Abstract
Go is the most abundant G protein in the central nervous system, where it comprises about 1% of membrane protein in mammalian brains. It functions to couple cell surface receptors to intercellular effectors, which is a critical process for cells to receive, interpret and respond to extracellular signals. Go protein belongs to the pertussis toxin-sensitive Gi/Go subfamily of G proteins. A number of G-protein-coupled receptors transmit stimuli to intercellular effectors through Go. Go regulates several cellular effectors, including ion channels, enzymes, and even small GTPases to modulate cellular function. This review summarizes some of the advances in Go research and proposes areas to be further addressed in exploring the functional role of Go.
Key Words: G protein, Go, G-protein-coupled receptors, Signal transduction, Effectors, Central nervous system, Alzheimer's disease
Introduction
In the late 1950s and early 1960s, Earl Sutherland discovered that cyclic AMP (cAMP) was a second messenger in hormone signaling while studying how epinephrine, a stress hormone, mobilized blood glucose by degrading glycogen to glucose in the liver. Epinephrine increased the accumulation of cAMP in cells by stimulating adenylyl cyclase. These findings opened the door for research on the mechanisms that control hormone action. In the early 1970s, Martin Rodbell and Lutz Birnbaumer [1] demonstrated that guanosine nucleotide triphosphate (GTP) is required for stimulation of adenylyl cyclase activity in adipocyte membrane preparations. Further investigation revealed that GTP affects hormone receptor-binding properties. The discovery of a GTP requirement for transducing extracellular signals into cells provided the basis for the existence of GTP-binding G proteins (formally referred to as N proteins for nucleotide-binding protein). In the late 1970s, Alfred Gilman and associates [2, 3] isolated and identified the first GTP-binding protein, Gs (stimulatory regulator of adenylyl cyclase G protein) and provided direct evidence that the GTP-binding protein acts as a signaling molecule in hormone signaling. Meanwhile, another GTP-binding protein, transducin (Gt), was identified as a signal transducer in bovine retina [4]. Transducins, abundant in the outer retinal segments, couple rhodopsin to cyclic GMP-dependent phosphodiesterase and transmit incoming photons into chemical signals.
The use of two bacterial toxins has greatly facilitated the identification and characterization of G proteins. Cholera toxin, from Vibrio cholerae bacteria, mediates ADP ribosylation of the Gs protein and was used in the early characterization and purification of Gs [5,6,7]. Bordetellapertussis toxin (PTX), discovered as the islet-activating protein, mediates the ADP ribosylation of Gi proteins (which inhibit adenylyl cyclase activity) [8, 9]. Treatment of cells or membranes with PTX results in the loss of adenylyl cyclase inhibition. Adenylyl cyclase inhibitory G proteins, Gi proteins, were first purified and identified by the Gilman and Birnbaumer groups independently [10, 11]. Go protein, the fourth member of the G protein family, was discovered during purification of Gi from the bovine brain. Go was named as the ‘other’ GTP-binding protein after Gs, Gi, and transducin [12,13,14]. The brain contains abundant Go protein as demonstrated by PTX-mediated ADP-ribosylation labeling. The first partial cDNA clone of Go was isolated by screening a rat glioma cDNA library for Gi cDNA using oligonucleotide probes derived from the Gi peptide sequence [15]. Since these initial findings, Go has been identified as the most abundant brain G protein and has received considerable attention to determine its physiological role in the body.
Biophysical Properties, Distribution, and Expression
Abundant Expression in Brain Tissue
Go protein was discovered serendipitously during attempts to purify Gi protein from the brain. GTPγS-binding and ADP-ribosylation assays were well established for purifying and characterizing the Gs and Gi proteins. A high level of GTPγS-binding activity was observed in brain preparations from two species and was severalfold higher than in other tissues. PTX-mediated ADP ribosylation and electrophoresis of brain samples revealed the abundant presence of a 39-kDa protein in addition to the 41-kDa Giα subunit. Go contains three subunits with molecular weights of 39-kDa (α subunit), 36-kDa (β subunit), and 10-kDa (γ subunit). Its βγ complex appears indistinguishable from that of Gi's. Go is 5- to 10-fold more abundant than other Gi proteins in the brain and accounts for an estimated 0.5–1% of total membrane protein. Like Gi, Go is a substrate of PTX ADP ribosylation. PTX-mediated ADP ribosylation requires the integrity of the heterotrimeric complex as the βγ subunit is needed for ADP ribosylation of the α subunit.
Affinity to Guanine Nucleotides and GTPase Activity
Study of purified Gi/o proteins from bovine brain revealed that the GDP/Go protein ratio is around 0.9 while the GDP/Gi protein ratio is 0.6 [16]. The GDP/G protein ratio may reflect the stability of the individual G proteins and it suggests that the Go-GDP complex is relatively more stable than the Gi-GDP complex. GDP-GTP exchange is critical for the activation of G protein signaling, while the dissociation of GDP from the α subunit is the rate-limiting step [3]. Binding studies have revealed that the kinetics of GTPγS association with either the heterotrimeric Go or Goα subunit is rapid. The rate of dissociation of GDP from Go as well as the association of GTPγS with Go are about 3 times faster than those with Gi protein. In addition, the intrinsic GTPase activity of Go is about 3–7 times higher than that of Gi. The GTPase of the α subunit hydrolyzes GTP to GDP, which converts G proteins from an active to an inactive state, and subsequently controls signal duration. Taken together, Go is a highly effective molecular signaling transducer (i.e. a fast on-off switch). How this physical property correlates to functional signaling has not been elucidated.
Many protein factors are involved in signal transduction through G proteins to regulate the strength, duration, efficiency, and specificity of signaling. These factors can be classified into two major groups based on their effect on G protein activity: either influencing the rate of guanine nucleotide exchange or the rate of hydrolysis of bound GTP. Guanine nucleotide exchange factors (GEF) modulate the guanine nucleotide exchange rate, while GTPaseactivating proteins (GAP), and regulators of G-protein signaling (RGS) influence the rate of GTP hydrolysis by the Gα subunit. Together they facilitate the ‘on-off’ switch of the G protein signal.
G-protein-coupled receptors (GPCRs) are widely recognized as GEFs for heterotrimeric G proteins. Many receptors have been identified to couple to Go protein. In addition to coupled receptors, several non-receptor proteins have been shown to influence nucleotide binding by the Goα subunit, including the growth cone-associated protein with molecular weight of 43 kDa (GAP-43), amyloid precursor protein (APP), and Presenilin I. GAP-43 was originally identified as an axonally transported protein. It is expressed at high levels in the developing nervous system of vertebrates and is localized in the inner surface of growth cone membranes. GAP-43 binds to and interacts with Goα, where it increases the rate of GDP dissociation and GTP association as was demonstrated with purified brain heterotrimeric G proteins as well as with purified Goα subunits [17, 18]. PTX does not alter the activation of Go protein by GAP-43. Activator of G-protein signaling (AGS) is another GEF involved in regulating Go-mediated signaling. AGS was identified using a modified yeast pheromone response pathway which is mediated by G protein [19]. In addition, AGS binds to the Goα subunit in protein-binding assays. In vitro, AGS greatly enhances GTPγS binding to purified Go/Gi proteins [20]. The paralog of human AGS1 in mice, Dexras1, was identified by rapid mRNA upregulation in AtT-20 corticotrophin cells after glucocorticoid treatment [21]. Overexpression of a constitutively active form of Dexras1/AGS1 greatly inhibits peptide hormone release from corticotrophin cells. The removal of the C-terminal prenylation site of Dexras1/AGS1 diminishes these inhibitory effects [22]. This implicates AGS as a GEF for Gi/o proteins, adding another mechanism that can modulate Go signaling.
In addition to the enhancing modulators of guanine nucleotide exchange, protein factors that decrease the guanine nucleotide exchange rate have also been identified. These are known as the GRP (G protein regulatory) or GoLoco motifs and can bind to Goα and stabilize it in the GDP-bound conformation while simultaneously competing with Gβγ for Gα binding [23, 24]. RGS proteins, on the other hand, are responsible for the rapid deactivation of receptor-stimulated G protein signals. RGS proteins negatively regulate G protein signaling by accelerating α subunit GTPase activity, physically blocking G protein effector binding and altering the level of free βγ subunits available to their downstream effectors [25]. The RGS4 protein and the Gα-interacting protein (GAIP) can selectively accelerate the deactivation of Gi and Go protein, respectively [26]. When the N-terminus of GAIP was deleted, the selectivity towards Goα was lost [27]. Recombinant RGS1, RGS16, and GAIP can stimulate the GTPase activity of Go1α following its activation by an agonist of the α2A-adrenoreceptor [28]. These studies demonstrate that the duration and specificity of receptor-stimulated Go protein signaling is also regulated by RGS proteins.
Conserved across Many Species
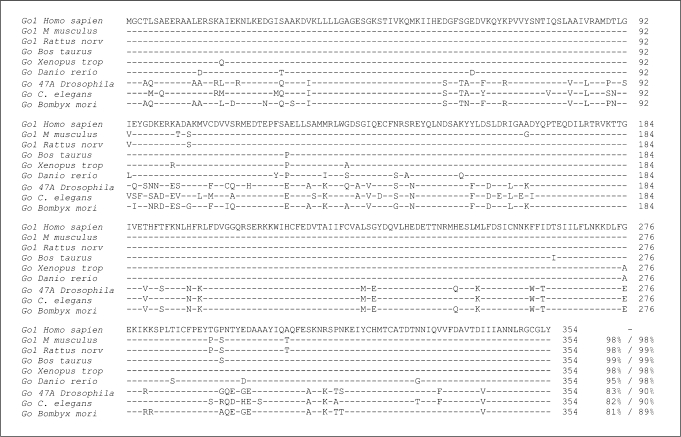
After the partial cDNA sequence for rat Go was first reported [15], cDNAs encoding Goα have been cloned and identified in many species, including human [29], rat [30], mouse [31], hamster [32], bovine [33], zebra fish [34], Xenopus[35], Drosophila[36,37,38], nematode [39] and silkmoth [40]. Goα is highly conserved across species, including fruit flies and Caenorhabditis elegans which share more than 80% identity to human Go (fig. 1). This high degree of similarity in protein sequence suggests that Go-mediated signaling pathways are critical for organisms in receiving, integrating, and executing the transduction of extracellular stimuli across species.
Fig. 1.
Protein sequence alignment of the human Gi/o family. Gly2/Ser6 motif for myristoylation and Cys3 for palmitoylation (bold), GTP-binding motif (GAGESGKS), Mg2+ -binding domain (DVGG), guanine ring-binding motif (NKKD), cysteine (∗) (at −4 from carboxyl terminal) for PTX ADP ribosylation on Gi/o proteins. Ggust is gustducin α subunit (gnat3). Gt sequence from cone transducin. Percentage of identity/homology. Spaces for alignment.
Two Isoforms, Generated via Alternative Splicing
The abundance of Go protein in the brain made it easier to purify, allowing biochemical characterization of the α subunit in functional reconstitution experiments with receptors. However, during the course of purification, heterogeneity of the Goα subunit samples was observed. A novel and less abundant form of Goα was observed and purified with the 39-kDa Goα [41]. In situ peptide mapping revealed sequence differences between each isoform [42]. This heterogeneity of the Goα subunit was also observed in membrane preparations from canine cardiac sarcolemma [43]. cDNA clones encoding both Goα forms were subsequently identified from both a HIT (hamster insulin-secreting tumor) cell cDNA library [32] and a mouse brain cDNA library [44]. The deduced amino acid sequences of the cDNAs revealed that the first two-thirds of the N-terminal residues (248 of 354 amino acids) of Gαo1 (GoAα) and Gαo2 (GoBα) are identical. Of the remaining C-terminal residues (116 out of 354), the two forms differ by only 25 or 26 amino acids (25 in mouse, 26 in hamster and 20 in human). At the level of nucleotide sequence, Gαo1 (GoAα) and Gαo2 (GoBα) are identical up to codon 242 and differ thereafter, including the 3′ untranslated region. The chromosomal gene for human Goα was identified later and analysis of genomic clones revealed that the human Goα gene spans more than 100 kb on chromosome 16q13 and contains 11 exons [45]. Gαo1 and Gαo2 are products of alternative splicing using different sets of exons for the carboxyl terminal and 3′ untranslated region. The two isoforms share the first 6 exons that encode the 241 N-terminal amino acids, and each isoform has unique exons 7, 8, and 9.
Member of the Gi/o Subfamily
Since the discovery of the first four G proteins (Gs, Gi, Gt and Go), numerous genes encoding additional G proteins have been cloned and identified based on nucleic acid sequence homology with the previously characterized G proteins. 17 mammalian G proteins have been identified in humans, and they can be classified into four subfamilies – Gs, Gi/o, Gq/11, and G12/13 – according to their sequence similarity and the functional relevance of their coupled effectors. The Gi/o subfamily contains two Go members, three Gi, Gz, two transducins (rod and cone), and gustducin. All members in the subfamily, except Gz, are substrates of PTX, which mediates ADP ribosylation at the carboxyl terminal cysteine residue (–4 position). As shown in figure 2, all members in the Gi/o family are highly homologous with more than 60% identity and greater than 70% similarity, especially among the non-sensory, PTX-sensitive members (Gi and Go). The three Giα and two Goα subunits display extensive homology in their primary sequence and are functionally similar in vitro, though they differ both in cellular and subcellular distribution. The high similarity in the protein sequence raised the question whether the individual proteins function distinctively to transmit information between different receptors and effectors or whether they are simply isoforms of one another. The similarity in the primary sequence among Gi/o subunits suggests that they may be functionally coupled to the same (or similar) receptors or may signal to the same effectors. However, the divergence in primary sequence and their unique patterns of tissue distribution strongly suggest that each member may contribute distinctive biological functions in the body. Each gene encoding an α subunit in the Gi/o family has been knocked out in mice [46], and studies of these animals reveal that each knockout has unique deficiencies or abnormalities. These knockout mouse studies support the notion that each member mediates distinctive biological functions in the body.
Fig. 2.
Protein sequence alignment of Go protein across species. Percentage of identity/homology.
Lipid Modification
G proteins, unlike their coupled receptors, are not transmembrane polypeptides. Therefore, mechanisms that bring G proteins close to receptors at the plasma membrane are critical for G protein signaling. Lipid modification and association with βγ subunits are the generally accepted mechanisms for anchoring the α subunit to the plasma membrane. Lipid modification has been observed in both the α and γ subunits of G proteins with N-myristoylation and/or palmitoylation on the α subunits and prenylation on γ subunits. Go protein purified from brain tissue is myristoylated [47]. The glycine at the N-terminus (G2) of G protein is the residue for myristoylation. Substitution of this glycine with an alanine residue (G2A) eliminated incorporation of myristate and resulted in cytosolic retention and no plasma membrane localization of the G protein [48, 49]. Furthermore, the constitutively active form of Gi2α is oncogenic with transforming activity that is sensitive to lipid modification. The non-myristoylated G2A mutation of this active form is incapable of transforming Rat 1a fibroblasts [50]. This emphasizes that lipid modification of Gα is important for anchoring G protein to the plasma membrane as well as maintaining its signaling capabilities.
In addition to myristoylation, Go and Gi α subunits are also palmitoylated [51], although myristoylation is a prerequisite to palmitoylation [52]. Palmitoylation of Goα is dynamic and highly regulated. The addition of the agonist adrenaline dramatically reduced palmitoylation of Goα1 in a fusion protein with the α2A-adrenoceptor. The reduction in palmitoylation is concentration-dependent and correlates with the occupancy of the ligand-binding site [53]. This suggests that palmitoylation is directly associated with agonist stimulation and may be involved in regulating signals. In this manner, dynamic palmitoylation could provide both temporal and spatial regulation of G protein signaling. Questions regarding how palmitoylation is regulated, whether de-palmitoylation is critical for permitting Goα-specific protein interactions and subcellular localization, and how it correlates with Go-mediated signaling need to be addressed.
The Gβγ subunits not only act as a signaling moiety, but also play an important role in controlling α subunit signaling. The binding of βγ has two functions in Goα signaling: it constitutes an anchorage point on the plasma membrane and assists in coupling the G protein to the proper receptors. Interestingly, the docking site of the Gβγ subunit is also in the N-terminus of the Gα subunit, adjacent to the myristoylation and palmitoylation sites [54]. Palmitoylation, association with Gβγ, and retention of Goα on the plasma membrane act synergistically to maintain proper G protein localization for signaling [55].
Deamidation of Go, GαoC
An additional isoform of Goα in bovine brain, GαoC (or Go3), was observed during purification and characterization of Gi/Go proteins [56]. In the mammalian genome, only one gene encodes Goα, and alternative splicing generates two mRNA transcripts, Gαo1 and Gαo2. Mass spectrometry sequencing revealed that GαoC is identical to Gαo1, except for a single difference at the carboxyl terminus [57]. GαoC differs from Gαo1 by substitution of an Asn347 residue with Asp near the C-terminus in a region involved in receptor activation [58, 59]. The substitution is a result of a posttranslational modification, deamidation of Asn to Asp. This modification may change the signaling properties since the region is critical for receptor discrimination and binding as well as guanine nucleotide binding. GαoC is widely distributed throughout the brain, and its prevalence is estimated at more than 30% of Go1 protein [60]. The modification might be important for the regulation of Go-mediated signaling, but the biological relevance and possible regulatory mechanisms remain unclear.
Go Expression Pattern
Go has a less ubiquitous expression pattern than Gi or Gs. Go, originally identified from bovine brain tissue, was estimated to compose around 0.5–1% of all brain membrane proteins. As expected Go is widely expressed in the central nervous system (CNS), including the retina and the olfactory bulb [61,62,63] as well as in the peripheral nervous system, including the superior cervical ganglia and sciatic nerve [64]. In addition, Go expression has been observed in heart tissue [61,65,66,67,68] and in the endocrine system, including the pituitary gland and pancreatic islets. Go is expressed widely in the brain, however the regional distribution of Go protein is not homogeneous. High densities of Go protein have been observed in the frontal cortex, cerebellum, hypothalamus, hippocampus, and substantia nigra [69]. At the cellular level, high concentrations of Go protein were detected in synaptic-rich neuropils, such as the molecular layer of the cerebral cortex, neuropil of hippocampus, and posterior pituitary. Go was mainly distributed in cell bodies and neuritic cytoplasm as demonstrated in ultrastructural localization studies. Go protein was principally found on the cytoplasmic face of the plasma membrane lining the cell body and the neurites, especially at ‘cell-to-cell’ contacts. Go was also detected in the cytoplasmic matrix, between the endoplasmic reticulum and Golgi cisternae [70]. These findings strongly suggest that the Go protein is involved in transducing chemical signals at the synaptic level and likely modulates synaptic functions by controlling the activity of effectors localized away from the synaptic membrane.
Protein Stability and Expression during Development
Changes in Goα protein expression were observed in the chick heart during embryonic development. A muscarinic agonist had little or no effect on heart rate 2.5 days in ovo, even though muscarinic receptors are already present on the cell membrane as measured by ligand binding of a muscarinic antagonist [71]. The expression level of Goα protein doubled in 1 day (day 3.5) and doubled again by the end of day 10 in ovo [65]. The muscarinic effect on adenylyl cyclase activity increased as a function of the Goα protein expression level. This developmental regulation of Goα protein was also observed in mammals [66, 72, 73]. In the feline heart, the concentration of Goα increases just after birth, remains elevated until postnatal day 10, and then gradually decreases [66]. Goα is detected in both atria and ventricles of rabbit, and interestingly, the concentration of Goα is higher in atria than in the ventricles [72]. Go1α is predominant in atrial samples, and there is no detectable Go2α mRNA in atria or ventricles [73]. However, the developmental pattern of Goα protein expression in atria does not match absolutely with the pattern of mRNA expression.
Studies of neuroblastoma × glioma hybrid cells (NG108-15) demonstrated that Go protein expression undergoes a dynamic change that is dependent on the stage of differentiation [74]. Very little Go was detected in membrane preparations from undifferentiated NG108-15 cells, while high levels of Go were observed after cells were induced to differentiate. Comparison of the expression levels of Go proteins in undifferentiated and differentiated neuroblastoma cells N1E-115 as well as in primary cultures of cerebellar granule cells, revealed that Go2 predominated in Go expression in undifferentiated neuroblastoma cells, whereas both isoforms, Go1 and Go2 were expressed in differentiated cells. Moreover, Go1 is predominately expressed in well-differentiated cerebellar granule cells [75]. Studies of Goα turnover using pulse-chase labeling in neuroblastoma cells showed that the half-life of total Go protein increased from 28 to 58 h when neuroblastoma cells were differentiated. The half-life of Go reached 154 h in primary cultures of cerebellar granule cells. Each isoform of Goα exhibited similar degradation rates in the same cells at varying stages of differentiation; however, the rate of each isoform's synthesis varies in those cells and at different stages of differentiation [76]. This suggests that the rate of degradation of the Go protein is not a characteristic of the protein itself but rather dependent on the state of cell differentiation. The synthesis rate of Goα1 is increased in differentiated cells while the rate for Goα2 is decreased. The mechanisms that control differential expression of each isoform, including the regulation of the alternative splicing that generates both isoforms, require further study.
Receptors Couple to Effectors through Go
Heptahelical GPCRs represent the largest family of transmembrane receptors. GPCRs constitute the most common targets for therapeutic drugs and many of these receptors are coupled to their effectors through Go or Gi proteins. The human genome project revealed that there are >400 GPCR genes (excluding genes for odorant receptors) in the human genome [77]. Go has been shown to couple to many receptors, including muscarinic cholinergic receptors, GABAb receptors, α2-adrenergic receptors, and somatostatin receptors. In earlier studies, receptor coupling to Go was identified by receptor- G-protein reconstitution, which assays the receptor ligand(s)-binding properties, the agonists stimulating GTPase activity, and those inhibiting adenylyl cyclase activity. Addition of Go proteins to a muscarinic receptor preparation enhanced the agonist's affinity to the receptors by at least 10- to 20-fold, and low concentrations of guanine nucleotides specifically reversed this effect [78, 79]. This result suggested that muscarinic receptors are coupled to their effectors by the Go protein. Similar experiments demonstrated that the μ-opioid receptor is coupled to both Gi and Go [80]. In reconstitution experiments, purified Go proteins exhibit a similar efficiency as purified Gi proteins for coupling with most receptors tested. Reconstitution is a useful in vitro assay to demonstrate receptor-G-protein coupling, however, in vivo assays in cells or whole organisms (e.g. gene knockout animals) are required to verify receptor-G protein functional coupling. PTX catalyzes the ADP ribosylation of Gi/Go α subunits and hence uncouples Go and Gi from their receptors, consequently disrupting Gi/Go signaling. Many receptors have been identified as Go or Gi coupled by PTX uncoupling, though to which member in this family they couple to could not be discriminated.
Somatostatin is a peptide hormone produced in the hypothalamus and peripheral endocrine δ cells. It can reduce voltage-dependent Ca2+ currents and lower intracellular free Ca2+ in pituitary cells in a reversible manner. Treatment of pituitary cells with PTX abolished the action of somatostatin on both Ca2+ currents and intracellular free Ca2+. Intracellular application of GTPγS changed the effects of somatostatin from reversible to irreversible inhibition. Intracellular cAMP clamped at 100 mM in cells, a concentration two magnitudes greater than is needed for maximal activation of PKA, had no effect on either the action of somatostatin on Ca2+ currents or effect of GTPγS on the inhibition of Ca2+ currents by somatostatin [81]. This suggests that Go and/or Gi proteins are directly involved in cAMP-independent somatostatin receptor-mediated inhibition of voltage-dependent Ca2+ channels in endocrine cells. Furthermore, immunoprecipitation with anti-Go, -Gi1, and -Gi3 antibodies can precipitate the somatostatin receptor complex along with the G protein, suggesting that Go, Gi1, and Gi3 couple to somatostatin receptors [82].
Like somatostatin inhibition of voltage-dependent Ca2+ currents, opioid peptides and opiates inhibit neurotransmitter release, which is a Ca2+-dependent, PTX-sensitive process. Pretreatment of neuroblastoma-glioma hybrid cells with PTX completely abolishes opioid inhibitory effects on the Ca2+ current. This inhibitory effect is restored by intracellular application of Gi and Go. However, Go (with or without the βγ complex) is 10 times more potent than Gi protein in this restoration [83]. In dorsal root ganglion neurons, reconstitution experiments demonstrate that the Go protein also couples neuropeptide Y (NPY) receptors to Ca2+ channels [84]. Microinjection of antibodies and antisense oligonucleotides against G protein subunits can specifically downregulate or block G protein-signaling pathways in cells. This approach has been used in combination with functional assays, such as electrophysiological recording of Ca2+ currents, to identify receptors that are coupled to Go. Acetylcholine M4 muscarinic receptors couple to Go1 (α1β3γ4), while somatostatin receptors couple to Go2 (α2β1γ3) as demonstrated by microinjection of antisense oligonucleotides into GH3 cells [85,86,87].
Recently, animals lacking both Go protein isoforms have been generated [88,89,90]. Studies of receptor signaling in tissues and cells derived from the Go knockout animals have provided direct genetic evidence to verify receptor coupling. Go couples opioid receptors to N-type Ca2+ channels in dorsal root ganglion neurons [89]. In addition, M2 muscarinic receptors have been shown to regulate L-type Ca2+ currents in ventricular cardiac myocytes through Go [90]. In order to test receptor-G protein coupling in the native CNS environment, agonists of several Go/Gi-coupled receptors have been tested for their ability to enhance GTPγS binding in mouse brain sections from Go knockout and control animals. Studies have demonstrated that many receptors, including dopamine, serotonin, opioid, glutamine, and muscarinic receptors, couple effectively to their effectors through the Go protein in the CNS. Furthermore, dopamine D2 receptor populations achieve their high-affinity conformational state by coupling to G proteins, and they predominantly couple to Go proteins in the brain [46].
Go-Mediated Signaling Transduction
The signaling transduction mechanism of Go protein and its intracellular effectors have received intensive scrutiny. Go proteins, comprised of the α subunit and its βγ moiety, regulate several intercellular effectors in their functional signaling pathways.
Calcium Channels
The inhibitory neurotransmitters, such as 5-HT, GABA, and noradrenaline, which reduce the duration of action potentials in dorsal root ganglion neurons, were identified before the discovery of Gi and Go proteins [91]. The reduction in action potential duration was demonstrated as result of the inhibition of voltage-gated Ca2+ channels by these neurotransmitters [92]. Since then, it has become evident that numerous hormones or neurotransmitters, including somatostatin and muscarinic agonists, suppress Ca2+ channel currents. Treatment of dorsal root ganglion neurons with PTX blocks noradrenaline and GABA-mediated inhibitory effects on Ca2+ channels. GTPγS irreversibly potentiates, while GDPβS blocks the agonist-mediated inhibitory effects on Ca2+ channels [93, 94]. These experiments demonstrated that G proteins, specifically PTX-sensitive Go or Gi subtypes, could modulate neuronal Ca2+ channel activity. Subsequently, many hormones and neurotransmitters acting on inhibitory receptors – including muscarinic acetylcholine, opioid, somatostatin, NPY, prostaglandin, adrenergic, and adenosine receptors – have been shown to inhibit Ca2+ channels through the PTX-sensitive Go/Gi G protein subtypes [95]. In rat dorsal root ganglion neurons, NPY effectively suppressed the N-type Ca2+ currents, while PTX treatment could completely abolish this inhibition. Addition of purified bovine Goα subunits effectively restored the NPY modulation of Ca2+ currents while purified Gi1α or Gi2α had little or no effect [96]. Interestingly, the bradykinin inhibitory effects on Ca2+ currents in PTX-treated cells was only partially restored by the addition of either purified Goα or Gi2α, even at high concentrations. Complete restoration required the combination of both Goα and Gi2α proteins. This suggests that Go and Gi2 proteins may couple to distinctive subpopulations of bradykinin receptors since Go nor Gi2 protein alone cannot compensate the loss of the other. The injection of anti-Goα antisense oligonucleotides into rat dorsal root ganglion neurons or into the pituitary neurosecretory GH3 cell line reduced the Goα protein levels and diminished Ca2+ channel inhibition by GABA, somatostatin, and carbachol [85, 97]. In addition, injections of anti-Goα antibodies into rat sympathetic neurons reduced inhibition mediated by noradrenaline [98]. In mouse dorsal root ganglion neurons, opioids function through their receptors to suppress N-type Ca2+ channels. Neurons lacking Go protein lose opioid inhibitory effects [89]. In ventricular cardiac myocytes, carbachol acts as a M2 muscarinic receptor agonist, thereby inhibiting L-type Ca2+ currents. This muscarinic inhibition of Ca2+ currents is completely missing in myocytes isolated from Goα gene knockout mice [90]. These findings suggest that the voltage-gated Ca2+ channel is an intracellular effector of the Go-signaling pathway.
Potassium Channels
The K+ channel on the plasma membrane is also one of the intracellular effectors of Go-mediated signaling. This has been demonstrated in several systems by electrophysiological studies. An inward rectifying K+ channel was first detected in frog skeletal muscles in 1949. Subsequently, its presence has been demonstrated in various cell types including egg, heart muscle, neurons in vertebrates as well as invertebrates [99]. In vertebrate cells, most of the resting membrane conductance has been attributed to inward K+ channel activity. Owing to its physiological significance, the regulation of K+ currents in heart muscle preparations and neurons received exhaustive attention. Binding of agonists to cardiac muscarinic acetylcholine and adenosine receptors slows pacemaker activity by activating K+ channels. The K+ currents in embryonic chick atrial cells were stimulated by acetylcholine as shown by electrophysiological recordings [100]. Atropine, an antagonist of muscarinic receptors, effectively prevented the induction of K+ conductance by acetylcholine. This suggests acetylcholine action was receptor-mediated. In fact, the acetylcholine-induced K+ current is GTP-dependent. Without GTP in the pipettes, cells did not show any response to acetylcholine. Cells pretreated with PTX lost acetylcholine-potentiated K+ currents. Similar mechanisms have been found in neural cells. In hippocampal pyramidal cells, serotonin and the selective GABAb agonist baclofen hyperpolarize cells by increasing K+ channel conductance. The 5-HT and baclofen responses are completely ablated in PTX-treated cells. Addition of a stable form of GDP, GDPβS, to cells reduced responses to 5-HT and baclofen, while addition of GTPγS, a non-hydrolysable GTP which binds to and activates G proteins, mimicked the action of 5-HT and baclofen. This suggests that Go and Gi proteins mediate the effects of serotonin and GABAb receptor signaling on K+ channels in hippocampal pyramidal cells [101]. Since Go is the predominant form of the PTX-sensitive G proteins in the brain, Go protein regulation of K+ channels would be logical. A reconstitution assay combined with single channel recording experiments provided convincing evidence for this assumption [102]. Both purified bovine brain Go proteins as well as a recombinant form of Goα proteins activated K+ channels in membrane patches excised from hippocampal pyramidal cells.
Many receptors including the somatostatin receptor, purinergic receptor, opioid receptor, and 5-HT receptor have been identified in heart muscle tissue and in peripheral and central neurons that activate K+ channels through Go and/or Gi proteins. Ligand receptor binding activates heterotrimeric G proteins which exchange GDP for GTP on the Gα subunit. The subsequent dissociation of the trimeric complex releases the Gβγ subunit and the Gα-GTP complex, which are both capable of signaling to downstream effectors. Purified and recombinant Gβγ subunits can activate K+ channels when applied to the cytosolic side of excised K+ channel-containing membrane patches [103,104,105]. The binding of Gβγ subunits at the N- and C-termini of K+ channels may be the mechanism of ligand-gating [106]. It seems that both Goα and Gβγ subunits can activate K+ channels. However, Gβγ subunits have less specificity for activating K+ channels; different combinations of the β and γ subunits can activate K+ channel almost equipotently [107]. It appears that the Goα subunit may act as a chaperone for Gβγ subunit receptor-targeting and K+ channel specificity. Expression of Gi1 or Gi3 α subunits in Xenopus oocytes reduced excessive G-protein-regulated inwardly rectifying K+ (GIRK) activity. The α subunits can also physically bind to GIRK channels [108], suggesting that the α subunits of Go/i may also be involved in channel gating. The exact mechanism by which Go proteins gate K+ channels in cardiac atrial cells and neurons requires further investigation.
Sodium Channels
In addition to the regulation of Ca2+ and K+ channels, Go proteins may also regulate Na+ channels in cells. Depolarization of the cell membrane in rat brainstem synaptoneurosomes activates Go protein and modulates Na+ channel gating [109, 110]. In addition, cross-linking and co-immunoprecipitation experiments in depolarized brainstem synaptoneurosomes demonstrated a direct physical interaction between Go proteins and the Na+ channel α subunit [111]. In mouse mandibular gland duct cells, increasing cytosolic Na+ concentration inhibits Na+ currents. This effect is blocked by the addition of GDPβS, treatment with PTX, or antibodies against the Goα protein [112, 113]. These studies indicate that active Go may mediate the effects of cytosolic Na+ on Na+ channels. Interestingly, Gi proteins can mediate the cytosolic anions inhibitory effects on Na+ channels. However, receptors responsible for this action have not been identified.
Adenylyl Cyclase
Although Go protein was originally identified as an additional form of Gi protein, a difference in their inhibitory effect has been observed in reconstituted adenylyl cyclase assay systems. The addition of purified GTPγS-bound Giα inhibits the stimulation of adenylyl cyclase induced by Gs, while the addition of purified GTPγS-bound Goα proteins (Go1) has no inhibitory effect on adenylyl cyclase activity [114]. Studies of constitutively active mutants of Gi and Go on adenylyl cyclase also support this finding [115]. The constitutively active mutants of the Giα (1, 2 and 3) subunits inhibit cAMP accumulation in stably transfected NIH-3T3 cells, while the active Go1α mutant lacks inhibitory effects on adenylyl cyclase activity. However, the other isoform of Go, Go2α can inhibit adenylyl cyclase similar to the Giα subunits in reconstituted adenylyl cyclase assay systems [116]. Interestingly, recent studies have demonstrated that Goα directly interacts with cAMP-dependent protein kinase (PKA) through its GTPase domain. This interaction did not inhibit the kinase function of PKA but interfered with the nuclear translocation of PKA, sparing its cytosolic function [117]. Both Gβγ subunits associated with Go and Gi proteins showed equal efficiency in inhibiting adenylyl cyclase activity. It is technically difficult to demonstrate inhibitory effects on adenylyl cyclase. The assay requires a large amount of highly purified components, including receptors, G proteins, and adenylyl cyclase. The proper lipid modification of the Gα and γ subunits is also required. Furthermore, there are more than nine different forms of adenylyl cyclase with selective responses. Finally, the inhibitory effects need to be observed in an activated background from which differences are often not profound.
DAG Kinase and Gq-PLC-β
As in mammalian species, Go protein is highly expressed in the neurons of C. elegans. Serotonin signaling controls several C. elegans behaviors, including locomotion, feeding, and egg laying. Go was identified genetically as the transducer for serotonin-controlled behavioral effects in C. elegans[118, 119]. Genetic studies demonstrated that animals that lost Goα or lost diacylglycerol kinase (DAG kinase) shared the same phenotype [120, 121]. Furthermore, the phenotype caused by constitutive activation of Goα in C. elegans was suppressed by mutations in DAG kinase. This suggests that Goα acts upstream of DAG kinase in the serotonin-signaling pathway. Mutants of Gq and mutants of PLC-β share the same phenotype, which is an opposite phenotype of animals lacking Go or DAG kinase. At the molecular level, activation of the Gq, PLC-β pathway results in production of diacylglycerol (DAG), while the activation of the Go-mediated pathway reduces levels of DAG in cells. Animals lacking Go, goa-1 mutants, accumulated high levels of DAG-binding protein UNC-13 at motor neuron nerve terminals, suggesting Go decreases the abundance of UNC-13 at release sites [121]. The Go-mediated pathway antagonizes the Gq-PLC-β, DAG-signaling pathway and plays an important role in controlling worm movement and olfactory adaptation [122, 123]. However, the precise mechanism has not been elucidated and unlike mammals that have two isotypes of Go protein, there is only a single orthologue of Go encoded by the goa-1 gene in the C. elegans. The role of Go in antagonizing the Gq pathway in C. elegans is not known to occur in mammalian systems and therefore needs to be addressed.
Mitogenic Activation Signaling
Evidence for Go protein's involvement in mitogen activation primarily comes from studies using constitutively activated mutants [124, 125]. Microinjection of a constitutively active, GTPase-deficient form of Goα into Xenopus oocytes resulted in resumption of the cell cycle after meiotic maturation of the oocytes. Furthermore, the expression of a constitutively active form of Goα in the mammalian cell line NIH-3T3 fibroblasts, lead to cellular transformation. Oocyte maturation mediated by active Goα occurs through the activation of protein kinase C and c-mos, while the transforming capability of Goα in NIH-3T3 fibroblasts is protein kinase C independent. Microinjection of anti-Go antibodies into Chinese hamster lung fibroblast CCL39 cells inhibited thrombin receptor-induced calcium signals and DNA synthesis, suggesting that Go protein could mediate mitogenic signaling [126]. In M1 muscarinic receptor transfected Chinese hamster ovary (CHO) cells, application of an M1-receptor agonist resulted in PTX-sensitive, PKC-dependent MAPK activation. In PTX-treated cells, only a PTX-insensitive mutant of Goα could restore the M1 muscarinic receptor-mediated MAPK activation, whereas the PTX-insensitive α subunit mutants of Gi1, Gi2, and Gi3 could not [127]. Using Goα as bait in a yeast two-hybrid screen, rap1GAPII, a GTPaseactivating protein for the small GTPase Rap1, and several other proteins including Gz-GAP and RGS17, were shown to physically interact with Go protein [128]. In a transfected PC12 cell system, expression of Goα results in the upregulation of Rap1 and leads to an increase in the activated state of the mitogen-activated kinase 2. Expression of the active Q to L Goα mutant in NIH-3T3 cells resulted in cellular transformation through activation of the protein kinase Src and subsequent activation of the signal transducer and activator of transcription 3 (Stat3), but not of the MAP kinases, suggesting that other mechanisms may be involved [129]. In neuro2A cells, activation of CB1 cannabinoid receptors mediates neurite outgrowth. Overexpression of Goα in neuro2A cells reduces the stability of rap1GAPII, which results in the activation of Rap1 [130]. Activation of Rap1 leads to the activation of Src and Stat3 that mediate gene expression that promotes neurite outgrowth [131].
Other lines of evidence suggest that a mechanism independent of Rap1 may activate MAPK through Goα signaling. Expression of an active mutant of Goα in CHO cells is not sufficient to induce MAPK activation but greatly potentiates the MAPK response to epidermal growth factor stimulation. The active Goα mutant can activate the B-Raf kinase and MAPK through protein kinase C, a PI3K-dependent mechanism [132]. Furthermore, expression of an active Goα mutant can abolish the activation of Rap1 induced by EGF, suggesting the activation of MAPK activity by Goα is independent of Rap1 in this cell system [133]. Since most results were obtained by transfection experiments with an active mutant Goα, activation of the MAPK pathway or STAT3 in the body remains to be explored. Interestingly, several lines of evidence suggest that the Gβγ complex is also involved in PTX-sensitive, MAPK activation [134, 135]. The role of Goα and its βγ subunit in the modulation of the MAPK pathway still remains unclear.
PI4-Kinase – Rho
Go protein not only regulates the intracellular effectors at the cytoplasmic membrane but also controls secretory granule movement by regulating the small GTPase Rho and phosphatidylinositol 4-kinase (PI4-kinase) activity. The identification of Go protein as a chromaffin cell granule membrane associated protein by PTX ADP ribosylation suggested a possible role for Go protein in regulating exocytosis [136]. In addition, the Goα subunit was indicated to be directly involved in the control of exocytosis in pancreatic β cells [137]. Mastoparan, a tetradecapeptide that selectively activates Gi and Go proteins, inhibits the Ca2+-evoked reorganization of the cortical actin network in chromaffin cells. Specific anti-Goα antibodies or peptides derived from the C terminus of Goα selectively reversed the effect of mastoparan on the actin network, suggesting Go protein's involvement [138, 139]. Furthermore, mastoparan-stimulated actin-network reorganization is PI4-kinase-dependent. Antibodies against Goα specifically inhibited mastoparan-stimulated PI4-kinase activity. Clostridium botulinum C3 exoenzyme, which specifically inhibits the small GTPase Rho activity, completely blocked the activation of PI4-kinase by mastoparan [140]. These results suggest that Rho and PI4-kinase are involved in the pathway by which Goα controls reorganization of the actin network and thus the exocytosis process in chromaffin cells.
Vesicular Transporters
As described above, Go proteins modulate Rho and PI4-kinase to control reorganization of the actin network. Recent studies suggest that regulation of Rho and PI4-kinase by Go may modulate neuronal excitability by controlling neurotransmitter uptake and secretion. Biochemical analysis has demonstrated that Go protein is present on secretory granules or synaptic vesicles [141]. In the rat pheochromocytoma PC12 cells and in human pancreatic neuroendocrine tumor BON cells, GTP analogs and AlF4–, which both activate G proteins, downregulated vesicular transporters. This effect was sensitive to treatment with pertussis toxin, suggesting Gi or Go protein involvement [142, 143]. Inhibition of vesicular transporters was suppressed by Goα-specific antibodies and mimicked by purified activated Goα2. Analysis of vesicular glutamate transporter activity in the mouse brain showed that Go2 exerts its action by affecting the chloride dependence of vesicular glutamate transporters. Glutamate uptake by vesicles isolated from Go2 knockout mice, but not from other Go/Gi knockouts, completely lost chloride activation [144]. C. botulinum C3 exoenzyme, which inhibits Rho activity, increased both uptake and secretion of glutamate in cultivated mouse astrocytes. The upregulation of glutamate transporters is responsible for the enhanced glutamate uptake. In addition, C. botulinum C3 exoenzyme promotes the Ca2+-dependent exocytosis process [145]. Taken together, Go protein may mediate a novel signal cascade to control neurotransmitter or hormone storage and release, which in turn would modulate neuronal and endocrine cell excitability. The signaling mechanism, by which factors or receptors either on the cell surface or within a novel intercellular location control the effect of Go protein on vesicular transporter activity, remains to be elucidated.
Go Protein Function
Considering that Go is the most abundant G protein in the brain, the functional importance of Go signaling in the CNS is evident but largely undefined. The Gi/o proteins couple to serotonin, dopamine, GABAb, opioid, glutamate, and cholinergic receptors among others. Studies of mice lacking both isoforms of Goα have reported several neurological deficits, including tremors, seizures, and a repetitive turning behavior [89, 90]. In addition to the animals having poor motor coordination, Goα knockouts are also hyperactive and exhibit abnormal exploratory behavior as well as hyperalgesia when subjected to a hot plate test [89]. Other knockout mice of the Gi/o family of G proteins do not display any observable neurological deficiencies.
GAP-43 and Neuronal Development
Go protein's localization to neuronal synapses and growth cones alludes to a role for Go in transducing signals from neurotransmitters and hormones as well as in chemosensing during axonal outgrowth. The ability of GAP-43 to enhance binding of GTP to Go protein in the distal tips of growth cones suggested that Go may have a role in neurite extension [146]. GAP-43 is highly expressed in the growth cones of developing and regenerating neurons [147,148,149,150]. Unlike GPCRs, GAP-43 is a cytosolic protein found at the leading edge of growth cones, however, like GPCRs, GAP-43 can promote guanine nucleotide exchange by accelerating GDP release from Go [151]. Transfection of an activated Go1 in NGF differentiated PC12 cells doubled the number of neurite outgrowths per cell, and this action occurred via inhibition of protein kinase C and modulation of intracellular Ca2+[152, 153]. In C. elegans, misdirected neuronal migration appears in serotonin-deficient mutants as well as in the Go protein homolog, goa-1, loss-of-function mutants [154]. Double loss-of-function analysis suggests that goa-1 is an effector of serotonin signaling, and in accordance, the N-type Ca2+ channel homolog, unc-2, appears to act downstream of goa-1 in neuronal guidance signaling [154]. These studies agree that Go-signaling is utilized in neuronal outgrowth. Studies of developing neurons from the moth (Manduca sexta) found that Go-mediated signaling could inhibit neuronal migration by modulating intracellular Ca2+ levels, and chelation of the intracellular Ca2+ would result in misguidance of neurons [155]. A body of literature identifies intracellular Ca2+ levels as a key modulator of axonal extension, cell targeting, cytoskeleton dynamics, and synaptic plasticity [156]. A possible role for Go protein in neuronal motility may be to translate gradients of extracellular factors into attractant or repellent signals by altering Ca2+ homeostasis.
The role of the Go protein homolog in Drosophila, Goα47A, has been broadly studied in the CNS and during development. Goα47A is the only substrate for PTX in fruit flies. PTX-labeling as well as immunological data have shown that Go protein expression increases at the 10-hour embryonic stage, coinciding with the commencement of neuronal axon development [157, 158]. Interestingly, elevated levels of Go protein have been reported in mutants having reduced short-term memory [159]. In behavioral studies, exclusive PTX expression in mushroom body neurons led to a reduction in associative learning ability in flies, and the effect appeared independent of the rutabaga memory mutation, which confers a defective type I adenylyl cyclase [160]. The other memory mutants – dunce, a cAMP-phosphodiesterase, and turnip, which affects protein kinase C activation – are also regulators of signal transduction, and it appears Go protein's observed upregulation is compensatory to these mutations [159]. The Drosophila Go homolog has also been shown to play a role in the polarization of sensory organ precursor cells [161]. Simply, Go protein transduces signals from the Frizzled receptor, which directs the asymmetric distribution of effectors and establishes the axis for cell polarization [162]. These studies depict Go protein as a versatile component in neural embryogenesis, learning and memory, and sensory organ development, though our understanding of the mechanisms and effectors involved in Go signaling remains incomplete.
Alzheimer's Disease
Go protein may be involved in the pathogenesis of Alzheimer's disease (AD). The progressive neurodegeneration in AD is pathologically characterized by the presence of senile plaques, neurofibrillary tangles, and the subsequent loss of neurons. Genes for APP, Presenilin 1 (PS1), and Presenilin 2 (PS2) have been identified as heritable familial AD (FAD) genes. A physical interaction between APP and Go protein has been demonstrated in synthetic vesicle preparations [163, 164]. The APP FAD mutants, V642I/F/G, can specifically activate Go protein in the absence of ligand [165]. This effect can be blocked by PTX treatment or by application of monoclonal antibodies against the APP cytoplasmic domain. Furthermore, these and other APP mutants have been shown to induce apoptosis in neuronal cell culture [166, 167]. The induction of apoptosis by these mutants as well as by amyloid-β (Aβ) peptides is mediated through Go signaling or the p21-activated kinase (PAK) 3 [168, 169]. PS1 has also been shown to directly interact with Go but not with Gi proteins [170]. In addition, PS2 protein can induce apoptosis through a PTX-sensitive pathway in PC12 cells [171]. These in vitro studies suggest that Go may be involved in neuronal loss in AD through apoptotic signaling mediated by either a pathogenic substrate, Aβ, or a mutant APP or PS-1/2. The extent to which apoptosis contributes to the cognitive decline in AD is debated, although evidence of increased DNA fragmentation and activation of multiple caspases, including −3 and −9, have been shown in the presence of Aβ peptides [172]. A defined biological function for a possible APP or PS1/2 receptor-Go complex and the relationship between Go and the pathogenesis of AD in vivo remain to be established.
Parkinson's Disease
The role of Go in Parkinson's disease (PD) pathogenesis has not been established. PD is a chronic, progressive neurodegenerative disorder that results primarily from the loss of dopaminergic neurons in the substantia nigra. Decreased dopaminergic signaling to the basal ganglia manifests itself as dysregulated movement in patients. The inhibitory D2-like dopamine receptors (D2, D3, and D4) are coupled to the Gi/o family of G proteins. Our group has shown that the majority of the effects of D2-like receptor signaling are mediated specifically by the Go protein [46]. Dopamine has shown to have both neurotoxic and neuroprotective effects in various cell types through D2 receptor signaling [173]. Further study will be necessary to determine if the D2 receptor-Go protein-signaling pathway could induce toxicity to neurons in PD.
Schizophrenia
Schizophrenia is a psychiatric disorder whose symptoms include delusional and disorganized thought, catatonic behavior, and sensory hallucinations. Unlike neurodegenerative diseases, schizophrenia is difficult to define due to the apparent comorbidity with other mental illnesses, inconsistency of symptoms, and lack of neuropathological hallmarks. Much research has been focused on the imbalance of neurotransmission. In the earliest studies of G proteins and schizophrenia, PTX labeling of brains uncovered a decreased level of Gi/o proteins in the left side of the hippocampus and putamen of schizophrenics [174, 175]. Go protein was specifically reduced across the hippocampus and caudate head of the right hemisphere in schizophrenic patients [176]. Dopaminergic hyperactivity in mesolimbic regions is prevalent in the schizophrenic brain, and thus D2-coupled signaling machinery, such as Go, are likely candidates for downregulation in regions with dopaminergic innervation. Further analysis of schizophrenic brains has shown decreased Gi/o and Gq protein levels in the left side of the superior temporal cortex versus non-diseased brains, and interestingly, significantly lower levels of Gi/o protein were detected in the amygdala and nucleus accumbens of the paranoid schizophrenic subtype than in the disorganized subtype [177]. Recently, mutational analysis in 175 unrelated schizophrenics revealed a missense mutation in Goα protein, ATG→ACG (M129T), which significantly correlated with schizophrenic patients albeit with a low allele frequency (0.031 vs. 0.006 control) [178]. These findings are indicative of deviant signaling in the schizophrenic brain. How Go protein compensates or contributes to signaling dysfunction is not clear, and it will be important to determine whether regional changes in neurotransmission can mirror changes in G protein function and expression.
Visual Signal Transduction
Visual signal transduction is one of the best-characterized signaling cascades in the body. Retina bipolar cells are retinal interneurons that receive and integrate glutamatergic input from photoreceptors, and transmit these signals to ganglion neurons. Visual information is segregated into parallel ON and OFF pathways at the bipolar cell level through two types of bipolar neurons, ON and OFF. This is critical for detecting weak contrast as well as rapid changes in light intensity [179]. The ON bipolar cells become depolarized, and the OFF bipolar cells hyperpolarized when photoreceptors receive photon stimuli. The majority of bipolar cells are the ON-type bipolar cells. The glutamate receptor (mGluR6) is expressed restrictively in ON-type bipolar cells and serves as their primary postsynaptic receptor [180]. The b-wave in electroretinogram (ERG) recordings which reflects ON bipolar depolarization, is completely missing in mice lacking mGluR6, suggesting that mGluR6 is the receptor for synaptic transmission in ON bipolar cells [181]. Go protein is expressed in bipolar cells that also express mGluR6 [62]. Similar to mGluR6 knockout mutants, mice lacking the Goα protein also have a no b-wave phenotype, suggesting Go protein couples mGluR6 for signal transduction [182]. Furthermore, analysis of subtype-specific Go knockout mice we have generated demonstrates that the Go1α protein is specific for mGluR6-mediated signal transduction in retinal ON bipolar cells [183]. However, the downstream effectors and the signaling mechanism have yet to be elucidated.
Vagal Regulation in Heart
In the heart, Go protein is preferentially expressed in the atrium [72]. Detection of Go protein has also been observed in peripheral neurons and in granules of atrial endocrine cardiomyoctyes [68]. Studies of Go protein knockout mice have revealed that Go signaling controls muscarinic regulation of L-type Ca2+ channels in the heart [90]. Ca2+ currents in myocytes from control mice had a reduction in conductance after treatment with the muscarinic agonist, carbachol, whereas myocytes from Goα(–/–) mice were significantly unaltered. In addition, recent studies have also shown reduced effectiveness of muscarinic agonists in lowering heart rate in addition to decreased heart rate variability in Gαo-deficient hearts [184]. These studies show that Go protein is responsible for certain negative chronotropic effects of parasympathetic stimulation in the heart. This mechanism seems to be independent of the IKACh channel [90]. The inability of Go protein-deficient animals to inhibit Ca2+ channel conductance at the plasma membrane may alter intracellular Ca2+ levels, which are critical to the induction of contraction. In fact, a transgenic animal expressing the constitutively active Q205L Gαo mutant in the heart has recently shown increased expression of L-type Ca2+ channels and enhanced contractility [185]. Interestingly, enhanced phosphorylation of ryanodine receptor 2 and phospholamban was also observed in these hearts, an effect that allows greater Ca2+ storage in the sarcoplasmic reticulum and consequently more robust contractility. The mechanism or Go subunits responsible for these downstream effects remains unclear.
Conclusion
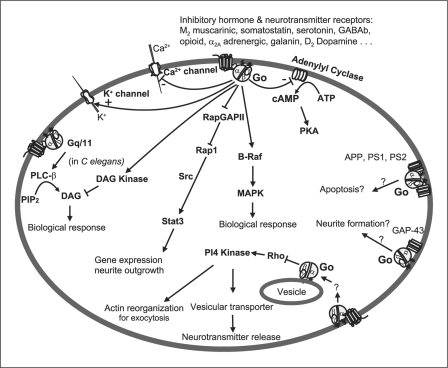
Proposed Model of Signaling (fig. 3)
Fig. 3.
Model of Go protein-mediated signal transduction.
As a signaling transducer with a high level of expression in the CNS, Go protein has received intensive attention. Great progress has been made in exploring the functional role of Go protein in the body and in revealing the mechanism for its actions. Among the PTX-sensitive Gi/Go family members, study of Go protein function is of special interest for several reasons. In contrast to Gi proteins, which are expressed fairly ubiquitously, the expression of Go is restricted in the central and peripheral nervous systems, endocrine cells, and cardiomyocytes. By various means in vitro and in vivo, including biochemical reconstitution, electrical cell recording, and whole animal genetic studies, Go protein has been shown to interact with many neurotransmitter and hormone GPCRs. Besides coupling to the classic seven-transmembrane receptors, Go also associates with GAP-43 and the APP, indicating that Go protein may possess novel neurological functions in the body.
Several effectors with which Go protein interacts have been identified. Agonist-mediated activation of Go protein inhibits the voltage-gated L-type and N-type Ca2+ channel currents and potentiates K+ channel currents and possibly Na+ channel currents as well. Go2 protein can inhibit adenylyl cyclase activity in vitro. Activation of the Go pathway in C. elegans antagonizes the Gq-DAG-signaling pathway by activating DAG kinase. Go protein-mediated signaling may interact with MAP kinase or STAT to modulate cellular functions. In addition, Go protein regulates Rho and PI4-kinase in controlling the reorganization of the actin network or in activating neurotransmitter vesicular transporters. Great progress has been made in exploring the signal mechanism of Go since its discovery two decades ago. However, many important questions still need to be answered including identification of effectors of the Go-mediated signaling pathways, as well as its role in the pathogenesis of disease. Addressing the physiological role of Go in the body, especially in the CNS, where Go protein was first identified, will be a central issue for Go protein research in the future.
Acknowledgement
This study was supported by research grant DK069771 (M.J.) from the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Birnbaumer L, Pohl SL, Rodbell M, Sundby F. The glucagon-sensitive adenylate cyclase system in plasma membranes of rat liver. VII. Hormonal stimulation: reversibility and dependence on concentration of free hormone. J Biol Chem. 1972;247:2038–2043. [PubMed] [Google Scholar]
- 2.Ross EM, Gilman AG. Resolution of some components of adenylate cyclase necessary for catalytic activity. J Biol Chem. 1977;252:6966–6969. [PubMed] [Google Scholar]
- 3.Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 4.Fung BK, Hurley JB, Stryer L. Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc Natl Acad Sci USA. 1981;78:152–156. doi: 10.1073/pnas.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moss J, Manganiello VC, Vaughan M. Substrate and effector specificity of a guanosine 3′,5′-monophosphate phosphodiesterase from rat liver. J Biol Chem. 1977;252:5211–5215. [PubMed] [Google Scholar]
- 6.Gill DM, Meren R. ADP ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci USA. 1978;75:3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassel D, Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci USA. 1978;75:2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katada T, Amano T, Ui M. Modulation by islet-activating protein of adenylate cyclase activity in C6 glioma cells. J Biol Chem. 1982;257:3739–3746. [PubMed] [Google Scholar]
- 9.Katada T, Ui M. Direct modification of the membrane adenylate cyclase system by islet-activating protein due to ADP ribosylation of a membrane protein. Proc Natl Acad Sci USA. 1982;79:3129–3133. doi: 10.1073/pnas.79.10.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Codina J, Hildebrandt J, Iyengar R, Birnbaumer L, Sekura RD, Manclark CR. Pertussis toxin substrate, the putative Ni component of adenylyl cyclases, is an alpha beta heterodimer regulated by guanine nucleotide and magnesium. Proc Natl Acad Sci USA. 1983;80:4276–4280. doi: 10.1073/pnas.80.14.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bokoch GM, Katada T, Northup JK, Hewlett EL, Gilman AG. Identification of the predominant substrate for ADP ribosylation by islet-activating protein. J Biol Chem. 1983;258:2072–2075. [PubMed] [Google Scholar]
- 12.Neer EJ, Lok JM, Wolf LG. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J Biol Chem. 1984;259:14222–14229. [PubMed] [Google Scholar]
- 13.Sternweis PC, Robishaw JD. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984;259:13806–13813. [PubMed] [Google Scholar]
- 14.Milligan G, Klee WA. The inhibitory guanine nucleotide-binding protein (Ni) purified from bovine brain is a high affinity GTPase. J Biol Chem. 1985;260:2057–2063. [PubMed] [Google Scholar]
- 15.Itoh H, Kozasa T, Nagata S, Nakamura S, Katada T, Ui M, Iwai S, Ohtsuka E, Kawasaki H, Suzuki K, et al. Molecular cloning and sequence determination of cDNAs for alpha subunits of the guanine nucleotide-binding proteins Gs, Gi, and Go from rat brain. Proc Natl Acad Sci USA. 1986;83:3776–3780. doi: 10.1073/pnas.83.11.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson KM, Higashijima T, Smigel MD, Gilman AG. The influence of bound GDP on the kinetics of guanine nucleotide binding to G proteins. J Biol Chem. 1986;261:7393–7399. [PubMed] [Google Scholar]
- 17.Strittmatter SM, Valenzuela D, Sudo Y, Linder ME, Fishman MC. An intracellular guanine nucleotide release protein for G0. GAP-43 stimulates isolated alpha subunits by a novel mechanism. J Biol Chem. 1991;266:22465–22471. [PubMed] [Google Scholar]
- 18.Strittmatter SM, Valenzuela D, Vartanian T, Sudo Y, Zuber MX, Fishman MC. Growth cone transduction: Go and GAP-43. J Cell Sci Suppl. 1991;15:27–33. doi: 10.1242/jcs.1991.supplement_15.5. [DOI] [PubMed] [Google Scholar]
- 19.Cismowski MJ, Takesono A, Ma C, Lizano JS, Xie X, Fuernkranz H, Lanier SM, Duzic E. Genetic screens in yeast to identify mammalian nonreceptor modulators of G-protein signaling. Nat Biotechnol. 1999;17:878–883. doi: 10.1038/12867. [DOI] [PubMed] [Google Scholar]
- 20.Cismowski MJ, Ma C, Ribas C, Xie X, Spruyt M, Lizano JS, Lanier SM, Duzic E. Activation of heterotrimeric G-protein signaling by a ras-related protein. Implications for signal integration. J Biol Chem. 2000;275:23421–23424. doi: 10.1074/jbc.C000322200. [DOI] [PubMed] [Google Scholar]
- 21.Kemppainen RJ, Behrend EN. Dexamethasone rapidly induces a novel ras superfamily member-related gene in AtT-20 cells. J Biol Chem. 1998;273:3129–3131. doi: 10.1074/jbc.273.6.3129. [DOI] [PubMed] [Google Scholar]
- 22.Graham TE, Prossnitz ER, Dorin RI. Dexras1/AGS-1 inhibits signal transduction from the Gi-coupled formyl peptide receptor to Erk-1/2 MAP kinases. J Biol Chem. 2002;277:10876–10882. doi: 10.1074/jbc.M110397200. [DOI] [PubMed] [Google Scholar]
- 23.Takesono A, Zahner J, Blumer KJ, Nagao T, Kurose H. Negative regulation of alpha-2-adrenergic receptor-mediated Gi signalling by a novel pathway. Biochem J. 1999;343(Pt 1):77–85. [PMC free article] [PubMed] [Google Scholar]
- 24.Siderovski DP, Diverse-Pierluissi M, De Vries L. The GoLoco motif: a Galphai/o binding motif and potential guanine-nucleotide exchange factor. Trends Biochem Sci. 1999;24:340–341. doi: 10.1016/s0968-0004(99)01441-3. [DOI] [PubMed] [Google Scholar]
- 25.L De Vries, Zheng B, Fischer T, Elenko E, Farquhar MG. The regulator of G protein signaling family. Annu Rev Pharmacol Toxicol. 2000;40:235–271. doi: 10.1146/annurev.pharmtox.40.1.235. [DOI] [PubMed] [Google Scholar]
- 26.Diverse-Pierluissi MA, Fischer T, Jordan JD, Schiff M, Ortiz DF, Farquhar MG, De Vries L. Regulators of G protein signaling proteins as determinants of the rate of desensitization of presynaptic calcium channels. J Biol Chem. 1999;274:14490–14494. doi: 10.1074/jbc.274.20.14490. [DOI] [PubMed] [Google Scholar]
- 27.Xu X, Zeng W, Popov S, Berman DM, Davignon I, Yu K, Yowe D, Offermanns S, Muallem S, Wilkie TM. RGS proteins determine signaling specificity of Gq-coupled receptors. J Biol Chem. 1999;274:3549–3556. doi: 10.1074/jbc.274.6.3549. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann M, Ward RJ, Cavalli A, Carr IC, Milligan G. Differential capacities of the RGS1, RGS16 and RGS-GAIP regulators of G protein signaling to enhance alpha-2A-adrenoreceptor agonist-stimulated GTPase activity of G(o1)alpha. J Neurochem. 2001;78:797–806. doi: 10.1046/j.1471-4159.2001.00479.x. [DOI] [PubMed] [Google Scholar]
- 29.Murtagh JJ, Jr, Eddy R, Shows TB, Moss J, Vaughan M. Different forms of Go alpha mRNA arise by alternative splicing of transcripts from a single gene on human chromosome 16. Mol Cell Biol. 1991;11:1146–1155. doi: 10.1128/mcb.11.2.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones DT, Reed RR. Molecular cloning of five GTP-binding protein cDNA species from rat olfactory neuroepithelium. J Biol Chem. 1987;262:14241–14249. [PubMed] [Google Scholar]
- 31.Strathmann M, Wilkie TM, Simon MI. Alternative splicing produces transcripts encoding two forms of the alpha subunit of GTP-binding protein Go. Proc Natl Acad Sci USA. 1990;87:6477–6481. doi: 10.1073/pnas.87.17.6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu WH, Rudolph U, Sanford J, Bertrand P, Olate J, Nelson C, Moss LG, Boyd AE, Codina J, Birnbaumer L. Molecular cloning of a novel splice variant of the alpha subunit of the mammalian Go protein. J Biol Chem. 1990;265:11220–11226. [PubMed] [Google Scholar]
- 33.Van Meurs KP, Angus CW, Lavu S, Kung HF, Czarnecki SK, Moss J, Vaughan M. Deduced amino acid sequence of bovine retinal Go alpha: similarities to other guanine nucleotide-binding proteins. Proc Natl Acad Sci USA. 1987;84:3107–3111. doi: 10.1073/pnas.84.10.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Damodaran S, Dlugos CA, Wood TD, Rabin RA. Effects of chronic ethanol administration on brain protein levels: a proteomic investigation using 2-D DIGE system. Eur J Pharmacol. 2006;547:75–82. doi: 10.1016/j.ejphar.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Olate J, Martinez S, Purcell P, Jorquera H, Codina J, Birnbaumer L, Allende J. Molecular cloning and sequence determination of four different cDNA species coding for alpha-subunits of G proteins from Xenopus laevis oocytes. FEBS Lett. 1990;268:27–31. doi: 10.1016/0014-5793(90)80964-k. [DOI] [PubMed] [Google Scholar]
- 36.Yoon J, Shortridge RD, Bloomquist BT, Schneuwly S, Perdew MH, Pak WL. Molecular characterization of Drosophila gene encoding G0 alpha subunit homolog. J Biol Chem. 1989;264:18536–18543. [PubMed] [Google Scholar]
- 37.De Sousa SM, Hoveland LL, Yarfitz S, Hurley JB. The Drosophila Go alpha-like G protein gene produces multiple transcripts and is expressed in the nervous system and in ovaries. J Biol Chem. 1989;264:18544–18551. [PubMed] [Google Scholar]
- 38.Thambi NC, Quan F, Wolfgang WJ, Spiegel A, Forte M. Immunological and molecular characterization of Go alpha-like proteins in the Drosophila central nervous system. J Biol Chem. 1989;264:18552–18560. [PubMed] [Google Scholar]
- 39.Lochrie MA, Mendel JE, Sternberg PW, Simon MI. Homologous and unique G protein alpha subunits in the nematode Caenorhabditis elegans. Cell Regul. 1991;2:135–154. doi: 10.1091/mbc.2.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miura N, Atsumi S, Tabunoki H, Sato R. Expression and localization of three G protein alpha subunits, Go, Gq, and Gs, in adult antennae of the silkmoth (Bombyx mori) J Comp Neurol. 2005;485:143–152. doi: 10.1002/cne.20488. [DOI] [PubMed] [Google Scholar]
- 41.Goldsmith P, Backlund PS, Jr, Rossiter K, Carter A, Milligan G, Unson CG, Spiegel A. Purification of heterotrimeric GTP-binding proteins from brain: identification of a novel form of Go. Biochemistry. 1988;27:7085–7090. doi: 10.1021/bi00418a062. [DOI] [PubMed] [Google Scholar]
- 42.Lang J. Purification and characterization of subforms of the guanine-nucleotide-binding proteins G alpha i and G alpha o. Eur J Biochem. 1989;183:687–692. doi: 10.1111/j.1432-1033.1989.tb21099.x. [DOI] [PubMed] [Google Scholar]
- 43.Scherer NM, Toro MJ, Entman ML, Birnbaumer L. G-protein distribution in canine cardiac sarcoplasmic reticulum and sarcolemma: comparison to rabbit skeletal muscle membranes and to brain and erythrocyte G-proteins. Arch Biochem Biophys. 1987;259:431–440. doi: 10.1016/0003-9861(87)90509-1. [DOI] [PubMed] [Google Scholar]
- 44.Strathmann M, Simon MI. G protein diversity: a distinct class of alpha subunits is present in vertebrates and invertebrates. Proc Natl Acad Sci USA. 1990;87:9113–9117. doi: 10.1073/pnas.87.23.9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsukamoto T, Toyama R, Itoh H, Kozasa T, Matsuoka M, Kaziro Y. Structure of the human gene and two rat cDNAs encoding the alpha chain of GTP-binding regulatory protein Go: two different mRNAs are generated by alternative splicing. Proc Natl Acad Sci USA. 1991;88:2974–2978. doi: 10.1073/pnas.88.8.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang M, Spicher K, Boulay G, Wang Y, Birnbaumer L. Most central nervous system D2 dopamine receptors are coupled to their effectors by Go. Proc Natl Acad Sci USA. 2001;98:3577–3582. doi: 10.1073/pnas.051632598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linder ME, Pang IH, Duronio RJ, Gordon JI, Sternweis PC, Gilman AG. Lipid modifications of G protein subunits. Myristoylation of Go alpha increases its affinity for beta gamma. J Biol Chem. 1991;266:4654–4659. [PubMed] [Google Scholar]
- 48.Mumby SM, Heukeroth RO, Gordon JI, Gilman AG. G-protein alpha-subunit expression, myristoylation, and membrane association in COS cells. Proc Natl Acad Sci USA. 1990;87:728–732. doi: 10.1073/pnas.87.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones TL, Simonds WF, Merendino JJ, Jr, Brann MR, Spiegel AM. Myristoylation of an inhibitory GTP-binding protein alpha subunit is essential for its membrane attachment. Proc Natl Acad Sci USA. 1990;87:568–572. doi: 10.1073/pnas.87.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gallego C, Gupta SK, Winitz S, Eisfelder BJ, Johnson GL. Myristoylation of the G alpha i2 polypeptide, a G protein alpha subunit, is required for its signaling and transformation functions. Proc Natl Acad Sci USA. 1992;89:9695–9699. doi: 10.1073/pnas.89.20.9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Linder ME, Middleton P, Hepler JR, Taussig R, Gilman AG, Mumby SM. Lipid modifications of G proteins: alpha subunits are palmitoylated. Proc Natl Acad Sci USA. 1993;90:3675–3679. doi: 10.1073/pnas.90.8.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mumby SM, Kleuss C, Gilman AG. Receptor regulation of G-protein palmitoylation. Proc Natl Acad Sci USA. 1994;91:2800–2804. doi: 10.1073/pnas.91.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barclay E, O'Reilly M, Milligan G. Activation of an alpha-2A-adrenoceptor-Galphao1 fusion protein dynamically regulates the palmitoylation status of the G protein but not of the receptor. Biochem J. 2005;385:197–206. doi: 10.1042/BJ20041432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Busconi L, Boutin PM, Denker BM. N-terminal binding domain of Galpha subunits: involvement of amino acids 11–14 of Galphao in membrane attachment. Biochem J. 1997;323:239–244. doi: 10.1042/bj3230239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen CA, Manning DR. Regulation of G proteins by covalent modification. Oncogene. 2001;20:1643–1652. doi: 10.1038/sj.onc.1204185. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi I, Shibasaki H, Takahashi K, Kikkawa S, Ui M, Katada T. Purification of GTP-binding proteins from bovine brain membranes. Identification of heterogeneity of the alpha-subunit of Go proteins. FEBS Lett. 1989;257:177–180. doi: 10.1016/0014-5793(89)81815-0. [DOI] [PubMed] [Google Scholar]
- 57.McIntire WE, Dingus J, Schey KL, Hildebrandt JD. Characterization of the major bovine brain Go alpha isoforms. Mapping the structural differences between the alpha subunit isoforms identifies a variable region of the protein involved in receptor interactions. J Biol Chem. 1998;273:33135–33141. doi: 10.1074/jbc.273.50.33135. [DOI] [PubMed] [Google Scholar]
- 58.McIntire WE, Schey KL, Knapp DR, Hildebrandt JD. A major G protein alpha O isoform in bovine brain is deamidated at Asn346 and Asn347, residues involved in receptor coupling. Biochemistry. 1998;37:14651–14658. doi: 10.1021/bi981642q. [DOI] [PubMed] [Google Scholar]
- 59.Exner T, Jensen ON, Mann M, Kleuss C, Nurnberg B. Posttranslational modification of Galphao1 generates Galphao3, an abundant G protein in brain. Proc Natl Acad Sci USA. 1999;96:1327–1332. doi: 10.1073/pnas.96.4.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McIntire WE, Dingus J, Wilcox MD, Hildebrandt JD. The relationship of G(o)alpha subunit deamidation to the tissue distribution, nucleotide binding properties, and betagamma dimer interactions of G(o)alpha subunit isoforms. J Neurochem. 1999;73:633–640. doi: 10.1046/j.1471-4159.1999.0730633.x. [DOI] [PubMed] [Google Scholar]
- 61.Huff RM, Axton JM, Neer EJ. Physical and immunological characterization of a guanine nucleotide-binding protein purified from bovine cerebral cortex. J Biol Chem. 1985;260:10864–10871. [PubMed] [Google Scholar]
- 62.Vardi N. Alpha subunit of Go localizes in the dendritic tips of ON bipolar cells. J Comp Neurol. 1998;395:43–52. [PubMed] [Google Scholar]
- 63.Shinohara H, Kato K, Asano T. Differential localization of G proteins, Gi and Go, in the olfactory epithelium and the main olfactory bulb of the rat. Acta Anat (Basel) 1992;144:167–171. doi: 10.1159/000147301. [DOI] [PubMed] [Google Scholar]
- 64.Homburger V, Brabet P, Audigier Y, Pantaloni C, Bockaert J, Rouot B. Immunological localization of the GTP-binding protein Go in different tissues of vertebrates and invertebrates. Mol Pharmacol. 1987;31:313–319. [PubMed] [Google Scholar]
- 65.Liang BT, Hellmich MR, Neer EJ, Galper JB. Development of muscarinic cholinergic inhibition of adenylate cyclase in embryonic chick heart. Its relationship to changes in the inhibitory guanine nucleotide regulatory protein. J Biol Chem. 1986;261:9011–9021. [PubMed] [Google Scholar]
- 66.Asano T, Kamiya N, Semba R, Kato K. Ontogeny of the GTP-binding protein Go in rat brain and heart. J Neurochem. 1988;51:1711–1716. doi: 10.1111/j.1471-4159.1988.tb01149.x. [DOI] [PubMed] [Google Scholar]
- 67.Milligan G, Tanfin Z, Goureau O, Unson C, Harbon S. Identification of both Gi2 and a novel, immunologically distinct, form of Go in rat myometrial membranes. FEBS Lett. 1989;244:411–416. doi: 10.1016/0014-5793(89)80574-5. [DOI] [PubMed] [Google Scholar]
- 68.Wolf WP, Spicher K, Haase H, Schulze W. Immunocytochemical localization of the G-protein subunit, G(o) alpha, in rat heart. Implications for a role of G(o) alpha in secretion of cardiac hormones. J Mol Cell Cardiol. 1998;30:1149–1162. doi: 10.1006/jmcc.1998.0679. [DOI] [PubMed] [Google Scholar]
- 69.Worley PF, Baraban JM, Van Dop C, Neer EJ, Snyder SH. Go, a guanine nucleotide-binding protein: immunohistochemical localization in rat brain resembles distribution of second messenger systems. Proc Natl Acad Sci USA. 1986;83:4561–4565. doi: 10.1073/pnas.83.12.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gabrion J, Brabet P, Nguyen Than Dao B, Homburger V, Dumuis A, Sebben M, Rouot B, Bockaert J. Ultrastructural localization of the GTP-binding protein Go in neurons. Cell Signal. 1989;1:107–123. doi: 10.1016/0898-6568(89)90025-9. [DOI] [PubMed] [Google Scholar]
- 71.Galper JB, Klein W, Catterall WA. Muscarinic acetylcholine receptors in developing chick heart. J Biol Chem. 1977;252:8692–8699. [PubMed] [Google Scholar]
- 72.Luetje CW, Tietje KM, Christian JL, Nathanson NM. Differential tissue expression and developmental regulation of guanine nucleotide binding regulatory proteins and their messenger RNAs in rat heart. J Biol Chem. 1988;263:13357–13365. [PubMed] [Google Scholar]
- 73.Kawai Y, Arinze IJ. Differential localization and development-dependent expression of G-protein subunits, Go alpha and G beta, in rabbit heart. J Mol Cell Cardiol. 1996;28:1555–1564. doi: 10.1006/jmcc.1996.0146. [DOI] [PubMed] [Google Scholar]
- 74.Mullaney I, Milligan G. Elevated levels of the guanine nucleotide binding protein, Go, are associated with differentiation of neuroblastoma × glioma hybrid cells. FEBS Lett. 1989;244:113–118. doi: 10.1016/0014-5793(89)81174-3. [DOI] [PubMed] [Google Scholar]
- 75.Brabet P, Pantaloni C, Rodriguez M, Martinez J, Bockaert J, Homburger V. Neuroblastoma differentiation involves the expression of two isoforms of the alpha-subunit of Go. J Neurochem. 1990;54:1310–1320. doi: 10.1111/j.1471-4159.1990.tb01964.x. [DOI] [PubMed] [Google Scholar]
- 76.Brabet P, Pantaloni C, Bockaert J, Homburger V. Metabolism of two Go alpha isoforms in neuronal cells during differentiation. J Biol Chem. 1991;266:12825–12828. [PubMed] [Google Scholar]
- 77.Ben-Shlomo I, S Yu Hsu, Rauch R, Kowalski HW, Hsueh AJ. Signaling receptome: a genomic and evolutionary perspective of plasma membrane receptors involved in signal transduction. Sci STKE. 2003;2003:RE9. doi: 10.1126/stke.2003.187.re9. [DOI] [PubMed] [Google Scholar]
- 78.Florio VA, Sternweis PC. Reconstitution of resolved muscarinic cholinergic receptors with purified GTP-binding proteins. J Biol Chem. 1985;260:3477–3483. [PubMed] [Google Scholar]
- 79.Kurose H, Katada T, Haga T, Haga K, Ichiyama A, Ui M. Functional interaction of purified muscarinic receptors with purified inhibitory guanine nucleotide regulatory proteins reconstituted in phospholipid vesicles. J Biol Chem. 1986;261:6423–6428. [PubMed] [Google Scholar]
- 80.Ueda H, Harada H, Nozaki M, Katada T, Ui M, Satoh M, Takagi H. Reconstitution of rat brain mu opioid receptors with purified guanine nucleotide-binding regulatory proteins, Gi and Go. Proc Natl Acad Sci USA. 1988;85:7013–7017. doi: 10.1073/pnas.85.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lewis DL, Weight FF, Luini A. A guanine nucleotide-binding protein mediates the inhibition of voltage-dependent calcium current by somatostatin in a pituitary cell line. Proc Natl Acad Sci USA. 1986;83:9035–9039. doi: 10.1073/pnas.83.23.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Law SF, Manning D, Reisine T. Identification of the subunits of GTP-binding proteins coupled to somatostatin receptors. J Biol Chem. 1991;266:17885–17897. [PubMed] [Google Scholar]
- 83.Hescheler J, Rosenthal W, Trautwein W, Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. Nature. 1987;325:445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- 84.Ewald DA, Sternweis PC, Miller RJ. Guanine nucleotide-binding protein Go-induced coupling of neuropeptide Y receptors to Ca2+ channels in sensory neurons. Proc Natl Acad Sci USA. 1988;85:3633–3637. doi: 10.1073/pnas.85.10.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kleuss C, Hescheler J, Ewel C, Rosenthal W, Schultz G, Wittig B. Assignment of G-protein subtypes to specific receptors inducing inhibition of calcium currents. Nature. 1991;353:43–48. doi: 10.1038/353043a0. [DOI] [PubMed] [Google Scholar]
- 86.Kleuss C, Scherubl H, Hescheler J, Schultz G, Wittig B. Different beta-subunits determine G-protein interaction with transmembrane receptors. Nature. 1992;358:424–426. doi: 10.1038/358424a0. [DOI] [PubMed] [Google Scholar]
- 87.Kleuss C, Scherubl H, Hescheler J, Schultz G, Wittig B. Selectivity in signal transduction determined by gamma subunits of heterotrimeric G proteins. Science. 1993;259:832–834. doi: 10.1126/science.8094261. [DOI] [PubMed] [Google Scholar]
- 88.Jiang M, Boulay G, Spicher K, Peyton MJ, Brabet P, Birnbaumer L, Rudolph U. Inactivation of the G alpha i2 and G alpha o genes by homologous recombination. Receptors Channels. 1997;5:187–192. [PubMed] [Google Scholar]
- 89.Jiang M, Gold MS, Boulay G, Spicher K, Peyton M, Brabet P, Srinivasan Y, Rudolph U, Ellison G, Birnbaumer L. Multiple neurological abnormalities in mice deficient in the G protein Go. Proc Natl Acad Sci USA. 1998;95:3269–3274. doi: 10.1073/pnas.95.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Valenzuela D, Han X, Mende U, Fankhauser C, Mashimo H, Huang P, Pfeffer J, Neer EJ, Fishman MC. G alpha(o) is necessary for muscarinic regulation of Ca2+ channels in mouse heart. Proc Natl Acad Sci USA. 1997;94:1727–1732. doi: 10.1073/pnas.94.5.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dunlap K, Fischbach GD. Neurotransmitters decrease the calcium component of sensory neurone action potentials. Nature. 1978;276:837–839. doi: 10.1038/276837a0. [DOI] [PubMed] [Google Scholar]
- 92.Dunlap K, Fischbach GD. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol. 1981;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holz GG, 4th, Rane SG, Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature. 1986;319:670–672. doi: 10.1038/319670a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scott RH, Dolphin AC. Regulation of calcium currents by a GTP analogue: potentiation of (–)-baclofen-mediated inhibition. Neurosci Lett. 1986;69:59–64. doi: 10.1016/0304-3940(86)90414-3. [DOI] [PubMed] [Google Scholar]
- 95.Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 96.Ewald DA, Pang IH, Sternweis PC, Miller RJ. Differential G protein-mediated coupling of neurotransmitter receptors to Ca2+ channels in rat dorsal root ganglion neurons in vitro. Neuron. 1989;2:1185–1193. doi: 10.1016/0896-6273(89)90185-2. [DOI] [PubMed] [Google Scholar]
- 97.Campbell V, Berrow N, Dolphin AC. GABAB receptor modulation of Ca2+ currents in rat sensory neurones by the G protein G(0): antisense oligonucleotide studies. J Physiol. 1993;470:1–11. doi: 10.1113/jphysiol.1993.sp019842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Caulfield MP, Jones S, Vallis Y, Buckley NJ, Kim GD, Milligan G, Brown DA. Muscarinic M-current inhibition via G alpha q/11 and alpha-adrenoceptor inhibition of Ca2+ current via G alpha o in rat sympathetic neurones. J Physiol. 1994;477:415–422. doi: 10.1113/jphysiol.1994.sp020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jan LY, Jan YN. How might the diversity of potassium channels be generated? Trends Neurosci. 1990;13:415–419. doi: 10.1016/0166-2236(90)90123-r. [DOI] [PubMed] [Google Scholar]
- 100.Pfaffinger PJ, Martin JM, Hunter DD, Nathanson NM, Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985;317:536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- 101.Andrade R, Malenka RC, Nicoll RA. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986;234:1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- 102.VanDongen AM, Codina J, Olate J, Mattera R, Joho R, Birnbaumer L, Brown AM. Newly identified brain potassium channels gated by the guanine nucleotide binding protein Go. Science. 1988;242:1433–1437. doi: 10.1126/science.3144040. [DOI] [PubMed] [Google Scholar]
- 103.Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- 104.Wickman KD, Iniguez-Lluhl JA, Davenport PA, Taussig R, Krapivinsky GB, Linder ME, Gilman AG, Clapham DE. Recombinant G-protein beta gamma-subunits activate the muscarinic-gated atrial potassium channel. Nature. 1994;368:255–257. doi: 10.1038/368255a0. [DOI] [PubMed] [Google Scholar]
- 105.Reuveny E, Slesinger PA, Inglese J, Morales JM, Iniguez-Lluhi JA, Lefkowitz RJ, Bourne HR, Jan YN, Jan LY. Activation of the cloned muscarinic potassium channel by G protein beta gamma subunits. Nature. 1994;370:143–146. doi: 10.1038/370143a0. [DOI] [PubMed] [Google Scholar]
- 106.Huang CL, Jan YN, Jan LY. Binding of the G protein betagamma subunit to multiple regions of G protein-gated inward-rectifying K+ channels. FEBS Lett. 1997;405:291–298. doi: 10.1016/s0014-5793(97)00197-x. [DOI] [PubMed] [Google Scholar]
- 107.Lei Q, Jones MB, Talley EM, Schrier AD, McIntire WE, Garrison JC, Bayliss DA. Activation and inhibition of G protein-coupled inwardly rectifying potassium (Kir3) channels by G protein beta gamma subunits. Proc Natl Acad Sci USA. 2000;97:9771–9776. doi: 10.1073/pnas.97.17.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peleg S, Varon D, Ivanina T, Dessauer CW, Dascal N. G-alpha-i controls the gating of the G protein-activated K+ channel, GIRK. Neuron. 2002;33:87–99. doi: 10.1016/s0896-6273(01)00567-0. [DOI] [PubMed] [Google Scholar]
- 109.Cohen-Armon M, Sokolovsky M. Depolarization-induced changes in the muscarinic receptor in rat brain and heart are mediated by pertussis-toxin-sensitive G-proteins. J Biol Chem. 1991;266:2595–2605. [PubMed] [Google Scholar]
- 110.Cohen-Armon M, Sokolovsky M. Evidence for involvement of the voltage-dependent Na+ channel gating in depolarization-induced activation of G-proteins. J Biol Chem. 1993;268:9824–9838. [PubMed] [Google Scholar]
- 111.Anis Y, Nurnberg B, Visochek L, Reiss N, Naor Z, Cohen-Armon M. Activation of Go-proteins by membrane depolarization traced by in situ photoaffinity labeling of galphao-proteins with [α32P]GTP-azidoanilide. J Biol Chem. 1999;274:7431–7440. doi: 10.1074/jbc.274.11.7431. [DOI] [PubMed] [Google Scholar]
- 112.Komwatana P, Dinudom A, Young JA, Cook DI. Cytosolic Na+ controls and epithelial Na+ channel via the Go guanine nucleotide-binding regulatory protein. Proc Natl Acad Sci USA. 1996;93:8107–8111. doi: 10.1073/pnas.93.15.8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Komwatana P, Dinudom A, Young JA, Cook DI. Activators of epithelial Na+ channels inhibit cytosolic feedback control. Evidence for the existence of a G protein-coupled receptor for cytosolic Na+ J Membr Biol. 1998;162:225–232. doi: 10.1007/s002329900360. [DOI] [PubMed] [Google Scholar]
- 114.Katada T, Oinuma M, Ui M. Two guanine nucleotide-binding proteins in rat brain serving as the specific substrate of islet- activating protein, pertussis toxin. Interaction of the alpha-subunits with beta gamma-subunits in development of their biological activities. J Biol Chem. 1986;261:8182–8191. [PubMed] [Google Scholar]
- 115.Wong YH, Conklin BR, Bourne HR. Gz-mediated hormonal inhibition of cyclic AMP accumulation. Science. 1992;255:339–342. doi: 10.1126/science.1347957. [DOI] [PubMed] [Google Scholar]
- 116.Kobayashi I, Shibasaki H, Takahashi K, Tohyama K, Kurachi Y, Ito H, Ui M, Katada T. Purification and characterization of five different alpha subunits of guanine-nucleotide-binding proteins in bovine brain membranes. Their physiological properties concerning the activities of adenylate cyclase and atrial muscarinic K+ channels. Eur J Biochem. 1990;191:499–506. doi: 10.1111/j.1432-1033.1990.tb19149.x. [DOI] [PubMed] [Google Scholar]
- 117.Ghil S, Choi JM, Kim SS, Lee YD, Liao Y, Birnbaumer L, Suh-Kim H. Compartmentalization of protein kinase A signaling by the heterotrimeric G protein Go. Proc Natl Acad Sci USA. 2006;103:19158–19163. doi: 10.1073/pnas.0609392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mendel JE, Korswagen HC, Liu KS, Hajdu-Cronin YM, Simon MI, Plasterk RH, Sternberg PW. Participation of the protein Go in multiple aspects of behavior in C. elegans. Science. 1995;267:1652–1655. doi: 10.1126/science.7886455. [DOI] [PubMed] [Google Scholar]
- 119.Segalat L, Elkes DA, Kaplan JM. Modulation of serotonin-controlled behaviors by Go in Caenorhabditis elegans. Science. 1995;267:1648–1651. doi: 10.1126/science.7886454. [DOI] [PubMed] [Google Scholar]
- 120.Miller KG, Emerson MD, Rand JB. Goalpha and diacylglycerol kinase negatively regulate the Gqalpha pathway in C. elegans. Neuron. 1999;24:323–333. doi: 10.1016/s0896-6273(00)80847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nurrish S, Segalat L, Kaplan JM. Serotonin inhibition of synaptic transmission: Galpha(0) decreases the abundance of UNC-13 at release sites. Neuron. 1999;24:231–242. doi: 10.1016/s0896-6273(00)80835-1. [DOI] [PubMed] [Google Scholar]
- 122.Matsuki M, Kunitomo H, Iino Y. Goalpha regulates olfactory adaptation by antagonizing Gqalpha-DAG signaling in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2006;103:1112–1117. doi: 10.1073/pnas.0506954103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mendel J. Go directly (or indirectly) to Gq. Neuron. 1999;24:287–288. doi: 10.1016/s0896-6273(00)80840-5. [DOI] [PubMed] [Google Scholar]
- 124.Kroll SD, Chen J, De Vivo M, Carty DJ, Buku A, Premont RT, Iyengar R. The Q205LGo-alpha subunit expressed in NIH-3T3 cells induces transformation. J Biol Chem. 1992;267:23183–23188. [PubMed] [Google Scholar]
- 125.Kroll SD, Omri G, Landau EM, Iyengar R. Activated alpha subunit of Go protein induces oocyte maturation. Proc Natl Acad Sci USA. 1991;88:5182–5186. doi: 10.1073/pnas.88.12.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Baffy G, Yang L, Raj S, Manning DR, Williamson JR. G protein coupling to the thrombin receptor in Chinese hamster lung fibroblasts. J Biol Chem. 1994;269:8483–8487. [PubMed] [Google Scholar]
- 127.Van Biesen T, Hawes BE, Raymond JR, Luttrell LM, Koch WJ, Lefkowitz RJ. G(o)-protein alpha-subunits activate mitogen-activated protein kinase via a novel protein kinase C-dependent mechanism. J Biol Chem. 1996;271:1266–1269. doi: 10.1074/jbc.271.3.1266. [DOI] [PubMed] [Google Scholar]
- 128.Jordan JD, Carey KD, Stork PJ, Iyengar R. Modulation of rap activity by direct interaction of Galpha(o) with Rap1 GTPaseactivating protein. J Biol Chem. 1999;274:21507–21510. doi: 10.1074/jbc.274.31.21507. [DOI] [PubMed] [Google Scholar]
- 129.Ram PT, Horvath CM, Iyengar R. Stat3-mediated transformation of NIH-3T3 cells by the constitutively active Q205L Galphao protein. Science. 2000;287:142–144. doi: 10.1126/science.287.5450.142. [DOI] [PubMed] [Google Scholar]
- 130.Jordan JD, He JC, Eungdamrong NJ, Gomes I, Ali W, Nguyen T, Bivona TG, Philips MR, Devi LA, Iyengar R. Cannabinoid receptor-induced neurite outgrowth is mediated by Rap1 activation through G(alpha)o/i-triggered proteasomal degradation of Rap1GAPII. J Biol Chem. 2005;280:11413–11421. doi: 10.1074/jbc.M411521200. [DOI] [PubMed] [Google Scholar]
- 131.He JC, Gomes I, Nguyen T, Jayaram G, Ram PT, Devi LA, Iyengar R. The G alpha(o/i)-coupled cannabinoid receptor-mediated neurite outgrowth involves Rap regulation of Src and Stat3. J Biol Chem. 2005;280:33426–33434. doi: 10.1074/jbc.M502812200. [DOI] [PubMed] [Google Scholar]
- 132.Antonelli V, Bernasconi F, Wong YH, Vallar L. Activation of B-Raf and regulation of the mitogen-activated protein kinase pathway by the G(o) alpha chain. Mol Biol Cell. 2000;11:1129–1142. doi: 10.1091/mbc.11.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bernasconi F, Malgaroli A, Vallar L. Independent regulation of Rap1 and mitogen-activated protein kinase by the alpha chain of Go. Neurosignals. 2006;15:180–189. doi: 10.1159/000096734. [DOI] [PubMed] [Google Scholar]
- 134.Crespo P, Xu N, Simonds WF, Gutkind JS. Ras-dependent activation of MAP kinase pathway mediated by G-protein beta gamma subunits. Nature. 1994;369:418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- 135.Gutkind JS. The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J Biol Chem. 1998;273:1839–1842. doi: 10.1074/jbc.273.4.1839. [DOI] [PubMed] [Google Scholar]
- 136.Toutant M, Aunis D, Bockaert J, Homburger V, Rouot B. Presence of three pertussis toxin substrates and Go alpha immunoreactivity in both plasma and granule membranes of chromaffin cells. FEBS Lett. 1987;215:339–344. doi: 10.1016/0014-5793(87)80174-6. [DOI] [PubMed] [Google Scholar]
- 137.Lang J, Nishimoto I, Okamoto T, Regazzi R, Kiraly C, Weller U, Wollheim CB. Direct control of exocytosis by receptor-mediated activation of the heterotrimeric GTPases Gi and G(o) or by the expression of their active G alpha subunits. EMBO J. 1995;14:3635–3644. doi: 10.1002/j.1460-2075.1995.tb00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gasman S, Chasserot-Golaz S, Popoff MR, Aunis D, Bader MF. Trimeric G proteins control exocytosis in chromaffin cells. Go regulates the peripheral actin network and catecholamine secretion by a mechanism involving the small GTP-binding protein Rho. J Biol Chem. 1997;272:20564–20571. doi: 10.1074/jbc.272.33.20564. [DOI] [PubMed] [Google Scholar]
- 139.Vitale N, Gensse M, Chasserot-Golaz S, Aunis D, Bader MF. Trimeric G proteins control regulated exocytosis in bovine chromaffin cells: sequential involvement of Go associated with secretory granules and Gi3 bound to the plasma membrane. Eur J Neurosci. 1996;8:1275–1285. doi: 10.1111/j.1460-9568.1996.tb01296.x. [DOI] [PubMed] [Google Scholar]
- 140.Gasman S, Chasserot-Golaz S, Hubert P, Aunis D, Bader MF. Identification of a potential effector pathway for the trimeric Go protein associated with secretory granules. Go stimulates a granule-bound phosphatidylinositol 4-kinase by activating RhoA in chromaffin cells. J Biol Chem. 1998;273:16913–16920. doi: 10.1074/jbc.273.27.16913. [DOI] [PubMed] [Google Scholar]
- 141.Ahnert-Hilger G, Schafer T, Spicher K, Grund C, Schultz G, Wiedenmann B. Detection of G-protein heterotrimers on large dense core and small synaptic vesicles of neuroendocrine and neuronal cells. Eur J Cell Biol. 1994;65:26–38. [PubMed] [Google Scholar]
- 142.Ahnert-Hilger G, Nurnberg B, Exner T, Schafer T, Jahn R. The heterotrimeric G protein Go2 regulates catecholamine uptake by secretory vesicles. EMBO J. 1998;17:406–413. doi: 10.1093/emboj/17.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Holtje M, von Jagow B, Pahner I, Lautenschlager M, Hortnagl H, Nurnberg B, Jahn R, Ahnert-Hilger G. The neuronal monoamine transporter VMAT2 is regulated by the trimeric GTPase Go(2) J Neurosci. 2000;20:2131–2141. doi: 10.1523/JNEUROSCI.20-06-02131.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Winter S, Brunk I, Walther DJ, Holtje M, Jiang M, Peter JU, Takamori S, Jahn R, Birnbaumer L, Ahnert-Hilger G. Galphao2 regulates vesicular glutamate transporter activity by changing its chloride dependence. J Neurosci. 2005;25:4672–4680. doi: 10.1523/JNEUROSCI.0549-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Holtje M, Hofmann F, Lux R, Veh RW, Just I, Ahnert-Hilger G. Glutamate uptake and release by astrocytes is enhanced by Clostridium botulinum C3 protein. J Biol Chem. 2008;283:9289–9299. doi: 10.1074/jbc.M706499200. [DOI] [PubMed] [Google Scholar]
- 146.Strittmatter SM, Valenzuela D, Kennedy TE, Neer EJ, Fishman MC. G0 is a major growth cone protein subject to regulation by GAP-43. Nature. 1990;344:836–841. doi: 10.1038/344836a0. [DOI] [PubMed] [Google Scholar]
- 147.Meiri KF, Pfenninger KH, Willard MB. Growth-associated protein, GAP-43, a polypeptide that is induced when neurons extend axons, is a component of growth cones and corresponds to pp46, a major polypeptide of a subcellular fraction enriched in growth cones. Proc Natl Acad Sci USA. 1986;83:3537–3541. doi: 10.1073/pnas.83.10.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Skene JH, Jacobson RD, Snipes GJ, McGuire CB, Norden JJ, Freeman JA. A protein induced during nerve growth (GAP-43) is a major component of growth-cone membranes. Science. 1986;233:783–786. doi: 10.1126/science.3738509. [DOI] [PubMed] [Google Scholar]
- 149.Benowitz LI, Shashoua VE, Yoon MG. Specific changes in rapidly transported proteins during regeneration of the goldfish optic nerve. J Neurosci. 1981;1:300–307. doi: 10.1523/JNEUROSCI.01-03-00300.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Skene JH, Willard M. Changes in axonally transported proteins during axon regeneration in toad retinal ganglion cells. J Cell Biol. 1981;89:86–95. doi: 10.1083/jcb.89.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Strittmatter SM, Fishman MC. The neuronal growth cone as a specialized transduction system. Bioessays. 1991;13:127–134. doi: 10.1002/bies.950130306. [DOI] [PubMed] [Google Scholar]
- 152.Strittmatter SM, Fishman MC, Zhu XP. Activated mutants of the alpha subunit of G(o) promote an increased number of neurites per cell. J Neurosci. 1994;14:2327–2338. doi: 10.1523/JNEUROSCI.14-04-02327.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xie R, Li L, Goshima Y, Strittmatter SM. An activated mutant of the alpha subunit of G(o) increases neurite outgrowth via protein kinase C. Brain Res Dev Brain Res. 1995;87:77–86. doi: 10.1016/0165-3806(95)00061-h. [DOI] [PubMed] [Google Scholar]
- 154.Kindt KS, Tam T, Whiteman S, Schafer WR. Serotonin promotes G(o)-dependent neuronal migration in Caenorhabditis elegans. Curr Biol. 2002;12:1738–1747. doi: 10.1016/s0960-9822(02)01199-5. [DOI] [PubMed] [Google Scholar]
- 155.Horgan AM, Copenhaver PF. G protein-mediated inhibition of neuronal migration requires calcium influx. J Neurosci. 1998;18:4189–4200. doi: 10.1523/JNEUROSCI.18-11-04189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zheng JQ, Poo MM. Calcium signaling in neuronal motility. Annu Rev Cell Dev Biol. 2007;23:375–404. doi: 10.1146/annurev.cellbio.23.090506.123221. [DOI] [PubMed] [Google Scholar]
- 157.Guillen A, Semeriva M, Bockaert J, Homburger V. The transduction signalling protein Go during embryonic development of Drosophila melanogaster. Cell Signal. 1991;3:341–352. doi: 10.1016/0898-6568(91)90063-z. [DOI] [PubMed] [Google Scholar]
- 158.Wolfgang WJ, Quan F, Thambi N, Forte M. Restricted spatial and temporal expression of G-protein alpha subunits during Drosophila embryogenesis. Development. 1991;113:527–538. doi: 10.1242/dev.113.2.527. [DOI] [PubMed] [Google Scholar]
- 159.Guillen A, Jallon JM, Fehrentz JA, Pantaloni C, Bockaert J, Homburger V. A Go-like protein in Drosophila melanogaster and its expression in memory mutants. Embo J. 1990;9:1449–1455. doi: 10.1002/j.1460-2075.1990.tb08261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ferris J, Ge H, Liu L, Roman G. G(o) signaling is required for Drosophila associative learning. Nat Neurosci. 2006;9:1036–1040. doi: 10.1038/nn1738. [DOI] [PubMed] [Google Scholar]
- 161.Katanaev VL, Ponzielli R, Semeriva M, Tomlinson A. Trimeric G protein-dependent frizzled signaling in Drosophila. Cell. 2005;120:111–122. doi: 10.1016/j.cell.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 162.Katanaev VL, Tomlinson A. Dual roles for the trimeric G protein Go in asymmetric cell division in Drosophila. Proc Natl Acad Sci USA. 2006;103:6524–6529. doi: 10.1073/pnas.0601853103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Brouillet E, Trembleau A, Galanaud D, Volovitch M, Bouillot C, Valenza C, Prochiantz A, Allinquant B. The amyloid precursor protein interacts with Go heterotrimeric protein within a cell compartment specialized in signal transduction. J Neurosci. 1999;19:1717–1727. doi: 10.1523/JNEUROSCI.19-05-01717.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nishimoto I, Okamoto T, Matsuura Y, Takahashi S, Okamoto T, Murayama Y, Ogata E. Alzheimer amyloid protein precursor complexes with brain GTP-binding protein G(o) Nature. 1993;362:75–79. doi: 10.1038/362075a0. [DOI] [PubMed] [Google Scholar]
- 165.Okamoto T, Takeda S, Giambarella U, Murayama Y, Matsui T, Katada T, Matsuura Y, Nishimoto I. Intrinsic signaling function of APP as a novel target of three V642 mutations linked to familial Alzheimer's disease. Embo J. 1996;15:3769–3777. [PMC free article] [PubMed] [Google Scholar]
- 166.Yamatsuji T, Okamoto T, Takeda S, Murayama Y, Tanaka N, Nishimoto I. Expression of V642 APP mutant causes cellular apoptosis as Alzheimer trait-linked phenotype. Embo J. 1996;15:498–509. [PMC free article] [PubMed] [Google Scholar]
- 167.Zhao B, Chrest FJ, Horton WE, Jr, Sisodia SS, Kusiak JW. Expression of mutant amyloid precursor proteins induces apoptosis in PC12 cells. J Neurosci Res. 1997;47:253–263. [PubMed] [Google Scholar]
- 168.Giambarella U, Yamatsuji T, Okamoto T, Matsui T, Ikezu T, Murayama Y, Levine MA, Katz A, Gautam N, Nishimoto I. G protein betagamma complex-mediated apoptosis by familial Alzheimer's disease mutant of APP. EMBO J. 1997;16:4897–4907. doi: 10.1093/emboj/16.16.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.McPhie DL, Coopersmith R, Hines-Peralta A, Chen Y, Ivins KJ, Manly SP, Kozlowski MR, Neve KA, Neve RL. DNA synthesis and neuronal apoptosis caused by familial Alzheimer disease mutants of the amyloid precursor protein are mediated by the p21-activated kinase PAK3. J Neurosci. 2003;23:6914–6927. doi: 10.1523/JNEUROSCI.23-17-06914.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Smine A, Xu X, Nishiyama K, Katada T, Gambetti P, Yadav SP, Wu X, Shi YC, Yasuhara S, Homburger V, Okamoto T. Regulation of brain G-protein go by Alzheimer's disease gene presenilin-1. J Biol Chem. 1998;273:16281–16288. doi: 10.1074/jbc.273.26.16281. [DOI] [PubMed] [Google Scholar]
- 171.Wolozin B, Iwasaki K, Vito P, Ganjei JK, Lacana E, Sunderland T, Zhao B, Kusiak JW, Wasco W, D'Adamio L. Participation of presenilin 2 in apoptosis: enhanced basal activity conferred by an Alzheimer mutation. Science. 1996;274:1710–1713. doi: 10.1126/science.274.5293.1710. [DOI] [PubMed] [Google Scholar]
- 172.Roth KA. Caspases, apoptosis, and Alzheimer disease: causation, correlation, and confusion. J Neuropathol Exp Neurol. 2001;60:829–838. doi: 10.1093/jnen/60.9.829. [DOI] [PubMed] [Google Scholar]
- 173.Bozzi Y, Borrelli E. Dopamine in neurotoxicity and neuroprotection: what do D2 receptors have to do with it? Trends Neurosci. 2006;29:167–174. doi: 10.1016/j.tins.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 174.Okada F, Crow TJ, Roberts GW. G-proteins (Gi, Go) in the basal ganglia of control and schizophrenic brain. J Neural Transm Gen Sect. 1990;79:227–234. doi: 10.1007/BF01245133. [DOI] [PubMed] [Google Scholar]
- 175.Okada F, Crow TJ, Roberts GW. G proteins (Gi, Go) in the medial temporal lobe in schizophrenia: preliminary report of a neurochemical correlate of structural change. J Neural Transm Gen Sect. 1991;84:147–153. doi: 10.1007/BF01249119. [DOI] [PubMed] [Google Scholar]
- 176.Okada F, Tokumitsu Y, Takahashi N, Crow TJ, Roberts GW. Reduced concentrations of the alpha-subunit of GTP-binding protein Go in schizophrenic brain. J Neural Transm Gen Sect. 1994;95:95–104. doi: 10.1007/BF01276428. [DOI] [PubMed] [Google Scholar]
- 177.Yang CQ, Kitamura N, Nishino N, Shirakawa O, Nakai H. Isotype-specific G protein abnormalities in the left superior temporal cortex and limbic structures of patients with chronic schizophrenia. Biol Psychiatry. 1998;43:12–19. doi: 10.1016/s0006-3223(97)80250-8. [DOI] [PubMed] [Google Scholar]
- 178.Tani M, Mui K, Minami Y, Kiriike N. Association of a GTP-binding protein Go alpha subunit mutation with schizophrenia. Mol Psychiatry. 2001;6:359. doi: 10.1038/sj.mp.4000913. [DOI] [PubMed] [Google Scholar]
- 179.Schiller PH. The ON and OFF channels of the visual system. Trends Neurosci. 1992;15:86–92. doi: 10.1016/0166-2236(92)90017-3. [DOI] [PubMed] [Google Scholar]
- 180.Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J Biol Chem. 1993;268:11868–11873. [PubMed] [Google Scholar]
- 181.Masu M, Iwakabe H, Tagawa Y, Miyoshi T, Yamashita M, Fukuda Y, Sasaki H, Hiroi K, Nakamura Y, Shigemoto R, et al. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell. 1995;80:757–765. doi: 10.1016/0092-8674(95)90354-2. [DOI] [PubMed] [Google Scholar]
- 182.Dhingra A, Lyubarsky A, Jiang M, Pugh EN, Jr, Birnbaumer L, Sterling P, Vardi N. The light response of ON bipolar neurons requires G(alpha)o. J Neurosci. 2000;20:9053–9058. doi: 10.1523/JNEUROSCI.20-24-09053.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dhingra A, Jiang M, Wang TL, Lyubarsky A, Savchenko A, Bar-Yehuda T, Sterling P, Birnbaumer L, Vardi N. Light response of retinal ON bipolar cells requires a specific splice variant of Galpha(o) J Neurosci. 2002;22:4878–4884. doi: 10.1523/JNEUROSCI.22-12-04878.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Duan SZ, Christe M, Milstone DS, Mortensen RM. Go but not Gi2 or Gi3 is required for muscarinic regulation of heart rate and heart rate variability in mice. Biochem Biophys Res Commun. 2007;357:139–143. doi: 10.1016/j.bbrc.2007.03.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhu M, Gach AA, Liu G, Xu X, Lim CC, Zhang JX, Mao L, Chuprun K, Koch WJ, Liao R, Koren G, Blaxall BC, Mende U. Enhanced calcium cycling and contractile function in transgenic hearts expressing constitutively active G(alpha)o* protein. Am J Physiol. 2008;294:H1335–H1347. doi: 10.1152/ajpheart.00584.2007. [DOI] [PubMed] [Google Scholar]