Abstract
T cell tolerance depends on the T cell receptor's affinity for peptide/major histocompatibility complex (MHC) ligand; this critical parameter determines whether a thymocyte will be included (positive selection) or excluded (negative selection) from the T cell repertoire. A quantitative analysis of ligand binding was performed using an experimental system permitting receptor–coreceptor interactions on live cells under physiological conditions. Using three transgenic mouse strains expressing distinct class I MHC–restricted T cell receptors, we determined the affinity that defines the threshold for negative selection. The affinity threshold for self-tolerance appears to be a constant for cytotoxic T lymphocytes.
Selection of mature T cells from a pool of immature CD4+CD8+ double-positive (DP) thymocytes is dependent on how their TCRs interact with self-peptide/MHC (pMHC) ligands (1–5). Thymocytes expressing TCRs that fail to recognize any self-pMHC ligand die from “neglect,” whereas weak recognition of self-pMHC complexes by the TCR and coreceptor results in the development of mature, single-positive (SP) T cells (positive selection). Strong recognition of self-pMHC leads to thymocyte death or lineage deviation, removing self-reactive cells from the T cell repertoire (negative selection). Therefore, the peripheral T cell repertoire is both self-pMHC restricted and self-tolerant.
These distinct cell fates are critically dependent on the affinity of TCR–ligand interactions (6–9). Surface plasmon resonance (BiaCore) allows the quantification of bimolecular TCR/pMHC affinities, but does not account for the contribution of the coreceptor (CD4 or CD8α/β) in the context of a living cell. This is an essential point because collaboration of TCR and coreceptor in pMHC binding is crucial for ligand discrimination and thymocyte selection (10–14). To circumvent these limitations, we made use of a TCR photoaffinity labeling system (15), where the antigenic pMHC complex, SYIPSAEK(ABA)I/H-2Kd, carries a photoreactive azidobenzoic acid (ABA) linked to the lysine present in the peptide. After specific binding of pMHC monomers to the appropriate T cells, photoactivation of the ABA group results in cross-linking of monomeric pMHC complexes to the TCR, allowing for quantitative analysis of pMHC monomer binding (16–18). Our results indicate that thymocytes expressing MHC class I–restricted TCRs use the same affinity threshold to initiate negative selection.
RESULTS AND DISCUSSION
Transgenic mice expressing the T1 TCR, which is a receptor specific for the SYIPSAEK(ABA)I–H-2Kd complex, were generated and backcrossed for at least 15 generations onto β2m−/− Rag−/− or Rag−/−-only genetic backgrounds. Peptide variants were created by replacing proline at position 4 of the agonist peptide, SYIPSAEK(ABA)I, (referred to as 4P) with leucine (4L), valine (4V), alanine (4A), serine (4S), asparagine (4N), or histidine (4H). Variant peptides bound H-2Kd with a similar affinity (within twofold), except for 4L, which bound H-2Kd with fivefold lower affinity than 4P (unpublished data).
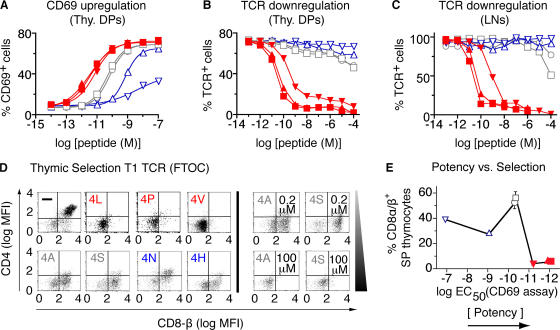
A potency hierarchy for these SYIPSAEK(ABA)I peptide variants was determined by their ability to induce CD69 expression (Fig. 1 A) or TCR down-regulation (Fig. 1, B and C). Peptide potencies were quantified by calculating EC50 values in the CD69 assay and normalizing these values for small differences in H-2Kd binding. These ligands could be classified into the following hierarchy: 4L > 4P > 4V > 4A, 4S > 4N > 4H. Selection properties of these peptide variants were determined using fetal thymic organ cultures (2) from T1 β2m−/− Rag−/− mice. Positive selection was monitored by the development of CD4− CD8αβ+ SP cells (Fig. 1 D). Negative selection was measured by the percentage of CD4−/CD8− (DN; Fig. 1 D) and CD4−/CD8αα+ thymocytes (not depicted); these cell populations are predominant in fetal thymus organ cultures (FTOCs) undergoing negative selection (2, 19). Using these criteria, the 4L, 4P, and 4V peptides induced negative selection, and the 4A, 4S, 4N, and 4H peptides induced positive selection (Fig. 1 D). To examine whether selection outcome was dependent on peptide concentration, FTOC experiments were repeated with varying doses of peptides (unpublished data). These results show that for strong (4L, 4P, and 4V) and weak (4N or 4H) ligands, selection outcomes are relatively independent of peptide concentration. However, peptides of moderate potency (4A and 4S) showed substantial variation in their selecting potential as a function of concentration (Fig. 1 D), suggesting that the 4A and 4S peptides were close to the boundary between positive and negative selection (11) for the T1 TCR. Plotting selection outcome as a function of peptide potency (Fig. 1 E and Table S1, available at http://www.jem.org/cgi/content/full/jem.20070254/DC1) showed that the threshold for unambiguous negative selection of T1 thymocytes was defined by a ligand potency slightly higher than the potencies of 4A and 4S peptide variants (EC50 < 5 × 10−11 M), which is similar to what was observed with the OT-1 TCR (11).
Figure 1.
T cell activation and thymic selection in T1 TCR transgenic mice. (A–C) Functional responses of DP thymocytes (A and B) or LN T cells (C) to SYIPSAEK(ABA)I peptide (4P; ▴) and six variants (4L [▪], 4V [▾], 4A [□], 4S [○], 4N [▵], and 4H [▿]) presented by T-2H-2Kd cells. CD69 up-regulation (A) and TCR down-regulation (B and C) were analyzed. (D) Representative dot blots from FTOC experiments showing CD4 and CD8β expression on thymocytes cultured for 7 d in the absence (−) or presence of 1 μM exogenous peptide (0.2 μM and 100 μM for 4A and 4S are also shown). (E) Percentages of positively selected (CD4−CD8αβ+) thymocytes plotted versus ligand potency (EC50 values determined from data in A; Table S1). Red, negative-selecting ligands; gray, threshold ligands; blue, positive-selecting ligands. Table S1 is available at http://www.jem.org/cgi/content/full/jem.20070254/DC1.
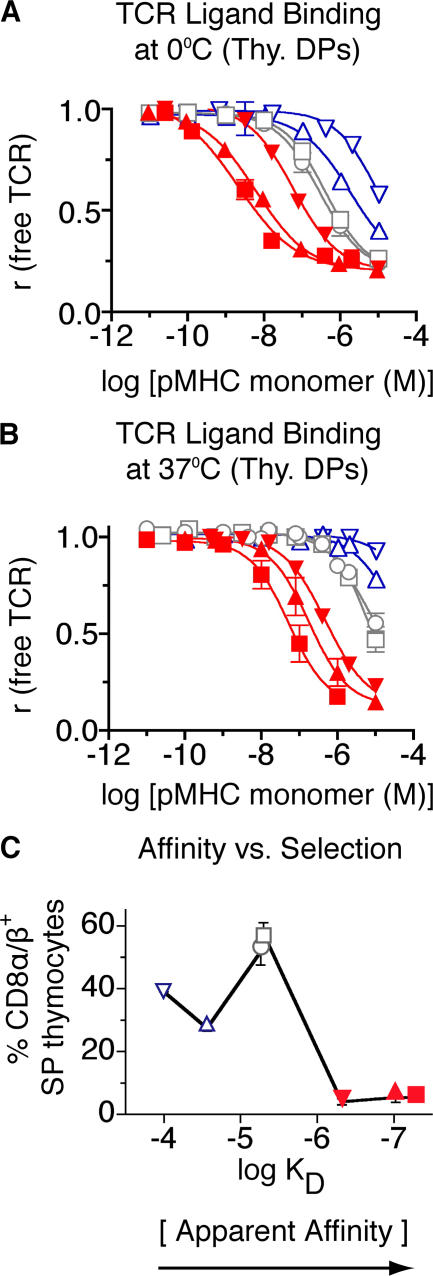
Photoaffinity labeling in this system provides a measure of the binding strength of a monomeric pMHC ligand for a docking site formed by the TCR and CD8. Because three binding partners are involved, affinity is not the appropriate term to describe this interaction; affinity is applied to bimolecular interactions. Avidity describes the binding of a ligand to multiple partners, but is also commonly used for the binding of a multivalent receptor with a multivalent ligand, e.g., an antibody. Therefore, to best describe the binding of a monomeric ligand to a combining site composed of two distinct molecular species, the term apparent affinity may be most suitable. This term has previously been used to describe the affinity of a substrate for a complex of enzyme and coenzyme (20). This is particularly relevant to self-tolerance because the induction of negative selection is dependent on the interactions of pMHC ligand with the TCR and coreceptor (11, 13, 19, 21–23). Binding measurements were performed on live T1 thymocytes using seven different monomeric pMHC ligands (Fig. 2, A and B), and K d values were determined using nonlinear regression and Scatchard analysis (Table II and Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20070254/DC1). The observed apparent affinities were decreased (higher K d values) at 37°C, compared with 0°C, because of temperature-dependent differences in the contribution of CD8 coreceptors to TCR/pMHC binding (Table II and Fig. S2). Nevertheless, the apparent affinity hierarchy was the same at both temperatures.
Figure 2.
Binding of soluble, monomeric pMHC ligands to live thymocytes from T1 TCR transgenic mice. Cells were incubated with several concentrations of pMHC monomers (4P-H-2Kd [▴], 4L-H-2Kd [▪], 4V-H-2Kd [▾], 4A-H-2Kd [□], 4S-H-2Kd [○], 4N-H-2Kd [▵], and 4H-H-2Kd [▿]) at 0°C (A) or 37°C (B), followed by photochemical cross-linking; the fraction of remaining free TCR was determined in a second step (two-step photoaffinity labeling method; see Material and methods and Fig. S1). (C) Selection properties of peptide variants as a function of apparent affinity [K d]. Percentages of positively selected T1 thymocytes induced by each ligand in FTOC are plotted versus their apparent affinity (Table II) for the T1 TCR. Red, negative-selecting ligands; gray, threshold ligands; blue, positive-selecting ligands. Fig. S1 is available at http://www.jem.org/cgi/content/full/jem.20070254/DC1.
Table II.
K d values (M) determined for pMHC monomers bound to T1 thymocytes and lymph node T cells
| Cell type | Ligands (pMHC monomers)
|
||||||
|---|---|---|---|---|---|---|---|
| 4L-Kd
K d (M) |
4P-Kd
K d (M) |
4V-Kd
K d (M) |
4A-Kd
K d (M) |
4S-Kd
K d (M) |
4N-Kd
K d (M) |
4H-Kd
K d (M) |
|
| 0°C | |||||||
| Preselection DP thymocyte |
6.8 × 10−10 | 2.9 × 10−9 | 3.2 × 10−8 | 2.2 × 10−7 | 2.2 × 10−7 | 7.1 × 10−7 | 3.5 × 10−6 |
| Postselection DP thymocyte |
1.8 × 10−9 | 6.1 × 10−9 | 5.9 × 10−8 | 5.0 × 10−7 | 5.2 × 10−7 | 2.2 × 10−6 | 5.9 × 10−6 |
| SP thymocyte | 1.4 × 10−9 | 5.3 × 10−9 | 3.3 × 10−8 | 5.6 × 10−7 | 3.7 × 10−7 | 2.3 × 10−6 | 7.8 × 10−6 |
| LN T cell | 1.5 × 10−9 | 6.8 × 10−9 | 3.0 × 10−8 | 4.5 × 10−7 | 3.3 × 10−7 | 1.6 × 10−6 | 6.0 × 10−6 |
| Δ Kd (SP/DP) | 0.8 | 0.9 | 0.6 | 1.1 | 0.7 | 1.0 | 1.3 |
| Δ Kd (LN/DP) | 0.8 | 1.1 | 0.5 | 0.9 | 0.6 | 0.7 | 1.0 |
| 37°C | |||||||
| Preselection DP thymocyte |
2.8 × 10−8 | 8.8 × 10−8 | 2.6 × 10−7 | 2.2 × 10−6 | 2.9 × 10−6 | 2.9 × 10−5 a | >6 × 10−5 a |
| Postselection DP thymocyte |
6.1 × 10−8 | 1.7 × 10−7 | 4.3 × 10−7 | 5.4 × 10−6 | 6.1 × 10−6 | 5.5 × 10−5 a | >6 × 10−5 a |
| SP thymocyte | 4.0 × 10−8 | 1.1 × 10−7 | 3.5 × 10−7 | 5.5 × 10−6 | 7.9 × 10−6 | 5.8 × 10−5 a | >6 × 10−5 a |
| LN T cell | 2.5 × 10−9 | 1.2 × 10−7 | 3.6 × 10−7 | 4.7 × 10−6 | 5.8 × 10−6 | 3.1 × 10−5 a | >6 × 10−5 a |
| Δ Kd (SP/DP) | 0.7 | 0.6 | 0.8 | 1.0 | 1.3 | nd | nd |
| Δ K d (LN/DP) | 0.4 | 0.7 | 0.8 | 0.9 | 0.9 | nd | nd |
Mean values of at least three independent experiments are shown. nd, not determined. DK d represents the fold difference in ligand affinities between T lineage cells at the indicated developmental stages.
Extrapolated values.
A clear correlation between selection potential and apparent affinity was observed (Fig. 2 C). At 37°C, ligands with a K d > 6.1 μM induced positive selection, whereas higher affinity ligands (K d ≤ 6.1 μM) induced negative selection. These data indicated that negative selection is first attainable with a TCR-CD8/pMHC (apparent) affinity, where the K d < 6 μM. This requirement was met by peptides 4S and 4A (Fig. 2 B and Table S2, available at http://www.jem.org/cgi/content/full/jem.20070254/DC1). These ligands were positive selectors, but could induce negative selection when used at high concentrations (Fig. 1 D). The threshold for unambiguous negative selection is likely to be slightly higher (K d between 5.4 μM [4A] and 0.4 μM [4V]). Examining the binding of negatively selecting pMHC monomers lacking the CD8-binding site (H-2Kd Δ223/227) revealed that CD8 participation increases the affinity of pMHC binding to the T1 TCR by 10–15-fold (Fig. S2) (17). In the absence of the CD8 contribution, the T1 TCR/peptide-H-2Kd bimolecular affinity at the selection threshold had a K d of ∼70 μM (Fig. S2); this agrees with previous BiaCore affinity measurements showing that weak agonist ligands exhibit K d values in this range (24). It should be noted that CD8 affinity varies for different class I MHC alleles; therefore, CD8's contribution to apparent affinity varies for different class I MHC ligands as well.
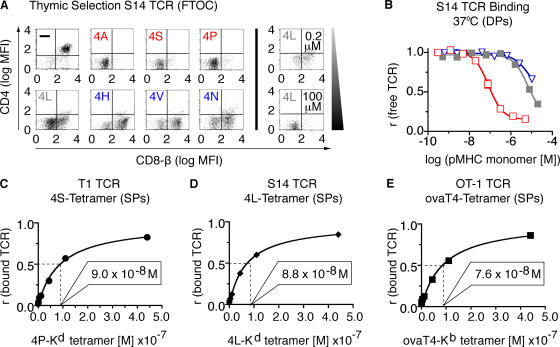
To test whether the apparent affinity threshold for negative selection was a particular feature of the T1 TCR or represented a general feature of negatively selecting ligands, we generated transgenic mice expressing a related TCR, S14. The potencies of these ligands with S14 thymocytes and T cells were measured (unpublished data) and fell into the following hierarchy: 4A > 4P > 4S > 4L > 4N > 4V > 4H. The selecting potential of these variant peptides for the S14 TCR were also determined in FTOC (Fig. 3 A), and 4L was found to be the threshold ligand for S14 thymocytes. Photoaffinity labeling was performed with S14 thymocytes using representative ligands (Fig. 3 B) and the apparent affinity for the threshold ligand, 4L was determined (Table I). The threshold affinities for the S14 (K d = 6.4 ± 1.5 μM) and T1 (K d = 6.1 ± 1.1 μM) receptors were strikingly similar (Table I). To confirm the apparent affinity of the selection threshold using a third unrelated TCR, we used the threshold ligand for the OT-1 receptor SIITFEKL/Kb (11). Because OT-1 ligands cannot be photo–cross-linked to the TCR, these threshold pMHC monomers were converted into tetramers, which were used in binding studies with their corresponding thymocytes (Fig. 3, C–E). As tetramer binding is dependent on CD8 and TCR specificity, it reflects apparent affinity. Tetramer binding of all three threshold ligands to thymocytes expressing the corresponding cognate TCR was remarkably similar (Fig. 3, C–E, and Table I), leading us to conclude that the apparent affinity threshold for negative selection of class I MHC–restricted thymocytes is a constant.
Figure 3.
T1, S14, and OT-1 TCR transgenic animals exhibit a similar affinity threshold for negative selection. (A) Thymic selection properties of peptide variants specific for S14 TCR transgenic thymocytes were determined by FTOC. Positive and negative selection were assessed as in Fig. 1 D. (B) Binding of pMHC monomers (4A-H-2Kd [□], 4L-H-2Kd [▪], and 4H-H-2Kd [▿]) to thymocytes from S14 TCR transgenic mice at 37°C using the two-step photoaffinity labeling method. (C–E) Tetramer binding of threshold ligands to thymocytes from T1, S14, or OT-1 TCR transgenic mice at 37°C. (C) 4S-H-2Kd tetramers (•) applied to T1 thymocytes. (D) 4L-H-2Kd tetramers (♦) applied to S14 thymocytes. (E) SIITFEKL-Kb tetramers (▪) applied to OT-1 thymocytes. Tetramer concentrations resulting in half-maximal TCR saturation are indicated in each panel.
Table I.
Binding of threshold ligands to thymocytes from three different TCR transgenic mouse strains (T1, S14, and OT-1 TCR)
| TCR /ligand | pMHC monomers
|
pMHC tetramers
|
|
|---|---|---|---|
| DP thymocytes (K d [M]) |
SP thymocytes (K d [M]) |
SP thymocytes (r 1/2 [M]) |
|
| T1/4A-Kd | 5.4 ± 1.6 × 10−6 | 5.5 ± 1.8 × 10−6 | Not done |
| T1/4S-Kd | 6.1 ± 1.1 × 10−6 | 7.9 ± 2.0 × 10−6 | 9.0 ± 1.5 × 10−8 |
| S14/4L-Kd | 6.4 ± 1.5 × 10−6 | 6.2 ± 1.7 × 10−6 | 8.8 ± 1.6 × 10−8 |
| OT-1/OvaT4-Kb | Not done | Not done | 7.6 ± 0.7 × 10−8 |
Mean values and SEMs of at least three independent experiments are shown.
We also measured the apparent affinity on various cell populations in T1 mice (Table II). Postselection DP thymocytes had two- to threefold lower apparent affinity compared with their preselection DP counterparts, indicating that for all ligands there is a decrease of TCR/coreceptor affinity at this stage of thymocyte development. This is consistent with work from Kao et al. (25), who demonstrated that the transition from pre- to postselection DP thymocytes is accompanied by a reduction of noncognate tetramer binding, and likely involves changes in surface glycosylation of CD8. On the other hand, for each T1 pMHC ligand, apparent affinity remained unchanged on postselection DP thymocytes, SP thymocytes, and LN T cells (Table II). This is similar to noncognate tetramer binding, which is similar on postselection DP and SP thymocytes (25). It is interesting that SP thymocytes and LN T cells from T1 mice, lose their responsiveness to low-affinity ligands (compared with postselection DP thymocytes; Table S1). As these three cell populations have similar apparent affinities, this change in responsiveness must have another basis. In this regard, the control of phosphatase activity is important in determining T cell sensitivity to antigens (26, 27).
Only negative-selecting ligands were able to fully activate naive peripheral T cells (11, 28, 29). With OT-I, T1, and S14 T cells, only high-affinity pMHC ligands (over the selection threshold) were able to induce Ca2+ fluxes, cytotoxic responses, or proliferation (unpublished data) (11, 28). Therefore, the affinity threshold for negative selection seems to be a functional threshold, at least for the activation of naive peripheral CD8+ T cells. Nonetheless, low-affinity ligands induced some responses from peripheral T cells, such as CD69 up-regulation (Table S1). Low-affinity, endogenous peptides play an important role as coagonists, enhancing a T cell's sensitivity to very small numbers of agonist pMHCs (30). This might be the case during negative selection as well, where the copy number of most self-antigens expressed on the surface of thymic APCs is likely to be very low (31). Therefore, the presence of low-affinity endogenous ligands may increase the thymocyte's sensitivity to the copy number of its cognate self-antigen, provided its apparent affinity is above the threshold for negative selection (K d ≤ 6 μM).
That class I MHC–restricted TCRs use a fixed threshold to induce negative selection allows the thymocyte to unambiguously interpret ligand affinity, i.e., the “rules” for inducing negative selection are likely to be the same for all thymocytes expressing class I MHC–restricted TCRs. Threshold affinity is precisely linked to the signaling pathway, which mediates negative selection (11). This affinity and its corresponding “off-rate” for ligand dissociation (Table S2) (6, 8, 9, 32) are essential quantitative parameters needed to understand the basis of ligand discrimination, on which T cell specificity and self -tolerance depend.
MATERIALS AND METHODS
Antibodies.
The following fluorescently labeled mAbs were purchased from BD PharMingen: α-TCR-Cβ (H57-597), α-CD4 (RM4-5), α-CD8α (53–6.7), α-CD8β (53–5.8), and α-CD69 (H1.2F3). The α-CD8β (H35-17) mAb and the α-H-2Kd(α1) mAb (20–8-4S) (American Type Culture Collection) were produced by hybridomas and purified by protein G chromatography.
Mice and cells.
S14 TCR (17) transgenic mice were produced as previously described for T1 TCR transgenic mice (33). Founders were backcrossed to BALB/c Rag−/− or B10.D2 Rag−/− β2m−/− mice for at least 15 generations. Similarly, C57BL/6 OT-1 Rag−/− mice were bred in our colony. Thymi or lymph nodes from these mice were harvested, and single-cell suspensions were used in the experiments described. Animal experiments were performed in accordance with the federal laws in Switzerland, and they were approved by the veterinary office of the canton of Basel.
CD69 and TCR down-regulation assays.
Freshly isolated thymocytes or lymphocytes from T1 or S14 Rag−/− mice were stimulated with peptide-loaded T2-H-2Kd cells, and EC50 values were determined as previously described (11).
Fetal thymic organ culture.
FTOCs were performed using thymi of fetuses (E15) from Rag−/− β2m−/− T1 or S14 TCR transgenic mice, as previously described (11). Thymocytes were harvested after 7 d, stained with α-CD4, α-CD8α, and/or α-CD8β mAbs, and analyzed by flow cytometry.
Ligand-binding measurements.
Soluble peptide-MHC monomers and tetramers were produced as previously described (11, 16). Direct photoaffinity labeling experiments were performed as previously described using Cy5-labeled H-2Kd/Plasmodium berghei circumsporozoite (PbCS)252-260SYIPSAEK(ABA)I complexes (15, 16). For two-step photoaffinity labeling experiments, thymocytes or lymph node T cells (105 cells) were incubated in DME complete media (+10% FCS) supplemented with indicated concentrations of unlabeled H-2Kd/PbCS252-260SYIPSAEK(ABA)I or variant peptide monomers. Samples were irradiated with UV light (312 ± 40 nm; 90 Watts) to photochemically cross-link cell-bound monomers (referred to as Step 1). Cells were washed three times at room temperature to remove monomers which were not covalently bound to TCR. The fraction of remaining free TCR was measured using a constant concentration (10−6 M) of Cy5-labeled H-2Kd/PbCS252-260SYIPSAEK(ABA)I monomers. Samples were subjected to a second round of UV irradiation (312 ± 40 nm [90 W]; Step 2), washed, and stained with α-TCR-Cβ, α-CD4, and α-CD8α mAbs, followed by flow cytometric analysis. In contrast to the direct photoaffinity labeling technique, Cy5 signals gained by the two-step method represented the fraction of free TCR (r (free TCR)) after the first round of ligand binding. Ligand concentration–dependent binding curves were fitted using Prism software performing nonlinear regression analysis (using the four-parameter equation). K d values were determined from fitted curves representing free TCR (r (free TCR)) or by Scatchard analysis. Tetramer binding assays were performed, as previously described (11). r1/2 represents tetramer concentration to achieve 50% binding.
TCR-CD8/pMHC dissociation (off-rate) measurements.
Thymocytes from T1 Rag−/− mice were subjected to saturating concentrations of unlabeled peptide–H-2Kd complexes for defined periods of time, allowing for steady-state binding to TCR. Samples were diluted 500-fold with medium containing 40 μg/ml α-H-2Kd(α1) mAb (20–8-4S) to prevent ligand rebinding, and UV was irradiated at defined intervals (Step 1), followed by Step 2 and flow cytometric analysis as described for the two-step photoaffinity labeling method. t 1/2 represents the time at which 50% of bound ligands dissociated from the TCR.
Online supplemental material.
Fig. S1 shows that determination of K d values for TCR-CD8/pMHC interactions was very similar using direct photoaffinity labeling or the two-step photoaffinity labeling methods (A–C). K d values are similar when determined by nonlinear regression or Scatchard analysis (compare B and D). In the direct-binding assay, 4P-H-2Kd-Cy5 monomers >1 μM resulted in a small amount of TCR-independent binding, which was not a feature of the two-step assay used for the experiments described in this report. Fig. S2 shows the contribution of CD8 to TCR/pMHC binding at 37°C (A–D) and the contribution of CD8 (E) to the temperature dependence of TCR-CD8–pMHC interactions (F). Table S1 displays the potencies of variant peptides recognized by T1 thymocytes and lymph node T cells. Table S2 displays TCR-CD8–pMHC t 1/2 experimentally determined with DP thymocytes from T1 TCR mice. The online version of this article is available at http://www.jem.org/cgi/content/full/jem.20070254/DC1.
Supplemental Material
Acknowledgments
We thank E. Wagner for animal husbandry and Dr. G. DeLibero for critical comments on the manuscript.
This work was supported by grants from the Swiss National Science Foundation, Novartis, the US Cancer Research Institute (to M.A. Daniels), European Association of Plastic Surgeons, and Hoffmann La Roche.
The authors have no conflicting financial interests.
Abbreviations used: ABA, azidobenzoic acid; DP, double-positive; FTOC, fetal thymus organ culture; PbCS, Plasmodium berghei circumsporozoite; pMHC, peptide/MHC; SP, single-positive.
References
- 1.Fink, P.J., and M.J. Bevan. 1978. H-2 antigens of the thymus determine lymphocyte specificity. J. Exp. Med. 148:766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogquist, K.A., S.C. Jameson, W.R. Heath, J.L. Howard, M.J. Bevan, and F.R. Carbone. 1994. T cell receptor antagonist peptides induce positive selection. Cell. 76:17–27. [DOI] [PubMed] [Google Scholar]
- 3.Kappler, J.W., N. Roehm, and P. Marrack. 1987. T cell tolerance by clonal elimination in the thymus. Cell. 49:273–280. [DOI] [PubMed] [Google Scholar]
- 4.Kisielow, P., H. Bluthmann, U.D. Staerz, M. Steinmetz, and H. von Boehmer. 1988. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 333:742–746. [DOI] [PubMed] [Google Scholar]
- 5.Zinkernagel, R.M., and P.C. Doherty. 1974. Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytic choriomeningitis. Nature. 251:547–548. [DOI] [PubMed] [Google Scholar]
- 6.Alam, S., P. Travers, J. Wung, W. Nasholds, S. Redpath, S. Jameson, and N. Gascoigne. 1996. T-cell-receptor affinity and thymocyte positive selection. Nature. 381:616–620. [DOI] [PubMed] [Google Scholar]
- 7.Gronski, M.A., J.M. Boulter, D. Moskophidis, L.T. Nguyen, K. Holmberg, A.R. Elford, E.K. Deenick, H.O. Kim, J.M. Penninger, B. Odermatt, et al. 2004. TCR affinity and negative regulation limit autoimmunity. Nat. Med. 10:1234–1239. [DOI] [PubMed] [Google Scholar]
- 8.Savage, P.A., and M.M. Davis. 2001. A kinetic window constricts the T cell receptor repertoire in the thymus. Immunity. 14:243–252. [DOI] [PubMed] [Google Scholar]
- 9.Williams, C.B., D.L. Engle, G.J. Kersh, J. Michael White, and P.M. Allen. 1999. A kinetic threshold between negative and positive selection based on the longevity of the T cell receptor–ligand complex. J. Exp. Med. 189:1531–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosselut, R., S. Kubo, T. Guinter, J.L. Kopacz, J.D. Altman, L. Feigenbaum, and A. Singer. 2000. Role of CD8beta domains in CD8 coreceptor function: importance for MHC I binding, signaling, and positive selection of CD8+ T cells in the thymus. Immunity. 12:409–418. [DOI] [PubMed] [Google Scholar]
- 11.Daniels, M.A., E. Teixeiro, J. Gill, B. Hausmann, D. Roubaty, K. Holmberg, G. Werlen, G.A. Hollander, N.R. Gascoigne, and E. Palmer. 2006. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 444:724–729. [DOI] [PubMed] [Google Scholar]
- 12.Holler, P.D., and D.M. Kranz. 2003. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity. 18:255–264. [DOI] [PubMed] [Google Scholar]
- 13.Ingold, A.L., C. Landel, C. Knall, G.A. Evans, and T.A. Potter. 1991. Co-engagement of CD8 with the T cell receptor is required for negative selection. Nature. 352:721–723. [DOI] [PubMed] [Google Scholar]
- 14.Nakayama, K., I. Negishi, K. Kuida, M.C. Louie, O. Kanagawa, H. Nakauchi, and D.Y. Loh. 1994. Requirement for CD8 beta chain in positive selection of CD8-lineage T cells. Science. 263:1131–1133. [DOI] [PubMed] [Google Scholar]
- 15.Luescher, I.F., J.C. Cerottini, and P. Romero. 1994. Photoaffinity labeling of the T cell receptor on cloned cytotoxic T lymphocytes by covalent photoreactive ligand. J. Biol. Chem. 269:5574–5582. [PubMed] [Google Scholar]
- 16.Cebecauer, M., P. Guillaume, S. Mark, O. Michielin, N. Boucheron, M. Bezard, B.H. Meyer, J.M. Segura, H. Vogel, and I.F. Luescher. 2005. CD8+ cytotoxic T lymphocyte activation by soluble major histocompatibility complex-peptide dimers. J. Biol. Chem. 280:23820–23828. [DOI] [PubMed] [Google Scholar]
- 17.Hudrisier, D., B. Kessler, S. Valitutti, C. Horvath, J.C. Cerottini, and I.F. Luescher. 1998. The efficiency of antigen recognition by CD8+ CTL clones is determined by the frequency of serial TCR engagement. J. Immunol. 161:553–562. [PubMed] [Google Scholar]
- 18.Kessler, B.M., P. Bassanini, J.C. Cerottini, and I.F. Luescher. 1997. Effects of epitope modification on T cell receptor-ligand binding and antigen recognition by seven H-2Kd-restricted cytotoxic T lymphocyte clones specific for a photoreactive peptide derivative. J. Exp. Med. 185:629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnden, M.J., W.R. Heath, and F.R. Carbone. 1997. Down-modulation of CD8 beta-chain in response to an altered peptide ligand enables developing thymocytes to escape negative selection. Cell. Immunol. 175:111–119. [DOI] [PubMed] [Google Scholar]
- 20.Klein, P., T. Pawson, and M. Tyers. 2003. Mathematical modeling suggests cooperative interactions between a disordered polyvalent ligand and a single receptor site. Curr. Biol. 13:1669–1678. [DOI] [PubMed] [Google Scholar]
- 21.Garcia, K.C., C.A. Scott, A. Brunmark, F.R. Carbone, P.A. Peterson, I.A. Wilson, and L. Teyton. 1996. CD8 enhances formation of stable T-cell receptor/MHC class I molecule complexes. Nature. 384:577–581. [DOI] [PubMed] [Google Scholar]
- 22.Goldrath, A.W., K.A. Hogquist, and M.J. Bevan. 1997. CD8 lineage commitment in the absence of CD8. Immunity. 6:633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yachi, P.P., J. Ampudia, T. Zal, and N.R. Gascoigne. 2006. Altered peptide ligands induce delayed CD8-T cell receptor interaction–a role for CD8 in distinguishing antigen quality. Immunity. 25:203–211. [DOI] [PubMed] [Google Scholar]
- 24.Gascoigne, N.R., T. Zal, and S.M. Alam. 2001. T-cell receptor binding kinetics in T-cell development and activation. Expert Rev. Mol. Med. 2001:1–17. [DOI] [PubMed] [Google Scholar]
- 25.Kao, C., M.M. Sandau, M.A. Daniels, and S.C. Jameson. 2006. The sialyltransferase ST3Gal-I is not required for regulation of CD8-class I MHC binding during T cell development. J. Immunol. 176:7421–7430. [DOI] [PubMed] [Google Scholar]
- 26.Altan-Bonnet, G., and R.N. Germain. 2005. Modeling T cell antigen discrimination based on feedback control of digital ERK responses. PLoS Biol. 3:e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, Q.J., J. Chau, P.J. Ebert, G. Sylvester, H. Min, G. Liu, R. Braich, M. Manoharan, J. Soutschek, P. Skare, et al. 2007. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 129:147–161. [DOI] [PubMed] [Google Scholar]
- 28.Davey, G.M., S.L. Schober, B.T. Endrizzi, A.K. Dutcher, S.C. Jameson, and K.A. Hogquist. 1998. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J. Exp. Med. 188:1867–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucas, B., I. Stefanova, K. Yasutomo, N. Dautigny, and R.N. Germain. 1999. Divergent changes in the sensitivity of maturing T cells to structurally related ligands underlies formation of a useful T cell repertoire. Immunity. 10:367–376. [DOI] [PubMed] [Google Scholar]
- 30.Krogsgaard, M., Q.J. Li, C. Sumen, J.B. Huppa, M. Huse, and M.M. Davis. 2005. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 434:238–243. [DOI] [PubMed] [Google Scholar]
- 31.Davis, M.M., M. Krogsgaard, M. Huse, J. Huppa, B.F. Lillemeier, and Q.J. Li. 2007. T cells as a self-referential, sensory organ. Annu. Rev. Immunol. 25:681–695. [DOI] [PubMed] [Google Scholar]
- 32.McKeithan, T.W. 1995. Kinetic proofreading in T-cell receptor signal transduction. Proc. Natl. Acad. Sci. USA. 92:5042–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doucey, M.A., L. Goffin, D. Naeher, O. Michielin, P. Baumgartner, P. Guillaume, E. Palmer, and I.F. Luescher. 2003. CD3 delta establishes a functional link between the T cell receptor and CD8. J. Biol. Chem. 278:3257–3264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.