Abstract
Reactive oxygen species (ROS) are implicated in plant innate immunity. NADPH oxidase (RBOH; for Respiratory Burst Oxidase Homolog) plays a central role in the oxidative burst, and EF-hand motifs in the N terminus of this protein suggest possible regulation by Ca2+. However, regulatory mechanisms are largely unknown. We identified Ser-82 and Ser-97 in the N terminus of potato (Solanum tuberosum) St RBOHB as potential phosphorylation sites. An anti-phosphopeptide antibody (pSer82) indicated that Ser-82 was phosphorylated by pathogen signals in planta. We cloned two potato calcium-dependent protein kinases, St CDPK4 and St CDPK5, and mass spectrometry analyses showed that these CDPKs phosphorylated only Ser-82 and Ser-97 in the N terminus of St RBOHB in a calcium-dependent manner. Ectopic expression of the constitutively active mutant of St CDPK5, St CDPK5VK, provoked ROS production in Nicotiana benthamiana leaves. The CDPK-mediated ROS production was disrupted by knockdown of Nb RBOHB in N. benthamiana. The loss of function was complemented by heterologous expression of wild-type potato St RBOHB but not by a mutant (S82A/S97A). Furthermore, the heterologous expression of St CDPK5VK phosphorylated Ser-82 of St RBOHB in N. benthamiana. These results suggest that St CDPK5 induces the phosphorylation of St RBOHB and regulates the oxidative burst.
INTRODUCTION
Rapid production of reactive oxygen species (ROS), called the oxidative burst, is one of the hallmarks in the early events during incompatible interactions between plants and pathogens (Doke, 1983; Lamb and Dixon, 1997). ROS generated by plasma membrane NADPH oxidase (Nox) play pivotal roles in the defense against pathogen attack. NADPH oxidase was first identified in phagocytic cells including gp91phox (phox for phagocyte oxidase) as a catalytic subunit. Recently, a new Nox family was discovered and shown to function in nonphagocytic mammalian cells (Bokoch and Knaus, 2003; Lambeth, 2004), in biotic and abiotic stresses or development in higher plants (Torres and Dangl, 2005), and in a mutualistic fungus–plant interaction (Tanaka et al., 2006). Plant NADPH oxidases designated as RBOH (for respiratory burst oxidase homolog) are predicted to have six transmembrane-spanning domains that correspond to those identified in gp91phox and to carry an N-terminal extension comprising two EF-hand motifs, suggesting that Ca2+ regulates its activity.
RBOH was first isolated from rice (Oryza sativa) as a homolog of gp91phox (Groom et al., 1996) and then identified in various plant species (Keller et al., 1998; Torres et al., 1998; Amicucci et al., 1999; Yoshioka et al., 2001, 2003; Yoshie et al., 2005). Earlier work has shown that RBOH is a main component in ROS production during biotic and abiotic stresses. The Arabidopsis thaliana double mutant rbohD rbohF in the dSpm insertion mutagenesis system produces greatly decreased ROS against infection with avirulent Pseudomonas syringae pv tomato DC3000 or Hyaloperonospora parasitica (Torres et al., 2002). Arabidopsis rbohD rbohF also shows decreased ROS production in response to abscisic acid (ABA) and is impaired in ABA-activated stomatal closure (Kwak et al., 2003). Tobacco (Nicotiana tabacum) cells transformed with antisense constructs of Nt RBOHD lose ROS production to elicitor treatment (Simon-Plas et al., 2002). Nb RBOHA/Nb RBOHB–silenced Nicotiana benthamiana plants show a reduced oxidative burst and reduced disease resistance to Phytophthora infestans (Yoshioka et al., 2003). Arabidopsis rbohC has a defect in Ca2+ uptake and ROS accumulation during root hair formation (Foreman et al., 2003). These reports suggest that RBOH is a key regulator of ROS production and displays pleiotropic functions in plants.
The phagocyte enzymatic complex of NADPH oxidase consists of two plasma membrane proteins, gp91phox (known as Nox2) and p22phox. Several cytosolic regulatory proteins, p47phox, p67phox, p40phox, and Rac2, translocate to the plasma membrane to form the active complex after stimulation (Cross and Segal, 2004; Lambeth, 2004). Noxo1 (for Nox organizer1) and Noxa1 (for Nox activator1) are homologs of p47phox and p67phox, respectively, and are required for Nox1 activation (Takeya et al., 2003). However, no homologs of the p22phox, p67phox, p47phox, and p40phox regulators of the phagocyte NADPH oxidase were found in the Arabidopsis genome (Dangl and Jones, 2001). Nox5, Duox1, and Duox2 have N-terminal extensions with four or two EF-hand motifs. They might be activated by an increase in cytosolic Ca2+ concentration ([Ca2+]cyt) (Dupuy et al., 1999; Bánfi et al., 2001, 2004; Sumimoto et al., 2005). These reports indicate that these Nox/Duox have specific regulatory mechanisms that differ from the phagocyte NADPH oxidase.
The regulatory mechanisms of RBOH also remain unknown, while several lines of evidence indicate the importance of Ca2+ and protein kinases in ROS production. Because overexpression of At RBOHD does not result in constitutive ROS production, RBOH may require posttranscriptional regulation for its activation (Torres et al., 2005). Ca2+ influx into the cytoplasm (Chandra and Low, 1997; Piedras et al., 1998; Grant et al., 2000) and changes in protein phosphorylation (Kauss and Jeblick, 1995; Miura et al., 1995) are implicated in the activation process of the RBOH. It was reported that RBOH-like proteins in the plasma membrane fractions of tomato (Solanum lycopersicum) and tobacco show NADPH oxidase activity without any cytosolic components in the renatured gel after SDS-PAGE, and the activity increases upon addition of Ca2+ (Sagi and Fluhr, 2001). On the other hand, a homolog of human Rac GTPase has been isolated from rice, and the constitutively active mutant of Rac activates ROS production (Kawasaki et al., 1999). These reports suggest that the extended N-terminal region may play a key role in the regulation of the RBOH enzyme.
We previously isolated St RBOHA to RBOHD from potato (Solanum tuberosum) plants (Yoshioka et al., 2001; Yamamizo et al., 2006). Treatment of potato tubers with hyphal wall components (HWC) from P. infestans causes a rapid and transient accumulation of H2O2 (phase I), followed by a massive oxidative burst at 6 to 9 h after the treatment (phase II). RNA gel blot analyses indicate that St RBOHA is constitutively expressed at a low level, whereas St RBOHB, St RBOHC, and St RBOHD are upregulated during the phase II burst. The NADPH oxidase inhibitor diphenylene iodonium blocked both bursts, while pretreatment of the tuber with the protein synthesis inhibitor cycloheximide abolished only the second burst. These data suggest that St RBOHA and St RBOHB to RBOHD contribute to the phase I and phase II bursts, respectively (Yoshioka et al., 2001; Yamamizo et al., 2006). We found that both bursts are also inhibited by a protein kinase inhibitor or a calcium inhibitor (Figure 1). These findings let us investigate the direct phosphorylation of the N-terminal region of St RBOH protein by certain protein kinases for the activation of the enzymes. Here, we show the potential phosphorylation sites in the N terminus of St RBOHB by in-gel kinase assay and mass spectrometry analysis. Moreover, we identify two calcium-dependent protein kinases (CDPKs) as protein kinases of the St RBOHB N-terminal region. Heterologous expression of the constitutively active mutant of CDPK induced Nb RBOHB–dependent ROS production in N. benthamiana leaves. These results demonstrate that the CDPK activates St RBOHB protein by phosphorylation of the N-terminal region.
Figure 1.
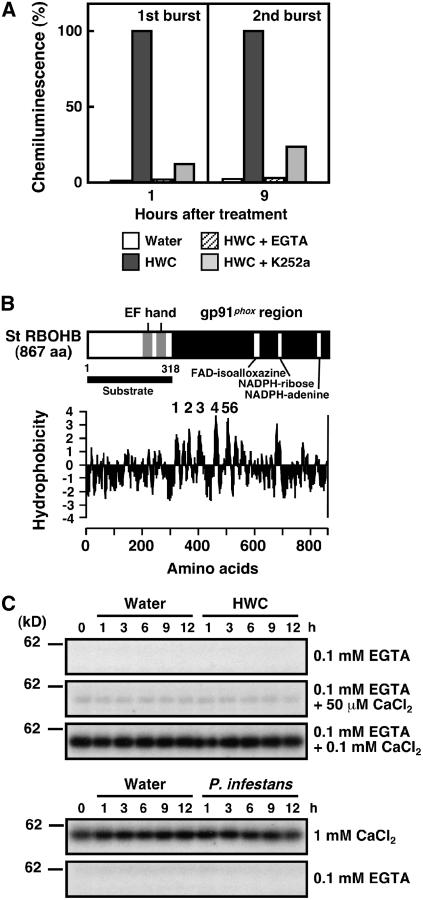
Calcium-Dependent Protein Phosphorylation Is Involved in the Oxidative Burst in Potato Tubers.
(A) Effects of K252a and EGTA on HWC-mediated first and second bursts. Chemiluminescence was counted at various times after treatment with water, 1 mg/mL HWC, HWC plus 10 μM K252a, or HWC plus 1 mM EGTA.
(B) Structure and hydrophilicity plots of St RBOHB. Gray-shaded area, EF-hand motifs; black-shaded area, gp91phox region; white vertical bars, FAD isoalloxazine, NADPH ribose, and NADPH binding sites; black horizontal bar, N-terminal region used for substrates. Numbers in hydrophilicity plots (Kyte and Doolittle, 1982) indicate putative membrane-spanning domains. aa, amino acids.
(C) Detection of St RBOHB kinase activity in potato tuber discs. Discs were treated with water or 1 mg/mL HWC or inoculated with P. infestans (1.0 × 105 zoospores/mL). Soluble proteins were extracted at the indicated times. Kinase activity was assayed with in-gel kinase assay using the N-terminal region of St RBOHB as a substrate in the presence of EGTA, CaCl2, or EGTA plus CaCl2.
RESULTS
Calcium-Dependent St RBOHB Kinase Activities in Potato Tuber Proteins
Treatment with HWC elicitor induces a biphasic oxidative burst in potato tuber tissues (Yoshioka et al., 2001). The first and second bursts (1 and 9 h after the elicitor treatment) were significantly suppressed by the addition of the Ser/Thr protein kinase inhibitor K252a or the extracellular Ca2+ chelator EGTA (Figure 1A). RBOH carries the N-terminal extension in addition to the gp91phox region, suggesting that the N terminus may be phosphorylated by St RBOHB kinase in a Ca2+-dependent manner. Because there are too many Ser/Thr residues as phosphorylation candidates in the St RBOHA N terminus, we used the St RBOHB N terminus for the analysis. First, we performed an in-gel kinase assay using recombinant St RBOHB N-terminal protein (St RBOHB1-318; Figure 1B) as a substrate and analyzed microsomal and soluble proteins from potato tubers. We detected Ca2+-dependent St RBOHB kinase activity in the soluble protein fraction (Figure 1C) as well as in the microsomal fraction (data not shown). We used the soluble proteins for subsequent analyses because enough soluble protein was available from a small amount of plant tissue.
In the experiments, Ca2+-dependent St RBOHB kinase activity was detected in all treatments, such as water, HWC, and Phytophthora inoculation (Figure 1C). We tested in vitro phosphorylation with different Ca2+ concentrations, as shown in Figure 1C. The St RBOHB kinase activity in the in-gel kinase assay did not change with Ca2+ concentration regardless of treatment. The activity depends on Ca2+ concentration in the reaction buffer, indicating that it is difficult to estimate changes in enzymatic activity in planta.
Phosphorylation of Ser-82 and Ser-97 in St RBOHB by Potato Soluble Proteins
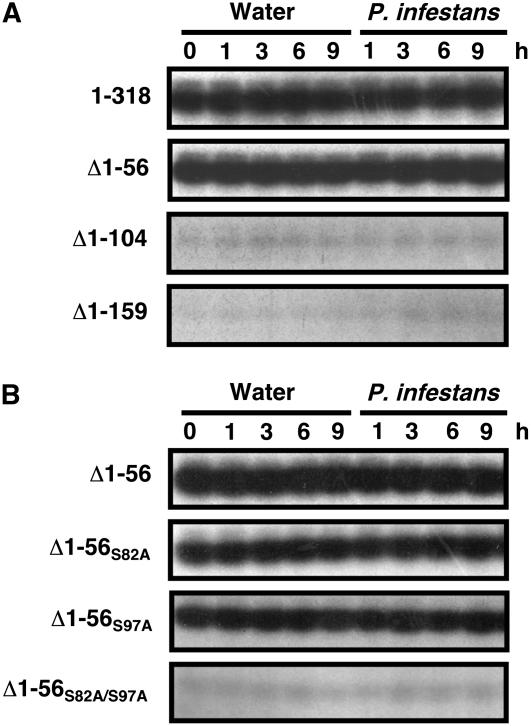
To investigate the phosphorylation site, we subjected the truncated N-terminal fragments to an in-gel kinase assay. Truncation of amino acids 1 to 104 (Δ1-104) as well as 1 to 159 (Δ1-159) largely reduced St RBOHB kinase activity, whereas truncation of 1 to 56 (Δ1-56) showed no difference from St RBOHB1-318 (Figure 2A). The fragment St RBOHB1-159 was phosphorylated at the same level as St RBOHB1-318 (data not shown). These results indicate that St RBOHB was phosphorylated in the area from amino acid 57 to 104. Δ1-56 fragments with Ala substituted for Ser or Thr were used as substrates (Figure 2B). S82A or S97A slightly reduced the activity; however, S82A/S97A double mutations significantly reduced kinase activity (Figure 2B). Mutations at other sites had no significant effect on activity (data not shown). These results suggest that Ser-82 and Ser-97 are phosphorylated by the Ca2+-dependent St RBOHB kinase in the potato proteins.
Figure 2.
Effects of N-Terminal Deletion and Mutation of Ser-82 and Ser-97 Residues in the St RBOHB N Terminus on Kinase Activities.
Soluble proteins were prepared from potato tubers inoculated with P. infestans (1.0 × 105 zoospores/mL). In-gel kinase assay was performed using the N-terminal region of St RBOHB with introduced N-terminal deletion (A) or site-directed mutagenesis of Ser-82 and/or Ser-97 as substrate (B).
Ser-82 Is Phosphorylated by Pathogen Signals in Potato Tubers
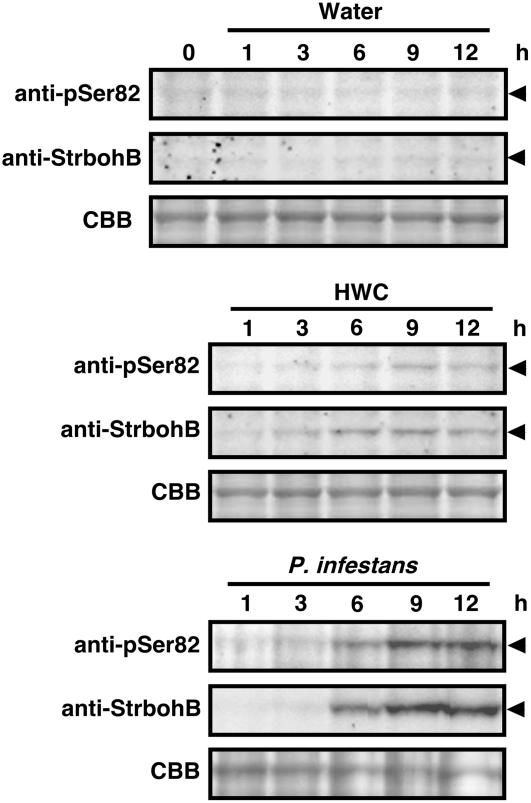
To determine whether Ser-82 and Ser-97 are phosphorylated in potato tuber tissues, we prepared anti-phosphopeptide antibody against peptides including phospho-Ser-82 (pSer82) or phospho-Ser-97 (pSer97). Immunoblot analyses using anti-St RBOHB antiserum indicated that HWC treatment and inoculation with an avirulent race of P. infestans induced the accumulation of St RBOHB proteins in potato tuber tissues at a peak at 9 h after the treatments (Figure 3). Anti-pSer82 antibody detected the immunostained bands corresponding to St RBOHB at 6 to 9 h after HWC treatment or Phytophthora inoculation, in agreement with the second burst (Figure 1A). On the other hand, anti-pSer97 antibody gave no specific signal in this study, although the antibody recognizes at least the original antigen phosphopeptide at a level similar to that of the anti-pSer82 antibody (data not shown).
Figure 3.
Phosphorylation of Ser-82 in Potato Tuber Tissues.
Microsomal proteins were prepared from potato tuber tissues treated with water or 1 mg/mL HWC or inoculated with P. infestans. Equal amounts of proteins were used for immunoblot analyses. Phosphorylation of Ser-82 and St RBOHB protein were detected by anti-pSer82 antibody and anti-St RBOHB antiserum, respectively. Protein loads were monitored by Coomassie Brilliant Blue (CBB) staining.
Screening of St RBOHB Kinase and Mass Spectrometry Analyses of the Phosphorylation Sites by St CDPK4 and St CDPK5
For further investigation, we attempted to isolate the St RBOHB kinase. The cloning was performed according to the method described by Matsuo et al. (2001) using Escherichia coli expressing St RBOHB1-159, anti-pSer82 antibody, and the potato λ phage cDNA expression library. From 4 × 105 plaques screened, seven positive clones including two CDPKs were isolated. CDPK is a Ser/Thr protein kinase that is activated by Ca2+ binding to EF-hand motifs of the C-terminal calmodulin-like domain and functions in biotic/abiotic stress responses (Harmon et al., 2000; Ludwig et al., 2004). We designated these two CDPKs as St CDPK4 and St CDPK5, and they were subjected to further analyses. St CDPK4 and St CDPK5 are 83.2 and 86.2% identical at the nucleotide and amino acid levels, respectively. The genes encode proteins of 557 and 535 amino acids with predicted molecular masses of 62.2 and 60.0 kD, respectively. The Gly residue at the second position and the Cys residue at the fifth position are predicted myristoylation and palmitoylation sites for membrane association, respectively. The closest homologs to St CDPK4 and St CDPK5 are Cm CPK2 of Cucurbita maxima and Ca CDPK3 of Capsicum annuum, respectively (84.4 and 91.3% amino acid identity). The closest homolog of St CDPK4 and St CDPK5 in Arabidopsis is At CPK6 (81.6 and 81.4% amino acid identity). The amino acid alignments and phylogenetic tree of the CDPKs are shown in Supplemental Figures 1 and 2 online, respectively.
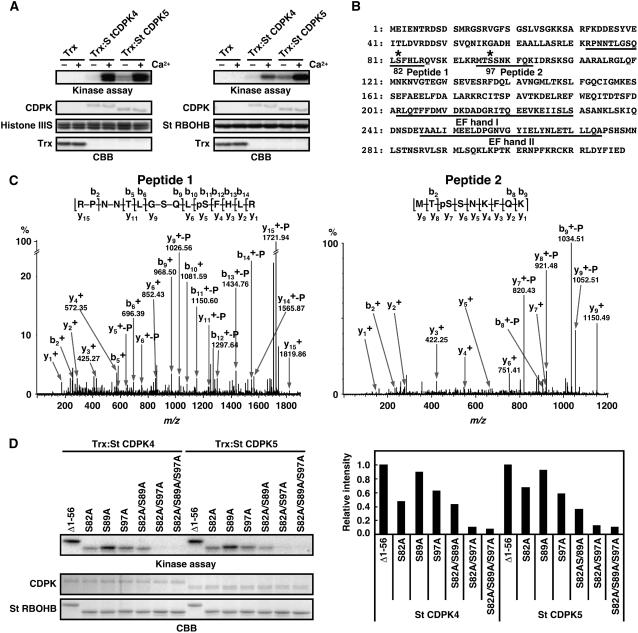
We tested whether St CDPK4 and St CDPK5 phosphorylate St RBOHB proteins using recombinant thioredoxin (Trx)-fused CDPKs expressed in E. coli. Trx:St CDPK4 and Trx:St CDPK5 phosphorylated the N-terminal region of St RBOHB1-318 and histone III-S, a model substrate for CDPK, in a Ca2+-dependent manner (Figure 4A). Trx:St CDPK4 and Trx:St CDPK5 also phosphorylated N-terminal proteins of St RBOHA, St RBOHC, and St RBOHD (see Supplemental Figure 3A online). Furthermore, liquid chromatography–tandem mass spectrometry (LC-MS/MS) identified phosphorylation of Ser-82 and Ser-97 in the N-terminal peptides of St RBOHB1-318 (Figures 4B and 4C).
Figure 4.
Phosphorylation of Ser-82 and Ser-97 of St RBOHB by Recombinant St CDPK4 and St CDPK5 Proteins.
(A) Purified N-terminal peptides of St RBOHB1-318 (right) or histone III-S (left) were used as substrates for Trx-fused St CDPK4 and St CDPK5. Phosphorylation of St RBOHB and histone III-S was detected by x-ray film. Protein loads were monitored by CBB staining.
(B) Amino acid sequence of St RBOHB. Fragments confirmed as phosphopeptide and EF-hand motifs are underlined. Asterisks indicate phosphorylation sites.
(C) Hybrid quadrupole/time-of-flight tandem mass spectrometry spectra identifying phosphorylation sites of N-terminal peptides of St RBOHB1-318 in vitro. The phosphorylated proteins were separated by SDS-PAGE and in-gel digested with trypsin. Resulting peptides were extracted and analyzed by LC-MS/MS analysis on a capillary LC system coupled directly to a Waters Q-ToF Ultima mass spectrometer. Identified phosphoseryl residues are denoted as pS. The superscript + indicates singly protonated ions. −P indicates neutral loss of H3PO4 from phosphorylated peptides.
(D) Effects of mutations of Ser-82 and Ser-97 residues on phosphorylation in vitro. N-terminal peptides of St RBOHB (Δ1-56)–introduced amino acid substitution in Ser-82, Ser-89, and/or Ser-97 were used as substrates for Trx-fused St CDPK4 and St CDPK5. The difference in size between Δ1-56 and Δ1-56 carrying point mutations is derived from different lengths of additional vector sequences by use of distinct restriction sites. Phosphorylation was detected by x-ray film (top panel). Protein loads were determined by CBB staining (middle panel). Incorporation of radioactivities into the various N-terminal regions of St RBOHB was measured using a phosphor imager (bottom panel).
We further analyzed the efficiency of phosphorylation of various St RBOHB mutants of Δ1-56 by recombinant St CDPK4 and St CDPK5. Mutation of either Ser-82 or Ser-97 to Ala partially reduced the phosphorylation efficiency, and mutation of both residues significantly reduced the efficiency, whereas mutation of Ser-89 did not affect the intensity of the phosphorylation (Figure 4D). These results indicate that St CDPK4 and St CDPK5 are activated by Ca2+ and phosphorylate Ser-82 and Ser-97 of St RBOHB in vitro.
Nb RBOH–Dependent ROS Production by Constitutively Active St CDPK5 in N. benthamiana Leaves
N. benthamiana, like potato, belongs to the Solanaceae and is thus useful for the dissection of biological functions by virus-induced gene silencing (VIGS) and Agrobacterium tumefaciens–mediated transient expression. N. benthamiana also includes at least two RBOH genes, Nb RBOHA and Nb RBOHB, corresponding homologs of St RBOHA and St RBOHB, respectively. An in vitro kinase assay indicated that Trx:St CDPK4 and Trx:St CDPK5 phosphorylated the N-terminal recombinant proteins Nb RBOHA and Nb RBOHB (see Supplemental Figure 3B online).
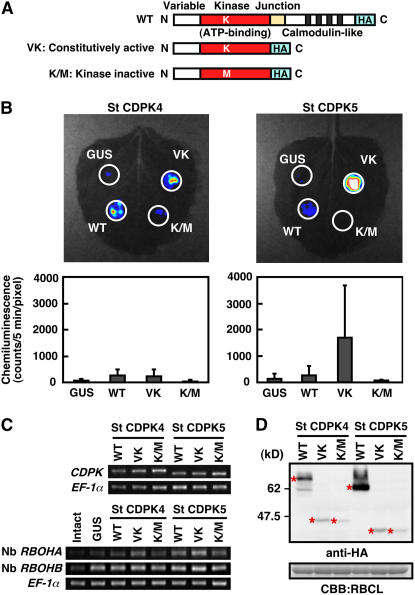
We performed Agrobacterium-mediated transient expression of CDPK variants (WT, VK, and K/M) and β-glucuronidase (GUS) proteins in N. benthamiana leaves and then measured ROS production using the photon-counting system (Figure 5). The CDPK structure consists of an N-terminal variable (V) domain, a protein kinase (K) domain, a junction (J) domain, and a calmodulin-like (C) domain including four EF-hand motifs (Harper et al., 2004; Ludwig et al., 2004). VK is a truncated variant lacking J and C domains and is expected to act like a constitutively active kinase (Harper et al., 1994; Ludwig et al., 2005). K/M is a VK variant with a Lys-to-Met mutation at the ATP binding site that disrupts the protein kinase activity (Kapiloff et al., 1991; Sheen, 1996). Expression of St CDPK4WT, St CDPK4VK, and St CDPK5WT slightly induced ROS production compared with GUS, St CDPK4K/M, and St CDPK5K/M. St CDPK5VK induced a significantly larger amount of ROS production than other variants (Figure 5B). Twenty leaves from six plants were analyzed for the quantification. To rule out the possibility that the truncation of the J and C domains eliminates the substrate specificity of the CDPKs, we used another type of constitutively active mutant that has a six-residue substitution in the J domain (Harper et al., 1994). The transient expression of these mutants also showed that St CDPK5 provoked a significant amount of ROS production compared with St CDPK4 (see Supplemental Figure 4 online). RT-PCR analyses showed that CDPK, Nb RBOHA, and Nb RBOHB were transcribed at similar levels. Nb RBOHB expression is known to increase in response to INF1 elicitor treatment and defense-related St MEK1DD expression (Yoshioka et al., 2003). St MEK1DD, which is a constitutively active mutant of the potato ortholog of tobacco Nt MEK2, induced hypersensitive response (HR)–like cell death and ROS production in N. benthamiana leaves (Katou et al., 2003, 2005). Although agroinfiltration also induced some expression of Nb RBOHB compared with intact leaves, the agroinfiltration-induced expression of Nb RBOHB was less than that of Nt MEK2DD (see Supplemental Figure 5 online). Protein accumulation of each variant was analyzed by immunoblot analysis using anti-hemagglutinin (HA) antibody (Figure 5D). Levels of protein accumulation between St CDPK4VK and St CDPK5VK were almost identical, suggesting that St CDPK5VK has a more important function in ROS production. We selected St CDPK5 for further study. However, St CDPK4 also may contribute to ROS production, because St CDPK4VK accumulates at a much lower level than St CDPK4WT.
Figure 5.
ROS Production Mediated by St CDPK4 and St CDPK5 Variants in N. benthamiana Leaves.
(A) Schematic structures of St CDPK4 and St CDPK5 with variable, kinase, junction, and calmodulin-like domains (WT). K indicates the Lys residue for ATP binding in the kinase domain. VK is a truncated variant lacking junction and calmodulin-like domains. K/M is a truncated kinase-inactive variant with substitution in Lys (K) of the ATP binding motif to Met (M).
(B) ROS production in N. benthamiana leaves expressing St CDPK4 or St CDPK5 variants. Two days after infiltration of Agrobacterium harboring St CDPK variants or GUS for agroinfiltration control, ROS production was measured by chemiluminescence mediated by L-012. Data are means ± sd from six experiments.
(C) Transcript accumulation was monitored by RT-PCR. Total RNA was extracted from the leaves shown in (B).
(D) Immunoblot analysis using anti-HA antibody. Total protein was prepared from the leaves shown in (B). Protein loads were monitored by CBB staining of the bands corresponding to ribulose-1,5-bisphosphate carboxylase large subunit (RBCL).
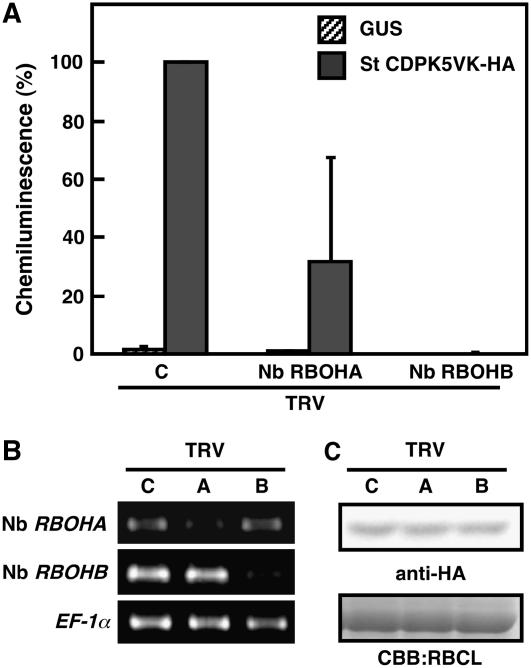
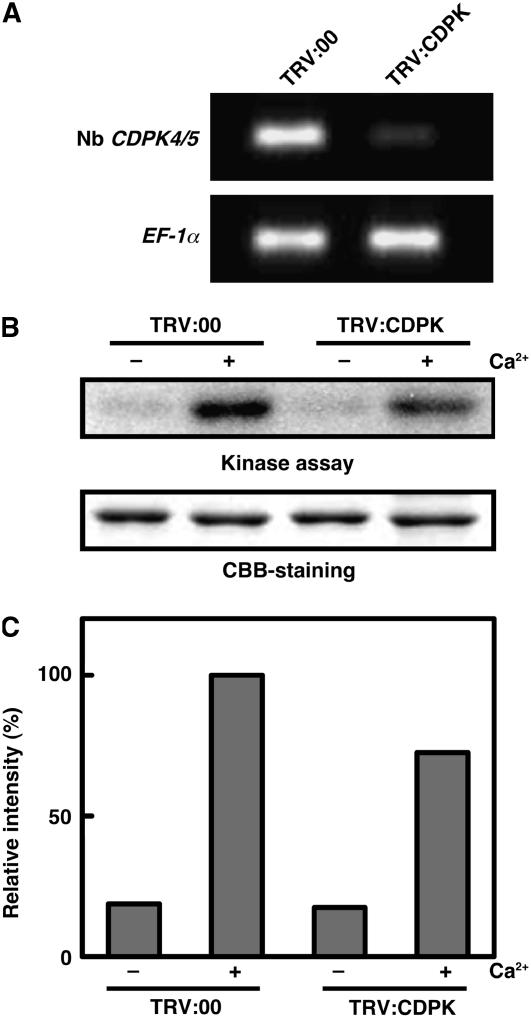
We next investigated whether ROS production is dependent on either Nb RBOHA or Nb RBOHB. VIGS of Nb RBOHA partially suppressed ROS production, whereas Nb RBOHB silencing abolished the oxidative burst to the control level (Figure 6A). The effect of VIGS was monitored by RT-PCR analysis. Each target gene was well silenced (Figure 6B). To rule out the possibility that VIGS interfered with St CDPK5VK transgene expression by agroinfiltration, we performed immunoblot analysis and confirmed that St CDPK5VK protein accumulation was not affected by the VIGS (Figure 6C). These results indicate that St CDPK5VK-mediated ROS production is largely dependent on Nb RBOHB.
Figure 6.
Disruption of St CDPK5VK-Mediated ROS Production by Nb RBOH Silencing.
N. benthamiana leaves were inoculated with TRV:00 (C), TRV:Nb RBOHA (A), or TRV:Nb RBOHB (B). After 3 weeks of inoculation, Agrobacterium harboring St CDPK5VK was infiltrated.
(A) ROS production was measured at 2 d after agroinfiltration. Data are means ± sd from four experiments.
(B) Gene silencing was monitored by RT-PCR.
(C) Protein expression of St CDPK5VK was detected by immunoblot analysis using anti-HA antibody.
Complementation of ROS Production by St RBOHB but Not by St RBOHB (S82A/S97A) in Nb RBOHB–Silenced Leaves
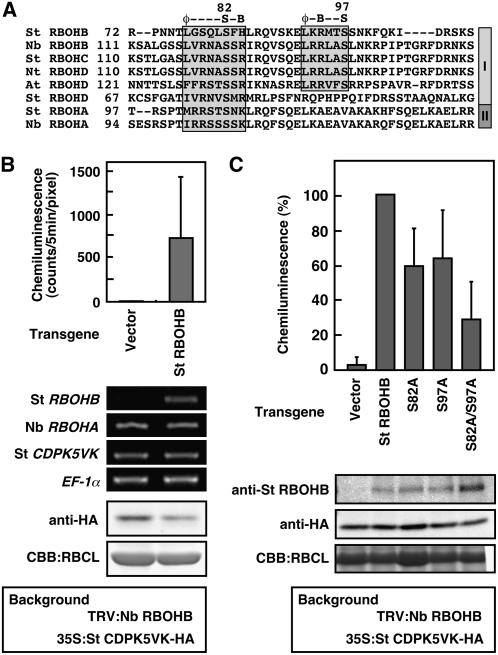
LC-MS/MS analyses indicated that St CDPK5 phosphorylates both Ser-82 and Ser-97 residues in a calcium-dependent manner in vitro (Figure 4). Although limited information is available about CDPK substrate specificity and phosphorylation motifs (Huang et al., 2001; Huang and Huber, 2001), we found the potential phosphorylation motifs at Ser-82 and Ser-97 of St RBOHB and related RBOHs used in the experiments (Figure 7A). Interestingly, the motif at Ser-82 was conserved in all RBOHs tested here, and the motif at Ser-97 was found in stress-inducible RBOHs (group I) except for St RBOHD.
Figure 7.
Complementation of ROS Production by Heterologous Expression of St RBOHB but Not by St RBOHBS82A/S97A in Nb RBOHB–Silenced Leaves.
(A) Partial amino acid alignment of St RBOHA to RBOHD, Nt RBOHD, At RBOHD, Nb RBOHA, and Nb RBOHB. Shaded boxes indicate the predicted target motifs by CDPK. φ, a hydrophobic residue; S, a Ser residue; B, a basic residue. RBOH genes in groups I and II are induced by pathogen signals and expressed constitutively, respectively.
(B) Activation of St RBOHB by St CDPK5VK. St CDPK5VK was coexpressed with or without St RBOHB by agroinfiltration in Nb RBOHB–silenced leaves. Two days after agroinfiltration, ROS production was measured (top panel). Mean values from triplicate experiments are shown. Vertical bars indicate sd. Accumulation of transcripts for St RBOHB, Nb RBOHA, and St CDPK5VK was monitored by RT-PCR (middle panels). Protein accumulation of St CDPK5VK was detected by immunoblot analysis using anti-HA antibody (bottom panels).
(C) Effects of Ser-82 and Ser-97 mutations on St RBOHB-mediated ROS production. St RBOHB variants were coexpressed with St CDPK5VK in Nb RBOHB–silenced leaves. Two days after agroinfiltration, ROS production was measured (top panel). Data are means ± sd from six experiments. Protein accumulation of St RBOHB variants and St CDPK5VK was detected by immunoblot analyses using anti-St RBOHB antiserum and anti-HA antibody, respectively (bottom panels).
Nb RBOHB and St RBOHB are 62.3% identical at the nucleotide level and do not share the continuous stretch of 23 nucleotides required for posttranscriptional gene silencing (Thomas et al., 2001). Thus, it is expected that VIGS of Nb RBOHB will not interfere with St RBOHB transgene expression by agroinfiltration. In Nb RBOHB–silenced leaves, ROS production was induced by coexpression of St RBOHB and St CDPK5VK (Figure 7B), indicating that the loss of function was complemented by heterologous expression of St RBOHB. RT-PCR analyses showed that St RBOHB mRNAs accumulate in the Nb RBOHB–silenced leaves. Nb RBOHA and St CDPK5VK mRNAs and St CDPK5VK proteins accumulate regardless of the VIGS of Nb RBOHB. These results suggest that St CDPK5VK activated St RBOHB–mediated ROS production.
Next, the mutated St RBOHB variants (S82A, S97A, and S82A/S97A) were used in place of St RBOHB. Coexpression of St RBOHB (S82A) or St RBOHB (S97A) with St CDPK5VK induced the oxidative burst to some extent; however, double mutated St RBOHB (S82A/S97A) showed ∼70% lower activity than did the wild type (Figure 7C). These mutations did not affect the accumulation of St CDPK5VK and St RBOHB variants. These data suggest that phosphorylation of Ser-82 and Ser-97 by St CDPK5 is required for full activity of St RBOHB.
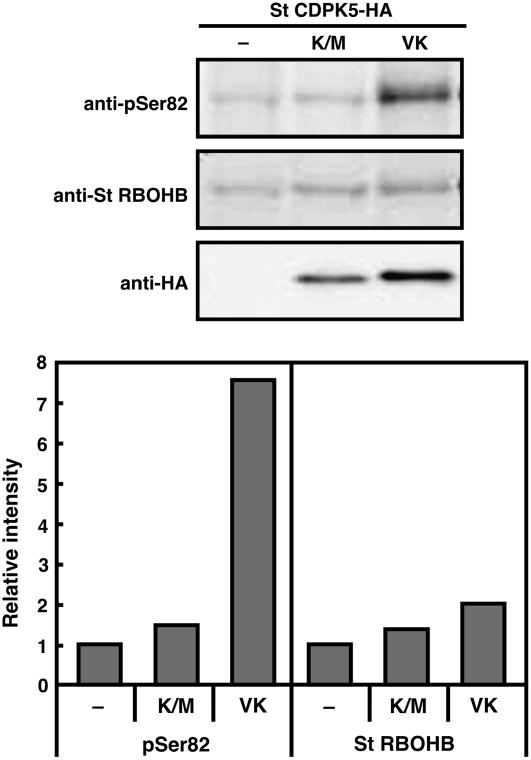
Phosphorylation of Ser-82 by St CDPK5VK in N. benthamiana Leaves
To confirm the phosphorylation of Ser-82 and Ser-97 when St RBOHB and St CDPK5VK are coexpressed in N. benthamiana, we prepared plasma membrane proteins from leaves expressing St RBOHB with or without St CDPK5VK or St CDPK5K/M. Immunoblot analysis using anti-pSer82 antibody revealed that much more phosphorylation of Ser-82 was detected in St CDPK5VK–expressed leaves than in St CDPK5K/M or St RBOHB alone (Figure 8). Phosphorylation of Ser-97 was not detected by anti-pSer97 antibody (data not shown). Immunoblot analysis using the anti-St RBOHB N-terminal antiserum showed that the amounts of St RBOHB are almost identical when coexpressed with St CDPK5VK and St CDPK5K/M. These results indicate that St CDPK5VK phosphorylates Ser-82 of St RBOHB in planta.
Figure 8.
Phosphorylation of Ser-82 in St RBOHB by Constitutively Active St CDPK5 in Planta.
St RBOHB was expressed with or without St CDPK5VK or St CDPK5K/M via agroinfiltration in N. benthamiana leaves. Plasma membrane proteins were prepared at 2 d after agroinfiltration. Immunoblot analyses were performed using anti-pSer82 antibody, anti-St RBOHB antiserum, or anti-HA antibody. The chemiluminescent signals in the immunoblot analyses were detected using a CCD camera.
St CDPK5 Is Responsible for St RBOHB Kinase Activity in Planta
To investigate whether St CDPK5 accounts for St RBOHB kinase activity in planta, we performed VIGS of St CDPK5 homologs in N. benthamiana. The cDNA fragment of Nb CDPK5 was isolated from N. benthamiana on the basis of the St CDPK5 sequence. The expression of the St CDPK4 homolog (Nb CDPK4) could be repressed by the VIGS of Nb CDPK5 because of the close identity between these sequences (see Supplemental Figure 6 online). RT-PCR with the universal primers for Nb CDPK4/5 showed that VIGS of Nb CDPK5 efficiently suppressed the accumulation of transcripts for Nb CDPK4/5 (Figure 9A). Ca2+-dependent St RBOHB kinase activity was partially reduced in the extracts from the Nb CDPK4/5–silenced leaves (Figures 9B and 9C). This indicates that Nb CDPK4/5 is responsible for St RBOHB kinase activity in planta and that other CDPKs are capable of St RBOHB phosphorylation in addition to Nb CDPK4/5.
Figure 9.
St RBOHB Kinase Activity Is Partially Dependent on Nb CDPK4/5.
CDPK-silenced leaves were treated with 10 μg/mL INF1 elicitin for 12 h.
(A) Gene silencing of Nb CDPK4/5 was monitored by RT-PCR using universal primers for Nb CDPK4 and Nb CDPK5.
(B) Total proteins were extracted and incubated with St RBOHB1-318 and [γ-32P]ATP with Ca2+ or EGTA (Ca2+ −). Phosphorylation was detected by x-ray film (top panel). Protein loads were determined by CBB staining (bottom panel).
(C) Incorporation of radioactivity into the N-terminal region of St RBOHB was measured using a phosphor imager. Relative kinase activities are expressed as percentage of the maximum activity.
DISCUSSION
ROS are generated by RBOH during defense responses in plants. Pharmacological studies indicated that protein kinase and Ca2+ influx are important in the oxidative burst (Figure 1A). Earlier works indicate that ectopic expression of the CDPK induces the oxidative burst (Xing et al., 2001). Together, these results implied the involvement of CDPK in the activation process of RBOH. Plants likely have no regulatory subunit homologs of phagocyte NADPH oxidase with the exception of gp91phox and Rac. Because RBOH has an N-terminal extension in addition to the gp91phox region, the N terminus is expected to play an important role in the regulation of RBOH. Considering all of the above, we hypothesized that the N-terminal phosphorylation by CDPK regulates the activity of RBOH proteins. Here, we demonstrated that St CDPK4 and St CDPK5 phosphorylate Ser-82 and Ser-97 of the St RBOHB N terminus by LC-MS/MS analysis. Both CDPKs phosphorylate St RBOHB in vitro; however, St CDPK5 was more functional in ROS generation from the transient expression analyses of constitutively active CDPKs in N. benthamiana leaves. We also demonstrated that heterologous expression of the active St CDPK5VK and St RBOHB in Nb RBOHB–silenced N. benthamiana plants caused a massive oxidative burst. Although immunoblot analyses using anti-phosphopeptide antibody indicated in vivo phosphorylation of Ser-82, in agreement with a second burst (Figure 3), replacement of Ser-82 and Ser-97 with Ala in the heterologous expression analysis of St RBOHB demonstrated the importance of phosphorylation of both Ser-82 and Ser-97 for the activation of St RBOHB protein (Figure 7).
Most Arabidopsis CDPKs possess predicted acylation sites such as myristoylation and palmitoylation (Cheng et al., 2002). Several CDPKs are reported to associate with various membranes, and the N-terminal acylation is required for their correct localization (Martín and Busconi, 2000; Lu and Hrabak, 2002; Rutschmann et al., 2002; Dammann et al., 2003). St CDPK5 also has a Gly residue at the second position and a Cys residue at the fifth position, which are predicted myristoylation and palmitoylation sites, respectively. Because St RBOHB localizes to the plasma membrane (Kobayashi et al., 2006), St CDPK5 is expected to function there. Although N-terminal acylation of St CDPK5 is unclear, ectopically expressed St CDPK5VK proteins were detected in the plasma membrane fraction (Figure 8), and we obtained the intriguing result that amino acid substitution of Gly-2 or Cys-5 for Ala eliminates ROS production by St CDPK5VK (our unpublished data). These findings suggest that plasma membrane targeting by N-terminal myristoylation and palmitoylation is required for St RBOHB activation by St CDPK5.
Potential Phosphorylation Motifs of CDPKs in the St RBOHB N-Terminal Region
Although limited information is available about substrate specificity and phosphorylation motifs of CDPKs, several attempts have been made to define specific motifs using synthetic peptides as substrates (Huang et al., 2001; Huang and Huber, 2001). Ser-82 applies to the motif [B-B-X-B]-φ-X-X-X-X-S/T-X-B (where S/T is the phosphorylated Ser/Thr, B is a basic residue, φ is a hydrophobic residue, and X is any residue), while Ser-82 lacks [B-B-X-B] at the N-terminal side of the phosphorylation site (Huang et al., 2001). Ser-97 applies to the motif φ-X-B-X-X-S-X-X-X-φ (Huang and Huber, 2001). These revealed that both Ser-82 and Ser-97 are potential phosphorylation sites for the CDPKs. Failure of detection by anti-pSer97 antibody might be caused by the protein conformation around Ser-97 so that the antibody might not be able to approach the site.
Substitution of Ser-82 and Ser-97 by Ala drastically diminished St RBOHB activity (Figure 7B); however, substitution by Asp produced only a small change in ROS production (data not shown). It is possible that substitution by Asp does not always result in conditions favorable to phosphorylation. For other reasons, it is thought that additional phosphorylation sites or other cofactors might be required for full activity of St RBOHB. In this study, although the mutated St RBOHB (S82A/S97A) produced <30% of wild-type St RBOHB activity, there was still some activity compared with the vector control (Figure 7B). There might be additional phosphorylation sites that are not identified by in vitro phosphorylation experiments.
Are Other RBOH Proteins Regulated by Phosphorylation?
Treatment with HWC elicitor or inoculation with the avirulent race of P. infestans induces biphasic oxidative burst in potato tubers, and St RBOHA and St RBOHB are suggested to function in the first and second bursts, respectively (Yoshioka et al., 2001). St CDPK5 might be involved in the regulation of the first burst as well as the second, because St CDPK5 phosphorylates both St RBOHA and St RBOHB (see Supplemental Figure 3A online). Does St CDPK5 activate not only St RBOHB but also other St RBOH proteins? RBOHs can be divided into two groups according to amino acid identity of the whole protein. One group (group I in Figure 7A) includes St RBOHB to RBOHD, Nb RBOHB, Nt RBOHD, and At RBOHD; the other group (group II in Figure 7A) includes St RBOHA and Nb RBOHA. RBOH genes in group I are induced by pathogen signals, while group II genes are expressed constitutively. Amino acid sequences around Ser residues corresponding to Ser-82 and Ser-97 are conserved in group I but not in group II (Figure 7A). According to the in vitro kinase assay using the N-terminal proteins of St RBOHs and Nb RBOHs (see Supplemental Figure 3 online), St RBOHB to RBOHD and Nb RBOHB were phosphorylated more intensively than St RBOHA and Nb RBOHA. Considering these findings, St CDPK5 likely prefers to regulate group I RBOHs. However, our preliminary experiments indicated that heterologous coexpression of not only St RBOHB but also St RBOHA, RBOHC, and RBOHD with the active St CDPK5VK in N. benthamiana plants caused the oxidative burst (our unpublished data). These results are consistent with the fact that St CDPK5VK–mediated ROS production was partially reduced by the VIGS of Nb RBOHA (Figure 6A). St RBOHA, Nb RBOHA, and St RBOHD do not have the conserved amino acid sequence around Ser-97 similar to St RBOHB; however, we found a CDPK phosphorylation motif similar to that of Ser-82 (Figure 7A) and another motif, B-X-X-S, the simplest CDPK phosphorylation motif (Roberts and Harmon, 1992), in their N-terminal regions.
The B-X-X-S motif is also targeted by the SNF1-related protein kinase (SNRK) family. In kinase assays using synthetic peptides as substrates, SNRK1 recognized φ-X-B-X-X-S-X-X-X-φ (Dale et al., 1995). Kelner et al. (2004) reported that the tobacco SNRK2 Nt OSAK recognized the sequence motif [KR]-[QMTAS]-X-[ST]-[VILMF]-[SQN]-[FLIRK] predicted by the program PREDIKIN developed for the prediction of substrate specificity of protein Ser/Thr kinases (Brinkworth et al., 2003). ABA-activated SAPK10, rice SNRK2, phosphorylates TRAB1 at the R-Q-G-S-L-T-L-P and R-T-L-S-V-K-T-V-D-E-V-W-R motifs (Kobayashi et al., 2005). Considering the data from these reports, plant SNRK family enzymes might recognize similar motifs, including at least B-X-X-S. Recently, Arabidopsis SNRK2.6/OST1/SRK2E was reported to be activated by ABA treatment and to function during stomatal closure (Mustilli et al., 2002; Yoshida et al., 2002). Interestingly, ROS generated by At RBOHD and At RBOHF in guard cells trigger stomatal closure followed by ABA treatment (Kwak et al., 2003). Mustilli et al. (2002) also suggested that OST1 acts at the interval between ABA perception and ROS production. Recently, it was reported that Arabidopsis CDPK genes, CPK6 and CPK3, are expressed in guard cells and mediate stomatal closure and S-type anion- and Ca2+-permeable channels following ABA treatment (Mori et al., 2006). These reports support the idea that SNRK2 and CDPK may regulate RBOH during ABA-mediated stomatal closure.
Mitogen-Activated Protein Kinase and CDPK Play Central Roles in the Oxidative Burst
Protein kinases are activated during plant defense responses (Peck, 2003). The mitogen-activated protein kinase (MAPK) cascade transduces extracellular stimuli to intracellular signals by phosphorylation cascades (MAPK Group, 2002). Previously, it was reported that Nt MEK2DD, a constitutively active mutant of Nt MEK2, a tobacco MAPK kinase, induced HR-like cell death and ROS production (Ren et al., 2002). Transient expression of St MEK1DD (St MEK1 is a potato ortholog of Nt MEK2) also induced HR-like cell death and ROS production in N. benthamiana leaves (Katou et al., 2003, 2005). The transient expression of St MEK1DD increased the accumulation of Nb RBOHB mRNA (Yoshioka et al., 2003), suggesting that the transcriptional activation of RBOH is one of the functions of the MAPK cascade in ROS production. We show here that St CDPK5VK activates RBOH-dependent ROS production by direct phosphorylation of their N-terminal regions. Together, MAPK and CDPK seem to play central roles in the regulation of pathogen-responsive RBOH at the transcriptional and posttranscriptional levels, respectively. However, VIGS of the St CDPK5 homolog in N. benthamiana partially repressed St RBOHB kinase activity in planta (Figure 9) and did not suppress the INF1-mediated ROS production (data not shown), possibly because of gene redundancy of CDPK. Two CDPKs were cloned in the experiments; however, other related CDPKs, such as Nt CDPK2 (described below), also might phosphorylate the RBOHs. In fact, no CDPK knockout mutant that loses resistance to pathogens has been identified in Arabidopsis.
Based upon amino acid sequence, 34 Arabidopsis CDPKs can be divided into four subgroups (Cheng et al., 2002) (see Supplemental Figure 2 online). Both Nt CDPK2 and St CDPK5 belong to subgroup I; however, sequence identity between St CDPK5 and Nt CDPK2 is only 62.4% (see Supplemental Figure 1 online). In tobacco cells expressing Cf-9, a resistance gene against the tomato pathogen Cladosporium fuluvum, a 68/70-kD CDPK was activated in response to the corresponding avirulence product Avr9 (Romeis et al., 2000). Nt CDPK2 expressed in N. tabacum and N. benthamiana leaves was activated by hypoosmotic stress and Cf-9/Avr9–dependent elicitation (Romeis et al., 2001). Furthermore, the transient expression of the constitutively active mutant of Nt CDPK2 in N. benthamiana leaves induced ROS production, defense genes, and HR-like cell death against additional abiotic stresses such as water injection and wounding (Ludwig et al., 2005). VIGS of Nt CDPK2 or Nt CDPK3 compromises Cf-9/Avr9–dependent cell death (Romeis et al., 2001). These reports suggest that Nt CDPK2 has multiple functions leading to cell death. On the other hand, St CDPK5 could function specifically in RBOH activation, and the transient expression of St CDPK5VK did not show any HR-like cell death phenotype.
Calcium May Function Not Only as an Inducer but Also Downstream of the Oxidative Burst
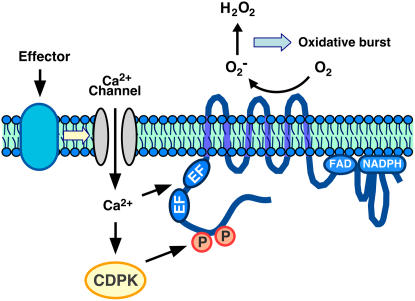
A model for St RBOHB activation mediated by St CDPK5 is shown in Figure 10. Cells stimulated by the elicitors seem to activate plasma membrane Ca2+ channels, and the increase in [Ca2+]cyt may contribute to the oxidative burst. Recently, it was reported that overexpression of Os TPC1, a putative voltage-gated Ca2+-permeable channel, which localizes to the plasma membrane, enhances an elicitor-induced oxidative burst (Kurusu et al., 2005). That report suggests that the Ca2+ ions bind directly to EF-hand motifs of RBOH and also activate CDPK to phosphorylate the St RBOHB.
Figure 10.
Model for St RBOHB Regulation by CDPK.
The elicitor induces Ca2+ influx. Increase of intracellular Ca2+ concentration provokes Ca2+ binding to EF-hand motifs of CDPK and the RBOH N-terminal region. Phosphorylation of St RBOHB by CDPK results in ROS production.
Calcium may function not only as an inducer of the oxidative burst but also as a signaling molecule downstream of the oxidative burst. ROS generated at the plasma membrane are presumably released into the apoplast and activate the Ca2+ channel to increase the level of cytosolic [Ca2+]. It has been shown that Ca2+ influx induced by cryptogein in Nicotiana plumbaginifolia cells activates the NADPH oxidase, which, in turn, leads to an increase in [Ca2+]cyt, mainly from extracellular Ca2+ influx (Lecourieux et al., 2002). In stomatal closure, At RBOHF and At RBOHD expression is induced by ABA in guard cells, and the resulting ROS activate the plasma membrane Ca2+ channel (Pei et al., 2000).
The small GTPase Rop is also considered to be an RBOH activator in ROS production, because a constitutively active Rac induces ROS generation (Kawasaki et al., 1999) and a RhoGTPase GDP dissociation inhibitor controls spatial ROS production during root hair development (Carol et al., 2005). The constitutively active Rac protein induces ROS production and HR-like cell death; however, the detailed mechanisms of Rac remain to be elucidated. Recently, direct interaction between the constitutively active Os Rac protein and the N terminus of Os RBOHs or St RBOHB was verified by yeast two-hybrid analyses (H.L. Wong, R. Pinontoan, K. Hayashi, R. Tabata, T. Yaeno, K. Hasegawa, C. Kojima, H. Yoshioka, K. Iba, T. Kawasaki, and K. Shimamoto, unpublished data). It will be interesting to see further studies on the relationship between Rac–RBOH interaction and the phosphorylation of Ser-82 and Ser-97.
METHODS
Plant Materials and Treatments
Tubers of potato (Solanum tuberosum cv Rishiri) were stored at 4°C until use. The tuber discs, 2.0 cm in diameter and 2 mm thick, were aged for 24 h and treated with 100 μL of various solutions. HWC elicitor was prepared from mycelium of Phytophthora infestans as described previously (Doke and Tomiyama, 1980). P. infestans (race 0) was maintained on susceptible potato (cv Irish Cobbler) tubers, and suspensions of Phytophthora zoospores were prepared as described previously (Yoshioka et al., 2003). Potato tuber discs were inoculated with 104 zoospores or treated with 1 mg/mL HWC elicitor per disc. Nicotiana benthamiana plants were grown in environmentally controlled growth cabinets under light conditions at 25°C. The P. infestans elicitin INF1 was prepared as described by Kamoun et al. (1997) and Schenke et al. (2005). N. benthamiana leaves were treated with INF1 by infiltration using a needleless syringe.
Preparation of Protein Extracts
Potato tuber discs were ground for 1 min with a blender in extraction buffer (50 mM MOPS-KOH, pH 7.6, 0.5 M sorbitol, 20 mM 2-mercaptoethanol, 5 mM EGTA, 5 mM EDTA, 0.1 M NaF, 1 mM Na3VO4, and 50 mM β-glycerophosphate) containing 0.1 mM 4-(2-aminoethyl) benzenesulfonyl fluoride (AEBSF), 1 μM E-64, and 10% Polyclar VT. The homogenate was filtered through four layers of gauze. The filtrate was centrifuged at 10,000g for 15 min at 4°C, and the resulting supernatant was centrifuged at 150,000g for 20 min at 4°C. The soluble fraction was used for soluble proteins. The pellet was suspended in suspension buffer (20 mM Tris-HCl, pH 7.5, 0.25 M sucrose, 5 mM 2-mercaptoethanol, 10 mM NaF, 1 mM Na3VO4, 10 mM β-glycerophosphate, 0.1 mM AEBSF, and 1 μM E-64). The suspension was centrifuged at 150,000g for 20 min at 4°C, and the pellet was suspended in suspension buffer. The suspension was used for microsomal proteins.
Total protein extracts from N. benthamiana leaves were prepared as follows. Leaves were ground in liquid nitrogen with pestle and mortar, thawed in extraction buffer containing 1% protease inhibitor cocktail for plant cell and tissue extracts (Sigma-Aldrich) and 0.5% Triton X-100, and centrifuged at 14,000g for 10 min at 4°C. The supernatant was used for total protein extracts.
Microsomal proteins from N. benthamiana leaves were prepared as follows. Leaves were ground with pestle and mortar in extraction buffer containing 0.1 mM AEBSF and 1 μM E-64, filtered through four layers of gauze, and centrifuged at 10,000g for 15 min at 4°C. The supernatant was centrifuged at 150,000g for 20 min at 4°C. The pellet was suspended in suspension buffer, and the suspension was used for microsomal proteins. Plasma membrane–rich fraction was fractionated by the aqueous two-phase partitioning method described previously (Yoshida et al., 1983). Phase preparation were performed in a 10-g phase system with a final composition of 6.0% (w/w) dextran T500 (GE Healthcare), 6.0% (w/w) polyethylene glycol 3350 (Sigma-Aldrich), 0.25 M sucrose, 28.8 mM NaCl, and 10 mM potassium phosphate, pH 7.5. The final upper phase from two rounds of partitioning was diluted fourfold in suspension buffer and centrifuged at 150,000g for 20 min at 4°C. The pellet was suspended in suspension buffer, and the suspension was used for plasma membrane proteins.
The protein concentration was determined using the Protein Assay Dye Reagent (Bio-Rad Laboratories) with BSA as a standard.
In-Gel Kinase Assay
In-gel kinase assays were performed as described previously (Katou et al., 1999). Briefly, 20 μg of potato soluble proteins was separated on a 12% SDS-polyacrylamide gel in the presence of 0.25 mg/mL St RBOHB N-terminal recombinant proteins. After electrophoresis, SDS was removed by washing the gel in 20 mM Tris-HCl, pH 8.0, containing 20% 2-propanol four times for 30 min each. After washing in buffer A (20 mM Tris-HCl, pH 8.0, and 5 mM 2-mercaptoethanol) twice for 30 min each, the proteins were denatured in buffer A containing 6 M guanidine hydrochloride twice for 30 min each. The proteins were renatured overnight at 4°C by incubating the gel in buffer A containing 0.03% Tween 20 with four changes of the solution. After equilibration in 20 mM HEPES-KOH, pH 7.6, 10 mM MgCl2, and 5 mM 2-mercaptoethanol with 1 mM CaCl2 or 0.1 mM EGTA, the gel was incubated in the same buffer containing 25 μM ATP and 0.5 μCi/mL [γ-32P]ATP (4000 Ci/mmol) for 1 h at room temperature. The reaction was stopped by washing the gel in 5% trichloroacetic acid and 1% sodium pyrophosphate. The gel was washed extensively with this solution, washed in 20% methanol, dried under vacuum, and autoradiographed with an intensifying screen.
Antibody Production and Immunoblotting
Preparation of anti-St RBOHB N-terminal antiserum was described previously (Kobayashi et al., 2006). The peptide for pSer82 (CGSQLS[PO3H2]FHLR) was synthesized and conjugated to keyhole limpet hemocyanin carrier, and polyclonal antiserum was raised in rabbits (Immunobion). The pSer82-specific antibody was purified by affinity chromatography using pSer82 peptide–coupled Cellufine Formyl. The eluate was then passed through the affinity column coupled with nonphosphorylated peptide. HA-tagged proteins were detected by monoclonal anti-HA antibody (clone HA-7; Sigma-Aldrich).
For immunoblotting, equal amounts of proteins were separated on a SDS-polyacrylamide gel and transferred to a nitrocellulose membrane (Schleicher and Schuell). After blocking in TBS-T (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.05% Tween 20) with 5% nonfat dry milk for 1 h at room temperature or overnight at 4°C, the membranes were incubated with anti-St RBOHB, anti-pSer82, or anti-HA antibodies diluted with TBS-T at room temperature for 1 h or at 4°C overnight. After washing with TBS-T, the membranes were incubated with horseradish peroxidase–conjugated anti-rabbit Ig or anti-mouse Ig antibody (GE Healthcare) diluted with TBS-T for 30 min at room temperature. Antibody–antigen complex was detected using the ECL protein gel blot detection kit (GE Healthcare) and Light-Capture equipped with a CCD camera (ATTO), and immunostained bands were analyzed by the CS Analyzer 2.1 (ATTO).
Expression Cloning of St RBOHB Kinases
Screening of kinases that phosphorylate St RBOHB at Ser-82 was performed according to Matsuo et al. (2001). A 477-bp cDNA fragment from the St RBOHB N terminus was cloned into BamHI/XhoI sites of pGEX-4T-1 vector (GE Healthcare). The plasmid was transformed into Escherichia coli strain LE392 cells. Overnight culture was harbored by centrifugation and resuspended with a half-volume of SM buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 10 mM MgSO4, and 0.01% gelatin) for infection with expression libraries from potato tubers (Yoshioka et al., 2001). After 3 h of incubation of the infected bacteria at 42°C, St RBOHB N-terminal protein and library-originated proteins were expressed in the host bacteria by overlaying the membrane (Protran BA 85; Schleicher and Schuell), which had been presoaked in 20 mM isopropylthio-β-galactoside (IPTG), onto the plates at 37°C for an additional 4 h. The membranes were washed twice for 5 min with TBS-T and blocked with 5% nonfat dry milk in TBS-T at room temperature for 1 h. The membranes were incubated in anti-pSer82 antibody diluted with TBS-T at 4°C overnight. The membranes were washed with TBS-T five times for 5 min, incubated with horseradish peroxidase–conjugated anti-rabbit Ig (GE Healthcare) diluted with TBS-T at room temperature for 1 h, and washed in TBS-T five times for 5 min. Antibody–antigen complex was detected using the ECL protein gel blot detection kit (GE Healthcare).
RNA Isolation and RT-PCR
Total RNA from N. benthamiana leaves was prepared using the TRIzol reagent according to the procedure described by the manufacturer (Invitrogen). RT-PCR was conducted using a commercial kit (rTth DNA Polymerase RT-PCR High Plus; TOYOBO). cDNA was synthesized from total RNA (1 μg). After the cDNA synthesis reaction, PCR was performed with a denaturing temperature of 94°C for 1 min and an annealing plus extension temperature of 52°C for 1.5 min for 26 to 35 cycles. Gene-specific primers of each sequence were as follows: Nb RBOHA (5′-CTGCTCAGGGCTGACGAAAACACCAAGAAA-3′ and 5′-AGTACTCTTTCTCCTTTTCGGAGAAATCTT-3′), Nb RBOHB (5′-TTTCTCTGAGGTTTGCCAGCCACCACCTAA-3′ and 5′-GCCTTCATGTTGTTGACAATGTCTTTAACA-3′). As a control for equal cDNA amounts in each reaction, PCR was performed with primers for EF-1α (5′-CTTCCTACCTCAAGAAGGTAGGATACAAC-3′ and 5′-TGCCTCCTGAAGAGCTTCGTGGTGCAT-3′).
In the case of transcript amplification of ectopically expressed genes by agroinfiltration, cDNA was synthesized with oligo(dT) primer to distinguish plant RNA from bacterial RNA. RT-PCR was conducted using a commercial kit (ReverTra Ace α; TOYOBO). PCR was performed by ExTaq (TaKaRa) with denaturing, annealing, and extension temperatures of 94°C for 30 s, 52°C for 30 s, and 72°C for 30 s, respectively, for 24 to 35 cycles. PCR was performed using primers annealed to vector sequence and gene-specific primers. The sequences were as follows: St CDPK4 (5′-AGAGGACACGCTCGAGTATAAGAGCTCATT-3′ and 5′-GAGATTGATTTACAGGCATACTCAGCACC-3′), St CDPK5 (5′-AGAGGACACGCT-CGAGTATAAGAGCTCATT-3′ and 5′-CTTAGAGATGAGTTTTCTCTTGGCAATA-3′), St RBOHB (5′-TCAATCTGTGCAGGACTCTTTACATGGAAA-3′ and 5′-CTGCTATTCCAAATGCAATTACCTTGTGGA-3′), Nb RBOHA (described above). The universal primers for Nb CDPK4 and Nb CDPK5 were designated Nb CDPK4/5 (5′-TCTCAAACCTGAGAATTTCTTGTTGGTTAA-3′ and 5′-TCAAAATCAATGTGCCCTTTCAGA-3′). As a control for equal cDNA amounts in each reaction, PCR was performed with primers for EF-1α (described above). PCR products were separated on a 1.8% agarose gel and visualized after ethidium bromide staining.
Agrobacterium tumefaciens–Mediated Transient Expression (Agroinfiltration) in N. benthamiana
cDNA fragments of full-length and truncated (VK) variants of St CDPK4 and St CDPK5 were generated by PCR and cloned into pGreen binary vector (Hellens et al., 2000). Amino acid substitution of kinase-inactivated variants (K/M) was introduced by PCR-based, site-directed mutagenesis. HA tag was added to the C-terminal ends. All variants of CDPK were preceded by the 35S promoter of Cauliflower mosaic virus, the 5′ untranslated region was replaced with the ω sequence from Tobacco mosaic virus, and the nopaline synthase terminator region was on the 3′ end of the gene. cDNA fragments of St RBOHB and mutated St RBOHB (S82A, S97A, and S82A/S97A) were generated by PCR and cloned into pGD binary vector (Goodin et al., 2002). These amino acid substitutions were introduced by PCR-based, site-directed mutagenesis.
The binary plasmids were transformed into Agrobacterium strain GV3101, which harbors the transformation helper plasmid pSoup (Hellens et al., 2000), by electroporation. The overnight culture was diluted in 10-fold Luria-Bertani medium (LB)/kanamycin/rifampicin/tetracycline and cultured until OD600 was 0.6. Cells were harvested by centrifugation and resuspended in 10 mM MES-NaOH, pH 5.6, and 10 mM MgCl2. Suspensions were adjusted to an OD600 of 0.5, and acetosyringone was added to a final concentration of 150 μM. Bacterial suspensions were incubated for 2 to 3 h at 22°C and then infiltrated into leaves of 4- to 5-week-old N. benthamiana plants using a needleless syringe (Romeis et al., 2001). Two days after infiltration, leaves were subjected to various experiments. Agrobacterium carrying GUS was used as a control for the effect of agroinfiltration.
VIGS
VIGS was performed according to Ratcliff et al. (2001). The 500-bp cDNA fragments of the paralog-specific region of Nb RBOHA and Nb RBOHB were subcloned into TRV vector pTV00 (RNA2). The following primers were used to amplify cDNA fragments from plasmid containing each full-length cDNA as a template: Nb RBOHA (5′-CCCAAGCTTCTGTGATTAACATTGACGCTC-3′ and 5′-CGGGATCCGCATTGTGCGAAATCGGAAC-3′) and Nb RBOHB (5′-CCATCGATGTTAAACAAACGAGGCGGCA-3′ and 5′-CCCAAGCTTTACATTCTCCAAATTTGGCAC-3′). A 450-bp cDNA fragment of the C-terminal region of Nb CDPK5 was amplified from a N. benthamiana cDNA expression library (Yoshioka et al., 2003) as a template with the following primers: Nb CDPK5 (5′-CCATCGATACATGGATTTCCTTTTTGCAT-3′ and 5′-CGGGATCCGTGATCGCTGAAAGCTTGTC-3′). Virus infections on N. benthamiana were achieved by Agrobacterium-mediated transient expression of infectious constructs. pBINTRA6 (RNA1) and pTV00 containing the inserts (RNA2) were transformed into Agrobacterium strain GV3101, which harbors the transformation helper plasmid pSoup, by electroporation separately. A mixture of equal parts of Agrobacterium suspensions of RNA1 and RNA2 was infiltrated into N. benthamiana seedlings (2 to 3 weeks old). Infected plants were grown under a 16-h photoperiod and an 8-h dark period at 22°C. Three to 4 weeks after infection, upper leaves of the infected plants were used for transient expression assays.
Plasmid Constructs for Recombinant Proteins
The PCR fragment of the St RBOHB N terminus was inserted into the NcoI/XhoI or BamHI/XhoI sites of pET-30a(+) vector (Novagen). The following primers were used: St RBOHB (1-318) (5′-CCATGGCTATGGAGATCGAAAACACGAGGG-3′ and 5′-CTCGAGCTAGTCCTCAATGAAATAGTCTAGCCTT-3′), Δ1-56-forward (5′-CGCGGATCCATGGGAGCTGATCATGAAGC-3′), Δ1-104-forward (5′-CGCGGATCCATGGATAGGAGTAAGTCTGG-3′), Δ1-159-forward (5′-CGCGGATCCATGTCAAGTGAGTTTGCTGAG-3′), Δ1-56-, Δ1-104-, and Δ1-159-reverse (5′-CCGCTCGAGCTAGTCCTCAATGAAATAGTC-3′). Amino acid substitutions were introduced by Mutan-Super Express Km site-directed mutagenesis (TaKaRa) using the following primers: S82A (5′-TGGATCACAACTTGCGTTTCATCTGAG-3′), S89A (5′-AGGCAAGTTGCGAAGGAATTG-3′), and S97A (5′-AAAGAATGACTGCTTCTAATAAGTTTCAG-3′). The PCR fragments of St CDPK4 and St CDPK5 were inserted into the KpnI/XhoI sites of the pET-32a(+) vector (Novagen).
Expression and Purification of Recombinant Proteins
pET30a(+) vectors containing St RBOHB N-terminal fragments were transformed into E. coli cells of strain BL21-CodonPlus (DE3)-RIL (Stratagene). The overnight culture at 37°C was transferred to 100-fold LB/kanamycin medium and cultured until OD600 was 0.6 to 0.8 at 37°C. Expression was induced by the addition of 1 mM IPTG for 3 h at 37°C. Cells were harbored and resuspended in buffer B (20 mM Tris-HCl, pH 8.0, and 0.5 M NaCl). The suspension was sonicated and centrifuged at 12,000g for 10 min at 4°C. The pellet was resuspended in buffer B containing 6 M urea and centrifuged at 12,000g for 10 min at 4°C. The supernatant was dialyzed against 10 mM Tris-HCl, pH 8.0.
pET32a(+) vectors containing St CDPK4 and St CDPK5 were transformed into E. coli cells of strain BL21-Gold (DE3) pLysS (Stratagene). The overnight culture at 37°C was transferred to 100-fold LB/ampicillin medium and cultured until OD600 was 0.6 to 0.8 at 37°C. Expression was induced by the addition of 0.4 mM IPTG for 3 h at 28°C. Extraction and purification of CDPK proteins and Trx proteins were performed with the MagneHis protein purification system (Promega).
In Vitro Kinase Assay
Kinase activity was determined in 15 μL of buffer (20 mM HEPES-KOH, pH 7.6, 1 mM 2-mercaptoethanol, and 5 mM MgCl2, with 0.1 mM CaCl2 or 2 mM EGTA) containing 1 to 3 μg of substrate, St RBOHB N-terminal peptides, or histone III-S (Sigma-Aldrich) and 0.25 to 1 μg of enzyme. Reactions were started by the addition of 50 μM ATP and 50 μCi/mL [γ-32P]ATP for 30 min at room temperature. In kinase assays for plant extracts, 1.5 μg of St RBOHB N-terminal peptides and 0.3 μg of plant extracts were used and incubated for 5 min at room temperature. The reactions were stopped by the addition of SDS-PAGE sample buffer and incubation at 95°C for 5 min. Each sample was separated on a 10% SDS-polyacrylamide gel. After electrophoresis, the gels were washed in 5% trichloroacetic acid and 1% sodium pyrophosphate for 15 min followed by washing in 20% methanol. The gels were dried under vacuum and autoradiographed with an intensifying screen. The relative radiation dose was counted by BAS-5000 (Fujifilm).
Mass Spectrometric Analysis
Protein bands excised from Sypru ruby (Invitrogen)–stained gels were excised and subjected to in-gel digestion (Shevchenko et al., 1996; Wilm and Mann, 1996). The trypsin-digested peptides were loaded on the column (NanoEase Atlantis dc18, 3 μm, 75 μm internal diameter, 15 cm; Waters) using the CapLC system (Waters), and the eluted peptides from the column were introduced directly into a nanoESI-Qq-TOF Ultima mass spectrometer (Waters-Micromass). The top two ions in each survey scan were subjected to automatic MS/MS fragmentation analysis in low-energy collision-induced dissociation. The MS/MS spectra were subjected to the MASCOT server (search parameter, variable modifications, Ser/Thr phosphorylation) against a protein database from the National Center for Biotechnology Information. The peak data of phosphopeptides were confirmed manually.
Determination of ROS
The relative intensity of ROS generation was principally determined by counting photons from luminol- or L-012–mediated chemiluminescence. In the case of potato tuber discs, 2.5 mM luminol in 10 mM Tris-HCl, pH 7.4, was treated on the discs. In the case of N. benthamiana leaves, 0.5 mM L-012 in 10 mM MOPS-KOH, pH 7.4, was infiltrated into N. benthamiana leaves using a needleless syringe. The chemiluminescence was monitored continuously by a photon image processor equipped with a sensitive CCD camera (ARGUS-50 or Aquacosmos 2.5; Hamamatsu Photonics). Photons were integrally incorporated for 5 min after the treatment.
Accession Numbers
Sequence data from this article have been deposited in the GenBank/DDBJ/EMBL data libraries under accession numbers AB279737 (St CDPK4) and AB279738 (St CDPK5). The accession numbers for the other sequences mentioned in this article are as follows: St RBOHA, AB050660; St RBOHB, AB050661; St RBOHC, AB198716; St RBOHD, AB198717; Nb RBOHA, AB079498; and Nb RBOHB, AB079499.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Alignment of the Predicted Amino Acid Sequences of St CDPK4, St CDPK5, Cm CPK2, Ca CDPK3, At CPK6, and Nt CDPK2.
Supplemental Figure 2. Phylogenetic Tree of Various Plant CDPKs.
Supplemental Figure 3. Phosphorylation of N-Terminal Proteins of St RBOHs and Nb RBOHs by Recombinant St CDPK4 and St CDPK5 Proteins in Vitro.
Supplemental Figure 4. ROS Production Mediated by St CDPK4 and St CDPK5 Full-Length Variants in N. benthamiana Leaves.
Supplemental Figure 5. Induction of Nb RBOHB Expression by Agrobacterium Infection and Agrobacterium-Mediated Nt MEK2DD Expression.
Supplemental Figure 6. Alignment of the Nucleotide Sequences of St CDPK4, St CDPK5, and Nb CDPK5.
Supplementary Material
Acknowledgments
We thank Jonathan D.G. Jones for pSLJ4K1 vector, Philip M. Mullineaux and Roger P. Hellens for providing pGreen binary vector, Andrew O. Jackson for pGD binary vector, David C. Baulcombe for pTV00 vector, Sophien Kamoun for INF1 elicitin, Yuko Ohashi for Nt MEK2WT, Nt MEK2KR, and Nt MEK2DD, and the Leaf Tobacco Research Center, Japan Tobacco, Inc., for N. benthamiana seeds. We also thank Jeff Dangl and Owen Rowland for critical reading and English correction of the manuscript, the members of the Radioisotope Research Center, Nagoya University, for technical assistance, and Yuhko Kobayashi for valuable suggestions. This work was supported in part by Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists, by a Grant-in-Aid for Scientific Research (S) (Grant 14104004) from the Ministry of Education, Science, and Culture of Japan and the 21st Century Center of Excellence Program from the Ministry of Education, Science, Sports, and Culture of Japan, and by a Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Hirofumi Yoshioka (hyoshiok@agr.nagoya-u.ac.jp).
Online version contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Amicucci, E., Gaschler, K., and Ward, J.M. (1999). NADPH oxidase genes from tomato (Lycopersicon esculentum) and curly-leaf pondweed (Potamogeton crispus). Plant Biol. 1 524–528. [Google Scholar]
- Bánfi, B., Molnár, G., Maturana, A., Steger, K., Hegedûs, B., Demaurex, N., and Krause, K.-H. (2001). A Ca2+-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 276 37594–37601. [DOI] [PubMed] [Google Scholar]
- Bánfi, B., Tirone, F., Durussel, I., Knisz, J., Moskwa, P., Molnár, G.Z., Krause, K.-H., and Cox, J.A. (2004). Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5). J. Biol. Chem. 279 18583–18591. [DOI] [PubMed] [Google Scholar]
- Bokoch, G.M., and Knaus, U.G. (2003). NADPH oxidases: Not just for leukocytes anymore! Trends Biochem. Sci. 28 502–508. [DOI] [PubMed] [Google Scholar]
- Brinkworth, R.I., Breinl, R.A., and Kobe, B. (2003). Structural basis and prediction of substrate specificity in protein serine/threonine kinases. Proc. Natl. Acad. Sci. USA 100 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol, R.J., Takeda, S., Linstead, P., Durrant, M.C., Kakesova, H., Derbyshire, P., Drea, S., Zarsky, V., and Dolan, L. (2005). A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature 438 1013–1016. [DOI] [PubMed] [Google Scholar]
- Chandra, S., and Low, P.S. (1997). Measurement of Ca2+ fluxes during elicitation of the oxidative burst in aequorin-transformed tobacco cells. J. Biol. Chem. 272 28274–28280. [DOI] [PubMed] [Google Scholar]
- Cheng, S.-H., Willmann, M.R., Chen, H.-C., and Sheen, J. (2002). Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol. 129 469–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross, A.R., and Segal, A.W. (2004). The NADPH oxidase of professional phagocytes—Prototype of the NOX electron transport chain systems. Biochim. Biophys. Acta 1657 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale, S., Wilson, W.A., Edelman, A.M., and Hardie, D.G. (1995). Similar substrate recognition motifs for mammalian AMP-activated protein kinase, higher plant HMG-CoA reductase-A, yeast SNF1, and mammalian calmodulin-dependent protein kinase I. FEBS Lett. 361 191–195. [DOI] [PubMed] [Google Scholar]
- Dammann, C., Ichida, A., Hong, B., Romanowsky, S.M., Hrabak, E.M., Harmon, A.C., Pickard, B.G., and Harper, J.F. (2003). Subcellular targeting of nine calcium-dependent protein kinase isoforms from Arabidopsis. Plant Physiol. 132 1840–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl, J.L., and Jones, J.D.G. (2001). Plant pathogens and integrated defense responses to infection. Nature 411 826–833. [DOI] [PubMed] [Google Scholar]
- Doke, N. (1983). Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol. Plant Pathol. 23 345–357. [Google Scholar]
- Doke, N., and Tomiyama, K. (1980). Effect of hyphal wall components from Phytophthora infestans on protoplasts of potato tuber tissues. Physiol. Plant Pathol. 16 169–176. [Google Scholar]
- Dupuy, C., Ohayon, R., Valent, A., Noël-Hudson, M.-S., Déme, D., and Virion, A. (1999). Purification of a novel flavoprotein involved in the thyroid NADPH oxidase: Cloning of the porcine and human cDNAs. J. Biol. Chem. 274 37265–37269. [DOI] [PubMed] [Google Scholar]
- Foreman, J., Demidchik, V., Bothwell, J.H.F., Mylona, P., Miedema, H., Torres, M.A., Linstead, P., Costa, S., Brownlee, C., Jones, J.D.G., Davies, J.M., and Dolan, L. (2003). Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422 442–446. [DOI] [PubMed] [Google Scholar]
- Goodin, M.M., Dietzgen, R.G., Schichnes, D., Ruzin, S., and Jackson, A.O. (2002). pGD vectors: Versatile tools for the expression of green and red fluorescent protein fusions in agroinfiltrated plant leaves. Plant J. 31 375–383. [DOI] [PubMed] [Google Scholar]
- Grant, M., Brown, I., Adams, S., Knight, M., Ainslie, A., and Mansfield, J. (2000). The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. Plant J. 23 441–450. [DOI] [PubMed] [Google Scholar]
- Groom, Q.J., Torres, M.A., Fordham-Skelton, A.P., Hammond-Kosack, K.E., Robinson, N.J., and Jones, J.D.G. (1996). rbohA, a rice homologue of the mammalian gp91phox respiratory burst oxidase gene. Plant J. 10 515–522. [DOI] [PubMed] [Google Scholar]
- Harmon, A.C., Gribskov, M., and Harper, J.F. (2000). CDPKs—A kinase for every Ca2+ signal? Trends Plant Sci. 5 154–159. [DOI] [PubMed] [Google Scholar]
- Harper, J.F., Breton, G., and Harmon, A. (2004). Decoding Ca2+ signals through plant protein kinases. Annu. Rev. Plant Physiol. Plant Mol. Biol. 55 263–288. [DOI] [PubMed] [Google Scholar]
- Harper, J.F., Huang, J.-F., and Lloyd, S.J. (1994). Genetic identification of an autoinhibitor in CDPK, a protein kinase with a calmodulin-like domain. Biochemistry 33 7267–7277. [DOI] [PubMed] [Google Scholar]
- Hellens, R.P., Edwards, E.A., Leyland, N.R., Bean, S., and Mullineaux, P.M. (2000). pGreen: A versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol. Biol. 42 819–832. [DOI] [PubMed] [Google Scholar]
- Huang, J.-Z., Hardin, S.C., and Huber, S.C. (2001). Identification of a novel phosphorylation motif for CDPKs: Phosphorylation of synthetic peptides lacking basic residues at P-3/P-4. Arch. Biochem. Biophys. 393 61–66. [DOI] [PubMed] [Google Scholar]
- Huang, J.-Z., and Huber, S.C. (2001). Phosphorylation of synthetic peptides by a CDPK and plant SNF1-related protein kinase. Influence of proline and basic amino acid residues at selected positions. Plant Cell Physiol. 42 1079–1087. [DOI] [PubMed] [Google Scholar]
- Kamoun, S., van West, P., de Jong, A.J., de Groot, K.E., Vleeshouwers, V.G.A.A., and Govers, F. (1997). A gene encoding a protein elicitor of Phytophthora infestans is down-regulated during infection of potato. Mol. Plant Microbe Interact. 10 13–20. [DOI] [PubMed] [Google Scholar]
- Kapiloff, M.S., Mathis, J.M., Nelson, C.A., Lin, C.R., and Rosenfeld, M.G. (1991). Calcium/calmodulin-dependent protein kinase mediates a pathway for transcriptional regulation. Proc. Natl. Acad. Sci. USA 88 3710–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katou, S., Senda, K., Yoshioka, H., Doke, N., and Kawakita, K. (1999). A 51 kDa protein kinase of potato activated with hyphal wall components from Phytophthora infestans. Plant Cell Physiol. 40 825–831. [Google Scholar]
- Katou, S., Yamamoto, A., Yoshioka, H., Kawakita, K., and Doke, N. (2003). Functional analysis of potato mitogen-activated protein kinase kinase, StMEK1. J. Gen. Plant Pathol. 69 161–168. [Google Scholar]
- Katou, S., Yoshioka, H., Kawakita, K., Rowland, O., Jones, J.D.G., Mori, H., and Doke, N. (2005). Involvement of PPS3 phosphorylated by elicitor-responsive mitogen-activated protein kinases in the regulation of plant cell death. Plant Physiol. 139 1914–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss, H., and Jeblick, W. (1995). Pretreatment of parsley suspension cultures with salicylic acid enhances spontaneous and elicited production of H2O2. Plant Physiol. 108 1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki, T., Henmi, K., Ono, E., Hatakeyama, S., Iwano, M., Satoh, H., and Shimamoto, K. (1999). The small GTP-binding protein Rac is a regulator of cell death in plants. Proc. Natl. Acad. Sci. USA 96 10922–10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, T., Damude, H.G., Werner, D., Doerner, P., Dixon, R.A., and Lamb, C. (1998). A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell 10 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelner, A., Pekala, I., Kaczanowski, S., Muszynska, G., Hardie, D.G., and Dobrowolska, G. (2004). Biochemical characterization of the tobacco 42-kD protein kinase activated by osmotic stress. Plant Physiol. 136 3255–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
Kobayashi, M., Kawakita, K., Maeshima, M., Doke, N., and Yoshioka, H. (2006). Subcellular localization of Strboh proteins and NADPH-dependent
 -generating activity in potato tuber tissues. J. Exp. Bot. 57 1373–1379. [DOI] [PubMed] [Google Scholar]
-generating activity in potato tuber tissues. J. Exp. Bot. 57 1373–1379. [DOI] [PubMed] [Google Scholar] - Kobayashi, Y., Murata, M., Minami, H., Yamamoto, S., Kagaya, Y., Hobo, T., Yamamoto, A., and Hattori, T. (2005). Abscisic acid-activated SNRK2 protein kinases function in the gene-regulation pathway of ABA signal transduction by phosphorylating ABA response element-binding factors. Plant J. 44 939–949. [DOI] [PubMed] [Google Scholar]
- Kurusu, T., Yagala, T., Miyao, A., Hirochika, H., and Kuchitsu, K. (2005). Identification of a putative voltage-gated Ca2+ channel as a key regulator of elicitor-induced hypersensitive cell death and mitogen-activated protein kinase activation in rice. Plant J. 42 798–809. [DOI] [PubMed] [Google Scholar]
- Kwak, J.M., Mori, I.C., Pei, Z.-M., Leonhardt, N., Torres, M.A., Dangl, J.L., Bloom, R.E., Bodde, S., Jones, J.D.G., and Schroeder, J.I. (2003). NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 22 2623–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte, J., and Doolittle, R.F. (1982). A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157 105–132. [DOI] [PubMed] [Google Scholar]
- Lamb, C., and Dixon, R.A. (1997). The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48 251–275. [DOI] [PubMed] [Google Scholar]
- Lambeth, J.D. (2004). NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4 181–189. [DOI] [PubMed] [Google Scholar]
- Lecourieux, D., Mazars, C., Pauly, N., Ranjeva, R., and Pugin, A. (2002). Analysis and effects of cytosolic free calcium increases in response to elicitors in Nicotiana plumbaginifolia cells. Plant Cell 14 2627–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, S.X., and Hrabak, E.M. (2002). An Arabidopsis calcium-dependent protein kinase is associated with the endoplasmic reticulum. Plant Physiol. 128 1008–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig, A.A., Romeis, T., and Jones, J.D.G. (2004). CDPK-mediated signalling pathways: Specificity and cross-talk. J. Exp. Bot. 55 181–188. [DOI] [PubMed] [Google Scholar]
- Ludwig, A.A., Saitoh, H., Felix, G., Freymark, G., Miersch, O., Wasternack, C., Boller, T., Jones, J.D.G., and Romeis, T. (2005). Ethylene-mediated cross-talk between calcium-dependent protein kinase and MAPK signaling controls stress responses in plants. Proc. Natl. Acad. Sci. USA 102 10736–10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAPK Group (2002). Mitogen-activated protein kinase cascades in plants: A new nomenclature. Trends Plant Sci. 7 301–308. [DOI] [PubMed] [Google Scholar]
- Martín, M.L., and Busconi, L. (2000). Membrane localization of a rice calcium-dependent protein kinase (CDPK) is mediated by myristoylation and palmitoylation. Plant J. 24 429–435. [DOI] [PubMed] [Google Scholar]
- Matsuo, R., Ochiai, W., Nakashima, K., and Taga, T. (2001). A new expression cloning strategy for isolation of substrate-specific kinases by using phosphorylation site-specific antibody. J. Immunol. Methods 247 141–151. [DOI] [PubMed] [Google Scholar]
- Miura, Y., Yoshioka, H., and Doke, N. (1995). An autophotographic determination of the active oxygen generation in potato tuber discs during hypersensitive response to fungal infection or elicitor. Plant Sci. 105 45–52. [Google Scholar]
- Mori, I.C., Murata, Y., Yang, Y., Munemasa, S., Wang, Y.-F., Andreoli, S., Tiriac, H., Alonso, J.M., Harper, J.F., Ecker, J.R., Kwak, J.M., and Schroeder, J.I. (2006). CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol. 4 1749–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustilli, A.-C., Merlot, S., Vavasseur, A., Fenzi, F., and Giraudat, J. (2002). Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14 3089–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck, S.C. (2003). Early phosphorylation events in biotic stress. Curr. Opin. Plant Biol. 6 334–338. [DOI] [PubMed] [Google Scholar]
- Pei, Z.M., Murata, Y., Benning, G., Thomine, S., Klüsener, B., Allen, G.J., Grill, E., and Schroeder, J.I. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406 731–734. [DOI] [PubMed] [Google Scholar]
- Piedras, P., Hammond-Kosack, K.E., Harrison, K., and Jones, J.D.G. (1998). Rapid, Cf-9- and Avr9-dependent, production of active oxygen species in tobacco suspension cultures. Mol. Plant Microbe Interact. 11 1155–1166. [Google Scholar]
- Ratcliff, F., Martin-Hernandez, A.M., and Baulcombe, D.C. (2001). Tobacco rattle virus as a vector for analysis of gene function by silencing. Plant J. 25 237–245. [DOI] [PubMed] [Google Scholar]
- Ren, D., Yang, H., and Zhang, S. (2002). Cell death mediated by MAPK is associated with hydrogen peroxide production in Arabidopsis. J. Biol. Chem. 277 559–565. [DOI] [PubMed] [Google Scholar]
- Roberts, D.M., and Harmon, A.C. (1992). Calcium-modulated proteins: Targets of intracellular calcium signals in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 43 375–414. [Google Scholar]
- Romeis, T., Ludwig, A.A., Martin, R., and Jones, J.D.G. (2001). Calcium-dependent protein kinases play an essential role in a plant defence response. EMBO J. 20 5556–5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis, T., Piedras, P., and Jones, J.D.G. (2000). Resistance gene-dependent activation of a calcium-dependent protein kinase in the plant defense response. Plant Cell 12 803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutschmann, F., Stalder, U., Piotrowski, M., Oecking, C., and Schaller, A. (2002). LeCPK1, a calcium-dependent protein kinase from tomato. Plasma membrane targeting and biochemical characterization. Plant Physiol. 129 156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi, M., and Fluhr, R. (2001). Superoxide production by plant homologues of the gp91phox NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol. 126 1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenke, D., Naito, K., Toyoda, K., Inagaki, Y., Shiraishi, T., and Ichinose, Y. (2005). Regulation of elicitin-induced ethylene production in suspension-cultured tobacco BY-2 cells. J. Gen. Plant Pathol. 71 273–279. [Google Scholar]
- Sheen, J. (1996). Ca2+-dependent protein kinases and stress signal transduction in plants. Science 274 1900–1902. [DOI] [PubMed] [Google Scholar]
- Shevchenko, A., Wilm, M., Vorm, O., and Mann, M. (1996). Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal. Chem. 68 850–858. [DOI] [PubMed] [Google Scholar]
- Simon-Plas, F., Elmayan, T., and Blein, J.-P. (2002). The plasma membrane oxidase NtrbohD is responsible for AOS production in elicited tobacco cells. Plant J. 31 137–147. [DOI] [PubMed] [Google Scholar]
- Sumimoto, H., Miyano, K., and Takeya, R. (2005). Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem. Biophys. Res. Commun. 338 677–686. [DOI] [PubMed] [Google Scholar]
- Takeya, R., Ueno, N., Kami, K., Taura, M., Kohjima, M., Izaki, T., Nunoi, H., and Sumimoto, H. (2003). Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J. Biol. Chem. 278 25234–25246. [DOI] [PubMed] [Google Scholar]
- Tanaka, A., Christensen, M.J., Takemoto, D., Park, P., and Scott, B. (2006). Reactive oxygen species play a role in regulating a fungus–perennial ryegrass mutualistic interaction. Plant Cell 18 1052–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, C.L., Jones, L., Baulcombe, D.C., and Maule, A.J. (2001). Size constraints for targeting post-transcriptional gene silencing and for RNA-directed methylation in Nicotiana benthamiana using a potato virus X vector. Plant J. 25 417–425. [DOI] [PubMed] [Google Scholar]
- Torres, M.A., and Dangl, J.L. (2005). Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr. Opin. Plant Biol. 8 397–403. [DOI] [PubMed] [Google Scholar]
- Torres, M.A., Dangl, J.L., and Jones, J.D.G. (2002). Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. USA 99 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres, M.A., Jones, J.D.G., and Dangl, J.L. (2005). Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat. Genet. 37 1130–1134. [DOI] [PubMed] [Google Scholar]
- Torres, M.A., Onouchi, H., Hamada, S., Machida, C., Hammond-Kosack, K.E., and Jones, J.D.G. (1998). Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox). Plant J. 14 365–370. [DOI] [PubMed] [Google Scholar]
- Wilm, M., and Mann, M. (1996). Analytical properties of the nanoelectrospray ion source. Anal. Chem. 68 1–8. [DOI] [PubMed] [Google Scholar]
- Xing, T., Wang, X.J., Malik, K., and Miki, B.L. (2001). Ectopic expression of an Arabidopsis calmodulin-like domain protein kinase-enhanced NADPH oxidase activity and oxidative burst in tomato protoplasts. Mol. Plant Microbe Interact. 14 1261–1264. [DOI] [PubMed] [Google Scholar]
- Yamamizo, C., Kuchimura, K., Kobayashi, A., Katou, S., Kawakita, K., Jones, J.D.G., Doke, N., and Yoshioka, H. (2006). Rewiring mitogen-activated protein kinase cascade by positive feedback confers potato blight resistance. Plant Physiol. 140 681–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, R., Hobo, T., Ichimura, K., Mizoguchi, T., Takahashi, F., Alonso, J., Ecker, J.R., and Shinozaki, K. (2002). ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol. 43 1473–1483. [DOI] [PubMed] [Google Scholar]
- Yoshida, S., Uemura, M., Niki, T., Sakai, A., and Gusta, L.V. (1983). Partition of membrane particles in aqueous two-polymer phase system and its practical use for purification of plasma membranes from plants. Plant Physiol. 72 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshie, Y., Goto, K., Takai, R., Iwano, M., Takayama, S., Isogai, A., and Che, F.-S. (2005). Function of the rice gp91phox homologs OsrbohA and OsrbohE genes in ROS-dependent plant immune responses. Plant Biotechnol. 22 127–135. [Google Scholar]
- Yoshioka, H., Numata, N., Nakajima, K., Katou, S., Kawakita, K., Rowland, O., Jones, J.D.G., and Doke, N. (2003). Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell 15 706–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka, H., Sugie, K., Park, H.-J., Maeda, H., Tsuda, N., Kawakita, K., and Doke, N. (2001). Induction of plant gp91 phox homolog by fungal cell wall, arachidonic acid, and salicylic acid in potato. Mol. Plant Microbe Interact. 14 725–736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.