Abstract
Maternal immune responses can promote allergy development in offspring, as shown in a model of increased susceptibility to asthma in babies of ovalbumin (OVA)-sensitized and -challenged mother mice. We investigated whether inflammatory responses to air pollution particles (diesel exhaust particles, DEP) or control “inert” titanium dioxide (TiO2) particles are enhanced during pregnancy and whether exposure to particles can cause increased neonatal susceptibility to asthma. Pregnant BALB/c mice (or nonpregnant controls) received particle suspensions intranasally at Day 14 of pregnancy. Lung inflammatory responses were evaluated 48 hours after exposure. Offspring of particle- or buffer-treated mothers were sensitized and aerosolized with OVA, followed by assays of airway hyperresponsiveness (AHR) and allergic inflammation (AI). Nonpregnant females had the expected minimal response to “inert” TiO2. In contrast, pregnant mice showed robust and persistent acute inflammation after both TiO2 and DEP. Genomic profiling identified genes differentially expressed in pregnant lungs exposed to TiO2. Neonates of mothers exposed to TiO2 (and DEP, but not PBS) developed AHR and AI, indicating that pregnancy exposure to both “inert” TiO2 and DEP caused increased asthma susceptibility in offspring. We conclude that (1) pregnancy enhances lung inflammatory responses to otherwise relatively innocuous inert particles; and (2) exposures of nonallergic pregnant females to inert or toxic environmental air particles can cause increased allergic susceptibility in offspring.
Keywords: maternal asthma, environmental particles, titanuim dioxide, diesel exhaust particles, susceptibility
CLINICAL RELEVANCE
A novel model allowing analysis of environmental exposures in pregnancy on offspring susceptibility to allergy identifies titanium dioxide particles as pro-inflammatory in pregnancy and pro-allergic for neonates.
The increased prevalence of asthma is a major public health problem (1–4). Asthma is a disease that primarily begins in early life, but can persist into adult life. One strong risk factor for asthma is maternal asthma (more so than paternal) (5, 6). Multiple mechanisms may contribute to the maternal effect, including genetic, environmental, and maternal immune system factors.
We have developed a murine model in which an identical genetic background allows experiments focused on maternal immunity and how it can affect susceptibility of offspring to allergy (7–9). In this model of maternal transmission of asthma risk, mother mice are sensitized and challenged with chicken ovalbumin (OVA) and their offspring are subjected to an “intentionally suboptimal” OVA sensitization and challenge protocol. An asthma-like phenotype of airway hyperresponsiveness (AHR) and allergic inflammation (AI) is seen only in offspring from asthmatic, but not normal, mothers.
Air pollution is well known to exacerbate existing asthma (10). The role of air pollution in the initiation of asthma is more controversial. Arguments against a link include epidemiologic data showing less asthma in highly polluted East Germany compared with West Germany (11) and the increase in asthma in Western countries where air pollution has in general been decreasing. On the other side of the controversy are epidemiologic data showing increased incidence of asthma in high-traffic areas (12, 13).
Some air pollutants—for example, diesel exhaust particles (DEP)—have been used extensively to address this question experimentally in people and in animal models. DEP can exacerbate established asthma in mice (14, 15) and nasal allergy outcomes in human studies (16). DEP can act as a strong adjuvant or co-factor in the initiation phase or sensitization to allergen in both mice and people (17, 18) and up-regulate production of pro-allergic cytokines (19, 20). Other air pollutants, like the titanium dioxide particles (TiO2) or carbon black particles (CB) are known to be immunologically “inert” and typically used as control substances in immunotoxicity studies.
Since asthma begins in early life, we sought to determine if our model could be used to detect and analyze increased susceptibility arising from environmental exposure of pregnant mice. Our pilot studies indicated that a single intratracheal instillation of DEP into normal, nonallergic mother mice during pregnancy results in increased susceptibility to allergy in their offspring. We hypothesized that in pregnancy the response to particles is enhanced and that this may influence the offspring allergic susceptibility. In addition, we were interested in effects of immunologically “inert” particles (e.g., TiO2) on both local pulmonary inflammation in the lungs of pregnant mice and on susceptibility of the offspring of exposed mothers to allergic sensitization.
MATERIALS AND METHODS
Animals
BALB/c mice were obtained from Charles River Laboratories (Cambridge, MA). All mice were housed in a clean barrier facility where animals are maintained at 22 to 24°C with a 12-hour dark/light cycle with an independent pressure-gradient–enabled ventilation system. Animal care complied with the Guide for the Care and Use of Laboratory Animals, and all experiments were approved by the Institutional Review Board.
Exposure to Environmental Particles
Respirable-size DEP, TiO2, and CB particles were generously provided by Dr. Ian Gilmour (U.S. E.P.A.) and Dr. Joseph Brain (Harvard University). Particle samples were baked at 165°C for 3 hours to eliminate endotoxin, aliquoted and stored frozen at −80°C. Particle suspensions (50 μg in 50 μl for DEP and TiO2, and 250 μg in 50 μl for CB) or PBS solution (vehicle) were administered by single intranasal insufflation of pregnant or normal BALB/c mice under light halothane anesthesia (21). We used two different protocols of particle exposure.
Protocol 1A: Comparison of innate immune response to particles in normal versus pregnant mice.
To test whether pregnancy alters the normally minimal inflammatory response to “inert” particles, we administered TiO2 and DEP suspensions (50 μg/mouse) or PBS solution by intranasal insufflation to normal or pregnant E14 mice (see Figure 1B). The mice were subjected to pathologic analysis 48 hours later.
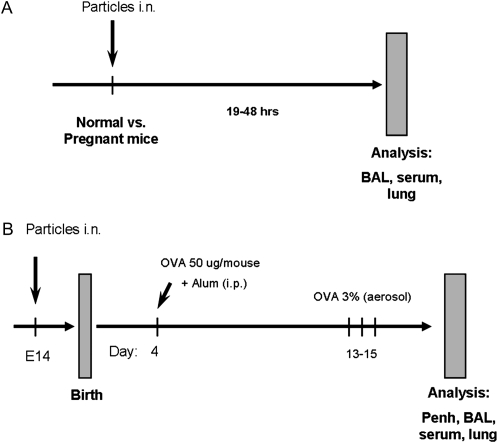
Figure 1.
Experimental protocols. (A) Particles exposure protocol. Normal females or pregnant mice were treated with diesel exhaust particle (DEP) or titanium dioxide (TiO2) particle suspensions (50 ug/mouse) and analyzed 19 or 48 hours later. (B) Maternal particles exposure + single intraperitoneal neonatal sensitization period. Pregnant mothers at Day 14 of pregnancy (E14) received 50 μg/mouse intranasally of DEP, carbon black (CB), or TiO2 particle suspensions or PBS buffer (negative control). Offspring of these mothers were injected once with 0.1 ml of 50 μg/ml ovalbumin (OVA) + alum (“suboptimal”) sensitization and challenged three times with 3% OVA aerosol.
Protocol 1B: Particle exposure during pregnancy and asthma susceptibility in offspring.
The protocol is based on our prior studies showing that maternal immune events can influence the susceptibility of offspring's immune system to allergy (7). The model uses an “intentionally suboptimal” allergen (OVA, grade III; Sigma-Aldrich, St. Louis, MO) sensitization and challenge protocol in the newborn mice, as detailed in (7). Briefly, female mice received two intraperitoneal injections of 5 μg OVA with 1 mg alum in 0.1 ml PBS at 3 and 7 days of age, and after weaning are exposed to aerosols of allergen (3% OVA [wt/vol] in PBS [pH 7.4]) for 10 minutes on 3 consecutive days at 4, 8, and 12 weeks of age. These “asthmatic” and normal control mothers are mated with normal males and the offspring receive “suboptimal” protocol. In this study we replaced prior maternal sensitization with particle exposure (Figure 1A).
Offspring Allergen Sensitization and Challenge
On Day 4 after birth, newborns from particle-exposed and normal control mother mice received a single intraperitoneal injection of OVA with alum. On Days 12 to 14 of life, these baby mice were exposed to aerosolized 3% OVA within individual compartments of a mouse pie chamber (Braintree Scientific, Braintree, MA) using a Pari IS2 nebulizer (Sun Medical Supply, Kansas City, KS) connected to air compressor (PulmoAID; DeVilbiss, Somerset, PA). After this challenge, the mice were subjected to pulmonary function and pathologic analysis.
Pulmonary Function Testing
Airway responsiveness of mice to increasing concentrations of aerosolized methacholine was measured using whole body plethysmography (Buxco, Sharon, CT). Briefly, each mouse was placed in a chamber, and continuous measurements of box pressure/time wave were calculated via a connected transducer and associated computer data acquisition system. The main indicator of airflow obstruction, enhanced pause (Penh), which shows strong correlation in BALB/c mice with the airway resistance examined by standard evaluation methods, was calculated from the box waveform. After measurement of baseline Penh, aerosolized PBS or methacholine (MCh, acetyl-methylcholine chloride; Sigma-Aldrich) in increasing concentrations (6, 12, 25, 50, and 100 mg/ml) was nebulized through an inlet of the chamber for 1 minute, and Penh measurements were taken for 9 minutes after each dose. Penh values for the first 2 and the last 2 minutes after each nebulization were discarded, and the values for 5 minutes in between were averaged and used to compare results. Increased Penh was interpreted as evidence of increased AHR.
Lipopolysaccharide Exposure
To test whether pregnancy alters inflammatory response to a nonspecific agent, pregnant mice and normal controls were place in individually labeled compartments of a pie chamber and exposed to 2 μg/ml lipopolysaccharide (LPS) (serotype 055:B5, CAT:L2880, LOT:110K4046; Sigma-Aldrich) nebulized aerosol for 10 minutes. Bronchoalveolar lavage (BAL) samples were collected 24 hours later. We chose this time point based on abundant work from other labs (53) and our own prior experience in working with inhaled LPS exposure, showing optimal detection of peak BAL polymorphonuclear leukocytes (PMN) responses at this time point.
Pathologic Analysis
Animals were killed with sodium pentobarbital (Veterinary Laboratories, Lenexa, KS). The chest wall was opened and the animals were exsanguinated by cardiac puncture. The trachea was cannulated after blood collection. BAL was performed five times with 0.3 ml of sterile PBS instilled and harvested gently. Lavage fluid (recovery volume was ∼ 90% of instilled) was collected and centrifuged at 1200 rpm (300 × g) for 10 minutes, and the cell pellet was resuspended in 0.1 ml PBS. Total cell yield was quantified by hemocytometer. BAL differential cell counts were performed on cytocentrifuge slides prepared by centrifugation of samples at 800 rpm for 5 minutes (Cytospin 2; Shandon, Pittsburgh, PA). These slides were fixed in 95% methanol and stained with Diff-Quik (VWR, Boston, MA), a modified Wright-Giemsa stain, and a total of 200 cells were counted for each sample by microscopy. Macrophages, lymphocytes, neutrophils, and eosinophils were enumerated. After lavage, the lungs were instilled with 10% buffered formalin, removed, and fixed in the same solution. After paraffin embedding, sections for microscopy were stained with hematoxylin and eosin (H&E). For allergy responses, an index of pathologic changes in coded H&E slides was derived by scoring the inflammatory cell infiltrates around airways and vessels for greatest severity (0, normal; 1, <3 cell diameter thick; 2, 4–10 cells thick; 3, >10 cells thick) and overall prevalence (0, normal; 1, <25% of sample; 2, 25–50%; 3, 51–75%; 4, >75%). The index was calculated by multiplying severity by prevalence, with a maximum possible score of 9.
Cytokine Detection
Levels of cytokines in BAL fluid, serum or cell culture supernatants were measured via the multiplexed Luminex xMAP assay (Luminex, Austin, TX). LINCOplex kits were obtained from Linco Research (St. Charles, MI). The sensitivity of the kit varied between 0.3 to 20 pg/ml for serum/plasma samples depending on the cytokine. Samples were tested in duplicates.
Gene Chip Microarray and Data Analysis
Total lung RNA extraction and isolation was performed using a Qiagen RNAeasy Mini kit according to manufacturer's instructions (Qiagen, Valencia, CA). RNA purity and quality were analyzed by Agilent Bioanalyzer 2100 scan (Agilent, Santa Clara, CA). The hybridization was carried out at the Harvard Partners Genomic Center Microarray facility (Cambridge, MA) using the Affymetrix GeneChip platform and Affymetrix mouse 430 2.0 chips (Affymetrix, Santa Clara, CA). Signal intensities and detection calls were extracted using dChip (v. 2006). Chip images were evaluated for overall quality; PM/MM pairs were evaluated for outliers to judge on hybridization performance. Hybridization quality was found to be consistent with the manufacturer's requirements. Probesets were filtered based on detection call to exclude ones in which “P” call was not present in all four samples in any one group, this also excluded probesets with all “A” calls. The filtration resulted in about 24,000 probesets. RMA values for this list were extracted using RMAExpress (v. 0.4.1) with background correction, normalization, and log2 transformation and were analyzed using tMEV (v. 4.0). Resampling with bootstrapping using the Support Tree feature indicated appropriate sample clustering with 90 to 100% support level (not shown). High-level analysis was performed in tMEV 4.0 and included Significance Analysis for Microarrays (SAM) at false-discovery rate (FDR) of 0, ANOVA, and t test with Welch approximation. Fold change was calculated from corresponding natural, not log2 values. Meta-analysis was carried out using the Expression Analysis Systematic Explorer (EASE v. 2.0)
General Statistical Methods
Data are presented as mean ± SEM. Data analysis was performed using Microsoft Excel from Microsoft Office 2003 Pro (Microsoft Corporation, Redmond, WA) and GraphPad Prism version 4.0 for Windows (GraphPad Software, San Diego, CA). Statistical significance was accepted when P < 0.05. To estimate significance of differences between groups in multiple comparisons ANOVA with Tukey's Honest Significant Differences for unequal N post hoc test and Kruskal-Wallis test with Dunn's post-test were used, as appropriate. For pairwise comparisons nonparametric Mann-Whitney U test was used. For repeated measurements in the plethysmography procedure we used repeated-measures ANOVA.
RESULTS
Inflammatory Response to Inhaled Particles Is Enhanced in Pregnancy
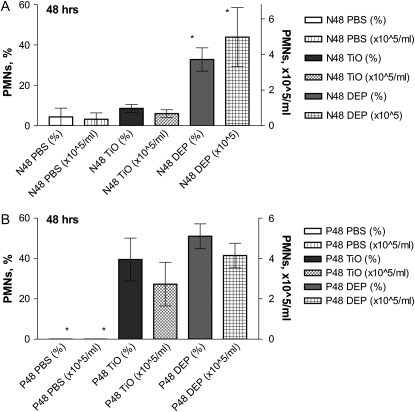
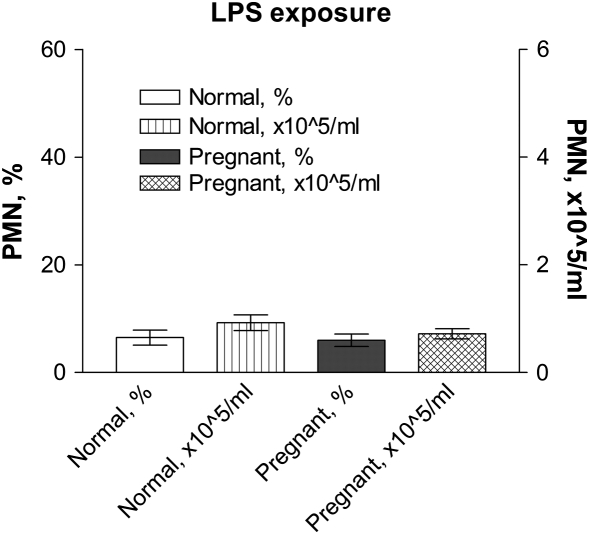
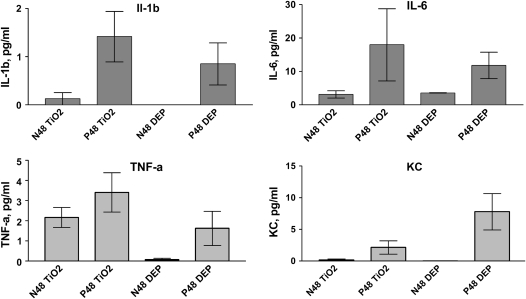
To investigate whether pregnancy alters the inflammatory response to particles, we exposed pregnant and control normal female mice to particle suspensions of DEP or vehicle (PBS) (Figure 1, Protocol 1A). We initially analyzed TiO2 as an “inert” negative control particle. In both normal and pregnant mice, BAL PMN counts were significantly increased at 48 hours after exposure to DEP, but not to PBS (Figure 2). Nonpregnant mice treated with TiO2 displayed minimal increases in BAL PMN counts 48 hours after exposure (Figure 2A). In contrast, pregnant mice exhibited a robust acute neutrophilic inflammation (Figure 2B). No significant changes were noted in any other cell type (e.g., lymphocytes) (data not shown). The specificity of the enhanced inflammatory response to TiO2 was tested by comparing responses to inhaled LPS. Both pregnant and nonpregnant females showed similar acute PMN influx into the lungs after exposure to aerosolized LPS (Figure 3). We used an exposure that causes mild inflammation in normal nonpregnant females (e.g., ∼ 10% PMNs in BAL samples) so as to allow sensitive detection of increased inflammation in pregnant mice. To investigate whether responses to particles in the lungs of pregnant mice were associated with systemic effects, serum samples from DEP- and TiO2-treated pregnant and normal animals were analyzed for cytokine levels via a multiplex assay (Luminex). The data show that pregnant mice exposed to both DEP and TiO2 had elevated levels of IL-1β, TNF-α, IL-6, and KC levels 48 hours after exposure, compared to nonpregnant controls (Figure 4).
Figure 2.
Direct analysis of bronchoalveolar lavage (BAL) responses in pregnant versus control females. Pregnant or normal mice were exposed to either DEP or TiO2 particle suspension or PBS alone and BALs were obtained 48 hours later. Normal mice exposed to TiO2 reveal minimal airway inflammation at 48 hours (A) after exposure. In contrast, pregnant mice reveal enhanced and prolonged inflammation seen even 48 hours after exposure to TiO2 (B). Mean ± SEM (n > 9 each group). *P < 0.05.
Figure 3.
Inflammatory BAL response to LPS challenge. Pregnant mice or normal controls were exposed to LPS aerosol, and BALs were obtained 24 hours later. There is no significant difference in PMN counts in these groups. Mean ± SEM (n = 8).
Figure 4.
Serum cytokine levels after particle exposure. Pregnant (P) or normal (N) mice were exposed to either DEP or TiO2 particle suspension and sera were obtained 48 hours later. Levels of proinflammatory cytokines are increased in pregnant mice compared with nonpregnant controls after both TiO2 and DEP exposure (with P < 0.05). Mean ± SEM (n = 9 each group).
Gene Expression Changes in Response to Inhaled Particles Are Different in Pregnant versus Normal Mice
To identify genes involved in the unexpected response of pregnant lungs to inert TiO2 particles, we performed microarray analysis of mRNA gene expression patterns in pregnant and nonpregnant females treated with TiO2 or PBS vehicle. Data analysis used significance analysis for microarrays (SAM). At false-discovery rate (FDR) of 0, SAM identified 130 probesets significantly different across the four groups (see Figure EA in the online supplement A). Pathway analysis indicated that most of these genes are involved in inflammatory response and immune regulation, cell proliferation/DNA metabolism, and metabolic processes (Table EA in online supplement B).
Using t test with Welch approximation and ANOVA, we identified a cluster of genes that were changed only upon exposure to TiO2 in pregnant mice (were significantly different between Pregnant PBS and Pregnant TiO2 groups) (Figure EB, left, in online supplement A). From this list we excluded genes that were significantly changed in normal mice upon TiO2 exposure, or were changed in pregnant mice compared with normals. We also excluded noncoding sequences. Expression of these 80 genes (see Table 1) is changed (increased or decreased) only in response to TiO2 on the background of pregnancy. We also identified genes that were changed upon exposure to TiO2 in normal mice, but were not significantly different between pregnant mice exposed to PBS versus TiO2 (Figure EB, right, in online supplement A; Table 2). Absence of change in these 108 genes in pregnant mice exposed to TiO2 compared to PBS may also contribute to the studied phenomenon. Detailed pathway analysis with EASE (Expression Analysis Systematic Explorer, a functional enrichment analysis that identifies groups of genes based on their involvement in various processes) for the genes in Tables 1 and 2 is presented in online supplement B. Genomic data has been submitted to Gene Expression Omnibus (GEO) database and has been assigned Series Record # GSE7475.
TABLE 1.
GENES POTENTIALLY INVOLVED IN TiO2 RESPONSE IN PREGNANT MICE
| Probe Set ID | Representative Public ID | Gene Title | Gene Symbol | Process | Adjusted P Value | Fold Ti > PBS |
|---|---|---|---|---|---|---|
| 1450920_at | AK013312 | Cyclin B2 | Ccnb2 | Cell division and cell cycle regulation, cytokinesis, apoptosis | 0.0082 | 1.489921 |
| 1423774_a_at | BC005475 | Protein regulator of cytokinesis 1 | Prc1 | 0.0039 | 1.328305 | |
| 1437716_x_at | BB251322 | Kinesin family member 22 | Kif22 | 0.0079 | 1.299987 | |
| 1449171_at | NM_009445 | Ttk protein kinase | Ttk | 0.0061 | 1.294891 | |
| 1423775_s_at | BC005475 | Protein regulator of cytokinesis 1 | Prc1 | 0.0038 | 1.287928 | |
| 1428104_at | AK011311 | TPX2, microtubule-associated protein homolog | Tpx2 | 0.0052 | 1.257099 | |
| 1460238_at | NM_018857 | Mesothelin | Msln | 0.0017 | 1.256257 | |
| 1428480_at | AV307110 | Cell division cycle associated 8 | Cdca8 | 0.0059 | 1.248081 | |
| 1417251_at | NM_023245 | Palmdelphin | Palmd | 0.0082 | 1.213763 | |
| 1422498_at | AF319981 | Melanoma antigen, family H, 1 | Mageh1 | 0.0081 | 1.191047 | |
| 1433543_at | BI690018 | Anillin, actin binding protein (scraps homolog, Drosophila) | Anln | 0.0086 | 1.179205 | |
| 1449249_at | NM_018764 | Protocadherin 7 | Pcdh7 | 0.0059 | 1.154676 | |
| 1439436_x_at | BB418702 | Inner centromere protein | Incenp | 0.0089 | 1.14459 | |
| 1438951_x_at | BB168451 | Nucleoporin 54 | Nup54 | 0.0040 | 1.142115 | |
| 1429594_at | BB030482 | Solute carrier family 38, member 2 | Slc38a2 | 0.0092 | 1.139607 | |
| 1416114_at | NM_010097 | SPARC-like 1 (mast9, hevin) | Sparcl1 | 0.0061 | 1.122001 | |
| 1449445_x_at | BB436326 | Microfibrillar-associated protein 1 | Mfap1 | 0.0089 | 1.10335 | |
| 1426129_at | BC003485 | Breast cancer metastasis-suppressor 1 | Brms1 | 0.0094 | 0.814778 | |
| 1450060_at | NM_011082 | Polymeric immunoglobulin receptor | Pigr | Immune response and regulation, complement cascade, adhesion, proteolysis | 0.0084 | 1.75463 |
| 1427747_a_at | X14607 | Lipocalin 2 | Lcn2 | 0.0075 | 1.736284 | |
| 1438148_at | BB829808 | Gene model 1960, (NCBI) | Gm1960 | 0.0061 | 1.670798 | |
| 1442187_at | AW490711 | Bradykinin receptor, beta 2 | Bdkrb2 | 0.0041 | 1.337381 | |
| 1450652_at | NM_007802 | Cathepsin K | Ctsk | 0.0080 | 1.291786 | |
| 1457664_x_at | AV227574 | Complement component 2 (within H-2S) | C2 | 0.0094 | 1.280136 | |
| 1417009_at | NM_023143 | Complement component 1, r subcomponent | C1r | 0.0012 | 1.260038 | |
| 1416051_at | NM_013484 | Complement component 2 (within H-2S) | C2 | 0.0046 | 1.214211 | |
| 1456532_at | BB428671 | Platelet-derived growth factor, D polypeptide | Pdgfd | 0.0015 | 1.202271 | |
| 1421812_at | AF043943 | TAP binding protein | Tapbp | 0.0091 | 1.113042 | |
| 1455327_at | BI684973 | SUMO/sentrin specific peptidase 2 | Senp2 | 0.0033 | 0.889723 | |
| 1435943_at | AI647687 | Dipeptidase 1 (renal) | Dpep1 | 0.0090 | 0.840445 | |
| 1435560_at | BI554446 | Integrin alpha L (CD11a antigen) | Itgal | 0.0036 | 0.757596 | |
| 1421546_a_at | NM_012025 | Rac GTPase-activating protein 1 | Racgap1 | Intracellular transport, cell metabolism | 0.0074 | 1.447467 |
| 1438773_at | BB817972 | Six transmembrane epithelial antigen of prostate 2 | Steap2 | 0.0062 | 1.360801 | |
| 1449203_at | NM_130861 | Solute carrier organic anion transporter family, member 1a5 | Slco1a5 | 0.0046 | 1.319134 | |
| 1417381_at | NM_007572 | Complement component 1, q subcomponent, alpha polypeptide | C1qa | 0.0046 | 1.300391 | |
| 1447234_s_at | AU018928 | Sorting nexin 6 | Snx6 | 0.0059 | 1.113238 | |
| 1417039_a_at | NM_025611 | Cullin 7 | Cul7 | 0.0075 | 1.104416 | |
| 1421594_a_at | NM_031394 | Synaptotagmin-like 2 | Sytl2 | 0.0017 | 0.87377 | |
| 1449227_at | NM_009890 | Cholesterol 25-hydroxylase | Ch25h | Metabolism | 0.0028 | 1.563081 |
| 1423256_a_at | BI154058 | ATPase, H+ transporting, lysosomal V1 subunit G1 /// | Atp6v1g1 | 0.0031 | 1.115357 | |
| 1455824_x_at | BB724781 | STT3, subunit of the oligosaccharyltransferase complex, homolog A (S. cerevisiae) | Stt3a | 0.0074 | 1.101699 | |
| 1427128_at | BM195862 | Protein tyrosine phosphatase, non-receptor type 23 | Ptpn23 | 0.0096 | 1.093921 | |
| 1422092_at | BC018418 | 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 2 | Pfkfb2 | 0.0050 | 0.838748 | |
| 1435893_at | BB127955 | Very low density lipoprotein receptor | Vldlr | 0.0068 | 0.81228 | |
| 1438338_at | BB318769 | Malate dehydrogenase 1, NAD (soluble) | Mdh1 | 0.0050 | 0.806002 | |
| 1434437_x_at | AV301324 | Ribonucleotide reductase M2 | Rrm2 | Transcription, DNA replication, metabolism and repair | 0.0087 | 1.494858 |
| 1448535_at | NM_023876 | Elongation protein 4 homolog (S. cerevisiae) | Elp4 | 0.0006 | 1.219804 | |
| 1455490_at | AV027632 | Upstream binding transcription factor, RNA polymerase I | Ubtf | 0.0006 | 1.64884 | |
| 1454694_a_at | BM211413 | Topoisomerase (DNA) II alpha | Top2a | 0.0080 | 1.535987 | |
| 1424105_a_at | AF069051 | Pituitary tumor-transforming 1 | Pttg1 | 0.0096 | 1.302197 | |
| 1418036_at | NM_008922 | DNA primase, p58 subunit | Prim2 | 0.0054 | 1.177537 | |
| 1416641_at | NM_010715 | Ligase I, DNA, ATP-dependent | Lig1 | 0.0094 | 1.176809 | |
| 1426484_at | AI788596 | UBX domain containing 2 | Ubxd2 | 0.0083 | 1.160992 | |
| 1449295_at | NM_020483 | SAP30 binding protein | Sap30bp | 0.0052 | 1.123169 | |
| 1435303_at | AV373814 | TAF4B RNA polymerase II, TATA box binding protein (TBP)-associated factor | Taf4b | 0.0051 | 1.078127 | |
| 1455323_at | BB446066 | RB-associated KRAB repressor | Rbak | 0.0099 | 0.941894 | |
| 1418397_at | BC019962 | Zinc finger protein 275 | Zfp275 | 0.0062 | 0.876814 | |
| 1436360_at | BB811893 | GLI-Kruppel family member HKR2 | Hkr2 | 0.0087 | 0.859708 | |
| 1452617_at | BG073014 | Single-stranded DNA binding protein 1 | Ssbp1 | 0.0028 | 0.820666 | |
| 1447198_at | AI853438 | RecQ protein-like | Recql | 0.0019 | 0.820491 | |
| 1438766_at | AV001197 | Proline-rich nuclear receptor coactivator 2 | Pnrc2 | 0.0046 | 0.791521 | |
| 1443867_at | BB320633 | Ankyrin repeat domain 12 | Ankrd12 | Other | 0.0087 | 1.512112 |
| 1416299_at | NM_011369 | Shc SH2-domain binding protein 1 | Shcbp1 | 0.0048 | 1.43836 | |
| 1429411_a_at | AI595744 | Enhancer of yellow 2 homolog (Drosophila) | Eny2 | 0.0072 | 1.372424 | |
| 1422430_at | NM_021891 | Fidgetin-like 1 | Fignl1 | 0.0049 | 1.327348 | |
| 1417926_at | NM_133762 | Leucine zipper protein 5 | Luzp5 | 0.0091 | 1.30666 | |
| 1449015_at | NM_020509 | Resistin like alpha | Retnla | 0.0023 | 1.303933 | |
| 1448894_at | NM_008012 | Aldo-keto reductase family 1, member B8 | Akr1b8 | 0.0086 | 1.252814 | |
| 1454630_at | BB282890 | Sterile alpha motif domain containing 14 | Samd14 | 0.0097 | 1.191833 | |
| 1429474_at | BE283373 | Zinc binding alcohol dehydrogenase, domain containing 1 | Zadh1 | 0.0030 | 1.188432 | |
| 1424292_at | BC005799 | DEP domain containing 1a | Depdc1a | 0.0078 | 1.185658 | |
| 1442059_at | BB385925 | Fragile X mental retardation gene 1, autosomal homolog | Fxr1h | 0.0070 | 1.140154 | |
| 1452042_a_at | AV306255 | Transmembrane protein 144 | Tmem144 | 0.0011 | 1.125372 | |
| 1416779_at | BE197945 | Serum deprivation response | Sdpr | 0.0084 | 1.115135 | |
| 1424049_at | BC027203 | Leucine rich repeat containing 42 | Lrrc42 | 0.0082 | 1.100444 | |
| 1417073_a_at | NM_021881 | Quaking | Qk | 0.0085 | 1.078538 | |
| 1453848_s_at | AK002774 | Zinc finger, BED domain containing 3 | Zbed3 | 0.0052 | 1.075243 | |
| 1425026_at | BC017549 | SFT2 domain containing 2 | Sft2d2 | 0.0099 | 1.055601 | |
| 1454794_at | AV298495 | Spastin | Spast | 0.0053 | 0.940109 | |
| 1420112_at | AI503516 | Phosphofurin acidic cluster sorting protein 1 | Pacs1 | 0.0015 | 0.763026 |
Meta-analysis on the list of genes significantly different in the group Preg TiO2 versus Preg PBS, with subtraction of genes significantly different in Norm TiO2 versus Norm PBS and of genes significantly different in all Preg versus all Norm. See heatmap in the online supplement, left.
TABLE 2.
GENES POTENTIALLY INVOLVED IN TiO2 RESPONSE IN NORMAL MICE
| Probe Set ID | Representative Public ID | Gene Title | Gene Symbol | Process | Adjusted P Value | Fold Ti > PBS |
|---|---|---|---|---|---|---|
| 1421394_a_at | BF137345 | Baculoviral IAP repeat-containing 4 | Birc4 | Cell cycle regulation, cell division and motility | 0.0048 | 1.709305 |
| 1426720_at | BG067463 | Amyloid beta (A4) precursor protein-binding, family B, member 2 | Apbb2 | 0.0044 | 1.418741 | |
| 1417086_at | BE688382 | Platelet-activating factor acetylhydrolase, isoform 1b, beta1 subunit | Pafah1b1 | 0.0029 | 1.26618 | |
| 1450784_at | NM_016678 | Reversion-inducing-cysteine-rich protein with kazal motifs | Reck | 0.0099 | 1.178259 | |
| 1434775_at | AW543460 | par-3 (partitioning defective 3) homolog (C. elegans) | Pard3 | 0.0005 | 1.168492 | |
| 1423663_at | BC025820 | Folliculin | Flcn | 0.0015 | 1.154846 | |
| 1449491_at | NM_130859 | Caspase recruitment domain family, member 10 | Card10 | 0.0058 | 1.120665 | |
| 1434000_at | BQ176608 | v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog | Kras | 0.0082 | 1.118953 | |
| 1423136_at | AI649186 | Fibroblast growth factor 1 | Fgf1 | 0.0012 | 1.113597 | |
| 1426110_a_at | U48235 | Endothelial differentiation, lysophosphatidic acid G-protein-coupled receptor, 2 | Edg2 | Immune response and regulaiton, cell adhesion, proteolysis and protein biosynthesis | 0.0043 | 1.51332 |
| 1419204_at | NM_007865 | Delta-like 1 (Drosophila) | Dll1 | 0.0041 | 1.491792 | |
| 1421319_at | NM_011197 | Prostaglandin F2 receptor negative regulator | Ptgfrn | 0.0083 | 1.352902 | |
| 1418634_at | NM_008714 | Notch gene homolog 1 (Drosophila) | Notch1 | 0.0022 | 1.251013 | |
| 1450817_at | NM_015830 | Small optic lobes homolog (Drosophila) | Solh | 0.0069 | 1.234057 | |
| 1455137_at | AW536472 | Rap guanine nucleotide exchange factor (GEF) 5 | Rapgef5 | 0.0043 | 1.213749 | |
| 1455251_at | AV071536 | Integrin alpha 1 | Itga1 | 0.0094 | 1.212642 | |
| 1418901_at | NM_009883 | CCAAT/enhancer binding protein (C/EBP), beta | Cebpb | 0.0090 | 1.178609 | |
| 1423902_s_at | AF467766 | Rho guanine nucleotide exchange factor (GEF) 12 | Arhgef12 | 0.0048 | 1.15206 | |
| 1437277_x_at | BB550124 | Transglutaminase 2, C polypeptide | Tgm2 | 0.0026 | 1.115566 | |
| 1418674_at | AB015978 | Oncostatin M receptor | Osmr | 0.0070 | 1.115232 | |
| 1448590_at | NM_009933 | Procollagen, type VI, alpha 1 | Col6a1 | 0.0011 | 1.108705 | |
| 1428622_at | AK014624 | DEP domain containing 6 | Depdc6 | 0.0043 | 1.091745 | |
| 1452143_at | BQ174069 | Spectrin beta 2 | Spnb2 | 0.0093 | 1.060853 | |
| 1453728_a_at | AK003008 | Mitochondrial ribosomal protein S17 | Mrps17 | 0.0085 | 0.909486 | |
| 1423254_x_at | BB836796 | Ribosomal protein S27-like | Rps27l | 0.0069 | 0.884305 | |
| 1434159_at | BG069810 | Serine/threonine kinase 4 | Stk4 | 0.0042 | 0.843541 | |
| 1450925_a_at | BB836796 | Ribosomal protein S27-like /// similar to 40S ribosomal protein S27-like protein | Rps27l /// LOC667571 | 0.0064 | 0.83938 | |
| 1433825_at | BM245880 | Neurotrophic tyrosine kinase, receptor, type 3 | Ntrk3 | 0.0061 | 0.83153 | |
| 1426898_at | AK009321 | Mitogen-activated protein kinase kinase kinase 7 interacting protein 1 | Map3k7ip1 | 0.0099 | 0.830648 | |
| 1418642_at | BC006948 | Lymphocyte cytosolic protein 2 | Lcp2 | 0.0063 | 0.809947 | |
| 1425294_at | BC024587 | SLAM family member 8 | Slamf8 | 0.0004 | 0.782785 | |
| 1420657_at | AF053352 | Uncoupling protein 3 (mitochondrial, proton carrier) | Ucp3 | 0.0061 | 0.766881 | |
| 1420659_at | NM_030710 | SLAM family member 6 | Slamf6 | 0.0026 | 0.745503 | |
| 1422707_at | BB205102 | Phosphoinositide-3-kinase, catalytic, gamma polypeptide | Pik3cg | 0.0077 | 0.684983 | |
| 1425871_a_at | AB007986 | Similar to immunoglobulin light chain variable region | LOC384413 | 0.0094 | 0.634865 | |
| 1424931_s_at | M94350 | Immunoglobulin lambda chain, variable 1 | Igl-V1 | 0.0074 | 0.574379 | |
| 1452292_at | AV271093 | Adaptor-related protein complex 2, beta 1 subunit | Ap2b1 | Inracellular transport and cell metabolism | 0.0028 | 1.487326 |
| 1450283_at | NM_007511 | ATPase, Cu++ transporting, beta polypeptide | Atp7b | 0.0064 | 1.471984 | |
| 1434140_at | AV293368 | mcf.2 transforming sequence-like | Mcf2l | 0.0067 | 1.450361 | |
| 1455285_at | BB771765 | Solute carrier family 31, member 1 | Slc31a1 | 0.0080 | 1.393235 | |
| 1420959_at | NM_023066 | Aspartate-beta-hydroxylase | Asph | 0.0099 | 1.393208 | |
| 1449943_at | NM_008494 | Lunatic fringe gene homolog (Drosophila) | Lfng | 0.0082 | 1.381953 | |
| 1451424_at | BC027245 | Gamma-aminobutyric acid (GABA-A) receptor, pi | Gabrp | 0.0026 | 1.375588 | |
| 1426343_at | AK018758 | STT3, subunit of the oligosaccharyltransferase complex, homolog B (S. cerevisiae) | Stt3b | 0.0075 | 1.347831 | |
| 1434939_at | BB437522 | Forkhead box F1a | Foxf1a | 0.0056 | 1.3441 | |
| 1444279_at | BB531571 | HECT, UBA and WWE domain containing 1 | Huwe1 | 0.0051 | 1.33872 | |
| 1423368_at | BI695636 | Lysosomal-associated protein transmembrane 4A | Laptm4a | 0.0099 | 1.253873 | |
| 1435432_at | BE688580 | Centaurin, gamma 2 | Centg2 | 0.0094 | 1.238072 | |
| 1429325_at | BB667633 | WD repeat domain 51B | Wdr51b | 0.0093 | 1.230761 | |
| 1423981_x_at | BC006711 | Solute carrier family 25 (mitochondrial carrier, palmitoylcarnitine transporter), member 29 | Slc25a29 | 0.0007 | 1.220603 | |
| 1455711_at | AW122183 | Deltex 4 homolog (Drosophila) | Dtx4 | 0.0088 | 1.204474 | |
| 1427035_at | BB399837 | Solute carrier family 39 (zinc transporter), member 14 | Slc39a14 | 0.0018 | 1.184887 | |
| 1435553_at | AV376136 | PDZ domain containing 2 | Pdzd2 | 0.0094 | 1.172223 | |
| 1450395_at | NM_011396 | Solute carrier family 22 (organic cation transporter), member 5 | Slc22a5 | 0.0058 | 1.165831 | |
| 1436103_at | AV235634 | RAB3A interacting protein | Rab3ip | 0.0049 | 1.162922 | |
| 1443830_x_at | AV337847 | Ring finger protein 103 | Rnf103 | 0.0052 | 1.156454 | |
| 1443332_at | BB157520 | Solute carrier family 12, member 2 | Slc12a2 | 0.0040 | 0.877155 | |
| 1439367_x_at | AV148210 | ADP-ribosylation factor 4 | Arf4 | 0.0049 | 0.819864 | |
| 1448687_at | NM_026125 | C1q domain containing 2 | C1qdc2 | 0.0095 | 0.813187 | |
| 1456071_a_at | AV155488 | Cytochrome c, somatic /// similar to Cytochrome c, somatic /// similar to Cytochrome c, somatic | Cycs /// LOC670717 /// | 0.0098 | 0.777892 | |
| 1431705_a_at | AK014467 | Mucolipin 2 | Mcoln2 | 0.0055 | 0.749568 | |
| 1424967_x_at | L47552 | Troponin T2, cardiac | Tnnt2 | 0.0053 | 0.720553 | |
| 1434342_at | BB316114 | S100 protein, beta polypeptide, neural | S100b | 0.0060 | 0.702169 | |
| 1419063_at | NM_011674 | UDP galactosyltransferase 8A | Ugt8a | 0.0038 | 0.696748 | |
| 1426225_at | U63146 | Retinol binding protein 4, plasma | Rbp4 | 0.0084 | 0.648824 | |
| 1451054_at | BE628912 | Orosomucoid 1 | Orm1 | 0.0098 | 0.25717 | |
| 1426342_at | AK018758 | STT3, subunit of the oligosaccharyltransferase complex, homolog B (S. cerevisiae) | Stt3b | Metabolism | 0.0065 | 1.155948 |
| 1449417_at | NM_009664 | Ameloblastin | Ambn | 0.0069 | 0.864413 | |
| 1430889_a_at | AK002335 | Thiopurine methyltransferase | Tpmt | 0.0054 | 0.848695 | |
| 1422033_a_at | NM_053007 | Ciliary neurotrophic factor /// zinc finger protein 91 | Cntf /// Zfp91 | 0.0034 | 0.800306 | |
| 1417741_at | NM_133198 | Liver glycogen phosphorylase | Pygl | 0.0075 | 0.78752 | |
| 1460316_at | BI413218 | Acyl-CoA synthetase long-chain family member 1 | Acsl1 | 0.0048 | 0.767281 | |
| 1430584_s_at | BB213876 | Carbonic anhydrase 3 | Car3 | 0.0089 | 0.662411 | |
| 1415964_at | NM_009127 | Stearoyl-Coenzyme A desaturase 1 | Scd1 | 0.0049 | 0.595407 | |
| 1460256_at | NM_007606 | Carbonic anhydrase 3 | Car3 | 0.0048 | 0.580866 | |
| 1416487_a_at | NM_009534 | Yes-associated protein 1 | Yap1 | Transcription, DNA replication, metabolism and repair | 0.0067 | 1.379506 |
| 1421604_a_at | NM_008453 | Kruppel-like factor 3 (basic) | Klf3 | 0.0070 | 1.376898 | |
| 1418366_at | BC010564 | Histone 2, H3c1, H2aa2, etc | Hist2h3c1 ///2 | 0.0024 | 1.337869 | |
| 1428354_at | BM206907 | Forkhead box K2 | Foxk2 | 0.0068 | 1.328746 | |
| 1450333_a_at | NM_008090 | GATA binding protein 2 | Gata2 | 0.0008 | 1.316352 | |
| 1425988_a_at | AF071071 | Homeodomain interacting protein kinase 1 /// similar to homeodomain-interacting protein kinase 1 | Hipk1 /// LOC634033 | 0.0069 | 1.27636 | |
| 1426358_at | BB272466 | TAO kinase 1 | Taok1 | 0.0054 | 1.266734 | |
| 1415834_at | NM_026268 | Dual specificity phosphatase 6 | Dusp6 | 0.0031 | 1.224709 | |
| 1454785_at | BE951717 | Dual specificity phosphatase 11 (RNA/RNP complex 1-interacting) | Dusp11 | 0.0075 | 1.183246 | |
| 1420628_at | NM_008989 | Purine rich element binding protein A | Pura | 0.0092 | 1.117373 | |
| 1420811_a_at | NM_007614 | Catenin (cadherin associated protein), beta 1 | Ctnnb1 | 0.0005 | 1.114722 | |
| 1435251_at | AV377013 | Sorting nexin 13 | Snx13 | 0.0080 | 1.114005 | |
| 1452460_at | BF134412 | Ankyrin repeat domain 26 /// similar to ankyrin repeat domain 26 | Ankrd26 /// LOC669838 | 0.0002 | 0.918573 | |
| 1450576_a_at | NM_013651 | Splicing factor 3a, subunit 2 | Sf3a2 | 0.0086 | 0.842407 | |
| 1448986_x_at | NM_010062 | Deoxyribonuclease II alpha | Dnase2a | 0.0029 | 0.789776 | |
| 1432646_a_at | BE859789 | Hypothetical LOC640370 /// | LOC640370 /// | Other | 0.0007 | 1.324163 |
| 1458358_at | BB402666 | Pantothenate kinase 2 (Hallervorden-Spatz syndrome) | Pank2 | 0.0099 | 1.30126 | |
| 1422609_at | BE648432 | cAMP-regulated phosphoprotein 19 | Arpp19 | 0.0060 | 1.267996 | |
| 1437856_at | BM225636 | Inositol polyphosphate multikinase | Ipmk | 0.0078 | 1.224059 | |
| 1451584_at | AF450241 | Hepatitis A virus cellular receptor 2 | Havcr2 | 0.0075 | 1.220239 | |
| 1460580_at | BB772192 | Pecanex homolog (Drosophila) | Pcnx | 0.0079 | 1.219831 | |
| 1429044_at | AK005444 | Calmodulin regulated spectrin-associated protein 1-like 1 | Camsap1l1 | 0.0019 | 1.194988 | |
| 1424280_at | BC018329 | Motile sperm domain containing 1 | Mospd1 | 0.0050 | 1.189681 | |
| 1447624_s_at | BB174262 | Storkhead box 2 | Stox2 | 0.0059 | 1.170559 | |
| 1417965_at | NM_133942 | Pleckstrin homology domain containing, family A (phosphoinositide binding specific) member 1 | Plekha1 | 0.0085 | 1.155582 | |
| 1435096_at | BB667093 | Resistance to inhibitors of cholinesterase 8 homolog B (C. elegans) | Ric8b | 0.0027 | 1.155356 | |
| 1436215_at | BB081797 | Inositol polyphosphate multikinase | Ipmk | 0.0091 | 1.133207 | |
| 1428197_at | AK020159 | Tetraspanin 9 | Tspan9 | 0.0084 | 1.107396 | |
| 1427773_a_at | L40934 | Rab acceptor 1 (prenylated) | Rabac1 | 0.0035 | 1.079644 | |
| 1458308_at | BF148215 | Strawberry notch homolog (Drosophila) | Stno | 0.0076 | 0.882994 | |
| 1442786_s_at | BB461022 | RUN and FYVE domain containing 3 | Rufy3 | 0.0021 | 0.850871 | |
| 1455927_x_at | AV216677 | Similar to non-SMC element 1 homolog /// similar to non-SMC element 1 homolog | LOC623809 /// LOC677159 | 0.0047 | 0.81128 | |
| 1417668_at | NM_130892 | Reticulon 4 interacting protein 1 | Rtn4ip1 | 0.0045 | 0.803591 |
Genes significantly different in the group Norm TiO2 versus Norm PBS with exclusion of those genes significantly different in Preg TiO2 versus Preg PBS as well as of genes significantly changed by pregnancy. See heatmap in the online supplement, right.
Enhanced Response in Pregnancy Leads to Increased Allergic Susceptibility in Offspring
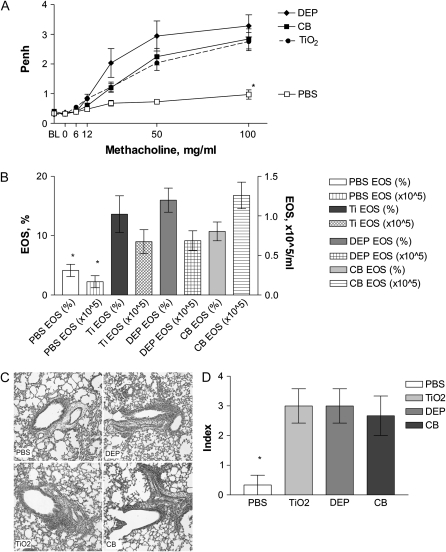
We investigated whether pregnancy-enhanced response to particles could influence the allergic susceptibility of the offspring. Offspring of mice exposed to DEP during pregnancy (Figure 1, Protocol 1B) showed increased AHR (Figure 5A) and allergic airway inflammation (AI) (Figures 5B–5D) compared with offspring of vehicle (PBS)-treated mice, indicating increased allergic susceptibility. We also analyzed offspring from pregnant mice treated with “inert” TiO2 and CB particles. These offspring also showed increased susceptibility to allergy, manifesting as increased AHR and AI (Figure 5).
Figure 5.
Neonatal susceptibility in OVA protocol. Mother mice were exposed during pregnancy to 50 μg/mouse of either DEP or TiO2, or 250 μg/mouse of CB particle suspension or PBS (Protocol 1B, Figure 1). Newborns were injected once with 0.1 ml of 50 μg/ml OVA plus alum and challenged three times with 3% OVA aerosol. Offspring of mice exposed during pregnancy to DEP showed increased airway hyperresponsiveness (AHR) seen as response to methacholine via whole-body plethysmography (Penh at 100 mg/ml Mch of 3.3±0.4) (A) and increased eosinophilic AI in BAL (B) as well as increased pulmonary infiltration (C, D), indicating that allergic susceptibility was induced. Surprisingly, neonates from mice exposed to “inert” TiO2 and CB also showed similarly increased AHR (Penh at 100 mg/ml Mch of 2.8 ± 0.3 and 2.8 ± 0.3, respectively, versus 1.0 ± 0.2 in PBS controls, P < 0.05) (A). BAL eosinophilia was also increased (TiO2 13.6 ± 3.1% and CB 10.7 ± 1.2% versus 4.1 ± 1.0% in PBS controls) (B), as well as pulmonary inflammation (C, D). Mean ± SEM (n = 17–21 each group). *P < 0.05.
DISCUSSION
Our findings indicate that in pregnancy both local and systemic inflammatory responses to immunologically “inert” environmental particles are enhanced compared to the normal nonpregnant state. This phenomenon is associated with differential activation of multiple genes involved in immune response and regulation, cell metabolism and proliferation. An important biological effect is increased allergic susceptibility in offspring of mothers exposed during pregnancy.
TiO2 (and CB) particles are a prototypical “inert” particle in pulmonary toxicology studies because of the minimal inflammatory response usually seen in vivo in animal models; they do not have soluble components. However, they are not completely innocuous. For example, specially coated TiO2 particles were shown to cause pulmonary inflammation (31). Moreover, TiO2 particles were shown to cause pulmonary inflammation with activation of antigen-presenting cells and production of certain chemokines (32, 33). They were also associated with increased production of IL-13 by mast cells (34) and, potentially germane to our study, were shown to cause increase IL-25 and IL-13 production by lung antigen-presenting cells (35). Similarly, there are a few studies showing that another generally “inert” particle type, CB particles may have also minor immune system effects (36).
Specific information on the subject of exposure to particles during pregnancy remains scarce. However, previous observations include findings that pulmonary immune response to certain environmental factors (e.g., ozone) (37, 38) can be enhanced in the already Th2-deviated milieu of pregnancy (39, 40). We compared the local pulmonary response of pregnant versus normal females to TiO2 particles. Normal nonpregnant females showed minimal residual inflammation 48 hours after particle treatment, the expected finding with “inert” particles. In contrast, at the 48-hour analysis point, pregnant mice reveal persistence of enhanced inflammation, a finding not seen in nonpregnant females (Figure 2). These data indicate that “inert” particles are no longer innocuous and noninflammatory in the setting of pregnancy. At the same time exposure to nonparticulate inflammatory agent LPS did not cause enhanced responses in pregnancy as compared with nonpregnant mice (Figure 3), indicating that not all inflammatory responses are altered in pregnancy in our model. We are aware of a discordant finding of enhanced inflammation after a higher dose of LPS in pregnant rats (37), therefore this issue requires further study.
We speculate that several factors may be involved in the mechanism, including alteration of innate and adaptive immune responses under the influence of estrogen and progesterone, the essential hormones of pregnancy that are produced in increasing concentrations (41–43). These hormones induce a pro-Th2 skewing of immunity (as reviewed in Ref. 44). More interestingly, it was shown that estrogens and progesterone can alter function of macrophages (45, 46) and regulate macrophage cytokine production (47, 48). Similar data applies to DCs located in the reproductive organs (49, 50), and recent reports suggest that DCs have estrogen receptors and respond to estrogen stimulation (51). Other possible mechanisms include alteration of the placental milieu in an inflamed organism towards production of Th2-skewing products (52).
The postulate that innate immune responses to “inert” particles are altered predicts selective activation or deactivation of gene transcription. Indeed, genomic profiling of total lung RNA from normal and pregnant females exposed to either TiO2 particles or PBS control identified several clusters of genes that may potentially be involved in the mechanism. We initially used a more stringent SAM analysis across all four groups and identified 130 sequences (mostly involved in inflammatory response and immune regulation, cell proliferation, DNA metabolism, and metabolic processes) to be differentially expressed (see online supplement). We then applied a more selective approach using less stringent ANOVA-based analysis to identify genes that are only changed in pregnant mice upon exposure to TiO2, as well as those that are only changed in normal mice upon TiO2 exposure. While these gene lists somewhat overlapped, after mutual subtraction we identified two separate gene sets (see online supplement), which indicates that possibly different genes are responsible for lung TiO2 response in pregnant and in normal mice. Further investigation including PCR validation and mechanistic studies is underway.
We found that newborns from DEP-exposed mothers had significantly higher AHR and AI (Figure 2) than PBS controls. Moreover, the offspring of TiO2- and CB particle–exposed mothers (Figure 2) also showed increased susceptibility, an unexpected finding that was replicated in four separate experiments. It has been concurrently shown using the same model that maternal exposure to residual oil fly ash (ROFA) increases offspring susceptibility (22). Here, we demonstrate that maternal exposure to particles considered immunologically innocuous, TiO2 and CB, can also cause increased allergic susceptibility in offspring. This finding identifies a functionally important consequence of the differential response to particles in pregnancy, and this may ultimately help identify mechanisms of the phenomenon. The data suggest that exposures of nonallergic pregnant females to environmental air particles under some conditions may cause increased allergic susceptibility in offspring.
The mechanisms by which pregnancy exposure caused increased susceptibility to allergy in offspring remain unknown. One possibility is suggested by previous findings that components of DEP can mediate pro-allergic effects. The organic components, especially polycyclic aromatic hydrocarbons (PAH), cause increased production of Th2 cytokines (e.g., IL-4), known to be important mediators of allergy and asthma (14–16). Studies found that pyrene, an abundant component in DEP, has caused specific and robust induction of IL-4 gene expression by T cells, but only as a co-factor in the presence of antigen (30). However, we sought but did not find evidence of Th2 cytokines in the lavage fluids and serum samples from DEP-treated pregnant mice (no detectable IL-4, -5, or -13; data not shown). Rather, multiplexed cytokine analysis of serum show that pregnant mice exposed to either DEP or TiO2 had elevated levels of IL-1β, TNF-α, IL-6, and KC levels 48 hours after exposure, as opposed to nonpregnant controls (Figure 4). These findings are consistent with the greater acute cellular inflammation observed in BAL samples, including after treatment with the “inert” particle TiO2. The discordance between similar levels of PMNs in the normal DEP versus pregnant DEP control groups and different levels of cytokines in these groups may be caused by “saturation” of bronchoalveolar inflammation locally but not systemically. Further studies are necessary to address this issue in more detail.
Some limitations of our study merit discussion. First, we used a single bolus dose of particles via intranasal insufflation of pregnant mice. While this strategy provides proof-of-principle, additional studies using aerosol exposures and dose–response analysis would allow more realistic comparison to actual human exposures. Second, the study uses one strain of mice (BALB/c). Additional studies are needed to determine if similar findings occur in other mouse strains. Finally, in our mouse model, we use noninvasive plethysmography to evaluate pulmonary function in very young mice (15 days old). We are aware of the ongoing discussion in the literature about whether Penh measurement via whole-body plethysmography truly represents AHR and whether it is a valid technique for different strains of laboratory animals (23). However, it is worth noting that analysis of responses to aerosolized OVA in sensitized, BALB/c strain mice (i.e., as in our model) is the experimental setting in which Penh values correlate best and to an (arguably) acceptable degree with more invasive measures (24–29). We also point out that the more invasive testing is technically impractical, given the small size of young mice in our model. Finally, in an earlier study we were able to find similar trends in Penh and basal pulmonary function tests using the invasive Flexivent approach in older, larger mice studied in a similar protocol (8).
In conclusion, we have developed a mouse model for analysis of environmental exposures during pregnancy and their effect on susceptibility of offspring to allergy. We showed using this model that maternal exposure to TiO2 and CB particles, previously considered immunologically “inert,” causes enhanced immune response in pregnancy and, similarly to DEP exposure results in increased allergic susceptibility in offspring. This model may be useful for toxicology screening and for further mechanistic analysis.
Supplementary Material
This work was supported by NIH HL69760 (to L.K.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2007-0124OC on July 26, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.von Mutius E, Schmid S, the PASTURE Study Group. The PASTURE project: EU support for the improvement of knowledge about risk factors and preventive factors for atopy in Europe. Allergy 2006;61:407–413. [DOI] [PubMed] [Google Scholar]
- 2.von Mutius E. The burden of childhood asthma. Arch Dis Child 2000;82:II2–II5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wills-Karp M. Murine models of asthma in understanding immune dysregulation in human asthma. Immunopharmacology 2000;48:263–268. [DOI] [PubMed] [Google Scholar]
- 4.Weiss ST. Epidemiology and heterogeneity of asthma. Annals of Allergy, Asthma, &. Immunology 2001;87:5–8. [DOI] [PubMed] [Google Scholar]
- 5.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med 1995;332:133–138. (see comments). [DOI] [PubMed] [Google Scholar]
- 6.Prescott SL. Maternal allergen exposure as a risk factor for childhood asthma. Curr Allergy Asthma Rep 2006;6:75–80. [DOI] [PubMed] [Google Scholar]
- 7.Hamada K, Suzaki Y, Goldman A, Ning YY, Goldsmith C, Palecanda A, Coull B, Hubeau C, Kobzik L. Allergen-independent maternal transmission of asthma susceptibility. J Immunol 2003;170:1683–1689. [DOI] [PubMed] [Google Scholar]
- 8.Fedulov A, Silverman E, Xiang Y, Leme A, Kobzik L. Immunostimulatory CpG oligonucleotides abrogate allergic susceptibility in a murine model of maternal asthma transmission. J Immunol 2005;175:4292–4300. [DOI] [PubMed] [Google Scholar]
- 9.Leme AS, Hubeau C, Xiang Y, Goldman A, Hamada K, Suzaki Y, Kobzik L. Role of breast milk in a mouse model of maternal transmission of asthma susceptibility. J Immunol 2006;176:762–769. [DOI] [PubMed] [Google Scholar]
- 10.Goldsmith C, Kobzik L. Particulate air pollution and asthma: a review of the epidemiological and biological studies. Rev Environ Health 1999;14:121–134. [DOI] [PubMed] [Google Scholar]
- 11.von Mutius E, Martinez FD, Fritzsch C, Nicolai T, Roell G, Thiemann HH. Prevalence of asthma and atopy in two areas of West and East Germany. Am J Respir Crit Care Med 1994;149:358–364. [DOI] [PubMed] [Google Scholar]
- 12.Venn AJ, Lewis SA, Cooper M, Hubbard R, Britton J. Living near a main road and the risk of wheezing illness in children. Am J Respir Crit Care Med 2001;164:2177–2180. [DOI] [PubMed] [Google Scholar]
- 13.Miyake Y, Yura A, Iki M. Relationship between distance from major roads and adolescent health in Japan. J Epidemiol 2002;12:418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takano H, Ichinose T, Miyabara Y, Yoshikawa T, Sagai M. Diesel exhaust particles enhance airway responsiveness following allergen exposure in mice. Immunopharmacol Immunotoxicol 1998;20:329–336. [DOI] [PubMed] [Google Scholar]
- 15.Miyabara Y, Takano H, Ichinose T, Lim HB, Sagai M. Diesel exhaust enhances allergic airway inflammation and hyperresponsiveness in mice. Am J Respir Crit Care Med 1998;157:1138–1144. [DOI] [PubMed] [Google Scholar]
- 16.Diaz-Sanchez D, Tsien A, Fleming J, Saxon A. Combined diesel exhaust particulate and ragweed allergen challenge markedly enchance human in vivo nasal ragweed-specific IgE and skews cytokine production to a T-helper cell 2-type pattern. J Immunol 1997;158:2406–2413. [PubMed] [Google Scholar]
- 17.Fujimaki H, Saneyoshi K, Shiraishi F, Imai T, Endo T. Inhalation of diesel exhaust enhances antigen-specific IgE antibody production in mice. Toxicology 1997;116:227–233. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki T, Kanoh T, Kanbayashi M, Todome Y, Ohkuni H. The adjuvant activity of pyrene in diesel exhaust on IgE antibody production in mice. Arerugi 1993;42:963–968. [PubMed] [Google Scholar]
- 19.Pauwels RA, Brusselle GG, Tournoy KG, Lambrecht BN, Kips JC. Cytokines and their receptors as therapeutic targets in asthma. Clin Exp Allergy 1998;28:1–5. [PubMed] [Google Scholar]
- 20.Cohn L, Herrick C, Niu N, Homer R, Bottomly K. IL-4 promotes airway eosinophilia by suppressing IFN-gamma production: defining a novel role for IFN-gamma in the regulation of allergic airway inflammation. J Immunol 2001;166:2760–2767. [DOI] [PubMed] [Google Scholar]
- 21.Southam DS, Dolovich M, O'Byrne PM, Inman M. D. Distribution of intranasal instillations in mice: effects of volume, time, body position, and anesthesia. Am J Physiol Lung Cell Mol Physiol 2002;282:L833–L839. [DOI] [PubMed] [Google Scholar]
- 22.Hamada K, Suzaki Y, Leme A, Ito T, Myamoto K, Kobzik L, Kimura H. Exposure of pregnant mice to an air pollutant aerosol increases asthma susceptibility in offspring. J Toxicol Environ Health 2006; (In Press). [DOI] [PubMed]
- 23.Adler A, Cieslewicz G, Irvin CG. Unrestrained plethysmography is an unreliable measure of airway responsiveness in BALB/c and C57BL/6 mice. J Appl Physiol 2004;97:286–292. [DOI] [PubMed] [Google Scholar]
- 24.Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen G, Irvin C, Gelfand E. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med 1997;156:766–775. [DOI] [PubMed] [Google Scholar]
- 25.Lutchen KR, Yang K, Kaczka DW, Suki B. Optimal ventilation waveforms for estimating low-frequency respiratory impedance. J Appl Physiol 1993;75:478–488. [DOI] [PubMed] [Google Scholar]
- 26.Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 1992;72:168–178. [DOI] [PubMed] [Google Scholar]
- 27.Lomax RG. Statistical concepts: a second course for education and the behavioral sciences, 2nd ed. Mahwah, NJ: Lawrence Erlbaum Associates; 2001.
- 28.Dohi M, Tsukamoto S, Nagahori T, Shinagawa K, Saitoh K, Tanaka Y, Kobayashi S, Tanaka R, To Y, Yamamoto K. Noninvasive system for evaluating the allergen-specific airway response in a murine model of asthma. Lab Invest 1999;79:1559–1571. [PubMed] [Google Scholar]
- 29.Gomes R, Shen X, Ramchandani R, Tepper RS, Bates JHT. Comparative respiratory system mechanics in rodents. J Appl Physiol 2000;89:908–916. [DOI] [PubMed] [Google Scholar]
- 30.Bommel H, Li-Weber M, Serfling E, Duschl A. The environmental pollutant pyrene induces the production of IL-4. J Allergy Clin Immunol 2000;105:796–802. [DOI] [PubMed] [Google Scholar]
- 31.Warheit DB, Brock WJ, Lee KP, Webb TR, Reed KL. Comparative pulmonary toxicity inhalation and instillation studies with different TiO2 particle formulations: impact of surface treatments on particle toxicity. Toxicol Sci 2005;88:514–524. [DOI] [PubMed] [Google Scholar]
- 32.Renwick LC, Brown D, Clouter A, Donaldson K. Increased inflammation and altered macrophage chemotactic responses caused by two ultrafine particle types. Occup Environ Med 2004;61:442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drumm K, Schindler H, Buhl R, Kustner E, Smolarski R, Kienast K. Indoor air pollutants stimulate interleukin-8-specific mRNA expression and protein secretion of alveolar macrophages. Lung 1999;177:9–19. [DOI] [PubMed] [Google Scholar]
- 34.Ahn MH, Kang CM, Park CS, Park SJ, Rhim T, Yoon PO, Chang HS, Kim SH, Kyono H, Kim KC. Titanium dioxide particle-induced goblet cell hyperplasia: association with mast cells and IL-13. Respir Res 2005;6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang CM, Jang AS, Ahn MH, Shin JA, Kim JH, Choi YS, Rhim TY, Park CS. Interleukin-25 and interleukin-13 production by alveolar macrophages in response to particles. Am J Respir Cell Mol Biol 2005;33:290–296. [DOI] [PubMed] [Google Scholar]
- 36.van Zijverden M, van der Pijl A, Bol M, van Pinxteren FA, de Haar C, Penninks AH, van Loveren H, Pieters R. Diesel exhaust, carbon black, and silica particles display distinct Th1/Th2 modulating activity. Toxicol Appl Pharmacol 2000;168:131–139. [DOI] [PubMed] [Google Scholar]
- 37.Huffman LJ, Frazer DG, Prugh D, Brumbaugh K, Platania C, Reynolds JS, Goldsmith WT. Enhanced pulmonary inflammatory response to inhaled endotoxin in pregnant rats. J Toxicol Environ Health A 2004;67:125–144. [DOI] [PubMed] [Google Scholar]
- 38.Gunnison AF, Weideman PA, Sobo M. Enhanced inflammatory response to acute ozone exposure in rats during pregnancy and lactation. Fundam Appl Toxicol 1992;19:607–612. [DOI] [PubMed] [Google Scholar]
- 39.Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol 1993;151:4562–4573. [PubMed] [Google Scholar]
- 40.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today 1993;14:353–356. (see comments). [DOI] [PubMed] [Google Scholar]
- 41.Albrecht ED, Aberdeen GW, Pepe GJ. The role of estrogen in the maintenance of primate pregnancy. Am J Obstet Gynecol 2000;182:432–438. [DOI] [PubMed] [Google Scholar]
- 42.Cahill M. Handbook of diagnostic tests. Springhouse, PA: Springhouse Corporation; 1995.
- 43.Arredondo F, Noble LS. Endocrinology of recurrent pregnancy loss. Semin Reprod Med 2006;24:33–39. [DOI] [PubMed] [Google Scholar]
- 44.Piccinni MP, Scaletti C, Maggi E, Romagnani S. Role of hormone-controlled Th1- and Th2-type cytokines in successful pregnancy. J Neuroimmunol 2000;109:30–33. [DOI] [PubMed] [Google Scholar]
- 45.Chao TC, Phuangsab A, Van Alten PJ, Walter RJ. Steroid sex hormones and macrophage function: regulation of chemiluminescence and phagocytosis. Am J Reprod Immunol 1996;35:106–113. [DOI] [PubMed] [Google Scholar]
- 46.Chao TC, Van Alten PJ, Walter RJ. Steroid sex hormones and macrophage function: modulation of reactive oxygen intermediates and nitrite release. Am J Reprod Immunol 1994;32:43–52. [DOI] [PubMed] [Google Scholar]
- 47.Chao TC, Van Alten PJ, Greager JA, Walter RJ. Steroid sex hormones regulate the release of tumor necrosis factor by macrophages. Cell Immunol 1995;160:43–49. [DOI] [PubMed] [Google Scholar]
- 48.Chao TC, Chao HH, Chen MF, Greager JA, Walter RJ. Female sex hormones modulate the function of LPS-treated macrophages. Am J Reprod Immunol 2000;44:310–318. [DOI] [PubMed] [Google Scholar]
- 49.Wieser F, Hosmann J, Tschugguel W, Czerwenka K, Sedivy R, Huber JC. Progesterone increases the number of Langerhans cells in human vaginal epithelium. Fertil Steril 2001;75:1234–1235. [DOI] [PubMed] [Google Scholar]
- 50.Ivanova E, Kyurkchiev D, Altankova I, Dimitrov J, Binakova E, Kyurkchiev S. CD83 monocyte-derived dendritic cells are present in human decidua and progesterone induces their differentiation in vitro. Am J Reprod Immunol 2005;53:199–205. [DOI] [PubMed] [Google Scholar]
- 51.Mao A, Paharkova-Vatchkova V, Hardy J, Miller MM, Kovats S. Estrogen selectively promotes the differentiation of dendritic cells with characteristics of Langerhans cells. J Immunol 2005;175:5146–5151. [DOI] [PubMed] [Google Scholar]
- 52.Hubeau C, Apostolou I, Kobzik L. Adoptively transferred allergen-specific T cells cause maternal transmission of asthma risk. Am J Pathol 2006;168:1931–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baron RM, Carvajal IM, Fredenburgh LE, Liu X, Porrata Y, Cullivan ML, Haley KJ, Sonna LA, De Sanctis GT, Ingenito EP, et al. Nitric oxide synthase-2 down-regulates surfactant protein-B expression and enhances endotoxin-induced lung injury in mice. FASEB J 2004;18:1276–1278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.