Abstract
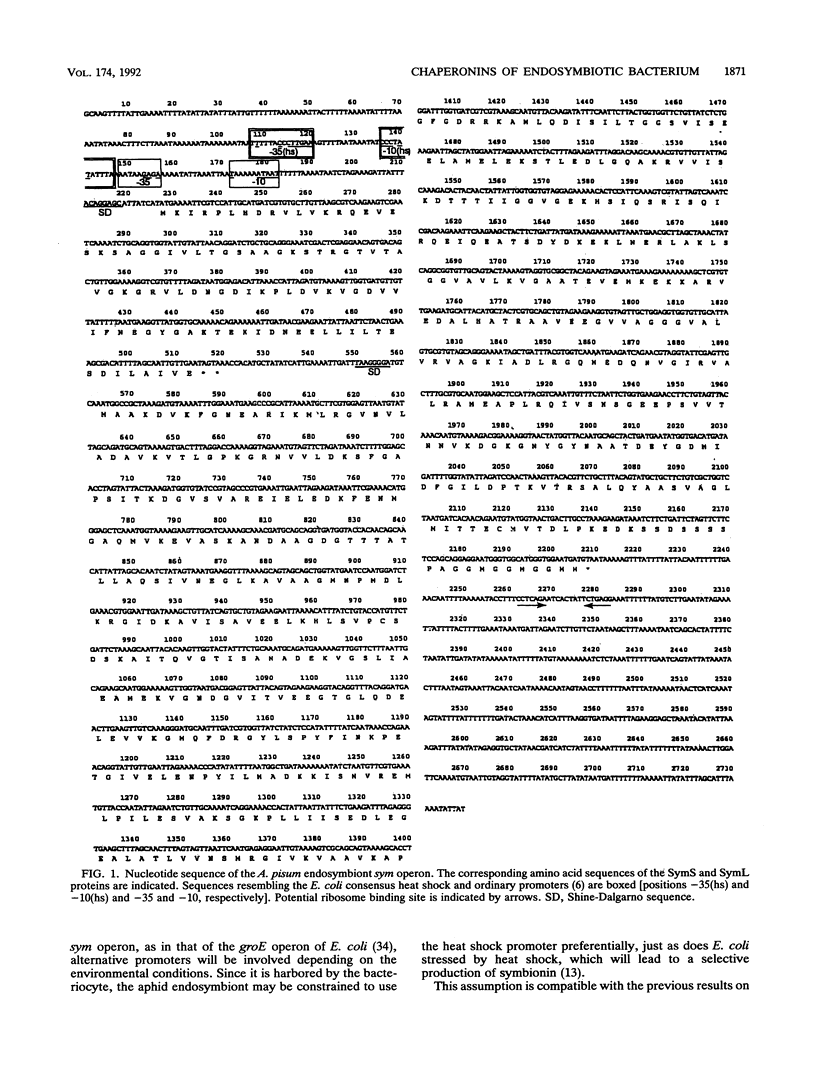
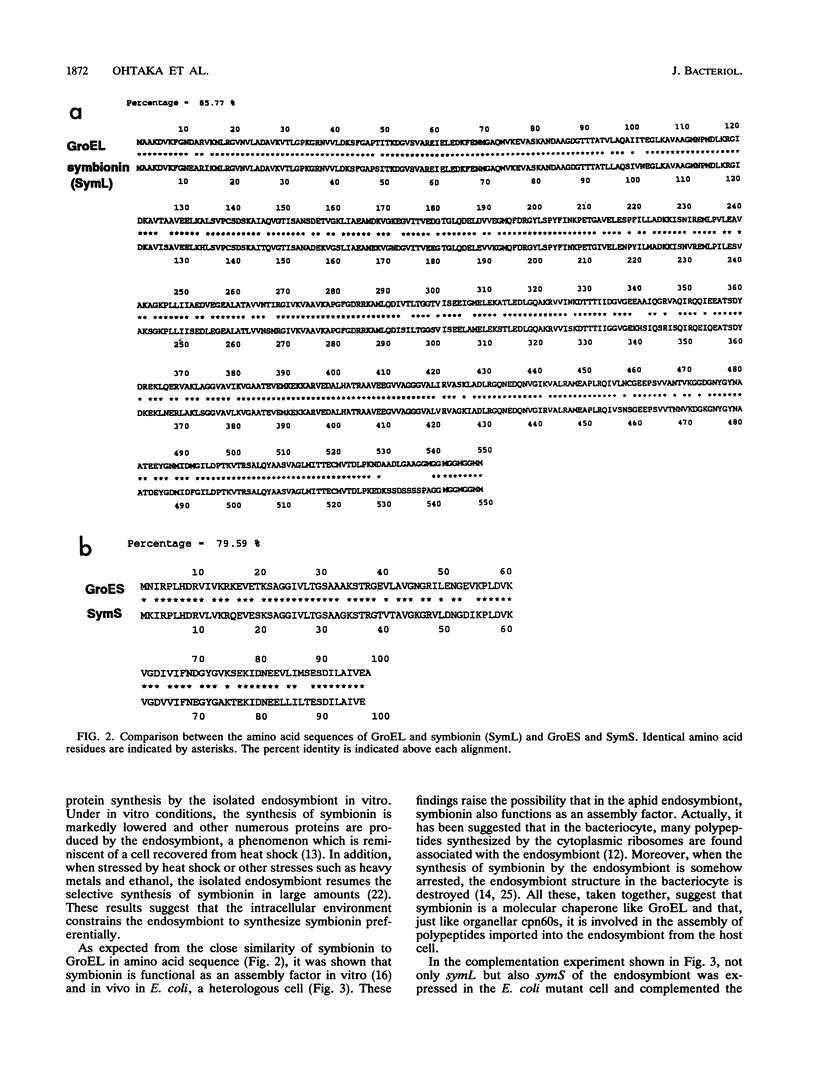
An intracellular symbiont harbored by the aphid bacteriocyte, a specialized fat body cell, synthesizes in vivo substantially only one protein, symbionin, which is a member of the chaperonin-60 family of molecular chaperones. Nucleotide sequence determination of the symbionin region of the endosymbiont genome revealed that it contains the two-cistron operon sym. Just like the Escherichia coli groE operon, the sym operon was dually led by a heat shock and an ordinary promoter sequence. According to the nucleotide sequence, symbionin was 85.5% identical to GroEL of E. coli at the amino acid sequence level. SymS, another protein encoded in the sym operon, which is a member of chaperonin-10, was 79.6% identical to GroES. Complementation experiments with E. coli groE mutants showed that the chaperonin-10 and chaperonin-60 genes from the endosymbiont are expressed in E. coli and that they can function as molecular chaperones together with endogenous GroEL and GroES, respectively.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bochkareva E. S., Lissin N. M., Girshovich A. S. Transient association of newly synthesized unfolded proteins with the heat-shock GroEL protein. Nature. 1988 Nov 17;336(6196):254–257. doi: 10.1038/336254a0. [DOI] [PubMed] [Google Scholar]
- Goloubinoff P., Christeller J. T., Gatenby A. A., Lorimer G. H. Reconstitution of active dimeric ribulose bisphosphate carboxylase from an unfoleded state depends on two chaperonin proteins and Mg-ATP. Nature. 1989 Dec 21;342(6252):884–889. doi: 10.1038/342884a0. [DOI] [PubMed] [Google Scholar]
- Goloubinoff P., Gatenby A. A., Lorimer G. H. GroE heat-shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carboxylase oligomers in Escherichia coli. Nature. 1989 Jan 5;337(6202):44–47. doi: 10.1038/337044a0. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Picketts D. J., Ahmad S. A novel ubiquitous protein 'chaperonin' supports the endosymbiotic origin of mitochondrion and plant chloroplast. Biochem Biophys Res Commun. 1989 Sep 15;163(2):780–787. doi: 10.1016/0006-291x(89)92290-0. [DOI] [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Jindal S., Dudani A. K., Singh B., Harley C. B., Gupta R. S. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989 May;9(5):2279–2283. doi: 10.1128/mcb.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakeda K., Ishikawa H. Molecular chaperon produced by an intracellular symbiont. J Biochem. 1991 Oct;110(4):583–587. doi: 10.1093/oxfordjournals.jbchem.a123623. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Kusukawa N., Yura T., Ueguchi C., Akiyama Y., Ito K. Effects of mutations in heat-shock genes groES and groEL on protein export in Escherichia coli. EMBO J. 1989 Nov;8(11):3517–3521. doi: 10.1002/j.1460-2075.1989.tb08517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecker S., Lill R., Ziegelhoffer T., Georgopoulos C., Bassford P. J., Jr, Kumamoto C. A., Wickner W. Three pure chaperone proteins of Escherichia coli--SecB, trigger factor and GroEL--form soluble complexes with precursor proteins in vitro. EMBO J. 1989 Sep;8(9):2703–2709. doi: 10.1002/j.1460-2075.1989.tb08411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubben T. H., Gatenby A. A., Donaldson G. K., Lorimer G. H., Viitanen P. V. Identification of a groES-like chaperonin in mitochondria that facilitates protein folding. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7683–7687. doi: 10.1073/pnas.87.19.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J. E. Expression of the unc genes in Escherichia coli. J Bioenerg Biomembr. 1988 Feb;20(1):19–39. doi: 10.1007/BF00762136. [DOI] [PubMed] [Google Scholar]
- McMullin T. W., Hallberg R. L. A highly evolutionarily conserved mitochondrial protein is structurally related to the protein encoded by the Escherichia coli groEL gene. Mol Cell Biol. 1988 Jan;8(1):371–380. doi: 10.1128/mcb.8.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Vaughn V. The genetics and regulation of heat-shock proteins. Annu Rev Genet. 1984;18:295–329. doi: 10.1146/annurev.ge.18.120184.001455. [DOI] [PubMed] [Google Scholar]
- Picketts D. J., Mayanil C. S., Gupta R. S. Molecular cloning of a Chinese hamster mitochondrial protein related to the "chaperonin" family of bacterial and plant proteins. J Biol Chem. 1989 Jul 15;264(20):12001–12008. [PubMed] [Google Scholar]
- Reading D. S., Hallberg R. L., Myers A. M. Characterization of the yeast HSP60 gene coding for a mitochondrial assembly factor. Nature. 1989 Feb 16;337(6208):655–659. doi: 10.1038/337655a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K., Georgopoulos C. Evidence that the two Escherichia coli groE morphogenetic gene products interact in vivo. J Bacteriol. 1982 Mar;149(3):1082–1088. doi: 10.1128/jb.149.3.1082-1088.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K., Murialdo H., Georgopoulos C. Identification of a second Escherichia coli groE gene whose product is necessary for bacteriophage morphogenesis. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1629–1633. doi: 10.1073/pnas.78.3.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterman B. M., Baumann P., McLean D. L. Pea aphid symbiont relationships established by analysis of 16S rRNAs. J Bacteriol. 1989 Jun;171(6):2970–2974. doi: 10.1128/jb.171.6.2970-2974.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb R., Reddy K. J., Sherman L. A. Regulation and sequence of the Synechococcus sp. strain PCC 7942 groESL operon, encoding a cyanobacterial chaperonin. J Bacteriol. 1990 Sep;172(9):5079–5088. doi: 10.1128/jb.172.9.5079-5088.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. N., Kusukawa N., Erickson J. W., Gross C. A., Yura T. Isolation and characterization of Escherichia coli mutants that lack the heat shock sigma factor sigma 32. J Bacteriol. 1988 Aug;170(8):3640–3649. doi: 10.1128/jb.170.8.3640-3649.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]