Abstract
Anaplasma phagocytophilum has long been known to cause tick-borne fever in ruminants and has been identified more recently as the causative agent of the emerging disease human granulocytic anaplasmosis. The related organism Anaplasma marginale uses gene conversion of the expression site for two major outer membrane proteins (OMPs) to generate extensive sequence and antigenic variation in these OMPs. This is thought to present a continuously varying repertoire of epitopes to the mammalian host and allow disease persistence. Recent genomic and structural data on human strains of A. phagocytophilum, together with animal studies in model systems, have implicated an orthologous OMP of A. phagocytophilum in a similar mechanism of variation. However, to date there has been little investigation of the mechanisms of antigenic variation or disease persistence in hosts naturally infected with field strains of A. phagocytophilum. Approximately 300,000 lambs in Norway suffer severe disease caused by A. phagocytophilum annually. We show here the persistent and cyclic nature of infection in these animals that is accompanied by loosely programmed sequence variation of the major OMP expression site in each rickettsemic peak. These data will allow analysis of interactions between A. phagocytophilum and the host immune system in naturally occurring persistent infections and provide an important comparison with enduring infections of cattle caused by A. marginale.
Anaplasma phagocytophilum (formerly Ehrlichia phagocytophila) is an obligate intracellular rickettsia that primarily infects the polymorphonuclear fraction of the white blood cells (12, 13). It causes tick-borne fever in ruminants and is known as the causative agent of the emerging tick-borne zoonosis human granulocytic anaplasmosis (10, 12, 32). The disease in ruminants has been recorded for at least 200 years in Norway and has been estimated to cause disease in 300,000 lambs annually (31). The disease in experimentally infected sheep is characterized by an incubation period of 4 to 8 days, followed by an early febrile reaction of often >41°C for around 1 week. The main problems with the disease are immune suppression and the appearance of secondary infections such as pneumonia, septicemia, and arthritis. In the early phase of the infection, A. phagocytophilum can be detected as cytoplasmic inclusions in phagocytes, especially neutrophils, by blood smear microscopy (34, 35).
Tick-borne Anaplasma and Ehrlichia species have the ability to cause persistent infections that may last for many years (1, 2, 20, 21). Antigenic variation of two major outer membrane proteins (OMPs), MSP2 and MSP3, is believed to be responsible for the persistence of Anaplasma marginale (2, 7, 8, 28). The phagocytophilic anaplasma is closely related to A. marginale in both genetic structure and infection pattern. They also have in common an orthologous OMP known as MSP2(P44) (4). A recent study revealed global diversity in MSP2(P44) variants of A. phagocytophilum in samples collected from geographically distinct locations (3) and identified a syntenic expression site for msp2(p44) present in all A. phagocytophilum strains. The genome of A. phagocytophilum consists of >100 msp2(p44) pseudogenes dispersed along a single chromosome (18). These pseudogenes are believed to be inserted into the msp2(p44) expression site by gene combinatorial conversion mechanisms (4). It has also previously been suggested that segmental gene conversion of the genomic expression site by partial sequences of truncated pseudogenes occurs in A. phagocytophilum, thereby creating a mosaic or chimera, as is the case with antigenic variation of MSP2 in A. marginale (3, 9). This suggestion is further discussed in the work presented here.
In this paper, we present data showing that cycling of A. phagocytophilum in blood from experimentally infected sheep occurs concurrently with sequence variation in the msp2(p44) expression site. By performing extensive cloning of the msp2(p44) expression site, we found evidence that sequence variation occurs in a loosely programmed fashion during persistent infection of experimentally infected lambs.
MATERIALS AND METHODS
Experimental infection of naive lambs and blood sampling.
Four lambs were raised in an indoor environment with barriers against tick entry and tick infestation. Two lambs, 4203 and 4210, were experimentally infected with a Norwegian 16S rRNA variant (GenBank accession no. M73220) of A. phagocytophilum. The inoculum had been stored in 10% dimethyl sulfoxide at −80°C. Two lambs were kept as negative controls throughout the sampling period of 3 months. All four lambs were examined and found negative for Mycoplasma ovis (formerly Eperythrozoon ovis) by blood smear analysis before inoculation. Rectal temperature and clinical status were recorded daily throughout the experimental period. EDTA blood samples were collected from the jugular vein. Samples were taken on day 0 of infection and every second day for a 3-month period. One EDTA blood sample from each animal was used for blood smear analysis and differential blood cell counts (ADVIA; Bayer). Another blood sample was frozen at −75°C for later PCR analysis. The blood smears were stained with May-Grünwald Giemsa for visual confirmation and quantitative assessment of infection under an oil immersion microscope (32). Serum samples were collected on the day of infection and then weekly during the whole experimental period for indirect fluorescent-antibody assay and immunoblotting (data not presented).
Real-time PCR for relative quantification and identification of positive samples, targeting the msp2(p44) gene.
DNA from EDTA blood was isolated with a QIAamp Mini kit (QIAGEN). Detection and selection of samples positive by PCR for further cloning and restriction fragment length polymorphism (RFLP) analyses was performed by real-time PCR (LightCycler 2.0; Roche). For relative quantification of rickettsemia in our samples, Fast Start MASTERPLUS SYBR green I Taq polymerase mix was used with fluorescence detection. The DNA template volume was 2.0 μl and contained 17 ng of total DNA.
Primers Apmsp2f (5′-ATGGAA GGT AGT GTT GGT TAT GGT ATT-3′) and Apmsp2r (5′-TTGGTC TTG AAG CGC TCG TA-3′) were designed to amplify a 77-bp segment at the conserved amino-terminal coding region of the msp2 gene and its multiple paralogs in the A. phagocytophilum genome for better sensitivity (11). All samples and a positive control were run in triplicate, and samples with a Cp (crossing point of the amplification curve) above 35 cycles were considered negative. The following formula was used to calculate the relative quantity of msp2(p44) copies in each sample: Quantity in sample = 2 − [Cp (sample) − Cp (standard)] (26, 27).
Endpoint PCR for amplification of the 2-kb expression site of msp2(p44) of A. phagocytophilum.
DNA was isolated with a QIAamp DNA Blood Mini Kit according to the manufacturer's protocol. A 2-kb sequence of the msp2(p44) expression site was amplified that included the central hypervariable region flanked by semiconserved and conserved N and C termini (3). The primers used, AB1221 (5′-ATAGAA CAA GAG CAG GGA GAA GAC-3′) and AB1227 (5′-TCTGTC TTG GAG AGT ATT GAG TC-3′), were diluted to a concentration of 4 μM. A high-fidelity proofreading polymerase (Phusion; New England BioLabs) was used. Thermocycling was done with Perkin-Elmer GeneAmp PCR system 9600. Samples were run at 8 V/cm on 1.5% agarose gels with 1-kb and 100-bp standards. The gel was stained with 25 μl of GelStar (Lonza, Rockland, ME) in 250 μl of Tris-borate-EDTA for 20 min.
The remaining volumes of PCR products were loaded for separation on individual 1% GTG agarose minigels for DNA recovery. These gels were run at 6 V/cm and stained with GelStar. Gel slices containing the 2-kb bands were cut out under UV light and stored in a preweighed 2-ml low-adhesion collection tube at 4°C overnight for further processing.
Purification and isolation of DNA from the agarose gels were performed with the S.N.A.P. gel purification kit (Invitrogen).
Cloning and analysis of the 2-kb msp2(p44) expression site.
3′ A-overhanging ends were created on the gel-purified, blunt-ended DNA fragments in order to perform TA cloning. Four microliters of template DNA, 1 μl of Applied Biosystems 5× Taq buffer, 0.1 μl of Applied Biosystems Taq polymerase at 5 U/μl, and 0.1 μl of Invitrogen dATP were mixed and incubated at 72°C for 15 min.
The DNA fragments were then rehydrated and ethanol precipitated to increase the concentration of template DNA for cloning. Activated plasmid vector pCR4-TOPO was used in the cloning reaction as described by the manufacturer. The 2-kb cloning products were analyzed by single digestion with EcoRI to release inserted DNA and determine the presence and size of the cloned fragments and then by double digestion with EcoRI and RsaI in combination to generate diagnostic fragments (RFLP) from each clone according to the protocol (Invitrogen). Ninety-six bacterial clones from each of the nine individual peaks of lambs 4203 and 4210 were analyzed for RFLP patterns as previously described (4). Clones were selected for DNA sequencing on the basis of the RFLP patterns obtained.
DNA sequencing.
Sequencing was performed at the University of Florida DNA Sequencing Core Laboratory (Gainesville) by using ABI Prism dye terminator cycle sequencing protocols from Applied Biosystems (Foster City, CA). The extension products were fluorescence labeled and analyzed on an Applied Biosystems model 373 Stretch DNA sequencer.
Nucleotide sequences were analyzed with the Wisconsin Package, version 10.3 Unix (Accelrys, Inc., San Diego, CA), running under SunOS 5.8, available through the Biological Computing Core facilities of the Interdisciplinary Center for Biotechnology Research at the University of Florida. Sequence alignments were done initially with PILEUP, and similarities were displayed with PLOTSIMILARITY and PRETTYBOX. The sequences were then trimmed to the LAKT residues present on both sides of the hypervariable region and aligned with PILEUP or MUSCLE (14). The aligned multiple-sequence file was then visualized with CHROMA (17).
RESULTS
Clinical manifestation and infection pattern.
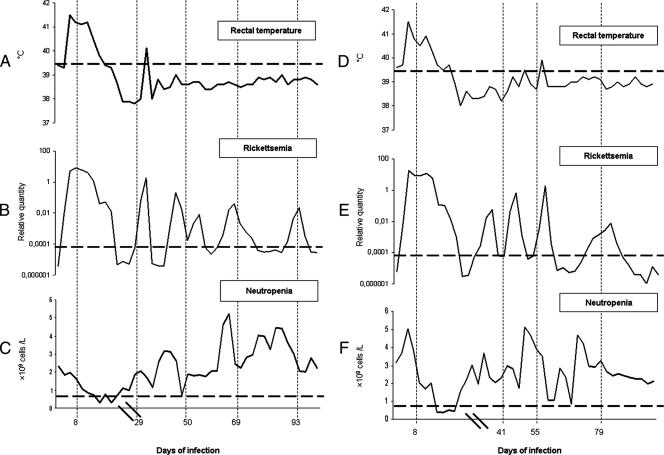
Lambs 4203 and 4210, together with the uninfected controls, were observed for 100 days. During the early phase of infection, the lambs showed signs of inappetence and depression for 1 to 2 days. The infected lambs had fever from day 3 postinoculation (p.i.), which lasted for about 6 days. The maximum temperatures recorded were 41.8°C in lamb 4203 (Fig. 1A) and 41.5°C in lamb 4210 (Fig. 1D) at days 4 and 5, respectively. In addition, neutropenia (<0.7 × 109 cells/liter) was observed for 10 days from about 13 days p.i. (Fig. 1C and F).
FIG. 1.
Clinical parameters of lambs 4203 and 4210 infected with A. phagocytophilum. (A to F) The rickettsemias in lambs 4203 (B) and lamb 4210 (E), presented as relative quantitative data on a logarithmic graph, showed little association with rectal temperature (A and D) or neutropenia (C and F), except during the first phase of infection. The horizontal dotted lines in panels A and D represent the temperature (39.5°C) above which fever was recorded in this study. The horizontal dotted lines in panels B and E illustrate the threshold level (Ct) for positive rickettsemia (more than 35 cycles by real-time PCR). The horizontal dotted lines in panels C and F represent the value (<0.7 × 109 cells/liter) below which significant neutropenia was recorded in this study. The vertical dotted lines show the sampling times that corresponded to days 8, 29, 50, 69, and 93 postinfection for lamb 4203 or days 8, 41, 55, and 79 postinfection for lamb 4210. Hash marks on the x axis indicate a scale change.
The investigation of EDTA blood samples collected over a 3-month time period showed a cyclic pattern when analyzed by real-time PCR. The peaks of rickettsemia lasted for several days interrupted by negative samples (Cp, >35). Lambs 4203 and 4210 showed five clearly distinct peaks of rickettsemia (Fig. 1B and E); blood samples were analyzed from time points corresponding to five of the rickettsemic peaks from animal 4203 and four of the rickettsemic peaks from animal 4210 (represented by vertical dotted lines in Fig. 1).
RFLP analysis of the msp2(p44) expression site.
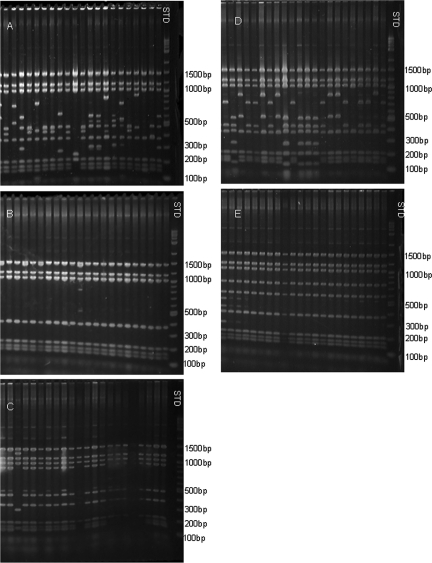
RFLP patterns obtained by digestion of expression site clones provided a preliminary estimate of the diversity of organisms present in each rickettsemic peak. These patterns showed great variation when obtained from the initial peaks of infection (Fig. 2A shows data from animal 4203). The second peak in lamb 4203 was evident by real-time PCR (Fig. 1) from 27 days postinfection and showed a more uniform band pattern with a single variant present when analyzed by RFLP (Fig. 2B). The third peak in the same animal, which may represent two peaks closely spaced in time, was evident by PCR from 41 days p.i. and showed the same uniformity of band patterns when sampled at day 50 p.i. (Fig. 2C). When the fourth peak, evident from day 62 p.i., was analyzed, multiple different band patterns each representing a low percentage of the total population were present again (Fig. 2D), while the fifth peak, present from 88 days p.i., again showed the reappearance of a more uniform band pattern (Fig. 2E). This tendency was similar in the other animal (data not shown). In the first peak of lamb 4210, analyzed at 8 days p.i., multiple band patterns were present, while only three different patterns were observed during the third peak (analyzed on day 41 p.i.). Two predominant band patterns were evident in the fourth peak, (analyzed on day 55 p.i.), with the major one representing 96% of the clones, and again in the fifth peak (analyzed on day 79 p.i.), few different patterns were observed. Figure 2 indicates a large diversity of sequences present during the initial peaks of rickettsemia and a possible selection of certain variants during the subsequent phases of infection. Interesting features are (i) the reappearance of great variation in the band patterns during the fourth peak of rickettsemia in animal 4203 and (ii) the band pattern uniformity as the fifth peak appeared 24 days later.
FIG. 2.
Complexity of msp2(p44) expression site clones from each rickettsemic peak by RFLP mapping. (A to E) Sequence diversity among a representation of the different clones of the msp2(p44) expression site from the infection in lamb 4203 only, sampled on days 8 (panel A), 29 (panel B), 50 (panel C), 69 (panel D), and 93 (panel E), respectively, by RFLP analysis. Note the large number of different variants at the initial stage of infection compared to later stages and the apparent increase in sequence diversity between days 50 and 69. The positions of molecular size standards (lane STD) are indicated on the right.
Sequential appearance of MSP2(P44) variants during persistent infection and recurrent bacteremia in infected lambs.
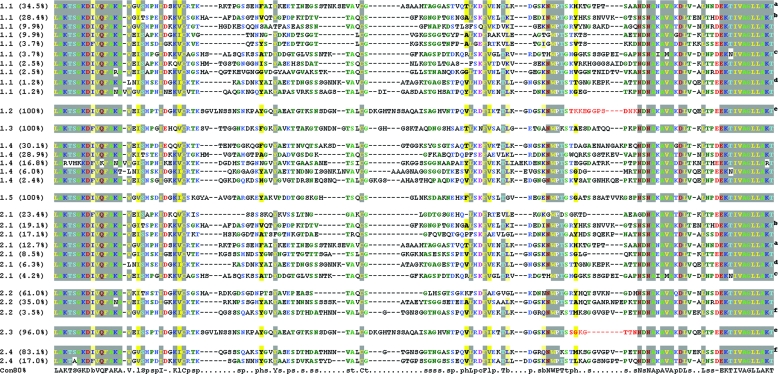
The different clones from the individual peaks of rickettsemia were analyzed by DNA sequencing of the 2-kb expression site clones, based on the band patterns obtained by prior RFLP analysis. The greatest sequence variation was observed in the central hypervariable region of MSP2(P44) between the LAKT-LAKT amino acid sequences. In cases where clones from the rickettsemic peaks showed great variation, i.e., 8 days p.i. in animals 4203 (Fig. 3, variants 1.1) and 4210 (Fig. 3, variants 2.1) and 69 days p.i. in animal 4203 (Fig. 3, variants 1.4), only the major band patterns were selected for DNA sequencing. As shown in Fig. 3, there is sequence variation between the different peaks of rickettsemia during the infection period in both animals. There appeared to be greater variation in the initial peaks and more uniform distribution of variants in the later peaks of the persistent phase of infection. During the first peak (day 8 p.i.) in animal 4203, >10 different sequence variants were present in different quantities (Fig. 3, variants 1.1), while in the second peak (day 29 p.i.), only 1 variant was present among the 96 selected clones (Fig. 3, variant 1.2). The same was true for the third peak of bacteremia (day 50 p.i.), where a single sequence variant (Fig. 3, variant 1.3) was present in 100% of the 96 clones analyzed by RFLP and DNA sequencing. During the fourth peak (day 69 p.i.) (Fig. 3, variants 1.4), five different major variants were present in variable numbers in the selected clones. The fifth peak (day 93 p.i.) had a single variant (Fig. 3, variant 1.5) among the 96 clones analyzed. In animal 4210, there were more than seven different sequence variants in the first peak of infection and four of these were also found in the first peak of animal 4203. In the third peak (second sampling, day 41 p.i.) of animal 4210, three different variants were detected by RFLP and DNA sequencing, while in the fourth peak (third sampling, day 55 p.i.), one major variant was present among 96% of the selected clones. The fifth peak (fourth sampling, day 79 p.i.) had two sequence variants present in 83.1% and 17% of the 96 selected clones. Interestingly, a 2.2 variant (Fig. 3) was present on day 41 p.i. of animal 4210 and accounted for 3.5% of the 96 clones and reappeared on day 79 p.i. in the same animal (Fig. 3, variant 2.4) but then accounted for 83.1% of the 96 clones. Another interesting feature is seen in variant 1.2 from day 29 p.i. of animal 4203 and variant 2.3 from day 55 p.i. of animal 4210, where only a small segment of the hypervariable region is different between the two.
FIG. 3.
MSP2(P44) hypervariable-region sequences from the predominant variants present in the rickettsemic peaks from sheep 4203 and 4210. Shown is a MUSCLE/CHROMA alignment of the sequence variants present at each sampling time analyzed here (Fig. 1) from lamb 4203 (variants 1) and lamb 4210 (variants 2). That is, variants 1.1 through 1.5 represent the variants present in lamb 4203 at sampling times of 8, 29, 50, 69, and 93 days p.i. and variants 2.1 through 2.4 represent the variants present in lamb 4210 at sampling times of 8, 41, 55, and 79 days p.i. The percentage of a variant in each sampled population is given next to the variant number designation. Identical variants that are present in different populations are indicated by the same superscript letter to the right, except for the two variants indicated by a superscript e. These variants (1.2 and 2.3) are identical except for a short region of difference that is highlighted in red. A consensus sequence for all variants is shown at the bottom, where lowercase letters indicate the following: b, big (EFHIKLMQRWY); c, charged (DEHKR); h, hydrophobic (ACFGHILMTVWY); p, polar (CDEHKNQRST); s, small (ACDGNPSTV); t, tiny (AGS).
DISCUSSION
In contrast to A. marginale infections of cattle, there is a lack of suitable experimental systems for investigating the mechanisms of persistence of A. phagocytophilum. To date, most studies have used U.S. human strains of the organism that either are not persistent or are difficult to recover and analyze after the early period of infection. Infections of sheep by strains of A. phagocytophilum found in Norway are naturally persistent, and we have shown previously that these strains possess an msp2(p44) expression site syntenic to that of U.S. human strains of A. phagocytophilum and to that which encodes antigenically variable MSP2 of A. marginale (4). We investigated here the structure and variability of this expression site at different times, up to 3 months postinfection.
After inoculation, lambs reacted with typical signs of A. phagocytophilum infection, i.e., high fever (>41°C), neutropenia (<0.7 × 109 cells/liter), and cytoplasmic inclusions in neutrophil granulocytes (35). None of these reactions were detected in the control (uninfected) animals. Analysis of blood samples by real-time PCR showed a characteristic cyclic appearance of rickettsiae in the blood interrupted by samples not considered positive by PCR, indicating clearance of the vast majority of the rickettsiae from the blood.
The RFLP and sequence data presented reveal variation in the central region which encodes MSP2(P44) in a Norwegian strain of A. phagocytophilum during persistent rickettsemia in experimentally infected lambs. In U.S. strains, this msp2(p44) expression site is used for the expression of multiple mRNAs with diverse central variable regions (CVRs) (4) and the sequence variation identified here suggests that antigenic variation of this major OMP occurs in sheep during persistent infection with A. phagocytophilum. Comparison of these sequences with the GenBank database showed little similarity between the CVRs from this Norwegian strain with those of U.S. strains, except in the conserved framework residues (25). The cyclic rickettsemias observed suggest a mechanism by which the pathogen escapes the cellular and humoral immune responses by switching surface antigens and so being unrecognized for reappearance at a later stage of infection. It is known that tick-borne pathogens have the ability to persist in the body in order to be transmitted between hosts by feeding ticks (5, 23, 29, 30). Earlier studies suggested that differential msp2(p44) expression is associated with host adaptive immunity (24). In the present study, we reveal the expression of diverse msp2(p44) paralogs without a single majority in the early phase of infection and with single predominant variants in some, but not all, of the later peaks of rickettsemia. However, it must be noted that these infections were initiated by experimental inoculation and not by tick challenge. These results show extensive variation of the msp2(p44) expression site in A. phagocytophilum infections of sheep that is similar to that in A. marginale infections of cattle (15, 16). As in A. marginale, some variants reappeared in different peaks. The same variants were sometimes present in different animals at similar times postinfection, indicating a loosely programmed order of expression. The A. phagocytophilum variants observed exhibited greater diversity in A. phagocytophilum than in A. marginale, which is likely related to the larger repertoire of available donor pseudogenes in A. phagocytophilum (approximately 100 compared to 7 in A. marginale) (6, 18). The selection and phenotypic expression of single variants observed in the later peaks of rickettsemia could be mediated in infected lambs by acquired immune responses, as is suggested by evidence that antibodies to MSP2(P44) have protective capacity, at least against identical variants (19, 22). Wang and coinvestigators have previously suggested that the rapid and synchronized switching of expression is an intrinsic property of MSP2(P44) proteins and that the coordination necessary for this change might be provided by the host (33). This may explain the multivariate presentation of MSP2(P44) during the initial peaks of rickettsemia and the selection of predominant variants in subsequent peaks of infection observed here. However, any synchronized switching of expression is not dependent on rickettsial density as quantitative PCR analyses show that the overall number of organisms is decreasing during the persistent phase of infection analyzed here.
It has previously been proposed that segmental gene conversion of multiple MSP2 or MSP2(p44) proteins at a single expression locus may allow largely unrestricted variability in the expressed variable region (2, 4). This could be achieved if the expressed msp2(p44) gene is a chimera or mosaic of two or more donor copies from pseudogenes elsewhere in the genome of A. phagocytophilum. In this study, we present results that indicate that this phenomenon may be occurring in the Norwegian sheep strain of A. phagocytophilum. This is observed in variant 1.2 from the second peak of animal 4203 and variant 2.3 from the third peak of animal 4210, which are identical except for a short segment of the hypervariable region (Fig. 3). As the inocula for the two animals had the same origin, a probable explanation for this would be a short gene conversion of an expression site hypervariable region from a segment of a new donor pseudogene. This is known to occur in A. marginale (9). However, this explanation cannot be proven without knowledge of the genome sequence of this Norwegian strain of A. phagocytophilum and elimination of alternative possibilities, such as expression of complete CVRs from two closely related donor pseudogenes. Our study indicates that knowledge of more complete genome sequences from European ruminant strains would help to elucidate in detail the mechanisms for generating the enormous diversity observed in single infections caused by A. phagocytophilum.
The level of rickettsemia showed no direct association with temperature or neutropenia during the persistent phase of infection (Fig. 1). This indicates that animals are more affected during the initial phase and thus more prone to suffer from secondary infections early in A. phagocytophilum infection. The persistent phase seems to be a dynamic relationship between the rickettsia and its host that enables organisms to be available for onward transmission to ticks such as Ixodes ricinus. The lambs stayed more or less within the normal temperature range from day 10 p.i. on and showed only minor fluctuations in rectal temperature. The marked neutropenia observed during the early phase of infection may explain why lambs often suffer from secondary infections during this early period. However, several other factors are involved in the immunosuppression of A. phagocytophilum-infected lambs (35). In conclusion, the data presented reveal recurrent peaks of rickettsemia in sheep infected by a Norwegian strain of A. phagocytophilum. These peaks are associated with the presence of multiple different sequence variants of msp2(p44) in the locus that is syntenic with the expression locus of U.S. strains of A. phagocytophilum and with that of A. marginale. The number of discreet variants is different in each peak, with extensive diversity observed in the first peak and less diversity in some subsequent peaks. In general, there was very limited identity between the different hypervariable regions of different variants, except in the previously defined framework residues. Similar to A. marginale infections of cattle, some variants reappeared in different peaks and in different animals, suggesting a mechanism of loosely programmed diversity followed by selection. This system will be of value in assessing the presence and significance of immune responses to conserved and different hypervariable regions of A. phagocytophilum MSP2(P44) in infected animals.
Acknowledgments
This investigation received support from NIH grant AI45580 and Hålands legat.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 29 October 2007.
REFERENCES
- 1.Andrew, H. R., and R. A. Norval. 1989. The carrier status of sheep, cattle and African buffalo recovered from heartwater. Vet. Parasitol. 34261-266. [DOI] [PubMed] [Google Scholar]
- 2.Barbet, A. F., A. Lundgren, J. Yi, F. R. Rurangirwa, and G. H. Palmer. 2000. Antigenic variation of Anaplasma marginale by expression of MSP2 mosaics. Infect. Immun. 686133-6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbet, A. F., A. M. Lundgren, A. R. Alleman, S. Stuen, A. Bjoersdorff, R. N. Brown, N. L. Drazenovich, and J. E. Foley. 2006. Structure of the expression site reveals global diversity in MSP2 (P44) variants in Anaplasma phagocytophilum. Infect. Immun. 746429-6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbet, A. F., P. F. Meeus, M. Belanger, M. V. Bowie, J. Yi, A. M. Lundgren, A. R. Alleman, S. J. Wong, F. K. Chu, U. G. Munderloh, and S. D. Jauron. 2003. Expression of multiple outer membrane protein sequence variants from a single genomic locus of Anaplasma phagocytophilum. Infect. Immun. 711706-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbour, A. G., Q. Dai, B. I. Restrepo, H. G. Stoenner, and S. A. Frank. 2006. Pathogen escape from host immunity by a genome program for antigenic variation. Proc. Natl. Acad. Sci. USA 10318290-18295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brayton, K. A., L. S. Kappmeyer, D. R. Herndon, M. J. Dark, D. L. Tibbals, G. H. Palmer, T. C. McGuire, and D. P. Knowles, Jr. 2005. Complete genome sequencing of Anaplasma marginale reveals that the surface is skewed to two superfamilies of outer membrane proteins. Proc. Natl. Acad. Sci. USA 102844-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brayton, K. A., D. P. Knowles, T. C. McGuire, and G. H. Palmer. 2001. Efficient use of a small genome to generate antigenic diversity in tick-borne ehrlichial pathogens. Proc. Natl. Acad. Sci. USA 984130-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brayton, K. A., P. F. Meeus, A. F. Barbet, and G. H. Palmer. 2003. Simultaneous variation of the immunodominant outer membrane proteins, MSP2 and MSP3, during Anaplasma marginale persistence in vivo. Infect. Immun. 716627-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brayton, K. A., G. H. Palmer, A. Lundgren, J. Yi, and A. F. Barbet. 2002. Antigenic variation of Anaplasma marginale msp2 occurs by combinatorial gene conversion. Mol. Microbiol. 431151-1159. [DOI] [PubMed] [Google Scholar]
- 10.Chen, S. M., J. S. Dumler, J. S. Bakken, and D. H. Walker. 1994. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 32589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Courtney, J. W., L. M. Kostelnik, N. S. Zeidner, and R. F. Massung. 2004. Multiplex real-time PCR for detection of Anaplasma phagocytophilum and Borrelia burgdorferi. J. Clin. Microbiol. 423164-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dumler, J. S., K. M. Asanovich, and J. S. Bakken. 2003. Analysis of genetic identity of North American Anaplasma phagocytophilum strains by pulsed-field gel electrophoresis. J. Clin. Microbiol. 413392-3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumler, J. S., A. F. Barbet, C. P. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 512145-2165. [DOI] [PubMed] [Google Scholar]
- 14.Edgar, R. C. 2004. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.French, D. M. 1999. Emergence of Anaplasma marginale antigenic variants during persistent rickettsemia. Infect. Immun. 675834-5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.French, D. M., T. F. McElwain, T. C. McGuire, and G. H. Palmer. 1998. Expression of Anaplasma marginale major surface protein 2 variants during persistent cyclic rickettsemia. Infect. Immun. 661200-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodstadt, L., and C. P. Ponting. 2001. CHROMA: consensus-based colouring of multiple alignments for publication. Bioinformatics (Oxford) 17845-846. [DOI] [PubMed] [Google Scholar]
- 18.Hotopp, J. C., M. Lin, R. Madupu, J. Crabtree, S. V. Angiuoli, J. Eisen, R. Seshadri, Q. Ren, M. Wu, T. R. Utterback, S. Smith, M. Lewis, H. Khouri, C. Zhang, H. Niu, Q. Lin, N. Ohashi, N. Zhi, W. Nelson, L. M. Brinkac, R. J. Dodson, M. J. Rosovitz, J. Sundaram, S. C. Daugherty, T. Davidsen, A. S. Durkin, M. Gwinn, D. H. Haft, J. D. Selengut, S. A. Sullivan, N. Zafar, L. Zhou, F. Benahmed, H. Forberger, R. Halpin, S. Mulligan, J. Robinson, O. White, Y. Rikihisa, and H. Tettelin. 2006. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. 2e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.IJdo, J., C. Wu, S. R. Telford III, and E. Fikrig. 2002. Differential expression of the p44 gene family in the agent of human granulocytic ehrlichiosis. Infect. Immun. 705295-5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iqbal, Z., and Y. Rikihisa. 1994. Reisolation of Ehrlichia canis from blood and tissues of dogs after doxycycline treatment. J. Clin. Microbiol. 321644-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kieser, S. T., I. S. Eriks, and G. H. Palmer. 1990. Cyclic rickettsemia during persistent Anaplasma marginale infection of cattle. Infect. Immun. 581117-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, H. Y., and Y. Rikihisa. 1998. Characterization of monoclonal antibodies to the 44-kilodalton major outer membrane protein of the human granulocytic ehrlichiosis agent. J. Clin. Microbiol. 363278-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsson, C., M. Andersson, J. Pelkonen, B. P. Guo, A. Nordstrand, and S. Bergstrom. 2006. Persistent brain infection and disease reactivation in relapsing fever borreliosis. Microbes Infect. 82213-2219. [DOI] [PubMed] [Google Scholar]
- 24.Lin, Q., Y. Rikihisa, S. Felek, X. Wang, R. F. Massung, and Z. Woldehiwet. 2004. Anaplasma phagocytophilum has a functional msp2 gene that is distinct from p44. Infect. Immun. 723883-3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin, Q., N. Zhi, N. Ohashi, H. W. Horowitz, M. E. Aguero-Rosenfeld, J. Raffalli, G. P. Wormser, and Y. Rikihisa. 2002. Analysis of sequences and loci of p44 homologs expressed by Anaplasma phagocytophila in acutely infected patients. J. Clin. Microbiol. 402981-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak, K. J. 1997, 2001. Relative quantification of gene expression. ABI Prism 7700 sequence detection system, user bulletin no. 2. Applied Biosystems, Foster City, CA.
- 27.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 28.Meeus, P. F., K. A. Brayton, G. H. Palmer, and A. F. Barbet. 2003. Conservation of a gene conversion mechanism in two distantly related paralogues of Anaplasma marginale. Mol. Microbiol. 47633-643. [DOI] [PubMed] [Google Scholar]
- 29.Palmer, G. H., and K. A. Brayton. 2007. Gene conversion is a convergent strategy for pathogen antigenic variation. Trends Parasitol. 23408-413. [DOI] [PubMed] [Google Scholar]
- 30.Palmer, G. H., W. C. Brown, and F. R. Rurangirwa. 2000. Antigenic variation in the persistence and transmission of the ehrlichia Anaplasma marginale. Microbes Infect. 2167-176. [DOI] [PubMed] [Google Scholar]
- 31.Stuen, S. 1998. Sjodogg (tick-borne fever)—a historical review. Norsk Veterinaertidsskr. 110703-706. [Google Scholar]
- 32.Stuen, S., K. Bergstrom, M. Petrovec, I. Van de Pol, and L. M. Schouls. 2003. Differences in clinical manifestations and hematological and serological responses after experimental infection with genetic variants of Anaplasma phagocytophilum in sheep. Clin. Diagn. Lab. Immunol. 10692-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, X., Y. Rikihisa, T. H. Lai, Y. Kumagai, N. Zhi, and S. M. Reed. 2004. Rapid sequential changeover of expressed p44 genes during the acute phase of Anaplasma phagocytophilum infection in horses. Infect. Immun. 726852-6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woldehiwet, Z., C. Dare, and S. D. Carter. 1993. The effects of tick-borne fever on the susceptibility of ovine polymorphonuclear cells to P. haemolytica cytotoxin. J. Comp. Pathol. 109303-307. [DOI] [PubMed] [Google Scholar]
- 35.Woldehiwet, Z., and G. R. Scott. 1993. Tick-borne (pasture) fever, 1st ed., vol. 1. Pergamon Press, Oxford, United Kingdom.