Abstract
Context
Blood levels of homocysteine may be elevated in Alzheimer’s disease (AD), and hyperhomocysteinemia may contribute to disease pathophysiology by vascular and direct neurotoxic mechanisms. Even in the absence of vitamin deficiency, homocysteine levels can be reduced by administration of high-dose supplements of folic acid and vitamins B6 and B12. Prior studies of B vitamins to reduce homocysteine in AD have not had sufficient size or duration to assess impact on cognitive decline.
Objective
To determine the efficacy and safety of B vitamin supplementation in the treatment of AD.
Design
We conducted a multicenter, randomized, double-blind controlled clinical trial of high-dose folate/B6/B12 supplementation in individuals with AD.
Setting
The study was conducted between March, 2003 and February, 2007 at clinical research sites of the Alzheimer’s Disease Cooperative Study located throughout the US.
Patients
A total of 409 participants (out of 601 screened) with mild to moderate AD (Mini-Mental Status Scores between 14 and 26, inclusive) and normal folic acid, B12 and homocysteine levels were enrolled in this trial; 340 completed the trial on study medication.
Intervention
Participants were randomly assigned to two groups of unequal size: 60% were treated with daily high-dose supplements (folate 5mg, vitamin B6 25mg, vitamin B12 1 mg), and 40% were treated with identical placebo; the duration of treatment was 18 months.
Main Outcome Measure
The primary outcome measure was the change in the cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAScog).
Results
Although the vitamin supplement regimen was effective in reducing homocysteine levels (active −2.42±3.35; placebo -0.86±2.59; p<0.001), it had no beneficial effect on the primary cognitive measure, rate of change in ADAS-Cog over 18 months (placebo: 0.372 point/month vs active: 0.401 point/month, p-value=0.522, CI of rate difference: (−0.06, 0.12), based on the Generalized Estimating Equations (GEE) model), or on any secondary measures. A higher rate of adverse events involving depression was observed in the group treated with vitamin supplements.
Conclusion
This regimen of high dose B vitamin supplements does not slow cognitive decline in individuals with mild to moderate AD.
Introduction
Alzheimer’s disease (AD) is among the most important health problems worldwide, and while advances in symptomatic treatments targeting cognitive function provide meaningful benefits, effective disease-modifying treatment is needed. Toward this goal, active drug development strategies aim to reduce amyloid accumulation and toxicity,1 slow tau phosphorylation and tangle formation,2 and/or promote neuronal survival and synaptic function.3 Evidence of homocysteine elevation in AD and the involvement of homocysteine in neuropathological mechanisms suggest that reduction of homocysteine may offer an approach to disease modification.4, 5 Indeed, B vitamins that influence homocysteine metabolism have been considered as a therapeutic option to reduce risk of AD or slow its progression.6
Homocysteine is a sulfur amino acid involved in essential metabolic pathways, including methylation reactions. Elevation of homocysteine in blood is a marker of genetic disorders and deficiencies of vitamins B12 and folate (cofactors in homocysteine pathways); homocysteine elevation is associated with endothelial dysfunction and vascular disease, as well as neuropsychiatric disorders.7 Homocysteine is associated with neurovascular ischemic disease, including stroke, silent infarctions and white matter disease.8, 9 Studies have linked homocysteine to amyloid and glutamate neurotoxicity, and to cognitive dysfunction and AD. For example, homocysteine elevation induces hippocampal neuron loss in transgenic mice with brain amyloid deposition;5 the mechanisms are incompletely understood, but may involve impaired DNA repair and induction of apoptotic cell death.4, 5
Studies have demonstrated a relationship between plasma homocysteine level and AD (including neuropathologically confirmed AD), and cognitive function in non-demented individuals;10, 11 this relationship spans the normal range of homocysteine levels.11 Reduction of homocysteine levels can be readily achieved with high doses of folic acid, vitamin B12, and vitamin B6 in the absence of vitamin B deficiency in the general population12 and in AD,13 and could plausibly represent a disease-modifying intervention in AD.
Randomized controlled trials of homocysteine reduction by B-vitamin supplementation have yielded conflicting results. A recent systematic review considered fourteen trials, concluding that there is insufficient evidence of a beneficial effect on cognition of such supplementation in people with normal or impaired cognition.14 A study of supplementation over three years in non-demented older individuals with a plasma homocysteine concentration of at least 13 μmol/L indicated a favorable influence on cognitive function,15 while a two-year study in older individuals not selected on the basis of homocysteine levels did not.16 Short-term (two to six months) B-vitamin supplementation does not influence cognition in AD,13, 17, 18 except perhaps when homocysteine levels are elevated;19 long-term treatment studies in AD have not previously been reported. There is intriguing evidence that homocysteine levels may be related to plasma levels of amyloid peptides in individuals with AD,20, 21 and that reduction of homocysteine levels may lower amyloid levels.22
We conducted a multicenter, randomized, placebo-controlled clinical trial to determine if reduction of homocysteine levels with high-dose folic acid/B6/B12 supplementation for 18 months would slow the rate of cognitive decline in individuals with mild to moderate AD.
Methods
Study Design
The study, conducted by the Alzheimer’s Disease Cooperative Study (ADCS), a consortium of US centers funded by the National Institute on Aging to conduct therapeutic trials, utilized a randomized, double-blind, two group parallel design comparing high-dose vitamin supplements to placebo. The treatment period was 18 months. Forty sites participated in this trial after obtaining approval from their local Institutional Review Boards. Informed consent was obtained from participants and/or legally authorized representatives, according to local guidelines.
Individuals with probable AD,23 recruited primarily from the sites’ clinic populations, were eligible if they were medically stable. Inclusion criteria included age greater than 50, and Mini-mental State Examination (MMSE)24 score within the range 14–26. Individuals were excluded if they had levels of vitamin B12 or folate below normal (B12less than 175 pg/ml; folate less than 4.2 ng/ml), or renal insufficiency (serum creatinine greater than or equal to 2.0). Individuals were also excluded if within the prior two months they had regularly used drugs with significant central anticholinergic effects, sedatives, anti-Parkinsonian medications, or any investigational treatment for AD. Stable use (for at least 3 months) of cholinesterase inhibitors and memantine was allowed.
The randomization process used a permuted block design with a block size of 5 (3 active, 2 placebo). Unequal group assignment, with a greater likelihood of assignment to active treatment, was intended to improve the recruitment rate.
Because individuals using multivitamin tablets, which typically contain 400mcg of folic acid, show a smaller homocysteine reduction in response to high-dose supplements compared to non-vitamin users,13 we restricted enrollment of multivitamin users to no more than 2 individuals in each block of 5. Individuals taking daily vitamin supplements containing more than 400mcg of folic acid were excluded.
Study medication, assignment and masking
The active study medication consisted of folic acid 5mg, vitamin B12 1mg plus vitamin B6 25mg, administered once daily; a placebo tablet was identical in appearance. The active regimen was selected to maximize reduction of fasting and post-prandial homocysteine levels with minimal risk; though the regimen assessed in a pilot AD study included 50mg vitamin B6,13 this was reduced to 25mg to reduce risk of neuropathy.25 Each individual received a bottle of study medication with a coded label at baseline, and at the 3, 6, 9, 12 and 15 month visits. The randomization sequence was generated by the ADCS data center. “Scratch-off” codebreakers were used so that instances of unblinding would be documented; all codebreakers were collected at the end of the trial. Adequacy of masking was assessed by questionnaires completed by participants, caregivers, psychometrists and site investigators.
Safety assessments, including vital signs, physical examination, urinalysis, and hematology and chemistry blood tests, were performed at each visit. Cognitive and behavioral assessments were performed at baseline, and at months 3, 6, 9, 12, 15 and 18.
Outcome measures
The primary outcome measure for this trial was the 18 month change score on the cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-cog),26 an instrument that evaluates memory, attention, language, orientation and praxis; it is a 70 point scale, with higher scores indicating greater impairment. Considering the expected safety and tolerability of the intervention, we considered a significant benefit to be a 25% reduction (in comparison to the placebo group) in cognitive decline as indicated by change in ADAS-cog score.
Secondary outcome measures included the Mini-Mental State Examination (MMSE),24 Clinical Dementia Rating sum of boxes (CDR-SOB),27 Alzheimer’s Disease Cooperative Study Activities of Daily Living (ADCS-ADL) scale,28 Neuropsychiatric Inventory (NPI),29 Quality of Life-AD (QOL-AD)30 and the time to attainment of significant endpoints (4-point decline from baseline ADAS-cog score, death, institutionalization, 1 stage worsening on the global CDR scale, 15 point decline on the ADCS-ADL).31
Assays
Plasma total homocysteine (tHcy)
Total homocysteine in plasma was measured using a high performance liquid chromatography (HPLC) method with fluorescence detection.32 Briefly the method consists of reduction of the sample with tri-n-butylphosphine, precipitation of proteins with perchloric acid, and derivitization with 7-fluorobenzo-2-oxa-1,3-diazole-4-sulphonate (SBDF). The derivatized samples were injected directly into the HPLC system.
Plasma pyridoxal phosphate (vitamin B6)
Plasma pyridoxal phosphate was determined by HPLC with fluorescence detection as previously described.33 Plasma was deproteinized with 2 volumes of perchloric acid and 20 μl of the clear extract obtained after centrifugation was injected directly into the HPLC-fluorescence detection system.
Genotyping of MTHF C677T (accession number U09806)
Genomic DNA was extracted from peripheral blood using QIAamp DNA blood minikit (Qiagen, Valencia, CA) and stored at −20°C. The extracted DNA was then assayed for the mutation in the MTHFR gene using the polymerase chain reaction for DNA amplification and restriction digestion of PCR products with HinfI for the 677C→T as previously described.34
Statistical analysis
The primary analysis aimed to determine if the rate of cognitive decline differed between the group assigned to high-dose supplement treatment and the group assigned to receive placebo. The method of generalized estimation equations (GEE)35 was used for the primary analysis.
Power calculations were based on GEE analysis of repeated ADAS-cog data from participants in the ADCS Prednisone Study36 (visit to visit correlation=.853, mean ADAS-cog standard deviation=11.5). A sample size of 240 in the active drug group and 160 in the placebo group, assuming a 20% attrition rate and a 10% drop-in rate (the rate among those assigned to placebo of starting high dose B vitamins in violation of the protocol) evenly dispersed along the 18 months of treatment and an alpha of 0.05, would provide greater than 80% power to detect a 25% reduction in the rate of ADAS-cog decline. This effect size is appropriate considering the safety and minimal expense associated with the treatment.
The primary analysis was an intent-to-treat (ITT) analysis including all randomized participants. Individual participants were analyzed in the group to which they were randomized regardless of medication compliance. Additionally, all available ADAS-cog assessments were used in the analysis for participants who discontinued medication but continued to be followed.
A list of covariates anticipated to be associated with rate of change in homocysteine and/or ADAS-cog at 18 months in this study population was compiled prior to study initiation. This list consisted of apolipoprotein ε4 allele count methylene tetrahydrofolate reductase (MTHFR) T677C allele count baseline plasma homocysteine, age, gender, baseline serum creatinine, smoking status and baseline ADAScog. The assessment of covariates consisted of a univariate two-sample test comparing baseline values between treatment groups, and a bivariate measure of association between the baseline variable value and the rate of change measure. If for any particular variable, the first test, assessing the equivalence of the baseline distributions was significant at the alpha =.1 level, and the second test, measuring association with response, was significant at the .15 level, the variable would be included as a covariate in the GEE model. For the primary analysis, age was found to be unbalanced and correlated to the rate of change in the ADAS-cog; therefore age was included in the model as a confounding variable for the primary analysis.
A planned secondary analysis of time to reach any one of five endpoints considered to be clinically significant (death, institutionalization, change in global CDR score, loss of 4 points on the ADAS-cog, loss of 15 points on the ADCS-ADL) used a Cox Proportional Hazards model; this analysis has been used previously (NS). Analysis of secondary outcome measures followed the method of the primary analysis.
No interim analysis was performed.
The study design was approved by an oversight committee of the National Institute on Aging. Roche, Inc. supplied vitamins, but did not participate in the design of the study, analysis or interpretation of the data, or preparation of the manuscript.
Statistical software R version 2.6.2 (2008-02-08 http://www.r-project.org) was used. For the primary hypothesis, analysis was duplicated using SAS version 9.1 for verification purpose. The a priori level of significance was 0.05.
Results
Participant flow and follow-up
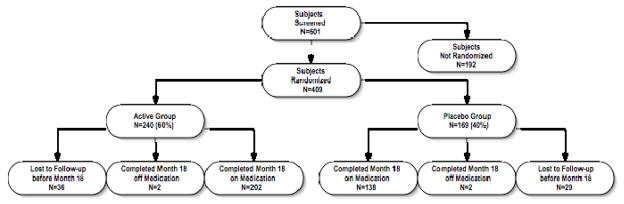
The flow of participants through the study protocol is shown in Figure 1. Participants were recruited between 2/20/2003 and 5/19/2005. From a total of 601 participants screened, 409 met the study criteria and were randomized to one of the two treatment groups. The median (Q1, Q3) length of follow up was 17.9 (17.7, 18.1) months.
Figure 1.
Participant flow. The reasons for screening failures were MMSE score out of range (n=47), excluded or unstable medications (n=38), unstable medical condition (n=29), abnormal screening laboratory results (n=29);,withdrawn consent (n=12), abnormal MRI findings (n=10), caregiver issues (n=10) and diagnostic uncertainty (n=4); the reason was unknown in 13 cases. The reasons for dropping out of the study were caregiver unwillingness to continue (active 13, placebo 13), travel difficulties (active 5, placebo 7), non-compliance with study medication (active 4, placebo 6), decisions to try alternate therapy (active 6, placebo 3), concern about potential adverse effects (active 3, placebo 2), frequency of assessments (active 1, placebo 4), safety concerns (active 1, placebo 3), length of protocol (active 1, placebo 3), concern regarding placebo assignment (active 2, placebo 2), protocol violations (active 1, placebo 1), and unspecified (active 18, placebo 14); participants were allowed to specify multiple reasons. All subjects in the active treatment arm were included in the primary analysis; three subjects in the placebo group were excluded because of missing data.
Protocol alteration in response to DSMB concern
During the course of the trial, the DSMB indicated that there was an excess of adverse events related to depression in the active treatment group. With DSMB concurrence, the protocol was altered to increase surveillance of depressive symptoms and to include NPI testing at each study visit; the informed consent documents were modified to indicate a possible association of depressive symptoms with study medication.
The randomization code was not broken during the trial in any instance.
The final study visit was completed for 204 participants (85%) in the active treatment group, and 140 (82%) in the placebo group. The predominant reasons for early discontinuation were caregiver issues (e.g., caregiver unwilling or unable to continue participation, caregiver-perceived lack of efficacy).
There were not statistically significant differences in baseline characteristics between participants who discontinued early and study completers.
Dropout rates in the active group and placebo group were similar (p=0.421, Fisher’s Exact test).
Correlation coefficient analysis indicated that baseline plasma homocysteine levels were correlated to age (coefficient 0.2, p<0.001 Spearman), creatinine (coefficient 0.4, p<0.001 Spearman), NPI total score (coefficient 0.1, p=0.138 Spearman), NPI depression subscore (coefficient 0.1, p=0.034 Spearman), plasma levels of vitamin B12 (coefficient −0.3, p<0.001 Spearman) and folate (coefficient −0.3, p<0.001 Spearman) and multivitamin use (coefficient −1.34, p<0.001 LM). Mean baseline homocysteine levels did not vary by MTHFR genotype (CC: 8.92±2.75, n=133; CT 9.18±3.29, n=142; TT 9.71±3.33, n=50; p=0.30, ANOVA). Homocysteine levels were not related to ADAScog or CDR-SOB scores. A total of 371 (91.2%) participants were taking cholinesterase inhibitors at the time of enrollment. There were 7 participants (5 in the active treatment group; 2 in the placebo group) who started treatment with cholinesterase inhibitors prior to the month 18 visit. These participants were included in the primary intent-to-treat analyses; in a secondary analysis excluding these participants, the results were similar. A total of 166/409 (40.6%) participants were taking multivitamins at enrollment; the proportion of multivitamin users did not differ across treatment groups (p=0.102, Fisher’s Exact Test).
The results of questionnaires administered at the month 18 visit indicated that the percentage of participants who believed that they were taking active study medication did not differ across the treatment group (active treatment group 68.0%, placebo group 71.4%; p = 0.532, Fisher’s Exact Test), indicating that blinding was adequately maintained. Similarly, survey results indicated that the blinding was maintained among informant, study coordinators and study physicians at the participating sites.
The demographic and clinical characteristics of the two treatment groups at baseline are shown in Table 1. There were no significant differences between the groups on any of the demographic or baseline characteristics.
Table 1.
Baseline Characteristics of the Subjects
| Variable | Placebo Group (N=169) |
B Vitamins Group (N=240) |
All Subjects (N=409) |
|---|---|---|---|
| Age – yr | 77.3±7.9 | 75.7±8.0 | 76.3±8.0 |
| Female sex – no. (%) | 91 (53.9) | 138 (57.5) | 229 (56.0) |
| Education – yr | 13.9±3.2 | 14.0±2.9 | 13.9±3.1 |
| APOE ε4 carrier – no. (%) | 105 (70.0) | 141 (64.7) | 246 (66.9) |
| MTHFR genotype – no. (%) | |||
| CC | 54(40.6) | 80(41.5) | 134(41.1) |
| CT | 56(42.1) | 86(44.6) | 142(43.6) |
| TT | 23(17.3) | 27(14.0) | 50(15.3) |
| Homocysteine level | 9.10±2.8 | 9.20±3.4 | 9.16±3.2 |
| Folate level | 23.59±14.35 | 25.74±17.65 | 24.85±16.38 |
| Vitamin B12 level | 577.46±281.08 | 605.68±304.10 | 593.99±294.76 |
| Vitamin B6 level* | 6.55(5, 81.85) | 5.00(5, 67.65) | 5.00(5, 71.95) |
| Smoker – no. (%) | 49 (29.0) | 79 (32.9) | 128 (31.3) |
| ADAS-Cog score | 22.63±8.6 | 22.43±9.0 | 22.51±8.8 |
| MMSE score | 20.91±3.7 | 20.98±3.4 | 20.95±3.5 |
| CDR sum-of-boxes score | 5.85±2.9 | 5.61±2.7 | 5.71±2.8 |
| Score on Neuropsychiatric Inventory* | 5.00(1, 11) | 6.00(0, 13) | 6.00(0, 12) |
| Score on Activities of Daily Living Scale | 59.66±12.9 | 61.31±11.58 | 60.63±12.15 |
Note: Median(Q1,Q3)
Analysis of outcomes
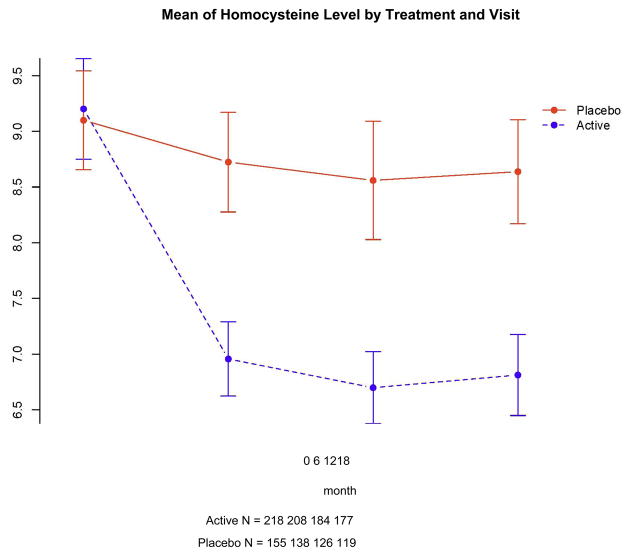
As expected, there was an increase over baseline levels of each vitamin at 18 months in the active treatment group, but no changes in the placebo group (data not shown). Homocysteine levels fell during the trial in the active treatment group (Figure 2); the decline from baseline at 12 months (when change was greatest) was 27% (2.52 μmol/L) overall, 19% (1.52 μmol/L) among individuals taking multivitamins, and 31% (3.10 μmol/L) in individuals not taking multivitamins. In the placebo group, homocysteine levels at 12 months fell 7% overall, 9% among individuals taking multivitamins, and 1% in individuals not taking multivitamins.
Figure 2.
Mean homocysteine level (μmol/L) for the two treatment groups at each visit. Error bars indicate standard error of the mean.
Primary outcome measure
The effect of treatment on the primary and secondary measures is shown in Figures 3–5, and Table 2.
Figure 3.
Effect of treatment on ADAScog score. Error bars indicate standard error of the mean.
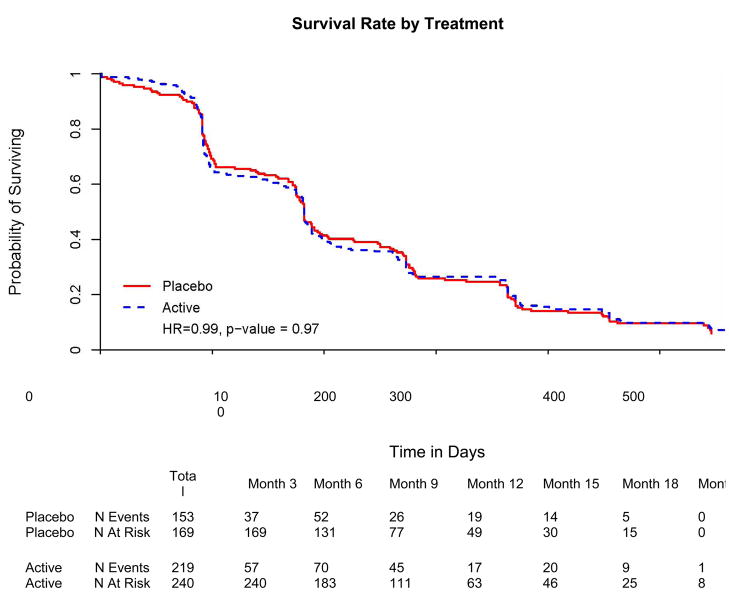
Figure 5.
Survival to any one of five clinically relevant endpoints (loss of 4 points on the ADAScog, increase in global CDR score, loss of 15 points on the ADCS-ADL scale, institutionalization or death).
Table 2.
Changes from Baseline in Cognitive and Functional Measures
| Test | Change in Score from Baseline | |||||
|---|---|---|---|---|---|---|
| 3 mo | 6mo | 9mo | 12mo | 15mo | 18mo | |
| Cognitive and functional measures | ||||||
| ADAS-Cog | ||||||
| Placebo | 1.51±4.68 | 1.72±4.74 | 2.71±6.63 | 4.46±6.32 | 5.80±6.34 | 6.54±8.17 |
| B Vitamins | 1.58±5.61 | 2.44±6.04 | 2.97±6.57 | 4.42±6.61 | 6.31±7.71 | 7.38±9.72 |
| MMSE | ||||||
| Placebo | −0.67±2.89 | −1.13±3.13 | −1.70±3.83 | −1.80±3.56 | −2.51±3.59 | −3.08±4.46 |
| B Vitamins | −0.17±3.02 | −0.44±3.19 | −0.89±3.26 | −1.64±3.84 | −2.15±4.21 | −2.65±4.56 |
| CDR sum of boxes | ||||||
| Placebo | - | 0.79±1.65 | - | 1.60±2.12 | - | 2.51±2.57 |
| B Vitamins | - | 0.69±1.67 | - | 1.50±1.92 | - | 2.58±2.45 |
| Neuropsychiatric Inventory | ||||||
| Placebo | 0.64±4.83 | 1.03±8.82 | 0.76±8.90 | 1.19±9.31 | 3.83±13.74 | 2.20±11.12 |
| B Vitamins | 0.71±6.31 | 0.97±10.82 | 0.12±11.27 | 1.03±10.53 | 2.97±11.20 | 3.31±12.84 |
| Activities of Daily Living Scale | ||||||
| Placebo | - | −2.86±7.80 | - | −7.82±10.00 | - | −10.00±11.09 |
| B Vitamins | - | −3.28±7.99 | - | −7.38±9.97 | - | −10.96±12.36 |
Note: ADAS total score rage: 0–70, 70 being the most impaired; MMSE total score range: 0–30, 30 being the least impaired; CDR sum of boxes range: 0–18, 18 being the most impaired; Neuropsychiatric Inventory range: 0–144, 144 being the most impaired; Activities of Daily Living Scale range: 0–78, 78 being the most impaired.
For the primary GEE analysis of the effect of supplementation on change in ADAScog, the covariates included in the model were treatment, month, age, and treatment and month interaction. The rate of change in ADAS-cog did not differ across treatment groups (placebo: 0.372 points/month, active: 0.401 points/month, p=0.52, CI of rate difference: −0.06, 0.12, based on GEE model).
A confirmatory ANCOVA analysis yielded similar results: with baseline ADAScog and age included as covariates, there was no effect of treatment arm on change in ADAScog at 18 months (p=0.56).
Secondary outcome measures
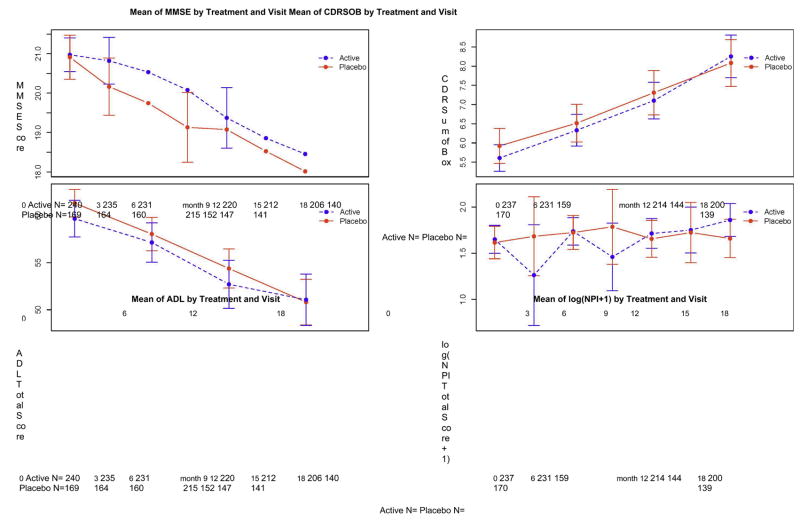
GEE analysis revealed no difference in rate of decline on the CDR-SOB in the two treatment groups (p=0.57).
The planned survival analysis considered the time interval from the baseline visit to the first among five possible endpoints: death, institutionalization, increase in global CDR score, 15 point decline on the ADCS-ADL scale, or 4 point decline on ADAS-cog. A total of 372 participants (91.0 %) reached at least one end-point (active treatment group: 219 (91.3 %); placebo group: 153 (91.0 %). There was no difference between treatment groups in time to first endpoint ((hazard ratio [HR]=0.99, p=0.97, 95% CI (0.798, 1.21), Cox proportional hazards model). When time to individual endpoints was examined, again there was no significant difference between groups (p>0.5 for each endpoint).
Adverse events
Numbers of adverse events (active: 224/240, 93%, placebo: 161/169, 95%; p=0.52, Fisher Exact ), serious adverse events (active: 123, 51%, placebo: 95, 56%; p=0.37), hospitalizations (active 111/240, 46%, placebo: 87/169, 51% ; p=0.30) and deaths (active: 3/240, 1%, placebo: 4/169, 2%; p=0.39) were similar in the active and placebo groups. Treatment emergent adverse events were grouped into categories for analysis; those categories with more frequent reports in the active treatment or placebo group (p < 0.1) are shown in Table 3.
Table 3.
Adverse Events
| Adverse Event | B Vitamins Group | Placebo Group |
|---|---|---|
| N (Percent) | ||
| Arthralgia | 16 (6.7) | 7 (4.1) |
| Depressed mood | 45 (18.8) | 20 (11.8) |
| Depression | 16 (6.7) | 8 (4.7) |
| Dyspnoea | 18 (7.5) | 9 (5.3) |
| Headache | 20 (8.3) | 9 (5.3) |
| Hyperhidrosis | 23 (9.6) | 7 (4.1) |
| Joint swelling | 17 (7.1) | 6 (3.6) |
| Restlessness | 29 (12.1) | 14 (8.3) |
| Upper respiratory tract infection | 16 (6.7) | 6 (3.6) |
| Urinary tract infection | 16 (6.7) | 8 (4.7) |
| Vision blurred | 13 (5.4) | 3 (1.8) |
Table lists adverse events that occurred in at least 5 percent of participants in either treatment group. In two instances, the differences in occurrence rates between groups approached significance: hyperhidrosis: p-value=.053, and vision blurred: p-value=.072 using Fisher’s Exact test.
There was an excess number of adverse events involving depression in the high-dose supplement group (67/240, 28% v. 29/169, 18%; p=0.024, Fisher’s Exact Test, unadjusted for multiple comparisons). The percentage of participants starting antidepressants during the trial was similar in the two groups (placebo group: 21%, active treatment: 26%, p=0.24 Fisher’s Exact Test).
The differences across groups (total adverse events, and each category individually) did not reach significance (p > 0.1 for each comparison, Fisher’s Exact Test) except hyperhidrosis (p=.053) and blurred vision (p=.072).
Subgroup analyses
A planned analysis assessed the impact of treatment on change in ADAS-cog within quartiles based on baseline homocysteine levels; there was no effect of treatment in the highest quartile (18 month change in ADAScog 6.55±8.75 active treatment arm, 5.13±8.45 placebo arm, p=0.65, GEE) or the lowest quartile (18 month change in ADAScog 7.49±10.92 active treatment arm, 10.64±9.82 placebo arm, p=0.15, GEE). Additional planned subgroup analyses among multivitamin non-users and groups defined by apolipoprotein E genotype were also negative (data not shown).
Planned subgroup ADAScog change analyses of participants above and below the median MMSE score at baseline numerically favored placebo in the lower MMSE group and favored active treatment in the higher MMSE group at each time point; these treatment effects were only significant at the 15 month timepoint (lower MMSE group: placebo change 5.18±6.57, active treatment change 8.63±8.19, p = 0.004; higher MMSE group: placebo change 6.43±6.08, active treatment change 3.93±6.40, p = 0.001, by Wilcoxson Rank Sum test unadjusted for multiple comparisons). The GEE analysis showed a significant interaction between baseline MMSE stratum and treatment effect on ADAS-cog change (p=0.018, unadjusted for multiple analyses).
Discussion
This study was designed to determine if use of high-dose supplements to maximally reduce homocysteine levels in individuals with mild to moderate AD would slow the decline in cognition, clinical status, function and behavior. The intervention was successful in reducing homocysteine levels, but in the study population as a whole, there was no evidence of benefit on any outcome measure. The general recommendation for B vitamin supplementation cannot be supported in patients with mild to moderate AD in the absence of B vitamin deficiency.
The study included multivitamin users and non-users, since high-dose supplementation reduces homocysteine in both groups. Because homocysteine reduction is greater in non-users of multivitamins, this subgroup was examined separately in a planned secondary analysis. Though homocysteine levels declined by 31%, there was no impact of supplementation on any outcome measure in this subgroup. Similarly, among participants with homocysteine levels in the highest quartile at baseline, there was no apparent benefit of high-dose B vitamin supplementation.
Apart from replacement therapy in individuals with low vitamin levels, the value of high dose B vitamin supplementation to reduce normal homocysteine levels has not been demonstrated unequivocally in any clinical setting. Randomized studies in non-demented individuals have yielded conflicting results; supplementation may be useful in older individuals with relatively high homocysteine levels. The identification of groups that may benefit from such treatment remains an important goal. The trend toward opposite effects in milder versus more moderately impaired individuals suggests that studies in more narrowly defined groups of individuals with AD, or perhaps amnestic mild cognitive impairment, may be warranted.
In addition to the stage of dementia of participants, other issues that may have influenced the negative outcome of this trial should be considered. While the active intervention aimed at a 25% reduction in HC level, this target was selected on the basis of feasibility in a North American population. Populations in countries that do not supplement grain products with folic acid have higher HC levels and generally see a greater reduction with supplementation. Thus supplementation could be clinically useful in other countries, or in participants selected on the basis of relatively high HC levels. However, the absence of any evidence of benefit in the participants in this trial with the highest baseline HC levels is not encouraging in this regard. It is also plausible that HC reduction might be effective in AD patients with significant concomitant cerebrovascular disease; such individuals were excluded from this trial.
Aside from the issue of lack of cognitive or clinical benefit, these results raise a safety issue regarding the vitamin supplementation. Adverse events involving depression were more common in the active treatment group. This is particularly notable in view of the association noted between elevated homocysteine levels and depression in Parkinson’s disease patients,37 and the observation that folate augmentation may increase the efficacy of antidepressant medications.38, 39 However, the adverse event finding reached marginal significance without adjustment for multiple categorical adverse event analyses, and analysis of change on the depression item of the NPI scale yielded only a trend toward support of the finding. Further, no difference was evident in the number of antidepressants prescribed to individuals in the two groups. Attention to this possible adverse effect in other trials of such treatment may be appropriate.
Many studies suggest that relative elevation of homocysteine is characteristic of AD, and laboratory research implicates homocysteine in neurodegenerative mechanisms. Supplementation with high dose B vitamins is effective in reducing homocysteine levels. But the present study does not support the treatment of individuals with mild to moderate AD and normal vitamin levels with B vitamin supplements.
Figure 4.
Changes in secondary outcome measures during the course of the trial. A. CDR-SB. B. ADCS-ADL. C. NPI., D. MMSE. Error bars indicate standard error of the mean.
Acknowledgments
This study was supported by a grant (U01-AG10483) from the National Institute on Aging, as well as grants from the General Clinical Research Center Program of the National Center for Research resources, National Institutes of health. Folic acid and vitamin B6 and B12 were donated by Roche. Participating investigators, data and safety monitoring board members and clinical monitors for the study are listed at the end of this article. None of the authors of this manuscript have any potential conflicts of interest. The corresponding author had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Participating Investigators and Staff: Southern Illinois University, Springfield: Sandra Vicari, PhD, Thomas Ala, MD, Karen Luster, RN; Oregon Health & Science University, Portland: Joseph Quinn, MD, Joyce Lear, RN, MN, Tera Clay; University of Southern California, Los Angeles: Lon S. Schneider, MD, Sonia Pawluczyk, MD, Karen Dagerman, MA, Shirley Sian; University of California, San Diego: Adam Fleisher, MD, MAS, Mary M. Pay, APRN; University of Michigan, Ann Arbor: Nancy Barbas, MD, MSW, Joanne Lord, LPN, BA, CCRC; University of Washington, Seattle: Elaine Peskind, MD, Murray A. Raskind, MD, Linda Mandelco; Baylor College of Medicine, Houston: Rachelle S. Doody, MD, PhD, Jamie Sims, BS, Susan Roundtree, MD; Columbia University, New York: Karen Bell, MD, Ruth Tejeda, MD, Evelyn Dominguez-Rivera, MD; University of Alabama at Birmingham: Linda Harrell, MD, PhD, Daniel Marson, JD, PhD, Edward Zamrini, MD; Mount Sinai School of Medicine, Bronx: Hillel Grossman, MD, George Marzloff, BS, Sirisha Nandipait, BA; Rush University Medical Center, Chicago: Neelum Aggarwal, MD, Raj Shah, MD, Julie Bach, CSW; University of South Florida, Tampa: Eric Pfeiffer, MD, Barbara Luhn, MSW, CCRC, Jennifer Hunter, MA; New York University Medical Center, New York: Steven H. Ferris, PhD, Isabel Monteiro, MD, Erica Maya, BA; University of Pennsylvania, Philadelphia: Christopher Clark, MD, Monica Han, BA, Cassie Pham, BS, Marianne Watson, RN; University of Pittsburgh: Steven T. DeKosky, MD, Donna Simpson CRNP, MPH; University of Rochester: Saleem M. Ismail, MD, Bonnie Goldstein, RN, Coleen McCallum, MSW; University of California, Irvine: Ruth A. Mulnard, RN, DNSc, FAAN, Catherine McAdams-Ortiz, RN, MSN, A/GNP, Beatriz Yanez, BA; University of Texas, Southwestern Medical Center at Dallas: Myron Weiner, MD, Kathleen Koch, RN, MN, CCRC, Carol Moore, MA; Emory University, Atlanta: Allan Levey, MD, PhD, Janet Cellar, RN, MSN, Andrea Kippels, RN, MSN; University of California, Los Angeles: John Ringman, MD, Jenny Bardens, RN, Alice Yau; Mayo Clinic, Jacksonville: Neill R. Graff-Radford, MD, Francine Parfitt, MS; Indiana University, Indianapolis: Martin R. Farlow, MD, Ann Hake, MD, Patricia Numberger, RN; Yale University, New Haven: Christopher van Dyck, MD, Martha MacAvoy, PhD, Nicole Black, BA, Pamela Hoffman, BS; University of California, Davis: Charles DeCarli, MD, William Seavey, MD, Cassandra Conover, RN; University of Arizona, Tucson: Geoffrey Ahern, MD, PhD, Marjorie Baldwin, RN, BSN, Mike Malek-Ahmadi, BA; University of Nevada, Las Vegas: Charles Bernick, MD, Donna Munic, PhD, Gail Vranesh, RN; Northwestern University, Chicago: Marsel Marek-Mesulam, MD, Nancy Johnson, PhD, Stephanie Epstein-Fields, MA; Medical University of South Carolina, North Charleston: Jacobo E. Mintzer, MD, Fay Ann Davis, LPN; ClinSearch, Inc., Kenilworth: Justine Kent, MD, Mark Roffman, PhD; Premiere Research Institute, West Palm Beach: Carl Sadowdky, MD, Teresa Villena, MD, Walter C. Martinez, MD; Georgetown University, Washington: Kathleen Johnson, NP, Brigid Reynolds, NP, Carolyn Ward, MSPH; Brigham and Women’s Hospital, Boston: Sibyl Salisbury, BS; Stanford/VA Aging Clinical Research Center, Stanford: Jerome A. Yesavage, MD, Wesson J. Ashford, MD, Alena Pechonkina, MD, Lisa Kinoshita, PhD; Sun Health Research Institute, Sun City: Marwan Sabbagh, MD, FAAN, Donald Connor, PhD, Sanja Obradov, CCRD; Boston University SOM, Boston: Robert C. Green, MD, MPH, Robert A. Stern, PhD, Anil Nair, MD, Margarget Brickley, MS, ACNP, GNP-BC; Mayo Clinic, Scottsdale: Richard Caselli, MD, Judy Lawrence, RN; Howard University, Washington DC: Thomas Obisesan, MD, MPH, Odunayo A. Obisesan, PharmD, Ezenwanyi Ahaghotu, MS; Case Western Reserve University, Cleveland: Paul Ogrocki, PhD, Marianne Sanders, RN, Elaine Ziol, MHS, CCRC; Rhode Island Hospital, Providence: Brian R. Ott, MD, Janet Grace, PhD, Teresa Erbozkurt, MA.
Data and Safety Monitoring Board: University of Rochester School of Medicine and Dentistry: Karl Kieburtz, MD; University of California, San Francisco: Bruce Miller, MD; University of Kentucky, Lexington: Richard Kryscio, PhD; Weill Cornell Medical College: George Alexopoulos, MD.
Clinical Monitors: University California, San Diego: Karen Croot, BA, Viviana Messick, BS, Alan Pamoleras, BA, Rebecca Ryan-Jones, PhD; Mount Sinai School of Medicine: Gina Garcia-Camilo, MD, Mario Schittini, MD, MPH; Georgetown University: Kris Gravanda Brugger, BA, Pamela A. Saunders, PhD; New York University: Janet Kastelan, MA.
Footnotes
Disclosures:
Paul S. Aisen has been, within the past five years, a consultant to the following pharmaceutical companies involved in the development of potential treatments for Alzheimer’s disease: Pfizer, Novartis, Janssen, Elan, Wyeth, Roche, Merck, Lilly, Bristol Myers Squibb, Schering Plough, Neurochem, Medivation, GlaxoSmithKline, PamLab, Adlyfe. He has received grant support from Pfizer, Elan-Wyeth, Merck, Lilly, Neurochem, Neuro-Hitech, Myriad. He holds stock options in Medivation.
Lon S. Schneider, within the past five years, has been a consultant to Abbott, Accera, AstraZeneca, Bristol Myers Squibb, Eli Lilly, Forest Pharmaceuticals, Lundbeck, Merz, Novartis, Pfizer, Johnson & Johnson, Wilmar Schwabe, all manufacturers of products used in the treatment of Alzheimer’s disease, and with Roche who provided the vitamin preparations for this trial. He has provided expert testimony in litigation involving Eli Lilly and Lundbeck, both manufacturers of products used in the treatment of Alzheimer’s disease.
Mary Sano, with the past five years, has been a consultant to the following companies involved in potential treatments for AD: Bristol Meyer Squibb, Elan, Esai, Forest, Galaxo Smith Kline Janssen, Novartis, Pfizer, Medivation, Takeda. She has received grant support from the Alzheimer Association and from the NIH.
Ramon Diaz-Arrastia, within the past five years, has had no potential conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject of this manuscript.
Christopher H. van Dyck, within the past five years, has been a consultant to Bristol-Myers Squibb and Forest Laboratories, Inc pertaining to Alzheimer’s disease therapeutics. He has received grant support from Elan Pharmaceuticals, Pfizer Inc, GlaxoSmithKline, Myriad Pharmaceuticals, Eli Lilly and Co, and Wyeth Pharmaceuticals pertaining to Alzheimer’s disease therapeutics.
Myron F. Weiner, within the past five years, has had no potential conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject of this manuscript.
Teodoro Bottiglieri, within the past five years, has been the Chairman of the Advisory Board for Methylation Sciences Inc. He also holds stock options in Methylation Sciences Inc. He has received grant/research funding from PamLab LLC, distributor of B-vitamins as a medical food.
Shelia Jin, within the past five years, has had no potential conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject of this manuscript.
Karen T. Stokes, within the past five years, has had no potential conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject of this manuscript.
Ronald G. Thomas, within the past five years, has had no potential conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject of this manuscript.
Leon J. Thal, within the past five years, had no potential conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject of this manuscript.
References
- 1.Aisen PS. The development of anti-amyloid therapy for Alzheimer’s disease : from secretase modulators to polymerisation inhibitors. CNS Drugs. 2005;19(12):989–996. doi: 10.2165/00023210-200519120-00002. [DOI] [PubMed] [Google Scholar]
- 2.Fillit HM, Refolo LM. Tau and Alzheimer’s disease: the long road to anti-tangle therapeutics. J Mol Neurosci. 2002 Dec;19(3):249–250. doi: 10.1007/s12031-002-0001-y. [DOI] [PubMed] [Google Scholar]
- 3.Longo FM, Massa SM. Neuroprotective strategies in Alzheimer’s disease. NeuroRx. 2004 Jan;1(1):117–127. doi: 10.1602/neurorx.1.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kruman II, Culmsee C, Chan SL, et al. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci. 2000;20(18):6920–6926. doi: 10.1523/JNEUROSCI.20-18-06920.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kruman II, Kumaravel TS, Lohani A, et al. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J Neurosci. 2002 Mar 1;22(5):1752–1762. doi: 10.1523/JNEUROSCI.22-05-01752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malouf M, Grimley EJ, Areosa SA. Folic acid with or without vitamin B12 for cognition and dementia. Cochrane Database Syst Rev. 2003;(4):CD004514. doi: 10.1002/14651858.CD004514. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006 Nov;5(11):949–960. doi: 10.1016/S1474-4422(06)70598-1. [DOI] [PubMed] [Google Scholar]
- 8.Wright CB, Paik MC, Brown TR, et al. Total homocysteine is associated with white matter hyperintensity volume: the Northern Manhattan Study. Stroke. 2005 Jun;36(6):1207–1211. doi: 10.1161/01.STR.0000165923.02318.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selhub J. The many facets of hyperhomocysteinemia: studies from the Framingham cohorts. J Nutr. 2006 Jun;136(6 Suppl):1726S–1730S. doi: 10.1093/jn/136.6.1726S. [DOI] [PubMed] [Google Scholar]
- 10.Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998;55:1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- 11.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002 Feb 14;346(7):476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 12.Homocysteine_Lowering_Trialists’_Collaboration. Lowering blood homocysteine with folic acid based supplements: meta- analysis of randomised trials. Brit Med J. 1998;316(7135):894–898. [PMC free article] [PubMed] [Google Scholar]
- 13.Aisen PS, Egelko S, Andrews H, et al. A pilot study of vitamins to lower plasma homocysteine levels in Alzheimer disease. Am J Geriatr Psychiatry. 2003 Mar-Apr;11(2):246–249. [PubMed] [Google Scholar]
- 14.Balk EM, Raman G, Tatsioni A, Chung M, Lau J, Rosenberg IH. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007 Jan 8;167(1):21–30. doi: 10.1001/archinte.167.1.21. [DOI] [PubMed] [Google Scholar]
- 15.Durga J, van Boxtel MP, Schouten EG, et al. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet. 2007 Jan 20;369(9557):208–216. doi: 10.1016/S0140-6736(07)60109-3. [DOI] [PubMed] [Google Scholar]
- 16.McMahon JA, Green TJ, Skeaff CM, Knight RG, Mann JI, Williams SM. A controlled trial of homocysteine lowering and cognitive performance. N Engl J Med. 2006 Jun 29;354(26):2764–2772. doi: 10.1056/NEJMoa054025. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y, Lu CJ, Chien KL, Chen ST, Chen RC. Efficacy of multivitamin supplementation containing vitamins B6 and B12 and folic acid as adjunctive treatment with a cholinesterase inhibitor in Alzheimer’s disease: a 26-week, randomized, double-blind, placebo-controlled study in Taiwanese patients. Clin Ther. 2007 Oct;29(10):2204–2214. doi: 10.1016/j.clinthera.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Connelly PJ, Prentice NP, Cousland G, Bonham J. A randomised double-blind placebo-controlled trial of folic acid supplementation of cholinesterase inhibitors in Alzheimer’s disease. Int J Geriatr Psychiatry. 2008 Feb;23(2):155–160. doi: 10.1002/gps.1856. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson K, Gustafson L, Hultberg B. Improvement of cognitive functions after cobalamin/folate supplementation in elderly patients with dementia and elevated plasma homocysteine. Int J Geriatr Psychiatry. 2001 Jun;16(6):609–614. doi: 10.1002/gps.388. [DOI] [PubMed] [Google Scholar]
- 20.Irizarry MC, Gurol ME, Raju S, et al. Association of homocysteine with plasma amyloid beta protein in aging and neurodegenerative disease. Neurology. 2005 Nov 8;65(9):1402–1408. doi: 10.1212/01.wnl.0000183063.99107.5c. [DOI] [PubMed] [Google Scholar]
- 21.Luchsinger JA, Tang MX, Miller J, Green R, Mehta PD, Mayeux R. Relation of plasma homocysteine to plasma amyloid beta levels. Neurochem Res. 2007 Apr-May;32(4–5):775–781. doi: 10.1007/s11064-006-9207-7. [DOI] [PubMed] [Google Scholar]
- 22.Flicker L, Martins RN, Thomas J, et al. B-vitamins reduce plasma levels of beta amyloid. Neurobiol Aging. 2006 Nov 17; doi: 10.1016/j.neurobiolaging.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 23.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 24.Folstein M, Folstein S, Mchugh P. The Mini-Mental State Examination. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Schaumburg H, Kaplan J, Windebank A, et al. Sensory neuropathy from pyridoxine abuse. A new megavitamin syndrome. N Engl J Med. 1983 Aug 25;309(8):445–448. doi: 10.1056/NEJM198308253090801. [DOI] [PubMed] [Google Scholar]
- 26.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. American Journal of Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 27.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 28.Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S33–S39. [PubMed] [Google Scholar]
- 29.Cummings J. The neuropsychiatric inventory: Assessing psychopathology in dementia patients. Neurology. 1997;48(Suppl 6):S10–S16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- 30.Logsdon RG, Gibbons LE, McCurry SM, Teri L. Quality of life in Alzheimer’s disease: Patient and caregiver reports. Journal of Mental Health and Aging. 1999;5:21–32. [Google Scholar]
- 31.Aisen PS, Schafer KA, Grundman M, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. Jama. 2003 Jun 4;289(21):2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- 32.Ubbink JB, Vermaak WJ, Bennett JM, Becker PJ, van Staden DA, Bissbort S. The prevalence of homocysteinemia and hypercholesterolemia in angiographically defined coronary heart disease. Klin Wochenschr. 1991 Aug 16;69(12):527–534. doi: 10.1007/BF01649290. [DOI] [PubMed] [Google Scholar]
- 33.Bisp MR, Bor MV, Heinsvig EM, Kall MA, Nexo E. Determination of vitamin B6 vitamers and pyridoxic acid in plasma: development and evaluation of a high-performance liquid chromatographic assay. Anal Biochem. 2002 Jun 1;305(1):82–89. doi: 10.1006/abio.2002.5638. [DOI] [PubMed] [Google Scholar]
- 34.Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995 May;10(1):111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 35.Zeger S, Liang K-Y. Longitudinal data analysis fordiscrete and continuous data. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 36.Aisen PS, Davis KL, Berg JD, et al. A randomized controlled trial of prednisone in Alzheimer’s disease. Alzheimer’s Disease Cooperative Study. Neurology. 2000;54(3):588–593. doi: 10.1212/wnl.54.3.588. [DOI] [PubMed] [Google Scholar]
- 37.O’Suilleabhain PE, Sung V, Hernandez C, et al. Elevated plasma homocysteine level in patients with Parkinson disease: motor, affective, and cognitive associations. Arch Neurol. 2004 Jun;61(6):865–868. doi: 10.1001/archneur.61.6.865. [DOI] [PubMed] [Google Scholar]
- 38.Folstein M, Liu T, Peter I, et al. The homocysteine hypothesis of depression. Am J Psychiatry. 2007 Jun;164(6):861–867. doi: 10.1176/ajp.2007.164.6.861. [DOI] [PubMed] [Google Scholar]
- 39.Fava M. Augmenting antidepressants with folate: a clinical perspective. J Clin Psychiatry. 2007;68 (Suppl 10):4–7. [PubMed] [Google Scholar]