Abstract
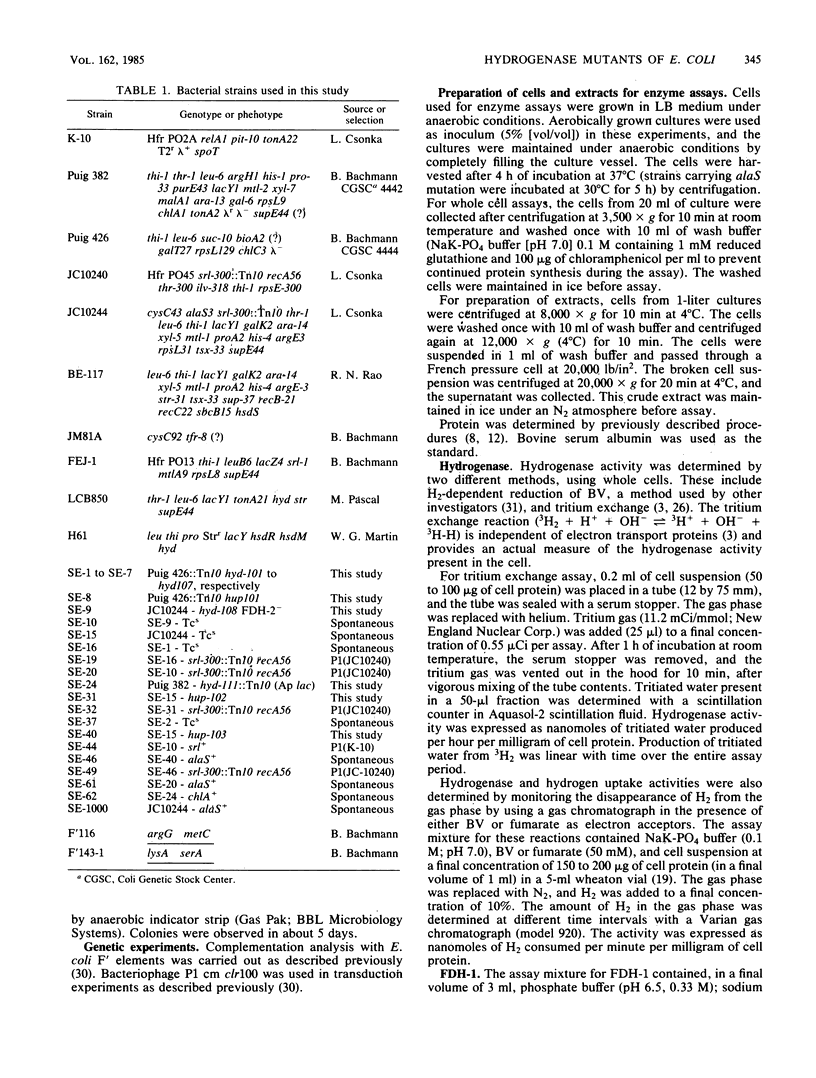
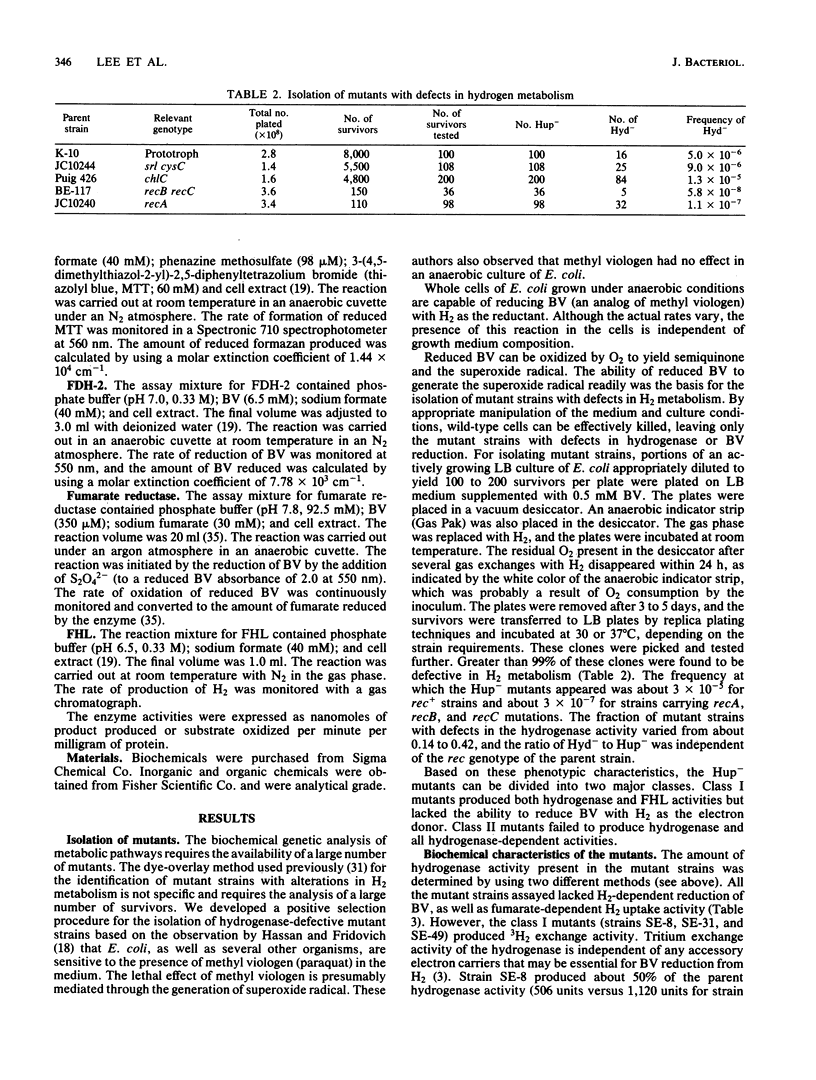
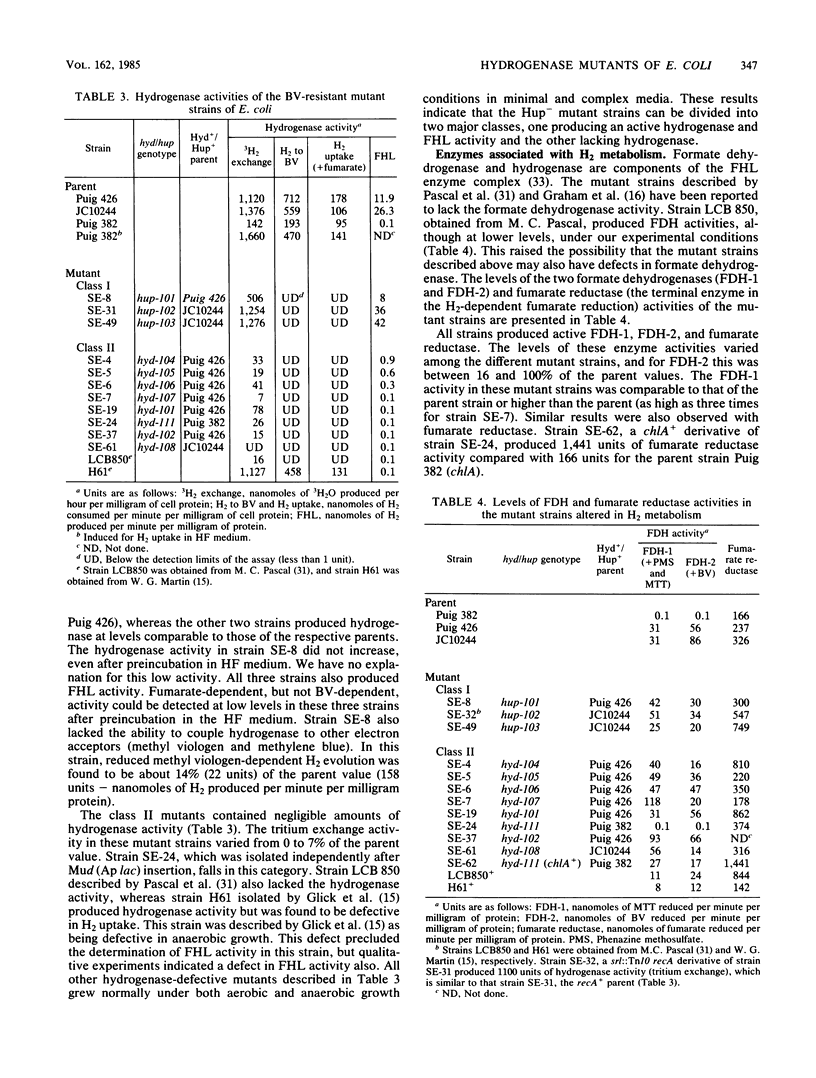
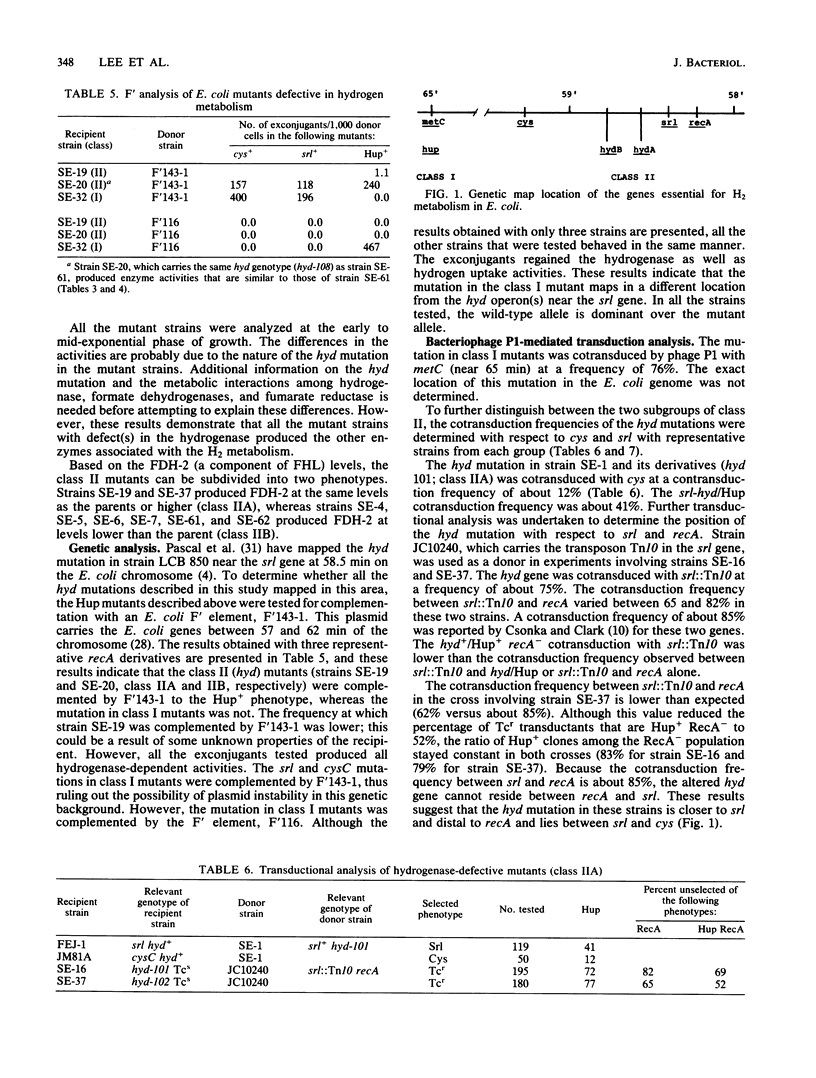
A positive selection procedure is described for the isolation of hydrogenase-defective mutant strains of Escherichia coli. Mutant strains isolated by this procedure can be divided into two major classes. Class I mutants produced hydrogenase activity (determined by using a tritium-exchange assay) and formate hydrogenlyase activity but lacked the ability to reduce benzyl viologen or fumarate with H2 as the electron donor. Class II mutants failed to produce active hydrogenase and hydrogenase-dependent activities. All the mutant strains produced detectable levels of formate dehydrogenase-1 and -2 and fumarate reductase. The mutation in class I mutants mapped near 65 min of the E. coli chromosome, whereas the mutation in class II mutants mapped between srl and cys operons (58 and 59 min, respectively) in the genome. The class II Hyd mutants can be further subdivided into two groups (hydA and hydB) based on the cotransduction characteristics with cys and srl. These results indicate that there are two hyd operons and one hup operon in the E. coli chromosome. The two hyd operons are needed for the production of active hydrogenase, and all three are essential for hydrogen-dependent growth of the cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. W., Hall D. O. Purification of the membrane-bound hydrogenase of Escherichia coli. Biochem J. 1979 Oct 1;183(1):11–22. doi: 10.1042/bj1830011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand S. R., Krasna A. I. Catalysis of the H2-HTO exchange by hydrogenase. A new assay for hydrogenase. Biochemistry. 1965 Dec;4(12):2747–2753. doi: 10.1021/bi00888a027. [DOI] [PubMed] [Google Scholar]
- Ananthaswamy H. N., Eisenstark A. Repair of hydrogen peroxide-induced single-strand breaks in Escherichia coli deoxyribonucleic acid. J Bacteriol. 1977 Apr;130(1):187–191. doi: 10.1128/jb.130.1.187-191.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. L., Kwan H. S., Macy J. Anaerobiosis, formate, nitrate, and pyrA are involved in the regulation of formate hydrogenlyase in Salmonella typhimurium. J Bacteriol. 1984 Jun;158(3):972–977. doi: 10.1128/jb.158.3.972-977.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard T., Gottschalk G. Cell yields of Escherichia coli during anaerobic growth on fumarate and molecular hydrogen. Arch Microbiol. 1978 Mar;116(3):235–238. doi: 10.1007/BF00417845. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Csonka L. N., Clark A. J. Deletions generated by the transposon Tn10 in the srl recA region of the Escherichia coli K-12 chromosome. Genetics. 1979 Oct;93(2):321–343. doi: 10.1093/genetics/93.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Halbrook J. Inducible repair of oxidative DNA damage in Escherichia coli. Nature. 1983 Aug 4;304(5925):466–468. doi: 10.1038/304466a0. [DOI] [PubMed] [Google Scholar]
- GEST H., GIBBS M. Preparation and properties of cell-free "formic hydrogenlyase" from escherichia coli. J Bacteriol. 1952 May;63(5):661–664. doi: 10.1128/jb.63.5.661-664.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B. R., Wang P. Y., Schneider H., Martin W. G. Identification and partial characterization of an Escherichia coli mutant with altered hydrogenase activity. Can J Biochem. 1980 Apr;58(4):361–367. doi: 10.1139/o80-047. [DOI] [PubMed] [Google Scholar]
- Graham A., Boxer D. H., Haddock B. A., Mandrand-Berthelot A. M., Jones R. W. Immunochemical analysis of the membrane-bound hydrogenase of Escherichia coli. FEBS Lett. 1980 May 5;113(2):167–172. doi: 10.1016/0014-5793(80)80584-9. [DOI] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Superoxide radical and the oxygen enhancement of the toxicity of paraquat in Escherichia coli. J Biol Chem. 1978 Nov 25;253(22):8143–8148. [PubMed] [Google Scholar]
- Hom S. S., Hennecke H., Shanmugam K. T. Regulation of nitrogenase biosynthesis in Klebsiella pneumoniae: effect of nitrate. J Gen Microbiol. 1980 Mar;117(1):169–179. doi: 10.1099/00221287-117-1-169. [DOI] [PubMed] [Google Scholar]
- Jones R. W. The role of the membrane-bound hydrogenase in the energy-conserving oxidation of molecular hydrogen by Escherichia coli. Biochem J. 1980 May 15;188(2):345–350. doi: 10.1042/bj1880345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karube I., Urano N., Yamada T., Hirochika H., Sakaguchi K. Cloning and expression of the hydrogenase gene from Clostridium butyricum in Escherichia coli. FEBS Lett. 1983 Jul 11;158(1):119–122. doi: 10.1016/0014-5793(83)80689-9. [DOI] [PubMed] [Google Scholar]
- Kong S., Davison A. J. The role of interactions between O2, H2O2, .OH,e- and O2- in free radical damage to biological systems. Arch Biochem Biophys. 1980 Oct 1;204(1):18–29. doi: 10.1016/0003-9861(80)90003-x. [DOI] [PubMed] [Google Scholar]
- Krasna A. I. Mutants of Escherichia coli with altered hydrogenase activity. J Gen Microbiol. 1984 Apr;130(4):779–787. doi: 10.1099/00221287-130-4-779. [DOI] [PubMed] [Google Scholar]
- Krasna A. I. Regulation of hydrogenase activity in enterobacteria. J Bacteriol. 1980 Dec;144(3):1094–1097. doi: 10.1128/jb.144.3.1094-1097.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. T. Determination of Hydrogenase in Free-living Cultures of Rhizobium japonicum and Energy Efficiency of Soybean Nodules. Plant Physiol. 1978 Oct;62(4):609–611. doi: 10.1104/pp.62.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen P. C. Isolation of catalase-deficient Escherichia coli mutants and genetic mapping of katE, a locus that affects catalase activity. J Bacteriol. 1984 Feb;157(2):622–626. doi: 10.1128/jb.157.2.622-626.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macy J., Kulla H., Gottschalk G. H2-dependent anaerobic growth of Escherichia coli on L-malate: succinate formation. J Bacteriol. 1976 Feb;125(2):423–428. doi: 10.1128/jb.125.2.423-428.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PECK H. D., Jr, GEST H. Formic dehydrogenase and the hydrogenlyase enzyme complex in coli-aerogenes bacteria. J Bacteriol. 1957 Jun;73(6):706–721. doi: 10.1128/jb.73.6.706-721.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascal M. C., Casse F., Chippaux M., Lepelletier M. Genetic analysis of mutants of Escherichia coli K12 and Salmonella typhimurium LT2 deficient in hydrogenase activity. Mol Gen Genet. 1975 Nov 24;141(2):173–179. doi: 10.1007/BF00267682. [DOI] [PubMed] [Google Scholar]
- Pecher A., Zinoni F., Jatisatienr C., Wirth R., Hennecke H., Böck A. On the redox control of synthesis of anaerobically induced enzymes in enterobacteriaceae. Arch Microbiol. 1983 Nov;136(2):131–136. doi: 10.1007/BF00404787. [DOI] [PubMed] [Google Scholar]
- Spencer M. E., Guest J. R. Isolation and properties of fumarate reductase mutants of Escherichia coli. J Bacteriol. 1973 May;114(2):563–570. doi: 10.1128/jb.114.2.563-570.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson M., Stickland L. H. Hydrogenase: a bacterial enzyme activating molecular hydrogen: The properties of the enzyme. Biochem J. 1931;25(1):205–214. doi: 10.1042/bj0250205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait R. C., Andersen K., Cangelosi G., Shanmugam K. T. Hydrogenase genes. Basic Life Sci. 1981;18:279–303. doi: 10.1007/978-1-4684-3980-9_17. [DOI] [PubMed] [Google Scholar]
- Uhlin B. E., Clark A. J. Overproduction of the Escherichia coli recA protein without stimulation of its proteolytic activity. J Bacteriol. 1981 Oct;148(1):386–390. doi: 10.1128/jb.148.1.386-390.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


