Abstract
Pathogenic spirochetes are the causative agents of several important diseases including syphilis, Lyme disease, leptospirosis, swine dysentery, periodontal disease and some forms of relapsing fever. Spirochetal bacteria possess two membranes and the proteins present in the outer membrane are at the site of interaction with host tissue and the immune system. This review describes the current knowledge in the field of spirochetal outer membrane protein (OMP) biology. What is known concerning biogenesis and structure of OMPs, with particular regard to the atypical signal peptide cleavage sites observed amongst the spirochetes, is discussed. We examine the functions that have been determined for several spirochetal OMPs including those that have been demonstrated to function as adhesins, porins or to have roles in complement resistance. A detailed description of the role of spirochetal OMPs in immunity, including those that stimulate protective immunity or that are involved in antigenic variation, is given. A final section is included which covers experimental considerations in spirochetal outer membrane biology. This section covers contentious issues concerning cellular localization of putative OMPs, including determination of surface exposure. A more detailed knowledge of spirochetal OMP biology will hopefully lead to the design of new vaccines and a better understanding of spirochetal pathogenesis.
Keywords: Pathogenic spirochetes, Outer membrane proteins, Lipoproteins, Immunity, Pathogenesis, Surface exposure
1. Introduction
Spirochetes are the etiological agents of several important animal and human diseases, such as syphilis, Lyme disease and leptospirosis. The genomic sequences of three pathogenic spirochetes (Treponema pallidum, Borrelia burgdorferi and Leptospira interrogans) have recently been determined, providing insights and facilitating the development of tools for molecular analysis. The spirochetal outer membrane forms the interface between the cell and its external environment. The outer membrane thus serves as a selective barrier that excludes some molecules, whilst simultaneously allowing entry of appropriate nutrient solutes. The selective permeability of the outer membrane is due to the inherent physico-chemical properties of the membrane itself and to the action of specific binding and uptake molecules associated with the outer membrane. Amongst pathogenic spirochetes the outer membrane is also the part of the cell that comes into direct contact with the host. During infection the spirochetes must contend with the hostile defenses of the host and be able to colonize the host tissues. The molecules associated with the outer membrane, particularly those exposed on the bacterial surface, must mediate these interactions with the host. Spirochetal outer membranes are composed primarily of phospholipids, outer membrane proteins (OMPs) and in some instances lipopolysaccharide (LPS). This review will focus on spirochetal OMPs, in particular aspects of their biogenesis, their functional significance with particular regard to pathogenesis, and their role in immunity. The final section of the review is dedicated to experimental considerations in spirochetal outer membrane biology.
2. Biogenesis and structure of OMPs
Escherichia coli has served as a paradigm for the study of protein secretion in bacteria. However, the absence of genetic exchange systems for some spirochetal species and the difficulty in generating isogenic mutants in others have hindered the investigation of protein secretion in spirochetes. Homologues of the E. coli sec and signal recognition particle (SRP)-related genes that encode proteins involved in secretion have been found in every bacterial genome sequenced, including those of the pathogenic spirochetes. Analysis of spirochetal transmembrane proteins and lipoproteins suggests that biosynthesis and secretion of membrane proteins in spirochetes is not vastly different from those of other bacteria.
2.1. The signal peptide and Sec machinery
All the spirochetal OMPs studied to date are synthesized with a signal peptide at their N-terminus, which initiates export of the protein and is subsequently cleaved following translocation. Although signal peptides do not share sequence homology, they share common structural features. Signal peptides of both lipoproteins and nonlipoproteins possess 1–3 positively charged residues at their N termini followed by approximately 9–15 mixed hydrophobic and neutral amino acids and a consensus cleavage site for a signal peptidase [1]. Signal peptides of lipoproteins are cleaved by lipoprotein signal peptidase (Lsp) and transmembrane OMPs are cleaved by leader peptidase (Lep); thus, the respective signal peptides possess different consensus sequences for their corresponding enzymes [2]. Ala-X -Ala at positions −3 to −1 is the most commonly observed sequence preceding the cleavage site used by Lep, with other small amino acids with neutral side chains also apparent [3]. Genetic studies in E. coli suggest that substitution of alanine at position −3 of the Lep cleavage site with glycine, serine, leucine, threonine, valine or cysteine will also permit cleavage [4]. In addition, substitution of alanine at position −1 of the Lep cleavage site with serine, glycine or cysteine was also shown to result in a cleavable signal peptide. It should be noted that the substitution of cysteine in the −1 and −3 positions might have resulted in cleavage of the signal peptide by the inadvertent construction of an Lsp cleavage site. Several OMPs that are processed by Lep also harbor the sequence Ala-X -Ala-X -Ala at positions −5 to −1, constituting an alternate processing site, which is of unknown significance [5]. Features of signal peptides from some well characterized nonlipoprotein OMPs or transmembrane OMPs of spirochetes are shown in Table 1.
Table 1.
Signal peptides of some well-characterized spirochetal transmembrane OMPs
| Transmembrane OMP (Accession number) | Species | Signal peptidea |
|---|---|---|
| Msp (JC6005) | T. denticola |
|
| OmpL1(AAA74591) | L. kirschneri |
|
| Oms28 (AAC66258) | B. burgdorferi |
|
| Oms66/P66 (AAC66949) | B. burgdorferi |
|
| p13 (AAD28360) | B. burgdorferi |
|
| TROMP1 (AAA92353) | T. pallidum |
|
Positively charged residues at the N-terminus are indicated in bold, strongly hydrophobic amino acids comprising the hydrophobic region of the signal peptide are underlined and the consensus sites for Lep cleavage are double underlined.
The Lsp cleavage site always precedes a cysteine and possesses the consensus sequence [L,V,I]−3[A,S,T,G,]−2 [G,A]−1C+1, which was established based on comparison of 26 lipoprotein sequences from a diverse group of bacteria [6]. This four-residue consensus specifies at least one match to the first two positions and precise matches to the final two. Subsequent studies of spirochetal lipoproteins revealed that proteins with disparate Lsp cleavage sites could still be lipid modified. The sequences of 26 spirochetal lipoproteins with experimental evidence of lipid modification were used to define a spirochete Lsp consensus sequence [L,A,S]−4[L,V,F,I]−3 [I,V,G]−2[A,S,G]−1C+1 [7]. More generally, a small neutral amino acid in the −1 position and a hydrophobic residue at −2, −3 or −4 seems to suffice for processing. The spirochetal Lsp consensus sequence is thus less stringent, with the absence of hydrophobic residues in the −3 position compensated for by the substitution of hydrophobic residues in the −4 and also variably in the −2 position. It has been postulated that a lower rate of protein synthesis in spirochetes may have permitted genetic drift of the spirochetal Lsp cleavage site [7]. Alternatively, the specificity of the spirochetal Lsp may simply differ from that of other bacteria. Predictions of lipoprotein signal peptidase cleavage sites by Psort are inaccurate for spirochetal lipoproteins because the Psort algorithm was based on E. coli lipoprotein sequences. Recently, an improved lipoprotein signal peptide prediction algorithm, LipoP, has been described which has improved performance for spirochetal lipoproteins [8] and is available on the internet (www.cbs.dtu.dk/services/LipoP/). Features of signal peptides from some well-characterized spirochetal outer membrane lipoproteins, that have been demonstrated experimentally to be lipid modified, are shown in Table 2.
Table 2.
Signal peptides of some well-characterized spirochetal outer membrane lipoproteins
| Lipoprotein (Accession number) | Species | Signal peptidea |
|---|---|---|
| DbpA (NP_045697) | B. burgdorferi |
|
| LipL21 (AAO88274) | L. kirschneri |
|
| LipL32 (AAF60198) | L. kirschneri |
|
| LipL36 (AAC12923) | L. kirschneri |
|
| LipL41 (AAB06799) | L. kirschneri |
|
| OspA(AAC66260) | B. burgdorferi |
|
| OspB (AAC66243) | B. burgdorferi |
|
| OspC (AAC66329) | B. burgdorferi |
|
| OspE (AAB63439) | B. burgdorferi |
|
| SmpA (S33585) | B. hyodysenteriae |
|
Positively charged residues at the N-terminus are indicated in bold, strongly hydrophobic amino acids comprising the hydrophobic region of the signal peptide are underlined and the consensus sites for Lsp are double underlined.
The molecular mechanisms of protein secretion have not been elucidated in spirochetes. However, the genome sequences of B. burgdorferi, T. pallidum and L. interrogans encode homologues of the proteins involved in secretion, suggesting that a similar secretion pathway exists in the spirochetes. As nascent E. coli OMPs emerge from the ribosome their signal peptides are targeted by a ribonucleotide complex called the SRP, which is composed of the Ffh protein and a 4.5S-RNA species [9]. The mature parts of the proteins are bound by chaperones such as SecB, which together with SRP target the nascent OMPs to the preprotein translocase within the cytoplasmic membrane [10]. The preprotein translocase is a protein complex comprised of a dimer of SecA–ATPase on the inner side of the cytoplasmic membrane, a heterotrimeric core of SecY, SecE and SecG that spans the membrane, and SecD and SecF situated on the periplasmic side of the cytoplasmic membrane [11]. On arrival of the nascent protein at the preprotein translocase it binds to SecA via interaction with the signal peptide [12], an interaction which may be additionally mediated by the affinity of SecA for the SecB chaperone [13]. SecB is then dispelled and the SecA–ATPase sequentially pushes small stretches of the protein through the SecYEG transmembrane channel, whilst SecD and SecF contribute by stabilizing SecA in the membrane [13].
2.2. Biogenesis of outer membrane lipoproteins
In E. coli, after cytoplasmic membrane translocation, proteins are lipid modified and cleaved in a three step biosynthetic pathway by enzymes located in the cytoplasmic membrane [14]. Initially, prolipoprotein diacylglyceryl transferase (Lgt) transfers a diacylglyceryl group (containing two fatty acids) from phosphatidyl-glycerol to the sulfur atom of the cysteine at position +1 relative to the Lsp cleavage site. Subsequently, Lsp cleaves the signal peptide making the cysteine the new N-terminal amino acid. Finally, a third fatty acid is transferred from a membrane phospholipid to the nitrogen atom of the cysteine by apolipoprotein transacylase (Lnt). Homologues of the lipoprotein biosynthetic enzymes Lgt, Lsp and Lnt can be found in the spirochetal genomes sequenced thus far, suggesting that biosynthesis of lipoproteins is similar amongst the spirochetes [7]. However, most bacteria analyzed have lipoproteins with homologous acylation profiles [15,16], whereas the spirochetal lipoproteins examined appear to have heterogeneous fatty acid compositions [17]. This seems to suggest that the spirochetal lipoprotein biosynthetic enzymes Lgt and Lnt may have different specificities for fatty acids. B. burgdorferi lipoproteins possess glyceryl residues acylated predominantly with oleic and linoleic acid, whilst the amide-linked fatty acid on the cysteine is predominantly stearic acid [17]. Interestingly, lipid modification of B. burgdorferi lipoproteins has been shown to be important in pathogenesis and immunity [18–21], a property which may well be attributable to their unique lipid composition.
Lipoproteins become associated with membranes via hydrophobic interaction between the lipid moiety and the membrane. Lipoproteins may be sorted to one or more locations including the periplasmic face of the cytoplasmic membrane, the inner leaflet of the outer membrane and the outer leaflet of the outer membrane. The mechanism of sorting of lipoproteins to these sub-cellular locations has been examined in E. coli and appears to be mediated by the amino acid residue in the +2 position relative to the Lsp cleavage site [22]. It is presumed that the +2 amino acid determines the ultimate cellular location of the nascent lipoprotein by interaction with the lipoprotein shuttle LolA [23]. In E. coli, aspartic acid, proline and tryptophan in the +2 position are thought to cause retention in the cytoplasmic membrane, whilst phenylalanine, glycine and tyrosine serve as ambiguous signals, with the resultant lipoprotein distributed in both the cytoplasmic and outer membranes [22]. All the other residues appear to direct lipoproteins to the outer membrane. However, very recently it was shown that the environment around the +2 sorting signal can influence the ultimate cellular localization of the protein, thus demonstrating that the +2 amino acid cannot always be relied upon to determine lipoprotein localization [24]. Nascent lipoproteins form a complex with LolA [23,25]. On recognition of the outer membrane sorting signal, lipoproteins are released in an ATP-dependent manner from the cytoplasmic membrane, by a process mediated by an ATP-binding cassette transporter LolCDE [26,27]. The resultant LolA-lipoprotein complex is hydrophilic and can traverse the periplasm [23] and interact with an outer membrane lipoprotein receptor LolB, which mediates anchoring in the outer membrane [25]. Interestingly, sequences with significant similarity to the lol genes can be identified only in L. interrogans, suggesting that lipoprotein sorting may differ between species of spirochetes. In addition, examination of known spirochetal lipoproteins revealed no clear correlation between ultimate cellular location and the amino acid in the +2 position. Possibly, conformation plays a greater role than amino acid sequence in determining the localization of spirochetal membrane lipoproteins.
2.3. Biogenesis of transmembrane OMPs
After translocation of transmembrane OMPs across the cytoplasmic membrane by the Sec machinery and cleavage of their signal peptides by Lep, they arrive at the outer membrane. In contrast to the lipoproteins, trafficking of nonlipoprotein OMPs beyond the cytoplasmic membrane and their subsequent integration into the outer membrane is not well understood. What little work has been done on outer membrane trafficking and insertion has been carried out in E. coli, which can only serve as a starting point for investigations of these processes in the spirochetes. One hypothesis explaining the translocation of transmembrane OMPs from the cytoplasmic to the outer membrane involves passage through the periplasm rather than probably artifactual Bayer junctions [28].
It has been speculated that LPS is intimately involved in OMP assembly and thus insertion into the bacterial outer membrane, a contention that is supported by some of the experimental data. LPS has been shown to be essential for the trimerization of porins in outer membrane vesicles [29,30] and E. coli rough mutants display inefficient porin trimerization [31,32]. However, neither B. burgdorferi nor T. pallidum have LPS, but they still possess transmembrane OMPs, suggesting that LPS may not be essential for folding and insertion of these proteins into the outer membranes of spirochetes. These spirochetes have been noted to have a paucity of transmembrane OMPs, which may be due to a hampered ability to assemble and insert these proteins into the outer membrane in the absence of LPS. Recently, several proteins thought to be involved in OMP assembly in E. coli have been identified. Some of these proteins such as Skp and OstA/Imp cannot be detected in any of the published spirochetal genome sequences, whilst others like SurA are variably present [33–39]. One exception is the highly conserved Omp85 of which clear homologues are apparent in all the published spirochetal genome sequences [40]. The absence of several of these proteins seems to suggest that assembly and insertion of OMPs into the outer membrane is at least partly different in the spirochetes.
2.4. Spirochetal OMP structure
Spirochetal outer membranes contain at least three kinds of proteins: lipoproteins tethered to either side of the membrane via their lipids, transmembrane proteins that span the membrane and recently described peripheral OMPs, which are associated with the outer membrane but are not transmembrane membrane proteins. Transmembrane proteins situated in bacterial cytoplasmic membranes span the membrane via transmembrane α-helices, whereas all characterized outer membrane transmembrane proteins are predicted to span the membrane by forming structures called β-barrels [41]. Notable exceptions are p13 and p66 of B. burgdorferi, which are predicted to span the outer membrane with hydrophobic α-helices [42,43].
The structures of several spirochetal outer membrane lipoproteins thought to be important in infection and immunity have been solved (Fig. 1). OspA is an abundant immunogenic lipoprotein of B. burgdorferi. Un-lipidated recombinant OspA was co-crystallized with the Fab from a murine monoclonal antibody (MAb) [44] and the structure was elucidated [45]. OspA consists of four β-sheets, formed by 21 antiparallel β-strands and a C-terminal α-helix. However, the three-dimensional structure of another B. burgdorferi lipoprotein, OspC, revealed a dimer of two protein molecules each comprising five parallel α-helices and two short β-strands [46,47]. The preponderance of helical structure in OspC is striking in contrast to OspA, which is mostly β-sheet (Fig. 1). The structure of VlsE, a B. burgdorferi lipoprotein that undergoes antigenic variation through an elaborate gene conversion mechanism, was recently determined [48]. The VlsE structure was different again from the other two structurally characterized spirochetal lipoproteins, containing eleven α-helices and four short β-strands. Although the VlsE structure is distinct from that of OspC, the structures share general features including neighbored N/C termini and long helices forming the proximal portion of the molecule (Fig. 1). Most notably, OspC exhibits heterogeneity in the sequence corresponding to the surface of the membrane distal region, a pattern also found for the VlsE molecule [48].
Fig. 1.
The crystal structures of three spirochetal (B. burgdorferi) outer membrane lipoproteins: OspA (A), VlsE (B) and OspC (C). β-Strands are represented as yellow arrows and α-helices are shown as red helices. This image was generated using the published PDB file for each structure (available from the Protein Data Bank) and the computer program DeepView [300].
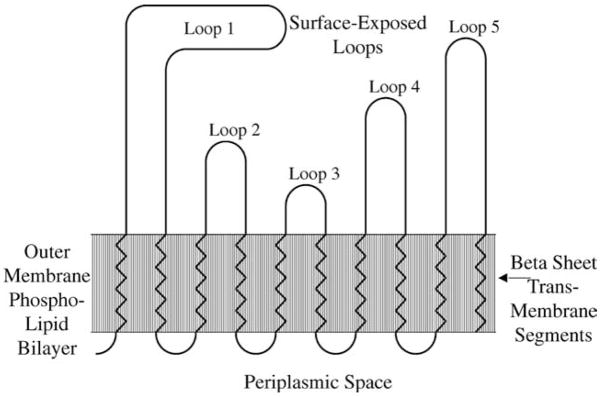
To date, no structural characterization of any spirochetal transmembrane OMPs has been performed. However, all structurally characterized bacterial transmembrane OMPs characterized thus far have been found to share a β-barrel structure [41,49]. The β-barrel consists of antiparallel β-sheets possessing alternating hydrophobic and hydrophilic residues, which can span the membrane in as few as ten amino acids [50]. The β-barrel structure is able to traverse the outer membrane since the hydrophobic residues of the amphipathic β-sheets are exposed on the exterior of the molecule, whilst the hydrophilic residues are concealed within the interior of the structure. Periplasmic and surface-exposed loops connect the membrane spanning β-sheets [51]. It is generally assumed that outer membrane transmembrane proteins all share this β-barrel structure, because the presence of transmembrane α-helices would prevent these proteins from traveling beyond the cytoplasmic membrane during biogenesis. Several bioinformatic approaches to identifying β-barrels have been developed [52,53]. For example, a topological model of the leptospiral transmembrane OMP OmpL1 revealed predicted transmembrane amphipathic β-sheets, short periplasmic turns and surface exposed loops (Fig. 2).
Fig. 2.
Predicted topological model of OmpL1. Ten predicted transmembrane regions, with alternating hydrophobic amino acids typical of the amphipathic β-sheets belonging to proteins with β-barrel structures, are depicted. Five loops of various sizes predicted to be exposed on the cell surface and four short periplasmic turns (containing the turn promoting amino acids proline, glycine, serine or asparagine) are also depicted.
3. OMP function in pathogenic spirochetes
OMPs, in particular those exposed on the cell surface, are in position to interact with the environment or to contribute to pathogenesis by interacting with the host. In other bacterial pathogens functional characterization of OMPs has revealed diverse roles for these molecules, including iron uptake [54–57], antimicrobial peptide resistance [58,59], multi-drug resistance [60,61], bile resistance [62,63], diffusion pores [64], adhesion [65], invasion [66] and serum resistance [67–70]. Several studies have revealed differential expression of OMPs when spirochetes are shifted between conditions representative of the environment or the arthropod vector and the mammalian host. These expression differences are presumably for the purpose of adapting to different environmental conditions and thus it seems reasonable to assume that the proteins up regulated in the mammalian host either aid survival of the spirochetes in vivo and/or contribute to pathogenesis. For example, when B. burgdorferi is shifted between temperatures, representative of a shift between the tick vector and the mammalian host, the expression of several OMPs thought to be involved in infection is increased [71–74]. Global expression analysis of the L. interrogans outer membrane proteome also revealed changes in expression in response to simulated in vivo conditions [75]. These ‘in vivo expressed’ OMPs are thought to be involved in pathogenesis though their functions have yet to be determined; it is anticipated that further characterization will reveal completely novel functions for some of these proteins.
3.1. Adhesins
To initiate infection bacteria must adhere to tissues in order not to be removed by the physical defenses of the host, such as ciliary action in the respiratory tract. Once adhered, bacteria can begin colonization of the host tissues. Adhesins are bacterial proteins, glycoconjugates or lipids that initiate colonization by mediating adhesion between the bacteria and the host cell surface [76,77]. These adhesins can have affinity for specific host cell receptors (which upon binding can induce secondary effects), plasma proteins or components of the extra-cellular matrix (ECM).
3.1.1. B. burgdorferi decorin binding proteins
B. burgdorferi is commonly found in association with collagen fibers in the ECM [78,79]. Initial analysis [80] suggested that colonization of these collagenous tissues may be mediated by spirochetal surface adhesins binding specifically to the collagen-associated proteoglycan decorin [81]. Two adhesins of B. burgdorferi recognizing decorin, lipoproteins with apparent masses of 18–20 kDa called decorin-binding proteins A and B (DbpA and DbpB, respectively), were identified [82]. Subsequently, analysis of 30 DbpA sequences from different isolates revealed five conserved lysine residues [83]. When these individual lysine residues were mutated to alanine, recombinant DbpA was no longer able to bind decorin, suggesting that three of the conserved residues are required for decorin binding. It is interesting to hypothesize that the decorin binding proteins might be involved in B. burgdorferi tissue tropism as decorin is found in various tissues, including the skin and joints, which are typically associated with Lyme disease. Significantly, DbpA antibodies can prevent infection, illustrating the importance of adhesion in the pathogenesis of Lyme disease [84,85].
3.1.2. B. burgdorferi fibronectin binding protein
When B. burgdorferi lysates were separated by SDS–PAGE and probed with alkaline phosphatase-labeled fibronectin, a 47-kDa fibronectin-binding protein was identified [62]. This protein was localized to the outer membrane, based on susceptibility to proteinase K, and the fibronectin region bound by the 47-kDa fibronectin-binding protein was mapped to a gelatin/collagen-binding domain. The 47-kDa fibronectin-binding protein was then purified by affinity chromatography and subjected to mass spectrometry, revealing that the protein was encoded by the bbk32 gene of B. burgdorferi. Recombinant BBK32 was found to bind fibronectin and inhibit the attachment of B. burgdorferi to fibronectin-coated slides. Later studies mapped the ligand-binding domain of BBK32 to 32 amino acids [62]. Analysis of BBK32 from several B. burgdorferi isolates revealed that the ligand-binding domains shared sequence identity with each other and with the fibronectin-binding peptide defined for protein F1 of Streptococcus pyogenes. The similarity between the ligand-binding regions of these proteins suggests a common mechanism of cellular adhesion for B. burgdorferi and S. pyogenes, in which binding of fibronectin deposited on cell surfaces or of soluble fibronectin that subsequently binds host cell surface molecules, such as integrins, facilitates adherence [86–88].
3.1.3. B. burgdorferi hemagglutinin
B. burgdorferi demonstrates high hemagglutinating activity [89] and hemagglutination has often been associated with lectin (sugar-binding) activity [90]. In an attempt to identify proteins involved in binding glycosaminoglycans (GAGs)/proteoglycans (molecules containing long disaccharide repeats of hexosamine and highly sulphated galactose or hexuronic acids attached to a protein core) cell fractions with hemolytic capacity were analyzed for proteins with GAG-binding activity [89]. A 26-kDa protein, Bgp, was identified on the basis of its ability to bind heparin and erythrocytes. Bgp was localized to the B. burgdorferi cell surface. Recombinant Bgp agglutinated erythrocytes and inhibited binding of B. burgdorferi to either purified GAGs or cultured mammalian cells. GAGs are expressed by virtually all eukaryotic nucleated cells [91] and the presence of a GAG binding ligand in B. burgdorferi is consistent with the multisystemic nature of Lyme disease.
3.1.4. B. burgdorferi β3-chain integrin binding protein
Using a filamentous phage display library of B. burgdorferi, Oms66 a transmembrane OMP previously described as a porin [92], was identified as a ligand for β3-chain integrins [93]. In this study, E. coli cells expressing surface exposed Oms66 were found to attach more readily to cultured cell lines expressing β3-chain integrins and binding of B. burgdorferi and recombinant Oms66 to β3-chain integrins were found to be mutually exclusive. It seems plausible that Oms66/P66 would play a role in adhesion early during infection, because integrins would be exposed on the surface of uninflammed tissue, whilst ECM components would not become accessible to the other B. burgdorferi adhesins until later during infection.
3.1.5. B. burgdorferi outer surface protein A
B. burgdorferi inside the midgut of unfed ticks expresses the lipoprotein OspA [94]. When ticks begin feeding on the mammalian host, expression of OspA decreases, whilst the expression of another lipoprotein OspC increases [95]. The crystal structure of OspA reveals a cavity with partially buried charges in the C-terminus, which may be a binding site for a negatively charged ligand with some hydrophobic character [45]. In agreement with the structural analysis performed, it was suggested that OspA functions as a plasminogen receptor [96]. It is postulated that when OspA binds plasminogen it accelerates the formation of active plasmin (which cannot be regulated by host serpins) that degrades high-molecular-weight glycoproteins such as fibronectin [97]. Seemingly, this resultant degradation of host molecules would endow the organism with increased invasive ability. Although infections of plasminogen knock out mice revealed that B. burgdorferi disseminated equally well in these animals, it was determined that plasminogen is required for efficient dissemination in feeding ticks and for enhancement of spirochetemia in mice [98]. This finding is not surprising given that expression of OspA is down-regulated during the early stages of contact with the host. Subsequent analysis has suggested that OspA also binds the tick midgut, which may facilitate attachment of B. burgdorferi to the midgut, whilst conversely repression of OspA may instigate detachment during tick feeding thus mediating transmission from the tick vector to the mammalian host [99]. Another Borrelia lipoprotein OspB shares 53% identity with OspA [100] and may share a similar function.
3.1.6. T. pallidum laminin binding protein
The T. pallidum genome was analyzed for predicted open reading frames (orfs) that encode putative OMPs and recombinant proteins corresponding to these orfs were analyzed for their ability to bind the ECM protein laminin [101]. The protein encoded by the T. pallidum orf Tp0751 was found to have specific laminin binding activity higher than that of the S. aureus laminin-binding protein [102], and to not have affinity for other ECM components. To determine the contribution of carbohydrate moieties to Tp0751-laminin attachment, the oxidative effect of sodium metaperiodate was exploited and it was found that laminin oligosaccharides play a role in interaction of Tp0751 with laminin. In addition, it was found that serum from natural syphilis infections recognized Tp0751, indicating that this protein was expressed during infection, consistent with the notion that Tp0751 functions as an adhesin during T. pallidum infection.
3.1.7. T. denticola major surface protein
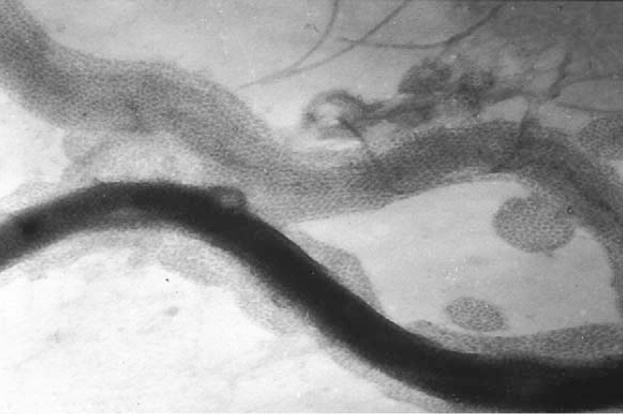
T. denticola Msp is 116–200 kDa oligomer, comprised of a 53 or 64 kDa protein (depending on the strain), that has been demonstrated to be highly surface exposed and which can be observed by electron microscopy to form hexagonal arrays covering the T. denticola surface (Fig. 3) [103,104]. Msp appears to play a role in adherence of T. denticola to host cells, as Fabs derived from antibodies raised against Msp prevent adhesion of T. denticola to gingival fibroblasts [104]. In addition, recombinant Msp has been shown to adhere to the ECM components fibronectin and laminin [105]. Subsequently, it was argued that Msp has a predominantly periplasmic location whilst exhibiting only limited surface exposure [106], a viewpoint which is hard to reconcile given the prior evidence of surface exposure and the adhesive function associated with this protein.
Fig. 3.
Electron micrograph of T. denticola revealing hexagonal arrays of the Msp surface exposed OMP. Kindly provided by Dr. J. Christopher Fenno.
3.1.8. Leptospiral immunoglobulin-like (Lig) proteins
A family of surface-exposed leptospiral proteins has recently been described with a structural organization similar to that of bacterial adhesins [107,108]. Like the Yersinia pseudotuberculosis invasin and E. coli intimin proteins, the leptospiral proteins have repeated immunoglobulin-like domains, and for this reason have been designated Lig (leptospiral Ig-like) proteins. LigA and LigB appear to be inserted into the outer membrane as lipoproteins and have 10–11 immunoglobulin-like domains. LigB has a unique carboxyterminal nonrepeat domain. Loss of Lig protein and lig RNA transcript expression is correlated with loss of virulence during culture attenuation of pathogenic strains. Thin section immunoelectron microscopy was performed, demonstrating surface-exposure of the Lig proteins. Studies are ongoing to determine whether the Lig proteins play a role in host cell attachment and invasion during leptospiral pathogenesis.
3.2. Serum resistance
Human and animal sera exhibit bactericidal activity primarily through the action of the complement system [109]. Complement is a system of plasma proteins that can be activated by antibody or lectins binding to the pathogen surface or by the pathogen surface itself. Once the complement system is activated it leads to a cascade of reactions resulting in the generation of active components with various effector functions. Bound complement components such as C3b are recognized by complement receptors on phagocytic cells and thus serve to opsonize pathogens. The cleavage fragments of complement proteins C3, C4 and C5 also serve to recruit phagocytes to the site of infection. The larger C5b fragment triggers the assembly of the membrane attack complex that can result in lysis of bacterial cells. The activity of complement components is modulated by a system of regulatory proteins that prevent tissue damage as a result of inadvertent activation of the complement system. Factor H controls activation of complement by acting as a co-factor in the cleavage of C3b, accelerating the decay of C3 convertase and preventing the formation of C3 convertase by competing with factor B for binding to C3b. Gram-negative bacteria have developed multiple strategies to protect themselves from the bactericidal effects of complement, utilizing LPS, capsule or OMPs [110–113]. OMPs generally promote resistance to complement-mediated killing by preventing the activation of complement cascades and/or by blocking the formation of the membrane attack complex.
3.2.1. B. burgdorferi Erp lipoprotein family
The Erps constitute a family of surface-exposed lipoproteins that are defined by sharing sequence homology with OspE and OspF proteins. A panel of B. burgdorferi surface proteins was analyzed by surface plasmon resonance to search for ligands for the complement regulatory protein factor H [114]. OspE was identified as a specific ligand for factor H. Using recombinant constructs of factor H, the binding site for OspE was localized to the C-terminal short consensus repeat domains 15–20. Binding of factor H to OspE on the B. burgdorferi surface may help the organism to evade complement-mediated opsonophagocytosis and direct killing. Interestingly, it has been suggested that the ability of B. burgdorferi to express a range of Erps with specificities for factor H of different animal species may provide a mechanism for its wide mammalian host range [115].
3.2.2. B. afzelli complement resistance proteins
Two OMPs that bind factor H and factor H-like protein 1 (FHL-1) were identified in complement-resistant B. afzelli by ligand blotting [116]. These were termed complement regulator acquiring surface proteins (CRASPs) and further characterized as CRASP-1, a 27.5-kDa molecule which interacts preferentially with FHL-1 present in all borreliae tested, and CRASP-2, an approximately 20-kDa protein which interacts preferentially with factor H. Subsequent studies suggest that each borrelial isolate expresses distinct CRASPs, which can be differentiated by their mobility and binding phenotypes [117]. Borrelia can be classified as resistant, intermediate-sensitive or sensitive to the bactericidal effects of normal human serum [113]. Differences in complement sensitivity amongst B. burgdorferi sensu lato have been attributed to the relative capacity of the different species to bind factor H [118]. It is interesting to speculate whether the sensitivities of different Borrelia isolates to normal human sera correlate with the possession of one or more of the CRASPs and OspE. Conspicuously, the molecular weight of OspE approximates that predicted for CRASP-2 suggesting that these proteins may be the same.
3.3. Porins
Porins form water-filled pores that permit passive diffusion of solutes across the outer membrane lipid bilayer [64]. Porin proteins, like other transmembrane OMPs, have a β-barrel structure and depending on their physicochemical properties take up either a monomeric or trimeric conformation in the outer membrane [49]. Variation in their structures confers specificity, with some functioning as general diffusion pores and others as substrate-specific channels [64,119]. Porin activity is usually confirmed by adding the protein under investigation to lipid bilayer membranes and observing stepwise increases in membrane conductance, a technique referred to as ‘black lipid bilayer experimentation’ [120]. Using experimentally derived values, the diameter of the porin pore can estimated using the equation Λ = σπr2/l, where Λ is the single channel conductance in nanosiemens, σ is the specific conductivity determined, r is the radius of the channel, and l is the length of the channel [121].
Porins often have other activities in addition to their porin function. Surface exposed porin loops can activate host signal transduction pathways [122], serve as adhesins [123,124] or act as receptors for bacteriophages [125]. One porin has also been shown to incorporate into the cytoplasmic membranes of host cells, which is thought to facilitate uptake of intracellular bacteria [126]. Variation in porin expression or sequence has been demonstrated to play an important role in antimicrobial resistance [127–129]. Porins have also been shown to activate complement [130,131], modulate inflammatory and immunological responses [132,133] and to enhance immune evasion by variation of their surface exposed loops [134].
Several spirochetal porins have been characterized; the properties attributed to them are detailed in Table 3. The spirochetal porins can be divided into two classes, those which exhibit small single channel conductance, as is the case for TROMP1, OmpL1, Oms28, or large single channel conductance, as observed for p13, Oms66 and Msp (Table 3). It seems feasible that the possession of porins with different selectivity may allow organisms such as Borrelia to contend with different environmental milieu, such as the tick midgut and the mammalian host. By this logic, Leptospira that has to contend with an aquatic environment as well as that of the mammalian host should possess additional, as yet undefined, porins. As observed for other bacteria, several spirochetal porins have additional functions attributed to them and some appear to play a part in immunity to infection (Table 3).
Table 3.
Properties of porins from spirochetal pathogens
| Protein (species) | Properties | Reference(s) |
|---|---|---|
| TROMP1 (T. pallidum) |
|
[244,301,302] |
| OmpL1 (Pathogenic Leptospira) |
|
[185,253,303] |
| p13 (B. burgdorferi sensu lato) |
|
[43,304–306] |
| Oms28 (B. burgdorferi sensu stricto) |
|
[307] |
| Oms66 (P66) (B. burgdorferi) |
|
[92,93,308] |
| Msp (T. denticola) |
|
[105,155] |
3.4. Spirochetal OMPs with other functions
3.4.1. LipL32/Hap-1 of pathogenic Leptospira spp
It has been suggested that hemolysins are important in the pathogenesis of leptospirosis [135,136]. Some of these hemolysins have been cloned, sequenced and found to encode phospholipases or sphingomyelinases, which act on erythrocytes and possibly other cell membranes containing phospholipids [137–139]. Using a portion of the sequence encoding sphingomyelinase C in plaque hybridization experiments, a clone containing two genes with hemolytic capacity was isolated from an L. interrogans serovar Lai genomic library [140]. One of the genes was termed sphH and was postulated to encode a pore-forming hemolysin. The other gene termed hap-1 exhibited minimal hemolysis, but when expressed alongside sphH exhibited a higher hemolytic activity than either gene alone. At approximately the same time an identical gene to hap-1 was found to encode the leptospiral MOMP LipL32 [141].
3.4.2. Vsp–OspC of Borrelia
The etiological agent of relapsing fever B. turicatae is classified into the serotypes A and B solely on the basis of its expressed variable membrane proteins (VMPs) VspA and VspB, respectively [142–144]. The B. turicatae Vsp proteins are related to the Vsps of Borrelia hermsii and the OspC proteins of B. burgdorferi [145]. When severe combined immunodeficient (SCID) mice were infected with serotype A strains of B. turicatae, early invasion of the brain by spirochetes was observed [146]. In contrast, infection with serotype B strains resulted in increased numbers of spirochetes in the blood and joints [142,146]. This may be explained by the different physicochemical properties of the VspA and VspB proteins. VspA, which is associated with invasion of the central nervous system, is more hydrophobic than other members of the Vsp–OspC family, whilst VspB, which is associated with high numbers of spirochetes in the blood, has a more basic isoelectric point (pI) than other members of this protein family [143]. Hence, it has been postulated that the Vsp–OspC family of proteins may be involved in tissue tropism of Borrelia species [147].
3.4.3. CTLP/dentilisin of T. denticola
T. denticola expresses a chymotrypsin-like protease (CTLP), whose native form has a mass of approximately 100 kDa and consists of three proteins of 72, 43 and 38 kDa [148]. The 72-kDa protein contains the protease domain and has been designated dentilisin (PrpT) [149]. Recently the two smaller proteins were found to be the cleavage products of a single 70-kDa protein designated PrcA [150]. CTLP functions as a propyl-phenylalanine-specific protease and degrades the ECM components collagen IV, fibronectin and laminin [151,152]. It is suspected that CTLP plays a key role in tissue invasion by hydrolyzing the surrounding substances and mediating migration through the basement membrane. In the mouse abscess model, PrpT mutants induce smaller abscesses, which resolve faster than those induced by wild-type T. denticola [153]. The PrpT mutants also possess less MSP oligomer and do not exhibit their typical surface hexagonal array, suggesting that dentilisin may also play a role in the biosynthesis and assembly of Msp [154].
3.4.4. Msp of T. denticola
The T. denticola OMP Msp has been demonstrated to have porin activity and to play a role in adhesion [105,155]. Patch clamp recordings of HeLa cells exposed to purified T. denticola outer membrane complexes, containing Msp, revealed depolarization and increased conductance of the HeLa cell membranes [156]. This suggests that Msp can insert into host cell membranes and form diffusion channels. Subsequent analysis has shown that Msp lyses erythrocytes and is cytotoxic for a variety of cell types, including periodontal ligament epithelial cells [157]. Using a series of elegant experiments it was demonstrated that Msp exerts its effects on fibroblasts by retarding Ca2+ release from endoplasmic reticulum stores and inhibiting subsequent Ca2+ influx by uncoupling store-operated channels, which is thought to instigate actin filament rearrangement [158]. These properties of Msp have been suggested to contribute to the cytopathic effects of T. denticola on host epithelial cells that are observed in periodontal disease.
3.4.5. HbpA and HbpB of T. denticola
Iron is an essential nutrient for most bacteria, with B. burgdorferi and T. pallidum being notable exceptions [159,160]. T. denticola does not appear to use siderophores to sequester iron [161] and thus may rely on other mechanisms for iron acquisition. Some gram-negative organisms utilize surface hemin-binding proteins to bind heme, which is subsequently transported into the cells by a protein complex containing TonB [162]. When T. denticola is grown under iron limiting conditions it expresses a 44-kDa OMP with hemin binding capacity, HbpA [163]. Subsequent analysis revealed a gene encoding another hemin binding protein HbpB downstream of hbpA, that was also induced under iron limiting conditions [164]. Neither the HbpA or HbpB sequences contain TonB boxes, suggesting that the iron acquisition pathway in which these proteins participate is TonB independent. These novel hemin-binding proteins may work in concert with the T. denticola hemolysin [165,166], binding hemin released from lysed erythrocytes, thereby acquiring iron from the host and thus contributing to pathogenesis.
4. The role of spirochetal OMPs in immunity
Although it is a topic of much controversy, it is generally accepted that the pathogenic spirochetes are extracellular pathogens, which are only transiently observed inside cells during translocation through epithelial cell barriers [167–170]. In addition, work investigating immunity to spirochetal infection has revealed that immune clearance is largely mediated through the actions of complement and antibody, which either directly kill spirochetes or target them for subsequent opsonophagocytosis [171–175]. In the case of syphilis there appears to be a concomitant role for adaptive cellular immunity [176], but the basis of immunity to T. pallidum remains controversial [172]. On account of these findings, stimulation of protective immunity to spirochetal infection is generally reliant on the identification of surface antigens, which are targets for bactericidal and/or opsonic antibody.
To elicit protective immunity to spirochetal infection it is considered a necessity that any putative protective antigens be exposed on the spirochetal surface so that antibody can bind the intact viable organisms and target them for immune clearance. Consequently, much heated debate surrounds the identity of antigens which are surface exposed and what techniques can legitimately be used to demonstrate surface exposure [7,106,177–180]. It has been suggested that the reason T. pallidum is particularly effective at evading the host immune response is due to an absence of surface exposed lipoproteins and a paucity of transmembrane OMPs [181]. It has been suggested by the same group that B. burgdorferi evades the host immune response by also having a paucity of transmembrane OMPs, but instead of having no surface exposed lipoproteins, possesses lipoproteins with limited surface exposure [180,181]. They have speculated that during infection a global down-regulation of surface antigenic sites occurs [178]. The suggested lack of surface exposure of these antigens provides an overly pessimistic viewpoint, which can be quelled by the knowledge that several of these proteins must be exposed on the cell surface to fulfill their required functions and by the fact that vaccination with some of these OMPs can elicit various degrees of immunoprotection [182–186].
As well as being accessible to antibody on the spirochetal surface, any putative OMP-based vaccinogen must be expressed during either initial colonization of the host or during subsequent stages of infection. For example, the leptospiral outer membrane lipoprotein LipL36, which is exclusively located on the periplasmic face of the outer membrane [187] and down-regulated during infection [188] would be an unlikely protective immunogen. Similarly, the surface-exposed brachyspiral outer membrane lipoprotein SmpA [189] that is also down-regulated during infection [190] is unlikely to elicit immunoprotection.
In addition to these requirements, an ideal vaccinogen would be conserved amongst different strains of an infective spirochete and not be subject to antigenic variation. For T. pallidum and B. burgdorferi that do not possess LOS or LPS [191–193], OMPs are the sole targets for protective immunity. However, other pathogenic spirochetes such as Brachyspira and Leptospira possess LOS or LPS and antibody directed to these molecules is either protective or correlated with protective immunity [194–196]. LOS or LPS are unsuitable vaccinogens for several reasons, one being that these molecules are extremely heterogeneous and stimulate immunity only against homologous strains [195,197–200]. Akin to the variation exhibited by LOS or LPS, some OMPs exhibit significant sequence heterogeneity [134,201,202]. For example, antibodies to OspC are able to prevent as well as resolve B. burgdorferi infection, making this protein a particularly attractive vaccine candidate [203]. However, this protein exhibits considerable sequence heterogeneity and thus may not be able to provide heterologous protection [202]. The problems associated with the design of vaccines based on heterogeneous molecules may sometimes be circumvented in two ways: by finding an epitope of the molecule conserved between strains or by designing a multi-valent vaccine, in which a discrete number of components is utilized to avoid problems associated with antigenic competition [204–207]. OMPs that are subject to antigenic variation, where the organism continually varies its surface antigens during an infection to prolong survival and avoid host antibody [208], are also unlikely to effectively stimulate protective immunity.
4.1. Spirochetal OMPs with putative roles in stimulating protective immunity
4.1.1. B. burgdorferi Osps
Early studies revealed that vaccination of mice with recombinant OspA, OspB or OspC elicited immuno-protection to challenge with B. burgdorferi [184]. Antiserum raised to each recombinant protein revealed that only OspA and OspB antibodies had borreliacidal activity. Subsequently, there were several reports of MAbs against OspB, which exhibited complement-independent bactericidal properties [209–212]. Interestingly, Fabs isolated from these MAbs retained bactericidal activity, suggesting that their killing ability was not mediated by agglutination. Binding of one of these MAbs, designated CB2, was observed to result in the formation of polar blebs (comprised of antigen–antibody complexes) followed by the formation of spheroplasts and cell lysis [211]. Later studies demonstrated that the CB2 MAb induces a conformational change in OspB that by unknown means triggers cell lysis [213]. Subsequently, isolates were discovered containing mutations in the OspB coding sequence that resulted in the loss of the neutralizing epitopes [214–216]. More recent investigations have revealed that the B. burgdorferi strains expressing the mutated OspB protein were still susceptible to the bactericidal effects of OspB antibody [217]. It has been shown that OspB is expressed in the tick midgut, but is subsequently cleared from the spirochete surface on contact with the host [218]. On first analysis, the absence of expression within the host would seem to suggest that this molecule would be unsuitable for use as a vaccinogen. However, antibodies to another B. burgdorferi OMP, OspA, have been shown to block transmission of spirochetes from the tick to the mammalian host [219].
Inside the tick midgut B. burgdorferi expresses OspA in large amounts [94], but as the tick begins feeding the expression of OspA is down-regulated and rapid synthesis of another outer membrane lipoprotein OspC begins [95]. A human trial of an OspA based vaccine (LYMERix™) found it to be safe and 76–92% effective [220,221]. It is believed that the protection afforded by this vaccine results from the action of anti-OspA antibodies, which on taking of the blood meal by the tick, appear to kill B. burgdorferi in the tick midgut and prevent dissemination to the tick salivary glands [222]. Direct killing of spirochetes in the tick occurs in the absence of complement, which has been demonstrated inside ticks, offering an explanation of why vaccine efficacy is critically dependent on anti-OspA antibody titer [223,224].
Misperceptions that LYMERix™ had a high incidence of serious side effects, despite findings to the contrary by the Food and Drug Administration (FDA), resulted in reduced use of the vaccine. The first year LYMERix™ was available on the market (it was approved by the FDA in December 1998), hundreds of thousands of people were vaccinated, yet as misperceptions spread it was predicted that fewer than 10,000 people would seek vaccination in 2002 and due to a lack of demand GlaxoSmithKline stopped production of LYMERix™ in February 2002 [225]. It has been postulated that OspA may trigger an autoimmune response against the leukocyte antigen LFA-1 in certain individuals, which could lead to the development of autoimmune arthritis [226], thereby raising concerns regarding the use of OspA as a vaccine antigen.
One reason that OspA made such a suitable vaccine was that it shows very little heterogeneity within geno-species of Borrelia [227–229], perhaps because it was not subject to the same immune selection in the invertebrate host that OspC is subjected to in the mammalian host. Several interesting immunological findings have resulted from investigations of OspA. For example, it was found that lipidated OspA was more immunogenic in animals [21] and humans [230], illustrating that post-translational modification of some proteins may be required for them to effectively stimulate immunoprotection. Even more interestingly, one investigation revealed that OspA functioned as a potential mucosal adjuvant, being more than 500-fold more effective than lipopeptides or the lipid alone, suggesting that there is something particularly immunostimulatory about the OspA molecule [231]. It has also been found that the OspA protein, which is protease resistant, shields the transmembrane OMP Oms66/P66 from anti-Oms66/P66 antibodies and proteases [232]. Hence, OspA may serve to protect Oms66/P66 from the action of proteases or host antibody whilst inside the tick, and later during infection, when Oms66/P66 would be exposed it would be able to fulfill its function of binding β3-integrins.
Initial experiments demonstrated that purified native OspC was capable of eliciting immunoprotection in gerbils [233]. Subsequently, Probert and LeFebvre [184] demonstrated that immunization of mice with recombinant OspC also stimulated homologous protection to challenge with cultured B. burgdorferi. In another study it was demonstrated that recombinant OspC could protect mice from challenge with B. burgdorferi via tick transmission [234]. Following feeding on immunized hosts, ticks were found to still harbor B. burgdorferi, suggesting that the mechanism of protection was not a result of destruction of the spirochetes within the tick. In addition, it was found that administration of gel-purified recombinant OspC was no longer able to afford protection, suggesting a requirement for conformational epitopes in OspC-mediated immunoprotection. The OspC sequence was known to be highly polymorphic amongst different isolates [235–238] and it was thought that this may preclude its use as a vaccinogen, particularly if isolates from the same locality exhibited diverse OspC sequences. A study investigating the variation in OspC sequence from B. burgdorferi isolated from 40 infected deer ticks in a single locality revealed that 97 out of 189 OspC amino acid residues were polymorphic [202], seeming to suggest that OspC might exhibit too much variation to ever be useful as a vaccinogen.
4.1.2. B. burgdorferi DbpA
The B. burgdorferi surface exposed lipoprotein, DbpA, that functions as an adhesin [82] is expressed in vivo and has been shown to protect mice from infection with culture-derived spirochetes in several studies [84,85]. DbpA antiserum also afforded passive protection in mice, even when administered four days after challenge [183]. In addition, vaccination with recombinant DbpA, but not OspA, was able to afford mice immunoprotection to challenge with plasma-derived B. burgdorferi [239]. An analysis of DbpA sequence heterogeneity from B. burgdorferi sensu lato isolates, revealed 58.3–100% similarity amongst the 30 strains analyzed, with 26 of 30 sharing greater than 90% similarity [240]. Despite the heterogeneity observed in the DbpA sequences of different isolates, most of the strains were vulnerable to killing by the same DbpA antiserum, suggesting that DbpA may be a suitable candidate for inclusion in a vaccine that would provide heterologous protection. In a promising step towards a second generation Lyme disease vaccine, a synergistic response was noted when both OspA and DbpA were used together. Mice immunized with OspA/DbpA and challenged with culture-derived B. burgdorferi exhibited protection against 100-fold higher challenge doses than when either antigen was administered alone [241].
4.1.3. T. pallidum TROMPs
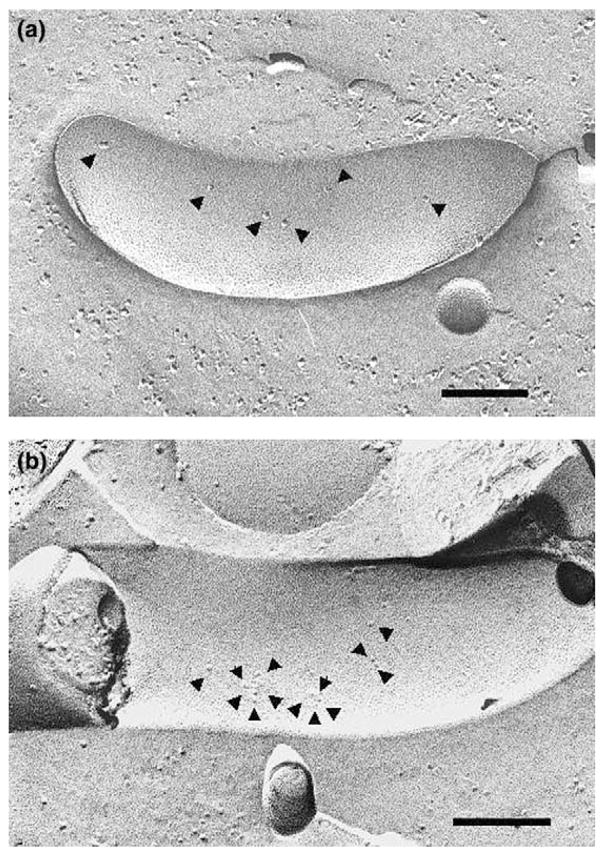
Serum derived from immunization of a mouse with outer membrane vesicles of T. pallidum was demonstrated to kill treponemes in a complement-dependent killing assay at titers of up to 44 [242]. Absorption of this antiserum to remove antibody reacting with outer membrane associated lipoproteins, resulted in an even higher killing titer of 1408, suggesting that antibodies directed towards TROMPs (Treponemal Rare Outer Membrane Proteins) were mediating killing. In support of this suggestion, both immune rabbit serum and the absorbed mouse serum raised against outer membrane vesicles were observed by freeze-fracture analysis to aggregate transmembrane OMPs (Fig. 4). In a subsequent study, aggregation of TROMPs was correlated with immunity to T. pallidum by assessing the degree of TROMP aggregation caused by sera from curatively treated rabbits exhibiting various degrees of immunity [243]. Interestingly, it was noted that antibody-mediated aggregation of the TROMPs, which takes 8–16 h in vitro, is the rate-limiting step for complement activation and killing [244]. Aggregation of TROMPS in vivo would presumably occur over an extensive period, which may account for the protracted onset of protective immunity observed during T. pallidum infection. Antiserum to recombinant TROMP1 has not been found to elicit significant levels of killing activity [242], which may be due to the absence of conformational epitopes present in the native TROMP1 protein.
Fig. 4.
Freeze-fracture electron micrograph of T. pallidum. (a) Absence of TROMP aggregation apparent after incubation with normal rabbit serum. (b) Aggregation of TROMP molecules after incubation with immune rabbit serum. Arrows point to individual TROMP molecules and TROMP aggregates. Bar = 100 nm. Kindly provided by Dr. David R. Blanco and Dr. Michael A. Lovett.
4.1.4. T. pallidum TprK
There has been much controversy over whether the TprK protein of T. pallidum is surface-exposed, the target of opsonic antibody and a protective immunogen [179,245]. In a recent study, TprK fragments found to be consistently recognized by antisera from infected rabbits were used to immunize rabbits that were subsequently subjected to intradermal challenge with T. pallidum [246]. Rabbits immunized with a fragment corresponding to the N-terminus of TprK had lesions that were 75% less likely to contain treponemes and 63% less likely to ulcerate. These results do not clarify whether TprK is a surface protein as other sub-surface proteins have been shown to elicit similar responses [247–249]. Comparison of the TprK sequences of several isolates of T. pallidum has revealed seven discrete variable regions in all but one isolate [250]. It has recently been demonstrated that antibody responses to TprK during infection are primarily directed against these variable regions [251], seeming to suggest that TprK is under immune selection as a result of antibodies binding viable and intact cells during infection. Not surprisingly, it has been hypothesized that the TprK variable regions may be involved in antigenic heterogeneity and in evasion of the immune response [250].
4.1.5. OmpL1/LipL41 leptospiral surface proteins
Co-administration of the surface-exposed leptospiral OMPs OmpL1 and LipL41 was shown to mediate partial synergistic immunoprotection in the hamster model of leptospirosis [185]. In contrast to the vaccine synergism observed between OspA and DbpA [241], neither leptospiral antigen alone was able to elicit protective immunity. LipL41 is an outer membrane lipoprotein [252] and OmpL1 is a transmembrane porin [253], making it tempting to speculate that the observed synergy is a result of the unmasking of sterically hindered protective epitopes on the surface exposed loops of OmpL1 by binding of LipL41 antibodies. In B. burgdorferi, the outer membrane lipoprotein OspA has been shown to prevent access of antibody and proteases to the transmembrane OMP Oms66/P66 [232]. However, until a method for developing isogenic mutants in pathogenic leptospires is developed, any steric hindrance of OmpL1 by LipL41 remains speculative. One of the interesting findings of this study was that the observed synergistic immunoprotection could not be elicited without using membrane forms of the proteins. The reasons for requirement of membrane forms may be 2-fold, with the immunostimulatory properties of LipL41-attached lipid being required for immunogenicity and/or the membrane conformation of the OmpL1 porin being required to conserve conformational epitopes. Future analysis of these proteins should involve a comprehensive assessment of sequence heterogeneity amongst different leptospiral isolates to see if these proteins would be likely to stimulate heterologous immunity.
4.1.6. Leptospiral LipL32/Hap-1
Adenovirus containing the ompL1 or lipL32/hap-1 genes was accessed for its ability to stimulate immunoprotection in gerbils, either singly or in combination [186]. The adenovirus vector containing the lipL32/hap-1 gene stimulated partial, but significant, protection which was not enhanced by the addition of adenovirus carrying the ompL1 gene; virus bearing ompL1 alone was not protective. Hence, in this study no vaccine synergy was noted when an outer membrane lipoprotein (LipL32/Hap-1) and a transmembrane OMP (OmpL1) were administered together. This lends credence to the hypothesis that the synergy observed in the study by Haake et al. [185], was a result of a specific interaction between LipL41 and OmpL1. However, the adenovirus-encoded LipL32/Hap-1 and OmpL1 would undoubtedly not contain the post-translational lipid modification and any conformational epitopes, which are likely to be associated with the membrane forms of these proteins, and which were found to be critical for immunoprotection in the study performed by Haake et al. [185].
4.2. Antigenic variation of spirochetal OMPs
4.2.1. Variable membrane proteins of relapsing fever borreliae
B. hermsii, a causative agent of relapsing fever, exhibits multiphasic antigenic variation [254]. The cyclic acute and afebrile episodes exhibited during relapsing fever are correlated with bouts of spirochetemia, followed by clearance of spirochetes and re-emergence in the blood of spirochetes exhibiting a different serotype [255]. The serotype of a B. hermsii cell is dependent on its major surface antigen, of which there are approximately 30 that can be divided equally into two families: variable large proteins (Vlps) and variable small proteins (Vsps) [256–258]. B. hermsii utilizes at least three mechanisms to vary its surface proteins whilst circulating in the blood [208]. One mechanism involves recombination between a linear plasmid containing silent vls and vsp genes, and another linear plasmid containing an active vls or vsp gene, resulting in the replacement of the gene currently in the active expression site with another [259,260]. The gene replacement is thought to be mediated by recombination between homologous sequences at the 5′ and 3′ ends of all silent and expressed vmp loci. In some instances incomplete recombination occurs and chimeric vmp genes are formed through genomic rearrangement, constituting another mechanism for varying B. hermsii surface proteins [261,262]. Additional sequence heterogeneity may also be derived from hyper-mutation of the 5′ end of vmp genes that is apparent following certain genomic rearrangements [263]. It has been postulated that B. hermsii may also vary the expression of its surface antigens by switching the active expression site. A second expression site has been found [264], whose activation is concomitant with silencing of the first expression site [265]. However, activation of the second expression site has been associated with tick, but not mammalian infection [266].
Other relapsing fever Borreliae, including B. recurrentis [267], B. crocidurae [268] and B. turicatae [144] also have vlp and vsp genes, but less is known about the mechanisms of antigenic variation amongst these organisms.
4.2.2. B. burgdorferi VMP like system (Vls)
Akin to relapsing fever borreliae, B. burgdorferi also has a system of recombining an expression locus with silent loci. It possesses a Vls comprising 15 silent vls loci (vls cassette) adjacent to a single expression locus (vlsE) [269]. During infection gene replacement events occur, in which portions of the silent vls loci recombine with corresponding regions within the vlsE expression locus, with the potential to produce millions of antigenic variants [270,271]. Analysis of three-dimensional structure of the VlsE outer membrane lipoprotein demonstrates that the membrane-distal surface of the protein is comprised primarily of the variable regions, thus masking the invariant regions [48] and presumably preventing antibodies from binding the more conserved parts of the molecule. Interestingly, antigenic recombination of the Vls was found to occur at a faster rate in immunologically intact mice than in SCID mice [271]. It is tempting to speculate that the antigenic variation endowed by the Vls may account for the persistence of B. burgdorferi in the mammalian host.
4.2.3. Scope for antigenic variation in B. hyodysenteriae
The tandem arrangement of paralogous genes encoding membrane proteins is a common theme in B. hyodysenteriae. Two vsp loci encode eight 39-kDa proteins which share high levels of similarity [272–274]. One of these, Vsp39, has been shown to be the predominant B. hyodysenteriae surface protein. The blpG-FEA operon encodes four paralogous outer membrane lipoproteins, of which only one has been shown to be expressed [275]. The presence of tandem paralogous genes in bacteria is rare and the presence of three loci containing paralogous genes in B. hyodysenteriae is conspicuous, particularly in light of the small portion of the genome that has been sequenced. The retention of these paralogues over time suggests that under certain conditions these proteins are expressed and serve essential functions, suggestive of antigenic variation.
4.3. Spirochetal OMPs and inflammation
Spirochetal OMPs, particularly lipoproteins, play a major role in the activation of the host inflammatory response. As in many infectious diseases, inflammation during spirochetal infections is a double-edged sword. Inflammation is essential for defense against infection, yet on the other hand, much of the host tissue damage is a result of the inflammatory response. Inflammation of tissues at sites of infection is a unifying feature in the manifestation of spirochetal diseases. Invasion of periodontal tissues by oral treponemes results in periodontitis, dissemination of organisms to the kidney in leptospirosis results in interstitial nephritis, the inflammatory skin lesions of primary and secondary syphilis are highly infectious, and B. burgdorferi can be isolated from cutaneous erythema migrans in Lyme disease. During spirochetal infections, inflammation results from both the innate and acquired immune response. The innate immune system provides a rapid response to a variety of bacterial products, including LPS, lipoproteins, peptidoglycan, and DNA. Lipoproteins appear to play a dominant role in activation of innate immunity during spirochetal infections. Peptidoglycan, DNA, and LPS probably play a secondary role, if any. LPS is absent from the outer membranes of T. pallidum and B. burgdorferi, and the LPS of pathogenic Leptospira species is a much less potent mediator of the innate immunity than LPS of typical gram-negative bacteria [276].
Spirochetal membranes are richly endowed with lipoproteins, which activate the innate immune response via the CD14 receptor and Toll-like receptor 2 (TLR2). Initial studies demonstrating reactivity to spirochetal lipoproteins were performed in vitro using cell lines transfected with genes encoding CD14 and TLR2 [276–278]. The importance of TLR2 in activation of innate immunity by lipoproteins has been confirmed more recently in studies showing that cells from TLR2-deficient mice are unable to respond to B. burgdorferi lipoproteins, despite exposure to 1000-fold greater concentrations than those necessary for activation of cells from wild-type mice [279]. Cells from TLR2-deficient mice are able to respond to sonicated B. burgdorferi, indicating a role for nonlipoprotein components of these bacteria [279]. Activation of cells from TLR2-deficient mice required 100-fold greater concentrations of sonicated B. burgdorferi, indicating the critical role of lipoproteins in activating innate immunity. The innate immune response appears to play a critical role in defense against B. burgdorferi infection. In B. burgdorferi infection of TLR2-deficient mice there was a 100-fold greater bacterial burden in tissues and a much greater amount of ankle swelling compared to wild-type mice [279]. These differences persisted long after development of the humoral immune response, which appeared to be similar in TLR2-deficient and wild-type mice.
TLR2 is also important, though not essential, for the humoral immune response to OspA [280]. Immunization of TLR2-deficient mice with lipidated OspA resulted in significantly lower levels of OspA-specific total IgG, as measured by OspA ELISA. However, this generalization did not apply to the IgG1 isotype, and OspA immunization protected TLR2-deficient mice from B. burgdorferi infection to a similar degree as in wild-type mice. The adjuvant property of OspA lipid modification was required for TLR2-independent antibody production, as there was no humoral immune response to unlipidated OspA in either wild-type or TLR2-deficient mice.
5. Spirochetal outer membrane biology: experimental considerations
5.1. Introduction
5.1.1. Architectural considerations and localization of spirochetal OMPs
The principles underlying outer membrane isolation in spirochetes rely on an understanding of their unique architecture. In spirochetes, the peptidoglycan cell wall is more closely related to the cytoplasmic membrane than the outer membrane [281]. This unique spirochetal architecture results in a much more unstable outer membrane than in typical gram-negative bacteria, where the outer membrane is stabilized by its close association with the cell wall. In enteric gram-negative bacteria, lipoproteins, such as the murein lipoprotein of E. coli in the periplasmic face of the outer membrane, stabilize the outer membrane by tethering it to the cell wall. In contrast, little is known about what features of the spirochetal outer membrane allow it to remain intact (see below). The instability of the spirochetal outer membrane is evident from micrographs of cultivated spirochetes showing spontaneous formation of outer membrane blebs.
One of the most fundamental questions to be addressed regarding any bacterial protein is its cellular location. Double-membrane bacteria, such as spirochetes, have at least five compartments. Working from the inside out, these are: the cytoplasm, the cytoplasmic membrane, the periplasm, the outer membrane, and the extracellular space. The properties of spirochetal proteins that determine the compartment in which they are found have been addressed earlier in this review. This section is devoted to a discussion of approaches to spirochetal fractionation, and determination of whether a protein is a component of the spirochetal outer membrane.
5.1.2. Is it an outer membrane protein?
Given the emergence of large amounts of genomic sequence data, the researcher is frequently faced with knowing relatively little about a putative spirochetal OMP, other than its sequence. Hydropathy analysis of protein sequences can be carried out with programs such as TMpred (www.ch.embnet.org/software/TMPRED_form.html) and THHMM (www.cbs.dtu.dk/services/TMHMM/). Proteins containing excessive predicted α-helical structure are unlikely to be exported to the outer membrane and can usually be discounted. Subsequent analysis using bioinformatic software, such as Psort, is useful in identification of signal peptides by detection of an aminoterminal hydrophobic alpha-helical region. However, Psort cannot be relied on to determine whether signal peptides of spirochetal proteins are cleavable, and if so, by which signal peptidase. The current generation of outer membrane prediction tools such as LipoP (www.cbs.dtu.dk/services/LipoP/) and Psort-b (www.psort.org/psortb/) make predictions of cellular location based on several criteria such as the presence of appropriate signal sequences and their corresponding cleavage sites, homology to other proteins of known location, presence of certain motifs associated with OMPs and amino acid composition.
Lipoproteins can be found either in the cytoplasmic membrane, the outer membrane, or in both membranes. Moreover, they may be anchored in the inner or outer leaflet of the outer membrane and thus be located predominantly in the periplasm or on the outer surface. Initially, it is important to determine whether the protein in question is, in fact, a membrane protein. The first step in the process is to produce purified recombinant protein for use in generating specific antiserum. This antiserum is essential to assess location of the protein by immunoblot.
5.2. Isolation of the spirochetal outer membrane
5.2.1. Isolation of the total membrane fraction
All bacteria are easily separated into membrane and soluble fractions. Disruption techniques such as sonication, freeze-thaw, or passage through a French pressure cell are followed by low speed centrifugation to remove incompletely fragmented organisms. Ultracentrifugation of the supernatant should then be performed in the presence of protease inhibitors [282]. The ultracentrifugation pellet is the total membrane fraction containing the sum of the cytoplasmic and outer membranes, including the peptidoglycan if lysozyme is omitted from the lysis buffer. The soluble supernatant fraction consists of the cytoplasm and periplasm. Isolation of the total membrane fraction in the presence of lysozyme allows further discrimination of integral membrane proteins from peripheral membrane proteins. Treatment of the total membrane fraction with buffers containing high salt, high pH (Na2CO3), or urea is used to remove proteins that are membrane-associated, but not integrated into the hydrophobic core of the phospholipid bilayer.
5.2.2. Detergent-based approaches for isolation of the outer membrane
Treatment of spirochetes with detergents is a convenient way to solubilize OMPs. When used at a sufficiently high concentration, almost any ionic or nonionic detergent is effective in the solubilization of spirochetal OMPs. Nonionic detergents have an advantage over ionic detergents, such as SDS, in that they are less likely to denature the protein. At low concentrations, nonionic detergents may selectively solubilize the spirochetal outer membrane, and leave the cytoplasmic membrane (stabilized by the cell wall) intact. It is thought that the nonionic detergents Triton X-100 and Triton X-114 are able to solubilize membrane proteins at low concentrations because their hydrocarbon tails are able to substitute for phospholipids in their interaction with membrane proteins. The importance of the cell wall in the possible selectivity of nonionic detergents for solubilization of the spirochetal outer membrane is consistent with their selective solubilization of the cytoplasmic membrane of typical gram-negative bacteria [283]. Triton X-114 has the unique feature of a low cloud point that, upon warming the detergent extract to 37 °C, allows phase partitioning into a detergent-rich hydrophobic fraction and a detergent-poor hydrophilic fraction. Membrane proteins typically partition into the Triton X-114 hydrophobic fraction, which is useful in distinguishing between periplasmic and outer membrane proteins. However, it is important to remember that some integral membrane proteins fractionate aberrantly into the Triton X-114 hydrophilic fraction [284].
5.2.3. Caveats
It is essential to be aware of several caveats when utilizing detergents for isolation of spirochetal outer membranes. The first caveat is that some nonionic detergents are able to efficiently solubilize cytoplasmic membrane proteins, even at low concentrations. For example, treatment of T. pallidum with Triton X-114 results in solubilization of its 47-kDa lipoprotein, Tpp47, initially thought to be an outer membrane protein. However, subsequent thin-section immunogold electron microscopy studies and isolation of the outer membrane in the form of membrane vesicles (see below) demonstrated that Tpp47 is actually a cytoplasmic membrane protein [285–287]. For this reason, detergent fractionation of T. pallidum membranes should be considered unreliable. In other spirochetes, such as Leptospira species, the results obtained with detergent fractionation are comparable to other methods [288]. However, in any organism, it is essential to examine the behavior of known cytoplasmic membrane and outer membrane controls to establish the selectivity of the membrane fractionation experiment. Another caveat is that treatment of spirochetal membranes with nonionic detergents may trigger proteolysis of membrane proteins. The leptospiral lipoproteins LipL32 and LipL48 are completely digested, possibly by an endogenous protease, during the phase partitioning step after Triton X-114 solubilization. A third caveat, that should be taken into account in studies requiring intact secondary structure, is that membrane proteins may be partially or totally denatured after detergent solubilization.
5.2.4. Isolation of the spirochetal outer membrane vesicles
Efforts to establish more reliable membrane fractionation approaches have resulted in development of methods for isolation of spirochetal outer membranes in the form of membrane vesicles. These methods have been developed for isolation of the outer membrane of T. pallidum [285,287], T. vincentii [285], B. burgdorferi [289–291], and L. kirschneri [288]. All of these methods rely on the relatively low buoyant density of spirochetal outer membranes for separation from the denser cytoplasmic membrane by sucrose density gradient ultracentrifugation. Unlike similar approaches utilized for typical gram-negative bacteria, these outer membrane vesicle isolation techniques do not rely on passage through a French pressure cell, and instead rely on the inherent instability of the spirochetal outer membrane. Buffers used in the spirochetal outer membrane isolation techniques appear to destabilize the spirochetal outer membrane and favor its release in the form of vesicles. Spontaneous release of outer membrane vesicles avoids the artifactual formation of hybrid inner membrane-outer membrane vesicles as a result of the high sheer forces involved in passage through a French pressure cell. An essential component of the buffers used in spirochetal outer membrane vesicle isolation techniques are chelators of divalent cations, either in the form of EDTA or citrate. This is consistent with the known property of divalent cations, particularly magnesium or calcium, to stabilize the spirochetal outer membrane. Some of these methods utilize low or high pH bufers to destabilize the outer membrane, suggesting that salt bridges are also important in stabilizing the spirochetal outer membrane. Those who utilize the hypotonic citrate approach should be aware that low pH causes endoflagellar disruption. Another caveat is that acidic and alkaline pH conditions have the potential of releasing lipoproteins from their lipid anchors. Aside from these caveats, isolation of spirochetal outer membranes in the form of membrane vesicles is superior to the detergent based approaches. Combined with genomic sequence data, proteomic studies of outer membrane vesicle components are poised to usher in a much richer understanding of the spirochetal outer membrane.
5.3. Assessment of OMP surface exposure
5.3.1. Rationale for obtaining empirical evidence
Knowing that a protein is a component of the outer membrane is not necessarily predictive of surface-exposure. OMPs that are surface-exposed are more likely to be relevant to mechanisms of pathogenesis and/or serve as a target for protective immunity. Lipoproteins or peripheral membrane proteins may be restricted to either the outer face or the inner (periplasmic) face of the outer membrane. We do not yet understand the signals that determine whether, and under what circumstances, proteins are sorted to one face of the outer membrane or the other. The structure of transmembrane OMPs would imply exposure on both faces of the outer membrane. However, some transmembrane OMPs may be shielded by other outer membrane components. For example, access of antibody and trypsin to the transmembrane protein p66 on the B. burgdorferi surface is restricted by outer surface lipoproteins [232]. For these reasons it is essential to rely on empirical evidence for surface exposure. There is a number of useful approaches to assessment of surface exposure of spirochetal OMPs. These surface-exposure assays have different advantages and disadvantages. An important principle underlying these studies is to keep in mind that the spirochetal outer membrane is easily disrupted by manipulation of the organism. For this reason it is essential to identify subsurface control proteins to demonstrate that the outer membrane is intact. Another principle is to utilize complementary measures of surface-exposure to help validate conclusions.
5.3.2. Thin-section immunogold electron microscopy
Thin-section immunogold electron microscopy should probably be considered the gold standard for assessment of surface-exposure [108,292]. Organisms are centrifuged, fixed, embedded, and sectioned using methods designed to preserve the integrity of the outer membrane. The sections are placed onto electron microscopy grids and exposed to primary antibody followed by secondary antibody conjugated to gold particles. The advantage of thin-section electron microscopy is that it allows antibody to bind to all parts of the organism, both on the surface and in subsurface locations, offering a relatively unbiased approach to assessment of the distribution of the antigen in the organism. The power of this approach is offset by several disadvantages. If the protein of interest is not abundant, high antibody titers must be used (which creates the potential for nonspecific binding of gold particles), and if there are relatively few gold particles bound per organism, a significant number of organisms must be examined to obtain a statistical assessment of location. Conclusions should never be based on the location of gold particle binding in only a few organisms. It is essential to examine the location of a large number of gold particles and organisms, and ideally have the results scored by a blinded observer. It should be kept in mind that each immunoglobulin molecule is 8 nm in length. With a primary antibody, secondary antibody, and 10 nm gold particle, the distance from the antigen to the gold particle can be up to 25 nm, which corresponds to the thickness of the outer membrane. Gold particles observed on the outer surface of the outer membrane could potentially be the result of antibody binding to an antigen on the inner surface of the outer membrane. A further constraint is that some proteins may be denatured by the fixative used (commonly glutaraldehyde), thereby diminishing their reactivity with antibodies.
5.3.3. Whole cell immunogold electron microscopy
Thin-section immunogold electron microscopy requires expert electron microscopy technical support. Whole-cell immunogold electron microscopy is less technically demanding, and therefore more accessible. Both of these electron microscopy approaches benefit from the advantage that an assessment of whether the outer membrane of the organism in question is intact can be made from the images. If the controls are included and the results are unambiguous, conclusions regarding surface-exposure are certainly valid, though one needs to remember that this may not reflect the total distribution of the protein in the organism. An example is the finding that even though OspA had been shown to be surface exposed, subsequent thin-section IEM studies showed that the protein is distributed between the inner and outer membranes [292].
5.3.4. Immunofluorescence (IF)
Another assay of surface-exposure requiring OMP-specific immunologic reagents is immunofluorescence. Immunofluorescence is especially useful when assessing surface-exposure of relatively abundant antigens. An advantage of immunofluorescence is that a larger number of organisms can be rapidly assessed. Images obtained by immunofluoresence are able to validate whether the organism in question is relatively intact, but unlike electron microscopy, immunofluoresence is unable to evaluate the integrity of the outer membrane. For this reason, care should be taken in organism preparation and in binding organisms to a microscope slide. Centrifugation should be avoided, since this can result in disruption of the outer membrane. Organisms are exposed to primary antibody, which is then washed away, followed by fluorescent-labeled secondary antibody. To evaluate the integrity of the organism, it is essential to include antibody to subsurface control antigens. A counterstain with a different emission wavelength is used to demonstrate that organisms are present in the field, but are not bound with antibody to the subsurface control antigen. Separate experiments should be performed with disrupted organisms to demonstrate that antibody to the subsurface control antigen is able to bind to native antigen in the format of the immunofluorescence experiment. A variation of IF has been developed that minimizes manipulation and potential OM disruption, involving encapsulation of organisms in agarose beads. Beads are then exposed sequentially to primary and secondary antibody. This approach has been used to visualize binding to subsurface antigens after exposure of agarose-encapsulated organisms to detergent [178]. It should be kept in mind that if the antibody was raised to a recombinant (nonnative) antigen, increased reactivity could be due to binding to nonnative epitopes resulting from detergent denaturation rather than exposure of a subsurface antigen. For this reason it is essential to determine whether antibody to subsurface control antigens is able to bind to the native protein.
5.3.5. Surface-immunoprecipitation
IEM and IF rely on having either OMP-specific antiserum produced by immunizing animals with recombinant protein, or MAbs. Surface-immunoprecipitation is a technique that also relies on binding of antibody to antigen on the spirochetal surface. However, unlike the previous approaches, surface immunoprecipitation can be performed with antiserum containing antibody to a pool of antigens. This allows the antiserum to be obtained from animals immunized with native antigen in the form of whole spirochetes. Use of antiserum containing antibodies to native surface and subsurface antigens ensures that when negative results are obtained, it is not because the antibody is unable to bind to the native antigen. Another advantage of this approach is that antibody can be added directly to a growing culture that has not been manipulated in any way. Unbound antibody is then washed away, the outer membrane solubilized in nonionic detergent (usually Triton X-100), and the antibody-antigen complexes isolated using staphylococcal protein A Sepharose [293]. Control experiments should be performed using disrupted organisms to demonstrate that subsurface control antigens can also be immunoprecipitated [252].
5.3.6. Nonimmunologic approaches to assessment of surface exposure
Several nonimmunologic approaches are available for assessment of surface exposure. One of these approaches is treatment of intact organisms with proteases to demonstrate selective proteolysis of surface-exposed proteins. This has been used successfully in demonstrating surface-exposure of a number of B. burgdorferi outer surface proteins, including OspA and OspB [294], OspC [238], OspD [295], OspE and OspF [296], p66 [232], and decorin-binding protein [82,85]. When performing this procedure it is useful to perform the procedure with a range of protease concentrations. Surface-exposed proteins should be fully digested at a relatively low protease concentration. If relatively high protease concentrations are required to digest a protein, or only partial digestion is observed, it is possible that the result is an artifact produced by disruption of some of the spirochetes, or incomplete inactivation of the protease when preparing the organisms for SDS–PAGE at the end of the experiment. Another pitfall of this approach is that not all proteins are equally susceptible to even relatively nonspecific proteases, such as Proteinase K. This concern can be controlled for by treating mechanically disrupted organisms with equal concentrations of Proteinase K to demonstrate digestion of subsurface control proteins.
Another approach to identification of surface-exposed proteins is the use of biotin cross-linking agents [297]. Biotin cross-linking agents typically label primary amines which are located on the side chain of lysine residues and at the amino terminus. This feature means that biotin cross-linking agents can identify a broader range of surface-exposed proteins than proteases, including relatively nonspecific proteases such as Proteinase K, which rely on exposure of a protease cleavage site. Since biotin cross-linking agents are relatively small molecules, these agents are more sensitive than antibodies or proteases for detection of OMPs with limited surface-exposure. For this reason, small perturbations in outer membrane integrity could result in labeling of subsurface proteins, even when using nonmembrane permeable biotin cross-linking agents. A useful control is to react the biotin cross-linking agent with disrupted organisms to demonstrate reactivity with subsurface proteins not identified in intact organisms (Fig. 5). Immunoprecipitation of biotinylated protein from a lysate of surface labeled cells adds the specificity required to demonstrate surface exposure of an individual protein. A good negative control for this kind of experiment is immunoprecipitation of labeled subsurface protein from a biotinylated cell lysate and unlabeled subsurface protein from a lysate of surface biotinylated cells.
Fig. 5.

Blot of biotinylated L. interrogans proteins separated by SDS–PAGE and probed with horseradish peroxidase-conjugated streptavidin. (Lane 1) A cell lysate of unlabeled leptospires revealing the absence of endogenously biotinylated proteins. (Lane 2) A lepto-spiral cell lysate labeled with biotin after cell lysis, demonstrating reactivity of sub-surface proteins with the biotinylation reagent. (Lane 3) A cell lysate of surface biotinylated leptospires showing selective labeling of the surface proteins. The positions of relative molecular mass standards are indicated on the left (kDa).
Surface exposure can also be examined using assays relevant to pathogenesis and/or immunoprotection. For example, evidence for a role of OMPs in adhesion strongly implicates surface exposure [82,298]. Evidence for OMPs as immunoprotective targets has been obtained using OMP-specific antibodies with bactericidal activity [299] (with or without complement), opsonophagocytosis, blockade of attachment, aggregation of outer membrane particles [244], and passive immunity [183]. Although these assays are indirect measures, they are powerful surrogates for assays of surface-exposure, and strongly implicate outer membrane proteins as targets of protective immunity.
Acknowledgments
Original work in the authors’ laboratories was supported by grants (to B.A.) from the National Health and Medical Research Council, Canberra, Australia and by VA Medical Research Funds (to D.A.H.) and a Public Health Service Grant AI-34431 (to D.A.H.). We are especially grateful to Dr. David R. Blanco, Dr. Michael A. Lovett and Dr. J. Christopher Fenno for providing electron micrographs.
References
- 1.von Heijne G. Signal sequences. The limits of variation J Mol Biol. 1985;184:99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi S, Wu HC. Lipoproteins in bacteria. J Bioenerg Biomemb. 1990;22:451–471. doi: 10.1007/BF00763177. [DOI] [PubMed] [Google Scholar]
- 3.von Heijne G. A new method for predicting signal sequence cleavage sites. Nucl Acid Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fikes JD, Barkocy-Gallagher GA, Klapper DG, Bassford PJ., Jr Maturation of Escherichia coli maltose-binding protein by signal peptidase I in vivo. Sequence requirements for efficient processing and demonstration of an alternate cleavage site. J Biol Chem. 1990;265:3417– 3423. [PubMed] [Google Scholar]
- 5.Sjostrom M, Wold S, Wieslander A, Rilfors L. Signal peptide amino acid sequences in Escherichia coli contain information related to final protein localization. A multivariate data analysis. EMBO J. 1987;6:823–831. doi: 10.1002/j.1460-2075.1987.tb04825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Heijne G. The structure of signal peptides from bacterial lipoproteins. Protein Eng. 1989;2:531–534. doi: 10.1093/protein/2.7.531. [DOI] [PubMed] [Google Scholar]
- 7.Haake DA. Spirochaetal lipoproteins and pathogenesis. Microbiology. 2000;146:1491–1504. doi: 10.1099/00221287-146-7-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juncker AS, Willenbrock H, Von Heijne G, Brunak S, Nielsen H, Krogh A. Prediction of lipoprotein signal peptides in gram-negative bacteria. Protein Sci. 2003;12:1652–1662. doi: 10.1110/ps.0303703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valent QA, Kendall DA, High S, Kusters R, Oudega B, Luirink J. Early events in preprotein recognition in E. coli: interaction of SRP and trigger factor with nascent polypeptides. EMBO J. 1995;14:5494–5505. doi: 10.1002/j.1460-2075.1995.tb00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valent QA, et al. The Escherichia coli SRP and SecB targeting pathways converge at the translocon. EMBO J. 1998;17:2504–2512. doi: 10.1093/emboj/17.9.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Economou A. Bacterial preprotein translocase: mechanism and conformational dynamics of a processive enzyme. Mol Microbiol. 1998;27:511–518. doi: 10.1046/j.1365-2958.1998.00713.x. [DOI] [PubMed] [Google Scholar]
- 12.Miller A, Wang L, Kendall DA. Synthetic signal peptides specifically recognize SecA and stimulate ATPase activity in the absence of preprotein. J Biol Chem. 1998;273:11409–11412. doi: 10.1074/jbc.273.19.11409. [DOI] [PubMed] [Google Scholar]
- 13.Fekkes P, van der Does C, Driessen AJ. The molecular chaperone SecB is released from the carboxy-terminus of SecA during initiation of precursor protein translocation. EMBO J. 1997;16:6105–6113. doi: 10.1093/emboj/16.20.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu HC. Biosynthesis of lipoproteins. In: FCNa, editor. Escherichia coli and Salmonella: Cellular and Molecular Biology. American Society for Microbiology; Washington, DC: 1996. pp. 1005–1014. [Google Scholar]
- 15.Zlotnick GW, Sanfilippo VT, Mattler JA, Kirkley DH, Boykins RA, Seid RC., Jr Purification and characterization of a peptidoglycan-associated lipoprotein from Haemophilus influenzae. J Biol Chem. 1988;263:9790–9794. [PubMed] [Google Scholar]
- 16.Mizuno T. A novel peptidoglycan-associated lipoprotein found in the cell envelope of Pseudomonas aeruginosa and Escherichia coli. J Biochem. 1979;86:991–1000. doi: 10.1093/oxfordjournals.jbchem.a132631. [DOI] [PubMed] [Google Scholar]
- 17.Beermann C, Wunderli-Allenspach H, Groscurth P, Filgueira L. Lipoproteins from Borrelia burgdorferi applied in liposomes and presented by dendritic cells induce CD8(+) T-lymphocytes in vitro. Cell Immunol. 2000;201:124– 131. doi: 10.1006/cimm.2000.1640. [DOI] [PubMed] [Google Scholar]
- 18.Radolf JD, Arndt LL, Akins DR, Curetty LL, Levi ME, Shen Y, Davis LS, Norgard MV. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J Immunol. 1995;154:2866–2877. [PubMed] [Google Scholar]
- 19.Sellati TJ, Abrescia LD, Radolf JD, Furie MB. Outer surface lipoproteins of Borrelia burgdorferi activate vascular endothelium in vitro. Infect Immun. 1996;64:3180–3187. doi: 10.1128/iai.64.8.3180-3187.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morr M, Takeuchi O, Akira S, Simon MM, Muhlradt PF. Differential recognition of structural details of bacterial lipopeptides by toll-like receptors. Eur J Immunol. 2002;32:3337–3347. doi: 10.1002/1521-4141(200212)32:12<3337::AID-IMMU3337>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Erdile LF, Brandt MA, Warakomski DJ, Westrack GJ, Sadziene A, Barbour AG, Mays JP. Role of attached lipid in immunogenicity of Borrelia burgdorferi OspA. Infect Immun. 1993;61:81–90. doi: 10.1128/iai.61.1.81-90.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seydel A, Gounon P, Pugsley AP. Testing the ‘+2 rule’ for lipoprotein sorting in the Escherichia coli cell envelope with a new genetic selection. Mol Microbiol. 1999;34:810–821. doi: 10.1046/j.1365-2958.1999.01647.x. [DOI] [PubMed] [Google Scholar]
- 23.Matsuyama S, Tajima T, Tokuda H. A novel periplasmic carrier protein involved in the sorting and transport of Escherichia coli lipoproteins destined for the outer membrane. EMBO J. 1995;14:3365–3372. doi: 10.1002/j.1460-2075.1995.tb07342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robichon C, Bonhivers M, Pugsley AP. An intramolecular disulphide bond reduces the efficacy of a lipoprotein plasma membrane sorting signal. Mol Microbiol. 2003;49:1145–1154. doi: 10.1046/j.1365-2958.2003.03654.x. [DOI] [PubMed] [Google Scholar]
- 25.Matsuyama S, Yokota N, Tokuda H. A novel outer membrane lipoprotein, LolB (HemM), involved in the LolA (p20)-dependent localization of lipoproteins to the outer membrane of Escherichia coli. EMBO J. 1997;16:6947–6955. doi: 10.1093/emboj/16.23.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narita S, Tanaka K, Matsuyama S, Tokuda H. Disruption of lolCDE, encoding an ATP-binding cassette transporter, is lethal for Escherichia coli and prevents release of lipoproteins from the inner membrane. J Bacteriol. 2002;184:1417–1422. doi: 10.1128/JB.184.5.1417-1422.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yakushi T, Masuda K, Narita S, Matsuyama S, Tokuda H. A new ABC transporter mediating the detachment of lipid-modified proteins from membranes. Nat Cell Biol. 2000;2:212–218. doi: 10.1038/35008635. [DOI] [PubMed] [Google Scholar]
- 28.Kellenberger E. The ‘Bayer bridges’ confronted with results from improved electron microscopy methods. Mol Microbiol. 1990;4:697–705. doi: 10.1111/j.1365-2958.1990.tb00640.x. [DOI] [PubMed] [Google Scholar]
- 29.Sen K, Nikaido H. In vitro trimerization of OmpF porin secreted by spheroplasts of Escherichia coli. Proc Natl Acad Sci USA. 1990;87:743–747. doi: 10.1073/pnas.87.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Cock H, Tommassen J. Lipopolysaccharides and divalent cations are involved in the formation of an assembly-competent intermediate of outer-membrane protein PhoE of E. coli. EMBO J. 1996;15:5567–5573. [PMC free article] [PubMed] [Google Scholar]
- 31.Laird MW, Kloser AW, Misra R. Assembly of LamB and OmpF in deep rough lipopolysaccharide mutants of Escherichia coli K-12. J Bacteriol. 1994;176:2259–2264. doi: 10.1128/jb.176.8.2259-2264.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ried G, Hindennach I, Henning U. Role of lipopolysaccharide in assembly of Escherichia coli outer membrane proteins OmpA, OmpC, and OmpF. J Bacteriol. 1990;172:6048–6053. doi: 10.1128/jb.172.10.6048-6053.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braun M, Silhavy TJ. Imp/OstA is required for cell envelope biogenesis in Escherichia coli. Mol Microbiol. 2002;45:1289–1302. doi: 10.1046/j.1365-2958.2002.03091.x. [DOI] [PubMed] [Google Scholar]
- 34.Chen R, Henning U. A periplasmic protein (Skp) of Escherichia coli selectively binds a class of outer membrane proteins. Mol Microbiol. 1996;19:1287–1294. doi: 10.1111/j.1365-2958.1996.tb02473.x. [DOI] [PubMed] [Google Scholar]
- 35.de Cock H, Schafer U, Potgeter M, Demel R, Muller M, Tommassen J. Affinity of the periplasmic chaperone Skp of Escherichia coli for phospholipids, lipopolysaccharides and nonnative outer membrane proteins. Role of Skp in the biogenesis of outer membrane protein. Eur J Biochem. 1999;259:96–103. doi: 10.1046/j.1432-1327.1999.00010.x. [DOI] [PubMed] [Google Scholar]
- 36.Lazar SW, Kolter R. SurA assists the folding of Escherichia coli outer membrane proteins. J Bacteriol. 1996;178:1770–1773. doi: 10.1128/jb.178.6.1770-1773.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Missiakas D, Betton JM, Raina S. New components of protein folding in extracytoplasmic compartments of Escherichia coli SurA, FkpA and Skp/OmpH. Mol Microbiol. 1996;21:871–884. doi: 10.1046/j.1365-2958.1996.561412.x. [DOI] [PubMed] [Google Scholar]
- 38.Rouviere PE, Gross CA. SurA, a periplasmic protein with peptidyl-prolyl isomerase activity, participates in the assembly of outer membrane porins. Gen Dev. 1996;10:3170–3182. doi: 10.1101/gad.10.24.3170. [DOI] [PubMed] [Google Scholar]
- 39.Thome BM, Hoffschulte HK, Schiltz E, Muller M. A protein with sequence identity to Skp (FirA) supports protein translocation into plasma membrane vesicles of Escherichia coli. FEBS Lett. 1990;269:113–116. doi: 10.1016/0014-5793(90)81132-8. [DOI] [PubMed] [Google Scholar]
- 40.Voulhoux R, Bos MP, Geurtsen J, Mols M, Tommassen J. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science. 2003;299:262–265. doi: 10.1126/science.1078973. [DOI] [PubMed] [Google Scholar]
- 41.Schulz GE. The structure of bacterial outer membrane proteins. Biochem Biophys Acta. 2002;1565:308–317. doi: 10.1016/s0005-2736(02)00577-1. [DOI] [PubMed] [Google Scholar]
- 42.Bunikis J, Noppa L, Bergstrom S. Molecular analysis of a 66-kDa protein associated with the outer membrane of Lyme disease Borrelia. FEMS Microbiol Lett. 1995;131:139–145. doi: 10.1111/j.1574-6968.1995.tb07768.x. [DOI] [PubMed] [Google Scholar]
- 43.Noppa L, Ostberg Y, Lavrinovicha M, Bergstrom S. P13, an integral membrane protein of Borrelia burgdorferi, is C-terminally processed and contains surface-exposed domains. Infect Immun. 2001;69:3323–3334. doi: 10.1128/IAI.69.5.3323-3334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, Lawson CL. Crystallization and preliminary X-ray analysis of Borrelia burgdorferi outer surface protein A (OspA) complexed with a murine monoclonal antibody Fab fragment. J Struct Biol. 1995;115:335–337. doi: 10.1006/jsbi.1995.1058. [DOI] [PubMed] [Google Scholar]
- 45.Li H, Dunn JJ, Luft BJ, Lawson CL. Crystal structure of Lyme disease antigen outer surface protein A complexed with an Fab. Proc Natl Acad Sci USA. 1997;94:3584–3589. doi: 10.1073/pnas.94.8.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eicken C, Sharma V, Klabunde T, Owens RT, Pikas DS, Hook M, Sacchettini JC. Crystal structure of Lyme disease antigen outer surface protein C from Borrelia burgdorferi. J Biol Chem. 2001;276:10010–10015. doi: 10.1074/jbc.M010062200. [DOI] [PubMed] [Google Scholar]
- 47.Kumaran D, Eswaramoorthy S, Luft BJ, Koide S, Dunn JJ, Lawson CL, Swaminathan S. Crystal structure of outer surface protein C (OspC) from the Lyme disease spirochete, Borrelia burgdorferi. EMBO J. 2001;20:971–978. doi: 10.1093/emboj/20.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eicken C, Sharma V, Klabunde T, Lawrenz MB, Hardham JM, Norris SJ, Sacchettini JC. Crystal structure of Lyme disease variable surface antigen VlsE of Borrelia burgdorferi. J Biol Chem. 2002;277:21691–21696. doi: 10.1074/jbc.M201547200. [DOI] [PubMed] [Google Scholar]
- 49.Koebnik R, Locher KP, Van Gelder P. Structure and function of bacterial outer membrane proteins: barrels in a nutshell. Mol Microbiol. 2000;37:239–253. doi: 10.1046/j.1365-2958.2000.01983.x. [DOI] [PubMed] [Google Scholar]
- 50.Tommassen J. Biogenesis and topology of outer membrane proteins in Escherichia coli. In: Op den Kamp JAF, editor. Membrane Biogenesis. Springer-Verlag KG; Berlin: 1988. pp. 351–373. [Google Scholar]
- 51.Koebnik R. Structural and functional roles of the surface-exposed loops of the beta-barrel membrane protein OmpA from Escherichia coli. J Bacteriol. 1999;181:3688–3694. doi: 10.1128/jb.181.12.3688-3694.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martelli PL, Fariselli P, Krogh A, Casadio R. A sequence-profile-based HMM for predicting and discriminating beta barrel membrane proteins. Bioinformatics. 2002;18 (Suppl 1):46–53. doi: 10.1093/bioinformatics/18.suppl_1.s46. [DOI] [PubMed] [Google Scholar]
- 53.Zhai Y, Saier MH., Jr The beta-barrel finder (BBF) program, allowing identification of outer membrane beta-barrel proteins encoded within prokaryotic genomes. Protein Sci. 2002;11:2196–2207. doi: 10.1110/ps.0209002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cornelissen CN, Sparling PF. Iron piracy: acquisition of transferrin-bound iron by bacterial pathogens. Mol Microbiol. 1994;14:843–850. doi: 10.1111/j.1365-2958.1994.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 55.Moeck GS, Coulton JW. TonB-dependent iron acquisition: mechanisms of siderophore-mediated active transport. Mol Microbiol. 1998;28:675–681. doi: 10.1046/j.1365-2958.1998.00817.x. [DOI] [PubMed] [Google Scholar]
- 56.Wandersman C, Stojiljkovic I. Bacterial heme sources: the role of heme, hemoprotein receptors and hemophores. Curr Opin Microbiol. 2000;3:215–220. doi: 10.1016/s1369-5274(00)00078-3. [DOI] [PubMed] [Google Scholar]
- 57.Braun V, Hantke K, Koster W. Bacterial iron transport: mechanisms, genetics, and regulation. Metal Ions Biol Syst. 1998;35:67–145. [PubMed] [Google Scholar]
- 58.Stumpe S, Schmid R, Stephens DL, Georgiou G, Bakker EP. Identification of OmpT as the protease that hydrolyzes the antimicrobial peptide protamine before it enters growing cells of Escherichia coli. J Bacteriol. 1998;180:4002–4006. doi: 10.1128/jb.180.15.4002-4006.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guina T, Yi EC, Wang H, Hackett M, Miller SI. A PhoP-regulated outer membrane protease of Salmonella enterica serovar typhimurium promotes resistance to alphahelical antimicrobial peptides. J Bacteriol. 2000;182:4077–4086. doi: 10.1128/jb.182.14.4077-4086.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Masuda N, Sakagawa E, Ohya S. Outer membrane proteins responsible for multiple drug resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:645–649. doi: 10.1128/AAC.39.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aires JR, Kohler T, Nikaido H, Plesiat P. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob Agents Chemother. 1999;43:2624–2628. doi: 10.1128/aac.43.11.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Provenzano D, Klose KE. Altered expression of the ToxR-regulated porins OmpU and OmpT diminishes Vibrio cholerae bile resistance, virulence factor expression, and intestinal colonization. Proc Natl Acad Sci USA. 2000;97:10220–10224. doi: 10.1073/pnas.170219997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thanassi DG, Cheng LW, Nikaido H. Active efflux of bile salts by Escherichia coli. J Bacteriol. 1997;179:2512–2518. doi: 10.1128/jb.179.8.2512-2518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Achouak W, Heulin T, Pages JM. Multiple facets of bacterial porins. FEMS Microbiol Lett. 2001;199:1–7. doi: 10.1111/j.1574-6968.2001.tb10642.x. [DOI] [PubMed] [Google Scholar]
- 65.Kronvall G, Jonsson K. Receptins: a novel term for an expanding spectrum of natural and engineered microbial proteins with binding properties for mammalian proteins. J Mol Recognit. 1999;12:38–44. doi: 10.1002/(SICI)1099-1352(199901/02)12:1<38::AID-JMR378>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 66.Dehio C, Gray-Owen SD, Meyer TF. The role of neisserial Opa proteins in interactions with host cells. Trends Microbiol. 1998;6:489–495. doi: 10.1016/s0966-842x(98)01365-1. [DOI] [PubMed] [Google Scholar]
- 67.Ram S, McQuillen DP, Gulati S, Elkins C, Pangburn MK, Rice PA. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. J Exp Med. 1998;188:671–680. doi: 10.1084/jem.188.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ram S, et al. C4bp binding to porin mediates stable serum resistance of Neisseria gonorrhoeae. Int Immunopharmacol. 2001;1:423–432. doi: 10.1016/s1567-5769(00)00037-0. [DOI] [PubMed] [Google Scholar]
- 69.Heffernan EJ, Wu L, Louie J, Okamoto S, Fierer J, Guiney DG. Specificity of the complement resistance and cell association phenotypes encoded by the outer membrane protein genes rck from Salmonella typhimurium and ail from Yersinia enterocolitica. Infect Immun. 1994;62:5183–5186. doi: 10.1128/iai.62.11.5183-5186.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vogt J, Schulz GE. The structure of the outer membrane protein OmpX from Escherichia coli reveals possible mechanisms of virulence. Struct Fold Des. 1999;7:1301–1309. doi: 10.1016/s0969-2126(00)80063-5. [DOI] [PubMed] [Google Scholar]
- 71.Stevenson B, Schwan TG, Rosa PA. Temperature- related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Porcella SF, Fitzpatrick CA, Bono JL. Expression and immunological analysis of the plasmid-borne mlp genes of Borrelia burgdorferi strain B31. Infect Immun. 2000;68:4992–5001. doi: 10.1128/iai.68.9.4992-5001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bono JL, Tilly K, Stevenson B, Hogan D, Rosa P. Oligopeptide permease in Borrelia burgdorferi: putative peptide-binding components encoded by both chromosomal and plasmid loci. Microbiology. 1998;144:1033–1044. doi: 10.1099/00221287-144-4-1033. [DOI] [PubMed] [Google Scholar]
- 74.Stevenson B, Bono JL, Schwan TG, Rosa P. Borrelia burgdorferi Erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect Immun. 1998;66:2648–2654. doi: 10.1128/iai.66.6.2648-2654.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cullen PA, Cordwell SJ, Bulach DM, Haake DA, Adler B. Global analysis of outer membrane proteins from Leptospira interrogans serovar Lai. Infect Immun. 2002;70:2311–2318. doi: 10.1128/IAI.70.5.2311-2318.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patti JM, Allen BL, McGavin MJ, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 77.Westerlund B, Korhonen TK. Bacterial proteins binding to the mammalian extracellular matrix. Mol Microbiol. 1993;9:687–694. doi: 10.1111/j.1365-2958.1993.tb01729.x. [DOI] [PubMed] [Google Scholar]
- 78.van Mierlo P, Jacob W, Dockx P. Erythema chronicum migrans: an electron-microscopic study. Dermatology. 1993;186:306–310. doi: 10.1159/000247384. [DOI] [PubMed] [Google Scholar]
- 79.Barthold SW, de Souza MS, Janotka JL, Smith AL, Persing DH. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:959–971. [PMC free article] [PubMed] [Google Scholar]
- 80.Guo BP, Norris SJ, Rosenberg LC, Hook M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–3472. doi: 10.1128/iai.63.9.3467-3472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choi HU, Johnson TL, Pal S, Tang LH, Rosenberg L, Neame PJ. Characterization of the dermatan sulfate proteoglycans, DS-PGI and DS-PGII, from bovine articular cartilage and skin isolated by octyl-Sepharose chromatography. J Biol Chem. 1989;264:2876–2884. [PubMed] [Google Scholar]
- 82.Guo BP, Brown EL, Dorward DW, Rosenberg LC, Hook M. Decorin-binding adhesins from Borrelia burgdorferi. Mol Microbiol. 1998;30:711–723. doi: 10.1046/j.1365-2958.1998.01103.x. [DOI] [PubMed] [Google Scholar]
- 83.Brown EL, Guo BP, O’Neal P, Hook M. Adherence of Borrelia burgdorferi. Identification of critical lysine residues in DbpA required for decorin binding. J Biol Chem. 1999;274:26272–26278. doi: 10.1074/jbc.274.37.26272. [DOI] [PubMed] [Google Scholar]
- 84.Feng S, Hodzic E, Stevenson B, Barthold SW. Humoral immunity to Borrelia burgdorferi N40 decorin binding proteins during infection of laboratory mice. Infect Immun. 1998;66:2827–2835. doi: 10.1128/iai.66.6.2827-2835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hagman KE, Lahdenne P, Popova TG, Porcella SF, Akins DR, Radolf JD, Norgard MV. Decorin-binding protein of Borrelia burgdorferi is encoded within a two-gene operon and is protective in the murine model of Lyme borreliosis. Infect Immun. 1998;66:2674–2683. doi: 10.1128/iai.66.6.2674-2683.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jadoun J, Ozeri V, Burstein E, Skutelsky E, Hanski E, Sela S. Protein F1 is required for efficient entry of Streptococcus pyogenes into epithelial cells. J Infect Dis. 1998;178:147–158. doi: 10.1086/515589. [DOI] [PubMed] [Google Scholar]
- 87.Molinari G, Talay SR, Valentin-Weigand P, Rohde M, Chhatwal GS. The fibronectin-binding protein of Streptococcus pyogenes, SfbI, is involved in the internalization of group A streptococci by epithelial cells. Infect Immun. 1997;65:1357–1363. doi: 10.1128/iai.65.4.1357-1363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ozeri V, Rosenshine I, Mosher DF, Fassler R, Hanski E. Roles of integrins and fibronectin in the entry of Streptococcus pyogenes into cells via protein F1. Mol Microbiol. 1998;30:625–637. doi: 10.1046/j.1365-2958.1998.01097.x. [DOI] [PubMed] [Google Scholar]
- 89.Leong JM, Morrissey PE, Ortega-Barria E, Pereira ME, Coburn J. Hemagglutination and proteoglycan binding by the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:874–883. doi: 10.1128/iai.63.3.874-883.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beeley J. Laboratory Techniques in Biochemistry and Molecular Biology: Glycoprotein and Proteoglycan Techniques. Elsevier Science; New York: 1985. [Google Scholar]
- 91.Kjellen L, Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- 92.Skare JT, et al. The Oms66 (p66) protein is a Borrelia burgdorferi porin. Infect Immun. 1997;65:3654–3661. doi: 10.1128/iai.65.9.3654-3661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Coburn J, Chege W, Magoun L, Bodary SC, Leong JM. Characterization of a candidate Borrelia burgdorferi β3-chain integrin ligand identified using a phage display library. Mol Microbiol. 1999;34:926–940. doi: 10.1046/j.1365-2958.1999.01654.x. [DOI] [PubMed] [Google Scholar]
- 94.Burkot TR, Piesman J, Wirtz RA. Quantitation of the Borrelia burgdorferi outer surface protein A in Ixodes scapularis: fluctuations during the tick life cycle, doubling times, and loss while feeding. J Infect Dis. 1994;170:883–889. doi: 10.1093/infdis/170.4.883. [DOI] [PubMed] [Google Scholar]
- 95.Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fuchs H, Wallich R, Simon MM, Kramer MD. The outer surface protein A of the spirochete Borrelia burgdorferi is a plasmin(ogen) receptor. Proc Natl Acad Sci USA. 1994;91:12594–12598. doi: 10.1073/pnas.91.26.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boyle MD, Lottenberg R. Plasminogen activation by invasive human pathogens. Thromb Haemost. 1997;77:1–10. [PubMed] [Google Scholar]
- 98.Coleman JL, Gebbia JA, Piesman J, Degen JL, Bugge TH, Benach JL. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell. 1997;89:1111–1119. doi: 10.1016/s0092-8674(00)80298-6. [DOI] [PubMed] [Google Scholar]
- 99.Pal U, de Silva AM, Montgomery RR, Fish D, Anguita J, Anderson JF, Lobet Y, Fikrig E. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J. Clin. Invest. 2000;106:561–569. doi: 10.1172/JCI9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bergstrom S, Bundoc VG, Barbour AG. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989;3:479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 101.Cameron CE. Identification of a Treponema pallidum Laminin-binding protein. Infect Immun. 2003;71:2525–2533. doi: 10.1128/IAI.71.5.2525-2533.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lopes JD, dos Reis M, Brentani RR. Presence of laminin receptors in Staphylococcus aureus. Science. 1985;229:275–277. doi: 10.1126/science.3160113. [DOI] [PubMed] [Google Scholar]
- 103.Masuda K, Kawata T. Isolation, properties, and reassembly of outer sheath carrying a polygonal array from an oral treponeme. J Bacteriol. 1982;150:1405–1413. doi: 10.1128/jb.150.3.1405-1413.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weinberg A, Holt SC. Chemical and biological activities of a 64-kDa outer sheath protein from Treponema denticola strains. J Bacteriol. 1991;173:6935–6947. doi: 10.1128/jb.173.21.6935-6947.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fenno JC, Muller KH, McBride BC. Sequence analysis, expression, and binding activity of recombinant major outer sheath protein (Msp) of Treponema denticola. J Bacteriol. 1996;178:2489–2497. doi: 10.1128/jb.178.9.2489-2497.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Caimano MJ, Bourell KW, Bannister TD, Cox DL, Radolf JD. The Treponema denticola major sheath protein is predominantly periplasmic and has only limited surface exposure. Infect Immun. 1999;67:4072–4083. doi: 10.1128/iai.67.8.4072-4083.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Palaniappan RU, et al. Cloning and molecular characterization of an immunogenic LigA protein of Leptospira interrogans. Infect Immun. 2002;70:5924–5930. doi: 10.1128/IAI.70.11.5924-5930.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Matsunaga J, et al. Pathogenic Leptospira species express surface-exposed proteins belonging to the bacterial immunoglobulin superfamily. Mol Microbiol. 2003;49:929–945. doi: 10.1046/j.1365-2958.2003.03619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Medzhitov R, Janeway C., Jr Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- 110.Rautemaa R, Meri S. Complement-resistance mechanisms of bacteria. Microbiol Infect. 1999;1:785–794. doi: 10.1016/s1286-4579(99)80081-1. [DOI] [PubMed] [Google Scholar]
- 111.Ram S, et al. The contrasting mechanisms of serum resistance of Neisseria gonorrhoeae and group B Neisseria meningitidis. Mol Immunol. 1999;36:915–928. doi: 10.1016/s0161-5890(99)00114-5. [DOI] [PubMed] [Google Scholar]
- 112.Vogel U, Frosch M. Mechanisms of neisserial serum resistance. Mol Microbiol. 1999;32:1133–1139. doi: 10.1046/j.1365-2958.1999.01469.x. [DOI] [PubMed] [Google Scholar]
- 113.Kraiczy P, Skerka C, Kirschfink M, Zipfel PF, Brade V. Mechanism of complement resistance of pathogenic Borrelia burgdorferi isolates. Int Immunopharmacol. 2001;1:393–401. doi: 10.1016/s1567-5769(00)00041-2. [DOI] [PubMed] [Google Scholar]
- 114.Hellwage J, Meri T, Heikkila T, Alitalo A, Panelius J, Lahdenne P, Seppala IJ, Meri S. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J Biol Chem. 2001;276:8427–8435. doi: 10.1074/jbc.M007994200. [DOI] [PubMed] [Google Scholar]
- 115.Stevenson B, El-Hage N, Hines MA, Miller JC, Babb K. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect Immun. 2002;70:491–497. doi: 10.1128/IAI.70.2.491-497.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kraiczy P, Skerka C, Kirschfink M, Brade V, Zipfel PF. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and Factor H. Eur. J Immunol. 2001;31:1674–1684. doi: 10.1002/1521-4141(200106)31:6<1674::aid-immu1674>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 117.Kraiczy P, Skerka C, Brade V, Zipfel PF. Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect Immun. 2001;69:7800–7809. doi: 10.1128/IAI.69.12.7800-7809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McDowell JV, Wolfgang J, Tran E, Metts MS, Hamilton D, Marconi RT. Comprehensive analysis of the factor H binding capabilities of Borrelia species associated with lyme disease: delineation of two distinct classes of factor H binding proteins. Infect Immun. 2003;71:3597–3602. doi: 10.1128/IAI.71.6.3597-3602.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Benz R. Porins – structure and function. In: Winkelmann G, editor. Microbial transport systems. Wiley-VCH Verlag GmbH; Weinheim, Germany: 2001. pp. 227–246. [Google Scholar]
- 120.Benz R, Janko K, Boos W, Lauger P. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochem Biophys Acta. 1978;511:305–319. doi: 10.1016/0005-2736(78)90269-9. [DOI] [PubMed] [Google Scholar]
- 121.Hancock RE. Model membrane studies of porin function. In: Inouye M, editor. Bacterial Outer Membranes as Model Systems. Wiley; New York: 1986. pp. 187–225. [Google Scholar]
- 122.Galdiero S, Capasso D, Vitiello M, D’Isanto M, Pedone C, Galdiero M. Role of surface-exposed loops of Haemophilus influenzae protein P2 in the mitogen-activated protein kinase cascade. Infect Immun. 2003;71:2798–2809. doi: 10.1128/IAI.71.5.2798-2809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bernardini ML, Sanna MG, Fontaine A, Sansonetti PJ. OmpC is involved in invasion of epithelial cells by Shigella flexneri. Infect Immun. 1993;61:3625–3635. doi: 10.1128/iai.61.9.3625-3635.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Moser I, Schroder WF, Hellmann E. In vitro binding of Campylobacter jejuni/coli outer membrane preparations to INT 407 cell membranes. Med Microbiol Immunol. 1992;180:289–303. doi: 10.1007/BF00191550. [DOI] [PubMed] [Google Scholar]
- 125.Yu S, Ding H, Seah J, Wu K, Chang Y, Chang KS, Tam MF, Syu W. Characterization of a phage specific to hemorrhagic Escherichia coli O157:H7 and disclosure of variations in host outer membrane protein OmpC. J Biomed Sci. 1998;5:370–382. doi: 10.1007/BF02253447. [DOI] [PubMed] [Google Scholar]
- 126.Rudel T, Schmid A, Benz R, Kolb HA, Lang F, Meyer TF. Modulation of Neisseria porin (PorB) by cytosolic ATP/GTP of target cells: parallels between pathogen accommodation and mitochondrial endosymbiosis. Cell. 1996;85:391–402. doi: 10.1016/s0092-8674(00)81117-4. [DOI] [PubMed] [Google Scholar]
- 127.Gill MJ, Simjee S, Al-Hattawi K, Robertson BD, Easmon CS, Ison CA. Gonococcal resistance to betalactams and tetracycline involves mutation in loop 3 of the porin encoded at the penB locus. Antimicrob Agents Chemother. 1998;42:2799–2803. doi: 10.1128/aac.42.11.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chevalier J, Pages JM, Mallea M. In vivo modification of porin activity conferring antibiotic resistance to Enterobacter aerogenes. Biochem Biophys Res Commun. 1999;266:248–251. doi: 10.1006/bbrc.1999.1795. [DOI] [PubMed] [Google Scholar]
- 129.Domenech-Sanchez A, Hernandez-Alles S, Martinez-Martinez L, Benedi VJ, Alberti S. Identification and characterization of a new porin gene of Klebsiella pneumoniae: its role in beta-lactam antibiotic resistance. J Bacteriol. 1999;181:2726–2732. doi: 10.1128/jb.181.9.2726-2732.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Alberti S, Marques G, Hernandez-Alles S, Rubires X, Tomas JM, Vivanco F, Benedi VJ. Interaction between complement subcomponent C1q and the Klebsiella pneumoniae porin OmpK36. Infect Immun. 1996;64:4719–4725. doi: 10.1128/iai.64.11.4719-4725.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Merino S, Nogueras MM, Aguilar A, Rubires X, Alberti S, Benedi VJ, Tomas JM. Activation of the complement classical pathway (C1q binding) by mesophilic Aeromonas hydrophila outer membrane protein. Infect Immun. 1998;66:3825–3831. doi: 10.1128/iai.66.8.3825-3831.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Iovane G, Pagnini P, Galdiero M, Cipollaro de l’Ero G, Vitiello M, D’Isanto M, Marcatili A. Role of Pasteurella multocida porin on cytokine expression and release by murine splenocytes. Vet Immunol Immunopathol. 1998;66:391–404. doi: 10.1016/s0165-2427(98)00183-4. [DOI] [PubMed] [Google Scholar]
- 133.Galdiero M, Folgore A, Molitierno M, Greco R. Porins and lipopolysaccharide (LPS) from Salmonella typhimurium induce leucocyte transmigration through human endothelial cells in vitro. Clin Exp Immunol. 1999;116:453–461. doi: 10.1046/j.1365-2249.1999.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jelfs J, Munro R, Wedege E, Caugant DA. Sequence variation in the porA gene of a clone of Neisseria meningitidis during epidemic spread. Clin Diag Lab Immunol. 2000;7:390–395. doi: 10.1128/cdli.7.3.390-395.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thompson JC. Morphological changes in red blood cells of calves caused by Leptospira interrogans serovar Pomona. J Comp Pathol. 1986;96:517–527. doi: 10.1016/0021-9975(86)90072-1. [DOI] [PubMed] [Google Scholar]
- 136.Faine S, Adler B, Bolin C, Perolat P. Leptospira and Leptospirosis. MediSci; Melbourne, Australia: 1999. [Google Scholar]
- 137.Segers RP, van Gestel JA, van Eys GJ, van der Zeijst BA, Gaastra W. Presence of putative sphingomyelinase genes among members of the family Leptospiraceae. Infect Immun. 1992;60:1707–1710. doi: 10.1128/iai.60.4.1707-1710.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Segers RPAM, van dDA, de NA, Corcione P, van dZBAM, Gaastra W. Molecular analysis of a sphingomyelinase C gene from Leptospira interrogans serovar Hardjo. Infect Immun. 1990;58:2177–2185. doi: 10.1128/iai.58.7.2177-2185.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Segers RPAM. Rijksuniversiteit te Utrecht; Netherlands: 1991. p. 147. [Google Scholar]
- 140.Lee SH, Kim KA, Park YG, Seong IW, Kim MJ, Lee YJ. Identification and partial characterization of a novel hemolysin from Leptospira interrogans serovar Lai. Gene. 2000;254:19–28. doi: 10.1016/s0378-1119(00)00293-6. [DOI] [PubMed] [Google Scholar]
- 141.Haake DA, et al. The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection. Infect Immun. 2000;68:2276–2285. doi: 10.1128/iai.68.4.2276-2285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pennington PM, Allred CD, West CS, Alvarez R, Barbour AG. Arthritis severity and spirochete burden are determined by serotype in the Borrelia turicatae-mouse model of Lyme disease. Infect Immun. 1997;65:285–292. doi: 10.1128/iai.65.1.285-292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pennington PM, Cadavid D, Barbour AG. Characterization of VspB of Borrelia turicatae, a major outer membrane protein expressed in blood and tissues of mice. Infect Immun. 1999;67:4637–4645. doi: 10.1128/iai.67.9.4637-4645.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pennington PM, Cadavid D, Bunikis J, Norris SJ, Barbour AG. Extensive interplasmidic duplications change the virulence phenotype of the relapsing fever agent Borrelia turicatae. Mol Microbiol. 1999;34:1120–1132. doi: 10.1046/j.1365-2958.1999.01675.x. [DOI] [PubMed] [Google Scholar]
- 145.Cadavid D, Pennington PM, Kerentseva TA, Bergstrom S, Barbour AG. Immunologic and genetic analyses of VmpA of a neurotropic strain of Borrelia turicatae. Infect Immun. 1997;65:3352–3360. doi: 10.1128/iai.65.8.3352-3360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cadavid D, Thomas DD, Crawley R, Barbour AG. Variability of a bacterial surface protein and disease expression in a possible mouse model of systemic Lyme borreliosis. J Exp Med. 1994;179:631–642. doi: 10.1084/jem.179.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nordstrand A, Barbour AG, Bergstrom S. Borrelia pathogenesis research in the post-genomic and postvaccine era. Curr Opin Microbiol. 2000;3:86–92. doi: 10.1016/s1369-5274(99)00056-9. [DOI] [PubMed] [Google Scholar]
- 148.Ishihara K, Okuda K. Molecular pathogenesis of the cell surface proteins and lipids from Treponema denticola. FEMS Microbiol Lett. 1999;181:199–204. doi: 10.1111/j.1574-6968.1999.tb08844.x. [DOI] [PubMed] [Google Scholar]
- 149.Ishihara K, Miura T, Kuramitsu HK, Okuda K. Characterization of the Treponema denticola prtP gene encoding a prolyl-phenylalanine-specific protease (dentilisin) Infect Immun. 1996;64:5178–5186. doi: 10.1128/iai.64.12.5178-5186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee SY, Bian XL, Wong GW, Hannam PM, McBride BC, Fenno JC. Cleavage of Treponema denticola PrcA polypeptide to yield protease complex-associated proteins Prca1 and Prca2 is dependent on PrtP. J Bacteriol. 2002;184:3864–3870. doi: 10.1128/JB.184.14.3864-3870.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Grenier D, Uitto VJ, McBride BC. Cellular location of a Treponema denticola chymotrypsinlike protease and importance of the protease in migration through the basement membrane. Infect Immun. 1990;58:347–351. doi: 10.1128/iai.58.2.347-351.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Uitto VJ, Grenier D, Chan EC, McBride BC. Isolation of a chymotrypsinlike enzyme from Treponema denticola. Infect Immun. 1988;56:2717–2722. doi: 10.1128/iai.56.10.2717-2722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ishihara K, Kuramitsu HK, Miura T, Okuda K. Dentilisin activity affects the organization of the outer sheath of Treponema denticola. J Bacteriol. 1998;180:3837–3844. doi: 10.1128/jb.180.15.3837-3844.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fenno JC, Wong GW, Hannam PM, McBride BC. Mutagenesis of outer membrane virulence determinants of the oral spirochete Treponema denticola. FEMS Microbiol Lett. 1998;163:209–215. doi: 10.1111/j.1574-6968.1998.tb13047.x. [DOI] [PubMed] [Google Scholar]
- 155.Egli C, Leung WK, Muller KH, Hancock RE, McBride BC. Pore-forming properties of the major 53-kDa surface antigen from the outer sheath of Treponema denticola. Infect Immun. 1993;61:1694–1699. doi: 10.1128/iai.61.5.1694-1699.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mathers DA, Leung WK, Fenno JC, Hong Y, McBride BC. The major surface protein complex of Treponema denticola depolarizes and induces ion channels in HeLa cell membranes. Infect Immun. 1996;64:2904–2910. doi: 10.1128/iai.64.8.2904-2910.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fenno JC, Hannam PM, Leung WK, Tamura M, Uitto VJ, McBride BC. Cytopathic effects of the major surface protein and the chymotrypsinlike protease of Treponema denticola. Infect Immun. 1998;66:1869–1877. doi: 10.1128/iai.66.5.1869-1877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang Q, Ko KS, Kapus A, McCulloch CA, Ellen RP. A spirochete surface protein uncouples storeoperated calcium channels in fibroblasts: a novel cytotoxic mechanism. J Biol Chem. 2001;276:23056–23064. doi: 10.1074/jbc.M011735200. [DOI] [PubMed] [Google Scholar]
- 159.Posey JE, Gherardini FC. Lack of a role for iron in the Lyme disease pathogen. Science. 2000;288:1651–1653. doi: 10.1126/science.288.5471.1651. [DOI] [PubMed] [Google Scholar]
- 160.Posey JE, Hardham JM, Norris SJ, Gherardini FC. Characterization of a manganese-dependent regulatory protein, TroR, from Treponema pallidum. Proc Natl Acad Sci USA. 1999;96:10887–10892. doi: 10.1073/pnas.96.19.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Scott D, Siboo IR, Chan EC, Klitorinos A, Siboo R. Binding of hemin and congo red by oral hemolytic spirochetes. Oral Microbiol Immunol. 1993;8:245–250. doi: 10.1111/j.1399-302x.1993.tb00568.x. [DOI] [PubMed] [Google Scholar]
- 162.Otto BR, Verweij-van Vught AM, MacLaren DM. Transferrins and heme-compounds as iron sources for pathogenic bacteria. Crit Rev Microbiol. 1992;18:217–233. doi: 10.3109/10408419209114559. [DOI] [PubMed] [Google Scholar]
- 163.Chu L, Song M, Holt SC. Effect of iron regulationon expression and hemin-binding function of outer-sheath proteins from Treponema denticola. Microb Pathogenesis. 1994;16:321–335. doi: 10.1006/mpat.1994.1033. [DOI] [PubMed] [Google Scholar]
- 164.Xu X, Holt SC, Kolodrubetz D. Cloning and expression of two novel hemin binding protein genes from Treponema denticola. Infect Immun. 2001;69:4465–4472. doi: 10.1128/IAI.69.7.4465-4472.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chu L, Burgum A, Kolodrubetz D, Holt SC. The 46-kDa-hemolysin gene from Treponema denticola encodes a novel hemolysin homologous to aminotransferases. Infect Immun. 1995;63:4448–4455. doi: 10.1128/iai.63.11.4448-4455.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Grenier D. Characteristics of hemolytic and hemagglutinating activities of Treponema denticola. Oral Microbiol Immunol. 1991;6:246–249. doi: 10.1111/j.1399-302x.1991.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 167.Riviere GR, Thomas DD, Cobb CM. In vitro model of Treponema pallidum invasiveness. Infect Immun. 1989;57:2267–2271. doi: 10.1128/iai.57.8.2267-2271.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Comstock LE, Thomas DD. Penetration of endothelial cell monolayers by Borrelia burgdorferi. Infect Immun. 1989;57:1626–1628. doi: 10.1128/iai.57.5.1626-1628.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Barocchi MA, Ko AI, Reis MG, McDonald KL, Riley LW. Rapid translocation of polarized MDCK cell monolayers by Leptospira interrogans, an invasive but nonintracellular pathogen. Infect Immun. 2002;70:6926–6932. doi: 10.1128/IAI.70.12.6926-6932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Merien F, Baranton G, Perolat P. Invasion of Vero cells and induction of apoptosis in macrophages by pathogenic Leptospira interrogans are correlated with virulence. Infect Immun. 1997;65:729–738. doi: 10.1128/iai.65.2.729-738.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Seiler KP, Weis JJ. Immunity to Lyme disease: protection, pathology and persistence. Curr Opin Immunol. 1996;8:503–509. doi: 10.1016/s0952-7915(96)80038-0. [DOI] [PubMed] [Google Scholar]
- 172.Salazar JC, Hazlett KR, Radolf JD. The immune response to infection with Treponema pallidum, the stealth pathogen. Microb Infect. 2002;4:1133–1140. doi: 10.1016/s1286-4579(02)01638-6. [DOI] [PubMed] [Google Scholar]
- 173.Adler B, Faine S. A pomona serogroup-specific, agglutinating antigen in Leptospira, identified by monoclonal antibodies. Pathology. 1983;15:247–250. doi: 10.3109/00313028309083501. [DOI] [PubMed] [Google Scholar]
- 174.Farrelly HE, Adler B, Faine S. Opsonic monoclonal antibodies against lipopolysaccharide antigens of Leptospira interrogans serovar hardjo. J Med Microbiol. 1987;23:1–7. doi: 10.1099/00222615-23-1-1. [DOI] [PubMed] [Google Scholar]
- 175.Joens LA, Nuessen ME. Bactericidal effect of normal swine sera on Treponema hyodysenteriae. Infect Immun. 1986;51:282–285. doi: 10.1128/iai.51.1.282-285.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Miller JN. Immunity in experimental syphilis. VI Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by -irradiation. J Immunol. 1973;110:1206–1215. [PubMed] [Google Scholar]
- 177.Cox DL, Akins DR, Porcella SF, Norgard MV, Radolf JD. Treponema pallidum in gel microdroplets: a novel strategy for investigation of treponemal molecular architecture. Mol Microbiol. 1995;15:1151–1164. doi: 10.1111/j.1365-2958.1995.tb02288.x. [DOI] [PubMed] [Google Scholar]
- 178.Cox DL, Akins DR, Bourell KW, Lahdenne P, Norgard MV, Radolf JD. Limited surface exposure of Borrelia burgdorferi outer surface lipoproteins. Proc Natl Acad Sci USA. 1996;93:7973–7978. doi: 10.1073/pnas.93.15.7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hazlett KR, Sellati TJ, Nguyen TT, Cox DL, Clawson ML, Caimano MJ, Radolf JD. The TprK protein of Treponema pallidum is periplasmic and is not a target of opsonic antibody or protective immunity. J Exp Med. 2001;193:1015–1026. doi: 10.1084/jem.193.9.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Radolf JD. Treponema pallidum and the quest for outer membrane proteins. Mol Microbiol. 1995;16:1067–1073. doi: 10.1111/j.1365-2958.1995.tb02332.x. [DOI] [PubMed] [Google Scholar]
- 181.Radolf JD. Role of outer membrane architecture in immune evasion by Treponema pallidum and Borrelia burgdorferi. Trends Microbiol. 1994;2:307–311. doi: 10.1016/0966-842x(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 182.Fikrig E, Barthold SW, Kantor FS, Flavell RA. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990;250:553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- 183.Hanson MS, Cassatt DR, Guo BP, Patel NK, McCarthy MP, Dorward DW, Hook M. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect Immun. 1998;66:2143–2153. doi: 10.1128/iai.66.5.2143-2153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Probert WS, LeFebvre RB. Protection of C3H/HeN mice from challenge with Borrelia burgdorferi through active immunization with OspA, OspB, or OspC, but not with OspD or the 83-kDa antigen. Infect Immun. 1994;62:1920–1926. doi: 10.1128/iai.62.5.1920-1926.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Haake DA, Mazel MK, McCoy AM, Milward F, Chao G, Matsunaga J, Wagar EA. Leptospiral outer membrane proteins OmpL1 and LipL41 exhibit synergistic immunoprotection. Infect Immun. 1999;67:6572–6582. doi: 10.1128/iai.67.12.6572-6582.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Branger C, Sonrier C, Chatrenet B, Klonjkowski B, Ruvoen-Clouet N, Aubert A, Andre-Fontaine G, Eloit M. Identification of the hemolysis-associated protein 1 as a cross-protective immunogen of Leptospira interrogans by adenovirus-mediated vaccination. Infect Immun. 2001;69:6831–6838. doi: 10.1128/IAI.69.11.6831-6838.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Haake DA, Martinich C, Summers TA, Shang ES, Pruetz JD, McCoy AM, Mazel MK, Bolin CA. Characterization of leptospiral outer membrane lipoprotein LipL36: downregulation associated with late-log-phase growth and mammalian infection. Infect Immun. 1998;66:1579–1587. doi: 10.1128/iai.66.4.1579-1587.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Barnett JK, Barnett D, Bolin CA, Summers TA, Wagar EA, Cheville NF, Hartskeerl RA, Haake DA. Expression and distribution of leptospiral outer membrane components during renal infection of hamsters. Infect Immun. 1999;67:853–861. doi: 10.1128/iai.67.2.853-861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Thomas W, Sellwood R. Molecular cloning, expression, and DNA sequence analysis of the gene that encodes the 16-kDa outer membrane lipoprotein of Serpulina hyodysenteriae. Infect Immun. 1993;61:1136–1140. doi: 10.1128/iai.61.3.1136-1140.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sellwood R, Walton F, Thomas W, Burrows MR, Chesham J. Expression of the SmpA outer membrane lipoprotein of Serpulina hyodysenteriae strain P18A in vivo. Vet Microbiol. 1995;44:25–35. doi: 10.1016/0378-1135(94)00108-9. [DOI] [PubMed] [Google Scholar]
- 191.Belisle JT, Brandt ME, Radolf JD, Norgard MV. Fatty acids of Treponema pallidum and Borrelia burgdorferi lipoproteins. J Bacteriol. 1994;176:2151–2157. doi: 10.1128/jb.176.8.2151-2157.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fraser CM, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 193.Fraser CM, et al. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science. 1998;281:375–388. doi: 10.1126/science.281.5375.375. [DOI] [PubMed] [Google Scholar]
- 194.Joens LA, Whipp SC, Glock RD, Neussen ME. Serotype-specific protection against Treponema hyodysenteriae infection in ligated colonic loops of pigs recovered from swine dysentery. Infect Immun. 1983;39:460–462. doi: 10.1128/iai.39.1.460-462.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jost BH, Adler B, Faine S. Experimental immunisation of hamsters with lipopolysaccharide antigens of Leptospira interrogans. J Med Microbiol. 1989;29:115–120. doi: 10.1099/00222615-29-2-115. [DOI] [PubMed] [Google Scholar]
- 196.Schoone GJ, Everard COR, Korver H, Carrington DG, Inniss VA, Baulu J, Terpstra WJ. An immunoprotective monoclonal antibody directed against Leptospira interrogans serovar Copenhageni. J Gen Microbiol. 1989;135:73–78. doi: 10.1099/00221287-135-1-73. [DOI] [PubMed] [Google Scholar]
- 197.Midwinter A, Faine S, Adler B. Vaccination of mice with lipopolysaccharide (LPS) and LPS-derived immunoconjugates from Leptospira interrogans. J Med Microbiol. 1990;33:199–204. doi: 10.1099/00222615-33-3-199. [DOI] [PubMed] [Google Scholar]
- 198.Parizek R, Stewart R, Brown K, Blevins D. Protection against swine dysentery with an inactivated Treponema hyodysenteriae bacterin. Vet Med. 1985;80:80–86. [Google Scholar]
- 199.Jenkins EM. Development of resistance to swine dysentery. Vet Med Small Anim Clin. 1978;73:931–936. [PubMed] [Google Scholar]
- 200.Fernie DS, Ripley PH, Walker PD. Swine dysentery: protection against experimental challenge following single dose parenteral immunisation with inactivated Treponema hyodysenteriae. Res Vet Sci. 1983;35:217–221. [PubMed] [Google Scholar]
- 201.Stamm LV, Greene SR, Bergen HL, Hardham JM, Barnes NY. Identification and sequence analysis of Treponema pallidum tprJ, a member of a polymorphic multigene family. FEMS Microbiol Lett. 1998;169:155–163. doi: 10.1111/j.1574-6968.1998.tb13312.x. [DOI] [PubMed] [Google Scholar]
- 202.Wang IN, Dykhuizen DE, Qiu W, Dunn JJ, Bosler EM, Luft BJ. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics. 1999;151:15–30. doi: 10.1093/genetics/151.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhong W, Gern L, Stehle T, Museteanu C, Kramer M, Wallich R, Simon MM. Resolution of experimental and tick-borne Borrelia burgdorferi infection in mice by passive, but not active immunization using recombinant OspC. Eur J Immunol. 1999;29:946–957. doi: 10.1002/(SICI)1521-4141(199903)29:03<946::AID-IMMU946>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 204.Raadsma HW, McEwan JC, Stear MJ, Crawford AM. Genetic characterisation of protective vaccine responses in sheep using multi-valent Dichelobacter nodosus vaccines. Vet Immunol Immunopathol. 1999;72:219–229. doi: 10.1016/s0165-2427(99)00135-x. [DOI] [PubMed] [Google Scholar]
- 205.Busch RA. Polyvalent vaccines in fish: the interactive effects of multiple antigens. Dev Biol Stand. 1997;90:245–256. [PubMed] [Google Scholar]
- 206.Requejo HI. Polyvalent pneumococcal polysaccharide vaccines; a review of the literature. Rev Hosp Clin. 1993;48:130–138. [PubMed] [Google Scholar]
- 207.Winterfield RW, Fadly AM. Potential for polyvalent infectious bronchitis vaccines. Am J Vet Res. 1975;36:524–526. [PubMed] [Google Scholar]
- 208.Barbour AG, Restrepo BI. Antigenic variation in vector-borne pathogens. Emerg Infect Dis. 2000;6:449–457. doi: 10.3201/eid0605.000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Coleman JL, Rogers RC, Benach JL. Selection of an escape variant of Borrelia burgdorferi by use of bactericidal monoclonal antibodies to OspB. Infect Immun. 1992;60:3098–3104. doi: 10.1128/iai.60.8.3098-3104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Coleman JL, Rogers RC, Rosa PA, Benach JL. Variations in the ospB gene of Borrelia burgdorferi result in differences in monoclonal antibody reactivity and in production of escape variants. Infect Immun. 1994;62:303–307. doi: 10.1128/iai.62.1.303-307.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Escudero R, Halluska ML, Backenson PB, Coleman JL, Benach JL. Characterization of the physiological requirements for the bactericidal effects of a monoclonal antibody to OspB of Borrelia burgdorferi by confocal microscopy. Infect Immun. 1997;65:1908–1915. doi: 10.1128/iai.65.5.1908-1915.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sadziene A, Jonsson M, Bergstrom S, Bright RK, Kennedy RC, Barbour AG. A bactericidal antibody to Borrelia burgdorferi is directed against a variable region of the OspB protein. Infect Immun. 1994;62:2037–2045. doi: 10.1128/iai.62.5.2037-2045.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Katona LI, Ayalew S, Coleman JL, Benach JL. A bactericidal monoclonal antibody elicits a change in its antigen, OspB of Borrelia burgdorferi, that can be detected by limited proteolysis. J Immunol. 2000;164:1425–1431. doi: 10.4049/jimmunol.164.3.1425. [DOI] [PubMed] [Google Scholar]
- 214.Fikrig E, Tao H, Barthold SW, Flavell RA. Selection of variant Borrelia burgdorferi isolates from mice immunized with outer surface protein A or B. Infect. Immun. 1995;63:1658–1662. doi: 10.1128/iai.63.5.1658-1662.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Schwan TG, Schrumpf ME, Karstens RH, Clover JR, Wong J, Daugherty M, Struthers M, Rosa PA. Distribution and molecular analysis of Lyme disease spirochetes, Borrelia burgdorferi, isolated from ticks throughout California. J Clin Microbiol. 1993;31:3096–3108. doi: 10.1128/jcm.31.12.3096-3108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Fikrig E, Tao H, Kantor FS, Barthold SW, Flavell RA. Evasion of protective immunity by Borrelia burgdorferi by truncation of outer surface protein B. Proc. Natl Acad Sci USA. 1993;90:4092–4096. doi: 10.1073/pnas.90.9.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Probert WS, Crawford M, LeFebvre RB. Antibodies to OspB prevent infection of C3H mice challenged with Borrelia burgdorferi isolates expressing truncated OspB antigens. Vaccine. 1997;15:15–19. doi: 10.1016/s0264-410x(96)00123-5. [DOI] [PubMed] [Google Scholar]
- 218.de Silva AM, Fikrig E. Arthropod- and host-specific gene expression by Borrelia burgdorferi. J Clin Invest. 1997;99:377–379. doi: 10.1172/JCI119169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.de Silva AM, Telford SR, 3rd, Brunet LR, Barthold SW, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sigal LH, et al. A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. Recombinant Outer-Surface Protein A Lyme Disease Vaccine Study Consortium. N Engl J Med. 1998;339:216–222. doi: 10.1056/NEJM199807233390402. [DOI] [PubMed] [Google Scholar]
- 221.Steere AC, et al. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. Lyme Disease Vaccine Study Group. N Engl J Med. 1998;339:209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 222.de Silva AM, Zeidner NS, Zhang Y, Dolan MC, Piesman J, Fikrig E. Influence of outer surface protein A antibody on Borrelia burgdorferi within feeding ticks. Infect Immun. 1999;67:30–35. doi: 10.1128/iai.67.1.30-35.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rathinavelu S, Broadwater A, de Silva AM. Does host complement kill Borrelia burgdorferi within ticks? Infect Immun. 2003;71:822–829. doi: 10.1128/IAI.71.2.822-829.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Nowling JM, Philipp MT. Killing of Borrelia burgdorferi by antibody elicited by OspA vaccine is inefficient in the absence of complement. Infect Immun. 1999;67:443–445. doi: 10.1128/iai.67.1.443-445.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hitt E. Poor sales trigger vaccine withdrawal. Nat Med. 2002;8:311–312. doi: 10.1038/nm0402-311b. [DOI] [PubMed] [Google Scholar]
- 226.Gross DM, et al. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science. 1998;281:703–706. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- 227.Gern L, Hu CM, Voet P, Hauser P, Lobet Y. Immunization with a polyvalent OspA vaccine protects mice against Ixodes ricinus tick bites infected by Borrelia burgdorferi ss, Borrelia garinii and Borrelia afzelii. Vaccine. 1997;15:1551–1557. doi: 10.1016/s0264-410x(97)00066-2. [DOI] [PubMed] [Google Scholar]
- 228.Padilla ML, Callister SM, Schell RF, Bryant GL, Jobe DA, Lovrich SD, DuChateau BK, Jensen JR. Characterization of the protective borreliacidal antibody response in humans and hamsters after vaccination with a Borrelia burgdorferi outer surface protein A vaccine. J Infect Dis. 1996;174:739–746. doi: 10.1093/infdis/174.4.739. [DOI] [PubMed] [Google Scholar]
- 229.Zumstein G, Fuchs R, Hofmann A, Preac-Mursic V, Soutschek E, Wilske B. Genetic polymorphism of the gene encoding the outer surface protein A (OspA) of Borrelia burgdorferi. Med Microbiol Immunol. 1992;181:57–70. doi: 10.1007/BF00189424. [DOI] [PubMed] [Google Scholar]
- 230.van Hoecke C, et al. Evaluation of the safety, reactogenicity and immunogenicity of three recombinant outer surface protein (OspA) Lyme vaccines in healthy adults. Vaccine. 1996;14:1620–1626. doi: 10.1016/s0264-410x(96)00146-6. [DOI] [PubMed] [Google Scholar]
- 231.Erdile LF, Guy B. OspA lipoprotein of Borrelia burgdorferi is a mucosal immunogen and adjuvant. Vaccine. 1997;15:988–996. doi: 10.1016/s0264-410x(96)00295-2. [DOI] [PubMed] [Google Scholar]
- 232.Bunikis J, Barbour AG. Access of antibody or trypsin to an integral outer membrane protein (P66) of Borrelia burgdorferi is hindered by Osp lipoproteins. Infect Immun. 1999;67:2874–2883. doi: 10.1128/iai.67.6.2874-2883.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Preac-Mursic V, et al. Active immunization with pC protein of Borrelia burgdorferi protects gerbils against B. burgdorferi infection. Infection. 1992;20:342–349. doi: 10.1007/BF01710681. [DOI] [PubMed] [Google Scholar]
- 234.Gilmore RD, Jr, Kappel KJ, Dolan MC, Burkot TR, Johnson BJ. Outer surface protein C (OspC), but not P39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect Immun. 1996;64:2234–2239. doi: 10.1128/iai.64.6.2234-2239.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Livey I, Gibbs CP, Schuster R, Dorner F. Evidence for lateral transfer and recombination in OspC variation in Lyme disease Borrelia. Mol Microbiol. 1995;18:257–269. doi: 10.1111/j.1365-2958.1995.mmi_18020257.x. [DOI] [PubMed] [Google Scholar]
- 236.Theisen M, Frederiksen B, Lebech AM, Vuust J, Hansen K. Polymorphism in ospC gene of Borrelia burgdorferi and immunoreactivity of OspC protein: implications for taxonomy and for use of OspC protein as a diagnostic antigen. J Clin Microbiol. 1993;31:2570–2576. doi: 10.1128/jcm.31.10.2570-2576.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Theisen M, Borre M, Mathiesen MJ, Mikkelsen B, Lebech AM, Hansen K. Evolution of the Borrelia burgdorferi outer surface protein OspC. J Bacteriol. 1995;177:3036–3044. doi: 10.1128/jb.177.11.3036-3044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wilske B, et al. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun. 1993;61:2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Cassatt DR, Patel NK, Ulbrandt ND, Hanson MS. DbpA, but not OspA, is expressed by Borrelia burgdorferi during spirochetemia and is a target for protective antibodies. Infect Immun. 1998;66:5379–5387. doi: 10.1128/iai.66.11.5379-5387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Roberts WC, Mullikin BA, Lathigra R, Hanson MS. Molecular analysis of sequence heterogeneity among genes encoding decorin binding proteins A and B of Borrelia burgdorferi sensu lato. Infect Immun. 1998;66:5275–5285. doi: 10.1128/iai.66.11.5275-5285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hanson MS, Patel NK, Cassatt DR, Ulbrandt ND. Evidence for vaccine synergy between Borrelia burgdorferi decorin binding protein A and outer surface protein A in the mouse model of Lyme borreliosis. Infect Immun. 2000;68:6457–6460. doi: 10.1128/iai.68.11.6457-6460.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Blanco DR, Champion CI, Lewinski MA, Shang ES, Simkins SG, Miller JN, Lovett MA. Immunization with Treponema pallidum outer membrane vesicles induces high-titer complement-dependent treponemicidal activity and aggregation of T. pallidum rare outer membrane proteins (TROMPs) J Immunol. 1999;163:2741–2746. [PubMed] [Google Scholar]
- 243.Lewinski MA, Miller JN, Lovett MA, Blanco DR. Correlation of immunity in experimental syphilis with serum-mediated aggregation of Treponema pallidum rare outer membrane proteins. Infect Immun. 1999;67:3631–3636. doi: 10.1128/iai.67.7.3631-3636.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Blanco DR, Walker EM, Haake DA, Champion CI, Miller JN, Lovett MA. Complement activation limits the rate of in vitro treponemicidal activity and correlates with antibody-mediated aggregation of Treponema pallidum rare outer membrane protein. J Immunol. 1990;144:1914–1921. [PubMed] [Google Scholar]
- 245.Centurion-Lara A, Castro C, Barrett L, Cameron C, Mostow M, Van Voorhis WC, Lukehart SA. Treponema pallidum major sheath protein homologue TprK is a target of opsonic antibody and the protective immune response. J Exp Med. 1999;189:647–656. doi: 10.1084/jem.189.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Morgan CA, Lukehart SA, Van Voorhis WC. Immunization with the N-terminal portion of Treponema pallidum repeat protein K attenuates syphilitic lesion development in the rabbit model. Infect Immun. 2002;70:6811–6816. doi: 10.1128/IAI.70.12.6811-6816.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Borenstein LA, Radolf JD, Fehniger TE, Blanco DR, Miller JN, Lovett MA. Immunization of rabbits with recombinant Treponema pallidum surface antigen 4D alters the course of experimental syphilis. J Immunol. 1988;140:2415–2421. [PubMed] [Google Scholar]
- 248.Cameron CE, Castro C, Lukehart SA, Van Voorhis WC. Function and protective capacity of Treponema pallidum subsp. pallidum glycerophosphodiester phosphodiesterase. Infect Immun. 1998;66:5763–5770. doi: 10.1128/iai.66.12.5763-5770.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Champion CI, Miller JN, Borenstein LA, Lovett MA, Blanco DR. Immunization with Treponema pallidum endoflagella alters the course of experimental rabbit syphilis. Infect Immun. 1990;58:3158–3161. doi: 10.1128/iai.58.9.3158-3161.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Centurion-Lara A, Godornes C, Castro C, Van Voorhis WC, Lukehart SA. The tprK gene is heterogeneous among Treponema pallidum strains and has multiple alleles. Infect Immun. 2000;68:824–831. doi: 10.1128/iai.68.2.824-831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Morgan CA, Molini BJ, Lukehart SA, Van Voorhis WC. Segregation of B and T cell epitopes of Treponema pallidum repeat protein K to variable and conserved regions during experimental syphilis infection. J Immunol. 2002;169:952–957. doi: 10.4049/jimmunol.169.2.952. [DOI] [PubMed] [Google Scholar]
- 252.Shang ES, Summers TA, Haake DA. Molecular cloning and sequence analysis of the gene encoding LipL41, a surface-exposed lipoprotein of pathogenic Leptospira species. Infect Immun. 1996;64:2322–2330. doi: 10.1128/iai.64.6.2322-2330.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Shang ES, Exner MM, Summers TA, Martinich C, Champion CI, Hancock RE, Haake DA. The rare outer membrane protein, OmpL1, of pathogenic Leptospira species is a heat-modifiable porin. Infect Immun. 1995;63:3174–3181. doi: 10.1128/iai.63.8.3174-3181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Barbour AG, Hayes SF. Biology of Borrelia species. Microbiol Rev. 1986;50:381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Barbour AG. Immunobiology of relapsing fever. Contrib Microbiol Immunol. 1987;8:125–137. [PubMed] [Google Scholar]
- 256.Barbour AG, Tessier SL, Stoenner HG. Variable major proteins of Borrellia hermsii. J Exp Med. 1982;156:1312–1324. doi: 10.1084/jem.156.5.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Burman N, Bergstrom S, Restrepo BI, Barbour AG. The variable antigens Vmp7 and Vmp21 of the relapsing fever bacterium Borrelia hermsii are structurally analogous to the VSG proteins of the African trypanosome. Mol Microbiol. 1990;4:1715–1726. doi: 10.1111/j.1365-2958.1990.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 258.Restrepo BI, Kitten T, Carter CJ, Infante D, Barbour AG. Subtelomeric expression regions of Borrelia hermsii linear plasmids are highly polymorphic. Mol Microbiol. 1992;6:3299–3311. doi: 10.1111/j.1365-2958.1992.tb02198.x. [DOI] [PubMed] [Google Scholar]
- 259.Barbour AG, Burman N, Carter CJ, Kitten T, Bergstrom S. Variable antigen genes of the relapsing fever agent Borrelia hermsii are activated by promoter addition. Mol Microbiol. 1991;5:489–493. doi: 10.1111/j.1365-2958.1991.tb02132.x. [DOI] [PubMed] [Google Scholar]
- 260.Kitten T, Barbour AG. Juxtaposition of expressed variable antigen genes with a conserved telomere in the bacterium Borrelia hermsii. Proc Natl Acad Sci USA. 1990;87:6077–6081. doi: 10.1073/pnas.87.16.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kitten T, Barrera AV, Barbour AG. Intragenic recombination and a chimeric outer membrane protein in the relapsing fever agent Borrelia hermsii. J Bacteriol. 1993;175:2516–2522. doi: 10.1128/jb.175.9.2516-2522.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Restrepo BI, Carter CJ, Barbour AG. Activation of a vmp pseudogene in Borrelia hermsii: an alternate mechanism of antigenic variation during relapsing fever. Mol Microbiol. 1994;13:287–299. doi: 10.1111/j.1365-2958.1994.tb00423.x. [DOI] [PubMed] [Google Scholar]
- 263.Restrepo BI, Barbour AG. Antigen diversity in the bacterium B. hermsii through somatic mutations in rearranged vmp genes. Cell. 1994;78:867–876. doi: 10.1016/s0092-8674(94)90642-4. [DOI] [PubMed] [Google Scholar]
- 264.Carter CJ, Bergstrom S, Norris SJ, Barbour AG. A family of surface-exposed proteins of 20 kDa in the genus Borrelia. Infect Immun. 1994;62:2792–2799. doi: 10.1128/iai.62.7.2792-2799.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Barbour AG, Carter CJ, Sohaskey CD. Surface protein variation by expression site switching in the relapsing fever agent Borrelia hermsii. Infect Immun. 2000;68:7114–7121. doi: 10.1128/iai.68.12.7114-7121.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Schwan TG, Hinnebusch BJ. Bloodstream- versus tick-associated variants of a relapsing fever bacterium. Science. 1998;280:1938–1940. doi: 10.1126/science.280.5371.1938. [DOI] [PubMed] [Google Scholar]
- 267.Vidal V, Scragg IG, Cutler SJ, Rockett KA, Fekade D, Warrell DA, Wright DJ, Kwiatkowski D. Variable major lipoprotein is a principal TNF-inducing factor of louse-borne relapsing fever. Nat Med. 1998;4:1416–1420. doi: 10.1038/4007. [DOI] [PubMed] [Google Scholar]
- 268.Shamaei-Tousi A, Martin P, Bergh A, Burman N, Brannstrom T, Bergstrom S. Erythrocyte-aggregating relapsing fever spirochete Borrelia crocidurae induces formation of microemboli. J Infect Dis. 1999;180:1929–1938. doi: 10.1086/315118. [DOI] [PubMed] [Google Scholar]
- 269.Zhang JR, Hardham JM, Barbour AG, Norris SJ. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
- 270.Zhang JR, Norris SJ. Genetic variation of the Borrelia burgdorferi gene vlsE involves cassette-specific, segmental gene conversion. Infect Immun. 1998;66:3698–3704. doi: 10.1128/iai.66.8.3698-3704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zhang JR, Norris SJ. Kinetics and in vivo induction of genetic variation of vlsE in Borrelia burgdorferi. Infect Immun. 1998;66:3689–3697. doi: 10.1128/iai.66.8.3689-3697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Gabe JD, Dragon E, Chang RJ, McCaman MT. Identification of a linked set of genes in Serpulina hyodysenteriae (B204) predicted to encode closely related 39-kDa extracytoplasmic proteins. J Bacteriol. 1998;180:444–448. doi: 10.1128/jb.180.2.444-448.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.McCaman MT, Auer K, Foley W, Gabe JD. Sequence characterization of two new members of a multi-gene family in Serpulina hyodysenteriae (B204) with homology to a 39 kDa surface exposed protein: vspC and D. Vet. Microbiol. 1999;68:273–283. doi: 10.1016/s0378-1135(99)00104-2. [DOI] [PubMed] [Google Scholar]
- 274.McCaman MT, Auer K, Foley W, Gabe JD. Brachyspira hyodysenteriae contains eight linked gene copies related to an expressed 39-kDa surface protein. Microb Infect. 2003;5:1–6. doi: 10.1016/s1286-4579(02)00048-5. [DOI] [PubMed] [Google Scholar]
- 275.Cullen PA, Coutts SA, Cordwell SJ, Bulach DM, Adler B. Characterization of a locus encoding four paralogous outer membrane lipoproteins of Brachyspira hyodysenteriae. Microb Infect. 2003;5:275–283. doi: 10.1016/s1286-4579(03)00027-3. [DOI] [PubMed] [Google Scholar]
- 276.Werts C, et al. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat Immunol. 2001;2:346–352. doi: 10.1038/86354. [DOI] [PubMed] [Google Scholar]
- 277.Hirschfeld M, Kirschning CJ, Schwandner R, Wesche H, Weis JH, Wooten RM, Weis JJ. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J Immunol. 1999;163:2382–2386. [PubMed] [Google Scholar]
- 278.Aliprantis AO, et al. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 279.Wooten RM, Ying MA, Yoder RA, Brown JP, Weis JH, Zachary JF, Kirschning CJ, Weis JJ. Toll-like receptor 2 plays a pivotal role in host defense and inflammatory response to Borrelia burgdorferi. Vector Borne Zoon Dis. 2002;2:275–278. doi: 10.1089/153036602321653860. [DOI] [PubMed] [Google Scholar]
- 280.Yoder A, et al. Tripalmitoyl-S-glyceryl-cysteine-dependent OspA vaccination of toll-like receptor 2-deficient mice results in effective protection from Borrelia burgdorferi challenge. Infect Immun. 2003;71:3894–3900. doi: 10.1128/IAI.71.7.3894-3900.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Holt SC. Anatomy and chemistry of spirochetes. Microbiol Rev. 1978;42:114–160. doi: 10.1128/mr.42.1.114-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Matsunaga J, Young TA, Barnett JK, Barnett D, Bolin CA, Haake DA. Novel 45-kDa leptospiral protein that is processed to a 31-kDa growth-phase-regulated peripheral membrane protein. Infect Immun. 2002;70:323–334. doi: 10.1128/IAI.70.1.323-334.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Schnaitman CA. Cell fractionation. In: Gerhardt P, Murray RGE, Costilow RN, Nester E, Wood WA, Krieg NR, Phillips GB, editors. Manual of Methods for General Bacteriology. American Society for Microbiol; Washington, DC: 1981. pp. 52–61. [Google Scholar]
- 284.Maher PA, Singer SJ. Anomalous interaction of the acetylcholine receptor protein with the nonionic detergent Triton X-114. Proc Natl Acad Sci USA. 1985;82:958–962. doi: 10.1073/pnas.82.4.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Blanco DR, et al. Isolation of the outer membranes from Treponema pallidum and Treponema vincentii. J Bacteriol. 1994;176:6088–6099. doi: 10.1128/jb.176.19.6088-6099.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Cox DL, Chang P, McDowall AW, Radolf JD. The outer membrane, not a coat of host proteins, limits antigenicity of virulent Treponema pallidum. Infect Immun. 1992;60:1076–1083. doi: 10.1128/iai.60.3.1076-1083.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Radolf JD, Robinson EJ, Bourell KW, Akins DR, Porcella SF, Weigel LM, Jones JD, Norgard MV. Characterization of outer membranes isolated from Treponema pallidum, the syphilis spirochete. Infect Immun. 1995;63:4244–4252. doi: 10.1128/iai.63.11.4244-4252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Haake DA, Matsunaga J. Characterization of the leptospiral outer membrane and description of three novel leptospiral membrane proteins. Infect Immun. 2002;70:4936–4945. doi: 10.1128/IAI.70.9.4936-4945.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Skare JT, et al. Virulent strain associated outer membrane proteins of Borrelia burgdorferi. J Clin Invest. 1995;96:2380–2392. doi: 10.1172/JCI118295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Radolf JD, Goldberg MS, Bourell K, Baker SI, Jones JD, Norgard MV. Characterization of outer membranes isolated from Borrelia burgdorferi, the Lyme disease spirochete. Infect Immun. 1995;63:2154–2163. doi: 10.1128/iai.63.6.2154-2163.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Bledsoe HA, Carroll JA, Whelchel TR, Farmer MA, Dorward DW, Gherardini FC. Isolation and partial characterization of Borrelia burgdorferi inner and outer membranes by using isopycnic centrifugation. J Bacteriol. 1994;176:7447–7455. doi: 10.1128/jb.176.24.7447-7455.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Brusca JS, McDowall AW, Norgard MV, Radolf JD. Localization of outer surface proteins A and B in both the outer membrane and intracellular compartments of Borrelia burgdorferi. J Bacteriol. 1991;173:8004–8008. doi: 10.1128/jb.173.24.8004-8008.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Haake DA, Walker EM, Blanco DR, Bolin CA, Miller JN, Lovett MA. Changes in the surface of Leptospira interrogans serovar grippotyphosa during in vitro cultivation. Infect Immun. 1991;59:1131–1140. doi: 10.1128/iai.59.3.1131-1140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Barbour AG, Tessier SL, Hayes SF. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984;45:94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Norris SJ, Carter CJ, Howell JK, Barbour AG. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect Immun. 1992;60:4662–4672. doi: 10.1128/iai.60.11.4662-4672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Lam TT, Nguyen TP, Montgomery RR, Kantor FS, Fikrig E, Flavell RA. Outer surface proteins E and F of Borrelia burgdorferi, the agent of Lyme disease. Infect Immun. 1994;62:290–298. doi: 10.1128/iai.62.1.290-298.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Cullen PA, Haake DA, Bulach DM, Zuerner RL, Adler B. LipL21 is a novel surface-exposed lipoprotein of pathogenic Leptospira species. Infect Immun. 2003;71:2414–2421. doi: 10.1128/IAI.71.5.2414-2421.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Probert WS, Johnson BJ. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol Microbiol. 1998;30:1003–1015. doi: 10.1046/j.1365-2958.1998.01127.x. [DOI] [PubMed] [Google Scholar]
- 299.Sadziene A, Thompson PA, Barbour AG. In vitro inhibition of Borrelia burgdorferi growth by antibodies. J Infect Dis. 1993;167:165–172. doi: 10.1093/infdis/167.1.165. [DOI] [PubMed] [Google Scholar]
- 300.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 301.Blanco DR, Champion CI, Exner MM, Erdjument-Bromage H, Hancock RE, Tempst P, Miller JN, Lovett MA. Porin activity and sequence analysis of a 31-kDa Treponema pallidum subsp. pallidum rare outer membrane protein (Tromp1) J Bacteriol. 1995;177:3556–3562. doi: 10.1128/jb.177.12.3556-3562.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Blanco DR, Miller JN, Lovett MA. Surface antigens of the syphilis spirochete and their potential as virulence determinants. Emerg Infect Dis. 1997;3:11–20. doi: 10.3201/eid0301.970102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Haake DA, Champion CI, Martinich C, Shang ES, Blanco DR, Miller JN, Lovett MA. Molecular cloning and sequence analysis of the gene encoding OmpL1, a transmembrane outer membrane protein of pathogenic Leptospira spp. J Bacteriol. 1993;175:4225–4234. doi: 10.1128/jb.175.13.4225-4234.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Sadziene A, Thomas DD, Barbour AG. Borrelia burgdorferi mutant lacking Osp: biological and immunological characterization. Infect Immun. 1995;63:1573–1580. doi: 10.1128/iai.63.4.1573-1580.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Nilsson CL, Cooper HJ, Hakansson K, Marshall AG, Ostberg Y, Lavrinovicha M, Bergstrom S. Characterization of the P13 membrane protein of Borrelia burgdorferi by mass spectrometry. J Am Soc Mass Spectrosc. 2002;13:295–299. doi: 10.1016/S1044-0305(01)00365-8. [DOI] [PubMed] [Google Scholar]
- 306.Ostberg Y, Pinne M, Benz R, Rosa P, Bergstrom S. Elimination of channel-forming activity by insertional inactivation of the p13 gene in Borrelia burgdorferi. J Bacteriol. 2002;184:6811–6819. doi: 10.1128/JB.184.24.6811-6819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Skare JT, et al. Porin activity of the native and recombinant outer membrane protein Oms28 of Borrelia burgdorferi. J Bacteriol. 1996;178:4909–4918. doi: 10.1128/jb.178.16.4909-4918.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Cugini C, Medrano M, Schwan TG, Coburn J. Regulation of expression of the Borrelia burgdorferi beta(3)-chain integrin ligand, P66, in ticks and in culture. Infect Immun. 2003;71:1001–1007. doi: 10.1128/IAI.71.2.1001-1007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]