Abstract
Insect-borne diseases cause significant human morbidity and mortality. Current control and preventive methods against vector-borne diseases rely mainly on insecticides. The emergence of insecticide resistance in many disease vectors highlights the necessity to develop new strategies to control these insects. Vector transgenesis and paratransgenesis are novel strategies that aim at reducing insect vectorial capacity, or seek to eliminate transmission of pathogens such as Plasmodium sp., Trypanosoma sp., and Dengue virus currently being developed. Vector transgenesis relies on direct genetic manipulation of disease vectors making them incapable of functioning as vectors of a given pathogen. Paratransgenesis focuses on utilizing genetically modified insect symbionts to express molecules within the vector that are deleterious to pathogens they transmit. Despite the many successes achieved in developing such techniques in the last several years, many significant barriers remain and need to be overcome prior to any of these approaches become a reality. Here, we highlight the current status of these strategies, pointing out advantages and constraints, and also explore issues that need to be resolved before the establishment of transgenesis and paratransgenesis as tools to prevent vector-borne diseases.
Keywords: Transgenesis, Paratransgenesis, Gene drive systems, Transposons, Symbionts, Vector-borne disease control
1. Introduction
Insect-borne diseases are responsible for severely affecting human life around the world, causing significant morbidity and mortality. Malaria alone is responsible for 1-2 million deaths annually, and approximately 300 million are at risk of becoming infected. The lack of effective vaccines to control the spread of a significant number of vector-borne diseases makes control mainly dependent on insecticides. However, the appearance of insecticide resistance requires the development of new strategies to reduce pathogen transmission in the field [1]. Novel approaches against vector borne diseases include transgenesis and paratransgenesis to reduce vector competence.
For vector transgenesis, the goal is to transform vectors with a gene (or genes) whose protein(s) impair pathogen development. A few stably transformed insect germ lines using transposons are available [2], and mosquitoes such as Aedes [3-6], Anopheles [7-16], and Culex [17] were genetically altered or transformed (Table 1). For some transformed mosquitoes, proteins targeting development of Plasmodium sp. and Dengue virus were successfully expressed [12, 13, 18-20].
Table 1.
Germ line transformed mosquitoes
| Insect species | Transposon | Promoter | Reporter gene | Transgene | Targeted pathogen | Reference |
|---|---|---|---|---|---|---|
| Ae. aegypti | Hermes |
D. melanogaster Cinnabar |
D. melanogaster Cinnabar |
Cinnabar | - | [3] |
| Mos1 |
D. melanogaster Cinnabar |
D. melanogaster Cinnabar |
Cinnabar | - | [5] | |
| Hermes |
Ae. aegypti maltase-like 1 / apyrase |
Firefly luciferase | Firefly luciferase | - | [37] | |
| Hermes |
D. melanogaster Actin 5C |
EGFP | EGFP | - | [6] | |
| Hermes |
Ae. aegypti vitellogenin |
D. melanogaster Cinnabar |
Defensin A | Micrococcus luteus | [28] | |
| Hermes |
Ae. aegypti carboxypeptidase |
Firefly luciferase | Firefly luciferase | - | [29] | |
| Mos1 |
An. gambiae carboxypeptidase |
Firefly luciferase | Firefly luciferase | - | [29] | |
| piggyBac |
Ae. aegypti vitellogenin |
EGFP | Defensin A |
Enterobacter cloacae/ P. gallinaceum |
[20, 30] | |
| Minos |
D. melanogaster Actin 5C |
dsRED | dsRED | - | [9] | |
| piggyBac |
D. melanogaster Actin 5C |
dsRED | dsRED | - | [9] | |
| piggyBac |
D. melanogaster Cinnabar |
D. melanogaster Cinnabar |
Cinnabar | - | [27] | |
| Mos1 |
D. melanogaster Cinnabar/hsp70 |
D. melanogaster Cinnabar/dsRED |
Cinnabar/dsRED | - | [80] | |
| piggyBac |
D. pseudoobscura hsp82 |
EGFP |
Mos1 transposase |
- | [80] | |
| piggyBac |
Ae. aegypti vitellogenin |
EGFP | ΔRELa | - | [36] | |
| piggyBac |
Ae. aegypti vitellogenin |
EGFP | Cecropin A | Enterobacter cloacae | [36] | |
| piggyBac |
Ae. aegypti vitellogenin |
EGFP | ΔREL1-Ab | - | [35] | |
| piggyBac |
Ae. aegypti vitellogenin |
EGFP |
dsRNA against REL1 transcripts |
- | [35] | |
| Ae. aegypti | Mos1 |
An. gambiae carboxypeptidase |
EGFP |
dsRNA against prM of DENV-2 |
Dengue-2 virus | [18] |
| Mos1 |
Ae. aegypti nos |
dsRED | Mos1 transposase | - | [48] | |
| Mos1 |
Ae. aegypti D7 |
EGFP | Gal4 | - | ||
| Ae. fluviatilis | piggyBac |
An. gambiae peritrophin |
EGFP | mPLA2 | P. gallinaceum | [19] |
| An. albimanus | piggyBac |
D. melanogaster polyubiquitin |
EGFP | EGFP | - | [8] |
| An. gambiae | piggyBac |
Baculovirus hr5-ie1 |
EGFP | EGFP | - | [7] |
| piggyBac |
Ae. aegypti carboxypeptidase |
EGFP | Cecropin A | P. berghei | [14] | |
| An. stephensi | minos |
D. melanogaster actin 5C |
EGFP | EGFP | - | [16] |
| piggyBac | D. melanogaster | dsRED | dsRED | - | [9] | |
| piggyBac |
An. gambiae carboxypeptidase |
EGFP | PLA2 | P. berghei | [13] | |
| piggyBac |
An. gambiae carboxypeptidase |
EGFP | [SM1]4 | P. berghei | [12] | |
| minos |
D. melanogaster actin 5C |
dsRED |
dsRNA against EGFP |
- | [11] | |
| minos |
An. gambiae apyrase An. gambiae D7r4 |
EGFP | LacZ | - | [34] | |
| piggyBac |
An. gambiae peritrophin |
EGFP | PLA2 | P. berghei | [10] | |
| piggyBac |
D. melanogaster β2-tubulin |
dsRED | EGFP | - | [15] | |
| piggyBac |
An. stephensi vitellogenin |
EGFP | CFP | - | [31] | |
| An. stephensi | minos |
An. stephensi antiplatelet |
EGFP | dsRED | - | [32] |
| piggyBac |
An. gambiae vitellogenin |
dsRED | EGFP | - | [33] | |
| piggyBac |
D. melanogaster hsp70 |
dsRED |
minos transposase |
- | [81] | |
| minos |
D. melanogaster actin 5C |
EGFP | EGFP | - | ||
|
Cx. quinquefasciatus |
Hermes |
D. melanogaster actin 5C |
EGFP | EGFP | - | [17] |
Drosophila Relish-related gene lacking the transactivator domain.
REL1-A lacking a C-terminal domain.
Paratransgenesis aims at reducing vector competence by genetically manipulating symbionts. Transformed symbionts are spread maternally or via coprophagy across an insect population [21]. Symbionts currently targeted in paratransgenesis include bacteria found within triatomine [21-23], tsetse fly [24], and mosquito [25, 26] tissues, and densoviruses infecting An. gambiae and Ae. aegypti mosquitoes [27, 28].
This review explores technical issues, future challenges and potential applications of transgenesis and paratrangenesis against vector-transmitted pathogens.
2. Transgenesis in insect disease vectors
The main objective of vector transgenesis is interruption of pathogen transmission via the introduction of exogenous DNA fragment (i.e., gene) into an insect genome, followed by expression of the gene to inhibit pathogen development within the vector. Several mosquitoes species vectors of different parasites and viruses have been transformed (Table 1). Some of the transformed mosquitoes were shown capable of blocking pathogen development via tissue-specific expression of molecules that impair pathogen attachment to the midgut [12], or activate biochemical pathways detrimental to pathogen survival [18]. However, vector transgenesis is still a somewhat complex approach, highlighted by the fact that insect germ line transformation technique is only successfully performed by a handful of laboratories. Various issues associated with development of transgenic vectors including stability of the transgene in the genome and fitness of the transformed insect in the field are significant hurdles to be overcome before transgenic insects can be applied against insect-borne diseases. Some of the issues involved in development of transgenic vectors, how interference or blockage of parasite is achieved, and what may lie ahead regarding challenges and perspectives for vector transgenesis are discussed below.
2.1. Germ line transformation
Stable genetic transformation of insects is accomplished by inserting an exogenous gene into the insect genome via the inoculation of plasmids containing the transgene (donor plasmids) into insect eggs (Figure 1). Donor plasmids are constructed to carry an engineered transposon element lacking the gene that encodes the transposase, the enzyme that mediates transposon activity by a cut and paste mechanism. Hermes, Mariner (Mos1), minus, and piggyBac, for the most part, have been the transposable elements of choice. Transposases are then supplied in trans (expressed in a separate plasmid) by the co-inoculation of a transposase-encoding helper plasmid along with the donor plasmid. Transposase expression is usually driven by a heat shock protein promoter that is activated upon raising the temperature, as it is frequently performed with injected mosquito eggs[2].
Figure 1. Transgenesis.

The procedure to generate transgenic insects by germ line transformation is depicted. (I) Insect eggs are microinjected with a donor plasmid, expressing a transgene (in orange) and a reporter gene (in green), and with a helper plasmid, expressing a transposase (purple). (II) After inoculation into the eggs, plasmids are taking up by some (or all) of the germ line cells. (III) Transformed larvae can be distinguished from non-transformed due to the expression of the reporter gene (green eye phenotype), which can be controlled by an eye specific promoter. (IV) Transformed insects are crossed with wild type specimens so as to confirm that the transposon-carrying transgene was inserted into the insect chromosome. (V) Transgenic adult insect expressing the transgene (e.g., orange proteins in the insect midgut) is depicted.
Transformed offspring can be identified by a specific phenotype alteration, mediated by the expression of a reporter protein encoded by the donor vector, such as eye color [2]. For example, the Ae. aegypti khW strain displays white eyes; upon transformation with the transposable element Hermes or piggyBac expressing the Drosophila melanogaster cinnabar gene (cn+), mosquito eye color (brown) was rescued [3, 29, 30]. However, this strategy is restricted to insect species displaying polymorphic eye phenotypes, such as Ae. aegypti.
Alternatively, phenotypic markers such as firefly luciferase, or green (EGFP), red (dsRED) and cyan fluorescent (CFP) proteins can be used as transformation markers [8, 9, 31-33]. Luciferase as reporter gene was specifically expressed in midguts of adult Ae. aegypti controlled by a carboxypeptidase promoter [31], while Anopheles albimanus displayed green fluorescence in whole body of larvae and pupae, and in the abdomen of adults following expression of GFP via piggyBac [8]. Anopheles stephensi also were modified to express dsRED and cyan fluorescent protein either second and third-instar larvae and in adult female midguts (dsRED) [9], or in fat bodies (cyan) [33].
2.2. Tissue-specific transgene expression
Within the insect vector, a pathogen may interact with specific tissues, such as the midgut (as is the case for Trypanosoma cruzi and Leishmania sp.), or midgut, hemocoel and salivary glands (as is the case for Plasmodium sp). Frequently, molecules that can block pathogen development within its vector can be expressed in a tissue-specific manner to increase effectiveness. Tissue-specific expression of a transgene is accomplished by the use of a tissue-specific promoter (Table 1). Most promoters used in vector transgenesis drive protein expression specifically within the midgut, the hemocoel, or the salivary glands, as these are sites where pathogens are commonly found within an infected vector [10, 19, 30, 31, 34-36].
In mosquitoes, promoters for carboxypeptidase and peritrophin have been widely used to drive midgut-specific expression of several transgenes. In Ae. aegypti, expression of luciferase under the control of the carboxypeptidase promoter was similar to the expression profile detected for the native carboxypeptidase: expressed in the midgut, after a blood-meal, and only in females [31]. Similar results were obtained with an An. gambiae peritrophin promoter driving expression of a transgenic protein in An. stephensi and Aedes fluviatilis [10, 19].
In Ae. aegypti, a vitellogenin promoter specifically drives expression of transgenes in the fat body. This promoter was used to express innate immune defense-related genes [30, 32, 37, 38], as well as dsRNA targeting REL1 transcripts [37]. The same promoter was used to express CFP in An. stephensi [33]. The robustness of the vitellogenin promoter was confirmed by its capacity to function following multiple gonotrophic cycles in transgenic An. stephensi [35].
Salivary gland-specific promoters also have been used in germ-line transformation of mosquitoes. D7 and apyrase promoters from An. gambiae and antiplatelet from An. stephensi were used to transform An. stephensi [34, 36]. Ae. aegypti also was transformed and successfully expressed luciferase within salivary glands using maltase-like 1 and apyrase promoters [39].
2.3. Transgenes targeting Plasmodium development
In spite of the many mosquito species successfully transformed (Table 1), only a handful has been transformed with molecules that impair pathogen development [4, 10, 12-14, 18]. A list of genetically modified mosquitoes obtained to date, including the transposon and reporter genes used, tissue-expression specificity and target pathogens, among others is seen on Table 1.
In one example of a transgene targeting Plasmodium, expression of phospholipase-2 (PLA2) in An. stephensi [13] led to an 87% reduction of P. berghei oocyst intensity compared to non-PLA2-expressing controls. Moreover, parasite transmission to naïve mice was completely inhibited in three out of four experiments [13]. When a peritrophin promoter was used to drive the expression of PLA2 in An. stephensi, inhibition of P. berghei oocyst intensity ranged from 74% to 94% [10]. Nevertheless, expression of PLA2 in the mosquito midgut did not exert a direct effect on the parasite, but rather led to structural damage of the midgut epithelium [10, 13]. When an inactive form of PLA2 (mPLA2) was used to transform Ae. fluviatilis using piggyBac under the control of a peritrophin promoter from An. gambiae, reduction in P. gallinaceum oocyst intensity ranged from 17.5% to 68.5% [19]. Here, despite the fact that inhibition of P. gallinaceum oocysts was not as accentuated as the inhibition detected for P. berghei oocysts using the active PLA2, the mPLA2 transgenic Ae. fluviatilis displayed greater survival rate than wild type mosquitoes [19].
Synthetic peptides that block Plasmodium development in mosquitoes also have been identified. Using a phage display library Gosh et al [40] identified SM1, a peptide that blocked P. berghei invasion of An. stephensi midgut and salivary glands. In An. stephensi transformed with piggyBac expressing a four tandem repeat of SM1 under a carboxypeptidase promoter, P. berghei intensity was inhibited by 81.6%. Interestingly, these transgenic mosquitoes even with sporozoites in their salivary glands were unable to transmit P. berghei to mice [12].
Besides PLA2 and SM1, mosquitoes also were transformed with antimicrobial peptides [14, 30, 37]. An. gambiae transformed with a cecropin A driven by an Ae. aegypti carboxypeptidase promoter inhibited P. berghei intensity by 61% on average when compared with non-transformed controls [14]. However, this study failed to demonstrate expression of cecropin A within the midguts of transgenic mosquitoes [14]. Similarly, Ae. aegypti transformed with piggyBac expressing defensin A under a peritrophin promoter, inhibited P. gallinaceum oocyst intensity by 65% to 70% [20].
2.4. Transgenes inducing gene silencing and targeting viral transmission
Stable transformation of mosquitoes with two inverted repeats of the same gene to induce assembly of double-strand RNAs (dsRNAs) and activation of the RNAi pathway has also been obtained [11, 37]. This strategy takes advantage of the RNAi mechanism to block expression of insect molecules associated with vectorial competence [37], or it can directly target viral replication within insect tissues [18]. In spite of the fact that both approaches are technically feasible, only the latter has led to substantial reduction in the development of any human pathogen [18].
Gene silencing via dsRNA was first demonstrated with An. stephensi expressing sense and anti-sense RNAs targeting EGFP [11]. EGFP dsRNA-expressing mosquitoes were crossed with a transgenic mosquito line that expresses EGFP. The double transgenic offspring displayed lower level of EGFP expression than the parental line expressing it, indicating the effect of the RNAi machinery reducing the expression of the EGFP transgene [11]. Ae. aegypti expressing dsRNA targeting REL1, a gene involved in innate immune response, also inhibited expression of REL1 via RNAi [37].
As indicated, the activation of the RNAi pathway is intended to affect the replication of infecting RNA viruses transmitted by mosquitoes. Ae. aegypti expressing DEN2 sense and antisense RNAs reduced viral load by fivefold, confirming the effectiveness of the RNAi in controlling virus replication in disease vectors [18].
2.5. Challenges and perspectives on vector transgenesis
Despite advances in the development of stable lines of genetically modified disease vectors [8, 12, 29, 31], many challenges exist to the application of transgenesis to control vector-borne diseases outside the laboratory. Beyond issues dealing with social and environmental impact(s) that are inherent to the potential use of genetically modified organisms (not the scope of this review), of significance also is the fact that, for example, blockage of Plasmodium development by transgenic mosquitoes was achieved only for non-human parasites. One such case involved a non-natural mosquito-parasite pair (An. stephensi-P. berghei) [12, 13], while other were obtained using the avian malaria P. gallinaceum transmitted by Ae. aegypti or Ae. fluviatilis [19, 20]. Strains of transgenic mosquitoes blocking development of human malaria parasites (such as P. falciparum and P. vivax) have yet to be developed [41]. The only exception of a transgenic insect robustly impairing development of a human pathogen is the transgenic strain of Ae. aegypti capable of inhibiting DEN virus development [18].
Fitness of transgenic mosquitoes in natural habitats is also an important issue. Laboratory tests demonstrated that in four lines of transgenic An. stephensi the frequency of transgenic individuals declined over time [42]. Although SM1-transgenic hemizygous An. stephensi, carrying a single transgene copy in the genome, exhibited higher fitness than wild type when fed on infected mice [43], transgenic homozygous An. stephensi (harboring two transgene copies), possibly advantageous for field releases, exhibited lower fitness than non-transformed mosquitoes [44]. Transgenic lines of Ae. aegypti expressing either EGFP or a transposase also exhibited lower fitness than wild type [45].
Although fitness in natural habitats is one of the main constraints of transgenic disease vectors, mathematical models suggest that a highly efficient transposon can spread through natural populations if it affects fitness by less than 50% [46, 47]. Nevertheless, pathogen refractoriness needs to be at or very close to 100% to substantially decrease disease prevalence in high endemic areas [48]. Future studies mimicking field conditions likely will uncover the importance of fitness to the establishment of transgenic mosquitoes in natural habitats.
Problems also are associated with transposons as genetic drive systems for transgenes. Transposons can remobilize in somatic tissues possibly causing damage in regions of the genome [49]. A mechanism to drive transposase expression and restrict gene drive system activity to germ-line tissues was created using the regulatory sequence of nanos, a gene involved in early embryonic development [50]. Interestingly, none of the transposable elements (Hermes, Mos1, minos, and piggyBac) appears to remobilize in Ae. aegypti germ line, possibly reflecting a resistance mechanism, since the same elements can remobilize in Drosophila germ line tissues [51].
Further issues regarding the design and potential field release of transgenic disease vectors include: i) non-canonical transposition reactions, such as transgene insertion by a mechanism other than cut-and-paste, resulting in integration of donor plasmid fragments into the insect genome, as observed in transposition events accomplished by the transposons Hermes, Mos1, and piggyBac in Ae. aegypti [51]; and ii) transgene size influencing transposon activity, as shown for Mariner [52]. Another issue is the possibility of horizontal transfer of the transgene between mosquito sibling species, as proposed for the introgression of the P-element between Drosophila lines [53]. Horizontal transfer also can be virus mediated, such as the case of piggyBac, initially identified in a Tricoplusia ni virus [54]. Technology to prevent the potential horizontal transposon transfer by viruses and to inhibit transposition activity mediated by endogenous transposases still needs to be developed.
Other gene-drive mechanisms have been developed to assist with problems associated with transposon elements in insect germ-line transformation [55]. Recently, a driving mechanism known as Medea (maternal-effect dominant embryonic arrest) was shown capable of driving population replacement in Drosophila without an apparent fitness cost [56]. This gene drive system consists of a DNA segment encoding a protein lethal to insects and an antidote that neutralize the lethal protein. A heterozygous female (Medea/+) expresses the toxin within all oocytes, killing all the +/+ offspring as they do not express the antidote to neutralize the maternal toxin. Medea can be designed to restrict transgene activity to the host species through the utilization of siRNAs-encoding genes as toxin genes [56]. Although Medea has been postulated to function in An. gambiae population replacement, it has yet to be developed for mosquitoes [57].
Transgenic insects also can be developed to express female dominant-lethal genes to reduce the number of females in an insect population [58, 59]. RIDL, or release of insects carrying a dominant lethal, was originally designed to overcome issues associated with SIT (sterile insect technique). Although SIT was successfully applied against the screwworm fly Cochliomyia homonivorax [60], the fruit fly Ceratitis capitata [61], and the tsetse fly G. austeni [62], drawbacks such as reduced sterile male fitness and sterile female contamination, were detected [59]. RIDL consists of release of transgenic male insects expressing the female dominant-lethal genes, causing a reduction on the numbers of females in the following generations [61]. Robust transgenic vectors approaches could also be used with RIDL [59]. In fact, a transgenic mosquito sexing-system has already been developed [15]; however, RIDL requires a reliable genetic transformation system not yet available for mosquitoes.
2.6. Future directions on vector transgenesis
In our view, two hurdles to the establishment of an efficient transgenic vector approach are the lack of a transgene(s) that effectively reduce pathogen load, and the inefficiency of transposons as gene-drive mechanism(s). Further studies utilizing QTL mapping, reverse genetics, gene knockdown, or other techniques to identify traits associated with vector competence can unveil candidate genes that, when targeted, may effectively block pathogen development and transmission. The availability of gene drive mechanisms to overcome issues associated with the use of transposons, such as remobilization, fitness load, and the potential to introgress to closely related species, also is of interest. Medea was suggested as a gene drive that could overcome such issues, but it is yet to be developed for insect vectors. As for RIDL, the current absence of a gene drive mechanism also prevents its application against insect vectors.
Clearly, much work remains before genetically modified insect vectors are released into natural habitats. When realized transgenesis may provide a significant tool in the fight against vector-borne diseases.
3. Paratransgenesis to reduce vector competence
Paratransgenesis refers to the use of genetically modified symbiotic organisms expressing molecules that can block pathogen development or transmission by vectors. Bacteria symbionts of blood sucking bugs [21-23], tsetse flies [24], and mosquitoes [25, 26], and symbiotic viruses of An. gambiae [27] and Ae. aegypti [28, 63, 64], have been used (Figure 2). Current data indicate that symbionts expressing molecules targeting pathogen development have the potential to reduce transmission in endemic regions, and appear unrelated to any fitness load [21, 24]. As with transgenesis, spread of transformed symbionts also would benefit from the availability of a gene drive system to replace non-transformed symbionts present in natural vector populations.
Figure 2. Paratransgenesis.
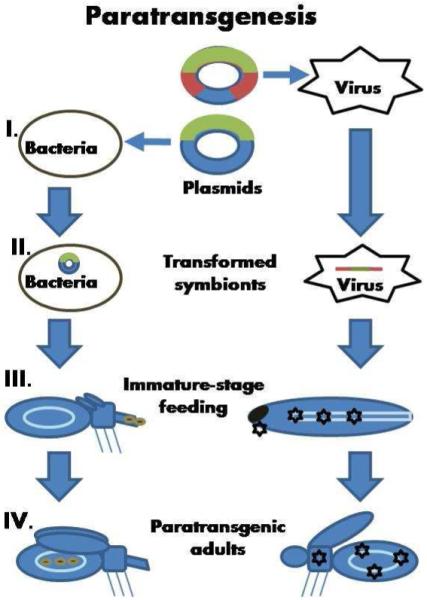
(B) Paratransgenesis. The procedure of insect transformation via transgenic symbionts is depicted. Here two orders are represented: reduviids (Hemiptera) on the left panel and mosquitoes (Diptera) on the right panel. (I) Bacteria or viruses symbionts can be genetically modified to express a gene blocking parasite development in vectors’ tissues. (II) Symbiotic bacteria are transformed with plasmids (blue) expressing a gene (green) to inhibit parasite development in insect gut. Alternatively, viral genome (red) is inserted into a plasmid (blue) and manipulated to express a transgene (green). Viral particles can be generated by expression of such a plasmid in insect cells. (III) The transformed symbionts are acquired by insect hosts through larvae or nymph feeding, or through thoracic injection. (IV) Once insects acquire the transformed symbionts, these microorganisms can express proteins to inhibit pathogen development.
3.1. Transformation of reduviids, tsetse, and mosquitoes with bacterial symbionts
Paratransgenesis in disease vectors was demonstrated through the expression of cecropin A by Rhodococcus rhodnni within the midgut of the kissing bug (reduviid) Rhodnius prolixus [21]. A 99% reduction in the intensity of Trypanosoma cruzi infection in the hindgut of R. prolixus was observed without interfering with insect fitness. Additionally, transformed symbionts were shown to be horizontally transmitted to R. prolixus carrying non-transformed symbionts via reduviid coprophagic habits [21]. Subsequently, functionally active antibody fragments also were successfully expressed in the guts of R. prolixus [22] and Triatoma infestans [23] utilizing symbionts. Transformed symbionts were stably maintained within the gut of the insects without need for antibiotic selection [21-23].
Paratransgenesis also appears to be a promising strategy to reduce African trypanosomes transmission by tsetse flies. Genetically transformed Sodalis, a symbiont of tsetse flies commonly found in the midgut and hemolymph of Glossina m. morsitans, Glossina p. palpalis, Glossina austeni, and Glossina brevipalpis, and the salivary glands of Gl. p. palpalis, is transmitted vertically via the female milk glands [24, 65, 66]. Vertical transmission was demonstrated with GFP-transformed (recSodalis) that was detected in 9 out of 12 F1 offspring and eight out of 12 F2 descendents, indicating the ability of the transformed symbiont to be spread across tsetse populations [24]. In addition, when Sodalis originally isolated from of Gl. m. morsitans and Gl. fuscipes was transformed with GFP, the recSodalis obtained colonized septic non-native tsetse host species at a density similar to a native colonization and without reducing host fitness [66].
Recently, symbiotic bacteria also were isolated from An. stephensi and their application in paratransgenesis was suggested [25, 67]. In one case, Asaia sp. bacteria were successfully transformed with GFP-expressing plasmids and used to re-infect adult mosquitoes through sugar or blood meals mixed with the transformed bacteria [25]. Larval stages also were able to pick up the transformed bacteria from their aquatic environment in the laboratory. Transformed Asaia were found in mosquito organs which are sites for pathogen development, such as midgut and salivary gland, as well as in male and female reproductive tracts, thus Asaia may represent a candidate for malaria control [25]. Further, Damiani et al [68] demonstrated that Asaia are transmitted from male to female An. stephensi during mating (venereal transmission), and the bacteria acquired are vertically transmitted to offspring. This transmission route was confirmed by the use of Asaia carrying either a DsRed or GFP fluorescent protein gene inserted into the bacteria genome. GFP and Ds-Red-labeled Asaia were fed (mixed with the sugar meal) to male mosquitoes that were later transferred to a cage with virgin females. After mating, the females were transferred to a new cage; blood fed and allowed to oviposit. Resulting male and females (F1) were later investigated and found to carry the DsRed (63%) or GFP (27%) labeled bacteria. Besides the fact that this study unequivocally demonstrated paternal transmission of symbionts in An. stephensi, it also lends significant support to the possibility of using paratrangenesis with male, non-biting mosquitoes to spread anti-pathogen gene-expressing symbionts to female mosquitoes [68].
In a separate study, bacteria were tested for their ability to express anti-plasmodium molecules in mosquito midgut l [67]. Firstly, Escherichia coli expressing the anti-plasmodium molecules SM1 and phospholipase-A (PLA2) were successfully produced and able to inhibit P. berghei development in An. stephensi midguts. However, survival of the transformed bacteria was poor, and did not last more than three days after a blood meal. According to the same report [67], Enterobacter agglomerans, a bacteria associated with An. stephensi, was isolated and selected for survival in the mosquito midgut by multiple passages, increasing their survival from 2 days to two weeks [67]. As non-pathogenic, wide spread bacteria, A. agglomerans can potentially applied in paratransgenesis approaches against Plasmodium.
3.2. Transformation of mosquito viral symbionts
Symbiotic densovirus also can be genetically manipulated to express molecules to reduce vector competence. Densoviruses are linear single-stranded DNA viruses with the genome packaged in a non-enveloped particle. These viruses are suitable vectors for expression of foreign genes in mosquitoes because they are highly specific, environmentally stable, kill mosquito larvae in a dose-dependent manner, decrease lifespan of surviving adults, and are transmitted vertically [63, 64]. In Ae. aegypti, densoviruses can spread to fat body, muscles, and nerves [28] following infection through the anal papillae. Densoviruses infecting Ae. aegypti (AeDNV) and An. gambiae (AgDNV) were isolated and modified to express GFP [27, 28]. The green phenotype obtained by the expression of GFP in recombinant AgDNV-infecting An. gambiae was observed in 20% of F2 and F3 generations, suggesting that transformed densoviruses may be used to express molecules targeting pathogen development in mosquitoes [27].
3.3. Challenges and perspectives on vector paratransgenesis
Although transformation of species-specific bacteria symbionts can overcome the potential problems associated with transgene introgression and horizontal transgene transfer, only symbionts of obligatory blood-sucking insects, such as tsetse and triatomine bugs, have been transformed [21, 24]. In spite of the low number of bacteria symbionts transformed to date, a potential advantage of this approach over transgenesis is lack of fitness load [21, 66]. Species-specificity and fitness load associated with mosquito bacterial symbionts have yet to be assessed [25, 26].
Alternatively to the transformation of bacterial symbionts, genetically modified symbiotic viruses can be used against pathogen development. Densoviruses efficiently expresses heterologous proteins in An. gambiae and Ae. aegypti and are transmitted vertically [27, 63]. As demonstrated for bacterial symbionts [22, 23], viral symbionts can be engineered to express single chain antibodies (scFv), blocking pathogen development [69]. Sindbis virus expressing a scFv targeting the P. gallinaceum circunsporozoite (SZ) protein reduced Ae. aegypti salivary gland infection by 96.8% [69].
Recombinant Sindbis expressing transcripts from an infecting virus genome can reduce viral load (of the infecting virus) in mosquitoes [70, 71]. The results from Sindbis expressing transcripts from LaCrosse (LAC), dengue (DEN), or yellow fever (YF) viruses indicated a substantial interference with the replication of these viruses in Aedes triseriatus (LAC) and in Ae. aegypti (DEN and YF) [70, 72-74].
Although the use of Sindbis may not be feasible in paratransgenesis due to its reduced specificity (Sindbis is capable of infecting a broad range of insect and vertebrate species) [64, 75], scFVs or transcripts from infecting viruses can be used to transform species-specific viruses, such as densovirus.
Viral paratransgenesis also can be used to target endogenous genes involved in pathogen development, as demonstrated by Armigeres subalbatus infected with Sindbis virus transiently expressing RNA complementary to the mosquito phenoloxidase gene [76]. Phenoloxidase is one of the enzymes of the melanization cascade [77] and is upregulated upon infection. In A. subalbatus, upregulation of phenoloxidase kills infecting Dirofilaria immitis [76]. In contrast, inhibition of phenoloxydase expression by Sindbis virus expressing phenoloxydase complementary RNA, allowed for the development of D. immitis in these mosquitoes [76].
3.4. Future directions on vector paratransgenesis
Despite early successes with transformation of insect vector symbionts, it is not known if transformed symbionts can replace non-transformed in natural insect populations, and potentially affect pathogen development and transmission in natural habitats. Symbionts seem to have no fitness load on insect hosts and are capable of being transmitted vertically (via trans-ovarian transmission) or laterally (due to feeding habits). Thus, a strong gene drive system can potentiate the effectiveness of paratransgenesis. Wolbachia endosymbionts have been proposed as such gene drive system [65].
Wolbachia are intracellular, maternally inherited bacteria that manipulate reproduction of insects via cytoplasmatic incompatibility (CI) [55]. Due to the effects of CI, a Wolbachia-uninfected female will not breed with infected males, reducing the frequency of uninfected individuals and increasing the frequency of Wolbachia-infected insects in a population [55]. Thus, other maternally inherited transformed symbionts would be spread within an insect population in association with Wolbachia [65], increasing the frequency of the transformed symbiont. This mechanism has been observed in Ae. aegypti, Aedes albopictus, and Culex quinquefasciatus [55], representing a potential manner to spread transformed symbionts, such as densovirus, in natural populations of mosquitoes.
Recently, a life-shortening strain of Wolbachia (wMelPop) identified in D. melanogaster was used in Ae. aegypti and An. gambiae [26, 78]. Beyond promoting the spread of the transformed symbiont across the mosquito population (i.e., acting as a gene drive mechanism), this strain of Wolbachia would also reduce the time frame available (i.e., mosquito life span) for pathogen development within the mosquito (known as the extrinsic incubation period or EIP) [78]. However, a potential application of wMelPop to eliminate disease vectors may be faced with a significant drawback: this approach might be associated with the ability of the pathogen to overcome the reduced vector life span for its own development, thus adapting a shorter developmental time [79]. In contrast to this suggestion, Wolbachia, as well as densovirus, potentially target older mosquitoes over younger ones, and are considered evolution-proof mosquitocidal biocontrols agents [80]. For Anopheles, due to high mortality in natural conditions (from 20% to 40% per gonotrophic cycle, [81]), a selective pressure on pathogen developmental time already exists, especially in the case of Plasmodium-infected mosquitoes [80]. Thus, such selective pressure from the additional of Wolbachia is likely not strong enough to shorten the parasite life cycle within the vector.
Reduction of vector survival interferes exponentially with vectorial capacity [82, 83] as the time for pathogen development within the vector is significantly shortened. An ideal approach should reduce vector competence (linear parameter), and vector survivorship (exponential parameter). These two effects combined may significantly reduce vectorial capacity and disease burden in endemic areas. A symbiont, such as wMelPop, in combination with a genetically modified densovirus capable of inhibiting pathogen development may provide just the double-whammy needed to prevent transmission.
4. Conclusion
Here we described the current status of two molecular strategies to control vector borne diseases and highlighted many challenges facing these approaches. Several studies still need to be performed before transgenesis and paratransgenesis can be considered against vector-borne diseases. Regardless of the approach, it is clear to many investigators that new technologies must be added to current applications if we are to be successful in preventing or significantly reducing the burden of vector-borne diseases.
Acknowledgment
Dr. Yoonseong Park (Kansas State University) for critical review of the manuscript. I.V.C-A. is grateful to the Department of Entomology of Kansas State University for a graduate research assistantship. MR-O is supported by National Institutes of Health grant 7RO1AI074691-02 and by the Kansas Agricultural Experiment Station (KAES, Kansas State University). KYZ is supported by NIH P20 grant RR016475 and KAES.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hill CA, et al. Arthropod-borne diseases: vector control in the genomics era. Nat Rev Microbiol. 2005;3(3):262–8. doi: 10.1038/nrmicro1101. [DOI] [PubMed] [Google Scholar]
- 2.Wimmer EA. Innovations: applications of insect transgenesis. Nat Rev Genet. 2003;4(3):225–32. doi: 10.1038/nrg1021. [DOI] [PubMed] [Google Scholar]
- 3.Jasinskiene N, et al. Stable transformation of the yellow fever mosquito, Aedes aegypti, with the Hermes element from the housefly. Proc Natl Acad Sci U S A. 1998;95(7):3743–7. doi: 10.1073/pnas.95.7.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodrigues FG, et al. Germline transformation of Aedes fluviatilis (Diptera:Culicidae) with the piggyBac transposable element. Mem Inst Oswaldo Cruz. 2006;101(7):755–7. doi: 10.1590/s0074-02762006000700008. [DOI] [PubMed] [Google Scholar]
- 5.Coates CJ, et al. Mariner transposition and transformation of the yellow fever mosquito, Aedes aegypti. Proc Natl Acad Sci U S A. 1998;95(7):3748–51. doi: 10.1073/pnas.95.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinkerton AC, et al. Green fluorescent protein as a genetic marker in transgenic Aedes aegypti. Insect Mol Biol. 2000;9(1):1–10. doi: 10.1046/j.1365-2583.2000.00133.x. [DOI] [PubMed] [Google Scholar]
- 7.Grossman GL, et al. Germline transformation of the malaria vector, Anopheles gambiae, with the piggyBac transposable element. Insect Mol Biol. 2001;10(6):597–604. doi: 10.1046/j.0962-1075.2001.00299.x. [DOI] [PubMed] [Google Scholar]
- 8.Perera OP, Harrell IR, Handler AM. Germ-line transformation of the South American malaria vector, Anopheles albimanus, with a piggyBac/EGFP transposon vector is routine and highly efficient. Insect Mol Biol. 2002;11(4):291–7. doi: 10.1046/j.1365-2583.2002.00336.x. [DOI] [PubMed] [Google Scholar]
- 9.Nolan T, et al. piggyBac-mediated germline transformation of the malaria mosquito Anopheles stephensi using the red fluorescent protein dsRED as a selectable marker. J Biol Chem. 2002;277(11):8759–62. doi: 10.1074/jbc.C100766200. [DOI] [PubMed] [Google Scholar]
- 10.Abraham EG, et al. Driving midgut-specific expression and secretion of a foreign protein in transgenic mosquitoes with AgAper1 regulatory elements. Insect Mol Biol. 2005;14(3):271–9. doi: 10.1111/j.1365-2583.2004.00557.x. [DOI] [PubMed] [Google Scholar]
- 11.Brown AE, et al. Stable and heritable gene silencing in the malaria vector Anopheles stephensi. Nucleic Acids Res. 2003;31(15):e85. doi: 10.1093/nar/gng085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito J, et al. Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature. 2002;417(6887):452–5. doi: 10.1038/417452a. [DOI] [PubMed] [Google Scholar]
- 13.Moreira LA, et al. Bee venom phospholipase inhibits malaria parasite development in transgenic mosquitoes. J Biol Chem. 2002;277(43):40839–43. doi: 10.1074/jbc.M206647200. [DOI] [PubMed] [Google Scholar]
- 14.Kim W, et al. Ectopic expression of a cecropin transgene in the human malaria vector mosquito Anopheles gambiae (Diptera: Culicidae): effects on susceptibility to Plasmodium. J Med Entomol. 2004;41(3):447–55. doi: 10.1603/0022-2585-41.3.447. [DOI] [PubMed] [Google Scholar]
- 15.Catteruccia F, Benton JP, Crisanti A. An Anopheles transgenic sexing strain for vector control. Nat Biotechnol. 2005;23(11):1414–7. doi: 10.1038/nbt1152. [DOI] [PubMed] [Google Scholar]
- 16.Catteruccia F, et al. Stable germline transformation of the malaria mosquito Anopheles stephensi. Nature. 2000;405(6789):959–62. doi: 10.1038/35016096. [DOI] [PubMed] [Google Scholar]
- 17.Allen ML, et al. Stable, germ-line transformation of Culex quinquefasciatus (Diptera: Culicidae) J Med Entomol. 2001;38(5):701–10. doi: 10.1603/0022-2585-38.5.701. [DOI] [PubMed] [Google Scholar]
- 18.Franz AW, et al. Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proc Natl Acad Sci U S A. 2006;103(11):4198–203. doi: 10.1073/pnas.0600479103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodrigues FG, et al. Expression of a mutated phospholipase A2 in transgenic Aedes fluviatilis mosquitoes impacts Plasmodium gallinaceum development. Insect Mol Biol. 2008;17(2):175–83. doi: 10.1111/j.1365-2583.2008.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin SW, Kokoza VA, Raikhel AS. Transgenesis and reverse genetics of mosquito innate immunity. J Exp Biol. 2003;206(Pt 21):3835–43. doi: 10.1242/jeb.00640. [DOI] [PubMed] [Google Scholar]
- 21.Durvasula RV, et al. Prevention of insect-borne disease: an approach using transgenic symbiotic bacteria. Proc Natl Acad Sci U S A. 1997;94(7):3274–8. doi: 10.1073/pnas.94.7.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durvasula RV, et al. Expression of a functional antibody fragment in the gut of Rhodnius prolixus via transgenic bacterial symbiont Rhodococcus rhodnii. Med Vet Entomol. 1999;13(2):115–9. doi: 10.1046/j.1365-2915.1999.00175.x. [DOI] [PubMed] [Google Scholar]
- 23.Durvasula RV, et al. Genetic transformation of a Corynebacterial symbiont from the Chagas disease vector Triatoma infestans. Exp Parasitol. 2008;119(1):94–8. doi: 10.1016/j.exppara.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng Q, Aksoy S. Tissue tropism, transmission and expression of foreign genes in vivo in midgut symbionts of tsetse flies. Insect Mol Biol. 1999;8(1):125–32. doi: 10.1046/j.1365-2583.1999.810125.x. [DOI] [PubMed] [Google Scholar]
- 25.Favia G, et al. Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proc Natl Acad Sci U S A. 2007;104(21):9047–51. doi: 10.1073/pnas.0610451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin C, Ren X, Rasgon JL. The virulent Wolbachia strain wMelPop efficiently establishes somatic infections in the malaria vector Anopheles gambiae. Appl Environ Microbiol. 2009;75(10):3373–6. doi: 10.1128/AEM.00207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren X, Hoiczyk E, Rasgon JL. Viral paratransgenesis in the malaria vector Anopheles gambiae. PLoS Pathog. 2008;4(8):e1000135. doi: 10.1371/journal.ppat.1000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward TW, et al. Aedes aegypti transducing densovirus pathogenesis and expression in Aedes aegypti and Anopheles gambiae larvae. Insect Mol Biol. 2001;10(5):397–405. doi: 10.1046/j.0962-1075.2001.00276.x. [DOI] [PubMed] [Google Scholar]
- 29.Lobo NF, et al. Germ line transformation of the yellow fever mosquito, Aedes aegypti, mediated by transpositional insertion of a piggyBac vector. Insect Mol Biol. 2002;11(2):133–9. doi: 10.1046/j.1365-2583.2002.00317.x. [DOI] [PubMed] [Google Scholar]
- 30.Kokoza V, et al. Engineering blood meal-activated systemic immunity in the yellow fever mosquito, Aedes aegypti. Proc Natl Acad Sci U S A. 2000;97(16):9144–9. doi: 10.1073/pnas.160258197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreira LA, et al. Robust gut-specific gene expression in transgenic Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A. 2000;97(20):10895–8. doi: 10.1073/pnas.97.20.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kokoza V, et al. Efficient transformation of the yellow fever mosquito Aedes aegypti using the piggyBac transposable element vector pBac[3xP3-EGFP afm] Insect Biochem Mol Biol. 2001;31(12):1137–43. doi: 10.1016/s0965-1748(01)00120-5. [DOI] [PubMed] [Google Scholar]
- 33.Nirmala X, et al. Functional characterization of the promoter of the vitellogenin gene, AsVg1, of the malaria vector, Anopheles stephensi. Insect Biochem Mol Biol. 2006;36(9):694–700. doi: 10.1016/j.ibmb.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida S, Watanabe H. Robust salivary gland-specific transgene expression in Anopheles stephensi mosquito. Insect Mol Biol. 2006;15(4):403–10. doi: 10.1111/j.1365-2583.2006.00645.x. [DOI] [PubMed] [Google Scholar]
- 35.Chen XG, et al. The Anopheles gambiae vitellogenin gene (VGT2) promoter directs persistent accumulation of a reporter gene product in transgenic Anopheles stephensi following multiple bloodmeals. Am J Trop Med Hyg. 2007;76(6):1118–24. [PubMed] [Google Scholar]
- 36.Lombardo F, et al. An Anopheles gambiae salivary gland promoter analysis in Drosophila melanogaster and Anopheles stephensi. Insect Mol Biol. 2005;14(2):207–16. doi: 10.1111/j.1365-2583.2004.00549.x. [DOI] [PubMed] [Google Scholar]
- 37.Bian G, et al. Transgenic alteration of Toll immune pathway in the female mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 2005;102(38):13568–73. doi: 10.1073/pnas.0502815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin SW, et al. Relish-mediated immune deficiency in the transgenic mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 2003;100(5):2616–21. doi: 10.1073/pnas.0537347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coates CJ, et al. Promoter-directed expression of recombinant fire-fly luciferase in the salivary glands of Hermes-transformed Aedes aegypti. Gene. 1999;226(2):317–25. doi: 10.1016/s0378-1119(98)00557-5. [DOI] [PubMed] [Google Scholar]
- 40.Ghosh AK, Ribolla PE, Jacobs-Lorena M. Targeting Plasmodium ligands on mosquito salivary glands and midgut with a phage display peptide library. Proc Natl Acad Sci U S A. 2001;98(23):13278–81. doi: 10.1073/pnas.241491198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lycett GJ, Kafatos FC. Anti-malarial mosquitoes? Nature. 2002;417(6887):387–8. doi: 10.1038/417387a. [DOI] [PubMed] [Google Scholar]
- 42.Catteruccia F, Godfray HC, Crisanti A. Impact of genetic manipulation on the fitness of Anopheles stephensi mosquitoes. Science. 2003;299(5610):1225–7. doi: 10.1126/science.1081453. [DOI] [PubMed] [Google Scholar]
- 43.Marrelli MT, et al. Transgenic malaria-resistant mosquitoes have a fitness advantage when feeding on Plasmodium-infected blood. Proc Natl Acad Sci U S A. 2007;104(13):5580–3. doi: 10.1073/pnas.0609809104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li C, et al. Fitness of transgenic Anopheles stephensi mosquitoes expressing the SM1 peptide under the control of a vitellogenin promoter. J Hered. 2008;99(3):275–82. doi: 10.1093/jhered/esn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Irvin N, et al. Assessing fitness costs for transgenic Aedes aegypti expressing the GFP marker and transposase genes. Proc Natl Acad Sci U S A. 2004;101(3):891–6. doi: 10.1073/pnas.0305511101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hickey DA. Selfish DNA: a sexually-transmitted nuclear parasite. Genetics. 1982;101(34):519–31. doi: 10.1093/genetics/101.3-4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ribeiro JM, Kidwell MG. Transposable elements as population drive mechanisms: specification of critical parameter values. J Med Entomol. 1994;31(1):10–6. doi: 10.1093/jmedent/31.1.10. [DOI] [PubMed] [Google Scholar]
- 48.Boete C, Koella JC. A theoretical approach to predicting the success of genetic manipulation of malaria mosquitoes in malaria control. Malar J. 2002;1:3. doi: 10.1186/1475-2875-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atkinson PW. Trangenic Mosquitoes and DNA Research Safeguards. In: Marquardt WC, editor. Biology of Disease Vectors. Elservier Academic Press; Burlington: 2004. p. 785. [Google Scholar]
- 50.Adelman ZN, et al. nanos gene control DNA mediates developmentally regulated transposition in the yellow fever mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 2007;104(24):9970–5. doi: 10.1073/pnas.0701515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Brochta DA, et al. Gene vector and transposable element behavior in mosquitoes. J Exp Biol. 2003;206(Pt 21):3823–34. doi: 10.1242/jeb.00638. [DOI] [PubMed] [Google Scholar]
- 52.Lampe DJ, Grant TE, Robertson HM. Factors affecting transposition of the Himar1 mariner transposon in vitro. Genetics. 1998;149(1):179–87. doi: 10.1093/genetics/149.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Engels WR. Invasions of P elements. Genetics. 1997;145(1):11–5. doi: 10.1093/genetics/145.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fraser MJ, Smith GE, Summers MD. Acquisition of Host Cell DNA Sequences by Baculoviruses: Relationship Between Host DNA Insertions and FP Mutants of Autographa californica and Galleria mellonella Nuclear Polyhedrosis Viruses. J Virol. 1983;47(2):287–300. doi: 10.1128/jvi.47.2.287-300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinkins SP, Gould F. Gene drive systems for insect disease vectors. Nat Rev Genet. 2006;7(6):427–35. doi: 10.1038/nrg1870. [DOI] [PubMed] [Google Scholar]
- 56.Chen CH, et al. A synthetic maternal-effect selfish genetic element drives population replacement in Drosophila. Science. 2007;316(5824):597–600. doi: 10.1126/science.1138595. [DOI] [PubMed] [Google Scholar]
- 57.Marshall JM, Taylor CE. Malaria control with transgenic mosquitoes. PLoS Med. 2009;6(2):e20. doi: 10.1371/journal.pmed.1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horn C, Wimmer EA. A transgene-based, embryo-specific lethality system for insect pest management. Nat Biotechnol. 2003;21(1):64–70. doi: 10.1038/nbt769. [DOI] [PubMed] [Google Scholar]
- 59.Thomas DD, et al. Insect population control using a dominant, repressible, lethal genetic system. Science. 2000;287(5462):2474–6. doi: 10.1126/science.287.5462.2474. [DOI] [PubMed] [Google Scholar]
- 60.Krafsur ES, Whitten CJ, Novy JE. Screwworm eradication in North and Central America. Parasitol Today. 1987;3(5):131–7. doi: 10.1016/0169-4758(87)90196-7. [DOI] [PubMed] [Google Scholar]
- 61.Robinson AS, Franz G, Fisher K. Genetic sexing strains in the medfly, Ceratitis capitata: Development, mass rearing and field application. Trends in Entomology. 1999;2:81–104. [Google Scholar]
- 62.Vreysen MJ, et al. Glossina austeni (Diptera: Glossinidae) eradicated on the island of Unguja, Zanzibar, using the sterile insect technique. J Econ Entomol. 2000;93(1):123–35. doi: 10.1603/0022-0493-93.1.123. [DOI] [PubMed] [Google Scholar]
- 63.Carlson J, Suchman E, Buchatsky L. Densoviruses for control and genetic manipulation of mosquitoes. Adv Virus Res. 2006;68:361–92. doi: 10.1016/S0065-3527(06)68010-X. [DOI] [PubMed] [Google Scholar]
- 64.Carlson J, et al. Molecular genetic manipulation of mosquito vectors. Annu Rev Entomol. 1995;40:359–88. doi: 10.1146/annurev.en.40.010195.002043. [DOI] [PubMed] [Google Scholar]
- 65.Aksoy S, Weiss B, Attardo G. Paratransgenesis applied for control of tsetse transmitted sleeping sickness. Adv Exp Med Biol. 2008;627:35–48. doi: 10.1007/978-0-387-78225-6_3. [DOI] [PubMed] [Google Scholar]
- 66.Weiss BL, et al. Interspecific transfer of bacterial endosymbionts between tsetse fly species: infection establishment and effect on host fitness. Appl Environ Microbiol. 2006;72(11):7013–21. doi: 10.1128/AEM.01507-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riehle MA, et al. Using bacteria to express and display anti-Plasmodium molecules in the mosquito midgut. Int J Parasitol. 2007;37(6):595–603. doi: 10.1016/j.ijpara.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 68.Damiani C, et al. Paternal transmission of symbiotic bacteria in malaria vectors. Curr Biol. 2008;18(23):R1087–8. doi: 10.1016/j.cub.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 69.de Lara Capurro M, et al. Virus-expressed, recombinant single-chain antibody blocks sporozoite infection of salivary glands in Plasmodium gallinaceum-infected Aedes aegypti. Am J Trop Med Hyg. 2000;62(4):427–33. doi: 10.4269/ajtmh.2000.62.427. [DOI] [PubMed] [Google Scholar]
- 70.Powers AM, et al. Molecularly engineered resistance to California serogroup virus replication in mosquito cells and mosquitoes. Proc Natl Acad Sci U S A. 1996;93(9):4187–91. doi: 10.1073/pnas.93.9.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Travanty EA, et al. Using RNA interference to develop dengue virus resistance in genetically modified Aedes aegypti. Insect Biochem Mol Biol. 2004;34(7):607–13. doi: 10.1016/j.ibmb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 72.Adelman ZN, et al. Sindbis virus-induced silencing of dengue viruses in mosquitoes. Insect Mol Biol. 2001;10(3):265–73. doi: 10.1046/j.1365-2583.2001.00267.x. [DOI] [PubMed] [Google Scholar]
- 73.Higgs S, et al. Engineered resistance in Aedes aegypti to a West African and a South American strain of yellow fever virus. Am J Trop Med Hyg. 1998;58(5):663–70. doi: 10.4269/ajtmh.1998.58.663. [DOI] [PubMed] [Google Scholar]
- 74.Olson KE, et al. Genetically engineered resistance to dengue-2 virus transmission in mosquitoes. Science. 1996;272(5263):884–6. doi: 10.1126/science.272.5263.884. [DOI] [PubMed] [Google Scholar]
- 75.Blair CD, Adelman ZN, Olson KE. Molecular strategies for interrupting arthropod-borne virus transmission by mosquitoes. Clin Microbiol Rev. 2000;13(4):651–61. doi: 10.1128/cmr.13.4.651-661.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shiao SH, et al. Effect of prophenoloxidase expression knockout on the melanization of microfilariae in the mosquito Armigeres subalbatus. Insect Mol Biol. 2001;10(4):315–21. doi: 10.1046/j.0962-1075.2001.00268.x. [DOI] [PubMed] [Google Scholar]
- 77.Barrillas-Mury C, Paskewitz S, Kanost MR. Immune response of vectors. In: Marquardt WC, editor. Biology of Disease Vectors. Elservier Academic Press; Burlington: 2005. p. 785. [Google Scholar]
- 78.McMeniman CJ, et al. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 2009;323(5910):141–4. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- 79.Read AF, Thomas MB. Microbiology. Mosquitoes cut short. Science. 2009;323(5910):51–2. doi: 10.1126/science.1168659. [DOI] [PubMed] [Google Scholar]
- 80.Read AF, Lynch PA, Thomas MB. How to make evolution-proof insecticides for malaria control. PLoS Biol. 2009;7(4):e1000058. doi: 10.1371/journal.pbio.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Killeen GF, et al. A simplified model for predicting malaria entomologic inoculation rates based on entomologic and parasitologic parameters relevant to control. Am J Trop Med Hyg. 2000;62(5):535–44. doi: 10.4269/ajtmh.2000.62.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Black WC, Moore CG. Population biology as a tool to study vector-borne diseases. In: Marquardt WC, editor. Biology of disease vectors. Elservier Academic Press; Burlington: 2004. pp. 187–206. IV. [Google Scholar]
- 83.Garrett-Jones C. Prognosis for Interruption of Malaria Transmission through Assessment of the Mosquito’s Vectorial Capacity. Nature. 1964;204:1173–5. doi: 10.1038/2041173a0. [DOI] [PubMed] [Google Scholar]


