. 2001. Temporal and spatial regulation of Rho-type guanine-nucleotide exchange factors: the yeast perspective. Genes Dev. 15:365-379. [DOI] [PubMed] [Google Scholar]
- 198.Guo, W., D. Roth, C. Walch-Solimena, and P. Novick. 1999. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 18:1071-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Guo, W., M. Sacher, J. Barrowman, S. Ferro-Novick, and P. Novick. 2000. Protein complexes in transport vesicle targeting. Trends Cell Biol. 10:251-255. [DOI] [PubMed] [Google Scholar]
- 200.Guo, W., F. Tamanoi, and P. Novick. 2001. Spatial regulation of the exocyst complex by Rho1 GTPase. Nat. Cell Biol. 3:353-360. [DOI] [PubMed] [Google Scholar]
- 201.Haarer, B. K., S. H. Lillie, A. E. Adams, V. Magdolen, W. Bandlow, and S. S. Brown. 1990. Purification of profilin from Saccharomyces cerevisiae and analysis of profilin-deficient cells. J. Cell Biol. 110:105-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Haarer, B. K., and J. R. Pringle. 1987. Immunofluorescence localization of the Saccharomyces cerevisiae CDC12 gene product to the vicinity of the 10 nm filaments in the mother-bud neck. Mol. Cell. Biol. 7:3678-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509-514. [DOI] [PubMed] [Google Scholar]
- 204.Hall, D. D., D. D. Markwardt, F. Parviz, and W. Heideman. 1998. Regulation of the Cln3-Cdc28 kinase by cAMP in Saccharomyces cerevisiae. EMBO J. 17:4370-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hall, P. A., and S. E. Russell. 2004. The pathobiology of the septin gene family. J. Pathol. 204:489-505. [DOI] [PubMed] [Google Scholar]
- 206.Hallett, M. A., H. S. Lo, and A. Bender. 2002. Probing the importance and potential roles of the binding of the PH-domain protein Boi1 to acidic phospholipids. BMC Cell Biol. 3:16-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Halme, A., M. Michelitch, E. L. Mitchell, and J. Chant. 1996. Bud10p directs axial cell polarization in budding yeast and resembles a transmembrane receptor. Curr. Biol. 6:570-579. [DOI] [PubMed] [Google Scholar]
- 208.Hancock, J. F., and A. Hall. 1993. A novel role for RhoGDI as an inhibitor of GAP proteins. EMBO J. 12:1915-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Haney, S. A., and J. R. Broach. 1994. Cdc25p, the guanine nucleotide exchange factor for the ras proteins of Saccharomyces cerevisiae, promotes exchange by stabilizing Ras in a nucleotide-free state. J. Biol. Chem. 269:16541-16548. [PubMed] [Google Scholar]
- 210.Harkins, H. A., N. Pagé, L. R. Schenkman, C. De Virgilio, S. Shaw, H. Bussey, and J. R. Pringle. 2001. Bud8p and Bud9p, proteins that may mark the sites for bipolar budding in yeast. Mol. Biol. Cell 12:2497-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Harris, E. S., and H. N. Higgs. 2004. Actin cytoskeleton: formins lead the way. Curr. Biol. 14:R520-R522. [DOI] [PubMed] [Google Scholar]
- 212.Harris, S. D., and M. Momany. 2004. Polarity in filamentous fungi: moving beyond the yeast paradigm. Fungal Genet. Biol. 41:391-400. [DOI] [PubMed] [Google Scholar]
- 213.Harrison, J. C., E. S. Bardes, Y. Ohya, and D. J. Lew. 2001. A role for the Pkc1p/Mpk1p kinase cascade in the morphogenesis checkpoint. Nat. Cell Biol. 3:417-420. [DOI] [PubMed] [Google Scholar]
- 214.Hart, M. J., Y. Maru, D. Leonard, O. N. Witte, T. Evans, and R. A. Cerione. 1992. A GDP dissociation inhibitor that serves as a GTPase inhibitor for the Ras-like protein CDC42Hs. Science 258:812-815. [DOI] [PubMed] [Google Scholar]
- 215.Hartwell, L. H. 1971. Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp. Cell Res. 69:265-276. [DOI] [PubMed] [Google Scholar]
- 216.Hartwell, L. H., J. Culotti, J. R. Pringle, and B. J. Reid. 1974. Genetic control of the cell division cycle in yeast. Science 183:46-51. [DOI] [PubMed] [Google Scholar]
- 217.Hartwell, L. H., R. K. Mortimer, J. Culotti, and M. Culotti. 1973. Genetic control of the cell division cycle in yeast. V. Genetic analysis of cdc mutants. Genetics 74:267-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Heinisch, J. J., A. Lorberg, H. P. Schmitz, and J. J. Jacoby. 1999. The protein kinase C-mediated MAP kinase pathway involved in the maintenance of cellular integrity in Saccharomyces cerevisiae. Mol. Microbiol. 32:671-680. [DOI] [PubMed] [Google Scholar]
- 219.Helliwell, S. B., A. Schmidt, Y. Ohya, and M. N. Hall. 1998. The Rho1 effector Pkc1, but not Bni1, mediates signalling from Tor2 to the actin cytoskeleton. Curr. Biol. 8:1211-1214. [DOI] [PubMed] [Google Scholar]
- 220.Herskowitz, I. 1988. Life cycle of the budding yeast Saccharomyces cerevisiae. Microbiol. Rev. 52:536-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Herskowitz, I., H.-O. Park, S. Sanders, N. Valtz, and M. Peter. 1995. Programming of cell polarity in budding yeast by endogenous and exogenous signals. Cold Spring Harbor Symp. Quant. Biol. 60:717-727. [DOI] [PubMed] [Google Scholar]
- 222.Hicks, J. B., J. N. Strathern, and I. Herskowitz. 1977. Interconversion of yeast mating types. III. Action of the homothallism (HO) gene in cells homozygous for the mating type locus. Genetics 85:395-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Higgs, H. N. 2005. Formin proteins: a domain-based approach. Trends Biochem. Sci. 30:342-353. [DOI] [PubMed] [Google Scholar]
- 224.Hill, K. L., N. L. Catlett, and L. S. Weisman. 1996. Actin and myosin function in directed vacuole movement during cell division in Saccharomyces cerevisiae. J. Cell Biol. 135:1535-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ho, J., and A. Bretscher. 2001. Ras regulates the polarity of the yeast actin cytoskeleton through the stress response pathway. Mol. Biol. Cell 12:1541-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hoch, H. C., and R. J. Howard. 1980. Ultrastructure of freeze-substituted hyphae of the basidiomycete Laetisaria arvalis. Protoplasma 103:281-297. [Google Scholar]
- 227.Hoepfner, D., M. van den Berg, P. Philippsen, H. F. Tabak, and E. H. Hettema. 2001. A role for Vps1p, actin, and the Myo2p motor in peroxisome abundance and inheritance in Saccharomyces cerevisiae. J. Cell Biol. 155:979-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hoffman, G. R., and R. A. Cerione. 2002. Signaling to the Rho GTPases: networking with the DH domain. FEBS Lett. 513:85-91. [DOI] [PubMed] [Google Scholar]
- 229.Hoffman, G. R., N. Nassar, and R. A. Cerione. 2000. Structure of the Rho family GTP-binding protein Cdc42 in complex with the multifunctional regulator RhoGDI. Cell 100:345-356. [DOI] [PubMed] [Google Scholar]
- 230.Hofken, T., and E. Schiebel. 2002. A role for cell polarity proteins in mitotic exit. EMBO J. 21:4851-4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hofken, T., and E. Schiebel. 2004. Novel regulation of mitotic exit by the Cdc42 effectors Gic1 and Gic2. J. Cell Biol. 164:219-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hori, Y., A. Kikuchi, M. Isomura, M. Katayama, Y. Miura, H. Fujioka, K. Kaibuchi, and Y. Takai. 1991. Post-translational modifications of the C-terminal region of the rho protein are important for its interaction with membranes and the stimulatory and inhibitory GDP/GTP exchange proteins. Oncogene 6:515-522. [PubMed] [Google Scholar]
- 233.Howard, M., and K. Kruse. 2005. Cellular organization by self-organization: mechanisms and models for Min protein dynamics. J. Cell Biol. 168:533-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hsu, S.-C., C. D. Hazuka, R. Roth, D. L. Foletti, J. Heuser, and R. H. Scheller. 1998. Subunit composition, protein interactions, and structures of the mammalian brain sec6/8 complex and septin filaments. Neuron 20:1111-1122. [DOI] [PubMed] [Google Scholar]
- 235.Hu, F., Y. Wang, D. Liu, Y. Li, J. Qin, and S. J. Elledge. 2001. Regulation of the Bub2/Bfa1 GAP complex by Cdc5 and cell cycle checkpoints. Cell 107:655-665. [DOI] [PubMed] [Google Scholar]
- 236.Huckaba, T. M., A. C. Gay, L. F. Pantalena, H. C. Yang, and L. A. Pon. 2004. Live cell imaging of the assembly, disassembly, and actin cable-dependent movement of endosomes and actin patches in the budding yeast, Saccharomyces cerevisiae. J. Cell Biol. 167:519-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Huh, W. K., J. V. Falvo, L. C. Gerke, A. S. Carroll, R. W. Howson, J. S. Weissman, and E. K. O'Shea. 2003. Global analysis of protein localization in budding yeast. Nature 425:686-691. [DOI] [PubMed] [Google Scholar]
- 238.Icking, A., K. Schilling, A. Wiesenthal, N. Opitz, and W. Müller-Esterl. 2006. FCH/Cdc15 domain determines distinct subcellular localization of NOSTRIN. FEBS Lett. 580:223-228. [DOI] [PubMed] [Google Scholar]
- 239.Iglesias, P. A., and A. Levchenko. 2002. Modeling the cell's guidance system. Sci. STKE 2002:RE12. [DOI] [PubMed] [Google Scholar]
- 240.Ihara, M., A. Kinoshita, S. Yamada, H. Tanaka, A. Tanigaki, A. Kitano, M. Goto, K. Okubo, H. Nishiyama, O. Ogawa, C. Takahashi, S. Itohara, Y. Nishimune, M. Noda, and M. Kinoshita. 2005. Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev. Cell 8:343-352. [DOI] [PubMed] [Google Scholar]
- 241.Imai, J., A. Toh-e, and Y. Matsui. 1996. Genetic analysis of the Saccharomyces cerevisiae RHO3 gene, encoding a rho-type small GTPase, provides evidence for a role in bud formation. Genetics 142:359-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Imamura, H., K. Tanaka, T. Hihara, M. Umikawa, T. Kamei, K. Takahashi, T. Sasaki, and Y. Takai. 1997. Bni1p and Bnr1p: downstream targets of the Rho family small G-proteins which interact with profilin and regulate actin cytoskeleton in Saccharomyces cerevisiae. EMBO J. 16:2745-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Inoue, S., B. G. Turgeon, O. C. Yoder, and J. R. Aist. 1998. Role of fungal dynein in hyphal growth, microtubule organization, spindle pole body motility and nuclear migration. J. Cell Sci. 111:1555-1566. [DOI] [PubMed] [Google Scholar]
- 244.Inoue, S. B., N. Takewaki, T. Takasuka, T. Mio, M. Adachi, Y. Fujii, C. Miyamoto, M. Arisawa, Y. Furuichi, and T. Watanabe. 1995. Characterization and gene cloning of 1,3-beta-D-glucan synthase from Saccharomyces cerevisiae. Eur. J. Biochem. 231:845-854. [DOI] [PubMed] [Google Scholar]
- 245.Irazoqui, J. E., A. S. Gladfelter, and D. J. Lew. 2003. Scaffold-mediated symmetry breaking by Cdc42p. Nat. Cell Biol. 5:1062-1070. [DOI] [PubMed] [Google Scholar]
- 246.Irazoqui, J. E., A. S. Howell, C. L. Theesfeld, and D. J. Lew. 2005. Opposing roles for actin in Cdc42p polarization. Mol. Biol. Cell 16:1296-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Irazoqui, J. E., and D. J. Lew. 2004. Polarity establishment in yeast. J. Cell Sci. 117:2169-2171. [DOI] [PubMed] [Google Scholar]
- 248.Ishikawa, K., N. L. Catlett, J. L. Novak, F. Tang, J. J. Nau, and L. S. Weisman. 2003. Identification of an organelle-specific myosin V receptor. J. Cell Biol. 160:887-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ito, T., Y. Matsui, T. Ago, K. Ota, and H. Sumimoto. 2001. Novel modular domain PB1 recognizes PC motif to mediate functional protein-protein interactions. EMBO J. 20:3938-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Itoh, T., S. Koshiba, T. Kigawa, A. Kikuchi, S. Yokoyama, and T. Takenawa. 2001. Role of the ENTH domain in phosphatidylinositol-4,5-bisphosphate binding and endocytosis. Science 291:1047-1051. [DOI] [PubMed] [Google Scholar]
- 251.Itoh, T., and T. Takenawa. 2004. Regulation of endocytosis by phosphatidylinositol 4,5-bisphosphate and ENTH proteins. Curr. Top. Microbiol. Immunol. 282:31-47. [DOI] [PubMed] [Google Scholar]
- 252.Itoh, T., A. Watabe, E. A. Toh, and Y. Matsui. 2002. Complex formation with Ypt11p, a rab-type small GTPase, is essential to facilitate the function of Myo2p, a class V myosin, in mitochondrial distribution in Saccharomyces cerevisiae. Mol. Cell. Biol. 22:7744-7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Iwase, M., J. Luo, S. Nagaraj, M. Longtine, H. B. Kim, B. K. Haarer, C. Caruso, Z. Tong, J. R. Pringle, and E. Bi. 2006. Role of a Cdc42p effector pathway in recruitment of the yeast septins to the presumptive bud site. Mol. Biol. Cell 17:1110-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Jansen, R. P., C. Dowzer, C. Michaelis, M. Galova, and K. Nasmyth. 1996. Mother cell-specific HO expression in budding yeast depends on the unconventional myosin Myo4p and other cytoplasmic proteins. Cell 84:687-697. [DOI] [PubMed] [Google Scholar]
- 255.Jaquenoud, M., and M. Peter. 2000. Gic2p may link activated Cdc42p to components involved in actin polarization, including Bni1p and Bud6p (Aip3p). Mol. Cell. Biol. 20:6244-6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Jaspersen, S. L., J. F. Charles, and D. O. Morgan. 1999. Inhibitory phosphorylation of the APC regulator Hct1 is controlled by the kinase Cdc28 and the phosphatase Cdc14. Curr. Biol. 9:227-236. [DOI] [PubMed] [Google Scholar]
- 257.Jensen, S., M. Geymonat, A. L. Johnson, M. Segal, and L. H. Johnston. 2002. Spatial regulation of the guanine nucleotide exchange factor Lte1 in Saccharomyces cerevisiae. J. Cell Sci. 115:4977-4991. [DOI] [PubMed] [Google Scholar]
- 258.Joberty, G., R. R. Perlungher, P. J. Sheffield, M. Kinoshita, M. Noda, T. Haystead, and I. G. Macara. 2001. Borg proteins control septin organization and are negatively regulated by Cdc42. Nat. Cell Biol. 3:861-866. [DOI] [PubMed] [Google Scholar]
- 259.Johnson, D. I. 1999. Cdc42: an essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol. Mol. Biol. Rev. 63:54-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Johnson, D. I., and J. R. Pringle. 1990. Molecular characterization of CDC42, a Saccharomyces cerevisiae gene involved in the development of cell polarity. J. Cell Biol. 111:143-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Johnston, G. C., J. A. Prendergast, and R. A. Singer. 1991. The Saccharomyces cerevisiae MYO2 gene encodes an essential myosin for vectorial transport of vesicles. J. Cell Biol. 113:539-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Jones, S., M. L. Vignais, and J. R. Broach. 1991. The CDC25 protein of Saccharomyces cerevisiae promotes exchange of guanine nucleotides bound to Ras. Mol. Cell. Biol. 11:2641-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Jonsdottir, G. A., and R. Li. 2004. Dynamics of yeast myosin I: evidence for a possible role in scission of endocytic vesicles. Curr. Biol. 14:1604-1609. [DOI] [PubMed] [Google Scholar]
- 264.Jorgensen, P., I. Rupes, J. R. Sharom, L. Schneper, J. R. Broach, and M. Tyers. 2004. A dynamic transcriptional network communicates growth potential to ribosome synthesis and critical cell size. Genes Dev. 18:2491-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kadota, J., T. Yamamoto, S. Yoshiuchi, E. Bi, and K. Tanaka. 2004. Septin ring assembly requires concerted action of polarisome components, a PAK kinase Cla4p, and the actin cytoskeleton in Saccharomyces cerevisiae. Mol. Biol. Cell 15:5329-5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kagami, M., A. Toh-e, and Y. Matsui. 1998. Sro7p, a Saccharomyces cerevisiae counterpart of the tumor suppressor l(2)gl protein, is related to myosins in function. Genetics 149:1717-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kaksonen, M., Y. Sun, and D. G. Drubin. 2003. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell 115:475-487. [DOI] [PubMed] [Google Scholar]
- 268.Kaksonen, M., C. P. Toret, and D. G. Drubin. 2005. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell 123:305-320. [DOI] [PubMed] [Google Scholar]
- 269.Kamada, Y., U. S. Jung, J. Piotrowski, and D. E. Levin. 1995. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 9:1559-1571. [DOI] [PubMed] [Google Scholar]
- 270.Kamei, T., K. Tanaka, T. Hihara, M. Umikawa, H. Imamura, M. Kikyo, K. Ozaki, and Y. Takai. 1998. Interaction of Bnr1p with a novel Src homology 3 domain-containing Hof1p. Implication in cytokinesis in Saccharomyces cerevisiae. J. Biol. Chem. 273:28341-28345. [DOI] [PubMed] [Google Scholar]
- 271.Kang, P. J., E. Angerman, K. Nakashima, J. R. Pringle, and H.-O. Park. 2004. Interactions among Rax1p, Rax2p, Bud8p, and Bud9p in marking cortical sites for bipolar bud-site selection in yeast. Mol. Biol. Cell 15:5145-5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Kang, P. J., B. Lee, and H.-O. Park. 2004. Specific residues of the GDP/GTP exchange factor Bud5p are involved in establishment of the cell type-specific budding pattern in yeast. J. Biol. Chem. 279:27980-27985. [DOI] [PubMed] [Google Scholar]
- 273.Kang, P. J., A. Sanson, B. Lee, and H.-O. Park. 2001. A GDP/GTP exchange factor involved in linking a spatial landmark to cell polarity. Science 292:1376-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Karpova, T. S., J. G. McNally, S. L. Moltz, and J. A. Cooper. 1998. Assembly and function of the actin cytoskeleton of yeast: relationships between cables and patches. J. Cell Biol. 142:1501-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Karpova, T. S., S. L. Reck-Peterson, N. B. Elkind, M. S. Mooseker, P. J. Novick, and J. A. Cooper. 2000. Role of actin and Myo2p in polarized secretion and growth of Saccharomyces cerevisiae. Mol. Biol. Cell 11:1727-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kartmann, B., and D. Roth. 2001. Novel roles for mammalian septins: from vesicle trafficking to oncogenesis. J. Cell Sci. 114:839-844. [DOI] [PubMed] [Google Scholar]
- 277.Kataoka, T., S. Powers, C. McGill, O. Fasano, J. Strathern, J. Broach, and M. Wigler. 1984. Genetic analysis of yeast RAS1 and RAS2 genes. Cell 37:437-445. [DOI] [PubMed] [Google Scholar]
- 278.Kawasaki, R., K. Fujimura-Kamada, H. Toi, H. Kato, and K. Tanaka. 2003. The upstream regulator, Rsr1p, and downstream effector, Gic1p and Gic2p, of the Cdc42p small GTPase coordinately regulate initiation of budding in Saccharomyces cerevisiae. Genes Cells 8:235-250. [DOI] [PubMed] [Google Scholar]
- 279.Kim, H. B., B. K. Haarer, and J. R. Pringle. 1991. Cellular morphogenesis in the Saccharomyces cerevisiae cell cycle: localization of the CDC3 gene product and the timing of events at the budding site. J. Cell Biol. 112:535-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Kim, J. H., and S. Powers. 1991. Overexpression of RPI1, a novel inhibitor of the yeast Ras-cyclic AMP pathway, down-regulates normal but not mutationally activated ras function. Mol. Cell. Biol. 11:3894-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kim, K., A. Yamashita, M. A. Wear, Y. Maeda, and J. A. Cooper. 2004. Capping protein binding to actin in yeast: biochemical mechanism and physiological relevance. J. Cell Biol. 164:567-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Kim, Y.-J., L. Francisco, G.-C. Chen, E. Marcotte, and C. S. M. Chan. 1994. Control of cellular morphogenesis by the Ipl2/Bem2 GTPase-activating protein: possible role of protein phosphorylation. J. Cell Biol. 127:1381-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Kinoshita, M. 2006. Diversity of septin scaffolds. Curr. Opin. Cell Biol. 18:54-60. [DOI] [PubMed] [Google Scholar]
- 284.Kinoshita, M., C. M. Field, M. L. Coughlin, A. F. Straight, and T. M. Mitchison. 2002. Self- and actin-templated assembly of mammalian septins. Dev. Cell 3:791-802. [DOI] [PubMed] [Google Scholar]
- 285.Kinoshita, M., S. Kumar, A. Mizoguchi, C. Ide, A. Kinoshita, T. Haraguchi, Y. Hiraoka, and M. Noda. 1997. Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev. 11:1535-1547. [DOI] [PubMed] [Google Scholar]
- 286.Kissel, H., M. M. Georgescu, S. Larisch, K. Manova, G. R. Hunnicutt, and H. Steller. 2005. The Sept4 septin locus is required for sperm terminal differentiation in mice. Dev. Cell 8:353-364. [DOI] [PubMed] [Google Scholar]
- 287.Klis, F. M., P. Mol, K. Hellingwerf, and S. Brul. 2002. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 26:239-256. [DOI] [PubMed] [Google Scholar]
- 288.Koch, G., K. Tanaka, T. Masuda, W. Yamochi, H. Nonaka, and Y. Takai. 1997. Association of the Rho family small GTP-binding proteins with Rho GDP dissociation inhibitor (Rho GDI) in Saccharomyces cerevisiae. Oncogene 15:417-422. [DOI] [PubMed] [Google Scholar]
- 289.Kohno, H., K. Tanaka, A. Mino, M. Umikawa, H. Imamura, T. Fujiwara, Y. Fujita, K. Hotta, H. Qadota, T. Watanabe, Y. Ohya, and Y. Takai. 1996. Bni1p implicated in cytoskeletal control is a putative target of Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15:6060-6068. [PMC free article] [PubMed] [Google Scholar]
- 290.Komarnitsky, S. I., Y. C. Chiang, F. C. Luca, J. Chen, J. H. Toyn, M. Winey, L. H. Johnston, and C. L. Denis. 1998. DBF2 protein kinase binds to and acts through the cell cycle-regulated MOB1 protein. Mol. Cell. Biol. 18:2100-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Kovar, D. R., J. R. Kuhn, A. L. Tichy, and T. D. Pollard. 2003. The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin. J. Cell Biol. 161:875-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Kozminski, K. G., L. Beven, E. Angerman, A. H. Y. Tong, C. Boone, and H.-O. Park. 2003. Interaction between a Ras and a Rho GTPase couples selection of a growth site to the development of cell polarity in yeast. Mol. Biol. Cell 14:4958-4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Kozminski, K. G., A. J. Chen, A. A. Rodal, and D. G. Drubin. 2000. Functions and functional domains of the GTPase Cdc42p. Mol. Biol. Cell 11:339-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kozubowski, L., J. R. Larson, and K. Tatchell. 2005. Role of the septin ring in the asymmetric localization of proteins at the mother-bud neck in Saccharomyces cerevisiae. Mol. Biol. Cell 16:3455-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Kremer, B. E., T. Haystead, and I. G. Macara. 2005. Mammalian septins regulate microtubule stability through interaction with the microtubule-binding protein MAP4. Mol. Biol. Cell 16:4648-4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Kron, S. J., C. A. Styles, and G. R. Fink. 1994. Symmetric cell division in pseudohyphae of the yeast Saccharomyces cerevisiae. Mol. Biol. Cell 5:1003-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Kroschewski, R., A. Hall, and I. Mellman. 1999. Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat. Cell Biol. 1:8-13. [DOI] [PubMed] [Google Scholar]
- 298.Kubler, E., H. U. Mosch, S. Rupp, and M. P. Lisanti. 1997. Gpa2p, a G-protein alpha-subunit, regulates growth and pseudohyphal development in Saccharomyces cerevisiae via a cAMP-dependent mechanism. J. Biol. Chem. 272:20321-20323. [DOI] [PubMed] [Google Scholar]
- 299.Kubler, E., and H. Riezman. 1993. Actin and fimbrin are required for the internalization step of endocytosis in yeast. EMBO J. 12:2855-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Larisch, S., Y. Yi, R. Lotan, H. Kerner, S. Eimerl, W. T. Parks, Y. Gottfried, S. B. Reffey, M. P. de Caestecker, D. Danielpour, N. Book-Melamed, R. Timberg, C. S. Duckett, R. J. Lechleider, H. Steller, J. Orly, S. J. Kim, and A. B. Roberts. 2000. A novel mitochondrial septin-like protein, ARTS, mediates apoptosis dependent on its P-loop motif. Nat. Cell Biol. 2:915-921. [DOI] [PubMed] [Google Scholar]
- 301.Larsson, K., F. Bohl, I. Sjostrom, N. Akhtar, D. Strand, B. M. Mechler, R. Grabowski, and L. Adler. 1998. The Saccharomyces cerevisiae SOP1 and SOP2 genes, which act in cation homeostasis, can be functionally substituted by the Drosophila lethal(2)giant larvae tumor suppressor gene. J. Biol. Chem. 273:33610-33618. [DOI] [PubMed] [Google Scholar]
- 302.Lechler, T., G. A. Jonsdottir, S. K. Klee, D. Pellman, and R. Li. 2001. A two-tiered mechanism by which Cdc42 controls the localization and activation of an Arp2/3-activating motor complex in yeast. J. Cell Biol. 155:261-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Lechler, T., A. Shevchenko, and R. Li. 2000. Direct involvement of yeast type I myosins in Cdc42-dependent actin polymerization. J. Cell Biol. 148:363-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Lee, K. S., and D. E. Levin. 1992. Dominant mutations in a gene encoding a putative protein kinase (BCK1) bypass the requirement for a Saccharomyces cerevisiae protein kinase C homolog. Mol. Cell. Biol. 12:172-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Lee, L., S. K. Klee, M. Evangelista, C. Boone, and D. Pellman. 1999. Control of mitotic spindle position by the Saccharomyces cerevisiae formin Bni1p. J. Cell Biol. 144:947-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Lee, P. R., S. Song, H. S. Ro, C. J. Park, J. Lippincott, R. Li, J. R. Pringle, C. De Virgilio, M. S. Longtine, and K. S. Lee. 2002. Bni5p, a septin-interacting protein, is required for normal septin function and cytokinesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 22:6906-6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Leeuw, T., A. Fourest-Lieuvin, C. Wu, J. Chenevert, K. Clark, M. Whiteway, D. Y. Thomas, and E. Leberer. 1995. Pheromone response in yeast: association of Bem1p with proteins of the MAP kinase cascade and actin. Science 270:1210-1213. [DOI] [PubMed] [Google Scholar]
- 308.Leeuw, T., C. Wu, J. D. Schrag, M. Whiteway, D. Y. Thomas, and E. Leberer. 1998. Interaction of a G-protein β-subunit with a conserved sequence in Ste20/PAK family protein kinases. Nature 391:191-195. [DOI] [PubMed] [Google Scholar]
- 309.Lehman, K., G. Rossi, J. E. Adamo, and P. Brennwald. 1999. Yeast homologues of tomosyn and lethal giant larvae function in exocytosis and are associated with the plasma membrane SNARE, Sec9. J. Cell Biol. 146:125-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Lemmon, M. A., and K. M. Ferguson. 2000. Signal-dependent membrane targeting by pleckstrin homology (PH) domains. Biochem. J. 350:1-18. [PMC free article] [PubMed] [Google Scholar]
- 311.Lengeler, K. B., R. C. Davidson, C. D'Souza, T. Harashima, W. C. Shen, P. Wang, X. Pan, M. Waugh, and J. Heitman. 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64:746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Leonard, D., M. J. Hart, J. V. Platko, A. Eva, W. Henzel, T. Evans, and R. A. Cerione. 1992. The identification and characterization of a GDP-dissociation inhibitor (GDI) for the CDC42Hs protein. J. Biol. Chem. 267:22860-22868. [PubMed] [Google Scholar]
- 313.Levin, D. E. 2005. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69:262-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Levin, D. E., and E. Bartlett-Heubusch. 1992. Mutants in the S. cerevisiae PKC1 gene display a cell cycle-specific osmotic stability defect. J. Cell Biol. 116:1221-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Lew, D., and S. Reed. 1995. Cell cycle control of morphogenesis in budding yeast. Curr. Opin. Genet. Dev. 5:17-23. [DOI] [PubMed] [Google Scholar]
- 316.Lew, D. J., and S. I. Reed. 1993. Morphogenesis in the yeast cell cycle: regulation by Cdc28 and cyclins. J. Cell Biol. 120:1305-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Lew, D. J., T. Weinert, and J. R. Pringle. 1997. Cell cycle control in Saccharomyces cerevisiae, p. 607-695. In J. R. Pringle, J. R. Broach, and E. W. Jones (ed.), The molecular and cellular biology of the yeast Saccharomyces, vol. 3. Cell cycle and cell biology. Cold Spring Harbor Laboratory Press, Plainview, NY. [Google Scholar]
- 318.Lewis, M. J., B. J. Nichols, C. Prescianotto-Baschong, H. Riezman, and H. R. Pelham. 2000. Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol. Biol. Cell 11:23-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Li, R. 1997. Bee1, a yeast protein with homology to Wiscott-Aldrich syndrome protein, is critical for the assembly of cortical actin cytoskeleton. J. Cell Biol. 136:649-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Li, R., Y. Zheng, and D. G. Drubin. 1995. Regulation of cortical actin cytoskeleton assembly during polarized cell growth in budding yeast. J. Cell Biol. 128:599-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Li, Z., X. Dong, Z. Wang, W. Liu, N. Deng, Y. Ding, L. Tang, T. Hla, R. Zeng, L. Li, and D. Wu. 2005. Regulation of PTEN by Rho small GTPases. Nat. Cell Biol. 7:399-404. [DOI] [PubMed] [Google Scholar]
- 322.Lillie, S. H., and S. S. Brown. 1994. Immunofluorescence localization of the unconventional myosin, Myo2p, and the putative kinesin-related protein, Smy1p, to the same regions of polarized growth in Saccharomyces cerevisiae. J. Cell Biol. 125:825-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Lim, C. J., G. B. Spiegelman, and G. Weeks. 2002. Cytoskeletal regulation by Dictyostelium Ras subfamily proteins. J. Muscle Res. Cell Motil. 23:729-736. [DOI] [PubMed] [Google Scholar]
- 324.Lim, H. H., F. M. Yeong, and U. Surana. 2003. Inactivation of mitotic kinase triggers translocation of MEN components to mother-daughter neck in yeast. Mol. Biol. Cell 14:4734-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Lippincott, J., and R. Li. 1998. Dual function of Cyk2, a cdc15/PSTPIP family protein, in regulating actomyosin ring dynamics and septin distribution. J. Cell Biol. 143:1947-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Lippincott, J., and R. Li. 1998. Sequential assembly of myosin II, an IQGAP-like protein, and filamentous actin to a ring structure involved in budding yeast cytokinesis. J. Cell Biol. 140:355-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Lippincott, J., K. B. Shannon, W. Shou, R. J. Deshaies, and R. Li. 2001. The Tem1 small GTPase controls actomyosin and septin dynamics during cytokinesis. J. Cell Sci. 114:1379-1386. [DOI] [PubMed] [Google Scholar]
- 328.Liu, H., and A. Bretscher. 1992. Characterization of TPM1 disrupted yeast cells indicates an involvement of tropomyosin in directed vesicular transport. J. Cell Biol. 118:285-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Liu, H., C. A. Styles, and G. R. Fink. 1993. Elements of the yeast pheromone response pathway required for filamentous growth of diploids. Science 262:1741-1744. [DOI] [PubMed] [Google Scholar]
- 330.Liu, H. P., and A. Bretscher. 1989. Disruption of the single tropomyosin gene in yeast results in the disappearance of actin cables from the cytoskeleton. Cell 57:233-242. [DOI] [PubMed] [Google Scholar]
- 331.Liu, H. P., and A. Bretscher. 1989. Purification of tropomyosin from Saccharomyces cerevisiae and identification of related proteins in Schizosaccharomyces and Physarum. Proc. Natl. Acad. Sci. USA 86:90-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Lo, W. S., and A. M. Dranginis. 1996. FLO11, a yeast gene related to the STA genes, encodes a novel cell surface flocculin. J. Bacteriol. 178:7144-7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Lo, W. S., and A. M. Dranginis. 1998. The cell surface flocculin Flo11 is required for pseudohyphae formation and invasion by Saccharomyces cerevisiae. Mol. Biol. Cell 9:161-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Lombardi, R., and H. Riezman. 2001. Rvs161p and Rvs167p, the two yeast amphiphysin homologs, function together in vivo. J. Biol. Chem. 276:6016-6022. [DOI] [PubMed] [Google Scholar]
- 335.Longtine, M., and E. Bi. 2003. Regulation of septin organization and function in yeast. Trends Cell Biol. 13:403-409. [DOI] [PubMed] [Google Scholar]
- 336.Longtine, M. S., D. J. DeMarini, M. L. Valencik, O. S. Al-Awar, H. Fares, C. De Virgilio, and J. R. Pringle. 1996. The septins: roles in cytokinesis and other processes. Curr. Opin. Cell Biol. 8:106-119. [DOI] [PubMed] [Google Scholar]
- 337.Longtine, M. S., H. Fares, and J. R. Pringle. 1998. Role of the yeast Gin4p protein kinase in septin assembly and the relationship between septin assembly and septin function. J. Cell Biol. 143:719-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Longtine, M. S., C. L. Theesfeld, J. N. McMillan, E. Weaver, J. R. Pringle, and D. J. Lew. 2000. Septin-dependent assembly of a cell cycle-regulatory module in Saccharomyces cerevisiae. Mol. Cell. Biol. 20:4049-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Lorberg, A., and M. N. Hall. 2004. TOR: the first 10 years. Curr. Top. Microbiol. Immunol. 279:1-18. [DOI] [PubMed] [Google Scholar]
- 340.Lord, M., F. Inose, T. Hiroko, T. Hata, A. Fujita, and J. Chant. 2002. Subcellular localization of Axl1, the cell type-specific regulator of polarity. Curr. Biol. 12:1347-1352. [DOI] [PubMed] [Google Scholar]
- 341.Lord, M., M. C. Yang, M. Mischke, and J. Chant. 2000. Cell cycle programs of gene expression control morphogenetic protein localization. J. Cell Biol. 151:1501-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Lorenz, M. C., and J. Heitman. 1997. Yeast pseudohyphal growth is regulated by GPA2, a G protein alpha homolog. EMBO J. 16:7008-7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Luca, F. C., M. Mody, C. Kurischko, D. M. Roof, T. H. Giddings, and M. Winey. 2001. Saccharomyces cerevisiae Mob1p is required for cytokinesis and mitotic exit. Mol. Cell. Biol. 21:6972-6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Luedeke, C., S. B. Frei, I. Sbalzarini, H. Schwarz, A. Spang, and Y. Barral. 2005. Septin-dependent compartmentalization of the endoplasmic reticulum during yeast polarized growth. J. Cell Biol. 169:897-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Lyons, D. M., S. K. Mahanty, K.-Y. Choi, M. Manandhar, and E. A. Elion. 1996. The SH3 domain protein Bem1 coordinates mitogen-activated protein kinase cascade activation with cell cycle control in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:4095-4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Ma, P., S. Wera, P. Van Dijck, and J. M. Thevelein. 1999. The PDE1-encoded low-affinity phosphodiesterase in the yeast Saccharomyces cerevisiae has a specific function in controlling agonist-induced cAMP signaling. Mol. Biol. Cell 10:91-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Macara, I. G., R. Baldarelli, C. M. Field, M. Glotzer, Y. Hayashi, S.-C. Hsu, M. B. Kennedy, M. Kinoshita, M. Longtine, C. Low, L. J. Maltais, L. McKenzie, T. J. Mitchison, T. Nishikawa, M. Noda, E. M. Petty, M. Peifer, J. R. Pringle, P. J. Robinson, D. Roth, S. E. H. Russell, H. Stuhlmann, M. Tanaka, T. Tanaka, W. S. Trimble, J. Ware, N. J. Zeleznik-Le, and B. Zieger. 2002. Mammalian septins nomenclature. Mol. Biol. Cell 13:4111-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Mack, D., K. Nishimura, B. K. Dennehey, T. Arbogast, J. Parkinson, A. Toh-e, J. R. Pringle, A. Bender, and Y. Matsui. 1996. Identification of the bud emergence gene BEM4 and its interactions with rho-type GTPases in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:4387-4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Mackin, N. A., T. J. Sousou, and S. E. Erdman. 2004. The PXL1 gene of Saccharomyces cerevisiae encodes a paxillin-like protein functioning in polarized cell growth. Mol. Biol. Cell 15:1904-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Madania, A., P. Dumoulin, S. Grava, H. Kitamoto, C. Schärer-Brodbeck, A. Soulard, V. Moreau, and B. Winsor. 1999. The Saccharomyces cerevisiae homologue of human Wiskott-Aldrich syndrome protein Las17p interacts with the Arp2/3 complex. Mol. Biol. Cell 10:3521-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Madaule, P., R. Axel, and A. M. Myers. 1987. Characterization of two members of the rho gene family from the yeast Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 84:779-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Madhani, H. D., C. A. Styles, and G. R. Fink. 1997. MAP kinases with distinct inhibitory functions impart signaling specificity during yeast differentiation. Cell 91:673-684. [DOI] [PubMed] [Google Scholar]
- 353.Mah, A. S., J. Jang, and R. J. Deshaies. 2001. Protein kinase Cdc15 activates the Dbf2-Mob1 kinase complex. Proc. Natl. Acad. Sci. USA 98:7325-7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Manning, B. D., J. G. Barrett, J. A. Wallace, H. Granok, and M. Snyder. 1999. Differential regulation of the Kar3p kinesin-related protein by two associated proteins, Cik1p and Vik1p. J. Cell Biol. 144:1219-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Manning, B. D., R. Padmanabha, and M. Snyder. 1997. The Rho-GEF Rom2p localizes to sites of polarized cell growth and participates in cytoskeletal functions in Saccharomyces cerevisiae. Mol. Biol. Cell 8:1829-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Marcoux, N., S. Cloutier, E. Zakrzewska, P. M. Charest, Y. Bourbonnais, and D. Pallotta. 2000. Suppression of the profilin-deficient phenotype by the RHO2 signaling pathway in Saccharomyces cerevisiae. Genetics 156:579-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Marquitz, A. R., J. C. Harrison, I. Bose, T. R. Zyla, J. N. McMillan, and D. J. Lew. 2002. The Rho-GAP Bem2p plays a GAP-independent role in the morphogenesis checkpoint. EMBO J. 21:4012-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Marston, A. L., T. Chen, M. C. Yang, P. Belhumeur, and J. Chant. 2001. A localized GTPase exchange factor, Bud5, determines the orientation of division axes in yeast. Curr. Biol. 11:803-807. [DOI] [PubMed] [Google Scholar]
- 359.Martemyanov, K. A., P. V. Lishko, N. Calero, G. Keresztes, M. Sokolov, K. J. Strissel, I. B. Leskov, J. A. Hopp, A. V. Kolesnikov, C.-K. Chen, J. Lem, S. Heller, M. E. Burns, and V. Y. Arshavsky. 2003. The DEP domain determines subcellular targeting of the GTPase activating protein RGS9 in vivo. J. Neurosci. 23:10175-10181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Martin, D. E., and M. N. Hall. 2005. The expanding TOR signaling network. Curr. Opin. Cell Biol. 17:158-166. [DOI] [PubMed] [Google Scholar]
- 361.Martin, H., A. Mendoza, J. M. Rodriguez-Pachon, M. Molina, and C. Nombela. 1997. Characterization of SKM1, a Saccharomyces cerevisiae gene encoding a novel Ste20/PAK-like protein kinase. Mol. Microbiol. 23:431-444. [DOI] [PubMed] [Google Scholar]
- 362.Martin, H., J. M. Rodriguez-Pachon, C. Ruiz, C. Nombela, and M. Molina. 2000. Regulatory mechanisms for modulation of signaling through the cell integrity Slt2-mediated pathway in Saccharomyces cerevisiae. J. Biol. Chem. 275:1511-1519. [DOI] [PubMed] [Google Scholar]
- 363.Martin, S. G., and F. Chang. 2005. New end take off: regulating cell polarity during the fission yeast cell cycle. Cell Cycle 4:1046-1049. [PubMed] [Google Scholar]
- 364.Martin-Cuadrado, A. B., J. L. Morrell, M. Konomi, H. An, C. Petit, M. Osumi, M. Balasubramanian, K. L. Gould, F. Del Rey, and C. R. de Aldana. 2005. Role of septins and the exocyst complex in the function of hydrolytic enzymes responsible for fission yeast cell separation. Mol. Biol. Cell 16:4867-4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Masuda, T., K. Tanaka, H. Nonaka, W. Yamochi, A. Maeda, and Y. Takai. 1994. Molecular cloning and characterization of yeast rho GDP dissociation inhibitor. J. Biol. Chem. 269:19713-19718. [PubMed] [Google Scholar]
- 366.Mata, J., and P. Nurse. 1997. tea1 and the microtubular cytoskeleton are important for generating global spatial order within the fission yeast cell. Cell 89:939-949. [DOI] [PubMed] [Google Scholar]
- 367.Matheos, D., M. Metodiev, E. Muller, D. Stone, and M. D. Rose. 2004. Pheromone-induced polarization is dependent on the Fus3p MAPK acting through the formin Bni1p. J. Cell Biol. 165:99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Matsui, Y., R. Matsui, R. Akada, and A. Toh-e. 1996. Yeast src homology region 3 domain-binding proteins involved in bud formation. J. Cell Biol. 133:865-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Matsui, Y., and A. Toh-e. 1992. Isolation and characterization of two novel ras superfamily genes in Saccharomyces cerevisiae. Gene 114:43-49. [DOI] [PubMed] [Google Scholar]
- 370.Matsui, Y., and A. Toh-e. 1992. Yeast RHO3 and RHO4 ras superfamily genes are necessary for bud growth, and their defect is suppressed by a high dose of bud formation genes CDC42 and BEM1. Mol. Cell. Biol. 12:5690-5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Mazur, P., and W. Baginsky. 1996. In vitro activity of 1,3-beta-D-glucan synthase requires the GTP-binding protein Rho1. J. Biol. Chem. 271:14604-14609. [DOI] [PubMed] [Google Scholar]
- 372.Mazur, P., N. Morin, W. Baginsky, M. el-Sherbeini, J. A. Clemas, J. B. Nielsen, and F. Foor. 1995. Differential expression and function of two homologous subunits of yeast 1,3-beta-D-glucan synthase. Mol. Cell. Biol. 15:5671-5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Mazzoni, C., P. Zarzov, A. Rambourg, and C. Mann. 1993. The SLT2 (MPK1) MAP kinase homolog is involved in polarized cell growth in Saccharomyces cerevisiae. J. Cell Biol. 123:1821-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.McCaffrey, M., J. S. Johnson, B. Goud, A. M. Myers, J. Rossier, M. R. Popoff, P. Madaule, and P. Boquet. 1991. The small GTP-binding protein Rho1p is localized on the Golgi apparatus and post-Golgi vesicles in Saccharomyces cerevisiae. J. Cell Biol. 115:309-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.McCallum, S. J., W. J. Wu, and R. A. Cerione. 1996. Identification of a putative effector for Cdc42Hs with high sequence similarity to the RasGAP-related protein IQGAP1 and a Cdc42Hs binding partner with similarity to IQGAP2. J. Biol. Chem. 271:21732-21737. [DOI] [PubMed] [Google Scholar]
- 376.McCollum, D., and K. L. Gould. 2001. Timing is everything: regulation of mitotic exit and cytokinesis by the MEN and SIN. Trends Cell Biol. 11:89-95. [DOI] [PubMed] [Google Scholar]
- 377.Meili, R., and R. A. Firtel. 2003. Two poles and a compass. Cell 114:153-156. [DOI] [PubMed] [Google Scholar]
- 378.Meili, R., A. T. Sasaki, and R. A. Firtel. 2005. Rho rocks PTEN. Nat. Cell Biol. 7:334-335. [DOI] [PubMed] [Google Scholar]
- 379.Meinhardt, H. 1999. Orientation of chemotactic cells and growth cones: models and mechanisms. J. Cell Sci. 112:2867-2874. [DOI] [PubMed] [Google Scholar]
- 380.Meluh, P. B., and M. D. Rose. 1990. KAR3, a kinesin-related gene required for yeast nuclear fusion. Cell 60:1029-1041. [DOI] [PubMed] [Google Scholar]
- 381.Mendoza, M., A. A. Hyman, and M. Glotzer. 2002. GTP binding induces filament assembly of a recombinant septin. Curr. Biol. 12:1858-1863. [DOI] [PubMed] [Google Scholar]
- 382.Merrifield, C. J., D. Perrais, and D. Zenisek. 2005. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell 121:593-606. [DOI] [PubMed] [Google Scholar]
- 383.Michelitch, M., and J. Chant. 1996. A mechanism of Bud1p GTPase action suggested by mutational analysis and immunolocalization. Curr. Biol. 6:446-454. [DOI] [PubMed] [Google Scholar]
- 384.Miliaras, N. B., and B. Wendland. 2004. EH proteins: multivalent regulators of endocytosis (and other pathways). Cell Biochem. Biophys. 41:295-318. [DOI] [PubMed] [Google Scholar]
- 385.Misteli, T. 2001. The concept of self-organization in cellular architecture. J. Cell Biol. 155:181-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Mitchison, T. J. 1992. Self-organization of polymer-motor systems in the cytoskeleton. Philos. Trans. R. Soc. Lond. B Biol. Sci. 336:99-106. [DOI] [PubMed] [Google Scholar]
- 387.Mitchison, T. J., and C. M. Field. 2002. Cytoskeleton: what does GTP do for septins? Curr. Biol. 12:R788-R790. [DOI] [PubMed] [Google Scholar]
- 388.Miyamoto, S., Y. Ohya, Y. Sano, S. Sakaguchi, H. Iida, and Y. Anraku. 1991. A DBL-homologous region of the yeast CLS4/CDC24 gene product is important for Ca2+-modulated bud assembly. Biochem. Biophys. Res. Commun. 181:604-610. [DOI] [PubMed] [Google Scholar]
- 389.Mochida, J., T. Yamamoto, K. Fujimura-Kamada, and K. Tanaka. 2002. The novel adaptor protein, Mti1p, and Vrp1p, a homolog of Wiskott-Aldrich syndrome protein-interacting protein (WIP), may antagonistically regulate type I myosins in Saccharomyces cerevisiae. Genetics 160:923-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Moffat, J., and B. Andrews. 2004. Late-G1 cyclin-CDK activity is essential for control of cell morphogenesis in budding yeast. Nat. Cell Biol. 6:59-66. [DOI] [PubMed] [Google Scholar]
- 391.Momany, M., J. Zhao, R. Lindsey, and P. J. Westfall. 2001. Characterization of the Aspergillus nidulans septin (asp) gene family. Genetics 157:969-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Mortensen, E. M., H. McDonald, J. Yates III, and D. R. Kellogg. 2002. Cell cycle-dependent assembly of a Gin4-septin complex. Mol. Biol. Cell 13:2091-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Mösch, H.-U., and G. R. Fink. 1997. Dissection of filamentous growth by transposon mutagenesis in Saccharomyces cerevisiae. Genetics 145:671-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Mösch, H.-U., T. Köhler, and G. H. Braus. 2001. Different domains of the essential GTPase Cdc42p required for growth and development of Saccharomyces cerevisiae. Mol. Cell. Biol. 21:235-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Mösch, H.-U., E. Kubler, S. Krappmann, G. R. Fink, and G. H. Braus. 1999. Crosstalk between the Ras2p-controlled mitogen-activated protein kinase and cAMP pathways during invasive growth of Saccharomyces cerevisiae. Mol. Biol. Cell 10:1325-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Mösch, H.-U., R. L. Roberts, and G. R. Fink. 1996. Ras2 signals via the Cdc42/Ste20/mitogen-activated protein kinase module to induce filamentous growth in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 93:5352-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Moseley, J. B., and B. L. Goode. 2005. Differential activities and regulation of Saccharomyces cerevisiae formin proteins Bni1 and Bnr1 by Bud6. J. Biol. Chem. 280:28023-28033. [DOI] [PubMed] [Google Scholar]
- 398.Moseley, J. B., and B. L. Goode. 2006. The yeast actin cytoskeleton: from cellular function to biochemical mechanism. Microbiol. Mol. Biol. Rev. 70:605-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Moseley, J. B., I. Sagot, A. L. Manning, Y. Xu, M. J. Eck, D. Pellman, and B. L. Goode. 2004. A conserved mechanism for Bni1- and mDia1-induced actin assembly and dual regulation of Bni1 by Bud6 and profilin. Mol. Biol. Cell 15:896-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Moskow, J. J., A. S. Gladfelter, R. E. Lamson, P. M. Pryciak, and D. J. Lew. 2000. Role of Cdc42p in pheromone-stimulated signal transduction in Saccharomyces cerevisiae. Mol. Cell. Biol. 20:7559-7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Mulholland, J., D. Preuss, A. Moon, A. Wong, D. Drubin, and D. Botstein. 1994. Ultrastructure of the yeast actin cytoskeleton and its association with the plasma membrane. J. Cell Biol. 125:381-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Muller, O., D. I. Johnson, and A. Mayer. 2001. Cdc42p functions at the docking stage of yeast vacuole membrane fusion. EMBO J. 20:5657-5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Munn, A. L. 2001. Molecular requirements for the internalisation step of endocytosis: insights from yeast. Biochim. Biophys. Acta 1535:236-257. [DOI] [PubMed] [Google Scholar]
- 404.Naqvi, S. N., R. Zahn, D. A. Mitchell, B. J. Stevenson, and A. L. Munn. 1998. The WASp homologue Las17p functions with the WIP homologue End5p/verprolin and is essential for endocytosis in yeast. Curr. Biol. 8:959-962. [DOI] [PubMed] [Google Scholar]
- 405.Nedelec, F. J., T. Surrey, A. C. Maggs, and S. Leibler. 1997. Self-organization of microtubules and motors. Nature 389:305-308. [DOI] [PubMed] [Google Scholar]
- 406.Nelson, B., A. B. Parsons, M. Evangelista, K. Schaefer, K. Kennedy, S. Ritchie, T. L. Petryshen, and C. Boone. 2004. Fus1p interacts with components of the Hog1p mitogen-activated protein kinase and Cdc42p morphogenesis signaling pathways to control cell fusion during yeast mating. Genetics 166:67-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Nelson, W. J. 2003. Adaptation of core mechanisms to generate cell polarity. Nature 422:766-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Nern, A., and R. A. Arkowitz. 1999. A Cdc24p-Far1p-Gβγ protein complex required for yeast orientation during mating. J. Cell Biol. 144:1187-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Nern, A., and R. A. Arkowitz. 1998. A GTP-exchange factor required for cell orientation. Nature 391:195-198. [DOI] [PubMed] [Google Scholar]
- 410.Nern, A., and R. A. Arkowitz. 2000. G proteins mediate changes in cell shape by stabilizing the axis of polarity. Mol. Cell 5:853-864. [DOI] [PubMed] [Google Scholar]
- 411.Nern, A., and R. A. Arkowitz. 2000. Nucleocytoplasmic shuttling of the Cdc42p exchange factor Cdc24p. J. Cell Biol. 148:1115-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Neufeld, T. P., and G. M. Rubin. 1994. The Drosophila peanut gene is required for cytokinesis and encodes a protein similar to yeast putative bud neck filament proteins. Cell 77:371-379. [DOI] [PubMed] [Google Scholar]
- 413.Newpher, T. M., R. P. Smith, V. Lemmon, and S. K. Lemmon. 2005. In vivo dynamics of clathrin and its adaptor-dependent recruitment to the actin-based endocytic machinery in yeast. Dev. Cell 9:87-98. [DOI] [PubMed] [Google Scholar]
- 414.Nguyen, T. Q., H. Sawa, H. Okano, and J. G. White. 2000. The C. elegans septin genes, unc-59 and unc-61, are required for normal postembryonic cytokineses and morphogenesis but have no essential function in embryogenesis. J. Cell Sci. 113:3825-3837. [DOI] [PubMed] [Google Scholar]
- 415.Ni, L., and M. Snyder. 2001. A genomic study of the bipolar bud site selection pattern in Saccharomyces cerevisiae. Mol. Biol. Cell 12:2147-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Nomanbhoy, T. K., and R. Cerione. 1996. Characterization of the interaction between RhoGDI and Cdc42Hs using fluorescence spectroscopy. J. Biol. Chem. 271:10004-10009. [DOI] [PubMed] [Google Scholar]
- 417.Norden, C., M. Mendoza, J. Dobbelaere, C. V. Kotwaliwale, S. Biggins, and Y. Barral. 2006. The NoCut pathway links completion of cytokinesis to spindle midzone function to prevent chromosome breakage. Cell 125:85-98. [DOI] [PubMed] [Google Scholar]
- 418.Novick, P., and D. Botstein. 1985. Phenotypic analysis of temperature-sensitive yeast actin mutants. Cell 40:405-416. [DOI] [PubMed] [Google Scholar]
- 419.Novick, P., and W. Guo. 2002. Ras family therapy: Rab, Rho and Ral talk to the exocyst. Trends Cell Biol. 12:247-249. [DOI] [PubMed] [Google Scholar]
- 420.Novick, P., and R. Schekman. 1979. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 76:1858-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Ohya, Y., S. Miyamoto, Y. Ohsumi, and Y. Anraku. 1986. Calcium-sensitive cls4 mutant of Saccharomyces cerevisiae with a defect in bud formation. J. Bacteriol. 165:28-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Ohya, Y., Y. Ohsumi, and Y. Anraku. 1986. Isolation and characterization of Ca2+-sensitive mutants of Saccharomyces cerevisiae. J. Gen. Microbiol. 132:979-988. [DOI] [PubMed] [Google Scholar]
- 423.Olson, M. F. 2003. GTPase signalling: new functions for diaphanous-related formins. Curr. Biol. 13:R360-R362. [DOI] [PubMed] [Google Scholar]
- 424.Ozaki, K., K. Tanaka, H. Imamura, T. Hihara, T. Kameyama, H. Nonaka, H. Hirano, Y. Matsuura, and Y. Takai. 1996. Rom1p and Rom2p are GDP/GTP exchange proteins (GEPs) for the Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15:2196-2207. [PMC free article] [PubMed] [Google Scholar]
- 425.Ozaki-Kuroda, K., Y. Yamamoto, H. Nohara, M. Kinoshita, T. Fujiwara, K. Irie, and Y. Takai. 2001. Dynamic localization and function of Bni1p at the sites of directed growth in Saccharomyces cerevisiae. Mol. Cell. Biol. 21:827-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Ozbudak, E. M., A. Becskei, and A. van Oudenaarden. 2005. A system of counteracting feedback loops regulates Cdc42p activity during spontaneous cell polarization. Dev. Cell 9:565-571. [DOI] [PubMed] [Google Scholar]
- 427.Page, B. D., L. L. Satterwhite, M. D. Rose, and M. Snyder. 1994. Localization of the Kar3 kinesin heavy chain-related protein requires the Cik1 interacting protein. J. Cell Biol. 124:507-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Pan, X., T. Harashima, and J. Heitman. 2000. Signal transduction cascades regulating pseudohyphal differentiation of Saccharomyces cerevisiae. Curr. Opin. Microbiol. 3:567-572. [DOI] [PubMed] [Google Scholar]
- 429.Pan, X., and J. Heitman. 1999. Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:4874-4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Pan, X., and J. Heitman. 2002. Protein kinase A operates a molecular switch that governs yeast pseudohyphal differentiation. Mol. Cell. Biol. 22:3981-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Papadaki, P., V. Pizon, B. Onken, and E. C. Chang. 2002. Two Ras pathways in fission yeast are differentially regulated by two Ras guanine nucleotide exchange factors. Mol. Cell. Biol. 22:4598-4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Paravicini, G., M. Cooper, L. Friedli, D. J. Smith, J. L. Carpentier, L. S. Klig, and M. A. Payton. 1992. The osmotic integrity of the yeast cell requires a functional PKC1 gene product. Mol. Cell. Biol. 12:4896-4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Parent, C. A., and P. N. Devreotes. 1999. A cell's sense of direction. Science 284:765-770. [DOI] [PubMed] [Google Scholar]
- 434.Park, H.-O., E. Bi, J. R. Pringle, and I. Herskowitz. 1997. Two active states of the Ras-related Bud1/Rsr1 protein bind to different effectors to determine yeast cell polarity. Proc. Natl. Acad. Sci. USA 94:4463-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Park, H.-O., J. Chant, and I. Herskowitz. 1993. BUD2 encodes a GTPase-activating protein for Bud1/Rsr1 necessary for proper bud-site selection in yeast. Nature 365:269-274. [DOI] [PubMed] [Google Scholar]
- 436.Park, H.-O., P. J. Kang, and A. W. Rachfal. 2002. Localization of the Rsr1/Bud1 GTPase involved in selection of a proper growth site in yeast. J. Biol. Chem. 277:26721-26724. [DOI] [PubMed] [Google Scholar]
- 437.Park, H.-O., A. Sanson, and I. Herskowitz. 1999. Localization of Bud2p, a GTPase-activating protein necessary for programming cell polarity in yeast to the presumptive bud site. Genes Dev. 13:1912-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Park, H. O., and J. Chant. 1996. Bud2 protein, p. 200-203. In M. Zerial and L. Huber (ed.), The guidebook to the small GTPases. Oxford University Press, New York, NY.
- 439.Pereira, G., T. Hofken, J. Grindlay, C. Manson, and E. Schiebel. 2000. The Bub2p spindle checkpoint links nuclear migration with mitotic exit. Mol. Cell 6:1-10. [PubMed] [Google Scholar]
- 440.Pereira, G., and E. Schiebel. 2005. Kin4 kinase delays mitotic exit in response to spindle alignment defects. Mol. Cell 19:209-221. [DOI] [PubMed] [Google Scholar]
- 441.Pereira, G., T. U. Tanaka, K. Nasmyth, and E. Schiebel. 2001. Modes of spindle pole body inheritance and segregation of the Bfa1p-Bub2p checkpoint protein complex. EMBO J. 20:6359-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Peter, B. J., H. M. Kent, I. G. Mills, Y. Vallis, P. J. Butler, P. R. Evans, and H. T. McMahon. 2004. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303:495-499. [DOI] [PubMed] [Google Scholar]
- 443.Peter, M., A. Gartner, J. Horecka, G. Ammerer, and I. Herskowitz. 1993. FAR1 links the signal transduction pathway to the cell cycle machinery in yeast. Cell 73:747-760. [DOI] [PubMed] [Google Scholar]
- 444.Peter, M., and I. Herskowitz. 1994. Direct inhibition of the yeast cyclin-dependent kinase Cdc28-Cln by Far1. Science 265:1228-1231. [DOI] [PubMed] [Google Scholar]
- 445.Peter, M., A. Neiman, H.-O. Park, M. van Lohuizen, and I. Herskowitz. 1996. Interaction between the small GTP-binding protein CDC42 and the protein kinase STE20 is necessary for signal transduction in the yeast pheromone pathway. EMBO J. 15:7046-7059. [PMC free article] [PubMed] [Google Scholar]
- 446.Peterson, J., Y. Zheng, L. Bender, A. Myers, R. Cerione, and A. Bender. 1994. Interactions between the bud emergence proteins Bem1p and Bem2p and Rho-type GTPases in yeast. J. Cell Biol. 127:1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Pfeffer, S. 2005. A model for Rab GTPase localization. Biochem. Soc. Trans. 33:627-630. [DOI] [PubMed] [Google Scholar]
- 448.Pfeffer, S., and D. Aivazian. 2004. Targeting Rab GTPases to distinct membrane compartments. Nat. Rev. Mol. Cell Biol. 5:886-896. [DOI] [PubMed] [Google Scholar]
- 449.Philip, B., and D. E. Levin. 2001. Wsc1 and Mid2 are cell surface sensors for cell wall integrity signaling that act through Rom2, a guanine nucleotide exchange factor for Rho1. Mol. Cell. Biol. 21:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Philippsen, P., A. Kaufmann, and H. P. Schmitz. 2005. Homologues of yeast polarity genes control the development of multinucleated hyphae in Ashbya gossypii. Curr. Opin. Microbiol. 8:370-377. [DOI] [PubMed] [Google Scholar]
- 451.Pollard, T. D., and G. G. Borisy. 2003. Cellular motility driven by assembly and disassembly of actin filaments. Cell 113:453-465. [DOI] [PubMed] [Google Scholar]
- 452.Powers, J., and C. Barlowe. 2002. Erv14p directs a transmembrane secretory protein into COPII-coated transport vesicles. Mol. Biol. Cell 142:1209-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Powers, S., E. Gonzales, T. Christensen, J. Cubert, and D. Broek. 1991. Functional cloning of BUD5, a CDC25-related gene from S. cerevisiae that can suppress a dominant-negative RAS2 mutant. Cell 65:1225-1231. [DOI] [PubMed] [Google Scholar]
- 454.Pringle, J. R., E. Bi, H. A. Harkins, J. E. Zahner, C. De Virgilio, J. Chant, K. Corrado, and H. Fares. 1995. Establishment of cell polarity in yeast. Cold Spring Harbor Symp. Quant. Biol. 60:729-744. [DOI] [PubMed] [Google Scholar]
- 455.Protopopov, V., B. Govindan, P. Novick, and J. E. Gerst. 1993. Homologs of the synaptobrevin/VAMP family of synaptic vesicle proteins function on the late secretory pathway in S. cerevisiae. Cell 74:855-861. [DOI] [PubMed] [Google Scholar]
- 456.Pruyne, D., and A. Bretscher. 2000. Polarization of cell growth in yeast. I. Establishment and maintenance of polarity states. J. Cell Sci. 113:365-375. [DOI] [PubMed] [Google Scholar]
- 457.Pruyne, D., and A. Bretscher. 2000. Polarization of cell growth in yeast. II. The role of the cortical actin cytoskeleton. J. Cell Sci. 113:571-585. [DOI] [PubMed] [Google Scholar]
- 458.Pruyne, D., M. Evangelista, C. Yang, E. Bi, S. Zigmond, A. Bretscher, and C. Boone. 2002. Role of formins in actin assembly: nucleation and barbed-end association. Science 297:612-615. [DOI] [PubMed] [Google Scholar]
- 459.Pruyne, D., L. Gao, E. Bi, and A. Bretscher. 2004. Stable and dynamic axes of polarity use distinct formin isoforms in budding yeast. Mol. Biol. Cell 15:4971-4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Pruyne, D., A. Legesse-Miller, L. Gao, Y. Dong, and A. Bretscher. 2004. Mechanisms of polarized growth and organelle segregation in yeast. Annu. Rev. Cell Dev. Biol. 20:559-591. [DOI] [PubMed] [Google Scholar]
- 461.Pruyne, D. W., D. H. Schott, and A. Bretscher. 1998. Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J. Cell Biol. 143:1931-1945. [DOI] [PubMed] [Google Scholar]
- 462.Qadota, H., C. P. Python, S. B. Inoue, M. Arisawa, Y. Anraku, Y. Zheng, T. Watanabe, D. E. Levin, and Y. Ohya. 1996. Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-beta-glucan synthase. Science 272:279-281. [DOI] [PubMed] [Google Scholar]
- 463.Rajavel, M., B. Philip, B. M. Buehrer, B. Errede, and D. E. Levin. 1999. Mid2 is a putative sensor for cell integrity signaling in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:3969-3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Reynaga-Pena, C. G., G. Gierz, and S. Bartnicki-Garcia. 1997. Analysis of the role of the Spitzenkorper in fungal morphogenesis by computer simulation of apical branching in Aspergillus niger. Proc. Natl. Acad. Sci. USA 94:9096-9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Richman, T. J., and D. I. Johnson. 2000. Saccharomyces cerevisiae Cdc42p GTPase is involved in preventing the recurrence of bud emergence during the cell cycle. Mol. Cell. Biol. 20:8548-8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Richman, T. J., M. M. Sawyer, and D. I. Johnson. 2002. Saccharomyces cerevisiae Cdc42p localizes to cellular membranes and clusters at sites of polarized growth. Eukaryot. Cell 1:458-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Richman, T. J., M. M. Sawyer, and D. I. Johnson. 1999. The Cdc42p GTPase is involved in a G2/M morphogenetic checkpoint regulating the apical-isotropic switch and nuclear division in yeast. J. Biol. Chem. 274:16861-16870. [DOI] [PubMed] [Google Scholar]
- 468.Richman, T. J., K. A. Toenjes, S. E. Morales, K. C. Cole, B. T. Wasserman, C. M. Taylor, J. A. Koster, M. F. Whelihan, and D. I. Johnson. 2004. Analysis of cell-cycle specific localization of the Rdi1p RhoGDI and the structural determinants required for Cdc42p membrane localization and clustering at sites of polarized growth. Curr. Genet. 45:339-349. [DOI] [PubMed] [Google Scholar]
- 469.Rickert, P., O. D. Weiner, F. Wang, H. R. Bourne, and G. Servant. 2000. Leukocytes navigate by compass: roles of PI3Kgamma and its lipid products. Trends Cell Biol. 10:466-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Rida, C. G., and U. Surana. 2005. Cdc42-dependent localization of polarisome component Spa2 to the incipient bud site is independent of the GDP/GTP exchange factor Cdc24. Eur. J. Cell Biol. 84:939-949. [DOI] [PubMed] [Google Scholar]
- 471.Roberson, R. W. 1992. The actin cytoskeleton in hyphal cells of Sclerotium rolfsii. Mycologia 84:41-51. [Google Scholar]
- 472.Roberts, C. J., B. Nelson, M. J. Marton, R. Stoughton, M. R. Meyer, H. A. Bennett, Y. D. He, H. Dai, W. L. Walker, T. R. Hughes, M. Tyers, C. Boone, and S. H. Friend. 2000. Signaling and circuitry of multiple MAPK pathways revealed by a matrix of global gene expression profiles. Science 287:873-880. [DOI] [PubMed] [Google Scholar]
- 473.Roberts, R. L., B. Bowers, M. L. Slater, and E. Cabib. 1983. Chitin synthesis and localization in cell division cycle mutants of Saccharomyces cerevisiae. Mol. Cell. Biol. 3:922-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Robinson, N. G., L. Guo, J. Imai, E. A. Toh, Y. Matsui, and F. Tamanoi. 1999. Rho3 of Saccharomyces cerevisiae, which regulates the actin cytoskeleton and exocytosis, is a GTPase which interacts with Myo2 and Exo70. Mol. Cell. Biol. 19:3580-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Rodal, A. A., L. Kozubowski, B. L. Goode, D. G. Drubin, and J. H. Hartwig. 2005. Actin and septin ultrastructures at the budding yeast cell cortex. Mol. Biol. Cell 16:372-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Roemer, T., K. Madden, J. Chang, and M. Snyder. 1996. Selection of axial growth sites in yeast requires Axl2p, a novel plasma membrane glycoprotein. Genes Dev. 10:777-793. [DOI] [PubMed] [Google Scholar]
- 477.Roh, D. H., B. Bowers, M. Schmidt, and E. Cabib. 2002. The septation apparatus, an autonomous system in budding yeast. Mol. Biol. Cell 13:2747-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Rossanese, O. W., and B. S. Glick. 2001. Deconstructing Golgi inheritance. Traffic 2:589-596. [DOI] [PubMed] [Google Scholar]
- 479.Rossanese, O. W., C. A. Reinke, B. J. Bevis, A. T. Hammond, I. B. Sears, J. O'Connor, and B. S. Glick. 2001. A role for actin, Cdc1p, and Myo2p in the inheritance of late Golgi elements in Saccharomyces cerevisiae. J. Cell Biol. 153:47-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Rothman, J. E. 1996. The protein machinery of vesicle budding and fusion. Protein Sci. 5:185-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Roumanie, O., C. Weinachter, I. Larrieu, M. Crouzet, and F. Doignon. 2001. Functional characterization of the Bag7, Lrg1 and Rgd2 RhoGAP proteins from Saccharomyces cerevisiae. FEBS Lett. 506:149-156. [DOI] [PubMed] [Google Scholar]
- 482.Roumanie, O., H. Wu, J. N. Molk, G. Rossi, K. Bloom, and P. Brennwald. 2005. Rho GTPase regulation of exocytosis in yeast is independent of GTP hydrolysis and polarization of the exocyst complex. J. Cell Biol. 170:583-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Rua, D., B. T. Tobe, and S. J. Kron. 2001. Cell cycle control of yeast filamentous growth. Curr. Opin. Microbiol. 4:720-727. [DOI] [PubMed] [Google Scholar]
- 484.Ruggieri, R., A. Bender, Y. Matsui, S. Powers, Y. Takai, J. R. Pringle, and K. Matsumoto. 1992. RSR1, a ras-like gene homologous to Krev-1 (smg21A/rap1A): role in the development of cell polarity and interactions with the Ras pathway in Saccharomyces cerevisiae. Mol. Cell. Biol. 12:758-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Russell, S. E., and P. A. Hall. 2005. Do septins have a role in cancer? Br. J. Cancer 93:499-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Sagot, I., S. K. Klee, and D. Pellman. 2002. Yeast formins regulate cell polarity by controlling the assembly of actin cables. Nat. Cell Biol. 4:42-50. [DOI] [PubMed] [Google Scholar]
- 487.Sagot, I., A. A. Rodal, J. Moseley, B. L. Goode, and D. Pellman. 2002. An actin nucleation mechanism mediated by Bni1 and profilin. Nat. Cell Biol. 4:626-631. [DOI] [PubMed] [Google Scholar]
- 488.Saka, A., M. Abe, H. Okano, M. Minemura, H. Qadota, T. Utsugi, A. Mino, K. Tanaka, Y. Takai, and Y. Ohya. 2001. Complementing yeast rho1 mutation groups with distinct functional defects. J. Biol. Chem. 276:46165-46171. [DOI] [PubMed] [Google Scholar]
- 489.Sambeth, R., and A. Baumgaertner. 2001. Autocatalytic polymerization generates persistent random walk of crawling cells. Phys. Rev. Lett. 86:5196-5199. [DOI] [PubMed] [Google Scholar]
- 490.Sanders, S. L., M. Gentzsch, W. Tanner, and I. Herskowitz. 1999. O-glycosylation of Axl2/Bud10p by Pmt4p is required for its stability, localization, and function in daughter cells. J. Cell Biol. 145:1177-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Sanders, S. L., and I. Herskowitz. 1996. The Bud4 protein of yeast, required for axial budding, is localized to the mother/bud neck in a cell cycle-dependent manner. J. Cell Biol. 134:413-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Santos, B., A. Duran, and M. H. Valdivieso. 1997. CHS5, a gene involved in chitin synthesis and mating in Saccharomyces cerevisiae. Mol. Cell. Biol. 17:2485-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Santos, B., and M. Snyder. 2003. Specific protein targeting during cell differentiation: polarized localization of Fus1p during mating depends on Chs5p in Saccharomyces cerevisiae. Eukaryot. Cell 2:821-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Santos, B., and M. Snyder. 1997. Targeting of chitin synthase 3 to polarized growth sites in yeast requires Chs5p and Myo2p. J. Cell Biol. 136:95-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Schenkman, L. R., C. Caruso, N. Pagé, and J. R. Pringle. 2002. The role of cell cycle-regulated expression in the localization of spatial landmark proteins in yeast. J. Cell Biol. 156:829-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Schmelzle, T., S. B. Helliwell, and M. N. Hall. 2002. Yeast protein kinases and the RHO1 exchange factor TUS1 are novel components of the cell integrity pathway in yeast. Mol. Cell. Biol. 22:1329-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Schmidt, A., M. Bickle, T. Beck, and M. N. Hall. 1997. The yeast phosphatidylinositol kinase homolog TOR2 activates RHO1 and RHO2 via the exchange factor ROM2. Cell 88:531-542. [DOI] [PubMed] [Google Scholar]
- 498.Schmidt, A., J. Kunz, and M. N. Hall. 1996. TOR2 is required for organization of the actin cytoskeleton in yeast. Proc. Natl. Acad. Sci. USA 93:13780-13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Schmidt, A., T. Schmelzle, and M. N. Hall. 2002. The RHO1-GAPs SAC7, BEM2 and BAG7 control distinct RHO1 functions in Saccharomyces cerevisiae. Mol. Microbiol. 45:1433-1441. [DOI] [PubMed] [Google Scholar]
- 500.Schmidt, M., B. Bowers, A. Varma, D.-H. Roh, and E. Cabib. 2002. In budding yeast, contraction of the actomyosin ring and formation of the primary septum at cytokinesis depend on each other. J. Cell Sci. 115:293-302. [DOI] [PubMed] [Google Scholar]
- 501.Schmitz, H. P., S. Huppert, A. Lorberg, and J. J. Heinisch. 2002. Rho5p downregulates the yeast cell integrity pathway. J. Cell Sci. 115:3139-3148. [DOI] [PubMed] [Google Scholar]
- 502.Schneper, L., K. Duvel, and J. R. Broach. 2004. Sense and sensibility: nutritional response and signal integration in yeast. Curr. Opin. Microbiol. 7:624-630. [DOI] [PubMed] [Google Scholar]
- 503.Schott, D., J. Ho, D. Pruyne, and A. Bretscher. 1999. The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J. Cell Biol. 147:791-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Schott, D., T. Huffaker, and A. Bretscher. 2002. Microfilaments and microtubules: the news from yeast. Curr. Opin. Microbiol. 5:564-574. [DOI] [PubMed] [Google Scholar]
- 505.Schott, D. H., R. N. Collins, and A. Bretscher. 2002. Secretory vesicle transport velocity in living cells depends on the myosin-V lever arm length. J. Cell Biol. 156:35-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Schultz, J., F. Milpetz, P. Bork, and C. P. Ponting. 1998. SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. USA 95:5857-5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Schwartz, M. A., and H. D. Madhani. 2004. Principles of MAP kinase signaling specificity in Saccharomyces cerevisiae. Annu. Rev. Genet. 38:725-748. [DOI] [PubMed] [Google Scholar]
- 508.Scott, B. L., J. S. Van Komen, H. Irshad, S. Liu, K. A. Wilson, and J. A. McNew. 2004. Sec1p directly stimulates SNARE-mediated membrane fusion in vitro. J. Cell Biol. 167:75-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Scott, M., P. L. Hyland, G. McGregor, K. J. Hillan, S. E. Russell, and P. A. Hall. 2005. Multimodality expression profiling shows SEPT9 to be overexpressed in a wide range of human tumours. Oncogene 24:4688-4700. [DOI] [PubMed] [Google Scholar]
- 510.Segall, J. E. 1993. Polarization of yeast cells in spatial gradients of alpha mating factor. Proc. Natl. Acad. Sci. USA 90:8332-8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Seiler, S., F. E. Nargang, G. Steinberg, and M. Schliwa. 1997. Kinesin is essential for cell morphogenesis and polarized secretion in Neurospora crassa. EMBO J. 16:3025-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Seiler, S., and M. Plamann. 2003. The genetic basis of cellular morphogenesis in the filamentous fungus Neurospora crassa. Mol. Biol. Cell 14:4352-4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Seiler, S., M. Plamann, and M. Schliwa. 1999. Kinesin and dynein mutants provide novel insights into the roles of vesicle traffic during cell morphogenesis in Neurospora. Curr. Biol. 9:779-785. [DOI] [PubMed] [Google Scholar]
- 514.Sekiya-Kawasaki, M., M. Abe, A. Saka, D. Watanabe, K. Kono, M. Minemura-Asakawa, S. Ishihara, T. Watanabe, and Y. Ohya. 2002. Dissection of upstream regulatory components of the Rho1p effector, 1,3-beta-glucan synthase, in Saccharomyces cerevisiae. Genetics 162:663-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Sells, M. A., and J. Chernoff. 1997. Emerging from the Pak: the p21-activated protein kinase family. Trends Cell Biol. 7:162-167. [DOI] [PubMed] [Google Scholar]
- 516.Seshan, A., and A. Amon. 2005. Ras and the Rho effector Cla4 collaborate to target and anchor Lte1 at the bud cortex. Cell Cycle 4:940-946. [DOI] [PubMed] [Google Scholar]
- 517.Seshan, A., A. J. Bardin, and A. Amon. 2002. Control of Ite1 localization by cell polarity determinants and Cdc14. Curr. Biol. 12:2098-2110. [DOI] [PubMed] [Google Scholar]
- 518.Shan, S., R. M. Stroud, and P. Walter. 2004. Mechanism of association and reciprocal activation of two GTPases. PLoS Biol. 2:e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Sheffield, P. J., C. J. Oliver, B. E. Kremer, S. Sheng, Z. Shao, and I. G. Macara. 2003. Borg/Septin interactions and the assembly of mammalian septin heterodimers, trimers, and filaments. J. Biol. Chem. 278:3483-3488. [DOI] [PubMed] [Google Scholar]
- 520.Sheu, Y. J., Y. Barral, and M. Snyder. 2000. Polarized growth controls cell shape and bipolar bud site selection in Saccharomyces cerevisiae. Mol. Cell. Biol. 20:5235-5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Sheu, Y. J., B. Santos, N. Fortin, C. Costigan, and M. Snyder. 1998. Spa2p interacts with cell polarity proteins and signaling components involved in yeast cell morphogenesis. Mol. Cell. Biol. 18:4053-4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Shimada, Y., M.-P. Gulli, and M. Peter. 2000. Nuclear sequestration of the exchange factor Cdc24p by Far1 regulates cell polarity during mating. Nat. Cell Biol. 2:117-124. [DOI] [PubMed] [Google Scholar]
- 523.Shimada, Y., P. Wiget, M. P. Gulli, E. Bi, and M. Peter. 2004. The nucleotide exchange factor Cdc24p may be regulated by auto-inhibition. EMBO J. 23:1051-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Shirayama, M., Y. Matsui, K. Tanaka, and A. Toh-e. 1994. Isolation of a CDC25 family gene, MSI2/LTE1, as a multicopy suppressor of ira1. Yeast 10:451-461. [DOI] [PubMed] [Google Scholar]
- 525.Shirayama, M., Y. Matsui, and A. Toh-e. 1996. Dominant mutant alleles of yeast protein kinase gene CDC15 suppress the lte1 defect in termination of M phase and genetically interact with CDC14. Mol. Gen. Genet. 251:176-185. [DOI] [PubMed] [Google Scholar]
- 526.Shirayama, M., Y. Matsui, and A. Toh-e. 1994. The yeast TEM1 gene, which encodes a GTP-binding protein, is involved in termination of M phase. Mol. Cell. Biol. 14:7476-7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Shou, W., J. H. Seol, A. Shevchenko, C. Baskerville, D. Moazed, Z. W. Chen, J. Jang, H. Charbonneau, and R. J. Deshaies. 1999. Exit from mitosis is triggered by Tem1-dependent release of the protein phosphatase Cdc14 from nucleolar RENT complex. Cell 97:233-244. [DOI] [PubMed] [Google Scholar]
- 528.Shulewitz, M. J., C. J. Inouye, and J. Thorner. 1999. Hsl7 localizes to a septin ring and serves as an adaptor in a regulatory pathway that relieves tyrosine phosphorylation of Cdc28 protein kinase in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:7123-7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Sietsma, J. H., H. A. B. Wosten, and J. G. H. Wessels. 1995. Cell wall growth and protein secretion in fungi. Can. J. Bot. 73(Suppl. 1):S388-S395. [Google Scholar]
- 530.Sil, A., and I. Herskowitz. 1996. Identification of an asymmetrically localized determinant, Ash1, required for lineage-specific transcription of the yeast HO gene. Cell 84:711-722. [DOI] [PubMed] [Google Scholar]
- 531.Simanis, V. 2003. Events at the end of mitosis in the budding and fission yeasts. J. Cell Sci. 116:4263-4275. [DOI] [PubMed] [Google Scholar]
- 532.Simon, M. N., C. De Virgilio, B. Souza, J. R. Pringle, A. Abo, and S. I. Reed. 1995. Role for the Rho-family GTPase Cdc42 in yeast mating-pheromone signal pathway. Nature 376:702-705. [DOI] [PubMed] [Google Scholar]
- 533.Sivadon, P., F. Bauer, M. Aigle, and M. Crouzet. 1995. Actin cytoskeleton and budding pattern are altered in the yeast rvs161 mutant: the Rvs161 protein shares common domains with the brain protein amphiphysin. Mol. Gen. Genet. 246:485-495. [DOI] [PubMed] [Google Scholar]
- 534.Sloat, B., A. Adams, and J. R. Pringle. 1981. Roles of the CDC24 gene product in cellular morphogenesis during the Saccharomyces cerevisiae cell cycle. J. Cell Biol. 89:395-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Sloat, B. F., and J. R. Pringle. 1978. A mutant of yeast defective in cellular morphogenesis. Science 200:1171-1173. [DOI] [PubMed] [Google Scholar]
- 536.Smith, G. R., S. A. Givan, P. Cullen, and G. F. Sprague, Jr. 2002. GTPase-activating proteins for Cdc42. Eukaryot. Cell 1:469-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Smith, M. G., S. R. Swamy, and L. A. Pon. 2001. The life cycle of actin patches in mating yeast. J. Cell Sci. 114:1505-1513. [DOI] [PubMed] [Google Scholar]
- 538.Snyder, M. 1989. The SPA2 protein of yeast localizes to sites of cell growth. J. Cell Biol. 108:1419-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Sobering, A. K., U. S. Jung, K. S. Lee, and D. E. Levin. 2002. Yeast Rpi1 is a putative transcriptional regulator that contributes to preparation for stationary phase. Eukaryot. Cell 1:56-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Soulard, A., S. Friant, C. Fitterer, C. Orange, G. Kaneva, G. Mirey, and B. Winsor. 2005. The WASP/Las17p-interacting protein Bzz1p functions with Myo5p in an early stage of endocytosis. Protoplasma 226:89-101. [DOI] [PubMed] [Google Scholar]
- 541.Soulard, A., T. Lechler, V. Spiridonov, A. Shevchenko, R. Li, and B. Winsor. 2002. Saccharomyces cerevisiae Bzz1p is implicated with type I myosins in actin patch polarization and is able to recruit actin-polymerizing machinery in vitro. Mol. Cell. Biol. 22:7889-7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Spiliotis, E. T., M. Kinoshita, and W. J. Nelson. 2005. A mitotic septin scaffold required for mammalian chromosome congression and segregation. Science 307:1781-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Spiliotis, E. T., and W. J. Nelson. 2006. Here come the septins: novel polymers that coordinate intracellular functions and organization. J. Cell Sci. 119:4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Spiliotis, E. T., and W. J. Nelson. 2003. Spatial control of exocytosis. Curr. Opin. Cell Biol. 15:430-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Sprague, G. F., Jr., and J. W. Thorner. 1992. Pheromone response and signal transduction during the mating process of Saccharomyces cerevisiae, p. 657-744. In E. W. Jones, J. R. Pringle, and J. R. Broach (ed.), The molecular and cellular biology of the yeast Saccharomyces, vol. 2. Gene expression. Cold Spring Harbor Laboratory Press, Plainview, NY. [Google Scholar]
- 546.Sproul, L. R., D. J. Anderson, A. T. Mackey, W. S. Saunders, and S. P. Gilbert. 2005. Cik1 targets the minus-end kinesin depolymerase Kar3 to microtubule plus ends. Curr. Biol. 15:1420-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Stamnes, M. 2002. Regulating the actin cytoskeleton during vesicular transport. Curr. Opin. Cell Biol. 14:428-433. [DOI] [PubMed] [Google Scholar]
- 548.Stefan, C. J., S. M. Padilla, A. Audhya, and S. D. Emr. 2005. The phosphoinositide phosphatase Sjl2 is recruited to cortical actin patches in the control of vesicle formation and fission during endocytosis. Mol. Cell. Biol. 25:2910-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Stegmeier, F., and A. Amon. 2004. Closing mitosis: the functions of the Cdc14 phosphatase and its regulation. Annu. Rev. Genet. 38:203-232. [DOI] [PubMed] [Google Scholar]
- 550.Stevenson, B. J., B. Ferguson, C. De Virgilio, E. Bi, J. R. Pringle, G. Ammerer, and G. F. J. Sprague. 1995. Mutation of RGA1, which encodes a putative GTPase-activating protein for the polarity-establishment protein Cdc42p, activates the pheromone-response pathway in the yeast Saccharomyces cerevisiae. Genes Dev. 9:2949-2963. [DOI] [PubMed] [Google Scholar]
- 551.Stradal, T., W. Kranewitter, S. J. Winder, and M. Gimona. 1998. CH domains revisited. FEBS Lett. 431:134-137. [DOI] [PubMed] [Google Scholar]
- 552.Surka, M. C., C. W. Tsang, and W. S. Trimble. 2002. The mammalian septin MSF localizes with microtubules and is required for the completion of cytokinesis. Mol. Biol. Cell 13:3532-3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Taheri, N., T. Köhler, G. H. Braus, and H.-U. Mösch. 2000. Asymmetrically localized Bud8p and Bud9p proteins control yeast cell polarity and development. EMBO J. 19:6686-6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Takizawa, P. A., J. L. DeRisi, J. E. Wilhelm, and R. D. Vale. 2000. Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science 290:341-344. [DOI] [PubMed] [Google Scholar]
- 555.Takizawa, P. A., A. Sil, J. R. Swedlow, I. Herskowitz, and R. D. Vale. 1997. Actin-dependent localization of an RNA encoding a cell-fate determinant in yeast. Nature 389:90-93. [DOI] [PubMed] [Google Scholar]
- 556.Tan, A. L., P. C. Rida, and U. Surana. 2005. Essential tension and constructive destruction: the spindle checkpoint and its regulatory links with mitotic exit. Biochem. J. 386:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Tanaka, K., B. K. Lin, D. R. Wood, and F. Tamanoi. 1991. IRA2, an upstream negative regulator of RAS in yeast, is a RAS GTPase-activating protein. Proc. Natl. Acad. Sci. USA 88:468-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Tanaka, K., M. Nakafuku, T. Satoh, M. S. Marshall, J. B. Gibbs, K. Matsumoto, Y. Kaziro, and A. Toh-e. 1990. S. cerevisiae genes IRA1 and IRA2 encode proteins that may be functionally equivalent to mammalian ras GTPase activating protein. Cell 60:803-807. [DOI] [PubMed] [Google Scholar]
- 559.Tanaka, K., M. Nakafuku, F. Tamanoi, Y. Kaziro, K. Matsumoto, and A. Toh-e. 1990. IRA2, a second gene of Saccharomyces cerevisiae that encodes a protein with a domain homologous to mammalian ras GTPase-activating protein. Mol. Cell. Biol. 10:4303-4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Tang, H. Y., J. Xu, and M. Cai. 2000. Pan1p, End3p, and S1a1p, three yeast proteins required for normal cortical actin cytoskeleton organization, associate with each other and play essential roles in cell wall morphogenesis. Mol. Cell. Biol. 20:12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Tasto, J. J., J. L. Morrell, and K. L. Gould. 2003. An anillin homologue, Mid2p, acts during fission yeast cytokinesis to organize the septin ring and promote cell separation. J. Cell Biol. 160:1093-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Tatchell, K., D. T. Chaleff, D. DeFeo-Jones, and E. M. Scolnick. 1984. Requirement of either of a pair of ras-related genes of Saccharomyces cerevisiae for spore viability. Nature 309:523-527. [DOI] [PubMed] [Google Scholar]
- 563.Tcheperegine, S. E., X.-D. Gao, and E. Bi. 2005. Regulation of cell polarity by interactions of Msb3 and Msb4 with Cdc42 and polarisome components. Mol. Cell. Biol. 25:8567-8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Tennyson, C. N., J. Lee, and B. Andrews. 1998. A role for the Pcl9-Pho85 cyclin-cdk complex at the M/G1 boundary in Saccharomyces cerevisiae. Mol. Microbiol. 28:69-79. [DOI] [PubMed] [Google Scholar]
- 565.Terasawa, H., Y. Noda, T. Ito, H. Hatanaka, S. Ichikawa, K. Ogura, H. Sumimoto, and F. Inagaki. 2001. Structure and ligand recognition of the PB1 domain: a novel protein module binding to the PC motif. EMBO J. 20:3947-3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.TerBush, D. R., T. Maurice, D. Roth, and P. Novick. 1996. The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 15:6483-6494. [PMC free article] [PubMed] [Google Scholar]
- 567.Theesfeld, C. L., J. E. Irazoqui, K. Bloom, and D. J. Lew. 1999. The role of actin in spindle orientation changes during the Saccharomyces cerevisiae cell cycle. J. Cell Biol. 146:1019-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Tjandra, H., J. Compton, and D. Kellogg. 1998. Control of mitotic events by the Cdc42 GTPase, the Clb2 cyclin and a member of the PAK kinase family. Curr. Biol. 8:991-1000. [DOI] [PubMed] [Google Scholar]
- 569.Toda, T., S. Cameron, P. Sass, M. Zoller, J. D. Scott, B. McMullen, M. Hurwitz, E. G. Krebs, and M. Wigler. 1987. Cloning and characterization of BCY1, a locus encoding a regulatory subunit of the cyclic AMP-dependent protein kinase in Saccharomyces cerevisiae. Mol. Cell. Biol. 7:1371-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Toda, T., S. Cameron, P. Sass, M. Zoller, and M. Wigler. 1987. Three different genes in S. cerevisiae encode the catalytic subunits of the cAMP-dependent protein kinase. Cell 50:277-287. [DOI] [PubMed] [Google Scholar]
- 571.Toenjes, K. A., M. M. Sawyer, and D. I. Johnson. 1999. The guanine-nucleotide-exchange factor Cdc24p is targeted to the nucleus and polarized growth sites. Curr. Biol. 9:1183-1186. [DOI] [PubMed] [Google Scholar]
- 572.Toenjes, K. A., D. Simpson, and D. I. Johnson. 2004. Separate membrane targeting and anchoring domains function in the localization of the S. cerevisiae Cdc24p guanine nucleotide exchange factor. Curr. Genet. 45:257-264. [DOI] [PubMed] [Google Scholar]
- 573.Tolliday, N., L. VerPlank, and R. Li. 2002. Rho1 directs formin-mediated actin ring assembly during budding yeast cytokinesis. Curr. Biol. 12:1864-1870. [DOI] [PubMed] [Google Scholar]
- 574.Tong, A. H. Y., M. Evangelista, A. B. Parsons, H. Xu, G. D. Bader, N. Pagé, M. Robinson, S. Raghibizadeh, C. W. V. Hogue, H. Bussey, B. Andrews, M. Tyers, and C. Boone. 2001. Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science 294:2364-2368. [DOI] [PubMed] [Google Scholar]
- 575.Trimble, W. S. 1999. Septins: a highly conserved family of membrane-associated GTPases with functions in cell division and beyond. J. Membr. Biol. 169:75-81. [DOI] [PubMed] [Google Scholar]
- 576.Truckses, D. M., J. E. Bloomekatz, and J. Thorner. 2006. The RA domain of Ste50 adaptor protein is required for delivery of Ste11 to the plasma membrane in the filamentous growth signaling pathway of the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 26:912-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Trueheart, J., and G. R. Fink. 1989. The yeast cell fusion protein FUS1 is O-glycosylated and spans the plasma membrane. Proc. Natl. Acad. Sci. USA 86:9916-9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Tsujita, K., S. Suetsugu, N. Sasaki, M. Furutani, T. Oikawa, and T. Takenawa. 2006. Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis. J. Cell Biol. 172:269-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Tucker, B. E., H. C. Hoch, and R. C. Staples. 1984. The involvement of F-actin in Uromyces cell differentiation: the effects of cytochalasin E and phalloidin. Protoplasma 135:88-101. [Google Scholar]
- 580.Ubersax, J. A., E. L. Woodbury, P. N. Quang, M. Paraz, J. D. Blethrow, K. Shah, K. M. Shokat, and D. O. Morgan. 2003. Targets of the cyclin-dependent kinase Cdk1. Nature 425:859-864. [DOI] [PubMed] [Google Scholar]
- 581.Uetz, P., L. Giot, G. Cagney, T. A. Mansfield, R. S. Judson, J. R. Knight, D. Lockshon, V. Narayan, M. Srinivasan, P. Pochart, A. Qureshi-Emili, Y. Li, B. Godwin, D. Conover, T. Kalbfleisch, G. Vijayadamodar, M. Yang, M. Johnston, S. Fields, and J. M. Rothberg. 2000. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature 403:623-627. [DOI] [PubMed] [Google Scholar]
- 582.Umikawa, M., K. Tanaka, T. Kamei, K. Shimizu, H. Imamura, T. Sasaki, and Y. Takai. 1998. Interaction of Rho1p target Bni1p with F-actin-binding elongation factor 1α: implication in Rho1p-regulated reorganization of the actin cytoskeleton in Saccharomyces cerevisiae. Oncogene 16:2011-2016. [DOI] [PubMed] [Google Scholar]
- 583.Utsugi, T., M. Minemura, A. Hirata, M. Abe, D. Watanabe, and Y. Ohya. 2002. Movement of yeast 1,3-beta-glucan synthase is essential for uniform cell wall synthesis. Genes Cells 7:1-9. [DOI] [PubMed] [Google Scholar]
- 584.Vaduva, G., N. C. Martin, and A. K. Hopper. 1997. Actin-binding verprolin is a polarity development protein required for the morphogenesis and function of the yeast actin cytoskeleton. J. Cell Biol. 139:1821-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Valdivia, R. H., D. Baggott, J. S. Chuang, and R. W. Schekman. 2002. The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late Golgi membrane proteins. Dev. Cell 2:283-294. [DOI] [PubMed] [Google Scholar]
- 586.Valdivia, R. H., and R. Schekman. 2003. The yeasts Rho1p and Pkc1p regulate the transport of chitin synthase III (Chs3p) from internal stores to the plasma membrane. Proc. Natl. Acad. Sci. USA 100:10287-10292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Vallen, E. A., J. Caviston, and E. Bi. 2000. Roles of Hof1p, Bni1p, Bnr1p, and Myo1p in cytokinesis in Saccharomyces cerevisiae. Mol. Biol. Cell 11:593-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Valtz, N., and I. Herskowitz. 1996. Pea2 protein of yeast is localized to sites of polarized growth and is required for efficient mating and bipolar budding. J. Cell Biol. 135:725-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Valtz, N., M. Peter, and I. Herskowitz. 1995. FAR1 is required for oriented polarization of yeast cells in response to mating pheromones. J. Cell Biol. 131:863-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.van Drogen, F., and M. Peter. 2002. Spa2p functions as a scaffold-like protein to recruit the Mpk1p MAP kinase module to sites of polarized growth. Curr. Biol. 12:1698-1703. [DOI] [PubMed] [Google Scholar]
- 591.Van Komen, J. S., X. Bai, T. L. Rodkey, J. Schaub, and J. A. McNew. 2005. The polybasic juxtamembrane region of Sso1p is required for SNARE function in vivo. Eukaryot. Cell 4:2017-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Versele, M., B. Gullbrand, M. J. Shulewitz, V. J. Cid, S. Bahmanyar, R. E. Chen, P. Barth, T. Alber, and J. Thorner. 2004. Protein-protein interactions governing septin heteropentamer assembly and septin filament organization in Saccharomyces cerevisiae. Mol. Biol. Cell 15:4568-4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Versele, M., and J. Thorner. 2004. Septin collar formation in budding yeast requires GTP binding and direct phosphorylation by the PAK, Cla4. J. Cell Biol. 164:701-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Versele, M., and J. Thorner. 2005. Some assembly required: yeast septins provide the instruction manual. Trends Cell Biol. 15:414-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Vink, E., R. J. Rodriguez-Suarez, M. Gerard-Vincent, J. C. Ribas, H. de Nobel, H. van den Ende, A. Duran, F. M. Klis, and H. Bussey. 2004. An in vitro assay for (1→6)-beta-D-glucan synthesis in Saccharomyces cerevisiae. Yeast 21:1121-1131. [DOI] [PubMed] [Google Scholar]
- 596.Visintin, R., K. Craig, E. S. Hwang, S. Prinz, M. Tyers, and A. Amon. 1998. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol. Cell 2:709-718. [DOI] [PubMed] [Google Scholar]
- 597.Visintin, R., E. S. Hwang, and A. Amon. 1999. Cfi1 prevents premature exit from mitosis by anchoring Cdc14 phosphatase in the nucleolus. Nature 398:818-823. [DOI] [PubMed] [Google Scholar]
- 598.Vrabioiu, A. M., S. A. Gerber, S. P. Gygi, C. M. Field, and T. J. Mitchison. 2004. The majority of the Saccharomyces cerevisiae septin complexes do not exchange guanine nucleotides. J. Biol. Chem. 279:3111-3118. [DOI] [PubMed] [Google Scholar]
- 599.Waddle, J. A., T. S. Karpova, R. H. Waterston, and J. A. Cooper. 1996. Movement of cortical actin patches in yeast. J. Cell Biol. 132:861-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Walch-Solimena, C., R. N. Collins, and P. J. Novick. 1997. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J. Cell Biol. 137:1495-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Walworth, N. C., B. Goud, A. K. Kabcenell, and P. J. Novick. 1989. Mutational analysis of SEC4 suggests a cyclical mechanism for the regulation of vesicular traffic. EMBO J. 8:1685-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Wasch, R., and F. R. Cross. 2002. APC-dependent proteolysis of the mitotic cyclin Clb2 is essential for mitotic exit. Nature 418:556-562. [DOI] [PubMed] [Google Scholar]
- 603.Watanabe, D., M. Abe, and Y. Ohya. 2001. Yeast Lrg1p acts as a specialized RhoGAP regulating 1,3-beta-glucan synthesis. Yeast 18:943-951. [DOI] [PubMed] [Google Scholar]
- 604.Wedlich-Soldner, R., S. Altschler, L. Wu, and R. Li. 2003. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science 299:1231-1235. [DOI] [PubMed] [Google Scholar]
- 605.Wedlich-Soldner, R., S. C. Wai, T. Schmidt, and R. Li. 2004. Robust cell polarity is a dynamic state established by coupling transport and GTPase signaling. J. Cell Biol. 166:889-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Weeks, G., and G. B. Spiegelman. 2003. Roles played by Ras subfamily proteins in the cell and developmental biology of microorganisms. Cell. Signal. 15:901-909. [DOI] [PubMed] [Google Scholar]
- 607.Weibel, P., A. Hiltbrunner, L. Brand, and F. Kessler. 2003. Dimerization of Toc-GTPases at the chloroplast protein import machinery. J. Biol. Chem. 278:37321-37329. [DOI] [PubMed] [Google Scholar]
- 608.Weiss, E. L., A. C. Bishop, K. M. Shokat, and D. G. Drubin. 2000. Chemical genetic analysis of the budding-yeast p21-activated kinase Cla4p. Nat. Cell Biol. 2:677-685. [DOI] [PubMed] [Google Scholar]
- 609.Wendland, J. 2001. Comparison of morphogenetic networks of filamentous fungi and yeast. Fungal Genet. Biol. 34:63-82. [DOI] [PubMed] [Google Scholar]
- 610.Wendland, J., and A. Walther. 2005. Ashbya gossypii: a model for fungal developmental biology. Nat. Rev. Microbiol. 3:421-429. [DOI] [PubMed] [Google Scholar]
- 611.White, W. H., and D. I. Johnson. 1997. Characterization of synthetic-lethal mutants reveals a role for the Saccharomyces cerevisiae guanine-nucleotide exchange factor Cdc24p in vacuole function and Na+ tolerance. Genetics 147:43-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Wiederkehr, A., J. O. De Craene, S. Ferro-Novick, and P. Novick. 2004. Functional specialization within a vesicle tethering complex: bypass of a subset of exocyst deletion mutants by Sec1p or Sec4p. J. Cell Biol. 167:875-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Wiederkehr, A., Y. Du, M. Pypaert, S. Ferro-Novick, and P. Novick. 2003. Sec3p is needed for the spatial regulation of secretion and for the inheritance of the cortical endoplasmic reticulum. Mol. Biol. Cell 14:4770-4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Wiget, P., Y. Shimada, A. C. Butty, E. Bi, and M. Peter. 2004. Site-specific regulation of the GEF Cdc24p by the scaffold protein Far1p during yeast mating. EMBO J. 23:1063-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Winter, D., T. Lechler, and R. Li. 1999. Activation of the yeast Arp2/3 complex by Bee1p, a WASP-family protein. Curr. Biol. 9:501-504. [DOI] [PubMed] [Google Scholar]
- 616.Winter, D., A. V. Podtelejnikov, M. Mann, and R. Li. 1997. The complex containing actin-related proteins Arp2 and Arp3 is required for the motility and integrity of yeast actin patches. Curr. Biol. 7:519-529. [DOI] [PubMed] [Google Scholar]
- 617.Winter, D. C., E. Y. Choe, and R. Li. 1999. Genetic dissection of the budding yeast Arp2/3 complex: a comparison of the in vivo and structural roles of individual subunits. Proc. Natl. Acad. Sci. USA 96:7288-7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Wu, C., S. F. Lee, E. Furmaniak-Kazmierczak, G. P. Cote, D. Y. Thomas, and E. Leberer. 1996. Activation of myosin-I by members of the Ste20p protein kinase family. J. Biol. Chem. 271:31787-31790. [DOI] [PubMed] [Google Scholar]
- 619.Wu, C., V. Lytvyn, D. Y. Thomas, and E. Leberer. 1997. The phosphorylation site for Ste20p-like protein kinases is essential for the function of myosin-I in yeast. J. Biol. Chem. 272:30623-30626. [DOI] [PubMed] [Google Scholar]
- 620.Wu, D. 2005. Signaling mechanisms for regulation of chemotaxis. Cell Res. 15:52-56. [DOI] [PubMed] [Google Scholar]
- 621.Wu, W.-J., D. A. Leonard, R. A. Cerione, and D. Manor. 1997. Interaction between Cdc42Hs and RhoGDI is mediated through the Rho insert region. J. Biol. Chem. 272:26153-26158. [DOI] [PubMed] [Google Scholar]
- 622.Wu, W. J., J. W. Erickson, R. Lin, and R. A. Cerione. 2000. The gamma-subunit of the coatomer complex binds Cdc42 to mediate transformation. Nature 405:800-804. [DOI] [PubMed] [Google Scholar]
- 623.Wullschleger, S., R. Loewith, and M. N. Hall. 2006. TOR signaling in growth and metabolism. Cell 124:471-484. [DOI] [PubMed] [Google Scholar]
- 624.Xu, J., F. Wang, A. Van Keymeulen, P. Herzmark, A. F. Straight, K. Kelly, Y. Takuwa, N. Sugimoto, T. J. Mitchison, and H. R. Bourne. 2003. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell 114:201-214. [DOI] [PubMed] [Google Scholar]
- 625.Xu, S., H. K. Huang, P. Kaiser, M. Latterich, and T. Hunter. 2000. Phosphorylation and spindle pole body localization of the Cdc15p mitotic regulatory protein kinase in budding yeast. Curr. Biol. 10:329-332. [DOI] [PubMed] [Google Scholar]
- 626.Xu, Y., J. B. Moseley, I. Sagot, F. Poy, D. Pellman, B. L. Goode, and M. J. Eck. 2004. Crystal structures of a formin homology-2 domain reveal a tethered dimer architecture. Cell 116:711-723. [DOI] [PubMed] [Google Scholar]
- 627.Yamochi, W., K. Tanaka, H. Nonaka, A. Maeda, T. Musha, and Y. Takai. 1994. Growth site localization of Rho1 small GTP-binding protein and its involvement in bud formation in Saccharomyces cerevisiae. J. Cell Biol. 125:1077-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Yang, H. C., and L. A. Pon. 2002. Actin cable dynamics in budding yeast. Proc. Natl. Acad. Sci. USA 99:751-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Yang, S., K. R. Ayscough, and D. G. Drubin. 1997. A role for the actin cytoskeleton of Saccharomyces cerevisiae in bipolar bud-site selection. J. Cell Biol. 136:111-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Yin, H., D. Pruyne, T. C. Huffaker, and A. Bretscher. 2000. Myosin V orientates the mitotic spindle in yeast. Nature 406:1013-1015. [DOI] [PubMed] [Google Scholar]
- 631.Yokoyama, K., H. Kaji, K. Nishimura, and M. Miyaji. 1990. The role of microfilaments and microtubules in apical growth and dimorphism of Candida albicans. J. Gen. Microbiol. 136:1067-1075. [DOI] [PubMed] [Google Scholar]
- 632.Yoshida, S., R. Ichihashi, and A. Toh-e. 2003. Ras recruits mitotic exit regulator Lte1 to the bud cortex in budding yeast. J. Cell Biol. 161:889-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Yoshida, S., and A. Toh-e. 2001. Regulation of the localization of Dbf2 and Mob1 during cell division of Saccharomyces cerevisiae. Genes Genet. Syst. 76:141-147. [DOI] [PubMed] [Google Scholar]
- 634.Young, M. E., J. A. Cooper, and P. C. Bridgman. 2004. Yeast actin patches are networks of branched actin filaments. J. Cell Biol. 166:629-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Yu, J. W., J. M. Mendrola, A. Audhya, S. Singh, D. Keleti, D. B. DeWald, D. Murray, S. D. Emr, and M. A. Lemmon. 2004. Genome-wide analysis of membrane targeting by S. cerevisiae pleckstrin homology domains. Mol. Cell 13:677-688. [DOI] [PubMed] [Google Scholar]
- 636.Zahner, J. E., H. A. Harkins, and J. R. Pringle. 1996. Genetic analysis of the bipolar pattern of bud-site selection in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 16:1857-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Zajac, A., X. Sun, J. Zhang, and W. Guo. 2005. Cyclical regulation of the exocyst and cell polarity determinants for polarized cell growth. Mol. Biol. Cell 16:1500-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Zanelli, C. F., and S. R. Valentini. 2005. Pkc1 acts through Zds1 and Gic1 to suppress growth and cell polarity defects of a yeast eIF5A mutant. Genetics 171:1571-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Zeitlinger, J., I. Simon, C. T. Harbison, N. M. Hannett, T. L. Volkert, G. R. Fink, and R. A. Young. 2003. Program-specific distribution of a transcription factor dependent on partner transcription factor and MAPK signaling. Cell 113:395-404. [DOI] [PubMed] [Google Scholar]
- 640.Zhang, J., C. Kong, H. Xie, P. S. McPherson, S. Grinstein, and W. S. Trimble. 1999. Phosphatidylinositol polyphosphate binding to the mammalian septin H5 is modulated by GTP. Curr. Biol. 9:1458-1467. [DOI] [PubMed] [Google Scholar]
- 641.Zhang, X., E. Bi, P. Novick, L. Du, K. G. Kozminski, J. H. Lipschutz, and W. Guo. 2001. Cdc42 interacts with the exocyst and regulates polarized secretion. J. Biol. Chem. 276:46745-46750. [DOI] [PubMed] [Google Scholar]
- 642.Zhang, X., P. Wang, A. Gangar, J. Zhang, P. Brennwald, D. TerBush, and W. Guo. 2005. Lethal giant larvae proteins interact with the exocyst complex and are involved in polarized exocytosis. J. Cell Biol. 170:273-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Zhao, Z. S., T. Leung, E. Manser, and L. Lim. 1995. Pheromone signalling in Saccharomyces cerevisiae requires the small GTP-binding protein Cdc42p and its activator CDC24. Mol. Cell. Biol. 15:5246-5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Zheng, Y., A. Bender, and R. A. Cerione. 1995. Interactions among proteins involved in bud-site selection and bud-site assembly in Saccharomyces cerevisiae. J. Biol. Chem. 270:626-630. [DOI] [PubMed] [Google Scholar]
- 645.Zheng, Y., R. Cerione, and A. Bender. 1994. Control of the yeast bud-site assembly GTPase Cdc42: catalysis of guanine nucleotide exchange by Cdc24 and stimulation of GTPase activity by Bem3. J. Biol. Chem. 269:2369-2372. [PubMed] [Google Scholar]
- 646.Zheng, Y., M. J. Hart, K. Shinjo, T. Evans, A. Bender, and R. A. Cerione. 1993. Biochemical comparisons of the Saccharomyces cerevisiae Bem2 and Bem3 proteins. Delineation of a limit Cdc42 GTPase-activating protein domain. J. Biol. Chem. 268:24629-24634. [PubMed] [Google Scholar]
- 647.Zigmond, S. H. 2004. Formin-induced nucleation of actin filaments. Curr. Opin. Cell Biol. 16:99-105. [DOI] [PubMed] [Google Scholar]
- 648.Zigmond, S. H., M. Evangelista, C. Boone, C. Yang, A. C. Dar, F. Sicheri, J. Forkey, and M. Pring. 2003. Formin leaky cap allows elongation in the presence of tight capping proteins. Curr. Biol. 13:1820-1823. [DOI] [PubMed] [Google Scholar]
- 649.Ziman, M., J. S. Chuang, and R. W. Schekman. 1996. Chs1p and Chs3p, two proteins involved in chitin synthesis, populate a compartment of the Saccharomyces cerevisiae endocytic pathway. Mol. Biol. Cell 7:1909-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Ziman, M., J. M. O'Brien, L. A. Ouellette, W. R. Church, and D. I. Johnson. 1991. Mutational analysis of CDC42Sc, a Saccharomyces cerevisiae gene that encodes a putative GTP-binding protein involved in the control of cell polarity. Mol. Cell. Biol. 11:3537-3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Ziman, M., D. Preuss, J. Mulholland, J. M. O'Brien, D. Botstein, and D. I. Johnson. 1993. Subcellular localization of Cdc42p, a Saccharomyces cerevisiae GTP-binding protein involved in the control of cell polarity. Mol. Biol. Cell 4:1307-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]