Abstract
RGS4, a mammalian GTPase activating protein for G protein α subunits, was identified by its ability to inhibit the pheromone response pathway in Saccharomyces cerevisiae. To define regions of RGS4 necessary for its function in vivo, we assayed mutants for activity in this system. Deletion of the N-terminal 33 aa of RGS4 (Δ1–33) yielded a nonfunctional protein and loss of plasma membrane localization. These functions were restored by addition of a C-terminal membrane-targeting sequence to RGS4 (Δ1–33). Thus, plasma membrane localization is tightly coupled with the ability of RGS4 to inhibit signaling. Fusion of the N-terminal 33 aa of RGS4 to green fluorescent protein was sufficient to localize an otherwise soluble protein to the plasma membrane, defining this N-terminal region as a plasma membrane anchorage domain. RGS4 is palmitoylated, with Cys-2 and Cys-12 the likely sites of palmitoylation. Surprisingly, mutation of the cysteine residues within the N-terminal domain of RGS4 did not affect plasma membrane localization in yeast or the ability to inhibit signaling. Features of the N-terminal domain other than palmitoylation are responsible for the plasma membrane association of RGS4 and its ability to inhibit pheromone response in yeast.
Heterotrimeric G proteins couple receptors for hormones, neurotransmitters, and sensory signals to intracellular effector molecules, thereby eliciting cellular responses (1, 2). Guanine nucleotide exchange and hydrolysis on the G protein α subunit drives the cycle of activation and deactivation of these signaling pathways. The duration of G protein-mediated responses is subject to the intrinsic GTPase rate of the G protein α subunit, but is also modulated by extrinsic factors. A recently appreciated form of regulation has come from the discovery that members of a protein family called regulators of G protein signaling, or RGS proteins, stimulate the rate of GTP hydrolysis by G protein α subunits (3–5). RGS proteins are found in species ranging from yeast to mammals and constitute a family of at least 20 mammalian proteins (6–8).
All RGS family members share sequence similarity that extends over approximately 130 aa, separated in some cases by insertions of varying length (9–11). This conserved RGS domain is sufficient to stimulate GTPase activity of G protein α subunits in vitro (12–14). Expression of the RGS homology domain of RET-RGS1 or RGS4 yields a recombinant protein that is a functional GAP (GTPase-activating protein). In the crystal structure of RGS4 bound to AlF4−-activated Giα1, only the core domain is visible (15). The RGS homology domain binds to the switch regions of Giα1 and appears to catalyze GTP hydrolysis by stabilizing the switch regions of the G protein in a conformation that favors the transition state of the reactants (14, 15). Sequences outside the RGS homology domain exhibit considerable diversity among family members.
Although rapid progress has been made in dissecting the biochemical mechanism by which RGS proteins regulate G protein activity in vitro, less is understood about RGS localization and regulation in vivo. At least one RGS protein, RET-RGS1, has a transmembrane domain predicted from its primary amino acid sequence (12). However, the other mammalian RGS proteins characterized to date (RGS1, 2, 4, 5, 10, 16‡, and GAIP) behave as soluble proteins when expressed in Escherichia coli and no obvious membrane anchoring domains are predicted from their sequences. The subcellular localization of RGS proteins in eukaryotic cells has not been examined in detail. The SST2 gene product in Saccharomyces cerevisiae, the prototypic member of the RGS family, is a negative regulator of the pheromone response pathway. Sst2p colocalizes with Gpa1p, the G protein α subunit of the pheromone response pathway, at the plasma membrane as assessed by density gradient sedimentation (16). GAIP, an RGS protein identified by its interaction with Giα3 in a two-hybrid screen (17), is found both in soluble and particulate fractions when expressed in COS and AtT-20 cells (18). The membrane-associated form of the protein is palmitoylated, providing a potential mechanism for membrane anchoring of GAIP (18). The role of membrane localization of RGS proteins in regulating G protein activity in vivo has not been characterized.
We sought to understand how sequences outside the RGS homology domain contribute to the localization and function of RGS proteins in vivo. Certain mammalian RGS family members, including RGS4, are able to complement an SST2 deletion in S. cerevisiae. The complementation assay is a convenient means of defining regions of the protein necessary for function in vivo. Here we report that the ability of RGS4 to inhibit pheromone signaling in yeast requires an N-terminal domain of the protein that is both necessary and sufficient for plasma membrane targeting.
EXPERIMENTAL PROCEDURES
Yeast Strains, Media, and Pheromone Response Assays.
The yeast strain used in the pheromone response assay was BC180 (MATa leu2–3, 112 ura3–52 his3Δ1 ade2–1 sst2-Δ2) and in microscopy was SWY518 ADE2+ (MATa ura3–1 his3–11, 15 leu2–3, 115 trp1–1 can1–100) (19). Yeast cells were grown at 30°C in synthetic dextrose medium lacking uracil (SD-ura). Pheromone responsiveness was determined by halo assay in which cells were embedded in agar containing SD-ura and exposed to various amounts of synthetic pheromone (α-factor) on sterile filter disks (11).
Plasmids and Mutagenesis Methods.
RGS4 was expressed in yeast from the constitutive ADH promoter. The entire coding region of the rat RGS4 cDNA (11) generated by PCR as a BamHI-XbaI fragment was subcloned into pVT102U, creating pVT-RGS4. Deletion mutants of RGS4 were generated by PCR and inserted into pVT102U as BamHI-XbaI fragments. To generate a chimeric protein of RGS4 in-frame with green fluorescent protein (GFP), the coding region of GFP was amplified as an XbaI-XhoI fragment from pRSET B-GFP (obtained from L. Robinson, Louisiana State University). pVT-RGS4 was digested with XbaI and XhoI and ligated with the GFP fragment, creating pVT-RGS4-GFP. Similarly, pVT-GFP was created by inserting the GFP PCR fragment at the XbaI and XhoI sites of pVT102U. To create a single-copy plasmid, an SphI fragment containing the ADH promoter, RGS4-GFP coding region, and ADH1 3′ region from the multicopy plasmid pVT-RGS4-GFP was subcloned into the SphI site of YCp50, creating YCp50-RGS4-GFP. To create RGS4 N-terminal fusion constructs [except (1–33)-GFP] with GFP, complementary oligonucleotides corresponding to the relevant amino acids of RGS4 were ligated in-frame to the beginning of the coding region of GFP. Fusion of the N-terminal 33 aa of RGS4 with GFP was achieved by ligating a corresponding PCR fragment to the start of the GFP coding region in pVT-GFP plasmid at BamHI-XbaI sites. Oligonucleotides encoding the C-terminal 9 aa of Ste18p (SNSVCCTLM) or Ras2p (GSGGCCIIS) were fused to the 3′ end of the (Δ1–33)RGS4-GFP coding region by using a PCR-based strategy. Point mutations in the RGS4 coding region were generated by using the Quikchange mutagenesis kit (Stratagene). An epitope-tagged form of RGS4 was created by ligating an XbaI fragment containing the triple myc epitope from pUC119–3x-myc (obtained from D. Pellman, Whitehead Institute, Cambridge, MA) with pVT-RGS4 digested with XbaI. The resulting plasmid, pVT-RGS4-myc, has the sequence encoding the epitope tag fused in-frame at the 3′ end of RGS4 sequence. The entire RGS4 coding region was sequenced in all plasmids to verify that the expected constructs or mutations had been generated.
Construction of Recombinant Baculovirus.
RGS4-myc was subcloned as a BamHI fragment from pVT-RGS4-myc into pBluescript. This construct served as the parental plasmid for subsequent subcloning and mutagenesis. Single cysteine point mutations, C2A, C12A, or C33A, were introduced by using PCR. C2A was constructed by PCR using a mutagenic primer for the coding strand that encompassed the 5′ BamHI cloning site to change codons 2 and 3, TGCAAA, to GCCAAG, substituting an alanine for the cysteine at amino acid 2 and introducing a StyI site as a silent mutation in the third codon. C12A was constructed by using inverse PCR, mutating the Cys12 codon TGC, to the Ala12 codon GCA, which disrupts the Bsu36I site. C33A was constructed by using a modified inverse PCR strategy to change the cysteine 33 codon TGT to GCG, introducing a SacII site. Wild-type and mutant constructs were subcloned from pBluescript into the Baculovirus vector pBAKPAK8 (CLONTECH) as BamHI fragments. Orientation of the insert was verified by restriction mapping. Recombinant Baculoviruses were generated as described (20).
Confocal Microscopy.
Yeast cells carrying the relevant plasmids were grown to mid-logarithmic phase in SD-ura liquid medium. Confocal microscopy was performed on live cells by using a Zeiss Axioplan microscope coupled to an MRC-1000 Laser Scanning Confocal Microscope (Bio-Rad). The images represent single planes obtained from the middle part of the cell with a ×63 objective. Confocal images were processed using adobe photoshop 4.0.
Insect Cell Culture and Radiolabeling.
Sf9 insect cells were cultured as described (20). Cells were infected at a density of 1.5 × 106 cells/ml with Baculoviruses encoding wild type, C2A-, C12A-, or C33A-RGS4-myc at 1/100 dilution of virus. After 36–42 hr of infection, cells were incubated with [3H]palmitate (DuPont/NEN, 35 Ci/mmol; 1 Ci = 37 GBq) at 0.5–1 mCi/ml for 2 hr or with [35S]methionine (Amersham [35S] In Vitro Cell Labeling Mix, >1,000 Ci/mmol) at 50 μCi/ml for 3 hr as described (21). Cells were harvested by centrifugation, washed once with cold PBS, and suspended in 10 mM sodium phosphate (pH 7.4), 1 mM EDTA, 1 mM DTT, and protease inhibitors [0.1 mM PMSF (phenylmethylsulfonyl fluoride), 21 μg/ml TPCK (N-tosyl-l-phenylalanine chloromethyl ketone), 21 μg/ml TLCK (Nα-p-tosyl-l-lysine chloromethyl ketone), 3.2 μg/ml leupeptin, 3.2 μg/ml lima bean trypsin inhibitor]. Lysis was achieved by three cycles of flash freezing in liquid nitrogen and thawing at 30°C. The lysate was centrifuged at 100,000 × g to yield particulate (P100) and soluble (S100) fractions. The P100 fraction was solubilized in RIPA buffer (PBS containing 0.1% SDS, 1% Nonidet P-40, 0.5% deoxycholate) as described (22). An equal volume of 2× RIPA buffer was added to the S100 fraction. A mAb against the c-myc epitope was used for immunoprecipitation of RGS4-myc proteins (23). The myc antibody was purified from the tissue culture supernatants of hybridoma MYC1–9E10.2 (obtained from American Type Culture Collection) and covalently coupled to Protein A-Sepharose CL-4B beads (Pharmacia) (24). Fractions from radiolabeled insect cells were incubated at 4°C for 2–16 hr with antibody-coupled beads. After separation from unbound proteins, immunoprecipitated RGS4-myc was eluted from the antibody beads with sample buffer and resolved by SDS/PAGE. Gels (13% acrylamide) were stained with Coomassie blue, treated with Amplify (Amersham) for 30 min, dried, and exposed to Kodak X-AR film at −80°C.
Hydroxylamine Treatment of SDS/PAGE Gels.
Radiolabeled immunoprecipitates were resolved on duplicate gels. After fixation in 30% methanol/10% acetic acid, the gels were soaked in 1 M hydroxylamine (pH 7) or 1 M Tris (pH 7) in 50% isopropanol for 12–18 hr. The gels were washed for 48 hr in five changes of 50% isopropanol, stained with Coomassie blue, and processed for fluorography as described (25).
Fatty Acid Analysis of Radiolabeled Proteins.
Immunoprecipitated RGS4 in a polyacrylamide gel slice was hydrolyzed with 1.5 M NaOH. Radioactive fatty acids were extracted with chloroform/methanol and analyzed as described (21).
RESULTS
Plasma Membrane Localization of RGS4 Is Required for Its Function in Yeast.
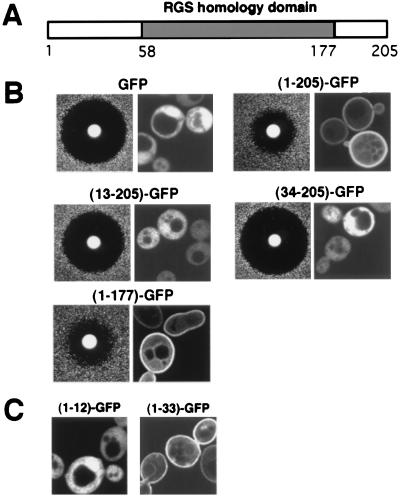
The RGS homology domain in RGS4, extending from amino acids 58–177 (Fig. 1A), is sufficient for the GAP activity of RGS4 in vitro (12–14). To define the minimal sequences required for RGS4 function in vivo, we used the ability of RGS4 protein to inhibit pheromone signaling in the budding yeast S. cerevisiae as an assay (11). In response to mating pheromone, yeast cells arrest late in the G1 phase of the cell cycle. This response can be measured by the size of the halo or zone of growth inhibition of cell lawns grown on an agar plate. We expressed RGS4 as a chimeric protein with GFP at the carboxyl terminus to examine both the function and localization of RGS4 in yeast (Fig. 1B). RGS4-GFP inhibited the growth arrest response (Fig. 1B), similar to the wild-type protein (data not shown). Expression of GFP alone did not affect the responsiveness of the cells to pheromone (Fig. 1B). Deletion of the first 12 aa of RGS4 resulted in a partial loss of function, and deletion of the first 33 aa from the N terminus completely inactivated RGS4 in vivo. However, RGS4 lacking 28 aa from the C terminus appeared fully functional. Thus, an intact amino-terminal region of RGS4 is required for its ability to function in yeast. Confocal microscopy of living cells expressing RGS4-GFP showed that the chimeric protein was localized at the plasma membrane (Fig. 1B). Mutant RGS4-GFP fusion proteins lacking the N-terminal 12 and 33 aa were mislocalized to the cytoplasm, whereas the functional C-terminal deletion mutant was found at the plasma membrane. Expression levels of all the deletion mutants were similar to the intact protein (data not shown). The strong correlation between plasma membrane localization of RGS4 and its ability to inhibit G protein signaling suggests that the function of RGS4 in yeast depends on its localization at the plasma membrane.
Figure 1.
Subcellular localization and function of RGS4 in yeast cells requires the amino-terminal 33 residues of the protein. (A) A schematic diagram of the primary structure of RGS4 protein. Shaded box represents the RGS homology domain. Numbers below the box correspond to amino acid residues in the protein. (B) Effects of deletion mutations in RGS4-GFP on its ability to inhibit pheromone signaling and its subcellular localization. For each pair, the left panel shows halo assays measuring pheromone response of an sst2Δ mutant (BC180) expressing the indicated constructs fromYCp50, a single-copy vector. The right panel represents confocal images of wild-type yeast cells (SWY518) harboring the same constructs in pVT102U (multicopy) vector. Numbers in parentheses in front of GFP represent the corresponding amino acids of RGS4. (C) Confocal images of cells expressing GFP fusions containing the first 12 or first 33 aa of RGS4.
The N-Terminal Domain of RGS4 Is Responsible for Its Plasma Membrane Localization.
To determine whether the N-terminal domain of RGS4 is sufficient to direct a heterologous protein to the plasma membrane, we created in-frame fusions of the N-terminal 12 or 33 aa of RGS4 with GFP. The first 33 aa of RGS4 conferred plasma membrane localization on GFP (Fig. 1C). The chimeric protein with only 12 aa of RGS4 was cytosolic (Fig. 1C). Thus, an N-terminal domain of 33 aa of RGS4 is both necessary and sufficient to target a heterologous cytosolic protein to the plasma membrane.
RGS4 Targeted to the Plasma Membrane by a Heterologous C-Terminal Membrane Anchor Is Functional in Vivo.
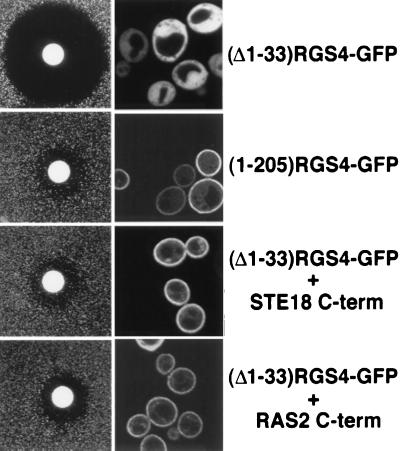
If the primary function of the N-terminal domain is to target RGS4 to the plasma membrane, then membrane localization of (Δ1–33)RGS4 by another mechanism should restore the ability of the deletion mutant to inhibit signaling. The C-terminal sequence of Ras that encodes the prenylation and palmitoylation sites can be used to direct heterologous cytoplasmic proteins to the plasma membrane (26). In yeast, the Ras2 protein and the G protein γ subunit encoded by the STE18 gene have C-terminal sequences that undergo prenylation and palmitoylation (refs. 27 and 28; C. L. Manahan and M.E.L., unpublished results). By analogy to mammalian Ras, we assumed that these sequences also would serve as plasma membrane targeting signals. Therefore, we fused the last 9 aa of Ras2p or Ste18p to the C terminus of (Δ1–33)RGS4-GFP. Addition of either membrane-targeting sequence to (Δ1–33)RGS4-GFP fully restored its ability to inhibit signaling (Fig. 2 Left). Furthermore, the chimeric proteins exhibited striking plasma membrane localization (Fig. 2 Right). Hence, we conclude that the primary role of the N-terminal 33 aa of RGS4 is to localize the protein at the plasma membrane where it can encounter its target G protein.
Figure 2.
C-terminal prenylation/palmitoylation sequences functionally replace the N-terminal 33 aa of RGS4. Halo assays of an sst2Δ mutant (BC180) expressing the indicated constructs from the pVT102U vector are shown in the left column. Confocal images of SWY518 cells harboring the corresponding constructs are shown in the right column. The smaller halo size for functional RGS4 in this figure compared with Fig. 1 is because of higher protein expression from a multicopy plasmid.
RGS4 Is Palmitoylated.
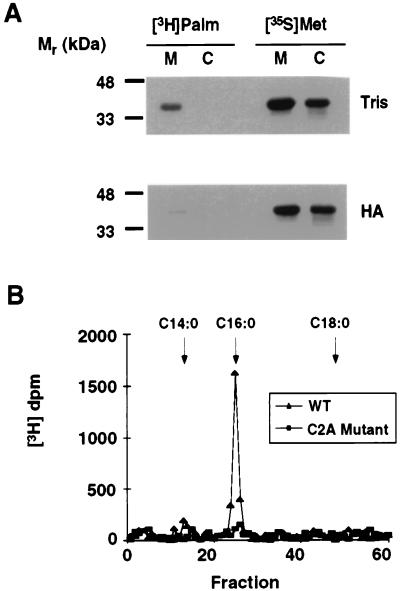
To determine how the N-terminal domain of RGS4 might target the protein to membranes, we examined the hypothesis that the protein was covalently modified with lipids. Many signaling proteins lacking transmembrane domains associate with the cytoplasmic surface of the plasma membrane by the covalent attachment of lipids (29). RGS4 does not have a transmembrane domain, nor does it have consensus sites for N-myristoylation or prenylation. However, there are three cysteine residues within the first 33 aa that are potential sites for the attachment of thioester-linked fatty acids. To determine whether RGS4 is palmitoylated, we generated recombinant Baculovirus encoding RGS4 with a triple myc epitope tag added at the carboxyl terminus. Insect cells infected with recombinant Baculovirus provide robust expression of lipid-modified proteins that facilitate detailed analysis of the lipid moiety. Both mammalian and yeast G protein subunits are appropriately modified with fatty acyl or prenyl groups when expressed in this system (refs. 20 and 21; C. L. Manahan and M.E.L., unpublished results). [35S]Methionine-labeled RGS4 was found in both the cytosol and membranes of Sf9 cells (Fig. 3A). However, labeling with [3H]palmitate was observed only in the membrane-bound pool. Incorporation of tritium into RGS4 was sensitive to neutral hydroxylamine, consistent with attachment of radioactive palmitate through a thioester linkage (Fig. 3A). [3H]Palmitate was released from RGS4 by alkaline hydrolysis as seen in the HPLC liquid chromatography profile in Fig. 3B, confirming that RGS4 is palmitoylated.
Figure 3.
RGS4 incorporates [3H]palmitate in a hydroxylamine-sensitive linkage. (A) Sf9 cells expressing RGS4-myc were metabolically labeled with [3H]palmitate or with [35S]methionine. The protein was immunoprecipitated from detergent extracts of membranes (M) or from cytosol (C) with a mAb against the myc epitope. Immunoprecipitates were resolved by SDS/PAGE, and the gels were treated with either 1 M Tris (pH 7) (Tris) or 1 M hydroxylamine (pH 7) (HA). The fluorograph was exposed to film for 3 days. (B) Sf9 cells expressing RGS4-myc or C2A RGS4-myc were labeled with [3H]palmitate and immunoprecipitated from detergent extracts of membranes. After resolution by SDS/PAGE, the RGS4 bands were excised from the gel and subjected to base hydrolysis. Base hydrolysates were extracted into organic solvent and chromatographed over a reversed-phase Ultrasphere C18 column. Fractions were collected and quantitated by scintillation counting. Elution positions of standards [myristate (C14:0), palmitate (C16:0), and stearate (C18:0)] are indicated with arrows.
Cys-2 and Cys-12 of RGS4 Are Candidate Sites of Palmitoylation.
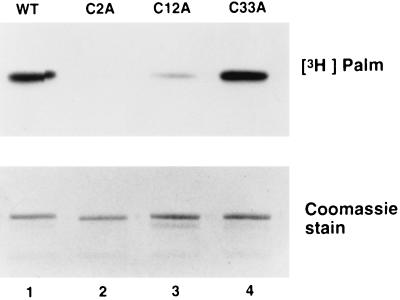
Having determined that RGS4 is a palmitoylated protein, we next examined which cysteines are the likely sites for palmitate attachment. No consensus sequence is associated with palmitoylation; thus any cysteine residue is a potential site. Because the N-terminal domain confers membrane localization of RGS4, cysteine residues within the first 33 aa at positions 2, 12, and 33 were examined. We assayed [3H]palmitate incorporation into RGS4 with alanine substitutions of each of these cysteine residues. The C2A mutant of RGS4-myc did not incorporate [3H]palmitate (Fig. 3B and Fig. 4). Thus, palmitoylation of RGS4 is dependent on a cysteine residue at position 2. Mutation of Cys-12 in RGS4-myc resulted in a substantial reduction of palmitate incorporation (Fig. 4), suggesting that this residue is also modified. Mutation of Cys-33 had little effect on [3H]palmitate incorporation, and thus this residue is not likely to be acylated (Fig. 4). The data are consistent with the primary site of palmitoylation as Cys-2, with Cys-12 as a secondary site. It is formally possible that other cysteine residues within the protein are also modified, but their modification is also dependent on Cys-2.
Figure 4.
Cysteines 2 and 12 of RGS4 are the likely sites of palmitate incorporation. Sf9 cells expressing wild type (WT), C2A-, C12A-, or C33A- RGS4-myc were labeled with [3H]palmitate. The labeled protein was immunoprecipitated from detergent extracts of membranes and subjected to SDS/PAGE and fluorography (Upper). Because C2A RGS4-myc was expressed at lower levels than wild type or the other cysteine substitution mutants, the amount of detergent extract immunoprecipitated for each sample was adjusted to produce equal amounts of RGS4 protein as indicated in the Coomassie blue-stained gel (Lower).
Palmitoylation Is Not Required for Plasma Membrane Localization or Function of RGS4.
To evaluate the role of palmitoylation of RGS4 in its membrane association, we examined the subcellular distribution of palmitoylated and nonpalmitoylated RGS4 by using immunoblots (data not shown). Quantitation of immunoblots revealed that for wild-type RGS4, the distribution was P1(27 ± 3%), P100 (23 ± 3%), and S100 (50 ± 6%). The C2A mutant of RGS4 was distributed as follows: P1 (24 ± 5%), P100 (26 ± 7%), and S100 (50 ± 10%). Distributions of the C12A and C33A mutants of RGS4 were similar. Thus, when expressed in Sf9 cells, palmitoylation of RGS4 has little effect on membrane localization.
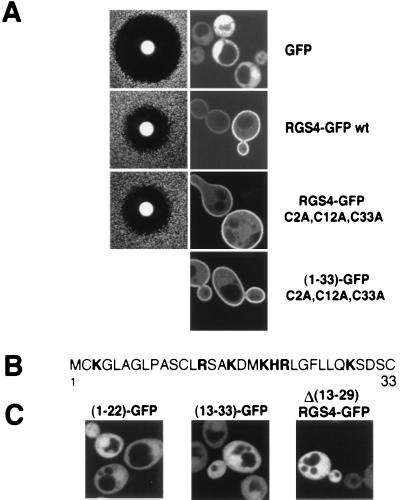
To test whether palmitoylation of RGS4 is responsible for plasma membrane localization in yeast, a mutant protein was generated with alanine substitutions at all three cysteine residues within the N-terminal domain (positions 2, 12, and 33). The triple cysteine mutant was found at the plasma membrane when expressed in yeast (Fig. 5A). Furthermore, consistent with the strong correlation between plasma membrane localization and function of RGS4, substituting all three N-terminal cysteine residues of RGS4-GFP with alanine residues resulted in a fully functional protein in the pheromone response assay (Fig. 5A). Mutation of cysteine residues also did not affect the ability of the N-terminal domain to localize the chimeric GFP to the plasma membrane (Fig. 5A).
Figure 5.
Analysis of the N-terminal domain of RGS4. (A) Mutation of cysteine residues within the amino terminus of RGS4 has no effect on the ability of RGS4 to inhibit pheromone response or its localization. The column on the left represents halo assays of an sst2Δ mutant (BC180) expressing the indicated constructs in a low-copy (YCp50-based) vector. The column on the right shows confocal images of wild-type cells harboring the respective constructs in a high-copy (pVT102U-based) vector. RGS4-GFP represents the entire coding region of RGS4 fused to GFP, whereas (1–33)-GFP represents the first 33 aa of RGS4 fused to GFP. (B) Sequence of the first 33 aa of RGS4 protein. Basic amino acids are indicated in bold letters. (C) Confocal images of SWY518 cells expressing the indicated constructs in the high-copy vector. Numbers in parentheses represent the first 22 (Left) or first 33 (Center) aa of RGS4 fused to GFP. In Δ(13–29)RGS4-GFP, the indicated residues are deleted from an otherwise full-length protein.
The surprising finding that palmitoylation of RGS4 had little effect on its membrane localization led us to look for other features of the N-terminal domain of RGS4 that would target the protein to membranes. A cluster of basic residues is found in RGS4 between amino acids 13 and 22 (Fig. 5B). Polybasic domains can act cooperatively with a myristoyl group or farnesyl group to target proteins to the plasma membrane including the MARCKS protein, p60Src kinase, and Ki-Ras (30). To test the role of the RGS4 polybasic domain in plasma membrane targeting, we analyzed GFP fusions with N-terminal subdomains. Smaller segments of the N terminus inclusive of the polybasic stretch of amino acids are not sufficient to target GFP to the plasma membrane (Fig. 5C). Furthermore, deletion of residues 13 through 29, which removes all but one basic residue, abolishes plasma membrane localization (Fig. 5C) and function (data not shown) of an otherwise full-length RGS4-GFP fusion protein. Thus, the region containing basic residues is necessary but not sufficient for plasma membrane targeting. We conclude that an intact amino-terminal domain of RGS4 comprising at least 30 aa is likely to be the signal for plasma membrane localization of RGS4.
DISCUSSION
Structure–function analysis of RGS proteins has revealed that the RGS homology domain or core domain is sufficient for GAP activity in vitro (12–14). In this study, we define a second functional domain of RGS4. The first 33 aa of RGS4 act as a transplantable plasma membrane targeting sequence that is required for the activity of RGS4 in vivo. A primary function of the N-terminal domain is its membrane-localizing activity. A heterologous C-terminal membrane-anchoring signal restores the function and localization of a mutant RGS4 that lacks the N-terminal 33 aa. Thus, RGS4 appears to rely on localization for function. Other RGS proteins may contain a similar functional N-terminal domain. Although localization of the mutant protein was not reported, deletion of the N-terminal 13-aa residues of mouse RGS16 renders the protein nonfunctional in yeast (8). Indeed, the sequence conservation in the N-terminal 30 aa of RGS4, RGS5, and RGS16 suggests that this region codes for a functionally important domain (Fig. 6). Fourteen of 33 residues are identical, including the cysteine residues that have been mapped as the palmitoylation sites of RGS4, and 5 additional positions have conservative substitutions. The conserved N-terminal region is likely to represent a plasma membrane targeting sequence for this subclass of RGS proteins.
Figure 6.
Conservation of N-terminal amino acid sequences of RGS4, RGS5, and RGS 16. The N-terminal sequences of rat RGS4, mouse RGS5, and mouse RGS16 are aligned by the clustal method (DNAStar). Conserved residues are boxed in black. Numbers refer to the position of the corresponding amino acid in the sequence.
Palmitoylation of RGS4 within the N-terminal domain of the protein suggests a mechanism for its membrane association. However, inactivation of the putative palmitoylation sites has no measurable effect on plasma membrane localization in yeast or insect cells or on its ability to inhibit signaling in yeast. How does the N-terminal domain associate with the plasma membrane in the absence of palmitoylation? The entire N-terminal domain consisting of residues 1–33 appears to contribute targeting information because attempts to subdivide it resulted in a loss of plasma membrane localization. From protein secondary structure modeling, the 33 aa sequence is predicted to form an α helix. A cluster of positive charges is found within residues 13–29 that may promote membrane interaction by neutralizing negative charges on phospholipid head groups. A cooperative interaction between positively charged amino acids and a myristoyl group promotes membrane interaction of the MARCKS protein or p60Src (30). In RGS4, the amphipathic nature of the proposed α-helical domain may be sufficient for plasma membrane association in the absence of palmitoylation. Examples of proteins that associate with membrane surfaces through amphipathic helices include CTP:phosphocholine cytidylyltransferase (31) and prostaglandin H synthase (32). Alternatively, the N-terminal domain may interact with other proteins to promote plasma membrane localization.
The finding that palmitoylation is not absolutely required for membrane association is surprising, but not without precedent. The palmitoylated form of glutamic acid decarboxylase (GAD65) is associated with membranes of microvesicles in pancreatic beta cells and synaptic vesicles in γ-aminobutyric acid-secreting neurons. Membrane localization of GAD65 requires amino acid residues 24–31 of the protein, but not cysteine residues 30 and 45, which are palmitoylated (33). Protein palmitoyltransferase activities are localized in membranes (34–36), and acylation of RGS4 or GAD65 may require the protein to first bind to membranes before it can be palmitoylated. In both proteins, the sites of palmitoylation are in the vicinity of sequences required for membrane targeting.
Two sequence motifs for palmitoylation of RGS proteins are suggested by the data currently available. GAIP was the first RGS family member reported to be palmitoylated (18). The proposed sites of palmitoylation are within a cysteine-rich region of GAIP (18). The cysteine-rich region of GAIP is analogous to that found in cysteine string proteins (37), a family of synaptic vesicle proteins that are palmitoylated with a stoichiometry of greater than 10 mol palmitate/mol protein (38). A similar cysteine string motif is present in RET-RGS1 (12), suggesting that it may also be posttranslationally acylated. Our data suggest that a second type of palmitoylation signal occurs in RGS4 at cysteine residues 2 and 12. As noted earlier, these sites are conserved in RGS5 and RGS16, and we predict that these proteins are also palmitoylated.
Palmitoylation of RGS4 and other RGS proteins probably subserves functions in mammalian cells other than or in addition to membrane targeting. These remain to be elucidated. Although best characterized for promoting membrane association (30), palmitoylation also affects trafficking of proteins (39), accessibility of proteins to kinases (40), and protein–protein interactions (41, 42). It was reported recently that the GAP activity of RGS4 and other RGS proteins is inhibited in vitro by palmitoylation of Giα (43). It will be of obvious interest to determine whether palmitoylation of RGS4 influences its protein–protein interactions. The reversibility of palmitoylation provides an additional level of control for any function that it influences. Thus, posttranslational fatty acylation is an attractive mechanism for regulating the regulator.
Acknowledgments
We thank Alison Woodworth for assistance with the initial phases of this work and Dr. Haibing Teng for assistance with confocal microscopy. This work was supported by the Monsanto-Searle/Washington University Biomedical Research Program (K.J.B. and M.E.L.) and United States Public Health Service Grants GM50556 (M.E.L.) and GM44592 (K.J.B.). L.S.B. was supported by Training Grant 5T32GM8151. K.J.B. is an Established Investigator of the American Heart Association.
ABBREVIATIONS
- RGS
regulator of G protein signaling
- GFP
green fluorescent protein
- GAP
GTPase-activating protein
Footnotes
References
- 1.Gilman A G. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 2.Neer E J. Cell. 1995;80:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 3.Berman D M, Wilkie T M, Gilman A G. Cell. 1996;86:445–452. doi: 10.1016/s0092-8674(00)80117-8. [DOI] [PubMed] [Google Scholar]
- 4.Watson N, Linder M E, Druey K M, Kehrl J, Blumer K J. Nature (London) 1996;383:172–175. doi: 10.1038/383172a0. [DOI] [PubMed] [Google Scholar]
- 5.Hunt T W, Fields T A, Casey P J, Peralta E G. Nature (London) 1996;383:175–177. doi: 10.1038/383175a0. [DOI] [PubMed] [Google Scholar]
- 6.Dohlman H G, Thorner J. J Biol Chem. 1997;272:3871–3874. doi: 10.1074/jbc.272.7.3871. [DOI] [PubMed] [Google Scholar]
- 7.Koelle M R. Curr Opin Cell Biol. 1997;9:143–147. doi: 10.1016/s0955-0674(97)80055-5. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Zheng B, Han J, Lin S-C. J Biol Chem. 1997;272:8679–8685. doi: 10.1074/jbc.272.13.8679. [DOI] [PubMed] [Google Scholar]
- 9.Siderovski D P, Hessel A, Chung S, Mak T W, Tyers M. Curr Biol. 1996;6:211–212. doi: 10.1016/s0960-9822(02)00454-2. [DOI] [PubMed] [Google Scholar]
- 10.Koelle M R, Horvitz H R. Cell. 1996;84:115–125. doi: 10.1016/s0092-8674(00)80998-8. [DOI] [PubMed] [Google Scholar]
- 11.Druey K M, Blumer K J, Kang V H, Kehrl J H. Nature (London) 1996;379:742–746. doi: 10.1038/379742a0. [DOI] [PubMed] [Google Scholar]
- 12.Faurobert E, Hurley J B. Proc Natl Acad Sci USA. 1997;94:2945–2950. doi: 10.1073/pnas.94.7.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Popov S, Yu K, Kozasa T, Wilkie T M. Proc Natl Acad Sci USA. 1997;94:7216–7220. doi: 10.1073/pnas.94.14.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srinivasa S P, Watson N, Overton M C, Blumer K J. J Biol Chem. 1998;273:1529–1533. doi: 10.1074/jbc.273.3.1529. [DOI] [PubMed] [Google Scholar]
- 15.Tesmer J J G, Berman D M, Gilman A G, Sprang S R. Cell. 1997;89:251–261. doi: 10.1016/s0092-8674(00)80204-4. [DOI] [PubMed] [Google Scholar]
- 16.Dohlman H G, Song J, Ma D, Courchesne W E, Thorner J. Mol Cell Biol. 1996;16:5914–5209. doi: 10.1128/mcb.16.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeVries L, Mousli M, Wurmser A, Farquhar M G. Proc Natl Acad Sci USA. 1995;92:11916–11920. doi: 10.1073/pnas.92.25.11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeVries L, Elenko E, Hubler L, Jones T L Z, Farquhar M G. Proc Natl Acad Sci USA. 1996;93:15203–15208. doi: 10.1073/pnas.93.26.15203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bucci M, Wente S R. J Cell Biol. 1997;136:1185–1199. doi: 10.1083/jcb.136.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iniguez-Lluhi J, Simon M I, Robishaw J D, Gilman A G. J Biol Chem. 1992;267:23409–23417. [PubMed] [Google Scholar]
- 21.Linder M E, Middleton P, Hepler J R, Taussig R, Gilman A G, Mumby S M. Proc Natl Acad Sci USA. 1993;90:3675–3679. doi: 10.1073/pnas.90.8.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linder M E, Kleuss C, Mumby S M. Methods Enzymol. 1995;250:314–330. doi: 10.1016/0076-6879(95)50081-2. [DOI] [PubMed] [Google Scholar]
- 23.Evan G I, Lewis G K, Ramsay G, Bishop J M. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harlow E, Lane D. Antibodies: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. pp. 521–523. [Google Scholar]
- 25.Bizzozero O, McGarry J, Lees M. J Biol Chem. 1987;262:13550–13357. [PubMed] [Google Scholar]
- 26.Hancock J F, Magee J I, Childs J E, Marshall C J. Cell. 1989;57:1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- 27.Deschenes R, Broach J. Mol Cell Biol. 1987;7:2344–2351. doi: 10.1128/mcb.7.7.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finegold A A, Schafer W R, Rine J, Whiteway M, Tamanoi F. Science. 1990;249:165–169. doi: 10.1126/science.1695391. [DOI] [PubMed] [Google Scholar]
- 29.Casey P. Science. 1995;268:221–225. doi: 10.1126/science.7716512. [DOI] [PubMed] [Google Scholar]
- 30.Bhatnagar R S, Gordon J I. Trends Cell Biol. 1997;7:14–20. doi: 10.1016/S0962-8924(97)10044-7. [DOI] [PubMed] [Google Scholar]
- 31.Dunne S J, Cornell R B, Johnson J E, Glover N R, Tracey A S. Biochemistry. 1996;35:11975–11984. doi: 10.1021/bi960821+. [DOI] [PubMed] [Google Scholar]
- 32.Picot D, Garavito R M. FEBS Lett. 1994;346:21–25. doi: 10.1016/0014-5793(94)00314-9. [DOI] [PubMed] [Google Scholar]
- 33.Shi Y, Veit B, Baekkeskov S. J Cell Biol. 1994;124:927–934. doi: 10.1083/jcb.124.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlesinger M J, Veit M, Schmidt M F G. In: Lipid Modifications of Proteins. Schlesinger M J, editor. Boca Raton, FL: CRC; 1993. pp. 2–19. [Google Scholar]
- 35.Dunphy J D, Greentree W K, Manahan C L, Linder M E. J Biol Chem. 1996;271:7154–7159. doi: 10.1074/jbc.271.12.7154. [DOI] [PubMed] [Google Scholar]
- 36.Berthiaume L, Resh M. J Biol Chem. 1995;270:22399–22405. doi: 10.1074/jbc.270.38.22399. [DOI] [PubMed] [Google Scholar]
- 37.Zinsmaier K E, Eberle K K, Buchner E, Walter N, Benzer S. Science. 1994;263:977–980. doi: 10.1126/science.8310297. [DOI] [PubMed] [Google Scholar]
- 38.Gundersen C B, Mastrogiacomo A, Faull K, Umbach J A. J Biol Chem. 1994;269:19197–19199. [PubMed] [Google Scholar]
- 39.Schweizer A, Kornfeld S, Rohrer J. J Cell Biol. 1996;132:577–584. doi: 10.1083/jcb.132.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moffett S, Adam L, Bonin H, Loisel T, Bouvier M, Mouillac B. J Biol Chem. 1996;271:21490–21497. doi: 10.1074/jbc.271.35.21490. [DOI] [PubMed] [Google Scholar]
- 41.Sudo Y, Valenzuela D, Becksickinger A G, Fishman M C, Strittmatter S M. EMBO J. 1992;11:2095–2102. doi: 10.1002/j.1460-2075.1992.tb05268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iiri T, Backlund P S, Jr, Jones T L Z, Wedegaertner P B, Bourne H R. Proc Natl Acad Sci USA. 1996;93:14592–14597. doi: 10.1073/pnas.93.25.14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tu Y, Wang J, Ross E M. Science. 1997;278:1132–1135. doi: 10.1126/science.278.5340.1132. [DOI] [PubMed] [Google Scholar]
- 44.Chen C K, Wieland T, Simon M I. Proc Natl Acad Sci USA. 1996;93:12885–12889. doi: 10.1073/pnas.93.23.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]