Abstract
Purpose
Listing’s law (LL) constrains the rotational axes of saccades and pursuit eye movements to Listing’s plane (LP). In the velocity domain, LL is ordinarily equivalent to a tilt in the ocular velocity axis equal to half the change in eye position, giving a tilt angle ratio (TAR) of 0.5. This study was undertaken to investigate vertical saccade behavior after the yaw vestibulo-ocular reflex (VOR) had driven eye torsion out of LP, an initial condition causing the position and velocity domain formulations of LL to differ.
Methods
Binocular eye and head motions were recorded with magnetic search coils in eight humans. With the head immobile, LP was determined for each eye, and mean TAR was 0.50 ± 0.07 (mean ± SD) for horizontal and 0.45 ± 0.11 for vertical saccades. The VOR was evoked by transient, whole-body yaw at 2800 deg/s2 peak acceleration, capable of evoking large, uninterrupted VOR slow phases. Before rotation, subjects viewed a target at eye level, 20° up, or 20° down. In two thirds of the trials, the target moved upward or downward at systematically varying times, triggering a vertical saccade during the horizontal VOR slow phase.
Results
Because the head rotation axis was generally misaligned with LP, the eye averaged 3.6° out of LP at vertical saccade onset. During the saccade, eye position continued to depart LP by an average 0.8°. The horizontal TAR at saccade onset was 0.29 ± 0.07. At peak saccade velocity 35 ± 3 ms later, the vertical TAR was 0.45 ± 0.07, statistically similar to that of head fixed saccades. Saccades did not return to LP.
Conclusions
Although they did not observe the position domain formulation of LL, vertical saccades, during the VOR, observed the half-angle velocity domain formulation of LL.
The eye has three degrees of freedom (DF), corresponding to pitch, yaw, and torsion. However, when the head is stationary during visual pursuit or saccades, eye torsion is constrained by Listing’s law (LL), so that eye position has only two DF.1,2 The classic Euler angle formulation of LL states that, with the head upright and immobile, any eye position can be reached from the primary position by rotation about a single axis lying in Listing’s plane (LP). Conformity with LL can be demonstrated by expressing ocular rotational axes as quaternions that, when plotted, are found to lie in LP.3 Tweed et al.4 have pointed out that an alternative but ordinarily equivalent formulation of LL can be expressed in the velocity domain. Unlike one-dimensional (1-D) velocity that is simply the time derivative of position, 3-D eye velocity is a function both of eye position and its derivative. The ocular rotational axis is constrained to a plane if, in the velocity domain, the ocular velocity axis changes by half the amount of eye position change. This change can be expressed as a tilt angle ratio (TAR) of 0.5. Because with the head upright and stationary it is safely assumed that the eye begins in LP, a TAR of 0.5 constrains the eye to remain in LP, and so satisfies LL. However, if eye position were somehow to begin outside LP at the onset of an eye movement that subsequently conforms to the velocity domain formulation of LL, eye position would remain in a plane parallel to but displaced from LP.
Although LL is well known to prevail during saccades and pursuit with the head immobile,5 LL is violated by the vestibulo-ocular reflex (VOR).6–8 Violation of LL during the VOR is not surprising, given that the VOR’s role is to compensate for head rotation about any arbitrary axis and so stabilize images of fixed objects on the retina. Because natural head motion occurs in all three DF,9 an ideal VOR should compensate for head rotation about any axis, without dependency of ocular torsion on eye position in the orbit as implied by LL. The ideal TAR for the VOR would be zero. When tested in the position domain, eye rotation roughly mirrors head rotation around a generous range of axes in both monkey10 and human.11,12 However, ocular torsion during the VOR depends on eye position in the orbit. When eye rotation is examined in the velocity domain, it is apparent that VOR axis shifts by approximately one quarter of the angle of eye position change relative to the head, corresponding to a TAR near 0.25.6–8 This quarter-angle shift in the velocity domain is only half the shift predicted by LL; hence, the VOR violates LL in both the position and velocity domains.
The roles of neural and mechanical factors in determination of ocular kinematics are not fully understood. Before modern descriptions of orbital connective tissue anatomy, it seemed likely that all aspects of LL were implemented neurally in premotor circuits as an intrinsic feature of central ocular motor control.13–15 Miller and Demer16 proposed that pulleys could constrain rectus extraocular muscle (EOM) paths so as to make EOM pulling directions dependent on eye position. It has subsequently emerged that the rectus EOMs have connective tissue soft pulleys17 that receive insertions from the orbital layer of each rectus EOM, so that the pulley moves in coordination with the globe to change the EOM pulling direction by half the change in eye position.18,19 Because 3-D eye velocity is imparted by the direction of EOM force, the mechanical arrangement of the rectus EOM pulleys appears to account for the TAR of 0.5 required by the velocity domain formulation of LL without any explicit central computation of eye torsion.20,21 However, physiologic violations of LL have continued to suggest a role for central neural control of 3-D ocular kinematics.22 For example, during convergence, both eyes extort,23 and LP for each eye rotates temporally.24,25 The ocular extorsion in central gaze is associated with torsional repositioning of the rectus EOM pulley array by the oblique EOMs, and the temporal rotation of LP appears to require that this effect be neurally modulated as a function of vertical gaze position.23 The finding that the eye velocity axis follows half-angle behavior during pursuit and saccades but quarter-angle behavior during the VOR also implies that there must also be neuronal control of the ocular axis. The mechanism of this interaction has yet to be clarified.
Ocular kinematics have been extensively studied during saccades, fixation, and visual pursuit in which torsion is governed by LL,5,13,22,26,27 and during the VOR, which violates LL.6,7,28,29 However, the transition between the VOR’s approximate quarter-angle strategy6,12 and saccades’ half-angle behavior22,27 has received little prior attention. This transition from LL to non-LL behavior is informative about the basis of LL. We know that ocular torsion is driven out of LP by the angular VOR when the axis of head rotation lies outside LP.12 If a saccade were initiated from a non-LP torsional eye position, how would saccade kinematics conform to LL? This is an unusual situation in which the velocity and position domain formulations of LL are no longer equivalent. To return the saccade’s position domain rotational axis to LP would require that the saccade’s velocity axis violate the half angle rule in the process of canceling the initial non-LP torsion. We term this behavior to be in compliance with the position domain formulation of LL. If instead the saccade’s velocity axis conformed to the half-angle rule, the saccade would begin and end with the non-LP torsion induced by the VOR. We term this alternative behavior to be in compliance with the velocity domain formulation of LL. In this sense, half-angle behavior is necessary but not sufficient to assure conformity to LL. To appreciate how these two formulations differ, it should be recognized that half-angle velocity domain behavior always constrains the ocular rotational axis to some plane, but that plane is identical with LP only if initial eye position is Listing’s primary position. It should be apparent that when a saccade is made during a VOR that evokes eye torsion, the ocular motor system can satisfy only one of the two formulations of LL and must violate the other. This dilemma permits determination of which formulation of LL is the underlying organizational principle of the ocular motor system. In other words, is the absolute position of LP of fundamental importance to the ocular motor system or is the half-angle velocity relationship the fundamental principle?
Prior experiments provide contradictory suggestions about which kinematic formulation of LL is observed when the two formulations are in conflict. Intrasaccadic torsion returning the eye to LP has been observed for both horizontal and vertical saccades after torsional optokinetic nystagmus (OKN) had driven the eye out of LP.30 This behavior supports the more fundamental nature of the position domain formulation of LL, because to return to LP, these saccades must have corrected the preexisting non-LL torsion and thus violated the half-angle velocity domain formulation. Such behavior has been presented as evidence for neural control of LL, because it seems impossible to implement with a purely mechanical system.30
Evidence favoring the more fundamental nature of the velocity domain formulation of LL comes from experiments using ocular counterrolling (OCR) to drive initial torsional eye position out of the LP defined with the head upright. Saccades and fixations during OCR have rotational axes confined to planes parallel to, but torsionally offset from, LP.10,31–35 Saccades during OCR obey the half-angle velocity axis formation of LL, but do not return to the LP defined with the head upright, so in this sense the saccades violate the position domain formulation of LL. This behavior has been taken as evidence of a mechanical basis of LL, since it would be explained by torsional shift of the rectus EOM pulley array during OCR.36 No data are available, however, on visually guided saccade kinematics during the angular VOR. The present study was conducted to clarify this issue.
It is also unclear whether the half-angle velocity axis dependency demanded by LL is a property of the entire ocular motor system at a given time or the behavior is axis dependent. Tilt of the ocular axis can, for example, be projected in both the torsional–horizontal, and torsional–vertical planes. Suppose that the head were being rotated around the yaw axis and a saccade were initiated in the orthogonal (pitch) direction. If the saccade were to observe half-angle LL kinematics, would the horizontal VOR slow phase component of eye movement also observe half-angle behavior, or would quarter-angle behavior be maintained? One goal of the current investigation was to answer this question by using a transient rotational stimulus capable of evoking large, horizontal VOR slow phases uninterrupted by quick phases or horizontal saccades.
METHODS
Subjects
Eight normal adult human volunteers gave written consent to participate in these experiments according to a protocol approved by the University of California, Los Angeles Human Subjects Protection Committee in conformity with the tenets of the Declaration of Helsinki. The subjects consisted of seven women and one man of average age 23 ± 4 (± SD; range, 19–29) years. All subjects underwent ophthalmic examination to verify that they were free of ocular disease and would be able to see the targets clearly without corrective lenses. Subjects were monitored during experiments via infrared closed-circuit television and with a duplex intercom.
Apparatus
Angular binocular eye and head positions were sampled at 1.2 kHz with dual-winding scleral magnetic search coils (Skalar Medical, Delft, The Netherlands),37 as previously used in the current laboratory.38 Reference magnetic fields were generated by three pairs of solenoid coils, each 2 m in diameter, and arranged to form the sides of a cube (C-N-C Engineering, Seattle, WA). This configuration placed the center of the cube near eye level. The two vertically oriented coil pairs were driven by 60-kHz sinusoidal currents in phase quadrature.37 The horizontally oriented coil pair was driven by a 120-kHz sinusoidal current.39 Dual-winding scleral magnetic search coil annuli were placed on both eyes of each subject under topical anesthesia with proparacaine 0.5%. Because of coil failures or other technical problems, only monocular eye movement was recorded in three subjects. The subjects in whom only the right eye was recorded were AJ and GA, whereas only the left eye was recorded in subject SY. Angular head position was measured by dual search coils mounted on a bite bar, custom molded to the upper teeth of each subject so that the teeth were rigidly coupled to skull motion. Preliminary experiments indicated that search coils affixed to a headband register head velocity with a significant delay compared with those affixed to a dental appliance, so only the latter were regarded as accurate. Search coils were connected to external detectors (C-N-C Engineering) incorporating single-pole, low-pass filters with a cutoff frequency of 167 Hz.38 Pitch-and-roll coil signals were demodulated by an amplitude-sensitive method, necessitating consideration of a sine nonlinearity. Thus, the pitch angle φ in the Fick sequence (yaw, followed by pitch, followed by roll), can be calculated from the vertical search coil demodulator output (v):
The torsion coil signal (t) can similarly be demodulated to calculate roll, in the Fick sequence:
Fick sequence angles can be used to calculate a rotation matrix3:
Calibration of the search coil system and experimental data acquisition at 1.2 kHz were performed as previously described in the current laboratory.38
Subjects were rotated by a 500 N-m stepper motor (Compumotor; Parker Hannifin Corp., Rohnert Park, CA) with a dedicated driver and position feedback digital controller, as previously described.38 Head position was adjusted so that the axis of rotation was located between the external auditory canals, which were approximately 7 cm posterior to the eyes. This axis was chosen to minimize the translational stimulus to the otoliths. The rotational stimulus had a peak acceleration of 2800 deg/s2 to a velocity of 190 deg/s, which rotated the head a total of 60° in 300 ms.
The visual stimulus consisted of a red laser spot back projected on a screen located 175 cm anterior to the subject. Laser spot position was precisely determined by a two-axis mirror galvanometer (General Scanning, Watertown, MA) synchronously controlled by the data-acquisition computer.
Measurement Conditions
During each trial, the subject sat with the head comfortably upright in a hardwood chair fabricated with nonmetallic fasteners, as previously described.12,38 Every rotational trial was preceded by a 2-second reference position recording in which the subject aligned the eyes toward a centered target 175 cm away.
In 90-second recordings downsampled to 120 Hz, LP was defined for each eye with the head immobile as the subject tracked the movements of the back projected laser target. The target moved in a radial pattern with a sinusoidal velocity peaking at 60 deg/s that could be tracked by visual pursuit, with only a few catch-up saccades per cycle. The velocity axis of vertical saccades was then determined with the head immobile as the subject followed a laser target that moved from center to positions ± 20° vertically eccentric. The center target position was then moved horizontally to the left and right in increments of 5°, so that vertical saccades could be studied with starting positions in the horizontal range of ± 20°. The velocity axis of horizontal saccades was similarly studied in an additional trial by rotating the previously described stimulus 90°. The head-fixed saccade trials provided a stimulus for 22 saccades of 20° (11 in each direction). Because experiment time was limited and the focus of the study was on saccades during the VOR, the head-fixed saccade trials were performed only once horizontally and once vertically by each subject.
Angular VOR trials consisted of 20 directionally randomized, transient yaw rotations (10 in each direction) delivered over a 50-second period. Trials were repeated for each of the three target locations and for each of the approximately five to seven chosen asynchronies between saccade target motion and rotation onset. Before each rotation, subjects were instructed in separate trials to fixate the projected laser target located at eye level, approximately 20° up or down. Onset of rotations varied randomly by ≤ 250 ms, to avoid predictive effects. Except for the laser target, the laboratory was dimly lit.
During a randomly selected third of trials for each initial target location, the target remained immobile throughout the rotation, to discourage anticipatory saccades (Fig. 1). In the remaining two thirds of the trials, the target jumped to a new location just before or during head rotation. Target motion was randomly up or down, with equal probability. In trials in which the target was initially centered, the new location was either 20° up or down. In trials in which the target began 20° eccentric (either up or down), the target moved to the center or moved farther to 25° eccentric. Both the 20° and 5° saccades were analyzed.
Figure 1.
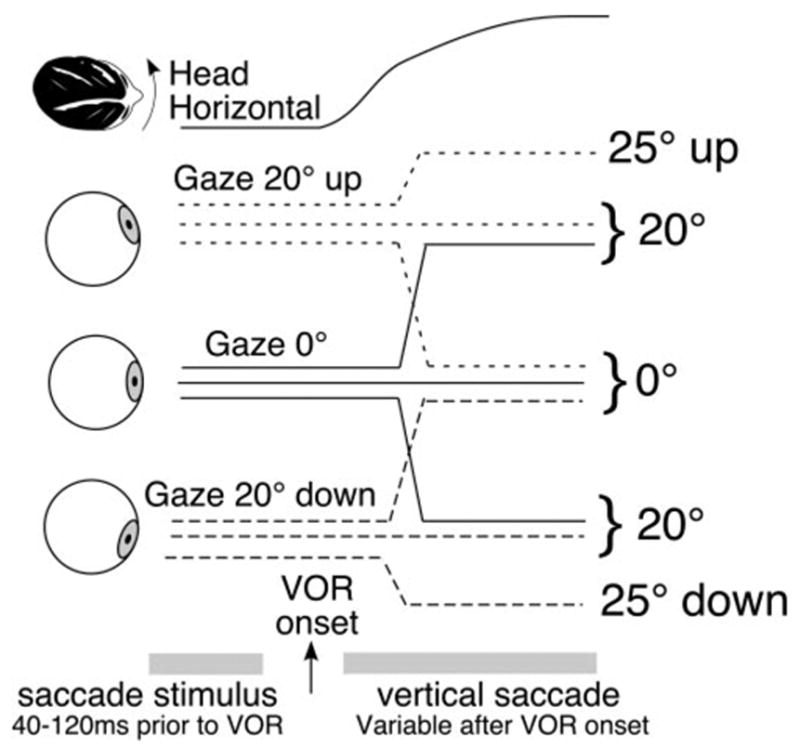
Depiction of the experimental timing during trials involving head motion. At the start of each trial, gaze was centered at 0°, 20° up, or 20° down. In a randomly chosen two thirds of the trials for each starting vertical eye position, the visual target moved randomly up or down with equal probability at a selected time interval before or slightly after onset of whole-body rotation, to evoke a saccade during the horizontal VOR slow phase. The interval between target motion and body rotation was systematically varied to obtain vertical saccades occurring at various horizontal eccentricities evoked by the horizontal VOR. To avoid predictive effects, the visual target remained motionless in a randomly chosen one third of trials.
Data acquisition involving variation of all the preceding conditions was repeated for five to seven different values of asynchrony between saccade target motion and rotation onset. Timing of target motion was in separate trials varied in 40-ms increments, from 280 ms before onset of head motion to 120 ms after onset of head motion. Data were monitored online with a digital polygraph, to assure that saccade target asynchronies were chosen so that for every subject, a large number of vertical saccades occurred over a broad range of initial horizontal eye positions during the VOR slow phase. Most of the data were collected for target motion occurring 40 to 120 ms before head rotation, because this evoked saccades during the first 100 ms of the VOR slow phase in most subjects. Thus, neural saccade programming generally began before onset of the vestibular stimulus, although all saccades that occurred during the VOR were analyzed.
Data Analysis
Data from LP definition and VOR trials were first corrected for orientation of search coils on the eye in central gaze during the immediately preceding reference trial, as previously described.12 Fick angles were converted to rotation matrices as previously described.3,9 The orientation of LP was determined using the best fit plane to quaternion eye positions collected during the LP definition trial, using a published analytical method.4
Data were analyzed automatically on computer, with custom software written in a commercial package (LabView 7.1; National Instruments, Austin TX; Macintosh G4 and G5 computers; Apple Computer, Cupertino, CA). For each subject, rotational transients were grouped based on direction of rotation, direction of gaze, and saccade stimulus. Transient rotations in which eye position varied by more than 0.2° in the 80 ms before rotation were discarded as failures of fixation. Events were also discarded when there were saccades or blink artifacts within the 100 ms before the onset of head rotation.
Eye and head position relative to LP were determined in each trial for each eye and viewing condition. The head stimulus and ocular response during VOR initiation were converted to quaternions in Listing’s coordinates. This allowed the deviation from LP for the eye and head to be determined at any point in the time course of the response. Velocity vectors (ω) for the eyes and head were computed from the corrected quaternion position (q) and derivative (q), as previously described3,29,40,41:
The quaternion derivative (q̇) was calculated from position (q) by taking the derivative of each component of the quaternion and filtering with a third-order Butterworth filter with a cutoff frequency of 50 Hz. The tilt angle of the velocity vector out of LP, φ (in the sagittal plane), was determined from the horizontal (h) and torsional (t) components of velocity as previously described29:
The tilt angle of the velocity vector out of LP in the horizontal plane (θ) was calculated using the vertical (v) and torsional (t) components of the eye velocity vector:
Linear regression was used to calculate the ratio of the tilt of the VOR velocity axis in the sagittal plane, φ, relative to vertical ocular displacement. This value is reported as the TAR of the VOR. Linear regression was performed on sets of three similar trials when the eye was in central, up, and down gazes, as previously described.12 Regression was performed separately for each sampled time point after onset of head motion. After the initial fit, data points lying more than two standard deviations from the fit were removed. This process was then repeated once to determine the final slope and correlation coefficient (R) for the linear regression.
Saccades with the head immobile were analyzed to find the dependence of eye velocity rotational axis on eye position. This value is reported as the TAR for saccades. Previously described visual stimuli were designed to elicit 20° vertical saccades over a range of horizontal starting positions or 20° horizontal saccades over a range of vertical starting positions. Velocity was determined from the quaternion position as described earlier. Saccades were automatically identified by finding the time at which eye velocity exceeded 250 deg/s for at least 25 ms. Data were then examined forward and backward from this time to identify the onset of the saccade, the duration of which was defined as the most recent time at which eye velocity was less than 50 deg/s, continuing until the next time that eye velocity decreased to less than 50 deg/s. The experimental design dictated that most saccades were 20°; however, subjects also occasionally made small corrective saccades. To exclude these small saccades from analysis, only saccades exceeding 10° amplitude were considered. Peak saccade velocity was identified, and the time of peak velocity was used to determine the velocity axis at the corresponding eye position. The slope of the linear regression of the saccade velocity axis against eye position was taken as the TAR. Linear regressions were considered in the overall analysis if R exceeded 0.85, based on a regression including at least 10 saccades.
Saccades that occurred during VOR slow phase were also analyzed. These saccades were identified by finding the time at which vertical eye velocity exceeded 150 deg/s in space-fixed coordinates for at least 25 ms. By using space-fixed coordinates to identify the saccade (but not for analysis of the saccade dynamics) the VOR contribution to eye velocity could be nearly eliminated. A slightly lower threshold was used for saccades during the VOR than for head-fixed saccades, so that smaller saccades could be included in the former analysis. Saccade onset time was defined as the time when vertical eye velocity first exceeded zero. Saccade end time was defined as the next time when the velocity returned to zero. The VOR axis in the sagittal plane was determined at saccade onset, which was considered the last possible time to observe slow-phase behavior. Saccade axis was determined in the horizontal plane at peak saccade velocity.
RESULTS
Listing’s Plane
The thickness and orientation of LP were measured in both eyes of five subjects, and in one eye of three subjects while they sat immobile and regarded a moving target on a screen 175 cm distant. For each subject, LP was defined during three separate trials: one in which the subject pursued a target that moved around a screen in a radial pattern with a sinusoidal peak velocity of 60 deg/s and an amplitude of 20° and two in which the subject made 20° saccades in the horizontal or vertical directions (Fig. 2). The thickness of LP was determined by taking the SD of torsion after the plane had been rotated into Listing’s coordinates. With the pursuit stimulus, the thickness of LP was 2.0 ± 0.6° (mean ± SD). When a horizontal or vertical saccade stimulus was used, LP thickness was 1.1 ± 0.4°, which was significantly thinner than for pursuit (P < 0.01). The orientation of LP measured using each of the stimuli was consistent, with the difference in orientation averaging only 2.6 ± 2.1° (mean ± SD, P > 0.1). However, LP orientation demonstrated a considerable variation both between eyes of the same individual and among individuals. In the right eye, the average LP was oriented 7 ± 5° upward and 6 ± 4° to the left. In the left eye, the orientation of LP averaged 8 ± 4° downward and 22 ± 11° to the right.
Figure 2.
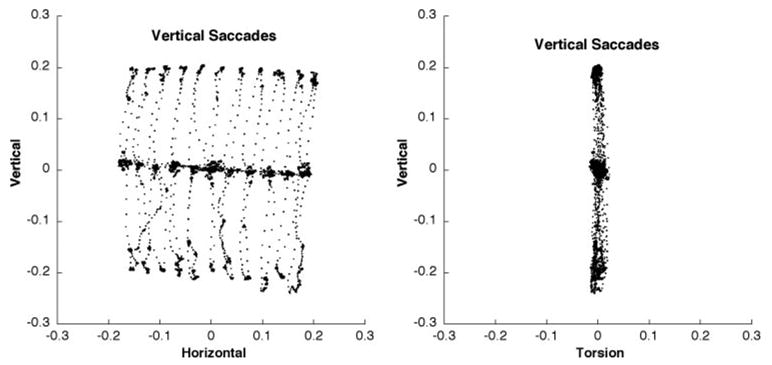
Listing’s plane defined during ± 20° vertical saccades over a ± 20° range of horizontal positions. Data are plotted in quaternions (similar to rotation vectors) that were rotated into Listing’s coordinates so that the thickness of the plane can be easily visualized. Horizontal and vertical offsets have been subtracted for clarity.
Saccades with Head Immobile
Saccades were studied with the head immobile while subjects tracked a target that moved from center to ± 20° eccentric from a range of horizontal or vertical positions (Fig. 2). Saccades with peak velocity of at least 250 deg/s and displacement of at least 10° were selected for analysis. The TAR was calculated at the time of peak saccade velocity and plotted on the orthogonal axis against eye position at saccade onset. Linear regression was performed independently for each subject and each eye to determine the slope of the eye position dependence that defined the TAR (Fig. 3). Only trials in which R ≥ 0.85 (R2 ≥ 0.72) were considered, to ensure that the linear regression accurately represented the data. All subjects could make horizontal saccades meeting this criterion, but only four subjects made vertical saccades that did. The mean TAR was 0.45 ± 0.11 for vertical and 0.50 ± 0.07 for horizontal saccades. These values do not differ significantly from the 0.5 value specified by LL, nor do they differ from one another (P > 0.1). The average R2 for the trials included was 0.90 ± 0.02 for vertical saccades and 0.88 ± 0.05 (mean ± SD) for horizontal saccades. The average R2 for trials excluded was 0.4 ± 0.3.
Figure 3.
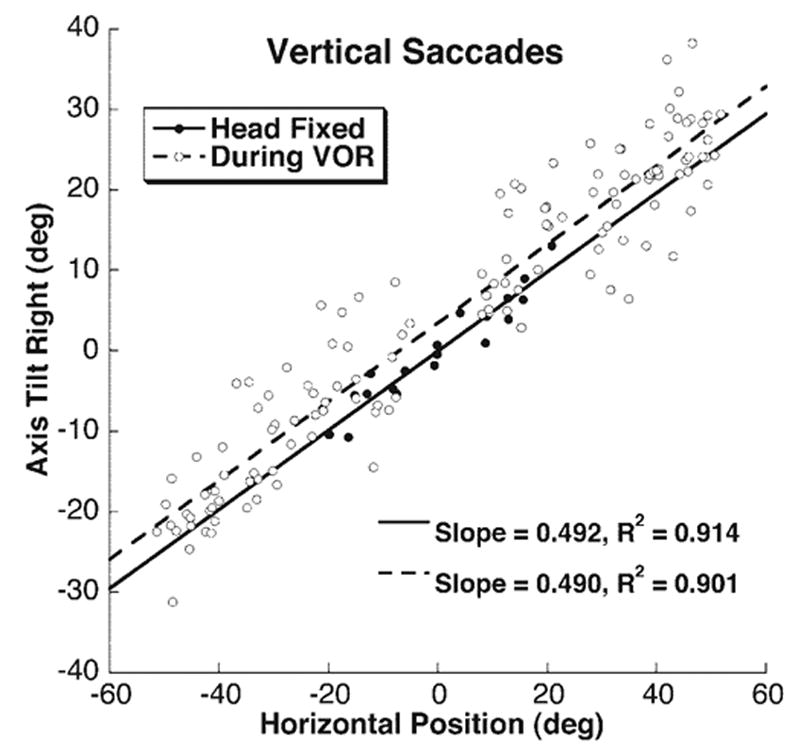
Dependence of the velocity axis of vertical saccades on starting horizontal eye position in a representative subject. Solid symbols: saccades with the head immobilized, derived from the data shown in Figure 2. Open symbols: saccades initiated during the horizontal VOR slow phase. Slopes and R2 values were similar for both conditions.
Saccades during the VOR
Position Domain Analysis
The VOR was evoked by a highly repeatable but directionally unpredictable, whole-body yaw stimulus (Fig. 4). Before onset of head motion, subjects viewed a laser target at eye level, 20° up, or 20° down. Timing of target motion relative to rotation onset was deliberately varied so that saccades would be initiated during a ± 50° range of horizontal eye positions during the VOR slow phase (Fig. 3). Most saccades were evoked by a target motion stimulus 40 to 120 ms before onset of head rotation. Saccades occurred from 4 to 760 ms after VOR onset, averaging 220 ms, for an average saccade latency from visual target motion averaging approximately 300 ms. The eye reached peak vertical velocity of approximately 500 deg/s and did so an average of 35 ± 3 ms (± SD of averages for each subject) after saccade onset. Mean vertical saccade duration was 68 ± 5 ms. A typical set of trials is shown in Figure 4 in which the subject began viewing a target located 20° down, then made a large upward saccade to follow the laser target during the VOR slow phase. There were also a few saccades evoked when target motion occurred 20 to 50 ms after onset of head rotation. Although these saccades tended to occur later and at more eccentric horizontal eye positions, they were qualitatively similar to the early saccades.
Figure 4.
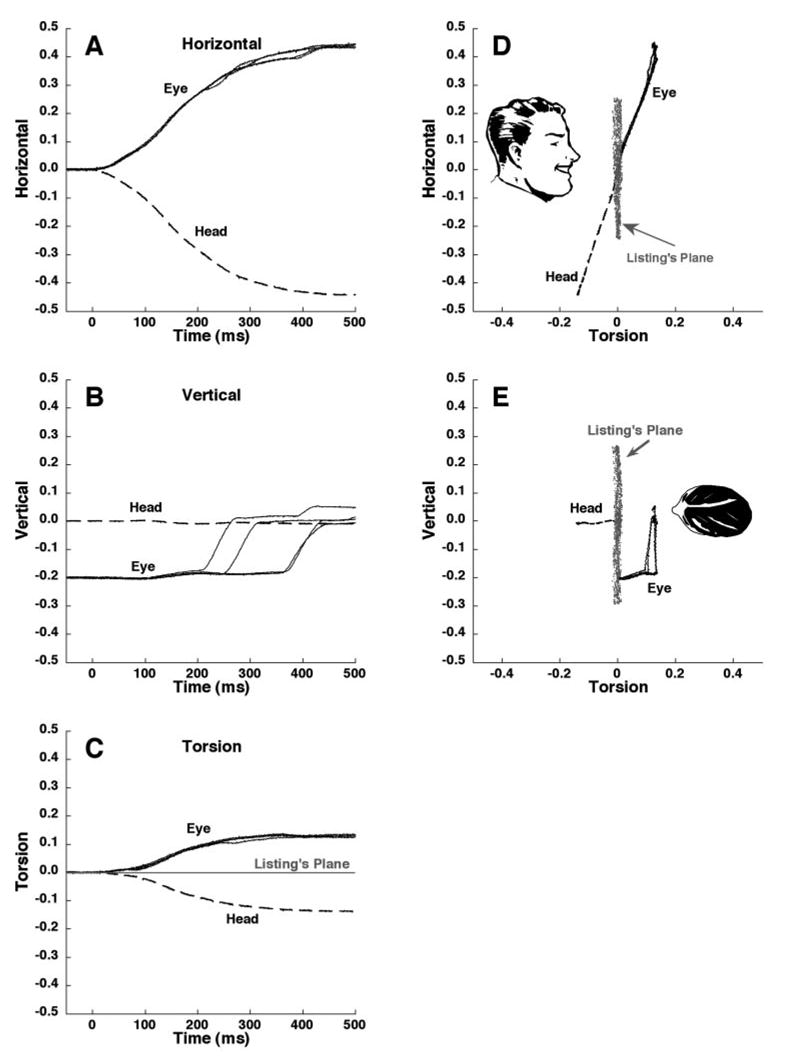
Head and eye position in quaternions, rotated into Listing’s coordinates. Horizontal and vertical offsets have been subtracted to indicate a starting position of zero for clarity. A set of four trials is shown in which the head moved to the left, initial gaze was 20° down, and saccades were made upward to the 0° position. Dashed line: head position, which was identical in each trial. Single dark line: eye responses. (A–C) Responses as a function of time; (D, E) previously defined LP in earth-fixed coordinates, in its orientation before head motion. Note that the eye position remains out of LP after saccades.
The position of the eye and head relative to LP could be represented by rotating eye and head data into Listing’s coordinates. By definition, the quaternion torsion component of LP is zero in Listing’s coordinates, so in this coordinate system, torsion indicates the distance from LP (Fig. 4C). As anticipated for the immobile head condition, eye position began in LP during target fixation before head rotation. Because LP and the axis of head rotation were generally misaligned, the VOR tended to drive eye position out of LP, as seen when the data are superimposed on data for LP plotted with the head immobile (Figs. 4C–E). Thus, a head rotation that is purely horizontal in earth-fixed coordinates typically includes significant torsion when considered in Listing’s coordinates. At the start of the saccade, head position averaged 3 ± 2° (SD) out of LP, and at saccade completion the head was 4 ± 3° out of LP. This effect did not vary significantly with gaze position (P > 0.1; Fig. 5). At the start of the saccade, eye position averaged 4 ± 2° out of LP. Eye position moved a mean of 1.7 ± 1.5° farther out of LP during the course of upward saccades and during maintained central gaze (not significantly different, P > 0.1). Eye position moved 0.5 ± 1.1° farther out of LP during the course of downward saccades, significantly less than for the other two conditions (P < 0.01, Fig. 5). Thus, under all conditions, mean eye position was farther from LP at the end of the saccade than it had been at the start. In no subject did the saccade return the eye to LP.
Figure 5.
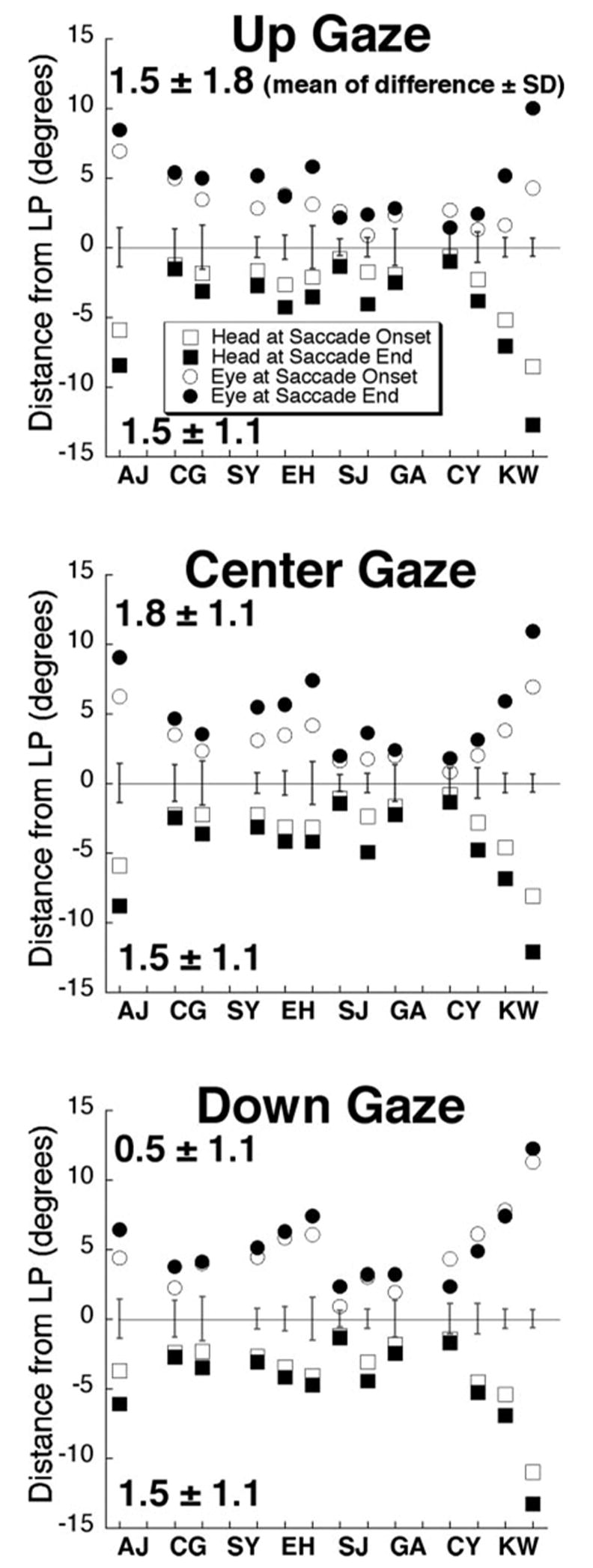
Mean distance of eye (plotted positive) and head (plotted negative) from LP at the onset and end of vertical saccades executed during the horizontal VOR for three vertical starting positions: ~20° up, center, and ~20° down. Two-letter codes designate individual subjects with data presented for both eyes when available. Data are plotted relative to LP determined with the head stationary in space-fixed coordinates so that the head torsion stimulus to the VOR can be easily assessed. Open symbols: the distance from LP of eye (circles) and head (squares) at saccade onset. Filled symbols: distance of the eye and head from LP at saccade end. Mean and SD values denote increase in distance of eye and head from LP during saccade. Two major factors contributed to variability in distance of eye and head from LP: saccade timing was deliberately varied relative to VOR onset, and LP orientation naturally varied relative to head rotation axis. More extreme values, such as in subjects AJ and KW due to more tilted orientation of LP. Error bars: LP thickness (± 1 SD of torsion in degrees). Head positions are shown as negative values for clarity.
Velocity Domain Analysis
Vertical saccades during the horizontal VOR were identified by the requirement that minimum head-fixed vertical peak velocity reach at least 150 deg/s. Compliance with LL in the velocity domain was assessed by measuring the TAR as demonstrated for static saccades in Figure 3. By design, the VOR was predominately in the horizontal (yaw) plane, and the visually triggered saccades were predominately in the vertical (pitch) plane. When measured at saccade onset, the TAR for the VOR, which depended on initial vertical eye position, was 0.29 ± 0.07 (mean ± SD; Fig. 6), based on an average R2 of 0.32 ± 0.16. Axis tilt of the vertical saccade, which depended on instantaneous yaw eye position, was most reliably analyzed at the time of peak saccade velocity, which averaged 35 ± 3 ms (mean ± SD) from saccade onset. The vertical saccade TAR was 0.45 ± 0.07 (mean ± SD; Fig. 6), based on an average R2 of 0.79 ± 0.13 (mean ± SD). The TAR for the vertical saccade was significantly greater than that for the VOR at saccade onset (P < 0.01) and was statistically identical with the TAR of saccades measured with the head immobile (P = 0.94). As seen in Figure 6, there was some individual variability, but the TAR for vertical saccades was greater than for the VOR in seven of eight subjects.
Figure 6.
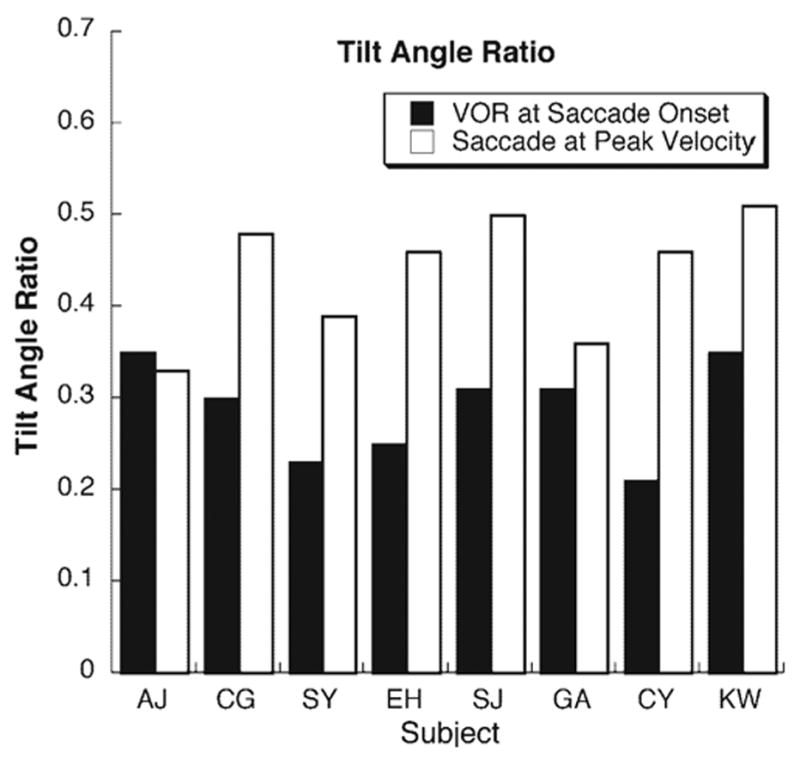
TAR for each subject at the start and at velocity peak of vertical saccades during the VOR. Data represents the average of both eyes of each subject when binocular data was available.
DISCUSSION
The present data confirm in humans conformity to LL under conditions when it is expected to prevail. Saccades with the head immobile have been shown to obey LL in the position domain,42 although slight deviations from the predicted velocity axes of saccades have been observed.27,43 Our data, collected with the head immobile, confirm these observations. The LP defined during saccade trials was slightly but significantly thinner than that defined during visual pursuit at 1.1 ± 0.4° versus 2.0 ± 0.6°. Others also have reported LP thickness in the range of 0.9 to 1.2° in monkeys10 and 1.2° in humans.6 The thickness of LP recorded in the current experiment during pursuit was slightly greater at 2.0 ± 0.6°, perhaps because the search coil annulus slipped slightly more over the eye during pursuit.44 A second possibility is that when saccades define LP, most of the data were collected during fixations, which obey LL more precisely than pursuit.27 One unexpected finding was that there was a systematic difference in vertical LP orientation between the two eyes, for reasons not clear. The orientation of LP did not depend on whether saccades or pursuit were used for definition. In the present study, the average TAR for vertical saccades was 0.45 ± 0.11 (mean ± SD) and 0.50 ± 0.07 for horizontal saccades. These values are consistent with the half-angle velocity domain requirement of LL. Taken together, these observations not only support the robustness of LL, but also provide confidence in the recording and analytical methods used in the present study.
The present study also confirms the non-LL behavior reported somewhat more variably for the VOR. During head motion, eye position has been described to correct for head position without regard to the orientation of LP.10,12 In the more sensitive velocity domain, the ocular rotational axis reportedly deviates from the head axis by roughly one quarter the angle of eye position, both during low-frequency, sinusoidal head rotation6,29 and rotational transients.7,8,12 The present study confirms this finding, extending its temporal resolution to include the time of initiation of vertical saccade onset. Even as the saccade was about to begin, the mean TAR of the VOR slow phase was 0.29 ± 0.07, not significantly different from quarter-angle behavior.
Conformity to LL in the angular position domain implies in the velocity domain a half-angle dependence of the ocular axis on eye position.4 By visually triggering vertical saccades during large horizontal VOR slow phases, however, the current experiment demonstrates that the converse of the preceding statement is not always true. Whereas the Euler angle and velocity domain formulations of LL are theoretically identical when eye position begins in LP,4 they are no longer equivalent when eye position begins out of LP. To obey LL in the position domain in such a case, a saccade beginning from a position out of LP would have had to return to LP by correcting the preexisting torsion. That correction would require rotation about an axis not changing by half of eye orientation and so violating the half-angle rule. To conform to the velocity axis definition of LL, the preexisting non-Listing torsion cannot be canceled, requiring eye position to follow a trajectory paralleling but displaced from LP. The VOR slow phase, as observed in this study, did not conform to LL and so generated a torsional eye position displacement out of LP that was present when the visually generated saccade occurred. Despite this initial condition of non-LL torsion, the resultant visually guided saccade conformed to the velocity domain formulation of LL, because the eye’s rotational velocity axis changed by half the angle of horizontal eye position. The saccade made during the VOR slow phase had a TAR of 0.45 ± 0.07 (mean ± SD), identical with the 0.45 ± 0.11 observed during vertical saccades with the head immobile and not significantly different from 0.5. Moreover, the visually guided vertical saccade exhibited this half-angle behavior in the velocity domain despite progressively increasing non-LL torsion due to the ongoing VOR slow phase. Rather than returning the eye to LP, the vertical saccade during the horizontal VOR continued to depart LP in about the same way as for the VOR alone. While failing to observe the position domain formulation of LL, the vertical saccades observed the velocity domain formulation of LL.
The kinematic result just described is seemingly at odds with the study of Lee et al.,30 who examined saccade kinematics from non-LL starting torsion induced by torsional OKN. The optokinetic system accesses vestibular velocity storage and can also produce ocular torsion out of LP. They found that saccades during torsional OKN returned eye position to LP.30 Although the velocity domain formulation of LL was not explicitly examined by Lee et al., such a saccade would necessarily violate LL in the velocity domain, since the torsional component of eye velocity must correct the initial non-LL torsion induced by the OKN stimulus. However, during normal torsional OKN, a slow-phase component brings the eye out of LP and is followed by a torsional quick phase that returns the eye back to LP.30 Given the similarities between the VOR and OKN, one might have incorrectly expected the saccade in the current experiment to have returned eye position to LP. The difference in outcomes may be related to the reflexive coupling between the slow and quick phases of nystagmus. During OKN, quick phases reflexively return eye position to LP, even in the absence of any voluntary saccade. It has been reported that during vestibular nystagmus, quick phases even drive the eye out of LP, allowing slow phases to return the eye to LP.10 One must presume that the neural commands for slow and quick phases of nystagmus are not independent45,46 and that saccades may trigger quick phases. Vestibular nystagmus, with its repetitive alternation of slow and quick phases, has an element of predictability that may confound the study of VOR kinematics. It is possible that in the experiments of Lee et al.,30 the experimentally imposed saccade entrained an OKN quick phase that was responsible for bringing eye position back into LP. The current experiment altogether avoided predictability and quick phases and indeed any saccades besides the desired vertical one. Unlike the experiment of Lee et al., in the present study, vertical saccades during the angular VOR never returned the eye to LP.
The transition in kinematics between the quarter angle of the VOR and the half angle of saccades occurred in the current study without measurable delay. It has been demonstrated that the TAR for the VOR is near 0.25 as early as 20 ms after head rotation onset.12 Because computation of the TAR requires sufficiently high eye velocity to compute the rotational axis in the presence of baseline noise, 20 ms is as early as any VOR axis can be determined at all and is very close to human angular VOR latency, as measured under these conditions.38 This suggests that quarter-angle kinematics are an intrinsic property of disynaptic VOR. The current results indicate that the TAR remained near quarter angle up to saccade onset and thus was not modified in anticipation of the saccade. By the time peak velocity was reached only 35 ms later, the TAR for the saccade was 0.5, as required by the velocity domain formulation of LL. Thus, the velocity domain formulation of LL seems to be an inherent property of the visually guided saccade, irrespective of head motion. We would therefore propose that the underlying rationale for LL originates in the velocity rather than in the position domain.
The purpose and underlying basis of LL in the oculomotor system remain controversial. Some favor a neuronal implementation of LL in the premotor neural circuits,15,30,47,48 whereas others have suggested that the origin of LL lies in the mechanical action of rectus EOM pulleys.20,36,49 The finding of Lee et al.30 that saccades beginning out of LP return the eye to LP has been taken as support for a neural origin of LL, but that finding is contradicted by the current results for the VOR. If a saccade always ended in LP, it would imply that the saccade was programmed to do so based on knowledge of starting eye position relative to LP. If such behavior had been observed, it would have been difficult to explain on the basis of mechanical factors. The current findings support a mechanical basis for LL. These results indicate that if the VOR has moved the eye out of LP at the beginning of a visually guided saccade, the eye remains out of LP at the end of the saccade. Thus, in the current paradigm, the absolute location of the eye relative to LP does not seem crucial to the oculomotor system. It is rather the half-angle velocity domain relationship that apparently provides the more general basis for LL.
Most of the vertical saccades studied were in response to target motion that occurred 40 to 120 ms before head rotation began. Saccade planning began, but may have been incomplete, when head rotation started. It should be noted that this is a major difference between the present study and the study of Lee et al.30 in which saccades were initiated during OKN. In that study, saccade programming began while relatively low velocity torsional OKN slow phases out of LP were already under way. For most saccades studied in the current experiment, the eye was still quietly in LP when saccade planning began. Thus, it is possible that in the saccades studied by Lee et al. during steady state OKN, neural programming could compensate for predictable and slowly accumulating ocular torsion, while neural programming could not anticipate the torsion later to develop due to the current unpredictable, high-acceleration VOR.
Perhaps in the current experiment saccade innervation was adjusted during saccade planning, or intrasaccadically, to fit the velocity domain definition of LL, and this computation was updated by ongoing vestibular input. Such a dynamic 3-D neural computation would be complex and serve no obvious purpose. A sensory purpose of LL is not evident, despite the suggestion that LL simplifies perception of orientation for eccentric gaze locations.50 Perception is not a factor during the flight of a saccade,51 although visual perception is vital during postsaccadic fixation.49 Perception does not explain why the saccade follows the velocity domain definition of LL despite initial torsion out of LP. The currently observed saccade behavior seems aimed to preserve the non-Listing’s torsion driven by the VOR slow phase. The torsional VOR slow phase itself is perceptually valuable to compensate for head rotation, since the VOR stabilizes images of the world on the retina to enable clear vision. Another possible rationale for neural implementation of LL is decreased energy expenditure,6 but the negligible energy expenditure associated with rotating the eye a few degrees in torsion hardly seems a rationale for such complex neural processing.
The current observations appear consistent with peripheral mechanical constraints on ocular rotation. The model of Quaia and Optican49 demonstrates that if the half-angle eye velocity dependence on eye position corresponding to LL is implemented in orbital mechanics, the oculomotor plant appears commutative to the brain and can be commanded by signals corresponding to time derivatives of eye position without measurable errors in velocity–position matching.20 This facilitates the matching of the saccadic pulse and step commands, so that perceptually important postsaccadic drift is avoided. Strong functional anatomic evidence from orbital magnetic resonance imaging indicates that the rectus EOMs change their pulling directions by half the change in eye orientation across a broad range of secondary and tertiary gaze positions.52,53 This mechanical behavior intrinsically implements half-angle behavior, corresponding to the velocity domain formulation of LL. The active-pulley hypothesis proposes that the half-angle kinematics of the rectus and inferior oblique EOMs is due to their path constraint by connective tissue rings comprising pulleys. These pulleys receive insertions from the orbital layers of their respective EOMs, permitting (for all pulleys except the trochlea of the superior oblique) active control of pulley position and hence EOM pulling direction.52–54 Thus, it appears that the velocity domain formulation of LL is an emergent property of the orbital mechanics and is a sort of default mode for the ocular motor apparatus. With such plant mechanics, visual fixation and pursuit can be commanded by 2-D retinal error signals without central neural representation of 3-D eye position19; and, although a 3-D representation of eye position is necessary at a higher level for the sensorimotor transformation that allows accurate target localization in space,55 peripheral orbital mechanics constrain the torsional DF.
The observation that the quarter-angle VOR behavior of the VOR and half-angle behavior of saccades can occur within a few milliseconds of each other provides some clues to the underlying basis of how the oculomotor system changes between these behaviors. It has been suggested that the quarter-angle behavior of the VOR could be caused by anteroposterior displacement of the pulleys.19,56 However, no such movement of the pulleys has been observed, and quarter-angle VOR kinematics may be more simply explained by weak torsional VOR gain.15 The current findings provide further evidence that the pulleys do not shift anteroposteriorly during the VOR, since the transition between quarter- and half-angle kinematics observed in our study would require such a hypothetical shift to occur in the unrealistically short time frame of 20 ms or less. Neural control of the VOR axis has been offered as a plausible alternative.15 If quarter-angle VOR kinematics is a neural strategy, it implies an underlying purpose. Quarter-angle VOR behavior has been suggested as a strategy for maintaining eye position within the oculomotor range and as a compromise between limiting optic flow at the fovea and the periphery.15 However, the current results indicate that a vertical saccade superimposed on the horizontal VOR does not bring eye position closer to LP, and so it does not confine the limit of eye position.
This evidence argues for reconsideration of mechanical factors in ocular kinematics, because rectus EOM paths are the determinants of the velocity axes the EOMs impose on the eye. The current experiment demonstrated that the quarter-angle VOR kinematics and half-angle kinematics of saccades could follow within a few milliseconds of each other. Quarter-angle VOR behavior that violates LL may be explained by oblique EOM action on rectus EOM pulling directions. The orbital layers of the inferior57 and superior oblique EOMs insert directly and indirectly on the rectus pulleys, so that oblique EOM activity can alter the torsional orientation of the rectus pulley array during the VOR.23,36 This imposes a torsional offset in the mechanically implemented LP, as is observed during static head tilts.33,58 Consistent with the noncommutative demands of the VOR,59 torsional repositioning of the rectus pulley array influences the directional response of the rectus EOMs to subsequent activation by other inputs, including saccadic target programming. It appears likely that vestibular inputs drive the instantaneous torsional position of the rectus pulley array. This torsion is mechanically superimposed on the mechanical configuration of the rectus EOM pulleys necessary to implement the half-angle dependency of ocular rotational axis on eye position required by LL. This would explain the current finding that vertical saccades during the horizontal VOR slow phase not only maintain the non-LL starting torsion imposed by the VOR, but also the current finding that this torsion increases intrasaccadically as the VOR continues. This arrangement also explains the absence of observable delay in shift between quarter-angle VOR kinematics and the half-angle kinematics of the saccade. The current findings correspond to those predicted by the “displacement-feedback” model of Crawford and Guitton, in which visual retinal error can be mapped only onto corresponding zero-torsion motor error commands within LP.60 The displacement-feedback model, with either a mechanical or neural basis for half-angle behavior, can simulate the visuomotor transformations necessary for accurate and kinematically correct saccades within a reasonable oculomotor range, but was rejected by Crawford and Guitton on the basis of their supposition of empirical findings contrary to those of the current experiment.60 The current findings suggest that the “displacement-feedback” model, lacking in a neural representation of LL, is plausible for control of saccades to visual targets, and that in context of realistic mechanical properties of EOM pulleys,53 control of visual saccades does not require explicit neural computation of ocular torsion. The quarter-angle dependence of the VOR axis on eye orientation, however, is a complex behavior that may result from both mechanical and central neural causes yet to be clarified.
Acknowledgments
The authors thank Nicolasa de Salles for help in recruiting and organizing the subjects and Frank Enriquez and David Burgess for providing technical support.
Footnotes
Disclosure: B.T. Crane, None; J. Tian, None; J.L. Demer, None
Supported by NIDCD Grant DC005224. JLD received an unrestricted award from Research to Prevent Blindness and is Leonard Apt Professor of Ophthalmology.
References
- 1.Ferman L, Collewijn H, Van dBA. A direct test of Listing’s law. I. Human ocular torsion measured in static tertiary positions. Vision Res. 1987;27:929–938. doi: 10.1016/0042-6989(87)90009-5. [DOI] [PubMed] [Google Scholar]
- 2.van Rijn LJ, van den Berg AV. Binocular eye orientation during fixations: Listing’s law extended to include eye vergence. Vision Res. 1993;33:691–708. doi: 10.1016/0042-6989(93)90189-4. [DOI] [PubMed] [Google Scholar]
- 3.Haslwanter T. Mathematics of three-dimensional eye rotations. Vision Res. 1995;35:1727–1739. doi: 10.1016/0042-6989(94)00257-m. [DOI] [PubMed] [Google Scholar]
- 4.Tweed D, Cadera W, Vilis T. Computing three-dimensional eye position quaternions and eye velocity from search coil signals. Vision Res. 1990;30:97–110. doi: 10.1016/0042-6989(90)90130-d. [DOI] [PubMed] [Google Scholar]
- 5.Tweed D, Fetter M, Andreadaki S, Koenig E, Dichgans J. Three-dimensional properties of human pursuit eye movements. Vision Res. 1992;32:1225–1238. doi: 10.1016/0042-6989(92)90217-7. [DOI] [PubMed] [Google Scholar]
- 6.Misslisch H, Tweed D, Fetter M, Sievering D, Koenig E. Rotational kinematics of the human vesibuloocular reflex. III. Listing’s law. J Neurophysiol. 1994;72:2490–2502. doi: 10.1152/jn.1994.72.5.2490. [DOI] [PubMed] [Google Scholar]
- 7.Thurtell MJ, Black RA, Halmagyi GM, Curthoys IA, Aw ST. Vertical eye position-dependence of the human vestibuloocular reflex during passive and active yaw head rotations. J Neurophysiol. 1999;81:2415–2428. doi: 10.1152/jn.1999.81.5.2415. [DOI] [PubMed] [Google Scholar]
- 8.Migliaccio AA, Cremer PD, Aw ST, et al. Vergence-mediated changes in the axis of eye rotation during the human vestibulo-ocular reflex can occur independent of eye position. Exp Brain Res. 2003;151:238–248. doi: 10.1007/s00221-003-1447-z. [DOI] [PubMed] [Google Scholar]
- 9.Crane BT, Demer JL. Human gaze stabilization during natural activities: translation, rotation, magnification, and target distance effects. J Neurophysiol. 1997;78:2129–2144. doi: 10.1152/jn.1997.78.4.2129. [DOI] [PubMed] [Google Scholar]
- 10.Crawford JD, Vilis T. Axes of eye rotation and Listing’s law during rotations of the head. J Neurophysiol. 1991;65:407–423. doi: 10.1152/jn.1991.65.3.407. [DOI] [PubMed] [Google Scholar]
- 11.Tweed D, Fetter M, Sievering D, Misslisch H, Koenig E. Rotational kinematics of the human vestibuloocular reflex. II. Velocity steps. J Neurophysiol. 1994;72:2480–2489. doi: 10.1152/jn.1994.72.5.2480. [DOI] [PubMed] [Google Scholar]
- 12.Crane BT, Tian JR, Demer JL. Human angular vestibulo-ocular reflex initiation: relationship to Listing’s Law. Ann NY Acad Sci. 2004 doi: 10.1196/annals.1325.004. [DOI] [PubMed] [Google Scholar]
- 13.Crawford JD, Vilis T. Symmetry of oculomotor burst neuron coordinates about Listing’s plane. J Neurophysiol. 1992;68:432.4–48. doi: 10.1152/jn.1992.68.2.432. [DOI] [PubMed] [Google Scholar]
- 14.Tweed D. Three-dimensional model of the human eye-head saccadic system. J Neurophysiol. 1997;77:654–666. doi: 10.1152/jn.1997.77.2.654. [DOI] [PubMed] [Google Scholar]
- 15.Misslisch H, Tweed D. Neural and mechanical factors in eye control. J Neurophysiol. 2001;86:1877–1883. doi: 10.1152/jn.2001.86.4.1877. [DOI] [PubMed] [Google Scholar]
- 16.Miller JM, Demer JL. New orbital constraints on eye rotation. In: Fetter M, Misslisch H, Tweed D, editors. Three-Dimensional Kinematic Principles of Eye-, Head-, and Limb Movements in Health and Disease. Tübingen, Germany: University of Tübingen; 1995. pp. 349–357. [Google Scholar]
- 17.Demer JL, Miller JM, Poukens V, Vinters HV, Glasgow BJ. Evidence for fibromuscular pulleys of the recti extraocular muscles. Invest Ophthalmol Vis Sci. 1995;36:1125–1136. [PubMed] [Google Scholar]
- 18.Clark RA, Miller JM, Demer JL. Location and stability of rectus muscle pulleys inferred from muscle paths. Invest Ophthalmol Vis Sci. 1997;38:227–240. [PubMed] [Google Scholar]
- 19.Demer JL, Oh SY, Poukens V. Evidence for active control of rectus extraocular muscle pulleys. Invest Ophthalmol Vis Sci. 2000;41:1280–1290. [PubMed] [Google Scholar]
- 20.Raphan T. Modeling control of eye orientation in three dimensions. I. Role of muscle pulleys in determining saccadic trajectory. J Neurophysiol. 1998;79:2653–2667. doi: 10.1152/jn.1998.79.5.2653. [DOI] [PubMed] [Google Scholar]
- 21.Demer JL. The orbital pulley system: a revolution in concepts of orbital anatomy. Ann NY Acad Sci. 2002;956:17–32. doi: 10.1111/j.1749-6632.2002.tb02805.x. [DOI] [PubMed] [Google Scholar]
- 22.Klier EM, Crawford JD. Human oculomotor system accounts for 3-D eye orientation in the visual-moor transformation for saccades. J Neurophysiol. 1998;80:2274–2294. doi: 10.1152/jn.1998.80.5.2274. [DOI] [PubMed] [Google Scholar]
- 23.Demer JL, Kono R, Wright W. Magnetic resonance imaging of human extraocular muscles in convergence. J Neurophysiol. 2003;89:2072–2085. doi: 10.1152/jn.00636.2002. [DOI] [PubMed] [Google Scholar]
- 24.Kapoula Z, Bernotas M, Haslwanter T. Listing’s plane rotation with convergence: role of disparity, accommodation, and depth perception. Exp Brain Res. 1999;126:175–186. doi: 10.1007/s002210050727. [DOI] [PubMed] [Google Scholar]
- 25.Mok D, Ro A, Cadera W, Crawford JD, Vilis T. Rotation of Listing’s plane during vergence. Vision Res. 1992;32:2055–2064. doi: 10.1016/0042-6989(92)90067-s. [DOI] [PubMed] [Google Scholar]
- 26.Haslwanter T, Straumann D, Hepp K, Hess BJ, Henn V. Smooth pursuit eye movements obey Listing’s law in the monkey. Exp Brain Res. 1991;87:470–472. doi: 10.1007/BF00231865. [DOI] [PubMed] [Google Scholar]
- 27.Straumann D, Zee DS, Solomon D, Kramer PD. Validity of Listing’s law during fixations, saccades, smooth pursuit eye movements, and blinks. Exp Brain Res. 1996;112:135–146. doi: 10.1007/BF00227187. [DOI] [PubMed] [Google Scholar]
- 28.Palla A, Straumann D, Obzina H. Eye-position dependence of three-dimensional ocular rotation axis orientation during head impulses in humans. Exp Brain Res. 1999;129:127–133. doi: 10.1007/s002210050943. [DOI] [PubMed] [Google Scholar]
- 29.Walker MF, Shelhamer M, Zee DS. Eye-position dependence of torsional velocity during interaural translation, horizontal pursuit, and yaw-axis rotation in humans. Vision Res. 2004;44:613–620. doi: 10.1016/j.visres.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Lee C, Zee DS, Straumann D. Saccades from torsional offset positions back to listing’s plane. J Neurophysiol. 2000;83:3241–3253. doi: 10.1152/jn.2000.83.6.3241. [DOI] [PubMed] [Google Scholar]
- 31.Haslwanter T, Straumann D, Hess BJM, Henn V. Static roll and pitch in the monkey: shift and rotation of Listing’s plane. Vision Res. 1992;23:1341–1348. doi: 10.1016/0042-6989(92)90226-9. [DOI] [PubMed] [Google Scholar]
- 32.Bockisch CJ, Haslwanter T. Three-dimensional eye position during static roll and pitch in humans. Vision Res. 2001;41:2127–2137. doi: 10.1016/s0042-6989(01)00094-3. [DOI] [PubMed] [Google Scholar]
- 33.Furman JM, Schor RH. Orientation of Listing’s plane during static tilt in young and older human subjects. Vision Res. 2003;43:67–76. doi: 10.1016/s0042-6989(02)00385-1. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki Y, Kase M, Kato H, Fukushima K. Stability of ocular counterrolling and Listing’s plane during static roll-tilts. Invest Ophthalmol Vis Sci. 1997;38:2103–2111. [PubMed] [Google Scholar]
- 35.Hess BJM, Angelaki DE. Gravity modulates Listing’s plane orientation during both pursuit and saccades. J Neurophysiol. 2003;90:1340–1345. doi: 10.1152/jn.00167.2003. [DOI] [PubMed] [Google Scholar]
- 36.Demer JL. Pivotal role of orbital connective tissues in binocular alignment and strabismus: The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 2004;45:729–738. doi: 10.1167/iovs.03-0464. [DOI] [PubMed] [Google Scholar]
- 37.Collewijn H, van der Mark F, Jansen TC. Precise recording of human eye movements. Vision Res. 1975;15:447–450. doi: 10.1016/0042-6989(75)90098-x. [DOI] [PubMed] [Google Scholar]
- 38.Crane BT, Demer JL. Human horizontal vestibulo-ocular reflex initiation: effects of angular acceleration, linear acceleration, stimulus intensity, target distance, and unilateral lesions. J Neurophysiol. 1998;80:1151–1166. doi: 10.1152/jn.1998.80.3.1151. [DOI] [PubMed] [Google Scholar]
- 39.Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Electr. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- 40.Hepp K. On Listing’s Law. Communications in Mathematical Physics. 1990;132:285–292. [Google Scholar]
- 41.Tweed D, Vilis T. Implications of rotational kinematics for the oculomotor system in three dimensions. J Neurophysiol. 1987;58:832–849. doi: 10.1152/jn.1987.58.4.832. [DOI] [PubMed] [Google Scholar]
- 42.Misslisch H, Tweed D, Vilis T. Neural constraints on eye motion in human eye-head saccades. J Neurophysiol. 1998;79:859–869. doi: 10.1152/jn.1998.79.2.859. [DOI] [PubMed] [Google Scholar]
- 43.Bruno P, Van den Berg AV. Torsion during saccades between tertiary positions. Exp Brain Res. 1997;117:251–265. doi: 10.1007/s002210050220. [DOI] [PubMed] [Google Scholar]
- 44.Bergamin O, Ramat S, Straumann D, Zee DS. Influence of orientation of exiting wire of search coil annulus on torsion after saccades. Invest Ophthalmol Vis Sci. 2004;45:131–137. doi: 10.1167/iovs.03-0615. [DOI] [PubMed] [Google Scholar]
- 45.Sylvestre PA, Galiana HL, Cullen KE. Conjugate and vergence oscillations during saccades and gaze shifts: implications for integrated control of binocular movement. J Neurophysiol. 2002;87:257–272. doi: 10.1152/jn.00919.2000. [DOI] [PubMed] [Google Scholar]
- 46.Tian JR, Crane BT, Demer JL. Vestibular catch-up saccades in labyrinthine deficiency. Exp Brain Res. 2000;131:448–457. doi: 10.1007/s002219900320. [DOI] [PubMed] [Google Scholar]
- 47.Angelaki DE, Hess BJ, Crawford JD, Martinez-Trujillo JC, Klier EM. Control of eye orientation: where does the brain’s role end and the muscle’s begin?—neural control of three-dimensional eye and head movements. Eur J Neurosci. 2004;19:1–10. doi: 10.1111/j.1460-9568.2004.03068.x. [DOI] [PubMed] [Google Scholar]
- 48.Crawford JD, Martinez-Trujillo JC, Klier EM. Neural control of three-dimensional eye and head movements. Curr Opin Neurobiol. 2003;13:655–662. doi: 10.1016/j.conb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 49.Quaia C, Optican LM. Commutative saccadic generator is sufficient to control a 3-D ocular plant with pulleys. J Neurophysiol. 1998;79:3197–3215. doi: 10.1152/jn.1998.79.6.3197. [DOI] [PubMed] [Google Scholar]
- 50.Radau P, Tweed D, Vilis T. Three-dimensional eye, head, and chest orientations after large gaze shifts and the underlying neural strategies. J Neurophysiol. 1994;72:2840–2852. doi: 10.1152/jn.1994.72.6.2840. [DOI] [PubMed] [Google Scholar]
- 51.Mateeff S. Saccadic eye movements and localization of visual stimuli. Percept Psychophys. 1978;24:215–224. doi: 10.3758/bf03206092. [DOI] [PubMed] [Google Scholar]
- 52.Clark RA, Miller JM, Demer JL. Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000;41:3787–3797. [PubMed] [Google Scholar]
- 53.Kono R, Clark RA, Demer JL. Active pulleys: magnetic resonance imaging of rectus muscle paths in tertiary gazes. Invest Ophthalmol Vis Sci. 2002;43:2179–2188. [PubMed] [Google Scholar]
- 54.Kono R, Poukens V, Demer JL. Quantitative analysis of the structure of the human extraocular muscle pulley system. Invest Ophthalmol Vis Sci. 2002;43:2923–2932. [PubMed] [Google Scholar]
- 55.Smith MA, Crawford JD. Distributed population mechanism for the 3-D oculomotor reference frame transformation. J Neurophysiol. 2005;93:1742–1761. doi: 10.1152/jn.00306.2004. [DOI] [PubMed] [Google Scholar]
- 56.Thurtell MJ, Kunin M, Raphan T. Role of muscle pulleys in producing eye position-dependence in the angular vestibuloocular reflex: a model-based study. J Neurophysiol. 2000;84:639–650. doi: 10.1152/jn.2000.84.2.639. [DOI] [PubMed] [Google Scholar]
- 57.Demer JL, Oh SY, Clark RA, Poukens V. Evidence for a pulley of the inferior oblique muscle. Invest Ophthalmol Vis Sci. 2003;44:3856–3865. doi: 10.1167/iovs.03-0160. [DOI] [PubMed] [Google Scholar]
- 58.Haslwanter T, Straumann D, Hess BJ, Henn V. Static roll and pitch in the monkey: shift and rotation of Listing’s plane. Vision Res. 1992;32:1341–1348. doi: 10.1016/0042-6989(92)90226-9. [DOI] [PubMed] [Google Scholar]
- 59.Tweed DB, Haslwanter TP, Happe V, Fetter M. Non-commutativity in the brain. Nature. 1999;399:261–263. doi: 10.1038/20441. [DOI] [PubMed] [Google Scholar]
- 60.Crawford JD, Guitton D. Visual-motor transformations required for accurate and kinematically correct saccades. J Neurophysiol. 1997;78:1447–1467. doi: 10.1152/jn.1997.78.3.1447. [DOI] [PubMed] [Google Scholar]


