Abstract
Modifier screens have been powerful genetic tools to define signaling pathways in lower organisms. The identification of modifier loci in mice has begun to allow a similar dissection of mammalian signaling pathways. Transgenic mice (Btklo) expressing 25% of endogenous levels of Bruton's tyrosine kinase (Btk) have B cell functional responses between those of wild-type and Btk−/− mice. We asked whether reduced dosage or complete deficiency of genes previously implicated as Btk regulators would modify the Btklo phenotype. We used two independent assays of Btk-dependent B cell function. Proliferative response to B cell antigen receptor cross-linking in vitro was chosen as an example of a relatively simple, well-defined signaling system. In vivo response to type II T-independent antigens (TI-II) measures complex interactions among multiple cell types over time and may identify additional Btk pathways. All modifiers identified differentially affected these two assays, indicating that Btk mediates these processes via distinct mechanisms. Loss of Lyn, PTEN (phosphatase and tensin homolog), or SH2-containing inositol phosphatase suppressed the Btklo phenotype in vitro but not in vivo, whereas CD19 and the p85α form of phosphoinositide 3-kinase behaved as Btklo enhancers in vivo but not in vitro. Effects of Lyn, PTEN, or p85α haploinsufficiency were observed. Haploinsufficiency or complete deficiency of protein kinase C β, Fyn, CD22, Gαq, or Gα11 had no detectable effect on the function of Btklo B cells. A transgenic system creating a reduction in dosage of Btk can therefore be used to identify modifier loci that affect B cell responses and quantitatively rank their contribution to Btk-mediated processes.
IIn vivo genetic analysis is a powerful approach to define the relative contribution of signaling interactions to physiological processes. Small genomes, ease of mutagenesis, and rapid generation time have made Drosophila melanogaster, Caenorhabditis elegans, and Saccharomyces cerevisiae the major models for genetic dissection of eukaryotic signaling pathways. Although there are some examples of large-scale genetic screens in mice (1–3), time, space, and financial resources generally limit mammalian genetic studies to targeted mutations in known genes or the characterization of naturally occurring mutations.
In a commonly used type of genetic screen, organisms carrying a defined mutation affecting the process of interest are further mutagenized and screened for second site mutations, or modifiers, that enhance (increase) or suppress (reduce) the severity of the parental phenotype (4, 5). The initial mutation often renders the system responsive to subtle changes in modifiers that would not be penetrant on an otherwise wild-type background. This sensitivity often permits a dominant screen, decreasing the time and resources required to identify new mutations.
Complete deficiency of many signaling molecules results in loss of the cell type of interest or pleiotropic or lethal phenotypes, making the effects of additional mutations difficult to interpret. This can be addressed by starting a modifier screen with a weak allele that either (i) reduces the signal strength to just above the threshold for a productive response or (ii) presents with a phenotype intermediate between wild-type and complete deficiency (4, 5). Both enhancers and suppressors can thus be characterized, and the pathway of interest studied in isolation. This approach has been used in large scale, random screens (4, 5) and to probe interactions between previously defined mutations. Many of the latter studies have been performed in mice. These have identified interactions between such genes as SOS and the epidermal growth factor receptor (6), Apc, and Dpc4(Smad4) (7), the basic helix–loop–helix transcription factors E2A, HEB, and EBF (8, 9), and components of the Lyn/SHP-1/CD22 B cell inhibitory pathway (10).
The importance of studying modifier effects in mammals is illustrated by the effect of genetic background on the severity of many human diseases. Mouse models have been developed for several such diseases, including but not limited to systemic lupus erythematosus (NZB x NZW), polycystic kidney disease (pcy), and familial adenomatous polyposis (Min). Intraspecific backcrosses have identified naturally occurring modifiers of each of these disease phenotypes (11–13).
The severity of X-linked agammaglobulinemia (XLA) and its murine counterpart X-linked immunodeficiency (xid) also depends on genetic background (14, 15). XLA and xid result from loss of function of Bruton's tyrosine kinase (Btk) (reviewed in refs. 16–18) and are characterized by blocks in B cell development at the pre-B stage in XLA and at the immature to mature transition in xid. The remaining B cells have impaired responses to several cell surface receptors including the B cell antigen receptor (BCR), CD38, RP-105, and the IL-5 receptor. Type II T-independent antigen responses are impaired, and B-1 cell numbers are reduced.
Many studies have begun to define mechanisms by which Btk mediates B cell development and function (reviewed in refs. 17–19). Conflicting results regarding the nature of the interaction between Btk and several other signaling molecules have been reported. Protein kinase Cβ (PKCβ) binds to and phosphorylates Btk in vitro, reducing Btk kinase activity (20). However, PKCβ-deficient mice have an xid-like phenotype, suggesting a positive interaction between these two proteins (21). Similarly, although the Src family kinase Lyn activates Btk in vitro (22, 23), Lyn has a net negative effect on Btk (24).
Several known genes have been identified as modifiers of xid or Btk deficiency, including Lyn, CD40, MHC class II, and OCA-B (refs. 24 and 25; reviewed in ref. 19). These studies can only address whether these genes have a role independent of or redundant with Btk because no functional Btk is expressed in the mice carrying the second mutation. To look more directly and quantitatively at the effect of mutations in various signaling molecules on Btk-dependent pathways, we used a transgenic mouse model in which a low dose of Btk (Btklo) behaves as a partial loss of function mutant (26). Twenty-five percent of endogenous Btk levels is sufficient to restore the IgMloIgDhi mature B cells that are absent in Btk−/− or xid mice. However, BCR-induced proliferation and response to type II T cell independent (TI-II) antigens (26, 27) are only partially rescued to levels between those of xid/Btk−/− and wild-type mice. A 2-fold increase in Btk expression in homozygous transgenic mice leads to a significant increase in responses, indicating that Btk is a limiting component of BCR signaling pathways. We asked whether reduction in dosage or complete deficiency of a panel of gene products (Table 1 and Fig. 1) implicated as Btk regulators by either the xid-like phenotypes of their deficiencies or whether in vitro physical and/or functional interactions would modify the Btklo response to BCR cross-linking in vitro and the TI-II antigen 2,4,6-trinitrophenyl (TNP)-Ficoll in vivo. Enhancers, suppressors, and genes with no effect were identified and could be quantitatively ranked.
Table 1.
B cell phenotype of candidate gene knockouts
| Gene | Viability | Knockout phenotype | Ref. |
|---|---|---|---|
| Lyn | Viable | ↓ B cell numbers, ↓ surface IgM levels, ↑ BCR responses, ↑ serum IgM levels, slightly ↓ T-dependent and T-independent responses, ↑ B-1 cells, autoantibodies with age | 24, 28–32 |
| Fyn | Viable | Normal B cell numbers, normal BCR responses, ↓ IL-5 response | 33, 34 |
| p85α | Perinatal lethality | Like xid with more severe block at proB to preB stage | 35, 36 |
| CD19 | Viable | ↓ B cell numbers, ↓ BCR responses, ↓ serum Ig levels, ↓ T-dependent responses, normal or ↑ T-independent responses, ↓ B-1 cells | 37–39 |
| Gαq | Viable | No B cell phenotype described | 40 |
| Gα11 | Viable | No B cell phenotype described | 41 |
| SHIP | Viable | ↓ B cell numbers, ↓ surface IgM levels, ↑ BCR responses, ↓ FcγRIIb-mediated inhibition of BCR, ↑ serum Ig levels, normal T-dependent responses, ↑ T-independent responses | 42–44 |
| PTEN | Embryonic lethal | In +/− mice: ↑ number of activated B cells (polyclonal expansion), ↑ autoantibodies | 45–49 |
| CD22 | Viable | ↓ B cell numbers, ↓ surface IgM levels, ↑ BCR responses, ↑ serum IgM levels, ↓ T-independent responses, normal T-dependent responses, ↑ B-1 cells, autoantibodies with age | 38, 50–52 |
| PKCβ | Viable | Like xid | 21 |
The B cell phenotypes of mice deficient in each candidate gene tested are indicated.
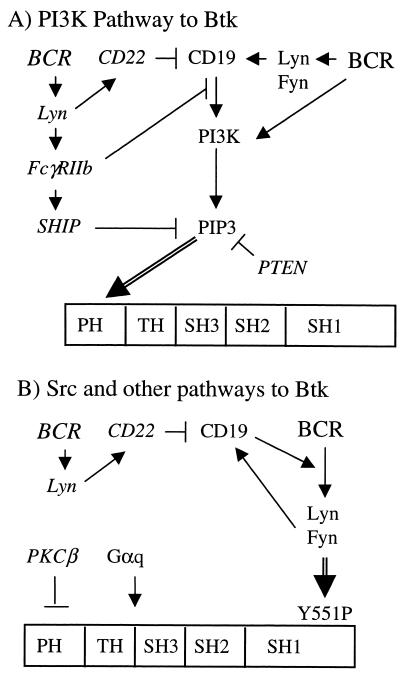
Figure 1.
Interactions of candidate modifiers with Btk. The major pathways for Btk activation, mediated by PI3K (A) and Src family kinases (B), are indicated. Double arrows represent interactions critical for Btk activation. Pathways that inhibit, or are predicted to inhibit, Btk are indicated by ⊣.
Materials and Methods
Mice.
Btklo mice carry a murine Btk cDNA transgene driven by the Ig heavy chain promoter and enhancer on a Btk−/− or Btk−/Y background (26). The transgene expresses about 25% of endogenous Btk protein levels in splenic B cells. Btklo females were mated with mice deficient in each candidate gene (C) to generate pups that were C+/− and either Btk−/Y, Btk−/Y tg (Btklo), Btk+/−, or Btk+/− tg. Mice were genotyped by PCR or Southern blot analysis as described (21, 26, 29, 34, 36, 40–42, 48) except for CD19 (37) and CD22 (38) mutant mice that were identified by FACS analysis of peripheral blood. Males and females from these crosses were then mated to each other. Informative progeny were Btklo (Btk−/Y tg or Btk−/− tg) and C+/+, C+/−, or C−/−. Wild-type and Btk-deficient mice were used as controls. Mice were on a mixed (C57BL/6 × 129 x BALB/c) background. Because the phenotype of Btk deficiency varies with genetic background (14, 15), littermates were compared within each experiment, and all results were confirmed with mice generated from independent crosses. Mice were 6–12 weeks old when used in experiments.
Flow Cytometry: Phenotypic Analysis.
Single-cell suspensions of spleens or peripheral blood were depleted of red blood cells by a 5-min incubation in 0.15 M NH4Cl, 1 mM KHCO3, and 0.1 mM Na2EDTA and stained with combinations of the following Abs: anti-B220 Tri-Color (Caltag, South San Francisco, CA), anti-IgD FITC (PharMingen), anti-IgM biotin (PharMingen), streptavidin-phycoerythrin (PE) (Dako), anti-CD19 FITC (PharMingen), anti-CD22 FITC (PharMingen), and anti-B220-PE (PharMingen). Data were acquired on a FACScan (Becton Dickinson) and analyzed by using cellquest software. Live cells were gated based on forward and side scatter.
B Cell Proliferation Assays.
Total splenocytes were depleted of red blood cells and plated in RPMI 1640 with 10% heat-inactivated FCS at 106/ml. Where indicated, goat anti-mouse IgM F(ab′)2 fragments (Jackson ImmunoResearch Laboratories) were added at either 2 or 20 μg/ml. At 24 h, BrdUrd (Sigma) was added to a final concentration of 10 μM. Cells were harvested at 48 h, stained with anti-BrdUrd FITC (Becton Dickinson) and anti-B220 PE (PharMingen) as described (24), and analyzed on a FACScan (Becton Dickinson) by using cellquest software.
ELISA.
Mice were immunized i.p. with 10 μg TNP-Ficoll (Biosearch Technologies) and bled 6 days later. The 96-well plates were coated with 25 μg/ml TNP-BSA (Biosearch Technologies) in PBS. Serum was diluted serially into PBS, 0.1% BSA, and 0.05% Tween-20 and added to wells in duplicate. Plates were washed, incubated with secondary Ab (goat anti-mouse IgM-alkaline phosphatase; Southern Biotechnology Associates) diluted 1:500 in PBS, 0.1% BSA, and 0.05% Tween-20, and developed with an alkaline phosphatase substrate kit (Bio-Rad). OD405 was read on a Vmax kinetic microplate reader (Molecular Devices).
Results
General Experimental Strategy.
We asked whether a reduction in dosage or complete deficiency of a panel of genes shown to regulate Btk (Fig. 1, Table 1) would modify the Btklo phenotype. In a two-step cross, mice carrying a targeted mutation in each candidate gene (21, 29, 34, 36–38, 40–42, 48) were mated with Btklo mice (24, 26) to generate progeny that were Btklo and either C+/+, C+/−, or C−/−. Wild-type and Btk-deficient mice were used as positive and negative controls, respectively, for Btk-dependent responses. In the case of p85α (36) or phosphatase and tensin homolog (PTEN), where complete deficiency is lethal (45–48), and PKCβ where loss of function mimics xid (21), only C+/− mice were tested. Because the phenotype of Btk deficiency varies with genetic background (14, 15), littermates were compared within each experiment and all results were confirmed with mice generated from independent crosses. We chose two independent assays of Btk-dependent processes, BCR-induced B cell proliferation in vitro and TNP-Ficoll response in vivo. The first measures a relatively well-defined process (53), whereas the second involves complex, kinetic interactions between multiple cell types and their microenviroment that are likely regulated by a network of signaling pathways (54). To control for effects of modifiers that did not involve regulation of Btk signaling pathways, we measured B cell numbers and Btk expression in resting splenic B cells purified by anti-B220 Ab bound to magnetic beads. In the case of CD19−/− Btklo mice, a slight reduction in Btk protein levels was observed (data not shown). No other changes in transgene-directed protein expression were seen in resting cells.
Selection of Candidate Genes.
Two major pathways are required for the activation of Btk on BCR cross-linking (Fig. 1). First, phosphatidylinositol 3,4,5-triphosphate (PIP3), the product of phosphoinositide 3-kinase (PI3K), binds to the pleckstrin homology domain of Btk (55, 56) and targets Btk to the membrane (Fig. 1A) (57–60). This pathway is stimulated by CD19 (61, 62), a cell surface molecule that functions to reduce B cell signaling thresholds and amplify BCR signals. PI3K-mediated Btk membrane association is inhibited by FcγRIIb via two mechanisms. The first is mediated by SH2-containing inositol phosphatase (SHIP) (57, 58), which dephosphorylates PIP3. The second prevents the CD19/PI3K association (63). CD22, a cell surface protein that generally inhibits BCR signaling, opposes CD19 (64–66) and therefore may inhibit the PI3K/Btk pathway as well.
Once at the membrane, Btk is transphosphorylated at Y551 by Src family kinases (Fig. 1B) (22, 23). Btk then autophosphorylates at Y223 in the SH3 domain (23, 67) and becomes fully active. CD19 feeds into this pathway by amplifying BCR-induced activation of Src family kinases (68). CD22 may interfere with this stage of Btk activation through its opposition of CD19 (64–66).
Other proteins also regulate Btk via different pathways (Fig. 1B). Btk and PKCβ have a complex interaction. PKCβ inhibits Btk activity in vitro (20), but the phenotype of PKCβ−/− mice resembles xid (21). Btk may also transmit G protein-coupled receptor initiated signals. Members of the q and 12 families of heterotrimeric G protein α subunits have been claimed to directly activate Btk in vitro (69, 70). There is no direct genetic evidence to support this observation.
Based on this model, we predicted that reduced dosage or loss of function of the positive regulators Lyn and Fyn (Src family kinases), p85α, CD19, Gαq, and its close relative Gα11 would enhance the Btklo phenotype, shifting it toward xid or Btk deficiency. In contrast, mutation of CD22 and molecules that down-regulate PIP3 levels such as the inositol phosphatases SHIP and PTEN (ref. 48; reviewed in ref. 71) would likely suppress the Btklo phenotype and shift it toward wild type. The net effect of PKCβ haploinsufficiency could not be predicted.
SHIP, PTEN, and Lyn Are BtkloSuppressors in the in Vitro Proliferation Assay.
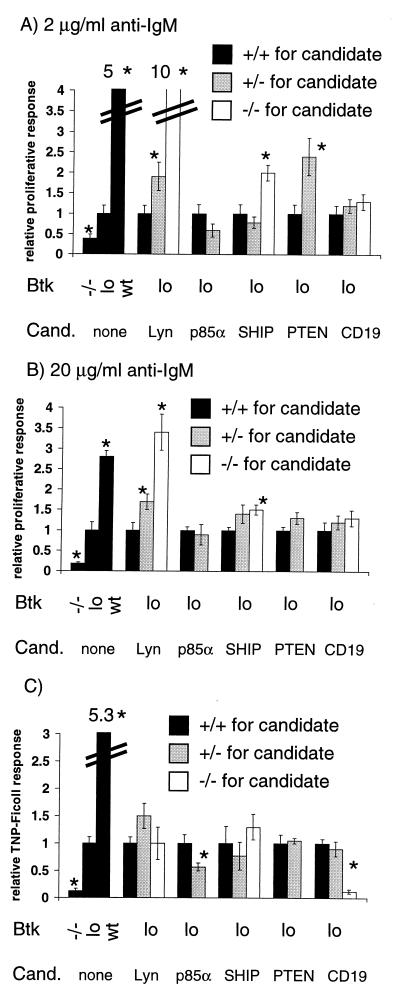
We first tested whether we could identify modifiers that regulated the mitogenic response of Btklo B cells to BCR cross-linking in vitro. As predicted, all regulators of PIP3 levels tested affected the performance of Btklo cells in this assay to some degree. SHIP and PTEN were Btklo suppressors, as B cells from PTEN+/−Btklo and SHIP−/−Btklo mice proliferated significantly better than Btklo cells (Fig. 2A), even in the absence of FcγRIIb engagement. In both cases, the effect was mild (2-fold) and observed only at low doses of anti-IgM (Fig. 2 A and B). A small enhancing effect of p85α haploinsufficiency was observed in some, but not all, animals at low doses of cross-linking Ab (Fig. 2A). However, this was not statistically significant.
Figure 2.
(A and B) Lyn, SHIP, and PTEN modify the Btklo response to BCR cross-linking in vitro. Splenocytes from 6- to 12-week-old Btklo mice either +/+ (■), +/− (░⃞), or −/− (□) for each candidate gene were incubated for 48 h with 2 (A) or 20 (B) μg/ml of goat anti-mouse IgM F(ab′)2 fragments. Cells were labeled with BrdUrd for the final 24 h. The percentage of B220+ cells labeled with BrdUrd was determined by flow cytometry. (C) CD19 and p85α modify the Btklo response to TNP-Ficoll in vivo. Six- to 12-week-old Btklo mice either +/+ (■), +/− (░⃞), or −/− (□) for each candidate gene were immunized with 10 μg TNP-Ficoll and bled 6 days later. Serum was diluted serially, and anti-TNP IgM was measured by ELISA. The results shown are from a 1:400 dilution of serum, which was within the linear range.(A–C) To compare results from several experiments, data were normalized to the average response of Btklo cells, which was arbitrarily set to 1. Data are presented as mean ± SEM (n >= 4). Results significantly different from Btklo by Student's t test (P < 0.05) are indicated by asterisks above the appropriate bars. p85α, PTEN, and PKCβ−/− mice were not tested because of lethality or xid-like phenotype.
Surprisingly, Src family kinases did not behave as expected. Lyn, which was predicted to be an enhancer based on its activation of Btk in biochemical studies (22, 23), was instead an extremely strong suppressor. Both Lyn+/−Btklo and Lyn−/−Btklo B cells responded better (up to 10-fold) to BCR cross-linking than Btklo cells (ref. 24; Fig. 2 A and B). At low doses of anti-IgM, Lyn−/−Btklo cells proliferated even better than wild-type cells. This activity was specific to Lyn, as Fyn loss of function did not change the response of either Btklo or Btkwt cells to BCR cross-linking.
In addition to Fyn, CD19, CD22, PKCβ, Gαq, and Gα11 failed to modify this aspect of the Btklo phenotype (Fig. 2 A and B, and data not shown).
CD19 and p85α Are Btklo Enhancers in the in Vivo Immune Response Assay.
It was unexpected that only 3 of 10 candidate genes had significant effects on the transmission of BCR signals by Btk in vitro. We also tested each candidate gene in an alternative measurement of Btk-mediated B cell function, the production of Abs in vivo in response to the TI-II antigen TNP-Ficoll. This response depends on both an antigen-induced proliferative burst and a spectrum of additional complex events (54). It was modified quite differently than BCR-stimulated mitogenesis in vitro. None of the suppressors identified in vitro affected the TNP-Ficoll response of Btklo cells (Fig. 2), despite the fact that SHIP−/− mice display an enhanced IgG response to this antigen (44). Fyn, CD22, PKCβ, Gαq, and Gα11 also had no effect (data not shown). In contrast, CD19 deficiency and p85α haploinsufficiency enhanced the Btklo phenotype in the TI-II antigen response assay (Fig. 2C). The effect of CD19 was particularly striking. CD19−/−Btklo mice resembled Btk−/− mice in their complete failure to mount a TNP-Ficoll response (Fig. 2C). In neither case could the reduced response be attributed to altered B cell numbers. Btklo mice and p85α+/−Btklo mice had similar numbers of mature splenic B cells (7.0 vs. 6.7 × 106). CD19−/−Btklo mice did have a 2-fold reduction in the size of the mature B cell population relative to Btklo mice. However, mature B cell numbers were increased 2-fold compared with CD19−/− mice, which have relatively normal or even enhanced responses to TNP-Ficoll (refs. 39 and 72; data not shown).
Discussion
Insights into Btk Signaling.
Although this study does not address the mechanism of modification by the genes identified here, it begins to define the relative contribution of these molecules to Btk-dependent processes. In some cases, the nature of these genetic interactions could not have been predicted by data from in vitro, biochemical, or cell culture studies.
Strikingly, all suppressors affected the response to BCR cross-linking in vitro but not TNP-Ficoll immunization in vivo. In contrast, enhancers altered only the in vivo response. This finding suggests that TI-II responses require a stronger Btk signal than anti-IgM-induced proliferation. Consistent with this idea, the amount of Btk protein required for an efficient TNP-Ficoll response is higher than that needed for a mitogenic response to BCR cross-linking (26). Alternatively, Btk may participate in non-BCR-mediated, or nonproliferation-dependent, processes that regulate TI-II responses. These might include the development of an appropriate B cell repertoire, migration of antigen-specific B cells to the correct microenvironment, cytokine-dependent B cell proliferation and differentiation, or antigen presentation (54).
The strong suppression of some aspects of the Btklo phenotype by Lyn (ref. 24, Fig. 2A) was unexpected. Several Src family kinases may be redundant for the activation of Btk (22, 23, 33, 34), whereas Lyn has a unique inhibitory role (24, 29). Down-regulation of BCR signals by Lyn can occur via CD22/SHP-1-mediated (10, 28, 73, 74) and FcγRIIb/SHIP-mediated (28, 30, 74) pathways. CD22 was not a Btklo modifier, suggesting that the Lyn/CD22/SHP-1 inhibitory pathway is redundant or does not impinge on Btk. In contrast, some SHIP-dependent inhibition of Btk signaling was seen. This did not account for all of the inhibition observed with Lyn, suggesting that additional mechanisms contribute to Lyn-mediated down-regulation of Btk pathways. These may include inhibition by the cell surface receptor PIR-B, a pathway shown to involve both Btk and Lyn (75, 76).
The mild suppression of the Btklo phenotype by both SHIP deficiency and PTEN haploinsufficiency suggests that SHIP and PTEN might be partially redundant in down-regulating BCR signaling pathways. PTEN is likely more dominant, at least in the absence of FcγRIIb engagement, based on the observed gene dosage effect. SHIP and PTEN may also have indirect effects on the proliferation of Btklo B cells. It has recently been shown that the absence of SHIP results in increased Akt phosphorylation and enhances the survival of B cells following stimulation with the F(ab′)2 fragment of anti-IgM (44). PTEN is known to inhibit the anti-apoptotic PI3K/PIP3/Akt pathway (48) (reviewed in ref. 71). Impaired down-regulation of Akt may increase survival of PTEN+/−Btklo or SHIP−/−Btklo B cells, indirectly enhancing proliferation.
The reduced TNP-Ficoll responses in both CD19−/−Btklo and p85α+/−Btklo mice supports a model in which CD19 amplifies Btk activation via PI3K (61, 62). p85α was more penetrant than CD19, however. An effect of haploinsufficiency of p85α, but not CD19, was observed in the context of a limiting dose of Btk. In addition, the consequences of p85α deficiency for B cell proliferation and response to TI-II antigens (35, 36) are much more severe than those of CD19 deficiency (37–39) in the presence of normal levels of Btk. PI3K is likely activated by both CD19-dependent and CD19-independent mechanisms in response to BCR cross-linking.
PKCβ, Fyn, CD22, Gαq, and Gα11 failed to modify the Btklo phenotype in either assay. A similar lack of genetic interaction between known components of a biochemical pathway was observed in a screen for modifiers of the rough-eye phenotype produced by an activated form of Ras expressed in the Drosophila eye (5). Although both Raf and mitogen-activated protein (MAP) kinase are known to be downstream of Ras, only 1 allele of Raf was identified out of 850,000 mutagenized progeny in comparison to more than 100 alleles of MAP kinase. This type of result could indicate that genes that do not modify as expected are redundant, not limiting, or have balanced activating and inhibitory effects on the pathway of interest. The latter scenario is particularly likely in the case of PKCβ, given that both positive (21) and negative (20) interactions between PKCβ and Btk have been shown in other systems. It is also possible that these genes do interact with Btk, but in pathways used by receptor systems not tested here.
A Mammalian, Sensitized Genetic System for the Identification of New Mutations.
Btklo mice are an excellent sensitized genetic system with which to identify functional interactions between components of B cell signaling pathways. Because the mechanism by which Btk signaling is modified varies among functional responses, an extension of these studies to other Btk-dependent pathways such as CD38, RP105, and IL-5 (reviewed in refs. 17–19) would likely demonstrate additional types of interactions.
Most importantly, haploinsufficiency of Lyn, PTEN, or p85α modified the Btklo phenotype. A dominant screen can therefore be undertaken for additional strong modifiers. Screening for phenotypes of heterozygous mutations would, like the cre-lox (77) or Rag2-complementation (78) systems, circumvent the lethal or pleiotropic effects of some complete deficiencies. This would allow the identification of a role for these genes within B cells. These observations set the stage for a genome-wide, random mutagenesis approach to identify novel modifiers by using the serological immune response to TI-II antigens as an assay.
This approach is not limited to the study of Btk-mediated processes. Reduction in dosage or functional activity of any combination of gene products by heterozygous mutation, transgene replacement, or “knocking-in” point mutations or small deletions by using homologous recombination in embryonic stem cells could reveal genetic interactions. These types of studies will be valuable for defining physiologically relevant signaling pathways and quantifying the processes affected in polygenic disorders.
Acknowledgments
We thank Dr. Roger Perlmutter for providing fyn−/− mice, Drs. Larry Zipursky and Naomi Rosenberg for critical reading of the manuscript and helpful suggestions, and Jamie White for assistance in the preparation of this manuscript. O.N.W. is an Investigator of the Howard Hughes Medical Institute. A.B.S. and D.F. are Special Fellows of the Leukemia and Lymphoma Society of America. This work was partially supported by National Institutes of Health Grants CA-81776 and CA-54464 (to T.F.T.), DK50267 (to C.A.L.), and GM41890 (to L.C.C.), and funds from the National Cancer Institute of Canada (to R.K.H. and C.D.H.).
Abbreviations
- Btk
Bruton's tyrosine kinase
- Btklo
Btk transgenic mice expressing 25% of endogenous Btk levels
- TI-II
type II T cell independent antigen
- xid
X-linked immunodeficiency
- BCR
B cell antigen receptor
- TNP
2,4,6-trinitrophenyl
- PI3K
phosphoinositide 3-kinase
- PIP3
phosphatidylinositol 3,4,5-triphosphate
- FITC
fluorescein isothiocyanate
- PE
phycoerythrin
- SHIP
SH2-containing inositol phosphatase
- PTEN
phosphatase and tensin homolog
- PKCβ
protein kinase Cβ
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.110146697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.110146697
References
- 1.Zambrowicz B P, Friedrich G A, Buxton E C, Lilleberg S L, Person C, Sands A T. Nature (London) 1998;392:608–611. doi: 10.1038/33423. [DOI] [PubMed] [Google Scholar]
- 2.Nolan P M, Kapfhamer D, Bucan M. Methods. 1997;13:379–395. doi: 10.1006/meth.1997.0545. [DOI] [PubMed] [Google Scholar]
- 3.Vitaterna M H, King D P, Chang A M, Kornhauser J M, Lowrey P L, McDonald J D, Dove W F, Pinto L H, Turek F W, Takahashi J S. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon M A, Bowtell D D L, Dodson G S, Laverty T R, Rubin G M. Cell. 1991;67:701–716. doi: 10.1016/0092-8674(91)90065-7. [DOI] [PubMed] [Google Scholar]
- 5.Karim F D, Chang H C, Therrien M, Wassarman D A, Laverty T, Rubin G M. Genetics. 1996;143:315–329. doi: 10.1093/genetics/143.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D Z, Hammond V E, Abud H E, Bertoncello I, McAvoy J W, Bowtell D D. Genes Dev. 1997;11:309–320. doi: 10.1101/gad.11.3.309. [DOI] [PubMed] [Google Scholar]
- 7.Takaku K, Oshima M, Miyoshi H, Matsui M, Seldin M F, Taketo M M. Cell. 1998;92:645–656. doi: 10.1016/s0092-8674(00)81132-0. [DOI] [PubMed] [Google Scholar]
- 8.Zhuang Y, Cheng P, Weintraub H. Mol Cell Biol. 1996;16:2898–2905. doi: 10.1128/mcb.16.6.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Riordan M, Grosschedl R. Immunity. 1999;11:21–31. doi: 10.1016/s1074-7613(00)80078-3. [DOI] [PubMed] [Google Scholar]
- 10.Cornall R J, Cyster J G, Hibbs M L, Dunn A R, Otipoby K L, Clark E A, Goodnow C C. Immunity. 1999;8:497–508. doi: 10.1016/s1074-7613(00)80554-3. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich W F, Lander E S, Smith J S, Moser A R, Gould K A, Luongo C, Borenstein N, Dove W. Cell. 1993;75:631–639. doi: 10.1016/0092-8674(93)90484-8. [DOI] [PubMed] [Google Scholar]
- 12.Woo D D, Nguyen D K, Khatibi N, Olsen P. J Clin Invest. 1997;100:1934–1940. doi: 10.1172/JCI119724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morel L, Tian X H, Croker B P, Wakeland E K. Immunity. 1999;11:131–139. doi: 10.1016/s1074-7613(00)80088-6. [DOI] [PubMed] [Google Scholar]
- 14.Bona C, Mond J J, Paul W E. J Exp Med. 1980;151:224–234. doi: 10.1084/jem.151.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bykowsky M J, Haire R N, Ohta Y, Tang H, Sung S S, Veksler E S, Greene J M, Fu S M, Litman G W, Sullivan K E. Am J Hum Genet. 1996;58:477–483. [PMC free article] [PubMed] [Google Scholar]
- 16.Wicker L S, Scher I. Curr Top Microbiol Immunol. 1986;124:87–101. doi: 10.1007/978-3-642-70986-9_6. [DOI] [PubMed] [Google Scholar]
- 17.Desiderio S. Curr Opin Immunol. 1997;9:534–540. doi: 10.1016/s0952-7915(97)80107-0. [DOI] [PubMed] [Google Scholar]
- 18.Satterthwaite A B, Li Z, Witte O N. Semin Immunol. 1998;10:309–316. doi: 10.1006/smim.1998.0123. [DOI] [PubMed] [Google Scholar]
- 19.Satterthwaite, A. B. & Witte, O. N. (2000) Immunol. Rev., in press. [PubMed]
- 20.Yao L, Kawakami Y, Kawakami T. Proc Natl Acad Sci USA. 1994;91:9175–9179. doi: 10.1073/pnas.91.19.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leitges M, Schmedt C, Guinamard R, Davoust J, Schaal S, Stabel S, Tarakhovsky A. Science. 1996;273:788–791. doi: 10.1126/science.273.5276.788. [DOI] [PubMed] [Google Scholar]
- 22.Mahajan S, Fargnoli J, Burkhardt A L, Kut S A, Saouaf S J, Bolen J B. Mol Cell Biol. 1995;15:5304–5311. doi: 10.1128/mcb.15.10.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rawlings D J, Scharenberg A M, Park H, Wahl M I, Lin S, Kato R M, Fluckiger A C, Witte O N, Kinet J P. Science. 1996;271:822–825. doi: 10.1126/science.271.5250.822. [DOI] [PubMed] [Google Scholar]
- 24.Satterthwaite A B, Lowell C A, Khan W N, Sideras P, Alt F W, Witte O N. J Exp Med. 1998;188:833–844. doi: 10.1084/jem.188.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schubart D B, Rolink A, Schubart K, Matthias P. J Immunol. 2000;164:18–22. doi: 10.4049/jimmunol.164.1.18. [DOI] [PubMed] [Google Scholar]
- 26.Satterthwaite A, Cheroutre H, Khan W N, Sideras P, Witte O N. Proc Natl Acad Sci USA. 1997;94:13152–13157. doi: 10.1073/pnas.94.24.13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinschewer D D, Ochsenbein A F, Satterthwaite A B, Witte O N, Hengartner H, Zinkernagel R M. Eur J Immunol. 1999;29:2981–2987. doi: 10.1002/(SICI)1521-4141(199909)29:09<2981::AID-IMMU2981>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 28.Nishizumi H, Horikawa K, Mlinaric-Rascan I, Yamamoto T. J Exp Med. 1998;187:1343–1348. doi: 10.1084/jem.187.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan V W F, Meng F, Soriano P, DeFranco A L, Lowell C A. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Koizumi T, Watanabe T. J Exp Med. 1996;184:831–838. doi: 10.1084/jem.184.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Llic D, Mori S, Watanabe T, Yamamoto T. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 32.Hibbs M L, Tarlinton D M, Armes J, Grail D, Hodgson G, Maglitto R, Stacker S A, Dunn A R. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 33.Sillman A L, Monroe J G. J Leukocyte Biol. 1994;56:812–816. doi: 10.1002/jlb.56.6.812. [DOI] [PubMed] [Google Scholar]
- 34.Appleby M W, Kerner J D, Chien S, Maliszewski C R, Bondada S, Perlmutter R M. J Exp Med. 1995;182:811–820. doi: 10.1084/jem.182.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki H, Terauchi Y, Fujiwara M, Aizawa S, Yazaki Y, Kadowaki T, Koyasu S. Science. 1999;283:390–392. doi: 10.1126/science.283.5400.390. [DOI] [PubMed] [Google Scholar]
- 36.Fruman D A, Snapper S B, Yballe C M, Davidson L, Yu J Y, Alt F W, Cantley L C. Science. 1999;283:393–397. doi: 10.1126/science.283.5400.393. [DOI] [PubMed] [Google Scholar]
- 37.Engel P, Zhou L-J, Ord D C, Sato S, Koller B, Tedder T F. Immunity. 1995;3:39–50. doi: 10.1016/1074-7613(95)90157-4. [DOI] [PubMed] [Google Scholar]
- 38.Sato S, Miller A S, Inaoki M, Bock C B, Jansen P J, Tang M L K, Tedder T F. Immunity. 1996;5:551–562. doi: 10.1016/s1074-7613(00)80270-8. [DOI] [PubMed] [Google Scholar]
- 39.Rickert R C, Rajewsky K, Roes J r. Nature (London) 1995;376:352–355. doi: 10.1038/376352a0. [DOI] [PubMed] [Google Scholar]
- 40.Offermanns S, Toombs C F, Hu Y-H, Simon M I. Nature (London) 1997;389:183–186. doi: 10.1038/38284. [DOI] [PubMed] [Google Scholar]
- 41.Offermanns S, Zhao L P, Gohla A, Sarosi I, Simon M I, Wilkie T M. EMBO J. 1999;17:4304–4312. doi: 10.1093/emboj/17.15.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Helgason C D, Damen J E, Rosten P, Grewal R, Sorensen P, Chappel S M, Borowski A, Jirik F, Krystal G, Humphries R K. Genes Dev. 1999;12:1610–1620. doi: 10.1101/gad.12.11.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Q, Oliveira-Dos-Santos A J, Mariathasan S, Bouchard D, Jones J, Sarao R, Kozieradzki I, Ohashi P S, Penninger J M, Dumont D J. J Exp Med. 1998;188:1333–1342. doi: 10.1084/jem.188.7.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Helgason C D, Kalberer C P, Damen J E, Chappel S M, Pineault N, Krystal G, Humphries R K. J Exp Med. 2000;191:781–794. doi: 10.1084/jem.191.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi P P. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki A, de la Pompa J L, Stambolic V, Elia A J, Sasaki T, del Barco Barrantes I, Ho A, Wakeham A, Itie A, Khoo W, et al. Curr Biol. 1998;8:1169–1178. doi: 10.1016/s0960-9822(07)00488-5. [DOI] [PubMed] [Google Scholar]
- 47.Podsypanina K, Ellenson L H, Nemes A, Gu J, Tamura M, Yamada K M, Cordon-Cardo C, Catoretti G, Fisher P E, Parsons R. Proc Natl Acad Sci USA. 1999;96:1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun H, Lesche R, Li D M, Liliental J, Zhang H, Gao J, Gavrilova N, Mueller B, Liu X, Wu H. Proc Natl Acad Sci USA. 1999;96:6199–6204. doi: 10.1073/pnas.96.11.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Cristofano A, Kotsi P, Peng Y F, Cordon-Cardo C, Elkon K B, Pandolfi P P. Science. 1999;285:2122–2125. doi: 10.1126/science.285.5436.2122. [DOI] [PubMed] [Google Scholar]
- 50.Nitschke L, Carsetti R, Ocker B, Kohler G, Lamers M C. Curr Biol. 1997;7:133–143. doi: 10.1016/s0960-9822(06)00057-1. [DOI] [PubMed] [Google Scholar]
- 51.Otipoby K L, Andersson K B, Draves K E, Klaus S J, Farr A G, Kerner J D, Perlmutter R M, Law C-L, Clark E A. Nature (London) 1996;384:634–637. doi: 10.1038/384634a0. [DOI] [PubMed] [Google Scholar]
- 52.O'Keefe T L, Williams G T, Davies S L, Neuberger M S. Science. 1996;274:798–801. doi: 10.1126/science.274.5288.798. [DOI] [PubMed] [Google Scholar]
- 53.DeFranco A L. Curr Opin Immunol. 1997;9:296–308. doi: 10.1016/s0952-7915(97)80074-x. [DOI] [PubMed] [Google Scholar]
- 54.Mond J J, Lees A, Snapper C M. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 55.Salim K, Bottomley M J, Querfurth E, Zvelebil M J, Gout I, Scaife R, Margolis R L, Gigg R, Smith C I E, Driscoll P C, et al. Oncogene. 1996;15:6241–6250. [PMC free article] [PubMed] [Google Scholar]
- 56.Rameh L E, Arvidsson A-k, Carraway III, K L, Couvillon A D, Rathbun G, Crompton A, VanRenterghem B, Czech M P, Ravichandran K S, Burakoff S J, et al. J Biol Chem. 1997;272:22059–22066. doi: 10.1074/jbc.272.35.22059. [DOI] [PubMed] [Google Scholar]
- 57.Bolland S, Pearse R N, Kurosaki T, Ravetch J V. Immunity. 1998;8:509–516. doi: 10.1016/s1074-7613(00)80555-5. [DOI] [PubMed] [Google Scholar]
- 58.Scharenberg A M, El-Hillal O, Fruman D A, Beitz L O, Li Z, Lin S, Gout I, Cantley L C, Rawlings D J, Kinet J P. EMBO J. 1998;17:1961–1972. doi: 10.1093/emboj/17.7.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Z, Wahl M I, Eguinoa A, Stephens L R, Hawkins P T, Witte O N. Proc Natl Acad Sci USA. 1997;94:13820–13825. doi: 10.1073/pnas.94.25.13820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li T, Rawlings D J, Park H, Kato R M, Witte O N, Satterthwaite A B. Oncogene. 1997;15:1375–1383. doi: 10.1038/sj.onc.1201308. [DOI] [PubMed] [Google Scholar]
- 61.Buhl A M, Pleiman C M, Rickert R C, Cambier J C. J Exp Med. 1997;186:1897–1910. doi: 10.1084/jem.186.11.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buhl A M, Cambier J C. J Immunol. 1999;162:4438–4446. [PubMed] [Google Scholar]
- 63.Hippen K L, Buhl A M, D'Ambrosio D, Nakamura K, Persin C, Cambier J C. Immunity. 1997;7:49–58. doi: 10.1016/s1074-7613(00)80509-9. [DOI] [PubMed] [Google Scholar]
- 64.Tooze R M, Doody G M, Fearon D T. Immunity. 1997;7:59–67. doi: 10.1016/s1074-7613(00)80510-5. [DOI] [PubMed] [Google Scholar]
- 65.Sato S, Jansen P J, Tedder T F. Proc Natl Acad Sci USA. 1997;94:13158–13162. doi: 10.1073/pnas.94.24.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fujimoto M, Poe J C, Jansen P J, Sato S, Tedder T F. J Immunol. 1999;162:7088–7094. [PubMed] [Google Scholar]
- 67.Park H, Wahl M I, Afar D E, Turck C W, Rawlings D J, Tam C, Scharenberg A M, Kinet J-P, Witte O N. Immunity. 1996;4:515–525. doi: 10.1016/s1074-7613(00)80417-3. [DOI] [PubMed] [Google Scholar]
- 68.Fujimoto M, Bradney A P, Poe J C, Steeber D A, Tedder T F. Immunity. 1999;11:191–200. doi: 10.1016/s1074-7613(00)80094-1. [DOI] [PubMed] [Google Scholar]
- 69.Bence K, Ma W, Kozasa T, Huang X-Y. Nature (London) 1997;389:296–299. doi: 10.1038/38520. [DOI] [PubMed] [Google Scholar]
- 70.Jiang Y, Ma W, Wan Y, Kozasa T, Hattori S, Huang X-Y. Nature (London) 1998;395:808–813. doi: 10.1038/27454. [DOI] [PubMed] [Google Scholar]
- 71.Cantley L C, Neel B G. Proc Natl Acad Sci USA. 1999;96:4240–4250. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sato S, Steeber D A, Tedder T F. Proc Natl Acad Sci USA. 1995;92:11558–11562. doi: 10.1073/pnas.92.25.11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith K G C, Tarlinton D M, Doody G M, Hibbs M L, Fearon D T. J Exp Med. 1998;187:807–811. doi: 10.1084/jem.187.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chan V W, Lowell C A, DeFranco A L. Curr Biol. 1998;8:545–553. doi: 10.1016/s0960-9822(98)70223-4. [DOI] [PubMed] [Google Scholar]
- 75.Ho L H, Uehara T, Chen C C, Kubagawa H, Cooper M D. Proc Natl Acad Sci USA. 1999;96:15086–15090. doi: 10.1073/pnas.96.26.15086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maeda A, Scharenberg A M, Tsukada S, Bolen J B, Kinet J P, Kurosaki T. Oncogene. 1999;18:2291–2297. doi: 10.1038/sj.onc.1202552. [DOI] [PubMed] [Google Scholar]
- 77.Gu H, Marth J D, Orban P C, Mossmann H, Rajewsky K. Science. 1994;265:103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- 78.Chen J, Lansford R, Stewart V, Young F, Alt F W. Proc Natl Acad Sci USA. 1993;90:4528–4532. doi: 10.1073/pnas.90.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]