Abstract
Oxidative stress may alter the functions of many proteins including the Slo1 large conductance calcium-activated potassium channel (BKCa). Previous results demonstrated that in the virtual absence of Ca2+, the oxidant chloramine-T (Ch-T), without the involvement of cysteine oxidation, increases the open probability and slows the deactivation of BKCa channels formed by human Slo1 (hSlo1) α subunits alone. Because native BKCa channel complexes may include the auxiliary subunit β1, we investigated whether β1 influences the oxidative regulation of hSlo1. Oxidation by Ch-T with β1 present shifted the half-activation voltage much further in the hyperpolarizing direction (−75 mV) as compared with that with α alone (−30 mV). This shift was eliminated in the presence of high [Ca2+]i, but the increase in open probability in the virtual absence of Ca2+ remained significant at physiologically relevant voltages. Furthermore, the slowing of channel deactivation after oxidation was even more dramatic in the presence of β1. Oxidation of cysteine and methionine residues within β1 was not involved in these potentiated effects because expression of mutant β1 subunits lacking cysteine or methionine residues produced results similar to those with wild-type β1. Unlike the results with α alone, oxidation by Ch-T caused a significant acceleration of channel activation only when β1 was present. The β1 M177 mutation disrupted normal channel activation and prevented the Ch-T–induced acceleration of activation. Overall, the functional effects of oxidation of the hSlo1 pore-forming α subunit are greatly amplified by the presence of β1, which leads to the additional increase in channel open probability and the slowing of deactivation. Furthermore, M177 within β1 is a critical structural determinant of channel activation and oxidative sensitivity. Together, the oxidized BKCa channel complex with β1 has a considerable chance of being open within the physiological voltage range even at low [Ca2+]i.
Keywords: BKCa, hSlo, chloramine-T, methionine, cysteine
INTRODUCTION
The large conductance calcium-activated potassium channel (BKCa) exists in various types of cells and tissues including smooth muscle and brain. In response to depolarization and/or a rise in intracellular Ca2+ ([Ca2+]i), BKCa channels mediate net K+ efflux to repolarize the membrane potential to the resting state. This function serves an important role in muscle contraction—during which Ca2+ sparks activate the BKCa channels leading to vasorelaxation (Nelson et al., 1995; Jaggar et al., 2000)—and the afterhyperpolarization phase of the action potential in select neurons (Storm, 1987). Furthermore, the impairments exhibited by mice lacking the channel indicate that BKCa channels influence normal urinary bladder (Meredith et al., 2004) and cerebellar functions (Sausbier et al., 2004).
The human BKCa channel pore-forming α subunit (hSlo1) contains seven putative transmembrane-spanning regions (Dworetzky et al., 1994; Pallanck and Ganetzky, 1994; Tseng-Crank et al., 1994). The S0 transmembrane domain, which distinguishes the Slo from the Shaker family of voltage-dependent potassium channels, is thought to be a site of interaction with auxiliary β subunits (Wallner et al., 1996; Meera et al., 1997). Multiple types of β subunits (β1–4) have been isolated in mammals, each with a different tissue distribution and function (Knaus et al., 1994; Xia et al., 1999; Brenner et al., 2000a; Uebele et al., 2000).
The β1 subunit is a 25-kD membrane protein consisting of two transmembrane domains connected by a large extracellular loop, such that both the NH2 and COOH termini are intracellularly located (Knaus et al., 1994; Orio et al., 2002; Patterson et al., 2002). The β1 subunit is present in the brain, particularly in the hippocampus and corpus callosum (Tseng-Crank et al., 1996), but is predominantly expressed in smooth muscle (Garcia-Calvo et al., 1994; Tanaka et al., 1997). The impaired vasorelaxation found in β1 knockout mice (Brenner et al., 2000b; Pluger et al., 2000) and the down-regulation of β1 expression associated with some forms of hypertension (Gollasch et al., 2002; Amberg et al., 2003; Amberg and Santana, 2003) clearly underscore the important physiological role of β1 in the BKCa channel regulation of vascular function. The presence of β1 modulates BKCa channel activity by enhancing the apparent Ca2+ sensitivity of the pore-forming subunit and also by slowing the activation/deactivation kinetics, even in the virtual absence of Ca2+ (McManus et al., 1995; Wallner et al., 1995; Meera et al., 1996; Nimigean and Magleby, 1999, 2000; Cox and Aldrich, 2000; Qian and Magleby, 2003). The structural determinants within β1 responsible for these critical modulatory properties are just beginning to be identified (Fernandez-Fernandez et al., 2004).
Other regulatory mechanisms such as phosphorylation, pH, and the cellular redox state influence BKCa channel activity (Weiger et al., 2002). During oxidative stress, cellular reactive oxygen/nitrogen species (ROS/RNS) readily modify cysteine and methionine residues in proteins. Oxidation of cysteine typically leads to the formation of disulfides, whereas oxidation of methionine residues creates the polar methionine sulfoxide (met-O). Oxidative modifications of amino acids differentially influence BKCa channel function depending on the ROS/RNS, the residues modified within the channel, as well as the experimental model system (DiChiara and Reinhart, 1997; Sobey et al., 1997; Wang and Wu, 1997; Wang et al., 1997; Barlow et al., 2000; Gong et al., 2000; Soh et al., 2001; Brakemeier et al., 2003). Studies using heterologously expressed hSlo1 indicate that oxidation of cysteine residues typically decreases the channel open probability (DiChiara and Reinhart, 1997; Soto et al., 2002; Tang et al., 2004). In contrast, methionine oxidation of the hSlo1 pore-forming subunit that is promoted by the oxidant chloramine-T (Ch-T) increases the channel open probability (Tang et al., 2001).
Oxidative stress is prominently involved in many disease states such as vascular dysfunction (Taniyama and Griendling, 2003) and neurodegenerative diseases (Knight, 1997; Markesbery, 1997; Butterfield et al., 2001). These physiological systems that are affected by oxidative stress depend on BKCa channel activity for normal function. Therefore, determining the effect of oxidative modification of BKCa channel complexes that closely resemble native channels is important to understand and possibly treat or prevent these diseases. Native BKCa channels are often multi-subunit complexes containing both Slo1 and auxiliary β subunits (Garcia-Calvo et al., 1994; Knaus et al., 1994; Giangiacomo et al., 1995; Vogalis et al., 1996; Tanaka et al., 1997; Wanner et al., 1999; Weiger et al., 2000). However, the influence of β subunits on the oxidative regulation of Slo1 function has not been thoroughly examined.
The purpose of the present work was to determine whether the presence of β1 alters the functional effects of hSlo1 oxidation. Methionine oxidation of hSlo1 alone causes a shift in the macroscopic G-V curve by −30 mV and slows deactivation without any appreciable effect on the activation kinetics at depolarized voltages (Tang et al., 2001). We show that, in the virtual absence of Ca2+, the auxiliary subunit β1 dramatically potentiates the effect of methionine oxidation in the hSlo1 pore-forming protein. This is demonstrated by a further increase in the open probability and even greater slowing of the deactivation kinetics. Furthermore, β1 confers novel oxidation sensitivity to the channel activation kinetics that is mediated largely by a single methionine residue located in the second transmembrane domain (TM2) of β1.
MATERIALS AND METHODS
Channel Expression and Mutagenesis
hSlo1 (U11058, hbr1; Tseng-Crank et al., 1994) channel alone, or hSlo1 and β1 (1:1 weight ratio) were transiently expressed in HEK-tsA cells using FuGENE 6 (Roche Applied Science) as described previously (Avdonin et al., 2003). The mouse Slo β1 (mβ1; AF020711; Jiang et al., 1999) in pEGFP-N1 (BD Biosciences) was obtained from the laboratory of R. Aldrich (Stanford University, Stanford, CA). The mβ1 mutants M7L, M23L, M177L, and Triple (M7L:M23L:M177L) were constructed using PCR-based mutagenesis, and the sequences were verified. “Cysless” bβ1, in which every cysteine in bovine β1 (bβ1; L26101; Knaus et al., 1994) was replaced with alanine (C18A, C53A, C76A, C103A, and C135A; Hanner et al., 1998), was obtained from the laboratory of M.L. Garcia (Merck Research Laboratories, Rahway, NJ).
Electrophysiology and Data Analysis
Currents were recorded from excised inside-out patches at room temperature essentially as described previously (Tang et al., 2001). Patch electrodes (Warner) had a typical initial resistance of 2.5–3 MΩ when filled with solutions (described in the next section); the series resistance, ∼90% of the input resistance, was electronically compensated. The current signal was filtered at 10 kHz through the built-in filter of the patch-clamp amplifier (model AxoPatch 200A; Axon Instruments). Data were acquired and analyzed using Pulse/PulseFit (HEKA), PatchMachine (Avdonin et al., 2003), and IgorPro (WaveMetrics) as described for single-channel data (Avdonin and Hoshi, 2001) and macroscopic current data (Tang et al., 2001; Avdonin et al., 2003). In brief, normalized macroscopic conductance was estimated from single exponential fits to the tail currents recorded at −50 mV excluding the initial 180 μs after pulses to different voltages from the holding voltage of 0 mV. The apparent equivalent charge movement (Qapp) was derived from the simple Boltzmann function used to describe the average G-V curve. Activation and deactivation time courses were fitted by single exponentials excluding the initial 150- and 180-μs segments, respectively. A single exponential fit to the voltage dependence of the time constant provided the value of the equivalent charge movement (z).
In some patches, the tail currents after Ch-T treatment contained a minor fast component. The fractional amplitude of this component was typically small (<10%), and the time constant estimated from single-exponential fits was essentially the same as that of the slow component estimated from two-exponential fits. Thus, single-exponential fits were used throughout to quantify the tail current kinetics. Because the time constant of the tail current before modification and that of the minor fast component after Ch-T treatment were similar, the fast component likely reflects the kinetics of unmodified channels.
The change in free energy associated with Ca2+ binding (ΔGCa) was determined based on the ΔGCa contribution to channel open probability (P O) as described previously (Tang et al., 2004). The values of P O, ΔGo, ΔGV, and ΔGCa were estimated by fitting the G-V curves obtained in 0 and 2.1 μM Ca2+.
Statistical comparisons were made using the paired t test. In some cases, the t test and ANOVA followed by the Bonferroni post hoc test were used as specifically indicated (DataDesk; Data Description). Statistical significance was assumed at P ≤ 0.05. Where appropriate, data are presented as mean ± SEM.
Reagents and Solutions
Both the external and internal recording solutions contained the following (mM): 140 KCl, 11 EGTA, and 10 HEPES, pH 7.2 adjusted with NMDG. The free Ca2+ concentration for these solutions was estimated at <1 nM assuming 20 μM contaminating Ca2+ (Patcher's Power Tools v1.0, F. Mendez; http://www.mpibpc.gwdg.de/abteilungen/140/software/). The external solution used to reduce the size of inward K+ currents for experiments involving 2.1 μM [Ca2+]i contained the following (mM): 70 KCl, 70 NaCl, 2 MgCl2, and 10 HEPES, pH 7.2 adjusted with NMDG. The 2.1-μM free Ca2+ internal solution contained the following (mM): 120 KCl, 20 KOH, 1 MgCl2, 2.2 CaCl2, 4 HEDTA, and 10 HEPES, pH 7.4 adjusted with NMDG. The external solution used for experiments involving 120 μM [Ca2+]i contained the following reagents (mM): 140 KCl, 2 MgCl2, and 10 HEPES, pH 7.2 adjusted with NMDG. The 120-μM free Ca2+ internal solution contained the following reagents (mM): 140 KCl, 10 MgCl2, 0.1 CaCl2, and 10 HEPES, pH 7.2 adjusted with NMDG.
Chloramine-T (Ch-T; Sigma-Aldrich) was dissolved in the internal solution immediately before use. In every experiment, 2 mM Ch-T was manually applied with a pipette to ensure the addition of six times the bath volume (∼150 μl). With Ch-T present, channel current in response to a pulse to 120 mV was monitored every 5 s for the following three features of oxidation by Ch-T: increased current amplitude, slowed deactivation, and accelerated activation. Once these characteristic changes reached steady-state levels (≤8 min), Ch-T was subsequently washed out with 1 ml of recording solution. The time courses of modification of channels, composed of either hSlo1 alone or hSlo1 and β1 together, were indistinguishable.
RESULTS
Oxidation by Ch-T More Dramatically Enhances hSlo1 Currents When β1 Is Present
To determine if the presence of β1 influences the functional effects of hSlo1 oxidation by Ch-T, ionic currents through hSlo1 or hSlo1 + mβ1 channels were recorded in the inside-out patch-clamp configuration from transiently transfected HEK-tsA cells. All recordings were initially made in the virtual absence of Ca2+, essentially permitting the Slo channel to act as a voltage-dependent channel to simplify the data analysis (Meera et al., 1996; Horrigan and Aldrich, 1999; Horrigan et al., 1999). Currents elicited by pulses to 120 mV in patches containing either hSlo1 or hSlo1 + mβ1 are shown in Fig. 1 A (thin sweeps). The hSlo1 + mβ1 currents displayed slow activation and deactivation (also see Fig. 2), a hallmark of the functional presence of the β1 subunit. After bath application of 2 mM Ch-T to the cytoplasmic side, hSlo1 and hSlo1 + mβ1 exhibited similar modification time courses (P = 0.08, t test) that resulted in larger current amplitudes (Fig. 1 A, thick sweeps). The current enhancement remained after Ch-T washout, consistent with the oxidative modification of the channel protein complex by Ch-T.
Figure 1.

Oxidation by Ch-T enhances hSlo1 + mβ1 currents to a greater extent than hSlo1 currents. (A) Representative currents before (thin sweep) and after (thick sweep) 2 mM Ch-T treatment. The currents were elicited in response to pulses from 0 to 120 mV. Mean times to reach 50% of final current amplitude in the presence of Ch-T for hSlo1 and hSlo1 + mβ1 were 5.64 ± 0.34 min and 4.68 ± 0.3 min, respectively (P = 0.08, n = 4). (B) Peak I-V curves before (open symbols) and after (closed symbols) modification by Ch-T. Continuous curves represent relative increases in current amplitude as a function of voltage (right axis). (C) G-V curves before (open symbols) and after (closed symbols) modification by Ch-T. The macroscopic currents were elicited by pulses to different test voltages from the holding voltage of 0 mV. The hSlo1 V0.5 values for the results obtained before and after Ch-T application were 171.9 ± 4.5 mV and 140.6 ± 6.1 mV (ΔV0.5 range, −19 to −42 mV; P < 0.0001, n = 7), respectively. The hSlo1 + mβ1 V0.5 values for the results obtained before and after Ch-T application were 163.8 ± 3.8 mV and 89.2 ± 4.1 mV (ΔV0.5 range −50 to −99 mV; P < 0.0001, n = 14), respectively. The hSlo1 Qapp values for the results obtained before and after Ch-T application were 1.09 ± 0.16e and 0.84 ± 0.09e (P = 0.036, n = 7), respectively. The hSlo1 + mβ1 Qapp values for the results obtained before and after Ch-T application were 0.86 ± 0.02e and 0.88 ± 0.04e , respectively, (P = 0.65, n = 14). (D) V0.5 and Qapp values before and after oxidation by Ch-T from individual experiments (open circles) and mean values (closed circles). (E) Representative hSlo1 + mβ1 channel openings at −40 mV before and after treatment with Ch-T. Data were filtered at 10 kHz and sampled at 83 kHz, but are shown filtered at 1 kHz for display purpose. Typically, 3-min segments were analyzed in each condition.
Figure 2.
Ch-T treatment slows deactivation of hSlo1 + mβ1 to a greater extent than hSlo1 deactivation. (A) Tail currents recorded at −40 mV after pulses to 180 mV before (thin sweep) and after (thick sweep) Ch-T treatment. (B) Voltage dependence of the deactivation time constant for hSlo1 control (open circles; n = 7), hSlo1 after Ch-T (closed circles; n = 7), hSlo1 + mβ1 control (open squares; n = 5), and hSlo1 + mβ1 after Ch-T (closed squares; n = 5). The hSlo1 τ(0) and z values obtained before and after Ch-T application were 0.35 ± 0.04 ms and 0.19 ± 0.01e, and 0.63 ± 0.06 ms and 0.21 ± 0.01e, respectively. The hSlo1 + mβ1 τ(0) and z values obtained before and after Ch-T application were 3.96 ± 0.52 ms and 0.38 ± 0.02e, and 10.9 ± 2.1 ms and 0.34 ± 0.01e, respectively. The relative increase in the value of the deactivation time constant as a function of voltage (right axis) is shown for hSlo1 (dashed line) and hSlo1 + mβ1 (continuous line).
Treatment with Ch-T shifted the peak I-V curves from both hSlo1 and hSlo1 + mβ1 to more negative voltages, such that at a given voltage, the current size was greater (Fig. 1 B). However, the current enhancement was drastically larger in hSlo1 + mβ1 than in hSlo1 alone, especially at moderately depolarizing voltages (50–100 mV; Fig. 1 B). The relative increase in current amplitude due to oxidation became progressively smaller at more depolarizing voltages where the channel open probability is saturated. This voltage dependence is consistent with Ch-T increasing the open channel probability as shown for hSlo1 (Tang et al., 2001).
The voltage dependence of the probability of the channel being open inferred from normalized macroscopic G-V curves confirmed that treatment with Ch-T enhanced the open probability of hSlo1 + mβ1 more profoundly than that of hSlo1 alone. The G-V curves estimated from tail current measurements were fit by a simple Boltzmann function as a data descriptor to describe the overall voltage dependence of the Ch-T effect (Fig. 1 C). After Ch-T treatment, the hSlo1 half-activation voltage (V0.5) shifted by ∼30 mV in the hyperpolarizing direction. However, for hSlo1 + mβ1, oxidation by Ch-T produced the strikingly greater shift of −75 mV. The mean shift in V0.5 for hSlo1 + mβ1 (ΔV0.5 = −74.6 ± 3.5 mV, n = 14) was more than twice as great as ΔV0.5 for hSlo1 alone (ΔV0.5 = −31.3 ± 3.3 mV, n = 7) (Fig. 1 D; P < 0.0001, t test). These results suggest that treatment with Ch-T leads to an increase in the open probability that is markedly potentiated with mβ1 present.
The apparent equivalent charge movement (Qapp) of hSlo1 activation, inferred from the steepness of the G-V curve, decreased by ∼23% (ΔQapp= −0.25 ± 0.07e, P = 0.036, n = 7) after Ch-T treatment (Fig. 1 D). In contrast, the ΔQapp for hSlo1 + mβ1 demonstrated no significant change after modification (ΔQapp= 0.02 ± 0.02e; P = 0.65, n = 14).
The increase in the channel open probability caused by Ch-T was maintained at more negative, physiological voltages. At −40 mV in the virtual absence of Ca2+, treatment with Ch-T markedly increased the number of hSlo1 + mβ1 channel openings (Fig. 1 E). Indeed, the mean open probability at this voltage increased by a factor of 12.0 ± 3.0 relative to control. In contrast with the dramatic changes in the gating properties of the hSlo1 + mβ1 channel, the open channel current-conductance characteristic estimated using voltage ramps (0–250 mV) in single-channel patches remained unaltered by Ch-T treatment (unpublished data).
Modification by Ch-T Drastically Slows hSlo1 + mβ1 Deactivation
To assess whether treatment with Ch-T affects hSlo1 deactivation differently when the β1 subunit is present, hSlo1 and hSlo1 + mβ1 tail currents were recorded before and after Ch-T treatment (Fig. 2 A). After Ch-T exposure, the mean deactivation time constant at −40 mV increased by ∼70% (from 0.26 to 0.45 ms) for hSlo1, whereas the increase for hSlo1 + mβ1 was ∼180% (from 2.12 to 6.06 ms). This appreciably greater slowing of hSlo1 + mβ1 deactivation was observed at every voltage examined (Fig. 2 B). Single exponential fits to the voltage dependence of the deactivation time constants in the voltage range of −150 to −50 mV indicated that oxidation by Ch-T specifically increased the time constant values at 0 mV, τ(0), for hSlo1 and hSlo1 + mβ1 (P = 0.0021 and 0.015, respectively, n = 5) without significantly affecting their equivalent charge movement (P = 0.17 and 0.08, respectively, n = 5). In fact, the change in τ(0) for hSlo1 + mβ1 is approximated by a voltage shift of −75 mV, which is similar in value to the voltage shift of the G-V curve after oxidation by Ch-T.
Activation Kinetics of hSlo1 + mβ1 Accelerates after Ch-T Treatment
The activation kinetics of hSlo1 alone remained unaltered after modification by Ch-T (Fig. 3 A; Tang et al., 2001). In contrast, Ch-T markedly accelerated the mean activation kinetics of hSlo1 + mβ1 by 68% at each voltage tested (130–240 mV; Fig. 3 B). Single exponential fits to the voltage dependence of the activation time constant within this voltage range demonstrated that treatment with Ch-T decreased τ(0) for hSlo1 + mβ1 (P = 0.002, n = 7), but not for hSlo1 (P = 0.23, n = 4), without affecting the equivalent charge movement (hSlo1: P = 0.14, n = 4; hSlo1 + mβ1: P = 0.92, n = 7). This change in τ(0) could be accounted for by a voltage shift of more than −150 mV, which is much larger in value than the voltage shift of the G-V curve after oxidation by Ch-T.
Figure 3.
Modification by Ch-T accelerates activation of hSlo1 + mβ1. (A) Normalized currents recorded at 240 mV from the holding voltage of 0 mV before (thin sweep) and after (thick sweep) Ch-T treatment. (B) Voltage dependence of the activation time constant for hSlo1 control (open circles; n = 7), hSlo1 after Ch-T (closed circles; n = 7), hSlo1 + mβ1 control (open squares; n = 14), and hSlo1 + mβ1 after Ch-T (closed squares; 5 ≤ n ≤ 12). The hSlo1 τ(0) and z values obtained before and after Ch-T application were 7.0 ± 1.2 ms and 0.28 ± 0.05e, and 4.4 ± 1.0 ms and 0.22 ± 0.02e, respectively. The hSlo1 + mβ1 τ(0) and z values obtained before and after Ch-T application were 0.082 ± 0.01 s and 0.171 ± 0.02e, and 0.026 ± 0.003 s and 0.174 ± 0.02e, respectively.
Altogether, modification by Ch-T caused a much greater increase in hSlo1 open probability, an enhanced slowing of deactivation, and a distinct acceleration of activation kinetics when β1 was coexpressed. These Ch-T–induced changes specific to hSlo1 + mβ1 may involve any of the following possible mechanisms. First, given that cysteine residues are also potential targets of Ch-T under physiological conditions, oxidation of cysteine within β1 may account for the enhanced oxidative regulation of hSlo1 + mβ1. Second, oxidation of methionine within β1 may synergistically enhance the functional effects of hSlo1 oxidation. Third, the mere presence of β1 may potentiate the functional outcome of oxidation within the hSlo1 pore-forming subunit. These possible mechanisms are addressed in the next sections.
Cysless β1 Produces Results Similar to Wild-type β1
Previous results demonstrated that cysteine modification within hSlo1 is not involved in the Ch-T–mediated response (Tang et al., 2001). To test whether the enhanced effects of Ch-T on channel behavior in the presence of β1 involve modification of cysteine residues within the β1 subunit, we used a mutant bβ1 subunit devoid of any cysteine named Cysless bβ1 (Fig. 4 A; Hanner et al., 1998). Because this mutant was derived from bovine β1, we compared the results from hSlo1 + bβ1 with hSlo1 + Cysless bβ1.
Figure 4.

Cysless bβ1 resembles wild-type bβ1. (A) A schematic representation of cysteine residues (open circles) in mβ1. C26 does not exist in bβ1. Closed circles represent methionine residues. (B) Representative currents before (thin sweep) and after (thick sweep) Ch-T treatment. The currents were elicited in response to pulses from 0 to 140 mV. (C) G-V curves before and after modification by Ch-T. The hSlo1 + bβ1 V0.5 values for the results obtained before (open circles) and after (closed circles) Ch-T application were 145.5 ± 8.2 mV and 91.4 ± 9.6 mV (ΔV0.5 range, −51 to −56 mV; P = 0.0009; n = 3), respectively. The hSlo1 + Cysless bβ1 V0.5 values for the results obtained before (open squares) and after (closed squares) Ch-T application were 156.6 ± 4.5 mV and 98.5 ± 7.4 mV (ΔV0.5 range, −39 to −77 mV; P < 0.0001, n = 10), respectively. The hSlo1 + bβ1 Qapp values for the results obtained before and after Ch-T application were 0.82 ± 0.06e and 0.8 ± 0.04e (P = 0.8, n = 3), respectively. The hSlo1 + Cysless bβ1 Qapp values for the results obtained before and after Ch-T application were 0.88 ± 0.03e and 0.74 ± 0.05e, respectively (P = 0.009, n = 10). (D) Voltage dependence of the deactivation and activation time constants. Symbols are the same as in C.
Expression of Cysless bβ1 slowed the hSlo1 activation and deactivation kinetics in the control condition essentially as observed with wild-type bβ1 (Fig. 4 B), confirming that Cysless bβ1 functionally interacts with hSlo1. Ch-T treatment enhanced the currents through both hSlo1 + bβ1 and hSlo1 + Cysless bβ1 in a similar manner. After modification by Ch-T, the hSlo1 + bβ1 and hSlo1 + Cysless bβ1 G-V curves shifted leftward (Fig. 4 C), such that the mean ΔV0.5 values were indistinguishable (−54.1 ± 1.7 mV and −58.1 ± 3.6 mV, n = 3 and 10, respectively). Importantly, these ΔV0.5 values were markedly greater than those found with hSlo1 alone (ΔV0.5 ≈ −30 mV; Fig. 1 D) (P < 0.05; Bonferroni test). The ΔV0.5 values of hSlo1 + bβ1 and hSlo1 + Cysless bβ1 were smaller than that of hSlo1 + mβ1 (ΔV0.5 ≈ −75 mV; Fig. 1 D) probably because the control V0.5 values before treatment with Ch-T for the bβ1 channel complexes (∼145 and 156 mV, respectively) were already less depolarized than that of mβ1 (∼164 mV); yet, all V0.5 values after treatment with Ch-T were ∼90 mV. Nevertheless, the removal of all cysteine residues within the β1 subunit still permitted the enhanced ΔV0.5 after modification by Ch-T. Furthermore, the activation and deactivation time courses of hSlo1 + Cysless bβ1 before and after treatment with the oxidant resembled those of hSlo1 + bβ1 (Fig. 4 D). Therefore, the kinetic and G-V results for hSlo1 + mβ1, hSlo1 + bβ1, and hSlo1 + Cysless bβ1 are largely similar and suggest that oxidation of cysteine residues within β1 is not responsible for the enhanced oxidative regulation of hSlo1 in the presence of the β1 subunit.
The Greater Increase in Open Probability Does Not Depend on Methionine Oxidation within β1
The Ch-T effect on hSlo1 + β1 function did not involve cysteine oxidation but the oxidation of methionine residues within β1 may be responsible. Each mβ1 contains five methionine residues: M1, M7, M23, M89, and M177 (Fig. 5 A); the contribution of these methionines to the enhanced shift of the G-V curve and further slowing of deactivation, as well as acceleration of the activation kinetics was assessed in the following manner. Because M1 is obligatory for normal β1 synthesis, we could not readily test its role. M89 is present in mβ1 but absent in bβ1. However, both mβ1 and bβ1 confer to hSlo1 the enhanced Ch-T sensitivity, thereby excluding the critical involvement of M89 (Fig. 4). Thus, M7, M23, and M177 were individually mutated to leucine which is much less susceptible to oxidation by Ch-T than methionine (Ciorba et al., 1997). In addition, a triple mβ1 mutant (Fig. 5, Triple) in which M7, M23, and M177 were all replaced by leucine was constructed. When coexpressed with hSlo1, each mβ1 mutant channel complex exhibited currents with wild-type β1-like characteristics including slower activation and deactivation compared with hSlo1 alone, thus confirming that these mβ1 mutants functionally associated with hSlo1.
Figure 5.

Methionine mutations within mβ1 permit oxidation-related increases in hSlo1 open probability similar to wild-type mβ1. (A) A schematic representation of methionine residues (closed circles) in mβ1. Open circles represent cysteine residues. (B) G-V curves before and after modification by Ch-T. The hSlo1 + M7L, M23L, M177L, or Triple V0.5 values for the results obtained before Ch-T application (open circles) were 153.9 ± 12.5 mV (n = 4), 147.1 ± 3.2 mV (n = 5), 164.1 ± 8.3 mV (n = 5), and 153.8 ± 6.2 mV (n = 6), respectively. After Ch-T application (closed circles), the hSlo1 + M7L, M23L, M177L, or Triple V0.5 values were 85.5 ± 9 mV (ΔV0.5 range, −54 to −78 mV; P = 0.001, n = 4), 96.9 ± 6.4 mV (ΔV0.5 range, −42 to −60 mV; P = 0.0002, n = 5), 105 ± 5.9 mV (ΔV0.5 range, −43 to −71 mV; P = 0.0005, n = 5), and 103.1 ± 7.3 mV (ΔV0.5 range, −45 to −60 mV; P < 0.0001, n = 6), respectively. (C) The mean ΔV0.5 values (left) for hSlo1 + M7L, M23L, M177L, and Triple mβ1 were −68.4 ± 5.2 mV, −50.2 ± 4.1 mV, −59.0 ± 5.8 mV, and −50.7 ± 2.6 mV, respectively. The mean ΔQapp values (right) for hSlo1 + M7L, M23L, M177L, and Triple mβ1 were −0.11 ± 0.03e (P = 0.04, n = 4), −0.14 ± 0.08e (P = 0.15, n = 5), −0.019 ± 0.03e (P = 0.6, n = 5), and −0.12 ± 0.04e (P = 0.02, n = 6), respectively. A negative ΔV0.5 indicates a leftward shift of the G-V curve, and a negative ΔQapp value indicates a decrease in the slope of the G-V curve after Ch-T modification.
After modification by Ch-T, each mβ1 mutant channel complex (M7L, M23L, M177L, and Triple) showed a large leftward shift in V0.5 (Fig. 5 B). The mean V0.5 for hSlo1 + M7L, M23L, M177L, or Triple mβ1 shifted by −68 ± 5.2 mV, −50 ± 4.1 mV, −59 ± 5.7 mV, and −50.6 ± 2.6 mV, respectively (Fig. 5 C); these ΔV0.5 were significantly larger than that found with hSlo1 alone (ΔV0.5 ≈ −30 mV; Fig. 1 D) (P < 0.05, Bonferroni test). These results indicated that oxidation of methionine residues within mβ1 is not necessary to produce the enhanced G-V curve shift after modification by Ch-T.
Methionine Oxidation within β1 Is Not Required for the Greater Slowing of hSlo1 Deactivation
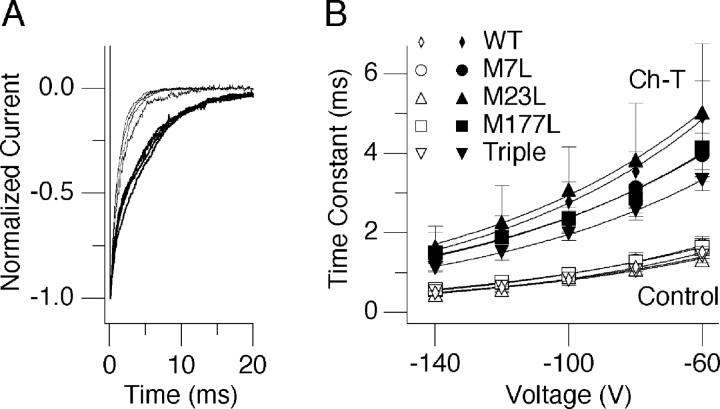
Treatment with Ch-T slowed the deactivation time course of every hSlo1+ mutant mβ1 complex examined (Fig. 6 A). The extent of this slowing of hSlo1 deactivation with any of the mβ1 mutants was indistinguishable from the slowing of hSlo1 + mβ1 (Fig. 6 B). The voltage dependence of the deactivation kinetics was also unaltered by Ch-T treatment with the equivalent charge movement remaining at ∼0.3e in all cases. Thus, the Ch-T–induced slowing of channel deactivation did not specifically require M7, M23, M177, or the presence of all three residues together in β1.
Figure 6.
Slowing of channel deactivation after oxidation by Ch-T is maintained in all mβ1 methionine mutant channel complexes. (A) Superimposed hSlo1 + M7L, M23L, M177L, or Triple mβ1 normalized tail currents recorded at −40 mV after pulses to 180 mV before (thin sweeps) and after (thick sweeps) treatment with Ch-T. (B) Voltage dependence of the deactivation time constant for hSlo1 + wild type (diamonds; n = 5), M7L (circles; n = 4), M23L (triangles; n = 5), M177L (squares; n = 5), or Triple (inverted triangles; n = 6) mβ1 before (open symbols) and after (closed symbols) Ch-T treatment.
M177 in mβ1 Is Critical for Typical hSlo1 Activation Properties
Oxidative modification by Ch-T accelerated the activation kinetics of hSlo1 + mβ1 but not of hSlo1 alone (Fig. 3). For channel complexes that included an mβ1 methionine point mutant, there was a trend for the activation kinetics to be faster than hSlo1+ wild-type mβ1 even before Ch-T treatment (Fig. 7 A). However, a difference in the activation time course was statistically significant only in hSlo1 + M177L mβ1 (220 mV; P = 0.002, Bonferroni test). In fact, the activation kinetics of hSlo1 + M177L mβ1 before Ch-T treatment was similar to that of the hSlo1+ wild-type mβ1 complex after modification by Ch-T.
Figure 7.
M177 in mβ1 specifically affects channel activation. (A) Voltage dependence of the activation time constant before treatment with Ch-T for hSlo1 + wild type (diamonds; n = 14), M7L (circles; n = 4), M23L (triangles; n = 5), or M177L (squares; n = 5) mβ1. (B) Currents recorded at 220 mV from the holding voltage of 0 mV before (thin sweep) and after (thick sweep) modification by Ch-T. (C) Activation time constant values at 220 mV before and after oxidation by Ch-T from individual experiments.
The activation kinetics of hSlo1 + M7L mβ1 and hSlo1 + M23L mβ1 after Ch-T treatment were significantly faster, as found with wild-type mβ1 (Fig. 7, B and C; P = 0.005 and 0.03, respectively). However, Ch-T failed to accelerate the activation time course of hSlo1 + M177L mβ1 in an appreciable manner (P ≥ 0.085). M177L mβ1 does associate with hSlo1 because the deactivation kinetics of hSlo1 + M177L mβ1 was indistinguishable from hSlo1 + mβ1 (Fig. 6 B). Thus, M177 in TM2 of β1 is a key determinant of the activation kinetics and the oxidative sensitivity of hSlo1 + mβ1.
The Effect of Ch-T Treatment with β1 Present Is Ca2+ Dependent
The hyperpolarizing shift in V0.5 caused by treatment with Ch-T was essentially eliminated by increasing [Ca2+]i to 2.1 μM (Fig. 8 A). Similar results were obtained with saturating levels of divalent ions, [Ca2+]i = 120 μM and [Mg2+]i = 10 mM (Fig. 8 B). In the presence of elevated [Ca2+]i, cysteine oxidation is capable of shifting the G-V curve to the right (Tang et al., 2004), which may account for the depolarizing shift seen here after treatment with Ch-T. With the assumption that the free energy changes associated with the BKCa channel intrinsic opening process, voltage-dependent activation, and Ca2+ binding together contribute to the overall open probability in a linearly additive manner (Cui and Aldrich, 2000), the measured G-V parameters were used to infer the free energy contributions of Ca2+ to channel opening (Tang et al., 2004) in the control and Ch-T treated conditions. The decrease in ΔGCa (∼50%) after Ch-T modification of hSlo1 + mβ1 in 2.1 μM [Ca2+]i indicates that Ca2+ makes a smaller free energy contribution to overall channel opening after oxidation (Fig. 8 A, inset).
Figure 8.
The effect of Ch-T on channel open probability depends on Ca2+. (A) G-V curves before and after modification by Ch-T. Currents were first generated by pulsing to different test potentials from a holding voltage of 0 mV in the virtual absence of Ca2+. This recording protocol was repeated after bath application of 2.1 μM Ca2+. Tail currents were measured at −50 mV in zero [Ca2+]i or −100 mV in high [Ca2+]i. After a return to zero Ca2+ for treatment with 2 mM Ch-T, the recording protocol was again repeated in zero and then 2.1 μM Ca2+. The zero [Ca2+]i V0.5 values for the results obtained before (open circles) and after (closed circles) Ch-T application were 155.3 ± 5.1 mV and 103.5 ± 7.2 mV (ΔV0.5 range, −47 to −56 mV; P = 0.002, n = 3), respectively. The smaller ΔV0.5 value (approximately −50 vs. −75 mV; Fig. 1) was most likely because of the use of an external recording solution containing less K+ than that previously used in the zero [Ca2+]i experiments. The 2.1 μM [Ca2+]i V0.5 values for the results obtained before (open squares) and after (closed squares) Ch-T application were 9.1 ± 11.2 mV and 29.6 ± 12.5 mV (ΔV0.5 range, 15–25 mV; P = 0.018, n = 3), respectively. The zero [Ca2+]i Qapp values for the results obtained before and after Ch-T application were 0.84 ± 0.04e and 0.79 ± 0.02e (P = 0.13, n = 3), respectively. The 2.1 μM [Ca2+]i Qapp values for the results obtained before and after Ch-T application were 1.03 ± 0.07e and 0.88 ± 0.03e (P = 0.1, n = 3), respectively. (inset) The contribution of Ca2+-dependent gating to overall channel opening (ΔGCa) before and after oxidation by Ch-T from individual experiments. (B) Representative currents from a single patch (n = 5) in the presence of 120 μM [Ca2+]i before (thin sweeps) and after (thick sweeps) Ch-T treatment. The currents were elicited from a holding voltage of −200 mV.
Biophysical Model Simulation
The functional effects of oxidation by Ch-T of the hSlo1 + β1 complex in the virtual absence of Ca2+ may be interpreted using the HCA allosteric gating model (Fig. 9 A; Horrigan et al., 1999) as performed for the hSlo1 channel without β1 (Tang et al., 2001). The voltage dependence of hSlo1 alone shifts by −30 mV, and the deactivation time course slows after oxidation by Ch-T. To account for these alterations, Tang et al. (2001) increased the value of the strongly voltage-dependent parameter α(0), which may correspond to movement of the voltage sensor (Horrigan et al., 1999), by 2.3-fold and decreased the rate constant of the closing transition dominant at negative voltages (γ0) by 60% (Fig. 9 B; Tang et al., 2001, Fig. 14). We simulated the potentiated effects of Ch-T treatment in the presence of β1 in the following manner. First, the value of α(0) is further increased (about twofold) to account for the larger shift, −75 mV, of the G-V curve (Fig. 9 B). Second, the closing rate constant γ0 decreases by an additional 40% to account for the greater slowing of the tail kinetics. Third, in addition to the two quantitative changes listed, the rate constant for the opening transition dominant at positive voltages (δ4) is increased by 2.1-fold to account for the unique acceleration of the activation kinetics observed in hSlo1 + β1 but not in hSlo1 alone. Simulated data produced from this model that account for the effect of Ch-T on hSlo1 function with β1 present match the experimental data (Fig. 9, C–E).
Figure 9.
Simulation of oxidation by Ch-T on hSlo1 + β1 function based on the HCA model. (A) The HCA allosteric gating model (Horrigan et al., 1999). The most probable opening of the channel at strongly depolarized voltages involves transitions from C0 to C1, C2, C3, C4, and then O4. Likewise, channel closing at negative voltages entails transitions from O4 to O3, O2, O1, O0, and then C0. (B) Adjustments in average parameter values from the HCA model required to simulate the effect of modification by Ch-T on hSlo1 (Tang et al., 2001) or hSlo1 + β1 function. HCA model values are as follows: α(0) = 1,500 s−1, β(0) = 35,370 s−1, δ0(0) = 0.007 s−1, δ1(0) = 0.154 s−1, δ2(0) = 3.39 s−1, δ3(0) = 52 s−1, δ4(0) = 65 s−1, D = 22, f = D 0.5, and L(0) = δ0(0)/γ0(0) = 2 × 10−6. L(0) represents the open-to-closed equilibrium constant in the absence of an applied voltage. (C) Experimental hSlo1 + mβ1 currents (continuous sweeps) recorded at 160 mV before (thin) and after (thick) oxidation by Ch-T and simulated currents (dashed lines) from the HCA model adjusted for the effect of Ch-T in the presence of β1. (D) G-V curves from a patch containing hSlo1 + mβ1 before (open circles) and after (closed circles) treatment with Ch-T. Data simulated from the hSlo1 + β1 model before (dotted line) and after (continuous line) Ch-T are superimposed. (E) Activation/deactivation time constants. Symbols are the same as in D.
DISCUSSION
Methionine Oxidation Leads to Distinct Alterations in hSlo1 + β1 Function
Coexpression of β1 with hSlo1 is known to affect the activation/deactivation kinetics and apparent Ca2+ sensitivity of the channel (Orio et al., 2002). Here, we have demonstrated that oxidation of hSlo1 in the presence of the auxiliary subunit β1 leads to functional effects clearly distinguishable from those observed with hSlo1 alone. Oxidation of hSlo1 promoted by Ch-T causes a leftward shift of the G-V curve by ∼30 mV. However, this hyperpolarizing shift is more than twice as great (∼75 mV) in the presence of β1. Furthermore, the Ch-T–induced slowing of hSlo1 deactivation is even more dramatic with β1 present. In addition, β1 confers a novel effect of oxidation not observed with hSlo1 alone; modification by Ch-T leads to the distinct acceleration of hSlo1 activation evident at each depolarized voltage only with the inclusion of β1 into the channel complex. These unique features of oxidative modification in the presence of the β1 subunit overall cannot be accounted for by a difference in the modification rate as compared with hSlo1 alone or a simple voltage-dependent shift in the open probability and activation/deactivation kinetics.
Role of Cysteine and Methionine Residues in β1
Because Ch-T preferentially oxidizes methionine residues under physiological conditions (Levine et al., 1996), methionine is implicated as the main target of oxidation by Ch-T that is responsible for the observed functional alterations in both hSlo1 and hSlo1 + β1. However, protein-modifying agents may not be perfectly specific for one particular amino acid. In fact, both cysteine and methionine are possible physiological targets of Ch-T. To determine if cysteine oxidation plays a role in the Ch-T effect on hSlo1 alone, Tang et al. (2001) previously showed that cysteine-specific reagents (5,5′-dithio-bis (2-nitrobenzoic acid), methanethiosulfonate ethylammonium, and p-chloromercuribenzoic acid) actually decreased channel activity, thereby demonstrating that cysteine oxidation has opposite effects on channel function than methionine oxidation. Furthermore, the Ch-T–induced potentiation was maintained in hSlo1 mutants that lacked most of the cysteine residues within the channel. Finally, peptide methionine sulfoxide reductase, an enzyme that catalyzes the reduction of met-O (Weissbach et al., 2002), partially reversed the effect of Ch-T treatment. Therefore, the functional alterations caused by Ch-T were attributed to methionine oxidation within hSlo1.
Cysteine residues within β1 are not required for typical regulation of hSlo1 kinetics or the enhanced effects on channel function after oxidation. The bβ1 subunit devoid of any cysteine residues behaves much like wild-type bβ1 in terms of slowing hSlo1 activation and deactivation. Furthermore, after oxidation by Ch-T, the cysteine mutations still permit the significantly larger ΔV0.5 value, the slower deactivation kinetics and the accelerated channel activation similar to those observed with wild-type bβ1. These results indicate that cysteine is not the likely Ch-T target responsible for causing the functional changes after oxidation.
Similar to the bβ1 cysteine mutant, the mβ1 methionine mutants regulate hSlo1 kinetics much like wild-type β1. Moreover, all mβ1 methionine mutants including the triple mutant maintain the dramatic shift of the G-V curve toward the hyperpolarizing direction and the enhanced slowing of channel deactivation after oxidation by Ch-T. Evaluation of the role of the initial β1 methionine residue (M1) is not straightforward. However, its contribution to the enhanced oxidative regulation of hSlo1, although a possibility, is unlikely due to potential removal by posttranslational processing of the mature protein (Creighton, 1993). Therefore, the presence of the β1 subunit provides the possibility to amplify the functional effects of methionine oxidation within the hSlo1 pore-forming subunit with regard to channel open probability and deactivation.
β1 M177 Involvement in the Functional Interaction with Slo1
Although the enhanced shift of the G-V curve and slowing of hSlo1 deactivation does not require cysteine or methionine residues within β1, the effect of oxidation on hSlo1 activation critically depends on M177 in TM2 of mβ1. In the control condition, only M177L mβ1 causes a significant difference in the channel activation time course. Furthermore, the M177L mutation eliminates the oxidative sensitivity of channel activation typically observed with β1 present. Thus, M177 controls the hSlo1 activation kinetics at very positive voltages, which is described by the rate constant δ4 in the HCA model (Fig. 9), and oxidation of M177 to met-O most likely mediates the Ch-T–induced acceleration of activation kinetics. However, the possibility that the M177L mutation hinders the access of Ch-T to its target elsewhere cannot be completely eliminated.
The mutant-specific effect on channel activation suggests a partial uncoupling of hSlo1 and β1 because of mutation or oxidation at the M177 position. Because the activation kinetics of hSlo1 is faster without β1, oxidation of hSlo1 + β1 may cause channel activation to be more like hSlo1 alone by removal of the β1 influence. The hydrophobic leucine mutation at M177 mimics the presence of met-O at that location because the control activation kinetics of hSlo1 + M177L mβ1 resembles that of oxidized hSlo1+ wild-type mβ1. Indeed, an increase in surface hydrophobicity, while somewhat paradoxical, has been shown after oxidation of methionine residues within the enzyme glutamine synthetase (Levine et al., 1996). Perhaps oxidation of M177 to met-O, whereby acting as the sensor or switch, partially disrupts an interaction between the β1 subunit and the structural elements in or near the RCK (regulator of K+ conductance) domains within hSlo1 that are specifically responsible for controlling activation kinetics. Similar to the effect of β1 M177 on channel activation, other residues within different β subunits influence functional coupling of the auxiliary subunit and hSlo1. For example, the phosphorylation states of T11/S17 in the cytoplasmic NH2 terminus and S210 in the cytoplasmic COOH terminus within β4 affect the functional coupling between hSlo1 and β4, as determined by changes in channel voltage dependence and activation/deactivation kinetics specific to modification of the different residues (Jin et al., 2002).
Physiological Implications
As found with hSlo1 alone, the effect of methionine oxidation on hSlo1 function in the presence of β1 is sensitive to [Ca2+]i. In the virtual absence of [Ca2+]i, hSlo1 + mβ1 displays a hyperpolarizing shift of V0.5 that is twice as great as hSlo1 alone after oxidation by Ch-T. This Ch-T–induced shift resembles the presence of ∼0.4 μM [Ca2+]i (Cox and Aldrich, 2000). The oxidized channel complex can open in the physiological voltage range (<50 mV) without [Ca2+]i as further evidenced by the increase in open probability observed at −40 mV. An increase in channel open probability at low [Ca2+]i could have an impact on resting BKCa channel activity in smooth muscle cells, thereby influencing vascular tone. Because BKCa channels crucially shape the action potential posthyperpolarization phase in certain cell types, this increase in channel open probability may prevent unregulated neuronal firing (Lancaster and Nicoll, 1987; Storm, 1987; Marsh and Brown, 1991; Zhang and McBain, 1995; Pedarzani et al., 2000; Faber and Sah, 2002; Edgerton and Reinhart, 2003).
The concept that the binding of Ca2+ performs mechanical work to open the Slo1 channel (Jiang et al., 2002) has been further developed into a spring-based gating mechanism in which the diameter of the gating ring, formed by the RCK domains from each Slo1 subunit, expands upon Ca2+ binding, thereby generating an active force that pulls the S6-RCK1 linker regions that act as the springs, thus opening the channel gates (Niu et al., 2004). This proposed gating process might be similarly affected by methionine oxidation, which biases the open channel state. In the absence of [Ca2+]i, oxidation of methionine residues to met-O within the hSlo1 pore-forming subunit may likewise affect the structure or position of the gating ring ultimately influencing gating of the channel. The lack of a hyperpolarizing shift of V0.5 in response to modification by Ch-T at high [Ca2+]i indicates that the effects of Ca2+ and methionine oxidation on channel gating are not additive and may in fact operate on the same effectors. In the case of the hSlo1 + β1 channel complex, the presence of β1 may cause a unique conformational change in hSlo1, such that additional methionine residues in hSlo1 are exposed and able to react with Ch-T, thereby accounting for the enhanced functional effects of oxidation. However, the similarity in the modification time courses of hSlo1 and hSlo1 + mβ1 argues against this possibility.
Modification of ion channels by ROS/RNS during oxidative stress could alter channel function and eventually disrupt normal [Ca2+]i and other homeostatic parameters (Kourie, 1998). Potential consequences of oxidative stress include accelerated aging (Hensley and Floyd, 2002), as well as pathophysiological conditions such as various neurodegenerative disorders (Coyle and Puttfarcken, 1993) and ischemia-reperfusion injury after stroke (Babbs, 1988; Rubanyi, 1988). However, certain ion channel modifications by ROS/RNS may serve as compensatory mechanisms to oxidative assault. One such example involves the mitochondrial ATP-sensitive K+ channel (mitoKATP) that is activated by ROS during initial, mild ischemia; as a result, the heart is preconditioned to future ischemic attacks and infarctions (Szewczyk and Marban, 1999; Grover and Garlid, 2000; Zhang et al., 2001). Much like the mitoKATP channel, the BKCa channel clearly represents a prime candidate for aiding in the recovery from ROS/RNS attack given its localization in brain and smooth muscle, as well as the documented oxidation-related alteration of its function (DiChiara and Reinhart, 1997; Sobey et al., 1997; Wang and Wu, 1997; Wang et al., 1997; Barlow et al., 2000; Gong et al., 2000; Soh et al., 2001; Tang et al., 2001, 2004; Brakemeier et al., 2003). Whether the BKCa channel contributes to the progression of oxidative stress-related conditions or instead serves a more compensatory role—such as maintaining resting membrane potential if [Ca2+]i is disrupted—remains to be determined.
In summary, we showed that in the virtual absence of Ca2+, methionine oxidation by Ch-T dramatically alters hSlo1 function with the association of the β1 subunit. The presence of β1 as opposed to methionine and/or cysteine oxidation within this auxiliary subunit greatly amplifies the increase in channel open probability and the slowing of deactivation derived from oxidation of the hSlo1 pore-forming subunit. The target methionine residues within hSlo1 are not yet known, but may be found in the S5/P/S6 segments (Tang et al., 2001) and/or the gating ring region (Niu et al., 2004). In contrast, M177 within β1 influences hSlo1 activation and most likely serves as the methionine target responsible for the acceleration in channel activation after methionine oxidation in the presence of the β1 subunit. Testing the oxidative effects with β subunits present provides more relevant results that can then be readily extended to physiological or pathophysiological conditions. Whether the effect of methionine oxidation on hSlo function in the presence of other β subunits (β2–4) also occurs remains to be determined, but β1 clearly facilitates unique modulation of channel function in the face of oxidation.
Acknowledgments
We thank Dr. D.R. Wassef and P. Rothenberg for help with mutant construction, Dr. R. Xu for cell culture, Dr. M.L. Garcia for the generous gift of the Cysless β1 mutant as well as helpful discussions, Dr. X.D. Tang for physiology discussions, and D. Armstrong and V.P. Santarelli for their views and advice.
This work was supported in part by grants from the National Institutes of Health (to L.C. Santarelli and T. Hoshi), Thüringer Ministerium für Wissenschaft B307-04004 (to S.H. Heinemann), and the National Natural Science Foundation of China (grant 30270351 to J. Chen).
Olaf S. Andersen served as editor.
Abbreviations used in this paper: BKCa channel, large conductance calcium-activated potassium channel; Ch-T, chloramine-T; ΔGCa, change in free energy change associated with Ca2+ binding; met-O, methionine sulfoxide; Qapp, apparent equivalent charge movement; ROS/RNS, reactive oxygen/nitrogen species; V0.5, half-activation voltage; z, equivalent charge.
References
- Amberg, G.C., and L.F. Santana. 2003. Downregulation of the BK channel β1 subunit in genetic hypertension. Circ. Res. 93:965–971. [DOI] [PubMed] [Google Scholar]
- Amberg, G.C., A.D. Bonev, C.F. Rossow, M.T. Nelson, and L.F. Santana. 2003. Modulation of the molecular composition of large conductance, Ca2+ activated K+ channels in vascular smooth muscle during hypertension. J. Clin. Invest. 112:717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avdonin, V., and T. Hoshi. 2001. Modification of voltage-dependent gating of potassium channels by free form of tryptophan side chain. Biophys. J. 81:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avdonin, V., X.D. Tang, and T. Hoshi. 2003. Stimulatory action of internal protons on Slo1 BK channels. Biophys. J. 84:2969–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbs, C.F. 1988. Reperfusion injury of postischemic tissues. Ann. Emerg. Med. 17:1148–1157. [DOI] [PubMed] [Google Scholar]
- Barlow, R.S., A.M. El-Mowafy, and R.E. White. 2000. H2O2 opens BKCa channels via the PLA2-arachidonic acid signaling cascade in coronary artery smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 279:H475–H483. [DOI] [PubMed] [Google Scholar]
- Brakemeier, S., I. Eichler, A. Knorr, T. Fassheber, R. Kohler, and J. Hoyer. 2003. Modulation of Ca2+-activated K+ channel in renal artery endothelium in situ by nitric oxide and reactive oxygen species. Kidney Int. 64:199–207. [DOI] [PubMed] [Google Scholar]
- Brenner, R., T.J. Jegla, A. Wickenden, Y. Liu, and R.W. Aldrich. 2000. a. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J. Biol. Chem. 275:6453–6461. [DOI] [PubMed] [Google Scholar]
- Brenner, R., G.J. Perez, A.D. Bonev, D.M. Eckman, J.C. Kosek, S.W. Wiler, A.J. Patterson, M.T. Nelson, and R.W. Aldrich. 2000. b. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature. 407:870–876. [DOI] [PubMed] [Google Scholar]
- Butterfield, D.A., B.J. Howard, and M.A. LaFontaine. 2001. Brain oxidative stress in animal models of accelerated aging and the age-related neurodegenerative disorders, Alzheimer's disease and Huntington's disease. Curr. Med. Chem. 8:815–828. [DOI] [PubMed] [Google Scholar]
- Ciorba, M.A., S.H. Heinemann, H. Weissbach, N. Brot, and T. Hoshi. 1997. Modulation of potassium channel function by methionine oxidation and reduction. Proc. Natl. Acad. Sci. USA. 94:9932–9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, D.H., and R.W. Aldrich. 2000. Role of the β1 subunit in large-conductance Ca2+-activated K+ channel gating energetics. Mechanisms of enhanced Ca2+ sensitivity. J. Gen. Physiol. 116:411–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle, J.T., and P. Puttfarcken. 1993. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 262:689–695. [DOI] [PubMed] [Google Scholar]
- Creighton, T.E. 1993. Proteins: Structures and Molecular Properties. 2nd edition. W.H. Freeman Publishers, New York. 507 pp.
- Cui, J., and R.W. Aldrich. 2000. Allosteric linkage between voltage and Ca2+-dependent activation of BK-type mslo1 K+ channels. Biochemistry. 39:15612–15619. [DOI] [PubMed] [Google Scholar]
- DiChiara, T.J., and P.H. Reinhart. 1997. Redox modulation of hslo Ca2+-activated K+ channels. J. Neurosci. 17:4942–4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworetzky, S.I., J.T. Trojnacki, and V.K. Gribkoff. 1994. Cloning and expression of a human large-conductance calcium-activated potassium channel. Brain Res. Mol. Brain Res. 27:189–193. [DOI] [PubMed] [Google Scholar]
- Edgerton, J.R., and P.H. Reinhart. 2003. Distinct contributions of small and large conductance Ca2+-activated K+ channels to rat Purkinje neuron function. J. Physiol. 548:53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber, E.S., and P. Sah. 2002. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J. Neurosci. 22:1618–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Fernandez, J.M., M. Tomas, E. Vazquez, P. Orio, R. Latorre, M. Senti, J. Marrugat, and M.A. Valverde. 2004. Gain-of-function mutation in the KCNMB1 potassium channel subunit is associated with low prevalence of diastolic hypertension. J. Clin. Invest. 113:1032–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Calvo, M., H.G. Knaus, O.B. McManus, K.M. Giangiacomo, G.J. Kaczorowski, and M.L. Garcia. 1994. Purification and reconstitution of the high-conductance, calcium-activated potassium channel from tracheal smooth muscle. J. Biol. Chem. 269:676–682. [PubMed] [Google Scholar]
- Giangiacomo, K.M., M. Garcia-Calvo, K. Hans-Gunther, T.J. Mullmann, M.L. Garcia, and O. McManus. 1995. Functional reconstitution of the large-conductance, calcium-activated potassium channel purified from bovine aortic smooth muscle. Biochemistry. 34:15849–15862. [DOI] [PubMed] [Google Scholar]
- Gollasch, M., J. Tank, F.C. Luft, J. Jordan, P. Maass, C. Krasko, A.M. Sharma, A. Busjahn, and S. Bahring. 2002. The BK channel β1 subunit gene is associated with human baroreflex and blood pressure regulation. J. Hypertens. 20:927–933. [DOI] [PubMed] [Google Scholar]
- Gong, L., T.M. Gao, H. Huang, and Z. Tong. 2000. Redox modulation of large conductance calcium-activated potassium channels in CA1 pyramidal neurons from adult rat hippocampus. Neurosci. Lett. 286:191–194. [DOI] [PubMed] [Google Scholar]
- Grover, G.J., and K.D. Garlid. 2000. ATP-sensitive potassium channels: a review of their cardioprotective pharmacology. J. Mol. Cell. Cardiol. 32:677–695. [DOI] [PubMed] [Google Scholar]
- Hanner, M., R. Vianna-Jorge, A. Kamassah, W.A. Schmalhofer, H.G. Knaus, G.J. Kaczorowski, and M.L. Garcia. 1998. The β subunit of the high conductance calcium-activated potassium channel. Identification of residues involved in charybdotoxin binding. J. Biol. Chem. 273:16289–16296. [DOI] [PubMed] [Google Scholar]
- Hensley, K., and R.A. Floyd. 2002. Reactive oxygen species and protein oxidation in aging: a look back, a look ahead. Arch. Biochem. Biophys. 397:377–383. [DOI] [PubMed] [Google Scholar]
- Horrigan, F.T., and R.W. Aldrich. 1999. Allosteric voltage gating of potassium channels II. mslo channel gating charge movement in the absence of Ca2+. J. Gen. Physiol. 114:305–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan, F.T., J. Cui, and R.W. Aldrich. 1999. Allosteric voltage gating of potassium channels I. mslo ionic currents in the absence of Ca2+. J. Gen. Physiol. 114:277–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar, J.H., V.A. Porter, W.J. Lederer, and M.T. Nelson. 2000. Calcium sparks in smooth muscle. Am. J. Physiol. Cell Physiol. 278:C235–C256. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., A. Lee, J. Chen, M. Cadene, B.T. Chait, and R. MacKinnon. 2002. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 417:515–522. [DOI] [PubMed] [Google Scholar]
- Jiang, Z., M. Wallner, P. Meera, and L. Toro. 1999. Human and rodent MaxiK channel β-subunit genes: cloning and characterization. Genomics. 55:57–67. [DOI] [PubMed] [Google Scholar]
- Jin, P., T.M. Weiger, Y. Wu, and I.B. Levitan. 2002. Phosphorylation-dependent functional coupling of hSlo calcium-dependent potassium channel and its hβ4 subunit. J. Biol. Chem. 277:10014–10020. [DOI] [PubMed] [Google Scholar]
- Knaus, H.G., K. Folander, M. Garcia-Calvo, M.L. Garcia, G.J. Kaczorowski, M. Smith, and R. Swanson. 1994. Primary sequence and immunological characterization of β-subunit of high conductance Ca2+-activated K+ channel from smooth muscle. J. Biol. Chem. 269:17274–17278. [PubMed] [Google Scholar]
- Knight, J.A. 1997. Reactive oxygen species and the neurodegenerative disorders. Ann. Clin. Lab. Sci. 27:11–25. [PubMed] [Google Scholar]
- Kourie, J.I. 1998. Interaction of reactive oxygen species with ion transport mechanisms. Am. J. Physiol. 275:C1–C24. [DOI] [PubMed] [Google Scholar]
- Lancaster, B., and R.A. Nicoll. 1987. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J. Physiol. 389:187–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, R.L., L. Mosoni, B.S. Berlett, and E.R. Stadtman. 1996. Methionine residues as endogenous antioxidants in proteins. Proc. Natl. Acad. Sci. USA. 93:15036–15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markesbery, W.R. 1997. Oxidative stress hypothesis in Alzheimer's disease. Free Radic. Biol. Med. 23:134–147. [DOI] [PubMed] [Google Scholar]
- Marsh, S.J., and D.A. Brown. 1991. Potassium currents contributing to action potential repolarization in dissociated cultured rat superior cervical sympathetic neurones. Neurosci. Lett. 133:298–302. [DOI] [PubMed] [Google Scholar]
- McManus, O.B., L.M. Helms, L. Pallanck, B. Ganetzky, R. Swanson, and R.J. Leonard. 1995. Functional role of the β subunit of high conductance calcium-activated potassium channels. Neuron. 14:645–650. [DOI] [PubMed] [Google Scholar]
- Meera, P., M. Wallner, Z. Jiang, and L. Toro. 1996. A calcium switch for the functional coupling between α (hslo) and β subunits (KV,Ca β) of maxi K channels. FEBS Lett. 382:84–88. [DOI] [PubMed] [Google Scholar]
- Meera, P., M. Wallner, M. Song, and L. Toro. 1997. Large conductance voltage- and calcium-dependent K+ channel, a distinct member of voltage-dependent ion channels with seven N-terminal transmembrane segments (S0-S6), an extracellular N terminus, and an intracellular (S9-S10) C terminus. Proc. Natl. Acad. Sci. USA. 94:14066–14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith, A.L., K.S. Thorneloe, M.E. Werner, M.T. Nelson, and R.W. Aldrich. 2004. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J. Biol. Chem. 10.1074/jbc.M405621200. [DOI] [PubMed]
- Nelson, M.T., H. Cheng, M. Rubart, L.F. Santana, A.D. Bonev, H.J. Knot, and W.J. Lederer. 1995. Relaxation of arterial smooth muscle by calcium sparks. Science. 270:633–637. [DOI] [PubMed] [Google Scholar]
- Nimigean, C.M., and K.L. Magleby. 1999. The β subunit increases the Ca2+ sensitivity of large conductance Ca2+-activated potassium channels by retaining the gating in the bursting states. J. Gen. Physiol. 113:425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimigean, C.M., and K.M. Magleby. 2000. Functional coupling of the β1 subunit to the large conductance Ca2+-activated K+ channel in the absence of Ca2+: increased Ca2+ sensitivity from a Ca2+-independent mechanism. J. Gen. Physiol. 115:719–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu, X., X. Qian, and K.L. Magleby. 2004. Linker-gating ring complex as passive spring and Ca2+-dependent machine for a voltage- and Ca2+-activated potassium channel. Neuron. 42:745–756. [DOI] [PubMed] [Google Scholar]
- Orio, P., P. Rojas, G. Ferreira, and R. Latorre. 2002. New disguises for an old channel: MaxiK channel β-subunits. News Physiol. Sci. 17:156–161. [DOI] [PubMed] [Google Scholar]
- Pallanck, L., and B. Ganetzky. 1994. Cloning and characterization of human and mouse homologs of the Drosophila calcium-activated potassium channel gene, slowpoke. Hum. Mol. Genet. 3:1239–1243. [DOI] [PubMed] [Google Scholar]
- Patterson, A.J., J. Henrie-Olson, and R. Brenner. 2002. Vasoregulation at the molecular level: a role for the β1 subunit of the calcium-activated potassium (BK) channel. Trends Cardiovasc. Med. 12:78–82. [DOI] [PubMed] [Google Scholar]
- Pedarzani, P., A. Kulik, M. Muller, K. Ballanyi, and M. Stocker. 2000. Molecular determinants of Ca2+-dependent K+ channel function in rat dorsal vagal neurones. J. Physiol. 527:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluger, S., J. Faulhaber, M. Furstenau, M. Lohn, R. Waldschutz, M. Gollasch, H. Haller, F.C. Luft, H. Ehmke, and O. Pongs. 2000. Mice with disrupted BK channel β1 subunit gene feature abnormal Ca2+ Spark/STOC coupling and elevated blood pressure. Circ. Res. 87:E53–E60. [DOI] [PubMed] [Google Scholar]
- Qian, X., and K.L. Magleby. 2003. β1 subunits facilitate gating of BK channels by acting through the Ca2+, but not the Mg2+, activating mechanisms. Proc. Natl. Acad. Sci. USA. 100:10061–10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi, G.M. 1988. Vascular effects of oxygen-derived free radicals. Free Radic. Biol. Med. 4:107–120. [DOI] [PubMed] [Google Scholar]
- Sausbier, M., H. Hu, C. Arntz, S. Feil, S. Kamm, H. Adelsberger, U. Sausbier, C.A. Sailer, R. Feil, F. Hofmann, et al. 2004. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc. Natl. Acad. Sci. USA. 101:9474–9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobey, C.G., D.D. Heistad, and F.M. Faraci. 1997. Mechanisms of bradykinin-induced cerebral vasodilatation in rats. Evidence that reactive oxygen species activate K+ channels. Stroke. 28:2290–2294. [DOI] [PubMed] [Google Scholar]
- Soh, H., W. Jung, D.Y. Uhm, and S. Chung. 2001. Modulation of large conductance calcium-activated potassium channels from rat hippocampal neurons by glutathione. Neurosci. Lett. 298:115–118. [DOI] [PubMed] [Google Scholar]
- Soto, M.A., C. Gonzalez, E. Lissi, C. Vergara, and R. Latorre. 2002. Ca2+-activated K+ channel inhibition by reactive oxygen species. Am. J. Physiol. Cell Physiol. 282:C461–C471. [DOI] [PubMed] [Google Scholar]
- Storm, J.F. 1987. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J. Physiol. 385:733–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk, A., and E. Marban. 1999. Mitochondria: a new target for K channel openers? Trends Pharmacol. Sci. 20:157–161. [DOI] [PubMed] [Google Scholar]
- Tanaka, Y., P. Meera, M. Song, H.G. Knaus, and L. Toro. 1997. Molecular constituents of maxi KCa channels in human coronary smooth muscle: predominant α + β subunit complexes. J. Physiol. 502:545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, X.D., H. Daggett, M. Hanner, M.L. Garcia, O.B. McManus, N. Brot, H. Weissbach, S.H. Heinemann, and T. Hoshi. 2001. Oxidative regulation of large conductance calcium-activated potassium channels. J. Gen. Physiol. 117:253–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, X.D., M.L. Garcia, S.H. Heinemann, and T. Hoshi. 2004. Reactive oxygen species impair Slo1 BK channel function by altering cysteine-mediated calcium sensing. Nat. Struct. Mol. Biol. 11:171–178. [DOI] [PubMed] [Google Scholar]
- Taniyama, Y., and K.K. Griendling. 2003. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 42:1075–1081. [DOI] [PubMed] [Google Scholar]
- Tseng-Crank, J., C.D. Foster, J.D. Krause, R. Mertz, N. Godinot, T.J. DiChiara, and P.H. Reinhart. 1994. Cloning, expression, and distribution of functionally distinct Ca2+- activated K+ channel isoforms from human brain. Neuron. 13:1315–1330. [DOI] [PubMed] [Google Scholar]
- Tseng-Crank, J., N. Godinot, T.E. Johansen, P.K. Ahring, D. Strobaek, R. Mertz, C.D. Foster, S.P. Olesen, and P.H. Reinhart. 1996. Cloning, expression, and distribution of a Ca2+-activated K+ channel β-subunit from human brain. Proc. Natl. Acad. Sci. USA. 93:9200–9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebele, V.N., A. Lagrutta, T. Wade, D.J. Figueroa, Y. Liu, E. McKenna, C.P. Austin, P.B. Bennett, and R. Swanson. 2000. Cloning and functional expression of two families of β-subunits of the large conductance calcium-activated K+ channel. J. Biol. Chem. 275:23211–23218. [DOI] [PubMed] [Google Scholar]
- Vogalis, F., T. Vincent, I. Qureshi, F. Schmalz, M.W. Ward, K.M. Sanders, and B. Horowitz. 1996. Cloning and expression of the large-conductance Ca2+-activated K+ channel from colonic smooth muscle. Am. J. Physiol. 271:G629–G639. [DOI] [PubMed] [Google Scholar]
- Wallner, M., P. Meera, M. Ottolia, G.J. Kaczorowski, R. Latorre, M.L. Garcia, E. Stefani, and L. Toro. 1995. Characterization of and modulation by a β-subunit of a human maxi KCa channel cloned from myometrium. Receptors Channels. 3:185–199. [PubMed] [Google Scholar]
- Wallner, M., P. Meera, and L. Toro. 1996. Determinant for β-subunit regulation in high-conductance voltage-activated and Ca2+-sensitive K+ channels: an additional transmembrane region at the N terminus. Proc. Natl. Acad. Sci. USA. 93:14922–14927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R., and L. Wu. 1997. The chemical modification of KCa channels by carbon monoxide in vascular smooth muscle cells. J. Biol. Chem. 272:8222–8226. [DOI] [PubMed] [Google Scholar]
- Wang, Z.W., M. Nara, Y.X. Wang, and M.I. Kotlikoff. 1997. Redox regulation of large conductance Ca2+-activated K+ channels in smooth muscle cells. J. Gen. Physiol. 110:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner, S.G., R.O. Koch, A. Koschak, M. Trieb, M.L. Garcia, G.J. Kaczorowski, and H.G. Knaus. 1999. High-conductance calcium-activated potassium channels in rat brain: pharmacology, distribution, and subunit composition. Biochemistry. 38:5392–5400. [DOI] [PubMed] [Google Scholar]
- Weiger, T.M., A. Hermann, and I.B. Levitan. 2002. Modulation of calcium-activated potassium channels. J. Comp. Physiol. A Neuroethol. Sens Neural. Behav. Physiol. 188:79–87. [DOI] [PubMed] [Google Scholar]
- Weiger, T.M., M.H. Holmqvist, I.B. Levitan, F.T. Clark, S. Sprague, W.J. Huang, P. Ge, C. Wang, D. Lawson, M.E. Jurman, et al. 2000. A novel nervous system β subunit that downregulates human large conductance calcium-dependent potassium channels. J. Neurosci. 20:3563–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbach, H., F. Etienne, T. Hoshi, S.H. Heinemann, W.T. Lowther, B. Matthews, G. St. John, C. Nathan, and N. Brot. 2002. Peptide methionine sulfoxide reductase: structure, mechanism of action, and biological function. Arch. Biochem. Biophys. 397:172–178. [DOI] [PubMed] [Google Scholar]
- Xia, X.M., J.P. Ding, and C.J. Lingle. 1999. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J. Neurosci. 19:5255–5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D.X., Y.F. Chen, W.B. Campbell, A.P. Zou, G.J. Gross, and P.L. Li. 2001. Characteristics and superoxide-induced activation of reconstituted myocardial mitochondrial ATP-sensitive potassium channels. Circ. Res. 89:1177–1183. [DOI] [PubMed] [Google Scholar]
- Zhang, L., and C.J. McBain. 1995. Potassium conductances underlying repolarization and after-hyperpolarization in rat CA1 hippocampal interneurones. J. Physiol. 488:661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]