I. Introduction
Arterial biomineralization processes have been afflicting humans for at least 5 millenia, as realized in 2003 via the computed tomographic imaging of Ötzi, the intriguing “Ice Mummy” discovered in the Tyrolean Alps1. Patchy abdominal atherosclerotic calcification was readily detected in the post mortem of this 40-ish year old hunter of the early Copper Age – by 2000 years a predecessor of King Tutankhamen1. Today, an epidemic of vascular calcification is emerging within our aging and dysmetabolic populace2, 3. Although vascular calcification was once considered only a passive process of dead and dying cells, work from laboratories worldwide has now highlighted that arterial biomineralization is an actively regulated form of calcified tissue metabolism4, 5. Moreover, as in skeletal development – where unique biology controls matrix mineralization in membranous bone, endochondral bone, dentin, and enamel6, 7 -- mechanistic diversity exists in the pathobiology of vascular calcium deposition 2, 4, 5, 8. Five common forms of vascular calcification -- each possessing unique histoanatomic characteristics and clinical settings with overlapping yet distinct molecular mechanisms -- have been described to date4, 5, 9 (Table 1). Although we touch upon the subject, the reader is referred to other contemporary reviews for in-depth consideration of pathogenic differences2, 4, 5.
Table 1. Common Histoanatomic Forms of Vascular Calcification & Clinical Settings 2, 4, 5, 11, 99.
| Type | Characteristics | Histopathology | Disease Biology | Risk Factors |
|---|---|---|---|---|
|
Calcific Aortic Valve Disease
(CAVD) a.k.a. calcific aortic stenosis or aortic valve calcification |
|
|
|
|
|
Arterial Medial
Calcification (AMC) a.k.a. medial artery calcification, Mönckeberg sclerosis, or medial calcific sclerosis |
|
|
|
|
|
Atherosclerotic Intimal
Calcification (AIC) |
|
|
|
|
| Vascular calcification of end-stage kidney disease CKD5 |
|
|
||
|
Calcific Uremic
Arteriolopathy (CUA) a.k.a. calciphylaxis |
|
|
|
AKP2, bone alkaline phosphatase; BMP, bone morphogenetic protein; CKD, chronic kidney disease; CRP, C reactive protein; CUA, calcific uremic arteriolopathy; CVC, calcifying vascular cell of Demer; ESR, erthyrocyte sedimentation rate; GFR, glomerular filtration rate; MGP, matrix gla protein; MI, myocardial infarction; Msx2, osteoblast transcription factor muscle segment homeobox homolog 2; NOS, nitric oxide synthase; Osx, osteoblast transcription factor osterix; Pit1, phosphate transporter SLC20A1; PTH, parathyroid hormone; RANKL, receptor activator of NF-kappaB ligand; Runx2, osteoblast transcription factor runt related transcription factor 2; TNF, tumor necrosis factor; VIC, valve interstitial cell; VSMC, vascular smooth muscle cells.
In this brief review and perspective, we recount recent data that emphasize inflammation and oxidative stress signaling as key contributors to the pathogenesis of vascular mineral deposition10. Furthermore, we highlight differences between the low density lipoprotein receptor (LDLR)-deficient and apolipoprotein E (apoE)-deficient murine models (Table 2) that help articulate the multifaceted contributions of dyslipidemia, diabetes, and uremia to arterial calcium deposition2, 4, 11. We end by summarizing the importance of considering these disease stage- and context-specific contributions arterial mineralization when crafting therapeutic strategies to address the disease burden of vascular calcification that increasingly afflicts our patients5, 12.
Table 2. Features of ApoE-/- and LDLR-/- Murine Models of Arterial Calcification*.
| Murine Disease Model | ||
|---|---|---|
| Parameter | ApoE-/- Mouse | LDLR-/- mouse |
| Diet-induced hypercholesterolemia | Yes33, 185 | Yes33, 186 |
| Diet-induced atherosclerosis | Yes185 | Yes187 |
| Diet–induced diabetes | No33 | Yes33, 44 |
| Diet-induce obesity | No33 | Yes33 |
| “Spontaneous” arterial chondroid metaplasia | Yes54, 98 (accelerated by drug-induced diabetes) |
No29, 44 |
| Early diet-induced non-endochondral7 medial artery calcification | No54 | Yes29, 168 |
| Late diet-induced endochondral7 atherosclerotic calcification | Yes54 | Yes28, 29, 168 |
| Hemodynamically -significant calcific aortic valve disease occurs with progression | Not known (valve thickening seen with CRI)188 |
Yes126, 189 (concomitant ApoB100/100 genotype) |
| Exaggerated inflammatory response and susceptibility to mortality with gram-negative sepsis | Yes53, 190 | Less so**53, 191 |
| Arterial calcification accelerated by chronic renal insufficiency (CRI) | Yes141 | Yes118 |
II. Inflammatory cytokines in the initiation and progression of arterial calcification: Lessons learned from LDLR-/- and apoE-/- mice
Some degree of vascular inflammation is a frequent concomitant of most forms of arterial calcification13, 14 Sites of inflammation relevant to disease biology may not only include the atherosclerotic intima and media, but also the tunica adventitia15-18. Of note, calcification of the elastic lamina with elastinolysis in the absence of overt histologic inflammation has been reported,19-23 and intimal CD68+ macrophage accumulation is more commonly associated with atherosclerotic vs. medial calcification24. However, because calcium phosphate mineral deposition itself elicits inflammatory responses25 -- including tumor necrosis factor (TNF) production by macrophages26, 27 -- a primary role for inflammation in the pathogenesis of clinically relevant vascular calcification was unproven until very recently28-32. In this section, we review this new data -- and also highlight distinctions between the LDLR-/- and apoE-/- murine disease models33 (Table 2) that provide insights into the mechanistic complexities of inflammation-dependent arterial calcium accumulation.
II.A.1 RANKL/OPG signaling and atherosclerotic calcification
The first robust evidence for the primary contributions of inflammatory cytokine signaling to pathogenesis of vascular calcification arose from the generation and evaluation of the osteoprotegerin (OPG)-/- mouse34. OPG-deficient mice develop severe medial and intimal arterial calcification in conjunction with high-turnover osteoporosis driven by excessive osteoclast formation34. OPG was first shown to function as an antagonistic “faux receptor” of RANKL (receptor activator of NFκB ligand), the TNF superfamily member that signals via its receptor RANK on monocyte/macrophage progenitors to promote the formation of bone-resorbing osteoclasts7, 35. In bone, the antagonist OPG is expressed alongside RANKL in the osteoblast lineage, However, OPG is also expressed in vascular smooth muscle cells and endothelial cells of large arteries – a venue where RANKL is normally absent but induced with inflammation35. RANKL expression is readily detected in T-cells and macrophages near atherosclerotic lesions, and within cytokine-stimulated endothelium35. Intriguingly, RANKL has been recently shown to promote osteochondrogenic mineralization of VSMCs (vascular smooth muscle cells) 36 and aortic VICs (valve interstitial cells)37 in vitro. Via the RANK expressed in VSMCs, RANKL upregulates BMP4 (bone morphogenetic protein 4) expression, thus providing an autocrine stimulus for osteogenic differentiation (see also section IV below)36. These dual and disparate actions of RANKL upon the skeletal monocyte/macrophage lineage vs. VSMCs likely explain the intriguing phenotype of OPG-null mice34. Of note, although the vascular calcification of OPG deficiency occurs in the complete absence of atheroma formation34, calcified lesions begin to form in arteries only in the post-partum period with copious CD3+ T-cell infiltrates, a few F4/80+ macrophages, and cathepsin K+ osteoclast-like cells 34, 38. This suggests that, in vivo, inflammatory signals absent in utero are necessary for vascular disease initiation and progression in OPG-/- animals. Additionally, as first observed in the diabetic LDLR-/- mouse39, serum levels of OPG are higher in patients with diabetes40, 41. Since OPG is expressed in VSMCs42, such increases in the setting of type II diabetes presumably reflect a vascular defense that helps prevents excessive RANKL signaling via negative feedback regulation28.
II.A.2 Perturbations in RANKL/OPG signaling and the pathobiology of arteriosclerosis
Although compelling, the “spontaneous” vascular calcification observed in response to the genetic lesioning in OPG deficient mice did not ensure contributions to the pathobiology of arteriosclerosis34; however, this caveat has been recently addressed.28 Inhibition of RANKL via administration of recombinant OPG has been evaluated in two very different murine models of vascular disease33 -- the LDLR-/-mouse28 and the apoE-/- mouse43. It is important to highlight that, while both models encompass impaired cholesterol metabolism and atherosis on the C56Bl/6 background, the arteriosclerotic disease processes exhibited by these two preclinical models are very distinct (Table 2)33. As LeBoeuf first showed, while both models develop atheroma in response to cholesterol-containing fatty diets, the apoE-null mouse never develops the clinically relevant contributions of insulin-resistant diabetes and obesity33. However, the male LDLR-/- mouse develops both of these relevant characteristics alongside arterial calcification in response to challenge with fatty diet possessing compositions typical of Westernized societies33, 44 – a clinically important stimulus for vascular disease45, 46. Early medial artery calcification is followed by progressively severe atherosclerotic disease in this model (see below)29. Furthermore, the diet- induced systemic low-grade inflammation -- characterized by low but measurable levels of circulating TNF in obese LDLR-/- mice29, 47 and diabetic humans48-50 -- is not seen and apparently does not contribute to vascular inflammation in the apoE-/- model43, 51 even when streptozotocin is administered to induce diabetes52. However, in response to other stimuli such as lipopolysaccharide administration or Klebsiella infection, apoE-/-mice exhibit exaggerated TNF induction and increased mortality53. Finally, in the apoE-null mouse, vascular calcification quickly evolves upon the backdrop of VSMC chondroid metaplasia8 that is observed over time even on mouse chow – i.e., in the absence of cholesterol-rich dietary challenge54. By comparison, evolution of arterial calcification in the LDLR-/- mouse is more protracted and elicited by the clinically relevant Western diet (42% of calories from fat, 0.15% cholesterol), accruing vascular mineral deposition via sequentially distinct mechanisms28, 29. At early stages, vascular calcification can be histologically detected by Alizarin red staining within the tunica media of major conduit arteries of diabetic, male LDLR-/- mice -- biochemically quantifiable following acid extraction.29 Atheromata are not uniformly present at this early stage, and if present do not stain for calcium. As with atherosclerosis, the initial calcium deposition within the tunica media may be elastin organized phospholipid vesicles55, 56, since very little inorganic phosphate staining is evident by von Kossa at this stage29. Similar observations have been described in human specimens57. With progression, however, massive aortic sinus and subintimal cholesterol deposits accrue, with atherosclerotic calcification visualized within the cholesterol clefts and degenerating atheromata29. During this second phase, chondroid metaplasia clearly contributes to vascular calcium accrual in male LDLR-/- mice28 as observed in apoE-/- mice8. The extent of medial calcium is thus increased upon Alizarin red staining29, 57, and the von Kossa method for visualizing inorganic phosphate now reveals massive medial and atherosclerotic calcium phosphate deposition in male LDLR-/- mice fed fatty diets28, 29. Thus, when place on high fat westernized diets, the male LDLR-/- mouse sequentially elaborates an early arterial medial calcification program (Table 1) that with disease progression is augmented by processes of atherosclerotic intimal calcification (Table 1; see also Table 2).
II.A.3 Inhibition of RANKL signaling as a therapeutic approach to arteriosclerotic calcification
As noted above, OPG is an endogenous inhibitor of RANKL signaling that limits arterial calcium accumulation during development. Recently, the impact of pharmacologic inhibition of RANKL by OPG has been evaluated in the above preclinical models of atherosclerosis and arterial calcification. Interestingly, very distinct responses are observed with OPG administration in LDLR-/- and apoE-/- mice28, 43. Demer and colleagues first evaluated the male LDLR-/- mouse, the dynamics of endogenous RANKL/OPG signaling during disease initiation and progression, and the impact of exogenous OPG administration28. Serum RANKL measurements demonstrated that progression of vascular disease over 5 months of dietary cholesterol challenge closely tracks the progressive recovery of circulating RANKL following an early phase of diet-induced suppression 28. Early diet-induced increases in OPG – a presumed adaptive mechanism to protect against untoward RANKL signaling36 – exhibited no dynamic change with progression28. As predicted from studies of OPG-/-mice34, male LDLR-/- mice treated with exogenous OPG exhibit reduced arterial calcification and diminished aortic osteochondrogenic differentiation28. However, no change in atherosis – i.e. the size of arterial atheroma – was observed28. Intriguingly, three sources of vascular RANKL production were identified in this LDLR-/- model: (i) the F4/80+ monocyte-macrophage population in closest proximity to lesions undergoing chondroid metaplasia; (ii) the endothelial cells overlying atheroma; and (iii) the CD3+ T-cells at the adventitial-medial junction28. Whether any one source of RANKL production represents the lynchpin for the OPG-dependent inhibition of progressive vascular mineral accrual in this model remains to be determined.
In apoE-/- mice, as in LDLR-/- mice, OPG administration apparently does not affect atheroma lesion size43. However, OPG significantly increases fibrous cap size and thickness and reduces MMP12 levels, potentially stabilizing the lesion but not directly assessed43 (see below). Non-significant, tantalizing trends for reductions in numbers of macrophages and T-cells were also observed in response to OPG administration. Unlike male LDLR-/- mice -- where diet-induced obesity increases circulating TNF levels29 -- basal TNF levels are below the limits of detection in apoE-/- animals and thus not measurably changed by OPG administration 43. Calcification was, unfortunately, not scored in this recent study43. However, Bennett and colleagues have applied murine genetics to carefully detail the important role for endogenous OPG in the calcification of advanced atherosclerotic lesions of apoE-/- mice by generating and evaluating OPG-/-;apoE-/- mice31. In this model, congenitally deficient OPG-/-;apoE-/- mice exhibit atherosclerotic lesions of increased size in the innominate artery, with significantly increased areas of calcification and aortic calcium accumulation measured during disease progression31. Plaque stability was not assessed in OPG-/-;apoE-/- mice, but OPG was shown to increase MMP9 (matrix metalloproteinase 9) activity in vitro31, and MMP9 promotes intraplaque hemorrhage in vivo in advanced atherosclerotic lesions of apoE-null animals58, 59. However, congenitally deficient MMP9-/-;apoE-/- mice exhibit increased lesion size following disease initiation vs. MMP9-replete siblings60, suggesting that stage-specific roles of MMP9 exist in atherosis and sclerosis58. As a modulator of MMP9, OPG could potentially exert adverse as well as beneficial arteriosclerotic actions during pharmacological manipulation of RANKL signaling 31. Thus, as in the LDLR-/- mouse, OPG limits arterial calcium accumulation in the apoE – null mouse. OPG may regulate plaque stability -- but the differential responses of pharmacologic vs. genetic manipulation of OPG on vascular histopathology in apoE-/- mice highlight the need for more a detailed assessment of impact upon plaque formation, stability, and regression.
In summary, antagonism of RANKL signaling cascades holds much promise for modulation of atherosclerotic calcification61. Of note, a humanized antibody that antagonizes human RANKL has been developed for prevention of fractures in osteoporosis62; based upon preclinical studies of Hofbauer et al using a “humanized RANKL” murine model32, this same reagent might be useful in treatment of cardiovascular calcification. However, the net impact on vascular physiology – vascular compliance, Windkessel-dependent conduit function, distal tissue perfusion, arterial remodeling and plaque stability – has yet to be determined.
II.B.1. Medial artery calcification, arteriosclerosis, and lower extremity amputation risk in T2DM
The relationship between arteriosclerotic medial artery calcification (AMC; Table 1) and the risk of lower extremity amputation in T2DM has been appreciated for 2 decades63, 64. The earliest studies were reported for Pima Indians, a native American population with increased risk for T2DM63, 64. Subsequent studies from Finland identified that radiographic femoral medial artery calcification – not atherosclerotic calcification – was the single best predictor of lower extremity amputation in T2DM65. Why, then, does increased arterial stiffness (arteriosclerosis) in T2DM -- arising from AMC without peripheral atherosclerosis -- contribute to the increased risk for lower extremity amputation?66 Conduit vessel stiffening from any cause67 compromises normal arterial Windkessel physiology67, 68, thus impairing uniform distal tissue perfusion throughout the cardiac cycle69, 70.
At this point, however, it should be re-emphasized that critical limb ischemia (CLI) arising from atherosclerotic plaque formation and arterial stenosis in the femoropopliteal bed is a well-recognized contributor to lower extremity amputation risk; moreover, atherosclerotic calcification also contributes to conduit vessel stiffness71-74. Medical strategies such as statins that reduce atherosclerotic disease burden also improve outcomes in patients with peripheral arterial disease (PAD)71, 75. Reductions in ankle-brachial indices (ABIs) provide a clinically useful tool for identifying symptomatic individuals at risk72, 73. Increased mobility, reduced claudication, limb salvage, and improved ABIs can often be achieved by surgical or percutaneous vascular interventions71 -- more successfully so in stenosed distal femoropopliteal segments76, 77 than proximal segments78, and less successfully so in patients with diabetes79-81. However, in the setting of T2DM, PAD arises with contributions from both medial artery calcification and atherosclerosis74. Furthermore, in T2DM, ABIs are frequently elevated82 – not reduced – due to medial calcific sclerosis74, 82. While elevated ABIs do not necessarily convey increased risk for atherosclerotic disease83, an ABI ≥ 1.3 does indicate the presence of arteriosclerosis – i.e., arterial stiffening -- and concomitantly portends lower extremity amputation84. In summary, the clinical evaluation of PAD in patients with T2DM requires special consideration, including assessment of toe-brachial indices in lieu of ABIs82.
II.B.2. Mechanisms of medial artery calcification in T2DM: Clues from the field of bone biology and the LDLR-/- mouse
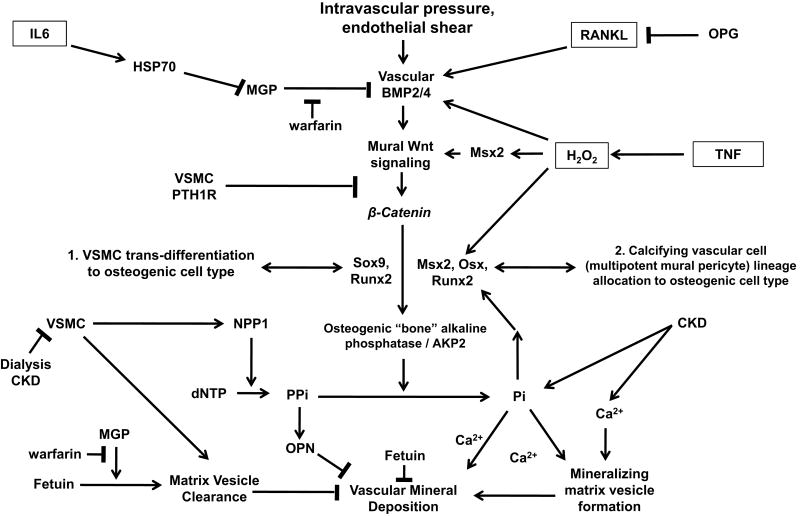
During skeletal mineralization, bone formation can occur via either endochondral (preceding cartilage template required) or membranous (non-endochondral; no cartilage required) processes7. Osteo/chondrocytic transcription factors such as Sox9, Runx2/Cbfa1, Msx2, Msx1, and Osx play critical roles in promoting either endochondral (Sox9, Runx2, Osx) or membranous (Msx2, Msx1, Runx2 and Osx) bone formation7. In bone, polypeptide morphogens such at BMPs (bone morphogenetic proteins) and Wnts (wingless/mouse mammary tumor virus integration site family) induce these osteoblast DNA binding proteins along with β-catenin, a transcription co-adapter indispensible for bone formation7, 85. A common feature of active osteogenic mineralization is induction of AKP2, the “bone” alkaline phosphatase that degrades the plentiful and endogenous mineralization inhibitor, inorganic pyrophosphate (PPi) (Figure 1)7. Of note, Sox9, Runx2, Msx2, and AKP2 have all been described as being expressed in calcifying human arterial segments86, and are upregulated by stimuli that promote arterial calcification (Figure 1).
Figure 1. Inflammation and Osteogenic Regulation of Vascular Calcification: A Review and Working Model.
Osteochondrocytic cells that promote vascular matrix mineralization can arise from at least two sources: (1) trans-differentiation of VSMCs – i.e., a type of phenotypic modulation in which the mature VSMC phenotype is replaced, and reprogrammed to that of an osteochondrocytic cell; or (2) osteogenic lineage allocation from a multipotent mesenchymal progenitor – i.e., a cell that has the potential to become an osteoblast, chondrocyte, VSMC, or adipocyte. Both processes are triggered by key inflammatory cytokines and oxidative stress signaling (boxed). VSMCs also elaborate apoptotic bodies and matrix vesicles that can nucleate mineral deposition – but also may play a role in removing vascular calciprotein particles via fetuin and MGP-dependent cellular uptake. Thus, apoptosis of VSMC not only provides substrate for nucleation, but also loss of cellular defenses. Multiple paracrine inhibitors control (a) pro-osteogenic signals provided by BMP/Wnt signaling, RANKL and TNF actions; and (b) nucleation/aggregation/epitaxial propagation of apatitic calcium phosphate deposition. Via HSP70-mediated inhibition of MGP and AKP2-mediated PPi degradation, inflammatory cytokines such as IL6 and TNF impair MGP and PPi defense mechanisms, respectively. Inflammation also down-regulates expression of serum fetuin, an import hepatocyte-derived inhibitor of soft tissue mineral deposition. Not shown are the enzymatic defense mechanisms such as catalase and glutathione peroxidase that reduce vascular oxidative stress10, 88, 169. Although clearly an important stimulus for vascular BMP2 expression132, remarkably few studies have examined the molecular mechanisms whereby hypertension activates vascular osteogenic signaling cascades. Of note, contribution of marrow-derived osteogenic endothelial progenitor cells as an additional source of mineralizing vascular mesenchymal progenitors has been recently posited, but has yet to be established153. See text for details and additional references.
The molecular mechanisms controlling initiation and progression of medial artery calcification in T2DM have recently been studied in detail in the male LDLR-/- mouse (Table 2) – a model in which obesity, diabetes, and osteogenic arterial calcification programs are induced in response to high fat diets possessing compositions characteristic of westernized societies 2, 29, 39, 44, 87. Importantly, diet-induced disease in male LDLR-/- mice2, 29, 44, 87 closely tracks molecular and physiological characteristics of T2DM patients afflicted with valve88, 89 and arterial86, 90 calcification. A critical clue to the pathogenesis of AMC in this setting arose from recognition that T2DM induces a low grade systemic inflammatory state, programmed in part by adipokines – i.e., fat-derived cytokines48-50 91, 92. TNF is the prototypic inflammatory cytokine, elaborated not only by adipocytes but also by adipose tissue macrophages (ATM) that infiltrate fat with “diabesity.”48, 93, 94. Demer et al first identified that TNF and a macrophage-derived signal stimulated the mineralization of aortic calcifying vascular cells (CVCs) in vitro95. Subsequently, we demonstrated that infliximab-mediated inhibition of TNF signaling in vivo in the LDLR-/- mouse down-regulated osteogenic Msx2-Wnt gene regulatory program in aortas of diabetic LDLR-/- mice29. Concomitant reductions in early vascular calcium load was also observed with infliximab29. While diet-induced abnormalities in fasting glucose and lipid profiles were not improved, dosing with infliximab did decrease serum 8-F-isoprostane levels, an oxylipid and marker of oxidative stress in T2DM29. Conversely, local augmentation of TNF tone in the aortic wall with a SM22-TNF transgene activated aortic Msx2-Wnt signaling in the absence of diet-induced disease, demonstrating the important role of TNF in the initiation of macrovascular disease in T2DM29. Others have now also confirmed the important role for Msx2 in TNF-dependent induction of AKP2 and mineralization in VSMCs (Figure 1)96. Koleganova highlighted the significance of these preclinical studies to human disease biology in those afflicted with arterial calcification of renal failure90; TNF, Msx2, and BMP2 expression were correlated with osteogenic differentiation in both calcified and non-calcified vessel segments of patients with CKD5 (chronic kidney disease)90.
Thus, in summary, these data90 and others86, 88, 89 confirm the clinical relevance of the osteogenic relationships established in the LDLR-/- murine model of calcific vasculopathy (Figure 1). Obligatory diet-induced “diabesity” in the LDLR-/- model is an important feature of this model that is highly relevant to the burgeoning disease burden of westernized societies. As in diseased humans vessels, osteogenic transcription factors (Msx2, Runx2, Osx, Sox9) are ectopically induced in the arteries and valves of diabetic LDLR-/- mice. Mechanistic insights possible via preclinical studies point to both (a) trans-differentiation of VSMCs; and (b) osteochondrogenic lineage allocation of multipotent mesenchymal progenitors by these osteogenic transcription factors4, 97. Since streptozotocin drug-induced diabetes also accelerates osteochondral metaplasia in the apoE-/- mouse98, further evaluation of this model may help elucidate the pathobiological mechanisms whereby hyperglycemia promotes arterial mineralization.
II.C. Inflammation, fetuin, and matrix vesicle metabolism: Novel insights into the calcific vasculopathy of chronic kidney disease (CKD)
CKD, particularly CKD5, represents a “perfect storm” of calcific vasculopathy (Table 1)11. Antecedent diabetes, hypertension, and dyslipidemia intersect with phosphate retention, low turnover bone disease, and dialysis-induced systemic inflammation and VSMC apoptosis synergize to drive ferocious arterial calcium accrual (Figure 1)11. Arterial calcification of CKD5 and in calcific uremic arteriolopathy (also called calciphylaxis; Table 1) have been reviewed in detail, and the reader is referred to these excellent manuscripts11, 99, 100. However, fetuin biology as relevant to the arterial calcification in CKD5 is worthy of special consideration, particularly within the context of inflammation-mediated vascular disease.
Fetuin - a.k.a. fetuin A, alpha-2-Heremans-Schmid glycoprotein, AHSG -- is a serum protein synthesized by the liver99. As first demonstrated by Jahnen-Dechent, fetuin avidly binds amorphous calcium phosphate, and maintains the solubility of supersaturated serum calcium phosphate101 via the formation of calciprotein particles that inhibit insoluble calcium phosphate crystal aggregate formation (Figure 1) 102-106. Consistent with these observations, Jahnen-Dechent, Ketteler, and colleagues demonstrated widespread soft tissue calcification in fetuin-deficient mice106. Shanahan recently identified that fetuin also plays a critical role in VSMC-mediated removal of pro-calcific matrix vesicles107, 108. In response to hypercalcemia and hyperphosphatemia – common stimuli in dialysis patients101 – VSMCs elaborate matrix vesicle and apoptotic bodies that not only can nucleate extracellular matrix deposition but might also help facilitate clearance of vascular calciprotein particles107, 108. Serum-derived fetuin and matrix vesicle-associated matrix Gla protein (MGP) are required for VSMC-mediated uptake and clearance of vesicles (Figure 1)107, 108. Importantly, fetuin is an “inverse” acute phase reactant, decreased by inflammation via inhibition of the CCAAT/enhancer binding protein-DNA interactions that support fetuin gene transcription in hepatocytes109, 110. In ESRD, fetuin levels are inversely related to extent of coronary calcification, providing yet another link between inflammation, oxidative stress, and arterial calcium accumulation111, 112. Similar results have been noted in patients with calcific aortic stenosis113.
In summary, the cumulative evidence overwhelmingly points to an important role of fetuin in limiting arterial calcium deposition. It remains to be determined if normalization or augmentation of serum fetuin reduces vascular calcification in a model of inflammation-induced vascular disease. Of note, increased patient mortality in CKD5 – an outcome related to the extent of vascular calcification114 – is associated with reductions in fetuin but is significant only in the setting of inflammation115. This suggests that other signals elaborated by inflammation independent of fetuin suppression – such as reactive oxygen species – must play an important pathophysiological role. Finally, the reader is referred to outstanding recent reviews highlighting the critical contributions of hyperphosphatemia11, 116, VSMC BMP2-dependent phosphate transport117, and BMP7-corrected hyperphosphatemia118, 119 to the pathobiology of vascular calcification in the setting of CKD.
III. Oxidative stress signaling and vascular calcification: Peroxide paves an osteogenic path
As noted above, when coupled with the clinical setting, histoanatomic and molecular characteristics distinguish aortic valve calcification, atherosclerotic calcification, diabetic medial artery calcification, vascular calcification of ESRD, and calcific uremic arteriolopathy (Table 1)120. However, over the past two years multiple groups have newly identified the important role of oxidative stress signaling in vascular activation of osteogenic gene regulatory programs88, 121. Chen first demonstrated that the osteochondrocytic transcription factor Runx2/Cbfa1 is activated by hydrogen peroxide (H202) and supports bone alkaline phosphatase (AKP2) expression and matrix mineralization in cultured vascular smooth muscle cells (Figure 1)121. Similarly, membranous ossification programs122, 123 elaborated by Msx2-Wnt signaling cascades are also dependent upon peroxide signals elaborated from mitochondrial activity and downstream of TNF stimulated NADPH oxidases124, 125. Very recently, Miller, Heistad, and colleagues demonstrated co-localization of oxidative stress signaling and the osteogenic transcription factors Msx2 and Runx2/Cbfa1 in calcifying human aortic valves88. However, the sources of oxidative stress were shown to arise from uncoupling of nitric oxide synthase (NOS) and failures in the enzymatic defenses (e.g., catalase) that restrain peroxide accumulation88. Using the “Reversa” mouse model, they subsequently demonstrated that elevated cholesterol levels are required for calcification and sustained vascular induction of the osteogenic transcription factors Msx2 and Runx2/Cbfa1126. This suggests that an oxylipid127 -- in addition to oxylipid-responsive cytokines such as TNF29, 95 and RANKL28, 128 -- is required for vascular calcification, as first proposed by Demer95, 127, 128.
In summary, although sources of oxidative stress may differ with vascular venue and disease state88 -- and the signaling cascades have yet to be fully elucidated124 -- oxidative stress signals provide important stimuli. With inflammatory cytokine signals, this helps provide a unifying theme for arterial elaboration of osteogenic mineralization processes.
IV. Of BMPs and Wnts: Osteogenic morphogens as proximal mediators of vascular calcification
BMP2 is a powerful osteogenic morphogen that promotes bone formation during skeletal development and also maintains skeletal integrity and supports fracture repair during post-natal life129. Via an autocrine Wnt signaling loop, BMP2 promotes osteoblast commitment and the induction of the bone alkaline phosphatase (AKP2) 130, the latter an important enzymatic mediator of osteogenic matrix mineralization (Figure 1)130. Almost two decades ago, Bostrom, Demer and colleagues identified the presence of BMP2 in calcified atherosclerotic plaques and demonstrated the important role for BMP2 in CVC mineralization131. With Anderson's studies55, this provided the first molecular insights into the biology of vascular calcium deposition and vascular BMP2 expression -- now mechanistically integrated with pathophysiological states that initiate calcific vasculopathy124. Ungvari and colleagues recently demonstrated that TNF, H202, and high intravascular pressure –stimuli commonly encountered with diabetes, hypertension, and the metabolic syndrome -- all upregulate the expression of BMP2 in endothelial cells132. This provides a morphogenetic cue that reinforces osteogenic differentiation of multipotent vascular mesenchymal cells such as pericytes and CVCs that reside within the vascular wall 131, 133, 134(Figure 1). Moreover, as mentioned above, RANKL stimulates BMP4 production by VSMCs, providing an autocrine stimulus for osteochondrogenic transdifferentiation -- if not held in check by BMP4 antagonists such as noggin36 or MGP30. Importantly, in addition to being entrained to TNF29, the vascular osteogenic Wnt signaling cascades previously discussed87, 124, 125 (Figure 1) are also activated downstream of BMP2 in vivo2. Of note, these canonical Wnt signals drive osteochondrocytic differentiation of multipotent vascular pericytes in vitro135 as well as promote the arterial calcification of type II diabetes in vivo87; via multiprotein cell surface receptor complexes containing LRP5 or LRP6, the Wnt polypeptide family contributes to bone morphogenesis and skeletal integrity136 in conjunction with the BMPs137. Intriguingly, along with many Wnt ligands, LRP5 and LRP6 are expressed in endothelial cells and VSMCs138. The precise reasons why vascular expression of these osteogenic morphogens does not always lead to arterial mineralization is still unclear. This presumably reflects the local balance between agonists and antagonists of BMP/Wnt signaling124 (e.g., MGP and Dkk1, respectively; Figure 1); the important roles of inorganic pyrophosphate139, fetuin107, and osteopontin140 as osteogeonic mineralization inhibitors; elastin metabolism141-144; and the impact of matrix stiffness145 upon the osteogenic potential of vascular mesenchymal progenitors. Nevertheless, strategies that can selectively (a) inhibit the activation or the actions of vascular osteogenic BMP/Wnt signaling; or (b) augment vascular defenses that prevent mineralization hold promise for limiting arterial calcium accumulation.
V. Inorganic pyrophosphate (PPi) and Matrix Gla protein (MGP): Overlapping consequences of genetic and inflammation-induced deficiency in two key vascular defenses
Elegant genetic studies in mice and humans have highlighted the important roles for inorganic pyrophosphate and MGP as non-inflammatory inhibitors of vascular mineralization. Karsenty and colleagues first identified that murine deficiency in the BMP2/4 antagonist MGP133 results in chondroid metaplasia of the arterial tunica media, pan-arterial calcification, and vascular rupture146. Similarly, Terkeltaub showed that murine deficiency in the mineralization inhibitor PPi, arising from genetic disruption of the ectoezyme NPP1 (ectonucleotide pyrophosphatase/phosphodiesterase I) also results in arterial calcification with chondroid metaplasia139. The relevance of the PPi/NPP1 axis to human genetic disease is established via identification that generalized arterial calcification of infancy (OMIM #208000), a rare congenital disorder, arises from PPi deficiency due to NPP1 loss-of-function mutations147. How, then, is inflammation connected to these critically important inhibitors of mineralization? Overtly, the induction of AKP2 by TNF95 and down-stream osteogenic BMP-Wnt pathways29, 87, 136 (described above) hydrolyzes PPi to destroy this inhibitor during vascular mineralization (Figure 1)148. Moreover, depletion of PPi markedly down-regulates osteopontin149, the even more potent inducible inhibitor of vascular mineralization140. However, until very recently, the relationship between inflammation and MGP insufficiency was less clear. In a series of very insightful studies, Bostrom identified that MGP is a Gla-dependent inhibitor of BMP2 and BMP4150, osteogenic morphogens that upregulate AKP2 expression30, 133, 150. Bostrom went on to show that IL6, an inflammatory cytokine important in diabetic vascular disease, increases the expression and secretion of HSP70, an endogenous MGP binding protein and antagonist of MGP function that is highly expressed in calcifying atherosclerotic plaques30. Thus, by inducing HSP70, inflammatory signals provided by IL6 potentiate vascular BMP2/4 actions by nullifying MGP30 (Figure 1). Whether other inflammatory cytokines participate in this hierarchy of regulated mineralization is unknown. Nevertheless, these newer data point to how two axes – genetic defenses against vascular mineralization and inflammation –induced arterial osteogenic programs – functionally intersect to regulate arterial calcification4.
VI. Summary and Future Directions
The fund of knowledge available to the field of arterial calcification and vascular mineral metabolism has dramatically grown in recent years. Our understanding of this disease biology has been enabled by incredible advancements in bone and mineral research that occurred alongside innovative investigation in cardiovascular medicine and insightful human studies from astute clinician-scientists. As in bone, mechanistic heterogeneity exists in the different forms of vascular mineral deposition, and also during stages of disease initiation and disease progression. Moreover, there is heterogeneity in the sources and mechanisms of mineralizing vascular cell types; osteochondrocytic VSMC trans-differentiation, VSMC apoptosis, and osteochondrocytic lineage allocation of multipotent mesenchymal cells all contribute, but to varying extents dependent upon pathophysiologic setting and disease stage (Figure 1). It has been posited that marrow derived circulating osteoprogenitors may also contribute to vascular mineralizing cell types151-153, but this has yet to be unambiguously established.
To date, approaches to prevent and/or reverse macrovascular calcification have largely been unsuccessful154, due in part to this mechanistic heterogeneity and the intrinsic pro-inflammatory actions of vascular calcium phosphate that provide a “feed-forward” stimulus for disease25, 26. In addition, clinical setting dramatically alters the metabolic milieu and rate-limiting pathophysiology of calcific vasculopathy (Table 1) – risk factors that differentially impact disease initiation and progression155. Moreover, not all human medial calcific sclerosis may be associated with overt inflammation; indeed, use of oral calcium-based phosphate binders alone increases coronary artery medial calcification in CKD – a vascular bed not usually afflicted by medial sclerosis24. However, recent data highlight the fundamental contributions of inflammation, oxidative stress, and osteogenic morphogen signaling in this vascular disease. With careful patient selection and consideration of the diseased vascular segment, intervention with a potent statin may yet play a clinically important role via LDL cholesterol reduction and anti-inflammatory actions12, 156, 157. Unfortunately, simple strategies that seek to “scavenge” redox signals elaborated by inflammation do not offer significant clinical benefits for most individuals at risk80. Approaches that target the OPG/RANKL pathway61, the calcium sensing receptor158 and other calciotropic signals39,116, 119, or key vascular proteases141, 144 offer hope --- but individually may be insufficient in some clinical settings, particularly dialysis-dependent renal failure100,159. Except for a few prescient reports on the relationships between arterial pressure and vascular BMP-Wnt signaling132, 160-164, remarkably few studies have examined the mechanistic links between hypertension and signals that regulate arterial calcification165. Given that endothelin-dependent signals control both vascular calcium homeostasis and blood pressure166, 167, the paucity of such studies represents an unmet scientific need. Moving forward with mechanistic insights, pharmacological strategies can be crafted that newly acknowledge disease complexity4, 5, and thus antagonize with sophistication the combination of pathobiological processes that promote vascular mineralization during disease initiation and progression168. The future holds great promise for the development of these successful therapeutics and the medical management necessary to address a burgeoning clinical need5.
Acknowledgments
Sources of Funding: Supported by NIH R01 grants HL069229, HL088138, and HL088651 to D.A.T., and the Barnes-Jewish Hospital Foundation.
Footnotes
Disclosures
J.S.S., S.L.C. & D.A.T. receive support from the National Institutes of Health. D.A.T receives support from the Barnes-Jewish Hospital Foundation, and serves as a paid consultant for the UCLA Department of Medicine Program Project P01HL030568 and for the Institute of Medicine. J.Sadhu has no disclosures.
References
- 1.Murphy WA, Jr, Nedden Dz D, Gostner P, Knapp R, Recheis W, Seidler H. The iceman: discovery and imaging. Radiology. 2003;226:614–629. doi: 10.1148/radiol.2263020338. [DOI] [PubMed] [Google Scholar]
- 2.Shao JS, Cai J, Towler DA. Molecular mechanisms of vascular calcification: lessons learned from the aorta. Arterioscler Thromb Vasc Biol. 2006;26:1423–1430. doi: 10.1161/01.ATV.0000220441.42041.20. [DOI] [PubMed] [Google Scholar]
- 3.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 4.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–2948. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Towler DA. Vascular Calcification: A Perspective On An Imminent Disease Epidemic. IBMS BoneKEy. 2008;5:41–58. doi: 10.1138/20080298. [DOI] [Google Scholar]
- 6.Chung UI, Kawaguchi H, Takato T, Nakamura K. Distinct osteogenic mechanisms of bones of distinct origins. J Orthop Sci. 2004;9:410–414. doi: 10.1007/s00776-004-0786-3. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MM., Jr The new bone biology: pathologic, molecular, and clinical correlates. Am J Med Genet A. 2006;140:2646–2706. doi: 10.1002/ajmg.a.31368. [DOI] [PubMed] [Google Scholar]
- 8.Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, Frutkin A, Dichek D, Giachelli CM. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–741. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Towler DA, Demer LL. Chapter 93: Vascular Calcification. American Society For Bone and Mineral Research; 2008. [Google Scholar]
- 10.Towler DA. Oxidation, inflammation, and aortic valve calcification peroxide paves an osteogenic path. J Am Coll Cardiol. 2008;52:851–854. doi: 10.1016/j.jacc.2008.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moe SM, Chen NX. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2008;19:213–216. doi: 10.1681/ASN.2007080854. [DOI] [PubMed] [Google Scholar]
- 12.Rajamannan NM. Calcific aortic stenosis: lessons learned from experimental and clinical studies. Arterioscler Thromb Vasc Biol. 2009;29:162–168. doi: 10.1161/ATVBAHA.107.156752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virchow R. Cellular pathology. As based upon physiological and pathological histology. Lecture XVI--Atheromatous affection of arteries. 1858. Nutr Rev. 1989;47:23–25. doi: 10.1111/j.1753-4887.1989.tb02747.x. [DOI] [PubMed] [Google Scholar]
- 14.Timio M. Historical Archives of Italian Nephrology. Virchow's theories on atherosclerosis and related kidney disease. G Ital Nefrol. 2003;20:393–399. [PubMed] [Google Scholar]
- 15.Takaoka M, Nagata D, Kihara S, Shimomura I, Kimura Y, Tabata Y, Saito Y, Nagai R, Sata M. Periadventitial adipose tissue plays a critical role in vascular remodeling. Circ Res. 2009;105:906–911. doi: 10.1161/CIRCRESAHA.109.199653. [DOI] [PubMed] [Google Scholar]
- 16.Tieu BC, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, Brasier AR. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayranpaa MI, Trosien JA, Fontaine V, Folkesson M, Kazi M, Eriksson P, Swedenborg J, Hedin U. Mast cells associate with neovessels in the media and adventitia of abdominal aortic aneurysms. J Vasc Surg. 2009;50:388–395. doi: 10.1016/j.jvs.2009.03.055. discussion 395-386. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Zalewski A, Liu Y, Mazurek T, Cowan S, Martin JL, Hofmann SM, Vlassara H, Shi Y. Diabetes-induced oxidative stress and low-grade inflammation in porcine coronary arteries. Circulation. 2003;108:472–478. doi: 10.1161/01.CIR.0000080378.96063.23. [DOI] [PubMed] [Google Scholar]
- 19.Micheletti RG, Fishbein GA, Currier JS, Singer EJ, Fishbein MC. Calcification of the internal elastic lamina of coronary arteries. Mod Pathol. 2008;21:1019–1028. doi: 10.1038/modpathol.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miki K, Yuri T, Takeda N, Takehana K, Iwasaka T, Tsubura A. An autopsy case of pseudoxanthoma elasticum: histochemical characteristics. Med Mol Morphol. 2007;40:172–177. doi: 10.1007/s00795-007-0368-5. [DOI] [PubMed] [Google Scholar]
- 21.Mendelsohn G, Bulkley BH, Hutchins GM. Cardiovascular manifestations of Pseudoxanthoma elasticum. Arch Pathol Lab Med. 1978;102:298–302. [PubMed] [Google Scholar]
- 22.Truter S, Rosenbaum-Fiedler J, Sapadin A, Lebwohl M. Calcification of elastic fibers in pseudoxanthoma elasticum. Mt Sinai J Med. 1996;63:210–215. [PubMed] [Google Scholar]
- 23.Rayssiguier Y. Role of magnesium and potassium in the pathogenesis of arteriosclerosis. Magnesium. 1984;3:226–238. [PubMed] [Google Scholar]
- 24.Nakamura S, Ishibashi-Ueda H, Niizuma S, Yoshihara F, Horio T, Kawano Y. Coronary calcification in patients with chronic kidney disease and coronary artery disease. Clin J Am Soc Nephrol. 2009;4:1892–1900. doi: 10.2215/CJN.04320709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bostrom K. Proinflammatory vascular calcification. Circ Res. 2005;96:1219–1220. doi: 10.1161/01.RES.0000172407.20974.e5. [DOI] [PubMed] [Google Scholar]
- 26.Nadra I, Mason JC, Philippidis P, Florey O, Smythe CD, McCarthy GM, Landis RC, Haskard DO. Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase C and MAP kinase pathways: a vicious cycle of inflammation and arterial calcification? Circ Res. 2005;96:1248–1256. doi: 10.1161/01.RES.0000171451.88616.c2. [DOI] [PubMed] [Google Scholar]
- 27.Nadra I, Boccaccini AR, Philippidis P, Whelan LC, McCarthy GM, Haskard DO, Landis RC. Effect of particle size on hydroxyapatite crystal-induced tumor necrosis factor alpha secretion by macrophages. Atherosclerosis. 2008;196:98–105. doi: 10.1016/j.atherosclerosis.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Morony S, Tintut Y, Zhang Z, Cattley RC, Van G, Dwyer D, Stolina M, Kostenuik PJ, Demer LL. Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(-/-) mice. Circulation. 2008;117:411–420. doi: 10.1161/CIRCULATIONAHA.107.707380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Aly Z, Shao JS, Lai CF, Huang E, Cai J, Behrmann A, Cheng SL, Towler DA. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr-/-mice. Arterioscler Thromb Vasc Biol. 2007;27:2589–2596. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 30.Yao Y, Watson AD, Ji S, Bostrom KI. Heat shock protein 70 enhances vascular bone morphogenetic protein-4 signaling by binding matrix Gla protein. Circ Res. 2009;105:575–584. doi: 10.1161/CIRCRESAHA.109.202333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennett BJ, Scatena M, Kirk EA, Rattazzi M, Varon RM, Averill M, Schwartz SM, Giachelli CM, Rosenfeld ME. Osteoprotegerin inactivation accelerates advanced atherosclerotic lesion progression and calcification in older ApoE-/- mice. Arterioscler Thromb Vasc Biol. 2006;26:2117–2124. doi: 10.1161/01.ATV.0000236428.91125.e6. [DOI] [PubMed] [Google Scholar]
- 32.Helas S, Goettsch C, Schoppet M, Zeitz U, Hempel U, Morawietz H, Kostenuik PJ, Erben RG, Hofbauer LC. Inhibition of receptor activator of NF-kappaB ligand by denosumab attenuates vascular calcium deposition in mice. Am J Pathol. 2009;175:473–478. doi: 10.2353/ajpath.2009.080957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schreyer SA, Vick C, Lystig TC, Mystkowski P, LeBoeuf RC. LDL receptor but not apolipoprotein E deficiency increases diet-induced obesity and diabetes in mice. Am J Physiol Endocrinol Metab. 2002;282:E207–214. doi: 10.1152/ajpendo.2002.282.1.E207. [DOI] [PubMed] [Google Scholar]
- 34.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collin-Osdoby P. Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ Res. 2004;95:1046–1057. doi: 10.1161/01.RES.0000149165.99974.12. [DOI] [PubMed] [Google Scholar]
- 36.Panizo S, Cardus A, Encinas M, Parisi E, Valcheva P, Lopez-Ongil S, Coll B, Fernandez E, Valdivielso JM. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ Res. 2009;104:1041–1048. doi: 10.1161/CIRCRESAHA.108.189001. [DOI] [PubMed] [Google Scholar]
- 37.Kaden JJ, Bickelhaupt S, Grobholz R, Haase KK, Sarikoc A, Kilic R, Brueckmann M, Lang S, Zahn I, Vahl C, Hagl S, Dempfle CE, Borggrefe M. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulate aortic valve calcification. J Mol Cell Cardiol. 2004;36:57–66. doi: 10.1016/j.yjmcc.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 38.Min H, Morony S, Sarosi I, Dunstan CR, Capparelli C, Scully S, Van G, Kaufman S, Kostenuik PJ, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med. 2000;192:463–474. doi: 10.1084/jem.192.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shao JS, Cheng SL, Charlton-Kachigian N, Loewy AP, Towler DA. Teriparatide (human parathyroid hormone (1-34)) inhibits osteogenic vascular calcification in diabetic low density lipoprotein receptor-deficient mice. J Biol Chem. 2003;278:50195–50202. doi: 10.1074/jbc.M308825200. [DOI] [PubMed] [Google Scholar]
- 40.Browner WS, Lui LY, Cummings SR. Associations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women. J Clin Endocrinol Metab. 2001;86:631–637. doi: 10.1210/jcem.86.2.7192. [DOI] [PubMed] [Google Scholar]
- 41.Terekeci HM, Senol MG, Top C, Sahan B, Celik S, Sayan O, Kucukardali Y, Ipcioglu O, Cagiltay E, Oktenli C, Ozata M. Plasma osteoprotegerin concentrations in type 2 diabetic patients and its association with neuropathy. Exp Clin Endocrinol Diabetes. 2009;117:119–123. doi: 10.1055/s-0028-1085425. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, Fu M, Myles D, Zhu X, Du J, Cao X, Chen YE. PDGF induces osteoprotegerin expression in vascular smooth muscle cells by multiple signal pathways. FEBS Lett. 2002;521:180–184. doi: 10.1016/s0014-5793(02)02872-7. [DOI] [PubMed] [Google Scholar]
- 43.Ovchinnikova O, Gylfe A, Bailey L, Nordstrom A, Rudling M, Jung C, Bergstrom S, Waldenstrom A, Hansson GK, Nordstrom P. Osteoprotegerin promotes fibrous cap formation in atherosclerotic lesions of ApoE-deficient mice--brief report. Arterioscler Thromb Vasc Biol. 2009;29:1478–1480. doi: 10.1161/ATVBAHA.109.188185. [DOI] [PubMed] [Google Scholar]
- 44.Towler DA, Bidder M, Latifi T, Coleman T, Semenkovich CF. Diet-induced diabetes activates an osteogenic gene regulatory program in the aortas of low density lipoprotein receptor-deficient mice. J Biol Chem. 1998;273:30427–30434. doi: 10.1074/jbc.273.46.30427. [DOI] [PubMed] [Google Scholar]
- 45.Katz R, Budoff MJ, Takasu J, Shavelle DM, Bertoni A, Blumenthal RS, Ouyang P, Wong ND, O'Brien KD. Relationship of metabolic syndrome with incident aortic valve calcium and aortic valve calcium progression: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes. 2009;58:813–819. doi: 10.2337/db08-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katz R, Wong ND, Kronmal R, Takasu J, Shavelle DM, Probstfield JL, Bertoni AG, Budoff MJ, O'Brien KD. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Circulation. 2006;113:2113–2119. doi: 10.1161/CIRCULATIONAHA.105.598086. [DOI] [PubMed] [Google Scholar]
- 47.Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, Lichtman AH. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J Clin Invest. 2007;117:2974–2982. doi: 10.1172/JCI31344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mishima Y, Kuyama A, Tada A, Takahashi K, Ishioka T, Kibata M. Relationship between serum tumor necrosis factor-alpha and insulin resistance in obese men with Type 2 diabetes mellitus. Diabetes Res Clin Pract. 2001;52:119–123. doi: 10.1016/s0168-8227(00)00247-3. [DOI] [PubMed] [Google Scholar]
- 49.Katsuki A, Sumida Y, Murashima S, Murata K, Takarada Y, Ito K, Fujii M, Tsuchihashi K, Goto H, Nakatani K, Yano Y. Serum levels of tumor necrosis factor-alpha are increased in obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1998;83:859–862. doi: 10.1210/jcem.83.3.4618. [DOI] [PubMed] [Google Scholar]
- 50.Mavridis G, Souliou E, Diza E, Symeonidis G, Pastore F, Vassiliou AM, Karamitsos D. Inflammatory cytokines in insulin-treated patients with type 2 diabetes. Nutr Metab Cardiovasc Dis. 2008;18:471–476. doi: 10.1016/j.numecd.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 51.Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 52.Nilsson-Ohman J, Fredrikson GN, Nilsson-Berglund LM, Gustavsson C, Bengtsson E, Smith ML, Agardh CD, Agardh E, Jovinge S, Gomez MF, Nilsson J. Tumor necrosis factor-alpha does not mediate diabetes-induced vascular inflammation in mice. Arterioscler Thromb Vasc Biol. 2009;29:1465–1470. doi: 10.1161/ATVBAHA.109.193862. [DOI] [PubMed] [Google Scholar]
- 53.de Bont N, Netea MG, Demacker PN, Verschueren I, Kullberg BJ, van Dijk KW, van der Meer JW, Stalenhoef AF. Apolipoprotein E knock-out mice are highly susceptible to endotoxemia and Klebsiella pneumoniae infection. J Lipid Res. 1999;40:680–685. [PubMed] [Google Scholar]
- 54.Qiao JH, Fishbein MC, Demer LL, Lusis AJ. Genetic determination of cartilaginous metaplasia in mouse aorta. Arterioscler Thromb Vasc Biol. 1995;15:2265–2272. doi: 10.1161/01.atv.15.12.2265. [DOI] [PubMed] [Google Scholar]
- 55.Tanimura A, McGregor DH, Anderson HC. Calcification in atherosclerosis. I. Human studies. J Exp Pathol. 1986;2:261–273. [PubMed] [Google Scholar]
- 56.Hsu HH, Tawfik O, Sun F. Mechanism of dystrophic calcification in rabbit aortas: temporal and spatial distributions of calcifying vesicles and calcification-related structural proteins. Cardiovasc Pathol. 2004;13:3–10. doi: 10.1016/S1054-8807(03)00093-0. [DOI] [PubMed] [Google Scholar]
- 57.Puchtler H, Meloan SN, Terry MS. On the history and mechanism of alizarin and alizarin red S stains for calcium. J Histochem Cytochem. 1969;17:110–124. doi: 10.1177/17.2.110. [DOI] [PubMed] [Google Scholar]
- 58.de Nooijer R, Verkleij CJ, von der Thusen JH, Jukema JW, van der Wall EE, van Berkel TJ, Baker AH, Biessen EA. Lesional overexpression of matrix metalloproteinase-9 promotes intraplaque hemorrhage in advanced lesions but not at earlier stages of atherogenesis. Arterioscler Thromb Vasc Biol. 2006;26:340–346. doi: 10.1161/01.ATV.0000197795.56960.64. [DOI] [PubMed] [Google Scholar]
- 59.Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 2006;116:59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson JL, George SJ, Newby AC, Jackson CL. Divergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proc Natl Acad Sci U S A. 2005;102:15575–15580. doi: 10.1073/pnas.0506201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tintut Y, Demer L. Role of osteoprotegerin and its ligands and competing receptors in atherosclerotic calcification. J Investig Med. 2006;54:395–401. doi: 10.2310/6650.2006.06019. [DOI] [PubMed] [Google Scholar]
- 62.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 63.Everhart JE, Pettitt DJ, Knowler WC, Rose FA, Bennett PH. Medial arterial calcification and its association with mortality and complications of diabetes. Diabetologia. 1988;31:16–23. doi: 10.1007/BF00279127. [DOI] [PubMed] [Google Scholar]
- 64.Nelson RG, Gohdes DM, Everhart JE, Hartner JA, Zwemer FL, Pettitt DJ, Knowler WC. Lower-extremity amputations in NIDDM. 12-yr follow-up study in Pima Indians. Diabetes Care. 1988;11:8–16. doi: 10.2337/diacare.11.1.8. [DOI] [PubMed] [Google Scholar]
- 65.Lehto S, Niskanen L, Suhonen M, Ronnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 66.Leskinen Y, Salenius JP, Lehtimaki T, Huhtala H, Saha H. The prevalence of peripheral arterial disease and medial arterial calcification in patients with chronic renal failure: requirements for diagnostics. Am J Kidney Dis. 2002;40:472–479. doi: 10.1053/ajkd.2002.34885. [DOI] [PubMed] [Google Scholar]
- 67.Greenwald SE. Ageing of the conduit arteries. J Pathol. 2007;211:157–172. doi: 10.1002/path.2268. [DOI] [PubMed] [Google Scholar]
- 68.Westerhof N, Lankhaar JW, Westerhof BE. The arterial Windkessel. Med Biol Eng Comput. 2009;47:131–141. doi: 10.1007/s11517-008-0359-2. [DOI] [PubMed] [Google Scholar]
- 69.O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 70.Yoshimura T, Suzuki E, Ito I, Sakaguchi M, Uzu T, Nishio Y, Maegawa H, Morikawa S, Inubushi T, Hisatomi A, Fujimoto K, Takeda J, Kashiwagi A. Impaired peripheral circulation in lower-leg arteries caused by higher arterial stiffness and greater vascular resistance associates with nephropathy in type 2 diabetic patients with normal ankle-brachial indices. Diabetes Res Clin Pract. 2008;80:416–423. doi: 10.1016/j.diabres.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 71.Mohler ER., 3rd Therapy insight: peripheral arterial disease and diabetes--from pathogenesis to treatment guidelines. Nat Clin Pract Cardiovasc Med. 2007;4:151–162. doi: 10.1038/ncpcardio0823. [DOI] [PubMed] [Google Scholar]
- 72.Halperin JL. Evaluation of patients with peripheral vascular disease. Thromb Res. 2002;106:V303–311. doi: 10.1016/s0049-3848(01)00366-8. [DOI] [PubMed] [Google Scholar]
- 73.Guzman RJ, Brinkley DM, Schumacher PM, Donahue RM, Beavers H, Qin X. Tibial artery calcification as a marker of amputation risk in patients with peripheral arterial disease. J Am Coll Cardiol. 2008;51:1967–1974. doi: 10.1016/j.jacc.2007.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soor GS, Vukin I, Leong SW, Oreopoulos G, Butany J. Peripheral vascular disease: who gets it and why? A histomorphological analysis of 261 arterial segments from 58 cases. Pathology. 2008;40:385–391. doi: 10.1080/00313020802036764. [DOI] [PubMed] [Google Scholar]
- 75.Alnaeb ME, Alobaid N, Seifalian AM, Mikhailidis DP, Hamilton G. Statins and peripheral arterial disease: potential mechanisms and clinical benefits. Ann Vasc Surg. 2006;20:696–705. doi: 10.1007/s10016-006-9104-1. [DOI] [PubMed] [Google Scholar]
- 76.Semaan E, Hamburg N, Nasr W, Shaw P, Eberhardt R, Woodson J, Doros G, Rybin D, Farber A. Endovascular Management of the Popliteal Artery: Comparison of Atherectomy and Angioplasty. Vasc Endovascular Surg. 2009 doi: 10.1177/1538574409345028. E-pub ahead of print. available at http://ves.sagepub.com/cgi/rapidpdf/1538574409345028v1. [DOI] [PMC free article] [PubMed]
- 77.Keeling AN, Khalidi K, Leong S, Wang TT, Ayyoub AS, McGrath FP, Athanasiou T, Lee MJ. Below knee angioplasty in elderly patients: Predictors of major adverse clinical outcomes. Eur J Radiol. 2009 doi: 10.1016/j.ejrad.2009.08.011. E-pub ahead of print. available at http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T6F-4X7YX8W-1&_user=10&_rdoc=1&_fmt=&_orig=search&_sort=d&_docanchor=&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=434e668d557e857159ce95af013d9bed. [DOI] [PubMed]
- 78.Dick P, Mlekusch W, Sabeti S, Amighi J, Schlager O, Haumer M, Minar E, Schillinger M. Outcome after endovascular treatment of deep femoral artery stenosis: results in a consecutive patient series and systematic review of the literature. J Endovasc Ther. 2006;13:221–228. doi: 10.1583/05-1766R.1. [DOI] [PubMed] [Google Scholar]
- 79.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. Jama. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 80.Belch J, MacCuish A, Campbell I, Cobbe S, Taylor R, Prescott R, Lee R, Bancroft J, MacEwan S, Shepherd J, Macfarlane P, Morris A, Jung R, Kelly C, Connacher A, Peden N, Jamieson A, Matthews D, Leese G, McKnight J, O'Brien I, Semple C, Petrie J, Gordon D, Pringle S, MacWalter R. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. Bmj. 2008;337:a1840. doi: 10.1136/bmj.a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paraskevas KI, Baker DM, Pompella A, Mikhailidis DP. Does diabetes mellitus play a role in restenosis and patency rates following lower extremity peripheral arterial revascularization? A critical overview. Ann Vasc Surg. 2008;22:481–491. doi: 10.1016/j.avsg.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 82.Brooks B, Dean R, Patel S, Wu B, Molyneaux L, Yue DK. TBI or not TBI: that is the question. Is it better to measure toe pressure than ankle pressure in diabetic patients? Diabet Med. 2001;18:528–532. doi: 10.1046/j.1464-5491.2001.00493.x. [DOI] [PubMed] [Google Scholar]
- 83.Wattanakit K, Folsom AR, Duprez DA, Weatherley BD, Hirsch AT. Clinical significance of a high ankle-brachial index: insights from the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 2007;190:459–464. doi: 10.1016/j.atherosclerosis.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 84.Silvestro A, Diehm N, Savolainen H, Do DD, Vogelea J, Mahler F, Zwicky S, Baumgartner I. Falsely high ankle-brachial index predicts major amputation in critical limb ischemia. Vasc Med. 2006;11:69–74. doi: 10.1191/1358863x06vm678oa. [DOI] [PubMed] [Google Scholar]
- 85.Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005;132:49–60. doi: 10.1242/dev.01564. [DOI] [PubMed] [Google Scholar]
- 86.Tyson KL, Reynolds JL, McNair R, Zhang Q, Weissberg PL, Shanahan CM. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol. 2003;23:489–494. doi: 10.1161/01.ATV.0000059406.92165.31. [DOI] [PubMed] [Google Scholar]
- 87.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol. 2008;52:843–850. doi: 10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Caira FC, Stock SR, Gleason TG, McGee EC, Huang J, Bonow RO, Spelsberg TC, McCarthy PM, Rahimtoola SH, Rajamannan NM. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol. 2006;47:1707–1712. doi: 10.1016/j.jacc.2006.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koleganova N, Piecha G, Ritz E, Schirmacher P, Muller A, Meyer HP, Gross ML. Arterial calcification in patients with chronic kidney disease. Nephrol Dial Transplant. 2009;24:2488–2496. doi: 10.1093/ndt/gfp137. [DOI] [PubMed] [Google Scholar]
- 91.Trayhurn P, Wood IS. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans. 2005;33:1078–1081. doi: 10.1042/BST0331078. [DOI] [PubMed] [Google Scholar]
- 92.Schulze MB, Rimm EB, Shai I, Rifai N, Hu FB. Relationship between adiponectin and glycemic control, blood lipids, and inflammatory markers in men with type 2 diabetes. Diabetes Care. 2004;27:1680–1687. doi: 10.2337/diacare.27.7.1680. [DOI] [PubMed] [Google Scholar]
- 93.Bassaganya-Riera J, Misyak S, Guri AJ, Hontecillas R. PPAR gamma is highly expressed in F4/80(hi) adipose tissue macrophages and dampens adipose-tissue inflammation. Cell Immunol. 2009;258:138–146. doi: 10.1016/j.cellimm.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol. 2005;25:2062–2068. doi: 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- 95.Tintut Y, Patel J, Territo M, Saini T, Parhami F, Demer LL. Monocyte/macrophage regulation of vascular calcification in vitro. Circulation. 2002;105:650–655. doi: 10.1161/hc0502.102969. [DOI] [PubMed] [Google Scholar]
- 96.Lee HL, Woo KM, Ryoo HM, Baek JH. Tumor necrosis factor-alpha increases alkaline phosphatase expression in vascular smooth muscle cells via MSX2 induction. Biochem Biophys Res Commun. 2009 doi: 10.1016/j.bbrc.2009.12.027. E-pub ahead of print. available at http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WBK-4XWMN99-4&_user=10&_rdoc=1&_fmt=&_orig=search&_sort=d&_docanchor=&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=f4fdf323ff5434ff0872eaf54aadba10. [DOI] [PubMed]
- 97.Mizobuchi M, Towler D, Slatopolsky E. Vascular calcification: the killer of patients with chronic kidney disease. J Am Soc Nephrol. 2009;20:1453–1464. doi: 10.1681/ASN.2008070692. [DOI] [PubMed] [Google Scholar]
- 98.Tse J, Martin-McNaulty B, Halks-Miller M, Kauser K, DelVecchio V, Vergona R, Sullivan ME, Rubanyi GM. Accelerated atherosclerosis and premature calcified cartilaginous metaplasia in the aorta of diabetic male Apo E knockout mice can be prevented by chronic treatment with 17 beta-estradiol. Atherosclerosis. 1999;144:303–313. doi: 10.1016/s0021-9150(98)00325-6. [DOI] [PubMed] [Google Scholar]
- 99.Hruska KA, Mathew S, Lund RJ, Memon I, Saab G. The pathogenesis of vascular calcification in the chronic kidney disease mineral bone disorder: the links between bone and the vasculature. Semin Nephrol. 2009;29:156–165. doi: 10.1016/j.semnephrol.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hayden MR, Goldsmith D, Sowers JR, Khanna R. Calciphylaxis: calcific uremic arteriolopathy and the emerging role of sodium thiosulfate. Int Urol Nephrol. 2008;40:443–451. doi: 10.1007/s11255-008-9373-4. [DOI] [PubMed] [Google Scholar]
- 101.O'Neill WC. The fallacy of the calcium-phosphorus product. Kidney Int. 2007;72:792–796. doi: 10.1038/sj.ki.5002412. [DOI] [PubMed] [Google Scholar]
- 102.Rochette CN, Rosenfeldt S, Heiss A, Narayanan T, Ballauff M, Jahnen-Dechent W. A shielding topology stabilizes the early stage protein-mineral complexes of fetuin-A and calcium phosphate: a time-resolved small-angle X-ray study. Chembiochem. 2009;10:735–740. doi: 10.1002/cbic.200800719. [DOI] [PubMed] [Google Scholar]
- 103.Heiss A, Eckert T, Aretz A, Richtering W, van Dorp W, Schafer C, Jahnen-Dechent W. Hierarchical role of fetuin-A and acidic serum proteins in the formation and stabilization of calcium phosphate particles. J Biol Chem. 2008;283:14815–14825. doi: 10.1074/jbc.M709938200. [DOI] [PubMed] [Google Scholar]
- 104.Heiss A, DuChesne A, Denecke B, Grotzinger J, Yamamoto K, Renne T, Jahnen-Dechent W. Structural basis of calcification inhibition by alpha 2-HS glycoprotein/fetuin-A. Formation of colloidal calciprotein particles. J Biol Chem. 2003;278:13333–13341. doi: 10.1074/jbc.M210868200. [DOI] [PubMed] [Google Scholar]
- 105.Schinke T, Amendt C, Trindl A, Poschke O, Muller-Esterl W, Jahnen-Dechent W. The serum protein alpha2-HS glycoprotein/fetuin inhibits apatite formation in vitro and in mineralizing calvaria cells. A possible role in mineralization and calcium homeostasis. J Biol Chem. 1996;271:20789–20796. doi: 10.1074/jbc.271.34.20789. [DOI] [PubMed] [Google Scholar]
- 106.Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T, Jahnen-Dechent W. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357–366. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reynolds JL, Skepper JN, McNair R, Kasama T, Gupta K, Weissberg PL, Jahnen-Dechent W, Shanahan CM. Multifunctional roles for serum protein fetuin-a in inhibition of human vascular smooth muscle cell calcification. J Am Soc Nephrol. 2005;16:2920–2930. doi: 10.1681/ASN.2004100895. [DOI] [PubMed] [Google Scholar]
- 108.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–2867. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 109.Woltje M, Tschoke B, von Bulow V, Westenfeld R, Denecke B, Graber S, Jahnen-Dechent W. CCAAT enhancer binding protein beta and hepatocyte nuclear factor 3beta are necessary and sufficient to mediate dexamethasone-induced up-regulation of alpha2HS-glycoprotein/fetuin-A gene expression. J Mol Endocrinol. 2006;36:261–277. doi: 10.1677/jme.1.02001. [DOI] [PubMed] [Google Scholar]
- 110.Gangneux C, Daveau M, Hiron M, Derambure C, Papaconstantinou J, Salier JP. The inflammation-induced down-regulation of plasma Fetuin-A (alpha2HS-Glycoprotein) in liver results from the loss of interaction between long C/EBP isoforms at two neighbouring binding sites. Nucleic Acids Res. 2003;31:5957–5970. doi: 10.1093/nar/gkg788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, Himmelfarb J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009–1016. doi: 10.1111/j.1523-1755.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- 112.Zheng S, de Las Fuentes L, Bierhals A, Ash-Bernal R, Spence K, Slatopolsky E, Davila-Roman VG, Delmez J. Relation of serum fetuin-A levels to coronary artery calcium in African-American patients on chronic hemodialysis. Am J Cardiol. 2009;103:46–49. doi: 10.1016/j.amjcard.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kaden JJ, Reinohl JO, Blesch B, Brueckmann M, Haghi D, Borggrefe M, Schmitz F, Klomfass S, Pillich M, Ortlepp JR. Systemic and local levels of fetuin-A in calcific aortic valve stenosis. Int J Mol Med. 2007;20:193–197. [PubMed] [Google Scholar]
- 114.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 115.Metry G, Stenvinkel P, Qureshi AR, Carrero JJ, Yilmaz MI, Barany P, Snaedal S, Heimburger O, Lindholm B, Suliman ME. Low serum fetuin-A concentration predicts poor outcome only in the presence of inflammation in prevalent haemodialysis patients. Eur J Clin Invest. 2008;38:804–811. doi: 10.1111/j.1365-2362.2008.02032.x. [DOI] [PubMed] [Google Scholar]
- 116.Li X, Giachelli CM. Sodium-dependent phosphate cotransporters and vascular calcification. Curr Opin Nephrol Hypertens. 2007;16:325–328. doi: 10.1097/MNH.0b013e3281c55ef1. [DOI] [PubMed] [Google Scholar]
- 117.Li X, Yang HY, Giachelli CM. BMP-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis. 2008;199:271–277. doi: 10.1016/j.atherosclerosis.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Davies MR, Lund RJ, Mathew S, Hruska KA. Low turnover osteodystrophy and vascular calcification are amenable to skeletal anabolism in an animal model of chronic kidney disease and the metabolic syndrome. J Am Soc Nephrol. 2005;16:917–928. doi: 10.1681/ASN.2004100835. [DOI] [PubMed] [Google Scholar]
- 119.Hruska KA, Mathew S, Saab G. Bone morphogenetic proteins in vascular calcification. Circ Res. 2005;97:105–114. doi: 10.1161/01.RES.00000175571.53833.6c. [DOI] [PubMed] [Google Scholar]
- 120.Towler DA. Vascular Calcification: A Perspective On An Imminent Disease Epidemic. IBMS BoneKEy. 2008;5:41–58. doi: 10.1138/20080298. [DOI] [Google Scholar]
- 121.Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283:15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dodig M, Tadic T, Kronenberg MS, Dacic S, Liu YH, Maxson R, Rowe DW, Lichtler AC. Ectopic Msx2 overexpression inhibits and Msx2 antisense stimulates calvarial osteoblast differentiation. Dev Biol. 1999;209:298–307. doi: 10.1006/dbio.1999.9258. [DOI] [PubMed] [Google Scholar]
- 123.Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, Maeda T, Takano Y, Uchiyama M, Heaney S, Peters H, Tang Z, Maxson R, Maas R. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 2000;24:391–395. doi: 10.1038/74231. [DOI] [PubMed] [Google Scholar]
- 124.Shao JS, Aly ZA, Lai CF, Cheng SL, Cai J, Huang E, Behrmann A, Towler DA. Vascular Bmp Msx2 Wnt signaling and oxidative stress in arterial calcification. Ann N Y Acad Sci. 2007;1117:40–50. doi: 10.1196/annals.1402.075. [DOI] [PubMed] [Google Scholar]
- 125.Lai Huang C, Huang A, Behrmann A, Cheng SL, Towler DA. Activation of Msx2-Wnt Signaling By TNF-alpha Requires Peroxide Derived From NADPH Oxidase and Mitochondrial Oxidative Metabolism. J Bone Miner Res. 2008;24 http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=28cc91f22-54de-4526-a4934-4527bc9437b4529e4528a.
- 126.Miller JD, Weiss RM, Serrano KM, Brooks RM, 2nd, Berry CJ, Zimmerman K, Young SG, Heistad DD. Lowering plasma cholesterol levels halts progression of aortic valve disease in mice. Circulation. 2009;119:2693–2701. doi: 10.1161/CIRCULATIONAHA.108.834614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, Berliner JA, Demer LL. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–687. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 128.Graham LS, Parhami F, Tintut Y, Kitchen CM, Demer LL, Effros RB. Oxidized lipids enhance RANKL production by T lymphocytes: Implications for lipid-induced bone loss. Clin Immunol. 2009;133:265–275. doi: 10.1016/j.clim.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–1429. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 130.Rawadi G, Vayssiere B, Dunn F, Baron R, Roman-Roman S. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res. 2003;18:1842–1853. doi: 10.1359/jbmr.2003.18.10.1842. [DOI] [PubMed] [Google Scholar]
- 131.Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–1809. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Csiszar A, Smith KE, Koller A, Kaley G, Edwards JG, Ungvari Z. Regulation of bone morphogenetic protein-2 expression in endothelial cells: role of nuclear factor-kappaB activation by tumor necrosis factor-alpha, H2O2, and high intravascular pressure. Circulation. 2005;111:2364–2372. doi: 10.1161/01.CIR.0000164201.40634.1D. [DOI] [PubMed] [Google Scholar]
- 133.Zebboudj AF, Shin V, Bostrom K. Matrix GLA protein and BMP-2 regulate osteoinduction in calcifying vascular cells. J Cell Biochem. 2003;90:756–765. doi: 10.1002/jcb.10669. [DOI] [PubMed] [Google Scholar]
- 134.Hadfield KD, Rock CF, Inkson CA, Dallas SL, Sudre L, Wallis GA, Boot-Handford RP, Canfield AE. HtrA1 inhibits mineral deposition by osteoblasts: requirement for the protease and PDZ domains. J Biol Chem. 2008;283:5928–5938. doi: 10.1074/jbc.M709299200. [DOI] [PubMed] [Google Scholar]
- 135.Kirton JP, Crofts NJ, George SJ, Brennan K, Canfield AE. Wnt/beta-catenin signaling stimulates chondrogenic and inhibits adipogenic differentiation of pericytes: potential relevance to vascular disease? Circ Res. 2007;101:581–589. doi: 10.1161/CIRCRESAHA.107.156372. [DOI] [PubMed] [Google Scholar]
- 136.Johnson ML, Rajamannan N. Diseases of Wnt signaling. Rev Endocr Metab Disord. 2006;7:41–49. doi: 10.1007/s11154-006-9003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Baron R, Rawadi G, Roman-Roman S. Wnt signaling: a key regulator of bone mass. Curr Top Dev Biol. 2006;76:103–127. doi: 10.1016/S0070-2153(06)76004-5. [DOI] [PubMed] [Google Scholar]
- 138.Goodwin AM, Sullivan KM, D'Amore PA. Cultured endothelial cells display endogenous activation of the canonical Wnt signaling pathway and express multiple ligands, receptors, and secreted modulators of Wnt signaling. Dev Dyn. 2006;235:3110–3120. doi: 10.1002/dvdy.20939. [DOI] [PubMed] [Google Scholar]
- 139.Johnson K, Polewski M, van Etten D, Terkeltaub R. Chondrogenesis mediated by PPi depletion promotes spontaneous aortic calcification in NPP1-/- mice. Arterioscler Thromb Vasc Biol. 2005;25:686–691. doi: 10.1161/01.ATV.0000154774.71187.f0. [DOI] [PubMed] [Google Scholar]
- 140.Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302–2309. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- 141.Aikawa E, Aikawa M, Libby P, Figueiredo JL, Rusanescu G, Iwamoto Y, Fukuda D, Kohler RH, Shi GP, Jaffer FA, Weissleder R. Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease. Circulation. 2009;119:1785–1794. doi: 10.1161/CIRCULATIONAHA.108.827972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Price PA, Chan WS, Jolson DM, Williamson MK. The elastic lamellae of devitalized arteries calcify when incubated in serum: evidence for a serum calcification factor. Arterioscler Thromb Vasc Biol. 2006;26:1079–1085. doi: 10.1161/01.ATV.0000216406.44762.7c. [DOI] [PubMed] [Google Scholar]
- 143.Simionescu A, Simionescu DT, Vyavahare NR. Osteogenic responses in fibroblasts activated by elastin degradation products and transforming growth factor-beta1: role of myofibroblasts in vascular calcification. Am J Pathol. 2007;171:116–123. doi: 10.2353/ajpath.2007.060930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Qin X, Corriere MA, Matrisian LM, Guzman RJ. Matrix metalloproteinase inhibition attenuates aortic calcification. Arterioscler Thromb Vasc Biol. 2006;26:1510–1516. doi: 10.1161/01.ATV.0000225807.76419.a7. [DOI] [PubMed] [Google Scholar]
- 145.Yip CY, Chen JH, Zhao R, Simmons CA. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler Thromb Vasc Biol. 2009;29:936–942. doi: 10.1161/ATVBAHA.108.182394. [DOI] [PubMed] [Google Scholar]
- 146.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 147.Ruf N, Uhlenberg B, Terkeltaub R, Nurnberg P, Rutsch F. The mutational spectrum of ENPP1 as arising after the analysis of 23 unrelated patients with generalized arterial calcification of infancy (GACI) Hum Mutat. 2005;25:98. doi: 10.1002/humu.9297. [DOI] [PubMed] [Google Scholar]
- 148.Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, Terkeltaub R, Millan JL. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci U S A. 2002;99:9445–9449. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Harmey D, Hessle L, Narisawa S, Johnson KA, Terkeltaub R, Millan JL. Concerted regulation of inorganic pyrophosphate and osteopontin by akp2, enpp1, and ank: an integrated model of the pathogenesis of mineralization disorders. Am J Pathol. 2004;164:1199–1209. doi: 10.1016/S0002-9440(10)63208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yao Y, Shahbazian A, Bostrom KI. Proline and gamma-carboxylated glutamate residues in matrix Gla protein are critical for binding of bone morphogenetic protein-4. Circ Res. 2008;102:1065–1074. doi: 10.1161/CIRCRESAHA.107.166124. [DOI] [PubMed] [Google Scholar]
- 151.Eghbali-Fatourechi GZ, Modder UI, Charatcharoenwitthaya N, Sanyal A, Undale AH, Clowes JA, Tarara JE, Khosla S. Characterization of circulating osteoblast lineage cells in humans. Bone. 2007;40:1370–1377. doi: 10.1016/j.bone.2006.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gossl M, Modder UI, Atkinson EJ, Lerman A, Khosla S. Osteocalcin expression by circulating endothelial progenitor cells in patients with coronary atherosclerosis. J Am Coll Cardiol. 2008;52:1314–1325. doi: 10.1016/j.jacc.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Khosla S, Eghbali-Fatourechi GZ. Circulating cells with osteogenic potential. Ann N Y Acad Sci. 2006;1068:489–497. doi: 10.1196/annals.1346.022. [DOI] [PubMed] [Google Scholar]
- 154.Rajamannan NM, Bonow RO, Rahimtoola SH. Calcific aortic stenosis: an update. Nat Clin Pract Cardiovasc Med. 2007;4:254–262. doi: 10.1038/ncpcardio0827. [DOI] [PubMed] [Google Scholar]
- 155.Messika-Zeitoun D, Bielak LF, Peyser PA, Sheedy PF, Turner ST, Nkomo VT, Breen JF, Maalouf J, Scott C, Tajik AJ, Enriquez-Sarano M. Aortic valve calcification: determinants and progression in the population. Arterioscler Thromb Vasc Biol. 2007;27:642–648. doi: 10.1161/01.ATV.0000255952.47980.c2. [DOI] [PubMed] [Google Scholar]
- 156.Moura LM, Ramos SF, Zamorano JL, Barros IM, Azevedo LF, Rocha-Goncalves F, Rajamannan NM. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol. 2007;49:554–561. doi: 10.1016/j.jacc.2006.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shinohara K, Shoji T, Kimoto E, Yokoyama H, Fujiwara S, Hatsuda S, Maeno T, Fukumoto S, Emoto M, Koyama H, Nishizawa Y. Effect of atorvastatin on regional arterial stiffness in patients with type 2 diabetes mellitus. J Atheroscler Thromb. 2005;12:205–210. doi: 10.5551/jat.12.205. [DOI] [PubMed] [Google Scholar]
- 158.Alam MU, Kirton JP, Wilkinson FL, Towers E, Sinha S, Rouhi M, Vizard TN, Sage AP, Martin D, Ward DT, Alexander MY, Riccardi D, Canfield AE. Calcification is associated with loss of functional calcium-sensing receptor in vascular smooth muscle cells. Cardiovasc Res. 2009;81:260–268. doi: 10.1093/cvr/cvn279. [DOI] [PubMed] [Google Scholar]
- 159.Shroff RC, McNair R, Figg N, Skepper JN, Schurgers L, Gupta A, Hiorns M, Donald AE, Deanfield J, Rees L, Shanahan CM. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118:1748–1757. doi: 10.1161/CIRCULATIONAHA.108.783738. [DOI] [PubMed] [Google Scholar]
- 160.de Jesus Perez VA, Alastalo TP, Wu JC, Axelrod JD, Cooke JP, Amieva M, Rabinovitch M. Bone morphogenetic protein 2 induces pulmonary angiogenesis via Wnt-beta-catenin and Wnt-RhoA-Rac1 pathways. J Cell Biol. 2009;184:83–99. doi: 10.1083/jcb.200806049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Laumanns IP, Fink L, Wilhelm J, Wolff JC, Mitnacht-Kraus R, Graef-Hoechst S, Stein MM, Bohle RM, Klepetko W, Hoda MA, Schermuly RT, Grimminger F, Seeger W, Voswinckel R. The noncanonical WNT pathway is operative in idiopathic pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2009;40:683–691. doi: 10.1165/rcmb.2008-0153OC. [DOI] [PubMed] [Google Scholar]
- 162.Cohen ED, Ihida-Stansbury K, Lu MM, Panettieri RA, Jones PL, Morrisey EE. Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway. J Clin Invest. 2009;119:2538–2549. doi: 10.1172/JCI38079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Qu J, Zhou J, Yi XP, Dong B, Zheng H, Miller LM, Wang X, Schneider MD, Li F. Cardiac-specific haploinsufficiency of beta-catenin attenuates cardiac hypertrophy but enhances fetal gene expression in response to aortic constriction. J Mol Cell Cardiol. 2007;43:319–326. doi: 10.1016/j.yjmcc.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.van de Schans VA, van den Borne SW, Strzelecka AE, Janssen BJ, van der Velden JL, Langen RC, Wynshaw-Boris A, Smits JF, Blankesteijn WM. Interruption of Wnt signaling attenuates the onset of pressure overload-induced cardiac hypertrophy. Hypertension. 2007;49:473–480. doi: 10.1161/01.HYP.0000255946.55091.24. [DOI] [PubMed] [Google Scholar]
- 165.Essalihi R, Zandvliet ML, Moreau S, Gilbert LA, Bouvet C, Lenoel C, Nekka F, McKee MD, Moreau P. Distinct effects of amlodipine treatment on vascular elastocalcinosis and stiffness in a rat model of isolated systolic hypertension. J Hypertens. 2007;25:1879–1886. doi: 10.1097/HJH.0b013e328255e906. [DOI] [PubMed] [Google Scholar]
- 166.Essalihi R, Ouellette V, Dao HH, McKee MD, Moreau P. Phenotypic modulation of vascular smooth muscle cells during medial arterial calcification: a role for endothelin? J Cardiovasc Pharmacol. 2004;44 1:S147–150. doi: 10.1097/01.fjc.0000166250.81733.a5. [DOI] [PubMed] [Google Scholar]
- 167.Dao HH, Essalihi R, Graillon JF, Lariviere R, De Champlain J, Moreau P. Pharmacological prevention and regression of arterial remodeling in a rat model of isolated systolic hypertension. J Hypertens. 2002;20:1597–1606. doi: 10.1097/00004872-200208000-00023. [DOI] [PubMed] [Google Scholar]
- 168.Shao JS, Sierra OL, Cohen R, Kovacs A, Halstead LR, Towler DA. Vascular Calcification and Aortic Fibrosis: Bifunctional Role of Osteopontin in Diabetic Arteriosclerosis. J Bone Miner Res. 2009;24 doi: 10.1161/ATVBAHA.111.230011. Available at http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=acf98212-98279fc-98214a98213f-a98164-b98214e683190270. [DOI] [PMC free article] [PubMed]
- 169.Csiszar A, Lehoux S, Ungvari Z. Hemodynamic forces, vascular oxidative stress, and regulation of BMP-2/4 expression. Antioxid Redox Signal. 2009;11:1683–1697. doi: 10.1089/ars.2008.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mohler ER, 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 171.Tadros TM, Klein MD, Shapira OM. Ascending aortic dilatation associated with bicuspid aortic valve: pathophysiology, molecular biology, and clinical implications. Circulation. 2009;119:880–890. doi: 10.1161/CIRCULATIONAHA.108.795401. [DOI] [PubMed] [Google Scholar]
- 172.Goebel FD, Fuessl HS. Monckeberg's sclerosis after sympathetic denervation in diabetic and non-diabetic subjects. Diabetologia. 1983;24:347–350. doi: 10.1007/BF00251822. [DOI] [PubMed] [Google Scholar]
- 173.Allison MA, Cheung P, Criqui MH, Langer RD, Wright CM. Mitral and aortic annular calcification are highly associated with systemic calcified atherosclerosis. Circulation. 2006;113:861–866. doi: 10.1161/CIRCULATIONAHA.105.552844. [DOI] [PubMed] [Google Scholar]
- 174.Doherty TM, Fitzpatrick LA, Inoue D, Qiao JH, Fishbein MC, Detrano RC, Shah PK, Rajavashisth TB. Molecular, endocrine, and genetic mechanisms of arterial calcification. Endocr Rev. 2004;25:629–672. doi: 10.1210/er.2003-0015. [DOI] [PubMed] [Google Scholar]
- 175.Graham LS, Parhami F, Tintut Y, Kitchen CM, Demer LL, Effros RB. Oxidized lipids enhance RANKL production by T lymphocytes: implications for lipid-induced bone loss. Clin Immunol. 2009;133:265–275. doi: 10.1016/j.clim.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang S, Yiu KH, Mok MY, Ooi GC, Khong PL, Mak KF, Lau CP, Lam KF, Lau CS, Tse HF. Prevalence and extent of calcification over aorta, coronary and carotid arteries in patients with rheumatoid arthritis. J Intern Med. 2009;266:445–452. doi: 10.1111/j.1365-2796.2009.02123.x. [DOI] [PubMed] [Google Scholar]
- 177.Yiu KH, Wang S, Mok MY, Ooi GC, Khong PL, Mak KF, Lam KF, Lau CS, Tse HF. Pattern of arterial calcification in patients with systemic lupus erythematosus. J Rheumatol. 2009;36:2212–2217. doi: 10.3899/jrheum.090312. [DOI] [PubMed] [Google Scholar]
- 178.Pereira RM, Ribeiro GG, Bonfa E, Neto RS, Abe J, Caparbo VF, Borba EF, Lopes JB, Gebrim E. Premature coronary artery calcification is associated with disease duration and bone mineral density in young female systemic lupus erythematosus patients. Lupus. 2009 doi: 10.1177/0961203309345778. E-pub ahead of print. available at http://lup.sagepub.com/cgi/rapidpdf/0961203309345778v1. [DOI] [PubMed]
- 179.Villa-Bellosta R, Levi M, Sorribas V. Vascular smooth muscle cell calcification and SLC20 inorganic phosphate transporters: effects of PDGF, TNF-alpha, and Pi. Pflugers Arch. 2009;458:1151–1161. doi: 10.1007/s00424-009-0688-5. [DOI] [PubMed] [Google Scholar]
- 180.London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, de Vernejoul MC. Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol. 2004;15:1943–1951. doi: 10.1097/01.asn.0000129337.50739.48. [DOI] [PubMed] [Google Scholar]
- 181.Raggi P, James G, Burke SK, Bommer J, Chasan-Taber S, Holzer H, Braun J, Chertow GM. Decrease in thoracic vertebral bone attenuation with calcium-based phosphate binders in hemodialysis. J Bone Miner Res. 2005;20:764–772. doi: 10.1359/JBMR.041221. [DOI] [PubMed] [Google Scholar]
- 182.Janigan DT, Hirsch DJ, Klassen GA, MacDonald AS. Calcified subcutaneous arterioles with infarcts of the subcutis and skin (“calciphylaxis”) in chronic renal failure. Am J Kidney Dis. 2000;35:588–597. doi: 10.1016/s0272-6386(00)70003-5. [DOI] [PubMed] [Google Scholar]
- 183.Schlieper G, Brandenburg V, Ketteler M, Floege J. Sodium thiosulfate in the treatment of calcific uremic arteriolopathy. Nat Rev Nephrol. 2009;5:539–543. doi: 10.1038/nrneph.2009.99. [DOI] [PubMed] [Google Scholar]
- 184.Griethe W, Schmitt R, Jurgensen JS, Bachmann S, Eckardt KU, Schindler R. Bone morphogenic protein-4 expression in vascular lesions of calciphylaxis. J Nephrol. 2003;16:728–732. [PubMed] [Google Scholar]
- 185.Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 186.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ishibashi S, Goldstein JL, Brown MS, Herz J, Burns DK. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J Clin Invest. 1994;93:1885–1893. doi: 10.1172/JCI117179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Simolin MA, Pedersen TX, Bro S, Mayranpaa MI, Helske S, Nielsen LB, Kovanen PT. ACE inhibition attenuates uremia-induced aortic valve thickening in a novel mouse model. BMC Cardiovasc Disord. 2009;9:10. doi: 10.1186/1471-2261-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Weiss RM, Ohashi M, Miller JD, Young SG, Heistad DD. Calcific aortic valve stenosis in old hypercholesterolemic mice. Circulation. 2006;114:2065–2069. doi: 10.1161/CIRCULATIONAHA.106.634139. [DOI] [PubMed] [Google Scholar]
- 190.de Bont N, Netea MG, Demacker PN, Kullberg BJ, van der Meer JW, Stalenhoef AF. Apolipoprotein E-deficient mice have an impaired immune response to Klebsiella pneumoniae. Eur J Clin Invest. 2000;30:818–822. doi: 10.1046/j.1365-2362.2000.00715.x. [DOI] [PubMed] [Google Scholar]
- 191.Lanza-Jacoby S, Miller S, Jacob S, Heumann D, Minchenko AG, Flynn JT. Hyperlipoproteinemic low-density lipoprotein receptor-deficient mice are more susceptible to sepsis than corresponding wild-type mice. J Endotoxin Res. 2003;9:341–347. doi: 10.1179/096805103225002782. [DOI] [PubMed] [Google Scholar]
- 192.McRobb L, Handelsman DJ, Heather AK. Androgen-induced progression of arterial calcification in apolipoprotein E-null mice is uncoupled from plaque growth and lipid levels. Endocrinology. 2009;150:841–848. doi: 10.1210/en.2008-0760. [DOI] [PubMed] [Google Scholar]