Abstract
Small molecules that inhibit the geranylgeranylation of K-Ras4B and RhoA by protein geranylgeranyltransferase type I (GGTase-I) were identified from chemical genetic screens of heterocycles synthesized through phosphine catalysis of allenes. To further improve the efficacy of the GGTase-I inhibitors (GGTIs), 4288 related compounds bearing core dihydropyrrole/pyrrolidine and tetrahydropyrdine/piperidine scaffolds were synthesized on SynPhase Lanterns in a split-pool manner through phosphine-catalyzed [3+2] and [4+2] annulations of resin-bound allenoates. Testing of the 4288 analogs resulted in several GGTIs exhibiting submicromolar IC50 values. Because proteins such as Ras and Rho GTPases are implicated in oncogenesis and metastasis, these GGTIs might ultimately lead to the development of novel antitumor therapeutics.
Although library approaches to the discovery of small-molecule enzyme inhibitors or receptor ligands are well established,1 many reactions continue to pose challenges when applied to solid phase synthesis for the generation of compound libraries. From our development of phosphine catalysis of allenoates,2 we envisioned that these reactions might be adaptable to solid phase synthesis for the generation of heterocycle libraries using resin-bound allenoates. Before embarking on the potentially time-consuming development of solid phase processes, however, we decided to screen our model compounds synthesized through solution-phase reactions. If we could identify a biologically important molecule from the preliminary screen, it would then be worthwhile pursuing a library generated through solid phase split-pool synthesis. Herein, we report the first example of phosphine catalysis of polymer-bound allenoates and a combinatorial library approach to the development of potent inhibitors of protein geranylgeranyltransferase type I (GGTase-I).
Protein prenylation, a posttranslational modification of nascent proteins by either the farnesyl or geranylgeranyl isoprenoid at the C-terminus cysteine residue, is a key event in the regulation of many protein functions.3 Of particular interest is the farnesylation of the oncogenic forms of Ras proteins, which is required for their membrane association and cell transforming activity.4 Constitutively activated mutant Ras proteins are found in ca. 30% of human tumors.5 Consequently, the development of FTase inhibitors (FTIs) as anticancer agents has been the focus of much academic and industrial research.6 Upon blocking FTase, however, the human oncogenic Ras isoform K-RasB is geranylgeranylated by protein GGTase-I.7 Geranylgeranylation functionally substitutes the farnesylation of Ras proteins. This phenomenon suggests that to effectively block Ras processing, the development of selective inhibitors of GGTase-I (GGTIs) is required just as importantly as the development of FTIs.8
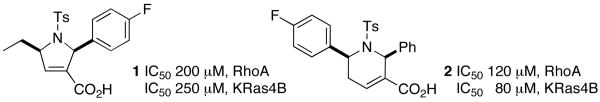
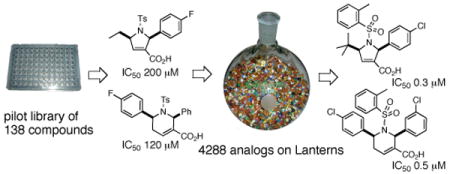
With this premise in mind, we screened a collection of 138 heterocycles9 for their ability to inhibit the activity of human GGTase-I to geranylgeranylate K-Ras4B or RhoA. Purified GGTase-I was incubated with its substrate protein K-Ras4B or RhoA, [3H]GGPP, and the 138 compounds. After 30 min, the degree of incorporation of tritiated geranylgeranyl groups was measured using a scintillation counter. We identified a number of compounds as GGTIs (Figure 1).
Figure 1.
Protein GGTase-I inhibitors 1 and 2.
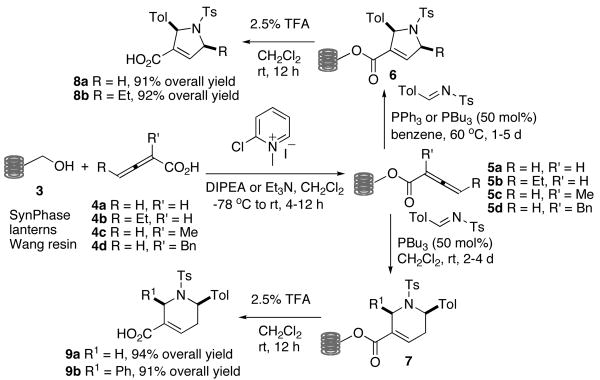
This discovery of promising lead GGTI compounds and their moderate activity warranted the development of efficient and rapid syntheses and evaluations of analogous structures in the search for better inhibitors; we envisioned a short, modular synthetic route (Scheme 1), using SynPhase™ lanterns as the solid support.10 Validation of the synthetic route on the polymer support commenced with formation of resin-bound allenoates 5. The loading of allenoic acids onto solid supports has not been reported previously. The allenoic acids 4 were coupled to the benzyl alcohol units of the SynPhase-PS lanterns grafted with Wang resin 3 using Mukaiyama's reagent and Hünig's base for 4a/b or Et3N for 4c/d.11 The direct use of an unmodified Wang resin minimizes the number of synthetic operations run on solid support. In addition, our strategy enabled simple trifluoroacetic acid (TFA)-mediated cleavage to release the carboxylic acid group, a key functional group in our GGTIs.
Scheme 1.
Solid phase syntheses of dihydropyrroles 8 and tetrahydropyridines 9.
Because we were unaware of any previous examples of phosphine catalysis of solid-bound allenoates, we were pleased to discover that the phosphine-catalyzed annulation between polymer-supported allenoates 5 and N-tosylimines proceeded smoothly. The allenoates 5a and 5b were treated with N-tosyltolualdimine and 50 mol% of PPh3 (for 5a) or PBu3 (for 5b) in benzene at 60 °C to provide the polymer-bound dihydropyrroles 6.2b Tetrahydropyridines 7 were formed from the reactions of 5c and 5d with N-tosyltolualdimine in the presence of 50 mol% of PBu3 at room temperature for 2 and 4 days, respectively.2a Heterocycles 6 and 7 were cleaved from the resin using 2.5% TFA in DCM to provide the carboxylic acids 8 and 9 in 91–94% yield (based on a theoretical loading of 15 μmol/lantern) with high diastereoselectivities (dr = 99:1 for 8b; 93:7 for 9b) after chromatographic purification.
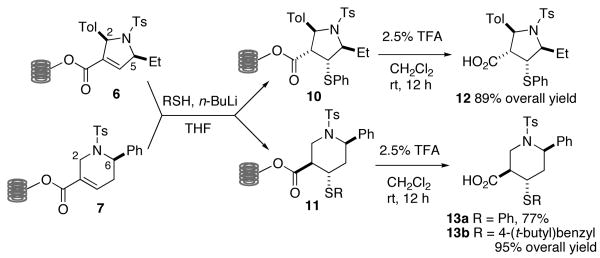
The α,β-unsaturated enoate functionalities in 6 and 7 were utilized to further increase the modularity and number of analogs. For example, the Michael additions of thiols to 6 and 7 using n-butyllithium as base12 provided 10 and 11, respectively, which upon TFA-mediated cleavage yielded 12 and 13, respectively, in 77–95% yield (Scheme 2). These two-step sequences occurred with high diastereoselectivities, providing the pentasubstituted pyrrolidine 12 and the tetrasubstituted piperidine 13 as single diastereoisomeric products.13 Apparently, thiols added opposite to the preexisting substituents in both the dihydropyrrole 6 and the tetrahydropyridine 7.15 Interestingly, however, the protonation of the resulting α-carbanions occurred anti (for 10) and syn (for 11) to the added β-mercapto groups.
Scheme 2.
Solid phase diastereoselective Michael additions.
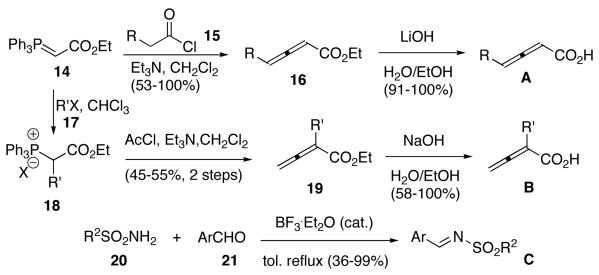
Having successfully established the solid phase reaction conditions, we next prepared the α- and γ-substituted allenoic acids building blocks (Scheme 3). The reactions between the phosphorane 14 and the acid chlorides 15 (1 equiv) in the presence of Et3N (1 equiv) provided the allenoates 16,16 which were hydrolyzed into γ-substituted allenoic acids A.17 Phosphorane 14 was treated with the alkyl halides 17 to give the phosphonium salts 18, which we converted to the α-substituted allenoates 19 upon treatment with Et3N (2 equiv) and acetyl chloride (1 equiv).18 α-Substituted allenoic acids B were prepared through saponification of the esters 19. The N-sulfonylimines C were formed simply through azeotropic removal of water from a mixture of the appropriate sulfonamide 20, aldehyde 21, and BF3·OEt2 under reflux in toluene.19 Chart 1 presents the building blocks synthesized as illustrated in Scheme 3 and the commercially available thiol building blocks D.
Scheme 3.
Building block preparation.
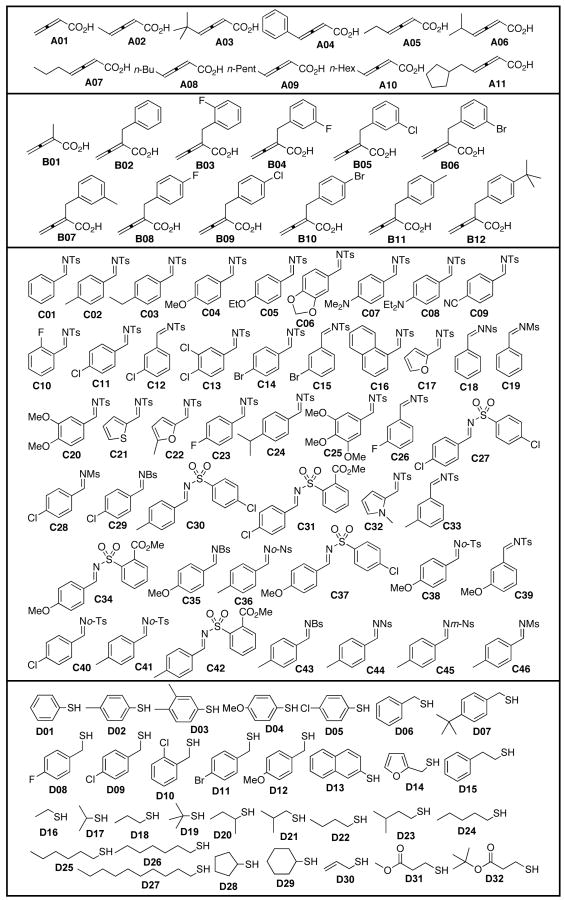
Chart 1.
Eleven γ-substituted allenoic acids A, 12 α-substituted allenoic acids B, 46 N-sulfonimines C, and 32 thiols D.
The building blocks were tested so that only the ones that provided high purity and stereoselectivity (as judged from 1H NMR spectra and LCMS analyses) for their crude cleavage products would be used in the synthesis of the GGTI analog library.14 Eleven γ-substituted allenoic acids A and 12 α-substituted allenoic acids B were loaded and reacted with imine C02 in the presence of a catalytic amount of phosphine, as indicated in Scheme 1. After cleavage with TFA and analysis (1H NMR and LCMS spectra) of the purity and diastereoselectivity of the 23 annulation products 6 and 7, we found that each of the allenoic acids, except for A10, yielded a single identifiable compound (dr ≥ 12:1; 1H NMR) in high purity (72–100%; LCMS/UV210). The resin-bound allenoates derived from allenoic acids A01, A05, B01, and B05 were selected to assess the reactivity and stereoselectivity of 46 imines in phosphine-catalyzed annulations (because A01, A02–A11, B01, and B02–B12 required different annulation reaction conditions). Using the criteria of >70% purity and >9:1 dr, we selected 30 (of 46) imine building blocks for allenoate A01, 21 for A05, 25 for B01, and 31 for B05.
For Michael addition of the thiols, all three building blocks were tested as follows: To select suitable allenoic acids, the 23 annulation products synthesized above were subjected to Michael addition using benzenethiol, toluenethiol, or benzyl thiol. Analysis of the cleaved products indicated that eight (of 23) allenoic acids were suitable candidates. For imine selection, the annulation products of 46 imines with A05 and B01 were tested because the allenoic acids A01–04 and B02–B12 were excluded from the Michael addition sequence. The number of imines selected was 25 (of 46) for A05 and 21 for B01. The annulation products 6 and 7 (Scheme 2) were used to select the thiols; the various thiols required different reaction times and temperatures. Upon extensive optimization, 19 (of 32) thiols for dihydropyrrole 6 and 17 for tetrahydropyridine 7 were selected. The combination of these selected building blocks resulted in the preparation of 4288 compounds.14
Using the chosen building blocks, we commenced the split-pool syntheses of the 4288 GGTI analogs on the SynPhase lanterns. Tagging was performed by inserting colored spindles and cogs into the lanterns prior to synthesis of the library.14 Twenty-three allenoic acids A and B were loaded onto the Wang resin 3 using Mukaiyama's reagent (Scheme 1). The resulting allenoate-loaded lanterns 5 were pooled and split into a number of flasks corresponding to the number of imines for each group of allenoic acids (A01, A02–A11, B01, and B02–B12). Sets of 240 dihydropyrrole-loaded lanterns 6 and 366 tetrahydropyridine-loaded lanterns 7 were placed aside for cleavage. Sets of 3325 dihydropyrrole-bound lanterns 6 and 357 tetrahydropyridine-bound lanterns 7 were further divided into 19 and 17 flasks, respectively, and subjected to the thiol Michael reactions (Scheme 2).
The 4288 lanterns were inserted into 4288 vials and treated with 2.5% TFA in CH2Cl2 for 12 h; the lanterns were then removed and rinsed with CH2Cl2. The resulting solution was concentrated and further co-evaporated with CHCl3 to effectively remove TFA. The cleaved compounds were weighed and redissolved in CHCl3; a portion (2 μmol) of each compound was transferred into 54 96-well plates (80 compounds per well; two columns of wells in each plate were left empty to accommodate controls in subsequent assays) and the solvents were left to evaporate. The products were redissolved in DMSO and analyzed in the same assay for activity against GGTase-I.
In the in vitro assay, active compounds were sought for their ability to inhibit the geranylgeranylation of both RhoA and K-Ras4B. Figure 2a displays the two compounds (22 and 23) that exhibited the highest activities obtained so far. These compounds exhibit specific inhibition of GGTase-I; i.e., they did not inhibit FTase at concentrations at which they inhibited GGTase-I by more than 90%.14
Figure 2.
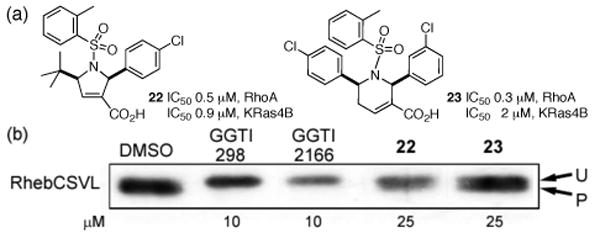
(a) GGTIs 22 and 23 that exhibited the highest potency. (b) Western blot of the cell (treated with 22 or 23 at 25 μM concentration) lysate qualitatively detecting the amount of geranylgeranylated Rheb. The descriptors P and U designate the processed and unprocessed Rheb, respectively.
Finally, we investigated in vivo effects of 22 and 23. Human embryonic kidney (HEK) 293 cells were transfected with the Rheb-CSVL construct that expresses the geranylgeranylated form of the Rheb protein.20 Inhibition of the geranylgeranylation of this protein can be detected from a shift in its mobility on SDS polyacrylamide gel; the unprocessed form appears as a slowly migrating band. Figure 2b indicates that treatment of the cells with 22 or 23 resulted in the appearance of a slowly migrating band (cf. the DMSO lane with lanes 22 and 23); known GGTIs (GGTI298, GGTI2166) were used as controls. These results suggest that compounds 22 and 23 inhibit geranylgeranylation within the cell. These dihydropyrrole and tetrahydropyridine-based GGTIs differ from the previously reported GGTIs, which, with the exception of Casey's GGTI-DU40, have been peptidomimetic compounds.8
In conclusion, small-molecule inhibitors of GGTase-I were identified through chemical genetic screens of the heterocycles produced through allene phosphine catalysis. This discovery instigated the development of the first solid phase phosphine catalysis of resin-bound allenoates. To further improve the efficacy of the GGTIs and to explore their structure–activity relationships, 4288 GGTI analogs were synthesized on SynPhase Lanterns in a split-pool fashion. Screening the 4288 analogs resulted in the identification of GGTIs 22 and 23 having submicromolar IC50 values. These powerful GGTIs should be useful for studies of the protein geranylgeranylation process and might ultimately lead to novel therapeutic leads.
Supplementary Material
Representative experimental procedures and spectral data for all new compounds (PDF). 1H NMR spectroscopic and LCMS data for 251 library members (PDF). Crystallographic data for compounds 12 and 13a (CIF). This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We are grateful to UCLA, Amgen, Pfizer, UCLA-JCCC, the Center for Biological Modulators of the 21st Century Frontier R&D Program (CBM-01-B-5) of the Korean Ministry of Science and Technology (O.K.), NIH (CA32737), and the Susan E. Riley Family Foundation (F.T.) for financial support. S.C. and S.S.K. thank the Italian Government (MIUR) for a research grant and the Nederlandse Organisatie voor Wetenschappelijk Onderzoek for a TALENT fellowship, respectively. We thank Drs. Matt Renner and Saeed Khan for performing the LCMS and the X-ray crystallographic analyses, respectively. O.K. thanks Professor Chulbom Lee for editorial assistance.
References
- 1.Tan DS. Nature Chem Biol. 2005;1:74. doi: 10.1038/nchembio0705-74., and references therein.
- 2.a Zhu XF, Lan J, Kwon O. J Am Chem Soc. 2003;125:4716. doi: 10.1021/ja0344009. [DOI] [PubMed] [Google Scholar]; b Zhu XF, Henry CE, Kwon O. Tetrahedron. 2005;61:6276. [Google Scholar]; c Zhu XF, Henry CE, Wang J, Dudding T, Kwon O. Org Lett. 2005;7:1387. doi: 10.1021/ol050203y. [DOI] [PubMed] [Google Scholar]; d Zhu XF, Schaffner AP, Li RC, Kwon O. Org Lett. 2005;7:2977. doi: 10.1021/ol050946j. [DOI] [PubMed] [Google Scholar]; e Tran YS, Kwon O. Org Lett. 2005;7:4289. doi: 10.1021/ol051799s. [DOI] [PubMed] [Google Scholar]; f Dudding T, Kwon O, Mercier E. Org Lett. 2006;8:3643. doi: 10.1021/ol061095y. [DOI] [PubMed] [Google Scholar]
- 3.Tamanoi F, Sigman DS. Editors of the Enzymes. 3rd. Vol. 21 Academic Press; San Diego, CA: 2001. [Google Scholar]
- 4.a Hancock JF, Magee AI, Childs JE, Marshall CJ. Cell. 1989;57:1167. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]; b Lowy DR, Willumsen BM. Annu Rev Biochem. 1993;62:851. doi: 10.1146/annurev.bi.62.070193.004223. [DOI] [PubMed] [Google Scholar]
- 5.Sebti SM, Hamilton AD. In: Farnesyltransferase Inhibitors in Cancer Therapy. Sebti SM, Hamilton AD, editors. Humana Press; Totowa, New Jersey: 2001. pp. 197–219. [Google Scholar]
- 6.O'Regan RM, Khuri FR. Endocr Relat Cancer. 2004;11:191. doi: 10.1677/erc.0.0110191.. Several FTIs are currently in clinical trials as antitumor agents; see: ClinicalTrials.gov: http://www.clinicaltrials.gov/
- 7.a James GL, Goldstein JL, Brown MS. J Biol Chem. 1995;270:6221. doi: 10.1074/jbc.270.11.6221. [DOI] [PubMed] [Google Scholar]; b Lerner EC, Qian Y, Hamilton AD, Sebti SM. J Biol Chem. 1995;270:26770. doi: 10.1074/jbc.270.45.26770. [DOI] [PubMed] [Google Scholar]
- 8.Unlike FTIs, it was not until 2006 that the first non-peptidomimetic small-molecule GGTI was developed; see: Peterson YK, Kelly P, Weinbaum CA, Casey PJ. J Biol Chem. 2006;281:12445. doi: 10.1074/jbc.M600168200.. For the generation of a combinatorial library of peptide-based GGTIs, see: Oualid FE, van den Elst H, Leroy IM, Pieterman E, Cohen LH, Burm BEA, Overkleeft HS, van der Marel GA, Overhand M. J Comb Chem. 2005;7:703. doi: 10.1021/cc0500203.
- 9.The structures of 138 compounds are provided in the Supporting Information. For the synthesis of the compounds, see ref. 2.
- 10.The SynPhase™ Lantern consists of a mobile surface polymer (e.g., polystyrene) grafted onto a rigid and unreactive base polymer of cylindrical shape. The rigid polymeric support beneath the grafted mobile phase makes weighing unnecessary and handling easier than that of resins. The SynPhase™ Lanterns are available in three different sizes, with loadings of 15, 35, and 75 μmol, providing more material for a given compound than beads when used in a split-pool library synthesis. Non-chemical tagging methods, such as radio-labeling (similar to the IRORI system) or color-coding using colored spindles and cogs, are available, alleviating the need for chemical encoding.
- 11.Mukaiyama T, Usui M, Shimada E, Saigo K. Chem Lett. 1975:1045. [Google Scholar]
- 12.Miyata O, Shinada T, Ninomiya I, Naito T, Date T, Okamura K, Inagaki S. J Org Chem. 1991;56:6556. [Google Scholar]
- 13.The relative stereochemistry of 12 and 13a was confirmed through X-ray crystallographic analyses.14
- 14.See the Supporting Information for details.
- 15.Dihydropyrroles 6 with C5-H, C5-Me, and C5-Ph substituents provided mixtures of diastereoisomers. On the other hand, dihydropyrroles with C5-t-Bu substituents were recalcitrant to the 1,4-addition conditions. For the tetrahydropyridines 7, only those lacking a C2 substituent underwent the 1,4-addition of the thiol. Remarkably, the remote C6 substituent directed the Michael addition from the opposite side.
- 16.Lang RW, Hansen HJ. Org Synth Coll. 1990;7:232. [Google Scholar]
- 17.The saponification of allenoates 16 provided mixtures of allenoic and alkynoic acids, both of which could be used to produce resin-bound allenoates.14
- 18.Scholz D, Weber-Roth S, Macoratti E, Francotte E. Synth Commun. 1999;29:1143. [Google Scholar]
- 19.McKay WR, Proctor GR. J Chem Soc, Perkin Trans. 1981;1:2435. [Google Scholar]
- 20.Gau CL, Kato-Stankiewicz J, Jiang C, Miyamoto S, Guo L, Tamanoi F. Mol Cancer Ther. 2005;4:918. doi: 10.1158/1535-7163.MCT-04-0347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative experimental procedures and spectral data for all new compounds (PDF). 1H NMR spectroscopic and LCMS data for 251 library members (PDF). Crystallographic data for compounds 12 and 13a (CIF). This material is available free of charge via the Internet at http://pubs.acs.org.