Abstract
Background
Many placebo-controlled trials have demonstrated the efficacy of individual pharmacotherapies approved for smoking cessation. However, few direct or indirect comparisons of such interventions have been conducted. We performed a meta-analysis to compare the treatment effects of 7 approved pharmacologic interventions for smoking cessation.
Methods
We searched the US Centers for Disease Control and Prevention's Tobacco Information and Prevention database as well as MEDLINE, EMBASE and the Cochrane Library for published reports of placebo-controlled, double-blind randomized controlled trials of pharmacotherapies for smoking cessation. We included studies that reported biochemically validated measures of abstinence at 6 and 12 months. We used a hierarchical Bayesian random-effects model to summarize the results for each intervention.
Results
We identified 70 published reports of 69 trials involving a total of 32 908 patients. Six of the 7 pharmacotherapies studied were found to be more efficacious than placebo: varenicline (odds ratio [OR] 2.41, 95% credible interval [CrI] 1.91–3.12), nicotine nasal spray (OR 2.37, 95% CrI 1.12–5.13), bupropion (OR 2.07, 95% CrI 1.73–2.55), transdermal nicotine (OR 2.07, 95% CrI 1.69–2.62), nicotine tablet (OR 2.06, 95% CrI 1.12–5.13) and nicotine gum (OR 1.71, 95% CrI 1.35–2.21). Similar results were obtained regardless of which measure of abstinence was used. Although the point estimate favoured nicotine inhaler over placebo (OR 2.17), these results were not conclusive because the credible interval included unity (95% CrI 0.95–5.43). When all 7 interventions were included in the same model, all were more efficacious than placebo. In our analysis of data from the varenicline trials that included bupropion control arms, we found that varenicline was superior to bupropion (OR 2.18, 95% CrI 1.09–4.08).
Interpretation
Varenicline, bupropion and the 5 nicotine replacement therapies were all more efficacious than placebo at promoting smoking abstinence at 6 and 12 months.
Health Canada recently approved the use of varenicline as a pharmacotherapy for smoking cessation. Varenicline works by stimulating dopamine, which results in reduced cravings and withdrawal symptoms. The drug also blocks nicotine receptors, which prevents the dopamine release associated with nicotine consumption.1 The drug has been examined in a few small randomized controlled trials.2–5 Despite limited evidence concerning its use, varenicline is viewed by many clinicians and researchers as the most effective smoking cessation aid. Consequently, there is a need for a systematic assessment of the effectiveness of varenicline relative to placebo. Furthermore, there is a need to compare the efficacy of varenicline with that of existing pharmacotherapies, including sustained-release bupropion and approved nicotine replacement therapies.
We undertook a meta-analysis of placebo-controlled randomized controlled trials of the efficacy of 7 pharmacotherapies approved for smoking cessation. We had 3 objectives: to summarize the efficacy of each pharmacotherapy; to undertake a direct comparison of varenicline and bupropion by analyzing trials that contained both varenicline and bupropion treatment arms; and to undertake an indirect comparison of all 7 pharmacotherapies using the results of the individual trials.
Methods
Search strategy
We conducted a literature search in January 2008 of the following databases: the US Centers for Disease Control and Prevention's Tobacco Information and Prevention database as well as MEDLINE (through PubMed), EMBASE and the Cochrane Library. We identified English-language reports of randomized controlled trials of 7 pharmacotherapies for smoking cessation: varenicline, bupropion and 5 formulations of nicotine replacement therapy (gum, inhaler, nasal spray, tablet and transdermal patch). We used the keywords “smoking,” “varenicline,” “bupropion,” “Zyban,” “nicotine gum,” “nicotine inhaler,” “nicotine lozenge,” “nicotine nasal spray,” “nicotine patch,” “nicotine replacement therapy,” “nicotine tablet” and “transdermal nicotine.” We reviewed the bibliographies of identified studies and recent reviews of smoking cessation pharmacotherapies for additional reports not found through the database searches.
Study selection
We included in our analysis all placebo-controlled, double-blind randomized controlled trials that reported biochemically validated measures of abstinence at 6 and 12 months. We included trials irrespective of setting (e.g., hospital, smoking cessation clinic). Trials in which the intervention and control groups received adjunctive support (e.g., counselling, group therapy) were included irrespective of the intensity of support, provided that patients in the 2 groups were exposed to the same level of adjunctive support. We included factorial-designed trials and treated them as 2 separate trials provided that appropriate placebos were used.
We excluded unblinded trials and those designed to evaluate whether an intervention reduced cigarette use or spontaneous cessation among smokers unwilling to quit. We also excluded trials involving smokers who had chronic disease.
Although we did not formally assess trial quality, we limited our study to double-blind, placebo-controlled randomized controlled trials with biochemically validated outcomes. The use of these strict inclusion criteria suggests that included trials were of high quality.
Data abstraction
Two reviewers performed the data abstraction independently. Abstracted information included demographic and clinical characteristics of the study groups, dosage regimens, adverse events, and outcomes of smoking abstinence at 6 and 12 months. Disagreements were resolved by consensus or by a third reviewer. If the required information was not available in the published article, we obtained additional information in correspondence with the authors.
Classification of outcomes
We defined abstinence as either continuous abstinence from cigarette smoking or point prevalence of abstinence. Continuous abstinence was defined as no smoking from the initial target quit date until follow-up. Point prevalence was defined as no smoking over a given period, usually 7 days, directly before the follow-up appointment. We included in the continuous abstinence category outcomes that were reported in terms of repeated point prevalence (subjects who were abstinent for a given period immediately before 2 or more follow-up visits) and outcomes that required subjects to abstain from smoking only after a 2- to 3-week grace period following the target quit date.
The variety of outcomes reported in the trials meant that, for any single outcome, we would have to exclude many studies from the meta-analysis. We therefore examined smoking abstinence with respect to the “most rigorous criterion” of abstinence reported.6 This criterion was defined as the most conservative outcome reported in any given randomized controlled trial, based on the following ranking: 1) continuous abstinence at 12 months; 2) continuous abstinence at 6 months; 3) point prevalence of abstinence at 12 months; and 4) point prevalence of abstinence at 6 months.
For our analysis, we included only smoking outcomes from trials that reported biochemically validated measures of abstinence. We assessed outcomes using the intention-to-treat principle. We classified as smokers all patients (excluding those who died before follow-up) who were randomly assigned to a study group but were unavailable at follow-up.
Statistical analysis
We used Bayesian hierarchical meta-analysis models to account for variations in outcomes between trials. To adjust for variations in patient characteristics, trial methodologies, settings and intensities of adjunctive support across trials, we used meta-regression. These models do not assume homogeneity of treatment effects. The probability of abstinence from smoking varied both between treatment and control groups within each study, and between studies. We assumed that the within-study logarithms of the odds ratios for each outcome would follow a normal distribution, whose mean represents mean treatment effects across trials and the variance represents the between-trial variability of the odds ratios.
We used normal prior distributions for each hierarchical mean treatment effect (on the logit scale), with a mean of 0 and a variance of 1 000 000. Similarly, we used normal prior distributions for placebo, with a mean of 0 and a variance of 10 000. The same prior distribution was used for regression parameters in models that included a meta-regression component. The hierarchical standard deviation followed a uniform distribution, with a minimum of 0.001 and a maximum of 10.
We created separate models for outcomes at 6 months and 12 months, and for both point prevalence and continuous abstinence outcomes. This resulted in 4 models for each of the 7 pharmacotherapies studied. We compared the efficacy of the pharmacotherapies to each other by running a single, large hierarchical meta-analysis model across all trials, where a log odds ratio was modelled as a separate parameter for each pharmacotherapy. We used a further hierarchical regression component to model the log odds ratio as a function of trial-level characteristics, including smoking cessation pharmacotherapy, age, sex and mean number of cigarettes per day. We created indicator variables for each pharmacotherapy and compared pharmacotherapies by calculating a ratio of odds ratios between each pair of interventions. We also directly compared varenicline and bupropion in a meta-analysis of trials that had both varencline and bupropion treatment arms.
Results
Through our literature search we identified 70 published reports of 69 placebo-controlled randomized trials that met our inclusion criteria (Figure 1; Appendices 1–5, available at www.cmaj.ca/cgi/content/full/179/2/135/DC2). The trials involved a total of 32 908 participants. There were 16 trials of bupropion (6653 patients), 22 of nicotine gum (5200 patients), 4 of nicotine inhaler (976 patients), 4 of nicotine nasal spray (887 patients), 30 of transdermal nicotine (14 459 patients), 6 of nicotine tablet (2306 patients) and 13 of varenicline (3395 patients). There were 45 comparisons of point prevalence of smoking abstinence at 6 months and 40 comparisons at 12 months. There were 49 comparisons of continuous abstinence at 6 months and 55 at 12 months.
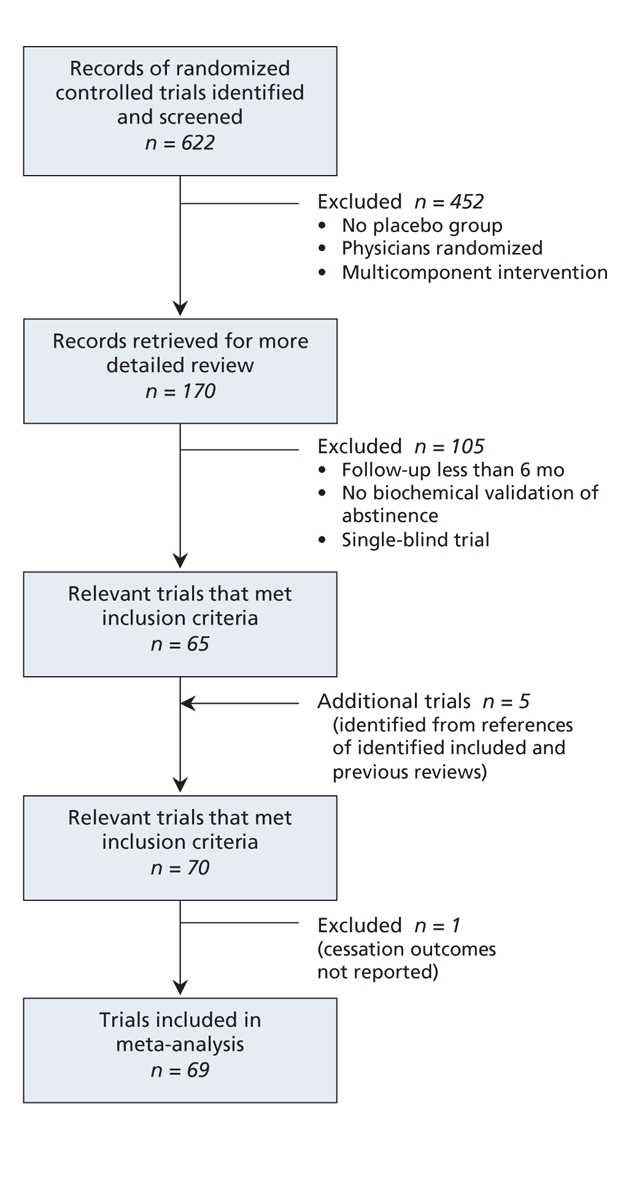
Figure 1: Retrieval and selection of randomized controlled trials of pharmacotherapies for smoking cessation.
Safety data were reported in most trials. These data primarily consisted of treatment discontinuation and nuisance side effects.
Efficacy of smoking cessation pharmacotherapies
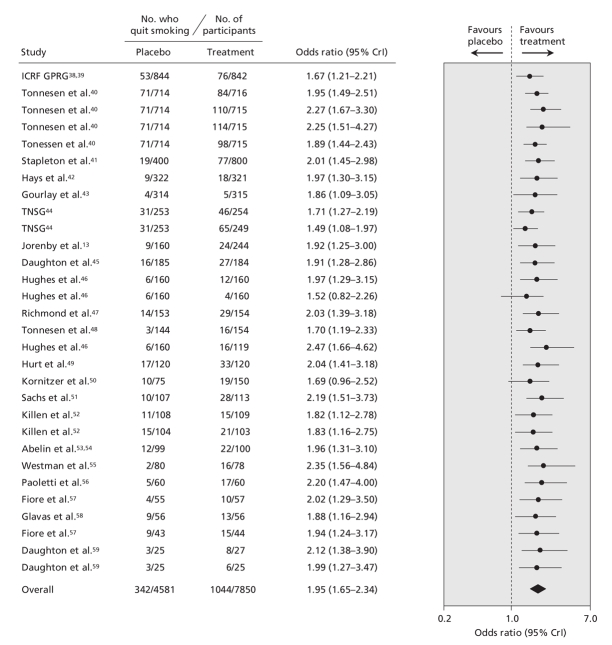
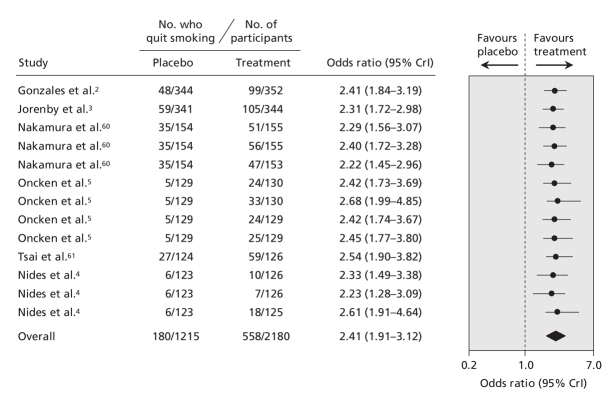
The pooled data for each smoking cessation pharmacotherapy, with smoking abstinence defined using the most rigorous criterion available, are shown in Figure 2 (bupropion2–4,7–18), Figure 3 (nicotine gum19–37), Figure 4 (transdermal nicotine13,38–59) and Figure 5 (varenicline2–5,60,61) and in online Appendix 6 (nicotine inhaler), Appendix 7 (nicotine nasal spray) and Appendix 8 (nicotine tablet). (The appendices are available at www.cmaj.ca/cgi/content/full/179/2/135/DC2). Data for bupropion were adjusted for treatment dosage and duration. Data for nicotine gum and tablet were adjusted for dosage. Data for transdermal nicotine were adjusted for differences in constant versus tapered therapy, as well as 16-hour versus 24-hour therapy.
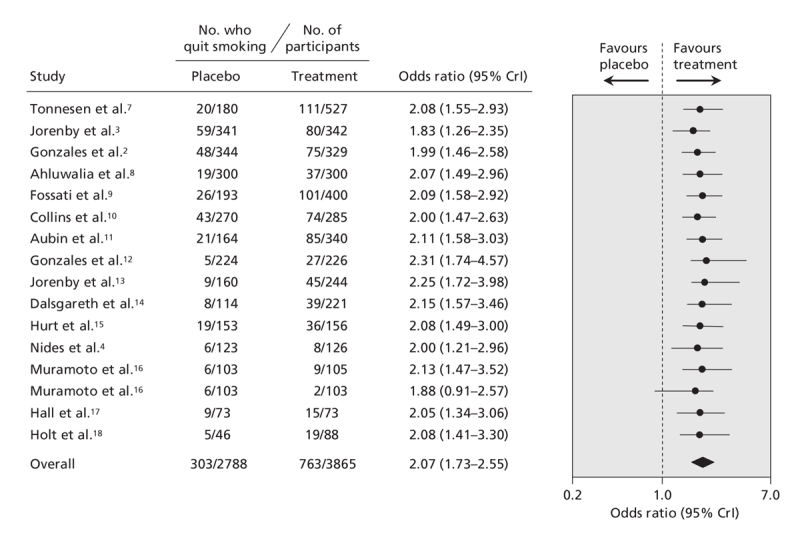
Figure 2: Effect of bupropion on smoking cessation. Smoking cessation is defined by the most rigorous criterion of abstinence (the most conservative outcome reported in any given trial), based on the following ranking: continuous abstinence at 12 months; continuous abstinence at 6 months; point prevalence of abstinence at 12 months; point prevalence of abstinence at 6 months. The data have been adjusted for length of treatment and dosage. Trials are ordered according to the number of patients analyzed using the most rigorous criteria. The total number of patients represents the number of unique patients and thus is less than the sum of the individual studies. Details of the individual trials are summarized in Appendix 1 (available at www.cmaj.ca/cgi/content/full/179/2/135/DC2). CrI = credible interval.
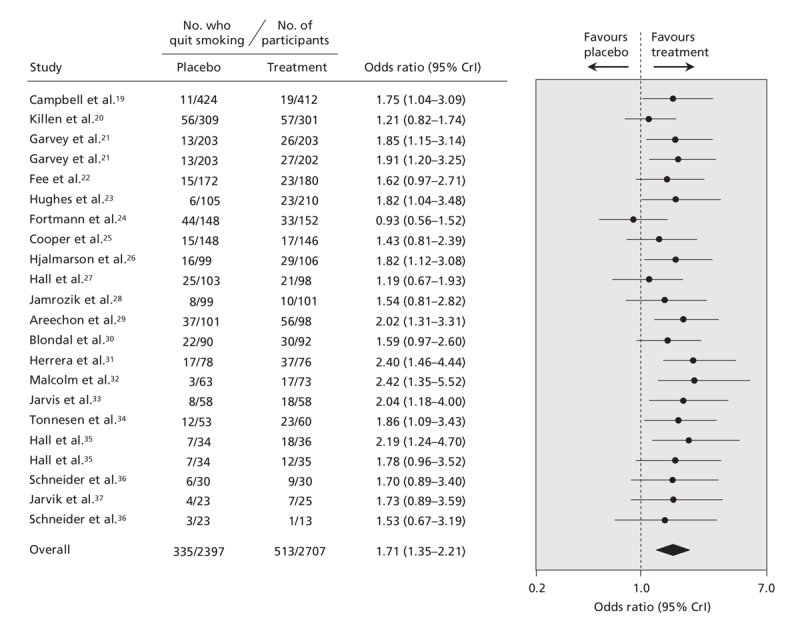
Figure 3: Effect of nicotine gum on smoking cessation. Smoking cessation is defined by the most rigorous criterion of abstinence (see Figure 2 caption for definition and ranking). The data have been adjusted for dosage. Trials are ordered according to the number of patients analyzed using the most rigorous criteria. The total number of patients represents the number of unique patients and thus is less than the sum of the individual studies. Details of the individual trials are summarized in Appendix 2 (available at www.cmaj.ca/cgi/content/full/179/2/135/DC2). CrI = credible interval.
Figure 4: Effect of transdermal nicotine on smoking cessation. Smoking cessation is defined by the most rigorous criterion of abstinence (see Figure 2 caption for definition and ranking). Trials are ordered according to the number of patients analyzed using the most rigorous criteria. The total number of patients represents the number of unique patients and thus is less than the sum of the individual studies. Details of the individual trials are summarized in Appendix 3 (available at www.cmaj.ca/cgi/content/full/179/2/135/DC2). CrI = credible interval, ICRF GPRG = Imperial Cancer Research Fund General Practice Research Group, TNSG = Transdermal Nicotine Study Group.
Figure 5: Effect of varenicline on smoking cessation. Smoking cessation is defined by the most rigorous criterion of abstinence (see Figure 2 caption for definition and ranking). Trials are ordered according to the number of patients analyzed using the most rigorous criteria. The total number of patients represents the number of unique patients and thus is less than the sum of the individual studies. Details of the individual trials are summarized in Appendix 4 (available at www.cmaj.ca/cgi/content/full/179/2/135/DC2). CrI = credible interval.
We found that bupropion, nicotine gum, nicotine nasal spray, transdermal nicotine, nicotine tablet and varenicline were all more efficacious than placebo. Similar results were obtained regardless of which measure of abstinence was used (data not shown). Although the point estimate favoured nicotine inhalers over placebo (odds ratio 2.17), these results were not conclusive because the 95% credible interval included unity (95% CrI 0.95–5.43) (Appendix 6, available at www.cmaj.ca/cgi/content/full/179/2/135/DC2). However, when we included all of the trials in the single hierarchical meta-analysis, we found that all 7 pharmacotherapies were more efficacious than placebo (Figure 6).
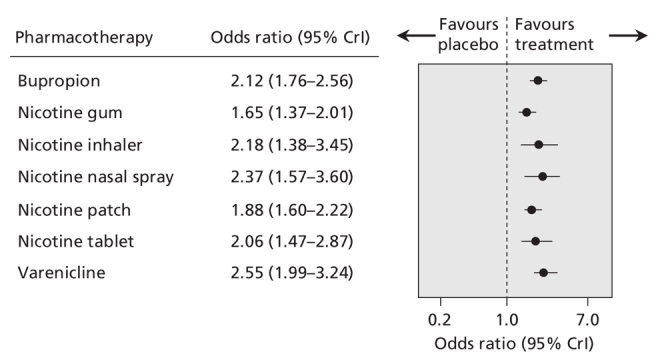
Figure 6: Summary estimates of the effect of pharmacotherapies for smoking cessation on the odds of smoking cessation. Smoking cessation is defined by the most rigorous criterion of abstinence (see Figure 2 caption for definition and ranking). Data have been adjusted for mean age, sex and mean number of cigarettes per day. CrI = credible interval.
Efficacy of varenicline versus bupropion
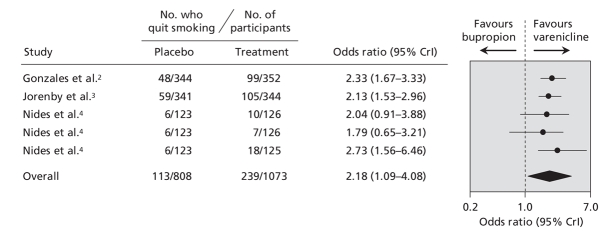
In the single hierarchical meta-analysis of all of the trials, we found that the point estimate favoured varenicline over the other pharmacotherapies (Figure 6). However, we cannot draw definitive conclusions from this indirect comparison because of the overlapping wide credible intervals. In the direct comparison in which we used data from the 3 trials of varenicline that had an active bupropion arm (1881 patients),2–4 we found that varenicline was superior to bupropion (OR 2.18, 95% CrI 1.09–4.08) (Figure 7).
Figure 7: Direct comparison of the effect of varenicline and bupropion on smoking cessation, based on results from varenicline trials that had a bupropion control arm. Smoking cessation is defined by the most rigorous criterion of abstinence (see Figure 2 caption for definition and ranking). Trials are ordered based on the number of patients analyzed using the most rigorous criteria. CrI = credible interval.
Interpretation
In our meta-analysis of randomized controlled trials of 7 pharmacotherapies for smoking cessation, we found that varenicline, bupropion and the 5 nicotine replacement therapies (gum, inhaler, nasal spray, tablet and patch) were all more efficacious than placebo, with ORs of about 2. In the direct comparison of varenicline and bupropion using data from trials with both varenicline and bupropion arms, we found that varenicline was about twice as efficacious as bupropion.
The efficacies of pharmacotherapies for smoking cessation have been examined in 3 previous meta-analyses.6,62,63 In one, the Tobacco Use and Dependence Guideline Panel performed a meta-analysis of both pharmacologic and behavioural interventions to provide the necessary evidence to update the Smoking Cessation Clinical Practice Guideline of the Agency for Healthcare Research and Quality (AHRQ).6 The authors did not limit their analysis to studies in which smoking abstinence was validated biochemically. They identified more than 180 articles for possible inclusion in their meta-analysis. Based on these studies, they found that bupropion, nicotine gum, nicotine inhaler, nicotine nasal spray and transdermal nicotine were more efficacious than placebo and recommended their use as first-line therapies for smoking cessation.
In the second meta-analysis, Hughes and colleagues62 pooled the results of 30 randomized controlled trials to examine the use of antidepressants (non-nicotine-replacement therapy) for smoking cessation. They found that nortriptyline and bupropion were both associated with increased long-term (≥ 6 months) smoking abstinence.
In the third meta-analysis, Silagy and colleagues63 identified 123 trials through the Cochrane Tobacco Addiction Group trials register. They found that all nicotine replacement therapies included in their analysis were superior to control. Our meta-analysis provided similar results.
Few randomized controlled trials of pharmacotherapies for smoking cessation have been head-to-head comparisons. Jorenby and colleagues13 conducted a direct comparison of sustained-release bupropion and transdermal nicotine in a small randomized trial. In this study, 893 patients were randomly assigned to receive sustained-release bupropion, transdermal nicotine patch, combination therapy or double placebo. The authors found significantly higher rates of smoking abstinence at 12 months with the combination therapy (35.5%) and bupropion alone (30.3%) than with transdermal nicotine alone (16.4%) or placebo (15.6%).
Bupropion has also been compared with varenicline in recent head-to-head randomized controlled trials.2–4 These trials, which we included in our study, consistently favoured varenicline. After pooling these data, we found that rates of smoking abstinence associated with varenicline were about twice those associated with bupropion.
Recently, the US Food and Drug Administration issued an alert concerning an increase in serious neuropsychiatric symptoms in patients taking varenicline.64 This alert highlights the need for an in-depth analysis of the safety of these pharmacotherapies. However, with relatively modest sample sizes and strict inclusion criteria, the randomized controlled trials identified in our study provided minimal information regarding safety. Despite the observed increase in neuropsychiatric symptoms that led to the US Food and Drug Administration's warning, only 2 serious neuropsychiatric events (emotional liability and acute psychosis) were observed in the varenicline trials. Only 1 death was reported in the varenicline trials, and only 2 deaths and 2 seizures were reported in the bupropion trials. The small number of observed seizures is likely due to the exclusion of patients at risk for seizures before randomization.
The safety data for the different pharmacotherapies were limited by the inconsistency and quality of reporting in the trials, particularly the older studies. Most studies reported the number of patients who stopped treatment because of adverse events as well as the occurrence of nuisance side effects. However, the definitions used in reporting adverse events varied greatly. For example, in the bupropion trial conducted by Jorenby and colleagues,13 over 30% of the patients randomly assigned to the placebo group reported headaches as adverse events. In a bupropion trial by Ahluwalia and colleagues,8 only 4% of the patients randomly assigned to the placebo group reported headaches. This heterogeneity is likely due to differences in definitions of adverse events and procedures for assessing adverse events. The inconsistency in reporting of adverse events is further highlighted by 2 recent trials of varenicline.4,5 In these trials, over 85% of the patients in the placebo group reported an adverse event, which indicates that these trials may not have used appropriate definitions of adverse events. The interpretation of these data is further complicated by the presence of nicotine withdrawal symptoms. Consequently, there remains a need for continued postmarketing surveillance of these agents.
Despite the efficacy of these pharmacotherapies, the number of patients who remained abstinent from smoking at follow-up was low. Most of the randomized controlled trials in our study reported the point prevalence of abstinence at 12 months to be well under 30% among patients in the treatment groups. With continuous abstinence as the outcome measure, the rate of abstinence was even lower. Consequently, further research into smoking cessation and the development of improved pharmacotherapies is needed. Promising agents include new non-nicotine-replacement pharmacotherapies such as selegiline and reboxetine, the development of a vaccine against nicotine dependence65 and pharmacogenetic approaches to smoking cessation.66 In addition, studies have shown that rimonabant, a cannabinoid receptor antagonist that has been approved by the US Food and Drug Administration for the treatment of obesity,67 may be effective for smoking cessation.68,69 There is also a need to identify under which circumstances each pharmacotherapy is most helpful to patients. Finally, future randomized controlled trials could focus on alternative ways to use existing agents, including combination therapy and prolonged or sequential use of pharmacotherapies.
Our study has limitations. First, although we used stricter inclusion and exclusion criteria than those used in previous meta-analyses, heterogeneity between various variables of the trials was still present. There were notable variations in duration of treatment and dosages. There were also differences in the assessment of abstinence; however, when we analyzed data separately by measure of smoking abstinence, the results were similar regardless of which outcome measure was used.
Second, randomized controlled trials in general involve highly selected patients who may not be representative of patients in actual practice. Trial participants are generally healthier and are likely to be more motivated to quit smoking than patients in actual practice. We limited our meta-analysis to randomized controlled trials involving otherwise “healthy” smokers to provide the cleanest comparison possible. Thus, patient selection may limit the generalizability of our results. Furthermore, these trials involved the use of pharmacotherapies in a setting in which dosing and patterns of use were tightly controlled. Consequently, the effectiveness of these pharmacotherapies when used by smokers in the real world remains poorly understood. Our meta-analysis also does not address the effectiveness of pharmacotherapies relative to well-conducted cognitive support therapy, or self-help, non-pharmacological cessation.
Third, we limited our search to randomized controlled trials published in English. Although the exclusion of studies in other languages could result in a potential selection bias, these studies likely did not differ substantially from their English-language counterparts. Furthermore, less than 5% of the randomized controlled trials identified in MEDLINE using our search strategy were published in a language other than English.
Fourth, with 7 interventions, 2 measures of smoking abstinence (continuous and point prevalence) and outcome assessment at 2 follow-up points (6 and 12 months), we conducted a number of statistical comparisons. Although we did not adjust for multiple comparisons, the potential effects of multiple comparisons should be considered when interpreting these results.
Finally, our exclusion of patients who died during the trial breaks the integrity of the randomization of the trial and may result in an underestimation of the effect of these pharmacotherapies. However, we included only trials involving otherwise healthy individuals, and thus very few deaths were reported.
Conclusion
We found that varenicline, bupropion and the 5 nicotine replacement therapies studied (gum, inhaler, nasal spray, tablet and and patch) were more efficacious than placebo at promoting smoking cessation. In addition, our findings suggest that varenicline may be superior to bupropion. Despite the documented efficacy of these agents, the absolute number of patients who were abstinent from smoking at 12 months was low. Consequently, there remains a need to develop improved smoking cessation agents and to identify optimal cessation strategies, including alternative ways to use existing agents.
@@ See related research paper by Cunningham and Selby, page 145, and related commentary by Ebbert and Hays, page 123
Supplementary Material
Acknowledgments
We thank Susan Wakil for her help with data abstraction.
Footnotes
Une version française de ce résumé est disponible à l'adresse www.cmaj.ca/cgi/content/full/179/2/135/DC1
This article has been peer reviewed.
Contributors: Mark Eisenberg, Kristian Filion, Daniel Yavin, Lawrence Joseph, André Gervais, Jennifer O'Loughlin, Gilles Paradis, Stephane Rinfret and Louise Pilote made substantial contributions to the study conception and design and drafted the manuscript. Mark Eisenberg, Kristian Filion and Salvatore Mottillo contributed to the acquisition and interpretation of data. Daniel Yavin and Lawrence Joseph contributed to the analysis and interpretation of data. Patrick Bélisle, André Gervais, Jennifer O'Loughlin, Gilles Paradis, Stephane Rinfret and Louise Pilote contributed to the interpretation of data. All of the authors revised the manuscript critically for important intellectual content and approved the final version to be published.
Funding: This work was supported by the Canadian Institutes of Health Research (Grant number 74651). Mark Eisenberg is a Senior Physician–Scientist of the Fonds de la recherche en santé du Québec. Lawrence Joseph is a Scientist of the Canadian Institutes for Health Research. Jennifer O'Loughlin holds a Canada Research Chair in the Childhood Determinants of Adult Chronic Disease. Stephane Rinfret is a Junior Physician–Scientist of the Fonds de la recherche en santé du Québec. Louise Pilote is a Physician–Scientist of the Canadian Institutes for Health Research.
Competing interests: Mark Eisenberg is a member of the Varenicline Advisory Board of Pfizer Canada Inc. André Gervais has received speaker fees and consultant fees from Pfizer Canada Inc., as well as travel assistance from Pfizer Canada Inc. to attend a conference on the treatment of tobacco dependence. No competing interests declared by Kristian Filion, Daniel Yavin, Patrick Bélisle, Salvatore Mottillo, Lawrence Joseph, Jennifer O'Loughlin, Gilles Paradis, Stephane Rinfret or Louise Pilote.
Correspondence to: Dr. Mark J. Eisenberg, Divisions of Cardiology and Clinical Epidemiology, Sir Mortimer B. Davis Jewish General Hospital, Ste. A-118, 3755 Cote Ste-Catherine Rd., Montréal QC H3T 1E2; fax 514 340-7564; mark.eisenberg@mcgill.ca
REFERENCES
- 1.Foulds J. The neurobiological basis for partial agonist treatment of nicotine dependence: varenicline. Int J Clin Pract 2006;60:571-6. [DOI] [PubMed]
- 2.Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 2006;296:47-55. [DOI] [PubMed]
- 3.Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 2006;296: 56-63. [DOI] [PubMed]
- 4.Nides M, Oncken C, Gonzales D, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo-and bupropion-controlled trial with 1-year follow-up. Arch Intern Med 2006;166:1561-8. [DOI] [PubMed]
- 5.Oncken C, Gonzales D, Nides M, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med 2006;166:1571-7. [DOI] [PubMed]
- 6.US Department of Health and Human Services. Quick reference guide for clinicians: treating tobacco use and dependence. Washington (DC): The Department; 2000. Available: www.ahrq.gov/clinic/tobacco/tobaqrg.pdf (accessed 2008 May 12).
- 7.Tonnesen P, Tonstad S, Hjalmarson A, et al. A multicentre, randomized, double-blind, placebo-controlled, 1-year study of bupropion SR for smoking cessation. J Intern Med 2003;254:184-92. [DOI] [PubMed]
- 8.Ahluwalia JS, Harris KJ, Catley D, et al. Sustained-release bupropion for smoking cessation in African Americans: a randomized controlled trial. JAMA 2002;288:468-74. [DOI] [PubMed]
- 9.Fossati R, Apolone G, Negri E, et al. A double-blind, placebo-controlled, randomized trial of bupropion for smoking cessation in primary care. Arch Intern Med 2007;167:1791-7. [DOI] [PubMed]
- 10.Collins BN, Wileyto EP, Patterson F, et al. Gender differences in smoking cessation in a placebo-controlled trial of bupropion with behavioral counseling. Nicotine Tob Res 2004;6:27-37. [DOI] [PubMed]
- 11.Aubin HJ, Lebargy F, Berlin I, et al. Efficacy of bupropion and predictors of successful outcome in a sample of French smokers: a randomized placebo-controlled trial. Addiction 2004;99:1206-18. [DOI] [PubMed]
- 12.Gonzales DH, Nides MA, Ferry LH, et al. Bupropion SR as an aid to smoking cessation in smokers treated previously with bupropion: a randomized placebo-controlled study. Clin Pharmacol Ther 2001;69:438-44. [DOI] [PubMed]
- 13.Jorenby DE, Leischow SJ, Nides MA, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med 1999;340:685-91. [DOI] [PubMed]
- 14.Dalsgareth OJ, Hansen NC, Soes-Petersen U, et al. A multicenter, randomized, double-blind, placebo-controlled, 6-month trial of bupropion hydrochloride sustained-release tablets as an aid to smoking cessation in hospital employees. Nicotine Tob Res 2004;6:55-61. [DOI] [PubMed]
- 15.Hurt RD, Sachs DP, Glover ED, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med 1997;337:1195-202. [DOI] [PubMed]
- 16.Muramoto ML, Leischow SJ, Sherrill D, et al. Randomized, double-blind, placebo-controlled trial of 2 dosages of sustained-release bupropion for adolescent smoking cessation. Arch Pediatr Adolesc Med 2007;161:1068-74. [DOI] [PubMed]
- 17.Hall SM, Humfleet GL, Reus VI, et al. Psychological intervention and antidepressant treatment in smoking cessation. Arch Gen Psychiatry 2002;59:930-6. [DOI] [PubMed]
- 18.Holt S, Timu-Parata C, Ryder-Lewis S, et al. Efficacy of bupropion in the indigenous Maori population in New Zealand. Thorax 2005;60:120-3. [DOI] [PMC free article] [PubMed]
- 19.Campbell IA, Lyons E, Prescott RJ. Stopping smoking. Do nicotine chewing-gum and postal encouragement add to doctors' advice. Practitioner 1987;231:114-7. [PubMed]
- 20.Killen JD, Fortmann SP, Newman B, et al. Evaluation of a treatment approach combining nicotine gum with self-guided behavioral treatments for smoking relapse prevention. J Consult Clin Psychol 1990;58:85-92. [DOI] [PubMed]
- 21.Garvey AJ, Kinnunen T, Nordstrom BL, et al. Effects of nicotine gum dose by level of nicotine dependence. Nicotine Tob Res 2000;2:53-63. [DOI] [PubMed]
- 22.Fee WM, Stewart MJ. A controlled trial of nicotine chewing gum in a smoking withdrawal clinic. Practitioner 1982;226:148-51. [PubMed]
- 23.Hughes JR, Gust SW, Keenan RM, et al. Nicotine vs placebo gum in general medical practice. JAMA 1989;261:1300-5. [PubMed]
- 24.Fortmann SP, Killen JD, Telch MJ, et al. Minimal contact treatment for smoking cessation. A placebo controlled trial of nicotine polacrilex and self-directed relapse prevention: initial results of the Stanford Stop Smoking Project. JAMA 1988;260:1575-80. [DOI] [PubMed]
- 25.Cooper TV, Klesges RC, Debon MW, et al. A placebo controlled randomized trial of the effects of phenylpropanolamine and nicotine gum on cessation rates and postcessation weight gain in women. Addict Behav 2005;30:61-75. [DOI] [PubMed]
- 26.Hjalmarson AI. Effect of nicotine chewing gum in smoking cessation. A randomized, placebo-controlled, double-blind study. JAMA 1984;252:2835-8. [PubMed]
- 27.Hall SM, Munoz RF, Reus VI, et al. Mood management and nicotine gum in smoking treatment: a therapeutic contact and placebo-controlled study. J Consult Clin Psychol 1996;64:1003-9. [DOI] [PubMed]
- 28.Jamrozik K, Fowler G, Vessey M, et al. Placebo controlled trial of nicotine chewing gum in general practice. Br Med J (Clin Res Ed) 1984;289:794-7. [DOI] [PMC free article] [PubMed]
- 29.Areechon W, Punnotok J. Smoking cessation through the use of nicotine chewing gum: a double-blind trial in Thailand. Clin Ther 1988;10:183-6. [PubMed]
- 30.Blondal T. Controlled trial of nicotine polacrilex gum with supportive measures. Arch Intern Med 1989;149:1818-21. [DOI] [PubMed]
- 31.Herrera N, Franco R, Herrera L, et al. Nicotine gum, 2 and 4 mg, for nicotine dependence. A double-blind placebo-controlled trial within a behavior modification support program. Chest 1995;108:447-51. [DOI] [PubMed]
- 32.Malcolm RE, Sillett RW, Turner JA, et al. The use of nicotine chewing gum as an aid to stopping smoking. Psychopharmacology (Berl) 1980;70:295-6. [DOI] [PubMed]
- 33.Jarvis MJ, Raw M, Russell MA, et al. Randomised controlled trial of nicotine chewing-gum. Br Med J (Clin Res Ed) 1982;285:537-40. [DOI] [PMC free article] [PubMed]
- 34.Tonnesen P, Fryd V, Hansen M, et al. Effect of nicotine chewing gum in combination with group counseling on the cessation of smoking. N Engl J Med 1988;318:15-8. [DOI] [PubMed]
- 35.Hall SM, Tunstall CD, Ginsberg D, et al. Nicotine gum and behavioral treatment: a placebo controlled trial. J Consult Clin Psychol 1987;55:603-5. [DOI] [PubMed]
- 36.Schneider NG, Jarvik ME, Forsythe AB, et al. Nicotine gum in smoking cessation: a placebo-controlled, double-blind trial. Addict Behav 1983;8:253-61. [DOI] [PubMed]
- 37.Jarvik ME, Schneider NG. Degree of addiction and effectiveness of nicotine gum therapy for smoking. Am J Psychiatry 1984;141:790-1. [DOI] [PubMed]
- 38.Effectiveness of a nicotine patch in helping people stop smoking: results of a randomised trial in general practice. Imperial Cancer Research Fund General Practice Research Group. BMJ 1993;306:1304-8. [DOI] [PMC free article] [PubMed]
- 39.Randomised trial of nicotine patches in general practice: results at one year. Imperial Cancer Research Fund General Practice Research Group. BMJ 1994;308:1476-7. [PMC free article] [PubMed]
- 40.Tonnesen P, Paoletti P, Gustavsson G, et al. Higher dosage nicotine patches increase one-year smoking cessation rates: results from the European CEASE trial. Collaborative European Anti-Smoking Evaluation. European Respiratory Society. Eur Respir J 1999;13:238-46. [DOI] [PubMed]
- 41.Stapleton JA, Russell MA, Feyerabend C, et al. Dose effects and predictors of outcome in a randomized trial of transdermal nicotine patches in general practice. Addiction 1995;90:31-42. [DOI] [PubMed]
- 42.Hays JT, Croghan IT, Schroeder DR, et al. Over-the-counter nicotine patch therapy for smoking cessation: results from randomized, double-blind, placebo-controlled, and open label trials. Am J Public Health 1999;89:1701-7. [DOI] [PMC free article] [PubMed]
- 43.Gourlay SG, Forbes A, Marriner T, et al. Double blind trial of repeated treatment with transdermal nicotine for relapsed smokers. BMJ 1995;311:363-6. [DOI] [PMC free article] [PubMed]
- 44.Transdermal nicotine for smoking cessation. Six-month results from two multicenter controlled clinical trials. Transdermal Nicotine Study Group. JAMA 1991;266:3133-8. [PubMed]
- 45.Daughton D, Susman J, Sitorius M, et al. Transdermal nicotine therapy and primary care. Importance of counseling, demographic, and participant selection factors on 1-year quit rates. The Nebraska Primary Practice Smoking Cessation Trial Group. Arch Fam Med 1998;7:425-30. [DOI] [PubMed]
- 46.Hughes JR, Lesmes GR, Hatsukami DK, et al. Are higher doses of nicotine replacement more effective for smoking cessation? Nicotine Tob Res 1999;1:169-74. [DOI] [PubMed]
- 47.Richmond RL, Harris K, de Almeida NA. The transdermal nicotine patch: results of a randomised placebo-controlled trial. Med J Aust 1994;161:130-5. [DOI] [PubMed]
- 48.Tonnesen P, Norregaard J, Simonsen K, et al. A double-blind trial of a 16-hour transdermal nicotine patch in smoking cessation. N Engl J Med 1991;325:311-5. [DOI] [PubMed]
- 49.Hurt RD, Dale LC, Fredrickson PA, et al. Nicotine patch therapy for smoking cessation combined with physician advice and nurse follow-up. One-year outcome and percentage of nicotine replacement. JAMA 1994;271:595-600. [PubMed]
- 50.Kornitzer M, Boutsen M, Dramaix M, et al. Combined use of nicotine patch and gum in smoking cessation: a placebo-controlled clinical trial. Prev Med 1995;24:41-7. [DOI] [PubMed]
- 51.Sachs DP, Sawe U, Leischow SJ. Effectiveness of a 16-hour transdermal nicotine patch in a medical practice setting, without intensive group counseling. Arch Intern Med 1993;153:1881-90. [PubMed]
- 52.Killen JD, Fortmann SP, Davis L, et al. Nicotine patch and self-help video for cigarette smoking cessation. J Consult Clin Psychol 1997;65:663-72. [DOI] [PubMed]
- 53.Abelin T, Buehler A, Muller P, et al. Controlled trial of transdermal nicotine patch in tobacco withdrawal. Lancet 1989;1:7-10. [DOI] [PubMed]
- 54.Abelin T, Ehrsam R, Buhler-Reichert A, et al. Effectiveness of a transdermal nicotine system in smoking cessation studies. Methods Find Exp Clin Pharmacol 1989;11:205-14. [PubMed]
- 55.Westman EC, Levin ED, Rose JE. The nicotine patch in smoking cessation. A randomized trial with telephone counseling. Arch Intern Med 1993;153:1917-23. [PubMed]
- 56.Paoletti P, Fornai E, Maggiorelli F, et al. Importance of baseline cotinine plasma values in smoking cessation: results from a double-blind study with nicotine patch. Eur Respir J 1996;9:643-51. [DOI] [PubMed]
- 57.Fiore MC, Kenford SL, Jorenby DE, et al. Two studies of the clinical effectiveness of the nicotine patch with different counseling treatments. Chest 1994;105:524-33. [DOI] [PubMed]
- 58.Glavas D, Rumboldt M, Rumboldt Z. Smoking cessation with nicotine replacement therapy among health care workers: randomized double-blind study. Croat Med J 2003;44:219-24. [PubMed]
- 59.Daughton DM, Heatley SA, Prendergast JJ, et al. Effect of transdermal nicotine delivery as an adjunct to low-intervention smoking cessation therapy. A randomized, placebo-controlled, double-blind study. Arch Intern Med 1991;151:749-52. [PubMed]
- 60.Nakamura M, Oshima A, Fujimoto Y, et al. Efficacy and tolerability of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40-week follow-up for smoking cessation in Japanese smokers. Clin Ther 2007;29:1040-56. [DOI] [PubMed]
- 61.Tsai ST, Cho HJ, Cheng HS, et al. A randomized, placebo-controlled trial of varenicline, a selective alpha4beta2 nicotinic acetylcholine receptor partial agonist, as a new therapy for smoking cessation in Asian smokers. Clin Ther 2007;29:1027-39. [DOI] [PubMed]
- 62.Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation [review]. Cochrane Database Syst Rev 2003;(2):CD000031. [DOI] [PubMed]
- 63.Silagy C, Lancaster T, Stead L, et al. Nicotine replacement therapy for smoking cessation [review]. Cochrane Database Syst Rev 2004;(3):CD000146. [DOI] [PubMed]
- 64.US Food and Drig Administration, Center for Drug Evaluation and Research. Varenicline (marketed as Chantix) information. Washington (DC): US Department of Health and Human Services; 2008 Feb 1. Available: www.fda.gov/CDER/Drug/infopage/varenicline/default.htm (accessed 2008 May 12).
- 65.Maurer P, Jennings GT, Willers J, et al. A therapeutic vaccine for nicotine dependence: preclinical efficacy, and phase I safety and immunogenicity. Eur J Immunol 2005;35:2031-40. [DOI] [PubMed]
- 66.Berrettini WH, Lerman CE. Pharmacotherapy and pharmacogenetics of nicotine dependence. Am J Psychiatry 2005;162:1441-51. [DOI] [PubMed]
- 67.Rimonabant: new drug. Obesity: loss of a few kilos, many questions. Prescrire Int 2006;15:123-6. [PubMed]
- 68.Boyd ST, Fremming BA. Rimonabant — a selective CB1 antagonist. Ann Pharmacother 2005;39:684-90. [DOI] [PubMed]
- 69.Cahill K, Ussher M. Cannabinoid type 1 receptor antagonists (rimonabant) for smoking cessation [review]. Cochrane Database Syst Rev 2007;(4):CD005353. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.