Abstract
Agrobacterium tumefaciens–mediated genetic transformation is an efficient tool for genetic engineering of plants. VirE2 is a single-stranded DNA binding Agrobacterium protein that is transported into the plant cell and presumably protects the T-DNA from degradation. Using a yeast two-hybrid system, we identified Arabidopsis thaliana VIRE2-INTERACTING PROTEIN2 (VIP2) with a NOT domain that is conserved in both plants and animals. Furthermore, we provide evidence supporting VIP2 interaction with VIP1, a basic domain/leucine zipper motif–containing protein required for nuclear import and integration of T-DNA. Virus-induced gene silencing of VIP2 in Nicotiana benthamiana and characterization of the Arabidopsis vip2 mutant (At vip2) demonstrate that VIP2 is required for Agrobacterium-mediated stable transformation but not for transient transformation. Assays based upon a promoter-trap vector and quantification of T-DNA integration further confirmed VIP2 involvement in T-DNA integration. Interestingly, VIP2 transcripts were induced to a greater extent over prolonged periods after infection with a T-DNA transfer-competent Agrobacterium strain compared with the transfer-deficient Agrobacterium strain. Transcriptome analyses of At vip2 suggest that VIP2 is likely a transcriptional regulator, and the recalcitrancy to transformation in At vip2 is probably due to the combination of muted gene expression response upon Agrobacterium infection and repression of histone genes resulting in decreased T-DNA integration events.
INTRODUCTION
Agrobacterium tumefaciens is a soil-borne phytopathogen that causes crown gall disease in plants. This disease is the manifestation of transfer, integration, and expression of oncogenes on a specific region of the T-DNA in susceptible hosts (reviewed in Gelvin, 2003; Anand and Mysore, 2006; Tzfira and Citovsky, 2006). Apart from T-DNA, several Agrobacterium encoded proteins, such as VirD2, VirE2, VirE3, and VirF, are also translocated into plants (Vergunst et al., 2003; Cascales and Christie, 2004; Christie, 2004). The current consensus is that Agrobacterium separately translocates the VirD2–T-strand and VirE2 and that the VirD2–T-strand–VirE2 complex (T-complex) assembles in the plant cell (Vergunst et al., 2000; Cascales and Christie, 2004). VirD2 remains tightly attached to the 5′ end of the nicked T-DNA region, while the remaining single-stranded DNA is covered stoichiometrically with VirE2, protecting the T-strand from exonucleolytic degradation in planta. The T-complex is subsequently imported into the nucleus most likely through interactions with other host proteins, such as VIP1 (Tzfira et al., 2001) and importin α (Ballas and Citovsky, 1997). Once inside the plant nucleus, the T-complex is stripped of its proteins probably through targeted proteolysis involving the SCFvirF ubiquitin complex (Tzfira et al., 2004). The T-DNA most likely relies on host DNA repair machinery for its conversion into double-stranded T-DNA intermediates and their recognition by proteins such as histone H2A (Mysore et al., 2000; Li et al., 2005a), histone H3 (Anand et al., 2007), and KU80 (Li et al., 2005b) for integration into the host chromosome.
To better characterize the functions of VirE2 in T-DNA transfer and integration, plant proteins that specifically associate with VirE2 were identified by screening the Arabidopsis thaliana cDNA library against VirE2 in the yeast two-hybrid system (Tzfira et al., 2001). Two VirE2-interacting proteins (VIPs) were identified and designated as VIP1 and VIP2 (Tzfira et al., 2000). Functional characterization of VIP1 through antisense and overexpression approaches implicated its requirement for T-DNA and VirE2 nuclear import via the importin α-dependent pathway (Tzfira et al., 2001; Tzfira and Citovsky, 2002). Here, we report the involvement of VIP2 in T-DNA integration. VIP2 encodes a NOT (for negative on TATA-less) domain–containing protein that interacts with VirE2 and is required for Agrobacterium-mediated plant transformation. VIP2 silenced and knockout plants are defective in stable T-DNA transformation but not in transient transformation. The amount of integrated T-DNAs in VIP2-silenced Nicotiana benthamiana plants was significantly less than in nonsilenced plants. On the basis of the above observations, we conclude that VIP2 plays an important role in Agrobacterium-mediated plant transformation by facilitating T-DNA integration into plant chromosomes. Furthermore, transcriptome analyses showed that many genes were constitutively differentially expressed in the At vip2 knockout, and gene expression response to Agrobacterium infection was muted in At vip2 compared with wild-type Arabidopsis plants. These data provided insights into the possible role of VIP2 as a transcription regulator.
RESULTS
Identification of At VIP2
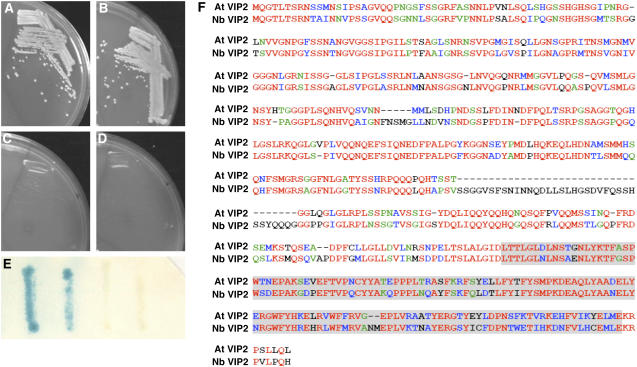
VIPs were identified using the yeast two-hybrid screen with an Arabidopsis cDNA library as prey and the Agrobacterium VirE2 protein as bait as described (Tzfira et al., 2001). Three VirE2-interacting clones belonged to the same cDNA, which was designated At VIP2. Coexpression of the largest clone of At VIP2 and VirE2 (Figure 1A), but not of lamin C (Figure 1C) or topoisomerase I (Figure 1D), indicated that only At VIP2 and VirE2 coexpression activated the HIS3 and β-galactosidase (Figure 1E) reporter genes. The interaction of At VIP2 with VirE2 was specific because it did not occur with lamin C and DNA topoisomerase I, known as nonspecific activators in the two-hybrid system best suited to eliminate false positive interactions (Bartel et al., 1993; Park and Sternglanz, 1998). At VIP2 did not interact with VirD2 (data not shown) that is thought to function differently from VirE2 during the T-DNA nuclear import (Guralnick et al., 1996), which further reinforces its specific interaction. Interestingly, At VIP2 also interacted with At VIP1, a previously identified VirE2-interacting protein (Tzfira et al., 2001), in the yeast two-hybrid system (Figures 1B and 1E).
Figure 1.
At VIP2–VirE2 and At VIP2–At VIP1 interactions in the Two-Hybrid System and Amino Acid Sequences of At VIP2 and Nb VIP2.
(A) At VIP2 + VirE2.
(B) At VIP2 + At VIP1.
(C) At VIP2 + human lamin C.
(D) At VIP2 + topoisomerase I.
(E) β-Galactosidase assay. From left to right: At VIP2 + VirE2, At VIP2 + At VIP1, At VIP2 + human lamin C, and At VIP2 + topoisomerase I.
Cells shown in (A) to (D) were grown in the absence of His, Trp, and Leu, and cells shown in (E) were grown in the absence of Trp and Leu.
(F) Multiple sequence alignment by ClustalW (1.81) of amino acid sequences of full-length proteins for At VIP2 and Nb VIP2. The identical amino acids are shown in red, conserved amino acids in blue, semiconserved amino acids in green, and the divergent amino acids in black. The shaded area represents the C-terminal NOT domain between the two proteins.
Sequence analysis of the At VIP2 cDNA predicted a single open reading frame (ORF) encoding a protein of 556 amino acids. The deduced amino acid sequence of At VIP2 contains a conserved C-terminal domain for NOT genes (NOT2/NOT3/NOT5; Collart and Struhl, 1994; Oberholzer and Collart, 1998) (Figure 1F). The VIP2 gene (At5g59710; genomic sequence of At VIP2 carries 11 exons and 10 introns) in the Arabidopsis database is represented by two splice variant cDNAs (GenBank accession numbers AK117230 and AF295433; Wang and Brendel, 2006). At VIP2 is also annotated as a transcription regulator NOT2/NOT3/NOT5 protein (GenBank accession number NM_125363). There are at least two other proteins with a NOT domain in Arabidopsis (GenBank accession numbers NM_100644 and NM_121828) that have 15 and 61% similarity to At VIP2, respectively (see Supplemental Figure 1 online). The NOT domain of VIP2 is conserved among plants and animals (see Supplemental Figure 1 online).
At VIP2 Is Imported into the Plant Cell Nucleus
We examined the subcellular localization of GFP-tagged At VIP2 in epidermal cells of tobacco and onion along with another fluorescent reporter, DsRed2 (known to partition between the cell cytoplasm and the nucleus; Dietrich and Maiss, 2002; Goodin et al., 2002; Schultheiss et al., 2003). GFP-At VIP2 was imported into the nucleus of onion (Allium cepa) and tobacco (Nicotiana tabacum) cells displaying a predominantly intranuclear accumulation as determined by confocal microscopy with optical sections through the cell nucleus (see Supplemental Figures 2A and 2D online). Combined image of GFP-At VIP2 and DsRed2 fluorescence showed overlapping signal (yellow color) within the cell nucleus, confirming GFP-At VIP2 localization within the nucleus (see Supplemental Figures 2C and 2F online). These results were consistent with the previous report that showed that in transgenic Arabidopsis plants, the yellow fluorescent protein (YFP)–tagged At VIP2, expressed under its native promoter and terminator sequences, accumulated within the cell nucleus (Tian et al., 2004).
Silencing of Nb VIP2 by Virus-Induced Gene Silencing in N. benthamiana Results in Smaller Crown Galls
Due to the unavailability of an Arabidopsis vip2 mutant at the initial stages of this study, we used a virus-induced gene silencing (VIGS)–based reverse genetics approach (Burch-Smith et al., 2004; Anand et al., 2007) to investigate whether VIP2 is required for Agrobacterium-mediated plant transformation. A fragment representing part of the Nb VIP2 gene (414 bp in length) was amplified by PCR from N. benthamiana cDNA, using primers specific to tomato (Solanum lycopersicum) VIP2 (Sl VIP2; GenBank accession number BG130671), and cloned into tobacco rattle virus (TRV)–based VIGS vectors (Liu et al., 2002a, 2002b). The reduction of Nb VIP2 transcripts was quantified by semiquantitative RT-PCR (see Supplemental Figure 3A online) and by real-time quantitative RT-PCR (qRT-PCR) analyses. Only 23% ± 4% mRNA of Nb VIP2 was detected in gene-silenced plants compared with TRV:00 (virus without the insert) inoculated plants.
To test whether VIP2 is required for Agrobacterium infectivity, the stems of Nb VIP2–silenced, TRV:00-inoculated, and wild-type (no virus inoculation) plants were infected with oncogenic strain A. tumefaciens A348 as described (Anand et al., 2007). We observed relatively smaller tumors incited on the shoots of Nb VIP2–silenced plants compared with the tumors on the TRV:00-inoculated plants or wild-type plants (see Supplemental Figure 3B online).
Nb VIP2 Is Required for Stable Transformation
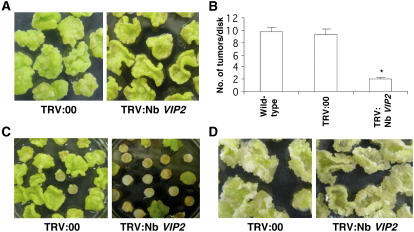
The ability of Nb VIP2–silenced plants to develop tumors on leaf disks was tested following inoculation with strain A. tumefaciens A348. Tumors were quantified by counting the number of tumors/leaf disk and by measuring the weight of leaf disks with tumors (Figures 2A and 2B; see Supplemental Figure 4 online). The tumor-inducing capability was severely attenuated in Nb VIP2–silenced plants compared with TRV:00 and wild-type plants.
Figure 2.
Agrobacterium Transformation Assays in Nb VIP2–Silenced Plants.
(A) Leaf disk tumorigenesis assay. Leaf disks of the Nb VIP2–silenced plants and TRV:00 (control) plants were inoculated with tumorigenic strain A. tumefaciens A348 and incubated on hormone-free Murashige and Skoog (MS) medium.
(B) Quantification of tumors. The number of tumors produced per leaf disk was counted 3 weeks after inoculation. Data represent the mean of two experiments with a minimum of 150 leaf disks each per treatment with their se values shown as error bars. Asterisk denotes significant difference compared with controls using Fisher's least significant difference test at P = 0.05.
(C) Stable transformation assay. Leaf disks from the silenced and TRV:00 plants were infected with a nontumorigenic strain A. tumefaciens GV2260 harboring the binary vector pCAS1 and incubated on CIM with GF.
(D) Effect of VIP2 gene silencing on cell division. The effect of gene silencing on cell division was evaluated by placing uninoculated leaf disks from the silenced and TRV:00 plants on a nonselective CIM. All the experiments were done with at least five biological replicates and repeated two times, and the results were consistent among the replicates. Photographs shown in (A), (C), and (D) were taken 4 weeks after Agrobacterium inoculation.
To rule out the possibility that the reduction in number of tumors produced in Nb VIP2–silenced plants could have resulted from the downregulation of genes involved in phytohormone responses, we inoculated leaf disks from the Nb VIP2–silenced and TRV:00 plants with a disarmed strain A. tumefaciens GV2260 containing the binary vector pCAS1 (Nam et al., 1999) that contains a nos-bar gene as a selectable marker. Approximately 33% of the leaf disks derived from the Nb VIP2–silenced plants survived the glufosinate ammonium (GF) selection and produced small transgenic GF-resistant calli on callus-inducing medium (CIM). In the case of TRV:00 and wild-type control plants, 100% of the leaf disks survived GF selection and produced predominantly large GF-resistant calli (Figure 2C).
Uninfected leaf disks of Nb VIP2–silenced plants were able to form calli, at an equal efficiency as that of TRV:00 plants, on nonselective CIM (Figure 2D). Thus, silencing of the VIP2 gene apparently does not interfere with essential plant cellular functions pertaining to cell division. These data clearly indicate that silencing of VIP2 in N. benthamiana attenuates Agrobacterium-mediated stable transformation.
We investigated whether the Nb VIP2–silenced plants can be efficiently transformed by Agrobacterium-independent transformation techniques. Leaf disks from the TRV:00 and Nb VIP2–silenced plants were biolistically transformed with 35S:gus (uidA-intron) or Ubi:bar constructs for transient and stable transformation, respectively. No differences were detected for the transient expression of β-glucuronidase (GUS) in the Nb VIP2–silenced plants and TRV:00-infected plants (see Supplemental Figure 5A online). No significant differences were also seen in the number of leaf disks producing GF-resistant calli on TRV:00-inoculated (76% ± 9%) and Nb VIP2–silenced plants (67% ± 7%). The presence of the bar gene in the GF-resistant calli was confirmed by PCR (data not shown). These results suggest that VIP2 gene silencing in N. benthamiana did not affect both transient and stable transformation by particle bombardment.
Nb VIP2–Silenced Plants Are Partially Blocked at the T-DNA Integration Step
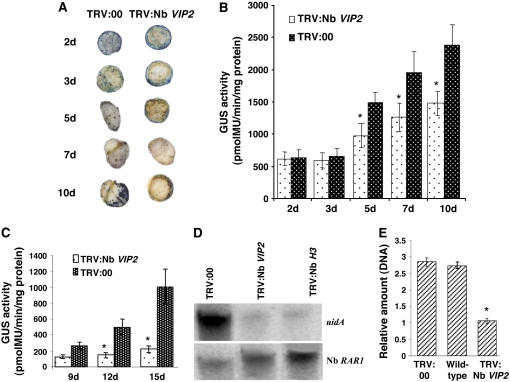
To identify the step at which VIP2 is involved in Agrobacterium-mediated transformation, we inoculated leaf disks derived from the Nb VIP2–silenced N. benthamiana and TRV:00 plants with a disarmed strain A. tumefaciens GV2260 containing the binary vector pBISN1 (carries on its T-DNA a uidA-intron gene encoding GUS; Nam et al., 1999). The 5-bromo-4-chloro-3-indolyl β-d-glucuronide (X-Gluc) staining and GUS activity on the leaf disks of Nb VIP2–silenced plants were not significantly different than TRV:00 plants at 2 and 3 d after inoculation (DAI; Figures 3A and 3B), suggesting that there was no deficiency in transient transformation in the silenced plants. Also, no qualitative differences in the transient GUS expression were detected, when the uidA-intron gene was delivered by agroinfiltration, in the Nb VIP2–silenced and TRV:00 plants (see Supplemental Figure 5B online). Leaf disks from Nb VIP2–silenced plants showed less X-Gluc staining and only 61 to 65% GUS activity compared with leaf disks derived from the TRV:00 plants at 5 to 10 DAI (Figures 3A and 3B). This represents a combination of both transient and stable GUS expression. Thus, we concluded that Nb VIP2 gene silencing partially blocked the later stages (T-DNA integration) of Agrobacterium-mediated transformation.
Figure 3.
Transient Transformation and T-DNA Integration Assays in Nb VIP2–Silenced Plants.
(A) Transient transformation assay. Leaf disks of the Nb VIP2–silenced and TRV:00 plants were inoculated with nontumorigenic strain A. tumefaciens GV2260 carrying pBISN1 (has the uidA-intron gene within the T-DNA). The inoculated leaves were periodically collected and stained with X-Gluc.
(B) Quantification of GUS activity. Leaf disks from the experiment in (A) were collected periodically and were used for measuring the fluorescence of 4-methylumbelliferone (4-MU).
(C) T-DNA integration assay. Leaf disks from TRV:00 and Nb VIP2–silenced plants were inoculated with Agrobacterium strain carrying a promoterless uidA-intron gene and 35S:luc-intron gene within the T-DNA. Leaf disks were periodically collected, and GUS activity was measured as described above.
(D) T-DNA integration in the Nb VIP2–silenced and TRV:00-infected plants. Suspension cells were derived from the calli generated from Nb VIP2–silenced and TRV:00-infected leaf segments infected with the nontumorigenic strain A.tumefaciens GV2260 carrying pBISN1. The suspension cell lines were grown for 8 weeks in nonselective medium. Genomic DNA was isolated from these cells, subjected to electrophoresis through a 0.8% agarose gel, blotted onto a nylon membrane, and hybridized with a uidA gene probe. After autoradiography, the membrane was stripped and rehybridized with the Nb RAR1 gene probe to compare the amount of DNA in each lane.
(E) Quantification of T-DNA integration. The amounts of integrated T-DNA molecules in the genomic DNA extracted from calli that were generated from leaf disks transformed with the Agrobacterium strain carrying the uidA-intron gene within the T-DNA were measured by quantitative PCR. The uidA gene transcripts in calli derived from Nb VIP2–silenced plants are represented in relative amounts in comparison to an average T-DNA amount in the calli derived from wild-type and TRV:00 plants. All the experiments were done with at least five biological replicates and repeated two times. Asterisks in (C) and (E) denote value that are significantly different between the two treatments by analysis of variance at P = 0.05. The data represent the average of five biological replicates in two experiments with se values shown as error bars.
To provide additional evidence that the T-DNA integration was blocked in Nb VIP2–silenced plants, we inoculated the leaf disks derived from Nb VIP2–silenced and TRV:00 plants with a disarmed strain A. tumefaciens GV2260 containing the binary vector pKM1 (Mysore et al., 1998) carrying a promoterless uidA-intron gene and a 35S:luciferase (luc)-intron gene within the T-DNA. Here, the expression of the uidA gene in plants is dependent upon T-DNA integration downstream of a plant promoter, while the luc gene can express transiently irrespective of T-DNA integration. Significantly less GUS activity was detected on the leaf disks of Nb VIP2–silenced plants at 9 to 15 DAI compared with the TRV:00 plants (Figure 3C; see Supplemental Figure 6A online). As a positive control for Agrobacterium infectivity, we detected the expression of the luc gene in the representative leaf disks derived from the same experiment for Nb VIP2–silenced and TRV:00 plants by semiquantitative RT-PCR (see Supplemental Figure 6B online).
To provide direct evidence for deficiency in T-DNA integration in Nb VIP2–silenced plants, DNA gel blot analyses (Mysore et al., 2000) were performed on high molecular weight DNA extracted from cell cultures of Nb VIP2–silenced and TRV:00 plants infected with a disarmed strain A. tumefaciens GV2260 containing the binary vector pBISN1 (Nam et al., 1999). The differences in the amount of T-DNA, containing uidA-intron gene, integrated into the genomes of Nb VIP2–silenced and TRV:00 plants was determined by hybridizing the above-mentioned DNA blot with radiolabeled uidA gene. DNA from Nb VIP2–silenced plants showed weaker signals compared with DNA from TRV:00 plants (Figure 3D). Nb H3–silenced plants recently have been shown to be deficient in T-DNA integration (Anand et al., 2007). DNA from Nb H3–silenced plants infected with A. tumefaciens GV2260 containing pBISN1 was used as control. We confirmed that the plant DNA samples were free of contaminating Agrobacterium DNA by performing quantitative DNA PCR (qPCR) using the bacterial chromosomal gene Atu0972 as previously described (Anand et al., 2007). The same DNA gel blot was stripped and rehybridized with the radiolabeled Nb RAR1 gene to demonstrate that similar amounts of DNA were loaded in lanes with DNA from Nb VIP2 and Nb H3 cultures with respect to DNA from TRV:00 cultures (Figure 3D). Furthermore, we support the above results by quantifying the relative amount of T-DNA integrated into the genome by real-time qPCR as described (Li et al., 2005b; Anand et al., 2007) on genomic DNA extracted from the calli generated on leaf disks infected with the disarmed strain of Agrobacterium containing the binary vector pBISN1. The amount of PCR products specific to the uidA gene, determined by qPCR, was ∼63% less in Nb VIP2–silenced plants compared with TRV:00 plants (Figure 3E). Semiquantitative PCR amplifications were also performed using primers specific to uidA exons bordering an intron and primers specific to a bacterial chromosome to show the specific amplification of the integrated T-DNA molecule (see Supplemental Figure 6C online). Based on these results, we suggest that VIP2 plays a crucial role in T-DNA integration.
Nb VIP2 Is Induced by Agrobacterium Infection
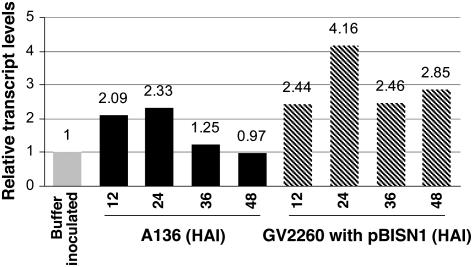
The Nb VIP2 gene was induced up to twofold 12 h after infection (HAI) with both an avirulent strain A. tumefaciens A136 (lacks Ti plasmid) and a T-DNA transfer competent strain A. tumefaciens GV2260, carrying pBISN1 when compared with the mock-inoculated N. benthamiana (Figure 4). Nb VIP2 transcripts remained elevated up to 36 HAI in leaves inoculated with A136 but decreased to basal levels at 48 HAI. In the leaves infected with GV2260, elevated transcript levels of Nb VIP2 were maintained up to 48 HAI and were twofold to threefold more than those detected in A136-infected leaves (Figure 4). These results suggest that the transfer-competent strain of Agrobacterium induces VIP2 gene expression to a greater extent than the avirulent strain.
Figure 4.
Differential Gene Expression of Nb VIP2 upon Infection with Agrobacterium.
Individual leaves of two separate N. benthamiana plants were syringe (needleless) infiltrated with either an avirulent strain Agrobacterium A136 (lacks Ti plasmid; cannot transfer T-DNA) or a T-DNA transfer-competent strain A. tumefaciens GV2260 carrying pBISN1. Leaf samples from the infiltrated area were collected at different times after inoculation, and total RNA was isolated for real-time quantitative PCR. RNA from the buffer-infiltrated N. benthamiana leaves collected at 12 HAI was used as a calibrator to determine the relative amount of Nb VIP2 transcripts. Samples were pooled together from two independent experiments, and the average of two technical replicates is shown.
Nb VIP2 Interacts with VirE2 Both in Vitro and in Planta
The N. benthamiana gene corresponding to Nb VIP2 was cloned by rapid amplification of cDNA ends (see Supplemental Methods online). The ORF of Nb VIP2 is 1812 bp in length, encoding a protein of 603 amino acid residues (GenBank accession number DQ000202). Sequence alignment of the Nb VIP2 and At VIP2 protein sequences showed 69% sequence identity with a conserved C-terminal NOT domain (Figure 1F). Nb VIP2 carries two in-frame insertions of five and 32 amino acids that are lacking in At VIP2 (Figure 1F).
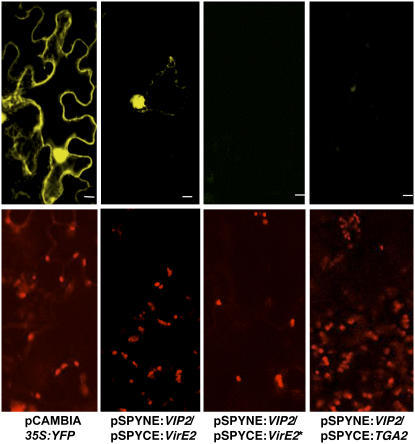
Nb VIP2 also interacted with VirE2 in a yeast two-hybrid system, and this interaction was specific since the Nb VIP2 interaction did not occur with the nonspecific interactors such as DNA topoisomerase I and lamin C (see Supplemental Figure 7 online). We further demonstrated that Nb VIP2 can interact with VirE2 in planta using biomolecular fluorescence complementation (BiFC; Walter et al., 2004). BiFC vectors were modified to make it GATEWAY ready (see Methods). The interaction between N-tagged Nb VIP2 (pSPYNE:Nb VIP2) and C-tagged VirE2 (pSPYCE:VirE2) was observed as yellow fluorescence from the reconstitution of YFP (Figure 5). Two different controls were used for BiFC: first, we made a translational fusion of full-length VirE2 including the stop codon (designated as VirE2*) with cYFP in pSPYCE; second, we cloned the full-length transcriptional factor TGA2 into pSPYCE. In the first control, no VirE2-cYFP fusion protein would be synthesized, resulting in the failure of the reconstitution of YFP fluorescence when the two interactors are brought together. The second control facilitates identification of nonspecific interaction of VIP2 with transcription factors. YFP fluorescence was not detected in leaves coinfiltrated with pSPYNE:Nb VIP2 and pSPYCE:VirE2* or pSPYNE:Nb VIP2 and pSPYCE:TGA2 (Figure 5).
Figure 5.
In Planta Interaction of Nb VIP2 with VirE2.
The top panels depict the YFP fluorescence, and the bottom panels represent the epifluorescence images of epidermal leaf cells from the same leaf infiltrated with Agrobacterium suspension cultures harboring the indicated proteins. Individual leaves of N. benthamiana plants were syringe (needleless) infiltrated with Agrobacterium suspension cultures singly or in the following combinations: pCAMBIA1390-35S:YFP, pSPYNE:VIP2, pSPYCE:VirE2, pSPYNE:VIP2/pSPYCE:VirE2, pSPYNE:VIP2/pSPYCE:TGA2, and pSPYNE:VIP2/pSPYCE:VirE2*. Wild-type 35S:YFP and fusion protein pSPYNE:VIP2/pSPYCE:VirE2 are both localized to the nucleus of plant cells, while pSPYNE:VIP2/pSPYCE:VirE2* carrying the VirE2 stop codon and pSPYNE:VIP2/pSPYCE:TGA2 did not produce any fluorescence. All the images are from a single confocal section. Bars = 10 μm.
The Arabidopsis vip2 Mutant Is Defective in T-DNA Integration but Not in Transient T-DNA Expression
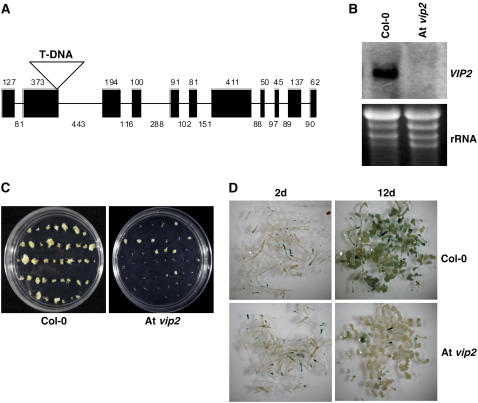
Recently, we were able to identify an Arabidopsis T-DNA mutant line (At vip2; GABI_676A06; T-DNA insertion in the second exon) (Rosso et al., 2003) (Figure 6A) that does not produce At VIP2 transcripts (Figure 6B). To further confirm the results obtained from Nb VIP2–silenced plants, we performed root transformation assays on At vip2 plants (Nam et al., 1999; Mysore et al., 2000). Upon infection with an oncogenic strain A. tumefaciens A208, At vip2 produced fewer tumors (38% ± 3% of the infected roots formed tumors) compared with the wild-type plants (87% ± 5% of the infected roots formed tumors) (Figure 6C; see Supplemental Table 1 online). However, no significant differences were observed between the wild type and At vip2 for transient GUS expression at 2 DAI (Figure 6D). Stable GUS expression in At vip2 was only 25% ± 6% of the GUS expression observed in the wild-type plants (Figure 6D; see Supplemental Table 1 online). We also performed stable transformation assay with the strain A. tumefaciens GV3101 containing pCAS1. Significantly reduced numbers of GF-resistant calli were observed in the At vip2 mutant (33% ± 1% of infected roots formed GF-resistant calli) relative to the wild-type plants (83% ± 3% of infected roots formed GF-resistant calli). Root segments derived from both the wild-type and At vip2 mutant plants were able to form calli at similar frequencies on nonselective CIM (data not shown). These results further support the role of VIP2 in T-DNA integration in another plant species.
Figure 6.
Identification of At vip2 and Transformation Assays in the Mutant.
(A) The full-length genomic sequence of the At VIP2 gene (exons are shaded) showing the T-DNA insertion in the second exon of the Arabidopsis T-DNA mutant line (At vip2; GABI_676A06).
(B) RNA gel blot analysis confirms the absence of At VIP2 transcripts in At vip2. Five micrograms of RNA extracted from leaves was fractionated on a formaldehyde-agarose gel, blotted onto a nylon membrane, and probed with 32P-labeled At VIP2 gene (top panel). Ethidium bromide–stained gel showing rRNA suggests equal amounts of total RNA were loaded in each lane (bottom panel).
(C) Roots of wild-type and vip2 mutant plants were infected with a tumorigenic strain A. tumefaciens A208 (nopaline strain), and tumors incited on the roots were visualized and scored 4 weeks after infection.
(D) Transient and stable GUS expression. Roots of the wild-type and At vip2 plants were inoculated with a strain A. tumefaciens GV3101 carrying the uidA-intron gene within the T-DNA. The inoculated roots were periodically collected and stained with X-Gluc. All the experiments were repeated two times.
Transcriptome Analyses Suggest That VIP2 Plays a Role in Transcription Regulation
To gain insight on the biological role of VIP2 in plants, a comprehensive survey of global gene expression was done using the Arabidopsis whole-genome Affymetrix gene chip (ATH1) to quantify the spatio-temporal variations in transcript abundance between wild-type Columbia-0 (Col-0) and At vip2. Comparative analyses between Col-0 and At vip2 showed 4241 genes to be constitutively differentially expressed with a false discovery rate (FDR) <10%. Out of the 4241 differentially expressed genes, 2157 genes had more transcript abundance in At vip2 compared with Col-0, whereas 2084 genes had more transcript abundance in Col-0 compared with At vip2 (see Supplemental Table 2 online). Functional classification of the 4241 differentially expressed genes indicated genes involved in a variety of functions, and the majority (28.7%) of them encode proteins of unknown function (see Supplemental Figure 8 online). These data support our hypothesis that VIP2 plays a direct or indirect role in transcription regulation of many genes. Interestingly, upon careful examination of the transcriptome data, we found a majority of the 52 genes encoding histones or histone-associated proteins to be constitutively repressed in the At vip2 compared with Col-0 plants (Figure 7; see Supplemental Table 3 online). Although the transcript differences of some of the histone genes were less than twofold, their expression profiles were obviously different in At vip2 and Col-0 plants (Figure 7). The exact expression values of these genes are shown in Supplemental Table 3 online. Histones have already been implicated in Agrobacterium-mediated plant transformation (Mysore et al., 2000; Yi et al., 2002, 2006; Li et al., 2005a; Anand et al., 2007).
Figure 7.
Expression Profile for the 52 Differentially Expressed Histone or Histone-Associated Genes Represented in the ATH1 Gene Chips in Col-0 and At vip2.
Color code represents expression values of ratio between At vip2 and Col-0, wherein red and green indicate up- and downregulation of genes, respectively. Each horizontal line displays the expression data for one gene. Data were clustered with correlation using the TIGR Multiple Experiment Viewer.
We monitored the differential expression of genes in the At vip2 mutant and Col-0 in response to Agrobacterium infection by infiltrating the leaves with a disarmed strain A. tumefaciens GV3101 harboring the uidA-intron gene as described (Wroblewski et al., 2005). Strikingly, under the same selection condition, fewer genes were differentially expressed in At vip2, at 48 and 72 HAI, compared with the number of genes that were differentially expressed in the Col-0 plant at the same time points (see Supplemental Table 4 online). The fact that we were not able to achieve 100% transformation in the infiltrated plants, based on the GUS histochemical staining (see Supplemental Figure 9 online), could have diluted the effect on differential gene expression upon Agrobacterium infection. Nevertheless, our data suggest that At vip2 is significantly muted in its response, based on differential gene expression, to Agrobacterium infection. These results further validate the role of VIP2 in transcriptional regulation and Agrobacterium-mediated plant transformation.
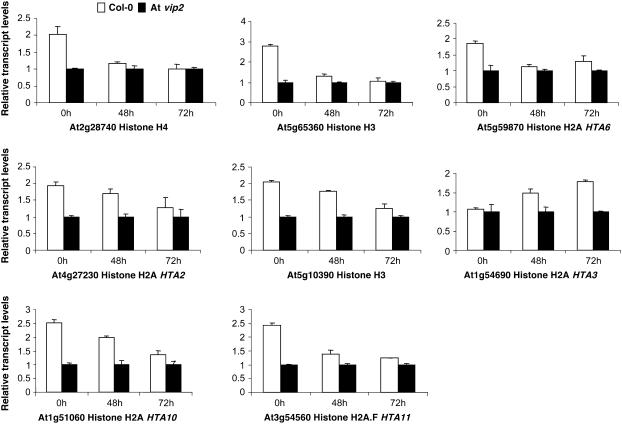
Real-Time Quantitative RT-PCR Validates Microarray Data and Shows Reduced Transcript Levels of Several Histone Genes in the At vip2 Mutant Compared with Wild-Type Arabidopsis
We validated the microarray results for a number of histone genes that showed differential expression between wild-type Col-0 and At vip2. Eight histone genes that showed less transcript abundance in At vip2 compared with Col-0 were selected for the qRT-PCR analysis. Five members of histone H2A, namely, HTA10 (At1g51060), HTA3 (At1g54690), HTA6 (At5g59870), HTA2 (At4g27230), and HTA11 (At3g54560); two histone H3 genes (At5g65360 and At5g10390); and one histone H4 gene (At2g28740) were selected. The transcript abundance of all eight genes tested were significantly less in At vip2 compared with Col-0 at one or the other time point (Figure 8). For all the histone genes tested, qRT-PCR results strongly correlated with the microarray data except for HTA3 (At1g54690), which did not show any significant difference in the expression between Col-0 and At vip2 at 0 HAI. However, less transcripts of HTA3 in the At vip2 mutant compared with Col-0 were observed at 48 and 72 h after Agrobacterium infection. These results further imply that VIP2 may play a role in Agrobacterium-mediated plant transformation by modulating the expression of several plant histone genes.
Figure 8.
Validation of the Microarray Data by Real-Time qRT-PCR.
Eight different histone genes that had less transcript abundance in At vip2 compared with Col-0, based on microarray experiments, were selected for validation. Total RNA was extracted from leaves of wild-type Col-0 and At vip2 following agroinfiltration at 0, 48, and 72 h. The first-strand cDNA was synthesized and used for qRT-PCR using gene-specific primers (see Methods). The amount of elongation factor-1-α transcripts was determined and used for normalization. cDNA extracted from Col-0 and At vip2 at 0 HAI was used as calibrator to obtain the relative transcript levels for each gene following agroinfiltration. The data represent the average of three biological replicates, including three technical replicates for each biological replicate with se values shown as error bars.
DISCUSSION
Here, we report the identification of VIP2 and show that it plays an important role in Agrobacterium-mediated plant transformation. VIP2 protein contains a conserved C-terminal domain of NOT2/NOT3/NOT5 proteins (Figure 1F). NOT2/NOT3/NOT5 domain–containing genes were identified from Saccharomyces cerevisiae via genetic screens for increased transcription from TATA-less promoters (Oberholzer and Collart, 1998; Collart, 2003). The NOT proteins are an integral component of the CCR4 (for carbon catabolite repression) transcriptional complex sharing overlapping functions (Liu et al., 1998) and are believed to be involved in both positive and negative regulation of gene expression in yeast (Collart, 2003; Collart and Timmers, 2004). The yeast Not2p and Drosophila Rga proteins that contain NOT domains are well studied and are thought to mediate intranuclear interactions between chromatin components and the transcriptional complex (Collart and Struhl, 1993; Frolov et al., 1998; Collart, 2003). The function of NOT domain–containing proteins in plants is not known. On the basis of its similarity to yeast and animal NOT proteins, we speculate VIP2 to be a transcriptional regulator.
The involvement of VIP2 in Agrobacterium-mediated plant transformation was shown using Nb VIP2–silenced N. benthamiana and an Arabidopsis T-DNA knockout, At vip2. Our observations that Nb VIP2–silenced and At vip2 knockout plants were recalcitrant to Agrobacterium-mediated stable transformation but not to biolistic transformation further suggest that VIP2 is specifically required for Agrobacterium-mediated stable plant transformation. Even though the amount of stably integrated T-DNA in the Nb VIP2–silenced plants was significantly lower compared with the wild-type plants, the Nb VIP2–silenced plants did not show any deficiency for transient transformation. These results implicated a role of VIP2 in T-DNA integration. The prolonged induction of the VIP2 transcripts by the T-DNA transfer competent strain of Agrobacterium compared with the induction by the avirulent strain is consistent with the hypothesis that Agrobacterium modulates host gene expression to facilitate the genetic transformation event (Ditt et al., 2001; Veena et al., 2003; Hwang and Gelvin, 2004). The magnitude of Nb VIP2 induction reported is likely an underestimation due to the fact that not all cells in the infiltrated area were transformed. The specific interaction of VirE2 and Nb VIP2 was confirmed in planta by BiFC. Even though we predominantly detected YFP fluorescence in the nucleus, we seldom observed YFP fluorescence in the cytoplasm due to VirE2–Nb VIP2 interaction. It is likely that the VirE2–Nb VIP2 interaction happens in the cytoplasm and then the protein complex along with other interactors gets localized to the nucleus. Nevertheless, our data suggest that VirE2 interacts with Nb VIP2 in planta, and further studies are needed to precisely identify the cellular compartment where the initial interaction takes place.
The transcriptome analyses of wild-type Arabidopsis and At vip2 identified 4241 differentially expressed genes with an FDR of 0.1, spanned across different functional groups. This was not a big surprise to us, and the data fit well with our speculation that VIP2 is likely a transcriptional regulator. This conclusion is based on circumstantial evidence, and we have not provided direct evidences to suggest its role as a transcriptional regulator. Nevertheless, At vip2 was muted in its response (based on differential gene expression) to Agrobacterium infection compared with wild-type plants. This muted response of At vip2 to Agrobacterium infection probably contributes to its recalcitrance to Agrobacterium-mediated stable plant transformation. It is important to note that At vip2 did not have any deficiency in transient transformation (2 to 3 DAI); therefore, the differential gene expression that we observed at 2 and 3 d after Agrobacterium infection was not due to differences in transformation efficiency between At vip2 and Col-0 plants. Strikingly, we found that transcripts of many histone genes were less abundant in At vip2 compared with wild-type plants (Figures 7 and 8), which could be another factor contributing to the transformation recalcitrance phenotype of At vip2. Our data support earlier speculation that histone proteins are involved in Agrobacterium-mediated transformation, especially in the step of T-DNA integration (Mysore et al., 2000; Yi et al., 2002, 2006; Li et al., 2005a; Anand et al., 2007).
Based on the pfam domain prediction, VIP2 from Arabidopsis and N. benthamiana have a conserved NOT2/NOT3/NOT5 C-terminal domain, which we speculate forms complexes similar to Ccr4-Not in plants, and participates in diverse cellular functions in plant development, including chromatin remodeling, that will be addressed elsewhere. The core histones (H2A, H2B, H3, and H4) form the building blocks of the nucleosome, which are the fundamental repeating units of chromatin. Earlier observations have suggested that once inside the nucleus, the Agrobacterium T-DNA–associated proteins likely interact with the nucleosome assembly. VIP1, a bZIP transcription factor, was shown to interact with Arabidopsis histone H2A in planta (Li et al., 2005a). Furthermore, it has been shown that chromatin-associated proteins, such as histone H2A (Mysore et al., 2000), and chromatin assembly factor 1 (Endo et al., 2006; Kirik et al., 2006) play a role in T-DNA integration in plants. Core histone proteins are evolutionarily conserved and undergo posttranslational modification, implicating them in regulating gene expression (for review, see Fischle et al., 2003). The fact that NOT2/NOT3/NOT5 proteins form a nuclear complex that mediates intranuclear interactions between chromatin components and the transcriptional complex in many eukaryotes (Collart and Struhl, 1993; Frolov et al., 1998; Collart, 2003) and our finding that the histone gene transcripts are less abundant in At vip2 further provides evidence for the biological role of VIP2 in Agrobacterium-mediated plant transformation. We hypothesize that Agrobacterium takes advantage of VIP2 to integrate its T-DNA into the plant chromosome. It is tempting to speculate that VIP1, VIP2, and VirE2 function as a multiprotein complex that plays a crucial role in T-DNA nuclear import, intranuclear transport of the T-complex, and integration of T-DNA into host genome. The above findings open up a new area of research that could be directed toward the functional characterization of VIP2 in plants and its biological relevance to plant development and transformation.
METHODS
Yeast Two-Hybrid Assay
The yeast two-hybrid assays were performed as previously described (Tzfira et al., 2001) and are detailed in the Supplemental Methods online. The Nb VIP2 gene was cloned into pGAD424 as a PstI fragment, and its product was shown to interact with pBTM116-VirE2 in a yeast two-hybrid system.
VIGS and in Planta Tumor Assay
Plant material, bacterial culture conditions, cloning of the Nb VIP2 gene into the TRV-VIGS vector, and sequence conformation and protocols for VIGS were performed as described (Ryu et al., 2004; Anand et al., 2007) with minor modifications (see Supplemental Methods online). Shoots of the gene-silenced plants and empty vector control plants (TRV:00), 3 weeks after TRV infection, were inoculated by puncturing the stem using a needle with a suspension culture of a tumorigenic strain Agrobacterium tumefaciens (A348) containing the octopine type Ti plasmid (pTiA6). Tumors on shoots were observed 4 weeks after Agrobacterium infection.
Leaf Disk Transformation Assays
Leaf disk transformation assays were performed as described (Anand et al., 2007). Briefly, axenic leaf disks (15 to 20 for each plant) were incubated with different strains of Agrobacterium (see Results) for 15 min, blotted on sterile filter paper, cocultivated with the bacteria at 25°C for 2 d in the dark, and transferred onto either MS medium (Gibco-BRL) for tumorigenesis assay or to CIM (4.32 g/L MS minimal salts, 1 mL/L vitamin stock, 100 mg/L myo-inositol, 20 g/L glucose, 0.5 mg/L 2,4-D, 0.3 mg/mL kinetin, 5 mg/L indole-3-acetic acid, and 0.8% phytagar with antibiotics) containing cefotaxamine (200 μg/mL) and tricarcillin (100 μg/mL) for stable and transient transformation assays. GF (5 μg/mL) was included in CIM for stable transformation assay. The cultures were incubated at 25.0 ± 2.0°C with a 16-h photoperiod at 70% humidity at 150 μE s−1 m−2 light intensity. Transient transformation assays (histochemical GUS staining and quantification of GUS activity) were performed as described (Jefferson et al., 1987; Anand et al., 2007).
Agrobacterium-Independent Transformation Assays
The efficacy of Agrobacterium independent transformation methods in the Nb VIP2–silenced leaves was tested by particle bombardment. DNA (1 μg) was adsorbed onto 10 mg of 1-μm gold particles (Bio-Rad) and bombarded at 150 p.s.i. into the leaf epidermis of greenhouse-grown Nicotiana benthamiana plants, followed by incubation for 24 to 48 h at 25°C in dark. For the transient transformation assay, leaf disks from the TRV:00-inoculated and Nb vip2–silenced plants were biolistically transformed with a 35S:uidA (pAHC20) construct. Bombarded leaves were stained with X-Gluc staining solution (50 mM NaH2PO4, 10 mM Na2·EDTA, 300 mM mannitol, and 2 mM X-Gluc, pH 7.0) 48 to 72 h after bombardment and viewed under a Bio-Rad confocal microscope for GUS-expressing spots. For the stable transformation assay, the leaf disks from the TRV:00 and Nb VIP2–silenced plants were biolistically transformed with Ubi:bar (pAHC20 cassette). The transformed leaf disks were selected on media supplemented with GF for 8 weeks, and the presence of the bar gene was detected by PCR in a few representative GF-resistant calli as described earlier (Anand et al., 2003b)
RNA Extraction, PCR, T-DNA Integration Assay, and Differential Gene Expression
RNA extraction, first-strand cDNA synthesis, semiquantitative RT-PCR, and qRT-PCR were performed using standard protocols as described (Ryu et al., 2004; see Supplemental Methods online). The RNA gel blot analyses were performed using standard protocols (Anand et al., 2003a) on total RNA extracted from leaves of Col-0 and the At vip2 mutant. For the T-DNA integration assay, we performed DNA gel blot analysis (Mysore et al., 2000) on the genomic DNA extracted from suspension cell lines generated from the calli produced on nonselective medium by leaf disks of Nb VIP2–silenced plants and TRV:00 plants infected with disarmed strain A. tumefaciens GV2260 carrying pBISN1 (see Results). Suspension cells were cultured for 8 weeks, in the presence of timentin, by periodic transferring into fresh media to remove bacterial contamination. Representative suspension cells were collected and stained with X-Gluc solution to check for the presence and expression of the uidA-gene in the transgenic cells. The DNA gel blots were hybridized with radiolabeled probes of the uidA and Nb RAR1 genes for detecting the integrated T-DNA and as control for DNA loading, respectively. For real-time quantification of integrated T-DNA, genomic DNA was extracted from calli (collected from a pool of two independent experiments, with five biological replicates each) produced on leaf disks of Nb VIP2–silenced and TRV:00 plants that were transformed with strain A. tumefaciens GV2260 carrying pBISN1. Calli were washed with dimethyl sulfoxide (15% [w/v]) several times by vortexing to remove any attached bacteria, and DNA was extracted using DNAzol (Invitrogen) according to the manufacturer's instructions. Quantitative DNA PCRs were performed on duplicate DNA samples using the GUS gene and Agrobacterium chromosomal gene (Atu0792) specific primers (for primer details, see Anand et al., 2007) to check for the presence of the uidA gene and bacterial DNA contamination. Duplicate samples were analyzed by qPCR with the primers GUS-FP (5′-AGGTGCACGGGAATATTTCG-3′) and GUS-RP (5′-ACGCGTCGGGTCGAGTT-3′) to determine the abundance of integrated T-DNA. As a loading control for silenced and nonsilenced plants, parallel qPCR reactions using Nb Ef1α primers Nb Ef1F (5′-TGAGGCTCTTGACCAGATTAATGA-3′) and Nb Ef1R (5′-GTAAACATCCTGAAGTGGAAGACGTA-3′) were performed.
For differential gene expression analyses of Nb VIP2, individual leaves of two separate N. benthamiana plants were infiltrated (using a needleless syringe) with an avirulent strain Agrobacterium A136 or a T-DNA transfer competent strain A. tumefaciens GV2260 carrying pBISN1 or the infiltration buffer. Samples were collected at different time points after inoculation and were subjected to qRT-PCR using primers Nb VIP2F (5′-AAGGTGGGAATGCTGATTATGC-3′) and Nb VIP2R (5′-TCTTCCCATTGAGAAGTGTTGCT-3′), in parallel with Nb Ef1α primers as loading control. The experiments were repeated twice.
Characterization of At vip2
Seeds of Arabidopsis thaliana wild-type Col-0 and T-DNA insertion mutant GABI_676A06 (At vip2) were germinated, and the roots were subjected to transient and stable Agrobacterium-mediated transformation assays as described (Nam et al., 1999; Zhu et al., 2003). RNA gel blot analyses were performed on RNA extracted from homozygous At vip2 plants and wild-type Col-0 plants using the radiolabeled probe of the At VIP2 gene. RT-PCR reactions were performed on cDNAs prepared from the mutant using primer combinations (At VIP2F, 5′-TGGTTCGGGCAGATCGTTTACTGC-3′; At VIP2R, 5′-GCAAGCTTGGTCTCTTTTCC-3′) to determine the presence of At VIP2 transcript. In vitro tumorigenesis assays were performed on the axenic root segments by infecting with oncogenic strain A. tumefaciens A208 containing a nopaline-type Ti plasmid (pTiT37), cocultivated for 48 h in dark at room temperature, transferred to a hormone-free MS media supplemented with cefotaxamine and tricarcillin, and the tumor numbers and phenotypes were recorded 4 to 5 weeks after infection. Transient and stable GUS expression assays and the GF-resistant calli assay were performed as detailed earlier (Li et al., 2005b) using the disarmed strain A. tumefaciens GV3101 containing either pBISN1 or pCAS1.
Expression Profiling in the vip2 Mutant in Response to Agrobacterium Infection
The Affymetrix microarrays (Arabidopsis ATH1 genome array) were used in the expression profiling study involving At vip2 and Col-0 plants. The wild-type Col-0 and At vip2 mutant plants were syringe (needleless) infiltrated with the disarmed strain A. tumefaciens GV3101 (OD600 ∼0.2). Samples were collected at 0, 48, and 72 HAI based on the previous report (Ditt et al., 2006) showing strong differential gene expression at 48 HAI in Arabidopsis suspension cell cultures following Agrobacterium infection. Preliminary experiments on agroinfiltration in Arabidopsis indicated that the earliest GUS expression was observed at 48 HAI, with the GUS expression even stronger at 72 HAI, which is indicative of higher transformation frequency. Leaf samples were individually pooled from the infected plants (10 plants for each time point) for RNA extraction, and few representative leaves were stained with X-Gluc to confirm GUS expression. Total RNA was extracted from two independent biological replicates as described earlier (Ryu et al., 2004). RNA was further cleaned with the RNeasy mini kit (Qiagen) following the manufacturer's instructions, and the quality check was performed using the Bioanalyzer 2100 (Agilent Technologies). Affymetrix chip labeling, hybridization, and scanning procedures followed the instructions provided in the Affymetrix manual (www.affymetrix.com/support/technical/manual/expression_manual.affx).
Data Analysis, Gene Clustering, and Validation of the Microarray Data
Leaf disk transformation data were subjected to analysis of variance using JMP software version 4.0.4 (SAS Institute) or by analysis of variance. When a significant result using F-test was obtained at P = 0.05, separation of treatment means was determined by Fisher's protected least significant difference.
To obtain genes differentially expressed between the wild type and mutant, pairwise comparisons were performed for microarray data obtained from Col-0 and At vip2 at the same treatment conditions. Genes responsive to Agrobacterium treatment in both Col-0 and At vip2 were identified by comparing the treated samples with their 0 h control accordingly. For each comparison, the normalized data were imported into an Excel sheet, and differential genes were selected using the Associative Analysis algorithm developed by Dozmorov and Centola (2003). In this analysis, the Bonferroni adjusted P value threshold for Student's t test was set at 0.05/N, n = 22,000, the number of probe sets in the reference group (Dozmorov and Centola, 2003). The corrected P value ensures that the overall false positive among the multiple comparisons are controlled under 0.05. To account for multiple hypotheses testing, Q values, an estimation of FDR, were also calculated for each probe set using EDGE software (http://www.biostat.washington.edu/software/jstorey/edge/; Storey and Tibshirani, 2003). The Q value for a particular probe set reflects the proportion of false positives incurred among all probe sets as or more significant than the one being measured (Storey and Tibshirani, 2003). For comparative gene expression analyses of histone and histone-related genes between Col-0 and the At vip2 mutant following Agrobacterium treatment, all 52 histones and histone-related genes known to be expressed (having the presence of calli in both replicates) were clustered and visualized using TIGR Multiple Experiment Viewer (http://www.tm4.org/mev.html).
A few differentially regulated histone genes identified from the transcriptome analysis were selected for validation of the results by qRT-PCR (see Supplemental Table 5 online for primer details). The data from two of the biological replicates used for microarray analysis and an independent third biological replicate each with three technical replicates were analyzed to quantify the relative transcript levels in At vip2 and Col-0 plants.
BiFC Assay
Agrobacterium binary BiFC vectors (Walter et al., 2004), pSPYNE-35S and pSPYCE-35S with YFP dissected into two parts (the N-terminal [nYFP] and the C-terminal [cYFP]) were used to generate GATEWAY-compatible derivatives. A blunt-end GATEWAY cassette, reading frame B (Invitrogen), was inserted into the EcoRV site of pBluescript SKII+ (Stratagene) to obtain pBGB-EH. Subsequently, an XbaI-XhoI fragment of pBGB-EH was transferred into the XbaI-XhoI sites of pSPYNE-35S and pSPYCE-35S to obtain pSPYNE-35S_GW and pSPYCE-35S_GW, respectively. For fusion protein analyses, the full-length Nb VIP2 ORF was cloned into the nYFP construct (pSPYNE:VIP2) and the translational fusion of VirE2 into the cYFP construct with (pSPYCE:VirE2*) and without (pSPYCE:VirE2) the stop codon. We also cloned the transcriptional factor TGA2 gene into the cYFP construct (pSPYCE:TGA2). These constructs were transformed into strain A. tumefaciens GV2260 by electroporation. We used pCAMBIA1390 harboring the 35S:YFP in GV2260 as a positive control. All the strains of Agrobacterium were grown in Luria-Bertani media under appropriate antibiotics overnight, induced with acetosyringone (100 μg/mL) for 4 h at room temperature. Agrobacterium strains containing individual constructs were mixed at a 1:1 ratio and infiltrated (∼1.0 OD) into the leaves of 3- to 4-week-old N. benthamiana plants. The infiltrated plants were placed in the dark for 72 h, and leaf sections were examined by a Leica TCS SP2 AOBS confocal laser scanning microscope with the samples excited at 514 nm at 72 HAI. These experiments were repeated twice.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AF295433 (At VIP2) and DQ000202 (Nb VIP2).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Alignment of Proteins with the NOT Domain.
Supplemental Figure 2. Nuclear Localization of At VIP2 in Plant Cells.
Supplemental Figure 3. Semiquantitative RT-PCR Analyses and in Planta Tumor Assays.
Supplemental Figure 4. Quantification of Tumors in Nb VIP2–Silenced Plants.
Supplemental Figure 5. Nb VIP2–Silenced Plants Transformed by Alternate Methods.
Supplemental Figure 6. T-DNA Integration Assay.
Supplementaly Figure 7. Nb Vip2–VirE2 Interaction.
Supplemental Figure 8. Classification of the 4241 Differentially Expressed Genes.
Supplemental Figure 9. Transient Expression of the uidA-Intron Gene in Leaves of Arabidopsis Plants.
Supplemental Table 1. Quantitative Analyses of Transient and Stable Transformations in Arabidopsis At vip2 Mutant and Wild-Type Plants.
Supplemental Table 2. Differentially Expressed Genes between Col-0 and At vip2 at the Same Treatment Conditions and between 0 h Control and Treatments in Either Col-0 or At vip2.
Supplemental Table 3. Differentially Expressed Histone and Histone-Associated Genes in At vip2 and Col-0 in Response to Agrobacterium Infection.
Supplemental Table 4. Differential Gene Expression in the Wild-Type Col-0 and the At vip2 Mutant in Response to Agrobacterium Infection.
Supplemental Table 5. Primer Sequences of Genes Used for Revalidation of the Microarray Data by qRT-PCR.
Supplemental Methods.
Supplementary Material
Acknowledgments
We thank S. Gelvin, S. Uppalapati, and M. Udvardi for critical reading of the manuscript. We also thank S.P. Dinesh-Kumar for providing GATEWAY ready TRV-VIGS vectors, Stan Gelvin for many strains of Agrobacterium, Miki Hartwell and Candice Jones for their assistance with in vitro assays, and Stacy Allen for his assistance with the microarray experiments. This work was supported by Noble Foundation and by National Science Foundation Award 0445799 (K.S.M.). The work in the V.C. laboratory is supported by grants from the National Institutes of Health, the National Science Foundation, the USDA, the U.S.–Israel Binational Agricultural Research and Development Fund, and the U.S.–Israel Binational Science Foundation. The confocal system used at the Noble Foundation was from an equipment grant from the National Science Foundation (DBI 0400580).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Kirankumar S. Mysore (ksmysore@noble.org).
Online version contains Web-only data.
References
- Anand, A., and Mysore, K.S. (2006). Agrobacterium-biology and crown gall disease. In Plant-Associated Bacteria, S.S. Gnanamanickam, ed (Dordrecht, The Netherlands: Springer Science), pp. 359–384.
- Anand, A., Trick, H.N., Gill, B.S., and Muthukrishnan, S. (2003. b). Stable transgene expression and random gene silencing in wheat. Plant Biotechnol. J. 1 241–252. [DOI] [PubMed] [Google Scholar]
- Anand, A., Zarir, V., Ryu, C.M., Kang, L., del-Pozo, O., Martin, G.B., and Mysore, K.S. (2007). Identification of plant genes involved in Agrobacterium-mediated transformation by using virus-induced gene silencing as a functional genomics tool. Mol. Plant Microbe Interact. 20 41–52. [DOI] [PubMed] [Google Scholar]
- Anand, A., Zhou, T., Trick, H.N., Gill, B.S., Bockus, W.W., and Muthukrishnan, S. (2003. a). Greenhouse and field testing of transgenic wheat plants stably expressing genes for thaumatin-like protein, chitinase and glucanase against Fusarium graminearum. J. Exp. Bot. 54 1101–1111. [DOI] [PubMed] [Google Scholar]
- Ballas, N., and Citovsky, V. (1997). Nuclear localization signal binding protein from Arabidopsis mediates nuclear import of Agrobacterium VirD2 protein. Proc. Natl. Acad. Sci. USA 94 10723–10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel, P., Chien, C., Sternglanz, R., and Fields, S. (1993). Elimination of false positives that arise in using the two-hybrid system. Biotechniques 14 920–924. [PubMed] [Google Scholar]
- Burch-Smith, T.M., Anderson, J.C., Martin, G.B., and Dinesh-Kumar, S.P. (2004). Applications and advantages of virus-induced gene silencing for gene function studies in plants. Plant J. 39 734–746. [DOI] [PubMed] [Google Scholar]
- Cascales, E., and Christie, P.J. (2004). Definition of a bacterial type IV secretion pathway for a DNA substrate. Science 304 1170–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie, P.J. (2004). Type IV secretion: The Agrobacterium VirB/D4 and related conjugation systems. Biochim. Biophys. Acta 1694 219–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart, M.A. (2003). Global control of gene expression in yeast by the Ccr4-Not complex. Gene 313 1–16. [DOI] [PubMed] [Google Scholar]
- Collart, M.A., and Struhl, K. (1993). CDC39 an essential nuclear protein that negatively regulates transcription and differentially affects the constitutive and inducible his3 promoters. EMBO J. 12 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart, M.A., and Struhl, K. (1994). NOT1 (CDC39), NOT2 (CDC35), NOT3 and NOT4 encode a global-negative regulator of transcription that differentially affects TATA-element utilization. Genes Dev. 8 525–537. [DOI] [PubMed] [Google Scholar]
- Collart, M.A., and Timmers, H.T. (2004). The eukaryotic Ccr4-Not complex: A regulatory platform integrating mRNA metabolism with cellular signaling pathways? Prog. Nucleic Acid Res. Mol. Biol. 77 289–322. [DOI] [PubMed] [Google Scholar]
- Dietrich, C., and Maiss, E. (2002). Red fluorescent protein DsRed from Discosoma sp. as a reporter protein in higher plants. Biotechniques 32 286–293. [DOI] [PubMed] [Google Scholar]
- Ditt, R.F., Kerr, F.K., de Figueiredo, P., Delrow, J., Comai, L., and Nester, E.W. (2006). The Arabidopsis thaliana transcriptome in response to Agrobacterium tumefaciens. Mol. Plant Microbe Interact. 19 665–681. [DOI] [PubMed] [Google Scholar]
- Ditt, R.F., Nester, E.W., and Comai, L. (2001). Plant gene expression response to Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA 98 10954–10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozmorov, I., and Centola, M. (2003). An associative analysis of gene expression array data. Bioinformatics 22 204–211. [DOI] [PubMed] [Google Scholar]
- Endo, M., Ishikawa, Y., Osakabe, K., Nakayama, S., Kaya, H., Araki, T., Shibahara, K., Abe, K., Ichikawa, H., Valentine, L., Hohn, B., and Toki, S. (2006). Increased frequency of homologous recombination and T-DNA integration in Arabidopsis CAF-1 mutants. EMBO J. 25 5579–5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischle, W., Wang, Y., and Allis, C.D. (2003). Histone and chromatin cross-talk. Curr. Opin. Cell Biol. 15 172–183. [DOI] [PubMed] [Google Scholar]
- Frolov, M.V., Benevolenskaya, E.V., and Birchler, J.A. (1998). Regena (Rga), a Drosophila homolog of the global negative transcriptional regulator CDC36 (NOT2) from yeast, modifies gene expression and suppresses position effect variegation. Genetics 148 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelvin, S.B. (2003). Agrobacterium-mediated plant transformation: The biology behind the “gene-jockeying” tool. Microbiol. Mol. Biol. Rev. 67 16–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodin, M.M., Dietzgen, R.G., Schichnes, D., Ruzin, S., and Jackson, A.O. (2002). pGD vectors: Versatile tools for the expression of green and red fluorescent protein fusions in agroinfiltrated plant leaves. Plant J. 31 375–383. [DOI] [PubMed] [Google Scholar]
- Guralnick, B., Thomsen, G., and Citovsky, V. (1996). Transport of DNA into the nuclei of Xenopus oocytes by a modified VirE2 protein of Agrobacterium. Plant Cell 8 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, H.-H., and Gelvin, S.B. (2004). Plant proteins that interact with VirB2, the Agrobacterium tumefaciens pilin protein, mediate plant transformation. Plant Cell 16 3148–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson, R.A., Kavanagh, T.A., and Bevan, M.W. (1987). GUS fusions: β-Glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6 901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik, A., Pecinka, A., Wendeler, E., and Reiss, B. (2006). The chromatin assembly factor subunit FASCIATA1 is involved in homologous recombination in plants. Plant Cell 18 2431–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Krichevsky, A., Vaidya, M., Tzfira, T., and Citovsky, V. (2005. a). Uncoupling of the functions of the Arabidopsis VIP1 protein in transient and stable plant genetic transformation by Agrobacterium. Proc. Natl. Acad. Sci. USA 102 5733–5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Vaidya, M., White, C., Vainstein, A., Citovsky, V., and Tzfira, T. (2005. b). Involvement of KU80 in T-DNA integration in plant cells. Proc. Natl. Acad. Sci. USA 102 19231–19236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H.-Y., Badarinarayana, V., Audino, D.C., Rappsilber, J., Mann, M., and Denis, C.L. (1998). The NOT proteins are part of the CCR4 transcriptional complex and affect gene expression both positively and negatively. EMBO J. 17 1096–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., Schiff, M., and Dinesh-Kumar, P. (2002. a). Virus-induced gene silencing in tomato. Plant J. 31 777–786. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Schiff, M., Marathe, R., and Dinesh-Kumar, P. (2002. b). Tobacco Rar1, EDS1 and NPR1/NIMI1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J. 30 415–429. [DOI] [PubMed] [Google Scholar]
- Mysore, K.S., Bassuner, B., Deng, X.B., Darbinian, N.S., Motchoulski, A., Ream, W., and Gelvin, S.B. (1998). Role of the Agrobacterium tumefaciens VirD2 protein in T-DNA transfer and integration. Mol. Plant Microbe Interact. 11 668–683. [DOI] [PubMed] [Google Scholar]
- Mysore, K.S., Nam, J., and Gelvin, S.B. (2000). An Arabidopsis histone H2A mutant is deficient in Agrobacterium T-DNA integration. Proc. Natl. Acad. Sci. USA 97 948–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam, J., Mysore, K.S., Zheng, C., Knue, M.K., Matthysse, A.G., and Gelvin, G.B. (1999). Identification of T-DNA tagged Arabidopsis mutants that are resistant to transformation by Agrobacterium. Mol. Gen. Genet. 261 429–438. [DOI] [PubMed] [Google Scholar]
- Oberholzer, U., and Collart, M.A. (1998). Characterization of NOT5 that encodes a new component of the Not protein complex. Gene 207 61–69. [DOI] [PubMed] [Google Scholar]
- Park, H., and Sternglanz, R. (1998). Two separate conserved domains of eukaryotic DNA topoisomerase I bind to each other and reconstitute enzymatic activity. Chromosoma 107 211–215. [DOI] [PubMed] [Google Scholar]
- Rosso, M.G., Li, Y., Strizhov, N., Reiss, B., Dekker, K., and Weisshaar, B. (2003). An Arabidopsis thaliana T-DNA mutagenized population (GABI-Kat) for flanking sequence tag-based reverse genetics. Plant Mol. Biol. 53 247–259. [DOI] [PubMed] [Google Scholar]
- Ryu, C.-M., Anand, A., Kang, L., and Mysore, K.S. (2004). Agrodrench: A novel and effective agroinoculation method for virus-induced gene silencing in roots and diverse Solanaceous species. Plant J. 40 322–331. [DOI] [PubMed] [Google Scholar]
- Schultheiss, H., Dechert, C., Kogel, K.H., and Hückelhoven, R. (2003). Functional analysis of barely RAC/ROP G-protein family members in susceptibility to the powdery mildew fungus. Plant J. 36 589–601. [DOI] [PubMed] [Google Scholar]
- Storey, J.D., and Tibshirani, R. (2003). Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 100 9440–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, G.-W., et al. (2004). High-throughput fluorescent tagging of full-length Arabidopsis gene products in planta. Plant Physiol. 135 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzfira, T., and Citovsky, V. (2002). Partners-in-infection: Host proteins involved in the transformation of plant cells by Agrobacterium. Trends Cell Biol. 12 121–129. [DOI] [PubMed] [Google Scholar]
- Tzfira, T., and Citovsky, V. (2006). Agrobacterium-mediated genetic transformation of plants: Biology and biotechnology. Curr. Opin. Biotechnol. 17 147–154. [DOI] [PubMed] [Google Scholar]
- Tzfira, T., Li, J., Lacroix, B., and Citovsky, V. (2004). Agrobacterium T-DNA integration: Molecules and models. Trends Genet. 20 375–383. [DOI] [PubMed] [Google Scholar]
- Tzfira, T., Rhee, Y., Chen, M.-H., Kunik, T., and Citovsky, V. (2000). Nucleic acid transport in plant-microbe interactions: The molecules that walk through the Walls. Annu. Rev. Microbiol. 54 187–219. [DOI] [PubMed] [Google Scholar]
- Tzfira, T., Vaidya, M., and Citovsky, V. (2001). VIP1, an Arabidopsis protein that interacts with Agrobacterium VirE2, is involved in VirE2 nuclear import and Agrobacterium infectivity. EMBO J. 20 3596–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veena, Jiang, H., Doerge, R.W., and Gelvin, S.B. (2003). Transfer of T-DNA and Vir proteins to plant cells by Agrobacterium tumefaciens induces expression of host genes involved in mediating transformation and suppresses host defense gene expression. Plant J. 35 219–226. [DOI] [PubMed] [Google Scholar]
- Vergunst, A.C., Schrammeijer, B., den Dulk-Ras, A., de Vlaam, C.M.T., Regensburg-Tuink, T.J., and Hooykaas, P.J. (2000). VirB/D4-dependent protein translocation from Agrobacterium into plant cells. Science 290 979–982. [DOI] [PubMed] [Google Scholar]
- Vergunst, A.C., van Lier, M.C.M., den Dulk-Ras, A., and Hooykaas, P.J. (2003). Recognition of the Agrobacterium VirE2 translocation signal by the VirB/D4 transport system does not require VirE1. Plant Physiol. 133 978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, M., Chaban, C., Schutze, K., Batistic, O., Weckermann, K., Nake, C., Blazevic, D., Grefen, C., Schumacher, K., Oecking, C., Harter, K., and Kudla, J. (2004). Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40 428–438. [DOI] [PubMed] [Google Scholar]
- Wang, B.-B., and Brendel, V. (2006). Genomewide comparative analysis of alternative splicing in plants. Proc. Natl. Acad. Sci. USA 103 7175–7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wroblewski, T., Tomczak, A., and Michelmore, R. (2005). Optimization of Agrobacterium-mediated transient assays of gene expression in lettuce, tomato and Arabidopsis. Plant Biotechnol. J. 3 259–273. [DOI] [PubMed] [Google Scholar]
- Yi, H., Sardesai, N., Fujinuma, T., Chan, C.W., Veena, and Gelvin, S.B. (2006). Constitutive expression exposes functional redundancy between the Arabidopsis histone H2A gene HTA1 and other H2A gene family members. Plant Cell 18 1575–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, H.C., Mysore, K.S., and Gelvin, S.B. (2002). Expression of the Arabidopsis histone H2A-1 gene correlates with susceptibility to Agrobacterium transformation. Plant J. 32 285–298. [DOI] [PubMed] [Google Scholar]
- Zhu, Y., et al. (2003). Identification of Arabidopsis rat mutants. Plant Physiol. 132 494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.