Abstract
Fas receptor is a member of the tumor necrosis factor-α family of death receptors that mediate physiologic apoptotic signaling. To investigate the molecular mechanisms regulating calcium mobilization during Fas-mediated apoptosis, we have analyzed the sequential steps leading to altered calcium homeostasis and cell death in response to activation of the Fas receptor. We show that Fas-mediated apoptosis requires endoplasmic reticulum–mediated calcium release in a mechanism dependent on phospholipase C-γ1 (PLC-γ1) activation and Ca2+ release from inositol 1,4,5-trisphosphate receptor (IP3R) channels. The kinetics of Ca2+ release were biphasic, demonstrating a rapid elevation caused by PLC-γ1 activation and a delayed and sustained increase caused by cytochrome c binding to IP3R. Blocking either phase of Ca2+ mobilization was cytoprotective, highlighting PLC-γ1 and IP3R as possible therapeutic targets for disorders associated with Fas signaling.
Introduction
Fas receptor apoptotic signaling is critical for normal development and tissue homeostasis (Nagata, 1999). At the molecular level, Fas receptor is activated by the binding of either membrane-bound or -soluble Fas ligand (FasL), which induces receptor oligomerization (Schneider et al., 1998; Algeciras-Schimnich et al., 2002). Fas receptor oligomerization stimulates formation of the death-induced signaling complex (DISC), which is minimally composed of the adaptor Fas-associated death domain protein and caspase 8/10 (Worth et al., 2006). Caspase 8/10 activation can lead to direct activation of the effector caspases, such as caspase 3. In some cells, the activation of effector caspases is amplified by engaging the mitochondrial pathway via the Bcl-2 family member Bid (Barnhart et al., 2003). Caspase-dependent Bid truncation leads to translocation of the protein to the mitochondria, where it promotes the release of proapoptotic factors such as cytochrome c. Proapoptotic proteins released from mitochondria stimulate formation of the apoptosome and efficient caspase 3 activation (Jiang and Wang, 2004).
In addition to activating the apoptosome, cytochrome c released from mitochondria can bind and modulate the activity of the inositol 1,4,5-trisphosphate receptor (IP3R), which is an ER-resident calcium channel (Boehning et al., 2003; Beresewicz et al., 2006). As such, Fas-mediated apoptosis requiring engagement of the mitochondrial pathway may be associated with IP3R-dependent signaling. However, although it has been suggested that elevated cytosolic calcium may contribute to the progression of Fas apoptosis (Oshimi and Miyazaki, 1995; Scoltock et al., 2000; Ayub et al., 2004; Boehning et al., 2005), the molecular mechanisms are unknown.
We show that efficient Fas signaling requires calcium release from the ER in two separate phases mediated by distinct but interdependent mechanisms. The first phase of calcium elevation is rapid and associated with activation of PLC-γ1, subsequent IP3 generation, and calcium release via IP3R channels. The second phase of calcium elevation occurs over the course of hours and is mediated by cytochrome c binding to IP3R. Blocking either phase of calcium elevation inhibits FasL-mediated apoptosis, highlighting possible therapeutic targets for disorders associated with this apoptotic pathway.
Results and discussion
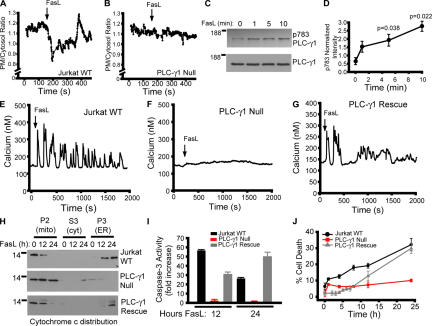
Jurkat T-lymphoma cells are sensitive to FasL-induced apoptosis in a manner dependent on engagement of the mitochondrial pathway (Scaffidi et al., 1998). To determine whether IP3R activation contributes to FasL-mediated cell death in Jurkat cells, we first examined whether IP3, which is the obligate ligand of IP3R, is produced after treatment with FasL. Jurkat cells were transfected with the phosphoinositide 4,5-bisphophate biosensor comprised of the pleckstrin homology domain of PLC-δ1 coupled to GFP (PLCδ1PH-GFP; Varnai and Balla, 1998). This protein localizes to the plasma membrane under basal conditions, but rapidly redistributes to the cytosol after treatment with agonists that stimulate IP3 production (Varnai and Balla, 1998; Holz et al., 2000). FasL stimulation of Jurkat cells resulted in rapid dissipation of PLCδ1PH-GFP fluorescence from the plasma membrane, indicating that FasL stimulates IP3 production (Fig. 1 A). The ratio of plasma membrane to cytosol fluorescence oscillates after an initial sharp decrease, which is consistent with other agonists coupled to PLC activation and oscillatory calcium release events (Hirose et al., 1999; Young et al., 2003). This redistribution is not seen in Jurkat cells lacking PLC-γ1 expression (PLC-γ1 null; Fig. 1 B; Irvin et al., 2000). A marker of PLC-γ1 activation is phosphorylation of tyrosine residue 783 (Kim et al., 1990; Kim et al., 1991). FasL stimulation resulted in a time-dependent increase in phosphorylation of tyrosine 783 (Fig. 1, C and D), indicating that PLC-γ1 is activated in response to FasL. There was no PLC-γ1 immunoreactivity in lysates prepared from PLC-γ1–null cells (not depicted).
Figure 1.
Requirement of PLC-δ1 for FasL-mediated apoptosis in Jurkat cells. (A) Plasma membrane (PM) to cytosol translocation of the phosphoinositide 4,5-bisphophate biosensor PLCδ-PH-GFP in wild-type Jurkat cells. (B) Jurkat cells lacking PLC-γ1 expression (Irvin et al., 2000). (C) PLC-γ1 activation indicated by phosphotyrosine 783 immunoblotting (p783 PLC-γ1) and total PLC-γ1 as a function of time. (D) Quantification of PLC-γ1 phosphorylation normalized to control. (E) Calcium response of Jurkat cells treated with 10 ng/ml FasL. Shown is a single-cell response representative of multiple determinations. Single-cell calcium responses of PLC-γ1–null (F) and PLC-γ1–rescued cells (G). (H) Cytochrome c release from mitochondria and translocation to the ER in response to FasL stimulation for 0, 12, or 24 h in WT, PLC-γ1–null, or PLC-γ1–rescued cells. Mito/P2, mitochondrial-enriched fraction; cyt/S3, cytosol fraction; ER/P3, ER-enriched fraction. (I) Caspase-3 activity in WT, PLC-γ1–null, and PLC-γ1–rescued cells. (J) Cell death (propidium iodide–positive cells as a percentage of the total) in WT, PLC-γ1–null, and PLC-γ1–rescued cells. Data is presented as the mean ± the SEM.
To examine whether PLC-γ1 activation is associated with calcium release, we monitored alterations in intracellular calcium in response to FasL stimulation in wild-type and PLC-γ1–null Jurkat cells. FasL stimulated immediate oscillations in cytosolic calcium (Fig. 1 E). Importantly, PLC-γ1–null cells did not release calcium in response to FasL treatment (Fig. 1 F). Stable expression of wild-type PLC-γ1 in PLC-γ1–null cells (Irvin et al., 2000) rescued calcium release in response to FasL application (Fig. 1 G).
FasL stimulation of Jurkat cells causes release from mitochondria in cytochrome c, which subsequently binds IP3R channels (Boehning et al., 2005). In subcellular fractionation experiments, this is observed as a translocation of cytochrome c immunoreactivity from a 10,000 g mitochondrial-enriched pellet to a 100,000 g ER-enriched and mitochondria-free pellet (Fig. 1 H; Jurkat WT; Boehning et al., 2003; Boehning et al., 2005). Cytochrome c release was not observed in PLC-γ1–null cells upon FasL treatment, suggesting that PLC-γ1 activation and calcium release are upstream of mitochondrial permeabilization (Fig. 1 H; PLC-γ1 null). Consistent with this observation, PLC-γ1–null cells were defective in caspase-3 activation (Fig. 1 I) and were resistant to cell death induced by FasL (Fig. 1 J). Stable expression of wild-type PLC-γ1 in PLC-γ1–null cells restored cytochrome c release, caspase 3 activation, and cell death induced by FasL (Fig. 1, H to J).
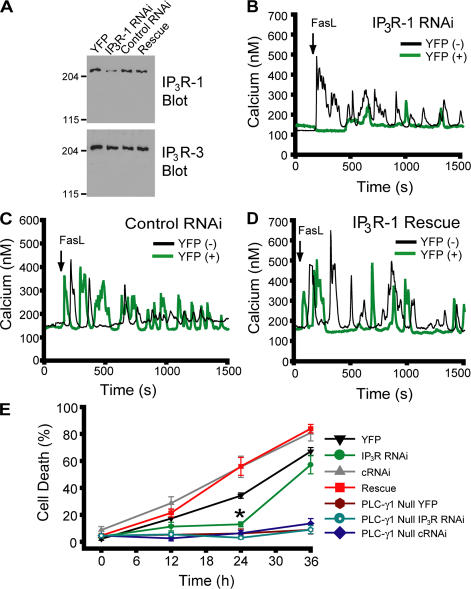
To investigate if IP3R channels are responsible for calcium mobilization downstream of PLC-dependent IP3 production, we transiently knocked down the expression of IP3R-1 by RNAi. This isoform is the predominant isoform expressed in Jurkat cells (Jayaraman and Marks, 1997). Because of the low (∼10%) transfection efficiency of Jurkat cells, biochemical determination of RNAi efficacy was first tested in HeLa cells (Fig. 2 A). RNAi transfection resulted in a substantial reduction in IP3R-1 levels, which could be rescued by transfection of the rat IP3R-1 gene, which has several nucleotide changes within the RNAi-targeted sequence (see Materials and methods). Expression of another closely related IP3R isoform (IP3R-3) abundantly expressed in HeLa cells was unaffected, demonstrating specificity. To test whether knockdown of IP3R-1 in Jurkat cells affected calcium release induced by FasL stimulation, we cotransfected RNAi and YFP and examined single-cell calcium responses simultaneously in RNAi-transfected and nontransfected cells. IP3R-1 knockdown cells were resistant to calcium release induced by FasL (Fig. 2 B). Control RNAi-transfected cells responded similarly to untransfected controls (Fig. 2 C). Overexpression of rat IP3R-1 rescued calcium release induced by FasL in IP3R-1 knockdown Jurkat cells (Fig. 2 D). IP3R-1 knockdown cells were also resistant to cell death induced by exposure to FasL, an effect rescued by overexpression of rat IP3R-1 (Fig. 2 E). Longer incubations of IP3R knockdown cells with FasL resulted in increased cell death, suggesting delayed activation of alternative pathways, such as mitochondrial-independent caspase activation (Scaffidi et al., 1998), residual IP3R activities, or RNAi turnover and loss of down-regulation. PLC-γ1–null cells, with or without treatment with IP3R RNAi, are refractory to FasL-induced cell death at all time points, suggesting that recovery of cell death in IP3R RNAi-treated cells is caused by loss of down-regulation (Fig. 2 E). These results indicate that activation of IP3R channels and calcium release from internal stores are downstream effectors of PLC-γ1 activation after FasL stimulation.
Figure 2.
Knockdown of IP3R-1 abrogates calcium release and apoptosis induced by Fas ligand. (A) Knockdown of IP3R-1 expression in HeLa cells by RNAi and rescue by overexpression of the rat IP3R-1 gene. IP3R-3 expression serves as a loading control. (B–D) Cytosolic calcium responses to 10 ng/ml FasL in RNAi, control RNAi, or RNAi-rescued Jurkat cells. Black traces, nontransfected (YFP-negative) cells; green traces, transfected (YFP-positive) cells from the same field. (E) Cell death in wild-type and PLC-γ1–null YFP-positive cells ± indicated RNAi. cRNAi, control RNAi. *, P = 0.0002 versus YFP only. Although not indicated on the graph, PLC-γ1–null treatment groups had significantly lower cell death at 24 and 36 h versus YFP only (P < 0.0001). Data is presented as the mean ± the SEM.
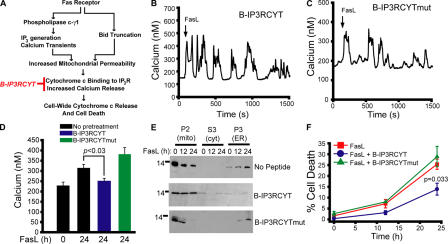
We hypothesized that cytochrome c binding to IP3R contributes to changes in calcium levels during FasL-mediated apoptosis, as we have previously shown for staurosporine-induced apoptosis (Boehning et al., 2003). It can be predicted that cytochrome c binding to IP3R would occur downstream of PLC-γ1 activation and mitochondrial permeabilization with kinetics similar to those observed biochemically for cytochrome c release (12–24 h; Fig. 1 H). Blocking cytochrome c binding to IP3R with a dominant-negative peptide (B-IP3RCYT) inhibits staurosporine-induced caspase activation and cell death (Boehning et al., 2005). We predicted B-IP3RCYT would not affect rapid PLC-γ1–dependent calcium release events, but would block potential sustained elevations in cytosolic calcium (Fig. 3 A). Consistent with these hypotheses, B-IP3RCYT or a peptide with two point-mutations eliminating cytochrome c binding (B-IP3RCYTmut) had no effect on initial calcium release events induced by FasL (Fig. 3, B and C). Treatment of Jurkat cells for 24 h with FasL resulted in increased basal cytosolic calcium (Fig. 3 D), which is consistent with previous studies (Scoltock et al., 2000). Pretreatment with B-IP3RCYT, but not B-IP3RCYTmut, blocked this late elevation in cytosolic calcium (Fig. 3 D). FasL treatment of Jurkat cells is also associated with rapid alterations in mitochondrial calcium immediately after FasL treatment, and sustained increases in mitochondrial calcium 24 h after FasL treatment (Fig. S1 and Video 1, available at http://www.jcb.org/cgi/content/full/jcb.200608035/DC1). B-IP3RCYT selectively inhibited the sustained increases in mitochondrial calcium at 24 h. Finally, preincubation with B-IP3RCYT, but not B-IP3RCYTmut, attenuated cytochrome c release and cell death in response to FasL stimulation (Fig. 3, E and F). Although not detectable in Fig. 3 E, we predict that small amounts of cytochrome c are released in the presence of B-IP3RCYT, even though cell-wide cytochrome c release is blocked. These results suggest that PLC-γ1 activation is critical to the initial oscillatory phase of calcium elevation, and cytochrome c binding to IP3R is necessary for a second, temporally delayed increase in cytosolic and mitochondrial calcium. It appears that both phases are required for optimal Fas signaling, as specific blockage of either pathway is cytoprotective. It has been shown that caspase-3 cleavage of the IP3R is associated with apoptotic calcium release (Hirota et al., 1999; Assefa et al., 2004). Treatment of Jurkat cells with the caspase-3 inhibitor z-DEVD-fmk had no effect on calcium mobilization in response to FasL, suggesting this pathway is not a required component of FasL-induced calcium release (Fig. S2).
Figure 3.
Cytochrome c binding to IP3R contributes to a late phase of calcium elevation in response to Fas ligand. (A) Schematic diagram depicting the predicted steps leading to calcium mobilization during FasL stimulation. B-IP3RCYT (in red) blocks cytochrome c binding to IP3R (Boehning et al., 2005). (B and C) Calcium responses in cells pretreated with 400 nM B-IP3RCYT or B-IP3RCYTmut, which does not bind cytochrome c. (D) Cytoplasmic calcium concentration in cells treated for 24 h with FasL in the presence or absence of 400 nM of the indicated peptide. (E) Subcellular fractionation of cells stimulated with FasL for 12 or 24 h and pretreated with vehicle or 400 nM peptide. (F) Cell death curve of control or peptide-pretreated cells. Data is presented as the mean ± the SEM.
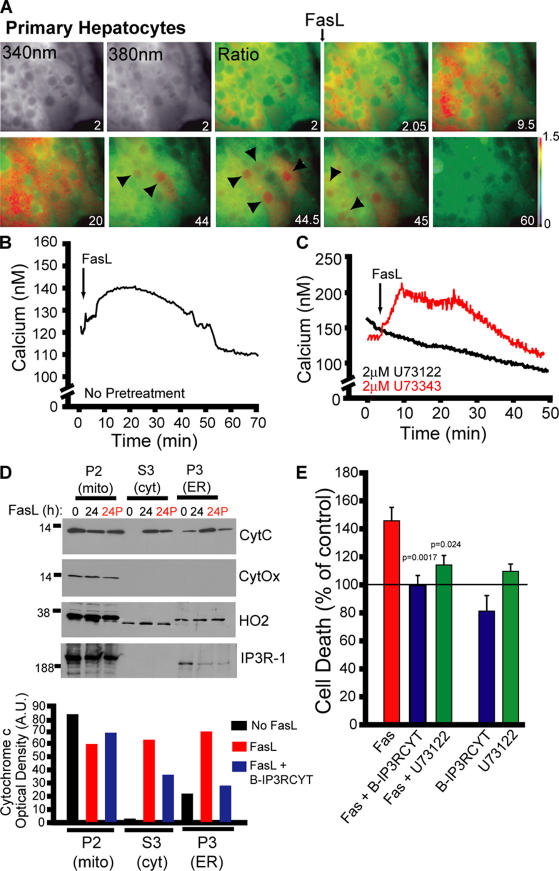
To determine if calcium is required for other models of FasL-mediated apoptosis, we tested whether primary mouse hepatocytes released calcium in response to FasL stimulation. FasL treatment of hepatocytes was associated with calcium release that could be inhibited with the PLC inhibitor U73122, but not the inactive analogue U73343 (Fig. 4, A–C). The kinetics of release were distinct from those observed in Jurkat cells, with a nonoscillatory rise which diminished after ∼50 min. Interestingly, we observed a spike in cytosolic and in particular nuclear calcium in individual cells after the initial rise in cytosolic calcium (Fig. 4 A, arrowheads), which would likely influence FasL-dependent nuclear events such as gene transcription. FasL treatment of hepatocytes for 24 h was associated with cytochrome c release and translocation to both the ER and cytosol, and these effects were reversed by B-IP3RCYT treatment (Fig. 4 D; compare 24 and 24P, where P indicates peptide treatment). The presence of cytochrome c in the cytosol in hepatocytes may reflect saturation of IP3R channels with cytochrome c at 24 h caused by the low density of IP3Rs in this cell type (Wojcikiewicz, 1995). We also observed a partial loss of IP3R-1 immunoreactivity after FasL treatment (Fig. 4 D), which may be caused by caspase-3 cleavage. Cell death induced by FasL was inhibited by U73122 or B-IP3RCYT, suggesting that the PLC activation and cytochrome c binding to IP3R are both required for FasL-mediated apoptosis in hepatocytes (Fig. 4 E).
Figure 4.
FasL-mediated apoptosis in primary hepatocytes requires PLC activity and cytochrome c binding to IP3R. (A) Fura-2 images (340 nm, 380 nm, and pseudocolored ratio) of a cluster of hepatocytes. Increases in nuclear calcium are indicated by black arrowheads. Time in minutes is indicated on each image. (B) Quantified FasL-stimulated calcium release in primary hepatocytes. Data presented is the mean of 40 cells from a single experiment, representative of three separate determinations. (C) Calcium responses in hepatocytes pretreated (30 min) with the PLC inhibitor U73122 (2 μM) or inactive control U73343 (in red). (D) Cytochrome c release and translocation to ER in primary hepatocytes treated for 24 h with FasL in the presence or absence of B-IP3RCYT (400 nM, indicated in red as 24P). Densitometry of cytochrome c immunoreactivity is given below the blots. CytOx, mitochondrial marker cytochrome c oxidase; HO2, microsomal marker heme oxygenase-2. (E) Cell death of primary hepatocytes treated with FasL in the presence or absence of 2 μM U73122 or 400 nM B-IP3RCYT. Indicated P values are versus FasL only. Data is normalized to basal cell death (indicated by a line at 100%). Data is presented as the mean ± the SEM.
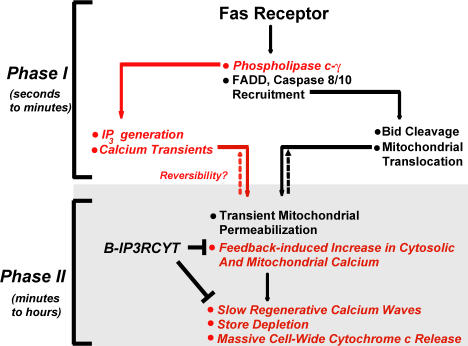
The molecular basis for calcium mobilization during apoptosis has remained enigmatic. We suggest a linear sequence of events, which lead to calcium-dependent mitochondrial permeabilization, caspase activation, and cell death during FasL-mediated apoptosis (Fig. 5). FasL binding to the Fas receptor recruits the canonical components of the DISC complex, and, concurrently, PLC-γ1 is activated by an unknown mechanism. These two events occur on the order of seconds to minutes, leading to calcium release from internal stores, elevated mitochondrial calcium, and Bid-mediated increases in mitochondrial permeability (Csordas et al., 2002). FasL-mediated apoptosis might be reversible at this stage, providing a critical control point. The second step occurs over the course of minutes to hours, and is characterized by limited cytochrome c release from mitochondria and binding to IP3R. This would sensitize the channel to increased calcium release (Boehning et al., 2003), ultimately resulting in depletion of ER calcium, mitochondrial calcium overload, and global cytochrome c release from all mitochondria. Consistent with the critical role of calcium in regulating FasL-mediated apoptosis, the calcium chelator BAPTA-AM suppresses cytochrome c release, caspase activation, and cell death in response to FasL (Fig. S3, A–C, available at http://www.jcb.org/cgi/content/full/jcb.200608035/DC1). Furthermore, if ER-derived calcium was critical for cytochrome c release from mitochondria and cell death in Jurkat cells, it would be expected that passive depletion of ER stores by thapsigargin would also cause cytochrome c release and cell death. We find that thapsigargin dose-dependently results in cytochrome c release, caspase activation, and cell death in Jurkat cells (Fig. S3, D–F). Finally, reducing the functional expression of PLC-γ1 and IP3R or blocking cytochrome c to IP3R is cytoprotective, suggesting that these proteins may be useful drug targets for treating disorders in Fas or other calcium-dependent apoptotic pathways.
Figure 5.
Model integrating calcium mobilization into the Fas signaling pathway. Fas receptor stimulation causes rapid formation of the DISC complex, minimally comprising Fas-associated death domain protein (FADD) and initiator caspases 8/10. Our results suggest a rapid activation of PLC-γ1, leading to IP3 generation and calcium release from IP3R channels (indicated in red). Calcium release from IP3R stores combined with increased mitochondrial permeability further amplify cytochrome c release. A temporally distinct chronic elevation of cytoplasmic calcium also occurs over the course of hours, mediated by cytochrome c binding to IP3R.
Materials and methods
Cell lines
Jurkat T cell leukemia and Jurkat derivatives j.gamma1 and j.gammaWT were obtained from the American Type Culture Collection. Primary mouse hepatocytes were prepared as described elsewhere (Park et al., 2005) and plated on collagen-coated plates or coverslips. Primary hepatocytes were used within 24 h to limit dedifferentiation.
Caspase activity
Caspase activity was determined as previously described (Boehning et al., 2003) using z-DEVD-R110 as the protease substrate at a final concentration of 50 μM (American Peptide Company).
PLC activity
Cells were transiently transfected with PLCδ-PH-GFP (Varnai and Balla, 1998), plated on poly-l-lysine–coated coverslips, and imaged at 25°C. PLCδ-PH-GFP was a gift from T. Balla (National Institutes of Health, Bethesda, MD). Images were acquired every 5 s using MetaFluor imaging software (Molecular Devices). Fas ligand-bearing vesicles (Millipore) were administered at 1 ng/ml. Quantification of PLC activity was calculated as a ratio of plasma membrane to cytosol fluorescence over time, as described elsewhere (Balla and Varnai, 2002). For immunoblot analysis, Jurkat cells were treated with 1 ng/ml FasL for the indicated times, and lysates were probed using phosphotyrosine 783-PLC-γ1 and PLC-γ1 antibodies (Cell Signaling Technologies).
Calcium imaging
Calcium measurements were performed as previously described (Boehning et al., 2005). Fura-2–loaded cells were allowed to attach to poly-l-lysine–coated coverslips and imaged at 25°C. Cells were treated with 10 ng/ml FasL vesicles, and images were acquired every 3 s for 2,000 s. Jurkat cells respond heterogeneously to FasL (Boehning et al., 2005), thus, we displayed representative single-cell traces. Cells with spontaneous release activity in the absence of FasL were identified by imaging at least 100 s before FasL addition and were eliminated from analysis. In experiments where DNA or RNAi were transfected into Jurkat cells, expressing cells were identified by cotransfecting YFP. Nonexpressing cells were imaged simultaneously with expressing cells as internal controls. Each experiment was repeated a minimum of five times, comprising hundreds of single-cell traces. In Figs. 3 D and S2 B, cells treated for 24 h with FasL were loaded with Fura-2 and cytoplasmic calcium imaged in three separate fields, each comprising 50–100 cells. The mean of the three fields comprised one data point. The experiment was repeated an additional two times and presented as the SEM of three experiments. Our previous study indicated that B-IP3RCYT loading into HeLa cells may augment staurosporine-induced calcium elevations (Boehning et al., 2005). Testing B-IP3RCYT in multiple apoptosis model systems, including Jurkat cells in this study, indicated that this effect is unique to that particular model system.
Image acquisition and manipulation
Fura-2 and GFP images were acquired on an inverted microscope (TE2000; Nikon) using a 60× oil immersion objective (SuperFluor; Nikon) with a 1.3 NA. All imaging was performed at 25°C in 107 mM NaCl, 7.2 mM KCl, 1.2 mM MgCl2, 1 mM CaCl2, 11.5 mM glucose, 0.1% bovine serum albumin, and 20 mM Hepes 7.2. Images were captured with a camera (CoolSNAP HQ; Roper Scientific). Rapid filter changes for ratiometric imaging were computer controlled via a 10–2 filter wheel controller (Lambda; Sutter) and MetaFluor data acquisition and analysis software. Raw data was acquired with MetaFluor and graphed in Sigma Plot (SPSS Scientific). Fluorescent images were pseudocolored using the IMD display mode in MetaFluor for display purposes in Fig. 4, and assembled without further manipulation in Photoshop (Adobe).
Subcellular fractionation
Subcellular fractionation was performed as described previously (Boehning et al., 2003). Fractionation purity in each experiment was determined by blotting with cytochrome c oxidase (mitochondria), heme oxygenase (ER), and lactate dehydrogenase (cytosol).
Cell death
Cell death was quantified as previously described (Boehning et al., 2005), either by propidium iodide staining, or, in the case of BODIPY-labeled peptide-treated samples, by trypan blue staining. The number of dead cells was determined either by manual counting in a light microscope or by flow cytometry. Cell death in transfected cells was determined by cotransfecting YFP. YFP was retained in dying cells, including propidium iodide–positive cells, allowing the determination of the effects of transfection on cell death. As such, there was no significant difference in the relative amount of cell death between nontransfected and YFP only–transfected cells (compare Fig. 2 E, YFP, with Fig. 1 J, Jurkat WT, or Fig. 3 F, FasL).
RNAi
Stealth-modified (Invitrogen) double-stranded RNA against the human IP3R-1 gene (sense sequence, 5′-GAGGGAUCGACAAAUGGAUUUAUUA-3′) targeting ORF nucleotides 314–338 was purchased from Invitrogen. Control RNA was similar, but with several deletions and insertions (sequence, 5′-GAGUAGCCAAAUAGGUAUUAGGUUA-3′). Transfection with Lipofectamine 2000 (Invitrogen) of various doses of RNA was used to determine that 100 pmol of double-stranded RNA complex per well of a six-well dish gave maximum knockdown. Knockdown was readily evident at 24 and 48 h. The blot in Fig. 2 A is 24 h after transfection. Rescue of RNAi knockdown was accomplished by overexpression of the rat IP3R-1 gene, which has three substitutions within the targeted sequence (rat sequence, with sequence changes in bold, 5′-GAGGGAUCUACGAAUGGAUUUAUCA-3′).
Peptide synthesis
The IP3RCYT sequence is DNKTVTFEEHIKEEHN, comprising amino acids 2,567–2,582 of human IP3R-1. IP3RCYTmut replaces two glutamic acid residues critical for binding (Boehning et al., 2005) with glutamine, DNKTVTFQQHIKEEHN. A C-terminal cysteine was added during synthesis to facilitate coupling to BODIPY 577/618 via a maleimide linkage, as previously described (Boehning et al., 2005). IP3RCYT and IP3RCYTmut were synthesized by the Protein Chemistry Laboratory core facility at the University of Texas Medical Branch.
Statistical analysis
All data is presented as the mean ± the SEM. Statistical significance was examined with a t test. P < 0.05 was determined to be significant. Actual P values are listed in each figure.
Online supplemental material
Fig. S1 shows mitochondrial calcium levels early and late after FasL stimulation, and the effect of B-IP3RCYT. Fig. S2 shows the effect of z-DEVD-fmk on FasL-induced calcium signals. Fig. S3 shows the effects of BAPTA and thapsigargin on cytochrome c release and cell death. Video 1 depicts mitochondrial calcium dynamics in response to FasL. There is also a referenced Supplemental materials and methods. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200608035/DC1.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the critical insights of Drs. Randen L. Patterson and Damian van Rossum (Penn State University), Dr. Solomon H. Snyder (Johns Hopkins University), and Drs. José M. Barral, Zhihua Zou, and Henry F. Epstein (University of Texas Medical Branch). D. Boehning thanks T.N.C. for helpful discussions.
This work was supported by National Institutes of Health grant R01ES012018 (C.J. Elferink) and funds provided by the University of Texas Medical Branch (D. Boehning).
Abbreviations used in this paper: DISC, death-induced signaling complex; IP3, inositol 1,4,5-trisphosphate; IP3R, IP3 receptor.
References
- Algeciras-Schimnich, A., L. Shen, B.C. Barnhart, A.E. Murmann, J.K. Burkhardt, and M.E. Peter. 2002. Molecular ordering of the initial signaling events of CD95. Mol. Cell. Biol. 22:207–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assefa, Z., G. Bultynck, K. Szlufcik, N. Nadif Kasri, E. Vermassen, J. Goris, L. Missiaen, G. Callewaert, J.B. Parys, and H. De Smedt. 2004. Caspase-3-induced truncation of type 1 inositol trisphosphate receptor accelerates apoptotic cell death and induces inositol trisphosphate-independent calcium release during apoptosis. J. Biol. Chem. 279:43227–43236. [DOI] [PubMed] [Google Scholar]
- Ayub, K., I. Laffafian, S. Dewitt, and M.B. Hallett. 2004. Ca influx shutdown in neutrophils induced by Fas (CD95) cross-linking. Immunology. 112:454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla, T., and P. Varnai. 2002. Visualizing cellular phosphoinositide pools with GFP-fused protein-modules. Sci. STKE. 2002:PL3. [DOI] [PubMed]
- Barnhart, B.C., E.C. Alappat, and M.E. Peter. 2003. The CD95 type I/type II model. Semin. Immunol. 15:185–193. [DOI] [PubMed] [Google Scholar]
- Beresewicz, M., J.E. Kowalczyk, and B. Zablocka. 2006. Cytochrome c binds to inositol (1,4,5) trisphosphate and ryanodine receptors in vivo after transient brain ischemia in gerbils. Neurochem. Int. 48:568–571. [DOI] [PubMed] [Google Scholar]
- Boehning, D., R.L. Patterson, L. Sedaghat, N.O. Glebova, T. Kurosaki, and S.H. Snyder. 2003. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat. Cell Biol. 5:1051–1061. [DOI] [PubMed] [Google Scholar]
- Boehning, D., D.B. van Rossum, R.L. Patterson, and S.H. Snyder. 2005. A peptide inhibitor of cytochrome c/inositol 1,4,5-trisphosphate receptor binding blocks intrinsic and extrinsic cell death pathways. Proc. Natl. Acad. Sci. USA. 102:1466–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas, G., M. Madesh, B. Antonsson, and G. Hajnoczky. 2002. tcBid promotes Ca(2+) signal propagation to the mitochondria: control of Ca(2+) permeation through the outer mitochondrial membrane. EMBO J. 21:2198–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose, K., S. Kadowaki, M. Tanabe, H. Takeshima, and M. Iino. 1999. Spatiotemporal dynamics of inositol 1,4,5-trisphosphate that underlies complex Ca2+ mobilization patterns. Science. 284:1527–1530. [DOI] [PubMed] [Google Scholar]
- Hirota, J., T. Furuichi, and K. Mikoshiba. 1999. Inositol 1,4,5-trisphosphate receptor type 1 is a substrate for caspase-3 and is cleaved during apoptosis in a caspase-3-dependent manner. J. Biol. Chem. 274:34433–34437. [DOI] [PubMed] [Google Scholar]
- Holz, R.W., M.D. Hlubek, S.D. Sorensen, S.K. Fisher, T. Balla, S. Ozaki, G.D. Prestwich, E.L. Stuenkel, and M.A. Bittner. 2000. A pleckstrin homology domain specific for phosphatidylinositol 4, 5-bisphosphate (PtdIns-4,5-P2) and fused to green fluorescent protein identifies plasma membrane PtdIns-4,5-P2 as being important in exocytosis. J. Biol. Chem. 275:17878–17885. [DOI] [PubMed] [Google Scholar]
- Irvin, B.J., B.L. Williams, A.E. Nilson, H.O. Maynor, and R.T. Abraham. 2000. Pleiotropic contributions of phospholipase C-gamma1 (PLC-gamma1) to T-cell antigen receptor-mediated signaling: reconstitution studies of a PLC-gamma1-deficient Jurkat T-cell line. Mol. Cell. Biol. 20:9149–9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman, T., and A.R. Marks. 1997. T cells deficient in inositol 1,4,5-trisphosphate receptor are resistant to apoptosis. Mol. Cell. Biol. 17:3005–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, X., and X. Wang. 2004. Cytochrome c-mediated apoptosis. Annu. Rev. Biochem. 73:87–106. [DOI] [PubMed] [Google Scholar]
- Kim, H.K., J.W. Kim, A. Zilberstein, B. Margolis, J.G. Kim, J. Schlessinger, and S.G. Rhee. 1991. PDGF stimulation of inositol phospholipid hydrolysis requires PLC-gamma 1 phosphorylation on tyrosine residues 783 and 1254. Cell. 65:435–441. [DOI] [PubMed] [Google Scholar]
- Kim, J.W., S.S. Sim, U.H. Kim, S. Nishibe, M.I. Wahl, G. Carpenter, and S.G. Rhee. 1990. Tyrosine residues in bovine phospholipase C-gamma phosphorylated by the epidermal growth factor receptor in vitro. J. Biol. Chem. 265:3940–3943. [PubMed] [Google Scholar]
- Nagata, S. 1999. Fas ligand-induced apoptosis. Annu. Rev. Genet. 33:29–55. [DOI] [PubMed] [Google Scholar]
- Oshimi, Y., and S. Miyazaki. 1995. Fas antigen-mediated DNA fragmentation and apoptotic morphologic changes are regulated by elevated cytosolic Ca2+ level. J. Immunol. 154:599–609. [PubMed] [Google Scholar]
- Park, K.T., K.A. Mitchell, G. Huang, and C.J. Elferink. 2005. The aryl hydrocarbon receptor predisposes hepatocytes to Fas-mediated apoptosis. Mol. Pharmacol. 67:612–622. [DOI] [PubMed] [Google Scholar]
- Scaffidi, C., S. Fulda, A. Srinivasan, C. Friesen, F. Li, K.J. Tomaselli, K.M. Debatin, P.H. Krammer, and M.E. Peter. 1998. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17:1675–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, P., N. Holler, J.L. Bodmer, M. Hahne, K. Frei, A. Fontana, and J. Tschopp. 1998. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J. Exp. Med. 187:1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoltock, A.B., C.D. Bortner, J.B.G. St, J.W. Putney Jr., and J.A. Cidlowski. 2000. A selective requirement for elevated calcium in DNA degradation, but not early events in anti-Fas-induced apoptosis. J. Biol. Chem. 275:30586–30596. [DOI] [PubMed] [Google Scholar]
- Varnai, P., and T. Balla. 1998. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol–labeled phosphoinositide pools. J. Cell Biol. 143:501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcikiewicz, R.J. 1995. Type I, II, and III inositol 1,4,5-trisphosphate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. J. Biol. Chem. 270:11678–11683. [DOI] [PubMed] [Google Scholar]
- Worth, A., A.J. Thrasher, and H.B. Gaspar. 2006. Autoimmune lymphoproliferative syndrome: molecular basis of disease and clinical phenotype. Br. J. Haematol. 133:124–140. [DOI] [PubMed] [Google Scholar]
- Young, K.W., M.S. Nash, R.A. Challiss, and S.R. Nahorski. 2003. Role of Ca2+ feedback on single cell inositol 1,4,5-trisphosphate oscillations mediated by G-protein-coupled receptors. J. Biol. Chem. 278:20753–20760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.