Abstract
The resolution of inflammation is an active process controlled by endogenous mediators with selective actions on neutrophils and monocytes. The initial phase of the acute inflammatory response is characterized by the production of pro-inflammatory mediators followed by a second phase in which lipid mediators with pro-resolution activities may be generated. The identification of these mediators has provided evidence for the dynamic regulation of the resolution of inflammation. Among these endogenous local mediators of resolution, lipoxins (LXs), lipid mediators typically formed during cell–cell interaction, were the first to be recognized. More recently, families of endogenous chemical mediators, termed resolvins and protectins, were discovered. LXs and aspirin-triggered LXs are considered to act as ‘braking signals’ in inflammation, limiting the trafficking of leukocytes to the inflammatory site. LXs are actively involved in the resolution of inflammation stimulating non-phlogistic phagocytosis of apoptotic cells by macrophages. Furthermore, LXs have emerged as potential anti-fibrotic mediators that may influence pro-fibrotic cytokines and matrix-associated gene expression in response to growth factors. Here, we provide a review and an update of the biosynthesis, metabolism and bioactions of LXs and LX analogues, and the recent studies on their therapeutic potential as promoters of resolution and fibro-suppressants.
This article is part of a themed issue on Mediators and Receptors in the Resolution of Inflammation. To view this issue visit http://www3.interscience.wiley.com/journal/121548564/issueyear?year=2009
Keywords: lipoxin, anti-inflammatory, resolution of inflammation, anti-fibrotic, leukocytes, mesangial cells
Introduction
Inflammation is a key process in effective host defence. It is a critical response to microbial invasion and tissue injury, and is characterized by site-specific accumulation and activation of leukocytes. The resolution of such inflammatory responses is necessary to re-establish homeostasis, limiting excessive tissue injury and minimizing the development of chronic inflammation, and depends on the biological actions of several anti-inflammatory and pro-resolving mediators, expressed by various cell types, as well as on apoptosis and clearance of inflammatory cells (Lawrence et al., 2002; Serhan and Savill, 2005; Serhan, 2007; Serhan et al., 2007). A failure of any step in this process may lead to chronic inflammation with possible further tissue destruction, fibrosis and eventually organ failure. The first evidence that the resolution of inflammation is an active rather than a passive process came with the discovery of pro-resolution biochemical signalling circuits (Serhan et al., 2000; 2007; Bannenberg et al., 2005). During the initial phase of inflammation, eicosanoids including prostaglandins and leukotrienes (LTs) play important role as local mediators in the development of an inflammatory condition, evoking potent chemotactic responses of leukocytes whose activation is coupled to the production of proinflammatory (Th1-derived cytokines) at sites of inflammation (Borgeat and Naccache, 1990). This is a biphasic process; the second stage is coupled to the biosynthesis of lipid mediators that actively limit inflammation and promote resolution. The new genus of pro-resolving mediators of molecules include lipoxins (LXs) and their aspirin-triggered carbon-15 epimers (ATL) (Levy et al., 2001; Serhan, 2005), as well as the recently discovered resolvins and protectins which are derived from ω-3 fatty acids (Serhan et al., 2000; 2008a,b;). Resolvins and protectins were first identified in self-resolving murine exudates using the murine dorsal air pouch model of inflammation (Serhan et al., 2000). In parallel studies, it was demonstrated that prostaglandin E2 and D2 stimulate the translation of neutrophil 15-lipoxygenase (LO) involved in LX biosynthesis, providing evidence for class switching within the eicosanoid pathways during the evolution of an inflammatory exudate (Levy et al., 2001). Figure 1 shows the cellular and molecular mechanisms involved in the onset and resolution of inflammation.
Figure 1.
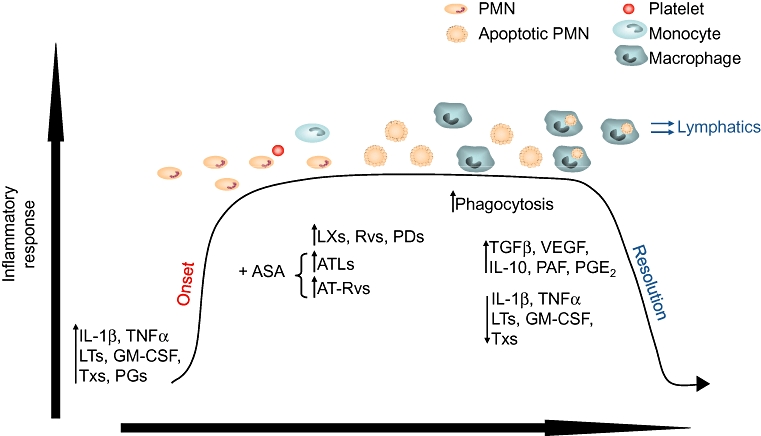
Representation of the temporal cellular and biochemical events in the onset and resolution of inflammation. The early phase of inflammation is characterized by the release of pro-inflammatory mediators and extravascular accumulation of neutrophils, followed by infiltration of monocytes that differentiate into macrophage. This phase is characterized by the formation of anti-inflammatory and pro-resolution mediators (LXs, resolvins). These mediators stop further neutrophil trafficking and facilitate the removal of apoptotic cells. The ingestion of apoptotic cells results in potent anti-inflammatory effects through the production of anti-inflammatory cytokines such as TGF-β1, IL-10 and PGE2, and the decrease of release of pro-inflammatory mediators, including IL-8, TNF-α and TXA2. This figure is adapted from Serhan et al. (2007). IL = interleukin; TNF-α= tumour necrosis factor-α; LTs = leukotrienes; Tx = thromboxane; GM-CSF = granulocyte–macrophage colony-stimulating factor; PGs = prostaglandins; ASA = aspirin; LXs = lipoxins; Rvs = resolvins; PDs = protectins; ATL = aspirin-triggered lipoxins; ATRv = aspirin-triggered resolvins; TGF-β= transforming growth factor β; VEGF = vascular endothelial growth factor; PAF = platelet-activating factor; PGE2= prostaglandin E2.
In this review, we will give an overview and an update of the role of LXs as pro-resolution and anti-fibrotic agents with particular focus on the potential development of LX analogues as therapeutics.
Biosynthesis of LXs
The term LXs is an acronym for LO interaction products. These lipid mediators were first recognized to have dual anti-inflammatory and pro-resolution activities (Maderna and Godson, 2003; Kieran et al., 2004; McMahon and Godson, 2004; Serhan, 2005). 5S,6R,15S-trihydroxy-7,9,13-trans-11-cis-eicosatetraenoicacid (LXA4) and its positional isomer 5S,14R,15S-trihy-droxy-6,10,12-trans-8-cis-eicosatetraenoic acid (LXB4) are the principal species formed in mammals (Serhan et al., 1986a,b;). LXs are typically formed by transcellular metabolism through distinct biosynthetic pathways depending on the cellular context (Kieran et al., 2004; McMahon and Godson, 2004; Chiang et al., 2005; Serhan, 2005). There are two main LO-mediated pathways of LX biosynthesis in human cells and tissues. The first of these involves the sequential lipoxygenation of arachidonic acid by 15-LO in epithelial cells and monocytes, and 5-LO in neutrophils (Serhan et al., 1984a,b;). This pathway not only leads to LX biosynthesis, but also reduces LT formation, resulting in an inverse relationship between LT and LX byosynthesis in human leukocytes (Serhan, 1989). Indeed, it has recently been shown that in acute post-streptococcal glomerulonephritis up-regulation of 15-LO and subsequent LX biosynthesis supersede production of proinflammatory LTB4 (Wu et al., 2009). The second major route of LX formation involves platelet/leukocyte or platelet/leukocyte microaggregate interactions that promote LX formation by transcellular conversion of the 5-LO epoxide product, LTA4 to LXA4 and LXB4 by the LX-synthetase activity of the 12-LO in platelets (Serhan and Sheppard, 1990). Interestingly, platelets are not able to produce LXs on their own, but this pathway has been highlighted as a major route for LX formation within the vasculature where activated platelets become a major source of LXs after adhesion to neutrophils (Chiang et al., 2005; Serhan, 2005).
In addition to the transcellular routes, another recognized source of LX biosynthesis involves a form of cellular ‘priming’ with the esterification of 15-HETE in inositol-containing phospholipids within the membranes of human neutrophils (Brezinski and Serhan, 1990). Discovery of this pathway suggests that during disease or host defence, precursors of LX biosynthesis might be stored within the membranes of the inflammatory cells and released after stimulation (Brezinski and Serhan, 1990).
The signalling networks involved in LX formation show even greater complexity given the potential regulation of biosynthetic enzymes by specific cytokines (Serhan et al., 1996). For example, interleukin 4 (IL-4) and IL-13, putative negative regulators of inflammatory and immune responses, promote transcellular LX generation through enhanced expression of 15-LO in monocytes and epithelial cells (Nassar et al., 1994; Munger et al., 1999). Cytokines such as granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-3 up-regulate 5-LO transcripts (Ring et al., 1996), while pro-inflammatory cytokines such as IL-1β, IL-6 and tumour necrosis factor (TNF-α) have been shown to induce cyclooxygenase-2 (COX-2), thus potentially contributing to the formation of ATLs in vivo (Parente and Perretti, 2003).
LXs are generated in vivo within an inflammatory milieu, and it has been suggested that an impaired LX biosynthesis may correlate with an inability to resolve the acute inflammatory reaction contributing to a more chronic inflammatory phenotype (Lee et al., 1990; Brezinski et al., 1992; Chiang et al., 1999; Munger et al., 1999; Bandeira-Melo et al., 2000; Pouliot et al., 2000; Bonnans et al., 2002; Karp et al., 2004). Recently, it has been described that exogenous resolvin E1 stimulated the production of endogenous LXA4 during the resolution of allergic airway (Haworth et al., 2008). There is a growing body of evidence that indicates an immunomodulatory role for LXs during infections. Toxoplasma gondii, a protozoan parasite, which encode their own 15-LO, has been shown to activate LXA4 biosynthesis, resulting in increased evasion of the parasite from host defence (Aliberti et al., 2002; Bannenberg et al., 2004b).
ATLs
Aspirin triggers the generation of epimeric forms of LXs (Claria and Serhan, 1995). Cells that express COX-2 (i.e. vascular endothelial cells, epithelial cells, macrophages, neutrophils) are able to produce ATLs by the actions of aspirin that triggers the endogenous formation of carbon-15 epimeric LXs, namely ATL (Claria and Serhan, 1995). In particular, in a cytokine primed milieu, aspirin acetylation of COX-2 switches the catalytic activity of the enzyme to an R-LO with the formation of 15R-HETE that is rapidly converted by 5-LO to 15-epimeric-LXA4 or 15-epimeric LXB4 (Claria and Serhan, 1995). Interestingly, ATL formation has been detected in vivo in various murine models of inflammation such as peritonitis (Chiang et al., 1998), dorsal air pouches (Perretti et al., 2002) and in aspirin-intolerant asthmatics (Sanak et al., 2000). Administration of low doses of aspirin to healthy subjects significantly increases plasma levels of ATL with a concomitant inhibition of thromboxane biosynthesis, suggesting that ATL may account for some of the beneficial effects of aspirin that are not strictly related to its anti-thrombotic actions (Chiang et al., 2004). A further synthetic route for the production of 15-epi LXA4 has been demonstrated in rat myocardium in response to statins and the PPAR-γ ligand pioglitazone (Birnbaum et al., 2006; 2007;), providing a novel mechanism for immune regulation by statins.
Metabolic inactivation of LXs
LXs are rapidly generated in response to stimuli, act locally and undergo rapid metabolic inactivation. Using monocytes or isolated enzymes, it has been possible to demonstrate that the major route of LXs degradation is via dehydrogenation at C-15 and possibly by ω-oxidation at C-20 (Serhan et al., 1995; Clish et al., 2000). A similar inactivation pathway was also shown for LXB4 (Maddox et al., 1998). ATLs are converted in vitro to their 15-oxo-metabolite with a slower rate compared to native LXs, indicating that the hydrogenation step is highly specific (Serhan et al., 1995). Furthermore, ATLs, when generated in vivo, display longer biological half-life than native compounds and enhanced ability to evoke bioactions (Serhan et al., 1995; Maddox et al., 1997; Clish et al., 1999).
Synthetic LX analogues
The rapid inactivation and short half-life of LXs in vivo have prompted the development of novel analogues designed to resist metabolism, maintain their structural integrity and bioavailability and their potential beneficial bioactions. The initial design of metabolically stable LXA4 analogues focused on identifying poor substrates for PGDH, which maintained potency in in vitro assays. The discovery that 15-epi-LXA4 was equipotent in in vitro assays to LXA4, but was a poorer substrate for PGDH, provided support for exploiting these observations in novel analogue design. However, although 15-epi-LXA4 has enhanced metabolic stability over LXA4in vivo, its pharmacokinetics remain poor, which, in addition to low chemical stability, creates challenges for development of analogues with better therapeutic potential. Therefore, a series of LX and ATL analogues were designed with specific modifications of the native structures of LXA4 and LXB4, such as the addition of methyl groups on C-15 and C-5 of LXA4 and LXB4, respectively (Serhan et al., 1995), and phenoxyl or para-fluoro-phenoxyl groups at C-16 of both LXA4 and 15-epi ATL, protecting the molecules from the ω-oxidation and dehydrogenation in vivo (Serhan et al., 1995; Maddox et al., 1997; Clish et al., 1999). Consequently, these analogues were widely used in a number of studies exploring the biological functions of LX and ATL in experimental models of disease (Scalia et al., 1997; Takano et al., 1997; Filep et al., 1999; Hachicha et al., 1999; Jozsef et al., 2002; Ariel et al., 2003). A second generation of LX stable analogues, 3-oxa-LXA4 analogues, with enhanced chemical and metabolic stability, has shown potency and efficacy comparable to ATL in diverse animal models after topical, intravenous or oral delivery (Bannenberg et al., 2004a; Guilford et al., 2004). More recently, we have developed a stereoselective synthesis of chemically stable aromatic LXA4 and LXB4 analogues (O'Sullivan et al., 2007). This synthetic route establishes the required stereochemistry by way of Sharpless epoxidation, Pd-mediated Heck coupling and diastereoselective reduction reactions (Figure 2). LXs, ATL and their stable analogues share potent protective actions in controlling inflammation, and provide new opportunities to explore the actions and therapeutic potential for LXs and ATL as it will be outlined later in this review.
Figure 2.
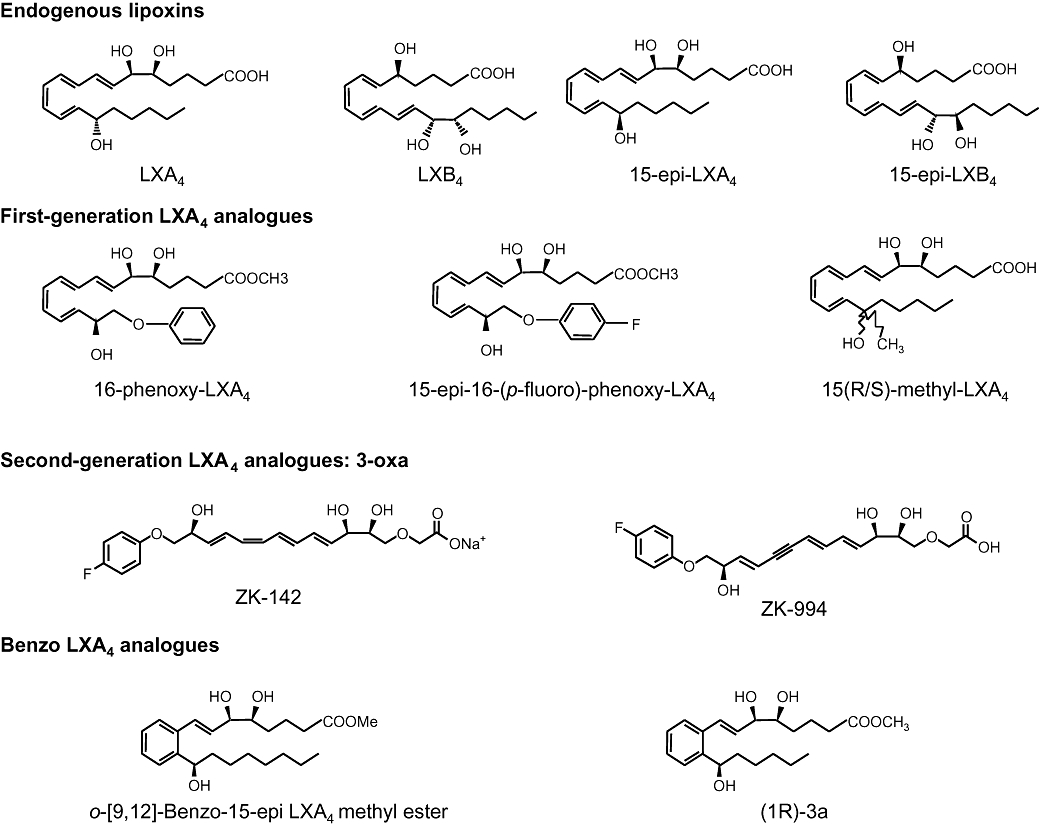
Structure of native lipoxins (LXs), 15-epi-LXs and synthetic analogues. The figure shows the structure of the native LXs (LXA4 and LXB4), aspirin-triggered LXs [aspirin-triggered lipoxin (ATL); 15-epi-LXA4 and 15-epi LXB4]. In order to increase the half-life of LXA4, and ATL, analogues resistant to enzymatic conversion by ω-oxidation and PGDH were designed (e.g. 16-p-fluorophenoxy-15-epi-LXA4 methyl ester) (Takano et al., 1998; Clish et al., 1999; Gewirtz et al., 1999; Karp et al., 2004). Modification of the tetraene structure to a trienyne further enhanced chemical stability as depicted here in the structure of the 3-oxa LX analogue ZK-142 and its 11-dehydro analogue ZK-994, which are topically and orally active anti-inflammatory agents (Guilford et al., 2004). More recently, a new class of lipoxin analogues featuring a benzo-fused ring system have been designed and proved to be as potent as native LXA4 in a series of in vitro and in vivo studies (O'Sullivan et al., 2007; Petasis et al., 2008).
LXA4 and ATL receptors
Several mechanisms have been proposed to underlie the bioactions of LXs as shown in Figure 3. These include activation of a high-affinity LX-specific G-protein coupled receptor, activation of subclasses of cysteinyl peptide receptors and/or cellular uptake of LX which in turn facilitates interactions with intracellular targets such as nuclear receptors (Fiore et al., 1992; Simchowitz et al., 1994; Schaldach et al., 1999; Chiang et al., 2000; 2004; McMahon et al., 2001; Planaguma et al., 2002).
Figure 3.
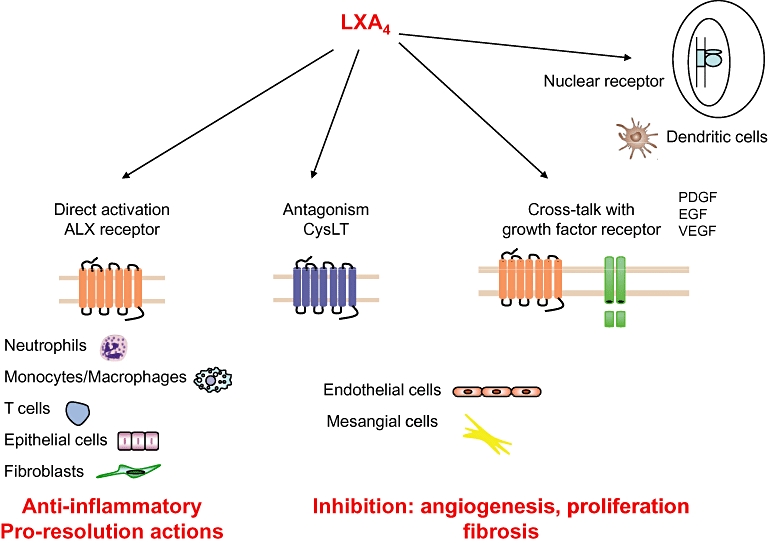
Lipoxin (LX) and receptors. The actions of LXs and aspirin-triggered lipoxins are mediated through several mechanisms. These include activation of high-affinity, LX-specific G-protein coupled receptor (ALXR), interaction of subclasses of cysteinyl peptide–LTs receptor. Direct activation of the lipoxin receptor results in anti-inflammatory and pro-resolution activities. Indirect inhibition, through other receptors such as CysLT and growth-factor receptors (such as vascular endothelial growth factor and platelet-derived growth factor receptors), reduces angiogenesis, and mesangial cell proliferation and fibrosis. Another potential receptor of LXA4 is the nuclear receptor aryl hydrocarbon receptor, which triggers expression of suppressor of cytokine signalling 2 in LX-stimulated DC.
A specific LX recognition site was first described in human neutrophils, and demonstrated to be responsible for the specific LXA4-evoked actions on these cells (Fiore et al., 1992). This G-protein coupled receptor was later designated as ALXR (FPRL-1) (Serhan, 1997; Chiang et al., 2005; 2006;). Although LXA4 and LXB4 share many of the biological activities, LXB4 does not bind ALXR, and the LXB4 receptor remains to be identified. Human ALXR belongs to a family of three members (FPR1, FPRL-1/ALXR and FPR3), and is expressed in several types of leukocytes such as neutrophils (Fiore et al., 1994), monocytes (Maddox et al., 1997), activated T cells (Ariel et al., 2003), as well as resident cells such as intestinal epithelial cells (Kucharzik et al., 2003), synovial fibroblasts (Sodin-Semrl et al., 2000), bronchial epithelial cells (Bonnans et al., 2003), astrocytes (Decker et al., 2009) and renal mesangial cells (McMahon et al., 2000). Transcription of ALXR had been shown to be up-regulated by various cytokines, suggesting regulation of receptor expression in an inflammatory milieu (Gronert et al., 1998; Sodin-Semrl et al., 2000). It has recently been shown that gene and cell surface expression of ALXR are significantly decreased in peripheral blood leukocytes of asthmatic subjects compared to healthy individuals (Planaguma et al., 2008).
The GPCR-designated ALXR can bind pleiotropic ligands, that is, both lipid and peptides eliciting either pro-inflammatory or anti-inflammatory responses (Chiang et al., 2000). Among the various ligands are MHC binding peptide (a potent necrotactic peptide derived from NADH dehydrogenase subunit 1 from mitochondria) (Chiang et al., 2000), antimicrobial peptides (e.g. LL37 and temporin A) (De et al., 2000; Chen et al., 2004), truncated chemotactic peptides (e.g. CKbeta8-1) (Elagoz et al., 2004), a urokinase-type plasminogen activator receptor fragment (Resnati et al., 2002) and the HIV envelope peptides (Su et al., 1999a; Le et al., 2000). ALXR can also bind prion protein (Le et al., 2001b), serum amyloid A (Su et al., 1999b) and amyloid β42 (Le et al., 2001a).
Another ligand of particular interest is annexin 1, a glucocorticoid-inducible protein (Perretti et al., 2002) that mediates many of the anti-inflammatory actions of glucocorticoids in models of acute and chronic inflammation (reviewed in Perretti and Flower, 2004; Lim and Pervaiz, 2007; Perretti and D'Acquisto, 2009). Interestingly, glucocorticoids induce up-regulation of the expression of ALXR by leukocytes and in in vivo model of dermatitis (Sawmynaden and Perretti, 2006; Hashimoto et al., 2007). Recently, a novel peptide agonist of ALXR with potent anti-inflammatory and cardioprotective effects was identified using a computational platform (Hecht et al., 2009). These data highlight the therapeutic potential of ligands designed as agonists of the ALXR in applications such as acute and chronic inflammation.
The binding of lipids and small peptides to the receptor occurs with different affinities and/or at discrete interaction sites, facilitating activation of distinct signalling pathways that depends on the cell type and system (Bae et al., 2003). N-glycosylation of ALXR is proposed to be important for ligand specificity and may play a role in switching receptor functions at local host defence sites, suggesting receptor versatility (Chiang et al., 2000).
Mouse and rat ALXR homologues have been cloned from a spleen cDNA library (Takano et al., 1997) and from peripheral blood leukocytes, respectively (Chiang et al., 2003). The overall homology between the human, murine and rat receptors is relatively high in particular in their second intracellular loop (100%) and between the sixth transmembrane domain (97%), suggesting important roles for these regions in ligand recognition and functional G protein coupling.
The partial antagonism of a subclass of peptide-LT receptors (CysLTs) is a potential mechanism through which LXs may contribute to the anti-inflammatory bioactions of LXs in several tissues and cell types other than leukocytes (Badr et al., 1989; McMahon et al., 2000; Gronert et al., 2001; Chiang et al., 2006). In mesangial cells, LXs (nanomolar) are potent inhibitors of proliferative responses to LTD4 by modulating LTD4-induced transactivation of the platelet-derived growth factor (PDGF) receptor and subsequent phosphotidylinositol 3-kinase activation and mitogenic responses (McMahon et al., 2000). The counter-regulatory responses identified for LX were insensitive to a CysLT1-specific receptor antagonist, but blocked by a non-selective antagonist (McMahon et al., 2002). These data are intriguing given the proposal that the interaction between CysLT1 and CysLT2 receptors regulates inflammatory responses such that activation of the CysLT2 receptor can exert a net inhibitory response on CysLT1 receptor responses (Jiang et al., 2007). By analogy, it might be proposed that LXA4 activation of the CysLT2 receptor regulates the pro-inflammatory response of the CysLT1; however, this has not been definitively demonstrated.
Further studies show that LXs inhibit proliferation induced by growth factors such as PDGF, epidermal growth factor (EGF) and connective tissue growth factor (CTGF) with a mechanism that involves cross-talk between AXLR and receptor tyrosine kinases (McMahon et al., 2000; 2002; Wu et al., 2006). This inactivation seems to be mediated through the coupling of the ALXR to the activation of the protein tyrosine phosphatase, SHP-2, and it is proposed that the association of the PDGF receptor β within lipid raft microdomains renders it susceptible to LXA4-mediated dephosphorylation by possible reactivation of oxidatively inactivated SHP-2 (Mitchell et al., 2007). The ALXR ligand annexin-1 also regulates protein phosphorylation of EGF and PDGF receptors (Mitchell et al., 2007).
It is noteworthy that LX-mediated dephosphorylation of intracellular proteins seems to be a recurrent feature of LXA4 signalling. In addition to dephosphorylation of receptor tyrosine kinases, LX-stimulated phagocytosis of apoptotic leukocytes as described below is dependent on dephosphorylation of myosin IIA (Reville et al., 2006). Recent evidence highlights the importance of LXA4 as regulators of eosinophil responses to GM-CSF through inhibition of protein tyrosine phosphorylation (Starosta et al., 2008). Additionally, LXA4 and ATL have been shown to regulate vascular endothelial growth factor (VEGF) receptor-2 phosphorylation in endothelial cells (Fierro et al., 2002; Cezar-de-Mello et al., 2006; 2008; Baker et al., 2009).
Another potential receptor for LXA4 is the aryl hydrocarbon receptor (AhR), a ligand-activated transcription factor. In a murine hepatoma cell line, LXA4 has been shown to bind and activate AhR (Schaldach et al., 1999). In dendritic cells, LXA4, signalling through AhR and ALXR modulate innate and acquired immune responses (Machado et al., 2006). It has recently been demonstrated that both LXA4 and ATL acting via the AhR inhibit innate immune responses of dendritic cells by up-regulating suppressor of cytokine signalling 2 (SOCS-2), which in turn promotes ubiquitinylation and degradation of TNF receptor-associated factor-6, a component of TNF-α, TLR signalling pathways (Machado et al., 2008). It should be noted that responses to the AhR require concentrations of LXA4 in the micromolar range, whereas cellular responses generated through ALXR (or CysLT) are typically maximal in the nanomolar range, and the Kd of the ALXR is subnanomolar (Fiore et al., 1994).
Anti-inflammatory, pro-resolution and anti-fibrotic effects of LXs
LXs and ATLs have been shown to modulate specific actions in cells involved in the immune–inflammatory response (Figure 4) (for extensive reviews, see: McMahon et al., 2001; Kieran et al., 2004; Maderna and Godson, 2005; Serhan, 2005; Serhan et al., 2007; 2008b;). The role for LXs as anti-inflammatory molecules is well defined, with bioactions involving the inhibition of neutrophil and eosinophil recruitment and activation (Lee et al., 1989; Colgan et al., 1993; Soyombo et al., 1994; Papayianni et al., 1995; 1996; Filep et al., 1999). In addition, LXs and ATLs are proposed to directly stimulate gene expression (i.e. NAB1) that is involved in endogenous anti-inflammation and resolution (Qiu et al., 2001) and to regulate NF-kB activation (Decker et al., 2009).
Figure 4.
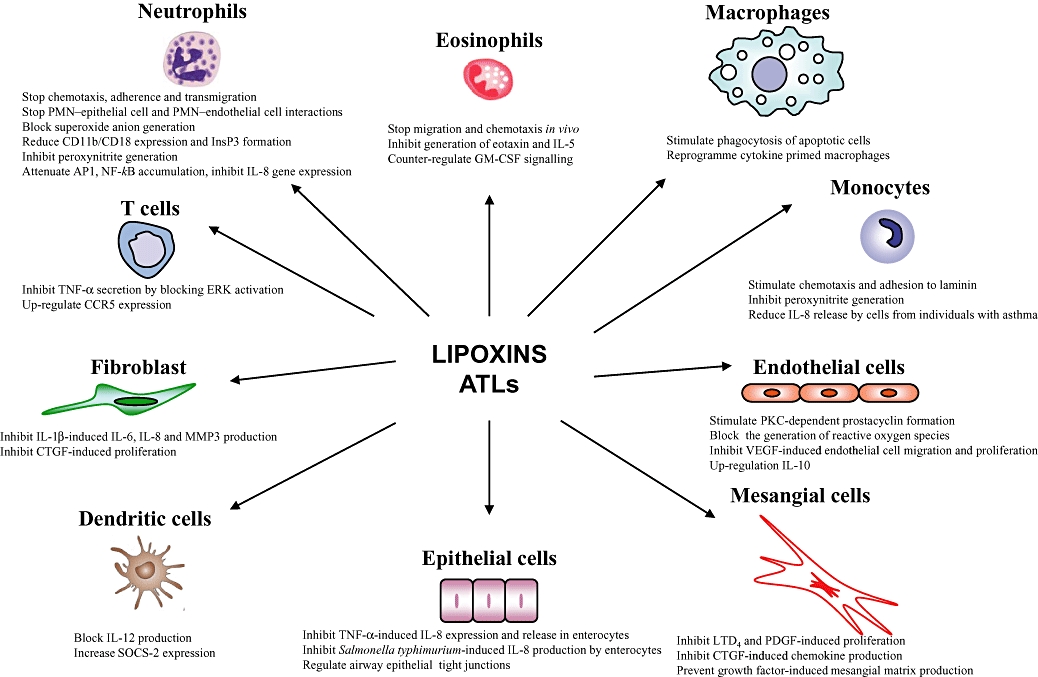
Target cells for lipoxin A4 and aspirin-triggered lipoxin bioactions.
The actions of LXs and ATL are not limited to counter-regulating the evolution of inflammation, but also to promote resolution at different levels. LXs stimulate monocyte chemotaxis and adherence, without causing degranulation or release of reactive oxygen species (Maddox et al., 1997), suggesting that the actions of LXs are related to the recruitment of monocytes to sites of injury. These monocyte activities may be host protective in view of the important role of these cells in wound healing and resolution at inflammatory sites. Indeed, LXs and ATLs stimulate the in vitro clearance of apoptotic cells by human monocyte-derived macrophages in a non-phlogistic manner (Godson et al., 2000; Mitchell et al., 2002; Reville et al., 2006). LXs stimulate phagocytosis of exogenously administered excess apoptotic PMN in a murine model of thioglycollate-induced peritonitis in vivo, suggesting that LXs rapidly promote the clearance of apoptotic leukocytes within an inflammatory milieu (Mitchell et al., 2002). Consistent with a role for LX promoting the resolution of inflammation are the observations that LX-stimulated phagocytosis is associated with increased transforming growth factor-β1 (TGF-β1) release from macrophages, and a decrease of IL-8 and monocyte chemoattractant protein-1 (MCP-1) release (Godson et al., 2000). The effect of LXs on phagocytosis of apoptotic cells by macrophages is mediated by protein kinase C and PI-3-kinase (Godson et al., 2000; Mitchell et al., 2002). A modulatory role for cAMP is suggested by the observation that LX-induced phagocytosis is inhibited by a cell permeant cAMP analogue, and mimicked by a protein kinase A inhibitor (Godson et al., 2000). Furthermore, LXs prime macrophages for chemotaxis and phagocytosis, through myosin IIa assembly, re-organization of the cytoskeleton, promoting the cell polarization and formation of actin filaments and pseudopodia (Maderna et al., 2002; Reville et al., 2006). Assembly of non-muscle myosin is coupled to serine dephosphorylation, a process stimulated by LXA4 through a process that may involve phosphatase activation as described in mesangial cells (Mitchell et al., 2007).
Other ligands of ALXR, and in particular endogenous annexin-1 and its peptidomimetic Ac2-26, promote phagocytosis of apoptotic cells through a mechanism involving ALXR and changes in F-actin re-organization (Maderna et al., 2005). Interestingly, we have shown that cells undergoing apoptosis release annexin-1 that can then stimulate phagocytosis through ALXR (Scannell et al., 2007), demonstrating that the ALXR is activated by soluble ‘eat me’ signals released from apoptotic cells. Figure 5 illustrates the possible mechanisms of LXs in the phagocytosis of apoptotic cells and resolution of inflammation.
Figure 5.
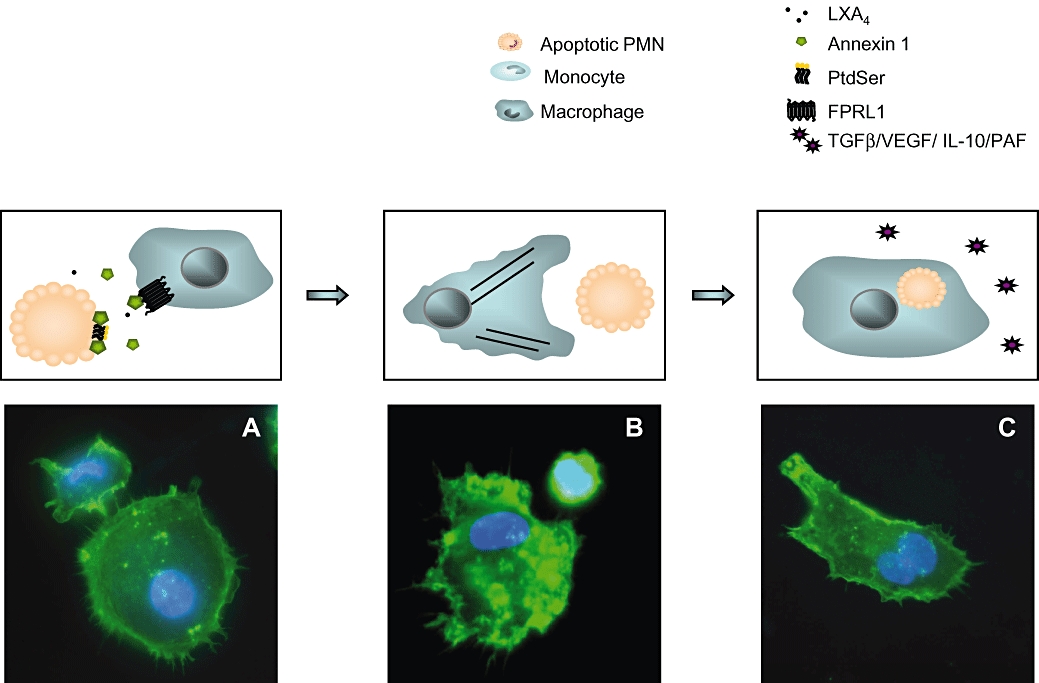
Phagocytosis of apoptotic cells by macrophages is augmented by ligands of the lipoxin (LX) receptor. LXs and other lipoxin A4 receptor (ALXR) ligands (i.e. aspirin-triggered lipoxins and annexin-1) engage ALXR on the macrophages, leading to intracellular signalling events, including activation of the small GTPases RhoA, Rac and Cdc42; myosin assembly; and actin rearrangement, priming the macrophages for the phagocytosis of apoptotic cells. Following ingestion, the production of anti-inflammatory cytokines is increased, whereas the release of pro-inflammatory mediators is decreased as depicted in schematic. Images depict human monocyte-derived macrophages and apoptotic neutrophil (A); after stimulation with LXA4 (1 nM), rearrangement of actin cytoskeleton is observed (B) and phagocytosis ensues (note two DAPI-stained nuclei in C).These conclusions are based on Maderna et al. (2002) and Reville et al. (2006).
In addition to promoting resolution by non-phlogistic phagocytosis of apoptotic cells, LX can act to reprogramme cytokine-primed macrophages from a classic pro-inflammatory (M1) phenotype to an alternatively activated phenotype demonstrating enhanced phagocytic capacity for apoptotic cells (Mitchell et al., 2002). This feature may suggest novel therapeutic strategies in chronic inflammation characterized by massive macrophage infiltration.
As discussed earlier, LXs are potent inhibitors of mesangial cell proliferation in response to LTD4 and growth factors with a mechanism that involves cross-talk between AXL and receptor tyrosine kinases (McMahon et al., 2000; 2002; Mitchell et al., 2004). In addition, LXA4 can counteract PDGF-induced, fibrosis-related gene expression in mesangial cells, suggesting that LXA4 might act as a potential anti-fibrotic agent, preventing growth factor-induced mesangial matrix production and the progression of renal disease (Rodgers et al., 2005). PDGF-treated renal mesangial cells were shown to secrete factors that promote the onset of tubulointerstitial damage, as observed by epithelial-to-mesenchymal transformation in proximal tubular epithelial cells, an effect attenuated by pretreatment with LXA4 (Rodgers et al., 2005). Further to these data, Wu et al. demonstrated that TNF-α-induced proliferation and cytokine release, as well as CTGF-mediated release of fractalkine, MCP-1 and RANTES, were modulated by LXA4 in rat mesangial cells (Wu et al., 2005; 2006;). In addition to evidence that LX can maintain the integrity of renal epithelia are data demonstrating that LXA4 stimulates the expression of ZO-1, claudin and occludin, and the maintenance of transepithelial resistance in cultured bronchial epithelial cells (Grumbach et al., 2009).
The synthetic LX analogue 15-epi-16-(para-fluoro)-phenoxy-LXA4 inhibits VEGF-induced endothelial cell proliferation and migration via inhibition of actin polymerization and assembly of focal adhesions (Fierro et al., 2002; Cezar-de-Mello et al., 2006). In addition, in endothelial cells, the 15-epi-16-(para-fluoro)-phenoxy-LXA4 induces the gene and protein expression of heme oxygenase-1 (HO-1), a key modulator of both innate and adaptative immunity (Nascimento-Silva et al., 2005). The pathophysiological importance of this finding is reflected by the fact that HO-1 synthesis triggered by ATL is required for the inhibition of TNF-α-induced adhesion molecule expression on endothelial cells which may impair leukocyte influx during the resolution phase of inflammation. Mice lacking 15-LO type I have an impaired HO-1 response. Topical application of LXA4 in these mice restores HO-1 expression and protects them from inflammatory challenge (Biteman et al., 2007).
The powerful anti-inflammatory, pro-resolution and more recently appreciated potential anti-fibrotic properties of LXs contribute to the overall anti-inflammatory mechanisms of LXs that can modulate the activation and migration of inflammatory cells.
LXs, ATLs and LX analogues in experimental model of diseases
There is reliable evidence that demonstrates that LXs or their stable analogue mimetics can reduce inflammation and symptoms in several experimental models of inflammatory disorders. As discussed earlier, the metabolism of LXs suggests that these molecules are highly susceptible to rapid inactivation; therefore, the availability of stable analogues has been a useful tool to extend the beneficial anti-inflammatory role of LXs to possible therapeutic applications.
One of the first analogues to be synthesized was 15-epi-16-(para-fluoro)-phenoxy-LXA4, an ATL analogue, widely used in systemic or topical therapy for a number of inflammatory conditions (Takano et al., 1998; Clish et al., 1999; Gewirtz et al., 1999; Karp et al., 2004). A role for LXA4 in reducing cutaneous inflammation has been shown in a variety of skin inflammation models, including psoriasis, atopic dermatitis and allergic contact dermatitis (Takano et al., 1997; Schottelius et al., 2002; Guilford et al., 2004). Topical application of LX analogues to mouse ear skin prevented vascular leakage and neutrophil infiltration in LTB4/PGE2-stimulated ear skin inflammation (Takano et al., 1997; Schottelius et al., 2002; Bannenberg et al., 2004a).
The anti-inflammatory spectrum of activity of LXs is well documented in in vivo models of glomerulonephritis and acute renal failure (Badr et al., 1989; Papayianni et al., 1995; Ohse et al., 2004), as well as in in vitro models (McMahon et al., 2000; 2002; Mitchell et al., 2004; Rodgers et al., 2005). In a murine model of ischaemic renal injury (IRI) disease, administration of 15-epi-16-(para-fluoro)-phenoxy-LXA4, before onset of experimental ischaemia, resulted in a significant functional and morphological protection with a markedly reduced neutrophil infiltration to the IRI kidney, while maintaining glomerular function and morphology, and attenuating chemokine and cytokine responses including up-regulation of SOCS-2 (Leonard et al., 2002). Using a transcriptomic approach to investigate the mechanism underlying the protective action of LXA4, specific cohorts of genes whose expression was altered in renal IRI and modulated by 15-epi-16-(para-fluoro)-phenoxy-LXA4 were identified (Kieran et al., 2003). Some of these genes included chemoattractants, cytokines, chemokines and chemokine receptors, growth factors and their receptors, adhesion molecules and molecules implicated in maintaining epithelial barrier function such as claudins (Kieran et al., 2003). These data are especially noteworthy given the evidence that LXA4 regulates transepithelial resistance in bronchial epithelia by a mechanism that includes up-regulation of claudin expression. It has been proposed that defective LX biosynthesis in cystic fibrosis (Karp et al., 2004) and asthma (Levy, 2005) could contribute to compromised epithelial barrier function (Grumbach et al., 2009). In vivo models of peritonitis have frequently been used to highlight anti-PMN trafficking effects of LX analogues (O'Sullivan et al., 2007). Interestingly, a recent report demonstrated the effect of ajulemic acid (AjA), a synthetic cannabinoid, on enhanced LXA4 production, an effect attributed to the observed reduction in peritoneal infiltration in a mouse model where AjA treatment before zymosan-induced peritonitis was associated with LO-dependent LX generation (Zurier et al., 2009).
A second generation of LX/ATL analogues was designed to subvert metabolism by β-oxidation through insertion of a 3-oxa group and to have improved chemical stability (Guilford et al., 2004). The changes resulted in significantly enhanced stability and plasma half-life, maintaining similar biological activity with a better pharmacokinetic profile over the 15-epi analogue (Fiorucci et al., 2004; Guilford et al., 2004; Levy et al., 2007). The potent anti-inflammatory and protective actions of LXs in intestinal inflammation make them an attractive candidate as a potential therapy for various inflammatory conditions of the digestive system, including Crohn's disease and ulcerative colitis. Indeed, ATL is protective in intestinal inflammation in a mouse model of dextran sodium sulphate-induced colitis (Gewirtz et al., 2002), and the β-oxidation resistant 3-oxa-ATL (ZK-192) has been shown to potently attenuate trinitrobenzene sulphonic acid (TNBS)-induced colitis, a Crohn's disease model (Fiorucci et al., 2004). When orally administered, ZK-192 reduced TNBS colitis both in preventive and therapeutic regimens, attenuating weight loss, macroscopic and histological colon injury, mucosal neutrophil infiltration, colon wall thickening, as well as decreasing mucosal mRNA levels for several inflammatory mediators (Fiorucci et al., 2004).
In asthma, the ZK-994 LX/ATL analogue (5S,6R,7E,9E,13E,15S)-16-(4-fluoro-phenoxy)-3-oxa-5,6,15-trihydroxy-7,9,13-hexadecatrien-11-ynoic acid was effective in reducing airway inflammation and airway bronchoconstriction (Levy et al., 2007).
Recently, we have described the activity of new LX analogues that show the substitution of the reactive hexatriene system with an aromatic ring. Beside a capacity to stimulate in vitro phagocytosis of apoptotic cells by macrophages, these LX analogues show potent anti-inflammatory activity in vivo (O'Sullivan et al., 2007). We used an in vivo model of mouse peritonitis, and examined neutrophil trafficking to the peritoneal cavity in response to zymosan A challenge. When administered intravenously, (1R)-3a inhibited the acute inflammatory cell recruitment into mouse peritoneum.
Summary
The successful resolution of inflammation is an integral component of effective host defence. The various steps of resolution are regulated by endogenous mediators and by clearance of apoptotic cells by phagocytes. In this context, LXs are a class of lipid mediators that serve as local endogenous anti-inflammatory and pro-resolution signals. The potential therapeutic applications of LXs and their stable synthetic analogues are significant; it will be of interest to learn whether these or related agonists of resolution can be exploited in a therapeutic context to ensure the effective restoration of tissue homeostasis and prevention of fibrosis subsequent to an inflammatory response.
Acknowledgments
Work in the authors' laboratory is supported by the Science Foundation Ireland, EU FP6 Eicosanox Programme LSHM-CT-2004-005033 and The Government of Ireland Programme for Research in Third Level Institutions.
Glossary
Abbreviations:
- 15-HETE
15-eicosatetraenoic acid
- AA
arachidonic acid
- AhR
aryl hydrocarbon receptor
- AjA
ajulemic acid
- ALXR
lipoxin A4 receptor
- ATL
aspirin-triggered lipoxin; 15-epi-LXA4 (5S,6R,15R-trihydroxyl-7,9,13-trans-11-cis-eicosatetraenoic acid)
- COX-2
cyclooxygenase-2
- CTGF
connective tissue growth factor
- CysLT
cysteinyl leukotriene receptor
- EGF
epidermal growth factor
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- IL
interleukin
- LO
lipoxygenase
- LT
leukotriene
- LX
lipoxin
- LXA4
lipoxin A4 (5S, 6R,15S-trihydroxyl-7,9,13-trans-11-cis-eicosatetraenoic acid)
- LXB4
lipoxin B4 (5S,14R,15S-trihydroxyl-7,9,13-trans-11-cis-eicosatetraenoic acid)
- PDGF
platelet-derived growth factor
- SOCS-2
suppressor of cytokine signalling 2
- TGF-β
transforming growth factor-β
- TNF-α
tumour necrosis factor-α
- VEGF
vascular endothelial growth factor
Note added in proof
During the final revision of this manuscript a new nomenclature for the FPR family of receptors was recommended by the International Union of Basic and Clinical Pharmacology LXXIII. On the basis of this classification, LXA4 is defined as an endogenous ligand for FPR2/ALX, instead of the previously used nomenclature of FPRL1/ALXR as used in this review (Ye et al., 2009).
References
- Aliberti J, Hieny S, Reis e Sousa C, Serhan CN, Sher A. Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity. Nat Immunol. 2002;3:76–82. doi: 10.1038/ni745. [DOI] [PubMed] [Google Scholar]
- Ariel A, Chiang N, Arita M, Petasis NA, Serhan CN. Aspirin-triggered lipoxin A4 and B4 analogs block extracellular signal-regulated kinase-dependent TNF-alpha secretion from human T cells. J Immunol. 2003;170:6266–6272. doi: 10.4049/jimmunol.170.12.6266. [DOI] [PubMed] [Google Scholar]
- Badr KF, DeBoer DK, Schwartzberg M, Serhan CN. Lipoxin A4 antagonizes cellular and in vivo actions of leukotriene D4 in rat glomerular mesangial cells: evidence for competition at a common receptor. Proc Natl Acad Sci USA. 1989;86:3438–3442. doi: 10.1073/pnas.86.9.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae YS, Yi HJ, Lee HY, Jo EJ, Kim JI, Lee TG, et al. Differential activation of formyl peptide receptor-like 1 by peptide ligands. J Immunol. 2003;171:6807–6813. doi: 10.4049/jimmunol.171.12.6807. [DOI] [PubMed] [Google Scholar]
- Baker N, O'Meara SJ, Scannell M, Maderna P, Godson C. Lipoxin A4: anti-inflammatory and anti-angiogenic impact on endothelial cells. J Immunol. 2009;182:3819–3826. doi: 10.4049/jimmunol.0803175. [DOI] [PubMed] [Google Scholar]
- Bandeira-Melo C, Bozza PT, Diaz BL, Cordeiro RS, Jose PJ, Martins MA, et al. Cutting edge: lipoxin (LX) A4 and aspirin-triggered 15-epi-LXA4 block allergen-induced eosinophil trafficking. J Immunol. 2000;164:2267–2271. doi: 10.4049/jimmunol.164.5.2267. [DOI] [PubMed] [Google Scholar]
- Bannenberg G, Moussignac RL, Gronert K, Devchand PR, Schmidt BA, Guilford WJ, et al. Lipoxins and novel 15-epi-lipoxin analogs display potent anti-inflammatory actions after oral administration. Br J Pharmacol. 2004a;143:43–52. doi: 10.1038/sj.bjp.0705912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannenberg GL, Aliberti J, Hong S, Sher A, Serhan C. Exogenous pathogen and plant 15-lipoxygenase initiate endogenous lipoxin A4 biosynthesis. J Exp Med. 2004b;199:515–523. doi: 10.1084/jem.20031325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- Birnbaum Y, Ye Y, Lin Y, Freeberg SY, Nishi SP, Martinez JD, et al. Augmentation of myocardial production of 15-epi-lipoxin-a4 by pioglitazone and atorvastatin in the rat. Circulation. 2006;114:929–935. doi: 10.1161/CIRCULATIONAHA.106.629907. [DOI] [PubMed] [Google Scholar]
- Birnbaum Y, Ye Y, Lin Y, Freeberg SY, Huang MH, Perez-Polo JR, et al. Aspirin augments 15-epi-lipoxin A4 production by lipopolysaccharide, but blocks the pioglitazone and atorvastatin induction of 15-epi-lipoxin A4 in the rat heart. Prostaglandins Other Lipid Mediat. 2007;83:89–98. doi: 10.1016/j.prostaglandins.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Biteman B, Hassan IR, Walker E, Leedom AJ, Dunn M, Seta F, et al. Interdependence of lipoxin A4 and heme-oxygenase in counter-regulating inflammation during corneal wound healing. FASEB J. 2007;21:2257–2266. doi: 10.1096/fj.06-7918com. [DOI] [PubMed] [Google Scholar]
- Bonnans C, Vachier I, Chavis C, Godard P, Bousquet J, Chanez P. Lipoxins are potential endogenous antiinflammatory mediators in asthma. Am J Respir Crit Care Med. 2002;165:1531–1535. doi: 10.1164/rccm.200201-053OC. [DOI] [PubMed] [Google Scholar]
- Bonnans C, Mainprice B, Chanez P, Bousquet J, Urbach V. Lipoxin A4 stimulates a cytosolic Ca2+ increase in human bronchial epithelium. J Biol Chem. 2003;278:10879–10884. doi: 10.1074/jbc.M210294200. [DOI] [PubMed] [Google Scholar]
- Borgeat P, Naccache PH. Biosynthesis and biological activity of leukotriene B4. Clin Biochem. 1990;23:459–468. doi: 10.1016/0009-9120(90)90272-v. [DOI] [PubMed] [Google Scholar]
- Brezinski ME, Serhan CN. Selective incorporation of (15S)-hydroxyeicosatetraenoic acid in phosphatidylinositol of human neutrophils: agonist-induced deacylation and transformation of stored hydroxyeicosanoids. Proc Natl Acad Sci USA. 1990;87:6248–6252. doi: 10.1073/pnas.87.16.6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezinski DA, Nesto RW, Serhan CN. Angioplasty triggers intracoronary leukotrienes and lipoxin A4. Impact of aspirin therapy. Circulation. 1992;86:56–63. doi: 10.1161/01.cir.86.1.56. [DOI] [PubMed] [Google Scholar]
- Cezar-de-Mello PF, Nascimento-Silva V, Villela CG, Fierro IM. Aspirin-triggered lipoxin A4 inhibition of VEGF-induced endothelial cell migration involves actin polymerization and focal adhesion assembly. Oncogene. 2006;25:122–129. doi: 10.1038/sj.onc.1209002. [DOI] [PubMed] [Google Scholar]
- Cezar-de-Mello PF, Vieira AM, Nascimento-Silva V, Villela CG, Barja-Fidalgo C, Fierro IM. ATL-1, an analogue of aspirin-triggered lipoxin A4, is a potent inhibitor of several steps in angiogenesis induced by vascular endothelial growth factor. Br J Pharmacol. 2008;153:956–965. doi: 10.1038/sj.bjp.0707650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Wade D, Kurosaka K, Wang ZY, Oppenheim JJ, Yang D. Temporin A and related frog antimicrobial peptides use formyl peptide receptor-like 1 as a receptor to chemoattract phagocytes. J Immunol. 2004;173:2652–2659. doi: 10.4049/jimmunol.173.4.2652. [DOI] [PubMed] [Google Scholar]
- Chiang N, Takano T, Clish CB, Petasis NA, Tai HH, Serhan CN. Aspirin-triggered 15-epi-lipoxin A4 (ATL) generation by human leukocytes and murine peritonitis exudates: development of a specific 15-epi-LXA4 elisa. J Pharmacol Exp Ther. 1998;287:779–790. [PubMed] [Google Scholar]
- Chiang N, Gronert K, Clish CB, O'Brien JA, Freeman MW, Serhan CN. Leukotriene B4 receptor transgenic mice reveal novel protective roles for lipoxins and aspirin-triggered lipoxins in reperfusion. J Clin Invest. 1999;104:309–316. doi: 10.1172/JCI7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Fierro IM, Gronert K, Serhan CN. Activation of lipoxin A(4) receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J Exp Med. 2000;191:1197–1208. doi: 10.1084/jem.191.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Takano T, Arita M, Watanabe S, Serhan CN. A novel rat lipoxin A4 receptor that is conserved in structure and function. Br J Pharmacol. 2003;139:89–98. doi: 10.1038/sj.bjp.0705220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Bermudez EA, Ridker PM, Hurwitz S, Serhan CN. Aspirin triggers antiinflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci USA. 2004;101:15178–15183. doi: 10.1073/pnas.0405445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Arita M, Serhan CN. Anti-inflammatory circuitry: lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins Leukot Essent Fatty Acids. 2005;73:163–177. doi: 10.1016/j.plefa.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DW, Rovati GE, et al. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- Claria J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell–leukocyte interactions. Proc Natl Acad Sci USA. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clish CB, O'Brien JA, Gronert K, Stahl GL, Petasis NA, Serhan CN. Local and systemic delivery of a stable aspirin-triggered lipoxin prevents neutrophil recruitment in vivo. Proc Natl Acad Sci USA. 1999;96:8247–8252. doi: 10.1073/pnas.96.14.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clish CB, Levy BD, Chiang N, Tai HH, Serhan CN. Oxidoreductases in lipoxin A4 metabolic inactivation: a novel role for 15-onoprostaglandin 13-reductase/leukotriene B4 12-hydroxydehydrogenase in inflammation. J Biol Chem. 2000;275:25372–25380. doi: 10.1074/jbc.M002863200. [DOI] [PubMed] [Google Scholar]
- Colgan SP, Serhan CN, Parkos CA, Delp-Archer C, Madara JL. Lipoxin A4 modulates transmigration of human neutrophils across intestinal epithelial monolayers. J Clin Invest. 1993;92:75–82. doi: 10.1172/JCI116601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker Y, McBean G, Godson C. Lipoxin A4 inhibits IL-1{beta}-induced IL-8 and ICAM-1 expression in 1321N1 human astrocytoma cells. Am J Physiol Cell Physiol. 2009;296:C1420–C1427. doi: 10.1152/ajpcell.00380.2008. [DOI] [PubMed] [Google Scholar]
- Elagoz A, Henderson D, Babu PS, Salter S, Grahames C, Bowers L, et al. A truncated form of CKbeta8-1 is a potent agonist for human formyl peptide-receptor-like 1 receptor. Br J Pharmacol. 2004;141:37–46. doi: 10.1038/sj.bjp.0705592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro IM, Kutok JL, Serhan CN. Novel lipid mediator regulators of endothelial cell proliferation and migration: aspirin-triggered-15R-lipoxin A(4) and lipoxin A(4) J Pharmacol Exp Ther. 2002;300:385–392. doi: 10.1124/jpet.300.2.385. [DOI] [PubMed] [Google Scholar]
- Filep JG, Zouki C, Petasis NA, Hachicha M, Serhan CN. Anti-inflammatory actions of lipoxin A(4) stable analogs are demonstrable in human whole blood: modulation of leukocyte adhesion molecules and inhibition of neutrophil–endothelial interactions. Blood. 1999;94:4132–4142. [PubMed] [Google Scholar]
- Fiore S, Ryeom SW, Weller PF, Serhan CN. Lipoxin recognition sites. Specific binding of labeled lipoxin A4 with human neutrophils. J Biol Chem. 1992;267:16168–16176. [PubMed] [Google Scholar]
- Fiore S, Maddox JF, Perez HD, Serhan CN. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J Exp Med. 1994;180:253–260. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorucci S, Wallace JL, Mencarelli A, Distrutti E, Rizzo G, Farneti S, et al. A beta-oxidation-resistant lipoxin A4 analog treats hapten-induced colitis by attenuating inflammation and immune dysfunction. Proc Natl Acad Sci USA. 2004;101:15736–15741. doi: 10.1073/pnas.0404722101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz AT, Fokin VV, Petasis NA, Serhan CN, Madara JL. LXA4, aspirin-triggered 15-epi-LXA4, and their analogs selectively downregulate PMN azurophilic degranulation. Am J Physiol. 1999;276:C988–C994. doi: 10.1152/ajpcell.1999.276.4.C988. [DOI] [PubMed] [Google Scholar]
- Gewirtz AT, Collier-Hyams LS, Young AN, Kucharzik T, Guilford WJ, Parkinson JF, et al. Lipoxin a4 analogs attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of dextran sodium sulfate-induced colitis. J Immunol. 2002;168:5260–5267. doi: 10.4049/jimmunol.168.10.5260. [DOI] [PubMed] [Google Scholar]
- Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- Gronert K, Gewirtz A, Madara JL, Serhan CN. Identification of a human enterocyte lipoxin A4 receptor that is regulated by interleukin (IL)-13 and interferon gamma and inhibits tumor necrosis factor alpha-induced IL-8 release. J Exp Med. 1998;187:1285–1294. doi: 10.1084/jem.187.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronert K, Martinsson-Niskanen T, Ravasi S, Chiang N, Serhan CN. Selectivity of recombinant human leukotriene D(4), leukotriene B(4), and lipoxin A(4) receptors with aspirin-triggered 15-epi-LXA(4) and regulation of vascular and inflammatory responses. Am J Pathol. 2001;158:3–9. doi: 10.1016/S0002-9440(10)63937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumbach Y, Quynh NV, Chiron R, Urbach V. LXA4 stimulates ZO-1 expression and transepithelial electrical resistance in human airway epithelial (16HBE14o-) cells. Am J Physiol. 2009;296:L101–L108. doi: 10.1152/ajplung.00018.2008. [DOI] [PubMed] [Google Scholar]
- Guilford WJ, Bauman JG, Skuballa W, Bauer S, Wei GP, Davey D, et al. Novel 3-oxa lipoxin A4 analogues with enhanced chemical and metabolic stability have anti-inflammatory activity in vivo. J Med Chem. 2004;47:2157–2165. doi: 10.1021/jm030569l. [DOI] [PubMed] [Google Scholar]
- Hachicha M, Pouliot M, Petasis NA, Serhan CN. Lipoxin (LX)A4 and aspirin-triggered 15-epi-LXA4 inhibit tumor necrosis factor 1 alpha-initiated neutrophil responses and trafficking: regulators of a cytokine–chemokine axis. J Exp Med. 1999;189:1923–1930. doi: 10.1084/jem.189.12.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto A, Murakami Y, Kitasato H, Hayashi I, Endo H. Glucocorticoids co-interact with lipoxin A4 via lipoxin A4 receptor (ALX) up-regulation. Biomed Pharmacother. 2007;61:81–85. doi: 10.1016/j.biopha.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht I, Rong J, Sampaio AL, Hermesh C, Rutledge C, Shemesh R, et al. A novel peptide agonist of formyl-peptide receptor-like 1 (ALX) displays anti-inflammatory and cardioprotective effects. J Pharmacol Exp Ther. 2009;328:426–434. doi: 10.1124/jpet.108.145821. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Borrelli LA, Kanaoka Y, Bacskai BJ, Boyce JA. CysLT2 receptors interact with CysLT1 receptors and down-modulate cysteinyl leukotriene dependent mitogenic responses of mast cells. Blood. 2007;110:3263–3270. doi: 10.1182/blood-2007-07-100453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozsef L, Zouki C, Petasis NA, Serhan CN, Filep JG. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit peroxynitrite formation, NF-kappa B and AP-1 activation, and IL-8 gene expression in human leukocytes. Proc Natl Acad Sci USA. 2002;99:13266–13271. doi: 10.1073/pnas.202296999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp CL, Flick LM, Park KW, Softic S, Greer TM, Keledjian R, et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat Immunol. 2004;5:388–392. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- Kieran NE, Doran PP, Connolly SB, Greenan MC, Higgins DF, Leonard M, et al. Modification of the transcriptomic response to renal ischemia/reperfusion injury by lipoxin analog. Kidney Int. 2003;64:480–492. doi: 10.1046/j.1523-1755.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- Kieran NE, Maderna P, Godson C. Lipoxins: potential anti-inflammatory, proresolution, and antifibrotic mediators in renal disease. Kidney Int. 2004;65:1145–1154. doi: 10.1111/j.1523-1755.2004.00487.x. [DOI] [PubMed] [Google Scholar]
- Kucharzik T, Gewirtz AT, Merlin D, Madara JL, Williams IR. Lateral membrane LXA4 receptors mediate LXA4's anti-inflammatory actions on intestinal epithelium. Am J Physiol. 2003;284:C888–C896. doi: 10.1152/ajpcell.00507.2001. [DOI] [PubMed] [Google Scholar]
- Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev. 2002;2:787–795. doi: 10.1038/nri915. [DOI] [PubMed] [Google Scholar]
- Le Y, Jiang S, Hu J, Gong W, Su S, Dunlop NM, et al. N36, a synthetic N-terminal heptad repeat domain of the HIV-1 envelope protein gp41, is an activator of human phagocytes. Clin Immunol. 2000;96:236–242. doi: 10.1006/clim.2000.4896. [DOI] [PubMed] [Google Scholar]
- Le Y, Gong W, Tiffany HL, Tumanov A, Nedospasov S, Shen W, et al. Amyloid (beta)42 activates a G-protein-coupled chemoattractant receptor, FPR-like-1. J Neurosci. 2001a;21:1–5. doi: 10.1523/JNEUROSCI.21-02-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Y, Yazawa H, Gong W, Yu Z, Ferrans VJ, Murphy PM, et al. The neurotoxic prion peptide fragment PrP(106–126) is a chemotactic agonist for the G protein-coupled receptor formyl peptide receptor-like 1. J Immunol. 2001b;166:1448–1451. doi: 10.4049/jimmunol.166.3.1448. [DOI] [PubMed] [Google Scholar]
- Lee TH, Horton CE, Kyan-Aung U, Haskard D, Crea AE, Spur BW. Lipoxin A4 and lipoxin B4 inhibit chemotactic responses of human neutrophils stimulated by leukotriene B4 and N-formyl-l-methionyl-l-leucyl-l-phenylalanine. Clin Sci (Lond) 1989;77:195–203. doi: 10.1042/cs0770195. [DOI] [PubMed] [Google Scholar]
- Lee TH, Crea AE, Gant V, Spur BW, Marron BE, Nicolaou KC, et al. Identification of lipoxin A4 and its relationship to the sulfidopeptide leukotrienes C4, D4, and E4 in the bronchoalveolar lavage fluids obtained from patients with selected pulmonary diseases. Am Rev Respir Dis. 1990;141:1453–1458. doi: 10.1164/ajrccm/141.6.1453. [DOI] [PubMed] [Google Scholar]
- Leonard MO, Hannan K, Burne MJ, Lappin DW, Doran P, Coleman P, et al. 15-Epi-16-(para-fluorophenoxy)-lipoxin A(4)-methyl ester, a synthetic analogue of 15-epi-lipoxin A(4), is protective in experimental ischemic acute renal failure. J Am Soc Nephrol. 2002;13:1657–1662. doi: 10.1097/01.asn.0000015795.74094.91. [DOI] [PubMed] [Google Scholar]
- Levy BD. Lipoxins and lipoxin analogs in asthma. Prostaglandins Leukot Essent Fatty Acids. 2005;73:231–237. doi: 10.1016/j.plefa.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- Levy BD, Lukacs NW, Berlin AA, Schmidt B, Guilford WJ, Serhan CN, et al. Lipoxin A4 stable analogs reduce allergic airway responses via mechanisms distinct from CysLT1 receptor antagonism. FASEB J. 2007;21:3877–3884. doi: 10.1096/fj.07-8653com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LH, Pervaiz S. Annexin 1: the new face of an old molecule. FASEB J. 2007;21:968–975. doi: 10.1096/fj.06-7464rev. [DOI] [PubMed] [Google Scholar]
- Machado FS, Johndrow JE, Esper L, Dias A, Bafica A, Serhan CN, et al. Anti-inflammatory actions of lipoxin A4 and aspirin-triggered lipoxin are SOCS-2 dependent. Nat Med. 2006;12:330–334. doi: 10.1038/nm1355. [DOI] [PubMed] [Google Scholar]
- Machado FS, Esper L, Dias A, Madan R, Gu Y, Hildeman D, et al. Native and aspirin-triggered lipoxins control innate immunity by inducing proteasomal degradation of TRAF6. J Exp Med. 2008;205:1077–1086. doi: 10.1084/jem.20072416. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- McMahon B, Godson C. Lipoxins: endogenous regulators of inflammation. Am J Physiol Renal Physiol. 2004;286:F189–F201. doi: 10.1152/ajprenal.00224.2003. [DOI] [PubMed] [Google Scholar]
- McMahon B, Stenson C, McPhillips F, Fanning A, Brady HR, Godson C. Lipoxin A4 antagonizes the mitogenic effects of leukotriene D4 in human renal mesangial cells. Differential activation of MAP kinases through distinct receptors. J Biol Chem. 2000;275:27566–27575. doi: 10.1074/jbc.M001015200. [DOI] [PubMed] [Google Scholar]
- McMahon B, Mitchell S, Brady HR, Godson C. Lipoxins: revelations on resolution. Trends Pharmacol Sci. 2001;22:391–395. doi: 10.1016/s0165-6147(00)01771-5. [DOI] [PubMed] [Google Scholar]
- McMahon B, Mitchell D, Shattock R, Martin F, Brady HR, Godson C. Lipoxin, leukotriene, and PDGF receptors cross-talk to regulate mesangial cell proliferation. FASEB J. 2002;16:1817–1819. doi: 10.1096/fj.02-0416fje. [DOI] [PubMed] [Google Scholar]
- Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. J Biol Chem. 1997;272:6972–6978. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- Maddox JF, Colgan SP, Clish CB, Petasis NA, Fokin VV, Serhan CN. Lipoxin B4 regulates human monocyte/neutrophil adherence and motility: design of stable lipoxin B4 analogs with increased biologic activity. FASEB J. 1998;12:487–494. doi: 10.1096/fasebj.12.6.487. [DOI] [PubMed] [Google Scholar]
- Maderna P, Godson C. Phagocytosis of apoptotic cells and the resolution of inflammation. Biochim Biophys Acta. 2003;1639:141–151. doi: 10.1016/j.bbadis.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Maderna P, Godson C. Taking insult from injury: lipoxins and lipoxin receptor agonists and phagocytosis of apoptotic cells. Prostaglandins Leukot Essent Fatty Acids. 2005;73:179–187. doi: 10.1016/j.plefa.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Maderna P, Cottell DC, Berlasconi G, Petasis NA, Brady HR, Godson C. Lipoxins induce actin reorganization in monocytes and macrophages but not in neutrophils: differential involvement of rho GTPases. Am J Pathol. 2002;160:2275–2283. doi: 10.1016/S0002-9440(10)61175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maderna P, Yona S, Perretti M, Godson C. Modulation of phagocytosis of apoptotic neutrophils by supernatant from dexamethasone-treated macrophages and annexin-derived peptide Ac(2-26) J Immunol. 2005;174:3727–3733. doi: 10.4049/jimmunol.174.6.3727. [DOI] [PubMed] [Google Scholar]
- Mitchell S, Thomas G, Harvey K, Cottell D, Reville K, Berlasconi G, et al. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J Am Soc Nephrol. 2002;13:2497–2507. doi: 10.1097/01.asn.0000032417.73640.72. [DOI] [PubMed] [Google Scholar]
- Mitchell D, Rodgers K, Hanly J, McMahon B, Brady HR, Martin F, et al. Lipoxins inhibit Akt/PKB activation and cell cycle progression in human mesangial cells. Am J Pathol. 2004;164:937–946. doi: 10.1016/S0002-9440(10)63181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D, O'Meara SJ, Gaffney A, Crean JK, Kinsella BT, Godson C. The lipoxin A4 receptor is coupled to SHP-2 activation: implications for regulation of receptor tyrosine kinases. J Biol Chem. 2007;282:15606–15618. doi: 10.1074/jbc.M611004200. [DOI] [PubMed] [Google Scholar]
- Munger KA, Montero A, Fukunaga M, Uda S, Yura T, Imai E, et al. Transfection of rat kidney with human 15-lipoxygenase suppresses inflammation and preserves function in experimental glomerulonephritis. Proc Natl Acad Sci USA. 1999;96:13375–13380. doi: 10.1073/pnas.96.23.13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento-Silva V, Arruda MA, Barja-Fidalgo C, Villela CG, Fierro IM. Novel lipid mediator aspirin-triggered lipoxin A4 induces heme oxygenase-1 in endothelial cells. Am J Physiol. 2005;289:C557–C563. doi: 10.1152/ajpcell.00045.2005. [DOI] [PubMed] [Google Scholar]
- Nassar GM, Morrow JD, Roberts LJ, 2nd, Lakkis FG, Badr KF. Induction of 15-lipoxygenase by interleukin-13 in human blood monocytes. J Biol Chem. 1994;269:27631–27634. [PubMed] [Google Scholar]
- Ohse T, Ota T, Kieran N, Godson C, Yamada K, Tanaka T, et al. Modulation of interferon-induced genes by lipoxin analogue in anti-glomerular basement membrane nephritis. J Am Soc Nephrol. 2004;15:919–927. doi: 10.1097/01.asn.0000119962.69573.cc. [DOI] [PubMed] [Google Scholar]
- O'Sullivan TP, Vallin KS, Shah ST, Fakhry J, Maderna P, Scannell M, et al. Aromatic lipoxin A4 and lipoxin B4 analogues display potent biological activities. J Med Chem. 2007;50:5894–5902. doi: 10.1021/jm060270d. [DOI] [PubMed] [Google Scholar]
- Papayianni A, Serhan CN, Phillips ML, Rennke HG, Brady HR. Transcellular biosynthesis of lipoxin A4 during adhesion of platelets and neutrophils in experimental immune complex glomerulonephritis. Kidney Int. 1995;47:1295–1302. doi: 10.1038/ki.1995.184. [DOI] [PubMed] [Google Scholar]
- Papayianni A, Serhan CN, Brady HR. Lipoxin A4 and B4 inhibit leukotriene-stimulated interactions of human neutrophils and endothelial cells. J Immunol. 1996;156:2264–2272. [PubMed] [Google Scholar]
- Parente L, Perretti M. Advances in the pathophysiology of constitutive and inducible cyclooxygenases: two enzymes in the spotlight. Biochem Pharmacol. 2003;65:153–159. doi: 10.1016/s0006-2952(02)01422-3. [DOI] [PubMed] [Google Scholar]
- Perretti M, D'Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- Perretti M, Flower RJ. Annexin 1 and the biology of the neutrophil. J Leukoc Biol. 2004;76:25–29. doi: 10.1189/jlb.1103552. [DOI] [PubMed] [Google Scholar]
- Perretti M, Chiang N, La M, Fierro IM, Marullo S, Getting SJ, et al. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat Med. 2002;8:1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petasis NA, Keledjian R, Sun YP, Nagulapalli KC, Tjonahen E, Yang R, et al. Design and synthesis of benzo-lipoxin A4 analogs with enhanced stability and potent anti-inflammatory properties. Bioorg Med Chem Lett. 2008;18:1382–1387. doi: 10.1016/j.bmcl.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Planaguma A, Titos E, Lopez-Parra M, Gaya J, Pueyo G, Arroyo V, et al. Aspirin (ASA) regulates 5-lipoxygenase activity and peroxisome proliferator-activated receptor alpha-mediated CINC-1 release in rat liver cells: novel actions of lipoxin A4 (LXA4) and ASA-triggered 15-epi-LXA4. FASEB J. 2002;16:1937–1939. doi: 10.1096/fj.02-0224fje. [DOI] [PubMed] [Google Scholar]
- Planaguma A, Kazani S, Marigowda G, Haworth O, Mariani TJ, Israel E, et al. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. Am J Respir Crit Care Med. 2008;178:574–582. doi: 10.1164/rccm.200801-061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliot M, Clish CB, Petasis NA, Van Dyke TE, Serhan CN. Lipoxin A(4) analogues inhibit leukocyte recruitment to Porphyromonas gingivalis: a role for cyclooxygenase-2 and lipoxins in periodontal disease. Biochemistry. 2000;39:4761–4768. doi: 10.1021/bi992551b. [DOI] [PubMed] [Google Scholar]
- Qiu FH, Devchand PR, Wada K, Serhan CN. Aspirin-triggered lipoxin A4 and lipoxin A4 up-regulate transcriptional corepressor NAB1 in human neutrophils. FASEB J. 2001;15:2736–2738. doi: 10.1096/fj.01-0576fje. [DOI] [PubMed] [Google Scholar]
- Resnati M, Pallavicini I, Wang JM, Oppenheim J, Serhan CN, Romano M, et al. The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1/LXA4R. Proc Natl Acad Sci USA. 2002;99:1359–1364. doi: 10.1073/pnas.022652999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reville K, Crean JK, Vivers S, Dransfield I, Godson C. Lipoxin A4 redistributes myosin IIA and Cdc42 in macrophages: implications for phagocytosis of apoptotic leukocytes. J Immunol. 2006;176:1878–1888. doi: 10.4049/jimmunol.176.3.1878. [DOI] [PubMed] [Google Scholar]
- Ring WL, Riddick CA, Baker JR, Munafo DA, Bigby TD. Lymphocytes stimulate expression of 5-lipoxygenase and its activating protein in monocytes in vitro via granulocyte macrophage colony-stimulating factor and interleukin 3. J Clin Invest. 1996;97:1293–1301. doi: 10.1172/JCI118545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers K, McMahon B, Mitchell D, Sadlier D, Godson C. Lipoxin A4 modifies platelet-derived growth factor-induced pro-fibrotic gene expression in human renal mesangial cells. Am J Pathol. 2005;167:683–694. doi: 10.1016/S0002-9440(10)62043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanak M, Levy BD, Clish CB, Chiang N, Gronert K, Mastalerz L, et al. Aspirin-tolerant asthmatics generate more lipoxins than aspirin-intolerant asthmatics. Eur Respir J. 2000;16:44–49. doi: 10.1034/j.1399-3003.2000.16a08.x. [DOI] [PubMed] [Google Scholar]
- Sawmynaden P, Perretti M. Glucocorticoid upregulation of the annexin-A1 receptor in leukocytes. Biochem Biophys Res Commun. 2006;349:1351–1355. doi: 10.1016/j.bbrc.2006.08.179. [DOI] [PubMed] [Google Scholar]
- Scalia R, Gefen J, Petasis NA, Serhan CN, Lefer AM. Lipoxin A4 stable analogs inhibit leukocyte rolling and adherence in the rat mesenteric microvasculature: role of P-selectin. Proc Natl Acad Sci USA. 1997;94:9967–9972. doi: 10.1073/pnas.94.18.9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannell M, Flanagan MB, deStefani A, Wynne KJ, Cagney G, Godson C, et al. Annexin-1 and peptide derivatives are released by apoptotic cells and stimulate phagocytosis of apoptotic neutrophils by macrophages. J Immunol. 2007;178:4595–4605. doi: 10.4049/jimmunol.178.7.4595. [DOI] [PubMed] [Google Scholar]
- Schaldach CM, Riby J, Bjeldanes LF. Lipoxin A4: a new class of ligand for the Ah receptor. Biochemistry. 1999;38:7594–7600. doi: 10.1021/bi982861e. [DOI] [PubMed] [Google Scholar]
- Schottelius AJ, Giesen C, Asadullah K, Fierro IM, Colgan SP, Bauman J, et al. An aspirin-triggered lipoxin A4 stable analog displays a unique topical anti-inflammatory profile. J Immunol. 2002;169:7063–7070. doi: 10.4049/jimmunol.169.12.7063. [DOI] [PubMed] [Google Scholar]
- Serhan CN. On the relationship between leukotriene and lipoxin production by human neutrophils: evidence for differential metabolism of 15-HETE and 5-HETE. Biochim Biophys Acta. 1989;1004:158–168. doi: 10.1016/0005-2760(89)90264-6. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Lipoxins and novel aspirin-triggered 15-epi-lipoxins (ATL): a jungle of cell–cell interactions or a therapeutic opportunity? Prostaglandins. 1997;53:107–137. doi: 10.1016/s0090-6980(97)00001-4. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot Essent Fatty Acids. 2005;73:141–162. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Sheppard KA. Lipoxin formation during human neutrophil-platelet interactions. Evidence for the transformation of leukotriene A4 by platelet 12-lipoxygenase in vitro. J Clin Invest. 1990;85:772–780. doi: 10.1172/JCI114503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Hamberg M, Samuelsson B. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc Natl Acad Sci USA. 1984a;81:5335–5339. doi: 10.1073/pnas.81.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Hamberg M, Samuelsson B. Trihydroxytetraenes: a novel series of compounds formed from arachidonic acid in human leukocytes. Biochem Biophys Res Commun. 1984b;118:943–949. doi: 10.1016/0006-291x(84)91486-4. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Hamberg M, Samuelsson B, Morris J, Wishka DG. On the stereochemistry and biosynthesis of lipoxin B. Proc Natl Acad Sci USA. 1986a;83:1983–1987. doi: 10.1073/pnas.83.7.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Nicolaou KC, Webber SE, Veale CA, Dahlen SE, Puustinen TJ, et al. Lipoxin A. Stereochemistry and biosynthesis. J Biol Chem. 1986b;261:16340–16345. [PubMed] [Google Scholar]
- Serhan CN, Maddox JF, Petasis NA, Akritopoulou-Zanze I, Papayianni A, Brady HR, et al. Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry. 1995;34:14609–14615. doi: 10.1021/bi00044a041. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Haeggstrom JZ, Leslie CC. Lipid mediator networks in cell signaling: update and impact of cytokines. FASEB J. 1996;10:1147–1158. doi: 10.1096/fasebj.10.10.8751717. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O'Neill LA, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev. 2008a;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008b;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchowitz L, Fiore S, Serhan CN. Carrier-mediated transport of lipoxin A4 in human neutrophils. Am J Physiol. 1994;267:C1525–C1534. doi: 10.1152/ajpcell.1994.267.6.C1525. [DOI] [PubMed] [Google Scholar]
- Sodin-Semrl S, Taddeo B, Tseng D, Varga J, Fiore S. Lipoxin A4 inhibits IL-1 beta-induced IL-6, IL-8, and matrix metalloproteinase-3 production in human synovial fibroblasts and enhances synthesis of tissue inhibitors of metalloproteinases. J Immunol. 2000;164:2660–2666. doi: 10.4049/jimmunol.164.5.2660. [DOI] [PubMed] [Google Scholar]
- Soyombo O, Spur BW, Lee TH. Effects of lipoxin A4 on chemotaxis and degranulation of human eosinophils stimulated by platelet-activating factor and N-formyl-l-methionyl-l-leucyl-l-phenylalanine. Allergy. 1994;49:230–234. doi: 10.1111/j.1398-9995.1994.tb02654.x. [DOI] [PubMed] [Google Scholar]
- Starosta V, Pazdrak K, Boldogh I, Svider T, Kurosky A. Lipoxin A4 counterregulates GM-CSF signaling in eosinophilic granulocytes. J Immunol. 2008;181:8688–8699. doi: 10.4049/jimmunol.181.12.8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SB, Gao J, Gong W, Dunlop NM, Murphy PM, Oppenheim JJ, et al. T21/DP107, A synthetic leucine zipper-like domain of the HIV-1 envelope gp41, attracts and activates human phagocytes by using G-protein-coupled formyl peptide receptors. J Immunol. 1999a;162:5924–5930. [PubMed] [Google Scholar]
- Su SB, Gong W, Gao JL, Shen W, Murphy PM, Oppenheim JJ, et al. A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J Exp Med. 1999b;189:395–402. doi: 10.1084/jem.189.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Fiore S, Maddox JF, Brady HR, Petasis NA, Serhan CN. Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: evidence for anti-inflammatory receptors. J Exp Med. 1997;185:1693–1704. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Clish CB, Gronert K, Petasis N, Serhan CN. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J Clin Invest. 1998;101:819–826. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SH, Lu C, Dong L, Zhou GP, He ZG, Chen ZQ. Lipoxin A inhibits TNF-alpha-induced production of interleukins and proliferation of rat mesangial cells. Kidney Int. 2005;68:35–46. doi: 10.1111/j.1523-1755.2005.00379.x. [DOI] [PubMed] [Google Scholar]
- Wu SH, Wu XH, Lu C, Dong L, Zhou GP, Chen ZQ. Lipoxin A4 inhibits connective tissue growth factor-induced production of chemokines in rat mesangial cells. Kidney Int. 2006;69:248–256. doi: 10.1038/sj.ki.5000025. [DOI] [PubMed] [Google Scholar]
- Wu SH, Liao PY, Yin PL, Zhang YM, Dong L. Elevated expressions of 15-lipoxygenase and lipoxin A4 in children with acute poststreptococcal glomerulonephritis. Am J Pathol. 2009;174:115–122. doi: 10.2353/ajpath.2009.080671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M, et al. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009;61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurier RB, Sun YP, George KL, Stebulis JA, Rossetti RG, Skulas A, et al. Ajulemic acid, a synthetic cannabinoid, increases formation of the endogenous proresolving and anti-inflammatory eicosanoid, lipoxin A4. FASEB J. 2009;23:1503–1509. doi: 10.1096/fj.08-118323. [DOI] [PMC free article] [PubMed] [Google Scholar]


